Abstract
We recently generated an advanced mouse model of Alzheimer’s disease (AD) by targeted knock-in of single-copy mutated human amyloid precursor-protein (APP) and tau genes, crossed with a non-symptomatic presenilin (PS1A246E) over-expressing mouse line. These PLB1Triple mice presented with age-dependent and AD-relevant phenotypes. Homozygous PLB1Triple mice aged 4–12 months were assessed here in a battery of spatial learning tasks: Exp.1 radial-arm water maze (spatial reference and working memory) Exp.2 open-field water maze (spatial reference memory); Exp.3 home cage observation system with spatial learning (IntelliCage); Exp.4 spontaneous object recognition (SOR; novel object and spatial object shift). A separate test with high-expression transgenic APP mice matching the design of experiment 1 was also performed. Spatial deficits in PLB1Triple mice were confirmed at 12, but not 4 months in both water maze tasks. PSAPP mice, by contrast, presented with severe yet non-progressive spatial learning deficits already at 4 months. During tests of spatial learning in SOR and IntelliCage, PLB1Triple mice neither acquired the location of the water-rewarded corner, nor recognize novel or spatially shifted objects at 4 months, indicating these protocols to be more sensitive than the water maze. Collectively and in line with AD symptomatology, PLB1Triple mice present with a graded and progressive age-dependent loss of spatial memory that can be revealed by the use of a battery of tasks. With the emergence of subtle deficits progressively increasing in severity, PLB1Triple mice may offer a more patho-physiologically relevant model of dementia than aggressive expression models.
Keywords: Knock-in mouse, Amyloid, Tau, Spatial cognition, Learning, Memory
Introduction
Alzheimer’s disease (AD) is the most common age-related neurodegenerative disease and the primary cause of dementia. With the number of sufferers and the associated healthcare burden continuously rising, effective treatment strategies are urgently required. The identification of specific mutations in familial forms of AD has led to a greater understanding of the disease process, and owing to the development of transgene technology, numerous experimental models expressing human mutant amyloid-precursor protein (APP), presenilin 1 or 2 (PS-1 or 2), and tau protein have met with some degrees of success in mimicking hallmarks of the disease (for review, see [1–3]). Single transgenic mouse models aimed at replicating (i) beta-amyloid containing plaques through over-expression of APP containing the Swedish (KM670/671NL: [4, 5]) or London mutation (V642I; [6]), and (ii) hyperphosphorylated tau aggregated into neurofibrillary tangles through over-expression of either human short-length tau [7], full-length tau replacing Pro301 by Leu (P301L: termed JNPL3—[8]; or pR5—[9]) or by Ser (P301S: [10, 11]), or at R406W mutation [12, 13]. Such models recapitulate elements of AD histopathology, often dependent on the regulatory element and background strain used for their generation, and frequently preceded by physiological and behavioral deficits.
Poly-transgenic models have widely reported the enhancement of amyloid pathology in both APPSWE or APPLON and PS1 co-expressing mice [14, 15]. Interestingly, amyloid pathology is not altered in APPSWE and TauP301L bigenic strains, but tau pathology is amplified [16, 17]. Recently, a triple transgenic model (3xTgAD) harboring APPSWE, TauP301L, and PS1M146V mutations has resulted in a more realistic modeling of AD-like pathology [18–20] and its corresponding cognitive decline [21, 22]. This includes deficits in spatial learning paradigms [1, 23] as a critical test that reflects some aspects of episodic-like and semantic-like memory in rodents [24].
These models, however, possess a number of drawbacks. For example, insertion of transgenes by pronuclear injection (as seen in Tg2576 and 3xTgAD) offers no control over the number of transgenes inserted into the mouse genome, nor is there any control over the location of the insertion site(s) of the transgene(s). In general, these models tend to rely upon a gross over-expression of the aberrant genes, thus often demonstrating marked AD-like pathologies as young as 3 months of age [18, 19, 25]. Crossing different lines to generate poly-transgenic mice is further complicated by different background strains [20, 26] with unpredictable behavioral consequences. Though existing models are valuable scientific tools, early and excessive expression of AD-like pathology draws into question whether this is ideal for modeling a disease of old-age.
In addressing some of these issues, we here characterize the novel PLB1Triple mouse model [27, 28] of AD, which carries a single copy of human mutated APPSWE/LON and tauP301L/R406W genes, inserted into a specific locus (Hprt) in the mouse genome [29] and was crossed with a mutant PS1 mouse line [30]. These mice show a slow but progressive AD-like pathology leading to translational phenotypes such as cortical hypometabolism, abnormal activity cycles, and sleep fragmentation. With its seemingly more physiological expression level and a known insertion site, we set out to determine whether cognitive phenotypes in PLB1Triple mice resemble more closely the progression of AD compared with high-amyloid expression models. Based on a companion study, [31], which describes a progressive increase of AD-like histo-pathology coupled with deficits in synaptic plasticity, we here present an in-depth behavioral analysis of age-related cognitive deficits in PLB1Triple mice using a battery of spatial learning and memory tests, these including different versions of the water maze, novel and spatial object recognition paradigms, as well as an automated home cage observation system with spatial conditioning training (IntelliCage).
This novel strain was in some experiments compared with a high-expression double-transgenic AD mouse model termed PSAPP, generated from Tg2576 mice, which co-expresses mutant PS1 (M146L) and mutant APPSWE (K670D/M671L) [26]. PSAPP mice have been well characterized in terms of amyloid deposition and behavioral deficits [26, 32–34] and present with accelerated amyloid deposits in cortex and hippocampus relative to single parental strains, detectable at about 4 months of age. Subtle cognitive deficits may precede this pathology, but are non-progressive despite increased severity of amyloid deposition at 9 months of age [26, 32]. By contrast, spatial deficits in both open field and radial arm water maze seem to appear after 12 months of age [35].
Materials and methods
Transgenic animals
For all animals, a 12-h day–night cycle was maintained (lights on at 7:00 am) with ad libitum access to standard food and water in a controlled environment (20–21 °C, 60–65 % relative humidity). All animals were tested at 4 ± 1, 8 ± 1, and 12 ± 1 months of age during the light phase of the cycle and in keeping with the Federation of European Laboratory Animal Science Associations (FELASA) guidelines as well as following Home Office regulations as described in the Animals (Scientific Procedures) Act 1986.
PLB1: as described previously [27] transgenic mice were generated using targeted knock-in into the HPRT targeting vector of a human APP–Tau cDNA construct (hAPP: containing Swedish and London mutations; hTau: with P301L and R406 W mutations) under control of the forebrain and neurone-specific CaMKIIα promoter. Genotypes were confirmed using PCR with primer combinations detecting either wild-type or genetically altered Hprt alleles. Offspring were crossed with a presenilin transgenic mouse (PS1A246E, Jackson Laboratories) to generate triple transgenic mice. PLB1Triple mice and their non-transgenic wild-type controls (PLB1WT) were housed in groups of 5–10. In this study, a mixture of male and female PLB1Triple mice were used. Due to the fact that the APP and tau transgenes are inserted into a locus on the X chromosome, male PLB1Triple mice were hemizygous for the transgene and female mice were homozygous (for further details, see [27]).
PSAPP: double-transgenic mice were generated by crossing a heterozygous mutant presenilin-1 line (PS1M146L) [36] with heterozygous Tg2576 mice [5] to create PSAPP offspring alongside non-transgenic wild-type littermates (generously provided by Wyeth Pharmaceuticals). Animals were delivered to the animal facility and tested blind with respect to phenotype after at least 2 weeks of recovery. Mice carrying retina degeneration (RD) mutations were identified by genotyping and excluded. All animals were tested longitudinally (i.e., the same animals were tested at both 4 ± 1, 8 ± 1, and 12 ± 1 months of age) with only male mice being used. Subjects were required to be singly housed due to high levels of aggression and fighting between mice.
Behavioral tests
Radial arm water maze (RAWM): we tested both transgenic AD models in this paradigm longitudinally at 4, 8, and 12 months, with n = 14 for PLB1WT and n = 25 for PLB1Triple (mixed gender); for comparison and to determine the sensitivity of the behavioral test in a severe amyloid over-expression model, male PSAPP n = 17 and their corresponding wild types (n = 18) were also studied.
By incorporating elements of the open field water maze and the radial arm maze, the radial arm water maze allows the evaluation of not only escape latency/path length but also the simultaneous assessment of both reference and working memory errors [37]. A 150-cm white Perspex pool (50 cm deep) was used, in which dividers were introduced to leave a small central region (diameter: 380 mm) and eight radiating arms projecting symmetrically to the pool walls (length of arms: 560 mm). It was filled with water to a depth of 35 cm at a temperature of 21 ± 2 °C. A number of salient distal extra-maze cues surrounded the pool and colorless plastic hydraulic platforms (Hydraulic Atlantis Platforms, Ugo Basile, Comero, Italy) submerged to the bottom of the pool were located in the center of each quadrant. Mice were required to find a submerged platform (as above) located at the end of one of the arms (NE, SE, SW, and NW). This was repeated for a total of three platform positions (‘three problems’), with the completion of a problem based on a ‘trials-to-criterion’ assessment (criterion: two consecutive trials with no more than two errors, or three trials with less than or equal to three errors. If an animal had zero and two errors in two consecutive trials, a third trial was required to confirm the criterion). An error was counted when either an animal entered or re-entered a wrong arm (all four limbs entering), or made no decision over a period of 15 s. Animals were given a maximum of eight trials per day and allowed 24 trials to complete each problem, following which the animal would be progressed to the next problem (N.B., animals were required to perform at least five trials for each problem and a minimum of two trials on any given day). Animals were pseudo-randomly released from one of four locations (N, E, S, or W), with trials lasting a maximum of 60 s (ITI of 10 min). If the animal found the platform in this time, it remained on the platform for 30 s, while any animal failing to find the platform within the allotted time was directed to the platform and also remained there for 30 s. Repeated testing of the animals at the different ages was performed in identical water mazes positioned in different rooms (at 4 and 12 months, room 1, 8 months, room 2) and the sequence of platform locations was randomly changed.
On day 1, curtains were drawn around the pool and the platform location was indicated with a distinctly colored flag. All animals performed eight trials on this day during which the platform location was constant, but release sites pseudo-randomly changed between trials. From day 2, curtains were opened and training commenced with the platform moved to a novel arm and submerged. Swim paths were monitored using an overhead CCTV camera and Any-Maze tracking software; the number of errors committed was manually scored. At the completion of each problem, a probe trial was performed 1–1.5 h later, where the platform was lowered to the floor of the pool (i.e., no escape platform present) and the animal placed in the water maze for 60 s. The time spent in the target arm was recorded and compared to time spent in the non-target arms.
Data were expressed as group mean ± SEM and analyzed using analysis of variance (ANOVA) with genotype as between-subject and problem as within-subject repeated measures factor followed by post hoc analyses on selected data sets (paired or un-paired t tests). A confidence level of 95 % (p < 0.05) was set for all comparisons and, for simplicity, only reliable differences are reported.
Open field water maze (OFWM): in a cross-sectional assessment, four mixed gender cohorts were tested consisting of PLB1WT n = 16 and PLB1Triple n = 16 (at 4 months); PLB1WT n = 14 and PLB1Triple n = 15 (at 12 months).
The water maze protocol used was modified compared to previous protocols [38, 39] and matched our recent work on these mice [27]. For details of the apparatus, see above. No inserts were available. Each animal was allocated a target platform (either NE or SW—fully counterbalanced with respect to age and genotype) and the appropriate platform raised to 1 cm below the water’s surface. Mice were alternately released from either NW or SE, facing the pool wall, in order to maintain equidistant release sites relative to the target. Animals were allowed 90 s to find the platform and remained on it for 10 s. Any mouse not locating the platform in the allotted time was directed to it. All animals received four trials on each day for five consecutive days (with the inter-trial interval (ITI) set at 30 min). Swim paths were monitored using video tracking software (Any-Maze; Ugo Basile) and stored as MPEG files.
On day 1, curtains were drawn around the pool to mask distal cues and the platform was made visible (by elevating above the water level and coloring it distinctly). The platform remained in the same location throughout this day, but release sites during the four trials varied. On days 2–5, the curtains were withdrawn and the target platform moved to the opposite quadrant from day 1 and was submerged. This platform location was maintained for the remainder of the experiment. On day 5, a probe trial was administered 1 h post-training with the platform lowered and each subject freely swimming for 60 s after release from a point opposite to the target quadrant.
Data gathered from swim paths allowed the following parameters to be extracted: (a) path length to reach and climb onto the platform; (b) swim speed; (c) thigmotaxis (time spent hugging the pool wall in the outer 10 % of the water maze), and (d) time spent in target quadrant during probe test. Each of these were expressed as group mean ± SEM and analyzed using analysis of variance (ANOVA) with genotype as between-subject and day as within-subject repeated measures factor. Again, post hoc parametric t tests were employed to contrast specific data sets. A confidence level of 95 % (p < 0.05) was set for all comparisons and, for simplicity, only reliable differences are reported.
IntelliCage: separate female cohorts of both PLB1WT and PLB1Triple mice were tested at 4 months (n = 11 each) and at 12 months (PLB1WT n = 15; PLB1Triple n = 13).
The IntelliCage (New-Behavior AG; Zurich, Switzerland) allows group-housed mice to be behaviorally assessed in both their spontaneous behavior and spatial learning [40]. Briefly, the apparatus consists of four recording chambers located in the corners of the housing cage. Access to each of these corners is via a small opening which contains an antenna, detecting each animal’s unique implanted transponder, thus recording individual visits to each corner (and, as required, granting or denying access to water in a corner selected for each particular mouse). Within each corner are two access doors to water, both of which have a motorized door, which can be opened in response to a mouse nose-poking through a photo-beam within the door’s aperture. Access to food (ad libitum) was available on top of a shelter placed in the center and fixed to the floor of the cage.
Experiments in the IntelliCage lasted for a total of 3 days; animals were introduced to the IntelliCage at approximately 4:00 pm. During the first 2 days (habituation phase), all corners were accessible and all water-access doors were open. For spatial training (day 3), several protocols have been proposed and we followed the original design of [40], for an alternative method, see [41]. Starting at 5:00 pm, animals were trained to obtain water in only one corner of the apparatus. Although all corners could still be accessed, the water-access doors were all closed except for the target corner of each individual mouse. The least preferred corner for each animal throughout habituation (as far as counter-balancing of corners would allow) was selected as the training corner. In addition to the number of visits, licking events were also monitored by a sensor on the water bottles.
Animals were examined twice daily for general well-being and retention of transponder. The 12-h light–dark cycle was maintained and general activity levels were monitored over all days of testing and compared for each day (unpaired t tests). Learning was assessed by analyzing total visits made to the trained corner (in %), and compared with visits to non-target corners (average). Target corner entries were also compared with chance (25 %; one-sample t test). Purposeful corner visits (i.e., those with licks and thus drinking) were also determined and expressed as a percentage of total visits.
All data are summarized as group mean and analyzed separately for the different age groups. Analysis of variance was employed initially with either training day or hour as within-subject and genotype as between-subject factor followed by Bonferroni-corrected post hoc comparisons. All other data were examined between genotypes using parametric statistics (Student’s t test) within each age range with alpha set to 0.05 for significance limit.
Object recognition: We set out to determine both memory for object novelty and spatial novelty in PLB1WT (n = 18 and n = 44) and PLB1Triple (n = 21 and n = 24) at 4 and 12 months, respectively, in mixed-gender cohorts.
Overall, we followed the protocol of [42] with minor modifications (see [27]). A number of cards to serve as visual cues were placed along the top rim of a white Perspex cylinder (diameter: 50 cm, height: 50 cm), which was located in a quiet experimental room. The animals’ activity was monitored by an overhead camera with data capture controlled by a PC (Ethovision Pro 3.1; Noldus, Wageningen, The Netherlands). Tall (15–20 cm) objects of different materials (e.g., glass, plastic, metal) color and shape were selected. Objects and arena were cleaned with 70 % ethanol prior to each trial (to eliminate any odor cues). Testing consisted of three distinct phases: (a) habituation, in which each animal was allowed to explore the apparatus for two trials of 5 min each (2-min inter-trial interval). In the first trial, the arena was empty (not recorded) and during the second trial one object was placed in the center of the apparatus. This phase lasted 2 days with the same procedure performed on each day. (b) Object novelty (day 3), in which each mouse was exposed to two identical objects (object A & A′) placed in opposing halves of the arena (sample phase). Mice were allowed 5 min of exploration and then, following a 5-min ITI, A′ was replaced with a novel object (object B) and the mouse given another 5 min to explore the arena (test phase). (c) Object displacement (day 4), whereby the mouse was presented with two novel sample objects (object C and D) placed similarly to above. Again, 5 min of exploration was allowed (sample phase), followed by a 5-min ITI and another 5 min of exploration during which one of the objects was moved to a different location (counterbalanced design) in the arena (test phase).
Exploration of an object was assessed as the time within the target zone (4 cm) around the respective object (expressed as group mean + SEM) and recorded and analyzed using Ethovision Pro 3.1. From these data, we calculated a discrimination index based on the formula
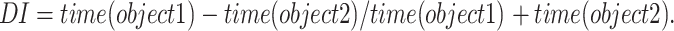 |
Object one was set as the novel or shifted object so that positive values indicate correct discriminations. Age and genotype comparisons were conducted using Student’s t test; performance was assessed relative to chance level (0) and deemed significant if p < 0.05. Exclusion criteria applied were: object bias during the sample phase at ratio 10:1; no exploration during any phase of testing or total object exploration <20 s; outlying data points (>2 SD from mean); discrimination index of 1/−1. There was no genotype or age bias for exclusions.
Tissue harvesting and histology
As in previous publications, mice were terminally anesthetized and tissue-fixed in situ by intra-aortic perfusions of 4 % paraformaldehyde in 0.1 M phosphate buffer. Brains were then removed and subsequently wax embedded and sectioned, e.g., [27, 43]. Slide-mounted 5-μm-thick coronial sections were used for DAB-based immunochemical staining conducted with a Leica/Bond autostainer (Leica Microsystems, Milton Keynes, UK). Sections underwent automated dewaxing, acidic antigen retrieval and antibody application. Immunolabeling for amyloid was conducted using 6E10, (dilution 1:250, Cambridge Bioscience, Cambridge, UK) and visualized using Bond Polymer Refine DAB staining kit (Leica Microsystems), nuclei were counterstained using hematoxylin. All images were taken via a digital camera (Axiocam) connected to a Zeiss microscope (Axioskop 2 plus) with water immersion lens using Axiovision software (Zeiss, Hertfordshire, UK).
Results
In all experiments, test cohorts were of mixed gender except the IntelliCage where only females were tested. In these cohorts, n’s of females and males were variable. We have not observed any evidence for behavioral deficits that are gender specific; contrary, in all tests, in which gender cohort sizes enabled separate statistical analysis, we have confirmed that PLB1Triple mice presented with a similar behavioral pattern independent of sex. Consequently, groups were combined for presentation and clarity.
An age-dependent impairment in recall in PLB1Triple mice tested in the radial arm water maze problem-solving task (Exp. 1a)
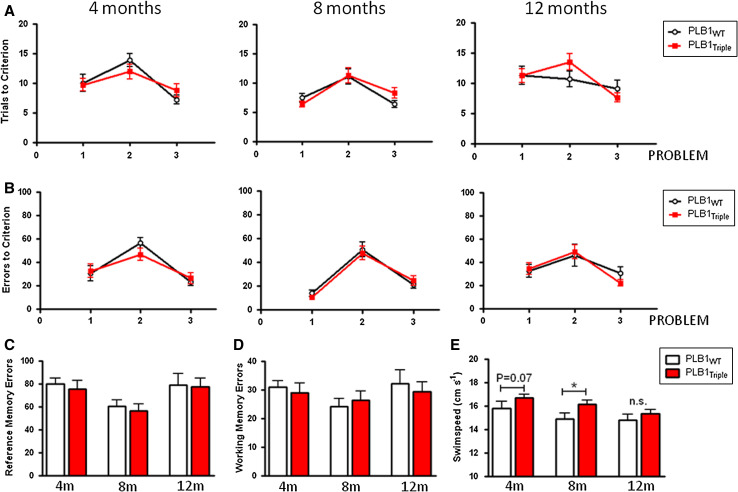
The RAWM combines features of the water maze and the radial arm maze, but has not been as widely used in AD models. It nevertheless offers the possibility to distinguish between reference memory errors (entry into non-rewarded arms), and working memory errors (repeated entry into a previously visited non-rewarded arm). On the first day of training, when required to locate a visible platform in the absence of spatial cues, PLB1WT and PLB1Triple mice performed equally well, confirming that PLB1Triple mice have no obvious deficit in sensory-motor ability (data not shown). No difference in performance (trials to criterion—Fig. 1a; errors to criterion, Fig. 1b) was seen between genotypes at any problem or any age group tested (Fig. 1a). This was also the case when the more traditional parameter, path length required to reach criterion, was considered (data not shown). In all age groups, it appeared that problem two was most difficult to solve as it constitutes reversal learning and was the first time the location of the platform was changed but comparisons did not attain reliability.
Fig. 1.
Longitudinal testing of PLB1Triple and PLB1WT mice in the radial arm water maze protocol in a problem-solving task aged 4, 8, and 12 months. Group mean ± SEM. No significant difference was found between transgenic and wild-type mice in (a) number of trials required to reach criterion or (b) number of errors committed before reaching criterion. Dissection of the errors committed into (c) reference and (d) working memory errors also did not yield any difference. However, swim speed (e) differed between genotypes and there was an age-related decline in swimming velocity, which was genotype-independent. *p < 0.05, n.s. not statistically significant
Separate analysis of reference or working memory errors (Fig. 1c, d) for the different age groups also did not yield any phenotype in the PLB1Triple mice. However, elevated swim speed in PLB1Triple mice was observed in the RAWM, with a strong trend apparent at 4 months (p = 0.07), and significance reached at 8 but not at 12 months (Fig. 1e). Both wild-type and transgenic PLB1 mice progressively reduced their swim speed over the course of the study (effect of age, PLB1WT: p < 0.05; PLB1Triple: p < 0.001).
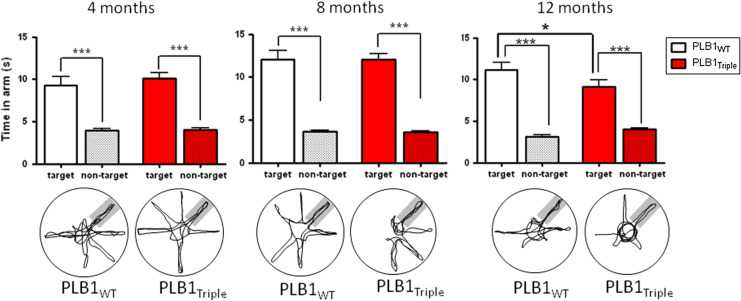
After achieving criterion in each of the problems, a probe test was conducted with the platform lowered to the floor. At all age groups, both genotypes presented with spatial memory indexed as preference for the arm formerly containing the platform (p < 0.001 in each case, Fig. 2). However, at 12 months, the PLB1Triple mice spent significantly less time in the target arm during the probe trials (9.1 ± 0.9 s) than their wild-type counterparts (11.1 ± 0.9 s; p < 0.05, Fig. 2).
Fig. 2.
Probe trial performance in the radial arm water maze problem solving task of PLB1Triple and PLB1WT mice. Both genotypes showed a statistically significant preference for the target arm at 4, 8 and 12 months of age as also indicated by the representative swim paths given below (target arm indicated as grey underlay). In the 12 month group, PLB1WT mice spent longer in the target arm than their transgenic counterparts. Group means + SEM. *p < 0.05 and ***p < 0.001, respectively
Severe impairments, but no age-dependence, in PSAPP mice in the radial arm water maze problem solving task (Exp. 1b)
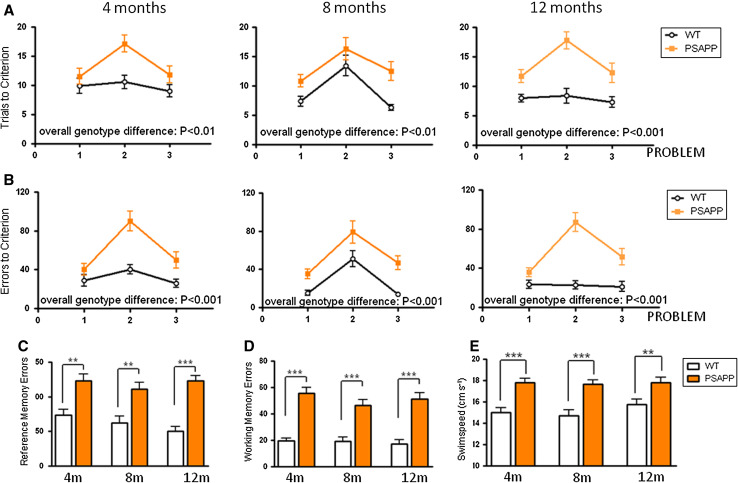
More subtle deficits in PLB1Triple mice in the RAWM than published previously for the OFWM [27] suggest that this task may not be as sensitive in revealing spatial deficits in PLB1Triple mice. We therefore investigated whether highly pathological mouse models present with an earlier phenotype. Such a more aggressive mutant is the PSAPP mouse, which was employed here as a comparator and to confirm the usefulness of this protocol. As for the PLB1 mice, the absence of apparent sensory-motor deficits was confirmed by equal performance in locating the visible platform on day 1 of testing (data not shown). Already at 4 months of age, transgenic PSAPP animals required markedly more trials to reach criterion than their wild-type counterparts (F(1,66) = 9.69, p < 0.01), with comparable deficits at 8 months (F(1,58) = 10.73, p < 0.01) and at 12 months (F(1,66) = 47.53, p < 0.0001; Fig. 3a). This pattern was also evident when the total number of errors committed before reaching criterion was considered (4 months: F(1,66) = 21.2, p < 0.0001; 8 months: F(1,58) = 19.42, p < 0.0001; 12 months: F(1,66) = 49.12, p < 0.0001; Fig. 3b). Again, problem two appeared to be particularly challenging as the submerged platform was re-located for the first time. The deficit in PSAPP mice was attributed to both reference and working memory errors; these were heightened cf. wild-types at all ages (p < 0.01 in each case, Fig. 3c, d). Much more marked than seen for the PLB1Triple mice, transgenic PSAPP mice had a consistently elevated swim speed compared with wild-types at all ages tested (p < 0.01 at 4, 8 and 12 months), with little/no change over time (Fig. 3e).
Fig. 3.
PSAPP mice are impaired in a problem solving task in the radial arm water maze when tested longitudinally at 4, 8 and 12 months. Reliable differences were obtained for a number of trials required to reach criterion and b number of errors committed before reaching criterion (overall genotype difference = p < 0.01 for each comparison). The deficit was due to impairments in both reference (c) and working memory errors (d) (p < 0.01 for each). Swim speed e was significantly elevated in PSAPP transgenic animals at all ages (p < 0.01 in each case) with no obvious decay over time. Group mean ± SEM. **p < 0.01 and ***p < 0.001, respectively
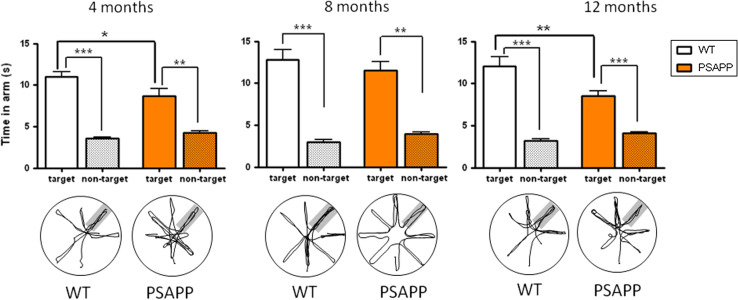
During the probe trial (Fig. 4), both transgenic and wild-type animals demonstrated a preference for the target arm compared with the mean time spent in non-target arms (all p’s < 0.01). For this transgenic line, the time spent in the target arm was significantly lower in PSAPP transgenic mice than their wild-type littermates at both 4 (11.0 ± 0.6 versus 8.6 ± 0.9 s; p < 0.05) and 12 months (12.1 ± 1.1 versus 8.5 ± 0.6 s; p < 0.01) (Fig. 4) and constitutes a deficit similar to the one seen in 12 month old PLB1Triple mice. This deficit did not reach significance in the 8 month PSAPP groups. Overall, the more aggressive amyloid pathology seems to have caused severe spatial learning deficits as early as 4 months, with no progressive worsening during ageing.
Fig. 4.
Memory impairment in PSAPP transgenic mice tested after achieving criterion in a problem solving task. Although both transgenic and wild-type mice presented with a statistically significant preference for the target arm at 4, 8 and 12 months of age (p < 0.01 in each case) PSAPP mice spent reliably less time in the target arm than WT mice at 4 and 12 months, but not at 8 months (p < 0.05 and p < 0.01). For each age group and genotype, probe trial sample traces are shown below the corresponding graph. Group means + SEM. *p < 0.05, **p < 0.01, and ***p < 0.001, respectively
PLB1Triple mice show an age-related decline in the open-field water maze (Exp. 2)
The open-field version of the water maze is well established to identify spatial learning and/or memory deficits and has been a popular test for AD mouse models [5, 21, 34, 44]. Since PLB1Triple mice presented with a mild phenotype in RAWM, we returned to our original protocol and repeated the experiment using 12-month-old mice. A cohort of 4-month-old subjects had been tested previously with no apparent spatial deficit [27]; these data, including a more detailed analysis, are also illustrated here for comparison and summarized in Fig. 5.
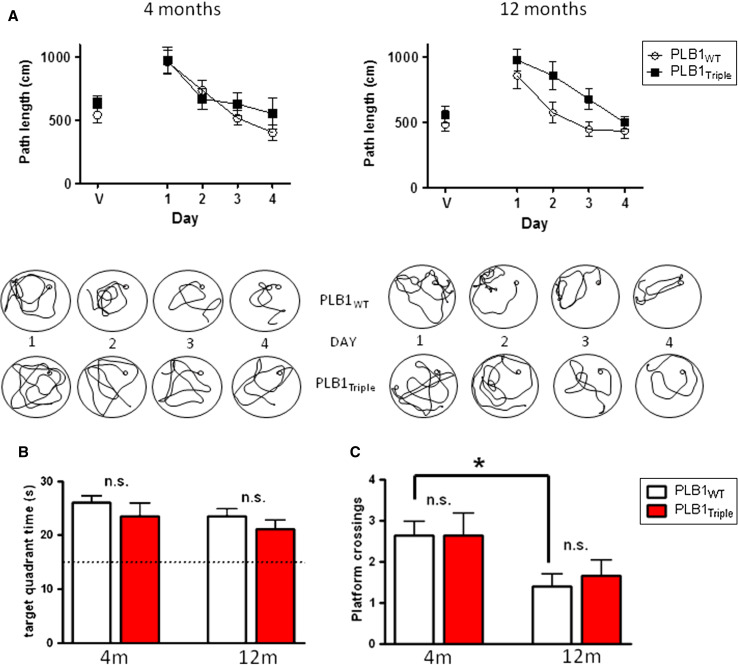
Fig. 5.
Assessment of PLB1Triple and PLB1WT in the open-field water maze. Mean ± SEM. a Acquisition phase for 4- and 12-month-old mice showing the mean distance required to find the platform on each day of testing including training with a visible platform (V). Note the markedly slower rate of learning in PLB1Triple mice at 12 months (right). Beneath each graph are representative sample swim paths from individuals over the course of the training days. b Time in target quadrant during the probe trial (60–90 min post-training) was not different between genotypes at any age. c Number of crossings of former platform location during probe test also did not show a phenotype. Note, older animals presented with lower number of platform crossings compared to young mice, but this was only reliable for the WT group (*p < 0.05). n.s. not significant
The path length to find the platform declined in both wild-type and transgenic mice progressively, and they improved over the course of training at both 4 (F(4,120) = 9.82, p < 0.0001) and 12 months (F(4,108) = 11.7, p < 0.0001). There was no observable difference between genotypes for the visible platform test (day 1) or during spatial learning in 4-month-old mice, indicative of intact sensory-motor functions. However, PLB1Triple mice covered a significantly longer distance than the wild-type group at 12 months (genotype effect: F(1, 108) = 10, p < 0.01; Fig. 5a). Representative swim paths underline this observation. Although 12-month-old PLB1Triple mice were slower in learning the task, overtraining on day 4 led to floor level performance with no difference between groups. Consequently, spatial memory assessed in a probe trial was not different between genotypes at 4 or 12 months of age. This was equally observed for the time spent in the training quadrant (Fig. 5b) or number of platform crossings (Fig. 5c) as a more stringent parameter of spatial memory. An age-related decline in spatial accuracy (platform crossings) was significant for PLB1WT (t = 2.8; df = 29; p = 0.009), but not for transgenic mice due to a somewhat higher variability.
Marked differences in swim speed were observed between genotypes at 4 and 12 months of age, with the PLB1Triple mice swimming considerably faster than PLB1WT (p’s < 0.05; Fig. 6a). However, PLB1Triple mice swam slower at 12 months relative to their younger counterparts (t = 5; df = 30; p < 0.0001) but swim speed in PLB1WT remained unchanged. Thigmotaxic swimming patterns were not overall different between genotypes at either age group (Fig. 6b, c); yet, on the very first day of testing in the 4-month group (visible platform), PLB1Triple spent markedly more time in the thigmotaxis zone than PLB1WT mice (p < 0.05).
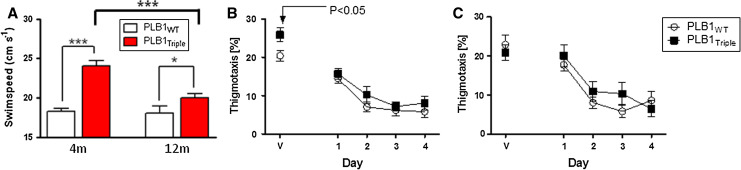
Fig. 6.
Non-spatial parameters recorded in the open-field water maze in PLB1Triple and PLB1WT mice at 4 and 12 months, respectively. a PLB1Triple mice presented with elevated swim speed compared with wild types (p < 0.001 and p < 0.05, respectively), which decayed by age. No age-related change was found in PLB1WT. Group means over all training days ± SEM. b, c Mean daily time (±SEM) spent in the thigmotaxis zone of the pool was greater on the first day of experimentation in transgenic mice (b) at 4 months (p < 0.05), but not on any other day or at 12 months (c). *p < 0.05 and ***p < 0.001, respectively
PLB1Triple mice show spatial deficits in the IntelliCage (Exp. 3)
In the IntelliCage, motor activity expressed as the total number of corner visits was evaluated over 2 days of habituation (H1, H2) and during testing (T) (Fig. 7a, b). This offers an alternative means of determining spatial learning with two elements different from the water maze. First, spatial learning took place on dry land in a home cage, in which multiple animals were housed as a cohort with regular social contacts, and positively rewarded by water. Second, there is no predetermined trial structure: each subject enters the activity corners without researcher’s influence.
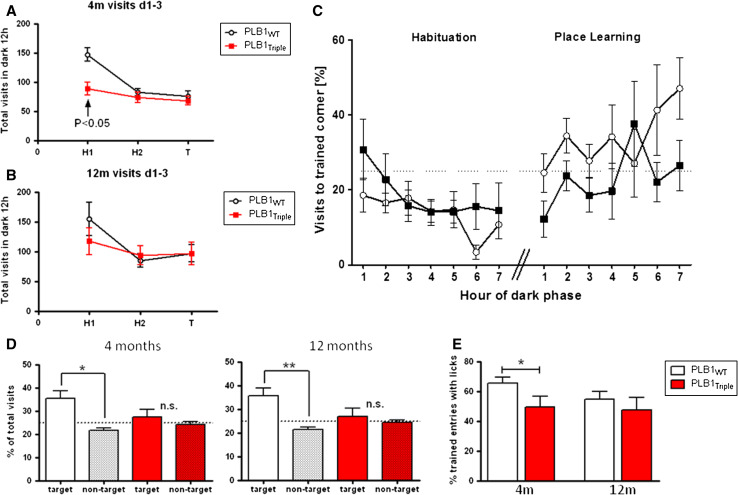
Fig. 7.
Performance of PLB1Triple and PLB1WT mice during habituation and spatial learning in the IntelliCage. Group mean ± SEM. Overall activity during 2 days of habituation (H1, H2) and 1 day of spatial training T in 4- (a) and 12-month-old (b) PLB1 mice. Note the higher activity in PLB1Triple mice during H1, which ceased on H2 and T so that genotypes and ages no longer differed. Hourly time-course of percent corner visits (c) to the ‘training corner’ in 12-month-old mice during the first 7 h of the dark phase of H2 of habituation and spatial learning. Percentage of entries into the target corner during the dark phase (d) in both genotypes at 4 and 12 months revealed a significant preference for the target corner relative to non-target corners in PLB1WT, but not PLB1Triple mice. Similarly, PLB1WT conducted more purposeful visits to the target corner accompanied with licking (e). *p < 0.05 and **p < 0.01, n.s. not statistically significant
When 4 months old, PLB1WT mice were significantly more active than PLB1Triple mice (F(1,40) = 5.56, p < 0.05), most notably on the first day of habituation (Fig. 7a). Despite a similar trend in the 12-month groups, higher variability in both groups prevented achievement of significance (Fig. 7b). Nevertheless, as all groups and ages were similarly active on habituation day 2 (H2), we progressed to spatial learning. Spatial learning towards the least favorite corner was expected to be rapid and completed within hours of nocturnal activity. A progressive hourly learning curve is depicted in Fig. 7c comparing 7 h of darkness during H2 and testing (T) in 12-month-old mice. PLB1WT animals progressively acquired the spatial task so that 40–45 % of entries were into the correct (rewarded) corner; PLB1Triple mice in contrast were impaired at both 4 and 12 months (Fig. 7d) by showing equal preference for rewarded and non-rewarded corners (PLB1WT at 4 months: p < 0.05; 12 months: p < 0.01; PLB1Triple: p > 0.05 at both age groups). Target visits in PLB1WT also differed significantly from the chance level of 25 % (ps < 0.05; Fig. 7d). Conversely, PLB1Triple mice did not show a significant preference for the training corner over the same 12 h of testing, nor was the percentage of visits to the trained corner significantly different from the hypothetical mean.
Finally, the percentage of purposeful versus exploratory visits to the training corner was considered. This was expressed as those visits to the training corner in which the mouse drank (licked). At 4 months, PLB1WT mice made a higher proportion of drinking visits than the PLB1Triple group (p < 0.05), while at 12 months there was no significant difference between genotypes (Fig. 7e) due to reduced licks in the wild-type group. It may be argued that PLB1Triple mice compensate the fewer visits to the target corner by heightened water intake such that similar amounts are consumed by both genotypes. Although the system does not enable direct measure of water intake by each mouse, we assumed that each lick would deliver a similar content of water for each subject and calculated the mean number of licks per visit for each phase. During H2, the mean licking rate per visit in 4-month-old mice was 15.5 for PLB1WT and 15.9 for PLB1Triple. In spatial learning (T), water was available in the target corner only and licks recorded per visit again did not differ between genotypes (50.5 ± 9.6 for PLB1WT; 36.9 ± 7.3 for PLB1Triple). Similar results were obtained for 12-month cohorts (not shown). These data exclude the notion that fewer visits to the target corner by PLB1Triple mice led to compensatory drinking patterns. Furthermore, there was no weight difference between PLB1WT (19.1 g) and PLB1Triple (19.2 g) mice.
Deficits in novel object recognition and object shift in PLB1Triple mice (Exp. 4)
In a between-subject design, cohorts were tested for object recognition in a mixed paradigm for a novel object first, and 24 h later for spatial recognition of object shift. Data are summarized in Fig. 8.
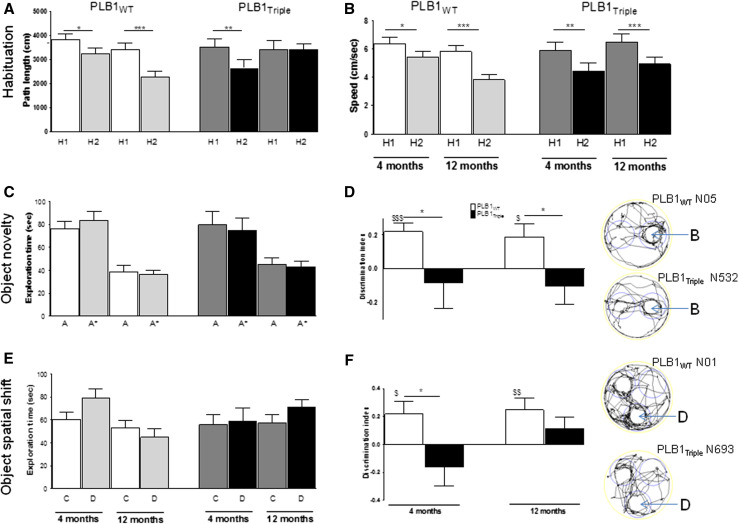
Fig. 8.
Performance of PLB1WT and PLB1Triple mice in two object recognition tasks. a Overall locomotor activity during habituation session H1 and H2: Activity was lower in H2 than H1 for all cohorts except PLB1Triple mice aged 12 months. b Ambulatory velocity was different between H1 and H2 at all age groups, but not related to factor phenotype. c Novel object recognition—sample phase: independent of genotype, animals spent equal amounts of time with objects A and A*. Note the age-related reduction in object exploration to about 50 % in 12 month old subjects. This was independent of genotype. d Novel object recognition—test phase: Object A* was replaced by object B. Discrimination index confirms that while PLB1WT mice prefer object B (positive index), PLB1Triple mice clearly did not (asterisks)—there was a small bias for the familiar object. e Object recognition, spatial shift sample phase: exploration was equal for objects C and D independent of age and genotype. f Object recognition, spatial shift test phase: Object D was shifted to a novel location. Discrimination index confirms that while PLB1WT mice prefer object D (positive index), PLB1Triple mice clearly did not (asterisk). The positive index of PLB1Triple mice at 12 months of age was due to two subjects. Note that index is not different from chance (0). All data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001 between genotype (t test). $ p < 0.05, $$ p < 0.01, $$$ p < 0.001 relative to chance. Tracks of representative animals aged 4 months undertaking object novelty test (top) and spatial shift (bottom) are also indicated. Circles indicate in-object zones, in which tracking was recorded as exploration. B novel object, D shifted object
During habituation, exploration of the open field with a central object was high on day 1 and all test groups lowered their ambulatory activity on day 2 apart from the 12-month-old PLB1Triple mice (Fig. 8a) so that the overall distance moved was significantly higher in PLB1Triple mice compared to PLB1WT (t = 2.9; df = 55, p = 0.005). As for the overall speed, which was higher in PLB1Triple mice during water maze procedures, there was no such phenotype recorded during habituation (Fig. 8b). Independent of age and genotype, the overall velocity was lower in the second session and in conjunction with reduced ambulation indicates that all animals habituated to the novel environment. Exploration of the central object was high on day 1 and lower on the day exactly matching the activity profile and its significance levels described above (data not presented).
No difference appeared between genotypes and between the two identical objects A and A* during the sample phase of object exploration (Fig. 8c); yet, 12-month-old animals explored the objects less than younger mice (t’s > 2.6; p’s < 0.05). At the test phase, two 4-month-old wild-type mice spent the majority of time sitting next to object A, not exploring the environment and the novel object (‘B’ in Fig. 8d); these mice were excluded from the analysis.
As indicated by the discrimination index, both PLB1WT groups showed a strong bias towards exploration of the novel object B whereas the PLB1Triple spent equal amounts of time with each object. This difference was reliable for both age groups (Fig. 8d) corroborating an object recognition deficit in PLB1Triple mice as early as 4 months. Furthermore, wild-type cohorts but not PLB1Triple groups performed significantly above the level of chance.
Similarly, no bias for any of the two objects C or D was observed during the second sampling phase for the ‘object shift’ stage. This was true for both genotypes and age cohorts investigated (Fig. 8e). However, after object displacement, PLB1Triple mice had a significant deficit in spatial recognition memory compared to PLB1WT, which was present already at 4 months of age, but did not attain reliability at 12 months, due to two transgenic subjects that persistently explored the shifted object (Fig. 8f). Nevertheless, PLB1WT mice performed significantly above chance at both ages, while PLB1Triple cohorts did not, indicating an overall lack of preference for the spatially altered object.
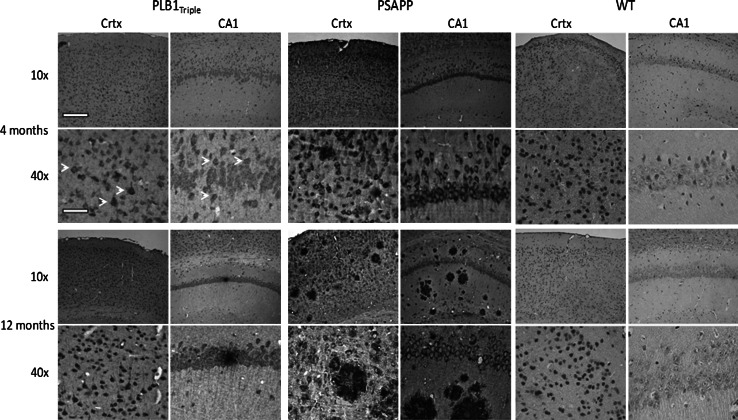
Amyloid pathology is exaggerated in PSAPP compared with PLB1Triple mice
Comparison of the amyloid pathology at 4 and 12 months of age confirmed an overall exaggerated level of amyloid expression and plaque deposition in PSAPP mice relative to PLB1Triple (Fig. 9). In repeating our previous results [27], PLB1Triple mice presented with a low number of plaques up to 12 months of age, yet a progressive increase in intracellular amyloid is apparent between 4 and 12 months (for a full time course, see accompanying paper [31]). In contrast, plaque deposition increased dramatically with age in PSAPP mice while intracellular amyloid, though overall more intense, remained at a constant level. There was no amyloid labeling in wild-type controls.
Fig. 9.
Amyloid pathology in PLB1Triple and PSAPP mice at 4 and 12 months old. PLB1Triple mice demonstrate intracellular amyloid in both the cortex (Crtx) and hippocampus (CA1), with infrequent extracellular plaque deposition and increasing amyloid reactivity with age. In comparison, PSAPP mice demonstrate a more aggressive expression of intracellular amyloid in both regions with a striking increase in plaque frequency with age. Age-matched wild types (WT) shown for comparison. Note the subtle intracellular staining for amyloid at 4 months in PLB1Triples (white arrows). Amyloid detected with 6E10 antibody (see methods for details). Scale bars represent 200 and 50 μm at 10× and 40× images, respectively
Discussion
The overall behavioral profile of PLB1Triple mice obtained in this study is summarized in Table 1. PLB1Triple mice consistently demonstrated spatial learning and memory deficits at 12 months in four different paradigms including aversive (water maze), neutral (object displacement), and positive reinforcement (IntelliCage). This strongly supports the notion that low transgene expression is sufficient for pathological proteins to accumulate over time and cause cognitive impairments, in keeping with ageing as the primary risk factor for dementia. Additionally, deficits in PLB1Triple mice can be attributed to abnormal forebrain function and likely include altered hippocampal firing patterns at rest and during task performance [27] and/or compromised synaptic plasticity (see [31] accompanying paper).
Table 1.
Simplified summary of behavioral deficits (↓) established for PLB1Triple mice compared to age-matched PLB1WT controls
| Age | 5 | 12 |
|---|---|---|
| Task | ||
| RAWM | n.d. | ↓ |
| OFWM | n.d. | ↓ |
| IC | ↓ | ↓ |
| SOR | ↓ | ↓ |
| NOR | ↓ | ↓ |
The spatial tasks appear to have differential sensitivity to the progression of the AD-like phenotype
RAWM radial arm water maze, OFWM open field water maze, IC IntelliCage, SOR spatial object recognition, NOR novel object recognition, n.d. not different
Slow progression of spatial water maze deficits in PLB1Triple mice
Many experimental mouse models of AD have been assessed in the open-field water maze and this has become the ‘gold standard’ in terms of spatial testing in rodents [24]. Deficits in this task are reported for single transgenic amyloid bearing mice, but also in bigenic and in triple APP–Tau–PS1 (3xTg) mice [45, 46]. We here implemented two different versions of the water maze: (1) the radial arm water maze [37] and (2) the open-field water maze [47], and compared these with other spatial learning tasks such as a spatial shift in the novel object recognition paradigm [48] and spatial training with water reward in the IntelliCage [40].
It is clear from this comparison that PLB1Triple mice represent with a more subtle and late-onset spatial phenotype than some of the established aggressive over-expression models such as the 5xFAD mouse [25], at least in terms of water maze-based protocols. This can be explained by the obvious impact of the genetic design, as the selective knock-in of mutant APP–Tau construct resulted in one copy placed within the HPRT locus. These two transgenes have never been expressed in one single construct and their individual vs. joint impact remains to be assessed. Low transgene copy numbers may delay the onset of the genotype and thus explain the slower but possibly more patho-physiologically relevant onset of cognitive decline in PLB1Triple mice in the water maze; this contrasts with an onset at 4 months in the other available triple AD model (3xTg) [21]. In addition, expression of our construct was tailored towards the forebrain by insertion of the CAMKIIα regulatory element in an attempt to mimic both progression and anatomy of human AD pathology. In these structures, expression of transgenes in PLB1 mice remain below native rodent APP/Tau levels and thus supports a slow patho-physiological disease onset at ~4 months (Fig. 9) when APP or Tau-labeling is faint [27, 31].
Spatial learning deficits in PLB1Triple mice in the open-field water maze evident at 12 but not at 4 months was also observed in experiments using the radial arm water maze, albeit with differing profiles: while acquisition was impaired in the former, there were deficits in retention in the latter. A number of possible explanations for this difference have been proposed (for review, see [45, 49]). In our specific case, (1) the experimental design in the radial arm water maze was longitudinal and animals were trained as early as 4 months. At this stage, PLB1Triple did not display spatial learning deficits in either task. Repeated testing at 8 and 12 months did not reveal any deficits in problem-solving, raising the possibility that a measure of retention for the procedure is retained from the previous time-point of training. Such repeated testing in the water maze can indeed render the protocol less sensitive to differences between groups (e.g., [50]), a characteristic that has also been observed in transgenic mice [51]. Our data are in line with previous observations by Billings and coworkers [52] in which early learning and repeated rehearsal of the same task benefited transgenic 3xTg-AD mice in particular while relatively little improvement was observed in wild-type controls. (2) The task in the RAWM was conceptually different and allowed segregation of reference and working memory errors; working memory in the open-field water maze would require different protocols (see [53]). Therefore, dependent variables in the radial arm maze were number of errors for working and reference memory errors (and their sum), as well as trials to a set criterion, which had to be met before progressing into the next learning phase. These measures are different from path length in the open-field variant, which is taken as an index of reference memory only and for which no criterion was set. (3) Acquisition learning in the RAWM is different in that the principle choice point is located in the center and once the correct alley has been selected, swimming towards the distal end will guarantee reward (finding the platform). By contrast, the open-field water maze offers an infinite number of choice points and requires higher accuracy in spatial knowledge to successfully complete the task. Consequently, having no barriers seems to make the task more difficult, a view consistent with our data set. (4) It might be argued that due to its greater simplicity, the radial arm maze may not be sensitive enough to reveal deficits in AD models, especially because it is not widely applied in its current version. However, this is unlikely given our observation that PSAPP mice already are deficient in this test at 4 months, and that a RAWM is typically considered more sensitive than the open-field water maze ([45] for review).
Others have used the radial arm version of the water maze to characterize the deficit in spatial learning in PSAPP mice and have shown somewhat divergent results from those described here. For example, a series of studies by Arendash and colleagues found no impairment in working or reference memory in PSAPP mice until 15–17 months of age [33, 34, 54]. These studies are also at odds with the pathology of PSAPP brains reported here, as they would suggest a long process of progressive plaque formation during which there is no cognitive phenotype. We, however, reveal an early onset of spatial deficit congruent with the emergence of intracellular amyloid labeling in both hippocampus and cortex of PSAPP mice. One explanation for this apparent discrepancy may lie in the difference of apparatus (we used eight arms, not six). The greater number of arms equates to a greater number of options and therefore adds a greater complexity to the task. A second marked difference is the training protocol: In our study, mice swam freely for the duration of each trial, whereas in the studies cited above, animals were immediately returned to the starting arm upon making an error. It is possible that the self-correction protocol used in our study places a higher demand upon hippocampus-dependent function (especially in terms of working memory) and that return to the start position favors egocentric route learning. Such a free-swimming protocol in the RAWM has been applied in a treatment study [35], but no data for a non-transgenic group is presented, precluding a meaningful comparison with our data.
Early onset of spatial deficits in PLB1Triple mice in spontaneous learning tasks
An earlier onset of deficits in PLB1Triple mice was revealed in a spatial (and non-spatial) version of the spontaneous object recognition test. In a series of elegant studies, the laboratory of Good conducted experiments using Tg2576 mice and confirmed a selective spatial deficit in aged (14 months) mice while at the same time novel object recognition was unimpaired [42, 55, 56]. Similarly, 18-month-old 3xTg–AD mice have spatial deficits in this task, but normal visual recognition after an interval of 3 min [57]. Although Good and colleagues did not detect deficits in object recognition, such deficits have been repeatedly found in APP transgenic mice inclusive of Tg2576 [46, 58–60], other bigenic lines over-expressing mutant human APP and presenilin genes [61–66], and also mice genetically modified to express human tau [67, 68]. It is likely that neurodegeneration of hippocampal-cortical connections may be responsible for deficits in spatial object shift. By contrast, novel object recognition may depend on prefrontal and perirhinal cortex [69], so that early loss of recognition memory could also impinge on spatial aspects of recognition. Moreover, this correlates with the more readily apparent pathology in cortex in PLB1Triple [27] and strongly indicates that object recognition provides a more sensitive early onset measure for spatial deficits in our novel model.
Similarly, an early onset of spatial memory deficits was also revealed in spontaneous behavior in the IntelliCage. Previous studies using this apparatus have found it to be sufficiently sensitive to detect cognitive deficits in a Huntington’s disease model [70] and in mice treated with methylmercury [71], and work by Codita and colleagues [41] has also found phenotypes in spatial learning in mice carrying transgenes for APP with both Swedish and Arctic mutations. Like the tg-ArcSwe mice, PLB1Triple presented with a reduction of activity (corner visits) during free exploration on day 1 in the IntelliCage, but were not different from controls once completely habituated to the test environment. The higher activity in PLB1WT mice at 4 months is unlikely at odds with greater swim speeds in PLB1Triple mice in both water maze procedures used, since the number of corner entries in the IntelliCage is not a reliable marker for speed, but reflects a proxy of exploratory behavior. In agreement with these data, young PLB1Triple mice have reduced global home cage activity [27] and this underlines general differences between tasks performed on dry land and in water. Generally, activity changes in over-expression models of AD have been well catalogued (e.g., [72]; for review, see [23]) and the presence of this attribute further confirms PLB1Triple mice as a realistic model of AD. Since PLB1Triple mice already presented with impairments in spatial acquisition learning, we did not implement a reversal/extinction protocol. Common to all tasks is the likely requirement of hippocampal-prefrontal system activation, and the occurrence of plasticity during and after task performance. The observation that PLB1Triple mice present with deficits in synaptic short- and long-term plasticity [31] appears therefore as an important physiological correlate for the cognitive decline reported here.
Acknowledgments
The authors acknowledge the help of Svetlana Wittnerova with experimental elements, and part-funding from KHIDI for the initial generation of the PLB1 mice. Some experimental components were supported by an award (NS-AU-098) from the Translational Medicine Research Collaboration—a consortium made up of the Universities of Aberdeen, Dundee, Edinburgh and Glasgow, the four associated NHS Health Boards (Grampian, Tayside, Lothian and Greater Glasgow and Clyde), Scottish Enterprise and Pfizer (formerly Wyeth). The CaMKII promoter was a generous gift from Dr. Mark Mayford. Knock-in of transgenes was conducted by genOway (France).
References
- 1.Götz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- 2.Gama Sosa MJ, De Gasperi R, Elder GA. Modeling human neurodegenerative diseases in transgenic systems. Hum Genet. 2012;131:535–563. doi: 10.1007/s00439-011-1119-1. [DOI] [PubMed] [Google Scholar]
- 3.Spires-Jones T, Knafo S. Spines, plasticity, and cognition in Alzheimer’s model mice. Neural Plast. 2012;2012:10. doi: 10.1155/2012/319836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Games D, Adams D, Alessandrini R, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 5.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, a-beta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 6.Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, Van Leuven F. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24(3):751–762. doi: 10.1016/S0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- 8.Lewis J, McGowan E, Rockwood J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25(4):402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 9.Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293(5534):1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 10.Allen B, Ingram E, Takao M, et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22(21):9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53(3):337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Tatebayashi Y, Miyasaka T, Chui DH, Akagi T, Mishima K, Iwasaki K, Fujiwara M, Tanemura K, Murayama M, Ishiguro K, Planel E, Sato S, Hashikawa T, Takashima A. Tau filament formation and associative memory deficit in aged mice expressing mutant (R406W) human tau. Proc Natl Acad Sci USA. 2002;99(21):13896–13901. doi: 10.1073/pnas.202205599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda M, Shoji M, Kawarai T, et al. Accumulation of filamentous tau in the cerebral cortex of human tau R406W transgenic mice. Am J Pathol. 2005;166(2):521–531. doi: 10.1016/S0002-9440(10)62274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borchelt DR, Thinakaran G, Eckman CB, et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17(5):1005–1013. doi: 10.1016/S0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 15.Dewachter I, van Dorpe J, Spittaels K, Tesseur I, Van Den Haute C, Moechars D, Van Leuven F. Modeling Alzheimer’s disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice. Exp Gerontol. 2000;35(6–7):831–841. doi: 10.1016/S0531-5565(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 16.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 17.Bolmont T, Clavaguera F, Meyer-Luehmann M, et al. Induction of tau pathology by intracerebral infusion of amyloid-beta -containing brain extract and by amyloid-beta deposition in APP × Tau transgenic mice. Am J Pathol. 2007;171(6):2012–2020. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular A-beta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 20.Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Dröse S, Brandt U, Savaskan E, Czech C, Götz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci USA. 2009;106(47):20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 22.Clinton LK, Billings LM, Green KN, Caccamo A, Ngo J, Oddo S, McGaugh JL, LaFerla FM. Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol Dis. 2007;28(1):76–82. doi: 10.1016/j.nbd.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi DT, Chen KS. Behavioral phenotypes of amyloid-based genetically modified mouse models of Alzheimer’s disease. Genes Brain Behav. 2005;4:173–196. doi: 10.1111/j.1601-183X.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- 24.Morris RGM, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O’Carroll C. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Phil Trans R Soc Lond B Biol Ser. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26(40):10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holcomb L, Gordon MN, McGowan E, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 27.Platt B, Drever B, Koss D, Stoppelkamp S, Jyoti A, Plano A, Utan A, Merrick G, Ryan D, Melis V, Wan H, Mingarelli M, Porcu E, Scrocchi L, Welch A, Riedel G. Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, PLB1. PLoS ONE. 2011;6(11):e27068. doi: 10.1371/journal.pone.0027068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Platt B, Welch A, Riedel G. FDG-PET imaging, EEG and sleep phenotypes as translational biomarkers for research in Alzheimer’s disease. Biochem Soc Trans. 2011;9(4):874–880. doi: 10.1042/BST0390874. [DOI] [PubMed] [Google Scholar]
- 29.Bronson SK, Plaehn EG, Kluckman KD, Hagaman JR, Maeda N, Smithies O. Single-copy transgenic mice with chosen-site integration. Proc Natl Acad Sci USA. 1996;93:9067–9072. doi: 10.1073/pnas.93.17.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17(1):181–190. doi: 10.1016/S0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 31.Koss DJ, Drever BD, Stoppelkamp S, Riedel G, Platt B (2013) Age-dependent changes in hippocampal synaptic transmission and plasticity in the PLB1Triple Alzheimer mouse. Cell Mol Life Sci. doi:10.1007/s00018-013-1273-9 [DOI] [PMC free article] [PubMed]
- 32.Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D. Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav Genet. 1999;29:177–185. doi: 10.1023/A:1021691918517. [DOI] [PubMed] [Google Scholar]
- 33.Gordon MN, King DL, Diamond DM, Jantzen PT, Boyett KV, Hope CE, Hatcher JM, DiCarlo G, Gottschall WP, Morgan D, Arendash GW. Correlation between cognitive deficits and abeta deposits in transgenic APP + PS1 mice. Neurobiol Aging. 2001;22:377–385. doi: 10.1016/S0197-4580(00)00249-9. [DOI] [PubMed] [Google Scholar]
- 34.Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/S0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 35.Todd Roach J, Volmar CH, Dwivedi S, Town T, Crescentini R, Crawford F, Tan J, Mullan M. Behavioral effects of CD40-CD40L pathway disruption in aged PSAPP mice. Brain Res. 2004;1015:161–168. doi: 10.1016/j.brainres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Duff K, Eckman C, Zehr C, Yu X, et al. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 37.Buresova O, Bures J, Oitzl MS, Zahalka A. Radial maze in the water tank: an aversively motivated spatial working memory task. Physiol Behav. 1985;34:1003–1005. doi: 10.1016/0031-9384(85)90028-9. [DOI] [PubMed] [Google Scholar]
- 38.Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- 39.Deiana S, Harrington CR, Wischik CM, Riedel G. Methylthioninium chloride reverses cognitive deficits induced by scopolamine: comparison with rivastigmine. Psychopharmacology. 2009;202:53–65. doi: 10.1007/s00213-008-1394-2. [DOI] [PubMed] [Google Scholar]
- 40.Galsworthy MJ, Amrein I, Kuptsov PA, Poletaeva II, Zinn P, Rau A, Vyssotski A, Lipp HP. A comparison of wild-caught wood mice and bank voles in the IntelliCage: assessing exploration, daily activity patterns and place learning paradigms. Behav Brain Res. 2005;157:211–217. doi: 10.1016/j.bbr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Codita A, Gumucio A, Lannfelt L, Gellerfors P, Winblad B, Mohammed AH, Nilsson LN. Impaired behavior of female tg-ArcSwe APP mice in the IntelliCage: a longitudinal study. Behav Brain Res. 2010;215(1):83–94. doi: 10.1016/j.bbr.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 42.Good MA, Hale G. The “Swedish” mutation of the amyloid precursor protein (APPswe) dissociates components of object-location memory in aged Tg2576 mice. Behav Neurosci. 2007;121(6):1180–1191. doi: 10.1037/0735-7044.121.6.1180. [DOI] [PubMed] [Google Scholar]
- 43.Jyoti A, Plano A, Riedel G, Platt B. EEG, activity, and sleep architecture in a transgenic AβPPSWE/PSEN1A246E Alzheimer’s disease mouse. J Alzheimers Dis. 2011;22(3):873–887. doi: 10.3233/JAD-2010-100879. [DOI] [PubMed] [Google Scholar]
- 44.Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408:975–979. doi: 10.1038/35046031. [DOI] [PubMed] [Google Scholar]
- 45.Stewart S, Cacucci F, Lever C. Which memory task for my mouse? a systematic review of spatial memory performance in the Tg2576 Alzheimer’s mouse model. J Alzheimer’s Dis. 2011;26:105–126. doi: 10.3233/JAD-2011-101827. [DOI] [PubMed] [Google Scholar]
- 46.Filali M, Lalonde R, Theriault P, Julien C, Calon F, Planel E. Cognitive and non-cognitive behaviours in the triple transgenic mouse model of Alzheimer’s disease expressing mutated APP, PS1, and Mapt (3xTg-AD) Behav Brain Res. 2012;234:334–342. doi: 10.1016/j.bbr.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 48.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-X. [DOI] [PubMed] [Google Scholar]
- 49.Tanila H. Wading pools, fading memories-place navigation in transgenic mouse models of Alzheimer’s disease. Front Aging Neurosci. 2012;4:11. doi: 10.3389/fnagi.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Groen T, Kadish I, Wyss JM. Old rats remember old tricks; memories of the water maze persist for 12 months. Behav Brain Res. 2002;136:247–255. doi: 10.1016/S0166-4328(02)00137-7. [DOI] [PubMed] [Google Scholar]
- 51.Janus C, D’Amelio S, Amitay O, Chishti MA, Strome R, Fraser P, Carlson GA, Roder JC, George-Hyslop P, Westaway D. Spatial learning in transgenic mice expressing human presenilin 1 (PS1) transgenes. Neurobiol Aging. 2000;21:541–549. doi: 10.1016/S0197-4580(00)00107-X. [DOI] [PubMed] [Google Scholar]
- 52.Billings LM, Green KN, McGaugh JL, LaFerla FM. Learning decreases A beta*56 and tau pathology and ameliorates behavioral decline in 3xTg-AD mice. J Neurosci. 2007;27(4):751–761. doi: 10.1523/JNEUROSCI.4800-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.EvL Roloff, Harbaran D, Micheau J, Platt B, Riedel G. Dissociation of cholinergic function in spatial and procedural learning in rats. Neuroscience. 2007;146(3):875–889. doi: 10.1016/j.neuroscience.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 54.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 55.Hale G, Good M. Impaired visuospatial recognition memory but normal object novelty detection and relative familiarity judgments in adult mice expressing the APPswe Alzheimer’s disease mutation. Behav Neurosci. 2005;119:884–891. doi: 10.1037/0735-7044.119.4.884. [DOI] [PubMed] [Google Scholar]
- 56.Good MA, Hale G, Staal V. Impaired “episodic-like” object memory in adult APPswe transgenic mice. Behav Neurosci. 2007;121(2):443–448. doi: 10.1037/0735-7044.121.2.443. [DOI] [PubMed] [Google Scholar]
- 57.Gulinello M, Gertner M, Mendoza G, Schoenfeld BP, Oddo S, LaFerla F, Choi CH, McBride SM, Faber DS. Validation of a 2-day water maze protocol in mice. Behav Brain Res. 2009;196(2):220–227. doi: 10.1016/j.bbr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bardgett ME, Davis NN, Schultheis PJ, Griffith MS (2010) Ciproxifan, an H(3) receptor antagonist, alleviates hyperactivity and cognitive deficits in the APP(Tg2576) mouse model of Alzheimer’s disease. Neurobiol Learn Mem. (epub ahead of print) [DOI] [PMC free article] [PubMed]
- 59.Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM, Timson BF, Csernansky JG. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;35(3):426–432. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;200(1):95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scullion GA, Kendall DA, Marsden CA, Sunter D, Pardon MC. Chronic treatment with the α(2)-adrenoceptor antagonist fluparoxan prevents age-related deficits in spatial working memory in APP × PS1 transgenic mice without altering β-amyloid plaque load or astrocytosis. Neuropharmacology. 2011;60:223–234. doi: 10.1016/j.neuropharm.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Woo DC, Lee SH, Lee DW, Kim SY, Kim GY, Rhim HS, Choi CB, Kim HY, Lee CU, Choe BY. Regional metabolic alteration of Alzheimer’s disease in mouse brain expressing mutant human APP-PS1 by 1H HR-MAS. Behav Brain Res. 2010;211(1):125–131. doi: 10.1016/j.bbr.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 63.Heneka MT, Ramanathan M, Jacobs AH, et al. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J Neurosci. 2006;26:1343–1354. doi: 10.1523/JNEUROSCI.4236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jardanhazi-Kurutz D, Kummer MP, Terwel D, Vogel K, Dyrks T, Thiele A, Heneka MT. Induced LC degeneration in APP/PS1 transgenic mice accelerates early cerebral amyloidosis and cognitive deficits. Neurochem Int. 2010;57(4):375–382. doi: 10.1016/j.neuint.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Dewachter I, Reversé D, Caluwaerts N, Ris L, Kuipéri C, Van den Haute C, Spittaels K, Umans L, Serneels L, Thiry E, Moechars D, Mercken M, Godaux E, Van Leuven F. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein (V717I) transgenic mice. J Neurosci. 2002;22(9):3445–3453. doi: 10.1523/JNEUROSCI.22-09-03445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mori T, Rezai-Zadeh K, Koyama N, Arendash GW, Yamaguchi H, Kakuda N, Horikoshi-Sakuraba Y, Tan J, Town T. Tannic acid is a natural secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice. J Biol Chem. 2012;287(9):6912–6927. doi: 10.1074/jbc.M111.294025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Polydoro M, Acker CM, Duff K, Castillo PE, Davies P. Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J Neurosci. 2009;29(34):10741–10749. doi: 10.1523/JNEUROSCI.1065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boekhoorn K, Terwel D, Biemans B, et al. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci. 2006;26(13):3514–3523. doi: 10.1523/JNEUROSCI.5425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rudenko O, Tkach V, Berezin V, Bock E. Detection of early behavioral markers of Huntington’s disease in R6/2 mice employing an automated social home cage. Behav Brain Res. 2009;203:188–199. doi: 10.1016/j.bbr.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 71.Onishchenko N, Tamm C, Vahter M, Hokfelt T, Johnson JA, Johnson DA, Ceccatelli S. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol Sci. 2007;97:428–437. doi: 10.1093/toxsci/kfl199. [DOI] [PubMed] [Google Scholar]
- 72.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]