Abstract
Approximately, up to 70 % of the human population is infected with cytomegalovirus (CMV) that persists for life in a latent state. In healthy people, CMV reactivation induces the expansion of CMV-specific T cells up to 10 % of the entire T cell repertoire. On the contrary, CMV infection is a major opportunistic viral pathogen that remains a leading cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation. Due to the delayed CMV-specific immune recovery, the incidence of CMV reactivation during post-transplant period is very high. Several methods are currently available for the monitoring of CMV-specific responses that help in clinical monitoring. In this review, essential aspects in the immune recovery against CMV are discussed to improve the better understanding of the immune system relying on CMV infection and, thereby, helping the avoidance of CMV disease or reactivation following hematopoietic stem cell transplantation with severe consequences for the transplanted patients.
Keywords: CMV-specific T cells, Allogeneic HSCT, Immune monitoring
Introduction
Human cytomegalovirus (CMV) is a member of the β-herpesvirus family present in approximately 70 % of the adult population [1, 2]. In healthy immunocompetent individuals, following primary infection, the virus and the immune system reach a homeostatic balance, and life-long asymptomatic latency is established predominantly in cells of the myeloid lineage where intermittent sub-clinical reactivations are successfully controlled by the immune system [3]. By contrast, CMV infection/reactivation can cause severe disease and even mortality in the absence of an effective immune response including immunocompromised individuals and immunologically immature neonates or newborns [4, 5]. In this sense, congenital CMV infection is the most frequent and important intrauterine infection that occurs during pregnancy [6]. On the other hand, recipients of solid organ or allogeneic hematopoietic stem cell are treated with immunosuppressive drugs which target both the CD8+ and CD4+ T cell compartments that are critical for the CMV immune control. This deficit allows uncontrolled CMV replication, and may lead to the development of life-threatening end-organ damage.
This is an overview of the most relevant findings regarding the host immune response following CMV infection in healthy individuals and immunocompromised patients, the diagnostic and treatment options, immune-monitoring tools and the attempts to develop protective anti-CMV immune strategies.
CMV immune responses
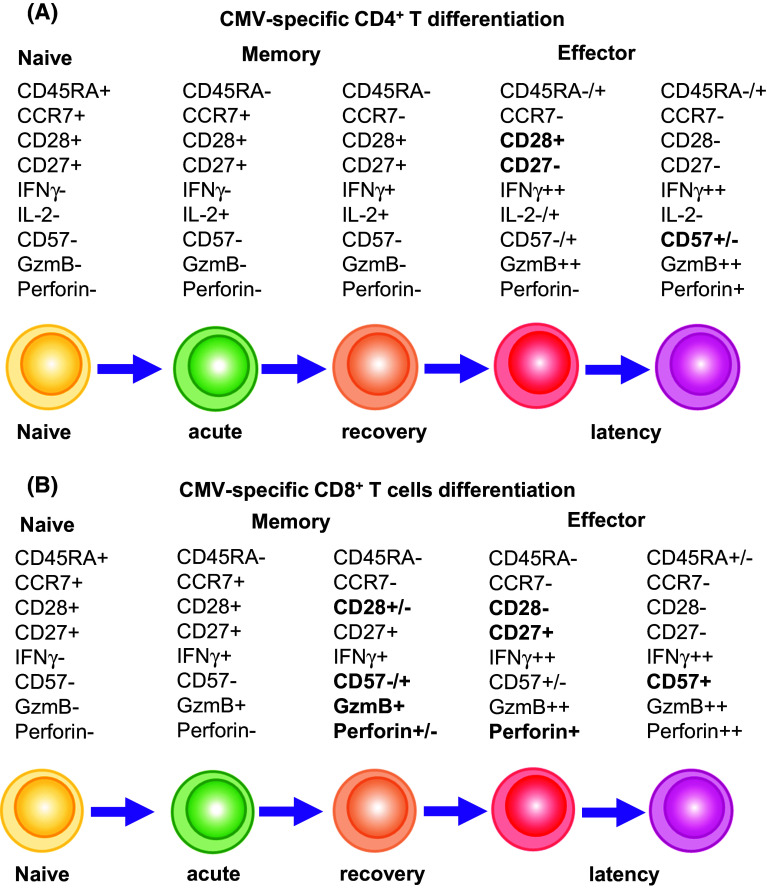
The time course of the appearance of CMV-specific immune responses in healthy individuals is difficult to follow, as the beginning of primary infection normally goes unnoticed. Once CMV establishes primary infection, several mechanisms and pathways of the innate immune response are activated. After CMV infection, monocytes, macrophages and dendritic cells (DCs) release inflammatory cytokines and upregulate co-stimulatory molecules that slow down the pathogen before an adequate adaptive immune response is developed [7]. Once the virus disseminates to cells of myeloid lineage including monocytes and CD34 cells, it establishes latent infection [8, 9]. Some authors have suggested that CD4+ T cells are essential for the initiation of virus-specific CD8+ T cell expansion after primary infection [10, 11], which eliminate the virus and are thought to be the major effector cells in controlling persistent infections. Van der Berg et al. [2] have studied CMV-naïve individuals that received donor kidney from CMV carriers as a model for analysing primary CMV infection. According to their results, they proposed that CMV-specific CD4+ T cells appear 1 week after the peak of CMV replication and synthesize T helper 1 type (Th1) cytokines (IFNγ and TNFα) [8, 12, 13]. Following primary CMV infection, CMV-specific CD4+ T cells show a phenotype of recently activated naïve T cells co-expressing CD45RA and CD45RO surface markers, co-stimulatory receptors CD27 and CD28 and the cell cycle-associated nuclear marker ki67 (Fig. 1a). Then, CMV-specific CD8+ T cells become detectable in peripheral blood and have an effector-memory (TEM) phenotype characterised by the loss of CD45RA and CCR7 cell surface markers [14]. These virus-specific CD8+ T cells express perforin, granzyme B and CD95 and have the capacity of lysing CMV-peptide presenting target cells [15, 16]. In the following months after primary infection, CMV-specific CD8+ T cells gradually lose CD27 and re-acquire CD45RA expression [known as terminally differentiated effector-memory CD45RA T cells (TEMRA)] which seems to increase with age, but still maintain their cytolytic potential (Fig. 1b). On the contrary, CMV-specific CD4+ T cells keep an effector-memory phenotype which is not related to age [17]. During latency, CMV-specific cells express CD57 but lose ki67 expression. However, these specific cells are not exhausted and can respond to reactivation of latent virus in vivo [18, 19]. T cell exhaustion is characterised by a progressive loss in the ability of CD8+ T cells to produce pro-inflammatory cytokines (IL-2, TNFα, IFNγ), as well as to survive, proliferate and kill targets. Notably, during chronic CMV infection in healthy individuals, CMV-specific T cells do not upregulate programmed cell death (PD)-1, an inhibitory molecule which is strongly associated with antigen-driven T cell exhaustion [20–22]. Indeed, even in the absence of CD4+ T cells, which accelerates CD8+ T cell exhaustion after chronic CMV infection [23, 24], only a small CD8+ T cell proportion is dysfunctional [21, 25]. Importantly, the released Th1 cytokines characterise early and late virus-specific T cells that accumulate during latency.
Fig. 1.
T cell differentiation. Phenotypic evolution of CD4+ (a) and CD8+ (b) T cells. CMV-experienced T cytotoxic cells exhibit a different functional phenotype compared with naïve cells. Expression of different molecules such as CD45RA, CCR7, CD28, CD27, CD57 or mediators of cytotoxicity such as IFNγ, granzyme B (GzmB) or perforin
It is noteworthy to mention that frequencies of CMV-specific T cells in healthy individuals are much higher than those observed for other human viruses, such as Epstein–Barr virus and Adenovirus [26]. It has been demonstrated by Sylwester et al. [27] that CMV-specific T cells dominate the memory compartment of infected subjects, where CD4+ and CD8+ cells comprise approximately 10 % of the memory compartments in the peripheral blood. However, these T cells, despite restraining viral replication and preventing disease, do not eliminate the virus which persists as a latent infection in the host.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a well-established clinical procedure used to treat haematological malignancies and inherited or acquired non-malignant disorders of blood cells, such as sickle-cell anaemia, aplastic anaemia, leukaemias, lymphomas, inherited diseases, immunodeficiencies and congenital or acquired severe disorders of the hematopoietic system [28–30].
The procedure of allo-HSCT requires partial or total elimination of the recipient’s hematopoietic and immune systems through pre-transplant chemotherapy that is sometimes combined with radiotherapy and T cell depleting antibodies. This conditioning treatment provides space for incoming cells to engraft, helps in preventing graft rejection and kills most residual cancer cells. Then, immunosuppressed recipients are infused with hematopoietic stem cell obtained from the donor, predominantly by direct collection from the bone marrow or by peripheral blood stem cell (PBSC) harvest following granulocyte colony-stimulating factor (G-CSF) treatment [31]. Donor bone marrow or PBSCs are enriched in hematopoietic progenitors, but they also contain mature CD4+ and CD8+ αβT cells that promote hematopoietic engraftment, help T cell immunity reconstitution and mediate a potent beneficial anti-tumour effect that is known as graft versus leukaemia (GvL).
In this microenvironment, restoration of adaptive immunity following allo-HSCT is a slow process. Naïve (CD45RA+CCR7+) T cells first appear at about 4 months after allo-HSCT, and the complete recovery of naïve T cell pool may require 1 to 2 years. T cell counts recover much earlier by peripheral expansion [32]. Post-thymic donor-derived T cells expand rapidly after allo-HSCT. These cells are central memory (CD45−CCR7+) (TCM) and TEM cells which are crucial for the success or failure of allo-HSCT due to their impact on the engraftment, graft versus host disease (GvHD), GvL and antiviral therapy [33]. In early stages post-transplant, the mature repertoire from the donor interacts with the new recipient’s environment, leading to clonal expansion against diverse antigens driven by lymphopenia and cytokines. Early clonal expansion of CMV-specific cytotoxic T cells (CTLs) depends on the presence of cells with these specificities in the donor [34].
Most studies have focused on CD8+ T cell immunity and have shown that after primary infection there is an increase of CMV-specific CD8+ T cells which is related to a reduction in both CMV reactivation and disease [35–38]. Later on during persistent infection, there is an equilibrium between viral replication and cellular immune responses.
However, several studies suggest that functional CMV-specific CD8+ T cells are not sufficient to control viral replication and effector-memory CD4+ T cells are necessary for the recovery of infection. In a study by Sester M et al., it was observed that during the steady state of latent CMV infection, CMV-specific CD4+ T cells may play a role in maintaining the state of antiviral immunity [39]. Gamadia et al. found that whereas in asymptomatic CMV infection CMV-specific CD4+ T cell responses precede CMV-specific CD8+ T cell responses, in symptomatic CMV infection CMV-specific effector-memory CD4+ T cell responses are delayed and only detectable after antiviral therapy [11]. Recent studies developed by Flinsenberg and collaborators supported these findings [40]. They also reported that cognate interaction between CD4+ T helper cells and DCs would enable DCs to stimulate more effective CTL responses. It has been demonstrated that CMV-specific CD4+ T cell response is dominated by large oligoclonal expansions of cells with cytotoxic activity and predominant production of IFNγ [41]. The impaired control of viral replication can be explained by the lack of IFNγ secreting effector-memory CD4+ T cells at the side of infection in allo-HSCT patients [11]. In addition, Gamadia et al. have demonstrated that in CMV reactivation, CMV-specific CD4+ T cell response, present before reactivation, was not detectable during viral replication, indicating that these cells moved to peripheral target sites of infection. It has been found that functional recovery of specific CD8+ T cell-mediated cytotoxicity after transplantation may require expansion and activation of virus-specific T helper cells [42]. So, CD4+ T cells seem to be necessary for the regulation of cell-mediated immunity, promoting cytotoxic T cell activity through Th1 cytokine elaboration and activation of antigen-presenting cells [43–45].
Pourgherysari et al. [46] found that patients with poor CMV-specific CD4+ and CD8+ T cell responses within the first 100 days post-transplantation were at very high risk of recurrent viral reactivation. They also described that T cell lymphopenia observed during this time is associated with recurrent viral activation.
In summary, immunity against CMV in patients after allo-HSCT seems to reduce the risk of CMV infection [47, 48]. Patients with a positive response against CMV have seemed to harbour lower virus load and more rapid clearance of CMV compared to their negative counterparts, suggesting the importance of CMV immunity in clearing the infection in those patients [49]. An early recovery of CMV-specific CTLs correlates with a rapid resolution of CMV infection and thus short duration of antiviral therapy [50]. On the contrary, late reconstitution of CMV-specific CTLs is associated with prolonged antiviral treatment and a major risk for developing late CMV disease [51]. Therefore, reconstitution of both functional CMV-specific CD4+ and CD8+ T cells after allo-HSCT seems to be protective against the development of CMV, and it is associated with the resolution of the CMV infection [52].
Factors that may modify responses in patients following allogeneic hematopoietic stem cell transplantation
Patients following allo-HSCT are at higher risk of active CMV infection due to the delayed recovery of T and B cell functions leading to an impaired CTL response [53–56]. This delay is influenced by the type of conditioning regimen, prophylaxis and treatment to prevent GvHD that induce a profound immunosuppression in the patient.
Donor and recipient serostatus
It has been established that the CMV serostatus of the donor affects the outcome of the allo-HSCT [57]. In that sense, the selection of the donor may depend on the serostatus of the allo-HSCT recipient [58]. For a CMV-seronegative recipient (R-), it is preferable to use a CMV-seronegative donor (D-) to reduce the possibility of primary CMV infection associated with a seropositive allograft [59]. On the contrary, stem cells from a seropositive donor (D+) are preferred for a seropositive recipient (R+) [60]. Lilleri et al. [52] have reported that CMV-seropositive patients showed a much higher incidence of CMV infection than CMV-seronegative recipients. However, this post-transplant CMV reactivation represents the major factor driving CMV-specific immune reconstitution [61, 62]. There are some cases in which immunity was reconstituted in the absence of detected infection, probably due to a silent infection occurring in a target organ. This mechanism may be similar for the immune recovery in seropositive donors and seronegative patients. However, an antigen-dependent, cytokine-driven expansion may help immune reconstitution [63].
Recent studies suggest that D+/R+ transplants generate higher levels of multifunctional CMV-specific T cells even in the absence of detectable CMV reactivation and also require less antiviral therapy compared with D−/R+, in which the reconstitution of CMV-specific cellular immunity is dependent on CMV antigen exposure during CMV reactivation [5, 64]. This fact may be explained by the transfer of T cells present in the allograft from a CMV-seropositive donor, in which both naïve and memory/effector CMV-specific T cells are transferred to the recipient. In grafts from seronegative donors, only naïve CMV-specific T cells are transferred. Approximately, 30 % of D+/R− develop primary CMV infection. Although the risk of CMV disease is low because of the pre-emptive treatment of CMV infection, the mortality caused by fungal or bacterial infections is higher in D+/R− compared to D−/R− (18.3 vs. 9.7 %, respectively), possibly because of the immunosuppressive effects of CMV therapy [65]. Borchers et al. have shown that the kinetic of CMV-CTL reconstitution is different in CMV-seropositive recipients receiving a transplant from either a CMV-seropositive or a seronegative donor. D-/R+ showed a delayed reconstitution of CMV immunity compared to D+/R+ (day +120 vs. day +30, respectively) [66]. Besides, they observed that the highest number of CMV-CTLs was found in the D+/R+ group compared with significantly lower numbers in the other groups (D−/R+, D−/R−, D+/R−) [67].
Degree of HLA disparity
Human leukocyte antigen (HLA) disparity between the host and the donor is another factor that may contribute to high risk of CMV infection. Based on HLA high-resolution typing for class I (HLA-A, -B, -C) and class II (HLA-DRB1, -DQB1), a well-matched donor is defined as 10/10 or 8/8 (when DQ is not taken into account) identical donor. If there is any difference, it is considered as mismatched. Borchers and colleagues have found that CMV reactivation was more frequent in patients transplanted from related or unrelated mismatched donors; most of them had GvHD and, therefore, they received steroids treatment that may led to a delay on CMV-specific CD4+ and CD8+ T cell immune recovery [67]. These observations agree with the results obtained by Mead et al. [68] in which they observe that CMV infection was more frequent in mismatched unrelated donors compared to matched unrelated donors (56 vs. 30 %). Jaskula and collaborators found that a lack of optimal donor/recipient HLA matching was associated with a higher risk of acute GvHD and with a higher rate of CMV reactivation/infection [69].
Immunosuppression
The immunosuppressive regimens that allow the recipient to keep the graft and avoid GvHD complications also play a role in CMV replication. Allo-HSCT recipients treated with high-dose corticosteroids (>1 mg/kg/day), mycophenolate mofetil (MMF) and certain anti-T cell strategies are considered at high risk for CMV disease. Hambach et al. reported that allo-HSCT patients treated with MMF had an increased risk of CMV disease or CMV-related complications as it seems by an upregulated CMV replication [70]. Besides, viral load may increase in highly immunosuppressed individuals receiving antiviral drugs [71]. Lilleri and coworkers found that in the presence of acute GvHD and chronic GvHD requiring steroid treatment, specific immune reconstitution did not protect against CMV infection, which required antiviral treatment [52].
Conditioning regimens
In allo-HSCT, the intensity of the conditioning regimens can be nonmyeloablative (NMA) or myeloablative (MA) conditioning. NMA conditioning regimen is based on combination of different drugs, such as fludarabine/busulfan, fludarabine/melfalan and fludarabine/cyclophosphamide in all situations with or without combination with alentuzumab. On the contrary, MA conditioning regimen is a combination of cyclophosphamide/total body irradiation, busulfan/cyclophosphamide and sometimes either alentuzumab or anti-thymocyte globuline. Some groups have studied the CMV immune recovery after allo-HSCT of patients according to pre-allo-HSCT treatment. Nakamae and colleagues have shown that NMA-allo-HSCT was associated with a lower risk of high-grade CMV infection; however, this effect does not appear to protect against complications of CMV [72]. Another recent study developed by Kim et al. showed that CMV reactivation was less common in NMA-HSCT patients compared to MA early after HSCT, while there is no difference during the late-recovery period [73]. Patients that have received alemtuzumab treatment have shown a high rate of early CMV reactivation; the numbers of CD4+ and CD8+ T cells remained low within the first 3 months but they started recovering on day 90. However, the number of CMV-specific T cells increased after 180 days post-HSCT [74]. In summary, the higher incidence of CMV reactivation has been related to an inadequate or defective recovery of CD8+ T cells as a consequence of the conditioning regimen [38].
Prophylactic and pre-emptive treatment
Antiviral drugs have been developed and employed for either prophylactic or pre-emptive therapies and for direct treatment for CMV disease. The aim of prophylaxis therapy is to reduce the incidence of CMV infection or disease after transplantation while the purpose of pre-emptive therapy consists of monitoring the CMV reactivation and early intervention when CMV reactivation is detected.
The use of immune globulin as prophylaxis has no effect in reducing the incidence of CMV disease or CMV infection in allo-HSCT recipients [59, 75, 76]. Prophylaxis with acyclovir and valacyclovir has been shown to reduce the risk of CMV infection or disease improving survival within 100 days after transplantation as Ljungman et al. reported [77]. However, it should be combined with a pre-emptive strategy using sensitive assays to monitor CMV.
The most common antiviral drugs used to treat CMV pre-emptively are ganciclovir (GCV), valganciclovir (VGC) and foscarnet. Although these drugs are considered effective strategies for preventing CMV disease early after transplantation, they are associated with drug-related toxicities (myelosuppressive and immunosuppressive effects) [78] and they have been found to impair and delay the development of CMV-specific CTLs [50, 79]. Lilleri et al. have shown that patients treated pre-emptively developed protection against CMV at a median time of 139 days compared to those that resolved CMV infection or did not have infection (median time 70 days) [52, 80]. It is possible to use cidofovir when patients fail the first-line of antiviral treatment [81, 82].
Despite the need of prophylactic or pre-emptive treatment for CMV infection early after transplantation, life-threatening complications such as secondary bacterial and fungal infections are related to them [83, 84]. The incidence of CMV disease within the first 4 months in patients following HSCT has been reduced due to prophylactic and pre-emptive strategies to less than 5 % [85]. Nevertheless, approximately between 10 and 30 % of the patients develop a delayed onset of CMV disease after 100 days post-transplant because of anti-viral prophylaxis and early intervention [51, 53, 86, 87]. It may be due to the resistance to the drugs as they share similar mechanisms of action [88] or to delayed reconstitution of CMV-specific T cell responses, representing one of the leading causes of mortality after allo-HSCT [50, 89, 90].
Graft manipulations
The use of T cell depletion and G-CSF-mobilised stem cells conditioning protocol are used to improve engraftment and reduce GvHD. However, as these manipulations may be involved in the risk of CMV infection, non-T cell depleted transplants are preferred when possible.
The CD34-positive selection of PBSCs allows GvHD to be minimised by effective reduction of T cells in the graft. A study by Lilleri et al. [80] reported that CMV-specific T cell reconstitution was significantly delayed in those patients receiving T cell depleted grafts by collection of CD34+ progenitor cells compared to those who received unmanipulated HSCT (median time of CMV-specific T cell reconstitution of 75 vs. 47 days, respectively).
In contrast to CD34-positive selection, CD3/CD19-depleted peripheral allografts contain other immune components, such as natural killer (NK) cells, DCs and monocytes that may be used to generate anti-leukaemic, anti-viral or graft-facilitating effects. Recent studies have shown that T cell recovery after CD3/CD19-depleted graft achieves normal values within the first 60 days after transplantation [91, 92]. However, when CD3/CD19-depletion is used in combination with NMA regimen, T cell reconstitution is delayed to 3 months after allo-HSCT [93]. Marek et al. found higher incidence of CMV reactivation in patients receiving depleted allografts [94].
A recently developed method is based on the depletion of αβ T lymphocytes coupled with B-cell depletion. This approach allows to transfer to the recipient not only high numbers of CD34+ cells and mature donor NK cells, but also γδ T cells which can exert their protective effect against both leukaemia cell regrowth and life-threatening infections. γδ T cells participate in anti-CMV responses, particularly when conventional adaptive immunity is insufficient to clear the viral infection. Airoldi and collaborators reported that there is a significant expansion of cytotoxic γδ T cells in patients with CMV reactivation after allo-HSCT compared to recipients who did not reactivate [95]. Scheper and coworkers stated that γδ T cells can cross react with tumour cells of hematopoietic origin, explaining the protecting effect of CMV reactivation on the risk of leukaemia relapse [96].
Gene polymorphisms
There are increasing evidences indicating that single nucleotide polymorphisms (SNPs) in several genes may modulate the susceptibility to, and the dynamics and outcomes of, chronic viral infections. Corrales et al. reported that patients carrying the donor (but not the recipient) CCR5 A/A genotype displayed episodes of active CMV infection with higher CMV load [97]. Suboptimal expression of CCR5 on CMV-specific T cells would likely result in an impairment in trafficking of these cells to mucosa and parenchyma tissues, thereby facilitating local viral replication and dissemination to the systemic compartment, despite treatment with antiviral agents. Increased expression of CCR5 would promote local inflammatory responses that trigger CMV reactivation and promote CMV replication. Therefore, donor SNPs in CCR5 gene may modulate the dynamics of CMV replication in allo-HSCT recipients. Other authors have found that heterozygosity for the toll-like receptor (TLR)2 and TLR4 SNPs was associated with lower risk of CMV infection and lower level of viremia, respectively [98] and, therefore, those polymorphisms appear to be protective factors in CMV replication. Saadi and colleagues found association between gene polymorphisms of co-stimulatory molecules and active CMV infection in HSCT recipients [99]. They reported that allo-HSCT patients with active CMV infection that experienced low-grade acute GvHD presented the genotype G/G of the cytotoxic T lymphocyte antigen-4 (CTLA-4). Allo-HSCT recipients that experienced acute GvHD and active CMV infection presented higher frequencies of the genotype T/T of the CD28 molecule. On the contrary, data from Bravo and collaborators seemed to point to a protective effect of the donor homozygous T allele of the IL-28B against CMV infection in allo-HSCT [100], which was supported by the study of Egli et al. [101].
CMV-specific immune response monitoring
The development of an effective T cell immunity is important for the control of CMV infection/disease in patients following allo-HSCT. Previous studies have found a strong association between the lack of CMV-specific CD8+ T cells and the development of CMV disease after transplantation [79, 102] and, therefore, the identification of the protective cell number against CMV infection and reactivation is of special interest.
Several studies have proposed the protective number of total CD4+ and CD8+ T cells with no clear agreement. A low lymphocyte count and less than 40 CD4+ T cells/μL at 3 months post-transplant have been demonstrated as risk factors for late CMV disease development [103]. Other authors such as Hakki and colleagues have shown that low T cell counts of both CD4+ (less than 100 cells/μL) and CD8+ (less than 50 cells/μL) T cells at 3 months after transplant are associated with poor CMV-specific immunity [86]. These results agree with the ones obtained by Moins-Teisserenc and colleagues in which they suggest that T cell counts higher than 100 cells/μL for both CD4+ and CD8+ may prevent the risk of CMV reactivation [104].
During the past years, several assays have been developed to measure CMV-specific T cell responses in patients following allo-HSCT.
Intracellular cytokine staining
The intracellular cytokine staining (ICS) is based on the detection by flow cytometry of diverse Th1 effector cytokines (usually IFNγ and TNFα) in peripheral blood mononuclear cells (PBMCs) or whole blood after stimulation with viral peptides, whole virus lysate or CMV-infected immature DCs. ICS is widely used by researchers to study functional recovery of CMV-specific T cells after allo-HSCT. The ICS method has been demonstrated to be useful in predicting the occurrence of CMV disease/reactivation after allo-HSCT [105]. Gratama et al. reported that the absence of CMV-specific CD4+ T cell responses, measured by IFNγ production, is related to an increased risk of CMV disease despite a vigorous CMV-specific CD8+ T cell response [106]. Based on IFNγ production by CD8+ and CD4+ T cells, Tormo and coworkers described different kinetics of CMV-specific T cells and CMV viremia during episodes of active CMV infection as well as cutoff cell levels of CMV-specific INFγ-producing T cells affording protection from CMV viremia [107, 108]. Giménez et al. reported that functional CMV-specific CD8+ T cell responses, measured by IFNγ and TNFα production, were found in patients without CMV viremia compared to recipients who developed it after allo-HSCT [109]. Besides, polyfunctional CD8+ T cells were associated with lower levels of CMV replication within patients with CMV viremia. They also found that these polyfunctional CD8+T cells were highly effective in controlling CMV replication. Lilleri and coworkers used immature DCs infected with an endotheliotropic and leukotropic CMV strain [110] to stimulate PBMCs and suggested that both CD4+ and CD8+ CMV-specific T cells are required to confer protection against CMV reactivation [52]. By looking at the frequency of CMV-specific CD4+ and CD8+ T cells producing IFNγ and IL-2, Lilleri et al. have proposed that levels of more than 1 CMV-specific CD4+ T cells/μL and more than 3 CMV-specific CD8+ T cells/μL at day 60 may confer protection against viremia [111]. In later studies, they have observed that after reaching levels of both CMV-specific CD4+ and CD8+ T cells similar to those found to be protective in immunocompetent subjects (more than 0.4 CMV-specific T cells/μL of blood), patients were able to control CMV infection without need of antiviral treatment [52]. These cutoff levels are close to the threshold value of 1.3 cells/μL for both T cell populations proposed by Tormo and coworkers [107] providing protection against development of CMV viremia. They also found that patients who experienced CMV reactivation had lower cytokine production by both CD4+ and CD8+ T cells 1 month after transplantation compared to recipients who did not [108].
ELISpot
The enzyme-linked immunosorbent spot (ELISpot) assay detects the release of IFNγ by CD4+ and CD8+ T cells in CMV antigen stimulated PBMCs. The clinical experience with ELISpot is relatively limited although it has also been used to monitor the reconstitution of CMV-specific T cells in allogeneic transplanted patients’ blood [112]. Ohnishi et al. found that CMV antigenemia was not observed in patients who had over 1 cell/μL when using pp65 peptides as antigen [113]. Sukdolak and colleagues have shown that the detection of memory T cells with IFNγ-ELISpot assay correlates with the detection of IFNγ after stimulation with CMV pp65 epitopes [26]. Abate et al. concluded in their study that the evaluation of the antiviral immune reconstitution is a promising and appealing system for identifying patients at higher risk of CMV infection [114].
MHC-multimer staining
Main histocompatibility complex (MHC)-multimers are complexes formed between HLA class I molecules and antigenic peptides covalently linked to fluorochromes, to allow direct visualisation, enumeration, phenotypic characterisation and isolation of virus-specific CD8+ T cells [115]. This technology has been largely developed to monitor immune responses by flow cytometry [116], allowing us to determine the frequencies of antigen-specific T cells. The most common format in use is the tetramer [66] although there are other forms of multimerization that can be used, such as pentamers [117] and streptamers [118]. According to Borchers et al. study, this technology may allow the prediction of CMV reactivation after allo-HSCT and they have shown that 1 cell/μL 2 months after allo-HSCT marks the beginning of an immune response against CMV, and following CMV-specific CD8+ T cell expansion thereafter indicated successful resolution of the CMV reactivation [66]. Gratama and coworkers reported that immune monitoring with tetramers was useful for the prediction of recurrent or persistent CMV infection or disease in allo-HSCT recipients [119].
Unfortunately, it has not been established a protective value of both CMV-specific CD4+ and CD8+ T cells that avoid CMV reactivation and disease. As it has been mentioned, several groups focus their interest on this purpose but a consensus has not been reached yet, mainly due to the variety of techniques used to monitor the immune response in patients following HSCT. Besides, it is not possible to definitively recommend one assay over the others due to the benefits each assay offers; nevertheless, a combination of some of them could give a better idea of the behaviour of these cells.
Future directions
New drugs’ approaches
Because of the safety profile of currently available drugs for CMV treatment, new available compounds are being studied with similar or improved efficacy and improved toxicity. Maribavir showed to effectively reduce CMV infection after allo-HSCT in a phase II study, but it failed to prevent CMV disease in a subsequent phase III study [120, 121]. However, two ongoing phase II trials are examining higher doses of this drug for the treatment of refractory or resistant CMV disease (clinicaltrials.gov NCT01611974) and as pre-emptive therapy (EudraCT: 2010-024247-32). In a phase II study, Brincidofovir (CMX001) has been shown to reduce CMV infection while not having evidences of increased myelosuppression or nephrotoxicity [122]. A phase III randomised multicentre trial is currently ongoing using a similar trial design as the phase II trial (clinicaltrials.gov NCT01769170). Finally, Letermovir seems to be a highly active anti-CMV agent and it is a potential new treatment option for patients infected with CMV strains that are resistant to currently approved antiviral drugs [123]. A phase III trial is currently ongoing (clinicaltrials.gov NCT02137772). However, because these drugs are currently in a phase II or III study, there is still no evidence of their effect on CMV immune recovery.
γδ T cells and NK cells
The fact that γδ T cells are not restricted by MHC molecules in combination with their potent antiviral and anti-tumour functions makes γδ T cells valuable, potential immune effector cells during the lymphopenic post-transplantation phase. Vδ1+ T cells are able to kill CMV-infected target cells and limit CMV propagation in vitro. Besides, significant long-term expansions of Vδ1+ T cells were observed during CMV reactivation early after allo-HSCT [124].
In addition, CMV infection inhibits the expression of MHC class I molecules in infected cells as an escape mechanism from CD8+ cytotoxic T cell-mediated control; however, infected cells become potentially susceptible to NK cells. It has been reported that KIR+NKG2C+ NK cells can control CMV infection in the absence of T cells [125]. Some studies have shown that increased proportions of these NK cells persisted and even increased 1 year after HSCT in patients who reactivates CMV but not in those with no CMV reactivation [126, 127]. Thus, CMV-induced NK cells may contribute not only to the control of the virus infection but also to the protection from disease relapses after HSCT.
New approaches to identify and quantify CMV-specific CD8+ T cells
Development of a reliable method to evaluate CMV-specific immunity may facilitate the management of patients at risk for CMV infection/reactivation allowing prompt initiation of antiviral treatment. The use of new available approaches of multimerization strategies is nowadays under evaluation to implement their use into clinical monitoring practice. These molecules have higher concentrations of MHC that can, to some extent, aid staining where T-cell receptor (TCR)-MHC interactions are weak. Pentamers have been used to monitor CMV immunity in kidney transplant patients [117]. Streptamers offer the advantage of dissociation from the T cell and it can be used in immunotherapy [128]. There are also some kits available to detect CMV-specific T cells in whole blood using dextramer technology that has been compared to tetramer technique [129]. A new whole blood assay, referred to as QuantiFERON-CMV®, has been recently developed to determine the clinical utility of measuring CMV-specific CD8+ T cell responses as a prognostic tool [130]. The use of QuantiFERON-CMV® in the week following CMV viremia can be useful in identifying HSCT recipients at risk of complicated reactivation [131].
Viral-specific adoptive immunotherapy after allogeneic HSCT
Previous studies have demonstrated a correlation between levels of CMV-specific CTL responses and improved control of CMV viremia in immunocompromised HSCT patients. Looking at these observations, many groups have focused their interest in developing strategies for adoptive transfer of CMV-specific T cells. Previous studies have demonstrated that infusion of donor-derived CMV-specific CD8+ T cell clones or cell lines can successfully transfer protective immunity [132–134] and numerous in vitro studies have defined the best methodology for the expansion and selection of virus-specific T lymphocytes for clinical use, which includes: (1) classic ex vivo expansion, where T cells are stimulated with antigen-presenting cells (APC) that have been transduced with either a viral vector or plasmids encoding the antigens of interest; (2) multimer selection of the specific T cells with magnetic beads; (3) capture of T cells that secrete cytokines after stimulation with viral antigens allowing antigen-specific T cell isolation by magnetic selection [135–139]; (4) methods based on genetic engineering that redirect the specificity of T cells to make them recognise the antigen of interest, by the introduction of TCR genes or chimeric antigen receptors (CAR) [140]. Studies of Feuchtinger et al. and Einsele et al. have indicated the significance of antiviral effector functions of T helper cells in maintaining CTL responses after adoptive transfer [133, 141]. There are few studies in Phase I/II that have demonstrated the feasibility of transferring CMV-specific T cells [133, 134, 142–144]. They have indicated that restoration of CMV-specific immunity can be accelerated without serious immediate infusion-related toxicities, although some patients can develop GvHD after infusion, which is correlated with clinical protection.
These promising results confirm that cellular immunotherapy can accelerate recovery of antiviral immunity in allogeneic HSCT recipients, with important clinical benefits (such as reduction of secondary viral infection episodes) [145, 146].
Mesenchymal stem cells for CMV infection treatment
One of the complications in patients following allo-HSCT is GvHD. Due to the lack of efficiency of existing methods for GvHD prophylaxis, new methods are being actively explored, including the use of donors’ multipotent mesenchymal stem cells (MSCs). These cells are fibroblast that can differentiate into osteoblasts, chondrocytes, adipocytes and myoblasts. MSCs exhibit extensive immunomodulatory activities and they affect a broad panel of immune cells of the innate and adaptive immunity. In a study developed by Meisel et al. [147], they demonstrate that on stimulation with inflammatory cytokines, MSCs exhibit broad-spectrum antimicrobial effector function directed against a range of clinically relevant bacteria, protozoal parasites and viruses. They report that MSCs exhibit potent antimicrobial effector function. However, a later study has shown that CMV-infected MSCs lose their cytokine-induced immunosuppressive capacity and are no longer able to restrict microbial growth [148]. Nevertheless, some researchers are interested in the use of MSC as treatment for viral infections and the efficacy of MSCs in the treatment of refractory CMV infection after allo-HSCT is currently being evaluated in a clinical trial (clinicaltrials.gov NCT02083731).
The adaptive immune response to CMV is the strongest document in human so far. The immune response in immunocompetent individuals protects them from CMV pathology which is a major problem in immunocompromised patients. A rapid CMV immune recovery helps them to protect from CMV reactivation/disease. The development of immune-monitoring techniques documented the importance of both CMV-specific CD4+ and CD8+ T cells to protect the immunosuppressed patients against CMV reactivation. In the future, it will be necessary to implement a robust, reproducible and rapid method to detect and measure the phenotype and function of the CMV-specific T cells. This method would allow to individualise the pre-emptive strategy against CMV, patients at low risk, with an adequate CMV-specific cell-mediated immunity, and those recipients unable to mount (in case of primary infection) or reconstitute (if reactivation) a proper response.
Acknowledgments
This work was supported by a research Grant (PI10/00136) from the Fondo de Investigaciones Sanitarias (FIS) granted by the Instituto de Salud Carlos III (ISCIII) issued to E. Olavarría. M. Ciáurriz is a recipient of PFIS Predoctoral Fellowship from ISCIII. E. Pérez-Valderrama is a recipient of the MINECO fellowship from the Ministerio de Educación. M. Lachén is a recipient of Tecnólogos Fellowship from the Gobierno de Navarra.
References
- 1.Landolfo S, Gariglio M, Gribaudo G, et al. The human cytomegalovirus. Pharmacol Ther. 2003;98(3):269–297. doi: 10.1016/S0163-7258(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 2.van de Berg PJ, van Stijn A, Ten Berge IJ, et al. A fingerprint left by cytomegalovirus infection in the human T cell compartment. J Clin Virol. 2008;41(3):213–217. doi: 10.1016/j.jcv.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Braendstrup P, Mortensen BK, Justesen S, et al. Identification and HLA-tetramer-validation of human CD4+ and CD8+ T cell responses against HCMV proteins IE1 and IE2. PLoS One. 2014;9(4):e94892. doi: 10.1371/journal.pone.0094892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70(11):7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Rosa C, Diamond DJ. The immune response to human CMV. Future Virol. 2012;7(3):279–293. doi: 10.2217/fvl.12.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev. 2002;15(4):680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossini G, Cerboni C, Santoni A, et al. Interplay between human cytomegalovirus and intrinsic/innate host responses: a complex bidirectional relationship. Mediat Inflamm. 2012;2012:607276. doi: 10.1155/2012/607276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldrop SL, Pitcher CJ, Peterson DM, et al. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99(7):1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kern F, Bunde T, Faulhaber N, et al. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J Infect Dis. 2002;185(12):1709–1716. doi: 10.1086/340637. [DOI] [PubMed] [Google Scholar]
- 10.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188(12):2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gamadia LE, Rentenaar RJ, van Lier RA, et al. Properties of CD4(+) T cells in human cytomegalovirus infection. Hum Immunol. 2004;65(5):486–492. doi: 10.1016/j.humimm.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 12.van Leeuwen EM, Remmerswaal EB, Vossen MT, et al. Emergence of a CD4+ CD28- granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J Immunol. 2004;173(3):1834–1841. doi: 10.4049/jimmunol.173.3.1834. [DOI] [PubMed] [Google Scholar]
- 13.van Leeuwen EM, ten Berge IJ, van Lier RA. Induction and maintenance of CD8+ T cells specific for persistent viruses. Adv Exp Med Biol. 2007;590:121–137. doi: 10.1007/978-0-387-34814-8_9. [DOI] [PubMed] [Google Scholar]
- 14.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie GM, Wills MR, Appay V, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J Virol. 2000;74(17):8140–8150. doi: 10.1128/JVI.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamadia LE, Remmerswaal EB, Weel JF, et al. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101(7):2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 17.Chidrawar S, Khan N, Wei W, et al. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol. 2009;155(3):423–432. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Leeuwen EM, Remmerswaal EB, Heemskerk MH, et al. Strong selection of virus-specific cytotoxic CD4+ T-cell clones during primary human cytomegalovirus infection. Blood. 2006;108(9):3121–3127. doi: 10.1182/blood-2006-03-006809. [DOI] [PubMed] [Google Scholar]
- 19.van Lier RA, ten Berge IJ, Gamadia LE. Human CD8(+) T-cell differentiation in response to viruses. Nat Rev Immunol. 2003;3(12):931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 20.Snyder CM. Buffered memory: a hypothesis for the maintenance of functional, virus-specific CD8(+) T cells during cytomegalovirus infection. Immunol Res. 2011;51(2–3):195–204. doi: 10.1007/s12026-011-8251-9. [DOI] [PubMed] [Google Scholar]
- 21.Snyder CM, Loewendorf A, Bonnett EL, et al. CD4+ T cell help has an epitope-dependent impact on CD8+ T cell memory inflation during murine cytomegalovirus infection. J Immunol. 2009;183(6):3932–3941. doi: 10.4049/jimmunol.0900227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hertoghs KM, Moerland PD, van Stijn A, et al. Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation. J Clin Invest. 2010;120(11):4077–4090. doi: 10.1172/JCI42758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78(11):5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walton SM, Torti N, Mandaric S, et al. T-cell help permits memory CD8(+) T-cell inflation during cytomegalovirus latency. Eur J Immunol. 2011;41(8):2248–2259. doi: 10.1002/eji.201141575. [DOI] [PubMed] [Google Scholar]
- 26.Sukdolak C, Tischer S, Dieks D, et al. CMV-, EBV- and ADV-specific T cell immunity: screening and monitoring of potential third-party donors to improve post-transplantation outcome. Biol Blood Marrow Transplant. 2013;19(10):1480–1492. doi: 10.1016/j.bbmt.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ljungman P, Bregni M, Brune M, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant. 2010;45(2):219–234. doi: 10.1038/bmt.2009.141. [DOI] [PubMed] [Google Scholar]
- 29.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 30.Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303(16):1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Passweg JR, Baldomero H, Bregni M, et al. Hematopoietic SCT in Europe: data and trends in 2011. Bone Marrow Transplant. 2013;48(9):1161–1167. doi: 10.1038/bmt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krol L, Stuchly J, Hubacek P, et al. Signature profiles of CMV-specific T-cells in patients with CMV reactivation after hematopoietic SCT. Bone Marrow Transplant. 2011;46(8):1089–1098. doi: 10.1038/bmt.2010.261. [DOI] [PubMed] [Google Scholar]
- 33.Dolstra H, Preijers F, Van de Wiel-van Kemenade E, et al. Expansion of CD8+ CD57+ T cells after allogeneic BMT is related with a low incidence of relapse and with cytomegalovirus infection. Br J Haematol. 1995;90(2):300–307. doi: 10.1111/j.1365-2141.1995.tb05150.x. [DOI] [PubMed] [Google Scholar]
- 34.Tuthill M, Chen F, Paston S, et al. The prevention and treatment of cytomegalovirus infection in haematopoietic stem cell transplantation. Cancer Immunol Immunother. 2009;58(9):1481–1488. doi: 10.1007/s00262-009-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cwynarski K, Ainsworth J, Cobbold M, et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97(5):1232–1240. doi: 10.1182/blood.V97.5.1232. [DOI] [PubMed] [Google Scholar]
- 36.Reusser P, Riddell SR, Meyers JD, et al. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78(5):1373–1380. [PubMed] [Google Scholar]
- 37.Gratama JW, van Esser JW, Lamers CH, et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98(5):1358–1364. doi: 10.1182/blood.V98.5.1358. [DOI] [PubMed] [Google Scholar]
- 38.Moss P, Khan N. CD8(+) T-cell immunity to cytomegalovirus. Hum Immunol. 2004;65(5):456–464. doi: 10.1016/j.humimm.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Sester M, Sester U, Gartner B, et al. Sustained high frequencies of specific CD4 T cells restricted to a single persistent virus. J Virol. 2002;76(8):3748–3755. doi: 10.1128/JVI.76.8.3748-3755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flinsenberg TW, Spel L, Jansen M, et al. Cognate CD4 T-cell licensing of dendritic cells heralds anti-cytomegalovirus CD8 T-cell immunity after human allogeneic umbilical cord blood transplantation. J Virol. 2015;89(2):1058–1069. doi: 10.1128/JVI.01850-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bitmansour AD, Waldrop SL, Pitcher CJ, et al. Clonotypic structure of the human CD4+ memory T cell response to cytomegalovirus. J Immunol. 2001;167(3):1151–1163. doi: 10.4049/jimmunol.167.3.1151. [DOI] [PubMed] [Google Scholar]
- 42.Foster AE, Gottlieb DJ, Sartor M, et al. Cytomegalovirus-specific CD4+ and CD8+ T-cells follow a similar reconstitution pattern after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2002;8(9):501–511. doi: 10.1053/bbmt.2002.v8.pm12374455. [DOI] [PubMed] [Google Scholar]
- 43.Elson LH, Nutman TB, Metcalfe DD, et al. Flow cytometric analysis for cytokine production identifies T helper 1, T helper 2, and T helper 0 cells within the human CD4+ CD27- lymphocyte subpopulation. J Immunol. 1995;154(9):4294–4301. [PubMed] [Google Scholar]
- 44.Openshaw P, Murphy EE, Hosken NA, et al. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182(5):1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290(5489):92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 46.Pourgheysari B, Piper KP, McLarnon A, et al. Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant. 2009;43(11):853–861. doi: 10.1038/bmt.2008.403. [DOI] [PubMed] [Google Scholar]
- 47.Aubert G, Hassan-Walker AF, Madrigal JA, et al. Cytomegalovirus-specific cellular immune responses and viremia in recipients of allogeneic stem cell transplants. J Infect Dis. 2001;184(8):955–963. doi: 10.1086/323354. [DOI] [PubMed] [Google Scholar]
- 48.Lidehall AK, Sund F, Lundberg T, et al. T cell control of primary and latent cytomegalovirus infections in healthy subjects. J Clin Immunol. 2005;25(5):473–481. doi: 10.1007/s10875-005-5372-8. [DOI] [PubMed] [Google Scholar]
- 49.Schulenburg A, Watkins-Riedel T, Greinix HT, et al. CMV monitoring after peripheral blood stem cell and bone marrow transplantation by pp65 antigen and quantitative PCR. Bone Marrow Transplant. 2001;28(8):765–768. doi: 10.1038/sj.bmt.1703227. [DOI] [PubMed] [Google Scholar]
- 50.Hebart H, Daginik S, Stevanovic S, et al. Sensitive detection of human cytomegalovirus peptide-specific cytotoxic T-lymphocyte responses by interferon-gamma-enzyme-linked immunospot assay and flow cytometry in healthy individuals and in patients after allogeneic stem cell transplantation. Blood. 2002;99(10):3830–3837. doi: 10.1182/blood.V99.10.3830. [DOI] [PubMed] [Google Scholar]
- 51.Ozdemir E, Saliba RM, Champlin RE, et al. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;40(2):125–136. doi: 10.1038/sj.bmt.1705699. [DOI] [PubMed] [Google Scholar]
- 52.Lilleri D, Gerna G, Zelini P, et al. Monitoring of human cytomegalovirus and virus-specific T-cell response in young patients receiving allogeneic hematopoietic stem cell transplantation. PLoS One. 2012;7(7):e41648. doi: 10.1371/journal.pone.0041648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hebart H, Einsele H. Clinical aspects of CMV infection after stem cell transplantation. Hum Immunol. 2004;65(5):432–436. doi: 10.1016/j.humimm.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 54.Meyers JD, Bowden RA, Counts GW. Infectious complications of marrow transplant: risk factors for infection. Prog Clin Biol Res. 1989;309:357–366. [PubMed] [Google Scholar]
- 55.Maury S, Mary JY, Rabian C, et al. Prolonged immune deficiency following allogeneic stem cell transplantation: risk factors and complications in adult patients. Br J Haematol. 2001;115(3):630–641. doi: 10.1046/j.1365-2141.2001.03135.x. [DOI] [PubMed] [Google Scholar]
- 56.Junghanss C, Boeckh M, Carter RA, et al. Incidence and outcome of cytomegalovirus infections following nonmyeloablative compared with myeloablative allogeneic stem cell transplantation, a matched control study. Blood. 2002;99(6):1978–1985. doi: 10.1182/blood.V99.6.1978. [DOI] [PubMed] [Google Scholar]
- 57.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. doi: 10.1182/blood.V98.7.2043. [DOI] [PubMed] [Google Scholar]
- 58.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103(6):2003–2008. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 59.Bowden RA, Sayers M, Flournoy N, et al. Cytomegalovirus immune globulin and seronegative blood products to prevent primary cytomegalovirus infection after marrow transplantation. N Engl J Med. 1986;314(16):1006–1010. doi: 10.1056/NEJM198604173141602. [DOI] [PubMed] [Google Scholar]
- 60.Nichols WG, Boeckh M, Carter RA, et al. Transferred herpes simplex virus immunity after stem-cell transplantation: clinical implications. J Infect Dis. 2003;187(5):801–808. doi: 10.1086/367894. [DOI] [PubMed] [Google Scholar]
- 61.Lacey SF, Gallez-Hawkins G, Crooks M, et al. Characterization of cytotoxic function of CMV-pp65-specific CD8+ T-lymphocytes identified by HLA tetramers in recipients and donors of stem-cell transplants. Transplantation. 2002;74(5):722–732. doi: 10.1097/00007890-200209150-00023. [DOI] [PubMed] [Google Scholar]
- 62.Ljungman P, Brand R, Einsele H, et al. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003;102(13):4255–4260. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- 63.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194(12):1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou W, Longmate J, Lacey SF, et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood. 2009;113(25):6465–6476. doi: 10.1182/blood-2009-02-203307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nichols WG, Corey L, Gooley T, et al. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002;185(3):273–282. doi: 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 66.Borchers S, Bremm M, Lehrnbecher T, et al. Sequential anti-cytomegalovirus response monitoring may allow prediction of cytomegalovirus reactivation after allogeneic stem cell transplantation. PLoS One. 2012;7(12):e50248. doi: 10.1371/journal.pone.0050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borchers S, Luther S, Lips U, et al. Tetramer monitoring to assess risk factors for recurrent cytomegalovirus reactivation and reconstitution of antiviral immunity post allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2011;13(3):222–236. doi: 10.1111/j.1399-3062.2011.00626.x. [DOI] [PubMed] [Google Scholar]
- 68.Mead AJ, Thomson KJ, Morris EC, et al. HLA-mismatched unrelated donors are a viable alternate graft source for allogeneic transplantation following alemtuzumab-based reduced-intensity conditioning. Blood. 2010;115(25):5147–5153. doi: 10.1182/blood-2010-01-265413. [DOI] [PubMed] [Google Scholar]
- 69.Jaskula E, Bochenska J, Kocwin E, et al. CMV Serostatus of donor-recipient pairs influences the risk of CMV infection/reactivation in HSCT patients. Bone Marrow Res. 2012;2012:375075. doi: 10.1155/2012/375075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hambach L, Stadler M, Dammann E, et al. Increased risk of complicated CMV infection with the use of mycophenolate mofetil in allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;29(11):903–906. doi: 10.1038/sj.bmt.1703583. [DOI] [PubMed] [Google Scholar]
- 71.Nichols WG, Corey L, Gooley T, et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood. 2001;97(4):867–874. doi: 10.1182/blood.V97.4.867. [DOI] [PubMed] [Google Scholar]
- 72.Nakamae H, Kirby KA, Sandmaier BM, et al. Effect of conditioning regimen intensity on CMV infection in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(6):694–703. doi: 10.1016/j.bbmt.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim SH, Kee SY, Lee DG, et al. Infectious complications following allogeneic stem cell transplantation: reduced-intensity vs. myeloablative conditioning regimens. Transpl Infect Dis. 2013;15(1):49–59. doi: 10.1111/tid.12003. [DOI] [PubMed] [Google Scholar]
- 74.Kanda Y, Oshima K, Kako S, et al. In vivo T-cell depletion with alemtuzumab in allogeneic hematopoietic stem cell transplantation: combined results of two studies on aplastic anemia and HLA-mismatched haploidentical transplantation. Am J Hematol. 2013;88(4):294–300. doi: 10.1002/ajh.23392. [DOI] [PubMed] [Google Scholar]
- 75.Ruutu T, Ljungman P, Brinch L, et al. No prevention of cytomegalovirus infection by anti-cytomegalovirus hyperimmune globulin in seronegative bone marrow transplant recipients. The Nordic BMT Group. Bone Marrow Transplant. 1997;19(3):233–236. doi: 10.1038/sj.bmt.1700649. [DOI] [PubMed] [Google Scholar]
- 76.Ichihara H, Nakamae H, Hirose A, et al. Immunoglobulin prophylaxis against cytomegalovirus infection in patients at high risk of infection following allogeneic hematopoietic cell transplantation. Transplant Proc. 2011;43(10):3927–3932. doi: 10.1016/j.transproceed.2011.08.104. [DOI] [PubMed] [Google Scholar]
- 77.Ljungman P, de La Camara R, Milpied N, et al. Randomized study of valacyclovir as prophylaxis against cytomegalovirus reactivation in recipients of allogeneic bone marrow transplants. Blood. 2002;99(8):3050–3056. doi: 10.1182/blood.V99.8.3050. [DOI] [PubMed] [Google Scholar]
- 78.Venton G, Crocchiolo R, Furst S, et al. Risk factors of Ganciclovir-related neutropenia after allogeneic stem cell transplantation: a retrospective monocentre study on 547 patients. Clin Microbiol Infect. 2014;20(2):160–166. doi: 10.1111/1469-0691.12222. [DOI] [PubMed] [Google Scholar]
- 79.Li CR, Greenberg PD, Gilbert MJ, et al. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83(7):1971–1979. [PubMed] [Google Scholar]
- 80.Lilleri D, Gerna G, Fornara C, et al. Human cytomegalovirus-specific T cell reconstitution in young patients receiving T cell-depleted, allogeneic hematopoietic stem cell transplantation. J Infect Dis. 2009;199(6):829–836. doi: 10.1086/597123. [DOI] [PubMed] [Google Scholar]
- 81.Oshima K, Kanda Y, Kako S, et al. Case report: persistent cytomegalovirus (CMV) infection after haploidentical hematopoietic stem cell transplantation using in vivo alemtuzumab: emergence of resistant CMV due to mutations in the UL97 and UL54 genes. J Med Virol. 2008;80(10):1769–1775. doi: 10.1002/jmv.21277. [DOI] [PubMed] [Google Scholar]
- 82.Busca A. Cytomegalovirus (CMV) infection after hematopoietic stem cell transplantation: significant progress, but many unresolved problems. Expert Opin Biol Ther. 2009;9(4):383–385. doi: 10.1517/14712590902854448. [DOI] [PubMed] [Google Scholar]
- 83.Martin DF, Sierra-Madero J, Walmsley S, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346(15):1119–1126. doi: 10.1056/NEJMoa011759. [DOI] [PubMed] [Google Scholar]
- 84.Busca A, de Fabritiis P, Ghisetti V, et al. Oral valganciclovir as preemptive therapy for cytomegalovirus infection post allogeneic stem cell transplantation. Transpl Infect Dis. 2007;9(2):102–107. doi: 10.1111/j.1399-3062.2006.00183.x. [DOI] [PubMed] [Google Scholar]
- 85.Gerna G, Lilleri D, Caldera D, et al. Validation of a DNAemia cutoff for preemptive therapy of cytomegalovirus infection in adult hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2008;41(10):873–879. doi: 10.1038/sj.bmt.1705986. [DOI] [PubMed] [Google Scholar]
- 86.Hakki M, Riddell SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102(8):3060–3067. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]
- 87.Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101(2):407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 88.Mercorelli B, Sinigalia E, Loregian A, et al. Human cytomegalovirus DNA replication: antiviral targets and drugs. Rev Med Virol. 2008;18(3):177–210. doi: 10.1002/rmv.558. [DOI] [PubMed] [Google Scholar]
- 89.Krause H, Hebart H, Jahn G, et al. Screening for CMV-specific T cell proliferation to identify patients at risk of developing late onset CMV disease. Bone Marrow Transplant. 1997;19(11):1111–1116. doi: 10.1038/sj.bmt.1700801. [DOI] [PubMed] [Google Scholar]
- 90.Einsele H, Hebart H, Kauffmann-Schneider C, et al. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone Marrow Transplant. 2000;25(7):757–763. doi: 10.1038/sj.bmt.1702226. [DOI] [PubMed] [Google Scholar]
- 91.Perez-Martinez A, Gonzalez-Vicent M, Valentin J, et al. Early evaluation of immune reconstitution following allogeneic CD3/CD19-depleted grafts from alternative donors in childhood acute leukemia. Bone Marrow Transplant. 2012;47(11):1419–1427. doi: 10.1038/bmt.2012.43. [DOI] [PubMed] [Google Scholar]
- 92.Lang P, Teltschik HM, Feuchtinger T, et al. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. Br J Haematol. 2014;165(5):688–698. doi: 10.1111/bjh.12810. [DOI] [PubMed] [Google Scholar]
- 93.Federmann B, Hagele M, Pfeiffer M, et al. Immune reconstitution after haploidentical hematopoietic cell transplantation: impact of reduced intensity conditioning and CD3/CD19 depleted grafts. Leukemia. 2010;25(1):121–129. doi: 10.1038/leu.2010.235. [DOI] [PubMed] [Google Scholar]
- 94.Marek A, Stern M, Chalandon Y, et al. The impact of T-cell depletion techniques on the outcome after haploidentical hematopoietic SCT. Bone Marrow Transplant. 2014;49(1):55–61. doi: 10.1038/bmt.2013.132. [DOI] [PubMed] [Google Scholar]
- 95.Airoldi I, Bertaina A, Prigione I, et al. gammadelta T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood. 2015;125(15):2349–2358. doi: 10.1182/blood-2014-09-599423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scheper W, van Dorp S, Kersting S, et al. gammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia. 2013;27(6):1328–1338. doi: 10.1038/leu.2012.374. [DOI] [PubMed] [Google Scholar]
- 97.Corrales I, Gimenez E, Solano C, et al. Incidence and dynamics of active cytomegalovirus infection in allogeneic stem cell transplant patients according to single nucleotide polymorphisms in donor and recipient CCR5, MCP-1, IL-10, and TLR9 genes. J Med Virol. 2015;87(2):248–255. doi: 10.1002/jmv.24050. [DOI] [PubMed] [Google Scholar]
- 98.Jablonska A, Paradowska E, Studzinska M, et al. Relationship between toll-like receptor 2 Arg677Trp and Arg753Gln and toll-like receptor 4 Asp299Gly polymorphisms and cytomegalovirus infection. Int J Infect Dis. 2014;25:11–15. doi: 10.1016/j.ijid.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Saadi MI, Yaghobi R, Karimi MH, et al. Association of the costimulatory molecule gene polymorphisms and active cytomegalovirus infection in hematopoietic stem cell transplant patients. Mol Biol Rep. 2013;40(10):5833–5842. doi: 10.1007/s11033-013-2689-x. [DOI] [PubMed] [Google Scholar]
- 100.Bravo D, Solano C, Gimenez E, et al. Effect of the IL28B Rs12979860 C/T polymorphism on the incidence and features of active cytomegalovirus infection in allogeneic stem cell transplant patients. J Med Virol. 2014;86(5):838–844. doi: 10.1002/jmv.23865. [DOI] [PubMed] [Google Scholar]
- 101.Egli A, Levin A, Santer DM, et al. Immunomodulatory function of Interleukin 28B during primary infection with cytomegalovirus. J Infect Dis. 2014;210(5):717–727. doi: 10.1093/infdis/jiu144. [DOI] [PubMed] [Google Scholar]
- 102.Goodrich JM, Mori M, Gleaves CA, et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med. 1991;325(23):1601–1607. doi: 10.1056/NEJM199112053252303. [DOI] [PubMed] [Google Scholar]
- 103.Boeckh M, Gooley TA, Myerson D, et al. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88(10):4063–4071. [PubMed] [Google Scholar]
- 104.Moins-Teisserenc H, Busson M, Scieux C, et al. Patterns of cytomegalovirus reactivation are associated with distinct evolutive profiles of immune reconstitution after allogeneic hematopoietic stem cell transplantation. J Infect Dis. 2008;198(6):818–826. doi: 10.1086/591185. [DOI] [PubMed] [Google Scholar]
- 105.Ozdemir E, St John LS, Gillespie G, et al. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood. 2002;100(10):3690–3697. doi: 10.1182/blood-2002-05-1387. [DOI] [PubMed] [Google Scholar]
- 106.Gratama JW, Brooimans RA, van der Holt B, et al. Monitoring cytomegalovirus IE-1 and pp65-specific CD4+ and CD8+ T-cell responses after allogeneic stem cell transplantation may identify patients at risk for recurrent CMV reactivations. Cytometry B Clin Cytom. 2008;74(4):211–220. doi: 10.1002/cyto.b.20420. [DOI] [PubMed] [Google Scholar]
- 107.Tormo N, Solano C, Benet I, et al. Kinetics of cytomegalovirus (CMV) pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells during episodes of viral DNAemia in allogeneic stem cell transplant recipients: potential implications for the management of active CMV infection. J Med Virol. 2010;82(7):1208–1215. doi: 10.1002/jmv.21799. [DOI] [PubMed] [Google Scholar]
- 108.Tormo N, Solano C, Benet I, et al. Reconstitution of CMV pp65 and IE-1-specific IFN-gamma CD8(+) and CD4(+) T-cell responses affording protection from CMV DNAemia following allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46(11):1437–1443. doi: 10.1038/bmt.2010.330. [DOI] [PubMed] [Google Scholar]
- 109.Gimenez E, Munoz-Cobo B, Solano C, et al. Functional patterns of cytomegalovirus (CMV) pp65 and immediate early-1-specific CD8 T cells that are associated with protection from and control of CMV DNAemia after allogeneic stem cell transplantation. Transpl Infect Dis. 2015 doi: 10.1111/tid.12391. [DOI] [PubMed] [Google Scholar]
- 110.Gerna G, Percivalle E, Lilleri D, et al. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J Gen Virol. 2005;86(Pt 2):275–284. doi: 10.1099/vir.0.80474-0. [DOI] [PubMed] [Google Scholar]
- 111.Lilleri D, Gerna G, Fornara C, et al. Prospective simultaneous quantification of human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in young recipients of allogeneic hematopoietic stem cell transplants. Blood. 2006;108(4):1406–1412. doi: 10.1182/blood-2005-11-012864. [DOI] [PubMed] [Google Scholar]
- 112.Lehmann PV, Zhang W. Unique strengths of ELISPOT for T cell diagnostics. Methods Mol Biol. 2012;792:3–23. doi: 10.1007/978-1-61779-325-7_1. [DOI] [PubMed] [Google Scholar]
- 113.Ohnishi M, Sakurai T, Heike Y, et al. Evaluation of cytomegalovirus-specific T-cell reconstitution in patients after various allogeneic haematopoietic stem cell transplantation using interferon-gamma-enzyme-linked immunospot and human leucocyte antigen tetramer assays with an immunodominant T-cell epitope. Br J Haematol. 2005;131(4):472–479. doi: 10.1111/j.1365-2141.2005.05800.x. [DOI] [PubMed] [Google Scholar]
- 114.Abate D, Cesaro S, Cofano S, et al. Diagnostic utility of human cytomegalovirus-specific T-cell response monitoring in predicting viremia in pediatric allogeneic stem-cell transplant patients. Transplantation. 2012;93(5):536–542. doi: 10.1097/TP.0b013e31824215db. [DOI] [PubMed] [Google Scholar]
- 115.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274(5284):94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 116.Doherty PC, Christensen JP. Accessing complexity: the dynamics of virus-specific T cell responses. Annu Rev Immunol. 2000;18:561–592. doi: 10.1146/annurev.immunol.18.1.561. [DOI] [PubMed] [Google Scholar]
- 117.Lee S, Park JB, Kim EY, et al. Monitoring of cytomegalovirus-specific CD8+ T-cell response with major histocompatibility complex pentamers in kidney transplant recipients. Transplant Proc. 2011;43(7):2636–2640. doi: 10.1016/j.transproceed.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 118.Knabel M, Franz TJ, Schiemann M, et al. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat Med. 2002;8(6):631–637. doi: 10.1038/nm0602-631. [DOI] [PubMed] [Google Scholar]
- 119.Gratama JW, Boeckh M, Nakamura R, et al. Immune monitoring with iTAg MHC Tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood. 2010;116(10):1655–1662. doi: 10.1182/blood-2010-03-273508. [DOI] [PubMed] [Google Scholar]
- 120.Winston DJ, Young JA, Pullarkat V, et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood. 2008;111(11):5403–5410. doi: 10.1182/blood-2007-11-121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marty FM, Ljungman P, Papanicolaou GA, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011;11(4):284–292. doi: 10.1016/S1473-3099(11)70024-X. [DOI] [PubMed] [Google Scholar]
- 122.Marty FM, Winston DJ, Rowley SD, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369(13):1227–1236. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 123.Chemaly RF, Ullmann AJ, Stoelben S, et al. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med. 2014;370(19):1781–1789. doi: 10.1056/NEJMoa1309533. [DOI] [PubMed] [Google Scholar]
- 124.Knight A, Madrigal AJ, Grace S, et al. The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood. 2010;116(12):2164–2172. doi: 10.1182/blood-2010-01-255166. [DOI] [PubMed] [Google Scholar]
- 125.Kuijpers TW, Baars PA, Dantin C, et al. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112(3):914–915. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- 126.Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Della Chiesa M, Falco M, Podesta M, et al. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood. 2012;119(2):399–410. doi: 10.1182/blood-2011-08-372003. [DOI] [PubMed] [Google Scholar]
- 128.Casalegno-Garduno R, Schmitt A, Yao J, et al. Multimer technologies for detection and adoptive transfer of antigen-specific T cells. Cancer Immunol Immunother. 2010;59(2):195–202. doi: 10.1007/s00262-009-0778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dolton G, Lissina A, Skowera A, et al. Comparison of peptide-major histocompatibility complex tetramers and dextramers for the identification of antigen-specific T cells. Clin Exp Immunol. 2014;177(1):47–63. doi: 10.1111/cei.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clari MA, Munoz-Cobo B, Solano C, et al. Performance of the QuantiFERON-cytomegalovirus (CMV) assay for detection and estimation of the magnitude and functionality of the CMV-specific gamma interferon-producing CD8(+) T-cell response in allogeneic stem cell transplant recipients. CVI. 2012;19(5):791–796. doi: 10.1128/CVI.05633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tey SK, Kennedy GA, Cromer D, et al. Clinical assessment of anti-viral CD8+ T cell immune monitoring using QuantiFERON-CMV(R) assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications. PLoS One. 2013;8(10):e74744. doi: 10.1371/journal.pone.0074744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Riddell SR, Walter BA, Gilbert MJ, et al. Selective reconstitution of CD8+ cytotoxic T lymphocyte responses in immunodeficient bone marrow transplant recipients by the adoptive transfer of T cell clones. Bone Marrow Transplant. 1994;14(Suppl 4):S78–S84. [PubMed] [Google Scholar]
- 133.Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116(20):4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 134.Peggs KS. Adoptive T cell immunotherapy for cytomegalovirus. Expert Opin Biol Ther. 2009;9(6):725–736. doi: 10.1517/14712590902967588. [DOI] [PubMed] [Google Scholar]
- 135.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202(3):379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peggs KS, Thomson K, Samuel E, et al. Directly Selected cytomegalovirus-reactive donor T Cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis. 2011;52(1):49–57. doi: 10.1093/cid/ciq042. [DOI] [PubMed] [Google Scholar]
- 137.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12(10):1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 138.Gerdemann U, Keirnan JM, Katari UL, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20(8):1622–1632. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hanley PJ, Cruz CR, Savoldo B, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114(9):1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maus MV, Fraietta JA, Levine BL, et al. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Einsele H. Immunotherapy for CMV infection. Cytotherapy. 2002;4(5):435–436. doi: 10.1080/146532402320776080. [DOI] [PubMed] [Google Scholar]
- 142.Hill GR, Tey SK, Beagley L, et al. Successful immunotherapy of HCMV disease using virus-specific T cells expanded from an allogeneic stem cell transplant recipient. Am J Transplant. 2010;10(1):173–179. doi: 10.1111/j.1600-6143.2009.02872.x. [DOI] [PubMed] [Google Scholar]
- 143.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745–3758. doi: 10.1182/blood-2012-08-448977. [DOI] [PubMed] [Google Scholar]
- 144.Clancy LE, Blyth E, Simms RM, et al. Cytomegalovirus-specific cytotoxic T lymphocytes can be efficiently expanded from granulocyte colony-stimulating factor-mobilized hemopoietic progenitor cell products ex vivo and safely transferred to stem cell transplantation recipients to facilitate immune reconstitution. Biol Blood Marrow Transplant. 2013;19(5):725–734. doi: 10.1016/j.bbmt.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 145.Saglio F, Hanley PJ, Bollard CM. The time is now: moving toward virus-specific T cells after allogeneic hematopoietic stem cell transplantation as the standard of care. Cytotherapy. 2014;16(2):149–159. doi: 10.1016/j.jcyt.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ramirez N, Beloki L, Ciaurriz M. Impact of T cell selection methods in the success of clinical adoptive immunotherapy. Cell Mol Life Sci. 2014;71(7):1211–1224. doi: 10.1007/s00018-013-1463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meisel R, Brockers S, Heseler K, et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia. 2011;25(4):648–654. doi: 10.1038/leu.2010.310. [DOI] [PubMed] [Google Scholar]
- 148.Meisel R, Heseler K, Nau J, et al. Cytomegalovirus infection impairs immunosuppressive and antimicrobial effector functions of human multipotent mesenchymal stromal cells. Mediators Inflamm. 2014;2014:898630. doi: 10.1155/2014/898630. [DOI] [PMC free article] [PubMed] [Google Scholar]