Abstract
SoxAX cytochromes are heme-thiolate proteins that play a key role in bacterial thiosulfate oxidation, where they initiate the reaction cycle of a multi-enzyme complex by catalyzing the attachment of sulfur substrates such as thiosulfate to a conserved cysteine present in a carrier protein. SoxAX proteins have a wide phylogenetic distribution and form a family with at least three distinct types of SoxAX protein. The types of SoxAX cytochromes differ in terms of the number of heme groups present in the proteins (there are diheme and triheme versions) as well as in their subunit structure. While two of the SoxAX protein types are heterodimers, the third group contains an additional subunit, SoxK, that stabilizes the complex of the SoxA and SoxX proteins. Crystal structures are available for representatives of the two heterodimeric SoxAX protein types and both of these have shown that the cysteine ligand to the SoxA active site heme carries a modification to a cysteine persulfide that implicates this ligand in catalysis. EPR studies of SoxAX proteins have also revealed a high complexity of heme dependent signals associated with this active site heme; however, the exact mechanism of catalysis is still unclear at present, as is the exact number and types of redox centres involved in the reaction.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-012-1098-y) contains supplementary material, which is available to authorized users.
Keywords: SoxAX cytochromes, Cytochromes, Heme thiolate proteins, Sulfur oxidation, Crystal structure, Redox centres
Introduction
The so-called SoxAX cytochromes are a group of c-type cytochromes that catalyze the formation of heterodisulfide bonds between inorganic sulfur compounds and a conserved cysteine on a sulfur carrier protein [1, 2]. The reaction involves a heme group located in the SoxAX active site, which has a His/Cys axial ligation. Unlike the well-known His/Met ligated hemes present in many pro- and eukaryotic cytochromes, proteins containing His/Cys ligated heme groups are relatively rare in nature, and fulfill a range of special and diverse functions [3]. A well-known example of heme thiolate proteins are the cytochrome P 450s that play a role in xenobiotic metabolism; however, in these proteins, the heme group is essentially five coordinate, which increases its catalytic reactivity [3, 4]. There are several examples of proteins that contain six coordinate heme thiolate groups, including human cystathionine beta synthase, an enzyme involved in the formation of cystathionine from homocysteine where the His/Cys ligated heme has been proposed to play a role in redox sensing and also influences catalytic activity [5]. Other examples are the bacterial CooA carbon monoxide sensor protein in which the heme group acts as a redox sensor [6, 7], a reaction centre cytochrome (PufC) from the phototrophic bacterium Rhodovulum sulfidophilum [8], and the DsrJ triheme cytochrome that is part of a membrane protein complex found in both dissimilatory sulfate reducing and sulfur oxidizing bacteria [9, 10].
The SoxAX heme thiolate proteins also belong to this diverse group of proteins and play a key role in bacterial sulfur oxidizing photo- and chemolithotrophs, i.e. bacteria that are capable of using inorganic sulfur compounds as electron donors for photosynthesis or for energy generation. Several related but structurally distinct types of SoxAX cytochromes are known, but the properties and types of redox centres present in these enzymes as well as their respective roles in catalysis are still only partly understood.
Discovery of SoxAX cytochromes
The SoxAX cytochromes were first discovered by Lu and Kelly [11–13] during studies of sulfur oxidation in the facultatively chemolithotrophic bacterium Paracoccus versutus (known at the time as Thiobacillus versutus or Thiobacillus A2 [14]). Kelly and coworkers were the first to isolate and partially characterize the constituents of a bacterial thiosulfate oxidizing enzyme complex to which the SoxAX cytochromes belong. This enzyme complex was called either the Paracoccus sulfur oxidation system (PSO), or the thiosulfate oxidizing multi-enzyme system (TOMES) (today it is often simply referred to as the Sox system). Kelly referred to the components of the complex as enzymes A and B (equivalent to SoxYZ and SoxB), a cytochrome c 552.5 (eq. to SoxAX) and a sulfite: cytochrome c oxidoreductase/cytochrome c 551 complex (eq. to SoxCD). In these early studies, many of the properties of the individual components of the enzyme system, such as the ability of enzyme A to bind thiosulfate or the fact that the cytochrome c 552.5 is an essential component of the enzyme complex, were correctly inferred although the molecular details underlying these observations could not be analyzed in depth. Characterization of the TOMES also included the first EPR spectroscopic studies of the metal centres present in the different enzymes (summarized in [15]).
In the early 1990s, the group of Friedrich used transposon mutagenesis to identify and then sequence a gene region involved in thiosulfate oxidation in another bacterium that is today known as Paracoccus pantotrophus GB17 [16, 17] (at the time: Thiosphaera pantotropha GB17, then Paracoccus denitrificans GB17 [18]). This gene region turned out to encode proteins of an enzyme complex similar to the one that had been studied by Kelly and coworkers. The identification of the gene region encoding the TOMES opened up many possibilities for comparative and in depth molecular and biological studies, and the group of Friedrich subsequently reported the purification and characterization of several TOMES components [19–24]. More than 15 genes have today been identified as belonging to the sox gene region in P. pantotrophus, but the core enzyme complex only requires the SoxAX, SoxYZ, SoxB and SoxCD proteins for function while the remaining genes encode proteins that are involved in regulation and (re-)activation of core Sox proteins [21, 25–29]. The fact that the genes found in the P. pantotrophus sulfur oxidation gene region were referred to as ‘sox’ genes laid the foundation for the current nomenclature of the encoded proteins, all of which are today referred to as ‘Sox’ proteins.
While initial studies of the Sox system all focussed on chemolithotrophic sulfur oxidizing bacteria, it was soon recognized that not only these bacteria, but also the phototrophic purple and green sulfur bacteria contained homologues of Sox proteins [30]. In the phototrophic bacteria, the Sox proteins occur in addition to the proteins of the dissimilatory sulfite reductase (Dsr) complex that have been known to be essential for sulfur oxidation processes in these bacteria for over 40 years [31–34]. The combination of Dsr and Sox proteins can occur in both photo- and chemotrophic bacteria that form sulfur deposits as an intermediate of the sulfur oxidation process. In these dsr-gene cluster containing photo- and chemotrophic bacteria the Sox system is thought to be specifically involved in the utilization of thiosulfate but not sulfide as an electron donor [33]. In contrast, chemotrophic bacteria that contain only a Sox system appear to be capable of using thiosulfate as well as sulfide and sulfite as substrates for Sox system mediated sulfur oxidation [35]. In one case even sulfur and tetrathionate have been reported to be substrates of a Sox multi-enzyme complex [36].
SoxAX cytochromes and their roles in different pathways for dissimilatory sulfur oxidation
The functions of the homologous Sox proteins from bacteria relying solely on the Sox pathway and those using the Dsr/Sox pathway are conserved, and although more than 15 individual Sox proteins are known at present, only three or four protein complexes, respectively, are essential for the functioning of the enzyme system, depending on whether a bacterium uses the Dsr/Sox or the Sox pathway. The three essential protein complexes common to all Sox systems are the SoxAX cytochromes, the cofactor-less SoxYZ proteins that carry a conserved ‘GGCGG’ motif [35] and the SoxB proteins that contain a dimanganese centre [37], while the molybdenum- and heme-containing SoxCD sulfur dehydrogenase is only found in bacteria using the Sox pathway but is essential for sulfur oxidation in these microorganisms [38].
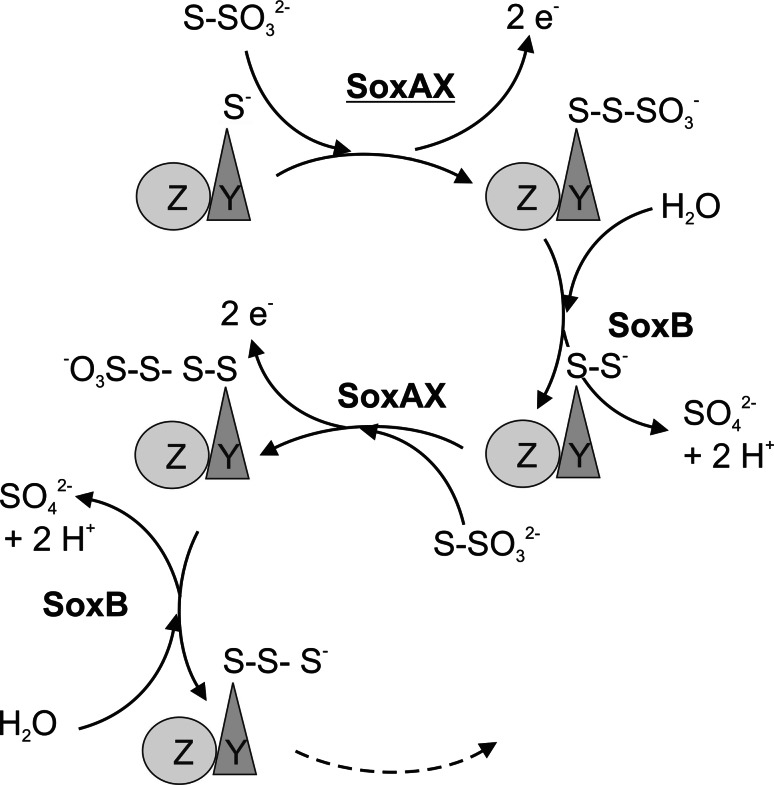
Based on the properties of the essential complex proteins and the observation that no free sulfur intermediates appear to occur during thiosulfate oxidation, the following model for the Sox pathway reactions has been proposed (Fig. 1) [30]. Throughout the Sox system reaction cycle, the heterodimeric SoxYZ protein acts as a ‘carrier protein’ to which the reduced sulfur compounds remain attached at all stages of the oxidation process (Fig. 1) [30, 35]. The attachment of the sulfur compounds to a conserved ‘GGCGG’ motif at the C-terminus of SoxY is mediated by the SoxAX cytochrome, which is thus essential for initiating the sulfur compound oxidation process [1, 35]. If the sulfur substrate is thiosulfate, the next reaction is a SoxB mediated hydrolysis of the sulfone group of the bound thiosulfate molecule, leading to the formation of one molecule of sulfate, the ultimate product of thiosulfate oxidation. Following the hydrolytic step, the SoxCD sulfur deydrogenase catalyzes the six electron oxidation of the remaining thiosulfate ‘sulfane’ sulfur atom to a sulfone group, which is then liberated from the SoxYZ carrier protein as a second molecule of sulfate through a second reaction with SoxB [23, 35, 37] (Fig. 1).
Fig. 1.
Schematic representation of the Sox pathway reaction cycle with different sulfur substrates, a thiosulfate as substrate, b sulfide as substrate, c sulfite as substrate
In cases where other sulfur substrates are being oxidized, the reaction cycle has been proposed to be shorter [35], e.g. if the reaction substrate were sulfide, then the reaction cycle would start with SoxAX mediated attachment of the substrate to SoxYZ, followed by the SoxCD mediated six electron oxidation of the SoxYZ bound sulfane sulfur molecules and a reaction with SoxB (Fig. 1) [35], while in the case of sulfite, only one SoxB mediated hydrolysis reaction would be predicted to take place (Fig. 1).
In bacteria using the Dsr/Sox pathway the absence of SoxCD requires a further modification of the Sox reaction cycle (Fig. 2). The current view is that following the initial SoxAX and SoxB mediated reactions, the sulfane sulfur atom of the thiosulfate molecule will remain bound to SoxYZ [33, 34, 39]. The cycle will then repeat and incoming, additional thiosulfate molecules will be attached to these SoxY bound sulfur atoms rather than directly to the conserved SoxY cysteine. This process is thought to lead to the formation of chains of sulfur atoms, that can eventually be transferred to the sulfur globules formed by many of these bacteria (Fig. 2), although the mechanism by which the ‘sulfur chain’ is transferred from SoxYZ to the sulfur globules as well as the length of the sulfur atom chain required for the transfer reaction is currently unknown.
Fig. 2.
Schematic representation of the reaction cycle of Sox proteins involved in thiosulfate oxidation via the Dsr/Sox pathway
In summary, in both types of Sox systems, the role of the SoxAX cytochromes in sulfur oxidation is to catalyze the formation of a heterodisulfide bond between the conserved SoxY cysteine (‘GGCGG’ motif) and thiosulfate (eq. 1) or other sulfur substrates.
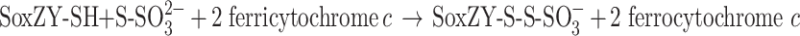 |
1 |
In each case, SoxAX proteins have been postulated to be able to interact with a variety of sulfur substrates or modified version of the SoxYZ protein; however, it is unknown at present whether differences in substrate specificities exist between SoxAX proteins from different bacteria or those employing different pathways for sulfur oxidation.
The heterodisulfide bond formation leads to the liberation of two electrons, which are thought to be transferred from the SoxAX cytochrome to a cytochrome c that channels them into the electron transport chain, thus contributing to energy conservation [1].
Types of SoxAX proteins and their encoding operons
Although the SoxAX proteins from bacteria employing the Sox and the Dsr/Sox pathway are thought to catalyze the same or similar reactions, differences exist in the basic structure of these proteins. As their name suggests, most SoxAX proteins are heterodimeric complexes of two c-type heme bearing subunits, where the SoxA subunits generally have a molecular mass of around 29 kDa and contain either one or two heme groups, while the SoxX subunits can vary in their molecular mass, from ~11–20 kDa for the mature proteins [40]. A further difference between the subunits is that the SoxA heme groups always have a His/Cys axial ligation while the single SoxX heme has a His/Met axial ligand pair.
In addition to heterodimeric forms of SoxAX there also exist heterotrimeric forms which possess a third, low molecular weight subunit (‘SoxK’, 9.4 kDa, also known as the ‘SoxAX binding protein’) that stabilizes the complex of the associated SoxA and SoxX subunits [41]. At present there is no data suggesting that the SoxK subunit has additional functions beyond stabilization of the SoxAX complex.
The structure of the gene clusters (or regions) encoding the Sox proteins also appears to differ depending on the type of bacteria and/or the sulfur oxidation pathways present. In sox gene clusters from most chemolithotrophic sulfur oxidizers such as P. pantotrophus, the genes encoding the core components of the enzyme complex occur together with genes encoding specialized proteins involved in maintaining the complex components in their active state as well as other proteins that regulate expression of the gene cluster (SoxR) [26, 27, 42]. In bacteria with Dsr/Sox pathways, however, the genes encoding these sox–specific accessory proteins appear to be absent or are located elsewhere on the chromosome, and the sox genes themselves either form shortened sox gene clusters (soxJXYZAKBW, found especially in members of the Chlorobi [43]) that lack soxCD genes or can be located in several independent loci [30, 41, 44]. As already indicated above, the absence of soxCD genes is thought to be typical of bacteria that oxidize reduced sulfur compounds with elemental sulfur as an intermediate [33].
Phylogeny of SoxAX proteins
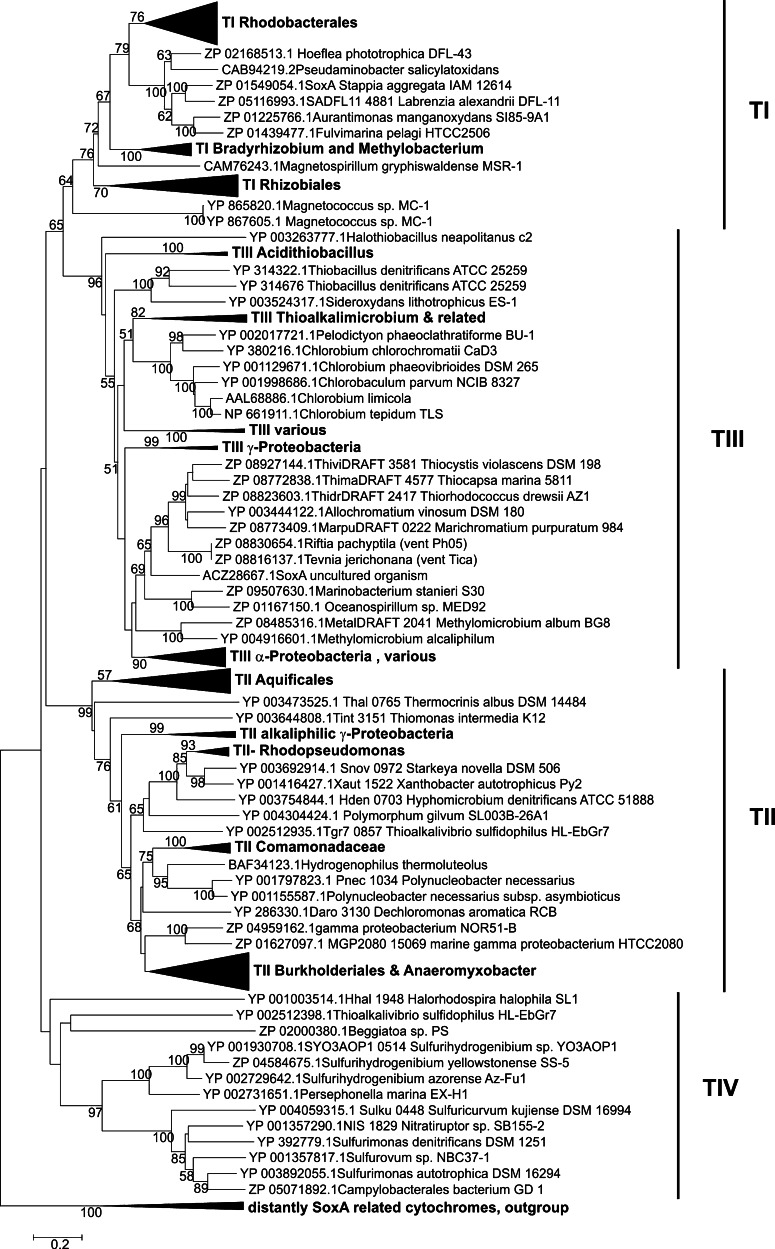
The phylogenetic relationships between the different forms of SoxAX proteins have been explored [40, 41] and while initially only a significant divergence of sequences originating from Starkeya and Ralstonia species relative to those from Paracoccus and Chlorobium sp. was apparent. Due to the limited number of available sequences [40], recent analyses by Ogawa and coworkers [41] clearly demonstrated the existence of three distinct types of SoxAX proteins. Type I and II SoxAX proteins are heterodimers, while the heterotrimeric SoxAXK proteins form the third group. Type I SoxAX proteins have SoxA subunits with two heme groups and SoxX subunits with a molecular mass of ~14 kDa, while the Type II SoxA proteins only contain one heme group, and are associated with SoxX proteins that have a molecular mass of approximately 20 kDa. The increased molecular mass in these SoxX proteins is largely due to the presence of an N-terminal extension [45]. The Type III SoxAXK proteins also have single heme group SoxA subunits and distinct SoxX subunits with a molecular mass of ~10 kDa. According to Ogawa and coworkers [41], the loss of 20–30 N-terminal amino acids distinguishes the single heme SoxA subunits of Type II from Type III SoxA proteins. Despite these differences, the overall levels of amino acid sequence identity and similarity are similar for the three groups.
The phylogenetic analysis carried out by Ogawa [41] focusses on the SoxAXK proteins and also includes a number of sequence alignments showing differences and conserved regions in the primary sequence of the SoxAX(K) proteins; however, the analysis was limited to sequences from ~40 bacterial species in total.
A recent database search revealed that, at present, there are over 200 SoxA related protein sequences available, and we have conducted an analysis of 216 SoxA amino acid sequences originating from a wide variety of bacterial species including not only all groups of Proteobacteria, green sulfur bacteria and Aquificales, but also members of the Cytophaga group, the Firmicutes and many general members of the domain bacteria. Five SoxA related protein sequences were used as an outgroup (Figs. 3, S1). Phylogenetic trees were generated using neighbor-joining, minimum evolution and maximum likelihood algorithms as integrated into Mega 5.0 [46] and indicated that in addition to the three types of SoxA proteins described above, a fourth group of SoxA proteins may be emerging as more sequences are available in the database (Fig. 3). At present the putative fourth SoxA group comprises 13 sequences from several ε-Proteobacteria such as Sulfurimonas and Sulfurihydrogenibium sp. as well as SoxA from the haloalkaliphilic γ-Proteobacterium Thioalkalivibrio sulfidophilus. The soxA genes encoding these proteins are mostly located in soxXYZAB gene clusters, with the exception of the sequences from Beggiatoa and Halomonas halophila, where the soxA encoding genes are not found in the vicinity of a soxX gene, and in fact, genes encoding the second subunit of the SoxAX protein appear to be absent from the genomes of these bacteria. It is possible that these two ‘SoxA’ proteins have a different function from the remaining proteins present in the phylogenetic tree; however, as they are clearly related to the new group of SoxAX proteins it was decided not to remove them from the alignment (Fig. 3). The soxX genes associated with the genes encoding the tentatively named ‘Type IV’ SoxA proteins encode proteins with ~150 amino acids (mature protein, mol. mass ~16.7 kDa) and contain an N-terminal extension region. However, database searches using the ‘Type IV’ SoxX proteins did not reveal any close relationship of these proteins to the SoxX proteins found in Type II SoxAX proteins. The properties of the putative ‘Type IV’ SoxAX proteins will need to be evaluated in more depth as more and especially biochemical data become available.
Fig. 3.
Phylogenetic relationship of SoxA proteins. Two hundred sixteen SoxA amino acid sequences were analyzed using Mega 5.0. The tree shown was generated using the Neighbor-joining algorithm (Poisson model; uniform rate of evolution for all sites; gap treatment: pairwise-deletion; robustness testing: bootstrap method with 500 resampling cycles). The different types of SoxAX proteins are indicated by black bars and labels TI-TIV. A group of several SoxA related cytochromes was used as the outgroup
Another feature that becomes apparent when examining the SoxA phylogenetic tree (Fig. 3) is that several bacteria contain multiple copies of SoxAX which can belong to several SoxAX types. An example are the Bradyrhizobium species, which contain at least two Type I and one Type III SoxAX protein (Figs. 3, S1), but multiple copies of SoxAX proteins are also found in Starkeya novella (Types I and II), Thiobacillus denitrificans (2 × Type III), Thioalkalivibrio sulfidophilus (2 × Type II, 1 × Type ‘IV’) and other bacteria. The genes encoding these proteins can either be located in different gene loci or form concatenations, as is the case in Thioalkalivibrio sulfidophilus where genes Tgr7_853-858 correspond to three consecutive copies of SoxAX genes encoding the two different types of SoxAX proteins found in this organism. The biological significance of these multiple soxAX gene copies is unknown at present, but could be indicative of either specialized functions associated with the different copies of soxAX genes or could indicate biological redundancy within the Sox systems of different organisms.
Ogawa et al. [41] also analyzed the structure of gene loci encoding SoxAX proteins with a special emphasis on genes encoding Type III SoxAX proteins for which they uncovered a remarkable diversity in the organization of gene clusters while only a limited analysis of gene clusters encoding Type I and Type II SoxAX proteins was carried out [41]. For Type I SoxAX proteins, the P. pantotrophus gene cluster is shown, while for the Type II proteins two sox gene clusters with an organization of the core sox genes (in bold) in two transciptional units-soxWV XA-sox YZBCD xF are presented. It should be noted, however, that in particular the genes encoding Type II SoxAX proteins are not always found in gene clusters consisting of two transcriptional units (this is the case for S. novella and several Rhodopseudomonas sp. as shown by [41]). For example, in Comamonas species the genes encoding Type II SoxAX proteins are part of a sox CDYZAXB gene cluster which forms only one transcriptional unit and also differs from the ‘canonical’ organization of the core sox genes (sox XYZABCD ) seen, e.g., in P. pantotrophus [47].
An interesting detail of Ogawa’s extensive analysis of the Type III soxAX gene clusters is that the SoxK subunit is not always encoded in close proximity to the soxA genes (e.g. in Thiobacillus denitrificans) and in one case (Thiomicrospira crunogena) it appears to be absent from the genome [41]. It would be interesting to investigate whether a SoxK subunit is an essential component of all Type III SoxAX proteins or not, and whether other small proteins can take over the function of SoxK in bacteria that contain Type III SoxAX proteins but lack a SoxK homologue.
Crystal structures of SoxAX proteins
At present, crystal structures have been solved for two Type I SoxAX proteins from R. sulfidophilum (RsSoxAX, PDB code 1H33, 1.75 Å resolution; [1]) and P. pantotrophus (PpSoxAX, PDB code 2C1D, 1.92 Å resolution; [48]), respectively and one Type II protein from S. novella (SnSoxAX, PDB code 3OA8, 1.77 Å resolution; [45]).
The SoxA structures
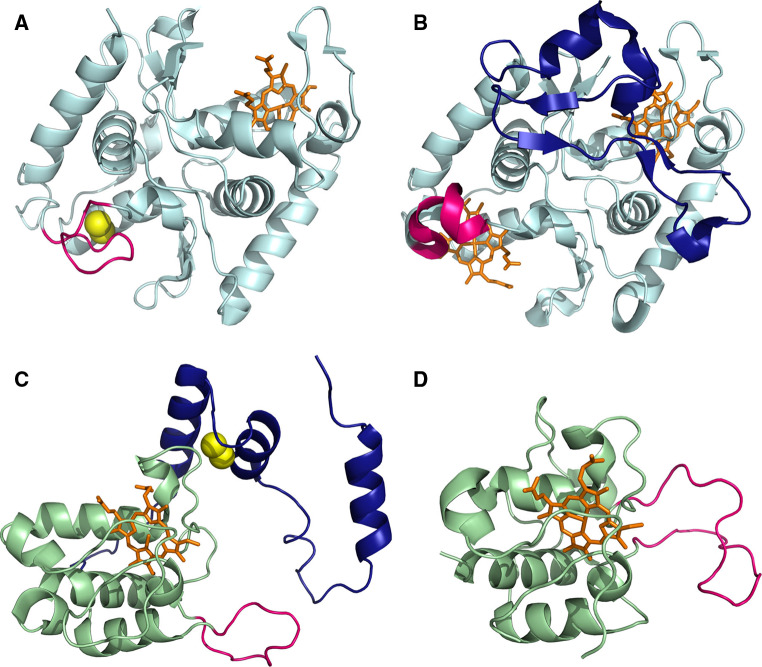
In all three structures, the SoxA subunits have a two-domain structure, with pseudo twofold symmetry between the domains. Each domain has a mitochondrial c-type cytochrome fold. In the case of the Type I proteins, each domain binds a heme cofactor (hemes 1 and 2, in the nomenclature used for the RsSoxAX structure) with His/Cys axial coordination. The structure of the SnSoxA subunit has a similar domain configuration, but binds only a single heme cofactor (the equivalent of Type I, heme 2). In the place of heme 1, there is a disulfide bond (Cys A74-Cys A110) (Fig. 4). The presence of the disulfide bond in the SnSoxA structure accompanies a difference in the conformation of a loop comprised of residues 73–83, when compared with the RsSoxA protein. This loop reaches in toward the core of the molecule in the SnSoxA structure, but faces away and forms part of the surface of the protein in the RsSoxA model (Fig. 4).
Fig. 4.
Comparison of the SoxA and SoxX structures from Starkeya novella and Rhodovulum sulfidophilum. a The structure of SnSoxA. The heme cofactor (heme 2, using RsSoxA nomenclature) is highlighted in orange and the Cys A74 and Cys A110 Sγ atoms, which participate in the disulfide linkage, which replaces the heme 1 site, are shown as yellow spheres. b The structure of RsSoxA. Heme cofactors 1 and 2 are highlighted in orange. The N-terminal extension (residues 1–51), which ‘caps’ the heme 2 binding site is represented in dark blue. For both structures, the loop (residues 73–83, SnSoxA; residues 79–87, RsSoxA) which shows a different conformation in the SnSoxA and RsSoxA structures is represented in pink. c The structure of SnSoxX. The N-terminal extension present in the SnSoxX structure is represented in blue and the disulfide linkage, between residues Cys B64 and Cys B175 is shown as yellow spheres (the positions of the Cys Sγ atoms are represented). d The structure of RsSoxX. For both c and d, the heme cofactors and their axial Met and His ligands are shown in orange. Features, which show different conformations in the two structures are highlighted in pink (residues B109–B121 for SnSoxX and residues B97–B119 for RsSoxX)
The Type I SoxA structures are distinguished by the presence of a significant N-terminal extension of approximately 50 residues, which ‘caps’ the heme 2 binding site (Fig. 4) and restricts the solvent accessibility to the heme cofactor. Significantly, the propionate groups of the heme 2 cofactor are solvent-accessible in the SnSoxA protein, but buried in the RsSoxA and PpSoxA structures (Fig. 5). This potentially restricts access to the active site, and may influence the (as yet undefined) substrate specificities of the respective proteins.
Fig. 5.
Active sites of the SnSoxAX and RsSoxAX structures. Panels a and b SnSoxAX with and without surface representations, respectively; Panels c and d RsSoxAX. In all panels the heme 2 cofactors is represented in orange and the Cys active site residue in hot pink. In the SnSoxAX and RsSoxAX structures, residues Gln 197 and Asp 192, respectively (indicated in stick representations) ‘gate’ access to the active site
Despite the differences in the polypeptide structures of the SoxA subunits between proteins from different families, the coordination structures of the heme 2 binding sites are remarkably conserved. However, while in the two crystal structures of Type I SoxAX proteins the cysteine ligands to the active site hemes were found to be quantitatively modified to a cysteine persulfide (CSS) [1, 48], in the SnSoxAX structure, the coordinating residue A236 was modelled as an equal mixture of Cys and post-translationally modified cysteine persulfide (CSS) [45]. The CSS modification has been suggested to result from incomplete catalysis, where only the thiosulfate sulfone sulfur (rather than the entire thiosulfate moiety) is transferred to SoxYZ [1]. Given that the SnSoxAX protein was produced recombinantly and not exposed to the SoxYZ protein during its production for crystallisation [45], the reason for the presence of a partial modification of the active site cysteine residue in the SnSoxAX structure is presently unclear.
The SoxX structures
At the core of all three SoxX structures is a tightly folded heme-binding domain, with the heme ligated by His and Met axial ligands (Fig. 4). In all three structures the heme cofactor and propionate groups form part of the solvent-exposed surface of the protein (Fig. 6). This correlates with the proposal that the SoxX heme is the site of electron storage and transfer to the electron-transfer partner cytochrome c during the turnover of the enzyme. The exposed part of the SoxX heme cofactor is surrounded by a ring of hydrophobic surface residues, which is bordered by regions of negative potential (Fig. 6). The sequence of the putative electron acceptor for SnSoxAX, cytochrome c 550 from S. novella, predicts a basic pI (~8.5) for the protein, in addition, both the assay for the entire Sox system and the SoxAX assay can be carried out using horse heart cytochrome c (pI ~10) [49, 50]. It is likely that the region described represents a docking site between SoxAX and the external, electron accepting cytochrome c during electron transfer.
Fig. 6.
Dimeric structures of the SnSoxAX and RsSoxAX structures. a SnSoxAX; b RsSoxAX. For both structures, the SoxA subunit is shown as a surface representation in light blue. The SoxX subunit is shown as a cartoon in light green and the SoxX heme in orange. c and d Electrostatic surfaces of the SnSoxAX dimer. Regions of positive charge are coloured blue and regions of negative charge, red. Hydrophobic surfaces are represented in white. The heme cofactors are represented in orange. Panel d is related to c by a 90° rotation about the x axis
The SoxAX dimer
The structure of the SnSoxX protein (Type II) shows an N-terminal extension of the SnSoxX subunit by ~65 residues (residues B29-B95 in SnSoxX) that is not present in the RsSoxAX and PpSoxAX structures and is tethered to the heme-binding domain through a disulfide bridge (SnSoxX residues Cys B64–Cys B175) and three hydrogen-bonding interactions. The presence of this N-terminal extension correlates with the buried surface area in the dimer being significantly greater for the Type II protein than for the Type I proteins (19 and 16 % buried surface areas on complex formation for the Type II and Type I proteins, respectively). Presumably, this results in increased dimer stability for the Type II proteins (Fig. 6).
In all structures the SoxAX dimer features a deep trough along the SoxA protein, near the interface between subunits. The heme 2 cofactor and the active site Cys/CSS residue lie at the bottom of this trough. An electrostatic surface calculation indicates that this area is positively charged (Fig. 6). This region of the structure has been proposed to represent the docking site for the ‘swinging arm’ of the SoxYZ protein [35] and other substrates [45], such as thiosulfate, sulfide (HS−) and sulfite (HSO3 −) [35, 47]. Many of these substrates are negatively charged at neutral pH, so that the positively charged binding pocket would help to attract the substrate molecule.
Interestingly, a single residue (Gln 197 in SnSoxAX and Asp 192 in RsSoxAX) seems to ‘gate’ access to the active site (Fig. 5). In fact, the sequence alignments presented by Ogawa et al. [41], indicate that the identity of this residue is different, depending on the classification of the SoxAX protein: the sequences of the Type I proteins show an aspartate residue in this position, with glutamine and glutamic acid residues for the Type II and Type III proteins, respectively. The consequences of this observation for the activities and substrate specificities of these enzymes have not been investigated.
Properties of the SoxAX redox centres
An important parameter for the biological function of heme groups and their ability to participate in electron transfer or other reactions is their redox potential, which is influenced to a large extent by the axial ligands present [51]. The His/Cys axial ligation of the SoxA hemes confers an extremely low redox potential on these heme groups, with values of −432 ± 15 and −479 ± 10 mV versus NHE at pH 7.0 having been reported for the P. pantotrophus Type I and the S. novella Type II SoxAX proteins, respectively [20, 50]. The SoxX heme groups of these two proteins had potentials of 189 ± 15 and 183 ± 10 mV versus NHE at the same pH, which is typical for His/Met ligated heme groups [20, 50]. While for both proteins the SoxX heme potential was essentially invariant, the potential of the SoxA heme was reported to change by approximately 45 mV/pH unit for the P. pantotrophus SoxA heme, and a similar trend was observed for the S. novella protein [20, 50].
The low redox potential of the SoxA heme means that at physiological pH values, this heme group is very unlikely to participate in electron transfer reactions, i.e. the heme will not be reduced during the reaction catalyzed by the SoxAX proteins [20, 50]. This poses a potential problem, as the SoxAX reaction (Eq. 1) requires the transient storage of two electrons in the SoxAX redox centres, but only one of the two heme groups will be capable of storing electrons. As so far no evidence for the presence of a radical species in any of the SoxAX proteins has been found, this suggests that additional redox centres may be present/required for the SoxAX reaction. The S. novella SoxAX protein (Type II) has recently been shown to specifically bind 1 equivalent of Cu/protein molecule, and this Cu centre was reported to have a redox potential of +196 ± 18 mV versus NHE at pH 8.0 which is similar to the redox potential of the SoxX heme [50]. The exact location of the Cu centre is unknown at present and could not be determined from the crystal structure of the corresponding protein as only the ‘as prepared’ form of the protein gave rise to crystals with suitable diffraction properties [45]. However, the Cu centre appears to be located in the vicinity of the SoxA heme, as Cu-loading of the S. novella SoxAX caused changes in the EPR properties of the SoxA heme (see below) as well as to its redox potential: for Cu-loaded S. novella SoxAX the redox potential at pH 7 was −455 ± 10 mV versus NHE as opposed to −479 ± 10 mV versus NHE for the as prepared protein [50]. At present, however, the S. novella SoxAX protein is the only SoxAX protein that has been shown to bind Cu and thus to contain a non-heme redox centre and investigation of other SoxAX proteins for their ability to bind Cu or other metal centres will be required to confirm the general presence/role of a Cu centre in SoxAX cytochromes.
Spectroscopy of the SoxAX redox centres
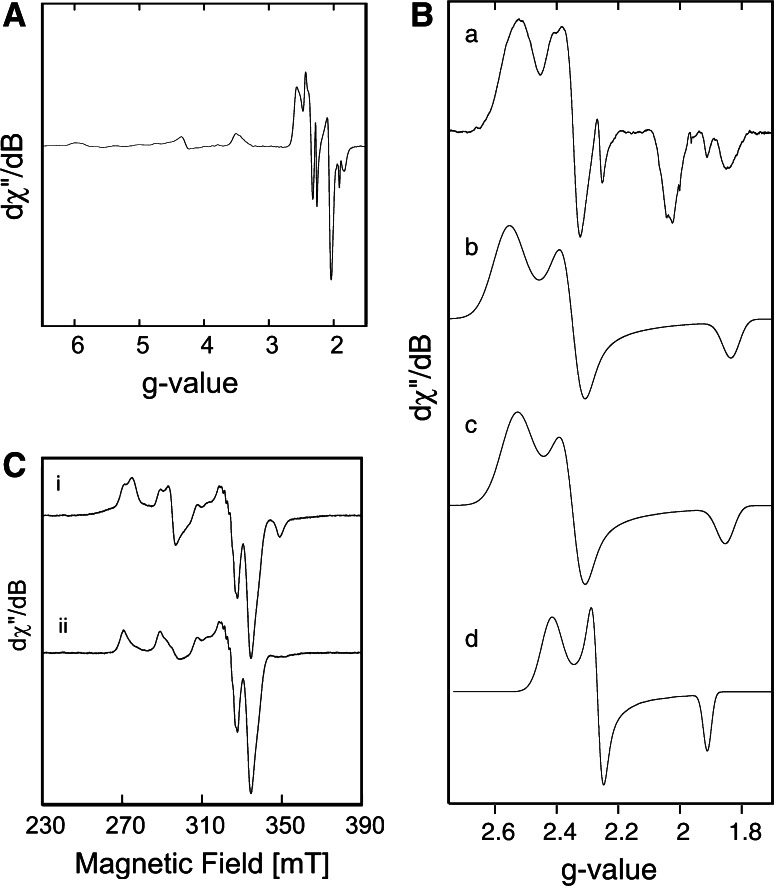
From the beginning the SoxAX cytochromes have been noted for the complexity of their EPR spectra. The first EPR spectra of a SoxAX cytochrome were published by Kelly [15], who identified several regions containing heme-dependent EPR signals for the SoxAX protein from Paracoccus versutus. These included a high spin signal (g ~ 6), some signals in the g ~ 3 region, that would most likely correspond to a His/Met ligated heme such as the one found in SoxX, and three additional, overlapping spectra in the g ~ 2 region. Kelly showed that following reduction of the protein with dithionite, only the signals in the g ~ 2 region remained, which is in keeping with the low redox potentials of the SoxA hemes that have been determined since then [20, 50]. Of the three heme-related features present in the g ~ 2 region, one did not undergo changes, while the relative proportions of the other two varied, e.g. in response to changes in the buffering system used [15] (Table 1). This led to the suggestion that the variable components were linked to the same heme group and would most likely arise from changes in the coordination environment of the central heme Fe atom. Kelly also concluded that at least one of the heme groups present in the P. versutus SoxAX protein might undergo a ligand switch from a six to a five coordinate state, giving rise to the observed high spin feature [15].
Table 1.
g-values associated with heme derived EPR species in different SoxAX cytochromes
| Heme | Type | g z | g x | g y | Ref |
|---|---|---|---|---|---|
| S. nov. rSoxAX LS1a | II | 1.859 | 2.531 | 2.349 | [53] |
| S. nov. rSoxAX LS1b | II | 1.835 | 2.574 | 2.348 | [53] |
| S. nov. rSoxAX LS2 | II | 1.913 | 2.433 | 2.271 | [53] |
| S. nov. rSoxAX LS3 | I | 3.502 | – | – | [53] |
| S. nov. SoxAX LS1a | II | 1.853 | 2.531 | 2.348 | [40] |
| S. nov. SoxAX LS1b | II | 1.835 | 2.556 | 2.348 | [40] |
| S. nov. SoxAX LS2 | II | 1.912 | 2.417 | 2.268 | [40] |
| S. nov. SoxAX LS3 | I | 3.502 | – | – | [40] |
| R. sulf. SoxAX LS1a | II | 1.870 | 2.580 | 2.300 | [52] |
| R. sulf. SoxAX LS1b | II | 1.840 | 2.520 | 2.230 | [52] |
| R. sulf. SoxAX LS2 | II | 1.910 | 2.420 | 2.260 | [52] |
| R. sulf. SoxAX LS3 | I | 3.50 | – | – | [52] |
| P. vers. SoxAX LS1 | II | 1.86 | 2.55 | 2.31 | [15] |
| P. vers. SoxAX LS2 | II | 1.89 | 2.43 | 2.27 | [15] |
| P. vers. SoxAX LS3 | I | 3.5 | – | – | [15] |
| P. vers.B SoxAX LS1a | II | 1.834 | 2.583 | 2.395 | [15] |
| P. vers.B SoxAX LS1b | II | 1.875 | 2.516 | 2.302 | [15] |
| P. vers.B SoxAX LS2 | II | 1.915 | 2.40 | 2.245 | [15] |
| P. vers.B SoxAX LS3 | I | 3.5 | – | – | [15] |
| P. panto. SoxAX LS1a | II | 1.87 | 2.54 | 2.3 | [20] |
| P. panto. SoxAX LS2 | II | 1.9 | 2.43 | 2.26 | [20] |
| P. panto. SoxAX LS3 | I | 3.45 | – | – | [20] |
| S. nov. C236 M 1 | II | 1.634 | 2.879 | 2.25 | [45] |
| S. nov. C236 M 2 | II | 1.379 | 2.979 | 2.3 | [45] |
| S. nov. C236 M 3 | I | 3.174 | – | – | [45] |
| S. nov. C236 M LS3 | I | 3.495 | – | – | [45] |
S. nov., Starkeya novella; R. sulf., Rhodovulum sulfidophilum; P. vers., Paracoccus versutus samples (no mark, B) for this organism differ in the buffering systems used; P. panto., Paracoccus pantotrophus
Subsequent studies of the EPR and MCD properties of the three heme, Type I SoxAX protein from R. sulfidophilum largely confirmed these findings and extended the analyses to an identification of the heme axial ligands through a combination of EPR and MCD spectroscopy [52]. In addition to a high spin signal at g ~ 5.8, the three heme groups of the R. sulfidophilum protein gave rise to four heme dependent EPR spectra, a readily reducible HALS (high anisotropy low spin) heme signal at g ~ 3.5 (LS3) and three additional heme species in the g ~ 2 region, that were shown to correspond to hemes with a histidine/thiolate axial ligation [52] (Table 1). In keeping with the suggestions of Kelly, it was postulated that the single heme group giving rise to two preparation-dependent EPR signals (LS1A and B), was likely to undergo a ligand switch [52]. The alternative ligand was suggested to be a cysteine-persulfide [52], which was later confirmed to exist in the crystal structure of the same protein [1]. The invariant signal in the g ~ 2 region (LS2) was thought to be linked to the second His/Cys ligated heme group present in the R. sulfidophilum diheme SoxA subunit [52].
The strong influence of the exact experimental conditions as well as the preparation method on the relative abundance of different EPR active forms of the SoxA thiolate ligated hemes was highlighted by studies of the triheme Type I SoxAX protein from P. pantotrophus, in which a high spin heme component was nearly absent, and the g ~ 2 region only contained two heme dependent spectra, LS1 and LS2 [20].
The assignment of the LS1 and LS2 components of the SoxA EPR spectra to the two heme groups present in the SoxA subunits of the triheme Type I SoxAX proteins, however, appears to be an oversimplification. EPR spectra of the diheme, Type II SoxAX protein from S. novella also contained EPR active components corresponding to LS1A and B, LS2 and LS3 [53] although this protein contains only a single active site SoxA heme group [40] (Fig. 7). If the LS2 spectrum was exclusively caused by the second SoxA heme it should have been absent from EPR spectra of a Type II SoxAX protein. This result clearly demonstrated that the active site SoxA heme group contributes not only to the LS1 but also the LS2 component of the EPR spectra, and indicated that this heme group can adopt a larger number of conformations than previously thought [40].
Fig. 7.
EPR spectra of the Type II SoxAX protein from Starkeya novella Panel a CW-EPR spectrum of S. novella SoxAX (50 mM, in 20 mM Tris–HCl, pH 8.0, T = 2 K), Panel b S. novella SoxAX CW-EPR signals in the g ~ 2 region (50 mM, in 20 mM Tris–HCl, pH 8.0, T = 2 K), a experimental spectrum, b,c,d simulations of LS1A, LS1B and LS2, respectively (adapted from [53]) Panel c Spectrum i, experimental CW-EPR spectrum of Cu(II)-loaded S. novella SoxAX, (T = 60.0 K), ii spectrum i corrected for heme dependent components (adapted from [50]
This conformational flexibility of the SoxA active site heme was also evident in EPR studies of a variant of the S. novella SoxAX protein (SnSoxAXC236M), in which the SoxA heme ligating cysteine was replaced by a methionine in an attempt to create a stable ligand environment around the SoxA heme [45]. Instead of the intended simplification of the SnSoxAXC236M EPR properties, the EPR spectra were found to be more complex, and contained four new signals (three in the g ~ 2 region, one in the g ~ 3 region) in addition to the LS3 SoxX heme signal and an intense high spin signal in the g ~ 6 region [45] (Table 1). The crystal structure of this protein showed that the heme ligating Met236 was in an unusual rotamer conformation, which would influence the EPR properties of the system due to changes in the orbital interactions between the Fe atom and the axial ligand [45]. The distances of the heme ligands to the central Fe atom also varied considerably (e.g. 2.56–2.9 Å for the Fe-Met bondlength) between the two SnSoxAXC236M structures present in one asymmetric unit [45], with a bondlength of 2.9 Å for the Fe-Met bond in one of these two structures essentially suggesting a heme group in transition to a high spin state. These variations were observed despite the fact that the amino acid substitution did not affect the structure of the backbone of the SnSoxAXC236M protein [45].
Together, all these observations indicate that the SoxA heme site has a significant inherent structural flexibility, which in turn gives rise to the observed microheterogeneity in the EPR spectra associated with this heme group [15, 20, 40, 45, 50, 52, 53]. The varying extents of flexibility observed for SoxAX proteins isolated from different species could either reflect differences in the preparation methods and buffering systems used, or might be caused by subtle changes in the protein environment of the SoxA active site heme although this is not apparent from the currently available crystal structures. Whether the structural flexibility of the SoxA site is important for catalysis and the proposed ligand exchange reaction that might underlie the formation of the heterodisulfide bond remains to be elucidated, although the structural flexibility may be necessary as SoxAX proteins are required to interact with a variety of substrates.
EPR studies of the Type II SoxAX Cu centre
Cu related resonances have been observed in the EPR spectra of as prepared SoxAX proteins from R. sulfidophilum, P. pantotrophus and S. novella, and in all cases were initially attributed to adventitiously bound Cu(II) [20, 40, 52]. However, following the observation that the S. novella SoxAX binds one equivalent of Cu per molecule EPR studies of the Cu centre in this protein were carried out [45, 50] and suggest that it is a tetragonally distorted, square planar Cu(II) centre [50]. Partially resolved nitrogen hyperfine resonances were also present, and the authors concluded that the Cu was most likely coordinated by three nitrogen and one oxygen ligand, or four nitrogen ligands [50] (Fig. 7). EPR spectra of Cu-loaded S. novella SoxAX showed line broadening for the LS2 species associated with the SoxA heme, indicating the presence of dipole–dipole interactions between the Cu centre and the SoxA heme, which would suggest a distance of 10–15 Å between the two redox centres [50]. Changing the axial ligand field of the SoxA heme, e.g. in the SoxAXC236M protein, resulted in changes of the EPR properties of the Cu centre, which was also taken as evidence that the two centres would most likely be located in close proximity [45]. The differences in the SnSoxAXWT and SnSoxAXC236M Cu EPR were attributed either to a change in the number of coordinated nitrogen nuclei or a change in the charge state of the Cu centre [50].
The most important issue regarding the SoxAX Cu centre at this stage is the identification of its exact location and the ligands involved in binding the Cu. Histidine residues are typical ligands for Cu centres, but the S. novella SoxA subunit contains only two His residues that are not axial ligands for heme groups, and only one of these His residues is located in proximity to the SoxA heme. While this histidine residue that could potentially be involved in binding the Cu centre is not conserved in the Type I SoxAX proteins, it is found in the majority of Type II SoxAX proteins that are currently in the database.
SoxAX activity measurements
The proposed reaction for SoxAX in all pathways in which it has been shown to play a role is the formation of a heterodisulfide bond between an incoming sulfur substrate molecule (e.g. thiosulfate) and the conserved cysteine residue present at the C-terminus of the SoxY subunit of the SoxYZ carrier protein (Eq. 1). Details of the reaction as well as the SoxAX reaction mechanism, however, remain to be elucidated. Efforts to study details of the SoxAX reaction have been hampered by the absence of a suitable assay for the SoxAX catalyzed reaction. Most assays of SoxAX activity have been carried out using a reconstituted Sox system, in which all essential Sox proteins are present in small amounts, and such assays have clearly shown that in photo- and chemotrophic sulfur oxidizers, the reaction catalyzed by SoxAX is essential for the function of the Sox pathway [41, 49] as in its absence only a residual amount of activity (~4.5 %) remains [49].
In some cases a reconstituted Sox system containing a mixture of components from a photo- and a chemotrophic bacterium representing the Dsr/Sox and the Sox pathway, respectively, may also be functional [54]. However, this depended strongly on which components of the system originated from the photo- or chemotroph: While the phototroph SoxYZ interacted well with the proteins derived from a chemotrophic bacterium, the SoxB protein from the phototrophic bacterium did not [54]. This work highlights the difficulties inherent in developing assays for the activities of individual Sox proteins–either purified samples of all the core Sox proteins from the same organism are required, or, in order to create a system that isolates a single reaction, large amounts of purified SoxYZ, which participates in all reactions of the Sox system as a substrate will be needed (Figs. 1, 2).
Some efforts have been made to create an in vitro assay system for SoxAX. The first assay system reported contained 20 mM of a suitable buffer (MES pH 6.0 or Tris–acetate pH 7.0), 0.04 mM cytochrome c from horse heart, a catalytic amount of purified SoxAX and 1 mM reduced glutathione [50]. In this assay, the reduced glutathione was proposed to take the place of both thiosulfate and SoxYZ as sulfur substrates (Eq. 2), and the reaction is thought to lead to the formation of oxidized glutathione and reduced cytochrome c, with the latter being monitored spectrophotometrically (Eq. 3). Combinations of GSH with, e.g. thiosulfate or other second sulfur substrates could not be used in this assay system due to high background activity being observed.
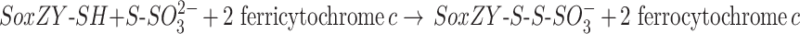 |
2 |
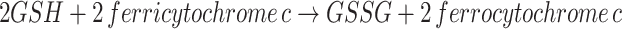 |
3 |
Using this system the activity of SoxAX from S. novella was assayed, and revealed K M_GSH values of 0.49 ± 0.12 mM and 0.195 ± 0.012 mM at pH 6 and 7, respectively (Table 2). The corresponding turnover numbers (k cat) were 8.72 ± 0.84 s−1 and 3.7 ± 0.25 s−1 [45, 50]. The assays were conducted using Cu-loaded SoxAX protein after it was observed that relative to the ‘as prepared’ protein, Cu-loaded protein had a 14 times increased activity in the assay system (1.54 U/mg vs. 0.124 U/mg for the as prepared protein) [50].
Table 2.
Activity of the Type II SoxAX protein from Starkeya novella in in vitro SoxAX activity assays
| S. novella SoxAXWT | S. novella SoxAXC236M | ||
|---|---|---|---|
| GSH-based assay, pH 7.0 | |||
| KM_GSH (mM) | 0.49 ± 0.12 | n.r. | [50] |
| k cat (s−1) | 8.72 ± 0.84 | n.r. | [50] |
| GSH-based assay, pH 6.0 | |||
| KM_GSH (mM) | 0.195 ± 0.012 | 0.228 ± 0.027 | [45] |
| k cat (s−1) | 3.7 ± 0.3 | 2.0 ± 0.5 | [45] |
| SoxYZ assay, pH 6 | |||
| U/mg | 0.165 ± 0.021 | 0.114 ± 0.022 | [45] |
n.r. not reported
A second assay system was also developed where thiosulfate and purified, recombinant SoxYZ were used as the sulfur substrates to mimic the in vivo reaction of SoxAX more closely. However, as SoxYZ proteins easily undergo oxidation and thus inactivation following purification [19], a major issue with such a system is how SoxYZ can be kept in a stable and active redox state, and how this redox state can be consistently reproduced. In vivo this is accomplished by the action of various accessory Sox proteins [25, 54]; for the in vitro assay the amount of free SH groups present on SoxYZ was used as a guide to the amount of ‘active’ SoxYZ in the preparation [45]. The limited availability of recombinant SoxYZ, however, precluded the determination of catalytic parameters using this assay system [45]. Using a tenfold excess of SoxYZ over SoxAX (4.86 μM and 0.048 μM concentrations were used, respectively), the activity of Cu-loaded S. novella wild type SoxAX was 0.165 ± 0.021 U/mg (Table 2).
The SoxAX reaction mechanism
Several proposals for the catalytic mechanism of SoxAX cytochromes have been made, and the suggestions vary largely depending on whether additional redox centres (such as a Cu centre) are thought to be present in the SoxAX protein or not.
The first proposal for a SoxAX reaction mechanism was made based on the crystal structure of the Type I SoxAX protein from R. sulfidophilum [1] and used elements of the reaction mechanisms of sulfur transferase/rhodanese enzymes as a model. Sulfurtransferases [EC 2.8.1.-] catalyze the transfer of a sulfane sulfur atom between substrate molecules.
The R. sulfidophilum SoxAX crystal structure revealed important similarities between the active sites of sulfur transferases and the one found in SoxAX, namely the presence of a strongly positively charged environment and a catalytically active cysteine residue, i.e. the cysteine ligand to the SoxA active site heme [1]. The fact that this heme group carries a cysteine-persulfide modification (which has also been identified in all subsequently solved structures of SoxAX proteins [45, 48]) was taken as evidence for its catalytic function; however, it was noted that a main difference between the sulfur transferase mechanism and that of SoxAX is that SoxAX is a redox active enzyme, while rhodaneses are non redox active enzymes that only perform a group transfer reaction [1]. An arginine (Arg218, R. sulfidophilum numbering) close to the SoxA active site heme and the cysteine ligand were proposed to be involved in orienting the incoming thiosulfate molecule so that the thiosulfate sulfane sulfur would be in close proximity to the catalytic cysteine residue [1]. Initially, a covalent bond would be formed between the cysteine and the thiosulfate molecule, leading to a two electron reduction of the SoxAX protein, and it was proposed that the two electrons liberated by the reaction would be stored in the SoxX and the SoxA active site heme [1], (hemes 2 and 3 in the nomenclature of [1]).
In a second reaction an incoming SoxYZ molecule would react with the SoxA-thiocysteine-S-sulfate complex, which could result either in a transfer of the entire thiosulfate moiety to SoxYZ, or could lead to an incomplete reaction where only the thiosulfate sulfone sulfur would be transferred, leaving the crystallographically observed persulfide modified cysteine which could be regenerated in a second reaction with another SoxYZ molecule [1].
The fact that the SoxA heme cysteine ligand is known to undergo modification on incubation with different sulfur substrates supports the suggestion that the heme-ligating cysteine is active in catalysis [53].
This is a very elegant suggestion for a potential reaction mechanism, however, it requires that the SoxA heme participates in the transient storage of one of the two electrons that are liberated during the formation of the disulfide bond, and as has subsequently been shown [20, 50], the extremely low redox potential of this heme group prevents it from storing electrons under physiological conditions.
This then leads to the question of what could be happening to the second electron liberated during heterodisulfide bond formation and how it could be stored in the SoxAX protein. The additional heme group that is present in Type I SoxAX proteins has the same axial ligation as the active site heme and thus will also have an extremely low redox potential that would stop it from acting as an electron sink even if it was located close enough to the active site to easily accept electrons which is not the case [1, 48].
Based on the observation that the S. novella Type II SoxAX protein is capable of binding exactly one equivalent of Cu and that Cu-loading of this protein resulted in changes to the EPR properties of the SoxA active site heme, it has been suggested that the Cu centre could be important in SoxAX catalysis [50]. The Cu atom would provide a redox centre capable of storing one electron and Cu atoms have also been noted for their reactivity towards sulfur compounds [55], which could enhance SoxAX catalysis. In the presence of a catalytically active Cu centre, the proposed SoxA thiocysteine–S-sulfate form of the protein might be an intermediate that would ‘trap’ thiosulfate inside the SoxA protein, ready for reaction with an incoming SoxYZ protein. Given that SoxYZ is a protein complex (~28 kDa) and thus interaction not only requires proximity to SoxAX but also the correct orientation of both proteins as well as a suitable position of the mobile GGCGG motif [35], it is to be expected that interactions with SoxYZ would form on a different timescale and be less frequent than interactions with a small molecule such as thiosulfate that can easily diffuse into the SoxAX active site. Thus, the interaction with SoxYZ might be a rate-limiting step in SoxAX catalysis. If the Cu centre were located within 10–15 Å of the SoxA active site heme it could be involved in promoting the formation of either modifications to the SoxA heme cysteine ligand and/or the subsequent reaction with the incoming SoxYZ protein.
This mechanism would also explain why in the GSH-based assay system the Cu-loaded S. novella SoxAX protein had a higher activity than the ‘as prepared’ SoxAX that only contained about 10–15 % Cu. Further evidence in favor of the involvement of the Cu centre comes from SoxAX activity assays with the already mentioned S. novella SoxAXC236M protein in which the cysteine heme ligand has been replaced. It was expected that if the cysteine were crucial to the reaction mechanism as it would be if no additional redox centres were involved in the reaction, this protein should have no catalytic activity. However, Cu–loaded SnSoxAXC236M was catalytically active although turnover was reduced by nearly 50 % (Table 2). Binding of the artificial sulfur substrate GSH was not significantly affected (K M_GSH values were similar for both SnSoxAXC236M and SoxAXWT protein). In the SoxYZ based assay, SnSoxAXC236M had ~70 % of the activity of the wild type enzyme (0.114 U/mg) when assayed under the same conditions as the wild type protein [45]. Together these observations suggest that the SoxA heme cysteine is important but not crucial for SoxAX activity and that the proposed Cu centre could be involved in speeding up the heterodisulfide bond formation [45].
Further work is clearly needed, however, to unravel the molecular details of the SoxAX reaction, including the exact location and properties of the Cu centre, its presence in other types of SoxAX proteins as well as experiments detailing the role of the active site arginine in catalysis and the actual products produced in a SoxYZ based assay system.
Concluding remarks
SoxAX cytochromes are a unique type of heme-containing enzymes that are essential for the bacterial oxidation of thiosulfate because they initiate the reaction of the Sox system in both photo- and chemotrophic sulfur oxidizing bacteria. They are found in nearly all known groups of bacteria, and additional forms of these proteins may be discovered as more genome sequences become available. The structural features underlying the formation of the SoxAX complex are worth investigating as in some cases stable complexes are formed between the SoxA and SoxX subunits while in other cases a third protein, SoxK (or SAXB) is required to achieve complex formation.
Features of central interest are the redox centres of SoxAX and how they shape the catalytic mechanism of these proteins. There is consensus regarding the nature of the reaction catalyzed by SoxAX, namely the formation of a heterodisulfide bond between the SoxYZ carrier protein and sulfur substrates such as thiosulfate. Details of the mechanism are unclear, however, partly due to the absence of an assay system that would be readily available and closely mimic the interactions of SoxAX with both its protein and its inorganic sulfur substrate. Another feature that requires further investigation to confirm or disprove its general role in sulfur oxidation is the Cu centre that appears to be present in the Type II SoxAX protein from S. novella. Further work should focus on establishing the presence of this redox centre in other SoxAX proteins and also aim to more clearly define the binding site required. If the SoxAX reaction mechanism would involve this Cu centre, the binding site would have to be capable of accommodating both Cu(I) and Cu(II), and it could also be expected to be conserved in other SoxAX proteins.
Another open issue is the exact role of the SoxAX active site heme and its cysteine ligand. The emerging picture is that the SoxA heme site, which is clearly implicated in catalysis is also a site of inherent structural flexibility that underlies the complexity of the EPR signals observed for all SoxAX proteins studied to date. Whether this flexibility is a prerequisite for catalysis is unknown, but it is possible that this is the feature enabling the formation of the high-spin heme signals that have been observed in EPR studies of many SoxAX proteins. Based on current knowledge it seems reasonable to suggest that if the heme-ligating cysteine is catalytically active it could at least temporarily cease to be a direct axial ligand to the SoxA heme.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1 Phylogenetic relationship of SoxA proteins. Two hundred sixteen SoxA amino acid sequences were analyzed using Mega 5.0. The tree shown was generated using the Neighbor-joining algorithm (Poisson model; uniform rate of evolution for all sites; gap treatment: pairwise-deletion; robustness testing: bootstrap method with 500 resampling cycles). This figure shows a tree that is identical to that shown in Fig. 3 but all branches are fully expanded. (PDF 58 kb)
Acknowledgments
This work was supported by a fellowship (Australian Research Fellowship, DP0878525) from the Australian Research Council to UK. MJM is supported by a La Trobe Institute for Molecular Science Senior Research Fellowship.
References
- 1.Bamford VA, Bruno S, Rasmussen T, Appia-Ayme C, Cheesman MR, Berks BC, Hemmings AM. Structural basis for the oxidation of thiosulfate by a sulfur cycle enzyme. EMBO J. 2002;21(21):5599–5610. doi: 10.1093/emboj/cdf566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu WP, Kelly DP. Respiration-driven proton translocation in Thiobacillus versutus and the role of the periplasmic thiosulphate-oxidizing enzyme system. Arch Microbiol. 1988;149:297–302. doi: 10.1007/BF00411645. [DOI] [Google Scholar]
- 3.Omura T. Heme-thiolate proteins. Biochem Biophys Res Comm. 2005;338(1):404–409. doi: 10.1016/j.bbrc.2005.08.267. [DOI] [PubMed] [Google Scholar]
- 4.Green MT. CH bond activation in heme proteins: the role of thiolate ligation in cytochrome P450. Curr Opin Chem Biol. 2009;13(1):84–88. doi: 10.1016/j.cbpa.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Pazicni S, Cherney MM, Lukat-Rodgers GS, Oliveriusova J, Rodgers KR, Kraus JP, Burstyn JN. The heme of cystathionine beta-synthase likely undergoes a thermally induced redox-mediated ligand switch. Biochemistry. 2005;44(51):16785–16795. doi: 10.1021/bi051305z. [DOI] [PubMed] [Google Scholar]
- 6.Youn H, Conrad M, Chung SY, Roberts GP. Roles of the heme and heme ligands in the activation of CooA, the CO-sensing transcriptional activator. Biochem Biophys Res Comm. 2006;348(2):345–350. doi: 10.1016/j.bbrc.2006.06.200. [DOI] [PubMed] [Google Scholar]
- 7.Dhawan IK, Shelver D, Thorsteinsson MV, Roberts GP, Johnson MK. Probing the heme axial ligation in the CO-sensing CooA protein with magnetic circular dichroism spectroscopy. Biochemistry. 1999;38(39):12805–12813. doi: 10.1021/bi991303c. [DOI] [PubMed] [Google Scholar]
- 8.Alric J, Tsukatani Y, Yoshida M, Matsuura K, Shimada K, Hienerwadel R, Schoepp-Cothenet B, Nitschke W, Nagashima KVP, Vermeglio A. Structural and functional characterization of the unusual triheme cytochrome bound to the reaction center of Rhodovulum sulfidophilum . J Biol Chem. 2004;279(25):26090–26097. doi: 10.1074/jbc.M400361200. [DOI] [PubMed] [Google Scholar]
- 9.Grein F, Venceslau SS, Schneider L, Hildebrandt P, Todorovic S, Pereira IAC, Dahl C. DsrJ, an essential part of the DsrMKJOP transmembrane complex in the purple sulfur bacterium Allochromatium vinosum, is an unusual triheme cytochrome c . Biochemistry. 2010;49(38):8290–8299. doi: 10.1021/bi1007673. [DOI] [PubMed] [Google Scholar]
- 10.Grein F, Pereira IAC, Dahl C. Biochemical characterization of individual components of the Allochromatium vinosum DsrMKJOP transmembrane complex aids understanding of complex function in vivo. J Bacteriol. 2010;192(24):6369–6377. doi: 10.1128/JB.00849-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu WP, Swoboda BEP, Kelly DP. Properties of the thiosulphate oxidising multi-enzyme system from Thiobacillus versutus . Biochim Biophys Acta. 1985;828:116–122. doi: 10.1016/0167-4838(85)90046-9. [DOI] [Google Scholar]
- 12.Lu WP, Kelly DP. Properties and role of sulphite:cytochrome c oxidoreductase purified from Thiobacillus versutus . J Gen Microbiol. 1984;130:1683–1692. [Google Scholar]
- 13.Lu W-P, Kelly DP. Purification and characterization of two essential cytochromes of the thiosulphate-oxidizing multi-enzyme system from Thiobacillus A2 (Thiobacillus versutus) Biochim Biophys Acta. 1984;765(2):106–117. doi: 10.1016/0005-2728(84)90003-3. [DOI] [Google Scholar]
- 14.Katayama Y, Hiraishi A, Kuraishi H. Paracoccus thiocyanatus sp. nov., a new species of thiocyanate- utilizing facultative chemolithotroph, and transfer of Thiobacillus versutus to the genus Paracoccus as Paracoccus versutus comb. nov. with emendation of the genus. Microbiology. 1995;141:1469–1477. doi: 10.1099/13500872-141-6-1469. [DOI] [PubMed] [Google Scholar]
- 15.Kelly DP, Shergill JK, Lu WP, Wood AP. Oxidative metabolism of inorganic sulfur compounds by bacteria. Antonie Van Leeuwenhoek. 1997;71(1–2):95–107. doi: 10.1023/A:1000135707181. [DOI] [PubMed] [Google Scholar]
- 16.Mittenhuber G, Sonomoto K, Egert M, Friedrich CG. Identification of the DNA region responsible for sulfur-oxidizing ability of Thiosphaera pantotropha . J Bacteriol. 1991;173(2):7340–7344. doi: 10.1128/jb.173.22.7340-7344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wodara C, Kostka S, Egert M, Kelly DP, Friedrich CG. Identification and sequence analysis of the soxB gene essential for sulfur oxidation of Paracoccus denitrificans GB17. J Bacteriol. 1994;176:6188–6191. doi: 10.1128/jb.176.20.6188-6191.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig W, Mittenhuber G, Friedrich CG. Transfer of Thiosphaera pantotropha to Paracoccus denitrificans . Int J Sys Bacteriol. 1993;43(2):363–367. doi: 10.1099/00207713-43-2-363. [DOI] [PubMed] [Google Scholar]
- 19.Quentmeier A, Li L, Friedrich CG. Identification of two inactive forms of the central sulfur cycle protein SoxYZ of Paracoccus pantotrophus . FEBS Lett. 2008;582(25–26):3701–3704. doi: 10.1016/j.febslet.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 20.Reijerse EJ, Sommerhalter M, Hellwig P, Quentmeier A, Rother D, Laurich C, Bothe E, Lubitz W, Friedrich CG. The unusual redox properties of SoxXA, a novel c-type heme-enzyme essential for chemotrophic sulfur-oxidation of Paracoccus pantotrophus . Biochemistry. 2007;46(26):7804–7810. doi: 10.1021/bi7003526. [DOI] [PubMed] [Google Scholar]
- 21.Bardischewsky F, Quentmeier A, Friedrich CG. The flavoprotein SoxF functions in chemotrophic thiosulfate oxidation of Paracoccus pantotrophus in vivo and in vitro. FEMS Microbiol Lett. 2006;258(1):121–126. doi: 10.1111/j.1574-6968.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 22.Bardischewsky F, Quentmeier A, Rother D, Hellwig P, Kostka S, Friedrich CG. Sulfur dehydrogenase of Paracoccus pantotrophus : the heme-2 domain of the molybdoprotein cytochrome c complex is dispensable for catalytic activity. Biochemistry. 2005;44(18):7024–7034. doi: 10.1021/bi047334b. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich CG, Rother D, Bardischewsky F, Quentmeier A, Fischer J. Oxidation of reduced inorganic sulfur compounds by bacteria: emergence of a common mechanisms? Appl Environ Microbiol. 2001;67(7):2873–2882. doi: 10.1128/AEM.67.7.2873-2882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quentmeier A, Kraft R, Kostka S, Klockenkamper R, Friedrich CG. Characterization of a new type of sulfite dehydrogenase from Paracoccus pantotrophus GB17. Arch Microbiol. 2000;173(2):117–125. doi: 10.1007/s002039900118. [DOI] [PubMed] [Google Scholar]
- 25.Rother D, Ringk J, Friedrich CG. Sulfur oxidation of Paracoccus pantotrophus: the sulfur-binding protein SoxYZ is the target of the periplasmic thiol-disulfide oxidoreductase SoxS. Microbiology. 2008;154:1980–1988. doi: 10.1099/mic.0.2008/018655-0. [DOI] [PubMed] [Google Scholar]
- 26.Bardischewsky F, Fischer J, Holler B, Friedrich CG. SoxV transfers electrons to the periplasm of Paracoccus pantotrophus—an essential reaction for chemotrophic sulfur oxidation. Microbiology. 2006;152:465–472. doi: 10.1099/mic.0.28523-0. [DOI] [PubMed] [Google Scholar]
- 27.Rother D, Orawski G, Bardischewsky F, Friedrich CG. SoxRS-mediated regulation of chemotrophic sulfur oxidation in Paracoccus pantotrophus . Microbiology. 2005;151:1707–1716. doi: 10.1099/mic.0.27724-0. [DOI] [PubMed] [Google Scholar]
- 28.Bardischewsky F, Friedrich CG. Identification of ccdA in Paracoccus pantotrophus GB17: disruption of ccdA causes complete deficiency in c-type cytochromes. J Bacteriol. 2001;183(1):257–263. doi: 10.1128/JB.183.1.257-263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardischewsky F, Friedrich CG. The shxVW locus is essential for oxidation of inorganic sulfur and molecular hydrogen by Paracoccus pantotrophus GB17: a novel function for lithotrophy. FEMS Microbiol Lett. 2001;202:215–220. doi: 10.1111/j.1574-6968.2001.tb10806.x. [DOI] [PubMed] [Google Scholar]
- 30.Friedrich CG, Bardischewsky F, Rother D, Quentmeier A, Fischer J. Prokaryotic sulfur oxidation. Curr Opin Microbiol. 2005;8(3):253–259. doi: 10.1016/j.mib.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Verte F, Kostanjevecki V, De Smet L, Meyer TE, Cusanovich MA, Van Beeumen JJ. Identification of a thiosulfate utilization gene cluster from the green phototrophic bacterium Chlorobium limicola . Biochemistry. 2002;41(9):2932–2945. doi: 10.1021/bi011404m. [DOI] [PubMed] [Google Scholar]
- 32.Klarskov K, Verte F, VanDriessche G, Meyer TE, Cusanovich MA, Van Beeumen J. The primary structure of soluble cytochrome c 551 from the photographic green sulfur bacterium Chlorobium limicola, strain Tassajara, reveals a novel c-type cytochrome. Biochemistry. 1998;37(30):10555–10562. doi: 10.1021/bi9806706. [DOI] [PubMed] [Google Scholar]
- 33.Frigaard NU, Dahl C, Robert KP (2008) Sulfur metabolism in phototrophic sulfur bacteria. In: Advances in Microbial Physiology, vol 54. Academic Press, pp 103–200 [DOI] [PubMed]
- 34.Hensen D, Sperling D, Truper HG, Brune DC, Dahl C. Thiosulphate oxidation in the phototrophic sulphur bacterium Allochromatium vinosum . Mol Microbiol. 2006;62(3):794–810. doi: 10.1111/j.1365-2958.2006.05408.x. [DOI] [PubMed] [Google Scholar]
- 35.Sauve V, Bruno S, Berks BC, Hemmings AM. The SoxYZ complex carries sulfur cycle intermediates on a peptide swinging arm. J Biol Chem. 2007;282(32):23194–23204. doi: 10.1074/jbc.M701602200. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh W, Roy P. Chemolithoautotrophic oxidation of thiosulfate, tetrathionate and thiocyanate by a novel rhizobacterium belonging to the genus Paracoccus . FEMS Microbiol Lett. 2007;270(1):124–131. doi: 10.1111/j.1574-6968.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 37.Sauve V, Roversi P, Leath KJ, Garman EF, Antrobus R, Lea SM, Berks BC. Mechanism for the hydrolysis of a sulfur–sulfur bond based on the crystal structure of the thiosulfohydrolase SoxB. J Biol Chem. 2009;284(32):21707–21718. doi: 10.1074/jbc.M109.002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zander U, Faust A, Klink BU, de Sanctis D, Panjikar S, Quentmeier A, Bardischewsky F, Friedrich CG, Scheidig AJ. Structural basis for the oxidation of protein-bound sulfur by the sulfur cycle molybdohemo-enzyme sulfane dehydrogenase SoxCD. J Biol Chem. 2011;286(10):8349–8360. doi: 10.1074/jbc.M110.193631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frigaard NU, Dahl C. Sulfur metabolism in phototrophic sulfur bacteria. Adv Microb Phys. 2009;54:103–200. doi: 10.1016/S0065-2911(08)00002-7. [DOI] [PubMed] [Google Scholar]
- 40.Kappler U, Aguey-Zinsou KF, Hanson GR, Bernhardt PV, McEwan AG. Cytochrome c551 from Starkeya novella: characterization, spectroscopic properties, and phylogeny of a diheme protein of the SoxAX family. J Biol Chem. 2004;279(8):6252–6260. doi: 10.1074/jbc.M310644200. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa T, Furusawa T, Nomura R, Seo D, Hosoya-Matsuda N, Sakurai H, Inoue K. SoxAX binding protein, a novel component of the thiosulfate-oxidizing multienzyme system in the green sulfur bacterium Chlorobium tepidum . J Bacteriol. 2008;190(18):6097–6110. doi: 10.1128/JB.00634-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orawski G, Bardischewsky F, Quentmeier A, Rother D, Friedrich CG. The periplasmic thioredoxin SoxS plays a key role in activation in vivo of chemotrophic sulfur oxidation of Paracoccus pantotrophus . Microbiology. 2007;153:1081–1086. doi: 10.1099/mic.0.2006/004143-0. [DOI] [PubMed] [Google Scholar]
- 43.Gregersen LH, Bryant DA, Frigaard N-U. Mechanisms and evolution of oxidative sulfur metabolism in green sulfur bacteria. Frontiers Microbiol. 2011;2:116. doi: 10.3389/fmicb.2011.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakurai H, Ogawa T, Shiga M, Inoue K. Inorganic sulfur oxidizing system in green sulfur bacteria. Photosynth Res. 2010;104(2–3):163–176. doi: 10.1007/s11120-010-9531-2. [DOI] [PubMed] [Google Scholar]
- 45.Kilmartin JR, Maher MJ, Krusong K, Noble CJ, Hanson GR, Bernhardt PV, Riley MJ, Kappler U. Insights into structure and function of the active site of SoxAX cytochromes. J Biol Chem. 2011;286(28):24872–24881. doi: 10.1074/jbc.M110.212183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rother D, Henrich HJ, Quentmeier A, Bardischewsky F, Friedrich CG. Novel genes of the sox gene cluster, mutagenesis of the flavoprotein SoxF, and evidence for a general sulfur-oxidizing system in Paracoccus pantotrophus GB17. J Bacteriol. 2001;183(15):4499–4508. doi: 10.1128/JB.183.15.4499-4508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dambe T, Quentmeier A, Rother D, Friedrich C, Scheidig AJ. Structure of the cytochrome complex SoxXA of Paracoccus pantotrophus, a heme enzyme initiating chemotrophic sulfur oxidation. J Struct Biol. 2005;152(3):229–234. doi: 10.1016/j.jsb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Friedrich CG, Quentmeier A, Bardischewsky F, Rother D, Kraft R, Kostka S, Prinz H. Novel genes coding for lithotrophic sulfur oxidation of Paracoccus pantotrophus GB17. J Bacteriol. 2000;182(17):4677–4687. doi: 10.1128/JB.182.17.4677-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kappler U, Bernhardt PV, Kilmartin J, Riley MJ, Teschner J, McKenzie KJ, Hanson GR. SoxAX cytochromes, a new type of heme copper protein involved in bacterial energy generation from sulfur compounds. J Biol Chem. 2008;283(32):22206–22214. doi: 10.1074/jbc.M800315200. [DOI] [PubMed] [Google Scholar]
- 51.Frausto da Silva JJR, Williams RJP. The biological chemistry of the elements—the inorganic chemistry of life. Oxford: Oxford University Press; 2001. [Google Scholar]
- 52.Cheesman MR, Little PJ, Berks BC. Novel heme ligation in a c-type cytochrome involved in thiosulfate oxidation: EPR and MCD of SoxAX from Rhodovulum sulfidophilum . Biochemistry. 2001;40:10562–10569. doi: 10.1021/bi0100081. [DOI] [PubMed] [Google Scholar]
- 53.Kappler U, Hanson GR, Jones A, McEwan AG. A recombinant diheme SoxAX cytochrome-implications for the relationship between EPR signals and modified heme-ligands. FEBS Lett. 2005;579:2491–2498. doi: 10.1016/j.febslet.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 54.Welte C, Hafner S, Kratzer C, Quentmeier A, Friedrich CG, Dahl C. Interaction between Sox proteins of two physiologically distinct bacteria and a new protein involved in thiosulfate oxidation. FEBS Lett. 2009;583(8):1281–1286. doi: 10.1016/j.febslet.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 55.Multhaup G, Schlicksupp A, Hesse L, Beher D, Ruppert T, Masters CL, Beyreuther K. The amyloid precursor protein of Alzheimer’s disease in the reduction of copper(II) to copper(I) Science. 1996;271(5254):1406–1409. doi: 10.1126/science.271.5254.1406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Phylogenetic relationship of SoxA proteins. Two hundred sixteen SoxA amino acid sequences were analyzed using Mega 5.0. The tree shown was generated using the Neighbor-joining algorithm (Poisson model; uniform rate of evolution for all sites; gap treatment: pairwise-deletion; robustness testing: bootstrap method with 500 resampling cycles). This figure shows a tree that is identical to that shown in Fig. 3 but all branches are fully expanded. (PDF 58 kb)