Abstract
The time of onset of cardiovascular disorders such as myocardial infarctions or ventricular arrhythmias exhibits a circadian rhythm. Diurnal variations in autonomic nervous activity, plasma cortisol level or renin–angiotensin activity underlie the pathogenesis of cardiovascular diseases. Transcriptional–translational feedback loop of the clock genes constitute a molecular clock system. In addition to the central clock in the suprachiasmatic nucleus, clock genes are also expressed in a circadian fashion in each organ to make up the peripheral clock. The peripheral clock seems to be beneficial for anticipating external stimuli and thus contributes to the maintenance of organ homeostasis. Loss of synchronization between the central and peripheral clocks also augments disease progression. Moreover, accumulating evidence shows that clock genes affect inflammatory and intracellular metabolic signaling. Elucidating the roles of the molecular clock in cardiovascular pathology through the identification of clock controlled genes will help to establish a novel therapeutic approach for cardiovascular disorders.
Keywords: Biological clock, Molecular clock, Myocardial infarction, Endothelial function, Heart, Vasculature, Arrhythmia
Introduction
The time of onset of cardiovascular diseases exhibits circadian variation [1]. For example, acute myocardial infarctions (MIs) or pulmonary embolisms mostly occur in the early morning [2]. Ventricular tachycardia/fibrillation (VT/VF) and cerebral infarction also have a tendency to develop in the morning [3]. In addition to the diurnal rhythmicity of autonomic nervous activity or the endocrine system, a molecular clock also exists in each organ or tissue, including the heart and vasculature [4]. Accumulating studies have led to the elucidation of the physiological roles of the intrinsic clock and demonstrated its contribution to the maintenance of tissue homeostasis [5]. Recent evidence has also revealed the direct roles of clock genes in inflammatory processes and cellular metabolism [6, 7]. Thus, understanding the molecular pathways of the peripheral clock will help the development of novel therapeutic approaches for cardiovascular diseases.
Molecular clock in mammalian cells
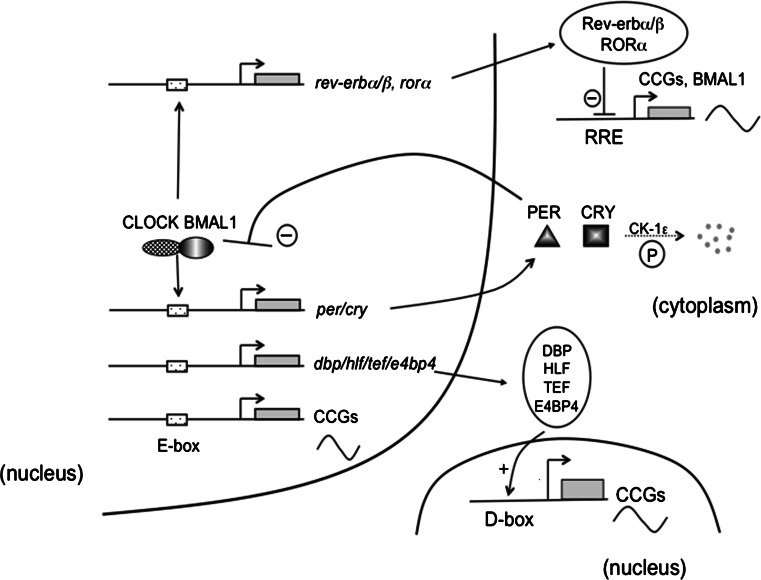
The molecular mechanisms underlying mammalian circadian regulation have been extensively studied [8–10]. Positive and negative arms constitute a transcriptional–translational loop of core clock genes. The positive arm includes CLOCK, NPAS2, BMAL1 and CLIF/BMAL2, whereas the negative arm comprises three period (PER1, PER2 and PER3) and two cryptochrome (CRY1, CRY2) proteins. CLOCK, NPAS2, BMAL1/2 and PER1/2/3 belong to a group of helix-loop-helix/per-arnt-sim domain-containing transcription factors. CLOCK or NPAS2 forms a heterodimer with BMAL1 or CLIF/BMAL2 via the PAS domain and bind to the E-box upstream of clock genes such as period or cry. PER1/2/3 and CRY1/2 proteins are phosphorylated in the cytoplasm via the serine–threonine kinase, casein kinase (CK)-1ε, and degraded through subsequent proteasomal pathways. PER and CRY proteins, however, gradually accumulate in amount and inhibit the transcriptional activity of CLOCK/BMAL1, thereby suppressing the transcription of per and cry gene themselves to form a negative feedback loop (Fig. 1). This negative feedback loop is considered to account for the autonomous 24-h oscillation of the molecular clock.
Fig. 1.
CLOCK and BMAL form a heterodimer through PAS domain, and bind to the E-box upstream of per and cry genes, and activates their transcription. PER and CRY proteins are phosphorylated by casein kinase 1 epsilon (CK-1ε) and degraded through the proteasomal pathway. PER and CRY proteins, however, gradually accumulate and inhibit CLOCK/BMAL-mediated transcription of per and cry genes. This negative feedback loop accounts for an approximate 24-h cycle of internal rhythm. Heterodimer of CLOCK and BMAL1 also activates the transcription of clock controlled genes (CCGs) directly, or via DBP/HLF/TEF mediated pathway
The CLOCK/BMAL1 heterodimer also binds to the promoters of other target genes such as arginine vasopressin and wee1, which are termed clock-controlled genes (CCGs). In addition, the CLOCK/BMAL1 heterodimer binds to the E-box upstream of the following proline and acid-rich (PAR) basic leucine zipper transcription factors: D-element binding proteins (dbp), hepatic leukemia factor (hlf) and thyrotrophic embryonic factor (tef) [11]. DBP, HLF, TEF and E4 promoter-binding Protein 4 (E4BP4) in turn bind to D-box elements, thereby eliciting the diurnal expression of CCGs. The CLOCK-BMAL1 heterodimer also activates the transcription of Rev-erbα/β and Rorα genes. REV-ERBα/β and RORα bind to the REV-ERB/ROR-binding element (RRE) and subsequently drive the cyclic expression of CCGs such as Bmal1.
We have previously identified Clif/Bmal2 as a clock gene in vascular endothelial cells [12]. However, it has also been suggested that Bmal1 is the essential clock gene and that Clif/Bmal2 may only play a minimal role in the clock system, since Bmal1 deficient mice displayed arrhythmic behavior, altered longevity and metabolic disorder [13]. This contrasts with evidence provided by recent studies that re-evaluated the roles of Clif/Bmal2 in the core clock system and showed that constitutive expression of CLIF/BMAL2 could rescue most of the behavioral or metabolic alterations in Bmal1 deficient mice [14]. Importantly, CLIF/BMAL2 expression is strikingly decreased in Bmal1-deficient mice [13], further illuminating the role of CLIF/BMAL2 in intrinsic circadian rhythm. The possibility that CLIF/BMAL2 may work as a tissue specific clock gene is supported by findings showing that it displays a circadian expression in the liver but not in the colon [15]. Circadian variation of the Bmal1 promoter activity was blunted not only in Bmal1-deficient fibroblasts, but also in Clif/Bmal2-deficient cells [16]. Therefore, further studies are needed to fully elucidate the contribution of CLIF/BMAL2 to tissue- or organ-specific circadian rhythm.
Post-translational modification also contributes to the biological clock. Proteins can be modified by monosaccharides of O-linked β–N-acetylglucosamine (O-GlcNAc) in a process termed O-GlcNAcylation. O-GlcNAcylation competes with phosphorylation reactions for the same serine or threonine residue. The level of O-GlcNAcylation modification is determined by the balance between two enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA). Importantly, both OGT and OGA transcript levels show circadian oscillation, thereby resulting in the diurnal variation of protein O-GlcNAcylation modifications in the heart [17].
In addition, there exists an epigenetic regulatory mechanism that contributes to the maintenance of the molecular clock [18]. Lys4 (K4) trimethylation of histone H3, a mark associated with transcriptional activation, of the promoter of clock genes exhibits circadian oscillation [19]. Mixed lineage leukemia 1 (MLL1), a mammalian homolog of Drosophila trithorax, works as an H3K4-specific methyltransferase. MLL1 interacts with the CLOCK-BMAL1 complex and contributes to the rhythmic recruitment of the CLOCK-BMAL1 heterodimer to the promoter region of its target genes. Furthermore, histone lysine demethylase 1a (JARID1a) also forms a complex with CLOCK-BMAL1. JARID1a inhibits the activity of histone deacetylase 1 (HDAC1) in a demethylase-independent manner and accelerates the transcription of the period gene [20]. Intriguingly, the CLOCK protein itself has intrinsic histone acetyl-transferase (HAT) activity [21]. BMAL1 enhances the HAT activity of CLOCK, thus modulating chromatin remodeling by inducing a transcription-permissive state. Moreover, CLOCK also acetylates BMAL1 as a non-histone substrate [22]. The acetylation of BMAL1 exhibits circadian variation and plays an essential role in maintaining circadian rhythmicity.
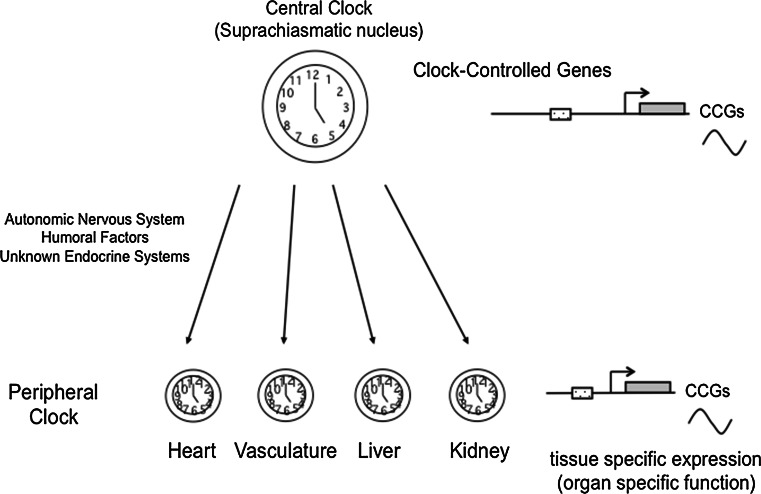
In mammals, the circadian clock in vivo can be divided into two components, a central clock and a peripheral clock. The central clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus; it receives light or other physiological signals and entrains the phase of its circadian rhythm [23]. In addition to the central clock, each cell in peripheral tissues or organs also possesses an intrinsic rhythm termed the peripheral clock [24]. The central clock orchestrates the phase of each peripheral clock through the autonomic nervous system, circulating hormones or other metabolic cues (Fig. 2). Each cell in the peripheral organs is also equipped with the molecular components required for the maintenance of an autonomous rhythm. One key feature of the central clock is the intercellular coupling that contributes to the robustness of the internal rhythm [25]. The peripheral clock in each organ directs the expression of its own target genes and is believed to play an important role in the maintenance of organ or tissue homeostasis [26].
Fig. 2.
The central clock exists in the suprachiasmatic nucleus (SCN) in the hypothalamus. In addition, clock genes expressed in a circadian fashion in each organ, thus called as peripheral clock. The central clock orchestrates the phase of each peripheral clock through the autonomic nervous system or humoral factors. Each peripheral clock plays an integral role in maintaining the tissue homeostasis
The molecular clock in the heart
Cardiomyocytes possess a peripheral clock [12]. Similar to SCN cells, a cardiomyocyte exhibits circadian expression of clock genes in response to serum shock or norepinephrine, a sympathetic neurotransmitter [27]. Microarray analysis showed that around 8–10 % of the transcripts showed circadian expression in heart and liver tissue [28]. Importantly, most of these genes are organ specific, thereby further illuminating the organ specificity of each peripheral clock.
Several genes related to intracellular metabolism or electrophysiological activity exhibit circadian expression in cardiomyocytes, including pyruvate dehydrogenase kinase isozyme-4 (pdk-4), glucose transporter 1,4 (glut-1, 4), and potassium channels Kv1.5 and Kv4.2 [5, 29, 30].
The activity of the autonomic nervous system, plasma cortisol level [31] and renin–aldosterone activity [32, 33] show intraday diurnal variation, resulting in the blood pressure (BP) varying over 24 h with a peak value occurring in the morning [34]. While a BP rise in the morning could induce an increase in afterload on cardiomyocytes, the likewise circadian expression of a cardioprotective agent, atrial natriuretic peptide (anp), may offset this consequence since diurnal variations in anp expression may be beneficial for anticipating changes in, and adapting to, the external environment (BP rise).
One of the key questions is whether the peripheral clock is altered under disease conditions. In rat heart tissue with pressure-overload hypertrophy, circadian expressions of PAR transcription factors (dbp, hlf) and anp were significantly attenuated [29]. Expression of core clock genes is also strikingly affected in myocardial ischemia. A clock gene, E4BP4, significantly accumulates during myocardial ischemia/reperfusion (I/R) and subsequently antagonizes the circadian function of the PAR family transcription factors (dbp, hlf and tef) [35]. In addition, the phase of diurnal variation has also been reported to be altered in the diabetic heart [36].
The next question is whether alterations within the internal clock could precipitate the onset of cardiovascular diseases. One study showed that repeated phase shift of light/dark (L/D) cycles in cardiomyopathic hamsters significantly compromised their survival [37]. A change in the L/D cycle length from 24-h (12/12-h L/D) to 20-h (10/10-h L/D) augmented disease severity of mice that had been subjected to the pressure-overload cardiac hypertrophy (transverse aortic constriction, TAC) model [38]. In rhythm-disturbed TAC mice, left ventricular end-systolic and diastolic dimensions were increased while contractility was decreased. Even a short-term rhythm disruption exacerbated the maladaptive post-myocardial left ventricular (LV) remodeling. Compared with control mice that were kept in a 12/12-h L/D environment, both MI area and left ventricular diameter were strikingly increased in rhythm disrupted mice that were maintained under a 10/10-h L/D cycle for 5 days immediately after being subjected to the MI model [39]. In addition to the decrease in LV systolic function and vascular density, serum levels of inflammatory cytokines were also increased in rhythm disrupted mice. These results clearly showed that disruption of external rhythmicity significantly affects longevity and cardiovascular pathology.
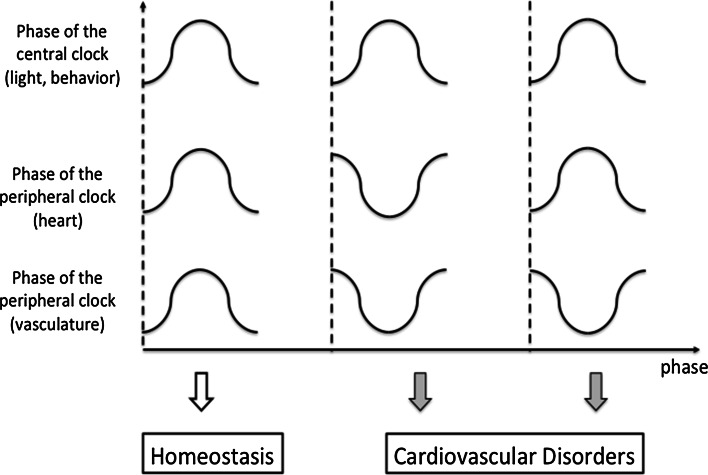
Loss of synchronization between the central and peripheral clocks may also underlie the pathogenesis of cardiovascular disorders. Casein kinase-1ε is a component of the core clock loop. Hamsters having a mutation in casein kinase-1ε are termed tau mutants, and instead of a 24-h circadian period as seen in wild type hamsters, +/tau heterozygotes have shorter (22-h) cycles. Hamsters having the +/tau mutation develop cardiomyopathy with excessive fibrosis and impaired systolic function, and die at a younger age [40]. More importantly, cardiac dysfunction was rescued when +/tau mutants were kept under a 22-hour cycle environment that fits their intrinsic rhythm. In addition, SCN ablation at a young age also prevented the development of cardiac dysfunction. These data suggest that, in addition to the disruption of the peripheral clock, dyssynchrony between the central and peripheral clocks also exacerbates cardiac dysfunction (Fig. 3).
Fig. 3.
Synchronization between the central and peripheral clock seems to be essential in keeping the physiological function and organ homeostasis. Dyssynchrony between the central and peripheral clock, or disharmonization among the peripheral clocks could exaggerate the severity of cardiovascular disorders
The roles of the internal clock in cardiovascular pathology have been examined using genetically engineered mice. Mice lacking Bmal1 have arrhythmic behavior. In addition to the alteration in circadian rhythm, Bmal1-deficient mice display a wide range of organ disorders such as infertility [41], structural and functional alterations in skeletal muscle [42], arthropathy [43], sleep disorder [44] and renal dysfunction [45]. Moreover, Bmal1-deficient mice develop age-associated dilated cardiomyopathy, characterized by a thinning of the myocardial wall and decreased cardiac function, as well as disruptions in sarcomere structure histologically [46].
To further elucidate the roles of the peripheral clock in cardiomyocytes, Young et al. generated a transgenic mouse line called the cardiomyocyte-specific Clock mutant (CCM). In order to disrupt the internal clock solely in cardiomyocytes, mutant CLOCK protein was overexpressed under the α-myosin heavy chain promoter. Through the studies using CCM mice, it became clear that intracellular metabolism of energy stores (triglyceride and glycogen) exhibits a clear circadian oscillation in a peripheral clock-dependent manner [47, 48]. Loss of synchronization between the central and peripheral clocks also augments the severity of cardiovascular disorders in CCM mice. Compared with the CCM mice that were maintained under a regular L/D (12/12 h) cycle, CCM mice kept under conditions of chronic dyssynchrony (12-h phase shift biweekly) exhibited higher expression of cardiac hypertrophy markers [49].
Cardiac dysfunction not only develops in global Bmal1-deficient mice [46], but also in cardiomyocyte-specific Bmal1-deficient mice [50]. Young et al. identified two genes, β-hydroxybutyrate dehydrogenase 1 (Bdh1) and p85α regulatory subunit of phosphatidylinositol 3-kinase (Pik3r1), as target genes of BMAL1 in cardiomyocytes. BDH1 catabolizes acetoacetate during fatty acid oxidation reactions, and while Bdh1 expression did not exhibit circadian oscillation, both Bdh1 mRNA and protein abundance were strongly reduced in the absence of Bmal1. Pik3r1 encodes a subunit of phosphatidylinositol 3-kinase (PI3 K) and regulates its intracellular signaling. In contrast to Bdh1, the Pik3r1 transcript showed circadian expression and its diurnal rhythm was lost in Bmal1 deficient mice. Intriguingly, Bmal1-deficient mice exhibit increased fatty acid oxidation and decreased glucose oxidation and glycolysis. Furthermore, heart failure with decreased ejection fraction (EF) eventually developed in cardiomyocyte-specific Bmal1-deficient mice. These results clearly demonstrate the essential role of Bmal1 for cardiomyocyte homeostasis. Moreover, Bmal1 seems to play important roles in intracellular metabolism and growth factor signaling in addition to its traditional role in intrinsic circadian rhythm. Further studies are required to fully elucidate the molecular processes of how BMAL1 protects cardiac function.
In addition to BMAL1, PER2 also has a cardioprotective function [51]. Adaptation of cardiomyocytes to hypoxia is termed ischemic preconditioning. For example, once the heart has suffered an ischemic insult, cardiomyocytes become more resistant to MI. The adenosine receptor ADORA2B plays an important role during ischemic preconditioning. Intriguingly, adora2b-mediated stabilization of PER2 contributes to its cardioprotective effects by stabilizing the glycolytic transcription factor, hypoxia inducible factor (HIF)-1α, where HIF-1α-mediated glycolytic reprogramming seems to underlie the protective effects on cardiomyocytes.
Circadian rhythm in the vasculature
One function of the vasculature is to regulate contractility in response to the external environment. Rat endothelium-dependent vasodilatory function shows clear diurnal oscillation in vitro [52]. Moreover, human endothelial vasodilative activity decreases in the early morning [53], whereas coronary artery tone increases in the morning [54]. On the other hand, blood pressure and heart rate increase in the early morning [55]. This mismatch between oxygen demand and supply is considered to underlie the morning onset of thrombotic events.
The peripheral clock also exists in the vasculature, including in endothelial cells and smooth muscle cells [56–58]. We previously identified thrombomodulin (TM), a membrane protein with anti-coagulant activity, as a clock controlled gene in vascular endothelial cells [57]. In addition, tissue inhibitor of metalloproteinase 1 and 3, collagen 3a1, transgelin1 (sm22α) and calponin1 are also known to be clock-controlled genes in vascular smooth muscle cells [59]. Phosphorylation of myosin light chain in cultured vascular smooth muscle cells also exhibited clear circadian oscillation with a 25.4-h cycle length. As an underlying mechanism, a clock gene, RORα, activates the transcription of ROCK2 gene, resulting in the circadian oscillation of ROCK2 expression and activity [60]. ROCK2 in turn phosphorylates myosin light chain, leading to the diurnal variation in vascular contractility.
The roles of clock genes in vascular physiology and nitric oxide production have been studied using mice with genetically engineered clock genes. Aortic rings from per2 mutant mice had decreased endothelium-dependent relaxation activity [61]. Aortic endothelial cells from per2 mutant mice produced less nitric oxide (NO) and vasodilatory prostaglandins whereas cyclooxygenase-1-derived vasoconstrictor production was increased, resulting in impaired vasodilatory function. BMAL1 or CLOCK also plays an essential role in normal vascular function. Akt and subsequent nitric oxide signaling in Bmal1-deficient or Clock mutant mice were strikingly attenuated, resulting in vascular injury and pathological remodeling [62]. The coupling of endothelial NO synthase (eNOS) is influenced by the ratio between tetrahydrobiopterin (BH4) and dihydrobiopterin (BH2), and the balance between BH4 and BH2 levels is in turn regulated by two key enzymes, GTP cyclochydrolase-1 (GTPCH-1) and dihydrofolate reductase (DHFR). Importantly, the expression of GTPCH1 and DHFR exhibits circadian oscillation in a Bmal1-dependent manner [63], which culminates in the diurnal oscillation of eNOS uncoupling.
Clock genes regulate arterial compliance, as demonstrated by the fact that Bmal1-deficient or Per triple knockout mice had impaired control of extracellular matrix composition, which subsequently underlies the hardening of the arteries [64]. In addition, the heterodimer of BMAL1 and NPAS2 regulates the promoter activity of NADPH oxidase 4 (NOX4) and induces its circadian expression. NOX4 expression as well as hydrogen peroxide levels was significantly increased in Bmal1 deficient mice [65], which illustrates the role of clock genes in vascular integrity.
In addition to vasodilatory function, the activities of coagulation cascades in the vasculature also show diurnal variation [66]. The balance between coagulation and fibrinolytic activity maintains the homeostasis of the coagulation cascade. Since the abundance of the plasmin–plasmin inhibitor complex decreases in the morning, this results in reduced fibrinolytic activity [67]. Plasminogen activator inhibitor-1 (PAI-1) regulates the activity of tissue plasminogen activator and affects fibrinolytic activity. It is well known that Pai-1 mRNA and protein levels in the plasma or aorta show a clear circadian oscillation [68]. We and other groups previously showed that the heterodimer of CLOCK/BMAL1/2 binds to the E-box upstream of the Pai-1 gene, thereby activating its transcription [12, 69].
In line with these findings, thrombogenesis in response to a photochemical injury shows diurnal oscillation in a clock-dependent manner [70]. The circadian variation in thrombosis was lost in Clock mutant or endothelial cell-specific Bmal1-deficient mice, therefore showing that the peripheral clock in vascular endothelial cells regulates diurnal thrombogenicity. In addition, plasma fibrinogen, factor VII and platelet counts were elevated in Bmal1-deficient mice [71]. The occlusion times during FeCl3-induced venous or arterial injury were shortened in Bmal1-deficient mice, suggesting that Bmal1-deficient mice have higher thrombogenicity. Intriguingly, the diurnal variation in plasma PAI-1 activity was preserved in endothelial cell-specific Bmal1-deficient mice, suggesting that clock controlled genes other than Pai-1 regulate the circadian rhythmicity of the thrombotic events.
Intermittent hypoxia affects coagulation processes. Diurnal oscillation of Pai-1 and tissue-type plasminogen activator (t-PA) activities was maintained in patients with obstructive sleep apnea (OSAS). The balance, however, between PAI-1 and t-PA was significantly altered in OSAS patients. PAI-1 activity was enhanced, whereas t-PA activity was decreased in OSAS patients, accounting for the increased incidence of thrombotic events in OSAS patients [72].
Clock genes also play an important role in transplant arteriosclerosis and angiogenesis. Arterial isografts obtained from wild type, Bmal1 deficient or Per triple knockout mice were anastomosed to the carotid arteries of the wild type recipient mice. Compared with grafts from the wild type donor, grafts from Bmal1-deficient mice displayed striking atherosclerotic lesions together with inflammatory cell accumulation [73]. These data suggest the involvement of the tissue intrinsic clock system in organ transplantation. A recent study in zebrafish showed a direct effect of clock genes on Vascular endothelial cell growth factor (VEGF) expression. BMAL1 binds to the E-box upstream of the VEGF gene and induces its transcription [74]. Morpholino to Bmal1 inhibited the processes of developmental angiogenesis, whereas Per2 Morpholino enhanced angiogenesis instead.
Arrhythmia and the molecular clock
The internal clock also plays an integral role in the electrophysiological function of cardiomyocytes. Basic electrophysiological parameters exhibit circadian variation, including AV nodal function and the QT interval [75–77]. Fluctuations in autonomic nerve activity contribute to the oscillation in the refractory period. In addition, the expression of two voltage-gated K channels, Kv1.5 and Kv4.2, also shows diurnal oscillation, thus leading to variations in the cellular refractory period [30]. Moreover, BMAL1 also regulates the expression of the ion channel gene sodium channel, voltage-gated, type V, alpha subunit (Scn5a), which controls Na+ current in ventricular myocytes [78]. The expression of Scn5a shows diurnal variation, thereby resulting in the variability in heart rate. Since BMAL1 directly activates the transcription of the Scn5a gene, mice with an inducible cardiomyocyte-specific deletion of Bmal1 showed slowed heart rate and prolonged RR and QRS intervals together with an increased incidence of arrhythmia. Consistent with these observations, the occurrence of ventricular premature beats (VPBs) peak in the morning in normal human subjects [3], although interestingly the diurnal variation in VPBs is lost in patients whose ejection fractions are lower than 30 % [79].
The onset of VT and VF also displays a morning peak between 7 a.m. and 11 a.m., and this is accompanied by a second peak in the afternoon [80, 81]. Circadian onset of VT/VF can be seen in both ischemic and non-ischemic heart disease [82]. The shortest refractory period in the morning seems to underlie the morning onset of VT/VF, resulting in the highest incidence of cardiac deaths in the morning [83]. As an underlying mechanism, clock genes may regulate QT-interval duration in cardiomyocytes. Kv channel-interacting protein 2 (KChIP2) encodes a subunit protein that contributes to the transient outward potassium current in cardiomyocytes, and KChIP2 is in turn transactivated by the transcription factor kruppel-like factor 15 (Klf15). BMAL1 is reported to induce the circadian expression of Klf15 [84], which leads to KLF15 transactivating the promoter of KChIP2 in a circadian fashion and results in the subsequent diurnal variation in KChIP2 expression. The variation in KChIP2 expression seems to underlie the circadian vulnerability to ventricular arrhythmia.
Circadian onset of VT/VF may be lost in patients receiving beta-blocker therapy. The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) examined the onset of ventricular arrhythmias in a population of patients with implantable cardioverter defibrillators (ICDs), and found that the onset of ventricular arrhythmias did not exhibit the typical morning peaks or events on Monday [85]. In particular, the variation in the time of onset was lost in patients receiving beta-blocker therapy.
Novel functions of clock genes: inflammation and metabolism
Accumulating evidence suggests that core clock genes also possess clock-independent functions such as in inflammation and intracellular metabolism. For example, BMAL1 strongly influences systemic inflammatory responses [7]. A population of inflammatory monocytes, Ly6Chi monocytes, expresses CCR2, a receptor for the chemokine CCL2. The number of Ly6Chi monocytes in peripheral blood and spleen shows diurnal oscillation. Intriguingly, the pathogenicity of Listeria monocytogenes infected at ZT8 was higher than that when infected at ZT0, suggesting that inflammatory responses to external pathogens also have a diurnal rhythm. BMAL1 significantly represses Ccl2 mRNA expression via the recruitment of polycomb repressive complex 2 and, consistent with this observation, Ccl2 expression was enhanced in myeloid-specific Bmal1-deficient mice. As a result, the severity of Listeria monocytogenes infection was significantly higher in Bmal1-deficient mice. It should be noted that the increase in systemic inflammation was not related to the number of Listeria infection, since bacterial colony forming units were not increased in Bmal1 deficient mice. These results show that BMAL1 mediated suppression of systemic inflammation contributes to the suppression of excessive responses to bacterial infections.
After vascular injury, progenitor cells seem to migrate into the sites of injury and promote reparative processes. The number of circulating progenitor cells, defined as those having the surface markers CD45med, CD34+, CD133+, exhibit diurnal variation [86]. REV-ERBα also contributes to inflammatory processes. For example, REV-ERBα directly represses the transcription of Ccl2 [87]. In Rev-erbα deficient mice, atheroma formation was augmented together with an increase in pro-inflammatory M1 macrophages [88]. In contrast, the overexpression of REV-ERBα reduced the number of M1 macrophages and thus resulting in the amelioration of atherosclerosis.
In addition to the immunomodulatory roles of clock genes, core clock genes also affect intracellular metabolism. PER2 directly binds to and suppresses the binding of peroxisome proliferator-activated receptor (PPAR)γ to its PPAR response elements (PPREs), thus inhibiting its function [6]. As a result, Per2 deficient fibroblasts have a higher potential to differentiate into adipocytes. Furthermore, fatty acid oxidation was increased whereas triacylglycerol accumulation was reduced in Per2 deficient mice. These anti-PPARγ roles of PER2 are considered to be independent from its function in circadian rhythm.
Conclusion
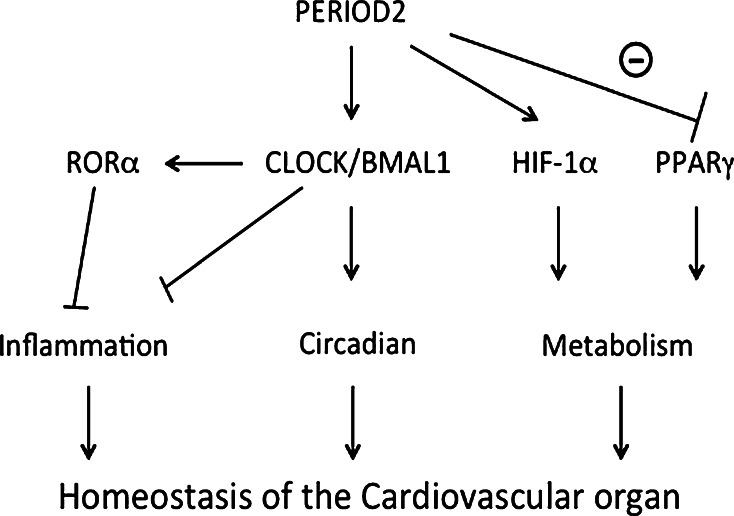
The peripheral clock in cardiovascular organs seems to play an integral role in maintaining organ homeostasis. Studies using tissue- or cell type-specific clock gene-deficient mice have allowed the elucidation of the functions of each peripheral clock. On the other hand, these approaches also uncovered unexpected functions of clock genes in inflammatory processes and intracellular metabolism (Fig. 4). As these cellular reactions have to be tightly linked for the proper maintenance of tissue homeostasis, clock genes may work to facilitate the coordination between these biological processes. Further studies are needed to fully elucidate how clock genes and the peripheral clock system coordinate the maintenance of organ homeostasis.
Fig. 4.
In addition to the maintenance of circadian rhythm, clock gene plays an integral role in inflammatory processes or intracellular metabolism. Clock genes may work to facilitate the coordination among those biological processes
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, Japan (to K.M. and N.T. 25461113).
Abbreviations
- CCG
Clock-controlled gene
- SCN
Suprachiasmatic nucleus
- ANP
Atrial natriuretic peptide
- PAR
Proline and acid-rich
- LV
Left ventricle
- MI
Myocardial infarction
- L/D
Light/dark
- TAC
Transverse aortic constriction
- CCM
Cardiomyocyte-specific clock mutant
- NO
Nitric oxide
- eNOS
Endothelial NO synthase
- PAI-1
Plasminogen activator inhibitor-1
- VT/VF
Ventricular tachycardia/fibrillation
Contributor Information
Norihiko Takeda, Phone: +81-3-3815-5411, Email: ntakeda-tky@umin.ac.jp.
Koji Maemura, Phone: +81-95-819-7286, Email: maemura@nagasaki-u.ac.jp.
References
- 1.Takeda N, Maemura K. Circadian clock and cardiovascular disease. J Cardiol. 2011;57:249–256. doi: 10.1016/j.jjcc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 3.Steinbach K, Glogar D, Weber H, Joskowicz G, Kaindl F. Frequency and variability of ventricular premature contractions–the influence of heart rate and circadian rhythms. Pacing Clin Electrophysiol. 1982;5:38–51. doi: 10.1111/j.1540-8159.1982.tb02190.x. [DOI] [PubMed] [Google Scholar]
- 4.Takeda N, Maemura K. Circadian clock and vascular disease. Hypertens Res. 2010;33:645–651. doi: 10.1038/hr.2010.68. [DOI] [PubMed] [Google Scholar]
- 5.Young ME, Razeghi P, Cedars AM, Guthrie PH, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res. 2001;89:1199–1208. doi: 10.1161/hh2401.100741. [DOI] [PubMed] [Google Scholar]
- 6.Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirayama J, Sassone-Corsi P. Structural and functional features of transcription factors controlling the circadian clock. Curr Opin Genet Dev. 2005;15:548–556. doi: 10.1016/j.gde.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Schibler U (2005) The daily rhythms of genes, cells and organs. Biological clocks and circadian timing in cells. EMBO Rep 6 Spec No:S9–S13 [DOI] [PMC free article] [PubMed]
- 11.Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol. 2006;290:H1–H16. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- 12.Maemura K, de la Monte SM, Chin MT, Layne MD, Hsieh CM, Yet SF, Perrella MA, Lee ME. CLIF, a novel cycle-like factor, regulates the circadian oscillation of plasminogen activator inhibitor-1 gene expression. J Biol Chem. 2000;275:36847–36851. doi: 10.1074/jbc.C000629200. [DOI] [PubMed] [Google Scholar]
- 13.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/S0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH. Circadian clock gene Bmal1 is not essential; functional replacement with its paralog, Bmal2. Curr Biol. 2010;20:316–321. doi: 10.1016/j.cub.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polidarova L, Sladek M, Novakova M, Parkanova D, Sumova A. Increased sensitivity of the circadian system to temporal changes in the feeding regime of spontaneously hypertensive rats—a potential role for Bmal2 in the liver. PLoS One. 2013;8:e75690. doi: 10.1371/journal.pone.0075690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki M, Yoshitane H, Du NH, Okano T, Fukada Y. Preferential inhibition of BMAL2-CLOCK activity by PER2 reemphasizes its negative role and a positive role of BMAL2 in the circadian transcription. J Biol Chem. 2009;284:25149–25159. doi: 10.1074/jbc.M109.040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durgan DJ, Pat BM, Laczy B, Bradley JA, Tsai JY, Grenett MH, Ratcliffe WF, Brewer RA, Nagendran J, Villegas-Montoya C, Zou C, Zou L, Johnson RL, Jr, Dyck JR, Bray MS, Gamble KL, Chatham JC, Young ME. O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J Biol Chem. 2011;286:44606–44619. doi: 10.1074/jbc.M111.278903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguilar-Arnal L, Sassone-Corsi P. The circadian epigenome: how metabolism talks to chromatin remodeling. Curr Opin Cell Biol. 2013;25:170–176. doi: 10.1016/j.ceb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katada S, Sassone-Corsi P. The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat Struct Mol Biol. 2010;17:1414–1421. doi: 10.1038/nsmb.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiTacchio L, Le HD, Vollmers C, Hatori M, Witcher M, Secombe J, Panda S. Histone lysine demethylase JARID1a activates CLOCK-BMAL1 and influences the circadian clock. Science. 2011;333:1881–1885. doi: 10.1126/science.1206022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- 23.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/S0092-8674(00)81199-X. [DOI] [PubMed] [Google Scholar]
- 25.Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ, 3rd, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maemura K, Layne MD, Watanabe M, Perrell MA, Nagai R, Lee ME. Molecular mechanisms of morning onset of myocardial infarction. Ann N Y Acad Sci. 2001;947:398–402. doi: 10.1111/j.1749-6632.2001.tb03972.x. [DOI] [PubMed] [Google Scholar]
- 27.Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, Shaw CA, Bray MS, Hardin PE, Young ME. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289:H1530–H1541. doi: 10.1152/ajpheart.00406.2005. [DOI] [PubMed] [Google Scholar]
- 28.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 29.Young ME, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res. 2001;88:1142–1150. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita T, Sekiguchi A, Iwasaki YK, Sagara K, Iinuma H, Hatano S, Fu LT, Watanabe H. Circadian variation of cardiac K + channel gene expression. Circulation. 2003;107:1917–1922. doi: 10.1161/01.CIR.0000058752.79734.F0. [DOI] [PubMed] [Google Scholar]
- 31.Orth DN, Island DP, Liddle GW. Experimental alteration of the circadian rhythm in plasma cortisol (17-OHCS) concentration in man. J Clin Endocrinol Metab. 1967;27:549–555. doi: 10.1210/jcem-27-4-549. [DOI] [PubMed] [Google Scholar]
- 32.Charloux A, Gronfier C, Lonsdorfer-Wolf E, Piquard F, Brandenberger G. Aldosterone release during the sleep-wake cycle in humans. Am J Physiol. 1999;276:E43–E49. doi: 10.1152/ajpendo.1999.276.1.E43. [DOI] [PubMed] [Google Scholar]
- 33.Stern N, Sowers JR, McGinty D, Beahm E, Littner M, Catania R, Eggena P. Circadian rhythm of plasma renin activity in older normal and essential hypertensive men: relation with inactive renin, aldosterone, cortisol and REM sleep. J Hypertens. 1986;4:543–550. doi: 10.1097/00004872-198610000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Hartikainen J, Tarkiainen I, Tahvanainen K, Mantysaari M, Lansimies E, Pyorala K. Circadian variation of cardiac autonomic regulation during 24-h bed rest. Clin Physiol. 1993;13:185–196. doi: 10.1111/j.1475-097X.1993.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 35.Kung TA, Egbejimi O, Cui J, Ha NP, Durgan DJ, Essop MF, Bray MS, Shaw CA, Hardin PE, Stanley WC, Young ME. Rapid attenuation of circadian clock gene oscillations in the rat heart following ischemia-reperfusion. J Mol Cell Cardiol. 2007;43:744–753. doi: 10.1016/j.yjmcc.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H. Alterations of the circadian clock in the heart by streptozotocin-induced diabetes. J Mol Cell Cardiol. 2002;34:223–231. doi: 10.1006/jmcc.2001.1504. [DOI] [PubMed] [Google Scholar]
- 37.Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13:430–436. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- 38.Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 1998;275:H2334–H2337. doi: 10.1152/ajpheart.1998.275.6.H2334. [DOI] [PubMed] [Google Scholar]
- 39.Alibhai FJ, Tsimakouridze EV, Chinnappareddy N, Wright DC, Billia F, O’Sullivan ML, Pyle WG, Sole MJ, Martino TA. Short-term disruption of diurnal rhythms after murine myocardial infarction adversely affects long-term myocardial structure and function. Circ Res. 2014;114:1713–1722. doi: 10.1161/CIRCRESAHA.114.302995. [DOI] [PubMed] [Google Scholar]
- 40.Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, Belsham DD, Backx PH, Ralph MR, Sole MJ. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1675–R1683. doi: 10.1152/ajpregu.00829.2007. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23:26–36. doi: 10.1177/0748730407311254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci USA. 2010;107:19090–19095. doi: 10.1073/pnas.1014523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, Bradfield CA. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 2005;41:122–132. doi: 10.1002/gene.20102. [DOI] [PubMed] [Google Scholar]
- 44.Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28:395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Yang Z, Niu Z, Wang W, Peng J, Li Q, Ma MY, Zhao Y. The mortality of MOP3 deficient mice with a systemic functional failure. J Biomed Sci. 2006;13:845–851. doi: 10.1007/s11373-006-9108-4. [DOI] [PubMed] [Google Scholar]
- 46.Lefta M, Campbell KS, Feng HZ, Jin JP, Esser KA. Development of dilated cardiomyopathy in Bmal1-deficient mice. Am J Physiol Heart Circ Physiol. 2012;303:H475–H485. doi: 10.1152/ajpheart.00238.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, Shaw CA, Hardin PE, Bray MS, Chandler MP, Chow CW, Young ME. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254–24269. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 48.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 49.Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, Garvey ME, Nagendran J, Dyck JR, Bray MS, Gamble KL, Gimble JM, Young ME. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol Int. 2011;28:187–203. doi: 10.3109/07420528.2010.550406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young ME, Brewer RA, Peliciari-Garcia RA, Collins HE, He L, Birky TL, Peden BW, Thompson EG, Ammons BJ, Bray MS, Chatham JC, Wende AR, Yang Q, Chow CW, Martino TA, Gamble KL. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms. 2014;29:257–276. doi: 10.1177/0748730414543141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guney HZ, Hodoglugil U, Uluoglu C, Gorgun CZ, Ercan ZS, Abacioglu N, Zengil H. In vitro susceptibility rhythms. II. Biological-time-dependent differences in effect of beta 1- and beta 2-adrenergic agonists of rat aorta and influence of endothelium. Chronobiol Int. 1998;15:159–172. doi: 10.3109/07420529808998680. [DOI] [PubMed] [Google Scholar]
- 53.Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V. Early morning attenuation of endothelial function in healthy humans. Circulation. 2004;109:2507–2510. doi: 10.1161/01.CIR.0000128207.26863.C4. [DOI] [PubMed] [Google Scholar]
- 54.Fujita M, Franklin D. Diurnal changes in coronary blood flow in conscious dogs. Circulation. 1987;76:488–491. doi: 10.1161/01.CIR.76.2.488. [DOI] [PubMed] [Google Scholar]
- 55.Kobrin I, Oigman W, Kumar A, Ventura HO, Messerli FH, Frohlich ED, Dunn FG. Diurnal variation of blood pressure in elderly patients with essential hypertension. J Am Geriatr Soc. 1984;32:896–899. doi: 10.1111/j.1532-5415.1984.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 56.McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/S0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 57.Takeda N, Maemura K, Horie S, Oishi K, Imai Y, Harada T, Saito T, Shiga T, Amiya E, Manabe I, Ishida N, Nagai R. Thrombomodulin is a clock-controlled gene in vascular endothelial cells. J Biol Chem. 2007;282:32561–32567. doi: 10.1074/jbc.M705692200. [DOI] [PubMed] [Google Scholar]
- 58.Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, Yagita K, Okamura H, Yokoyama M. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation. 2001;104:1746–1748. doi: 10.1161/hc4001.098048. [DOI] [PubMed] [Google Scholar]
- 59.Chalmers JA, Martino TA, Tata N, Ralph MR, Sole MJ, Belsham DD. Vascular circadian rhythms in a mouse vascular smooth muscle cell line (Movas-1) Am J Physiol Regul Integr Comp Physiol. 2008;295:R1529–R1538. doi: 10.1152/ajpregu.90572.2008. [DOI] [PubMed] [Google Scholar]
- 60.Saito T, Hirano M, Ide T, Ichiki T, Koibuchi N, Sunagawa K, Hirano K. Pivotal role of Rho-associated kinase 2 in generating the intrinsic circadian rhythm of vascular contractility. Circulation. 2013;127:104–114. doi: 10.1161/CIRCULATIONAHA.112.135608. [DOI] [PubMed] [Google Scholar]
- 61.Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, Zaugg CE, Ming XF, Montani JP, Albrecht U, Yang Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation. 2007;115:2188–2195. doi: 10.1161/CIRCULATIONAHA.106.653303. [DOI] [PubMed] [Google Scholar]
- 62.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anea CB, Cheng B, Sharma S, Kumar S, Caldwell RW, Yao L, Ali MI, Merloiu AM, Stepp DW, Black SM, Fulton DJ, Rudic RD. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ Res. 2012;111:1157–1165. doi: 10.1161/CIRCRESAHA.111.261750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anea CB, Ali MI, Osmond JM, Sullivan JC, Stepp DW, Merloiu AM, Rudic RD. Matrix metalloproteinase 2 and 9 dysfunction underlie vascular stiffness in circadian clock mutant mice. Arterioscler Thromb Vasc Biol. 2010;30:2535–2543. doi: 10.1161/ATVBAHA.110.214379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anea CB, Zhang M, Chen F, Ali MI, Hart CM, Stepp DW, Kovalenkov YO, Merloiu AM, Pati P, Fulton D, Rudic RD. Circadian clock control of Nox4 and reactive oxygen species in the vasculature. PLoS One. 2013;8:e78626. doi: 10.1371/journal.pone.0078626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bridges AB, McLaren M, Scott NA, Pringle TH, McNeill GP, Belch JJ. Circadian variation of tissue plasminogen activator and its inhibitor, von Willebrand factor antigen, and prostacyclin stimulating factor in men with ischaemic heart disease. Br Heart J. 1993;69:121–124. doi: 10.1136/hrt.69.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kurnik PB. Circadian variation in the efficacy of tissue-type plasminogen activator. Circulation. 1995;91:1341–1346. doi: 10.1161/01.CIR.91.5.1341. [DOI] [PubMed] [Google Scholar]
- 68.Naito Y, Tsujino T, Kawasaki D, Okumura T, Morimoto S, Masai M, Sakoda T, Fujioka Y, Ohyanagi M, Iwasaki T. Circadian gene expression of clock genes and plasminogen activator inhibitor-1 in heart and aorta of spontaneously hypertensive and Wistar-Kyoto rats. J Hypertens. 2003;21:1107–1115. doi: 10.1097/00004872-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 69.Schoenhard JA, Smith LH, Painter CA, Eren M, Johnson CH, Vaughan DE. Regulation of the PAI-1 promoter by circadian clock components: differential activation by BMAL1 and BMAL2. J Mol Cell Cardiol. 2003;35:473–481. doi: 10.1016/S0022-2828(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 70.Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, Bradfield CA, FitzGerald GA. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117:2087–2095. doi: 10.1161/CIRCULATIONAHA.107.739227. [DOI] [PubMed] [Google Scholar]
- 71.Hemmeryckx B, Van Hove CE, Fransen P, Emmerechts J, Kauskot A, Bult H, Lijnen HR, Hoylaerts MF. Progression of the prothrombotic state in aging Bmal1-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:2552–2559. doi: 10.1161/ATVBAHA.111.229062. [DOI] [PubMed] [Google Scholar]
- 72.Bagai K, Muldowney JA, 3rd, Song Y, Wang L, Bagai J, Artibee KJ, Vaughan DE, Malow BA. Circadian variability of fibrinolytic markers and endothelial function in patients with obstructive sleep apnea. Sleep. 2014;37:359–367. doi: 10.5665/sleep.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng B, Anea CB, Yao L, Chen F, Patel V, Merloiu A, Pati P, Caldwell RW, Fulton DJ, Rudic RD. Tissue-intrinsic dysfunction of circadian clock confers transplant arteriosclerosis. Proc Natl Acad Sci USA. 2011;108:17147–17152. doi: 10.1073/pnas.1112998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jensen LD, Cao Z, Nakamura M, Yang Y, Brautigam L, Andersson P, Zhang Y, Wahlberg E, Lanne T, Hosaka K, Cao Y. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Rep. 2012;2:231–241. doi: 10.1016/j.celrep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Bexton RS, Vallin HO, Camm AJ. Diurnal variation of the QT interval–influence of the autonomic nervous system. Br Heart J. 1986;55:253–258. doi: 10.1136/hrt.55.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cinca J, Moya A, Bardaji A, Rius J, Soler-Soler J. Circadian variations of electrical properties of the heart. Ann N Y Acad Sci. 1990;601:222–233. doi: 10.1111/j.1749-6632.1990.tb37303.x. [DOI] [PubMed] [Google Scholar]
- 77.Oda E, Aizawa Y, Arai Y, Shibata A. Diurnal variation of QT interval in patients with VVI pacemaker. Tohoku J Exp Med. 1985;145:419–426. doi: 10.1620/tjem.145.419. [DOI] [PubMed] [Google Scholar]
- 78.Schroder EA, Lefta M, Zhang X, Bartos DC, Feng HZ, Zhao Y, Patwardhan A, Jin JP, Esser KA, Delisle BP. The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility. Am J Physiol Cell Physiol. 2013;304:C954–C965. doi: 10.1152/ajpcell.00383.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gillis AM, Peters RW, Mitchell LB, Duff HJ, McDonald M, Wyse DG. Effects of left ventricular dysfunction on the circadian variation of ventricular premature complexes in healed myocardial infarction. Am J Cardiol. 1992;69:1009–1014. doi: 10.1016/0002-9149(92)90855-S. [DOI] [PubMed] [Google Scholar]
- 80.Behrens S, Galecka M, Bruggemann T, Ehlers C, Willich SN, Ziss W, Dissmann R, Andresen D. Circadian variation of sustained ventricular tachyarrhythmias terminated by appropriate shocks in patients with an implantable cardioverter defibrillator. Am Heart J. 1995;130:79–84. doi: 10.1016/0002-8703(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 81.Kozak M, Krivan L, Semrad B. Circadian variations in the occurrence of ventricular tachyarrhythmias in patients with implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2003;26:731–735. doi: 10.1046/j.1460-9592.2003.00124.x. [DOI] [PubMed] [Google Scholar]
- 82.Englund A, Behrens S, Wegscheider K, Rowland E. Circadian variation of malignant ventricular arrhythmias in patients with ischemic and nonischemic heart disease after cardioverter defibrillator implantation. European 7219 Jewel Investigators. J Am Coll Cardiol. 1999;34:1560–1568. doi: 10.1016/S0735-1097(99)00369-1. [DOI] [PubMed] [Google Scholar]
- 83.Willich SN, Maclure M, Mittleman M, Arntz HR, Muller JE. Sudden cardiac death. Support for a role of triggering in causation. Circulation. 1993;87:1442–1450. doi: 10.1161/01.CIR.87.5.1442. [DOI] [PubMed] [Google Scholar]
- 84.Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS, Jain MK. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–99. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patton KK, Hellkamp AS, Lee KL, Mark DB, Johnson GW, Anderson J, Bardy GH, Poole JE. Unexpected deviation in circadian variation of ventricular arrhythmias: the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) J Am Coll Cardiol. 2014;63:2702–2708. doi: 10.1016/j.jacc.2013.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al Mheid I, Corrigan F, Shirazi F, Veledar E, Li Q, Alexander WR, Taylor WR, Waller EK, Quyyumi AA. Circadian variation in vascular function and regenerative capacity in healthy humans. J Am Heart Assoc. 2014;3:e000845. doi: 10.1161/JAHA.114.000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192:407–417. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 88.Ma H, Zhong W, Jiang Y, Fontaine C, Li S, Fu J, Olkkonen VM, Staels B, Yan D. Increased atherosclerotic lesions in LDL receptor deficient mice with hematopoietic nuclear receptor Rev-erbalpha knock- down. J Am Heart Assoc. 2013;2:e000235. doi: 10.1161/JAHA.113.000235. [DOI] [PMC free article] [PubMed] [Google Scholar]