Abstract
The mammalian genome is transcribed in a developmentally regulated manner, generating RNA strands ranging from long to short non-coding RNA (ncRNAs). NcRNAs generated by intergenic sequences and protein-coding loci, represent up to 98 % of the human transcriptome. Non-coding transcripts comprise short ncRNAs such as microRNAs, piwi-interacting RNAs, small nucleolar RNAs and long intergenic RNAs, most of which exercise a strictly controlled negative regulation of expression of protein-coding genes. In humans, the DLK1-DIO3 genomic region, located on human chromosome 14 (14q32) contains the paternally expressed imprinted genes DLK1, RTL1, and DIO3 and the maternally expressed imprinted genes MEG3 (Gtl2), MEG8 (RIAN), and antisense RTL1 (asRTL1). This region hosts, in addition to two long intergenic RNAs, the MEG3 and MEG8, one of the largest microRNA clusters in the genome, with 53 miRNAs in the forward strand and one (mir-1247) in the reverse strand. Many of these miRNAs are differentially expressed in several pathologic processes and various cancers. A better understanding of the pathophysiologic importance of the DLK1-DIO3 domain-containing microRNA cluster may contribute to innovative therapeutic strategies in a range of diseases. Here we present an in-depth review of this vital genomic region, and examine the role the microRNAs of this region may play in controlling tissue homeostasis and in the pathogenesis of some human diseases, mostly cancer, when aberrantly expressed. The potential clinical implications of this data are also discussed.
Keywords: DLK1-DIO3, Long non-coding RNA, microRNAs, Cancer, Signaling pathways, Transcriptional regulatory pathways
Introduction
The mammalian genome is transcribed in a developmentally regulated manner, generating RNA transcripts ranging from long to short non-coding RNA (ncRNAs). NcRNAs represent up to 98 % of the human transcriptome and can be considered as a molecular marker in the evolution of complex organisms, since there is an association between organism complexity and non-coding transcripts [1]. Additionally, recent work has implicated ncRNAs in many aspects of gene regulation and various cellular processes [2]. The ncRNAs can be generated by both intergenic sequences and protein-coding loci [3] and are arbitrarily subdivided in long ncRNAs (lncRNAs) (longer than 200 nucleotides) and short ncRNAs (<200 nucleotides long).
Short non-coding RNAs include microRNAs (miRNAs), piwi-interacting RNAs (piRNAs), small nucleolar RNAs (snoRNAs) and other categories [4]. Short ncRNAs are involved in the regulation of protein biosynthesis, removal of intronic sequences, telomere synthesis, transcription control and posttranscriptional gene silencing [5]. SnoRNAs are highly conserved, intermediate-sized RNAs (60–300 nucleotides long) that are predominantly found in the nucleus [6]. Two major classes of snoRNAs have been identified, each possessing distinctive, evolutionary conserved sequence elements: the C/D box group and the H/ACA box group. SnoRNAs are components of the small nucleolar ribonucleoproteins (snoRNPs) and are involved in the post-transcriptional modification of rRNAs and some spliceosomal RNAs [7]. A large number of lncRNAs have also been identified and constitute the largest portion of the mammalian non-coding transcriptome. The biological role of lncRNAs as a class remains largely elusive, however several of them have been shown to be involved in the regulation of the epigenetic machinery by recruiting DNA methyltransferases, histone modifying enzymes, and chromatin remodeling complexes to their sites of action in specific cells at specific stages of differentiation [8]. LncRNAs are also involved in genomic imprinting [9]. Long intergenic RNAs (lincRNAs), and others with enhancer-like functions were described only recently [10, 11]. LincRNAs are transcripts that have been generated from intergenic regions with a specific chromatin signature. This signature consists of a short stretch of trimethylation of histone protein H3 at the lysine in position 4 (H3K4me3), which corresponds to promoter regions, followed by a longer stretch of trimethylation of histone H3 at the lysine in position 36 (H3K36me3), which covers the entire transcribed region, also known as “K4–K36 signature” [10]. In primary and metastatic tumors, lincRΝΑs have been found to be differentially expressed, and this aberrant expression was also found to be a powerful predictor of disease outcome [12, 13]. LncRNAs overlapping or antisense to protein-coding gene promoters may also control the expression of their cognate protein-coding genes through epigenetic modifications contributing to carcinogenesis [2]. NcRNAs can function either in cis or in trans. Trans-acting function of ncRNAs is associated with both lincRNAs and short ncRNAs, while cis-acting functions are associated with long ncRNAs. In fact, the majority of imprinted lncRNAs serve as host transcripts for trans-acting microRNAs (miRNAs) [9, 14].
microRNA biology
MiRNAs are the best-studied class of ncRNAs. MiRNAs are single-stranded RNAs of approximately 22 nucleotides long that act as gene regulators at a post-transcription level. As part of a protein-to-RNA complex known as RISC or miRNP, they provide sequence specificity and by pairing with their target mRNAs anywhere along the length commonly but not only in the 3′untranslated region leading mainly to translational repression [15]. Occasionally some miRNAs, such as miR369-3 can also activate translation of target mRNAs on cell cycle arrest [16]. While miRNAs’ loci are predominantly found in non-coding and intronic regions of the genome, recent work has identified miRNAs encoded from within protein-coding exons [17]. Additionally, miRNAs are sometimes arranged as clusters with the multiple members of the cluster being transcribed simultaneously [18]. MiRNAs’ biogenesis is a multistep process involving a complex circuit of proteins; the biogenesis typically requires the enzymes Drosha, DGCR8 and Dicer, although recently alternative pathways have also been described [19, 20].
MiRNAs have been shown to regulate complex cell signaling networks [21] in the context of different diseases including cancers [22, 23]. A widespread down-regulation of miRNAs is generally observed in human neoplasias [24]. On a related note, many miRNAs are located in cancer-associated genomic regions or in regions of chromosomal instability that are frequently affected in human cancer, suggesting potential roles in the onset and progression of cancer [25]. Deregulated miRNAs expression profiles have been observed in both solid and blood cancers [26–31] and can distinguish among human cancers according to the developmental lineage and differentiation status of the cancer [32].
The DLK1-DIO3 genomic region
DLK1 and MEG3 (Gtl2 in mouse) are imprinted genes located on mouse chromosome 12 and on human chromosome 14 (Fig. 1), which are paternally and maternally expressed, respectively [33]. In humans, the DLK1-DIO3 genomic region contains the paternally expressed genes DLK1, RTL1, and DIO3, and, the maternally expressed genes MEG3 (Gtl2), MEG8 (RIAN), and antisense RTL1 (RTL1as) [34].
Fig. 1.
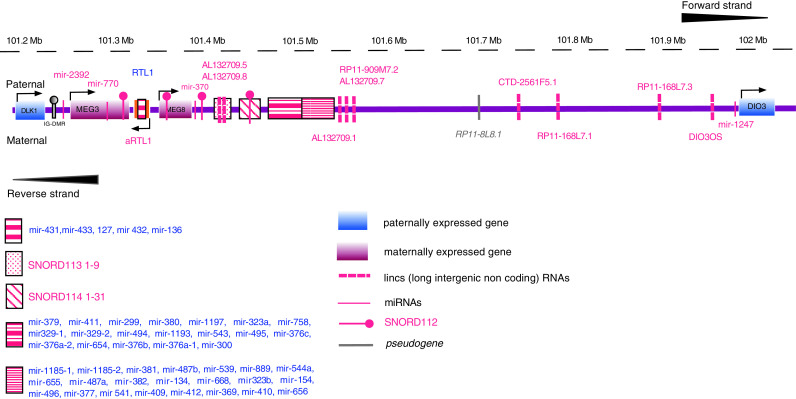
Schematic representation of the DLK1-DIO3 genomic region in the parental and maternal chromosomes. Genes, miRNAs, snoRNAs, lincRNAs, and pseudogenes are noted
This region has undergone important changes during the course of evolution, the most important being the acquisition of the Imprinting Control Region (ICR). In the process, the genes of this region have acquired their own distinct and important functions. For example, RTL1 has evolved into a new gene with important functions in growth and development, while miRNAs and snoRNAs also evolved in this specific region. Finally, the intron size of DLK1 was reduced and the entire imprinted cluster gained a sub-telomeric chromosomal position in eutherians [35]. Two distinct, differentially methylated regions (DMR) are present in the region and have separate functional properties: a primary, germline-derived DLK1-MEG3 intergenic DMR (IG-DMR) and a secondary, post-fertilization-derived MEG3-DMR. Both DMRs are hyper-methylated after paternal transmission and hypo-methylated after maternal transmission in somatic cells. Functionally, IG-DMR seems to at the top of the hierarchy and controls the methylation pattern of the imprinting regulator of both paternally and maternally expressed genes, i.e., of the secondary MEG3-DMR on the maternally transmitted chromosome [36] (see also Fig. 1). Both MEG3 and MEG8 are lincRNAs.
DLK1 is a member of the Notch signaling pathway and is involved in cell differentiation of several tissues [37]. RTL1 is on the opposite strand from DLK1 and is a retrotransposon-like gene. RTL1 is essential for the normal development of placental and embryonic tissues whereas DIO3 encodes a type 3-iodothyronine deiodinase protecting developing tissues from excessive amounts of thyroid hormone [37].
This region also hosts 53 miRNAs (forward strand) and one more (mir-1247) on the reverse strand. This cluster of 54 miRNAs is the largest one currently known in the human genome (Fig. 1; Table 1). MiR-2392 and miR-770 are in the DLK1-MEG3 region whereas the rest of the miRNAs are located downstream of MEG3 (Fig. 1; Table 1). The region downstream of MEG3 also includes many snoRNAs, and in particular one, nine, and 31 paralogous copies of SNORD112 [14q(0)], SNORD113 [14q(I)] and SNORD114 [14q(II)], respectively. In addition to MEG3 and MEG8, nine more lincs are found in the DLK1-DIO3 locus, six of which are on the forward strand (AL132709.5, AL132709.7, AL132709.8, CTD-2561F5.1, RP11-168L7.3, RP11-909M7.2) and three on the reverse strand (RP11-168L7.1, AL132709.1 and DIO3OS). A pseudogene, RP11-8L8.1 and a spliceosomal small nuclear RNA, U3 also lie in the reverse strand of DLK1-DIO3 locus (ENSEMBL release 67).
Table 1.
MiRNAs located in the DLK1-DIO3 region (MiRBase v. 18) (http://www.mirbase.org/), the predicted pathways that are involved in, GO terms analysis and also diseases that published work has shown they have been implicated in (PubMed search)
| microRNA (strand) | Predicted pathways involved | GO TERMS | Disease | References |
|---|---|---|---|---|
| miR-127-5p (+) | Type II diabetes mellitus, renal cell carcinoma, Toll-like receptor signaling pathway, natural killer cell-mediated cytotoxicity, T cell receptor signaling pathway, JAK-STAT signaling pathway | M band, ER-to-Golgi transport, regulation of microtubule depolymerization, negative regulation of microtubule depolymerization, calcium | AML, lymphomas, squamous esophageal carcinoma, colorectal Ca, hepatocellular carcinoma, ovarian Ca, cervical carcinoma, oligodendrogliomas, lung cancer, breast cancer, renal interstitial fibrosis, prostate, osteosarcoma, papillary thyroid carcinoma, bladder Ca, medulloblastoma, atherosclerosis, muscular dystrophy, gastric Ca | [73, 74, 77, 79–82, 85, 95, 97, 99, 101–103, 105, 110–119] |
| miR-134 (+) | Focal adhesion, endometrial cancer, prostate cancer, adherens junction | Cytoplasmic microtubule organization, regulation of microtubule depolymerization, negative regulation of microtubule depolymerization, dendrite development, response to axon injury | Lymphoma, tongue squamous carcinoma, esophageal squamous carcinoma, gastric cancer, colorectal Ca, GISTs, endometrial carcinosarcomas, oligodendrogliomas, glioblastomas, lung Ca, osteosarcomas, fragile-X mental retardation, lupus nephritis, coronary artery disease, acute pulmonary embolism, mastocytosis | [68, 74–77, 84, 95, 110, 113, 120–125] |
| miR-136-5p (+) | Viral myocarditis, non-small cell lung cancer, glioma, ECM receptor interaction, prostate cancer, neurotrophin signaling pathway, adherens junction, small cell lung cancer, focal adhesion, tight junction, pathways in cancer | Substrate adhesion-dependent cell spreading, microfilament motor activity, actin filament-based movement, MAP kinase activity, cell–cell adhesion junction | Leukemia, glioblastomas, lung neoplasms, heart failure, muscular dystrophy, pheochromocytomas | [90, 97, 104, 118, 126, 127] |
| miR-154-5p (+) | Viral myocarditis, prostate cancer, ECM–receptor interaction, ABC transporters, glioma, adherens junction, pancreatic cancer, small cell lung cancer, focal adhesion, pathways in cancer, regulation of actin cytoskeleton | Protein phosphatase 2A binding, M band, myosin filament, microfilament motor activity, A band | AML, tongue squamous carcinoma, esophageal Ca, colorectal Ca, hepatocellular carcinoma, ovarian Ca, endometrial carcinosarcomas, breast Ca, muscular disorders, salivary gland pleomorphic adenomas | [73, 74, 77, 80, 82, 84, 124, 128, 129] |
| miR-299-5p (+) | Notch signaling pathway, ECM–receptor interaction, regulation of actin cytoskeleton, focal adhesion | Dendrite morphogenesis, integrin complex, neuromuscular process controlling balance, basal plasma membrane, cerebellum development | Heart failure, muscular disorders AML, salivary gland adenomas, gastric Ca, colorectal Ca, ovarian Ca, lung Ca, breast Ca | [73, 75, 77, 95, 100, 126, 129, 130] |
| miR-300 (+) | Citrate cycle, regulation of actin cytoskeleton, focal adhesion, pathways in cancer | Protein phosphatase 2A binding, phospholipid-translocating ATPase activity, aminophospholipid transporter activity, extracellular-glutamate-gated ion channel activity, A band | Heart failure, ovarian Ca | [126, 131] |
| miR-323a-5p (+) | Endometrial cancer, ECM–receptor interaction, ABC transporters, small cell lung cancer, non-small cell lung cancer | Microfilament motor activity, fascia adherens, regulation of mitochondrial membrane permeability, Rac GTPase activator activity, sodium channel complex | AML, colorectal cancer, traumatic brain injury, papillary thyroid carcinoma, medulloblastoma, renal interstitial fibrosis | [73, 77, 103, 114, 116, 132] |
| miR-323b-5p (+) | ABC transporters, endometrial cancer, ECM–receptor interaction, arrhythmogenic right ventricular cardiomyopathy (ARVC), viral myocarditis | Intercalated disc, neuromuscular junction, microfilament motor activity, phospholipid-translocating ATPase activity, aminophospholipid transporter activity | ||
| miR-329 (+) | Pancreatic cancer, nuclear receptors in lipid metabolism and toxicity, progesterone-mediated oocyte maturation, pathways in cancer | Guanyl-nucleotide exchange factor activity, DNA replication, small GTPase regulator activity, nucleoside-triphosphatase regulator activity, GTPase regulator activity | GISTs, lung Ca | [76, 95] |
| miR-337-5p (+) | Beta-alanine metabolism, pathogenic Escherichia coli infection, butanoate metabolism | Apolipoprotein binding, neuromuscular process controlling balance, positive regulation of neurological system process, lamellipodium, microtubule-based movement | CLL, salivary gland pleomorphic adenomas, gastric Ca, colorectal Ca, ovarian Ca, lung Ca, breast Ca, prion disease | [77, 82, 97, 101, 102, 129, 133–135] |
| miR-369-5p (+) | Ribosome, intestinal immune network for IgA production | Myosin II complex, immune response-regulating cell surface receptor signaling pathway, myosin complex, cytosolic ribosome, actin binding | Gastric Ca, endometrial carcinosarcomas, renal interstitial fibrosis | [75, 84, 103] |
| miR-370 (+) | Role of Erk5 in neuronal survival, EPO signaling pathway, endometrial cancer, ECM–receptor interaction, arrhythmogenic right ventricular cardiomyopathy (ARVC) | Establishment or maintenance of epithelial cell apical/basal polarity, microfilament motor activity, actin filament-based movement, actin cytoskeleton reorganization, basal plasma membrane | AML, squamous esophageal carcinoma, gastric Ca, GISTs, colorectal Ca, cholangiocarcinoma, endometrial carcinosarcomas, prion disease, breast Ca, coronary artery disease |
[73] |
| miR-376a-5p (+) | Role of ERBB2 in signal transduction and oncology, long-term potentiation, Alzheimer’s disease | Photoreceptor outer segment, regulation of microtubule depolymerization, negative regulation of microtubule depolymerization, negative regulation of microtubule polymerization or depolymerization, microfilament motor activity | Squamous esophageal carcinoma, GISTs, pancreatic Ca, endometrial carcinosarcomas, traumatic brain injury, lung Ca, breast Ca, pleomorphic adenomas of the salivary gland, primary pigmented nodular adrenocortical disease, medulloblastoma | [74, 76, 84, 95, 97, 101, 102, 116, 129, 132, 137, 138] |
| miR-376b (+) | Human cytomegalovirus and map kinase pathways, purine metabolism | Human cytomegalovirus and map kinase pathways, 3′,5′-cyclic-nucleotide phosphodiesterase activity, cyclic-nucleotide phosphodiesterase activity, endocytic vesicle membrane, phosphoric diester hydrolase activity | Primary pigmented nodular adrenocortical disease, ovarian Ca, breast Ca | [82, 100, 137] |
| miR-376c (+) | 81.Structure_of_Caps_and_SMACs, neuroactive ligand–receptor interaction | Guanyl-nucleotide exchange factor activity, regulation of small GTPase mediated signal transduction, GTPase regulator activity, nucleoside-triphosphatase regulator activity, ion binding | AML, GISTs, colorectal Ca, ovarian Ca, lung Ca, renal interstitial fibrosis, osteosarcoma, hepatocellular carcinoma, chronic hepatitis, muscular disorders, primary pigmented nodular adrenocortical disease, muscular dystrophy, medulloblastoma | [72, 73, 76, 77, 83, 95, 103, 115, 118, 130, 137, 139] |
| miR-377-5p (+) | Primary immunodeficiency, Rho cell motility signaling pathway, endometrial cancer, apoptosis, focal adhesion | Histone methyltransferase activity (H3-K4 specific), smooth muscle tissue development, voltage-gated sodium channel complex, sodium channel complex, stereocilium | Gastric Ca, ovarian Ca, lung Ca, mesothelioma, breast Ca, diabetic nephropathy, bladder Ca, heart failure | [82, 98, 100, 105, 126, 134, 140, 141] |
| miR-379-5p (+) | ECM–receptor interaction, cytosolic DNA-sensing pathway, focal adhesion, natural killer cell mediated cytotoxicity | Detection of mechanical stimulus involved in sensory perception of sound, inner ear receptor stereocilium organization, transforming growth factor beta binding, detection of mechanical stimulus involved in sensory perception, endochondral ossification | Gastric Ca, glioblastomas, traumatic brain injury, lung Ca, renal interstitial fibrosis, muscular disorders, medulloblastoma hepatocellular carcinoma | [75, 90, 95, 97, 103, 116, 130, 132, 136, 142] |
| miR-380-5p (+) | Role of PPAR-gamma coactivators in obesity and thermogenesis, growth hormone signaling pathway, ECM–receptor interaction, Jak-STAT signaling pathway, axon guidance | Auditory receptor cell differentiation, inner ear receptor cell differentiation, I band, microtubule basal body, Z disc | Neuroblastoma | [89] |
| miR-381 (+) | Sonic Hedgehog (Shh) pathway, Hedgehog signaling pathway, neurotrophin signaling pathway, focal adhesion, calcium signaling pathway | Regulation of cholesterol storage, dendritic spine, Rho guanyl-nucleotide exchange factor activity, Ras guanyl-nucleotide exchange factor activity, regulation of Rho protein signal transduction | Mastocytosis, ovarian Ca, gliomas, heart failure, muscular disorders, autism | [68, 82, 88, 126, 130, 143] |
| miR-382-5p (+) | Inositol phosphate metabolism, ECM–receptor interaction, Focal adhesion, Regulation of actin cytoskeleton | Gamma-tubulin complex, negative regulation of translational initiation, cortical actin cytoskeleton, positive regulation of multicellular organism growth, cellular component maintenance | AML, ovarian Ca, glioblastomas, lung Ca, breast Ca, renal interstitial fibrosis, osteosarcoma, heart failure, massive macronodular adrenocortical disease, HIV infections | [72, 82, 90, 95, 103, 111, 115, 126, 144, 145] |
| miR-409-5p (+) | 99.NF-κB_activation, type II diabetes mellitus, Apoptosis | Cytoskeletal anchoring at plasma membrane, regulation of microtubule depolymerization, negative regulation of microtubule depolymerization, negative regulation of microtubule polymerization or depolymerization, lateral plasma membrane | Salivary gland pleomorphic adenomas, medulloblastoma, gastric Ca, GISTs, ovarian Ca, glioblastomas, oligodendrogliomas, lung Ca, breast Ca | [75, 76, 82, 95, 99, 113, 116, 119, 129] |
| miR-410 (+) | Focal adhesion | Ligand-gated ion channel activity, ligand-gated channel activity, apoptosis, programmed cell death, cell death | Gastric Ca, ovarian Ca, lung Ca, pheochromocytomas, androgenetic alopecia | [75, 82, 97, 104, 146] |
| miR-411-5p (+) | Actions of nitric oxide in the heart, induction of apoptosis through DR3 and DR4/5 death receptors, small cell lung cancer, cell adhesion molecules (CAMs), ubiquitin mediated proteolysis | Anaphase-promoting complex, cell–matrix adhesion, cell-substrate adhesion, amine transport, cation channel activity | Preeclampsia, glioblastomas, breast Ca | [90, 111, 147] |
| miR-412 (+) | Keratan sulfate biosynthesis, ECM–receptor interaction, viral myocarditis, B cell receptor signaling pathway, prostate cancer | Vascular endothelial growth factor receptor activity, laminin complex, microfilament motor activity, regulation of embryonic development, sodium channel complex | Lung Ca, breast Ca | [128, 146] |
| miR-431-5p (+) | ABC transporters, ECM–receptor interaction, Inositol phosphate metabolism, control of gene expression by vitamin D receptor, viral myocarditis | Uropod, trailing edge, cytoskeletal anchoring at plasma membrane, laminin complex, metalloenzyme inhibitor activity | Hepatocellular carcinoma, lung Ca, malignant pheochromocytomas | [80, 97, 104] |
| miR-432-5p (+) | ECM–receptor interaction, ABC transporters, non-small cell lung cancer, small cell lung cancer, focal adhesion | Sodium channel complex, voltage-gated sodium channel complex, microfilament motor activity, regulation of Cdc42 protein signal transduction, regulation of Cdc42 GTPase activity | Squamous esophageal carcinoma, schizophrenia, lung Ca, osteosarcoma, heart failure, muscular disorders, autism | [74, 95, 122, 126, 130, 143, 148] |
| miR-433 (+) | Adherens junction, focal adhesion, prostate cancer, tight junction, regulation of actin cytoskeleton | Lamellar body, voltage-gated sodium channel complex, microfilament motor activity, sodium channel complex, intercalated disc | Ovarian Ca, gastric Ca, mesothelioma, breast Ca, traumatic brain injury, lupus nephritis, adenoviral infections, X-linked chondrodysplasia | [75, 82, 98, 121, 128, 132, 136, 141, 149, 150] |
| miR-485-p (+) | 79.B_cell_activation, ABC transporters, arrhythmogenic right ventricular cardiomyopathy (ARVC), axon guidance, ECM–receptor interaction | Voltage-gated sodium channel complex, sodium channel complex, pronucleus, voltage-gated sodium channel activity, negative regulation of actin filament depolymerization | ALL, ovarian adenocarcinoma, traumatic brain injury, lung Ca, mesothelioma, breast cancer, renal interstitial fibrosis, endometriosis | [69, 95, 98, 101–103, 111, 128, 132, 151, 152] |
| miR-487a (+) | Toll-like receptor signaling pathway, RIG-I-like receptor signaling pathway | Nuclear export, nucleocytoplasmic transport, nuclear transport, alkali metal ion binding, induction of apoptosis | – | |
| miR-487b (+) | CD40L signaling pathway, EGF signaling pathway, signal transduction through IL1R, angiotensin II-mediated activation of JNK pathway via Pyk2 dependent signaling, keratinocyte differentiation | Activation of MAPKK activity, positive regulation of nitric oxide biosynthetic process, SH2 domain binding, regulation of nitric oxide biosynthetic process, histone acetyltransferase activity | Glioblastomas, lung Ca, osteosarcomas, muscular disorders | [90, 95, 122, 130] |
| miR-493-5p (+) | ECM–receptor interaction, focal adhesion, small cell lung cancer, regulation of actin cytoskeleton, pathways in cancer | Smoothened signaling pathway, Rho guanyl-nucleotide exchange factor activity, Ras guanyl-nucleotide exchange factor activity, regulation of Rho protein signal transduction, basement membrane | Esophageal Ca, renal interstitial fibrosis, male breast Ca | [74, 103, 128] |
| miR-494 (+) | AKT signaling pathway, non-small cell lung cancer, ErbB signaling pathway, viral myocarditis, chronic myeloid leukemia | Regulation of cytokine biosynthetic process, positive regulation of cell differentiation, positive regulation of developmental process | Lymphomas, squamous esophageal carcinoma, gastric Ca, colorectal Ca, cholangiocarcinoma, lung Ca, breast Ca, bladder Ca, heart failure, lupus nephritis, WM, prion disease, intestinal-type gastric Ca, medulloblastoma, cervical carcinoma | [61, 71, 74, 75, 79, 95, 97, 101, 102, 105, 116, 121, 122, 153–156] |
| miR-495 (+) | Phosphatidylinositol signaling system, ECM–receptor interaction, progesterone-mediated oocyte maturation, GnRH signaling pathway, oocyte meiosis | Focal adhesion, cell-substrate adherens junction, cell-substrate junction, adherens junction, anchoring junction | Gastric Ca, glioblastomas, traumatic brain injury, lung Ca, breast Ca, bladder Ca, muscular disorders, salivary gland adenomas, medulloblastoma |
[75] |
| miR-496 (+) | Cytosolic DNA-sensing pathway, Small cell lung cancer, ECM-receptor interaction, focal adhesion, natural killer cell mediated cytotoxicity | Catenin complex, negative regulation of lymphocyte differentiation, cell–cell adherens junction, DNA-dependent ATPase activity, gliogenesis | Gastric cancer, fetal alcohol syndrome | [75, 87] |
| miR-539 (+) | Pathways in cancer | Tumor necrosis factor binding, gamma-tubulin complex, regulation of interleukin-2 biosynthetic process, basal plasma membrane, hindlimb morphogenesis | Mastocytosis, autism, medulloblastoma | [68, 116, 143] |
| miR-541-5p (+) | Apoptosis, calcium signaling pathway | Lamin binding, protein export from nucleus, cell–cell adherens junction, dendrite development, negative regulation of protein complex disassembly | Pheochromocytomas | [104] |
| miR-543 (+) | Cytokine–cytokine receptor interaction, axon guidance, nuclear receptors in lipid metabolism and toxicity, dilated cardiomyopathy, neuroactive ligand–receptor interaction | Transmembrane receptor protein tyrosine kinase activator activity, protein tyrosine kinase activator activity, kinase activator activity, receptor activator activity, protein kinase activator activity | Glioblastomas, mesothelioma | [90, 98] |
| miR-544a (+) | Hypertrophic cardiomyopathy (HCM), viral myocarditis, NOD-like receptor signaling pathway, dilated cardiomyopathy, epithelial cell signaling in Helicobacter pylori infection | Negative regulation of muscle cell apoptosis, muscle filament sliding, actin-myosin filament sliding, actin-mediated cell contraction, myosin filament | Breast Ca | [99, 128] |
| miR-654-5p (+) | Keratan sulfate biosynthesis, thyroid cancer, dorso-ventral axis formation, nuclear receptors in lipid metabolism and toxicity, ABC transporters | Plasma membrane part, cell junction, cytoskeleton, cytoskeletal protein binding, metal ion binding | Lupus nephritis, lung Ca | [95, 121] |
| miR-655 (+) | – | Helicase activity, purine NTP-dependent helicase activity, ATP-dependent helicase activity, stem cell differentiation | Adenoviral infections | [149] |
| miR-656 (+) | Role of Ran in mitotic spindle regulation, mismatch repair, eicosanoid metabolism | Cytosolic calcium ion homeostasis | Gastric Ca | [134] |
| miR-665 (+) | Multi-drug resistance factors, hypoxia and p53 in the cardiovascular system, ABC transporters, ECM–receptor interaction, non-small cell lung cancer | Transforming growth factor beta binding, cell-substrate junction assembly, patterning of blood vessels, collagen, regulation of mitotic metaphase/anaphase transition | GISTs | [76] |
| miR-668 (+) | ECM-receptor interaction, focal adhesion, calcium signaling pathway | Apoptotic cell clearance, calcium-transporting ATPase activity, calcium ion transmembrane transporter activity, regulation of dendrite development, regulation of dendrite morphogenesis | – | |
| miR-758 (+) | ECM-receptor interaction, amyotrophic lateral sclerosis (ALS), viral myocarditis, focal adhesion, prostate cancer | N-methyl-d-aspartate selective glutamate receptor complex, endoplasmic reticulum calcium ion homeostasis, ionotropic glutamate receptor signaling pathway, suckling behavior, ionotropic glutamate receptor complex | Esophageal carcinoma | [153] |
| miR-770-5p (+) | ECM-receptor interaction, focal adhesion, ABC transporters, phosphatidylinositol signaling system, glioma | Actin-dependent ATPase activity, microfilament motor activity, sodium channel complex, spectrin, voltage-gated sodium channel complex | Glioblastomas | [90] |
| miR-889 (+) | – | – | Glioblastomas | [90] |
|
miR-1185-5p (+) miR-1185-2 (+) |
Calcium signaling pathway, regulation of actin cytoskeleton | Calmodulin-dependent cyclic-nucleotide phosphodiesterase activity, homotypic cell–cell adhesion, Golgi-to-endosome transport, positive regulation of cell–matrix adhesion, organic anion transport | – | |
| miR-1193 (+) | ABC transporters, control of gene expression by vitamin D receptor, ECM–receptor interaction, nuclear receptors in lipid metabolism and toxicity, IL-2 receptor beta chain in T cell activation | Sodium channel complex, voltage-gated sodium channel complex, ionotropic glutamate receptor signaling pathway, cytoplasmic microtubule, voltage-gated sodium channel activity | – | |
| miR-1197 (+) | Heparan sulfate biosynthesis, ECM–receptor interaction, arrhythmogenic right ventricular cardiomyopathy (ARVC), dilated cardiomyopathy, hypertrophic cardiomyopathy (HCM) | Heparan sulfate proteoglycan biosynthetic process, collagen, clathrin-coated vesicle membrane, extracellular matrix part, extracellular matrix structural constituent | – | |
| miR-1247-5p (-) | Regulation of actin cytoskeleton, calcium signaling pathway | 1-phosphatidylinositol-4-phosphate 5-kinase activity, photoreceptor inner segment, sulfate transport, A band, negative regulation of angiogenesis | Adenoviral infections | [149] |
MiRNA targets were predicted using RNA22 (MiRBase v. 18, ENSEMBL release 65) [108]. Prediction of pathways and GO terms analaysis was performed using DAVID 6.7 (http://david.abcc.ncifcrf.gov/home.jsp) [109], with default settings and EASE 0.01 (in case there were no results, EASE 0.1 was allowed). Only the top 5 results based on fold enrichment are listed for both predicted pathways and GO terms
GO gene ontology, CLL chronic lymphocytic leukemia, GISTs gastrointestinal stromal tumors, AML acute myeloid leukemia, WM Waldenström’s macroglobulinemia, ALL acute lymphoblastic leukemia, Ca cancer
MEG3 is currently the most studied transcript of the region, however its function remains poorly understood. It is believed to be involved in both physiological and pathological processes of cell biology, to possess tumor suppressor properties [38], and to exert anti-proliferative function through interactions with p53 and MDM2, and also by regulating directly or indirectly the phosphorylation of Rb [39]. MEG3 also interacts with crucial signaling pathways involved in angiogenesis, cell proliferation and differentiation, survival regulation, and has been found deregulated in several types of tumors [39].
Recent studies in knock-out mice attempted to delineate the function of some of the components of DLK1-DIO3 region. Takahashi et al. [40] produced Gtl2-KO mutants harboring a deletion of exons 1–5 including Gtl2-DMR in an effort to gain a better understanding of the function of maternally expressed ncRNAs in this domain. They observed that the deletion of the Gtl2 imprinted ncRNA with its DMR induced parental origin-dependent lethality in these mutants. Zhou et al. [41] also generated a knockout mouse model, in which the first five exons and adjacent promoter region of the Gtl2 gene were deleted. In this study, maternal deletion of Gtl2 resulted in perinatal death and skeletal muscle defects, completely abolished expression of downstream maternally expressed genes, activated expression of silenced paternally expressed genes and resulted in the methylation of the IG-DMR. By contrast, the paternally inherited deletion did not demonstrate such an effect. Such findings indicate that Gtl2 plays an important role in embryonic development and in regulating Dlk1-Gtl2 imprinting [41]. Gordon et al. [42] used Meg3 knockout mice in order to identify targets and potential functions of this gene in embryonic development and tumorigenesis. They observed differences in signaling pathways and ontologies related to angiogenesis between wild-type and knock-out embryos. In addition, MEG3-null embryos were shown to have increased expression of vascular endothelial growth factor (VEGF) pathway genes and increased cortical microvessel density, suggesting important roles for Meg3 in vascularization in the brain and in tumor suppression partly through angiogenesis inhibition [42].
The degree of activation of the DLK1-DIO3 region has also been found to correlate with pluripotency levels of mouse stem cells. It has been found activated in fully pluripotent mouse stem cells and repressed in partially pluripotent cells. Additionally, several miRNAs from this cluster potentially target components of the Polycomb repressive complex 2 (PRC2) and therefore may form a feedback regulatory loop resulting in the expression of all genes and non-coding RNAs encoded by this region in full pluripotent stem cells [43].
It has been demonstrated that MEG3 is under-expressed in hepatocellular carcinoma cells compared to normal hepatocytes or adjacent cirrhotic tissue, due to altered DNA methylation, which is consistently found in several types of cancer [39, 44]. Transfection of miR-134, which was one of the significantly downregulated miRNAs, resulted in cell cycle arrest in the human pituitary-derived cell line (PDFS). Below, we examine the function of these miRNAs in disease etiology and possible implications as a result of their proximity to the imprinted MEG3 lncRNA.
DLK1-DIO3 clustered microRNAs biology and involvement in disease pathogenesis
The clustered miRNAs of the DLK1-DIO3 genomic region
As already mentioned, the DLK1-DIO3 genomic region contains 54 miRNAs and represents one of the largest miRNA-containing clusters of the human genome. In mouse, some of these miRNAs are expressed as products of a large non-coding transcript named Mirg [45, 46]. Hagan et al. [47] have reported the presence of at least ten genes in the Dlk1-Meg3 mouse imprinted domain and cloned for the first time a novel RNA named Irm (Imprinted RNA near Meg3); Irm partially overlaps Rian (the mouse homologue of Meg8), raising questions about the in vivo existence of Rian based on their results [47].
MiR-127 and miR-433, which belong to the same cluster, are transcribed from independent promoters in overlapping genomic regions in a 5′ → 3′ direction [48], and are under a common transcriptional regulatory mechanism among different mammalian species depending on the transcription factors nuclear estrogen-related receptor gamma (ERRγ) and small heterodimer partner (SHP) [49, 50]. As both miRNAs possess a conserved gene structure, it seems that the miR-433/127 loci might have evolved from an ancient common origin in mammals and are now transcribed from two separate promoters among species.
Biology and functions of the DLK1-DIO3 region miRNAs
Collective data suggest that the miR-127/136 cluster has a potential role in the silencing of the Rtl gene. Seitz et al. [51] originally showed that miR-127 and miR-136 display perfect complementarities with the Rtl mRNA [51]; subsequently, it was shown that silencing of the miR-127/136 cluster correlated with an increase in the expression of Rtl1. Moreover, in knock-out mice lacking expression of miR-127/136, an increased expression of Rtl1 was noted [52]. Study of the expression levels of miR-127 and miR-136 in fertilized mouse embryos, parthenotes, androgenotes, and cloned embryos developing in vitro, showed the two miRNAs to be highly expressed in parthenotes, but rarely in androgenotes. The Rtl1 promoter region was hyper-methylated in blastocyst stage parthenotes and its methylation decreased during development. Conversely, the promoter region of miR-127 was hypo-methylated in parthenogenetically activated embryos but heavily methylated in cloned blastocysts, suggesting a role for miR-127 and miR-136 in the epigenetic reprogramming of the Rtl1 imprinting process [53]. Moreover, Seitz et al. [51] suggested that miR-136 also acts as a small interfering RNA silencing Rtl1 expression in a mouse model.
MiR-134 is specifically expressed in the brain and controls dendritic spine formation in vitro. It also regulates the expression of CREB through a post-transcriptional mechanism and thus is linked to synaptic plasticity. MiR-134 over-expression in specific brain areas of mutant mice contributed to the impaired long-term memory formation in the contextual fear-conditioning paradigm, negatively regulating synaptic plasticity through translational inhibition of target genes such as CREB in mice [54]. MiR-134 also acts through inhibition of the translation of Lim-domain-containing-protein-kinase 1 (Limk1) responsible for spine development, thus indicating miRNA are important players in the maturation process of the mammalian brain [55]. MiR-134 expression increased in mouse embryonic stem cells (mESC) after treatment with retinoic acid. It also significantly reduced the promoter activity of LRH1, Sox2, Oct4, and Nanog promoters, which are known essential transcription factors for the maintenance and differentiation of embryonic stem cells status, acting at a post-transcription control level [15, 56]. MiR-134 could modulate mESC differentiation irrespectively of Oct4 and Nanog, and together with miR-470 could regulate pluripotency, self-renewal, and differentiation of stem cells [15, 56].
MiR-495 was the only miRNA that presented a similar expression in prefrontal and parietal cortex and exhibited laminar specificity in human prefrontal cortex [57]. MiR-541 over-expression negatively affected cell proliferation and differentiation, neurite outgrowth, synapse formation and modulated expression levels of the target mRNA synapsin I, conferring a role in neurocyte differentiation and axon maintenance [58]. Fiore et al. [59] demonstrated that the entire miR-379/410 cluster is most likely transcribed as single polycistronic unit, and is co-regulated by neuronal activity in a Mef 2-dependent manner. In particular, miR-329, miR-381, and miR-134 promoted dendritogenesis and dendritic growth in hippocampal neurons by down-regulating the RNA-binding protein Pum2 [59]. MiR-495 expression was also observed in embryonic liver and pancreas cells where it represses the expression of the HNF-6/Onecut transcription factor, suggesting a role in liver and pancreas development [60]. MiR-494 behaves as a pro-apoptotic post-transcriptional nucleolin regulator, thus probably possessing tumor suppressor properties, and competes with the RNA-binding protein HuR involved in nucleolin expression, therefore modulating cell proliferation and survival [61].
A subset of the miRNAs located in the DLK1-DIO3 genomic region, including miR-134, miR-154, miR-299, miR-323, miR-337, miR-370, and miR-376c, have been shown to be differentially expressed during human and mouse lung development [62]. MiRNAs of this particular domain are also involved in erythropoiesis [63]. In CD34+ cells derived from umbilical cord blood, miR-127, miR-136, miR-154, and miR-379 exhibited deregulated expression throughout erythropoiesis showing lineage commitment [63]. MiR-376a negatively modulated early human erythropoiesis in the K562 cell line with the ability to erythroid differentiation [64]. Enforced expression of miR-376a suppressed the hemin-induced erythropoiesis by repressing the erythroid differentiation of CD34+ stem/progenitor cells, while inhibition of the aforementioned miRNA promoted erythroid differentiation [64]. Lastly, miR-376a over-expression also negatively regulated Argonaute 2 (Ago2) and cyclin-dependent kinase (CDK) 2, two factors known to affect erythropoiesis, and to result in the arrest of erythroid differentiation [64]. MiR-377 in combination with miR-217 are involved in the regulation of the expression of the heme oxygenase-1 enzyme, which metabolizes the excess heme generated during hemolysis [65].
DLK1-DIO3 region miRNAs in disease pathogenesis
In vitro
Teferedegne et al. [66], using microarray-expression and qRT-PCR assays, found that increases in the expression of miR-376a, miR-543, miR-654-3p, miR-382 correlated with the acquisition of tumorigenic phenotypes in cell lines of non-human primates. In another in vitro study, it was found that the overexpressed p16ink4a down-regulated CDK1 through modulation of miR-410 expression [67]. It also seems that miR-410 expression is under the negative control of the E2F4 transcription factor, and its expression may increase in response to p16ink4a overexpression and consequent inhibition of the Rb/E2F pathway [67].
In recent animal model studies, it was reported that miRNAs jointly with KIT and the microphthalmia-associated transcription factor (MITF) comprise a novel regulatory pathway in mast cell transformation [68]. Among the miRNAs whose expression is repressed by KIT activation are miR-134, miR-539, and miR-381. Also, retroviral expression of miR-539 and miR-381 resulted in decreased expression of MITF, suggesting that KIT indirectly modulates MITF protein levels through regulation of these particular miRNAs [68].
MiR-485-3p was significantly under-expressed in the human lymphoblastic leukemia teniposide-resistant cell line compared to the sensitive counterpart. Decreased miR-485-3p expression inversely correlated to the NF-Y transcription factor increased expression, which in turn was accompanied by down-regulation of DNA topoisomerase IIα [69]. In cholangiocarcinoma cell lines, miR-370 and miR-494 were found down-regulated [70, 71].
Blood cancers
Li et al. [72], conducted a genome-wide miRNA expression analysis in 52 acute myeloid leukemia (AML) samples with common translocations, including t(8;21)/AML1(RUNX1)-ETO(RUNX1T1), inv(16)/CBFB-MYH11, t(15;17)/PML-RARA, and MLL rearrangements. Several miRNAs were differentially expressed and clustered in the various AML subtypes. Among them, miR-382 was found over-expressed almost exclusively in leukemias harboring the t(15;17) translocation. Moreover miR-382 and miR-376c, along with miR-224, were found to represent a signature of t(15;17) AML with a very high diagnostic accuracy, suggesting their involvement in the pathogenesis of acute promyelocytic leukemia (APL). Similar findings were also reported by another group who performed quantitative expression profile analysis of 157 mature miRNAs on 100 AML patients of a whole spectrum of known karyotypes common in AML [73]. The study identified a set of miRNAs, all of which located in the DLK1-MEG3 cluster, to be significantly associated with APL cases. The set comprised the up-regulated miR-127, miR-154, miR-370, miR-299, miR-376c and miR-323, which suggests that miRNAs hosted in the DLK1-MEG3 domain are highly specific for the APL subtype.
Solid cancers
Serum miRNA levels have been evaluated in esophageal squamous cell carcinoma and at least nine miRNAs of the DLK1-MEG3 cluster (miR-127-3p, miR-134, miR-154, miR-370, miR-376a, miR-493, miR-494, miR-432, and miR-665) were found deregulated. MiR-127-3p was among a panel of seven serum miRNAs found up-regulated in the blood of patients with esophageal squamous cell carcinoma (ESCC) and could classify esophageal squamous cell carcinoma [74]. Several other miRNAs of the cluster have been implicated in the pathogenesis and progression of gastric cancer. In particular, miR-495, miR-134, miR-409-3p, miR-496, miR-379, miR-369-3p are linked to the tumor depth of invasion [75], while miR-376a expression was associated with nodal metastasis [75]. MiR-494 expression was found to correlate significantly with both staging variables [75]. Peritoneal dissemination was also associated with the deregulated expression of miR-299-5p, miR-409-3p, and miR-410. Moreover, miR-495, miR-433, and miR-410 predicted both disease-free survival (DFS) in gastric cancer patients and overall survival (OS) [75]. Such findings further emphasize the potential for miRNAs to serve as prognostic markers in cancer [31, 75].
In a recent study on gastrointestinal stromal tumors (GIST), Haller et al. [76] profiled miRNA expression for a relatively small series of GIST samples. They found 32 of 734 studied miRNAs to be differentially expressed according to localization and mutation status. Over-expression of miR-376c, miR-376a, miR-370, miR-409-3p, miR-665, and miR-329 was found to be correlated with the presence of KIT or PDGFR mutations and also gastric or intestinal localization. Moreover, decreased expression of miR-134 and miR-370 was associated with loss of the 14q chromosomal region. Although the single 14q loss does not confer shorter DFS in GISTs, it seems that low expression of miR-370 and miR-134 is associated with tumor progression and a shorter DFS irrespective of the 14q status [76].
In colon cancer, miR-154-3p, miR-323, miR-134, miR-376c, and miR-337 were all down-regulated in colorectal cancer cell lines and patient samples, probably indicating specific biological markers for such type of tumors [77]. On the contrary, miR-134 was not differentially expressed in the plasma of colorectal cancer patients compared to the control subjects [78]. MiR-494 had the highest fold change among over-expressed miRNAs in colorectal cancers whereas miR-127-3p was significantly over-expressed in colorectal cancer samples harboring KRAS mutations compared to wild-type KRAS, known downstream target of the EGFR signaling pathway [79].
Regarding hepatocellular carcinoma, Luk et al. [80], using a TRE-c-Met-driven transgenic HCC mouse model identified a cluster of 23 miRNAs that is encoded within the Dlk1-Gtl2 imprinted region on chromosome 12qF1 overexpressed in all of the isolated liver tumors. Further to that, they examined the expression the miRNA cluster of the DLK1-DIO3 on chromosome 14q32.2, which corresponds to Dlk1-Gtl2 imprinted region in mouse, in a cohort of 97 hepatitis B virus-associated HCC patients. Interestingly, overexpression of the DLK1-DIO3 miRNA cluster was positively correlated with HCC stem cell markers (CD133, CD90, EpCAM, Nestin) and associated with a high level of serum alpha-fetoprotein, a conventional biomarker for liver cancer, and poor survival rate in HCC patients [80]. The data are also supported by a rat model study showing that down-regulation of expression of miR-127 and consequent decreased apoptosis rates resulted in the formation of hepatocellular carcinoma. The expression of miR-127 was not restored in methyl re-fed rats, suggesting a non-methylation-dependent control mechanism, and also correlated with the different stages of rat hepatocarcinogenesis [81].
Recently, Shih et al. [82] evaluated the prognostic value of 470 miRNAs in advanced serous ovarian cancer. They found 29 miRNAs that were associated with disease outcome. Interestingly, 11 of the 29 miRNAs were located in the DLK1-MEG3 cluster, and included miR-433, miR-337, miR-127, miR-410, miR-381, miR-377, miR-299-3p, miR-409-3p, miR-154, miR-382, and miR-376b. Among them, three miRNAs (miR-337, miR-410, and miR-645) were negatively associated with overall survival. In another study, overexpression of miR-376c in ovarian cancer cells was found to block cisplatin-induced cell death: patients with serous ovarian cancer who responded well to chemotherapy had low miR-376c expression whereas chemotherapy resistant patients had high levels of miR-376c [83]. The investigators suggested that miR-376c enhances proliferation, survival, and chemoresistance by targeting, at least in part, activin receptor-like kinase 7 (ALK7) [83].
MiR-370, miR-369-5p, miR-154, mir-376a, and miR-134 were all up-regulated in the sarcomatous component of endometrial carcinosarcomas compared to the epithelial component, and could represent a potential miRNAs signature, for distinguishing epithelial-to-mesenchymal transdifferentiation in this type of tumor [84]. In invasive cervical cancer, miR-127 and miR-134 were found significantly over-expressed compared with normal samples and miR-127 was significantly associated with lymph node metastasis making it a good candidate as a prognostic biomarker [85]. Zhang et al. [86] also showed that down-regulation of the chromosome 14 miRNA cluster is an event common to many human epithelial tumors and suggested that eight miRNAs (mir-337, mir-432, mir-495, mir-368, mir-376a, mir-376b, mir-377, and mir-419) located in DLK1-DIO3 domain could play a role as tumor suppressor genes in solid tumors.
MiRNAs clustered in the DLK1-DIO3 locus are also involved in central nervous system diseases [87]. MiR-381 has been found to play a major role in human glioma progression through suppression of the tumor suppressor LRRC4, which is associated with decreased inhibition of MEK/ERK and AKT signaling [88]. MiR-380-5p has been found to be developmentally restricted, characteristic of undifferentiated cells, also expressed through neuronal specification, and possess oncogenic properties in vivo through interaction with H-RAS, p53, and p21waf1 [89]. Inhibition of miR-380-5p in embryonic stem cells leads to up-regulation of p53 enhancing apoptosis whereas ectopic expression of the specific miRNA results in direct translation suppression of p53. MiR-380-5p expression was high in primary human neuroblastoma tumors, suppressed p53 expression, and predicted a poor outcome [89]. By high-throughput sequencing, nine miRNAs were found down-regulated in glioblastomas compared to normal brain tissues and included miR-495, miR-543, miR-770-5p, miR-379, miR-487b, miR-889, miR-382, miR-136, and miR-411-3p [90]. On the contrary, no miRNAs of the specific cluster were found up-regulated. Again, some of these miRNAs demonstrated 5′-end structural modifications reflecting fluctuations in strand dominance and likely processing variations [90]. Recently, differential expression of a set of 17 miRNAs was found to discriminate high-risk from low-risk neuroblastoma [91]. Notably, a total of 15 of the 17 miRNAs belonged to the imprinted human 14q32.31 miRNA cluster and two, miR-487b and miR-410, were significantly down-regulated in the high-risk group. Multivariate analysis showed that expression of miR-487b and miR-410 carried a predictive value beyond the classical high-/low-risk stratification and therefore could be a potential biomarker of relapse in favorable neuroblastoma [91]. The silencing of 18 genes, including 13 miRNAs at the DLK1-MEG3 locus has also been reported in human clinically non-functioning pituitary adenomas [92].
In human bronchial epithelial cell lines, mir-494 was shown to target and regulate PTEN. Overexpression of miR-494 led to a decreased expression of PTEN resulting in attenuated apoptosis, and also directly promoted colony formation acting as an oncomir [93]. Similarly, over-expressed levels of miR-494 were observed in primary murine bronchial epithelial cells exposed to the carcinogen benzo[a]pyrene, and resulted in G1 arrest of the cell cycle [94].
Guo et al. [95] evaluated the expression of 856 miRNAs and approximately 22,000 genes using miRNA microarray and cDNA microarray in sensitive (NCI-H69) and resistant to chemotherapy cell lines (NCI-H69AR) of small cell lung carcinoma (SCLC). They found 24 up-regulated and 37 down-regulated miRNAs. Among the miRNAs in the latter group, miR-134, miR-299, miR-329, miR-376a, miR-376c, miR-379, miR-382, miR-409, miR-485, miR-487b, miR-494, miR-495, miR-654, miR-127, miR-432, were found to be down-regulated with a concomitant modulation of the expression of their target mRNA [95]. In addition, miR-495 was found significantly up-regulated in K-RAS mutated lung adenocarcinomas compared to healthy lung tissue [96]. A novel 10 over-expressed miRNAs signature in murine lung cancers has been recently reported [97]. Interestingly, seven out of the 10 miRNAs belong to the DLK1-DIO3 domain and included miR-136, miR-376a, miR-337, miR-410, miR-127, miR-379, and miR-431. However, in human non-small cell lung cancers only miR-136 and miR-376a were over-expressed in contrast to murine [97]. MiR-377, miR-433, miR-543 and miR-485 have been identified in malignant mesothelioma differentially expressed in each histopathological subtype [98].
Five miRNAs including miR-485-5p, miR-544, miR-409-3p, miR-127, and miR-495 were up-regulated in two different breast cancer stem cell sub-populations. Most interestingly, miR-495 was the only one over-expressed in both breast cancer stem cell sub-populations evaluated while the remaining four were unique to each sub-population. MiR-495 has been suggested to promote colony formation, tumor growth, and cancer cell invasion through downregulation of E-cadherin expression [99]. It has also been shown to inhibit REDD1 and therefore promote cancer cell proliferation under hypoxia [99]. MiR-495 expression is under the positive control of the transcription factors E12 and E47 involved in the cell cycle regulation of the hematopoietic stem cells [99]. Three distinct miRNAs signatures have been proposed in order to differentiate estrogen receptor, progesterone receptor, and HER2/Neu breast cancer. In all three instances, miRNAs of the DLK1-DIO3 cluster were identified, including miR-299-3p, miR-377, and miR-376b, respectively [100]. MiR-127 and miR-376a were found down-regulated in breast cancer samples compared to adjacent normal tissues, while miR-127-3p presented higher expression in basal cells than luminal cells and was significantly elevated in malignant myoepithelioma compared to luminal and basal-like cancer subtypes. Differentially expressed miRNAs in basal cells and luminal cells included miR-337-3p, miR-485-3p, and miR-494 [101, 102].
In an effort to better understand the development of renal interstitial fibrosis, Kriegel et al. [103] evaluated the association of TGFβ1 and miRNAs expression. Half of the up-regulated miRNAs after treatment with TGFβ1 belonged to the DLK1-DIO3 domain such as miR-127-5p, miR-493, miR-376c, miR-379, miR-323-3p, miR-382, miR485-3p, and miR-369, while no miRNA of the cluster was down-regulated.
Lastly, miR-541 over-expression was observed in von Hippel-Lindau disease-associated pheochromocytomas compared to sporadic benign pheochromocytomas, and miR-376b was overexpressed in recurrent or metastatic/malignant pheochromocytomas. On the contrary, miR-431, miR-541, miR-410, and miR-136 were under-expressed in malignant pheochromocytoma [104]. Microarray analysis on bladder cancer cells and on normal fibroblast cells after treatment with azacytidine and/or phenylbutyric acid identified over-expression of miR-127, miR-134, miR-494, miR-495, and miR-377 [105]. In particular, miR-127 showed prominent up-regulation compared to the other miRNAs of the same cluster, after combinational therapy, and negatively regulated its target, the protoncogene BCL6. The particular miRNA was also down-regulated in primary human cancers of the colon, bladder and prostate as well as the other counterparts of the cluster [105].
Conclusions
A growing body of evidence suggests that ncRNAs are molecules of previously unrecognized major biological importance. LncRNAs can induce local gene silencing in cis, or repress multiple genes activity throughout the genome in trans, bind chromatin modifiers complexes, and be implicated in genomic reprogramming of somatic cells to induced pluripotent stem cells through global remodeling of the epigenome [106]. They can also activate transcription factors expression and enhance stem cell differentiation by recruiting epigenetic activators to chromatin [107].
MiRNAs are recognized as essential post-transcriptional regulators of gene expression in both physiological and pathological processes. Their expression is deregulated in many diseases and in cancer [2, 24, 26]. Deregulated miRNA expression affects most of the hallmarks of cancer and could also influence cancer cell chemosensitivity and therefore cancer curability.
The DLK1-DIO3 genomic region is dominated by the lncRNA MEG3, which is involved in several important signaling cascades and comprises one of the largest miRNA clusters encoded from non-coding transcription units. The findings reported here indicate that these miRNAs are involved in a wide spectrum of human diseases, mostly cancers. They may modulate important signaling pathways like mTOR, MAPK, Wnt, JAK-STAT, p53, and they are related to cytokine signaling cascades, DNA methylation, oncogenic kinases expression, and many more. Given the quantitative assessment of these miRNAs, they can be used as biomarkers for the diagnosis, progression and prognosis of several cancers and can identify cancer sub-classes of different clinical behavior. Moreover, they are capable of early characterizing latent malignant cells in the bone marrow stroma that can pass through the gap junctional intercellular communication and move from stroma to cells altering cell cycle. The fact that some miRNAs (e.g., miR-127) are more up-regulated when azacytidine is co-administered with phenylbutyric acid [105] might in part explain the synergistic therapeutic effect observed in diseases such as myelodysplastic syndromes. Perhaps, strong re-expression of the suppressed miRNA might lead in higher down-regulation of the target oncogenes [105].
Our knowledge on long and short ncRNAs biology and function is increasing every day. A better understanding of miRNA clusters that are implicated in human diseases and malignant cell transformation could help lead to the discovery of new therapeutic strategies.
Acknowledgments
E.H. is a scholar of the Hellenic Society of Haematology Foundation. P.L. and I.R. are partially supported by a William M. Keck Foundation grant.
Conflict of interest
The authors declare no potential conflicts of interest.
Footnotes
L. Benetatos, E. Hatzimichael contributed equally to this work.
I. Rigoutsos and E. Briasoulis contributed equally to this work.
References
- 1.Shabalina SA, Spiridonov NA. The mammalian transcriptome and the function of non-coding DNA sequences. Genome Biol. 2004;5(4):105. doi: 10.1186/gb-2004-5-4-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol. 2010;220(2):126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- 3.Mattick JS. The genetic signatures of noncoding RNAs. PLoS Genet. 2009;5(4):e1000459. doi: 10.1371/journal.pgen.1000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10(2):126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 5.Rother S, Meister G. Small RNAs derived from longer non-coding RNAs. Biochimie. 2011;93(11):1905–1915. doi: 10.1016/j.biochi.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 6.Tollervey D, Kiss T. Function and synthesis of small nucleolar RNAs. Curr Opin Cell Biol. 1997;9(3):337–342. doi: 10.1016/S0955-0674(97)80005-1. [DOI] [PubMed] [Google Scholar]
- 7.Kiss T. Small nucleolar RNAs: an abundant group of noncoding RNAs with diverse cellular functions. Cell. 2002;109(2):145–148. doi: 10.1016/S0092-8674(02)00718-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Hu H, Lai M. Non-coding RNAs and their epigenetic regulatory mechanisms. Biol Cell Auspices Eur Cell Biol Organ. 2010;102(12):645–655. doi: 10.1042/BC20100029. [DOI] [PubMed] [Google Scholar]
- 9.Koerner MV, Pauler FM, Huang R, Barlow DP. The function of non-coding RNAs in genomic imprinting. Development. 2009;136(11):1771–1783. doi: 10.1242/dev.030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458(7235):223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, Guigo R, Shiekhattar R. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143(1):46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai MC, Spitale RC, Chang HY. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res. 2011;71(1):3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, Yang X, Amit I, Meissner A, Regev A, Rinn JL, Root DE, Lander ES. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477(7364):295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455(7216):1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 16.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 17.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 18.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires ago catalysis. Nature. 2010;465(7298):584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Mol Cell. 2007;28(2):328–336. doi: 10.1016/j.molcel.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichimura A, Ruike Y, Terasawa K, Tsujimoto G. miRNAs and regulation of cell signaling. FEBS J. 2011;278(10):1610–1618. doi: 10.1111/j.1742-4658.2011.08087.x. [DOI] [PubMed] [Google Scholar]
- 22.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467(7311):86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 24.Melo SA, Esteller M. Dysregulation of microRNAs in cancer: playing with fire. FEBS Lett. 2011;585(13):2087–2099. doi: 10.1016/j.febslet.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 27.Benetatos L, Vartholomatos G. Deregulated microRNAs in multiple myeloma. Cancer. 2012;118(4):878–887. doi: 10.1002/cncr.26297. [DOI] [PubMed] [Google Scholar]
- 28.Calvo KR, Landgren O, Roccaro AM, Ghobrial IM. Role of microRNAs from monoclonal gammopathy of undetermined significance to multiple myeloma. Semin Hematol. 2011;48(1):39–45. doi: 10.1053/j.seminhematol.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcucci G, Mrozek K, Radmacher MD, Garzon R, Bloomfield CD. The prognostic and functional role of microRNAs in acute myeloid leukemia. Blood. 2011;117(4):1121–1129. doi: 10.1182/blood-2010-09-191312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhyasen GW, Starczynowski DT. Deregulation of microRNAs in myelodysplastic syndrome. Leuk Off J Leuk Soc Am Leuk Res Fund UK. 2012;26(1):13–22. doi: 10.1038/leu.2011.221. [DOI] [PubMed] [Google Scholar]
- 31.Chira P, Vareli K, Sainis I, Papandreou C, Briasoulis E. Alterations of MicroRNAs in solid cancers and their prognostic value. Cancers. 2010;2(2):1328–1353. doi: 10.3390/cancers2021328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt JV, Matteson PG, Jones BK, Guan XJ, Tilghman SM. The Dlk1 and Gtl2 genes are linked and reciprocally imprinted. Gene Dev. 2000;14(16):1997–2002. [PMC free article] [PubMed] [Google Scholar]
- 34.Irving MD, Buiting K, Kanber D, Donaghue C, Schulz R, Offiah A, Mohammed SN, Oakey RJ. Segmental paternal uniparental disomy (patUPD) of 14q32 with abnormal methylation elicits the characteristic features of complete patUPD14. Am J Med Genet Part A. 2010;152A(8):1942–1950. doi: 10.1002/ajmg.a.33449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards CA, Mungall AJ, Matthews L, Ryder E, Gray DJ, Pask AJ, Shaw G, Graves JA, Rogers J, Dunham I, Renfree MB, Ferguson-Smith AC. The evolution of the DLK1-DIO3 imprinted domain in mammals. PLoS Biol. 2008;6(6):e135. doi: 10.1371/journal.pbio.0060135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagami M, O’Sullivan MJ, Green AJ, Watabe Y, Arisaka O, Masawa N, Matsuoka K, Fukami M, Matsubara K, Kato F, Ferguson-Smith AC, Ogata T. The IG-DMR and the MEG3-DMR at human chromosome 14q32.2: hierarchical interaction and distinct functional properties as imprinting control centers. PLoS Genet. 2010;6(6):e1000992. doi: 10.1371/journal.pgen.1000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.da Rocha ST, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet TIG. 2008;24(6):306–316. doi: 10.1016/j.tig.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol. 2012;48(3):R45–R53. doi: 10.1530/JME-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benetatos L, Vartholomatos G, Hatzimichael E. MEG3 imprinted gene contribution in tumorigenesis. Int J Cancer J Int du cancer. 2011;129(4):773–779. doi: 10.1002/ijc.26052. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi N, Okamoto A, Kobayashi R, Shirai M, Obata Y, Ogawa H, Sotomaru Y, Kono T. Deletion of Gtl2, imprinted non-coding RNA, with its differentially methylated region induces lethal parent-origin-dependent defects in mice. Hum Mol Genet. 2009;18(10):1879–1888. doi: 10.1093/hmg/ddp108. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Cheunsuchon P, Nakayama Y, Lawlor MW, Zhong Y, Rice KA, Zhang L, Zhang X, Gordon FE, Lidov HG, Bronson RT, Klibanski A. Activation of paternally expressed genes and perinatal death caused by deletion of the Gtl2 gene. Development. 2010;137(16):2643–2652. doi: 10.1242/dev.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon FE, Nutt CL, Cheunsuchon P, Nakayama Y, Provencher KA, Rice KA, Zhou Y, Zhang X, Klibanski A. Increased expression of angiogenic genes in the brains of mouse meg3-null embryos. Endocrinology. 2010;151(6):2443–2452. doi: 10.1210/en.2009-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Luo GZ, Yang W, Zhao X, Zheng Q, Lv Z, Li W, Wu HJ, Wang L, Wang XJ, Zhou Q. Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells. J Biol Chem. 2010;285(25):19483–19490. doi: 10.1074/jbc.M110.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braconi C, Kogure T, Valeri N, Huang N, Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM, Patel T. microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene. 2011;30(47):4750–4756. doi: 10.1038/onc.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glazov EA, McWilliam S, Barris WC, Dalrymple BP. Origin, evolution, and biological role of miRNA cluster in DLK-DIO3 genomic region in placental mammals. Mol Biol Evol. 2008;25(5):939–948. doi: 10.1093/molbev/msn045. [DOI] [PubMed] [Google Scholar]
- 46.Seitz H, Royo H, Bortolin ML, Lin SP, Ferguson-Smith AC, Cavaille J. A large imprinted microRNA gene cluster at the mouse Dlk1-Gtl2 domain. Genome Res. 2004;14(9):1741–1748. doi: 10.1101/gr.2743304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagan JP, O’Neill BL, Stewart CL, Kozlov SV, Croce CM. At least ten genes define the imprinted Dlk1-Dio3 cluster on mouse chromosome 12qF1. PLoS One. 2009;4(2):e4352. doi: 10.1371/journal.pone.0004352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song G, Wang L. MiR-433 and miR-127 arise from independent overlapping primary transcripts encoded by the miR-433-127 locus. PLoS One. 2008;3(10):e3574. doi: 10.1371/journal.pone.0003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song G, Wang L. Transcriptional mechanism for the paired miR-433 and miR-127 genes by nuclear receptors SHP and ERRgamma. Nucleic Acids Res. 2008;36(18):5727–5735. doi: 10.1093/nar/gkn567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song G, Wang L. A conserved gene structure and expression regulation of miR-433 and miR-127 in mammals. PLoS One. 2009;4(11):e7829. doi: 10.1371/journal.pone.0007829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seitz H, Youngson N, Lin SP, Dalbert S, Paulsen M, Bachellerie JP, Ferguson-Smith AC, Cavaille J. Imprinted microRNA genes transcribed antisense to a reciprocally imprinted retrotransposon-like gene. Nat Genet. 2003;34(3):261–262. doi: 10.1038/ng1171. [DOI] [PubMed] [Google Scholar]
- 52.Sekita Y, Wagatsuma H, Nakamura K, Ono R, Kagami M, Wakisaka N, Hino T, Suzuki-Migishima R, Kohda T, Ogura A, Ogata T, Yokoyama M, Kaneko-Ishino T, Ishino F. Role of retrotransposon-derived imprinted gene, Rtl1, in the feto-maternal interface of mouse placenta. Nat Genet. 2008;40(2):243–248. doi: 10.1038/ng.2007.51. [DOI] [PubMed] [Google Scholar]
- 53.Cui XS, Zhang DX, Ko YG, Kim NH. Aberrant epigenetic reprogramming of imprinted microRNA-127 and Rtl1 in cloned mouse embryos. Biochem Biophys Res Commun. 2009;379(2):390–394. doi: 10.1016/j.bbrc.2008.12.148. [DOI] [PubMed] [Google Scholar]
- 54.Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466(7310):1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439(7074):283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 56.Tay YM, Tam WL, Ang YS, Gaughwin PM, Yang H, Wang W, Liu R, George J, Ng HH, Perera RJ, Lufkin T, Rigoutsos I, Thomson AM, Lim B. MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem Cells. 2008;26(1):17–29. doi: 10.1634/stemcells.2007-0295. [DOI] [PubMed] [Google Scholar]
- 57.Mellios N, Huang HS, Grigorenko A, Rogaev E, Akbarian S. A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Hum Mol Genet. 2008;17(19):3030–3042. doi: 10.1093/hmg/ddn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Zhang J, Liu LH, Zhou Y, Li YP, Shao ZH, Wu YJ, Li MJ, Fan YY, Shi HJ. Effects of miR-541 on neurite outgrowth during neuronal differentiation. Cell Biochem Funct. 2011;29(4):279–286. doi: 10.1002/cbf.1747. [DOI] [PubMed] [Google Scholar]
- 59.Fiore R, Khudayberdiev S, Christensen M, Siegel G, Flavell SW, Kim TK, Greenberg ME, Schratt G. Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 2009;28(6):697–710. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simion A, Laudadio I, Prevot PP, Raynaud P, Lemaigre FP, Jacquemin P. MiR-495 and miR-218 regulate the expression of the Onecut transcription factors HNF-6 and OC-2. Biochem Biophys Res Commun. 2010;391(1):293–298. doi: 10.1016/j.bbrc.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 61.Tominaga K, Srikantan S, Lee EK, Subaran SS, Martindale JL, Abdelmohsen K, Gorospe M. Competitive regulation of nucleolin expression by HuR and miR-494. Mol Cell Biol. 2011;31(20):4219–4231. doi: 10.1128/MCB.05955-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams AE, Moschos SA, Perry MM, Barnes PJ, Lindsay MA. Maternally imprinted microRNAs are differentially expressed during mouse and human lung development. Dev Dyn Off Publ Am Assoc Anat. 2007;236(2):572–580. doi: 10.1002/dvdy.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choong ML, Yang HH, McNiece I. MicroRNA expression profiling during human cord blood-derived CD34 cell erythropoiesis. Exp Hematol. 2007;35(4):551–564. doi: 10.1016/j.exphem.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Wang F, Yu J, Yang GH, Wang XS, Zhang JW. Regulation of erythroid differentiation by miR-376a and its targets. Cell Res. 2011;21(8):1196–1209. doi: 10.1038/cr.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beckman JD, Chen C, Nguyen J, Thayanithy V, Subramanian S, Steer CJ, Vercellotti GM. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J Biol Chem. 2011;286(5):3194–3202. doi: 10.1074/jbc.M110.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teferedegne B, Murata H, Quinones M, Peden K, Lewis AM. Patterns of microRNA expression in non-human primate cells correlate with neoplastic development in vitro. PLoS One. 2010;5(12):e14416. doi: 10.1371/journal.pone.0014416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chien WW, Domenech C, Catallo R, Kaddar T, Magaud JP, Salles G, Ffrench M. Cyclin-dependent kinase 1 expression is inhibited by p16(INK4a) at the post-transcriptional level through the microRNA pathway. Oncogene. 2011;30(16):1880–1891. doi: 10.1038/onc.2010.570. [DOI] [PubMed] [Google Scholar]
- 68.Lee YN, Brandal S, Noel P, Wentzel E, Mendell JT, McDevitt MA, Kapur R, Carter M, Metcalfe DD, Takemoto CM. KIT signaling regulates MITF expression through miRNAs in normal and malignant mast cell proliferation. Blood. 2011;117(13):3629–3640. doi: 10.1182/blood-2010-07-293548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen CF, He X, Arslan AD, Mo YY, Reinhold WC, Pommier Y, Beck WT. Novel regulation of nuclear factor-YB by miR-485-3p affects the expression of DNA topoisomerase IIalpha and drug responsiveness. Mol Pharmacol. 2011;79(4):735–741. doi: 10.1124/mol.110.069633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meng F, Wehbe-Janek H, Henson R, Smith H, Patel T. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene. 2008;27(3):378–386. doi: 10.1038/sj.onc.1210648. [DOI] [PubMed] [Google Scholar]
- 71.Olaru AV, Ghiaur G, Yamanaka S, Luvsanjav D, An F, Popescu I, Alexandrescu S, Allen S, Pawlik TM, Torbenson M, Georgiades C, Roberts LR, Gores GJ, Ferguson-Smith A, Almeida MI, Calin GA, Mezey E, Selaru FM. MicroRNA down-regulated in human cholangiocarcinoma control cell cycle through multiple targets involved in the G1/S checkpoint. Hepatology. 2011;54(6):2089–2098. doi: 10.1002/hep.24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Z, Lu J, Sun M, Mi S, Zhang H, Luo RT, Chen P, Wang Y, Yan M, Qian Z, Neilly MB, Jin J, Zhang Y, Bohlander SK, Zhang DE, Larson RA, Le Beau MM, Thirman MJ, Golub TR, Rowley JD, Chen J. Distinct microRNA expression profiles in acute myeloid leukemia with common translocations. Proc Natl Acad Sci USA. 2008;105(40):15535–15540. doi: 10.1073/pnas.0808266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dixon-McIver A, East P, Mein CA, Cazier JB, Molloy G, Chaplin T, Andrew Lister T, Young BD, Debernardi S. Distinctive patterns of microRNA expression associated with karyotype in acute myeloid leukaemia. PLoS One. 2008;3(5):e2141. doi: 10.1371/journal.pone.0002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang C, Wang C, Chen X, Yang C, Li K, Wang J, Dai J, Hu Z, Zhou X, Chen L, Zhang Y, Li Y, Qiu H, Xing J, Liang Z, Ren B, Yang C, Zen K, Zhang CY. Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin Chem. 2010;56(12):1871–1879. doi: 10.1373/clinchem.2010.147553. [DOI] [PubMed] [Google Scholar]
- 75.Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, Yoshida K, Sasaki H, Nomura S, Seto Y, Kaminishi M, Calin GA, Croce CM. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11(2):136–146. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haller F, von Heydebreck A, Zhang JD, Gunawan B, Langer C, Ramadori G, Wiemann S, Sahin O. Localization- and mutation-dependent microRNA (miRNA) expression signatures in gastrointestinal stromal tumours (GISTs), with a cluster of co-expressed miRNAs located at 14q32.31. J Pathol. 2010;220(1):71–86. doi: 10.1002/path.2610. [DOI] [PubMed] [Google Scholar]
- 77.Bandres E, Cubedo E, Agirre X, Malumbres R, Zarate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzo M, Garcia-Foncillas J. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol Cancer. 2006;5:29. doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer J Int du Cancer. 2010;127(1):118–126. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 79.Mosakhani N, Sarhadi VK, Borze I, Karjalainen-Lindsberg ML, Sundstrom J, Ristamaki R, Osterlund P, Knuutila S. MicroRNA profiling differentiates colorectal cancer according to KRAS status. Genes Chromosom Cancer. 2012;51(1):1–9. doi: 10.1002/gcc.20925. [DOI] [PubMed] [Google Scholar]
- 80.Luk JM, Burchard J, Zhang C, Liu AM, Wong KF, Shek FH, Lee NP, Fan ST, Poon RT, Ivanovska I, Philippar U, Cleary MA, Buser CA, Shaw PM, Lee CN, Tenen DG, Dai H, Mao M. DLK1-DIO3 genomic imprinted microRNA cluster at 14q32.2 defines a stemlike subtype of hepatocellular carcinoma associated with poor survival. J Biol Chem. 2011;286(35):30706–30713. doi: 10.1074/jbc.M111.229831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tryndyak VP, Ross SA, Beland FA, Pogribny IP. Down-regulation of the microRNAs miR-34a, miR-127, and miR-200b in rat liver during hepatocarcinogenesis induced by a methyl-deficient diet. Mol Carcinog. 2009;48(6):479–487. doi: 10.1002/mc.20484. [DOI] [PubMed] [Google Scholar]
- 82.Shih KK, Qin LX, Tanner EJ, Zhou Q, Bisogna M, Dao F, Olvera N, Viale A, Barakat RR, Levine DA. A microRNA survival signature (MiSS) for advanced ovarian cancer. Gynecol Oncol. 2011;121(3):444–450. doi: 10.1016/j.ygyno.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 83.Ye G, Fu G, Cui S, Zhao S, Bernaudo S, Bai Y, Ding Y, Zhang Y, Yang BB, Peng C. MicroRNA 376c enhances ovarian cancer cell survival by targeting activin receptor-like kinase 7: implications for chemoresistance. J Cell Sci. 2011;124(Pt 3):359–368. doi: 10.1242/jcs.072223. [DOI] [PubMed] [Google Scholar]
- 84.Castilla MA, Moreno-Bueno G, Romero-Perez L, Van De Vijver K, Biscuola M, Lopez-Garcia MA, Prat J, Matias-Guiu X, Cano A, Oliva E, Palacios J. Micro-RNA signature of the epithelial-mesenchymal transition in endometrial carcinosarcoma. J Pathol. 2011;223(1):72–80. doi: 10.1002/path.2802. [DOI] [PubMed] [Google Scholar]
- 85.Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ, Hwang SY, Kim WY, Kim TJ, Lee JH, Kim BG, Bae DS. Altered microRNA expression in cervical carcinomas. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14(9):2535–2542. doi: 10.1158/1078-0432.CCR-07-1231. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N, Liu CG, Giannakakis A, Alexiou P, Hasegawa K, Johnstone CN, Megraw MS, Adams S, Lassus H, Huang J, Kaur S, Liang S, Sethupathy P, Leminen A, Simossis VA, Sandaltzopoulos R, Naomoto Y, Katsaros D, Gimotty PA, DeMichele A, Huang Q, Butzow R, Rustgi AK, Weber BL, Birrer MJ, Hatzigeorgiou AG, Croce CM, Coukos G. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci USA. 2008;105(19):7004–7009. doi: 10.1073/pnas.0801615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang LL, Zhang Z, Li Q, Yang R, Pei X, Xu Y, Wang J, Zhou SF, Li Y. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum Reprod. 2009;24(3):562–579. doi: 10.1093/humrep/den439. [DOI] [PubMed] [Google Scholar]
- 88.Tang H, Liu X, Wang Z, She X, Zeng X, Deng M, Liao Q, Guo X, Wang R, Li X, Zeng F, Wu M, Li G. Interaction of hsa-miR-381 and glioma suppressor LRRC4 is involved in glioma growth. Brain Res. 2011;1390:21–32. doi: 10.1016/j.brainres.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 89.Swarbrick A, Woods SL, Shaw A, Balakrishnan A, Phua Y, Nguyen A, Chanthery Y, Lim L, Ashton LJ, Judson RL, Huskey N, Blelloch R, Haber M, Norris MD, Lengyel P, Hackett CS, Preiss T, Chetcuti A, Sullivan CS, Marcusson EG, Weiss W, L’Etoile N, Goga A. miR-380-5p represses p53 to control cellular survival and is associated with poor outcome in MYCN-amplified neuroblastoma. Nat Med. 2010;16(10):1134–1140. doi: 10.1038/nm.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skalsky RL, Cullen BR. Reduced expression of brain-enriched microRNAs in glioblastomas permits targeted regulation of a cell death gene. PLoS One. 2011;6(9):e24248. doi: 10.1371/journal.pone.0024248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gattolliat CH, Thomas L, Ciafre SA, Meurice G, Le Teuff G, Job B, Richon C, Combaret V, Dessen P, Valteau-Couanet D, May E, Busson P, Douc-Rasy S, Benard J. Expression of miR-487b and miR-410 encoded by 14q32.31 locus is a prognostic marker in neuroblastoma. Br J Cancer. 2011;105(9):1352–1361. doi: 10.1038/bjc.2011.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheunsuchon P, Zhou Y, Zhang X, Lee H, Chen W, Nakayama Y, Rice KA, Tessa Hedley-Whyte E, Swearingen B, Klibanski A. Silencing of the imprinted DLK1-MEG3 locus in human clinically nonfunctioning pituitary adenomas. Am J Pathol. 2011;179(4):2120–2130. doi: 10.1016/j.ajpath.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu L, Jiang Y, Zhang H, Greenlee AR, Han Z. Overexpressed miR-494 down-regulates PTEN gene expression in cells transformed by anti-benzo(a)pyrene-trans-7,8-dihydrodiol-9,10-epoxide. Life Sci. 2010;86(5–6):192–198. doi: 10.1016/j.lfs.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Duan H, Jiang Y, Zhang H, Wu Y. MiR-320 and miR-494 affect cell cycles of primary murine bronchial epithelial cells exposed to benzo[a]pyrene. Toxicol In vitro Int J publ Assoc BIBRA. 2010;24(3):928–935. doi: 10.1016/j.tiv.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 95.Guo L, Liu Y, Bai Y, Sun Y, Xiao F, Guo Y. Gene expression profiling of drug-resistant small cell lung cancer cells by combining microRNA and cDNA expression analysis. Eur J Cancer. 2010;46(9):1692–1702. doi: 10.1016/j.ejca.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 96.Dacic S, Kelly L, Shuai Y, Nikiforova MN. miRNA expression profiling of lung adenocarcinomas: correlation with mutational status. Mod Pathol Off J USA Can Acad Pathol Inc. 2010;23(12):1577–1582. doi: 10.1038/modpathol.2010.152. [DOI] [PubMed] [Google Scholar]
- 97.Liu X, Sempere LF, Ouyang H, Memoli VA, Andrew AS, Luo Y, Demidenko E, Korc M, Shi W, Preis M, Dragnev KH, Li H, Direnzo J, Bak M, Freemantle SJ, Kauppinen S, Dmitrovsky E. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J Clin Investig. 2010;120(4):1298–1309. doi: 10.1172/JCI39566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guled M, Lahti L, Lindholm PM, Salmenkivi K, Bagwan I, Nicholson AG, Knuutila S. CDKN2A, NF2, and JUN are dysregulated among other genes by miRNAs in malignant mesothelioma-A miRNA microarray analysis. Genes Chromosom Cancer. 2009;48(7):615–623. doi: 10.1002/gcc.20669. [DOI] [PubMed] [Google Scholar]
- 99.Hwang-Verslues WW, Chang PH, Wei PC, Yang CY, Huang CK, Kuo WH, Shew JY, Chang KJ, Lee EY, Lee WH. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene. 2011;30(21):2463–2474. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- 100.Lowery AJ, Miller N, Devaney A, McNeill RE, Davoren PA, Lemetre C, Benes V, Schmidt S, Blake J, Ball G, Kerin MJ. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res BCR. 2009;11(3):R27. doi: 10.1186/bcr2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, Zeng YX, Shao JY. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14(11):2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bockmeyer CL, Christgen M, Muller M, Fischer S, Ahrens P, Langer F, Kreipe H, Lehmann U. MicroRNA profiles of healthy basal and luminal mammary epithelial cells are distinct and reflected in different breast cancer subtypes. Breast Cancer Res Treat. 2011;130(3):735–745. doi: 10.1007/s10549-010-1303-3. [DOI] [PubMed] [Google Scholar]
- 103.Kriegel AJ, Fang Y, Liu Y, Tian Z, Mladinov D, Matus IR, Ding X, Greene AS, Liang M. MicroRNA-target pairs in human renal epithelial cells treated with transforming growth factor beta 1: a novel role of miR-382. Nucleic Acids Res. 2010;38(22):8338–8347. doi: 10.1093/nar/gkq718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tombol Z, Eder K, Kovacs A, Szabo PM, Kulka J, Liko I, Zalatnai A, Racz G, Toth M, Patocs A, Falus A, Racz K, Igaz P. MicroRNA expression profiling in benign (sporadic and hereditary) and recurring adrenal pheochromocytomas. Mod Pathol Off J USA Canad Acad Pathol Inc. 2010;23(12):1583–1595. doi: 10.1038/modpathol.2010.164. [DOI] [PubMed] [Google Scholar]
- 105.Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, Jones PA. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9(6):435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 106.Nagano T, Fraser P. No nonsense functions for long noncoding RNAs. Cell. 2011;145(2):178–181. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 107.Bertani S, Sauer S, Bolotin E, Sauer F. The noncoding RNA mistral activates Hoxa6 and Hoxa7 expression and stem cell differentiation by recruiting MLL1 to chromatin. Mol Cell. 2011;43(6):1040–1046. doi: 10.1016/j.molcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 109.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 110.Robertus JL, Harms G, Blokzijl T, Booman M, de Jong D, van Imhoff G, Rosati S, Schuuring E, Kluin P, van den Berg A. Specific expression of miR-17-5p and miR-127 in testicular and central nervous system diffuse large B-cell lymphoma. Mod Pathol Off J US Can Acad Pathol Inc. 2009;22(4):547–555. doi: 10.1038/modpathol.2009.10. [DOI] [PubMed] [Google Scholar]
- 111.Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS, Rameshwar P. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71(5):1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 112.Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol. 2009;112(1):55–59. doi: 10.1016/j.ygyno.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 113.Lages E, Guttin A, El Atifi M, Ramus C, Ipas H, Dupre I, Rolland D, Salon C, Godfraind C, deFraipont F, Dhobb M, Pelletier L, Wion D, Gay E, Berger F, Issartel JP. MicroRNA and target protein patterns reveal physiopathological features of glioma subtypes. PLoS One. 2011;6(5):e20600. doi: 10.1371/journal.pone.0020600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cahill S, Smyth P, Denning K, Flavin R, Li J, Potratz A, Guenther SM, Henfrey R, O’Leary JJ, Sheils O. Effect of BRAFV600E mutation on transcription and post-transcriptional regulation in a papillary thyroid carcinoma model. Mol Cancer. 2007;6:21. doi: 10.1186/1476-4598-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duan Z, Choy E, Harmon D, Liu X, Susa M, Mankin H, Hornicek F. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol Cancer Ther. 2011;10(8):1337–1345. doi: 10.1158/1535-7163.MCT-11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Genovesi LA, Carter KW, Gottardo NG, Giles KM, Dallas PB. Integrated analysis of miRNA and mRNA expression in childhood medulloblastoma compared with neural stem cells. PLoS One. 2011;6(9):e23935. doi: 10.1371/journal.pone.0023935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cipollone F, Felicioni L, Sarzani R, Ucchino S, Spigonardo F, Mandolini C, Malatesta S, Bucci M, Mammarella C, Santovito D, de Lutiis F, Marchetti A, Mezzetti A, Buttitta F. A unique microRNA signature associated with plaque instability in humans. Stroke J Cereb Circ. 2011;42(9):2556–2563. doi: 10.1161/STROKEAHA.110.597575. [DOI] [PubMed] [Google Scholar]
- 118.Sylvius N, Bonne G, Straatman K, Reddy T, Gant TW, Shackleton S. MicroRNA expression profiling in patients with lamin A/C-associated muscular dystrophy. FASEB J Off Publ Fed Am Soc Exp Biol. 2011;25(11):3966–3978. doi: 10.1096/fj.11-182915. [DOI] [PubMed] [Google Scholar]
- 119.Tsai KW, Wu CW, Hu LY, Li SC, Liao YL, Lai CH, Kao HW, Fang WL, Huang KH, Chan WC, Lin WC. Epigenetic regulation of miR-34b and miR-129 expression in gastric cancer. Int J Cancer J Int du Cancer. 2011;129(11):2600–2610. doi: 10.1002/ijc.25919. [DOI] [PubMed] [Google Scholar]
- 120.Hamano R, Miyata H, Yamasaki M, Kurokawa Y, Hara J, Moon JH, Nakajima K, Takiguchi S, Fujiwara Y, Mori M, Doki Y. Overexpression of miR-200c induces chemoresistance in esophageal cancers mediated through activation of the Akt signaling pathway. Clin Cancer Res Off J Am Assoc Cancer Res. 2011;17(9):3029–3038. doi: 10.1158/1078-0432.CCR-10-2532. [DOI] [PubMed] [Google Scholar]
- 121.Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y. Comprehensive analysis of microRNA expression patterns in renal biopsies of lupus nephritis patients. Rheumatol Int. 2009;29(7):749–754. doi: 10.1007/s00296-008-0758-6. [DOI] [PubMed] [Google Scholar]
- 122.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116(3):258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 123.Haenisch S, Laechelt S, Bruckmueller H, Werk A, Noack A, Bruhn O, Remmler C, Cascorbi I. Down-regulation of ATP-binding cassette C2 protein expression in HepG2 cells after rifampicin treatment is mediated by microRNA-379. Mol Pharmacol. 2011;80(2):314–320. doi: 10.1124/mol.110.070714. [DOI] [PubMed] [Google Scholar]
- 124.Xiao J, Jing ZC, Ellinor PT, Liang D, Zhang H, Liu Y, Chen X, Pan L, Lyon R, Liu Y, Peng LY, Liang X, Sun Y, Popescu LM, Condorelli G, Chen YH. MicroRNA-134 as a potential plasma biomarker for the diagnosis of acute pulmonary embolism. J Transl Med. 2011;9:159. doi: 10.1186/1479-5876-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim TH, Kim YK, Kwon Y, Heo JH, Kang H, Kim G, An HJ. Deregulation of miR-519a, 153, and 485–5p and its clinicopathological relevance in ovarian epithelial tumours. Histopathology. 2010;57(5):734–743. doi: 10.1111/j.1365-2559.2010.03686.x. [DOI] [PubMed] [Google Scholar]
- 126.Eisenberg I, Eran A, Nishino I, Moggio M, Lamperti C, Amato AA, Lidov HG, Kang PB, North KN, Mitrani-Rosenbaum S, Flanigan KM, Neely LA, Whitney D, Beggs AH, Kohane IS, Kunkel LM. Distinctive patterns of microRNA expression in primary muscular disorders. Proc Natl Acad Sci USA. 2007;104(43):17016–17021. doi: 10.1073/pnas.0708115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hoekstra M, van der Lans CA, Halvorsen B, Gullestad L, Kuiper J, Aukrust P, van Berkel TJ, Biessen EA. The peripheral blood mononuclear cell microRNA signature of coronary artery disease. Biochem Biophys Res Commun. 2010;394(3):792–797. doi: 10.1016/j.bbrc.2010.03.075. [DOI] [PubMed] [Google Scholar]
- 128.Lehmann U, Streichert T, Otto B, Albat C, Hasemeier B, Christgen H, Schipper E, Hille U, Kreipe HH, Langer F. Identification of differentially expressed microRNAs in human male breast cancer. BMC Cancer. 2010;10:109. doi: 10.1186/1471-2407-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang X, Cairns M, Rose B, O’Brien C, Shannon K, Clark J, Gamble J, Tran N. Alterations in miRNA processing and expression in pleomorphic adenomas of the salivary gland. Int J Cancer J Int du Cancer. 2009;124(12):2855–2863. doi: 10.1002/ijc.24298. [DOI] [PubMed] [Google Scholar]
- 130.Abu-Elneel K, Liu T, Gazzaniga FS, Nishimura Y, Wall DP, Geschwind DH, Lao K, Kosik KS. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics. 2008;9(3):153–161. doi: 10.1007/s10048-008-0133-5. [DOI] [PubMed] [Google Scholar]
- 131.Bimpaki EI, Iliopoulos D, Moraitis A, Stratakis CA. MicroRNA signature in massive macronodular adrenocortical disease and implications for adrenocortical tumourigenesis. Clin Endocrinol. 2010;72(6):744–751. doi: 10.1111/j.1365-2265.2009.03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Redell JB, Liu Y, Dash PK. Traumatic brain injury alters expression of hippocampal microRNAs: potential regulators of multiple pathophysiological processes. J Neurosci Res. 2009;87(6):1435–1448. doi: 10.1002/jnr.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hussein K, von Neuhoff N, Busche G, Buhr T, Kreipe H, Bock O. Opposite expression pattern of Src kinase Lyn in acute and chronic haematological malignancies. Ann Hematol. 2009;88(11):1059–1067. doi: 10.1007/s00277-009-0727-5. [DOI] [PubMed] [Google Scholar]
- 134.Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni L, Zhang WG, Nan KJ, Song TS, Huang C. MicroRNA profiling of human gastric cancer. Mol Med Rep. 2009;2(6):963–970. doi: 10.3892/mmr_00000199. [DOI] [PubMed] [Google Scholar]
- 135.Saba R, Goodman CD, Huzarewich RL, Robertson C, Booth SA. A miRNA signature of prion induced neurodegeneration. PLoS One. 2008;3(11):e3652. doi: 10.1371/journal.pone.0003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luo H, Zhang H, Zhang Z, Zhang X, Ning B, Guo J, Nie N, Liu B, Wu X. Down-regulated miR-9 and miR-433 in human gastric carcinoma. J Exp Clin Cancer Res CR. 2009;28:82. doi: 10.1186/1756-9966-28-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ, Schmittgen TD. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer J Int du Cancer. 2007;120(5):1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kumar S, Kumar A, Shah PP, Rai SN, Panguluri SK, Kakar SS. MicroRNA signature of cis-platin resistant vs. cis-platin sensitive ovarian cancer cell lines. J Ovarian Res. 2011;4(1):17. doi: 10.1186/1757-2215-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iliopoulos D, Bimpaki EI, Nesterova M, Stratakis CA. MicroRNA signature of primary pigmented nodular adrenocortical disease: clinical correlations and regulation of Wnt signaling. Cancer Res. 2009;69(8):3278–3282. doi: 10.1158/0008-5472.CAN-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao W, Shen H, Liu L, Xu J, Xu J, Shu Y. MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol. 2011;137(4):557–566. doi: 10.1007/s00432-010-0918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Q, Wang Y, Minto AW, Wang J, Shi Q, Li X, Quigg RJ. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J Off Publ Fed Am Soc Exp Biol. 2008;22(12):4126–4135. doi: 10.1096/fj.08-112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol. 2009;200(6):661–667. doi: 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 143.Yu J, Wang F, Yang GH, Wang FL, Ma YN, Du ZW, Zhang JW. Human microRNA clusters: genomic organization and expression profile in leukemia cell lines. Biochem Biophys Res Commun. 2006;349(1):59–68. doi: 10.1016/j.bbrc.2006.07.207. [DOI] [PubMed] [Google Scholar]
- 144.Wang X, Ye L, Zhou Y, Liu MQ, Zhou DJ, Ho WZ. Inhibition of anti-HIV microRNA expression: a mechanism for opioid-mediated enhancement of HIV infection of monocytes. Am J Pathol. 2011;178(1):41–47. doi: 10.1016/j.ajpath.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP, Wei WI. Mature miR-184 as Potential Oncogenic microRNA of Squamous Cell Carcinoma of Tongue. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14(9):2588–2592. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 146.Goodarzi HR, Abbasi A, Saffari M, Tabei MB, Noori Daloii MR. MicroRNAs take part in pathophysiology and pathogenesis of male pattern baldness. Mol Biol Rep. 2010;37(6):2959–2965. doi: 10.1007/s11033-009-9862-2. [DOI] [PubMed] [Google Scholar]
- 147.Hummel R, Wang T, Watson DI, Michael MZ, Van der Hoek M, Haier J, Hussey DJ. Chemotherapy-induced modification of microRNA expression in esophageal cancer. Oncol Rep. 2011;26(4):1011–1017. doi: 10.3892/or.2011.1381. [DOI] [PubMed] [Google Scholar]
- 148.Lai CY, Yu SL, Hsieh MH, Chen CH, Chen HY, Wen CC, Huang YH, Hsiao PC, Hsiao CK, Liu CM, Yang PC, Hwu HG, Chen WJ. MicroRNA expression aberration as potential peripheral blood biomarkers for schizophrenia. PLoS One. 2011;6(6):e21635. doi: 10.1371/journal.pone.0021635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Qi Y, Tu J, Cui L, Guo X, Shi Z, Li S, Shi W, Shan Y, Ge Y, Shan J, Wang H, Lu Z. High-throughput sequencing of microRNAs in adenovirus type 3 infected human laryngeal epithelial cells. J Biomed Biotechnol. 2010;2010:915980. doi: 10.1155/2010/915980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Simon D, Laloo B, Barillot M, Barnetche T, Blanchard C, Rooryck C, Marche M, Burgelin I, Coupry I, Chassaing N, Gilbert-Dussardier B, Lacombe D, Grosset C, Arveiler B. A mutation in the 3′-UTR of the HDAC6 gene abolishing the post-transcriptional regulation mediated by hsa-miR-433 is linked to a new form of dominant X-linked chondrodysplasia. Hum Mol Genet. 2010;19(10):2015–2027. doi: 10.1093/hmg/ddq083. [DOI] [PubMed] [Google Scholar]
- 151.Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25(5):821–832. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Melkamu T, Zhang X, Tan J, Zeng Y, Kassie F. Alteration of microRNA expression in vinyl carbamate-induced mouse lung tumors and modulation by the chemopreventive agent indole-3-carbinol. Carcinogenesis. 2010;31(2):252–258. doi: 10.1093/carcin/bgp208. [DOI] [PubMed] [Google Scholar]
- 153.Sander S, Bullinger L, Klapproth K, Fiedler K, Kestler HA, Barth TF, Moller P, Stilgenbauer S, Pollack JR, Wirth T. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112(10):4202–4212. doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- 154.Roccaro AM, Sacco A, Chen C, Runnels J, Leleu X, Azab F, Azab AK, Jia X, Ngo HT, Melhem MR, Burwick N, Varticovski L, Novina CD, Rollins BJ, Anderson KC, Ghobrial IM. MicroRNA expression in the biology, prognosis, and therapy of Waldenstrom macroglobulinemia. Blood. 2009;113(18):4391–4402. doi: 10.1182/blood-2008-09-178228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Montag J, Hitt R, Opitz L, Schulz-Schaeffer WJ, Hunsmann G, Motzkus D. Upregulation of miRNA hsa-miR-342-3p in experimental and idiopathic prion disease. Mol Neurodegener. 2009;4:36. doi: 10.1186/1750-1326-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li X, Luo F, Li Q, Xu M, Feng D, Zhang G, Wu W. Identification of new aberrantly expressed miRNAs in intestinal-type gastric cancer and its clinical significance. Oncol Rep. 2011;26(6):1431–1439. doi: 10.3892/or.2011.1437. [DOI] [PubMed] [Google Scholar]


