Abstract
Chicken ovalbumin upstream promoter transcription factors (COUP-TFs) are nuclear receptors belonging to the superfamily of the steroid/thyroid hormone receptors. Members of this family are internalized to the nucleus both in a ligand-dependent or -independent manner and act as strong transcriptional regulators by binding to the DNA of their target genes. COUP-TFs are defined as orphan receptors, since ligands regulating their activity have not so far been identified. From the very beginning of metazoan evolution, these molecules have been involved in various key events during embryonic development and organogenesis. In this review, we will mainly focus on their function during development and maturation of the central nervous system, which has been well characterized in various animal classes ranging from ctenophores to mammals. We will start by introducing the current knowledge on COUP-TF mechanisms of action and then focus our discussion on the crucial processes underlying forebrain ontogenesis, with special emphasis on mammalian development. Finally, the conserved roles of COUP-TFs along phylogenesis will be highlighted, and some hypotheses, worth exploring in future years to gain more insight into the mechanisms controlled by these factors, will be proposed.
Keywords: COUP-TF, Orphan nuclear receptors, Forebrain, Cerebral cortex, Neurogenesis, Differentiation, Neuron migration
Introduction
In vertebrates, the forebrain, the anterior-most portion of the central nervous system, encompasses the telencephalon, constituted dorsally by the pallium (allo- and isocortex), and the pallial nuclei (calustro–amygdaloid complex), and ventrally by the subpallium (striatal, pallidal, diagonal-innominate and preoptic nuclei)and the diencephalon (prethalamus, thalamus, hypothalamus, subthalamus, epithalamus, and pretectum) (Fig. 1). The cerebral cortex in mammals is the structure which has undergone the most dramatic changes during vertebrate evolution [1–3], and it is subdivided into the archicortex (hippocampus), neocortex, and paleocortex (piriform or olfactory cortex). In mammals, the pallial nuclei have common origins with the dorsal ventricular ridge (DVR), a periventricular structure of sauropsid telencephalon responsible for the elaboration of auditory and tecto-visual inputs. In reptiles and birds (sauropsids), the cortex is subdivided into medial, dorsal, and lateral cortex, whereas in amphibians and fishes, these subdivisions are more blurred, since, for example, amphibian cortices can be subdivided into just a medial pallium (hodologically corresponding to the medial and dorsal cortex of sauropsids) and a dorsal domain which receives olfactory afferences [2].
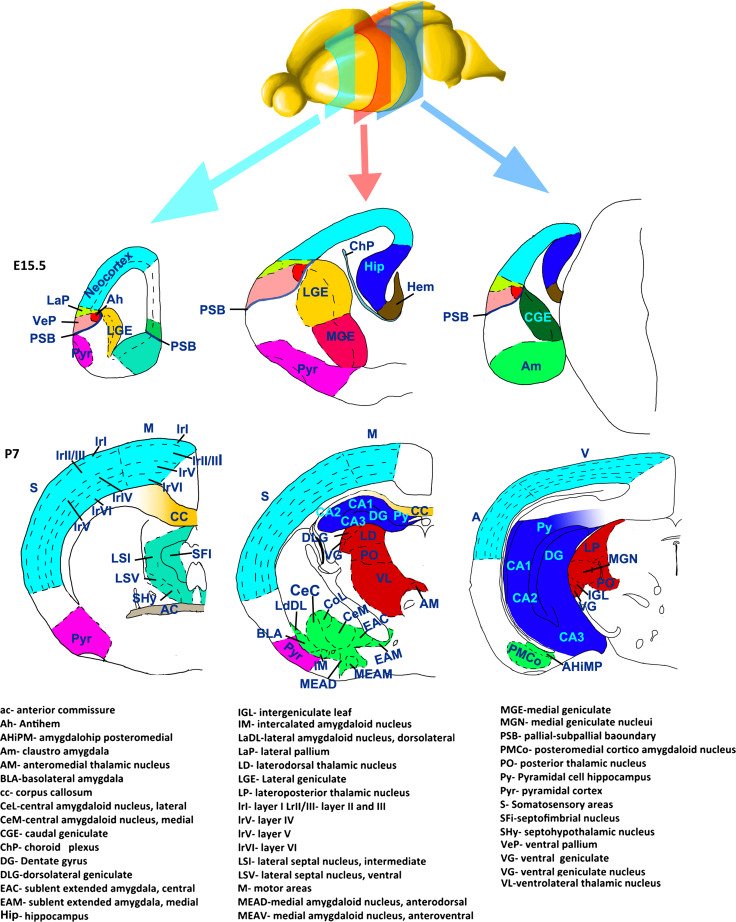
Fig. 1.
Schematic representation of the main subdivisions of the forebrain at E15 and P7. Imaginary coronal sections of rostral (green square), medial (red square), and caudal (light blue square) regions of the embryonic and postnatal forebrain. Different colours correspond to different structures constituting the forebrain: neocortex (light blue), hippocampal complex (blue), lateral (light green) and ventral (rose) pallium, hem (brown), antihem (orange), dorsal thalamic nuclei (red), septal nuclei (marine blue), pyriform cortex (violet), amygdalar complex (green), lateral (yellow), medial (fuchsia) and caudal (dark green) ganglionic eminences
All these structures have different and crucial functions in the vertebrate central nervous system (CNS). One of the main functions of the thalamus, for example, which is a major component of the diencephalon, is to receive sensory and motor afferences from peripheral systems and to relay them to the main elaboration centers, i.e. to the cerebral cortex in mammals, the dorsal cortex and the DVR in sauropsids, or the medial cortex and the striatum in amphibians [2, 4, 5]. The striatum has important roles in the elaboration and implementation of movements, especially in reptiles and birds, whereas in mammals it contributes to the elaboration of voluntary movements and modulates their implementation by sending motor inputs (via the ventrolateral thalamic nuclei) to the frontal/motor regions of the cortex [6]. The cerebral cortex has gained an increasing relevance during vertebrate evolution, particularly in the elaboration of sensory and motor inputs. In the last branched class of vertebrates, the mammals, this structure represents the prevalent center for the elaboration of peripheral inputs, the seat of higher functions, like memory, language, and voluntary movements, and, in higher primates, thoughts and consciousness [1–3, 7].
The main constituents of the cerebral cortex are excitatory and inhibitory neurons and glia cells. The first two categories of cells convey and modulate motor and sensory inputs, favoring their integration and elaboration and, ultimately, the implementation of motor plans. In contrast, the glia cellsare mainly involved in a variety of functions such as cell feeding, cell homeostasis, axon myelination and structural support, even if a growing body of evidence also demonstrates the involvement of astrocytes in the modulation of neuronal activity [8, 9]. Generally, neurons and glial cells are born from common precursors (through the neurogenic and gliogenic processes), acquire their identity, and either migrate to different, sometimes remarkably far regions of the forebrain, or elongate their axons to convey information to a given target region.
The chicken ovalbumin upstream transcription factors (COUP-TFs) are orphan nuclear receptors belonging to the superfamily of steroid/thyroid hormone receptors [10], and are involved, from the earliest branched phyla of metazoan evolution (ctenophores), in many, if not all, the above-mentioned processes during nervous system development [11–27]. Most of the data that we will present in this review on COUP-TFs function in neurogenesis, neuronal cell specification, migration, and axonal pathfinding were mainly obtained by a plethora of studies on mouse brains. However, several studies have also been conducted on other model systems, such as Drosophila melanogaster [14, 18, 21] and Hydra [16, 17], revealing an impressive degree of conservation of COUP-TFs functions and mechanisms of action during phylogenesis. This is confirmed by the high sequence homology of these nuclear receptors in vertebrate and non-vertebrate subphyla [28]. Moreover, two mammalian homologues of COUP-TFs (namely COUP-TFI and II) are expressed in almost all of the mentioned structures of the forebrain at prenatal stages, suggesting a strong involvement of these transcriptional regulators during forebrain development [26, 29].
In this review, we will briefly introduce the genetic mechanisms regulated by COUP-TFs and those modulating their action, then we will analyze their specific functions in neurogenesis, gliogenesis, neuronal differentiation, axonal pathfinding and neuronal migration. Where possible, we will compare the data obtained by different model systems and highlight the unsolved questions on COUP-TFs’ role in the different events underlying forebrain formation.
COUP-TFs: function and modulation in vertebrates
Sequence conservation among vertebrates and pattern of expression in mammalian forebrain
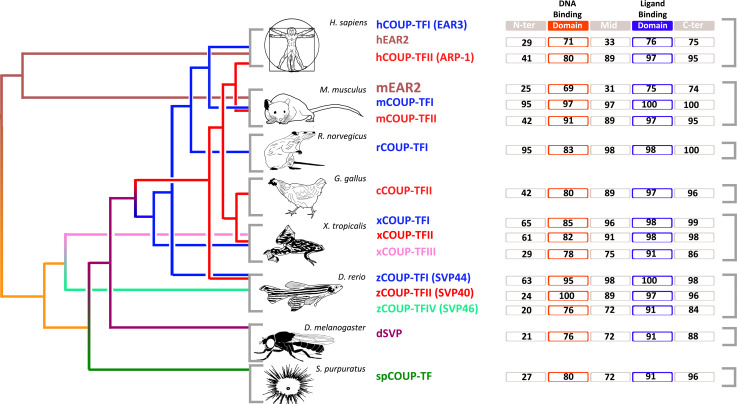
Two major homologues of COUP-TFs have been described in vertebrates, named COUP-TFI and COUP-TFII (Fig. 2), also known as nuclear receptor 2 family 1 and 2 (NR2F1 and 2) [28]. Little is known about EAR2, the other COUP-TF member in vertebrates(Fig. 2) [30], which is expressed in the brain and can form heterodimers with COUP-TFII [31]. The family of COUP-TFs has a highly conserved modular structure, comprising a central DNA binding domain (DBD), a putative C terminal ligand binding domain (LBD), and two activation function domains, AF-1 and AF-2, necessary for co-factor recruitment [28]. The homology between COUP-TFI and COUP-TFII in humans is very high in the DBD (80 %), and even more in the LBD (97 %), which comprises the activation function-2 (AF-2) [32]. Interestingly, crystallographic analyses show that the AF-2 domain is involved in the regulation of COUP-TFII function [33]. However, the N-terminal domain, comprising the AF-1, has only 45 % homology between COUP-TFI and II [34, 35], suggesting that, while these two factors share similar activation mechanisms, they might differ remarkably in their molecular interactions. These findings are further supported by their overlapping but distinct expression patterns in all three germ layers during mouse development. Indeed, while COUP-TFI has its highest expression in the nervous system, COUP-TFII is predominantly expressed in the mesenchyme of internal organs [29, 36]. Finally, COUP-TFs sequences are remarkably conserved through many distant vertebrate species like human, mouse, rat, hamster, chicken, Xenopus, and zebrafish, and to invertebrate species such as the sea urchin [28]. Using the improved quality of COUP-TF sequences from different species available online, we have generated an updated alignment of their sequences (Fig. 2). With the exception of the N-terminal domain, COUP-TF homologues share a high degree of sequence conservation, strongly suggesting similar functions in different vertebrate species, and probably between vertebrates and invertebrates.
Fig. 2.
Schematic representation of the homologies of COUP-TF genes in different classes of animals. Distinct colours in the phylogenetic tree indicate divergence in different types of proteins within the COUP-TF family. Numbers in the protein domains indicate score of sequence homology with respect to the corresponding human COUP-TFI (EAR3) domains. The comparison was performed using Clustalw alignment tool. The N-terminal, DBD (DNA binding domain), middle, LBD (ligand binding domain) and C-Terminal domains of each COUP-TF homologous sequence were compared to the human COUP-TFI homologue. Homology score percentages are reported for every domain of the proteins in the respective rectangle. The references of the sequences used in this analysis are the following: hCOUP-TFI (EAR3) = NP_005645.1; hEAR2 = CAA54097.1; hCOUP-TFII (ARP-1) = AAH42897.1; mEAR2 = CAA54097.1; mCOUP-TFI = AAA19853.1; mCOUP-TFII = AAA19854.1; rCOUP-TFI = NP_112392.1; cCOUP-TFII = NP_989752.1; xCOUP-TFI = NP_001093677.1; xCOUP-TFII = NP_001087950.1; xCOUP-TFIII = CAA44806.1; zCOUP-TFI = CAA49780.1; zCOUP-TFII = NP_571258.1; zCOUP-TFIV = Q06726.1; dSVP = AAA62770.1; spCOUP-TF = AAA30041.1. The subdivision of the proteins in domains was obtained by the means of “Conserved Domains Search Tool” available on NCBI web site (http://www.ncbi.nlm.nih.gov/Structure/cdd/docs/cdd_search.html)
During development COUP-TFI and II are first detected at E8.5 in the mouse head mesenchyme. Up to E10.5, they share common expression patterns in the optic stalk, in the dorsocaudal region of the telencephalon and in the diencephalon [29]. However, while COUP-TFI is expressed in a rostral low to caudal high gradient in the telencephalon from E9.5 onwards [26, 29, 37], COUP-TFII expression remains limited to its caudalmost regions in both dorsal and ventral telencephalon [29]. In addition, slight differences in the pattern of COUP-TFI and II expression in the thalamus have also been described [29]. Starting from E11.5, their expression profile becomes increasingly complementary in the cerebral cortex, in the ganglionic eminences (precursors of the striatum), and in the thalamus [26, 29]. At E13.5, COUP-TFs are both expressed in the cerebral cortex (in the archicortex, neocortex, and paleocortex primordia), in the preoptic area (POA), in the lateral and caudal ganglionic eminences (LGE and CGE, respectively), in the dorsalmost region of the medial ganglionic eminence (MGE), called dMGE, and in scattered cells of the ventral telencephalon (see Fig. 1 for details on the position of these regions) [26]. However, COUP-TFII is generally expressed in more restricted regions than COUP-TFI, and, most importantly, the characteristic rostromedial-low to caudolateral-high gradient of COUP-TFI expression is only partially mirrored by COUP-TFII, which remains primordially limited to the caudalmost region of the telencephalon [26, 29].
Molecular mechanisms underlying COUP-TFs function
The DNA binding domains (DBD) of COUP-TFs contain two zinc finger domains, similarly to the DBD of other members of the steroid/thyroid hormone receptor superfamily [38]. Members of this family usually bind to DNA as dimers and recognize sequences containing two GGTCA half repeats, named DR, separated by a variable number of nucleotides (1–5), called spatial variants. COUP-TFs are able to bind to oligonucleotides containing both direct and palindromic GGTCA repeats, undergoing a remarkable structural change to adapt to different spatial variants but also showing the highest affinity for 2-bp-spaced direct repeats [38]. This remarkable structural change to adapt to different spatial variants is favored by their relatively short N-terminal domains, which may provide less steric hindrance and allow promiscuous DNA binding [38].
The steroid/thyroid hormone receptors show different mechanisms of action. They can either bind the DNA in the absence of a ligand (similarly to the thyroid hormone receptors), or be present in the nucleus free from any interactors, or alternatively be bound to heat shock proteins (HSP) in the cytoplasm (steroid hormone receptors) [39]. In this latter case, nuclear receptors are prevented from binding the DNA until they are no longer bound to their ligand, allowing them to release the HSP and translocate as homodimers to the nucleus [39]. Although COUP-TFs belong to the family of steroid/thyroid hormone receptors, their exact cellular localization and the dynamics underlying their action are still largely unknown.
Members of the COUP-TF family are prevalently transcriptional repressors [22, 40–44]. They accomplish this function by different mechanisms. COUP-TFs can either repress gene expression, outcompeting other hormone receptors, such as the thyroid hormone receptor (TR), the retinoic acid receptors (RAR), and the vitamin D receptor (VDR), for the binding of hormone response elements (HRE) [45, 46], or directly interact with them through the LBD domain [47]. COUP-TFs can also subtract common co-factors from other hormone receptors, such as the retinoid X receptor (RXR), inhibiting their heterodimerization and consequently repressing transcription of their target genes [45, 47–51]. These mechanisms normally work on hormone-induced gene transcription in a passive way; however, COUP-TFI can actively repress its target genes by interacting with co-repressors, such as the nuclear co-repressor (NCoR) and the silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) [52]. These molecules can recruit many histone deacetylases to a given promoter region and inactivate its function [53, 54]. Finally, COUP-TFs can also interact with transcription factor II B (TFIIB) [55], a component of the basal transcriptional machinery, and most likely freeze the pre-initiation complex in an inactive form, in this way inhibiting transactivator-dependent activation [47].
Although COUP-TFs are primordially considered strong transcriptional repressors, it has been described that COUP-TFs can also positively regulate a considerable number of genes [56–58]. Their transcription is either regulated by sequences containing GGTCA direct repeats with 2-bp spacers, such as the rat and human apolipoprotein CIII [34, 59, 60], the human apolipoprotein AI [34], and the mouse lactoferrin [61], or alternatively by regulatory elements containing direct and palindromic GGTCA repeats separated by a variable number of nucleotides, such as the rat insulin II promoter [62, 63], the human immunodeficiency virus type 1 long terminal repeat negative response element [64, 65], and the thyroid hormone response element (TRE) [34]. Moreover, in vitro, COUP-TFII promotes the transcription of genes under the control of the human apolipoprotein AI enhancer [66], while p62, a ligand of the tyrosine kinase signaling molecule p56lck, works as a co-adjuvant of COUP-TFII in its transactivation activity [67].
The general idea is that COUP-TFs act differently on transcriptional activity depending on the “genoarchitecture” (the structural architecture of the DNA binding site) of different genetic loci, the cell context [57, 66] and chiefly, on the presence of distinct co-activators or co-repressors.
Mechanisms regulating COUP-TFs expression and activity
Many reports have shown that COUP-TF genes are regulated by several signalling molecules, such as Sonic hedgehog (Shh) [68] and retinoids [29, 69, 70], but also by mitogen-activated protein kinase (MAPK) and protein kinase C (PKC) signalling pathways [71]. There is also some evidence that physiological levels of dopamine may initiate COUP-TF activity [72].
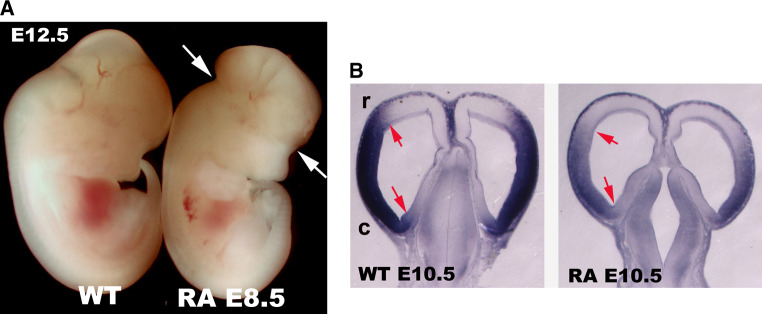
A functional cross-talk between COUP-TFs and retinoid-signaling pathways during development has been proposed; this is mainly based on the antagonistic effects of COUP-TFs on retinoid-dependent gene expression, and on the observation that COUP-TF genes are themselves targeted by retinoids [69, 73–76]. In this respect, it is not surprising that the COUP-TFI-, RARα- and RARβ-independent knockouts share common phenotypes, such as similar bone fusions [77, 78]. However, the effects of retinoids on COUP-TFs action and/or their expression pattern are still unclear. Previous reports showed that, in vitro, both COUP-TF genes are up-regulated during retinoid-induced differentiation of P19 EC cells [28, 36, 69, 73]. Moreover, overexpression of COUP-TFI in murine embryonic stem cells reduces retinoic acid growth arrest and increases gene expression in extra-embryonic tissues [70]. In vivo, retinoids seem to induce COUP-TFs expression in zebrafish and mouse hindbrains [69, 79]; however, we found that high doses of retinoic acid in E8.5 old mouse embryos lead to malformed forebrains and down-regulation of COUP-TFI in the cortical neuroepithelium (Fig. 3; unpublished data). Thus, COUP-TFs might be key regulators of the retinoid signalling pathway during embryonic development and play distinct functions in a context- and time-dependent manner.
Fig. 3.
Control of COUP-TFI expression by retinoic acid in the forebrain. a Lateral views of E12.5 mouse embryos after gavage of all-trans retinoic acid (RA) (final dose of 20 mg/kg) to pregnant females at 8.5 days post-coitum (dpc). Note that the shape of the forebrain is malformed (arrows) in RA-treated embryos, as previously reported [208]. b Horizontal forebrain sections of E10.5 control and RA-treated embryos indicate strong down-regulation of COUP-TFI in the cortical primordium and loss of clear rostral (r) and caudal (c) expression boundaries (arrows)
In addition, COUP-TFI sequence contains several putative phosphorylation sites recognized and phosphorylated in vivo by mitogen-activated protein kinase (MAPK) and protein kinase C (PKC) [71]. Interestingly, MAPK- and PKC-mediated phosphorylation modulates COUP-TFI transcriptional activity differently. While PKC-mediated phosphorylation enhances COUP-TFI affinity for DNA, MAPK-mediated phosphorylation positively regulates the transactivating function of COUP-TFI, possibly by recruiting specific co-activators [71]. Hence, a variety of extracellular stimuli transduced by MAPK and PKC signalingmight influence COUP-TFI control of different biological mechanisms [71].
Little is known about upstream regulators of COUP-TFs expression. During forebrain development, COUP-TFI and COUP-TF II expression levels are modulated by two important morphogens involved in antero-posterior and dorso-ventral patterning: Fgf8 and Shh, respectively [80, 81]. As we will see in the next sections, Fgf8 represses COUP-TFI expression in the rostral regions of the mammalian embryonic neocortex [82, 83]. This is a crucial event during the process of subdivision of the neocortex into tangential domains (areas) with particular functions [80]. Moreover, a sonic hedgehog response element (Shh-RE) was identified in the COUP-TFII promoter [68], suggesting that Shh-mediated modulation of COUP-TFII might be one of the mechanisms underlying the well-known function of Shh in subpallial patterning [81, 84, 85].
Finally, an old study based on in vitro experiments indicated a role for dopamine in the transcriptional activity of human COUP-TF [72]. The authors showed that various concentrations of dopamine and dopamine receptor agonists were sufficient to induce the expression of COUP-TF target genes, and that deletion in the COOH-terminal domain of human COUP-TF abolished this activation [72]. The hypothesis that dopamine regulates COUP-TFs during brain development is extremely interesting. Indeed, since COUP-TFI expression in the brain is maintained from early stages of embryogenesis to adulthood in several structures producing dopamine [86, 87], further studies on this topic might contribute to clarifying COUP-TF action on dopamine signaling and dopaminergic cell differentiation in brain development.
COUP-TFs in neuronal migration
Neocortical “glia-guided” radial migration
One of the most important processes in forebrain development is cell migration. Cohorts of neurons, once born, migrate to reach their final destinations, which in some cases can be considerably distant from their site of birth. In the cerebral cortex, newborn projection neurons leave the ventricular zone (VZ) and move radially to give rise to the cortical plate while starting their specification (Fig. 4). In reptiles, and during early stages of mammalian corticogenesis (E11.0–E13.5), newborn neurons leave the ventricular zone (VZ) by somal translocation. At this stage, neurons inherit from their progenitors a process in contact with the pial surface and use it as a puller, which progressively translocates the cell body towards the primordial cortical plate [88–91]. In mammals, this process of radial translocation becomes remarkably complex during late corticogenesis (from E13.5 onward) [89, 92–94]. Late-born neurons migrate along the glia scaffold generated by neuronal apical progenitors (also called radial glia cells, or RGC), which is the reason why this type of migration is called “glia-guided” [93, 94]. Once moved from the VZ to the sub-ventricular zone (SVZ), the neurons adopt a multipolar shape (MSC), elongate and retract thin neurites, detach from the glia, and continue their migration both radially and tangentially with respect to the ventricular surface (Fig. 4a). Then, once the upper limit of the intermediate zone (IZ) is reached, they re-attach to the glia scaffold, assume a bipolar shape (BSC), and enter the CP [93, 94]. Finally, late-born neurons contact the pial surface with their leading process, leave the glia scaffold, and move towards the pia by somal translocation [89]. This last step of radial migration is crucial for the inside–out order of layer formation in the neocortex, since it allows the incoming neurons to settle beyond their predecessors [89, 95]. Such a mechanism allows the subdivision of mammalian neocortex into six layers constituted by particular neuronal cell-types and connectivity [3].
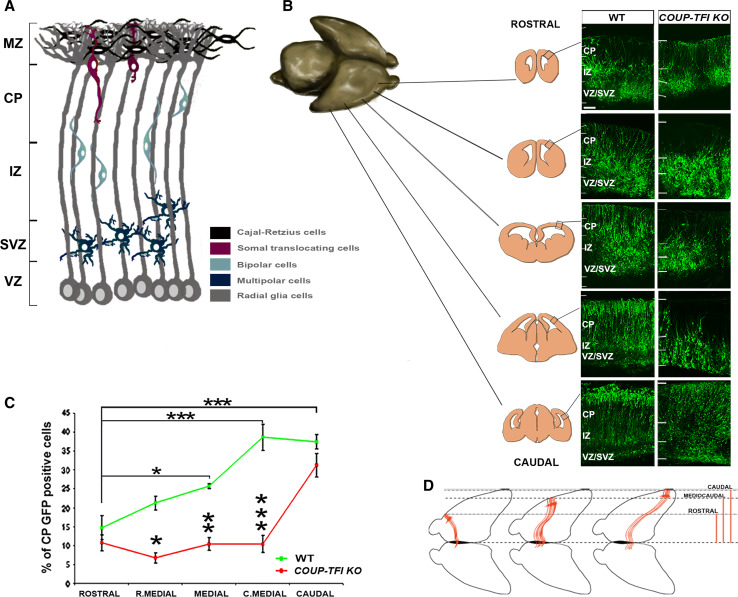
Fig. 4.
Gradient of cortical radial migration. a Schematic model of cortical radial migration representing the different phases of the “glia-guided” type of migration. Newborn neurons acquire a multipolar shape, detach from the glia scaffold and migrate both tangentially and radially. Then, they adopt a bipolar shape, attach to the glia scaffold, and radially migrate toward the cortical plate. Finally, migrating neurons contact the pial surface and detach from the radial glia through somal translocation. This final phase of “glia-guided” migration is modulated by Reelin, a glycoprotein released by Cajal-Retzius cells. VZ ventricular zone, SVZ sub-ventricular zone, IZ intermediate zone, CP cortical plate, MZ marginal zone. b Ex vivo electroporation of a GFP-expressing vector in E14.5 wt and COUP-TFI KO neocortical VZ and organotypic slices from representative regions along the rostro-caudal axis after 4 days in culture (DIV) (figure adapted from [11]). Note that the rate of radial migration along the rostro-caudal axis of the cortex follows a rostral-low to caudal-high gradient, which is strongly impaired in absence of COUP-TFI function. c Plot comparing the number of GFP-positive cells reaching the CP (from rostral to caudal cortical regions) in wt and COUP-TFI KO brains (taken from [11]). d The migratory gradient may regulate the formation of the corpus callosum. Axon elongation begins during radial migration and is modulated by the same mechanisms underlying neuron migration (see text). If the rate of migration correlates with the rate of elongation of axons, as suggested by Alfano et al. [11], an increasingly higher rate of neuronal radial migration from rostral to caudal regions of the cortex may correlate with the increasing rate of axon elongation going from rostral to caudal callosal neurons. This rostro-caudal gradient might be crucial for the correct formation of the corpus callosum. The midline is permissive to callosal axon crossing for a limited amount of time. Since caudal callosal neurons are considerably distant from this structure, they will need to elongate their axons before, or faster than the rostral callosal neurons to cross the midline during permissive stages. Accordingly, the rate of their migration to the CP seems to be faster in caudal regions than in rostral ones
A study from our group showed that COUP-TFI plays an important role in each step of glia-guided migration during cortical development, mainly through transcriptional regulation of a small Rho GTPase: Rnd2 [11]. Previous reports had shown that, in contrast to other members belonging to the Rho-GTPase family, Rnd2 activity is controlled transcriptionally and that precise Rnd2 expression levels are necessary for proper radial migration [96, 97]. Our data showed that COUP-TFI and Rnd2 have opposite expression gradients in migrating neurons and that COUP-TFI negatively regulates Rnd2 expression in migrating post-mitotic cells, thus favoring newborn neurons to reach the CP [11]. In the absence of COUP-TFI, an abnormally higher number of MSC and BSC failed to leave the IZ and were found between the upper SVZ and lower IZ (Fig. 4b). The rescue of correct levels of Rnd2 in COUP-TFI mutant brains partially recovered the balance between MSC and BSC and strongly promoted BSC migration to the CP [11].
Since BSC migration along the glia scaffold consists in the sequential elongation and retraction of their leading processes, it seems conceivable that the defective migration of COUP-TFI-deficient BSC neurons might be due to impairments in their cytoskeletal machinery. Indeed, at the end of their migration, COUP-TFI mutant neurons failed to be pulled by their leading process toward the pia, even if they were able to contact the MZ [11]. Furthermore, they remained quite far from the pial surface and showed abnormally long apical dendrites (which developed from the BSC leading process). Similarly, we showed in a previous study that a high proportion of COUP-TFI-deficient hippocampal cells had an abnormal distribution of actin- and tubulin-rich structures around their nuclei, which led to poorly formed axons and to problems in neurite elongation in vitro [13]. This was correlated with a significant decrease in MAP1B expression (an important cytoskeleton-associated protein involved in microtubule dynamics) and with an abnormal localization of Rnd2, which was ectopically up-regulated along the whole length of mutant neurons [13]. It is known that the interaction of Rnd2 with Plexin D1 delays neurite outgrowth [98], and that interaction of Rnd2 with WASP protein modulates actin dynamics, which are important for axonal branching [99]. Thus, abnormal Rnd2 levels might alter biochemical interactions amongst cytoskeletal proteins ultimately leading to the defective axonal growth and morphology observed in COUP-TFI mutant cells.
In vivo, we showed that abnormal axonal morphology due to the absence of COUP-TFI can lead to strong impairments in cortico-cortical commissures (e.g., corpus callosum, and anterior and hippocampal commissures) [13]. Similarly, acute inactivation of COUP-TFI in single newborn neurons not only impaired their migrationbut strongly delayed the formation of the corpus callosum [11], supporting the involvement of COUP-TFI in the modulation of cytoskeletal remodeling. Restoring correct levels of Rnd2 not only rescued neuronal radial migrationbut also midline crossing by callosal axons [11]. These data correlate well with previous studies indicating that commissural axon elongation occurs from the very beginning of radial neuronal migration and that these two processes might rely on similar mechanisms [100–102].
Neuronal tangential migration
Different neuronal populations reach their targets by tangential (parallel with respect to the ventricular surface) migration, travelling in some cases for very long distances (reviewed in [103–105]). Cajal-Retzius (CR) cells, for example, originate in different regions of the brain and constitute a transient population of neurons invading the cortex as early as E10.5 by tangential migration (reviewed in [7, 106–108]). These cells promote the radial alignment of the RGC, the inside–out radial migration of neocortical neurons and seem to control the position and size of cortical areas [2, 3, 7, 108, 109]. Amongst COUP-TF genes, COUP-TFII but not COUP-TFI is expressed in the caudomedial wall of telencephalic vesicles (also called hem and choroidal roof), which is a major source of CR cells in the brain, and in calretinin-positive CR cells in the MZ of the cerebral cortex [26, 110]. Thus, it would be interesting to understand whether and how COUP-TFII is involved in the specification and/or migration of CR cells invading the neocortical primordium.
In addition to CR cells, the other cell population migrating tangentially from the ganglionic eminences to different districts of the developing forebrain (especially to the neocortex) is constituted by cortical interneurons [111–113]. These GABAergic neurons create both local and long-range synaptic connections, either with projection (excitatory) neurons or with other interneurons [114], to inhibit their activity. In doing so, interneurons influence the response of excitatory neurons to incoming inputs and control neuronal activity, contributing to the assembly of local circuits, to the shaping of neocortical receptive fields, and to the synchronization of brain rhythms [115, 116]. Eventual impairment in their migration, positioning, or specification is associated with the onset of epileptic seizures [117, 118] or with other psychiatric disorders [119, 120].
The first evidence of a correlation between COUP-TFI and II expression in cortical interneuron tangential migration came from Tripodi et al. [26]. Although both genes are expressed at high levels in the CGE (in addition to the dorsal MGE for COUP-TFI and the interganglionic sulcus for COUP-TFII), they seem to be expressed in different cortical interneuron populations and to follow distinct migratory paths, dorsal and ventral for COUP-TFI [26] and caudal for COUP-TFII [121, 122]. Fate mapping and transplantation analyses demonstrated that COUP-TFII is highly expressed in the caudal migratory stream (CMS) constituted mainly by CGE-derived cells directed towards the neocortex, amygdala, and hippocampus (Fig. 5) [23, 25, 123–126]. The morphology adopted by these cells during caudal migration is similar to that of cells migrating radially to upper layers of the neocortex, hence they might exploit similar mechanisms for their locomotion (Fig. 4) [124, 126]. These neurons begin their migration as early as E13.5, and probably require external signaling, since grafting CGE cells into the MGE impairs migration [126], and ectopic expression of COUP-TFII in the MGE with subsequent grafting into the CGE allows MGE cells to migrate caudally [123]. This is also consistent with the observation that a small population of cells in the dorsal MGE expressing COUP-TFII migrate caudally in the CGE, which strongly supports the role for COUP-TFII in promoting caudal migration of GE cells in a cell-autonomous way [123].
Fig. 5.
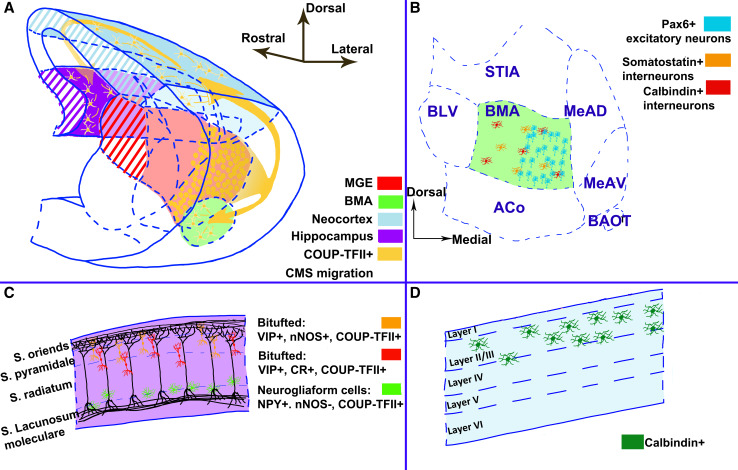
Schematic representation of the caudal migratory stream (CMS) and its derivatives in the mature brain. a 3D reconstruction of the caudal half of the mouse brain at E14.5; b–d indicate the major contributions of COUP-TFII-expressing neurons (yellow in a) in the mature target structures. b Schematics of the BMA nucleus showing the high number of Pax6-positive neurons localized in the medial domain, and the Somatostatin- and Calbindin-expressing interneurons scattered throughout the whole nucleus. c Molecular profile and localization of COUP-TFII-expressing interneurons in the hippocampus. d In the neocortex, derivatives of the CMS will express calbindin and localize primordially in upper layers. ACo anterior cortical amygdaloid nucleus, BAOT bed nucleus of the accessory olfactory tract, BLV basolateral amygdala ventral, BMA basomedial amygdala, MeAD medial amygdala nucleus anterior dorsal, MeAV medial amygdala nucleus anterior ventral, STIA bed nucleus of the stria terminalis intraamygdaloid division
Contrary to COUP-TFII, the influence of COUP-TFI in directing interneuron migration is still not clear and requires further study. In contrast, COUP-TFI plays a crucial role in the specification of distinct cortical interneuron subpopulations [20]. By abolishing COUP-TFI function in intermediate progenitors and early post-mitotic interneurons derived from the MGE and CGE, the total number of GABAergic interneurons reaching the cerebral cortex remained normal; however, the proportion of GE-derived specific interneuronal sub-types was altered. The VIP (vasoactive intestinal polipeptide)- and calretinin-expressing interneurons, originating from the CGE, were significantly decreased with a corresponding increase in PV (parvalbumin)-expressing neurons derived from the MGE [20]. This phenotype correlated with an increased proliferation of intermediate progenitor cells, indicating that COUP-TFI might control cell fate specification by modulating cell-cycle progression during interneuron specification. Strikingly, in contrast with other genetic mouse models, which normally show a higher predisposition to epileptic seizures when interneuron subpopulations are unbalanced, COUP-TFI mutants were more resistant to pharmacologically induced seizures when compared to control mice, possibly due to an overproduction of PV-expressing interneurons [20]. How this excess of PV interneurons affects the local cortical circuits and neuronal activity is still unknown and might be worth analyzing in future studies.
Whether COUP-TFII can play a role in cortical interneuron specification is, to date, still unclear. At present, COUP-TFII is mainly used as a CGE marker and it is abnormally expressed in the MGE of E10.5 Nkx2.1 mutants in accordance with an MGE to CGE fate switch [127], suggesting that COUP-TFII might play a role in early regionalization of the GE during interneuron development. Two recent studies found expression of COUP-TFII in distinct GABAergic cortical interneuron subtypes; however, their conclusions were not always univocal. On the one hand, Tang et al. [23] used the lacZ reporter gene inserted in the COUP-TFII flox allele and observed co-localization of lacZ with calretinin- and VIP-positive cells (CGE-derived), but not with somatostatin- and PV-expressing interneurons (MGE-derived cells). On the other hand, immunofluorescence with a monoclonal anti-COUP-TFII antibody labeled mainly somatostatin- and Sox6-expressing cells in layer V- and Sp8-expressing cells, originating from the dorsal LGE and CGE, in upper cortical layers. However, no calretinin-positive cells co-localized with COUP-TFII in this study [128]. Surprisingly, conditional inactivation of COUP-TFII by using a RxCre mouse line, normally active in the ventral forebrain and developing retina [129], did not alter the distribution and number of cortical interneurons [23], suggesting that either the Cre line was not appropriate for inactivating COUP-TFII in cortical interneuronsor that COUP-TFI might compensate for the absence of COUP-TFII during interneuron specification and migration. Nevertheless, various reports demonstrate an important role for COUP-TFII in amygdala development and, possibly, in the hippocampus, where it becomes a reliable marker for neurogliaform cells (see also below).
COUP-TFII and CMS cells directed to the amygdala
The amygdala is a structure located in the caudo-ventral regions of the cortex (Fig. 1) and is involved both in the processing of emotional responses and behavior, and in the regulation of vital functions, such as heart and breathing rates [130]. This structure develops with the contribution of cells derived from different sources, such as the GE, the lateral and ventral pallium and the POA (reviewed in [2, 7, 131, 132]), ultimately leading to a structure composed of different nuclei subdivided on the basis of their pallial or subpallial origins. The lateral (LA), baso-lateral (BLA) and the baso-medial amygdala (BMA) are part of the pallial amygdala while the central (CeN) and medial (MeN) nuclei are part of the subpallial amygdala, although some cells of the BMA also derive from subpallial domains [23, 133–135]. The cells that reach the amygdala from the CGE, through the CMS, are in part interneurons expressing mainly calbindin and somatostatin, and in part excitatory neurons expressing Pax6 [23, 136]. Tang et al. [23] demonstrated that inactivation of COUP-TFII in the ventral telencephalon and, particularly in the amygdala primordium altered the formation of the amygdala complex, including the LA, BLAn and BMA nuclei. Molecular analysis showed that the migration of CGE-derived excitatory Pax6-expressing neurons failed to settle into the BMA nucleus, owing to reduced expression of two semaphorin receptors, neuropilin 1 (Nrp1) and Nrp2, described to be direct targets of COUP-TFII and known to regulate neuronal cell migration and axon guidance [23]. No differences were observed in the calretinin- and VIP-expressing cortical interneuron populations in the cortex of COUP-TFII mutant animals and COUP-TFI was abnormally maintained in COUP-TFII-deficient interneurons, suggesting that COUP-TFI and COUP-TFII might compensate for each other during cortical interneuron development. Hence, the authors conclude that COUP-TFII is a crucial regulator for the amygdala morphogenesis, but not for the development of cortical interneurons [23].
COUP-TFII and CMS cells directed to the hippocampus
The hippocampus is a region of the brain located in the caudo-medial part of the cortex and is involved in the process of learning and coordinating spatial and temporal memories. The main sources of interneurons of the hippocampus are the MGE and the CGE [136–143]. Of all the different subsets of interneurons, somatostatin- and PV-expressing ones derive exclusively from the MGE, whereas calretinin- and VIP-positive interneurons derive from the CGE. Neural nitric oxide synthase is a marker that identifies a subset of interneurons that derives largely from the MGE and, only in a small part, from the CGE, and it co-localizes with neuropeptide Y (NPY)-expressing interneurons and with a subset of Ivy and neurogliaform cells [25]. COUP-TFII expression in the hippocampus is limited to interneurons originating from the CGE and reaching this region through the CMS, as assessed by the lack of expression of COUP-TFII in MGE-derived labeled cells [25]. Moreover, COUP-TFII was shown to be expressed in a very large population in the hippocampus (30–40 % of the total interneurons) constituted of Ivy, neurogliaform, and calbindin-expressing cells [123, 144]. Contrary to what was observed in the embryonic mouse cortex [26], COUP-TFII does not seem to be expressed in Cajal-Retzius in the hippocampus.
COUP-TFII and CMS cells directed to the neocortex
The neocortex is one of the main targets of CMS cells (in the mouse 75 % of neurons from the CGE migrate to layers I to III of the neocortex); their settlement in upper layers, in contrast to projection neurons born in the neocortical ventricular zone, does not seem to be correlated with their time of birth [121]. CMS neurons reaching the neocortex are mainly calbindin-expressing interneurons (61.3 %) [126]; however, to date, there is no evidence that COUP-TFII is involved in the tangential migration of this cell population directed to the cortex. There is, however, a correlation between COUP-TFII expression in the cortex and the localization of CGE-derived interneurons, as previously discussed. Moreover, a population of calretinin-positive interneurons maintaining COUP-TFII expression was identified in the visual cortex at post-natal stages [123]. Finally, COUP-TFII expression was analyzed in samples of fetal human brain, and a sparse expression of this gene was found in 9 gestational weeks (GW) human forebrains [125]. Similarly to rodents [29], COUP-TFII is expressed in the GE, and, at 15 GW, at a higher percentage in the CGE with respect to the LGE and the MGE [125]. In summary, all these data suggest, but still do not confirm, a potential role for COUP-TFII in the migration and specification of CGE-derived cortical interneurons.
Final remarks
COUP-TFs play key roles in radial and tangential cell migration, even if their importance in this process and the mechanisms underlying their function are still largely unknown. As mentioned above, COUP-TFI and II have complementary patterns of expression in the cortex, GE, POA, and other territories at early stages of development and their overexpression seems to promote different routes of migration [26]. This implies that these factors might be involved in different pathways and might contribute to corticogenesis through complementary processes. Single and double COUP-TF mutants, where both genes become inactivated in the same cortical cell types, would be extremely informative for clarifying some of the unresolved issues raised above. Moreover, the identification and characterization of novel target genes involved in their pathways (since only few downstream genes have been found and well characterized [11, 13, 23]), might also clarify how COUP-TFs regulate the different steps of neuronal migration taking place during forebrain development.
COUP-TFs in neurogenesis, gliogenesis, and neuronal fate specification
Cnidarians: the very beginning
The appearance of COUP-TFs during evolution is tightly linked to neurogenesis. The first members of this family of nuclear receptors appeared together with the first metazoans developing a primordial nervous system. Indeed, a homologue of COUP-TF is already present in the cnidarians, which were the first phylum to develop specialized neuronal cells deputed to animal defence, active movement, and feeding [16, 17]. Strikingly, COUP-TFs, together with ParaHox, Gsx, Pax, Six, and Twist-type regulators, are among the few families of neurogenic genes born at the very beginning of metazoan phylogenesis, while all other bilaterian neurogenic factors seemingly appeared as particular features of eumetazoans [16]. In the Hydra, hyCOUP-TF is expressed in the nematoblasts, which are progenitors of the cnidarian sensory mechanoreceptor cells (nematocytes), and in the precursors of other neurons constituting the CNS of these animals [17]. The expression of hyCOUP-TF is maintained in neuronal precursors during adulthood, since, in cnidarians, neurogenesis lasts throughout life, and the loss of the cell lineage expressing this factor completely ablates nerve formation in Hydra [17]. Moreover, hyCOUP-TF binds the evolutionary conserved DR1 and DR5 response elements and, if overexpressed in mammalian cells, is able to inhibit RAR:RXR-mediated transactivation, suggesting a high conservation of both DNA- and protein-binding sequences of COUP-TFs during evolution [17]. This is striking if we think that coelenterates, a group of animals comprising ctenophores and cnidarians, emerged before the Cambrian era (more than 500 Mya) [145, 146].
Drosophila: the temporal windows
The homologue of COUP-TFs in the arthropod D. melanogaster (class: Insecta), namely Seven-up (Svp), was first identified as a crucial factor in the cell fate specification of fly compound eye photoreceptors [147]. In Svp mutants different typology of photoreceptors (R1, R2, R3…) undergo a switch of fate, which triggers an R7-like specification program (hence, this COUP-TF homologue was named Seven-up) [147]. The advantage of Drosophila, as a model for developmental biology, is that it enabled the study of the role of Svp at different stages of neurogenesis and in cell fate specification [14, 18, 21, 148, 149]. Neuroblasts are neuronal stem cells giving rise to different lineages of neural and glia cells in the ventral nerve cord of Drosophila. The neuroblast expression profile changes according to a well-defined temporal order which resembles that of mammalian corticogenesis, in which lower and upper layer neurons are sequentially produced at given time-points and always in the same temporal sequence [150–155]. In Drosophila, these time-points are marked by the expression of different transcription factors. Going from early to late cell lineage specification, neuroblasts sequentially express Hunchback (Hb), Krüppel (Kr), Pdm1/Pdm2, Castor (Cas), and Grainyhead (Grh) [156–159]. After each neuroblast division, these factors remain expressed in the founder of the newborn cell lineage (ganglion mother cell, or GMC), while they are silenced in those cells that switch to a new cell fate specification program. The transition from one temporal window to another is regulated by the action of the so-called “switching (or temporal) genes”, such as Svp, which is able to promote the switch from Hb to Kr expression stage by inhibiting Hb during mitosis [18, 21]. Since Svp is expressed both in the neuroblast and in the GMC, the expression of Prospero (a homeodomain protein) in the GMC suppresses Svp function in these cells, which continue to express Hb [21]. Interestingly, Svp has a second wave of expression at later stages of neurogenesis. In double Cas/Grh-positive neuroblasts, Svp has a “sub-temporal” function, contributing to the fine specification of a subgroup of interneurons (namely Ap neurons) through selective inactivation of specific genes in early- and late-generated neurons [14]. Finally, Svp also has a role in the adult, where it limits neuroblast proliferation to avoid abnormal expansion of proliferating precursors in ectopic territories [148]. In summary, Svp is a strong transcriptional regulator during temporal specification of cell lineage formation at early and late stages of development.
Mammals: old solutions for new challenges
Interestingly, COUP-TFs seem to have maintained similar functions in vertebrates with respect to their invertebrate homologues. Knocking down COUP-TFI and II in neurospheres (NS) obtained from mice embryonic stem cells (ESC) delays the onset of gliogenesis [22]. Normally, primary NS differentiate exclusively into neurons, whereas gliogenesis starts from the second generation of NS. After combined inactivation of COUP-TFI and II function, the time of neurogenesis is prolonged to the third stage of NS. In the cerebral cortex, knockdown of COUP-TFs leads to an increase of early-born neurons (resembling Svp inactivation in Drosophila) and to the production of neurons at the expense of glia cells. This would suggest that COUP-TFs impinge on progenitor cell neuropotency. A molecular analysis performed on the promoter of GFAP (an important molecule for glia cell specification) in COUP-TFI/II-knockdown cells revealed an altered methylation pattern, indicating that COUP-TFs promote gliogenesis through epigenetic modifications [22]. Taken together, COUP-TFs appeared to be necessary but not sufficient to induce gliogenesis, suggesting that they limit the neurogenic temporal window rather than promoting gliogenesis.
In mammals, the neurogenic and cell fate specification programs became, especially in the neocortex, much more complex than in invertebrates (and even with respect to all other vertebrate classes), and the mechanisms underlying these processes are still under intense investigation (for reviews, see [152, 153]). Hence, the studies on the role of mammalian COUP-TFs in these events, which were mainly analyzed in mice, sometimes gave contradictory results [12, 15, 22, 24, 27, 160].
To date, except for the study of Naka et al. [22], only COUP-TFI has been investigated in depth in neurogenesis and in neuronal fate specification [12, 15, 24], while the role of COUP-TFII in these events is still poorly understood. In agreement with the phenotype observed in Drosophila, the COUP-TFI mutant neocortices showed a thinning of the upper layers and defective expression of lower layer markers [12, 15, 24]. Paradoxically, overexpression of COUP-TFI in the neocortex under the promoter of the mDach1 gene (D6/COUP-TFI transgenic mice)gave similar results, with an even stronger reduction of upper layers [15]. This apparent contradiction was partially solved by analyzing the effect of COUP-TFI expression on the balance between progenitor self-renewal and neurogenesis. Previous studies clearly showed the link between COUP-TFs function and cell cycle dynamics. In Drosophila, the Svp-mediated control of neuroblast temporal identity is connected with the mitotic event [21, 149], whereas in Strongylocentrotus purpuratus (a species of sea urchin), the distribution of spCOUP-TFI in nuclear sub-compartments of early blastomeres changes remarkably during the different stages of their mitosis, suggesting a role in the early transcription of genes modulating the mechanisms underlying cell cycle progression [161]. Accordingly, in COUP-TFI KO mice, the reduced number of progenitors exiting the cell cycle and undergoing the neurogenic program confirmed a role for this orphan receptor in cell cycle control, while in D6/COUP-TFI transgenic mice, the opposite phenotype was observed [15]. Thus, the strong decrease of upper layers in D6/COUP-TFI mice might be due to an early depletion of the progenitor pool, which would impinge on late-born neuron production. However, the increased proliferation of VZ progenitors in COUP-TFI KO mice falls short in explaining the impairment of upper layer neuron production. Various explanations could elucidate these differences. One may be the alteration of putative downstream signaling pathways controlled by COUP-TFI, such as Mapk/Erk, PI3 K/Akt, and ß-catenin signaling [15]. The Mapk/Erk pathway is involved in the G1- to S-phase transition of cell cycle in different systems [162]. Similarly, β-catenin, a downstream effector of Wnt signaling, controls, in a dose-dependent manner, the duration of the G1 phase in neuronal precursors of the cortical midline [163]. While a short G1 promotes proliferation, a prolonged G1 phase promotes cell responsiveness to intrinsic and extrinsic specification signals, which trigger the neurogenic program in mammals (reviewed in [164]). Thus, it is conceivable to hypothesize that COUP-TFI regulates cell responsiveness to cell-type specification signals and thus the specification of distinct neocortical subpopulations of upper and lower layers. This mis-specification might explain, for example, why layer IV is not maintained in COUP-TFI mutant cortices [160, 165]. Alternatively, the reduction of upper layers in COUP-TFI KO might be due to defective radial migration of late-born neurons, as described above and in [11].
Regarding lower layer specification, a study from our laboratory clearly demonstrates that COUP-TFI can regulate the temporal shift of a layer VI to layer V specification program [24]. After genetic ablation of COUP-TFI, Fezf2 and its downstream effector CTIP2, both involved in the specification of subcerebral cortical fate and connectivity, respectively (review in [151]), are strongly expanded in layer VI, which is normally constituted by Tbr1-positive cortico-thalamic projection neurons. In COUP-TFI mutants, layer VI projection neurons co-express Tbr1 and CTIP2 and project ectopically to the spinal cord, while CTIP2-positive neurons of layer V fail to do so. It is well accepted that, during early phases of differentiation, cortical neurons co-express transcription factors of different cell-type identity, whereas at later stages, the same neurons acquire and refine their final identity by expressing prevalently one factor and down-regulating the inappropriate one [166]. We hypothesize that COUP-TFI, which is expressed in progenitors and maintained in post-mitotic cells, might be required in this refinement process during early and late specification of distinct neuronal sub-types. This function would closely resemble that of Svp in Drosophila where it specifies Ap neurons subpopulations [14], and that of UNC55 (the Caenorhabditis elegans homologue of COUP-TFs) which is upstream of a specific motor neuron genetic program that patterns synaptic remodeling and enables the distinguishing of two motor neuron classes from each another [167–169].
Finally, samples of human fetal brain already display sparse COUP-TFII expression by 9 GW in the forebrain [125]. Up to 13 GW, COUP-TFII progressively increases in the VZ/SVZ of the developing human neocortex and labels a population of neurons in layer I at 20–22 GW. These cells might represent Cajal-Retzius expressing Reelin and Calretinin, as previously described in mice [26]. An interesting observation was that COUP-TFII is expressed in an abundant population of subplate cells, particularly in the caudal region of the developing cortex of human and primates [125]. These data may suggest important roles for COUP-TFII in neurogenesis and cell fate specification in primates.
COUP-TFI orchestrates neocortical arealization in mammalian brains
As predicted by its graded expression and by its role in neurogenic and specification processes, COUP-TFI controls the tangential subdivision of the neocortex into functional areas [27, 165], a particular feature of mammalian brains. These tangential domains are characterized by different cytoarchitecture and connectivity and are deputed to the elaboration of sensory and motor inputs, to voluntary movements and to the performance of high level cognitive functions, such as language and complex behaviours (reviewed in [80, 106, 170–173]). Normally, motor and pre-motor areas are positioned in the frontal regions of the neocortex, whereas sensory areas (elaborating somatosensory, auditory and visual inputs) are located in the parietal and occipital cortex (Fig. 6). The events leading to the specification and positioning of the cortical areas are still under intense debate, and a series of hypotheses have been formulated in recent years to explain these processes [80, 153, 171]. The general opinion is that different events co-ordinately contribute to the specification and refinement of neocortical areas. Different organizing centers surrounding the neocortex (e.g., the hem, the antihem, the septum) (Fig. 1) release, from early stages, several morphogens, such as Fgfs, Wnts, and Bmps. These molecules pattern the neocortex along the rostro-caudal and medio-lateral axes and either repress or promote expression of the so-called areal patterning genes, expressed in distinct gradients in neuronal progenitors. Both morphogens and patterning genes contribute to construct a primordial rough areal map. Subsequently, the arrival of thalamocortical afferences relaying sensory and motor inputs to their respective cortical targets contributes to finely shaped boundaries and connectivity of neocortical functional areas (Fig. 6) [80, 153, 171].
Fig. 6.
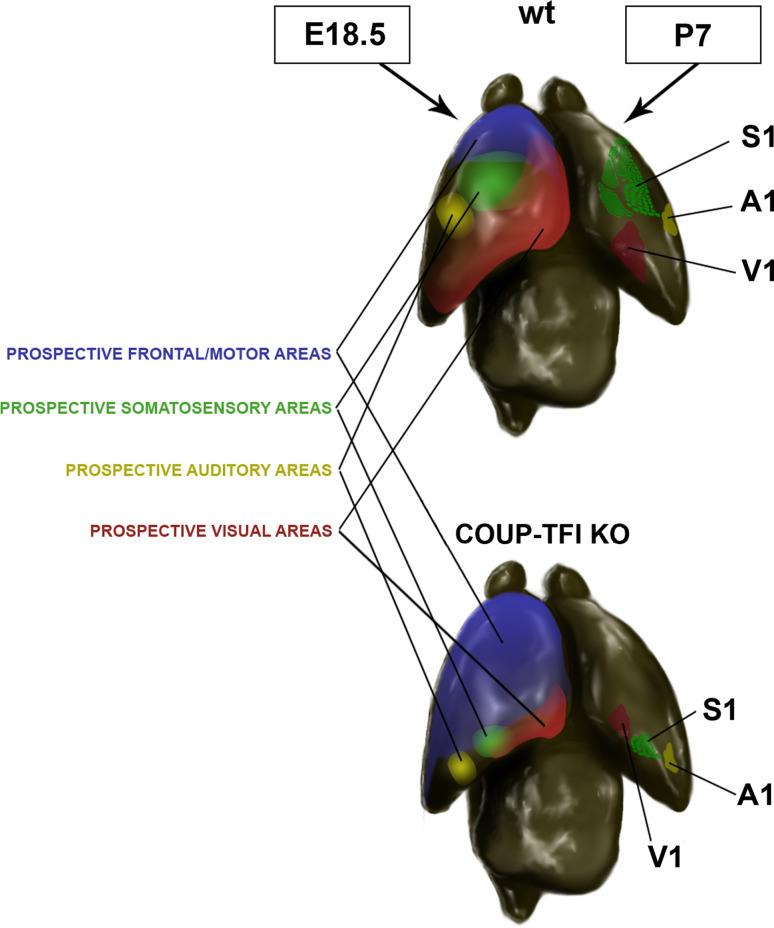
COUP-TFI orchestrates neocortical arealization. In this schematic representation of the neocortical partitioning into functional areas, the prospective areas are shown as continuous colour gradients on the right hemisphere of the mouse cerebral cortex. On the left hemisphere, the shapes of the sensory areas at postnatal stages (P7) in wt and COUP-TFI cortical mutants (KO) are depicted. The ablation of COUP-TFI in the cerebral cortex leads to a strong expansion of the motor at the expense of sensory areas. S1 primary somatosensory area, A1 primary auditory area, V1 primary visual area
Several studies have shown that COUP-TFI contributes both to the first partitioning of the neocortex and to the final refinement of functional areas. Indeed, COUP-TFI is one of the four patterning genes identified so far (the others are Pax6, Emx2, and Sp8), which promotes, together with Emx2, caudal fate specification [12, 27, 160, 174, 175]. After COUP-TFI inactivation, rostral/motor regions expand caudally at the expense of caudal/sensory areas, which are shrunken and shifted towards the occipital cortex [165]. The mechanisms underlying COUP-TFI functions are still under investigation; however, some evidence suggests that it out-competes with Fgf8 signalling in the balance between rostral and caudal fate specification. Fgf8 and its downstream targets (Fgf17, Sp8, Ets genes), which promote rostral identity [82, 83, 176–185], perform, at least in part, this function by negatively controlling COUP-TFI expression or its phosphorylation state [80]. Indeed, COUP-TFI expression becomes rostrally up-regulated in Fgf8 mutant mice [82], whereas Ets genes, Fgf8 downstream targets, can recognize binding sites which are upstream of the COUP-TFI gene and might negatively regulate its expression [186]. Moreover, one of the main effectors of Fgf8 signalling is the Mapk pathway [187–189], and, as previously mentioned, COUP-TFI activity can be regulated by Mapk-mediated phosphorylation in several putative sites [71]. However, the molecular mechanisms by which Fgf signalling controls COUP-TFI and/or vice versa are still unclear. For example, in vitro, Ets genes seem to activate rather than repress COUP-TFI expression [186]. Moreover, COUP-TFI appears to inhibit Ets expression through positive regulation of Sprouty genes [190]. However, both Ets genes and COUP-TFI promote glia-guided neuron radial migration in late corticogenesis, and their expression seem to co-localize in the parietal cortex [11, 191]; thus, it is unlikely that they perform their function by reciprocal inhibition. Finally, if on one side the Mapk/Erk cascade may influence COUP-TFI activity, this factor, in turn, negatively controls Erk phosphorylation and, thus, interferes with Fgf8 signalling during neocortical patterning [15]. In summary, all these data suggest a reciprocal negative feedback loop between COUP-TFI and Fgf8, which may partially contribute to influence the establishment of boundaries between rostral/motor and caudal/sensory areas, although they do not justify the huge arealization defect obtained after COUP-TFI cortical inactivation [165]. Even if an exhaustive answer to this question is still lacking, possible mechanisms have been proposed by a number of studies dissecting COUP-TFI function in different cell types and will be presented in the last section of this review.
Final remarks
From Drosophila [14, 18, 147], to amphioxus [19] and to vertebrates [10], COUP-TFs have always been described as important transcriptional regulators of neurogenesis and neuronal differentiation. This indicates the success of this class of nuclear receptors in regulating neural-specific processes throughout metazoan evolution. There is still much to discover about the mechanisms controlled by these orphan receptors, and the study of their functions in other model systems might help to shed new light in this field. For example, other homologues of COUP-TFs, namely xCOUP-TFA and B, have been identified in Xenopus laevi and play a clear role in antero-posterior patterning of the CNS, but their function in neurogenesis and in cell type specification processes has not yet been queried [76, 192]. As in the case of Drosophila and mouse, the comparison between different organisms may help to identify new mechanisms underlying COUP-TF functions in neurogenesis and cell fate specification.
Conclusions, perspectives and remarks
The data discussed above clearly qualify COUP-TF nuclear receptors as crucial regulators in forebrain development. In the last decade, comparisons between different model systems, genetic manipulations in different domains of the telencephalon, and biomolecular analyses, have allowed the further dissection of their role in brain development. The general outcome is that COUP-TFs regulate a crucial range of events, which are indispensable for the correct formation of several forebrain structures. For example, COUP-TFI regulates the expression of cytoskeletal-associated proteins, such as Rnd2 and Map1B, which in turn control cell migration and axonal elongation [11, 13]. The impairment of these processes might impinge on the formation of cortico-cortical commissures, as mentioned above, or of thalamocortical connections. Indeed, COUP-TFI is expressed in dorsal thalamic nuclei, and in COUP-TFI KO brains thalamocortical afferents fail to invade the cerebral cortex [160]. Since the selective ablation of COUP-TFI in the cerebral cortex does not impede thalamocortical afferents from entering the cortex [12], COUP-TFI might cell-autonomously regulate their elongation by modulating actin and tubulin dynamics in thalamic axons [11]. It has also been observed that COUP-TFI regulates the rate of radial neuron migration in the cortex (Fig. 4b, c). In good accordance with COUP-TFI gradient of expression, an increasingly higher number of cortical neurons migrate to the cortical platefrom rostral to caudal cortical regions. The biological meaning of this migratory gradient is still unknown. However, since migration and axon outgrowth rely on similar mechanisms (as discussed above), this gradient may suggest a similar trend for the rate of axon elongation by callosal neurons. This would well correlate with the dynamics of corpus callosum formation, since, despite different distances of callosal neurons from the midline, their axons do cross the midline at similar stages of development [193]. This is a crucial event, since the midline is permissive to the crossing axon just for a limited period of time. Thus, caudal callosal neurons, which are considerably distant from the midline, might begin to elongate their axons earlier or at a higher rate with respect to rostral ones (Fig. 4d). It would thus be interesting to understand whether the low rostral to high caudal COUP-TFI expression gradient [27, 165, 194] plays a direct role in this event.
Importantly, COUP-TFs are also involved in the neurogenic process by promoting cell cycle exit and modulating Erk, Akt and ß-catenin pathways [15], which normally sustain progenitor proliferation at the expense of neurogenesis [163, 195–198]. COUP-TFs also interact with SMRT [52], which plays a critical role in forebrain development and in the maintenance of the neural stem cell state [199]. SMRT can repress the expression of the jumonji domain containing gene JMJD3—a direct retinoic acid receptor target that functions as a histone H3 trimethyl K27 demethylase—which is capable of activating specific components of the neurogenic program [199]. A high degree of conservation of COUP-TFs functional domains, among metazoans, suggests that these factors modulate neurogenesis through similar mechanisms despite the progressive increase in nervous system complexity during phylogenesis. The Hydra homologue of COUP-TFs, indeed, can also work as a transcriptional repressor in mammalian cells [17]. This indicates that complex processes, such as neocortical arealization, might result from small changes in the modulation of several phylogenetic conserved processes. Accordingly, maintaining a correct balance between neurogenesis and progenitor self-renewal, a process likely regulated by COUP-TF genes, becomes crucial for cell-type specification, neocortical partitioning, and cytoarchitecture of neocortical areas [80, 152, 153, 164, 170, 200, 201]. For example, the “radial unit hypothesis” [153, 201, 202] states that, once a given progenitor acquires its areal identity, all derived neurons will maintain not only a specific areal fatebut also precise positional information, since they remain in the same position as their progenitors along the cortical radial axis. As a matter of fact, a higher number of asymmetric/neurogenic divisions at the level of VZ progenitors will increase the number of neurons along each radial glia filament (increasing the thickness of a given area), while an increase in symmetric/self-renewal divisions will amplify the pool of progenitors augmenting the tangential extension of functional areas. Hence, COUP-TFI might finely modulate the type and mode of cell divisions in neocortical progenitors and, thus, influence the shape and thickness of functional areas during cortical patterning.
In the last few years, transcription factors expressed post-mitotically have been shown to play a relevant role in the specification of different mammalian neurons subtypes, in neocortical lamination, and possibly in arealization [151, 200, 203]. Nevertheless, the number of factors so far identified is still small, and the mechanisms of how post-mitotic factors regulate each other have not been exhaustively investigated. COUP-TFI is expressed both in the mitotic and post-mitotic compartments of the cerebral cortex and of the ganglionic eminences, but its role in the post-mitotic phases of neurogenesis is still not well understood. Interestingly, COUP-TFI interacts with CTIP2, a post-mitotic transcription factor required in corticospinal motor neuron connectivity [204], and, accordingly, in both COUP-TFI and CTIP2 mutant mice, corticospinal motor neurons fail to project to their normal targets [24, 204]. Moreover, COUP-TFs also interact with the histone deacetylase 1 (HDAC1), an important component of the histone deacetylase complex involved in gene regulation as well as in cell proliferation and differentiation [205]. Thus, during cortical neuron subtype specification, COUP-TFI might regulate Satb2-mediated inhibition of CTIP2 expression [206, 207]. This event would be crucial for correct specification of callosal neurons (Satb2-positive) versus corticospinal motor neurons (CTIP2-positive)[204]. Moreover, since it is extremely unlikely that the strong impairment in arealization observed after cortical ablation of COUP-TFI is solely due to the enhancement of Fgf8 signalling, it might be conceivable to hypothesize that post-mitotic expression of COUP-TFI plays an important role in cortical subtype specification and, ultimately, cortical arealization.
The imbalance in interneuronal populations observed in COUP-TFI mutants, in which the gene is inactivated solely in intermediate progenitors and post-mitotic neurons, may further imply that COUP-TFI neuron specification is independent of its expression in progenitor cells. However, in this model, although COUP-TFI is still active in the ganglionic apical progenitors, it is inactivated in the proliferating intermediate progenitors. Thus, COUP-TFI function in the final specification of neuronal subtype is still questionable. Nonetheless, the study of this mouse model may be important to further understand the establishment of local and long-range circuits controlled by interneurons and the relevance of different interneuronal sub-populations in the regulation of brain rhythms. Thus, further studies on this unique mouse model may help in understanding the aetiology of epileptic seizures.
Finally, COUP-TF expression is not limited to a specific stage of neuronal developmentbut is also prolonged into adulthood [86, 87], implying that COUP-TFI and COUP-TFII might also be involved in the maintenance and control of adult corticogenesis and/or plasticity. We therefore think that the further dissection of COUP-TF functions in distinct regions and/or cell-types of the forebrain and at different stages of neurogenesis and neuronal differentiation would help in identifying novel functions of these exciting nuclear receptors; a real “coup de pouce” (boost) in the field of cerebral cortical development and maturation!
Acknowledgments
We thank Monica Courtney for her English editing and proofreading. Work in the laboratory is supported by the Agence Nationale Recherche (“ANR Chaire d’Excellence” Program) under grant number R09125AA, by the Fondation Recherche Medicale (“Equipe FRM 2011”) under grant number R11078AA to M.S., by an AXA Research Fund fellowship to E.M., and by a CNRS fellowship from Lebanon to K.H.
Footnotes
Elia Magrinelli and Kawssar Harb have contributed equally to this manuscript.
References
- 1.Aboitiz F. Genetic and developmental homology in amniote brains. Toward conciliating radical views of brain evolution. Brain Res Bull. 2011;84(2):125–136. doi: 10.1016/j.brainresbull.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Aboitiz F, Morales D, Montiel J. The evolutionary origin of the mammalian isocortex: towards an integrated developmental and functional approach. Behav Brain Sci. 2003;26(5):535–552. doi: 10.1017/s0140525x03000128. [DOI] [PubMed] [Google Scholar]
- 3.Super H, Uylings HB. The early differentiation of the neocortex: a hypothesis on neocortical evolution. Cereb Cortex. 2001;11(12):1101–1109. doi: 10.1093/cercor/11.12.1101. [DOI] [PubMed] [Google Scholar]
- 4.Jones EG. Lamination and differential distribution of thalamic afferents within the sensory-motor cortex of the squirrel monkey. J Comp Neurol. 1975;160(2):167–203. doi: 10.1002/cne.901600203. [DOI] [PubMed] [Google Scholar]
- 5.Jones EG. The thalamus. Berlin: Plenum Press; 1985. [Google Scholar]
- 6.Reiner A, Brauth SE, Karten HJ. Evolution of the amniote basal ganglia. Trends Neurosci. 1984;7(9):320–325. [Google Scholar]
- 7.Puelles L. Pallio-pallial tangential migrations and growth signaling: new scenario for cortical evolution? Brain Behav Evol. 2011;78:108–127. doi: 10.1159/000327905. [DOI] [PubMed] [Google Scholar]
- 8.Halassa M, Haydon P. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32(8):421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Pereira FA, Tsai MJ, Tsai SY. COUP-TF orphan nuclear receptors in development and differentiation. Cell Mol Life Sci. 2000;57(10):1388–1398. doi: 10.1007/PL00000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alfano C, et al. COUP-TFI promotes radial migration and proper morphology of callosal projection neurons by repressing Rnd2 expression. Development. 2011;138(21):4685–4697. doi: 10.1242/dev.068031. [DOI] [PubMed] [Google Scholar]
- 12.Armentano M, et al. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10(10):1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- 13.Armentano M, et al. COUP-TFI is required for the formation of commissural projections in the forebrain by regulating axonal growth. Development. 2006;133(21):4151–4162. doi: 10.1242/dev.02600. [DOI] [PubMed] [Google Scholar]
- 14.Benito-Sipos J, et al. Seven up acts as a temporal factor during two different stages of neuroblast 5–6 development. Development. 2011;138(24):5311–5320. doi: 10.1242/dev.070946. [DOI] [PubMed] [Google Scholar]
- 15.Faedo A, et al. COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and beta-catenin signaling. Cereb Cortex. 2008;18(9):2117–2131. doi: 10.1093/cercor/bhm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galliot B, Quiquand M. A two-step process in the emergence of neurogenesis. Eur J Neurosci. 2011;34(6):847–862. doi: 10.1111/j.1460-9568.2011.07829.x. [DOI] [PubMed] [Google Scholar]
- 17.Gauchat D, et al. The orphan COUP-TF nuclear receptors are markers for neurogenesis from cnidarians to vertebrates. Dev Biol. 2004;275(1):104–123. doi: 10.1016/j.ydbio.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 18.Kanai MI, Okabe M, Hiromi Y. Seven-up Controls switching of transcription factors that specify temporal identities of Drosophila neuroblasts. Dev Cell. 2005;8(2):203–213. doi: 10.1016/j.devcel.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Langlois MC, et al. Amphicoup-TF, a nuclear orphan receptor of the lancelet Branchiostoma floridae, is implicated in retinoic acid signalling pathways. Dev Genes Evol. 2000;210(10):471–482. doi: 10.1007/s004270000087. [DOI] [PubMed] [Google Scholar]
- 20.Lodato S, et al. Loss of COUP-TFI alters the balance between caudal ganglionic eminence- and medial ganglionic eminence-derived cortical interneurons and results in resistance to epilepsy. J Neurosci. 2011;31(12):4650–4662. doi: 10.1523/JNEUROSCI.6580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mettler U, Vogler G, Urban J. Timing of identity: spatiotemporal regulation of hunchback in neuroblast lineages of Drosophila by Seven-up and Prospero. Development. 2006;133(3):429–437. doi: 10.1242/dev.02229. [DOI] [PubMed] [Google Scholar]
- 22.Naka H, et al. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci. 2008;11(9):1014–1023. doi: 10.1038/nn.2168. [DOI] [PubMed] [Google Scholar]
- 23.Tang K, et al. COUP-TFII controls amygdala patterning by regulating neuropilin expression. Development. 2012;139(9):1630–1639. doi: 10.1242/dev.075564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomassy GS, et al. Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc Natl Acad Sci USA. 2010;107(8):3576–3581. doi: 10.1073/pnas.0911792107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tricoire L, et al. Common origins of hippocampal Ivy and nitric oxide synthase expressing neurogliaform cells. J Neurosci. 2010;30(6):2165–2176. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tripodi M, et al. The COUP-TF nuclear receptors regulate cell migration in the mammalian basal forebrain. Development. 2004;131(24):6119–6129. doi: 10.1242/dev.01530. [DOI] [PubMed] [Google Scholar]
- 27.Zhou C, Tsai SY, Tsai MJ. COUP-TFI: an intrinsic factor for early regionalization of the neocortex. Genes Dev. 2001;15(16):2054–2059. doi: 10.1101/gad.913601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai SY, Tsai MJ. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr Rev. 1997;18(2):229–240. doi: 10.1210/edrv.18.2.0294. [DOI] [PubMed] [Google Scholar]
- 29.Qiu Y, et al. Spatiotemporal expression patterns of chicken ovalbumin upstream promoter-transcription factors in the developing mouse central nervous system: evidence for a role in segmental patterning of the diencephalon. Proc Natl Acad Sci USA. 1994;91(10):4451–4455. doi: 10.1073/pnas.91.10.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyajima N, et al. Identification of two novel members of erbA superfamily by molecular cloning: the gene products of the two are highly related to each other. Nucleic Acids Res. 1988;16(23):11057–11074. doi: 10.1093/nar/16.23.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avram D, et al. Heterodimeric interactions between chicken ovalbumin upstream promoter-transcription factor family members ARP1 and ear2. J Biol Chem. 1999;274(20):14331–14336. doi: 10.1074/jbc.274.20.14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Lambert MH, Xu HE. Activation of nuclear receptors: a perspective from structural genomics. Structure. 2003;11(7):741–746. doi: 10.1016/s0969-2126(03)00133-3. [DOI] [PubMed] [Google Scholar]
- 33.Kruse SW, et al. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008;6(9):e227. doi: 10.1371/journal.pbio.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ladias JA, Karathanasis SK. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991;251(4993):561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- 35.Wang LH, et al. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- 36.Pereira FA, et al. Chicken ovalbumin upstream promoter transcription factor (COUP-TF): expression during mouse embryogenesis. J Steroid Biochem Mol Biol. 1995;53(1–6):503–508. doi: 10.1016/0960-0760(95)00097-j. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q, Dwyer N, O’Leary D. Differential expression of COUP-TFI, CHL1, and two novel genes in developing neocortex identified by differential display PCR. J Neurosci. 2000;20(20):7682–7690. doi: 10.1523/JNEUROSCI.20-20-07682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooney AJ, et al. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol. 1992;12(9):4153–4163. doi: 10.1128/mcb.12.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novac N, Heinzel T. Nuclear receptors: overview and classification. Curr Drug Targets Inflamm Allergy. 2004;3(4):335–346. doi: 10.2174/1568010042634541. [DOI] [PubMed] [Google Scholar]
- 40.Ladias JA, et al. Transcriptional regulation of human apolipoprotein genes ApoB, ApoCIII, and ApoAII by members of the steroid hormone receptor superfamily HNF-4, ARP-1, EAR-2, and EAR-3. J Biol Chem. 1992;267(22):15849–15860. [PubMed] [Google Scholar]
- 41.Paulweber B, et al. Identification of a negative regulatory region 5′ of the human apolipoprotein B promoter. J Biol Chem. 1991;266(32):21956–21961. [PubMed] [Google Scholar]
- 42.Rottman JN, et al. A retinoic acid-responsive element in the apolipoprotein AI gene distinguishes between two different retinoic acid response pathways. Mol Cell Biol. 1991;11(7):3814–3820. doi: 10.1128/mcb.11.7.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Widom RL, et al. Synergistic interactions between transcription factors control expression of the apolipoprotein AI gene in liver cells. Mol Cell Biol. 1991;11(2):677–687. doi: 10.1128/mcb.11.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Widom RL, Rhee M, Karathanasis SK. Repression by ARP-1 sensitizes apolipoprotein AI gene responsiveness to RXR alpha and retinoic acid. Mol Cell Biol. 1992;12(8):3380–3389. doi: 10.1128/mcb.12.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooney AJ, et al. Multiple mechanisms of chicken ovalbumin upstream promoter transcription factor-dependent repression of transactivation by the vitamin D, thyroid hormone, and retinoic acid receptors. J Biol Chem. 1993;268(6):4152–4160. [PubMed] [Google Scholar]
- 46.Tran P, et al. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol. 1992;12(10):4666–4676. doi: 10.1128/mcb.12.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leng X, et al. Molecular mechanisms of COUP-TF-mediated transcriptional repression: evidence for transrepression and active repression. Mol Cell Biol. 1996;16(5):2332–2340. doi: 10.1128/mcb.16.5.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berrodin TJ, et al. Heterodimerization among thyroid hormone receptor, retinoic acid receptor, retinoid X receptor, chicken ovalbumin upstream promoter transcription factor, and an endogenous liver protein. Mol Endocrinol. 1992;6(9):1468–1478. doi: 10.1210/mend.6.9.1331778. [DOI] [PubMed] [Google Scholar]
- 49.Kliewer SA, et al. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci USA. 1992;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kliewer SA, et al. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casanova J, et al. Functional evidence for ligand-dependent dissociation of thyroid hormone and retinoic acid receptors from an inhibitory cellular factor. Mol Cell Biol. 1994;14(9):5756–5765. doi: 10.1128/mcb.14.9.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibata H, et al. Gene silencing by chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) is mediated by transcriptional corepressors, nuclear receptor-corepressor (N-CoR) and silencing mediator for retinoic acid receptor and thyroid hormone receptor (SMRT) Mol Endocrinol. 1997;11(6):714–724. doi: 10.1210/mend.11.6.0002. [DOI] [PubMed] [Google Scholar]
- 53.Zamir I, et al. A nuclear hormone receptor corepressor mediates transcriptional silencing by receptors with distinct repression domains. Mol Cell Biol. 1996;16(10):5458–5465. doi: 10.1128/mcb.16.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perissi V, et al. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell. 2004;116(4):511–526. doi: 10.1016/s0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- 55.Ing NH, et al. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II) J Biol Chem. 1992;267(25):17617–17623. [PubMed] [Google Scholar]
- 56.Lu XP, Salbert G, Pfahl M. An evolutionary conserved COUP-TF binding element in a neural-specific gene and COUP-TF expression patterns support a major role for COUP-TF in neural development. Mol Endocrinol. 1994;8(12):1774–1788. doi: 10.1210/mend.8.12.7708064. [DOI] [PubMed] [Google Scholar]
- 57.Power SC, Cereghini S. Positive regulation of the vHNF1 promoter by the orphan receptors COUP-TF1/Ear3 and COUP-TFII/Arp1. Mol Cell Biol. 1996;16(3):778–791. doi: 10.1128/mcb.16.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall RK, Sladek FM, Granner DK. The orphan receptors COUP-TF and HNF-4 serve as accessory factors required for induction of phosphoenolpyruvate carboxykinase gene transcription by glucocorticoids. Proc Natl Acad Sci USA. 1995;92(2):412–416. doi: 10.1073/pnas.92.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haddad IA, et al. Linkage, evolution, and expression of the rat apolipoprotein A-I, C-III, and A-IV genes. J Biol Chem. 1986;261(28):13268–13277. [PubMed] [Google Scholar]
- 60.Reue K, Leff T, Breslow JL. Human apolipoprotein CIII gene expression is regulated by positive and negative cis-acting elements and tissue-specific protein factors. J Biol Chem. 1988;263(14):6857–6864. [PubMed] [Google Scholar]
- 61.Liu YH, Teng CT. Characterization of estrogen-responsive mouse lactoferrin promoter. J Biol Chem. 1991;266(32):21880–21885. [PubMed] [Google Scholar]
- 62.Hwung YP, et al. Differential binding of the chicken ovalbumin upstream promoter (COUP) transcription factor to two different promoters. J Biol Chem. 1988;263(26):13470–13474. [PubMed] [Google Scholar]
- 63.Hwung YP, et al. The COUP transcription factor binds to an upstream promoter element of the rat insulin II gene. Mol Cell Biol. 1988;8(5):2070–2077. doi: 10.1128/mcb.8.5.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooney AJ, et al. Chicken ovalbumin upstream promoter transcription factor binds to a negative regulatory region in the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1991;65(6):2853–2860. doi: 10.1128/jvi.65.6.2853-2860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orchard K, et al. A novel T-cell protein which recognizes a palindromic sequence in the negative regulatory element of the human immunodeficiency virus long terminal repeat. J Virol. 1990;64(7):3234–3239. doi: 10.1128/jvi.64.7.3234-3239.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malik S, Karathanasis S. Transcriptional activation by the orphan nuclear receptor ARP-1. Nucleic Acids Res. 1995;23(9):1536–1543. doi: 10.1093/nar/23.9.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marcus SL, et al. A p56(lck) ligand serves as a coactivator of an orphan nuclear hormone receptor. J Biol Chem. 1996;271(44):27197–27200. doi: 10.1074/jbc.271.44.27197. [DOI] [PubMed] [Google Scholar]
- 68.Krishnan V, et al. Mediation of Sonic hedgehog-induced expression of COUP-TFII by a protein phosphatase. Science. 1997;278(5345):1947–1950. doi: 10.1126/science.278.5345.1947. [DOI] [PubMed] [Google Scholar]
- 69.Fjose A, Weber U, Mlodzik M. A novel vertebrate svp-related nuclear receptor is expressed as a step gradient in developing rhombomeres and is affected by retinoic acid. Mech Dev. 1995;52(2–3):233–246. doi: 10.1016/0925-4773(95)00404-o. [DOI] [PubMed] [Google Scholar]
- 70.Zhuang Y, Gudas LJ. Overexpression of COUP-TF1 in murine embryonic stem cells reduces retinoic acid-associated growth arrest and increases extraembryonic endoderm gene expression. Differentiation. 2008;76(7):760–771. doi: 10.1111/j.1432-0436.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 71.Gay F, et al. Multiple phosphorylation events control chicken ovalbumin upstream promoter transcription factor I orphan nuclear receptor activity. Mol Endocrinol. 2002;16(6):1332–1351. doi: 10.1210/mend.16.6.0840. [DOI] [PubMed] [Google Scholar]
- 72.Power RF, et al. Dopamine activation of an orphan of the steroid receptor superfamily. Science. 1991;252(5012):1546–1548. doi: 10.1126/science.2047861. [DOI] [PubMed] [Google Scholar]
- 73.Jonk LJ, et al. Cloning and expression during development of three murine members of the COUP family of nuclear orphan receptors. Mech Dev. 1994;47(1):81–97. doi: 10.1016/0925-4773(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 74.Neuman K, et al. Orphan receptor COUP-TF I antagonizes retinoic acid-induced neuronal differentiation. J Neurosci Res. 1995;41(1):39–48. doi: 10.1002/jnr.490410106. [DOI] [PubMed] [Google Scholar]
- 75.Schuh TJ, Kimelman D. COUP-TFI is a potential regulator of retinoic acid-modulated development in Xenopus embryos. Mech Dev. 1995;51(1):39–49. doi: 10.1016/0925-4773(94)00346-o. [DOI] [PubMed] [Google Scholar]
- 76.van der Wees J, et al. Developmental expression and differential regulation by retinoic acid of Xenopus COUP-TF-A and COUP-TF-B. Mech Dev. 1996;54(2):173–184. doi: 10.1016/0925-4773(95)00471-8. [DOI] [PubMed] [Google Scholar]
- 77.Qiu Y, et al. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev. 1997;11(15):1925–1937. doi: 10.1101/gad.11.15.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luo J, et al. Compound mutants for retinoic acid receptor (RAR) beta and RAR alpha 1 reveal developmental functions for multiple RAR beta isoforms. Mech Dev. 1996;55(1):33–44. doi: 10.1016/0925-4773(95)00488-2. [DOI] [PubMed] [Google Scholar]
- 79.Clotman F, Van Maele-Fabry G, Picard JJ. All-trans-retinoic acid upregulates the expression of COUP-TFI in early-somite mouse embryos cultured in vitro. Neurotoxicol Teratol. 1998;20(6):591–599. doi: 10.1016/s0892-0362(98)00018-x. [DOI] [PubMed] [Google Scholar]
- 80.O’Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56(2):252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 81.Sousa VH, Fishell G. Sonic hedgehog functions through dynamic changes in temporal competence in the developing forebrain. Curr Opin Genet Dev. 2010;20(4):391–399. doi: 10.1016/j.gde.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130(9):1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- 83.Storm EE, et al. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133(9):1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- 84.Crossley PH, et al. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108(2):183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- 85.Shimamura K, et al. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121(12):3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- 86.Dye CA, El Shawa H, Huffman KJ. A lifespan analysis of intraneocortical connections and gene expression in the mouse II. Cereb Cortex. 2011;21(6):1331–1350. doi: 10.1093/cercor/bhq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dye CA, El Shawa H, Huffman KJ. A lifespan analysis of intraneocortical connections and gene expression in the mouse I. Cereb Cortex. 2011;21(6):1311–1330. doi: 10.1093/cercor/bhq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miyata T, et al. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131(13):3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- 89.Nadarajah B, et al. Ventricle-directed migration in the developing cerebral cortex. Nat Neurosci. 2002;5(3):218–224. doi: 10.1038/nn813. [DOI] [PubMed] [Google Scholar]
- 90.Nadarajah B, et al. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4(2):143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- 91.Noctor SC, et al. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7(2):136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 92.Kwan KY, Šestan N, Anton ES. Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development. 2012;139(9):1535–1546. doi: 10.1242/dev.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.LoTurco JJ, Bai J. The multipolar stage and disruptions in neuronal migration. Trends Neurosci. 2006;29(7):407–413. doi: 10.1016/j.tins.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 94.Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci. 2003;23(31):9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 96.Heng JI, et al. Neurogenin 2 controls cortical neuron migration through regulation of Rnd2. Nature. 2008;455(7209):114–118. doi: 10.1038/nature07198. [DOI] [PubMed] [Google Scholar]
- 97.Nakamura K, et al. In vivo function of Rnd2 in the development of neocortical pyramidal neurons. Neurosci Res. 2006;54(2):149–153. doi: 10.1016/j.neures.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 98.Uesugi K, et al. Different Requirement for Rnd GTPases of R-Ras GAP Activity of Plexin-C1 and Plexin-D1. J Biol Chem. 2009;284(11):6743–6751. doi: 10.1074/jbc.M805213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kakimoto T, Katoh H, Negishi M. Identification of splicing variants of Rapostlin, a novel RND2 effector that interacts with neural Wiskott-Aldrich syndrome protein and induces neurite branching. J Biol Chem. 2004;279(14):14104–14110. doi: 10.1074/jbc.M312763200. [DOI] [PubMed] [Google Scholar]
- 100.Lickiss T, et al. Examining the relationship between early axon growth and transcription factor expression in the developing cerebral cortex. J Anat. 2012;220(3):201–211. doi: 10.1111/j.1469-7580.2011.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shoukimas GM, Hinds JW. The development of the cerebral cortex in the embryonic mouse: an electron microscopic serial section analysis. J Comp Neurol. 1978;179(4):795–830. doi: 10.1002/cne.901790407. [DOI] [PubMed] [Google Scholar]
- 102.Norris CR, Kalil K. Guidance of callosal axons by radial glia in the developing cerebral cortex. J Neurosci. 1991;11(11):3481–3492. doi: 10.1523/JNEUROSCI.11-11-03481.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chedotal A, Rijli FM. Transcriptional regulation of tangential neuronal migration in the developing forebrain. Curr Opin Neurobiol. 2009;19(2):139–145. doi: 10.1016/j.conb.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 104.Corbin JG, Nery S, Fishell G. Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat Neurosci. 2001;4(Suppl):1177–1182. doi: 10.1038/nn749. [DOI] [PubMed] [Google Scholar]
- 105.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2(11):780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 106.Borello U, Pierani A. Patterning the cerebral cortex: traveling with morphogens. Curr Opin Genet Dev. 2010;20(4):408–415. doi: 10.1016/j.gde.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 107.Marin-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21(2):64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- 108.Soriano E, Del Rio JA. The cells of cajal-retzius: still a mystery one century after. Neuron. 2005;46(3):389–394. doi: 10.1016/j.neuron.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 109.Griveau A, et al. A novel role for Dbx1-derived Cajal-Retzius cells in early regionalization of the cerebral cortical neuroepithelium. PLoS Biol. 2010;8(7):e1000440. doi: 10.1371/journal.pbio.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takiguchi-Hayashi K, et al. Generation of reelin-positive marginal zone cells from the caudomedial wall of telencephalic vesicles. J Neurosci. 2004;24(9):2286–2295. doi: 10.1523/JNEUROSCI.4671-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Faux C, et al. Neurons on the move: migration and lamination of cortical interneurons. Neurosignals. 2012;20(3):168–189. doi: 10.1159/000334489. [DOI] [PubMed] [Google Scholar]
- 112.Tanaka DH, Nakajima K. Migratory pathways of GABAergic interneurons when they enter the neocortex. Eur J Neurosci. 2012;35(11):1655–1660. doi: 10.1111/j.1460-9568.2012.08111.x. [DOI] [PubMed] [Google Scholar]
- 113.Tanaka DH, Nakajima K. GABAergic interneuron migration and the evolution of the neocortex. Dev Growth Differ. 2012;54(3):366–372. doi: 10.1111/j.1440-169X.2012.01351.x. [DOI] [PubMed] [Google Scholar]
- 114.Helmstaedter M, et al. Reconstruction of an average cortical column in silico. Brain Res Rev. 2007;55(2):193–203. doi: 10.1016/j.brainresrev.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 115.Lee S, et al. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30(50):16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2(1):11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 117.Baraban SC. Emerging epilepsy models: insights from mice, flies, worms and fish. Curr Opin Neurol. 2007;20(2):164–168. doi: 10.1097/WCO.0b013e328042bae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cossart R, Bernard C, Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28(2):108–115. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 119.Genius J, et al. Disturbed function of GABAergic interneurons in schizophrenia: relevance for medical treatment? Curr Pharm Biotechnol. 2012;13:1549–1556. doi: 10.2174/138920112800784943. [DOI] [PubMed] [Google Scholar]
- 120.Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 121.Miyoshi G, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30(5):1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vucurovic K, et al. Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb Cortex. 2010;20(10):2333–2347. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kanatani S, et al. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J Neurosci. 2008;28(50):13582–13591. doi: 10.1523/JNEUROSCI.2132-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nadarajah B, et al. Neuronal migration in the developing cerebral cortex: observations based on real-time imaging. Cereb Cortex. 2003;13(6):607–611. doi: 10.1093/cercor/13.6.607. [DOI] [PubMed] [Google Scholar]
- 125.Reinchisi G, et al. COUP-TFII expressing interneurons in human fetal forebrain. Cereb Cortex. 2011;22(12):2820–30. doi: 10.1093/cercor/bhr359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yozu M, Tabata H, Nakajima K. The caudal migratory stream: a novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci. 2005;25(31):7268–7277. doi: 10.1523/JNEUROSCI.2072-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Butt SJ, et al. The requirement of Nk2–1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59(5):722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cai Y, et al. Nuclear receptor COUP-TFII-expressing neocortical interneurons are derived from the medial and lateral/caudal ganglionic eminence and define specific subsets of mature interneurons. J Comp Neurol. 2013;521(2):479–97. doi: 10.1002/cne.23186. [DOI] [PubMed] [Google Scholar]
- 129.Swindell EC, et al. Rx-Cre, a tool for inactivation of gene expression in the developing retina. Genesis. 2006;44(8):361–363. doi: 10.1002/dvg.20225. [DOI] [PubMed] [Google Scholar]
- 130.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 131.Adams RB, Jr, et al. Culture, gaze and the neural processing of fear expressions. Soc Cogn Affect Neurosci. 2010;5(2–3):340–348. doi: 10.1093/scan/nsp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Medina L, Bupesh M, Abellan A. Contribution of genoarchitecture to understanding forebrain evolution and development, with particular emphasis on the amygdala. Brain Behav Evol. 2011;78(3):216–236. doi: 10.1159/000330056. [DOI] [PubMed] [Google Scholar]
- 133.Medina L, Bupesh M, Abellàn A. Contribution of genoarchitecture to understanding forebrain evolution and development, with particular emphasis on the amygdala. Brain. 2011;3:11. doi: 10.1159/000330056. [DOI] [PubMed] [Google Scholar]
- 134.Soma M, et al. Development of the mouse amygdala as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J Comp Neurol. 2009;513(1):113–128. doi: 10.1002/cne.21945. [DOI] [PubMed] [Google Scholar]
- 135.Puelles L, et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424(3):409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 136.Nery S, FIshell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:9. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- 137.Anderson S, et al. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9(6):646–654. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]
- 138.Pleasure SJ, et al. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28(3):727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 139.Butt SJ, et al. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48(4):591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 140.Fogarty M, et al. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27(41):10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lavdas AA, et al. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19(18):7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sussel L, et al. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126(15):3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- 143.Wichterle H, et al. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128(19):3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 144.Fuentealba P, et al. Expression of COUP-TFII nuclear receptor in restricted GABAergic neuronal populations in the adult rat hippocampus. J Neurosci. 2010;30(5):1595–1609. doi: 10.1523/JNEUROSCI.4199-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Philippe H, et al. Phylogenomics revives traditional views on deep animal relationships. Curr Biol. 2009;19(8):706–712. doi: 10.1016/j.cub.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 146.De Robertis EM. Evo-devo: variations on ancestral themes. Cell. 2008;132(2):185–195. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mlodzik M, et al. The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell. 1990;60(2):211–224. doi: 10.1016/0092-8674(90)90737-y. [DOI] [PubMed] [Google Scholar]
- 148.Maurange C, Cheng L, Gould AP. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell. 2008;133(5):891–902. doi: 10.1016/j.cell.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 149.Urban J, Mettler U. Connecting temporal identity to mitosis: the regulation of Hunchback in Drosophila neuroblast lineages. Cell Cycle. 2006;5(9):950–952. doi: 10.4161/cc.5.9.2727. [DOI] [PubMed] [Google Scholar]
- 150.Bossing T, et al. The embryonic central nervous system lineages of Drosophila melanogaster. I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev Biol. 1996;179(1):41–64. doi: 10.1006/dbio.1996.0240. [DOI] [PubMed] [Google Scholar]
- 151.Molyneaux BJ, et al. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8(6):427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 152.Pierani A, Wassef M. Cerebral cortex development: from progenitors patterning to neocortical size during evolution. Dev Growth Differ. 2009;51(3):325–342. doi: 10.1111/j.1440-169X.2009.01095.x. [DOI] [PubMed] [Google Scholar]
- 153.Rakic P, et al. Decision by division: making cortical maps. Trends Neurosci. 2009;32(5):291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schmid A, Chiba A, Doe CQ. Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development. 1999;126(21):4653–4689. doi: 10.1242/dev.126.21.4653. [DOI] [PubMed] [Google Scholar]
- 155.Schmidt H, et al. The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev Biol. 1997;189(2):186–204. doi: 10.1006/dbio.1997.8660. [DOI] [PubMed] [Google Scholar]
- 156.Brody T, Odenwald WF. Programmed transformations in neuroblast gene expression during Drosophila CNS lineage development. Dev Biol. 2000;226(1):34–44. doi: 10.1006/dbio.2000.9829. [DOI] [PubMed] [Google Scholar]
- 157.Isshiki T, et al. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell. 2001;106(4):511–521. doi: 10.1016/s0092-8674(01)00465-2. [DOI] [PubMed] [Google Scholar]
- 158.Kambadur R, et al. Regulation of POU genes by castor and hunchback establishes layered compartments in the Drosophila CNS. Genes Dev. 1998;12(2):246–260. doi: 10.1101/gad.12.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Novotny T, Eiselt R, Urban J. Hunchback is required for the specification of the early sublineage of neuroblast 7–3 in the Drosophila central nervous system. Development. 2002;129(4):1027–1036. doi: 10.1242/dev.129.4.1027. [DOI] [PubMed] [Google Scholar]
- 160.Zhou C, et al. The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron. 1999;24(4):847–859. doi: 10.1016/s0896-6273(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 161.Vlahou A, Flytzanis CN. Subcellular trafficking of the nuclear receptor COUP-TF in the early embryonic cell cycle. Dev Biol. 2000;218(2):284–298. doi: 10.1006/dbio.1999.9456. [DOI] [PubMed] [Google Scholar]
- 162.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26(22):3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 163.Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129(9):2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 164.Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8(6):438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- 165.Armentano M, et al. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- 166.Azim E, et al. Lmo4 and Clim1 progressively delineate cortical projection neuron subtypes during development. Cereb Cortex. 2009;19(Suppl 1):i62–i69. doi: 10.1093/cercor/bhp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Petersen SC, et al. A transcriptional program promotes remodeling of GABAergic synapses in Caenorhabditis elegans. J Neurosci. 2011;31(43):15362–15375. doi: 10.1523/JNEUROSCI.3181-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou HM, Walthall WW. UNC-55, an orphan nuclear hormone receptor, orchestrates synaptic specificity among two classes of motor neurons in Caenorhabditis elegans. J Neurosci. 1998;18(24):10438–10444. doi: 10.1523/JNEUROSCI.18-24-10438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Walthall WW, Plunkett JA. Genetic transformation of the synaptic pattern of a motoneuron class in Caenorhabditis elegans. J Neurosci. 1995;15(2):1035–1043. doi: 10.1523/JNEUROSCI.15-02-01035.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18(1):90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sansom SN, Livesey FJ. Gradients in the brain: the control of the development of form and function in the cerebral cortex. Cold Spring Harb Perspect Biol. 2009;1(2):a002519. doi: 10.1101/cshperspect.a002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shepherd GM. Intracortical cartography in an agranular area. Front Neurosci. 2009;3(3):337–343. doi: 10.3389/neuro.01.030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shipp S. The importance of being agranular: a comparative account of visual and motor cortex. Philos Trans R Soc Lond B. 2005;360(1456):797–814. doi: 10.1098/rstb.2005.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288(5464):344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- 175.Mallamaci A, et al. Area identity shifts in the early cerebral cortex of Emx2−/− mutant mice. Nat Neurosci. 2000;3(7):679–686. doi: 10.1038/76630. [DOI] [PubMed] [Google Scholar]
- 176.Fukuchi-Shimogori T, Grove EA. Emx2 patterns the neocortex by regulating FGF positional signaling. Nat Neurosci. 2003;6(8):825–831. doi: 10.1038/nn1093. [DOI] [PubMed] [Google Scholar]
- 177.Huffman KJ, Garel S, Rubenstein JL. Fgf8 regulates the development of intra-neocortical projections. J Neurosci. 2004;24(41):8917–8923. doi: 10.1523/JNEUROSCI.2086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shimogori T, Grove EA. Fibroblast growth factor 8 regulates neocortical guidance of area-specific thalamic innervation. J Neurosci. 2005;25(28):6550–6560. doi: 10.1523/JNEUROSCI.0453-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cholfin JA, Rubenstein JL. Genetic regulation of prefrontal cortex development and function. Novartis Found Symp. 2007;288:165–173. [PubMed] [Google Scholar]
- 180.Cholfin JA, Rubenstein JL. Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci USA. 2007;104(18):7652–7657. doi: 10.1073/pnas.0702225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sahara S, et al. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2007;2:10. doi: 10.1186/1749-8104-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zembrzycki A, et al. Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Dev. 2007;2:8. doi: 10.1186/1749-8104-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cholfin JA, Rubenstein JL. Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17, and Emx2. J Comp Neurol. 2008;509(2):144–155. doi: 10.1002/cne.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Scearce-Levie K, et al. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2008;7(3):344–354. doi: 10.1111/j.1601-183X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 185.Assimacopoulos S, et al. Fibroblast growth factor 8 organizes the neocortical area map and regulates sensory map topography. J Neurosci. 2012;32(21):7191–7201. doi: 10.1523/JNEUROSCI.0071-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Salas R, et al. Induction of chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) gene expression is mediated by ETS factor binding sites. Eur J Biochem. 2002;269(1):317–325. doi: 10.1046/j.0014-2956.2001.02655.x. [DOI] [PubMed] [Google Scholar]
- 187.Guillemot F, Zimmer C. From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron. 2011;71(4):574–588. doi: 10.1016/j.neuron.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 188.Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev Neurosci. 2007;8(8):583–596. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]
- 189.Rubenstein JL. Annual research review: development of the cerebral cortex: implications for neurodevelopmental disorders. J Child Psychol Psychiatry. 2011;52(4):339–355. doi: 10.1111/j.1469-7610.2010.02307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Faedo A, Borello U, Rubenstein JL. Repression of Fgf signaling by sprouty1–2 regulates cortical patterning in two distinct regions and times. J Neurosci. 2010;30(11):4015–4023. doi: 10.1523/JNEUROSCI.0307-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hasegawa H, et al. Laminar patterning in the developing neocortex by temporally coordinated fibroblast growth factor signaling. J Neurosci. 2004;24(40):8711–8719. doi: 10.1523/JNEUROSCI.3070-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tanibe M, et al. xCOUP-TF-B regulates xCyp26 transcription and modulates retinoic acid signaling for anterior neural patterning in Xenopus. Int J Dev Biol. 2012;56(4):239–244. doi: 10.1387/ijdb.113482mt. [DOI] [PubMed] [Google Scholar]
- 193.Richards LJ, Plachez C, Ren T. Mechanisms regulating the development of the corpus callosum and its agenesis in mouse and human. Clin Genet. 2004;66(4):276–289. doi: 10.1111/j.1399-0004.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 194.Liu Q, Dwyer ND, O’Leary DD. Differential expression of COUP-TFI, CHL1, and two novel genes in developing neocortex identified by differential display PCR. J Neurosci. 2000;20(20):7682–7690. doi: 10.1523/JNEUROSCI.20-20-07682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vaccarino FM, et al. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci. 1999;2(3):246–253. doi: 10.1038/6350. [DOI] [PubMed] [Google Scholar]
- 196.Raballo R, et al. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000;20(13):5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297(5580):365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 198.Sinor AD, Lillien L. Akt-1 expression level regulates CNS precursors. J Neurosci. 2004;24(39):8531–8541. doi: 10.1523/JNEUROSCI.1470-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jepsen K, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450(7168):415–419. doi: 10.1038/nature06270. [DOI] [PubMed] [Google Scholar]
- 200.Alfano C, Studer M (2012) Neocortical arealization: evolution, mechanisms and open questions. Dev Neurobiol. doi:10.1002/dneu.22067 [DOI] [PubMed]
- 201.Rakic P. Specification of cerebral cortical areas. Science. 1988;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 202.Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18(9):383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 203.Fishell G, Hanashima C. Pyramidal neurons grow up and change their mind. Neuron. 2008;57(3):333–338. doi: 10.1016/j.neuron.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 204.Arlotta P, et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45(2):207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 205.Zhang LJ, et al. A chicken ovalbumin upstream promoter transcription factor I (COUP-TFI) complex represses expression of the gene encoding tumor necrosis factor alpha-induced protein 8 (TNFAIP8) J Biol Chem. 2009;284(10):6156–6168. doi: 10.1074/jbc.M807713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Alcamo EA, et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57(3):364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 207.Britanova O, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57(3):378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 208.Halilagic A, et al. Retinoids control anterior and dorsal properties in the developing forebrain. Dev Biol. 2007;303(1):362–375. doi: 10.1016/j.ydbio.2006.11.021. [DOI] [PubMed] [Google Scholar]