Abstract
Crohn’s disease (CD) is one of main disease entities under the umbrella term chronic inflammatory bowel disease. The etiology of CD involves alterations in genetic, microbiological, and immunological factors. This review is devoted to the role of the bacterial wall compound muramyl dipeptide (MDP) for the activation of inflammatory pathways involved in the pathogenesis of CD. The importance of this molecule is underscored by the fact that (1) MDP, which is found in most Gram-negative and -positive bacteria, is able to trigger several immunological responses in the intestinal system, and (2) that alterations in several mediators of the MDP response including—but not restricted to—nucleotide oligomerization domain 2 (NOD2) are associated with CD. The normalization of MDP signaling is one of several important factors that influence the intestinal inflammatory response, a fact which emphasizes the pathogenic importance of MDP signaling for the pathogenesis of CD. The important aspects of NOD2 and non-NOD2 mediated effects of MDP for the development of CD are highlighted, as well as how alterations in these pathways might translate into the development of new therapeutic strategies.
Keywords: ATG16L1, Autophagy, Crohn's disease (CD), Inflammatory bowel disease (IBD), Innate lymfoid cells, Inflammasome, Muramyl dipeptide (MDP), NF-κB, Nucleotide oligomerization domain 2, Paneth cells, Peptidoglycan, Toll-like receptor
Introduction
The innate immune system is the first line of defense in the intestinal tract, and it possesses a unique feature in recognizing microbial components. The pathogens are detected by pattern recognition receptors (PRRs), which recognize a wide array of conserved structures of microorganisms, called the pathogen-associated molecular patterns (PAMPs). The PRRs sense specific viral and bacterial structures, and subsequently initiate various immunological responses. PRRs are located on either the cell membrane surface, e.g., Toll-like receptors (TLRs), or inside the cytoplasm, e.g., NOD-like receptors (NLRs) [1].
Muramyl dipeptide (MDP) is an immunoreactive peptide found in the peptidoglycan (PGN) motif which encodes “the building blocks” of bacterial cell walls [2]. MDP can activate several immunological signaling pathways, including the nucleotide oligomerization domain 2 (NOD2, a NLR member) dependent pathway via specific interaction between NOD2 and MDP, resulting in activation of nuclear factor-κB (NF-κB), a ubiquitous transcription factor which induces expression of pro-inflammatory cytokines [2]. Multiple studies have linked NOD2 to severe immunological dysfunctions such as graft-versus-host disease [3], enhanced mortality during sepsis [4, 5], and Crohn’s disease (CD) [6, 7], indicating that MDP might play a pathophysiological role in these disorders. The NOD2 gene, which initially was identified as NOD1-related gene, was found to have a highly restricted expression in monocytes [8]. The major effects of NOD2 in the pathogenesis of CD were later revealed by Hugot et al. [6] and Ogura et al. [7], who identified three disease-linked polymorphisms in the NOD2 gene. As described below, MDP also affects PRRs other than NOD2.
CD is one of the two main entities of inflammatory bowel disease (IBD), potentially affecting any part of the gastrointestinal tract; however, most commonly the terminal ileum or the perianal region [9, 10]. The etiology of IBD is still unknown, but considering epidemiological, genetic and immunological data together, IBD has a multifactorial etiology in which genetic alternations and environmental factors (i.e. microbial behavior) interact to produce the immunological background of the disease [11]. Accordingly, it is becoming increasingly evident that an impaired innate immunity plays a crucial role in CD [12].
In this review, the recent studies on MDP-mediated pathways in the innate immunity of CD are summarized, covering both NOD2-dependent and NOD2-independent pathways. As described in detail below, MDP signaling is impaired on different levels in CD, and animal studies suggest that reversal of these pathways reduces intestinal inflammation. Revealing such mechanisms might lead to identification of new potential therapeutic approaches of this devastating disorder.
MDP intracellular delivery
In most Gram-negative and -positive bacteria, the PGN represents a key component of the cell wall with an important impact on maintaining the structural integrity of plasma membranes and anchoring cell components such as lipoproteins [2]. The pivotal role of PGN in the bacterial pathogenesis is clearly highlighted by the fact that the most frequently used antibiotics are targeting the PGN biosynthesis pathway [13]. MDP is a cleavage product, and the smallest bioactive motif of PGN that specifically binds to NOD2, and it leads to an activation of NOD2-mediated signal pathways and other pathways [14]. In general, the structure of PGN contains a repetition of disaccharides (glycans) cross-linked by short chains of amino acids (peptides) to form a lattice surrounding the entire cell. MDP consists of N-acetylmuramic acid linked to the two amino acids: D-Ala and D-isoGln (or D-Glu) [15]. The structure of MDP is unique in the way that MDP analogues composed of other amino acid lead to a reduced or inhibited ability to induce a biological response [2, 16].
There is accumulating evidence suggesting that NOD2 might be a selective bacterial sensor due to the fact that N-acetyl (A)-MDP, which is found in most bacteria, less efficiently activates NOD2 than N-glycolyl (G)-MDP—which is found in mycobacteria and related Actinomycetes species [17]. This is of interest since CD has been associated with mycobacteria infections [18]. Accordingly, Coulombe et al. [17] showed that G-MDP was more efficacious than A-MDP at inducing ovalbumin-specific T cell immunity in a model of adjuvancy. Further, G-MDP has been found to be more potent than A-MDP in NOD2-mediated activation of NF-κB, as well as c-Jun N-terminal kinase (JNK) [17]. A recent in vivo experiment has supported this finding in a setting where intravenously administrated MDP showed protective antiviral activity in mice exposed for influenza A virus (IAV). In this model, G-MDP showed more potency than A-MDP and with a greater antiviral activity against IAV [19].
In both Gram-negative and -positive bacteria, the PGN has to be cleaved during cell growth by autolysis which allows attaching/inserting of new material into the existing cell wall. The loss of PGN resulting from this cleavage is termed “PGN turnover”. However, these turnover products are normally captured and reutilized by the cell in another process named “PGN recycling”, which is typically observed in studies of Gram‐negative bacteria, e.g., Escherichia coli [20]. Whether PGN is also recycled in Gram-positive bacteria is basically unknown.
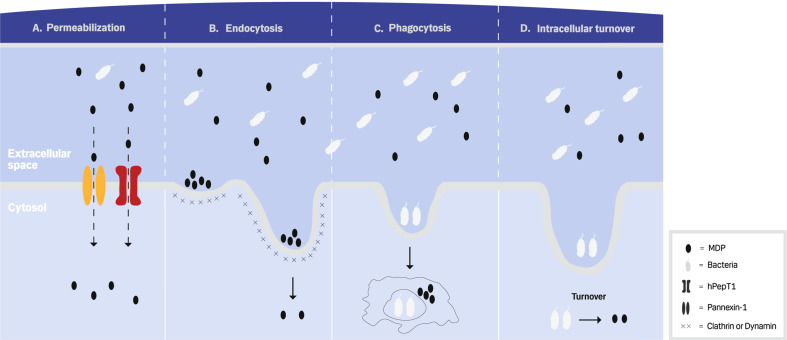
A number of studies have focused on identifying the mechanisms by which the extracellular NOD2 ligands reach the cytosolic receptor and trigger downstream effector functions (Fig. 1). The extracellular MDP can get access to the host cytosol through several ways. First, MDP can be taken up by the cell via membrane protein transporters, human peptide transporter 1 (hPepT1) [21] and pannexin-1 (Fig. 1a) [22]. Inhibition of these transporters leads to reduced localization of MDP from the extracellular space to the cytosol [22, 23]. Although hPepT1 is expressed in monocytes and human enterocyte cell lines like HT-29 cells, most protocols for stimulating primary cells with MDP rely on artificial permeabilization of the cell membranes with liposome forming reagents like lipofectamine, suggesting that the hPepT1 system might be of limited efficiency in human cells [24]. Second, MDP similar to lipopolysaccharide (LPS) may use a clathrin- and dynamin-dependent endocytic pathway to cross the host cell membrane (Fig. 1b) [25–27]. Clathrin and dynamin mediate a vesicle formation at the cell membrane, and an inhibition of clathrin or dynamin also leads to attenuated MDP-mediated signaling pathways [25]. The mechanism by which MDP is targeted to clathrin-coated pits for endocytosis remains unclear. Typically, endocytosis is thought to rely on adaptor transmembrane proteins, such as adaptor protein 2 (AP2), to mediate the recruiting process to coated pits by interaction to clathrin adaptors [28]. Third, it has been shown that phagocytic cells, interacting with the epithelium breaching microorganisms and microbial components, presents a key mechanism in allowing components of microorganisms to be exposed to intracellular receptors: Macrophages may generate such microbial components by ingesting whole bacteria and subsequently digesting them in the phagolysosomes (Fig. 1c) [29, 30]. Thus, Herskovits et al. [29] have shown that NOD2 is activated in stimulated macrophages by bacterial ligands generated in the phagosome and transported to the cytosol [29]. Finally, PGN containing MDP can be delivered into the cytosol by the previously mentioned intracellular PGN turnover after internalization of invasive bacteria into the cells (Fig. 1d) [31].
Fig. 1.
Mechanisms of MDP intracellular delivery. a Extracellular MDP can permeabilize the host cell through membrane receptors such as hPepT1 and Pennexin-1. b Extracellular MDP can also be transported into the host cell by clathrin- and dynamin-dependent endocytic pathways. c Moreover, MDP can be derived from bacteria by the intracellular phagocytic cleavage. d Finally, MDP can additionally be released to the cytosol through autolysis of internalized of invasive bacteria
MDP-NOD2-mediated pathway
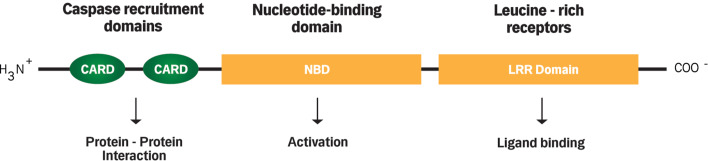
The NOD2 protein is located in the cytosol and is broadly expressed in macrophages, dendritic cells, and to a lower degree in intestinal epithelial cells (IECs), including Paneth cells of the small bowel [32]. The structure of NOD2 consists of two N-terminal caspase recruitment domains (CARDs), a centrally located nucleotide-binding domain (NBD), a C-terminal of site of ligand binding (e.g., MDP), and a leucine-rich repeat region (LRR) (Fig. 2) [8]. MDP binds to the LRR of NOD2, which subsequently activates NF-κB and mitogen-activated protein kinases (MAPK) [33]. When activated, NF-κB translocates into the nucleus to initiate cell-specific genetic programs, such as immune activation, inflammation, and cell development [34, 35]. Recent studies have shown that the intestinal MDP–NOD2 pathway is tightly controlled by epithelium-associated lymphocytes, which specifically cleave the NOD2-detected MDP structure [36, 37]. Pro-inflammatory mediators produced by T helper 1 (Th1) cells, i.e. interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), upregulate the expression of NOD2 in IECs [38]. This reflects the importance of NOD2 in the maintenance of the intestinal mucosal homeostasis, which actually links the innate and adaptive immunity.
Fig. 2.
Structure of NOD2. The structure of NOD2 consists of two N-terminal caspase recruitment domains (CARDs) which mediate protein–protein interactions, a centrally located nucleotide-binding domain (NBD) which mediates protein self-oligomerization required for activation, and C-terminal of site of ligand binding, i.e. leucine-rich repeats (LRRs)
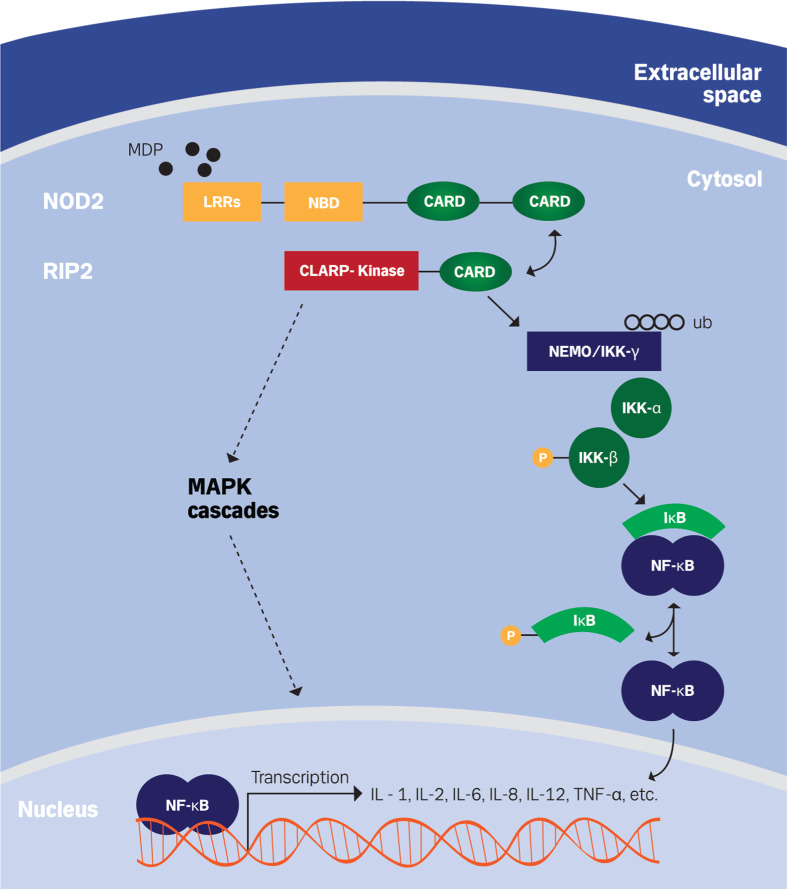
The specificity of interaction between MDP and NOD2 is not completely understood. However, a recent study found that MDP binds directly to NOD2 with high affinity at a pH range of 5.0–6.5, compared with a pH range of 7.0–7.5 [39], which is the estimation of human cytosolic pH [40]. A pH-dependent affinity of MDP–NOD2 interaction lines with earlier observations suggested that the internalization of MDP is optimal in the pH range from 5.5 to 6.5 [26]. MDP binding onto NOD2 causes a conformational change allowing a CARD–CARD interaction between NOD2 and RIP2 (receptor-interacting protein 2), a CARD-containing serine-threonine protein kinase [41, 42]. The activation of RIP2 results in polyubiquitination of NEMO (NF-κB essential modifier), also named IKKγ (inhibitor of IκB kinase γ), which is found in complex with IKKα and IKKβ [43, 44]. IKKγ polyubiquitylation leads to phosphorylation of IKKβ, which in turn phosphorylates IκB (inhibitor of NF-κB) resulting in dissociation of IκB from NF-κB. Subsequently NF-κB can translocate into the nucleus where it promotes transcription of several pro-inflammatory and anti-inflammatory cytokines, such as interleukin (IL)-1, IL-2, IL-6, IL-8, IL-12, and TNF-α [32, 33, 45, 46] (Fig. 3).
Fig. 3.
Signaling pathway of NOD2. Recognition of MDP through LRRs domain activates NOD2 and causes a conformational change, allowing a CARD–CARD interaction between NOD2 and RIP2, receptor-interacting protein 2 (also known as caspase like regulatory protein (CLARP) kinase). Activation of RIP2 upon binding with NOD2 results in activation of MAPK and NF-κB signaling pathways. RIP2 induces polyubiquitination (ub) of NEMO, NF-κB essential modifier (or IKKγ, inhibitor of IκB kinase γ), which is found in complex with IKKα and IKKβ. This is followed by the phosphorylation of IKKβ as well as the phosphorylation of IκB, inhibitor of NF-κB, and the release of NF-κB. NF-κB can subsequently translocate into the nucleus and promote transcription of many pro-inflammatory and anti-inflammatory cytokines, including interleukin-1 (IL-1), IL-2, IL-6, IL-8, IL-12, and TNF-α
NF-κB can additionally be activated by another pathway, known as “the alternative pathway”, which has been classified according to the complex structure of NF-κB [35, 47]. The alternative pathway is, however, activated with much slower kinetics than the classical pathway [48]: The alternative NF-κB pathway is linked to the adaptive immune system, and is involved in the development of lymphoid organs by regulating the generation of B and T lymphocytes [35]. This pathway is activated by a small number of stimuli, including lymphotoxins (α and β), B cell activating factor (BAFF), receptor activator of nuclear factor kappa-B ligand (RANKL), and NOD2 [35, 48, 49]. The alternative pathway induces NF-κB-inducing kinase (NIK) instead of NEMO to phosphorylate and activate the IKK complex, which facilitates induction of NF-κB dimers [34, 35]. The alternative pathway is a prerequisite for the development of secondary lymphoid tissues. Thus, mice with defects in the alternative pathway signaling are often associated with absence of lymph nodes, and a lack of specific lymphoid organization in spleen and thymus [50].
In IBD research, three independent gene variants of NOD2 exist, a frame-shift mutation [c3020insC (SNP13)] and two missense mutations [R702 W (SNP8); G908R (SNP12)]. Further, some rare variants have been associated with susceptibility to CD [6]. The frame-shift mutation results in an incompetent truncated NOD2 protein [7]. The NOD2 gene variants cause a reduced response to MDP by generating a loss-of-function phenotype [51]. Thus, human peripheral blood mononuclear cells (PBMCs) from CD patients with the frame-shift mutation (c3020insC) express reduced levels of pro-inflammatory cytokines, e.g., TNF-α, IL-6, and IL-8 in response to MDP [15, 52, 53].
In addition to activation of the NF-κB-mediated pathway, NOD2 stimulation further results in the activation of the MAPK: p38, JNK, and extracellular signal-regulated kinase (ERK), which are intracellular serine/threonine-specific kinases involved in activation of multiple cellular processes, including cell growth, proliferation, differentiation, migration, inflammation, and survival [54–58].
The MDP–NOD2 pathway and T helper cell regulation
During the last decade, a number of hypotheses have discussed the regulatory immunological role of the NOD2 mutations in skewing Th differentiation. One of these hypotheses claims, that MDP NOD2-activation results in a down-modulation of TLR2-mediated Th1 responses, in which antigen-presenting cells (APCs) from Nod2-deficient mice exert increased amounts of IL-12 supporting Th1 inflammatory responses [59]. This hypothesis is supported by observations that administration of MDP protects mice from acute experimental colitis [60, 61]. A second hypothesis suggests that defects in NOD2 signaling polarize the adoptive immune system towards the Th1/Th17-type, resulting in excessive Th1/Th17 cell-mediated inflammation, in which Th1 and Th17 cells are enriched at the inflamed gut together with increased levels of pro-inflammatory cytokines [62, 63]. In addition to the previous hypotheses, other studies indicate that activation of NOD2 signaling enhances the Th17 polarization. Thus, stimulation of human dendritic cells (DCs) with MDP has been shown to enhance NOD2-mediated production of IL-1β and IL-23 which in turn promotes IL-17 production by memory Th17 cells [64]. Moreover, a recently study reported by Geddes et al. [65] suggests a specific role for NOD2 in the early innate response. The authors used a mouse model in identifying an early NOD2-dependent Th17 response restricted to the acute phase of infections after treatment with pathogens, including Salmonella, Typhimurium, and Citrobacter rodentium. NOD2 expression by myeloid and somatic cells was a crucial factor in recognition of pathogens and thereby for a functional early Th17 response [65]. Further, impaired acute Th17 responses in Nod2 knockout mice were associated with unaffected colonic colonization and diminished colonic inflammation at early stages, which is in contrast to an enhanced tissue destruction and increased bacterial translocation later in the course of the disease, indicating a protective role of the acute Th17 response in infection control [65]. However, treatment with human anti-IL-17A monoclonal antibodies has been revealed to be without any clinical effects in CD [66].
NOD2 also seems to be involved in immune regulatory pathways for maintaining the Th1/Th2/Th17 immune balance. For instance, mutations of NOD2 lead to an inhibited IL-10 expression, which is assumed to suppress Th1 cells [53, 67]. NOD2 mutations suppress transcription of human IL-10 by inhibitory activity of the nuclear ribonucleoprotein hnRNP-A1, a nucleocytoplasmic shuttling heterogeneous nuclear ribonucleoprotein that accompanies eukaryotic mRNAs from the active site of transcription to that of translation [67]. Further, PBMCs with the 3020insC frame shift NOD2 mutation have an impaired production of the anti-inflammatory cytokine, IL-10 [53, 67]. Based on the observation that IL-10 knockout mice develop colitis, and that loss of function mutations in the IL-10 receptor gene lead to early onset of CD in humans, this finding adds another aspect to the importance of NOD2 for balancing various immune responses [68, 69]. Another hypothesis is based on observations where NOD2 is found to have a reciprocal interaction with transforming growth factor β-activated kinase 1, TAK1 [70]. NOD2 inhibits TAK1-induced activation of NF-κB [70], and TAK1 gene silencing decreases the frequency of Th1 and Th17 cells [71]. This indicates that a loss-of-function phenotype of NOD2-mutations can lead to an increased TAK1-mediated Th17 cell activation. Recently, short non-coding RNAs, known as microRNAs (miRNAs), were profiled in DCs from patients with CD-associated mutations of NOD2, and a downregulation in two miRNA clusters was revealed. Interestingly, these clusters were suggested to regulate a critical component of the Th1/Th17 immune response [72].
The dysregulation of the immune balance in Th1/Th2/Th17 is further considered to be of importance for a defective Th2 immune response. Magalhaes et al. [73] showed that MDP activation of the NOD2-mediated pathway in DCs is insufficient to initiate Th2 immunity in absence of stromal-derived mediators, such as thymic stromal lymphopoientin (TSLP). TSLP is thought to promote Th2 immunity by an upregulation of the expression of OX40 ligand (OX40L), which is a transmembrane protein expressed by APCs to recognize OX40, a receptor expressed by T cells [74, 75]. Magalhaes et al. found that NOD2 stimulation induced the upregulation of OX40L expression on DCs. Moreover, OX40-deficient mice had diminished capabilities in generating NOD2-drived Th2 immunity [73].
T-regulatory Foxp3+ cells are important for the homeostasis of the intestinal immune response, and a lack of T-regulatory cells has been associated with development of experimental colitis [76]. The dynamic of T-regulatory Foxp3+ cells in IBD is still incompletely understood. Several studies have revealed an increased number of mucosal T regulatory cells in active CD [77, 78]. On the other hand, the number of T-regulatory Foxp3+ cells has been found to be increased in the colonic lamina propria of children with CD treated with anti-TNF-α compared with both CD receiving conventional therapy and non-IBD controls patients with active CD [79]. Moreover, the number of T-regulator cells has also been reported to be higher in inflamed IBD mucosa than the peripheral blood [80]. It is therefore of interest that the MDP-NOD2 pathway seems to be necessary for survival signaling in T-regulatory Foxp3+ cells [81]. Further, T-regulatory Foxp3+ cells from CD patients with disease-associated gene variants of NOD2 were more susceptible to cell death than wild-type NOD2 T-regulatory Foxp3+ cells, and the number of T-regulatory Foxp3+ cells were substantially decreased in NOD2-mutated patients [81]. The MDP–NOD2 pathway might therefore also be crucial for the mucosal immune homeostasis.
MDP has been used as vaccine adjuvant, suggesting that MDP additionally stimulates B cell adaptive immunity [82]. Taken together, these observations reflect the impact of the NOD2 signaling pathway in the dysregulation of adaptive responses related to the CD pathogenesis, although further clarification of the pathways involved is needed.
The MDP–NOD2 pathway in mucosal immunity
In the mucosal immunity, IECs provide a physical barrier with elemental signal-transduction functions in the maintenance of gut homeostasis. IECs consist of a number of epithelial cell types including Paneth cells [83]. The loss-of-function phenotype of NOD2 mutations has been linked to deficiencies of the epithelial-barrier function, which promotes the intestinal bacterial flora invasion and inflammation into the intestinal walls [84]. In Paneth cells, the expression levels of NOD2 increases in response to the intestinal bacteria [85, 86]. MDP stimulation of Paneth cells leads to a NF-κB-dependent expression and secretion of α-defensins, i.e. anti-microbial peptides, into the intestinal lumen [87–90]. Thus, α-defensins levels in CD patients with NOD2 mutations are markedly reduced [91]. This reduction of α-defensins is consistent with activated intracytoplasmic digestion (i.e. crinophagy) of the secretory granules in CD Paneth cells [92]. In addition, Nod2 knockout mice have been reported to possess a reduced mRNA expression of Paneth cell-derived α-defensins. These mice have an impaired intestinal mucosal homeostasis reflected in a reduced intestinal bacterial clearance, and an increased bacterial load of the terminal ileum [93, 94].
In addition, an abnormal MDP-NOD2 pathway has been associated with intestinal disorders and higher bacterial translocation. Thus, MDP stimulation of PBMCs from healthy individuals results in a 2- to 3-fold enhancement of TLR9 agonist stimulation of PBMC production of TNF-α and IL-8, whereas such an enhancement is not seen in PBMCs from CD patients bearing NOD2 mutations [95], suggesting that the synergistic cytokine response between NOD2 and TLR9 might have a role in maintaining intestinal homeostasis. Moreover, it was recently found that MDP stimulation of murine colorectal epithelial cell lines leads to an enhanced TLR2 response in the shape of significantly increased expression levels of chemokines, cytokines, and tight junction molecules, e.g., claudin-3 and claudin-4, which are typically expressed from IECs in the colon to improve the barrier function [96]. Further, the NOD2 genotype has been evidenced to influence the bacterial translocation in CD patients due to the fact, that the intestinal endotoxin accumulation in heterozygous SNP8 and SNP13 is stronger than in homozygos SNP12 patients and wild-type patients [97]. In addition, Nod2 knockout mice are characterized by significantly higher tissue-associated intestinal bacterial levels [93, 94, 98]. Nod2 knockout mice suffered from an impaired bacterial clearance after exposing with pathogens, e.g., Listeria monocytogenes, Citrobacter rodentium, Salomonella, and Mycobacterium tuberculosis [99–101]. The increased bacterial colonization indicates a crucial role for NOD2 in the bacterial clearance. In a recent study, inhibition of NOD2 using carbamoyl phosphate synthetase/aspartate transcarbamylase/dihydroorotase (CAD) (a newly discovered NOD2-interacting protein) leads to a dramatic decrease in the clearance of Salmonella [102]. Thus, NOD2 mutations may play an essential role in the maintenance of intestinal homeostasis, and the results indicate that an impaired NOD2-mediated bacterial clearance might lead to accumulation of mucosal microbiota and secretion of higher levels of pro-inflammatory cytokines.
In mucosal immunity, innate lymphoid cell (ILC) populations, which are newly defined innate immune cells, have been shown to regulate the epithelial cell responses and maintain intestinal homeostasis [103]. ILCs are constituted of both well-established and recently identified T cell populations and can be grouped into three major groups depending on specific transcription factors for their development and function: Group 1 ILCs depends on expression of T-bet (T-box expressed in T cells); Group 2 ILCs relay typically on expression of GATA3 (GATA-binding protein 3); and Group 3 ILCs which require the expression of RORγt (retinoid-related orphan receptor γ) [103, 104]. Although only a few studies have investigated the MDP signaling in ILCs, the presence of this pathway might be involved in the developmental and functional characteristics of ILCs. For instance, Natural Killer (NK) cells, which belong to Group 1 ILCs and attend in the gut homeostasis by responding to commensally enteric bacteria through the innate immune system and cytolytic activity, express high levels of NOD2 [105], and MDP treatment of these cells leads directly to cell activation through the NOD2-mediated pathway [106]. In addition, MDP can also be sensed by TLR2 (described below) [107, 108], which is expressed on RORγt+ ILCs [109]. Therefore, MDP might have an indirectly influence on ILCs through modulation of other cellular components of the innate immunity. As previously mentioned, DCs stimulated with MDP express high levels of IL-23 and IL-1β [64], cytokines that can induce production of IL-22 from RORγt+ ILCs [110, 111]. IL-22 has been reported to ameliorate intestinal inflammation in a murine model of experimental colitis [112], and to bolster intestinal epithelial barrier function by upregulation of antimicrobial peptides [113]. Overall, additional studies are, however, required to clarify the MDP signaling and its influence on ILCs responses.
The MDP–NOD2 pathway and autophagy
The MDP–NOD2 signaling pathway might be a potential regulatory target for other upstream and downstream genes. An interesting illustration of such genes is the autophagy-related protein, 16-like 1 (ATG16L1), which has a key role in the process of cellular protein turnover as well as in handling the intracellular bacteria [90, 114]. The prevalence of ATG16L1 polymorphism is high, as it exists in around 50 % of the European population; however, the loss-of-function mutations in ATG16L1 are associated to CD in form of a two-fold increased susceptibility [115–117]. Moreover, NOD2 is linked to ATG16L1 in the autophagy pathway, and is of importance for the recruitment of ATG16L1 at the site of bacterial entry [118]. The link between NOD2 and ATG16L1 was shown by LPS and MDP stimulation of GFP-LC3-transduced bone marrow-derived macrophages (BMDMs), a cell population expressing functional Nod2: The MDP-treated, but not LPS-treated, BMDMs showed an activated autophagy in wild-type macrophages, whereas macrophages isolated from Nod2-deficent mice did not activate this pathway [118]. These observations indicate that the MDP–NOD2 signaling pathway is emerging as a crucial activator in autophagy, thereby linking important aspects of the pathogenesis of CD to disturbances of the innate immune system.
Cross-talk between NOD2 and other innate pathways
MDP sensing is not NOD2-restricted. Beyond the ability to activate NOD2-mediated signaling pathways, MDP can interact and signal through TLR2 [107, 108]. TLR2 recognizes MDP and activates the NEMO/NF-κB pathway through two independently upstream signaling pathways [119]. Further, TLR2 triggers the association with myeloid differentiation primary-response protein 88 (MyD88), which subsequently can signal either through tumor-necrosis factor receptor-associated factor 6 (TRAF6), or by binding to receptor-interacting serine/threonine kinase (RICK) activating pathways similar to those involved in NOD2-signaling [42, 120–122]. Activation of TLR2 also facilitates induction of MAPK signaling pathways [119, 123–125]. In vitro data on MDP stimulation of splenic macrophages isolated from Nod2-deficient mice have shown an excessive NF-κB-dependent IL-12 production, pointing to a possible NOD2-negative regulatory role of the TLR2-mediated Th1 response, whereas the expression levels of other inflammatory cytokines produced by TLR2-signaling, such as TNF-α and IL-10, were unaffected [59]. In contrast, MDP stimulation of NOD2 and TLRs, including TLR2 in human PBMCs, leads to a positive synergistic effect, while MDP stimulation of mutant NOD2 cells shows a lack of this immunological response, which results in an impaired NF-κB signaling pathway [52, 95]. This immunological response is, however, detrimental to the suggested pathogenesis of CD with elevated levels of NF-κB activation-dependent Th1 cytokines [126–128]. However, the effect of NOD2-activation on TLR2-mediated cytokine response has, in another study, been found to depend on the MDP activation dose and the NOD2-genotype: Stimulation of NOD2-mutant monocytes with low doses of MDP results in a significant increase in the TNF-α production, as opposed to a downregulated response at higher MDP doses. However, when monocytes isolated from NOD2-deficient patients are stimulated with high-dose MDP, divergent dynamics are revealed as the response of downregulation lacks [129].
In addition to the previously mentioned NOD2-interaction, it has further been reported that NOD2 could play a role in activation of innate immune antiviral responses. The mechanisms underlying NOD2-mediated activation of antiviral responses remain controversial and poorly understood. Sabbah et al. [130] concluded that NOD2 is the general activator of antiviral cytokines, including IFN-β, in response to external viral stimuli such as single-stranded RNA (ssRNA) and respiratory syncytial virus (RSV). The authors found that the activation mechanism requires NBD and the LRR domain of NOD2 interaction with the mitochondrial antiviral signaling (MAVS) protein. The NOD2–MAVS protein complex facilitates the NOD2-mediated response by activating interferon-regulatory factor 3 (IRF3), which subsequently translocates to the nucleus and activates production of cytokines such as IFNs [131]. However, the role of NOD2 in antiviral defense is questionable. Recent studies have reported that, although NOD2 has been implicated in the antiviral responses against infections with IAV or murine norovirus-1, no critical role of NOD2 itself in antiviral activity has been revealed [19, 132]. Recent studies have further shown a synergistic proinflammatory cytokine response in human PBMCs stimulated with RSV followed by MDP stimulation. Interestingly, this synergic response is absent in PBMCs isolated from CD patients homozygous for the 3020insC mutation in the NOD2 gene [133]. These results suggest a critical role of NOD2 in this synergy and add an interesting aspect in how the loss of function mutations in NOD2 can eliminate essential immune responses.
The inflammasome-mediated pathway
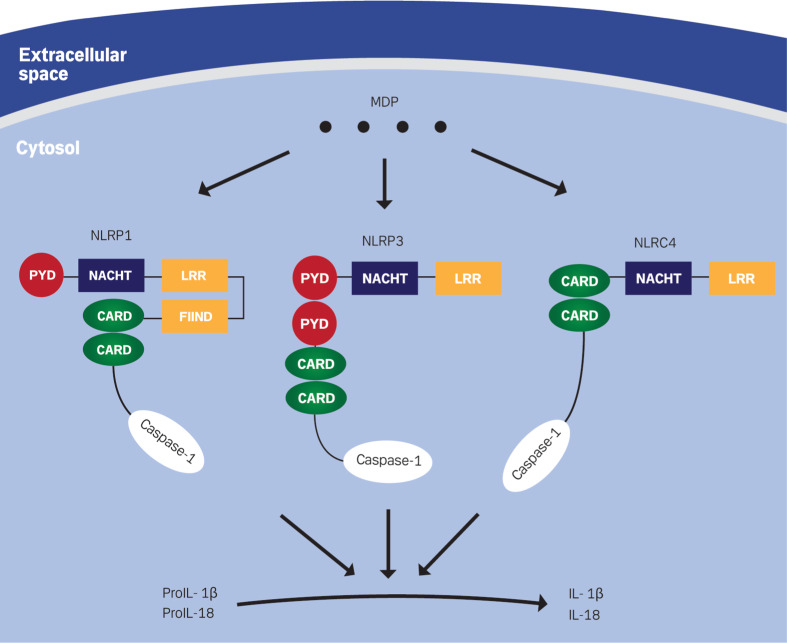
Another outcome of MDP recognizing by PRRs is the formation of inflammasomes, i.e. multiprotein complexes, which are involved in the conversion of pro-caspase-1 to caspase-1 through a CARD–CARD interaction. Active caspase-1 leads to cleavage of pro-inflammatory cytokines, such as pro-IL-1β and pro-IL-18, allowing more secretion of mature IL-1β and IL-18 [134, 135] (Fig. 4). Recently, Meinzer et al. [136]. showed that Yersinia pseudotuberculosis induces an intestinal barrier dysfunction by subverting signaling of Nod2. They reported that acetylation of RICK and TAK1 kinases resulted in a reduced affinity for Nod2. Thus, the free Nod2 interacts with and activates caspase-1 resulting in increased levels of IL-1β, which contributes to the induction of intestinal barrier dysfunction in Peyer’s patches [136].
Fig. 4.
MDP activation of pro-inflammatory cytokines through inflammasome-mediated pathway. The prototype structures of NLRP1, NLRP3 and NLRC4 consist of the following major interaction domains: The ligand sensing leucine-rich repeats (LRR), NACHT or the oligomerization domain, pyrin domain (PYD), or caspase recruitment domain (CARD), and function to find domain (FIIND). MDP interaction with these NLRs leads to the formation of inflammasomes, which are able to convert pro-inflammatory cytokines into their biologically active form
The NLR family encodes a key component in the innate immunity by microbe recognition and the activation of response signaling pathways. One of these pivotal pathways is the NLR control of inflammatory caspases, such as caspase-1 via formation of inflammasomes [137–139]. Four inflammasomes have been identified and named after the PRR that regulates their activity: NLRP1, NLRP3, NLRC4, and AIM2 (absent in melanoma 2), an intracellular DNA receptor [137, 140]. Three of them include PRRs from the NLR family: NLRP1, NLRP3, and NLRC4, which are composed of a C-terminal leucine-rich repeat domain, a central nucleotide-binding domain, and an N-terminal effector domain, CARD or pyrin domain (PYD) (Fig. 4) [141]. NLRPs contain PYD, while CARD mediates downstream protein–protein interaction in NLRCs [142]. Recently, AIM2, a non-NLR family member belonging to the interferon-inducible HIN-200 protein family, was identified as a cytosolic double-stranded DNA (dsDNA) sensor facilitating formation of the AIM2 inflammasome. The AIM2 PYD interacts with adaptor protein apoptosis-associated speck-like protein (ASC) to recruit caspase-1 via its CARD and results in caspase-1-dependent IL-1β induction [143–146].
The initial description of the inflammasome was established on observations showing that formation of the human NLRP1 inflammasome was required for activation of the pro-inflammatory protease, caspase-1 [137, 147]. MDP has been identified as inducer for the NLRP1 inflammasome and IL-1β secretion, in which NOD2/NLRP1 complexes are believed to mediate the process of caspase-1-dependent IL-1β secretion [148], although no evident MDP–NLRP1 interaction has been found [149]. In addition, MDP was found to induce the activation of NLRP3 inflammasome [22, 150]. The activation mechanism of the NLRP3 inflammasome requires induction of NLRP3 expression mediated by NF-κB [150–152]. A recent study revealed that NLRP3 variants contribute to the CD susceptibility, indicating an important interference of the inflammasome in the pathogenesis of CD [153]. The gain-of-function mutation in NLRP3 leads to elevated levels of IL-1β produced by human monocytes THP-1 cells [154]. This finding might also be associated with other severe inflammatory disorders, e.g., Muckle–Wells syndrome, familial cold autoinflammatory syndrome, and neonatal-onset multisystem inflammatory [155, 156].
Other MDP-dependent pathways and clinical implications
Decreased bone mineral density (BMD) is a common extraintestinal complication in IBD [157–159]. The balance between bone resorption and formation, i.e. the process of bone remodeling, is tightly regulated by osteoclasts and osteoblasts, respectively [160]. In recent studies, MDP has been found to contribute synergistically to the enhancement of osteoclasts formation through elevated NOD2-dependent expression of RANKL osteoblasts [161]. These observations suggest that MDP might play a key role in osteoclastic bone resorption in inflammatory bone diseases.
Moreover, MDP can elicit divergent biological effects dependent on the activated biological switch. For instance, a number of apoptotic pathways are induced by the MDP-dependent pathway, such as the calreticulin (CRT) and the ASC-mediated pathways [162, 163]. MDP activation of CRT, an endoplasmic reticulum Ca2+ binding chaperone with multiple functions [164], results in the induction of a conformational change of TNFR1-associated death domain protein (TRADD) or Fas-associated death domain protein (FADD), which subsequently activates caspase-8 and thereby cell death in cell lines such as rabbit kidney epithelial cells and Hela cells [163, 165]. Normalization of this apoptotic pathway might imply a promising treatment procedure for IBD. For instance, repressing of FADD in a mouse model of acute experimental colitis leads to a modulation of TNF-mediated intestinal epithelial apoptosis [166].
In addition to its role as an adaptor protein linking NLR proteins with caspases, the ASC protein interacts with MDP and activates caspase-8-mediated apoptosis [162]. Previously, ASC has been shown to be involved in the chemosensitivity of cancer cells [167], and the ASC-mediated apoptosis might thus be a potential target in oncology.
Concluding remarks
In combination with genetic and environmental factors, the luminal bacterial flora plays a pivotal role in the initiation and perpetuation of IBD. An analysis of published research shows that MDP induces a wide range of biological effects, and, further, that impairment of MDP-related pathways might contribute to the inflammatory process in CD. Thus, MDP can activate divergent extra- and intracellular PRRs and utilize several strategies for intracellular delivery. NOD2 is a well-known innate cytosolic receptor that senses the intracellular MDP. MDP–NOD2 interaction has been shown to activate NF-κB-dependent pro-inflammatory and antibacterial responses. Mutations in NOD2 are associated with an early onset and a more complicated clinical course of CD including fibrostenosis and fistulization, but the functional link between these mutations and CD has not been entirely elucidated. There are several possible mechanisms for a dysfunctional role of NOD2 in CD susceptibility. It has been suggested that defects in NOD2 signaling polarize the adoptive immune towards Th1/Th17-type, which leads to an excessive Th1/Th17 cell-mediated inflammation. In addition, NOD2 signaling has been reported as both a negative and a positive regulator of TLR responses. A NOD2 mutation can drive a Th1 immune response by negative regulation of TLR2, whereas NOD2 can also provide a synergistic effect with TLR9 in maintaining intestinal homeostasis. An impaired NOD2 interaction with ATG16L1, and thereby impaired autophagy causing defect bacterial handling, is another likely mechanism of CD-associated dysbiosis.
Recent studies have expanded the induction ability of MDP beyond interaction with NOD2. Indeed, MDP has been shown to interact with inflammasomes, through NLRP1 and NLRP3, and to induce production of the pro-inflammatory cytokines belonging to the IL-1 family of cytokines. Further, NOD2 signaling might be impaired even in CD patients with the wild-type NOD2 gene variant, suggesting that epigenetic changes and alternations downstream to NOD2 could play a key role in the bacteria–host interaction in CD [168]. The understanding of how interaction between MDP-producing bacteria and the innate immune system contributes to the pathogenesis of CD therefore relies on a better understanding of how shutting down of one part of the MDP response (e.g., by defect NOD2 signaling), poses imbalances towards other parts of the NOD2 response (e.g., non-NOD2-dependent signaling). Forcing or enhancing signaling through these pathways by stimulation with small molecules could in this way downgrade the imbalance and normalize the innate immune response, and thereby reduce the inflammatory burden in CD, as has been observed in experimental colitis [60, 169].
In this review, several findings related to the molecular mechanisms of intracellular delivery of MDP and its biological effect in the CD pathogenesis have been highlighted. Furthermore, the cross-talk between NOD2 and other innate signaling pathways of relevance to disease pathogenesis have been described, as well as the role of MDP in the inflammasome-mediated and various other pathways (e.g., NOD2 and TLR2). Altogether, these observations raise questions on the responsive roles of MDP for the innate immunity and CD pathogenesis, and it is assumed that clarification of the impact of MDP could possess major implications of importance for future treatment strategies for CD.
Acknowledgments
The authors wish to thank Dr. Marcel Behr from McGill University, Montreal, Canada, for critical reading and discussion of the manuscript. This study was supported by grants from Fonden til Lægevidenskabens Fremme (the A.P. Møller Foundation), the Family Erichsen Memorial Foundation, the Axel Muusfeldts Foundation, and the The Foundation of Aase and Ejnar Danielsen.
References
- 1.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa C, Liu YJ, Kobayashi KS. Muramyl dipeptide and its derivatives: peptide adjuvant in immunological disorders and cancer therapy. Curr Bioact Compd. 2011;7:180–197. doi: 10.2174/157340711796817913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holler E, Rogler G, Herfarth H, Brenmoehl J, Wild PJ, Hahn J, Eissner G, Scholmerich J, et al. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood. 2004;104:889–894. doi: 10.1182/blood-2003-10-3543. [DOI] [PubMed] [Google Scholar]
- 4.Kim YG, Shaw MH, Warner N, Park JH, Chen F, Ogura Y, Nunez G. Cutting edge: crohn’s disease-associated Nod2 mutation limits production of proinflammatory cytokines to protect the host from Enterococcus faecalis-induced lethality. J Immunol. 2011;187:2849–2852. doi: 10.4049/jimmunol.1001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenmoehl J, Herfarth H, Gluck T, Audebert F, Barlage S, Schmitz G, Froehlich D, Schreiber S, et al. Genetic variants in the NOD2/CARD15 gene are associated with early mortality in sepsis patients. Intensive Care Med. 2007;33:1541–1548. doi: 10.1007/s00134-007-0722-z. [DOI] [PubMed] [Google Scholar]
- 6.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 7.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 8.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 9.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 11.Tsianos EV, Katsanos KH, Tsianos VE. Role of genetics in the diagnosis and prognosis of Crohn’s disease. World J Gastroenterol. 2012;18:105–118. doi: 10.3748/wjg.v18.i2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braat H, Peppelenbosch MP, Hommes DW. Immunology of Crohn’s disease. Ann NY Acad Sci. 2006;1072:135–154. doi: 10.1196/annals.1326.039. [DOI] [PubMed] [Google Scholar]
- 13.Gautam A, Vyas R, Tewari R. Peptidoglycan biosynthesis machinery: a rich source of drug targets. Crit Rev Biotechnol. 2011;31:295–336. doi: 10.3109/07388551.2010.525498. [DOI] [PubMed] [Google Scholar]
- 14.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 15.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 16.Takada H, Kawabata Y, Kawata S, Kusumoto S. Structural characteristics of peptidoglycan fragments required to prime mice for induction of anaphylactoid reactions by lipopolysaccharides. Infect Immun. 1996;64:657–659. doi: 10.1128/iai.64.2.657-659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coulombe F, Divangahi M, Veyrier F, de Léséleuc L, Gleason JL, Yang Y, Kelliher MA, Pandey AK, et al. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. 2009;206(8):1709–1716. doi: 10.1084/jem.20081779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalande JD, Behr MA. Mycobacteria in Crohn’s disease: how innate immune deficiency may result in chronic inflammation. Expert Rev Clin Immunol. 2010;6:633–641. doi: 10.1586/eci.10.29. [DOI] [PubMed] [Google Scholar]
- 19.Coulombe F, Fiola S, Akira S, Cormier Y, Gosselin J. Muramyl dipeptide induces NOD2-dependent Ly6C(high) monocyte recruitment to the lungs and protects against influenza virus infection. PLoS One. 2012;7:e36734. doi: 10.1371/journal.pone.0036734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JT, Uehara T. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan) Microbiol Mol Biol Rev. 2008;72:211–227. doi: 10.1128/MMBR.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismair MG, Vavricka SR, Kullak-Ublick GA, Fried M, Mengin-Lecreulx D, Girardin SE. hPepT1 selectively transports muramyl dipeptide but not Nod1-activating muramyl peptides. Can J Physiol Pharmacol. 2006;84:1313–1319. doi: 10.1139/y06-076. [DOI] [PubMed] [Google Scholar]
- 22.Marina-Garcia N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, Nunez G. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 23.Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, et al. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology. 2004;127:1401–1409. doi: 10.1053/j.gastro.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Scharl M, Mwinyi J, Fischbeck A, Leucht K, Eloranta JJ, Arikkat J, Pesch T, Kellermeier S, et al. Crohn’s disease-associated polymorphism within the PTPN2 gene affects muramyl-dipeptide-induced cytokine secretion and autophagy. Inflamm Bowel Dis. 2012;18:900–912. doi: 10.1002/ibd.21913. [DOI] [PubMed] [Google Scholar]
- 25.Marina-Garcia N, Franchi L, Kim YG, Hu Y, Smith DE, Boons GJ, Nunez G. Clathrin- and dynamin-dependent endocytic pathway regulates muramyl dipeptide internalization and NOD2 activation. J Immunol. 2009;182:4321–4327. doi: 10.4049/jimmunol.0802197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Tattoli I, Wojtal KA, Vavricka SR, Philpott DJ, Girardin SE. pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling. J Biol Chem. 2009;284:23818–23829. doi: 10.1074/jbc.M109.033670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B, Brech A, Latz E, et al. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 29.Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 2007;3:e51. doi: 10.1371/journal.ppat.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith PD, Smythies LE, Shen R, Greenwell-Wild T, Gliozzi M, Wahl SM. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4:31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 32.Fritz T, Niederreiter L, Adolph T, Blumberg RS, Kaser A. Crohn’s disease: NOD2, autophagy and ER stress converge. Gut. 2011;60:1580–1588. doi: 10.1136/gut.2009.206466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correa RG, Milutinovic S, Reed JC. Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases. Biosci Rep. 2012;32:597–608. doi: 10.1042/BSR20120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Res. 2011;21:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duerr CU, Salzman NH, Dupont A, Szabo A, Normark BH, Normark S, Locksley RM, Mellroth P, et al. Control of intestinal Nod2-mediated peptidoglycan recognition by epithelium-associated lymphocytes. Mucosal Immunol. 2011;4:325–334. doi: 10.1038/mi.2010.71. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Geddes K, Streutker C, Philpott DJ, Girardin SE. Role of mouse peptidoglycan recognition protein PGLYRP2 in the innate immune response to salmonella enterica serovar typhimurium infection in vivo. Infect Immun. 2012;80:2645–2654. doi: 10.1128/IAI.00168-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenstiel P, Fantini M, Brautigam K, Kuhbacher T, Waetzig GH, Seegert D, Schreiber S. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–1009. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 39.Grimes CL, Ariyananda LD, Melnyk JE, O’Shea EK. The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J Am Chem Soc. 2012;134(33):13535–13537. doi: 10.1021/ja303883c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bright GR, Fisher GW, Rogowska J, Taylor DL. Fluorescence ratio imaging microscopy: temporal and spatial measurements of cytoplasmic pH. J Cell Biol. 1987;104:1019–1033. doi: 10.1083/jcb.104.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lecine P, Esmiol S, Metais JY, Nicoletti C, Nourry C, McDonald C, Nunez G, Hugot JP, et al. The NOD2-RICK complex signals from the plasma membrane. J Biol Chem. 2007;282:15197–15207. doi: 10.1074/jbc.M606242200. [DOI] [PubMed] [Google Scholar]
- 42.Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn’s disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–2227. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 43.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sebban-Benin H, Pescatore A, Fusco F, Pascuale V, Gautheron J, Yamaoka S, Moncla A, Ursini MV, et al. Identification of TRAF6-dependent NEMO polyubiquitination sites through analysis of a new NEMO mutation causing incontinentia pigmenti. Hum Mol Genet. 2007;16:2805–2815. doi: 10.1093/hmg/ddm237. [DOI] [PubMed] [Google Scholar]
- 45.Wullaert A, Bonnet MC, Pasparakis M. NF-kappaB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 47.Xiao G, Rabson AB, Young W, Qing G, Qu Z. Alternative pathways of NF-kappaB activation: a double-edged sword in health and disease. Cytokine Growth Factor Rev. 2006;17:281–293. doi: 10.1016/j.cytogfr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Meshcheriakova EA, Andronova TM, Ivanov VT. A protein interaction network and cell signaling pathways activated by muramyl peptides. Bioorg Khim. 2010;36:581–595. doi: 10.1134/s1068162010050018. [DOI] [PubMed] [Google Scholar]
- 49.Pan Q, Kravchenko V, Katz A, Huang S, Ii M, Mathison JC, Kobayashi K, Flavell RA, et al. NF-kappa B-inducing kinase regulates selected gene expression in the Nod2 signaling pathway. Infect Immun. 2006;74:2121–2127. doi: 10.1128/IAI.74.4.2121-2127.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 51.Strober W, Kitani A, Fuss I, Asano N, Watanabe T. The molecular basis of NOD2 susceptibility mutations in Crohn’s disease. Mucosal Immunol. 2008;1(suppl 1):S5–S9. doi: 10.1038/mi.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Heel DA, Ghosh S, Butler M, Hunt KA, Lundberg AM, Ahmad T, McGovern DP, Onnie C, et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn’s disease. Lancet. 2005;365:1794–1796. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- 53.Netea MG, Kullberg BJ, de Jong DJ, Franke B, Sprong T, Naber TH, Drenth JP, van der Meer JW. NOD2 mediates anti-inflammatory signals induced by TLR2 ligands: implications for Crohn’s disease. Eur J Immunol. 2004;34:2052–2059. doi: 10.1002/eji.200425229. [DOI] [PubMed] [Google Scholar]
- 54.Coskun M, Olsen J, Seidelin JB, Nielsen OH. MAP kinases in inflammatory bowel disease. Clin Chim Acta. 2011;412:513–520. doi: 10.1016/j.cca.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 55.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 56.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 58.Schieven GL. The p38alpha kinase plays a central role in inflammation. Curr Top Med Chem. 2009;9:1038–1048. doi: 10.2174/156802609789630974. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe T, Asano N, Murray PJ, Ozato K, Tailor P, Fuss IJ, Kitani A, Strober W. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bertrand MJ, Doiron K, Labbe K, Korneluk RG, Barker PA, Saleh M. Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity. 2009;30:789–801. doi: 10.1016/j.immuni.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macdonald TT, Bell I, Monteleone G. The opposing roles of IL-21 and TGFbeta1 in chronic inflammatory bowel disease. Biochem Soc Trans. 2011;39:1061–1066. doi: 10.1042/BST0391061. [DOI] [PubMed] [Google Scholar]
- 64.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 65.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le BL, Cho JH, Robertson SJ, Kim CJ, et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med. 2011;17:837–844. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- 66.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noguchi E, Homma Y, Kang X, Netea MG, Ma X. A Crohn’s disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat Immunol. 2009;10:471–479. doi: 10.1038/ni.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rennick DM, Fort MM. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10(−/−) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2000;278:G829–G833. doi: 10.1152/ajpgi.2000.278.6.G829. [DOI] [PubMed] [Google Scholar]
- 69.Biswas A, Petnicki-Ocwieja T, Kobayashi KS. Nod2: a key regulator linking microbiota to intestinal mucosal immunity. J Mol Med (Berl) 2012;90:15–24. doi: 10.1007/s00109-011-0802-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen CM, Gong Y, Zhang M, Chen JJ. Reciprocal cross-talk between Nod2 and TAK1 signaling pathways. J Biol Chem. 2004;279:25876–25882. doi: 10.1074/jbc.M400682200. [DOI] [PubMed] [Google Scholar]
- 71.Courties G, Seiffart V, Presumey J, Escriou V, Scherman D, Zwerina J, Ruiz G, Zietara N, et al. In vivo RNAi-mediated silencing of TAK1 decreases inflammatory Th1 and Th17 cells through targeting of myeloid cells. Blood. 2010;116:3505–3516. doi: 10.1182/blood-2010-02-269605. [DOI] [PubMed] [Google Scholar]
- 72.Brain O, Allan P, Pichulik T, Khatamzas E, Simpson P, Jewell D, Simmons A. NOD2 regulation of micrornas [Abstract] Gut. 2011;60:A37. [Google Scholar]
- 73.Magalhaes JG, Rubino SJ, Travassos LH, Le BL, Duan W, Sellge G, Geddes K, Reardon C, et al. Nucleotide oligomerization domain-containing proteins instruct T cell helper type 2 immunity through stromal activation. Proc Natl Acad Sci USA. 2011;108:14896–14901. doi: 10.1073/pnas.1015063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol. 2007;120:238–244. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 75.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 76.Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P, Neurath MF. Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671–680. doi: 10.1136/gut.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reikvam DH, Perminow G, Lyckander LG, Gran JM, Brandtzaeg P, Vatn M, Carlsen HS. Increase of regulatory T cells in ileal mucosa of untreated pediatric Crohn’s disease patients. Scand J Gastroenterol. 2011;46:550–560. doi: 10.3109/00365521.2011.551887. [DOI] [PubMed] [Google Scholar]
- 78.Ban H, Andoh A, Shioya M, Nishida A, Tsujikawa T, Fujiyama Y. Increased number of FoxP3+ CD4+ regulatory T cells in inflammatory bowel disease. Mol Med Rep. 2008;1:647–650. doi: 10.3892/mmr_00000006. [DOI] [PubMed] [Google Scholar]
- 79.Ricciardelli I, Lindley KJ, Londei M, Quaratino S. Anti tumour necrosis-alpha therapy increases the number of FOXP3 regulatory T cells in children affected by Crohn’s disease. Immunology. 2008;125:178–183. doi: 10.1111/j.1365-2567.2008.02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 81.Rahman MK, Midtling EH, Svingen PA, Xiong Y, Bell MP, Tung J, Smyrk T, Egan LJ, et al. The pathogen recognition receptor NOD2 regulates human FOXP3+ T cell survival. J Immunol. 2010;184:7247–7256. doi: 10.4049/jimmunol.0901479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iribe H, Koga T, Kotani S, Kusumoto S, Shiba T. Stimulating effect of MDP and its adjuvant-active analogues on guinea pig fibroblasts for the production of thymocyte-activating factor. J Exp Med. 1983;157:2190–2195. doi: 10.1084/jem.157.6.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gersemann M, Wehkamp J, Stange EF. Innate immune dysfunction in inflammatory bowel disease. J Intern Med. 2012;271:421–428. doi: 10.1111/j.1365-2796.2012.02515.x. [DOI] [PubMed] [Google Scholar]
- 84.Lecat A, Piette J, Legrand-Poels S. The protein Nod2: an innate receptor more complex than previously assumed. Biochem Pharmacol. 2010;80:2021–2031. doi: 10.1016/j.bcp.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 85.Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nunez G, et al. Crohn’s disease and the NOD2 gene: a role for paneth cells. Gastroenterology. 2003;125:47–57. doi: 10.1016/s0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 86.Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, Zimmermann E, Tretiakova M, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52:1591–1597. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 88.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schaffeler E, Schlee M, Herrlinger KR, Stallmach A, et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bevins CL, Stange EF, Wehkamp J. Decreased Paneth cell defensin expression in ileal Crohn’s disease is independent of inflammation, but linked to the NOD2 1007fs genotype. Gut. 2009;58:882–883. [PubMed] [Google Scholar]
- 90.Kaser A, Blumberg RS. Autophagy, microbial sensing, endoplasmic reticulum stress, and epithelial function in inflammatory bowel disease. Gastroenterology. 2011;140:1738–1747. doi: 10.1053/j.gastro.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci USA. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thachil E, Hugot JP, Arbeille B, Paris R, Grodet A, Peuchmaur M, Codogno P, Barreau F, et al. Abnormal activation of autophagy-induced crinophagy in paneth cells from patients with Crohn’s disease. Gastroenterology. 2012;142:1097–1099. doi: 10.1053/j.gastro.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 94.Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci USA. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Heel DA, Ghosh S, Hunt KA, Mathew CG, Forbes A, Jewell DP, Playford RJ. Synergy between TLR9 and NOD2 innate immune responses is lost in genetic Crohn’s disease. Gut. 2005;54:1553–1557. doi: 10.1136/gut.2005.065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hiemstra IH, Bouma G, Geerts D, Kraal G, den Haan JM. Nod2 improves barrier function of intestinal epithelial cells via enhancement of TLR responses. Mol Immunol. 2012;52:264–272. doi: 10.1016/j.molimm.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 97.Kosovac K, Brenmoehl J, Holler E, Falk W, Schoelmerich J, Hausmann M, Rogler G. Association of the NOD2 genotype with bacterial translocation via altered cell–cell contacts in Crohn’s disease patients. Inflamm Bowel Dis. 2010;16:1311–1321. doi: 10.1002/ibd.21223. [DOI] [PubMed] [Google Scholar]
- 98.Smith P, Siddharth J, Pearson R, Holway N, Shaxted M, Butler M, Clark N, Jamontt J, et al. Host genetics and environmental factors regulate ecological succession of the mouse colon tissue-associated microbiota. PLoS One. 2012;7:e30273. doi: 10.1371/journal.pone.0030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 100.Geddes K, Rubino S, Streutker C, Cho JH, Magalhaes JG, Le BL, Selvanantham T, Girardin SE, et al. Nod1 and Nod2 regulation of inflammation in the Salmonella colitis model. Infect Immun. 2010;78:5107–5115. doi: 10.1128/IAI.00759-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, Kobayashi KS, Flavell RA, et al. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol. 2008;181:7157–7165. doi: 10.4049/jimmunol.181.10.7157. [DOI] [PubMed] [Google Scholar]
- 102.Richmond AL, Kabi A, Homer CR, Marina-Garcia N, Nickerson KP, Nesvizhskii AI, Sreekumar A, Chinnaiyan AM, et al. The nucleotide synthesis enzyme CAD inhibits NOD2 antibacterial function in human intestinal epithelial cells. Gastroenterology. 2012;142:1483–1492. doi: 10.1053/j.gastro.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sonnenberg GF, Artis D. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity. 2012;37:601–610. doi: 10.1016/j.immuni.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 105.Qiu F, Maniar A, Diaz MQ, Chapoval AI, Medvedev AE. Activation of cytokine-producing and antitumor activities of natural killer cells and macrophages by engagement of Toll-like and NOD-like receptors. Innate Immun. 2011;17:375–387. doi: 10.1177/1753425910372000. [DOI] [PubMed] [Google Scholar]
- 106.Athie-Morales V, O’Connor GM, Gardiner CM. Activation of human NK cells by the bacterial pathogen-associated molecular pattern muramyl dipeptide. J Immunol. 2008;180:4082–4089. doi: 10.4049/jimmunol.180.6.4082. [DOI] [PubMed] [Google Scholar]
- 107.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 108.Uehori J, Fukase K, Akazawa T, Uematsu S, Akira S, Funami K, Shingai M, Matsumoto M, et al. Dendritic cell maturation induced by muramyl dipeptide (MDP) derivatives: monoacylated MDP confers TLR2/TLR4 activation. J Immunol. 2005;174:7096–7103. doi: 10.4049/jimmunol.174.11.7096. [DOI] [PubMed] [Google Scholar]
- 109.Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33:752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 110.Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 114.Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 115.Marquez A, Nunez C, Martinez A, Mendoza JL, Taxonera C, Fernandez-Arquero M, Diaz-Rubio M, de la Concha EG, et al. Role of ATG16L1 Thr300Ala polymorphism in inflammatory bowel disease: a study in the Spanish population and a meta-analysis. Inflamm Bowel Dis. 2009;15:1697–1704. doi: 10.1002/ibd.21001. [DOI] [PubMed] [Google Scholar]
- 116.Prescott NJ, Fisher SA, Franke A, Hampe J, Onnie CM, Soars D, Bagnall R, Mirza MM, et al. A nonsynonymous SNP in ATG16L1 predisposes to ileal Crohn’s disease and is independent of CARD15 and IBD5. Gastroenterology. 2007;132:1665–1671. doi: 10.1053/j.gastro.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 117.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 118.Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhaes JG, Yuan L, Soares F, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 119.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 120.Takeuchi O, Takeda K, Hoshino K, Adachi O, Ogawa T, Akira S. Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int Immunol. 2000;12:113–117. doi: 10.1093/intimm/12.1.113. [DOI] [PubMed] [Google Scholar]
- 121.Yang RB, Mark MR, Gurney AL, Godowski PJ. Signaling events induced by lipopolysaccharide-activated toll-like receptor 2. J Immunol. 1999;163:639–643. [PubMed] [Google Scholar]
- 122.Tang L, Zhou XD, Wang Q, Zhang L, Wang Y, Li XY, Huang DM. Expression of TRAF6 and pro-inflammatory cytokines through activation of TLR2, TLR4, NOD1, and NOD2 in human periodontal ligament fibroblasts. Arch Oral Biol. 2011;56:1064–1072. doi: 10.1016/j.archoralbio.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 123.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 124.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 125.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 126.Bene L, Falus A, Baffy N, Fulop AK. Cellular and molecular mechanisms in the two major forms of inflammatory bowel disease. Pathol Oncol Res. 2011;17:463–472. doi: 10.1007/s12253-011-9397-4. [DOI] [PubMed] [Google Scholar]
- 127.Wei J, Feng J. Signaling pathways associated with inflammatory bowel disease. Recent Pat Inflamm Allergy Drug Discov. 2010;4:105–117. doi: 10.2174/187221310791163071. [DOI] [PubMed] [Google Scholar]
- 128.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 129.Borm ME, van Bodegraven AA, Mulder CJ, Kraal G, Bouma G. The effect of NOD2 activation on TLR2-mediated cytokine responses is dependent on activation dose and NOD2 genotype. Genes Immun. 2008;9:274–278. doi: 10.1038/gene.2008.9. [DOI] [PubMed] [Google Scholar]
- 130.Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen H, Jiang Z (2012) The essential adaptors of innate immune signaling. Protein Cell [DOI] [PMC free article] [PubMed]
- 132.Kim YG, Park JH, Reimer T, Baker DP, Kawai T, Kumar H, Akira S, Wobus C, et al. Viral infection augments Nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe. 2011;9:496–507. doi: 10.1016/j.chom.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vissers M, Remijn T, Oosting M, de Jong DJ, Diavatopoulos DA, Hermans PW, Ferwerda G. Respiratory syncytial virus infection augments NOD2 signaling in an IFN-beta-dependent manner in human primary cells. Eur J Immunol. 2012;42:2727–2735. doi: 10.1002/eji.201242396. [DOI] [PubMed] [Google Scholar]
- 134.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 135.Rodriguez-Bores L, Fonseca GC, Villeda MA, Yamamoto-Furusho JK. Novel genetic markers in inflammatory bowel disease. World J Gastroenterol. 2007;13:5560–5570. doi: 10.3748/wjg.v13.i42.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meinzer U, Barreau F, Esmiol-Welterlin S, Jung C, Villard C, Leger T, Ben-Mkaddem S, Berrebi D, et al. Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe. 2012;11:337–351. doi: 10.1016/j.chom.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 137.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 141.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 144.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 146.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 148.Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci USA. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 150.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 151.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harder J, Franchi L, Munoz-Planillo R, Park JH, Reimer T, Nunez G. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J Immunol. 2009;183:5823–5829. doi: 10.4049/jimmunol.0900444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, Collette C, Baba N, Libioulle C, et al. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat Genet. 2009;41:71–76. doi: 10.1038/ng285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Verma D, Sarndahl E, Andersson H, Eriksson P, Fredrikson M, Jonsson JI, Lerm M, Soderkvist P. The Q705K polymorphism in NLRP3 is a gain-of-function alteration leading to excessive interleukin-1beta and IL-18 production. PLoS One. 2012;7:e34977. doi: 10.1371/journal.pone.0034977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 156.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 157.Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med. 2010;42:97–114. doi: 10.3109/07853890903559724. [DOI] [PubMed] [Google Scholar]
- 158.Lorinczy K, Lakatos G, Mullner K, Hritz I, Lakatos PL, Tulassay Z, Miheller P. Low bone mass in microscopic colitis. BMC Gastroenterol. 2011;11:58. doi: 10.1186/1471-230X-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Miheller P, Muzes G, Racz K, Blazovits A, Lakatos P, Herszenyi L, Tulassay Z. Changes of OPG and RANKL concentrations in Crohn’s disease after infliximab therapy. Inflamm Bowel Dis. 2007;13:1379–1384. doi: 10.1002/ibd.20234. [DOI] [PubMed] [Google Scholar]
- 160.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang S, Takahashi N, Yamashita T, Sato N, Takahashi M, Mogi M, Uematsu T, Kobayashi Y, et al. Muramyl dipeptide enhances osteoclast formation induced by lipopolysaccharide, IL-1 alpha, and TNF-alpha through nucleotide-binding oligomerization domain 2-mediated signaling in osteoblasts. J Immunol. 2005;175:1956–1964. doi: 10.4049/jimmunol.175.3.1956. [DOI] [PubMed] [Google Scholar]
- 162.Motani K, Kawase K, Imamura R, Kinoshita T, Kushiyama H, Suda T. Activation of ASC induces apoptosis or necrosis, depending on the cell type, and causes tumor eradication. Cancer Sci. 2010;101:1822–1827. doi: 10.1111/j.1349-7006.2010.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen D, Texada DE, Duggan C, Liang C, Reden TB, Kooragayala LM, Langford MP. Surface calreticulin mediates muramyl dipeptide-induced apoptosis in RK13 cells. J Biol Chem. 2005;280:22425–22436. doi: 10.1074/jbc.M413380200. [DOI] [PubMed] [Google Scholar]
- 164.Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, Michalak M, Murphy-Ullrich JE. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010;24:665–683. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bender LM, Morgan MJ, Thomas LR, Liu ZG, Thorburn A. The adaptor protein TRADD activates distinct mechanisms of apoptosis from the nucleus and the cytoplasm. Cell Death Differ. 2005;12:473–481. doi: 10.1038/sj.cdd.4401578. [DOI] [PubMed] [Google Scholar]
- 166.Hindryckx P, De VM, Jacques P, Ferdinande L, Peeters H, Olievier K, Bogaert S, Brinkman B, et al. Hydroxylase inhibition abrogates TNF-alpha-induced intestinal epithelial damage by hypoxia-inducible factor-1-dependent repression of FADD. J Immunol. 2010;185:6306–6316. doi: 10.4049/jimmunol.1002541. [DOI] [PubMed] [Google Scholar]
- 167.Ohtsuka T, Ryu H, Minamishima YA, Macip S, Sagara J, Nakayama KI, Aaronson SA, Lee SW. ASC is a Bax adaptor and regulates the p53-Bax mitochondrial apoptosis pathway. Nat Cell Biol. 2004;6:121–128. doi: 10.1038/ncb1087. [DOI] [PubMed] [Google Scholar]
- 168.Seidelin JB, Broom OJ, Olsen J, Nielsen OH. Evidence for impaired CARD15 signalling in Crohn’s disease without disease linked variants. PLoS One. 2009;4:e7794. doi: 10.1371/journal.pone.0007794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc Natl Acad Sci USA. 2007;104:19440–19445. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]