Abstract
Posttranslational modification of the neural cell adhesion molecule (NCAM) by polysialic acid (polySia) is well studied in the nervous system and described as a dynamic modulator of plastic processes like precursor cell migration, axon fasciculation, and synaptic plasticity. Here, we describe a novel function of polysialylated NCAM (polySia-NCAM) in innate immunity of the lung. In mature lung tissue of healthy donors, polySia was exclusively attached to the transmembrane isoform NCAM-140 and located to intracellular compartments of epithelial cells. In patients with chronic obstructive pulmonary disease, however, increased polySia levels and processing of the NCAM carrier were observed. Processing of polysialylated NCAM was reproduced in a mouse model by bleomycin administration leading to an activation of the inflammasome and secretion of interleukin (IL)-1β. As shown in a cell culture model, polySia-NCAM-140 was kept in the late trans-Golgi apparatus of lung epithelial cells and stimulation by IL-1β or lipopolysaccharide induced metalloprotease-mediated ectodomain shedding, resulting in the secretion of soluble polySia-NCAM. Interestingly, polySia chains of secreted NCAM neutralized the cytotoxic activity of extracellular histones as well as DNA/histone-network-containing “neutrophil extracellular traps”, which are formed during invasion of microorganisms. Thus, shedding of polySia-NCAM by lung epithelial cells may provide a host-protective mechanism to reduce tissue damage during inflammatory processes.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1342-0) contains supplementary material, which is available to authorized users.
Keywords: Polysialic acid, NCAM, Innate immune system, COPD, Lung
Introduction
In mammalian organisms, polysialic acid (polySia) consisting of α2,8-linked N-acetylneuraminic acid (Neu5Ac) residues is mainly present as a posttranslational modification of the neural cell adhesion molecule (NCAM). As a polyanion at cellular surfaces, polySia plays a crucial role in the regulation of cell–cell contact/repulsion mediating migration during neurogenesis, axonal guidance or synaptic plasticity, and appears to be essential for neuronal development and learning processes [1, 2]. As for the nervous system, polySia is also involved in the embryonic development of other organs derived from mesodermal and endodermal origin such as heart, kidney, pancreas, or the respiratory tract [3–5].
Two polysialyltransferases, ST8SiaII and ST8SiaIV, are responsible for posttranslational modification of NCAM with two or more polySia chains per N-glycan in the 5th immunoglobulin (Ig)-like domain as illustrated in Fig. 1 for NCAM-140 [6–8]. Thereby, polySia chains can gain a size of 50 or more Neu5Ac residues, resulting in an enormous increase of the hydrodynamic radius of polysialylated glycoproteins [9].
Fig. 1.
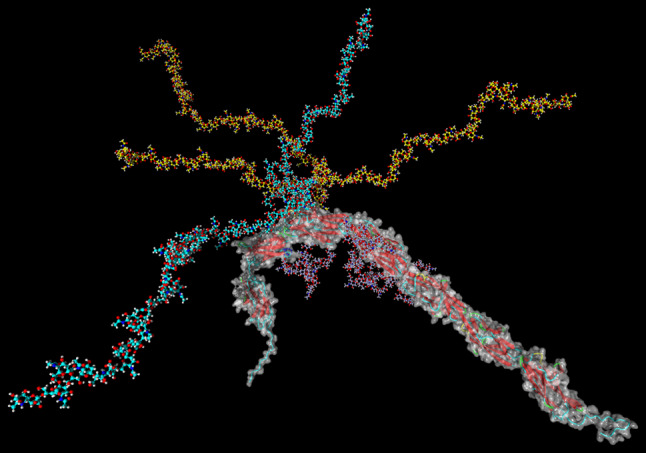
Model of polySia-NCAM-140. Complex-type N-glycan chains were computationally added to N-glycosylation sites N222, N315, N347, N423, N449, and N478 of a homology model of NCAM-140. The glycan chain at position N449 (cyan) contains two polySia chains, one consisting of 38 and one of 20 sialic acid residues. Three polySia chains are present in the N-glycan at position N478 (yellow), consisting of 38, 30, and 20 sialic acid residues. The figure was created with Yasara [59]
Besides NCAM, four other glycoproteins are known to carry polySia; these include a soluble form of CD36 [10] in human milk, the α-subunit of the voltage-gated sodium channel in adult rat brain [11], the synaptic cell adhesion molecule SynCAM1 in postnatal murine brain [12] as well as neuropilin-2 on human mature dendritic cells (mDC) [13]. As a consequence of its impact on diverse cellular functions and the widespread distribution of polySia during embryonic organogenesis, the lack of polysialylation due to genetic ablation of both polysialyltransferases results in a lethal phenotype [14].
Different from the nervous system where polySia functions have been intensively studied, the significance of polysialylation in immunological processes is poorly understood even though the presence of polySia on neuropilin-2 has a major impact on mDC migratory capacity [15, 16]. Moreover, polysialylated glycoproteins were identified in immune cells like monocytes, natural killer cells and multipotent hematopoietic progenitors [13, 17–20] and are discussed to influence the immune response and the progenitor access to the thymus.
The identification of polysialylated NCAM-140 in the trans-Golgi apparatus of lung epithelial cells, interleukin (IL-) 1β-mediated release of soluble polySia-NCAM-110 after ectodomain shedding in vitro and in vivo as well as polySia-NCAM-110 in lung tissue from patients with chronic obstructive pulmonary disease (COPD) prompted us to search for mechanistic relationships. Since IL-1β is an important cytokine of the innate immune system and polySia-NCAM shedding could be similarly induced with bacterial lipopolysaccharides (LPS), a possible role of polySia-NCAM in defense mechanisms of the lung was investigated. The results of our study provide compelling evidence that soluble polySia-NCAM-110 is able to neutralize the cytotoxic activity of extracellular histones [in isolated form or in complex with DNA, the latter designated as “neutrophil extracellular traps” (NET)] and thus may represent a novel endogenous factor to reduce lung tissue destruction or consequences thereof during inflammatory processes such that polySia may serve as a host protective component in innate immunity.
Materials and methods
Materials
NCAM-specific monoclonal antibody (mAb) 123C3 (human) and H28 (mouse) [21, 22] and polySia-specific mAb 735 as well as inactive and active endoN were purified as previously described [23, 24]. For Western blotting, horseradish peroxidase-conjugated secondary antibodies were used (Dako, Hamburg, Germany). All reagents used were of analytical grade.
Human lung samples
The use of donor as well as COPD lung samples was approved by the local research ethics committee, and written consent was obtained from all participants (no. 31/93, 84/93, 29/01). Explanted lungs (n = 16 for COPD) and non-utilized donor lungs or lobes fulfilling transplantation criteria (n = 8) were obtained from the Department of Thoracic Surgery in Vienna, Austria. Lung tissue was snap frozen and stored in −80 °C freezers. Information on mean age, gender, and smoking pack/years is given in Table 1. Out of 16 patients included in this study, 11 were categorized as GOLD IV, two patients as GOLD III, one patient was identified with α1-antitrypsin deficiency. Of note, Prednisone was not prescribed to the COPD patients included in our study.
Table 1.
Information—human lung samples
| Category | COPD | Donors |
|---|---|---|
| Mean age ± SEM | 56.5 ± 1.38 | 44.5 ± 3.31 |
| Sex male/female | 11/5 | 4/4 |
| Smoking, pack/years ± SEM | 46.8 ± 7.5 | Non smokers |
3D structure modeling
The three-dimensional structure of the NCAM-140 protein was established using the homology modeling software Modeler [25]. Template search in the Protein Data Bank [26] and generation of sequence alignments was done with the BLAST service [27]. PDB entries 1QZ1, 2XY1, 2JLL, 2XYC, and 3MTR were selected as templates for modeling. These entries cover the Ig and Fn3 domains of NCAM or NCAM-2 in an overlapping manner (see Fig. S1). N-glycan chains were modeled with Sweet-II [28] and added to the protein model with the GlyProt tool [29] of the glycosciences.de web portal [30]. The polySia chains of the glycan models are initially computed using identical torsion angles between each pair of sialic acid residues, resulting in regular helices. To obtain a model that resembles better the natural shape of the glycans, a molecular dynamics simulation using the Yasara software [31] with AMBER03 force field [32] was performed.
Tissue homogenization and purification of polysialylated proteins
Tissue samples were homogenized in lysis buffer consisting of 50 mM Tris/HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1 % Triton-X-100, 5 % sodium deoxycholate, 2 mM PMSF, 1,000 U/ml aprotinin, and 1 mM leupeptin. Extracts were centrifuged and protein concentrations of supernatants were determined by BCA protein assay (Thermo Scientific, Bonn, Germany). For purification of polysialylated proteins, inactivated enzyme endoN was coupled to magnetic dynabeads M-280 tosylactivated (Invitrogen, Darmstadt, Germany) according to the manufacturer’s instructions. Beads and extracted samples (always identical volume and protein concentrations for comparison measurements) were incubated overnight at 4 °C on a shaker. Beads were washed twice with washing buffer 1 [20 mM Tris/HCl (pH 7.5), containing 150 mM NaCl, 0.5 % Triton-X-100], twice with washing buffer 2 [20 mM Tris/HCl (pH 7.5), containing 150 mM NaCl], and finally bound polySia-carriers were eluted with 100 mM triethylamine. Thereafter, samples were directly dried in a SpeedVac concentrator for further analysis.
Western blotting
Purified polysialylated proteins (protein amount was too low for quantification) or complete tissue lysates (10 μg/lane) were separated by 10 % SDS-PAGE under reducing conditions, followed by transfer of samples onto a PVDF membrane. Immunostaining was carried out with anti-polySia (mAb 735; 1 μg/ml), anti-human NCAM [mAb 123C3, 1.5 μg/ml; mAb OB-11, 5 μg/ml (Sigma-Aldrich, Taufkirchen, Germany]; mAb 123A8, 0.5 μg/ml (Santa Cruz biotechnology, Heidelberg, Germany) or anti murine NCAM (mAb H28, 1 μg/ml) mAb, in association with labeled secondary Ab and chemiluminescence SuperSignal kit (Thermo Fisher, Kehl, Germany) for detection.
MS-based protein identification
Eluted polySia-carriers were separated by electrophoresis on 7.5 % ready-to-use SDS gels (Bio-Rad, Munich, Germany), followed by in-gel digestion with trypsin according to the manufacturer’s instructions (Promega, Mannheim, Germany) [12]. Extracted peptides were separated on a C18 column (PepMap, 3 μm, 75 μm × 100 mm, Dionex) using an Ultimate nanoLC system (Dionex, Idstein, Germany) with a linear gradient from 10 % acetonitrile/0.1 % formic acid to 60 % acetonitrile/0.1 % formic acid in 30 min at a flow rate of 0.2 ml/min. Peptides were directly spotted by a Probot (Dionex) onto a matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) steel target (Bruker Daltonics, Bremen, Germany) and mixed with an equal volume of 2,5-dihydrobenzoic acid (DHB) matrix (7.5 mg DHB/ml, 1 % phosphoric acid, 50 % acetonitrile). Peptide mass fingerprints of tryptic digests were obtained by MALDI-TOF-MS using an Ultraflex I TOF/TOF mass spectrometer (Bruker Daltonics). MS and MS/MS spectra were acquired in reflector mode using FlexControl 2.4 software, and analyzed by the FlexAnalysis software 3.0 (both Bruker Daltonics). External calibration of mass spectra was carried out using peptide calibration standard for MS (Bruker-Daltonics), and annotations of fragment ions in the MS/MS mode was performed according to [33].
Analysis of polySia by HPLC
To analyze the chain-length pattern of polySia, a DMB–HPLC method was used as described in detail previously [7, 34]. To this end, purified polySia-carriers were dissolved in 100 μl DMB reaction buffer, and incubated for 24 h at 4 °C. The reaction was stopped by adding 25 μl 1 mM NaOH, and released polySia chains were separated by HPLC on a DNAPac PA-100 column (Dionex); DMB-labeled polySia chains were detected with a fluorescence detector. MilliQ water (E1) and 1 M NaNO3 (E2) were used as eluents at a flow rate of 1 ml/min. Elution was performed by the following gradient: T 0 min = 0 % E2; T 5 min = 1 % E2; T 15 min = 10 % E2; and T 60 min = 50 % E2. The column was washed with 100 % E2 for 10 min.
Immunohistochemistry/immunofluorescence
Immunohistochemical staining was performed using the streptavidin–biotin–alkaline phosphatase method according to the manufacturer’s instructions (Zytomed Systems, Berlin, Germany). Paraffin-embedded lung tissue sections were cut into 5-μm serial sections. After rehydration in xylene and a following ascending ethanol series, lung sections were incubated with blocking solution for 5 min, followed by incubation overnight at 4 °C with the respective primary antibodies mAb 735 (10 μg/ml) or mAb 123C3 (20 μg/ml) diluted in PBS containing 2 % (w/v) bovine serum albumin (BSA).
As negative control of the polySia-staining, serial lung sections were pre-treated with endoN (3 μg/ml in PBS/0.1 % BSA) overnight at 37 °C before incubation with mAb 735. To identify polySia-positive cells, different cell markers were applied: Basal cells: polyclonal anti-KRT5 antibody (abcam, Cambridge, UK); ciliated bronchio–alveolar epithelial cells: anti-HFH4 mAb (abcam); Clara cells: anti-CC10 antibody (R&D systems); cell proliferating marker Ki-67 (Sigma). After three washes in PBS, a polyvalent secondary biotinylated antibody (ZytoChem-Plus AP Kit) was applied for 15 min, followed by incubation with AP-conjugated streptavidin for 15 min. Tissue sections were developed with Fast Red substrate solution (ZytoChem-Plus AP Kit) and the reaction was terminated by washing with distilled water. For staining of murine mouse sections the Envision+ System HRP Kit (Dako) was used. The stained sections were counterstained with hemalaun (Roth, Karlsruhe, Germany) and mounted in MOWIOL supplemented with 1,4-diazadicyclo(2)octane (DABCO, Fluka, Neu-Ulm, Germany). Lung tissue sections were scanned with a Mirax Desk slide scanning device (Zeiss, Oberkochen, Germany).
For immunofluorescence A549 cells were grown on coverslips and fixed with ice-cold methanol. Unspecific binding was blocked with 1 % BSA/PBS (blocking buffer), and the cells were labeled with biotinylated, inactive endoN as well as a polyclonal Ab against the trans-Golgi-network protein 38 (α-TGN 38) (Sigma) (10 μg/ml) for 1 h in blocking buffer. The staining was performed with FITC (fluoresceinisothiocyanate)-conjugated Streptavidin and Rhodamin-conjugated α-mouse IgG, respectively, for 45 min. The samples were embedded in MOWIOL/Dabco (Roth). Images were taken with a confocal laser-scanning microscope (Zeiss LSM510 Meta).
mRNA analysis of ST8SiaII and ST8SiaIV
Total cellular RNA was prepared from human lung tissue frozen at the time of explantation. Reverse transcription was performed using 1 μg total RNA, random hexamer primers (Applied Biosystems) and Superscript II reverse transcriptase (Invitrogen) according to the instructions of the manufacturer. Quantitative PCRs were performed with intron-spanning primers using the Platinum SYBR Green qPCR SuperMix (Invitrogen) according to the instructions of the manufacturer. Reaction mixtures were pre-heated at 50 °C for 2 min and at 95 °C for 2 min, followed by 40 cycles at 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. Emitted fluorescence was detected online using a Mx3000P real-time PCR system (Stratagene). For all primer pairs the amplification products were confirmed by sequencing, no template control and dissociation curve analysis. In addition, amplification efficiency was determined by analyzing the slope of a CT/log (template concentration) plot. For normalization, primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used (rE = 1/2ΔCT). Primers used in quantitative PCR expression analysis: GAPDH for 5′-AGTCAACGGATTTGGTCGTAT-3′; GAPDH rev 5′-ACCATGTAGTTGAGGTCAATGAAG-3′, ST8SiaII for 5′-CCAGCTGTTGTTGACAGAAGTAA-3′; ST8SiaII rev 5′-TAAAATCTGCTTCCTGATCCTC-3′; ST8SiaIV for 5′-CTTCCAGCACAATGTAGAAGGTTG-3′; ST8SiaIV rev 5′-GCTCTTGACCACTGACACATCTC-3′; NCAM for 5′-GTGTGGTTACAGGCGAGGAT-3; NCAM rev 5-GATGACATCTCGGCCTTTGT-3′.
RNAeasy micro kit (Qiagen, Hilden, Germany) was used to isolate RNA of lung epithelial cell lines. Eluted RNA was used as a template for the synthesis of first-strand cDNA using GeneAmp RNA PCR kit (Applied Biosystems, Darmstadt, Germany).
Intron spanning primers were used to amplify NCAM, ST8Sia II, ST8Sia IV, and GAPDH targets using AmpliTaq gold DNA polymerase (Applied Biosystems) according to the manufacturer’s introductions. cDNA samples were denatured at 94 °C for 12 min, followed by 40 cycles of each 30 s at 94 °C, 30 s at 58 °C and 30 s at 72 °C. PCR samples were kept at 72 °C for 10 min and stored at 4 °C. Amplified products were separated by gel electrophoresis using 2 % agarose gel.
Cell culture experiments
A549 cells were cultured in Dulbecco’s MEM, containing l-glutamine, 10 % FCS and 1 % Penicillin/Streptomycin (PAA Laboratories, Marburg, Germany). For the following experiments 105 cells/well were counted and seeded onto 24-well plates. A549 cells were incubated with different concentrations of LPS (from E. coli strain 0127:B8 Sigma) or IL-1β (R&D Systems, Wiesbaden, Germany), diluted in RPMI 1640 without phenol red (PAA) and FCS, for 5 h at 37 °C in 5 % CO2-atmosphere. To inhibit metalloprotease-dependent processes, cells were treated with (TNF-α Protease Inhibitor-2) in a final concentration of 50 μM.
For immunostaining against polySia, NCAM, and TGN38, cells were fixed with methanol at −20 °C for 10 min, blocked with 1 % BSA in PBS for 15 min, and incubation with appropriately diluted primary antibodies or biotinylated inactive endoN was continued for 1 h. After three washing steps with PBS and exposure to streptavidin–FITC conjugate and an anti-mouse-Rhodamine secondary antibody (Dianova, Hamburg, Germany), the final mounting VECTASHIELD with DAPI (Linaris, Munich, Germany) was added to the fixed cells.
Unstimulated and stimulated cells were incubated overnight with different concentrations of histones (5, 10, and 15 μg/ml) (Sigma-Aldrich) or NET. NET generation from neutrophils and subsequent isolation was described earlier in detail [35]. Cytotoxicity was determined with the lactate dehydrogenase (LDH) cytotoxicity assay (Roche Applied Science, Mannheim, Germany) according to the instructions of the manufacturer.
Bleomycin administration
Animal experimentation was approved by the local Committee on Animal Investigations. Adult C57Bl6/N mice weighting 18–20 g were obtained from Janvier, France. Animals were housed under room temperature and 12/12 h light/dark cycle. They had free access to drinking water and were fed ad libitum. At day 0 of the experiment mice were anesthetized with isoflurane (Baxter, Germany) and intubated with a 20-G tube under a binocular loupe; 200 μl of bleomycin (Bleomycin Hexal 15000 IE, N1) dissolved in sterile saline (5 U/kg bodyweight) or 200 μl saline (control group) was applied intratracheally via a microsprayer (Model IA-1C, Penn Century, Philadelphia, PA, USA). After application of bleomycin mice were ventilated, if necessary, then extubated and transferred back to their cages.
At day 7, the mice were killed by intraperitoneal injection of pentobarbital (Merial GmbH, Hallbergmoos, Germany) and the lungs were excised for histological and molecular biological analysis.
Results
NCAM-140 is polysialylated in adult human lungs
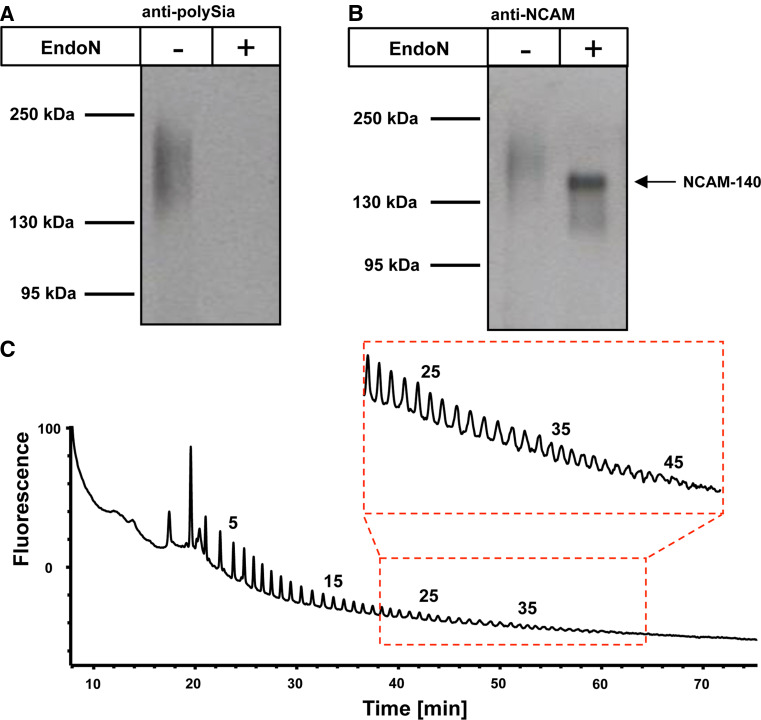
So far, pulmonary expression of polySia has been described exclusively in the developing but not in the mature lung [5]. However, using Western-blot analysis for sensitive detection of polysialylated proteins, we found polySia in protein-lysates of adult human lungs (Fig. 2a). Staining with a mAb against polySia revealed a diffuse band in a molecular mass region between 140–250 kDa. Pretreatment of the samples with endoneuraminidase (endoN)—an enzyme that specifically cleaves α2,8-linked polySia [24]—completely abolished immunostaining confirming the specificity of the polySia signal and the presence of polySia in mature lung tissue.
Fig. 2.
Detection of polySia-NCAM-140 in protein lysates of healthy human lung donors. a Lung homogenates of human tissue were separated by SDS-PAGE for Western blotting using 10 μg protein per lane with or without endoN pretreatment. Immunostaining was performed with an anti-polySia mAb. Apparent molecular weights of standard proteins are indicated in kDa. b Polysialylated NCAM was isolated using inactive endoN coupled to magnetic beads and visualized by Western blotting using a mAb against NCAM (mAb 123C3). De-polysialylated NCAM was additionally examined after endoN treatment. c PolySia chains were released from purified polySia-NCAM and directly labeled with DMB in situ. Resulting fluorescently tagged sialic acid polymers were separated on an anion exchange column according to polySia chain length. Respective chain length of polySia residues is given for selected peaks on top of the profiles
To identify the underlying polySia carrier, a glycoproteomics approach was performed. Polysialylated glycoproteins were isolated from lung lysates using an inactive form of endoN coupled to magnetic beads. EndoN has an extended polySia binding site and abolishing enzymatic activity by targeted mutation of active site residues gives rise to a high affinity lectin that specifically binds to α2,8-linked polySia [36, 37]. Purified polysialylated proteins were separated by SDS gel electrophoresis followed by tryptic in-gel digestion. The resulting peptide mass fingerprinting as well as peptide fragmentation analyses of extracted tryptic peptides led to the identification of NCAM as polySia carrier protein (Fig. S2). Subsequent Western-blot analysis with an anti-NCAM mAb that recognizes all three major isoforms of NCAM [the transmembrane isoforms NCAM-140 and NCAM-180 as well as the glycosyl-phosphatidylinositol (GPI)-anchored isoform NCAM-120] [38] revealed that only NCAM-140 served as polySia carrier. While polysialylated NCAM migrated as diffuse band centering at ~200 kDa, the signal collapsed after endoN treatment, and a single focused band at 140 kDa appeared, representing NCAM-140 (Fig. 2b). While the polysialylated glycoprotein was hardly detectable, the protein moiety itself could be very clearly visualized after removal of polySia by endoN treatment. The poor visibility of the polysialylated glycoprotein can be explained by the bulky and highly negative properties of polySia. The antibody binding to the protein might be similarly inhibited as the NCAM–NCAM interaction. Furthermore, the polysialylated glycoproteins are distributed to a greater area on the blotting membrane.
Since the biological function of polySia depends on its chain length [39], the degree of polymerization (DP) was analyzed. Therefore, polySia-chains of purified polySia-NCAM were released, labeled with 1,2-diamino-4,5-methylenedioxybenzene (DMB), and separated according to chain length by anion exchange chromatography. Obtained chromatograms revealed the presence of polySia chains with more than 40 sialic acid residues on N-glycans of NCAM-140 (Fig. 2c). The average chain length of polySia in human lungs could not be characterized by the applied DMB approach, since internal cleavages occur during fluorescence labeling.
Localization of polySia-NCAM-140 in lung epithelial cells
To investigate which respiratory tract cells express polySia-NCAM, serial sections of lung biopsies from healthy donors were stained for both polySia and NCAM. Besides a few scattered epithelial cells in the alveolar regions as well as peripheral immune cells, immunostaining against polySia and NCAM was preferentially concentrated in bronchial epithelial cells (Fig. 3) as confirmed by co-staining with ciliated bronchial epithelial cell markers (Fig. S3). In contrast, basal cells and proliferating cells (Ki-67-positive) were polySia-negative (Fig. S3). Notably, polySia-NCAM in the mature lung was localized to intracellular structures but not to the cell surface as during lung development [5].
Fig. 3.
Immunohistological localization of polySia-NCAM-140 in lung tissue of healthy human donors. Paraffin embedded serial human lung sections were stained with a mAb against NCAM (mAb 123C3) and polySia (arrows). For negative control of polySia-staining lung sections were exposed to endoN. Scale bars 50 μm
Up-regulation of polySia-NCAM and polysialyltransferases during COPD
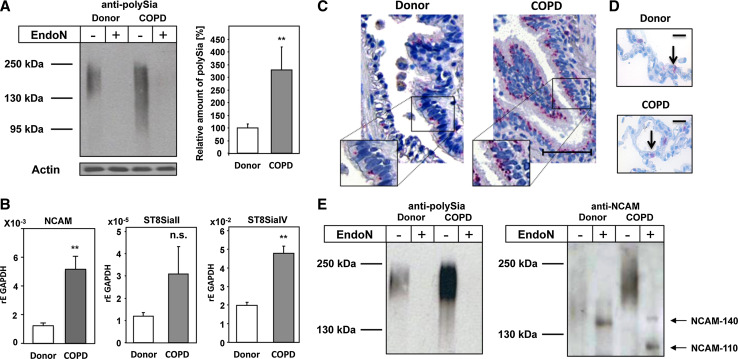
Compared to healthy donors, pulmonary expression of polySia was increased in COPD patients (Fig. 4a). The prominently elevated polySia levels observed under disease conditions were accompanied by significantly enhanced mRNA levels of NCAM (~4-fold) (Fig. 4b). In addition a ~3- or a ~2.5-fold up-regulation of the two polysialyltransferases ST8SiaII and ST8SiaIV was detected, respectively (Fig. 4b). However, only upregulation of ST8SiaIV appeared to be significant. Although transcripts of both enzymes were detected, a ~100-fold higher mRNA level was found for ST8SiaIV in comparison to ST8SiaII, suggesting that ST8SiaIV is the predominant polysialyltransferases in the respiratory system.
Fig. 4.
Expression and distribution of polySia-NCAM in lungs of COPD patients. a PolySia was analyzed in lung lysates of healthy donors and COPD patients by Western blotting. As specificity control, lysates were exposed to endoN prior to analysis. As loading control actin was used. Apparent molecular masses of standard proteins are indicated in kDa. Protein bands representing polySia were quantified by densitometry, and values are means of eight healthy donors and ten COPD patients each (100 % was set for healthy donors). b mRNA expression levels of NCAM, ST8SiaII and ST8SiaIV in lungs of donor and COPD patients were determined by quantitative real-time PCR; GAPDH was used as standard housekeeping gene. Values represent means of three donors and 11 COPD patients, respectively. The statistical evaluation was performed by Student’s t test (unequal variances, two-tailed). Significance levels are indicated by n.s. (not significant), p > 5 %, *p < 0.05, **p < 0.01, ***p < 0.001. c, d The distribution of polySia was visualized with anti-polySia mAb on paraffin embedded lung section from healthy donors and COPD patients; scale bar equals c 50 μm and d 25 μm. e Polysialylated NCAM were purified from lung lysates of donor and COPD patients and visualized by Western blotting using a mAb against polySia and an anti-NCAM (mAb 123C3) before and after polySia degradation by endoN
To study the spatial expression pattern of polySia in lungs of COPD patients, paraffin embedded sections were stained with an anti-polySia antibody. In tissue from both, healthy donors and COPD patients, polySia-positive immunostaining was prevalently located in bronchial epithelial cells in addition to scattered alveolar cells (Fig. 4c, d). However, in diseased tissue, more intense cell-associated polySia-positive granules in bronchial epithelial cells as well as luminal staining were observed. A real comparison of the staining intensity in alveolar cells was not possible, since the polySia staining was more scattered and more heterogeneous in comparison to polySia-positive bronchial cells.
Prior Western-blot analysis of tissue lysates before and after pretreatment with endoN demonstrated that polySia was attached to NCAM-140. In contrast to healthy donors, however, the signal intensity of NCAM-140 was reduced in COPD patients (Fig. 4e). Moreover, an additional NCAM signal with an apparent molecular mass of 110 kDa was observed (Fig. 4e), which might represent a proteolytically processed form of NCAM-140. This isoform was also detectable by a different mAb against all NCAM isoforms (mab 123A8) but not with a mAb against the cytosolic domain of NCAM-140 and NCAM-180 (mAb OB-11) (Fig. S4). These data demonstrate that the pathophysiological conditions in COPD patients promote up-regulation of polysialyltransferases, an increase in polySia and NCAM expression as well as the processing of the extracellular part of the polySia carrier NCAM-140.
Activation of the inflammasome leads to the generation of polySia-NCAM-110 in murine lung tissue
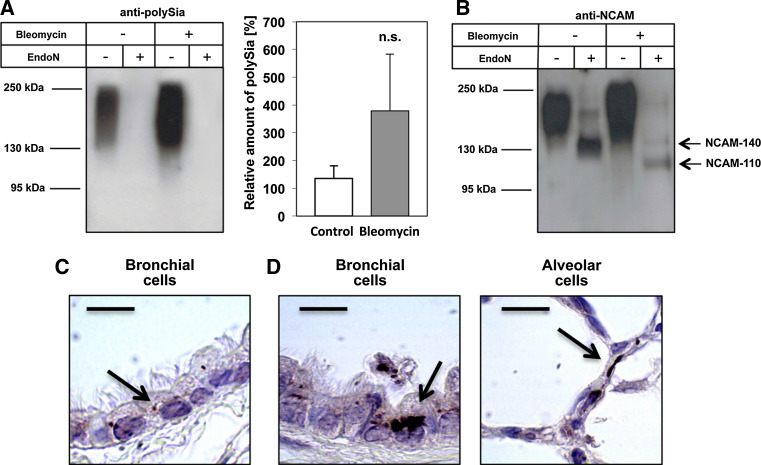
COPD is an inflammatory disease, characterized inter alia by an increased production of the cytokine IL-1β [40–43]. To investigate whether the observed increase in polySia expression in COPD patients is a response to inflammatory conditions, we used a mouse model of bleomycin-induced pulmonary inflammation and fibrosis [44, 45]. Bleomycin induces DNA strand breaks and cell injury, resulting in activation of the inflammasome and secretion of IL-1β. As demonstrated in Fig. 5a, single-dose intratracheal administration of bleomycin tended towards increased polySia expression. Furthermore, bleomycin-induced activation of the inflammasome was accompanied by appearance of NCAM-110 in addition to NCAM-140, which was also present in untreated mice (Fig. 5b). Analysis of lung sections revealed that in both control and bleomycin-treated mice, polySia was mainly expressed by bronchial epithelial cells (Fig. 5c, d). In bleomycin-treated mice, however, the level of pulmonary polySia was increased and polySia was also detected in alveolar cells (Fig. 5d). In the case of the applied mouse model, the upregulation in alveolar cells was detectable, since we observed no polySia-positive alveolar cells in untreated mice. These data indicate that activation of the inflammasome results in processing of the NCAM protein scaffold.
Fig. 5.
Activation of the inflammasome leads to increased levels of polySia-NCAM-110 in murine lung tissue. Western-blot analyses of lysated lung tissue of bleomycin-treated and control mice were performed using a mAb against a polySia and b NCAM (mAb H28) before and after degradation of polySia by endoN. To this end, polysialylated NCAM was isolated using inactive endoN coupled to magnetic beads. a Protein bands representing polySia were quantified by densitometry, and values are means of three NaCl-treated and three bleomycin-treated mice (100 % was set for three untreated mice). The statistical evaluation was performed by Student’s t test (unequal variances, two-tailed). Significance levels are indicated by n.s. (not significant), p > 5 %, *p < 0.05, **p < 0.01, ***p < 0.001. Serial murine lung sections of c untreated mice and d mice treated with bleomycin were stained with a mAb against polySia. Arrows indicate immunostaining. Scale bars 10 μm
IL-1β and LPS induce release of soluble polySia-NCAM
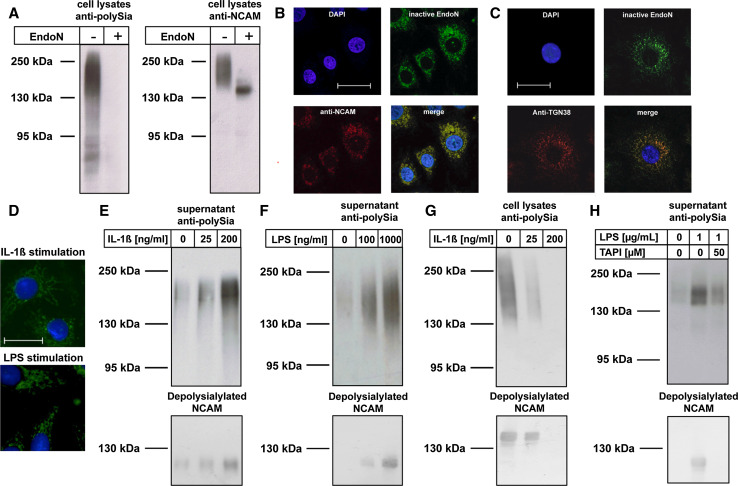
Epithelial cells express the IL-1β receptor and are part of the first line defense of the respiratory innate immune system [46]. To investigate whether IL-1β triggers processing of polySia-NCAM, we used the human lung epithelial cell line A549, which is discussed to be of alveolar origin and serves as an established cell culture model to study fundamental immunological processes of the lung epithelium [35, 47, 48]. The presence of polySia-associated NCAM in A549 cells was confirmed by Western blotting and demonstrated the predominant expression of polysialylated NCAM-140 (Fig. 6a). Moreover, as shown by RT-PCR, both polysialyltransferases, ST8SiaII and ST8SiaIV, were expressed with ST8SiaIV being the prevalent enzyme (Fig. S5). In fixed A549 cells, co-staining of polySia and NCAM as well as polySia and a trans-Golgi marker revealed the localization of polySia-NCAM in the late secretory sorting station of the Golgi apparatus (Fig. 6b, c, respectively). Treatment of A549 cells with IL-1β did not change the expression levels of NCAM, ST8SiaII and ST8SiaIV (Fig. S6) as well as the association of polySia-NCAM within the late trans-Golgi network (Fig. 6d). However, analysis of the cell culture supernatant revealed that administration of IL-1β resulted in secretion of soluble polySia-NCAM-110 (Fig. 6e), an effect that was also observed after stimulation of A549 cells with LPS (Fig. 6f). Interestingly, the increase of soluble polySia-NCAM correlates with a decrease of cell-associated polySia-NCAM-140 as exemplarily shown for IL-1β-treated cells (Fig. 6g).
Fig. 6.
The secretion of intracellularly localized polySia-NCAM of human lung epithelial cell line A549 is induced by IL-1β and LPS. a Western-blot analyses of cell lysates of human A549 cells were performed using a mAb against polySia and NCAM (123C3) before and after endoN treatment. b, c Cultured A549 cells were fixed with methanol and stained with fluorescently labeled inactive endoN and b anti-NCAM mAb 123C3 or c polyclonal antibodies to visualize the trans-Golgi apparatus (anti-TGN38). For nuclear staining DAPI was used. d In addition, polySia was visualized in cells after stimulation with IL-1β (200 ng/ml) or LPS (100 ng/ml). Scale bars for c and d = 25 μm. e, f Polysialylated proteins were purified from supernatants of differently treated A549 cells and visualized by Western blotting using an anti-polySia mAb and after depolysialylation with a mAb against NCAM (123C3). g In an analogous cell lysates of IL-1β-treated cells were analyzed. h Western blots against polySia and NCAM (after depolysialylation) were performed using supernatants of untreated A549 cells as well LPS-stimulated cells in the absence or presence of TAPI-2. Apparent molecular masses of standard proteins are indicated in kDa
In neuronal cells, proteolytic cleavage and ectodomain shedding of NCAM has been shown to be mediated by “a disintegrin and a metalloproteinase” (ADAM) variants [49, 50]. To find out whether IL-1β/LPS-induced secretion of polySia-NCAM depends on ADAMs activity, the metalloproteinase inhibitor TAPI-2 was added to the cell culture medium. Western-blot analysis of the cell supernatant demonstrated that secretion of polySia-NCAM was efficiently blocked by TAPI-2 (Fig. 6h), demonstrating that secretion of polySia-NCAM by bronchial epithelial cells is based on metalloproteinase-mediated ectodomain shedding of polysialylated NCAM-140.
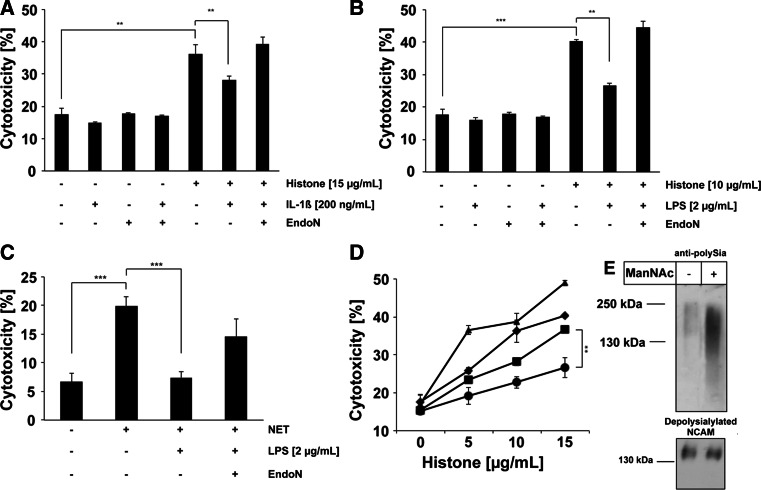
Soluble polySia-NCAM-110 compensates the cytotoxic activity of extracellular histones and neutrophil extracellular traps (NET)
Based on our previous finding that colominic acid [an α2,8-linked Neu5Ac-polymer of Escherichia coli (E. coli) K1, equivalent to polySia in mammals] can abolish the cytotoxic effect of extracellular histones [35], experiments with A549 cells were conducted to establish a possible protective role of soluble polySia-NCAM-110. When unstimulated A549 cells were treated with isolated extracellular histones, the cytotoxicity rate increased (Fig. 7a, b). However, cells pre-activated by IL-1β or LPS (which led to the release of soluble polySia-NCAM-110) were significantly protected against the cytotoxic activity of isolated histones. Since this resistance towards histone-mediated cytotoxicity was abolished by pretreatment with endoN, the cell-protective effect can be associated with the polySia chains of shedded NCAM (Fig. 7a, b). In an analogous manner, DNA/histone-containing NET induced and increased the mortality rate of A549 cells, an effect that was fully compensated by LPS-induced secretion of polySia-NCAM-110 (Fig. 7c). Parallel degradation of polySia by endoN restored NET-mediated cytotoxicity, demonstrating that the protective function of secreted NCAM relies on its modification by polySia.
Fig. 7.
Soluble polySia-NCAM decreases histone- and NET-mediated cytotoxicity and stimulation of polysialylation increases the protective capability of soluble polySia-NCAM. a, b A549 cells were exposed to histones (10 μg/ml) and the cytotoxicity was determined. In parallel, A549 cells were stimulated by a IL-1β (200 ng/ml) and b LPS (2 μg/ml). To calculate the impact of polySia, endoN was added to the cell culture medium. c Moreover, cells were exposed to NET with or without LPS (2 μg/ml) stimulation and/or additional supplemental degradation of polySia by endoN. d A549 cells were exposed to increasing amount of histones and the cytotoxicity was determined (diamonds). In parallel, A549 cells were stimulated by IL-1β (200 ng/ml) (squares). To examine a possible effect of Neu5Ac precursors on the protective capability of soluble polySia-NCAM, A549 cells were fed with ManNAc prior to IL-1β stimulation (circles). The impact of polySia after ManNAc supplemental was evaluated by endoN treatment during IL-1β stimulation (triangles). e The influence of ManNAc treatment on polySia biosynthesis was analyzed by Western blotting using antibodies against polySia. In addition, depolysialylated NCAM was visualized with mAb 123C3. All values ± SD in Fig. 7 are means of three independent experiments. The statistical evaluation was performed by Student’s t test (unequal variances, two-tailed). Significance levels are indicated by ns (not significant), p > 5 %, *p < 0.05, **p < 0.01, ***p < 0.001
Since the degree of polysialylation of NCAM can be increased by supplementing the cell culture medium with sialic acid precursors such as mannosamine (ManN) [51], this may represent a possibility to improve the cellular resistance against histone-mediated cytotoxicity. To optimize the cellular uptake of mannosamine, the respective per-acetylated form (ManNAc) was used [52]. As shown in Fig. 7d, addition of sialic acid precursors to the cell culture medium was found to effectively increase the resistance and protection against histone-mediated cytotoxicity due to a substantially elevated quantity of polySia per NCAM molecule (Fig. 7e). This effect was completely reversible by polySia degradation (Fig. 7d).
Taken together, these results indicate that the secretion of soluble polySia-NCAM-110 counteracts the cytotoxicity mediated by both, extracellular histones and NET, implicating a potent cytoprotective function for polySia.
Discussion
In the present study, we showed that epithelial cells of healthy adult humans expressed polysialylated NCAM-140. Besides polysialylated NCAM no further polysialylated glycoproteins were identified. However, a proteomics approach can never completely assure that all proteins were identified. Thus, the presence of small amount of further carriers cannot be totally excluded. In contrast to the organogenesis, polySia-NCAM was localized to intracellular structures and was not found at the cell surface [5]. Notably, elevated levels of polySia and a significant up-regulation of NCAM and ST8SiaIV were detected in lung tissue of COPD patient. Furthermore, polySia-NCAM was processed during this disease, which is characterized by chronic lung inflammation resulting in a permanent induction of IL-1β secretion [40]. In addition, all COPD patients were smokers and cigarette smoke has been reported to induce IL-1β formation [42, 43, 53].
In agreement with this observation, polySia-NCAM processing was also detected in murine lungs after the activation of the inflammasome by bleomycin. In contrast to COPD patients, however, the total quantity of polySia-NCAM was not significantly enhanced. The observed differences between COPD patients and bleomycin-treated mice could be the consequence of several reasons. For example, COPD is a chronic disease, whereas in the mouse model the reaction is a consequence of a single dose bleomycin treatment. Furthermore, in COPD patients the inflammasome is triggered by a combination of numerous impulses like pathogens, cigarette smoke, therapeutics, and environmental pollutants. In addition, a very recent study could show that especially during inflammatory processes rather diverse gene expression profiles were observed in man and mice [54]. Thus, mouse models are only comparable to a limited extend to the human situation.
In order to study the observed process, which occurs during inflammation, we used a human cell culture model demonstrating that treatment with LPS and/or IL-1β stimulation resulted in the secretion of soluble polySia-NCAM-110 by ectodomain shedding. The cleavage of polySia-NCAM could be prevented using metalloproteinase inhibitor TAPI-2. Thus, ectodomain shedding of polySia-NCAM was mediated by metalloproteinases. Interestingly, van Oosterhout et al. [55] detected a generally enhanced activity of the metalloproteinase ADAM 17 in bronchial epithelial cells of COPD patients, which is responsible for NCAM shedding on neuronal cells [56]. An upregulation of the expression levels of NCAM and the polysialyltransferases was not induced in the cell culture model by LPS and/or IL-1β treatment. Likely, the interplay with other -so far unknown- cytokines and/or pathological factors appears to be necessary for the observed phenotype in COPD patients. Taken together, our results demonstrated that ectodomain shedding of polySia-NCAM-140 is linked to mechanisms of the innate immune system.
For this reason, we aimed to elucidate a putative biological function of soluble polySia-NCAM-110 during invasion processes by pathogens in the lung. Thereby, we focused our experiments on polySia-dependent mechanisms because polySia chains represent the predominant domain of this glycoprotein, which is often neglected (see model of polySia-NCAM-140 in Fig. 1). An inflammatory response, e.g., during COPD, may lead inter alia to the recruitment of neutrophils which play an important role in the clearance of pathogens [57]. One mechanism of neutrophils against invading microorganisms is the formation of NET, whereby DNA in complex with histones and degranulated proteins form an anti-microbial meshwork to capture and kill pathogens [58]. However, NET and also extracellular histones alone are “double-edged swords” since their anti-microbial activity is combined with cytotoxicity to bystander host cells, such as bronchial epithelial cells [35, 59, 60].
Here, we demonstrated that the release of polySia-NCAM counteracts the cytotoxic effects of extracellular histones and NET. Thereby, polySia might play an important role in the protection of host cells during inflammatory processes as summarized in Fig. 7. Likewise, polysialylated glycoconjugates on the surface of monocytes and mDCs may provide a protective shield against the cytotoxicity of NET since these cells have to migrate into such hot spots of inflammation. The detailed binding properties of polySia-NCAM to histones and NET could not be clarified so far. Our findings show that the sialic acid polymers can consist of more than 40 sialic acid residues. The precise structural requirements for the cytoprotective effect (e.g., the polySia chain length and the number of polySia chains per N-glycans), however, need to be further investigated using defined polySia chains as well as polySia-glycopeptide standards. Two or more adjacent flexible chains—as illustrated in Fig. 1—may produce probably more efficient binding “pockets” than one chain alone.
Besides its protective functions, shedded polySia-NCAM-110 may also serve as modulator of immune cells by specific interaction with siglecs (sialic acid binding Ig-like lectins) that recognize α2,8-linked sialic acid residues [61, 62]. This type of interaction has been described for siglec-11 on microglia (tissue macrophages of the brain), recognizing polySia on neurons in trans leading to inhibition of pro-inflammatory pathways and a reduced IL-1β expression [63].
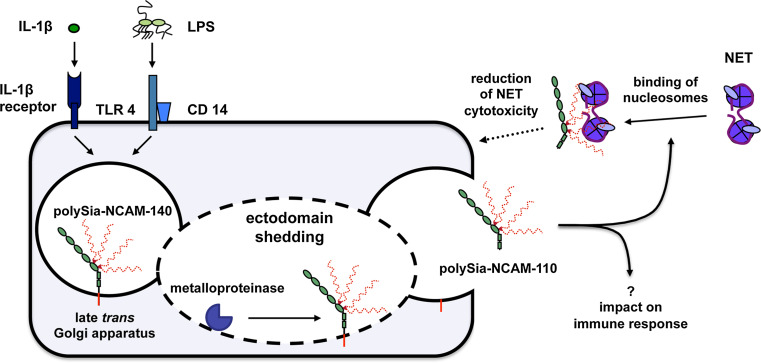
In summary, our data provide compelling evidence that the ectodomain of polysialylated NCAM-140 of lung epithelial cells is shedded by metalloproteinases in an IL-1β- and/or LPS-dependent manner and that soluble polySia-NCAM-110 will counteract the cytotoxicity of extracellular histones and NET (Fig. 8). Consequently, soluble polySia-NCAM may represent a novel player in the orchestra of the lung’s innate immune system and stimulation of polysialylation by sialic acid precursors or the direct administration of polySia might be useful strategies to combat diseases with strong exaggerated NET formation like sepsis, systemic lupus erythematosus, transfusion-related acute lung injury, deep vein thrombosis or cystic fibrosis [64–68].
Fig. 8.
Proposed model for the secretion of polySia-NCAM-110 and its subsequent cytoprotective function. Stimulation of lung epithelial cells by IL-1β or LPS induced ectodomain shedding of polySia-NCAM-140 by metalloproteinases resulting in the release of polySia-NCAM-110. PolySia chains of soluble NCAM-110 bind nucleosomes, thereby inhibiting their cytotoxic activity
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Christina Galuska and Kai Maass for many helpful discussions during preparation of the manuscript and proofreading as well as Werner Mink and Siegfried Kühnhardt for expert technical assistance. This work was supported by the Excellence Cluster Cardiopulmonary System (ECCPS) from the Deutsche Forschungsgemeinschaft (DFG) (Bonn, Germany), by the von Behring Röntgen Stiftung, by the BMBF-Clinical Research Group “Pneumonia” (Ministry for Education and Research, Berlin, Germany) as well as the LOEWE-program “Insect Biotechnology” (state of Hessen, Wiesbaden, Germany). RGS received financial support by the DFG in the framework of DFG Research Unit 548.
Footnotes
M. Saffarzadeh, P. Mahavadi, and S. Müller contributed equally to this work.
References
- 1.Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9(1):26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- 2.Hildebrandt H, Muhlenhoff M, Weinhold B, Gerardy-Schahn R. Dissecting polysialic acid and NCAM functions in brain development. J Neurochem. 2007;103(Suppl 1):56–64. doi: 10.1111/j.1471-4159.2007.04716.x. [DOI] [PubMed] [Google Scholar]
- 3.Lackie PM, Zuber C, Roth J. Polysialic acid and N-CAM localisation in embryonic rat kidney: mesenchymal and epithelial elements show different patterns of expression. Development. 1990;110(3):933–947. doi: 10.1242/dev.110.3.933. [DOI] [PubMed] [Google Scholar]
- 4.Lackie PM, Zuber C, Roth J. Expression of polysialylated N-CAM during rat heart development. Differentiation. 1991;47(2):85–98. doi: 10.1111/j.1432-0436.1991.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 5.Lackie PM, Zuber C, Roth J. Polysialic acid of the neural cell adhesion molecule (N-CAM) is widely expressed during organogenesis in mesodermal and endodermal derivatives. Differentiation. 1994;57(2):119–131. doi: 10.1046/j.1432-0436.1994.5720119.x. [DOI] [PubMed] [Google Scholar]
- 6.Galuska SP, Geyer R, Gerardy-Schahn R, Muhlenhoff M, Geyer H. Enzyme-dependent variations in the polysialylation of the neural cell adhesion molecule (NCAM) in vivo. J Biol Chem. 2008;283(1):17–28. doi: 10.1074/jbc.M707024200. [DOI] [PubMed] [Google Scholar]
- 7.Galuska SP, et al. Polysialic acid profiles of mice expressing variant allelic combinations of the polysialyltransferases ST8SiaII and ST8SiaIV. J Biol Chem. 2006;281(42):31605–31615. doi: 10.1074/jbc.M606516200. [DOI] [PubMed] [Google Scholar]
- 8.Colley KJ. Structural basis for the polysialylation of the neural cell adhesion molecule. Adv Exp Med Biol. 2010;663:111–126. doi: 10.1007/978-1-4419-1170-4_7. [DOI] [PubMed] [Google Scholar]
- 9.Rutishauser U. Polysialic acid at the cell surface: biophysics in service of cell interactions and tissue plasticity. J Cell Biochem. 1998;70(3):304–312. doi: 10.1002/(SICI)1097-4644(19980901)70:3<304::AID-JCB3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 10.Yabe U, Sato C, Matsuda T, Kitajima K. Polysialic acid in human milk. CD36 is a new member of mammalian polysialic acid-containing glycoprotein. J Biol Chem. 2003;278(16):13875–13880. doi: 10.1074/jbc.M300458200. [DOI] [PubMed] [Google Scholar]
- 11.Zuber C, Lackie PM, Catterall WA, Roth J. Polysialic acid is associated with sodium channels and the neural cell adhesion molecule N-CAM in adult rat brain. J Biol Chem. 1992;267(14):9965–9971. [PubMed] [Google Scholar]
- 12.Galuska SP, et al. Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc Natl Acad Sci USA. 2010;107(22):10250–10255. doi: 10.1073/pnas.0912103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curreli S, Arany Z, Gerardy-Schahn R, Mann D, Stamatos NM. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J Biol Chem. 2007;282:30346–30356. doi: 10.1074/jbc.M702965200. [DOI] [PubMed] [Google Scholar]
- 14.Weinhold B, et al. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280(52):42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- 15.Rey-Gallardo A, Delgado-Martin C, Gerardy-Schahn R, Rodriguez-Fernandez JL, Vega MA. Polysialic acid is required for neuropilin-2a/b-mediated control of CCL21-driven chemotaxis of mature dendritic cells and for their migration in vivo. Glycobiology. 2011;21(5):655–662. doi: 10.1093/glycob/cwq216. [DOI] [PubMed] [Google Scholar]
- 16.Rey-Gallardo A, et al. Polysialylated neuropilin-2 enhances human dendritic cell migration through the basic C-terminal region of CCL21. Glycobiology. 2010;20(9):1139–1146. doi: 10.1093/glycob/cwq078. [DOI] [PubMed] [Google Scholar]
- 17.Drake PM, et al. Polysialic acid, a glycan with highly restricted expression, is found on human and murine leukocytes and modulates immune responses. J Immunol. 2008;181(10):6850–6858. doi: 10.4049/jimmunol.181.10.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drake PM, et al. Polysialic acid governs T-cell development by regulating progenitor access to the thymus. Proc Natl Acad Sci USA. 2009;106(29):11995–12000. doi: 10.1073/pnas.0905188106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husmann M, Pietsch T, Fleischer B, Weisgerber C, Bitter-Suermann D. Embryonic neural cell adhesion molecules on human natural killer cells. Eur J Immunol. 1989;19(9):1761–1763. doi: 10.1002/eji.1830190935. [DOI] [PubMed] [Google Scholar]
- 20.Moebius JM, Widera D, Schmitz J, Kaltschmidt C, Piechaczek C. Impact of polysialylated CD56 on natural killer cell cytotoxicity. BMC Immunol. 2007;8:13. doi: 10.1186/1471-2172-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lantuejoul S, et al. NCAM (neural cell adhesion molecules) expression in malignant mesotheliomas. Hum Pathol. 2000;31(4):415–421. doi: 10.1053/hp.2000.6552. [DOI] [PubMed] [Google Scholar]
- 22.Hirn M, Pierres M, Deagostini-Bazin H, Hirsch M, Goridis C. Monoclonal antibody against cell surface glycoprotein of neurons. Brain Res. 1981;214(2):433–439. doi: 10.1016/0006-8993(81)91208-7. [DOI] [PubMed] [Google Scholar]
- 23.Frosch M, Gorgen I, Boulnois GJ, Timmis KN, Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci USA. 1985;82(4):1194–1198. doi: 10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stummeyer K, Dickmanns A, Muhlenhoff M, Gerardy-Schahn R, Ficner R. Crystal structure of the polysialic acid-degrading endosialidase of bacteriophage K1F. Nat Struct Mol Biol. 2005;12(1):90–96. doi: 10.1038/nsmb874. [DOI] [PubMed] [Google Scholar]
- 25.Eswar N et al (2006) Comparative protein structure modeling using Modeller. Curr Protoc Bioinforma (chapter 5: unit 5.6) [DOI] [PMC free article] [PubMed]
- 26.Berman H, Henrick K, Nakamura H. Announcing the worldwide Protein Data Bank. Nat Struct Biol. 2003;10(12):980. doi: 10.1038/nsb1203-980. [DOI] [PubMed] [Google Scholar]
- 27.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 28.Bohne A, Lang E, von der Lieth CW. SWEET—WWW-based rapid 3D construction of oligo- and polysaccharides. Bioinformatics. 1999;15(9):767–768. doi: 10.1093/bioinformatics/15.9.767. [DOI] [PubMed] [Google Scholar]
- 29.Bohne-Lang A, Lieth CW. GlyProt: in silico glycosylation of proteins. Nucleic Acids Res. 2005;33:W214–219. doi: 10.1093/nar/gki385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lütteke T, et al. GLYCOSCIENCES.de: an Internet portal to support glycomics and glycobiology research. Glycobiology. 2006;16(5):71R–81R. doi: 10.1093/glycob/cwj049. [DOI] [PubMed] [Google Scholar]
- 31.Krieger E, et al. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: four approaches that performed well in CASP8. Proteins. 2009;77(Suppl 9):114–122. doi: 10.1002/prot.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan Y, et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem. 2003;24(16):1999–2012. doi: 10.1002/jcc.10349. [DOI] [PubMed] [Google Scholar]
- 33.Roepstorff P, Fohlman J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed Mass Spectrom. 1984;11(11):601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- 34.Inoue S, Lin SL, Lee YC, Inoue Y. An ultrasensitive chemical method for polysialic acid analysis. Glycobiology. 2001;11(9):759–767. doi: 10.1093/glycob/11.9.759. [DOI] [PubMed] [Google Scholar]
- 35.Saffarzadeh M, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7(2):e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haselhorst T, et al. Endosialidase NF appears to bind polySia DP5 in a helical conformation. Chembiochem. 2006;7(12):1875–1877. doi: 10.1002/cbic.200600252. [DOI] [PubMed] [Google Scholar]
- 37.Schwarzer D, et al. Proteolytic release of the intramolecular chaperone domain confers processivity to endosialidase F. J Biol Chem. 2009;284(14):9465–9474. doi: 10.1074/jbc.M808475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci. 2004;5(3):195–208. doi: 10.1038/nrn1349. [DOI] [PubMed] [Google Scholar]
- 39.Isomura R, Kitajima K, Sato C. Structural and functional impairments of polysialic acid by a mutated polysialyltransferase found in schizophrenia. J Biol Chem. 2011;286(24):21535–21545. doi: 10.1074/jbc.M111.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50s–59s. doi: 10.1183/09031936.01.00229701. [DOI] [PubMed] [Google Scholar]
- 41.van Eeden SF, Yeung A, Quinlam K, Hogg JC. Systemic response to ambient particulate matter: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(1):61–67. doi: 10.1513/pats.200406-035MS. [DOI] [PubMed] [Google Scholar]
- 42.Rusznak C, et al. Effect of cigarette smoke on the permeability and IL-1beta and sICAM-1 release from cultured human bronchial epithelial cells of never-smokers, smokers, and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2000;23(4):530–536. doi: 10.1165/ajrcmb.23.4.3959. [DOI] [PubMed] [Google Scholar]
- 43.Doz E, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180(2):1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 44.Grande NR, Peao MND, Sa CMd, Aguas AP (1998) Lung fibrosis induced by bleomycin: structural changes and overview of recent advances. Scan Microsc 12(3):487–494
- 45.Gasse P, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117(12):3786–3799. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45(2):189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Wetering S, et al. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272(5 Pt 1):L888–L896. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- 48.Phung TT, et al. Key role of regulated upon activation normal T-cell expressed and secreted, nonstructural protein1 and myeloperoxidase in cytokine storm induced by influenza virus PR-8 (A/H1N1) infection in A549 bronchial epithelial cells. Microbiol Immunol. 2011;55(12):874–884. doi: 10.1111/j.1348-0421.2011.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Secher T. Soluble NCAM. Adv Exp Med Biol. 2010;663:227–242. doi: 10.1007/978-1-4419-1170-4_15. [DOI] [PubMed] [Google Scholar]
- 50.Bock E, et al. Characterization of soluble forms of NCAM. FEBS Lett. 1987;225(1–2):33–36. doi: 10.1016/0014-5793(87)81126-2. [DOI] [PubMed] [Google Scholar]
- 51.Bork K, Reutter W, Gerardy-Schahn R, Horstkorte R. The intracellular concentration of sialic acid regulates the polysialylation of the neural cell adhesion molecule. FEBS Lett. 2005;579(22):5079–5083. doi: 10.1016/j.febslet.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Du J, et al. Metabolic glycoengineering: sialic acid and beyond. Glycobiology. 2009;19(12):1382–1401. doi: 10.1093/glycob/cwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saetta M, et al. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med. 2000;161(3 Pt 1):1016–1021. doi: 10.1164/ajrccm.161.3.9907080. [DOI] [PubMed] [Google Scholar]
- 54.Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heijink IH, et al. Role of aberrant metalloproteinase activity in the pro-inflammatory phenotype of bronchial epithelium in COPD. Respir Res. 2011;12:110. doi: 10.1186/1465-9921-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalus I, Bormann U, Mzoughi M, Schachner M, Kleene R. Proteolytic cleavage of the neural cell adhesion molecule by ADAM17/TACE is involved in neurite outgrowth. J Neurochem. 2006;98(1):78–88. doi: 10.1111/j.1471-4159.2006.03847.x. [DOI] [PubMed] [Google Scholar]
- 57.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2010;17(3–4):293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 59.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118(13):3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crocker PR. Siglecs in innate immunity. Curr Opin Pharmacol. 2005;5(4):431–437. doi: 10.1016/j.coph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Neumann H. Alleviation of neurotoxicity by microglial human Siglec-11. J Neurosci. 2010;30(9):3482–3488. doi: 10.1523/JNEUROSCI.3940-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villanueva E, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187(1):538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marcos V, et al. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat Med. 2010;16(9):1018–1023. doi: 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- 66.Hakkim A, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. 2010;107(21):9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(8):1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caudrillier A, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122(7):2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.