Abstract
The research field of fetal programming has developed tremendously over the years and increasing knowledge suggests that both maternal and paternal unbalanced diet can have long-lasting effects on the health of offspring. Studies implicate that macronutrients play an important role in fetal programming, although the importance of micronutrients is also becoming increasingly apparent. Folic acid and vitamins B2, B6 and B12 are essential for one-carbon metabolism and are involved in DNA methylation. They can therefore influence the programming of the offspring’s epigenome. Also, other micronutrients such as vitamins A and C, iron, chromium, zinc and flavonoids play a role in fetal programming. Since it is estimated that approximately 78 % of pregnant women in the US take vitamin supplements during pregnancy, more attention should be given to the long-term effects of these supplements on offspring. In this review we address several different studies which illustrate that an unbalanced diet prior and during pregnancy, regarding the intake of micronutrients of both mother and father, can have long-lasting effects on the health of adult offspring.
Keywords: Fetal programming, Epigenetics, Maternal/paternal effect, Transgenerational inheritance, Micronutrients, Flavonoids
Introduction
In the late 1980s interest grew regarding the idea that maternal nutrition during pregnancy is associated with an increased risk for degenerative diseases later in life [1, 2]. A large number of studies on fetal over- and under-nourishment have been performed to investigate the adverse effects at adult age [1, 3–5]. A clear example is given by the Dutch famine, which occurred during the winter of 1944–1945. In this study population the long-term effect of reduced nutrient supply to the fetus during the first, second and third trimester of pregnancy was investigated. It was identified that, independent of the stage of fetal development, prenatal under-nourishment resulted in a reduced glucose tolerance in both male and female adults, at the age of 50 and 58 years [6, 7]. Under-nutrition during early pregnancy is also known to result in a higher prevalence of coronary heart diseases [8] and obesity [9], although the latter was only found for women. These studies support the fetal programming theory, also known as the ‘Barker hypothesis’; namely, an attempt of the fetus to adapt to adverse conditions in utero, resulting in adaptations that will be detrimental when these conditions will not prevail later in life [10].
Over the years, it was discovered that other diseases that develop in adulthood are linked to malnutrition in utero. Both animal and human studies have shown that low caloric diet of the mother during pregnancy are linked to an increased risk for adult offspring developing type 2 diabetes [11–14]. Several studies on fetal programming showed that fetal nutritional deprivation (maternal caloric or macronutrient deficiency during pregnancy) is a strong programming stimulus [1, 3, 4]. However, in many Western societies, maternal nutrition is sufficient, or even excessive. In mice, a diet high in saturated fat during pregnancy resulted in insulin resistance, obesity and hypertension in adult offspring [15], which indicates that increased health risk in adulthood may not only be a consequence of under-nourishment, but also the result of over-nourishment during pregnancy. Also, women may become obsessed with being healthy, to aid in the growth of the developing fetus, and they subsequently consume excessive amounts of supplements during pregnancy due to the misconception that for many dietary supplements ‘more is better’. In addition, to overcome certain deficiencies, such as folic acid deficiency, unbeknown to them, the general population, including pregnant women or women who are trying to conceive, is exposed to such micronutrients daily through fortification of food products. Additionally, it is obvious that paternal intake of supplements is not related to programming effects during pregnancy, although the father’s diet prior to conceiving may also play a role in fetal programming. Male rats exposed to a high-fat diet, which bred with females on a control diet, produced female offspring with an early onset of impaired insulin secretion and glucose tolerance, as well as altered gene expression in pancreatic islets, increasing their risk for diabetes later in life [16]. Therefore, it is important to understand how epigenetic programming of the developing fetus is affected by the micronutrient content of the diet of both parents.
Epigenetics: a tool for fetal programming
The first theory comprising the responsiveness of the genome to the environment, and its modifying capacity, was proposed by Jean-Baptiste de Lamarck in the early 19th century [17]. However, his ideas were neglected because of lack of evidence and aberrant conceptualization [17, 18]. Conrad Waddington was the first to follow in de Lamarck’s footsteps during the first half of the 20th century, introducing the term ‘epigenetics’, which combines the prefix ‘epi’, meaning ‘upon’ or ‘over’, with ‘genetics’ implying the involvement of genes, to define the study of events over or beyond genes themselves. The term ‘epigenetics’ was also derived from the Aristotelian word ‘epigenesis’, implying that developmental changes are gradual and qualitative, but that there is a link to heredity [19].
While the phenomena of epigenetic manifestations, namely changes in DNA methylation, chromatin remodeling and microRNA expression, are gaining increasing appreciation, researchers are becoming increasingly interested in understanding the control of gene expression and its connection with the underlying processes of disease development. Moreover, they seek to identify the origin of the disease in the epigenetic regulation within the placenta [20, 21]. Such epigenetic regulatory mechanisms include modifications in promoter DNA methylation, resulting in hypo- or hypermethylation of DNA CpG regions, and changes in histone proteins, chromatin conformation, as well as microRNA and non-coding RNA-mediated control of gene expression within the placenta [20–23]. Most studies regarding diet-induced fetal programming address DNA methylation as an epigenetic mechanism, and since several micronutrients are involved in the one-carbon metabolism, we mainly focus on the role of DNA methylation on fetal programming. Furthermore, it has been shown that modifications of histone proteins turnover more rapidly than cell division, thereby questioning the involvement of histone modifications in transmitting epigenetic information [24, 25].
During fetal development, epigenetic alterations are crucial in the orchestration of gene expression [26]. Incorporation of a methyl (CH3) group on the cytosine base of CpG islands, which are mainly located in gene promoter regions, the expression of a gene can be regulated [27]. During fetal development, the epigenome cycles through several precisely timed methylation changes to ensure proper development. Shortly after fertilization the paternal genome is actively demethylated, while the maternal genome is passively demethylated [26]; however, some epigenetic marks are maintained to allow proper expression of imprinted genes (parent-of-origin methylation marks) [26] (about 100 identified in mammals [28]), but also allele-specific expression is very common [29]. Subsequently, a new pattern of DNA methylation, which is also referred to as de novo methylation, is established predominantly at the blastocyst stage [20, 21, 30]. The incorporation of methyl groups during de novo methylation is carried out by DNA methyltransferases (DNMTs) 3A and 3B [21, 27]. This de novo methylation can be considered as a reprogramming of the DNA methylation patterns in the zygote, which are normally retained throughout the organisms’ life by the action of DNMT1, and are crucial for somatic cell viability [20, 21, 30]. The appropriate succession of the resetting and reprogramming phase of methylation is fundamental to post-gestational health and survival as aberrant methylation patterns can lead to the development or progression of human diseases. This signifies an important sensitive window within the development of an embryo and of its germ cells, during which the environment can have profound effects on the expression pattern of certain genes and persist throughout life [20, 31]. A clear example of fetal programming by diet-induced changes in methylation is given by Dolinoy et al. [32]. They showed that heterozygous agouti viable yellow (A vy) mice exposed to the flavonoid genistein in utero via the maternal diet resulted in an altered coat color of these mice, namely towards pseudo-agouti, and these mice were also protected against obesity later in life, which is usually associated with the agouti phenotype. These changes in phenotype were caused by hypermethylation of transposable repetitive elements, namely intracisternal A particles (IAP), upstream of the transcription start site of the Agouti gene which remained unaltered throughout life. Moreover, the study by Gordon et al. [33] also indicated how critical and sensitive this in utero period is for fetal programming, as they showed that within monozygotic twins at birth the expression of genes sensitive to the external environment differed. As monozygotic twins are genetically identical, they assumed that even the slightest difference in the intrauterine environment could affect epigenetic regulation.
Maternal and paternal influences on fetal programming
Regarding fetal programming, most studies focus on the maternal diet as being an important contributor since it can result in adaptations of the offspring via regulation of the in utero environment. The importance of the maternal diet during pregnancy has clearly been shown in the study of Persson et al. [34], who investigated the effect of supplementing poor, malnourished women in Bangladesh from early pregnancy onwards with multiple micronutrients, including iron, folic acid and food supplements, to increase both micronutrient and macronutrient intake. Although this intervention did not affect birth weight compared with the standard intervention of iron, folic acid and food supplements from mid-pregnancy onwards, it did result in a decreased 5-year mortality rate of their children. Also, the study of Carlsen et al. [35] showed that maternal diet during pregnancy could have an effect on the development of the fetus as they showed that maternal fatty acid intake during pregnancy affected fetal growth patterns. These investigators namely found that maternal blood n-3 polyunsaturated fatty acids during pregnancy were inversely associated with the length of the femu of the fetus, although no association was found with fetal weight. Moreover, maternal over- or under-nutrition has been shown to induce long-lasting health effects in several studies [1, 3–5]. In sheep, a 50 % global nutrition restriction during the first half of gestation resulted in a predisposition for the development of obesity and diabetes in aged female offspring [36]. Likewise, maternal caloric restriction during pregnancy resulted in an exaggerated pulmonary hypertension and right ventricular hypertrophy when offspring mice were exposed to hypoxia in vivo which is attributed to a change in DNA methylation status in the lung tissue of these offspring. Interestingly, when pups of mothers on caloric-restricted diets were given histone deacetylase inhibitors, lung DNA methylation status and pulmonary vascular function were normalized [37], thereby demonstrating the plasticity of epigenetic modifications.
Alternatively, the paternal influence on epigenetic alterations in offspring should not be neglected. Since the father solely transmits genetic and epigenetic factors to the oocyte, the father may serve as a better model to explore epigenetic involvement. For instance, paternal age seems to be associated with an increased risk for birth defects and DNA mutations in sperm cells [38]. Increasing age is also associated with alterations in DNA methylation patterns of somatic cells. Oakes et al. [39] demonstrated that age-dependent alterations in DNA methylation occur in rat spermatozoa. In addition, paternal lifestyle can play a role in fetal programming, as suggested in the study performed by Bielawski et al. [40], which showed that, in rat, paternal chronic alcohol intake decreased messenger RNA (mRNA) expression of cytosine methyltransferase in sperm, which could result in hypomethylated DNA which is then passed on to the offspring. Moreover, Ouko et al. [41] showed that chronic alcohol intake in men resulted in the hypomethylation of two imprinted genes, H19 and intergenic differentially-methylated region (IG-DMR), which are normally hypermethylated in sperm. However, in order to transmit the environmentally-induced epigenetic modifications to the offspring, and influence adult phenotypes, the active demethylation of the paternal genome shortly after fertilization and subsequent de novo methylation during embryogenesis need to be survived.
A recent study showed that offspring of male mice fed a protein-deficient diet, and female mice on a control diet throughout and after gestation, had an increased expression of genes involved in fat and cholesterol biosynthesis in the liver. Although they did not find significant changes in DNA methylation, there was a substantial increase in methylation at an intergenic CpG island upstream of Ppara, which is likely an enhancer for Ppara and could therefore regulate hepatic gene expression [42]. Additionally, fathers can also induce epigenetic alterations in their offspring via the RNA present in sperm cells; microinjection of total RNA or microRNA from sperm of Kit heterozygote males into fertilized eggs resulted in the birth of wild-type offspring mice with white spots on their tail, a characteristic of Kit mutant mice [43]. Moreover, in humans, the mRNA profile of sperm cells is known to be sensitive to environmental factors, including cigarette smoke [44], further supporting a possible mechanism by which the environment can affect the health of offspring via paternal lineage.
Diet effects across generations
Although it has been suggested that some epigenetic variations can be conserved over generations, most studies, both in humans and animals, address the effect of in utero nutrition on fetal programming in first- and second-generation offspring. For instance, Kaati et al. [45] showed that the grandparent’s diet during the slow growth period, namely before the prepubertal growth peak, had long-lasting effects on the longevity of their grandchildren, by gender-specific transgenerational inheritance.
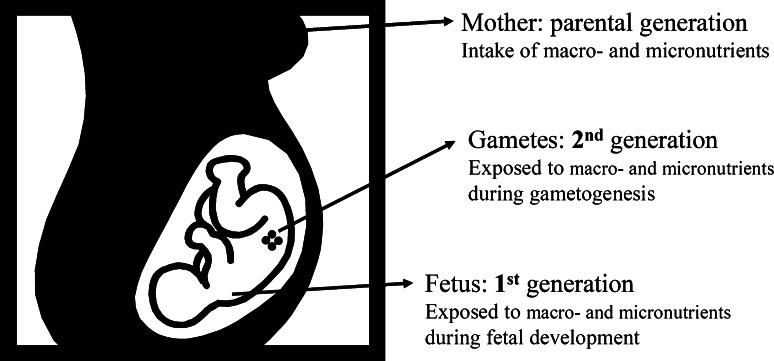
However, to examine true transgenerational inheritance in response to diet, the third generation, being the first ‘unexposed’ generation, should be studied. The first filial generation is directly exposed to the maternal diet, while the second generation results from gametes that were exposed in utero (Fig. 1). In order to transfer environmentally-induced epigenetic modifications to future generations, demethylation of the paternal and maternal genome shortly after fertilization, as well as demethylation of primordial germ cells, need to survive permanently.
Fig. 1.
Transgenerational inheritance of epigenetic modifications induced by exposure to macro- and micronutrients
Despite increased awareness that parental dietary intake can affect the health of future generations, the number of true transgenerational studies are rather limited, and not all studies labeled as being transgenerational studies actually investigate the effect on the F3 generation. One clear example of a transgenerational study is given by Dunn and Bale [46], who showed that in utero exposure to a high-fat diet resulted in increased body size in F3 female offspring. Interestingly, this transgenerational inheritance seemed to be transmitted via the paternal lineage. Dunn and Bale [46] assumed that the diet-induced effect on body size may result from changes in epigenetic status of imprinted genes, which could explain the sex-specific inheritance. Since obese adults are more likely to be insulin resistant, they also have an increased risk for chronic diseases, including cardiovascular diseases, type 2 diabetes mellitus and hypertension [47]. Also, in a rat model of carcinogen-induced breast cancer it has been shown that maternal high-fat intake during gestation increases the risk for breast cancer in the following two generations [48]. Moreover, the increased risk for breast cancer in the F2 generation can be transmitted by both parents who were exposed to a high-fat diet in utero, although this effect was not transmitted to the F3 generation and therefore cannot be seen as a true transgenerational inheritance. In the same rat model, maternal exposure to ethinylestradiol during gestation resulted in an increased breast cancer risk for F1 and F3 generations, whereas the F2 generation depended on whether or not the mother (increased risk) or the father (decreased risk) was prenatally exposed to ethinylestradiol [48]. Waterland et al. [49] also performed a transgenerational study, although more complicated than the study of Dunn and Bale [46] and de Assis et al. [48]. Waterland et al. [49] found that female heterozygous A vy mice, prone to hyperphagic obesity, placed on a diet supplemented with methyl-donors, namely folic acid, vitamin B12, betaine and choline, throughout life, were significantly leaner at adult age compared with the unsupplemented group. This was also observed for their daughters, granddaughters and great-granddaughters, whereas the unsupplemented offspring had an increase in body weight with successive generations. However, supplementation did not affect adult body weight of daughters and granddaughters, suggesting that the methyl-donor supplementation had a cumulative transgenerational effect and resulted in protection against obesity.
Nevertheless, not all environmentally-induced epigenetic modifications are inherited transgenerationally. Although dietary methyl-donor supplementation of female mice before and during gestation affected the methylation status of transposable repetitive elements upstream of the transcription start site in the Agouti gene in heterozygous A vy offspring mice [50] and successive generations of A vy mice placed on a diet supplemented with methyl-donors throughout life resulted in a cumulative transgenerational effect regarding the risk for obesity [49], it did not have a cumulative effect on diet-induced hypermethylation of the Agouti gene over three generations, suggesting that no transgenerational inheritance occurred [51].
The role of micronutrients in fetal programming
Importance of micronutrients involved in DNA methylation
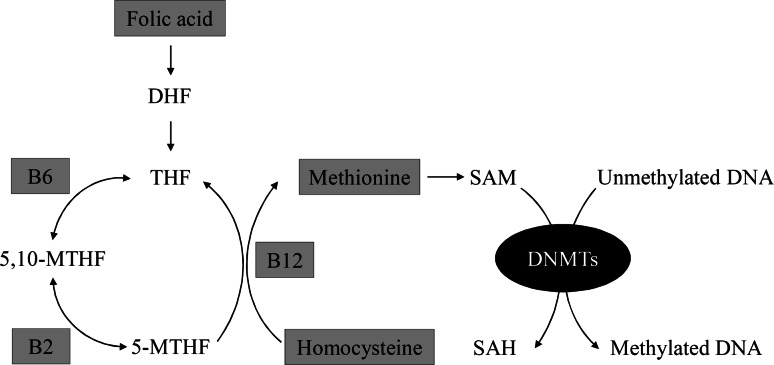
At the present time it seems clear that an unbalanced maternal diet plays a role in fetal programming of adult diseases. Although several studies implicate that macronutrients play a major role in fetal programming [1, 3, 4, 9, 52], micronutrients are also of interest since they are essential for the one-carbon metabolism involved in DNA methylation. The micronutrient folic acid, also known as vitamin B9, donates its methyl group to homocysteine to form methionine. Subsequently, methionine donates its methyl group to DNA via S-adenosyl-methionine (SAM) [Fig. 2]. Other important micronutrients involved in the one-carbon metabolism are vitamins B2, B6 and B12 [53]. This suggests that an unbalanced maternal micronutrient diet could also affect DNA methylation patterns of offspring and result in altered fetal programming. Indeed, an increased maternal serum vitamin B12 level during pregnancy has been associated with decreased global DNA methylation in newborns, while higher serum vitamin B12 concentration in newborns was associated with reduced methylation of the IGFBP3 gene, which is involved in intrauterine growth [54]. Yajnik et al. [55] showed that within the Pune Maternal Nutrition cohort, children born to mothers with low vitamin B12 and high folic acid levels were insulin resistant and had higher body fat percentage and higher abdominal fat at the age of 6 years, making them at increased risk of developing type 2 diabetes. A periconceptional methyl-deficient maternal diet also resulted in increased adiposity and insulin resistance in sheep offspring [56]. In addition, the offspring of ewes with reduced levels of vitamin B12, folic acid and methionine also had altered immune function and high blood pressure at adult age. The effects of the maternal diet were mainly found in male offspring and were thought to be the result of epigenetic mechanisms since 4 % of the 1,400 CpG islands examined showed an altered methylation status [56]. Furthermore, the tumor-suppressor gene p53 showed to be less methylated in the intestines of adult mice born from mothers fed a diet low on folate during pregnancy, compared with the intestines of adult mice born from mothers fed normal amounts of folate during pregnancy [57]. This research group also showed that, in mice, low folate intake by the mother during pregnancy resulted in global hypomethylation of the adult offspring, which is associated with higher risk of developing cancer, compared with adult offspring in utero exposed to normal levels of folate [58].
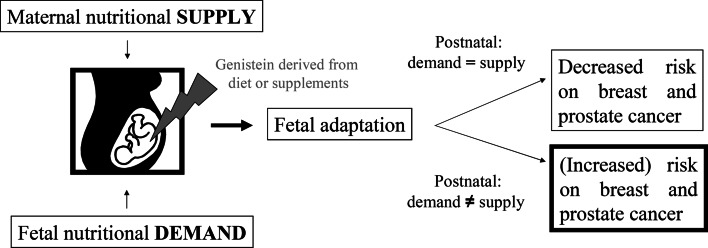
Fig. 2.
Hypothetical concept regarding the difference in fetal programming induced by genistein obtained from a Western or Asian diet. In the case of an Asian diet, genistein is taken up by the mother, via dietary sources prior to, during and after pregnancy, while the offspring remains exposed to dietary genistein throughout its life, resulting in a decreased risk for breast and prostate cancer. In the case of a Western diet, genistein levels are low, although they can be increased due to supplement intake. The fetus adapts to these low levels of genistein; however, once born these levels may not be attained via the diet, potentially affecting the risk for offspring to develop breast or prostate cancer later in life
Although these studies investigated the effect of maternal folic acid deficiency on the health of the offspring, folic acid supplementation during pregnancy is almost universally implemented to prevent neural tube defects [59, 60], as the normal Western diet contains about 0.2 mg of natural folic acid/day, while 0.4 mg folic acid/day is recommended [61]. Moreover, diet fortification with folic acid is also performed in several countries and has been shown to reduce the incidence of neural tube defects [62, 63]; therefore, it is reasonable to assume that in some cases folic acid ‘oversupplementation’ may occur. A study in which high-dose folic acid was supplemented (≥5 mg folic acid/day) during early pregnancy showed an association with increased neurodevelopment, resulting in enhanced vocabulary development, communicational skills and verbal comprehension at 18 months of age [64]. However, folic acid supplementation during late pregnancy seems to be associated with childhood asthma, while folic acid supplementation during early pregnancy was associated with wheezing [65]. These data are, however, still controversial [66, 67] and need further study, both in humans and animal models. Mice in utero exposed to a diet rich in folic acid and methyl donors, indeed more often develop severe allergic airway disease, partly due to the increased methylation of Runx3 [68]. In humans it has been shown that high folate and vitamin B12 intake during the first trimester of pregnancy was associated with the development of atopic dermatitis [69]. Moreover, maternal intake of the recommended amount of folic acid (0.4 mg folic acid/day) was found to be related to increased methylation of the IGF2 gene in children at 17 months of age. This increase in methylation was also associated with a decrease in birth weight [70], which is known to be associated with an increased risk of developing chronic diseases later in life [5, 10]. In addition, maternal folic acid supplementation during gestation, at a level equivalent to the average post-fortification of total folic acid intake in North America, increased the risk for mammary tumors in rat offspring [71]. Epigenetic mechanisms are thought to be involved since in utero exposure to increased levels of folic acid resulted in global DNA hypomethylation [71]. Interestingly, the risk of developing colorectal cancer was decreased in rat offspring exposed in utero to folic acid supplements at the level equivalent to the average post-fortification of total folic acid intake in North America [72]. On the other hand, maternal methyl-donor supplementation during gestation, consisting of folic acid, vitamin B12, betaine and choline, increased the susceptibility of mice offspring to develop colitis after dextran sulfate sodium exposure. In this case, epigenetic mechanisms were also involved since the onset of colitis was linked to persistent changes in DNA methylation of a selected number of genomic loci [73].
Role of other micronutrients in fetal programming
Insufficiency or excess of other micronutrients may also have long-lasting effects on the health of offspring as they are involved in cardiovascular, renal and pulmonary functioning at adult age. For instance, chronic maternal restriction of chromium, which is involved in glucose and fat metabolism, during gestation resulted in increased body adiposity in offspring rats [74]. This outcome was also found by the same research group for chronic restriction of magnesium [75], and for overall chronic mineral restriction, resulting in lower maternal levels of iron, zinc, magnesium and calcium [76]. Jou et al. [77] also found that, in rats, moderate maternal zinc restriction during gestation resulted in an increase in body weight of the offspring compared with offspring of mothers consuming normal levels of zinc, although no difference in birth weight was found between both groups. Moreover, these offspring also showed increased blood glucose levels and were less sensitive to insulin and glucose stimulation. Jou et al. [78] also showed that postnatal diet played an important role in the severity of the metabolic derangements as it was observed that increased body weight and insulin resistance was only found in the offspring of rats that received lower levels of prenatal zinc and adequate nutrition or excess nutrition postnatal. Moreover, poor nutrition postnatal even decreased the body weight of the offspring compared with the control offspring. Vitamin D supplementation during pregnancy is thought to protect against multiple sclerosis [79, 80] and osteoporosis [81]. However, male rats exposed in utero to high levels of multivitamins (tenfold higher levels than the recommended amount of vitamins) have a higher food intake, increased body weight, insulin resistance and elevated blood pressure at adult age, despite having normal birth weights [82]. Furthermore, low maternal vitamin D status during pregnancy seems to be associated with an increased risk of developing eczema in the first year of life [83].
In addition, micronutrients also serve as antioxidants or are essential cofactors for antioxidant enzymes [84], and therefore it is believed that the effects of micronutrients are linked to antioxidant systems. Deficiencies in antioxidants during pregnancy may induce organ damage and impair embryonic development due to increased levels of reactive oxygen species (ROS). Franco Mdo et al. [85] showed that prenatal supplementation with mixed antioxidant vitamins and minerals (selenium, folic acid, vitamin C and vitamin E) protected rat offspring from long-term cardiovascular injury, but not long-lasting renal injury, induced by in utero macronutrient restriction. In addition, in utero exposure to increased levels of antioxidants also protected the colon of 3-day-old piglets against iron-induced oxidative DNA damage [86].
Are flavonoids involved in fetal programming?
The micronutrients we focus on are the dietary flavonoids as they are known to be potent antioxidants. They, being the main component of a plant’s pigmentation and flavor, comprise a large group of polyphenolic compounds widely distributed throughout the diet [87]. Total flavonoid intake of humans varies considerably, due to the variability of foods consumed. For a Western population, it has been estimated that the average flavonoid intake ranges between 65 and 250 mg/day [88]. The most predominant flavonoid in the human diet is quercetin, which is mainly found in onions, apples, tea and red wine [87–89], the average daily intake of which is estimated to be between 10 and 100 mg [90]. Another important flavonoid is the isoflavone genistein, which is mainly found in soybeans. Due to the low consumption of soy-containing food in Western countries, the daily intake of isoflavones is in the range of several milligrams per day. However, for lactose intolerant people, who consume soy milk as a replacement for bovine milk, isoflavone intake can increase dramatically since soy milk contains between 30 and 175 mg/L of isoflavones. In Asian countries, the daily intake of isoflavones is much higher because of the high consumption of soy, and reaches levels of 25–40 mg/day [91]. Vegetarians and vegans, for whom soybeans are an important protein source, also have isoflavone plasma concentrations comparable with concentrations found in Asian populations [92]. Interestingly, the level of genistein, found in soy-based infant formula, exceeds the levels found in Asian adults [93]. Presently, studies regarding the antioxidant property of flavonoids claim that they provide several health benefits, such as anti-inflammatory effects [94, 95], and protection against several diseases, including cardiovascular diseases [96, 97], neurodegenerative diseases [98, 99], chronic obstructive pulmonary disease (progression) [100, 101] and ocular disease [102, 103]. This is also the main reason why flavonoids are gaining interest as treatment and prevention options for adult diseases [87, 104–106]. Flavonoids are therefore also commercially available as high-dose supplements in some countries, and to date no human data on the long-term effects of high-dose flavonoid supplementation are available [107, 108]. For instance, the daily dose of quercetin supplements lies between 200 and 1,200 mg [108]. Moreover, quercetin may also be used as a nutraceutical for functional foods [109]. Since, in Asian countries, the potential health benefits of genistein intake include a decreased risk for developing breast and prostate cancer [110], isoflavone supplements (daily doses ranging from 50 to 500 mg) are the most commonly used supplements[111]. However, high soy consumption, and therefore genistein intake, in Asian countries is part of the culture, and a high-exposure level of genistein not only occurs in utero but also throughout life (Fig. 3). It should also be considered that other nutritional and lifestyle factors may contribute to the lower incidence of breast and prostate cancer in Asian countries.
Fig. 3.
Simplified schematic of the folic acid metabolic pathway resulting in DNA methylation. Methylation of DNA occurs via the folic acid metabolic pathway. In this pathway the micronutrient folic acid (vitamin B9) is first reduced to dihydrofolate (DHF), which is then reduced to tetrahydrofolate (THF). 5,10-methylene-THF (5,10-MTHF) is formed by adding a methylene group to THF. In this step of the pathway, vitamin B6 (B6) serves as an essential co-enzyme. Next, 5,10-MTHF is reduced to 5-methyl THF (5-MTHF) with the aid of the essential co-enzyme vitamin B2 (B2). 5-MTHF then donates, with the co-enzyme, vitamin B12 (B12), its methyl group to homocysteine, resulting in the formation of methionine. Subsequently, methionine donates its methyl group to DNA via S-adenosyl-methionine (SAM). SAH s-adenosylhomocysteine
Despite the possible health benefits of flavonoids in adults, little is still known about their action on offspring during and after pregnancy, although it is known that flavonoids pass the placenta and can accumulate in the fetus [112, 113].
In utero exposure to genistein and other endocrine disrupters
As mentioned previously, a clear example of the effect on fetal programming by the flavonoid genistein was given by Dolinoy et al. [32], whereby they showed that prenatal exposure of heterozygous A vy mice to genistein resulted in a change in coat color and protection against adult obesity. Moreover, we have found that prenatal exposure to genistein produced long-lasting alterations on bone marrow gene expression of adult mice [114]. More specifically, these mice showed a pronounced downregulation of estrogen-responsive genes and of genes involved in inhibiting hematopoiesis, but also an upregulation of genes required for erythropoiesis and granulopoiesis [114]. The effect of genistein on estrogen-responsive genes is somewhat expected since genistein has structural similarities with 17β-estradiol and exerts its effect through the estrogen receptor [115]. Another estrogen-/endocrine-disrupting chemical, bisphenol A (BPA), present in many types of plastics, is involved in fetal programming as a result of unintentional exposure to pregnant woman and their fetuses. Prenatal exposure to BPA is thought to increase the onset of obesity [116], childhood asthma [117], breast cancer [118, 119] and diabetes [120]. As mentioned previously, de Assis et al. [48] showed that in utero exposure to the endocrine-disrupting agent ethinylestradiol is involved in fetal programming for up to three consecutive generations.
In utero exposure to genistein, quercetin and other antioxidant micronutrients
Oxidative stress is thought to play an important role in the development and progression of many chronic diseases, including neurodegenerative diseases [121–125], cardiovascular diseases [126, 127], diabetes [128] and cancer [129–131], but may also contribute to aging [132]. Oxidative stress occurs when an imbalance between ROS and antioxidants arises, resulting in ROS-induced damage to proteins, lipids and DNA [133]. Besides a native antioxidant system consisting of enzymatic antioxidants [such as catalase (Cat), glutathione peroxidase (Gpx) and superoxide dismutase (SOD)] and non-enzymatic antioxidants (for instance glutathione), humans can also enhance their antioxidant defense by increasing the intake of vitamin C, vitamin E, β-carotene and flavonoids. Does this enhancement of antioxidant defense also apply for in utero exposure to flavonoids? To assess this, we recently investigated the long-term effect of prenatal exposure to genistein and quercetin on antioxidants. Prenatal exposure to quercetin resulted in upregulation of Nrf2, a regulator of antioxidant gene transcription, and Sod2, responsible for enzymatic antioxidant defense in the liver of fetuses at day 14.5 of gestation and in both the liver and lung tissue of adult mice. Alternatively, prenatal exposure to genistein only resulted in increased gene expression of Nrf2 and enzymatic antioxidant genes in the liver of adult mice. Moreover, prenatal exposure to genistein and quercetin decreased the oxidative stress-induced DNA damage in the liver of these adult mice. This suggests that adaptations made by the fetus in response to the in utero conditions during maternal supplementation with increased level of genistein or quercetin are beneficial and protective against oxidative DNA damage in the liver of these mice as adults [134].
In addition, the effect of prenatal exposure to other micronutrients having antioxidant capacity has been investigated. For instance, supplementation of female rats, during gestation and lactation with vitamin A, a fat-soluble vitamin required for several processes, including reproduction, immune response, vision and maintenance of cellular differentiation, resulted in decreased glutathione-S-transferase activity in the kidneys of offspring. Moreover, the total antioxidant potential of the offspring’s liver was decreased. Interestingly, male offspring showed increased levels of lipid peroxidation, whereas this was decreased in female offspring [135]. Vitamin A supplementation during gestation and lactation has also been shown to induce oxidative stress in the lungs of rat offspring [136], making them more prone to develop lung diseases. Another lipid soluble antioxidant, vitamin E, enhanced the spontaneous tumor formation in both heterozygous and mutant p53 knockout offspring mice when exposed in utero, although it reduced fetal death presumably by decreasing ROS [137].
These findings show that in utero exposure to micronutrients with antioxidant capacity indeed have long-lasting effects on the offspring’s antioxidant defense system.
Fetal programming by iron
Oxidative stress and formation of ROS can also be induced by micronutrients, such as iron. Although it is known that the iron chelating property of quercetin helps to reduce oxidative stress [138, 139], we found in our studies that in utero exposure of mice to quercetin did not affect the total amount of iron in the amniotic fluid, or adjusted the switch in erythroid lineage or hemoglobin profile of fetuses at day 14.5 of gestation. However, prenatal exposure to quercetin did result in increased iron storage in the liver of mice at adult age. We showed that this was the result of upregulation of iron-associated cytokines (hepcidin, interleukin (IL)-1β, IL-6 and IL-10). Despite the increased iron levels, oxidative stress was significantly decreased in the liver of these animals. We assumed that there was probably less iron accessible for fetuses in utero, to which they adapted. Once born, quercetin exposure was ceased and offspring encountered normal levels of iron, which they presumably sensed as iron overload. Nevertheless, they did cope with the increased iron availability later in life. Likewise, they showed an increase in protection against iron-induced ROS formation, since we observed a decrease in oxidative stress-induced DNA damage in the liver of these mice as adults. However, the cytokines involved in iron homeostasis are also involved in regulating inflammation. Therefore, one could expect that induction of inflammatory pathways by an inflammatory trigger would result in an uncommon reaction from these mice [140]. As inflammation is involved in many chronic diseases, including cancer [133], it seems that although these animals appear to be protected against oxidative stress-induced diseases, an inflammatory stressor could alter this protective mechanism towards a harmful one, but this needs confirmation.
Nevertheless, iron remains the most commonly prescribed supplement during pregnancy to prevent pregnancy-related anemia, which could be harmful for both mother and child. However, it has also been reported that iron supplementation during pregnancy could suppress the antioxidant status of pregnant woman. This is thought to be the result of iron-induced formation of ROS [141]. Moreover, Lachili et al. [142] showed that when given high iron supplements combined with vitamin C during the last trimester of pregnancy, healthy, non-anemic pregnant woman produced an increase in lipid peroxidation and consequently decreased vitamin E levels. Preconceptional and early pregnancy exposure to high levels of dietary heme iron has also been suggested to increase the risk of gestational diabetes [143]. Therefore, instead of prophylactic iron supplementation of pregnant woman, a more individual approach may have better outcomes as iron overload could be harmful for both mother and child.
Role of natural aryl hydrocarbon receptor agonists in fetal programming
Throughout life, humans are constantly exposed to drugs and other xenobiotic compounds, which, if not excreted from the body, can induce damage resulting in chronic diseases. Environmental genotoxicants and mutagens cause about 80 % of human malignancies [144]. One important group of environmental contaminants is the polycyclic aromatic hydrocarbons (PAHs). Humans are exposed to PAHs through inhalation of smoke from coal, wood, diesel fuel and tobacco; but also through ingestion of roasted, smoked or charbroiled foods. However, to induce damage, PAHs first need to be metabolically activated. The best-studied PAH is benzo[a]pyrene (B[a]P). An important player in the metabolization of B[a]P is the aryl hydrocarbon receptor (AhR). Binding of B[a]P to AhR will result in the transcription of phase I and phase II enzymes and consequently in the metabolism and excretion of B[a]P [145]. Therefore, AhR signaling plays an important role in determining the capacity to detoxify genotoxicants and mutagens, and may therefore contribute to an individual’s risk of developing cancer after exposure [146]. Besides playing a role in the metabolism of genotoxicans and mutagens, phase I and phase II enzymes are also involved in the metabolism of exogenous compounds that can affect fetal programming, for instance 17β-estradiol [147] and BPA [148]. However, dietary components can also influence AhR activity, for instance quercetin [145]. Moreover, the intake of natural AhR agonists in the Dutch population seems to be much higher compared with the intake of xenobiotic AhR agonists [149]; therefore we investigated whether in utero exposure to quercetin could alter B[a]P metabolism of the adult liver. We found that exposure to quercetin induced gene expression of cytochrome P450 (CYP) 1A1 and CYP1B1, both involved in the metabolic activation of B[a]P, and of phase II enzymes (Nqo1, Ugt1a6 and Gstp1), in mice fetuses at day 14.5 of gestation. Moreover, increased expression of CYP1B1 was maintained throughout life, while AhR gene expression was acquired. Ex vivo induction of B[a]P diolepoxide-DNA (BPDE-DNA) adducts was decreased when liver microsomes of mice were prenatally exposed to quercetin, suggesting that prenatal exposure to quercetin could protect the liver from B[a]P-induced DNA damage, which we suspect is due to the upregulation of phase I enzymes. We also compared male and female littermates and found that when prenatally exposed to quercetin, female mice developed a more masculine profile regarding the phase I and II enzymes compared with control female mice [150]. Moreover, Makaji et al. [151] also investigated the effect of prenatal exposure to quercetin on the activity of biotransforming enzymes at adult age. Maternal quercetin intake during gestation resulted in an increased biotransformation of several fluorogenic substrates by liver microsomes of adult offspring. Here, female offspring also had a more masculine profile.
Role for DNA methylation in genistein- and quercetin-induced fetal programming
The changes in liver and bone marrow gene expression which resulted from in utero exposure to genistein or quercetin, as discussed above, are maintained throughout life. We therefore investigated whether prenatal exposure to genistein or quercetin could affect methylation status of repetitive elements, since the mouse genome constitutes almost 37.5 % of these repetitive elements [152]. Results showed that neither flavonoid induce changes in global methylation (namely, methylation of SINEB1, SINEB2, LINE1, IAP, major and minor satellites) in the liver of fetuses at gestational day 14.5 [114, 140, 150]. However, when investigating methylation patterns in the liver of adult mice prenatally exposed to genistein or quercetin, mild hypomethylation of the repetitive elements SINEB1, SINEB2 and LINE1 was observed [150]. On the other hand, in bone marrow of mice prenatally exposed to genistein, hypermethylation of repetitive elements (SINEB1, SINEB2, LINE1, IAP, minor and major satellites) could be distinguished [114, 140], suggesting that prenatal exposure to flavonoids results in organ-specific alteration in DNA methylation.
The fact that no changes in methylation could be found in fetuses at day 14.5 of gestation suggests that de novo methylation induced by flavonoids took place at a later timepoint in gestation, or even after birth. This is plausible as during the late gestational and early postnatal period, tissue maturation can result in epigenetic modifications.
Conclusion and future directions
The field of fetal programming, both by macronutrients and micronutrients, is still developing, although increasing knowledge suggests that an unbalanced diet of both mother and father can have long-lasting effects on the health of their offspring, which is shown by its association with the onset of diabetes [11–14, 16, 55, 56], obesity [55, 56, 74–76], cancer [71, 137] and colitis [73], and the ability to increase oxidative stress [136, 141, 142]. However, our studies suggest that prenatal exposure to flavonoids is beneficial as it resulted in increased protection against ROS and environmental carcinogens at adult age [140, 150]. As vitamins and minerals continue to play a critical role in the function of organ systems later in life [153] and antioxidants remain important in coping with the onset of adult chronic diseases induced by oxidative stress [133, 154, 155], extensive future research is needed. Special attention should be given to prenatal diet and subsequent disease triggers later in life to see whether prenatal diet can have a pre-emptive effect (see Fig. 4).
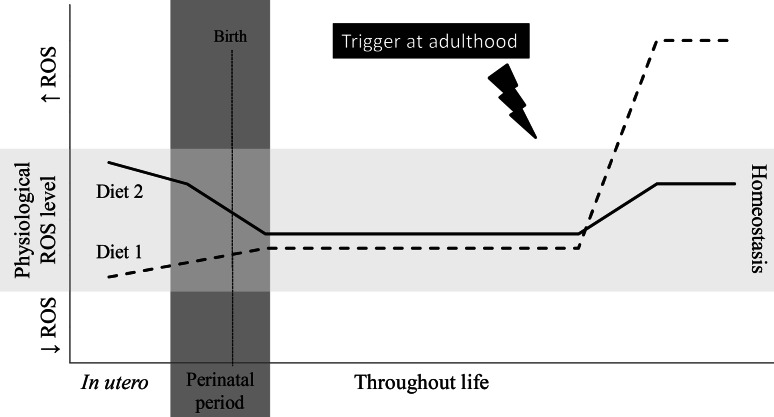
Fig. 4.
Hypothetical health consequences of prenatal diets upon trigger at adulthood. Whether or not the prenatal diet will have a pre-emptive effect and be beneficial at adult age, or whether it will have deleterious effects later in life may depend upon the composition of the diet, the fetal adaptations and subsequent disease triggers later in life. For instance, in the case of diet 1, an increase in antioxidant intake by the mother during pregnancy decreased the ROS levels in the fetus, to which it adapts. If during the offspring’s adult life, its antioxidant defense system is triggered, excessive oxidative stress can occur because the offspring did not ‘learn’ in utero how to cope with these increased levels of ROS. In the case of diet 2, the maternal diet during pregnancy consisted of lower levels of antioxidants or increased triggers of the Nrf2 pathway. Again, the fetus will adapt to these conditions, and in adulthood, when the antioxidant defense system of this offspring is triggered, this can result in an improved response since the offspring adapted and ‘learned’ in utero how to cope with such a trigger
In the US, as much as 47 % of males and 59 % of females regularly use dietary supplements [156]. Although studies concerning the use of dietary supplements during pregnancy are limited, it has been reported, for the US, that 78 % of woman take vitamins during pregnancy [157]. For instance, 72 % of pregnant women and 60 % of lactating women consume iron supplements, versus 9–23 % of non-pregnant, non-lactating women [158]. Women who are trying to conceive are advised to take, in addition to iron supplements, folic acid supplements, preferably prior to and during the first trimester of pregnancy, as folic acid deficiency is related to neural tube defects. However, since women do not always anticipate their pregnancy, many countries fortified food with folic acid to reduce the risk of folic acid deficiency [159]. Additionally, it has also been put forward that folic acid should be added to the contraceptive pill to prevent neural tube defects [160]. Nevertheless, the data presented in this review indicates that ‘over-supplementation’, resulting from both supplementation and/or food fortification, could also have long-term effects on the unborn child, which may be harmful; therefore, the long-term effects need more attention, especially since adequate levels of folic acid, needed to prevent neural tube defects, only applies during the first trimester of pregnancy [63]. Hence, folic acid supplementation during the remainder of pregnancy could be unnecessary and may even be associated with a higher risk of developing mammary cancer at adult age [71]. Moreover, supplement intake can be regulated, although this cannot be done in the case of fortified foods. Fortification of food not only has an effect on the nutritional status of the mother, but also of the father, for whom supplementation may not be necessary. Although increased intake of folic acid by males may increase sperm quality [61], it could also affect DNA methylation status of sperm cells. Overall, the ideal diet composition for pregnant women and future fathers still needs to be established, especially in regards to micronutrient supplementation, as the intake may not directly be toxic to the developing child, but may affect fetal programming resulting in physiological alterations that persist into adulthood. Moreover, the dietary status of both parents may not only affect the health outcome of their children but may also have an influence on multiple future generations.
Studies concerning micronutrient fortified foods or supplement use during pregnancy mainly focus on maternal status (maternal mortality and anemia) and pregnancy outcome (pre-eclampsia, birth weight and length, and premature birth) and are often shown to be beneficial for both mother and child [161]. Birth weight is an important marker used in these studies to determine the safety of compounds for the unborn child as low birth weight is thought to be associated with increased risk of developing chronic diseases later in life [5, 10]. However, this is not always true; for instance, people exposed to the Dutch famine during early gestation had a normal birth weight, while later in life they had a higher body mass index (BMI) and risk of developing cardiovascular diseases. This was thought to be the result of a weight catch-up by the fetus when World War II ended and food supply was restored [52]. Persistent epigenetic changes are again thought to be involved as Heijmans et al. [162] showed that the imprinted IGF2 gene was less methylated in 60-year-old humans conceived during the ‘Dutch hunger winter’ compared with siblings conceived before or after the hunger winter. Hence, more careful use of biomarkers such as DNA methylation to determine the risk for development of adult chronic diseases is required.
From this, one can conclude that a balanced intake of micronutrients is just as important as the balanced intake of macronutrients. Moreover, an unbalanced micronutrient intake by both parents can have long-lasting consequences for the health of offspring and future generations.
Acknowledgments
This review was partly supported by the Network of Excellence ECNIS2, EU-FP7-KBBE-2010-4266198. We would also like to thank Matt Randall for carefully reading and editing the manuscript.
Conflicts of interest
All authors have read and approved the manuscript and declare no conflicts of interest.
Abbreviations
- AhR
Aryl hydrocarbon receptor
- Avy
Agouti viable yellow
- B[a]P
Benzo[a]pyrene
- BPA
Bisphenol A
- Cat
Catalase
- DNMTs
DNA methyltransferases
- Gpx
Glutathione peroxidase
- Gstp1
Glutathione S-transferase pi 1
- IAP
Intracisternal A particles
- IL
Interleukin
- LINE1
Long interspersed nucleotide element
- Nqo1
NAD(P)H dehydrogenase, quinone 1
- PAHs
Polycyclic aromatic hydrocarbons
- ROS
Reactive oxygen species
- SAM
S-adenosyl-methionine
- SINEB1/2
Short interspersed nuclear element B1/2
- SOD
Superoxide dismutase
- Ugt1a6
UDP glucuronosyltransferase 1 family, polypeptide A6
References
- 1.Barker DJ. The intrauterine environment and adult cardiovascular disease. Ciba Found Symp. 1991;156:3–10. doi: 10.1002/9780470514047.ch2. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Osmond C, Law CM. The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J Epidemiol Community Health. 1989;43:237–240. doi: 10.1136/jech.43.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Jaddoe VW, Qi L, He Y, Lai J, et al. Exposure to the Chinese famine in early life and the risk of hypertension in adulthood. J Hypertens. 2011;29:1085–1092. doi: 10.1097/HJH.0b013e328345d969. [DOI] [PubMed] [Google Scholar]
- 4.McMillen IC, MacLaughlin SM, Muhlhausler BS, Gentili S, Duffield JL, et al. Developmental origins of adult health and disease: the role of periconceptional and foetal nutrition. Basic Clin Pharmacol Toxicol. 2008;102:82–89. doi: 10.1111/j.1742-7843.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 5.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 6.de Rooij SR, Painter RC, Roseboom TJ, Phillips DI, Osmond C, et al. Glucose tolerance at age 58 and the decline of glucose tolerance in comparison with age 50 in people prenatally exposed to the Dutch famine. Diabetologia. 2006;49:637–643. doi: 10.1007/s00125-005-0136-9. [DOI] [PubMed] [Google Scholar]
- 7.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 8.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84:595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 year in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 10.Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod. 1997;2:105–112. doi: 10.1530/ror.0.0020105. [DOI] [PubMed] [Google Scholar]
- 11.Dumortier O, Blondeau B, Duvillie B, Reusens B, Breant B, et al. Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia. 2007;50:2495–2503. doi: 10.1007/s00125-007-0811-0. [DOI] [PubMed] [Google Scholar]
- 12.Garofano A, Czernichow P, Breant B. In utero undernutrition impairs rat beta-cell development. Diabetologia. 1997;40:1231–1234. doi: 10.1007/s001250050812. [DOI] [PubMed] [Google Scholar]
- 13.Inoue T, Kido Y, Asahara S, Matsuda T, Shibutani Y, et al. Effect of intrauterine undernutrition during late gestation on pancreatic beta cell mass. Biomed Res. 2009;30:325–330. doi: 10.2220/biomedres.30.325. [DOI] [PubMed] [Google Scholar]
- 14.Prentice AM, Moore SE. Early programming of adult diseases in resource poor countries. Arch Dis Child. 2005;90:429–432. doi: 10.1136/adc.2004.059030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang C, Oest ME, Prater MR. Intrauterine exposure to high saturated fat diet elevates risk of adult-onset chronic diseases in C57BL/6 mice. Birth Defects Res B Dev Reprod Toxicol. 2009;86:377–384. doi: 10.1002/bdrb.20206. [DOI] [PubMed] [Google Scholar]
- 16.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, et al. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 17.Jablonka E, Lamb MJ. Precis of evolution in four dimensions. Behav Brain Sci. 2007;30:353–365. doi: 10.1017/S0140525X07002221. [DOI] [PubMed] [Google Scholar]
- 18.Holliday R. Epigenetics: a historical overview. Epigenetics. 2006;1:76–80. doi: 10.4161/epi.1.2.2762. [DOI] [PubMed] [Google Scholar]
- 19.Jablonka E, Lamb MJ. The changing concept of epigenetics. Ann N Y Acad Sci. 2002;981:82–96. doi: 10.1111/j.1749-6632.2002.tb04913.x. [DOI] [PubMed] [Google Scholar]
- 20.Maccani MA, Marsit CJ. Epigenetics in the placenta. Am J Reprod Immunol. 2009;62:78–89. doi: 10.1111/j.1600-0897.2009.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelissen EC, van Montfoort AP, Dumoulin JC, Evers JL. Epigenetics and the placenta. Hum Reprod Update. 2011;17:397–417. doi: 10.1093/humupd/dmq052. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson SP, Keith WN. Epigenetic control of cellular senescence in disease: opportunities for therapeutic intervention. Expert Rev Mol Med. 2007;9:1–26. doi: 10.1017/S1462399407000269. [DOI] [PubMed] [Google Scholar]
- 23.Szyf M. The early life environment and the epigenome. Biochim Biophys Acta. 2009;1790:878–885. doi: 10.1016/j.bbagen.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Deal RB, Henikoff JG, Henikoff S. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science. 2010;328:1161–1164. doi: 10.1126/science.1186777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dion MF, Kaplan T, Kim M, Buratowski S, Friedman N, et al. Dynamics of replication-independent histone turnover in budding yeast. Science. 2007;315:1405–1408. doi: 10.1126/science.1134053. [DOI] [PubMed] [Google Scholar]
- 26.Bernal AJ, Jirtle RL. Epigenomic disruption: the effects of early developmental exposures. Birth Defects Res A Clin Mol Teratol. 2010;88:938–944. doi: 10.1002/bdra.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 28.Verona RI, Mann MR, Bartolomei MS. Genomic imprinting: intricacies of epigenetic regulation in clusters. Annu Rev Cell Dev Biol. 2003;19:237–259. doi: 10.1146/annurev.cellbio.19.111401.092717. [DOI] [PubMed] [Google Scholar]
- 29.Palacios R, Gazave E, Goni J, Piedrafita G, Fernando O, et al. Allele-specific gene expression is widespread across the genome and biological processes. PLoS One. 2009;4:e4150. doi: 10.1371/journal.pone.0004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szyf M. The dynamic epigenome and its implications in toxicology. Toxicol Sci. 2007;100:7–23. doi: 10.1093/toxsci/kfm177. [DOI] [PubMed] [Google Scholar]
- 31.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(Suppl 3):451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon L, Joo JH, Andronikos R, Ollikainen M, Wallace EM, et al. Expression discordance of monozygotic twins at birth: effect of intrauterine environment and a possible mechanism for fetal programming. Epigenetics. 2011;6:579–592. doi: 10.4161/epi.6.5.15072. [DOI] [PubMed] [Google Scholar]
- 34.Persson LA, Arifeen S, Ekstrom EC, Rasmussen KM, Frongillo EA, et al. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh: the MINIMat randomized trial. JAMA, J Am Med Assoc. 2012;307:2050–2059. doi: 10.1001/jama.2012.4061. [DOI] [PubMed] [Google Scholar]
- 35.Carlsen K, Pedersen L, Bonnelykke K, Stark KD, Lauritzen L, et al. Association between whole-blood polyunsaturated fatty acids in pregnant women and early fetal weight. Eur J Clin Nutr. 2013 doi: 10.1038/ejcn.2013.108. [DOI] [PubMed] [Google Scholar]
- 36.George LA, Zhang L, Tuersunjiang N, Ma Y, Long NM, et al. Early maternal undernutrition programs increased feed intake, altered glucose metabolism and insulin secretion, and liver function, in aged female offspring. Am J Physiol Regul Integr Comp Physiol. 2012;302(7):R795–R804. doi: 10.1152/ajpregu.00241.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rexhaj E, Bloch J, Jayet PY, Rimoldi SF, Dessen P, et al. Fetal programming of pulmonary vascular dysfunction in mice: role of epigenetic mechanisms. Am J Physiol Heart Circ Physiol. 2011;301:H247–H252. doi: 10.1152/ajpheart.01309.2010. [DOI] [PubMed] [Google Scholar]
- 38.Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update. 2010;16:65–79. doi: 10.1093/humupd/dmp027. [DOI] [PubMed] [Google Scholar]
- 39.Oakes CC, Smiraglia DJ, Plass C, Trasler JM, Robaire B. Aging results in hypermethylation of ribosomal DNA in sperm and liver of male rats. Proc Natl Acad Sci USA. 2003;100:1775–1780. doi: 10.1073/pnas.0437971100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bielawski DM, Zaher FM, Svinarich DM, Abel EL. Paternal alcohol exposure affects sperm cytosine methyltransferase messenger RNA levels. Alcohol Clin Exp Res. 2002;26:347–351. [PubMed] [Google Scholar]
- 41.Ouko LA, Shantikumar K, Knezovich J, Haycock P, Schnugh DJ, et al. Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes: implications for fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1615–1627. doi: 10.1111/j.1530-0277.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 42.Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rassoulzadegan M, Grandjean V, Gounon P, Vincent S, Gillot I, et al. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–474. doi: 10.1038/nature04674. [DOI] [PubMed] [Google Scholar]
- 44.Linschooten JO, Van Schooten FJ, Baumgartner A, Cemeli E, Van Delft J, et al. Use of spermatozoal mRNA profiles to study gene-environment interactions in human germ cells. Mutat Res. 2009;667:70–76. doi: 10.1016/j.mrfmmm.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Kaati G, Bygren LO, Pembrey M, Sjostrom M. Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet:EJHG. 2007;15:784–790. doi: 10.1038/sj.ejhg.5201832. [DOI] [PubMed] [Google Scholar]
- 46.Dunn GA, Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology. 2011;152:2228–2236. doi: 10.1210/en.2010-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am. 2011;95:875–892. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 48.de Assis S, Warri A, Cruz MI, Laja O, Tian Y, et al. High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring. Nat Commun. 2012;3:1053. doi: 10.1038/ncomms2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond) 2008;32:1373–1379. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waterland RA, Travisano M, Tahiliani KG. Diet-induced hypermethylation at agouti viable yellow is not inherited transgenerationally through the female. FASEB J. 2007;21:3380–3385. doi: 10.1096/fj.07-8229com. [DOI] [PubMed] [Google Scholar]
- 52.Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, et al. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Twin Res. 2001;4:293–298. doi: 10.1375/1369052012605. [DOI] [PubMed] [Google Scholar]
- 53.Christensen BC, Marsit CJ. Epigenomics in environmental health. Front Genet. 2011;2:84. doi: 10.3389/fgene.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKay JA, Groom A, Potter C, Coneyworth LJ, Ford D, et al. Genetic and non-genetic influences during pregnancy on infant global and site specific DNA methylation: role for folate gene variants and vitamin B12. PLoS One. 2012;7:e33290. doi: 10.1371/journal.pone.0033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38. doi: 10.1007/s00125-007-0793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA. 2007;104:19351–19356. doi: 10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKay JA, Williams EA, Mathers JC. Effect of maternal and post-weaning folate supply on gene-specific DNA methylation in the small intestine of weaning and adult apc and wild type mice. Front Genet. 2011;2:23. doi: 10.3389/fgene.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKay JA, Waltham KJ, Williams EA, Mathers JC. Folate depletion during pregnancy and lactation reduces genomic DNA methylation in murine adult offspring. Genes Nutr. 2011;6:189–196. doi: 10.1007/s12263-010-0199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.(1991) Prevention of neural tube defects: results of the medical research council vitamin study MRC vitamin study research group. Lancet 338:131–137 [PubMed]
- 60.Botto LD, Lisi A, Robert-Gnansia E, Erickson JD, Vollset SE, et al. International retrospective cohort study of neural tube defects in relation to folic acid recommendations: are the recommendations working? BMJ. 2005;330:571. doi: 10.1136/bmj.38336.664352.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shelke N, Keith L. Folic acid supplementation for women of childbearing age versus supplementation for the general population: a review of the known advantages and risks. Int J Family Med. 2011;2011:173705. doi: 10.1155/2011/173705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Persad VL, Van den Hof MC, Dube JM, Zimmer P. Incidence of open neural tube defects in Nova Scotia after folic acid fortification. CMAJ. 2002;167:241–245. [PMC free article] [PubMed] [Google Scholar]
- 63.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981–2986. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- 64.Chatzi L, Papadopoulou E, Koutra K, Roumeliotaki T, Georgiou V, et al. Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age: the mother-child cohort ‘Rhea’ study in Crete, Greece. Public Health Nutr. 2012;15(9):1728–1736. doi: 10.1017/S1368980012000067. [DOI] [PubMed] [Google Scholar]
- 65.Sharland E, Montgomery B, Granell R. Folic acid in pregnancy—is there a link with childhood asthma or wheeze? Aust Fam Physician. 2011;40:421–424. [PubMed] [Google Scholar]
- 66.Bekkers MB, Elstgeest LE, Scholtens S, Haveman A, de Jongste JC, et al. Maternal use of folic acid supplements during pregnancy and childhood respiratory health and atopy: the PIAMA birth cohort study. Eur Respir J. 2011;39(6):1468–1474. doi: 10.1183/09031936.00094511. [DOI] [PubMed] [Google Scholar]
- 67.Magdelijns FJ, Mommers M, Penders J, Smits L, Thijs C. Folic acid use in pregnancy and the development of atopy, asthma, and lung function in childhood. Pediatrics. 2011;128:e135–e144. doi: 10.1542/peds.2010-1690. [DOI] [PubMed] [Google Scholar]
- 68.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Investig. 2008;118:3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Kiefte-de Jong JC, Timmermans S, Jaddoe VW, Hofman A, Tiemeier H, et al. High circulating folate and vitamin B-12 concentrations in women during pregnancy are associated with increased prevalence of atopic dermatitis in their offspring. J Nutr. 2012;142:731–738. doi: 10.3945/jn.111.154948. [DOI] [PubMed] [Google Scholar]
- 70.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, et al. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One. 2009;4:e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ly A, Lee H, Chen J, Sie KK, Renlund R, et al. Effect of maternal and postweaning folic acid supplementation on mammary tumor risk in the offspring. Cancer Res. 2011;71:988–997. doi: 10.1158/0008-5472.CAN-10-2379. [DOI] [PubMed] [Google Scholar]
- 72.Sie KK, Medline A, van Weel J, Sohn KJ, Choi SW, et al. Effect of maternal and postweaning folic acid supplementation on colorectal cancer risk in the offspring. Gut. 2011;60:1687–1694. doi: 10.1136/gut.2011.238782. [DOI] [PubMed] [Google Scholar]
- 73.Schaible TD, Harris RA, Dowd SE, Smith CW, Kellermayer R. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Hum Mol Genet. 2011;20:1687–1696. doi: 10.1093/hmg/ddr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Padmavathi IJ, Rao KR, Venu L, Ganeshan M, Kumar KA, et al. Chronic maternal dietary chromium restriction modulates visceral adiposity: probable underlying mechanisms. Diabetes. 2010;59:98–104. doi: 10.2337/db09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Venu L, Padmavathi IJ, Kishore YD, Bhanu NV, Rao KR, et al. Long-term effects of maternal magnesium restriction on adiposity and insulin resistance in rat pups. Obesity (Silver Spring) 2008;16:1270–1276. doi: 10.1038/oby.2008.72. [DOI] [PubMed] [Google Scholar]
- 76.Venu L, Harishankar N, Krishna TP, Raghunath M. Does maternal dietary mineral restriction per se predispose the offspring to insulin resistance? Eur J Endocrinol. 2004;151:287–294. doi: 10.1530/eje.0.1510287. [DOI] [PubMed] [Google Scholar]
- 77.Jou MY, Philipps AF, Lonnerdal B. Maternal zinc deficiency in rats affects growth and glucose metabolism in the offspring by inducing insulin resistance postnatally. J Nutr. 2010;140:1621–1627. doi: 10.3945/jn.109.119677. [DOI] [PubMed] [Google Scholar]
- 78.Jou MY, Lonnerdal B, Philipps AF. Maternal zinc restriction affects postnatal growth and glucose homeostasis in rat offspring differently depending upon adequacy of their nutrient intake. Pediatr Res. 2012;71:228–234. doi: 10.1038/pr.2011.44. [DOI] [PubMed] [Google Scholar]
- 79.Chaudhuri A. Why we should offer routine vitamin D supplementation in pregnancy and childhood to prevent multiple sclerosis. Med Hypotheses. 2005;64:608–618. doi: 10.1016/j.mehy.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 80.Hanwell HE, Banwell B. Assessment of evidence for a protective role of vitamin D in multiple sclerosis. Biochim Biophys Acta. 2011;1812:202–212. doi: 10.1016/j.bbadis.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 81.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 82.Szeto IM, Aziz A, Das PJ, Taha AY, Okubo N, et al. High multivitamin intake by Wistar rats during pregnancy results in increased food intake and components of the metabolic syndrome in male offspring. Am J Physiol Regul Integr Comp Physiol. 2008;295:R575–R582. doi: 10.1152/ajpregu.90354.2008. [DOI] [PubMed] [Google Scholar]
- 83.Jones AP, Palmer D, Zhang G, Prescott SL. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics. 2012;130:e1128–e1135. doi: 10.1542/peds.2012-1172. [DOI] [PubMed] [Google Scholar]
- 84.Mistry HD, Williams PJ. The importance of antioxidant micronutrients in pregnancy. Oxid Med Cell Longev. 2011;2011:841749. doi: 10.1155/2011/841749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Franco Mdo C, Ponzio BF, Gomes GN, Gil FZ, Tostes R, et al. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009;85:327–333. doi: 10.1016/j.lfs.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 86.Langie SA, Kowalczyk P, Tudek B, Zabielski R, Dziaman T, et al. The effect of oxidative stress on nucleotide-excision repair in colon tissue of newborn piglets. Mutat Res. 2010;695:75–80. doi: 10.1016/j.mrgentox.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 88.Erdman JW, Jr, Balentine D, Arab L, Beecher G, Dwyer JT, et al. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31–June 1, 2005, Washington, DC. J Nutr. 2007;137:718S–737S. doi: 10.1093/jn/137.3.718S. [DOI] [PubMed] [Google Scholar]
- 89.Aherne SA, O’Brien NM. Dietary flavonols: chemistry, food content, and metabolism. Nutrition. 2002;18:75–81. doi: 10.1016/s0899-9007(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 90.Egert S, Rimbach G. Which sources of flavonoids: complex diets or dietary supplements? Adv Nutr. 2011;2:8–14. doi: 10.3945/an.110.000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 92.Verkasalo PK, Appleby PN, Allen NE, Davey G, Adlercreutz H, et al. Soya intake and plasma concentrations of daidzein and genistein: validity of dietary assessment among eighty British women (Oxford arm of the European prospective investigation into cancer and nutrition) Br J Nutr. 2001;86:415–421. doi: 10.1079/bjn2001424. [DOI] [PubMed] [Google Scholar]
- 93.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998;68:1453S–1461S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- 94.Boots AW, Drent M, de Boer VC, Bast A, Haenen GR. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr. 2011;30:506–512. doi: 10.1016/j.clnu.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 95.Valsecchi AE, Franchi S, Panerai AE, Rossi A, Sacerdote P, et al. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol. 2011;650:694–702. doi: 10.1016/j.ejphar.2010.10.060. [DOI] [PubMed] [Google Scholar]
- 96.Loke WM, Proudfoot JM, Hodgson JM, McKinley AJ, Hime N, et al. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2010;30:749–757. doi: 10.1161/ATVBAHA.109.199687. [DOI] [PubMed] [Google Scholar]
- 97.Mahn K, Borras C, Knock GA, Taylor P, Khan IY, et al. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005;19:1755–1757. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- 98.Heo HJ, Lee CY. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J Agric Food Chem. 2004;52:7514–7517. doi: 10.1021/jf049243r. [DOI] [PubMed] [Google Scholar]
- 99.Mercer LD, Kelly BL, Horne MK, Beart PM. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: investigations in primary rat mesencephalic cultures. Biochem Pharmacol. 2005;69:339–345. doi: 10.1016/j.bcp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 100.Ganesan S, Faris AN, Comstock AT, Chattoraj SS, Chattoraj A, et al. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir Res. 2010;11:131. doi: 10.1186/1465-9921-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirayama F, Lee AH, Binns CW, Hiramatsu N, Mori M, et al. Dietary intake of isoflavones and polyunsaturated fatty acids associated with lung function, breathlessness and the prevalence of chronic obstructive pulmonary disease: possible protective effect of traditional Japanese diet. Mol Nutr Food Res. 2010;54:909–917. doi: 10.1002/mnfr.200900316. [DOI] [PubMed] [Google Scholar]
- 102.Cornish KM, Williamson G, Sanderson J. Quercetin metabolism in the lens: role in inhibition of hydrogen peroxide induced cataract. Free Radic Biol Med. 2002;33:63–70. doi: 10.1016/s0891-5849(02)00843-2. [DOI] [PubMed] [Google Scholar]
- 103.Miyamoto N, Izumi H, Miyamoto R, Kondo H, Tawara A, et al. Quercetin induces the expression of peroxiredoxins 3 and 5 via the Nrf2/NRF1 transcription pathway. Invest Ophthalmol Vis Sci. 2011;52:1055–1063. doi: 10.1167/iovs.10-5777. [DOI] [PubMed] [Google Scholar]
- 104.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 105.Hollman PC, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–942. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 106.Yao LH, Jiang YM, Shi J, Tomas-Barberan FA, Datta N, et al. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004;59:113–122. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- 107.Skibola CF, Smith MT. Potential health impacts of excessive flavonoid intake. Free Radic Biol Med. 2000;29:375–383. doi: 10.1016/s0891-5849(00)00304-x. [DOI] [PubMed] [Google Scholar]
- 108.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, et al. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 109.Egert S, Wolffram S, Bosy-Westphal A, Boesch-Saadatmandi C, Wagner AE, et al. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J Nutr. 2008;138:1615–1621. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- 110.Coward L, Barnes N, Setchell K, Barnes S. Genistein, daidzein, and their.beta.-glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J Agric Food Chem. 1993;41:1961–1967. [Google Scholar]
- 111.Espin JC, Garcia-Conesa MT, Tomas-Barberan FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 112.Bonacasa B, Siow RC, Mann GE. Impact of dietary soy isoflavones in pregnancy on fetal programming of endothelial function in offspring. Microcirculation. 2011;18:270–285. doi: 10.1111/j.1549-8719.2011.00088.x. [DOI] [PubMed] [Google Scholar]
- 113.Schroder-van der Elst JP, van der Heide D, Rokos H, Morreale de Escobar G, Kohrle J. Synthetic flavonoids cross the placenta in the rat and are found in fetal brain. Am J Physiol. 1998;274:E253–E256. doi: 10.1152/ajpendo.1998.274.2.E253. [DOI] [PubMed] [Google Scholar]
- 114.Vanhees K, Coort S, Ruijters EJ, Godschalk RW, van Schooten FJ, et al. Epigenetics: prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J. 2011;25:797–807. doi: 10.1096/fj.10-172155. [DOI] [PubMed] [Google Scholar]
- 115.Miodini P, Fioravanti L, Di Fronzo G, Cappelletti V. The two phyto-oestrogens genistein and quercetin exert different effects on oestrogen receptor function. Br J Cancer. 1999;80:1150–1155. doi: 10.1038/sj.bjc.6690479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol. 2012;354(1–2):74–84. doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakajima Y, Goldblum RM, Midoro-Horiuti T. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: an animal model study. Environ Health. 2012;11:8. doi: 10.1186/1476-069X-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer. 2010;1:146–155. doi: 10.1007/s12672-010-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weber Lozada K, Keri RA. Bisphenol A increases mammary cancer risk in two distinct mouse models of breast cancer. Biol Reprod. 2011;85:490–497. doi: 10.1095/biolreprod.110.090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118:1243–1250. doi: 10.1289/ehp.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bolanos JP, Moro MA, Lizasoain I, Almeida A. Mitochondria and reactive oxygen and nitrogen species in neurological disorders and stroke: therapeutic implications. Adv Drug Deliv Rev. 2009;61:1299–1315. doi: 10.1016/j.addr.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 122.Hensley K, Butterfield DA, Hall N, Cole P, Subramaniam R, et al. Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer’s disease-associated amyloid beta peptide. Ann N Y Acad Sci. 1996;786:120–134. doi: 10.1111/j.1749-6632.1996.tb39057.x. [DOI] [PubMed] [Google Scholar]
- 123.Multhaup G, Ruppert T, Schlicksupp A, Hesse L, Beher D, et al. Reactive oxygen species and Alzheimer’s disease. Biochem Pharmacol. 1997;54:533–539. doi: 10.1016/s0006-2952(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 124.Tabner BJ, Turnbull S, El-Agnaf O, Allsop D. Production of reactive oxygen species from aggregating proteins implicated in Alzheimer’s disease, Parkinson’s disease and other neurodegenerative diseases. Curr Top Med Chem. 2001;1:507–517. doi: 10.2174/1568026013394822. [DOI] [PubMed] [Google Scholar]
- 125.Tieu K, Ischiropoulos H, Przedborski S. Nitric oxide and reactive oxygen species in Parkinson’s disease. IUBMB Life. 2003;55:329–335. doi: 10.1080/1521654032000114320. [DOI] [PubMed] [Google Scholar]
- 126.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells: implications in cardiovascular disease. Braz J Med Biol Res. 2004;37:1263–1273. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- 127.Yoshizumi M, Tsuchiya K, Tamaki T. Signal transduction of reactive oxygen species and mitogen-activated protein kinases in cardiovascular disease. J Med Invest. 2001;48:11–24. [PubMed] [Google Scholar]
- 128.Muhammad S, Bierhaus A, Schwaninger M. Reactive oxygen species in diabetes-induced vascular damage, stroke, and Alzheimer’s disease. J Alzheimers Dis. 2009;16:775–785. doi: 10.3233/JAD-2009-0982. [DOI] [PubMed] [Google Scholar]
- 129.Lau AT, Wang Y, Chiu JF. Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J Cell Biochem. 2008;104:657–667. doi: 10.1002/jcb.21655. [DOI] [PubMed] [Google Scholar]
- 130.Renschler MF. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004;40:1934–1940. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 131.Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dugan LL, Quick KL. Reactive oxygen species and aging: evolving questions. Sci Aging Knowledge Environ. 2005;2005:pe20. doi: 10.1126/sageke.2005.26.pe20. [DOI] [PubMed] [Google Scholar]
- 133.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vanhees K, van Schooten FJ, Doorn-Khosrovani SB, van Helden S, Munnia A, et al. Intrauterine exposure to flavonoids modifies antioxidant status at adulthood and decreases oxidative stress induced DNA damage. Free Radic Biol Med. 2013;57:154–161. doi: 10.1016/j.freeradbiomed.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 135.Schnorr CE, Morrone Mda S, Weber MH, Lorenzi R, Behr GA, et al. The effects of vitamin A supplementation to rats during gestation and lactation upon redox parameters: increased oxidative stress and redox modulation in mothers and their offspring. Food Chem Toxicol. 2011;49:2645–2654. doi: 10.1016/j.fct.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 136.Pasquali MA, Schnorr CE, Feistauer LB, Gelain DP, Moreira JC. Vitamin A supplementation to pregnant and breastfeeding female rats induces oxidative stress in the neonatal lung. Reprod Toxicol. 2010;30:452–456. doi: 10.1016/j.reprotox.2010.05.085. [DOI] [PubMed] [Google Scholar]
- 137.Chen CS, Wells PG. Enhanced tumorigenesis in p53 knockout mice exposed in utero to high-dose vitamin E. Carcinogenesis. 2006;27:1358–1368. doi: 10.1093/carcin/bgi325. [DOI] [PubMed] [Google Scholar]
- 138.Ferrali M, Signorini C, Caciotti B, Sugherini L, Ciccoli L, et al. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997;416:123–129. doi: 10.1016/s0014-5793(97)01182-4. [DOI] [PubMed] [Google Scholar]
- 139.Kitagawa S, Sakamoto H, Tano H. Inhibitory effects of flavonoids on free radical-induced hemolysis and their oxidative effects on hemoglobin. Chem Pharm Bull (Tokyo) 2004;52:999–1001. doi: 10.1248/cpb.52.999. [DOI] [PubMed] [Google Scholar]
- 140.Vanhees K, Godschalk RW, Sanders A, van Waalwijk van Doorn-Khosrovani SB, van Schooten FJ. Maternal quercetin intake during pregnancy results in an adapted iron homeostasis at adulthood. Toxicology. 2011;290:350–358. doi: 10.1016/j.tox.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 141.Anetor JI, Ajose OA, Adeleke FN, Olaniyan-Taylor GO, Fasola FA. Depressed antioxidant status in pregnant women on iron supplements: pathologic and clinical correlates. Biol Trace Elem Res. 2010;136:157–170. doi: 10.1007/s12011-009-8534-3. [DOI] [PubMed] [Google Scholar]
- 142.Lachili B, Hininger I, Faure H, Arnaud J, Richard MJ, et al. Increased lipid peroxidation in pregnant women after iron and vitamin C supplementation. Biol Trace Elem Res. 2001;83:103–110. doi: 10.1385/BTER:83:2:103. [DOI] [PubMed] [Google Scholar]
- 143.Qiu C, Zhang C, Gelaye B, Enquobahrie DA, Frederick IO, et al. Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron intake. Diabetes Care. 2011;34:1564–1569. doi: 10.2337/dc11-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khan TH, Jahangir T, Prasad L, Sultana S. Inhibitory effect of apigenin on benzo(a)pyrene-mediated genotoxicity in Swiss albino mice. J Pharm Pharmacol. 2006;58:1655–1660. doi: 10.1211/jpp.58.12.0013. [DOI] [PubMed] [Google Scholar]
- 145.Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J. 1999;340(Pt 3):715–722. [PMC free article] [PubMed] [Google Scholar]
- 146.Gelboin HV. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980;60:1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- 147.Hanna IH, Dawling S, Roodi N, Guengerich FP, Parl FF. Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res. 2000;60:3440–3444. [PubMed] [Google Scholar]
- 148.Moors S, Diel P, Degen GH. Toxicokinetics of bisphenol A in pregnant DA/Han rats after single i.v. application. Arch Toxicol. 2006;80:647–655. doi: 10.1007/s00204-006-0097-x. [DOI] [PubMed] [Google Scholar]
- 149.De Waard WJ, Aarts JM, Peijnenburg AC, De Kok TM, Van Schooten FJ, et al. Ah receptor agonist activity in frequently consumed food items. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25:779–787. doi: 10.1080/02652030701798880. [DOI] [PubMed] [Google Scholar]
- 150.Vanhees K, van Schooten FJ, Moonen EJ, Maas LM, van Waalwijk van Doorn-Khosrovani SB, et al. Maternal intake of quercetin during gestation alters ex vivo benzo[a]pyrene metabolism and DNA adduct formation in adult offspring. Mutagenesis. 2012;27:445–451. doi: 10.1093/mutage/ges002. [DOI] [PubMed] [Google Scholar]
- 151.Makaji E, Ho SH, Holloway AC, Crankshaw DJ. Effects in rats of maternal exposure to raspberry leaf and its constituents on the activity of cytochrome p450 enzymes in the offspring. Int J Toxicol. 2011;30:216–224. doi: 10.1177/1091581810388307. [DOI] [PubMed] [Google Scholar]
- 152.Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 153.Christian P, Stewart CP. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr. 2010;140:437–445. doi: 10.3945/jn.109.116327. [DOI] [PubMed] [Google Scholar]
- 154.Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012;2012:941868. doi: 10.1155/2012/941868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 156.Soni MG, Thurmond TS, Miller ER, 3rd, Spriggs T, Bendich A, et al. Safety of vitamins and minerals: controversies and perspective. Toxicol Sci. 2010;118:348–355. doi: 10.1093/toxsci/kfq293. [DOI] [PubMed] [Google Scholar]
- 157.Sullivan KM, Ford ES, Azrak MF, Mokdad AH. Multivitamin use in pregnant and nonpregnant women: results from the behavioral risk factor surveillance system. Public Health Rep. 2009;124:384–390. doi: 10.1177/003335490912400307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Picciano MF, McGuire MK. Use of dietary supplements by pregnant and lactating women in North America. Am J Clin Nutr. 2009;89:663S–667S. doi: 10.3945/ajcn.2008.26811B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Crider KS, Bailey LB, Berry RJ. Folic Acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011;3:370–384. doi: 10.3390/nu3030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Holzgreve W, Pietrzik K, Koletzko B, Eckmann-Scholz C. Adding folate to the contraceptive pill: A new concept for the prevention of neural tube defects. J Matern Fetal Neonatal Med. 2012;25(9):1529–1536. doi: 10.3109/14767058.2011.648672. [DOI] [PubMed] [Google Scholar]
- 161.Yang Z, Huffman SL. Review of fortified food and beverage products for pregnant and lactating women and their impact on nutritional status. Matern Child Nutr. 2011;7(Suppl 3):19–43. doi: 10.1111/j.1740-8709.2011.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]