Abstract
Several metabolic, genetic and oncogenic bone diseases are characterized by defective or excessive bone formation. These abnormalities are caused by dysfunctions in the commitment, differentiation or survival of cells of the osteoblast lineage. During the recent years, significant advances have been made in our understanding of the cellular and molecular mechanisms underlying the osteoblast dysfunctions in osteoporosis, skeletal dysplasias and primary bone tumors. This led to suggest novel therapeutic approaches to correct these abnormalities such as the modulation of WNT signaling, the pharmacological modulation of proteasome-mediated protein degradation, the induction of osteoprogenitor cell differentiation, the repression of cancer cell proliferation and the manipulation of epigenetic mechanisms. This article reviews our current understanding of the major cellular and molecular mechanisms inducing osteoblastic cell abnormalities in age-related bone loss, genetic skeletal dysplasias and primary bone tumors, and discusses emerging therapeutic strategies to counteract the osteoblast abnormalities in these disorders of bone formation.
Keywords: Bone formation, Osteoporosis, Skeletal dysplasias, Genetic mutations, Osteosarcoma, Treatments
Introduction
In the adult skeleton, the maintenance of the bone structure and strength is dependent on the balance between bone resorption and formation during the physiological process of bone remodeling. Bone remodeling begins with the recruitment and differentiation of bone resorbing osteoclasts, the resorption of a fraction of the calcified bone matrix followed by the replacement of the resorbed bone by new bone formed by osteoblasts. Both the quantity and the quality of bone structures are dependent on the balance between bone resorption and formation, and any defective bone formation relative to bone resorption causes bone loss and defective skeletal integrity [1, 2]. The excessive bone resorption in diseases such as osteoporosis or lytic bone tumors can be reduced by anti-resorptive drugs that target osteoclast differentiation or activity [3]. In contrast, bone disorders characterized by too much or disorganized bone formation (such as skeletal dysplasias or osteogenic bone tumors), or by reduced bone formation (caused by disuse or aging) are much more difficult to treat because of the limited number of safe and efficient drugs promoting osteoblast number and activity [4, 5]. In this context, the two important challenges in metabolic, genetic and oncogenic disorders of bone formation and their treatment are to identify the cellular and molecular mechanisms underlying these pathologies and to translate this knowledge into efficient therapy.
During the recent years, studies in human and mouse genetics greatly improved our knowledge of the mechanisms that control osteoblastogenesis and bone formation [6, 7]. Moreover, the identification of osteoblast dysfunctions in human skeletal diseases and mouse models led to propose novel targeted therapeutic approaches in these bone disorders. This article summarizes our current understanding of the cellular and molecular mechanisms underlying the osteoblast dysfunctions in selected metabolic, genetic and bone tumor pathologies, and focuses on emerging targeted therapeutic strategies that can attenuate the osteoblastic cell abnormalities in these bone disorders.
Mechanisms of osteoblastogenesis
Bone formation is a complex process [8] which starts with the recruitment of mesenchymal skeletal (stromal) cells (MSCs) located within the bone marrow stroma, or of pericytes in sinusoids in the bone marrow [9]. The commitment and differentiation of these cells to osteoblasts is dependent on the expression of the osteoblast transcriptional factors Runx2 and Osterix, followed by the expression of alkaline phosphatase, type 1 collagen and non-collagenous proteins, leading to matrix deposition and mineralization [10–12]. At the end of the bone formation period, some osteoblasts die by apoptosis or become flattened lining cells, whereas others are embedded in the bone matrix as osteocytes which control bone remodeling [13]. The activity of bone formation at the tissue level is dependent on both the number and function of differentiated osteoblasts [14, 15]. The number of osteoblasts depends on the osteogenic commitment from precursor cells and on the life-span of mature osteoblasts, whereas the amount of bone matrix produced by mature osteoblasts is dependent on the functional activity of each osteoblast [15, 16]. Both the number of functional osteoblasts and the activity of each osteoblast are controlled by transcriptional and epigenetic mechanisms [12, 17, 18] and are regulated by hormonal and local regulatory proteins [19], mechanical strain [20], cell–cell [21] and cell–matrix interactions [22]. Thus, any cellular or molecular abnormality in the recruitment, function or life-span of osteoblasts induced by metabolic, genetic or oncogenic diseases may impact bone formation at the tissue level, as detailed below.
Mechanisms of osteoblast dysfunctions in osteoporosis
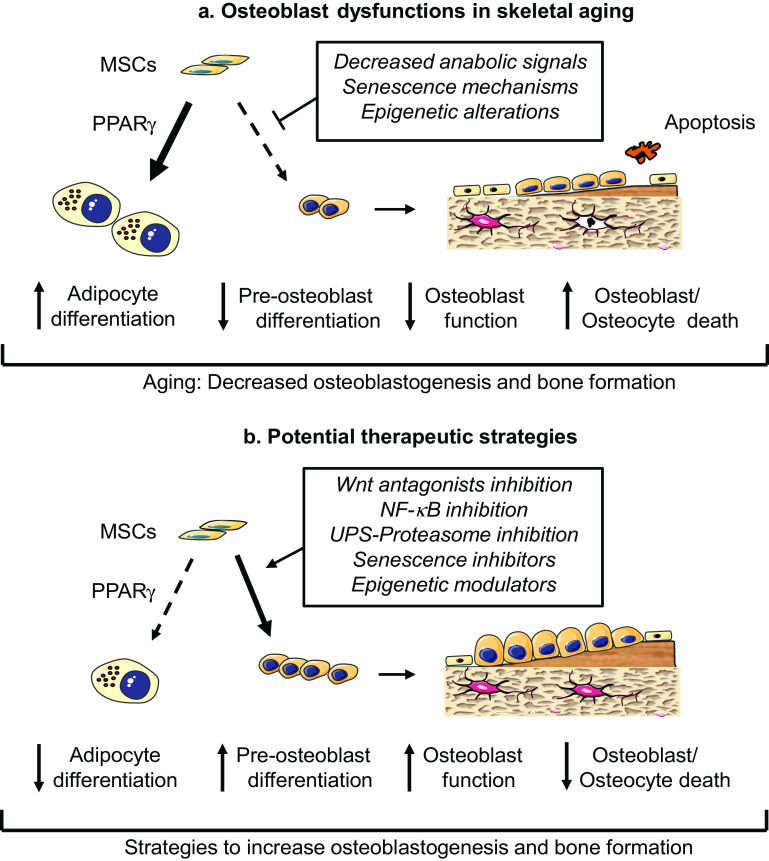
Sex hormone deficiency at the menopause is associated with increased bone resorption, resulting in trabecular bone perforation, bone loss and increased risk of fractures [23]. Mechanistically, bone formation is insufficient to compensate for the increased bone resorbing activity. In addition to this mechanism, age-related bone loss occurs in both women and men as a slow process characterized by decreased osteoblast number and activity. This leads to thinning of bone trabeculae and cortical thickness, which contributes to bone fragility. Thus, the insufficient bone formation relative to bone resorption is an important mechanism mediating bone loss associated with aging and sex hormone deficiency [1]. Studies in humans and animal models allowed identifying multiple cellular mechanisms that contribute to the osteoblast dysfunctions in age-related bone loss. These mechanisms include the reduced recruitment and differentiation of osteoblast progenitor cells and the decreased life-span of mature osteoblasts, in addition to the reduced bone-forming capacity of individual osteoblasts [24–26] (Fig. 1a). Several extrinsic factors can cause these osteoblast dysfunctions in osteoporosis [27]. In addition, intrinsic senescence-related mechanisms, such as oxidative damage and epigenetic mechanisms affecting osteoblast genes, may contribute to the age-related defective osteoblastogenesis [26, 28] (Fig. 1a). Now that these pathogenic mechanisms have been identified in cells of the osteoblast lineage, the current aim is to find ways to target these processes and to develop effective therapeutic strategies to promote bone formation during skeletal aging [28, 29].
Fig. 1.
Mechanisms underlying the osteoblast dysfunctions in skeletal aging and potential therapeutic strategies to attenuate these osteoblast abnormalities. The defective bone formation associated with aging is characterized by preferential differentiation of mesenchymal stromal cells (MSCs) into adipocytes instead of pre-osteoblasts, reduced osteoblast differentiation and function and increased osteoblast and octeocyte death. These abnormalities result from alterations in local signaling factors, senescence mechanisms and epigenetic alterations of genes controlling osteoblastogenesis (a). Potential therapeutic strategies to reduce the osteoblast dysfunctions and improve bone formation in the aging skeleton include promoting anabolic signaling pathways, inhibiting negative regulators of bone formation, and attenuating senescence and epigenetic mechanisms in cells of the osteoblast lineage (b)
Current anabolic strategies in osteoporosis
Current therapeutic strategies to promote osteoblastogenesis in osteoporosis consist in increasing osteoblast number (by promoting the recruitment of osteoblast precursor cells or reducing osteoblast apoptosis), and increasing osteoblast activity [5, 30] (Fig. 1b). Up to now, however, bone anabolic molecules are limited [31] (Table 1). Intermittent administration of parathyroid hormone (iPTH) was shown to enhance osteoblast differentiation, function and life-span through various signaling pathways [32]. These effects result in increased bone microarchitecture and bone mass in osteopenic animals and in osteoporosis resulting from aging, sex hormone deficiency and glucocorticoid therapy [33]. Recent studies suggest that PTH-related Peptide (PTHrP) may be a physiological regulator of bone formation [34]. Recombinant hPTHrP1–34 and 1–84 were found to increase osteogenic cell differentiation in vitro [35] and intermittent treatment with a synthetic PTHrP 1–34 analog was recently shown to increase lumbar spine bone density in osteoporotic patients [36]. However, the clinical use of iPTH (or potentially iPTHrP) remains limited and other anabolic agents are needed for improving bone formation in osteopenic disorders [37]. In this context, several factors and signaling pathways have been considered for promoting osteoblastogenesis. This includes Insulin Growth Factor-1 (IGF-1), Fibroblast Growth Factor (FGF), Transforming Growth Factor-β (TGFβ), Bone Morphogenetic Proteins (BMPs), NOTCH and WNT signaling [38]. Some BMPs are known to promote the differentiation of mesenchymal osteoprogenitor cells into osteoblasts and were approved for clinical use in orthopedic surgery for non-union fractures [39]. However, only one BMP was reported to promote bone formation and bone mass in aged osteopenic ovariectomized rats [40], and the short half-life and off-effects of BMPs prevent a systemic use in osteoporotic patients. FGF, TGF-β and IGF-1 were shown to increase bone formation and bone mass in animals [41–44] but their non-specific effect is a major drawback [5]. Among the multiple pathways that control bone formation, NOTCH signaling was found to control osteoblastogenesis through increased osteoblast precursor cell proliferation [45, 46], but the activation of this pathway may contribute to the development of bone cancer [47]. Some pathways mediated by integrins, G protein-coupled receptors, receptor tyrosine kinases (RTKs), stretch-activated Ca(2+) channels, and WNT signaling are activated by mechanical stimulation, and their stimulation results in increased osteoblastic cell growth, differentiation and activity [20, 48, 49]. One of these pathways, WNT signaling, is a potential targetable pathway for bone anabolics. However, other potential therapeutic strategies are also emerging (Table 1).
Table 1.
Current therapeutic strategies and potential approaches targeting Wnt signaling to promote osteoblastogenesis and bone formation in osteopenic disorders
| Therapeutic strategy | Osteoprogenitor cell number | Osteoblast differentiation and activity | Osteoblast/osteocyte survival |
|---|---|---|---|
| Current | |||
| BMP | + | ||
| iPTH | + | + | + |
| Potential | |||
| Anti-sclerostin Ab | + | + | + |
| Anti-DKK1, anti-sFRP1 | + | + | + |
| Anti-N-cadherin | + | + | + |
The reader is referred to the text for details and references
Emerging strategies to promote bone formation
Targeting WNT signaling
WNT signaling is a complex pathway that involves multiple ligands, receptors, extracellular and intracellular agonists and antagonists. The huge impact of WNT-β-catenin on bone homeostasis has been recently reviewed elsewhere [50]. Briefly, the original finding that mutations in Lrp5, a co-receptor of WNT signaling, cause abnormalities in bone mass [51] led to the discovery that WNT signaling is an important pathway controlling bone formation and bone mass. Mouse and human genetics as well as meta-analyses of genome-wide studies in humans showed that several WNT partners are involved in the control of bone formation and bone mass [7]. These findings support the concept that the WNT pathway could be targeted to modulate osteoblastogenesis in diseases where bone formation is compromised [52–54]. One current strategy is to target WNT signaling indirectly by antagonizing sclerostin, the product of the Sost gene, which is produced by mature osteocytes and antagonizes WNT binding to LRP4-6 co-receptors [55, 56] (Table 1). In line with this finding, blocking sclerostin using a monoclonal antibody was shown to increase bone formation, bone mass and strength in osteopenic animals [57]. This preclinical beneficial effect may translate into the clinics since an anti-sclerostin antibody was recently found to increase bone formation and bone mass in postmenopausal women [58]. Alternatively, other WNT signaling antagonists such as DKK-1 and sFRP1 [59] could be targeted since their ablation or inhibition results in increased bone formation and bone mass in osteopenic models [60, 61]. In this context, one potential strategy is to target miRNAs that repress sclerostin, DKK-1 or sFRP1 expression [62–64]. Another potential strategy is to target molecules that interact indirectly with WNT signaling. Notably, we showed that cadherin-2 (CDH2), a transmembrane homophilic protein that controls cell–cell adhesion, is a negative modulator of WNT signaling in osteoblasts. This effect results from both β-catenin sequestration at the cell membrane and CDH2 binding to LRP5 or LRP6, leading to reduced WNT-β-catenin signaling, decreased osteoblast differentiation and bone formation in mice [65, 66]. Consequently, blocking CDH2 and LRP5/6 interaction using a competitor peptide led to increased WNT-β catenin and enhanced periosteal bone formation in mice [67]. More importantly, we showed that peptide-mediated blockade of the CDH2-LRP5/6 interaction can increase WNT-β signaling, osteoblast differentiation, bone formation and bone mass in senescent osteopenic mice [68]. Others found that CDH2 impairs LRP6 availability to interact with the PTH receptor, and that ablation of CDH2 in osteoblasts can increase the bone anabolic response to iPTH in mice [69]. These findings support the concept that CDH2 in osteoblasts may be a potential pharmacologic target for enhancing the bone response to WNT-β signaling and iPTH in osteopenic disorders [70] (Table 1). Another example of a potential WNT signaling target is FHL2, a transcriptional cofactor which positively interacts with β-catenin. In mice, FHL2 deficiency results in osteopenia, whereas FHL2 transgenic mice exhibit increased bone mass [71, 72]. We showed that FHL2 positively controls the osteogenic differentiation of MSCs, bone formation and bone mass by promoting β-catenin nuclear translocation and modulating WNT ligand expression in mice [73, 74], suggesting another basis for therapeutic intervention. However, the ubiquitous role of WNT signaling as well as its potential implication in cancer development [75] may limit the use of direct WNT signaling activators in clinical settings.
Targeting NF-κB signaling
In addition to play an important role in osteoclastogenesis and inflammation [76], Nuclear factor kappaB (NF-κB) was recently found to control osteoblast differentiation and bone formation [77, 78]. In vitro, NF-κB activation inhibits osteoblast differentiation and activity through various mechanisms [78] including β-catenin degradation [79]. Consistently, specific pharmacological inhibition of IκB kinase (IKK)-NF-κB resulted in increased osteogenic capacity of MSCs and improved bone repair in a calvarial bone defect [79]. In mice, the inhibition of NF-κB in mature osteoblasts led to increased bone formation and bone mass with no change in bone resorption [80]. Similarly, the ablation of ReIA-mediated NF-κB signaling in mice resulted in increased osteoblast differentiation, bone formation and bone mass [81]. Collectively, these studies suggest that the inhibition of NF-κB signaling may be efficient to promote bone formation and repair in bone disorders. The recent findings that effective inhibition of NF-κB signaling in vivo can be achieved by noncanonical WNT4 [82] or by cell-mediated secretion of NF-kB inhibitors [83] may provide effective tools for targeting NF-κB signaling in bone and promoting bone formation in osteopenic disorders.
Targeting senescence mechanisms
Several senescence mechanisms were recently shown to negatively impact osteoblastogenesis and bone formation [24, 27]. These senescence mechanisms are not tissue specific and include telomere shortening, accumulation of oxidative damage, impaired DNA repair and altered epigenetic mechanisms. Interestingly, recent studies indicate that some of these mechanisms may be targeted to reduce osteoblast aging [27]. One promising target is the sirtuin deacetylase silent information regulator 1 (SIRT1), a transcription factor that counteracts cell senescence and decreases with aging [84]. SIRT1 upregulates osteoblast differentiation by downregulating the WNT antagonist gene Sost, by deacetylating β-catenin and by inhibiting NF-κB signaling [85–87]. Accordingly, Sirt1 overexpression [88] or pharmacological SIRT1 activation was found to protect against age-related bone loss [89]. Such strategy targeting a key senescence mechanism that affects both WNT and NF-κB signaling may be promising to promote bone formation and reduce bone loss associated with aging (Fig. 1a).
Targeting the ubiquitin-dependent proteolysis system
Another emerging approach to increase osteoblastogenesis is to target the ubiquitin-dependent proteolysis system (UPS), a physiological machinery that mediates the ubiquitination of proteins and their degradation by the proteasome [90]. In bone, UPS controls the degradation of key osteoblast modulators such as RTKs, BMP-2, RUNX2, β-catenin and GLI2 (a transcription factor regulating BMP-2 expression) [91]. Initial studies showed that proteasome inhibition led to increased osteoblast differentiation, trabecular bone formation and bone mass in ovariectomized mice [92–95]. This therapeutic approach is, however, limited by the non-specific effects of UPS inhibitors on overall protein degradation. A more selective approach may be to target key proteins involved in UPS-mediated protein degradation such as ubiquitin ligases that direct proteins to proteasomal degradation. Recent data indicate that some ubiquitin ligases may be targeted to promote bone formation [91]. One important ubiquitin ligase called SMAD ubiquitin regulatory factor 1 (SMURF1) controls the degradation of RUNX2 and SMAD1/5 which are critical signaling molecules involved in BMP signaling [96]. Consistently, silencing caseine-kinase 2 interacting protein-1 (Plekho1) that enhances SMURF1-mediated Smad1/5 degradation led to enhanced osteoblast differentiation, bone formation and bone mass in osteopenic rats [97]. Another E3 ubiquitin ligase, WWP1, interacts with RUNX2 and the zinc finger adapter protein SCHNURRI-3, leading to RUNX2 proteasome degradation. Schnurri-3 or Wwp1 deficiency in mice led to increased osteoblast activity and bone mass [98–100], suggesting that silencing these molecules may lead to promote bone formation. Other studies have highlighted the role of CBL ubiquitin ligases in osteoblastogenesis [91]. CBL proteins are scaffold proteins with multiple interaction domains that interact with a large number of proteins [101]. In bone-forming cells, CBL-b enhances RUNX2 stability and osteoblast-related genes [102], whereas c-CBL controls RTK ubiquitination and degradation [103, 104]. Recently, we showed that inhibiting the c-CBL-RTK interaction in human MSCs resulted in increased RTK expression and signaling causing enhanced osteoblast differentiation and survival [105]. Silencing c-CBL expression in MSCs also promoted osteoblast differentiation as a result of decreased ubiquitination of the transcription factor STAT5 that positively interacts with RUNX2 [106]. In vivo, the inhibition of c-CBL interaction with phosphatidylinositol-3′ kinase (PI3 K) led to increased PI3 K levels, bone formation and bone volume in mice [107]. Overall, these findings suggest that targeting selected ubiquitin ligases may lead to increased osteoblastogenesis, bone formation and bone mass in osteopenic disorders.
Targeting osteoblast progenitor cells
Mesenchymal cells present in the bone marrow stroma are able to differentiate into osteoblasts, adipocytes or chondrocytes under appropriate stimulation [108, 109]. Although MSCs have long been used as a source for bone regeneration in bone defects, the capacity of MSCs to differentiate and to generate new bone in vivo is limited [110, 111]. Several growth and differentiation factors were found to expand MSC proliferation or osteogenic differentiation in vitro [112] with limited impact on bone formation in vivo [113]. Novel targeted strategies have been recently developed to promote MSC osteogenic differentiation. Notably, targeting integrins, which are essential cell surface adhesion transmembrane molecules, proved to be effective to promote MSC osteogenic differentiation and bone formation [114]. We showed that lentiviral-mediated expression of α5β1 integrin, a cell surface receptor for fibronectin that is essential for osteoblast differentiation and survival [115, 116], promoted bone repair in two relevant critical-size bone defects in the mouse [117]. More importantly, an agonist of the α5 integrin subunit promoted osteoblast differentiation and survival, resulting in increased bone formation in mice [118]. These findings indicate that the α5 integrin subunit can be targeted to promote MSC osteogenic differentiation, bone formation and repair. Recent data indicate that the α4β1 integrin is another potential target for promoting MSC osteogenic differentiation. The transient expression of the α4 integrin subunit in MSCs was found to increase the homing of injected MSCs in the bone marrow, an effect that resulted in increased osteoblast formation [119]. Moreover, the injection of a chimeric molecule composed of a peptidomimetic which binds the α4β1 integrin coupled to the bone-seeking agent alendronate increased bone formation and bone mass in mice [120, 121]. Thus, direct targeting of α4β1 or α5β1 integrins appear to be promising therapies for promoting MSC osteogenic differentiation, bone formation and regeneration in age-related bone loss and repair [114].
One effective approach to promote MSC differentiation specifically at the bone surface is to silence genes that inhibit bone formation using small interference RNAs (siRNAs). Specifically, the systemic delivery of siRNA for Plekho1, a negative regulator of osteogenesis, using cationic liposomes attached to (AspSerSer)6 that preferentially binds to bone-forming surfaces, resulted in increased bone formation and bone mass in osteopenic rats [97]. This may provide a cell-selective delivery system for gene knockdown and bone anabolic therapy. An alternative indirect method to target MSC osteogenic differentiation is the use of polymeric nanoparticles delivering siRNAs. This delivery system was recently used to target semaphorin 4D expression in osteoclasts, resulting in increased number of osteoblasts and bone volume in ovariectomized mice [122]. Thus, the RNA interference-based approach appears to be another promising strategy for targeting cells at the bone surface and promoting bone formation (Fig. 1b).
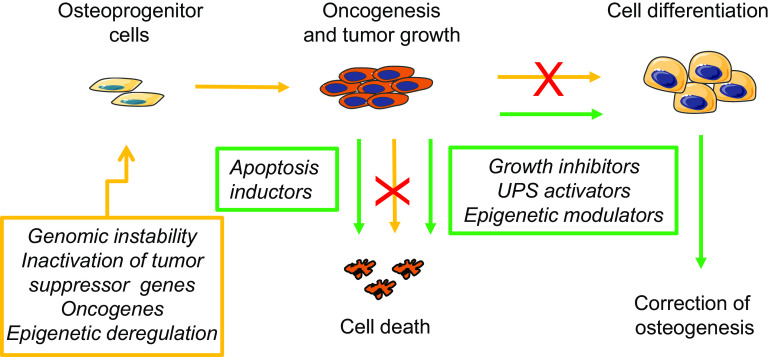
Fig. 2.
Osteoblast dysfunctions and potential targeted therapies in primary bone tumors. In primary bone tumors, genomic instability, aberrations in tumor suppressor genes, oncogenes and epigenetic deregulation of key transcription factors result in the transformation of osteoblast precursor cells into tumor cells which exhibit deregulated cell proliferation, defective terminal osteoblast differentiation and reduced cell apoptosis. These abnormalities can be specifically targeted to restore normal bone formation, as indicated
Osteoblast dysfunctions induced by genetic mutations in skeletal dysplasias
During the past years, the analysis of the genotype–phenotype relationship in skeletal dysplasias provided important information on the genetic control of bone formation [6, 123, 124]. This is best illustrated by the analysis of the skeletal phenotype in mice and humans induced by loss-of-function and gain-of-function mutations in the WNT-β-catenin signaling pathway [7, 125]. Studies of the osteoblast phenotype in genetic skeletal dysplasias unraveled some key pathophysiological mechanisms that may be targeted to correct the abnormalities in these bone diseases (Table 2), as detailed below.
Table 2.
Osteoblast dysfunctions and potential therapeutic strategies in skeletal dysplasias induced by genetic mutations
| Osteoprogenitor cell replication | Osteoblast differentiation and function | Osteoblast/osteocyte death | Skeletal phenotype | Potential therapeutic strategy | |
|---|---|---|---|---|---|
| Mutation | |||||
| Gsα (+) | ↑ | ↓ | ↑ | Accelerated (local) bone formation | Wnt signaling inhibition |
| FGFR2 (+) | ↔ | ↑ | ↑ | Increased cranial ossification | FGFR2-PDGERα signaling inhibition |
| Twist (−) | ↑ | ↑ | ↑ | Increased cranial ossification | FGFR2 signaling inhibition |
| CFTR (−) | ↔ | ↓ | ↔ | Decreased bone formation | CFTR correction |
(+) and (−) identify a gain- and loss-of-function, respectively, induced by the indicated genetic mutation in skeletal dysplasias
Gain-of-function mutations impacting bone formation
A typical example of gain-of-function mutation that affects osteoblastogenesis is provided by mutations in Gsα (R201C, R201H) causing fibrous dysplasia (FD), a rare disease characterized by immature bone deposition in dysplastic lesions [126]. The analysis of the osteoblast phenotype induced by Gsα mutations in FD provided insights into the role of Gsα in osteogenesis [127, 128]. The stimulatory α subunit of G protein (Gsα) binds and hydrolyses GTP, resulting in adenylate cyclase activation, cAMP production and protein kinase A (PKA) activation. In FD, Gsα mutations are expressed in osteoprogenitor cells in the bone marrow stroma in dysplastic lesions, causing excessive cAMP levels and alterations in the expression of c-Fos and c-Jun, resulting in increased proliferation of pre-osteoblastic cells, abnormal osteoblast maturation and deposition of an immature bone [126, 129, 130]. This phenotype can be reproduced in adult mice bearing the R201C mutation [131]. Conversely, selective silencing of the mutated Gsα allele in skeletal progenitor cells can revert the excessive cAMP production and the abnormal osteoblast differentiation in human FD cells [132]. A role for Gsα in bone formation is supported by the finding that activation of the Gsα-PKA signaling cascade mediates in part the anabolic action of iPTH in murine bone [133]. In contrast, the loss of Gsα led to the preferential commitment of bone marrow stromal cells to adipocytes [134]. Consistently, conditional deletion of Gsα in skeletal progenitors led to decreased osteoblast progenitor number, bone formation and bone mass in mice. This phenotype was associated with increased expression of the WNT antagonists, sclerostin and DKK-1, suggesting that Gsα interacts with WNT signaling to control cells of the osteoblast lineage [135]. In line with this finding, activating Gαs mutations in FD patients was found to potentiate WNT-β-catenin signaling and consistently, osteoblast differentiation abnormalities in FD can be attenuated by reducing β-catenin levels [136]. These findings suggest a potential therapeutic approach targeting WNT signaling to correct the osteoblast dysfunctions in FD patients with aberrant Gsα signaling (Table 2).
Another typical example of gain-of-function mutations affecting bone formation includes mutations in FGFR2 causing craniosynostosis (or premature cranial suture fusion) [137]. We initially showed that activating FGFR2 mutations in human osteoblasts promote osteoblast differentiation and osteogenesis [138, 139]. In mouse models, the phenotype induced by FGFR2 mutations is variable between the models [140–143] which reflects the complexity of FGF signaling in cells of the osteoblast lineage [144]. Mechanistically, the analysis of signaling pathways in human osteoblasts [43, 139] and mouse models [145, 146] revealed that MEK, PKC, SRC and WNT signaling pathways are implicated in the aberrant osteoblast phenotype induced by activating FGFR2 mutations. These findings led to propose selective therapeutic strategies in craniosynostosis [147, 148]. Specifically, the use of tyrosine kinase inhibitors was shown to attenuate FGFR signaling and to prevent the abnormal skeletal phenotype in mouse models of craniosynostosis [149, 150]. Recently, we and others showed that crosstalks between FGFR2 and platelet-derived growth factor receptor α (PDGFRα) signaling amplify the osteoblast abnormalities induced by FGFR2 activating mutations in vivo [151, 152]. Consistently, pharmacological inhibition of PDGFRα attenuated the abnormal bone formation induced by aberrant FGFR2 signaling in osteoblasts [153]. These findings suggest novel therapeutic strategies targeted to RTK signaling to attenuate the abnormal bone formation in this skeletal disorder (Table 2).
Loss-of-function mutations affecting bone formation
Some typical loss-of-function genetic mutations also cause abnormalities in bone formation. Notably, mutations in Twist1, a basic helix–loop–helix (bHLH) factor involved in mesodermal differentiation, induce the Saethre–Chotzen syndrome (SCS) which is characterized by premature fusion of coronal sutures [154]. TWIST1 heterodimerizes with bHLH E proteins that bind E-boxes present in the promoter of target genes. In SCS, Twist haploinsufficiency causes TWIST1 protein degradation which abolishes TWIST1 binding activity [155]. However, in the developing mouse, TWIST1 can inhibit the functional activity of RUNX2 independently of the bHLH domain [156]. In human SCS osteoblasts, Twist haploinsufficiency increased collagen expression and osteogenic capacity independently of Runx2 expression [157, 158]. This phenotype resulted from activated PI3 K/AKT-dependent osteoprogenitor cell growth and increased number of collagen-forming osteoblasts [159]. Another potential pathophysiological mechanism involved in SCS is the interaction between Twist1 and FGFR2 signaling [142, 160–162]. We showed that molecular or pharmacological inhibition of FGFR2 signaling can correct the abnormal osteoblast phenotype induced by Twist1 silencing in human MSCs [162]. Consistently, inhibition of FGF signaling was shown to prevent the premature cranial suture fusion in Twist1 heterozygotic mice [161]. Collectively, these studies indicate that targeting FGF/FGFR signaling may be a valuable option to prevent the abnormal osteoblastic cell replication and function induced by Twist1 genetic mutations [139] (Table 2).
Another example of a loss-of-function genetic mutation causing abnormalities in osteoblast function is the F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR). Patients with cystic fibrosis (CF) who exhibit this prominent CFTR mutation display low bone density and decreased bone formation [163, 164] but the underlying mechanism was unknown. We recently showed that the F508del CFTR mutation impacts osteoblast activity, bone formation and bone mass in a mouse model, independently of other etiological factors, suggesting a link between this CFTR mutation and bone formation in this disease [165]. Pharmacological correction of the mutant CFTR-dependent channel led to improve bone formation, bone mass and microarchitecture in F508del mice [166], suggesting a potential therapeutic strategy to reduce bone loss in this disorder (Table 2). Further investigation of the molecular mechanisms involved in the defective osteoblast function in CF is needed for developing a specific therapeutic strategy and improving bone formation in this genetic disorder affecting the skeleton.
Osteoblast dysfunctions in primary bone tumors
During the recent years, the analyses of the cellular and molecular dysfunctions in primary bone tumors led to propose novel targeted therapies capable of counteracting bone tumor development and metastasis. Osteosarcoma, the main primary bone tumor, results from genetic or epigenetic changes that cause uncontrolled osteoprogenitor cell proliferation, halted terminal differentiation and reduced apoptosis (Fig. 2). One current concept underlying tumor development is that osteosarcoma cells retain a high proliferative capacity and lose their potential to differentiate as the consequence of deregulations of various processes [167]. These dysfunctions may result from aberrations in tumor suppressor genes, such as p53 that is implicated in cell survival [168], or Rb that controls terminal osteoblast differentiation [169]. Overexpression of oncogenes such as Fos, Jun [170] or Met [171] also causes deregulated osteosarcoma cell proliferation. In addition, osteosarcoma development is often associated with RUNX2 deregulation [172], Osterix downregulation [173] and Sox2 overexpression [174], resulting in blockade of terminal osteoblast differentiation. Deregulation of growth factors and receptor RTKs may also contribute to the aberrant cell growth and differentiation in primary bone tumors [175]. Notably, primary bone tumorigenesis is often associated with increased expression of TGF-β1 [176], IGF-1 [177], EGFR [178, 179] and PDGFR [179, 180]. In line with these findings, we recently reported that ErbB3, a member of the EGF family which heterodimerises with ErbB2, is overexpressed in human osteosarcoma cell lines and tumors. Consistent with this finding, ErbB3 silencing reduced osteosarcoma cell proliferation and decreased tumor growth in mice, suggesting that ErbB3 can be targeted to reduce bone tumorigenesis [181]. One potential mechanism for the increased RTK signaling in bone tumors may be the reduction of RTK degradation by the ubiquitin–proteasome system [91]. In support of this concept, proteasome inhibition was found to suppress cell growth and to induce apoptosis in osteosarcoma [182]. Recently, we showed that the expression of the ubiquitin ligase c-CBL, which negatively controls RTK levels and signaling, is downregulated in osteosarcoma cells. Consistently, increasing c-Cbl expression reduced EGFR and PDGFRα levels and inhibited osteosarcoma cell growth and tumorigenesis in mice [179]. Taken together, these studies suggest an effective therapeutic approach targeting the proteasome to inhibit primary bone tumor development [91] (Fig. 2).
WNT signaling may also play a role in osteosarcoma tumorigenesis. Notably, osteosarcoma cells often exhibit overexpression of WNT ligands and receptors, downregulation of WNT inhibitors and increased β-catenin-mediated activity [75, 183–185]. This suggests that inhibiting WNT signaling may reduce osteosarcoma tumorigenesis [186]. Indeed, inhibition of WNT signaling decreased bone tumorigenesis in animal models [184, 187] and increased osteosarcoma cell sensitivity to chemotoxic drugs [188]. The inhibition of WNT signaling by silencing FHL2, a Wnt-β-catenin activator, also resulted in decreased cell growth and tumorigenesis in murine osteosarcoma [73]. However, downregulation of WNT signaling in osteosarcoma cells may block WNT-mediated osteoblast differentiation and thereby promote cell self-renewal [174], suggesting that WNT signaling in osteosarcoma may have pro-tumoral or anti-tumoral effects depending on the state of cell progression.
An alternative strategy to inhibit bone tumorigenesis may be to target the epigenetic mechanisms that are involved in the deregulation of tumor cell proliferation and survival in osteosarcoma [189–191]. In this context, the inhibition of histone deacetylase (HDAC) was shown to inhibit osteosarcoma cell growth and to induce terminal osteoblast differentiation [192]. Current evidence indicate that miRNAs play a role in the pathogenesis of bone tumors, suggesting that miRNAs could be targeted to reduce osteosarcoma tumorigenesis [191, 193, 194]. One promising method to deliver miRNAs or HDAC inhibitors locally in bone tumors may be the use of polymeric nanoparticles associated with bone-binding molecule (bisphosphonate), thus allowing bone homing and delivery of anticancer drugs [195]. A better understanding of the role of epigenetic mechanisms in gene deregulation in primary bone tumors is likely to result in selective therapeutic strategies to efficiently reduce primary bone tumor growth and to sensitize tumor cells to chemotherapy (Fig. 2).
Conclusion and perspectives
During the recent years, the phenotypic and functional analyses of human bone pathologies and mouse models allowed identifying some important mechanisms that are implicated in metabolic, genetic and oncogenic skeletal disorders. This led to propose novel therapeutic approaches such as the modulation of WNT signaling, the pharmacological modulation of proteasome-mediated protein degradation, the inhibition of senescence mechanisms, the induction of osteoblastic differentiation in osteoprogenitor cells and the inhibition of cell proliferation in osteosarcoma cells (Figs. 1, 2). The next step will be to improve the specificity and efficiency of these therapeutic approaches in preclinical studies, and to translate these strategies into effective therapies for correcting the abnormal osteoblastogenesis in human skeletal disorders.
In the future, a number of important issues need to be addressed in osteoblast biology and pathology. First, we need to identify specific markers of cells of the osteoblast lineage at different stages of differentiation to determine which cell(s) could be best targeted to correct the defective osteoblastogenesis in bone disorders. In this context, the lineage tracing methods that are currently available [196, 197] may help identifying the nature of cells in the bone marrow or periosteum to be targeted for selective therapeutic effects. Second, there is mounting evidence that dysfunctions in epigenetic mechanisms contribute to both age-related bone loss [198, 199] and primary bone tumors [191, 193, 194]. It will be of major interest to determine if the specific modulation of epigenetic mechanisms can attenuate the abnormal osteoblastic cell fate and function in disorders of bone formation. Finally, recent evidence indicate that cells of the osteoblast lineage control both hematopoietic stem cell differentiation [200, 201] and oncogenesis [202–205]. It will be thus essential to determine whether and how cells of the osteoblast lineage may be selectively targeted to improve or to correct bone marrow cell fate. It is hoped that such research in osteoblast biology and pathology will translate into efficient and safe therapeutics not only for correcting the abnormal osteoblastogenesis in human metabolic and genetic skeletal disorders, but also for preventing the development of oncogenic bone disorders.
Acknowledgments
The author thanks all members of his group Osteoblast Biology and Pathology who contributed to the work reviewed in this paper, and apologizes to the investigators whose work could not be cited due to space limitations. The author’s work was supported by Centre National de la Recherche Scientifique (CNRS), Institut National de la Recherche Médicale (Inserm), University Paris Diderot, European Commission programs (FP76, FP7), European Calcified Tissue Society (ECTS), Agence Nationale de la Recherche (ANR), Centre National d’Etudes Spatiales (CNES), DIM Stem Pôle Ile de France, Fondation de l’Avenir pour la Recherche Appliquée, Association Recherche contre le Cancer (ARC), Fondation pour la Recherche Médicale (FRM), Société Française de Rhumatologie (SFR), Association Prévention et Traitement des Décalcifications (PTD) and Association Rhumatisme et Travail (Paris, France). Figures were produced using Servier Medical Art.
References
- 1.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 2.Khosla S, Riggs BL. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin N Am. 2005;34:1015–1030. doi: 10.1016/j.ecl.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20:177–184. doi: 10.1359/JBMR.041114. [DOI] [PubMed] [Google Scholar]
- 4.Kawai M, Modder UI, Khosla S, Rosen CJ. Emerging therapeutic opportunities for skeletal restoration. Nat Rev Drug Discov. 2011;10:141–156. doi: 10.1038/nrd3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marie PJ, Kassem M. Osteoblasts in osteoporosis: past emerging and future anabolic targets. Eur J Endocrinol. 2011;165:1–10. doi: 10.1530/EJE-11-0132. [DOI] [PubMed] [Google Scholar]
- 6.Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- 7.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 8.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 9.Bianco P, Sacchetti B, Riminucci M. Stem cells in skeletal physiology and endocrine diseases of bone. Endocr Dev. 2011;21:91–101. doi: 10.1159/000328138. [DOI] [PubMed] [Google Scholar]
- 10.Karsenty G. Minireview: transcriptional control of osteoblast differentiation. Endocrinology. 2001;142:2731–2733. doi: 10.1210/endo.142.7.8306. [DOI] [PubMed] [Google Scholar]
- 11.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 12.Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98–105. doi: 10.1016/j.abb.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marie PJ. Cellular and molecular alterations of osteoblasts in human disorders of bone formation. Histol Histopathol. 1999;14:525–538. doi: 10.14670/HH-14.525. [DOI] [PubMed] [Google Scholar]
- 15.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 16.Marie PJ. Human osteoblastic cells: a potential tool to assess the etiology of pathologic bone formation. J Bone Miner Res. 1994;9:1847–1850. doi: 10.1002/jbmr.5650091202. [DOI] [PubMed] [Google Scholar]
- 17.Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8:212–227. doi: 10.1038/nrendo.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley EW, McGee-Lawrence ME, Westendorf JJ. Hdac-mediated control of endochondral and intramembranous ossification. Crit Rev Eukaryot Gene Expr. 2011;21:101–113. doi: 10.1615/critreveukargeneexpr.v21.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canalis E. The hormonal and local regulation of bone formation. Endocr Rev. 1983;4:62–77. doi: 10.1210/edrv-4-1-62. [DOI] [PubMed] [Google Scholar]
- 20.Marie PJ, Jones D, Vico L, Zallone A, Hinsenkamp M, Cancedda R. Osteobiology strain and microgravity: part I. Studies at the cellular level. Calcif Tissue Int. 2000;67:2–9. doi: 10.1007/s00223001088. [DOI] [PubMed] [Google Scholar]
- 21.Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3:481. doi: 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marie PJ. Bone cell-matrix protein interactions. Osteoporos Int. 2009;20:1037–1042. doi: 10.1007/s00198-009-0856-7. [DOI] [PubMed] [Google Scholar]
- 23.Khosla S. Pathogenesis of age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;38:1228–1235. doi: 10.1093/gerona/gls163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21:369–374. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahdjoudj S, Fromigué O, Marie PJ. Plasticity and regulation of human bone marrow stromal osteoprogenitor cells: potential implication in the treatment of age-related bone loss. Histol Histopathol. 2004;19:151–157. doi: 10.14670/HH-19.151. [DOI] [PubMed] [Google Scholar]
- 26.Kassem M, Marie PJ. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell. 2011;10:191–197. doi: 10.1111/j.1474-9726.2011.00669.x. [DOI] [PubMed] [Google Scholar]
- 27.Marie PJ, Kassem M. Extrinsic mechanisms involved in age-related defective bone formation. J Clin Endocrinol Metab. 2011;96:600–609. doi: 10.1210/jc.2010-2113. [DOI] [PubMed] [Google Scholar]
- 28.Marie PJ. Bone cell senescence: mechanisms and perspectives. J Bone Miner Res. 2014;29:1311–1321. doi: 10.1002/jbmr.2190. [DOI] [PubMed] [Google Scholar]
- 29.Marie PJ. The molecular genetics of bone formation: implications for therapeutic interventions in bone disorders. Am J Pharmacogenomics. 2001;1:175–187. doi: 10.2165/00129785-200101030-00003. [DOI] [PubMed] [Google Scholar]
- 30.Canalis E. New treatment modalities in osteoporosis. Endocr Pract. 2010;16:855–863. doi: 10.4158/EP10048.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin TJ. Bone biology and anabolic therapies for bone: current status and future prospects. J Bone Metab. 2014;21:8–20. doi: 10.11005/jbm.2014.21.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Compston JE. Skeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structure. Bone. 2007;40:1447–1452. doi: 10.1016/j.bone.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Martin TJ. Osteoblast-derived PTHrP is a physiological regulator of bone formation. J Clin Invest. 2005;115:2322–2324. doi: 10.1172/JCI26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Liu J, Yin Y, Wu J, Wang Z, Miao D, Sun W. Recombinant human parathyroid hormone related protein 1–34 and 1–84 and their roles in osteoporosis treatment. PLoS One. 2014;9:e88237. doi: 10.1371/journal.pone.0088237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horwitz MJ, Augustine M, Khan L, Martin E, Oakley CC, Carneiro RM, Tedesco MB, Laslavic A, Sereika SM, Bisello A, Garcia-Ocana A, Gundberg CM, Cauley JA, Stewart AF. A comparison of parathyroid hormone-related protein (1–36) and parathyroid hormone (1–34) on markers of bone turnover and bone density in postmenopausal women: the PrOP study. J Bone Miner Res. 2013;28:2266–2276. doi: 10.1002/jbmr.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 38.Marie PJ. Signaling pathways affecting skeletal health. Curr Osteoporos Rep. 2012;10:190–198. doi: 10.1007/s11914-012-0109-0. [DOI] [PubMed] [Google Scholar]
- 39.Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord. 2006;7:51–65. doi: 10.1007/s11154-006-9000-6. [DOI] [PubMed] [Google Scholar]
- 40.Simic P, Culej JB, Orlic I, Grgurevic L, Draca N, Spaventi R, Vukicevic S. Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem. 2006;281:25509–25521. doi: 10.1074/jbc.M513276200. [DOI] [PubMed] [Google Scholar]
- 41.Fromigué O, Modrowski D, Marie PJ. Growth factors and bone formation in osteoporosis: roles for fibroblast growth factor and transforming growth factor beta. Curr Pharm Des. 2004;10:2593–2603. doi: 10.2174/1381612043383773. [DOI] [PubMed] [Google Scholar]
- 42.Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-beta1 to the bone. Endocr Rev. 2005;26:743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 43.Marie PJ. Fibroblast growth factor signaling controlling bone formation: an update. Gene. 2012;498:1–4. doi: 10.1016/j.gene.2012.01.086. [DOI] [PubMed] [Google Scholar]
- 44.Kawai M, Rosen CJ. The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol Metab Clin North Am. 2012;41:323–333. doi: 10.1016/j.ecl.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, Boyce BF, Lee B. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao J, Jiang MM, Jiang L, Salvo JS, Zeng HC, Dawson B, Bertin TK, Rao PH, Chen R, Donehower LA, Gannon F, Lee BH. Notch activation as a driver of osteogenic sarcoma. Cancer Cell. 2014;26:390–401. doi: 10.1016/j.ccr.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Robling AG. The interaction of biological factors with mechanical signals in bone adaptation: recent developments. Curr Osteoporos Rep. 2012;10:126–131. doi: 10.1007/s11914-012-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 52.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 53.Canalis E. Wnt signaling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol. 2013;9:575–583. doi: 10.1038/nrendo.2013.154. [DOI] [PubMed] [Google Scholar]
- 54.Wagner ER, Zhu G, Zhang BQ, Luo Q, Shi Q, Huang E, Gao Y, Gao JL, Kim SH, Rastegar F, Yang K, He BC, Chen L, Zuo GW, Bi Y, Su Y, Luo J, Luo X, Huang J, Deng ZL, Reid RR, Luu HH, Haydon RC, He TC. The therapeutic potential of the Wnt signaling pathway in bone disorders. Curr Mol Pharmacol. 2011;4:14–25. [PubMed] [Google Scholar]
- 55.van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW. SOST/sclerostin an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16:319–327. doi: 10.1016/j.cytogfr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 57.Paszty C, Turner CH, Robinson MK. Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res. 2010;25:1897–1904. doi: 10.1002/jbmr.161. [DOI] [PubMed] [Google Scholar]
- 58.McClung MR, Grauer A. Romosozumab in postmenopausal women with osteopenia. N Engl J Med. 2014;370:1664–1665. doi: 10.1056/NEJMc1402396. [DOI] [PubMed] [Google Scholar]
- 59.Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev. 2012;33:747–783. doi: 10.1210/er.2011-1060. [DOI] [PubMed] [Google Scholar]
- 60.Morvan F, Boulukos K, Clément-Lacroix P, Roman Roman S, Suc-Royer I, Vayssière B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 61.Bodine PV, Stauffer B, Ponce-de-Leon H, Bhat RA, Mangine A, Seestaller-Wehr LM, Moran RA, Billiard J, Fukayama S, Komm BS, Pitts K, Krishnamurthy G, Gopalsamy A, Shi M, Kern JC, Commons TJ, Woodworth RP, Wilson MA, Welmaker GS, Trybulski EJ, Moore WJ. A small molecule inhibitor of the Wnt antagonist secreted frizzled-related protein-1 stimulates bone formation. Bone. 2009;44:1063–1068. doi: 10.1016/j.bone.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Tu Q, Bonewald LF, He X, Stein G, Lian J, Chen J. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res. 2011;26:1953–1963. doi: 10.1002/jbmr.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang W, Jafferji M, Gordon JA, Li Z, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem. 2012;287:42084–42092. doi: 10.1074/jbc.M112.377515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo D, Li Q, Lv Q, Wei Q, Cao S, Gu J. MiR-27a targets sFRP1 in hFOB cells to regulate proliferation apoptosis and differentiation. PLoS One. 2014;9:e91354. doi: 10.1371/journal.pone.0091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haÿ E, Laplantine E, Geoffroy V, Frain M, Kohler T, Müller R, Marie PJ. N-cadherin interacts with axin and LRP5 to negatively regulate Wnt/beta-catenin signaling osteoblast function and bone formation. Mol Cell Biol. 2009;29:953–964. doi: 10.1128/MCB.00349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haÿ E, Nouraud A, Marie PJ. N-cadherin negatively regulates osteoblast proliferation and survival by antagonizing Wnt ERK and PI3 K/Akt signaling. PLoS One. 2009;4:e8284. doi: 10.1371/journal.pone.0008284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haÿ E, Buczkowski T, Marty C, Da Nascimento S, Sonnet P, Marie PJ. Peptide-based mediated disruption of N-cadherin-LRP5/6 interaction promotes Wnt signaling and bone formation. J Bone Miner Res. 2012;27:1852–1863. doi: 10.1002/jbmr.1656. [DOI] [PubMed] [Google Scholar]
- 68.Haÿ E, Dieudonné FX, Saidak Z, Marty C, Brun J, Da Nascimento S, Sonnet P, Marie PJ. N-Cadherin/Wnt interaction controls bone marrow mesenchymal cell fate and bone mass during aging. J Cell Physiol. 2014;229:1765–1775. doi: 10.1002/jcp.24629. [DOI] [PubMed] [Google Scholar]
- 69.Revollo L, Kading J, Jeong SY, Li J, Salazar V, Mbalaviele G, Civitelli R. N-cadherin restrains PTH activation of Lrp6/beta-catenin signaling and osteoanabolic action. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marie PJ, Haÿ E, Saidak Z. Integrin and cadherin signaling in bone: role and potential therapeutic targets. Trends Endocrinol Metab. 2014;925:567–575. doi: 10.1016/j.tem.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 71.Gunther T, Poli C, Muller JM, Catala-Lehnen P, Schinke T, Yin N, Vomstein S, Amling M, Schüle R. Fhl2 deficiency results in osteopenia due to decreased activity of osteoblasts. EMBO J. 2005;24:3049–3056. doi: 10.1038/sj.emboj.7600773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Govoni KE, Baylink DJ, Chen J, Mohan S. Disruption of four-and-a-half LIM 2 decreases bone mineral content and bone mineral density in femur and tibia bones of female mice. Calcif Tissue Int. 2006;79:112–117. doi: 10.1007/s00223-006-0074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brun J, Dieudonné FX, Marty C, Muller J, Schüle R, Patino-Garcia A, Lecanda F, Fromigué O, Marie PJ. FHL2 silencing reduces Wnt signaling and osteosarcoma tumorigenesis in vitro and in vivo. PLoS One. 2013;8:e55034. doi: 10.1371/journal.pone.0055034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brun J, Fromigué O, Dieudonné FX, Marty C, Chen J, Dahan J, Wei Y, Marie PJ. The LIM-only protein FHL2 controls mesenchymal cell osteogenic differentiation and bone formation through Wnt5a and Wnt10b. Bone. 2013;53:6–12. doi: 10.1016/j.bone.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons PJ, Dawid IB, Thomas DM. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest. 2009;119:837–851. doi: 10.1172/JCI37175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boyce BF, Yao Z, Xing L. Functions of nuclear factor kappaB in bone. Ann N Y Acad Sci. 2010;1192:367–375. doi: 10.1111/j.1749-6632.2009.05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krum SA, Chang J, Miranda-Carboni G, Wang CY. Novel functions for NFkappaB: inhibition of bone formation. Nat Rev Rheumatol. 2010;6:607–611. doi: 10.1038/nrrheum.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Novack DV. Role of NF-kappaB in the skeleton. Cell Res. 2011;21:169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang J, Liu F, Lee M, Wu B, Ting K, Zara JN, Soo C, Al Hezaimi K, Zou W, Chen X, Mooney DJ, Wang CY. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc Natl Acad Sci USA. 2009;110:9469–9474. doi: 10.1073/pnas.1300532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao Z, Li Y, Yin X, Dong Y, Xing L, Boyce BF. NF-kappaB RelB negatively regulates osteoblast differentiation and bone formation. J Bone Miner Res. 2014;29:866–877. doi: 10.1002/jbmr.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu B, Chang J, Liu Y, Li J, Kevork K, Al-Hezaimi K, Graves DT, Park NH, Wang CY. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-kappaB. Nat Med. 2014;20:1009–1017. doi: 10.1038/nm.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koutsokeras A, Purkayashta N, Rigby A, Subang MC, Sclanders M, Vessillier S, Mullen L, Chernajovsky Y, Gould D. Generation of an efficiently secreted cell penetrating NF-kappaB inhibitor. FASEB J. 2014;28:373–381. doi: 10.1096/fj.13-236570. [DOI] [PubMed] [Google Scholar]
- 84.Herranz D, Serrano M. SIRT1: recent lessons from mouse models. Nat Rev Cancer. 2010;10:819–823. doi: 10.1038/nrc2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cohen-Kfir E, Artsi H, Levin A, Abramowitz E, Bajayo A, Gurt I, Zhong L, D’Urso A, Toiber D, Mostoslavsky R, Dresner-Pollak R. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin a bone formation inhibitor. Endocrinology. 2011;152:4514–4524. doi: 10.1210/en.2011-1128. [DOI] [PubMed] [Google Scholar]
- 86.Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, Baron R, Scadden D, Schipani E, Guarente L. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating beta-catenin. EMBO Mol Med. 2013;5:430–440. doi: 10.1002/emmm.201201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Edwards JR, Perrien DS, Fleming N, Nyman JS, Ono K, Connelly L, Moore MM, Lwin ST, Yull FE, Mundy GR, Elefteriou F. Silent information regulator (Sir)T1 inhibits NF-kappaB signaling to maintain normal skeletal remodeling. J Bone Miner Res. 2013;28:960–969. doi: 10.1002/jbmr.1824. [DOI] [PubMed] [Google Scholar]
- 88.Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Artsi H, Cohen-Kfir E, Gurt I, Shahar R, Bajayo A, Kalish N, Bellido TM, Gabet Y, Dresner-Pollak R. The sirtuin1 activator SRT3025 down-regulates sclerostin and rescues ovariectomy-induced bone loss and biomechanical deterioration in female mice. Endocrinology. 2014;155:3508–3515. doi: 10.1210/en.2014-1334. [DOI] [PubMed] [Google Scholar]
- 90.Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Cell Death Differ. 2005;12:1178–1190. doi: 10.1038/sj.cdd.4401692. [DOI] [PubMed] [Google Scholar]
- 91.Sévère N, Dieudonné FX, Marie PJ. E3 ubiquitin ligase-mediated regulation of bone formation and tumorigenesis. Cell Death Dis. 2013;4:e463. doi: 10.1038/cddis.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, Harris SE, Gallwitz W, Kim KB, Hu S, Crews CM, Mundy GR. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M, Mancini C, Martella E, Ferrari L, Tabilio A, Rizzoli V. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110:334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 94.Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, Jones DC, Vallet S, Bouxsein ML, Pozzi S, Chhetri S, Seo YD, Aronson JP, Patel C, Fulciniti M, Purton LE, Glimcher LH, Lian JB, Stein G, Anderson KC, Scadden DT. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest. 2008;118:491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khedgikar V, Kushwaha P, Gautam J, Verma A, Changkija B, Kumar A, Sharma S, Nagar GK, Singh D, Trivedi PK, Sangwan NS, Mishra PR, Trivedi R. Withaferin A: a proteasomal inhibitor promotes healing after injury and exerts anabolic effect on osteoporotic bone. Cell Death Dis. 2013;4:e778. doi: 10.1038/cddis.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xing L, Zhang M, Chen D. Smurf control in bone cells. J Cell Biochem. 2010;110:554–563. doi: 10.1002/jcb.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang G, Guo B, Wu H, Tang T, Zhang BT, Zheng L, He Y, Yang Z, Pan X, Chow H, To K, Li Y, Li D, Wang X, Wang Y, Lee K, Hou Z, Dong N, Li G, Leung K, Hung L, He F, Zhang L, Qin L. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat Med. 2012;18:307–314. doi: 10.1038/nm.2617. [DOI] [PubMed] [Google Scholar]
- 98.Jones DC, Wein MN, Oukka M, Hofstaetter JG, Glimcher MJ, Glimcher LH. Regulation of adult bone mass by the zinc finger adapter protein Schnurri-3. Science. 2006;312:1223–1227. doi: 10.1126/science.1126313. [DOI] [PubMed] [Google Scholar]
- 99.Glimcher LH, Jones DC, Wein MN. Control of postnatal bone mass by the zinc finger adapter protein Schnurri-3. Ann N Y Acad Sci. 2007;1116:174–181. doi: 10.1196/annals.1402.044. [DOI] [PubMed] [Google Scholar]
- 100.Shu L, Zhang H, Boyce BF, Xing L. Ubiquitin E3 ligase Wwp1 negatively regulates osteoblast function by inhibiting osteoblast differentiation and migration. J Bone Miner Res. 2013;28:1925–1935. doi: 10.1002/jbmr.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsygankov AY, Teckchandani AM, Feshchenko EA, Swaminathan G. Beyond the RING: CBL proteins as multivalent adapters. Oncogene. 2001;20:6382–6402. doi: 10.1038/sj.onc.1204781. [DOI] [PubMed] [Google Scholar]
- 102.Salingcarnboriboon RA, Pavasant P, Noda M. Cbl-b enhances Runx2 protein stability and augments osteocalcin promoter activity in osteoblastic cell lines. J Cell Physiol. 2010;224:743–747. doi: 10.1002/jcp.22176. [DOI] [PubMed] [Google Scholar]
- 103.Sanjay A, Horne WC, Baron R. The Cbl family: ubiquitin ligases regulating signaling by tyrosine kinases. Sci STKE. 2001;2001:40. doi: 10.1126/stke.2001.110.pe40. [DOI] [PubMed] [Google Scholar]
- 104.Thien CB, Langdon WY. Negative regulation of PTK signaling by Cbl proteins. Growth Factors. 2005;23:161–167. doi: 10.1080/08977190500153763. [DOI] [PubMed] [Google Scholar]
- 105.Sévère N, Miraoui H, Marie PJ. The Casitas B lineage lymphoma (Cbl) mutant G306E enhances osteogenic differentiation in human mesenchymal stromal cells in part by decreased Cbl-mediated platelet-derived growth factor receptor alpha and fibroblast growth factor receptor 2 ubiquitination. J Biol Chem. 2011;286:24443–24450. doi: 10.1074/jbc.M110.197525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dieudonné FX, Sévère N, Biosse-Duplan M, Weng JJ, Su Y, Marie PJ. Promotion of osteoblast differentiation in mesenchymal cells through Cbl-mediated control of STAT5 activity. Stem Cells. 2013;31:1340–1349. doi: 10.1002/stem.1380. [DOI] [PubMed] [Google Scholar]
- 107.Brennan T, Adapala NS, Barbe MF, Yingling V, Sanjay A. Abrogation of Cbl-PI3 K interaction increases bone formation and osteoblast prolifération. Calcif Tissue Int. 2011;89:396–410. doi: 10.1007/s00223-011-9531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2001;2:81–94. doi: 10.1023/a:1010011209064. [DOI] [PubMed] [Google Scholar]
- 109.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 110.Bianco P, Robey PG, Saggio I, Riminucci M. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature identity and significance in incurable skeletal disease. Hum Gene Ther. 2010;21:1057–1066. doi: 10.1089/hum.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 2003;100:11917–11923. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marie PJ, Fromigué O. Osteogenic differentiation of human marrow-derived mesenchymal stem cells. Regen Med. 2006;1:539–548. doi: 10.2217/17460751.1.4.539. [DOI] [PubMed] [Google Scholar]
- 113.Vilquin JT, Rosset P. Mesenchymal stem cells in bone and cartilage repair: current status. Regen Med. 2006;1:589–604. doi: 10.2217/17460751.1.4.589. [DOI] [PubMed] [Google Scholar]
- 114.Marie PJ. Targeting integrins to promote bone formation and repair. Nat Rev Endocrinol. 2013;9:288–295. doi: 10.1038/nrendo.2013.4. [DOI] [PubMed] [Google Scholar]
- 115.Hamidouche Z, Fromigué O, Ringe J, Haüpl T, Vaudin P, Pages JC, Srouji S, Livne E, Marie PJ. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc Natl Acad Sci USA. 2009;106:18587–18591. doi: 10.1073/pnas.0812334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaabeche K, Guénou H, Bouvard D, Didelot N, Listrat A, Marie PJ. Cbl-mediated ubiquitination of alpha5 integrin subunit mediates fibronectin-dependent osteoblast detachment and apoptosis induced by FGFR2 activation. J Cell Sci. 2005;118:1223–1232. doi: 10.1242/jcs.01679. [DOI] [PubMed] [Google Scholar]
- 117.Srouji S, Ben-David D, Fromigué O, Vaudin P, Kuhn G, Müller R, Livne E, Marie PJ. Lentiviral-mediated integrin alpha5 expression in human adult mesenchymal stromal cells promotes bone repair in mouse cranial and long-bone defects. Hum Gene Ther. 2012;23:167–172. doi: 10.1089/hum.2011.059. [DOI] [PubMed] [Google Scholar]
- 118.Fromigué O, Brun J, Marty C, Da Nascimento S, Sonnet P, Marie PJ. Peptide-based activation of alpha5 integrin for promoting osteogenesis. J Cell Biochem. 2012;113:3029–3038. doi: 10.1002/jcb.24181. [DOI] [PubMed] [Google Scholar]
- 119.Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007;21:3917–3927. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- 120.Yao W, Guan M, Jia J, Dai W, Lay YA, Amugongo S, Liu R, Olivos D, Saunders M, Lam KS, Nolta J, Olvera D, Ritchie RO, Lane NE. Reversing bone loss by directing mesenchymal stem cells to bone. Stem Cells. 2013;31:2003–2014. doi: 10.1002/stem.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, Panganiban B, Meng L, Zhou P, Shahnazari M, Ritchie RO, Lane NE. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med. 2012;18:456–462. doi: 10.1038/nm.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y, Wei L, Miron RJ, Shi B, Bian Z. Anabolic bone formation via a site specific bone targeting delivery system by interfering with semaphorin 4d expression. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2322. [DOI] [PubMed] [Google Scholar]
- 123.Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Genet Dev. 2001;11:527–532. doi: 10.1016/s0959-437x(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 124.Ralston SH, Uitterlinden AG. Genetics of osteoporosis. Endocr Rev. 2010;31:629–662. doi: 10.1210/er.2009-0044. [DOI] [PubMed] [Google Scholar]
- 125.Janssens K, Van Hul W. Molecular genetics of too much bone. Hum Mol Genet. 2002;11:2385–2393. doi: 10.1093/hmg/11.20.2385. [DOI] [PubMed] [Google Scholar]
- 126.Shenker A, Chanson P, Weinstein LS, Chi P, Spiegel AM, Lomri A, Marie PJ. Osteoblastic cells derived from isolated lesions of fibrous dysplasia contain activating somatic mutations of the Gs alpha gene. Hum Mol Genet. 1995;4:1675–1676. doi: 10.1093/hmg/4.9.1675. [DOI] [PubMed] [Google Scholar]
- 127.Marie PJ. Cellular and molecular basis of fibrous dysplasia. Histol Histopathol. 2001;16:981–988. doi: 10.14670/HH-16.981. [DOI] [PubMed] [Google Scholar]
- 128.Riminucci M, Robey PG, Bianco P. The pathology of fibrous dysplasia and the McCune-Albright syndrome. Pediatr Endocrinol Rev. 2007;4:401–411. [PubMed] [Google Scholar]
- 129.Marie PJ, de Pollak C, Chanson P, Lomri A. Increased proliferation of osteoblastic cells expressing the activating Gs alpha mutation in monostotic and polyostotic fibrous dysplasia. Am J Pathol. 1997;150:1059–1069. [PMC free article] [PubMed] [Google Scholar]
- 130.Riminucci M, Fisher LW, Shenker A, Spiegel AM, Bianco P, Gehron Robey P. Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation. Am J Pathol. 1997;151:1587–1600. [PMC free article] [PubMed] [Google Scholar]
- 131.Saggio I, Remoli C, Spica E, Cersosimo S, Sacchetti B, Robey PG, Holmbeck K, Cumano A, Boyde A, Bianco P, Riminucci M. Constitutive expression of Gsalpha in mice produces a heritable direct replica of human fibrous dysplasia bone pathology and demonstrates its natural history. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Piersanti S, Remoli C, Saggio I, Funari A, Michienzi S, Sacchetti B, Robey PG, Riminucci M, Bianco P. Transfer analysis and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors. J Bone Miner Res. 2010;25:1103–1116. doi: 10.1359/jbmr.091036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kronenberg HM. Gs signaling in osteoblasts and hematopoietic stem cells. Ann NY Acad Sci. 2010;1192:327–329. doi: 10.1111/j.1749-6632.2009.05251.x. [DOI] [PubMed] [Google Scholar]
- 134.Sinha P, Aarnisalo P, Chubb R, Ono N, Fulzele K, Selig M, Saeed H, Chen M, Weinstein LS, Divieti Pajevic P, Kronenberg HM, Wu JY. Loss of G alpha early in the osteoblast lineage favors adipogenic differentiation of mesenchymal progenitors and committed osteoblast precursors. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu JY, Aarnisalo P, Bastepe M, Sinha P, Fulzele K, Selig MK, Chen M, Poulton IJ, Purton LE, Sims NA, Weinstein LS, Kronenberg HM. Gsalpha enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 2011;121:3492–3504. doi: 10.1172/JCI46406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Regard JB, Cherman N, Palmer D, Kuznetsov SA, Celi FS, Guettier JM, Chen M, Bhattacharyya N, Wess J, Coughlin SR, Weinstein LS, Collins MT, Robey PG, Yang Y. Wnt/beta-catenin signaling is differentially regulated by Galpha proteins and contributes to fibrous dysplasia. Proc Natl Acad Sci USA. 2011;108:20101–20106. doi: 10.1073/pnas.1114656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- 138.Lomri A, Lemonnier J, Hott M, de Parseval N, Lajeunie E, Munnich A, Renier D, Marie PJ. Increased calvaria cell differentiation and bone matrix formation induced by fibroblast growth factor receptor 2 mutations in Apert syndrome. J Clin Invest. 1998;101:1310–1317. [PMC free article] [PubMed] [Google Scholar]
- 139.Marie PJ, Kaabeche K, Guénou H. Roles of FGFR2 and twist in human craniosynostosis: insights from genetic mutations in cranial osteoblasts. Front Oral Biol. 2008;12:144–159. doi: 10.1159/000115036. [DOI] [PubMed] [Google Scholar]
- 140.Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 141.Suzuki H, Suda N, Shiga M, Kobayashi Y, Nakamura M, Iseki S, Moriyama K. Apert syndrome mutant FGFR2 and its soluble form reciprocally alter osteogenesis of primary calvarial osteoblasts. J Cell Physiol. 2012;227:3267–3277. doi: 10.1002/jcp.24021. [DOI] [PubMed] [Google Scholar]
- 142.Rice DP, Aberg T, Chan Y, Tang Z, Kettunen PJ, Pakarinen L, Maxson RE, Thesleff I. Integration of FGF and TWIST in calvarial bone and suture development. Development. 2000;127:1845–1855. doi: 10.1242/dev.127.9.1845. [DOI] [PubMed] [Google Scholar]
- 143.Hajihosseini MK. Fibroblast growth factor signaling in cranial suture development and pathogenesis. Front Oral Biol. 2008;12:160–177. doi: 10.1159/000115037. [DOI] [PubMed] [Google Scholar]
- 144.Marie PJ, Coffin JD, Hurley MM. FGF and FGFR signaling in chondrodysplasias and craniosynostosis. J Cell Biochem. 2005;96:888–896. doi: 10.1002/jcb.20582. [DOI] [PubMed] [Google Scholar]
- 145.Mansukhani A, Bellosta P, Sahni M, Basilico C. Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol. 2000;149:1297–1308. doi: 10.1083/jcb.149.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mansukhani A, Ambrosetti D, Holmes G, Cornivelli L, Basilico C. Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. J Cell Biol. 2005;168:1065–1076. doi: 10.1083/jcb.200409182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wilkie AO. Cancer drugs to treat birth defects. Nat Genet. 2007;39:1057–1059. doi: 10.1038/ng0907-1057. [DOI] [PubMed] [Google Scholar]
- 148.Melville H, Wang Y, Taub PJ, Jabs EW. Genetic basis of potential therapeutic strategies for craniosynostosis. Am J Med Genet A. 2010;152A:3007–3015. doi: 10.1002/ajmg.a.33703. [DOI] [PubMed] [Google Scholar]
- 149.Eswarakumar VP, Ozcan F, Lew ED, Bae JH, Tome F, Booth CJ, Adams DJ, Lax I, Schlessinger J. Attenuation of signaling pathways stimulated by pathologically activated FGF-receptor 2 mutants prevents craniosynostosis. Proc Natl Acad Sci USA. 2006;103:18603–18608. doi: 10.1073/pnas.0609157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shukla V, Coumoul X, Wang RH, Kim HS, Deng CX. RNA interference and inhibition of MEK-ERK signaling prevent abnormal skeletal phenotypes in a mouse model of craniosynostosis. Nat Genet. 2007;39:1145–1150. doi: 10.1038/ng2096. [DOI] [PubMed] [Google Scholar]
- 151.Miraoui H, Ringe J, Haüpl T, Marie PJ. Increased EGF- and PDGFα-receptor signaling by mutant FGF-receptor 2 contributes to osteoblast dysfunction in Apert craniosynostosis. Hum Mol Genet. 2010;19:1678–1689. doi: 10.1093/hmg/ddq045. [DOI] [PubMed] [Google Scholar]
- 152.Moenning A, Jager R, Egert A, Kress W, Wardelmann E, Schorle H. Sustained platelet-derived growth factor receptor alpha signaling in osteoblasts results in craniosynostosis by overactivating the phospholipase C-gamma pathway. Mol Cell Biol. 2009;29:881–891. doi: 10.1128/MCB.00885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miraoui H, Marie PJ. Fibroblast growth factor receptor signaling crosstalk in skeletogenesis. Sci Signal. 2010;3:re9. doi: 10.1126/scisignal.3146re9. [DOI] [PubMed] [Google Scholar]
- 154.Howard TD, Paznekas WA, Green ED, Chiang LC, Ma N, Ortiz de Luna RI, Garcia Delgado C, Gonzalez-Ramos M, Kline AD, Jabs EW. Mutations in TWIST a basic helix-loop-helix transcription factor in Saethre-Chotzen syndrome. Nat Genet. 1997;15:36–41. doi: 10.1038/ng0197-36. [DOI] [PubMed] [Google Scholar]
- 155.El Ghouzzi V, Legeai-Mallet L, Aresta S, Benoist C, Munnich A, de Gunzburg J, Bonaventure J. Saethre-Chotzen mutations cause TWIST protein degradation or impaired nuclear location. Hum Mol Genet. 2000;9:813–819. doi: 10.1093/hmg/9.5.813. [DOI] [PubMed] [Google Scholar]
- 156.Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6:423–435. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- 157.Yousfi M, Lasmoles F, Lomri A, Delannoy P, Marie PJ. Increased bone formation and decreased osteocalcin expression induced by reduced Twist dosage in Saethre-Chotzen syndrome. J Clin Invest. 2001;107:1153–1161. doi: 10.1172/JCI11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yousfi M, Lasmoles F, Marie PJ. TWIST inactivation reduces CBFA1/RUNX2 expression and DNA binding to the osteocalcin promoter in osteoblasts. Biochem Biophys Res Commun. 2002;297:641–644. doi: 10.1016/s0006-291x(02)02260-x. [DOI] [PubMed] [Google Scholar]
- 159.Miraoui H, Marie PJ. Pivotal role of Twist in skeletal biology and pathology. Gene. 2010;468:1–7. doi: 10.1016/j.gene.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 160.Guénou H, Kaabeche K, le Mée SL, Marie PJ. A role for fibroblast growth factor receptor-2 in the altered osteoblast phenotype induced by Twist haploinsufficiency in the Saethre-Chotzen syndrome. Hum Mol Genet. 2005;14:1429–1439. doi: 10.1093/hmg/ddi152. [DOI] [PubMed] [Google Scholar]
- 161.Connerney J, Andreeva V, Leshem Y, Mercado MA, Dowell K, Yang X, Lindner V, Friesel RE, Spicer DB. Twist1 homodimers enhance FGF responsiveness of the cranial sutures and promote suture closure. Dev Biol. 2008;318:323–334. doi: 10.1016/j.ydbio.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miraoui H, Sévère N, Vaudin P, Pagès JC, Marie PJ. Molecular silencing of Twist1 enhances osteogenic differentiation of murine mesenchymal stem cells: implication of FGFR2 signaling. J Cell Biochem. 2010;110:1147–1154. doi: 10.1002/jcb.22628. [DOI] [PubMed] [Google Scholar]
- 163.Haworth CS, Webb AK, Elkin SL, Hodson ME, Compston JE, Selby PL. Cystic fibrosis related low bone density. Arch Dis Child. 2000;83:369. doi: 10.1136/adc.83.4.369a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Elkin SL, Vedi S, Bord S, Garrahan NJ, Hodson ME, Compston JE. Histomorphometric analysis of bone biopsies from the iliac crest of adults with cystic fibrosis. Am J Respir Crit Care Med. 2002;166:1470–1474. doi: 10.1164/rccm.200206-578OC. [DOI] [PubMed] [Google Scholar]
- 165.Le Henaff C, Gimenez A, Haÿ E, Marty C, Marie PJ, Jacquot J. The F508del mutation in cystic fibrosis transmembrane conductance regulator gene impacts bone formation. Am J Pathol. 2012;180:2068–2075. doi: 10.1016/j.ajpath.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 166.Le Henaff C, Haÿ E, Velard F, Marty C, Tabary O, Marie PJ, Jacquot JP. Enhanced F508del-CFTR channel activity ameliorates bone pathology in murine cystic fibrosis. Am J Pathol. 2014;184:1132–1141. doi: 10.1016/j.ajpath.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 167.Marie PJ, Fromigué O, Modrowski D. Deregulation of osteoblast differentiation in primary bone cancers. In: Heymann D, editor. Bone cancer: primary bone cancers and bone metastases. 2. New York: Elseiver; 2014. [Google Scholar]
- 168.Broadhead ML, Clark JC, Myers DE, Dass CR, Choong PF. The molecular pathogenesis of osteosarcoma: a review. Sarcoma. 2011;2011:959248. doi: 10.1155/2011/959248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thomas DM, Carty SA, Piscopo DM, Lee JS, Wang WF, Forrester WC, Hinds PW. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell. 2001;8:303–316. doi: 10.1016/s1097-2765(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 170.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 171.Dani N, Olivero M, Mareschi K, van Duist MM, Miretti S, Cuvertino S, Patane S, Calogero R, Ferracini R, Scotlandi K, Fagioli F, Di Renzo MF. The MET oncogene transforms human primary bone-derived cells into osteosarcomas by targeting committed osteo-progenitors. J Bone Miner Res. 2012;27:1322–1334. doi: 10.1002/jbmr.1578. [DOI] [PubMed] [Google Scholar]
- 172.Martin JW, Zielenska M, Stein GS, van Wijnen AJ, Squire JA. The role of RUNX2 in osteosarcoma oncogenesis. Sarcoma. 2011;2011:282745. doi: 10.1155/2011/282745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cao Y, Zhou Z, de Crombrugghe B, Nakashima K, Guan H, Duan X, Jia SF, Kleinerman ES. Osterix a transcription factor for osteoblast differentiation mediates antitumor activity in murine osteosarcoma. Cancer Res. 2005;65:1124–1128. doi: 10.1158/0008-5472.CAN-04-2128. [DOI] [PubMed] [Google Scholar]
- 174.Basu-Roy U, Seo E, Ramanathapuram L, Rapp TB, Perry JA, Orkin SH, Mansukhani A, Basilico C. Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene. 2012;31:2270–2282. doi: 10.1038/onc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rettew AN, Getty PJ, Greenfield EM. Receptor tyrosine kinases in osteosarcoma: not just the usual suspects. Adv Exp Med Biol. 2014;804:47–66. doi: 10.1007/978-3-319-04843-7_3. [DOI] [PubMed] [Google Scholar]
- 176.Zhang H, Wu H, Zheng J, Yu P, Xu L, Jiang P, Gao J, Wang H, Zhang Y. Transforming growth factor beta1 signal is crucial for dedifferentiation of cancer cells to cancer stem cells in osteosarcoma. Stem Cells. 2013;31:433–446. doi: 10.1002/stem.1298. [DOI] [PubMed] [Google Scholar]
- 177.Rikhof B, de Jong S, Suurmeijer AJ, Meijer C, van der Graaf WT. The insulin-like growth factor system and sarcomas. J Pathol. 2009;217:469–482. doi: 10.1002/path.2499. [DOI] [PubMed] [Google Scholar]
- 178.Lee JA, Ko Y, Kim DH, Lim JS, Kong CB, Cho WH, Jeon DG, Lee SY, Koh JS. Epidermal growth factor receptor: is it a feasible target for the treatment of osteosarcoma? Cancer Res Treat. 2012;44:202–209. doi: 10.4143/crt.2012.44.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sévère N, Dieudonné FX, Marty C, Modrowski D, Patino-Garcia A, Lecanda F, Fromigué O, Marie PJ. Targeting the E3 ubiquitin casitas B-lineage lymphoma decreases osteosarcoma cell growth and survival and reduces tumorigenesis. J Bone Miner Res. 2012;27:2108–2117. doi: 10.1002/jbmr.1667. [DOI] [PubMed] [Google Scholar]
- 180.Hassan SE, Bekarev M, Kim MY, Lin J, Piperdi S, Gorlick R, Geller DS. Cell surface receptor expression patterns in osteosarcoma. Cancer. 2012;118:740–749. doi: 10.1002/cncr.26339. [DOI] [PubMed] [Google Scholar]
- 181.Jullien N, Dieudonné FX, Habel N, Marty C, Modrowski D, Patino A, Lecanda F, Sévère N, Marie PJ. ErbB3 silencing reduces osteosarcoma cell proliferation and tumor growth in vivo. Gene. 2013;521:55–61. doi: 10.1016/j.gene.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 182.Shapovalov Y, Benavidez D, Zuch D, Eliseev RA. Proteasome inhibition with bortezomib suppresses growth and induces apoptosis in osteosarcoma. Int J Cancer. 2010;127:67–76. doi: 10.1002/ijc.25024. [DOI] [PubMed] [Google Scholar]
- 183.Haydon RC, Deyrup A, Ishikawa A, Heck R, Jiang W, Zhou L, Feng T, King D, Cheng H, Breyer B, Peabody T, Simon MA, Montag AG, He TC. Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer. 2002;102:338–342. doi: 10.1002/ijc.10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hoang BH, Kubo T, Healey JH, Sowers R, Mazza B, Yang R, Huvos AG, Meyers PA, Gorlick R. Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer. 2004;109:106–111. doi: 10.1002/ijc.11677. [DOI] [PubMed] [Google Scholar]
- 185.Dieudonné FX, Marion A, Haÿ E, Marie PJ, Modrowski D. High Wnt signaling represses the proapoptotic proteoglycan syndecan-2 in osteosarcoma cells. Cancer Res. 2010;70:5399–5408. doi: 10.1158/0008-5472.CAN-10-0090. [DOI] [PubMed] [Google Scholar]
- 186.McQueen P, Ghaffar S, Guo Y, Rubin EM, Zi X, Hoang BH. The Wnt signaling pathway: implications for therapy in osteosarcoma. Expert Rev Anticancer Ther. 2011;11:1223–1232. doi: 10.1586/era.11.94. [DOI] [PubMed] [Google Scholar]
- 187.Guo Y, Rubin EM, Xie J, Zi X, Hoang BH. Dominant negative LRP5 decreases tumorigenicity and metastasis of osteosarcoma in an animal model. Clin Orthop Relat Res. 2008;466:2039–2045. doi: 10.1007/s11999-008-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dieudonné FX, Marion A, Marie PJ, Modrowski D. Targeted inhibition of T-cell factor activity promotes syndecan-2 expression and sensitization to doxorubicin in osteosarcoma cells and bone tumors in mice. J Bone Miner Res. 2012;27:2118–2129. doi: 10.1002/jbmr.1650. [DOI] [PubMed] [Google Scholar]
- 189.Rao-Bindal K, Kleinerman ES. Epigenetic regulation of apoptosis and cell cycle in osteosarcoma. Sarcoma. 2011;2011:679457. doi: 10.1155/2011/679457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kresse SH, Rydbeck H, Skarn M, Namlos HM, Barragan-Polania AH, Cleton-Jansen AM, Serra M, Liestol K, Hogendoorn PC, Hovig E, Myklebost O, Meza-Zepeda LA. Integrative analysis reveals relationships of genetic and epigenetic alterations in osteosarcoma. PLoS One. 2012;7:e48262. doi: 10.1371/journal.pone.0048262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gordon JA, Montecino MA, Aqeilan RI, Stein JL, Stein GS, Lian JB. Epigenetic pathways regulating bone homeostasis: potential targeting for intervention of skeletal disorders. Curr Osteoporos Rep. 2014;12:496–506. doi: 10.1007/s11914-014-0240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cain JE, McCaw A, Jayasekara WS, Rossello FJ, Marini KD, Irving AT, Kansara M, Thomas DM, Ashley DM, Watkins DN. Sustained low-dose treatment with the histone deacetylase inhibitor LBH589 induces terminal differentiation of osteosarcoma cells. Sarcoma. 2013;2013:608964. doi: 10.1155/2013/608964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nugent M. MicroRNA function and dysregulation in bone tumors: the evidence to date. Cancer Manag Res. 2014;6:15–25. doi: 10.2147/CMAR.S53928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Miao J, Wu S, Peng Z, Tania M, Zhang C. MicroRNAs in osteosarcoma: diagnostic and therapeutic aspects. Tumour Biol. 2013;34:2093–2098. doi: 10.1007/s13277-013-0940-7. [DOI] [PubMed] [Google Scholar]
- 195.Swami A, Reagan MR, Basto P, Mishima Y, Kamaly N, Glavey S, Zhang S, Moschetta M, Seevaratnam D, Zhang Y, Liu J, Memarzadeh M, Wu J, Manier S, Shi J, Bertrand N, Lu ZN, Nagano K, Baron R, Sacco A, Roccaro AM, Farokhzad OC, Ghobrial IM. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc Natl Acad Sci USA. 2014;111:10287–10292. doi: 10.1073/pnas.1401337111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Magnon C, Lucas D, Frenette PS. Trafficking of stem cells. Methods Mol Biol. 2011;750:3–24. doi: 10.1007/978-1-61779-145-1_1. [DOI] [PubMed] [Google Scholar]
- 197.Lo Celso C, Lin CP, Scadden DT. In vivo imaging of transplanted hematopoietic stem and progenitor cells in mouse calvarium bone marrow. Nat Protoc. 2011;6:1–14. doi: 10.1038/nprot.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.McGee-Lawrence ME, Westendorf JJ. Histone deacetylases in skeletal development and bone mass maintenance. Gene. 2011;474:1–11. doi: 10.1016/j.gene.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.van Wijnen AJ, van de Peppel J, van Leeuwen JP, Lian JB, Stein GS, Westendorf JJ, Oursler MJ, Im HJ, Taipaleenmaki H, Hesse E, Riester S, Kakar S. MicroRNA functions in osteogenesis and dysfunctions in osteoporosis. Curr Osteoporos Rep. 2013;11:72–82. doi: 10.1007/s11914-013-0143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Askmyr M, Sims NA, Martin TJ, Purton LE. What is the true nature of the osteoblastic hematopoietic stem cell niche? Trends Endocrinol Metab. 2009;20:303–309. doi: 10.1016/j.tem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 201.Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009;24:759–764. doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shiozawa Y, Taichman RS. Getting blood from bone: an emerging understanding of the role that osteoblasts play in regulating hematopoietic stem cells within their niche. Exp Hematol. 2012;40:685–694. doi: 10.1016/j.exphem.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, Khiabanian H, Lee A, Murty VV, Friedman R, Brum A, Park D, Galili N, Mukherjee S, Teruya-Feldstein J, Raza A, Rabadan R, Berman E, Kousteni S. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 2014;506:240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Krevvata M, Silva BC, Manavalan JS, Galan-Diez M, Kode A, Matthews BG, Park D, Zhang CA, Galili N, Nickolas TL, Dempster DW, Dougall W, Teruya-Feldstein J, Economides AN, Kalajzic I, Raza A, Berman E, Mukherjee S, Bhagat G, Kousteni S (2014) Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood 124:2834–2846 [DOI] [PMC free article] [PubMed]