Abstract
Two different models describe the development of definitive hematopoiesis and hematopoietic stem cells (HSCs). In one of these, the visceral yolk sac serves as a starting point of relatively lengthy developmental process culminating in the fetal liver hematopoiesis. In another, the origin of adult hematopoiesis is split between the yolk sac and the dorsal aorta, which has a peculiar capacity to generate definitive HSCs. Despite a large amount of experimental data consistent with the latter view, it becomes increasingly unsustainable in the light of recent cell tracing studies. Moreover, analysis of the published studies supporting the aorta-centered version uncovers significant caveats in standard experimental approach and argumentation. As a result, the theory cannot offer feasible cellular mechanisms of the HSC emergence. This review summarizes key efforts to discern the developmental pathway of the adult-type HSCs and attempts to put forward a hypothesis on the inflammatory mechanisms of hematopoietic ontogenesis.
Keywords: Hematopoiesis, Development, Hematopoietic stem cells, Repopulation, Cell potential, Cell tracing
Introduction
All diverse cellular components of mammalian blood share a common ancestor—long-term hematopoietic stem cells (LT-HSC). It is thought that these cells preside over a vast hierarchy of hematopoietic progenitors that become progressively restricted in their capacity to give rise to multiple blood cell lineages. The progenitors ultimately differentiate into mature blood cells that include erythroid cells, megakaryocytes, monocytes/macrophages, granulocytes, and lymphocytes. In addition to the multipotency, HSCs are capable of self-renewal, which perpetuates the stem cell pool throughout life.
HSCs lack any specific morphological features. They are defined functionally and somewhat artificially as being capable of reconstituting the entire hematopoietic system of a conditioned transplant recipient. To do this, transplanted HSCs have to occupy the stem cell niches, maintain themselves through self-renewal, and give rise to the differentiated blood cell progeny. The functional definition is lengthy, cumbersome, and retrospective, and for practical purposes, it is often supplemented by a phenotypic definition, i.e., the antigen expression profile segregating with the highest repopulation capacity. Unfortunately, the surface marker profiling cannot completely replace the functional analysis since the marker expression is dependent on a number of circumstances including experimental stress, the avidity of the antibodies used for the test, and genetic background of the tested cell population.
Adult LT-HSCs, or simply HSCs, are distinct from misnomered short-term hematopoietic stem cells (ST-HSCs), which, in the strict sense, are not stem cells. From a conceptual standpoint, the population of LT-HSCs is functionally separated from the rest of blood cells—it is a unique autonomous blood tissue continuously supporting its multipotency by sustained self-renewal. However, despite sitting on the top of blood hierarchy, HSCs are not the first blood cells to emerge during ontogenesis. Contradictory to the essence of the HSC concept, differentiated blood cells and committed hematopoietic progenitors arise before HSCs [1]. One possible explanation for this paradoxical reversal is that the early conceptal, i.e., derived from the conceptus, “progenitors” most probably represent a functional capacity in particular circumstances rather than cells of a distinct phenotype. This review is an attempt to address the issue of nonhierarchical hematopoietic development during mammalian ontogenesis.
It is easy to imagine that due to their singularity, the HSCs emerge in a distinct way, at a specific location, and separately from other early conceptal hematopoietic cells. Alternatively, HSCs might develop alongside with other early blood cells, emerging at the culminating point of the entire hematopoietic lineage specification. From the mechanistic point of view, these possibilities can be formulated into two corresponding scenarios. In the first one, the stem cells arise through diminution in embryonic cell potency, which can be described as abrupt shutting down of all non-hematopoietic epigenetic programs in mesodermal precursors. Hematopoietic multipotency and self-renewal are sustained as a default endowment of the conceptal cells. In the second scenario, the multipotency is unfurled step-by-step in progressively specifying HSC precursors. At the same time, non-hematopoietic programs are gradually erased. The precursors that retain or modify their ancestors’ self-renewal capacity are selected to become HSCs. In both models, the final maturation should include the induction of adaptive and homing mechanisms that make HSCs capable to repopulate adult hematopoietic system.
In terms of conceptal anatomy, the first scheme can be initiated in multiple locations where pre-HSC precursors are capable of segregating from other mesodermal lineages. This inevitably implicates relatively late stages of development—the midgestation in mice—since the newly emerged mature HSCs have to migrate immediately into their fetal niches. The second variant has to be initiated at the earliest site of hematopoiesis in developing conceptus, i.e., the visceral yolk sac. Thus, our understanding of blood cell ontogenesis essentially fluctuates between “polyphyletic” and “monophyletic” models [2].
In the search of the HSC origin, the second, “monophyletic”, scheme is intuitive and correspondingly enjoyed the early recognition, or at least temporary acceptance [3, 4]. Possibly in all mammals the visceral or secondary yolk sac serves as the site of the embryo-type or primitive hematopoiesis. In the “monophyletic” model, all adult-type, or definitive, blood elements including stem cells or their precursors arise also in the yolk sac [5]. The “polyphyletic”, or originally “diphyletic”, scheme came to the fore later, and in earnest only about 20 years ago [6], but now it is generally accepted as predominant, or at least persistently reiterated as such in the new publications [7–9]. In this theory, adult-type HSCs emerge intraembryonically, within the aorta-gonad-mesonephros (AGM) region [10] and possibly in the proximal regions of umbilical and vitelline arteries [11] as well as in the vascular labyrinth region of placenta [12, 13].
In addition to discussing these opposite, but not entirely incompatible, theories of the HSC emergence, this review aims to compile a workable hypothesis on the cellular mechanism of hematopoietic development in mammals. Learning the “logic” of hematopoietic ontogenesis helps to gain a better insight into the regulation and functioning of the HSC hierarchy in adults. Furthermore, it is extremely instrumental for development of safe gene and stem cell therapies for hematopoietic disorders. Developmental hematopoiesis, however, is still a subject of animated debates, and it appears that this is because studying blood ontogenesis excessively relies on the standard methods of adult hematology.
Studying hematopoiesis in mammalian ontogenesis
Early mammalian development is highly regulative [14], which means that the embryo patterning is not defined by morphogenetic determinants that were set from the start of development. The preimplantation mouse embryo can be drastically reorganized by changing the arrangement or the number of cells without any effect on the success of the embryo development [15]. A substantial number of additional cells can be introduced into the embryo and become easily incorporated into normal development [16]. In mammals, cell fate determination is not fixed; it unrolls during the ontogenesis. Regulative development, however, is not restricted to mammals; it is operative to a certain extent throughout almost all vertebrates. Such a type of development probably enhances the competence to accommodate evolutionary changes at the embryonic stage.
In general terms, embryonic cells undergoing regulative development can be transplanted to another part of the embryo and form there a structure that belongs in that area instead of the structure that it would have originally formed. This happens when cells retain or develop the capacity to respond to the cues present in the new microenvironment. Highly regulative mode of mammalian development has an important corollary for developmental hematology. This means that when cells from early mammalian conceptus are transplanted into adult or newborn hematopoietic systems or introduced into hematopoietic tissue culture, they can generate corresponding hematopoietic progenitors instead of cell progeny expected to form during normal development.
The developmental flexibility of certain conceptus cells persist in the normal mammalian ontogenesis for a relatively long time. Cells of the anterior epiblast in gastrulating mouse embryo of embryonic day 6.75 (E6.75) are normally fated to give rise to neuroectoderm, but when recombined with visceral endoderm (VE), they adopt cell fates characteristic to posterior lateral mesoderm, i.e., hematopoietic and endothelial [17]. The endodermal explant could be replaced by beads soaked in Indian hedgehog, and Bmp4 was found to be a possible mediator in the regulative event [18]. In another example, ALCAM(CD166)highFlk1(Kdr)− cells of the E8.5 yolk sac, when cultured in Matrigel or on OP9 stroma, are capable to develop into cardiac troponin T (cTn-T)-positive functional cardiomyocytes [19]. Thus, extraembryonic mesoderm, which is not destined to contribute to heart development, still preserve the capacity to respond to appropriate external cardiomyogenic cues at E8.5. Interestingly, in the absence of the functional Scl/Tal1 gene, the cardiogenesis takes place ectopically in the yolk sac within the CD31-positive cell population [20]. Taken together, the data indicate that analysis of the conceptal cell potential in a bioassay can lead to profound epigenetic changes comparable to those induced by inactivation of a key regulatory gene.
Cells in mid-term conceptuses still have the regulative capacity to change their identity and potential (epigenetic reprogramming [21]). Even at the advanced stage of E11.5–E12.5, primordial germ cells (PGCs) placed on a feeder cell layer in the presence of LIF, steel factor and Fgf4 develop the pluripotency, which is normally characteristic of the inner cell mass (ICM) and early epiblast cells [16]. It appears that PGCs are not exceptional in terms of regulative development. Endothelial or endothelium-associated cells of the E9.5 dorsal aorta can spontaneously reprogram into skeletal muscle cells upon transplantation into newborn mice [22]. Midgestation neural tube cells were capable of doing the same in vitro without any genetic alterations involved, although in the absence of inductive growth factors the efficiency of this “transdifferentiation” was low [23].
Unless genetically manipulated, usually only a limited number of cells from early mammalian conceptus can successfully adapt to alternative or enhanced inductive microenvironment. In the search for the developmental source of hematopoiesis, cells “positively” responding to the artificial inductive conditions are considered to represent the earliest hematopoietic progenitors. However, the “responder” cells may become progenitors only during the hematopoietic progenitor assay. In an emblematic example, the clonogenic macrophage progenitors, macrophage colony-forming cells (M-CFCs), are first detected at E7.0 after transplanting conceptus cells into cytokine-rich methylcellulose culture [24]. The development of these progenitors is absolutely dependent on the cell-autonomous function of the hematopoietic ETS-related transcription factor PU.1 [25–27]. This factor is essential for fetal liver hematopoiesis and is not expressed anywhere in the mouse conceptus before E11.5 [28, 29]. Therefore, gastrulating conceptus cells that display the M-CFC potential have to undergo additional developmental steps to reach the phase at which PU.1 takes part in the macrophage lineage development. This phase would roughly correspond to the onset of hematopoiesis in the early fetal liver. Absence of PU.1 expression in the starting cell population suggests that there are no adult-type clonogenic macrophage progenitors in E7.0 conceptus. Rather, the functional in vitro assay demonstrates the presence of cells that are capable and selected to become M-CFCs in a particular artificial environment. Taking into consideration the inherently regulative manner of mammalian development, it is possible that some of these cells do not belong to the conceptal macrophage lineage. Moreover, it is hard to exclude a possibility that in the early conceptuses some of these “progenitors” actually develop into non-hematopoietic cells. Their development in vitro might be skewed towards macrophages as an adaptation to the changed microenvironment. This adaptation must include the induction of PU.1 and other macrophage lineage molecules such as c-fms (receptor for M-SCF, macrophage-colony stimulating factor), which started to be expressed in the yolk sac as late as E9.5 [29]. Similarly, the PU.1-dependent stages of B and T cell precursor development should be reached during the lymphoid progenitor assays conducted with cells of the pre-liver mouse conceptus.
The actual conditions of a bioassay determine the probability that selected conceptal cells will demonstrate a hematopoietic potential. Cells of the E6.5 conceptus lacked the capacity to give rise to M-CFCs in particular conditions of the in vitro assay [24]. These cells might be incompetent at this stage to respond to the cues presented in the clonogenic assay. The absence of M-CFC potential at the early stage can be therefore a technical problem, like a missing cytokine, growth factor, or an inducer molecule in the assay medium. Similarly, the E10.0 yolk sac explants cannot autonomously develop the HSC potential unless IL-3 is added to the culture medium [30]. The notion of the adaptive character of developmental assays is further supported by the outcome of multiple attempts during the last 25 years to clarify the origin of the lymphoid lineage in the early mouse conceptus. Different groups employing diverse culture systems have reached sometimes opposite results in terms of extraembryonic versus intraembryonic source of B and T cells [31–34]. For instance, replacing adult bone marrow stromal cell line S17 [35] for OP9 stromal cells derived from M-CSF—deficient newborn calvaria [36] as well as slight changes in cytokine composition resulted in detection of a strong lymphoid potential of the E7.5–E8.0 yolk sac [34], the capacity that was denied by the earlier S17-based assays [33]. One possible reason for the discrepancy is that the yolk sac precursor cells are responsive only to a certain combination of the factors promoting lymphoid differentiation.
In general, it would not be surprising if a proper combination of assay conditions detects “hematopoietic progenitors” and “pre-HSCs” in any part of the early embryo. Most likely, the non-hematopoietic candidates for the “progenitors” would be conditionally pluripotent PGCs, and cells of the late primitive streak, which is present in the mouse embryo until E10.5 [37]. The location of these cells in embryo proper can explain the prevalence of the intraembryonic sources in polyphyletic models of blood development.
Detection of conceptus cells that bear the potential to become the hematopoietic progenitors is important to get an insight into general epigenetic mechanisms underlying the process of cell commitment. In the reductionist set of coordinates, any such information would be valid and instrumental in explaining the molecular mechanisms of ontogenesis. It is presumed that one could simply shuffle the bits of mechanistic information in order to solve the whole puzzle. This logic is, however, questionable because the cell fate and potential often do not coincide, and the actual development may use altogether different molecular mechanisms. The reductionist approach is in danger of studying some molecular “shortcuts” that actually do not take place in development and are not ordained by natural selection. Thus, assembling the whole cellular and corresponding molecular pathways might become extremely problematic, with many “bits” that refuse to fit into the picture.
The most reliable alternative to recursive cell potential measurements would be analysis of developmental processes in situ using refined procedures of non-invasive long-term cell tracing and designing assay systems which would closely reproduce the conditions within developing conceptus. Genetic cell tracing is currently getting broader recognition in developmental hematology [38]. Despite some technical limitations, which are mainly associated with a narrow choice of good genetic models, the procedure can be dramatically improved in terms of the specificity and the level of cell labeling. In addition, the cell tracing can be used in the embryo or tissue rescue setup, which allows functional characterization of conceptal genetics [34]. Another direction that has to be exploited in studying hematopoietic development is the refinement and adaptation of the whole embryo culture for efficient ex utero cell labeling either by genetic constructions or vital dye injections. Direct embryo cell labeling can be also performed in utero [39]. Although this procedure is currently used to mark cells of relatively late embryos, more sophisticated approaches may push its operational limits to much earlier stages.
A number of model organisms are being used in the studies of blood development. Zebrafish (Danio rerio) is by far the most developed and studied of all non-mammalian models to date [40]. Zebrafish studies revealed that the molecular mechanisms of hematopoietic development seem to bear a similarity with those of mammals [41]. An important consideration that has to be taken into account, however, is that the mammals together with birds and reptiles are amniotes and their early development is drastically different from that of anamniote fish. Amniotes developed a number of supporting extraembryonic tissues, the key of which is the hypoblast or the VE in mice [42]. The VE is not only involved in the nourishment of the embryo but also participates in the primitive streak positioning as well as in separating neuroectodermal and mesendodermal domains of the amniote gastrula. Importantly, the VE is a major inducing tissue for both primitive and definitive hematopoiesis and vasculogenesis [17]. Therefore, the hematological data obtained in the anamniote vertebrate model such as zebrafish has to be considered through the prism of the absence of blood-inducing hypoblast. Furthermore, the invention of extraembryonic support tissues lengthened the embryogenesis, enabled the embryo to grow larger at early stages and allowed amniotes to erase the larval stage from their development [42]. These changes have profound implications for hematopoietic development since blood is one of the earliest functional tissues in the conceptus. Fast enlargement of the amniote embryo required coordinated, speedy, and life-saving induction of the vascular and hematopoietic development whereas zebrafish embryonic and larval hematopoiesis is essentially disposable [43]. Taking into account all these profound differences, one has to be careful in applying anamniote experimental data to the study of hematopoietic development in mammals. Some basic molecular mechanisms can indeed be similar due to conservatism of evolution: developing something new is much more difficult than modifying what already exists. Yet, for their blood formation, fish and mammals may use distinct combinations of similar conservative molecular protocols which results in altogether a very different hematopoietic development.
The singularity of amniote developmental hematopoiesis was well understood by the early researchers who essentially established the field. Seeking to decipher the blood development in birds and mammals, they turned their attention to the first clearly hematopoietic tissue in the conceptus—the yolk sac, and specifically to the structures commonly known as blood islands.
Blood island origin of definitive blood stem cells
It had been first formulated by Moore and Owen [5] in 1967 that developing hematopoietic organs within embryo are colonized by circulating HSCs, which originate in the yolk sac. Similar to avian yolk sac, the visceral (i.e., internal) yolk sac of mammals has been long known to contain “blood islands”, which are regarded as the first site of hematopoiesis and vascular development in the amniote conceptus. In mice, blood islands are localized cellular aggregates of extraembryonic mesoderm confined externally by the VE, and internally by a layer of mesothelium. Blood islands become detectable starting from E8.25–E8.5 when these structures indeed look like large isolated aggregates of reddish erythroid cells contrasted with the whitish background of the conceptus. Yolk sac blood islands are recognized as the exclusive source of the primitive hematopoiesis, which is mainly represented by large nucleated erythroblasts, or megaloblasts [44]. Initially, the primitive erythroblasts synthesize only embryonic globins then later start to express also the adult versions of these pivotal oxygen transporting proteins. The first primitive erythroid progenitors can be detected immediately after the onset of gastrulation and then abruptly disappear between E8.5 and E9.0 [24], although the primitive erythroblasts continue to divide within the circulation until E13.0 [44]. The primitive erythroid cells serve the immediate needs of developing conceptus and are the only oxygen transporters before E12.0 [45–47].
Histochemical examination of chicken and mouse blood islands reveals rather unelaborate cellular composition though already 100 years ago the heterogeneity of blood island cells had been noticed [48]. The most defining feature of blood islands is a close association of their endothelial and hematopoietic components. Using mostly chicken embryology data, it has long been hypothesized that blood islands derive from an early transient cell population of hemangioblasts, the common precursors for hematopoietic and endothelial lineages [49].
The first experimental evidence supporting the hemangioblast idea came from the clonal in vitro differentiation of mouse embryonic stem cells (ESCs) [50, 51]. It appears that at the early stages of ESC differentiation (2.5–4 days of embryoid body formation), a distinct type of precursor cells can be identified on the basis of its potential and phenotype. These transient precursors were found almost exclusively among the Brachyury+Flk1+ cells [52]. The precursors form colonies of blast-like cells in the methylcellulose culture, which made them designated as blast colony-forming cells (BL-CFCs). Blast colonies upon replating into cytokine-supplemented liquid medium display both hematopoietic and vascular potential. Precursors that are very similar to the BL-CFCs have been found in E7.5 mouse conceptuses [53]. Similar to their ESC-derived counterparts [52, 54], the conceptal BL-CFCs were capable of differentiating into primitive and definitive hematopoietic as well as endothelial and smooth muscle cells, but not cardiomyocytes [53]. The in vivo BL-CFCs therefore qualify as lateral mesoderm precursors rather than bipotential hemangioblasts. The latter, however, is already widely accepted as a traditional designation, and its broader than expected differentiation potential would remain a mere terminology issue unless the existence of strictly defined hemangioblasts is demonstrated.
The in vivo hemangioblasts or lateral mesoderm precursors seem to be more heterogeneous than their in vitro counterparts, so that some of them are Flk1-negative but majority segregates with the Brachyury+Flk1+ phenotype [53]. Correspondingly, most of the BL-CFC activity (75 %) is located around the primitive streak while the rest is spread around conceptus and exact location or final destination of the off-streak BL-CFCs remains to be envisaged. Although the number of BL-CFCs per conceptus drops significantly by the head fold stage, it is unclear where the remaining few might linger in older conceptuses and when the bipotent hemangioblast-like cells disappear completely.
During their differentiation and blast colony formation, the ESC-derived hemangioblasts transiently upregulate the receptor tyrosine kinase Tie2/Tek [55]. The transitional Tie2+c-Kit+CD41− population is probably responsible for the appearance of the tight adherence in the BL-CFC progeny. It is not incidental that this population expresses VE-cadherin (Cdh5) as well as a number of other endothelial markers. The in vivo counterparts of these cells localize in blood islands of neural plate and headfold stage conceptuses and display both primitive and definitive hematopoietic potential [55]. By the end of blast colony development, Tie2 is downregulated whereas the earliest hematopoietic marker CD41 [glycoprotein IIb (GPIIb) or integrin αIIb] become expressed by almost all cells of the colony [55]. In this regard, embryoid body-derived blast colonies resemble the mesodermal part of the blood island primordium, which steadily accumulates CD41 on the surface of its cells [56]. Furthermore, the blood island cells are related to the descendants of BL-CFCs in their expression of the same endothelial markers: VE-cadherin, Tie-2, endoglin, CD31, and CD34 [57]. Taken together, these observations indicated that in vitro differentiation of hemangioblasts resembles the blood islands development and supported the historic notion that hemangioblast-like cells are the founder population of yolk sac hematopoiesis and vasculogenesis.
However, despite being endowed with broad in vitro developmental potential, the hemangioblasts may not have an opportunity to give rise to both endothelial and hematopoietic cell lineages in vivo. It seems that the bipotent (or tripotent) hemangioblast potential of the nascent lateral mesoderm is exposed or induced with a limited efficiency by the artificial in vitro assay conditions. Such conditions, however, are unlikely to exist in developing conceptus. For the earliest cells of lateral mesoderm, it is the development into either one lineage or another. Indeed, cell tracing analysis [58, 59] and immunohistochemical staining [56] of the developing yolk sac revealed that the extraembryonic endothelium and hematopoiesis emerge from separate mesodermal precursors. The endothelial-hematopoietic dichotomy is apparently initiated in the early epiblast, and any bi- or tripotent precursors, which may exist in or around blood islands play only marginal, if any, role in blood islands formation [58]. It is therefore safe to conclude that even though blood islands are very similar to the progeny of hemangioblasts, they are formed by gradual commitment of divergent mesodermal precursors. Nevertheless, precursors that are capable of bipotent regulative development might persist in the early circulation, which can explain incorporation of blood-borne cells into endothelium of large conceptal vessels [59].
Analysis of CD41 expression in the early conceptus reveals that instead of isolated “islands” there is a continuous circumferential band of primitive hematopoietic precursors in the proximal extraembryonic mesoderm [56, 60]. The hematopoietic band arises through local thickening of the nascent extraembryonic mesoderm sheet at the neural plate stages. Accumulated mesodermal masses dramatically upregulate Gata1 expression and start to express embryonic globin genes before early headfold stage (around E7.75) [57]. The “islands” as semi-isolated patches of blood cells are formed when arising more distally extraembryonic vascular plexus expands into the hematopoietic band and then envelops and separates the blood cell aggregates. At this stage, the extraembryonic plexus fuses with the embryo proper vascular system and blood cells begin to gradually scatter around conceptus.
Morphological examination of the fully developed blood islands had suggested that their hematopoietic part consists exclusively of the primitive erythroblasts [3]. However, probing cell differentiation potential in the mid streak and neural plate yolk sac showed the presence of cells capable to develop into macrophage clonal progenitors [24]. Multi-color fluorescent immunohistochemical analysis revealed the appearance of non-erythroid hematopoietic precursors, which express high levels of CD41 and form clusters around primitive vessels of the yolk sac vascular plexus at E8.25. Very similar clusters of cells expressing high levels of Runx1 (AML1/CBF2α/PEBP2αB) (Fig. 1) were detected in the yolk sac at approximately the same stage [59]. The clusters that originate in the E7.5 hematopoietic band gradually dissociate and release their cells into embryonic circulation during the E9.0–E10.0 period. Some cells that secede from these Runx1high clusters are apparently capable to incorporate into endothelium of large conceptus vessels in vivo [59]. It remains to be determined whether these angioblasts or endothelial precursor cells have hematopoietic potential, but their origin in the hematopoietic band of yolk sac, intense Runx1 expression, and the ability to routinely enter the early circulation point to this probability. If so, the cells apparently represent a long-lasting population of bipotent hemangioblast-like cells, although the full regulative mesodermal potential might be broader for some of these cells and can be disclosed or induced in a suitable assay [22, 61]. The presence of the hemangioblast-like cells in conceptal blood may manifest the stepwise process of definitive hematopoietic lineage specification in which mesodermal precursors are programmed by default to develop into endothelium [62] but subverted to hematopoietic development by upregulation of hematopoietic transcriptional network [63].
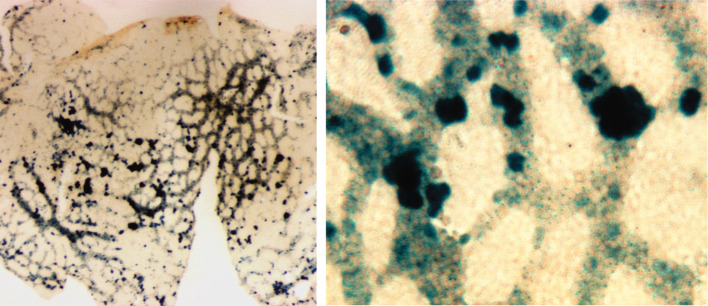
Fig. 1.
Runx1high clusters (β-galactosidase staining) in the E8.5–E9.0 yolk sac of Runx1-LacZ conceptuses [59], at lower magnification (left panel) and at higher magnification (right panel). The vitelline vascular plexus is filled with primitive erythroblasts that downregulate Runx1 at the time when their progenitors disappear. Note that even though all clusters are associated with nascent vasculature, some of them protrude into the avascular area. This is consistent with the model of separate emergence of blood and endothelial cells in the yolk sac
The endothelial differentiation potential of the blood-born Runx1high precursors is evidently crucial for the embryo survival. Severe cerebral hemorrhage develops in the Runx1-null midgestation embryos as an indication of pronounced vascular defects [64]. The causes of the fatal hemorrhage in Runx1-null embryos are still poorly understood. A non-autonomous mechanism for the vascular defects of Runx1 disruption has been proposed in which the absence of Runx1-dependent definitive hematopoietic progenitors (CD45+c-Kit+CD34+ cells) leads to the deficiency in angiopoietin-1 (Ang1) at the sites of angiogenesis [65]. However, it is unclear how diminished angiogenesis can lead to increased vascular leakiness and extensive hemorrhage not only in the embryonic parenchymal tissues but mainly in the central neural system of Runx1-deficient embryos. In an alternative, cell-autonomous model, early Runx1-dependent blood-borne endothelial precursors are able to restore occasional cell deficiencies in the endothelium of large vessels, and perhaps also in rapidly growing embryonic vascular plexus (Fig. 2). The deficiencies are likely to arise during vasculogenesis or result from intensive vascular remodeling in midgestation conceptuses. The inactivation of Runx1 exposes these temporary defects in the nascent vasculature. Interestingly, inactivation of several other key hematopoietic and endothelial genes leads to characteristic intensive hemorrhage, which settles in after the process of vasculogenesis is essentially completed [66–69]. This suggests that developing vasculature requires concerted and continuous molecular efforts to sustain its integrity. Although the cell-autonomous model remains to be supported by direct experimental evidence, the Runx1-null embryo rescue studies demonstrated that the progeny of E7.5 Runx1-positive yolk sac precursors restores the integrity of intraembryonic vasculature and prevents the development of the lethal hemorrhage [34]. Evidently, the cells migrating through circulation can invade the endothelial layer and then “plug” the structural deficiencies in the endothelial layer [59].
Fig. 2.
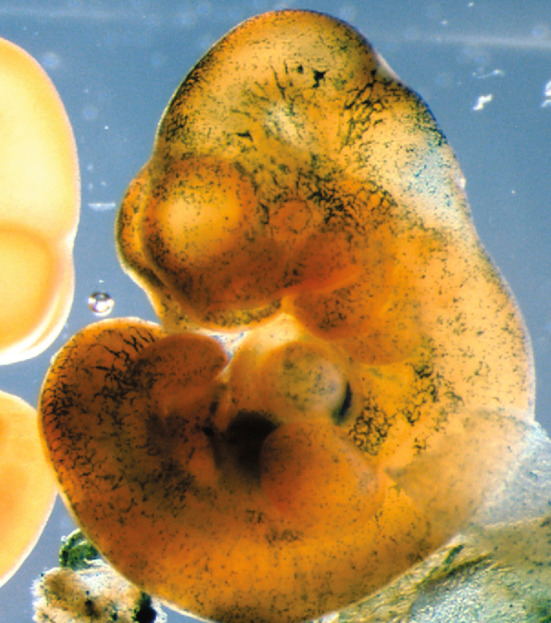
The β-gal-labeled (Rosa 26-LacZ+) progeny of Runx1-positive E7.5 yolk sac cells contributes to head capillaries of the E10.5–E11.0 embryo
Development of the Runx1-null embryos can also be rescued by restoration of Runx1 expression in cells expressing the Ang1 receptor tyrosine kinase Tie2/Tek [70]. This further indicates that the developmental function of Runx1 is required in Tie2+ cells [71]. It is reasonable to assume that these cells are located in the proximal yolk sac, since a large proportion of the hematopoietic band cells are Tie2-positive at E7.5-E8.0 [57] and Runx1-null embryos can be rescued only by pre-E8.0 gene reactivations in the proximal extraembryonic mesoderm [34]. In addition to Tie2 upregulation, the Runx1 + hematopoietic band cells that belong to the HSC lineage apparently express another vascular endothelial marker—VE-cadherin [34]. It therefore becomes evident that the hematopoietic band of the early yolk sac represents a unique transitory cell population, not fully hematopoietically specified but distinct from the endothelial lineage and undifferentiated extraembryonic mesoderm. The band apparently serves as a unique milieu for de novo generation of all definitive hematopoietic lineages including HSCs.
Recent cell tracing and embryo rescue experiments using the Runx1 genetic model have demonstrated that adult-type HSCs develop exclusively from a subpopulation of Runx1+ proximal yolk sac cells. The hematopoietic band of the early yolk sac contains the earliest precursors of the entire HSC lineage, but maturation of the pre-HSCs occurs practically around entire conceptus at the later stages of development. This is consistent with earlier cell potential studies in which CD34 + c-Kit + cells of E9.0 yolk sac were capable to efficiently repopulate adult hematopoietic system after being transplanted into the liver of myeloablated newborn mice [72, 73]. Importantly, the progenitors co-expressing CD34 and cKit were 37 times more abundant in yolk sac compared to the P-Sp, which suggests that the newborn—repopulating or immature HSCs originate in yolk sac and relatively slowly disperse around conceptus [74]. Moreover, orthotopic in utero transplantations of E8.5–E9.5 yolk sac cells led to the long-term repopulation of adult hematopoietic system [75, 76]. Blood circulation at these stages is largely inefficient [74] so that a large proportion of yolk sac precursors still reside at the site of their emergence.
Only complete absence of circulation, however, ensures that no intraembryonic cells arrive in the yolk sac at time of analysis. As it turns out, the ablation of cardiac contractions by Ncx1 (also called solute carrier family 8, member1 protein, Slc8a1) inactivation confirms the key role of the early yolk sac in definitive hematopoiesis. These mutants lack blood circulation between the yolk sac and embryo body though some cell locomotion and a minimal progenitor mixing might be still present. No definitive hematopoietic progenitors were found in the Ncx1−/− P-Sp, whereas the mutant yolk sac contained the normal number of the progenitors [77]. The lack of fluid shear stress itself cannot explain the absence of progenitors in the P-Sp, since the stress has stimulatory rather than inductive effect on definitive hematopoiesis [78, 79]. The blood flow promotes hematopoiesis, but it does not mean that its absence inhibits the generation of hematopoietic progenitors. The data corroborate the notion that definitive hematopoiesis originates in the yolk sac and the intraembryonic hematopoietic locations are seeded by immigrant progenitors. Indeed, despite having essentially normal circulatory system, the GFI1/GFI1b–null embryos completely lack definitive hematopoietic progenitors which, being unable to enter the circulation, become stuck in the yolk sac [80]. The autonomous hematopoietic failure of the P-Sp/AGM region suggests that the definitive progenitor potential observed in the normal regions at E9.5–E10.5 [81] is derived from circulating yolk sac precursors. The definitive hematopoietic potential, however, can be extracted from Ncx1-null caudal halves explanted and cultured on the OP9 stroma in the presence of exogenous cytokines [82], but it is unclear whether the progenitors derive from nascent HSCs or the complex culture system is able to induce ectopic hematopoiesis in the E8.5–E9.5 embryonic tissues. It is worth mentioning in this regard that HSCs fail to develop in the culture of P-Sp/AGM explants dissected at E9.5 [83].
The key element in understanding blood ontogenesis is the observation that progenitor and stem cells originate in vivo intravascularly but function extravascularly [84]. In order to reach the first location of the extravascular hematopoiesis, these emerging progenitor cells have to migrate over a long vascular distance into the venous plexus of fetal liver and extravasate there into the abluminal mesenchyme. It is not surprising that some local accumulations of the immature progenitors on their way to the fetal liver have been regarded as the candidate sites of the autonomous progenitor and HSC generation.
The P-Sp/AGM region: an intraembryonic source of HSCs?
Early transplantation experiments suggesting the yolk sac blood origin were performed with donor chick embryos that had their blood already in circulation [85]. Therefore, due to tremendous mixing of emerging hematopoietic precursors, no firm conclusion could be made. Even after the pivotal experiments of Moore and Metcalf [3], the concept of yolk sac origin of adult hematopoiesis was not unequivocally accepted. The progenitors studied in their work were not bona fide HSCs, and it was not clear whether the embryo culture conditions employed in the study could faithfully reflect the in vivo development. Furthermore, other researchers found that the yolk sac erythropoiesis is irresponsive to erythropoietin (Epo) treatment, whereas Epo alone efficiently induced proliferation and differentiation of intraembryonic erythroid progenitors [86]. Taken together, these observations casted some doubts on the proposed main role of the yolk sac in the establishment of definitive hematopoiesis. In an effort to clarify the situation, Françoise Dieterlen-Lièvre [87] performed grafting experiments with pre-circulation avian conceptuses and analyzed the hematopoietic system of the surviving chimeras. The results of this milestone research indicated the intraembryonic origin of adult-type blood. In a string of later publication, Dieterlen-Lièvre and her colleagues [88–90] further strengthened the original conclusion and suggested that the dorsal aorta is the ultimate source of “intraembryonic stem cells responsible for definitive hematopoiesis”.
The functional definition of LT-HSCs, which was elaborated by the early 1990s, created an obvious curiosity of when and where these strictly defined stem cells first emerge during ontogenesis. The earliest of these cells have been detected in the AGM region, rather than in the yolk sac, of the E10.5 mouse conceptus [6]. However, the efficiency of detecting cells with the adult-type HSC potential at this stage was so low that the authors could not rule out that at least some HSCs emerge in the yolk sac independently. Besides, direct measurement of HSC activity in various parts of the midgestation conceptus could not in principle determine the anatomic origin of these cells due to ongoing interchange of these cells via circulation. When the same researchers used the approach of Moore and Metcalf [10] and cultured the conceptus tissue explants, only the AGM region turned out to be capable to accumulate the HSC activity. Even though strictly defined HSCs have been found to appear at around E10.5 in umbilical and vitelline arteries as well as placental vasculature [11–13], only the AGM demonstrated a robust increase in the number of the HSCs after explant culture [1]. These crucial data laid the ground for currently dominant theory of the midgestation origin of adult-type HSCs [91].
The theory stipulates that adult-type HSCs emerge autonomously from mesodermal precursors of the AGM region and separately from the majority of hematopoietic progenitors. The progeny of the mesodermal precursors develop into adult-type HSCs within hematopoietic intra-aortic clusters (HIACs) when the P-Sp turns into the AGM region. HIACs emerge in the dorsal aorta after E9.5, their size and numbers peak at E10.5 and then drop to just a few single-cell attachments by E14.5 [92]. The presence of the essentially identical cell clusters in the umbilical and vitelline arteries is consistent with detecting an early HSC potential in these vessels [11]. HIAC formation provides a hint on cellular mechanisms of the postulated autonomous generation of adult-type HSCs in the large conceptal vessels.
The identity of the earliest members of the HSC lineage is still debated [1], though it is more frequently thought that they may belong to a particular type of endothelial precursor cells called hemogenic endothelium [7, 92, 93]. In the most accepted version, as long as it can be meaningfully compiled, the precursors of hemogenic endothelium locate in the pre-aortic mesenchyme in the P-Sp, and starting from E8.0 these mesenchymal cells turn into angioblasts (or hemangioblasts, in accord with the tradition) which coalesce and form hemogenic segments of the dorsal aorta endothelium by E10.5. These segments are regarded as being capable to give rise to HIACs which manifest the process of the HSC formation. The clusters therefore are immediate and direct hematopoietic progeny of transitory hemogenic endothelium and indicators of its activity [92].
Several lines of evidence support the described above chain of events. Dissected before the onset of circulation, the P-Sp demonstrates an exclusive lymphohematopoietic and a conditional HSC potential (with severely immunodeficient recipients) after a brief explant culture [33, 94]. The existence of vascular endothelial cells possessing hematopoietic differentiation potential had been proposed by Sabin in 1920 [48]. Cells with similar characteristics were found in E9.5 mouse conceptuses by Nishikawa’s laboratory some 15 years ago [95]. These hemogenic endothelial cells express VE-cadherin, CD34, Flk-1, PECAM (CD31), and turn out to be negative for pan-leucocyte and erythroid markers, CD45 and Ter119, respectively. Equivalent cell population has been found in the planar ES differentiation system, and clonal analysis suggested that hemogenic endothelial cells are derived from hemangioblasts [96]. This has been recently confirmed in the hemangioblast differentiation model using another set of markers [55]. It was shown that hemogenic endothelium is an intermediate step between hemangioblast and definitive hematopoietic cells, and SCL but not Runx1 is required for its formation.
Upregulation of the VE-cadherin expression has been regarded as the most characteristic event during hemogenic endothelium formation, and this assumption is being used to investigate the role of hemogenic endothelium in the HSC development in vivo. Runx1 ablation in VE-cadherin+conceptal domain resulted in complete loss of definitive hematopoiesis and development of the characteristic lethal phenotype of Runx1-deficient embryos [97]. Importantly, when Runx1 was deleted in fully committed Vav + hematopoietic cells, the hematopoietic progenitors and dHSC were still able to develop. These findings directly supported the notion that HSCs are descendants of hemogenic endothelium and suggested that Runx1 is required only for the endothelial to hematopoietic cell transition. Unfortunately, the published data do not provide information on the conceptal locations that accommodate the hemogenic endothelium phase of the HSC lineage. The data therefore are essentially consistent with the key role of VE-cadherin+ yolk sac hematopoietic band in the development of definitive hematopoiesis and HSCs.
In another study, the descendants of cells expressing VE-cadherin during midgestation were found in all major adult hematopoietic organs and blood cell lineages including definitive HSCs [98]. The results of these non-invasive, inducible cell labeling experiments were to offer the strongest argument in support of the hematopoietic endothelium concept. However, to drive the ligand-dependent Runx1 deletion, this study utilized a randomly integrated and leaky [99] Cre-ERT2 transgene under a minimal VE-cadherin promoter. The transgene expression might not reflect the VE-cadherin + domain with sufficient fidelity and its cell tracing specificity is therefore questionable. Moreover, it is getting clear that VE-cadherin is not a specific marker of vascular endothelial cells, since some non-vascular cells in the early conceptus and the fetal liver are also VE-cadherin-positive [34, 57, 100]. Thus, the cell tracing or conditional gene ablation experiments based on VE-cadherin promoter-driven Cre-recombinase constructs are not suitable for demonstrating the existence of hemogenic endothelium.
If non-vascular VE-cadherin-positive hematopoietic precursors in the early yolk sac [34] are the founding members of definitive hematopoiesis, it casts serious doubt over the validity of the entire concept of hemogenic endothelium. It is unclear what role, if any, VE-cadherin has in the hematopoietically committed non-vascular extraembryonic mesoderm, but it is known that the function of VE-cadherin is not restricted to determination of endothelial cell contact integrity and permeability of endothelium. The ectopic expression of VE-cadherin in non-endothelial cells has been shown to suppress cell proliferation [101]. It was suggested that VE-cadherin could transfer growth inhibitory signals through association with catenins, β-catenin in particular, or other cytoplasmic effector molecules [102]. In this regard, it is tempting to think of VE-cadherin expression in the yolk sac progenitors as one of the mechanisms for regulating the definitive wave of hematopoiesis. Although the VE-cadherin is not critical for the emergence definitive hematopoietic progenitors [103, 104], its expression may delay the advent of the definitive blood lineage in order to coordinate it with the development of the fetal liver. Further research would help to clarify this issue.
Since VE-cadherin-positive hematopoietic precursors of the yolk sac are not immobilized within endothelial layers, they can migrate through the circulation towards fetal liver and give rise there to the VE-cadherin+ HSCs [100]. The lineage relationship between these two cell populations should be, however, determined experimentally. It is necessary to mention in this regard that groups of cells expressing high levels of VE-cadherin can be seen in the intraluminal space of the yolk sac vascular plexus as late as E9.5, and these cells are distinct from primitive blood cells and the plexus endothelium [95 (Fig. 1F therein)]. This might be indicative of sustained expression of VE-cadherin in the hematopoietic precursors migrating towards their fetal liver destinations. The intraluminal aggregations of VE-cadherin+ cells in the yolk sac are reminiscent of HIACs and the cell clusters of vitelline and umbilical arteries.
Despite the substantial body of supporting experimental evidence, the hypothesis of direct derivation of HSCs from large midgestation vasculature or subjacent mesenchyme is increasingly vulnerable. The avian “yolk sac chimera” approach in addition to some technical uncertainties suffers from a major weakness that it does not take into account the developmental potential of the grafts. Similar to mammals the early avian development is largely regulative [105] meaning that gastrulating intraembryonic graft might grow its own extraembryonic mesoderm after transplantation. Proper cell tracing analysis has to be performed to make any reliable conclusions on the source of adult hematopoiesis in birds.
It is far from being certain that the hematopoietic progenitors emerge through HIACs. No direct measurements of the HSC potential of the clusters have been performed. It is still not very clear how these clusters look like in the live and intact mouse embryo. While only ventral domain of the dorsal aorta has a capacity to induce HSC formation, HIACs has been found attached to all aspects of the vessel [106]. The number of the dorsal aorta HIACs does not correlate with the repopulation potential of the AGM’s HSCs [107]. When the HSC activity peaks in the 47–48 somite pairs (sp) AGM region, the number of large (less accidental) aortic HIACs decreases [92, 107]. The HIAC dynamics fits better with the ability of the AGM cells to develop HSC activity in the explant cultures. The explant culture of the AGM region, however, seems to be a very crude surrogate of the in vivo development. For example, the ability of the AGM to form CFU-Cs during explant culture clearly drops in the older regions (45–49 sp) despite their relatively high content of mature HSCs and efficient expansion of their HSC potential by the ex vivo culture [107]. In other words, the AGM region’s HSCs lose their capacity to differentiate into hematopoietic progenitors during extended four-day culture. It is sensible to interpret these data as evidence that instead of mature HSCs the AGM region accommodate progressively specifying precursor cells which are competent to develop either into CFU-Cs or infrequently into HSC-like cells depending on the assay conditions. The difference between this interpretation and the “AGM theory” is that these precursors are pressed to become HSCs, and may never turn into stem cells in vivo.
Many HIACs and cell clusters inside of umbilical/vitelline arteries comprise so many cells after just a single day since their inception at E9.5 [92] that they are very unlikely to form through cell proliferation. The explosive HIAC budding suggests non-proliferative causes for their formation, either through sub-aortic mesenchyme migration and sprouting [108], vascular remodeling [109] or clustering of blood-born cells on the endothelial surface [59]. To keep up with the AGM-centered model of hematopoietic development, vascular remodeling might be considered as the preferred mechanism by which hemogenic endothelium can form HIACs, because the dorsal aorta endothelium in midgestation is clearly devoid of active cell proliferation [110, 111]. Recent reports on efficient expansion of the HSC potential in cultured dissociated-reaggregated AGM regions [108, 112] strongly suggest, however, that despite its evidently important role in the HIAC formation, vascular remodeling is not involved in the HSC generation. Indeed, even though reaggregated embryonic explants can partially restore the spatial organization of native tissues [113], the AGM region reaggregates apparently do not reinstate their vascular structures [108].
In an alternative AGM-compatible mechanism for the stem cell generation, the incorporation of subendothelial mesenchymal precursors into endothelial layer is followed by their non-proliferative protrusion into the lumen. Subaortic patches (SAPs) were implicated in the de novo generation of HSCs as starting point for pre-HSC migration towards HIACs [114]. However, it is hard to rule out migration in the opposite direction, so that SAPs are formed by blood-born hematopoietic precursors extravasating into the abluminal mesenchyme and acquiring there the pre-HSC phenotype [115]. The absence of the de novo generation of HSCs beyond E8.0 [34] indicates that SAPs are likely to be formed by descendants of the transmigrating precursors.
The intra-aortic clusters disappear in Runx1-deficient embryos and this evidence was used to invigorate the idea of hemogenic endothelium and to suggest the direct regulation of the hypothetical endothelial-hematopoietic transition (EHT) by Runx1 [111]. The EHT has been recently demonstrated in zebrafish development [109, 116], whereas it is difficult to prove the existence of such a process in mice [117]. A deeper insight into the cellular mechanisms of the EHT exposed its close relationship with negative remodeling (regression) of the dorsal aorta [109]. At the same time, inactivation of Runx1 in the zebrafish embryo caused an abrupt apoptosis of seceding endothelial cells, but it is hard to rule out a possibility that cells in the normal embryo can also enter a milder apoptotic pathway after their secession. In this regard it is worth to note that in a rare, if not unique, electronic microphotograph of the mouse HIACs, the “budding” endothelial cells clearly show the specific signs of progressive apoptosis: cell shrinkage, cytoplasm blebbing, nuclear pyknosis with dense chromatin patches against the nuclear envelope [111 (Fig. 3 therein)]. Although it remains to be investigated whether the aorta clustering is caused by negative remodeling and apoptosis, postnatal arterial regression in large mammals is manifested by the appearance of apoptotic cell clusters [118]. In the embryonic dorsal aorta, the apoptotic clusters could be further enlarged by retention of circulating immature macrophages.
Molecular studies of the midgestation AGM region were attempted to support the notion of the intraembryonic origin of HSCs. Bmp4, the early stimulator or inducer of hematopoietic lineage specification via Cdx-Hox pathway [119], shows strong localized expression in the ventral sub-aortic mesenchyme [120]. Furthermore, Gli1, the mediator of Hedgehog signaling is also expressed in the mesenchyme surrounding the dorsal aorta suggesting a role for Hedgehog in the polarized induction of the HSC emergence [121]. Considering that the Hedgehog-Foxf1-Bmp4 signaling cascade was postulated to operate in the murine vasculogenesis [122], the proposed origin of HSCs in the AGM region is seemingly substantiated by a feasible molecular induction mechanism. The experimental evidence pointing to the Bmp4-dependent generation of HSCs in the AGM region is, however, far from being compelling. Focal expression of Bmp4 underneath of the aorta is not necessarily connected to the HSC formation, because this growth factor, apart from its key role in the initiation of hematopoietic development, is known to participate in Müllerian duct regression, vasculogenesis, and intestinal epithelium maintenance [123]. Bmp4 itself had statistically unreliable effect on HSC production during explant culture [120]. The more pronounced inhibition of this production by highly concentrated Gremlin (Grem-1), a Bmp antagonist, is likely to be explained by Gremlin’s wide range of activity in mesenchymal cells including suppression of the Wnt/β-catenin signaling [124]. Moreover, Gremlin was recently found to be capable of binding the key provasculogenic/proangiogenic receptor Flk1 (KDR) and to induce its autophosphorylation, which led to Flk1-dependent endothelial cell responses in vivo and in vitro [125]. The dorsal aorta endothelium is Flk1-positive in the E11.5 AGM region [115] and therefore may respond to the Gremlin treatment by endothelial cell expansion at the expense of the HSC potential.
Runx1 as well as Sca-1 (Ly-6A/E), a GPI-linked cell surface glycoprotein, are expressed in non-polarized fashion in the dorsal aorta endothelium at the AGM stage [34, 59, 126]. Sca-1 is one of the cell surface makers that are most frequently used to enrich adult murine HSCs. The overlapping expression of the key hematopoietic transcription factor and the common HSC marker in the dorsal aorta is in line with the proposed aorta’s role in the autonomous HSC emergence. However, Runx1 is one of the iconic pleotropic transcription factors; it is expressed during specific stages of development of many tissues and cell populations [111] and plays developmental roles in several organs, by affecting cell survival, proliferation, and differentiation [127–130]. The HSCs in the dorsal aorta were shown to be Runx1+ [115], but most of the aortic Runx1 expression might not be associated with hematopoiesis. Indeed, when the hematopoietic progenitors and HSCs completely disappear due to arrest of their development at the yolk sac stage, the dorsal aorta endothelium continues to transcribe the Runx1 locus [34, 111]. In an alternative explanation, transiently Runx1-expressing endothelium of the aorta might represent a progeny of circulating hemangioblast-like precursor cells, which are engaged in vascular development and maintenance. These precursors can be retained by the endothelium using a number of adhesion molecules, and Sca-1 is one of them because it is widely believed that receptor-ligand interactions underlie the function of Ly6 proteins, potentially mediating cell–cell adhesion and signaling [131].
Notch signaling is also thought to be essential for HSC emergence in the dorsal aorta. Notch1-deficient E9.5 yolk sac and P-Sp cells failed to contribute to adult hematopoiesis through newborn recipients [132]. Correspondingly, Notch1−/− ES cells could not produce adult hematopoietic cells in wild-type chimeras [133]. However, the valuable information on the role of Notch receptors in the early hematopoiesis provided by Kumano and colleagues [132] can be interpreted differently compared to the published version. The hematopoietic progenitor potential measured in a two-step assay (OP9 stroma co-culture followed by methylcellulose assay) was almost completely extinguished in the Notch1-deficient P-Sp and only slightly decreased in the yolk sac. At the same time, the mutant conceptuses demonstrate a dramatic expansion (20-fold) of c-Kit+CD34− cells and concomitant fourfold increase of c-Kit+CD34+ hematopoietic progenitor population in the E9.5 yolk sac [132 (Fig. 3 therein)]. The same was true for the VE-cadherin-positive population (threefold increase) which contains, as discussed above, the earliest definitive hematopoietic progenitors. Notch1 therefore controls proliferation of extraembryonic mesodermal and endothelial cells as well as nascent definitive hematopoietic progenitors. This conclusion is supported by the observation that after acquisition of arterial identity by major embryonic vascular beds, the tissue-specific loss of Notch1 function leads to expansion of definitive hematopoietic progenitors (Ann Zovein, personal communication). It is conceivable that uncontrolled expansion of committed hematopoietic progenitors in the mutants leads to a loss of their newborn repopulating potential first in yolk sac and then in the P-Sp. This process is also manifested by loss of the methylcellulose progenitor activity during the lengthy OP9 culture of Notch1-null cells. All progenitors in the E9.5 P-Sp are immigrant and therefore they almost completely lose their clonogenic potential in the absence of external supply of undifferentiated progenitors. The moderate effect in the yolk sac culture can be explained by ongoing generation of definitive hematopoietic progenitors which to some extent compensates the Notch-dependent loss.
Definitive hematopoiesis of Notch1-null mutants can be rescued by retroviral Runx1 overexpression in the stromal culture of the E9.5 P-Sp cells [134]. This has been considered as a proof of independent origin of P-Sp/AGM hematopoiesis, in which the key hematopoietic factor Runx1 is regulated by Notch signaling [1]. Unexpectedly for the investigators, similar overexpression of SCL and GATA2 had no effect on the Notch1−/− P-Sp hematopoiesis [134], despite previous observations suggesting that these genes are also regulated by Notch pathway [132]. The failure of the GATA2 overexpression is puzzling since it has been demonstrated that the gene’s promoter is direct target of the Notch1 transcriptional complex [135]. Perhaps the hematopoietic defects cannot be rescued by overexpression of GATA2 alone because it is only one of several hematopoietic factors regulated by Notch signaling. However, complete rescue by overexpression of a single hematopoietic gene, Runx1, which in fact is the downstream direct target of the SCL-GATA2 multiprotein complex [136], suggests that a simple scheme of Notch signaling being the master regulator of developmental hematopoietic transcription network is too simplistic and has to be reevaluated.
Runx1-mediated hematopoietic rescue of the Notch1 deficiency does not necessarily suggest that Runx1 overexpression overcomes the block in autonomous generation of HSCs in the mutant P-Sp/AGM region. Overexpression of Runx1 has been shown to alter the normal course of DP thymocyte differentiation driving them to preferentially develop into immature CD8 SP thymocytes even in the circumstances in which these thymocytes do not arise [137]. The data suggest that the increased dose of Runx1 may lead to a partial epigenetic reprogramming of certain cell lineages. Similar reprogramming activity has been observed when PU.1, a direct target of Runx1, is ectopically expressed or overexpressed in mouse fibroblast lineages [138]. This highlights an increasingly common phenomenon in which the induction of key transcription factors has a potential to drastically change the epigenetic status of target cells [139–142]. Thus, when retroviral Runx1 overexpression “rescues” the hematopoietic development in the P-Sp/AGM region of Notch1-null or Runx1-null embryos [81, 143], it is more likely to be due to reprogramming of some endogenous mesenchymal precursors into hematopoietic progenitors. The retroviral transgene turns out to be much more efficient in changing cell fate than a tetracycline-regulated transgene lacking the coding sequence for N-terminal region of the factor [144].
In summary, it is difficult to find reliable cellular and molecular mechanisms for the proposed autonomous hematopoiesis and/or HSC generation in the AGM region. Nevertheless, it seems that the dorsal aorta, in contrast to other vascular territories which contain nascent HSCs, is not simply a conduit for hematopoietic progenitors. Special affinity of migrating progenitors to aortic endothelium can be seen as an atavism, a reminder of the important role of mesonephros as a niche for definitive hematopoiesis in fish. Mammals, however, adapted another fetal organ to serve as a central hematopoietic niche during ontogenesis—the fetal liver.
Fetal liver hematopoiesis
The fetal liver is the key hematopoietic organ and the main site for maturation, expansion, and differentiation of fetal HSCs; it is the first location of the adult-type hematopoiesis. The fetal liver hematopoiesis, however, is completely dependent on the immigrant hematopoietic precursor cells [145, 146], and it is generally believed that it cannot support de novo generation of hematopoietic progenitors and HSCs.
Fetal liver organogenesis starts in mouse embryos of 4–7 sp (E8.25–E8.5) [147], when the cells in ventral wall of the foregut endoderm receive the inductive signals from the developing heart and initiate their specialization toward hepatic fate. The primary liver bud can be identified as an anatomical outgrowth from the ventral wall of the foregut in 10–12 sp mouse embryos (around E8.5). During E8.75–E9.25 the hepatic epithelium thickens and transitions from a columnar to a pseudostratified epithelium. By E9.5, when mouse embryos contain 15–20 sp, the basement membrane of the endoderm epithelium disintegrates, and the liver bud cells delaminate into the surrounding septum transversum mesenchyme forming cords of primary hepatoblasts. The early hepatic morphogenesis is promoted by mesenchymal angioblasts and nascent vascular structures independently from their oxygenation or blood cell-providing function [148]. It seems that Bmp4, in concert with FGF8 (or some other FGFs), constitute morphogenic or growth promoting signals emanating from angioblasts and endothelial cells [149].
The fetal liver starts to be colonized by circulating hematopoietic progenitors at E9.5–E10.0, 22–28 sp [145], almost immediately after the hepatoblast cords start to form. Between E10.0 and E15.5, the liver bud experiences a period of accelerated growth as it becomes progressively vascularized and colonized by definitive hematopoietic progenitors, which undergo intensive proliferation starting from E11.5 [150]. The hematopoiesis in the early fetal liver, when HSC activity is not yet present or it is very low, is conspicuously dominated by definitive erythroid progenitors (CFU-Es and BFU-Es, burst forming unit—erythroid) and their descendants—proerythroblasts. This strongly suggests that these progenitors are not HSC-derived and represent a separate cell lineage originating in the yolk sac, which has been elegantly confirmed in the recent hematopoietic rescue experiments [151]. Myeloid and lymphoid cell lineages develop slower and never reach the same density as erythroid counterparts. The first HSCs appear in the fetal liver around E11.5 and then their numbers expand precipitously during the next day, reaching the level of 50 HSCs per liver [152]. Cell proliferation cannot explain such dramatic increase of stem cell numbers, and it has been presumed that some mature HSCs arrive in the fetal liver from the AGM region, placenta and yolk sac. However, these sources of the “ready-to-use” HSCs are not exhausted or attenuated on E12.5 as they should have been in order to provide for the overwhelming growth of HSC activity in the E12.5 fetal liver [12, 152]. This observation suggests that the fetal liver can create its own pool of HSCs from immigrant pre-HSCs. Notably, the generation of HSCs has been confirmed in the recent report that the fetal liver microenvironment supports maturation of pre-HSCs which originated from early hematopoietic sources of the conceptus [153].
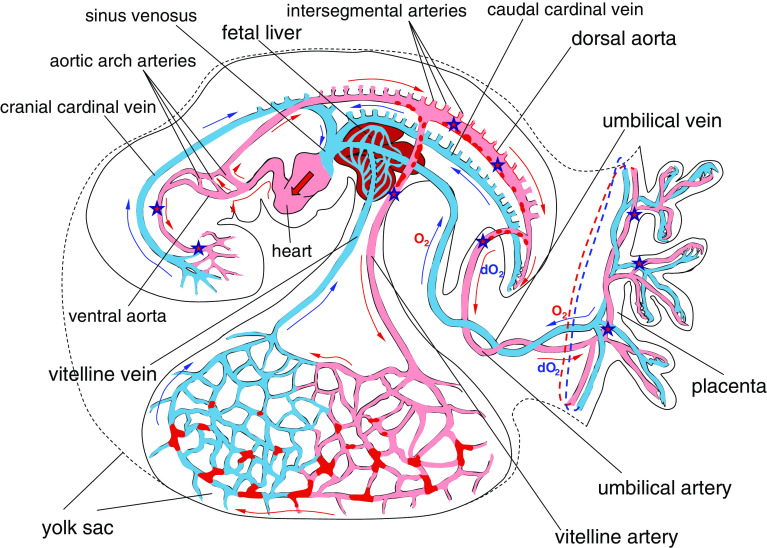
The routes for the fetal liver delivery of hematopoietic progenitors are vitelline and umbilical veins (Fig. 3). Vitelline veins play a primary and major role in the fetal liver vascularization, and it is thought that the liver sinusoids are largely derived from the paired vitelline veins [154]. Early and direct vascular connection between yolk sac and fetal liver is consistent with the idea that the fetal liver hematopoiesis is initiated by the yolk sac hematopoietic precursors which include immature HSC. Any cell transport from the AGM region toward the liver vasculature would require the migrating progenitors to traverse complex capillary networks of either placenta or the yolk sac (Fig. 3). Developing pre-HSCs are CD45 + VE-cadherin+ [108, 155] and also bear other endothelial adhesive molecules [83, 92] making them “endothelium-sticky” especially in the low shear-stress conditions of small vessels. In this circumstance, the capillary networks would seem to be poorly penetrable for the AGM-derived cell traffic.
Fig. 3.
Scheme of blood circulation in the E9.5–E10.5 mouse conceptus. Red patches in the yolk sac denote Runx1+ or CD41+ hematopoietic cell clusters; red dots in the dorsal aorta, umbilical and vitelline arteries—HIACs; red arrows show the blood flow in the arteries and blue arrows in the veins. O2 and dO2 denote oxygenated and deoxygenated blood, respectively. Star symbols designate the conceptal territories where the earliest HSCs were detected
In the yolk sac—primed scenario, a subset of yolk sac precursors becomes retained in early liver bud though some hematopoietic precursors escape due to yet poor branching of vitelline veins. The escapees are pumped by heart into head capillaries and settle there, giving rise to such cell lineages as microglia [156]. Not all precursors are lost in the head since there is a shortcut route of aortic arch arteries allowing them to enter the dorsal aorta (Fig. 3). Then the hematopoietic precursors interact with endothelium of the dorsal aorta, umbilical and vitelline arteries, forming arterial hematopoietic cell clusters on the way. Eventually they end up in the placental vasculature and in yolk sac capillaries, at which time some of the clustered precursors are mature enough to acquire the HSC potential before or during cell transplantation assays. The cycle is repeated until almost all progenitors colonize the fetal liver. Notably, the described pathway explains comparatively high levels of the stem cell activity in circulation and a surprising capacity of the E12.5 yolk sac to expand the HSC potential in the explant culture [152]. Moreover, a low number of hematopoietic precursors demonstrating HSC potential have been recently found in the E10.5–E11.5 embryo head [9], which is consistent the idea of gradual spread of pre-HSCs all over the developing conceptus.
The hallmark feature of the fetal liver hematopoiesis is that, for the first time in the mammalian ontogenesis, the blood cell generation is fully extravascular. The transition from the intravascular hematopoiesis of the yolk sac and the intraluminal modus vivendi of early developing progenitors towards the mesenchymal hematopoiesis of the fetal liver requires a special cellular mechanism for the efficient transendothelial migration of the progenitors. The traffic has to be initiated at the specialized endothelium of liver sinusoids which is not only adapted to intensive molecular exchange but also may facilitate the blood cell extravasation. Adult liver sinusoids are characterized by their fenestrated and even discontinuous endothelial lining, which is devoid of well-organized basement membrane. This specialization, however, occurs relatively late in development [157]. Transendothelial migration of definitive progenitors at the early stages of development has to be explained therefore as a manifestation of a process similar to acute inflammation. Moreover, the massive, selective and directed extravasation of hematopoietic progenitors in the liver bud can be reliably described only in terms of the inflammatory response. If putative ontogenic inflammation mechanisms are to be compiled in a hypothesis, one would assume that selective extravasation of multipotent definitive progenitors through the activated embryonic endothelium is one of the key events in the hematopoietic development. In fact, the inflammatory concept can explain the great majority of events during the establishment of definitive hematopoiesis.
Inflammation model of the developmental hematopoiesis
Accumulated to date experimental evidence offers a possibility to propose a mechanistic model of hematopoietic development in mammals. Its purpose is to explain and systematize diverse sets of data into a unifying testable hypothesis that can be used for predicting experimental observations and prospective technological applications. In this model, inflammatory and stress responses are the driving force for the hematopoietic and endothelial development. The ontogenic inflammation can be described as non-infectious (aseptic) systemic inflammatory response by innate immune cells to localized damage and stress caused by developmental apoptosis and hypoxia. It is induced by released intracellular constituents, proceeds as active phagocytosis and is followed by a resolution and tissue-repair phase. An important aspect of the ontogenic inflammation is that it has to be tightly controlled and progressively suppressed by negative regulators. The inflammation mechanisms are operative on the relatively early stages of hematopoietic ontogenesis when the available professional macrophages are at scarce and overwhelmed by massive developmental apoptosis [158–161]. At these stages, the hypoxic cellular stress is exacerbated in the absence of well-developed and organized vasculature.
A large body of direct evidence supports the proposed inflammatory model of blood cells ontogenesis. Inactivation of β1 integrin—a major modulator of inflammatory leukocyte extravasation—leads to the failure of embryonic hematopoietic progenitors to seed fetal hematopoietic organs [162]. Moreover, the lack of interaction with endothelium makes the β1 integrin-null hematopoietic progenitors unable to develop properly [162] so that they completely disappear in adult mice [163].
Prostaglandin E2 (PGE2), a potent vasodilation mediator and regulator of inflammatory cytokine production [164], was shown to stimulate generation of HSCs and multipotent hematopoietic progenitors in the zebra fish AGM region as well as in differentiating murine ESCs and in adult bone marrow [165]. Furthermore, the cyclooxygenases responsible for PGE2 synthesis were required for the progenitor formation in the mouse ESC differentiation assay. However, when the concentration of the PGE2 in the culture medium was increased beyond a certain threshold, the ESC-derived definitive hematopoiesis was apparently suppressed [165 (Fig. 3 therein)]. This is consistent with the inhibitory role of the high levels of PGE2 in inflammation [166] and with generally context-dependent mode of the PGE2 function [167]. It would be interesting to investigate which function of the vast PGE2 regulatory arsenal is responsible for the hematopoietic effect in the ESC differentiation context. PGE2 is produced in vivo by the cells of innate immunity [167], thereby it is reasonable to assume that its generation can be initiated by early hematopoietic progenitors of the developing conceptus.
One of the pro-inflammatory cytokines interleukin-1 (IL-1) and the molecules that are essential in the IL-1 signaling pathway were found to be expressed and regulate myeloid differentiation and the HSC activity in the E11–E12 AGM region [168]. IL-1 signaling was shown to induce matrix metallopeptidase 9 (MMP9) in human umbilical vein endothelium and vascular smooth muscle cells [169] and to upregulate the proteinase in the mouse AGM explant cultures [167]. MMP9, in turn, controls the ability of innate immunity cells to migrate across extracellular matrix [170].
ADAR1, an essential suppressor of interferon signaling was recently found to be required for fetal liver hematopoiesis [171]. It is well known that interferons play intricate roles either of support or inhibition of inflammatory reactions depending on the timing of their expression and the cellular context [166]. Eotaxin (CCL11), a potent leukocyte chemoattractant upregulated during inflammation, is expressed in midgestation yolk sac and fetal liver and promotes differentiation of embryonic myeloid progenitors [172]. Also it has long been known that the key inflammatory mediator TNF-α and its receptors, as well as some other key inflammation molecules, are expressed at high level in mouse embryo circulation, the early yolk sac and fetal liver [173]. It has been recently reported that GATA3-dependent stimulation of the HSC potential in the AGM region is associated with the role of GATA3 in the production of catecholamines, the mediators of sympathetic nervous system [8], and important modulators of immune and inflammatory cells [174]. It has to be noted that catecholamines are known to enhance inflammatory cell retention by murine vascular endothelium and stimulate the expression of cell adhesion molecules by innate immune cells [175].
A revealing evidence supporting the idea of inflammatory hematopoietic ontogenesis can be envisaged from recent publications on the role of hydrodynamic or shear stress in promoting embryo hematopoiesis [78, 79]. Possible mechanisms of the stimulation are thought to involve a powerful inflammation mediator NO (nitric oxide) [176], synthesized by endothelial cells in response to pulsative blood flow. Notably, endothelial NO synthase gene (Nos3) was found to be highly expressed in the endothelial cells lining the E11.5 dorsal aorta [79], suggesting the involvement of inflammatory mechanisms in the maturation of HSCs. Nitric oxide was found to be capable of direct stimulation of the hematopoietic progenitor expansion [177]. However, the NO-based model needs to be supplemented with more intricate molecular and cellular mechanisms to explain the requirement of a certain level of shear stress for efficient stimulation [78]. A more comprehensive explanation comes from the fact that the shear stress of the optimal strength is required for efficient selectin-dependent leukocyte tethering and stable “rolling” on endothelium [178, 179], which are the hallmarks of inflammation process. Too low or too high blood flow shear decreases the extent of leukocyte interaction with the activated endothelium. The stimulation of hematopoiesis by a certain sheer stress level therefore suggests that definitive hematopoiesis development depends on blood progenitor tethering and rolling. These cell–cell interactions might be mediated by inducible endothelial P-selectins and constitutive endothelial E-selectins, as well as L-selectins expressed on circulating conceptal progenitors in a similar way as on adult blood leukocytes [180]. It is conceivable that stabilized progenitor–endothelium interactions allow the dorsal aorta to retrieve considerable numbers of hematopoietic progenitors from circulation.
The inflammatory model of hematopoiesis helps to put forward another mechanism for the aggregation of blood-born progenitors on the dorsal aorta endothelium. In developing vessels with the optimal local blood pressure the progenitors roll stably, but when the pressure drops down below a critical level, the rolling progenitors form homotypic cell aggregations due to local low-shear environment [181]. Greatly dilated midgestation dorsal aorta which sprouted numerous intersegmental arteries by E10.5 provides such kind of environment because the blood flow velocity, and correspondingly the shear stress, is critically dependent on the sum of local vasculature cross sections. The aggregation–promoting environment is likely to spread to adjacent proximal regions of vitelline and umbilical arteries.
Non-invasive in vivo cell tracing experiments have directly demonstrated the attachment of developing circulatory hematopoietic precursors to vascular beds in midgestation conceptus [59]. The labeled progeny of blood islands cells was observed to extravasate through the endothelium into underlying mesenchyme in a process that is apparently reminiscent of leukocyte extravasation during inflammation (Fig. 4).
Fig. 4.
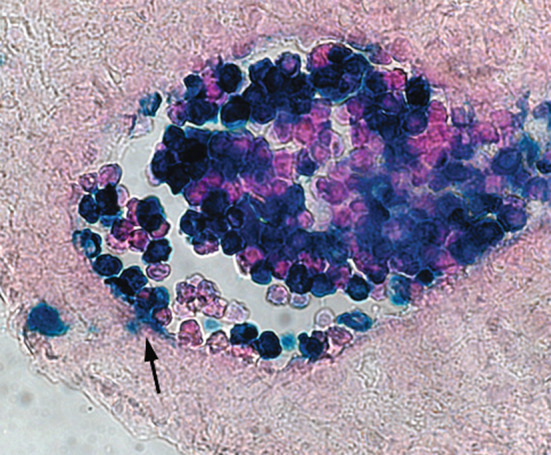
Extravasation (arrow) of the E7.5 yolk sac-derived blood cells (Rosa26-LacZ-labeled) in the proximal region of umbilical artery at E11.5
In the ontogenic inflammation model the driving force behind hemoendothelial development is a controlled inflammatory response to hypoxic condition [182, 183] and to massive morphogenetic apoptosis which overwhelms the macrophage resources [161] that are available in the midgestation conceptus. Hypoxia-inducible factor HIF1, the central regulator of hypoxia response [184], and NF-κB, the “smoke” detector of cellular stress [185] may play the role of molecular triggers, which help to initiate the following inflammatory ontogenic processes:
Cellular stress and extensive ontogenic apoptosis in extravascular regions of the early circulatory conceptus [186] activates chemokine production by mesenchyme cells which can function as non-professional phagocytes [187]. Increasing concentrations of the chemokines stimulate transmigration of first committed hematopoietic precursors into abluminal space.
Developing hematopoietic precursor cells are tethered and induced to stably “roll” on activated endothelial cells. The tethering provides the intravascular niche for the precursors, promotes proper maturation of their hematopoietic progenitor capability, and with the use of Notch signaling may prevent them from spontaneous terminal or abnormal differentiation.
Inflammation induces vascular remodeling and angiogenesis through upregulation of potent endothelial growth factors such as VEGF [188]. In the intraembryonic context, the local upsurge of the endothelial growth factors results in the incorporation of “rolling” precursors into expanding endothelium [59]. This type of vasculogenesis may assist angiogenesis if it proceeds at the sites of vessel sprouting, or it serves to prevent vessel clogging when excessive homotypic aggregation of circulating precursor cells occurs. It is conceivable that embryonic blood-borne precursors also contribute to pericyte or vascular smooth muscle cell populations.
Inflammatory blood vessel dilation promotes the establishment of blood circulation in early embryo. Also, the inflammatory chemokine gradient attracts motile yolk sac hematopoietic precursors directly into the fetal liver and then into intraembryonic vascular regions.
Increased density of the phagocytes in diverse mesenchymal sites such as the fetal liver bud or subaortic region further stimulate hematopoietic precursors in circulation to initiate selective extravasation. It first occurs in the liver bud since it is directly connected to the hematopoietic origin through afferent vitelline vein. Intensive diapedesis of developing hematopoietic progenitors through fetal liver sinusoid endothelium establishes the first active site of extravascular hematopoiesis. This response quickly accelerates and becomes self-perpetuating for some period of developmental time.
The remodeling-associated apoptosis in large conceptal arteries further promote the tethering of non-professional phagocytes or early macrophage cells. These cells might contribute to HIAC/arterial cluster formation at E10.5. On the other hand, blood-borne hematopoietic precursors tend to aggregate in the low-shear environment of extensive capillary network of head, placenta and eventually yolk sac, which leads to formation of the HSC potential there [9, 13, 152].
Extensive inflammatory cytokine and chemokine signaling facilitate the epigenetic changes in the early committed precursors. This leads to gradual differentiation of nascent hematopoietic cells and specialization of the cell lineages including the HSC lineage. Evidently, the adult-repopulating capacity of HSCs has a propensity to develop efficiently within large aggregations of hematopoietic precursors or progenitors in the presence of endothelial cells [108]. Such aggregations occur naturally in the fetal liver at the onset of the adult-type hematopoiesis.
Some hematopoietic precursors serving as non-professional phagocytes become more specialized and turn into resident tissue macrophages [156, 189]. These cells are better suited for cleaning up apoptotic and necrotic cells throughout the conceptus. Increasing numbers of macrophages in embryonic tissues helps to suppress inflammatory signaling in the later development.
Currently, the notion of the inflammatory blood development is little more than an educated conjecture, but analysis of available experimental data suggests that it can explain or agree with practically all observations of developmental hematology. Although further studies are required to determine the relevance of the ontogenic inflammation for the hematopoietic ontogenesis, a large amount of accumulating evidence in its support is highly unlikely to be a chain of mere coincidences.
Conclusion
In the quest to understand the principles of hematopoietic development, extraordinary insights have been made during the last century. However, at the next level of untangling complex cellular interactions and providing adequate models for molecular studies, the classical hematology approaches are getting increasingly unreliable. Numerous contradictions and inconsistencies in the field to a large extent result from unrestricted reliance on standard cell potential studies. A critical reappraisal of the published data revealed that the midgestation HSC origin model is vulnerable and essentially unsustainable. One of the important deficiencies of the model is the difficulty with ascertaining basic cellular mechanisms of HSC or progenitor emergence.
Recent cell-tracing experiments revived a historic understanding that the common origin of all blood cells locates in the early visceral yolk sac. In the current version of the yolk sac-centered concept, the precursor cells of the early conceptus are essentially committed to hematopoietic lineage, but their actual fate is not fixed up until the fetal liver stage. Gradual specialization and selection of the precursors lead to the formation of hematopoietic progenitors and HSCs at the stage when the fetal liver hematopoiesis starts to operate. Before this extravascular stage of hematopoiesis the precursors possess only a certain potential to become progenitors, and this potential is realized with varying penetrance and efficiency in the standard hematological assays. This concept resolves the paradoxical “reverse order” of hematopoietic progenitor development with the assumption that there are no bona fide adult-type hematopoietic progenitors before the onset of the fetal liver hematopoiesis. It is easier for the representatives of the same prospectively diversifying population of early precursor cells to form methylcellulose colonies than to repopulate a newborn or an adult recipient. Only gradually some precursors acquire the engraftment capacity and enter the higher level of the HSC specification [62].
Finally, the global shift to the extravascular niche during the establishment of the fetal liver hematopoiesis necessitates the involvement of inflammation-powered transmigration process. A substantial and still growing body of experimental evidences supports the inflammatory mechanism of the pre-liver developmental hematopoiesis. The hypothesis explains many controversial or poorly understood observations and puts forward a unifying cellular and molecular mechanism of blood tissue formation.
Acknowledgments
This work was supported by Science and Technology Planning Project of Guangdong Province, China (2011A060901019).
References
- 1.Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 2.Lensch MW, Daley GQ. Origins of mammalian hematopoiesis: in vivo paradigms and in vitro models. Curr Top Dev Biol. 2004;60:127–196. doi: 10.1016/S0070-2153(04)60005-6. [DOI] [PubMed] [Google Scholar]
- 3.Moore MAS, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony-forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 4.Metcalf D, Moore MAS. Haemopoietic cells: North-Holland research monographs. Front Biol. 1971;24:130. [Google Scholar]
- 5.Moore MAS, Owen JJT. Stem-cell migration in developing myeloid and lymphoid systems. Lancet. 1967;2:658–659. [Google Scholar]
- 6.Müller AM, Medvinsky AL, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 7.Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T, Vink CS, Bhandoola A, Dzierzak E, Speck NA. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9:541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitch SR, Kimber GM, Wilson NK, Parker A, Mirshekar-Syahkal B, Göttgens B, Medvinsky A, Dzierzak E, Ottersbach K. Signalling from the sympathetic nervous system regulates hematopoietic stem cell emergence during embryogenesis. Cell Stem Cell. 2012;11:554–566. doi: 10.1016/j.stem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Lan Y, He W, Chen D, Wang J, Zhou F, Wang Y, Sun H, Chen X, Xu C, Li S, Pang Y, Zhang G, Yang L, Zhu L, Fan M, Shang A, Ju Z, Luo L, Ding Y, Guo W, Yuan W, Yang X, Liu B. Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell. 2012;11:663–675. doi: 10.1016/j.stem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 11.de Bruijn M, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gekas C, Dieterlen-Lièvre F, Orkin SH, Mikkola HKA. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD (1994) Cell diversification in the early animal embryo. In: molecular biology of the cell, 3rd edn. Garland Science, New York
- 15.Zernicka-Goetz M. Cleavage pattern and emerging asymmetry of the mouse embryo. Nat Rev Mol Cell Biol. 2005;6:919–928. doi: 10.1038/nrm1782. [DOI] [PubMed] [Google Scholar]
- 16.Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Belaoussoff M, Farrington SM, Baron MH. Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development. 1998;125:5009–5018. doi: 10.1242/dev.125.24.5009. [DOI] [PubMed] [Google Scholar]
- 18.Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neuroectodermal cell fate in the mouse embryo. Development. 2001;128:1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- 19.Murakami Y, Hirata H, Miyamoto Y, Nagahashi A, Sawa Y, Jakt M, Asahara T, Kawamata S. Isolation of cardiac cells from E8.5 yolk sac by ALCAM (CD166) expression. Mech Dev. 2007;124:830–839. doi: 10.1016/j.mod.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Van Handel B, Montel-Hagen A, Sasidharan R, Nakano H, Ferrari R, Boogerd CJ, Schredelseker J, Wang Y, Hunter S, Org T, Zhou J, Li X, Pellegrini M, Chen J-N, Orkin SH, Kurdistani SK, Evans SM, Nakano A, Mikkola HKA. Scl represses cardiomyogenesis in prospective hemogenic endothelium and endocardium. Cell. 2012;150:590–605. doi: 10.1016/j.cell.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14:R47–R58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- 22.De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–877. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajbakhsh S, Vivarelli E, Cusella-De Angelis G, Rocancourt D, Buckingham M, Cossu G. A population of myogenic cells derived from the mouse neural tube. Neuron. 1994;13:813–821. doi: 10.1016/0896-6273(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 24.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5081. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 25.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 26.Scott EW, Fisher RC, Olson MC, Kehrli EW, Simon MC, Singh H. PU.1 functions in a cell-autonomous manner to control the differentiation of multipotential lymphoid-myeloid progenitors. Immunity. 1997;6:437–447. doi: 10.1016/s1074-7613(00)80287-3. [DOI] [PubMed] [Google Scholar]
- 27.Anderson KL, Smith KA, Conners K, McKercher SR, Maki RA, Torbett BE. Myeloid development is selectively disrupted in PU.1 null mice. Blood. 1998;91:3702–3710. [PubMed] [Google Scholar]
- 28.Fisher RC, Scott EW. Role of PU.1 in hematopoiesis. Stem cells. 1998;16:25–37. doi: 10.1002/stem.160025. [DOI] [PubMed] [Google Scholar]
- 29.Lichanska AM, Browne CM, Henkel GW, Murphy KM, Ostrowski MC, McKercher SR, Maki RA, Hume DA. Differentiation of the mononuclear phagocyte system during mouse embryogenesis: the role of transcription factor PU.1. Blood. 1999;94:127–138. [PubMed] [Google Scholar]
- 30.Robin C, Ottersbach K, Durand C, Peeters M, Vanes L, Tybulewicz V, Dzierzak E. An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev Cell. 2006;11:171–180. doi: 10.1016/j.devcel.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa M, Nishikawa S, Ikuta K, Yamamura F, Naito M, Takahashi K, Nishikawa S-I. B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. EMBO J. 1988;7:1337–1343. doi: 10.1002/j.1460-2075.1988.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palacios R, Imhof BA. At day 8–8.5 of mouse development the yolk sac, not the embryo proper, has lymphoid precursor potential in vivo and in vitro . Proc Natl Acad Sci USA. 1993;90:6581–6585. doi: 10.1073/pnas.90.14.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cumano A, Dieterlen-Lievre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka Y, Hayashi M, Kubota Y, Nagai H, Sheng G, Nishikawa S-I, Samokhvalov IM. Early ontogenic origin of the hematopoietic stem cell lineage. Proc Natl Acad Sci USA. 2012;109:4515–4520. doi: 10.1073/pnas.1115828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins LS, Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987;138:1082–1087. [PubMed] [Google Scholar]
- 36.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 37.Gofflot F, Hall M, Morriss-Kay GM. Genetic patterning of the developing mouse tail at the time of posterior neuropore closure. Dev Dyn. 1997;210:431–445. doi: 10.1002/(SICI)1097-0177(199712)210:4<431::AID-AJA7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimoto M, Porayette P, Yoder MC. Overcoming obstacles in the search for the site of hematopoietic stem cell emergence. Cell Stem Cell. 2008;3:583–586. doi: 10.1016/j.stem.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabata H, Nakajima K. Labeling embryonic mouse central nervous system cells by in utero electroporation. Dev Growth Differ. 2008;50:507–511. doi: 10.1111/j.1440-169X.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- 40.Martin CS, Moriyama A, Zon LI. Hematopoietic stem cells, hematopoiesis and disease: lessons from the zebrafish model. Genome Med. 2011;3:83. doi: 10.1186/gm299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol. 2008;18:1234–1240. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 42.Stern CD, Downs KM. The hypoblast (visceral endoderm): an evo-devo perspective. Development. 2012;139:1059–1069. doi: 10.1242/dev.070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sood R, Liu P. Novel insights into the genetic controls of primitive and definitive hematopoiesis from zebrafish models. Adv Hematol. 2012;2012:830703. doi: 10.1155/2012/830703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palis J, Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- 45.Brotherton TW, Chui DH, Gualdie J, Patterson M. Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci USA. 1979;76:2853–2857. doi: 10.1073/pnas.76.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufman MH. The atlas of mouse development. San Diego: Academic Press; 1992. p. 128. [Google Scholar]
- 47.Kingsley PD, Malik J, Fantauzzo KA, Palis J. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood. 2004;104:19–25. doi: 10.1182/blood-2003-12-4162. [DOI] [PubMed] [Google Scholar]
- 48.Sabin FR. Studies on the origin of blood vessels and of red corpuscles as seen in the living blastoderm of the chick during the second day of incubation. Carnegie Inst Wash Publ Contrib Embryol. 1920;9:213–262. [Google Scholar]
- 49.Murray P. The development in vitro of the blood of early chick embryo. Proc Royal Soc London. 1932;111:497–521. [Google Scholar]
- 50.Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller G. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 51.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 52.Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Valerie K. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 53.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 54.Ema M, Faloon P, Zhang WJ, Hirashima M, Reid T, Stanford WL, Orkin S, Choi K, Rossant J. Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse. Genes Dev. 2003;17:380–393. doi: 10.1101/gad.1049803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferkowicz MJ, Starr M, Xie X, Li W, Johnson SA, Shelley WC, Morrison PR, Yoder MC. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- 57.Ema M, Yokomizo T, Wakamatsu A, Terunuma T, Yamamoto M, Takahashi S. Primitive erythropoiesis from mesodermal precursors expressing VE-cadherin, PECAM-1, Tie2, endoglin, and CD34 in the mouse embryo. Blood. 2006;108:4018–4024. doi: 10.1182/blood-2006-03-012872. [DOI] [PubMed] [Google Scholar]
- 58.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Samokhvalov IM, Samokhvalova NI, Nishikawa S-I. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 60.Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–1047. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 62.Samokhvalov IM. A long way to stemness. Cell Cycle. 2012;11:2965–2966. doi: 10.4161/cc.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pimanda JE, Ottersbach K, Knezevic K, Kinston S, Chan WYI, Wilson NK, Landry J-R, Wood AD, Kolb-Kokocinski A, Green AR, Tannahill D, Lacaud G, Kouskoff V, Göttgens B. Gata2, Fli1, and Scl form a recursively wired gene-regulatory circuit during early hematopoietic development. Proc Natl Acad Sci USA. 2007;104:17692–17697. doi: 10.1073/pnas.0707045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okuda T, Van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 65.Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102:199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 66.Hart A, Melet F, Grossfeld P, Chien K, Jones C, Tunnacliffe A, Favier R, Bernstein A. Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity. 2000;13:167–177. doi: 10.1016/s1074-7613(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 67.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis and organogenesis precede lethality in mice lacking all αv integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 68.Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, Lupu F, Cummings RD, McEver RP. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J Cell Biol. 2004;164:451–459. doi: 10.1083/jcb.200311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14:5884–5891. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liakhovitskaia A, Gribi R, Stamateris E, Villain G, Jaffredo T, Wilkie R, Gilchrist D, Yang J, Ure J, Medvinsky A. Restoration of Runx1 expression in the Tie2 cell compartment rescues definitive hematopoietic stem cells and extends life of Runx1 knockout animals until birth. Stem Cells. 2009;27:1616–1624. doi: 10.1002/stem.71. [DOI] [PubMed] [Google Scholar]
- 71.Li Z, Chen MJ, Stacy T, Speck NA. Runx1 function in hematopoiesis is required in cells that express Tek. Blood. 2006;107:106–110. doi: 10.1182/blood-2005-05-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci USA. 1997;94:6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- 74.McGrath KE, Koniski AD, Malik J, Palis J. Circulation is established in a stepwise pattern in the mammalian embryo. Blood. 2003;101:1669–1676. doi: 10.1182/blood-2002-08-2531. [DOI] [PubMed] [Google Scholar]
- 75.Weissman I, Papaioannou V, Gardner R. Fetal hematopoietic origins of the adult hematolymphoid systems. New York: Cold Spring Harbor laboratory Press; 1978. [Google Scholar]
- 76.Toles JF, Chui DHK, Belbeck LW, Starr E, Barker JE. Hemopoietic stem cells in murine embryonic yolk sac and peripheral blood. Proc Natl Acad Sci USA. 1989;86:7456–7459. doi: 10.1073/pnas.86.19.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracio-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC, Garcia-Cardena G, Daley GQ. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, Weber GJ, Harris J, Cutting CC, Huang P, Dzierzak E, Zon LI. Hematopoietic stem cells development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lancrin C, Mazan M, Stefanska M, Patel R, Lichtinger M, Costa G, Vargel Ö, Wilson NK, Möröy T, Bonifer C, Göttgens B, Kouskoff V, Lacaud G. GFI1 and GFI1B control the loss of endothelial identity of hemogenic endothelium during hematopoietic commitment. Blood. 2012;120:314–322. doi: 10.1182/blood-2011-10-386094. [DOI] [PubMed] [Google Scholar]
- 81.Mukouyama Y, Chiba N, Hara T, Okada H, Ito Y, Kanamaru R, Miyajima A, Satake M, Watanabe T. The AML1 transcription factor function to develop and maintain hematogenic precursor cells in the embryonic aorta-gonad-mesonephros region. Dev Biol. 2000;220:27–36. doi: 10.1006/dbio.2000.9617. [DOI] [PubMed] [Google Scholar]
- 82.Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HKA. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cai Z, de Bruijn M, Ma X, Dortland B, Luteijn T, Downing JR, Dzierzak E. Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity. 2000;13:423–431. doi: 10.1016/s1074-7613(00)00042-x. [DOI] [PubMed] [Google Scholar]
- 84.Yoder MC. Hematopoietic regulation in the embryo. Haematol Rep. 2006;2:86–89. [Google Scholar]
- 85.Moore MAS, Owen JJT. Chromosome marker studies in the irradiated chick embryo. Nature. 1967;215:1081–1082. doi: 10.1038/2151081a0. [DOI] [PubMed] [Google Scholar]
- 86.Marks PA, Rifkind RA. Protein synthesis: its control in erythropoiesis. Science. 1972;175:955–961. doi: 10.1126/science.175.4025.955. [DOI] [PubMed] [Google Scholar]
- 87.Dieterlen-Lièvre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol. 1975;33:607–619. [PubMed] [Google Scholar]
- 88.Lassila O, Eskola J, Toivanen P, Martin C, Dieterlen-Lievre F. The origin of lymphoid stem cells studied in chick yolk sac-embryo chimaeras. Nature. 1978;272:353–354. doi: 10.1038/272353a0. [DOI] [PubMed] [Google Scholar]
- 89.Dieterlen-Lièvre F, Martin C. Diffuse intraembryonic hemopoiesis in normal and chimeric avian development. Dev Biol. 1981;88:180–191. doi: 10.1016/0012-1606(81)90228-1. [DOI] [PubMed] [Google Scholar]
- 90.Cormier F, Dieterlen-Lièvre F. The wall of the chick embryo aorta harbours M-CFC, G-CFC, GM-CFC and BFU-E. Development. 1988;102:272–285. doi: 10.1242/dev.102.2.279. [DOI] [PubMed] [Google Scholar]
- 91.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yokomizo T, Dzierzak E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development. 2010;137:3651–3661. doi: 10.1242/dev.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zape JP, Zovein AC. Hemogenic endothelium: origins, regulation, and implications for vascular biology. Semin Cell Dev Biol. 2011;22:1036–1047. doi: 10.1016/j.semcdb.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 94.Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15:477–485. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 95.Nishikawa S-I, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, Kataoka H, Katsura Y. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 96.Nishikawa S-I, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK + VE-cadherin + cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 97.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kellendonk C, Tronche F, Casanova E, Anlag K, Opherk C, Schütz G. Inducible site-specific recombination in the brain. J Mol Biol. 1999;285:175–182. doi: 10.1006/jmbi.1998.2307. [DOI] [PubMed] [Google Scholar]
- 100.Kim I, Yilmaz OH, Morrison SJ. CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood. 2005;106:903–905. doi: 10.1182/blood-2004-12-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caveda L, Martin-Padura I, Navarro P, Breviario F, Corada M, Gulino D, Lampugnani MG, Dejana E. Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin) J Clin Invest. 1996;98:886–893. doi: 10.1172/JCI118870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dejana E, Bazzoni G, Lampugnani MG. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp Cell Res. 1999;252:13–19. doi: 10.1006/excr.1999.4601. [DOI] [PubMed] [Google Scholar]
- 103.Carmeliet P, Lampugnani M-G, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert J-M, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 104.Gory-Fauré S, Prandini M-H, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126:2093–2102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- 105.Spratt NT, Haas H. Integrative mechanisms in development of the early chick blastoderm. I. Regulative potentiality of separated parts. J Exp Zool. 1960;145:97–137. [Google Scholar]
- 106.Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci USA. 2007;104:9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taylor E, Taoudi S, Medvinsky A. Hematopoietic stem cell activity in the aorta-gonad-mesonephros region enhances after mid-day 11 of mouse development. Int J Dev Biol. 2010;54:1055–1060. doi: 10.1387/ijdb.103152et. [DOI] [PubMed] [Google Scholar]
- 108.Taoudi S, Gonneau C, Moore K, Sheridan JM, Blackburn CC, Taylor E, Medvinsky A. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin + CD45 + pre-definitive HSCs. Cell Stem Cell. 2008;3:99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 109.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 110.Minot CS. Development of the blood, the vascular system and the spleen. In: Keibel F, Mall FP, editors. Manual of human embryology. Philadelphia: The Washington Square Press; 1912. [Google Scholar]
- 111.North T, Gu T-L, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M, Speck NA. Cbfa2 is required for the formation of the intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 112.Sheridan JM, Taoudi S, Medvinsky A, Blackburn CC. A novel method for the generation of reaggregated organotypic cultures that permits juxtaposition of defined cell populations. Genesis. 2009;47:346–351. doi: 10.1002/dvg.20505. [DOI] [PubMed] [Google Scholar]
- 113.Gyevai A, Chapple PJ, Douglas WH. Long-term maintenance of reaggregated hypothalamic cultures developed from embryonic rat hypothalamus: prostaglandin release during synaptogenesis in vitro . J Cell Sci. 1978;34:159–171. doi: 10.1242/jcs.34.1.159. [DOI] [PubMed] [Google Scholar]
- 114.Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, Godin I, Cumano A. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc Natl Acad Sci USA. 2005;102:134–139. doi: 10.1073/pnas.0402270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.North TE, de Bruijn MFTR, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E, Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 116.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DYR, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boisset J-C, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 118.Cho A, Courtman DW, Langille BL. Apoptosis (programmed cell death) in arteries of the neonatal lamb. Circ Res. 1995;76:168–175. doi: 10.1161/01.res.76.2.168. [DOI] [PubMed] [Google Scholar]
- 119.Lengerke C, Schmitt S, Bowman TV, Jang IH, Maouche-Chretien L, McKinney-Freeman S, Davidson AJ, Hammerschmidt M, Rentzsch F, Green JBA, Zon LI, Daley GQ. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 120.Durand C, Robin C, Bollerot K, Baron MH, Ottersbach K, Dzierzak E. Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. Proc Natl Acad Sci USA. 2007;104:20838–20843. doi: 10.1073/pnas.0706923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peeters M, Ottersbach K, Bollerot K, Orelio C, de Bruijn M, Wijgerde M, Dzierzak E. Ventral embryonic tissues and Hedgehog proteins induce early AGM hematopoietic stem cell development. Development. 2009;136:2613–2621. doi: 10.1242/dev.034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Astorga J, Carlsson P. Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development. 2007;134:3753–3761. doi: 10.1242/dev.004432. [DOI] [PubMed] [Google Scholar]
- 123.Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 124.Gazzerro E, Smerdel-Ramoya A, Zanotti S, Stadmeyer L, Durand D, Economides AN, Canalis E. Conditional deletion of gremlin causes a transient increase in bone formation and bone mass. J Biol Chem. 2007;282:31549–31557. doi: 10.1074/jbc.M701317200. [DOI] [PubMed] [Google Scholar]
- 125.Mitola S, Ravelli C, Moroni E, Salvi V, Leali D, Ballmer-Hofer K, Zammataro L, Presta M. Gremlin is a novel agonist of the major proangiogenic receptor VEGFR2. Blood. 2010;116:3677–3680. doi: 10.1182/blood-2010-06-291930. [DOI] [PubMed] [Google Scholar]
- 126.de Bruijn MFTR, Ma X, Robin C, Ottersbach K, Sanchez M-J, Dzierzak E. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 127.Stifani S, Ma Q. “Runxs and regulations” of sensory and motor neuron subtype differentiation: implications for hematopoietic development. Blood Cells Mol Dis. 2009;43:20–26. doi: 10.1016/j.bcmd.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liakhovitskaia A, Lana-Elola E, Stamateris E, Rice DP, van’t Hof RJ, Medvinsky A. the essential requirement for Runx1 in the development of the sternum. Dev Biol. 2010;340:539–546. doi: 10.1016/j.ydbio.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 129.Wang X, Blagden C, Fan J, Nowak SJ, Taniuchi I, Littman DR, Burden SJ. Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev. 2005;19:1715–1722. doi: 10.1101/gad.1318305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoi CSL, Lee SE, Lu S-Y, McDermitt DJ, Osorio KM, Piskun CM, Peters RM, Paus R, Tumbar T. Runx1 directly promotes proliferation of hair follicle stem cells and epithelial tumor formation in mouse skin. Mol Cell Biol. 2010;30:2518–2536. doi: 10.1128/MCB.01308-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 132.Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, Yamaguchi T, Masuda S, Shimizu K, Takahashi T, Ogawa S, Hamada Y, Hirai H. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 133.Hadland BK, Huppert SS, Kanungo J, Xue Y, Jiang R, Gridley T, Conlon RA, Cheng AM, Kopan R, Longmore GD. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood. 2004;104:3097–3105. doi: 10.1182/blood-2004-03-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakagawa M, Ichikawa M, Kumano K, Goyama S, Kawazu M, Asai T, Ogawa S, Kurokawa M, Chiba S. AML/Runx1 rescues Notch1-null mutation-induced deficiency of para-aortic splanchnopleural hematopoiesis. Blood. 2006;108:3329–3334. doi: 10.1182/blood-2006-04-019570. [DOI] [PubMed] [Google Scholar]
- 135.Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. RBPjκ-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. 2005;132:1117–1126. doi: 10.1242/dev.01660. [DOI] [PubMed] [Google Scholar]
- 136.Landry J-R, Kinston S, Knezevic K, de Bruijn MFTR, Wilson N, Nottingham WT, Peitz M, Edenhofer F, Pimanda JE, Ottersbach K, Göttgens B. Runx genes are direct targets of Scl/Tal1 in the yolk sac and fetal liver. Blood. 2008;111:3005–3014. doi: 10.1182/blood-2007-07-098830. [DOI] [PubMed] [Google Scholar]
- 137.Hayashi K, Abe N, Watanabe T, Obinata M, Ito M, Sato T, Habu S, Satake M. Overexpression of AML1 transcription factor drives thymocytes into the CD8 single-positive lineage. J Immunol. 2001;167:4957–4965. doi: 10.4049/jimmunol.167.9.4957. [DOI] [PubMed] [Google Scholar]
- 138.Feng R, Desbordes SC, Xie H, Tillo ES, Pixley F, Stanley ER, Graf T. PU.1 and C/EBPα/β converts fibroblasts into macrophage-like cells. Proc Natl Acad Sci USA. 2008;105:6057–6062. doi: 10.1073/pnas.0711961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 140.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marro S, Pang ZP, Yang N, Tsai M-C, Qu K, Chang HY, Südhof TC, Wernig M. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9:374–382. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Giorgetti A, Marchetto MCN, Li M, Yu D, Fazzina R, Mu Y, Adamo A, Paramonov I, Cardoso JC, Monasterio MB, Bardy C, Cassiani-Ingoni R, Liu G-H, Gage FH, Izpisua Belmonte JC. Cord blood-derived neuronal cells by ectopic expression of Sox2 and c-Myc. Proc Natl Acad Sci USA. 2012;109:12556–12561. doi: 10.1073/pnas.1209523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goyama S, Yamaguchi Y, Imai Y, Kawazu M, Nakagawa M, Asai T, Kumano K, Minati K, Ogawa S, Chiba S, Kurokawa M, Hirai H. The transcriptionally active form of AML1 is required for hematopoietic rescue of the AML1-deficient embryonic para-aortic splanchnopleural (P-Sp) region. Blood. 2004;104:3558–3564. doi: 10.1182/blood-2004-04-1535. [DOI] [PubMed] [Google Scholar]
- 144.Sakai E, Kitajima K, Sato A, Nakano T. Increase of hematopoietic progenitor and suppression of endothelial gene expression by Runx1 expression during in vitro ES differentiation. Exp Hematol. 2009;37:334–345. doi: 10.1016/j.exphem.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 145.Johnson GR, Moore MAS. Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature. 1975;258:726–728. doi: 10.1038/258726a0. [DOI] [PubMed] [Google Scholar]
- 146.Houssaint E. Differentiation of the mouse hepatic primordium. II. Extrinsic origin of the haemopoietic cell line. Cell Differ. 1981;10:243–252. doi: 10.1016/0045-6039(81)90007-5. [DOI] [PubMed] [Google Scholar]
- 147.Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 1996;10:1670–1682. doi: 10.1101/gad.10.13.1670. [DOI] [PubMed] [Google Scholar]
- 148.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 149.Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev. 2003;120:59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 150.Tavassoli M. Embryonic and fetal hemopoiesis: an overview. Blood Cells. 1991;17:269–281. [PubMed] [Google Scholar]
- 151.Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T, Vink CS, Bhandoola A, Dzierzak E, Speck NA. Erythroid/myeloid progenitors and hematopoietic stem cell originate from distinct populations of endothelial cells. Cell Stem Cell. 2012;9:541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J, Medvinsky A. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonization of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 153.Kieusseian A, Brunet de la Grange P, Burlen-Defranoux O, Godin I, Cumano A. Immature hematopoietic stem cells undergo maturation in the fetal liver. Development. 2012;139:3521–3530. doi: 10.1242/dev.079210. [DOI] [PubMed] [Google Scholar]
- 154.Collardeau-Frachon S, Scoazec J-Y. Vascular development and differentiation during human liver organogenesis. Anat Rec. 2008;291:614–627. doi: 10.1002/ar.20679. [DOI] [PubMed] [Google Scholar]
- 155.Rybtsov S, Sobiesiak M, Taoudi S, Souilhol S, Senserrich J, Liakhovitskaia A, Ivanovs A, Frampton J, Zhao S, Medvinsky A. Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J Exp Med. 2011;208:1305–1315. doi: 10.1084/jem.20102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Couvelard A, Scoazec J-Y, Dauge M-C, Bringuier A-F, Potet F, Feldmann G. Structural and functional differentiation of sinusoidal endothelial cells during liver organogenesis in human. Blood. 1996;87:4568–4580. [PubMed] [Google Scholar]
- 158.Lichanska AM, Hume DA. Origins and functions of phagocytes in the embryo. Exp Hematol. 2000;28:601–611. doi: 10.1016/s0301-472x(00)00157-0. [DOI] [PubMed] [Google Scholar]
- 159.Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106:3004–3011. doi: 10.1182/blood-2005-02-0461. [DOI] [PubMed] [Google Scholar]
- 160.Penaloza C, Lin L, Lockshin RA, Zakeri Z. Cell death in development: shaping the embryo. Histochem Cell Biol. 2006;126:149–158. doi: 10.1007/s00418-006-0214-1. [DOI] [PubMed] [Google Scholar]
- 161.Silva MT, do Vale A, dos Santos NMN. Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications. Apoptosis. 2008;13:436–482. doi: 10.1007/s10495-008-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Potocnik AJ, Brakebusch C, Fassler R. Fetal and adult hematopoietic stem cells require β1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- 163.Hirsh E, Iglesias A, Potocnik AJ, Hartmann U, Fässler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of β1 integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- 164.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 165.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang I-H, Grosser T, FitzGerald GA, Daley GQ, Orkin SH, Zon LI. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 167.Cabral GA. Lipids as bioeffectors in the immune system. Life Sci. 2005;77:1699–1710. doi: 10.1016/j.lfs.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 168.Orelio C, Haak E, Peeters M, Dzierzak E. Interleukin-1 mediated hematopoietic cell regulation in the aorta-gonad-mesonephros region of the mouse embryo. Blood. 2008;112:4895–4904. doi: 10.1182/blood-2007-12-123836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gurjar MV, Deleon J, Sharma RV, Bhalla RC. Role of reactive oxygen species in IL-1 beta-stimulated sustained ERK activation and MMP-9 induction. Am J Physiol Heart Circ Physiol. 2001;281:H2568–H2574. doi: 10.1152/ajpheart.2001.281.6.H2568. [DOI] [PubMed] [Google Scholar]
- 170.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008;118:3012–3024. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hartner JC, Walkley CR, Lu J, Orkin SH. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat Immunol. 2009;10:109–115. doi: 10.1038/ni.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Quackenbush EJ, Wershil BK, Aguirre V, Gutierrez-Ramos J-C. Eotaxin modulates myelopoiesis and mast cell development from embryonic hematopoietic progenitors. Blood. 2010;92:1887–1897. [PubMed] [Google Scholar]
- 173.Kohchi C, Noguchi K, Tanabe Y, Mizuno D-I, Soma G-I. Constitutive expression of TNF-α and -β genes in mouse embryo: roles of cytokines as regulator and effector on development. Int J Biochem. 1994;26:111–119. doi: 10.1016/0020-711x(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 174.Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening Pandora’s box. Mol Med. 2008;14:195–204. doi: 10.2119/2007-00105.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen Y, Ke Q, Xiao Y-F, Wu G, Kaplan E, Hampton TG, Malek S, Min J-Y, Amende I, Morgan JP. Cocaine and catecholamines enhance inflammatory cell retention in the coronary circulation of mice by upregulation of adhesion molecules. Am J Physiol Heart Circ Physiol. 2005;288:H2323–H2331. doi: 10.1152/ajpheart.00822.2004. [DOI] [PubMed] [Google Scholar]
- 176.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 177.Krasnov P, Michurina T, Packer MA, Stasiv Y, Nakaya N, Moore KA, Drazan KE, Enikolopov G. Neuronal nitric oxide synthase contributes to the regulation of hematopoiesis. Mol Med. 2008;14:141–149. doi: 10.2119/2007-00011.Krasnov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Beste MT, Hammer DA. Selectin catch-slip kinetics encode shear threshold adhesive behavior of rolling leukocytes. Proc Natl Acad Sci USA. 2008;105:20716–20721. doi: 10.1073/pnas.0808213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yago T, Zarnitsyna VI, Klopocki AG, McEver RP, Zhu C. Transport governs flow-enhanced cell tethering through L-selectin at threshold shear. Biophys J. 2007;92:330–342. doi: 10.1529/biophysj.106.090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 181.Finger EB, Puri KD, Alon R, Lawrence MB, von Andrian UH, Springer TA. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- 182.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Taylor CT, Cummins EP. The role of NF-κB in hypoxia-induced gene expression. Ann NY Acad Sci. 2009;1177:178–184. doi: 10.1111/j.1749-6632.2009.05024.x. [DOI] [PubMed] [Google Scholar]
- 184.Dehne N, Brüne B. HIF-1 in the inflammatory microenvironment. Exp Cell Res. 2009;315:1791–1797. doi: 10.1016/j.yexcr.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 185.Ahn KS, Aggarwal BB. Transcription factor NF-κB. A sensor for smoke and stress signal. Ann N Y Acad Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- 186.Orelio C, Harvey KN, Miles C, Oostendorp RAJ, van der Horn K, Dzierzak E. The role of apoptosis in the development of AGM hematopoietic stem cells revealed by Bcl-2 overexpression. Blood. 2004;103:4084–4092. doi: 10.1182/blood-2003-06-1827. [DOI] [PubMed] [Google Scholar]
- 187.Wood W, Turmaine M, Weber R, Camp V, Maki RA, McKercher SR, Martin P. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development. 2000;127:5245–5252. doi: 10.1242/dev.127.24.5245. [DOI] [PubMed] [Google Scholar]
- 188.Halin C, Detmar M. Chapter 1. Inflammation, angiogenesis and lymphangiogenesis. Methods Enzymol. 2008;445:1–25. doi: 10.1016/S0076-6879(08)03001-2. [DOI] [PubMed] [Google Scholar]
- 189.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SHY, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JKY, Ng LG, Samokhvalov IM, Merad M, Ginhoux F. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac–derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]