Abstract
Protein quality control is vital for all living cells and sophisticated molecular mechanisms have evolved to prevent the excessive accumulation of unfolded proteins. High-temperature requirement A (HtrA) proteases have been identified as important ATP-independent quality-control factors in most species. HtrA proteins harbor a serine-protease domain and at least one peptide-binding PDZ domain to ensure efficient removal of misfolded or damaged proteins. One distinctive property of HtrAs is their ability to assemble into complex oligomers. Whereas all examined HtrAs are capable of forming pyramidal 3-mers, higher-order complexes consisting of up to 24 molecules have been reported. Tight control of chaperone and protease function is of pivotal importance in preventing deleterious HtrA-protease activity. In recent years, structural biology provided detailed insights into the molecular basis of the regulatory mechanisms, which include unique intramolecular allosteric signaling cascades and the dynamic switching of oligomeric states of HtrA proteins. Based on these results, functional models for many family members have been developed. The HtrA protein family represents a remarkable example of how structural and functional diversity is attained from the assembly of simple molecular building blocks.
Keywords: X-ray crystallography, Protein quality control, Oligomerization, PDZ domain, Molecular switch
Introduction
HtrA (high-temperature requirement A) proteins are oligomeric proteases well conserved in many species, including Gram-positive and Gram-negative prokaryotes, plants, and mammals [1, 2]. According to the MEROPS nomenclature, HtrA proteases belong to the S1B subfamily of the clan PA [3]. Prokaryotic HtrAs are involved in the heat-shock response and contribute to tolerance of harsh conditions such as elevated temperatures, extreme pH values, and increased oxidative or osmotic stress [4–7]. In this context, members of the HtrA family counteract protein-folding stress and play important roles in quality control of proteins of the periplasmatic compartment. Unlike other heat-shock proteins, HtrAs fulfill this function independent of ATP. In many pathogenic bacteria, HtrA proteins represent important virulence factors promoting intra- and extracellular survival in hostile environments [8]. In eukaryotes, HtrA proteins are involved in numerous cellular processes such as extracellular matrix remodeling, apoptosis, and protein quality control. Human HtrAs play roles in cell development and ageing processes and are believed to contribute to a number of severe medical conditions such as arthritis, cancer, Parkinson’s disease, and Alzheimer’s disease [2].
Despite this diverse set of functions, HtrAs share a common modular domain architecture comprising a chymotrypsin-type serine protease domain and one or two C-terminal PDZ domains (named after the three proteins PSD-95, DLG1, and ZO-1, for which this domain was first described) (Fig. 1a). Depending on cellular localization and functionality, signal sequences, transmembrane regions, and/or additional domains may be present at the N-terminus of HtrA proteins (Fig. 1a). During the last decade, a number of structures determined by X-ray crystallography and cryo-electron microscopy (cryo-EM) revealed that all examined HtrAs share the same basic oligomerization mode. All members of the family form homotrimers that are stabilized by extensive contacts between the three protease domains. Interestingly, for several HtrA proteins, the formation of higher-order oligomers (6-mers to 24-mers; Fig. 1b) has been reported [9–18]. These oligomers consist of multiple 3-meric building blocks. Together with more subtle allosteric control mechanisms, the assembly and disassembly of higher-order oligomers allows for precise regulation of protease activity. In contrast, HtrA proteins such as DegS and possibly HtrA2 from Mycobacterium tuberculosis do not form higher-order assemblies and regulation of proteolytic activity is achieved by different means [19, 20]. Integrating biochemical data on HtrAs accumulated over several decades, the recently published three-dimensional structures promoted the development of detailed functional models for many members of this fascinating protein family. In this review, we discuss the organization of HtrA proteins together with mechanistic and regulatory aspects, with a focus on family members with known three-dimensional structure.
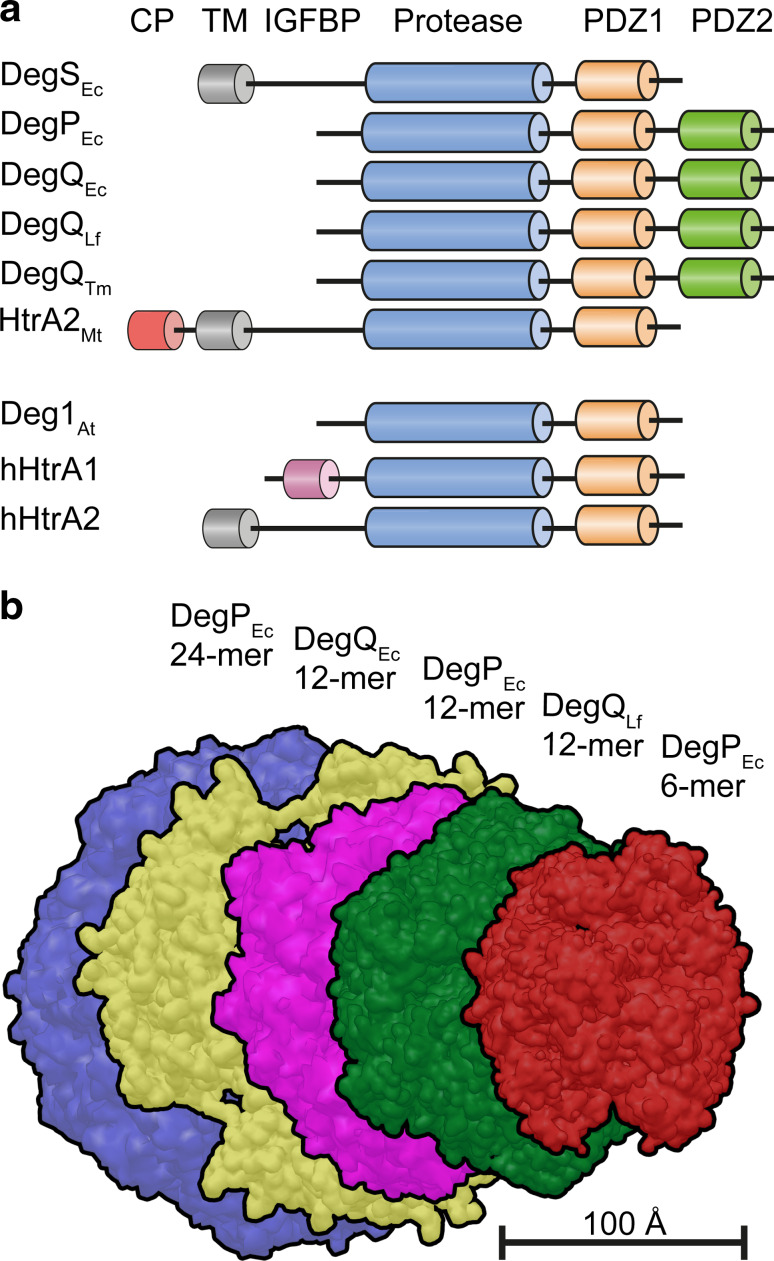
Fig. 1.
Domain organization and molecular assemblies of HtrA proteins. a Schematic representation of the distribution of domains in HtrA proteins discussed in this review. b Comparison of the overall dimensions of different higher-order HtrA particles. CP, cytoplasmic domain; TM, transmembrane domain; IGFBP, insulin-like growth-factor binding protein domain
Prokaryotic HtrAs
Over many years, research has been focused on HtrA homologues from Escherichia coli. In this species, three HtrA family members, DegS, DegP, and DegQ, have been identified [2, 21] and characterized. All three participate in protein quality control in the periplasmatic space. DegS is a tightly controlled sensory protease able to detect the accumulation of misfolded proteins and alert the cell [21]. DegP and DegQ are protective bifunctional protease-chaperones that refold or degrade accumulated misfolded proteins [9, 22, 23]. Although it has been demonstrated that HtrA proteins contribute to virulence in a number of bacterial species [24–30], in most cases detailed information on the molecular mechanisms is lacking. Only for HtrA proteins from E. coli, Legionella fallonii, Thermotoga maritima, and M. tuberculosis, biochemical as well as structural data (Fig. 2) are available, revealing a remarkable variation in overall organization, function, and regulation.
Fig. 2.
Three-dimensional structures of prokaryotic HtrA proteins. The proteins are shown in a surface representation with protease domain in blue, PDZ1 in orange, and PDZ2 in green. HtrA assemblies from top left to bottom right: DegS from E. coli (3-mer); DegQ from T. maritima (3-mer); HtrA2 from M. tuberculosis (3-mer); DegP from E. coli 6-mer, 12-mer, 24-mer; DegQ from L. fallonii (12-mer); DegQ from E. coli based on cryo-EM maps, 12-mer, 24-mer. The assemblies are not shown to scale relative to each other
DegS, a sensory protease triggering the σE stress-response pathway
In E. coli, DegS (DegSEc) acts as a folding-stress sensor anchored to the periplasmic face of the inner membrane. DegSEc includes an N-terminal transmembrane segment, a protease domain, and a single C-terminal PDZ domain (Fig. 1a). Upon accumulation of unfolded outer membrane proteins (OMPs) in the periplasm, DegSEc cleaves RseA, a negative regulator of the stress-response factor σE. RseA is a transmembrane protein with a cytoplasmic domain that binds σE and locks it in an inactive state. The cleavage of RseA by DegSEc triggers a proteolytic cascade that subsequently induces degradation of RseA by additional proteases. This in turn leads to the release of σE at the cytoplasmic face of the membrane and enables transcriptional activation of compartment-specific protein quality control genes including degP [31–33].
Recent experimental results suggest that in vivo, a second negative regulator, RseB, controls σE activation in addition to RseA. RseB effectively inhibits the DegSEc-mediated activation of the σE pathway by competing with DegSEc for RseA [34]. It has been proposed that under stress conditions, increased periplasmatic levels of lipopolysaccharide (LPS) or lipoprotein might relieve the inhibitory effect of RseB [34]. Whereas the latter mechanism has been discovered only very recently and many questions are still open, the activation of DegSEc by unfolded OMPs is well understood. Wild-type DegSEc, a number of engineered protein variants as well as DegSEc complexes with OMP-derived peptides have been structurally and biochemically characterized [19, 33, 35–38].
Like other HtrA proteins, DegSEc forms stable 3-mers (Fig. 2) supported by numerous interactions between protease domains of neighboring monomers [19]. All available crystal structures of DegSEc show an identical arrangement of the protease domains within the 3-mer with little variation in the position of the C-terminal PDZ domain with respect to the protease domain [36]. As the catalytic triad (His96, Asp126, and Ser201) is freely accessible, a tight regulation of proteolytic activity is of pivotal importance to prevent uncontrolled processing of RseA. Unligated structures of DegSEc display the protease in an inactive state, in which the oxyanion hole and the S1 specificity pocket are blocked by loop L1 and the catalytic triad is disrupted [19]. This inactive state is stabilized by interactions between PDZ and protease domain [37]. The binding of OMP-peptides to the PDZ domain leads to conformational changes in loop L3 and subsequently to a pronounced remodeling of L1, L2, and LD (Fig. 3a), enabling the active site to switch into a proteolytically competent ON state [19]. Intriguingly, very similar signaling cascades along PDZ → L3 → LD/L1/L2 are present in most, if not all, HtrA proteins. In the following, we will refer to this regulatory mechanism as allosteric activation cascade (AAC).
Fig. 3.
Allosteric activation cascade of HtrA proteins. a DegSEc monomer (gray) and protease domain of an adjacent monomer (blue) as observed in the DegSEc 3-mer. b DegPEc monomer (gray) and protease domain (brown) of an adjacent molecule taken from the DegPEc 24-mer. Activating peptides bound to the allosteric PDZ1 binding site are shown as sticks. Residues of the catalytic triad are shown in ball-and-stick representation (in b the catalytic Ser was replaced by Ala). Loops of the allosteric activation cascade are colored in red and the activated proteolytic sites are highlighted in green. Asterisks denote structural elements in neighboring DegSEc and DegPEc molecules in 3- and 24-mers, respectively
DegP, a protein quality control factor counteracting folding stress in the periplasm
DegP (protease Do) is a bifunctional protein with tightly regulated protease and chaperone activities, facilitating the degradation or refolding of misfolded periplasmatic proteins. Since the expression of degP is regulated by σE, the concerted action of DegS and DegP permits recognition and relief of periplasmatic protein-folding stress. DegP consists of an N-terminal protease domain and two PDZ domains (PDZ1 and PDZ2) (Fig. 1a). In E. coli, DegP (DegPEc) is responsible for maintaining periplasmatic protein homeostasis and thus is crucial for survival under stress conditions such as higher temperatures [39]. Under non-stress conditions, DegPEc participates in guiding unfolded OMPs through the periplasm before they are inserted into the outer membrane [40].
The first indication that DegP belongs to the family of self-compartmentizing proteases came from electron microscopy studies of DegPEc [41]. However, the limited resolution of the data did not allow an unambiguous determination of the oligomeric structure of DegPEc. Only after elucidation of higher-resolution structures by X-ray crystallography and cryo-EM, it became apparent that DegPEc is able to adopt multiple oligomeric states which determine the activity state of the protease domain [11, 14–16, 18]. In 2002, Clausen and coworkers presented the first DegPEc X-ray crystal structure, which revealed that the protein is able to form 6-meric assemblies consisting of two interlocked 3-mers arranged in a face-to-face manner [14] (Fig. 2). The 6-mer represents an inactive resting state of DegPEc, as in this assembly, all proteolytic sites are blocked by extended LA loops of opposite DegPEc monomers. As in DegSEc, each 3-mer displays extensive contacts between its protease domains, yet, the unique higher-order 6-mer is stabilized by a pillar-like arrangement of three pairs of intertwined LA loops. Two distinct DegPEc 6-mers adopting different conformations in the crystal suggest that in contrast to the protease domain, the positioning of the PDZ domains is flexible in this assembly [14]. Although the structure of the 6-mer supported the notion that DegPEc is a self-compartmentizing protease, it did not explain how refolding or degradation of substrate proteins are achieved. This puzzle was resolved in 2008, when two independent groups reported cryo-EM and X-ray structures of two large, cage-like particles consisting of 12 and 24 DegPEc molecules [15, 16]. Like 6-mers, these new oligomers consist of multiple 3-meric building blocks. However, the general shape of 6-mers is fundamentally different from 12- and 24-mers, which resemble hollow spheres with an outer diameter of approximately 160 and 195 Å, respectively (Figs. 1, 2). The protease-active sites line the inner wall of the particles and are accessible only from the interior. In 12- and 24-mers, PDZ1 and PDZ2 are integral parts of the protein shell and supply stability to the particle [15, 16]. The cage-like structures enclose internal cavities of considerable volume suitable to accommodate typical substrate proteins in an unfolded state. In fact, substrate molecules or peptide fragments have been detected in size-exclusion chromatography (SEC) fractions of 12- and 24-mers and in cryo-EM and X-ray crystal structures [11, 15, 16]. The inner cavity of the cage is connected to bulk solvent via lateral pores in the protein shell. The current model suggests that partially unfolded substrate proteins promote the dissociation of resting state DegPEc 6-mers into 3-mers, which then transiently assembly into proteolytically active 12- or 24-mers. Based on cryo-EM studies of DegPEc in the presence of lipid monolayers, it has been proposed that rapid formation of 12- or 24-mers might be influenced by bowl-shaped particles of 15 and 18 DegPEc molecules, which are associated with membrane lipids [18].
Similar to DegSEc, binding of substrate molecules to PDZ1 of DegPEc 12- and 24-mers triggers an AAC and activates the protease [42]. The activation signal is transmitted along PDZ → L3 → LD* → L1*/L2* to the protease site of an adjacent monomer of the particle (the asterisk denotes a contribution from a neighboring subunit) (Fig. 3b). In a series of well-designed Förster resonance energy transfer (FRET) experiments, Sauer and coworkers showed that after initial substrate binding, DegPEc 12-mers remain stable until all substrate molecules are depleted [11]. New substrate molecules enter the particle presumably through the lateral pores to gain access to the protease-active sites in the interior of the particle. These exciting results for the first time demonstrated that constant assembly and disassembly of DegP 12-mers is not necessary for efficient removal of misfolded proteins. Indeed, due to the restricted size of the pores, this mode of action also excludes chaperone-bound proteins and flexible loops of otherwise native proteins from entering the proteolytic cage.
In structures of DegPEc 12- or 24-mers, the LA loop is no longer blocking access to the active site and catalytic-triad residues adopt a protease-competent conformation. In consequence, substrate molecules encapsulated in the interior of the large oligomers are quickly degraded. As a prerequisite for efficient peptide-bond cleavage, a partially unfolded substrate molecule has to interact with the protease and the regulatory PDZ1 domain. This was recently corroborated by the X-ray crystal structure of a DegPEc 12-mer in complex with a lysozyme fragment of 41 amino-acid residues, which promotes the formation of higher-order DegPEc oligomers in solution [11]. Indeed, in this structure, the lysozyme fragment was found to simultaneously bind to the protease and the regulatory PDZ1 binding site of an adjacent molecule. The covalent linkage of substrate residues interacting with protease and PDZ1 domain is essential for full activation of DegPEc [11]. It has been proposed that binding of an unfolded substrate molecule with multiple cleavage sites might increase the protease activity of DegPEc, most likely since it effectively crosslinks the 3-meric building blocks of the 12-mer and prevents premature disassembly. Interestingly, most recent results indicate that formation of cage-like oligomers might not be required for proteolytic activity of DegPEc. Facilitating a DegPEc variant with an amino-acid substitution that abrogates 12- and 24-mer formation, Kim et al. [43] showed that DegPEc 3-mers can efficiently degrade the 41 residue lysozyme fragment. This observation is in line with previous results showing that 3-meric DegPEc variants lacking the PDZ2 domain retain proteolytic activity [16, 23, 44]. Strikingly, the assembly of higher-order oligomers is also not necessary for survival at elevated temperatures [43]. Although protease activation does not depend on higher-order oligomers in vitro, it is likely that cage-like particles play an important role in the selection of substrate molecules and regulation of chaperone activity in vivo.
DegQ, a periplasmatic chaperone-protease homologous to DegP
DegQ is another periplasmatic HtrA-family member with dual functions, combining chaperone and protease activities much like DegP [9, 22, 45]. Both proteins share a common domain organization (Fig. 1a) and a high sequence similarity (~60 % amino-acid identity in E. coli). However, the length of loop LA, which is important for the stabilization of the 6-meric resting state of DegP [44], is dramatically reduced in DegQ (for many species: ~20 residues in DegQ vs. ~40 residues in DegP). Whereas in E. coli DegP and DegQ are present, many prokaryotes encode only a DegQ homologue. This stresses the importance of DegQ for protein homeostasis in the periplasm of these species [5, 46].
Very recently, the functional and structural characterization of DegQ from L. fallonii (DegQLf) has been reported [9]. Like all intracellular pathogens, Legionella have to counteract protein folding stress caused by defense mechanisms of the host cell. Thus, in hostile environments, as encountered in phagosomes of professional macrophages, Legionella needs effective means to prevent the excessive accumulation of misfolded proteins in the periplasm. As expected from sequence similarity to DegPEc, the Legionella DegQ homologue functions as chaperone and protease [9]. Similar to DegPEc, purified DegQLf is able to form 12-meric complexes in solution. The DegQLf 12-mer determined by X-ray crystallography is composed of four tightly interlocked 3-mers that together form a hollow, sphere-like particle [9] (Fig. 2). Truncated protein variants that lack PDZ2 (DegQLfΔPDZ2) are unable to form 12-mers and assemble into proteolytically inactive 3-mers. Thus, in DegQLf, both PDZ domains are necessary for 12-mer formation and proteolytic activity. As in DegSEc and DegPEc, an AAC is also present in DegQLf. It can be assumed that regulation of protease activity after assembly of the active oligomeric form is very similar in all three HtrA proteins. However, despite its high sequence similarity, DegQLf does not form 6-meric resting states analogous to DegPEc. Instead, predominantly 12-mers and 3-mers were observed in the absence of unfolded proteins. This might be attributed to the reduced length of the LA loop in DegQLf. The protease-active sites in DegQLf 3-mers are easily accessible, and harmful, uncontrolled proteolytic activity needs to be prevented. The crystal structure of DegQLfΔPDZ2 revealed that the PDZ1 domain was rotated by approximately 180° with respect to the protease domain [9]. As this rotation places the protease active site and the allosteric PDZ1 binding site on opposite faces of the 3-mer, proteolytic activity is shut down. In addition, in this orientation the allosteric peptide-binding site of PDZ1 is blocked by a linker that connects protease and PDZ1 domain. It has been proposed that a similar mechanism ensures inactivation of full-length DegQLf 3-mers [9].
The genome of the hyperthermophilic Gram-negative bacterium T. maritima encodes only one HtrA protein, a DegQ homologue (DegQTm). In the absence of DegS or DegP, DegQTm alone is responsible for counteracting protein-folding stress in the periplasm at the extreme temperatures associated with the lifestyle of this organism. DegQTm is not very well characterized and to our knowledge, no experimental data on the oligomerization behavior of DegQTm in solution has been published. However, the crystal structure of a truncated variant lacking both PDZ domains (DegQTmΔPDZ1&2) confirmed the formation of 3-meric assemblies typical for HtrA proteins [47] (Fig. 2). Furthermore, in a 2005 review paper, Kim et al. [46] stated that at room temperature, DegQTm assembles into 6-meric complexes that form even after deletion of loop LA but depend on the PDZ2 domain for stabilization of the 6-mer. Interestingly, the crystal structure DegQTmΔPDZ1&2 revealed that the LA loop of DegQTm contains a unique α-helical region that has not been observed in other HtrAs [47]. Applying spin-labeling electron paramagnetic resonance and fluorescence spectroscopy techniques in a follow-up study, Kim et al. [47, 48] elegantly showed that this helix in DegQTm acts as a molecular lid that completely blocks the catalytic triad and the protease-specificity sites. At the same time, the oxyanion hole and proper alignment of catalytic triad residues are disrupted, thereby rendering DegQTm in a protease-inactive state. Upon activation, e.g., at higher temperatures, the lid undergoes a conformational change which facilitates the reorganization of the catalytic-triad residues and enables substrate molecules to access the functional proteolytic active site [48]. It is unknown if activation of full-length DegQTm coincides with the assembly of higher-order oligomers and if a functional AAC regulating proteolytic activity exists.
In contrast to many other prokaryotes, both DegQ and DegP are present in E. coli. It has been shown that DegQEc can complement DegPEc under certain conditions [22]. Very recently, two independent groups reported the structural and functional characterization of DegQEc [10, 13, 17]. Employing SEC and sedimentation velocity analysis, both groups demonstrated that DegQEc forms 12- and 24-mers (Fig. 2) in the presence of suitable substrate molecules. In addition to cryo-EM structures of DegQEc 12-mers and 24-mers [10, 17], X-ray crystal structures of truncated DegQEc variants forming 3- and 12-mers have been determined [13]. As expected, the cage-like oligomers are the proteolytically active forms of DegQEc. However, in certain aspects the results presented by different groups contradict each other. First, there is some controversy on the protease resting state of DegQEc. One group reported that in the absence of substrate, DegQEc forms proteolytically inactive 6-mers similar to DegPEc [13, 17]; whereas the other group observed mainly 3-mers but no 6-mers and thus proposed 3-mers to be the resting state of DegQEc [10]. Second, based on cryo-EM, data the PDZ2 domain should be important for the formation of DegQEc 12- and 24-mer [13, 17]. Surprisingly, according to SEC and X-ray crystallographic data, the DegQEcΔPDZ2 variant is able to form 12-mers, if suitable substrate or co-purified peptide ligands are present [13]. The reason for the conflicting results is unclear.
Cryo-EM structures of DegQEc 12- and 24-mers in complex with model substrate proteins provide the structural basis for the chaperone activity of HtrA proteins [17]. Of great interest is especially the complex of 12-meric DegQEc with lysozyme, which revealed for the first time that for refolding, multiple unfolded proteins are encapsulated in the interior of the particle. Furthermore, interaction sites for unfolded proteins on the inner surface of DegQEc 12- and 24-mers have been identified. These sites are symmetric composite interfaces comprising residues from two LA loops, two PDZ1 and two PDZ2 domains [17]. Thus, the interior of DegQEc cages represents a molecular reaction chamber where both refolding and degradation of unfolded proteins take place.
HtrA2, a potential virulence factor in Mycobacterium tuberculosis
The genome of M. tuberculosis encodes three HtrA homologues: HtrA1Mt (DegP or Rv1223), HtrA2Mt (PepD or Rv0983), and HtrA3Mt (PepA or Rv0215). All three proteins contain only one PDZ domain C-terminal to the protease domain. Although M. tuberculosis is a Gram-positive species, it shares many characteristics with Gram-negative bacteria, including a periplasmatic space [49]. Predicted N-terminal transmembrane regions suggest that HtrAs of M. tuberculosis are localized to the inner membrane of the periplasmic compartment. In addition, secreted forms of HtrA2Mt and HtrA3Mt have been detected in in vitro culture supernatants [50]. Although precise functions have not been assigned, it is likely that M. tuberculosis HtrA proteins allow prolonged survival of mycobacteria in macrophages and caseous granulomas that form after infection as a result of the cell-mediated immune response of the host. In this environment, the pathogen has to deal with stressful conditions that likely include acidic pH, low oxygen, poor nutrient availability, and reactive oxygen and nitrogen intermediates. HtrA2Mt is the only M. tuberculosis HtrA protein structurally characterized and the X-ray crystal structure of an N-terminally truncated variant lacking the cytoplasmic and transmembrane domains (mHtrA2Mt) is available [20] (Figs. 1a, 2). mHtrA2Mt displays protease and chaperone activities and forms 3-mers in solution. There is some indication that the chaperone activity is localized to the PDZ domain, which can be autoproteolytically released from mHtrA2Mt in vitro [20]. The formation of DegP-like 6-mers is impossible for HtrA2Mt, as the length of the LA loop is reduced to six amino-acid residues, and higher-order oligomers have not been detected. Interestingly, the structure revealed tetrapeptides binding to the protease-active site and to the allosteric binding site of the PDZ domain [20]. These peptides are most likely derived from autoproteolytic processing of the enzyme. The peptide-bound PDZ domain interacts via helix α4 with loop L3 of the AAC, which in turn might trigger the formation of the protease-competent active site observed in the HtrA2Mt structure. Based on these observations, it has been proposed that mHtrA2Mt might control its activity state via autoproteolysis [20]. However, it cannot be excluded that the observed mHtrA2Mt fragments bind to protease and PDZ domains in vitro because of their relatively high concentration during purification and crystallization. Thus, it is unclear if HtrA2Mt represents a constitutively active HtrA protein which is regulated by autoproteolysis or if protease activity is controlled by oligomerization and/or allosteric substrate-binding analogous to other HtrAs. As deletion of the htra2 Mt gene leads to attenuated virulence in a mouse model of tuberculosis, a thorough characterization of proteolysis regulation in HtrA2Mt is highly desirable.
Eukaryotic HtrAs
Eukaryotic HtrA proteins are structurally and biochemically not as well characterized as their prokaryotic counterparts. Until very recently, structural information was available only for human HtrA2/Omi, an HtrA-family member localized in the intermembrane compartment of mitochondria [51]. In 2011, Clausen and coworkers succeeded in determining the X-ray crystal structures of two additional eukaryotic HtrAs, Deg1 from Arabidopsis [12] and human HtrA1 [52] (Fig. 4). As eukaryotic HtrAs play roles in fundamental biological processes such as photosynthesis, cell proliferation, and apoptosis, the importance to understand the precise molecular mechanisms of these proteins cannot be overestimated.
Fig. 4.
Three-dimensional structures of eukaryotic HtrA proteins. Representation as in Fig. 2. Deg1 from A. thaliana (6-mer); human HtrA1 (3-mer); human HtrA2/Omi (3-mer). The assemblies are not shown to scale relative to each other
Deg1, a pH-sensor protease that maintains components of the photosynthetic machinery in plants
Deg1 is an HtrA protein present in all photosynthetic organisms. In higher plants, Deg1, Deg5, Deg7, Deg8, and FtsH are major players in the catabolism of photosystem II (PSII), which is indispensable for the generation of the pH-gradient across the thylakoid membrane. Deg1 is the best characterized HtrA-family member in plants. As a chaperone, Deg1 is involved in the assembly of PSII by interacting with the reaction-center component D1. Under intense light, D1 is damaged by reactive oxygen species. The protease activity of Deg1 leads to the specific cleavage of an exposed loop of photodamaged D1, thereby initiating the complete degradation of D1 by the concerted action of other thylakoid proteases [53, 54].
Deg1 harbors a protease domain and a single C-terminal PDZ domain (Fig. 1a). The X-ray crystal structure of the A. thaliana homologue (Deg1At) revealed 6-meric protein complexes in the crystal lattice (Fig. 4). Deg1At molecules contain co-purified peptides bound to the PDZ domain and display the protease-active site in a catalytically competent conformation [12]. Moreover, components of the AAC, i.e., L1, L2, and LD, adopt a conformation typically observed in proteolytically active HtrA proteins. Thus, in stark contrast to all other characterized HtrAs, 6-mers seem to represent protease-active assemblies in Deg1At. As in the DegPEc 6-mer, the LA loop is important for linking the two 3-meric building blocks of the Deg1At 6-mer. However, the pillar-like interaction of two LA loops observed in 6-mers of DegPEc is absent in Deg1At. Here, the LA loop forms a composite intertrimer interface with structural elements of PDZ domains from the two 3-mers. This arrangement wedges the L3 sensor loop between protease and PDZ domain and allows for hydrogen-bonding interactions between L3 and LD*, leading to a structural rearrangement of the latter. Thus, positioning of the PDZ domain during 6-mer formation is most likely the signal that triggers the AAC in Deg1At and leads to the activation of the protease active site [12].
The assembly of Deg1At is controlled by the pH value of the thylakoid lumen. SEC experiments revealed that at acidic pH and physiological concentrations, Deg1At forms mostly 6-mers, whereas at neutral and basic pH, monomers are the predominant species. Assays at different pH values confirmed that the proteolytic activity of Deg1At is higher at pH 6 than 8. It has been shown that the protonation state of a specific histidine (His244) in the intra-3-mer interface is critical for the transient formation of 3-mers, which readily assemble into 6-mers [12]. The reported results are consistent with the following model for the in vivo function of Deg1: at dark, when the pH within the thylakoid lumen is neutral to basic, Deg1 adopts a monomeric resting state. Under these conditions, the need for degradation of photosystem components is minimal. In strong sunlight, however, the pH of the thylakoid lumen drops and Deg1 assembles via transient 3-mers into proteolytically active 6-mers, facilitating the efficient removal of dysfunctional photosystem proteins damaged by oxidation.
Human HtrA1, a tumor suppressor regulated by a unique induced-fit mechanism
Human HtrA1 (hHtrA1) is ubiquitously expressed in all tissues and localized in the cytoplasm and in the extracellular space. Intracellular hHtrA1 is implicated in cell proliferation by acting on a number of proteins involved in cell division, maturation, development, and translation such as tuberous sclerosis complex 2 (TSC2) [55], insulin-like growth-factor (IGF) binding protein 5 (IGFBP5) [56], transforming growth factor-β (TGFβ) [57], and other proteins of the TGFβ family [58]. It has been suggested that cytoplasmic hHtrA1 acts as a tumor suppressor. In the extracellular matrix, hHtrA1 is processing numerous secreted proteins (e.g., fibronectin, type-II collagen, ADAM9, vitronectin, α2-macroglobulin, and the amyloid precursor protein fragment Aβ) [58–62] and has been associated with arthritis, cancer, familial ischemic cerebral small-vessel disease, age-related macular degeneration, and Alzheimer’s disease [59, 63–66].
Human HtrA1 consists of an N-terminal domain of unknown function (a fragment of IGFBP7), a protease domain, and a single C-terminal PDZ domain (Fig. 1a), and forms 3-mers that assemble into large multimers of approximately 600 kDa in the presence of unfolded substrate proteins [52]. As for large protein complexes, molecular-weight determination by SEC is often inaccurate; the large particle might consist of 12 or 24 hHtrA1 monomers. However, it cannot be excluded that the assembly mode of hHtrA1 and the number of monomers in the observed particle deviates from higher-order oligomers of other HtrAs. The two available crystal structures of hHtrA1 in an inactive and an active state display HtrA-typical 3-mers (Fig. 4) [52], and thus cannot clarify this ambiguity. The hHtrA1 variant used for crystallization incorporates the protease and the PDZ domain, yet, due to high flexibility, the PDZ domain is not visible. In the inactive form, the active-site loops L1, L2, L3, and LD are disordered rendering catalytic triad, oxyanion hole, and S1 specificity pocket dysfunctional. In the active form, which features a peptidic ligand bound to the protease site, all these elements display a protease-competent state. Here, the sensor loop L3 is directly interacting with the peptidic ligand, thereby rearranging neighboring loops LD and L1/2. Thus, it is likely that an induced-fit mechanism of substrate-binding controls the activity state of hHtrA1 and this is the signal for the protein to switch into the protease-active state. Consistent with this mechanism, deletion of the PDZ domain in HtrA1 does not impede proteolytic activity, and thus a PDZ-mediated AAC as observed in other HtrAs is not necessary for hHtrA1 [52]. However, as the length of cleavage products is affected by the PDZ domain, it most likely takes part in substrate processing. Additional experimental data is needed to elucidate details of the unique activation of hHtrA1, especially in the context of the higher-order oligomers observed in solution.
Human HtrA2/Omi, a mitochondrial HtrA homologue involved in apoptosis
Human HtrA2/Omi carries a transmembrane anchor and a mitochondrial localization signal, which targets the protein to the intermembrane space of mitochondria (Fig. 1a). The membrane anchor facilitates attachment to the mitochondrial inner membrane. Processed forms of HtrA2/Omi can enter the cytoplasm of the cell during apoptosis and cleave proteins such as XIAP (X-linked inhibitor of apoptosis protein) and other caspase inhibitors. Furthermore, HtrA2/Omi has been reported to cleave cytoskeletal proteins, components of the translation machinery, and additional apoptosis-associated proteins, and thus promotes cell death in a caspase-independent way (see [67] for an excellent review on HtrA2/Omi and apoptosis). In addition, HtrA2/Omi might function as a protein quality control factor in the mitochondrial intermembrane compartment similar to prokaryotic HtrAs in the periplasmic space. There are several indications that support this view: first, HtrA2/Omi protein levels are upregulated under protein folding-stress conditions [68] and upon activation of the p53 stress-response pathway [69]. Second, as observed for DegPEc, protease activity of HtrA2/Omi is enhanced at elevated temperatures [70]. Third, the protease substrate-specificity is similar in HtrA2/Omi and DegPEc [42, 70]. Fourth, HtrA/Omi is connected to neurodegenerative diseases caused by aggregation of misfolded protein, such as Parkinson’s [71] and Alzheimer’s [72, 73].
The processed, mature form of HtrA2/Omi (residues 134–458) contains a protease domain and a single PDZ domain (Fig. 1a). The crystal structure revealed HtrA2/Omi 3-mers (Fig. 4) with an active site in the proteolytically competent state [51]. However, the access to the catalytic cleft is restricted by the PDZ domain and the peptide-binding groove of the PDZ domain in turn is blocked by residues of the protease domain. Thus, both interaction sites are not compatible with efficient binding of effectors or substrates in this conformation. Based on these observations and complementary mutational studies it has been proposed that the PDZ domain controls protease activity by regulating access to the substrate-binding cleft of the protease domain [51]. According to this model, binding of substrate protein to the PDZ domain should replace protease residues and enable a repositioning of the PDZ to expose the catalytic site of the protease to the substrate. However, it cannot be fully excluded that the inhibitory position of the PDZ domain in the X-ray structure is influenced by crystal packing constraints. It is unknown if binding of allosteric effectors or the formation of higher-order oligomers is involved in regulating protease activity in HtrA2/Omi.
Common structural and functional aspects of HtrA proteins
The constituting feature of HtrA-family proteins is the common domain organization. Exhibiting an average rmsd of ~0.6 Å, the protease domain is structurally well conserved in all HtrAs [74]. It displays the canonical trypsin fold comprising two β-barrel lobes with additional α-helices attached. The catalytic Ser–His–Asp triad is positioned in a crevice between the two lobes. In HtrA proteins, interactions between three protease domains are responsible for 3-mer formation.
PDZ domains are highly abundant protein interaction modules of 80–100 amino-acid residues present in many metazoan genomes (number of PDZ-containing proteins in selected organisms: human, 151; Drosophila melanogaster, 65; Caenorhabditis elegans 64; [75]). Interestingly, in unicellular organisms, PDZ domain proteins are scarce (E. coli: five proteins, Saccharomyces cerevisiae: three proteins). Thus, it is tempting to speculate that PDZ domains have evolved to facilitate intercellular signaling in multi-cellular organisms [76]. Canonical PDZ domains usually consist of six β-strands (βA–βF) that form a β-sandwich structure, and two associated α-helices (αA and αB). The PDZ fold allows for the specific interaction with exposed C-termini of partner proteins by β-sheet augmentation with βB and interactions with αB. Typically, five to seven C-terminal residues of the substrate are recognized by the PDZ domain and provide specificity for the interaction [77]. The fold of PDZ domains in HtrA proteins shows some variation compared to that of canonical PDZ domains. The core of HtrA PDZ domains generally consists of five β-strands (β1–β5) and two α-helices (α2 and α3) equivalent to the secondary structural elements of canonical PDZ domains. Yet, in several HtrAs, two additional β-strands (βN and βC) are present at the N- and C-terminus of PDZ domains and a α-helix (α1) is inserted into the loop connecting β1 and β2 (for details on PDZ architecture see [78]). In contrast to the protease domain, HtrA PDZ domains are structurally less well conserved (average rmsd ~1.2 Å). This is consistent with the variety of roles that PDZ domains play in the context of different HtrA-family members.
Regulation of protease activity in HtrA proteins
Regulation of uncontrolled, potentially deleterious activity is vital for all proteases. Using PDZ domains, HtrAs have developed regulatory mechanisms that allow precise control of the activity state and influence substrate specificity as well as processivity of the protease domain.
Ligand binding to the allosteric site of the PDZ domain adjacent to the protease domain leads to a disorder-to-order transition of important components (L1, L2, L3, and LD) of the intrinsic allosteric regulation mechanism present in most HtrAs. Initiated by sensor loop L3, a cascade of conformational changes along L3 → LD → L1/L2 induces the remodeling and activation of the proteolytic site (Fig. 3). It can be assumed that the general mechanism is conserved in DegS, DegP, DegQ, HtrA2Mt, and Deg1At; however the activation signal detected by sensor loop L3 is different. In DegSEc, the binding of allosteric peptides to PDZ domains leads to the activation of the protease-active sites (Fig. 3a). Peptide-binding causes a reorientation of the PDZ domain, thereby either relieving inhibitory contacts between PDZ and protease domain (“inhibition-relief” model) or enabling a direct interaction of the bound peptide and L3 (“peptide-activation” model). In DegQ, DegP, and Deg1At, the assembly of active oligomeric forms leads to a reorientation of the regulatory PDZ domain. In this process, L3 is wedged in between protease and PDZ domain and is thus able to detect the locked position of PDZ1. As loop LD is located at the intratrimer interface, peptide binding to the PDZ domain leads to the activation of the protease site in an adjacent monomer (Fig. 3b). The activation mechanism of human HtrA1 is unique, as the interaction of the substrate protein with the proteolytic site stabilizes the conformation of loops L1, L2, and L3 and the PDZ domain is dispensable for protease activity. The protease regulation is not well understood in other HtrAs.
Binding sites on protease and PDZ domain of DegPEc exhibit identical substrate specificities. Therefore, it is likely that degradation intermediates released by the protease domain are recognized by the PDZ1 domain. Subsequently, the shortened PDZ-bound substrate is presented to the protease domain again and another cleavage occurs. This “hold-bite-and-rebind” mechanism leads to a processive degradation of the substrate molecule into peptides of 9–20 amino-acid residues [79]. The length of the degradation products is dictated by the distance between protease- and PDZ-binding sites within the active DegPEc oligomer. It is quite possible that similar “molecular ruler” mechanisms also exist in other HtrA proteins.
Intermolecular PDZ–PDZ interactions modulate the assembly of higher-order HtrA oligomers
Large molecular complexes perform tasks of critical importance for the cell. The DNA replication complex, the RNA polymerase, and the proteasome represent the most prominent examples for molecular machines assembled from individual cellular components which are interconnected by extensive protein–protein interactions. The formation of protein complexes offers numerous possibilities for intrinsic regulation and in many cases facilitate cooperative substrate binding and the coordination of enzymatic reactions.
In most HtrA proteins, peptide binding to PDZ domains is important for the assembly of protease-active oligomeric forms. Regulation of enzymatic activity by oligomer formation is exceptionally well understood in the DegP system, where in the presence of unfolded proteins, resting-state 6-mers disassemble into transient 3-mers that subsequently form active 12- and 24-mers. Oligomerization represents a simple and effective mechanism for controlling access to the proteolytic sites of active DegP particles, which are stable for many reaction cycles [11]. During the last year, a very similar picture emerged for DegQ. As for DegP, protease-active DegQ 12- and 24-mers have been reported [9, 11, 15, 17]; however, the nature of the resting state is still under debate and might in fact vary from species to species. Surprisingly, and in stark contrast to DegP and DegQ, in plant Deg1At 6-mers that assemble from inactive monomers represent protease-active oligomeric forms. The situation is less clear for other eukaryotic HtrA proteins. In human HtrA1 and HtrA2/Omi, it is likely that 3-mers are protease-resting states, but the stoichiometry of active particles is unknown.
In all higher-order HtrA assemblies, the oligomerization mode of the protease domains that stabilize the 3-meric building blocks is identical (average rmsd of ~1.1 Å for protease cores of 3-mers extracted from 6-, 12-, or 24-mers of DegPEc, DegQEc, DegQLf, and Deg1At). The assembly of higher-order particles is stabilized by PDZ–PDZ interactions between multiple 3-mers. Typically, PDZ domains interact with the C-terminus or internal sequence motifs of partner proteins [80]. In addition, PDZ domains can facilitate the formation of protein complexes via PDZ dimerization [78]. A recent systematic analysis of all mouse PDZ domains using protein microarrays revealed that as many as 30 % are involved in PDZ–PDZ contacts [81]. Thus, PDZ dimerization is more frequent than previously anticipated and many PDZ domains might have evolved to specifically guide the assembly of protein multimers. Prominent examples for structurally characterized PDZ–PDZ interactions are ZO-1 [82], tamalin [83], GRIP1 [84], and Shank1 [85]. Interestingly, the dimerization mode and the interfaces involved in the PDZ–PDZ contact are different in all four cases. To explore the assembly mode of higher-order HtrA oligomers, we conducted a detailed analysis of intertrimer PDZ–PDZ interactions observed in the available X-ray crystal structures. Structural models based on cryo-EM data were not included in this analysis due to ambiguities in placement and orientation of the PDZ domains at low resolution.
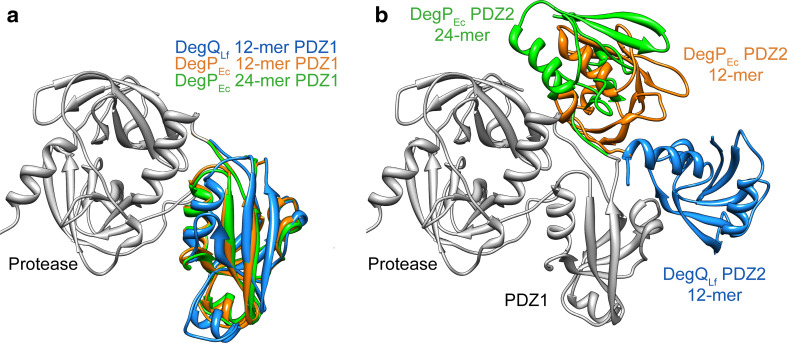
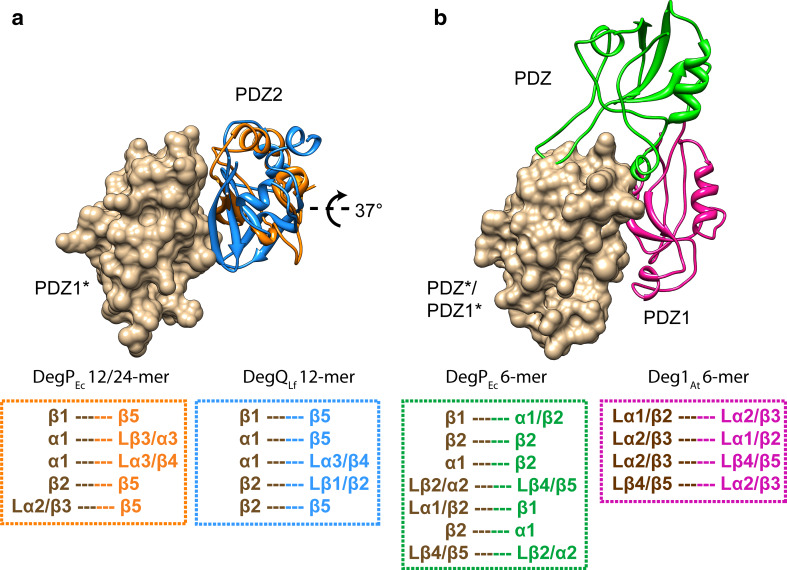
The protease domain and PDZ1 form a relatively rigid structural unit in 12- and 24-mers of DegPEc and DegQLf (Fig. 5a). This is not surprising, as the sensor loop L3 of the protease domain needs to specifically interact with the peptide-bound PDZ1 to trigger the AAC. PDZ1 and PDZ2 are connected by a flexible linker that allows the latter to occupy different positions with respect to the protease-PDZ1 unit (Fig. 5b). The position of PDZ2 determines the architecture of 12-mers as well as 24-mers. Whereas PDZ2 of the DegQLf 12-mer is contacting two PDZ2*, one PDZ1*, and one protease* domain [9], PDZ2 of DegPEc in 12- and 24-mers interacts with one PDZ1* only [11, 15]. It is interesting to note that interactions between PDZ2 and PDZ1* seem to be important for the stabilization of DegQLf and DegPEc complexes and that the buried surface area between PDZ2 and PDZ1* is comparable (500–560 Å2). A superimposition of the interfacing domains based on PDZ1* revealed that position and orientation of the contacting PDZ2 is very similar in DegPEc 12- and 24-mers. In both particles, regions involved in the contact are identical (PDZ1*: α1, β1, β2, loop α2/β3; PDZ2: β5, loop β3/α3, loop α3/β4). In contrast, PDZ2 of DegQLf is rotated by ~37° compared to DegPEc and the interaction pattern between PDZ1*(α1, β1, β2) and PDZ2 (β5, loop β1/β2, loop α3/β4) differs from that observed in DegPEc (Fig. 6a).
Fig. 5.
PDZ domains in higher-order HtrA oligomers. a Position of the PDZ1 from DegQLf 12-mer (blue), DegPEc 12-mer (orange), and DegPEc 24-mer (green) with respect to the superimposed protease domains (gray). b Position of PDZ2 with respect to superimposed protease and PDZ1 domains (gray). Colors as in a
Fig. 6.
Interaction of PDZ domains stabilizing intertrimer interfaces of higher-order HtrA assemblies. a Interaction of PDZ1* (brown, surface representation) and PDZ2 in DegPEc 12-mers (orange ribbons) and PDZ2 in DegQLf 12-mers (blue ribbons). b Interaction of PDZ1*/PDZ* (brown, surface representation) and PDZ1 in DegPEc 6-mers (purple ribbons) and PDZ in Deg1AtQLf 6-mers (green ribbons). PDZ1* (a) and PDZ1*/PDZ* (b) are shown in the same orientation. Boxes specify the interaction of structural elements responsible for stabilization of the PDZ–PDZ interaction in each case. Numbering of secondary structure elements and loops (L) as in [78]
Besides playing a pivotal role in assembly of large spherical HtrA particles, PDZ–PDZ interactions are also important for the stabilization of 6-meric forms of DegPEc and Deg1At [12, 14]. In DegPEc, PDZ1 domains of opposite 3-mers face each other with residues of α1, β1, β2, loop β2/α2, and loop α1/β2 constituting a symmetric interaction interface that exhibits a buried surface area of 570 Å2 (Fig. 6b). Deg1At harbors a single PDZ domain and Deg1At 6-mers display an intimate contact between two PDZ domains of opposite 3-mers via loops α1/β2, α2/β3, and β4/β5 that form a symmetric interface with a buried surface area of 630 Å2 (Fig. 6b). Compared to other HtrAs, the loops that mediate this interaction are elongated in Deg1At. A superimposition of DegPEc PDZ1* and Deg1At PDZ* domains shows that the positions and orientations of the interacting PDZ domains are different (~22 Å translation and ~37° rotation of Deg1At PDZ with respect to DegPEc PDZ1). Furthermore, the PDZ–PDZ interactions observed in HtrA 6-mers deviate from those found in 12- and 24-mers (Fig. 6a, b).
In conclusion, the comparison of PDZ–PDZ interfaces of HtrA complexes revealed a remarkable diversity of interaction modes. The PDZ domain itself provides a unique structural scaffold that tolerates numerous modifications and allows for the assembly of molecular complexes as diverse in size and architecture as HtrA 6-, 12-, and 24-mers.
Concluding remarks and future perspectives
Recent progress in the structural and functional characterization of HtrA proteins from prokaryotic and eukaryotic species has greatly enhanced our understanding of this intriguing protein family. Most prokaryotic HtrAs play critical roles in protein quality control within the periplasmic compartment. However, several members of the family have explicit functions in bacterial pathogenicity, e.g., supporting biofilm formation [86], secretion of virulence factors [87], and remodeling of cell–cell contacts within the host organism [88]. Eukaryotic HtrAs take care of damaged cellular components and protect the cell from the accumulation of toxic protein aggregates responsible for neurodegeneration in Alzheimer’s and Parkinson’s disease. Furthermore, mammalian HtrAs might act as tumor repressors in certain cancers and are associated with the induction of apoptosis. It is very likely that future research will reveal additional functions of eukaryotic HtrAs.
As several aspects of architecture and regulation are shared, there is no clear line that separates prokaryotic and eukaryotic HtrAs, indicating an evolutionary link between both subgroups. The detailed analysis of selected family members revealed that subtle modifications of various structural elements have a profound impact on oligomerization behavior and intrinsic regulation, which enable HtrA proteins to fulfill specific functions. In this respect, PDZ domains as widespread and versatile protein scaffolds play crucial roles. On the one hand, PDZ domains facilitate the specific activation of HtrAs by binding substrate proteins at the allosteric site. On the other hand, PDZ domains supply interaction surfaces suited to stabilize a diverse set of molecular assemblies. It is fascinating that in many HtrA proteins regulatory and structural capabilities of PDZ domains are elegantly interconnected to tailor functionality to the specific requirements of the cellular environment. However, we have only begun to understand the role of HtrA proteins in protein quality control and pathogenesis and important questions need to be addressed in the future.
Although in recent years a much clearer picture of structure and function of bacterial HtrAs has emerged, certain aspects of their molecular architecture are still not well understood. It is interesting to note that the reported HtrA 12-mers show different assembly modes. Whereas most structures of DegPEc and DegQEc display symmetric particles with relatively small pores in the protein shell [10, 16, 17], Sauer and coworkers recently presented an asymmetric DegPEc 12-mer with enlarged pores [11]. This distorted DegPEc structure might be a result of packing constraints within the protein crystals. However, it is also possible that 12-mers can adopt multiple conformational states and that the asymmetric DegPEc particle provides a snapshot of one of these states. The analysis of monomers from different HtrA particles showed multiple positions of PDZ2 in respect to the rather rigid protease-PDZ1 unit (Fig. 5b). Strikingly, the asymmetric DegPEc 12-mer is composed of monomers with two distinct conformations with respect to the position of PDZ2. Thus, it cannot be excluded that PDZ2 can change its position within the DegPEc 12-mer without destabilizing the particle. This would allow a “breathing” motion of the 12-mer profoundly affecting the intrinsic regulation and the access of substrates to the interior.
The recent studies by Malet et al. [17] on the chaperone activity of DegQEc are of ground-breaking importance. This publication shed light on the structural basis of the elusive refolding activity of HtrA proteins and for the first time revealed that both, protease and chaperone function, localize to the interior of the spherical HtrA particle. Although interaction surfaces have been identified that might support the rescue of misfolded proteins, the precise mechanism awaits characterization. We hope that the report on the structural basis of chaperone activity will spearhead research efforts focusing on this function of HtrA proteins in the future, just like the structural information on the higher-order oligomers of DegP greatly stimulated research into the protease activity of HtrAs in the past few years.
Acknowledgments
We thank Sen-Fang Sui and Xiaochen Bai for supplying the atomic coordinates of DegQEc models based on their cryo-EM data. RH is supported by a Chinese Academy of Sciences Visiting Professorship for Senior International Scientists, Grant no. 2010T1S6, by the DFG Cluster of Excellence “Inflammation at Interfaces” (EXC 306), as well as by the Fonds der Chemischen Industrie.
Abbreviations
- ADAM9
A disintegrin and metalloproteinase domain-containing 9
- FRET
Förster resonance energy transfer
- HtrA
High-temperature requirement A
- OMP
Outer membrane protein
- PDZ
Postsynaptic density of 95 kDa (PSD-95), discs large (DLG1), and zonula occludens 1 (ZO-1)
- rmsd
Root-mean-square deviation
- SEC
Size-exclusion chromatography
References
- 1.Page MJ, Di Cera E. Evolution of peptidase diversity. J Biol Chem. 2008;283:30010–30014. doi: 10.1074/jbc.M804650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clausen T, Kaiser M, Huber R, Ehrmann M. HtrA proteases: regulated proteolysis in protein quality control. Nat Rev Mol Cell Biol. 2011;12:152–162. doi: 10.1038/nrm3065. [DOI] [PubMed] [Google Scholar]
- 3.Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2008;36:D320–D325. doi: 10.1093/nar/gkm954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skorko-Glonek J, Zurawa D, Kuczwara E, Wozniak M, Wypych Z, Lipinska B. The Escherichia coli heat shock protease HtrA participates in defense against oxidative stress. Mol Gen Genet. 1999;262:342–350. doi: 10.1007/s004380051092. [DOI] [PubMed] [Google Scholar]
- 5.Önder Ö, Turkarslan S, Sun D, Daldal F. Overproduction or absence of the periplasmic protease DegP severely compromises bacterial growth in the absence of the dithiol: disulfide oxidoreductase DsbA. Mol Cell Proteomics. 2008;7:875–890. doi: 10.1074/mcp.M700433-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skorko-Glonek J, Wawrzynow A, Krzewski K, Kurpierz K, Lipinska B. Site-directed mutagenesis of the HtrA (DegP) serine protease, whose proteolytic activity is indispensable for Escherichia coli survival at elevated temperatures. Gene. 1995;163:47–52. doi: 10.1016/0378-1119(95)00406-V. [DOI] [PubMed] [Google Scholar]
- 7.Alba BM, Gross CA. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol Microbiol. 2004;52:613–619. doi: 10.1111/j.1365-2958.2003.03982.x. [DOI] [PubMed] [Google Scholar]
- 8.Ingmer H, Brøndsted L. Proteases in bacterial pathogenesis. Res Microbiol. 2009;160:704–710. doi: 10.1016/j.resmic.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Wrase R, Scott H, Hilgenfeld R, Hansen G. The Legionella HtrA homologue DegQ is a self-compartmentizing protease that forms large 12-meric assemblies. Proc Natl Acad Sci USA. 2011;108:10490–10495. doi: 10.1073/pnas.1101084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai XC, Pan XJ, Wang XJ, Ye YY, Chang LF, Leng D, Lei J, Sui SF. Characterization of the structure and function of Escherichia coli DegQ as a representative of the DegQ-like proteases of bacterial HtrA family proteins. Structure. 2011;19:1328–1337. doi: 10.1016/j.str.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Grant RA, Sauer RT. Covalent linkage of distinct substrate degrons controls assembly and disassembly of DegP proteolytic cages. Cell. 2011;145:67–78. doi: 10.1016/j.cell.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kley J, Schmidt B, Boyanov B, Stolt-Bergner PC, Kirk R, Ehrmann M, Knopf RR, Naveh L, Adam Z, Clausen T. Structural adaptation of the plant protease Deg1 to repair photosystem II during light exposure. Nat Struct Mol Biol. 2011;18:728–731. doi: 10.1038/nsmb.2055. [DOI] [PubMed] [Google Scholar]
- 13.Sawa J, Malet H, Krojer T, Canellas F, Ehrmann M, Clausen T. Molecular adaptation of the DegQ protease to exert protein quality control in the bacterial cell envelope. J Biol Chem. 2011;286:30680–30690. doi: 10.1074/jbc.M111.243832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krojer T, Garrido-Franco M, Huber R, Ehrmann M, Clausen T. Crystal structure of DegP (HtrA) reveals a new protease-chaperone machine. Nature. 2002;416:455–459. doi: 10.1038/416455a. [DOI] [PubMed] [Google Scholar]
- 15.Krojer T, Sawa J, Schäfer E, Saibil HR, Ehrmann M, Clausen T. Structural basis for the regulated protease and chaperone function of DegP. Nature. 2008;453:885–890. doi: 10.1038/nature07004. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Zhang X, Chen Y, Wu Y, Zhou ZH, Chang Z, Sui SF. Activation of DegP chaperone-protease via formation of large cage-like oligomers upon binding to substrate proteins. Proc Natl Acad Sci USA. 2008;105:11939–11944. doi: 10.1073/pnas.0805464105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malet H, Canellas F, Sawa J, Yan J, Thalassinos K, Ehrmann M, Clausen T, Saibil HR. Newly folded substrates inside the molecular cage of the HtrA chaperone DegQ. Nat Struct Mol Biol. 2012;19:152–157. doi: 10.1038/nsmb.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen QT, Bai XC, Chang LF, Wu Y, Wang HW, Sui SF. Bowl-shaped oligomeric structures on membranes as DegP’s new functional forms in protein quality control. Proc Natl Acad Sci USA. 2009;106:4858–4863. doi: 10.1073/pnas.0811780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilken C, Kitzing K, Kurzbauer R, Ehrmann M, Clausen T. Crystal structure of the DegS stress sensor: how a PDZ domain recognizes misfolded protein and activates a protease. Cell. 2004;117:483–494. doi: 10.1016/S0092-8674(04)00454-4. [DOI] [PubMed] [Google Scholar]
- 20.Mohamedmohaideen NN, Palaninathan SK, Morin PM, Williams BJ, Braunstein M, Tichy SE, Locker J, Russell DH, Jacobs WR, Jr, Sacchettini JC. Structure and function of the virulence-associated high-temperature requirement A of Mycobacterium tuberculosis . Biochemistry. 2008;47:6092–6102. doi: 10.1021/bi701929m. [DOI] [PubMed] [Google Scholar]
- 21.Alba BM, Leeds JA, Onufryk C, Lu CZ, Gross CA. DegS and YaeL participate sequentially in the cleavage of RseA to activate the σE-dependent extracytoplasmic stress response. Genes Dev. 2002;16:2156–2168. doi: 10.1101/gad.1008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waller PR, Sauer RT. Characterization of degQ and degS, Escherichia coli genes encoding homologs of the DegP protease. J Bacteriol. 1996;178:1146–1153. doi: 10.1128/jb.178.4.1146-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiess C, Beil A, Ehrmann M. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell. 1999;97:339–347. doi: 10.1016/S0092-8674(00)80743-6. [DOI] [PubMed] [Google Scholar]
- 24.Wilson RL, Brown LL, Kirkwood-Watts D, Warren TK, Lund SA, King DS, Jones KF, Hruby DE. Listeria monocytogenes 10403S HtrA is necessary for resistance to cellular stress and virulence. Infect Immun. 2006;74:765–768. doi: 10.1128/IAI.74.1.765-768.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stack HM, Sleator RD, Bowers M, Hill C, Gahan CG. Role for HtrA in stress induction and virulence potential in Listeria monocytogenes . Appl Environ Microbiol. 2005;71:4241–4247. doi: 10.1128/AEM.71.8.4241-4247.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowley G, Stevenson A, Kormanec J, Roberts M. Effect of inactivation of degS on Salmonella enterica serovar typhimurium in vitro and in vivo. Infect Immun. 2005;73:459–463. doi: 10.1128/IAI.73.1.459-463.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flannagan RS, Aubert D, Kooi C, Sokol PA, Valvano MA. Burkholderia cenocepacia requires a periplasmic HtrA protease for growth under thermal and osmotic stress and for survival in vivo. Infect Immun. 2007;75:1679–1689. doi: 10.1128/IAI.01581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farn J, Roberts M. Effect of inactivation of the HtrA-like serine protease DegQ on the virulence of Salmonella enterica serovar typhimurium in mice. Infect Immun. 2004;72:7357–7359. doi: 10.1128/IAI.72.12.7357-7359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chitlaru T, Zaide G, Ehrlich S, Inbar I, Cohen O, Shafferman A. HtrA is a major virulence determinant of Bacillus anthracis . Mol Microbiol. 2011;81:1542–1559. doi: 10.1111/j.1365-2958.2011.07790.x. [DOI] [PubMed] [Google Scholar]
- 30.Raivio TL. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol Microbiol. 2005;56:1119–1128. doi: 10.1111/j.1365-2958.2005.04625.x. [DOI] [PubMed] [Google Scholar]
- 31.Walsh NP, Alba BM, Bose B, Gross CA, Sauer RT. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell. 2003;113:61–71. doi: 10.1016/S0092-8674(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 32.Hasenbein S, Meltzer M, Hauske P, Kaiser M, Huber R, Clausen T, Ehrmann M. Conversion of a regulatory into a degradative protease. J Mol Biol. 2010;397:957–966. doi: 10.1016/j.jmb.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Sohn J, Grant RA, Sauer RT. Allosteric activation of DegS, a stress sensor PDZ protease. Cell. 2007;131:572–583. doi: 10.1016/j.cell.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 34.Chaba R, Alba BM, Guo MS, Sohn J, Ahuja N, Sauer RT, Gross CA. Signal integration by DegS and RseB governs the σE-mediated envelope stress response in Escherichia coli . Proc Natl Acad Sci USA. 2011;108:2106–2111. doi: 10.1073/pnas.1019277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeth K. Structural analysis of DegS, a stress sensor of the bacterial periplasm. FEBS Lett. 2004;569:351–358. doi: 10.1016/j.febslet.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Sohn J, Grant RA, Sauer RT. Allostery is an intrinsic property of the protease domain of DegS: implications for enzyme function and evolution. J Biol Chem. 2010;285:34039–34047. doi: 10.1074/jbc.M110.135541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohn J, Grant RA, Sauer RT. OMP peptides activate the DegS stress-sensor protease by a relief of inhibition mechanism. Structure. 2009;17:1411–1421. doi: 10.1016/j.str.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasselblatt H, Kurzbauer R, Wilken C, Krojer T, Sawa J, Kurt J, Kirk R, Hasenbein S, Ehrmann M, Clausen T. Regulation of the σE stress response by DegS: how the PDZ domain keeps the protease inactive in the resting state and allows integration of different OMP-derived stress signals upon folding stress. Genes Dev. 2007;21:2659–2670. doi: 10.1101/gad.445307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipinska B, Sharma S, Georgopoulos C. Sequence analysis and regulation of the HtrA gene of Escherichia coli: a σ32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988;16:10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawa J, Heuck A, Ehrmann M, Clausen T. Molecular transformers in the cell: lessons learned from the DegP protease-chaperone. Curr Opin Struct Biol. 2010;20:253–258. doi: 10.1016/j.sbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Kim KI, Park SC, Kang SH, Cheong GW, Chung CH. Selective degradation of unfolded proteins by the self-compartmentalizing HtrA protease, a periplasmic heat shock protein in Escherichia coli . J Mol Biol. 1999;294:1363–1374. doi: 10.1006/jmbi.1999.3320. [DOI] [PubMed] [Google Scholar]
- 42.Krojer T, Sawa J, Huber R, Clausen T. HtrA proteases have a conserved activation mechanism that can be triggered by distinct molecular cues. Nat Struct Mol Biol. 2010;17:844–852. doi: 10.1038/nsmb.1840. [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Sauer RT. Cage assembly of DegP protease is not required for substrate-dependent regulation of proteolytic activity or high-temperature cell survival. Proc Natl Acad Sci USA. 2012;109:7263–7268. doi: 10.1073/pnas.1204791109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jomaa A, Damjanovic D, Leong V, Ghirlando R, Iwanczyk J, Ortega J. The inner cavity of Escherichia coli DegP protein is not essential for molecular chaperone and proteolytic activity. J Bacteriol. 2007;189:706–716. doi: 10.1128/JB.01334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolmar H, Waller PR, Sauer RT. The DegP and DegQ periplasmic endoproteases of Escherichia coli: specificity for cleavage sites and substrate conformation. J Bacteriol. 1996;178:5925–5929. doi: 10.1128/jb.178.20.5925-5929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim DY, Kim KK. Structure and function of HtrA family proteins, the key players in protein quality control. J Biochem Mol Biol. 2005;38:266–274. doi: 10.5483/BMBRep.2005.38.3.266. [DOI] [PubMed] [Google Scholar]
- 47.Kim DY, Kim DR, Ha SC, Lokanath NK, Lee CJ, Hwang HY, Kim KK. Crystal structure of the protease domain of a heat-shock protein HtrA from Thermotoga maritima . J Biol Chem. 2003;278:6543–6551. doi: 10.1074/jbc.M208148200. [DOI] [PubMed] [Google Scholar]
- 48.Kim DY, Kwon E, Shin YK, Kweon DH, Kim KK. The mechanism of temperature-induced bacterial HtrA activation. J Mol Biol. 2008;377:410–420. doi: 10.1016/j.jmb.2007.12.078. [DOI] [PubMed] [Google Scholar]
- 49.Fu LM, Fu-Liu CS. Is Mycobacterium tuberculosis a closer relative to Gram-positive or Gram-negative bacterial pathogens? Tuberculosis (Edinb) 2002;82:85–90. doi: 10.1054/tube.2002.0328. [DOI] [PubMed] [Google Scholar]
- 50.Skeiky YA, Lodes MJ, Guderian JA, Mohamath R, Bement T, Alderson MR, Reed SG. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis . Infect Immun. 1999;67:3998–4007. doi: 10.1128/iai.67.8.3998-4007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W, Srinivasula SM, Chai J, Li P, Wu JW, Zhang Z, Alnemri ES, Shi Y. Structural insights into the pro-apoptotic function of mitochondrial serine protease HtrA2/Omi. Nat Struct Biol. 2002;9:436–441. doi: 10.1038/nsb795. [DOI] [PubMed] [Google Scholar]
- 52.Truebestein L, Tennstaedt A, Mönig T, Krojer T, Canellas F, Kaiser M, Clausen T, Ehrmann M. Substrate-induced remodeling of the active site regulates human HtrA1 activity. Nat Struct Mol Biol. 2011;18:386–388. doi: 10.1038/nsmb.2013. [DOI] [PubMed] [Google Scholar]
- 53.Edelman M, Mattoo AK. D1-protein dynamics in photosystem II: the lingering enigma. Photosynth Res. 2008;98:609–620. doi: 10.1007/s11120-008-9342-x. [DOI] [PubMed] [Google Scholar]
- 54.Huesgen PF, Schuhmann H, Adamska I. Deg/HtrA proteases as components of a network for photosystem II quality control in chloroplasts and cyanobacteria. Res Microbiol. 2009;160:726–732. doi: 10.1016/j.resmic.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Campioni M, Severino A, Manente L, Tuduce IL, Toldo S, Caraglia M, Crispi S, Ehrmann M, He X, Maguire J, De Falco M, De Luca A, Shridhar V, Baldi A. The serine protease HtrA1 specifically interacts and degrades the tuberous sclerosis complex 2 protein. Mol Cancer Res. 2010;8:1248–1260. doi: 10.1158/1541-7786.MCR-09-0473. [DOI] [PubMed] [Google Scholar]
- 56.Hou J, Clemmons DR, Smeekens S. Expression and characterization of a serine protease that preferentially cleaves insulin-like growth factor binding protein-5. J Cell Biochem. 2005;94:470–484. doi: 10.1002/jcb.20328. [DOI] [PubMed] [Google Scholar]
- 57.Launay S, Maubert E, Lebeurrier N, Tennstaedt A, Campioni M, Docagne F, Gabriel C, Dauphinot L, Potier MC, Ehrmann M, Baldi A, Vivien D. HtrA1-dependent proteolysis of TGF-β controls both neuronal maturation and developmental survival. Cell Death Differ. 2008;15:1408–1416. doi: 10.1038/cdd.2008.82. [DOI] [PubMed] [Google Scholar]
- 58.Oka C, Tsujimoto R, Kajikawa M, Koshiba-Takeuchi K, Ina J, Yano M, Tsuchiya A, Ueta Y, Soma A, Kanda H, Matsumoto M, Kawaichi M. HtrA1 serine protease inhibits signaling mediated by Tgfβ family proteins. Development. 2004;131:1041–1053. doi: 10.1242/dev.00999. [DOI] [PubMed] [Google Scholar]
- 59.Grau S, Baldi A, Bussani R, Tian X, Stefanescu R, Przybylski M, Richards P, Jones SA, Shridhar V, Clausen T, Ehrmann M. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc Natl Acad Sci USA. 2005;102:6021–6026. doi: 10.1073/pnas.0501823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grau S, Richards PJ, Kerr B, Hughes C, Caterson B, Williams AS, Junker U, Jones SA, Clausen T, Ehrmann M. The role of human HtrA1 in arthritic disease. J Biol Chem. 2006;281:6124–6129. doi: 10.1074/jbc.M500361200. [DOI] [PubMed] [Google Scholar]
- 61.An E, Sen S, Park SK, Gordish-Dressman H, Hathout Y. Identification of novel substrates for the serine protease HTRA1 in the human RPE secretome. Investig Ophthalmol Vis Sci. 2010;51:3379–3386. doi: 10.1167/iovs.09-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuchiya A, Yano M, Tocharus J, Kojima H, Fukumoto M, Kawaichi M, Oka C. Expression of mouse HtrA1 serine protease in normal bone and cartilage and its upregulation in joint cartilage damaged by experimental arthritis. Bone. 2005;37:323–336. doi: 10.1016/j.bone.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 63.Chien J, Campioni M, Shridhar V, Baldi A. HtrA serine proteases as potential therapeutic targets in cancer. Curr Cancer Drug Targets. 2009;9:451–468. doi: 10.2174/156800909788486704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coleman HR, Chan CC, Ferris FL, 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hara K, Shiga A, Fukutake T, Nozaki H, Miyashita A, Yokoseki A, Kawata H, Koyama A, Arima K, Takahashi T, Ikeda M, Shiota H, Tamura M, Shimoe Y, Hirayama M, Arisato T, Yanagawa S, Tanaka A, Nakano I, Ikeda S, Yoshida Y, Yamamoto T, Ikeuchi T, Kuwano R, Nishizawa M, Tsuji S, Onodera O. Association of HtrA1 mutations and familial ischemic cerebral small-vessel disease. N Engl J Med. 2009;360:1729–1739. doi: 10.1056/NEJMoa0801560. [DOI] [PubMed] [Google Scholar]
- 66.Milner JM, Patel A, Rowan AD. Emerging roles of serine proteinases in tissue turnover in arthritis. Arthr Rheum. 2008;58:3644–3656. doi: 10.1002/art.24046. [DOI] [PubMed] [Google Scholar]
- 67.Vande Walle L, Lamkanfi M, Vandenabeele P. The mitochondrial serine protease HtrA2/Omi: an overview. Cell Death Differ. 2008;15:453–460. doi: 10.1038/sj.cdd.4402291. [DOI] [PubMed] [Google Scholar]
- 68.Gray CW, Ward RV, Karran E, Turconi S, Rowles A, Viglienghi D, Southan C, Barton A, Fantom KG, West A, Savopoulos J, Hassan NJ, Clinkenbeard H, Hanning C, Amegadzie B, Davis JB, Dingwall C, Livi GP, Creasy CL. Characterization of human HtrA2, a novel serine protease involved in the mammalian cellular stress response. Eur J Biochem. 2000;267:5699–5710. doi: 10.1046/j.1432-1327.2000.01589.x. [DOI] [PubMed] [Google Scholar]
- 69.Jin S, Kalkum M, Overholtzer M, Stoffel A, Chait BT, Levine AJ. CIAP1 and the serine protease HTRA2 are involved in a novel p53-dependent apoptosis pathway in mammals. Genes Dev. 2003;17:359–367. doi: 10.1101/gad.1047003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martins LM, Turk BE, Cowling V, Borg A, Jarrell ET, Cantley LC, Downward J. Binding specificity and regulation of the serine protease and PDZ domains of HtrA2/Omi. J Biol Chem. 2003;278:49417–49427. doi: 10.1074/jbc.M308659200. [DOI] [PubMed] [Google Scholar]
- 71.Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Müller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Krüger R. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum Mol Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- 72.Lee MS, Jun DH, Hwang CI, Park SS, Kang JJ, Park HS, Kim J, Kim JH, Seo JS, Park WY. Selection of neural differentiation-specific genes by comparing profiles of random differentiation. Stem Cells. 2006;24:1946–1955. doi: 10.1634/stemcells.2005-0325. [DOI] [PubMed] [Google Scholar]
- 73.Huttunen HJ, Guenette SY, Peach C, Greco C, Xia W, Kim DY, Barren C, Tanzi RE, Kovacs DM. HtrA2 regulates beta-amyloid precursor protein (APP) metabolism through endoplasmic reticulum-associated degradation. J Biol Chem. 2007;282:28285–28295. doi: 10.1074/jbc.M702951200. [DOI] [PubMed] [Google Scholar]
- 74.Singh N, Kuppili RR, Bose K. The structural basis of mode of activation and functional diversity: a case study with HtrA family of serine proteases. Arch Biochem Biophys. 2011;516:85–96. doi: 10.1016/j.abb.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Letunic I, Doerks T, Bork P. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012;40:D302–D305. doi: 10.1093/nar/gkr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jelen F, Oleksy A, Smietana K, Otlewski J. PDZ domains-common players in the cell signaling. Acta Biochim Pol. 2003;50:985–1017. [PubMed] [Google Scholar]
- 77.Tonikian R, Zhang Y, Sazinsky SL, Currell B, Yeh JH, Reva B, Held HA, Appleton BA, Evangelista M, Wu Y, Xin X, Chan AC, Seshagiri S, Lasky LA, Sander C, Boone C, Bader GD, Sidhu SS. A specificity map for the PDZ domain family. PLoS Biol. 2008;6:e239. doi: 10.1371/journal.pbio.0060239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee HJ, Zheng JJ. PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun Signal. 2010;8:8. doi: 10.1186/1478-811X-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krojer T, Pangerl K, Kurt J, Sawa J, Stingl C, Mechtler K, Huber R, Ehrmann M, Clausen T. Interplay of PDZ and protease domain of DegP ensures efficient elimination of misfolded proteins. Proc Natl Acad Sci USA. 2008;105:7702–7707. doi: 10.1073/pnas.0803392105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang CK, Pan L, Chen J, Zhang M. Extensions of PDZ domains as important structural and functional elements. Protein Cell. 2011;1:737–751. doi: 10.1007/s13238-010-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang BH, Gujral TS, Karp ES, BuKhalid R, Grantcharova VP, MacBeath G. A systematic family-wide investigation reveals that ~ 30 % of mammalian PDZ domains engage in PDZ–PDZ interactions. Chem Biol. 2011;18:1143–1152. doi: 10.1016/j.chembiol.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fanning AS, Lye MF, Anderson JM, Lavie A. Domain swapping within PDZ2 is responsible for dimerization of ZO proteins. J Biol Chem. 2007;282:37710–37716. doi: 10.1074/jbc.M707255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sugi T, Oyama T, Muto T, Nakanishi S, Morikawa K, Jingami H. Crystal structures of autoinhibitory PDZ domain of Tamalin: implications for metabotropic glutamate receptor trafficking regulation. EMBO J. 2007;26:2192–2205. doi: 10.1038/sj.emboj.7601651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Im YJ, Park SH, Rho SH, Lee JH, Kang GB, Sheng M, Kim E, Eom SH. Crystal structure of GRIP1 PDZ6-peptide complex reveals the structural basis for class II PDZ target recognition and PDZ domain-mediated multimerization. J Biol Chem. 2003;278:8501–8507. doi: 10.1074/jbc.M212263200. [DOI] [PubMed] [Google Scholar]
- 85.Im YJ, Lee JH, Park SH, Park SJ, Rho SH, Kang GB, Kim E, Eom SH. Crystal structure of the Shank PDZ-ligand complex reveals a class I PDZ interaction and a novel PDZ–PDZ dimerization. J Biol Chem. 2003;278:48099–48104. doi: 10.1074/jbc.M306919200. [DOI] [PubMed] [Google Scholar]
- 86.Biswas S, Biswas I. Role of HtrA in surface protein expression and biofilm formation by Streptococcus mutans . Infect Immun. 2005;73:6923–6934. doi: 10.1128/IAI.73.10.6923-6934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baud C, Hodak H, Willery E, Drobecq H, Locht C, Jamin M, Jacob-Dubuisson F. Role of DegP for two-partner secretion in Bordetella . Mol Microbiol. 2009;74:315–329. doi: 10.1111/j.1365-2958.2009.06860.x. [DOI] [PubMed] [Google Scholar]
- 88.Hoy B, Löwer M, Weydig C, Carra G, Tegtmeyer N, Geppert T, Schröder P, Sewald N, Backert S, Schneider G, Wessler S. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010;11:798–804. doi: 10.1038/embor.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]