Abstract
Withania somnifera, also called ‘Indian ginseng’, is an important medicinal plant of the Indian subcontinent. It is widely used, singly or in combination, with other herbs against many ailments in Indian Systems of Medicine since time immemorial. Withania somnifera contains a spectrum of diverse phytochemicals enabling it to have a broad range of biological implications. In preclinical studies, it has shown anti-microbial, anti-inflammatory, anti-tumor, anti-stress, neuroprotective, cardioprotective, and anti-diabetic properties. Additionally, it has demonstrated the ability to reduce reactive oxygen species, modulate mitochondrial function, regulate apoptosis, and reduce inflammation and enhance endothelial function. In view of these pharmacologic properties, W. somnifera is a potential drug candidate to treat various clinical conditions, particularly related to the nervous system. In this review, we summarize the pharmacologic characteristics and discuss the mechanisms of action and potential therapeutic applications of the plant and its active constituents.
Keywords: Withania somnifera, Anti-bacterial, Anti-inflammatory, Anti-arthritic, Anti-cancer, Cardio-protective, Anti-diabetic, Anti-stress, Parkinson’s disease, Alzheimer’s disease, Stroke hypoxia
Introduction
Withania somnifera (W. Somnifera) is a small woody shrub commonly known as “Winter cherry” or “Indian Ginseng”. In Sanskrit it is known as ‘Ashwagandha’ and in Urdu as ‘Asgand’ [1, 2]. It belongs to the family Solanaceae and attains a height of 0.5–2 m. The plant is widely distributed in the drier parts of tropical and subtropical zones ranging from the Canary Islands, South Africa, Middle East, Sri Lanka, India and to China. It is cultivated in gardens in the warmer parts of Europe and has become a wild weed in some parts of Australia [3, 4]. However, in India it is grown as a medicinal crop [5]. The whole plant or its different parts are widely used in Ayurvedic and Unani systems of medicine (indigenous systems of medicine in India) for its medicinal properties and has been used since antiquity. The plant is mentioned as an official drug in Indian Pharmacopoeia-1985 [6, 7].
In Ayurveda it is a prominent herbal Rasayana and is known as “Sattvic Kapha Rasayana”. Rasayana is a herbal or metallic preparation that is used for pharmacologic properties such as/in adaptogenic, aphrodisiac, tonic, narcotic, diuretic, anti-helminthic, astringent, thermogenic and stimulant, anti-stress, anti-inflammation, anti-carbuncle, anti-ulcer, debility from old age, rheumatism, vitiated conditions of vata, leucoderma, constipation, insomnia, nervous breakdown, goiter, leucorrhoea, boils, pimples, flatulent colic, worms, piles, and oligospermia [8–13]. Additionally, it is prescribed for snake venom and scorpion sting [14–16]. In the Unani system of medicine, the plant has been mentioned in an old testament “Kitab-ul-Hashaish” by Dioscorides in 78 AD. In Unani, Asgand has various therapeutic uses. Asgand has been recommended for the treatment of various ailments, which include arthritis, lumbago, carbuncle, spermatorrhoea, asthma, leucoderma, general debility, sexual debility, anxiety, neurosis, scabies, ulcers, and leucorrhoea [6, 17–21]. Owing to its pronounced stress-busting qualities, the plant has been given its species name ‘somnifera’, which is a Latin word meaning ‘sleep-inducer’ [22, 23]. Pharmacologic effects and folkloric uses of W. somnifera are akin to that of Korean Ginseng tea, which furnishes a modest explanation for calling W. somnifera as Indian Ginseng [24].
In Unani and Ayurvedic systems of medicine, mostly roots of W. somnifera are used for the therapeutic purposes. The plant loses it pharmacologic activity after 2 years, therefore freshly dried roots are preferred for good results [6, 7]. The leaves of the plant are bitter and have some medicinal uses in fever and painful swelling. The flowers are astringent, depurative, diuretic, and aphrodisiac. The seeds are anti-helminthic, remove white spots from the cornea, increase sperm count, as well as testicular growth. The fruits have been traditionally used as a topical treatment for tumors and tubercular glands, carbuncles, and skin ulcers [7, 25, 26].
Chemical composition
Phytochemical studies have shown the presence of different chemical constituents in various regions of W. somnifera. More than 12 alkaloids, 40 withanolides and several sitoindosides have been isolated and reported from the plant [27]. The major chemical constituents of W. somnifera are (Table 1):
- Alkaloids
Withanine, withananine, withasomnine, somniferine, tropeltigloate, somniferinine, somninine and nicotine [28]
- Steroidal lactones
Withaferin-A, withanone, withanolide-E, withanolide-F, withanolide-A, withanolide-G, withanolide-H, withanolide-I, withanolide-J, withanolide-K, withanolide-L, withanolide-M [27, 28]
- Steroids
Cholesterol, β-sitosterol, stigmasterol, diosgenin, stigmastadien, sitoinosides VII, sitoinosides VIII, sitoinosides IX, sitoinosides X [27, 29]
- Salts
Cuscohygrine, anahygrine, tropine, pseudotropine, anaferine [30]
- Flavonoids
Kaempferol, quercetin [29]
- Nitrogen-containing compounds
Withanol, somnisol, and somnitol [28]
Table 1.
Bio-active compounds in the different parts of the plant
| Part of plant | Bio-active compounds present |
|---|---|
| Root | Vitoindosides VII, VIII (acylsteryl-glucoside) [31], sitoindosides IX, X (glycowithanolide) [32], withanine, withananine (alkaloids), withanolide-A, viscosa lactone-B, stigmasterol, stigmasterol [33–35] and ashwagandhanolide [27, 36] |
| Leaf | Withaferin [37], withaferin-A, withanone, withanolide-D, withanolide-E, withanolide-B, 27-deoxywithaferin-A, 2, 24-dienolide, trienolide (steroidal lactones), withanoside-IV, withanolide-Z, 7-hydroxywithanolide, 3α-methoxy-2, 3-dihydro, 4β, 17α-dihydroxy-1-1oxo,5β, 6β-epoxy-22R-witha, 4β-dihydroxy-5β, 6β-epoxy, 1-oxo-22R-witha-2, 14–24 [38–44] |
| Sitoindoside IX, 4-(1-hydroxy-2, 2-dimethylcyclo propanone, 2, 3-dihydrowithaferin-A, 2, 3-dihydrowithaferin-A, 24, 25-dihydro-27 desoxywithaferin-A, physagulin-d, physagulin-d (1–>6)-β-d-glucopyranosyl- (1–>4)-β-d-glucopyranoside, 27-O-β-d-glucopyranosylphysagulin-d, 27-O-β-d- lucopyranosylviscosalactone-B, 4, 16-dihydroxy-5β, 6β-epoxyphysagulin-d, viscosalactone–B [45, 46] | |
| 5, 20α (R)-dihydroxy-6α, 7α-epoxy-1-oxo- (5α) -witha-2, 24-dienolide (steroidal lactone)2, 3-dihydrowithaferin-A-3β-O-sulfate [47] | |
| Fruit | 5β, 6α, 14α, 17β, 20β-pentahydroxy-1-oxo-20S, 22R-witha-2,24-dienolide, 6α,7α-epoxy-5α,14α,17α,23β- tetrahydroxy-1-oxo-22R-witha-2,24-dienolide, 7α-hydroxy withanolide, withanolide glycosides, 17α- and 17β-withanolides, Withanone, 27-hydroxy withanolide- A [48–50] |
| Seed | Withanolide-WS-2 (aliphatic ester), withanolide-WS-1 (aliphatic ketone) [49, 51, 52] |
Toxicologic studies
Withania somnifera has been used for various pharmacological activities for very long time for all age groups and both sexes and even during pregnancy without toxic effects [13]. Prabu et al. [53] have evaluated hydro-alcoholic root extract of W. somnifera against acute and sub-acute oral toxicities in Wistar rats and found it non-toxic even at 2000 mg/kg body weight. The extract was administered at 2000 mg/kg and observed for 14 days for acute toxicity and at 500, 1000 and 2000 mg/kg and observed for 28 days for sub-acute toxicity, however there was no significant change in body weight, organ weight, and hemato-biochemical parameters. In addition, the toxicity profile of W. somnifera was assessed on the developing fetus of pregnant rats including mortality, structural abnormalities, and changes in growth but no evident changes were found in the mother or in the fetus. No changes were found in the body weight of prenatal females, number of corpora lutea, implantations, viable fetuses, and skeletal and visceral formations [54]. Acute and sub-acute toxicity studies in Swiss albino mice and Wistar rats administrated with intraperitoneal injections of 1100 mg/kg did not produce any deaths within 24 h but small increases have led to mortality with an LD50 of 1260 mg/kg of body weight. No changes were observed in peripheral blood constituents. However, significant reductions were found in the spleen, thymus, and adrenal weights [21, 55]. Hence, W. somnifera can be used as safe therapeutic agent for various clinical conditions.
Pharmacokinetic studies
Numerous studies have been carried out in different biological models to elucidate the pharmacokinetics of W. somnifera. Two major constituents—withaferin-A and withanolide-A have been observed after oral administration of standardized W. somnifera aqueous extract in mice using multiple reaction monitoring. A dose of 1000 mg/kg extract (equivalent to 0.4585 mg/kg of withaferin-A and 0.4785 mg/kg of withanolide-A) demonstrated almost similar pharmacokinetic patterns for both of these withanolides with mean plasma concentrations (C max) of 16.69 ± 4.02 and 26.59 ± 4.47 ng/ml for withaferin-A and withanolide-A, with T max (time taken to reach C max) of 10 and 20 min, respectively, indicating their rapid absorption. The area under the plasma concentration–time curve from 0 to 4 h (AUC0–4h) was 1572.27 ± 57.80 and 2458.47 ± 212.72 min ng/ml, respectively. The T 1/2 of 59.92 ± 15.90 min and 45.22 ± 9.95 min and clearance of 274.10 ± 9.10 and 191.10 ± 16.74 ml/min/kg for withaferin-A and withanolide-A, respectively, were observed. Overall relative oral bioavailability has been found to be 1.44 times greater for withaferin-A compared to withanolide-A [56]. In addition, Thaiparambil et al. [57] have shown that withaferin-A reaches peak concentrations up to 2 μM in plasma with a half-life of 1.36 h following a single 4 mg/kg dose in 7–8-week-old female Balb/C mice, whereas the clearance from plasma is rapid (0.151 ng/ml/min). Another study has demonstrated that at a single oral dose of 500 mg/kg in six healthy buffalo calves resulted in a mean peak plasma concentration at 0.75 h and was 248.16 ± 16.12 μg/ml. Further on, the mean plasma concentration of 6.55 ± 0.12 μg/ml was detected up to 3 h. The mean therapeutic concentration (≥0.1 mg/ml) of W. somnifera has been maintained from 10 min to 3 h in plasma of healthy buffalo calves. The mean elimination half-life (t 1/2) of W. somnifera was observed to be 0.92 ± 0.032 h. The total body clearance ranges from 2.26 to 3.09 l/kg/h with a mean value of 2.78 ± 0.12 l/kg/h [58]. In a study involving Albino rabbits (1.5–1.8 kg, either sex, n = 6) that were fasted overnight, a single oral dose of 0.42 g/kg. W. somnifera (obtained from two sources) was well absorbed with a peak plasma concentration (C max) of 18,317.8–21,360.7 ng/ml with a T max of 1–2 h. The biological half-life ranged from 18.29 to 27.69 h [59].
Anti-microbial activity
Consistent with the folkloric use of W. somnifera against infections, methanolic leaf extract of W. somnifera has shown marked anti-bacterial activity against Gram-positive clinical isolates of methicillin-resistant Staphylococcus aureus and Enterococcus spp. [60]. Additionally, W. somnifera demonstrated potent anti-microbial activities against Gram-negative species such as Escherichia coli, Salmonella typhi, Proteus mirabilis, Citrobacter freundii, Pseudomonas aeruginosa, and Klebsiella pneumonia [61, 62]. The potency of W. somnifera has been observed to vary in different studies against different organisms. The mechanism of anti-microbial activity was ascribed to cytotoxicity, gene silencing, and immunopotentiation [63]. W. somnifera has strong anti-Salmonella typhimurium activity in vitro [62]. Additionally, increased survival rate and reduced bacterial load of various vital organs of mice with salmonellosis has been reported after administration of W. somnifera [64]. W. somnifera extracts synergized increase the anti-bacterial effect of Tibrim (rifampicin and isoniazid) against Salmonella typhimurium and E. coli [65].
W. somnifera inhibited acid production, acid tolerance, and biofilm formation of oral bacteria, Streptococcus mutans, and Streptococcus sobrinus at even sub-minimum inhibitory concentration (MIC) levels. There was also a dose-related increase in doubling times of Streptococcus mutans and Streptococcus sobrinus up to 258 and 400 %, respectively [66]. Withanolides induces apoptosis-like death in Leishmania donovani in vitro by provoking DNA nicks, cell cycle arrest at the sub G0/G1 phase, and externalization of phosphatidylserine in a dose- as well as time-dependent manner through an increase in reactive oxygen species (ROS) and a decrease in mitochondrial potential [67] by blocking the protein kinase-C signaling pathway [68]. Importantly, anti-leishmanial activity was exhibited by W. somnifera against free-living promastigotes and intracellular amastigotes of Leishmania major with a maximum inhibitory effect of >50 % [69]. W. somnifera synergized protection in cisplatin-treated L. donovani-infected mice as compared to only W. somnifera-treated L. donovani-infected mice by enhancing the percentage of T cells (CD4+, CD8+) and natural killer cell-associated marker (NK1) [70]. W. somnifera dose-dependently reduced parasite load and protected packed cell volume drop effect in mice infected with malarial parasite. Maximum inhibition was seen at 600 mg/kg [71], while it produced a non-significant suppression (21 %) against a chloroquine-resistant Plasmodium berghei in mice [72].
A glycoprotein from W. somnifera exerts a fungistatic effect in phytopathogenic fungi by inhibiting spore germination and hyphal growth in the tested fungi Aspergillus flavus, Fusarium oxysporum and Fusarium verticilloides [73]. Furthermore, flavonoids extracted from W. somnifera have been reported to be effective against Candida albicans with MIC of 0.039 and minimum fungicidal concentration (MFC) of 0.039. Moreover, it was demonstrated that A. flavus and Aspergillus niger were resistant to W. somnifera [61].
Anti-inflammatory activity
Withania somnifera has exhibited marked anti-inflammatory effects in various disease models. Its root extract exhibited anti-inflammatory and muco-restorative activity by resolving necrosis, edema, neutrophil infiltration in trinitro-benzyl-sulfonic acid (TNBS) -induced inflammatory bowel disease [74]. Powder of its roots was found to have a potent inhibitory effect on proteinuria, nephritis, and other inflammatory markers such as cytokines including interleukin (IL)-6 and tumor necrosis factor (TNF)-α, nitric oxide (NO), and ROS in a mouse model of lupus [75, 76].
In human umbilical vein endothelial cells (HUVECs), withaferin-A was shown to inhibit phorbol-12-myristate-13-acetate (PMA)-induced shedding of endothelial cell protein-C -receptor (EPCR) by inhibiting TNF-α and interleukin (IL)-1β. Moreover, in mouse withaferin-A attenuated cecal ligation and puncture (CLP)-induced EPCR shedding by reducing the expression and activity of tumor necrosis factor-α (TNF-α) converting enzyme. Additionally, withaferin-A attenuated PMA-stimulated phosphorylation of p38, extracellular regulated kinases (ERK)-1/2, and c-Jun N-terminal kinase (JNK) [77]. Withaferin-A protects vascular barrier integrity in HUVECs and in mice, induced by high mobility group box-1-protein (HMGB1) by inhibiting hyperpermeability, expression of cell adhesion molecules (CAM)s, adhesion and migration of leukocytes, production of interleukin-6, TNF-α, and activation of nuclear factor κ-β (NFκ-β) [78]. Withaferin-A prevents Iκ-β phosphorylation and degradation, which subsequently blocks NFκ-β translocation, NFκ-β/DNA binding, and gene transcription in Murine fibrosarcoma L929sA cells and human embryonic kidney 293T cells [79]. It also inhibits TNF-α-induced expression of cell adhesion molecules by inactivation of AKT and NFκ-β in human pulmonary epithelial cells [80]. Additionally, withaferin-A hampers NFκ-β activation by targeting cysteine 179 located in catalytic site of IKK-β [81]. In cellular models of cystic fibrosis, Withaferin-A leads to inhibition of NFκ-β and IL-8 [82].
Anti-arthritic activity
Ample precedent suggests a major role for W. somnifera in arthritis. Aqueous extracts of W. somnifera root powder showed a transitory chondroprotective effect on damaged human osteoarthritic cartilage by significant and reproducible inhibition of the gelatinase activity of collagenase type-2 enzyme in vitro [83] and by significantly decreased NO release [84]. Additionally, the crude ethanol extract of W. somnifera significantly suppressed lipopolysaccharide (LPS)-induced production of pro-inflammatory cytokines TNF-α, IL-1β, and IL-12p40 in peripheral blood mononuclear cells from normal individuals and synovial fluid mononuclear cells from rheumatoid arthritis patients possibly by inhibiting nuclear translocation of the transcription factors NFκ-β and activator protein-1 (AP-1) and phosphorylation of Iκ-β as evidenced from mouse cell line data from the same study. Additionally, it normalized LPS-induced NO production in RAW 264.7 cells [85]. In a rat model of adjuvant-induced arthritis, W. somnifera root powder attenuated cartilage degradation as assessed by estimation of bone collagen [86]. Aqueous extract of W. somnifera root prevented increased arthritic index, autoantibodies, and C-reactive-protein-P in collagen-induced arthritic rats [87]. Administration of W. somnifera root powder to the arthritic rats significantly decreased the severity of arthritis by effectively improving the functional recovery of motor activity and radiological score [88]. Furthermore, W. somnifera as a constituent in a polyherbal formulation (BV-9238) reduced TNF-α and NO production, without any cytotoxic effects in Freund’s complete adjuvant-induced arthritis in rats and a mouse macrophage cell line [89]. More importantly, W. somnifera helps collagen stabilization by inhibiting collagenase [90].
Some studies have reported conflictual reports regarding Withaferin-A. In rabbit articular chondrocytes, Withaferin-A-induced loss of type collagen expression and inflammatory responses mediated up-regulation of cyclooxygenase-2 (COX-2) expression through activation of microRNA-25 [91, 92]. Moreover, marked exacerbation in the production of intracellular ROS accompanied by apoptosis and increased p53 expression were observed, and these effects were dependent on PI3 K/AKT and JNK pathways [92, 93].
Anti-cancer activity
Various types of cancers or cancer-related changes in cell lines have been attenuated by W. somnifera or its chemical constituents. Molecular docking analysis demonstrated the use of withaferin-A and withanone for cancer drug development [94]. Leaf extract of W. somnifera and its components kills cancer cells by at least five different pathways—p53 signaling, granulocyte–macrophage colony-stimulating factor (GM-CFS) signaling, death receptor signaling, apoptosis signaling and by G2-M DNA damage regulation pathway [95]. Withaferin-A exhibited anti-cancer activity by inducing ROS-induced apoptosis in melanoma cells by crashing Bcl-2/Bax and Bcl-2/Bim ratios. This apoptotic cascade employed the mitochondrial pathway and was associated with Bcl-2 down-regulation, translocation of Bax to the mitochondrial membrane, release of cytochrome-c into the cytosol, abrogation of transmembrane potential, and activation of caspases-9 and 3. The withanolide-induced early ROS generation and mitochondrial membrane potential disturbances followed by the release of cytochrome c, translocation of Bax to mitochondria, and apoptosis-inducing factor to cell nuclei. These events paralleled activation of caspases-9 and 3, Poly-(ADP-Ribose) Polymerase (PARP) fragmentation of DNA [96]. Withaferin-A also led to the overexpression of tumor necrosis factor receptor (TNFR)-1 and obliterated the expression of Bid. More importantly, withaferin-A blocked binding of NFκ-β to DNA and instigated nuclear cleavage of p65/Rel by activated caspase-3. These studies suggest that withaferin-A kills cancerous cells by apoptosis that can be dependent and/or independent of mitochondrial mechanisms [97]. Enhanced production of ROS, down-regulation of Bcl-2, cleavage of PARP, stimulation of caspase-3, and mitogen-activated protein kinase (MAPK) signaling cascade are critically involved in the apoptosis induced by withaferin-A and radiation in human lymphoma U937 cells [98]. However, MAPK has a cell line-specific role in cell death by withaferin-A [99]. Similarly, Withaferin-A exacerbated radiation-induced apoptosis in human renal cancer cells by excessive generation of ROS, and by inhibition of Bcl-2 and dephosphorylation of AKT [100], and by endoplasmic reticulum (ER) stress [101]. Development of mammary cancer in a transgenic mouse model was markedly inhibited by withaferin-A by reducing the population of breast cancer stem cells and tumor size and tumor area. Similarly, mammosphere formation was dose-dependently blocked by withaferin-A treatment in human breast cancer cells which accompanied induction of apoptosis and mitigation of complex-III activity [102–104]. All these effects are independent of autophagy [105]. However, it activates Notch-2 and Notch-4, which leads to the arrest of their migration [106]. Additionally, withaferin-A causes G2 and M phase cellcycle arrest in human breast cancer cells [107]. Nagalingam et al. [108] demonstrated that Withaferin-A application inhibited breast tumor progression in xenograft and transgenic mouse models that employed up-regulation of the ERK/RSK axis, activation of Death Receptor 5 (DR5), and high levels of nuclear ETS domain-containing protein-1 (Elk-1) and CAAT/enhancer-binding protein-homologous protein (CHOP). Withaferin-A treatment inhibits experimental mammary cancer growth through the suppression of vimentin protein expression [109] by interfering with β-tubulin of cytoskeletal architecture [110].
W. somnifera killed human laryngeal carcinoma Hep2 cells and led to the arrest of the cell cycle with concomitant blockade of angiogenesis [111]. Withaferin-A inhibits cell proliferation in human umbilical vein endothelial cell inhibition of cyclin-D1 expression and by ubiquitination of proteins and defects in ubiquitin-mediated proteasome pathway [112]. Similarly, withaferin-A inhibits the growth of patient-derived mesothelioma by inhibiting proteasome and by inducing apoptosis [113].
In a kidney cancer cell line, Withaferin-A induced dose-dependent apoptotic cell death and PARP cleavage through down-regulation of the STAT-3 pathway [101, 114]. Additionally, Choi et al. [101] demonstrated that this cell death was due to ER stress. They observed that Withaferin-A led to phosphorylation of eukaryotic initiation factor-2α (eIF-2 α), ER stress-specific X-box binding protein-1 (XBP-1) splicing, and up-regulation of glucose-regulated protein (GRP)-78 and that of CHOP.
Cardio-protective activity
Withania somnifera possesses cardio-protective activity [115]. W. somnifera demonstrated cardiotropic and cardioprotective properties in animal models [116, 117]. Polyherbal formulations which had W. somnifera as a component showed cardioprotection in animal models [118, 119] by activating nuclear factor-erythroid-2-related transcription factor (Nrf)-2, stimulating phase-II detoxification enzymes, abrogating apoptosis in a Nrf-2-dependent manner [120]. Furthermore, it improved hematopoiesis [121]. Prophylactic treatment with W. somnifera markedly restored the myocardial oxidant/anti-oxidant balance, anti-apoptotic/pro-apoptotic effects, and reduced TUNEL positivity and lessened histopathologic deterioration of myocardium in a rat model of coronary artery occlusion [122]. These effects were in addition to restoring oxidant/anti-oxidant balance [123–125]. Similarly, standardized extract of W. somnifera prevented doxorubicin-induced cardiotoxicity and restoration of biochemical changes [126].
Anti-diabetic activity
Various polyherbal formulations (Dianix, Trasina) of Indian Systems of Medicine showed strong anti-diabetic activity in human subjects [127–129]. In patients, W. somnifera root powder stabilized blood glucose that was comparable to that of an oral hypoglycemic drug daonil, when treated orally for 30 days [130]. Additionally, W. somnifera treatment significantly improved insulin sensitivity index and blocked the rise in homeostasis model assessment of insulin resistance in non-insulin-dependent diabetes mellitus in rats [131]. In agreement with these studies, W. somnifera leaf and root extracts improved glucose uptake in skeletal myotubes and adipocytes in a dose-dependent manner, with the leaf extract demonstrating more pronounced effects than the root extract [132]. Root and leaf extracts significantly normalized the levels of urine sugar, blood glucose, glucose-6-phosphatase, and tissue glycogen levels in alloxan-induced diabetes mellitus in rats. Additionally, attenuation of improving the non-enzymatic and enzymatic anti-oxidant defenses was also observed [133, 134]. Withaferin-A blocks inflammatory response in cytokine-induced damage to islets in culture and following transplantation [135] and exhibits potent anti-glycating activity [136].
Anti-stress activity
Withania somnifera resulted in better stress tolerance in animals [137–139]. The aqueous fraction of W. somnifera roots alleviated chronic stress-induced reduction of T cell population and up-regulated Th1 cytokines in mice [140]. In a clinical study for the safety and efficacy of a high-concentration full-spectrum extract of W. somnifera roots in human subjects, serum cortisol levels were reduced, without causing any major side effects [141]. Furthermore, EuMil, a poly herbal formulation markedly ameliorated cerebral monoamine (nor-adrenaline, dopamine, and 5-hydroxytryptamine) levels induced by chronic electroshock stress [142]. In another study, EuMil restored chronic stress-induced glucose intolerance and normalized male sexual behavior and behavioral despair. Additionally, it attenuated cognitive dysfunction, immunosuppression, gastric ulceration, and plasma corticosterone levels [143]. Another poly-herbal formulation (Perment®) exhibited anti-depressant and anxiolytic activity in rats, which was partly due to activation of adrenergic and serotonergic systems [144]. Glycowithanolides from W. somnifera produced an anxiolytic effect against pentylenetetrazole-induced anxiety in rats, which was comparable to that exhibited by well-known anti-depressants. In addition, it reduced rat brain levels of tribulin, an endocoid marker of clinical anxiety [145]. Further on, it normalized oxidative free radical scavenging enzymes and lipid peroxidation (LPO) in rat frontal cortex and striatum of chronically footshock stressed rats [146].
Neuroprotective activities
Many studies have documented the neuroprotective effects of W. somnifera [22, 147–150]. The leaf extract and its component withanone protect scopolamine-induced toxic changes in both neuronal and glial cells. Scopolamine-induced inactivation of neuronal cell markers such as NF-H, MAP-2, PSD-95, GAP-43, and glial cell marker glial fibrillary acidic protein (GFAP) and with DNA damage and oxidative stress markers was markedly attenuated by W. somnifera [151]. W. somnifera extract attenuated lead-induced toxicity in glial cells by balancing the expression of GFAP and heat shock protein (HSP70), mortalin, and neural cell adhesion molecule (NCAM) [152]. Glycowithanolides from W. somnifera exhibited significant anti-oxidant activity in cortex and striatum of rat brain by inducing a dose-related increase in superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activity [153]. Additionally, extract of W. somnifera prevented streptozotocin-induced oxidative damage in treated mice by mitigating oxidative stress [154]. W. somnifera root powder extract markedly rescued the number of degenerating cells in CA2 and CA3 sub-areas of rats hippocampus subjected to immobilization stress [155]. W. somnifera root extract or its derivatives promoted neurite outgrowth extensions in human neuroblastoma cell lines [156]. The axons are mainly extended by withanolide-A, and dendrites by withanolides-IV and VI while withanoside-IV induced both axonal and dendritic rejuvenation and synaptic restoration in rat cortical neurons damaged by amyloid-β (Aβ) [157, 158]. Kataria et al. [159] demonstrated that W. somnifera leaf extract rescued retinoic acid-differentiated C6 and IMR-32 cells from glutamate toxicity. W. somnifera leaf extract pre-treatment inhibited glutamate-induced cell death and reversed glutamate-evoked stress response by up-regulation of HSP70 and additionally it restored neuronal plasticity by neuronal plasticity markers, neural cell adhesion molecules, and its polysialylated form. W. somnifera extract also reduced kainic acid-induced excitotoxic damage by mitigating oxidative stress [160].
Anti-Parkinson activity
Precedent exists in literature for a major role for W. somnifera in Parkinson’s disease. W. somnifera have been shown to attenuate Parkinson symptoms and pathology in a 6-hydroxydopamine (6-OHDA) rat model for the disease. The study demonstrated the restoration of the content of striatal dopamine and its metabolites most likely via its pronounced anti-oxidant action as evidenced by the attenuation of LPO, reduced glutathione (GSH) content, and activities of glutathione-S-transferase (GST), glutathione reductase (GR), GPX, SOD, and CAT. Improvement of striatal catecholamine content due to W. somnifera might have reversed the functional impairments like locomotor activity and muscular coordination and drug-induced rotational behavior. This study also demonstrated up-regulation of dopaminergic D2 receptor populations in striatum, which acts as a compensatory mechanism after induction of Parkinsonism to grab every available dopamine molecule. Additionally, W. somnifera has led to an increase in the number of surviving dopaminergic neurons as estimated by tyrosine hydroxylase labeling [161]. W. somnifera root extract restored anti-oxidant status, reduced oxidant stress, and thus normalized catecholamine content in mid brain of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-intoxicated parkinsonian mice. These biochemical changes accompanied the betterment in functional activity of the model [162–164]. Standardized extract of W. somnifera significantly reduced rotenone-induced oxidative impairment and mitochondrial respiratory chain enzymes that in turn have attenuated disturbances in cholinergic function and repleted dopamine content. These changes were responsible for reduced locomotor deficits and lethality in a Drosophila melanogaster model of Parkinson induced by rotenone [165]. Additionally, rotenone toxicity in cerebellum and striatum of mouse brain was greatly decreased by W. somnifera root powder through its anti-oxidant and anti-inflammatory actions and by correcting mitochondrial dysfunctions. These changes brought about restoration of neurotransmitter functions and dopamine levels in striatum [166]. Maneb-paraquat-induced mouse model of Parkinson and ethanolic root extract of W. somnifera rescued dopaminergic neurons as measured by expression of tyrosine hydroxylase, replenished dopamine levels in the substantia nigra, and attenuated locomotor activity by reducing inflammation and apoptosis and various aspects of oxidative damage. In particular, W. somnifera reduced the expression of inducible NO synthase (iNOS), a measure of oxidative stress. W. somnifera deactivated pro-apoptotic Bax and activated anti-apoptotic Bcl-2 protein expression and down-regulated the activation of astrocytes and expression of GFAP [167, 168].
Anti-Alzheimer activity
Literature suggests a prominent role of W. somnifera in drug development against Alzheimer’s disease. Standardized aqueous extract of W. somnifera improved cognitive and psychomotor performance in healthy human participants [169]. W. somnifera root extract reversed the behavioral deficits and pathological clues as well as Aβ clearance in Alzheimer’s disease models by up-regulating lipoprotein receptor-related protein in liver [170]. Simulation studies have shown that withanamides-A and -C uniquely bind to the active motif of (Aβ)(25–35) and suggest that withanamides have the ability to prevent the fibril formation and thus protect cells from Aβ toxicity [171]. Furthermore, docking simulation studies have predicted inhibition of human acetyl cholinesterase by withanolide-A for Alzheimer’s treatment [172]. Withanoside-IV and its active metabolite, sominone, attenuated Aβ(25–35)-induced neurodegeneration by improving memory deficits in mice and preventing a loss of axons, dendrites, and synapses [158]. W. somnifera elicits a protective response and abolishes acetylcholine esterase (AChE) activity inhibition and cognitive impairment caused by sub-chronic exposure to propoxur to rats [173]. W. somnifera affords a beneficial effect on cognitive deficit by ameliorating oxidative damage induced by streptozotocin in a model of cognitive impairment [174]. W. somnifera restored cellular morphology in Aβ-treated SK-N-MC cell line by enhancing cell viability and the peroxisome proliferator-activated receptor-γ (PPAR-γ) levels [175]. Further on, it led to inhibition of acetylcholinesterase activity [176]. W. somnifera root extract concentration dependently exhibited protective effects against hydrogen peroxide and Aβ(1–42)-induced cytotoxicity in differentiated PC12 cells [177].
Anti-ischemic and anti-hypoxic activity
Withania somnifera attenuated middle cerebral artery occlusion-induced enhancement of the oxidative stress marker malondialdehyde, reduction in lesion area, and restoration of neurological deficits [178]. W. somnifera imparted functional restoration and attenuation of infarct volume in mice subjected to permanent distal middle cerebral artery occlusion (pMCAO). It led to recovery of hemeoxygenase-1 (HO-) expression and abated the up-regulation of the proapoptotic protein PARP-1 via the PARP-1 apoptosis-inducing factor (AIF) pathway that was altered by pMCAO in mouse cortex. This phenomenon led to a blockade of the apoptotic cascade by preventing nuclear translocation of AIF. Additionally, semaphorin-3A expression was increased by pMCAO and it initiates inhibitory signals that thwart repair. W. somnifera significantly reduced the expression of Semaphorin-3A and thus initiated repair mechanisms [179, 180]. W. somnifera root extract and withanolide-A attenuated hypobaric hypoxia-induced memory and hippocampal neurodegeneration by repleting reduced glutathione (GSH) levels through activation of the glutathione biosynthesis pathway in hippocampal cells. These effects were mediated by the Nrf-2 pathway and NO in a corticosterone-dependent manner [181, 182].
Conclusions
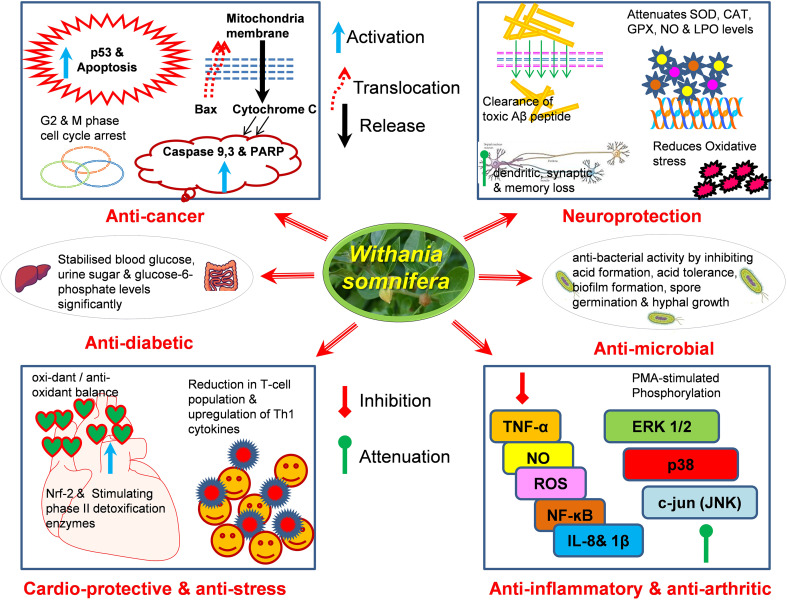
Withania somnifera is a natural product with promising pharmacological and pharmaceutical properties; it has extensive clinical applications in Indian Systems of Medicine (Fig. 1). In animal studies, it or its constituents exert multiple protective properties such as anti-inflammatory, anti-oxidant, inhibiting NFκ-β transcription, MAPK signaling pathways, anti-apoptotic, angiogenic, and ER stress reducing effects (Table 2). The claims for use of W. somnifera to improve a myriad of clinical conditions are overwhelmingly encouraging as a multi-purpose medicinal agent. More clinical validation needs to be performed for its general medical use.
Fig. 1.
Withania somnifera exerts multiple pharmacologic actions such as neuroprotection (reducing oxidative stress by restoring antioxidant levels, clearance of Aβ levels, attenuating synaptic and dendritic loss and reversing SOD, CAT, GPx, NO and LPO levels), cardio-protection (anti-oxidant balance and activating Nrf-2 and stimulating phase-II detoxification enzymes) anti-inflammatory, anti-oxidant and anti-stress (inhibiting NFκ-β transcription, MAPK signaling pathways, TNF-α, NO, ROS and IL-8, reducing T-cell population and up-regulating Th1 cytokines), anti-diabetic (stabilizing blood glucose, urine sugar, and glucose-6-phosphate levels significantly), anti-bacterial (inhibiting acid formation, acid tolerance, biofilm formation, spore germination, and hyphal growth) and anti-cancer (cell cycle arrest and activation of p53, loss of mitochondrial membrane potential, activation of caspase cascade and PARP-1)
Table 2.
Various pharmacological action of W. somnifera or its chemical constituents
| W. somnifera | Dosage and mode of administration | Diseased condition/model | Mechanism of action | References |
|---|---|---|---|---|
| Methanolic leaf extract | 2 mg/ml in vitro | Methicillin resistant Staphylococcus aureus and Enterococcus spp. | Anti-bacterial activity | [60] |
| Methanolic extract | 0.125–2 mg/ml in vitro | Oral infections by Streptococcus mutans and Streptococcus sobrinus | Inhibited acid production, acid tolerance, and biofilm formation of oral bacteria | [66] |
| Withanolides (F5 and F6 fractions) | 60 and 15 μg/ml in vitro | Leishmania donovani | Apoptosis, DNA nicks, cell cycle arrest and externalization of phosphatidylserine, increased ROS, and decreased mitochondrial potential | [67] |
| Glycoproteins | 20 µg/ml in vitro | Aspergillus flavus, Fusarium oxysporum, F. verticilloides | Inhibiting spore germination and hyphal growth | [73] |
| Root extract | 500 µg/ml and 1000 mg/kg b.wt. (rectal route) | TNBS-induced inflammatory bowel disease in rats | Mucorestorative and anti-inflammatory, resolved edema, neutrophil infiltration and necrosis | [74–76] |
| 500 and 1000 mg/kg b.wt. (orally) | Mouse model of lupus | Inhibited proteinuria, nephritis, TNF-α, NO and ROS | ||
| Withaferin-A | 1.882 µg per mouse (I.V.) | Human umbilical vein endothelial cells | Inhibited TNF-α and IL-1β | [77, 79, 82] |
| 2 µM in vitro | Murine fibrosarcoma | Attenuated p38, ERK-1/2, C jun JNK | ||
| 3 µM/ml in vitro | Cellular models of Cystic Fibrosis inflammation (KKLEB cells) | Inhibited Iκβ phosphorylation and degradation by blocking NFκ-β translocation, inhibited IL-8 | ||
| Aqueous extract of root powder | 10 mg/ml in vitro | Human osteoarthritis (cartilage damage explant models) | Chondroprotective actions by inhibiting gelatinase activity of collagenase type-2 enzyme and decreased NO | [83, 88] |
| 600 and 800 mg/kg (orally) | Collagen-induced arthritis in rats | Attenuated cartilage degradation, improved the functional recovery of motor activity and radiological score | ||
| Crude ethanolic extract | 1 mg/ml in vitro | Rheumatoid arthritis (PBM cells) | Suppression of LPS-induced production of cytokines, interleukins, and TNF-α | [85] |
| Leaf extract | 6, 15, 21, 25, and 32 µg/ml in vitro | Cancer cells (TIG1, U2OS, and HT1080) | Activated p53, apoptosis pathway, and arresting cell cycle | [95] |
| Withaferin-A | 3 µM/ml in vitro | Human melanoma cells (M14, Lu1205, and Sk28) | Promoted ROS-induced apoptosis by lowering Bax/Bcl2 and Bcl2/Bim ratio | [96, 107, 108, 111] |
| 2 and 3 µM/ml in vitro | Breast cancer cells (MDA-MB-231 and MCF-7) | Translocation of Bax to mitochondrial membrane resulting in cytochrome c release and activation of caspase-9 and 3 and PARP | ||
| 5 and 10 µM/L in vitro and injections of 4 mg/kg b.wt. 5 days/week for 5 weeks. (i.p.) | Breast tumor progression in xenograft and transgenic mouse models | G2 and M-phase cell cycle arrest, up-regulated ERK/RSK axis, activation of DR-5, Elk1 and CHOP | ||
| 25 µg/ml in vitro | Human laryngeal carcinoma Hep2 cells | Cell cycle arrest with concomitant blockade of angiogenesis | ||
| 4 µM/ml in vitro | Renal cancers (Caki cells) | PARP cleavage through down-regulation of STAT-3 pathway | [101, 114] | |
| 26 µM/ml in vitro | ER stress-specific XBP1 splicing, and up-regulation of GRP-78 and CHOP | |||
| Whole extract | 30, 60 and 90 mg/kg/day for 60 days (orally) | Myocardial infarction in rats | Cardiotropic and cardioprotective | [116, 117, 120, 122, 125] |
| 50, 75 and 100 mg/ml in vitro | Coronary artery occlusion in rats | Activated Nrf2, stimulated phase II detoxification enzymes, abrogated apoptosis in a Nrf2-dependent manner | ||
| 50 mg/kg b.wt. for 30 days (orally) | Anti-apoptotic/pro-apoptotic effects, and reduced TUNEL positivity and lessened histopathologic deterioration of myocardium | |||
| Root extract | 3 g/day human subjects (orally) | Diabetes | Stabilized blood glucose levels | [130, 131, 133, 134] |
| Aqueous extract | 200 and 400 mg/kg b.wt./day for 5 weeks (orally) | Non-insulin-dependent diabetes mellitus in rats | Improved insulin sensitivity index and blocked the rise in homeostasis model assessment of insulin resistance | |
| Root and leaf extract | 200 mg/kg b.wt. for 8 weeks(orally) | Alloxan-induced diabetes mellitus in rats | Normalized the urine sugar, blood glucose, glucose-6-phosphatase and tissue glycogen levels | |
| Aqueous fraction of roots | 25, 50, 100 and 200 mg/kg for 14 days (orally) | Mouse model of chronic stress | Reduced in T-cell population and up-regulated Th1 cytokines | [140] |
| EuMil, poly herbal formulation | 100 mg/kg for 14 days(orally) | Chronic electroshock stress in rats | Ameliorated cerebral monoamine levels | [142, 143] |
| 100 mg/kg for 14 days (orally) | Attenuated cognitive dysfunction, immunosuppression, gastric ulceration, and plasma corticosterone levels | |||
| Glycowithanolides | 20 and 50 mg/kg for 5 days (orally) | Pentylenetetrazole induced anxiety in rats | Anxiolytic effects and reduced rat brain levels of tribulin | [145] |
| Leaf extract and Withanone | 100, 200 and 300 mg/kg b.wt. for 7 days (orally) | Scopolamine induced toxicity in mice | Produced neuronal and glial protection cells by activating neuronal proteins, oxidative stress and DNA damage | [151] |
| Root extract | 20 mg/kg b.wt. for 30 days (orally) | Immobilization stress in albino rats | Markedly rescued the number of degenerating cells in CA2 and CA3 subareas of rat hippocampus | [155] |
| Withanolide-A, withanolides-IV, Withanoside-VI | 10 µM/kg/day (orally) | Amyloid- β toxicity (rat cortical neurons) | Promoted neurite outgrowth, axonal and dendritic and synaptic rejuvenation | [157, 158] |
| Water extract | 0.05 and 0.1 % in vitro | Glutamate induced excitotoxicity in IMR-32 and C6 cells | Reversed glutamate-evoked stress response by up-regulation of HSP70, restored neuronal plasticity, reduced kainic acid-induced excitotoxic damage by mitigating oxidative stress | [159, 160] |
| Whole extract | 100, 200 and 300 mg/kg b.wt. for 3 weeks (orally) | 6-OHDA induced toxicity in rats | Attenuated lipid peroxidation, reduced glutathione content, and activities of glutathione-S- transferase, glutathione reductase, glutathione peroxidase, superoxide dismutase and catalase, increased number of dopaminergic neurons | [161] |
| Root extract | 100 mg/kg b.wt. (orally) | MPTP induced toxicity in mice | Normalized catecholamine content, reduced oxidant stress, and functional activity | [162, 164] |
| Root powder | 100 and 400 mg/kg b.wt./day for 4 weeks (orally) | Rotenone-induced impairment in mice | Antioxidant and anti-inflammatory actions and corrected mitochondrial dysfunctions, normalized neurotransmitter function, and dopamine levels in striatum | [166] |
| Ethanolic extract | 100 mg/kg b.wt. for 3, 6, and 9 weeks (orally) | MBPQ-induced toxicity in mice | Rescued dopaminergic neurons, replenished dopamine levels in substantia nigra and attenuated locomotor activity and reduced oxidative stress and inflammation | [167, 168] |
| 100 mg/kg b.wt. for 9 weeks(i.p) | Activated anti-apoptotic Bcl-2 protein expression and down-regulated pro-apoptotic Bax and astrocytes and expression of GFAP | |||
| Standardized aqueous extract | 250 mg twice daily for 14 days to human subjects (orally) | Psychomotor functional disorders in healthy humans | Improved cognitive and psychomotor performance | [169] |
| Root extract | 1 g/kg b.wt. for 7–30 days (orally) | Alzheimer’s disease models | Reversed the behavioral deficits and pathological clues as well as Aβ clearance by up-regulating lipoprotein receptor-related protein in liver | [170] |
| Withanolides | 6.25, 12.5, 25, 50, 100 μg/ml in vitro | Alzheimer’s disease transgenic mice | Prevented the fibril formation and thus protect cells from amyloid- β toxicity | [171] |
| Withanoside-IV and Sominone | 10 µM/kg/day (orally) | Alzheimer’s disease mice | Attenuated Aβ(25,35) induced neurodegeneration and improved memory deficits in mice and prevented loss of axons, dendrites, and synapses | [158] |
| Whole extract | 0.15 and 0.3 µg/ml in vitro | Aβ toxicity in SK-N-MC cells | Enhanced cell viability and PPARγ levels, inhibited of acetyl- cholinesterase activity | [175, 176] |
| Whole extract | 1 g/kg for 15 and 30 days (orally) | Middle cerebral artery occlusion in rats | Attenuated oxidative stress marker malondialdehyde, reduced lesion area, and restoration of neurological deficits | [178] |
| Root extract | 50, 100, 150, 200 and 250 mg/kg b.wt. for 21 days (orally) | Hypoxia pathway in hippocampal cells | Enhanced memory and attenuated hippocampal neurodegeneration by repleting glutathione levels through activation of glutathione biosynthesis | [181, 182] |
Acknowledgments
Dr. Ahmad’s work was partly supported by Ramalingaswamy Fellowship of Department of Biotechnology and financial assistance (MLP6009) as well as logistic support from Council for Scientific and Industrial Research. Mr. Dar is thankful to University Grants Commission, India for Ph.D. research fellowship. The contents do not represent any governmental views of India. (Institutional publication number of this article is IIIM/1823/2015).
Abbreviations
- TNF-α
Tumor necrosis factor-α
- IL-1β
Interleukin-1β
- NFκ-β
Nuclear factor kappa-β
- NO
Nitric oxide
- ROS
Reactive oxygen species
- PARP-1
Poly(ADP-ribose) polymerase-1
- pMCAO
Permanent middle cerebral artery occlusion
- GFAP
Glial fibrillary acidic protein
- 6OHDA
6-hydroxydopamine
Compliance with ethical standards
Conflict of interest
Authors do not have any conflict of interest.
References
- 1.Dhuley JN. Effect of ashwagandha on lipid peroxidation in stress-induced animals. J Ethnopharmacol. 1998;60:173–178. doi: 10.1016/S0378-8741(97)00151-7. [DOI] [PubMed] [Google Scholar]
- 2.Ziauddin M, Phansalkar N, Patki P, Diwanay S, Patwardhan B. Studies on the immunomodulatory effects of Ashwagandha . J Ethnopharmacol. 1996;50:69–76. doi: 10.1016/0378-8741(95)01318-0. [DOI] [PubMed] [Google Scholar]
- 3.Hepper FN. Old World Withania (Solanaceae): a taxonomic review and key to the species. In: Hawkes JG, Lester RN, Nee M, Estrada N, editors. Solanaceae III: taxonomy, chemistry, evolution. London: Royal Botanic Gardens Kew and Linnean Society of London; 1991. [Google Scholar]
- 4.Purdie RW, Symon DE, Haegi L. Solanaceae. Flora Aust. 1982;29:184. [Google Scholar]
- 5.Van Wyk B-E, Wink M. Medicinal plants of the world. Pretoria: Briza Publications; 2004. [Google Scholar]
- 6.Uddin Q, Samiulla L, Singh V, Jamil S. Phytochemical and pharmacological profile of Withania somnifera dunal: a review. J Appl Pharm Sci. 2012;02(01):170–175. [Google Scholar]
- 7.Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8(S):208–213. doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Changhadi GS. Ashwagandharishta—Rastantra Sar Evam Sidhyaprayog Sangrah. Nagpur: Krishna-Gopal Ayurveda Bhawan (Dharmarth Trust); 1938. pp. 743–774. [Google Scholar]
- 9.Sharma PV. Ashwagandha. Chaukhambha Viashwabharti Varanasi: Dravyaguna Vijana; 1999. pp. 763–765. [Google Scholar]
- 10.Bhandari CR (1970) Ashwagandha (Withania somnifera) Vanaushadhi Chandroday (An Encyclopedia of Indian Herbs), vol 1. CS Series, Varanasi Vidyavilas Press, Varanasi, India, pp 96–97
- 11.Basu KA. Withania somnifera, Indian medicinal plants. 2. Allahabad: IIIrd Lalit Mohan Basu; 1935. pp. 1774–1776. [Google Scholar]
- 12.Mishra B. Ashwagandha—Bhavprakash Nigantu (Indian Materia Medica) Chaukhambha Bharti Academy: Varanasi; 2004. pp. 393–394. [Google Scholar]
- 13.Sharma S, Dahanukar S, Karandikar S. Effects of long-term administration of the roots of ashwagandha and shatavari in rats. Indian Drugs. 1985;22:133. [Google Scholar]
- 14.Machiah DK, Girish K, Gowda TV. A glycoprotein from a folk medicinal plant, Withania somnifera, inhibits hyaluronidase activity of snake venoms. Comp Biochem Physiol C Toxicol Pharmacol. 2006;143:158–161. doi: 10.1016/j.cbpc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Machiah DK, Gowda TV. Purification of a post-synaptic neurotoxic phospholipase A 2 from Naja naja venom and its inhibition by a glycoprotein from Withania somnifera . Biochimie. 2006;88:701–710. doi: 10.1016/j.biochi.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Diwanay S, Patki P, Patwardhan B. Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. J Ethnopharmacol. 1999;67:27–35. doi: 10.1016/S0378-8741(99)00065-3. [DOI] [PubMed] [Google Scholar]
- 17.Ali M, Shuaib M, Ansari SH. Withanolides from the stem bark of Withania somnifera . Phytochemistry. 1997;44:1163–1168. doi: 10.1016/S0031-9422(96)00656-5. [DOI] [Google Scholar]
- 18.Ghani N (1920) Khazain-ul-Adviyah, vol I. Munshi Nawal Kishore, Lucknow, pp 230–231
- 19.Kabiruddin M. Makhzan-ul-Mufradat. Lahore: Nadeem University Printers; 1955. pp. 75–76. [Google Scholar]
- 20.Nadkarni KM (1982) Indian Materia Medica, 3rd edn, vol I. Popular Prakashan Pvt Ltd, Bombay, pp 1292–1294
- 21.Tiwari R, Chakraborty S, Saminathan M, Dhama K, Singh SV. Ashwagandha (Withania somnifera): role in safeguarding health, immunomodulatory effects, combating infections and therapeutic applications: a review. J Biol Sci. 2014;14(2):77–94. doi: 10.3923/jbs.2014.77.94. [DOI] [Google Scholar]
- 22.Ven Murthy M, Ranjekar PK, Ramassamy C, Deshpande M. Scientific basis for the use of Indian Ayurvedic medicinal plants in the treatment of neurodegenerative disorders: 1. Ashwagandha. Cent Nerv Syst Agents Med Chem. 2010;10:238–246. doi: 10.2174/1871524911006030238. [DOI] [PubMed] [Google Scholar]
- 23.Seenivasagam R, Sathiyamoorthy S, Hemavathi K. Therapeutic impacts of Indian and Korean ginseng on human beings—a review. Int J Immunol Stud. 2011;1:297–317. doi: 10.1504/IJIS.2011.041727. [DOI] [Google Scholar]
- 24.Grandhi A, Mujumdar AM, Patwardhan B. A comparative pharmacological investigation of Ashwagandha and Ginseng. J Ethnopharmacol. 1994;44:131–135. doi: 10.1016/0378-8741(94)01119-2. [DOI] [PubMed] [Google Scholar]
- 25.Kaur K, Rani G, Widodo N, Nagpal A, Taira K, et al. Evaluation of the anti-proliferative and anti-oxidative activities of leaf extract from in vivo and in vitro raised Ashwagandha. Food Chem Toxicol. 2004;42:2015–2020. doi: 10.1016/j.fct.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Chopra A, Lavin P, Patwardhan B, Chitre D. A 32-week randomized, placebo-controlled clinical evaluation of RA-11, an Ayurvedic drug, on osteoarthritis of the knees. JCR J Clin Rheumatol. 2004;10:236–245. doi: 10.1097/01.rhu.0000138087.47382.6d. [DOI] [PubMed] [Google Scholar]
- 27.Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazon J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14:2373–2393. doi: 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 29.Matsuda H, Murakami T, Kishi A, Yoshikawa M. Structures of withanolides I, II, III, IV, V, VI, and VII, new withanolide glycosides, from the roots of Indian Withania somnifera DUNAL and inhibitory activity for tachyphylaxis to clonidine in isolated guinea-pig ileum. Bioorg Med Chem. 2001;9:1499–1507. doi: 10.1016/S0968-0896(01)00024-4. [DOI] [PubMed] [Google Scholar]
- 30.Singh G, Sharma P, Dudhe R, Singh S. Biological activities of Withania somnifera . Ann Biol Res. 2010;1:56–63. [Google Scholar]
- 31.Bhattacharya SK, Goel RK, Kaur R, Ghosal S. Anti-stress activity of sitoindosides VII and VIII, new acylsterylglucosides from Withania somnifera . Phytother Res. 1987;1:32–37. doi: 10.1002/ptr.2650010108. [DOI] [Google Scholar]
- 32.Ghosal S, Kaur R, Srivastava R. Sito-indosides IX and X, two new glycowithanolides from Withania somnifera . Indian J Nat Prod. 1988;4:12–13. [Google Scholar]
- 33.Majumdar D. Withania somnifera Dunal, Part II. Alkaloidal constituents and their chemical characterization. Indian J Pharm. 1955;17:158–161. [Google Scholar]
- 34.Praveen N, Murthy H. Production of withanolide-A from adventitious root cultures of Withania somnifera . Acta Physiol Plant. 2010;32:1017–1022. doi: 10.1007/s11738-010-0489-7. [DOI] [Google Scholar]
- 35.Misra L, Mishra P, Pandey A, Sangwan RS, Sangwan NS, et al. Withanolides from Withania somnifera roots. Phytochemistry. 2008;69:1000–1004. doi: 10.1016/j.phytochem.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Subbaraju GV, Vanisree M, Rao CV, Sivaramakrishna C, Sridhar P, et al. Ashwagandhanolide, a bioactive dimeric thiowithanolide isolated from the roots of Withania somnifera⊥. J Nat Prod. 2006;69:1790–1792. doi: 10.1021/np060147p. [DOI] [PubMed] [Google Scholar]
- 37.Anjaneyulu A, Rao D, Lequesne P. Withanolides, biologically active natural steroidal lactones. Struct Chem Part F. 1998;20:135. doi: 10.1016/S1572-5995(97)80032-4. [DOI] [Google Scholar]
- 38.Kirson I, Glotter E, Abraham A, Lavie D. Constituents of Withania somnifera dun—XI: the structure of three new withanolides. Tetrahedron. 1970;26:2209–2219. doi: 10.1016/S0040-4020(01)92800-5. [DOI] [Google Scholar]
- 39.Lavie D, Glotter E, Shvo Y. Constituents of Withania somnifera Dun. III. The side chain of withaferin A*, 1. J Org Chem. 1965;30:1774–1778. doi: 10.1021/jo01017a015. [DOI] [Google Scholar]
- 40.Lavie D, Kashman Y, Glotter E. Constituents of Withania somnifera dun—V: studies on some model steroidal epoxides. Tetrahedron. 1966;22:1103–1111. doi: 10.1016/0040-4020(66)80086-8. [DOI] [Google Scholar]
- 41.Glotter E, Abraham A, Günzberg G, Kirson I. Naturally occurring steroidal lactones with a 17α-oriented side chain. Structure of withanolide E and related compounds. J Chem Soc Perkin. 1977;1:341–346. doi: 10.1039/p19770000341. [DOI] [Google Scholar]
- 42.Kirson I, Glotter E, Lavie D, Abraham A (1971) Constituents of Withania somnifera Dun: part XII. The withanolides of an Indian chemotype. J Chem Soc 2032–2044
- 43.Dhalla N, Sastry M, Malhotra C. Chemical studies of the leaves of Withania somnifera . J Pharm Sci. 1961;50:876–877. doi: 10.1002/jps.2600501019. [DOI] [PubMed] [Google Scholar]
- 44.Pramanick S, Roy A, Ghosh S, Majumder HK, Mukhopadhyay S. Withanolide Z, a new chlorinated withanolide from Withania somnifera . Planta Med. 2008;74:1745–1748. doi: 10.1055/s-2008-1081357. [DOI] [PubMed] [Google Scholar]
- 45.Jayaprakasam B, Zhang Y, Seeram NP, Nair MG. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003;74:125–132. doi: 10.1016/j.lfs.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Jayaprakasam B, Nair MG. Cyclooxygenase-2 enzyme inhibitory withanolides from Withania somnifera leaves. Tetrahedron. 2003;59:841–849. doi: 10.1016/S0040-4020(02)01601-0. [DOI] [Google Scholar]
- 47.Menssen H, Stapel G (1973) Uber ein C28-Steroidlacton aus der Wurzel von Withania Somnifera. Plant Med [DOI] [PubMed]
- 48.Abou-Douh AM. New withanolides and other constituents from the fruit of Withania somnifera . Arch Pharm. 2002;335(6):267–276. doi: 10.1002/1521-4184(200208)335:6<267::AID-ARDP267>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 49.Kundu AB, Mukherjee A, Dey A. New Withanolide from seeds of Withania-somnifera dunal. Indian J Chem. 1976;14:434–435. [Google Scholar]
- 50.Jayaprakasam B, Strasburg GA, Nair MG. Potent lipid peroxidation inhibitors from Withania somnifera fruits. Tetrahedron. 2004;60:3109–3121. doi: 10.1016/j.tet.2004.01.016. [DOI] [Google Scholar]
- 51.Xu Y-M, Marron MT, Seddon E, McLaughlin SP, Ray DT, et al. 2, 3-Dihydrowithaferin A-3β-O-sulfate, a new potential prodrug of withaferin A from aeroponically grown Withania somnifera . Bioorg Med Chem. 2009;17:2210–2214. doi: 10.1016/j.bmc.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 52.Khan F, Saeed M, Alam M, Chaudhry A. Biological studies of indigenous medicinal plants III. Phytochemical and antimicrobial studies on the non-alkaloidal constituents of some solanaceous fruits. Eczacilik Fakultesi Dergisi-Gazi Universitesi. 1993;10:105. [Google Scholar]
- 53.Prabu PC, Panchapakesan S, Raj CD. Acute and sub-acute oral toxicity assessment of the hydroalcoholic extract of Withania somnifera roots in Wistar rats. Phytother Res. 2013;27:1169–1178. doi: 10.1002/ptr.4854. [DOI] [PubMed] [Google Scholar]
- 54.Prabu PC, Panchapakesan S. Prenatal developmental toxicity evaluation of Withania somnifera root extract in Wistar rats. Drug Chem Toxicol. 2015;38:50–56. doi: 10.3109/01480545.2014.900073. [DOI] [PubMed] [Google Scholar]
- 55.Sharada A, Solomon FE, Devi PU. Toxicity of Withania somnifera root extract in rats and mice. Pharm Biol. 1993;31:205–212. doi: 10.3109/13880209309082943. [DOI] [Google Scholar]
- 56.Patil D, Gautam M, Mishra S, Karupothula S, Gairola S, et al. Determination of withaferin A and withanolide A in mice plasma using high-performance liquid chromatography-tandem mass spectrometry: application to pharmacokinetics after oral administration of Withania somnifera aqueous extract. J Pharm Biomed Anal. 2013;80:203–212. doi: 10.1016/j.jpba.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Thaiparambil JT, Bender L, Ganesh T, Kline E, Patel P, et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;129:2744–2755. doi: 10.1002/ijc.25938. [DOI] [PubMed] [Google Scholar]
- 58.Dahikar PR, Kumar N, Sahni Y. Pharmacokinetics of Withania somnifera (ashwagandha) in healthy buffalo calves. Buffalo Bull. 2012;31:219. [Google Scholar]
- 59.Sumanth M, Nedunuri S. Comparison of bioavailability and bioequivalence of herbal anxiolytic drugs with marketed drug alprazolam. World J Pharm Res. 2014;3:1358–1366. [Google Scholar]
- 60.Bisht P, Rawat V. Antibacterial activity of Withania somnifera against Gram-positive isolates from pus samples. Ayu. 2014;35:330. doi: 10.4103/0974-8520.153757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh G, Kumar P. Evaluation of antimicrobial efficacy of flavonoids of Withania somnifera L. Indian J Pharm Sci. 2011;73:473. doi: 10.4103/0250-474X.99019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alam N, Hossain M, Mottalib MA, Sulaiman SA, Gan SH, et al. Methanolic extracts of Withania somnifera leaves, fruits and roots possess antioxidant properties and antibacterial activities. BMC Complement Altern Med. 2012;12:175. doi: 10.1186/1472-6882-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mwitari PG, Ayeka PA, Ondicho J, Matu EN, Bii CC. Antimicrobial activity and probable mechanisms of action of medicinal plants of Kenya: Withania somnifera, Warbugia ugandensis, Prunus africana and Plectrunthus barbatus . PLoS One. 2013;8(6):e65619. doi: 10.1371/journal.pone.0065619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Owais M, Sharad K, Shehbaz A, Saleemuddin M. Antibacterial efficacy of Withania somnifera (ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine. 2005;12:229–235. doi: 10.1016/j.phymed.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 65.Arora S, Dhillon S, Rani G, Nagpal A. The in vitro antibacterial/synergistic activities of Withania somnifera extracts. Fitoterapia. 2004;75:385–388. doi: 10.1016/j.fitote.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 66.Pandit S, Chang K-W, Jeon J-G. Effects of Withania somnifera on the growth and virulence properties of Streptococcus mutans and Streptococcus sobrinus at sub-MIC levels. Anaerobe. 2013;19:1–8. doi: 10.1016/j.anaerobe.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Chandrasekaran S, Dayakar A, Veronica J, Sundar S, Maurya R. An in vitro study of apoptotic like death in Leishmania donovani promastigotes by withanolides. Parasitol Int. 2013;62:253–261. doi: 10.1016/j.parint.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Grover A, Katiyar SP, Jeyakanthan J, Dubey VK, Sundar D. Blocking Protein kinase C signaling pathway: mechanistic insights into the anti-leishmanial activity of prospective herbal drugs from Withania somnifera . BMC Genom. 2012;13:S20. doi: 10.1186/1471-2164-13-S8-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El-On J, Ozer L, Gopas J, Sneir R, Enav H, et al. Antileishmanial activity in Israeli plants. Ann Trop Med Parasitol. 2009;103:297–306. doi: 10.1179/136485909X440827. [DOI] [PubMed] [Google Scholar]
- 70.Sachdeva H, Sehgal R, Kaur S. Studies on the protective and immunomodulatory efficacy of Withania somnifera along with cisplatin against experimental visceral leishmaniasis. Parasitol Res. 2013;112:2269–2280. doi: 10.1007/s00436-013-3387-2. [DOI] [PubMed] [Google Scholar]
- 71.Dikasso D, Makonnen E, Debella A, Abebe D, Urga K, et al. Anti-malarial activity of Withania somnifera L. Dunal extracts in mice. Ethiop Med J. 2006;44:279–285. [PubMed] [Google Scholar]
- 72.Muregi FW, Ishih A, Suzuki T, Kino H, Amano T, et al. In Vivo antimalarial activity of aqueous extracts from Kenyan medicinal plants and their Chloroquine (CQ) potentiation effects against a blood-induced CQ-resistant rodent parasite in mice. Phytother Res. 2007;21:337–343. doi: 10.1002/ptr.2067. [DOI] [PubMed] [Google Scholar]
- 73.Girish K, Machiah K, Ushanandini S, Harish Kumar K, Nagaraju S, et al. Antimicrobial properties of a non-toxic glycoprotein (WSG) from Withania somnifera (Ashwagandha) J Basic Microbiol. 2006;46:365–374. doi: 10.1002/jobm.200510108. [DOI] [PubMed] [Google Scholar]
- 74.Pawar P, Gilda S, Sharma S, Jagtap S, Paradkar A, et al. Rectal gel application of Withania somnifera root extract expounds anti-inflammatory and muco-restorative activity in TNBS-induced inflammatory bowel disease. BMC Complement Altern Med. 2011;11:34. doi: 10.1186/1472-6882-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Minhas U, Minz R, Bhatnagar A. Prophylactic effect of Withania somnifera on inflammation in a non-autoimmune prone murine model of lupus. Drug Discov Ther. 2011;5:195–201. doi: 10.5582/ddt.2011.v5.4.195. [DOI] [PubMed] [Google Scholar]
- 76.Minhas U, Minz R, Das P, Bhatnagar A. Therapeutic effect of Withania somnifera on pristane-induced model of SLE. Inflammopharmacology. 2012;20:195–205. doi: 10.1007/s10787-011-0102-8. [DOI] [PubMed] [Google Scholar]
- 77.Ku SK, Han MS, Bae JS. Withaferin A is an inhibitor of endothelial protein C receptor shedding in vitro and in vivo. Food Chem Toxicol. 2014;68:23–29. doi: 10.1016/j.fct.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 78.Lee W, Kim TH, Ku SK, Min KJ, Lee HS, et al. Barrier protective effects of withaferin A in HMGB1-induced inflammatory responses in both cellular and animal models. Toxicol Appl Pharmacol. 2012;262:91–98. doi: 10.1016/j.taap.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 79.Kaileh M, Vanden Berghe W, Heyerick A, Horion J, Piette J, et al. Withaferin a strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem. 2007;282:4253–4264. doi: 10.1074/jbc.M606728200. [DOI] [PubMed] [Google Scholar]
- 80.Oh JH, Kwon TK. Withaferin A inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules by inactivation of Akt and NF-kappaB in human pulmonary epithelial cells. Int Immunopharmacol. 2009;9:614–619. doi: 10.1016/j.intimp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Heyninck K, Lahtela-Kakkonen M, Van der Veken P, Haegeman G, Vanden Berghe W. Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKbeta. Biochem Pharmacol. 2014;91:501–509. doi: 10.1016/j.bcp.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Maitra R, Porter MA, Huang S, Gilmour BP. Inhibition of NFkappaB by the natural product Withaferin A in cellular models of Cystic Fibrosis inflammation. J Inflamm (Lond) 2009;6:15. doi: 10.1186/1476-9255-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sumantran VN, Kulkarni A, Boddul S, Chinchwade T, Koppikar SJ, et al. Chondroprotective potential of root extracts of Withania somnifera in osteoarthritis. J Biosci. 2007;32:299–307. doi: 10.1007/s12038-007-0030-3. [DOI] [PubMed] [Google Scholar]
- 84.Sumantran VN, Chandwaskar R, Joshi AK, Boddul S, Patwardhan B, et al. The relationship between chondroprotective and antiinflammatory effects of Withania somnifera root and glucosamine sulphate on human osteoarthritic cartilage in vitro. Phytother Res. 2008;22:1342–1348. doi: 10.1002/ptr.2498. [DOI] [PubMed] [Google Scholar]
- 85.Singh D, Aggarwal A, Maurya R, Naik S. Withania somnifera inhibits NF-kappaB and AP-1 transcription factors in human peripheral blood and synovial fluid mononuclear cells. Phytother Res. 2007;21:905–913. doi: 10.1002/ptr.2180. [DOI] [PubMed] [Google Scholar]
- 86.Rasool M, Varalakshmi P. Protective effect of Withania somnifera root powder in relation to lipid peroxidation, antioxidant status, glycoproteins and bone collagen on adjuvant-induced arthritis in rats. Fundam Clin Pharmacol. 2007;21:157–164. doi: 10.1111/j.1472-8206.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 87.Khan MA, Subramaneyaan M, Arora VK, Banerjee BD, Ahmed RS. Effect of Withania somnifera (Ashwagandha) root extract on amelioration of oxidative stress and autoantibodies production in collagen-induced arthritic rats. J Complement Integr Med. 2015;12:117–125. doi: 10.1515/jcim-2014-0075. [DOI] [PubMed] [Google Scholar]
- 88.Gupta A, Singh S. Evaluation of anti-inflammatory effect of Withania somnifera root on collagen-induced arthritis in rats. Pharm Biol. 2014;52:308–320. doi: 10.3109/13880209.2013.835325. [DOI] [PubMed] [Google Scholar]
- 89.Dey D, Chaskar S, Athavale N, Chitre D. Inhibition of LPS-induced TNF-alpha and NO production in mouse macrophage and inflammatory response in rat animal models by a novel Ayurvedic formulation, BV-9238. Phytother Res. 2014;28:1479–1485. doi: 10.1002/ptr.5151. [DOI] [PubMed] [Google Scholar]
- 90.Ganesan K, Sehgal PK, Mandal AB, Sayeed S. Protective effect of Withania somnifera and Cardiospermum halicacabum extracts against collagenolytic degradation of collagen. Appl Biochem Biotechnol. 2011;165:1075–1091. doi: 10.1007/s12010-011-9326-8. [DOI] [PubMed] [Google Scholar]
- 91.Kim JH, Kim SJ. Overexpression of microRNA-25 by withaferin A induces cyclooxygenase-2 expression in rabbit articular chondrocytes. J Pharmacol Sci. 2014;125:83–90. doi: 10.1254/jphs.13232FP. [DOI] [PubMed] [Google Scholar]
- 92.Yu SM, Kim SJ. Production of reactive oxygen species by withaferin A causes loss of type collagen expression and COX-2 expression through the PI3 K/Akt, p38, and JNK pathways in rabbit articular chondrocytes. Exp Cell Res. 2013;319:2822–2834. doi: 10.1016/j.yexcr.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 93.Yu SM, Kim SJ. Withaferin A-caused production of intracellular reactive oxygen species modulates apoptosis via PI3K/Akt and JNKinase in rabbit articular chondrocytes. J Korean Med Sci. 2014;29:1042–1053. doi: 10.3346/jkms.2014.29.8.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vaishnavi K, Saxena N, Shah N, Singh R, Manjunath K, et al. Differential activities of the two closely related withanolides, Withaferin A and Withanone: bioinformatics and experimental evidences. PLoS One. 2012;7:e44419. doi: 10.1371/journal.pone.0044419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Widodo N, Takagi Y, Shrestha BG, Ishii T, Kaul SC, et al. Selective killing of cancer cells by leaf extract of Ashwagandha: components, activity and pathway analyses. Cancer Lett. 2008;262:37–47. doi: 10.1016/j.canlet.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 96.Mayola E, Gallerne C, Esposti DD, Martel C, Pervaiz S, et al. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16:1014–1027. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- 97.Malik F, Kumar A, Bhushan S, Khan S, Bhatia A, et al. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis. 2007;12:2115–2133. doi: 10.1007/s10495-007-0129-x. [DOI] [PubMed] [Google Scholar]
- 98.Yang ES, Choi MJ, Kim JH, Choi KS, Kwon TK. Combination of withaferin A and X-ray irradiation enhances apoptosis in U937 cells. Toxicol In Vitro. 2011;25:1803–1810. doi: 10.1016/j.tiv.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 99.Hahm ER, Lee J, Singh SV. Role of mitogen-activated protein kinases and Mcl-1 in apoptosis induction by withaferin A in human breast cancer cells. Mol Carcinog. 2014;53:907–916. doi: 10.1002/mc.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang ES, Choi MJ, Kim JH, Choi KS, Kwon TK. Withaferin A enhances radiation-induced apoptosis in Caki cells through induction of reactive oxygen species, Bcl-2 downregulation and Akt inhibition. Chem Biol Interact. 2011;190:9–15. doi: 10.1016/j.cbi.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 101.Choi MJ, Park EJ, Min KJ, Park JW, Kwon TK. Endoplasmic reticulum stress mediates withaferin A-induced apoptosis in human renal carcinoma cells. Toxicol In Vitro. 2011;25:692–698. doi: 10.1016/j.tiv.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 102.Kim SH, Singh SV. Mammary cancer chemoprevention by withaferin A is accompanied by in vivo suppression of self-renewal of cancer stem cells. Cancer Prev Res (Phila) 2014;7:738–747. doi: 10.1158/1940-6207.CAPR-13-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hahm ER, Lee J, Kim SH, Sehrawat A, Arlotti JA, et al. Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J Natl Cancer Inst. 2013;105:1111–1122. doi: 10.1093/jnci/djt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, et al. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One. 2011;6:e23354. doi: 10.1371/journal.pone.0023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hahm ER, Singh SV. Autophagy fails to alter withaferin A-mediated lethality in human breast cancer cells. Curr Cancer Drug Targets. 2013;13:640–650. doi: 10.2174/15680096113139990039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee J, Sehrawat A, Singh SV. Withaferin A causes activation of Notch2 and Notch4 in human breast cancer cells. Breast Cancer Res Treat. 2012;136:45–56. doi: 10.1007/s10549-012-2239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer. 2008;60(Suppl 1):51–60. doi: 10.1080/01635580802381477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nagalingam A, Kuppusamy P, Singh SV, Sharma D, Saxena NK. Mechanistic elucidation of the antitumor properties of withaferin a in breast cancer. Cancer Res. 2014;74:2617–2629. doi: 10.1158/0008-5472.CAN-13-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee JH, Kim JE, Jang YJ, Lee CC, Lim TG, et al. Dehydroglyasperin C suppresses TPA-induced cell transformation through direct inhibition of MKK4 and PI3K. Mol Carcinog. 2015 doi: 10.1002/mc.22302. [DOI] [PubMed] [Google Scholar]
- 110.Antony ML, Lee J, Hahm ER, Kim SH, Marcus AI, et al. Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of beta-tubulin. J Biol Chem. 2014;289:1852–1865. doi: 10.1074/jbc.M113.496844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mathur R, Gupta SK, Singh N, Mathur S, Kochupillai V, et al. Evaluation of the effect of Withania somnifera root extracts on cell cycle and angiogenesis. J Ethnopharmacol. 2006;105:336–341. doi: 10.1016/j.jep.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 112.Mohan R, Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 113.Yang H, Wang Y, Cheryan VT, Wu W, Cui CQ, et al. Withaferin A inhibits the proteasome activity in mesothelioma in vitro and in vivo. PLoS One. 2012;7:e41214. doi: 10.1371/journal.pone.0041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Um HJ, Min KJ, Kim DE, Kwon TK. Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of human renal carcinoma Caki cells. Biochem Biophys Res Commun. 2012;427:24–29. doi: 10.1016/j.bbrc.2012.08.133. [DOI] [PubMed] [Google Scholar]
- 115.Das PK, Malhotra CL, Prasad K. Cardiotonic activity of Ashwagandhine and Ashwagandhinine, two alkaloids from Withania ashwagandha, Kaul. Arch Int Pharmacodyn Ther. 1964;150:356–362. [PubMed] [Google Scholar]
- 116.Ojha SK, Arya DS. Withania somnifera Dunal (Ashwagandha): a promising remedy for cardiovascular diseases. World J Med Sci. 2009;4:156–158. [Google Scholar]
- 117.Prince PSM, Suman S, Devika PT, Vaithianathan M. Cardioprotective effect of ‘Marutham’a polyherbal formulation on isoproterenol induced myocardial infarction in Wistar rats. Fitoterapia. 2008;79:433–438. doi: 10.1016/j.fitote.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 118.Thirunavukkarasu M, Penumathsa S, Juhasz B, Zhan L, Bagchi M, et al. Enhanced cardiovascular function and energy level by a novel chromium (III)-supplement. BioFactors. 2006;27:53–67. doi: 10.1002/biof.5520270106. [DOI] [PubMed] [Google Scholar]
- 119.Mohan IK, Kumar KV, Naidu MU, Khan M, Sundaram C. Protective effect of CardiPro against doxorubicin-induced cardiotoxicity in mice. Phytomedicine. 2006;13:222–229. doi: 10.1016/j.phymed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 120.Reuland DJ, Khademi S, Castle CJ, Irwin DC, McCord JM, et al. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic Biol Med. 2013;56:102–111. doi: 10.1016/j.freeradbiomed.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 121.Aphale AA, Chhibba AD, Kumbhakarna NR, Mateenuddin M, Dahat SH. Subacute toxicity study of the combination of ginseng (Panax ginseng) and ashwagandha (Withania somnifera) in rats: a safety assessment. Indian J Physiol Pharmacol. 1998;42:299–302. [PubMed] [Google Scholar]
- 122.Mohanty IR, Arya DS, Gupta SK. Withania somnifera provides cardioprotection and attenuates ischemia-reperfusion induced apoptosis. Clin Nutr. 2008;27:635–642. doi: 10.1016/j.clnu.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 123.Gupta SK, Mohanty I, Talwar KK, Dinda A, Joshi S, et al. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: a hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem. 2004;260:39–47. doi: 10.1023/B:MCBI.0000026051.16803.03. [DOI] [PubMed] [Google Scholar]
- 124.Mohanty I, Arya DS, Dinda A, Talwar KK, Joshi S, et al. Mechanisms of cardioprotective effect of Withania somnifera in experimentally induced myocardial infarction. Basic Clin Pharmacol Toxicol. 2004;94:184–190. doi: 10.1111/j.1742-7843.2004.pto940405.x. [DOI] [PubMed] [Google Scholar]
- 125.Ashour OM, Abdel-Naim AB, Abdallah HM, Nagy AA, Mohamadin AM, et al. Evaluation of the potential cardioprotective activity of some Saudi plants against doxorubicin toxicity. Z Naturforsch C. 2012;67:297–307. doi: 10.5560/ZNC.2012.67c0297. [DOI] [PubMed] [Google Scholar]
- 126.Hamza A, Amin A, Daoud S. The protective effect of a purified extract of Withania somnifera against doxorubicin-induced cardiac toxicity in rats. Cell Biol Toxicol. 2008;24:63–73. doi: 10.1007/s10565-007-9016-z. [DOI] [PubMed] [Google Scholar]
- 127.Gauttam VK, Kalia AN. Development of polyherbal antidiabetic formulation encapsulated in the phospholipids vesicle system. J Adv Pharm Technol Res. 2013;4:108–117. doi: 10.4103/2231-4040.111527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mutalik S, Chetana M, Sulochana B, Devi PU, Udupa N. Effect of Dianex, a herbal formulation on experimentally induced diabetes mellitus. Phytother Res. 2005;19:409–415. doi: 10.1002/ptr.1570. [DOI] [PubMed] [Google Scholar]
- 129.Bhattacharya SK, Satyan KS, Chakrabarti A. Effect of Trasina, an Ayurvedic herbal formulation, on pancreatic islet superoxide dismutase activity in hyperglycaemic rats. Indian J Exp Biol. 1997;35:297–299. [PubMed] [Google Scholar]
- 130.Andallu B, Radhika B. Hypoglycemic, diuretic and hypocholesterolemic effect of winter cherry (Withania somnifera, Dunal) root. Indian J Exp Biol. 2000;38:607–609. [PubMed] [Google Scholar]
- 131.Anwer T, Sharma M, Pillai KK, Iqbal M. Effect of Withania somnifera on insulin sensitivity in non-insulin-dependent diabetes mellitus rats. Basic Clin Pharmacol Toxicol. 2008;102:498–503. doi: 10.1111/j.1742-7843.2008.00223.x. [DOI] [PubMed] [Google Scholar]
- 132.Gorelick J, Rosenberg R, Smotrich A, Hanus L, Bernstein N (2015) Hypoglycemic activity of withanolides and elicitated Withania somnifera. Phytochemistry [DOI] [PubMed]
- 133.Udayakumar R, Kasthurirengan S, Mariashibu TS, Rajesh M, Anbazhagan VR, Kim SC, Ganapathi A, Choi CW. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int J Mol Sci. 2009;10(5):2367–2382. doi: 10.3390/ijms10052367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Udayakumar R, Kasthurirengan S, Vasudevan A, Mariashibu TS, Rayan JJ, et al. Antioxidant effect of dietary supplement Withania somnifera L. reduce blood glucose levels in alloxan-induced diabetic rats. Plant Foods Hum Nutr. 2010;65:91–98. doi: 10.1007/s11130-009-0146-8. [DOI] [PubMed] [Google Scholar]
- 135.SoRelle JA, Itoh T, Peng H, Kanak MA, Sugimoto K, et al. Withaferin A inhibits pro-inflammatory cytokine-induced damage to islets in culture and following transplantation. Diabetologia. 2013;56:814–824. doi: 10.1007/s00125-012-2813-9. [DOI] [PubMed] [Google Scholar]
- 136.Babu PV, Gokulakrishnan A, Dhandayuthabani R, Ameethkhan D, Kumar CV, et al. Protective effect of Withania somnifera (Solanaceae) on collagen glycation and cross-linking. Comp Biochem Physiol B Biochem Mol Biol. 2007;147:308–313. doi: 10.1016/j.cbpb.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 137.Bhattacharya SK, Kumar A, Ghosal S. Effects of glycowithanolides from Withania somnifera on an animal model of Alzheimer’s disease and perturbed central cholinergic markers of cognition in rats. Phytother Res. 1995;9:110–113. doi: 10.1002/ptr.2650090206. [DOI] [Google Scholar]
- 138.Kaur P, Mathur S, Sharma M, Tiwari M, Srivastava KK, et al. A biologically active constituent of Withania somnifera (ashwagandha) with antistress activity. Indian J Clin Biochem. 2001;16:195–198. doi: 10.1007/BF02864860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Singh B, Saxena AK, Chandan BK, Gupta DK, Bhutani KK, et al. Adaptogenic activity of a novel, withanolide-free aqueous fraction from the roots of Withania somnifera Dun. Phytother Res. 2001;15:311–318. doi: 10.1002/ptr.858. [DOI] [PubMed] [Google Scholar]
- 140.Khan B, Ahmad SF, Bani S, Kaul A, Suri KA, et al. Augmentation and proliferation of T lymphocytes and Th-1 cytokines by Withania somnifera in stressed mice. Int Immunopharmacol. 2006;6:1394–1403. doi: 10.1016/j.intimp.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 141.Chandrasekhar K, Kapoor J, Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med. 2012;34:255–262. doi: 10.4103/0253-7176.106022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bhattacharya A, Muruganandam AV, Kumar V, Bhattacharya SK. Effect of poly herbal formulation, EuMil, on neurochemical perturbations induced by chronic stress. Indian J Exp Biol. 2002;40:1161–1163. [PubMed] [Google Scholar]
- 143.Muruganandam AV, Kumar V, Bhattacharya SK. Effect of poly herbal formulation, EuMil, on chronic stress-induced homeostatic perturbations in rats. Indian J Exp Biol. 2002;40:1151–1160. [PubMed] [Google Scholar]
- 144.Ramanathan M, Balaji B, Justin A. Behavioural and neurochemical evaluation of Perment an herbal formulation in chronic unpredictable mild stress induced depressive model. Indian J Exp Biol. 2011;49:269–275. [PubMed] [Google Scholar]
- 145.Bhattacharya SK, Bhattacharya A, Sairam K, Ghosal S. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: an experimental study. Phytomedicine. 2000;7:463–469. doi: 10.1016/S0944-7113(00)80030-6. [DOI] [PubMed] [Google Scholar]
- 146.Bhattacharya A, Ghosal S, Bhattacharya SK. Anti-oxidant effect of Withania somnifera glycowithanolides in chronic footshock stress-induced perturbations of oxidative free radical scavenging enzymes and lipid peroxidation in rat frontal cortex and striatum. J Ethnopharmacol. 2001;74:1–6. doi: 10.1016/S0378-8741(00)00309-3. [DOI] [PubMed] [Google Scholar]
- 147.Durg S, Dhadde SB, Vandal R, Shivakumar BS, Charan CS (2015) Withania somnifera (Ashwagandha) in neurobehavioural disorders induced by brain oxidative stress in rodents: a systematic review and meta-analysis. J Pharm Pharmacol [DOI] [PubMed]
- 148.Wollen KA. Alzheimer’s disease: the pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern Med Rev. 2010;15(3):223–244. [PubMed] [Google Scholar]
- 149.Singh RH, Narsimhamurthy K, Singh G. Neuronutrient impact of Ayurvedic Rasayana therapy in brain aging. Biogerontology. 2008;9:369–374. doi: 10.1007/s10522-008-9185-z. [DOI] [PubMed] [Google Scholar]
- 150.Kuboyama T, Tohda C, Komatsu K. Effects of Ashwagandha (roots of Withania somnifera) on neurodegenerative diseases. Biol Pharm Bull. 2014;37:892–897. doi: 10.1248/bpb.b14-00022. [DOI] [PubMed] [Google Scholar]
- 151.Konar A, Shah N, Singh R, Saxena N, Kaul SC, et al. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One. 2011;6:e27265. doi: 10.1371/journal.pone.0027265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kumar P, Singh R, Nazmi A, Lakhanpal D, Kataria H, et al. Glioprotective effects of Ashwagandha leaf extract against lead induced toxicity. Biomed Res Int. 2014;2014:182029. doi: 10.1155/2014/182029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bhattacharya SK, Satyan KS. Experimental methods for evaluation of psychotropic agents in rodents: I-Anti-anxiety agents. Indian J Exp Biol. 1997;35:565–575. [PubMed] [Google Scholar]
- 154.Parihar MS, Hemnani T. Alzheimer’s disease pathogenesis and therapeutic interventions. J Clin Neurosci. 2004;11:456–467. doi: 10.1016/j.jocn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 155.Jain S, Shukla SD, Sharma K, Bhatnagar M. Neuroprotective effects of Withania somnifera Dunn in hippocampal sub-regions of female albino rat. Phytother Res. 2001;15:544–548. doi: 10.1002/ptr.802. [DOI] [PubMed] [Google Scholar]
- 156.Zhao J, Nakamura N, Hattori M, Kuboyama T, Tohda C, et al. Withanolide derivatives from the roots of Withania somnifera and their neurite outgrowth activities. Chem Pharm Bull (Tokyo) 2002;50:760–765. doi: 10.1248/cpb.50.760. [DOI] [PubMed] [Google Scholar]
- 157.Kuboyama T, Tohda C, Zhao J, Nakamura N, Hattori M, et al. Axon- or dendrite-predominant outgrowth induced by constituents from Ashwagandha. NeuroReport. 2002;13:1715–1720. doi: 10.1097/00001756-200210070-00005. [DOI] [PubMed] [Google Scholar]
- 158.Kuboyama T, Tohda C, Komatsu K. Withanoside IV and its active metabolite, sominone, attenuate Abeta(25–35)-induced neurodegeneration. Eur J Neurosci. 2006;23:1417–1426. doi: 10.1111/j.1460-9568.2006.04664.x. [DOI] [PubMed] [Google Scholar]
- 159.Kataria H, Wadhwa R, Kaul SC, Kaur G. Water extract from the leaves of Withania somnifera protect RA differentiated C6 and IMR-32 cells against glutamate-induced excitotoxicity. PLoS One. 2012;7:e37080. doi: 10.1371/journal.pone.0037080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Parihar MS, Hemnani T. Phenolic antioxidants attenuate hippocampal neuronal cell damage against kainic acid induced excitotoxicity. J Biosci. 2003;28:121–128. doi: 10.1007/BF02970142. [DOI] [PubMed] [Google Scholar]
- 161.Ahmad M, Saleem S, Ahmad AS, Ansari MA, Yousuf S, et al. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum Exp Toxicol. 2005;24:137–147. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- 162.Sankar SR, Manivasagam T, Krishnamurti A, Ramanathan M. The neuroprotective effect of Withania somnifera root extract in MPTP-intoxicated mice: an analysis of behavioral and biochemical variables. Cell Mol Biol Lett. 2007;12:473–481. doi: 10.2478/s11658-007-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.RajaSankar S, Manivasagam T, Sankar V, Prakash S, Muthusamy R, et al. Withania somnifera root extract improves catecholamines and physiological abnormalities seen in a Parkinson’s disease model mouse. J Ethnopharmacol. 2009;125:369–373. doi: 10.1016/j.jep.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 164.Rajasankar S, Manivasagam T, Surendran S. Ashwagandha leaf extract: a potential agent in treating oxidative damage and physiological abnormalities seen in a mouse model of Parkinson’s disease. Neurosci Lett. 2009;454:11–15. doi: 10.1016/j.neulet.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 165.Manjunath MJ, Muralidhara Standardized extract of Withania somnifera (Ashwagandha) markedly offsets rotenone-induced locomotor deficits, oxidative impairments and neurotoxicity in Drosophila melanogaster . J Food Sci Technol. 2015;52:1971–1981. doi: 10.1007/s13197-013-1219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Manjunath MJ, Muralidhara Effect of Withania somnifera supplementation on rotenone-induced oxidative damage in cerebellum and striatum of the male mice brain. Cent Nerv Syst Agents Med Chem. 2013;13:43–56. doi: 10.2174/1871524911313010007. [DOI] [PubMed] [Google Scholar]
- 167.Prakash J, Chouhan S, Yadav SK, Westfall S, Rai SN, et al. Withania somnifera alleviates parkinsonian phenotypes by inhibiting apoptotic pathways in dopaminergic neurons. Neurochem Res. 2014;39:2527–2536. doi: 10.1007/s11064-014-1443-7. [DOI] [PubMed] [Google Scholar]
- 168.Prakash J, Yadav SK, Chouhan S, Singh SP. Neuroprotective role of Withania somnifera root extract in maneb-paraquat induced mouse model of parkinsonism. Neurochem Res. 2013;38:972–980. doi: 10.1007/s11064-013-1005-4. [DOI] [PubMed] [Google Scholar]
- 169.Pingali U, Pilli R, Fatima N. Effect of standardized aqueous extract of Withania somnifera on tests of cognitive and psychomotor performance in healthy human participants. Pharmacognosy Res. 2014;6:12–18. doi: 10.4103/0974-8490.122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sehgal N, Gupta A, Valli RK, Joshi SD, Mills JT, et al. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc Natl Acad Sci USA. 2012;109:3510–3515. doi: 10.1073/pnas.1112209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jayaprakasam B, Padmanabhan K, Nair MG. Withanamides in Withania somnifera fruit protect PC-12 cells from beta-amyloid responsible for Alzheimer’s disease. Phytother Res. 2010;24:859–863. doi: 10.1002/ptr.3033. [DOI] [PubMed] [Google Scholar]
- 172.Grover A, Shandilya A, Agrawal V, Bisaria VS, Sundar D. Computational evidence to inhibition of human acetyl cholinesterase by withanolide a for Alzheimer treatment. J Biomol Struct Dyn. 2012;29:651–662. doi: 10.1080/07391102.2012.10507408. [DOI] [PubMed] [Google Scholar]
- 173.Yadav CS, Kumar V, Suke SG, Ahmed RS, Mediratta PK, et al. Propoxur-induced acetylcholine esterase inhibition and impairment of cognitive function: attenuation by Withania somnifera . Indian J Biochem Biophys. 2010;47:117–120. [PubMed] [Google Scholar]
- 174.Ahmed ME, Javed H, Khan MM, Vaibhav K, Ahmad A, et al. Attenuation of oxidative damage-associated cognitive decline by Withania somnifera in rat model of streptozotocin-induced cognitive impairment. Protoplasma. 2013;250:1067–1078. doi: 10.1007/s00709-013-0482-2. [DOI] [PubMed] [Google Scholar]
- 175.Kurapati KR, Atluri VS, Samikkannu T, Nair MP. Ashwagandha (Withania somnifera) reverses beta-amyloid1-42 induced toxicity in human neuronal cells: implications in HIV-associated neurocognitive disorders (HAND) PLoS One. 2013;8:e77624. doi: 10.1371/journal.pone.0077624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kurapati KR, Samikkannu T, Atluri VS, Kaftanovskaya E, Yndart A, et al. beta-Amyloid1-42, HIV-1Ba-L (clade B) infection and drugs of abuse induced degeneration in human neuronal cells and protective effects of ashwagandha (Withania somnifera) and its constituent Withanolide A. PLoS One. 2014;9:e112818. doi: 10.1371/journal.pone.0112818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kumar S, Seal CJ, Howes MJ, Kite GC, Okello EJ. In vitro protective effects of Withania somnifera (L.) dunal root extract against hydrogen peroxide and beta-amyloid(1-42)-induced cytotoxicity in differentiated PC12 cells. Phytother Res. 2010;24:1567–1574. doi: 10.1002/ptr.3261. [DOI] [PubMed] [Google Scholar]
- 178.Chaudhary G, Sharma U, Jagannathan NR, Gupta YK. Evaluation of Withania somnifera in a middle cerebral artery occlusion model of stroke in rats. Clin Exp Pharmacol Physiol. 2003;30:399–404. doi: 10.1046/j.1440-1681.2003.03849.x. [DOI] [PubMed] [Google Scholar]
- 179.Raghavan A, Shah ZA. Withania somnifera improves ischemic stroke outcomes by attenuating PARP1-AIF-mediated caspase-independent Apoptosis . Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8907-2. [DOI] [PubMed] [Google Scholar]
- 180.Raghavan A, Shah ZA. Withania somnifera: a pre-clinical study on neuroregenerative therapy for stroke. Neural Regen Res. 2015;10:183–185. doi: 10.4103/1673-5374.152362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baitharu I, Jain V, Deep SN, Hota KB, Hota SK, et al. Withania somnifera root extract ameliorates hypobaric hypoxia induced memory impairment in rats. J Ethnopharmacol. 2013;145:431–441. doi: 10.1016/j.jep.2012.10.063. [DOI] [PubMed] [Google Scholar]
- 182.Baitharu I, Jain V, Deep SN, Shroff S, Sahu JK, et al. Withanolide A prevents neurodegeneration by modulating hippocampal glutathione biosynthesis during hypoxia. PLoS One. 2014;9:e105311. doi: 10.1371/journal.pone.0105311. [DOI] [PMC free article] [PubMed] [Google Scholar]