Abstract
Crohn’s disease and ulcerative colitis are both associated with an increased risk of inflammation-associated colorectal carcinoma. Colitis-associated cancer (CAC) is one of the most important causes for morbidity and mortality in patients with inflammatory bowel diseases (IBD). Colitis-associated neoplasia distinctly differs from sporadic colorectal cancer in its biology and the underlying mechanisms. This review discusses the molecular mechanisms of CAC and summarizes the most important genetic alterations and signaling pathways involved in inflammatory carcinogenesis. Then, clinical translation is evaluated by discussing new endoscopic techniques and their contribution to surveillance and early detection of CAC. Last, we briefly address different types of concepts for prevention (i.e., anti-inflammatory therapeutics) and treatment (i.e., surgical intervention) of CAC and give an outlook on this important aspect of IBD.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Crohn’s disease, Colitis-associated cancer, Colorectal carcinoma, Endoscopy, Chemoprevention
Introduction
When Crohn and Rosenberg [1] published their first reports about inflammatory bowel disease (IBD) in the beginning of the last century, they already highlighted an association of IBD with the onset of colorectal neoplasia. Since then it has been established, that ulcerative colitis in particular [2], and also Crohn’s disease [3] pose an increased risk for patients to develop colorectal carcinoma (CRC). In fact, the two high-risk conditions for colorectal cancer are either hereditary diseases (i.e., familiar adenomatous polyposis or Lynch Syndrome) or chronic colitis. But while colitis-associated carcinoma (CAC) represents only a small fraction of all colorectal cancer cases, in patients with ulcerative colitis the development of a malignant disease is associated with high morbidity and mortality. In a landmark meta-analysis, Eaden et al. [4] evaluated the cumulative risk of colitis-associated cancer with 1.6 % at 10 years, 8.3 % at 20 years and 18.4 % at 30 years. Rutter and colleagues [5], who examined 600 patients from a 30-year colonoscopic surveillance program found a considerably lower incidence, but still 10.8 % of ulcerative colitis patients developed colitis-associated neoplasms after 30 years of disease duration. Alarmingly, more than half of the invasive carcinomas detected (16/30) were interval cancers. Tumor onset in IBD patients even increases when colitis is diagnosed at a young age and accompanied by prolonged disease duration, right-sided colitis, pancolitis [2] and/or primary sclerosing cholangitis [6]. Further contributing factors are colonic strictures and postinflammatory polyps [7, 8]. Interestingly, proctitis and proctosigmoiditis do not increase the chance of developing colonic neoplasia [9].
Pathophysiology and molecular basis
Sporadic and colitis-associated colorectal neoplasia
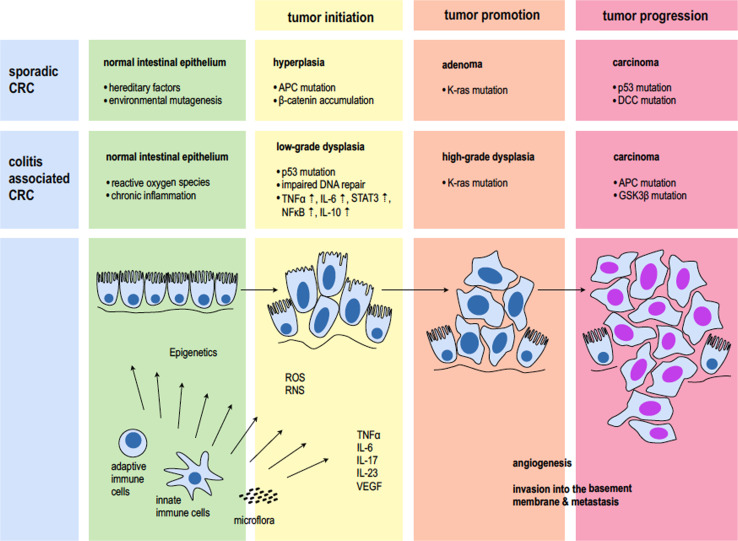
Most scientific data on the pathophysiology of colorectal tumorigenesis available today has been gathered from sporadic colorectal carcinoma. In various landmark trials—some of which are the most cited papers in recent history—Vogelstein and colleagues were the first to describe a series of molecular events that turn normal mucosa first into hyperplastic epithelium, then into adenomatous polyps with dysplastic cells and then into malignant carcinoma with invasion of the basement membrane and metastasis [10–13]. What has become known as the adenoma–carcinoma sequence (Vogelstein hypothesis) started with the discovery that mutations in the APC (adenomatous polyposis coli) gene initiate colorectal tumor development [14]. This gene had until then only been found to be altered in germline cells of patients with hereditary neoplasms of the colon, a condition called familiar adenomatous polyposis (FAP). Further mutational steps include alterations in the K-ras (Kirsten rat sarcoma viral oncogene homolog) oncogene, loss of function of the retinoblastoma (rb1) and the TP53 gene—two major tumor suppressors—and deletion of the DCC (deleted in colorectal cancer) gene. These genetic alterations are paralleled by distinct histopathologic changes. But while in sporadic cancer, genetic disposition and alterations acquired during aging are key factors that promote carcinogenesis, in colitis-associated cancer it is the inflammation which drives tumor development [15]. A constant inflammatory stimulus leads to lesions with different morphologic features such as flat and serrated structures, often times occurring simultaneously at multiple sites of inflamed mucosa. This is represented by the modified inflammation-low-grade/high-grade dysplasia–carcinoma sequence. Although similar mutations as in colorectal cancer are found in colitis-associated cancer, they occur in different stages of the disease and with different underlying etiology [16].
Mutations
For the TP53 gene, which encodes for the p53 protein and is regarded as the “guardian of the genome” [17], defined mutations and loss of heterozygosity (LOH) are observed early in inflammatory carcinogenesis. This is in contrast to its role in sporadic colorectal cancer, where it is regarded as one of the last protectors from invasive carcinoma. Loss of heterozygosity means that only one mutation can lead to total loss of gene function, which is the case if you have only one allele left due to prior mutations or inheritance [18]. It was found that 50–85 % of colitis-associated cancers have deletions in the TP53 gene [19]. Furthermore, in over 50 % of colonic tissue specimens of ulcerative colitis patients p53 deletions could be observed without any sign of dysplasia or neoplasia [20]. This was explored in further detail by Brentnall and colleagues [21], who carefully mapped whole colectomy specimen of ulcerative colitis patients. They could prove that, mutations in the p53 gene succeeded aneuploidy, which is then followed by LOH. LOH thereby seems to correlate with progression into invasive carcinoma. The importance of the TP53 gene in colitis-associated cancer is also highlighted by a growing amount of data from animal studies. For example, it has been shown that mutant prolongs chemically induced NFκB activation in mice harboring a germline p53 mutation. This causes severe damage to the mucosa and increases the risk to develop colitis-associated tumors. It further seems to rapidly enhance the progression from high-grade dysplasia to invasive carcinoma [22].
While in sporadic carcinoma mutations in the APC gene were described to initiate tumorigenesis, this does not seem to be the case in colitis-associated cancer [23]. LOH in the APC gene loci was found in 0 % of normal mucosa of ulcerative colitis patients (0/6) and also 0 % of specimen of active colitis (0/7). Only in high-grade dysplasia APC mutations start to occur in 27.3 % of patients (3/11) while 50 % of cases of colitis-associated carcinoma (3/6) display alterations in this tumor suppressor gene as examined by Fogt et al. [24]. In contrast, in sporadic benign adenomas already 21 % of the specimen (4/19) displayed APC mutations. The role of APC in colitis-associated cancer progression has been confirmed by others and is also supported by substantial evidence from animal studies [16]. For example, it has been shown that in APCmin (multiple intestinal neoplasia) mice chemically induced dextran sodium sulfate (DSS) colitis increases tumor frequency especially for later stages of dysplasia and carcinoma development [25]. Consistently, analysis of β-catenin, an important intracellular signaling molecule in the APC and Wnt pathway, which is mutated in many types of cancers, showed no alterations in most specimen of active ulcerative colitis, dysplasia and colitis-associated cancer [26]. This may be at least in part based on the fact, that mesalazine, a common treatment for IBD patients has been shown to inhibit in particular β-catenin metabolism [27]. Furthermore, other inflammatory pathways have been demonstrated to bypass various components of the Wnt/APC/β-catenin signaling axis and still promote intestinal tumorigenesis [28–30]. Data on K-ras alterations in colitis-associated cancer show mutations occur less frequently in inflammatory carcinogenesis [31]. In patients with ulcerative colitis, alterations in this gene tend to appear at more advanced stages, such as high-grade dysplasia and carcinoma. Furthermore, a risk factor for K-ras mutation seems to be a disease history longer than 10 years [32, 33]. Further steps in inflammatory carcinogenesis are the loss of tumor suppressor gene DCC resulting in low-grade dysplasia followed by the activation of other less frequent proto-oncogenes, such as src high-grade dysplasia [34].
While the majority of research on genetic alterations focused on specimens of ulcerative colitis patients, the situation in individuals with Crohn’s disease is less clear. This may be due to the fact, that tumor onset is less likely in this form of IBD. In one of the few studies on mutations in small bowel adenocarcinomas, it was shown that K-ras and p53 alterations occur early during inflammatory tumor development, while APC, DCC and TGF-β-RII mutations are rare, which contrasts with sporadic colorectal cancer [35]. Further studies are needed to evaluate similarities and differences in tumor genetics of ulcerative colitis and Crohn’s-associated neoplasia (Fig. 1).
Fig. 1.
Pathophysiology of sporadic and colitis-associated carcinoma
Oxidative stress
One major mechanism, which links inflammation to pro-neoplastic genetic alterations is oxidative stress [36–38]. Oxidative stress is mainly produced by cells of the innate immune system such as macrophages and granulocytes and includes the generation of various reactive oxygen and nitrogen species (ROS, NOS). This “respiratory/oxidative burst” is a crucial component of the unspecific defense orchestrated by innate immune cells. While effectively killing mucosal pathogens, ROS and NOS also pose a constant mutational challenge for the intestinal epithelium [39]. This results in DNA breaks, DNA adducts and damage to cellular lipids and proteins [40]. Consequently, direct or indirect inhibition of oxidative stress has been demonstrated to effectively delay DNA damage and decrease intestinal tumor development [41–43]. When oxidative stress is present, this not only leads to activation of the DNA damage response (DDR) [44] and but also effects particular regions of the genome, which are the telomeres. In fact, in a series of papers, the Rabinovitch group could show that colonocyte telomeres shorten with age almost twice as rapidly in ulcerative colitis patients as in normal controls. Furthermore, phospho-H2AX, a marker of the DDR, was significantly higher in colonocytes of ulcerative colitis patients than in control [45]. O’Sullivan et al. could show that genomic instability in patients with ulcerative colitis is related to telomere shortening and that this parameter can be used to identify patients with progressive disease and predisposition to colitis-associated cancer [19, 46, 47].
Soluble factors and signaling cascades
Apart from oxidative stress and subsequent genomic alterations induced by cells of the innate immune system, soluble factors also contribute to inflammation-associated carcinogenesis. These soluble factors include inflammatory cytokines, chemokines, growth- and transcription factors. In this regard, most of the data available today has been gathered from animal studies [48], which are a useful tool to mimic human disease. But despite preclinical advances translation into clinical application remains challenging nonetheless [49]. One key player of inflammation is the nuclear factor ‘κ-light-chain-enhancer’ of activated B cells, better known as NFκB. NFκB is activated not only in sites of inflammation, but interestingly also in many solid tumors and cancer cell lines [50]. Greten and colleagues examined the NFκB pathway in the most frequent mouse model of colitis-associated colorectal cancer, the so-called AOM + DSS model. Azoxymethane (AOM) is a potent mutagen and repeated cycles of dextran sodium sulfate induce colonic inflammation. After knocking down the IKKβ kinase, an important enzyme upstream of NFκB, mice were significantly protected against inflammatory tumor development [51, 52]. It was further shown that toll-like receptors (TLR) play an important role in the interaction between the intestinal microflora and the mucosal immune defense via NFκB activation [53].
Another molecule that has a pivotal role in inflammatory carcinogenesis of the colon is IL-6. IL-6 is produced predominantly by cells of the innate immune system such as monocytes and macrophages. In these cells, IL-6 expression is regulated through the activation of several transcription factors such as C/EBPβ (CAAT/enhancer-binding protein β), AP-1 (activator protein 1) and also NFκB [54]. In subsequent studies, Becker and colleagues were able to demonstrate, that it is not the membrane bound form of the IL-6 receptor, which is responsible for the tumor promoting effect, but rather a trans-signaling mechanism with soluble form of the IL-6 receptor. A dimer of IL-6 and its soluble receptor interacts with glycoprotein 130 (gp130, CD130), and thereby enables the activation of subsequent downstream signaling cascades [55, 56]. Major effector is the signal transducer and activator of transcription 3 (STAT3) through phosphorylation. STAT3 activation has been shown to be an important step for promotion and progression through the induction of various target genes. These target genes are involved in tumor cell survival, proliferation, metastasis and others [57–59]. The role of IL-6 in colitis-associated carcinogenesis has further been explored by Grivennikov and colleagues, who could show that both IL-6 and STAT3 are required for the survival of intestinal epithelial cells and development of colitis-associated cancer [60, 61]. Very recently, IL-11 was further identified as another key cytokine of the IL-6 family during gastrointestinal tumorigenesis [62]. Supporting these data, high levels of IL-6 could be found in patients with colon carcinoma [63]. Consequently, the anti-IL-6R antibody tocilizumab has been tested in clinical trials for both inflammatory diseases and cancer and shows promising results in some of these conditions [64].
Another important cytokine in the pathogenesis of both IBD and colitis-associated cancer is the tumor necrosis factor α (TNF α). TNF α is secreted predominantly by macrophages but other cells have the ability to release TNF α at the site of inflammation. The cytokine acts mainly through the TNF receptor 1 (TNF-R1) and has multiple effects depending on the tissue context. Its role as a pro-inflammatory mediator made it an attractive target in IBD therapy. In fact, TNF-targeting therapeutics are an integral part of the anti-inflammatory regime in Crohn’s disease as well as ulcerative colitis [65]. TNF α promotes inflammatory carcinogenesis by inducing DNA damage, proliferation and angiogenesis in the intestinal epithelium. When TNF-R1-deficient mice were exposed to AOM/DSS these mice showed reduced inflammation and fewer colitis-associated tumors. In addition, bone marrow chimera from TNF-R1-deficient mice or mice treated with etanercept, a soluble TNF receptor, developed fewer colonic neoplasms [66]. This is supported by newer data in mice receiving long-term DSS mimicking chronic colitis. When treated with infliximab, a monoclonal antibody targeting TNF α, only 16.7 % of mice developed tumors, compared to 75.0 % percent of control mice. Surprisingly, treatment was effective only at early time points of DSS colitis to prevent animals from developing inflammatory tumors. This is in favor of a ‘top-down’ treatment rather than a ‘step-up’ regimen [67]. Unfortunately, to date there are no good data dealing with a potential preventive effect on inflammatory carcinogenesis in patients receiving anti-TNF therapeutics.
Another important cytokine of the interleukin family for inflammatory bowel disease as well as colitis-associated colorectal carcinoma is IL-10. This has extensively been studied in IL-10-deficient mice, which spontaneously develop colitis mimicking inflammatory bowel disease in humans [68–70]. IL-10 is produced by monocytes and Th2 lymphocytes. Together with TGFβ it is a major anti-inflammatory cytokine [71]. It has been demonstrated, that variants of the IL-10 gene are linked to susceptibility to developing ulcerative colitis [72]. The cytokine has even been evaluated in various clinical trials but so far results with recombinant IL-10 do not fulfill the expectations [73]. It has further been shown, that IL-10 also promotes inflammatory carcinogenesis but the underlying mechanisms have not been elucidated entirely [74].
There are also other cytokines, which do not have a direct effect on the intestinal epithelium but still contribute to colitis-associated carcinogenesis. In this regard, the IL-12 family and IL-23 in particular seems to be a crucial molecule in IBD and colitis-associated cancer development [75, 76]. IL-23 levels are upregulated in various cancers including colorectal cancer and in preclinical animal studies, ablation of IL-23 and its receptor lead to protection against carcinogenesis in different inflammatory tumor models [77–79]. In this regard, IL-23 also influences other pro-inflammatory and thus pro-tumorigenic cytokines such as IL-6, IL-17 and IL-22.
Another signaling molecule that becomes increasingly important in colitis-associated carcinogenesis is the vascular endothelial growth factor VEGF and its main effector molecule the VEGFR-2. The VEGF/VEGFR-2 signaling axis and its role on angiogenesis have been studied extensively in sporadic colorectal cancer and led to the approval of Bevacizumab, a monoclonal antibody targeting VEGF, after it has been proven beneficial in a large phase III clinical trial [80]. The role of angiogenesis in chronic inflammation, however, is less clear [81]. Growing evidence suggests that VEGF is also secreted by various immune cells at the site of inflammation to induce neovascularization, enhance vascular permeability and also directly activate other cells of the immune system [82]. Scaldaferri and colleagues [83] could demonstrate that acute DSS colitis was aggravated in mice constitutively overexpressing VEGF, which could be reversed, by overexpressing a soluble VEGFR protein. Furthermore, it has recently been shown, that VEGFR-2 signaling links inflammation and carcinogenesis in a STAT3-dependent manner. Chronic inflammation leads to an upregulation of the VEGFR-2 directly on the intestinal epithelial cells, where it promotes proliferation and tumor development [59].
The importance of inflammatory mediators in the development of colorectal neoplasia is also supported by a different observation: Aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, were found to decrease the risk of developing sporadic colorectal carcinoma [84, 85]. These drugs even have a beneficial effect in patients with familiar adenomatous polyposis, the hereditary condition in which germline mutations in the APC gene lead to a very early onset of intestinal neoplasia [86]. These data were further supported by a data that demonstrated that 5-ASA (5-aminosalicylic acid) can decrease the risk of colitis-associated cancer in patients with IBD [87, 88]. In contrast, a new study by Ishikawa and Herschman [89] found that tumor formation not necessarily requires COX-1 or COX-2 expression in AOM/DSS-treated mice. Thus, further studies concerning the role of anti-inflammatory drugs such as anti-TNF α therapeutics, COX inhibitors and other NSAIDs in colitis-associated carcinogenesis are needed.
Cell types
Cells of the innate immune system (neutrophils, monocytes, macrophages, mast cells, dendritic cells, etc.) have been regarded mainly as pro-tumorigenic, as they facilitate an unspecific inflammatory response [90]. Among others this has been studied by Mangerich et al. [91] in a model of infection-induced IBD. The role of cells of the adaptive immune system however is more complex. CD4+ effector T cells, respectively various subsets of this cell population, were shown to be critical for the maintenance of chronic inflammation in patients with Crohn’s disease and ulcerative colitis [92]. This would also suggest some CD4+ T cell subsets as possible promoters of colitis-associated carcinoma. Indeed, a Th2 response has been attributed to the progression of experimental and human sporadic CRC, whereas a Th1 response has been associated with an improved prognosis in this disease [93–95]. CD8+ T cells play a very important role in cancer immunosurveillance. Regulatory T cells (Tregs) have been shown to have a protective effect on inflammation and autoimmunity, but this suppression of the immune system can be deleterious. In fact, Tregs have been demonstrated to reduce the host antitumor immune response mediated by CD8+ T cells [96]. Unfortunately, there are only limited data about the role of Tregs in the immunosurveillance of inflammation-associated cancer. Interestingly, in a study by Erdman and coworkers, Tregs were able to reduce tumor growth in APCmin mice, which spontaneously develop colon cancer and have been used for the study of sporadic CRC development, indicating that further data on Tregs are needed [97–99].
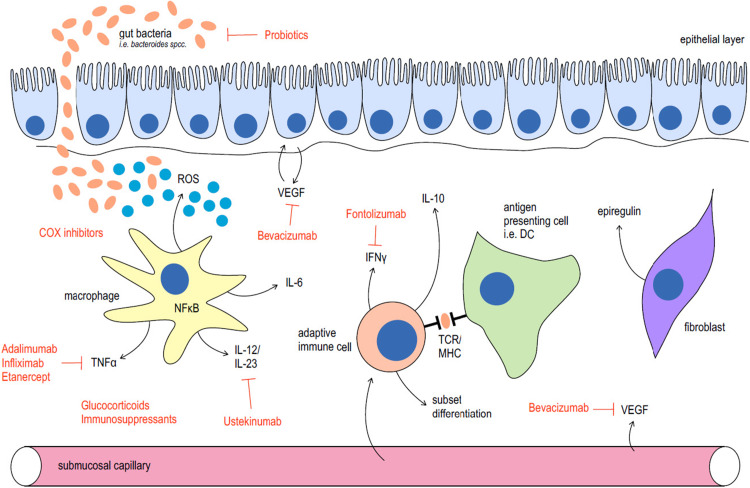
Apart from immune cells and inflammatory cytokines, it becomes more and more apparent, that other cell types have an important role in sporadic and colitis-associated carcinogenesis. In sporadic colorectal cancer it has been well established, that the cells of origin are the intestinal stem cells (ISCs) [100–103]. Using various sophisticated mouse models, it could be shown, that ISCs are located at the base of the intestinal crypt and express the marker Leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5). Targeted knockout of the APC gene in these stem cells resulted in tumor development, while this was not the case when APC was deleted in a non-stem cell population [103]. The influence of chronic colitis on these cells is less clear. In a very recent highly published study, the Greten group could show that during inflammation also non-stem cells of the intestinal epithelium can re-acquire stem like properties and that this leads to the development of colorectal cancer via NFκB and Wnt-signaling [104]. Not only the stem cell niche is in the focus of colorectal carcinogenesis but also the tumoral stroma seems to be of particular importance. For example, Neufert and colleagues [105] could demonstrate very recently, that tumor fibroblast-derived epiregulin promotes growth of colitis-associated neoplasms through ERK (Fig. 2).
Fig. 2.
Cells and mediators involved in inflammatory bowel disease and colitis-associated cancer and their therapeutic implications
Intestinal microflora
Having elucidated the role of cellular and soluble factors in the pathogenesis of IBD and colitis-associated cancer, it has to be discussed, that the human intestine is host of a highly complex microbiome with a variety of over 1,000 different species [106]. The intestinal microbiome fulfills numerous physiologic functions such as development and maturation of the immune system, vitamin synthesis and food digestion [107]. As for malignant diseases, a large body of evidence also shows that in various types of cancers different microorganisms and viruses can significantly contribute to tumor development and progression [107–109]. This seems to be of particular relevance in colitis-associated carcinogenesis. For example, germ-free mice, do not develop colitis and tumor frequency is dramatically reduced in the APCmin as well as the AOM + DSS model [110, 111]. In a study by Arthur and colleagues [112] it could further been demonstrated, that colitis can lead to alterations in microbial composition, which promote tumorigenesis by inducing the expansion of certain bacteria with genotoxic capabilities. But while some specific pathogens have been identified such as Helicobacter and Bacteroides spp [113–115], we are far from understanding the role of intestinal microbes in health and disease.
Epigenetic alterations in CAC
Apart from mutations and genetic factors influencing IBD and consequently CAC development, epigenetic factors could potentially mediate interactions between the environment and the genome in patients with CD and UC. The field of epigenetics subsumes all heritable genetic changes that are not due to changes in the DNA sequence itself. The three major epigenetic mechanisms are RNA interference by microRNAs, histone modification and DNA methylation [116, 117]. In resection specimen of patients with active UC, for example, a substantial number of loci have been demonstrated to be differentially methylated when compared to non-inflamed tissue. These loci include MYOD1, GDNF, HPP1 and CDH1 and are associated with cancer development [118]. Interestingly, a study by Dhir et al. [119] could show methylation of WNT signaling pathway genes which occur early in patients with IBD colitis and increase during inflammatory carcinogenesis. This is in contrast to actual mutations in this pathway, which are typical for late colitis-associated tumorigenesis. Furthermore, studies have shown that various DNA methylation patterns can be found in the colonic mucosa of IBD patients that are related to aging. This potentially reflects a higher proliferation rate in these tissues and could contribute to genomic instability [120]. Data on the role of histone modification in IBD and CAC are scarce and are mainly derived from the use of histone deacetylase inhibitors [121, 122]. For RNAi by microRNAs, several candidates could be identified, which are differentially expressed and seem to be involved in the pathogenesis of IBD such as miR-150 [123] or miR-192 [124]. In addition, when a molecule involved in processing miRs in the intestine is ablated this leads to spontaneous intestinal inflammation [125].
Translational approaches
Diagnosis of colitis-associated cancer
Endoscopic surveillance
With IBD patients being at high risk to develop colorectal cancer, surveillance colonoscopy is considered to be the gold standard in the diagnosis of dysplastic and neoplastic changes. Current guidelines recommend the first surveillance colonoscopy 8–10 years after onset of the first symptoms. Endoscopic examinations are then scheduled every 1–2 years. In high-risk patients with extraintestinal manifestations, screening should take place annually. But, because that risk depends on various factors, such as disease duration or anti-inflammatory therapy, the role of preventative surveillance programs remains controversial. Opponents argue that as many as 50–80 % of colitis-associated cancer lesions are not visible during colonoscopy [126] and point to a relatively high number of patients that need to be enrolled in these programs to identify a patient with colitis-associated cancer [5, 127]. This is even more relevant when taken into account that the usual endoscopic procedure in patients with IBD is to take multiple non-targeted biopsies. Rubin and colleagues [128] calculated that at least 56 of these biopsies needed to be taken at every single surveillance colonoscopy to rule out dysplasia with a 95 % confidence interval. Although in large part driven by technological advances, diagnosis of IBD and CRC is also highly dependent on our understanding of the pathophysiology and molecular events, which occur during inflammatory carcinogenesis.
Emerging technologies
Recently, new endoscopic imaging techniques have been introduced, which allowed a more detailed visual representation of mucosal and submucosal features. For example, high-definition (HD) technology can be applied for endoscopy to achieve a higher resolution and greater magnification. It has been shown that HD endoscopy could substantially contribute to detect and characterize flat neoplastic lesions as often found in patients with IBD [129]. Other technologies include the use of different filters to highlight various mucosal features. Enhancing the appearance of the crypt helps to differentiate neoplastic from non-neoplastic changes and enables the performance of targeted biopsies. In a meta-analysis of six randomized controlled trials it was found that the diagnostic accuracy of chromoendoscopy for colitis-associated cancer detection dysplastic lesions is considerably high [130]. It has been further shown that some of these techniques might be useful in the diagnosis of colitis-associated dysplasia and neoplasia [131–133].
Another promising diagnostic tool is denoted confocal laser endomicroscopy (CLE) [134]. It uses miniaturized confocal microscopic probes that are integrated in conventional endoscopes und thus provide the endoscopist with direct in vivo histologic imaging of the mucosa during ongoing examination. For precancerous lesions in IBD, Kiesslich et al. demonstrated that when CLE was combined with chromoendoscopy 4.75-fold more neoplasia could be detected in surveillance colonoscopies of patients with ulcerative colitis compared to conventional endoscopy. In addition, only half of the biopsies were necessary and CLE could predict neoplastic changes with high sensitivity and specificity. [135–137]. Only recently, CLE was even explored to perform molecular-targeted microscopic imaging in vivo in various models of sporadic colorectal cancer. This was achieved by aiming at different tumor epitopes such as EGFR (epithelial growth factor receptor) and VEGF (vascular endothelial growth factor) with fluorescently labeled antibodies [138, 139]. Also imaging of COX activity was feasible with an activate probe [140]. The first step from bench to bedside in molecular-targeted imaging with CLE in IBD patients was then achieved using fluorescently labeled anti-TNF α therapeutics in patients with ulcerative colitis. Specific imaging was not only feasible, but also it could be used to predict the response to therapy in a small cohort of patients receiving anti-TNF α treatment [141]. This is very promising, as these therapies are costly, not without side effects and there is a distinct group of non-responders. Further applications of this in vivo molecular-targeted imaging include early detection of colitis-associated carcinoma by targeting pre-neoplastic stages. In summary, the role of endoscopy and surveillance programs in patients with IBD remains a controversial issue, but recent technologic advances could potentially improve diagnosis of colorectal neoplasia in IBD patients [142].
Systemic treatment of colitis-associated cancer
Having examined recent diagnostic advances in IBD and colitis-associated cancer, which in part originated from growing knowledge about molecular mechanisms in the pathophysiology of IBD, we would like to close this review by looking at some important aspects in the current treatment of colitis-associated cancer. As described above, for sporadic colorectal cancer it has been shown that chemoprevention can lower the incidence and reduce the risk for individuals to develop colorectal cancer. Therapeutics that can be used in colorectal cancer prevention are drugs inhibiting inflammation (COX inhibitors, Salicylates etc.). This might result from the fact that sporadic colorectal cancer and inflammation-associated cancer are promoted by similar pathways. So the rationale in using anti-inflammatory drugs in IBD patients not only to treat their chronic disease, but to prevent development of colitis-associated cancer is very strong. While several studies support this concept, the evidence is somehow conflicting due to differences in study design and details of the medication regimen.
Chemoprevention of colitis-associated cancer
Aminosalicylates
Aminosalicylates are in integral part of maintenance therapy in IBD patients as they are inexpensive and fairly safe to use. Several studies have tried to elucidate the potential of sulfasalazine and mesalazine in the prevention of colitis-associated cancer development in patients with long-standing inflammation. It was shown, that colitis-associated cancer incidence can be reduced over 75 %, when mesalazine was used, while sulfasalazine was not as that much effective. The highest risk reduction was seen when 1 g/day was administered, but both drugs showed also dose-dependent effects [143–145]. This was supported by animal studies in which the administration of 5-ASA or 5-ASA prodrugs demonstrated a significant inhibitory effect on the development of intestinal tumors [146, 147]. One of the main influenced pathways is the NFκB signaling axis, for example. While several studies came to similar conclusions, some groups were not able to reproduce these findings and could not observe any significance when these drugs were used. However, the majority of the investigations have found at least a protecting effect, supporting the role of mesalazine and to some extent sulfasalazine as chemopreventive agents in colitis-associated cancer development [148, 149].
Ursodesoxycholic acid
In patients with the non-intestinal manifestation of primary sclerosing cholangitis, ursodesoxycholic acid is usually used to treat this liver condition. Surprisingly it could be observed, that especially in ulcerative colitis patients, that drug could prevent development of colitis-associated cancer and even dysplasia, when compared to untreated patients. It is unclear what the underlying molecular mechanisms are but it has been suggested that ursodesoxycholic acid acts antioxidative and thereby reduces mutational stress by ROS and RNS. Also bile acids, which are believed to have carcinogenic potential, are reduced [150–152]. Again, further studies are needed to clearly show a beneficial effect of these drugs [153].
Glucocorticoids
Glucocorticoids only play a minor role in chemoprevention of CAC. While these therapeutics are important in the treatment of acute flares of IBD, their adverse effects during long-term use are dreaded. So there are only few studies dealing with glucocorticoids and the risk of CRC development in patients with IBD. Among others, van Staa and colleagues [154] could show, that regular use of glucocorticoids and other immunosuppressants was associated with a reduced risk of CRC (adjusted OR 0.38), while only taking oral glucocorticoids even slightly increased the risk to develop CRC. The use of budesonide, a glucocorticoid with minimal systemic absorption, could pose a potential alternative, but has yet to be evaluated in this regard [155].
Biologicals
For treatment with neutralizing antibodies and other biologicals, there are unfortunately no solid data about preventive properties against dysplasia or colitis-associated cancer [156, 157]. Animal studies however suggested that chronic colitis mimicked by repeated cycles of DSS lead to a reduced onset of colitis-associated neoplasia, when animals were treated with infliximab compared to standard therapy without biologicals [67]. In a recent retrospective study on follow-up data of patients with infliximab, 4 out of 651 patients with IBF developed colorectal cancer, while probability calculations from epidemiologic data would have predicted a slightly higher number [158]. Other biologicals, such as anti-IL-6 receptor antibody Tocilizumab or anti VEGF antibody Bevacizumab have only recently been suggested for treatment of IBD and colitis-associated cancer patients, so there are no data in chemoprevention of colitis-associated cancer as of yet [73].
Conclusion
In summary, chronic inflammation is the key factor that creates a favorable environment for initiation and progression of colitis-associated carcinoma in patients with inflammatory bowel disease. A large amount of data has been obtained by drawing parallels to sporadic colorectal cancer, but the whole pathogenesis of ‘inflammatory carcinogenesis’ is not completely understood [159]. Studying colorectal cancer and colitis-associated neoplasia in particular can help to elucidate the important role of the immune system and microbiota in the development of solid tumors [94, 109]. Diagnosis of colitis-associated cancer is one of the big challenges in gastrointestinal endoscopy, which might be improved by new endoscopic imaging and resection techniques. Clear guidelines should be thoroughly adopted and applied in clinical practice to minimize the risk of tumor development for patients suffering from IBD [160].
Acknowledgments
SF was supported by the clinical research group KFO257 of the Deutsche Forschungsgemeinschaft (DFG), the Johannes and Frieda Marohn-Stiftung and the Endoscopy Research Award by the Olympus Europe Foundation.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Crohn B, Rosenberg H. The sigmoidoscopic picture of chronic ulcerative colitis (non-specific) Am J Med Sci. 1925;170:220–228. [Google Scholar]
- 2.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 3.Jess T, Gamborg M, Matzen P, et al. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724–2729. doi: 10.1111/j.1572-0241.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 4.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutter MD, Saunders BP, Wilkinson KH, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. YGAST. 2006;130:1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Soetikno RM, Lin OS, Heidenreich PA, et al. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48–54. doi: 10.1067/mge.2002.125367. [DOI] [PubMed] [Google Scholar]
- 7.Velayos FS, Loftus EV, Jess T, et al. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: a case-control study. YGAST. 2006;130:1941–1949. doi: 10.1053/j.gastro.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Lashner BA, Turner BC, Bostwick DG, et al. Dysplasia and cancer complicating strictures in ulcerative colitis. Dig Dis Sci. 1990;35:349–352. doi: 10.1007/BF01537413. [DOI] [PubMed] [Google Scholar]
- 9.Lukas M. Inflammatory bowel disease as a risk factor for colorectal cancer. Dig Dis. 2010;28:619–624. doi: 10.1159/000320276. [DOI] [PubMed] [Google Scholar]
- 10.Cho KR, Vogelstein B. Genetic alterations in the adenoma–carcinoma sequence. Cancer. 1992;70:1727–1731. doi: 10.1002/1097-0142(19920915)70:4+<1727::aid-cncr2820701613>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 12.Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87(2):159–170 [DOI] [PubMed]
- 13.Su LK, Kinzler KW, Vogelstein B, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science (New York, NY) 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 14.Powell SM, Zilz N, Beazer-Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 15.Foersch S, Waldner MJ, Neurath MF. Colitis and colorectal cancer. Dig Dis. 2012;30:469–476. doi: 10.1159/000341692. [DOI] [PubMed] [Google Scholar]
- 16.Kern SE, Redston M, Seymour AB, et al. Molecular genetic profiles of colitis-associated neoplasms. YGAST. 1994;107:420–428. doi: 10.1016/0016-5085(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 17.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 18.Gondek LP, Tiu R, O’Keefe CL, et al. Chromosomal lesions and uniparental disomy detected by SNP arrays in MDS, MDS/MPD, and MDS-derived AML. Blood. 2008;111:1534–1542. doi: 10.1182/blood-2007-05-092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burmer GC, Rabinovitch PS, Haggitt RC, et al. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. YGAST. 1992;103:1602–1610. doi: 10.1016/0016-5085(92)91184-6. [DOI] [PubMed] [Google Scholar]
- 20.Hussain SP, Amstad P, Raja K, et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- 21.Brentnall TA, Crispin DA, Rabinovitch PS, et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. YGAST. 1994;107:369–378. doi: 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 22.Cooks T, Pateras IS, Tarcic O, et al. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634–646. doi: 10.1016/j.ccr.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarmin L, Yin J, Harpaz N, et al. Adenomatous polyposis coli gene mutations in ulcerative colitis-associated dysplasias and cancers versus sporadic colon neoplasms. Cancer Res. 1995;55:2035–2038. [PubMed] [Google Scholar]
- 24.Fogt F, Vortmeyer AO, Goldman H, et al. Comparison of genetic alterations in colonic adenoma and ulcerative colitis-associated dysplasia and carcinoma. Hum Pathol. 1998;29:131–136. doi: 10.1016/s0046-8177(98)90222-2. [DOI] [PubMed] [Google Scholar]
- 25.Cooper HS, Everley L, Chang W, et al. The role of mutant Apc in the development of dysplasia and cancer in the mouse model of dextran sulfate sodium–induced colitis. Gastroenterology. 2001;121:1407–1416. doi: 10.1053/gast.2001.29609. [DOI] [PubMed] [Google Scholar]
- 26.Leedham S, Graham T, Oukrif D. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology. 2009;136(542):550.e6. doi: 10.1053/j.gastro.2008.10.086. [DOI] [PubMed] [Google Scholar]
- 27.Brown JB, Lee G, Managlia E, et al. Mesalamine inhibits epithelial beta-catenin activation in chronic ulcerative colitis. Gastroenterology. 2010;138:595–605, 605.e1–3. doi: 10.1053/j.gastro.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee G, Goretsky T, Managlia E, et al. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869. doi: 10.1053/j.gastro.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pozzi A, Yan X, Macias-Perez I, et al. Colon carcinoma cell growth is associated with prostaglandin E2/EP4 receptor-evoked ERK activation. J Biol Chem. 2004;279:29797–29804. doi: 10.1074/jbc.M313989200. [DOI] [PubMed] [Google Scholar]
- 30.Tessner TG, Muhale F, Riehl TE, et al. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest. 2004;114:1676–1685. doi: 10.1172/JCI22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rapozo DCM, Grinmann AB, Carvalho ATP, et al. Analysis of mutations in TP53, APC, K-ras, and DCC genes in the non-dysplastic mucosa of patients with inflammatory bowel disease. Int J Colorectal Dis. 2009;24:1141–1148. doi: 10.1007/s00384-009-0748-5. [DOI] [PubMed] [Google Scholar]
- 32.Lang SM, Stratakis DF, Heinzlmann M, et al. Molecular screening of patients with long standing extensive ulcerative colitis: detection of p53 and Ki-ras mutations by single strand conformation polymorphism analysis and differential hybridisation in colonic lavage fluid. Gut. 1999;44:822–825. doi: 10.1136/gut.44.6.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umetani N, Sasaki S, Masaki T, et al. Involvement of APC and K-ras mutation in non-polypoid colorectal tumorigenesis. Br J Cancer. 2000;82:9–15. doi: 10.1054/bjoc.1999.0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itzkowitz SH. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:553–571. doi: 10.1016/j.gtc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Rashid A, Hamilton SR. Genetic alterations in sporadic and Crohn’s-associated adenocarcinomas of the small intestine. Gastroenterology. 1997;113:127–135. doi: 10.1016/s0016-5085(97)70087-8. [DOI] [PubMed] [Google Scholar]
- 36.Hofseth LJ, Saito S, Hussain SP, et al. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci USA. 2003;100:143–148. doi: 10.1073/pnas.0237083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 38.Westbrook AM, Wei B, Braun J, Schiestl RH. Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Res. 2009;69:4827–4834. doi: 10.1158/0008-5472.CAN-08-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferguson LR. Chronic inflammation and mutagenesis. Mutat Res. 2010;690:3–11. doi: 10.1016/j.mrfmmm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Cook PJ, Ju BG, Telese F, et al. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature. 2009;458:591–596. doi: 10.1038/nature07849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaked H, Hofseth LJ, Chumanevich A, et al. Chronic epithelial NF-κB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc Natl Acad Sci USA. 2012;109:14007–14012. doi: 10.1073/pnas.1211509109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erdman SE, Rao VP, Poutahidis T, et al. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci USA. 2009;106:1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman JE, Hofseth LJ, Hussain SP, Harris CC. Nitric oxide and p53 in cancer-prone chronic inflammation and oxyradical overload disease. Environ Mol Mutagen. 2004;44:3–9. doi: 10.1002/em.20024. [DOI] [PubMed] [Google Scholar]
- 44.Bonner WM, Redon CE, Dickey JS, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Risques RA, Lai La, Brentnall Ta, et al. Ulcerative colitis is a disease of accelerated colon aging: evidence from telomere attrition and DNA damage. Gastroenterology. 2008;135:410–418. doi: 10.1053/j.gastro.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Sullivan JN, Bronner MP, Brentnall Ta, et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat Genet. 2002;32:280–284. doi: 10.1038/ng989. [DOI] [PubMed] [Google Scholar]
- 47.O’Sullivan J, Risques RA, Mandelson MT, et al. Telomere length in the colon declines with age: a relation to colorectal cancer? Cancer Epidemiol Biomarkers Prev. 2006;15:573–577. doi: 10.1158/1055-9965.EPI-05-0542. [DOI] [PubMed] [Google Scholar]
- 48.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 49.Neurath MF, Finotto S. Translating inflammatory bowel disease research into clinical medicine. Immunity. 2009;31:357–361. doi: 10.1016/j.immuni.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 50.Amit S, Ben-Neriah Y. NF-kappaB activation in cancer: a challenge for ubiquitination- and proteasome-based therapeutic approach. Semin Cancer Biol. 2003;13:15–28. doi: 10.1016/s1044-579x(02)00096-2. [DOI] [PubMed] [Google Scholar]
- 51.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Greten FR, Arkan MC, Bollrath J, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Waldner MJ, Foersch S, Neurath MF. Interleukin-6—a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8:1248–1253. doi: 10.7150/ijbs.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto K, Rose-John S. Therapeutic blockade of interleukin-6 in chronic inflammatory disease. Clin Pharmacol Ther. 2012;91:574–576. doi: 10.1038/clpt.2012.11. [DOI] [PubMed] [Google Scholar]
- 56.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22:83–89. doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Jarnicki A, Putoczki T, Ernst M. Stat3: linking inflammation to epithelial cancer—more than a “gut” feeling? Cell Div. 2010;5:14. doi: 10.1186/1747-1028-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waldner MJ, Wirtz S, Jefremow A, et al. VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. J Exp Med. 2010;207:2855–2868. doi: 10.1084/jem.20100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell. 2008;13:7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 62.Putoczki TL, Thiem S, Loving A, et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell. 2013;24:257–271. doi: 10.1016/j.ccr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Chung Y-C, Chang Y-F. Significance of inflammatory cytokines in the progression of colorectal cancer. Hepatogastroenterology. 2003;50:1910–1913. [PubMed] [Google Scholar]
- 64.Tanaka T, Narazaki M, Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol. 2012;52:199–219. doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]
- 65.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 66.Popivanova BK, Kitamura K, Wu Y, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim YJ, Hong KS, Chung JW, et al. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res (Phila) 2010;3:1314–1333. doi: 10.1158/1940-6207.CAPR-09-0272. [DOI] [PubMed] [Google Scholar]
- 68.Berg DJ, Davidson N, Kühn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rennick DM, Fort MM, Davidson NJ. Studies with IL-10-/- mice: an overview. J Leukoc Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 70.Kühn R, Löhler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 71.Grütz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol. 2005;77:3–15. doi: 10.1189/jlb.0904484. [DOI] [PubMed] [Google Scholar]
- 72.Franke A, Balschun T, Karlsen TH, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 73.Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut. 2012;61:918–932. doi: 10.1136/gutjnl-2011-300904. [DOI] [PubMed] [Google Scholar]
- 74.Sturlan S, Oberhuber G, Beinhauer BG, et al. Interleukin-10-deficient mice and inflammatory bowel disease associated cancer development. Carcinogenesis. 2001;22:665–671. doi: 10.1093/carcin/22.4.665. [DOI] [PubMed] [Google Scholar]
- 75.Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grivennikov SI, Wang K, Mucida D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 79.Hinoi T, Akyol A, Theisen BK, et al. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 2007;67:9721–9730. doi: 10.1158/0008-5472.CAN-07-2735. [DOI] [PubMed] [Google Scholar]
- 80.Hurwitz HI, Yi J, Ince W, et al. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14:22–28. doi: 10.1634/theoncologist.2008-0213. [DOI] [PubMed] [Google Scholar]
- 81.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–166. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 82.Yoo S-A, Kwok S-K, Kim W-U. Proinflammatory role of vascular endothelial growth factor in the pathogenesis of rheumatoid arthritis: prospects for therapeutic intervention. Mediators Inflamm. 2008;2008:129873. doi: 10.1155/2008/129873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scaldaferri F, Vetrano S, Sans M, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136(585):595.e5. doi: 10.1053/j.gastro.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 84.Jänne PA, Mayer RJ. Chemoprevention of colorectal cancer. N Engl J Med. 2000;342:1960–1968. doi: 10.1056/NEJM200006293422606. [DOI] [PubMed] [Google Scholar]
- 85.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 86.Raju R, Cruz-Correa M. Chemoprevention of colorectal cancer. Dis Colon Rectum. 2006;49:113–115. doi: 10.1007/s10350-005-0170-1. [DOI] [PubMed] [Google Scholar]
- 87.Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100(6):1345–1353. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 88.Rubin DT, Cruz-Correa MR, Gasche C, et al. Colorectal cancer prevention in inflammatory bowel disease and the role of 5-aminosalicylic acid: a clinical review and update. Inflamm Bowel Dis. 2008;14:265–274. doi: 10.1002/ibd.20297. [DOI] [PubMed] [Google Scholar]
- 89.Ishikawa T-O, Herschman HR. Tumor formation in a mouse model of colitis-associated colon cancer does not require COX-1 or COX-2 expression. Carcinogenesis. 2010;31:729–736. doi: 10.1093/carcin/bgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waldner MJ, Neurath MF. Colitis-associated cancer: the role of T cells in tumor development. Semin Immunopathol. 2009;31:249–256. doi: 10.1007/s00281-009-0161-8. [DOI] [PubMed] [Google Scholar]
- 91.Mangerich A, Knutson CG, Parry NM, et al. (2012) Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer. http://www.pnas.org/cgi/doi/10.1073/pnas.1207829109 [DOI] [PMC free article] [PubMed]
- 92.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 93.Shibata M, Nezu T, Kanou H, et al. Decreased production of interleukin-12 and type 2 immune responses are marked in cachectic patients with colorectal and gastric cancer. J Clin Gastroenterol. 2002;34:416–420. doi: 10.1097/00004836-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 94.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 95.Kettunen HL, Kettunen ASL, Rautonen NE. Intestinal immune responses in wild-type and Apcmin/+ mouse, a model for colon cancer. Cancer Res. 2003;63:5136–5142. [PubMed] [Google Scholar]
- 96.Wolf D, Wolf AM, Rumpold H, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 97.Erdman SE, Sohn JJ, Rao VP, et al. CD4+ CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 98.Erdman SE, Poutahidis T. Roles for inflammation and regulatory T cells in colon cancer. Toxicol Pathol. 2010;38:76–87. doi: 10.1177/0192623309354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Műzes G, Molnár B, Sipos F. Regulatory T cells in inflammatory bowel diseases and colorectal cancer. World J Gastroenterol. 2012;18:5688–5694. doi: 10.3748/wjg.v18.i40.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 102.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 103.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 104.Schwitalla S, Fingerle Aa, Cammareri P, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 105.Neufert C, Becker C, Türeci Ö, et al. Tumor fibroblast-derived epiregulin promotes growth of colitis-associated neoplasms through ERK. J Clin Invest. 2013;123:1428–1443. doi: 10.1172/JCI63748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guarner F, Malagelada J-R. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 107.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rous P, Beard JW. The progression to carcinoma of virus-induced rabbit papillomas (SHOPE) J Exp Med. 1935;62:523–548. doi: 10.1084/jem.62.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y, Kundu P, Seow SW, et al. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis. 2012;33:1231–1238. doi: 10.1093/carcin/bgs137. [DOI] [PubMed] [Google Scholar]
- 111.Strober W, Fuss IJ. Experimental models of mucosal inflammation. Adv Exp Med Biol. 2006;579:55–97. doi: 10.1007/0-387-33778-4_5. [DOI] [PubMed] [Google Scholar]
- 112.Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu S, Rhee K-J, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chichlowski M, Sharp JM, Vanderford DA, et al. Helicobacter typhlonius and Helicobacter rodentium differentially affect the severity of colon inflammation and inflammation-associated neoplasia in IL10-deficient mice. Comp Med. 2008;58:534–541. [PMC free article] [PubMed] [Google Scholar]
- 115.Iliev ID, Funari VA, Taylor KD, et al. Interactions between commensal fungi and the C-type lectin receptor dectin-1 influence colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Relton CL, Smith GD. Epigenetic epidemiology of common complex disease: prospects for prediction, prevention, and treatment. PLoS Med. 2010 doi: 10.1371/journal.pmed.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ventham NT, Kennedy Na, Nimmo ER, Satsangi J. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology. 2013;145:293–308. doi: 10.1053/j.gastro.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saito S, Kato J, Hiraoka S, et al. DNA methylation of colon mucosa in ulcerative colitis patients: correlation with inflammatory status. Inflamm Bowel Dis. 2011;17:1955–1965. doi: 10.1002/ibd.21573. [DOI] [PubMed] [Google Scholar]
- 119.Dhir M, Montgomery EA, Glöckner SC, et al. Epigenetic regulation of WNT signaling pathway genes in inflammatory bowel disease (IBD) associated neoplasia. J Gastrointest Surg. 2008;12:1745–1753. doi: 10.1007/s11605-008-0633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Issa JP, Ahuja N, Toyota M, et al. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001;61:3573–3577. [PubMed] [Google Scholar]
- 121.Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 122.Rosen MJ, Frey MR, Washington MK, et al. STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm Bowel Dis. 2011;17:2224–2234. doi: 10.1002/ibd.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bian Z, Li L, Cui J, et al. Role of miR-150-targeting c-Myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. J Pathol. 2011;225:544–553. doi: 10.1002/path.2907. [DOI] [PubMed] [Google Scholar]
- 124.Wu F, Zikusoka M, Trindade A, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2α. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 125.McKenna LB, Schug J, Vourekas A, et al. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71–74. doi: 10.1016/s0140-6736(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 127.Rutter MD, Saunders BP, Wilkinson KH, et al. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813–1816. doi: 10.1136/gut.2003.038505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rubin CE, Haggitt RC, Burmer GC, et al. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. YGAST. 1992;103:1611–1620. doi: 10.1016/0016-5085(92)91185-7. [DOI] [PubMed] [Google Scholar]
- 129.Hoffman A, Kagel C, Goetz M, et al. High definition colonoscopy (HD plus) with I-scan function allows to recognize and characterize flat neoplastic changes as precisely as chromoendoscopy. Gastrointest Endosc. 2008;67:AB125. [Google Scholar]
- 130.Wu L, Li P, Wu J, et al. The diagnostic accuracy of chromoendoscopy for dysplasia in ulcerative colitis: meta-analysis of six randomized controlled trials. Colorectal Dis. 2012;14:416–420. doi: 10.1111/j.1463-1318.2010.02505.x. [DOI] [PubMed] [Google Scholar]
- 131.Neumann H, Mönkemüller K, Günther C, et al. Advanced endoscopic imaging for diagnosis of Crohn’s disease. Gastroenterol Res Pract. 2012;2012:301541. doi: 10.1155/2012/301541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880–888. doi: 10.1053/gast.2003.50146. [DOI] [PubMed] [Google Scholar]
- 133.Rutter MD, Saunders BP, Schofield G, et al. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256–260. doi: 10.1136/gut.2003.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–713. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 135.Kiesslich R, Goetz M, Lammersdorf K, et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874–882. doi: 10.1053/j.gastro.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 136.Hurlstone DP, Thomson M, Brown S, et al. Confocal endomicroscopy in ulcerative colitis: differentiating dysplasia-associated lesional mass and adenoma-like mass. Clin Gastroenterol Hepatol. 2007;5:1235–1241. doi: 10.1016/j.cgh.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 137.Hurlstone DP, Kiesslich R, Thomson M, et al. Confocal chromoscopic endomicroscopy is superior to chromoscopy alone for the detection and characterisation of intraepithelial neoplasia in chronic ulcerative colitis. Gut. 2007;57:196–204. doi: 10.1136/gut.2007.131359. [DOI] [PubMed] [Google Scholar]
- 138.Goetz M, Ziebart A, Foersch S, et al. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology. 2010;138:435–446. doi: 10.1053/j.gastro.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 139.Foersch S, Kiesslich R, Waldner MJ, et al. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut. 2010;59:1046–1055. doi: 10.1136/gut.2009.202986. [DOI] [PubMed] [Google Scholar]
- 140.Foersch S, Neufert C, Neurath MF, Waldner MJ. Endomicroscopic imaging of COX-2 activity in murine sporadic and colitis-associated colorectal cancer. Diagn Ther Endosc. 2013;2013:250641. doi: 10.1155/2013/250641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Atreya R, Neumann H, Neufert C, et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med. 2014;20:313–318. doi: 10.1038/nm.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schürmann S, Foersch S, Atreya R, et al. Label-free imaging of inflammatory bowel disease using multiphoton microscopy. Gastroenterology. 2013;145:514–516. doi: 10.1053/j.gastro.2013.06.054. [DOI] [PubMed] [Google Scholar]
- 143.Eaden J, Abrams K, Ekbom A, et al. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145–153. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 144.Eaden J. Review article: the data supporting a role for aminosalicylates in the chemoprevention of colorectal cancer in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 2):15–21. doi: 10.1046/j.1365-2036.18.s2.3.x. [DOI] [PubMed] [Google Scholar]
- 145.Bernstein CN, Eaden J, Steinhart AH, et al. Cancer prevention in inflammatory bowel disease and the chemoprophylactic potential of 5-aminosalicylic acid. Inflamm Bowel Dis. 2002;8:356–361. doi: 10.1097/00054725-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 146.Brown WA, Farmer KC, Skinner SA, et al. 5-aminosalicyclic acid and olsalazine inhibit tumor growth in a rodent model of colorectal cancer. Dig Dis Sci. 2000;45:1578–1584. doi: 10.1023/a:1005517112039. [DOI] [PubMed] [Google Scholar]
- 147.MacGregor DJ, Kim YS, Sleisenger MH, Johnson LK. Chemoprevention of colon cancer carcinogenesis by balsalazide: inhibition of azoxymethane-induced aberrant crypt formation in the rat colon and intestinal tumor formation in the B6-Min/+ mouse. Int J Oncol. 2000;17:173–179. doi: 10.3892/ijo.17.1.173. [DOI] [PubMed] [Google Scholar]
- 148.Lindberg B, Persson B, Veress B, et al. Twenty years’ colonoscopic surveillance of patients with ulcerative colitis. Detection of dysplastic and malignant transformation. Scand J Gastroenterol. 1996;31:1195–1204. doi: 10.3109/00365529609036910. [DOI] [PubMed] [Google Scholar]
- 149.Herfarth H. The role of chemoprevention of colorectal cancer with 5-aminosalicylates in ulcerative colitis. Dig Dis. 2012;30(Suppl 2):55–59. doi: 10.1159/000341894. [DOI] [PubMed] [Google Scholar]
- 150.Tung BY, Emond MJ, Haggitt RC, et al. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med. 2001;134:89–95. doi: 10.7326/0003-4819-134-2-200101160-00008. [DOI] [PubMed] [Google Scholar]
- 151.Pardi DS, Loftus EV, Kremers WK, et al. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. YGAST. 2003;124:889–893. doi: 10.1053/gast.2003.50156. [DOI] [PubMed] [Google Scholar]
- 152.Ikegami T, Matsuzaki Y. Ursodeoxycholic acid: mechanism of action and novel clinical applications. Hepatol Res. 2008;38:123–131. doi: 10.1111/j.1872-034X.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 153.Eaton JE, Silveira MG, Pardi DS, et al. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011;106:1638–1645. doi: 10.1038/ajg.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Van Staa TP, Card T, Logan RF, Leufkens HGM. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573–1578. doi: 10.1136/gut.2005.070896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chan EP, Lichtenstein GR. Chemoprevention: risk reduction with medical therapy of inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:675–712. doi: 10.1016/j.gtc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 156.Biancone L, Petruzziello C, Calabrese E, et al. Long-term safety of Infliximab for the treatment of inflammatory bowel disease: does blocking TNFalpha reduce colitis-associated colorectal carcinogenesis? Gut. 2009;58:1703. doi: 10.1136/gut.2008.176461. [DOI] [PubMed] [Google Scholar]
- 157.Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–508. doi: 10.1136/gut.2008.163642. [DOI] [PubMed] [Google Scholar]
- 158.Caspersen S, Elkjaer M, Riis L, et al. Infliximab for inflammatory bowel disease in Denmark 1999–2005: clinical outcome and follow-up evaluation of malignancy and mortality. Clin Gastroenterol Hepatol. 2008;6:1212–1217. doi: 10.1016/j.cgh.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 159.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 160.Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–774. doi: 10.1053/j.gastro.2009.12.035. [DOI] [PubMed] [Google Scholar]