Abstract
The Na+,K+-ATPase, or sodium pump, is well known for its role in ion transport across the plasma membrane of animal cells. It carries out the transport of Na+ ions out of the cell and of K+ ions into the cell and thus maintains electrolyte and fluid balance. In addition to the fundamental ion-pumping function of the Na+,K+-ATPase, recent work has suggested additional roles for Na+,K+-ATPase in signal transduction and biomembrane structure. Several signaling pathways have been found to involve Na+,K+-ATPase, which serves as a docking station for a fast-growing number of protein interaction partners. In this review, we focus on Na+,K+-ATPase as a signal transducer, but also briefly discuss other Na+,K+-ATPase protein–protein interactions, providing a comprehensive overview of the diverse signaling functions ascribed to this well-known enzyme.
Keywords: Na+,K+-ATPase; Protein–protein complexes; Cardiotonic steroids; Src kinase; Signaling
Introduction
The Na+,K+-ATPase (EC 3.6.3.9) was first described by Jens Christian Skou in 1957 [1]. It is a plasma membrane protein found in animal cells and belongs to the so-called P-type ATPase family, whose family members have a phosphorylated (P) enzyme intermediate in common [2]. During one ATP-driven transport cycle, three Na+ ions are exported from the cell against their chemical gradients and also against the electrical potential. As a counter, two K+ ions are imported into the cell. The transport of Na+ and K+ is accomplished by multiple conformations of the enzyme. In general, two main states exist: the sodium-bound E1 state and the potassium-bound E2 state. The transition between these two states is accompanied by multiple intermediate states. The resultant ion and electrochemical gradients are essential for physiological processes such as electrical excitability, cellular uptake of ions, nutrients and neurotransmitters, as well as regulation of cell volume and intracellular pH. In the brain, about 50 % of the ATP or more is consumed by the Na+,K+-ATPase [3].
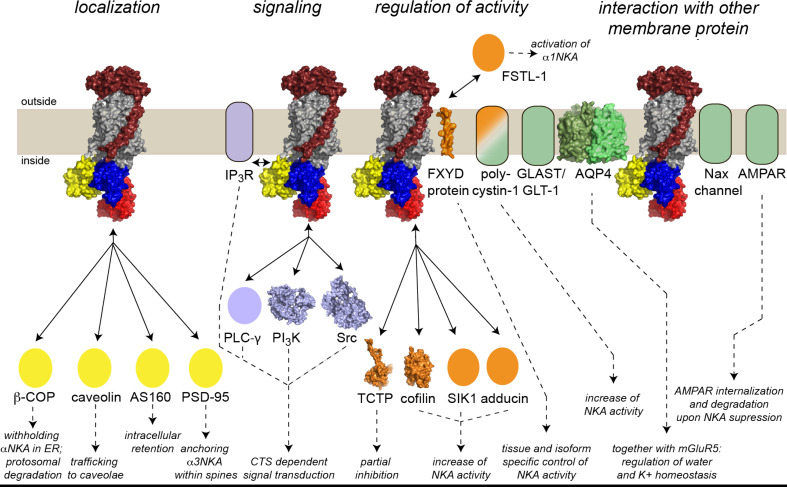
Besides the high physiological significance of the Na+,K+-ATPase as an ion pump, research in the last decade elucidated that the Na+,K+-ATPase is also capable of forming multiple protein–protein complexes. This review aims to give an overview of the variety of protein–protein interactions for which the α-subunit of Na+,K+-ATPase serves as an anchoring platform. An overview is presented in Fig. 1.
Fig. 1.
Overview of proteins interacting with Na+,K+-ATPase and downstream events. Na+,K+-ATPase is abbreviated by NKA. The actual size and oligomerization states of the individual proteins are not considered. Bi-directional and dashed arrows indicate interactions and downstream events, respectively. Proteins, for which three-dimensional structures are available, are presented in surface presentation using the pdb-entries: Na+,K+-ATPase (including FXYD): 3KDP [14]; PI3K: 1E8X [156]; Src: 2H8H [157]; TCTP: 2HR9 [158]; cofilin: 1Q8G [159]; AQP4: 3GD8 [160]
Structural organization of Na+,K+-ATPase
Na+,K+-ATPase is a multi-subunit P-type ATPase consisting of an α- and a β-subunit (Fig. 2) [4]. Human Na+,K+-ATPase contains four α-subunit isoforms (α1–α4). The mature human α1-isoform is 1,018 amino acid residues long, with slightly different numbers in other isoforms and species. The mRNA of the α1- and α2-isoform code for five additional residues at the N-terminus, which are removed by co- or post-translational modification to form the mature protein [5, 6]. In this article, all sequence numbering of amino acid residues is for the mature proteins. The human α1-, α2- and α3-isoforms exhibit a sequence identity of ~87 %, whereas the human α4-isoform is rather distinct as indicated by a sequence identity of only ~78 % towards the other three human α-subunit isoforms. Across species, the α-isoforms are at least 85 % identical.
Fig. 2.
a Schematic presentation of the structural arrangement of the αβ-complex of the Na+,K+-ATPase with the γ-subunit. The second cytoplasmatic domain (CD2) connecting TM helix 2 and 3 harbors the main part of the A-domain. The third cytoplasmatic domain (CD3) connecting TM helix 4 and 5 contains the P- and N-domain. b Illustration of Na+,K+-ATPase in the [Rb2]:E2:MgF4 2− state (PDB entry: 3KDP [14]). The actuator (A-; yellow), nucleotide binding (N-; red), phosphorylation (P-; blue) domain and the transmembrane region (TM; gray) of the α-subunit as well as the β- (red–brown) and γ-subunit (orange) are shown. Rb+ ions are shown as cyan spheres. c Illustration of Na+,K+-ATPase in the E2P state with bound high affinity ouabain (yellow spheres; PDB entry: 3N23 [17]). Color code as in b
The Na+,K+-ATPase α-subunit contains the major transmembrane (TM) region consisting of ten TM helices (TM1-10), which harbors the ion binding sites. In addition, three major cytoplasmatic domains are present: the actuator (A-) domain, the nucleotide binding (N-) domain, and the phosphorylation (P-) domain. The A-domain consists of the N-terminus and the second cytoplasmatic domain (CD2) connecting helices TM2 and TM3. The third cytoplasmatic domain (CD3) connecting helices TM4 and TM5 harbors the N- and P-domain, with the N-domain being inserted in the P-domain. The C-terminus of the Na+,K+-ATPase α-subunit regulates the entrance to an intracellular C-terminal pathway, probably for protons [7].
In humans, three β-subunit isoforms (β1-β3) exist, which are 279 (β3) to 303 (β1) amino acid residues in length. The β-subunit isoforms show a high degree of diversity, such that in humans a sequence identity between 35 and 47 % exists. Similarly, the sequence identity of the β-subunit isoforms across species is low, e.g., as low as 50 % for β3. The β-subunit is unique to K+-counter-transporting P-type ATPases and it is composed of one TM helix and a highly glycosylated extracellular domain. The ectodomain exhibits an immunoglobulin-like structure [16]. The β-subunit is important for the occlusion of the K+ ions and plays an essential role in trafficking of the functional αβ-complex of the Na+,K+-ATPase to the plasma membrane (PM) [8, 9]. A role for the β-subunit in signaling and cell–cell adhesion is also proposed [10], which will not be further discussed in this review.
Na+,K+-ATPase is usually also associated with so-called FXYD proteins often referred to as γ-subunits, which are small membrane proteins influencing Na+,K+-ATPase activity in a tissue- and isoform-specific manner [11]. In mammals, the FXYD protein family contains seven members [12, 13]. The family is characterized by the conserved FXYD motif (containing the amino acids phenylalanine, tyrosine, and aspartate), two conserved glycines, and a conserved serine residue. All human FXYD proteins are type I membrane proteins with one TM helix and an extracytoplasmic N-terminus [11]. In all crystal structures of Na+,K+-ATPase in various functional states determined to date, the αβ-complex of the Na+,K+-ATPase was associated with an FXYD protein [14–17], however, FXYD proteins are not required for the functional expression of Na+,K+-ATPase αβ-complex [13].
The expression profile and kinetic properties of the individual α- and β-isoforms vary from each other. Human Na+,K+-ATPase α1-isoform is ubiquitously expressed in all tissues and Na+,K+-ATPase α2-isoform is restricted to astrocytes, cardiac myocytes and a few more cell types. The α3-isoform is expressed in neurons and ovaries [18], whereas the α4-isoform is only present in spermatozoa. In human, the α1, -2, and -3 isoforms exhibit similar nanomolar affinities for the Na+,K+-ATPase-specific inhibitor ouabain, while α4 is considerably more sensitive [18–21].
Localization and trafficking of Na+,K+-ATPase
The Na+,K+-ATPase is localized in the plasma membrane (PM). The PM does not exhibit a homogenous lipid and protein composition, but rather a mosaic of microdomains with varying composition. The local variations in lipid and protein composition define and modulate the local shape and properties of the PM. In X-ray diffraction data of the sarco(endo)plasmatic reticulum Ca2+-ATPase (SERCA) crystallized in lipid bilayers, features indicating the position of phosphate head groups of the lipid bilayer are visible in the electron density map [22]. It was shown that SERCA is capable of adapting to different hydrophobic thicknesses by inducing local deformations in the lipid bilayers in combination with small rearrangements of the amino acid side chains and helix tilts. It is reasonable to assume that a similar observation can also be made for the Na+,K+-ATPase. However, the influence of composition and shape of the PM on Na+,K+-ATPase ion pumping activity remains to be elucidated in more detail, although important roles for phosphatidyl serine lipids and cholesterol have been identified [161, 162].
Trafficking and sorting of Na+,K+-ATPase
After biosynthesis, while residing in the endoplasmic reticulum (ER), the α- and β-subunit form a 1:1 complex mediated by both TM and extracellular interactions [23]. Before assembly, both subunits associate with the chaperone immunoglobulin-binding protein BiP, which act as a catalyst for correct folding, and as part of a quality control system that prevents misfolded pumps from exiting the ER [24]. From the time of synthesis and until it reaches the PM, the α-subunit passes through several maturation steps, each giving rise to unique trypsin digestion patterns, which probably correspond to progressing structural rearrangements [25]. Membrane insertion of newly synthesized α-subunits occur in a sequential order where for the first four TM helixes are fully implemented in the membrane, and the proceeding six only become completely integrated when as the extracellular loop between TM7 and TM8 gets stabilized upon interaction with the β-subunit [26].
To avoid unintended trafficking of unassembled α-subunit to the PM, a safety precaution retains the Na+,K+-ATPase α-subunit in the ER when it is not in complex with the β-subunit. It has been demonstrated through co-immunoprecipitation experiments that the α-subunit, in the absence of β, interacts with the β-COP component of the coating protein COP-1, and that this leads to withholding of the α-subunit in the ER and ultimately to its degradation by the proteasome [27]. It was also shown that the interaction between β-COP and Na+,K+-ATPase occurs via a N-terminal dibasic motif on Na+,K+-ATPase, and that mutational disruption of this motif allows trafficking of the α-subunit without the β-subunit to the PM [27]. Importantly, the mutated α-subunit could not catalyze ion exchange without association with a β-subunit.
By means of an elegant pulse-chase experiment where cohorts of newly synthesized Na+,K+-ATPases were collected in the Golgi network before their release, important insight in the trafficking of membrane proteins in polarized cells is now available [28]. When cultured under appropriate conditions, MDCK cells develop two distinct membrane domains: an apical and a basolateral. The Na+,K+-ATPase α1-subunit is found exclusively in the basolateral membrane, but in contrast to many other basolateral membrane proteins, Na+,K+-ATPase is transported to this domain via a direct pathway that skips passage through recycling endosomes [28]. Since the Na+,K+-ATPase exists as four different α-subunit isoforms, which can localize to specific subcellular compartments, an interesting question is: how are these isoforms sorted to their respective membrane domains?
Gating to caveolae
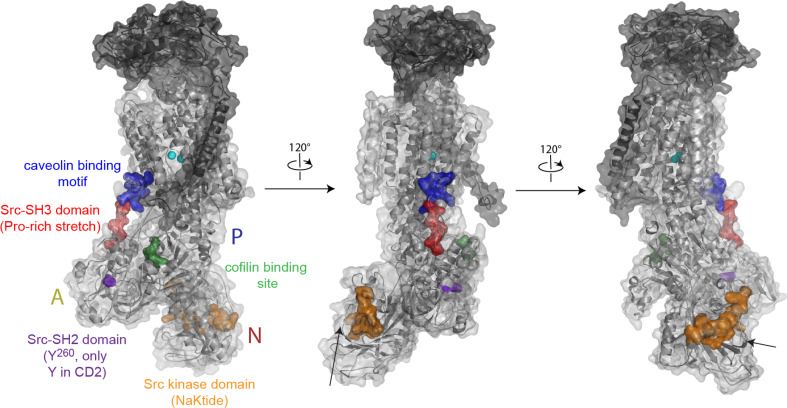
In addition to its general localization in the PM, Na+,K+-ATPase α1-subunit was also described to be present in caveolae—a subfraction of the PM exhibiting high levels of cholesterol, glycosphingolipids, and sphingomyelin [29]. Na+,K+-ATPase is gated to caveolae by caveolin, which is a 21 to 24-kDa membrane-associated scaffolding protein [29, 30]. In mammals, three different caveolin genes exist, resulting in five isoforms, which are expressed in a tissue-specific manner [31]. The Na+,K+-ATPase α1-subunit contains two conserved caveolin-binding motifs (CBM). One is located in the cytoplasmatic A-domain close to M1, and the second one on the extracellular side of M10 (F87CRQLFGGF95 and W982WFCAFPY989, respectively, in human Na+,K+-ATPase α1-subunit). Due to its intracellular location, the first CBM appears more likely as a relevant site of interaction (Fig. 3). Since the CBMs are located far away from the N- and P-domains, it was proposed that caveolin binding is unlikely to affect Na+,K+-ATPase activity [32], however, proteolytic cleavage and mutations in this region do affect function significantly [33, 34], so this assumption is not clear. Na+,K+-ATPase binds to the N-terminal portion of caveolin-1 [32, 35].
Fig. 3.
Potential binding sites located on the cytoplasmatic domains of Na+,K+-ATPase. For Na+,K+-ATPase αβγ-complex, an illustration and transparent surface are shown. The orientation of the left image is as in Fig. 2b. The A-, N-, and P-domains are indicated. Possible binding sites are shown by colored surface presentations. The NaKtide fragment is mainly located in the core of the N-domain (black arrows in middle and right figure) and hence are only partially accessible. Rb+ is shown in cyan spheres
In different cell types, Na+,K+-ATPase was found in caveolae and initially it was suggested that this caveolar Na+,K+-ATPase represents a non-pumping Na+,K+-ATPase pool, which is involved in the signal transduction processes (see below) due to its close localization to its signaling partners [36, 37]. A recent study, however, questions this hypothesis. An analysis of the separated caveolar and non-caveolar membrane fractions showed that Na+,K+-ATPase activity seems to be independent of its membrane localization [38].
Intracellular retention
While the majority of Na+,K+-ATPase is located in the PM, a latent pool of Na+,K+-ATPase has been found in intracellular compartments [39]. This intracellular retention presents a mode of regulation of Na+,K+-ATPase activity and can for instance be initiated by the direct interaction between Na+,K+-ATPase α-subunit and AS160 (Akt kinase substrate of 160 kDa), which is a Rab-GTPase-activating protein located in the cytoplasm [40]. It was demonstrated that AS160 predominantly interacts with CD3 of Na+,K+-ATPase α1-subunit, which contains the N- and P-domain. The interaction was analyzed using pull-down assays, co-immunoprecipitation, and immunofluorescence assays on COS cells, demonstrating that these proteins co-localize in the cytoplasm. The interaction between Na+,K+-ATPase α-subunit and AS160 is modulated by phosphorylation by Akt and adenosine monophosphate-stimulated protein kinase (AMPK). Compound C (Dorsomorphin), which is a specific inhibitor for AMPK, induced inhibition of AMPK in Madin–Darby canine kidney cells and resulted in an increase of Na+,K+-ATPase endocytosis [40].
Synaptic localization of Na+,K+-ATPase α3-subunit
In mammalian neurons, expression of both Na+,K+-ATPase α1- and α3-subunit is evident [41, 42], but the exact role of these isoforms is still elusive. In Drosophila neurons, the Na+,K+-ATPase is involved in short-term cellular memory formation by a mechanism that involves a direct influence on the amplitude of after-hyperpolarization following bursts of action potentials [43]. Interestingly, very similar results have been obtained from direct electrophysiological measurements of single presynaptic terminals from mice, and here the after-hyperpolarization was shown to be due to Na+,K+-ATPase α3-subunit, which is the only Na+,K+-ATPase isoform located in these synapses [44]. Studies with super-resolution stimulated emission depletion (STED) microscopy have shown that Na+,K+-ATPase α3-subunit is located in dendritic spines, and both pull-down and co-immunoprecipitation experiments confirmed that Na+,K+-ATPase α3-subunit interacts, via its N-terminal tail, with the Post-Synaptic-Density-95 (PSD-95) scaffolding protein [45]. PSD-95 is a key organizer of the complex assortment of the different proteins in synapses, and is a well-known interaction partner to, for example, voltage-gated potassium channels (Kv1) [46], NMDA receptors [47], and neuroligins [48]. With the proposed Na+,K+-ATPase α3-subunit/PSD-95 interaction in mind, it is an interesting observation that ouabain seems to stimulate allocation of Na+,K+-ATPase α3-subunit to synaptic PM fractions [49], which together with the role of Na+,K+-ATPase α3-subunit in after-hyperpolarization roughly sketches a novel mode of synaptic plasticity.
Na+,K+-ATPase as a signal transducer
Cardiotonic steroids (CTS) are well known to specifically inhibit Na+,K+-ATPase and have served as drugs in the treatment for congestive heart failure for hundreds of years. Plant-derived digitalis drugs such as digoxin and ouabain, as well as vertebrate-derived aglycones such as bufalin and marinobufagenin belong to the group of CTS [50]. Ouabain and marinobufagenin were identified to exist in humans where they function as endogenous hormones [51, 52]. CTS bind to an extracellular pocket of Na+,K+-ATPase, blocking the enzyme in the E2P state and causing the enzymatic transport cycle to be interrupted (Fig. 2c) [17]. However, at nano- and sub-nanomolar concentrations, insufficient to affect the general pool of Na+,K+-ATPase significantly, CTS were found to possess a hormone-like effect, initiating downstream phosphorylation events, which result in modulation of various cellular processes such as changes in gene expression, cell proliferation, apoptosis, and cell–cell contacts [32, 53–56]. As Na+,K+-ATPase is the only known CTS receptor, these observations lead to the hypothesis that Na+,K+-ATPase not only functions as an ion pump but also as a signal transducer.
Src kinase as a direct binding partner
A key player involved in the Na+,K+-ATPase signaling process is the Src kinase [53, 54]. Src family kinases are 52–62-kDa non-receptor tyrosine kinases that play a crucial role in several signal transduction pathways induced by extracellular stimuli such as cytokines, growth factors, and stress response [57]. They are present in many different cell types such as cardiac myocytes, smooth muscle, and kidney epithelial cells where they regulate cell adhesion, invasion, and motility. Malfunction of Src is often associated with the development of cancer [58].
All Src family kinases exhibit a similar structural organization: At the N-terminus, they contain an Src homology 4 (SH4) domain harboring a myristoylation site required for membrane anchoring, followed by a unique region of unknown function. The most important core domains are the SH3 domain, the SH2 domain, and the Src kinase domain (also termed SH1 domain), followed by a short C-terminal region containing a conserved tyrosine residue (Y530 in human cSrc). SH3 and SH2 domains are well known to interact with other proteins by recognizing poly-proline and phosphotyrosine sites, respectively [59–62]. The kinase activity of Src is regulated by phosphorylation of Y419 and Y530 (sequence numbering for human cSrc). Phosphorylation of Y530 is recognized intramolecularly by the Src-SH2 domain subsequently allowing the Src-SH3 domain to bind to the SH2—kinase domain linker containing a poly-proline type II helix, altogether resulting in a compact, inactive form of Src [63, 64]. Dephosphorylation of Y530 leads to an opening of the structural arrangement with a subsequent increase in kinase activity [65]. Phosphorylation of Y419, which is located in the active loop segment of the kinase domain, significantly increases Src kinase activity and is considered as a marker to analyze Src activity.
Multiple experiments indicate that Src directly interacts with the Na+,K+-ATPase α1-subunit [66–68]. It was shown by co-immunoprecipitation experiments from A7r5 and LLC-PK1 cells that ouabain stimulates binding of Src to Na+,K+-ATPase in a dose- and time-dependent manner as well as increases Src phosphorylation at residue Y419, which correlates to an increase in Src kinase activity [54]. Other reports, however, fail to observe a correlation between ouabain concentration and Src activity [38].
Based on pull-down and co-immunoprecipitation assays, the current working model suggests that the Src-SH2 domain is bound to the CD2 of Na+,K+-ATPase, which constitutes the major part of the A-domain, independent of the E1 or E2 state of Na+,K+-ATPase (Fig. 3). The Src kinase domain was shown to interact with the N-domain in the E1 state only, whereas an interaction causes inhibition of Src kinase activity. More precisely, in in vitro experiments, a 20-amino-acid-long polypeptide fragment of the N-domain (S410ATWLALSRIAGLCNRAVFQ429 in human Na+,K+-ATPase α1-subunit; also termed NaKtide) was shown to inhibit Src kinase activity (IC50 = 70 nM) in an ATP-concentration-independent manner [67]. Addition of a TAT leader sequence makes NaKtide (now also termed pNaKtide) cell permeable. In vivo experiments demonstrated that pNaKtide is able to induce apoptosis, inhibit growth, and decrease tumor angiogenesis in cultures of human cancer cells exhibiting low levels of Na+,K+-ATPase α1-subunit [69]. The binding affinity of the SH2 domains appears to be higher than the one of the Src kinase domain [66]. A direct interaction between the SH3 domain and Na+,K+-ATPase was not observed, though Na+,K+-ATPase exhibits a conserved proline-rich sequence within its A-domain (P77PPTTP82 in human Na+,K+-ATPase α1-subunit). Since the analyzed protein constructs Src-SH3-SH2 resulted in increased levels of pulled-down protein compared to Src-SH2 alone, it was suggested that the binding of Src-SH3 is weak but supports binding of Src-SH2 [67].
The deduced model for the signal transduction is that Src is more or less permanently attached to Na+,K+-ATPase via its SH2 domain. The Src kinase domain can only bind to the N-domain in the absence of ouabain, when Na+,K+-ATPase is in the E1 state. Once ouabain is bound to Na+,K+-ATPase, the E2P form is stabilized, the kinase domain is released, and the Src kinase is activated [66, 68]. For SERCA, it is well known that the E1–E2 transition goes along with large conformational rearrangement of the positions of the A- and N-domains [70]. For Na+,K+-ATPase, the three-dimensional structure of the E1 state is still unknown, but it can be assumed that, as for SERCA, large conformational changes occur during the E1–E2 transition.
The recently published crystal structure of E2P Na+,K+-ATPase in the high affinity complex with ouabain enlightens structural rearrangements, which occur upon ouabain binding. Ouabain binds to an extracellular cleft formed by TM1-6, subsequently plugging the ion pathway [17]. Compared to the [K2]E2:MgF4 2− Na+,K+-ATPase structures (where MgF4 2− mimics an associated, but not covalently attached phosphate) [14, 16], the position of the N- and P-domain as well as of the β- and γ-subunits is nearly identical. However, the A-domain rotates about 10° and TM1-2 move towards the ouabain molecule. This observation suggests that the extracellular-bound ouabain signal is transduced to the intracellular compartment by movement of the TM1-2 linker, a region that contains many interaction sites in its vicinity [17]. These conformational changes might be sufficient to cause a release of the Src kinase domain [68], resulting in Src kinase activation. Hence, it was suggested that Na+,K+-ATPase regulates the associated Src in a conformation-dependent manner. In addition, Src binding was shown to have no effect on the Na+,K+-ATPase ion transport activity [68]. This observation indicates that one of the domains (probably the Src kinase domain) is released from Na+,K+-ATPase during the ion transport cycle.
The interaction studies of Na+,K+-ATPase and Src domains were performed in vitro by mixing purified proteins. On the first view, these interactions seem to be rather plausible, however, a number of important questions concerning the exact mode of interaction remain to be answered. First, how exactly does the Src-SH2 domain recognize the Na+,K+-ATPase A-domain? SH2 domains are well known to recognize phosphotyrosine residues [59, 61], however, phosphotyrosine-independent recognition has also been reported [71–74]. The A-domain construct of the α1-subunit of Na+,K+-ATPase, which was used in the published binding studies [66, 68], contained only one possible tyrosine residue—Y255 in the human Na+,K+-ATPase α1-subunit. This tyrosine residue only exists in Na+,K+-ATPase α1-subunit, not in the other three human isoforms, where the corresponding residue is an alanine. Moreover, Y255 is only conserved in mammals, but not in reptiles and birds. The question of whether phosphorylation of Y255 is required for Src-SH2 recognition or whether a possible phosphotyrosine-independent recognition occurs, remains to be answered. Possible phosphorylation of Y255 in the CD2 constructs used in the in vitro experiments has not been reported [66, 68]. Moreover, in the analyzed CD2 construct, the first part of the A-domain was absent. It is not clear how the presence of this part might influence Src-SH2 or -SH3 binding in the full-length enzyme. If Src-SH2 is recognizing the Na+,K+-ATPase A-domain via a phosphotyrosine residue, it could be speculated that only Na+,K+-ATPase α1-subunit (but no other isoform) is capable of acting as a signal transducer. This, however, would be in contrast to recent studies, which proposed the interaction between Src and Na+,K+-ATPase α3- and α4-subunit [75, 76].
The second important question to be answered is how the interaction with the N-domain is capable of inhibiting the Src kinase activity. The NaKtide sequence involved in Src inhibition (S 410ATWLALSRIAGLCNRAVFQ429; conserved residues of human Na+,K+-ATPase isoforms in bold; sequence numbering for human Na+,K+-ATPase α1-subunit) is rather occluded in the crystal structure of pig Na+,K+-ATPase in the E2P state (Fig. 3). So far, experimental evidence for the identification of key residues involved in the N-domain–Src kinase domain interaction is missing. Since the NaKtide forms an α-helical structure, it was suggested that helix–helix contacts cause Src kinase inhibition [67]. However, the α-helical fragment of the NaKtide fragment is buried in the complete N-domain and hence this hypothesis seems to be unlikely. Furthermore, it was suggested that the NaKtide is neither an ATP mimic nor a substrate Src inhibitor such that a novel mode of Src inhibition might be possible [67]. Obviously, the mode of inhibition has to be analyzed in more detail on both a functional and a structural level.
It should be mentioned that several recent studies question a direct interaction of Src with native Na+,K+-ATPase [38, 77]. Liu et al. separated caveolar and noncaveolar Na+,K+-ATPase pools and compared their properties. Active endogenous Src was found in both preparations, which could, however, not be further activated by ouabain [38]. The absence of ouabain-induced Src activation as well as the lack of Na+,K+-ATPase/Src crosslinking products challenge the model of a direct Na+,K+-ATPase/Src interaction. In addition, a recent biochemical assay using highly purified Na+,K+-ATPase and commercially available Src showed that Src kinase activity is regulated by both ADP and ATP concentrations and provides no evidence for a direct Na+,K+-ATPase/Src interaction [77]. Any inhibition of Na+,K+-ATPase resulted in Src-activation, while a decrease in ATP level as well as the resulting increase in ADP level caused by Na+,K+-ATPase activity were unfavorable for Src activation [77]. These results indicate that the hydrolytic activity of Na+,K+-ATPase (via changes in the energetic status) rather than the induction of a certain conformation of the pump regulate Src activity. The activating effects of digitalis-like compounds on Src are thus proposed to have a functional but not structural origin.
Downstream signaling events
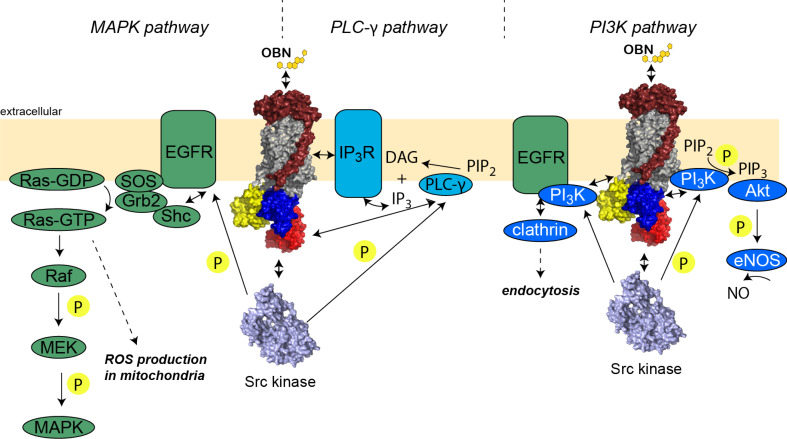
As discussed above, a current hypothesis is that binding of ouabain (which stabilizes the E2P state of Na+,K+-ATPase) releases the Src kinase domain from the N-domain, resulting in an activation of Src kinase, which is responsible for the communication between Na+,K+-ATPase and other proteins. Subsequently, several different signaling pathways are initiated in a cell-type-specific manner (Fig. 4).
Fig. 4.
Overview of ouabain-induced signaling cascades. Bi-directional arrows represent interactions and uni-directional arrows show reactions, with P indicating phosphorylation events
MAPK cascade
Activated Src transactivates the epidermal growth factor receptor (EGFR) by phosphorylation of multiple tyrosine residues other than the major phosphorylation site Y1173 in A7r5 cells [53, 78]. EGFR is a receptor tyrosine kinase and phosphorylation of EGFR in turn recruits the adaptor protein Shc, followed by binding of the GRB2-SOS (growth factor receptor-bound protein 2; Son of Sevenless) complex. Upon binding, SOS, a guanine nucleotide exchange factor, is activated and facilitates the GDP-to-GTP exchange in Ras. Active GTP-bound Ras starts the classical MAPK (mitogen-activated protein kinase) cascade, which is a serine/threonine protein kinase cascade involving Raf (MAP3K), MEK (MAP2K), and p42/p44 MAPK (also known as extracellular regulated kinases ERK1/2). Subsequently, MAPK influences cellular processes such as proliferation, differentiation, migration, and metabolism [79]. Activation of the MAPK signaling cascade upon ouabain binding was shown to occur in different cell types such as vascular smooth muscle cells, cardiac myocytes, and A7r5 cells [53, 54]. If Src is knocked out or inhibited, no activation of p42/44 MAPKs is observed upon ouabain treatment. Ras was not only shown to activate the MAPK cascade but also to be involved in the mitochondrial production of reactive oxygen species (ROS) in different cell types [80, 81].
Activation of phospholipase C-γ and IP3 receptor
CTS-induced Src activation can also initiate Phospholipase C-γ (PLC-γ) signaling cascades [82]. PLC-γ is usually activated through G protein-coupled receptors. Phosphorylation of rat PLC-γ at Y783 results in its activation leading to increased levels of 1,2-diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3) from phosphatidylinositol 4,5-biphosphate (PIP2) hydrolysis [83]. IP3 in turn activates the IP3 receptor (IP3R), which is a tetrameric calcium channel predominantly situated in the endoplasmic reticulum (ER) that functions to release calcium that is stored in the ER. Furthermore, low levels of IP3R can also be located in the PM [84].
It has been shown that both PLC-γ1 and IP3R were co-enriched with Na+,K+-ATPase, caveolin-1, and Src in the light fraction of Na+,K+-ATPase prepared from rat kidney outer medulla [82]. Subsequent GST pull-down assays identified the CD3 of Na+,K+-ATPase, which harbors the N- and P-domain, as the interaction site with PLC-γ1, while the Na+,K+-ATPase N-terminus binds to IP3R2 and IP3R3 [82, 85]. In particular, a conserved LKK motif, which is conserved in all Na+,K+-ATPase α-subunit isoforms, was found to be essential for binding to the IP3R N-terminus. Interestingly, the first five residues that are not present in the mature Na+,K+-ATPase α1-subunit, may block the Na+,K+-ATPase–IP3R interaction. The binding region within IP3R comprises the interface between the suppressor domain and the IP3-binding domain (as constructs containing both domains bind Na+,K+-ATPase, but individual domains do not bind Na+,K+-ATPase). The Na+,K+-ATPase α1-subunit N-terminal tail was further shown to mediate ouabain protection from apoptosis and activation of NF-κB [85].
These results indicate that Na+,K+-ATPase can tether PLC-γ1 and IP3R into a Ca2+-regulatory complex, where PLC-γ1 can transmit the signal directly to the effector IP3R. Ouabain was found to increase the interaction between PLC-γ1 and Na+,K+-ATPase, which subsequently leads to interaction with and tyrosine phosphorylation of IP3 receptors. The central role of Na+,K+-ATPase in this “signalosome” was confirmed by the fact that depletion of cholesterol reduced ouabain-induced interaction of Na+,K+-ATPase–PLC-γ1/IP3R, indicating that the caveolar Na+,K+-ATPase assembles the “signalosome” in response to ouabain [82]. The involvement of PLC-γ1 in this “signalosome” is still controversial. While Aperia’s laboratory discovered a direct Na+,K+-ATPase–IP3R interaction, causing characteristic calcium oscillations [86, 87], Xie’s laboratory discovered the additional involvement of PLC-γ1 and ouabain-stimulated calcium transients rather than oscillations (oscillations only observed in <1 % of cells) [82].
Apart from activation by IP3, an alternative mechanism of IP3R activation has been recently proposed. Ouabain was found to trigger the release of calcium from the intracellular stores [86]. Using fluorescence resonance energy transfer (FRET) it could be shown that Na+,K+-ATPase and IP3R form a signaling microdomain that can, in the presence of ouabain, generate slow Ca2+ oscillations, leading to downstream activation of NF-κB in renal cells [87]. Those oscillations were found to be independent of IP3 generation, indicating that no soluble messenger but rather direct protein–protein interactions are the basis for the signaling microdomain consisting of Na+,K+-ATPase and IP3R.
PI3K pathway
The Na+,K+-ATPase/Src complex has also been implicated in the PI3 kinase (PI3K) pathway. PI3Ks are a group of lipid kinases that phosphorylate phosphatidylinositol lipids at the 3-hydroxyl group [88]. Upon dopamine stimulation, PI3K was shown to directly interact with Na+,K+-ATPase α1-subunit via its SH3 domain, whereas the level of interaction is increased by serine phosphorylation in Na+,K+-ATPase (S18 in rodent Na+,K+-ATPase α1-subunit) [89]. The secondary messenger phosphatidylinositol 3,4,5-biphosphate (PIP3) is generated by phosphorylation of PIP2. PIP3 then interacts with downstream effector proteins including Akt (also known as protein kinase B (PKB)), anchoring it to the PM, where it can be activated by phosphorylation through phosphoinositol-kinase 1 (PDK1). Ouabain-activated Akt was reported to have a cytoprotective effect in LLC-PK1 cells and to cause hypertrophy in cardiac myocytes [90, 91]. In endothelial cells, Akt activates endothelial nitric oxide synthase (eNOS) by phosphorylation, resulting in NO production [92]. In LLC-PK1 cells, it was shown that the Na+,K+-ATPase/Src/EGFR/PI3K complex was also able to attract adaptor protein-2 (AP2) and clathrin, resulting in the formation of clathrin-coated pits [93]. As a consequence, endocytosis of Na+,K+-ATPase was observed, and hence a reduction in Na+,K+-ATPase activity.
Further modification through TCTP and adducin
The highly conserved translationally controlled tumor protein (TCTP) was shown to interact with Na+,K+-ATPase [94]. TCTP is abundantly expressed in a wide range of eukaryotes. Its cell-type-specific expression levels are influenced by external signals such as growth and stress factors, as well as cytotoxic signals. TCTP binding to Na+,K+-ATPase results in a partial inhibition of the Na+,K+-ATPase ion pumping activity [94]. Using human breast epithelial cells co-immunoprecipitation experiments, it was shown that TCTP interacts with Na+,K+-ATPase and that binding of TCTP decreases the amount of Src bound to Na+,K+-ATPase, which goes along with subsequent Src activation [95].
Adducin is a highly conserved heterodimeric cytoskeletal protein composed of related but non-identical subunits (α, β, and γ) coded by different genes (ADD1, ADD2, and ADD3) [96]. Adducin is involved in signal transduction via modulation of the actin cytoskeleton at cell–cell contact sites [97, 98], participates in cell migration [99], modulates actin–spectrin assembly [100] and influences cell surface exposure of integrins and adhesion molecules [101]. Adducin point mutations have been implicated in ion-transport dysfunctions associated with primary or essential hypertension [102].
Using recombinant as well as endogenous purified proteins and activity assays, a direct and specific interaction between adducin and Na+,K+-ATPase has been discovered. This interaction was suggested to be also present in intact renal membranes [103]. Mutant adducins implicated in hypertension show higher affinities for Na+,K+-ATPase and higher potentials for stimulation of Na+,K+-ATPase activity compared to wild-type adducin. It has recently been discovered that both mutant α-adducin variants as well as ouabain-bound Na+,K+-ATPase can interact with the Src-SH2 domain, leading to increased Src activity and Src-dependent Na+,K+-ATPase phosphorylation and activity [104]. In contrast, wild-type adducin or ouabain-free Na+,K+-ATPase were found to interact less robustly with Src-SH2. Rostafuroxin, a digitoxigenin derivative and ouabain antagonist [105–107], was shown to reverse Src activation and Na+,K+-ATPase phosphorylation by disrupting the interaction between Src-SH2 domain and mutant α-adducin or ouabain-bound Na+,K+-ATPase, respectively, as shown in HK2 cells, a cell-free system, and rats. Thus, rostafuroxin treatment may be a promising approach for normalizing blood pressure in hypertensive rats [104].
Influence of ion concentration
During ion transport, Na+,K+-ATPase cycles between the Na+-bound E1-state and the K+-bound E2-state. The intracellular Na+ and extracellular K+ concentrations influence the E1/E2 ratio. In vitro experiments demonstrated that Na+,K+-ATPase in the E1-form is able to bind the Src-SH2 and kinase domain, resulting in inhibition of Src kinase activity [108]. The transformation to the E2-form releases the kinase domain, with subsequent Src kinase activation [68]. It was furthermore demonstrated that lowering extracellular K+ concentrations can shift the E1/E2 equilibrium towards the E2 state and hence influence Src activity [68]. A downstream activation of MAPK was observed, similar to the effect observed by ouabain addition. This means that signaling via the Na+,K+-ATPase/Src complex might not only be regulated by ouabain but also by the cellular salt composition. On the other hand, low concentrations of ouabain, which are able to activate Src and downstream signaling events, have no influence on the overall Na+ and K+ ion concentrations [53, 80].
Regulation of Na+,K+-ATPase activity
The activity of Na+,K+-ATPase can be controlled at different levels, e.g., at the level of gene expression [109], the number of active molecules within the plasma membrane [110], and modulation of its intrinsic turnover rate [111]. The probably predominant mode of regulation is achieved through the control of the number of active molecules in the PM. More precisely, to archive a reduction in activity, endocytosis is observed [112], whereas increased activity levels can be obtained through recruitment of Na+,K+-ATPase from the latent intracellular pool to the plasma membrane [113]. As already mentioned above, AS160 is involved in such a regulatory mechanism.
Another way of regulation at the protein level is through changing the intrinsic turnover rate of Na+,K+-ATPase. As discussed, FXYD proteins, which tightly interact with Na+,K+-ATPase, influence Na+,K+-ATPase activity in a tissue- and isoform-specific manner. Furthermore, several other proteins have been reported to influence Na+,K+-ATPase activity through direct protein–protein interactions. A short (but by no means complete) overview will be given in the following sections. Similarly, post-translational modifications may change the activity of the present pool of Na+,K+-ATPase.
SIK1 and PP2A
Salt-inducible kinase 1 (SIK1) is a sucrose-nonfermenting-like kinase that belongs to the AMPK family. Three SIK isoforms with tissue-specific effects exist [114]. Unlike AMPK, the 86-kDa SIK1 consists of a single subunit containing three major domains. Recently, an important role of SIK1 in regulating Na+,K+-ATPase in the renal proximal tubule (RPT) has been discovered. SIK1 was found to be associated with Na+,K+-ATPase in the RPT opossum kidney cell line and elevated levels of intracellular Na+ lead to activation of SIK1 and subsequent increased Na+,K+-ATPase activity [115]. The detailed mechanism involves an indirect Na+,K+-ATPase activation by SIK1 via Protein Phosphatase 2A (PP2A), which is part of a multiprotein complex containing Na+,K+-ATPase, SIK1, PP2A, and PME-1 (Protein phosphatase methylesterase-1) [114, 115]. In addition, a role of SIK1 in transcriptional regulation of the Na+,K+-ATPase gene ATP1B1 (encoding Na+,K+-ATPase β-subunit) by TORCs (transducers of regulated CREB) has been described. Cytoplasmic SIK1 sequesters TORCs in the cytoplasm and thus prevents them from entering the nucleus, where they usually increase transcription levels of ATP1B1 [114, 116]. Recently, a single-nucleotide polymorphism (SNP) in the hSIK1 gene resulting in the SIK1-G15S mutations has been found to increase Na+,K+-ATPase activity in cultured vascular smooth muscle cells from rat aorta, indicating that this mutation is associated with lower blood pressure [117].
Cofilin
Cofilin is an actin-interacting protein that is regulated through reversible phosphorylation. By yeast two-hybrid systems cofilin was found to interact together with the triose-phosphate isomerase with the CD3 of the Na+,K+-ATPase α1- and α2-subunits [118, 119]. Rat Na+,K+-ATPase α2-subunit residues D672 and R700 were identified to be important for the interaction, as analyzed by mutational studies [120]. The binding region on rat cofilin essential for a functional association comprises residues 45–99 [118]. Cofilin binds to Na+,K+-ATPase upon phosphorylation by Rho-mediated signaling pathways, which results in an increased enzymatic activity of Na+,K+-ATPase [118, 119]. The observed activation is probably due to increased glycolytic levels fueling Na+,K+-ATPase. It was observed that the dephosphorylation of cofilin could be linked to ouabain signaling via Ras/Raf/MAPK such that cofilin is released from Na+,K+-ATPase [121]. Dephosphorylated cofilin seems to be involved in reorganization of the cytoskeletal structure and cell-volume regulation [121].
FSTL-1
Follistatin-like 1 (FSTL-1) is a secreted glycoprotein rich in cysteine that contains a follistatin-like domain and a pair of EF hands [122]. FSTL-1 was found to directly activate Na+,K+-ATPase α1-subunit [123]. FSTL-1-induced Na+,K+-ATPase α1-subunit activation was shown to regulate both membrane potential and neuronal excitability; both actions could be abolished by the Na+,K+-ATPase inhibitor ouabain. Using whole-cell recordings of rat spinal cord slices, it could be shown that FSTL-1 regulates synaptic transmission by activating Na+,K+-ATPase. Furthermore, FSTL-1 is required for maintaining the normal threshold of somatic sensation as Fstl-1 −/− mice displayed increased sensitivity to nociceptive stimuli. Thus, the Na+,K+-ATPase α1-subunit/FSTL-1 system seems to be of fundamental importance for somatic sensation with dysfunction of this system leading to abnormal sensation such as pain hypersensitivity [123].
It was suggested that the regions involved in the direct, high-affinity binding between FSTL-1 and Na+,K+-ATPase α1-subunit are the EF-hands in FSTL-1 (as ∆EF or mutants are inactive) and extracellular loops TM3–TM4 and TM7–TM8 or more specifically E309 and T885 in rat Na+,K+-ATPase α1-subunit, respectively [123]. However, T889 in rat Na+,K+-ATPase α1-subunit is not conserved in other species, e.g., human Na+,K+-ATPase α1-subunit exhibits a Val at the equivalent position, while the other three human isoforms contain a leucine residue. Thus, it will be interesting to see whether the interaction observed in rat can also be confirmed for FSTL-1 and Na+,K+-ATPase from other species. In addition, it would be interesting to investigate the structural consequences of FSTL-1 binding for the Na+,K+-ATPase β-subunit, which partly overlaps with the proposed TM7–TM8 binding site on Na+,K+-ATPase α1-subunit.
Interaction of Na+,K+-ATPase with other membrane proteins, transporters, and channels
Co-localization with glutamate transporters
Glutamate transporters are sodium-dependent proteins that rely on ion gradients generated by Na+,K+-ATPase. Its family members glutamate aspartate transporter (GLAST) and glutamate transporter 1 (GLT-1) are major players in the glutamate uptake in the mammalian central nervous system (CNS) [124–126]. A link between Na+,K+-ATPase and glutamate transporters has been previously proposed based on the observation that ouabain impairs glutamate transporter activity [127]. Rose et al. [128] investigated this putative connection in more detail and showed that glutamate transporters co-localize, co-purify, and co-immunoprecipitate with Na+,K+-ATPase α-subunit in rat brains. The activity of the glutamate transporters is regulated by Na+,K+-ATPase (as shown using the Na+,K+-ATPase inhibitor ouabain) with subtle differences between synaptosomes and astrocytes where ouabain displayed a bimodal effect on glutamate transporter activity [128]. Vice versa, glutamate transporters might be able to modulate Na+,K+-ATPase activity as increased expression of GLAST was shown to be linked to increased Na+,K+-ATPase activity [129]. Src inhibitors reduce glutamate transporter activity in synaptosomes to a level similar to that of ouabain [128]. Based on the observation that Na+,K+-ATPase forms a “signalosome” complex with Src that operated independent of ion pumping functions of Na+,K+-ATPase [37, 130], Rose et al. [128] propose the existence of a Na+,K+-ATPase/Src/glutamate transporter protein complex.
Nax channels
Nax channels are expressed in specific glial cells in the subfornical organ, which is responsible for monitoring the Na-levels in the human body. The Nax channels are sensitive to Na+ concentrations and they open upon increased levels of extracellular Na+. The resultant intracellular Na+ concentration is activating Na+,K+-ATPase, stimulating the anaerobic metabolism of glucose resulting in lactate production.
The Nax channel was shown to directly interact with the Na+,K+-ATPase α-subunit using a yeast two-hybrid system, pull-down, and co-immunoprecipitation assays as well as double-fluorescent immunostaining [131]. The C-terminal region (residues 1,489–1,681) of the mouse Nax channel interacts with the mouse α1-subunit segment 596–717, which is part of the P-domain. A similar interaction pattern was also observed between mouse Nax channel and mouse Na+,K+-ATPase α2-subunit, but not with mouse α3-subunit. Interestingly, on a sequence level, there are only very few differences between the mouse Na+,K+-ATPase α1-, α2- and α3-isoform in this region. Due to the close co-localization of Nax channel and Na+,K+-ATPase, it was suggested that Nax channels have a direct influence on molecular properties of Na+,K+-ATPase [131]. The interaction between the Nax channel and Na+,K+-ATPase was shown to play a crucial role for Na+-dependent stimulation of cellular glucose metabolism as both Na+ influx as well as stimulation of Na+,K+-ATPase by Nax channels are required for activation of glucose uptake [131].
Dopamine receptors
In striatal neurons, the neurotransmitter dopamine inhibits the activity of Na+,K+-ATPase, and apparently in a synergistic fashion, where activation of both the D1 and D2 receptor systems are required [132]. The coupling between the dopamine system and Na+,K+-ATPase has also been identified in photoreceptor cells, and appears to be isoform-specific, affecting only α3 [133, 134]. The dopamine system regulates the activity of Protein Kinase A by increasing (D1) or decreasing (D2) the level of cAMP, and it has been suggested that also Protein Kinase C activity is regulated by the dopamine system [135, 136]. Even though Na+,K+-ATPase is known to be regulated by phosphorylation [137], it was shown that dopamine inhibits the pump by a mechanism that does not involve phosphorylation [133]. Recently, the dopamine D1 receptor was found to interact with Na+,K+-ATPase α3-subunit in the spines of striatal neurons, either directly or through the scaffolding protein PSD-95 [138]. It is still not known how dopamine inhibits Na+,K+-ATPase α3-subunit without phosphorylation of Na+,K+-ATPase, but the physical linkage between the D1 receptor and Na+,K+-ATPase could be involved.
Somewhat surprisingly, co-immunoprecipitation of lysate from HEK cells transfected with either dopamine D1 or D2 receptors together with Na+,K+-ATPase α1-subunit have shown a direct interaction between both receptor types and the Na+,K+-ATPase α1-isoform [139]. Interestingly, Rb+ uptake experiments with HEK cells transfected with D1, D2, or control, and either treated with dopamine or a mock solution, indicated that both dopamine receptors could inhibit Na+,K+-ATPase α1-subunit by physical interaction. In the case of the D1 receptor, no inhibition was apparent after application of dopamine, while the D2-induced inhibition was not affected by dopamine [139].
In the striatum, the two-faced nature of dopamine action on the Na+,K+-ATPase is demonstrated by the influence of morphine treatment: short-term application stimulate Na+,K+-ATPase activity in a dose-dependent manner via a mechanism that depended on dopamine D2-like receptors, while long-term administration suppress Na+,K+-ATPase activity via the dopamine D1-like receptor system [140]. Sodium homeostasis is efficiently regulated upon variations in salt intake mainly by the coordination of sodium discharge or uptake through the action of hormones and peptides that control renal epithelia [141, 142]. Two main pathways implicated in the determination of blood pressure are linked to the activity of the Na+,K+-ATPase: the dopaminergic and the renin-angiotensin systems. In response to excessive salt consumption, the renal dopamine system up-regulates sodium excretion [143], and simultaneously inhibits the Na+,K+-ATPase [144, 145] and the Na+/H+ exchanger [146, 147], causing decreased vectorial sodium transport and reabsorption. The development of high blood pressure has been linked to decreased renal production of dopamine as well as to D1 receptor-G protein coupling defects [148]. In contrast to dopamine, which is thought to inhibit the Na+,K+-ATPase by internalization [149], angiotensin stimulates activity by recruitment of new pumps to the PM, possibly via a mechanism that regulates the phosphorylation pattern of the α-subunit of the Na+,K+-ATPase [150].
Aquaporin 4
Water molecules are able to pass through water channels—aquaporins—across biological membrane. Aquaporins are grouped into several subfamilies, and aquaporin 4 (AQP4) is the predominant water channel in the PM of astrocytes in the brain. AQP4 exists in multiple isoforms, of which the AQP4 M1 and M23 isoforms are most abundant in humans [151]. They differ in the N-terminal region, which is 22 amino acid residues longer in AQP4 M1.
So far, only a single study showed by co-immunoprecipitation, pull-down assays and photobleaching FRET (pbFRET) in rat brain tissue that the N-terminus of AQP4 interacts with Na+,K+-ATPase α1- and α2-subunits [152]. The cytoplasmic segment, comprising residues 23–32 (M23VAFKGVWTQ32) of rat AQP4 M1 and M23, was identified as the interaction site, with K27 and W30 being key residues for interaction. Furthermore, phosphorylation of T31 was found to further enhance the level of interaction. The proposed Na+,K+-ATPase binding site (residues 23–32) is unique for AQP4, where it is highly conserved between different species. Notably, the applied methods could not clarify whether the observed interaction is direct or mediated by scaffolding proteins. Hence it is also not known which part of Na+,K+-ATPase is involved in the possible interaction.
In addition, the same N-terminal fragment of AQP4 was also shown to interact with its regulator metabotropic glutamate receptor 5 (mGluR5) [152], suggesting the formation of an AQP4/Na+,K+-ATPase/mGluR5 macromolecular complex/transporting microdomain in astrocytes. This complex is suggested to be of functional importance for the regulation of water and K+ homeostasis in the brain, as well as for neuron-astrocyte metabolic crosstalk [152]. Obviously, further studies are required to confirm the presence of this complex and to elucidate its functionality in more detail.
AMPA receptor
AMPA (2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid)-type glutamatergic receptors (AMPARs) are heterotetrameric assemblies of GluR1-4 subunits that form Na+ permitting channels. They are major mediators of synaptic transmission and are critical for the formation of synaptic plasticity such as long-term potentiation and long-term depression [153], while the ion gradients maintained by Na+,K+-ATPase form a prerequisite for neuronal excitation. It was recently shown that Na+,K+-ATPase co-localizes and interacts with AMPARs in neurons and that Na+,K+-ATPase suppression causes internalization and proteasome-mediated degradation of AMPARs, leading to persistent suppression of AMPAR-mediated synaptic transmission [153]. A co-localization of Na+,K+-ATPase and AMPAR seems to be logical in a sense that in case of AMPAR activity, a fast Na+ clearance is required to maintain the physiological balance in the cell.
Polycystin-1
Polycystin-1 is a large membrane protein with 11 TM helices that has a large extracellular N-terminal domain, and a smaller ~200-aa cytoplasmic C-terminal tail. It is primarily expressed in epithelial cells of the developing renal tubules, but is also found in smaller amounts in other tissues. At the subcellular level, polycystin-1 is found in the primary cilium and lateral membrane of polarized epithelial cells. The cytoplasmic tail of polycystin-1 can be cleaved and liberated from the PM dormant part, and is then translocated to the nucleus where it may participate in gene regulation [154].
Following indications from yeast two-hybrid screens, that the C-terminal tail of polycystin-1 could interact with the Na+,K+-ATPase, pull-down experiments with GST fusion proteins further established a physical interaction between both A-domain and CD3 (P- and N-domain) of Na+,K+-ATPase. Co-immunoprecipitation with samples from MDCK cells or mouse kidney demonstrated that Na+,K+-ATPase interact with full-length polycystin-1 in vivo. A potential functional consequence of the interaction was demonstrated in CHO cells, where overexpression of polycystin-1 significantly upregulated ouabain sensitive ATPase activity, without affecting the apparent affinity for Na+ of the Na+,K+-ATPase. Since polycystin-1 is involved in the genetic disorder autosomal dominant polycystic kidney disease (ADPKD), which is characterized by accumulation of transepithelial fluid inside cysts, the interaction between polycystin-1 and the Na+,K+-ATPase could be of medical importance, since the Na+,K+-ATPase is a regulator of fluid balances [155].
Conclusions/future directions
It has become clear in the last years that Na+,K+-ATPase is not only an (fundamentally important) ion pump, but is also involved in many signaling events, most of them being regulated by protein–protein interactions involving Na+,K+-ATPase. We expect the number of discovered interaction partners to expand in the future.
Given the enormous variety of reported Na+,K+-ATPase interaction partners and the complexity of signaling cascades involved, it would be important to confirm and further characterize several Na+,K+-ATPase complexes initially discovered by pull-down assays using biophysical and structural methods. We envision a more detailed structural analysis of the Na+,K+-ATPase “signalosome” to shed light on the mechanistic details of Na+,K+-ATPase signaling in the near future.
A comprehensive understanding of Na+,K+-ATPase signaling would add another dimension to this fascinating enzyme and open the field for future interdisciplinary studies.
Acknowledgments
We apologize for publications that have not been cited due to space limitations. L.R. is supported by the Danish Council for Independent Research, Medical Sciences. H.T. is a Junior Research Fellow at Trinity College, Cambridge, and is supported by a HFSP Long-Term Fellowship. P.N. is supported by an ERC advanced grant (BIOMEMOS).
Abbreviations
- A-domain
Actuator domain
- ADP
Adenosine diphosphate
- ATP
Adenosine triphosphate
- AQP4
Aquaporin 4
- AMPAR
2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid (AMPA) receptor
- AMPK
Adenosine monophosphate-stimulated protein kinase
- AS160
Akt substrate of 160 kDa
- CBM
Caveolin binding motif
- CDx
x Cytoplasmatic domain of the Na+,K+-ATPase (x = 2, 3)
- CTS
Cardiotonic steroids
- DAG
1,2-diacylglycerol
- EGFR
Epidermal growth factor receptor
- ER
Endoplasmic reticulum
- ERK
Extracellular regulated kinases
- FSTL-1
Follistatin-like 1
- GLAST
Glutamate aspartate transporter
- GLT-1
Glutamate transporter 1
- GLUT4
Glucose transporter type 4
- IP3
Inositol 1,4,5-triphosphate
- IP3R
Inositol 1,4,5-triphosphate receptor
- MAPK
Mitogen-activated protein kinase
- mGluR5
Metabotropic glutamate receptor 5
- N-domain
Nucleotide binding domain
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- OBN
Ouabain
- P-domain
Phosphorylation domain
- pbFRET
Photobleaching fluorescence resonance energy transfer
- PIP2
Phosphatidylinositol 4,5-biphosphate
- PKC
Protein kinase C
- PLC-γ
Phospholipase C-γ
- PM
Plasma membrane
- PP2A
Protein phosphatase 2A
- ROS
Reactive oxygen species
- RPT
Renal proximal tubule
- PSD-95
Post-synaptic-density-95
- SERCA
Sarco(endo)plasmatic reticulum Ca2+-ATPase
- SHx domain
Src homology domain x (x = 1, 2, 3, 4)
- SIK1
Salt-induced kinase 1
- SNP
Single-nucleotide polymorphism
- SOS
Son of Sevenless
- TCTP
Translationally controlled tumor protein
- TM
Transmembrane
- TORCs
Transducers of regulated CREB (cAMP response element-binding)
References
- 1.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957;23:394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen PL, Carafoli E. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem Sci. 1987;12:146–150. doi: 10.1016/0968-0004(87)90071-5. [DOI] [Google Scholar]
- 3.Erecinska M, Silver IA. Ions and energy in mammalian brain. Prog Neurobiol. 1994;43:37–71. doi: 10.1016/0301-0082(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 4.Craig WS, Kyte J. Stoichiometry and molecular weight of the minimum asymmetric unit of canine renal sodium and potassium ion-activated adenosine triphosphatase. J Biol Chem. 1980;255:6262–6269. [PubMed] [Google Scholar]
- 5.Shull GE, Greeb J, Lingrel JB. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986;25:8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- 6.Pressley TA, Allen JC, Clarke CH, Odebunmi T, Higham SC. Amino-terminal processing of the catalytic subunit from Na(+)-K(+)-ATPase. Am J Physiol. 1996;271:C825–C832. doi: 10.1152/ajpcell.1996.271.3.C825. [DOI] [PubMed] [Google Scholar]
- 7.Poulsen H, Khandelia H, Morth JP, Bublitz M, Mouritsen OG, Egebjerg J, Nissen P. Neurological disease mutations compromise a C-terminal ion pathway in the Na(+)/K(+)-ATPase. Nature. 2010;467:99–102. doi: 10.1038/nature09309. [DOI] [PubMed] [Google Scholar]
- 8.Ackermann U, Geering K. Mutual dependence of Na, K-ATPase alpha- and beta-subunits for correct posttranslational processing and intracellular transport. FEBS Lett. 1990;269:105–108. doi: 10.1016/0014-5793(90)81130-G. [DOI] [PubMed] [Google Scholar]
- 9.Geering K. The functional role of beta subunits in oligomeric P-type ATPases. J Bioenerg Biomembr. 2001;33:425–438. doi: 10.1023/A:1010623724749. [DOI] [PubMed] [Google Scholar]
- 10.Tokhtaeva E, Sachs G, Sun H, Dada LA, Sznajder JI, Vagin O. Identification of the amino acid region involved in the intercellular interaction between the beta1 subunits of Na+/K+-ATPase. J Cell Sci. 2012;125:1605–1616. doi: 10.1242/jcs.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geering K. Function of FXYD proteins, regulators of Na, K-ATPase. J Bioenerg Biomembr. 2005;37:387–392. doi: 10.1007/s10863-005-9476-x. [DOI] [PubMed] [Google Scholar]
- 12.Sweadner KJ, Rael E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics. 2000;68:41–56. doi: 10.1006/geno.2000.6274. [DOI] [PubMed] [Google Scholar]
- 13.Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Ren Physiol. 2006;290:F241–F250. doi: 10.1152/ajprenal.00126.2005. [DOI] [PubMed] [Google Scholar]
- 14.Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TL, Petersen J, Andersen JP, Vilsen B, Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa H, Shinoda T, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc Natl Acad Sci USA. 2009;106:13742–13747. doi: 10.1073/pnas.0907054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinoda T, Ogawa H, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature. 2009;459:446–450. doi: 10.1038/nature07939. [DOI] [PubMed] [Google Scholar]
- 17.Yatime L, Laursen M, Morth JP, Esmann M, Nissen P, Fedosova NU. Structural insights into the high affinity binding of cardiotonic steroids to the Na+,K+-ATPase. J Struct Biol. 2011;174:296–306. doi: 10.1016/j.jsb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Lingrel JB. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na, K-ATPase. Annu Rev Physiol. 2010;72:395–412. doi: 10.1146/annurev-physiol-021909-135725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Lelievre L, Geering K. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J Biol Chem. 2000;275:1976–1986. doi: 10.1074/jbc.275.3.1976. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Velotta JB, McDonough AA, Farley RA. All human Na(+)-K(+)-ATPase alpha-subunit isoforms have a similar affinity for cardiac glycosides. Am J Physiol Cell Physiol. 2001;281:C1336–C1343. doi: 10.1152/ajpcell.2001.281.4.C1336. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez G, Nguyen AN, Timmerberg B, Tash JS, Blanco G. The Na, K-ATPase alpha4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol Hum Reprod. 2006;12:565–576. doi: 10.1093/molehr/gal062. [DOI] [PubMed] [Google Scholar]
- 22.Sonntag Y, Musgaard M, Olesen C, Schiott B, Moller JV, Nissen P, Thogersen L. Mutual adaptation of a membrane protein and its lipid bilayer during conformational changes. Nat Commun. 2011;2:304. doi: 10.1038/ncomms1307. [DOI] [PubMed] [Google Scholar]
- 23.Hasler U, Wang X, Crambert G, Beguin P, Jaisser F, Horisberger JD, Geering K. Role of beta-subunit domains in the assembly, stable expression, intracellular routing, and functional properties of Na, K-ATPase. J Biol Chem. 1998;273:30826–30835. doi: 10.1074/jbc.273.46.30826. [DOI] [PubMed] [Google Scholar]
- 24.Beggah A, Mathews P, Beguin P, Geering K. Degradation and endoplasmic reticulum retention of unassembled alpha- and beta-subunits of Na, K-ATPase correlate with interaction of BiP. J Biol Chem. 1996;271:20895–20902. doi: 10.1074/jbc.271.34.20895. [DOI] [PubMed] [Google Scholar]
- 25.Geering K, Kraehenbuhl JP, Rossier BC. Maturation of the catalytic alpha-subunit of Na, K-ATPase during intracellular transport. J Cell Biol. 1987;105:2613–2619. doi: 10.1083/jcb.105.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beguin P, Hasler U, Beggah A, Horisberger JD, Geering K. Membrane integration of Na, K-ATPase alpha-subunits and beta-subunit assembly. J Biol Chem. 1998;273:24921–24931. doi: 10.1074/jbc.273.38.24921. [DOI] [PubMed] [Google Scholar]
- 27.Morton MJ, Farr GA, Hull M, Capendeguy O, Horisberger JD, Caplan MJ. Association with beta-COP regulates the trafficking of the newly synthesized Na, K-ATPase. J Biol Chem. 2010;285:33737–33746. doi: 10.1074/jbc.M110.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farr GA, Hull M, Mellman I, Caplan MJ. Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J Cell Biol. 2009;186:269–282. doi: 10.1083/jcb.200901021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 30.Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 31.Williams TM, Lisanti MP. The caveolin proteins. Genome Biol. 2004;5:214. doi: 10.1186/gb-2004-5-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem. 2004;279:17250–17259. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 33.Einholm AP, Andersen JP, Vilsen B. Roles of transmembrane segment M1 of Na+,K+-ATPase and Ca2-ATPase, the gatekeeper and the pivot. J Bioenerg Biomembr. 2007;39:357–366. doi: 10.1007/s10863-007-9106-x. [DOI] [PubMed] [Google Scholar]
- 34.Daiho T, Danko S, Yamasaki K, Suzuki H. Stable structural analog of Ca2+-ATPase ADP-insensitive phosphoenzyme with occluded Ca2+ formed by elongation of A-domain/M1′-linker and beryllium fluoride binding. J Biol Chem. 2010;285:24538–24547. doi: 10.1074/jbc.M110.144535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai T, Wang H, Chen Y, Liu L, Gunning WT, Quintas LE, Xie ZJ. Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J Cell Biol. 2008;182:1153–1169. doi: 10.1083/jcb.200712022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Mohammadi K, Aynafshar B, Wang H, Li D, Liu J, Ivanov AV, Xie Z, Askari A. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol. 2003;284:C1550–C1560. doi: 10.1152/ajpcell.00555.2002. [DOI] [PubMed] [Google Scholar]
- 37.Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem. 2007;282:10585–10593. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Ivanov AV, Gable ME, Jolivel F, Morrill GA, Askari A. Comparative properties of caveolar and noncaveolar preparations of kidney Na(+)/K(+)-ATPase. Biochemistry. 2011;50:8664–8673. doi: 10.1021/bi2009008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barlet-Bas C, Khadouri C, Marsy S, Doucet A. Enhanced intracellular sodium concentration in kidney cells recruits a latent pool of Na-K-ATPase whose size is modulated by corticosteroids. J Biol Chem. 1990;265:7799–7803. [PubMed] [Google Scholar]
- 40.Alves DS, Farr GA, Seo-Mayer P, Caplan MJ. AS160 associates with the Na+,K+-ATPase and mediates the adenosine monophosphate-stimulated protein kinase-dependent regulation of sodium pump surface expression. Mol Biol Cell. 2010;21:4400–4408. doi: 10.1091/mbc.E10-06-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGrail K, Phillips J, Sweadner K. Immunofluorescent localization of three Na, K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na, K-ATPase. J Neurosci Off J Soc Neurosci. 1991;11:381–472. doi: 10.1523/JNEUROSCI.11-02-00381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bøttger P, Tracz Z, Heuck A, Nissen P, Romero-Ramos M, Lykke-Hartmann K. Distribution of Na/K-ATPase alpha 3 isoform, a sodium-potassium P-type pump associated with rapid-onset of dystonia parkinsonism (RDP) in the adult mouse brain. J Comp Neurol. 2011;519:376–780. doi: 10.1002/cne.22524. [DOI] [PubMed] [Google Scholar]
- 43.Pulver S, Griffith L. Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics. Nat Neurosci. 2010;13:53–62. doi: 10.1038/nn.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Sizov I, Dobretsov M, von Gersdorff H. Presynaptic Ca2+ buffers control the strength of a fast post-tetanic hyperpolarization mediated by the alpha3 Na(+)/K(+)-ATPase. Nat Neurosci. 2007;10:196–401. doi: 10.1038/nn1839. [DOI] [PubMed] [Google Scholar]
- 45.Blom H, Rönnlund D, Scott L, Spicarova Z, Widengren J, Bondar A, Aperia A, Brismar H. Spatial distribution of Na+-K+-ATPase in dendritic spines dissected by nanoscale superresolution STED microscopy. BMC Neurosci. 2011;12:16. doi: 10.1186/1471-2202-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim E, Niethammer M, Rothschild A, Jan Y, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–93. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 47.Kornau H, Schenker L, Kennedy M, Seeburg P (1995) Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science (New York, NY) 269:1737–1777 [DOI] [PubMed]
- 48.Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl T, Südhof T (1997) Binding of neuroligins to PSD-95. Science (New York, NY) 277:1511–1516 [DOI] [PubMed]
- 49.Taguchi K, Kumanogoh H, Nakamura S, Maekawa S. Ouabain-induced isoform-specific localization change of the Na+,K+-ATPase alpha subunit in the synaptic plasma membrane of rat brain. Neurosci Lett. 2007;413:42–47. doi: 10.1016/j.neulet.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 50.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs. 2007;7:173–189. doi: 10.2165/00129784-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 51.Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci USA. 1991;88:6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bagrov AY, Shapiro JI. Endogenous digitalis: pathophysiologic roles and therapeutic applications. Nat Clin Pract Nephrol. 2008;4:378–392. doi: 10.1038/ncpneph0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aydemir-Koksoy A, Abramowitz J, Allen JC. Ouabain-induced signaling and vascular smooth muscle cell proliferation. J Biol Chem. 2001;276:46605–46611. doi: 10.1074/jbc.M106178200. [DOI] [PubMed] [Google Scholar]
- 54.Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem. 2002;277:18694–18702. doi: 10.1074/jbc.M111357200. [DOI] [PubMed] [Google Scholar]
- 55.Kulikov A, Eva A, Kirch U, Boldyrev A, Scheiner-Bobis G. Ouabain activates signaling pathways associated with cell death in human neuroblastoma. Biochim Biophys Acta. 2007;1768:1691–1702. doi: 10.1016/j.bbamem.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Zheng M, Li Z, Li R, Jia L, Xiong X, Southall N, Wang S, Xia M, Austin CP, et al. Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Res. 2009;69:6556–6564. doi: 10.1158/0008-5472.CAN-09-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 58.Aleshin A, Finn RS. SRC: a century of science brought to the clinic. Neoplasia. 2010;12:599–607. doi: 10.1593/neo.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moran MF, Koch CA, Anderson D, Ellis C, England L, Martin GS, Pawson T. Src homology region 2 domains direct protein–protein interactions in signal transduction. Proc Natl Acad Sci USA. 1990;87:8622–8626. doi: 10.1073/pnas.87.21.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu H, Rosen MK, Shin TB, Seidel-Dugan C, Brugge JS, Schreiber SL. Solution structure of the SH3 domain of Src and identification of its ligand-binding site. Science. 1992;258:1665–1668. doi: 10.1126/science.1280858. [DOI] [PubMed] [Google Scholar]
- 61.Machida K, Mayer BJ. The SH2 domain: versatile signaling module and pharmaceutical target. Biochim Biophys Acta. 2005;1747:1–25. doi: 10.1016/j.bbapap.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Kaneko T, Li L, Li SS. The SH3 domain–a family of versatile peptide- and protein-recognition module. Front Biosci. 2008;13:4938–4952. doi: 10.2741/3053. [DOI] [PubMed] [Google Scholar]
- 63.MacAuley A, Cooper JA. Structural differences between repressed and derepressed forms of p60c-src. Mol Cell Biol. 1989;9:2648–2656. doi: 10.1128/mcb.9.6.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 65.Cowan-Jacob SW, Fendrich G, Manley PW, Jahnke W, Fabbro D, Liebetanz J, Meyer T. The crystal structure of a c-Src complex in an active conformation suggests possible steps in c-Src activation. Structure. 2005;13:861–871. doi: 10.1016/j.str.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 66.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006;17:317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Z, Cai T, Tian J, Xie JX, Zhao X, Liu L, Shapiro JI, Xie Z. NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J Biol Chem. 2009;284:21066–21076. doi: 10.1074/jbc.M109.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye Q, Li Z, Tian J, Xie JX, Liu L, Xie Z. Identification of a potential receptor that couples ion transport to protein kinase activity. J Biol Chem. 2011;286:6225–6232. doi: 10.1074/jbc.M110.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Zhang Z, Xie JX, Li X, Tian J, Cai T, Cui H, Ding H, Shapiro JI, Xie Z. Na/K-ATPase mimetic pNaKtide peptide inhibits the growth of human cancer cells. J Biol Chem. 2011;286:32394–32403. doi: 10.1074/jbc.M110.207597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olesen C, Picard M, Winther AM, Gyrup C, Morth JP, Oxvig C, Moller JV, Nissen P. The structural basis of calcium transport by the calcium pump. Nature. 2007;450:1036–1042. doi: 10.1038/nature06418. [DOI] [PubMed] [Google Scholar]
- 71.Muller AJ, Pendergast AM, Havlik MH, Puil L, Pawson T, Witte ON. A limited set of SH2 domains binds BCR through a high-affinity phosphotyrosine-independent interaction. Mol Cell Biol. 1992;12:5087–5093. doi: 10.1128/mcb.12.11.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raffel GD, Parmar K, Rosenberg N. In vivo association of v-Abl with Shc mediated by a non-phosphotyrosine-dependent SH2 interaction. J Biol Chem. 1996;271:4640–4645. doi: 10.1074/jbc.271.9.4640. [DOI] [PubMed] [Google Scholar]
- 73.Poy F, Yaffe MB, Sayos J, Saxena K, Morra M, Sumegi J, Cantley LC, Terhorst C, Eck MJ. Crystal structures of the XLP protein SAP reveal a class of SH2 domains with extended, phosphotyrosine-independent sequence recognition. Mol Cell. 1999;4:555–561. doi: 10.1016/S1097-2765(00)80206-3. [DOI] [PubMed] [Google Scholar]
- 74.Huang Q, Szebenyi DM. Structural basis for the interaction between the growth factor-binding protein GRB10 and the E3 ubiquitin ligase NEDD4. J Biol Chem. 2010;285:42130–42139. doi: 10.1074/jbc.M110.143412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karpova LV, Bulygina ER, Boldyrev AA. Different neuronal Na(+)/K(+)-ATPase isoforms are involved in diverse signaling pathways. Cell Biochem Funct. 2010;28:135–141. doi: 10.1002/cbf.1632. [DOI] [PubMed] [Google Scholar]
- 76.Konrad L, Dietze R, Kirch U, Kirch H, Eva A, Scheiner-Bobis G. Cardiotonic steroids trigger non-classical testosterone signaling in Sertoli cells via the alpha4 isoform of the sodium pump. Biochim Biophys Acta. 2011;1813:2118–2124. doi: 10.1016/j.bbamcr.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 77.Weigand KM, Swarts HG, Fedosova NU, Russel FG, Koenderink JB. Na, K-ATPase activity modulates Src activation: a role for ATP/ADP ratio. Biochim Biophys Acta. 2012;1818:1269–1273. doi: 10.1016/j.bbamem.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 78.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275:27832–27837. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 79.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293:C509–C536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 80.Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. 2000;275:27838–27844. doi: 10.1074/jbc.M002950200. [DOI] [PubMed] [Google Scholar]
- 81.Kominato R, Fujimoto S, Mukai E, Nakamura Y, Nabe K, Shimodahira M, Nishi Y, Funakoshi S, Seino Y, Inagaki N. Src activation generates reactive oxygen species and impairs metabolism-secretion coupling in diabetic Goto-Kakizaki and ouabain-treated rat pancreatic islets. Diabetologia. 2008;51:1226–1235. doi: 10.1007/s00125-008-1008-x. [DOI] [PubMed] [Google Scholar]
- 82.Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell. 2005;16:4034–4045. doi: 10.1091/mbc.E05-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung SH, Kim SK, Kim JK, Yang YR, Suh PG, Chang JS. A double point mutation in PCL-gamma1 (Y509A/F510A) enhances Y783 phosphorylation and inositol phospholipid-hydrolyzing activity upon EGF stimulation. Exp Mol Med. 2010;42:216–227. doi: 10.3858/emm.2010.42.3.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dellis O, Dedos SG, Tovey SC, Taufiq Ur R, Dubel SJ, Taylor CW. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313:229–233. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]
- 85.Zhang S, Malmersjo S, Li J, Ando H, Aizman O, Uhlen P, Mikoshiba K, Aperia A. Distinct role of the N-terminal tail of the Na, K-ATPase catalytic subunit as a signal transducer. J Biol Chem. 2006;281:21954–21962. doi: 10.1074/jbc.M601578200. [DOI] [PubMed] [Google Scholar]
- 86.Aperia A. New roles for an old enzyme: Na, K-ATPase emerges as an interesting drug target. J Intern Med. 2007;261:44–52. doi: 10.1111/j.1365-2796.2006.01745.x. [DOI] [PubMed] [Google Scholar]
- 87.Miyakawa-Naito A, Uhlen P, Lal M, Aizman O, Mikoshiba K, Brismar H, Zelenin S, Aperia A. Cell signaling microdomain with Na, K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. J Biol Chem. 2003;278:50355–50361. doi: 10.1074/jbc.M305378200. [DOI] [PubMed] [Google Scholar]
- 88.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 89.Yudowski GA, Efendiev R, Pedemonte CH, Katz AI, Berggren PO, Bertorello AM. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+,K+-ATPase alpha subunit and regulates its trafficking. Proc Natl Acad Sci USA. 2000;97:6556–6561. doi: 10.1073/pnas.100128297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou X, Jiang G, Zhao A, Bondeva T, Hirszel P, Balla T. Inhibition of Na, K-ATPase activates PI3 kinase and inhibits apoptosis in LLC-PK1 cells. Biochem Biophys Res Commun. 2001;285:46–51. doi: 10.1006/bbrc.2001.5126. [DOI] [PubMed] [Google Scholar]
- 91.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol. 2007;293:C1489–C1497. doi: 10.1152/ajpcell.00158.2007. [DOI] [PubMed] [Google Scholar]
- 92.Eva A, Kirch U, Scheiner-Bobis G. Signaling pathways involving the sodium pump stimulate NO production in endothelial cells. Biochim Biophys Acta. 2006;1758:1809–1814. doi: 10.1016/j.bbamem.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 93.Liu J, Kesiry R, Periyasamy SM, Malhotra D, Xie Z, Shapiro JI. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 2004;66:227–241. doi: 10.1111/j.1523-1755.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 94.Jung J, Kim M, Kim MJ, Kim J, Moon J, Lim JS, Lee K. Translationally controlled tumor protein interacts with the third cytoplasmic domain of Na, K-ATPase alpha subunit and inhibits the pump activity in HeLa cells. J Biol Chem. 2004;279:49868–49875. doi: 10.1074/jbc.M400895200. [DOI] [PubMed] [Google Scholar]
- 95.Jung J, Kim HY, Kim M, Sohn K, Lee K. Translationally controlled tumor protein induces human breast epithelial cell transformation through the activation of Src. Oncogene. 2011;30:2264–2274. doi: 10.1038/onc.2010.604. [DOI] [PubMed] [Google Scholar]
- 96.Matsuoka Y, Li X, Bennett V. Adducin: structure, function and regulation. Cell Mol Life Sci. 2000;57:884–895. doi: 10.1007/PL00000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bennett V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta. 1989;988:107–121. doi: 10.1016/0304-4157(89)90006-3. [DOI] [PubMed] [Google Scholar]
- 98.Kuhlman PA, Hughes CA, Bennett V, Fowler VM. A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. J Biol Chem. 1996;271:7986–7991. doi: 10.1074/jbc.271.14.7986. [DOI] [PubMed] [Google Scholar]
- 99.Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, Matsuura Y, Kaibuchi K. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J Cell Biol. 1999;145:347–361. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hughes CA, Bennett V. Adducin: a physical model with implications for function in assembly of spectrin-actin complexes. J Biol Chem. 1995;270:18990–18996. doi: 10.1074/jbc.270.32.18990. [DOI] [PubMed] [Google Scholar]
- 101.Tripodi G, Valtorta F, Torielli L, Chieregatti E, Salardi S, Trusolino L, Menegon A, Ferrari P, Marchisio PC, Bianchi G. Hypertension-associated point mutations in the adducin alpha and beta subunits affect actin cytoskeleton and ion transport. J Clin Invest. 1996;97:2815–2822. doi: 10.1172/JCI118737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bianchi G, Tripodi G, Casari G, Salardi S, Barber BR, Garcia R, Leoni P, Torielli L, Cusi D, Ferrandi M, et al. Two point mutations within the adducin genes are involved in blood pressure variation. Proc Natl Acad Sci USA. 1994;91:3999–4003. doi: 10.1073/pnas.91.9.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferrandi M, Salardi S, Tripodi G, Barassi P, Rivera R, Manunta P, Goldshleger R, Ferrari P, Bianchi G, Karlish SJ. Evidence for an interaction between adducin and Na(+)-K(+)-ATPase: relation to genetic hypertension. Am J Physiol. 1999;277:H1338–H1349. doi: 10.1152/ajpheart.1999.277.4.H1338. [DOI] [PubMed] [Google Scholar]
- 104.Ferrandi M, Molinari I, Torielli L, Padoani G, Salardi S, Rastaldi MP, Ferrari P, Bianchi G (2010) Adducin- and ouabain-related gene variants predict the antihypertensive activity of rostafuroxin, part 1: experimental studies. Sci Transl Med 2:59ra86. doi:10.1126/scitranslmed.3001815 [DOI] [PubMed]
- 105.Ferrari P, Torielli L, Ferrandi M, Padoani G, Duzzi L, Florio M, Conti F, Melloni P, Vesci L, Corsico N, et al. PST2238: a new antihypertensive compound that antagonizes the long-term pressor effect of ouabain. J Pharmacol Exp Ther. 1998;285:83–94. [PubMed] [Google Scholar]
- 106.Ferrari P, Ferrandi M, Tripodi G, Torielli L, Padoani G, Minotti E, Melloni P, Bianchi G. PST 2238: a new antihypertensive compound that modulates Na, K-ATPase in genetic hypertension. J Pharmacol Exp Ther. 1999;288:1074–1083. [PubMed] [Google Scholar]
- 107.Ferrari P, Ferrandi M, Valentini G, Bianchi G. Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na+-K+-ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R529–R535. doi: 10.1152/ajpregu.00518.2005. [DOI] [PubMed] [Google Scholar]
- 108.Ye Q, Li Z, Tian J, Xie JX, Liu L, Xie Z. Identification of a potential receptor that couples ion transport to protein kinase activity. J Biol Chem. 2011;286:6225–6232. doi: 10.1074/jbc.M110.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hatou S. Hormonal regulation of Na+/K+-dependent ATPase activity and pump function in corneal endothelial cells. Cornea. 2011;30(Suppl 1):S60–S66. doi: 10.1097/ICO.0b013e318227faab. [DOI] [PubMed] [Google Scholar]
- 110.Pierre SV, Belliard A, Sottejeau Y. Modulation of Na(+)-K(+)-ATPase cell surface abundance through structural determinants on the alpha1-subunit. Am J Physiol Cell Physiol. 2011;300:C42–C48. doi: 10.1152/ajpcell.00386.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arystarkhova E, Sweadner KJ. Splice variants of the gamma subunit (FXYD2) and their significance in regulation of the Na, K-ATPase in kidney. J Bioenerg Biomembr. 2005;37:381–386. doi: 10.1007/s10863-005-9475-y. [DOI] [PubMed] [Google Scholar]
- 112.Chibalin AV, Pedemonte CH, Katz AI, Feraille E, Berggren PO, Bertorello AM. Phosphorylation of the catalytic alpha-subunit constitutes a triggering signal for Na+,K+-ATPase endocytosis. J Biol Chem. 1998;273:8814–8819. doi: 10.1074/jbc.273.15.8814. [DOI] [PubMed] [Google Scholar]
- 113.Efendiev R, Bertorello AM, Pressley TA, Rousselot M, Feraille E, Pedemonte CH. Simultaneous phosphorylation of Ser11 and Ser18 in the alpha-subunit promotes the recruitment of Na(+), K(+)-ATPase molecules to the plasma membrane. Biochemistry. 2000;39:9884–9892. doi: 10.1021/bi0007831. [DOI] [PubMed] [Google Scholar]
- 114.Taub M, Springate JE, Cutuli F. Targeting of renal proximal tubule Na, K-ATPase by salt-inducible kinase. Biochem Biophys Res Commun. 2010;393:339–344. doi: 10.1016/j.bbrc.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sjostrom M, Stenstrom K, Eneling K, Zwiller J, Katz AI, Takemori H, Bertorello AM. SIK1 is part of a cell sodium-sensing network that regulates active sodium transport through a calcium-dependent process. Proc Natl Acad Sci USA. 2007;104:16922–16927. doi: 10.1073/pnas.0706838104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kovacs KA, Steullet P, Steinmann M, Do KQ, Magistretti PJ, Halfon O, Cardinaux JR. TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. Proc Natl Acad Sci USA. 2007;104:4700–4705. doi: 10.1073/pnas.0607524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Popov S, Silveira A, Wagsater D, Takemori H, Oguro R, Matsumoto S, Sugimoto K, Kamide K, Hirose T, Satoh M, et al. Salt-inducible kinase 1 influences Na+,K+-ATPase activity in vascular smooth muscle cells and associates with variations in blood pressure. J Hypertens. 2011;29:2395–2403. doi: 10.1097/HJH.0b013e32834d3d55. [DOI] [PubMed] [Google Scholar]
- 118.Lee K, Jung J, Kim M, Guidotti G. Interaction of the alpha subunit of Na, K-ATPase with cofilin. Biochem J. 2001;353:377–385. doi: 10.1042/0264-6021:3530377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jung J, Yoon T, Choi EC, Lee K. Interaction of cofilin with triose-phosphate isomerase contributes glycolytic fuel for Na, K-ATPase via Rho-mediated signaling pathway. J Biol Chem. 2002;277:48931–48937. doi: 10.1074/jbc.M208806200. [DOI] [PubMed] [Google Scholar]
- 120.Kim M, Jung J, Park CS, Lee K. Identification of the cofilin-binding sites in the large cytoplasmic domain of Na, K-ATPase. Biochimie. 2002;84:1021–1029. doi: 10.1016/S0300-9084(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 121.Jung J, Kim M, Choi S, Kim MJ, Suh JK, Choi EC, Lee K. Molecular mechanism of cofilin dephosphorylation by ouabain. Cell Signal. 2006;18:2033–2040. doi: 10.1016/j.cellsig.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 122.Grabarek Z. Structural basis for diversity of the EF-hand calcium-binding proteins. J Mol Biol. 2006;359:509–525. doi: 10.1016/j.jmb.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 123.Li KC, Zhang FX, Li CL, Wang F, Yu MY, Zhong YQ, Zhang KH, Lu YJ, Wang Q, Ma XL, et al. Follistatin-like 1 suppresses sensory afferent transmission by activating Na+,K+-ATPase. Neuron. 2011;69:974–987. doi: 10.1016/j.neuron.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 124.Amara SG, Fontana AC. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int. 2002;41:313–318. doi: 10.1016/S0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 125.Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev. 2004;45:250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 126.Kanner BI. Structure and function of sodium-coupled GABA and glutamate transporters. J Membr Biol. 2006;213:89–100. doi: 10.1007/s00232-006-0877-5. [DOI] [PubMed] [Google Scholar]
- 127.Li S, Stys PK. Na(+)-K(+)-ATPase inhibition and depolarization induce glutamate release via reverse Na(+)-dependent transport in spinal cord white matter. Neuroscience. 2001;107:675–683. doi: 10.1016/S0306-4522(01)00385-2. [DOI] [PubMed] [Google Scholar]
- 128.Rose EM, Koo JC, Antflick JE, Ahmed SM, Angers S, Hampson DR. Glutamate transporter coupling to Na, K-ATPase. J Neurosci. 2009;29:8143–8155. doi: 10.1523/JNEUROSCI.1081-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gegelashvili M, Rodriguez-Kern A, Sung L, Shimamoto K, Gegelashvili G. Glutamate transporter GLAST/EAAT1 directs cell surface expression of FXYD2/gamma subunit of Na, K-ATPase in human fetal astrocytes. Neurochem Int. 2007;50:916–920. doi: 10.1016/j.neuint.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 130.Xie Z. Molecular mechanisms of Na/K-ATPase-mediated signal transduction. Ann N Y Acad Sci. 2003;986:497–503. doi: 10.1111/j.1749-6632.2003.tb07234.x. [DOI] [PubMed] [Google Scholar]
- 131.Shimizu H, Watanabe E, Hiyama TY, Nagakura A, Fujikawa A, Okado H, Yanagawa Y, Obata K, Noda M. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron. 2007;54:59–72. doi: 10.1016/j.neuron.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 132.Bertorello A, Hopfield J, Aperia A, Greengard P. Inhibition by dopamine of (Na(+)+K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature. 1990;347:386–394. doi: 10.1038/347386a0. [DOI] [PubMed] [Google Scholar]
- 133.Nishi A, Fisone G, Snyder G, Dulubova I, Aperia A, Nairn A, Greengard P. Regulation of Na+,K+-ATPase isoforms in rat neostriatum by dopamine and protein kinase C. J Neurochem. 1999;73:1492–1993. doi: 10.1046/j.1471-4159.1999.0731492.x. [DOI] [PubMed] [Google Scholar]
- 134.Shulman L, Fox D. Dopamine inhibits mammalian photoreceptor Na+,K+-ATPase activity via a selective effect on the alpha3 isozyme. Proc Natl Acad Sci U S A. 1996;93:8034–8043. doi: 10.1073/pnas.93.15.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Callier S, Snapyan M, Le Crom S, Prou D, Vincent J-D, Vernier P. Evolution and cell biology of dopamine receptors in vertebrates. Biol Cell Under Auspices Eur Cell Biol Organ. 2003;95:489–991. doi: 10.1016/S0248-4900(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L, Guo F, Guo H, Wang H, Zhang Z, Liu X, Shi X, Gou X, Su Q, Yin J, et al. The paradox of dopamine and angiotensin II-mediated Na(+), K(+)-ATPase regulation in renal proximal tubules. Clin Exp Hypertens. 2010;32:464–468. doi: 10.3109/10641963.2010.496516. [DOI] [PubMed] [Google Scholar]
- 137.Poulsen H, Morth P, Egebjerg J, Nissen P. Phosphorylation of the Na+,K+-ATPase and the H+, K+-ATPase. FEBS Lett. 2010;584:2589–2684. doi: 10.1016/j.febslet.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 138.Hans B, Daniel R, Lena S, Zuzana S, Ville R, Jerker W, Anita A, Hjalmar B. Nearest neighbor analysis of dopamine D1 receptors and Na+-K+-ATPases in dendritic spines dissected by STED microscopy. Microsc Res Tech. 2011 doi: 10.1002/jemt.21046. [DOI] [PubMed] [Google Scholar]
- 139.Hazelwood L, Free R, Cabrera D, Skinbjerg M, Sibley D. Reciprocal modulation of function between the D1 and D2 dopamine receptors and the Na+,K+-ATPase. J Biol Chem. 2008;283:36441–36494. doi: 10.1074/jbc.M805520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu ZQ, Chen J, Chi ZQ, Liu JG. Involvement of dopamine system in regulation of Na+,K+-ATPase in the striatum upon activation of opioid receptors by morphine. Mol Pharmacol. 2007;71:519–530. doi: 10.1124/mol.106.029561. [DOI] [PubMed] [Google Scholar]
- 141.Bianchi G, Fox U, Di Francesco GF, Bardi U, Radice M. The hypertensive role of the kidney in spontaneously hypertensive rats. Clin Sci Mol Med Suppl. 1973;45(Suppl 1):135s–139s. doi: 10.1042/cs045135s. [DOI] [PubMed] [Google Scholar]
- 142.Dahl LK, Heine M, Thompson K. Genetic influence of renal homografts on the blood pressure of rats from different strains. Proc Soc Exp Biol Med. 1972;140:852–856. doi: 10.3181/00379727-140-36566. [DOI] [PubMed] [Google Scholar]
- 143.Alexander RW, Gill JR, Jr, Yamabe H, Lovenberg W, Keiser HR. Effects of dietary sodium and of acute saline infusion on the interrelationship between dopamine excretion and adrenergic activity in man. J Clin Invest. 1974;54:194–200. doi: 10.1172/JCI107743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bertorello AM, Katz AI. Short-term regulation of renal Na–K-ATPase activity: physiological relevance and cellular mechanisms. Am J Physiol. 1993;265:F743–F755. doi: 10.1152/ajprenal.1993.265.6.F743. [DOI] [PubMed] [Google Scholar]
- 145.Bertorello A, Hokfelt T, Goldstein M, Aperia A. Proximal tubule Na+-K+-ATPase activity is inhibited during high-salt diet: evidence for DA-mediated effect. Am J Physiol. 1988;254:F795–F801. doi: 10.1152/ajprenal.1988.254.6.F795. [DOI] [PubMed] [Google Scholar]
- 146.Bacic D, Kaissling B, McLeroy P, Zou L, Baum M, Moe OW. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int. 2003;64:2133–2141. doi: 10.1046/j.1523-1755.2003.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Felder CC, Albrecht FE, Campbell T, Eisner GM, Jose PA. cAMP-independent, G protein-linked inhibition of Na+/H+ exchange in renal brush border by D1 dopamine agonists. Am J Physiol. 1993;264:F1032–F1037. doi: 10.1152/ajprenal.1993.264.6.F1032. [DOI] [PubMed] [Google Scholar]
- 148.Carey RM. Theodore cooper lecture: renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension. 2001;38:297–302. doi: 10.1161/hy0901.096422. [DOI] [PubMed] [Google Scholar]
- 149.Pedemonte CH, Efendiev R, Bertorello AM. Inhibition of Na, K-ATPase by dopamine in proximal tubule epithelial cells. Semin Nephrol. 2005;25:322–327. doi: 10.1016/j.semnephrol.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 150.Yingst DR, Massey KJ, Rossi NF, Mohanty MJ, Mattingly RR. Angiotensin II directly stimulates activity and alters the phosphorylation of Na–K-ATPase in rat proximal tubule with a rapid time course. Am J Physiol Renal Physiol. 2004;287:F713–F721. doi: 10.1152/ajprenal.00065.2004. [DOI] [PubMed] [Google Scholar]
- 151.Lu M, Lee MD, Smith BL, Jung JS, Agre P, Verdijk MA, Merkx G, Rijss JP, Deen PM. The human AQP4 gene: definition of the locus encoding two water channel polypeptides in brain. Proc Natl Acad Sci USA. 1996;93:10908–10912. doi: 10.1073/pnas.93.20.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Illarionova NB, Gunnarson E, Li Y, Brismar H, Bondar A, Zelenin S, Aperia A. Functional and molecular interactions between aquaporins and Na, K-ATPase. Neuroscience. 2010;168:915–925. doi: 10.1016/j.neuroscience.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 153.Zhang D, Hou Q, Wang M, Lin A, Jarzylo L, Navis A, Raissi A, Liu F, Man HY. Na, K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J Neurosci. 2009;29:4498–4511. doi: 10.1523/JNEUROSCI.6094-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chapin HC, Caplan MJ. The cell biology of polycystic kidney disease. J Cell Biol. 2010;191:701–710. doi: 10.1083/jcb.201006173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zatti A, Chauvet V, Rajendran V, Kimura T, Pagel P, Caplan MJ. The C-terminal tail of the polycystin-1 protein interacts with the Na, K-ATPase alpha-subunit. Mol Biol Cell. 2005;16:5087–5093. doi: 10.1091/mbc.E05-03-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by Wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6:909–919. doi: 10.1016/S1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- 157.Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, Lambert-van der Brempt C, Morgentin R, Norman RA, Olivier A, et al. N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5-(tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem. 2006;49:6465–6488. doi: 10.1021/jm060434q. [DOI] [PubMed] [Google Scholar]
- 158.Feng Y, Liu D, Yao H, Wang J. Solution structure and mapping of a very weak calcium-binding site of human translationally controlled tumor protein by NMR. Arch Biochem Biophys. 2007;467:48–57. doi: 10.1016/j.abb.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 159.Pope BJ, Zierler-Gould KM, Kuhne R, Weeds AG, Ball LJ. Solution structure of human cofilin: actin binding, pH sensitivity, and relationship to actin-depolymerizing factor. J Biol Chem. 2004;279:4840–4848. doi: 10.1074/jbc.M310148200. [DOI] [PubMed] [Google Scholar]
- 160.Ho JD, Yeh R, Sandstrom A, Chorny I, Harries WE, Robbins RA, Miercke LJ, Stroud RM. Crystal structure of human aquaporin 4 at 1.8 A and its mechanism of conductance. Proc Natl Acad Sci USA. 2009;106:7437–7442. doi: 10.1073/pnas.0902725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lifshitz Y, Petrovich E, Haviv H, Goldshleger R, Tal DM, Garty H, Karlish SJD. Purification of the human alpha2 isoform of Na, K-ATPase expressed in Pichia pastoris. Stabilization by lipids and FXYD1. Biochemistry. 2007;46:14937–14950. doi: 10.1021/bi701812c. [DOI] [PubMed] [Google Scholar]
- 162.Cornelius F, Turner N, Christensen HR. Modulation of Na, K-ATPase by phospholipids and cholesterol. II. Steady-state and presteadystate kinetics. Biochemistry. 2003;42(28):8541–8549. doi: 10.1021/bi034532e. [DOI] [PubMed] [Google Scholar]