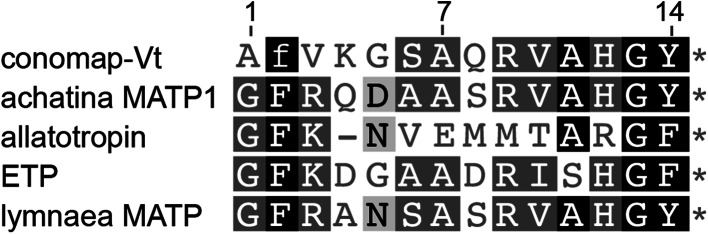Abstract
Pest insect species are a burden to humans as they destroy crops and serve as vectors for a wide range of diseases including malaria and dengue. Chemical insecticides are currently the dominant approach for combating these pests. However, the de-registration of key classes of chemical insecticides due to their perceived ecological and human health risks in combination with the development of insecticide resistance in many pest insect populations has created an urgent need for improved methods of insect pest control. The venoms of arthropod predators such as spiders and scorpions are a promising source of novel insecticidal peptides that often have different modes of action to extant chemical insecticides. These peptides have been optimized via a prey–predator arms race spanning hundreds of millions of years to target specific types of insect ion channels and receptors. Here we review the current literature on insecticidal venom peptides, with a particular focus on their structural and pharmacological diversity, and discuss their potential for deployment as insecticides.
Keywords: Insecticidal toxins, Venom peptides, Toxin structural folds, Spider, Scorpion, Cone snail, Sea anemone, Snake, Voltage-gated ion channels
Introduction
Although only a small minority of insects are classified as pests, they nevertheless destroy 10–14 % of the world’s food supply [1, 2] and transmit a diverse array of human and animal pathogens [3, 4]. Despite the introduction of transgenic crops and other biological control methods, chemical insecticides remain the dominant approach for combating insect pests. The major classes of chemical insecticides act on only six molecular targets in the insect nervous system, namely acetylcholinesterase, voltage-gated sodium (NaV) channels, nicotinic acetylcholine receptors, GABA- and glutamate-gated chloride channels, and ryanodine receptors (RyRs) [3, 5]. With the exception of the latter, resistance to these classes of insecticides has already developed in over 600 arthropod pest species [6]. Insecticides that target RyRs have only been on the market since 2008 and resistance has not yet emerged in the field. However, the appearance of insects resistant to RyR insecticides is inevitable, as suggested by a recent controlled study in a lab environment that found a 12-fold increase in the LC50 of the RyR insecticide chlorantraniliprole against lepidopteran pests after 22 generations under insecticidal selection pressure [5]. The increasing incidence of insecticide-resistant pest species, together with the limited range of molecular targets for extant insecticides, has created an urgent need for improved strategies for insect pest management [3, 7].
In addition, mounting evidence suggests that long-term exposure to certain insecticides may be detrimental to the health of humans and other vertebrates. Chronic exposure to organophosphates, which were widely used until recently, as well as pyrethroids have been linked to decreased male fertility and neurodevelopmental problems in children [8, 9]. These concerns have resulted in restrictions in use or de-registration of a number of insecticidal compounds. The US Environmental Protection Agency cancelled registrations for 169 insecticidal compounds during the 5-year period between January 2005 and December 2009, with only nine new insecticides registered during the same period [4]. With an increasing world population applying further pressure on the agricultural sector and the alarming decrease in commercially available insecticidal compounds, new ligands for existing molecular targets and the discovery of novel insect-specific targets are urgently required. There is no time to waste, as it can take 7–10 years to develop and register a new insecticide [10].
Fortunately, nature has provided us with a treasure trove of insecticidal toxins that have evolved within a diverse range of venomous animals, such as scorpions [11], spiders [12], centipedes [13], cone-snails [14], insects [15, 16], cnidarians (sea-anemone and jellyfish; [17, 18]), and snakes [19]. Due to millions of years of evolutionary fine-tuning, many of these insecticidal toxins exhibit remarkable selectivity and potency for their molecular targets. Since insecticidal toxins have arisen independently in various classes of venomous animals, there is a huge diversity of structural scaffolds for insecticidal toxins present in these organisms [20, 21]. In the venom of spiders alone, which are among the world’s most successful insect predators, it is estimated that millions of insecticidal toxins are yet to be discovered [12]. Consequently, this review can only provide a glimpse into the huge diversity of structural scaffolds found in insecticidal toxins derived from venomous animals (Table 1). We have therefore focused this review on peptide-toxin scaffolds that have the potential to be developed into novel insecticides with better properties than our current armamentarium of chemical insecticides.
Table 1.
Overview of structural folds adopted by insecticidal toxins
| Structural fold | Venomous animals | Targets | |
|---|---|---|---|
| Inhibitor cystine knot |
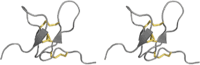
|
Spiders, scorpions, cone snails | Nav, Cav, BKCa Cl (?) channels |
| Cystine-stabilized αβ |
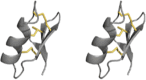
|
Scorpions | Nav, Kv channels |
| Disulfide-directed β-hairpin |

|
Spiders, scorpions | Unknown |
| Defensin-like |
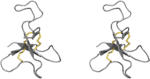
|
Sea anemones | Nav channels |
| Neuro-toxin III |

|
Sea anemones | Nav channels |
| Con-tryphan |
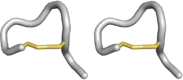
|
Cone snails | Kv, BKCa channels |
| Three-finger toxins |
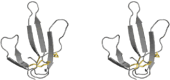
|
Snakes | Nicotinic acetylcholine receptor |
Stereoimages of ribbon representations of each fold are shown, with disulfide bonds highlighted in yellow
The representative toxins shown for each structural class are as follows, with PDB code given in parentheses: inhibitor cystine knot: κ-TRTX-Pg1a (2WH9); cystine-stabilized αβ: charybdotoxin (2CRD); disulfide-directed β-hairpin: U1-LITX-Lw1a (2KYJ); defensin-like: anthopleurin-A (1AHL); neurotoxin III: Av3 (1ANS); contryphan: contryphan-Vn (1NXN); three-finger toxins: cobratoxin (1COE)
ICK fold
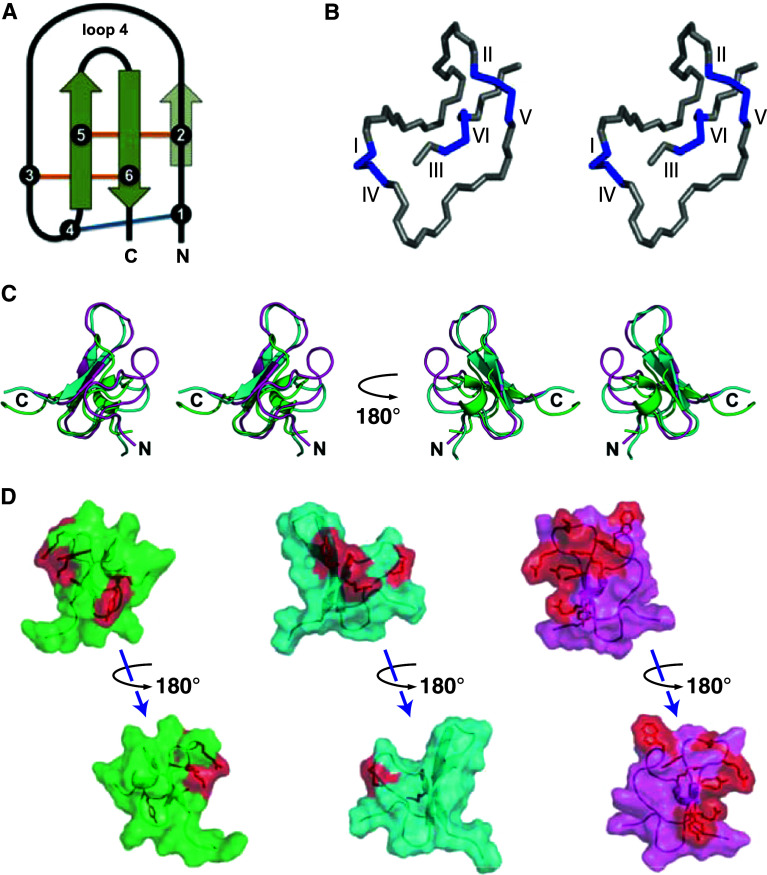
The inhibitor cystine knot motif is a structural scaffold composed of a ring formed by two disulfide bonds and the intervening peptide backbone, which is penetrated by a third disulfide to create a pseudo-knot [22] (Fig. 1a, b). This structural topology provides ICK-folded peptides with a high degree of stability and increased resistance to enzymatic degradation [23, 24], which are desirable features when engineering pesticides. Peptides from a wide range of evolutionarily unrelated organisms including plants, cone snails, and scorpions have been found to adopt the ICK motif [25]. Of all the organisms that utilize the ICK fold, spiders have indisputably produced the most numerous and chemically diverse array of ICK peptides [26, 27]. In spider venom peptides, the ICK configuration has been evolutionarily conserved as a framework on which a multitude of pharmacologically varied motifs have been grafted (Fig. 1c). As a result, the majority of spider-venom peptides contain an ICK-fold. Approximately 900 of the ~2,100 known ICK peptides are from spider venoms, of which about 116 are insect-selective [28]. Pharmacological targets have been identified for many of the insecticidal ICK toxins and include Nav channels, voltage-gated calcium (Cav) channels, Maxi-K calcium-activated potassium channels (BKca), and the NMDA-subtype of glutamate receptors. The structure–activity relationships for insect-selective ICK toxins at various receptors are discussed in the following sections.
Fig. 1.
Inhibitor cystine knot (ICK) fold. a Schematic of the ICK fold. The two disulfides present in the disulfide-directed β-hairpin (DDH) fold, the proposed precursor of the ICK motif, are colored orange, with the third disulfide necessary for the formation of the ICK fold colored blue. Solid green arrows represent the two requisite β strands, while the translucent arrow represents the third β strand that is present in some ICK peptides. Adapted from [65, 143]. b Stereoimage of the disulfide-bond configuration in the ICK motif. The disulfides between the first and fourth, and the second and fifth cystines, along with the intervening peptide backbone, create a ring that is pierced by the disulfide formed between the third and sixth cystines. Adapted from [4]. c Stereoview of an overlay of the ICK spider toxins κ-TRTX-Gr1a (KV channel blocker, PDB 1D1H, green), κ-HXTX-Hv1c (BKCa channel blocker, PDB1DL0, cyan), and δ-amaurobitoxin-Pl1b (NaV channel modulator, PDB 1V91, magenta). d Surface representations of the same three toxins showing the conserved ICK framework and the location of the toxin pharmacophores (red)
Spider ICK toxins targeting insect Nav channels
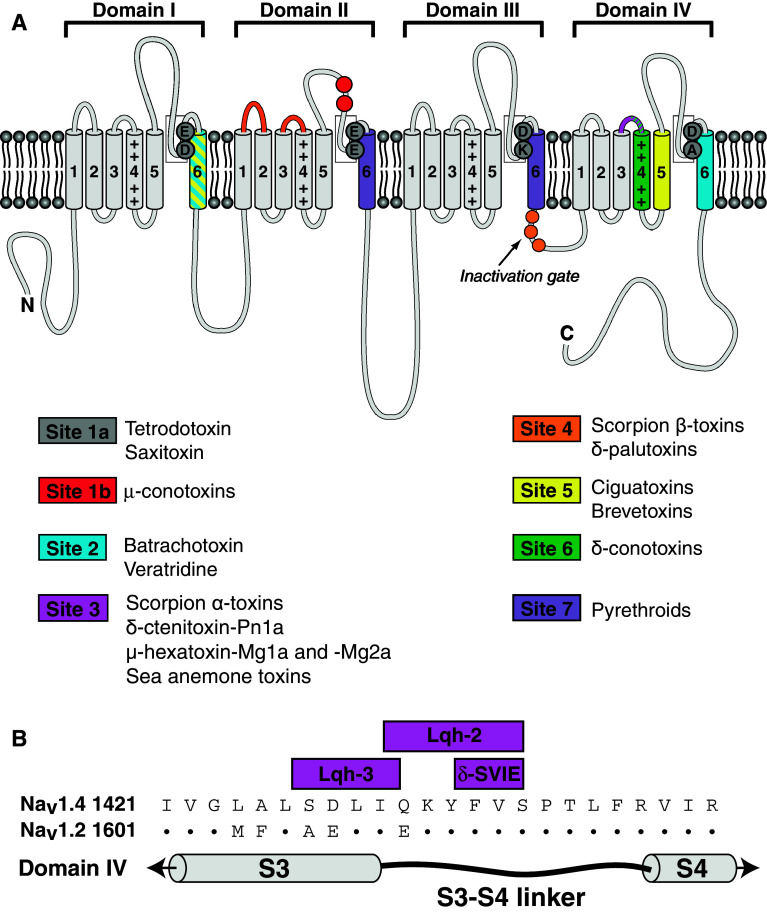
Nav channels are essential transmembrane proteins that mediate the intracellular influx of sodium ions during the initiation and propagation of action potentials [29]. Toxins that target Nav channels are therefore present in venoms from a variety of animals for the purposes of prey subjugation and self-defense [30]. Nav channels consist primarily of a single pore-forming α subunit composed of four homologous domains (I–IV). Each domain is comprised of six transmembrane helical segments (S1–S6), with voltage sensitivity conferred by the S4 segments (Fig. 2a). Ion selectivity is mediated by an inner and outer ring of amino acid residues located between the transmembrane segments five and six of each domain. While the overall domain architecture of insect and vertebrate Nav channels are very similar, there are considerable sequence differences; insect Nav channels are only ~60 % homologous to their human counterparts (Table 2) [31], which provides ample opportunity for producing Nav channel insecticides that are insect-specific.
Fig. 2.
Structure of voltage-gated sodium (NaV) channel. a Graphical representation of NaV channel showing the different neurotoxin receptor binding sites. Adapted from [31, 212]. b Sequence alignment of the S3–S4 region of NaV channel domain IV (i.e., neurotoxin receptor site 3) showing residues important for interaction with the scorpion toxins Lqh-3 and Lqh-2, and conotoxin δ-SVIE. Adapted from [95]
Table 2.
Homology between various insect NaV channels and between insect NaV channels and the nine human NaV channel subtypes
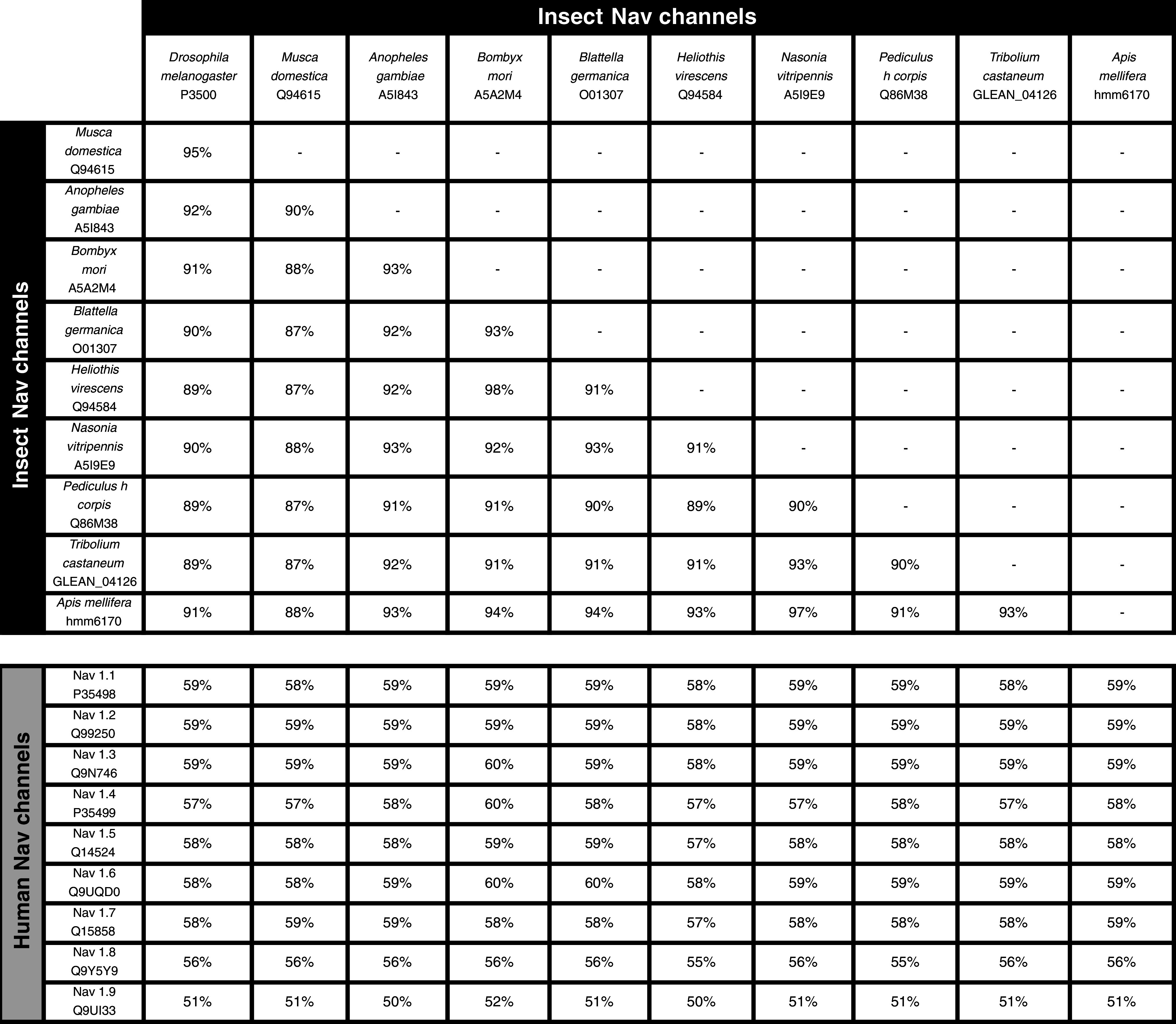
UniProt identifiers or genome accession numbers are given for each entry. Adapted from [31].
Although Nav channels are the target of extant insecticides such as pyrethroids, dihydropyrazoles, and oxadiazines [32], there is still significant potential for the development of bioinsecticides acting on Nav channels. Arachnid toxins that target Nav channel could even be useful in situations where an insect population has developed resistance to a Nav channel insecticide. This seemingly counterintuitive scenario is possible because arachnid venom peptides act at different sites to chemical insecticides. Thus, even though the scorpion toxin AaIT and pyrethroids both target Nav channels, a pyrethroid-resistant strain of H. virescens is more susceptible than non-resistant strains to a recombinant baculovirus expressing AaIT [33].
There are numerous spider-venom ICK toxins that selectively target insect Nav channels. Seven neurotoxin binding sites have been identified on Nav channels, with the site at which the toxin binds usually determining its effect on the channel. Spider ICK insecticidal toxins interact almost exclusively with neurotoxin receptor sites 3 and 4, which cause effects on channel inactivation and activation, respectively [4]. In the following sections, we review some of the insecticidal spider-venom peptides that act on Nav channels.
Spider ICK toxins potentially targeting neurotoxin receptor site 1
This recently described family of spider toxins is comprised of several members of the huwentoxin and hainantoxin groups isolated from the Chinese tarantulas Haplopelma huwenum and Haplopelma hainanum, respectively (Table 3). In contrast to all other spider toxins that target Nav channels, these toxins do not alter the kinetics of channel inactivation or the voltage-dependence of channel activation, but instead are proposed to be pore blockers that inhibit channel current [34].
Table 3.
Insecticidal venom peptides with an ICK fold
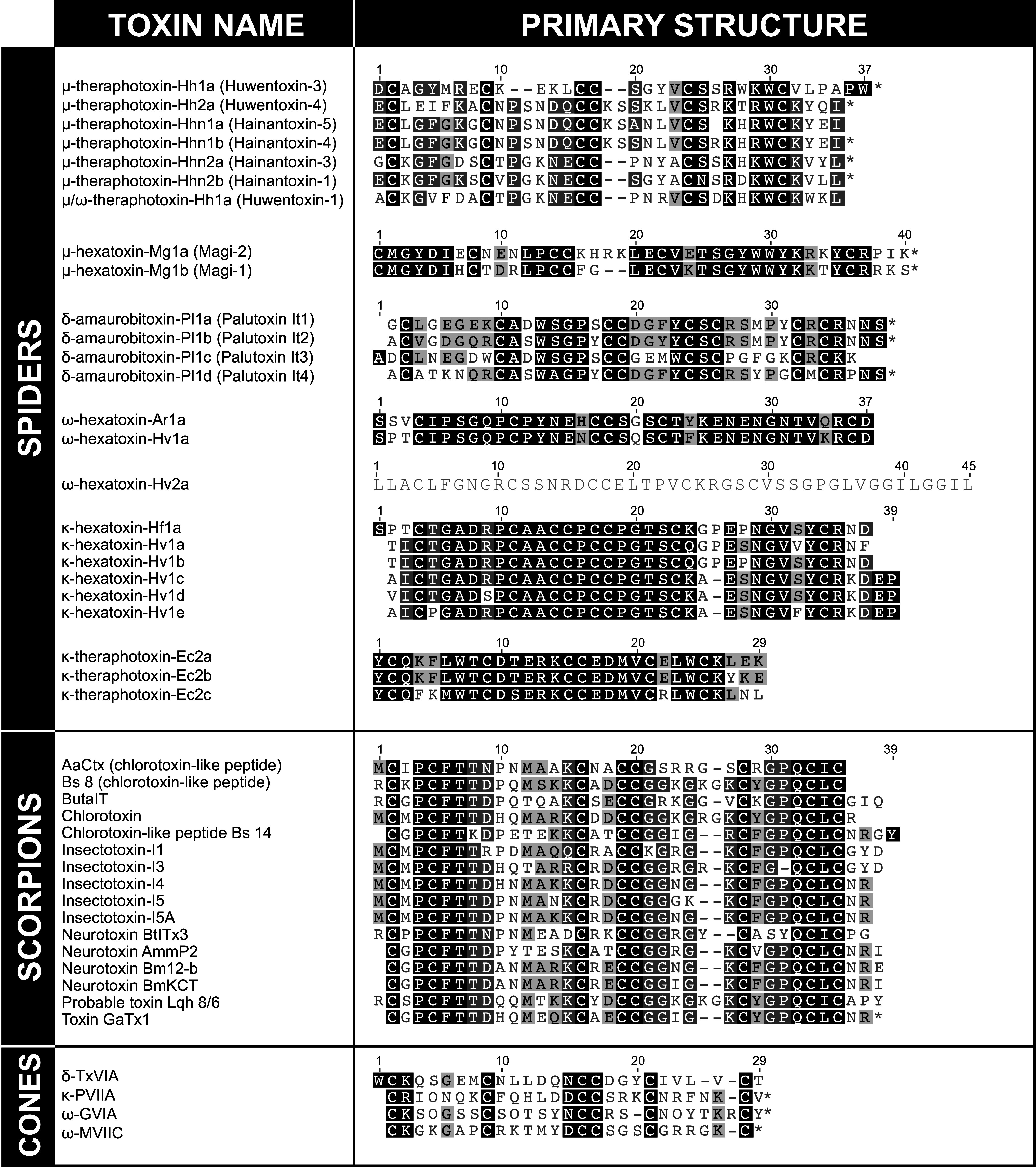
Asterisks indicate an amidated C-terminus
These toxins inhibit tetrodotoxin-sensitive (TTX-S) but not tetrodotoxin-resistant (TTX-R) currents in rat neurons. Due to their similar action to TTX, it was proposed that these toxins bind to the same neurotoxin receptor site on Nav channels as TTX (i.e., site 1) [34]. However, competition binding studies with TTX/saxitoxin have not been performed to confirm this hypothesis. Moreover, recent experiments suggest that these toxins may in fact bind to neurotoxin receptor site 4 [35, 36]. It was previously shown that the sensitivity of the vertebrate Nav1.7 channel to TTX was greatly decreased by a single Y632S mutation in site 1 [35] but the putative site 1-binding spider toxin μ-theraphotoxin-Hh2a (μ-TRTX-Hh2a; huwentoxin-IV) remained completely active at the mutant receptor. In contrast, the activity of μ-TRTX-Hh2a was almost abolished at channels with mutations in site 4, revealing an interaction between the toxin and this site. Thus, further studies probing the interaction of these toxins with vertebrate and invertebrate Nav channels may ultimately lead to their reclassification as site 4 ligands.
All toxins in this family are also active on vertebrate Nav channels, although two members (μ-TRTX-Hhn2b; hainantoxin-I, and μ/ω-theraphotoxin-Hh1a; huwentoxin-1) are over tenfold more potent on insect channels than rat channels. μ-TRTX-Hhn2b is 15-fold more potent on the insect Nav channel compared with the rat Nav1.2 channel when expressed in Xenopus laevis oocytes (IC50 values of 4.3 μM and 68 μM, respectively) [34]. μ/ω-theraphotoxin-Hh1a is 14 times more potent on sodium currents in cockroach DUM neurons than the TTX-S sodium currents of rat hippocampal neurons (IC50 values of 4.80 and 66.1 nM, respectively) [37]. μ/ω-theraphotoxin-Hh1a also inhibits high-voltage-activated Ca2+ channels in differentiated NG-108-15 rat glioma x mouse neuroblastoma hybrid cells, however the toxin has not been tested on insect Ca2+ currents [38]. Comparison of the sequences of μ-TRTX-Hhn2b and μ/ω-theraphotoxin-Hh1a with other toxins in this family suggest that variation in acidic residues and local structural differences play a role in determining phylum selectivity [34, 37]. However, mutational studies to explore the molecular epitopes underlying the preference of μ-TRTX-Hhn2b for insect Nav channels are still awaited.
Spider ICK toxins and toxins of unknown fold targeting neurotoxin receptor site 3
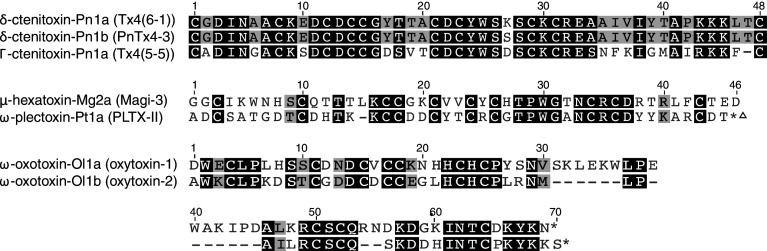
Spider toxins that bind to neurotoxin receptor site 3 inhibit the fast inactivation of Nav channels, thereby prolonging action potentials. They produce neuroexcitatory effects, leading to muscle fatigue and paralysis. δ-Ctenitoxin-Pn1a (Tx4(6-1)) (LD50 in Musca domestica of 36 pmol/g) is a disulfide-rich toxin that adopts an undefined fold from the venom of the highly venomous Brazilian spider Phoneutria nigriventer. It is lethal to a range of insects but has no effect on oocyte-expressed rat Nav channels or on mice when injected intracerebroventricularly [39]. δ-Ctenitoxin-Pn1a competes with scorpion α-like toxin Bom IV for binding to site 3 of cockroach axonal Nav channels [40]. Two additional homologues named δ-ctenitoxin-Pn1b (PnTx4-3, LD50 192 pmol g−1 in Musca domestica) and Γ-ctenitoxin-Pn1a (Tx4(5–5), LD50 90 pmol g−1 in Musca domestica) have been isolated from the same spider species (Fig. 3) [41, 42]. Although their molecular target has not been determined, their similarity in sequence to δ-ctenitoxin-Pn1a, excitatory effects upon injection into insects, and lack of toxicity in mice suggest both peptides probably also act on insect Nav channels. An additional action of this family of toxins is their ability to partially inhibit glutamate uptake in rat brain synaptosomes via an as-yet-unknown mechanism. They are also likely inhibitors of the NMDA-subtype of the ionotropic glutamate receptor as Γ-ctenitoxin-Pn1a inhibits NMDA-elicited currents without affecting GABA-, AMPA-, or kainate-induced currents [41, 42]. No experiments have been performed to ascertain structure–activity relationships for this family of toxins. However, inhibition of glutamate uptake by δ-ctenitoxin-Pn1b is 2.5-fold greater than seen with δ-ctenitoxin-Pn1a, which differs by only one amino acid at position 27 (Ser and Lys, respectively) [42]. It remains to be determined whether the chemical pharmacophore conferring insecticidal activity is distinct from that responsible for the inhibition of glutamate uptake in vertebrates.
Fig. 3.
Insecticidal spider venom peptides with an undefined fold. Asterisks indicate an amidated C-terminus. Triangle indicates an O-palmitoyl group
Numerous Nav toxins have been isolated from the Japanese funnel-web spider Macrothele gigas, two of which have insecticidal activity and no toxic effects in mice. One toxin adopts the ICK motif (μ-hexatoxin-Mg1a; Magi-2, LD50 17,600 pmol g−1 in Spodoptera litura), while the 3D fold of the other toxin (μ-hexatoxin-Mg2a; Magi-3) remains to be determined [43]. Both toxins compete with scorpion toxin LqhαIT for binding to Nav channel site 3 in cockroach synaptosomes, whereas neither toxin competes with radiolabeled toxins for binding to site 4 in cockroach synaptosomes, or sites 3, 4, and 6 in rat brain Nav channels. Interestingly, μ-hexatoxin-Mg1a shares 68 % sequence identity with μ-hexatoxin-Mg1b (Magi-1), a peptide with no activity in insects or mice (Table 3) [43]. The other insecticidal toxin from M. gigas, μ-hexatoxin-Mg2a, may also act on insect CaV channels since it is 43 % homologous to the insect CaV channel blocker ω-plectoxin-Pt1a (PLTX-II); however, this has not been experimentally verified [43]. Further investigations, including mutagenesis studies, are required to elucidate the residues necessary for activity and selectivity of these site 3 toxins.
Spider ICK toxins targeting neurotoxin receptor site 4
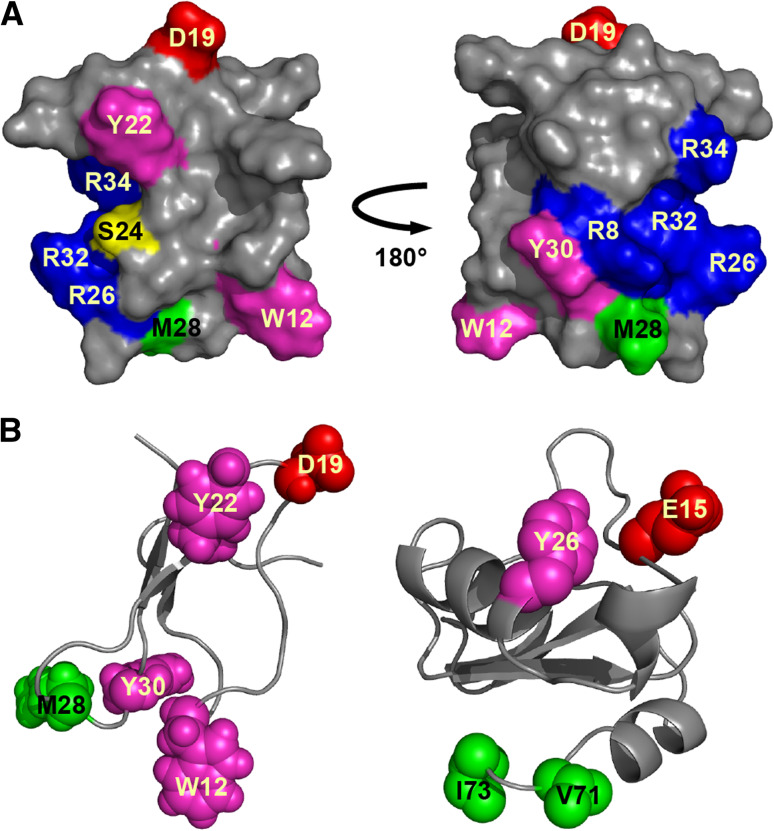
Toxins that target neurotoxin receptor site 4 classically affect Nav channels by causing a hyperpolarizing shift in the voltage dependence of activation, leading to a decrease in the threshold potential required for the generation of action potentials and the occurrence of spontaneous transient activity. This is the primary mode of action of spider toxins that target vertebrate NaV channels [44]. However, only four insecticidal spider toxins, the δ-palutoxins, have been shown to bind at site 4 of insect Nav channels (Table 3). They compete with the site-4 scorpion toxin Bj-xtrIT and do not compete with the site-3 scorpion toxin LqhαIT for binding to cockroach neuronal membranes [45]. However, the δ-palutoxins are unusual as they slow channel inactivation in a manner similar to toxins that bind to site 3. δ-Amaurobitoxin-Pl1b (δ-palutoxin IT2) is the only δ-palutoxin that also causes toxicity in mice, with the three other members of this family being specific for insects [46]. Although there are few sequence differences between δ-palutoxin paralogues, the residues conferring mammalian activity to δ-amaurobitoxin-Pl1b are not obviously apparent and remain to be determined. Alanine scanning mutagenesis of δ-amaurobitoxin-Pl1b revealed that the bioactive surface is comprised of a basic region that may help localize the toxin to the receptor, plus a hydrophobic cluster and a key aspartic acid residue at position 19 (Fig. 4a). Substitution of D19 with alanine resulted in a considerable decrease in toxin activity, without a concurrent reduction in binding affinity [45]. E15 mutants of the insect-selective scorpion toxin Bj-xtrIT presented a similar disconnect between binding and activity. Consequently, the acidic residue was proposed to be involved in voltage sensor trapping but not receptor binding [47].
Fig. 4.
Structure of site-4 NaV channel toxins. a Surface representation of the spider toxin δ-amaurobitoxin-Pl1b (PDB 1V91) showing key pharmacophore residues. Adapted from [45]. b Ribbon representation of δ-amaurobitoxin-Pl1b (left) and the scorpion toxin Bj-xtrIT (PDB 1BCG, right) showing the similar spatial positioning of key functional residues. In each panel, the chemical nature of amino acid residues is color coded as follows: aromatic, magenta; aliphatic, green; basic, blue; acidic, red; polar but uncharged, yellow
Comparison of the three-dimensional (3D) structures of δ-amaurobitoxin-Pl1b and Bj-xtrIT highlights the resemblance in spatial orientation of several key hydrophobic/aromatic residues that are important for activity [45, 48] (Fig. 4b). These hydrophobic residues, as well as many of the other residues comprising the bioactive surface of δ-amaurobitoxin-Pl1b, are highly conserved in other insect-selective spider toxins, namely the β/δ- and μ-agatoxins. Based on their sequence homology with the δ-palutoxins, the β/δ- and μ-agatoxins are thought to bind to receptor site 4, however confirmation is required via binding assays. The β/δ-agatoxins also have an unusual pharmacological profile, since they inhibit channel inactivation similar to site-3 toxins, as well as shifting the voltage dependence of channel activation to more hyperpolarized potentials analogous to classical site-4 toxins. Idiosyncratically, both of these effects are voltage dependent and display a bell-shaped curve between −80 and 0 mV. Although the effects of μ-agatoxins have not been as extensively explored, μ-agatoxin-Aa1a and -Aa1d also affect channel activation and inactivation in a manner qualitatively resembling β/δ-agatoxins [49, 50]. Additional studies into the mode of action of μ-agatoxins may result in their reclassification as β/δ-agatoxins. The diverse effects of these site-4 spider toxins have challenged the long-held presumption of a correlation between binding site and activity. Future studies are needed to uncover the molecular determinants of these various actions.
Spider ICK toxins and toxins of unknown fold targeting insect Cav channels
Like Nav channels, Cav channels play an essential role in action potential generation. However, Cav channel subtypes are not as highly conserved between different insect orders as Nav channels, with identity levels between 76–90 % compared to above 90 % for Nav channels [31]. This theoretically reduces the likelihood of finding toxins active against a broad spectrum of insect Cav channels, which may explain the lack of chemical insecticides that target Cav channels. However, this attribute could potentially be exploited to develop insecticides that target only pest insects without harming beneficial species.
While modulation of Cav channels is one of the dominant pharmacologies of spider-venom toxins, in-depth functional and biophysical characterization of insect-selective Cav toxins has been difficult due to the lack of a system by which to study insect Cav channels discretely [51]. Recombinant expression of a functional insect Cav channel has been a considerable challenge, with the only example described in a recent patent [52]. Despite this obstacle, numerous insect-selective Cav channel spider ICK toxins have been characterized via their ability to block Ca2+ currents in insect neurons.
Cockroach dorsal unpaired median (DUM) neurons have been a valuable tool and the archetypal system for the study of Ca2+ currents in insects. There are four different types of Ca2+ currents that can be evoked in DUM neurons: low-voltage activated currents that are either transient (tLVA) or maintained (mLVA), mid- and high-voltage activated currents (MVA and HVA, respectively). These four current types are thought to be mediated by channels belonging to the three subtype groups Cav1, Cav2 and Cav3, which are orthologues of the respective vertebrate families. Although the subtypes responsible for each current type have not been conclusively established, the pharmacological properties of the subtypes in conjunction with studies of the Drosophila Cav2 channels have lead to the proposal that Cav3 channels cause tLVA/mLVA currents, while MVA and HVA currents are produced primarily through Cav2 channels, with a minor component contributed by Cav1 channels [31, 51, 53].
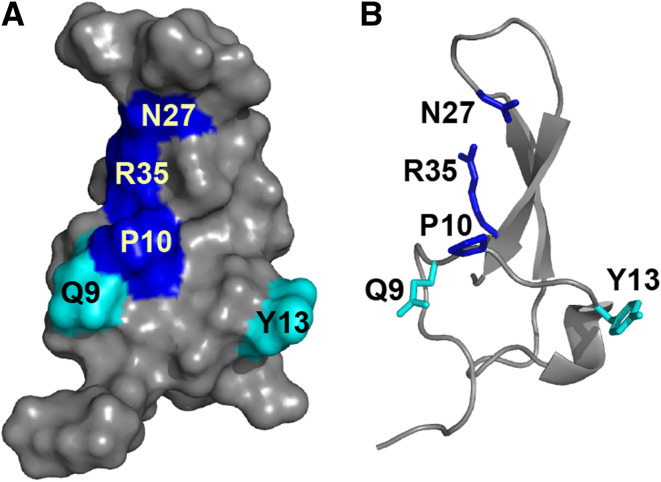
To date, all characterized spider toxins that target insect Cav channels act by blocking MVA and HVA currents, thus most likely targeting Cav1 and/or Cav2 channels. The most extensively analyzed of these toxins is ω-hexatoxin-Hv1a (ω-HXTX-Hv1a) from the Australian funnel-web Hadronyche versuta [54, 55], which has been proposed to act primarily on insect Cav1 channels [51]. ω-HXTX-Hv1a is lethal to a wide range of insect orders (LD50 77 pmol g−1 in Musca domestica, LD50 89 pmol g−1 in Acheta domestica) but does not affect vertebrates [54–56]. A panel of alanine mutants of ω-HXTX-Hv1a revealed that three spatially contiguous residues (P10, N27 and R35) form a major pharmacophore crucial for both insecticidal activity and binding to cockroach neurons. Two other residues (Q9 and Y13) comprising a minor pharmacophore also contribute to toxin binding and activity (Fig. 5) [57, 58]. Both the major and minor pharmacophores are highly conserved in ω-HXTX-Hv1a paralogues and orthologues. Whereas the major pharmacophore may bestow activity at insect Cav1 channels in general, the slight variations in primary structure between family members might confer selectivity between different insect orders. ω-HXTX-Ar1a is 84 % identical to ω-hexatoxin-Hv1a, however it is 2–3 times less potent in crickets (LD50 236 pmol g−1 in Acheta domestica) (Table 3) [59]. It is likely that this decrease in toxicity is due to the presence of His rather than Asn at position 16 since an N16A mutation in ω-HXTX-Hv1a caused a ~4-fold reduction in toxicity to crickets. Notably, the N16A mutation did not affect the activity of ω-HXTX-Hv1a in flies or its binding to cockroach neurons [58]. Testing other ω-HXTX-Hv1a orthologues for selectivity over different insect orders would be a worthwhile future study, as it may enable the development of insecticides that target specific pest orders, while leaving neutral or beneficial insect species unharmed.
Fig. 5.
Pharmacophore of ω-HXTX-Hv1a (PDB 1AXH). a Surface and b ribbon representations of ω-HXTX-Hv1a with the major pharmacophore residues shown in blue and less critical pharmacophore residues in cyan
Another insect-selective toxin from Hadronyche versuta, ω-hexatoxin-Hv2a (ω-HXTX-Hv2a), blocks CaV channel currents in bee brain neurons and is toxic to insects from a wide array of orders including Lepidoptera, Orthoptera and Diptera [60]. ω-HXTX-Hv2a shows no homology to the ω-HXTX-Hv1a toxin family and does not posses the ω-HXTX-Hv1a pharmacophore (Table 3). While both toxins contain an ICK motif, ω-HXTX-Hv2a differs considerably from ω-HXTX-Hv1a in containing a long, unstructured C-terminal “tail” [60]. Although the residues responsible for insecticidal activity have not been ascertained, synthetic analogues of ω-HXTX-Hv2a without the 12-residue tail were unable to inhibit Cav channels [60]. The C-terminal region is also important for the activity of other Cav channel spider toxins. The eight C-terminal residues of ω-agatoxin-Aa4a, the terminal Phe and Ser residues in ω-TRTX-Hh2a (huwentoxin-V, LD50 ≥ 24,339 pmol g−1 in Locusta migratoria manilensis), and an unusual O-palmitoyl threonine amide at the C-terminus of the non-ICK folded ω-plectoxin-Pt1a (PLTX-II) are essential for the activity of these toxins [60–63]. Although no significant sequence homology exists between these toxins, their C-terminal regions are lipophilic and structurally disordered. Since insect Cav channels have a largely polar surface that is unlikely to have any extensive favorable interaction with the hydrophobic tails of these toxins, it was proposed that the C-termini anchor the toxins in the cell membrane and direct the requisite toxin regions into the channel. It is also possible that the C-termini somehow cause a conformational change in the channel, thereby exposing a previously inaccessible high-affinity binding site to which other areas of the toxin bind [60]. Although a hydrophobic C-terminus is vital for the activity of these Cav channel blockers, it is most likely not a determinant of vertebrate versus invertebrate selectivity as ω-agatoxin-Aa4a targets both mammalian and insect channels. Much work remains to establish the structure–activity relationships between these spider toxins and Cav channels; nevertheless the ICK fold is a sound framework for engineering bioinsecticides that are specifically targeted against insect Cav channels.
The most recently characterized spider-venom peptides that inhibit insect Cav channels are the ω-oxytoxins, which have low sequence identity with the other spider-derived Cav channel toxins, do not adopt the ICK fold, nor possess lipophilic C-termini (Fig. 3). ω-Oxytoxins paralyze larvae of the lepidopteran pest Spodoptera frugiperda (army worms) with an ED50 of 5,000–6,200 pmol g−1 but they are non-toxic to mice. They do, however, block HVA currents of expressed rabbit Cav channels, and therefore additional toxicity assays in other vertebrate species are required to assess their suitability as prospective insecticides [64].
Spider ICK toxins targeting insect BKCa channels
BKCa channels, also known as Maxi-K or Slo1, are calcium-activated potassium channels important in the control of neuronal and muscle excitability. A lack of insect-selective ligands has meant the BKCa channel has not previously been considered a potential pesticidal target. However, in recent years, it has been established that two families of insecticidal spider toxins are selective for insect BKCa channels. The κ-hexatoxins (formerly J-ACTXs) are lethal to insects from an extensive array of taxonomic orders (LD50 167 pmol g−1 in Acheta domestica, LD50 91 pmol g−1 in Musca domestica), while being inactive on mouse, chick, rat and rabbit preparations [65, 66]. The prototypic family member, κ-HXTX-Hv1c, potently inhibits BKCa currents in cockroach DUM neurons with an IC50 of ~3 nM [67, 68]. κ-Hexatoxins act as blockers of channel current, probably interacting with the pore or turret residues located between transmembrane segment S5 and S6 of the BKCa channel [67]. A comparison of the region between S5 and S6 in insect and vertebrate BKCa channel reveals several amino acid differences that possibly underlie the phyletic discrimination of the κ-hexatoxins (Table 3) [67, 69]. It has been demonstrated that the sensitivity of insect BKCa channels to charybdotoxin, a pore blocking scorpion toxin with a preference for vertebrates, can be increased upon substitution of single residues in the S5-S6 region with the corresponding residues in vertebrate BKCa channels [70]. Therefore, there is ample variation in the amino acid sequences of the pore region between insect and vertebrate channels to warrant the insect-selectivity of the κ-hexatoxins.
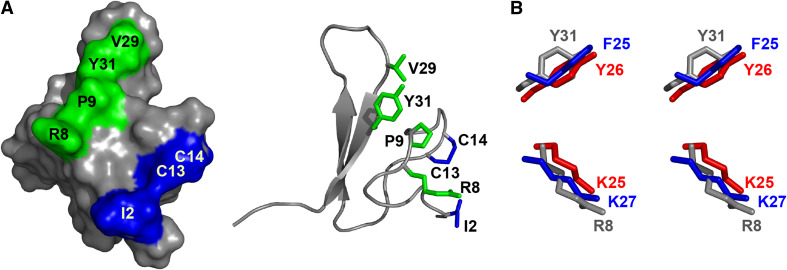
Mutagenesis studies have shown that the residues responsible for the activity of κ-hexatoxins are Ile2, Arg8, Pro9, Val29, Tyr31 and a rare vicinal disulfide bond between Cys13 and Cys14 (Fig. 6a) [65, 66]. Numerous vertebrate K+ channel toxins from different phyla interact with the channel via a functional dyad consisting of a Lys and Tyr/Phe 6.6 ± 1.0 Å apart [71]. The Arg8 and Tyr31 in κ-hexatoxins spatially overlay well with the functional dyad in other toxins, and it was thought that the Arg might have been analogous to Lys in the dyad (Fig. 6b). However, substitution of Arg8 with Lys in κ-HXTX-Hv1c resulted in a dramatic decrease in binding and activity [67]. Consequently, the essential Arg8 and Tyr31 are not likely to be synonymous to the dyad of other K+ channel toxins. The mode of interaction of κ-hexatoxins with BKCa channels therefore cannot be assumed to be similar to that of dyad-containing Kv channel toxins.
Fig. 6.
Pharmacophore of κ-HXTX-Hv1c. a Surface view (left) and ribbon representation (right) of κ-HXTX-Hv1c (PDB 1DL0) showing the bipartite pharmacophore in green and blue. b Stereoimage of the overlay of the side-chains forming the functional Kv-channel dyad of agitoxin 2 (blue, PDB 1AGT), BgK (red, PDB 1BGK) and the specious Kv-channel dyad of κ-hexatoxin-Hv1c (gray). Adapted from [67]
The second group of spider toxins that block BKCa channels are the κ-TRTX-Ec2 family from the African tarantula Eucratoscelus constrictus (Table 3). κ-TRTX-Ec2a and -Ec2b are insect-selective (LD50 1,100 pmol g−1 in Gryllus bimaculatus), while κ-TRTX-Ec2c is toxic to both insects and mice [21]. Although the pharmacophore of the κ-TRTX-Ec2 family has not been elucidated, sequence comparisons with homologous but functionally unrelated spider toxins have identified several residues that may determine their selectivity for insect BKCa channels. It is hypothesized that a Glu residue in the C-terminal region is responsible for the insect-selectivity of κ-TRTX-Ec2a and -Ec2b, since homologous toxins are mammalian-active and have a basic or hydrophobic residue at the corresponding location. A highly conserved Trp5 and Met6 motif in homologous toxins is not present in the κ-TRTX-Ec2 toxins and this difference may serve as the basis for their affinity at the BKCa channel [21].
Scorpion ICK toxins targeting unknown insect targets
Several scorpion toxins have been found to adopt the ICK fold, although this fold is not as abundant in the venom of scorpions in comparison to spiders. Many of the scorpion ICK toxins target mammalian RyRs [72], however their toxicity in insects have not been ascertained. Others appear to be insect selective as they are lethal to insects but not toxic to mice, though their molecular targets are unknown [73]. These putatively insect-specific toxins, termed small insectotoxins, share a conserved spatial arrangement of cystines and sequence homology with chlorotoxin, a scorpion ICK toxin that induces paralysis in crayfish and cockroaches and inhibits small conductance Cl− channels isolated from rat epithelia and brain. Due to the similarities with chlorotoxin (Table 3), it was hypothesized that the short insectotoxins also target Cl− channels, but this has never been experimentally validated [74, 75]. Furthermore, subsequent studies have revealed that chlorotoxin binds to the cell-surface molecules annexin A2 and matrix metalloprotease-2, and therefore these could also be molecular targets for the short insectotoxins [76, 77]. Based on the action of insectotoxin I5A, an additional target of the insectotoxins may be a glutamate receptor located on postsynaptic membranes [78]. The actual receptors with which the short insectotoxins interact therefore remain to be ascertained and it may eventuate that these toxins target different receptors to chlorotoxin despite their structural homology. Although detailed pharmacological analyses of the short insectotoxins are lacking, a considerable array of bioassays have been performed with the most studied of these toxins, ButaIT. This toxin is lethal to insect pests from a wide range of orders including Lepidoptera (a dose of 2593 pmol g−1 was lethal to Heliothis virescens), Coleoptera, Diptera, and Dictyoptera, making it a promising candidate for bioinsecticide development [73, 79].
Cone snail ICK toxins targeting various insect channels
While terrestrial venomous organisms produce insecticidal toxins for the benefit of prey capture and/or defense, the presence of such toxins in the venom of marine invertebrates such as cone snails seems paradoxical, since there are no marine insects described. However, a certain degree of homology is likely to exist between insect ion channels and those of the natural molluscan and crustacean prey targets of venomous marine invertebrates, as all belong to the phylum Arthropoda. Indeed, a BLAST search of the putative Nav channel sequence from the crab Cancer borealis reveals its closest homologue to be the para sodium channel from the German cockroach Blattella germanica, with which it is 69 % identical [80]. The activity of toxins from marine invertebrates on insects is thus almost certainly incidental, with their intended targets probably being molluscs, crustaceans, and/or annelids.
Although conotoxins have been studied for over 30 years, most research has focused on mammalian-active toxins and their potential as drug leads for human diseases [81–83]. There have been very few investigations into their activity on insects and, as a result, only a handful of conotoxins have been found to be insecticidal. The ω-conotoxins GVIA and MVIIC both inhibit Cav channels in cockroach DUM neurons [84] and κ-conotoxin PVIIA is a blocker of the insect shaker KV channel [85]. However, these conotoxins are not insect-specific, with ω-GVIA and ω-MVIIC potently active on rat Cav channels [86, 87] and κ-PVIIA causing hyperexcitability in mice when injected i.c.v. and ‘fin popping’ activity in fish [85, 88]. To date, the only conotoxin that seems inactive in vertebrates is δ-TxVIA, which is non-toxic in mouse, rat and fish [89, 90] while being lethal to the housefly Musca domestica (283436 pmol g−1 caused lethality in 40 % of tested insects) and larvae of the cabbage moth Mamestra brassicae (247,183 pmol g−1 caused lethality in 20 % of tested insects) [91]. δ-TxVIA is also known as the ‘King-Kong’ toxin as it has the uncanny ability of making submissive lobsters assume a dominant posture [89]. Similar to site-3 binding toxins, δ-TxVIA slows the rate of inactivation of Nav channels. However, δ-TxVIA does not compete with the site-3 toxin Av2 in channel binding assays, nor does it compete with toxins that bind site 1, site 4 or site 5. Thus, δ-conotoxins were deemed to bind at a novel location, termed site 6 [92, 93]. Scanning mutagenesis of residues in the rat Nav1.4 channel revealed that the main Nav channel interaction site of the mammalian active δ-conotoxin SVIA is a triad of hydrophobic residues (Y1433, F1434, V1435) in the S3/S4 linker of domain four. This triad of residues is also part of the channel epitope important for activity of the site-3 scorpion α-toxin Lqh-2, but not of α-toxin Lqh-3 (Fig. 2b) [94]. Based on these observations, it was proposed that site 6 overlaps with or may in fact be part of site 3. Moreover, site 3 may be considered a ‘macrosite’ since different toxins interacting with distinct regions within site 3 are all regarded as site-3 binders [95]. Additional studies are necessary to locate the binding epitope of other δ-conotoxins in order to unequivocally determine whether neurotoxin site 6 is distinct from site 3.
δ-TxVIA is an unusual toxin since despite its lack of activity on vertebrates, it nevertheless binds with high affinity to vertebrate Nav channels [92]. δ-conotoxins have a high proportion of hydrophobic residues and the solution structure of δ-TxVIA reveals that they are clustered in a hydrophobic patch on the surface of the molecule [96]. It is believed that this hydrophobic region mediates binding of δ-conotoxins to Nav channels [96], with phyletic selectivity determined by other residues as yet unknown.
CSαβ fold
The cystine-stabilized αβ (CSαβ) motif consists of a short α-helix connected to two or three antiparallel β-strands via three or four disulfide bonds (Table 1) [97, 98]. This fold is adopted by one of two structural classes of defensin molecules. CSαβ defensins are antimicrobial peptides involved in innate immunity in an assortment of plants, fungi, insects and arachnids [99, 100]. CSαβ peptides also dominate the venom peptidome of scorpions, with the majority of known scorpion toxins adopting this fold [101]. It is believed the CSαβ defensins were recruited and neofunctionalized in scorpion venoms, prompting an explosive proliferation of toxin genes [20]. The CSαβ motif serves as a framework for scorpion toxins that are ligands of Na+ and K+ channels, many of which are insect-selective. These toxins are discussed in the following sections.
Scorpion CSαβ toxins targeting insect Nav channels
The potential application of scorpion toxins as insecticides was first proposed more than 40 years ago, with the Nav channel toxin AahIT (PD50 0.64 pmol g−1 in Sarcophaga argyrostoma) being the first venom peptide seriously considered as a bioinsecticide lead [102]. The characterization of AahIT prompted the search for more insect-selective scorpion toxins, leading to the discovery of insecticidal toxins such as LqhαIT (PD50 16 pmol g−1 in Sarcophaga falculata), BjIT2, Cn10 (onset of toxicity at 4,047 pmol g−1 in Acheta spp.) and BotIT2 (LD50 196 pmol g−1 in Blattella germanica) [103–108]. Akin to spider ICK toxins, the majority of scorpion toxins that target Nav channels have been found to bind at either neurotoxin receptor site 3 or 4, with site-3 binders inhibiting fast inactivation and site-4 binders lowering the threshold potential required for channel activation [109].
CSαβ toxins targeting neurotoxin receptor site 3
Scorpion toxins that bind neurotoxin receptor site 3 on Nav channels are also known as α-toxins. They are divided into three classes based on their activity on vertebrate or invertebrate channels, namely classical, anti-insect, and α-like α-toxins. The classical α-toxins are highly active in mammals and exhibit low toxicity in insects. Anti-insect α-toxins are highly toxic to insects and also display low toxicity to mammals when injected i.c.v. α-Like toxins are toxic to both mammals and insects [110]. Numerous studies attempting to ascertain the pharmacophore of α-toxins have revealed two distinct regions pertinent to the interaction of the toxins with Nav channels and the partiality of toxins for insect or mammalian receptors. The first of these regions is a highly conserved core-domain, consisting of 4–5 positively charged and aromatic residues located on the loops between the secondary structure elements (Fig. 7a) [111, 112]. It is thought that the core-domain mediates the interaction of all α-toxins with a region of Nav channels conserved between insects and mammals. The indiscriminate interaction of this domain with phylogenetically different Nav channels may explain the ability of all α-toxins to bind at both insect and mammalian Nav channels, albeit with vastly different affinities [111].
Fig. 7.
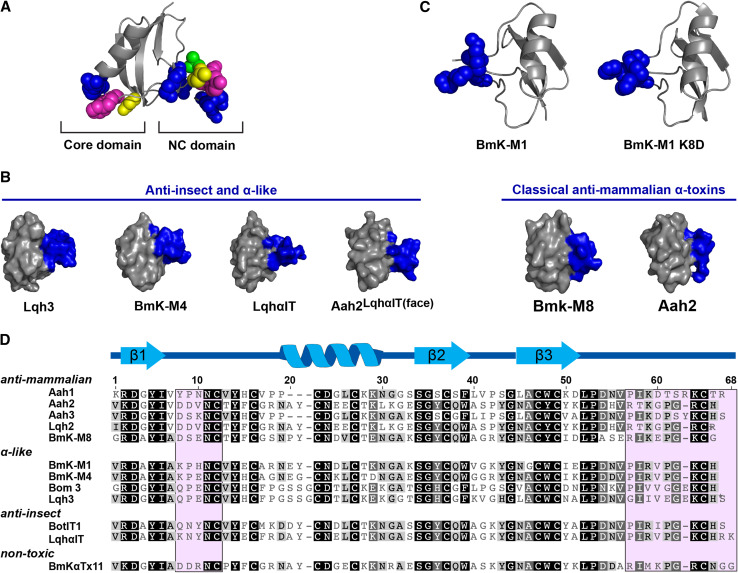
Scorpion α-toxins. a Location of the NC and Core domains in α-toxins. The chemical nature of amino acid residues is color coded as follows: aromatic, magenta; aliphatic, green; basic, blue; polar but uncharged, yellow. Adapted from [213]. b Spatial orientation of the NC-domains (colored blue) in anti-insect and mammalian-active scorpion α-toxins. c Orientation of the NC-domain (colored blue) of the α-like toxin BmK-M1 and a K8D mutant. d Representative sequences of scorpion α-toxins. Residues comprising the NC-domain are shaded in pink, and secondary structure elements are shown above the sequences. Adapted from [115]
The phyletic and channel subtype selectivity of many α-toxins is believed to arise from a second toxin region, termed the NC-domain (Fig. 7). This domain is comprised of the five-residue turn between the first β-strand and the α-helix of the CSαβ structure and the C-terminal tail [111]. In a key experiment, the entire NC-domain and residue 17 of the core-domain of the classical α-toxin Aah2 was replaced with that of the archetypal anti-insect α-toxin LqhαIT. The resulting chimeric toxin, named Aah2LqhαIT(face), was only threefold less active on blowfly larvae than LqhαIT, representing a 380-fold increase in insecticidal activity compared to Aah2. Additionally, the chimera did not bind to rat brain synaptosomes, the high affinity binding of which is characteristic of Aah2. It should be noted that a chimera in which only the NC-domain of Aah2 was substituted with that of LqhαIT was twofold less potent in insects than Aah2LqhαIT(face), hence phyletic selectivity cannot solely be attributed to residues of the NC-domain [111].
A comparison of the 3D structures of various α-toxins revealed that the spatial orientation of the NC-domain differs between insect- and mammalian-active toxins. In anti-insect and α-like α-toxins, the NC-domain protrudes into the solvent, while it is flat in classical α-toxins (Fig. 6b) [111, 113]. The protrusion may present residues of the NC-domain to a region or binding pocket on the Nav channel specific to insects. A protruding NC-domain is often associated with an uncommon non-proline cis peptide bond between residues nine and ten. In the flat configuration, this peptide bond is consistently trans [111]. Mutagenesis studies of the α-like toxin BmK M1 have highlighted residue eight as a potential ‘molecular switch’ that affects the conformation of this peptide bond in some α-toxins [114]. It was noticed that residue eight in many classical α-toxins is Asp, whereas Lys or Gln occupies this position in nearly all of the α-like and anti-insect α-toxins [115]. Residue eight of wild-type BmK M1 is Lys and a cis peptide bond exists between residues nine and ten. Upon substitution of Lys8 with Asp, the 9–10 peptide bond ‘switched’ to the trans conformation. While the NC-domain did not completely assume a flat topology, several residues of the five-residue turn in the NC-domain that protrude in the wild-type toxin adopted a flatter surface topology similar to classical mammalian-specific α-toxins (Fig. 6c) [114]. The flatter topology of the BmK M1 K8D mutant did not result in increased activity in mammals; in fact, the mutant completely lost mammalian activity, with no symptoms observed in mice at 47 times the LD50 of native BmK M1 [115]. The mutant was also 12-fold less toxic in insects compared to the wild-type toxin. Moreover, a K8Q mutant of BmK M1 that retained the cis 9–10 peptide bond conformation and protruding topology of the wild-type toxin was 200-fold less potent at insect Nav channels than the native toxin. The activities of the BmK M1 mutants demonstrate that phyletic selectivity is not solely determined by the conformation of the NC-domain, as suitable side-chain chemistries are required in order for the toxin to be able to interact with the target Nav channel.
Although the topology of the NC-domain is usually indicative of the residue at position 8 and the conformation of the 9–10 peptide bond, this is not always the case, as exemplified by the venom peptide BmKαTx11. The NC-domain of this peptide is protruding, even though the Asp at position 8 and the 9-10 trans peptide bond usually gives rise to a flat topology [116]. Closer examination of the 3D structure of BmKαTx11 and other α-toxins revealed that the hydrogen bonding properties of residue 58 (or equivalent position in relation to BmKαTx11) is another factor that can determine the orientation of the NC-domain. In anti-insect α-toxins and some α-like toxins, residue 58 is Arg compared to Lys in all classical α-toxins. A non-polar Val or Ile is also found in this position in various α-like toxins [116]. The side chains of Lys and Arg are both able to form hydrogen bonds and they indeed do so with the backbone of a Gly residue that is highly conserved within the C-terminus of all α-toxins. However, the slightly shorter side chain of Lys draws in the Gly residue to a greater extent than Arg, thus bringing the entire C-terminus closer to the bulk of the toxin [111, 113, 117]. This results in the flat topology of the classical α-toxins, while the less restrained C-terminus of toxins with Arg58 presents as a protrusion. Since Val or Ile cannot form hydrogen bonds, many α-like toxins with these residues at position 58 also have a protruding NC-domain [118].
Aside from the core and NC-domain, several additional functionally important sites have been identified. Many of these sites are toxin-specific and are not part of the functional surface of other α-toxins [119]. Furthermore, the five-residue turn in the NC-domain is not involved in the activity of some α-toxins. In the α-like toxin Lqh3, neutral or charge-inverted substitutions in the five-residue turn did not significantly decrease insecticidal activity, with the residues essential for activity located in the core and C-terminal segment [112]. While the functional surface of α-toxins are similar, these subtle site-specific differences may underlie the variation in activity between family members. Through further research, a more thorough understanding of the sites that increase toxin preference for insect rather than mammalian channels will hopefully allow molecular tuning of α-toxins to improve their selectivity for insects, thereby rendering them suitable for bioinsecticide development.
Recent mutagenesis studies [120] have identified residues within NaV channel site 3 that appear to be important for sensitivity toward either anti-insect or anti-mammalian α-toxins. Substitution of Glu1613 in the DIV/S3-S4 loop of the rat NaV1.2 channel with Asp, the residue in the equivalent position (1,701) of the Drosophila DmNav channel, rendered the channel ~1,000-fold more sensitive to LqhαIT without any loss of sensitivity to the classical α-toxin Lqh2. However, the reciprocal replacement of Asp1701 in DmNaV with Glu did not increase the sensitivity of the channel to Lqh2. DmNaV gained sensitivity to Lqh2 only upon replacement of external loops between segments of DIV and/or DI. These results suggest receptor site 3 is similar but not identical between mammalian and insect NaV channels. Further studies are required to pinpoint the insect receptor residues that are involved in interacting with anti-insect α-toxins and to determine whether these receptor residues are important for the activity of other site 3 toxins from scorpions and other organisms. Thus, only the DIV/S3-S4 loop in Fig. 2 is currently assigned as site 3. Future designations of site 3 may include regions of the external loops between segments of DI.
CSαβ toxins targeting neurotoxin receptor site 4
Also known as β-toxins, scorpion toxins that bind site 4 of Nav channels are divided into four categories based on their activity: (i) anti-mammalian toxins; (ii) β-like toxins that are active on both insect and mammalian channels; (iii) depressant anti-insect toxins; and (iv) excitatory anti-insect toxins. Depressant anti-insect β-toxins induce flaccid paralysis in insects, while excitatory anti-insect β-toxins cause immediate contractile paralysis upon injection [11]. Similar to the mode of channel interaction proposed for scorpion α-toxins, it is believed channel binding is mediated by a primary pharmacophore common to nearly all β-toxins, while a second cluster of variable amino acids determines phyletic specificity [48]. The solvent-exposed primary pharmacophore consists of a spatially conserved Glu residue located on the core α helix that is flanked by hydrophobic residues (Fig. 8a, b). Site-directed mutagenesis of the Glu and hydrophobic residues in this ‘hot spot’ region has been shown to severely decrease the binding affinity of various β-toxins [48, 121, 122]. It is thought that the hydrophobic residues act as a gasket, occluding bulk solvent and sealing the point of interaction between Glu and interacting residues on the channel, which are yet to be determined. This pharmacophore, which is common to most β-toxins, may explain their ability to competitively bind Nav channel types at which they show no activity. For example, the mammalian-specific β-toxin Cn2 is not toxic to insects, yet it competes with the excitatory insect toxin Bj-xtrIT (PD50 4.9 pmol g−1 in Sarcophaga falculata) for binding at insect Nav channels [48, 123]. It should be noted that several β-toxins do not have an acidic residue at the ‘hot spot’ region and further investigations are necessary to elucidate the functional surface of these toxins.
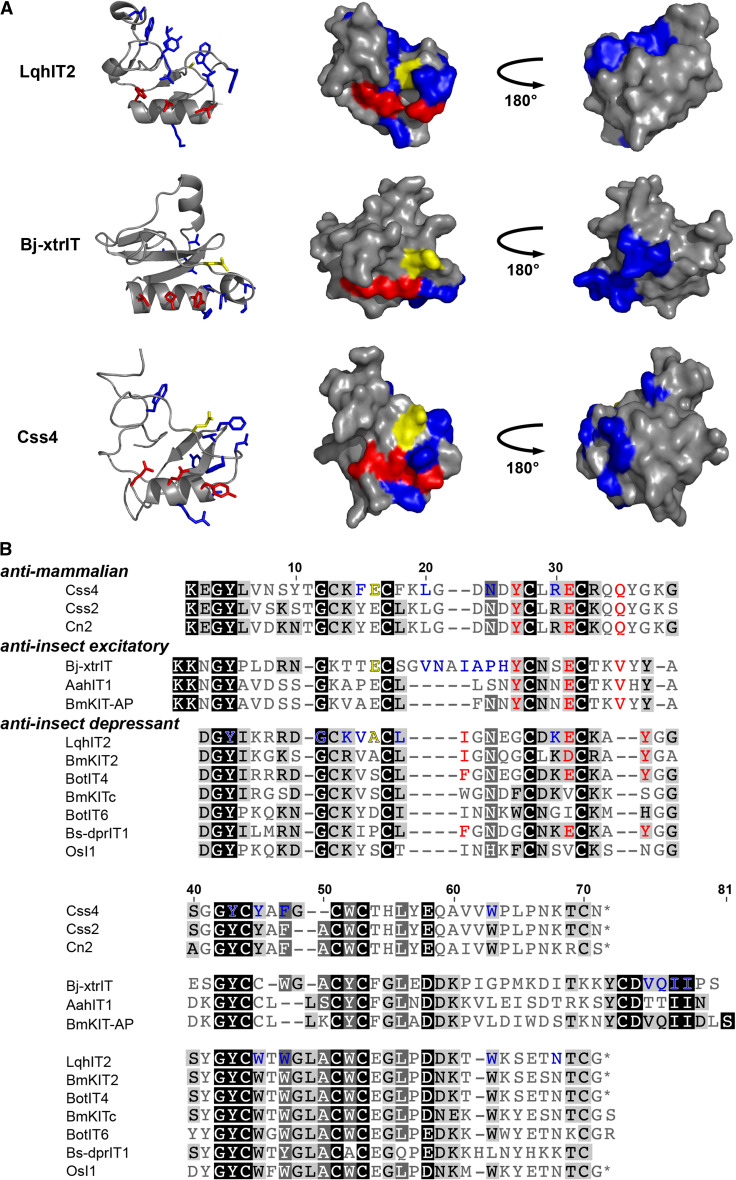
Fig. 8.
Scorpion β-toxins. a Structures of representative anti-mammalian (Css4, homology model from [48]), anti-insect excitatory (Bj-xtrIT, PDB 1BCG), and anti-insect depressant (LqhIT2, PDB 2I61) scorpion β-toxins. Red denotes the primary pharmacophore located on the α-helix consisting of a central Glu flanked by two hydrophobic residues. Blue residues comprise the secondary pharmacophore. Glu15 in Bj-xtrIT and Css4, and Ala13 in LqhIT2 are colored yellow. b Primary structures of representative scorpion β-toxins
The second bioactive surface believed to be responsible for phyletic selectivity is formed by a group of hydrophobic residues. Based on mutagenesis of Bj-xtrIT and Css4, this surface is proposed to be located at the C-terminus of excitatory anti-insect β-toxins and the β2 strand and loops connecting secondary structures in anti-mammalian toxins (Fig. 8a, b) [48, 121, 123]. In the depressant insect-selective toxin LqhIT2, this hydrophobic region is positioned in a groove preceding the α-helix near the N-terminus, termed the ‘N-groove’ [122]. Residue Ala13 in the N-groove of LqhIT2 has a particularly interesting role. Substitution of Ala13 with a charged residue (Glu or Arg) was detrimental to binding, however substitution with Trp dramatically increased toxin activity at the insect Nav channel with very little effect on binding affinity [122]. Glu15 in Bj-xtrIT and Css4, the residue in the same spatial position as Ala13, appears to play a similar functional role. An uncoupling of activity from binding was seen in E15R mutants of Bj-xtrIT and Css4, as both toxins were rendered inactive by this mutation while incurring only a slight decrease in binding affinity [48, 121]. As mentioned in the section above on ICK spider toxins that bind site 4 of Nav channels, Glu15 may be functionally analogous to Asp19 in δ-amaurobitoxin-Pl1b [45].
The chemical nature of residue 13 in LqhIT2 and its equivalent position in other toxins can also affect the overall topology of the N-groove. A structural comparison of potent and weak β-toxins revealed that highly active β-toxins possess a deep N-groove about 8 Å in width and depth, while the groove is shallow and less defined in weakly active toxins [122]. Both binding and activity were abrogated in an E15Q mutant of Bj-xtrIT, whose N-groove was distorted from the deep hollow of the wild-type toxin to a more protruding structure; thus, the loss of activity in toxins with mutations at this position might be attributed to structural perturbances of the N-groove [48, 112]. Additionally, subtle differences within the N-groove of potent β-toxins may contribute to phyletic selectivity. In the anti-insect toxin LqhIT2 and β-like toxin Ts1, a Lys side chain lies at the bed of the N-groove, while the equivalent Lys in the anti-mammalian toxin Cn2 forms an ionic interaction with Glu15 and bends back, revealing a narrow cavity [122, 124, 125]. A mutant of the Cn2 homologue Css4 was made in which Glu15 was replaced with Ala, thereby removing the interaction restraining Lys and allowing its side chain to assume a similar position in the N-groove as LqhIT2 and Ts1. While wild-type Css4 is non-toxic to insects, the E15A mutant gained activity at the insect Nav channel and induced contractile paralysis in blowfly larvae. The E15A Css4 mutant also retained high binding affinity and activity at mammalian Nav channels, suggesting that the residue at the bed of the N-groove may be important for activity in insects but not mammals [121, 122].
Excitatory and depressant insect β-toxins interact with different regions of Nav channel site 4. Depressant toxins bind at two non-overlapping regions, one with high affinity and low capacity, and the other with low affinity and high capacity [126, 127]. Excitatory toxins interact solely with the high affinity site, as demonstrated by competition binding assays using locust and cockroach membranes which revealed that excitatory toxins are only able to compete with depressant toxins for binding to the high affinity site [127–129]. The location of the common high affinity binding site is suggested by Nav channel mutagenesis studies that have shown both the excitatory toxin AahIT and the depressant toxin BmK IT2 interact with a region located on domain two of insect Nav channels, with the receptor site of BmK IT2 further isolated to the S3-S4 linker [130, 131]. This region is essential for the response of the channel to toxin as mutations of G904, E896 or L899 in the S3-S4 linker of domain two rendered the channel insensitive to BmK IT2 [131]. However, these residues are well conserved in mammalian Nav channels upon which BmK IT2 does not act, and therefore these residues likely mediate the interaction of the toxin with the voltage sensor after initial binding but not the phyletic preference of β-toxins. BmK IT2 also interacts with the N-part of the domain 3 SS2-S6 loop and residues Ile1529 and Arg1530 of the channel, and it is this region that may be the recognition epitope for insect-selective depressant β-toxins [131]. The DII S3-S4 and DIII SS2-S6 linkers of mammalian channels are also important for their interaction with anti-mammalian β-toxins, as well as the DII S1-S2 linker [132, 133]. However, the involvement of the DII S1-S2 linker in binding of scorpion toxins by insect Nav channels remains to be established. The endeavor to dissect the structure–activity relationships between β-toxins and the insect Nav channel has uncovered details that aid our understanding of the functional surfaces required for insect-specificity. Nevertheless, much remains unknown, including the molecular basis underlying the differences in sensitivity of various insect species to some β-toxins. For example, AahIT is at least 100-fold more toxic to Sarcophaga falculata blowflies (LD50 18 pmol g−1) than the larvae of the cotton leafworm Spodoptera littoralis (LD50 1791 pmol g−1), and it appears to be non-toxic to the tenebrionid beetle Trachyderma philistina [134–136]. This variable toxicity towards insects of different families may be employed to develop insecticides targeting pest species while reducing the negative impact on beneficial insects such as pollinators and parasitoids.
Scorpion CSαβ toxins targeting insect Kv channels
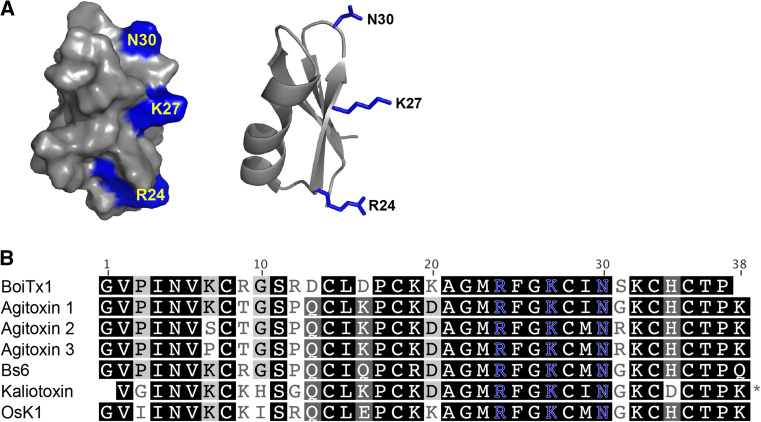
Kv channel blockers are the second most abundant peptides characterized from scorpion venoms after Nav channel toxins, with over 200 sequences described to date [80]. Despite such a large pool of scorpion-derived Kv channel toxins, only one shows high preference for insect over mammalian Kv channels. Named BoiTx1, this toxin is at least 100-fold more active on the Drosophila shaker Kv channel than a range of human and rat Kv channels, including the mammalian shaker homologues Kv1.1 and Kv1.3 [137]. The shaker channel is involved in cellular repolarization after the initial depolarization phase of an action potential [138], and its blockage leads to repetitive firing and persistent action potentials. The resulting phenotype includes sustained muscle contraction, which is observed in Drosophila larvae upon injection with BoiTx1 [137]. The closest orthologue of BoiTx1 with 84 % sequence identity is agitoxin 1, which is only ten times more potent on shaker than Kv1.3 [139]. Scorpion toxins that are active on Shaker and mammalian shaker-like channels are classified as members of the α-KTx3 family [140]. Scanning mutagenesis of agitoxin 2 revealed that residues Arg24, Lys27, and Asn30 are crucial for toxin binding to the shaker channel and that these residues are conserved throughout the α-KTx3 family (Fig. 9a, b) [141]. Although the structure–activity relationship between the insect Shaker channel and BoiTx1 has not been fully dissected, sequence comparison with other α-KTx3 toxins has highlighted a few residues that may provide BoiTx1 with its relatively marked preference for insect channels. This includes the lack of a C-terminal basic residue that is present in most other α-KTx3 toxins and which is hypothesized to affect the spatial position of the critical Arg24 and its predicted interaction with a Glu residue on the Shaker channel [137, 142]. The discovery of BoiTx1 and its preference for insect Kv channels has presented the Shaker channel as a novel insecticidal target. Simultaneous deployment of Shaker channel blockers and sodium channel modulators could potentiate their insecticidal actions [137] and reduce the likelihood of resistance arising. Future mutagenesis studies coupled with the discovery of insect-specific α-KTx3 toxins may reveal the functional surfaces required for Shaker channel specificity.
Fig. 9.
Scorpion KV toxins. a Surface (left) and ribbon (right) representations of agitoxin 2 (PDB 1AGT), with residues crucial for binding to KV1.3 channels highlighted in blue. b Representative sequences of members of the α-KTx3 scorpion toxin family
DDH fold
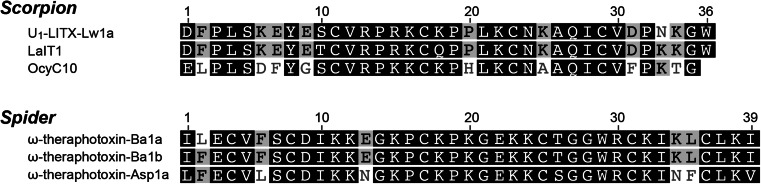
Composed of a double-stranded β-sheet core stabilized by two disulfide bonds, the disulfide-directed hairpin, or DDH fold, is considered the evolutionary precursor to the ICK fold (Table 1) [65, 143]. Several proteins from evolutionarily diverse organisms, such as the cellulose-binding domain of cellobiohydrolase I from the Trichoderma reesei fungus and the pancreatic lipase cofactor colipase from various vertebrates either contain or appear to have arisen through elaborations of the DDH fold [65]. In venomous species, it is hypothesized that the ICK fold arose from the addition of a single disulfide bond to the DDH fold (Fig. 1a), resulting in the characteristic disulfide-pierced ring structure [65, 143]. The dominance of the ICK fold over the DDH fold in venoms presumably results from the extraordinary chemical and thermal stability and greater resistance to enzymatic degradation conferred by the cystine knot [23]. Nevertheless, toxins that adopt the simpler DDH fold have been found in the venom of spiders and scorpions, though they are uncommon. ω-TRTX-Ba1a and ω-TRTX-Ba1b are two insect-selective DDH toxins from the venom of the Mexican golden redrump tarantula Brachypelma albiceps that are lethal to crickets (Ba1a: LD50 2,451 pmol g−1, Ba1b: 2,072 pmol g−1 in Acheta domestica) but non-toxic to mice when injected intracranially or intraperitoneally [144]. Although these toxins contain three disulfide bonds, they are not arranged in the ICK motif [144]. The molecular target for these toxins have not been elucidated, however they have been provisionally assigned the ω prefix based on sequence homology to the ICK toxin ω-TRTX-Asp1a which is active on vertebrate Cav channels (Fig. 10) [145]. Two-disulfide DDH peptides with activity across various insect families have also been isolated from the venom of Liocheles and Opisthacanthus sp. scorpions [143, 146, 147].
Fig. 10.
Arachnid venom peptides with a disulfide-directed β-hairpin (DDH) fold
Linear peptides
Non-disulfide bonded peptides are ubiquitous in nature and serve many purposes, from neurotransmission [148, 149] to forming spider silk [150]. A major property of linear peptides in venoms appears to be antimicrobial activity and it is has been proposed they may protect venom glands from microbial infection [151], although this seems unlikely in the case of spiders [152]. In communal arthropods such as ants and bees, antimicrobial excretions may protect the colony from pathogens and prevent fungal and bacterial outbreaks in the nest or hive [153, 154].
Linear venom peptides are usually amphipathic and although they are structurally disordered in aqueous solution, they typically adopt an α-helical conformation in the presence of membranes containing negatively charged lipids [24], thereby forming pores that result in cell lysis. It is unlikely that they target a specific receptor as they generally display broad-spectrum antimicrobial, antifungal, and cytolytic activity, though their activity profile varies [155–157]. Some linear antimicrobial venom peptides also have low levels of insecticidal activity, presumably due to local tissue damage caused by lysis [158, 159]. Their weak insecticidal activity and lack of a specific molecular target renders the linear venom peptides unsuitable as standalone insecticides.
It has been proposed that their primary role of cytolytic venom peptides is to potentiate the action of the disulfide-rich neurotoxins by breaking down anatomical barriers, dissipating transmembrane ion gradients, and/or perturbing the membrane potential across excitable cells [152]. Thus, they could potentially be used in combination with other venom peptides to enhance bioactivity. Consistent with this idea, it was demonstrated that the combined injection of a disulfide-rich spider-venom neurotoxin and the linear spider-venom peptide M-oxotoxin-Ot1a into tobacco cutworms resulted in over tenfold reduction in IC50 compared with injection of the neurotoxin alone [160]. Furthermore, the time required for the neurotoxin to exert its paralytic and lethal effects on the larvae was greatly reduced upon co-injection with the linear peptide. A synergistic effect was also observed between the linear scorpion-venom peptide pandinin-2 and spider neurotoxins [160]. These amphipathic linear peptides therefore have the potential to augment the efficacy of insecticidal neurotoxins. However, a targeted delivery approach may need to be implemented to ensure their cytotoxic effects are localized to pest invertebrates and do not adversely affect vertebrates or the crop that is being produced.
An unusual linear peptide named conomap-Vt from the venom of Conus vitulinus was found to be homologous to myoactive tetradecapeptides (MATPs) found in annelids, molluscs and insects (Fig. 11) [161]. MATPs are short linear peptides with excitatory or inhibitory activity in invertebrate muscles, however their pharmacological targets have not been ascertained [162, 163]. Conomap-Vt exerted a potent excitatory contractile effect on a range of molluscan muscle preparations without being active on isolated rat atria and ileum [161]. While the activity of conomap-Vt in insects is yet to be determined, its similarity to MATPs suggests it will be active in arthropods. Further characterization of this interesting peptide is required to determine its suitability as a candidate for insecticide development.
Fig. 11.
Homology between linear conomap venom peptides and myoactive tetradecapeptides (MATPs). Asterisks indicates an amidated C-terminus, while lowercase letters indicate d-amino acids
Defensin-like and neurotoxin III fold
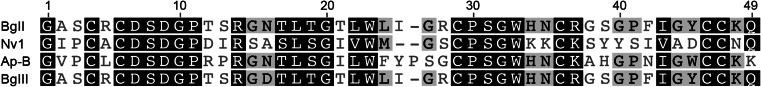
Peptides of the defensin-like structural fold are disulfide-rich and consist primarily of β strands (Table 1). Included in this fold are the human β-defensins involved in innate immunity and melanogenesis [164, 165], as well as the related bovine and murine defensins. Akin to the CSαβ defensins, the crotamine myotoxins in rattlesnake venoms and the β-defensin-like peptides in platypus venom arose from duplication and functional diversification of β-defensin genes [20, 166, 167]. Defensin-like toxins are also one of the major components of sea anemone venoms and comprise the type 1 class of anemone toxins active on Nav channels [168]. Although rich in structurally diverse peptides that target various Nav and Kv channels, the only sea anemone toxins found to show significant selectivity for insect over mammalian channels are the Nav channel ligands Nv1, BgII and Av3 (Fig. 12) [169–172]. Nv1 and BgII are defensin-like toxins belonging to the type 1 class of anemone toxins. Av3 is comprised of four reverse turns and two chain reversals with no α-helix or β-sheet structures [169, 173, 174]. Av3 has no structural homologues and thus defines the monoclastic neurotoxin III fold. Nv1 is hypothesized to bind Nav channels at site 3 as it presents the typical site 3 modulatory action of inhibiting channel inactivation [169]. Av3 and BgII also inhibit inactivation of insect Nav channels and were shown to compete with the site-3 scorpion toxins LqhαIT, Aah II, and the sea anemone toxin CgNa, respectively [171–173]. As mentioned in the previous section on ICK toxins from cone snails, the ability of sea anemone toxins to target insects may be due to conservation between insect and marine arthropod ion channels. Additionally, many insect larval stages subsist in water where they are likely to encounter sea anemones. Indeed, the anemone Nematostella vectensis from which the toxin Nv1 was discovered includes insect larvae in its diet [175].
Fig. 12.
Sea anemone toxins with defensin-like and neurotoxin III folds
Of the three described insecticidal sea anemone toxins, Av3 has the highest preference for insects, being at least 300-fold more toxic to blowfly larvae (PD50 11.5 pmol g−1 in Sarcophaga falculata) than mice and having a negligible effect at 10 μM on mammalian Nav1.2, Nav1.3, Nav1.5, and Nav1.6 channels expressed in Xenopus oocytes [172]. Although the activity of Nv1 in mammals has not been established, it had an insignificant effect on rat Nav1.2 and Nav1.4, as well as human Nav1.5 channels at 25 μM, while 1 μM completely abolished inactivation of the Drosophila DmNav channel [169]. BgII is at least 15-fold more potent on DmNav channels than Nav1.2, the most sensitive mammalian channel tested [171]. However, BgII is potent in mice upon i.c.v. injection, causing toxicity at 80 pmol/kg, and it binds rat brain synaptosomes with a K d of 9 nM [173]. Consequently, BgII is unlikely to be a useful insecticidal lead, although ascertaining the molecular epitopes that confer its toxicity to both insects and mammals might allow engineering of more insect-selective analogues. APETx3 is the most recently discovered insect selective sea anemone toxin; it is eightfold less potent at the most sensitive mammalian channel tested (Nav1.6) than at DmNav1 and the cockroach BgNav1.1 channel [170]. Additional structure–function studies of this group of toxins and testing over a more comprehensive array of channels, tissues, and whole organisms are necessary to better understand their mechanism of action and phyletic selectivity.
Most research on sea anemone toxins has focused on their interaction with mammalian channels, with very few studies investigating the basis of toxin preference for insect or mammalian targets. Alanine mutagenesis of surface residues of the insect and mammalian active type 1 toxin Av2 revealed that the anti-insect bioactive surface is comprised of residues Val2, Leu5, Asp9, Asn16, Leu18 and Ile41. Five of these residues were also important for activity of Av2 on the human Nav1.5 channel, and therefore the toxin surfaces involved in the insect and mammalian bioactivity of Av2 appear to be similar [176]. Additionally, the residues equivalent to Asp9, Asn16 and Leu18 in the highly potent mammalian type 1 toxin Anthopleurin-B (Ap-B) are also functionally significant [177–179]. The role of Asn16 in insect selectivity is further highlighted by comparison of the activities BgII and BgIII. BgII is at least 180-fold more active on insect Nav channels than BgIII, though differing by only a single residue at position 16; Asn in BgII and Asp in BgIII [171, 173]. The six residues important to the insecticidal activity of Av2 are not, however, conserved throughout all type 1 toxins that are able to affect insects. Four of the six residues are conserved in BgII while only three are present in Nv1 [169, 173, 176]. This suggests the toxin face that interacts with the receptor, as well as the exact location of the toxin binding site within neurotoxin receptor site 3 on the Nav channel varies between different type 1 toxins.
APETx3 differs from APETx1 by only one residue (position 3 in APETx1 is Thr, while it is a Pro in APETx3) (Fig. 12), yet the two toxins have vastly dissimilar pharmacological profiles. APETx1 is a promiscuous toxin, acting on the human ether-á-go-go related gene (hERG) K+ channel (KV11.1) and mammalian Nav channel subtypes 1.2, 1.4, 1.5, 1.6 and 1.8 [170]. Unlike APETx3 however, APETx1 does not affect the insect Nav channels DmNav1 and BgNav1.1. Moreover, APETx1 inhibits Nav channel conductance and interacts with neurotoxin binding site 1, while APETx3 locks the channel in the open state and binds to site 3 [170]. The manifestation of such remarkably different functional profiles caused by a single amino acid change is a structure–activity relationship enigma. It is thought the Pro3 residue of APETx3 may introduce a structural kink that is not present in APETx1, resulting in a conformational change that may explain the functional differences [170]; however, detailed structural analyses are required to examine this hypothesis.
Other potential insecticidal leads
The sections above highlight the folds adopted by the majority of venom-derived insecticidal toxins known to date. There are also numerous other venom components with insecticidal properties, however they have not been studied in as much detail or they are non-discriminant in their action towards vertebrates and invertebrates. Thus, in the following sections, we provide only a brief discussion of these toxins.
Contryphan fold
The contryphans are a group of 7–12-residue peptides from the venom of marine cone snails. Their 3D structure consists of a well-defined loop constrained by a single disulfide bridge (Table 1) and they are rich in unusual post-translational modifications including tryptophan bromination, proline hydroxylation and, characteristically, d-tryptophan or d-leucine [180]. Less than 20 contryphans have been discovered, with pharmacologically characterized members displaying mammalian toxicity (Fig. 13) [181]. However, Contryphan-Vn also affects voltage-gated and calcium-dependent K+ channel currents in cockroach DUM neurons at a concentration of 20 μM [182]. While Contryphan-Vn modulates K+ channels in cultured rat fetal chromaffin cells and very weakly binds to human Kv1.1 and 1.2 channels [182], further investigation into contryphans may uncover members with insect selectivity.
Fig. 13.
Contryphan peptides. Asterisks indicate amidated C-termini and lowercase letters indicate d-amino acids. B and γ denote bromotryptophan and γ-carboxyglutamic acid, respectively
Three-finger snake toxins
The three-finger toxins found in snake venoms contain 60–74 amino acid residues and are crosslinked by 4–5 disulfide bonds (Table 1) [183]. They are named after their 3D characteristic structure composed of three β-stranded loops resembling fingers extending from a hand formed by the small hydrophobic peptide core [184]. While these toxins have primarily evolved to target nicotinic acetylcholine receptors (nAChRs) in vertebrate prey, a recent study demonstrated that six members can also block cockroach nAChRs at low concentration (0.1 μM) (Fig. 14) [185]. With additional studies to dissect the molecular basis for their activity at invertebrate nAChRs, the three-finger toxin fold may become a useful scaffold for engineering insecticides.
Fig. 14.
Insecticidal three-finger toxins from snake venoms
Proteins
In addition to small neurotoxins, venoms from many organisms also contain larger proteins and enzymes that often have insecticidal activity. For example, the large latroinsectotoxins found in the venom of widow spiders (genera Latrodectus and Steatoda) are the most potent insecticidal toxins isolated to date from spider venoms. Latroinsectotoxins induce exhaustive neurotransmitter release at insect neuromuscular junctions, and they have extremely low LD50 values of <1 pmol g−1 in both lepidopterans and dipterans [186]. However, they have not been pursued as bioinsecticide leads due to their large size (110–140 kDa), complex mode of action, and the difficulty of producing them using synthetic or recombinant methods [24].
A number of proteins of mass 16 kDa and larger isolated from the venom of the small ectoparasitic wasp Bracon hebetor cause flaccid paralysis in invertebrate pest species including the tobacco budworm [187]. Many venoms also contain enzymes that are likely to be intrinsically insecticidal in addition to potentiating the activity of disulfide-rich neurotoxins found in venom [24]. For example, sphingomyelinase found in the venom of sicariid siders was recently shown to be lethal to crickets [188]. However, these enzymes are unlikely to be useful insecticidal leads as their activity often extends to vertebrates. For example, sphingomyelinase is responsible for the dermonecrotic lesions and serious systemic effects in humans (loxoscelism) that sometimes result from bites by sicariid spiders [189]. Moreover, unlike peptides, enzymes have the disadvantage of being more difficult and costly to produce on a commercial scale.
Deployment of insecticidal venom peptides
Orally active fusion proteins
In contrast with chemical insecticides, venom peptides are unlikely to be topically active since they would have to penetrate the insect exoskeleton in order to access their molecular targets in the insect nervous system. Thus, in order to be effective they must be delivered to insect pests via a vector such as an entomopathogen or ingested by the targeted insect pest if they have oral activity.
While some insecticidal venom peptides are orally active [190], most are ~90-fold less potent when fed to insects compared to when they are injected [24]. This lower activity results primarily from a slow rate of absorption in the insect gut, as observed for disulfide-rich peptides from scorpion and snake venoms [191]. Thus, the commercial potential of insecticidal venom peptides would be enhanced by any approach that significantly improved their oral activity. One promising option is to fuse venom peptides with a carrier protein that facilitates their transport across the insect gut. The best studied fusion protein for this purpose is Galanthus nivalis agglutinin (GNA), a mannose-specific lectin from the snowdrop plant. When ingested by insects, GNA binds to glycoproteins in the digestive tract and is subsequently transported across the gut epithelium into the hemolymph; over a period of several hours, the protein accumulates in the insect gut, Malpighian tubules, hemolymph, and central nervous system [192, 193]. Thus, GNA can be fused to insecticidal peptides to enhance their transport across the gut to sites of action in the nervous system, thereby enhancing their oral activity. This approach has been used to massively enhance the oral insecticidal activity of a variety of venom peptides from both spiders and scorpions [79, 193–195].
Enhanced entomopathogens
Since venom peptides are genetically encoded mini-proteins, an alternative method of peptide delivery is to engineer entomopathogens to express transgenes encoding these toxins. For example, transgenes encoding a variety of insecticidal arachnid and sea anemone toxins have been engineered into lepidopteran-specific baculoviruses. Wild-type baculovirus are generally not competitive with chemical insecticides because they typically take a week or longer to kill their host, during which time the insect continues to feed and cause crop damage [196]. In contrast, the time between virus application and the cessation of feeding was dramatically reduced in transgenic viruses engineered to express a variety of insecticidal venom peptides [12].
The potency and speed of kill of the entomopathogenic fungus Metarhizium anisopliae can also be enhanced by engineering it to express insecticidal venom peptides. For example, a transgenic fungus expressing the scorpion-venom peptide AahIT reduced the kill time as well as the dose required to kill the tobacco hornworm Manduca sexta and the dengue vector Aedes aegypti [197]. Moreover, mosquitoes infected with transgenic fungus had a reduced tendency to blood feed [197].
Engineering entompathogens to express insecticidal venom-peptide transgenes mitigates two of the potential disadvantages of venom peptides as bioinsecticides. First, in this scenario, the venom peptides are produced systemically in the insect host after viral/fungal infection, and hence lack of oral activity is not an impediment to toxin deployment. Second, the phylum selectivity of the venom peptide becomes unimportant as the range of affected insects will be determined largely by the host range of the entomopathogen. This should limit off-target effects, particularly on predators and parasitoids, since entomopathogens can be chosen that have a very restricted host range; for example, Metarhizium acridum exclusively infects grasshoppers in the suborder Caelifera [198] and hence it is ideal as a locust-specific bioinsecticide.
Transgenic plants
Transgenes encoding insecticidal venom peptides can also be engineered into crop plants. In 2010, 148 million hectares of genetically modified (GM) crops were planted in 29 countries, representing 10 % of all cropland [199]. The active transgene in all extant insect-resistant GM crops encodes an insecticidal protein known as δ-endotoxin, Cry toxin, or simply Bt from the bacterium Bacillus thuringiensis. Insecticidal venom peptides might represent viable alternatives to Bt transgenes as they have vastly different modes of action and different phylum selectivities.
A number of plants have been engineered to express insecticidal spider-venom peptides, beginning in 1996 with the demonstration that transgenic tobacco expressing ω-HXTX-Ar1a from the Australian funnel-web spider Atrax robustus have enhanced resistance to larvae (cotton bollworms) of the recalcitrant lepidopteran pest Helicoverpa armigera [200]. Transgenes encoding this venom peptide (or its orthologue ω-HXTX-Hv1a) have subsequently been engineered into cotton [201], tobacco [202], and poplar [203]. Tobacco plants have also been engineered to express Magi-6, a 36-residue insecticidal peptide from the venom of the related hexathelid spider Macrothele gigas [204]. All of these transgenic plants have enhanced resistance to lepidopteran pests and it has even been claimed that transgenic cotton expressing ω-HXTX-Hv1a is as effective as Monsanto’s pyramided Bollgard II® cotton for controlling major cotton pests [201].
Thus, transgenes encoding insecticidal venom peptides hold promise as a standalone insect-resistant plant traits. Moreover, these transgenes might be good candidates for trait stacking with Bt since they have completely different mechanisms of action and are likely to be synergized by Bt, which should facilitate movement of venom peptides into the insect hemocoel by virtue of its ability to induce lysis of midgut epithelial cells [205].
Future prospects and concluding remarks
The armamentarium of insecticides for control of insect pests is rapidly diminishing due to the evolution of insecticide resistance and the de-registration of key insecticide classes due to concerns about their impact on human health and the environment. Consequently, there is a pressing need for the development of novel ligands for current insecticide targets or, better still, ligands with novel modes of insecticidal action. Due to their high potency, stability, and molecular and organismal selectivity, a number of venom peptides are being used as leads for bioinsecticide development. The current review has showcased the multitude of insecticidal toxins present in animal venoms and their wide range of molecular targets. Although the putative functional surfaces of many insecticidal toxins have been deduced, there are clear knowledge gaps regarding how slight differences between toxins can result in large variations in channel selectivity. The answers to these questions undoubtedly lie in the further mapping of sites on receptors where toxins bind as well as studying the structural changes that occur in receptors upon toxin binding.
With few exceptions [206–211], methods to elucidate the structure of receptor–toxin complexes are mostly limited to computational studies. Technical advancements of existing methodologies such as NMR spectroscopy and X-ray crystallography are needed to expedite the study of such complexes. A better understanding of the dynamic interactions between toxins and receptors would bring us closer to the ultimate goal of predicting the selectivity of toxins from their sequence or structure alone, bypassing the often laborious and time consuming process of biological screens. Despite these current limitations, the numerous insecticidal venom peptides discussed herein provide ample leads for insecticidal development. While these peptides could be used in a similar manner as chemical insecticides, for example as foliar sprays, the fact that they are genetically encoded mini-proteins opens the door to a wider variety of deployment methods, such as incorporation of transgenes encoding these peptides into crop plants and entomopathogens. Thus, venom-derived insecticidal peptides appear to have immense potential for the development of novel bioinsecticides.
Abbreviations
- 3D
Three-dimensional
- BKCa
Maxi-K calcium-activated potassium channel
- CaV
Voltage-gated calcium channel
- CSα/β
Cystine-stabilized α/β
- DDH
Disulfide-directed β-hairpin
- DmNaV
Drosophila voltage-gated sodium channel
- GABA
γ-aminobutyric acid
- HVA
High-voltage activated
- HXTX
Hexatoxin
- ICK
Inhibitor cystine knot
- KV
Voltage-gated potassium channel
- MATP
Myoactive tetradecapeptide
- NaV
Voltage-gated sodium channel
- LVA
Low-voltage activated
- MVA
Mid-voltage activated
- nAChR
Nicotinic acetylcholine receptor
- NMDA
N-methyl-d-aspartic acid
- RyR
Ryanodine receptor
- TRTX
Theraphotoxin
- tLVA
Transient low-voltage activated
- TTX-R
Tetrodotoxin-resistant
- TTX-S
Tetrodotoxin-sensitive
Contributor Information
Glenn F. King, Email: glenn.king@imb.uq.edu.au
Paul F. Alewood, Email: p.alewood@imb.uq.edu.au
References
- 1.Oerke EC. Crop losses to pests. J Agric Sci. 2006;144(1):31–43. doi: 10.1017/S0021859605005708. [DOI] [Google Scholar]
- 2.Pimental D. Pesticides and pest control. In: Peshin R, Dhawan AK, editors. Integrated pest management: innovation-development process. Dordrecht: Springer; 2009. pp. 83–87. [Google Scholar]
- 3.Tedford HW, Sollod BL, Maggio F, King GF. Australian funnel-web spiders: master insecticide chemists. Toxicon. 2004;43(5):601–618. doi: 10.1016/j.toxicon.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Windley MJ, Herzig V, Dziemborowicz SA, Hardy MC, King GF, Nicholson GM. Spider-venom peptides as bioinsecticides. Toxins. 2012;4(3):191–227. doi: 10.3390/toxins4030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai TC, Su JY. Assessment of resistance risk in Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae) to chlorantraniliprole. Pest Manag Sci. 2011;67(11):1468–1472. doi: 10.1002/ps.2201. [DOI] [PubMed] [Google Scholar]
- 6.Bass C, Field LM. Gene amplification and insecticide resistance. Pest Manag Sci. 2011;67(8):886–890. doi: 10.1002/ps.2189. [DOI] [PubMed] [Google Scholar]
- 7.Bates SL, Zhao JZ, Roush RT, Shelton AM. Insect resistance management in GM crops: past, present and future. Nat Biotechnol. 2005;23(1):57–62. doi: 10.1038/nbt1056. [DOI] [PubMed] [Google Scholar]
- 8.Casida JE, Quistad GB. Golden age of insecticide research: past, present, or future? Annu Rev Entomol. 1998;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Koureas M, Tsakalof A, Tsatsakis A, Hadjichristodoulou C. Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicol Lett. 2012;210(2):155–168. doi: 10.1016/j.toxlet.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Zaim M, Guillet P. Alternative insecticides: an urgent need. Trends Parasitol. 2002;18(4):161–163. doi: 10.1016/S1471-4922(01)02220-6. [DOI] [PubMed] [Google Scholar]
- 11.Gurevitz M, Karbat I, Cohen L, Ilan N, Kahn R, Turkov M, Stankiewicz M, Stuhmer W, Dong K, Gordon D. The insecticidal potential of scorpion β-toxins. Toxicon. 2007;49(4):473–489. doi: 10.1016/j.toxicon.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Maggio F, Sollod BL, Tedford HW, King GF. Spider toxins and their potential for insect control. In: Gilbert LI, Gill SS, editors. Insect pharmacology: channels, receptors, toxins and enzymes. London: Academic Press; 2010. pp. 101–123. [Google Scholar]
- 13.Undheim EA, King GF. On the venom system of centipedes (Chilopoda), a neglected group of venomous animals. Toxicon. 2011;57(4):512–524. doi: 10.1016/j.toxicon.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Yang DM, Hu KP, Li CY, Wu CH, Zhou PA. Neural electrophysiological effect of crude venom of Conus textile from the South China Sea. Toxicon. 2000;38(11):1607–1612. doi: 10.1016/S0041-0101(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 15.Orivel J, Redeker V, Le Caer JP, Krier F, Revol-Junelles AM, Longeon A, Chaffotte A, Dejean A, Rossier J. Ponericins, new antibacterial and insecticidal peptides from the venom of the ant Pachycondyla goeldii . J Biol Chem. 2001;276(21):17823–17829. doi: 10.1074/jbc.M100216200. [DOI] [PubMed] [Google Scholar]
- 16.Quistad GB, Nguyen Q, Bernasconi P, Leisy DJ. Purification and characterization of insecticidal toxins from venom glands of the parasitic wasp Bracon hebetor . Insect Biochem Mol Biol. 1994;24(10):955–961. doi: 10.1016/0965-1748(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 17.Bosmans F, Tytgat J. Sea anemone venom as a source of insecticidal peptides acting on voltage-gated Na+ channels. Toxicon. 2007;49(4):550–560. doi: 10.1016/j.toxicon.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu HH, Liu XG, Dong XL, Li CP, Xing RE, Liu S, Li PC. Insecticidal activity of proteinous venom from tentacle of jellyfish Rhopilema esculentum Kishinouye. Bioorg Med Chem Lett. 2005;15(22):4949–4952. doi: 10.1016/j.bmcl.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Primor N, Teitelbaum Z, Zlotkin E. Penetrability of orally toxic protein from cobra venom through the gut of a blowfly. Biochim Biophys Acta. 1980;627(1):71–81. doi: 10.1016/0304-4165(80)90124-5. [DOI] [PubMed] [Google Scholar]
- 20.Fry BG, Roelants K, Champagne DE, Scheib H, Tyndall JD, King GF, Nevalainen TJ, Norman JA, Lewis RJ, Norton RS, Renjifo C, de la Vega RC. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu Rev Genomics Hum Genet. 2009;10:483–511. doi: 10.1146/annurev.genom.9.081307.164356. [DOI] [PubMed] [Google Scholar]
- 21.Windley MJ, Escoubas P, Valenzuela SM, Nicholson GM. A novel family of insect-selective peptide neurotoxins targeting insect large-conductance calcium-activated K+ channels isolated from the venom of the theraphosid spider Eucratoscelus constrictus . Mol Pharmacol. 2011;80(1):1–13. doi: 10.1124/mol.110.070540. [DOI] [PubMed] [Google Scholar]
- 22.Pallaghy PK, Nielsen KJ, Craik DJ, Norton RS. A common structural motif incorporating a cystine knot and a triple-stranded β-sheet in toxic and inhibitory polypeptides. Protein Sci. 1994;31:833–1839. doi: 10.1002/pro.5560031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saez NJ, Senff S, Jensen JE, Er SY, Herzig V, Rash LD, King GF. Spider-venom peptides as therapeutics. Toxins. 2010;2:2851–2871. doi: 10.3390/toxins2122851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King GF, Hardy MC. Spider-venom peptides: structure, pharmacology, and potential for control of insect pests. Annu Rev Entomol. 2013;58:475–496. doi: 10.1146/annurev-ento-120811-153650. [DOI] [PubMed] [Google Scholar]
- 25.Craik DJ, Daly NL, Waine C. The cystine knot motif in toxins and implications for drug design. Toxicon. 2001;39(1):43–60. doi: 10.1016/S0041-0101(00)00160-4. [DOI] [PubMed] [Google Scholar]
- 26.King GF, Tedford HW, Maggio F. Structure and function of insecticidal neurotoxins from Australian funnel-web spiders. J Toxicol-Toxin Rev. 2002;21(4):359–389. [Google Scholar]
- 27.Sollod BL, Wilson D, Zhaxybayeva O, Gogarten JP, Drinkwater R, King GF. Were arachnids the first to use combinatorial peptide libraries? Peptides. 2005;26:131–139. doi: 10.1016/j.peptides.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Herzig V, Wood DL, Newell F, Chaumeil PA, Kaas Q, Binford GJ, Nicholson GM, Gorse D, King GF. ArachnoServer 2.0, an updated online resource for spider toxin sequences and structures. Nucleic Acids Res. 2011;39((database issue)):D653–D657. doi: 10.1093/nar/gkq1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57(4):397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 30.Billen B, Bosmans F, Tytgat J. Animal peptides targeting voltage-activated sodium channels. Curr Pharm Des. 2008;14(24):2492–2502. doi: 10.2174/138161208785777423. [DOI] [PubMed] [Google Scholar]
- 31.King GF, Escoubas P, Nicholson GM. Peptide toxins that selectively target insect NaV and CaV channels. Channels (Austin) 2008;2(2):100–116. doi: 10.4161/chan.2.2.6022. [DOI] [PubMed] [Google Scholar]
- 32.Raymond-Delpech V, Matsuda K, Sattelle BM, Rauh JJ, Sattelle DB. Ion channels: molecular targets of neuroactive insecticides. Invertebr Neurosci. 2005;5(3–4):119–133. doi: 10.1007/s10158-005-0004-9. [DOI] [PubMed] [Google Scholar]
- 33.McCutchen BF, Choudary PV, Crenshaw R, Maddox D, Kamita SG, Palekar N, Volrath S, Fowler E, Hammock BD, Maeda S. Development of a recombinant baculovirus expressing an insect-selective neurotoxin: potential for pest control. Biotechnology. 1991;9:848–852. doi: 10.1038/nbt0991-848. [DOI] [PubMed] [Google Scholar]
- 34.Li D, Xiao Y, Hu W, Xie J, Bosmans F, Tytgat J, Liang S. Function and solution structure of hainantoxin-I, a novel insect sodium channel inhibitor from the Chinese bird spider Selenocosmia hainana . FEBS Lett. 2003;555(3):616–622. doi: 10.1016/S0014-5793(03)01303-6. [DOI] [PubMed] [Google Scholar]
- 35.Xiao Y, Bingham JP, Zhu W, Moczydlowski E, Liang S, Cummins TR. Tarantula huwentoxin-IV inhibits neuronal sodium channels by binding to receptor site 4 and trapping the domain II voltage sensor in the closed configuration. J Biol Chem. 2008;283(40):27300–27313. doi: 10.1074/jbc.M708447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Y, Blumenthal K, Jackson JO, 2nd, Liang S, Cummins TR. The tarantula toxins ProTx-II and huwentoxin-IV differentially interact with human Nav1.7 voltage sensors to inhibit channel activation and inactivation. Mol Pharmacol. 2010;78(6):1124–1134. doi: 10.1124/mol.110.066332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M, Rong M, Xiao Y, Liang S. The effects of huwentoxin-I on the voltage-gated sodium channels of rat hippocampal and cockroach dorsal unpaired median neurons. Peptides. 2012;34(1):19–25. doi: 10.1016/j.peptides.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Peng K, Chen XD, Liang SP. The effect of Huwentoxin-I on Ca2+ channels in differentiated NG108-15 cells, a patch-clamp study. Toxicon. 2001;39(4):491–498. doi: 10.1016/S0041-0101(00)00150-1. [DOI] [PubMed] [Google Scholar]
- 39.Figueiredo SG, Garcia ME, Valentim AC, Cordeiro MN, Diniz CR, Richardson M. Purification and amino acid sequence of the insecticidal neurotoxin Tx4(6–1) from the venom of the ‘armed’ spider Phoneutria nigriventer (Keys) Toxicon. 1995;33(1):83–93. doi: 10.1016/0041-0101(94)00130-Z. [DOI] [PubMed] [Google Scholar]
- 40.de Lima ME, Stankiewicz M, Hamon A, de Figueiredo SG, Cordeiro MN, Diniz CR, Martin-Eauclaire M, Pelhate M. The toxin Tx4(6–1) from the spider Phoneutria nigriventer slows down Na+ current inactivation in insect CNS via binding to receptor site 3. J Insect Physiol. 2002;48(1):53–61. doi: 10.1016/S0022-1910(01)00143-3. [DOI] [PubMed] [Google Scholar]
- 41.de Figueiredo SG, de Lima ME, Nascimento Cordeiro M, Diniz CR, Patten D, Halliwell RF, Gilroy J, Richardson M. Purification and amino acid sequence of a highly insecticidal toxin from the venom of the Brazilian spider Phoneutria nigriventer which inhibits NMDA-evoked currents in rat hippocampal neurones. Toxicon. 2001;39(2–3):309–317. doi: 10.1016/S0041-0101(00)00129-X. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira LC, De Lima ME, Pimenta AM, Mansuelle P, Rochat H, Cordeiro MN, Richardson M, Figueiredo SG. PnT4–3, a new insect toxin from Phoneutria nigriventer venom elicits the glutamate uptake inhibition exhibited by PhTx4 toxic fraction. Toxicon. 2003;42(7):793–800. doi: 10.1016/j.toxicon.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Corzo G, Gilles N, Satake H, Villegas E, Dai L, Nakajima T, Haupt J. Distinct primary structures of the major peptide toxins from the venom of the spider Macrothele gigas that bind to sites 3 and 4 in the sodium channel. FEBS Lett. 2003;547(1–3):43–50. doi: 10.1016/S0014-5793(03)00666-5. [DOI] [PubMed] [Google Scholar]
- 44.Klint JK, Senff S, Rupasinghe DB, Er SY, Herzig V, Nicholson GM, King GF. Spider-venom peptides that target voltage-gated sodium channels: pharmacological tools and potential therapeutic leads. Toxicon. 2012;60(4):478–491. doi: 10.1016/j.toxicon.2012.04.337. [DOI] [PubMed] [Google Scholar]
- 45.Corzo G, Escoubas P, Villegas E, Karbat I, Gordon D, Gurevitz M, Nakajima T, Gilles N. A spider toxin that induces a typical effect of scorpion α-toxins but competes with β-toxins on binding to insect sodium channels. Biochemistry. 2005;44(5):1542–1549. doi: 10.1021/bi048434k. [DOI] [PubMed] [Google Scholar]
- 46.Corzo G, Escoubas P, Stankiewicz M, Pelhate M, Kristensen CP, Nakajima T. Isolation, synthesis and pharmacological characterization of δ-palutoxins IT, novel insecticidal toxins from the spider Paracoelotes luctuosus (Amaurobiidae) Eur J Biochem. 2000;267(18):5783–5795. doi: 10.1046/j.1432-1327.2000.01653.x. [DOI] [PubMed] [Google Scholar]
- 47.Karbat I, Cohen L, Gilles N, Gordon D, Gurevitz M. Conversion of a scorpion toxin agonist into an antagonist highlights an acidic residue involved in voltage sensor trapping during activation of neuronal Na+ channels. Faseb J. 2004;18(6):683–689. doi: 10.1096/fj.03-0733com. [DOI] [PubMed] [Google Scholar]
- 48.Cohen L, Karbat I, Gilles N, Froy O, Corzo G, Angelovici R, Gordon D, Gurevitz M. Dissection of the functional surface of an anti-insect excitatory toxin illuminates a putative “hot spot” common to all scorpion β-toxins affecting Na+ channels. J Biol Chem. 2004;279(9):8206–8211. doi: 10.1074/jbc.M307531200. [DOI] [PubMed] [Google Scholar]
- 49.Billen B, Vassilevski A, Nikolsky A, Debaveye S, Tytgat J, Grishin E. Unique bell-shaped voltage-dependent modulation of Na+ channel gating by novel insect-selective toxins from the spider Agelena orientalis . J Biol Chem. 2010;285(24):18545–18554. doi: 10.1074/jbc.M110.125211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams ME. Agatoxins: ion channel specific toxins from the American funnel web spider, Agelenopsis aperta . Toxicon. 2004;43(5):509–525. doi: 10.1016/j.toxicon.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 51.King GF. Modulation of insect CaV channels by peptidic spider toxins. Toxicon. 2007;49(4):513–530. doi: 10.1016/j.toxicon.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 52.Wu S, Hayashi JH, Kinne LP, Dierks PM, Dierks CP (2004) New Lepidoptera calcium channel subunits, useful as active agents in agrochemical, veterinary or pharmaceutical fields, e.g. as insecticide, acaricide, or for treating infection, damage or discomfort caused by parasitic organisms. International Patent Application No. PCT/US2003/036001
- 53.Wicher D, Penzlin H. Ca2+ currents in central insect neurons: electrophysiological and pharmacological properties. J Neurophysiol. 1997;77(1):186–199. doi: 10.1152/jn.1997.77.1.186. [DOI] [PubMed] [Google Scholar]
- 54.Fletcher JI, Smith R, O’Donoghue SI, Nilges M, Connor M, Howden ME, Christie MJ, King GF. The structure of a novel insecticidal neurotoxin, ω-atracotoxin-HV1, from the venom of an Australian funnel web spider. Nat Struct Biol. 1997;4(7):559–566. doi: 10.1038/nsb0797-559. [DOI] [PubMed] [Google Scholar]
- 55.Atkinson RK, Howden MEH, Tyler MI, Vonarx EJ (1999) Insecticidal toxins derived from funnel web (Atrax or Hadronyche) spiders. US Patent 5,959,182, 28 Sept 1999
- 56.Wang X, Smith R, Fletcher JI, Wilson H, Wood CJ, Howden ME, King GF. Structure-function studies of ω-atracotoxin, a potent antagonist of insect voltage-gated calcium channels. Eur J Biochem. 1999;264(2):488–494. doi: 10.1046/j.1432-1327.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- 57.Tedford HW, Fletcher JI, King GF. Functional significance of the β-hairpin in the insecticidal neurotoxin ω-atracotoxin-Hv1a. J Biol Chem. 2001;276:26568–26576. doi: 10.1074/jbc.M102199200. [DOI] [PubMed] [Google Scholar]
- 58.Tedford HW, Gilles N, Menez A, Doering CJ, Zamponi GW, King GF. Scanning mutagenesis of ω-atracotoxin-Hv1a reveals a spatially restricted epitope that confers selective activity against insect calcium channels. J Biol Chem. 2004;279(42):44133–44140. doi: 10.1074/jbc.M404006200. [DOI] [PubMed] [Google Scholar]
- 59.Chong Y, Hayes JL, Sollod B, Wen S, Wilson DT, Hains PG, Hodgson WC, Broady KW, King GF, Nicholson GM. The ω-atracotoxins: selective blockers of insect M-LVA and HVA calcium channels. Biochem Pharmacol. 2007;74(4):623–638. doi: 10.1016/j.bcp.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 60.Wang XH, Connor M, Wilson D, Wilson HI, Nicholson GM, Smith R, Shaw D, Mackay JP, Alewood PF, Christie MJ, King GF. Discovery and structure of a potent and highly specific blocker of insect calcium channels. J Biol Chem. 2001;276(43):40306–40312. doi: 10.1074/jbc.M105206200. [DOI] [PubMed] [Google Scholar]
- 61.Kim JI, Konishi S, Iwai H, Kohno T, Gouda H, Shimada I, Sato K, Arata Y. Three-dimensional solution structure of the calcium channel antagonist ω-agatoxin IVA: consensus molecular folding of calcium channel blockers. J Mol Biol. 1995;250(5):659–671. doi: 10.1006/jmbi.1995.0406. [DOI] [PubMed] [Google Scholar]
- 62.Zhang PF, Chen P, Hu WJ, Liang SP. Huwentoxin-V, a novel insecticidal peptide toxin from the spider Selenocosmia huwena, and a natural mutant of the toxin: indicates the key amino acid residues related to the biological activity. Toxicon. 2003;42(1):15–20. doi: 10.1016/S0041-0101(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 63.Bodi J, Nishio H, Zhou Y, Branton WD, Kimura T, Sakakibara S. Synthesis of an O-palmitoylated 44-residue peptide amide (PLTX-II) blocking presynaptic calcium channels in Drosophila . Pept Res. 1995;8(4):228–235. [PubMed] [Google Scholar]
- 64.Villegas E, Adachi-Akahane S, Bosmans F, Tytgat J, Nakajima T, Corzo G. Biochemical characterization of cysteine-rich peptides from Oxyopes sp. venom that block calcium ion channels. Toxicon. 2008;52(2):228–236. doi: 10.1016/j.toxicon.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Connor M, Smith R, Maciejewski MW, Howden ME, Nicholson GM, Christie MJ, King GF. Discovery and characterization of a family of insecticidal neurotoxins with a rare vicinal disulfide bridge. Nat Struct Biol. 2000;7(6):505–513. doi: 10.1038/75921. [DOI] [PubMed] [Google Scholar]
- 66.Maggio F, King GF. Scanning mutagenesis of a Janus-faced atracotoxin reveals a bipartite surface patch that is essential for neurotoxic function. J Biol Chem. 2002;277(25):22806–22813. doi: 10.1074/jbc.M202297200. [DOI] [PubMed] [Google Scholar]
- 67.Gunning SJ, Maggio F, Windley MJ, Valenzuela SM, King GF, Nicholson GM. The Janus-faced atracotoxins are specific blockers of invertebrate KCa channels. FEBS J. 2008;275(16):4045–4059. doi: 10.1111/j.1742-4658.2008.06545.x. [DOI] [PubMed] [Google Scholar]
- 68.Mobli M, de Araujo AD, Lambert LK, Pierens GK, Windley MJ, Nicholson GM, Alewood PF, King GF. Direct visualization of disulfide bonds through diselenide proxies using 77Se NMR spectroscopy. Angew Chem Int Ed. 2009;48(49):9312–9314. doi: 10.1002/anie.200905206. [DOI] [PubMed] [Google Scholar]
- 69.Giangiacomo KM, Becker J, Garsky C, Schmalhofer W, Garcia ML, Mullmann TJ. Novel α-KTx sites in the BK channel and comparative sequence analysis reveal distinguishing features of the BK and KV channel outer pore. Cell Biochem Biophys. 2008;52(1):47–58. doi: 10.1007/s12013-008-9026-3. [DOI] [PubMed] [Google Scholar]
- 70.Myers MP, Stampe P. A point mutation in the maxi-K clone dSlo forms a high affinity site for charybdotoxin. Neuropharmacol. 2000;39(1):11–20. doi: 10.1016/S0028-3908(99)00074-X. [DOI] [PubMed] [Google Scholar]
- 71.Dauplais M, Lecoq A, Song J, Cotton J, Jamin N, Gilquin B, Roumestand C, Vita C, de Medeiros CL, Rowan EG, Harvey AL, Menez A. On the convergent evolution of animal toxins. Conservation of a diad of functional residues in potassium channel-blocking toxins with unrelated structures. J Biol Chem. 1997;272(7):4302–4309. doi: 10.1074/jbc.272.7.4302. [DOI] [PubMed] [Google Scholar]
- 72.Mosbah A, Kharrat R, Fajloun Z, Renisio JG, Blanc E, Sabatier JM, El Ayeb M, Darbon H. A new fold in the scorpion toxin family, associated with an activity on a ryanodine-sensitive calcium channel. Proteins. 2000;40(3):436–442. doi: 10.1002/1097-0134(20000815)40:3<436::AID-PROT90>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 73.Wudayagiri R, Inceoglu B, Herrmann R, Derbel M, Choudary PV, Hammock BD. Isolation and characterization of a novel lepidopteran-selective toxin from the venom of South Indian red scorpion, Mesobuthus tamulus . BMC Biochem. 2001;216(2):16. doi: 10.1186/1471-2091-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeBin JA, Maggio JE, Strichartz GR. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am J Physiol. 1993;264(2 Pt 1):C361–C369. doi: 10.1152/ajpcell.1993.264.2.C361. [DOI] [PubMed] [Google Scholar]
- 75.Tytgat J, Debont T, Rostoll K, Muller GJ, Verdonck F, Daenens P, van der Walt JJ, Possani LD. Purification and partial characterization of a ‘short’ insectotoxin-like peptide from the venom of the scorpion Parabuthus schlechteri . FEBS Lett. 1998;441(3):387–391. doi: 10.1016/S0014-5793(98)01589-0. [DOI] [PubMed] [Google Scholar]
- 76.Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278(6):4135–4144. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- 77.Kesavan K, Ratliff J, Johnson EW, Dahlberg W, Asara JM, Misra P, Frangioni JV, Jacoby DB. Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects. J Biol Chem. 2010;285(7):4366–4374. doi: 10.1074/jbc.M109.066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grishin EV, Soldatov NM, Ovchinnikov YA. The nature of the membrane-receptors for scorpion-venom neurotoxin. Bioorg Khim. 1980;6(6):914–922. [Google Scholar]
- 79.Fitches EC, Bell HA, Powell ME, Back E, Sargiotti C, Weaver RJ, Gatehouse JA. Insecticidal activity of scorpion toxin (ButaIT) and snowdrop lectin (GNA) containing fusion proteins towards pest species of different orders. Pest Manag Sci. 2010;66(1):74–83. doi: 10.1002/ps.1833. [DOI] [PubMed] [Google Scholar]
- 80.UniProt Consortium Reorganizing the protein space at the Universal Protein Resource (UniProt) Nucl Acids Res. 2012;40:D71–D75. doi: 10.1093/nar/gkr981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lewis RJ. Conotoxin venom peptide therapeutics. Adv Exp Med Biol. 2009;655:44–48. doi: 10.1007/978-1-4419-1132-2_5. [DOI] [PubMed] [Google Scholar]
- 82.Norton RS. μ-Conotoxins as leads in the development of new analgesics. Molecules. 2010;15(4):2825–2844. doi: 10.3390/molecules15042825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewis RJ, Dutertre S, Vetter I, Christie MJ. Conus venom peptide pharmacology. Pharmacol Rev. 2012;64(2):259–298. doi: 10.1124/pr.111.005322. [DOI] [PubMed] [Google Scholar]
- 84.Wicher D, Penzlin H. ω-Toxins affect Na+ currents in neurosecretory insect neurons. Receptors Channels. 1998;5(6):355–366. [PubMed] [Google Scholar]
- 85.Shon KJ, Stocker M, Terlau H, Stuhmer W, Jacobsen R, Walker C, Grilley M, Watkins M, Hillyard DR, Gray WR, Olivera BM. κ-Conotoxin PVIIA is a peptide inhibiting the shaker K+ channel. J Biol Chem. 1998;273(1):33–38. doi: 10.1074/jbc.273.1.33. [DOI] [PubMed] [Google Scholar]
- 86.McCleskey EW, Fox AP, Feldman DH, Cruz LJ, Olivera BM, Tsien RW, Yoshikami D. ω-Conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci USA. 1987;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hillyard DR, Monje VD, Mintz IM, Bean BP, Nadasdi L, Ramachandran J, Miljanich G, Azimi-Zoonooz A, McIntosh JM, Cruz LJ, et al. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992;9(1):69–77. doi: 10.1016/0896-6273(92)90221-X. [DOI] [PubMed] [Google Scholar]
- 88.Terlau H, Shon KJ, Grilley M, Stocker M, Stuhmer W, Olivera BM. Strategy for rapid immobilization of prey by a fish-hunting marine snail. Nature. 1996;381(6578):148–151. doi: 10.1038/381148a0. [DOI] [PubMed] [Google Scholar]
- 89.Hillyard DR, Olivera BM, Woodward S, Corpuz GP, Gray WR, Ramilo CA, Cruz LJ. A molluscivorous Conus toxin: conserved frameworks in conotoxins. Biochemistry. 1989;28(1):358–361. doi: 10.1021/bi00427a049. [DOI] [PubMed] [Google Scholar]
- 90.Fainzilber M, Gordon D, Hasson A, Spira ME, Zlotkin E. Mollusc-specific toxins from the venom of Conus textile neovicarius . Eur J Biochem. 1991;202(2):589–595. doi: 10.1111/j.1432-1033.1991.tb16412.x. [DOI] [PubMed] [Google Scholar]
- 91.Bruce C, Fitches EC, Chougule N, Bell HA, Gatehouse JA. Recombinant conotoxin, TxVIA, produced in yeast has insecticidal activity. Toxicon. 2011;58(1):93–100. doi: 10.1016/j.toxicon.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 92.Fainzilber M, Kofman O, Zlotkin E, Gordon D. A new neurotoxin receptor site on sodium channels is identified by a conotoxin that affects sodium channel inactivation in molluscs and acts as an antagonist in rat brain. J Biol Chem. 1994;269(4):2574–2580. [PubMed] [Google Scholar]
- 93.Fainzilber M, Lodder JC, Kits KS, Kofman O, Vinnitsky I, Van Rietschoten J, Zlotkin E, Gordon D. A new conotoxin affecting sodium current inactivation interacts with the δ-conotoxin receptor site. J Biol Chem. 1995;270(3):1123–1129. doi: 10.1074/jbc.270.3.1123. [DOI] [PubMed] [Google Scholar]
- 94.Leipold E, Hansel A, Olivera BM, Terlau H, Heinemann SH. Molecular interaction of δ-conotoxins with voltage-gated sodium channels. FEBS Lett. 2005;579(18):3881–3884. doi: 10.1016/j.febslet.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 95.Heinemann SH, Leipold E. Conotoxins of the O-superfamily affecting voltage-gated sodium channels. Cell Mol Life Sci. 2007;64(11):1329–1340. doi: 10.1007/s00018-007-6565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kohno T, Sasaki T, Kobayashi K, Fainzilber M, Sato K. Three-dimensional solution structure of the sodium channel agonist/antagonist δ-conotoxin TxVIA. J Biol Chem. 2002;277(39):36387–36391. doi: 10.1074/jbc.M206833200. [DOI] [PubMed] [Google Scholar]
- 97.Bontems F, Roumestand C, Gilquin B, Menez A, Toma F. Refined structure of charybdotoxin: common motifs in scorpion toxins and insect defensins. Science. 1991;254(5037):1521–1523. doi: 10.1126/science.1720574. [DOI] [PubMed] [Google Scholar]
- 98.Cornet B, Bonmatin JM, Hetru C, Hoffmann JA, Ptak M, Vovelle F. Refined three-dimensional solution structure of insect defensin A. Structure. 1995;3(5):435–448. doi: 10.1016/S0969-2126(01)00177-0. [DOI] [PubMed] [Google Scholar]
- 99.Wong JH, Xia L, Ng TB. A review of defensins of diverse origins. Curr Prot Pept Sci. 2007;8(5):446–459. doi: 10.2174/138920307782411446. [DOI] [PubMed] [Google Scholar]
- 100.Zhu S, Gao B, Tytgat J. Phylogenetic distribution, functional epitopes and evolution of the CSαβ superfamily. Cell Mol Life Sci. 2005;62(19–20):2257–2269. doi: 10.1007/s00018-005-5200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodriguez de la Vega RC, Possani LD. Current views on scorpion toxins specific for K+-channels. Toxicon. 2004;43(8):865–875. doi: 10.1016/j.toxicon.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 102.Zlotkin E, Rochat H, Miranda F, Kopeyan C, Lissitzky S. Purification and properties of the insect toxin from the venom of the scorpion Androctonus australis Hector . Biochimie. 1971;53(10):1073–1078. doi: 10.1016/S0300-9084(71)80195-5. [DOI] [PubMed] [Google Scholar]
- 103.Borchani L, Mansuelle P, Stankiewicz M, Grolleau F, Cestele S, Karoui H, Lapied B, Rochat H, Pelhate M, el Ayeb M. A new scorpion venom toxin paralytic to insects that affects Na+ channel activation. Purification, structure, antigenicity and mode of action. Eur J Biochem. 1996;241(2):525–532. doi: 10.1111/j.1432-1033.1996.00525.x. [DOI] [PubMed] [Google Scholar]
- 104.Zilberberg N, Gordon D, Pelhate M, Adams ME, Norris TM, Zlotkin E, Gurevitz M. Functional expression and genetic alteration of an alpha scorpion neurotoxin. Biochemistry. 1996;35(31):10215–10222. doi: 10.1021/bi9528309. [DOI] [PubMed] [Google Scholar]
- 105.Cestele S, Borchani L, El Ayeb M, Rochat H. Bot IT2: a new scorpion toxin to study receptor site on insect sodium channels. FEBS Lett. 1997;405(1):77–80. doi: 10.1016/S0014-5793(97)00160-9. [DOI] [PubMed] [Google Scholar]
- 106.Eitan M, Fowler E, Herrmann R, Duval A, Pelhate M, Zlotkin E. A scorpion venom neurotoxin paralytic to insects that affects sodium current inactivation: purification, primary structure, and mode of action. Biochemistry. 1990;29(25):5941–5947. doi: 10.1021/bi00477a009. [DOI] [PubMed] [Google Scholar]
- 107.Gurevitz M, Zlotkin E, Zilberberg N. Characterization of the transcript for a depressant insect selective neurotoxin gene with an isolated cDNA clone from the scorpion Buthotus judaicus . FEBS Lett. 1990;269(1):229–232. doi: 10.1016/0014-5793(90)81161-G. [DOI] [PubMed] [Google Scholar]
- 108.Selisko B, Garcia C, Becerril B, Delepierre M, Possani LD. An insect-specific toxin from Centruroides noxius Hoffmann. cDNA, primary structure, three-dimensional model and electrostatic surface potentials in comparison with other toxin variants. Eur J Biochem. 1996;242(2):235–242. doi: 10.1111/j.1432-1033.1996.0235r.x. [DOI] [PubMed] [Google Scholar]
- 109.Catterall WA, Cestele S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49(2):124–141. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 110.Bosmans F, Tytgat J. Voltage-gated sodium channel modulation by scorpion α-toxins. Toxicon. 2007;49(2):142–158. doi: 10.1016/j.toxicon.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karbat I, Frolow F, Froy O, Gilles N, Cohen L, Turkov M, Gordon D, Gurevitz M. Molecular basis of the high insecticidal potency of scorpion α-toxins. J Biol Chem. 2004;279(30):31679–31686. doi: 10.1074/jbc.M402048200. [DOI] [PubMed] [Google Scholar]
- 112.Karbat I, Kahn R, Cohen L, Ilan N, Gilles N, Corzo G, Froy O, Gur M, Albrecht G, Heinemann SH, Gordon D, Gurevitz M. The unique pharmacology of the scorpion α-like toxin Lqh3 is associated with its flexible C-tail. FEBS J. 2007;274(8):1918–1931. doi: 10.1111/j.1742-4658.2007.05737.x. [DOI] [PubMed] [Google Scholar]
- 113.He XL, Li HM, Zeng ZH, Liu XQ, Wang M, Wang DC. Crystal structures of two α-like scorpion toxins: non-proline cis peptide bonds and implications for new binding site selectivity on the sodium channel. J Mol Biol. 1999;292(1):125–135. doi: 10.1006/jmbi.1999.3036. [DOI] [PubMed] [Google Scholar]
- 114.Guan RJ, Xiang Y, He XL, Wang CG, Wang M, Zhang Y, Sundberg EJ, Wang DC. Structural mechanism governing cis and trans isomeric states and an intramolecular switch for cis/trans isomerization of a non-proline peptide bond observed in crystal structures of scorpion toxins. J Mol Biol. 2004;341(5):1189–1204. doi: 10.1016/j.jmb.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 115.Ye X, Bosmans F, Li C, Zhang Y, Wang DC, Tytgat J. Structural basis for the voltage-gated Na+ channel selectivity of the scorpion α-like toxin BmK M1. J Mol Biol. 2005;353(4):788–803. doi: 10.1016/j.jmb.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 116.Zhu J, Tong X, Cao C, Wu G, Zhang N, Wu H. Solution structure of BmKalphaTx11, a toxin from the venom of the Chinese scorpion Buthus martensii Karsch. Biochem Biophys Res Commun. 2010;391(1):627–633. doi: 10.1016/j.bbrc.2009.11.110. [DOI] [PubMed] [Google Scholar]
- 117.Li HM, Wang DC, Zeng ZH, Jin L, Hu RQ. Crystal structure of an acidic neurotoxin from scorpion Buthus martensii Karsch at 1.85 Å resolution. J Mol Biol. 1996;261(3):415–431. doi: 10.1006/jmbi.1996.0473. [DOI] [PubMed] [Google Scholar]
- 118.Krimm I, Gilles N, Sautiere P, Stankiewicz M, Pelhate M, Gordon D, Lancelin JM. NMR structures and activity of a novel α-like toxin from the scorpion Leiurus quinquestriatus hebraeus . J Mol Biol. 1999;285(4):1749–1763. doi: 10.1006/jmbi.1998.2418. [DOI] [PubMed] [Google Scholar]
- 119.Zhu S, Peigneur S, Gao B, Lu X, Cao C, Tytgat J. Evolutionary diversification of Mesobuthus α-scorpion toxins affecting sodium channels. Mol Cell Proteomics. 2012;11(1):1–18. doi: 10.1074/mcp.M111.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gur M, Kahn R, Karbat I, Regev N, Wang J, Catterall WA, Gordon D, Gurevitz M. Elucidation of the molecular basis of selective recognition uncovers the interaction site for the core domain of scorpion α-toxins on sodium channels. J Biol Chem. 2011;286(40):35209–35217. doi: 10.1074/jbc.M111.259507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cohen L, Karbat I, Gilles N, Ilan N, Benveniste M, Gordon D, Gurevitz M. Common features in the functional surface of scorpion β-toxins and elements that confer specificity for insect and mammalian voltage-gated sodium channels. J Biol Chem. 2005;280(6):5045–5053. doi: 10.1074/jbc.M408427200. [DOI] [PubMed] [Google Scholar]
- 122.Karbat I, Turkov M, Cohen L, Kahn R, Gordon D, Gurevitz M, Frolow F. X-ray structure and mutagenesis of the scorpion depressant toxin LqhIT2 reveals key determinants crucial for activity and anti-insect selectivity. J Mol Biol. 2007;366(2):586–601. doi: 10.1016/j.jmb.2006.10.085. [DOI] [PubMed] [Google Scholar]
- 123.Froy O, Zilberberg N, Gordon D, Turkov M, Gilles N, Stankiewicz M, Pelhate M, Loret E, Oren DA, Shaanan B, Gurevitz M. The putative bioactive surface of insect-selective scorpion excitatory neurotoxins. J Biol Chem. 1999;274(9):5769–5776. doi: 10.1074/jbc.274.9.5769. [DOI] [PubMed] [Google Scholar]
- 124.Pinheiro CB, Marangoni S, Toyama MH, Polikarpov I. Structural analysis of Tityus serrulatus Ts1 neurotoxin at atomic resolution: insights into interactions with Na+ channels. Acta Crystallogr Sect D. 2003;59(3):405–415. doi: 10.1107/S090744490202111X. [DOI] [PubMed] [Google Scholar]
- 125.Pintar A, Possani LD, Delepierre M. Solution structure of toxin 2 from Centruroides noxius Hoffmann, a β-scorpion neurotoxin acting on sodium channels. J Mol Biol. 1999;287(2):359–367. doi: 10.1006/jmbi.1999.2611. [DOI] [PubMed] [Google Scholar]
- 126.Li YJ, Tan ZY, Ji YH. The binding of BmK IT2, a depressant insect-selective scorpion toxin on mammal and insect sodium channels. Neurosci Res. 2000;38(3):257–264. doi: 10.1016/S0168-0102(00)00164-4. [DOI] [PubMed] [Google Scholar]
- 127.Gordon D, Moskowitz H, Eitan M, Warner C, Catterall WA, Zlotkin E. Localization of receptor sites for insect-selective toxins on sodium channels by site-directed antibodies. Biochemistry. 1992;31(33):7622–7628. doi: 10.1021/bi00148a025. [DOI] [PubMed] [Google Scholar]
- 128.Gordon D, Jover E, Couraud F, Zlotkin E. The binding of the insect-selective neurotoxin (AaIT) from scorpion-venom to locust synaptosomal membranes. Biochim Biophys Acta. 1984;778(2):349–358. doi: 10.1016/0005-2736(84)90379-1. [DOI] [Google Scholar]
- 129.Cestele S, Kopeyan C, Oughideni R, Mansuelle P, Granier C, Rochat H. Biochemical and pharmacological characterization of a depressant insect toxin from the venom of the scorpion Buthacus arenicola . Eur J Biochem. 1997;243(1–2):93–99. doi: 10.1111/j.1432-1033.1997.93_1a.x. [DOI] [PubMed] [Google Scholar]
- 130.Shichor I, Zlotkin E, Ilan N, Chikashvili D, Stuhmer W, Gordon D, Lotan I. Domain 2 of Drosophila para voltage-gated sodium channel confers insect properties to a rat brain channel. J Neurosci. 2002;22(11):4364–4371. doi: 10.1523/JNEUROSCI.22-11-04364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.He H, Liu Z, Dong B, Zhang J, Shu X, Zhou J, Ji Y. Localization of receptor site on insect sodium channel for depressant β-toxin BmK IT2. PLoS ONE. 2011;6(1):e14510. doi: 10.1371/journal.pone.0014510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J, Yarov-Yarovoy V, Kahn R, Gordon D, Gurevitz M, Scheuer T, Catterall WA. Mapping the receptor site for alpha-scorpion toxins on a Na+ channel voltage sensor. Proc Natl Acad Sci USA. 2011;108(37):15426–15431. doi: 10.1073/pnas.1112320108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang JZ, Yarov-Yarovoy V, Scheuer T, Karbat I, Cohen L, Gordon D, Gurevitz M, Catterall WA. Mapping the interaction site for a β-scorpion toxin in the pore module of domain III of voltage-gated Na+ channels. J Biol Chem. 2012;287(36):30719–30728. doi: 10.1074/jbc.M112.370742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Herrmann R, Fishman L, Zlotkin E. The tolerance of lepidopterous larvae to an insect selective neurotoxin. Insect Biochemistry. 1990;20(6):625–637. doi: 10.1016/0020-1790(90)90075-6. [DOI] [Google Scholar]
- 135.Zlotkin E, Fishman L, Shapiro JP. Oral toxicity to flesh flies of a neurotoxic polypeptide. Arch Insect Biochem Physiol. 1992;21(1):41–52.124. doi: 10.1002/arch.940210105. [DOI] [PubMed] [Google Scholar]
- 136.Fishman L, Herrmann R, Gordon D, Zlotkin E. Insect tolerance to a neurotoxic polypeptide: pharmacokinetic and pharmacodynamic aspects. J Exp Biol. 1997;200(Pt 7):1115–1123. doi: 10.1242/jeb.200.7.1115. [DOI] [PubMed] [Google Scholar]
- 137.Kozminsky-Atias A, Somech E, Zilberberg N. Isolation of the first toxin from the scorpion Buthus occitanus israelis showing preference for shaker potassium channels. FEBS Lett. 2007;581(13):2478–2484. doi: 10.1016/j.febslet.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 138.Tempel BL, Papazian DM, Schwarz TL, Jan YN, Jan LY. Sequence of a probable potassium channel component encoded at shaker locus of Drosophila . Science. 1987;237(4816):770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- 139.Garcia ML, Garcia-Calvo M, Hidalgo P, Lee A, MacKinnon R. Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus quinquestriatus var. hebraeus venom. Biochemistry. 1994;33(22):6834–6839. doi: 10.1021/bi00188a012. [DOI] [PubMed] [Google Scholar]
- 140.Tytgat J, Chandy KG, Garcia ML, Gutman GA, Martin-Eauclaire MF, van der Walt JJ, Possani LD. A unified nomenclature for short-chain peptides isolated from scorpion venoms: α-KTx molecular subfamilies. Trends Pharmacol Sci. 1999;20(11):444–447. doi: 10.1016/S0165-6147(99)01398-X. [DOI] [PubMed] [Google Scholar]
- 141.Ranganathan R, Lewis JH, MacKinnon R. Spatial localization of the K+ channel selectivity filter by mutant cycle-based structure analysis. Neuron. 1996;16(1):131–139. doi: 10.1016/S0896-6273(00)80030-6. [DOI] [PubMed] [Google Scholar]
- 142.Eriksson MA, Roux B. Modeling the structure of agitoxin in complex with the Shaker K+ channel: a computational approach based on experimental distance restraints extracted from thermodynamic mutant cycles. Biophys J. 2002;83(5):2595–2609. doi: 10.1016/S0006-3495(02)75270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Smith JJ, Hill JM, Little MJ, Nicholson GM, King GF, Alewood PF. Unique scorpion toxin with a putative ancestral fold provides insight into evolution of the inhibitor cystine knot motif. Proc Natl Acad Sci USA. 2011;108(26):10478–10483. doi: 10.1073/pnas.1103501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Corzo G, Bernard C, Clement H, Villegas E, Bosmans F, Tytgat J, Possani LD, Darbon H, Alagon A. Insecticidal peptides from the theraposid spider Brachypelma albiceps: an NMR-based model of Ba2. Biochim Biophys Acta. 2009;1794(8):1190–1196. doi: 10.1016/j.bbapap.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 145.Nason DM, Douglas P, Saccomano NA, Volkmann RA (1995) Calcium channel blocking polypeptides from Theraphosidae Aphonopelma. European Patent EP0668872, 13 May 1998
- 146.Matsushita N, Miyashita M, Sakai A, Nakagawa Y, Miyagawa H. Purification and characterization of a novel short-chain insecticidal toxin with two disulfide bridges from the venom of the scorpion Liocheles australasiae . Toxicon. 2007;50(6):861–867. doi: 10.1016/j.toxicon.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 147.Silva EC, Camargos TS, Maranhao AQ, Silva-Pereira I, Silva LP, Possani LD, Schwartz EF. Cloning and characterization of cDNA sequences encoding for new venom peptides of the Brazilian scorpion Opisthacanthus cayaporum . Toxicon. 2009;54(3):252–261. doi: 10.1016/j.toxicon.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 148.Grundemar L, Hakanson R. Neuropeptide Y effector systems: perspectives for drug development. Trends Pharmacol Sci. 1994;15(5):153–159. doi: 10.1016/0165-6147(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 149.Erdos EG. Hypotensive peptides: bradykinin, kallidin, and eledoisin. Adv Pharmacol. 1966;4:1–90. doi: 10.1016/S1054-3589(08)60097-6. [DOI] [PubMed] [Google Scholar]
- 150.Xu M, Lewis RV. Structure of a protein superfiber: spider dragline silk. Proc Natl Acad Sci USA. 1990;87(18):7120–7124. doi: 10.1073/pnas.87.18.7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gao B, Tian C, Zhu S. Inducible antibacterial response of scorpion venom gland. Peptides. 2007;28(12):2299–2305. doi: 10.1016/j.peptides.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 152.Kuhn-Nentwig L, Stöcklin R, Nentwig W (2011) Venom composition and strategies in spiders: is everything possible? In: Casas J (ed) Spider physiology and behaviour—physiology, vol 40. Advances in insect physiology. Elsevier, New York, pp 1–86.141
- 153.Veal DA, Trimble JE, Beattie AJ. Antimicrobial properties of secretions from the metapleural glands of Myrmecia gulosa (the Australian bull ant) J Appl Bacteriol. 1992;72(3):188–194. doi: 10.1111/j.1365-2672.1992.tb01822.x. [DOI] [PubMed] [Google Scholar]
- 154.Fujiwara S, Imai J, Fujiwara M, Yaeshima T, Kawashima T, Kobayashi K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J Biol Chem. 1990;265(19):11333–11337. [PubMed] [Google Scholar]
- 155.Bechinger B. The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochim Biophys Acta. 1999;1462(1–2):157–183. doi: 10.1016/s0005-2736(99)00205-9. [DOI] [PubMed] [Google Scholar]
- 156.Zeng XC, Corzo G, Hahin R. Scorpion venom peptides without disulfide bridges. IUBMB Life. 2005;57(1):13–21. doi: 10.1080/15216540500058899. [DOI] [PubMed] [Google Scholar]
- 157.Kuhn-Nentwig L. Antimicrobial and cytolytic peptides of venomous arthropods. Cell Mol Life Sci. 2003;60(12):2651–2668. doi: 10.1007/s00018-003-3106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vassilevski AA, Kozlov SA, Samsonova OV, Egorova NS, Karpunin DV, Pluzhnikov KA, Feofanov AV, Grishin EV. Cyto-insectotoxins, a novel class of cytolytic and insecticidal peptides from spider venom. Biochem J. 2008;411(3):687–696. doi: 10.1042/BJ20071123. [DOI] [PubMed] [Google Scholar]
- 159.Miyashita M, Sakai A, Matsushita N, Hanai Y, Nakagawa Y, Miyagawa H. A novel amphipathic linear peptide with both insect toxicity and antimicrobial activity from the venom of the scorpion Isometrus maculatus . Biosci Biotechnol Biochem. 2010;74(2):364–369. doi: 10.1271/bbb.90723. [DOI] [PubMed] [Google Scholar]
- 160.Corzo G, Villegas E, Gomez-Lagunas F, Possani LD, Belokoneva OS, Nakajima T. Oxyopinins, large amphipathic peptides isolated from the venom of the wolf spider Oxyopes kitabensis with cytolytic properties and positive insecticidal cooperativity with spider neurotoxins. J Biol Chem. 2002;277(26):23627–23637. doi: 10.1074/jbc.M200511200. [DOI] [PubMed] [Google Scholar]
- 161.Dutertre S, Lumsden NG, Alewood PF, Lewis RJ. Isolation and characterisation of conomap-Vt, a D-amino acid containing excitatory peptide from the venom of a vermivorous cone snail. FEBS Lett. 2006;580(16):3860–3866. doi: 10.1016/j.febslet.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 162.Paemen L, Tips A, Schoofs L, Proost P, Van Damme J, De Loof A. Lom-AG-myotropin: a novel myotropic peptide from the male accessory glands of Locusta migratoria . Peptides. 1991;12(1):7–10. doi: 10.1016/0196-9781(91)90158-L. [DOI] [PubMed] [Google Scholar]
- 163.Harada A, Yoshida M, Minakata H, Nomoto K, Muneoka Y, Kobayashi M. Structure and function of the molluscan myoactive tetradecapeptides. Zool Sci. 1993;10(2):257–265. [PubMed] [Google Scholar]
- 164.Prado-Montes de Oca E. Human β-defensin 1: a restless warrior against allergies, infections and cancer. Int J Biochem Cell Biol. 2010;42(6):800–804. doi: 10.1016/j.biocel.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 165.Beaumont KA, Smit DJ, Liu YY, Chai E, Patel MP, Millhauser GL, Smith JJ, Alewood PF, Sturm RA. Melanocortin-1 receptor-mediated signalling pathways activated by NDP-MSH and HBD3 ligands. Pigment Cell Melanoma Res. 2012;25(3):370–374. doi: 10.1111/j.1755-148X.2012.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nicastro G, Franzoni L, de Chiara C, Mancin AC, Giglio JR, Spisni A. Solution structure of crotamine, a Na+ channel affecting toxin from Crotalus durissus terrificus venom. Eur J Biochem. 2003;270(9):1969–1979. doi: 10.1046/j.1432-1033.2003.03563.x. [DOI] [PubMed] [Google Scholar]
- 167.Torres AM, Wang X, Fletcher JI, Alewood D, Alewood PF, Smith R, Simpson RJ, Nicholson GM, Sutherland SK, Gallagher CH, King GF, Kuchel PW. Solution structure of a defensin-like peptide from platypus venom. Biochem J. 1999;341(3):785–794. doi: 10.1042/0264-6021:3410785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Norton RS. Structures of sea anemone toxins. Toxicon. 2009;54(8):1075–1088. doi: 10.1016/j.toxicon.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 169.Moran Y, Weinberger H, Reitzel AM, Sullivan JC, Kahn R, Gordon D, Finnerty JR, Gurevitz M. Intron retention as a posttranscriptional regulatory mechanism of neurotoxin expression at early life stages of the starlet anemone Nematostella vectensis . J Mol Biol. 2008;380(3):437–443. doi: 10.1016/j.jmb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 170.Peigneur S, Beress L, Moller C, Mari F, Forssmann WG, Tytgat J. A natural point mutation changes both target selectivity and mechanism of action of sea anemone toxins. Faseb J. 2012;26(12):5141–5151. doi: 10.1096/fj.12-218479. [DOI] [PubMed] [Google Scholar]
- 171.Bosmans F, Aneiros A, Tytgat J. The sea anemone Bunodosoma granulifera contains surprisingly efficacious and potent insect-selective toxins. FEBS Lett. 2002;532(1–2):131–134. doi: 10.1016/S0014-5793(02)03653-0. [DOI] [PubMed] [Google Scholar]
- 172.Moran Y, Kahn R, Cohen L, Gur M, Karbat I, Gordon D, Gurevitz M. Molecular analysis of the sea anemone toxin Av3 reveals selectivity to insects and demonstrates the heterogeneity of receptor site-3 on voltage-gated Na+ channels. Biochem J. 2007;406(1):41–48. doi: 10.1042/BJ20070233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Loret EP, del Valle RM, Mansuelle P, Sampieri F, Rochat H. Positively charged amino acid residues located similarly in sea anemone and scorpion toxins. J Biol Chem. 1994;269(24):16785–16788. [PubMed] [Google Scholar]
- 174.Manoleras N, Norton RS. Three-dimensional structure in solution of neurotoxin III from the sea anemone Anemonia sulcata . Biochemistry. 1994;33(37):11051–11061. doi: 10.1021/bi00203a001. [DOI] [PubMed] [Google Scholar]
- 175.Frank PG, Bleakney JS. Asexual reproduction, diet, and anomalies of anemone Nematostella vectensis in Nova Scotia. Can Field Nat. 1978;92(3):259–263. [Google Scholar]
- 176.Moran Y, Cohen L, Kahn R, Karbat I, Gordon D, Gurevitz M. Expression and mutagenesis of the sea anemone toxin Av2 reveals key amino acid residues important for activity on voltage-gated sodium channels. Biochemistry. 2006;45(29):8864–8873. doi: 10.1021/bi060386b. [DOI] [PubMed] [Google Scholar]
- 177.Seibert AL, Liu J, Hanck DA, Blumenthal KM. Role of Asn-16 and Ser-19 in anthopleurin B binding. Implications for the electrostatic nature of NaV site 3. Biochemistry. 2004;43(22):7082–7089. doi: 10.1021/bi0496135. [DOI] [PubMed] [Google Scholar]
- 178.Dias-Kadambi BL, Drum CL, Hanck DA, Blumenthal KM. Leucine 18, a hydrophobic residue essential for high affinity binding of anthopleurin B to the voltage-sensitive sodium channel. J Biol Chem. 1996;271(16):9422–9428. doi: 10.1074/jbc.271.16.9422. [DOI] [PubMed] [Google Scholar]
- 179.Khera PK, Blumenthal KM. Importance of highly conserved anionic residues and electrostatic interactions in the activity and structure of the cardiotonic polypeptide anthopleurin B. Biochemistry. 1996;35(11):3503–3507. doi: 10.1021/bi9528457. [DOI] [PubMed] [Google Scholar]
- 180.Jimenez EC, Watkins M, Juszczak LJ, Cruz LJ, Olivera BM. Contryphans from Conus textile venom ducts. Toxicon. 2001;39(6):803–808. doi: 10.1016/S0041-0101(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 181.Kaas Q, Yu R, Jin AH, Dutertre S, Craik DJ. ConoServer: updated content, knowledge, and discovery tools in the conopeptide database. Nucl Acids Res. 2012;40:D325–D330. doi: 10.1093/nar/gkr886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Massilia GR, Eliseo T, Grolleau F, Lapied B, Barbier J, Bournaud R, Molgo J, Cicero DO, Paci M, Schinina ME, Ascenzi P, Polticelli F. Contryphan-Vn: a modulator of Ca2+-dependent K+ channels. Biochem Biophys Res Commun. 2003;303(1):238–246. doi: 10.1016/S0006-291X(03)00331-0. [DOI] [PubMed] [Google Scholar]
- 183.Kini RM, Doley R. Structure, function and evolution of three-finger toxins: mini proteins with multiple targets. Toxicon. 2010;56(6):855–867. doi: 10.1016/j.toxicon.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 184.Brown LR, Wuthrich K. Nuclear magnetic resonance solution structure of the α-neurotoxin from the black mamba (Dendroaspis polylepis polylepis) J Mol Biol. 1992;227(4):1118–1135. doi: 10.1016/0022-2836(92)90525-O. [DOI] [PubMed] [Google Scholar]
- 185.Hue B, Buckingham SD, Buckingham D, Sattelle DB. Actions of snake neurotoxins on an insect nicotinic cholinergic synapse. Invertebr Neurosci. 2007;7(3):173–178. doi: 10.1007/s10158-007-0053-3. [DOI] [PubMed] [Google Scholar]
- 186.Rohou A, Nield J, Ushkaryov YA. Insecticidal toxins from black widow spider venom. Toxicon. 2007;49(4):531–549. doi: 10.1016/j.toxicon.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Johnson JH, Kral RM, Krapcho K (2001) Insecticidal toxins from Bracon hebetor nucleic acid encoding said toxin and methods of use. US Patent 5,874,298, 17 Feb 1995
- 188.Zobel-Thropp PA, Kerins AE, Binford GJ. Sphingomyelinase D in sicariid spider venom is a potent insecticidal toxin. Toxicon. 2012;60(3):265–271. doi: 10.1016/j.toxicon.2012.04.350. [DOI] [PubMed] [Google Scholar]
- 189.Swanson DL, Vetter RS. Loxoscelism. Clin Dermatol. 2006;24(3):213–221. doi: 10.1016/j.clindermatol.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 190.Mukherjee AK, Sollod BL, Wikel SK, King GF. Orally active acaricidal peptide toxins from spider venom. Toxicon. 2006;47:182–187. doi: 10.1016/j.toxicon.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 191.Casartelli M, Corti P, Giovanna Leonardi M, Fiandra L, Burlini N, Pennacchio F, Giordana B. Absorption of albumin by the midgut of a lepidopteran larva. J Insect Physiol. 2005;51(8):933–940. doi: 10.1016/j.jinsphys.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 192.Fitches E, Woodhouse SD, Edwards JP, Gatehouse JA. In vitro and in vivo binding of snowdrop (Galanthus nivalis agglutinin; GNA) and jackbean (Canavalia ensiformis; Con A) lectins within tomato moth (Lacanobia oleracea) larvae; mechanisms of insecticidal action. J Insect Physiol. 2001;47:777–787. doi: 10.1016/S0022-1910(01)00068-3. [DOI] [PubMed] [Google Scholar]
- 193.Fitches EC, Pyati P, King GF, Gatehouse JA. Fusion to snowdrop lectin magnifies the oral activity of insecticidal ω-hexatoxin-Hv1a peptide by enabling its delivery to the central nervous system. PLoS ONE. 2012;7(6):e39389. doi: 10.1371/journal.pone.0039389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Down RE, Fitches EC, Wiles DP, Corti P, Bell HA, Gatehouse JA, Edwards JP. Insecticidal spider venom toxin fused to snowdrop lectin is toxic to the peach-potato aphid, Myzus persicae (Hemiptera: Aphididae) and the rice brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) Pest Manag Sci. 2006;62(1):77–85. doi: 10.1002/ps.1119. [DOI] [PubMed] [Google Scholar]
- 195.Fitches E, Edwards M, Mee C, Grishin E, Gatehouse A, Edwards J, Gatehouse J. Fusion proteins containing insect-specific toxins as pest control agents: snowdrop lectin delivers fused insecticidal spider venom toxin to insect haemolymph following oral ingestion. J Insect Physiol. 2004;50:61–71. doi: 10.1016/j.jinsphys.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 196.Bonning BC, Hammock BD. Development of recombinant baculoviruses for insect control. Annu Rev Entomol. 1996;41:191–210. doi: 10.1146/annurev.en.41.010196.001203. [DOI] [PubMed] [Google Scholar]
- 197.Wang C, St Leger RJ. A scorpion neurotoxin increases the potency of a fungal insecticide. Nat Biotechnol. 2007;25(12):1455–1456. doi: 10.1038/nbt1357. [DOI] [PubMed] [Google Scholar]
- 198.Charnley AK, Collins SA. Entomopathogenic fungi and their role in pest control. In: Kubicek CP, Druzhinina IS, editors. The Mycota IV: environmental and microbial relationships. 2. Berlin: Springer; 2007. pp. 159–187. [Google Scholar]
- 199.Gatehouse AM, Ferry N, Edwards MG, Bell HA. Insect-resistant biotech crops and their impacts on beneficial arthropods. Philos Trans R Soc Lond B Biol Sci. 2011;366(1569):1438–1452. doi: 10.1098/rstb.2010.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jiang H, Zhu YX, Che ZL. Insect resistance of transformed tobacco plants with the gene of the spider insecticidal peptide. J Integr Plant Biol (Acta Botan Sin) 1996;38(2):95–99. [Google Scholar]
- 201.Omar A, Ali Chatha K. National Institute for Biotechnology and Genetic Engineering (NIBGE): genetically modified spider cotton. Asian J Manag Cases. 2012;9:33–58. doi: 10.1177/097282011100900105. [DOI] [Google Scholar]
- 202.Khan SA, Zafar Y, Briddon RW, Malik KA, Mukhtar Z. Spider venom toxin protects plants from insect attack. Transgenic Res. 2006;15(3):349–357. doi: 10.1007/s11248-006-0007-2. [DOI] [PubMed] [Google Scholar]
- 203.Cao C-W, Liu G-F, Wang Z-Y, Yan S-C, Ma L, Yang C-P. Response of the gypsy moth, Lymantria dispar to transgenic poplar, Populus simonii × P. nigra, expressing fusion protein gene of the spider insecticidal peptide and Bt-toxin C-peptide. J Insect Sci. 2010;10(200):1–13. doi: 10.1673/031.010.20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hernández-Campuzano B, Suárez R, Lina L, Hernández V, Villegas E, Corzo G, Iturriaga G. Expression of a spider venom peptide in transgenic tobacco confers insect resistance. Toxicon. 2009;53(1):122–128. doi: 10.1016/j.toxicon.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 205.Soberon M, Fernandez LE, Perez C, Gill SS, Bravo A. Mode of action of mosquitocidal Bacillus thuringiensis toxins. Toxicon. 2007;49:597–600. doi: 10.1016/j.toxicon.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 206.Baconguis I, Gouaux E. Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature. 2012;489(7416):400–405. doi: 10.1038/nature11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dawson RJ, Benz J, Stohler P, Tetaz T, Joseph C, Huber S, Schmid G, Hügin D, Pflimlin P, Trube G, Rudolph MG, Hennig M, Ruf A. Structure of the acid-sensing ion channel 1 in complex with the gating modifier Psalmotoxin 1. Nat. Commun. 2012;3:936. doi: 10.1038/ncomms1917. [DOI] [PubMed] [Google Scholar]
- 208.Lange A, Giller K, Hornig S, Martin-Eauclaire MF, Pongs O, Becker S, Baldus M. Toxin-induced conformational changes in a potassium channel revealed by solid-state NMR. Nature. 2006;440(7086):959–962. doi: 10.1038/nature04649. [DOI] [PubMed] [Google Scholar]
- 209.Bourne Y, Talley TT, Hansen SB, Taylor P, Marchot P. Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake alpha- neurotoxins and nicotinic receptors. EMBO J. 2005;24(8):1512–1522. doi: 10.1038/sj.emboj.7600620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Celie PH, Kasheverov IE, Mordvintsev DY, Hogg RC, van Nierop P, van Elk R, van Rossum-Fikkert SE, Zhmak MN, Bertrand D, Tsetlin V, Sixma TK, Smit AB. Crystal structure of nicotinic acetylcholine receptor homolog AChBP in complex with an alpha-conotoxin PnIA variant. Nat Struct Mol Biol. 2005;12(7):582–588. doi: 10.1038/nsmb951. [DOI] [PubMed] [Google Scholar]
- 211.Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 A resolution. Nat Neurosci. 2007;10(8):953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- 212.Cestele S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82(9–10):883–892. doi: 10.1016/S0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- 213.Gordon D, Karbat I, Ilan N, Cohen L, Kahn R, Gilles N, Dong K, Stuhmer W, Tytgat J, Gurevitz M. The differential preference of scorpion α-toxins for insect or mammalian sodium channels: implications for improved insect control. Toxicon. 2007;49(4):452–472. doi: 10.1016/j.toxicon.2006.11.016. [DOI] [PubMed] [Google Scholar]