Abstract
Cell migration plays a central role in a variety of physiological and pathological processes during our whole life. Cellular movement is a complex, tightly regulated multistep process. Although the principle mechanisms of migration follow a defined general motility cycle, the cell type and the context of moving influences the detailed mode of migration. Endothelial cells migrate during vasculogenesis and angiogenesis but also in a damaged vessel to restore vessel integrity. Depending on the situation they migrate individually, in chains or sheets and complex signaling, intercellular signals as well as environmental cues modulate the process. Here, the different modes of cell migration, the peculiarities of endothelial cell migration and specific guidance molecules controlling this process will be reviewed.
Keywords: Angiogenesis, Guidance cues, Lamellipodium, Notch signaling, Motility cycle, Tip cell, VEGF, Vessel repair
Introduction
Cell migration is a fundamental process during our whole life. During development in gastrulation cells migrate to form the three layers of the embryo. Subsequently, migration to target locations is necessary to form tissues and organs. Cell migration is also a key component of the homeostasis in the adult individual. Failure of cells to migrate as well as inappropriate migration lead to severe consequences like immunosuppression and wound healing defects on the one hand and dissemination of tumors on the other. Almost all types of cells need to migrate either under physiological or pathological conditions. Leukocytes migrate to sites of inflammation, where they exert their phagocytic and immune function. Vascular smooth muscle cells move into the subintimal space during atherosclerotic plaque formation and migration of endothelial cells is essential for angiogenesis, the physiological and pathological formation of new blood vessels.
Depending on the cell type and the context of moving, different modes of migration are distinguished. Cells can move individually or in groups or sheets, their movement can be random or directional towards a chemotactic stimulus. Despite these differences, cell migration follows a defined motility cycle, with recurring typical features. The migrating cell has an asymmetric morphology with a leading and a trailing edge. The leading edge forms membrane protrusions and through new contacts attaches to the underlying substrate. Cellular contraction and traction forces release the cellular attachments at the distal end, leading to its retraction. In this review, first the general steps of cell movement will be detailed and subsequently endothelial specific aspects of cell migration will be discussed.
Principles of cell migration
Induction of cell migration
Single cells exhibit movement in the absence of any external stimuli. This as “random walk” [1] described phenomenon is characterized by spontaneous lamellipodium formation leading to random polarization and movement. The persistence of a migratory direction under such conditions is very low. Rather, cells only migrate a short net distance and exhibit equal preference for all directions. This form of movement resembles that of particles during diffusion. For effective migration, however, persistent movement towards a specific direction is required, which is the consequence of external stimuli that force the cell to prefer a particular direction. Such stimuli can be mechanic forces which guide the random migratory process or a chemotactic or chemokinetic signal.
A large number of extracellular molecules initiate and promote migration and two different characteristics of the migration process can be distinguished: The first is an increase in the movement rate, i.e. the speed of the random migration as induced by growth factors, cytokines and high glucose [2–4]. The second is that most growth factors, like platelet-derived growth factor (PDGF) [5], also stimulate cell directionality leading to directed, persistent cell migration. This intrinsic directionality is observed when cells respond to a non-directional motogenic stimulus like the uniform presence of PDGF with migration in a constant direction without turning. Importantly, this behavior occurs in the absence of a chemotactic gradient. When the motogenic signal is present as an external gradient a steering mechanism also becomes operative and by coupling signal sensing to the basic motility machinery, directional migration is established [6, 7]. Different guidance cues lead to directional migration: soluble factors induce chemotaxis and graded adhesion in the extracellular matrix leads to haptotaxis [8], electric fields can stimulate electrotaxis [9] and durotaxis is the directed cell migration in response to mechanical signals of the environment [10].
Importantly, the process of protrusion formation and thus initiation of movement is independent of the mechanism that induces directionality [11].
Cell polarization
Chemotactic stimuli lead to local activation of signaling pathways and polarization of the cell. This polarization results in the formation of a leading edge that forms protrusions such as broad, sheet-like lamellipodia and thin, needle-like filopodia (Fig. 1).
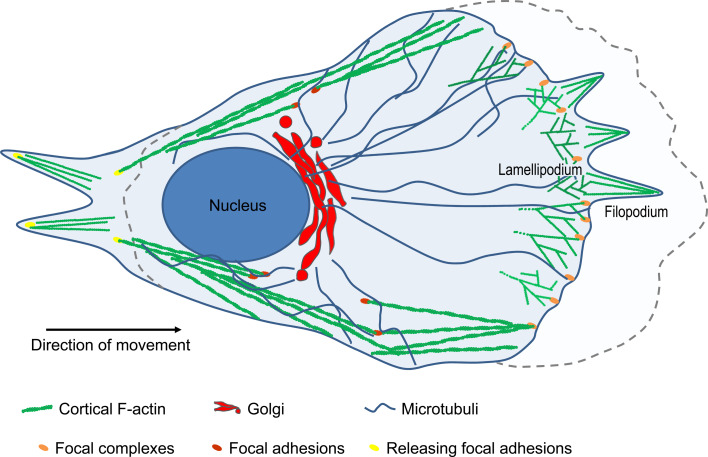
Fig. 1.
Schematic illustration of a migrating cell. At the cell front, actin assembly drives the extension of flat lamellipodia and fingerlike filopodia. In the lamellipodium branched actin filaments are generated and the cell forms adhesions that connect the extracellular matrix to the actin cytoskeleton to anchor the protrusion and tract the cell body. To move forward, the cell retracts its trailing edge by combining actomyosin contractility and disassembly of adhesions at the rear
Thus, the cellular motility cycle begins with polarization. One of the first molecules showing a polarized cellular distribution in response to a chemotactic agent is phosphatidylinositol (3,4,5)-trisphosphate (PIP3) [12, 13]. Localized activation of PI3-kinase and low level of lipid phosphatases, such as phosphatase and tensin homolog (PTEN), at the cell front produce a rapid accumulation of PIP3 at the leading edge. As a consequence, small GTPases of the Rho family become activated through their PIP3-dependent guanine nucleotide exchange factors (GEFs) and induce actin polymerization at the cell front [14].
One key regulator of cell polarization is the small GTPase Cdc42. Cdc42 is active at the cell front in migrating cells and regulates polarity by controlling where lamellipodia form [15]. Cdc42 also determines the localization of the microtubule-organization center (MTOC) and the Golgi apparatus. By localizing MTOC and the Golgi in front of the nucleus, Cdc42 facilitates microtubule growth into the lamella, the region behind the lamellipodium, and microtubule-mediated transport of Golgi-derived vesicles to the leading edge [16, 17].
Locally accumulated PIP3 also promotes the activation of Rac, in neutrophils via the GEF P-Rex1, at the cell front [18]. Rac activity is maintained at the lamellipodia by additional feedback loops. Microtubule growth activates Rac through additional GEFs [19, 20] and Rac in turn stabilizes microtubules [17, 21]. By regulating the formation of lamellipodia, Rac seems to be an important regulator of intrinsic-directed cell migration. Active Rac induces random migration by promoting peripheral lamellae oriented in directions different from the direction of migration along the main cell axis. Even small reductions in active Rac, therefore, increase directionality. In the absence of a chemotactic gradient, cells with reduced Rac activity migrate straighter due to the formation of only one stable protrusion in the direction of the main cell axis [22].
Protrusion formation
Localized activation of Rac and Cdc42 is a prerequisite for protrusion formation of the polarizing cell. RhoA, in contrast, was long thought to be selectively activated at the rear end of the cell to retract its posterior. Recent reports, however, suggest an involvement of RhoA also in protrusion formation [23].
Formation of membrane extensions like lamellipodia and filopodia is facilitated by cytoskeletal rearrangements. The major targets for Rac and Cdc42 mediating actin polymerization in protrusions are proteins of the WASP/WAVE family and activators of actin related proteins 2/3 (Arp2/3) complex. Cdc42 binds to WASP proteins [24] and Rac is thought to activate WAVE proteins [25]. Actin filaments are intrinsically polarized with fast growing plus/barbed ends and slow growing minus/pointed ends. In cell protrusions, the Arp2/3 complex facilitates branching polymerization of the actin filaments from monomeric or globular actin (G-actin) at their barbed end, while at the pointed end G-actin is liberated by depolymerisation. The orientation of the actin filaments with their barbed end to the membrane and the pointed end to the center of the cell leads to growing of the protrusion.
Adhesion formation and forward movement
To transform protrusion formation into forward movement, the cell has to anchor to a substrate at focal complexes. Once attached, the continuous addition of G-actin at the barbed ends will push the plasma membrane forward and the extended leading edge then stabilizes through the formation of new adhesion sites. Thus, focal complexes are small adhesions that drive migration.
Adhesion complexes are composed of a large number of proteins, including adhesion receptors, kinases, adapters and structural molecules. The formation of adhesion complexes at the leading edge is also a process dependent on small GTPases [26] orchestrating the guided sequential recruitment of proteins to a nucleation center. For example, paxillin is present in newly formed adhesion sites before α-actinin joins the complex. Subsequently integrin α5 enters the adhesions to form visible complexes stabilizing the adhesions.
Integrins, indeed, are a major family of migration-promoting receptors. These receptors support adhesion to the extracellular matrix (ECM) but also link the adhesion complexes to the actin filaments at the inside of the cell. Binding of ECM proteins to the extracellular part of an integrin induces its conformational change and leads to integrin clustering. Through the subsequent approximation of intracellular integrin-attached molecules signaling is initiated. Among these are tyrosine phosphorylation of proteins, activation of small GTPases, and changes in phospholipid biosynthesis. The consequence of these events is further growth and strengthening of adhesion sites and the organization and modulation of the cytoskeleton [27].
In the migrating cell, the focal complexes disassemble at the base of the protrusion as new adhesions are formed at the leading edge. Some of the small focal complexes can also mature to larger more organized focal adhesions which tend to inhibit migration [28]. Consequently, slower moving cells form more stabilized focal adhesions.
Contraction of the cells and detachment at the rear end
To allow forward movement of the whole cell, adhesions must disassemble at the rear part. High tension exerted on rear adhesions by myosin motors contributes to detachment [29, 30]. Myosin II forms bipolar filaments which can pull two actin filaments past one another leading to contraction and increased traction force. As mentioned above, an important factor mediating retraction is RhoA, which also mediates stress fiber formation [31, 32].
Migration in the multi-cellular context
The general elements of the migratory cycle are now well understood and are shared between different cell types; yet, the details vary to a high extent. The single steps of the cycle are most distinctly observed in slow-moving cells such as fibroblasts; fast-migrating cells like neutrophils in contrast appear to glide over the substrate.
In addition to cell-specific aspects, also the cellular context impacts on the migration mechanism. Cells not only migrate individually but during many biological processes move together as sheets, chains or clusters. This type of migration is characterized by a collective behavior of the cohort and thus characteristics of the individual cells become less relevant whereas reactions as a consequence of interactions with the neighbors predominate.
Collective cell movement
Collective migration ensures appropriate distribution of cells within development and maintenance of a tissue and serves to keep the tissue intact during remodeling. In this, the collective migration of cells is characterized by their reciprocal interaction with their surrounding neighbors [33].
A major difference between individual and collective migration is the interaction with the cellular environment. An epithelial cell starting to migrate as a single individual cell has to detach from neighboring, non-motile cells. This process involves down-regulation of specific adhesion molecules mediating cell–cell adhesion, changes in the cytoskeleton and expression of the ability to adhere to the ECM.
During the pre-migratory phase the cell begins to extend protrusions in a non-directed fashion. Collectively migrating cells stay physically and functionally connected and the integrity of cell–cell junctions, mostly via cadherins [34] is preserved during movement [35]. Cadherin-based junctions are important in branching morphogenesis of the mammary duct, in epithelial regeneration, in the sprouting of blood vessels and for invasive cancers [36, 37].
Collectively migrating cells also show differences with respect to their polarization. Not only the single cell is polarized under this condition but also a collective cellular polarization of the cohort is established. A front-rear asymmetry divides the cell group in ‘leaders’ and ‘followers’. Leader cells at the front row or in the tip position in a migrating chain of cells (therefore, also called tip cells) sense extracellular guidance cues, show a more polarized morphology and generate more cytoskeletal dynamics than the follower (to leaders in sheets) or stalk (to tip cells in chains) cells [38]. Leading cells also form a clear lamellipodium and are often less ordered and mesenchyme-like. Cells at the rear form a ‘cryptic’ lamellipodium against the substratum towards the cells in front of them [39] and have more tight junctions and tend to form tightly packed cell assemblies [40].
These differences in polarity are a consequence of a differential expression of surface receptors, like those for chemokines. For example, differential expression of CXCR4b in leading cells and CXCR7b in trailing cells is required for coordinated, directed cell migration [41]. Also, leading cells of primary melanoma cultures are characterized by preferential expression of integrin β1 generating polarized attachment and higher traction forces [42]. Specification in leader and follower cells can be genetically determined but it is often also dynamic as a result of a temporary, functional state induced by the position of the cell, the ECM and collective migration-inducing signals like stromal cell-derived factor 1 (SDF1), members of the fibroblast growth factor (FGF) and transforming growth factor-β (TGFβ) families [38, 43].
The molecular principles of actin turnover and polarized force generated by collectively moving cells are similar to those of the migrating individual cells, but additionally they are shared and coordinated between cells at different positions. The cortical actin network in the cell cohort is characterized by a supracellular organisation. Like the polarization over the whole cell group, the anterior protrusion activities and posterior retraction dynamics involve many cells [42, 44, 45]. The mechanisms of supracellular cytoskeletal organization are not clear yet. Probably, cadherin- and gap-junctional cell–cell coupling and paracrine release of cytokines and growth factors are involved [46].
2D versus 3D migration
Cell migration varies from cell type to cell type but also the environment of the cell regulates its migratory behavior. Depending on the context, cells migrate two dimensionally (2D) on tightly packed basement membrane, a thin, dense acellular layer or three dimensionally (3D) through loose or denser connective tissue. 3D migration of individual cells can be seen by primordial germ cells, leukocytes, hematopoietic stem cells and cancer cells during metastasis formation [47]. Also collective cell movement occurs in 3D. Movement of multicellular 3D strands can result in the formation of an inner lumen, as in morphogenic duct and gland formation or vascular sprouting during angiogenesis. Isolated groups or clusters of cells can migrate through tissue if they detach from their origins, e.g. border cells in Drosophila melanogaster egg chamber [11] or metastatic cancer cell clusters that penetrate a tissue.
2D migration in contrast, is seen predominantly by cell sheets. Cells migrate as tightly associated epithelial sheets or clusters or they possess a mesenchymal character as during neural crest migration. Sheet migration is also apparent during gastrulation [48], by the gut intestinal epithelium moving across the intact basement membrane or by epidermal keratinocytes during wound closure.
Collective migration in 3D
If a cell collective invades tissue in a 3D fashion, like during sprouting angiogenesis or invading cancer cells, the leading cells form filopodia or pseudopodia (morphologically dynamic, cylindrical cell protrusion) instead of flat lamellipodia [49, 50]. Cytoskeletal rearrangements as seen at the leading edge are not only important for forward movement but also regulate cell–cell contacts [51]. Under such conditions, p120 catenin acts as a linker between the leading edge and cell–cell junctions. It facilitates protrusion formation and the stabilization of cell–cell contacts through cortactin [52]. A cell collective can also migrate along or through multicellular tissue. In this case, interactions with the surrounding cells occur via E-cadherin–E-cadherin adhesions [53].
In 3D tissues, collective cell migration is more constrained than migration of individual cells. Single migrating cells are either native tissue resident or circulating cells that infiltrate the tissue.
Cells migrating in a 3D matrix express matrix adhesions on their whole surface and not only on the ventral surface directed towards the 2D support or basal membrane. Furthermore, the cells have to move through the often soft matrix. For this, the matrix usually has to be degraded and thus 3D migration absolutely depends on proteolytic activity of the migrating cells. Highly aggressive tumor cells were found to express membrane type 1-matrix metalloprotease (MT1-MMP) as well as active MMP-2 at the invasive front [54]. Migrating fibroblasts and tumor cells also exhibit pericellular collagenolytic activity that allows them to traverse the ECM. Particularly MT1-MMP seems to be the major protease mediating the invasive activity of normal and neoplastic cells [55].
Despite these considerations, the role of proteases in migration through ECM is not as clear as it seems. In vitro studies showed that tumor cells are still able to migrate after inhibition of proteases [56]. Clinical trials with MMP inhibitors as anti-cancer drugs were disappointing and some inhibitors even reduced patient survival. On this basis, another concept of migration through ECM has been proposed which suggests that during 3D-migration cells switch to an amoeboid mode, independent of MMP activity and matrix degradation, in which the cytosol is pushed into the surrounding matrix [56, 57].
Endothelial cell migration
A collectively migrating cell cohort shows similar characteristics to individually migrating cells scaled up over the whole group. A biological process can be dependent on both types of migration and one cell type can migrate in both ways depending on the context of migration. An example is migration of endothelial cells during the formation of blood vessels.
Endothelial cell migration is a crucial process throughout the whole life. It starts in the early embryo where the formation of the circulatory system precedes that of all other organ systems. After a primitive network has been formed, angiogenesis leads to the formation of a complex, functional vascular system. In the adult organism angiogenesis has a key role during several physiological as well as pathological processes including wound healing, tissue regeneration and cancer development. A key element of angiogenesis is endothelial cell migration. But also when a blood vessel is damaged, endothelial cells have to migrate to fill the open space and restore vessel integrity. During migration, endothelial cells pass through the previously described migratory cycle. Depending on the environment of the cells and the context of migration, endothelial cells migrate individually, as chains or in sheets in processes regulated by a multitude of different molecules and mechanisms.
Endothelial cell migration during embryogenesis
In the embryo the cardiovascular system is the first organ system to develop. The primary network of blood vessels is formed by the process of vasculogenesis. This is defined as the de novo formation of blood vessels by the aggregation of endothelial precursors, the angioblasts, which specialize from mesoderm. Formation of the embryonic vasculature involves multiple cellular processes including differentiation of angioblasts into endothelial cells, and the migration, proliferation, and assembly of angioblasts and endothelial cells into vessel-like structures. Angioblasts show the characteristics of individually migrating cells and multiple cell-autonomous and extrinsic signals control their specification, differentiation, and migration [58, 59]. Originating from hemangioblasts, angioblasts migrate throughout the whole body, invading embryonic mesenchymal and epithelial tissues and participate in blood vessel formation at distant sites [60]. Already at the earliest stages of vascular development, the vascular endothelial growth factor (VEGF) signaling pathway is essential for blood vessel formation as VEGF and its receptors are critical for angioblast migration. In xenopus larvae VEGF is expressed by the hypochord, which lies at the embryonic midline directly dorsal to the location of the future dorsal aorta. Here, VEGF induces the migration of angioblasts from the lateral plate mesoderm to the midline where they form a single dorsal aorta [61]. At this early stage of development, VEGF serves as a chemoattractant inducing directed single cell migration of the endothelial precursors. A later study shows that the transcription factor sonic hedgehog induces VEGF expression and that the Notch signaling pathway acts downstream of VEGF signaling in the control of angioblast migration [62].
Three different receptors for the VEGF family of proteins are known: VEGFR1 (Flt1), VEGFR2 (Flk1) and VEGFR3 (Flt4). VEGR2 is the earliest known specific marker for endothelial cells and amongst the earliest markers of embryonic angioblasts whereas VEGFR1 is expressed slightly later during development. VEGFR2 expression is essential for the chemoattractant effect of VEGF on angioblasts and also in vitro studies using VEGFR knockout embryonic stem cells indicate that VEGFR2 is indispensable for vascular precursor cell migration [63]. Targeted mutation of VEGFR2 in mice results in embryos that lack any organized blood vessels at any stage [64], whereas VEGR1 mutation leads to abnormalities in vessel organization [65] (for review see [66]).
Endothelial cell migration during vessel repair
Endothelial cell monolayers constitute the luminal surface of all blood and lymph vessels where they provide a barrier to retain plasma components in the circulation while regulating the exchange of molecules and cells between lumen and tissues. Dysfunction of this barrier has been implicated in a number of human diseases like atherosclerosis [67], edema and respiratory distress syndrome of the lung [68]. To maintain the integrity of the monolayer, endothelial cells show dynamic planar migration behavior of individual cells and cell groups [38].
If the endothelial monolayer is damaged, cells migrate in to fill the gap, a process which is promoted by growth factors like basic FGF (bFGF). Upon bFGF stimulation, cells near the wound edge exhibit directed migration towards the denuded area. The cells at the boundary function as leader or pioneer cells (similar to the tip cells in an angiogenic sprout) and pass the migratory signal to the follower cells (equivalent to stalk cells). The consequence is directed sheet migration. This regulation of the follower cells involves VE-cadherin- and α-catenin-mediated cell–cell coordination [69]. In the absence of any stimulating growth factor migration in the sheet is more random and filling the gap is the consequence of an inhibition of backwards migration to areas covered by cells (Fig. 2a).
Fig. 2.
Collective endothelial cell migration. a Endothelial sheet migration. If the endothelial monolayer is damaged, endothelial cells migrate to fill the gap. Leader cells are formed sensing the gradient of a chemoattractant and forming a lamellipodium. At the leading edge new focal contacts are formed. Stable focal adhesions in the center adhere the cell to the basement membrane. The following cells are characterized by cryptic lamellipodia. b Endothelial cell migration in an angiogenic sprout. In a newly forming blood vessel a tip cell is formed sensing the chemotactic gradient. It is characterized by filopodia and high activity of metalloproteases. Induction of Notch signaling in the following stalk abolishes the formation of additional tip cells
As described in the general part, Rac regulates lamellipodia formation and intrinsic directionality and this is also true for endothelial cells. In individually migrating cells an increase in Rac activity enhances the number of lamellipodia formed leading to a loss of directionality. One recently described regulator of Rac localization and lamellipodia formation in endothelial cells is the polarity protein Scrib, previously characterized in epithelial cells. In individually migrating endothelial cells, silencing Scrib results in a disoriented formation and increased number of Rac-positive lamellipodia [70]. Interestingly, in collectively migrating cells in an endothelial sheet, the orientation of the lamellipodia is still disturbed but the number of lamellipodia is not increased after silencing Scrib (unpublished data) suggesting that the contact to surrounding cells inhibits Rac activity and lamellipodia formation and increases directionality. This control of Rac activity by cell–cell contacts might explain the observed directionality of endothelial cells migrating in sheets (e.g. in the scratch wound assay).
Endothelial cell migration during angiogenesis
The vast majority of endothelial cells in blood vessels are quiescent with little migratory and proliferative activity. They show a regular cobblestone appearance and were termed “phalanx cells” [71]. When nutritional and oxygen demands within a tissue exceed the supply provided by existing blood vessels, the tissue sends out signals that stimulate the formation of new blood vessels (for review see [72]). Then, endothelial cells change into an ‘activated’ phenotype and start to migrate and proliferate to form new vessels, a process termed angiogenesis. Angiogenesis includes sprouting morphogenesis, intussuceptive growth, splitting, remodeling, stabilization and differentiation into arterioles, venules and capillaries. Complex cellular events are involved in the process comprising alterations in cell proliferation, survival, differentiation, and obviously migration. Each of these elements is subjected to tight control as excess or paucity of blood vessel formation contributes to cancer growth and metastasis, ischemic retinopathies and stroke as well as many metabolic disorders [73].
Endothelial cell migration in an angiogenic sprout is a guided and, therefore, directed process and guidance cues are provided by the local environment. These signals are often provided by surrounding cells like astrocytes in the retina, neuronal or tumor cells. The different guidance cues will be discussed below, whereas the general mechanism of directed endothelial cell migration will be described exemplified for VEGF the best investigated and potentially the most important stimulus for endothelial cell migration.
VEGF, the paradigm of an endothelial pro-migratory factor
In sprouting angiogenesis, the previously described concept of tip and stalk cells is operative. The first step is, therefore, the selection of the cell that initiates sprout formation and becomes the tip cell. This is necessary because if all cells reacted in the same way to the stimulus, they would migrate in a similar manner and the vessel would disintegrate. Thus, only one cell has to become privileged to migrate by a fine-tuned feedback loop entertained by VEGF and the Notch/Dll4 system [74–76]. The Notch pathway is an inter-endothelial signaling mechanism between adjacent cells. In cultured and non-polarized endothelial sheets, cells usually express both, the ligand, in endothelial cells delta-like 4 (Dll4), and the Notch receptor. VEGF present as a gradient in the angiogenic tissue binds to VEGFR2 on endothelial cells and enhances the expression of Dll4. Dll4 then binds the Notch receptor on adjacent cells and trigger its activation. Notch signaling is mediated by proteolytic cleavage of its intracellular domain (NICD), which translocates into the nucleus and alters gene expression [77, 78]. Due to stochastic differences in local VEGF concentrations, in filopodia elongation (and thus VEGF exposure) or in transcription rate, one cell will express slightly higher Dll4 levels and, thus, will dominate its neighbors by activating more Notch signaling. The cell with more Dll4, and less Notch activity, will be selected as the tip cell as Dll4-mediated activation of Notch inhibits VEGFR2, indirectly inhibiting Dll4 expression levels. This process reinforces the dominance of the selected tip cell and limits the number of tip cells induced by VEGF [75, 76]. Tip cells are migratory and polarized; they extend long filopodia that scan the environment for attractant or repellent signals, and hence serve to guide new blood vessels in the direction of the chemotactic stimulus (Fig. 2b) [79, 80]. Previously it was thought that once selected, the cells keep their fate and that the once selected tip cell stays at the leading position of the sprout. Now it is known that cells continually interchange their places, tip cells become stalk cells and vice versa [81–83].
Endothelial cell polarization and hence the directionality of filopodia extension during migration is dependent on Cdc42 activation [84]. We recently showed that the polarity protein Scrib is essential for polarization of migrating endothelial cells. Silencing of Scrib leads to mislocalization of Rac, to the formation of an increased number of lamellipodia and to accelerated integrin α5 degradation. These effects result in the loss of directionality of migrating endothelial cells and a reduced number of tip cells during sprouting angiogenesis at least in vitro [70].
The formation of branch-like filopodia by tip endothelial cells is mediated by remodeling of the actomyosin and microtubule cytoskeleton, similar to the way in which neurite extensions protrude from neuronal cell bodies [79, 85]. The formation of endothelial branches from lamellipodia has been described to be dependent on the local attenuation of myosin II-mediated contraction. The process is a consequence of a local loss of RhoA and RhoA kinase (ROCK) activity leading to reduced myosin light-chain phosphorylation. As a consequence, the cell branches by ‘‘escaping’’ retraction through cortical tension [86]. If the down-regulation of myosin II is not locally restricted, the cell forms an increased number of pseudopodia leading to a reduced directionality.
Interestingly, filopodia are dispensable for tip cell formation and integration of endothelial guidance cues. When filopodia formation is inhibited, the formation of lamellipodia is sufficient to drive endothelial cell migration. Nevertheless, filopodia facilitate endothelial cell migration and anastomosis formation [87].
Compared to stalk cells, tip cells show a differential gene expression profiles [88]. VEGFR2, PDGFB, the Netrin receptor unc-5 homolog B (Unc5B), the notch ligand Dll4, EC-specific molecule 1, the peptide ligand apelin and MT1-MMP [79, 88–90] are all enriched in tip cells. The selective expression of MT1-MMP at this location suggests that for sprouting angiogenesis, matrix degradation is essential [90]. It provides space into which endothelial cells can migrate, and also liberates key growth factors sequestered within the matrix, including bFGF, VEGF, and insulin-like growth factor [91]. For angiogenesis, MMP-2, MMP-9 and MT1-MMP are of particular interest [92]. Knockout of MMP-2 leads to markedly reduced tumor angiogenesis in mice [93] and MMP-9-deficient mice show abnormal growth plate vascularization [94]. MT1-MMP-knockout mice fail to respond to bFGF in the corneal angiogenesis assay [95]. The extracellular matrix has the capacity to store pro-angiogenic chemokines and cytokines, such as VEGF, that are released upon MMP activation [96, 97]. However, also the opposite is true: MMPs generate protein fragments of the extracellular matrix that may also limit angiogenesis, like endostatin and angiostatin [98, 99].
The complex role of integrins in endothelial cell migration
Integrins are important mediators of endothelial cell adhesion during migration. Among them, nine (α1β1, α2β1, α3β1, α4β1, α5β1, α6β1, α6β4, αvβ3, αvβ5) have been described to be expressed on endothelial cells and are implicated in angiogenesis [100, 101]. These include collagen receptors (α1β1, α2β1), laminin receptors (α3β1, α6β1, α6β4), fibronectin receptors (α4β1, α5β1) and the pair of αv receptors (αvβ3, αvβ5). αvβ3 mediates adhesion at the leading edge of migrating endothelial cells and, therefore, regulates directionality of endothelial cell movement [102]. VEGF-induced cell migration requires αvβ3, αvβ5 and β1 integrins [103] and also PDGF-induced migration depends on integrin αvβ3 [104]. Integrin β1-deficient endothelial cells are also defective in cell adhesion and migration [105].
We recently demonstrated that recycling of integrin α5β1 is essential for directed migration of endothelial cells. An increased lysosomal degradation of integrin α5β1 observed in cells not expressing the polarity protein Scrib contributes to a disorientation of chemoattracted endothelial cell [70]. Furthermore, a cross-talk between integrin αvβ3 and α5β1 regulates endothelial cell migration [106]. As a consequence of the regulation of endothelial cell migration, αv integrins and integrin α5β1 are implicated in angiogenesis. αvβ3 and αvβ5 are upregulated on cultured endothelial cells or in angiogenic blood vessels in response to several angiogenic growth factors and in certain tumors. Blockade of αvβ3 and/or αvβ5 by antibodies or peptide-based approaches therefore inhibited neovascularization in various systems [107].
Surprisingly, these findings could not be confirmed by genetic ablation studies; mice lacking αvβ3 and αvβ5 integrins are viable and fertile and blood vessel development in these animals is not disturbed. A possible explanation is a compensatory response mediated by enhanced VEGF/VEGFR2 signaling [108]. Nevertheless, the data concerning αvβ3 and αvβ5 integrins in neovascularization is conflicting and the true function of αv integrins in angiogenesis has to be further elucidated.
Also the role of integrin α5β1 in angiogenesis is not entirely understood. Although global knockout of integrin α5 causes early embryonic lethality with defects in vascular development [109, 110], an endothelial specific knockout of this integrin has no obvious effect on developmental angiogenesis [111]. Since an endothelial specific double knockout of integrin α5 and αv results in defects in vessel remodeling, a compensatory regulation between these integrins has been suggested. Albeit these integrins seem not to play a major role during developmental angiogenesis, but their function during vascular remodeling and pathological angiogenesis in the adult organism remains to be elucidated.
Mechanistically, integrins regulate not only focal adhesion formation in the migrating endothelial cell but also facilitate several additional steps of the migratory cycle through interaction with small GTPases. Rac, Cdc42 and Rho activity are all subject to integrin signaling affecting actin polymerization and cellular protrusion formation [112].
Being receptors for ECM proteins integrins transmit cues from the ECM to regulate outgrowth and maturation of sprouting endothelial cells. The vascular basal membrane is unique with respect to their laminin isoform composition as a consequence of endothelial matrix synthesis. Endothelial cells express only two laminin isoforms, laminin 8 and 10, and the relative proportion of the synthesis rate of the two proteins varies depending on vessel type, developmental stage and endothelial activation state [113]. Laminin 8 is expressed by all endothelial cells regardless of their state of development, whereas laminin 10 is detected only in the basement membrane of capillaries and some venules starting 3–4 weeks after birth. In in vitro assays endothelial cells bind to laminin 8 only with low affinity which suggests that this matrix protein might facilitate cell migration [114, 115]. But not only the intact laminin molecule is biologically active also fragments of the molecule generated by proteases in vivo induce cellular signaling. A laminin fragment of the last two globular domains inhibits endothelial cell migration and tube formation in vitro [116], which probably involves interference with integrin α6β1 and α3β1. Consistent with these data are the observations in laminin α4 (the α subunit of laminin 8) knockout mice, which exhibit hemorrhages during the late embryonic and neonatal periods. Furthermore, in the cornea angiogenesis model laminin 8 was required for vessel integrity and maturation. In this model, blood vessel formation is enhanced, with dilation of vessels, aberrant branching and edema formation [117]. In contrast to the important role of laminin 8 in angiogenesis, laminin 10 seems to be dispensable for angiogenesis and rather mediates vessel stability and barrier function [118]. Additionally, laminin-111 regulates Notch signaling and tip/stalk cell balance. It induces integrin α2β1 and α6β1-dependent signaling, which increases Dll4 expression and Notch pathway activation. Furthermore, VEGF induces laminin production and deposition and, therefore, impacts on the regulation of tip/stalk cell phenotypes at an additional level [119].
Apart from laminins also other ECM components regulate endothelial cell migration and morphogenesis, including fibrin and collagen I [120].
Endothelial cell migration within the vessels: cell rearrangements
Endothelial cell migration also occurs within the vessel. In the sprout of a newly formed vessel the tip cell migrates in the direction of vessel formation but also cell rearrangement occurs: the tip cell migrates retrograde and is replaced by a stalk cell. Also during anastomosis, the fusion of two tip cells or a tip cell and a pre-existing blood vessel, cell rearrangements can be detected.
Recent reports demonstrate that in a sprouting vessel heterogeneous collective cell migration occurs. Individual cells migrate forwards and backwards at different velocities, changing their relative position within the branch, even at the tip position. By live cell imaging it could be documented that the leading tip cell after some time in this position slows down and stops elongation and is, therefore, subsequently overtaken by another cell. This observation could be formulated into a novel model in which the tip cell creates the space for vessel invasion and serves as a rail for the prospective tip cell [121].
As described above, tip cell formation and position changes in the sprout are dependent on Dll4–Notch signaling. Position shuffling in the sprout ,therefore, constantly changes neighborhood relationships and should trigger recurrent Dll4–Notch-mediated competition. Cells with higher VEGFR2 expression and low-Notch signaling will overtake their neighbors in this process. The biological significance of cell shuffling is not clear. It could be speculated that cell shuffling improves directionality of migration [81] or prevents exhaustion of the cell at tip position.
Additionally, VE-cadherin signaling impacts on cell rearrangements. Motile cells, e.g. VEGF-stimulated cells, within sheets or sprouts form serrated junctions instead of straight junctions which are typical for quiescent cells. These serrated junctions are characterized by high protein turnover of VE-cadherin. Notch signaling inhibits VE-cadherin phosphorylation and turnover and attenuates serrate junction formation. Consequently, differential cell movement and position changes are abolished. These findings demonstrate that an active endothelial cell can either form a new branch or shuffle up through the existing branch. The process by which the cells take this decision, however, is still unclear [122].
Also during branch anastomosis cell rearrangements occur. In zebrafish the dorsal longitudinal anastomotic vessel (DLAV) forms from the connection of two tip cells of the intersegmental vessels. But also stalk cells migrate dorsally and contribute to parts of the DLAV [123]. Moreover, the transformation of a unicellular tube into a multicellular tube involves cell rearrangements, a typical step in the morphogenetic events of anastomosis [124].
Guidance cues
Endothelial cell migration depends on the environmental conditions. Under certain conditions, like in the Matrigel assay, migrating endothelial cells make up a network and it is unclear whether this process involves any guidance. Additionally, cell migration during cell rearrangement seems to be independent of the directional stimulus. But to adapt the vasculature to the surrounding tissue, the local environment regulates vessel formation by angiogenesis-regulating substances. These guide the developing vessel and facilitate the development of a tissue-adapted vascular pattern but how vascular patterning is achieved is not yet entirely understood. One mechanism is the guidance by factors similar to those involved in neuronal network patterning. Already five centuries ago Andreas Vesalius recognized the similarities of the two networks and noted that nerves and vessels are often aligned. In recent years, pathways shared by both systems have been identified and it is now clear that axons and vessels often take advantage of one another to follow the same path. Vessels produce axon-attracting signals [125, 126] and conversely, nerves produce pro-angiogenic and vascular patterning signals. Apart from this mutual guidance, a second mechanism explains that axons and vessels are aligned: neuronal cells and endothelial cells respond to common cues. Several molecules have been identified to guide nerves as well as blood vessels. In the nervous system, astrocytes and other glia cells generate these cues and, therefore, play an essential role in guiding endothelial cells in neuro-vascular development as observed in the retina.
Neuronal guidance cues in vessel formation
Although different at the macro-anatomical level, the neuronal growth cone and the endothelial tip cell share many similarities like the exploration of the environment for the correct migration path by extension and retraction of filopodia. Many guidance cues have been identified that stimulate both cell types. Astrocyte-derived epoxyeicosatrienoic acids, for example, induce endothelial cell migration and angiogenesis [127, 128] and recently it was realized that they also enhance axonal growth [129]. Guidance molecules are either matrix- or cell membrane bound or secreted. Importantly, they can attract as well as repel cells and this aspect depends on receptors expressed on the responsive cell [130, 131]. The majority of these cues is a part of four families: the Semaphorins, Ephrins, Netrins and Slits.
Semaphorins and their neuropilin and plexin receptors
The Semaphorins are a family of either secreted (class A Semaphorins) or membrane-bound proteins regulating axon growth, cell migration, cell death or synapse formation during nervous system development [132]. Initially, they were identified as neuronal growth cone-collapsing proteins involved in repulsive axon guidance but additional studies implicated Semaphorins in cellular events such as dendrite specification, axon sorting and synaptic specificity [133, 134]. Semaphorins bind two major receptor families; secreted class A Semaphorins bind neuropilins (Nrp) whereas membrane-bound forms bind plexins [135, 136]. Sema7A stimulates axon extension by activating integrins [137].
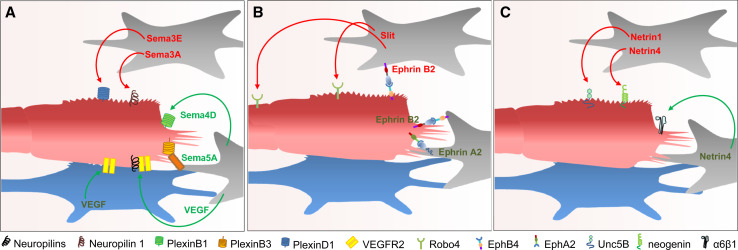
Semaphorins and their receptors also regulate vessel guidance and branching (Fig. 3a). Endothelial cells express various Nrp and plexin receptors mediating anti- and pro-angiogenic effects depending on the respective receptor. On endothelial cells, Nrps not only bind Semaphorins but also serve as VEGF co-receptors and are ,therefore, involved in vascular development [138, 139]. Knockdown of Nrp inhibits endothelial cell migration leading to impaired heart and blood vessel development and embryonic lethality [140, 141]. Initially, Sema3A has been demonstrated to inhibit endothelial cell migration and angiogenic sprouting via binding to neuropilin-1 [142]. It has been suggested that this effect is mediated by inhibition of VEGF signaling, since Nrp-1 also acts as a receptor for VEGF. Despite this finding, no obvious developmental vascular patterning defects were found in mice lacking Sema3A [143]. Therefore, Sema3A does not seem to be required for the early stages of developmental angiogenesis, but rather plays a role during vessel remodeling and pathological angiogenesis. Sema3E is the only class 3 semaphorin that binds Plexin D1 instead of the Nrp receptor. During developmental angiogenesis Sema3E is highly expressed in somites and acts as a repulsive cue to restrict vessel growth and branching to the intersomitic space [144]. Furthermore, Sema3E regulates the initial formation of the dorsal aorta. Sema3E knockout embryos develop an abnormally branched aortic plexus with a markedly narrowed avascular mid-line. Additionally, recent studies have demonstrated that exogenous Sema3E can inhibit tumor angiogenesis [145].
Fig. 3.
Regulation of endothelial cell migration by guidance cues. Guidance molecules released from astrocytes (gray) or neurons (blue) can induce or abolish endothelial cell migration depending on the receptor expressed on the endothelial cell. a Attractive and repellent signaling by Semaphorins and their receptors. b Ephrin-Eph forward and reverse signaling regulates endothelial cell migration. Binding of Slit to Robo4 inhibits endothelial cell migration and helps maintaining vascular integrity. c Binding of Netrin to Unc5B or neogenin inhibits endothelial cell migration whereas binding to integrin α6β1 attracts endothelial cells
The angiogenesis promoting effect of some Semaphorins is mediated by an activation of plexin receptors, with Sema4D being the best studied molecule. Binding of Sema4D to Plexin B1 increases endothelial cell migration and attraction by stimulating the interaction of the Plexin B1 receptor with the two GEFs, PDZ-RhoGEF and LARG. The subsequent activation of Rho results in migration, tubulogenesis and angiogenesis [146]. Plexin B1 signaling, however, is complex as RhoA also activates the tyrosine kinases Pyk2 and Src as well as the PI3K and Akt [147]. Furthermore, Plexin B1 activation results in phosphorylation of the receptor tyrosine kinases Met and Ron [148, 149] and as a crosstalk with integrin-dependent cell adhesion and phosphatidylinositol 4-phosphate 5-kinase-generated phosphatidyl-inositol 4,5-bisphosphate (PI(4,5)P2) has been suggested to contribute to the pro-migratory effect of Sema4D [150, 151]. In Plexin B1 receptor knockout mice tumor angiogenesis was not attenuated suggesting that other B-type plexins can substitute the PlexinB1 receptor [152].
An additional pro-angiogenic semaphorin is Sema5A, a membrane-anchored semaphorin that binds the Plexin B3 receptor [153]. Sema5A has been suggested to regulate remodeling of cranial blood vessels during development, and in Sema5A null mutants, the complexity of the hierarchically organized branches of the cranial cardinal veins is decreased [154]. Finally, Sema6D was found to activate VEGFR-2-mediated signal transduction via its receptor Plexin A1 forming complexes with VEGFR2 [155], but the angiogenic potential of this semaphorin has not yet been described.
Ephrins and Eph receptors
The Eph receptor tyrosine kinases and their Ephrin ligands provide another principal class of axon guidance molecules [156]. Both proteins are expressed on the cell surface of neighboring cells and the interaction results in bidirectional signaling from the Eph receptor (forward signaling) and from the Ephrin ligand (reverse signaling) [157, 158]. Initially identified as repellent guidance molecules [159, 160], Ephrins have emerged as positive and negative regulators in the axon wiring process, controlling dendritic spine formation, guidance of neuronal cells and synaptic plasticity [156].
Eph-Ephrin signaling also controls vascular development [161]. One important function of these guidance molecules is the determination of arterial–venous fate of endothelial cells (for reviews see [162, 163]).
Genetic experiments in mice have demonstrated that the global deletion of EphB4 or EphrinB2 leads to embryonic death with marked defects in the angiogenic growth of the primary vascular plexus [164, 165]. Further studies documented an important role of EphrinB2 signaling in sprouting angiogenesis, particularly in the regulation of tip cell function (Fig. 3b). EphrinB2 under this condition is phosphorylated and thus activated [166] and expression of a PDZ-mutant EphrinB2 reduced tip cell filopodia formation. Conversely, stimulation of endothelial EphrinB2 by an EphB4 mimicking antibody promoted filopodia formation. Similar to Semaphorins, also Ephrins mediate part of their action through VEGFRs. Silencing EphrinB2 attenuated VEGFR2 and VEGFR3 signaling and the activation of their downstream components Rac1, Erk1/2, and Akt [167]. Apart from serving as inducers of EphrinB2 reverse signaling, EphB receptors induce forward signaling in their own endothelial cell. This represses endothelial cell migration, adhesion, and proliferation in vitro by suppressing the activation of the small GTPase Ras and mitogen-activated protein (MAP) kinase [168, 169]. Thus, EphB4 forward signaling appears to have opposite effects to EphrinB2 reverse signaling, although also some pro-migratory and angiogenic effects have been described for EphB4 forward signaling [166, 170].
Additional Eph receptors have been implicated in angiogenic processes. For example, by activation of EphA2, EphrinA1 can stimulate angiogenesis [171, 172] but additional studies are required to better define the contribution of the other Ephrins to endothelial function and to reconcile the conflicting findings.
Netrins and their receptors
Also Netrins act as bifunctional guidance cues; they attract and repel axons. During brain development, Netrin-1 is secreted from cells at the ventral midline and guides axons to this site by binding receptors of the deleted in colorectal carcinoma (DCC)-family [173, 174]. To avoid commissural axon stalling at the midline, additional signals terminate the attractant activity of Netrin [175]. Repulsion in response to Netrin-1 requires signaling through Unc receptor homodimers or Unc-DCC receptor heterodimers [176, 177].
Similar to axon guidance, Netrins have bidirectional activities in angiogenesis. The Unc5b receptor which mediates repulsive activity is expressed on endothelial tip cells during development [89] and is reinduced during sprouting angiogenesis in various animal models including Matrigel implants and tumor xenografts. Unc5b activation by Netrin-1 leads to retraction of filopodia in endothelial tip cells, inhibition of migration and attenuation of angiogenic sprouting (Fig. 3c) [89, 178, 179]. Similar as Netrin-1 also Netrin-4 inhibits endothelial cell migration and angiogenesis but this involves binding to its receptor neogenin (another member of the DCC family) and co-activation of Unc5b [178]. Nevertheless, several investigations report a pro-angiogenic role of Netrins as Netrin-1 and -4 induce endothelial cell migration in vitro, but the receptor involved has not been identified yet [180–182]. One study suggests involvement of the DCC receptor but most of the other studies, failed to detect significant DCC expression in cultured endothelial cells [89, 178, 180, 182]. Another potential explanation for the differential response to Netrin-1 is that its concentration determines the quality of the response: low concentrations induce endothelial cell migration, proliferation and neovascularization, but higher concentrations have the opposite effect [183]. Alternatively, not all receptors for Netrin have yet been identified. For Netrin-4 integrin, α6β1 has recently been identified as a novel binding partner mediating the pro-migratory effect [184]. Together these data suggest a dual activity of Netrins during angiogenesis as they also have during axon guidance. The involved receptors and underlying mechanisms are, however, not yet completely understood.
Slits and roundabouts (Robos)
Slits are large secreted glycoproteins binding to robo receptors, which were identified as repellents for some axons [185, 186] and as stimulators of branching and elongation of others [187]. One important function of Slits, mediated via robo1 and 2, is preventing commissural axons to cross the midline [188]. Additionally, they are implicated in additional axon guidance processes and regulate differentiation and migration of diverse neuronal cell populations [189].
In endothelial cells, a vascular-specific robo receptor, Robo4, is expressed. In the zebrafish, Robo4 mediates correct path finding of developing intersomitic vessels [190]. Angioblasts isolated from zebrafish embryos treated with Robo4 morpholinos showed more active and extensive movement with less active Cdc42 and Rac [191] and activation of Robo4 by slit inhibits endothelial cell migration [192]. Surprisingly, Robo4 null mice displayed no vascular guidance defects and Robo4-deficient mice are viable and fertile. As a potential explanation for this result, Robo4 may regulate vascular stability rather than guidance. Robo4 expression is predominantly localized to stalk cells and recombinant slit protein can inhibit VEGF-induced cell migration and tube formation in a Robo4-dependent manner (Fig. 3b) [193]. Thus, Robo4 could help to maintain vascular integrity by preventing stalk cells from being activated by VEGF. Indeed, slit-deficient mice showed an increase in vascular network density and complexity in the mammary glands. Slits, derived from stromal cells of the vessel, inhibit vessel growth by down-regulating VEGFR signaling through Robo4 but not Robo1. Robo4 signaling suppresses cellular protrusive activity and cell migration through a direct interaction with the adaptor protein paxillin. Formation of the Robo4-paxillin complex blocks the activation of the small GTPase ADP-ribosylation factor Arf6 and, consequently that of Rac by recruitment of Arf GTPase-activating proteins (GAPs) such as GIT1. Consistent with these in vitro studies, inhibition of Arf6 activity in vivo phenocopies Robo4 activation—it reduces pathologic angiogenesis [194]. An additional receptor for Robo4 is Unc5b. By activating Unc5b, Robo4 inhibits VEGF signaling and maintains vessel integrity [195].
In contrast to these concepts, the slit-robo-system can also be pro-migratory [196]. It appears that cooperation between Slit2 and Ephrin-A1 regulates the balance between the pro- and anti-angiogenic functions of Slit2 [197]. While Slit2 promotes angiogenesis in culture and in vivo as a single agent, it potently inhibits angiogenic remodeling in the presence of Ephrin-A1. Indeed, EphrinA1 inhibits mammalian target of rapamycin (mTOR) activity in the endothelium, which impairs Slit2-induced activation of Akt and/or Rac. These data suggest that Slit2 differentially regulates angiogenesis depending on Ephrin-A1 abundance.
Conclusion
Endothelial cell migration is a complex process important during our whole life. Endothelial cells migrate similar to other cells but possess a specific arsenal of receptors and ligands to orchestrate the many aspects of vascular network formation. Endothelial cell migration not only contributes to tissue formation, it is also indispensable for wound healing and regeneration. Although the fundamental steps of cell migration are reasonably well understood the involved mechanisms and consequences vary according to the cellular context and these aspects are still uncertain for most situations. Endothelial cell migration is a therapeutic target for the treatment of different diseases like age-related macular degeneration, diabetic angiopathy, myocardial infarction and cancer. Exploitation of the subtle differences in endothelial cell migratory signaling may allow the development of anti-angiogenic drugs which do only block pathology-associated migration and angiogenesis but which do not interfere with physiological endothelial function.
Acknowledgments
This work was supported by the Excellence Cluster Pulmonary System (ECCPS) and the DFG SFB834 TP A10. The author thanks Ralf P. Brandes for editorial suggestions during the preparation of the manuscript.
Abbreviations
- Arf6
ADP-ribosylation factor 6
- Arp2/3
Activators of actin related proteins 2/3
- DCC
Deleted in colorectal carcinoma
- Dll4
Delta-like 4
- ECM
Extra cellular matrix
- bFGF
Basic fibroblast growth factor
- G-actin
Globular actin
- GAP
GTPase-activating protein
- GEF
Guanine nucleotide exchange factor
- MT1-MMP
Membrane type 1-matrix metalloprotease
- MTOC
Microtubule-organization center
- Nrp
Neuropilin
- PDGF
Platelet-derived growth factor
- PIP3
Phosphatidylinositol (3,4,5)-trisphosphate
- PI(4,5)P2
Phosphatidyl-inositol 4,5-bisphosphate
- PTEN
Phosphatase and tensin homolog
- Robo
Roundabout
- SDF1
Stromal cell-derived factor 1
- TGFβ
Transforming growth factor-β
- Unc5B
Unc-5 homolog B
- VEGF
Vascular endothelial growth factor
- VEGFR
VEGF receptor
References
- 1.Gail MH, Boone CW. The locomotion of mouse fibroblasts in tissue culture. Biophys J. 1970;10:980–993. doi: 10.1016/S0006-3495(70)86347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand-Apte B, Zetter B. Signaling mechanisms in growth factor-stimulated cell motility. Stem Cells. 1997;15:259–267. doi: 10.1002/stem.150259. [DOI] [PubMed] [Google Scholar]
- 3.Stoker M, Gherardi E. Regulation of cell movement: the motogenic cytokines. Biochim Biophys Acta. 1991;1072:81–102. doi: 10.1016/0304-419x(91)90008-9. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Peng W, Zhuang J, Lu Y, Jian W, Wei Y, Li W, Xu Y. Vaspin attenuates high glucose-induced vascular smooth muscle cells proliferation and chemokinesis by inhibiting the MAPK, PI3K/Akt, and NF-kappaB signaling pathways. Atherosclerosis. 2013;228:61–68. doi: 10.1016/j.atherosclerosis.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982;92:584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrieumerlou C, Meyer T. A local coupling model and compass parameter for eukaryotic chemotaxis. Dev Cell. 2005;8:215–227. doi: 10.1016/j.devcel.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Bourne HR, Weiner O. A chemical compass. Nature. 2002;419:21. doi: 10.1038/419021a. [DOI] [PubMed] [Google Scholar]
- 8.Carter SB. Principles of cell motility: the direction of cell movement and cancer invasion. Nature. 1965;208:1183–1187. doi: 10.1038/2081183a0. [DOI] [PubMed] [Google Scholar]
- 9.Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin Cell Dev Biol. 2009;20:674–682. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aman A, Piotrowski T. Cell migration during morphogenesis. Dev Biol. 2010;341:20–33. doi: 10.1016/j.ydbio.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 13.Weiner OD. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr Opin Cell Biol. 2002;14:196–202. doi: 10.1016/s0955-0674(02)00310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- 18.Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–821. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 19.Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- 20.Rooney C, White G, Nazgiewicz A, Woodcock SA, Anderson KI, Ballestrem C, Malliri A. The Rac activator STEF (Tiam2) regulates cell migration by microtubule-mediated focal adhesion disassembly. EMBO Rep. 2010;11:292–298. doi: 10.1038/embor.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etienne-Manneville S. Microtubules in cell migration. Annu Rev Cell Dev Biol. 2013;29:471–499. doi: 10.1146/annurev-cellbio-101011-155711. [DOI] [PubMed] [Google Scholar]
- 22.Pankov R, Endo Y, Even-Ram S, Araki M, Clark K, Cukierman E, Matsumoto K, Yamada KM. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connor K, Chen M. Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases. 2013;4:141–147. doi: 10.4161/sgtp.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welch MD, Mullins RD. Cellular control of actin nucleation. Annu Rev Cell Dev Biol. 2002;18:247–288. doi: 10.1146/annurev.cellbio.18.040202.112133. [DOI] [PubMed] [Google Scholar]
- 25.Cory GO, Ridley AJ. Cell motility: braking WAVEs. Nature. 2002;418:732–733. doi: 10.1038/418732a. [DOI] [PubMed] [Google Scholar]
- 26.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 27.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix—cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 28.Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 30.Jay PY, Pham PA, Wong SA, Elson EL. A mechanical function of myosin II in cell motility. J Cell Sci. 1995;108(Pt 1):387–393. doi: 10.1242/jcs.108.1.387. [DOI] [PubMed] [Google Scholar]
- 31.Spiering D, Hodgson L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adhes Migr. 2011;5:170–180. doi: 10.4161/cam.5.2.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gov NS. Traction forces during collective cell motion. HFSP J. 2009;3:223–227. doi: 10.2976/1.3185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 35.Friedl P, Hegerfeldt Y, Tusch M. Collective cell migration in morphogenesis and cancer. Int J Dev Biol. 2004;48:441–449. doi: 10.1387/ijdb.041821pf. [DOI] [PubMed] [Google Scholar]
- 36.van Kempen LC, van den Oord JJ, van Muijen GN, Weidle UH, Bloemers HP, Swart GW. Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am J Pathol. 2000;156:769–774. doi: 10.1016/S0002-9440(10)64943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vitorino P, Meyer T. Modular control of endothelial sheet migration. Genes Dev. 2008;22:3268–3281. doi: 10.1101/gad.1725808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- 40.Lecaudey V, Cakan-Akdogan G, Norton WH, Gilmour D. Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development. 2008;135:2695–2705. doi: 10.1242/dev.025981. [DOI] [PubMed] [Google Scholar]
- 41.Aman A, Piotrowski T. Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev Cell. 2008;15:749–761. doi: 10.1016/j.devcel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Hegerfeldt Y, Tusch M, Brocker EB, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell–cell interaction, beta1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–2130. [PubMed] [Google Scholar]
- 43.Valentin G, Haas P, Gilmour D. The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Curr Biol. 2007;17:1026–1031. doi: 10.1016/j.cub.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 44.Kolega J. The movement of cell clusters in vitro: morphology and directionality. J Cell Sci. 1981;49:15–32. doi: 10.1242/jcs.49.1.15. [DOI] [PubMed] [Google Scholar]
- 45.Friedl P, Noble PB, Walton PA, Laird DW, Chauvin PJ, Tabah RJ, Black M, Zanker KS. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res. 1995;55:4557–4560. [PubMed] [Google Scholar]
- 46.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 47.Friedl P, Borgmann S, Brocker EB. Amoeboid leukocyte crawling through extracellular matrix: lessons from the Dictyostelium paradigm of cell movement. J Leukoc Biol. 2001;70:491–509. [PubMed] [Google Scholar]
- 48.Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–R228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Le NF, Klein C, Tintu A, Pries A, Buschmann I. Neural guidance molecules, tip cells, and mechanical factors in vascular development. Cardiovasc Res. 2008;78:232–241. doi: 10.1093/cvr/cvn058. [DOI] [PubMed] [Google Scholar]
- 50.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 51.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 52.Boguslavsky S, Grosheva I, Landau E, Shtutman M, Cohen M, Arnold K, Feinstein E, Geiger B, Bershadsky A. p120 catenin regulates lamellipodial dynamics and cell adhesion in cooperation with cortactin. Proc Natl Acad Sci USA. 2007;104:10882–10887. doi: 10.1073/pnas.0702731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geisbrecht ER, Montell DJ. Myosin VI is required for E-cadherin-mediated border cell migration. Nat Cell Biol. 2002;4:616–620. doi: 10.1038/ncb830. [DOI] [PubMed] [Google Scholar]
- 54.Hofmann UB, Eggert AA, Blass K, Brocker EB, Becker JC. Expression of matrix metalloproteinases in the microenvironment of spontaneous and experimental melanoma metastases reflects the requirements for tumor formation. Cancer Res. 2003;63:8221–8225. [PubMed] [Google Scholar]
- 55.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 58.Ferguson JE, III, Kelley RW, Patterson C. Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2246–2254. doi: 10.1161/01.ATV.0000183609.55154.44. [DOI] [PubMed] [Google Scholar]
- 59.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 60.Noden DM. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103(Suppl):121–140. doi: 10.1242/dev.103.Supplement.121. [DOI] [PubMed] [Google Scholar]
- 61.Cleaver O, Krieg PA. VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development. 1998;125:3905–3914. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- 62.Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 63.Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 64.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 65.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 66.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakashima Y, Wight TN, Sueishi K. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res. 2008;79:14–23. doi: 10.1093/cvr/cvn099. [DOI] [PubMed] [Google Scholar]
- 68.Weis SM. Vascular permeability in cardiovascular disease and cancer. Curr Opin Hematol. 2008;15:243–249. doi: 10.1097/MOH.0b013e3282f97d86. [DOI] [PubMed] [Google Scholar]
- 69.Vitorino P, Hammer M, Kim J, Meyer T. A steering model of endothelial sheet migration recapitulates monolayer integrity and directed collective migration. Mol Cell Biol. 2011;31:342–350. doi: 10.1128/MCB.00800-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michaelis UR, Chavakis E, Kruse C, Jungblut B, Kaluza D, Wandzioch K, Manavski Y, Heide H, Santoni MJ, Potente M, Eble JA, Borg JP, Brandes RP. The polarity protein Scrib is essential for directed endothelial cell migration. Circ Res. 2013;112:924–934. doi: 10.1161/CIRCRESAHA.112.300592. [DOI] [PubMed] [Google Scholar]
- 71.Carmeliet P, De SF, Loges S, Mazzone M. Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat Rev Clin Oncol. 2009;6:315–326. doi: 10.1038/nrclinonc.2009.64. [DOI] [PubMed] [Google Scholar]
- 72.Germain S, Monnot C, Muller L, Eichmann A. Hypoxia-driven angiogenesis: role of tip cells and extracellular matrix scaffolding. Curr Opin Hematol. 2010;17:245–251. doi: 10.1097/MOH.0b013e32833865b9. [DOI] [PubMed] [Google Scholar]
- 73.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 74.Hellstrom M, Phng LK, Gerhardt H. VEGF and Notch signaling: the yin and yang of angiogenic sprouting. Cell Adhes Migr. 2007;1:133–136. doi: 10.4161/cam.1.3.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suchting S, Freitas C, Le NF, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 79.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De SF, Segura I, De BK, Hohensinner PJ, Carmeliet P. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol. 2009;29:639–649. doi: 10.1161/ATVBAHA.109.185165. [DOI] [PubMed] [Google Scholar]
- 81.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 82.Bentley K, Mariggi G, Gerhardt H, Bates PA. Tipping the balance: robustness of tip cell selection, migration and fusion in angiogenesis. PLoS Comput Biol. 2009;5:e1000549. doi: 10.1371/journal.pcbi.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4:241–246. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kater SB, Rehder V. The sensory-motor role of growth cone filopodia. Curr Opin Neurobiol. 1995;5:68–74. doi: 10.1016/0959-4388(95)80089-1. [DOI] [PubMed] [Google Scholar]
- 86.Fischer RS, Gardel M, Ma X, Adelstein RS, Waterman CM. Local cortical tension by myosin II guides 3D endothelial cell branching. Curr Biol. 2009;19:260–265. doi: 10.1016/j.cub.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phng LK, Stanchi F, Gerhardt H. Filopodia are dispensable for endothelial tip cell guidance. Development. 2013;140:4031–4040. doi: 10.1242/dev.097352. [DOI] [PubMed] [Google Scholar]
- 88.del Toro R, Prahst C, Mathivet T, Siegfried G, Kaminker JS, Larrivee B, Breant C, Duarte A, Takakura N, Fukamizu A, Penninger J, Eichmann A. Identification and functional analysis of endothelial tip cell-enriched genes. Blood. 2010;116:4025–4033. doi: 10.1182/blood-2010-02-270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu X, Le NF, Yuan L, Jiang Q, De LB, Sugiyama D, Breant C, Claes F, De SF, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 90.Yana I, Sagara H, Takaki S, Takatsu K, Nakamura K, Nakao K, Katsuki M, Taniguchi S, Aoki T, Sato H, Weiss SJ, Seiki M. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci. 2007;120:1607–1614. doi: 10.1242/jcs.000679. [DOI] [PubMed] [Google Scholar]
- 91.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen M, Arkell J, Jackson CJ. Human endothelial gelatinases and angiogenesis. Int J Biochem Cell Biol. 2001;33:960–970. doi: 10.1016/s1357-2725(01)00007-3. [DOI] [PubMed] [Google Scholar]
- 93.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 94.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000;151:879–889. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O’Reilly MS, Wiederschain D, Stetler-Stevenson WG, Folkman J, Moses MA. Regulation of angiostatin production by matrix metalloproteinase-2 in a model of concomitant resistance. J Biol Chem. 1999;274:29568–29571. doi: 10.1074/jbc.274.41.29568. [DOI] [PubMed] [Google Scholar]
- 99.Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- 100.Hynes RO, Bader BL, Hodivala-Dilke K. Integrins in vascular development. Braz J Med Biol Res. 1999;32:501–510. doi: 10.1590/s0100-879x1999000500002. [DOI] [PubMed] [Google Scholar]
- 101.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–658. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 102.Kiosses WB, Shattil SJ, Pampori N, Schwartz MA. Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat Cell Biol. 2001;3:316–320. doi: 10.1038/35060120. [DOI] [PubMed] [Google Scholar]
- 103.Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, Plow EF. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- 104.Woodard AS, Garcia-Cardena G, Leong M, Madri JA, Sessa WC, Languino LR. The synergistic activity of alphavbeta3 integrin and PDGF receptor increases cell migration. J Cell Sci. 1998;111(Pt 4):469–478. doi: 10.1242/jcs.111.4.469. [DOI] [PubMed] [Google Scholar]
- 105.Carlson TR, Hu H, Braren R, Kim YH, Wang RA. Cell-autonomous requirement for beta1 integrin in endothelial cell adhesion, migration and survival during angiogenesis in mice. Development. 2008;135:2193–2202. doi: 10.1242/dev.016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim S, Harris M, Varner JA. Regulation of integrin alpha vbeta 3-mediated endothelial cell migration and angiogenesis by integrin alpha5beta1 and protein kinase A. J Biol Chem. 2000;275:33920–33928. doi: 10.1074/jbc.M003668200. [DOI] [PubMed] [Google Scholar]
- 107.Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost. 2007;5(Suppl 1):32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- 108.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 109.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 110.Goh KL, Yang JT, Hynes RO. Mesodermal defects and cranial neural crest apoptosis in alpha5 integrin-null embryos. Development. 1997;124:4309–4319. doi: 10.1242/dev.124.21.4309. [DOI] [PubMed] [Google Scholar]
- 111.van der Flier A, Badu-Nkansah K, Whittaker CA, Crowley D, Bronson RT, Lacy-Hulbert A, Hynes RO. Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development. 2010;137:2439–2449. doi: 10.1242/dev.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 113.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 114.Fujiwara H, Kikkawa Y, Sanzen N, Sekiguchi K. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins. J Biol Chem. 2001;276:17550–17558. doi: 10.1074/jbc.M010155200. [DOI] [PubMed] [Google Scholar]
- 115.Fujiwara H, Gu J, Sekiguchi K. Rac regulates integrin-mediated endothelial cell adhesion and migration on laminin-8. Exp Cell Res. 2004;292:67–77. doi: 10.1016/j.yexcr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 116.Talts JF, Sasaki T, Miosge N, Gohring W, Mann K, Mayne R, Timpl R. Structural and functional analysis of the recombinant G domain of the laminin alpha4 chain and its proteolytic processing in tissues. J Biol Chem. 2000;275:35192–35199. doi: 10.1074/jbc.M003261200. [DOI] [PubMed] [Google Scholar]
- 117.Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22:1194–1202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sorokin LM, Pausch F, Frieser M, Kroger S, Ohage E, Deutzmann R. Developmental regulation of the laminin alpha5 chain suggests a role in epithelial and endothelial cell maturation. Dev Biol. 1997;189:285–300. doi: 10.1006/dbio.1997.8668. [DOI] [PubMed] [Google Scholar]
- 119.Estrach S, Cailleteau L, Franco CA, Gerhardt H, Stefani C, Lemichez E, Gagnoux-Palacios L, Meneguzzi G, Mettouchi A. Laminin-binding integrins induce Dll4 expression and Notch signaling in endothelial cells. Circ Res. 2011;109:172–182. doi: 10.1161/CIRCRESAHA.111.240622. [DOI] [PubMed] [Google Scholar]
- 120.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 121.Arima S, Nishiyama K, Ko T, Arima Y, Hakozaki Y, Sugihara K, Koseki H, Uchijima Y, Kurihara Y, Kurihara H. Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development. 2011;138:4763–4776. doi: 10.1242/dev.068023. [DOI] [PubMed] [Google Scholar]
- 122.Bentley K, Franco CA, Philippides A, Blanco R, Dierkes M, Gebala V, Stanchi F, Jones M, Aspalter IM, Cagna G, Westrom S, Claesson-Welsh L, Vestweber D, Gerhardt H. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol. 2014;16:309–321. doi: 10.1038/ncb2926. [DOI] [PubMed] [Google Scholar]
- 123.Blum Y, Belting HG, Ellertsdottir E, Herwig L, Luders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 124.Lenard A, Ellertsdottir E, Herwig L, Krudewig A, Sauteur L, Belting HG, Affolter M. In vivo analysis reveals a highly stereotypic morphogenetic pathway of vascular anastomosis. Dev Cell. 2013;25:492–506. doi: 10.1016/j.devcel.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 125.Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, Milbrandt J. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35:267–282. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- 126.Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 127.Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278:H1163–H1167. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- 128.Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J Cell Sci. 2005;118:5489–5498. doi: 10.1242/jcs.02674. [DOI] [PubMed] [Google Scholar]
- 129.Abdu E, Bruun DA, Yang D, Yang J, Inceoglu B, Hammock BD, Alkayed NJ, Lein PJ. Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures. J Neurochem. 2011;117:632–642. doi: 10.1111/j.1471-4159.2010.07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 131.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 132.Koncina E, Roth L, Gonthier B, Bagnard D. Role of semaphorins during axon growth and guidance. Adv Exp Med Biol. 2007;621:50–64. doi: 10.1007/978-0-387-76715-4_4. [DOI] [PubMed] [Google Scholar]
- 133.Kumanogoh A, Kikutani H. Semaphorins and their receptors: novel features of neural guidance molecules. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:611–620. doi: 10.2183/pjab.86.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263–274. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 136.Fujisawa H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol. 2004;59:24–33. doi: 10.1002/neu.10337. [DOI] [PubMed] [Google Scholar]
- 137.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 138.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165 [corrected] J Biol Chem. 2000;275:18040–18045. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 139.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 140.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 141.Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 2008;135:2479–2488. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 142.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vieira JM, Schwarz Q, Ruhrberg C. Role of the neuropilin ligands VEGF164 and SEMA3A in neuronal and vascular patterning in the mouse. Novartis Found Symp. 2007;283:230–235. doi: 10.1002/9780470319413.ch18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 145.Sakurai A, Gavard J, Annas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, Weigert R, Gutkind JS. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 2010;30:3086–3098. doi: 10.1128/MCB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- 147.Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25:6889–6898. doi: 10.1128/MCB.25.16.6889-6898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- 149.Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23:5131–5137. doi: 10.1038/sj.onc.1207650. [DOI] [PubMed] [Google Scholar]
- 150.Basile JR, Gavard J, Gutkind JS. Plexin-B1 utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK and endothelial cell motility. J Biol Chem. 2007;282:34888–34895. doi: 10.1074/jbc.M705467200. [DOI] [PubMed] [Google Scholar]
- 151.Binmadi NO, Proia P, Zhou H, Yang YH, Basile JR. Rho-mediated activation of PI(4)P5K and lipid second messengers is necessary for promotion of angiogenesis by Semaphorin 4D. Angiogenesis. 2011;14:309–319. doi: 10.1007/s10456-011-9214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fazzari P, Penachioni J, Gianola S, Rossi F, Eickholt BJ, Maina F, Alexopoulou L, Sottile A, Comoglio PM, Flavell RA, Tamagnone L. Plexin-B1 plays a redundant role during mouse development and in tumour angiogenesis. BMC Dev Biol. 2007;7:55. doi: 10.1186/1471-213X-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 2004;5:710–714. doi: 10.1038/sj.embor.7400189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fiore R, Rahim B, Christoffels VM, Moorman AF, Puschel AW. Inactivation of the Sema5a gene results in embryonic lethality and defective remodeling of the cranial vascular system. Mol Cell Biol. 2005;25:2310–2319. doi: 10.1128/MCB.25.6.2310-2319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Palmer A, Klein R. Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes Dev. 2003;17:1429–1450. doi: 10.1101/gad.1093703. [DOI] [PubMed] [Google Scholar]
- 157.Chrencik JE, Brooun A, Kraus ML, Recht MI, Kolatkar AR, Han GW, Seifert JM, Widmer H, Auer M, Kuhn P. Structural and biophysical characterization of the EphB4*ephrinB2 protein–protein interaction and receptor specificity. J Biol Chem. 2006;281:28185–28192. doi: 10.1074/jbc.M605766200. [DOI] [PubMed] [Google Scholar]
- 158.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 160.Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- 161.Augustin HG, Reiss Y. EphB receptors and ephrinB ligands: regulators of vascular assembly and homeostasis. Cell Tissue Res. 2003;314:25–31. doi: 10.1007/s00441-003-0770-9. [DOI] [PubMed] [Google Scholar]
- 162.Adams RH. Molecular control of arterial-venous blood vessel identity. J Anat. 2003;202:105–112. doi: 10.1046/j.1469-7580.2003.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aitsebaomo J, Portbury AL, Schisler JC, Patterson C. Brothers and sisters: molecular insights into arterial-venous heterogeneity. Circ Res. 2008;103:929–939. doi: 10.1161/CIRCRESAHA.108.184937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 166.Salvucci O, Tosato G. Essential roles of EphB receptors and EphrinB ligands in endothelial cell function and angiogenesis. Adv Cancer Res. 2012;114:21–57. doi: 10.1016/B978-0-12-386503-8.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 168.Fuller T, Korff T, Kilian A, Dandekar G, Augustin HG. Forward EphB4 signaling in endothelial cells controls cellular repulsion and segregation from ephrinB2 positive cells. J Cell Sci. 2003;116:2461–2470. doi: 10.1242/jcs.00426. [DOI] [PubMed] [Google Scholar]
- 169.Hamada K, Oike Y, Ito Y, Maekawa H, Miyata K, Shimomura T, Suda T. Distinct roles of ephrin-B2 forward and EphB4 reverse signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:190–197. doi: 10.1161/01.atv.0000055440.89758.c2. [DOI] [PubMed] [Google Scholar]
- 170.Steinle JJ, Meininger CJ, Forough R, Wu G, Wu MH, Granger HJ. Eph B4 receptor signaling mediates endothelial cell migration and proliferation via the phosphatidylinositol 3-kinase pathway. J Biol Chem. 2002;277:43830–43835. doi: 10.1074/jbc.M207221200. [DOI] [PubMed] [Google Scholar]
- 171.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science. 1995;268:567–569. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- 172.Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–6052. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 173.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 174.Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 175.Stein E, Schoecklmann H, Daniel TO. Eph family receptors and ligands in vascular cell targeting and assembly. Trends Cardiovasc Med. 1997;7:329–334. doi: 10.1016/S1050-1738(97)00095-9. [DOI] [PubMed] [Google Scholar]
- 176.Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 177.Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature. 1997;386:833–838. doi: 10.1038/386833a0. [DOI] [PubMed] [Google Scholar]
- 178.Lejmi E, Leconte L, Pedron-Mazoyer S, Ropert S, Raoul W, Lavalette S, Bouras I, Feron JG, Maitre-Boube M, Assayag F, Feumi C, Alemany M, Jie TX, Merkulova T, Poupon MF, Ruchoux MM, Tobelem G, Sennlaub F, Plouet J. Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc Natl Acad Sci USA. 2008;105:12491–12496. doi: 10.1073/pnas.0804008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bouvree K, Larrivee B, Lv X, Yuan L, DeLafarge B, Freitas C, Mathivet T, Breant C, Tessier-Lavigne M, Bikfalvi A, Eichmann A, Pardanaud L. Netrin-1 inhibits sprouting angiogenesis in developing avian embryos. Dev Biol. 2008;318:172–183. doi: 10.1016/j.ydbio.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 180.Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GA, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci USA. 2006;103:6530–6535. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, Kuo CJ, Li DY. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci USA. 2004;101:16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang Y, Zou L, Wang Y, Xu KS, Zhang JX, Zhang JH. Axon guidance cue Netrin-1 has dual function in angiogenesis. Cancer Biol Ther. 2007;6:743–748. doi: 10.4161/cbt.6.5.3976. [DOI] [PubMed] [Google Scholar]
- 184.Larrieu-Lahargue F, Welm AL, Thomas KR, Li DY. Netrin-4 activates endothelial integrin {alpha}6{beta}1. Circ Res. 2011;109:770–774. doi: 10.1161/CIRCRESAHA.111.247239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 186.Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, Ornitz DM, Wu JY, Rao Y. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 187.Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 188.Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- 189.Chedotal A. Slits and their receptors. Adv Exp Med Biol. 2007;621:65–80. doi: 10.1007/978-0-387-76715-4_5. [DOI] [PubMed] [Google Scholar]
- 190.Bedell VM, Yeo SY, Park KW, Chung J, Seth P, Shivalingappa V, Zhao J, Obara T, Sukhatme VP, Drummond IA, Li DY, Ramchandran R. Roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:6373–6378. doi: 10.1073/pnas.0408318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kaur S, Castellone MD, Bedell VM, Konar M, Gutkind JS, Ramchandran R. Robo4 signaling in endothelial cells implies attraction guidance mechanisms. J Biol Chem. 2006;281:11347–11356. doi: 10.1074/jbc.M508853200. [DOI] [PubMed] [Google Scholar]
- 192.Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–267. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 193.Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS, Lindblom P, Seth P, Frias A, Nishiya N, Ginsberg MH, Gerhardt H, Zhang K, Li DY. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jones CA, Nishiya N, London NR, Zhu W, Sorensen LK, Chan AC, Lim CJ, Chen H, Zhang Q, Schultz PG, Hayallah AM, Thomas KR, Famulok M, Zhang K, Ginsberg MH, Li DY. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol. 2009;11:1325–1331. doi: 10.1038/ncb1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Koch AW, Mathivet T, Larrivee B, Tong RK, Kowalski J, Pibouin-Fragner L, Bouvree K, Stawicki S, Nicholes K, Rathore N, Scales SJ, Luis E, del Toro R, Freitas C, Breant C, Michaud A, Corvol P, Thomas JL, Wu Y, Peale F, Watts RJ, Tessier-Lavigne M, Bagri A, Eichmann A. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev Cell. 2011;20:33–46. doi: 10.1016/j.devcel.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 196.Wang B, Xiao Y, Ding BB, Zhang N, Yuan X, Gui L, Qian KX, Duan S, Chen Z, Rao Y, Geng JG. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell. 2003;4:19–29. doi: 10.1016/s1535-6108(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 197.Dunaway CM, Hwang Y, Lindsley CW, Cook RS, Wu JY, Boothby M, Chen J, Brantley-Sieders DM. Cooperative signaling between Slit2 and Ephrin-A1 regulates a balance between angiogenesis and angiostasis. Mol Cell Biol. 2011;31:404–416. doi: 10.1128/MCB.00667-10. [DOI] [PMC free article] [PubMed] [Google Scholar]