Abstract
Calcium (Ca2+) is an universal second messenger that regulates the most important activities of all eukaryotic cells. It is of critical importance to neurons as it participates in the transmission of the depolarizing signal and contributes to synaptic activity. Neurons have thus developed extensive and intricate Ca2+ signaling pathways to couple the Ca2+ signal to their biochemical machinery. Ca2+ influx into neurons occurs through plasma membrane receptors and voltage-dependent ion channels. The release of Ca2+ from the intracellular stores, such as the endoplasmic reticulum, by intracellular channels also contributes to the elevation of cytosolic Ca2+. Inside the cell, Ca2+ is controlled by the buffering action of cytosolic Ca2+-binding proteins and by its uptake and release by mitochondria. The uptake of Ca2+ in the mitochondrial matrix stimulates the citric acid cycle, thus enhancing ATP production and the removal of Ca2+ from the cytosol by the ATP-driven pumps in the endoplasmic reticulum and the plasma membrane. A Na+/Ca2+ exchanger in the plasma membrane also participates in the control of neuronal Ca2+. The impaired ability of neurons to maintain an adequate energy level may impact Ca2+ signaling: this occurs during aging and in neurodegenerative disease processes. The focus of this review is on neuronal Ca2+ signaling and its involvement in synaptic signaling processes, neuronal energy metabolism, and neurotransmission. The contribution of altered Ca2+ signaling in the most important neurological disorders will then be considered.
Keywords: Calcium signaling, Calcium channels, Calcium pumps, Neurons, Neurodegenerative disorders, Migraine
Introduction: general principle of Ca2+ signaling
Cell life would not be possible without the information conveyed by calcium (Ca2+) to most of its essential processes. In essence, Ca2+ controls virtually all aspects of cell life—but does so only after its signal is suitably processed [1, 2]. The processing is performed by sensor proteins endowed with the ability to specifically complex Ca2+ and consists essentially in the conformational change of the complexing protein induced by the binding of Ca2+. Since the regulation of cellular functions is by definition a reversible process, the binding of Ca2+ also occurs reversibly. Sensor proteins must bind Ca2+ within cells in the presence of much larger concentrations of other potentially competing cations. They do so thanks to the peculiar flexibility of Ca2+ as a ligand. The coordination chemistry of Ca2+ indeed allows it to be accommodated within binding sites of irregular geometry, such as those that can be expected within proteins. In contrast, Mg2+ coordination, given its stricter requirement for a perfectly octahedral geometry of the binding sites, is hard to satisfy within the structure of proteins [2]. Once Ca2+ was chosen as a carrier of cellular information, its background concentration within cells had to be maintained at a level low enough to permit it to be significantly changed without prohibitive energy costs. In the course of evolution, systems were thus developed which maintain the concentration of Ca2+ within cells at adequately low levels. Families of soluble proteins are found in all eukaryotic cells whose task is to reversibly bind Ca2+: these proteins simply buffer Ca2+ to maintain its background concentration within the appropriately low range without decoding its message. However, the overall task of controlling the concentration of Ca2+ within cells is performed by families of proteins intrinsic to membranes that transport Ca2+ across membrane boundaries and/or mediate its passage across them down the steep concentration gradient (about 10,000-fold) between the extracellular ambient and the cell interior, and between the lumen of intracellular Ca2+-storing organelles and the cytosol. These proteinaceous systems are channels of various types, ATPases (colloquially called pumps), exchangers [mostly Na+/Ca2+ exchangers (NCXs)], and a complex electrophoretic system that mediates the import of Ca2+ across the inner mitochondrial membrane into the matrix. They differ in transport mechanism, affinity for Ca2+, and total transport capacity, and thus offer/provide the multiplicity of responses necessary for the efficient operation of the Ca2+ signaling system.
Neuronal Ca2+ signaling
Eukaryotic cells use Ca2+ as a signal carrier to regulate functions that are common to all of them: they include the control of metabolism by the process of enzyme phosphorylation and dephosphorylation, motility processes like those involving the cytoskeleton, the secretion of various molecules in the process of exocytosis, the transcription of numerous genes, the process of programmed cell death. Interestingly, Ca2+ signals have the peculiar property, which is distinctive among second messengers, of autoregulation: i.e., protein systems that control cellular Ca2+ homeostasis may be regulated by Ca2+ itself, both at the transcriptional and post-transcriptional level [3, 4]. Other important properties that are controlled by Ca2+ are cell specific, as for instance, the origin of life in the process of egg fertilization, and the contraction and relaxation of muscles. The signaling function of Ca2+ also has aspects that are peculiar and/or particularly important to neurons. Synaptic transmission, which is the form of secretion that leads to the release of neurotransmitters, the process of learning and the formation and consolidation of memory, also through the regulation of specific gene pools, the long-term potentiation (LTP) or depression of synaptic transmission, the direct coupling between the depolarization of the plasma membrane and the increase of intracellular Ca2+ are all specific neuronal processes that are under the control of Ca2+ signals. Neuronal life depends on the correct functioning of all these processes, and thus depends, to a degree that is perhaps greater than in most other eukaryotic cells, on the precise temporal and spatial regulation of the Ca2+ signals.
This review will concentrate on neuronal Ca2+ signaling, specifically mentioning the causative role of Ca2+ dysfunction in some of the most important neuronal pathologies.
The neuronal Ca2+ signaling toolkit
Ca2+ fluxes across the plasma membrane and between intracellular organelles integrate diverse cellular functions and support complex signaling processes that are of special importance to the activity of neuronal cells. Both the coordinated action of the systems that handle Ca2+ movements and the activity of a vast array of checkpoints that control them are essential to brain physiology.
Ion channels, exchangers, and pumps both in the plasma membrane and the membranes of mitochondria, endoplasmic reticulum (ER), Golgi apparatus, and nucleus (possibly in the acidic compartments as well) contribute to the Ca2+ toolkit that, together with the action of G protein-coupled receptors, Ca2+ binding proteins, and transcriptional networks, orchestrate neuronal Ca2+-regulated processes. They are important for neuronal plasticity (see below) and underlie critical neuronal functions, such as learning and memory. They also play a critical role in neuronal survival.
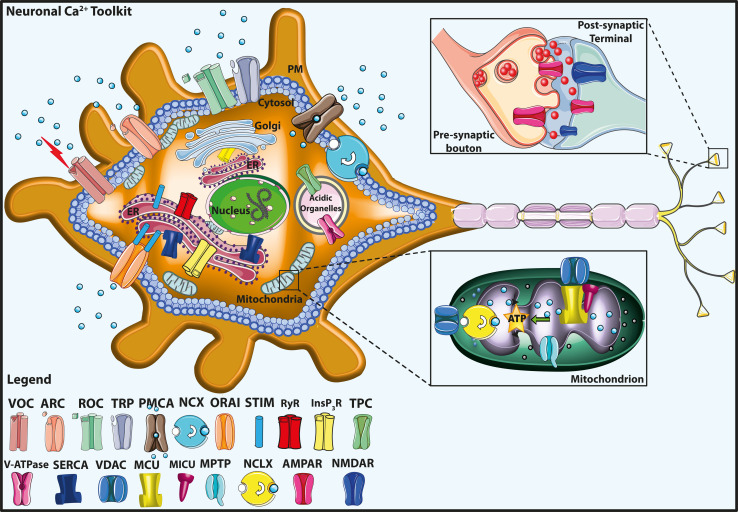
Figure 1 shows a cartoon representing the neuronal Ca2+ signaling toolkit that will be briefly reviewed in the following paragraphs.
Fig. 1.
Neuronal calcium (Ca2+) signaling toolkit. The Ca2+ transport proteins, the receptors of the plasma membrane (PM), and the intracellular organelles, including mitochondria, endoplasmic reticulum (ER), Golgi apparatus, and acidic organelles, are indicated. The mitochondrial Ca2+ handling systems (proteins) are shown in greater detail in the bottom right inset. The top right inset shows a schematic view of the pre-synaptic bouton and the post-synaptic termination. The legend on the bottom left indicates the Ca2+ transporter proteins. VOC Voltage-gated Ca2+ channel, ROC receptor-operated Ca2+ channel, ORAI the pore-forming subunit of store-operated Ca2+ entry channel (SOC), STIM the Ca2+ sensor, TPC two-pore channel, ARC arachidonic acid-regulated Ca2+ channel, TRP transient receptor potential channel, PMCA plasma membrane Ca2+ ATPase, V-ATPase vacuolar H+ ATPase, InsP3R inositol 1,4,5 tris–phosphate receptors, RyR ryanodine receptor, NCX plasma membrane Na+/Ca2+ exchanger, SERCA sarco-/endoplasmic reticulum Ca2+ ATPase, MCU mitochondrial Ca2+ uniporter, MICU mitochondrial Ca2+ uniporter regulator, NCLX mitochondrial Na+/Ca2+ exchanger, VDAC voltage-dependent anion channels, MPTP mitochondrial permeability transition pore, AMPAR 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, NMDAR N-methyl-d-aspartate receptor
Plasma membrane Ca2+ channels
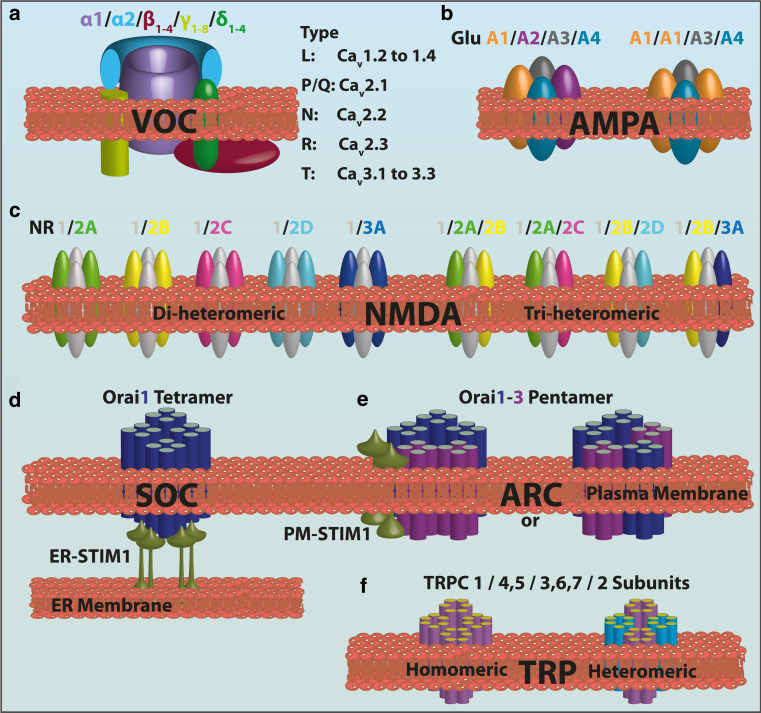
The plasma membrane Ca2+ channels are traditionally divided in three major groups according to their mechanism of opening: the voltage-gated Ca2+ channels (VOC), the receptor-operated Ca2+ channels (ROC), and the store-operated Ca2+ entry channels (SOC) which become activated by the emptying of the cellular Ca2+ stores. The first two channel types have been known about for a long time, but the molecular identity and the mechanism of action of the last channels were only resolved in 2006, when their pore-forming subunit ORAI was cloned [5]. The transient receptor potential (TRP) channels and the arachidonic acid-regulated Ca2+ (ARC) channels, even if not direct components of the SOC, interact with them. The SOC were initially observed in non-excitable cells, but numerous studies have now established their existence also in neuronal cells as well. The main Ca2+ channels types are schematically represented in Fig. 2.
Fig. 2.
Assembly and subtypes of the subunits of the main neuronal Ca2+ channels. a Graphic representation of the VOC complex consisting of the main pore forming α1-subunit plus ancillary β-, γ-, and α2-δ-subunits. Different neuronal α1-subunits correspond to the different types of Ca2+ channels identified in native neurons. b The AMPAR is a ionotropic transmembrane receptor for glutamate assembled from a pool of four subunits (GluA1–A4) which share a high degree of sequence identity. The number of subunits in the functional channels is unclear. The permeability to Ca2+ and other cations, such as sodium (Na+) and potassium (K+), is governed by the GluA2 subunit. An AMPAR lacking a GluA2 subunit will be permeable to Na+, K+, and Ca2+, while the presence of the GluA2 subunit will render the channel impermeable to Ca2+. c The NMDAR is a ionotropic glutamate receptor that allows the flow of Na+, and of smaller amounts of Ca2+, into the cell. Seven NMDAR subunits have been identified: GluNR1, GluNR2A–D, GluNR3A, and GluN3B (not shown). The various populations of di-heteromeric and tri-heteromeric NMDARs that are thought to exist in the central nervous system are shown. d, e The basic structure of ORAI1 and ORAI3 proteins. Each has four transmembrane-spanning domains: the different stoichiometry between SOC and ARCs is shown. The SOC pore is formed by a tetramer of ORAI1 subunits, while a pentameric assembly of three ORAI1 subunits and two ORAI3 subunits arranged in two possible conformations forms the ARC channel. Both SOC and ARC are regulated by STIM1; however, SOC activation is regulated by STIM1 in the endoplasmic reticulum (ER), while the pool of STIM1 residing in the plasma membrane regulates the ARC. f Transient receptor potential (TRP) channels (TRPC) are relatively non-selectively permeable to cations, including Na+, Ca2+, and Mg2+. They belong to a large superfamily of cation channels having six transmembrane-spanning segments, which presumably assemble in a ring-like structure with fourfold symmetry. The TRP protein assembles into homo-tetramers or hetero-tetramers. Here, only the “common” family of these TRPC, with its relative subunits, is shown for simplicity
The VOC transduce the electrical signals occurring at the cell surface membrane due to local rises of intracellular Ca2+. In neurons, VOC play a role in the generation and propagation of the nerve impulse and in cell homeostasis. The VOC are formed of five distinct subunits (α1, α2, β, γ, δ) encoded by different genes. Depending on the type of α1 pore-forming subunit, they are divided into three subfamilies, namely, Cav1, Cav2, and Cav3, and into six further classes, termed L, N, P, Q, R, and T, based on the physiological and pharmacological properties of the type of current they carry (Fig. 2a). Each of these channel types is indeed inhibited by specific toxins that have been used to characterize them. The α1 subunit is a 190- to 250-kDa transmembrane protein organized in four repeat domains (I–IV), each containing six transmembrane segments (S1–S6). The S4 segments comprise some positively charged residues and serve as the voltage sensor in the pore-containing subunit. The associated α2, β, γ, δ subunits have auxiliary functions, including the control of channel expression and the modulation of current kinetics. The β subunit is a hydrophilic protein of 50–65 kDa that binds to the intracellular loop connecting domains I and II of the α1 subunit. The α2 and δ subunits are encoded as pre-polypeptides by a single gene and undergo post-translational cleavage to yield two disulfide-bonded and lipid-anchored distinct subunits. The γ subunit has four transmembrane segments: it is an essential component of skeletal muscle Ca2+ channels, but it may not be included in the assembly of Ca2+ channels in the brain. The β subunits greatly enhance cell-surface expression of the α1 subunits and shift their kinetics and voltage dependence [6, 7]. The α2δ subunits enhance cell-surface expression of α1 subunits [8] and are important for efficiently coupling the entry of Ca2+ to exocytosis at active zones in nerve terminals [9].
The Cav1 subfamily mediates the L-type currents and initiates excitation–contraction coupling in skeletal and cardiac muscle. In neuronal cells, its activity generates Ca2+ transients in cell bodies and dendrites that in turn control processes like secretion and gene expression. Cav2 channels generate N-, P/Q-, and R-type currents and are mainly responsible for the initiation of synaptic transmission, neurotransmitter release, and the generation of dendritic Ca2+ transients. The Cav3 subfamily is responsible for the T-type current and is important for the pacemaking and repetitive firing of action potentials in cardiac myocytes and thalamic neurons [10].
The ROC are activated by the binding of specific ligands, such as neurotransmitters, to their extracellular domain. l-Glutamate is the main excitatory neurotransmitter in the mammalian brain, and it activates two classes of receptors, the ionotropic receptors (iGluRs), and the metabotropic receptors (mGluRs). Alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid-sensitive receptors (AMPARs) and N-methyl-d-aspartate sensitive receptors (NMDARs) are the two principal types of ionotropic glutamate receptors. AMPARs mediate fast excitatory synaptic transmission in the mammalian central nervous system (CNS) and are primarily permeable to Na+ and K+, but may also become permeable to Ca2+. NMDARs are permeable to both Na+, which contributes to postsynaptic depolarization, and Ca2+, which generates Ca2+ transients and determines intracellular physiological responses. NMDARs respond to glutamate more slowly than AMPARs. Their activation requires not only glutamate (ligand-gating), but also membrane depolarization (voltage dependence), which removes internal Mg2+ that normally blocks the channel. Interestingly, the coincidence detection required for the opening of NMDAR channels is particularly important in learning and memory, since the removal of the Mg2+ block of NMDARs is crucial to CREB (cAMP response element-binding protein)-dependent gene expression upon long-term memory induction. [11]. Both AMPARs and NMDARs are tetrameric structures. AMPARs are homo- or heterotetramers assembled from GluA1 to A4 subunits (Fig. 2b). The four subunits can combine in various stoichiometries to form receptor subtypes with distinct channel properties [12]. Almost all (99 %) GluA2s in the adult brain contain a positively charged arginine (R) in the M2 channel-forming segment at position 607 (Q/R site), while the other AMPAR subunits have a glutamine (Q) at this position [13]. This is dependent on a site-selective deamination of adenosine to inosine on the pre-mRNA by RNA-editing enzymes [14]. Edited R-containing subunits remain largely unassembled and are retained in the ER. The presence of a positively charged amino acid (R) in the channel-forming segment effectively blocks Ca2+ entry and results in AMPARs with low conductance. Unedited Q-containing subunits readily tetramerize and traffic to synapses where they generate high conductance channels which are Ca2+ permeable [15]. NMDARs are also assembled as heterotetramers of different subunits in specific brain areas and during development. Seven subunits encoded by different genes have been identified to date (Fig. 2c), and these are grouped into three subfamilies according to sequence homology: the GluN1 subunit, four distinct GluN2 subunits (GluN2A, GluN2B, GluN2C, GluN2D), and two GluN3 subunits (GluN3A and GluN3B). The size of each subunit varies from 900 to 1,480 amino acids due to differences in the length of the intracellular carboxyl-terminal domain, a region that is involved in receptor trafficking and in coupling receptors to signaling cascades. NMDARs typically associate GluN1 subunits with GluN2 subunits or a mixture of GluN2 and GluN3 subunits. The composition of the subunits of NMDARs is plastic, resulting in a large number of receptor subtypes with distinct biophysical, pharmacological, and signaling properties [16]. NMDARs are typically found at extra- and postsynaptic sites. In particular, NMDA-dependent Ca2+ influxes are known to regulate CREB-dependent gene transcription [17–21], which is important for the establishment of long-term synaptic plasticity, learning, and memory [22]. NMDARs have also been found in the presynaptic compartment, but their exact roles there are still being debated, although they may facilitate glutamatergic release by increasing both spontaneous and evoked excitatory postsynaptic currents. Their activation is also necessary for the induction of long-term depression (LTD) at both cerebellar parallel fiber–Purkinje cell synapses and neocortical synapses [23].
The mGluRs are coupled to G proteins and are organized with the canonical seven transmembrane domains. They are encoded by eight genes (mGluR1–8) and exist as homodimers that generate Ca2+ signals through the activation of distinct downstream signaling cascades that activate phospholipase C and activate, or inhibit, adenylyl cyclase. They are expressed in neuronal and glial cells in the brain, spinal cord, and peripheral neurons. mGluR1 is the most abundantly expressed metabotropic receptor in the mammalian CNS, with its highest expression in the Purkinje cells of the cerebellum. It produces two type of neuronal depolarization, a rapid transient depolarization related to the release of Ca2+ from intracellular stores and a prolonged and larger depolarization resulting from the activation of TRP channels (see below).
The purinergic signaling system has many physiological and pathological roles [24–27]. In the CNS, extracellular ATP released by neurons and glial cells (astrocytes) and the diversity of purinergic receptors allow the regulation of several neuronal relevant processes. In addition, different neuronal insults may result in the massive exit of ATP from damaged neural cells, and its consequent increase in the extracellular space may assume cytotoxic effects, ultimately leading to cell death [26]. The ionotropic receptors responding to extracellular ATP to induce membrane depolarization and Ca2+ influx [the so-called ionotropic P2X receptors (P2XRs)] deserve a special mention. Their activation has multiple modulatory effects on synaptic plasticity, either inhibiting or facilitating the long-term changes of synaptic strength depending on the physiological context [28]. ATP released during synaptic transmission activates astrocytic receptors, which in turn initiate Ca2+ signals and propagate Ca2+ waves in the astroglial networks through the activation of metabotropic P2Y receptors (P2YRs) and the diffusion of inositol 1,4,5 tris–phosphate through the gap-junctions [29]. The presence of both P2XRs and P2YRs at the same astrocyte permits a coordinated action of Ca2+ signaling: ionotropic P2XRs are responsible for rapid astrocytic signaling, whereas G protein-coupled P2YRs mediate long-term effects, including trophic responses, through a variety of intracellular pathways, among which gene activation [30, 31].
Ionotropic P2XRs are represented by seven different subtypes/subunits (P2X1–7) that contribute to the generation of heterotrimers or, with the exception of P2X6, homotrimers [32]. These subunits are widely distributed pre-and postsynaptically in different cell types in the brain and spinal cord [33]. They are the main postsynaptic Ca2+ entry channels at resting potential when NMDARs are blocked by Mg2+ [34]. P2XRs display very high Ca2+ permeability, the level of which depends upon the composition of the subunit. Importantly, the permeability of P2X2, P2X4, and P2X7Rs to larger cations increases following repetitive or longer lasting exposure to ATP and (particularly in the case of P2X7Rs) may result in the opening of large pores in the cell membrane and ultimately cell death by either apoptotic or necrotic pathways [35].
The SOC are activated by the release of Ca2+ from the ER. As mentioned, they were initially described in non-excitable cells, but they have now been documented in other cell types, such as neurons and skeletal muscle cells. Store-operated Ca2+ entry (SOCE) was originally proposed as a mechanism for assuring the refilling of intracellular stores following Ca2+ release from these [36]. More recently, the notion has emerged that the influx through this pathway may provide direct Ca2+ signals to targets localized to spatially restricted areas close to the sites of Ca2+ entry, thus initiating specific signaling pathways; for example, hereditary defects in SOCE lead to severe immunodeficiency [37] due to a perturbation of Ca2+ microdomains and to their effect on the generation of Ca2+ oscillations and nuclear factor of activated T-cell-regulated gene transcription [38]. The molecules constituting the SOCE pathway were identified only recently as Ca2+-binding transmembrane proteins of the EF-hand family; they are present in two different isoforms. The transmembrane stromal interaction molecules STIM (STIM 1 and 2 proteins) serve as sensors of Ca2+ within the ER. STIM communicates with the plasma membrane SOC that is composed of ORAI subunits (Fig. 2d). The ORAI protein family includes three isoforms [39], among which ORAI1 is the pore-forming subunit of the channels [5]. Evidence for the presence and function of ORAI/STIM in neurons has come from recent work in which their expression was abrogated by siRNA in Drosophila melanogaster. The abrogation impaired rhythmic firing of the flight motor neurons, resulting in defective flight [40]. By contrast with other tissues, STIM2 is the predominant isoform in mice brain. Cortical neurons isolated from STIM1- and STIM2-deficient mice showed no defects in STIM1-deficient mice, whereas the increase in intracellular Ca2+ following the induction of in vitro ischemia was slower, and neuronal recovery faster, in STIM2-deficient mice, suggesting a neuroprotective effect of STIM2 deficiency, which also correlated with similar effects in vivo [41].
The ARC channels are small conductance, highly Ca2+-selective ion channels [42]. They are widely distributed in different cell types (including neurons) where they provide an alternative, store-independent pathway for agonist-activated Ca2+ entry. Their activation is specifically dependent on low concentrations of arachidonic acid generated on the intracellular side after physiologically relevant activation of the appropriate receptors on the plasma membrane. Although biophysically similar to the SOC channels, ARC channels function as the predominant route of Ca2+ entry during the oscillatory signals generated at low agonist concentrations. Like the SOC, their activation is dependent on STIM1 protein. However, in their case the pool of STIM1 constitutively localized in the plasma membrane, rather than the STIM of the ER membranes, is responsible for the activation. These channels are also formed by ORAI proteins but, at variance with the SOC which are homotetramers of ORAI1 subunits, the pore is formed by a heteropentameric assembly of three ORAI1 subunits and two ORAI3 subunits (Fig. 2e).
The TRP channels are a class of channels that can generate changes in intracellular Ca2+ concentration either directly by acting as a Ca2+ entry pathway (even if their selectivity for Ca2+ changes in the different channels subtypes) or indirectly by causing cell depolarization that triggers the activation of the voltage-dependent ion channels. They are expressed in a large number of tissues and cell types (excitable and non-excitable) and may be activated by phosphatidylinositol phosphates, such as phosphatidylinositol 4,5 bisphosphate (PIP2) [43], and modulated by Ca2+. They are assembled as a tetramer, with each subunit containing six transmembrane domains (Fig. 2f). In mammals, 28 TRP channels have been described to date; these have been classified according to their homology into six different subtypes: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPML (mucolipin), and TRPP (polycistin). The TRPCs have a special relationship with the SOC since ORAI1 has been proposed to interact with TRPCs and to act as regulatory subunit that confers STIM1-mediated store depletion sensitivity to them [44–46]. However, in comparison to SOC, TRPC channels show high Ca2+ selectivity, very small single channel conductance and different Ca2+ modulation.
Finally, it must be mentioned that most of the TRP channels are also localized in the ER and Golgi membranes and that TRPMLs, also defined as TPC (two-pore channels), have been proposed to mediate the NAADP-activated intracellular Ca2+ release from endosomes and lysosomes [47]. This proposal, however, is controversial [48].
Na+/Ca2+ exchanger and plasma membrane Ca2+ ATPase
The plasma membrane Ca2+ ATPase (PMCA) and the plasma membrane Na+/Ca2+ exchanger (NCX) are the two systems responsible for Ca2+ extrusion to the extracellular environment. The NCX has a low Ca2+ affinity but a high capacity for Ca2+ transport, whereas the PMCA has the opposite properties. Thus, traditionally, a housekeeping role in maintaining cytosolic Ca2+ has been attributed to the PMCA pump, and a dynamic role of counteracting large cytosolic Ca2+ variations (especially in excitable cells) to the NCX. However, numerous studies have now provided evidence that the two systems are co-expressed in most cells, including neurons and astrocytes, but also that they may play different signaling role [49, 50].
The Ca2+ ATPase of the sarco-/endoplasmic reticulum (SERCA pump) and the electrophoretic uniporter in the mitochondria (MCU) also play important roles in removing excess cytosolic Ca2+, thereby restoring its basal levels.
NCX accomplishes Ca2+ extrusion by using the electrochemical gradient of Na+: during each cycle three Na+ ions enter the cell and one Ca2+ ion is extruded against its gradient. NCX can also work in the reverse mode: the direction of the movement of the transported ions depends entirely upon the electrochemical gradients of Na+ and Ca2+ and on the number of ions that bind to the molecule and are transported. Under the resting cellular ionic condition and membrane potential, the NCX extrudes Ca2+ from the cytoplasm. However, in the heart, when the plasma membrane becomes depolarized during systole and the Na+ levels rise as a consequence of the opening of the plasma membrane voltage-operated Na+ channels, the exchanger reverses its operation and mediates Ca2+ entry. The influx of Ca2+ through the NCX may play an important regulatory role in the excitation–contraction process as it now influences the gating of voltage-operated Ca+ channels and alters the sarcoplasmic reticulum Ca2+ load. This mechanism has also been proposed to occur in astrocytes and neuronal cells where NCX could regulate the state of Ca2+ refilling of the intracellular stores by acting at the plasma membrane–ER junctions [51]. Three different genes encoding NCX isoforms, NCX1, -2, and -3, have been identified. NCX1 was originally characterized and cloned from the heart, but its expression is widespread, with high levels in the brain and kidney. NCX2 and NCX3 are selectively expressed in different neuronal populations of the brain and in skeletal muscle. All three basic NCX isoforms are essentially co-expressed in brain neurons, thus making it difficult to attribute specific functions to each of them. However, the NCX2 isoform is particularly abundant and widespread, thus suggesting that it plays a key role in neuronal Ca2+ homeostasis. NCX3 also appears to be important to neurons: its specific cleavage during brain ischemia and in primary neurons undergoing excitotoxicity has shown that it plays a critical role in ischemic insults [52, 53].
PMCA pumps belong to the family of P-type ATPases, which are characterized by the temporary conservation of ATP energy in the form of a phosphorylated enzyme intermediate (hence P-type) formed between the γ-phosphate of hydrolyzed ATP and an invariant D-residue in a highly conserved sequence of the pump molecules. These pumps are ubiquitously expressed; however, the four basic isoforms (PMCA1, -2, -3, and -4) are specifically distributed among the different tissues, with isoforms 2 and 3 being particularly abundant in neurons.
The PMCA pump is a classical target of calmodulin (CaM) regulation. CaM interacts with a C-terminal domain of the pump [54], with a dissociation constant (K d) in the nanomolar range [55]. In the absence of CaM, the C-terminal tail of the pump folds over, binding to two sites in the main body of the enzyme and thus keeping it auto-inhibited. CaM binding to the C-terminal domain of the pump removes the C-terminal tail from the binding sites, thereby relieving the auto-inhibition [56, 57]. An important aspect of the regulation of the PMCA pump activity is the stimulation by acidic phospholipids, which decrease the Ca2+ affinity of the pump to values even lower than those achieved with optimal CaM (about 0.2 μM [58]), thus presumably contributing to pump regulation, possibly in a CaM-independent way [59, 60].
PMCA pumps have been traditionally considered to be housekeeping enzymes. However, the possibility that they may also have important signaling roles in specific cell types is now emerging. The first and well-characterized evidence comes from the action of isoform 4 in the heart. Studies on the phenotypes of transgenic mice in which PMCA4 was selectively overexpressed or totally ablated in the heart revealed that PMCA4 contributes to the modulation of heart contractility. Specifically, PMCA4 acts to tether membrane-associated neuronal nitric-oxide synthase (nNOS) to a highly compartmentalized PMCA4-associated microdomain formed by the cardiac cell membrane. Thus, in addition to the obvious role in extracellular Ca2+ transport, the PMCA4 pump may control heart contractility by regulating the activity of nNOS through the control of Ca2+ concentration in a restricted microdomain where it co-localizes with nNOS. PMCA4 overexpression reduces Ca2+ concentration in the proximity of the nNOS enzyme and thus reduces the contractility response to β-adrenergic stimulation [61]. Its ablation, perhaps not surprisingly, does not affect the beat-to-beat Ca2+ transport across the plasma membrane which is controlled mainly by NCX activity, but instead causes a delocalization of the nNOS protein to the cytosol, thus causing a significant decrease in the microdomain cGMP, which leads to a significant elevation in local cAMP levels. This increases L-type Ca2+ channel activity and ryanodine receptor phosphorylation (see below) and hence enhances contractility [62].
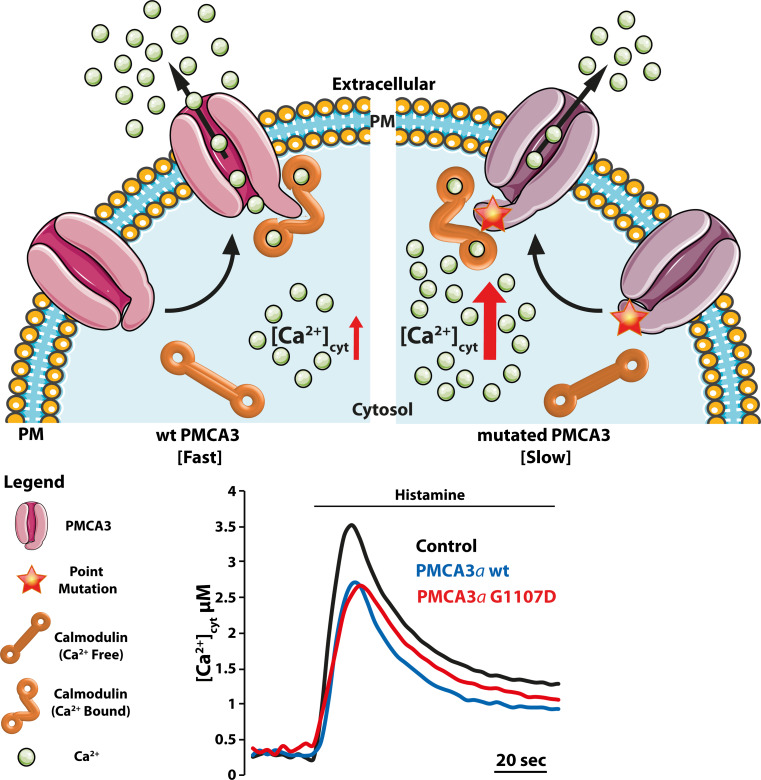
The identification of specific mutations in neuronal PMCA isoforms 2 and 3 and their association with neurodegenerative conditions indicates that they may also have relevance in the regulation of Ca2+ signaling in the brain. The PMCA2 isoform is abundant in the apical (stereociliar) membrane of the inner ear hair cells, and the PMCA3 pump is particularly abundant in the choroid plexus and some other brain regions. Genetic hearing loss associated with PMCA2 mutations has been reported both in mice and humans [63–68], and a point mutation in the PMCA3 pump gene has very recently been associated with X-linked congenital cerebellar ataxia in humans [69]. The study of the activity of recombinant PMCA2 and three mutants at the cellular level has revealed that in both cases the Ca2+ handling ability of the cells containing the mutated pumps was impaired and that the time necessary to restore basal Ca2+ levels after cell stimulation was compromised. The defect is not dramatic in terms of Ca2+ handling, thus leaving the possibility open that the disease phenotype may be linked to signaling defects rather than to general Ca2+ dyshomeostasis.
Endoplasmic reticulum and mitochondria
The release of Ca2+ from the ER occurs in neurons via two types of Ca2+ channels/receptors: ryanodine receptors (RyRs) and inositol-1,4,5-tris-phosphate receptors (InsP3Rs). InsP3Rs are ubiquitously expressed in many cell types, whereas RyRs are more characteristic of neurons and muscle cells. Ca2+ release through InsP3Rs requires the binding of the second messenger InsP3 in response to the activation of various G-protein-coupled receptors on the cell membrane. Increased cytoplasmic Ca2+ concentration is a major trigger for Ca2+ release via RyRs, the phenomenon known as Ca2+-induced Ca2+ release (CICR). RyRs are also regulated by other intraneuronal messengers, such as cyclic adenosine diphosphate ribose (cADP-ribose). They are only relatively selective for Ca2+, which is at variance with the voltage-gated and store-operated plasma membrane Ca2+ channels that are more selective. However, considering that Ca2+ is probably the only cation with an appreciable electrochemical gradient across the ER/SR membrane, the lack of selectivity is not detrimental to the cell.
The InsP3Rs are encoded by three different genes that have distinct patterns of tissue expression (however, some overlapping occurs, especially during differentiation). InsP3R1 is the predominant isoform and is widely expressed in tissues, particularly the brain and smooth muscle. The highest expression level of InsP3R1 among neurons occurs in the Purkinje cells of the cerebellum, where it localizes in the cell body, axons, and dendrites, especially in dendritic spines. InsP3R2 expression is lower than that of InsP3R1: its specific localization in the brain remains elusive, but in cerebellar granule cells it is expressed to the same extent as InsP3R1. InsP3R3 is highly expressed in the brain and gastrointestinal tract. The InsP3 channels consist of homo- or heterotetramers of a large (2,700 residues) protein spanning the membrane with a hydrophobic region containing six helices. Several molecules interact with the InsP3R and modulate its activity. Ca2+ itself has both stimulatory and inhibitory effects [70] depending on its concentration. Cytosolic Ca2+ is a co-agonist of the InsP3Rs, strongly increasing the activity of the latter at concentrations of up to about 300 nM. By contrast, at higher concentrations Ca2+ inhibits the receptor. Luminal Ca2+ also sensitizes the InsP3Rs, possibly by tuning its sensitivity to cytosolic InsP3. A Ca2+-mediated inhibition of the receptor is assumed to contribute to the termination of local cytosolic Ca2+ signals. However, the precise mechanism of the inhibition is not clear [71].
The RyRs are also encoded by three distinct genes with different tissue expression patterns: RyR1 is expressed in skeletal muscles and in the brain with an almost exclusive localization to cerebellar Purkinje cells; RyR2 is the predominant type in the brain (particularly in the cerebellum), and it is also expressed in the heart and in the cerebral cortex. RyR3 is expressed in various brain regions, albeit with low levels of expression, being most predominant in the hippocampus, striatum, and diencephalon. The RyR is also formed by homo-tetramers that associate to form the largest channel known to date (>2 MDa). The C-terminal portion of the protein forms the pore, and the large cytoplasmic region contains the sites where most RyR modulators interact. Excitation–contraction-mediated coupling with the voltage-dependent Ca2+ channel dihydropyridine receptor (DHPR; so defined from the inhibitor dihydropyridine) located in the T-tubules of the plasma membrane of muscle cells represents the major gating mechanism. The molecular gating mechanism differs between skeletal and cardiac muscle [72]. In skeletal muscles, a physical interaction (electromechanical coupling) between the Cav1.1 DHPR and RyR1 is required; in cardiac muscle, Ca2+ release by the RyR2 is initiated by Ca2+ influx via Cav1.2 (i.e., the CICR). cADPR, generated by ADP-ribosyl cyclases, in particular by the ectoenzyme [73], can also gate RyR2 and RyR3. Similar to the InsP3R, the channel activity of the RyR is also modulated by a number of molecules, such as PKA, FK506 binding proteins (FKBP12 and 12.6), CaM, Ca2+/CaM-dependent protein kinase II, calsequestrin, triadin, junctin, Mg2+, ATP, and Ca2+ itself.
Ca2+ reuptake in the ER/SR is mediated by the P-type Ca2+ ATPase SERCA pump which replenishes the ER/SR stores, and thus, together with the PMCA and the NCX, re-establishes resting cytosolic Ca2+ values after the Ca2+ transient induced by cell stimulation. The SERCA pump is encoded by three independent genes which generate primary transcripts that undergo processes of alternative splicing to generate additional isoforms. Since the pump is ubiquitously distributed in tissues and has no distinctive characteristics in neurons, it falls outside the scope of this review and will not be discussed here. Details on its structure and function can be found in a number of comprehensive reviews (e.g. [74]).
Mitochondria are the other major player in the regulation of Ca2+ signals. They participate both in Ca2+ buffering and in the formation of special communications with the ER, that permit them to take advantage of localized Ca2+ release by vicinal ER channels to accumulate Ca2+ into the matrix. Ca2+ regulates the ability of mitochondria to provide ATP to energy-demanding processes, including pumps that mediate its removal from the cytosol. Increases of Ca2+ concentration in the mitochondrial matrix boost the activity of three enzymes of the tricarboxylic acid cycle: pyruvate dehydrogenase (indirectly via its phosphatase activation), isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase (by binding directly to the enzymes). They are thus essential to the delivery of reducing equivalents to the respiratory chain and thus to the production of ATP. However, matrix Ca2+ also directly regulates the F1F0-ATPase [75–77]. In addition to regulating mitochondrial function, mitochondrial Ca2+ handling has an important signaling role. By controlling the Ca2+ microenvironment in the proximity of the ER or plasma membrane Ca2+ channels, mitochondria can positively or negatively control the feedback effect of Ca2+ on the channels [78, 79] with functional consequences on several biological processes.
MCU mediates the entry of Ca2+ into the mitochondrial matrix [80, 81] which is driven by the negative membrane potential generated by the activity of the respiratory chain components of the inner membrane. The MCU is formed by a 40-kDa pore-forming protein which contains two transmembrane domains and possibly assembles as oligomers. Recently, a second MCU isoform, MCUb, has been identified, which has been shown to act as an inhibitory subunit in the formation of MCU hetero-oligomers [82]. MCU associates with several regulators, among which MICU1 is the best characterized. This is an EF-hand protein that acts as MCU gatekeeper by regulating the threshold of MCU channel opening and allowing its cooperative activation [83, 84], Two paralogs of MICU1, namely, MICU2 and MICU3, and EMRE, an essential single transmembrane protein, have been recently identified; it has been suggested that these form a complex. MICU1 and MICU2 stabilize each other, and EMRE is required for the interaction of MCU with both of them [85, 86].
A mitochondrial NCX (NCLX) [87, 88] mediates the efflux of Ca2+ from mitochondria, thus preventing it from reaching thermodynamic equilibrium. Ca2+ efflux can be also mediated by the transient opening of the mitochondrial permeability transition pore (mPTP) [89, 90]. Its recent molecular identification has revealed that it is part of the ATP synthase complex, thus further strengthening the link between mitochondrial Ca2+ and energy balance [91, 92].
Ca2+ binding proteins: Ca2+ buffers and Ca2+ sensors
Several Ca2+ binding proteins are expressed in the nervous system. Their localization and their affinity for Ca2+ differ according to their specific role. Functionally, they can be divided into two groups: the Ca2+ buffers, which together with the Ca2+ clearance mechanisms control the duration and the spread of Ca2+ signals, and the Ca2+ sensors, which also translate the changes in Ca2+ concentration into specific signals. Both Ca2+ buffers and sensor proteins belong to various Ca2+-binding protein subtypes. The EF-hand proteins and C2 domain proteins are the most important. CaM is the best known among them: it has four EF-hand binding motifs, and the molecular mechanism by which it decodes the Ca2+ signal has been clarified. Fluctuations in intracellular Ca2+ concentration not only change its subcellular distribution but also induce different conformational states that result in target-specific activation [93]. Other major cytosolic EF-hand Ca2+ binding proteins are calbindin D-28k (CB-D28k), calretinin (CR), and parvalbumin (PV); all of these undergo conformational changes upon Ca2+ binding to conserved domains that permit the interaction with target proteins in a Ca2+-regulated manner. Proteins such as CaM thus do not act as pure Ca2+ chelators, but they do exert an important modulatory role by regulating the delivery of the Ca2+ signal to different substrates. However, a clear distinction between Ca2+ buffer and Ca2+ sensor function is not simple, since Ca2+ sensor proteins obviously also buffer Ca2+. CaM is perhaps the best example of this dual function, as it is present in the brain at high concentrations (up to 100 μM). It has numerous specific roles in neurons, among them the control of glutamate receptors, of ion channels, and of the NO synthase. One important direct function of CaM is possibly the control of VOCs through binding to channel subunits [94]. The major CaM target proteins are Ca2+/CaM-dependent kinases (CaMKs) and the phosphatase calcineurin; these contribute to a number of neuronal regulatory pathways, from synaptic plasticity to gene transcription.
The spatiotemporal aspects of Ca2+ signals depend on the kinetics of various Ca2+ buffers—in particular, in excitable cells [95–97]. The expression of Ca2+-buffering proteins varies according to cell types, with the highest Ca2+-buffering capacity being that of cerebellar Purkinje cells which express high levels of CB-D28k and PV [98]. The majority of Ca2+ buffers have dissociation constants in the low micromolar range. Thus, in a resting cell they are mostly in the Ca2+-free form. CR is defined as a fast Ca2+ buffer; in contrast, PV and CB-D28k are instead considered to be slow Ca2+ buffers. Several studies in knockout mice for different Ca2+ buffers have revealed that the deletion of one Ca2+ binding protein is not compensated by the overexpression of others, underlining the importance of each protein in the cell type in which it is particularly abundant.
Purkinje, stellate, and basket cells in the cerebellum can be defined as “PV-ergic” neurons: [99] the ablation of PV in these cells is compensated by an increase of mitochondrial mass and by a remodeling of mitochondria distribution that results in the accumulation of mitochondria beneath the plasma membrane. These mitochondria may specifically prevent or restrict the diffusion of Ca2+ signals from the plasma membrane to the center of the cell, thus acting as a “firewall.” CR- or CB-D28k-immunoreactive neurons are instead those of the cortex, hippocampus, and specific peripheral nervous systems. Cerebellar granule cells also express CR, and, as mentioned, Purkinje cells present high amounts of CB-D28k. The loss of CR affects the electron-responsiveness of cerebellar granule cells, and the decrease in Ca2+ buffering results in increased excitability: cerebellar granule cells have faster action potentials and generate repetitive spikes with enhanced frequency [100]. The loss of CB-D28k increases the Ca2+-dependent inactivation of high voltage-gated channels, thus reducing Ca2+ entry during the prolonged action potential trains that occur, for example, during epileptic seizures. The absence of CB-D28k induces alterations in the morphology of the spines in cerebellar cells, possibly accounting for the motor coordination impairment observed in KO mice [101].
Neuronal Ca2+ sensors (NCSs) are a family of EF-hand proteins which have an important role in synaptic function and in neuronal diseases. The NCSs show little sequence homology with CaM (<20 %). In mammals they are encoded by 14 different genes. Their restricted expression in particular classes of neurons and their high Ca2+ sensitivity contribute to their non-redundancy with respect to CaM. Additionally, posttranslational myristoylation or palmitoylation permits their alternative Ca2+-dependent membrane association, thus allowing changes in their subcellular locations and in binding specific targets for regulation. Unlike CaM, not all EF-hands of NCS proteins are functional, and unlike the dumbbell-like structure of CaM that undergoes conformational changes to bind the target, NCSs are compact and globular proteins that do not significantly change conformation upon Ca2+ and substrate binding.
Only two of the NCSs, namely, NCS-1 and calsenilin/DREAM/KchIP-3, will be briefly discussed in this review. NCS-1 is the most widely expressed and characterized NCS. It has a higher Ca2+ affinity than CaM, and therefore it may preferentially interact with some partners when the Ca2+-signals are below the threshold required for CaM activation. NCS-1 plays a role in the regulation of several neuronal activities, such as neurotransmitter release [102–104], voltage-gated Ca+ channel activity [105, 106], short-term synaptic plasticity [107], and the LTD [108]. A study in Drosophila has shown that the fly NCS-1 ortholog frequenin interacts with a VOC [109], equivalent to the mammalian P/Q-like Cav2.1 channel which is regulated by CaM [110]. Interestingly, NCS-1 expression is not confined to a specific neuronal population, and many of its regulated functions are evolutionary conserved. However, the appearance of other NCS members has revealed a more specialized function in specific neurons, suggesting that these proteins may have evolved to carry out separate roles with respect to CaM.
The calsenilin/DREAM/KChIP3 protein is not confined to neuronal cells, even if it is expressed predominantly in the brain. KChIP3 is one of the KChIP proteins that have been found to be associated with voltage-gated K+ channels [111]. KChIP3 is also known as DREAM or calsenilin and has a role both in the regulation of gene transcription [112] and in the binding and processing of presenilin [113], a component of the γ-secretase enzyme involved in amyloid precursor protein (APP) processing and in familial Alzheimer’s disease [114]. Interestingly, the expression of two genes encoding two Ca2+-regulating proteins, i.e., the NCX and one subunit of the L-type channels, is a target of Ca2+-dependent DREAM regulation [115, 116].
Analysis of DREAM knockout transgenic mice or of mice overexpressing a DREAM dominant negative mutant insensitive to Ca2+ regulation has resulted in the identification of additional physiological neuronal roles for KChIP3. There is evidence for an enhancement of learning (contextual fear memory) in the absence of KChIP3 [117, 118] and of an impairment of LTD in knockout mouse studies [119]. The effect of DREAM on LTD was suggested to be due to direct or indirect interaction with NMDAR function [119, 120]. Another important role for KChIP3 in neuronal physiology has been identified in mice expressing the Ca2+-insensitive constitutively active mutant. KChIP3 is involved in the mechanisms of pain sensation by acting on the expression of several genes related to pain, including prodynorphin and brain-derived neurotrophic factor receptor [121].
Why is Ca2+ especially important to neurons?
As mentioned, neurons depend on Ca2+ for the control of processes that are not common to all eukaryotic cells. One of these, the release of neurotransmitter(s), is the neuronal variant of the process of secretion that operates in the release of hormones by other cell types. The molecular mechanism of the process is best understood in neurons. Neurotransmitters convey signals from the axon terminal across the synapse to the next neuron or cell. They are packaged into synaptic vesicles which fuse with the presynaptic portion of the plasma membrane of the terminal, discharging their contents into the synaptic cleft to reach specific receptors on the postsynaptic membrane of the next neuron. Once fusing with the plasma membrane and releasing the neurotransmitter, the membrane is recycled to reform the synaptic vesicles. Ca2+ has a dual role in the process of neurotransmitter release: it penetrates into the axonal terminal as a result of the depolarization of its plasma membrane that opens VOC. Once in the terminal, it promotes the fusion of the synaptic vesicles with the presynaptic plasma membrane, in a process that involves a number of proteins of the large SNARE superfamily (see [122] for a comprehensive coverage of the topic). The Ca2+ sensor in the membrane of the synaptic vesicle is synaptotagmin, a protein that contains two cytoplasmic C2 Ca2+-binding domains: upon the binding of Ca2+ to these domains, synaptotagmin docks to the presynaptic membrane via interaction with the SNARE proteins synaptobrevin and SNAP 25, eventually discharging the content of the synaptic vesicle into the synaptic cleft.
LTP and LTD also are neuron-specific processes that make use of Ca2+ signals. LTP defines the long-lasting increase in synaptic transmission following high-frequency stimulation. Synapses have the ability to modify their strength, i.e., they display plasticity. Since learning and memory are generally related to modifications of the synaptic strength, LTP is widely assumed to be involved in these processes. LTP was originally discovered in the hippocampus, subsequently to be also found in a number of other neuronal types. The liberation of glutamate by the presynaptic terminal triggers its release. This neurotransmitter interacts first with postsynaptic AMPARs, inducing the penetration of Na+ into the postsynaptic neuron. Its depolarization removes the Mg2+ block of the postsynaptic NMDA receptors, permitting the influx of Ca2+ into the neuron and the activation of a number of kinases and other enzymes, such as the NO synthase. The role of CaM kinase II in the molecular mechanism of memory has been repeatedly discussed [123], and the activity of (nuclear) CaM kinase IV appears to be important for memory consolidation.
LTD is the opposite of LTP. It defines the persistent weakening of synaptic strength that, depending on the brain areas (e.g., cerebellum or hippocampus), may result from strong synaptic stimulation or from protracted weak stimulation. As for LTP, the neurotransmitter most commonly involved in LTD is glutamate. LTD appears to be linked to the reduced density of the postsynaptic AMPARs and NMDARs. It cooperates with LTP so that the ability to fine-tune their opposite functions will reflect synaptic plasticity.
One on-going problem in the general area of Ca2+ signaling is that of the transfer of Ca2+ from the cytosol to the nucleus: or, more specifically, of the transfer of Ca2+ signals from the plasma membrane to the nucleoplasm. The large pores of the nuclear envelope would logically suggest that cytoplasmic and nuclear Ca2+ are in continuous passive equilibrium. Yet a number of indications suggest that some form of gating may operate in the transmission of Ca2+ signals through the nuclear envelope. These indications are particularly convincing in neurons [124]. Ca2+ signals initiated by synaptic activity specifically propagate to the nucleus to switch on genetic programs: they are perhaps the most important path in the communication between the synapse and the nucleus. In the nucleus, Ca2+ targets the transcription factor CREB, but also the CREB binding protein (CBP), which is critical to the transcriptional activity of CREB. CBP also interacts with other transcription factors, thus conferring Ca2+ control to a number of target genes. Nuclear Ca2+ also targets the multifunctional transcriptional repressor DREAM (see above), causing its dissociation from DRE sites in DNA and reactivating the transcription of a number of genes. Finally, the path of Ca2+ transfer from the plasma membrane to the nucleus may trigger functionally different processes: Ca2+ coming specifically from extrasynaptic NMDARs may modulate death processes in hippocampal neurons by promoting the translocation of the Forkhead transcription factor FoxO3a into the nucleus [125]. This neuronal death program is counteracted by the activation of nuclear CaMKIV triggered by synaptic activity.
Dysregulation of Ca2+ signaling in the brain and neuronal degeneration
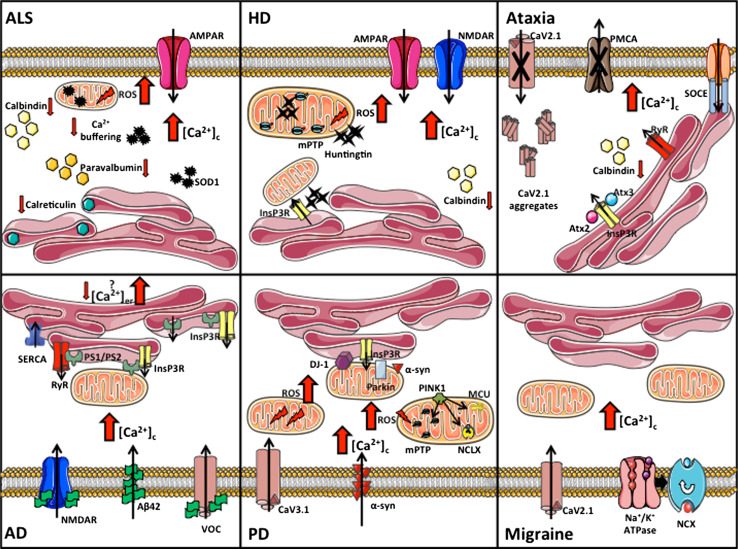
Considering the special importance of Ca2+ signaling to neuronal function, the involvement of its defects in neuronal diseases is hardly surprising. The causative role of the malfunction of Ca2+ regulation is now being increasingly documented in a vast array of important neuronal pathologies, particularly, but not exclusively, those characterized by neurodegenerative processes. A number of comprehensive reviews on the topic have appeared [126–129]: here, we will succinctly discuss the most important pathologies. Table 1 and the cartoon in Fig. 3 summarize the main pathways involved in the generation of the Ca2+ dyshomeostasis that eventually give rise the neuronal pathologies.
Table 1.
Summary of the information presented in this review
| Disease/pathology | Type of neuron/brain area principally affected by the specific pathology | Protein/organellea | Location | Effect on Ca2+ homeostasis or putative affected Ca2+ pathway in the pathologyb |
|---|---|---|---|---|
| Amyotrophic lateral sclerosis (ALS) | Upper and lower motor neurons of the motor cortex, brain stem and spinal cord | AMPAR | Plasma membrane | Excitotoxicity |
| GluR2 subunit (AMPAR) | Plasma membrane | |||
| Calbindin D-28k | Cytosol | Decreased Ca2+-buffering capacity | ||
| Paravalbumin | Cytosol | |||
| Calreticulin | ER | |||
| Mitochondria | ||||
| Huntington’s disease (HD) | Spiny neurons of striatum | Calbindin D-28k | Cytosol | Decreased Ca2+-buffering capacity |
| Calretinin | Cytosol | |||
| NMDAR | Plasma membrane | Excitotoxicity | ||
| Postsynaptic density 95 | Plasma membrane | Loss of NMDAR/AMPAR inhibition | ||
| Mitochondria | Ca2+ handling defects | |||
| ER | Increased release of Ca2+ | |||
| Cerebellar ataxia | Purkinje neurons of cerebellum. The spinal cord can also be affected | Calbindin D-28k | Cytosol | Decreased Ca2+-buffering capacity |
| Cav2.1 (SCA6) | Plasma membrane | Accumulation of mutant channel | ||
| Cav2.1 (EA2) | Plasma membrane | Loss of channel function | ||
| InsP3R (SCA2/3) | Endoplasmic reticulum | Increased InsP3 sensitivity | ||
| PMCA2 | Plasma membrane | Loss of function (ataxic phenotype) | ||
| PMCA3 (X-linked ataxia) | Plasma membrane | Loss of function | ||
| Alzheimer’s disease (AD) | Cortical neurons | CALHM1 | Plasma membrane | Regulation of amyloid metabolism |
| NMDAR | Plasma membrane | Aβ42-PP mediated increased Ca2+ entry | ||
| Aβ42 | Extracellular space | Increased membrane Ca2+ permeability | ||
| N-type Ca2+ channels | Plasma membrane | Aβ42 mediated increased Ca2+ entry | ||
| PS | ER | Altered ER Ca2+ level | ||
| InsP3R | ER | Postsynaptic-mediated altered activation | ||
| RyR | ER | |||
| SERCA | ER | |||
| PS/Aβ42 | Altered ER–mitochondria Ca2+ transfer | |||
| Parkinson’s disease (PD) | Dopaminergic neurons of the substantia nigra pars compacta | α-Synuclein | Cytosol/mitochondria | Modulation of ER–mitochondria Ca2+ transfer/increased membrane Ca2+ permeability |
| Parkin | Cytosol/mitochondria | Mitophagy/modulation of ER–mitochondria Ca2+ transfer | ||
| DJ-1 | Cytosol/mitochondria | Oxidative stress defense/modulation of ER–mitochondria Ca2+ transfer | ||
| Cav1.3 | Plasma membrane | Increased Ca2+-induced oxidative stress in SNPC neurons | ||
| PINK1 | Mitochondria | Mitochondrial dynamics/mitophagy/Ca2+ modulation (MCU, Na+/Ca2+ exchanger, mPTP) | ||
| Familial hemiplegic migraine (FHM) | Cortical neurons | Cav2.1 (FHM1) | Plasma membrane | Increased open probability |
| ATP1A2 (FHM2) | Plasma membrane | Glutamate clearance/regulation of Na+/Ca2+ exchanger |
ER Endoplasmic reticulum, AMPAR 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, NMDAR N-methyl-d-aspartate receptor; for other abbreviations, see text
aProteins or organelles involved in the process of Ca2+ homeostasis and in the pathogenesis of the specified disease/pathology. For the proteins (mostly, but not exclusively, Ca2+ channels or pumps) the principal location within the cells is indicated
bPossible effects on Ca2+ signaling described by the papers quoted in this review
Fig. 3.
Perturbed Ca2+ homeostasis in neuronal disorders. The cartoon summarizes the main pathways involved in the Ca2+ dyshomeostasis processes linked to the neuronal pathologies described in the text. ALS Amyotrophic lateral sclerosis, HD Huntington’s disease, AD Alzheimer’s disease, PD Parkinson’s disease, ROS reactive oxygen species, PS presenilin, SOD superoxide dismutase, mPTP mitochondrial permeability transition pore, ATX ataxin, PINK1 PTEN-induced kinase, α-syn alpha-synuclein
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS; also called Lou Gehrig’s disease from the name of a prominent USA baseball player who died of it) is a neurodegenerative disorder caused by the progressive and selective loss of motor neurons in the spinal cord and the brain that leads to paralysis of the voluntary muscles and eventual death. The disease typically affects men and women aged 50–60 years: the majority of cases are sporadic, but about 10 % are inherited. The clinical and pathological phenotype is the same in the sporadic and the inherited ALS: at the molecular level, mutations in the Zn–Cu superoxidase dismutase (SOD1) (more than 100 have been described) are present in about 20 % of the familial ALS cases [130], whereas autoimmunity and excitotoxicity processes are suggested to be causative of sporadic ALS. SOD1 is an ubiquitous enzyme that protects cells against oxidative stress. Interestingly, the mutations do not cause a defect of SOD1, but rather its gain-of-function. The mechanism by which its mutations induce the ALS phenotype has not yet been elucidated, but it is assumed to involve a number of phenomena including mitochondrial dysfunction, excess production of radical species, protein misfolding, and glutamate excitotoxicity. It has been suggested that the vulnerability of motor neurons in ALS may be linked to their selective susceptibility to excitotoxicity mediated by glutamate AMPARs [131]. The perturbation of cellular Ca2+ homeostasis is now increasingly considered to be important in the pathogenesis of ALS [132, 133]: it may involve malfunctions of membrane channels and/or of the mitochondrial Ca2+ controlling systems, as well as alterations in the Ca2+-buffering capacity of proteins. The expression of CB-D28k and of PV is significantly downregulated in the motor neurons that are lost early in ALS (e.g., spinal and cranial motor neurons), while it is normal in those that are damaged only late in the disease process [134]. A dramatic reduction in the level of CB-D28k has also been detected in several brain domains of ALS patients [135]. The importance of Ca2+-buffering proteins is also underlined by studies on animal models of ALS. The loss of motor neurons was found to be significantly lower in PV transgenic mice interbred with mutant SOD1 transgenic mice than in mutant SOD1 transgenic mice [136]. Expression of the ER Ca2+-buffering protein calreticulin has also been found to be decreased in neuronal models of ALS [137].
Mitochondrial alterations, both functional and ultrastructural, have been frequently described in both ALS patients and in mice models of ALS [138]. Decreased mitochondrial Ca2+-buffering capacity has been observed in the brain and spinal cord of mutant SOD1 transgenic mice [139], and the transfection of cultured neurons with mutant SOD1 has been found to disrupt the ability of mitochondria to buffer Ca2+ [140]. SOD1 is predominantly cytosolic, but it also localizes to mitochondria, and the intramitochondrial localization of mutant SOD1 has been observed to be correlated with damaged mitochondrial function [141, 142]. Defects in complex IV of the respiratory chain have been consistently observed [142–145], but mutant SOD 1 could also damage mitochondria from outside. Various aspects of mitochondrial bioenergetics can be affected in ALS, but Ca2+ handling has a special place, as its malfunction has a direct role on cytosolic Ca2+ overload and cell death. Defects of mitochondrial Ca2+ uptake have been observed in mitochondria isolated from the brain and spinal cord of ALS mice, but not from unaffected liver [139], in organotypic brainstem slices [146], or in cultured neurons [147].
Intracellular Ca2+ increases in motor neurons are mediated by VOC and ionotropic receptors. Hyperactivation of glutamate receptors is a leading cause of the overload of Ca2+ in ALS motor neurons [148]. Motor neurons are particularly vulnerable to the excitotoxity of AMPARs [149]: the defective editing of the GluR2 subunit specifically increases Ca2+ influx through the receptors in susceptible motor neurons, e.g., in the spinal cord of ALS patients [150]. The AMPA receptor-mediated excitotoxicity may be exacerbated by the lower cytosolic Ca2+ clearance that has been observed in mutant SOD1 motor neurons [151].
Huntington’s disease
Huntington’s disease (HD) is a genetic, autosomal-dominant neurodegenerative disorder that terminates with death within 15–20 years after its onset. Its phenotype is causally related to the loss of striatal GABAergic neurons [the medium spiny neurons which produce gamma-aminobutyric-acid (GABA)] that control brain development, movements, and psychiatric functions such as memory. Its symptoms thus include neuropsychiatric defects, such as chorea, motility impairments, and dementia. HD is one of the nine polyglutamine diseases caused by the expansion of CAG repeats encoding a polyglutamine tract in the genes of different proteins. In HD, the polyQ expansion occurs at the N-terminal portion of huntingtin (Htt), a ubiquitous protein that normally contains up to 35 Qs in the N-terminal portion: in HD, Htt may number more than 100 Qs, with their number being inversely correlated with the age of disease onset and its severity. The function of Htt is not fully understood, but one suggestion is that it acts as a scaffold in the control of numerous cellular processes, including gene transcription, vesicular and organellar trafficking, mitochondrial functions (including the handling of Ca2+ and the expression of respiratory chain components), the production/scavenging balance of reactive oxygen species, the balance of Ca2+ in the ER, and apoptosis [152]. The polyQ expansion in mutant Htt renders it susceptible to proteolytic cleavage, which appears to be executed by caspase 6 [153], producing N-terminal fragments that accumulate in the neuron as toxic mono/oligomers that could translocate to the nucleus to impair transcription and/or grow to aggregates that sequester important components such as transcription factors and CaM [154–156]. It has also been claimed that the toxic fragments associate with organelles, for example, the mitochondria, altering their function. The cellular/molecular etiology of the disease still has numerous unclear aspects, including the reason for the late onset even if the genetic defect is present at birth and the trigger leading neurons to die only after decades of coexistence with the anomalous protein.
Ca2+ dyshomeostasis as a causative factor in the cellular phenotype of HD is supported by several lines of evidence [157–159]. It may involve diverse mechanisms, including alterations of buffering capacity by Ca2+ binding proteins, malfunction of Ca2+ channels, particularly those involved in glutamate excitotoxicity, and disruption of the mitochondrial Ca2+ handling system. A specific loss of neurons containing CB-D28k has been observed in the brains of HD patients [160], which is in line with the decrease of Ca2+-buffering capacity in the specific brain areas that house these neurons. It has also been shown that CR interacts with Htt, with a slight preference for the protein that contains an expanded polyQ tract [161]. The overexpression of CR reduces the cytotoxicity caused by the mutant Htt in both neuronal and non-neuronal cells, whereas the downregulation of CR increases neuronal death caused by the mutant protein.
In terms of excitotoxicity, ample evidence has documented the excessive activation of glutamate-gated Ca2+ channels and its detrimental effects on neurons of HD patients, including the early loss of striatal neurons that express high levels of NMDARs [157] and the promotion of glutamate excitotoxicity by mutant Htt [162]. The latter disturbs the interaction of wild-type Htt with the postsynaptic density 95, which normally inhibits the activity of NMDARs and AMPARs.
Mitochondrial dysfunction is perhaps the most important factor in cellular HD etiology. As mentioned above, components of the respiratory chain, most notably complex II, are downregulated in cellular models of HD and in the brains of HD patients [163]. The HD phenotype has actually been reproduced in mice, and in cellular models, by the complex II inhibitor 3-nitropropionic acid [164]. The decreased efficiency of the respiratory chain is naturally expected to affect energy-linked mitochondrial reactions, including, and most importantly, Ca2+ uptake, and thus the ability of the organelles to control Ca2+ homeostasis in the cytosol. Defects in the handling of Ca2+ by mitochondria have indeed been repeatedly observed in cellular models of HD [159, 165–167] and in isolated mitochondria exposed to polyglutamine tracts. The permeability transition pore, which permits the exit of pro-apoptotic factors from mitochondria, appears to open at a lower Ca2+ concentration threshold in isolated mitochondria [167] and in mitochondria in HD model cells [159]. The mitochondrial Ca2+ dysfunction has been attributed to the direct interaction of mutant Htt with mitochondria [165], but could also be mediated by the Htt-promoted release of Ca2+ from neighboring ER [168], which would expose mitochondria to abnormally high, and thus harmful, concentrations of Ca2+.
Alzheimer’s disease
Alzheimer’s disease (AD) is a devastating neurological disorder characterized by the progressive decline of cognitive function and increased neuronal cell death. At the morphological level, the accumulation of abnormal fibers in neuronal bodies and the presence of senile plaques in their extracellular space represent the main hallmarks. These structures are formed by a hyperphosphorylated form of the microtubular protein tau and by peptide β-amyloid (Aβ) (in its most frequent forms, Aβ40 and Aβ42). Aβ40 and Aβ42 derive from the transmembrane protein amyloid precursor protein (APP) that is alternatively processed by α, β, and γ secretases. Although the majority of AD cases are sporadic, mutations in the genes encoding for APP and for presenilin-1 and -2 (PS1 and PS2), two proteins belonging to the γ-secretase enzymatic complex, have been linked to the autosomal-dominant familial form of AD (FAD). Since the majority of FAD mutations are associated with enhanced fibrillization of the amyloidogenic Aβ42 fragment, an increase in amyloid metabolism is generally considered to be the major mechanism in the pathogenesis of AD. However, the finding that the Ca2+ homeostasis modulator 1 (CALHM1), a voltage-gated ion channel which promotes Ca2+ entry from the extracellular ambient, may regulate amyloid metabolism [169], as well as the advances in the functional characterization of presenilins have led to the “calcium hypothesis” of AD [170–172]. A number of studies on cell or animal models of FAD have suggested that a dysregulation of Ca2+ release from the ER could be involved in the pathogenic mechanism of AD. However, the molecular details and the effectors of this dysregulation are still controversial. The different aspects of the Ca2+ hypothesis can be summarized as follows: Ca2+ concentration measurements in cortical neurons of 3xTg-AD animals [173] (a triple transgenic model for AD, having a homozygous mutation for PS1 and homozygous APPSwe and tauP301L transgenes) and in the spines or dendrites of cortical neurons located close to amyloid deposits [174] have revealed higher basal Ca2+ levels than in corresponding non-pathological neurons. Alternative mechanisms for the generation of the neuronal Ca2+ overload in AD neurons have been proposed. Some have focused on the Ca2+ entry pathway showing that Aβ42 oligomers, by binding to the cellular prion protein that may function as the receptor [175], may enhance Ca2+ entry through the ROC (e.g., the NMDAR [176]), increase the Ca2+ permeability of the membrane [177, 178], or increase the sensitivity of the VOC [179]. Other proposals have focused on the release of Ca2+ from internal stores, suggesting that presenilin (PS) may directly regulate the Ca2+ leakiness of the ER membrane by forming a novel Ca2+ permeable channel [180] or by interfering with the activity of the resident ER Ca2+ InsP3Rs and RyRs channels [181, 182]. These channels could in turn even interfere with the activity of the SERCA pump [183, 184] and with the ER–mitochondria relationship [185]. PS mutants have been shown to alter both the expression and the sensitivity of the ER Ca2+ release channels, i.e., RyRs and InsP3Rs, in different AD cell models [186–189] and in neurons from transgenic AD mice [190]. Several FAD-causing PS mutants indeed enhance the response of InsP3Rs to saturating and suboptimal levels of InsP3 [182, 191]. Similarly, neurons from 3xTg-AD mice have been shown to display enhanced caffeine-induced Ca2+ release from the RyRs [192] and to undergo changes in their expression (especially of the RyR3 isoform) [193]. In the latter study, RyR-mediated Ca2+ upregulation within synaptic compartments was associated with altered synaptic homeostasis and network depression at early (presymptomatic) AD stages [193]. RyRs have recently been proposed as targets to prevent disease progression: short-term sub-chronic treatment of AD mouse models with the RyR inhibitor dantrolene fully normalized ER Ca2+ signaling within somatic and dendritic compartments in hippocampal slices from mice with early and later stages of AD. In addition, the elevated RyR2 levels and the altered synaptic transmission and plasticity observed in the AD mice were restored to control levels by dantrolene. Aβ deposition within the cortex and hippocampus has also been found to be reduced by the drug [194].
As mentioned, it has also been proposed that PS can form Ca2+ permeable leak channels on the ER membrane and that FAD-linked mutations could impair the Ca2+ permeability of the ER [180]. This proposal is still controversial, as other data have instead shown that some FAD-linked PS2 mutations decrease the ER Ca2+ content rather than increase it [195–197]. An increase in ER Ca2+ leakage was observed in PS1- and PS2-silenced fibroblasts [184] and in different cell models expressing mutant PS2. The effect has been attributed to increased Ca2+ leakiness through InsP3 and RyR channels and also to the reduction of SERCA pumping activity by both wild-type and mutant PS [184]. Interestingly, it has recently been shown that nanomolar concentrations of the amyloid β-peptide upregulate the InsP3R and the VDAC and increase the number of ER–mitochondria contact points and the mitochondrial Ca2+ concentration. This suggests a role of ER–mitochondria contacts and cross-talk in AD pathology [185, 198], thus adding further complexity to the Ca2+ hypothesis of AD.
Parkinson’s disease
Parkinson’s disease (PD) is a progressive neurodegenerative condition clinically characterized by motor impairment involving resting tremor, bradykinesia, and rigidity. Motor deficits are due to the specific loss of dopaminergic neurons in the substantia nigra pars compacta (SNc). In the large majority of cases, these neurons also display typical cytoplasmic protein inclusions called Lewy bodies [199]. The oral administration of the dopamine precursor l-3,4 dihyroxyphenylalanine (l-DOPA) ameliorates the symptoms, at least in the early stages of the disease, but elevated cytosolic levels of dopamine (DA) and its metabolite are neurotoxic. Many factors are involved in the etiopathogenesis of the disease, age being the greatest risk factor [200], but environmental and genetic factors are also determinants [201]. In particular, exposure to pesticides and substances that are toxic to mitochondria, as well as mutations in several genes which encode proteins related to mitochondrial function, have been causally linked to PD. At the cellular level, PD is widely associated with defects in the respiratory chain complex I [202]. More recently, an important role has been attributed to the dysfunction of the mitochondrial quality control system [203]. This aspect will not be discussed in this review, but a number of recent reviews cover this topic extensively [204–206].
The possible involvement of Ca2+ in PD originates from the observation that the expression of the Ca2+-buffering protein CB-D28k is negatively correlated with neuronal vulnerability in PD [207, 208] and from the observation that the cytoplasmic Ca2+ concentration was higher in SNc dopaminergic neurons (which express Cav1.3 L-type Ca2+ channels that guarantee their autonomous pacemaking activity [209, 210] ) than in the neighboring dopaminergic neurons in the ventral tegmental area (VTA). Higher Ca2+ levels have been shown to correlate with a two- to threefold increase in cytosolic levels of DA in SNc neurons with respect to VTA neurons, thus explaining their greater susceptibility to l-DOPA-induced neurotoxicity [210].
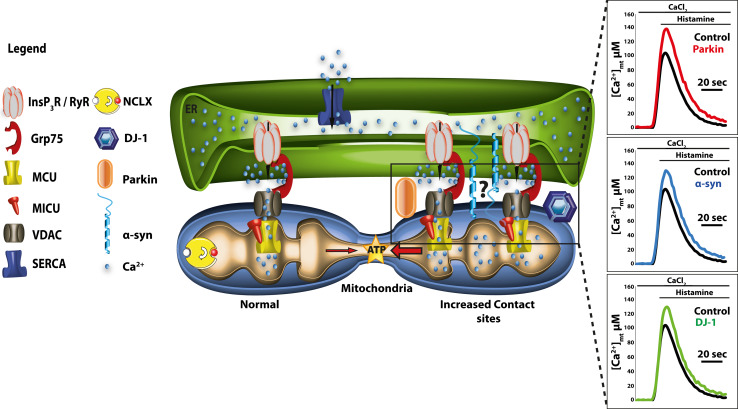
Recently, increased Ca2+ entry related to spontaneous pacemaking activity has been shown to contribute to the PD risk by increasing mitochondrial oxidative stress. The development of transgenic mice expressing the redox-sensitive variant of the green fluorescent protein with a mitochondrial-matrix targeting sequence under the control of the tyrosine hydroxylase promoter has enabled quantitative monitoring of the oxidation of mitochondrial matrix proteins, resulting in the finding that Ca2+ entry through the L-type channels is normally counteracted by a mild transient mitochondrial depolarization that parallels cytosolic Ca2+ oscillations. When mitochondrial function is compromised, i.e., by exposure to mitochondrial toxins or by mutations in PD-related genes, this protective role may be affected, selectively compromising SNc neurons [211, 212]. Other studies on model cells that overexpress familial PD-related proteins or which have been silenced for their expression have indicated the possibility that they may be involved in the modulation of Ca2+ signals. Our laboratory has recently shown that α-synuclein, parkin, and DJ-1 overexpression enhanced ER-mitochondrial Ca2+ transfer by increasing the tethering between the two organelles and that this privileged communication was required to guarantee mitochondrial physiology and morphology (Fig. 4) [213–215]. Other groups have shown the involvement of α-synuclein in the modulation of the plasma membrane Ca2+ entry pathway, suggesting that its aberrant expression, or the presence of aggregate forms, may enhance Ca2+ influx from the extracellular ambient, thus making the cells more susceptible to Ca2+ overload [216–218]. Finally, intriguing data supporting a role for the Ca2+/mitochondria connection in PD pathogenesis have been obtained in cell models in which the expression of the mitochondrial serine/threonine kinase PINK1 was silenced. Further investigations on this aspect are necessary, as the data currently available are still controversial. However, even if a disturbance in mitochondrial Ca2+ handling is involved, it is still not known whether it is due to the altered activity of the mitochondrial Ca2+ uniporter [219], of the mitochondrial Na/Ca2+ exchanger [220], or of the mPTP [221]. It could also be causatively linked to the general impairment of the mitochondrial respiratory chain [222].
Fig. 4.
Three Parkinson’s disease (PD)-related proteins, namely, α-synuclein (α-syn), parkin, and DJ-1, control mitochondrial Ca2+ uptake by modulating ER–mitochondria contact sites. Mitochondria accumulate Ca2+ into the matrix via the electrophoretic MCU using the electrochemical gradient generated by the respiratory chain. The VDAC controls Ca2+ diffusion through the outer mitochondrial membrane, facilitating Ca2+ entry into mitochondria by coupling with the InsP3R through the chaperone protein Grp75. The NCLX mediates Ca2+ extrusion from the mitochondria, thus preventing the attainment of the thermodynamic equilibrium. ER–mitochondria tethering has recently emerged as a key element in cell Ca2+ metabolism and mitochondrial physiology. Our recent findings provide evidence for a role of α-syn, parkin and DJ-1 in favoring ER–mitochondria Ca2+ transfer by enhancing the ER–mitochondria contact sites [213–215]. The traces (right) refer to mitochondrial Ca2+ transients generated by the stimulation of HeLa cells overexpressing parkin, α-syn, or DJ-1 with an InsP3-linked agonist (histamine). The peak amplitude of the mitochondrial Ca2+ transients was higher in overexpressing cells than in control cells
Ataxias
Ataxias are a large group of neurological disorders (more than 40 in the most recent classifications) characterized by the inability to coordinate muscle activity during voluntary movement. They are most frequently caused by disorders of the cerebellum or the posterior column of the spinal cord, with Purkinje neurons in the cerebellum being predominantly affected. Ataxias may be inherited, the genetic defect being either recessive or dominant, or caused by non-genetic factors/circumstances, such as injuries, surgery, exposure to toxicants, infections, and tumors. Some forms of ataxias, such as spinocerebellar ataxia 2 and 3 (also known as Machado–Joseph disease), belong to the group of polyglutamine diseases mentioned above, i.e., their origin can be traced back to the abnormal expansion of the polyQ tract in the cytosolic proteins ataxin2 in SCA2 (Atx2) and ataxin3 in SCA3 (Atx3), respectively. Ca2+ homeostasis dysregulation is common in ataxic neurons: here the most interesting cases will be discussed, as a comprehensive coverage of this topic will not be possible within the scope of this review.
As in the case of other neurodegenerative disorders, dysregulation of Ca2+ homeostasis in ataxias may be causatively related to deficient Ca2+ buffering by Ca2+-binding proteins. Purkinje neurons are known to contain high amounts of CB-D28k, which acts as a fast, high-affinity Ca2+ buffer that helps to control the synaptically evoked Ca2+ transients. Disruption of the calbindin gene in mice has been found to result in impaired motor coordination and to alter the fast—but not the slow—component of the postsynaptic Ca2+ transient induced in the Purkinje dendritic tree by stimulation of the climbing fibers in cerebellar slices [223]. Other studies in cultured cells have shown reductions in the peak amplitude of slower Ca2+ transients with increases in the level of calbindin in cultured cells [224, 225].
Several forms of ataxia are caused by mutations in Ca2+ channel genes and thus qualify as Ca2+ channelopathies. Among these, spinocerebellar ataxia 6 (SCA6) is both a Ca2+ channelopathy and a polyQ disease, as an expansion of the CAG repeat tract occurs in the gene encoding the Cav2.1 subunit of the P/Q voltage-sensitive Ca2+ channels which are abundantly expressed in Purkinje neurons, where they mediate up to 90 % of their high-voltage activated Ca2+ influx. Since the electrophysiological properties of the P/Q channels are not altered by the SCA6 mutation, the pathogenesis of SCA6 has been suggested to involve the toxic accumulation of the mutant channel subunit [226]. The gene of the Cav2.1 subunit of the P/Q channel is also involved in episodic ataxia type 2 (EA2): a large number of mutations cause truncation of the subunit, causing in this case loss of P/Q channel function.
The abnormal polyQ tract in Atx2 and Atx3 in SCA2 and SCA3 instead interferes with the interaction of the two proteins with isoform 1 of the InsP3R. The interaction of the mutant proteins (but not that of the wild-type proteins) with the receptor increases its sensitivity to InsP3 in lipid bilayer experiments [227, 228], favoring the release of Ca2+ from the reticular store, and at the same time promotes the influx of Ca2+ into the neuron through the SOC. To validate the physiological significance of the findings, the authors of these studies fed transgenic mice carrying abnormal Atx2 and Atx3 polyQ tracts with dantrolene (the inhibitor of the RyR which is activated by the release of Ca2+ from the ER and by Ca2+ entering through the SOC, see above) and found age-dependent alleviation of the ataxic symptoms.
Recent findings have revealed that yet another regulator of cellular Ca2+ homeostasis, the PMCA pump, is involved in the genesis of ataxias. As mentioned above, PMCA2 and PMCA3 isoforms are predominantly expressed in neurons. Mutations in the PMCA2 gene were originally described as causing hereditary deafness and vestibular abnormalities [229]. The special functional properties of the PMCA2 isoform, which is specifically able to efficiently extrude Ca2+ in the absence of the natural activator CaM, are evidently optimally suited to maintain the delicate Ca2+ balance requirements between the outer hair cells of the Corti organ and the endolymph [74]. However, PMCA2 is also the dominant pump isoform of the cerebellum and is very abundant in the soma, dendrites, and spines of Purkinje cells. In keeping with the role of Purkinje neurons in motor coordination, the genetic deletion of the pump is associated with an overt ataxic phenotype in mice [230]. The Purkinje neurons of PMCA2 knockout mice are morphologically damaged, with their dendritic tree stunted and disordered. The recovery time of their dendritic Ca2+ transients is greatly increased [230, 231], and their overall physiology is disturbed (e.g., hyperpolarized membrane potentials, prolonged Ca2+-dependent outward K+ current, abnormal action potential firing). Somehow, even in the presence of the severe disturbance of Ca2+ homeostasis, Purkinje neurons in the PMCA2 knockout mice nevertheless manage to survive. The compartmentalization of Ca2+ homeostasis mechanisms between Purkinje dendrites and soma may possibly play a role [232], and other Ca2+ controlling mechanisms could compensate for the lack of PMCA2 function [233, 234]. The PMCA3 pump, which is also expressed in Purkinje neurons [235], could, for example, provide a plausible compensatory mechanism, but it does not appear to be overexpressed in PMCA2 knockout Purkinje neurons [231]. The PMCA3 pump is not as well-known as the other isoforms of the PMCA family, and its role in Ca2+ homeostasis in neurons is not yet understood. It is thus of particular interest that genetic screening has recently identified a mutation in PMCA3 in a family with X-linked cerebellar ataxia [69]. The mutation replaces a Gly with an Asp in the C-terminal CaM binding domain of the pump. The speed at which the mutated pump, overexpressed in model cells, clears the Ca2+ transients induced by the appropriate agonists is much slower than that of the non-mutated pump, thus creating a situation of cytosolic Ca2+ overload. The molecular defect is comparatively modest with respect to the severe ataxic phenotype of the affected patient: work now in progress is using alternative protocols to clarify whether the mutation only affects the Ca2+ extrusion function of the PMCA3 pump, or whether, possibly by interfering with the autoinhibitory mechanism of the C-terminal CaM binding domain, the mutation has more dramatic consequences for the local intracellular Ca2+ signaling regulation (Fig. 5).
Fig. 5.
A mutation in the plasma membrane (PM) Ca2+ ATPase 3 (PMCA3) pump causes X-linked congenital cerebellar ataxia. The PMCA3 pump is highly expressed in the cerebellum, particularly in the presynaptic terminals of parallel fibers–Purkinje neurons. The cartoon in the figure summarizes the recent finding that a missense mutation of the X-linked PMCA3 pump gene in a family with congenital cerebellar ataxia impaired the Ca2+ extrusion ability of the pump. The figure also shows cytosolic Ca2+ measurements in model cells (HeLa) overexpressing the PMCA3a wild-type (wt) (blue trace) or PMCA3a G1107D mutant pump (red trace). Cells were stimulated with a Ca2+ mobilizing agonist (histamine). As mentioned in the text, the “a” isoform is a spliced variant of the PMCA3 pump. The black trace in the bottom panel shows the Ca2+ transient in untransfected control cells. The reduction of the peak amplitude in PMCA3-overexpressing cells is due to the Ca2+ extrusion activity of the overexpressed pump. The mutant PMCA3a pump (red trace) has a reduced ability to extrude Ca2+ from the cells: the decline of the red trace is slower than that of the blue trace, which corresponds to cells overexpressing the wt PMCA3a pump [69]
Migraine
Migraine is a common episodic neurological disorder that manifests itself as recurrent attacks of typically throbbing and unilateral, often severe, headache, with associated features such as nausea, phonophobia, and/or photophobia; in one-third of patients the headache is preceded by transient neurological symptoms that are most frequently visual, but may involve other senses [migraine with aura (MA)]. Migraine is a disorder of brain excitability which has complex physiopathological mechanisms [236] that have begun to be understood only recently thanks to the use of mouse models of familial hemiplegic migraine (FHM), a rare genetic autosomal-dominant form of MA. Three genes, all encoding ion channels or transporters, have been identified in FHM [237]. FHM1 is caused by missense mutations in CACNA1A, the gene encoding the pore-forming subunit of neuronal Cav2.1 (P/Q-type) voltage-gated Ca2+ channels [238]. These channels are widely expressed in the nervous system and play a dominant role in controlling neurotransmitter release, particularly at central synapses. Analysis of the properties of mutant recombinant human Cav2.1 channel currents in different neurons (including cortical and trigeminal ganglion neurons) of knock-in mice carrying FHM1 mutations have revealed that the mutations produce gain-of-function of the channels, mainly due to their increased open probability and activation at lower voltages. In the cerebral cortex of FHM1 knock-in mice, excitatory synaptic transmission was found to be enhanced [239].
FHM2 is caused by mutations (mainly missense) in ATP1A2, the gene encoding the α2 subunit of the Na+/K+ ATPase [240]. In the brain, this pump isoform is expressed primarily in neurons during embryonic development and at time of birth, but in adults it is almost exclusively expressed in astrocytes. It colocalizes with glutamate transporters in astrocytic processes surrounding glutamatergic synapses, suggesting a specific role in glutamate clearance [241]. It also colocalizes with the NCX in microdomains that overlie sub-plasma membrane ER, suggesting a possible role in the regulation of intracellular Ca2+. FHM2 mutations cause complete or partial loss-of-function of recombinant Na+/K+ ATPases due to the loss or reduction of its catalytic activity or to impairment of its delivery to the plasma membrane [242, 243].
FHM3 is caused by missense mutations in SCNA1A, the gene encoding the pore-forming subunit of neuronal NaV1.1 voltage-gated Na+ channels [244]. These channels are highly expressed in inhibitory inter-neurons where they play an important role in sustaining high-frequency firing [245]. Conflicting findings were obtained from the analysis of mutant recombinant human NaV1.1 channels expressed in non-neuronal cells, pointing to either gain- or loss-of-function effects [246, 247].
Although it is clear that most migraine attacks start in the brain and that they are essentially due to an imbalance between excitatory and inhibitory signals, the nature and mechanisms of the primary brain dysfunctions, as well as of the mechanisms that activate the trigeminovascular pain pathway, are still unsolved questions. FHM1 and -2 share features that suggest, as in other neurological disorders, exaggerated Ca2+ signals. Since the symptoms are not extended to all of the nervous system, but rather are confined to specific areas, specific characteristics of different neuronal populations may also play a role.
Concluding remarks
The development of genetic models and the improvement in technologies to image Ca2+ signals in vivo have greatly advanced the understanding of the molecular regulation of cellular Ca2+ homeostasis and its perturbation in neurological disorders. The dis-homeostasis of Ca2+ as a causative pathogenic mechanism of these disorders has now gained wide consensus. The identification of the defects affecting selective Ca2+ signaling processes is a necessary step in the road to therapeutic strategies that would correct the disturbances of neuronal Ca2+ signaling. Clearly, a number of questions still need to be solved, the foremost one being perhaps the understanding of the reasons for the specific vulnerability of different neuronal populations in the neurodegenerative processes. In this context the “Ca2+ hypothesis” of neurodegeneration can be considered to be a general background at the basis of different neuronal pathologies, which must be integrated with specific factors that determine the specificity of each disease process.
Acknowledgments
The original work by the authors has been supported over the years by grants from the Italian Ministry of University and Research (FIRB2001 to E.C., PRIN 2003, 2005 and 2008 to M.B), the Telethon Foundation (Project GGP04169 to M.B.), the FP6 program of the European Union (FP6 Network of Excellence NeuroNe, LSH-2003-2.1.3-3 to E.C. and Integrated Project Eurohear to E.C.), the Human Frontier Science Program Organization to E.C., the ERANet-Neuron (nEUROsyn), and CARIPARO Foundation to E.C, the Italian National Research Council (Agenzia 2000, CNR) and by grant from the University of Padova (Progetto di Ateneo 2008 CPDA082825) to M.B. Tito Calì is supported by the University of Padova (Progetto Giovani GRIC128SP0, Bando 2012). We apologize to many authors who have published substantial scientific contributions in the field of this specific Ca2+ research whose work could not or not sufficiently be cited here. This is due to space restrictions. Figures 1, 3 and 5 were produced using ServierMedical Art (http://www.servier.com/serviermedical-art/powerpoint-image-bank).
Contributor Information
Marisa Brini, Phone: +39-49-8276150, FAX: +39-49-8276125, Email: marisa.brini@unipd.it.
Ernesto Carafoli, Phone: +39-49-8276137, FAX: +39-49-8276125, Email: ernesto.carafoli@unipd.it.
References
- 1.Carafoli E, Malmstrom K, Sigel E, Crompton M. The regulation of intracellular calcium. Clin Endocrinol (Oxf) 1976;5(Suppl):49S–59S. doi: 10.1111/j.1365-2265.1976.tb03815.x. [DOI] [PubMed] [Google Scholar]
- 2.Brini M, Cali T, Ottolini D, Carafoli E. Intracellular calcium homeostasis and signaling. Met Ions Life Sci. 2013;12:119–168. doi: 10.1007/978-94-007-5561-1_5. [DOI] [PubMed] [Google Scholar]
- 3.Mellstrom B, Savignac M, Gomez-Villafuertes R, Naranjo JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev. 2008;88(2):421–449. doi: 10.1152/physrev.00041.2005. [DOI] [PubMed] [Google Scholar]
- 4.Carafoli E. The unusual history and unique properties of the calcium signal. In: Krebs J, Michalak M, editors. Calcium: a matter of life and death. Amsterdam: Elsevier; 2007. pp. 3–22. [Google Scholar]
- 5.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann F, Lacinova L, Klugbauer N. Voltage-dependent calcium channels: from structure to function. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- 7.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 8.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28(5):220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Hoppa MB, Lana B, Margas W, Dolphin AC, Ryan TA. Alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature. 2012;486(7401):122–125. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3(8):a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyashita T, Oda Y, Horiuchi J, Yin JC, Morimoto T, Saitoe M. Mg(2+) block of Drosophila NMDA receptors is required for long-term memory formation and CREB-dependent gene expression. Neuron. 2012;74(5):887–898. doi: 10.1016/j.neuron.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280(5369):1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- 13.Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40(4):763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406(6791):78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- 15.Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15(2):453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 16.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 17.Hardingham GE, Chawla S, Cruzalegui FH, Bading H. Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron. 1999;22(4):789–798. doi: 10.1016/s0896-6273(00)80737-0. [DOI] [PubMed] [Google Scholar]
- 18.Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4(3):261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 19.Hardingham GE, Arnold FJ, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001;4(6):565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- 20.Impey S, Goodman RH (2001) CREB signaling—timing is everything. Sci STKE 2001(82):pe1. doi:10.1126/stke.2001.82.pe1 [DOI] [PubMed]
- 21.Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 2001;98(5):2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108(5):689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- 23.Corlew R, Brasier DJ, Feldman DE, Philpot BD. Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist. 2008;14(6):609–625. doi: 10.1177/1073858408322675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnstock G, Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal. 2013 doi: 10.1007/s11302-013-9372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32(2):79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8(3):629–657. doi: 10.1007/s11302-012-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levano-Garcia J, Dluzewski AR, Markus RP, Garcia CR. Purinergic signalling is involved in the malaria parasite Plasmodium falciparum invasion to red blood cells. Purinergic Signal. 2010;6(4):365–372. doi: 10.1007/s11302-010-9202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A. P2X receptors and synaptic plasticity. Neuroscience. 2009;158(1):137–148. doi: 10.1016/j.neuroscience.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11(4):227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- 30.Burnstock G, Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1:e9. doi: 10.1038/cddis.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann H. Purinergic signaling in neural development. Semin Cell Dev Biol. 2011;22(2):194–204. doi: 10.1016/j.semcdb.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Browne LE, Jiang LH, North RA. New structure enlivens interest in P2X receptors. Trends Pharmacol Sci. 2010;31(5):229–237. doi: 10.1016/j.tips.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 34.Pankratov Y, Lalo U, Krishtal O, Verkhratsky A. P2X receptor-mediated excitatory synaptic currents in somatosensory cortex. Mol Cell Neurosci. 2003;24(3):842–849. doi: 10.1016/s1044-7431(03)00233-1. [DOI] [PubMed] [Google Scholar]
- 35.Duan S, Neary JT. P2X(7) receptors: properties and relevance to CNS function. Glia. 2006;54(7):738–746. doi: 10.1002/glia.20397. [DOI] [PubMed] [Google Scholar]
- 36.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 37.Feske S. Immunodeficiency due to defects in store-operated calcium entry. Ann N Y Acad Sci. 2011;1238:74–90. doi: 10.1111/j.1749-6632.2011.06240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putney JW. Calcium signaling: deciphering the calcium-NFAT pathway. Curr Biol. 2012;22(3):R87–R89. doi: 10.1016/j.cub.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 39.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282(22):16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 40.Venkiteswaran G, Hasan G. Intracellular Ca2+ signaling and store-operated Ca2+ entry are required in Drosophila neurons for flight. Proc Natl Acad Sci USA. 2009;106(25):10326–10331. doi: 10.1073/pnas.0902982106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, Nieswandt B. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal. 2009;2(93):ra67. doi: 10.1126/scisignal.2000522. [DOI] [PubMed] [Google Scholar]
- 42.Thompson JL, Mignen O, Shuttleworth TJ. The ARC channel—an endogenous store-independent Orai channel. Curr Top Membr. 2013;71:125–148. doi: 10.1016/B978-0-12-407870-3.00006-8. [DOI] [PubMed] [Google Scholar]
- 43.Rohacs T. Regulation of TRP channels by PIP(2) Pflugers Arch. 2007;453(6):753–762. doi: 10.1007/s00424-006-0153-7. [DOI] [PubMed] [Google Scholar]
- 44.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42(2):213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Lu M, Branstrom R, Berglund E, Hoog A, Bjorklund P, Westin G, Larsson C, Farnebo LO, Forsberg L. Expression and association of TRPC subtypes with Orai1 and STIM1 in human parathyroid. J Mol Endocrinol. 2010;44(5):285–294. doi: 10.1677/JME-09-0138. [DOI] [PubMed] [Google Scholar]
- 46.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci USA. 2007;104(11):4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galione A, Evans AM, Ma J, Parrington J, Arredouani A, Cheng X, Zhu MX. The acid test: the discovery of two-pore channels (TPCs) as NAADP-gated endolysosomal Ca(2+) release channels. Pflugers Arch. 2009;458(5):869–876. doi: 10.1007/s00424-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151(2):372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brini M, Carafoli E (2011) The plasma membrane Ca(2)+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb Perspect Biol 3(2). doi:10.1101/cshperspect.a004168 [DOI] [PMC free article] [PubMed]
- 50.Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann N Y Acad Sci. 2002;976:356–366. doi: 10.1111/j.1749-6632.2002.tb04762.x. [DOI] [PubMed] [Google Scholar]
- 51.Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca(2+) stores. Trends Neurosci. 2001;24(10):602–608. doi: 10.1016/s0166-2236(00)01891-9. [DOI] [PubMed] [Google Scholar]
- 52.Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120(2):275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 53.Annunziato L, Pignataro G, Boscia F, Sirabella R, Formisano L, Saggese M, Cuomo O, Gala R, Secondo A, Viggiano D, Molinaro P, Valsecchi V, Tortiglione A, Adornetto A, Scorziello A, Cataldi M, Di Renzo GF. ncx1, ncx2, and ncx3 gene product expression and function in neuronal anoxia and brain ischemia. Ann N Y Acad Sci. 2007;1099:413–426. doi: 10.1196/annals.1387.050. [DOI] [PubMed] [Google Scholar]
- 54.James P, Maeda M, Fischer R, Verma AK, Krebs J, Penniston JT, Carafoli E. Identification and primary structure of a calmodulin binding domain of the Ca2+ pump of human erythrocytes. J Biol Chem. 1988;263(6):2905–2910. [PubMed] [Google Scholar]
- 55.Enyedi A, Verma AK, Heim R, Adamo HP, Filoteo AG, Strehler EE, Penniston JT. The Ca2+ affinity of the plasma membrane Ca2+ pump is controlled by alternative splicing. J Biol Chem. 1994;269(1):41–43. [PubMed] [Google Scholar]
- 56.Falchetto R, Vorherr T, Brunner J, Carafoli E. The plasma membrane Ca2+ pump contains a site that interacts with its calmodulin-binding domain. J Biol Chem. 1991;266(5):2930–2936. [PubMed] [Google Scholar]
- 57.Falchetto R, Vorherr T, Carafoli E. The calmodulin-binding site of the plasma membrane Ca2+ pump interacts with the transduction domain of the enzyme. Protein Sci. 1992;1(12):1613–1621. doi: 10.1002/pro.5560011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Enyedi A, Vorherr T, James P, McCormick DJ, Filoteo AG, Carafoli E, Penniston JT. The calmodulin binding domain of the plasma membrane Ca2+ pump interacts both with calmodulin and with another part of the pump. J Biol Chem. 1989;264(21):12313–12321. [PubMed] [Google Scholar]
- 59.Zvaritch E, James P, Vorherr T, Falchetto R, Modyanov N, Carafoli E. Mapping of functional domains in the plasma membrane Ca2+ pump using trypsin proteolysis. Biochemistry. 1990;29(35):8070–8076. doi: 10.1021/bi00487a012. [DOI] [PubMed] [Google Scholar]
- 60.Brodin P, Falchetto R, Vorherr T, Carafoli E. Identification of two domains which mediate the binding of activating phospholipids to the plasma-membrane Ca2+ pump. Eur J Biochem. 1992;204(2):939–946. doi: 10.1111/j.1432-1033.1992.tb16715.x. [DOI] [PubMed] [Google Scholar]
- 61.Oceandy D, Cartwright EJ, Emerson M, Prehar S, Baudoin FM, Zi M, Alatwi N, Venetucci L, Schuh K, Williams JC, Armesilla AL, Neyses L. Neuronal nitric oxide synthase signaling in the heart is regulated by the sarcolemmal calcium pump 4b. Circulation. 2007;115(4):483–492. doi: 10.1161/CIRCULATIONAHA.106.643791. [DOI] [PubMed] [Google Scholar]
- 62.Mohamed TM, Oceandy D, Zi M, Prehar S, Alatwi N, Wang Y, Shaheen MA, Abou-Leisa R, Schelcher C, Hegab Z, Baudoin F, Emerson M, Mamas M, Di Benedetto G, Zaccolo M, Lei M, Cartwright EJ, Neyses L. Plasma membrane calcium pump (PMCA4)-neuronal nitric-oxide synthase complex regulates cardiac contractility through modulation of a compartmentalized cyclic nucleotide microdomain. J Biol Chem. 2011;286(48):41520–41529. doi: 10.1074/jbc.M111.290411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ficarella R, Di Leva F, Bortolozzi M, Ortolano S, Donaudy F, Petrillo M, Melchionda S, Lelli A, Domi T, Fedrizzi L, Lim D, Shull GE, Gasparini P, Brini M, Mammano F, Carafoli E. A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness. Proc Natl Acad Sci USA. 2007;104(5):1516–1521. doi: 10.1073/pnas.0609775104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spiden SL, Bortolozzi M, Di Leva F, de Angelis MH, Fuchs H, Lim D, Ortolano S, Ingham NJ, Brini M, Carafoli E, Mammano F, Steel KP. The novel mouse mutation Oblivion inactivates the PMCA2 pump and causes progressive hearing loss. PLoS Genet. 2008;4(10):e1000238. doi: 10.1371/journal.pgen.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat Genet. 1998;19(4):390–394. doi: 10.1038/1284. [DOI] [PubMed] [Google Scholar]
- 66.Bortolozzi M, Brini M, Parkinson N, Crispino G, Scimemi P, De Siati RD, Di Leva F, Parker A, Ortolano S, Arslan E, Brown SD, Carafoli E, Mammano F. The novel PMCA2 pump mutation Tommy impairs cytosolic calcium clearance in hair cells and links to deafness in mice. J Biol Chem. 2010;285(48):37693–37703. doi: 10.1074/jbc.M110.170092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi K, Kitamura K. A point mutation in a plasma membrane Ca(2+)-ATPase gene causes deafness in Wriggle Mouse Sagami. Biochem Biophys Res Commun. 1999;261(3):773–778. doi: 10.1006/bbrc.1999.1102. [DOI] [PubMed] [Google Scholar]
- 68.Schultz JM, Yang Y, Caride AJ, Filoteo AG, Penheiter AR, Lagziel A, Morell RJ, Mohiddin SA, Fananapazir L, Madeo AC, Penniston JT, Griffith AJ. Modification of human hearing loss by plasma-membrane calcium pump PMCA2. N Engl J Med. 2005;352(15):1557–1564. doi: 10.1056/NEJMoa043899. [DOI] [PubMed] [Google Scholar]
- 69.Zanni G, Cali T, Kalscheuer VM, Ottolini D, Barresi S, Lebrun N, Montecchi-Palazzi L, Hu H, Chelly J, Bertini E, Brini M, Carafoli E. Mutation of plasma membrane Ca2+ ATPase isoform 3 in a family with X-linked congenital cerebellar ataxia impairs Ca2+ homeostasis. Proc Natl Acad Sci USA. 2012;109(36):14514–14519. doi: 10.1073/pnas.1207488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor CW, Tovey SC. IP(3) receptors: toward understanding their activation. Cold Spring Harb Perspect Biol. 2010;2(12):a004010. doi: 10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor CW, Laude AJ. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+ Cell Calcium. 2002;32(5–6):321–334. doi: 10.1016/s0143416002001859. [DOI] [PubMed] [Google Scholar]
- 72.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2(11):a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Flora A, Franco L, Guida L, Bruzzone S, Zocchi E. Ectocellular CD38-catalyzed synthesis and intracellular Ca(2+)-mobilizing activity of cyclic ADP-ribose. Cell Biochem Biophys. 1998;28(1):45–62. doi: 10.1007/BF02738309. [DOI] [PubMed] [Google Scholar]
- 74.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89(4):1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 75.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96(24):13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787(11):1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82(3):415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 78.Hajnoczky G, Hager R, Thomas AP. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+ J Biol Chem. 1999;274(20):14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- 79.Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137(3):633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476(7360):341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476(7360):336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabo I, Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32(17):2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, Koteliansky V, Adijanto J, Mootha VK, Hajnoczky G. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter. Cell Metab. 2013;17(6):976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, Rothberg BS, Mak DO, Foskett JK, Madesh M. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 2012;151(3):630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, Oshea J, Koteliansky V, Mootha VK. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One. 2013;8(2):e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O, Mootha VK. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carafoli E, Tiozzo R, Lugli G, Crovetti F, Kratzing C. The release of calcium from heart mitochondria by sodium. J Mol Cell Cardiol. 1974;6(4):361–371. doi: 10.1016/0022-2828(74)90077-7. [DOI] [PubMed] [Google Scholar]
- 88.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA. 2010;107(1):436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bernardi P, von Stockum S. The permeability transition pore as a Ca(2+) release channel: new answers to an old question. Cell Calcium. 2012;52(1):22–27. doi: 10.1016/j.ceca.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bernardi P. The mitochondrial permeability transition pore: a mystery solved? Front Physiol. 2013;4:95. doi: 10.3389/fphys.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, Pinton P. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle. 2013;12(4):674–683. doi: 10.4161/cc.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA. 2013;110(15):5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10(8):322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 94.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59(6):882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 95.Schwaller B. The continuing disappearance of “pure” Ca2+ buffers. Cell Mol Life Sci. 2009;66(2):275–300. doi: 10.1007/s00018-008-8564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmidt H, Eilers J. Spine neck geometry determines spino-dendritic cross-talk in the presence of mobile endogenous calcium binding proteins. J Comput Neurosci. 2009;27(2):229–243. doi: 10.1007/s10827-009-0139-5. [DOI] [PubMed] [Google Scholar]
- 97.Schmidt H, Kunerth S, Wilms C, Strotmann R, Eilers J. Spino-dendritic cross-talk in rodent Purkinje neurons mediated by endogenous Ca2+-binding proteins. J Physiol. 2007;581(Pt 2):619–629. doi: 10.1113/jphysiol.2007.127860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fierro L, Llano I. High endogenous calcium buffering in Purkinje cells from rat cerebellar slices. J Physiol. 1996;496(Pt 3):617–625. doi: 10.1113/jphysiol.1996.sp021713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schwaller B, Tetko IV, Tandon P, Silveira DC, Vreugdenhil M, Henzi T, Potier MC, Celio MR, Villa AE. Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol Cell Neurosci. 2004;25(4):650–663. doi: 10.1016/j.mcn.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 100.Gall D, Roussel C, Susa I, D’Angelo E, Rossi P, Bearzatto B, Galas MC, Blum D, Schurmans S, Schiffmann SN. Altered neuronal excitability in cerebellar granule cells of mice lacking calretinin. J Neurosci. 2003;23(28):9320–9327. doi: 10.1523/JNEUROSCI.23-28-09320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moreno H, Burghardt NS, Vela-Duarte D, Masciotti J, Hua F, Fenton AA, Schwaller B, Small SA. The absence of the calcium-buffering protein calbindin is associated with faster age-related decline in hippocampal metabolism. Hippocampus. 2012;22(5):1107–1120. doi: 10.1002/hipo.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pongs O, Lindemeier J, Zhu XR, Theil T, Engelkamp D, Krah-Jentgens I, Lambrecht HG, Koch KW, Schwemer J, Rivosecchi R, et al. Frequenin—a novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron. 1993;11(1):15–28. doi: 10.1016/0896-6273(93)90267-u. [DOI] [PubMed] [Google Scholar]
- 103.McFerran BW, Graham ME, Burgoyne RD. Neuronal Ca2+ sensor 1, the mammalian homologue of frequenin, is expressed in chromaffin and PC12 cells and regulates neurosecretion from dense-core granules. J Biol Chem. 1998;273(35):22768–22772. doi: 10.1074/jbc.273.35.22768. [DOI] [PubMed] [Google Scholar]
- 104.McFerran BW, Weiss JL, Burgoyne RD. Neuronal Ca(2+) sensor 1. Characterization of the myristoylated protein, its cellular effects in permeabilized adrenal chromaffin cells, Ca(2+)-independent membrane association, and interaction with binding proteins, suggesting a role in rapid Ca(2+) signal transduction. J Biol Chem. 1999;274(42):30258–30265. doi: 10.1074/jbc.274.42.30258. [DOI] [PubMed] [Google Scholar]
- 105.Weiss JL, Archer DA, Burgoyne RD. Neuronal Ca2+ sensor-1/frequenin functions in an autocrine pathway regulating Ca2+ channels in bovine adrenal chromaffin cells. J Biol Chem. 2000;275(51):40082–40087. doi: 10.1074/jbc.M008603200. [DOI] [PubMed] [Google Scholar]
- 106.Tsujimoto T, Jeromin A, Saitoh N, Roder JC, Takahashi T. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science. 2002;295(5563):2276–2279. doi: 10.1126/science.1068278. [DOI] [PubMed] [Google Scholar]
- 107.Sippy T, Cruz-Martin A, Jeromin A, Schweizer FE. Acute changes in short-term plasticity at synapses with elevated levels of neuronal calcium sensor-1. Nat Neurosci. 2003;6(10):1031–1038. doi: 10.1038/nn1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jo J, Heon S, Kim MJ, Son GH, Park Y, Henley JM, Weiss JL, Sheng M, Collingridge GL, Cho K. Metabotropic glutamate receptor-mediated LTD involves two interacting Ca(2+) sensors, NCS-1 and PICK1. Neuron. 2008;60(6):1095–1111. doi: 10.1016/j.neuron.2008.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dason JS, Romero-Pozuelo J, Marin L, Iyengar BG, Klose MK, Ferrus A, Atwood HL. Frequenin/NCS-1 and the Ca2+-channel alpha1-subunit co-regulate synaptic transmission and nerve-terminal growth. J Cell Sci. 2009;122(Pt 22):4109–4121. doi: 10.1242/jcs.055095. [DOI] [PubMed] [Google Scholar]
- 110.Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T, Catterall WA. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;399(6732):155–159. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- 111.An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403(6769):553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 112.Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398(6722):80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- 113.Buxbaum JD, Choi EK, Luo Y, Lilliehook C, Crowley AC, Merriam DE, Wasco W. Calsenilin: a calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nat Med. 1998;4(10):1177–1181. doi: 10.1038/2673. [DOI] [PubMed] [Google Scholar]
- 114.De Strooper B, Iwatsubo T, Wolfe MS. Presenilins and gamma-secretase: structure, function, and role in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(1):a006304. doi: 10.1101/cshperspect.a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gomez-Villafuertes R, Torres B, Barrio J, Savignac M, Gabellini N, Rizzato F, Pintado B, Gutierrez-Adan A, Mellstrom B, Carafoli E, Naranjo JR. Downstream regulatory element antagonist modulator regulates Ca2+ homeostasis and viability in cerebellar neurons. J Neurosci. 2005;25(47):10822–10830. doi: 10.1523/JNEUROSCI.3912-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ronkainen JJ, Hanninen SL, Korhonen T, Koivumaki JT, Skoumal R, Rautio S, Ronkainen VP, Tavi P. Ca2+-calmodulin-dependent protein kinase II represses cardiac transcription of the L-type calcium channel alpha(1C)-subunit gene (Cacna1c) by DREAM translocation. J Physiol. 2011;589(Pt 11):2669–2686. doi: 10.1113/jphysiol.2010.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fontan-Lozano A, Romero-Granados R, del-Pozo-Martin Y, Suarez-Pereira I, Delgado-Garcia JM, Penninger JM, Carrion AM. Lack of DREAM protein enhances learning and memory and slows brain aging. Curr Biol. 2009;19(1):54–60. doi: 10.1016/j.cub.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 118.Alexander JC, McDermott CM, Tunur T, Rands V, Stelly C, Karhson D, Bowlby MR, An WF, Sweatt JD, Schrader LA. The role of calsenilin/DREAM/KChIP3 in contextual fear conditioning. Learn Mem. 2009;16(3):167–177. doi: 10.1101/lm.1261709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu LJ, Mellstrom B, Wang H, Ren M, Domingo S, Kim SS, Li XY, Chen T, Naranjo JR, Zhuo M. DREAM (downstream regulatory element antagonist modulator) contributes to synaptic depression and contextual fear memory. Mol Brain. 2010;3:3. doi: 10.1186/1756-6606-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Y, Su P, Liang P, Liu T, Liu X, Liu XY, Zhang B, Han T, Zhu YB, Yin DM, Li J, Zhou Z, Wang KW, Wang Y. The DREAM protein negatively regulates the NMDA receptor through interaction with the NR1 subunit. J Neurosci. 2010;30(22):7575–7586. doi: 10.1523/JNEUROSCI.1312-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rivera-Arconada I, Benedet T, Roza C, Torres B, Barrio J, Krzyzanowska A, Avendano C, Mellstrom B, Lopez-Garcia JA, Naranjo JR. DREAM regulates BDNF-dependent spinal sensitization. Mol Pain. 2010;6:95. doi: 10.1186/1744-8069-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karp G. Cell and molecular biology. New York: Wiley; 2002. [Google Scholar]
- 123.Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- 124.Bading H. Nuclear calcium signalling in the regulation of brain function. Nat Rev Neurosci. 2013;14(9):593–608. doi: 10.1038/nrn3531. [DOI] [PubMed] [Google Scholar]
- 125.Dick O, Bading H. Synaptic activity and nuclear calcium signaling protect hippocampal neurons from death signal-associated nuclear translocation of FoxO3a induced by extrasynaptic N-methyl-D-aspartate receptors. J Biol Chem. 2010;285(25):19354–19361. doi: 10.1074/jbc.M110.127654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Foskett JK. Inositol trisphosphate receptor Ca2+ release channels in neurological diseases. Pflugers Arch. 2010;460(2):481–494. doi: 10.1007/s00424-010-0826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Camandola S, Mattson MP. Aberrant subcellular neuronal calcium regulation in aging and Alzheimer’s disease. Biochim Biophys Acta. 2011;1813(5):965–973. doi: 10.1016/j.bbamcr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duchen MR. Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers Arch. 2012;464(1):111–121. doi: 10.1007/s00424-012-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cali T, Ottolini D, Brini M. Mitochondrial Ca(2+) and neurodegeneration. Cell Calcium. 2012;52(1):73–85. doi: 10.1016/j.ceca.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 131.Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci. 1996;16(13):4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 133.von Lewinski F, Keller BU. Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends Neurosci. 2005;28(9):494–500. doi: 10.1016/j.tins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 134.Alexianu ME, Ho BK, Mohamed AH, La Bella V, Smith RG, Appel SH. The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1994;36(6):846–858. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- 135.Iacopino AM, Christakos S. Corticosterone regulates calbindin-D28k mRNA and protein levels in rat hippocampus. J Biol Chem. 1990;265(18):10177–10180. [PubMed] [Google Scholar]
- 136.Beers DR, Ho BK, Siklos L, Alexianu ME, Mosier DR, Mohamed AH, Otsuka Y, Kozovska ME, McAlhany RE, Smith RG, Appel SH. Parvalbumin overexpression alters immune-mediated increases in intracellular calcium, and delays disease onset in a transgenic model of familial amyotrophic lateral sclerosis. J Neurochem. 2001;79(3):499–509. doi: 10.1046/j.1471-4159.2001.00582.x. [DOI] [PubMed] [Google Scholar]
- 137.Bernard-Marissal N, Moumen A, Sunyach C, Pellegrino C, Dudley K, Henderson CE, Raoul C, Pettmann B. Reduced calreticulin levels link endoplasmic reticulum stress and Fas-triggered cell death in motoneurons vulnerable to ALS. J Neurosci. 2012;32(14):4901–4912. doi: 10.1523/JNEUROSCI.5431-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Beal MF. Mitochondria and the pathogenesis of ALS. Brain. 2000;123(Pt 7):1291–1292. doi: 10.1093/brain/123.7.1291. [DOI] [PubMed] [Google Scholar]
- 139.Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, Flint Beal M, Manfredi G. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem. 2006;96(5):1349–1361. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- 140.Carri MT, Ferri A, Battistoni A, Famhy L, Gabbianelli R, Poccia F, Rotilio G. Expression of a Cu, Zn superoxide dismutase typical of familial amyotrophic lateral sclerosis induces mitochondrial alteration and increase of cytosolic Ca2+ concentration in transfected neuroblastoma SH-SY5Y cells. FEBS Lett. 1997;414(2):365–368. doi: 10.1016/s0014-5793(97)01051-x. [DOI] [PubMed] [Google Scholar]
- 141.Ferri A, Cozzolino M, Crosio C, Nencini M, Casciati A, Gralla EB, Rotilio G, Valentine JS, Carri MT. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc Natl Acad Sci USA. 2006;103(37):13860–13865. doi: 10.1073/pnas.0605814103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Son M, Leary SC, Romain N, Pierrel F, Winge DR, Haller RG, Elliott JL. Isolated cytochrome c oxidase deficiency in G93A SOD1 mice overexpressing CCS protein. J Biol Chem. 2008;283(18):12267–12275. doi: 10.1074/jbc.M708523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Son M, Puttaparthi K, Kawamata H, Rajendran B, Boyer PJ, Manfredi G, Elliott JL. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc Natl Acad Sci USA. 2007;104(14):6072–6077. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mattiazzi M, D’Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277(33):29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- 145.Jung C, Higgins CM, Xu Z. Mitochondrial electron transport chain complex dysfunction in a transgenic mouse model for amyotrophic lateral sclerosis. J Neurochem. 2002;83(3):535–545. doi: 10.1046/j.1471-4159.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- 146.Jaiswal MK, Keller BU. Cu/Zn superoxide dismutase typical for familial amyotrophic lateral sclerosis increases the vulnerability of mitochondria and perturbs Ca2+ homeostasis in SOD1G93A mice. Mol Pharmacol. 2009;75(3):478–489. doi: 10.1124/mol.108.050831. [DOI] [PubMed] [Google Scholar]
- 147.Jaiswal MK, Zech WD, Goos M, Leutbecher C, Ferri A, Zippelius A, Carri MT, Nau R, Keller BU. Impairment of mitochondrial calcium handling in a mtSOD1 cell culture model of motoneuron disease. BMC Neurosci. 2009;10:64. doi: 10.1186/1471-2202-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rothstein JD, Tsai G, Kuncl RW, Clawson L, Cornblath DR, Drachman DB, Pestronk A, Stauch BL, Coyle JT. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990;28(1):18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- 149.Carriedo SG, Sensi SL, Yin HZ, Weiss JH. AMPA exposures induce mitochondrial Ca(2+) overload and ROS generation in spinal motor neurons in vitro. J Neurosci. 2000;20(1):240–250. doi: 10.1523/JNEUROSCI.20-01-00240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Takuma H, Kwak S, Yoshizawa T, Kanazawa I. Reduction of GluR2 RNA editing, a molecular change that increases calcium influx through AMPA receptors, selective in the spinal ventral gray of patients with amyotrophic lateral sclerosis. Ann Neurol. 1999;46(6):806–815. doi: 10.1002/1531-8249(199912)46:6<806::aid-ana2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 151.Guatteo E, Carunchio I, Pieri M, Albo F, Canu N, Mercuri NB, Zona C. Altered calcium homeostasis in motor neurons following AMPA receptor but not voltage-dependent calcium channels’ activation in a genetic model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;28(1):90–100. doi: 10.1016/j.nbd.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 152.Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol Rev. 2010;90(3):905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
- 153.Graham RK, Deng Y, Slow EJ, Haigh B, Bissada N, Lu G, Pearson J, Shehadeh J, Bertram L, Murphy Z, Warby SC, Doty CN, Roy S, Wellington CL, Leavitt BR, Raymond LA, Nicholson DW, Hayden MR. Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin. Cell. 2006;125(6):1179–1191. doi: 10.1016/j.cell.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 154.Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, Vendruscolo M, Hayer-Hartl M, Hartl FU, Vabulas RM. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell. 2011;144(1):67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 155.Bao J, Sharp AH, Wagster MV, Becher M, Schilling G, Ross CA, Dawson VL, Dawson TM. Expansion of polyglutamine repeat in huntingtin leads to abnormal protein interactions involving calmodulin. Proc Natl Acad Sci USA. 1996;93(10):5037–5042. doi: 10.1073/pnas.93.10.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamanaka T, Nukina N. Transcription factor sequestration by polyglutamine proteins. Methods Mol Biol. 2010;648:215–229. doi: 10.1007/978-1-60761-756-3_14. [DOI] [PubMed] [Google Scholar]
- 157.Fan MM, Raymond LA. N-methyl-d-aspartate (NMDA) receptor function and excitotoxicity in Huntington’s disease. Prog Neurobiol. 2007;81(5–6):272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 158.Bezprozvanny I. Inositol 1,4,5-tripshosphate receptor, calcium signalling and Huntington’s disease. Subcell Biochem. 2007;45:323–335. doi: 10.1007/978-1-4020-6191-2_11. [DOI] [PubMed] [Google Scholar]
- 159.Lim D, Fedrizzi L, Tartari M, Zuccato C, Cattaneo E, Brini M, Carafoli E. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J Biol Chem. 2008;283(9):5780–5789. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- 160.Seto-Ohshima A, Emson PC, Lawson E, Mountjoy CQ, Carrasco LH. Loss of matrix calcium-binding protein-containing neurons in Huntington’s disease. Lancet. 1988;1(8597):1252–1255. doi: 10.1016/s0140-6736(88)92073-9. [DOI] [PubMed] [Google Scholar]
- 161.Dong G, Gross K, Qiao F, Ferguson J, Callegari EA, Rezvani K, Zhang D, Gloeckner CJ, Ueffing M, Wang H. Calretinin interacts with huntingtin and reduces mutant huntingtin-caused cytotoxicity. J Neurochem. 2012;123(3):437–446. doi: 10.1111/j.1471-4159.2012.07919.x. [DOI] [PubMed] [Google Scholar]
- 162.Sun Y, Savanenin A, Reddy PH, Liu YF. Polyglutamine-expanded huntingtin promotes sensitization of N-methyl-d-aspartate receptors via post-synaptic density 95. J Biol Chem. 2001;276(27):24713–24718. doi: 10.1074/jbc.M103501200. [DOI] [PubMed] [Google Scholar]
- 163.Benchoua A, Trioulier Y, Zala D, Gaillard MC, Lefort N, Dufour N, Saudou F, Elalouf JM, Hirsch E, Hantraye P, Deglon N, Brouillet E. Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol Biol Cell. 2006;17(4):1652–1663. doi: 10.1091/mbc.E05-07-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13(10):4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5(8):731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 166.Panov AV, Burke JR, Strittmatter WJ, Greenamyre JT. In vitro effects of polyglutamine tracts on Ca2+-dependent depolarization of rat and human mitochondria: relevance to Huntington’s disease. Arch Biochem Biophys. 2003;410(1):1–6. doi: 10.1016/s0003-9861(02)00585-4. [DOI] [PubMed] [Google Scholar]
- 167.Choo YS, Johnson GV, MacDonald M, Detloff PJ, Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum Mol Genet. 2004;13(14):1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- 168.Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39(2):227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dreses-Werringloer U, Lambert JC, Vingtdeux V, Zhao H, Vais H, Siebert A, Jain A, Koppel J, Rovelet-Lecrux A, Hannequin D, Pasquier F, Galimberti D, Scarpini E, Mann D, Lendon C, Campion D, Amouyel P, Davies P, Foskett JK, Campagne F, Marambaud P. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell. 2008;133(7):1149–1161. doi: 10.1016/j.cell.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31(9):454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Green KN, LaFerla FM. Linking calcium to Abeta and Alzheimer’s disease. Neuron. 2008;59(2):190–194. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 172.Berridge MJ. Calcium hypothesis of Alzheimer’s disease. Pflugers Arch. 2010;459(3):441–449. doi: 10.1007/s00424-009-0736-1. [DOI] [PubMed] [Google Scholar]
- 173.Lopez JR, Lyckman A, Oddo S, Laferla FM, Querfurth HW, Shtifman A. Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice. J Neurochem. 2008;105(1):262–271. doi: 10.1111/j.1471-4159.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- 174.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59(2):214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457(7233):1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kawahara M, Kuroda Y. Molecular mechanism of neurodegeneration induced by Alzheimer’s beta-amyloid protein: channel formation and disruption of calcium homeostasis. Brain Res Bull. 2000;53(4):389–397. doi: 10.1016/s0361-9230(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 178.Kagan BL, Hirakura Y, Azimov R, Azimova R, Lin MC. The channel hypothesis of Alzheimer’s disease: current status. Peptides. 2002;23(7):1311–1315. doi: 10.1016/s0196-9781(02)00067-0. [DOI] [PubMed] [Google Scholar]
- 179.Price SA, Held B, Pearson HA. Amyloid beta protein increases Ca2+ currents in rat cerebellar granule neurones. NeuroReport. 1998;9(3):539–545. [PubMed] [Google Scholar]
- 180.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126(5):981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J Neurosci. 2006;26(19):5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58(6):871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Brunello L, Zampese E, Florean C, Pozzan T, Pizzo P, Fasolato C. Presenilin-2 dampens intracellular Ca2+ stores by increasing Ca2+ leakage and reducing Ca2+ uptake. J Cell Mol Med. 2009;13(9B):3358–3369. doi: 10.1111/j.1582-4934.2009.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, LaFerla FM. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J Cell Biol. 2008;181(7):1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zampese E, Fasolato C, Kipanyula MJ, Bortolozzi M, Pozzan T, Pizzo P. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc Natl Acad Sci USA. 2011;108(7):2777–2782. doi: 10.1073/pnas.1100735108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Leissring MA, Parker I, LaFerla FM. Presenilin-2 mutations modulate amplitude and kinetics of inositol 1,4,5-trisphosphate-mediated calcium signals. J Biol Chem. 1999;274(46):32535–32538. doi: 10.1074/jbc.274.46.32535. [DOI] [PubMed] [Google Scholar]
- 187.Guo Q, Furukawa K, Sopher BL, Pham DG, Xie J, Robinson N, Martin GM, Mattson MP. Alzheimer’s PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid beta-peptide. NeuroReport. 1996;8(1):379–383. doi: 10.1097/00001756-199612200-00074. [DOI] [PubMed] [Google Scholar]
- 188.Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275(24):18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 189.Lee SY, Hwang DY, Kim YK, Lee JW, Shin IC, Oh KW, Lee MK, Lim JS, Yoon DY, Hwang SJ, Hong JT. PS2 mutation increases neuronal cell vulnerability to neurotoxicants through activation of caspase-3 by enhancing of ryanodine receptor-mediated calcium release. FASEB J. 2006;20(1):151–153. doi: 10.1096/fj.05-4017fje;1. [DOI] [PubMed] [Google Scholar]
- 190.Smith IF, Green KN, LaFerla FM. Calcium dysregulation in Alzheimer’s disease: recent advances gained from genetically modified animals. Cell Calcium. 2005;38(3–4):427–437. doi: 10.1016/j.ceca.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 191.Cheung KH, Mei L, Mak DO, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal. 2010;3(114):ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer’s disease. J Neurochem. 2005;94(6):1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 193.Supnet C, Grant J, Kong H, Westaway D, Mayne M. Amyloid-beta-(1–42) increases ryanodine receptor-3 expression and function in neurons of TgCRND8 mice. J Biol Chem. 2006;281(50):38440–38447. doi: 10.1074/jbc.M606736200. [DOI] [PubMed] [Google Scholar]
- 194.Chakroborty S, Briggs C, Miller MB, Goussakov I, Schneider C, Kim J, Wicks J, Richardson JC, Conklin V, Cameransi BG, Stutzmann GE. Stabilizing ER Ca2+ channel function as an early preventative strategy for Alzheimer’s disease. PLoS One. 2012;7(12):e52056. doi: 10.1371/journal.pone.0052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Giacomello M, Barbiero L, Zatti G, Squitti R, Binetti G, Pozzan T, Fasolato C, Ghidoni R, Pizzo P. Reduction of Ca2+ stores and capacitative Ca2+ entry is associated with the familial Alzheimer’s disease presenilin-2 T122R mutation and anticipates the onset of dementia. Neurobiol Dis. 2005;18(3):638–648. doi: 10.1016/j.nbd.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 196.Zatti G, Ghidoni R, Barbiero L, Binetti G, Pozzan T, Fasolato C, Pizzo P. The presenilin 2 M239I mutation associated with familial Alzheimer’s disease reduces Ca2+ release from intracellular stores. Neurobiol Dis. 2004;15(2):269–278. doi: 10.1016/j.nbd.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 197.Zatti G, Burgo A, Giacomello M, Barbiero L, Ghidoni R, Sinigaglia G, Florean C, Bagnoli S, Binetti G, Sorbi S, Pizzo P, Fasolato C. Presenilin mutations linked to familial Alzheimer’s disease reduce endoplasmic reticulum and Golgi apparatus calcium levels. Cell Calcium. 2006;39(6):539–550. doi: 10.1016/j.ceca.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 198.Hedskog L, Pinho CM, Filadi R, Ronnback A, Hertwig L, Wiehager B, Larssen P, Gellhaar S, Sandebring A, Westerlund M, Graff C, Winblad B, Galter D, Behbahani H, Pizzo P, Glaser E, Ankarcrona M. Modulation of the endoplasmic reticulum–mitochondria interface in Alzheimer’s disease and related models. Proc Natl Acad Sci USA. 2013;110(19):7916–7921. doi: 10.1073/pnas.1300677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 200.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 201.Trinh J, Farrer M. Advances in the genetics of Parkinson disease. Nat Rev Neurol. 2013;9(8):445–454. doi: 10.1038/nrneurol.2013.132. [DOI] [PubMed] [Google Scholar]
- 202.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58(4):495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 203.Vives-Bauza C, Przedborski S. Mitophagy: the latest problem for Parkinson’s disease. Trends Mol Med. 2011;17(3):158–165. doi: 10.1016/j.molmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 204.Corti O, Brice A. Mitochondrial quality control turns out to be the principal suspect in parkin and PINK1-related autosomal recessive Parkinson’s disease. Curr Opin Neurobiol. 2013;23(1):100–108. doi: 10.1016/j.conb.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 205.Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J. 2012;31(14):3038–3062. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.McCoy MK, Cookson MR (2012) Mitochondrial quality control and dynamics in Parkinson’s disease. Antioxid Redox Signal 16(9):869–882. doi:10.1089/ars.2011.4074; 10.1089/ars.2011.4019 [DOI] [PMC free article] [PubMed]
- 207.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain. 1999;122(Pt 8):1421–1436. doi: 10.1093/brain/122.8.1421. [DOI] [PubMed] [Google Scholar]
- 208.German DC, Manaye KF, Sonsalla PK, Brooks BA. Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann N Y Acad Sci. 1992;648:42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- 209.Nedergaard S, Flatman JA, Engberg I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. J Physiol. 1993;466:727–747. [PMC free article] [PubMed] [Google Scholar]
- 210.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62(2):218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468(7324):696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Surmeier DJ, Schumacker PT. Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J Biol Chem. 2013;288(15):10736–10741. doi: 10.1074/jbc.R112.410530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ottolini D, Cali T, Negro A, Brini M. The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum–mitochondria tethering. Hum Mol Genet. 2013;22(11):2152–2168. doi: 10.1093/hmg/ddt068. [DOI] [PubMed] [Google Scholar]
- 214.Cali T, Ottolini D, Negro A, Brini M. Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca(2+) transfer to sustain cell bioenergetics. Biochim Biophys Acta. 2013;1832(4):495–508. doi: 10.1016/j.bbadis.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 215.Cali T, Ottolini D, Negro A, Brini M. alpha-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J Biol Chem. 2012;287(22):17914–17929. doi: 10.1074/jbc.M111.302794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Melachroinou K, Xilouri M, Emmanouilidou E, Masgrau R, Papazafiri P, Stefanis L, Vekrellis K. Deregulation of calcium homeostasis mediates secreted alpha-synuclein-induced neurotoxicity. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 217.Hettiarachchi NT, Parker A, Dallas ML, Pennington K, Hung CC, Pearson HA, Boyle JP, Robinson P, Peers C. alpha-Synuclein modulation of Ca2+ signaling in human neuroblastoma (SH-SY5Y) cells. J Neurochem. 2009;111(5):1192–1201. doi: 10.1111/j.1471-4159.2009.06411.x. [DOI] [PubMed] [Google Scholar]
- 218.Furukawa K, Matsuzaki-Kobayashi M, Hasegawa T, Kikuchi A, Sugeno N, Itoyama Y, Wang Y, Yao PJ, Bushlin I, Takeda A. Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. J Neurochem. 2006;97(4):1071–1077. doi: 10.1111/j.1471-4159.2006.03803.x. [DOI] [PubMed] [Google Scholar]
- 219.Marongiu R, Spencer B, Crews L, Adame A, Patrick C, Trejo M, Dallapiccola B, Valente EM, Masliah E. Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson’s disease by disturbing calcium flux. J Neurochem. 2009;108(6):1561–1574. doi: 10.1111/j.1471-4159.2009.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33(5):627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gautier CA, Giaime E, Caballero E, Nunez L, Song Z, Chan D, Villalobos C, Shen J. Regulation of mitochondrial permeability transition pore by PINK1. Mol Neurodegener. 2012;7:22. doi: 10.1186/1750-1326-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Heeman B, Van den Haute C, Aelvoet SA, Valsecchi F, Rodenburg RJ, Reumers V, Debyser Z, Callewaert G, Koopman WJ, Willems PH, Baekelandt V. Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J Cell Sci. 2011;124(Pt 7):1115–1125. doi: 10.1242/jcs.078303. [DOI] [PubMed] [Google Scholar]
- 223.Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci USA. 1997;94(4):1488–1493. doi: 10.1073/pnas.94.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lledo PM, Somasundaram B, Morton AJ, Emson PC, Mason WT. Stable transfection of calbindin-D28k into the GH3 cell line alters calcium currents and intracellular calcium homeostasis. Neuron. 1992;9(5):943–954. doi: 10.1016/0896-6273(92)90246-a. [DOI] [PubMed] [Google Scholar]
- 225.Chard PS, Bleakman D, Christakos S, Fullmer CS, Miller RJ. Calcium buffering properties of calbindin D28k and parvalbumin in rat sensory neurones. J Physiol. 1993;472:341–357. doi: 10.1113/jphysiol.1993.sp019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Watase K, Barrett CF, Miyazaki T, Ishiguro T, Ishikawa K, Hu Y, Unno T, Sun Y, Kasai S, Watanabe M, Gomez CM, Mizusawa H, Tsien RW, Zoghbi HY. Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant CaV2.1 channels. Proc Natl Acad Sci USA. 2008;105(33):11987–11992. doi: 10.1073/pnas.0804350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chen X, Tang TS, Tu H, Nelson O, Pook M, Hammer R, Nukina N, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci. 2008;28(48):12713–12724. doi: 10.1523/JNEUROSCI.3909-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP, Pulst SM, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci. 2009;29(29):9148–9162. doi: 10.1523/JNEUROSCI.0660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Giacomello M, De Mario A, Primerano S, Brini M, Carafoli E. Hair cells, plasma membrane Ca(2)(+) ATPase and deafness. Int J Biochem Cell Biol. 2012;44(5):679–683. doi: 10.1016/j.biocel.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 230.Empson RM, Turner PR, Nagaraja RY, Beesley PW, Knopfel T. Reduced expression of the Ca(2+) transporter protein PMCA2 slows Ca(2+) dynamics in mouse cerebellar Purkinje neurones and alters the precision of motor coordination. J Physiol. 2010;588(Pt 6):907–922. doi: 10.1113/jphysiol.2009.182196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Empson RM, Akemann W, Knopfel T. The role of the calcium transporter protein plasma membrane calcium ATPase PMCA2 in cerebellar Purkinje neuron function. Funct Neurol. 2010;25(3):153–158. [PubMed] [Google Scholar]
- 232.Fierro L, DiPolo R, Llano I. Intracellular calcium clearance in Purkinje cell somata from rat cerebellar slices. J Physiol. 1998;510(Pt 2):499–512. doi: 10.1111/j.1469-7793.1998.499bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hartmann J, Konnerth A. Determinants of postsynaptic Ca2+ signaling in Purkinje neurons. Cell Calcium. 2005;37(5):459–466. doi: 10.1016/j.ceca.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 234.Zhao S, Chen N, Yang Z, Huang L, Zhu Y, Guan S, Chen Q, Wang JH. Ischemia deteriorates the spike encoding of rat cerebellar Purkinje cells by raising intracellular Ca2+ Biochem Biophys Res Commun. 2008;366(2):401–407. doi: 10.1016/j.bbrc.2007.11.173. [DOI] [PubMed] [Google Scholar]
- 235.Filoteo AG, Elwess NL, Enyedi A, Caride A, Aung HH, Penniston JT. Plasma membrane Ca2+ pump in rat brain. Patterns of alternative splices seen by isoform-specific antibodies. J Biol Chem. 1997;272(38):23741–23747. doi: 10.1074/jbc.272.38.23741. [DOI] [PubMed] [Google Scholar]
- 236.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- 237.de Vries B, Frants RR, Ferrari MD, van den Maagdenberg AM. Molecular genetics of migraine. Hum Genet. 2009;126(1):115–132. doi: 10.1007/s00439-009-0684-z. [DOI] [PubMed] [Google Scholar]
- 238.Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87(3):543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 239.Pietrobon D. Calcium channels and migraine. Biochim Biophys Acta. 2013;1828(7):1655–1665. doi: 10.1016/j.bbamem.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 240.De Fusco M, Marconi R, Silvestri L, Atorino L, Rampoldi L, Morgante L, Ballabio A, Aridon P, Casari G. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet. 2003;33(2):192–196. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- 241.Rose EM, Koo JC, Antflick JE, Ahmed SM, Angers S, Hampson DR. Glutamate transporter coupling to Na, K-ATPase. J Neurosci. 2009;29(25):8143–8155. doi: 10.1523/JNEUROSCI.1081-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tavraz NN, Friedrich T, Durr KL, Koenderink JB, Bamberg E, Freilinger T, Dichgans M. Diverse functional consequences of mutations in the Na+/K+-ATPase alpha2-subunit causing familial hemiplegic migraine type 2. J Biol Chem. 2008;283(45):31097–31106. doi: 10.1074/jbc.M802771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tavraz NN, Durr KL, Koenderink JB, Freilinger T, Bamberg E, Dichgans M, Friedrich T. Impaired plasma membrane targeting or protein stability by certain ATP1A2 mutations identified in sporadic or familial hemiplegic migraine. Channels (Austin) 2009;3(2):82–87. doi: 10.4161/chan.3.2.8085. [DOI] [PubMed] [Google Scholar]
- 244.Dichgans M, Freilinger T, Eckstein G, Babini E, Lorenz-Depiereux B, Biskup S, Ferrari MD, Herzog J, van den Maagdenberg AM, Pusch M, Strom TM. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet. 2005;366(9483):371–377. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- 245.Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol. 2010;588(Pt 11):1849–1859. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cestele S, Scalmani P, Rusconi R, Terragni B, Franceschetti S, Mantegazza M. Self-limited hyperexcitability: functional effect of a familial hemiplegic migraine mutation of the Nav1.1 (SCN1A) Na+ channel. J Neurosci. 2008;28(29):7273–7283. doi: 10.1523/JNEUROSCI.4453-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kahlig KM, Rhodes TH, Pusch M, Freilinger T, Pereira-Monteiro JM, Ferrari MD, van den Maagdenberg AM, Dichgans M, George AL., Jr Divergent sodium channel defects in familial hemiplegic migraine. Proc Natl Acad Sci USA. 2008;105(28):9799–9804. doi: 10.1073/pnas.0711717105. [DOI] [PMC free article] [PubMed] [Google Scholar]