Abstract
Snail belongs to the superfamily of zinc-finger transcription factors and plays a crucial role in processes regulating cell fate, such as the formation of mesoderm and initiation of epithelial–mesenchymal transition. We have previously discovered that Snail modulates adiponectin expression in 3T3-L1 cells during adipogenesis. In the present study, we elucidated the functional role of Snail in adipocyte differentiation and its underlying molecular mechanism. Snail expression was dramatically decreased during adipogenesis in 3T3-L1 cells. Overexpression of Snail blocked adipocyte differentiation by suppressing the expression of peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT-enhancer-binding protein alpha, while knockdown of Snail expression stimulated adipogenesis in 3T3-L1 cells. Chromatin immunoprecipitation assay and luciferase assay showed that Snail inhibits the transcriptional activity of the PPARγ gene by directly binding to the E-box motifs in the PPARγ promoter. Wnt10b induced phosphorylation of glycogen synthase kinase 3 beta (GSK3β), leading to inhibition of adipogenesis in 3T3-L1 cells in accordance with increased expression of Snail, whereas adipogenic capacity was restored in Snail siRNA-transfected preadipocytes. LiCl (a GSK3β inhibitor)-treated cells also showed increased expression of Snail, with a reduced adipogenic potential. Snail-overexpressing 3T3-F442A cells did not differentiate into mature adipocytes in immunodeficient nude mice. Taken together, Snail is a novel regulator of adipocyte differentiation, which acts by direct suppression of PPARγ expression. Our data also indicate that the expression of Snail is mediated by the Wnt–GSK3β signaling pathway.
Keywords: Adipogenesis, Snail, WNT signaling, PPARγ
Introduction
Obesity is a worldwide epidemic [1] and has an immense impact on the development of various diseases including cardiovascular diseases, diabetes, and cancer [2]. From a cell-biological aspect, obesity is defined as the expansion of adipose tissue in the body, which is caused by an increased size of adipocytes (hypertrophy) as well as an increased number of adipocytes (hyperplasia). Hyperplasia of the adipocytes is initiated by adipogenesis of the mesenchymal stem cells or preadipocytes. Therefore, understanding the molecular mechanism that regulates the development and growth of adipose tissues is essential to treat and prevent obesity.
Adipocyte differentiation involves a complex network of transcription factors that modulate the expression of various genes responsible for the development of mature adipocytes [3]. The members of the Wnt signaling pathway are known to be an important component of adipogenesis inhibition [4]. Although both canonical pathways (including β-catenin) and non-canonical pathways play an essential role in adipocyte differentiation, the downstream signaling cascades have not been fully elucidated.
The Snail superfamily of zinc-finger transcription factors plays a crucial role in processes regulating cell fate, such as the formation of mesoderm and initiation of epithelial–mesenchymal transition (EMT) during embryonic development and tumor metastasis or progression [5]. The Snail family of proteins is involved in neural differentiation, cell survival, and left–right asymmetry [5]. Snail (SNAI1) usually acts as a transcription repressor and triggers EMT by directly downregulating the expression of E-cadherin in the mesoderm [6]. In addition, Snail regulates osteoblast differentiation by repressing the expression of Runx2 and the vitamin D receptor in osteoblasts [7]. As osteogenesis is closely related to adipogenesis, Snail may play a certain role in adipocyte differentiation. The function of Slug (SNAI2), which is the second member of the Snail family, has been identified in transgenic mice, and the overexpression of Slug in these mice increased the amount of white adipose tissue by stimulating adipogenesis [8]. Recently, we have found that Snail inhibits the expression of adiponectin by directly binding to the E-box of the adiponectin promoter in 3T3-L1 adipocytes [9]. In this study, overexpression of Snail in 3T3-L1 adipocytes resulted in decreased levels of peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT-enhancer-binding protein alpha (C/EBPα), which are two major elements of adipogenic transcription factors, suggesting a plausible link between Snail and adipogenesis. However, to date, there has been no evidence regarding the functional role of Snail in adipocyte differentiation.
Furthermore, diverse signaling pathways such as fibroblast growth factor, transforming growth factor-β, and Wnt have been linked with the activation of Snail in the processes of EMT [5]. The role of the Wnt pathway in Snail activation is demonstrated by the finding that Snail expression is modulated by Wnt–GSK3β-dependent phosphorylation in breast carcinoma cells [10]. Therefore, the aim of the present study was to investigate the functional role of the transcription factor Snail in adipocyte differentiation using 3T3-L1 preadipocytes and a xenograft animal model, and to demonstrate that the Wnt–GSK3β signaling cascades regulate the expression of Snail.
Materials and methods
Cell culture and differentiation of preadipocytes into adipocytes
The 3T3-L1 and 3T3-F442A mouse preadipocyte cell lines were cultured according to the method described previously [9]. Two-day post-confluent cells (designated as day 0) were cultured in DMEM (WelGENE Inc., Daegu, Korea) supplemented with 10 % fetal bovine serum (FBS, WelGENE Inc.), 0.5 mM 3-isobutyl-1-methylxanthine (IBMX, Sigma-Aldrich, St. Louis, MO, USA), 2 μg/ml dexamethasone (Sigma-Aldrich), and 1 μg/ml insulin (Roche, Mannheim, Germany) for 2 days. Every 2 days thereafter, the cells were incubated with fresh DMEM supplemented with 10 % FBS and 1 μg/ml insulin. Fully differentiated adipocytes were used for experiments after 8 days. Lipid droplets were visualized by Oil Red O staining on day 8. To measure the quantification of lipid accumulation, Oil Red O was eluted by adding 100 % isopropanol and optical density was detected using a spectrophotometer at 520 nm.
Plasmid transfection and Snail silencing by lentiviral-delivered RNA interference
3T3-F442A and 3T3-L1 preadipocytes were grown in 12-well culture plates until confluence. To induce overexpression of Snail, C/EBPα or Wnt10b, the preadipocytes were transfected with the expression vector pcDNA3 that encoded Snail or C/EBPα and pCMV6-Entry vector with Wnt10b (Origene, Rockville, MD, USA), using Tfx-50 (Promega, Madison, WI, USA) and electroporation (model No. 640: BTX Harvard Apparatus, Holliston, MA, USA) in accordance with the manufacturer’s instructions. Following electroporation, the cells were replated on tissue culture plates and allowed to recover for 48 h. Snail and control small interfering RNAs (siRNAs, Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used for knockdown experiments. A mouse shRNA kit for silencing Snail expression was purchased from Santa Cruz and 3T3-L1 cells were infected with either control or Snail shRNA lentiviral particles according to the manufacturer’s instructions.
Site-directed mutagenesis on PPARγ promoter
To assess whether each E-box motif in the mouse PPARγ promoter is functional, we performed site-directed mutagenesis to destruct putative E-boxes in the -707 and -154 of the mouse PPARγ promoter. E-boxes in the -472 of the PPARγ promoter were excluded by the evidence that consensus sequence of E-box-472 was not found in rat or human. The plasmids containing the mouse PPARγ promoter followed by luciferase constructs were mutated with the following oligonucleotide primers: sense 5′- ATT GGA ATA CTA CTG TGGTAC CTA TTG ATA GAT AAA-3′ and antisense 5′-TTT ATC TAT CAA TAG GTACCA CAG TAG TAT TCC AAT-3 (mutant E-box-707), sense 5′-CAC ACC ATT TTG TCA TTACGC GCT CTC AGT CAG GAC-3′ and antisense 5′-GTC CTG ACT GAG AGC GCGTAA TGA CAA AAT GGT GTG-3′ (mutant E-box-154). PCR was conducted with using 2X EF-Taq DNA Premix (SolGent, Seoul, Korea) according to the manufacturer’s instructions. Cycle conditions were: 2 min hot start at 95 °C, 40 cycles of 20 s at 95 °C, 40 s at 60 °C, 7 min at 72 °C, and extension at 72 °C for 20 min. Digestion with DpnI was then proceeded in 26 μl of the reaction at 37 °C for 2 h, followed by transformation into DH5α Escherichia coli to select and amplify the mutated plasmids. All constructs were confirmed by sequencing (SolGent).
Luciferase reporter assay
Plasmids containing the mouse PPARγ promoter (−2,000 bp to +27 bp from transcription start site)-luciferase constructs were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Plasmids containing mouse C/EBPα and Snail were simultaneously co-transfected in 3T3-L1 preadipocytes. Assays were performed according to the method described previously [11] using the Dual-Luciferase Reporter Assay System (Promega). Luciferase activity was measured using a microplate luminometer (Centro XS3 LB960; Berthold Technologies, Bad Wildbad, Germany). The Renilla luciferase signals were normalized to the internal firefly luciferase transfection control. Transfections were performed in triplicate for each independent experiment.
RNA isolation and reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from cultured 3T3-L1 preadipocytes and adipocytes using TRIzol reagent (Invitrogen) as described by the manufacturer’s instructions. Twenty micrograms of RNA were treated with RNase-free DNase I (QIAGEN Korea Ltd., Seoul, Korea) for 10 min at 25 °C. Random hexamers were used to synthesize single-stranded complementary DNA (cDNA) using 2 μg of DNase I-treated RNA in a 20-μl reaction volume containing 50 mM of Tris–HCl (pH 8.3), 8 mM of MgCl2, 50 mM of NaCl, 1 mM of dithiothreitol, 1 mM of each dNTP (Bioneer, Seoul, Korea), 22 U of RNase inhibitor (Bioneer), and 10 U of Moloney murine leukemia virus RT (Promega) for 60 min at 37 °C. A portion (1/40) of the cDNA solution was treated with 2.5 U of Taq DNA polymerase (SolGent) for amplification of Snail and β-actin (used as an internal control). The PCR was performed under the following conditions: 15 min hot start at 95 °C, followed by 35 cycles, with each cycle programmed to 20 s at 95 °C, 40 s at 58 °C, followed by 30 s at 72 °C, and a final extension step at 72 °C for 5 min. An aliquot (20 %) of each PCR product was resolved by electrophoresis on a 1.5 % agarose gel, and the DNA product was visualized using ethidium bromide. Oligonucleotide primers of mouse used for PCR amplification were as follows: Snail, forward 5′- TTC CAG CAG CCC TAC GAC CAG -3′ and reverse 5′- CGG ACT CTT GGT GCT TGT GGA -3′; PPARγ, forward 5′-CTC CGT GAT GGA AGA CCA CT-3′ and reverse 5′-AAC CAT TGG GTC AGC TCT TG-3′; C/EBPα, forward 5′-TGG ACA AGA ACA GCA ACG AG-3′ and reverse 5′-TCA CTG GTC AAC TCC AGC AC-3′; β-actin, forward 5′-CCA GGG TGT GAT GGT GGG AAT G-3′ and reverse 5′-CGC ACG ATT TCC CTC TCA GCT G-3′.
Western blotting
Preadipocytes and adipocytes in 10-cm plates were lysed in RIPA buffer (Cell Signaling, Danvers, MA, USA), and the protein content was measured using the Coomassie (Bradford) Protein Assay Kit (Pierce, Rockford, IL, USA). Equal amounts of protein (60 μg) were heat-denatured in 4 × sample buffer (2 % sodium dodecyl sulfate, 62.5 mM Tris (pH 6.8), 0.01 % bromophenol blue, 1.43 mM mercaptoethanol, and 0.1 % glycerol), separated on 10 or 12 % sodium dodecyl sulfate–polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Roth, Karlsruhe, Germany). The membranes were treated with the appropriate antibodies against the following proteins: Snail (Abcam, Cambridge, MA, USA), C/EBPα, PPARγ (Santa Cruz), adiponectin (Affinity Bioreagents, Rockford, IL, USA), GSK3β (Cell Signaling, Danvers, MA, USA), β-actin (Sigma-Aldrich).
Immunofluorescence
To detect the expression of Snail in cultured 3T3-L1 preadipocytes and adipocytes, cells were plated on fibronectin-coated cover slips and washed with PBS twice and fixed with 4 % paraformaldehyde in PBS (pH 7.4) for 5 min. Cells were blocked in PBS containing 5 % bovine serum albumin for 2 h at room temperature and incubated with the primary rabbit anti-Snail antibody (1:200, Abcam) overnight at 4 °C, followed by incubation with the secondary goat anti-rabbit IgG-FITC (1:400, Invitrogen) for 2 h at room temperature. Propidium iodide (PI, 1:1,000, Invitrogen) was used as a nuclear counterstain. Images were obtained using a Zeiss microscope (Axioskop, Carl Zeiss Inc., Oberkochen, Germany).
Cell proliferation and survival assay
Cell survival was assessed using the MTT (3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyltetrazolium bromide) assay (Sigma-Aldrich). Cells were plated on 24-well plates (5 × 104 cells/well) and transfected at 70 % confluence with the pcDNA3 and pcDNA3-Snail plasmid using Lipofectamine 2000 reagent (Invitrogen). MTT was added at a final concentration of 0.5 mg/ml at 48 h after transfection, after which the cells were incubated for 4 h at 37 °C and suspended in 250 μl of dimethyl sulfoxide (Sigma-Aldrich). Optical density values were read at 540 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Each experiment was carried out in triplicate and three measurements were taken for each experiment. To confirm the total cell number, 1 × 105 cells were seeded into six-well plates. Forty-eight hours after transfection, cells were collected by trypsin–EDTA treatment and suspended in PBS containing 10 % FBS. Cell viability was determined by direct counting of cells on a hemocytometer in the presence of 0.5 % Trypan blue.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was carried out using the online protocol resource (http://mescaline.igh.cnrs.fr/EpiGeneSys/images/stories/protocols/pdf/20111025152730_p10.pdf; accessed February 4, 2009) provided by the Epigenome Network of Excellence (Institute of Human Genetics, CNRS UPR 1142, Montpellier, France) as follows: (A) cells were cultured to 90 % confluence in 150-mm culture dishes, (B) cells were collected by scraping in 20 ml of ice-cold PBS, (C) after sonication, the supernatant was centrifuged at 16,000 × g for 5 min, and (D) the supernatant was precleared by incubation with G-Sepharose (GE Healthcare, Waukesha, WI, USA). Antibodies against histone deacetylase (HDAC) 1 and 2, acetylated-H3, and histone H3 were purchased from Santa Cruz. The precipitated DNA was analyzed by 30 cycles of PCR and primers used for PCR amplification were as follows: ChIP primer 1, forward 5′-CAC TTA AAC ATC AAC CAT TGG -3′ and reverse 5′-GGA GTT TCA ACC AAA GAT AA-3′; ChIP primer 2, forward 5′- TTC ACG CCC CTC ACA GAA CA-3′ and reverse 5′- GTG CCA GCC AAT TCA GGC CTG-3′. The primers spanned the upstream region of the PPARγ promoter from −738 to −439 bp (ChIP primer 1) and −277 to −112 bp (ChIP primer 2).
Animals, histological analysis, and immunohistochemistry
Animal experiments were performed using 8-week-old male athymic immunodeficient nude mice (Charles River Japan, Inc., Yokohama, Japan). All animal studies were approved by the Animal Care and Use Committee of the Yonsei University College of Medicine. Each group had four animals and was housed in an animal room maintained at a temperature of 23 ± 2 °C and a humidity of 55 ± 5 %. The mice were exposed to a 12-h light, 12-h dark cycle and fed a standard unrestricted diet. Transfected 3T3-F442A preadipocytes were collected, washed twice with PBS, resuspended in DMEM, and injected (3 × 107 cells) into the flanks of mice at day 0. After 5 weeks from injection, the mice were euthanized and tumor masses were removed. The collected masses were fixed using 10 % neutral-buffered formalin and processed into paraffin blocks. Sections (4 μm) were stained with hematoxylin and eosin (H&E) and reviewed by a pathologist. Immunohistochemical study for the expression of Snail was performed as previously described [10]. After antigen retrieval process with citrate buffer (pH 6.0 at 90 °C), specimens were incubated with anti-Snail antibody (1:400; Abcam), specific biotinylated secondary antibodies (1:100; Vector Laboratories, Burlingame, CA, USA) and streptavidin-peroxidase (DAKO, Kyoto, Japan), sequentially. Diaminobenzidine (Vector Laboratories) was used as a chromogen, and counterstaining was conducted using hematoxylin.
Statistical analyses
The Mann–Whitney U test and Kruskal–Wallis test were applied to determine significant differences between the groups. The R software (version 2.15.0) was used for all statistical analyses. A p value less than 0.05 was considered statistically significant. All results are presented as representative data from three experiments.
Results
Expression of the transcription factor Snail during adipocyte differentiation
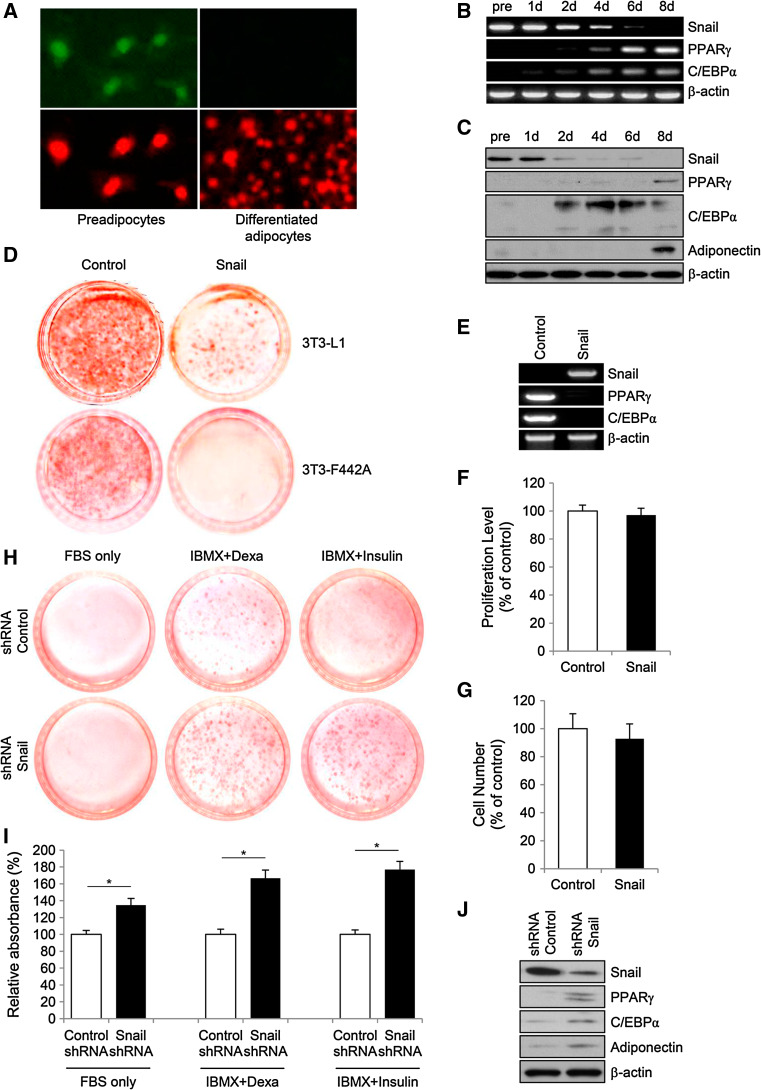
Immunofluorescent staining with anti-Snail antibody showed that Snail (stained green) is localized in the nucleus of 3T3-L1 preadipocytes (stained red), whereas Snail is not detected in differentiated adipocytes (Fig. 1a). To examine the time course of Snail expression during differentiation, 3T3-L1 cells were cultured for the indicated times from 1 to 8 days after initiating the treatment with adipogenic hormonal cocktails. RT-PCR and immunoblot analyses showed that the amount of Snail mRNA and protein were high in preadipocytes, whereas Snail expression dramatically decreased during adipogenesis after induction with adipogenic cocktails (Fig. 1b, c). Consistent with previous findings, the expression of PPARγ and C/EBPα, which are the two major transcription factors required for adipocyte differentiation, was observed within approximately 2 days of induction, followed by the expression of adiponectin.
Fig. 1.
The association between Snail and adipogenesis. The expression of transcription factor Snail during differentiation of 3T3-L1 preadipocytes. Immunofluorescent assay using anti-Snail antibody (stained green) was performed on 3T3-L1 preadipocytes and fully differentiated adipocytes. Propidium iodide (stained red) was used for nuclear stain. Magnification, ×400 (a). To examine the time course of the expression of Snail during differentiation of 3T3-L1 preadipocytes, 3T3-L1 cells cultured for the indicated times after initiating the treatment of adipogenic hormonal cocktail were subjected to RT-PCR (b), or to immunoblot analysis (c). All results are representative of three independent experiments. Overexpression of Snail inhibits the in vitro adipogenesis of murine preadipocytes. On day 8 after treatment with adipogenic cocktails, 3T3-L1 and 3T3-F442A cells transfected with plasmids harboring Snail-encoding DNA or backbone control plasmids were stained with Oil Red O (d). The data are representative of three independent experiments. RT-PCR analyses (e) of Snail, C/EBPα, and PPARγ expression in 3T3-L1 cells on day 8 after transfection with either a control plasmid or a plasmid expressing Snail (pcDNA3-Snail). Effect of Snail on the proliferation and survival of 3T3-L1 preadipocytes. Cells were transfected with either a control plasmid or a plasmid expressing Snail. After 48 h of transfection, proliferation of the 3T3-L1 cells was assessed by MTT assay (f), and cell viability was examined using tryptophan assay (g). The proliferation levels of cells harboring the Snail plasmids or the control plasmids were not significantly different. The viability of the Snail-overexpressing cells was similar to that of the control cells. The number of Snail-expressing cells is represented as a percentage of those in the control group. Results of six independent experiments were averaged and plotted as the mean ± SD. Knockdown of Snail stimulates the in vitro adipogenesis of murine preadipocytes. Snail or control shRNA were introduced into 3T3-L1 cells followed by treatment with indicated adipogenic inducers or FBS only. Oil Red O staining (h) and spectrophotometer analysis (i) were performed for quantification of lipid accumulation. Immunoblot analyses (j) of Snail, C/EBPα, PPARγ and adiponectin expression in 3T3-L1 cells on day 8 after treated with either Snail or control shRNA. The data are representative of three independent experiments. Results of three independent experiments were averaged and plotted as the mean ± SD
Snail inhibits adipogenesis in vitro
To investigate whether the constitutive expression of Snail affects adipocyte differentiation in vitro, the expression construct pcDNA3 Snail was transfected into preadipocytes. Oil Red O staining at 8 days after induction with adipogenic cocktails demonstrated that Snail overexpression significantly suppressed adipogenesis in the transfected 3T3-L1 and 3T3-F442A cells (Fig. 1d). This finding was further supported by the results from RT-PCR analysis, showing that cells transfected with a plasmid encoding Snail did not express mRNA for PPARγ and C/EBPα after 8-day treatment with adipogenic cocktails (Fig. 1e).
During adipocyte differentiation, impairment in the process of mitogenic clonal expansion can result in the failure of preadipocytes to effectively differentiate into mature adipocytes [12]. To explore whether Snail regulates the proliferation or viability of the preadipocytes in vitro, the 3T3-L1 preadipocytes were transfected with pcDNA3 Snail or control backbone plasmids. Forty-eight hours after transfection, MTT assay and direct cell counting were performed using the transfected preadipocytes (Fig. 1f, g). The proliferation of preadipocytes in the presence of the pcDNA3 Snail or the control plasmid was not significantly different, and the amount of cell death was similar in both the groups. We explored the capacity of adipogenic potentials in 3T3-L1 preadipocytes after knocked-down the expression of Snail using shRNA. Under the condition of FBS, IBMX + dexamethasone or IBMX + insulin, the proportion of differentiated adipocytes was significantly increased in cells with Snail shRNA comparing to those with control shRNA (Fig. 1h, i), which was further confirmed by immunoblots showing that the expression of PPARγ, C/EBPα and adiponectin were significantly increased in Snail shRNA-treated cells (Fig. 1j). These findings indicate that Snail blocks adipogenesis in 3T3-L1 preadipocytes without affecting cell proliferation or viability.
Snail suppresses the expression of PPARγ by directly binding to the E-box in the PPARγ gene promoter
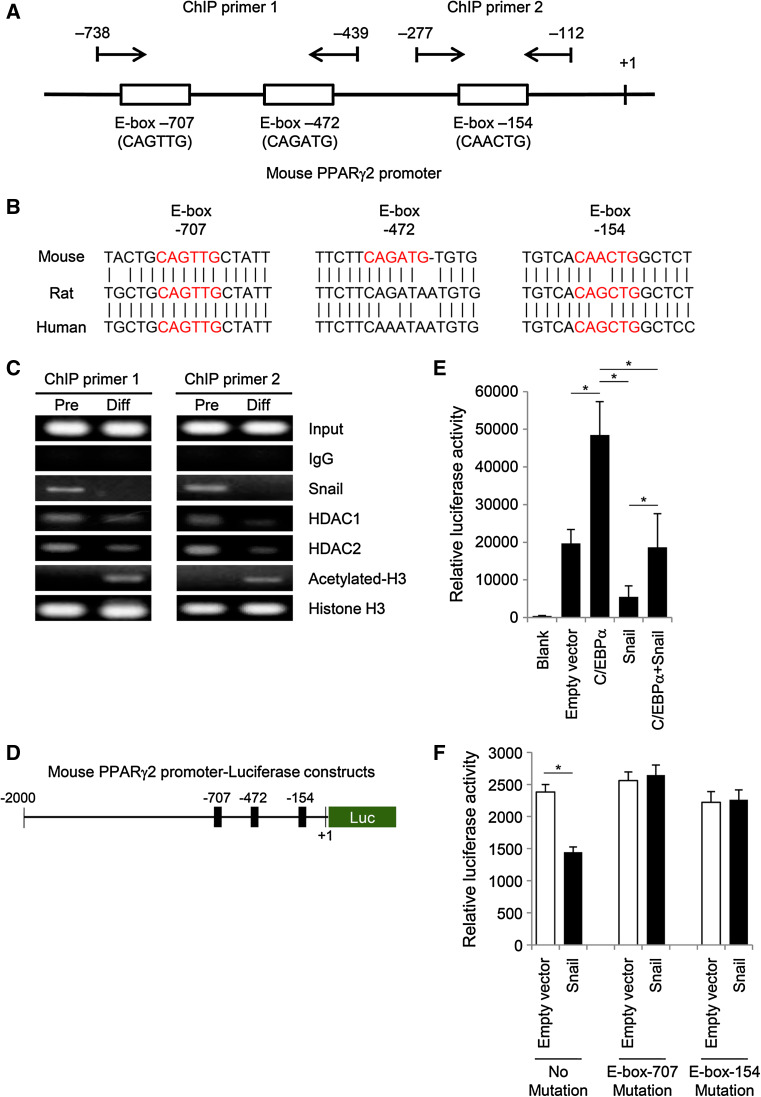
Overexpression of Snail significantly suppressed the expression of PPARγ, which is one of the most crucial transcription factors for induction of adipogenic differentiation. It is known that transcription factor Snail represses E-cadherin expression by directly binding to the E-box motifs in the E-cadherin promoter [6]. In a similar manner, we hypothesized that Snail might regulate the expression of PPARγ by directly binding to the E-box motifs in the PPARγ promoter region. A schematic diagram of the mouse PPARγ2 promoter sequence is depicted in Fig. 2a. Three putative E-box motifs are located in the 1-kb PPARγ2 promoter sequence. Each motif contains a slightly different DNA sequence, which is consistent with the consensus sequence of the E-box (CANNTG). Among three putative E-box motifs in the mouse PPARγ2 gene promoter, two E-box sequences (E-box-707 and -154) are conserved in rat and human PPARγ2 gene promoters (Fig. 2b). The presence of these conserved motifs suggests the possibility that E-boxes in the PPARγ2 gene promoter might be involved in regulation of PPARγ2 expression.
Fig. 2.
Snail inhibits PPARγ expression by directly binding to the E-box in the mouse PPARγ2 gene promoter. a Schematic diagram of the mouse PPARγ2 promoter sequence spanning from –1,021 bp to +1 bp (GenBank: NT_039353.7), with arrows indicating the forward and reverse primers used to amplify ChIP products. Pairs of ChIP primer 1 are located around the two distal E-boxes, which are the possible binding sites for Snail, and one proximal E-box is surrounded by pairs of ChIP primer 2. b E-box motifs in the mouse PPARγ2 gene promoter are conserved in rat and human PPARγ2 gene promoters. Mouse has three putative E-box regions, while rat and human have two E-box motifs (E-box-707 and -154). Predicted E-box motifs in each group are presented in red. c ChIP assay for the upstream region of the PPARγ2 promoter in 3T3-L1 cells was performed using antibodies against Snail, HDAC1, HDAC2, acetyl histone H3, or total histone H3. Presence of the promoter sequence prior to immunoprecipitation was confirmed by PCR (input sample), and immunoglobulin G antibody was used as a negative control. Snail is recruited to the E-box motif of the PPARγ2 promoter in 3T3-L1 preadipocytes, whereas the recruitment of Snail to the PPARγ2 promoter is decreased after differentiation of the preadipocytes into adipocytes. PCR products were separated by electrophoresis on 2 % agarose gels containing ethidium bromide. The results are representative of three independent experiments. d Schematic diagram of the luciferase reporter assay plasmid containing the mouse PPARγ2 promoter. The number indicates the 5′ end of the PPARγ2 promoter gene. e To confirm whether Snail acts as a transcriptional repressor of PPARγ2 expression by binding to the PPARγ2 promoter, plasmids containing Snail or C/EBPα were cotransfected into 3T3-L1 preadipocytes along with the reporter vector containing the PPARγ2 promoter. Reporter assay showed that Snail suppresses C/EBPα-induced transactivation of the PPARγ2 promoter as well as the basal activity of the PPARγ2 promoter. The pGL3-basic vector and the blank well were used as negative controls, whereas C/EBPα-expressing plasmid was used as a positive control. Luciferase activity was normalized using Renilla expression activity. The data are presented as the mean ± SD of four independent experiments. f To assess whether each putative E-box is functional in vitro, sequences of E-boxes in the mouse PPARγ2 promoter were replaced by site-directed mutagenesis. 3T3-L1 preadipocytes were cotransfected with reporter plasmids bearing mutant E-box-707 or -154 and either Snail plasmid or empty pcDNA3 vector (control). Luciferase activity was normalized using Renilla expression activity. The data are presented as the mean ± SD of three independent experiments *p < 0.001
To further confirm that Snail is recruited to the putative E-box motifs in the PPARγ2 promoter, a ChIP assay was performed using two primers that covered the region of the three putative E-boxes (Fig. 2c). Chromatin samples were prepared from 3T3-L1 preadipocytes and from differentiated adipocytes and immunoprecipitated with specific antibodies against Snail, HDAC1, HDAC2, acetylated histone H3 and total histone H3. In preadipocytes, both Snail and HDAC1/2 were recruited at the PPARγ2 promoter region containing the E-box motifs. However, the binding of Snail and HDAC1/2 was significantly decreased after adipogenic differentiation, whereas acetylation levels at histone H3 were reciprocally increased.
To determine whether Snail regulates PPARγ expression, the activity of the PPARγ2 promoter in 3T3-L1 preadipocytes was measured by a luciferase assay using reporter plasmids that contained a 2-kb proximal fragment of murine PPARγ2 promoter (pGL-PPARγ2, Fig. 2d) [13]. Cells were transfected with either C/EBPα or Snail expression vectors, or both. The expression of PPARγ2 was dramatically increased under the presence of C/EBPα expression, whereas the co-expression of Snail was able to inhibit the luciferase activity stimulated by C/EBPα (Fig. 2e). Similarly, the transfection of Snail significantly suppressed the luciferase activity when compared with luciferase activity in presence of the empty vector, implying an inhibitory effect of Snail on PPARγ2 transcription.
To further confirm whether each putative E-box motif is functionally acting as a negative cis element to suppress the promoter activity of PPARγ, mutation of putative E-boxes was generated by site-directed mutagenesis. After cotransfection with reporter plasmids bearing mutant E-box-707 or -154 and either Snail plasmid or empty pcDNA3 vector, mutation in either E-box-707 or -154 significantly restored the luciferase activity suppressed by Snail expression in 3T3-L1 cells (Fig. 2f). These findings indicate that Snail suppresses the expression of PPARγ2 by directly binding to the E-box in the PPARγ gene promoter.
Snail is involved in Wnt–GSK3β signaling-mediated inhibition of adipogenesis
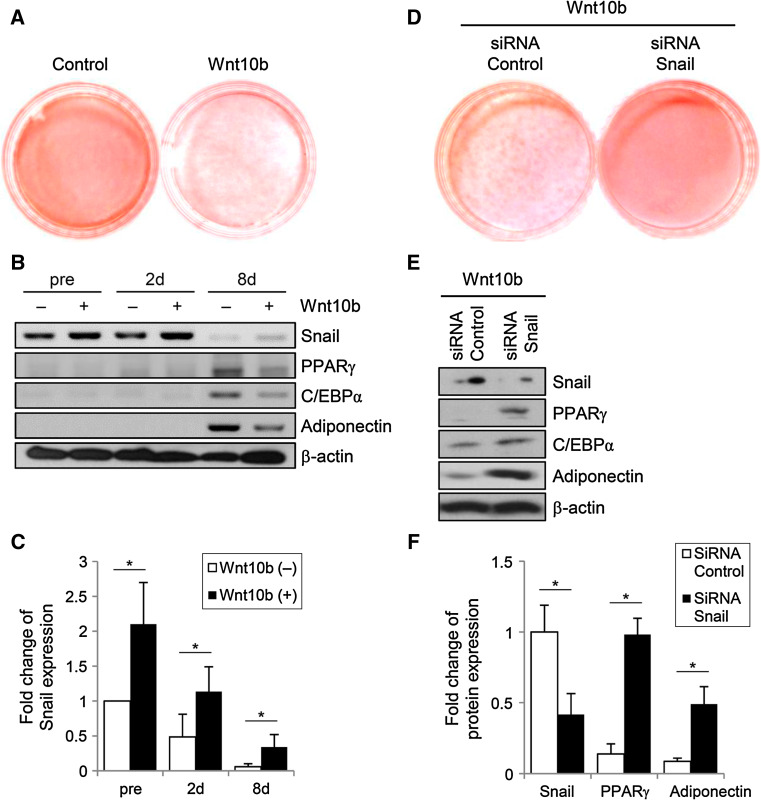
Wnt signaling is one of the most essential pathways that blocks adipocyte differentiation through inhibition of adipogenic transcription factors, such as C/EBPα and PPARγ [4]. Moreover, it has been established that Wnt regulates its downstream enzyme GSK3β, which mediates the phosphorylation of Snail to modulate the expression of E-cadherin [10]. Based on these findings, we investigated whether Snail expression is affected by the Wnt–GSK3β axis. Wnt10b-expressing cells were not able to differentiate into adipocytes, whereas control preadipocytes showed a normal adipogenic potential (Fig. 3a). Immunoblotting revealed that the expression of Snail was more than twofold higher in both Wnt10b-transfected preadipocytes and differentiated adipocytes when compared with control cells not transfected with Wnt10b (Fig. 3b, c).
Fig. 3.
Wnt10b blocks adipogenesis by induction of Snail expression in 3T3-L1 cells. a On day 8 after treatment with adipogenic cocktail, the control cells and cells transfected with plasmids harboring Wnt10b were stained with Oil Red O. These data are representative of three independent experiments. b Immunoblot analysis shows that Wnt10b increases the expression of Snail in preadipocytes and during adipogenic differentiation. The transfected 3T3-L1 cells were cultured for the indicated times before and after initiating the treatment with adipogenic hormonal cocktail. Actin was used as a loading control. c The graph shows densitometric analysis of the optical density-based data of the immunoblots shown in Fig. 4b. It can be seen that Wnt10b increases the expression of Snail in 3T3-L1 cells, regardless of differentiation. *p < 0.001 vs. compared to the cells without Wnt10b treatment (control). Data are presented as the mean ± SD of three independent experiments. d To assess the role of Snail in the Wnt-mediated suppression of adipogenesis, 3T3-L1 preadipocytes transfected with Wnt10b-expressing plasmids were treated with Snail siRNA or scrambled control siRNA. On day 8, after the treatment with adipogenic cocktails, Snail siRNA-transfected cells show full differentiation into adipocytes. e Immunoblot analysis of Snail, C/EBPα, PPARγ and adiponectin expression in 3T3-L1 cells on day 8 after treated with either Snail or control siRNA. The data are representative of three independent experiments. f Densitometric graph of the optical density-based data of the immunoblots. *p < 0.001 vs. compared to the cells with control siRNA treatment (control). Data are presented as the mean ± SD of three independent experiments
To examine whether Snail is involved in the pathway of Wnt-mediated suppression of adipogenesis, Snail expression was knocked down by using siRNA against Snail, followed by the cultivation of the preadipocytes in adipogenic media for 8 days. The capacity of adipogenic differentiation in Wnt10b-expressing preadipocytes was restored after treatment with siRNA against Snail, whereas control siRNA had no effect on the Wnt-mediated suppression of adipogenesis (Fig. 3d). This finding was further supported by the results from immunoblots, showing that cells treated with Snail siRNA had significantly higher levels of PPARγ, C/EBPα, and adiponectin (Fig. 3e, f).
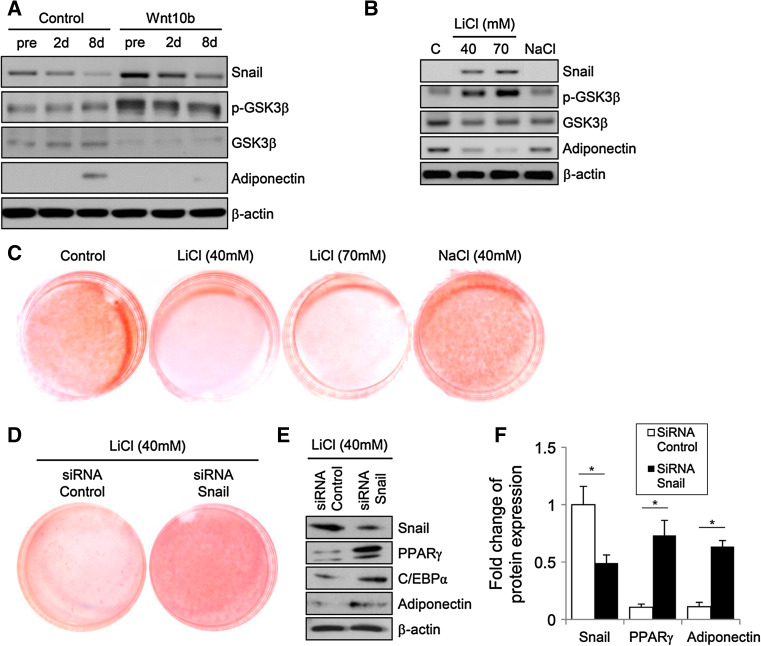
GSK3β mediates posttranslational regulation of Snail via phosphorylation; phosphorylated Snail is then targeted for degradation by the ubiquitin system. The kinase activity of GSK3β is abolished when Wnt signaling induces phosphorylation at Ser 9 in GSK3β. To test whether GSK3β regulates adipocyte differentiation via modulating the expression of Snail, immunoblotting was performed using antibodies against the phosphorylated form of GSK3β and total GSK3β protein after treatment with Wnt10b (Fig. 4a). Compared to the control group, Wnt10b-treated preadipocytes and adipocytes showed increased expression levels of phosphorylated GSK3β and Snail. To manipulate the activity of GSK3β, 3T3-L1 cells were cultured in the presence of either LiCl, which is a well-known inhibitor of GSK3, or NaCl (as a control), followed by Oil Red O staining. As expected, inhibition of GSK3β suppressed adipocyte differentiation (Fig. 4c), and the expression level of Snail significantly increased in a dose-dependent manner in LiCl-treated cells (Fig. 4b). During the treatment with LiCl, Snail expression was knocked down by using siRNA against Snail, followed by the cultivation of the preadipocytes in adipogenic media for 8 days. The capacity of adipogenic differentiation in LiCl-treated preadipocytes was restored after treatment with Snail siRNA (Fig. 4d), which was further confirmed by immunoblots showing that the expression of PPARγ, C/EBPα and adiponectin were significantly increased in Snail siRNA-treated cells (Fig. 4e, f). These results indicate that the Wnt–GSK3β pathway blocks adipogenesis via induction of Snail in 3T3-L1 cells.
Fig. 4.
Inhibition of GSK3β suppresses adipogenesis by induction of Snail expression in 3T3-L1 cells. a Immunoblot analysis shows that Wnt10b increases the expression of phosphorylated GSK3β and Snail in preadipocytes during adipogenic differentiation. Transfected 3T3-L1 cells were cultured for the indicated times before and after initiating the treatment with adipogenic hormonal cocktail. Actin was used as a loading control. b Immunoblot analysis of differentiated adipocytes treated with the indicated reagents on day 8. The expression levels of phosphorylated GSK3β and Snail are significantly increased in LiCl-treated cells. Cells with no reagent and NaCl-treated cells were used as controls. Actin was used as a loading control. c On day 8 after treatment with adipogenic cocktail, control and cells treated with LiCl (40 and 70 mM) or NaCl (40 mM) were stained with Oil Red O. Cells with no reagent and NaCl-treated cells were used as controls. To assess the role of Snail in the GSK3β-mediated suppression of adipogenesis, 3T3-L1 preadipocytes transfected with Snail siRNA or scrambled control siRNA were treated with LiCl (40 mM). On day 8, after the treatment with adipogenic cocktails, Snail siRNA-transfected cells differentiated into adipocytes, confirmed by Oil Red O staining (d) and immunoblots (e). The figures are representative of three independent experiments. f Densitometric graph of the optical density-based data of the immunoblots. *p < 0.001 vs. compared to the cells with control siRNA treatment (control). Data are presented as the mean ± SD of three independent experiments
Snail inhibits adipogenesis in vivo
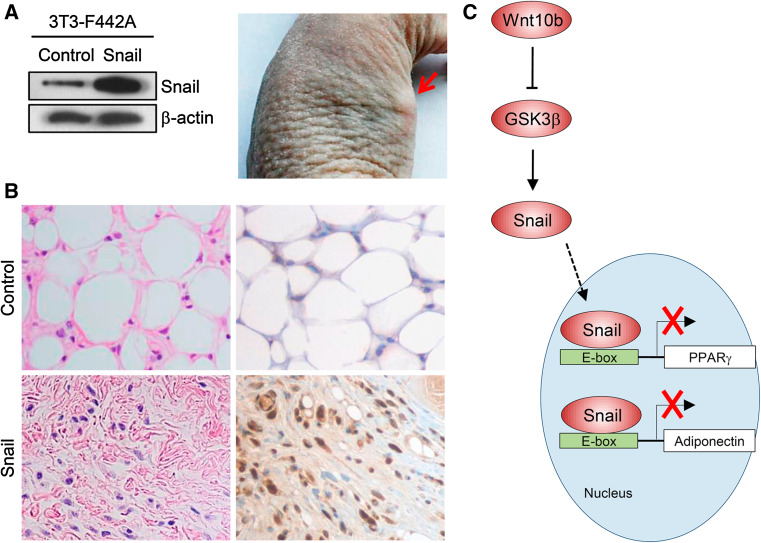
To support the in vitro evidence that Snail inhibits adipogenesis, we adopted an in vivo model of adipocyte differentiation [14]. The 3T3-F442A preadipocytes were transfected with either Snail-expressing or control backbone plasmids (Fig. 5a, left) and harvested with trypsin, after which 3 × 107 cells were injected subcutaneously into the flank area of athymic nude mice. Injected sites were initially swollen; however, the swelling disappeared shortly over a few days. After 7 days, a flat thickened mass began to appear in the injected flank area, and discrete fat pads were formed, which increased in size over the next several weeks (Fig. 5a, right). The mice were killed at 5 weeks after implantation of the transfected preadipocytes. A well-defined mass resembling fat tissue (indicative of normal adipocyte differentiation) was observed in the mice injected with cells containing a backbone control plasmid. Histology of the isolated tissues showed that the control plasmid-transfected 3T3-F442A cells were normally differentiated into adipocytes, whereas Snail plasmid-transfected cells were not differentiated into adipocytes, presenting as undifferentiated fibroblast-like cells (Fig. 5b, left column). Immunohistochemistry with anti-Snail antibody was also performed in the background stain of hematoxylin, showing that Snail expression was strongly positive (stained brown) in the tissues of mice which were injected with 3T3-F442A cells containing Snail plasmids, whereas differentiated adipocytes in the tissue of control mice demonstrated no expression of Snail (Fig. 5b, right column). These findings suggest that Snail also blocks adipocyte differentiation in a physiological in vivo system.
Fig. 5.
Overexpression of Snail inhibits adipogenesis in vivo. The 3T3-F442A cells were transfected with plasmids harboring Snail or backbone control plasmids, confirmed by immunoblots (a, left panel). After 2 days, preadipocytes (3 × 107 cells) were harvested with trypsin and were injected subcutaneously into the flank area of athymic mice. After 5 weeks of implantation, a mass was developed at the site of injection. Red arrows indicate mass formation in the flank (a, right panel). Histology of the tissues isolated from athymic mice xenografts (magnification, ×400). The dissected mass was fixed in formalin, followed by staining with hematoxylin and eosin (H&E) (b, left column). Immunohistochemistry with anti-Snail antibody was also performed in the background of hematoxylin (b, right column). The left upper figure shows normal adipogenesis in 3T3-F442A cells of the control group, whereas the left bottom figure indicates that Snail-overexpressing cells were not differentiated into adipocytes in vivo. Immunohistochemistry shows Snail-positive brown nuclei in the tissue of mice injected with Snail-overexpressing cells (right bottom), while differentiated adipocytes in control mice have no expression of Snail. Figures are representative of four tissues. c Schematic illustration showing the putative mechanism by which Snail inhibits adipocyte differentiation
Discussion
Recently, the obesity epidemic has drawn much attention and has persuaded research in the area of adipose cell biology, particularly the development and formation of white adipose tissue. Adipocyte differentiation is a complex process induced by external stimuli such as hormones and chemokines, followed by the precise coordination and control of transcriptional factors, cell-cycle regulators, and other cofactors [3]. During adipogenesis, the transcription factors PPARγ and C/EBPα are major determinants of cell differentiation [15]. PPARγ, a member of the nuclear receptor superfamily, is a well-established target of thiazolidinedione, which is an insulin-sensitizing antidiabetic drug [16]. A number of studies have shown that PPARγ is essential for adipogenesis both in vitro and in vivo, and that its expression is tightly regulated by various factors [3]. However, factors/effectors that control the expression of PPARγ have not been fully elucidated.
Our study is the first to identify the functional role of Snail in adipogenesis. The principal findings in the current study are: (1) the nuclear expression of Snail gradually decreases during adipocyte differentiation after exposure to an adipogenic cocktail, (2) Snail inhibits adipocyte differentiation by directly binding to the E-box motifs of the PPARγ promoter, without affecting the proliferation and survival of preadipocytes, and (3) Snail is involved in the Wnt–GSK3β signaling pathway-mediated suppression of adipogenesis.
It has been previously reported that Snail is expressed in NIH-3T3 fibroblasts [17], which lack the potential to differentiate in the presence of conventional adipogenic hormones. However, no research has been conducted to show the expression pattern of Snail in 3T3-L1 cells during adipocyte differentiation. Time-course analysis of the expression of Slug, which is the second member of the Snail superfamily, showed that its expression significantly increased in preadipocytes [8], which is consistent with our results. However, contrary to Snail, Slug expression was slightly diminished, but still observed in differentiated adipocytes, implying that their functions in the adipocytes might be different.
Consistent with our findings, Park et al. [18] also showed that Snail did not affect proliferation or viability of mesenchymal stem cells and preosteoblasts. This suggests that Snail is not involved in the process of mitotic clonal expansion during adipocyte differentiation. It is well established that the PPARγ promoter contains C/EBP binding sites, where C/EBPδ, C/EBPβ, and C/EBPα are able to bind and transactivate PPARγ gene expression during adipogenesis [19, 20]. Similar to Snail, GATA-2 and GATA-3, which belong to the zinc-finger family of transcription factors, directly suppress the expression of PPARγ by binding to the promoter region of the PPARγ gene [21]. These GATA transcription factors are initially found in preadipocytes, but dissipate after induction with adipogenic media [21], which is consistent with the expression pattern of Snail.
There are few published reports on the regulation of PPARγ expression by E-boxes, which are present in the proximal region of the PPARγ promoter. Fajas et al. [22] reported that PPARγ expression is regulated by ADD-1/SREBP-1 and SREBP-2 through the PPARγ1 and PPARγ3 promoters that contain a consensus E-box motif. Interestingly, genetic studies showed that polymorphism at the putative E-box in the PPARγ2 promoter region determined the promoter activity and was associated with obesity and type 2 diabetes in humans [23]. This suggests the clinical significance of E-box in the PPARγ gene promoter. Slug has been shown to bind to the PPARγ promoter and regulate PPARγ expression by modulating HDAC recruitment [8]. Similarly, our ChIP assay showed that the recruitment of HDAC and the status of the acetylated histone H3 were changed after differentiation of preadipocytes into adipocytes, indicating that Snail may be involved in HDAC-mediated transcriptional regulation process. This is a plausible hypothesis, particularly in light of previous reports that Snail suppresses E-cadherin expression by recruitment of HDAC and mSin3A complex in mammalian keratinocytes [24]. Therefore, the identification of putative Snail-binding sites in the PPARγ promoter provides further understanding of the complex regulation of PPARγ expression during adipogenesis.
Wnt10b, which is secreted from preadipocytes, inhibits the kinase activity of GSK3β. This is followed by the activation and accumulation of β-catenin, which translocates into the nucleus and stimulates the TCF/LEF transcription factors [15]. These events eventually inhibit the expression of PPARγ. However, further downstream effectors of this pathway have not been fully elucidated. It is known that Snail (SNAI1) usually acts as a transcription repressor and triggers EMT by directly downregulating the expression of E-cadherin in the mesoderm [6]. The Wnt–GSK3β-Snail axis has been identified in breast carcinoma cell lines by Yook et al. [10], indicating that Snail contains β-catenin-like canonical motifs that are regulated by Wnt–GSK3β-dependent phosphorylation. Based on this speculation and our current findings, it is reasonable for us to propose that Snail can also act as a mediator in the process of adipogenic differentiation under the control of the Wnt and GSK3β pathways (Fig. 5c).
We have previously demonstrated that Snail not only suppresses the expression of PPARγ but also inhibits adiponectin expression via directly binding to the E-box of the proximal region of the PPARγ [9]. Taken together, our data shows that Snail plays a crucial role during both early and late stages of adipocyte differentiation. Although we demonstrated the inhibitory effect of Snail on adipogenesis using an in vivo murine model, a previous study of transgenic animals expressing Snail reported that no abnormal phenotypes were observed [25]. This model showed only a mild increase (~20 % above normal) in Snail expression and was not examined for the purpose of adipose tissue research, such as effects of high-fat diet. Unfortunately, the effect of Snail on PPARγ expression in adipose tissue cannot be assessed in Snail knockout mice, because these mice show a lethal phenotype during gastrulation [26].
Recently, it was shown that human adipose tissue progenitor cells are not able to differentiate into normal fat cells through the action of TGFβ family members produced from macrophages within adipose tissues [27]. Progenitor cells affected by these macrophages were altered into myofibroblast-like cells, which expressed high levels of Snail. This decrease in the adipogenic potential of progenitor cells indicates a reduction in normal adipocyte hyperplasia, with a reciprocal increase in myofibroblasts. In addition, myofibroblasts can cause fibrosis in adipose tissue, which may inhibit adipocyte hypertrophy in mice [28]. Therefore, both decreased adipocyte hyperplasia and hypertrophy may reduce expandability of the subcutaneous fat tissue, leading to ectopic fat deposition [27]. Taken together, dysregulation of Snail in progenitor cells of adipose tissue by external factors, such as TGFβ, might affect the lipid-storing capacity of adipose tissues and promote a lipodystrophy-like condition.
The present study has some limitations, which should be addressed by further experiments. Further research is necessary to determine the association between Snail and β-catenin, which are both downstream mediator molecules of Wnt signaling and have inhibitory effects on adipogenesis. Moreover, experiments with genetically engineered animal models such as adipose tissue-specific Snail knockout mice or transgenic animals expressing Snail should be conducted in the future to confirm the putative role of Snail in adipocyte differentiation.
In conclusion, the present study demonstrated for the first time that Snail regulates adipocyte differentiation by binding to the E-box motif in the PPARγ promoter. Elucidation of the underlying mechanisms of Wnt/GSK3β-mediated suppression of adipogenesis may broaden the knowledge of the complex network of developmental processes in the adipose tissue and support the idea that Snail may be a promising candidate for the treatment of obesity and obesity-related diseases. Furthermore, identification of molecules that modulate the expression of Snail could be a rewarding approach for future obesity research.
Acknowledgments
We sincerely thank Chun Sik Park, MD, PhD, for his critical comments on the study. This study was supported by a faculty research grant of Yonsei University College of Medicine for 2012 (6-2012-2010).Y. L., S. H. K. and Y. J. L. researched data. Y. L., S. H. K. and H. C. L. wrote the manuscript. E. S. K., B–W. L., B. S. C. J. W. K., and D. H. S. contributed to the discussion and reviewed and edited the manuscript. H. C. L. is the guarantor of this work and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
None.
Abbreviations
- C/EBPα
CCAAT-enhancer-binding protein alpha
- ChIP
Chromatin immunoprecipitation
- EMT
Epithelial–mesenchymal transition
- HDAC
Histone deacetylase
- PPARγ
Peroxisome proliferator-activated receptor gamma
- siRNA
Small interfering RNA
Footnotes
Yong-ho Lee and Soo Hyun Kim contributed equally to this work and should be considered as the first authors.
References
- 1.Nguyen DM, El-Serag HB. The epidemiology of obesity. Gastroenterol Clin North Am. 2010;39:1–7. doi: 10.1016/j.gtc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 3.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 5.Nieto MA. The Snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 6.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 7.de Frutos CA, Dacquin R, Vega S, Jurdic P, Machuca Gayet I, Nieto MA. Snail1 controls bone mass by regulating Runx2 and VDR expression during osteoblast differentiation. EMBO J. 2009;28:686–696. doi: 10.1038/emboj.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Mancera PA, Bermejo-Rodriguez C, Gonzalez-Herrero I, Herranz M, Flores T, Jimenez R, Sanchez-Garcia I. Adipose tissue mass is modulated by SLUG (SNAI2) Hum Mol Genet. 2007;16:2972–2986. doi: 10.1093/hmg/ddm278. [DOI] [PubMed] [Google Scholar]
- 9.Park YM, Lee YH, Kim SH, Lee EY, Kim KS, Williams DR, Lee HC. Snail, a transcriptional regulator, represses adiponectin expression by directly binding to an E-box motif in the promoter. Metabolism. 2012;61:1622–1632. doi: 10.1016/j.metabol.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor Snail. J Biol Chem. 2005;280:11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Kim JM, Lee EJ. Functional expression of CXCR4 in somatotrophs: CXCL12 activates GH gene, GH production and secretion, and cellular proliferation. J Endocrinol. 2008;199:191–199. doi: 10.1677/JOE-08-0250. [DOI] [PubMed] [Google Scholar]
- 12.Lee H, Lee YJ, Choi H, Ko EH, Kim J. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem. 2009;284:10601–10609. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J. The organization, promoter analysis, and expression of the human PPARγ gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 14.Mandrup S, Loftus TM, MacDougald OA, Kuhajda FP, Lane MD. Obese gene expression at in vivo levels by fat pads derived from s.c. implanted 3T3-F442A preadipocytes. Proc Natl Acad Sci USA. 1997;94:4300–4305. doi: 10.1073/pnas.94.9.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20:107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Lehrke M, Lazar MA. The many faces of PPARγ. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Franc C, Takkunen M, Dave N, Alameda F, Gmez S, Rodrguez R, Escriv M, Montserrat-Sents B, Bar T, Garrido M, Bonilla F, Virtanen I, García de Herreros A. Expression of Snail protein in tumor-stroma interface. Oncogene. 2006;25:5134–5144. doi: 10.1038/sj.onc.1209519. [DOI] [PubMed] [Google Scholar]
- 18.Park SJ, Jung SH, Jogeswar G, Ryoo HM, Yook JI, Choi HS, Rhee Y, Kim CH, Lim SK. The transcription factor Snail regulates osteogenic differentiation by repressing Runx2 expression. Bone. 2010;46:1498–1507. doi: 10.1016/j.bone.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Saladin R, Fajas L, Dana S, Halvorsen YD, Auwerx J, Briggs M. Differential regulation of peroxisome proliferator activated receptor γ1 (PPARγ1) and PPARγ2 messenger RNA expression in the early stages of adipogenesis. Cell Growth Differ. 1999;10:43–48. [PubMed] [Google Scholar]
- 20.Shi XM, Blair HC, Yang X, McDonald JM, Cao X. Tandem repeat of C/EBP binding sites mediates PPARγ2 gene transcription in glucocorticoid-induced adipocyte differentiation. J Cell Biochem. 2000;76:518–527. doi: 10.1002/(SICI)1097-4644(20000301)76:3<518::AID-JCB18>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 21.Tong Q, Dalgin G, Xu H, Ting CN, Leiden JM, Hotamisligil GS. Function of GATA transcription factors in preadipocyte-adipocyte transition. Science. 2000;290:134–138. doi: 10.1126/science.290.5489.134. [DOI] [PubMed] [Google Scholar]
- 22.Fajas L, Schoonjans K, Gelman L, Kim JB, Najib J, Martin G, Fruchart JC, Briggs M, Spiegelman BM, Auwerx J. Regulation of peroxisome proliferator-activated receptor γ expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol. 1999;19:5495–5503. doi: 10.1128/mcb.19.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller YL, Bogardus C, Beamer BA, Shuldiner AR, Baier LJ. A functional variant in the peroxisome proliferator-activated receptor γ2 promoter is associated with predictors of obesity and type 2 diabetes in Pima Indians. Diabetes. 2003;52:1864–1871. doi: 10.2337/diabetes.52.7.1864. [DOI] [PubMed] [Google Scholar]
- 24.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez-Mancera PA, Pérez-Caro M, González-Herrero I, Flores T, Orfao A, de Herreros AG, Gutiérrez-Adán A, Pintado B, Sagrera A, Sánchez-Martín M, Sánchez-García I. Cancer development induced by graded expression of Snail in mice. Hum Mol Genet. 2005;14:3449–3461. doi: 10.1093/hmg/ddi373. [DOI] [PubMed] [Google Scholar]
- 26.Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse Snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourlier V, Sengenes C, Zakaroff-Girard A, Decaunes P, Wdziekonski B, Galitzky J, Villageois P, Esteve D, Chiotasso P, Dani C, Bouloumie A. TGFβ family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages. PLoS One. 2012;7:e31274. doi: 10.1371/journal.pone.0031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]