Abstract
The skeletal muscle has the capacity to repair damage by the activation and differentiation of fiber sub-laminar satellite cells. Regeneration impairment due to reduced satellite cells number and/or functional capacity leads to fiber substitution with ectopic tissues including fat and fibrous tissue and to the loss of muscle functions. Muscle mesenchymal cells that in physiological conditions sustain or directly contribute to regeneration differentiate in adipocytes in patients with persistent damage and inflammation of the skeletal muscle. These cells comprise the fibro-adipogenic precursors, the PW1-expressing cells and some interstitial cells associated with vessels (pericytes, mesoangioblasts and myoendothelial cells). Resident fibroblasts that are responsible for collagen deposition and extracellular matrix remodeling during regeneration yield fibrotic tissue and can differentiate into adipose cells. Some authors have also proposed that satellite cells themselves could transdifferentiate into adipocytes, although recent results by lineage tracing techniques seem to put this theory to discussion. This review summarizes findings about muscle resident mesenchymal cell differentiation in adipocytes and recapitulates the molecular mediators involved in intramuscular adipose tissue deposition.
Keywords: Skeletal muscle, Adipogenesis, Fibro-adipogenic precursor, Mesenchymal cells, Fat, Nitric oxide, Satellite cells
Satellite cells and skeletal muscle regeneration
Skeletal muscle physiologically responds to fiber degeneration with a complex and highly coordinated regenerative process. This leads to the repair of damaged tissue or to the establishment of new fibers that progressively substitute those damaged, restoring original integrity [1]. Satellite cells (SC) are localized between the sarcolemma and the basal lamina of healthy fibers in a resting, quiescent state; they are the protagonist of this regenerative process [2]. Following damage, they activate myogenic program, proliferate and differentiate into myoblasts that are able to fuse with themselves or with other fibers [3, 4]. The state of quiescence is characterized by a reversibly arrested G0 phase and expression of the paired-box protein Pax7 [5]. Activated, cell cycling SC express myogenic regulatory factors (MRF) (Myf-5, MyoD, and MRF4) [4, 6]. The down-regulation of Pax7 and the expression of the myogenic factor myogenin characterized the final commitment of these cells to myogenic differentiation [7, 8]. Terminally differentiated cells form multinucleated structures and express muscle proteins such as myosin heavy chain (MHC) [8]. During this process, a subset of SC progeny does not down-regulate Pax7 and returns to the quiescent state during the process of self-renewal [9].
Expression of Pax7 marks SC [5]. In the absence of Pax7, SC can be detected, but their maintenance and proliferation are defective: in mutant mice, postnatal growth and regeneration are severely compromised with progressive loss of SC after birth mainly because of apoptosis [10, 11]. Pax3, the Pax7 paralog, is transcribed in these cells at least in some anatomical districts [12], but it does not exert any anti-apoptotic role [11, 13].
MRF gene family encodes for nuclear proteins with a conserved basic helix–loop–helix domain responsible for dimerization, DNA binding and the establishment of myogenic lineage as well as the control of terminal differentiation. These proteins drive the myogenic program when ectopically expressed from a constitutive promoter in non-myogenic cell [14]. Mice with homozygous deletion of MyoD or Myf-5 have, however, fairly normal muscles indicating overlapping functions for these MRF [15, 16]. On the contrary, myogenesis is severely disrupted in double mutant mice that do not express MyoD and Myf-5, and Myogenin is not transcribed [17]. This may be explained considering that expression of MyoD depends on Pax7, whereas in this postnatal context expression of Myf-5 is Pax independent [11]. Similarly, MRF4 null mice have a normal muscle phenotype even if they are characterized by myogenin overexpression [18]. MRF myogenin has a unique function in the transition from myoblast to a fully differentiated myotube [19, 20].
In vitro studies demonstrate that, once expressed, MRF translocate to the nucleus and bind to DNA by heterodimerization with non-myogenic proteins encoded by the E1A and HEB genes [21, 22]. Their activity is indeed finely regulated: MRF are also subjected to negative control at the post-transcriptional level by direct interaction with repressors that block their binding to DNA or by indirect mechanisms (phosphorylation/dephosphorylation, acetylation, ubiquitination). Expression of Id protein induced by growth factors sequestrates the MRF dimers blocking their activity [23], and a similar control is exerted by cell cycle regulators [24, 25] and other repressors (MyoR, Mist1, ZEB, I-mfa) [26–29]. Protein kinases A and C [30, 31], cell cycle kinases [32, 33] and mitogen-activated protein kinases regulate MRF via phosphorylation that inhibits or promotes their activity [33, 34]. MyoD co-precipitates with co-activators that have acetyltransferase activity, suggesting that gene acetylation is an additional regulation mechanism [35]. Finally, MRF and in particular MyoD and myogenin have short half-lives and their degradation is regulated by the ubiquitin system [33].
A second class of transcription factors that controls myogenesis is the myogenic enhancer factor (MEF)-2 family, which transcriptionally activates various muscle-specific genes including creatine kinase and MHC, desmin and MRF [36, 37]. Although these factors are not muscle specific, mRNA splicing regulates their muscular expression [38]. Similarly to MRF, they initiate myogenic program when overexpressed in non-muscle cells [37]. Many studies have underlined a regulatory network between MFR and MEF: their DNA-binding sites are located in close proximity and overexpression of MRF induces MEF [39, 40].
These complex and highly regulated mechanisms of SC’s activation and differentiation are necessary for regeneration, since the muscle cannot restore its original integrity in the absence of SC [41, 42]. Nevertheless, the regenerative process involves additional cells. Immune cells, firstly dominated by neutrophils and subsequently by macrophages, rapidly infiltrate tissue upon damage. They remove debris, but also drive SC myogenesis [43, 44]. In the regeneration contest, endothelial/vascular cells are then responsible for the vasculature remodeling and supply of energy to newly formed fibers [45]. Angiogenesis and myogenesis proceed simultaneously and endothelial cells regulate SC’s activation [46]. Interstitial fibroblasts exert another important role in regeneration. They proliferate upon fiber damage and synthesize collagen and other extracellular matrix (ECM) components to provide a scaffold that supports SC migration and new fiber formation. ECM presents growth factors as well as other signaling molecules to fibers [47]. The subsequent degradation of ECM, driven by proteases (including matrix metalloproteinases, MMP), is required for normal tissue repair [48].
During the last decade, other muscle cells of mesenchymal origin that contribute to regeneration have been also identified: they reside in the interstitium between fibers eventually associated with vessels. They, in physiological conditions, sustain SC activation and differentiation and/or directly differentiate in myoblasts and form new fibers [49–51].
Muscle regeneration versus fat tissue deposition
Despite the regeneration ability of healthy skeletal muscle, extensive and widespread fiber destruction is a common feature of various diseases in which the trigger cannot be eliminated, such as skeletal muscular dystrophies. Genetic defects of different dystrophin complex proteins that lead to sarcolemma fragilities during contraction are the basis of these diseases; persistent injury jeopardizes the ability of the tissue to heal and progressively leads to fiber substitution with ectopic tissues such as bone, fibrotic tissue and fat [52, 53].
The muscle fat includes both acellular lipid droplets within the fibers and interstitial adipocytes characterized by drops of triglycerides and cholesterol ester (that can be stained by O-red oil) and by the expression of peroxisome proliferator-activated receptor γ2 (PPARγ2), perilipin, leptin, adiponectin and fatty acid-binding protein 4 [54]. Intra- and interfiber fat is usually white, but brown adipocytes have also been identified [55]. These cells that express uncoupled protein 1 (UCP1) and are characterized by uncoupled respiration could be useful when skeletal muscles fail to produce heat during physical exercise [56].
Intramuscular fat is a characteristic of muscles with impaired SC function as demonstrated in injured mutant mice with ablation of Pax7, myogenin or MyoD [13, 15, 19]. It is also a histopathological characteristic of muscle dystrophies [57, 58], inflammatory myopathies [59], sarcopenia due to disuse or nerve injury as well as aging [60–65], obesity, diabetes and other metabolic diseases [66–68]. In Duchenne muscular dystrophy (DMD), the most severe and prevalent among dystrophies, intramuscular fat can reach as much as 50 % of muscle mass in young boys [69]. Evaluation of muscular fat content by magnetic resonance imaging (MRI) represents, for DMD as well as for inflammatory myopathies, a biomarker of disease progression or activity. It also provides a potential outcome measure for the assessment of treatment efficacy in clinical trials [69–71]. In DMD, older boys show higher muscle fatty infiltration as measured by MRI; their characteristic pattern is the presence of fat mainly in the gluteus and the adductor magnus. Semimembranous muscles, biceps and rectus femoris, are fatty infiltrated too, while gracilis and sartorius muscles are usually spared [72]. Images show both intermuscular and intramuscular adipose depositions and seem to correlate [72]. MRI analyses of DMD muscles reveal that muscles showing clear signs of inflammation and edema are not always infiltrated by adipose tissue. In contrast, other muscles undergo marked fatty deposition in the absence of a prominent inflammatory reaction [73].
To investigate muscle adipose tissue deposition in experimental mouse model, glycerol injection is most commonly used [74], but adipogenesis can be also detected after cardiotoxin injection or freeze injury, depending on the genetic background of mice [75–77]. This indicates that activation of cells responsible for fat deposition could be a hallmark of regeneration processes regardless of the trigger, and that there is a strict connection between SC and adipocyte precursors. It seems important to take into consideration that some cytokines released by adipocyte precursors can positively regulate myogenic cell proliferation and differentiation [78], and ablation of muscle adipogenic cells impairs regeneration [79, 80]. This suggests that the intramuscular pre-adipocytes are per se important for muscle homeostasis and underlines the importance of investigating the factors that influence the choice between regeneration and ectopic tissue deposition.
Different cells of mesenchymal origin, including SC themselves, appear to be able to differentiate into adipocytes when cultured in vitro in the presence of adipogenic-inducing factor (including insulin, dexamethasone and 3-isobutyl-1-methylxanthine) [81] and, therefore, contribute to adipose tissue deposition in vivo. This review focuses on muscle resident cells responsible for intrafiber adipogenesis in damaged/inflamed muscle including recent findings about SC transdifferentiation obtained relying on innovative methods for cell isolation and lineage tracking. We also analyze molecular regulators of muscle adipogenic precursors in muscle and consider the meaning of adipose cells in the pathophysiology of the skeletal muscle. Finally, since fatty degeneration occurs and often, but not always, associates with skeletal muscle fibrosis in chronic neuromuscular diseases, we discuss differences and resemblances between the two events.
Skeletal muscle resident cells are sources of intramuscular adipose tissue deposition after damage
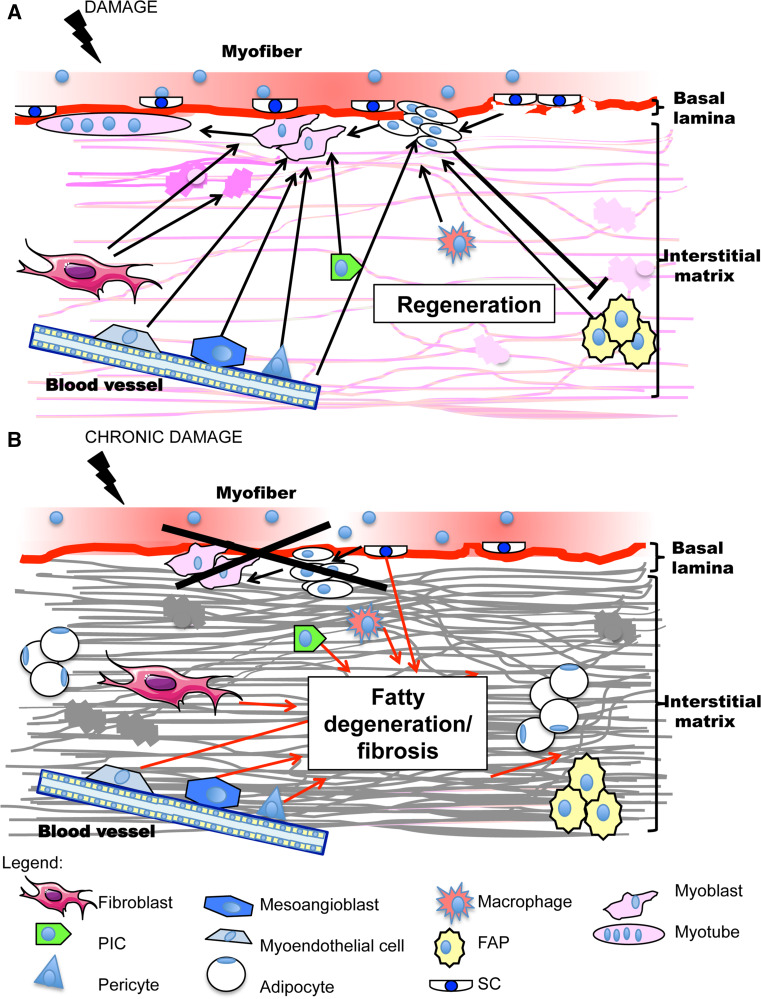
Many cells contribute to ectopic intrafiber adipose tissue formation, both resident in the skeletal muscle and originating from adipose tissue or bone marrow. Figure 1 depicts muscle resident cells involved in regeneration or in fibrosis and fatty degeneration tissue formation (SC, endothelium, interstitial cells and fibro-adipogenic precursors, matrix fibroblasts) and their interactions during the two events.
Fig. 1.
Schematic representation of muscle resident cells involved in regeneration or in adipo-fibrous tissue formation and of their interactions. a In physiological conditions, fiber damage induces satellite cells (SC) activation after basal lamina breakage, proliferation and differentiation into myoblasts that fuse to form new myotubes. Fibro-adipogenic precursors (FAP), located in the interstitial matrix, sustain SC cell regeneration by soluble factors and conversely SC inhibit their differentiation into adipocytes. Interstitial cells (PW1-expressing cells or PIC, pericytes, mesoangioblasts and myoendothelial cells) differentiate into myoblasts and contribute to regeneration. Fibroblasts, endothelium and infiltrating macrophages sustain myogenesis. Fibroblasts are also responsible of ECM remodeling, which in addition favors myoblast migration and fusion. b In a situation of persistent damage regeneration fails because of SC number reduction and/or functional impairment. In this case, ectopic fibro-adipose tissue deposition comes into view: it is sustained by interstitial mesenchymal cells, including FAP, as well as by fibroblasts and chronic inflammation. SC transdifferentiation along the adipocyte program also occurs
SC transdifferentiation
SC exist as heterogenic populations of precursor cells committed to the myogenic lineage and are endowed with “stem cell” properties, such as self-renewal. Upon damage, SC re-enter the cell cycle and, after some rounds of division, the majority of them proceed along the myogenic pathway. Some cells, however, down-regulate myogenic-specific proteins and revert to quiescence to maintain the stem cell compartment [82, 83]. Both quiescent and activated SC express two isoforms, different in length, of the stem cell marker CD34 [84]. Based on this stem cell potential, some authors suggest the possibility that SC transdifferentiate to non-myogenic cells including adipocytes. Several in vitro studies indicate that immortalized myoblasts (such as C2C12 and L6 myoblasts) and primary SC can differentiate into non-myogenic cells, including adipocytes. This SC transdifferentiation has been investigated using both isolate cells and single fibers maintained in culture [54, 85–88]. Differentiation appears more likely to occur in SC isolated from damaged muscle, such as in experimental models of obesity or aging, and in cells cultured in conditions of high oxygen pressure [89–91]. The SC isolation methods as well as the use of immortalized cell lines represent a potential criticism of these experiments. SC are usually isolated by enzymatic digestion of muscle followed by propagation in myogenic-selecting conditions or by clonal proliferation. This carries risk of contamination, depending on the method employed. Moreover, usually cell clones are cultured for long periods of time with a certain risk of spontaneous transformation with the generation of cells that are defective for differentiation [79].
Cell sorting by fluorescence-activated flow cytometry provides a powerful tool for cell isolation. The selection of specific SC markers (e.g., Pax7) limits contamination by non-myogenic cells [92, 93]. Similarly, quiescent SC can be also isolated using SM/C-2.6 [94, 95], anti-integrin α7 [96, 97] or anti-syndecan 3/4 [98] antibodies. Some authors have employed CD34 for myoblast isolation [84]. However, the use of this antigen for SC’s retrieval is debatable; in human muscle, CD34negative precursors show an in vivo and in vitro myogenic potential higher than CD34 positive cells [99]. These progenitors indeed comprise cells with adipogenic or myogenic commitment and myoadipogenic bipotent precursors and can be separated by the expression of CD56 and CD15 [100]. CD34 is also expressed, together with the stem cells antigen 1 (Sca-1), by a recently identified population resident in skeletal muscle, localized in the interstitial space, and able to differentiate into adipocyte, endothelial and myogenic cells. These cells are defined as myoendothelial cells [101]. It should be taken into account that all the isolation methods based on quiescence markers may need validation for SC purification from damaged muscles: activated cells have a reduced expression of Pax7 as well as of the other quiescence markers [6]. Some recent papers report investigations about SC transdifferentiation employing the Cre-loxP recombination system for lineage tracing. Using the Cre–loxP recombination system with the cre gene driven by the MyoD promoter, Goldhamer and colleagues demonstrated in vitro that the YFP clonal cells derived from MyoD (cre+)R26R(YFPpositive) muscles (that also represent 98 % of isolated Pax7positive cells) only undergo myogenic differentiation [102]. These cells accumulate lipids within the cytoplasm, but do not activate an adipogenic program and do not express terminal differentiation markers of mature adipocytes, such as adipsin or FAB4 [103]. The same group investigated the origin of isolated adipocytes in Tie2–Cre mice muscle fibers. Since Tie-2 identifies a population of interstitial cells with adipogenic potential, they demonstrated that the majority of adipocytes were Cre-recombinant, underlining that the origin of muscle adipocytes is different from that of SC. Consistently with the previous studies, using a Cre/LoxP tracing system for Pax3 (a paired-box gene expressed during muscle development), it has been demonstrated that intramuscular fat in leg muscles (e.g., soleus and extensor digitorum longus muscles) is derived from a lineage that is Pax3 negative, non-SC lineage [80].
While these recent studies seem to call the SC transdifferentiation theory into question, the possibility that SCs could adopt an adipogenic fate in vivo during repeated rounds of damage or under different death stimuli remains to be clarified.
Brown adipocytes are present in muscles [104]. SC and brown adipose tissue precursors derive from progenitors that have common gene determinants: both cells originate from a mesenchymal precursor that expresses Myf-5. In 2001, the group of Rudnicki demonstrated that lacZ+ cells isolated from Myf-5–nlacZ mice undergo myogenic, osteogenic and adipogenic differentiations [86]. More recently using a Cre/LoxP-based system for SC lineage tracing (Pax7–CreER; R26R-tdTomato), they showed that SC could become brown adipocytes. The choice between myogenic or brown adipose differentiation is tightly controlled in physiological conditions by regulation of the expression of nuclear Prdm16, a transcription factor required to establish brown adipocyte lineage [105]. Myf-5 expression in adipose tissue is heterogeneous and some Myf-5-positive cells differentiate in vitro and in vivo into adipocytes or myofibers according to the expression levels of stem cell antigen (Sca-1) [106]. Accordingly, mesenchymal stem cells derived from adipose tissue differentiate into functional skeletal muscle cells when intramuscularly or intravenously injected in murine dystrophic muscle [107].
Fibro-adipogenic precursors (FAP)
Back to back papers published in 2010 identified very similar cells located in the interstitial space between fibers (but outside of vessels) that have adipogenic potential and are important in skeletal muscle regeneration [78, 108]. These cells, called fibro/adipogenic precursor cells (FAP), can be identified and isolated from both wild-type (WT) and dystrophic mice muscles as CD45negative, CD31negative and Sca-1positive cells. They also express the platelet-derived growth factor receptor α (PDGFRα) in the absence of SC markers such as α-7 integrin, SMC 2.6 and Pax7 [78, 92, 108]. FAP and SC do not share common progenitors, reside in close proximity within damaged muscle and are both important for regeneration. Bromodeoxyuridine incorporation experiments reveal that FAP proliferate more quickly than SC during the first 72 h after injury. Their number returns to those measured in pre-damaged tissue 4–5 days later, concomitantly with SC-initiated muscle regeneration [78]. This suggests that, when regeneration physiologically occurs, FAP proliferate to sustain myogenesis. They produce soluble factors that stimulate SC. Among these, IL-6 has been identified in cells isolated from WT animals [78], while FAP from leg dystrophic muscles of young but not old mdx mice (a mouse model of DMD) produce follistatin [109]. FAP also seem to play a role in the phagocytic clearance of necrotic cells and debris. This event is essential for effective muscle regeneration [110].
FAP yield adipocytes in vitro when challenged with adipogenic factors and in vivo under conditions that favor adipogenesis, such as glycerol injection [79] or conditions characterized by SC failure (e.g., DMD) [92]. Conversely, efficiently proliferating myoblasts inhibit FAP differentiation into adipocytes [79].
FAP isolated from healthy animals can also differentiate into other cells of mesenchymal origin, including osteoblasts, chondroblasts and smooth muscle cells when cultured under appropriate conditions. They apparently never yield myogenic cells [78, 79]. Transforming growth factor (TGFβ1) acts on FAP to yield collagen expressing fibroblasts: they are abundant in the fibrotic area of the diaphragm muscle of mdx mice, indicating that FAP are the possible source of fibrosis in these muscles [111]. Sca-1positive, α-7 integrinnegative cells isolated from the leg muscle of mdx mice that received Tricostatin (TSA), a histone deacetylases inhibitor, acquired myogenic potential with the expression of MyoD and of the SWI/SNF chromatin-remodeling factor BAF60C (important for MyoD-mediated transcription). These cells form MHC-positive structures and less adipocytes when cultured for 6 days in adipogenic conditions [112] However the expression of PDGFRα, expressed by Sca-1positive-FAP, is not reported. These data indicate that in chronically damaged muscles, the environment profoundly modifies the FAP behavior. Interestingly, a human counterpart of PDGFRα+ progenitors described in mice has been recently identified and seems to aberrantly accumulate in muscle diseases [113]. This might open the possibility of developing new approaches for DMD patient therapy based on the regulation of FAP differentiation: the efficacy of treatment has been already demonstrated in pre-clinical studies [92, 109, 114].
Myoendothelial cells, pericytes and mesoangioblasts, and PW1-expressing cells (PIC)
Besides FAP, other mesenchymal cells that differentiate into adipocytes have been identified. This is a heterogeneous group of cells located in the interstitium between fibers (outside the basal lamina). Most of these cells are tightly associated with muscle vessels and express the platelet endothelial cell adhesion molecule. This stem cell population is distinct from SC, but may have a myogenic fate in vivo. It was identified in 1999 and purified based on fluorescent dye Hoechst 33342 efflux; these cells have been named side population (SP) [115]. SP cells express Sca-1 and c-Kit and may be either CD45negative or CD45positive: despite the absence of Pax7 and desmin expression, they can differentiate into myoblasts within muscle or in co-culture with SC. They become hematopoietic cells after intravenous injection [51, 115–119]. All these results indicate that SP cells, though possessing a constitutive haematopoietic potential, yield myoblasts upon appropriate conditions. Tamaki and co-workers showed that CD34positive cells are present outside the basal lamina. Among these, some CD34positive/Sca-1positive/CD45negative cells differentiate into myoblasts and endothelial cells when injected into a recipient muscle and become adipocytes in vitro. These cells have been named myoendothelial progenitors [101]. They have been recently characterized for the high expression of PDGFRβ and absence of PDGFRα, and have showed a multi-lineage potential (i.e., myogenic, endothelial and adipogenic) by clonal analysis. Hence, these cells represent a subpopulation of SP cells endowed with more pronounced myogenic potential. Myoendothelial cells are also able to inhibit in vivo, under physiological conditions, muscle adipogenesis via bone morphogenetic protein (BMP) [120]. A human putative counterpart able to differentiate in chondrocytes and osteoblasts has also recently been identified [121].
Pericytes, also known as aka mural cells, surround endothelial cells in capillaries and microvessels. Like myoendothelial cells, pericytes are characterized by the expression of the neural/glial antigen 2 (NG2), α-smooth muscle actin, CD146 and PDGFRβ. They exhibit multi-lineage developmental potential and differentiate into skeletal myofibers, bone and cartilage [122, 123]. They express PPARγ2 and form lipid droplets when cultured in adipogenic medium [124]. Proliferating pericytes do not express Pax7, Myf5 and MyoD, but up-regulate rapidly these pro-myogenic markers, together with myogenin before forming myotubes, in myogenic-inducing condition [123]. Recently, two pericyte subtypes have been identified using a double transgenic nestin–GFP/NG2-DsRed mouse. Type 1 cells express nestin and type 2 do not [125]. Both types re-enter into cell cycle and proliferate in vivo after damage; however only type 2 pericytes, which are characterized by the lack of CD34 and Sca-1 expression, generate muscle cells. In contrast, type 1 nestinnegative pericytes express Sca-1 and PDGFRα and differentiate into adipocytes, but not into myogenic cells. This indicates that a subset of pericytes may correspond to the previously described FAP [126].
Mesoangioblasts, possibly a sub-fraction of pericytes, have been first initially isolated from murine aorta and have been found in skeletal muscle vessels of different species [127]. Freshly isolated mesoangioblasts in culture express several early endothelial markers such as fetal liver kinase-1, Sca-1, CD34 and VE (vascular endothelial)-cadherin but not the von Willebrand factor. A fraction of mesoangioblasts consistently express smooth muscle actin [128]. Both murine and human mesoangioblasts differentiate into skeletal myoblasts under condition permissive for myogenesis, in osteoblasts after exposure to BMP-2, into adipocytes and into chondrocytes and in smooth muscle too [128, 129]. These cells express Pax3, which is required for both myogenic and adipogenic differentiation [130]. After long-term culture they lose myogenic differentiation capacity, but remain myogenesis inducible upon co-culture with myoblasts. Therefore, they have a therapeutic potential in the treatment of skeletal muscle disease [128, 131]. Interestingly, mesoangioblasts apparently depend on the interaction with polarized macrophages to yield effectively functional contractile tissue [132].
Finally, Mitchell and co-workers identified another mesenchymal population with adipogenic potential in the early mouse postnatal muscle interstitium. These cells, called PIC, express PW1, a protein coded by a zinc finger gene which as a role in the myogenic and neuronal lineage development [133]. PIC share with SC the PW1 expression, but do not express Pax3 or Pax7 and display myogenic potential in vitro. They form new fiber after in vivo engraftment while retaining the ability to differentiate into α-smooth muscle actin-positive myofibroblasts in vitro [133]. A recent paper by the same group of researches revealed that postnatal PIC were a heterogeneous population. They express different levels of PW1 and sca-1 and are endowed with different differentiation potentials [134]. A fraction of cells express a high level of sca-1 (Sca-1high), and 60 % of these positive for PW1 show also PDGFRα. Another group of PW1+ cells expressing low (or middle, Sca-1med) level of sca-1 is abundant in the early mouse postnatal stages, but rapidly declines later on. They are no longer detectable after 5–7 weeks of age. When analyzed in early postnatal stages, Sca-1medPW1+ cells have both myogenic and adipogenic potential, higher than that of PW1+/Sca-1high cells. In adult muscles, following a decline of Sca-1medPW1+, PW1+/Sca-1high cells increase their myogenic potential. It is important to note that the fraction of PDGFRα+/PW1+ cells, which is not myogenic, could overlap at least in part with already described FAP. All PIC seem to express mRNA for NG2, indicating a possible overlap of these cells also with muscle pericytes.
Fibroblasts
Stromal fibroblasts synthesize ECM proteins including elastin, laminin, fibronectin, proteoglycans (PG) and the various isoforms of collagen [135]. Fibroblasts are also sensitive to mechanical loading and synthesize different amounts and types of collagen. Moreover, they express different levels of MMP according to the need of each specific muscle [136]. They play an active role in reparative myogenesis through ECM remodeling; fibroblasts proliferate and migrate after damage and provide new ECM components. These elements stabilize the damaged tissue, provide a scaffold to new fibers and drive the formation of neuromuscular junction. Upon injury resolution, they undergo apoptosis. Concomitantly, they regulate matrix remodeling and degradation by the expression of proteases and regulation of their specific inhibitors [137].
Chronic diseases, including DMD, are characterized by recurring cycle of damage and regeneration, alongside persistent inflammation. Those events lead to the development of fibrosis, an accumulation of aberrant ECM within the tissue [138]. In non-muscle organs, activated/fibrogenic fibroblasts can be easily identified by the expression of vimentin and, in particular, of α-smooth muscle actin (αSMA), a contractile protein of stress fibers. It exerts mechanical tension on the ECM, providing a mechanically resistant support, and hence the name of ‘myofibroblasts’. In tissues such as of liver or lung, myofibroblasts may derive from various cells including resident mesenchymal cells and epithelial and endothelial cells or from circulating progenitors derived from the bone marrow. Myofibroblasts are activated by a variety of mechanisms, such as cytokines produced by lymphocytes and macrophages, autocrine factors and pathogen-associated molecular patterns [137]. Notably, one greatest limitation of the study of fibrosis in the muscle is the lack of markers for activated fibroblasts; vimentin or αSMA are also expressed by myoblasts, albeit at lower levels. Recently, transcription factor 4 (Tcf4) has been identified as a newly identified fibroblast marker. The key role of fibroblasts in regeneration has been recently underlined, inducing their ablation using a Tcf4CreERT2 system: this leads to depletion of the SC pool with premature differentiation and formation of smaller regenerated myofibers [139].
In muscle chronic disorders, fibroblasts continue to proliferate, leading to a progressive and self-perpetuating ECM deposition known as fibrosis, with a mechanism resembling and often accompanying muscle fat deposition [140, 141].
Fibroblasts could also directly convert to adipocytes. Green and Kehinde demonstrated this in 1974; when 3T3 fibroblasts are cultured in adipogenic medium they increase their content in fatty acid, precursors of triglyceride synthesis, and activity of lipogenic enzymes. This cellular differentiation occurs when cells stop growing [142, 143]. In 2006, these cells were shown to be pluripotent and could be reprogrammed to pluripotency by transduction of four stem cell-specific transcription factors. The discovery led to the award of the 2012 Nobel Prize to Prof. Takahashi [144]. The fate of skeletal muscle stromal fibroblasts in vivo remains unclear and the lack of specific markers for these cells increases the problem complexity. In human tissues including skeletal muscle, TE-7 has been recently validated as a fibroblast-specific protein [145]. TE-7+/CD56− cells have been isolated from muscle biopsies. They express collagen IV, fibronectin, vimentin and PDGFRα and respond to fatty acid treatment with full adipocyte differentiation [146]. Interestingly, FAP can be identified in mdx diaphragm fibrotic areas and differentiate in vitro into collagen type I-producing cells upon TGFβ stimulation [111]. Accordingly, fibroblast-activated protein-α+ stromal cells express PDGFRα and sca-1 like FAP [147], and PDGFRα knockin mice, characterized by chronic activation of the receptor, have diffuse fibrosis in the skeletal muscle, as well as other organs [148].
The interplay between muscle fibrosis and adipogenesis in pathological conditions as well as the possible connection of stromal skeletal muscle fibroblasts and FAP during physiological regeneration remain to be clarified.
Regulators of skeletal muscle adipocytes-generating cells
The differentiation of pre-adipocytes into fully differentiated adipocytes (endowed with lipid droplet and expressing adipocyte proteins) is finely regulated and already described in non-muscle tissue [149]. Some molecular regulators of muscle resident mesenchymal precursor differentiation in adipocytes have been identified. Table 1 summarizes the best-characterized pathways of intramuscular pre-adipocyte differentiation describing their suggested sources and signaling pathways.
Table 1.
Regulators of muscle pre-adipocyte differentiation
| Regulators | Source/origin | Molecular pathway | Effect | Experiments | References |
|---|---|---|---|---|---|
| High glucose | Diet | Oxidative stress, mTOR activation | Adipogenesis induction | In vitro | [151, 152] |
| Protein synthesis reduction | Dietary restriction | n.d. | Adipogenesis induction | In vitro | [153] |
| PPARγ | Expression/up-regulation | Gene transcription | Adipogenesis induction | In vitro | [54, 85] |
| WNT | Skeletal muscle | cCreB, PPARγ inhibition | Adipogenesis inhibition | In vitro/in vivo | [159, 160] |
| IL-6 | Skeletal muscle/adipose tissue | Akt phosphorylation/c-Jun terminal kinase | Lipolysis induction/adipogenesis inhibition | In vitro | [78] |
| Myostatin | Skeletal muscle/adipose tissue | Smad 3 | Adipogenesis induction/inhibition | In vitro | [170–173, 177] |
| Follistatin | Skeletal muscle/adipose tissue | n.d. | Brown adipogenesis induction | In vitro | [176] |
| BMP | Skeletal muscle/(other organs?) | Smad 1, 5, 8 | Adipogenesis induction/inhibition | In vitro/in vivo | [104, 180] |
| IGF-1 | Skeletal muscle | Rho GTPase | Adipogenesis inhibition | In vitro | [183] |
| MMP | Fibroblasts/other cells | n.d. | Adipogenesis inhibition | In vivo | [185] |
| Collagen (V and VI) | Fibroblasts/adipocytes | Adipose gene transcriptional inhibition | Adipogenesis induction | In vitro | [193, 196] |
| PG | Fibroblasts/adipocytes | n.d. | Adipogenesis induction/inhibition | In vitro | [197, 198] |
| Macrophages-secreted factors | Macrophages | n.d. | Adipogenesis inhibition | In vitro | [201] |
| NO | Skeletal muscle pharmacological | microRNA-27b | Inhibition of FAP differentiation | In vitro/in vivo | [92] |
| HDAC inhibitor (TSA) | Pharmacological | miR1. miR2, miR133, miR206 | Adipogenesis inhibition | In vivo/in vitro | [229, 236] |
Nutrient availability
Nutrient and energy availability is crucial for muscle homeostasis. Myofibers burn substrates to produce energy and have a relatively high content of mitochondria, depending on the type of fiber. Adipocytes are responsible for energy storage and are endowed with few oxidative organelles [54]. SC activation and differentiation is also hinged on mitochondrial biogenesis [150]. Muscle-derived stem cells and SC undergo adipogenic differentiation when exposed to persistent hyperglycemia via oxidative stress or mTOR activation [151, 152]. Moreover, restriction of protein synthesis by essential amino acid reduction seems to favor SC’s transdifferentiation into adipocytes, without affecting their viability [153]. The efficacy of dietary interventions in attenuating muscle loss and restoring muscle mass in sports and geriatric medicine and in the treatment of neuromuscular disease [154] may also be grounded on the regulation of muscle adipose tissue deposition.
PPARγ
PPARγ is a key regulator of adipogenesis. A number of diverse lipids, lipid-like compounds and drugs activate PPARγ and induce pre-adipocytes to generate fully differentiated adipose cells. Up-regulation and activation of PPARγ in mesenchymal cells induce adipogenesis and modulate insulin sensitivity [54, 85]. PPARγ expression and activation induce repression of MyoD [85]. Recent data indicate the existence of a mutual regulation between PPARγ and myogenic factors such as MyoD. The simultaneous expression of the proteins in mesenchymal cell generates myotubes or adipocytes, but not hybrid cells. In adipocytes, the ubiquitin–proteasome system induces MyoD degradation. In myotubes, PPARγ histone acetylation is inhibited in several loci including that of C/EBPα, the essential pro-adipogenesis PPARγ partner [155].
WNT
The wingless-type mouse mammary tumor virus integration site family (WNT) was first identified for a role in carcinogenesis. The proteins also have important function in myogenesis, where the integration of multiple WNT signals allows the self-renewal and the differentiation of muscle precursors. WNT signals deregulation leading to the disruption of muscle homeostasis and to fibrosis [156, 157]. The WNT-activated canonical-β catenin pathway negatively controls adipogenesis and favors myogenic differentiation [158]. Interestingly, WNT signaling is down-regulated in aging [159]. WNT 10 has an important role in the control of intramuscular adipogenesis. Its deficiency/inhibition is involved in adipose tissue deposition after injury caused by diverse triggers, including cardiotoxin, low temperatures and rotator cuff tear [159, 160].
Myokine and adipo-myokine
The skeletal muscle has been identified as an endocrine organ. It releases soluble factors called myokines, at least in part responsible for the beneficial effect of exercise in neuromuscular disorders [161]. They are synthesized by skeletal muscle tissue especially during contraction and exercise and can act within the muscles in an autocrine/paracrine manner and in distant tissues in an endocrine fashion. Most myokines are also secreted by adipose tissue and are therefore referred to as adipo-myokines [162]. The adipo-myokine family includes angiopoietin-like 4, fibroblast growth factor 21, follistatin-like 1, interleukin 6 (IL-6), interleukin 8, monocyte chemoattractant protein-1, myostatin, and vascular endothelial growth factor. IL-6, myostatin and follistatin may control differentiation of mesenchymal precursor in adipocytes within the muscle as described in this review.
Interleukin-6 (IL-6)
Mechanical load increases IL-6 production depending on exercise duration, intensity and the muscle mass. It is the best-characterized adipo-myokine: IL-6 may act locally within the muscle in an autocrine/paracrine manner or may be secreted. IL-6 is produced during inflammation. It has well-described roles in the adipose tissue and in the liver including the inhibition of insulin-signaling pathways [163, 164]. In myotubes, recombinant IL-6 enhances insulin-stimulated Akt phosphorylation and seems to have a beneficial effect on insulin-stimulated glucose disposal and fatty acid oxidation [165]. Muscle-derived IL-6 may locally inhibit the effects of other inflammatory cytokines such as tumor necrosis factor-alpha. Recently, it has received significant attention for its regulatory role in muscle wasting during cachexia [166]. Its role in muscle resident pre-adipocytes differentiation remains to be elucidated. Interestingly, FAP up-regulate expression of IL-6 after muscle damage. The cytokine is the possible regulator of FAP-induced stimulation of myogenic differentiation [78]. Production of not yet identified soluble factors (probably including IL-6) during pre-adipocytes/myoblasts interaction results in adipogenic inhibition via suppression of lipogenic genes such as lipoprotein lipase, adipsin and glycerol-3-phosphate dehydrogenase [167]. These findings support the hypothesis for a role of IL-6 in the control of metabolism during contraction with a pro-myogenic effect. Conversely, the chronic elevation of IL-6 released from adipocytes may induce muscle insulin resistance.
Myostatin/follistatin
Myostatin is a developmental protein which acts as a negative regulator in myogenesis. It is a member of the TGFβ protein family produced by both skeletal muscle cells and adipose tissue. It inhibits muscle differentiation and growth. Accordingly, myostatin knockdown accordingly promotes myogenesis [168]. Based on these observations during the last decade, experimental therapies with myostatin blockers to treat DMD have been developed [169]. Myostatin stimulation or inhibition of adipogenesis was alternatively described depending on cell types and culture conditions. Its expression in mesenchymal cells promotes its adipogenic differentiation via Smad3 with a negative cross talk with β catenin [170, 171]. Myostatin inhibits lipid accumulation in pre-adipocytes cell lines and fibroblasts [172, 173]. The inhibitory role of myostatin in muscle is counterbalanced by the endogenously produced follistatin [174]. White and brown adipocytes also produce follistatin [175, 176]. In particular in brown adipocytes, follistatin induces differentiation of pre-adipocyte cells. In sharp contrast, myostatin inhibits this process [176, 177]. Interestingly, FAP of young mice exposed to the histone deacetylase inhibitor TSA do not differentiate into adipocytes and efficiently stimulate SC differentiation producing a high level of follistatin [110].
Bone morphogenetic proteins (BMP)
Sequence similarities link TGFβ to the BMP family of proteins. In spite of this, the BMP pathway is a positive regulator of muscle mass [178]: BMP-2/4 up-regulates inhibitors of TGF-β-induced myogenesis repressors to block the TGF-β1-negative effect on myogenesis [179].
Like myostatin, BMP could both induce and repress adipogenesis. Ablation of BMP receptor 1 in mouse muscle mesenchymal precursors (myoendothelial progenitors) increases their adipogenic differentiation upon BMP4 stimulation, while the myogenic differentiation is reduced [180]. In apparent contrast, sca-1-positive cells isolated from skeletal muscle adopt an adipogenic fate after BMP7 treatment even if they seem to differentiate into brown instead of white adipocytes [104]. To gain a full appreciation of the role of TGFβ and BMP, further studies are needed. However, a crucial point has already emerged: both proteins regulate target genes via Smad. The manner by which the two subfamilies of ligands recruit different Smad proteins in various cell types is important for the skeletal muscle physiology [181]. Unraveling the mechanism may be advantageous for developing suitable inhibitors or mimetic agents to treat adipose tissue-related dysfunctions.
Insulin-like growth factor 1 (IGF1)
IGF1 is ubiquitously expressed in all tissues. Blood concentrations are high, because of its production by liver, bone and adipose tissue. The expression level of IGF-binding proteins is tissue specific. In the case of skeletal muscle, IGF-I signaling is a requisite for development [182]. Studies on precursor clonal cells have revealed that IGF1 is a crucial player in both adipocyte and myoblast differentiation. Although growth arrest is necessary for differentiation, IGF1 paradoxically stimulates both proliferation and differentiation of the cells. IGF-1 acts through Rho GTPase to switch in the adipogenesis–myogenesis fate. To alter the differentiation process it is sufficient to manipulate the activation of the Rho GTPases;.a reduction in the levels of the Rho inhibitory protein, p190-B RhoGAP, results in the reduction of adipogenesis and in increase of myogenesis. IGF-1 receptor directs the down-modulation of Rho GTPase activity by regulating the sub-cellular distribution of p190-B RhoGAP. This leads to increased IGF-1 signaling to downstream proteins previously implicated in adipogenesis [183].
Extracellular matrix
Muscle cells are surrounded by the basal lamina composed of collagen IV, laminin and heparin sulfate-containing PG. These are directly linked to sarcolemma and sustain muscle structural integrity. These enable the tissue mechano-transduction and act together with other components of muscle ECM named endomisium (around muscle cell) perimysium (around groups of muscle cells) and epimysium (around whole muscle) [184]. These elements have quite different compositions, but contain fibrils of collagen I and III in close association to collagen V, PG and glycoproteins (such as perlecan and fibronectin). ECM is mainly produced primary by fibroblasts: however other muscle cells within the muscle such as myoblasts are able to synthesize various ECM proteins upon activation [185].
Recent evidence pinpoints a role for ECM in the regulation of SC growth and differentiation. Data suggest that a tightly regulated dynamic interplay between intrinsic SC factors and extrinsic molecules of the microenvironment exists. Therefore, ECM, the adjacent vascular system, the intramuscular fibroblasts and preadipocytes are defined as SC niche [185, 186]. Isolated SC quickly change their fate and lose self-renewal capacity. Hence, the recent quest for appropriate scaffold to culture of SC [187]. Genetic and pharmacological studies in both animals and humans demonstrated that loss of almost any ECM components can lead to a myopathy often accompanied by fibrosis and to further fatty degeneration [138].
Differentiation of multipotent stem cells into various lineages is influenced by their interactions with ECM components. Muscle mesenchymal stem cells sense mechanical properties of their matrix (i.e., strain, shear stress, substrate rigidity and topography) and respond to environmental changes differentiating into mature myoblasts [188]. Activation of the muscle adipogenesis process occurs rapidly when ECM is disrupted. In healthy mice, ECM damage can be induced by nylon mesh material implanted to create space between fibers. The mice show abundant adipocytes at week 2 that invade and replace fibrous material. By week 4 granulation tissue typical of wound healing is detected [189].
MMP are a heterogeneous group of zinc-containing, calcium-dependent endopeptidases. They are part of the ECM and they have been extensively studied for their role in muscle regeneration. MMP differ in substrate specificity, cellular localization and regulation. After damage, their expression is rapidly up-regulated. Their activation favors a number of processes, namely myogenesis and angiogenesis. MMP control the migration of inflammatory and endothelial cells and fibroblast and the proliferation and differentiation of myogenic precursors. Specific inhibitors finely regulate them. MMP activation needs the cleavage by membrane-type 1 MMP or plasmin. Once activated, MMP are regulated through covalent binding by specific inhibitors. Collagenases and MMP further remodel ECM when fiber integrity is restored. [185].
Upon administration of GM6001, a broad-spectrum MMP inhibitor, in vitro muscle-derived stem cells display a reduced migration capacity as well as a reduced myogenic and adipogenic differentiation. Accordingly, in vivo treatment of injured mice with the MPP inhibitor jeopardizes skeletal muscle healing [190].
Another important ECM component is collagen. The muscles of DMD patients that show increased ECM deposition express lower levels of the PG decorin and significantly higher levels of collagens I and VI. Expression of collagen VI α5 and α6 chains has been recently identified [191]. Collagen’s role in regulating pre-adipocyte differentiation has been well investigated in vitro [192]. Pre-adipocytes synthesize various ECM proteins in vitro; they produce type II, V and VI collagens and some glycoproteins. When stimulated with adipogenic-inducing medium, these cells arrange type III and IV collagen and laminin in a non-fibrous structure, increase expression of type V and VI and reduce the expression of type II and type I collagen and of fibronectin [193–195]. Bovine pre-adipocytes derived from the muscle and 3T3-L1 cells have been treated with modulators of type V and VI collagen. As a consequence, they reduce triglyceride synthesis and their adipose differentiation is inhibited [193, 196]. In vivo, the role of collagen in the control of adipogenic differentiation in conditions of jeopardized repair is still unclear.
The role of PG in pre-adipocyte proliferation and differentiation has been studied in vitro using 3T3-L1 with dishes coated with biglycan and decorin and fibronectin. Biglycan and decorin reduce proliferation of pre-adipocytes, partly by induction of apoptosis. Co-treatment with fibronectin restores normal proliferation [197]. In vivo, co-injection of an extract of basement membrane proteins and basic fibroblast growth factor into mouse leg or masticatory muscle induced angiogenesis followed by fat pad formation [198]. Nevertheless, the exact role of muscle PG as a regulatory factor for muscular pre-adipocytes proliferation and differentiation remains to be investigated.
Inflammatory cells
Inflammatory cells including macrophages are recruited into the extra-fiber environment after injury to remove debris and sustain SC activity [43, 199]. Alterations of macrophage responses (e.g., an imbalance between the different macrophage sub-types) have profound effects on muscle regeneration and induce fibrosis as in DMD [138].
The absence or the alteration of macrophage metabolism has important consequences also for adipose tissue deposition within the muscle. The administration of an inhibitor of macrophage colony-stimulating factor and reduction of the differentiation and proliferation of macrophage/monocyte lineage induce intramuscular adipogenesis and abundant collagen deposition after cardiotoxin damage [200]. The role of macrophages is at least in part due to secreted factors; mesenchymal stem cells isolated from mouse skeletal muscle and incubated with macrophages conditioned medium show reduced tendency to differentiate into adipocytes [201]. Macrophages are also important for the storage and recycling of iron, a function that is tightly dependent on their polarization state [202] and that might play a role in the effective regeneration of the tissue (G. Corna and PRQ, unpublished results). Effective recycling of myoglobin-associated iron might be a relevant step to prevent fat deposition. It might require the effective recruitment and in situ activation of leukocytes able to properly support the reconstitution of effective vasculature in the regenerating tissue [203, 204].
Adipose tissue of obese subjects is infiltrated by inflammatory macrophages positive for F4/80, CD11b and CD11c which predominantly display a classically activation, (M1-like macrophages) [205, 206]. Skeletal muscle macrophages that classically infiltrate dystrophic muscle could contribute locally to the adipose intrafiber deposition: therapeutic interventions that control the shift between M1 and M2 macrophages exert a beneficial effect in animal models of dystrophy and might also regulate muscle adipogenesis [207, 208].
Heredia and co-workers have recently demonstrated that eosinophils are also important for muscle adipogenesis and in particular for FAP regulation. IL-4α receptor null mice are defective in muscle regeneration. Using a GPF reporter construct for IL-4 gene, they identified eosinophils as the dominant cell type producing the cytokine [110]. Accordingly, eosinophil-deficient mice showed defective regeneration after injury. A specific up-regulation of IL-4α in FAP is detected and inhibits their differentiation into adipocytes, thereby preventing muscle fatty degeneration. Finally, FAP stimulated via eosinophil-derived IL-4 show an increase ability to clear cell and tissue debris by phagocytosis [110].
Nitric oxide (NO)
NO is a key signaling molecule synthesized from l-arginine by a family of NO synthases (NOS). Neuronal NOS (called NOSμ) is the most important NOS in skeletal muscle and is located at the sarcolemma of fibers in the dystrophin complex. As a small, hydrophobic gas molecule NO readily diffuses into cells. It acts on different targets by cGMP-dependent or -independent pathways and in cross talks with other molecules [209–212]. In muscle, it controls the structure, bioenergetics, mitochondrial function and number, energy and oxygen supply, and excitation–contraction coupling [213–216]. Alteration of NO synthesis has an important role in the pathophysiology of muscle diseases and in particular of DMD [217, 218]. Restoration of NO generation, by genetic or pharmacological interventions with NO donating drugs, ameliorates dystrophic phenotype increasing regeneration and preserving muscle morphology in animals [219–223]. This intervention has high profiles of safety and tolerability with promising signs of efficacy in humans [224].
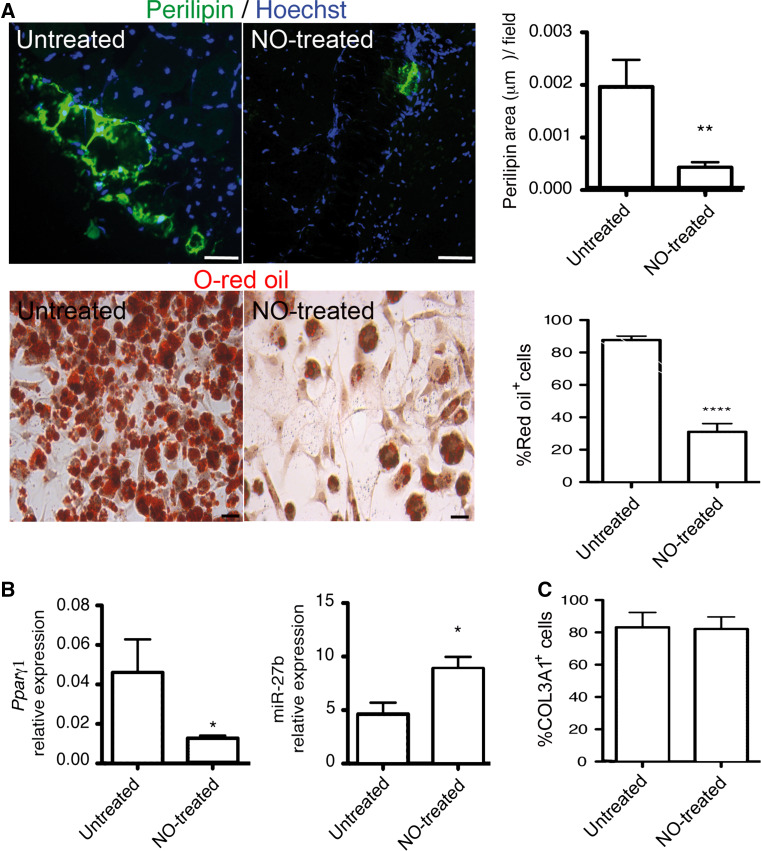
We and other groups have explored the mechanisms beyond the therapeutic potential of NO. It has been found that it has multiple actions on survival, self-renewal, activation and differentiation of SCs. Some of these effects depend on NO-induced increase of cGMP generation, while others are independent of it [222, 225–228]. We have recently demonstrated that NO influences FAP differentiation. Long-term treatment of mdx dystrophic mice with NO donor drugs inhibits adipose tissue deposition in tibialis anterior muscles in vivo and reduces the differentiation to adipocytes of both WT and mdx FAP [92] (Fig. 2a). NO inhibits the increase of PPARγ induced by adipogenic medium by controlling both its promoter activity and the expression of microRNA-27b (miR-27b), an important PPARγ post-transcriptional regulator [92] (Fig. 2b). In dystrophic muscles, the treatment with the drug, and the subsequent enhanced expression of miR-27b, reduced the expression of adipocyte markers [92]. These NO actions are cGMP independent and apparently not critically involved in the initial stages of FAP adipogenic differentiation; NO does not affect the expression of the early adipogenesis transcription factors KLF4, c-EBPβ and CHOP10. However, it regulates factors active at a later stage in adipogenesis, such as PPARγ, an adipogenic transcription factor active at later phases of the process. NO does not apparently affect the TGFβ-induced differentiation of FAP in fibroblasts (Fig. 2c). However, it regulates muscle fibrosis by controlling miR-133a, a known regulator of collagen type I expression [92]. The mechanism by which NO regulates microRNAs (miRNAs) 133a and 27b has not been clarified yet. NO might induce S-nitrosylation as demonstrated for other miRNAs (miR-1 and miR-29) and can function as a histone deacetylase (HDAC) inhibitor (see below) [229]. The role of NO in the control of adipose differentiation of other mesenchymal stem cells responsible for intrafiber adipose tissue deposition remains to be investigated.
Fig. 2.
Nitric oxide inhibits adipose tissue deposition and FAP adipocyte differentiation in dystrophic muscle. a Nitric oxide effect on adipose tissue deposition (upper panels) and on FAP differentiation in adipocytes (lower panels) for tibialis anterior muscles, cells isolated from untreated mdx mice (untreated) and from mice that received an NO donor drug (NO-treated). Adipose tissue in section was revealed using an anti-perilipin antibody (upper panels) while O-red oil was used to count adipocytes in culture (lower panels). Hoechst was employed for nuclei staining. Quantification of perilipin-positive area (upper) and adipocytes number (lower) are reported in the right graphs (mean ± S.D). **p < 0.001 and ****p < 0.0001 vs. untreated, scale bars are 20 μm. b Expression of PPARγ mRNA and mir27b (left and right, respectively) in tibialis anterior muscles obtained from untreated mdx mice (untreated) and from mice that received an NO donor drug (NO treated); mean ± S.D. *p < 0.05 vs. untreated. c Percentage of collagen-positive cells in FAPs cultured in the absence (untreated) or in the presence of an NO donor (NO treated) and stimulated with TGFβ; mean ± S.D
Another interesting issue to explore is NO capacity to induce mitochondrial biogenesis and to regulate mitochondrial function in these cells. These effects are critically dependent on its concentrations, NOS localization and exposure of the target; in isolated mitochondria, high concentrations of NO inhibit complexes of the mitochondrial respiratory chain irreversibly, whereas physiological lower levels of NO reversibly inhibit cytochrome c oxidase [230, 231]. In intact cells, physiological levels of NO stimulate the uptake and oxidation of glucose and fatty acids by skeletal muscle and adipose tissue, while they inhibit the synthesis of glucose, glycogen and fat, and enhance white adipocyte lipolysis [232]. These effects could also control brown adipocyte generation and exert beneficial effects on energy production in defective muscles. NO stimulates cell expression of both PPARα and PGC-1α [216, 233]. PGC-1α, the master regulator of UCP1 expression, is involved in the development of brown adipocytes as well as in mitochondrial biogenesis [234].
Histone deacetylases and micro-RNA
Histone deacetylases (HDAC) are enzymes that remove acetyl groups from lysine residues of histones, thus regulating gene expression. Blockade of HCAC by drugs such as valproic acid or Tricostatin A (TSA) results in chromatin expansion, facilitating transcription. Among the HDAC family, HDCA2 seems to control the expression of many skeletal muscle genes such as follistatin, the endogenous antagonist of myostatin [235]. HCDAC inhibitor treatment of mdx mice ameliorates dystrophy by enhancing regeneration and preventing fibrotic scars and fat deposition [229, 236]. HDAC are effective only when administered to young mice. This indicates that a permissive environment is essential. In isolated cells, TSA increases SC differentiation capacity similarly in young and old mice, whereas FAP response to the drug changes dramatically with the age of the donor. FAP isolated from young animals and exposed to TSA fail to differentiate into adipocytes, produce high level of follistatin and efficiently stimulate SC differentiation. On the contrary, the adipogenic potential of FAP obtained from 12 months old animals was unaffected by treatment with HDAC inhibitors and unable to stimulate SC [109]. Furthermore, HDAC inhibitor treatment of FAP isolated from dystrophic mice at the early stage of the disease de-repressed their latent myogenic program inducing myogenic transcriptional machinery. HDAC-induced up-regulation of myogenic miRNAs (mir1.2, miR133 and miR206) seems to mediate the effect [237].
Micro-RNA
Micro-RNAs (miRNAs) are small non-protein coding RNAs, some of which act as post-transcriptional gene regulators in muscle development and function [238]. Among miRNAs specifically expressed in muscles, miR-1/206 and miR-133 are the most studied. miR-1 and miR-133 have distinct roles in modulating proliferation and differentiation of cultured myoblasts; miR-1 promotes myogenesis by targeting HDAC4 (histone deacetylase 4). Unlike miR-1, miR-206 expression is restricted to skeletal muscles where it plays a crucial role in the differentiation of activated SCs, by targeting Pax7 mRNA, and in myoblast differentiation by targeting multiple genes [239]. Their expression is altered in muscle disorders including DMD [240, 241]. MiRNAs have recently emerged as crucial determinants for cellular lineage decision. By a peculiar repressing activity on the 3′ UTR (untranslated region) of target mRNAs, miRNAs have been reported to confer proper timing and robustness or differentiation program. Mir129a is highly expressed in SP interstitial muscle cells and contributes to maintain their quiescent state, blocking proliferation and differentiation in adipogenic, osteogenic and myogenic cells targeting PPARγ, Runx1 and Pax3 [242].
Fat deposition versus fibrosis
Fibrosis reflects excessive accumulation of ECM components, particularly of collagen. Stromal fibroblasts, which play a fundamental role in normal repair, are also crucial in fibrosis that is a hallmark of chronic neuromuscular disorders. When regeneration fails, fatty degeneration occurs. Fibrosis and fatty degeneration of muscle are certainly strictly linked and share common precursor cells [111]. Nevertheless, it is not entirely clear whether fibrosis and adipose degeneration in muscle damage are the two sides of the same coin or if independent/alternative pathways sustain them. Some issues should be highlighted.
The two processes occur often, but not always together during muscle degeneration. In experimental models of acute injury, the type of insult is relevant. For example after glycerol injection, adipogenesis is predominant, while after acute ischemia fibrosis occurs [108, 243].
The link between fibrosis and aberrant inflammatory reaction is well established in muscles: virtually no fibrotic tissue can be identified in the absence of inflammation. A link with the infiltration by inflammatory, M1-like, macrophages has been suggested in adipose tissue [206]. However, detailed MRI analyses of DMD muscles indicate that inflamed and edematous muscles are not always affected by adipose tissue infiltration, while other muscles display fatty deposition in the absence of a prominent inflammatory reaction [72].
Finally, fibrosis appears to correlate with the loss of muscle locomotor activity and the reduction of contractile fiber force, at least in DMD [244]. On the contrary, MRI investigations of DMD patients fail to demonstrate a correlation between intramuscular adipose tissue and muscle strength.
Signals for fibroblast or adipocyte differentiation seem to act at least in vitro. TGFβ and connective tissue factors as well as myostatin easily induce fibroblast activation and fibrotic features [138]. Although the effect of TGFβ/myostatin has not been completely clarified, mesenchymal stem cells fail to differentiate in adipocytes in the presence of those proteins [172, 245]. Similarly, PDGF receptor inhibition reduces fibrosis in mdx mice diaphragm, but promotes in vitro adipogenesis in mesenchymal stromal cells [114, 246].
Concluding remarks
Progressive loss of muscle mass and contractile function is a common feature of skeletal muscle diseases characterized by impairment of the tissue repair process. This process is consistently accompanied by adipose intrafiber deposition. SC play a central role in regeneration; alteration of their regulatory signals within the tissue explain muscle adipogenesis. The close anatomic contact between fat and muscle cells suggests a reciprocal influence. In fact, several molecules coming from the muscle or from the regenerative milieu in turn affect the deposition of adipose tissue. Mesenchymal stem/progenitor cells are reported to exist in almost all mammalian organs. The ability to differentiate toward adipogenic, osteogenic and chondrogenic lineages is a hallmark of these cells. Strong evidence indicates that differentiating into a certain lineage is not an intrinsic property of mesenchymal cells in physiological condition. Their differentiation fate changes during disease progression and might be detrimental. Dissecting the mechanism of mesenchymal cells interactions and differentiation ought to be an important object of future studies. A better understanding of the molecular pathways that regulate gain or loss of muscle mass and muscle-to-fat conversion is crucial for treating muscle wasting-associated disorders along with their physical and metabolic complications. Fibrosis and fatty degeneration are a consequence of regeneration failure. Fibrosis is induced in disease characterized by chronic inflammation. Fibrosis and adipogenesis are often simultaneous and share the some precursor cells. However, fibrosis and inflammation can occur in the absence of adipogenesis and in vitro the differentiation of precursors in adipocytes or fibrogenic cells seems to be regulated by different molecular pathways. These data suggest that fibrosis and fatty degeneration could be the outcome of independent programs recruited during skeletal muscle degeneration.
Acknowledgments
This work was supported by the Ministero della Salute “Ricerca corrente 2014” Grant to E.C.; “Ministero dell’Istruzione, Università e Ricerca”, PRIN2010-2011 Grant to E.C and PRIN 2010 Grant to A.A.M; “Fondo per gli Investimenti della Ricerca di Base-IDEAS” to P.R.-Q. and “Ricerca Finalizzata” to P.R.-Q. and A.A.M.); Associazione Italiana Ricerca sul Cancro (AIRC IG11761 to A.A.M.) and by the Italian Ministry of University and Research. We are grateful to Lara Campana and Ross Dobie (Queen’s Medical Research Institute, Edinburgh GB) for text revision.
Abbreviations
- SC
Satellite cells
- MRF
Myogenic regulatory factors
- MHC
Myosin heavy chain
- MEF
Myogenic enhancer factors
- EMC
Extracellular matrix components
- MMP
Matrix metalloproteinases
- PPARγ2
Peroxisome proliferator-activated receptor γ2
- UCP1
Uncoupling protein 1
- DMD
Duchenne muscular dystrophy
- MRI
Magnetic resonance imaging
- sca-1
Stem cell antigen 1
- PDGFR
Platelet-derived growth factor receptor
- FAP
Fibro-adipogenic precursors
- TGFβ1
Transforming growth factor beta
- SP
Side population
- PIC
PW1-expressing cells
- NG2
Neural/glial antigen 2
- PG
Proteoglycans
- miRNAs
MicroRNAs
- HDAC
Histone deacetylases
- WNT
Wingless-type mouse mammary tumor virus integration site family
- BMP
Bone morphogenetic proteins
- IGF1
Insulin-like growth factor 1
- IL
Interleukin
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- WT
Wild type
- TSA
Tricostatin A
- αSMA
α-smooth muscle actin
References
- 1.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 2.Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14:82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- 4.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 6.Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, Beauchamp JR. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- 7.Halevy O, Piestun Y, Allouh MZ, Rosser BW, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- 8.Cusella-De Angelis MG, Lyons G, Sonnino C, De Angelis L, Vivarelli E, Farmer K, Wright WE, Molinaro M, Bouche M, Buckingham M, et al. MyoD, myogenin independent differentiation of primordial myoblasts in mouse somites. J Cell Biol. 1992;116:1243–1255. doi: 10.1083/jcb.116.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins CA, Partridge TA. Self-renewal of the adult skeletal muscle satellite cell. Cell Cycle. 2005;4:1338–1341. doi: 10.4161/cc.4.10.2114. [DOI] [PubMed] [Google Scholar]
- 10.Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, Montarras D, Rocancourt D, Relaix F. The formation of skeletal muscle: from somite to limb. J Anat. 2003;202:59–68. doi: 10.1046/j.1469-7580.2003.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 15.Rudnicki MA, Braun T, Hinuma S, Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992;71:383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- 16.Braun T, Rudnicki MA, Arnold HH, Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992;71:369–382. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- 17.Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Behringer RR, Olson EN. Inactivation of the myogenic bHLH gene MRF4 results in up-regulation of myogenin and rib anomalies. Genes Dev. 1995;9:1388–1399. doi: 10.1101/gad.9.11.1388. [DOI] [PubMed] [Google Scholar]
- 19.Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- 20.Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- 21.Lassar AB, Davis RL, Wright WE, Kadesch T, Murre C, Voronova A, Baltimore D, Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991;66:305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- 22.Murre C, McCaw PS, Vaessin H, Caudy M, Jan LY, Jan YN, Cabrera CV, Buskin JN, Hauschka SD, Lassar AB, et al. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 23.Jen Y, Weintraub H, Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev. 1992;6:1466–1479. doi: 10.1101/gad.6.8.1466. [DOI] [PubMed] [Google Scholar]
- 24.Rao SS, Chu C, Kohtz DS. Ectopic expression of cyclin D1 prevents activation of gene transcription by myogenic basic helix-loop-helix regulators. Mol Cell Biol. 1994;14:5259–5267. doi: 10.1128/mcb.14.8.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 26.Lu J, Webb R, Richardson JA, Olson EN. MyoR: a muscle-restricted basic helix-loop-helix transcription factor that antagonizes the actions of MyoD. Proc Natl Acad Sci U S A. 1999;96:552–557. doi: 10.1073/pnas.96.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemercier C, To RQ, Carrasco RA, Konieczny SF. The basic helix-loop-helix transcription factor Mist1 functions as a transcriptional repressor of myoD. EMBO J. 1998;17:1412–1422. doi: 10.1093/emboj/17.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Postigo AA, Dean DC. ZEB, a vertebrate homolog of Drosophila Zfh-1, is a negative regulator of muscle differentiation. EMBO J. 1997;16:3935–3943. doi: 10.1093/emboj/16.13.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CM, Kraut N, Groudine M, Weintraub H. I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell. 1996;86:731–741. doi: 10.1016/s0092-8674(00)80148-8. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Heller-Harrison R, Czech M, Olson EN. Cyclic AMP-dependent protein kinase inhibits the activity of myogenic helix-loop-helix proteins. Mol Cell Biol. 1992;12:4478–4485. doi: 10.1128/mcb.12.10.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Zhou J, James G, Heller-Harrison R, Czech MP, Olson EN. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domains. Cell. 1992;71:1181–1194. doi: 10.1016/s0092-8674(05)80066-2. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto N, Ogashiwa M, Okumura E, Endo T, Iwashita S, Kishimoto T. Phosphorylation of a proline-directed kinase motif is responsible for structural changes in myogenin. FEBS Lett. 1994;352:236–242. doi: 10.1016/0014-5793(94)00964-3. [DOI] [PubMed] [Google Scholar]
- 33.Song A, Wang Q, Goebl MG, Harrington MA. Phosphorylation of nuclear MyoD is required for its rapid degradation. Mol Cell Biol. 1998;18:4994–4999. doi: 10.1128/mcb.18.9.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 35.Puri PL, Sartorelli V, Yang XJ, Hamamori Y, Ogryzko VV, Howard BH, Kedes L, Wang JY, Graessmann A, Nakatani Y, Levrero M. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell. 1997;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 36.Edmondson DG, Lyons GE, Martin JF, Olson EN. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120:1251–1263. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- 37.Kaushal S, Schneider JW, Nadal-Ginard B, Mahdavi V. Activation of the myogenic lineage by MEF2A, a factor that induces and cooperates with MyoD. Science. 1994;266:1236–1240. doi: 10.1126/science.7973707. [DOI] [PubMed] [Google Scholar]
- 38.Martin JF, Miano JM, Hustad CM, Copeland NG, Jenkins NA, Olson EN. A Mef2 gene that generates a muscle-specific isoform via alternative mRNA splicing. Mol Cell Biol. 1994;14:1647–1656. doi: 10.1128/mcb.14.3.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naidu PS, Ludolph DC, To RQ, Hinterberger TJ, Konieczny SF. Myogenin and MEF2 function synergistically to activate the MRF4 promoter during myogenesis. Mol Cell Biol. 1995;15:2707–2718. doi: 10.1128/mcb.15.5.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cserjesi P, Olson EN. Myogenin induces the myocyte-specific enhancer binding factor MEF-2 independently of other muscle-specific gene products. Mol Cell Biol. 1991;11:4854–4862. doi: 10.1128/mcb.11.10.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 42.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brunelli S, Rovere-Querini P. The immune system and the repair of skeletal muscle. Pharmacol Res. 2008;58:117–121. doi: 10.1016/j.phrs.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31:384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 45.Rhoads RP, Johnson RM, Rathbone CR, Liu X, Temm-Grove C, Sheehan SM, Hoying JB, Allen RE. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol Cell Physiol. 2009;296:C1321–C1328. doi: 10.1152/ajpcell.00391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abou-Khalil R, Mounier R, Chazaud B. Regulation of myogenic stem cell behavior by vessel cells: the “menage a trois” of satellite cells, periendothelial cells and endothelial cells. Cell Cycle. 2010;9:892–896. doi: 10.4161/cc.9.5.10851. [DOI] [PubMed] [Google Scholar]
- 47.DiMario J, Buffinger N, Yamada S, Strohman RC. Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science. 1989;244:688–690. doi: 10.1126/science.2717945. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adh Migr. 2009;3:337–341. doi: 10.4161/cam.3.4.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ceafalan LC, Popescu BO, Hinescu ME. Cellular players in skeletal muscle regeneration. Biomed Res Int. 2014;2014:957014. doi: 10.1155/2014/957014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rinaldi F, Perlingeiro RC. Stem cells for skeletal muscle regeneration: therapeutic potential and roadblocks. Transl Res. 2013;163:409–417. doi: 10.1016/j.trsl.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asakura A. Stem cells in adult skeletal muscle. Trends Cardiovasc Med. 2003;13:123–128. doi: 10.1016/s1050-1738(03)00024-0. [DOI] [PubMed] [Google Scholar]
- 52.Lewis SF, Haller RG. Skeletal muscle disorders and associated factors that limit exercise performance. Exerc Sport Sci Rev. 1989;17:67–113. [PubMed] [Google Scholar]
- 53.Barany M, Venkatasubramanian PN, Mok E, Siegel IM, Abraham E, Wycliffe ND, Mafee MF. Quantitative and qualitative fat analysis in human leg muscle of neuromuscular diseases by 1H MR spectroscopy in vivo. Magn Reson Med. 1989;10:210–226. doi: 10.1002/mrm.1910100206. [DOI] [PubMed] [Google Scholar]
- 54.Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, Federspil G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297:E987–E998. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 55.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, Schrier D, Falb D, Kirkland JL, Wagers AJ, Tseng YH. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2010;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Virtanen KA, Nuutila P. Brown adipose tissue in humans. Curr Opin Lipidol. 2010;22:49–54. doi: 10.1097/MOL.0b013e3283425243. [DOI] [PubMed] [Google Scholar]
- 57.Triplett WT, Baligand C, Forbes SC, Willcocks RJ, Lott DJ, DeVos S, Pollaro J, Rooney WD, Sweeney HL, Bonnemann CG, Wang DJ, Vandenborne K, Walter GA. Chemical shift-based MRI to measure fat fractions in dystrophic skeletal muscle. Magn Reson Med. 2013;72:8–19. doi: 10.1002/mrm.24917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan WP, Liu GC. MR imaging of primary skeletal muscle diseases in children. AJR. 2002;179:989–997. doi: 10.2214/ajr.179.4.1790989. [DOI] [PubMed] [Google Scholar]
- 59.Reimers CD, Finkenstaedt M. Muscle imaging in inflammatory myopathies. Curr Opin Rheumatol. 1997;9:475–485. doi: 10.1097/00002281-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85:377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 62.Gargiulo P, Kern H, Carraro U, Ingvarsson P, Knutsdottir S, Gudmundsdottir V, Yngvason S, Vatnsdal B, Helgason T. Quantitative color three-dimensional computer tomography imaging of human long-term denervated muscle. Neurol Res. 2010;32:13–19. doi: 10.1179/016164109X12536042424171. [DOI] [PubMed] [Google Scholar]
- 63.Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45:304–309. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- 64.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 65.Kohrt WM, Holloszy JO. Loss of skeletal muscle mass with aging: effect on glucose tolerance. J Gerontol A Biol Sci Med Sci. 1995;50:68–72. doi: 10.1093/gerona/50a.special_issue.68. [DOI] [PubMed] [Google Scholar]
- 66.Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, Granzotto M, Vettor R, Camastra S, Ferrannini E. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002;51:144–151. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]
- 68.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88:1336–1344. doi: 10.2522/ptj.20080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaeta M, Messina S, Mileto A, Vita GL, Ascenti G, Vinci S, Bottari A, Vita G, Settineri N, Bruschetta D, Racchiusa S, Minutoli F. Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments. Preliminary experience. Skeletal Radiol. 2010;41:955–961. doi: 10.1007/s00256-011-1301-5. [DOI] [PubMed] [Google Scholar]
- 70.Yao L, Gai N. Fat-corrected T2 measurement as a marker of active muscle disease in inflammatory myopathy. AJR. 2012;198:W475–W481. doi: 10.2214/AJR.11.7113. [DOI] [PubMed] [Google Scholar]
- 71.Kim HK, Merrow AC, Shiraj S, Wong BL, Horn PS, Laor T. Analysis of fatty infiltration and inflammation of the pelvic and thigh muscles in boys with Duchenne muscular dystrophy (DMD): grading of disease involvement on MR imaging and correlation with clinical assessments. Pediatr Radiol. 2013;43:1327–1335. doi: 10.1007/s00247-013-2696-z. [DOI] [PubMed] [Google Scholar]
- 72.Marden FA, Connolly AM, Siegel MJ, Rubin DA. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol. 2005;34:140–148. doi: 10.1007/s00256-004-0825-3. [DOI] [PubMed] [Google Scholar]
- 73.Wren TA, Bluml S, Tseng-Ong L, Gilsanz V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR. 2008;190:W8–12. doi: 10.2214/AJR.07.2732. [DOI] [PubMed] [Google Scholar]
- 74.Pisani DF, Bottema CD, Butori C, Dani C, Dechesne CA. Mouse model of skeletal muscle adiposity: a glycerol treatment approach. Biochem Biophys Res Commun. 2010;396:767–773. doi: 10.1016/j.bbrc.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 75.Fukada S, Morikawa D, Yamamoto Y, Yoshida T, Sumie N, Yamaguchi M, Ito T, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Genetic background affects properties of satellite cells and mdx phenotypes. Am J Pathol. 2010;176:2414–2424. doi: 10.2353/ajpath.2010.090887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lukjanenko L, Brachat S, Pierrel E, Lach-Trifilieff E, Feige JN. Genomic profiling reveals that transient adipogenic activation is a hallmark of mouse models of skeletal muscle regeneration. PLoS One. 2013;8:e71084. doi: 10.1371/journal.pone.0071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wagatsuma A. Adipogenic potential can be activated during muscle regeneration. Mol Cell Biochem. 2007;304:25–33. doi: 10.1007/s11010-007-9482-x. [DOI] [PubMed] [Google Scholar]
- 78.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uezumi A, Ikemoto-Uezumi M, Tsuchida K. Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Front Physiol. 2014;5:68. doi: 10.3389/fphys.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu W, Liu Y, Lai X, Kuang S. Intramuscular adipose is derived from a non-Pax3 lineage and required for efficient regeneration of skeletal muscles. Dev Biol. 2011;361:27–38. doi: 10.1016/j.ydbio.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun L, Nicholson AC, Hajjar DP, Gotto AM, Jr, Han J. Adipogenic differentiating agents regulate expression of fatty acid binding protein and CD36 in the J744 macrophage cell line. J Lipid Res. 2003;44:1877–1886. doi: 10.1194/jlr.M300084-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 83.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci U S A. 1995;92:9856–9860. doi: 10.1073/pnas.92.21.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 87.Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci. 2004;117:5393–5404. doi: 10.1242/jcs.01419. [DOI] [PubMed] [Google Scholar]
- 88.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Csete M, Walikonis J, Slawny N, Wei Y, Korsnes S, Doyle JC, Wold B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J Cell Physiol. 2001;189:189–196. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- 90.Scarda A, Franzin C, Milan G, Sanna M, Dal Pra C, Pagano C, Boldrin L, Piccoli M, Trevellin E, Granzotto M, Gamba P, Federspil G, De Coppi P, Vettor R. Increased adipogenic conversion of muscle satellite cells in obese Zucker rats. Int J Obes (Lond) 2010;34:1319–1327. doi: 10.1038/ijo.2010.47. [DOI] [PubMed] [Google Scholar]
- 91.Taylor-Jones JM, McGehee RE, Rando TA, Lecka-Czernik B, Lipschitz DA, Peterson CA. Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev. 2002;123:649–661. doi: 10.1016/s0047-6374(01)00411-0. [DOI] [PubMed] [Google Scholar]
- 92.Cordani N, Pisa V, Pozzi L, Sciorati C, Clementi E. Nitric oxide controls fat deposition in dystrophic skeletal muscle by regulating fibro-adipogenic precursor differentiation. Stem Cells. 2014;32:874–885. doi: 10.1002/stem.1587. [DOI] [PubMed] [Google Scholar]
- 93.Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, Perlingeiro RC, Kyba M. Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells. 2008;26:3194–3204. doi: 10.1634/stemcells.2007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fukada S, Higuchi S, Segawa M, Koda K, Yamamoto Y, Tsujikawa K, Kohama Y, Uezumi A, Imamura M, Miyagoe-Suzuki Y, Takeda S, Yamamoto H. Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp Cell Res. 2004;296:245–255. doi: 10.1016/j.yexcr.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 95.Fukada S, Ma Y, Ohtani T, Watanabe Y, Murakami S, Yamaguchi M. Isolation, characterization, and molecular regulation of muscle stem cells. Front Physiol. 2013;4:317. doi: 10.3389/fphys.2013.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blanco-Bose WE, Yao CC, Kramer RH, Blau HM. Purification of mouse primary myoblasts based on alpha 7 integrin expression. Exp Cell Res. 2001;265:212–220. doi: 10.1006/excr.2001.5191. [DOI] [PubMed] [Google Scholar]
- 97.Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 98.Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pisani DF, Dechesne CA, Sacconi S, Delplace S, Belmonte N, Cochet O, Clement N, Wdziekonski B, Villageois AP, Butori C, Bagnis C, Di Santo JP, Kurzenne JY, Desnuelle C, Dani C. Isolation of a highly myogenic CD34-negative subset of human skeletal muscle cells free of adipogenic potential. Stem Cells. 2010;28:753–764. doi: 10.1002/stem.317. [DOI] [PubMed] [Google Scholar]
- 100.Pisani DF, Clement N, Loubat A, Plaisant M, Sacconi S, Kurzenne JY, Desnuelle C, Dani C, Dechesne CA. Hierarchization of myogenic and adipogenic progenitors within human skeletal muscle. Stem Cells. 2010;28:2182–2194. doi: 10.1002/stem.537. [DOI] [PubMed] [Google Scholar]
- 101.Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen JC, Mortimer J, Marley J, Goldhamer DJ. MyoD-cre transgenic mice: a model for conditional mutagenesis and lineage tracing of skeletal muscle. Genesis. 2005;41:116–121. doi: 10.1002/gene.20104. [DOI] [PubMed] [Google Scholar]
- 103.Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J Histochem Cytochem. 2011;59:33–46. doi: 10.1369/jhc.2010.956995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, Schrier D, Falb D, Kirkland JL, Wagers AJ, Tseng YH. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2010;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S, Seale P, Fernando P, van Ijcken W, Grosveld F, Dekemp RA, Boushel R, Harper ME, Rudnicki MA. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013;17:210–224. doi: 10.1016/j.cmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shan T, Liang X, Bi P, Zhang P, Liu W, Kuang S. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J Lipid Res. 2013;54:2214–2224. doi: 10.1194/jlr.M038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mizuno H. The potential for treatment of skeletal muscle disorders with adipose-derived stem cells. Curr Stem Cell Res Ther. 2009;5:133–136. doi: 10.2174/157488810791268573. [DOI] [PubMed] [Google Scholar]
- 108.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 109.Mozzetta C, Consalvi S, Saccone V, Tierney M, Diamantini A, Mitchell KJ, Marazzi G, Borsellino G, Battistini L, Sassoon D, Sacco A, Puri PL. Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice. EMBO Mol Med. 2013;5:626–639. doi: 10.1002/emmm.201202096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 112.Saccone V, Consalvi S, Giordani L, Mozzetta C, Barozzi I, Sandona M, Ryan T, Rojas-Munoz A, Madaro L, Fasanaro P, Borsellino G, De Bardi M, Frige G, Termanini A, Sun X, Rossant J, Bruneau BG, Mercola M, Minucci S, Puri PL. HDAC-regulated myomiRs control BAF60 variant exchange and direct the functional phenotype of fibro-adipogenic progenitors in dystrophic muscles. Genes Dev. 2014;28:841–857. doi: 10.1101/gad.234468.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Uezumi A, Fukada S, Yamamoto N, Ikemoto-Uezumi M, Nakatani M, Morita M, Yamaguchi A, Yamada H, Nishino I, Hamada Y, Tsuchida K. Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5:e1186. doi: 10.1038/cddis.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ito T, Ogawa R, Uezumi A, Ohtani T, Watanabe Y, Tsujikawa K, Miyagoe-Suzuki Y, Takeda S, Yamamoto H, Fukada S. Imatinib attenuates severe mouse dystrophy and inhibits proliferation and fibrosis-marker expression in muscle mesenchymal progenitors. Neuromuscul Disord. 2013;23:349–356. doi: 10.1016/j.nmd.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 115.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 116.Muskiewicz KR, Frank NY, Flint AF, Gussoni E. Myogenic potential of muscle side and main population cells after intravenous injection into sub-lethally irradiated mdx mice. J Histochem Cytochem. 2005;53:861–873. doi: 10.1369/jhc.4A6573.2005. [DOI] [PubMed] [Google Scholar]
- 117.Pavlath GK, Gussoni E. Human myoblasts and muscle-derived SP cells. Methods Mol Med. 2005;107:97–110. doi: 10.1385/1-59259-861-7:097. [DOI] [PubMed] [Google Scholar]
- 118.Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang P, Schulz TJ, Beauvais A, Tseng YH, Gussoni E. Intramuscular adipogenesis is inhibited by myo-endothelial progenitors with functioning Bmpr1a signalling. Nat Commun. 2014;5:4063. doi: 10.1038/ncomms5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, Gharaibeh B, Deasy BM, Huard J, Peault B. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 122.Crisan M, Deasy B, Gavina M, Zheng B, Huard J, Lazzari L, Peault B. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods Cell Biol. 2008;86:295–309. doi: 10.1016/S0091-679X(08)00013-7. [DOI] [PubMed] [Google Scholar]
- 123.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 124.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 125.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res. 2012;10:67–84. doi: 10.1016/j.scr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013;22:2298–2314. doi: 10.1089/scd.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tonlorenzi R, Dellavalle A, Schnapp E, Cossu G, Sampaolesi M (2007) Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr Protoc Stem Cell Biol Chapter 2: Unit 2B 1 [DOI] [PubMed]
- 128.Cossu G, Bianco P. Mesoangioblasts–vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 129.Roobrouck VD, Clavel C, Jacobs SA, Ulloa-Montoya F, Crippa S, Sohni A, Roberts SJ, Luyten FP, Van Gool SW, Sampaolesi M, Delforge M, Luttun A, Verfaillie CM. Differentiation potential of human postnatal mesenchymal stem cells, mesoangioblasts, and multipotent adult progenitor cells reflected in their transcriptome and partially influenced by the culture conditions. Stem Cells. 2011;29:871–882. doi: 10.1002/stem.633. [DOI] [PubMed] [Google Scholar]
- 130.Messina G, Sirabella D, Monteverde S, Galvez BG, Tonlorenzi R, Schnapp E, De Angelis L, Brunelli S, Relaix F, Buckingham M, Cossu G. Skeletal muscle differentiation of embryonic mesoangioblasts requires pax3 activity. Stem Cells. 2009;27:157–164. doi: 10.1634/stemcells.2008-0503. [DOI] [PubMed] [Google Scholar]
- 131.Sciorati C, Galvez BG, Brunelli S, Tagliafico E, Ferrari S, Cossu G, Clementi E. Ex vivo treatment with nitric oxide increases mesoangioblast therapeutic efficacy in muscular dystrophy. J Cell Sci. 2006;119:5114–5123. doi: 10.1242/jcs.03300. [DOI] [PubMed] [Google Scholar]
- 132.Bosurgi L, Corna G, Vezzoli M, Touvier T, Cossu G, Manfredi AA, Brunelli S, Rovere-Querini P. Transplanted mesoangioblasts require macrophage IL-10 for survival in a mouse model of muscle injury. J Immunol. 2012;188:6267–6277. doi: 10.4049/jimmunol.1102680. [DOI] [PubMed] [Google Scholar]
- 133.Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 134.Pannerec A, Formicola L, Besson V, Marazzi G, Sassoon DA. Defining skeletal muscle resident progenitors and their cell fate potentials. Development. 2013;140:2879–2891. doi: 10.1242/dev.089326. [DOI] [PubMed] [Google Scholar]
- 135.Sanderson RD, Fitch JM, Linsenmayer TR, Mayne R. Fibroblasts promote the formation of a continuous basal lamina during myogenesis in vitro. J Cell Biol. 1986;102:740–747. doi: 10.1083/jcb.102.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Archile-Contreras AC, Mandell IB, Purslow PP. Phenotypic differences in matrix metalloproteinase 2 activity between fibroblasts from 3 bovine muscles. J Anim Sci. 2010;88:4006–4015. doi: 10.2527/jas.2010-3060. [DOI] [PubMed] [Google Scholar]
- 137.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 138.Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293:C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 141.Serrano AL, Munoz-Canoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res. 2010;316:3050–3058. doi: 10.1016/j.yexcr.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 142.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 143.O’Shea Alvarez MS. 3T3 cells in adipocytic conversion. Arch Invest Med (Mex) 1991;22:235–244. [PubMed] [Google Scholar]
- 144.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 145.Goodpaster T, Legesse-Miller A, Hameed MR, Aisner SC, Randolph-Habecker J, Coller HA. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem. 2008;56:347–358. doi: 10.1369/jhc.7A7287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Agley CC, Rowlerson AM, Velloso CP, Lazarus NR, Harridge SD. Human skeletal muscle fibroblasts, but not myogenic cells, readily undergo adipogenic differentiation. J Cell Sci. 2013;126:5610–5625. doi: 10.1242/jcs.132563. [DOI] [PubMed] [Google Scholar]
- 147.Roberts EW, Deonarine A, Jones JO, Denton AE, Feig C, Lyons SK, Espeli M, Kraman M, McKenna B, Wells RJ, Zhao Q, Caballero OL, Larder R, Coll AP, O’Rahilly S, Brindle KM, Teichmann SA, Tuveson DA, Fearon DT. Depletion of stromal cells expressing fibroblast activation protein-alpha from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med. 2013;210:1137–1151. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Olson LE, Soriano P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16:303–313. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2011;53:227–246. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Duguez S, Feasson L, Denis C, Freyssenet D. Mitochondrial biogenesis during skeletal muscle regeneration. Am J Physiol Endocrinol Metab. 2002;282:E802–E809. doi: 10.1152/ajpendo.00343.2001. [DOI] [PubMed] [Google Scholar]
- 151.Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, Abatangelo G, Pozzan T, Pinton P, Rizzuto R. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A. 2008;105:1226–1231. doi: 10.1073/pnas.0711402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yue T, Yin J, Li F, Li D, Du M. High glucose induces differentiation and adipogenesis in porcine muscle satellite cells via mTOR. BMB Rep. 2010;43:140–145. doi: 10.5483/bmbrep.2010.43.2.140. [DOI] [PubMed] [Google Scholar]
- 153.Powell DJ, McFarland DC, Cowieson AJ, Muir WI, Velleman SG. The effect of nutritional status and muscle fiber type on myogenic satellite cell fate and apoptosis. Poult Sci. 2014;93:163–173. doi: 10.3382/ps.2013-03450. [DOI] [PubMed] [Google Scholar]
- 154.Radley HG, De Luca A, Lynch GS, Grounds MD. Duchenne muscular dystrophy: focus on pharmaceutical and nutritional interventions. Int J Biochem Cell Biol. 2007;39:469–477. doi: 10.1016/j.biocel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 155.Sunadome K, Suzuki T, Usui M, Ashida Y, Nishida E. Antagonism between the master regulators of differentiation ensures the discreteness and robustness of cell fates. Mol Cell. 2014;54:526–535. doi: 10.1016/j.molcel.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 156.Cisternas P, Vio CP, Inestrosa NC. Role of Wnt signaling in tissue fibrosis, lessons from skeletal muscle and kidney. Curr Mol Med. 2014;14:510–522. doi: 10.2174/1566524014666140414210346. [DOI] [PubMed] [Google Scholar]
- 157.von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22:602–609. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 159.Vertino AM, Taylor-Jones JM, Longo KA, Bearden ED, Lane TF, McGehee RE, Jr, MacDougald OA, Peterson CA. Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol Biol Cell. 2005;16:2039–2048. doi: 10.1091/mbc.E04-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Itoigawa Y, Kishimoto KN, Sano H, Kaneko K, Itoi E. Molecular mechanism of fatty degeneration in rotator cuff muscle with tendon rupture. J Orthop Res. 2011;29:861–866. doi: 10.1002/jor.21317. [DOI] [PubMed] [Google Scholar]
- 161.Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011;25:811–816. doi: 10.1016/j.bbi.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 162.Raschke S, Eckardt K, Bjorklund Holven K, Jensen J, Eckel J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. Plos One. 2013;8:e62008. doi: 10.1371/journal.pone.0062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 164.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52:2784–2789. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- 165.Raschke S, Eckel J. Adipo-myokines: two sides of the same coin–mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013;2013:320724. doi: 10.1155/2013/320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536:329–337. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Park S, Baek K, Choi C. Suppression of adipogenic differentiation by muscle cell-induced decrease in genes related to lipogenesis in muscle and fat co-culture system. Cell Biol Int. 2013;37:1003–1009. doi: 10.1002/cbin.10150. [DOI] [PubMed] [Google Scholar]
- 168.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 169.Ruegg UT. Pharmacological prospects in the treatment of Duchenne muscular dystrophy. Curr Opin Neurol. 2013;26:577–584. doi: 10.1097/WCO.0b013e328364fbaf. [DOI] [PubMed] [Google Scholar]
- 170.Artaza JN, Bhasin S, Magee TR, Reisz-Porszasz S, Shen R, Groome NP, Meerasahib MF, Gonzalez-Cadavid NF. Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology. 2005;146:3547–3557. doi: 10.1210/en.2005-0362. [DOI] [PubMed] [Google Scholar]
- 171.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–7242. doi: 10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li F, Yang H, Duan Y, Yin Y. Myostatin regulates preadipocyte differentiation and lipid metabolism of adipocyte via ERK1/2. Cell Biol Int. 2011;35:1141–1146. doi: 10.1042/CBI20110112. [DOI] [PubMed] [Google Scholar]
- 174.Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab. 2009;297:E157–E164. doi: 10.1152/ajpendo.00193.2009. [DOI] [PubMed] [Google Scholar]
- 175.Flanagan JN, Linder K, Mejhert N, Dungner E, Wahlen K, Decaunes P, Ryden M, Bjorklund P, Arver S, Bhasin S, Bouloumie A, Arner P, Dahlman I. Role of follistatin in promoting adipogenesis in women. J Clin Endocrinol Metab. 2009;94:3003–3009. doi: 10.1210/jc.2008-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Braga M, Reddy ST, Vergnes L, Pervin S, Grijalva V, Stout D, David J, Li X, Tomasian V, Reid CB, Norris KC, Devaskar SU, Reue K, Singh R. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J Lipid Res. 2014;55:375–384. doi: 10.1194/jlr.M039719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Braga M, Pervin S, Norris K, Bhasin S, Singh R. Inhibition of in vitro and in vivo brown fat differentiation program by myostatin. Obesity (Silver Spring) 2013;21:1180–1188. doi: 10.1002/oby.20117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sartori R, Schirwis E, Blaauw B, Bortolanza S, Zhao J, Enzo E, Stantzou A, Mouisel E, Toniolo L, Ferry A, Stricker S, Goldberg AL, Dupont S, Piccolo S, Amthor H, Sandri M. BMP signaling controls muscle mass. Nat Genet. 2013;45:1309–1318. doi: 10.1038/ng.2772. [DOI] [PubMed] [Google Scholar]
- 179.Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- 180.Huang P, Schulz TJ, Beauvais A, Tseng YH, Gussoni E. Intramuscular adipogenesis is inhibited by myo-endothelial progenitors with functioning Bmpr1a signalling. Nat Commun. 2014;5:4063. doi: 10.1038/ncomms5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sartori R, Gregorevic P, Sandri M. TGFbeta and BMP signaling in skeletal muscle: potential significance for muscle-related disease. Trends Endocrinol Metab. 2014;25:464–471. doi: 10.1016/j.tem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 182.Kawai M, Rosen CJ. The IGF-I regulatory system and its impact on skeletal and energy homeostasis. J Cell Biochem. 2010;111:14–19. doi: 10.1002/jcb.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sordella R, Jiang W, Chen GC, Curto M, Settleman J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/s0092-8674(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 184.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 185.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44:318–331. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kaariainen M, Jarvinen T, Jarvinen M, Rantanen J, Kalimo H. Relation between myofibers and connective tissue during muscle injury repair. Scand J Med Sci Sports. 2000;10:332–337. doi: 10.1034/j.1600-0838.2000.010006332.x. [DOI] [PubMed] [Google Scholar]
- 187.Boldrin L, Elvassore N, Malerba A, Flaibani M, Cimetta E, Piccoli M, Baroni MD, Gazzola MV, Messina C, Gamba P, Vitiello L, De Coppi P. Satellite cells delivered by micro-patterned scaffolds: a new strategy for cell transplantation in muscle diseases. Tissue Eng. 2007;13:253–262. doi: 10.1089/ten.2006.0093. [DOI] [PubMed] [Google Scholar]
- 188.Kshitiz, Park J, Kim P, Helen W, Engler AJ, Levchenko A, Kim DH. Control of stem cell fate and function by engineering physical microenvironments. Integr Biol (Camb) 2012;4:1008–1018. doi: 10.1039/c2ib20080e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xaymardan M, Gibbins JR, Zoellne H. Adipogenic healing in adult mice by implantation of hollow devices in muscle. Anat Rec. 2002;267:28–36. doi: 10.1002/ar.10072. [DOI] [PubMed] [Google Scholar]
- 190.Bellayr I, Holden K, Mu X, Pan H, Li Y. Matrix metalloproteinase inhibition negatively affects muscle stem cell behavior. Int J Clin Exp Pathol. 2013;6:124–141. [PMC free article] [PubMed] [Google Scholar]
- 191.Sabatelli P, Gualandi F, Gara SK, Grumati P, Zamparelli A, Martoni E, Pellegrini C, Merlini L, Ferlini A, Bonaldo P, Maraldi NM, Paulsson M, Squarzoni S, Wagener R. Expression of collagen VI alpha5 and alpha6 chains in human muscle and in Duchenne muscular dystrophy-related muscle fibrosis. Matrix Biol. 2012;31:187–196. doi: 10.1016/j.matbio.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sul HS, Smas CM, Wang D, Chen L. Regulation of fat synthesis and adipose differentiation. Prog Nucleic Acid Res Mol Biol. 1998;60:317–345. doi: 10.1016/s0079-6603(08)60896-x. [DOI] [PubMed] [Google Scholar]
- 193.Nakajima I, Yamaguchi T, Ozutsumi K, Aso H. Adipose tissue extracellular matrix: newly organized by adipocytes during differentiation. Differentiation. 1998;63:193–200. doi: 10.1111/j.1432-0436.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- 194.Yi T, Choi HM, Park RW, Sohn KY, Kim IS. Transcriptional repression of type I procollagen genes during adipocyte differentiation. Exp Mol Med. 2001;33:269–275. doi: 10.1038/emm.2001.44. [DOI] [PubMed] [Google Scholar]
- 195.Kubo Y, Kaidzu S, Nakajima I, Takenouchi K, Nakamura F. Organization of extracellular matrix components during differentiation of adipocytes in long-term culture. In Vitro Cell Dev Biol Anim. 2000;36:38–44. doi: 10.1290/1071-2690(2000)036<0038:OOEMCD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 196.Ibrahimi A, Bonino F, Bardon S, Ailhaud G, Dani C. Essential role of collagens for terminal differentiation of preadipocytes. Biochem Biophys Res Commun. 1992;187:1314–1322. doi: 10.1016/0006-291x(92)90446-r. [DOI] [PubMed] [Google Scholar]
- 197.Ward M, Ajuwon KM. Regulation of pre-adipocyte proliferation and apoptosis by the small leucine-rich proteoglycans, biglycan and decorin. Cell Prolif. 2011;44:343–351. doi: 10.1111/j.1365-2184.2011.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1998;95:1062–1066. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rigamonti E, Zordan P, Sciorati C, Rovere-Querini P, Brunelli S. Macrophage plasticity in skeletal muscle repair. Biomed Res Int. 2014;2014:560629. doi: 10.1155/2014/560629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Segawa M, Fukada S, Yamamoto Y, Yahagi H, Kanematsu M, Sato M, Ito T, Uezumi A, Hayashi S, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp Cell Res. 2008;314:3232–3244. doi: 10.1016/j.yexcr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 201.Malerba A, Vitiello L, Segat D, Dazzo E, Frigo M, Scambi I, De Coppi P, Boldrin L, Martelli L, Pasut A, Romualdi C, Bellomo RG, Vecchiet J, Baroni MD. Selection of multipotent cells and enhanced muscle reconstruction by myogenic macrophage-secreted factors. Exp Cell Res. 2009;315:915–927. doi: 10.1016/j.yexcr.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 202.Corna G, Campana L, Pignatti E, Castiglioni A, Tagliafico E, Bosurgi L, Campanella A, Brunelli S, Manfredi AA, Apostoli P, Silvestri L, Camaschella C, Rovere-Querini P. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95:1814–1822. doi: 10.3324/haematol.2010.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Campana L, Santarella F, Esposito A, Maugeri N, Rigamonti E, Monno A, Canu T, Del Maschio A, Bianchi ME, Manfredi AA, Rovere-Querini P. Leukocyte HMGB1 is required for vessel remodeling in regenerating muscles. J Immunol. 2014;192:5257–5264. doi: 10.4049/jimmunol.1300938. [DOI] [PubMed] [Google Scholar]
- 204.Zordan P, Rigamonti E, Freudenberg K, Conti V, Azzoni E, Rovere-Querini P, Brunelli S. Macrophages commit postnatal endothelium-derived progenitors to angiogenesis and restrict endothelial to mesenchymal transition during muscle regeneration. Cell Death Dis. 2014;5:e1031. doi: 10.1038/cddis.2013.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev. 2014;262:134–152. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zordan P, Sciorati C, Campana L, Cottone L, Clementi E, Querini PR, Brunelli S. The nitric oxide-donor molsidomine modulates the innate inflammatory response in a mouse model of muscular dystrophy. Eur J Pharmacol. 2013;715:296–303. doi: 10.1016/j.ejphar.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 208.Rigamonti E, Touvier T, Clementi E, Manfredi AA, Brunelli S, Rovere-Querini P. Requirement of inducible nitric oxide synthase for skeletal muscle regeneration after acute damage. J Immunol. 2013;190:1767–1777. doi: 10.4049/jimmunol.1202903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 210.Clementi E, Meldolesi J. The cross-talk between nitric oxide and Ca2 + : a story with a complex past and a promising future. Trends Pharmacol Sci. 1997;18:266–269. doi: 10.1016/s0165-6147(97)01087-0. [DOI] [PubMed] [Google Scholar]
- 211.Clementi E, Borgese N, Meldolesi J. Interactions between nitric oxide and sphingolipids and the potential consequences in physiology and pathology. Trends Pharmacol Sci. 2003;24:518–523. doi: 10.1016/j.tips.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 212.Ascenzi P, di Masi A, Sciorati C, Clementi E. Peroxynitrite-An ugly biofactor? BioFactors. 2010;36:264–273. doi: 10.1002/biof.103. [DOI] [PubMed] [Google Scholar]
- 213.Filippin LI, Moreira AJ, Marroni NP, Xavier RM. Nitric oxide and repair of skeletal muscle injury. Nitric Oxide. 2009;21:157–163. doi: 10.1016/j.niox.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 214.De Palma C, Clementi E. Nitric oxide in myogenesis and therapeutic muscle repair. Mol Neurobiol. 2012;46:682–692. doi: 10.1007/s12035-012-8311-8. [DOI] [PubMed] [Google Scholar]
- 215.Kaminski HJ, Andrade FH. Nitric oxide: biologic effects on muscle and role in muscle diseases. Neuromuscul Disord. 2001;11:517–524. doi: 10.1016/s0960-8966(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 216.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119:2855–2862. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- 217.Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 218.Gucuyener K, Ergenekon E, Erbas D, Pinarli G, Serdaroglu A. The serum nitric oxide levels in patients with Duchenne muscular dystrophy. Brain Dev. 2000;22:181–183. doi: 10.1016/s0387-7604(00)00106-6. [DOI] [PubMed] [Google Scholar]
- 219.Sciorati C, Buono R, Azzoni E, Casati S, Ciuffreda P, D’Angelo G, Cattaneo D, Brunelli S, Clementi E. Co-administration of ibuprofen and nitric oxide is an effective experimental therapy for muscular dystrophy, with immediate applicability to humans. Br J Pharmacol. 2010;160:1550–1560. doi: 10.1111/j.1476-5381.2010.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tidball JG, Wehling-Henricks M. Expression of a NOS transgene in dystrophin-deficient muscle reduces muscle membrane damage without increasing the expression of membrane-associated cytoskeletal proteins. Mol Genet Metab. 2004;82:312–320. doi: 10.1016/j.ymgme.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 221.Brunelli S, Sciorati C, D’Antona G, Innocenzi A, Covarello D, Galvez BG, Perrotta C, Monopoli A, Sanvito F, Bottinelli R, Ongini E, Cossu G, Clementi E. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci U S A. 2007;104:264–269. doi: 10.1073/pnas.0608277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sciorati C, Miglietta D, Buono R, Pisa V, Cattaneo D, Azzoni E, Brunelli S, Clementi E. A dual acting compound releasing nitric oxide (NO) and ibuprofen, NCX 320, shows significant therapeutic effects in a mouse model of muscular dystrophy. Pharmacol Res. 2011;64:210–217. doi: 10.1016/j.phrs.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Brunelli S, Rovere-Querini P, Sciorati C, Manfredi AA, Clementi E. Nitric oxide: emerging concepts about its use in cell-based therapies. Expert Opin Investig Drugs. 2007;16:33–43. doi: 10.1517/13543784.16.1.33. [DOI] [PubMed] [Google Scholar]
- 224.D’Angelo MG, Gandossini S, Martinelli Boneschi F, Sciorati C, Bonato S, Brighina E, Comi GP, Turconi AC, Magri F, Stefanoni G, Brunelli S, Bresolin N, Cattaneo D, Clementi E. Nitric oxide donor and non steroidal anti inflammatory drugs as a therapy for muscular dystrophies: evidence from a safety study with pilot efficacy measures in adult dystrophic patients. Pharmacol Res. 2012;65:472–479. doi: 10.1016/j.phrs.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 225.Buono R, Vantaggiato C, Pisa V, Azzoni E, Bassi MT, Brunelli S, Sciorati C, Clementi E. Nitric oxide sustains long-term skeletal muscle regeneration by regulating fate of satellite cells via signaling pathways requiring Vangl2 and cyclic GMP. Stem Cells. 2011;30:197–209. doi: 10.1002/stem.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.De Palma C, Falcone S, Pisoni S, Cipolat S, Panzeri C, Pambianco S, Pisconti A, Allevi R, Bassi MT, Cossu G, Pozzan T, Moncada S, Scorrano L, Brunelli S, Clementi E. Nitric oxide inhibition of Drp1-mediated mitochondrial fission is critical for myogenic differentiation. Cell Death Differ. 2010;17:1684–1696. doi: 10.1038/cdd.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Anderson JE. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell. 2000;11:1859–1874. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Drenning JA, Lira VA, Simmons CG, Soltow QA, Sellman JE, Criswell DS. Nitric oxide facilitates NFAT-dependent transcription in mouse myotubes. Am J Physiol Cell Physiol. 2008;294:C1088–C1095. doi: 10.1152/ajpcell.00523.2007. [DOI] [PubMed] [Google Scholar]
- 229.Colussi C, Mozzetta C, Gurtner A, Illi B, Rosati J, Straino S, Ragone G, Pescatori M, Zaccagnini G, Antonini A, Minetti G, Martelli F, Piaggio G, Gallinari P, Steinkuhler C, Clementi E, Dell’Aversana C, Altucci L, Mai A, Capogrossi MC, Puri PL, Gaetano C. HDAC2 blockade by nitric oxide and histone deacetylase inhibitors reveals a common target in Duchenne muscular dystrophy treatment. Proc Natl Acad Sci U S A. 2008;105:19183–19187. doi: 10.1073/pnas.0805514105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Clementi E, Brown GC, Foxwell N, Moncada S. On the mechanism by which vascular endothelial cells regulate their oxygen consumption. Proc Natl Acad Sci U S A. 1999;96:1559–1562. doi: 10.1073/pnas.96.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dai Z, Wu Z, Yang Y, Wang J, Satterfield MC, Meininger CJ, Bazer FW, Wu G. Nitric oxide and energy metabolism in mammals. BioFactors. 2013;39:383–391. doi: 10.1002/biof.1099. [DOI] [PubMed] [Google Scholar]
- 233.Nisoli E, Clementi E, Tonello C, Sciorati C, Briscini L, Carruba MO. Effects of nitric oxide on proliferation and differentiation of rat brown adipocytes in primary cultures. Br J Pharmacol. 1998;125:888–894. doi: 10.1038/sj.bjp.0702131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kang C, Li Ji L. Role of PGC-1alpha signaling in skeletal muscle health and disease. Ann N Y Acad Sci. 2012;1271:110–117. doi: 10.1111/j.1749-6632.2012.06738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Iezzi S, Di Padova M, Serra C, Caretti G, Simone C, Maklan E, Minetti G, Zhao P, Hoffman EP, Puri PL, Sartorelli V. Deacetylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin. Dev Cell. 2004;6:673–684. doi: 10.1016/s1534-5807(04)00107-8. [DOI] [PubMed] [Google Scholar]
- 236.Minetti GC, Colussi C, Adami R, Serra C, Mozzetta C, Parente V, Fortuni S, Straino S, Sampaolesi M, Di Padova M, Illi B, Gallinari P, Steinkuhler C, Capogrossi MC, Sartorelli V, Bottinelli R, Gaetano C, Puri PL. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med. 2006;12:1147–1150. doi: 10.1038/nm1479. [DOI] [PubMed] [Google Scholar]
- 237.Saccone V, Consalvi S, Giordani L, Mozzetta C, Barozzi I, Sandona M, Ryan T, Rojas-Munoz A, Madaro L, Fasanaro P, Borsellino G, De Bardi M, Frige G, Termanini A, Sun X, Rossant J, Bruneau BG, Mercola M, Minucci S, Puri PL. HDAC-regulated myomiRs control BAF60 variant exchange and direct the functional phenotype of fibro-adipogenic progenitors in dystrophic muscles. Genes Dev. 2014;28:841–857. doi: 10.1101/gad.234468.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Twayana S, Legnini I, Cesana M, Cacchiarelli D, Morlando M, Bozzoni I. Biogenesis and function of non-coding RNAs in muscle differentiation and in Duchenne muscular dystrophy. Biochem Soc Trans. 2013;41:844–849. doi: 10.1042/BST20120353. [DOI] [PubMed] [Google Scholar]
- 240.Greco S, De Simone M, Colussi C, Zaccagnini G, Fasanaro P, Pescatori M, Cardani R, Perbellini R, Isaia E, Sale P, Meola G, Capogrossi MC, Gaetano C, Martelli F. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 2009;23:3335–3346. doi: 10.1096/fj.08-128579. [DOI] [PubMed] [Google Scholar]
- 241.Cacchiarelli D, Legnini I, Martone J, Cazzella V, D’Amico A, Bertini E, Bozzoni I. miRNAs as serum biomarkers for Duchenne muscular dystrophy. EMBO Mol Med. 2011;3:258–265. doi: 10.1002/emmm.201100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Motohashi N, Alexander MS, Casar JC, Kunkel LM. Identification of a novel microRNA that regulates the proliferation and differentiation in muscle side population cells. Stem Cells Dev. 2012;21:3031–3043. doi: 10.1089/scd.2011.0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10:844–854. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 244.Desguerre I, Mayer M, Leturcq F, Barbet JP, Gherardi RK, Christov C. Endomysial fibrosis in Duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol. 2009;68:762–773. doi: 10.1097/NEN.0b013e3181aa31c2. [DOI] [PubMed] [Google Scholar]
- 245.Tan JT, McLennan SV, Song WW, Lo LW, Bonner JG, Williams PF, Twigg SM. Connective tissue growth factor inhibits adipocyte differentiation. Am J Physiol Cell Physiol. 2008;295:C740–C751. doi: 10.1152/ajpcell.00333.2007. [DOI] [PubMed] [Google Scholar]
- 246.Fitter S, Vandyke K, Gronthos S, Zannettino AC. Suppression of PDGF-induced PI3 kinase activity by imatinib promotes adipogenesis and adiponectin secretion. J Mol Endocrinol. 2012;48:229–240. doi: 10.1530/JME-12-0003. [DOI] [PubMed] [Google Scholar]