Abstract
The voltage-gated Ca2+ (CaV) channel acts as a key player in β cell physiology and pathophysiology. β cell CaV channels undergo hyperactivation subsequent to exposure to type 1 diabetic (T1D) serum resulting in increased cytosolic free Ca2+ concentration and thereby Ca2+-triggered β cell apoptosis. The present study was aimed at revealing the subtypes of CaV1 channels hyperactivated by T1D serum as well as the biophysical mechanisms responsible for T1D serum-induced hyperactivation of β cell CaV1 channels. Patch-clamp recordings and single-cell RT-PCR analysis were performed in pancreatic β cells from CaV1 channel knockout and corresponding control mice. We now show that functional CaV1.3 channels are expressed in a subgroup of islet β cells from CaV1.2 knockout mice (CaV1.2−/−). T1D serum enhanced whole-cell CaV currents in islet β cells from CaV1.3 knockout mice (CaV1.3−/−). T1D serum increased the open probability and number of functional unitary CaV1 channels in CaV1.2−/− and CaV1.3−/− β cells. These data demonstrate that T1D serum hyperactivates both CaV1.2 and CaV1.3 channels by increasing their conductivity and number. These findings suggest CaV1.2 and CaV1.3 channels as potential targets for anti-diabetes therapy.
Keywords: Apolipoprotein, Calcium channel, Genetic ablation, Patch-clamp recording, Single-cell RT-PCR, Type 1 diabetes
Introduction
The voltage-gated calcium (CaV) channel CaV1 is critical to β cell physiology and pathophysiology [1–5]. The two CaV1 channel subtypes CaV1.2 and CaV1.3 are identified in β cells including human and rat islet β cells [1, 2]. The level of CaV1.3 subunit mRNA is 13.5 and 2.5 times higher than that of CaV1.2 subunit mRNA in human and rat islet β cells, respectively [6, 7]. CaV1 channel subtypes in mouse islet β cells are still a debated topic. Some studies claim that the mouse islet β cell only possesses CaV1.2 channels, since commonly used concentrations of dihydropyridines (DHPs) can no longer alter CaV currents when CaV1.2 subunits or their DHP sensitivity are genetically ablated [8, 9]. However, these studies ignore the fact that the DHP sensitivity of CaV1.3 channels is lower than that of CaV1.2 channels. For example, nifedipine at 10 μM only blocks about 40 % of the CaV1.3 channel-mediated Ca2+ currents, whereas nifedipine at 100 nM can completely ablate CaV1.2-mediated Ca2+ currents in the mouse hair cell [10]. Furthermore, CaV1.3 subunit mRNA and protein are convincingly detected in mouse islet β cells [11–13]. Genetic ablation of mouse CaV1.3 subunits abrogates basal insulin secretion [12]. The presence of functional CaV1.3 channels in mouse islet β cells is therefore still a matter of uncertainty [1, 2].
Dysregulation of β cell CaV1 channels impairs β cell function and even kills β cells resulting in diabetes [2, 8, 14–21]. The reduced expression of CaV1.2 and CaV1.3 subunits and the consequent decrease in CaV1 channel activity blunt stimulus–secretion coupling in Zucker diabetic fatty rat β cells [15]. β cell-specific CaV1.2−/− selectively abrogates the initial rapid component of insulin exocytosis and thereby first phase insulin secretion [8]. Trinucleotide expansion in the human CaV1.3 gene has been revealed in a subgroup of patients with type 2 diabetes [16, 17]. Point mutation of the human CaV1.2 subunit results in hyperactivation of β cell CaV1 channels, thereby causing excessive insulin secretion, episodic hypoglycemia and even death of some affected individuals [18]. Type 1 diabetic (T1D) serum hyperactivates β cell CaV1 channels, leading to increased cytosolic free Ca2+ concentration ([Ca2+]i) and thereby β cell apoptosis [2, 19, 20]. However, for a long time the lack of β cell-specific CaV1 knockout mouse models prevented us from understanding if T1D serum affects either Cav1.2 or Cav1.3 channels or both, and the underlying biophysical mechanisms.
In the present study, combined application of CaV1 knockout mouse models, patch-clamp techniques and single-cell RT-PCR analysis leads to satisfactory circumvention of the aforementioned issues and results in the following novel observations. First, a subgroup of mouse islet CaV1.2−/− β cells accommodate functional CaV1.3 channels. This solves the uncertainty about the presence and function of CaV1.3 channels in the mouse islet β cell and provides a convenient small animal model for further investigations of physiology and pathophysiology of β cell CaV1.3 channels. Second, T1D serum hyperactivates both CaV1.2 and CaV1.3 channels in the β cell. This adds a new dimension to the molecular pathogenesis of type 1 diabetes. Finally, T1D serum-induced hyperactivation of β cell CaV1 channels results from both increased activity and elevated number of the channels. This offers a mechanistic interpretation of T1D serum-induced hyperactivation of β cell CaV1 channels.
Materials and methods
Animals
β cell-specific CaV1.2 subunit-knockout (CaV1.2−/−) mice and their corresponding heterozygous (CaV1.2+/−) control mice were provided by Dr. Franz Hofmann (Institut für Pharmakologie und Toxikologie, Technische Universität München, Germany) [8]. General CaV1.3 subunit-knockout (CaV1.3−/−) mice and wild-type control (CaV1.3+/+) mice were provided by Dr. Jörg Striessnig (Institute of Pharmacy, Pharmacology and Toxicology, University of Innsbruck, Austria) [22]. The background mouse strain is C57BL/6.
Isolation of islets of Langerhans
Islets of Langerhans were isolated from adult control and mutant mice [23]. Briefly, the mice were killed by cervical dislocation. The pancreas was quickly dissected and cut into small pieces. Subsequently, the cut pancreatic tissue was digested with collagenase A (Roche, Basel, Switzerland) under vigorous shaking for 10–12 min at 37 °C. The digested pancreatic tissue was rinsed twice with a solution containing (in mM) 125 NaCl, 5.9 KCl, 1.28 CaCl2, 1.2 MgCl2, 10 HEPES, 0.1 % bovine albumin and 3 glucose, pH 7.4. Islets were hand picked-up under microscope.
Islet cell culture and treatments
The isolated islets of Langerhans were dispersed into single islet cells in a Ca2+-free medium containing (in mM) 125 NaCl, 5.9 KCl, 2 EGTA, 25 HEPES and 1 % bovine serum albumin, pH 7.4 [24]. The cells were cultured in RPMI 1640 medium supplemented with 10 % fetal bovine serum, 2 mM l-glutamine and 100 U/100 μg/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) at 37 °C in a humidified 5 % CO2 incubator for 2–3 days [25]. Subsequently, the cells were treated with 10 % healthy serum- or 10 % T1D serum-containing RPMI 1640 medium overnight before electrophysiological recordings.
Preparation of healthy serum or type 1 diabetic serum
Sera from healthy blood donors and T1D patients were collected and heat-inactivated at 56 °C for 30 min.
Electrophysiological recordings
Whole-cell and single CaV channel currents were recorded by using conventional whole-cell, perforated whole-cell and cell-attached configurations of the patch-clamp technique, respectively [4, 26–28]. Pipettes were pulled from borosilicate glass capillaries (Hilgenberg, Malsfeld, Germany) on a horizontal programmable puller (DMZ Universal Puller, Zeitz-Instrumente, Augsburg, Germany) and then fire-polished and coated with Sylgard close to their tips. Typical electrode resistance was 4–6 MΩ. For conventional whole-cell recordings, pipettes were filled with a solution consisting of (in mM) 150 N-methyl-d-glucamine, 125 HCl, 10 EGTA, 1.2 MgCl2, 3 MgATP, and 5 HEPES (pH 7.15). In perforated whole-cell patch-clamp experiments, the pipette solution contained (in mM) 76 Cs2SO4, 1 MgCl2, 10 KCl, 10 NaCl, and 5 HEPES (pH 7.35), as well as amphotericin B (0.24 mg/ml) to permeabilize the cell membrane and allow low-resistance electrical access without breaking the plasma membrane patch. Cells used for both conventional and perforated whole-cell recordings were bathed in a solution containing (in mM) 138 NaCl, 10 tetraethylammonium chloride, 10 CaCl2, 5.6 KCl, 1.2 MgCl2, 5 HEPES and 3 glucose (pH 7.4). After obtaining a seal, the holding potential was set at −70 mV during the course of an experiment. Depolarizing voltage pulses (100 ms) were made from a holding potential of −70 mV to a test potential of 0 mV or several test potentials from −60 to 50 mV in 10 mV increments at 0.5 Hz. The selective CaV1 channel blocker nimodipine (10 µM) was used to block whole-cell CaV1.3 currents. Cell-attached single-channel recordings were made with Ba2+ as the charge carrier. Pipettes were filled with a solution containing (in mM): 110 BaCl2, 10 TEA-Cl and 5 HEPES-Ba(OH)2 (pH 7.4). A depolarizing external recording solution, containing (in mM) 125 KCl, 30 KOH, 10 EGTA, 2 CaCl2, 1 MgCl2, 5 HEPES–KOH (pH 7.15), was used to bring the intracellular potential to ~0 mV. Voltage pulses (200 ms) were applied at a frequency of 0.5 Hz to depolarize cells from a holding potential of −70 mV to a membrane potential of 0 mV. Resulting currents were recorded with an Axopatch 200B amplifier (Molecular Devices, Foster City, CA), filtered at 1 kHz and digitized at 5 kHz. All recordings were made at room temperature (about 22 °C). Acquisition and analysis of data were done using the software program pCLAMP10 (Molecular Devices).
Single-cell RT-PCR
The entire islet cell was harvested with a glass pipette after a patch-clamp recording and stored in a PCR tube with 10 µl 1× Taq DNA polymerase reaction buffer containing MgCl2 (Promega, Madison, WI) at −70 °C for later use. The QIAGEN OneStep RT-PCR Kit (Valencia, CA) was used to detect insulin mRNA in harvested cells according to the manufacturer’s instructions. The RNasin Plus RNase Inhibitor (Promega) was added to avoid damaging mRNA. The insulin primer pair for RT-PCR analysis was synthesized by Sigma-Aldrich (St. Louis, MO) and consisted of the forward primer 5′-CAGCAAGCAGGTCATTGTTT-3′ and the reverse primer 5′-CAGTAGTTCTCCAGCTGGTAGA-3′. These primers were added at a final concentration of 0.6 µM to the reaction mix. The reaction was amplified for 35 cycles of 94 °C for 1 min, 62 °C for 1 min and 72 °C for 1 min. The amplified PCR products were detected by 2 % agarose gel electrophoresis and ethidium bromide staining.
Statistical analysis
Data are presented as mean ± SEM. The statistical significance of differences between multiple groups was assessed by one-way ANOVA, followed by least significant difference (LSD) test. The statistical difference between two groups was determined by unpaired Student’s t test or Mann–Whitney U test. The significance level was set to 0.05 or 0.01.
Results
Characterization of type 1 diabetic serum
Table 1 summarizes characterization of type 1 diabetic serum. All healthy sera are negative for antibodies to glutamic acid decarboxylase (GAD), islet cells (ICA), and tyrosine phosphatase IA2 (IA-2). T1D sera were pre-screened and all of them induced a higher increase in [Ca2+]i upon depolarization with KCl, compared to healthy sera [20].
Table 1.
Characterization of type 1 diabetic sera
| Sex | Age at onset of type 1 diabetes (years) | Medicationa | GAD | ICA | IA-2 |
|---|---|---|---|---|---|
| Male | 28 | No | + | nd | nd |
| Male | 34 | No | + | – | – |
| Male | 19 | No | + | + | + |
| Female | 34 | No | + | + | – |
| Male | 24 | No | + | – | – |
| Male | 27 | No | + | nd | nd |
+ presence, − absence, nd no data available, GAD antibodies to glutamic acid decarboxylase, ICA antibodies to islet cells, IA-2 antibodies to tyrosine phosphatase IA2
aInsulin was the only medication administered
Functional CaV1.3 channels are present in a subgroup of mouse islet CaV1.2−/− β cells
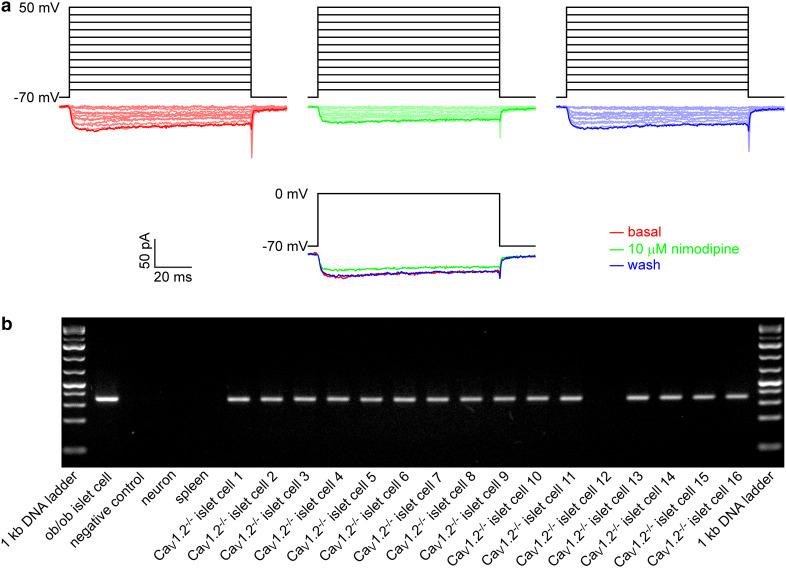
To test for the presence of functional CaV1.3 channels in the mouse islet CaV1.2−/− β cell, we examined if CaV1 currents are present in the mouse islet CaV1.2−/− β cell by combining whole-cell patch-clamp analysis and single-cell RT-PCR assay. Figure 1a shows that a β cell-specific CaV1.2−/− islet cell, which is insulin mRNA-positive (CaV1.2−/− islet cell 8), displayed clear CaV1 currents, which were blocked by the specific CaV1 channel blocker nimodipine. Figure 1b shows insulin mRNA expression in single islet cells from the β cell-specific CaV1.2−/− mouse and in control samples including the positive control ob/ob islet cell and the negative controls neuron, spleen and sterile ultrapure water. Sixteen CaV1.2−/− islet cells were subjected to nimodipine-sensitive current measurements and then single-cell RT-PCR assay. These cells did not display any detectable voltage-gated Na+ currents. Their capacitance is greater than 7 pF. Although these electrophysiological criteria strongly indicate that they are β cells, their β cell identity was still confirmed by insulin mRNA positivity. A standard RT-PCR protocol using insulin-specific primers revealed the expected 344-bp amplicon for insulin in 15 islet cells (Fig. 1b). Among them, three insulin mRNA-positive cells are sensitive to nimodipine. Hence, 20 % insulin mRNA-positive CaV1.2−/− islet cells (3 out of 15) were estimated to express functional CaV1.3 channels.
Fig. 1.
CaV1.3 channels are functionally expressed in a subgroup of mouse islet CaV1.2−/− β cells. a Examples of whole-cell CaV current traces evoked by a set of depolarizing voltage pulses (upper panel) and those generated by single voltage pulses (lower panel) in an islet cell from the β cell-specific CaV1.2−/− mouse before (red), during exposure to 10 µM nimodipine (green) and after washing treatment (blue). b RT-PCR analysis of cDNA obtained from single islet cells of the β cell-specific CaV1.2−/− mouse and from the positive control ob/ob islet cell and negative controls neuron, spleen and sterile ultrapure water with specific primers for insulin (344-bp amplicon)
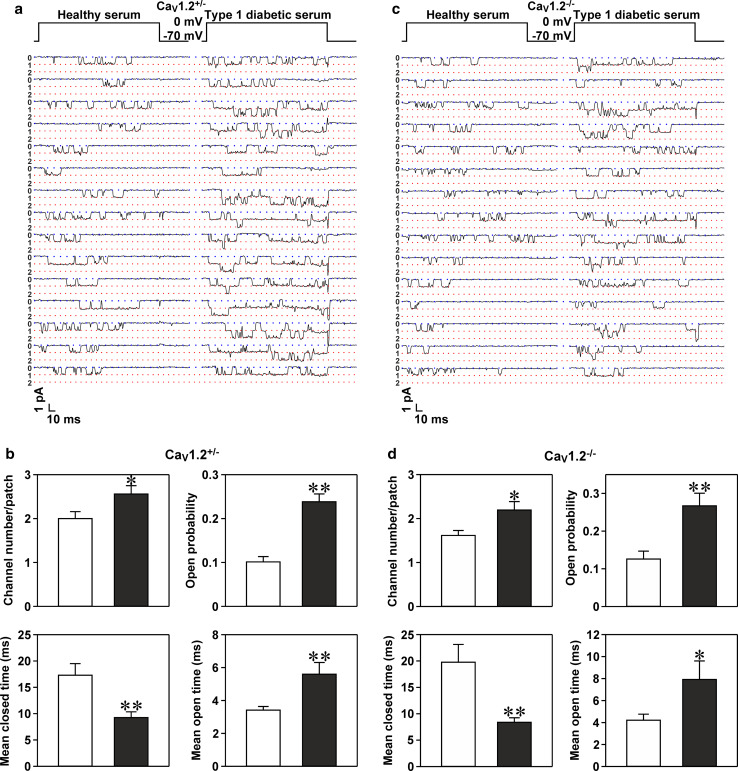
Type 1 diabetic serum increases the open probability and number of single CaV1 channels in mouse islet CaV1.2−/− β cells
In this set of experiments, we first validated the effectiveness of the T1D serum used on CaV1 channel hyperactivation in islet β cells isolated from control mice. Figure 2a shows representative Ba2+ currents mediated by single CaV1 channels in a control mouse islet β cell subjected to treatment with healthy serum and a control mouse islet β cell incubated with T1D serum. Incubation with T1D serum not only made CaV1 channels dwell longer in their open state, but also increased the number of functional CaV1 channels, reflected by more unitary conductance levels (more layers of unitary Ba2+ currents) (Fig. 2a). Statistical analysis revealed that treatment with T1D serum significantly altered four parameters of single CaV1 channels in control mouse islet β cells (Fig. 2b). Compared to healthy serum treatment, T1D serum significantly increased the number, open probability and mean open time of CaV1 channels and significantly decreased the mean closed time of these channels in control mouse islet β cells (Fig. 2b).
Fig. 2.
Type 1 diabetic serum hyperactivates CaV1 channels through elevating their open probability and number in CaV1.2+/− and CaV1.2−/− β cells. a Sample unitary CaV1 channel current traces obtained from a plasma membrane patch attached to a CaV1.2+/− β cell exposed to either healthy serum or T1D serum. The plasma membrane patch was held at −70 mV and depolarized for 200 ms to a test potential of 0 mV. b Average number, open probability, mean closed time and mean open time of single CaV1 channels registered in plasma membrane patches of CaV1.2+/− β cells incubated with healthy serum (open bars, n = 33 cells) and T1D serum (closed bars, n = 34 cells), respectively. c Representative unitary CaV1 channel current traces recorded in a plasma membrane patch of a CaV1.2−/− β cell following exposure to healthy serum and T1D serum, respectively. d Average number, open probability, mean closed time and mean open time of single CaV1 channels measured in plasma membrane patches attached to CaV1.2−/− β cells exposed to either healthy serum (open bars, n = 26 cells) or T1D serum (closed bars, n = 26 cells). *p < 0.05 and **p < 0.01 vs. healthy serum
The verification of the presence and function of CaV1.3 channels in mouse islet CaV1.2−/− β cells prompted us to clarify if T1D serum hyperactivates CaV1.3 channels in these cells. A prerequisite for tackling this issue is a simple and reliable discrimination between CaV1.3 channel-negative and CaV1.3 channel-positive β cells, since only a small proportion of mouse islet CaV1.2−/− β cells express functional CaV1.3 channels. It is difficult to identify CaV1.3 channel-positive β cells during a whole-cell patch-clamp recording. Unitary CaV1 channel currents, characterized by a large unitary Ba2+ conductance with long-lasting openings, can be convincingly recognized and discriminated from unitary CaV2 and CaV3 channel currents, exhibiting a smaller unitary Ba2+ conductance with shorter-lasting openings, during a cell-attached patch-clamp recording [1, 2]. The occurrence of such unitary CaV1 channel currents verifies the presence of functional CaV1.3 channels since the CaV1.2 channel was genetically ablated and two other members of the CaV1 family, i.e., CaV1.1 and CaV1.4, do not express in mouse islet β cells [1, 2]. Therefore, we performed cell-attached single-channel recording rather than whole-cell current measurements in CaV1.2−/− β cells. In the analysis, only the larger CaV1.2−/− β cells (capacitance >7 pF) displaying single CaV1 channel currents with a large unitary Ba2+ conductance and long-lasting openings were included in the analysis of CaV1.3 channel hyperactivation by T1D serum. Figure 2c represents sample unitary Ba2+ currents flowing through single CaV1 channels in a healthy serum-treated cell and in a cell exposed to T1D serum. These current traces clearly show that CaV1 channels stay open longer and more CaV1 channels appear in a plasma membrane patch attached to a cell treated with T1D serum. Summary data show that plasma membrane patches of T1D serum-treated cells display significantly more channels than those of healthy serum-treated cells (Fig. 2d). Moreover, treatment with T1D serum significantly increased the open probability, prolonged the mean open time and shortened the mean closed time of CaV1 channels (Fig. 2d).
Type 1 diabetic serum enhances whole-cell CaV currents in mouse islet CaV1.3−/− β cells
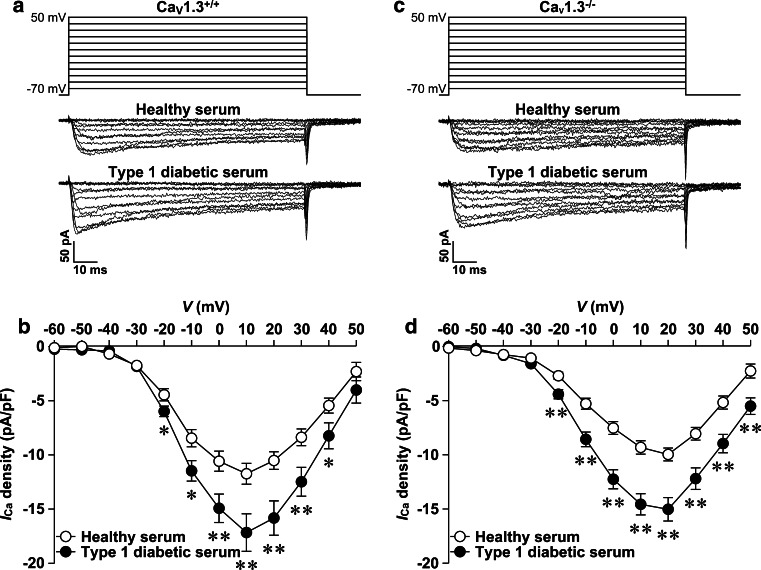
There is no doubt that every mouse islet β cell is equipped with CaV1.2 channels to mediate CaV1 currents. To determine if mouse β cell CaV1.2 channels undergo hyperactivation in response to T1D serum exposure, we characterized the effects of T1D serum on whole-cell CaV currents in mouse islet CaV1.3+/+ and CaV1.3−/− β cells. As aforementioned, mouse islet CaV1.3+/+ β cells were employed as positive control to assess the effectiveness of the T1D serum used in this set of experiments on CaV1 channel hyperactivation. Incubation with T1D serum dramatically increased whole-cell CaV currents in CaV1.3+/+ β cells, as manifested by representative whole-cell CaV current traces obtained from a control CaV1.3+/+ and a T1D serum-treated CaV1.3+/+ β cell (Fig. 3a). Compiled data show that T1D serum significantly elevated whole-cell CaV current density measured at depolarizations in the range of −20 to 40 mV from a holding potential of −70 mV compared to healthy serum in CaV1.3+/+ cells (Fig. 3b). Like CaV1.3+/+ β cells, CaV1.3−/− β cells show that their whole-cell CaV1 currents are hyperactivated by T1D serum (Fig. 3). A T1D serum-treated CaV1.3−/− β cell displays larger whole-cell CaV currents compared to a CaV1.3−/− β cell incubated with healthy serum (Fig. 3c). Statistical analysis reveals that T1D serum-treated cells exhibit significantly higher CaV current density at depolarizations from −20 to 50 mV compared to cells incubated with healthy serum (Fig. 3d).
Fig. 3.
Type 1 diabetic serum increases whole-cell CaV currents in CaV1.3+/+ and CaV1.3−/− β cells. a Sample whole-cell CaV current traces from a CaV1.3+/+ β cell incubated with either healthy serum (cell capacitance: 5.9 pF) or T1D serum (cell capacitance: 5.6 pF). b Average CaV current density–voltage relationships in CaV1.3+/+ cells exposed to healthy serum (open circles, n = 36 cells) and T1D serum (filled circles, n = 36 cells), respectively. c Representative whole-cell CaV current traces from a CaV1.3−/− β cell following treatment with healthy serum (cell capacitance: 5.4 pF) and T1D serum (cell capacitance: 5.5 pF), respectively. d Average CaV current density–voltage relationships in CaV1.3−/− β cells subjected to exposure to either healthy serum (open circles, n = 35 cells) or T1D serum (filled circles, n = 35 cells). *p < 0.05 and **p < 0.01 vs. healthy serum
To determine if T1D serum treatment or CaV1.3 knockout alters activation of β cell CaV channels, the midpoint (V1/2) of activation of whole-cell CaV currents was calculated by fitting whole-cell CaV current–voltage data with Boltzmann function. T1D serum treatment did not influence activation midpoint in either CaV1.3+/+ β cells (T1D serum: −16.2 ± 1.2 mV vs. healthy serum: −16.9 ± 1.1 mV, n = 36 and 36, p > 0.05) or CaV1.3−/− β cells (T1D serum: −12.1 ± 1.3 mV vs. healthy serum: −11.8 ± 1.0 mV, n = 35 and 35, p > 0.05). However, CaV1.3 knockout slightly but significantly shifted activation midpoint to more depolarized potentials in both healthy serum-treated cells (CaV1.3−/−: −11.8 ± 1.0 mV vs. CaV1.3+/+: −16.9 ± 1.1 mV, n = 35 and 36, p < 0.01) and T1D serum-treated cells (CaV1.3−/−:12.1 ± 1.3 mV vs. CaV1.3+/+:−16.2 ± 1.2 mV, n = 35 and 36, p < 0.05).
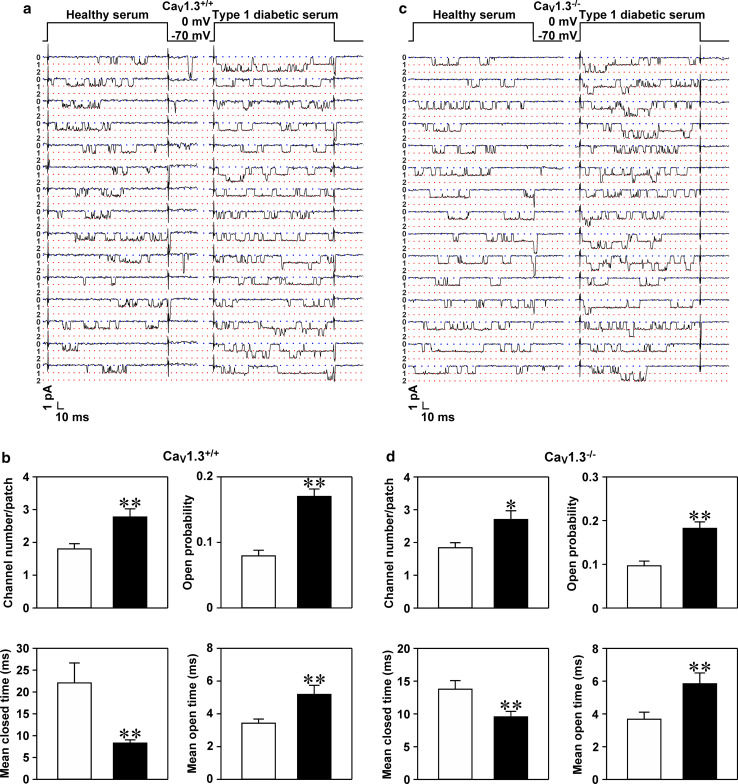
Type 1 diabetic serum elevates the open probability and number of single CaV1 channels in mouse islet CaV1.3−/− β cells
The above data obtained from whole-cell patch-clamp experiments suggest that CaV1.2 channels are hyperactivated by T1D serum. To further substantiate this suggestion, we selectively characterized single CaV1 channels exhibiting a large unitary Ba2+ conductance and long-lasting openings in mouse islet CaV1.3+/+ and CaV1.3−/− β cells following healthy serum and T1D serum treatments (Fig. 4a, c). We first characterized how the T1D serum used in this set of experiments affected biophysical properties of single CaV1 channels in mouse islet CaV1.3+/+ β cells as a positive control. Figure 4a illustrates unitary Ba2+ currents passing through CaV1 channels sampled from a plasma membrane patch of a CaV1.3+/+ β cell exposed to healthy serum and that incubated with T1D serum. The latter accommodated more single CaV1 channels, manifested by more layers of unitary Ba2+ currents, and longer open states (Fig. 4a). Figure 4b shows the detailed biophysical properties of the unitary CaV1 channels. The number, open probability and mean open time of single CaV1 channels following exposure to T1D serum were significantly greater than those incubated with healthy serum. The mean closed time of single CaV1 channels exposed to T1D serum was significantly shorter than that following healthy serum treatment (Fig. 4b).
Fig. 4.
Type 1 diabetic serum hyperactivates CaV1 channels through increasing their open probability and number in CaV1.3+/+ and CaV1.3−/− β cells. a Examples of unitary CaV1 channel currents registered in a plasma membrane patch of a CaV1.3+/+ β cell incubated with either healthy serum or T1D serum. The plasma membrane patch was held at −70 mV and depolarized for 200 ms to a test potential of 0 mV. b Average number, open probability, mean closed time and mean open time of single CaV1 channels acquired from plasma membrane patches attached to CaV1.3+/+ β cells following incubation with healthy serum (n = 30 cells) and T1D serum (n = 31 cells), respectively. c Samples of unitary CaV1 channel currents monitored in a plasma membrane patch attached to a CaV1.3−/− β cell exposed to healthy serum and T1D serum, respectively. d Average number, open probability, mean closed time and mean open time of single CaV1 channels detected in plasma membrane patches of CaV1.3−/− β cells subjected to exposure to either healthy serum (n = 31 cells) or T1D serum (n = 30 cells). *p < 0.05 and **p < 0.01 vs. healthy serum
Subsequently, we examined the effects of T1D serum on unitary CaV1 currents in CaV1.3−/− β cells. As above, CaV1 channel currents were verified with their fingerprint features, i.e., a large unitary Ba2+ conductance and long-lasting openings. Such features were clearly depicted in unitary Ba2+ current traces registered in a healthy serum-treated cell and in a cell treated with T1D serum (Fig. 4c). Figure 4c shows that unitary Ba2+ currents recorded in a plasma membrane patch of a T1D serum-treated cell display more layers, resulting from simultaneous opening of multiple single channels, and persist for long time periods. Detailed statistical analysis illustrated that the number, open probability and mean open time of single CaV1 channels significantly increased and the mean closed time of these channels significantly decreased subsequent to exposure to T1D serum (Fig. 4d).
Discussion
The presence of functional CaV1.3 channels in the mouse islet β cell remains controversial due to technical limitations [1, 2, 8, 9, 11–13]. The single-cell RT-PCR approach and immunofluorescence labeling can be used to detect CaV1.3 subunit mRNA and protein, respectively, but not functional CaV1.3 channels. In fact, we previously showed expression of CaV1.3 subunit mRNA in some mouse islet β cells, but could not clarify the presence of functional CaV1.3 channels [13]. All available anti-CaV1.3 subunit antibodies are not specific enough to solely recognize the CaV1.3 subunit and the β cell plasma membrane only accommodates about hundred native CaV channels, composed only partly of CaV1.3, which are too few to be detected by confocal scanning microscopy [29]. Moreover, CaV1.2 and CaV1.3 channels differ in activation threshold and DHP sensitivity when separately expressed in heterologous cells [30, 31]. Although a combination of the patch-clamp technique with application of DHPs appears to be the best choice for identification of functional CaV1.3 channels in the mouse islet β cell, the difference in activation threshold is too subtle to be used for convincing discrimination between native CaV1.2 and CaV1.3 channels. DHPs act on both CaV1.2 and CaV1.3 channels and are not suitable to reliably differentiate CaV1.3 channels from CaV1.2 channels. The conceptual novelty of the present work is that it overcomes the aforementioned technical limitations by combining whole-cell patch-clamp analysis, application of the selective CaV1 channel blocker, single-cell RT-PCR detection of insulin mRNAs and the CaV1.2−/− mouse model.
The obtained data reveal that some CaV1.2−/− islet cells, which are larger in size (capacitance > 7 pF), did not display Na+ currents, but express insulin mRNA and are equipped with the CaV channel having a low but true sensitivity to the selective CaV1 channel blocker nimodipine (10 µM). This demonstrates that a proportion of mouse islet CaV1.2−/− β cells express functional CaV1.3 channels. This makes the CaV1.2−/− mouse a convenient small animal model to clarify if T1D serum affects β cell CaV1.3 channels and to further investigate physiology and pathophysiology of these channels, which are predominantly expressed in human β cells and whose polymorphisms are closely associated with diabetes [1, 2, 16, 32].
The development of a novel treatment strategy for diabetes critically depends on understanding of the molecular pathogenesis of this disease [33–37]. We have previously shown that T1D serum and its diabetogenic factor apolipoprotein CIII selectively hyperactivate β cell CaV1 (L-type) channels by using the selective CaV1 channel blocker verapamil or nimodipine [19–21]. These studies demonstrated that T1D serum makes unphysiological amounts of Ca2+ enter pancreatic β cells through hyperactivation of β cell CaV1 channels resulting in β cell apoptosis [19]. Along the same line, we revealed that elevated apolipoprotein CIII in T1D serum serves as the diabetogenic serum factor to drive Ca2+-dependent β cell destruction via selective hyperactivation of β cell CaV1 channels [20]. In vivo down-regulation of this apolipoprotein delays the onset of diabetes in the BioBreeding rat, a rat model for human type 1 diabetes [38]. Mechanistically, apolipoprotein CIII hyperactivates β cell CaV1 channels through scavenger receptor class B type I/β1 integrin-dependent coactivation of protein kinase A and Src [21]. This process aggravates the disease development on top of the T-lymphocyte-mediated autoimmune attack [2, 39, 40]. To advance our understanding of the details of hyperactivated CaV1 channels in diabetes development, the present study examined if either CaV1.2 or CaV1.3 channels or both are hyperactivated by T1D serum. We now demonstrate that both CaV1.2 and CaV1.3 channels in the mouse β cell are vulnerable to the attack of T1D serum and their hyperactivation underlies the molecular pathogenesis of type 1 diabetes. Importantly, this finding highlights that both CaV1 channel subtypes are suitable for molecular intervention in diabetes mellitus. In fact, pharmacological intervention with the CaV1 channel blocker verapmil has been demonstrated to ameliorate and even prevent low-dose streptozotocin-induced progressive diabetes in mice through reduction of β cell apoptosis and promotion of β cell survival and function [41].
We previously found that T1D serum significantly increases the amplitude of whole-cell and average unitary CaV1 currents in the β cell [19]. Such a grossly increased activity can arise from enriched density and/or increased conductivity of these channels in the β cell plasma membrane. We have now carefully analyzed how T1D serum alters CaV1 channel behavior at both the single-channel and whole-cell levels. Our whole-cell patch-clamp recordings show that T1D serum significantly enhances whole-cell CaV currents in CaV1.3+/+ and CaV1.3−/− β cells. This is in line with previous findings from our group and others [19, 42–45]. More importantly, we are able to mechanistically interpret T1D serum-induced hyperactivation of β cell CaV1 channels by thoroughly examining the biophysical properties of single CaV1 channels in CaV1.2+/−, CaV1.2−/−, CaV1.3+/+ and CaV1.3−/− β cells. The CaV1 channel hyperactivation results from both increased activity and elevated number of functional single CaV1 channels in the recorded area of the β cell plasma membrane. The former is reflected by an increased open probability attributed to the prolonged mean open time and shortened mean closed time. The latter is verified by appearance of more levels of single CaV1 channel conductance.
Overall, our work verifies that functional CaV1.3 channels are expressed in a proportion of mouse islet CaV1.2−/− β cells. Importantly, T1D serum hyperactivates both CaV1.2 and CaV1.3 channels by increasing their conductivity and density. Intriguingly, our findings suggest CaV1.2 and CaV1.3 channels as potential druggable targets and pave the way for a novel diabetes therapy.
Acknowledgments
This work was supported by grants from Berth von Kantzow’s Foundation, Diabetes Research and Wellness Foundation, EuroDia (FP6-518153), European Research Council (ERC-2013-AdG), the Family Erling-Persson Foundation, Funds of Karolinska Institutet, the Knut and Alice Wallenberg Foundation, Skandia Insurance Company, Ltd., the Stichting af Jochnick Foundation, Strategic Research Program in Diabetes at Karolinska Institutet, the Swedish Diabetes Association, the Swedish Research Council and the Novo Nordisk Foundation. P-OB is the founder of the Biotech Company BioCrine AB and is also a member of the board of this company. S-NY is a consultant to BioCrine AB. Biocrine AB is pursuing ApoCIII as a novel druggable target in diabetes.
Abbreviations
- CaV
Voltage-gated calcium
- CaV1.2−/−
CaV1.2 subunit knockout
- CaV1.3−/−
CaV1.3 subunit knockout
- DHP
Dihydropyridine
- T1D
Type 1 diabetic
Footnotes
GY and YS contributed equally to this study.
Contributor Information
Per-Olof Berggren, Phone: +46 8 517 757 31, Email: per-olof.berggren@ki.se.
Shao-Nian Yang, Phone: +46 8 517 794 56, Email: shao-nian.yang@ki.se.
References
- 1.Yang SN, Berggren PO. β-Cell CaV channel regulation in physiology and pathophysiology. Am J Physiol. 2005;288:E16–E28. doi: 10.1152/ajpendo.00042.2004. [DOI] [PubMed] [Google Scholar]
- 2.Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic β-cell physiology and pathophysiology. Endocr Rev. 2006;27:621–676. doi: 10.1210/er.2005-0888. [DOI] [PubMed] [Google Scholar]
- 3.Trus M, Corkey RF, Nesher R, Richard AM, Deeney JT, Corkey BE, Atlas D. The L-type voltage-gated Ca2+ channel is the Ca2+ sensor protein of stimulus–secretion coupling in pancreatic beta cells. Biochemistry. 2007;46:14461–14467. doi: 10.1021/bi7016816. [DOI] [PubMed] [Google Scholar]
- 4.Berggren PO, Yang SN, Murakami M, Efanov AM, Uhles S, Kohler M, Moede T, Fernstrom A, Appelskog IB, Aspinwall CA, Zaitsev SV, Larsson O, Moitoso de Vargas L, Fecher-Trost C, Weissgerber P, Ludwig A, Leibiger B, Juntti-Berggren L, Barker CJ, Gromada J, Freichel M, Leibiger IB, Flockerzi V. Removal of Ca2+ channel β3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Cell. 2004;119:273–284. doi: 10.1016/j.cell.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Yang SN, Shi Y, Yang G, Li Y, Yu J, Berggren PO (2014) Ionic mechanisms in pancreatic β cell signaling. Cell Mol Life Sci. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 6.Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, Johnson PR, Rorsman P. Voltage-gated ion channels in human pancreatic β-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- 7.Iwashima Y, Pugh W, Depaoli AM, Takeda J, Seino S, Bell GI, Polonsky KS. Expression of calcium channel mRNAs in rat pancreatic islets and downregulation after glucose infusion. Diabetes. 1993;42:948–955. doi: 10.2337/diab.42.7.948. [DOI] [PubMed] [Google Scholar]
- 8.Schulla V, Renstrom E, Feil R, Feil S, Franklin I, Gjinovci A, Jing XJ, Laux D, Lundquist I, Magnuson MA, Obermuller S, Olofsson CS, Salehi A, Wendt A, Klugbauer N, Wollheim CB, Rorsman P, Hofmann F. Impaired insulin secretion and glucose tolerance in β cell-selective CaV1.2 Ca2+ channel null mice. Embo J. 2003;22:3844–3854. doi: 10.1093/emboj/cdg389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renstrom E, Wietzorrek G, Berjukov S, Cavalli M, Walter D, Koschak A, Waldschutz R, Hering S, Bova S, Rorsman P, Pongs O, Singewald N, Striessnig JJ. Isoform-specific regulation of mood behavior and pancreatic β cell and cardiovascular function by L-type Ca2+ channels. J Clin Invest. 2004;113:1430–1439. doi: 10.1172/JCI200420208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel J, Michna M, Platzer J, Striessnig J. Calcium channels in mouse hair cells: function, properties and pharmacology. Adv Otorhinolaryngol. 2002;59:35–41. doi: 10.1159/000059243. [DOI] [PubMed] [Google Scholar]
- 11.Yang SN, Larsson O, Branstrom R, Bertorello AM, Leibiger B, Leibiger IB, Moede T, Kohler M, Meister B, Berggren PO. Syntaxin 1 interacts with the LD subtype of voltage-gated Ca2+ channels in pancreatic β cells. Proc Natl Acad Sci USA. 1999;96:10164–10169. doi: 10.1073/pnas.96.18.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namkung Y, Skrypnyk N, Jeong MJ, Lee T, Lee MS, Kim HL, Chin H, Suh PG, Kim SS, Shin HS. Requirement for the L-type Ca2+ channel α1D subunit in postnatal pancreatic β cell generation. J Clin Invest. 2001;108:1015–1022. doi: 10.1172/JCI200113310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vignali S, Leiss V, Karl R, Hofmann F, Welling A. Characterization of voltage-dependent sodium and calcium channels in mouse pancreatic A- and B-cells. J Physiol. 2006;572:691–706. doi: 10.1113/jphysiol.2005.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AK, Yeung-Yam-Wah V, Tse FW, Tse A. Cholesterol elevation impairs glucose-stimulated Ca2+ signaling in mouse pancreatic β-cells. Endocrinology. 2011;152:3351–3361. doi: 10.1210/en.2011-0124. [DOI] [PubMed] [Google Scholar]
- 15.Roe MW, Worley JF, Tokuyama Y, Philipson LH, Sturis J, Tang J, Dukes ID, Bell GI, Polonsky KS. NIDDM is associated with loss of pancreatic β-cell L-type Ca2+ channel activity. Am J Physiol. 1996;270:E133–E140. doi: 10.1152/ajpendo.1996.270.1.E133. [DOI] [PubMed] [Google Scholar]
- 16.Yamada Y, Kuroe A, Li Q, Someya Y, Kubota A, Ihara Y, Tsuura Y, Seino Y. Genomic variation in pancreatic ion channel genes in Japanese type 2 diabetic patients. Diabetes Metab Res Rev. 2001;17:213–216. doi: 10.1002/dmrr.193. [DOI] [PubMed] [Google Scholar]
- 17.Yamada Y, Masuda K, Li Q, Ihara Y, Kubota A, Miura T, Nakamura K, Fujii Y, Seino S, Seino Y. The structures of the human calcium channel α1 subunit (CACNL1A2) and β subunit (CACNLB3) genes. Genomics. 1995;27:312–319. doi: 10.1006/geno.1995.1048. [DOI] [PubMed] [Google Scholar]
- 18.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. CaV1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Juntti-Berggren L, Larsson O, Rorsman P, Ammala C, Bokvist K, Wahlander K, Nicotera P, Dypbukt J, Orrenius S, Hallberg A, Berggren PO. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science. 1993;261:86–90. doi: 10.1126/science.7686306. [DOI] [PubMed] [Google Scholar]
- 20.Juntti-Berggren L, Refai E, Appelskog I, Andersson M, Imreh G, Dekki N, Uhles S, Yu L, Griffiths WJ, Zaitsev S, Leibiger I, Yang SN, Olivecrona G, Jornvall H, Berggren PO. Apolipoprotein CIII promotes Ca2+-dependent β cell death in type 1 diabetes. Proc Natl Acad Sci USA. 2004;101:10090–10094. doi: 10.1073/pnas.0403551101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y, Yang G, Yu J, Yu L, Westenbroek R, Catterall WA, Juntti-Berggren L, Berggren PO, Yang SN. Apolipoprotein CIII hyperactivates β cell CaV1 channels through SR-BI/β1 integrin-dependent coactivation of PKA and Src. Cell Mol Life Sci. 2014;71:1289–1303. doi: 10.1007/s00018-013-1442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/S0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 23.Yang SN, Wenna ND, Yu J, Yang G, Qiu H, Yu L, Juntti-Berggren L, Kohler M, Berggren PO. Glucose recruits KATP channels via non-insulin-containing dense-core granules. Cell Metab. 2007;6:217–228. doi: 10.1016/j.cmet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Refai E, Dekki N, Yang SN, Imreh G, Cabrera O, Yu L, Yang G, Norgren S, Rossner SM, Inverardi L, Ricordi C, Olivecrona G, Andersson M, Jornvall H, Berggren PO, Juntti-Berggren L. Transthyretin constitutes a functional component in pancreatic β-cell stimulus–secretion coupling. Proc Natl Acad Sci USA. 2005;102:17020–17025. doi: 10.1073/pnas.0503219102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, Efanov A, Yang SN, Fried G, Kolare S, Brown H, Zaitsev S, Berggren PO, Meister B. Munc-18 associates with syntaxin and serves as a negative regulator of exocytosis in the pancreatic β-cell. J Biol Chem. 2000;275:41521–41527. doi: 10.1074/jbc.M005479200. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Leibiger B, Yang SN, Caffery JJ, Shears SB, Leibiger IB, Barker CJ, Berggren PO. Cytosolic multiple inositol polyphosphate phosphatase in the regulation of cytoplasmic free Ca2+ concentration. J Biol Chem. 2003;278:46210–46218. doi: 10.1074/jbc.M303743200. [DOI] [PubMed] [Google Scholar]
- 27.Brown H, Larsson O, Branstrom R, Yang SN, Leibiger B, Leibiger I, Fried G, Moede T, Deeney JT, Brown GR, Jacobsson G, Rhodes CJ, Braun JEA, Scheller RH, Corkey BE, Berggren PO, Meister B. Cysteine string protein (CSP) is an insulin secretory granule-associated protein regulating β-cell exocytosis. Embo J. 1998;17:5048–5058. doi: 10.1093/emboj/17.17.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown H, Meister B, Deeney J, Corkey BE, Yang SN, Larsson O, Rhodes CJ, Seino S, Berggren PO, Fried G. Synaptotagmin III isoform is compartmentalized in pancreatic β-cells and has a functional role in exocytosis. Diabetes. 2000;49:383–391. doi: 10.2337/diabetes.49.3.383. [DOI] [PubMed] [Google Scholar]
- 29.Barg S, Eliasson L, Renstrom E, Rorsman P. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse β-cells. Diabetes. 2002;51:S74–S82. doi: 10.2337/diabetes.51.2007.S74. [DOI] [PubMed] [Google Scholar]
- 30.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. α1D (CaV1.3) Subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 31.Xu W, Lipscombe D. Neuronal CaV1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seino S, Chen L, Seino M, Blondel O, Takeda J, Johnson JH, Bell GI. Cloning of the α1 subunit of a voltage-dependent calcium channel expressed in pancreatic β cells. Proc Natl Acad Sci USA. 1992;89:584–588. doi: 10.1073/pnas.89.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herold KC, Vignali DA, Cooke A, Bluestone JA. Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nat Rev Immunol. 2013;13:243–256. doi: 10.1038/nri3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:139–148. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- 35.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 36.Lernmark A, Larsson HE. Immune therapy in type 1 diabetes mellitus. Nat Rev Endocrinol. 2013;9:92–103. doi: 10.1038/nrendo.2012.237. [DOI] [PubMed] [Google Scholar]
- 37.Lebovitz HE. Type 2 diabetes mellitus–current therapies and the emergence of surgical options. Nat Rev Endocrinol. 2011;7:408–419. doi: 10.1038/nrendo.2011.10. [DOI] [PubMed] [Google Scholar]
- 38.Holmberg R, Refai E, Höög A, Crooke RM, Graham M, Olivecrona G, Berggren PO, Juntti-Berggren L. Lowering apolipoprotein CIII delays onset of type 1 diabetes. Proc Natl Acad Sci USA. 2011;108:10685–10689. doi: 10.1073/pnas.1019553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghazarian L, Diana J, Simoni Y, Beaudoin L, Lehuen A. Prevention or acceleration of type 1 diabetes by viruses. Cell Mol Life Sci. 2013;70:239–255. doi: 10.1007/s00018-012-1042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jahromi MM, Eisenbarth GS. Cellular and molecular pathogenesis of type 1A diabetes. Cell Mol Life Sci. 2007;64:865–872. doi: 10.1007/s00018-007-6469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu G, Chen J, Jing G, Shalev A. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61:848–856. doi: 10.2337/db11-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ristic H, Srinivasan S, Hall KE, Sima AA, Wiley JW. Serum from diabetic BB/W rats enhances calcium currents in primary sensory neurons. J Neurophysiol. 1998;80:1236–1244. doi: 10.1152/jn.1998.80.3.1236. [DOI] [PubMed] [Google Scholar]
- 43.Hall KE, Sima AA, Wiley JW. Voltage-dependent calcium currents are enhanced in dorsal root ganglion neurones from the Bio Bred/Worchester diabetic rat. J Physiol. 1995;486:313–322. doi: 10.1113/jphysiol.1995.sp020814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall KE, Sima AA, Wiley JW. Opiate-mediated inhibition of calcium signaling is decreased in dorsal root ganglion neurons from the diabetic BB/W rat. J Clin Invest. 1996;97:1165–1172. doi: 10.1172/JCI118530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall KE, Liu J, Sima AA, Wiley JW. Impaired inhibitory G-protein function contributes to increased calcium currents in rats with diabetic neuropathy. J Neurophysiol. 2001;86:760–770. doi: 10.1152/jn.2001.86.2.760. [DOI] [PubMed] [Google Scholar]