Abstract
Connexins and pannexins form connexons, pannexons and membrane channels, which are critically involved in many aspects of cardiovascular physiology. For that reason, a vast number of studies have addressed the role of connexins and pannexins in the arterial and venous systems as well as in the heart. Moreover, a role for connexins in lymphatics has recently also been suggested. This review provides an overview of the current knowledge regarding the involvement of connexins and pannexins in cardiovascular physiology.
Keywords: Connexins, Pannexins, Gap junction channel, Hemichannel, Cardiovascular, Physiology
Introduction
Connexins and pannexins are families of transmembrane proteins that are expressed throughout the mammalian body. Both connexins and pannexins are inserted into the cell membrane as hexamers called connexons or pannexons, respectively. These structures can function as transmembrane channels that allow diffusion of small and soluble factors, such as calcium ions, adenosine triphosphate (ATP) and cyclic adenosine monophosphate, from the cytosol to the extracellular space or vice versa [1]. Connexons can dock to a connexon expressed by an adjacent cell, thereby forming a gap junction channel [2]. Gap junction channels are crucial for direct cell-to-cell communication and are thus critical for many aspects of mammalian physiology. In contrast, pannexons are more typical membrane channels, which open to the extracellular space. The role of pannexins in physiology is less clear, although new evidence is showing a role for pannexin channels in the immune response and inflammation in a number of systems [3–6].
Connexins in the heart
With the extensive focus on the role of gap junctions in the heart over the past decades [7], one would think that we know everything there is to know about them, but our knowledge of these structures continues to grow. Gap junctions were thought to be stand-alone structures that had the singular role of passing electrical current directly from the cytoplasm of a cardiomyocyte to the cytoplasm of the neighboring cardiomyocyte, thus propagating the electrical signal across the working myocardium. Studies have now shown that cardiac gap junctions form complexes with a myriad of other proteins to accomplish connexin localization, regulation, coordinated function and turnover. These complexes regulate every aspect of connexin life [8]. Therefore, it is not the connexin alone that accomplishes electrical propagation in the heart, but rather a coordinated interaction within cells between connexins and other proteins which, taken together, have the overarching role of maintaining electrical propagation and coordinated cardiac contraction. Much of the study of these complexes has focused on 1 of the cardiac connexins, connexin43 (Cx43), but based on the extensive roles that binding partners play, it seems likely that the other connexins will be found to utilize protein partners as well.
Over the years, every corner of the heart has been examined for the connexin isoform that regulates normal function in each particular compartment. It is generally agreed upon that if one were to grind up the whole heart and look for the types of connexins found, 3 individual isoforms would be found, namely Cx40, Cx43 and Cx45. In addition, there were early reports of Cx30.2 in the conduction system of the mouse, leading to discussion on whether this connexin regulated conduction velocity [9], but the human orthologue Cx31.9 was found to not occur in the human heart [10], indicating that it might play a species-specific role. The primary isoform in the heart, based solely on quantity, is Cx43. Like all cardiac connexins, Cx43 is not unique to the heart. In fact, this connexin isoform is almost ubiquitously expressed across the body systems, although not in every tissue type. Cx43 in the heart is found both in the ventricular myocytes in large quantities as well as in the atrial myocytes and the distal His Purkinje fibers [11], and is located at the intercalated disks of the individual myocytes in both compartments of the heart. Cx40 has a more restricted expression pattern, being found in the atria [12, 13] and Purkinje fibers [14], but not in the ventricular myocytes. The third connexin in the heart is Cx45, which is found in low levels primarily in the ventricular myocytes, where it appears to colocalize with Cx43 at the intercalated disk [15] and in the conduction system [12]. Early studies showed that gap junctions are clusters of channels located at the end of the cardiomyocytes in normal heart [16, 17]. Fluorescence imaging of normal atrial tissue shows that connexins appear to cover the surface of the cardiomyocyte when the cell is imaged in the longitudinal aspect, while a view of the transverse aspect of the cell shows that connexins are localized around the perimeter of the intercalated disk (Fig. 1). Ventricular connexins are localized similarly at the intercalated disks of the cardiomyocytes [17, 18]. During the early studies which looked at localization of cardiac gap junctions, researchers did not focus solely on the myocytes of the heart [19]. In fact, these studies specifically looked for junctional contacts in other cell types of the heart as well, including where fibroblasts and cardiomyocytes are in close contact. No other cell type except the cardiomyocyte showed gap junctions and no junction between heterologous cells types was found [19]. More recent studies have reviewed the possibility that junctions occur between cardiomyocytes and fibroblasts [20–23], but definitive proof of these interactions in vivo is still lacking [24, 25]. It is interesting to note that the basic structural studies were prescient of much of the focus of work today, including showing the first indication that cardiac gap junctions get disordered upon loss of normal cardiac function [26] and the presence of specialized membrane domains that were hypothesized at the time to be involved in formation of the junctional plaques [26]. It is these specialized membrane domains that are now the subject of great study, as we have begun to realize that connexins do not function alone at the intercalated disk within myocytes of the heart.
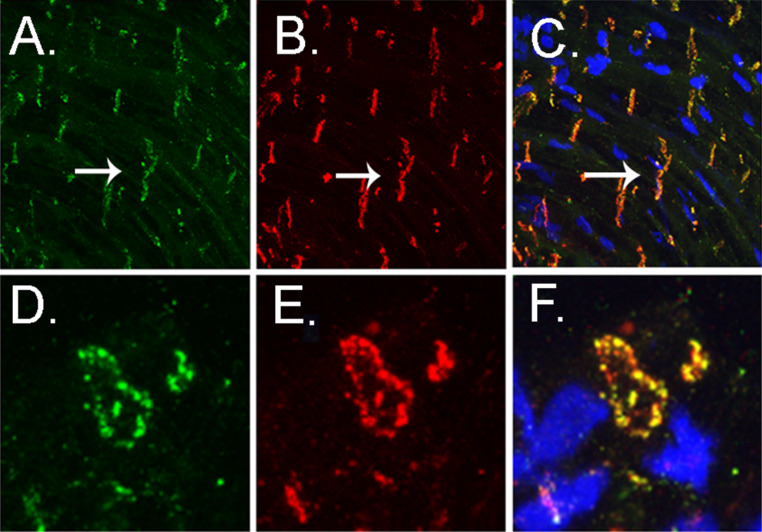
Fig. 1.
Immunostaining of Cx40 (a) and Cx43 (b) in atrial myocytes, showing in the longitudinal aspect that both connexins localize to the intercalated disk (arrow). Double labeling shows that they are highly colocalized in normal atria. (c) Transverse sections of atrial myocytes at the level of the intercalated disk show that the primary localization of both connexins in these myocytes is around the circumference of the cell (d, e) and that colocalization is extensive at this site (f). This suggests that the functional gap junctions in atria may have intermediate junctional properties when compared with pure Cx43- or pure Cx40-containing gap junctions
The role of these connexins in cardiac conduction has been studied extensively, written about exhaustively and described in excellent reviews over the years [7, 27, 28]. Therefore, the present paper will focus on the role of connexins within protein complexes within the heart and will discuss how these complexes play a role in normal cardiac physiology.
Discovering cardiac gap junctions
In 1952, Silvio Weidman showed that electrical current injected into a single cell in a Purkinje strand spread along a distance considerably larger than the length of a single cell, which suggested that electrical charge could move rapidly between 2 cells by a low resistance conduit [29]. Since that time, researchers have spent years examining the structure, composition and function of the gap junction channel [30]. Based on the offset positioning of cardiomyocytes in relation to each other, this perimeter localization suggests that the primary connections the connexin make is not to the cell that is attached at the site of the intercalated disk, but rather it is to the cells on either side. This suggests that conductance across the myocardium does not go in a direct line from site of stimulation to the final cardiomyocyte, but it spreads in an elliptical manner with a wave of conductance passing over the surface of the heart. This was predicted by the modeling of electrical conduction in the heart done by Madison Spach in the 1990s and early 2000s [31, 32], and elegantly visualized using cardiac optical mapping by José Jalife’s group [33].
A clear role for gap junction channels in electrical conduction emerged that included a role for gap junctions in regulating conduction from the nodal tissue down into the working myocardium both in the atria and in the ventricle. The tightly regulated junctional conduction allows for a uniform passage of current across the working myocardium in order to facilitate contraction in an orderly fashion. As posited by early localization studies in the heart [34], this orderly conduction is facilitated by the preferential localization of the gap junctions to the terminal ends of the cardiomyocytes, which allows for directed current flow [7, 35, 36]. All of the cardiac connexins have 3 channel states that affect the conductance of the channel. They can be fully open, in the residual state where they are almost closed yet have a lower conductance level, or fully closed [37], and the individual connexins each have a different regulation of this conductance [30, 38]. To further complicate the story, the cardiac connexins have the potential to form both homotypic gap junction channels, those made up of a single connexin isoform, as well as heterotypic channels that consist of more than 1 connexin isoform [36]. This sets the stage for complex conductance patterns in the heart. In areas where 1 connexin is dominant, such as found in the ventricular myocardium where Cx43 dominates, the conductance is based on the single connexin conductance for Cx43. In the atria, Cx40 is present, but Cx43 is there as well and studies have shown that these 2 connexins colocalize in atrial myocytes [39]. Electrophysiological studies suggest that these 2 connexins form rectifying heterotypic gap junctions, which could have a major impact on conduction through the atria [40]. In fact, it has been shown that changes in the make-up of connexins in atrial myocytes affects conduction through strands of atrial myocytes with a loss of Cx43 leading to an increase in rate of conduction when Cx40 is absent and a decreased rate in atrial myocytes lacking Cx43 [41]. These data suggest that the partnership between these 2 connexin isoforms is key in the formation of the normal conduction rate through the atrial compartment and loss of 1 connexin or the other during disease states may lead to abnormal conduction and potentially arrhythmias. Overall, it is clear that coupling of myocytes via gap junctions plays a key role in normal electrical propagation in the heart. Multiple studies have focused on the complexities in this process, and reviews [30] on the how, where and why of connexins in cardiac conduction are numerous and give more information on this topic than is allowable in the space provided here.
Connexin protein complexes in normal heart
A less studied role of connexins in cardiac cells is the potential role within protein complexes, although more studies are emerging on this topic each year. It appears that the normal function of gap junctions in the heart requires the coordinated interaction between the connexin subunits of the gap junction channel and numerous other binding partners within the cardiomyocyte. It has now been shown that connexins bind to a number of other cellular proteins, including scaffolding proteins, kinases, other junctional proteins and even to ion channels [42]. The roles that these interactions play in normal cardiac function are not yet clear, although a picture is emerging that the localization and function of connexins are regulated by these interactions and, in turn, connexins regulate the proteins they bind to. To date, studies have shown that binding partners are important in every aspect of the life cycle of Cx43, including its oligomerization and trafficking to cellular membranes, its insertion at the intercalated disk, its function as an ion channel and even its turnover rate [42]. To understand how these proteins are all coordinated is an ongoing project and likely to provide many more years of work for researchers in the field. To begin, formation of a gap junction channel from individual connexins has been shown to require the interaction of Cx43 with other proteins in the cell [42–45].
Formation of gap junction channels and their targeting to cardiomyocyte membranes requires that connexins interact with a number of protein partners in a stepwise fashion. Initial studies on trafficking of gap junctions suggested 2 mechanisms, 1 within the canonical Golgi pathway and 1 external to that pathway [46, 47], but only the non-cardiac Cx26 was suggested to use the external pathway. It is now clear that gap junction channels are formed and trafficked via the Golgi/endoplasmic reticulum pathway [48] and assembly of the oligomers of connexins occurs after exit from the endoplasmic reticulum [49]. Each of these steps requires that connexins interact with a different protein partner. These oligomers are thought to traffic to the membrane through interaction with lipid rafts containing caveolin-1 [50]. New information on how trafficking occurs in the polarized myocytes of the heart shows that binding to a chaperone protein end-binding protein 1 is required for normal localization of at least Cx43 to the intercalated disk [51] and that the actin cytoskeleton plays an important role as well [52]. This pathway is vital for the normal function of the heart, as it has been shown that the turnover time of Cx43 is quite rapid, necessitating a constant restocking of gap junctions in normal cardiomyocytes [53]. Degradation of gap junction channels has also been shown to require binding partners [54, 55]. These studies show that the formation of gap junction channels and their movement within cells is highly dependent on protein–protein interactions.
Other roles for protein–protein interactions of connexins in normal heart function have also been studied. The first connexin found to have regulating binding partners was Cx43. The story with Cx43 began with the observation that the tight junction associated protein zonula occludens-1 (ZO-1) interacted with the carboxyl terminal domain of Cx43 [44, 56]. ZO-1 is a scaffolding protein that contains multiple PSD-95, disk large, zonula occludens-1 (PDZ) domains, which serve in multiple systems to form functional protein complexes. This led to the idea that a protein complex maintained Cx43 at cell membranes [57, 58]. Further studies by these groups and others proceeded to show that the interaction between these 2 proteins was regulated by the non-receptor tyrosine kinase c-Src, with activation of c-Src leading to a loss of Cx43 at the cardiomyocyte cell membrane [43, 59–61]. Due to the fact that loss of Cx43 at myocyte membranes is strongly associated with the development of cardiac arrhythmias, the role of the regulation of Cx43-containing protein complexes has been an important topic of study [45, 62]. It is now clear that in the heart the interaction of Cx43 with ZO-1 forms an important functional complex of proteins that is required for normal physiology.
Studies have looked at other types of proteins that might be associated with the protein complex that contains Cx43, the so-called Cx43 interactome. An interesting finding was that the pore-forming subunit of the cardiac sodium channel Nav1.5 interacts with Cx43 [63–65]. Mario Delmar’s group carefully stepped off the changes in Cx43 with alterations of the desmosomal protein plakophilin-2 [66], interactions of desmosomal proteins plakophilin-2 and ankyrin-G with the sodium channel [67] and the association of Cx43 with the sodium channel within a single protein complex [65]. The finding that these 2 channels, which have both been found to be vital to cardiac conduction, work together has led to a unified theory of Cx43 and sodium channel function, suggesting that not only does Cx43 regulate electrical signals in the heart directly via gap junctional coupling, but also via regulation of the cardiac sodium channel [68].
Regulation of connexins by phosphorylation is equally important and the interaction of connexins with kinases has been studied extensively [69–71]. Cx43 was first discovered to be a phosphoprotein, suggesting that rather than being an unregulated channel that cellular activity may determine the level of coupling between myocytes [72]. It is well documented that Cx43 contains multiple phosphospecific residues, which have the potential to be regulated by numerous kinases [69, 70, 72, 73]. Some phosphorylation events work to close the gap junction channel [74–76], while others appear to maintain the channel in the open configuration [70, 77]. As phosphoproteins, connexins are regulated by dephosphorylation as well, with protein phosphatase-1 shown to interact with Cx43 to regulate its phosphorylation state [78, 79]. Thus, interactions with kinases and phosphatases are important in regulating whether the gap junction channel passes electrical signals. In normal heart, these phosphorylation and dephosphorylation events are kept in balance, allowing for maintenance of electrical propagation. A plethora of studies have shown that alteration of the phosphorylation status leads to gap junction dysfunction, indicating that when interactions of Cx43 with either kinases or phosphatases are altered, electrical coupling between cells is compromised [69–78, 80].
Finally, in a new chapter of understanding cardiac conduction, which is taken from an older chapter in neuroscience [81], ephaptic transmission has been resurrected as a possible alternative pathway for electrical passage through the heart. Ephaptic transmission is the transmission of the electrical signal from a cell to its neighbor in the absence of a functional gap junction or ion channel/synapse type process. It has been postulated in other systems that connexin hemichannels may be the conduit for the released electrical signal [82]. Traditional studies have shown that loss of connexins leads to a complete block of electrical transmission [83]. This has suggested that the gap junction is the only required structure, which has slowed interest in this type of electrical transmission. With the finding of the interaction of Cx43 with the sodium channel, suggesting microdomain involvement in conduction, interest has revived in this area of study. In a new study, these types of interactions were examined in a model of edema, which showed that along with gap junctional coupling and sodium channel function, regulated via the interaction between the 2 as discussed above. It was shown that increasing the distance between cells during edema decreased electrical transmission. Computer modeling suggested that the decrease was in part due to changes that could not be related to gap junction or sodium channel function and have now suggested that ephaptic transmission may play a role in slowing of conduction under disease conditions [84, 85]. As of yet, the role of ephapses in the heart needs, however, further investigation.
Taken together, it appears that the proteins which interact with Cx43 regulate trafficking to the membrane, localization at the intercalated disk, function of the gap junction channel, conduction and turnover. The goal of this is to ensure that cardiac conduction occurs unimpeded in normal heart. The key complex is the one in which Cx43 sits at the sarcolemma and where it may regulate conduction through separate mechanisms, namely direct gap junctional coupling and regulation of sodium channel activity, and possibly via formation of an ephapse. This tripartite mechanism makes sense when considering how critical it is for the heart to have a safety factor for conduction.
Pannexins in the heart
Pannexins are ATP-releasing pores that are activated under conditions of cellular stress [86]. Initial studies suggesting that pannexins were found in cultured cardiomyocytes sparked interest in a potential role for these channels in normal heart physiology [87]. It was suggested that the Panx1 isoform was the channel responsible for the large conductance chloride channel found in cardiomyocytes. The authors of that study showed that over time in culture, the chloride channel disappeared, but upon transfection with Panx1, it was seen again in the cells. It is not clear whether the Panx1 channel itself released the chloride or if the presence of Panx1 allowed for another channel to function, but in either case, it suggested a role for Panx1 in cardiomyocytes. Further experimentation has made it clear that pannexins are present in intact cardiac myocytes and that these channels play a role in cardiac pathology, specifically in the expansion of fibrosis following cardiac injury [88]. It was shown that ischemic/hypoxic conditions lead to the opening of Panx1 channels in cardiomyocytes and to the release of ATP that activated neighboring fibroblasts to differentiate towards myofibroblasts. This activation has been shown to be a key factor in the development of cardiac fibrosis in the injured myocardium [25]. Additionally, as pannexins were found to be localized to T-tubules where they interact with synapse-associated protein 97 [88], there may be a role for pannexins in the regulation of other synapse-associated protein 97-interacting ion channels [89, 90]. Further studies on this interesting channel in the heart are warranted to determine if there are functions for pannexins in healthy myocardium or if its role is solely restricted to injury responses.
Connexins in conduit arteries
Conduit arteries, including the aorta, carotid, iliac, femoral and brachial arteries, function as low resistance pathways to visceral organs and limbs. In healthy conduit arteries, endothelial cells (ECs) mainly express Cx37 and Cx40 [91], whereas vascular smooth muscle cells (VSMCs) express Cx43 [91]. Whether or not Cx45 is expressed by VSMCs of conduit arteries remains elusive. Studies using antibodies have reported expression of Cx45 in the aorta of rats and mice [92, 93], but a more recent study in which distribution of enhanced green fluorescent protein expressed under the control of the Cx45 promoter was used suggests that its expression is restricted to the femoral artery [94]. The physiological roles for connexins in conduit arteries seem to be limited to the endothelium. In ECs of conduit arteries, gap junctions synchronize agonist-induced calcium responses [95, 96]. One would, therefore, expect that Cx40-deficient mice, which exhibit a downregulation of endothelial Cx37 along with Cx40 [94, 97–99], would display reduced endothelium-dependent relaxation in response to acetylcholine and this is indeed the case [97]. Whether this endothelial dysfunction is due to a direct effect of Cx40-deficiency and Cx37 deficiency on synchronization of calcium or to indirect effects, such as effects mediated via the reported interactions between Cx40, Cx37 and endothelial nitric oxide synthase (eNOS) [97, 100], remains to be addressed. Furthermore, gap junctions have been shown to participate in relaxation due to endothelium-dependent hyperpolarization in rabbit iliac arteries [101]. Finally, studies performed with pharmacological inhibitors have suggested that gap junctions are involved in the release of contractile factors by ECs in the aorta of spontaneously hypertensive rats [102].
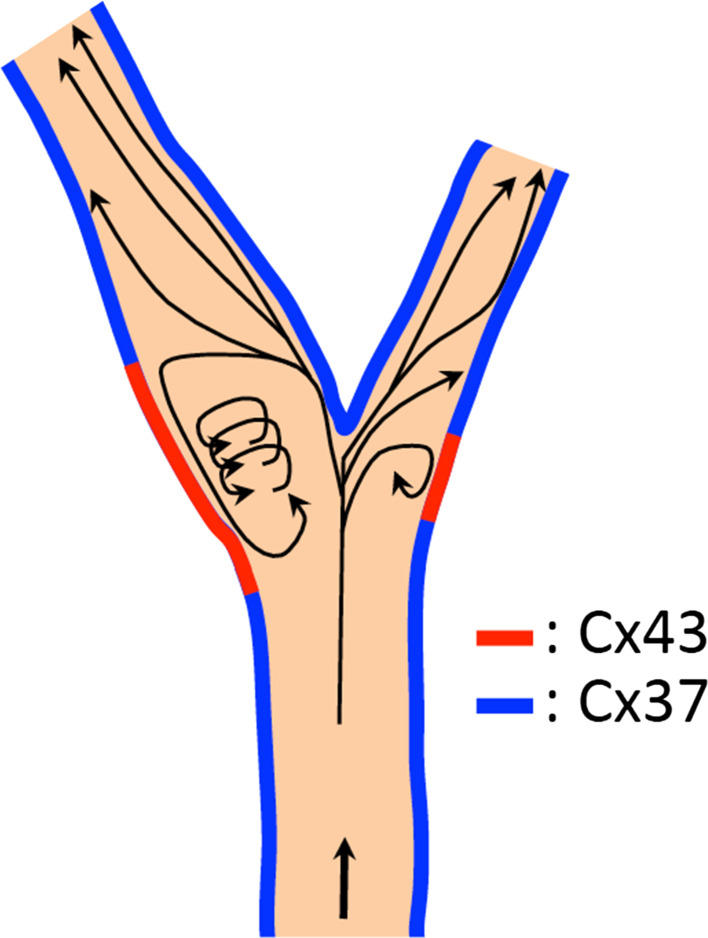
Endothelium lining conduit arteries is highly sensitive to hemodynamic shear stresses acting at the vessel luminal surface in the direction of blood flow [103]. Wall shear stress, the frictional force between blood and the endothelium, is an important determinant of endothelial cell function and also regulates connexin expression [104]. Indeed, Cx43 is moderately expressed or absent in quiescent arterial endothelia, but is induced locally in areas facing a disturbed blood flow pattern, such as arterial bifurcations or branch points [105]. Multiple in vitro studies have confirmed this correlation between Cx43 expression and disturbed flow [106–108]. Cx37 also appears to be a shear stress regulated gene. It is highly expressed in the endothelium in straight portions of the arterial tree, which are exposed to high laminar shear stress, and absent at arterial bifurcations and branch points that experience oscillatory shear stress [105, 109]. It has recently been demonstrated that the flow-responsive transcription factor Krüppel-like factor 2 mediates the induction of Cx37 expression by high laminar shear stress in ECs, which in turn allows for synchronization of physiological responses in the arterial endothelium [109]. Consequently, the opposite expression patterns of Cx37 and Cx43 in arterial regions exposed to different hemodynamic conditions induces endothelial communication compartments that may be relevant to the focal vulnerability of atherosclerosis (Fig. 2).
Fig. 2.
Schematic overview illustrating how shear stress patterns affect Cx37 or Cx43 expression at the carotid bifurcation
The pathophysiological roles of connexins in conduit arteries have been studied extensively. These studies have mostly focused on their contribution to atherosclerosis and neointima formation after injury. Similar to other inflammatory diseases, atherosclerotic plaque development is characterized by transmigration of leukocytes, such as monocytes, neutrophils and T-lymphocytes, over the endothelial barrier. This transmigration process is under the control of paracrine intercellular communication, involving adhesion molecules, cytokines and chemokines. It turns out that endothelial Cx40 and Cx37 in macrophages are also anti-atherogenic by inhibiting leukocyte adhesion resulting in reduced recruitment to the atherosclerotic lesion [99, 110]. In contrast, Cx43 is an atherogenic protein [111, 112]. Neointima formation may be considered as a wound healing response after arterial damage induced by balloon catheterization followed or not by the placement of a stent. The role of Cx43 has been extensively studied in this maladaptive repair process. It was already shown at the end of the previous century that balloon catheter injury in the rat carotid artery results in an upregulation of Cx43 in VSMCs in the neointima 9 days after the injury [113]. Interestingly, changes in the expression of Cx43 in the media preceded the changes in the intima and were already apparent at 1-day post-injury [113]. Remarkably, a global 50 % reduction of Cx43 expression reduced neointimal formation by decreasing the inflammatory response and, in addition, the migration of VSMCs after acute balloon injury [114]. However, specific deletion of Cx43 in VSMCs rather seems to enhance neointimal formation [115]. More recently, it has been suggested that blockade of Cx43 hemichannels reduces neointimal formation after vascular injury [116]. Nonetheless, this study relied on carbenoxelone to inhibit Cx43 hemichannels and did thus not directly yield insight into the contribution of hemichannels versus gap junction channels.
Pannexins in conduit arteries
Panx1 is expressed in the endothelium throughout the arterial tree [117]. Panx1 is also expressed in VSMCs. However, Panx1 expression is higher in VSMCs from smaller arteries and arterioles compared to large arteries [117]. Whether or not pannexins play a physiological role in conduit arteries is a matter of debate. It has been reported that conduit arteries from Panx1-deficient animals display slightly reduced endothelium-dependent relaxation [118]. One should, however, consider this conclusion with caution since these measurements have been performed on saphenous artery, which is generally considered to be a muscular resistance artery [119]. A definitive role for Panx1 in the maintenance of vasomotor function in conduit arteries thus remains to be proven. Panx2 and Panx3 are not expressed in conduit arteries [117]. Whether pannexins contribute to pathophysiological processes in conduit arteries has not yet been investigated.
Connexins in resistance arteries and arterioles
Resistance arteries are precapillary vessels with diameters ranging from 100 to 300 μm. These arteries passively regulate resting resistance and actively contract or relax to control organ perfusion [120]. Obviously, these processes require a large degree of synchronization between ECs and VSMCs, mostly since the ECs sense the need for contraction or relaxation and the VSMCs do the actual work. It is, therefore, interesting to note that the typical vascular connexins are present in homocellular, but also heterocellular gap junctions in various types of resistance arteries [121–124], suggesting that these may provide a pathway by which ECs can control the function of the adjacent VSMCs and thereby regulate vascular physiology. Indeed, it has been shown that gap junctions serve many physiological roles within the resistance vasculature. Thus, they are involved in relaxation induced by endothelium-dependent hyperpolarization [125–131]. Furthermore, hyperpolarization evoked in either ECs or VSMCs can spread a relatively large distance from its source to neighboring cells via gap junctions causing remote dilatation [132–136]. Importantly, gap junction-induced spread of dilatation is not only observed in the direction of blood flow, as would be the case for soluble factors secreted in blood, but also in retrograde direction. Moreover, such spreading responses are not restricted to dilatations. Indeed, they can also be evoked with contractile stimuli. Furthermore, they can be observed in resistance arteries, arterioles and capillaries. In this respect, arterioles of Cx40-deficient mice display irregular vasomotion and spontaneous vessel closure [137]. Conducted dilatation appeared to be reduced in arterioles from Cx40-deficient mice, suggesting that Cx40 is of critical importance for this phenomenon [133, 138]. A more recent study addressed possible differences between the spread of hyperpolarization induced by an agonist, such as acetylcholine, or by an opener of potassium channels, like pinacidil [135]. Spreading dilatation induced by acetylcholine was not affected by Cx37-deficiency and reduced by deletion of Cx40 [135]. However, spreading dilatation induced by pinacidil was not affected by either Cx37-deficiency or Cx40-deficiency [135]. In lung capillaries, Cx43 and Cx40 seem to be of vital importance for spreading responses. In this respect, Cx43 is implicated in the spread of pro-inflammatory calcium-dependent responses [139] and Cx40 seems involved in the spread of signals from oxygen sensing cells towards upstream arterioles [140]. In addition to their roles in relaxation mediated by endothelium-dependent hyperpolarization, it has also been shown that gap junctions act as pathways that allow for endothelial feedback on vasoconstriction [141, 142]. When an agonist, such as phenylephrine, activates VSMCs and thereby causes an increase in the cytosolic calcium concentrations, gap junctions between ECs and VSMCs allow for diffusion of calcium towards the ECs, where it activates eNOS to produce nitric oxide (NO), a well-known vasodilator. Finally, it has been suggested that gap junctions are involved in the spread of NO from ECs towards VSMCs in the rat aorta and in rat mesenteric vessels [143].
Given the roles of connexins in vasomotor responses, one could reason that vascular connexins may be involved in blood pressure regulation and hypertension. Cx37-deficient mice display normal blood pressure [135], thus making a role for this connexin in blood pressure homeostasis unlikely. The role of Cx43 is less clear, as blood pressure is not affected by specific removal of Cx43 from VSMCs [115] in mice, but both no effect [144] or a blood pressure-lowering effect [145] has been reported in mice with endothelial-specific deletion of Cx43. Finally, Cx40-deficient mice are hypertensive, but the contribution of arterial Cx40 to this hypertensive phenotype is probably marginal at best, since blood pressure in Cx40-deficient mice can be normalized by treatment with angiotensin receptor antagonists [146]. Moreover, restoration of Cx40 in renin-producing cells largely reduces the hypertension in Cx40-deficient mice [98]. Conversely, selective deletion of Cx40 from renin-producing cells induces hypertension [147] and finally selective deletion of Cx40 from the endothelium only does not affect blood pressure [99, 147].
Pannexins in resistance arteries and arterioles
In resistance arteries, Panx1, but not Panx2 or Panx3, is expressed by ECs and VSMCs, with the exception of arteries of less than 100 μm where Panx3 is detectable [117, 148]. Panx1 is important in the physiology of resistance arteries, mostly through its capacity to form channels that release purines, including ATP. Indeed, Panx1 channels were first shown to control blood flow indirectly by releasing ATP from erythrocytes in response to mechanical stress, initiating calcium elevation in ECs through the stimulation of purinergic receptors, which then induces the release of NO leading to relaxation of VSMCs and vasodilatation [149]. Panx1 also seems to play a role in phenylephrine-induced vasoconstriction of thoracodorsal resistance arteries through association of Panx1 and alpha1-adrenergic receptors in VSMCs [148]. The authors propose that activation of adrenoceptors expressed by VSMCs leads to contraction as a consequence of an increase in the intracellular calcium concentration and opening of Panx1 channels, which release ATP that can subsequently further enhance the contraction by activation of P2Y receptors [148]. Interestingly, experiments in which site-directed mutagenesis or mimetic peptides where used revealed that a specific sequence in the intracellular loop of Panx1 seems to be crucial for this link between noradrenergic and purinergic signaling [150]. However, contradicting results have been reported as well. First, a study using another muscular resistance artery of Panx1-deficient mice reported enhanced rather than reduced phenylephrine-induced contractions [118]. Moreover, there is also no evidence that Panx1 channels releasing ATP have any role in the constrictor actions of alpha1-adrenoceptor activation of small resistance arteries [151]. Clearly, more studies are needed before a consensus will be reached regarding involvement of Panx1 in alpha1-adrenergic receptor-mediated effects. It has also been suggested that release of calcitonin gene-related peptide (CGRP) from sensory–motor nerves can cause opening of Panx1 channels expressed by VSMCs in mouse mesenteric arteries [152]. However, as also noted by the authors, the physiological or pathophysiological relevance of CGRP-induced Panx1 channel opening remains to be determined [152]. Finally, Panx2 is expressed in the middle cerebral artery [153], but whether this or the expression of Panx3 in very small arteries has functional consequences is unknown.
Connexins in lymphatics
The lymphatic system is of crucial importance for tissue drainage, intestinal lipid absorption and the regulation of immune responses [154]. Interestingly, a few decades ago, gap junction-like structures, composed of at least Cx43 [155], were reported in the sinus wall of rabbit popliteal lymph nodes [156] and it is now clear that at least 3 connexins, namely Cx37, Cx43 and Cx47, are expressed in the lymphatic endothelium [157, 158]. With respect to the physiological role of gap junctions in the lymphatic system, it should be noted that early ex vivo experiments performed on isolated lymphatics and in vivo experiments performed on rat mesenteric lymphatics suggested that gap junctions are responsible for the coordination of retrogradely propagated contractile responses [156, 159]. More recently, 2 independent studies used genetically engineered mice to specifically study the role of connexins in lymphatic development [157, 158]. It appeared that Cx43 and Cx37 are implicated in the initial development of the jugular lymph sac and, importantly, in the formation of lymphatic valves. Interestingly, Cx37 seems to play a similar role in the development of venous valves [160]. Finally, mutations in Cx47 and Cx43 genes have been linked to diseases of lymphatic vasculature, most notably primary and secondary lymphedema [161]. Whether Cx43 or Cx47 play a role in lymphatic physiology is currently unclear. Furthermore, whether pannexins are expressed in lymphatic vessels and contribute to their physiological processes has not yet been investigated.
Conclusion
Connexins and pannexins are integral components of the cardiovascular system. While studies on pannexins are in the infant stage and so far only indicate a role for these channels in arterial physiology and in the diseased myocardium, it is clear that gap junctions and the connexins that form them form an integral part in normal cardiovascular physiology. The roles of connexins in resistance arteries and arterioles seem, at first sight, straightforward [132]. Thus, they permit exchange of small molecules between ECs and VSMCs via myoendothelial gap junctions and synchronize tissue responses, thereby allowing for retrograde spreading of dilatations and contractions. However, from recent work onto electrical conduction in the heart, we have learned that the role of connexins is more complex than originally anticipated. Rather than being simple passive electrical conduits, it appears as if they play a more active role in overall coordination of conduction through their involvement with protein–protein complexes [162, 163]. While the vast majority of studies have been done on cardiac Cx43, further studies on the other cardiovascular connexins may show that these connexins also coordinate electrical or metabolic signaling by working together with a compilation of protein partners rather than as individual channel units. In this light, Cx37 interacts with eNOS and via this interaction may regulate the activity of the enzyme or, conversely, the conductance of the Cx37 channel [100]. Additionally, it has been shown that Cx40 forms a complex with the tight junction associated protein ZO-1 [164]. Overall, these studies suggest that the connexin interactome is crucial for cardiovascular physiology, as it would allow for coordinated regulation of the connexin function, the function of sodium channels in the heart and the endothelial production of vasodilators. Thus, the coordinated electrical propagation in the heart as well as the coordinated vasomotor responses is likely not only due to a direct conduction via gap junctions, but also on the composition of proteins found within the scaffold at the cellular junctional membrane. This obviously has consequences for future connexin-targeted therapies in the setting of cardiovascular disease.
Acknowledgments
This work was supported by grants from the Swiss National Science Foundation (no. 310030_143343 and CRSII3_141811 to BRK).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Abbreviations
- ATP
Adenosine triphosphate
- CGRP
Calcitonin gene-related peptide
- Cx
Connexin
- ECs
Endothelial cells
- eNOS
Endothelial nitric oxide synthase
- NO
Nitric oxide
- Panx1
Pannexin1
- PDZ
PSD-95, disk large, zonula occludens-1
- VSMCs
Vascular smooth muscle cells
- ZO-1
Zonula occludens-1
References
- 1.Saez JC, Leybaert L. Hunting for connexin hemichannels. FEBS Lett. 2014;588(8):1205–1211. doi: 10.1016/j.febslet.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120(Pt 21):3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- 3.Riteau N, Baron L, Villeret B, Guillou N, Savigny F, Ryffel B, Rassendren F, Le Bert M, Gombault A, Couillin I. ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation. Cell Death Dis. 2012;3:e403. doi: 10.1038/cddis.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, Thompson RJ, Zhao HB, Dahl G. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011;5(3):193–197. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velasquez S, Eugenin EA. Role of Pannexin-1 hemichannels and purinergic receptors in the pathogenesis of human diseases. Front Physiol. 2014;5:96. doi: 10.3389/fphys.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamson SE, Leitinger N. The role of pannexin1 in the induction and resolution of inflammation. FEBS Lett. 2014;588(8):1416–1422. doi: 10.1016/j.febslet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen JA, van Veen TA, de Bakker JM, van Rijen HV. Cardiac connexins and impulse propagation. J Mol Cell Cardiol. 2010;48(1):76–82. doi: 10.1016/j.yjmcc.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Laird DW. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010;20(2):92–101. doi: 10.1016/j.tcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Duffy HS, Fort AG, Spray DC. Cardiac connexins: genes to nexus. Adv Cardiol. 2006;42:1–17. doi: 10.1159/000092550. [DOI] [PubMed] [Google Scholar]
- 10.Kreuzberg MM, Liebermann M, Segschneider S, Dobrowolski R, Dobrzynski H, Kaba R, Rowlinson G, Dupont E, Severs NJ, Willecke K. Human connexin31.9, unlike its orthologous protein connexin30.2 in the mouse, is not detectable in the human cardiac conduction system. J Mol Cell Cardiol. 2009;46(4):553–559. doi: 10.1016/j.yjmcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Davis LM, Kanter HL, Beyer EC, Saffitz JE. Distinct gap junction protein phenotypes in cardiac tissues with disparate conduction properties. J Am Coll Cardiol. 1994;24(4):1124–1132. doi: 10.1016/0735-1097(94)90879-6. [DOI] [PubMed] [Google Scholar]
- 12.Davis LM, Rodefeld ME, Green K, Beyer EC, Saffitz JE. Gap junction protein phenotypes of the human heart and conduction system. J Cardiovasc Electrophysiol. 1995;6(10 Pt 1):813–822. doi: 10.1111/j.1540-8167.1995.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 13.Beyer EC, Davis LM, Saffitz JE, Veenstra RD. Cardiac intercellular communication: consequences of connexin distribution and diversity. Braz J Med Biol Res. 1995;28(4):415–425. [PubMed] [Google Scholar]
- 14.Saffitz JE, Schuessler RB. Connexin-40, bundle-branch block, and propagation at the Purkinje-myocyte junction. Circ Res. 2000;87(10):835–836. doi: 10.1161/01.res.87.10.835. [DOI] [PubMed] [Google Scholar]
- 15.Yamada KA, Rogers JG, Sundset R, Steinberg TH, Saffitz J. Up-regulation of connexin45 in heart failure. J Cardiovasc Electrophysiol. 2003;14(11):1205–1212. doi: 10.1046/j.1540-8167.2003.03276.x. [DOI] [PubMed] [Google Scholar]
- 16.Gourdie RG, Harfst E, Severs NJ, Green CR. Cardiac gap junctions in rat ventricle: localization using site-directed antibodies and laser scanning confocal microscopy. Cardioscience. 1990;1(1):75–82. [PubMed] [Google Scholar]
- 17.Sepp R, Severs NJ, Gourdie RG. Altered patterns of cardiac intercellular junction distribution in hypertrophic cardiomyopathy. Heart. 1996;76(5):412–417. doi: 10.1136/hrt.76.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy HS. Inflammatory responses in the atria: should they stay or should they go? Heart Rhythm. 2011;8(2):286–287. doi: 10.1016/j.hrthm.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Palomo A, Benitez D, Alanis J. Selective deposition of lanthanum in mammalian cardiac cell membranes. Ultrastructural and electrophysiological evidence. J Cell Biol. 1973;58(1):1–10. doi: 10.1083/jcb.58.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohl P. Heterogeneous cell coupling in the heart: an electrophysiological role for fibroblasts. Circ Res. 2003;93(5):381–383. doi: 10.1161/01.RES.0000091364.90121.0C. [DOI] [PubMed] [Google Scholar]
- 21.Kohl P, Gourdie RG. Fibroblast-myocyte electrotonic coupling: does it occur in native cardiac tissue? J Mol Cell Cardiol. 2014;70:37–46. doi: 10.1016/j.yjmcc.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93(5):421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 23.Rook MB, Jongsma HJ, de Jonge B. Single channel currents of homo- and heterologous gap junctions between cardiac fibroblasts and myocytes. Pflugers Arch. 1989;414(1):95–98. doi: 10.1007/BF00585633. [DOI] [PubMed] [Google Scholar]
- 24.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57(4):376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffy HS. Fibroblasts, myofibroblasts, and fibrosis: fact, fiction, and the future. J Cardiovasc Pharmacol. 2011;57(4):373–375. doi: 10.1097/FJC.0b013e3182155a38. [DOI] [PubMed] [Google Scholar]
- 26.Green CR, Severs NJ. Connexon rearrangement in cardiac gap junctions: evidence for cytoskeletal control? Cell Tissue Res. 1984;237(1):185–186. doi: 10.1007/BF00229215. [DOI] [PubMed] [Google Scholar]
- 27.Kleber AG, Saffitz JE. Role of the intercalated disc in cardiac propagation and arrhythmogenesis. Front Physiol. 2014;5:404. doi: 10.3389/fphys.2014.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhein S, Seidel T, Salameh A, Jozwiak J, Hagen A, Kostelka M, Hindricks G, Mohr FW. Remodeling of cardiac passive electrical properties and susceptibility to ventricular and atrial arrhythmias. Front Physiol. 2014;5:424. doi: 10.3389/fphys.2014.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weidmann S. The electrical constants of Purkinje fibres. J Physiol. 1952;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34(3):325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 31.Spach MS, Heidlage JF. The stochastic nature of cardiac propagation at a microscopic level. Electrical description of myocardial architecture and its application to conduction. Circ Res. 1995;76(3):366–380. doi: 10.1161/01.res.76.3.366. [DOI] [PubMed] [Google Scholar]
- 32.Spach MS, Barr RC. Effects of cardiac microstructure on propagating electrical waveforms. Circ Res. 2000;86(2):E23–E28. doi: 10.1161/01.res.86.2.e23. [DOI] [PubMed] [Google Scholar]
- 33.Herron TJ, Lee P, Jalife J. Optical imaging of voltage and calcium in cardiac cells & tissues. Circ Res. 2012;110(4):609–623. doi: 10.1161/CIRCRESAHA.111.247494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luke RA, Beyer EC, Hoyt RH, Saffitz JE. Quantitative analysis of intercellular connections by immunohistochemistry of the cardiac gap junction protein connexin43. Circ Res. 1989;65(5):1450–1457. doi: 10.1161/01.res.65.5.1450. [DOI] [PubMed] [Google Scholar]
- 35.Dun W, Boyden PA. The Purkinje cell; 2008 style. J Mol Cell Cardiol. 2008;45(5):617–624. doi: 10.1016/j.yjmcc.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desplantez T, Dupont E, Severs NJ, Weingart R. Gap junction channels and cardiac impulse propagation. J Membr Biol. 2007;218(1–3):13–28. doi: 10.1007/s00232-007-9046-8. [DOI] [PubMed] [Google Scholar]
- 37.Moreno AP. Biophysical properties of homomeric and heteromultimeric channels formed by cardiac connexins. Cardiovasc Res. 2004;62(2):276–286. doi: 10.1016/j.cardiores.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Kwak BR, Hermans MM, De Jonge HR, Lohmann SM, Jongsma HJ, Chanson M. Differential regulation of distinct types of gap junction channels by similar phosphorylating conditions. Mol Biol Cell. 1995;6(12):1707–1719. doi: 10.1091/mbc.6.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verheule S, van Kempen MJ, te Welscher PH, Kwak BR, Jongsma HJ. Characterization of gap junction channels in adult rabbit atrial and ventricular myocardium. Circ Res. 1997;80(5):673–681. doi: 10.1161/01.res.80.5.673. [DOI] [PubMed] [Google Scholar]
- 40.Lin X, Xu Q, Veenstra RD. Functional formation of heterotypic gap junction channels by connexins-40 and -43. Channels (Austin) 2014;8(5):433–443. doi: 10.4161/19336950.2014.949188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beauchamp P, Yamada KA, Baertschi AJ, Green K, Kanter EM, Saffitz JE, Kleber AG. Relative contributions of connexins 40 and 43 to atrial impulse propagation in synthetic strands of neonatal and fetal murine cardiomyocytes. Circ Res. 2006;99(11):1216–1224. doi: 10.1161/01.RES.0000250607.34498.b4. [DOI] [PubMed] [Google Scholar]
- 42.Herve JC, Bourmeyster N, Sarrouilhe D, Duffy HS. Gap junctional complexes: from partners to functions. Prog Biophys Mol Biol. 2007;94(1–2):29–65. doi: 10.1016/j.pbiomolbio.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Giepmans BN, Hengeveld T, Postma FR, Moolenaar WH. Interaction of c-Src with gap junction protein connexin-43. Role in the regulation of cell-cell communication. J Biol Chem. 2001;276(11):8544–8549. doi: 10.1074/jbc.M005847200. [DOI] [PubMed] [Google Scholar]
- 44.Giepmans BN, Verlaan I, Hengeveld T, Janssen H, Calafat J, Falk MM, Moolenaar WH. Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol. 2001;11(17):1364–1368. doi: 10.1016/s0960-9822(01)00424-9. [DOI] [PubMed] [Google Scholar]
- 45.Sovari AA, Iravanian S, Dolmatova E, Jiao Z, Liu H, Zandieh S, Kumar V, Wang K, Bernstein KE, Bonini MG, Duffy HS, Dudley SC. Inhibition of c-Src tyrosine kinase prevents angiotensin II-mediated connexin-43 remodeling and sudden cardiac death. J Am Coll Cardiol. 2011;58(22):2332–2339. doi: 10.1016/j.jacc.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.George CH, Kendall JM, Evans WH. Intracellular trafficking pathways in the assembly of connexins into gap junctions. J Biol Chem. 1999;274(13):8678–8685. doi: 10.1074/jbc.274.13.8678. [DOI] [PubMed] [Google Scholar]
- 47.Evans WH, Ahmad S, Diez J, George CH, Kendall JM, Martin PE. Trafficking pathways leading to the formation of gap junctions. Novartis Found Symp. 1999;219:44–54. doi: 10.1002/9780470515587.ch4. [DOI] [PubMed] [Google Scholar]
- 48.Falk MM. Biosynthesis and structural composition of gap junction intercellular membrane channels. Eur J Cell Biol. 2000;79(8):564–574. doi: 10.1078/0171-9335-00080. [DOI] [PubMed] [Google Scholar]
- 49.Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74(6):1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- 50.Schubert AL, Schubert W, Spray DC, Lisanti MP. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41(18):5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 51.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128(3):547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smyth JW, Vogan JM, Buch PJ, Zhang SS, Fong TS, Hong TT, Shaw RM. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ Res. 2012;110(7):978–989. doi: 10.1161/CIRCRESAHA.111.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115(5):1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su V, Hoang C, Geerts D, Lau AF. CIP75 (connexin43-interacting protein of 75 kDa) mediates the endoplasmic reticulum dislocation of connexin43. Biochem J. 2014;458(1):57–67. doi: 10.1042/BJ20131247. [DOI] [PubMed] [Google Scholar]
- 55.Smyth JW, Zhang SS, Sanchez JM, Lamouille S, Vogan JM, Hesketh GG, Hong T, Tomaselli GF, Shaw RM. A 14-3-3 mode-1 binding motif initiates gap junction internalization during acute cardiac ischemia. Traffic. 2014;15(6):684–699. doi: 10.1111/tra.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem. 1998;273(21):12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- 57.Barker RJ, Price RL, Gourdie RG. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res. 2002;90(3):317–324. doi: 10.1161/hh0302.104471. [DOI] [PubMed] [Google Scholar]
- 58.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16(12):5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Tada M, Hori M. Functional role of c-Src in gap junctions of the cardiomyopathic heart. Circ Res. 1999;85(8):672–681. doi: 10.1161/01.res.85.8.672. [DOI] [PubMed] [Google Scholar]
- 60.Duffy HS, Ashton AW, O’Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. Regulation of connexin43 protein complexes by intracellular acidification. Circ Res. 2004;94(2):215–222. doi: 10.1161/01.RES.0000113924.06926.11. [DOI] [PubMed] [Google Scholar]
- 61.Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC. Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J Biol Chem. 2004;279(52):54695–54701. doi: 10.1074/jbc.M409552200. [DOI] [PubMed] [Google Scholar]
- 62.Rutledge CA, Ng FS, Sulkin MS, Greener ID, Sergeyenko AM, Liu H, Gemel J, Beyer EC, Sovari AA, Efimov IR, Dudley SC. c-Src kinase inhibition reduces arrhythmia inducibility and connexin43 dysregulation after myocardial infarction. J Am Coll Cardiol. 2014;63(9):928–934. doi: 10.1016/j.jacc.2013.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhett JM, Ongstad EL, Jourdan J, Gourdie RG. Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J Membr Biol. 2012;245(7):411–422. doi: 10.1007/s00232-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malhotra JD, Thyagarajan V, Chen C, Isom LL. Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. J Biol Chem. 2004;279(39):40748–40754. doi: 10.1074/jbc.M407243200. [DOI] [PubMed] [Google Scholar]
- 65.Sato PY, Coombs W, Lin X, Nekrasova O, Green KJ, Isom LL, Taffet SM, Delmar M. Interactions between ankyrin-G, Plakophilin-2, and Connexin43 at the cardiac intercalated disc. Circ Res. 2011;109(2):193–201. doi: 10.1161/CIRCRESAHA.111.247023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101(7):703–711. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- 67.Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patino GA, Taffet SM, Isom LL, Delmar M. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res. 2009;105(6):523–526. doi: 10.1161/CIRCRESAHA.109.201418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delmar M. Connexin43 regulates sodium current; ankyrin-G modulates gap junctions: the intercalated disc exchanger. Cardiovasc Res. 2012;93(2):220–222. doi: 10.1093/cvr/cvr343. [DOI] [PubMed] [Google Scholar]
- 69.Lampe PD, Cooper CD, King TJ, Burt JM. Analysis of Connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J Cell Sci. 2006;119(Pt 16):3435–3442. doi: 10.1242/jcs.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36(7):1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morel S, Kwak BR. Roles of connexins in atherosclerosis and ischemia-reperfusion injury. Curr Pharm Biotechnol. 2012;13(1):17–26. doi: 10.2174/138920112798868638. [DOI] [PubMed] [Google Scholar]
- 72.Kadle R, Zhang JT, Nicholson BJ. Tissue-specific distribution of differentially phosphorylated forms of Cx43. Mol Cell Biol. 1991;11(1):363–369. doi: 10.1128/mcb.11.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lau AF, Hatch-Pigott V, Crow DS. Evidence that heart connexin43 is a phosphoprotein. J Mol Cell Cardiol. 1991;23(6):659–663. doi: 10.1016/0022-2828(91)90975-r. [DOI] [PubMed] [Google Scholar]
- 74.Zhou L, Kasperek EM, Nicholson BJ. Dissection of the molecular basis of pp60(v-src) induced gating of connexin 43 gap junction channels. J Cell Biol. 1999;144(5):1033–1045. doi: 10.1083/jcb.144.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hyrc K, Rose B. The action of v-src on gap junctional permeability is modulated by pH. J Cell Biol. 1990;110(4):1217–1226. doi: 10.1083/jcb.110.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arellano RO, Rivera A, Ramon F. Protein phosphorylation and hydrogen ions modulate calcium-induced closure of gap junction channels. Biophys J. 1990;57(2):363–367. doi: 10.1016/S0006-3495(90)82537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD. Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC. J Cell Biol. 2007;179(6):1301–1309. doi: 10.1083/jcb.200707060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duthe F, Plaisance I, Sarrouilhe D, Herve JC. Endogenous protein phosphatase 1 runs down gap junctional communication of rat ventricular myocytes. Am J Physiol Cell Physiol. 2001;281(5):C1648–C1656. doi: 10.1152/ajpcell.2001.281.5.C1648. [DOI] [PubMed] [Google Scholar]
- 79.Kang M, Lin N, Li C, Meng Q, Zheng Y, Yan X, Deng J, Ou Y, Zhang C, He J, Luo D. Cx43 phosphorylation on S279/282 and intercellular communication are regulated by IP 3/IP 3 receptor signaling. Cell Commun Signal. 2014;12(1):58. doi: 10.1186/s12964-014-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang M, Lin N, Li C, Meng Q, Zheng Y, Yan X, Deng J, Ou Y, Zhang C, He J, Luo D. Cx43 phosphorylation on S279/282 and intercellular communication are regulated by IP3/IP3 receptor signaling. Cell Commun Signal. 2014;12:58. doi: 10.1186/s12964-014-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rasminsky M. Ephaptic transmission between single nerve fibres in the spinal nerve roots of dystrophic mice. J Physiol. 1980;305:151–169. doi: 10.1113/jphysiol.1980.sp013356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kamermans M, Fahrenfort I. Ephaptic interactions within a chemical synapse: hemichannel-mediated ephaptic inhibition in the retina. Curr Opin Neurobiol. 2004;14(5):531–541. doi: 10.1016/j.conb.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 83.Beauchamp P, Choby C, Desplantez T, de Peyer K, Green K, Yamada KA, Weingart R, Saffitz JE, Kleber AG. Electrical propagation in synthetic ventricular myocyte strands from germline connexin43 knockout mice. Circ Res. 2004;95(2):170–178. doi: 10.1161/01.RES.0000134923.05174.2f. [DOI] [PubMed] [Google Scholar]
- 84.Veeraraghavan R, Lin J, Hoeker GS, Keener JP, Gourdie RG, Poelzing S. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflugers Arch. 2015 doi: 10.1007/s00424-014-1675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin J, Keener JP. Ephaptic coupling in cardiac myocytes. IEEE Trans Biomed Eng. 2013;60(2):576–582. doi: 10.1109/TBME.2012.2226720. [DOI] [PubMed] [Google Scholar]
- 86.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2010;299(4):H1146–H1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kienitz MC, Bender K, Dermietzel R, Pott L, Zoidl G. Pannexin 1 constitutes the large conductance cation channel of cardiac myocytes. J Biol Chem. 2011;286(1):290–298. doi: 10.1074/jbc.M110.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dolmatova E, Spagnol G, Boassa D, Baum JR, Keith K, Ambrosi C, Kontaridis MI, Sorgen PL, Sosinsky GE, Duffy HS. Cardiomyocyte ATP release through pannexin 1 aids in early fibroblast activation. Am J Physiol Heart Circ Physiol. 2012;303(10):H1208–H1218. doi: 10.1152/ajpheart.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vikstrom KL, Vaidyanathan R, Levinsohn S, O’Connell RP, Qian Y, Crye M, Mills JH, Anumonwo JM. SAP97 regulates Kir2.3 channels by multiple mechanisms. Am J Physiol Heart Circ Physiol. 2009;297(4):H1387–H1397. doi: 10.1152/ajpheart.00638.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gillet L, Rougier JS, Shy D, Sonntag S, Mougenot N, Essers M, Shmerling D, Balse E, Hatem SN, Abriel H. Cardiac-specific ablation of synapse-associated protein SAP97 in mice decreases potassium currents but not sodium current. Heart Rhythm. 2015;12(1):181–192. doi: 10.1016/j.hrthm.2014.09.057. [DOI] [PubMed] [Google Scholar]
- 91.Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22(2):225–230. doi: 10.1161/hq0102.104125. [DOI] [PubMed] [Google Scholar]
- 92.Alonso F, Krattinger N, Mazzolai L, Simon A, Waeber G, Meda P, Haefliger JA. An angiotensin II- and NF-kappaB-dependent mechanism increases connexin 43 in murine arteries targeted by renin-dependent hypertension. Cardiovasc Res. 2010;87(1):166–176. doi: 10.1093/cvr/cvq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ko YS, Coppen SR, Dupont E, Rothery S, Severs NJ. Regional differentiation of desmin, connexin43, and connexin45 expression patterns in rat aortic smooth muscle. Arterioscler Thromb Vasc Biol. 2001;21(3):355–364. doi: 10.1161/01.atv.21.3.355. [DOI] [PubMed] [Google Scholar]
- 94.Jobs A, Schmidt K, Schmidt VJ, Lubkemeier I, van Veen TA, Kurtz A, Willecke K, de Wit C. Defective Cx40 maintains Cx37 expression but intact Cx40 is crucial for conducted dilations irrespective of hypertension. Hypertension. 2012;60(6):1422–1429. doi: 10.1161/HYPERTENSIONAHA.112.201194. [DOI] [PubMed] [Google Scholar]
- 95.Boittin FX, Alonso F, Le Gal L, Allagnat F, Beny JL, Haefliger JA. Connexins and M3 muscarinic receptors contribute to heterogeneous Ca(2+) signaling in mouse aortic endothelium. Cell Physiol Biochem. 2013;31(1):166–178. doi: 10.1159/000343358. [DOI] [PubMed] [Google Scholar]
- 96.Kameritsch P, Pogoda K, Ritter A, Munzing S, Pohl U. Gap junctional communication controls the overall endothelial calcium response to vasoactive agonists. Cardiovasc Res. 2012;93(3):508–515. doi: 10.1093/cvr/cvr345. [DOI] [PubMed] [Google Scholar]
- 97.Alonso F, Boittin FX, Beny JL, Haefliger JA. Loss of connexin40 is associated with decreased endothelium-dependent relaxations and eNOS levels in the mouse aorta. Am J Physiol Heart Circ Physiol. 2010;299(5):H1365–H1373. doi: 10.1152/ajpheart.00029.2010. [DOI] [PubMed] [Google Scholar]
- 98.Le Gal L, Alonso F, Wagner C, Germain S, Nardelli Haefliger D, Meda P, Haefliger JA. Restoration of connexin 40 (Cx40) in Renin-producing cells reduces the hypertension of Cx40 null mice. Hypertension. 2014;63(6):1198–1204. doi: 10.1161/HYPERTENSIONAHA.113.02976. [DOI] [PubMed] [Google Scholar]
- 99.Chadjichristos CE, Scheckenbach KE, van Veen TA, Richani Sarieddine MZ, de Wit C, Yang Z, Roth I, Bacchetta M, Viswambharan H, Foglia B, Dudez T, van Kempen MJ, Coenjaerts FE, Miquerol L, Deutsch U, Jongsma HJ, Chanson M, Kwak BR. Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation. 2010;121(1):123–131. doi: 10.1161/CIRCULATIONAHA.109.867176. [DOI] [PubMed] [Google Scholar]
- 100.Pfenniger A, Derouette JP, Verma V, Lin X, Foglia B, Coombs W, Roth I, Satta N, Dunoyer-Geindre S, Sorgen P, Taffet S, Kwak BR, Delmar M. Gap junction protein Cx37 interacts with endothelial nitric oxide synthase in endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30(4):827–834. doi: 10.1161/ATVBAHA.109.200816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Griffith TM, Chaytor AT, Taylor HJ, Giddings BD, Edwards DH. cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction via gap junctions. Proc Natl Acad Sci USA. 2002;99(9):6392–6397. doi: 10.1073/pnas.092089799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang EH, Vanhoutte PM. Gap junction inhibitors reduce endothelium-dependent contractions in the aorta of spontaneously hypertensive rats. J Pharmacol Exp Ther. 2008;327(1):148–153. doi: 10.1124/jpet.108.140046. [DOI] [PubMed] [Google Scholar]
- 103.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99(2):315–327. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meens MJ, Pfenniger A, Kwak BR, Delmar M. Regulation of cardiovascular connexins by mechanical forces and junctions. Cardiovasc Res. 2013;99(2):304–314. doi: 10.1093/cvr/cvt095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res. 1998;83(6):636–643. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- 106.Cowan DB, Lye SJ, Langille BL. Regulation of vascular connexin43 gene expression by mechanical loads. Circ Res. 1998;82(7):786–793. doi: 10.1161/01.res.82.7.786. [DOI] [PubMed] [Google Scholar]
- 107.DePaola N, Davies PF, Pritchard WF, Jr, Florez L, Harbeck N, Polacek DC. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci USA. 1999;96(6):3154–3159. doi: 10.1073/pnas.96.6.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kwak BR, Silacci P, Stergiopulos N, Hayoz D, Meda P. Shear stress and cyclic circumferential stretch, but not pressure, alter connexin43 expression in endothelial cells. Cell Commun Adhes. 2005;12(5–6):261–270. doi: 10.1080/15419060500514119. [DOI] [PubMed] [Google Scholar]
- 109.Pfenniger A, Wong C, Sutter E, Cuhlmann S, Dunoyer-Geindre S, Mach F, Horrevoets AJ, Evans PC, Krams R, Kwak BR. Shear stress modulates the expression of the atheroprotective protein Cx37 in endothelial cells. J Mol Cell Cardiol. 2012;53(2):299–309. doi: 10.1016/j.yjmcc.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 110.Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12(8):950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 111.Wong CW, Burger F, Pelli G, Mach F, Kwak BR. Dual benefit of reduced Cx43 on atherosclerosis in LDL receptor-deficient mice. Cell Commun Adhes. 2003;10(4–6):395–400. doi: 10.1080/cac.10.4-6.395.400. [DOI] [PubMed] [Google Scholar]
- 112.Kwak BR, Veillard N, Pelli G, Mulhaupt F, James RW, Chanson M, Mach F. Reduced connexin43 expression inhibits atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Circulation. 2003;107(7):1033–1039. doi: 10.1161/01.cir.0000051364.70064.d1. [DOI] [PubMed] [Google Scholar]
- 113.Yeh HI, Lupu F, Dupont E, Severs NJ. Upregulation of connexin43 gap junctions between smooth muscle cells after balloon catheter injury in the rat carotid artery. Arterioscler Thromb Vasc Biol. 1997;17(11):3174–3184. doi: 10.1161/01.atv.17.11.3174. [DOI] [PubMed] [Google Scholar]
- 114.Chadjichristos CE, Matter CM, Roth I, Sutter E, Pelli G, Luscher TF, Chanson M, Kwak BR. Reduced connexin43 expression limits neointima formation after balloon distension injury in hypercholesterolemic mice. Circulation. 2006;113(24):2835–2843. doi: 10.1161/CIRCULATIONAHA.106.627703. [DOI] [PubMed] [Google Scholar]
- 115.Liao Y, Regan CP, Manabe I, Owens GK, Day KH, Damon DN, Duling BR. Smooth muscle-targeted knockout of connexin43 enhances neointimal formation in response to vascular injury. Arterioscler Thromb Vasc Biol. 2007;27(5):1037–1042. doi: 10.1161/ATVBAHA.106.137182. [DOI] [PubMed] [Google Scholar]
- 116.Song M, Yu X, Cui X, Zhu G, Zhao G, Chen J, Huang L. Blockade of connexin 43 hemichannels reduces neointima formation after vascular injury by inhibiting proliferation and phenotypic modulation of smooth muscle cells. Exp Biol Med (Maywood) 2009;234(10):1192–1200. doi: 10.3181/0902-RM-80. [DOI] [PubMed] [Google Scholar]
- 117.Lohman AW, Billaud M, Straub AC, Johnstone SR, Best AK, Lee M, Barr K, Penuela S, Laird DW, Isakson BE. Expression of pannexin isoforms in the systemic murine arterial network. J Vasc Res. 2012;49(5):405–416. doi: 10.1159/000338758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gaynullina D, Tarasova OS, Kiryukhina OO, Shestopalov VI, Panchin Y. Endothelial function is impaired in conduit arteries of pannexin1 knockout mice. Biol Direct. 2014;9:8. doi: 10.1186/1745-6150-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Storkebaum E, Ruiz de Almodovar C, Meens M, Zacchigna S, Mazzone M, Vanhoutte G, Vinckier S, Miskiewicz K, Poesen K, Lambrechts D, Janssen GM, Fazzi GE, Verstreken P, Haigh J, Schiffers PM, Rohrer H, Van der Linden A, De Mey JG, Carmeliet P. Impaired autonomic regulation of resistance arteries in mice with low vascular endothelial growth factor or upon vascular endothelial growth factor trap delivery. Circulation. 2010;122(3):273–281. doi: 10.1161/CIRCULATIONAHA.109.929364. [DOI] [PubMed] [Google Scholar]
- 120.Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res. 2001;38(1):1–12. doi: 10.1159/000051024. [DOI] [PubMed] [Google Scholar]
- 121.Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. Myoendothelial contacts, gap junctions, and microdomains: anatomical links to function? Microcirculation. 2012;19(5):403–415. doi: 10.1111/j.1549-8719.2011.00146.x. [DOI] [PubMed] [Google Scholar]
- 122.Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol. 2006;291(5):H2047–H2056. doi: 10.1152/ajpheart.00484.2006. [DOI] [PubMed] [Google Scholar]
- 123.Isakson BE, Best AK, Duling BR. Incidence of protein on actin bridges between endothelium and smooth muscle in arterioles demonstrates heterogeneous connexin expression and phosphorylation. Am J Physiol Heart Circ Physiol. 2008;294(6):H2898–H2904. doi: 10.1152/ajpheart.91488.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KCa) and connexins: possible relationship to vasodilator function? J Anat. 2006;209(5):689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beny JL, Schaad O. An evaluation of potassium ions as endothelium-derived hyperpolarizing factor in porcine coronary arteries. Br J Pharmacol. 2000;131(5):965–973. doi: 10.1038/sj.bjp.0703658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396(6708):269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 127.Hutcheson IR, Chaytor AT, Evans WH, Griffith TM. Nitric oxide-independent relaxations to acetylcholine and A23187 involve different routes of heterocellular communication. Role of Gap junctions and phospholipase A2. Circ Res. 1999;84(1):53–63. doi: 10.1161/01.res.84.1.53. [DOI] [PubMed] [Google Scholar]
- 128.Kansui Y, Fujii K, Nakamura K, Goto K, Oniki H, Abe I, Shibata Y, Iida M. Angiotensin II receptor blockade corrects altered expression of gap junctions in vascular endothelial cells from hypertensive rats. Am J Physiol Heart Circ Physiol. 2004;287(1):H216–H224. doi: 10.1152/ajpheart.00915.2003. [DOI] [PubMed] [Google Scholar]
- 129.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97(4):399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 130.Rath G, Saliez J, Behets G, Romero-Perez M, Leon-Gomez E, Bouzin C, Vriens J, Nilius B, Feron O, Dessy C. Vascular hypoxic preconditioning relies on TRPV4-dependent calcium influx and proper intercellular gap junctions communication. Arterioscler Thromb Vasc Biol. 2012;32(9):2241–2249. doi: 10.1161/ATVBAHA.112.252783. [DOI] [PubMed] [Google Scholar]
- 131.Yamamoto Y, Imaeda K, Suzuki H. Endothelium-dependent hyperpolarization and intercellular electrical coupling in guinea-pig mesenteric arterioles. J Physiol. 1999;514(Pt 2):505–513. doi: 10.1111/j.1469-7793.1999.505ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dora KA. Coordination of vasomotor responses by the endothelium. Circ J. 2010;74(2):226–232. doi: 10.1253/circj.cj-09-0879. [DOI] [PubMed] [Google Scholar]
- 133.Howitt L, Chaston DJ, Sandow SL, Matthaei KI, Edwards FR, Hill CE. Spreading vasodilatation in the murine microcirculation: attenuation by oxidative stress-induced change in electromechanical coupling. J Physiol. 2013;591(Pt 8):2157–2173. doi: 10.1113/jphysiol.2013.250928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dora KA, Xia J, Duling BR. Endothelial cell signaling during conducted vasomotor responses. Am J Physiol Heart Circ Physiol. 2003;285(1):H119–H126. doi: 10.1152/ajpheart.00643.2002. [DOI] [PubMed] [Google Scholar]
- 135.Figueroa XF, Duling BR. Dissection of two Cx37-independent conducted vasodilator mechanisms by deletion of Cx40: electrotonic versus regenerative conduction. Am J Physiol Heart Circ Physiol. 2008;295(5):H2001–H2007. doi: 10.1152/ajpheart.00063.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wolfle SE, de Wit C. Intact endothelium-dependent dilation and conducted responses in resistance vessels of hypercholesterolemic mice in vivo. J Vasc Res. 2005;42(6):475–482. doi: 10.1159/000088101. [DOI] [PubMed] [Google Scholar]
- 137.de Wit C, Roos F, Bolz SS, Pohl U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics. 2003;13(2):169–177. doi: 10.1152/physiolgenomics.00169.2002. [DOI] [PubMed] [Google Scholar]
- 138.de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86(6):649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- 139.Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest. 2006;116(8):2193–2200. doi: 10.1172/JCI26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang L, Yin J, Nickles HT, Ranke H, Tabuchi A, Hoffmann J, Tabeling C, Barbosa-Sicard E, Chanson M, Kwak BR, Shin HS, Wu S, Isakson BE, Witzenrath M, de Wit C, Fleming I, Kuppe H, Kuebler WM. Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Invest. 2012;122(11):4218–4230. doi: 10.1172/JCI59176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci USA. 1997;94(12):6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler Thromb Vasc Biol. 2011;31(2):399–407. doi: 10.1161/ATVBAHA.110.215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Figueroa XF, Lillo MA, Gaete PS, Riquelme MA, Saez JC. Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. Neuropharmacology. 2013;75:471–478. doi: 10.1016/j.neuropharm.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 144.Theis M, de Wit C, Schlaeger TM, Eckardt D, Kruger O, Doring B, Risau W, Deutsch U, Pohl U, Willecke K. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis. 2001;29(1):1–13. doi: 10.1002/1526-968x(200101)29:1<1::aid-gene1000>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 145.Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci USA. 2001;98(17):9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int. 2007;72(7):814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 147.Wagner C, Jobs A, Schweda F, Kurtz L, Kurt B, Lopez ML, Gomez RA, van Veen TA, de Wit C, Kurtz A. Selective deletion of Connexin 40 in renin-producing cells impairs renal baroreceptor function and is associated with arterial hypertension. Kidney Int. 2010;78(8):762–768. doi: 10.1038/ki.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, Isakson BE. Pannexin1 regulates alpha1-adrenergic receptor- mediated vasoconstriction. Circ Res. 2011;109(1):80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006;103(20):7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Billaud M, Chiu YH, Lohman AW, Parpaite T, Butcher JT, Mutchler SM, DeLalio LJ, Artamonov MV, Sandilos JK, Best AK, Somlyo AV, Thompson RJ, Le TH, Ravichandran KS, Bayliss DA, Isakson BE. A molecular signature in the pannexin1 intracellular loop confers channel activation by the alpha1 adrenoreceptor in smooth muscle cells. Sci Signal. 2015;8(364):ra17. doi: 10.1126/scisignal.2005824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Angus JA, Betrie AH, Wright CE. Pannexin-1 channels do not regulate alpha-adrenoceptor-mediated vasoconstriction in resistance arteries. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 152.Gaete PS, Lillo MA, Figueroa XF. Functional role of connexins and pannexins in the interaction between vascular and nervous system. J Cell Physiol. 2014;229(10):1336–1345. doi: 10.1002/jcp.24563. [DOI] [PubMed] [Google Scholar]
- 153.Burns AR, Phillips SC, Sokoya EM. Pannexin protein expression in the rat middle cerebral artery. J Vasc Res. 2012;49(2):101–110. doi: 10.1159/000332329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kutkut I, Meens MJ, McKee TA, Bochaton-Piallat ML, Kwak BR. Lymphatic vessels: an emerging actor in atherosclerotic plaque development. Eur J Clin Invest. 2015;45(1):100–108. doi: 10.1111/eci.12372. [DOI] [PubMed] [Google Scholar]
- 155.Krenacs T, Rosendaal M. Immunohistological detection of gap junctions in human lymphoid tissue: connexin43 in follicular dendritic and lymphoendothelial cells. J Histochem Cytochem. 1995;43(11):1125–1137. doi: 10.1177/43.11.7560895. [DOI] [PubMed] [Google Scholar]
- 156.Compton CC, Raviola E. Structure of the sinus-lining cells in the popliteal lymph node of the rabbit. Anat Rec. 1985;212(4):408–423. doi: 10.1002/ar.1092120412. [DOI] [PubMed] [Google Scholar]
- 157.Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hagerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, Calmes JM, Adams RH, Makinen T, Kiefer F, Kwak BR, Petrova TV. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell. 2012;22(2):430–445. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 158.Kanady JD, Dellinger MT, Munger SJ, Witte MH, Simon AM. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev Biol. 2011;354(2):253–266. doi: 10.1016/j.ydbio.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol. 1989;257(6 Pt 2):H2059–H2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- 160.Munger SJ, Kanady JD, Simon AM. Absence of venous valves in mice lacking Connexin37. Dev Biol. 2013;373(2):338–348. doi: 10.1016/j.ydbio.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meens MJ, Sabine A, Petrova TV, Kwak BR. Connexins in lymphatic vessel physiology and disease. FEBS Lett. 2014;588(8):1271–1277. doi: 10.1016/j.febslet.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 162.Agullo-Pascual E, Cerrone M, Delmar M. Arrhythmogenic cardiomyopathy and Brugada syndrome: diseases of the connexome. FEBS Lett. 2014;588(8):1322–1330. doi: 10.1016/j.febslet.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Veeraraghavan R, Poelzing S, Gourdie RG. Old cogs, new tricks: a scaffolding role for connexin43 and a junctional role for sodium channels? FEBS Lett. 2014;588(8):1244–1248. doi: 10.1016/j.febslet.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bouvier D, Kieken F, Kellezi A, Sorgen PL. Structural changes in the carboxyl terminus of the gap junction protein connexin 40 caused by the interaction with c-Src and zonula occludens-1. Cell Commun Adhes. 2008;15(1):107–118. doi: 10.1080/15419060802014347. [DOI] [PMC free article] [PubMed] [Google Scholar]