Abstract
αA-Crystallin (αA) and αB-crystallin (αB) are small heat shock proteins responsible for the maintenance of transparency in the lens. In non-lenticular tissues, αB is involved in both maintenance of the cytoskeleton and suppression of neurodegeneration amongst other roles. Despite their importance in maintaining cellular health, modifications and mutations to αA and αB appear to play a role in disease states such as cataract and myopathies. The list of modifications that have been reported is extensive and include oxidation, disulphide bond formation, C- and N-terminal truncation, acetylation, carboxymethylation, carboxyethylation, carbamylation, deamidation, phosphorylation and methylation. Such modifications, notably phosphorylation, are alleged to cause changes to chaperone activity by inducing substructural changes and altering subunit exchange dynamics. Although the effect modification has on the activities of αA and αB is contentious, it has been proposed that these changes are responsible for the induction of hyperactivity and are thereby indirectly responsible for protein deposition characteristic of many diseases associated with αA and αB. This review compiles all reported sites of αA and αB modifications, and investigates the role phosphorylation, in particular, plays in cellular processes.
Keywords: Chaperones, PTMs, Structure, Proteostasis
Introduction
The human lens proteome is dominated by the crystallins, a long-lived family of proteins that encompasses the α-, β- and γ-crystallins. In the lenticular environment, the crystallins are susceptible to the accumulation of post-translational modifications, due largely to their extended life spans. Such modifications, notably phosphorylation, have also been reported in extralenticular αB, often being implicated in the regulation of the protein’s activity [3, 36–50]. Although such modifications have been extensively reported in the lens in recent years [23–25, 27–31], the implications, particularly for αA and αB functions, have yet to be characterised.
The crystallins
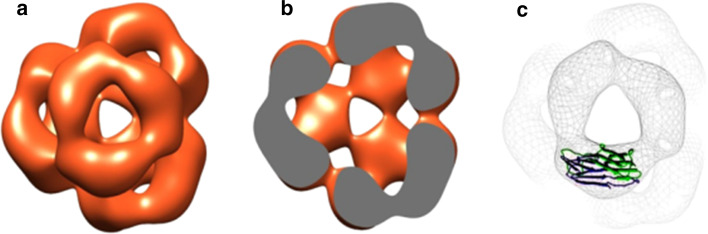
The two subunits of α-crystallin, αA and αB, also known as HSPB4 and HSPB5, respectively, both have an approximate molecular mass of 20 kDa [51], share 60 % sequence similarity [52], and exist in the cytoplasm of the lens fibres at a ratio of three A subunits to one B subunit [53] where they form a dynamic polydisperse population of heterooligomers with an average mass of ~800 kDa [51]. α-Crystallin oligomers exist in a state of dynamic equilibrium, undergoing subunit exchange where αA and αB subunits are readily interconverted [54]. As a member of the sHsp family [55–57], each subunit contains a highly conserved α-crystallin domain containing 5 β-strands labelled β2- β5, β6/7, β8 and β9 [58]. The α-crystallin domain is flanked by the C- and N-terminal domains [59]. The N-terminal domain contains an antiparallel β-sheet involving the residues 44–65 [60], while the C-terminal domain is highly variable and is capped with a flexible C-terminal tail [61]. Dimers, the building block of the oligomer [60, 62], are formed via interactions between the β6/7 strands of the α-crystallin domain of two monomers [60, 63, 64]. They form an antiparallel β-sheet at the interface [63, 64], while the β4 and β8 strands form a groove lined with charged amino acids [60, 65] that participate in ionic bonds that stabilise the complex [62–64]. Hydrophobic binding sites within the groove bind the C-terminal I-X-I motif of neighbouring dimers to form hexamers [60, 62–66]. Due to difficulty in achieving protein crystallisation, the structure of larger oligomers is based largely on conjecture with multiple models being proposed [60, 64–66]. However, it is generally believed that the hexamers interact to form larger structures that feature a roughly spherical shape with binding sites located either as pockets on the surface [60], or within the hollow interior accessible through openings in the shell [52, 64, 67], as depicted in Fig. 1. It has been reported that αB shows a bias towards a 24meric structure [64], that allows the formation of differently sized constructs via either the association of extra dimers to the external pockets [60] or the addition and subtraction of subunits to the shell itself [64, 66]. In the latter, the subtraction of subunits would lead to the exposure of hydrophobic regions found on the inner walls [64] and extensions [65], and the production of higher activity smaller oligomers [34] and, eventually, monomers [32, 68], allowing for the regulation of activity mediated by the dissociation of subunits. The molecular changes to dimers required for these dynamic interconversions, which involve the breakage of two C-terminal interactions, are reportedly minor, with only a slight change to the angle of interactions of the C-terminal regions and the groove observed [66]. Polydispersity is thought to be achieved as a result of the reverse and forward binding of the palindromic I-X-I motif to the β4–β8 groove, and the presence of 3 different registration shifts at the interface [58]. Considering the homogeneity of the oligomers, it is likely that no currently proposed model fully characterises the true quaternary structure of α-crystallin, especially as many focus on the 24meric oligomer [60, 64], potentially overstating the prevalence or importance of the hexameric substructure. Instead, it is more likely that the true structure resembles an amalgamation of various proposed models [69].
Fig. 1.
Model of recombinant human αB quaternary structure. Depicted as surface representation (a), density cross section (b) and with dimeric α-crystallin domain of Hsp16.5 from M. jannaschii overlay (c). Adapted from Peschek et al. [52]
Chaperone activity of α-crystallin
α-Crystallin oligomers have been reported to exist in two substructural formations: a high-affinity monomeric and a low-affinity dimeric substructures [68]. The major substrates of αA and αB in the lens fibre cell are λ- and β-crystallin [21], although interactions with ‘house-keeping’ enzymes such as enolase [70], cytoskeletal elements [71] and membrane-associated proteins also occur [72, 73]. It appears that the quaternary structure of the protein with a hydrophilic exterior and a hydrophobic interior, is vital to chaperone activity, as it has been observed that the structure is conserved [74] and resistant to changes in the amino acid sequence [61]. Furthermore, the addition of a negative charge to the protein surface such as through citraconylation has been seen to increase activity [75]. This is significant considering that various PTMs, which can alter surface charge, have also been found to affect the chaperone activities of αA and αB [57, 76–78].
α-Crystallins in health and disease
While αA is found almost exclusively in the lens [52] with trace amounts being detected in the thymus, spleen, retina [79], liver, kidney and pancreas [80], αB is also found in relatively high amounts in non-lenticular tissue, such as the brain, heart, muscle tissue [52], nervous system, retina, iris, thyroid, colon, squamous epithelia, placenta, spermatocytes and tissues subjected to high oxidative stress such as in the kidneys [79]. Expression of αB has been found to be induced by infection [81], oncogene expression [82], osmotic stress [83], heat shock [56], dexamethasone, cadmium, sodium arsenite [84] and muscle stretch [79, 85].
As αB is expressed ubiquitously throughout body tissues, the functions it serves in maintaining health are highly diverse. In the lens, the crystallins are vital in lens transparency by maintaining a medium that is optimal for light transmission [86]. In non-lenticular tissue, however, αB has often been associated with neurodegenerative disorders, for example being overexpressed and co-deposited in protein inclusion bodies such as Rosenthal fibres associated with Alexander’s disease [87], where it is believed to serve a neuroprotective role by preventing the aggregation of GFAP [6]. It has also been shown that αB has anti-apoptotic properties, preventing cell death in response to disease such as stroke [88] or infection [81], and preventing damage caused by the inflammation response itself [81]. αB is also known to be involved in maintenance of the cytoskeleton, binding to and stabilising components of the cytoskeleton in response to stress such as ischaemia [1]. Considering αA’s comparatively low abundance in non-lenticular tissues, its role outside the lens has not been as extensively studied. However, it has been implicated with cases of pancreatic cancer, where it has been proposed that it inhibits carcinogenesis by retarding cell migration, as well as activating protein-1 (AP-1) and upregulating components of the TGFβ signalling pathway [80]. These roles are summarised in Table 1.
Table 1.
Suggested roles of αA- and αB-crystallins in non-lenticular tissue
| Functions | References |
|---|---|
| αA | |
| Inhibition of carcinogenesis | [80] |
| αB | |
| Anti-apoptosis | [4, 42, 81, 89, 90] |
| Protection against oxidative damage | [4, 89] |
| Maintenance of cytoskeletal integrity | [1–4] |
| Suppression of neurodegeneration | [5, 6] |
| Formation of inclusion bodies in protein conformation diseases | [4, 10, 87, 91, 92] |
| Promotion of neoplastic changes and invasiveness of cancerous cells | [93, 94] |
| Anti-inflammation | [88, 90] |
Despite its overall role in maintaining the health of cells, overexpression or mutations in αB have been associated with particular disease states (Table 2) [87, 91]. For example, it is believed that the accumulation of αB may cause the deterioration of the microenvironment surrounding neurons associated with Alzheimer’s Disease [87]. αB has also been identified as an oncoprotein, where it promotes neoplastic changes and invasiveness and prevents apoptosis in basal-like breast carcinomas [93]. αB has also been implicated in the increased risk of recurrence in head and neck cancers, [94] as well as the inhibition of TRAIL-induced apoptosis in various cancer cell lines [95]. Mutations in αA and αB have been associated with cataract [7–16] and various myopathies [10, 17–20].
Table 2.
Pathologically significant mutations of αA and αB in humans
| Disease | Species | Mutation | References |
|---|---|---|---|
| Autosomal dominant congenital cataract | αB | D140N | [9] |
| 450delA | [8] | ||
| P20S | [11] | ||
| R120G | [10] | ||
| αA | R116C | [7, 12] | |
| R49C | [13] | ||
| R116H | [14] | ||
| R54P | [15] | ||
| Autosomal recessive congenital cataract | αA | W9X | [16] |
| Desmin-related myopathy | αB | R120G | [10] |
| Dilated cardiomyopathy | αB | R157H | [17] |
| G157S | [18] | ||
| Myofibrillar myopathy | αB | Q151X | [20] |
| 464delCT | [20] | ||
| Late-onset distal vacuolar myopathy | αB | G154S | [19] |
Central to all of these functions is the ability of αA and αB to act as a molecular chaperones, which in the lens is also essential for the maintenance of lens transparency.
Post-translational modification of α-Crystallin
Owing to the protein longevity and the negligible protein turnover of the lens, lenticular αA and αB are vulnerable to the accumulation of PTMs with age. Such modifications are first detectable in the foetal lens [96] with the modification profile changing little after 17 years of age [97]. The levels of intact αA and αB itself have been shown to decrease with age, with little full-length isoforms remaining after 75 years of age [53]. However, αA is lost at a greater rate, suggesting αB has a higher level of stability [53].
Modifications that have been reported are summarised in Table 3.
Table 3.
Modification sites identified in human lens αA and αB
| Subunit | Modification | Residue | References |
|---|---|---|---|
| αA | Oxidation |
Met1 Trp9 Met138 Tyr18 Tyr34 His154 |
[24] [24] [99] |
| Disulphide bond | Cys131–Cys142 | [21, 22] | |
| Deamidation |
Gln6 Gln50 Gln90 Asn101 Gln104 Asn123 Gln126 Gln147 |
||
| Truncation |
Met1–Gln50 Glu102–Ser173 Ser173 Met1–Arg65 Met1–Phe80 Ala152–Ser173 Ser169–Ser173 |
[22] [22] [22] [21] [21] [22] [22] |
|
| Acetylation |
Met1 Lys70 Lys78 Lys88 Lys145 |
[24] [24] [24] |
|
| Carboxymethylation | Lys11 | [23] | |
| Carboxyethylation | Lys11 | [23] | |
| Carbamylation | Lys99 | [23] | |
| Methylation |
His79 His154 Arg21 Lys88 Val89 Gln90 Ile146 Gln147 His154 Arg157 |
[99] [23] [24] [24] [99] [99] [99] [99] [99] [99] |
|
| Ethylation |
Thr13 His79 Val89 Ile146 |
[99] [99] [99] [99] |
|
| Formylation | His79 | [98] | |
| Conversion to DHA | Ser59 | [98] | |
| dkpD formation | Asp67 | [102] | |
| αB | Oxidation |
Met1 Trp9 Trp60 Met68 Tyr48 |
[24] |
| Deamidation |
Gln26 Asn78 Gln108 Asn146 |
||
| Carboxymethylation |
Lys82 Lys90 Lys92 Lys103 Lys174 |
[23] [23] [23] [23] [23] |
|
| Carboxyethylation |
Lys92 Lys175 |
[23] [23] |
|
| Acetylation |
Met1 Lys92 |
||
| Methylation |
His83 Arg22 Arg50 |
[24] [24] |
|
| Ethylation |
His8 Val93 Gln108 |
[99] [99] [99] |
|
| Carbamylation |
Met1 Glu164 Lys166 Lys92 Lys175 |
[99] [99] [23] [101] |
|
| Truncation |
Met1–Pro46 Gln151–Lys175 Val152–Lys175 Ser153–Lys175 Gly154–Lys175 Pro155–Lys175 Arg163–Lys175 Glu164–Lys175 Ala171–Lys175 Ala172–Lys175 Lys175 Pro130–Lys175 Met1–His7 |
[105] [103] [103] [103] [103] [103] [103] [103] [103] [25] [104] [22] |
When considering the effect such modifications have on the structure and function of αA and αB, it has become apparent that a holistic approach is required, treating it as a complex system of interacting modifications rather than standalone events. For example, in vitro experiments performed by Chaves et al. [107] and Asomugha et al. [108] showed that deamidation of αA and αB at N123 and N146, respectively, and truncation alter structure and function, leading to a decrease in chaperone function [107] and an increase in β-sheet formation and oligomeric size [108]. However, when present on the same protein, an ameliorating effect was seen with the loss of activity being limited [108]. Furthermore, while deamidation, C-terminal truncation and a combination of the two produced a compact tertiary structure, N-terminal truncation and a combination of deamidation and N-terminal truncation resulted in a relaxed tertiary structure [108].
In the context of the lens, N- and C-terminal truncation, oxidation of methionine residues, deamidation [21, 22], cleavage at deamidated residues [22], phosphorylation and intramolecular disulphide bond formation [21] have all been associated with the insoluble protein fraction. This has led to the implication of such modifications in protein insolubilisation events. One mechanism proposed involves deamidation-induced conformational changes that lead to disulphide bond formation between oxidised cysteine residues [21, 22] and exposure of the hydrophobic interior [21]. Insoluble complexes form as a consequence and are held together by strong hydrogen bonds [21]. Although various modifications have been associated with this process, this paper focuses on phosphorylation, as it is one of the most abundant and most widely researched modifications to both αA and αB.
Phosphorylation of αA and αB
33–50 % of lenticular αA and αB has been reported to undergo phosphorylation [74, 86], with phosphorylated products being present from birth [96]. The phosphorylation status of the crystallins is in dynamic equilibrium under physiological conditions [109] and in dividing, differentiating or newly differentiated cells, like that of the lenticular epithelium [96]. Phosphorylation of αA and αB can also be induced by oxidative agents such as Fenton reagent [32, 110, 111], and by stress conditions such as ischaemia [35] and heat stress [109].
In vivo, the majority of αA and αB is phosphorylated via the cAMP-dependent pathway in a reversible serine-specific manner [79]. It has been a long-held belief that the dominant sites of phosphorylation in α-crystallin are Ser19, Ser45 and Ser59 for αB, and Ser122 for αA. The enzymes believed to be responsible for enzymatic phosphorylation at these sites are p44/42 MAP kinase for the Ser19 and Ser45 residues of αB and MAPKAP kinase 2 for the Ser59 residue of αB and Ser122 residue of αA [112]. Despite the dominance of these sites in the literature, a multitude of other sites of phosphorylation, including at threonine and tyrosine residues, for both subunits have been recently reported (Table 4).
Table 4.
Phosphorylation sites of human lens αA and αB
| Subunit | Site | References |
|---|---|---|
| αA | S13 | [28] |
| S20 | [27, 28] | |
| S45 | [24, 27] | |
| S51 | [27] | |
| S59 | [27, 28] | |
| S62 | [27, 28] | |
| S66 | [27, 29] | |
| S81 | [27, 29] | |
| S122 | [23, 24, 27, 30, 31] | |
| S127 | [27] | |
| S130 | [27] | |
| S134 | Thornell, unpublished | |
| S140 | [24] | |
| S162 | [27, 28] | |
| S172 | [27] | |
| S173 | [27] | |
| T13 | [24, 27] | |
| T43 | [27] | |
| T55 | [27] | |
| T86 | [27] | |
| T140 | [24] | |
| T148 | [27–29] | |
| T168/S169 | [27] | |
| T153 | [27, 29] | |
| Y47 | [27] | |
| Y118 | [27] | |
| αB | S19 | [23, 24, 27, 29, 31, 103] |
| S21 | [24, 27, 29] | |
| S43 | [24, 27] | |
| S45 | [24, 27, 31] | |
| S53 | [24, 27, 28] | |
| S59 | [23–25, 27–31] | |
| S66 | [27] | |
| S76 | [23, 24, 27, 29] | |
| S85 | [27] | |
| S136 | [27] | |
| S138 | [27, 28] | |
| S139 | [27–29] | |
| S153 | [27] | |
| T63 | [27] | |
| T170 | [28] | |
| T132 | [27] | |
| T134 | [27] | |
| T158 | [27] |
Although much less prominent in the literature, non-enzymatic autophosphorylation has also been observed in lenticular αA and αB [79]. A little is known about the mechanism of autophosphorylation in the lens, but it has been shown in vitro that both αA and αB are able to undergo non-enzymatic phosphorylation in the presence of either Mn+ [113] or Mg+ [114] and ATP. Notably, it has been shown that under conditions of limited cAMP-dependent kinase concentration, αB experiences 10 × higher rates of phosphorylation than αA, while incubation with 1 % deoxycholate causes the disaggregation of αA into tetramers and a 10× higher rate of autophosphorylation compared to αB [115], suggesting preferences for different phosphorylation pathways. Recently, a mechanism of non-enzymatic dephosphorylation has also been proposed that involves the spontaneous β-elimination of phosphate groups from phosphoserine and phosphothreonine residues and the subsequent glutathionylation of the resultant dehydroalanine [116] intermediate [28]. The presence of such non-enzymatic pathways in the lens is significant as they provide a mechanism of reversible phosphorylation in a tissue that is generally believed to be largely metabolically and enzymatically inactive. Although it is as yet unclear what proportion of the phosphorylation observed in the lens is residual from early enzymatic phosphorylation events, and how much results from subsequent non-enzymatic events, it is likely that αA and αB enzymatically phosphorylated in the metabolically active cells of the outer cortex or gradually dephosphorylated non-enzymatically as it forms part of the metabolically inactive nucleus, where a dynamic balance of non-enzymatic phosphorylation and dephosphorylation events occur.
The effect phosphorylation has on the chaperone activity of αA and αB, if any, has met with contention in the literature. Earlier studies by Ito et al. [120] reported a decrease in chaperone activity when thermal aggregation assays were performed utilising phosphomimics. They attributed this to an observed decrease in oligomeric size. These findings were supported by Kamei et al. [117] who reported a 30 % decrease in the ability of bovine phosphoextracts to prevent the heat-induced aggregation of βL-crystallin. A complimentary study by Augusteyn et al. [118], however, reported no effect on chaperone activity. More recently, studies utilising phosphomimics have found that the addition of one or more negative charges onto αB acts to increase target protein affinity [33, 119], increase oligomeric polydispersity [33], increases the rate of subunit exchange, decreases stability [35] and decrease average oligomeric size [33, 120]. It has been proposed that such modifications to the N-terminal domain region of αA and αB induce flexibility and structural changes that lead to increased exposure of substrate binding sites [34]. Phosphorylation in this region has also been shown to interrupt intersubunit interactions, leading to the dissociation of larger oligomers to form smaller oligomers with higher activity [33, 34]. It has been suggested that this hyperactivity induces aggregation rather than prevents it, as αA and αB reach saturation and codeposit with the target proteins at an earlier stage [33]. This is supported by a study by Aquilina et al. [32] that reported both a change in oligomeric substructure i.e. a preference towards high-affinity monomeric substructure and away from low-affinity dimeric substructure, coupled with an earlier onset of the reduction-induced aggregation of α-lactalbumin. Taken together, it appears that the hyperactivity observed recently upon phosphorylation of αA and αB is a result of the increased exposure of substrate binding sites [34] brought about by the dissociation of dimeric oligomer substructures [32]. This process also provides a mechanism to regulate the activity of αA and αB in non-lenticular tissues.
Roles of phosphorylated αA and αB
In the context of the lens, a little is known of the significance of αA or αB phosphorylation. However, reports of the involvement of phosphorylated αB in various pathways including stress response [39, 40, 42, 44–46, 48, 49, 88], the regulation of apoptosis [38, 50], actin dynamics [3, 39, 40] and protein quality control [36, 41] suggest that it is pivotal in many cellular processes in non-lenticular tissues.
It has been widely reported that phosphorylation of αB is induced in response to various stresses via activation of the p38/MAPK pathway [3, 36, 45, 49]. Although this appears to often be an intrinsic step in the cytoprotection of cells by αB, the protective pathways that follow are wide and varied. In cardiomyocytes subjected to induced ischemic stress, phosphorylation of αB at the Ser59 residue [42, 44] and Ser45 [44] was accompanied by concurrent translocation of 15–20 % of the total αB pool to the z-lines of sarcomeres [37, 42–44]. Although it was reported that the translocation was not due exclusively to the phosphorylation event [44], inhibiting phosphorylation at Ser59 leads to cell death [42]. It was proposed that the cytoprotective properties of associated phosphorylated αB were due to the stabilisation of myofibrils [42] and the prevention of osmotic stress and associated swelling [44]. αB has also been reported to inhibit caspase-3 activation in both cardiomyocytes in response to hyperosmotic and hypoxic stress [46] and in differentiating myoblasts [50]. In the case of stressed cardiomyocytes, cell death was prevented by phosphorylation at Ser59 [46]. However, the cytoprotective properties of nonphosphorylated αB in preventing differentiation-induced apoptosis in myoblasts were lost following multiple phosphorylation [50], indicating that the phosphorylation is involved in both positive and negative regulation of apoptotic processes under different conditions. Phosphorylation at Ser59 has also been implicated in the expression and regulation of the cytoprotectant protein, Bcl2 [38, 45]. Exposure of myoblasts to TNF-α has been seen to induce phosphorylation of αB, leading to a cascade of events resulting in the nuclear uptake and increased expression of Bcl2 [45]. In conjunction with this, exposure to vinblastine, a microtubule-depolymerisation agent, has been shown to induce αB phosphorylation in breast epithelial carcinoma cell line MCF7, inducing association with Bcl2 [38]. It is believed that this prevents translocation of Bcl2 into the mitochondria, repressing Bcl2 anti-apoptotic ability [38], thereby limiting cell immortality and cancer pathogenesis. Dual phosphorylation at Ser59 in conjunction with Ser45 induced by PAR-2 activation has also been implicated in the protection of astrocytes against cytotoxic stress induced with C2-ceramide and staurosporine [49], and in the formation of nuclear speckles in HeLa cells [47, 48]. It was reported that αB phosphorylated at Ser59 interacts with Gemin-3, part of the Survival Motor Neuron complex [48], and is translocated into the nucleoplasm where phosphorylation at Ser45 is involved in speckle formation [47]. Nuclear speckles act as depots to ensure a readily available source of chaperone in the event of cellular stress, upon which αB is then released from the speckles to prevent cell death [48]. Interestingly, the hyperphosphorylated R120G αB mutant is unable to be translocated into the nucleus due to the formation of cytoplasmic inclusions [47]. It has been reported that αB which preferentially phosphorylated at Ser59 accumulates in brains from Alexander’s [121, 122] and Alzheimer’s disease patients [121]. However, as similar accumulation was also seen in aged control brains [121, 122], it remains unclear whether phosphorylation state is important for neuroprotective properties of αB in neurodegenerative disorders.
Observations of increased phosphorylation at the Ser59, and to a lesser extent the Ser45 residue and concurrent translocation of αB to the insoluble fraction of rat soleus muscle lead to the suggestion that phosphorylated αB is involved in the ubiquitin–proteasome system [36], which is involved in the degradation of proteins. This was supported by the observation that treatment of U373 MG human glioma cells with the proteasome inhibitor MG-132 induced phosphorylation of αB at Ser59 which was accompanied by translocation to aggresomes [41]. It was proposed that αB alters the structure of intermediate filaments, facilitating aggresome formation, in conjunction with targeting and degrading ubiquitinated proteins [41]. However, although phosphorylation was induced by MG-132 treatment, it was not seen to be required for aggresome association [41], implying a different role for the phosphorylated form.
It has been shown that any perturbation to the cytoskeletal structure i.e. actin, microfilaments, microtubules or intermediate filaments, induces the p38/MAPKAP-2 pathway and thereby phosphorylation of αB at Ser59 [3]. Phosphorylated αB is believed to associate with and stabilise cytoskeletal components [3], but in murine myoblasts treated with vinblastine and cytochalasin-D, known disrupters of the cytoskeleton, phosphorylated αB was also seen to relocate to cytoplasmic focal adhesions where actin stress fibres attach [3]. In the lens, both αA and αB have been seen to localise at the leading membrane of migrating epithelial cells, suggesting that they are involved in actin dynamics including those associated with membrane protrusion and cell adhesion in cell mobility [39].Unlike αA, however, the association of αB appears to be mediated by phosphorylation at Ser59, leading to interactions with the proteins Arp3, a component of the actin nucleation complex, WAVE-1, Abi and β-catenin, which is involved in cell adhesion, and the actin meshwork [39]. Actin polymerisation is also believed to be modulated by both αA and αB [40]. Unphosphorylated αA and αB have both been shown to inhibit the cytochalasin-D-induced polymerisation of actin, an ability that is lost upon phosphorylation [40]. This suggests that the dynamic phosphorylation state of both subunits is involved in the regulation of actin polymerisation and depolymerisation events during cellular remodelling processes such as those associated with epithelial cell differentiation in the lens [40]. Information on the significance of αA phosphorylation in non-lenticular tissue is sparse due to its relatively low abundance. However, if αA phosphorylation is of more import in the lens than in other tissues due to its higher abundance, it may also help explain why αA is relatively more susceptible to autophosphorylation compared to αB [115], as the enzymatic activity in the lens is somewhat suppressed.
These roles are summarised in Table 5.
Table 5.
Role of phosphorylated αA and αB in lenticular and non-lenticular tissues
| Role | Mechanism | Residues | References |
|---|---|---|---|
|
Cytoprotection (inhibition of apoptosis) |
Localisation to Z-bands of myocytes following ischaemia. |
Ser59 Ser45 and Ser59 |
[44] |
| Induces Bcl2 expression in myoblasts in presence of TNF-α. | Ser59 | [45] | |
| Inhibits caspase-3 activation in myocytes following hyperosmotic or hypoxic stress. | Ser59 | [46] | |
| Nuclear speckle formation following heat shock. | Ser45 and Ser59 | [47, 48] | |
| Activated by PAR-2 in astrocytes in presence of C2-ceramide and staurosporine. | Ser45 and Ser59 | [49] | |
| Regulation of apoptosis (promotes apoptosis) | Reduces anti-apoptotic activity of αB in differentiation-induced cell death of myoblasts. | Ser19, Ser45 and Ser59 | [50] |
| Associates with and inhibits anti-apoptotic activity of Bcl2 in vinblastine treated breast epithelial carcinoma cells. | Ser59 | [38] | |
| Modulation of actin dynamics | Associates with and stabilises cytoskeletal components at focal adhesions in myoblasts treated with vinblastine and cytochalasin-D. | Ser59 | [3] |
| Associates with proteins of the actin nucleation complex involved with membrane protrusion and cell migration in migratory lenticular epithelial cells. | Ser59 | [39] | |
| Negatively modulates actin depolymerisation in vitro. | [40] | ||
| Protein quality control | Participates in the ubiquitin–proteasome system to degrade excess protein in disused rat soleus muscle. | Ser59 | [36] |
| Accumulates in and potentially facilitates formation of aggresomes in MG-132-treated glioma cells. | Ser59 | [41] |
It has become clear that modifications to αA and αB such as phosphorylation not only are responsible for the regulation of chaperone activity of the proteins [32–34, 120], but it is greatly influential in the roles they take on in cellular processes [3, 36–50]. Although phosphorylation, particularly of αB, appears to be important in maintaining cellular health, it may also be involved in the disruption of proteostasis that can result in protein deposition diseases such as Alexander’s disease [121, 122]. Further understanding of the processes that contribute to these conditions is therefore vital, particularly when considering the longevity of the proteins involved and their propensity to be modified in such a way.
Acknowledgments
The help of Heath Ecroyd with the editing of this paper is gratefully acknowledged.
References
- 1.Bennardini F, Wrzosek A, Chiesi M. αB-Crystallin in cardiac tissue. Association with actin and desmin filaments. Circ Res. 1992;71:288–294. doi: 10.1161/01.RES.71.2.288. [DOI] [PubMed] [Google Scholar]
- 2.Djabali K, de Nechaud B, Landon F, Portier M-M. αB-Crystallin interacts with intermediate filaments in response to stress. J Cell Sci. 1997;110:2759–2769. doi: 10.1242/jcs.110.21.2759. [DOI] [PubMed] [Google Scholar]
- 3.Launay N, Goudeau B, Kato K, Vicart P, Lilienbaum A (2006) Cell signaling pathways to αB-crystallin following stresses of the cytoskeleton. Exp Cell Res 312:3570–3584 [DOI] [PubMed]
- 4.Arrigo A-P, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P. Hsp27 (HspB1) and αB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3874. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Martin E, Gonzales V, Borchelt DR, Lee MK. Differential regulation of sHsps in transgenic mouse models of neurodegenerative diseases. Neurobiol Aging. 2008;29:586–597. doi: 10.1016/j.neurobiolaging.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagemann TL, Boelens WC, Wawrousek EF, Messing A. Suppression of GFAP toxicity by αB-crystallin in mouse models of Alexander disease. Hum Mol Genet. 2009;18:1190–1199. doi: 10.1093/hmg/ddp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanita V, Singh JR, Hejtmancik JF, Nurnberg P, Hennies HC, Singh D, Sperling K. A novel fan-shaped cataract-microcornea syndrome caused by a mutation of CRYAA in an Indian family. Mol Vis. 2006;12:518–522. [PubMed] [Google Scholar]
- 8.Berry V, Francis P, Reddy MA, Collyer D, Vithana E, Mackay I, Dawson G, Carey AH, Moore A, Bhattacharya SS, Quinlan RA. αB-Crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in human. Am J Hum Genet. 2001;69:1141–1145. doi: 10.1086/324158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Zhang X, Luo L, Wu M, Zeng R, Cheng G, Hu B, Liu B, Liang JJ, Shang F. A novel αB-crystallin mutation associated with autosomal dominant congenital lamellar cataract. Invest Ophthalmol Vis Sci. 2006;47:1069–1075. doi: 10.1167/iovs.05-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vicart P, Caron A, Guicheney P, Li Z, Prevost M-C, Faure A, Chateau D, Chapon F, Tome F, Dupret J-M, Paulin D, Fardeau M. A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 11.Liu M, Ke T, Wang Z, Yang Q, Chang W, Jiang F, Tang Z, Li H, Ren X, Wang X, Wang T, Li Q, Yang J, Liu J, Wang QK. Identification of CRYAB mutation associated with autosomal dominant posterior polar cataract in a Chinese family. Invest Ophthalmol Vis Sci. 2006;47:3461–3466. doi: 10.1167/iovs.05-1438. [DOI] [PubMed] [Google Scholar]
- 12.Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missense mutation in the human α-crystallin gene CRYAA . Hum Mol Genet. 1998;7:471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- 13.Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the αA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- 14.Richter L, Flodman P, von-Bischhoffshausen FB, Burch D, Brown S, Nguyen L, Turner J, Spence MA, Bateman JB (2008) Clinical variability of autosomal dominant cataract, microcornea and corneal opacity and novel mutation in the αA-crystallin gene (CRYAA). Am J Med Genet 146:833–842 [DOI] [PubMed]
- 15.Su D, Guo Y, Li Q, Guan L, Zhu S, Ma X. A novel mutation in CRYAA is associated with autosomal dominant suture cataracts in a Chinese family. Mol Vis. 2012;18:3057–3063. [PMC free article] [PubMed] [Google Scholar]
- 16.Pras E, Frydman M, Levy-Nissenbaum E, Bakhan T, Raz J, Assia EI, Goldman B, Pras E (2000) A nonsense mutation (W9X) in CRYAA causes autosomal recessive cataract in an inbred Persian Jewish family. Invest Ophthalmol Vis Sci 41:3511–3515 [PubMed]
- 17.Inagaki N, Hayashi T, Arimura T, Koga Y, Takahashi M, Shibata H, Teraoka K, Chikamori T, Yamashina A, Kimura A. αB-Crystallin mutation in dilated cardiomyopathy. Biochem Biophys Res Commun. 2006;342:379–386. doi: 10.1016/j.bbrc.2006.01.154. [DOI] [PubMed] [Google Scholar]
- 18.Pilotto A, Marziliano N, Pasotti M, Grasso M, Costante AM, Arbustini E. αB-Crystallin mutation in dilated cardiomyopathies: low prevalence in a consecutive series of 200 unrelated probands. Biochem Biophys Res Commun. 2006;346:1115–1117. doi: 10.1016/j.bbrc.2006.05.203. [DOI] [PubMed] [Google Scholar]
- 19.Reilich P, Schoser B, Schramm N, Krause S, Schessl J, Kress W, Muller-Hocker J, Walter MC, Lochmuller H. The p. G154S mutation of the αB-crystallin gene (CRYAB) causes late-onset distal myopathy. Neuromuscul Disord. 2010;20:255–259. doi: 10.1016/j.nmd.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Selcen D, Engel AG. Myofibrillar myopathy caused by novel dominant negative αB-crystallin mutations. Ann Neurol. 2003;54:804–810. doi: 10.1002/ana.10767. [DOI] [PubMed] [Google Scholar]
- 21.Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-soluble lens crystallins are disulphide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res. 2000;71:195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- 22.Lund AL, Smith JB, Smith DL. Modifications of the water-insoluble human lens α-crystallins. Exp Eye Res. 1996;63:661–672. doi: 10.1006/exer.1996.0160. [DOI] [PubMed] [Google Scholar]
- 23.Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL. Age-related changes in humans crystallins determined from comparative analysis of post-translational modifications in young and aged lenses: does deamidation contribute to crystallin insolubility. J Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacCoss MJ, McDonald WH, Saraf A, Sadygov R, Clark JM, Tasto JJ, Gould KL, Wolters D, Washburn M, Weiss A, Clark JL, Yates JR. Shotgun identification of protein modifications from protein complexes and lens tissue. Proc Natl Acad Sci. 2002;99:7900–7905. doi: 10.1073/pnas.122231399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamei A, Hamaguchi T, Matsuura N, Iwase H, Masuda K. Post-translational modification of αB-crystallin of normal human lenses. Biol Pharm Bull. 2000;23:226–230. doi: 10.1248/bpb.23.226. [DOI] [PubMed] [Google Scholar]
- 26.Hains PG, Truscott RJW. Age-dependent deamidation of lifelong proteins in the human lens. Invest Ophthalmol Vis Sci. 2010;51:3107–3114. doi: 10.1167/iovs.09-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Han J, David LL, Schey KL. Proteomics and phosphoproteomics analysis of human lens fibre cell membranes. Invest Ophthalmol Vis Sci. 2013;54:1135–1143. doi: 10.1167/iovs.12-11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Lyons B, Truscott RJW, Schey KL. Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging Cell. 2013;13:226–234. doi: 10.1111/acel.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C-H, Wang Y-T, Tsai C-F, Chen Y-J, Lee J-S, Chiou S-H. Phosphoproteomics characterisation of novel phosphorylated sites of lens proteins fom normal and cataractous human eye lenses. Mol Vis. 2011;17:186–198. [PMC free article] [PubMed] [Google Scholar]
- 30.Searle BC, Dasari S, Wilmarth PA, Turner M, Reddy AP, David LL, Nagalla SR. Identification of protein modifications using MS/MS de novo sequencing and the open Sea Alignment algorithm. J Proteome Res. 2005;4:546–554. doi: 10.1021/pr049781j. [DOI] [PubMed] [Google Scholar]
- 31.Miesbauer LR, Zhou X, Yang Z, Yang Z, Sun Y, Smith DL, Smith JB. Post-translational modifications of water-soluble human lens crystallins from young adults. J Biol Chem. 1994;269:12494–12502. [PubMed] [Google Scholar]
- 32.Aquilina JA, Benesch JLP, Ding LL, Yaron O, Horwitz J, Robinson CV. Phosphorylation of αB-crystallin alters chaperone function through loss of dimeric substructure. J Biol Chem. 2004;279:28675–28680. doi: 10.1074/jbc.M403348200. [DOI] [PubMed] [Google Scholar]
- 33.Ecroyd H, Meehan S, Horwitz J, Aquilina JA, Benesch JLP, Robinson CV, Macphee CE, Carver JA. Mimicking phosphorylation of αB-crystallin affects its chaperone activity. Biochem J. 2007;401:129–141. doi: 10.1042/BJ20060981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peschek J, Braun N, Rohrburg J, Back KC, Kriehuber T, Kastenmuller A, Weinkauf S, Buchner J. Regulated structural transitions unleash the chaperone activity of αB-crystallin. Proc Natl Acad Sci. 2013;110:3780–3789. doi: 10.1073/pnas.1308898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad F, Raman B, Ramakrishna T, Rao M. Effect of phosphorylation of αB-crystallin: differences in stability, subunit exchange and chaperone activity of homo and mixed oligomers of αB-crystallin and its phosphorylation-mimicking mutant. J Mol Biol. 2008;375:1040–1051. doi: 10.1016/j.jmb.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Kato K, Ito H, Kamei A, Iwamoto Y., II Innervation-dependent phosphorylation and accumulation of αB-crystallin and Hsp27 as insoluble complexes in disused muscle. FASEB J. 2002;16:1432–1434. doi: 10.1096/fj.02-0129fje. [DOI] [PubMed] [Google Scholar]
- 37.Golenhofen N, Htun P, Ness W, Koob R, Schaper W, Drenckhahn D. Binding of the stress protein αB-crystallin to cardiac myofibrils correlates with the degree of myocardial damage during ischemia/reperfusion in vivo . J Mol Cell Cardiol. 1999;31:569–580. doi: 10.1006/jmcc.1998.0892. [DOI] [PubMed] [Google Scholar]
- 38.Launay N, Tarze A, Vicart P, Lilienbaum A. Serine 59 phosphorylation of αB-crystallin down-regulates its anti-apoptotic function by binding and sequestering Bcl-2 in breast cancer cells. J Biol Chem. 2010;285:37324–37332. doi: 10.1074/jbc.M110.124388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maddala R, Rao V. α-Crystallin localises to the leading edges of migrating lens epithelial cells. Exp Eye Res. 2005;306:203–215. doi: 10.1016/j.yexcr.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 40.Wang K, Spector A. α-Crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur J Biochem. 1996;242:56–66. doi: 10.1111/j.1432-1033.1996.0056r.x. [DOI] [PubMed] [Google Scholar]
- 41.Ito H, Kamei A, Iwamoto I, Inaguma Y, Garcia-Mata R, Sztul E, Kato K. Inhibition of proteasomes induces accumulation, phosphorylation, and recruitment of Hsp27and αB-crystallin to aggresomes. J Biochem (Tokyo) 2002;131:593–603. doi: 10.1093/oxfordjournals.jbchem.a003139. [DOI] [PubMed] [Google Scholar]
- 42.Hoover HE, Thuerauf DJ, Martindale JJ, Glembotski CC. αB-Crystallin gene induction and phosphorylation by MKK6-activated p38. J Biol Chem. 2000;275:23825–23833. doi: 10.1074/jbc.M003864200. [DOI] [PubMed] [Google Scholar]
- 43.Golenhofen N, Ness W, Koob R, Htun P, Schaper W, Drenckhahn D. Ischemia-induced phosphorylation and translocation of stress protein αB-crystallin to Z lines of myocardium. Am J Physiol. 1998;274:1457–1464. doi: 10.1152/ajpheart.1998.274.5.H1457. [DOI] [PubMed] [Google Scholar]
- 44.Eaton P, Fuller W, Bell JR, Shattock MJ. αB-crystallin translocation and phosphorylation: signal transduction pathways and preconditioning in the isolated rat heart. J Mol Cell Cardiol. 2001;33:1659–1671. doi: 10.1006/jmcc.2001.1418. [DOI] [PubMed] [Google Scholar]
- 45.Adhikari AS, Singh BN, Rao KS, Rao M. αB-Crystallin, a small heat shock protein, modulates NF-κB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-α induced toxicity. Biochim Biophys Acta. 2011;1813:1532–1542. doi: 10.1016/j.bbamcr.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Morrison LE, Hoover HE, Thuerauf DJ, Glembotski CC. Mimicking phosphorylation of αB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ Res. 2003;92:203–211. doi: 10.1161/01.RES.0000052989.83995.A5. [DOI] [PubMed] [Google Scholar]
- 47.den Engelsman J, Gerrits D, de Jong WW, Robbins J, Kato K, Boelens WC. Nuclear import of αB-crystallin is phosphorylation-dependent and hampered by hyperphosphorylation of the myopathy-related mutant R120G. J Biol Chem. 2005;280:37139–37148. doi: 10.1074/jbc.M504106200. [DOI] [PubMed] [Google Scholar]
- 48.den Engelsman J, van de Schootbrugge C, Yong J, Pruijn GJM, Boelens WC. Pseudophosphorylated αB-crystallin is a nuclear chaperone imported into the nucleus with help of the SMN complex. PLoS One. 2013;8:73489–73498. doi: 10.1371/journal.pone.0073489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li R, Reiser G. Phosphorylation of Ser45 and Ser59 of αB-crystallin and p38/extracellular regulated kinase activity determine αB-crystallin-mediated protection of rat brain astrocytes from C2-ceramide- and staurosporine-induced cell death. J Neurochem. 2011;118:354–364. doi: 10.1111/j.1471-4159.2011.07317.x. [DOI] [PubMed] [Google Scholar]
- 50.Kamradt MC, Chen F, Sam S, Cryns VL. The small heat shock protein αB-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277:38731–38736. doi: 10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- 51.Sun TX, Das BK, Liang JJN. Conformational and functional differences between recombinant human lens αA- and αB-crystallin. J Biol Chem. 1997;272:6220–6225. doi: 10.1074/jbc.272.10.6220. [DOI] [PubMed] [Google Scholar]
- 52.Peschek J, Braun N, Franzmann TM, Georgalis Y, Halsbeck M, Weinkaut S, Buchner J. The eye lens chaperone α-crystallin forms defined globular bodies. Proc Natl Acad Sci. 2009;106:13272–13277. doi: 10.1073/pnas.0902651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grey AC, Schey KL. Age-related changes in the spatial distribution of human lens α-crystallin products by MALDI imaging mass spectrometry. Invest Ophthalmol Vis Sci. 2009;50:4319–4329. doi: 10.1167/iovs.09-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bova MP, Mchaourab HS, Han Y, Fung BKK. Subunit exchange of small heat shock proteins: analysis of oligomer formation of αA-crystallin and Hsp27 by fluorescence resonance energy transfer and site-directed truncations. J Biol Chem. 2000;275:1035–1042. doi: 10.1074/jbc.275.2.1035. [DOI] [PubMed] [Google Scholar]
- 55.Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian α-crystallin. Proc Natl Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klemenz R, Frohli E, Steiger R, H., Schafer R, Aoyama A (1991b) αB-crystallin is a small heat shock protein. Proceedings of the National Academy of Science USA 88:3652-3656 [DOI] [PMC free article] [PubMed]
- 57.Horwitz J. α-Crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laganowsky A, Benesch JLP, Landau M, Ding L, Sawaya MR, Cascio D, Huang Q, Robinson CV, Horwitz J, Eisenberg D. Crystal structures of truncated αA- and αB-crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 2010;19:1031–1043. doi: 10.1002/pro.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Jong WW, Caspers G-J, Leunissen JAM. Genealogy of the α-crystallin–small heat-shock protein superfamily. Int JBiol Macromol. 1998;22:151–162. doi: 10.1016/S0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 60.Jehle S, Vollmar BS, Bardiaux B, Dove KK, Rajagopal P, Gonen T, Oschkinat H, Klevit RE. N-terminal domain of αB-crystallin provides a conformational switchfor multimerization and structural heterogeneity. Proc Natl Acad Sci. 2011;108:6409–6414. doi: 10.1073/pnas.1014656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smulders RHPH, Van Boekel MAM, De Jong WW. Mutations and modifications support a ‘pitted-flexiball’ model for α-crystallin. Int JBiol Macromol. 1998;22:187–196. doi: 10.1016/S0141-8130(98)00016-6. [DOI] [PubMed] [Google Scholar]
- 62.Jehle S, Rajagopal P, Bardiaux B, Markovic S, Kuhne R, Stout JR, Higman VA, Klevit RE, van Rossum B-J, Oschkinat H. Solid-state NMR and SAXS studies provide a structural basis for the activation of αB-crystallin oligomers. Nature. 2010;17:1037–1043. doi: 10.1038/nsmb.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clark AR, Naylor CE, Bagneris C, Keep NH, Slingsby C. Crystal structure of R120G disease mutant of human αB-crystallin domain dimer shows closure of a groove. J Mol Biol. 2011;408:118–134. doi: 10.1016/j.jmb.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braun N, Zacharias M, Peschek J, Kastenmuller A, Zou J, Hanzlik M, Haslbek M, Rappsilber J, Buchner J, Weinkauf S. Multiple molecular architectures of the eye lens chaperone αB-crystallin elucidated by a triple hybrid approach. Proc Natl Acad Sci. 2011;108:20491–20496. doi: 10.1073/pnas.1111014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bagneris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, Slingsby C. Crystal structures of α-crystallin domain dimers of αB-crystallin and Hsp20. J Mol Biol. 2009;392:1242–1252. doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 66.Baldwin AJ, Lioe H, Hilton GR, Baker LA, Rubinstein JL, Kay LE, Benesch JLP. The polydispersity of αB-crystallin is rationalised by an interconverting polyhedral architecture. Structure. 2011;19:1855–1863. doi: 10.1016/j.str.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 67.Haley DA, Horwitz J, Stewart PL. The small heat-shock protein, αB-crystallin, has a variable quaternary structure. J Mol Biol. 1998;277:27–35. doi: 10.1006/jmbi.1997.1611. [DOI] [PubMed] [Google Scholar]
- 68.Benesch JLP, Ayoub M, Robinson CV, Aquilina JA. Small heat shock protein activity is regulated by variable oligomeric substructure. J Biol Chem. 2008;283:28513–28517. doi: 10.1074/jbc.M804729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delbecq SP, Klevit RE. One size does not fit all: the oligomeric states of αB-crystallin. FEBS Lett. 2013;587:1073–1080. doi: 10.1016/j.febslet.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valasco PT, Lukas TJ, Murthy SNP, Duglas-Tabor Y, Garland DL, Lorand L. Hierarchy of lens proteins requiring protection against heat induced precipitation by the α-crystallin chaperone. Exp Eye Res. 1997;65:497–505. doi: 10.1006/exer.1997.0358. [DOI] [PubMed] [Google Scholar]
- 71.Quinlan R. Cytoskeletal competence requires protein chaperones. In: Arrigo AP, Muller WEG, editors. Small stress proteins. Berlin: Springer; 2002. pp. 219–228. [DOI] [PubMed] [Google Scholar]
- 72.Horwitz J. α-Crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/S0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 73.Cobb BA, Petrash JM. α-crystallin chaperone-like activity and membrane binding in age-related cataracts. Biochemistry (Mosc) 2002;41:483–490. doi: 10.1021/bi0112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farnsworth PN, Frauwith H, Groth-Vasselli B, Singh K. Refinement of 3D structure of bovine lens αA-crystallin. Int JBiol Macromol. 1998;22:175–185. doi: 10.1016/S0141-8130(98)00015-4. [DOI] [PubMed] [Google Scholar]
- 75.Bindels JG, Misdom LW, Hoenders HJ. The reaction of citraconic anhydride with bovine α-crystallin lysine residues: surface probing and dissociation-reassociation studies. Biochim Biophys Acta. 1985;828:255–260. doi: 10.1016/0167-4838(85)90305-X. [DOI] [PubMed] [Google Scholar]
- 76.Cherian M, Abraham EC. Decreased molecular chaperone property of α-crystallins due to posttranslational modifications. Biochem Biophys Res Commun. 1995;208:675–679. doi: 10.1006/bbrc.1995.1391. [DOI] [PubMed] [Google Scholar]
- 77.Derham BK, Harding JJ. Effect of aging on the chaperone-like function of human α-crystallin assessed by three methods. Biochem J. 1997;328:763–768. doi: 10.1042/bj3280763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soti C, Csermely P. Molecular chaperones and the aging process. Biogerontology. 2000;1:225–233. doi: 10.1023/A:1010082129022. [DOI] [PubMed] [Google Scholar]
- 79.Kantorow M, Piatigorsky J. Phosphorylations of αA- and αB-crystallin. Int JBiol Macromol. 1998;22:307–314. doi: 10.1016/S0141-8130(98)00028-2. [DOI] [PubMed] [Google Scholar]
- 80.Deng M, Chen P-C, Xie S, Zhao J, Gong L, Liu J, Zhang L, Sun S, Liu J, Ma H, Batra SK, Li D. The small heat shock protein αA-crystallin is expressed in pancreas and acts as a negative regulator of carcinogenesis. Biochim Biophys Acta. 2010;1802:621–631. doi: 10.1016/j.bbadis.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Whiston EA, Sugi N, Kamradt MC, Krevosky M, Sack C, Heimer SR, Engelbert M, Wawrousek EF, Gilmore MS, Ksander BR, Gregory MS. αB-Crystallin protects retinal tissue during Staphylococcus aureus-induced endophthalmitis. Infect Immun. 2008;76:1781–1790. doi: 10.1128/IAI.01285-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klemenz R, Frohli E, Aoyama A, Hoffmann S, Simpson RJ, Moritz RL, Schafer R. αB-accumulation is a specific response to Ha-ras and v-mos oncogene expression in mouse NIH 3T3 fibroblasts. Mol Cell Biol. 1991;11:803–812. doi: 10.1128/MCB.11.2.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dasgupta S, Hohman TC, Carper D. Hypertonic stress induces αB-crystallin expression. Exp Eye Res. 1992;54:461–470. doi: 10.1016/0014-4835(92)90058-Z. [DOI] [PubMed] [Google Scholar]
- 84.Klemenz R, Andres AC, Frohli E, Schafer R, Aoyama A. Expression of the murine small heat shock proteins Hsp25 and αB-crystallin in the absence of stress. J Cell Biol. 1993;120:639–645. doi: 10.1083/jcb.120.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atomi Y, Yamada S, Hong YM. Dynamic expression of αB-crystallin in skeletal muscle. Proc Jpn Acad Ser B. 1990;66:203–208. doi: 10.2183/pjab.66.203. [DOI] [Google Scholar]
- 86.Carver JA, Lindner RA. NMR spectroscopy of α-crystallin: insights into the structure, interactions and chaperone action of sHsps. Int JBiol Macromol. 1998;22:197–209. doi: 10.1016/S0141-8130(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 87.Shinohara H, Inaguma Y, Goto S, Inagaki T, Kato K. αB-Crystallin and Hsp28 are enhance in the cerebral cortex of patients with Alzheimer’s disease. J Neurol Sci. 1993;119:203–208. doi: 10.1016/0022-510X(93)90135-L. [DOI] [PubMed] [Google Scholar]
- 88.Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of αB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci. 2011;108:13287–13292. doi: 10.1073/pnas.1107368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mehlen P, Kretz-Remy C, Preville X, Arrigo A-P. Human hsp27, Drosophila hsp27 and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFα-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 90.Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for αB-crystallin in autoimmune demyelination. Nature. 2007;448:474–481. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 91.Head M, Corbin E, Goldman J. Overexpression and abnormal modification of the stress proteins αB-crystallin and Hsp27 in Alexanders disease. Am J Pathol. 1993;143:1743–1753. [PMC free article] [PubMed] [Google Scholar]
- 92.Iwaki T, Kume-Iwaki A, Liem RKH, Goldman JE. αB-Crystallin is expressed in non-lenticular tissues and accumulates in Alexander’s disease brain. Cell. 1989;57:71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- 93.Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, Diaz LK, Turbin D, Karaca G, Wiley E, Nielsen TO, Perou CM, Cryns VL. αB-Crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chin D, Boyle DM, Williams RM, Ferguson K, Pandeya N, Pedley J, Campbell CM, Theile DR, Parsons PG, Coman WB. αB-Crystallin, a new independent marker for poor prognosis in head and neck cancer. Laryngoscope. 2005;115:1239–1242. doi: 10.1097/01.MLG.0000164715.86240.55. [DOI] [PubMed] [Google Scholar]
- 95.Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A, Oshita S, Wilkinson JC, Yu C, Oliver PG, Duckett CS, Buchsbaum DJ, LoBuglio AF, Jordan VC, Cryns VL. The small heat shock protein αB-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase 3. J Biol Chem. 2005;280:11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- 96.Phelps Brown N, Bron AJ, editors. Lens disorders: a clinical manual of cataract diagnosis. Oxford: Butterworth-Heinemann; 1996. [Google Scholar]
- 97.Bloemendal H, de Jong WW, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 98.Srivastava OP, Kirk MC, Srivastava K. Characterization of covalent multimers of crystallins in aging human lenses. J Biol Chem. 2004;279:10901–10909. doi: 10.1074/jbc.M308884200. [DOI] [PubMed] [Google Scholar]
- 99.Asomugha CO, Gupta R, Srivastava OP. Identification of crystallin modifications in the human lens cortex and nucleus using laser capture microdissection and CyDye labelling. Mol Vis. 2010;16:476–494. [PMC free article] [PubMed] [Google Scholar]
- 100.Lin PP, Barry RC, Smith DL, Smith JB. In vivo acetylation identified at lysine 70 of human lens αA-crystallin. Protein Sci. 1998;7:1451–1457. doi: 10.1002/pro.5560070622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park Z-Y, Sadygov R, Clark JM, Clark JI, Yates JR. Assigning in vivo carbamylation and acetylation in human lens proteins using tandem mass spectrometry and database searching. Int J Mass Spectrom. 2007;259:161–173. doi: 10.1016/j.ijms.2006.08.013. [DOI] [Google Scholar]
- 102.Lyons B, Kwan AH, Truscott RJW. Spontaneous cyclization of polypeptides with a penultimate Asp, Asn or isoAsp at the N-terminus and implications for cleavage by aminopeptidase. FEBS J. 2014;281:2945–2955. doi: 10.1111/febs.12833. [DOI] [PubMed] [Google Scholar]
- 103.Colvis CM, Duglas-Tabor Y, Werth KB, Vieira NE, Kowalak JA, Janjani A, Yergey AL, Garland DL. Tracking pathology and proteomics: identification of in vivo degradation products of αB-crystallin. Electrophoresis. 2000;21:2219–2227. doi: 10.1002/1522-2683(20000601)21:11<2219::AID-ELPS2219>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 104.Srivastava OP, Srivastava K. Existence of deamidated αB-crystallin fragments in normal and cataractous human lenses. Mol Vis. 2003;9:110–118. [PubMed] [Google Scholar]
- 105.Jimenez-Asensio J, Colvis CM, Kowalak JA, Duglas-Tabor Y, Datiles MB, Moroni M, Rao M, Balasubramanian D, Janjani A, Garland D. An atypical form of αB-crystallin is present in high concentration in some human cataractous lenses. J Biol Chem. 1999;274:32287–32294. doi: 10.1074/jbc.274.45.32287. [DOI] [PubMed] [Google Scholar]
- 106.Lapko VN, Smith DL, Smith JB. In vivo carbamylation and acetylation of water-soluble human lens αB-crystallin lysine 92. Protein Sci. 2001;10:1130–1136. doi: 10.1110/ps.40901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaves JM, Srivastava K, Gupta R, Srivastava OP. Structural and functional roles of deamidation and/or truncation of N- or C-termini in human αA-crystallin. Biochemistry (Mosc) 2008;47:10069–10083. doi: 10.1021/bi8001902. [DOI] [PubMed] [Google Scholar]
- 108.Asomugha CO, Gupta R, Srivastava OP. Structural and functional roles of deamidation of N146 and/or truncation of NH2- or COOH-termini in human αB-crystallin. Mol Vis. 2011;17:2407–2420. [PMC free article] [PubMed] [Google Scholar]
- 109.Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. Phosphorylation of αB-crystallin in response to various types of stress. J Biol Chem. 1997;272:29934–29941. doi: 10.1074/jbc.272.47.29934. [DOI] [PubMed] [Google Scholar]
- 110.Wang K, Spector A, Ma W. Phosphorylation of α-crystallin in rat lenses is stimulated by H2O2 but phosphorylation has no effect on chaperone activity. Exp Eye Res. 1995;61:115–124. doi: 10.1016/S0014-4835(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 111.Takemoto L, Boyle D. The possible role of α-crystallins in human senile cataractogenesis. Int JBiol Macromol. 1998;22:331–337. doi: 10.1016/S0141-8130(98)00031-2. [DOI] [PubMed] [Google Scholar]
- 112.Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S. Phosphorylation of αB-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem. 1998;273:28346–28354. doi: 10.1074/jbc.273.43.28346. [DOI] [PubMed] [Google Scholar]
- 113.Schieven G, Martin GS. Nonenzymatic phosphorylation of tyrosine and serine by ATP is catalyzed by manganese but not magnesium. J Biol Chem. 1988;263:15590–15593. [PubMed] [Google Scholar]
- 114.Kantorow M, Piatigorsky J. α-Crystallin/small heat shock protein has autokinase activity. Biochemistry (Mosc) 1994;91:3112–3116. doi: 10.1073/pnas.91.8.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kantorow M, Horwitz J, Van Boekel MAM, De Jong WW, Piatigorsky J. Conversion from oligomers to tetramers enhances autophosphorylation by lens αA-crystallin. J Biol Chem. 1995;270:17215–17220. doi: 10.1074/jbc.270.29.17215. [DOI] [PubMed] [Google Scholar]
- 116.White JG, Amos WB, Fordham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol. 1987;105:41–48. doi: 10.1083/jcb.105.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kamei A, Hamaguchi T, Matsuura N, Masuda K. Does post-translational modification influence chaperone-like activity of α-crystallin? 1. study on phosphorylation. Biol Pharm Bull. 2001;24:96–99. doi: 10.1248/bpb.24.96. [DOI] [PubMed] [Google Scholar]
- 118.Augusteyn RC, Murnane L, Nicola A, Stevens A. Chaperone activity in the lens. Clin Exp Optom. 2002;85:83–90. doi: 10.1111/j.1444-0938.2002.tb03013.x. [DOI] [PubMed] [Google Scholar]
- 119.Koteiche HA, Mchaourab HS. Mechanism of chaperone function in small heat-shock proteins: phosphorylation-induced activation of two-mode binding in αB-crystallin. J Biol Chem. 2003;278:10361–10367. doi: 10.1074/jbc.M211851200. [DOI] [PubMed] [Google Scholar]
- 120.Ito H, Kamei K, Iwamoto I, Inaguma Y, Nohara D, Kato K. Phosphorylation-induced change of the oligomerization state of αB-crystallin. J Biol Chem. 2001;276:5346–5352. doi: 10.1074/jbc.M009004200. [DOI] [PubMed] [Google Scholar]
- 121.Kato K, Inaguma Y, Ito H, Iida K, Iwamoto I, Kamei A, Ochi N, Ohta H, Kishikawa M. Ser-59 is the major phosphorylation site of αB-crystallin accumulated in the brains of patients with Alexander’s disease. J Neurochem. 2001;76:730–736. doi: 10.1046/j.1471-4159.2001.00038.x. [DOI] [PubMed] [Google Scholar]
- 122.Mann E, McDermott MJ, Goldman J, Chiesa R, Spector A. Phosphorylation of α-crystallin B in Alexander’s disease brain. FEBS Lett. 1991;294:133–136. doi: 10.1016/0014-5793(91)81359-G. [DOI] [PubMed] [Google Scholar]