Abstract
Calorie restriction extends longevity and delays ageing in model organisms and mammals, opposing the onset and progression of an array of age-related diseases. These beneficial effects also extend to the maintenance of brain cognitive functions at later age and to the prevention, at least in rodents, of brain senescence and associated neurodegenerative disorders. In recent years, the molecular mechanisms underlying brain response to calorie restriction have begun to be elucidated, revealing the unanticipated role of a number of key nutrient sensors and nutrient-triggered signaling cascades in the translation of metabolic cues into cellular and molecular events that ultimately lead to increased cell resistance to stress, enhanced synaptic plasticity, and improved cognitive performance. Of note, the brain’s role in CR also includes the activation of nutrient-sensitive hypothalamic circuitries and the implementation of neuroendocrine responses that impact the entire organism. The present review addresses emerging molecular themes in brain response to dietary restriction, and the implications of this knowledge for the understanding and the prevention of brain disorders associated with ageing and metabolic disease.
Keywords: Calorie restriction, Brain ageing, Nutrient sensing, CREB, Stem cell, Neurodegenerative disease
Ageing and calorie restriction
Ageing is a process characterized by the progressive deterioration of biological functions and the development of diseases (such as hypertension, obesity, cardiovascular disease, and cancer) induced by environmental insults and genetic factors [1]. Life expectancy in the Western world has significantly increased in the last century, but simultaneously the need is emerging for interventions aimed at preventing or reducing the incidence of ageing-related diseases. To this end, scientific research in the coming decades will have as the primary goal to unravel the fundamental mechanisms underlying the time-dependent decline of health and to provide new strategies to arrest or delay them.
One of the most reliable and experimentally validated models for delayed ageing in mammals is represented by calorie restriction (CR): solid experimental evidence demonstrates that reducing the calorie intake of an organism by 20–40 % (maintaining an adequate intake of vitamins and essential elements) has the effect of extending maximal lifespan and ameliorating “healthspan” (i.e., the period of life free of chronic diseases). Since the pioneer studies conducted in the middle 1930s by McCay and coworkers [2] showing that calorie restriction was able to prolong the lifespan of rats, numerous studies have confirmed this observation in different organisms throughout the phylogenetic scale [3, 4]. The mechanism by which caloric restriction operates in delaying ageing appears to be manifold: CR increases cell ability to repair DNA damage and induces anti-stress proteins [5], improves the efficiency of glucose metabolism [6], slows the age-related decline of the immune system [7]; above all, however, reduction of oxidative stress and modulation of the neuroendocrine system are believed to play a key role in the beneficial effects of dietary restriction [8, 9]. Indeed, calorie-restricted animals have reduced levels of circulating inflammatory cytokines [10], and display metabolic and hormonal adaptations (increased insulin sensitivity, reduced glucose and insulin in the blood, and elevated levels of cortisol) [11–13] having the overall effect of reducing the risk of several age-related diseases.
Different levels of brain response to CR
The “hormesis hypothesis” posits that calorie restriction creates a physiological stress of moderate intensity that would fortify the organism against insults of greater intensity [14] by activating signals of longevity and survival [15].
This fundamental, cell-autonomous mechanism affects nearly all cells and tissues of the body, and likely participates in the beneficial CR action on a wide range of chronic diseases, including diabetes, cancer, cardiovascular, autoimmune, and neurodegenerative diseases in rodents [16–19].
Some tissues, additionally, are also able to sense the metabolic status, change their biological function, and orchestrate the adaptation of the whole organism through the release of hormones, neurotransmitters, and cytokines (cell non-autonomous or humoral mechanism): this is in fact the case of the brain, which integrates signals in the hypothalamus and processes information regarding the availability of nutrients and energy stores in order to adapt behavioral and metabolic responses of the whole body [20].
Schematically, brain response to calorie restriction appears to occur at three distinct but highly integrated levels: (a) detection of nutrient availability and activation of the feeding and neuroendocrine response; (b) behavioral changes and improved higher-order (memory, learning) functions; (c) increased resistance to brain damage and to senescence-associated pathology.
Brain as a nutrient sensor and regulator of metabolism
Peripheral organs cross-talk with the central nervous system in order to maintain metabolic homeostasis: hormones and nutrients come into contact with the hypothalamus (in particular with specialized fuel-sensing neurons in the arcuate nucleus) through a characteristic discontinuity of the blood–brain barrier [21], and emerging evidence shows that specialized neurons sensitive to nutrients can detect changes in glucose and lipids in the blood.
Fluctuations in blood glucose modify the electrical activity of various populations of hypothalamic neurons [22]: some neurons are activated by high concentrations of glucose, while others are inhibited; in both cases changes in electric potentials are translated into chemical signals that control the feeding behavior [23]. Nutrient-sensitive cells of the arcuate nucleus (ARC), the most studied and best characterized, belong to two functionally opposite populations: neurons expressing the peptide Agouti/neuropeptide Y (NPY and AgRP) and cells expressing proopiomelanocortin (POMC) and cocaine and amphetamine-related transcript (CART). The latter have anorexigenic action, reducing food intake and increasing energy expense [24, 25], and are activated by high concentrations of glucose; AgRP and NPY neurons, instead, are stimulated by hypoglycemia and have orexigenic action [26]. Accordingly, the infusion of glucose in the rodent hypothalamus induces satiety and loss of weight [27] while its deprivation, experimentally obtained by administration of 2-deoxy-glucose (a chemical analogue that blocks glucose metabolism), elicits food intake [28, 29]: importantly, several brainstem areas characterized by adult neurogenesis, such as the nucleus of the solitary tract (NTS) and dorsal motor nucleus of the vagus (DNMV), are extremely sensitive to minor changes of glycemia and can regulate food seeking through axonal projections to hypothalamic neurons [30].
As the efferent branch of this circuitry, the brain communicates through sympathetic neurons with peripheral organs such as brown adipose tissue and liver in order to maintain glucose homeostasis: in particular, sympathetic outflow directly activates thermogenesis in brown fat [31] and blocks hepatic gluconeogenesis [32].
Importantly, glucose is not the only nutrient monitored by the brain, and recent evidence shows that fatty acids also inform the hypothalamus of the metabolic state of the organism and regulate through lipids-sensitive neurons, food intake, hepatic synthesis of glucose, and insulin secretion [33]. Of note, glucose and lipid sensing are highly integrated in the central nervous system: in fact, hyperglycemia leads to an increase in intracellular malonyl-CoA, which inhibits the beta-oxidation of fats and in parallel promotes glucose oxidation [34]; conversely, a reduction of blood glucose results in the inhibition of the synthesis of malonyl-CoA and induction of fatty acid beta-oxidation [35]. The latter effect is largely mediated by the AMP-activated serine threonine kinase AMPK, an evolutionarily conserved nutrient sensor that monitors cellular energy status as reported by the ratio between AMP and ATP. AMPK phosphorylates and inhibits Acetyl-CoA carboxylase (ACC), thus reducing the biosynthesis of malonyl-CoA [35], in the context of a general metabolic cell reprogramming whereby catabolic reactions are favored at expenses of anabolic processes, to cope with nutrient shortage. In the hypothalamus in particular, AMPK sensitivity to energy fluctuations qualify this molecule as a global nutrient detector and key central regulator of the feeding behavior.
As a further layer of complexity, nutrient sensing by the hypothalamic neurons AgRP/NPY and POMC is also modulated by hormones such as insulin and leptin [36]. Central infusion of insulin in mice reduces appetite [37] and body weight [38] and inhibits hepatic gluconeogenesis [39]; this action is mediated by the activation of PI3 kinase (PI3 K) and the regulation of an ATP-sensitive potassium channel (K ATP), which translates nutrient signals into neuronal excitability [40].
Similarly, leptin (an adipokine produced by fat cells) acts as anorexigenic by modulating in the hypothalamus the activity of AMPK [41, 42].
Thus, AMPK seems to represent a point of convergence for both nutritional and hormonal signals that modulates the excitability of hypothalamic neurons. Although experiments conducted on murine models lacking AMPK only in AgRP and POMC cells have highlighted a more important role of for this enzyme in glucose sensing than in insulin/leptin signaling in the hypothalamus [43], current literature overall identifies this enzyme as a main nutrient sensor in neuronal cells, and by extension as a key molecular player in brain response to dietary restriction [44]. This idea is further supported by the functional linkage of AMPK with the nutrient-sensitive cascade triggered by the mammalian target of rapamycin (mTOR), another evolutionary conserved pathway that integrates inputs from nutrients (amino acids) and growth factors (insulin) to promote protein synthesis and cell growth/proliferation, and is inhibited by AMPK under nutrient shortage. In particular, recent data have revealed an important role of mTOR signal in the control of feeding behavior in the hypothalamus, as well as in promoting protein synthesis required for the “long-term potentiation” and memory formation in the hippocampus [45]. Thus, the AMPK-mTOR axis may be central not only to neuronal nutrient sensing in the hypothalamus but also for higher levels of brain adaptation to nutrient availability.
Similarly, another nutrient sensor and longevity determinant, the NAD+-dependent protein deacetylase Sirt1, has been shown to participate in the hypothalamic control of energy balance and feeding behavior. This enzyme, initially identified as the closest mammalian homolog of the yeast Sir2 longevity gene, regulates at gene transcription level cell response to metabolic and environmental stress, and is believed to mediate at least some of the beneficial effects of calorie restriction on age-related diseases in rodents and humans [46]. Consistent with its role as a detector of nutrient shortage, Sirt1 deacetylase activity is induced by an elevation of the intracellular NAD+/NADH ratio and by a reductive-to-oxidative shift in the intracellular redox balance as determined by limited nutrient availability. Accordingly, Sirt1 releases orexigenic signals in AgRP neurons, and its inhibition in this cell population leads to reduced appetite, negative energy balance and weight loss in mice [47]. On the other hand, Sirt1 genetic ablation from anorexigenic POMC neurons [48] or SF-1 (Steroidogenic Factor 1) neurons [49] impairs sympathetic outflow and adaptive energy expenditure without major effects on food intake, and exacerbates obesity and leptin resistance in mice fed a high-calorie (HC) diet. This partially conflicting evidence fully underscores the complexity of Sirt1 roles in hypothalamic circuitries regulating body metabolism. Importantly, as it is a recurrent theme in brain nutrient sensing, Sirt1’s neural roles are not limited to the regulation of feeding and energy balance, but also connect nutrient detection with higher-order functions including endocrine and behavioral response to CR [50], neuroprotection, and neural plasticity [51].
Not just hunger: behavioral and cognitive responses to nutrient restriction
Beside their important effect on appetite, nutrient restriction and the ensuing hormonal modifications are associated in several experimental models with cognitive and behavioral changes [52]. Rodents subjected to a calorie-restricted regimen display locomotor hyperactivity [53] and reduced mood alterations such as anxiety or depression [54], changes that are at least in part mediated by the action of caloric restriction on the hypothalamic orexigenic system [55], and that likely reflect increased awakeness and motivation related to food-seeking. Additionally, there is extensive evidence demonstrating that in rodents a reduced dietary regimen improves memory and learning [56, 57], in particular in murine models of neurodegenerative diseases [58, 59] or brain injury [60]. Interestingly, some of these effects may be sex-specific [61], and occur independently of CR action on the ARC neurons [62]. On the other hand, many data correlate with an increase in synaptic function in the hippocampus of animals subjected to a restricted diet: dietary restriction modulates the expression and distribution of NMDA and AMPA receptor subunits [63, 64] and prevents the age-dependent decline of synaptic plasticity [65].
Cognitive improvement by food restriction also seems to occur in human beings, based on the results of the CALERIE (Comprehensive Assessment of the Long-term Effect of Reducing Intake of Energy) study, performed on non-obese healthy subjects. These studies have revealed that also in humans a 20–30 % caloric restriction for at least 6 months increases specific markers of longevity (body temperature, concentration of glucose, insulin and lipoproteins in the blood), improves physical performances and, most relevant to our topic, reduces symptoms associated with eating or mood disorders including depression [66]. On the other hand, the effect of calorie restriction on cognitive performances in humans is still controversial [67, 68], probably due to differences in experimental group composition, dietary regimen, and cognitive assessment throughout the different studies.
Increased resistance to brain damage and senescence-associated pathology
Despite the existence of areas delegated to adult neurogenesis, the brain is mainly populated by terminally differentiated neurons, permanent cells that accumulate oxidative damage to a greater extent than the other organs [69].
DNA [70] and protein [71] damage occurs during ageing in a fashion that is amplified and/or anticipated in neurodegenerative diseases such as Alzheimer’s, Huntington’s, and Parkinson’s[72–74]. In addition, a depletion of neurotrophic factors is involved in the deterioration of cognitive functions observed in physiological and especially in pathological ageing [75].
Experimental evidence demonstrates that calorie restriction has significant effects on brain resistance to chronic, age-related injury. The beneficial effects of a dietary regimen are, at least in part, the result of a stress response that stimulates the expression of molecules offering resistance to oxidative and metabolic damage (see below, “cellular and molecular mechanisms”) [76]. Documented neuroprotective actions exerted by CR and relevant to brain ageing [77] include the reduced formation of Aß oligomers and plaques in murine models of Alzheimer’s disease [78], the attenuation of age-associated neuroinflammation [79], and the suppression of oxidative markers (lipid peroxidation, protein carbonyls, and nitrotyrosine) of neuronal senescence [80].
Moreover, besides the positive effects on chronic brain damage, dietary restriction also seems to improve neuronal survival and recovery in the context of acute damaging events, like seizures and ischemic stroke [81]; importantly, these events trigger pathogenic responses (excitotoxicity, mitochondrial and ER damage, oxidative stress and inflammation) that largely overlap, although with a different kinetic, those operating in brain ageing, thus suggesting a common mechanism of protection by CR from acute and chronic neuronal insults [82].
Cognitive enhancement and neuroprotection by CR: cellular and molecular mechanisms
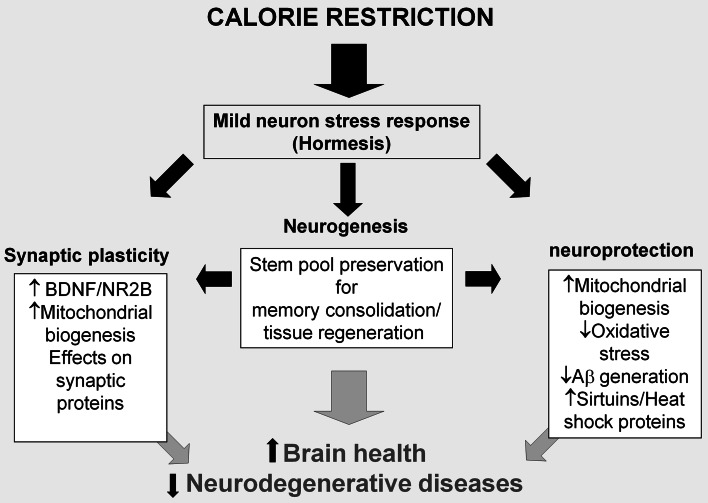
While the molecular mechanisms involved in the improvement of cognitive functions by caloric restriction are still largely elusive, most researchers agree on the role of improved neurogenesis, increased synaptic plasticity, and activation of stress-resistance signaling pathways as key cellular events at the base of the enhancement of the brain health by a calorie-restricted diet [83] (Fig. 1).
Fig. 1.
How calorie restriction prevents brain ageing. Improved neurogenesis, increased synaptic plasticity, and neuroprotection are the basis for the enhancement of brain health by a calorie-restricted diet. A nutrient-restricted regimen induces a mild stress able to modulate the expression of key molecules for both neuron activity as well as for the resistance to stronger stress that can induce nervous system damage. Moreover, dietary restriction preserves the neural stem cells pool, that contributes to formation of new neural circuits during memory consolidation, and attenuates age-dependent functional decline and neurodegeneration. Calorie restriction, through these cellular mechanisms, extends mindspan and prevents neurodegenerative diseases
CR and neurogenesis
The central nervous system contains neural precursor cells (NPCs) [84] that can proliferate and differentiate into new neurons and glial cells in adulthood: these newly formed elements are believed to be very important in learning and memory consolidation [85], as well as in tissue repair after a cerebral damage [86]. Importantly, animals fed a restricted diet exhibit increased neurogenesis in the dentate gyrus of the hippocampus in young age [87] and an attenuation of the age-related reduction of the stem cell pool [86]. Growth factors induced by caloric restriction [88], such as BDNF [89], may at least partly explain the trophic action of nutrient deprivation on the stem cell compartment in the central nervous system; in addition, the reduced availability of nutrients as monitored by a number of nutrient-sensitive cascades may directly exert beneficial effects on neuronal stem cell capacity to self-renew and differentiate.
Metabolic regulation of stem cell functions is an emerging theme in cell biology, at the crossroad of nutrition, ageing research, and cancer [90]. In particular, deregulated signaling through the mTOR/S6 kinase cascade, as induced by either genetic defects or excess nutrients, appears to promote mitogenic stimulation and rapid exhaustion of stem cells as diverse as hemopoietic stem cells (HSCs) [91–93] and epidermal stem cells [94, 95], leading to premature tissue ageing. Conversely, a blockade of this cascade by the specific inhibitor Rapamycin, or detoxification of harmful reactive oxygen species generated in mitochondria as a consequence of Tor-driven hypermetabolism, delays stem cell senescence and extends tissue regenerative capacity, anticipating a similar beneficial effect also from nutrient restriction. Accordingly, calorie restriction has been found to increase, through non-cell autonomous mechanisms involving soluble factors secreted by ancillary Paneth’s cells, the number and function of intestinal stem cells (ISC), as well as intestine regeneration following a severe inflammatory insult [96].
Neuronal stem cells may as well be subdued to nutrient and Tor-dependent metabolic regulation; by analyzing mice genetically deficient of the tuberous sclerosis complex protein Tsc1 (an mTOR inhibitor), Magri and colleagues have in fact observed that sustained activation of the mTOR pathway in embryonic neural stem cells leads to reduced self-renewal and earlier neuronal and astroglial differentiation of mutant NSC, resulting in impaired brain development [97].
The FoxO family of transcription factors, another class of nutrient- and insulin-regulated molecules involved in longevity determination and resistance to oxidative stress in model organisms [98], also appears to contribute to metabolic modulation of neural stem cell function. Similar to Tor-hyperstimulated NSC, in fact, FoxO3-deficient progenitor cells undergo unchecked proliferation and rapid and premature exhaustion both in vivo and in vitro [99]. FoxOs are fasting-responsive factors and are inhibited by insulin under nutrient replenishment; thus, absence of FoxOs mimics insulin signaling and accelerates stem cell senescence. Collectively, the above evidence suggests that calorie restriction, by reducing plasma insulin and restraining insulin and nutrient-activated signaling cascades in neuronal stem/progenitor cells, may preserve their number and functional capacity against metabolic attrition, thus delaying brain ageing [100].
In line with this view is also evidence linking stem cell renewal and differentiation in the SNC with the NAD+-dependent protein deacetylase and longevity factor Sirt1 [46]. As an epigenetic regulator, Sirt1 has been shown to promote neural progenitor cell (NPC) differentiation into neurons through the transcriptional repression of the Hes-1 gene, thus promoting neurogenesis under basal (unstressed) conditions [101]. Interestingly, Sirt1 seems to instead sustain glial differentiation of NPC under conditions of oxidative stress [102], indicating a context-dependent effect of the deacetylase on neural progenitor fate.
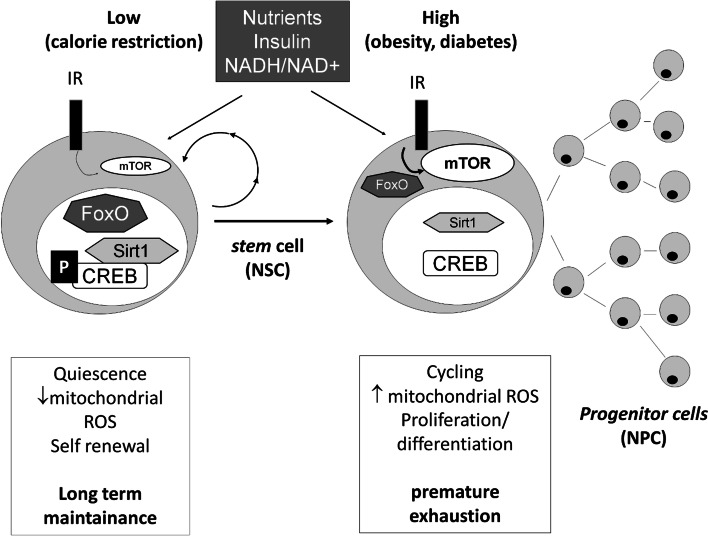
Together, this and the above evidence support the idea that nutrient-dependent metabolic control of stem cell fate may actively contribute to the complex brain changes related to feeding, both under healthy (calorie restriction) and pathologic (overnutrition/dysmetabolism) regimens (Fig. 2).
Fig. 2.
Metabolic regulation of stem cell fate by nutrients and insulin. Restricted nutrient availability, as under calorie restriction, reduces insulin and mTOR signaling and promotes “fasting-mode” responses by activating FoxO (by nuclear translocation) Sirt1 (by increasing the NAD+/NADH ratio) and CREB (by promoting its phosphorylation and association with Sirt1); this results in stem cell quiescence state with low oxidative burden and extended self-renewal. Conversely, chronic activation of the “feeding response” as in obesity and diabetes (high insulin and mTOR signaling, inhibition of FoxO, inactivation of Sirt1 and CREB) drives stem cells from quiescence to cycling, increases oxidative burden, and promotes stem cell proliferation and maturation at the expense of self-renewal, leading to premature exhaustion and tissue ageing. The scheme refers to a general stem cell model that also applies to neural stem cells. The possible involvement of CREB and Sirt1 in NSC self-renewal is still to be fully validated and is presented here as largely hypothetical
CR and cell protective responses
Mitochondrial biogenesis
Effects of calorie restriction on mitochondrial biogenesis and activity in the brain as well as in other organs has recently come under the spotlight as a general cell protective and anti-ageing mechanism [103]. During ageing, a deterioration of mitochondrial activity occurs, with increased formation of damaging free radical species [104] and alterations of mitochondrial respiratory chain being a common finding in several experimental models of neurodegenerative diseases [105]. Dysfunctional mitochondria and increased ROS burden may also link ageing to neuroinflammation, another hallmark of brain senescence that is ameliorated by CR [106].
A major mechanism for CR-dependent effects on mitochondria number and health has been identified in the induction of the transcription factor PGC-1 (coactivator of PPARγ) [107], a master controller of cellular respiratory function. Upregulation of neuronal PGC-1 by dietary restriction may have nitric oxide as an intermediate [108] and occurs through a humoral mechanism, since treatment of cultured neurons with serum of calorie restriction animals induces the expression of nitric oxide synthetases (eNOS and nNOS) and increases mitochondrial mass and cell survival in a fashion that can be recapitulated by the cell exposure to NO donors [109].
Importantly, PGC-1 is deacetylated and activated in several organs by the nutrient-sensitive deacetylase Sirt1 [110], a molecule endowed of well-established neuroprotective activities (see below); moreover, PGC-1 deletion in mice causes striatal degeneration [111] while its overexpression inhibits oxidative stress [112] and reduces the neurodegenerative changes in a model of Huntington disease [113].
Neurotrophins
As an additional neuroprotective mechanism, restricted dietary regimens (caloric restriction or intermittent fasting) also promote the synthesis of neurotrophins. Neurotrophins are neuronal trophic factors that enhance neuronal survival and stress resistance [114] and whose critical decrease during ageing and in age-related brain disorders has been largely established [115]. It is in fact conceivable that some of the described neuroprotective effects of CR [116] reflect, at least in part, a preserved and heightened action of these neuroprotective factors.
Sirt1
Converging lines of evidence also point to Sirtuin 1 as a key determinant of brain health and extended youthfulness in response to CR [117]: in fact, Sirt1 overexpression prevents the accumulation of beta amyloid in neurons [118, 119] and its pharmacological activation by the administration of resveratrol reduced neurodegeneration in murine models of Alzheimer’s disease and lateral amyotrophic sclerosis [120, 121]. Additionally, SIRT1 plays a neuroprotective role in models of Huntington’s and Parkinson disease [122–124].
SIRT1 operates in the cell by modulating the activity of several transcription factors, like FOXO1, TORC/CREB, NF-κB, and PGC-1 alpha, which in turn regulate cell metabolism stress resistance and inflammation [50, 110, 121–125]. Since these factors are also sensitive to neurotrophins and, at least in part, intrinsically responsive to nutrients and regulated by calorie restriction, Sirt1 appears to lay at the hub of the complex molecular network deputed to cell genetic reprogramming and stress adaptation induced by the reduction of nutrient availability. Accordingly, Sirt1 has been found necessary for brain response to CR, and mice lacking sirtuin 1 in the brain subjected to a restricted calorie regimen do not show the improvement in insulin sensitivity and the increase in locomotor activity observed in control animals [50].
mTOR/autophagy
Important evidence identifies (in signaling through the mTOR cascade) another main nutrient-regulated pathway involved in neurodegeneration and targeted by CR. Recent data link increased mTOR signaling to the accumulation of Abeta and Tau protein in neurons and to a worsening of Alzheimer’s pathology; in a possible model, mTOR may exacerbate protein misfolding and aggregation through both induction of endoplasmic reticulum (ER) overload and stress, and inhibition of autophagy [126], a self-eating cell process whereby cytosolic protein aggregates and damaged organelles are engulfed in double-membrane vacuoles and targeted to lysosomal degradation [127]; moreover, these detrimental effects on proteostasis would add to the above-mentioned mTOR-dependent impairment of NPC-driven neurogenesis. Accordingly, mTOR blockade by the specific inhibitor Rapamycin attenuates cognitive deficits in mouse models of AD disease [128]. Interestingly, rapamycin has been shown to mimic several other beneficial effects of calorie restriction in mice, including remarkably extended longevity [129]. In this regard, great expectations for the treatment of neurodegenerative diseases are pinned in the development of drug mimetics of caloric restriction: for instance animals treated with 2-deoxy-glucose (not metabolizable analog of glucose) show increased resistance to neuronal damage in experimental models of Alzheimer’s and Parkinson disease [130, 131].
Along similar lines, much attention has been paid in recent years to resveratrol and other sirtuin activators: these drugs have been to reduce beta amyloid neurotoxicity [132] at least in part by inducing mitochondrial biogenesis and activating AMPK [133], but some of the effects do not seem to involve sirtuins and a complete understanding of the mechanisms underlying their activity has yet to be reached [134].
CR and synaptic plasticity
The earliest and more debilitating sign of age-related neurodegenerative diseases is the diminished ability to retain new information: synaptic plasticity is the mechanism that underlies memory formation and it requires a proper synaptic transmission and adequate synaptogenesis (in addition to the above-mentioned hippocampal neurogenesis) [135].
In particular, the hippocampus is one of the most important brain areas involved in learning and novelty acquisition [136].
Ageing alters the expression of genes involved in synaptic transmission (such as the receptor of neurotrophins Trk-B, NR1 subunit of NMDA receptor, BDNF) while the caloric restriction counteracts this effect [137].
In addition, a restricted-calorie regimen induces the expression of BDNF and NR2B subunits of NMDA glutamate receptor [63] and prevents their time-dependent decline in the hippocampus [138, 139]: these actions promote synaptic plasticity and an improvement of cognitive function assessed by hippocampus dependent memory tasks. In addition, NMDA receptors are required for the activation of the hypothalamic AgRP neurons by fasting [140]: synaptogenesis induced by caloric restriction in these cells together with glutamatergic receptors are therefore essential for the hypothalamic response to fasting.
Of note, mitochondria play a key role in neuronal synaptic plasticity [141]: several studies have in fact shown that a tetanic stimulation triggers mitochondria to the synapse [142] and that the damage of these organelles impairs processes such learning and memory consolidation [143].
Interestingly, also nitric oxide has been involved in synapse formation in the hippocampus [144], and this gaseous mediator is increased in the brain by calorie restriction and promotes mitochondrial biogenesis.
Thus, maintenance of synaptic plasticity and resistance to neurodegeneration may both occur as a beneficial consequence of CR-dependent and NO-mediated increase in cell respiratory capacity and mitochondrial number and function; consistent with this idea, mice deficient of Sirt1 in the hippocampus display deficits in synaptic plasticity and memory [51], in parallel with impaired upregulation of nNOS [125].
CREB, a new player in the hungry brain
CREB in central nutrient sensing
The CREB (cAMP-responsive element binding) protein, an ubiquitous transcription factor exquisitely sensitive to cAMP and Ca+2 signals triggered by an array of hormones and growth factors [145, 146], has been extensively investigated both as a regulator of fasting-induced metabolic gene programs in liver and other peripheral tissues [147, 148], as well as a key neuroprotective factor involved in neuronal differentiation, survival, and plasticity in response to neurotrophic peptides [149]. In recent years, a role for this molecule as a nutrient and metabolic sensor in the hypothalamic areas deputed to the central regulation of appetite and energy expenditure [150] has also been revealed; in particular, CREB phosphorylation and increased transcriptional activity has been observed during fasting in neurons of the arcuate nucleus (ARC) that release the orexigenic peptide NPY [151]; accordingly, CREB activity was blocked by leptin [152], a fat-derived hormone that acts centrally by signaling satiety and increased energy expenditure. Similarly, VGF, an established CREB target gene, was induced during fasting [151] and repressed by leptin in the same hypothalamic area [152]; of note, VGF has a primary role in regulating feeding behavior and energy balance [153], and VGF genetic ablation prevents obesity in mice [154]. These observations suggest that CREB, while coordinating the metabolic adaptation to fasting (gluconeogenesis, lipolysis) in the periphery [155], may also participate in the central feeding response to starvation through the up-regulation of orexigenic neuropeptides.
In partial contradiction with this view, Altareyos and Montminy reported that the CREB co-factor Crtc1/TORC1 is phosphorylated and excluded from the nucleus (thus failing to co-activate CREB) in the hypothalamic arcuate nucleus of fasting mice, while it becomes dephosphorylated and promotes CREB-dependent transcription of the anorexigenic factors CART (cocaine and amphetamine-related transcript) and KISS1 in response to leptin [156]. Importantly, Crtc1 −/− mice are hyperphagic and obese, display low energy expenditure, and are infertile, confirming the relevance of the Crtc1/CREB axis to the maintenance of normal energy balance.
The bulk of the above information suggests that CREB affects central nutrient sensing and metabolic adaptation in response to fasting and feeding stimuli are complex and multifaceted likely depending on the hypothalamic neuronal population involved, the transcriptional co-activators CREB engages with, and the normal or pathologic (obesity/hyperinsulinemia/hyperleptinemia) context in which these feeding circuitries operate. Further research aimed at evaluating these diverse possibilities is therefore warranted.
CREB, CR, and higher brain functions
The direct involvement in central nutrient sensing combined with the established role in the development and maintenance of neuronal function and plasticity [157, 158], and in the pathogenesis of age-related neurodegenerative diseases [159], identify in CREB a key molecular player in brain response to calorie restriction. Accordingly, initial studies on Drosophila showed that mutant flies lacking the CREB co-activator TORC are exaggeratedly sensitive to starvation, and that starvation tolerance can be restored in those mutants by rescuing TORC expression exclusively in neuronal cells [160]. Moreover, in C. Elegans, a mutation phenocopying dietary restriction prevents the age-dependent decline of CRH-1, the worm’s homolog of CREB, and to preserve in parallel cognitive function (long-term memory) [161]. Surprisingly, however, worms lacking TORC or CRH-1 are long-lived, and dietary restriction extends lifespan through the AMPK-dependent phosphorylation of TORC and downregulation of TORC/CRH-1 activity [162]. A finding also at odds with the fact that insulin, whose signaling activity has an established negative effect on worm lifespan [163], also inhibits CREB activity and CREB-dependent stress responses [160]. While the above incongruences may be due to differences in experimental manipulations or reflect the complexity of the phenotypes under analysis as well as the peculiarity of the C. elegans model system, these findings are overall suggestive of an intriguing linkage between CREB signaling and dietary restriction effects on longevity and neuronal functions in model organisms.
We have taken advantage of the conditional CREB KO mouse strain to investigate the role of brain CREB in mammalian response to calorie restriction [125]. In this model, CREB1 gene inactivation occurs at adult age in neuronal cells of most of the forebrain areas, as well as in some lower areas including the hypothalamus.
Brain CREB KO (BCKO) mice, although phenotypically normal, failed to display the cognitive and behavioral improvement observed in control animals subdued to a 5-week calorie-restriction regimen. This remarkable finding was paralleled by electrophysiological evidence of no LTP enhancement in mutant mice, and by impaired hippocampal up-regulation of neuronal genes critical for stress resistance (PGC-1) and plasticity (nNOS); importantly, Sirt1, another longevity-related molecule involved in brain response to calorie restriction, was identified as a critical CREB target gene modulated by nutrients in cognitive (cortex, hippocampus) brain areas. This study thus revealed an important role for CREB in the molecular cascade linking nutrient availability to brain metabolic adaptation and to changes in brain plasticity and high-order functions.
CREB, CR, and neuroprotection
Along similar lines of evidence, a role for CREB in the neuroprotective action of CR against age-associated neurodegenerative disorders was demonstrated by Krainc and colleagues, who recently reported that Sirt1 mediates neuroprotection from mutant Huntingtin by activation of the TORC1 and CREB transcriptional pathway [164]. Importantly, this study also provided direct evidence that Sirt1 activates CREB by deacetylation of the co-activator TORC1, indicating a potential cell autonomous molecular switch for nutrient and metabolic sensing by brain CREB. It is however likely that this is not the only mechanism whereby brain CREB “senses” calorie restriction, and other potential signaling cascades, including those triggered by paracrine and humoral factors, are currently under active investigation.
Conclusions and perspectives
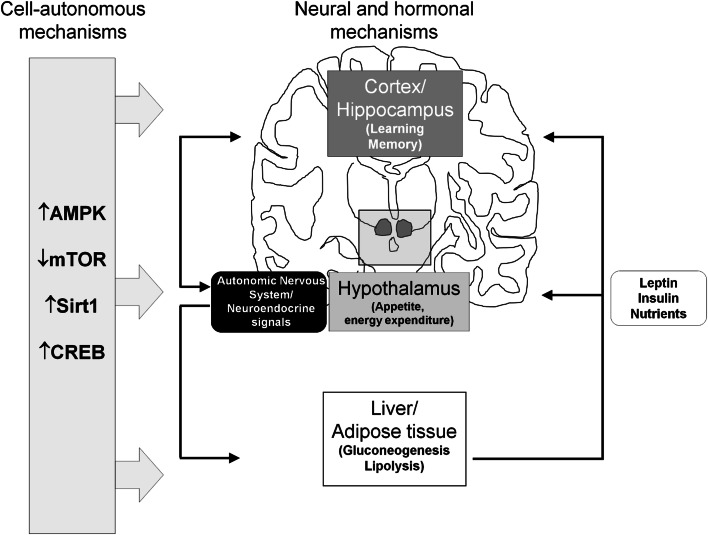
The involvement of several nutrient-sensitive molecules including CREB, Sirt1, AMPK, and mTOR in brain areas and neuronal circuitries as diverse as those underlying feeding behavior and learning/memory processes, suggest an integrated view for brain-centered control of organismal metabolism, whereby regulation of appetite and energy expenditure in the hypothalamus are tightly coupled with high-order cognitive and behavioral responses (alertness, increased physical activity) based in the forebrain and conceivably related, in a finalistic perspective, to food seeking. On the other hand, hypothalamic centers also control (via neuroendocrine signals and autonomic nervous outflows) metabolic responses in the periphery, impinging on the same biochemical cascades that operate in the SNC. In the case of CREB, for instance, cell autonomous (Sirt1-mediated?) activation by restricted nutrients could simultaneously lead to increased appetite in the hypothalamus, enhanced cognition in the forebrain, and fasting adaptation (gluconeogenesis, lipolysis) in the peripheral organs, with neuroendocrine/endocrine (GH, insulin, glucagon, leptin) as well as electrical signals further coordinating, in a cell non-autonomous fashion, the three levels of response (Fig. 3). Thus, in general terms, brain represents not only an important target but most likely also a major effector and regulator of the whole body adaptation and healthy response to CR.
Fig. 3.
Brain-centered control of organismal metabolism. Calorie restriction regulates nutrient-sensitive molecules including CREB, Sirt1, AMPK and mTOR in several tissues including brain (cell autonomous mechanism), thus promoting central behavioral adaptations (food seeking, appetite, alertness) and peripheral metabolic modifications (gluconeogenesis, lipolysis). In parallel, brain integrates nutrient-related cues and coordinates, through neuroendocrine and autonomic signals, organismal response to fasting (non cell-autonomous mechanism)
It is also intriguing to notice that nutrient sensors like CREB, AMPK, and Sirt1 have also been linked to the regulation of central and peripheral circadian rhythms [165], and that these oscillators are highly coordinated with each other and by nutrient availability [166, 167]. In this respect, it is known that calorie restriction switches circadian regulation from the light–dark to the fasting-feeding cycle likely by impinging on neurons of the hypothalamic suprachiasmatic nucleus where the hypothalamic biological clock resides; importantly, this central oscillator plays an important role in the process of memory consolidation [168], and alterations of the circuits that regulate circadian rhythms are involved in the pathogenesis of mood disorders [169]. It is therefore possible that brain and body response to calorie restriction reflect, at least in part, the global metabolic reprogramming of the central biological clock. Future research will tell whether this fascinating hypothesis holds, at least in part, true.
Finally, in a reductionistic perspective, calorie restriction could simply be viewed as an experimental approach to investigate the feeding-brain connection. With this respect, it should be kept in mind that laboratory rodents fed ad libitum without possibility or need for physical activity, accumulate fat and become obese in adulthood, thus dramatically resembling a human “Westernized” lifestyle. Is experimental “calorie restriction” simply a sort of normalization of an animal’s feeding behavior? If so, it is conceivable that investigation on CR mechanisms will help us to understand and prevent human disorders (including brain ageing and Alzheimer’s disease) [170, 171] that are induced and/or accelerated by obesity and diabetes.
This perspective should further stimulate the already intense research in the field, by encouraging, for instance, the application of the CR experimental paradigm to mutant murine strains harboring brain specific mutations in nutrient-sensitive pathways, in order to score for diet-related changes in brain functions. Optimism is however warranted, as we keep on learning more and more about the reasons why the way we eat changes the way we think, and vice versa.
Acknowledgments
The authors apologize to all the colleagues whose important work could not be properly cited in this review due to space constraints. The authors are indebted to professors Claudio Grassi (Institute of Human Physiology, Catholic University Medical School), and Achille Cittadini (Institute of General Pathology, Catholic University Medical School) and to members of the laboratory for their helpful comments and suggestions. Original work from the authors’ laboratory was funded by Catholic University Intramural Grants (linea D1 and linea D3.2) and by the Italian Ministry of University and Research (MIUR, ex 60 %).
References
- 1.Fontana L. The scientific basis of caloric restriction leading to longer life. Curr Opin Gastroenterol. 2009;25:144–150. doi: 10.1097/MOG.0b013e32831ef1ba. [DOI] [PubMed] [Google Scholar]
- 2.McCay CM, Crowel MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 3.Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield: Charles C Thomas Publisher; 1988. [Google Scholar]
- 4.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Weraarchakul N, Strong R, Wood WG, Richardson A. Effect of aging and dietary restriction on DNA repair. Exp Cell Res. 1989;181:197–204. doi: 10.1016/0014-4827(89)90193-6. [DOI] [PubMed] [Google Scholar]
- 6.Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordr) 2010;32(1):97–108. doi: 10.1007/s11357-009-9118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93(1–3):87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- 8.Pani G. P66SHC and ageing: ROS and TOR? Aging (Albany NY) 2010;8:514–518. doi: 10.18632/aging.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redman LM, Ravussin E. Endocrine alterations in response to calorie restriction in humans. Mol Cell Endocrinol. 2009;299(1):129–136. doi: 10.1016/j.mce.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuzaki J, Kuwamura M, Yamaji R, et al. Inflammatory responses to lipopolysaccharide are suppressed in 40% energy-restricted mice. J Nutr. 2001;131:2139–2144. doi: 10.1093/jn/131.8.2139. [DOI] [PubMed] [Google Scholar]
- 11.Barzilai N, Banerjee S, Hawkins M, et al. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest. 1998;101:1353–1361. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Luo L, Liu J, et al. Aging and caloric restriction: effects on Leydig cell steroidogenesis. Exp Gerontol. 2005;40:498–505. doi: 10.1016/j.exger.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Sabatino F, Masoro EJ, McMahan CA, Kuhn RW. Assessment of the role of the glucocorticoid system in aging processes and in the action of food restriction. J Gerontol. 1991;46:B171–B179. doi: 10.1093/geronj/46.5.b171. [DOI] [PubMed] [Google Scholar]
- 14.Masoro EJ. The role of hormesis in life extension by dietary restriction. Interdiscip Top Gerontol. 2007;35:1–17. doi: 10.1159/000096552. [DOI] [PubMed] [Google Scholar]
- 15.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285(5432):1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 16.Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7(1):43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hursting SD, Lavigne JA, Berrigan D, et al. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 18.Guo Z, Mitchell-Raymundo F, Yang H, et al. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein E-deficient mice. Mech Ageing Dev. 2002;123:1121–1131. doi: 10.1016/s0047-6374(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Piccio L, Stark JL, Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol. 2008;84:940–948. doi: 10.1189/jlb.0208133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan SD, Konner AC, Bruning JC. Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis. Cell Mol Life Sci. 2010;67:3255–3273. doi: 10.1007/s00018-010-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banks WA. Blood–brain barrier and energy balance. Obesity (Silver Spring) 2006;14(Suppl 5):234S–237S. doi: 10.1038/oby.2006.315. [DOI] [PubMed] [Google Scholar]
- 22.Oomura Y, Yoshimatsu H. Neural network of glucose monitoring system. J Auton Nerv Syst. 1984;10:359–372. doi: 10.1016/0165-1838(84)90033-x. [DOI] [PubMed] [Google Scholar]
- 23.Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- 24.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 25.Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, Wraight V, Sims M, Vatin V, Meyre D, Shield J, Burren C, Ibrahim Z, Cheetham T, Swift P, Blackwood A, Hung CC, Wareham NJ, Froguel P, Millhauser GL, O’Rahilly S, Farooqi IS. A POMC variant implicates beta-melanocyte stimulating hormone in the control of human energy balance. Cell Metab. 2006;3:135–140. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Bruning JC. Agouti-related peptide expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 27.Davis JD, Wirtshafter D, Asin KE, Brief D. Sustained intracerebroventricular infusion of brain fuels reduces body weight and food intake in rats. Science. 1981;212:81–83. doi: 10.1126/science.7193909. [DOI] [PubMed] [Google Scholar]
- 28.Berthoud HR, Mogenson GJ. Ingestive behavior after intracerebral and intracerebroventricular infusions of glucose and 2-deoxy-d-glucose. Am J Physiol. 1977;233:R127–R133. doi: 10.1152/ajpregu.1977.233.3.R127. [DOI] [PubMed] [Google Scholar]
- 29.Miselis RR, Epstein AN. Feeding induced by intracerebroventricular 2-deoxy-d-glucose in the rat. Am J Physiol. 1975;229:1438–1447. doi: 10.1152/ajplegacy.1975.229.5.1438. [DOI] [PubMed] [Google Scholar]
- 30.Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience. 1978;3:207–218. doi: 10.1016/0306-4522(78)90102-1. [DOI] [PubMed] [Google Scholar]
- 31.Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–947. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi T, Bray GA. The effect of intrahypothalamic injections of glucose on sympathetic efferent firing rate. Brain Res Bull. 1987;18:591–595. doi: 10.1016/0361-9230(87)90128-6. [DOI] [PubMed] [Google Scholar]
- 33.Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci. 2005;8(5):579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 34.Wolfgang MJ, Cha SH, Sidhaye A, Chohnan S, Cline G, Shulman GI, Lane MD. Regulation of hypothalamic malonyl-CoA by central glucose and leptin. Proc Natl Acad Sci USA. 2007;104(49):19285–19290. doi: 10.1073/pnas.0709778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol. 2006;574(Pt 1):73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belgardt BF, Okamura T, Bruning JC. Hormone and glucose signalling in POMC and AgRP neurons. J Physiol. 2009;587:5305–5314. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 38.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 39.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Ashcroft FM, Gribble FM. ATP-sensitive K? Channels and insulin secretion: their role in health and disease. Diabetologia. 1999;42:903–919. doi: 10.1007/s001250051247. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 43.Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantó C, Auwerx J. Calorie restriction: is AMPK a key sensor and effector? Physiology (Bethesda) 2011;26(4):214–224. doi: 10.1152/physiol.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garelick MG, Kennedy BK. TOR on the brain. Exp Gerontol. 2011;46(2–3):155–163. doi: 10.1016/j.exger.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fusco S, Maulucci G, Pani G (2012) Sirt1: def-eating senescence? Cell Cycle 11(22). [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 47.Dietrich MO, Antunes C, Geliang G, Liu ZW, Borok E, Nie Y, Xu AW, Souza DO, Gao Q, Diano S, Gao XB, Horvath TL. Agrp neurons mediate Sirt1′s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci. 2010;30(35):11815–11825. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12(1):78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michán S, Vianna CR, Sinclair DA, Elias CF, Coppari R. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011;14(3):301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009;23(24):2812–2817. doi: 10.1101/gad.1839209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michán S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30(29):9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallagher M, Stocker AM, Koh MT. Mindspan: lessons from rat models of neurocognitive aging. ILAR J. 2011;52(1):32–40. doi: 10.1093/ilar.52.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 54.Inoue K, Zorrilla EP, Tabarin A, Valdez GR, Iwasaki S, Kiriike N, Koob GF. Reduction of anxiety after restricted feeding in the rat: implication for eating disorders. Biol Psychiatry. 2004;55:1075–1081. doi: 10.1016/j.biopsych.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 55.Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28(12):3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hashimoto T, Watanabe S. Chronic food restriction enhances memory in mice–analysis with matched drive levels. NeuroReport. 2005;16(10):1129–1133. doi: 10.1097/00001756-200507130-00019. [DOI] [PubMed] [Google Scholar]
- 57.Mattson MP. The impact of dietary energy intake on cognitive aging. Front Aging Neurosci. 2010;2:5. doi: 10.3389/neuro.24.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu P, Shen Q, Dong S, Xu Z, Tsien JZ, Hu Y. Calorie restriction ameliorates neurodegenerative phenotypes in forebrain-specific presenilin-1 and presenilin-2 double knockout mice. Neurobiol Aging. 2008;29(10):1502–1511. doi: 10.1016/j.neurobiolaging.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 59.Qin W, Zhao W, Ho L, Wang J, Walsh K, Gandy S, Pasinetti GM. Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer’s disease-type amyloid neuropathology and spatial memory deterioration. Ann NY Acad Sci. 2008;1147:335–347. doi: 10.1196/annals.1427.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rich NJ, Van Landingham JW, Figueiroa S, Seth R, Corniola RS, Levenson CW. Chronic caloric restriction reduces tissue damage and improves spatial memory in a rat model of traumatic brain injury. J Neurosci Res. 2010;88(13):2933–2939. doi: 10.1002/jnr.22443. [DOI] [PubMed] [Google Scholar]
- 61.Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet JL, Becker KG, Wood W, Duffy K, Vinayakumar P, Maudsley S, Mattson MP. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148(9):4318–4333. doi: 10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minor RK, Villarreal J, McGraw M, Percival SS, Ingram DK, de Cabo R. Calorie restriction alters physical performance but not cognition in two models of altered neuroendocrine signaling. Behav Brain Res. 2008;189(1):202–211. doi: 10.1016/j.bbr.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 63.Fontán-Lozano A, Sáez-Cassanelli JL, Inda MC, de los Santos-Arteaga M, Sierra-Domínguez SA, López-Lluch G, Delgado-García JM, Carrión AM. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci. 2007;27(38):10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newton IG, Forbes ME, Linville MC, Pang H, Tucker EW, Riddle DR, Brunso-Bechtold JK. Effects of aging and caloric restriction on dentate gyrus synapses and glutamate receptor subunits. Neurobiol Aging. 2008;29(9):1308–1318. doi: 10.1016/j.neurobiolaging.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res Mol Brain Res. 2000;78(1–2):154–162. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 66.Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal. 2011;14(2):275–287. doi: 10.1089/ars.2010.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheatham RA, Roberts SB, Das SK, Gilhooly CH, Golden JK, Hyatt R, Lerner D, Saltzman E, Lieberman HR. Long-term effects of provided low and high glycemic load low energy diets on mood and cognition. Physiol Behav. 2009;98(3):374–379. doi: 10.1016/j.physbeh.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Witte AV, Fobker M, Gellner R, Knecht S, Flöel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci USA. 2009;106(4):1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Aging Res Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 70.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the aging human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 71.Trojanowski JQ, Mattson MP. Overview of protein aggregation in single, double, and triple neurodegenerative brain amyloidoses. Neuromol Med. 2003;4:1–6. doi: 10.1385/NMM:4:1-2:1. [DOI] [PubMed] [Google Scholar]
- 72.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sieradzan KA, Mann DM. The selective vulnerability of nerve cells in Huntington’s disease. Neuropathol Appl Neurobiol. 2001;27:1–21. doi: 10.1046/j.0305-1846.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 74.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53:S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 75.Balietti M, Tamagnini F, Fattoretti P, Burattini C, Casoli T, Platano D, Lattanzio F, Aicardi G. Impairments of synaptic plasticity in aged animals and in animal models of Alzheimer’s disease. Rejuvenation Res. 2012;15(2):235–238. doi: 10.1089/rej.2012.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park SK, Prolla TA. Lessons learned from gene expression profile of aging and caloric restriction. Aging Res Rev. 2005;4:55–65. doi: 10.1016/j.arr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Levenson CW, Rich NJ. Eat less, live longer? New insights into the role of caloric restriction in the brain. Nutr Rev. 2007;65:412–415. doi: 10.1111/j.1753-4887.2007.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Ho L, Qin W, et al. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- 79.Morgan TE, Wong AM, Finch CE. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdiscip Top Gerontol. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- 80.Hyun DH, Emerson SS, Jo DG, et al. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci USA. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45(1):8–15. [PubMed] [Google Scholar]
- 82.Manzanero S, Gelderblom M, Magnus T, Arumugam TV. Calorie restriction and stroke. Exp Transl Stroke Med. 2011;12(3):8. doi: 10.1186/2040-7378-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fontán-Lozano A, López-Lluch G, Delgado-García JM, Navas P, Carrión AM. Molecular bases of caloric restriction regulation of neuronal synaptic plasticity. Mol Neurobiol. 2008;38(2):167–177. doi: 10.1007/s12035-008-8040-1. [DOI] [PubMed] [Google Scholar]
- 84.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85(6):2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 86.Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63(4):313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 87.Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin -expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 88.Bondolfi L, Ermini F, Long JM, et al. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 89.Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram DK. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc Natl Acad Sci USA. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448(7155):767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 91.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110(8):3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205(10):2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2(98):ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5(3):279–289. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wood KC, Sabatini DM. Growth signaling at the nexus of stem cell life and death. Cell Stem Cell. 2009;5(3):232–234. doi: 10.1016/j.stem.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yilmaz OH, Katajisto P, Lamming DW, Gultekin Y, Bauer-Rowe KE, Sengupta S, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486(7404):490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Magri L, Cambiaghi M, Cominelli M, Alfaro-Cervello C, Cursi M, Pala M, Bulfone A, Garcìa-Verdugo JM, Leocani L, Minicucci F, Poliani PL, Galli R. Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex-associated lesions. Cell Stem Cell. 2011;9(5):447–462. doi: 10.1016/j.stem.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 98.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8(6):440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 99.Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, Palmer TD, Butte AJ, Brunet A. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog Neurobiol. 2011;93(2):182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 101.Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, Sato M, Horio Y. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci USA. 2008;105(40):15599–15604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schröter F, Ninnemann O, Siegert E, Bendix I, Brüstle O, Nitsch R, Zipp F, Aktas O. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10(4):385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- 103.Martin-Montalvo A, de Cabo R (2012) Mitochondrial metabolic reprogramming induced by calorie restriction. Antioxid Redox Signal [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 104.Sanz A, Scialo F, Mallikarjun V, Stefanatos R (2012) Regulation of lifespan by the mitochondrial electron transport chain: ROS-dependent and ROS-independent mechanisms. Antioxid Redox Signal [Epub ahead of print] [DOI] [PubMed]
- 105.Nakamura T, Cho DH, Lipton SA. Redox regulation of protein misfolding, mitochondrial dysfunction, synaptic damage, and cell death in neurodegenerative diseases. Exp Neurol. 2012;238(1):12–21. doi: 10.1016/j.expneurol.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chung HY, Kim HJ, Kim JW, Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann NY Acad Sci. 2001;928:327–335. [PubMed] [Google Scholar]
- 107.López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA. 2006;103(6):1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 109.Cerqueira FM, Cunha FM, Laurindo FR, Kowaltoswski AJ. Calorie restriction increase cerebral mithocondrial respiratory capacity in a NO·-mediated mechanism: impact on neuronal survival. Free Radic Biol Med. 2012;52(7):1236–1241. doi: 10.1016/j.freeradbiomed.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 110.Rasouri S, Lagouge M, Auwerx J. SIRT1/PGC-1: a neuroprotective axis? Med Sci (Paris) 2007;23(10):840–844. doi: 10.1051/medsci/20072310840. [DOI] [PubMed] [Google Scholar]
- 111.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 112.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 113.Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 114.Bedogni B, Pani G, Colavitti R, Riccio A, Borrello S, Murphy M, Smith R, Eboli ML, Galeotti T. Redox regulation of cAMP-responsive element-binding protein and induction of manganous superoxide dismutase in nerve growth factor-dependent cell survival. J Biol Chem. 2003;278(19):16510–16519. doi: 10.1074/jbc.M301089200. [DOI] [PubMed] [Google Scholar]
- 115.Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293(5529):493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- 116.Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang F, Wang S, Gan L, Vosler PS, Gao Y, Zigmond MJ, Chen J. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011;95(3):373–395. doi: 10.1016/j.pneurobio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281(31):21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 119.Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142(2):320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26(13):3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pallàs M, Casadesús G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, Camins A. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009;6(1):70–81. doi: 10.2174/156720209787466019. [DOI] [PubMed] [Google Scholar]
- 122.Jiang M, Wang J, Fu J, Du L, Jeong H, West T, Xiang L, Peng Q, Hou Z, Cai H, Seredenina T, Arbez N, Zhu S, Sommers K, Qian J, Zhang J, Mori S, Yang XW, Tamashiro KL, Aja S, Moran TH, Luthi-Carter R, Martin B, Maudsley S, Mattson MP, Cichewicz RH, Ross CA, Holtzman DM, Krainc D, Duan W. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med. 2011;18(1):153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, Mazzulli JR, Yates JR, 3rd, Bordone L, Guarente L, Krainc D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2011;18(1):159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mudò G, Mäkelä J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Mälkiä A, Bonomo A, Kairisalo M, Aguirre JA, Korhonen L, Belluardo N, Lindholm D. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci. 2012;69(7):1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fusco S, Ripoli C, Podda MV, Ranieri SC, Leone L, Toietta G, McBurney MW, Schütz G, Riccio A, Grassi C, Galeotti T, Pani G. A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proc Natl Acad Sci USA. 2012;109(2):621–626. doi: 10.1073/pnas.1109237109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23(6):744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 127.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 128.Oddo S. The role of mTOR signaling in Alzheimer disease. Front Biosci (Schol Ed) 2012;4:941–952. doi: 10.2741/s310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res. 1999;57:192–195. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 131.Lee J, Bruce-Keller AJ, Kruman Y, Chan SL, Mattson MP. 2-Deoxy-d-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J Neurosci Res. 1999;57:48–61. doi: 10.1002/(SICI)1097-4547(19990701)57:1<48::AID-JNR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 132.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-b toxicity through inhibiting NF-kB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 133.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Foti Cuzzola V, Ciurleo R, Giacoppo S, Marino S, Bramanti P. Role of resveratrol and its analogues in the treatment of neurodegenerative diseases: focus on recent discoveries. CNS Neurol Disord Drug Targets. 2011;10(7):849–862. doi: 10.2174/187152711798072310. [DOI] [PubMed] [Google Scholar]
- 135.Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem. 2003;10:456–465. doi: 10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- 136.Gruart A, Muñoz MD, Delgado-García JM. Involvement of the CA3–CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26:1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mattson MP, Duan W, Guo Z. Meal size and frequency affect neuronal plasticity and vulnerability to disease: cellular and molecular mechanisms. J Neurochem. 2003;84:417–431. doi: 10.1046/j.1471-4159.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- 138.Adams MM, Shi L, Linville MC, et al. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mockett BG, Hulme SR. Metaplasticity: new insights through electrophysiological investigations. J Integr Neurosci. 2008;7(2):315–336. doi: 10.1142/s0219635208001782. [DOI] [PubMed] [Google Scholar]
- 140.Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73(3):511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kann O, Kovács R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 142.Tong JJ. Mitochondrial delivery is essential for synaptic potentiation. Biol Bull. 2007;212:169–175. doi: 10.2307/25066594. [DOI] [PubMed] [Google Scholar]
- 143.Levy M, Faas GC, Saggau F, Craigen W, Sweatt JD. Mitochondrial regulation of synaptic plasticity in the hippocampus. J Biol Chem. 2003;278:17727–17738. doi: 10.1074/jbc.M212878200. [DOI] [PubMed] [Google Scholar]
- 144.Dinerman JL, Dawson TM, Schell MJ, Snowman A, Snyder SH. Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: implications for synaptic plasticity. Proc Natl Acad Sci USA. 1994;91(10):4214–4218. doi: 10.1073/pnas.91.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2(8):599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 146.Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004;16(11):1211–1227. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 147.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12(3):141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Noriega LG, Feige JN, Canto C, Yamamoto H, Yu J, Herman MA, Mataki C, Kahn BB, Auwerx J. CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep. 2011;12(10):1069–1076. doi: 10.1038/embor.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 150.Morikawa Y, Ueyama E, Senba E. Fasting-induced activation of mitogen-activated protein kinases (ERK/p38) in the mouse hypothalamus. J Neuroendocrinol. 2004;16(2):105–112. doi: 10.1111/j.0953-8194.2004.01135.x. [DOI] [PubMed] [Google Scholar]
- 151.Hahm S, Fekete C, Mizuno TM, Windsor J, Yan H, Boozer CN, Lee C, Elmquist JK, Lechan RM, Mobbs CV, Salton SR. VGF is required for obesity induced by diet, gold thioglucose treatment, and agouti and is differentially regulated in pro-opiomelanocortin- and neuropeptide Y-containing arcuate neurons in response to fasting. J Neurosci. 2002;22(16):6929–6938. doi: 10.1523/JNEUROSCI.22-16-06929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shimizu-Albergine M, Ippolito DL, Beavo JA. Downregulation of fasting induced cAMP response element-mediated gene induction by leptin in neuropeptide Y neurons of the arcuate nucleus. J Neurosci. 2001;21(4):1238–1246. doi: 10.1523/JNEUROSCI.21-04-01238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Watson E, Hahm S, Mizuno TM, Windsor J, Montgomery C, Scherer PE, Mobbs CV, Salton SR. VGF ablation blocks the development of hyperinsulinemia and hyperglycemia in several mouse models of obesity. Endocrinology. 2005;146(12):5151–5163. doi: 10.1210/en.2005-0588. [DOI] [PubMed] [Google Scholar]
- 154.Hahm S, Mizuno TM, Wu TJ, Wisor JP, Priest CA, Kozak CA, Boozer CN, Peng B, McEvoy RC, Good P, Kelley KA, Takahashi JS, Pintar JE, Roberts JL, Mobbs CV, Salton SR. Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron. 1999;23(3):537–548. doi: 10.1016/s0896-6273(00)80806-5. [DOI] [PubMed] [Google Scholar]
- 155.Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J, 3rd, Olefsky J, Guarente L, Montminy M. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456(7219):269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Altarejos JY, Goebel N, Conkright MD, Inoue H, Xie J, Arias CM, Sawchenko PE, Montminy M. The Creb1 coactivator is required for energy balance and fertility. Nat Med. 2008;14(10):1112–1117. doi: 10.1038/nm.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16(1):89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 158.Pittenger C, Kandel E. A genetic switch for long-term memory. CR Acad Sci III. 1998;321(2–3):91–96. doi: 10.1016/s0764-4469(97)89807-1. [DOI] [PubMed] [Google Scholar]
- 159.Saura CA, Valero J. The role of CREB signaling in Alzheimer’s disease and other cognitive disorders. Rev Neurosci. 2011;22(2):153–169. doi: 10.1515/RNS.2011.018. [DOI] [PubMed] [Google Scholar]
- 160.Wang B, Goode J, Best J, Meltzer J, Schilman PE, Chen J, Garza D, Thomas JB, Montminy M. The insulin-regulated CREB coactivator TORC promotes stress resistance in Drosophila. Cell Metab. 2008;7(5):434–444. doi: 10.1016/j.cmet.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kauffman AL, Ashraf JM, Corces-Zimmerman MR, Landis JN, Murphy CT. Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS Biol. 2010;8(5):e1000372. doi: 10.1371/journal.pbio.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470(7334):404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28(2):139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 164.Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, Mazzulli JR, Yates JR, 3rd, Bordone L, Guarente L, Krainc D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2011;18(1):159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13(2):125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 166.Challet E, Caldelas I, Graff C, Pevet P. Synchronization of the molecular clockwork by light- and food-related cues in mammals. Biol Chem. 2003;384:711–719. doi: 10.1515/BC.2003.079. [DOI] [PubMed] [Google Scholar]
- 167.Mendoza J, Graff C, Dardente H, Pevet P, Challet E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci. 2005;25:1514–1522. doi: 10.1523/JNEUROSCI.4397-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sakai T, Tamura T, Kitamoto T, Kidokoro Y. A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc Natl Acad Sci USA. 2004;101(45):16058–16063. doi: 10.1073/pnas.0401472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hampp G, Albrecht U. The circadian clock and mood-related behaviour. Commun Integr Biol. 2008;1(1):1–3. doi: 10.4161/cib.1.1.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307(5708):375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 171.Yau PL, Castro Bs MG, Tagani A, Tsui WH, Convit A (2012) Obesity and Metabolic Syndrome and Functional and Structural Brain Impairments in Adolescence. Pediatrics [Epub ahead of print] [DOI] [PMC free article] [PubMed]