Abstract
BCL2-associated athanogene 6 (BAG-6) (also Bat-3/Scythe) was discovered as a gene product of the major histocompatibility complex class III locus. The Xenopus ortholog Scythe was first identified to act as an anti-apoptotic protein. Subsequent studies unraveled that the large BAG-6 protein contributes to a number of cellular processes, including apoptosis, gene regulation, protein synthesis, protein quality control, and protein degradation. In this context, BAG-6 acts as a multifunctional chaperone, which interacts with its target proteins for shuttling to distinct destinations. Nonetheless, as anticipated from its genomic localization, BAG-6 is involved in a variety of immunological pathways such as macrophage function and TH1 response. Most recently, BAG-6 was identified on the plasma membrane of dendritic cells and malignantly transformed cells where it serves as cellular ligand for the activating natural killer (NK) cell receptor NKp30 triggering NK cell cytotoxicity. Moreover, target cells were found to secrete soluble variants of BAG-6 and release BAG-6 on the surface of exosomes, which inhibit or activate NK cell cytotoxicity, respectively. These data suggest that the BAG-6 antigen is an important target to shape a directed immune response or to overcome tumor-immune escape strategies established by soluble BAG-6. This review summarizes the currently known functions of BAG-6, a fascinating multicompetent protein, in health and disease.
Keywords: Bat-3, Scythe, NKp30 ligand, Natural killer cell, Natural cytotoxicity receptor, Immune regulation, Protein quality control
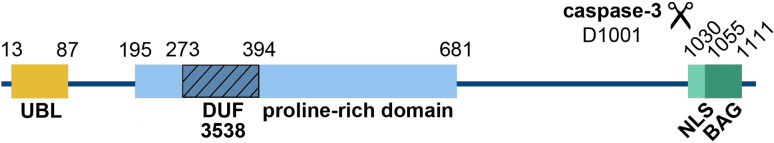
Originally, the BCL2-associated athanogene 6 (BAG-6) was identified as a gene located in a cluster of immune-relevant genes of the human major histocompatibility complex (MHC) III region on chromosome 6 [1], which encodes a ubiquitin-like protein leading to two major isoforms of 1,132 amino acids (isoform 1, P46379-1) and 1,126 amino acids (isoform 2, P46379-2) generated by alternative splicing. The functional significance of the two isoforms remains elusive. The high-resolution 3D structure of BAG-6 is unknown except for an N-terminal ubiquitin-like domain (UBL; PDB IDs: 4EEW and 4DWF). Additionally, a domain of unknown function (DUF3538, amino acids 273–394), a long proline-rich stretch (amino acids 195–681), which might be involved in intra- and intermolecular protein–protein interactions, and a C-terminal BAG domain were identified (Fig. 1). Notably, no transmembrane domain was predicted. BAG-6 is a multifunctional protein that fulfills important functions in a variety of cellular pathways in health and disease. An overview of these protein interaction networks and the related functions of BAG-6 is provided in Fig. 2; the molecular details of BAG-6 action are summarized in the following chapters.
Fig. 1.
Domain organization of human BAG-6. The domain borders are given by numbers corresponding to the amino acid positions. The caspase-3 cleavage site at position 1001 is indicated. Numbering refers to isoform 1 of BAG-6 (P46379-1). UBL ubiquitin-like domain, DUF domain of unknown function, NLS nuclear localization signal, BAG BCL2-associated athanogene domain
Fig. 2.
The functional BAG-6 interactome. BAG-6 is a multifunctional protein involved in a variety of non-related cellular pathways in health and disease. Consequently, BAG-6 has a diverse cellular localization. In the nucleus, BAG-6 associates with the nucleoprotein p300 in response to DNA damage, facilitating subsequent acetylation of p53 and DNA repair. Moreover, BAG-6 facilitates targeting of BRCA1 to sites of DNA damage for repair. In complex with the histone methyltransferases SET1A or DOT1L, BAG-6 is involved in regulation of gene expression. BAG-6 chaperones the cytosolic class II transactivator CIITA to the nucleus for regulation of gene expression of proteins of the HLA class II processing pathway. Phosphorylation of BAG-6 by ATM/ATR is a prerequisite for DNA damage-induced apoptosis. BAG-6 is retained in the cytosol by shielding of the nuclear localization signal by a complex which contains TRC35. In the cytosol, BAG-6 is cleaved by caspase-3 after induction of intrinsic or extrinsic apoptosis generating a C-terminal fragment of BAG-6, which triggers apoptosis. BAG-6 is part of a complex together with TRC35, TRC40, and Ubl4a, which shields the C-terminal transmembrane domain of tail anchored proteins until post-translational insertion into the ER membrane. BAG-6 associates with protein substrates dedicated to degradation and docks them to the 26S-proteasome subunit Rpn10c. Consequently, BAG-6 drives MHC class I antigen presentation and regulates the supply of antigenic peptides. Tumor cells and dendritic cells release BAG-6 on exosomes and/or as a soluble protein. Exosomal BAG-6 activates NK cells, whereas soluble BAG-6 inhibits NK cell cytotoxicity upon ligation to the activating NK cell receptor NKp30. Furthermore, BAG-6 is found on the plasma membrane of malignantly transformed cells and dendritic cells and triggers killing of the BAG-6 presenting cell. The way how BAG-6 is attached to the exosomal or plasma membrane remains obscure
BAG-6 in DNA damage response and gene regulation
In early studies, Scythe, the Xenopus laevis ortholog of BAG-6, was shown to sequester pro-apoptotic factors [2]. By contrast, BAG-6 binding to the Drosophila melanogaster protein Reaper leads to the release of these pro-apoptotic factors resulting in cytochrome c release, caspase-activation, and nuclear fragmentation [2, 3]. In addition to Reaper, BAG-6 interacts with the apoptotic proteins Hid and Grim of D. melanogaster [3]. Recombinant BAG-6 comprising only the last 312 C-terminal amino acids is unable to sequester pro-apoptotic factors [2]. Furthermore, BAG-6 counteracts apoptosis by binding via its 436 N-terminal residues to the Rpn10c subunit of the 26S-proteasome promoting proteasomal degradation of pro-apoptotic factors such as X. laevis inducer of apoptosis XEF1AO [4, 5]. Moreover, deletion of the XEF1AO binding site on BAG-6 (ΔN1-436) leads to caspase-3/7 activation.
Despite the anti-apoptotic regulator function under steady-state conditions, BAG-6 can induce apoptosis in case of cellular stress. Initially, the treatment of cells with the poison ricin, a ribosome inactivating protein, leads to BAG-6 cleavage behind the canonical cleavage site DEQD1001 by caspase-3, resulting in a C-terminal fragment comprising the last 131 amino acids, which triggers apoptosis [6]. Cleavage by caspase-3 occurs both after extrinsic (i.e. Fas/APO-1-mediated) and intrinsic (i.e. staurosporine-induced) activation of the apoptotic process [7]. In addition, it was shown that cytosolic BAG-6 interacts with apoptosis-inducing factor (AIF), regulating its stability and location after endoplasmic reticulum (ER) stress mediated apoptosis stimulation [8].
Based on genetic ablation of BAG-6 in 129SvJ × C57BL/6-derived mice, Desmots et al. [9] observed neonatal lethality associated with pronounced developmental defects due to apoptosis and aberrant cell proliferation. In contrast to that, Sasaki et al. [10] reported a rescue of the lethal phenotype of BAG-6 knock-down in mice with imprinting control region (ICR) genetic background.
BAG-6 possesses a nuclear localization signal (NLS) indicating a nuclear localization [11]. Indeed, BAG-6 forms a complex with the nucleoprotein p300 in response to DNA damage facilitating subsequent p53 acetylation [10]. Activated p53 induces the expression of p21, which stops the cell division by complexing CDK2 for DNA repair, and puma, which promote p53-dependent DNA damage-induced apoptosis. In consequence, thymocytes of BAG-6-deficient ICR-mice exhibit reduced expression of puma and p21, and show impaired apoptosis after γ-irradiation [10]. Interestingly, primary neuronal cells from 129SvJ × C57BL/6-derived BAG-6 knockout mice [9] were resistant to apoptosis as well.
The cellular localization of BAG-6 is diverse. Early studies showed that BAG-6 remains in the nucleus during staurosporine-induced apoptosis [11], whereas others have shown that re-localization of BAG-6 to the cytosol is required for apoptosis after different stimuli [6–8, 12].
Recently, Krenciute et al. [13] reported that nuclear localization of BAG-6 and phosphorylation of BAG-6 by ATM/ATR are required for induction of apoptosis following treatment with ionizing radiation or DNA-damaging agents. Furthermore, upon DNA damage, BAG-6/BRCA1 complexes translocate to sites of the damage, mediating DNA damage response signaling and homologous recombination-mediated repair. The formation of these BRCA1 foci is strongly dependent on BAG-6.
BAG-6 regulates gene expression by interaction with the two histone methyltransferases SET1A and DOT1L, which dimethylate histone H3K4 and H3K79, respectively [14, 15]. Interestingly, BAG-6 is able to induce expression of the DNA repair-promoting protein 53BP1 [15] and of its interaction partner BRCA1 providing a feedback loop to boost DNA repair [13–15].
Notably, the cellular localization of BAG-6 can be regulated by cell type-specific alternative RNA splicing [16]. Furthermore, masking of the NLS by interacting proteins such as TRC35 might lead to a preferential cytosolic localization of BAG-6 [17].
Recent reports show that BAG-6 can be targeted to the plasma membrane and to exosomes, which are released in response to cellular stress [18, 19]. Although these pathways play an important role in immunosurveillance of tumor cells and shaping of immune responses [20], the molecular details of BAG-6 re-routing are poorly understood.
BAG-6 in protein targeting and quality control
Recently, it has been demonstrated that BAG-6 plays a critical role in regulating a number of cellular processes, such as facilitating protein targeting, protein quality control, and protein degradation. The N-terminal UBL domain of BAG-6 suggests an involvement in protein degradation and the C-terminal BAG domain suggests the ability to interact with HSP70 [21]. Indeed, BAG-6 is required for the accumulation of HSP70 upon heat shock and, once accumulated, HSP70 leads to the degradation of BAG-6 through the ubiquitin–proteasome system [22]. These reciprocal influences suggest that BAG-6 is a central regulator of the cellular content of HSP70. Furthermore, BAG-6 has been shown to regulate the stability of HSP2A in the context of spermatogenesis [23].
Beside BAG-6, the BAG-family includes BAG-1 (four isoforms), BAG-2, BAG-3 (Bis, CAIR-1) and BAG-4 (SODD) containing a single common C-terminal BAG domain and BAG-5 which has four BAG repeats [24, 25]. By binding several other proteins via N-terminal domains, all of the BAG-family proteins function as adapter proteins forming complexes with signaling molecules and molecular chaperones. BAG-family proteins participate in a variety of cellular processes such as cell stress response, proliferation, migration [26], and apoptosis [27], while anti-apoptotic activities are strongly associated with carcinogenesis [24, 25, 28, 29].
In addition to its function as a co-chaperone, BAG-6 regulates tail-anchored (TA) protein biogenesis. In contrast to signal-recognition particle-mediated co-translational membrane insertion of the majority of membrane proteins, TA proteins are inserted post-translationally into the ER membrane by a single C-terminal transmembrane domain (TMD) [30]. During delivery through the cytosol, the hydrophobic TMDs are shielded by chaperones (TRC40 in mammals [31] or Get3 in yeast [32]) in order to prevent protein aggregation. The TA protein insertion pathway has been studied extensively in yeast. In a first step, the pre-targeting factor Sgt2, which assembles Get3, Get4, and Get5 to form the TMD recognition complex [33], and TA proteins are transferred to the homodimeric ATPase Get3 [30, 32, 34]. After transfer to the ER membrane, TA proteins are released ATP-dependently into the ER membrane via Get1 and Get2 [34]. Recently, BAG-6 was identified as a central TMD-specific chaperone acting in a complex with TRC40, TRC35, and Ubl4a, the mammalian homologues of Get3, Get4, and Get5 [35, 36], respectively. This BAG-6 complex is recruited to ribosomes synthesizing TA proteins and shields the released hydrophobic TMDs from the aqueous cytosol and transfers them to TRC40 for ER membrane targeting. Another component of the BAG-6 complex is SGTA, the homologue of yeast sgt2, which is recruited by Ubl4a [37, 38]. Notably, the chaperone system protecting nascent TA proteins from aggregation, inappropriate interactions, or sequestration by other chaperones for ER membrane integration is highly conserved.
BAG-6 in degradation of defective proteins
Unmistakably, there is a link between BAG-6 and protein quality control due to the fact that BAG-6 has an UBL domain, implicating a role in protein turnover by the ubiquitin–proteasome system. As mentioned before, BAG-6 interacts with the 26S-proteasome component Rpn10c mediating the degradation of pro-apoptotic proteins [5]. Previous studies have shown that BAG-6 binds to the proteasome substrate EGFP-CL1 and promotes its degradation. Consequently, the knockdown of BAG-6 led to the accumulation of EGFP-CL1 [39, 40]. Interestingly, also newly synthesized defective ribosomal products (DRiPs) were associated with BAG-6, suggesting that BAG-6 provides a transient platform for the delivery of substrates to the 26S-proteasome [41, 42]. DRiPs represent the major source of peptides for MHC class I antigen presentation [43]. Therefore, it is not surprising that the knockdown of BAG-6 led to limited supply of antigenic peptides and thus to reduced MHC class I antigen presentation [39, 43].
Misfolded and mislocalized proteins must be rapidly degraded to avoid aggregation and accumulation disturbing cell homeostasis. In this context, substrates of both, the co-translational and post-translational targeting pathways are captured by the BAG-6 complex and are either transferred for membrane insertion or targeted for proteasomal degradation following ubiquitination by a BAG-6-associated ubiquitin ligase [44].
An important role of the ubiquitination system is the regulation of the proteasome-dependent turnover of misfolded proteins from the ER by the ER-associated degradation (ERAD) [45]. Wang et al. [17] showed that ERAD employs the translocation-driving ATPase p97 to retrotranslocate misfolded proteins out of the ER and a ubiquitin E3 ligase gp78-associated multiprotein complex comprising BAG-6, Ubl4A, and TRC35, which chaperones retrotranslocated polypeptides for proteasomal degradation. The importance of this BAG-6 multiprotein complex “holdase” activity is underscored by the observation that ERAD substrates aggregate upon BAG-6 depletion. BAG-6 was also found to interact specifically with misfolded ER glycoproteins that have undergone retrotranslocation [46]. As an example, misfolded dislocated forms of the T cell receptor alpha (TCRα) chain are translocated to the proteasome by BAG-6, while exposed hydrophobic domains of the TCRα chain are shielded [46].
Taken together, these studies identify the BAG-6 complex as multifaceted chaperone machinery, acting at the interface of protein synthesis and degradation.
BAG-6 in disease and its role in immunoregulation
BAG-6 has an influence on tissue development and the microenvironment. Kwak et al. [47] showed that BAG-6 interacts with TGF-β receptors type I and type II in renal mesangial cells. TGF-β1 plays essential roles in a wide panel of cellular processes, such as in development and the pathogenesis of tissue fibrosis, which is associated with progressive kidney diseases. TGF-β1 stimulation resulted in enhanced expression of type I collagen in mouse mesangial cells when full-length BAG-6 was expressed. Full-length BAG-6 and C-terminal truncation variants, which arise by alternative RNA splicing [16], are differentially expressed in embryonic and adult kidney tissue. Notably, the C-terminal truncation variant of BAG-6 could not enhance TGF-β1-induced type I collagen expression, as well as RNAi-mediated knock-down of BAG-6 protein suppressed the expression of type I collagen induced by TGF-β1.
Interestingly, BAG-6 is found to be ubiquitinated in the course of a Legionella pneumophila infection [48]. F-box-domain-containing proteins injected by L. pneumophila into the host have eukaryotic-like motifs associated with ubiquitination. These proteins assemble in a multisubunit E3 ubiquitin ligase complex together with BAG-6 promoting ubiquitination and subsequent proteasomal degradation of host proteins to drive the bacterial infection cycle [48].
There are also studies providing a link between BAG-6 and cancer. Wu et al. [12] demonstrated that the interaction of BAG-6 with YWK-II/APLP2, a novel G0-protein-coupled receptor for Mullerian inhibiting substance in cell survival, enhances the stability of YWK-II/APLP2 by reducing its ubiquitination and degradation. Strikingly, elevated levels of BAG-6 and YWK-II/APLP2 were found in human colorectal cancer.
Human genetic studies have shown that polymorphisms in the HLA gene complex between HLA-B and BAG-6 are associated with an increased incidence of lung cancer [49], type 1 diabetes [50], Parkinson’s disease [51], and some autoimmune disorders such as Kawasaki syndrome [52], myasthenia gravis [53], and rheumatoid arthritis [54]. Furthermore, bi-allelic inactivating mutations were found in the BAG-6 gene [55].
Recent studies demonstrated that BAG-6 plays a critical role in the regulation of innate and adaptive immunity. In this context, BAG-6 protects T helper type 1 (TH1) cells [56], regulates antigen presentation on antigen-presenting cells [57], serves as a secreted and membrane-bound antigen of stressed macrophages [58] and transformed cells to target these for immune detection by natural killer (NK) cells [18], and serves as a regulator of dendritic cell maturation [19].
Rescue of a T helper type 1 (TH1) cell exhausted-like phenotype by BAG-6
Recently, Rangachari et al. [56] reported that BAG-6 represses the function of the T cell immunoglobulin and mucin domain-containing molecule-3 (Tim-3), which is an inhibitory receptor expressed on exhausted T cells during HIV-1 and hepatitis C infection, upon binding to its intracellular tail. Overexpression of BAG-6 protects TH1 cells from galectin-9-mediated cell death [59] and promotes both proliferation and pro-inflammatory cytokine production [56]. Notably, mice that received BAG-6 overexpressing TH1 cells developed earlier onset of experimental autoimmune encephalomyelitis (EAE) due to an increased TH1 cell response by BAG-6. Moreover, a model of BAG-6 deficiency was established by transferring Bag-6−/− fetal liver cells into Rag1−/−-deficient mice. Loss of function of BAG-6 led to reduced severity of EAE in mice chimera, and BAG-6-deficient TH1 cells exhibited an exhausted-like phenotype, characterized by high expression of Tim-3 and IL-10 and low frequency of interferon (IFN)-γ and IL-2 production. Finally, Rangachari et al. found that the active form of Lck kinase is recruited to the Tim-3 tail in a BAG-6-dependent manner, and upon Tim-3 ligation the catalytically active form of Lck is released from BAG-6. In conclusion, the Tim-3/BAG-6 interaction may thus represent a promising molecular target in autoimmune disorders and chronic infections.
HLA class II expression is influenced by BAG-6 on antigen-presenting cells
BAG-6 plays a pivotal role in the regulation of professional antigen-presenting cells (pAPCs) such as macrophages and dendritic cells. MHC class II molecules on pAPCs, encoded by the HLA class II-genes, present peptides derived from proteolytic processing of endocytosed particulate exogenous antigens. The expression of proteins of the HLA class II processing pathway is regulated by the IFN-γ-inducible class II transactivator (CIITA) on the transcription level [60]. Interestingly, IFN-γ stimulation leads to a synchronized up-regulation of CIITA and BAG-6 expression thereby coupling the expression of HLA class II molecules to BAG-6, as shown in HLA class II molecule-positive tumor cells and primary human macrophages [57]. The molecular mechanism of the HLA class II regulation by BAG-6 remains unknown. Notably, overexpression of BAG-6 did not alter CIITA expression and vice versa, indicating no transcriptional activation. Another possibility suggested by the authors is that BAG-6 chaperones the cytosolic CIITA to the nucleus. This co-migration of BAG-6/CIITA complexes upon IFN-γ treatment could be observed via immunofluorescence microscopy [60].
BAG-6 regulates the activity of macrophages
Surprisingly, besides being an intracellular protein, BAG-6 is secreted by cells [18, 19, 58]. Grover and Izzo [58] showed that BAG-6 is released by heat-shocked macrophages in vitro, which in turn down-regulated nitric oxide and pro-inflammatory cytokines' release of macrophages, which were stimulated by IFN-γ and LPS. However, the physiological significance of this phenomenon is unclear. Interestingly, Mycobacterium tuberculosis infection causes ESAT-6 (early secreted antigenic target-6)-induced apoptosis of macrophages and epithelial cells [61]. The ESAT-6 antigen likewise induces transient BAG-6 expression, which counteracts ESAT-6-induced apoptosis in macrophages by interacting with the anti-apoptotic protein BCL2 [58].
For engulfment of neighboring apoptotic cells, macrophages recognize externalized phosphatidylserine (PS) on the surface of the apoptotic cell [62, 63]. Mitochondrial release of AIF is essential for PS exposure during death-receptor-induced apoptosis. Preta and Fadeel [7] recently demonstrated that re-localization of BAG-6 from the nucleus to the cytosol is required for PS exposure. BAG-6 stabilizes AIF in the cytosol of cells subjected to ER stress [8], and the BAG-6 AIF interaction is critical for PS exposure and subsequent phagocytosis by macrophages [64].
These observations suggest that BAG-6 plays a role in the early immune response to M. tuberculosis infection and further supports the accurate removal of apoptotic cells by macrophages, thereby promoting the process of inflammation and preventing induction of harmful autoimmune responses.
BAG-6 modulates the crosstalk of NK cells and DCs
BAG-6 regulates dendritic cell maturation
Upon inflammatory and infectious stimuli, dendritic cells (DCs) are subjected to a differentiation process termed maturation. Mature DCs (mDCs) activate NK cells via inflammatory cytokines. These activated NK are in turn enabled to kill DCs that remain immature (iDCs) and thus express low levels of HLA class I molecules or promote maturation of these iDCs towards mDCs [65]. In contrast, mDCs escape from NK cell killing by upregulation of HLA class I expression. Recent studies suggest that BAG-6 serves as an activating cellular ligand on the plasma membrane of iDCs triggering NKp30-dependent NK cell killing [18, 19]. By killing of iDCs activated NK cells might select a more immunogenic subset of DCs during a protective immune response [20].
BAG-6 employs a dual mode of action in tumor immunosurveillance and tumor immune escape
Tumor cells have the ability to shed ligands from the cell surface, and the presence of these soluble molecules in patients’ sera correlates with an attenuated immune response and progression of disease [66–68]. This tumor immune escape mechanism is established for the MHC class I-related chain (MIC) A and MIC B and the unique long 16-binding proteins (ULBP) 1 and 2, which are all cellular ligands for the activating NK cell receptor NKG2D [69]. Furthermore, the allelic variant MICA*008 and ULBP3 are released from the tumor cell on exosomes [69, 70], which are bioactive (30–100 nm) vesicles formed through the fusion of multivesicular bodies with the plasma membrane.
Previous studies demonstrated that BAG-6 is released from heat-shocked tumor cells [18] and DCs [19]. Interestingly, the authors suggest that the interaction of target cells with NK cells induces re-routing of nuclear BAG-6 to the plasma membrane [18]. The molecular details of this phenomenon remain elusive. Culture supernatants of cells overexpressing BAG-6 could induce NKp30-dependent IFN-γ and TNF-α secretion by NK cells, and transient BAG-6 knockdown in HeLa cells or DCs resulted in reduced killing by NK cells. Further studies demonstrated that BAG-6-positive exosomes lead to reconstitution of cytotoxicity towards the leukemia cell lines 697 and NALM, which were initially resistant to NK cell killing [18, 19, 67]. In line with these results, inhibition of exosomal release by neutral sphingomyelinase 2 led to reduced killing of target cells [67, 71].
It is still puzzling how BAG-6 is anchored on the plasma membrane and on exosomes since no transmembrane segment or other anchoring moiety is predicted from primary sequence. One possibility could be recruitment via HSP70. However, it remains to be elucidated whether BAG-6 possesses a membrane attachment moiety on its own such as a GPI-anchor or whether the complex of BAG-6 and HSP70 binds to a membrane adaptor protein (which remains to be discovered). Since both membrane-associated and soluble BAG-6 coexist (see below), we speculate that membrane-associated BAG-6 arises from recruitment of secreted BAG-6 to the surface of exosomes or the plasma membrane. Thus, this membrane-associated BAG-6 would be able to activate NK cell cytotoxicity upon ligation to NKp30.
Recently, soluble BAG-6 released into the serum of patients suffering from Hodgkin lymphoma [68] or chronic lymphocytic leukemia (CLL) was found to inhibit NK cell cytotoxicity [67]. By contrast, exosomal BAG-6 could not inhibit NK cell cytotoxicity. The concentration of soluble BAG-6 in patients’ plasma was directly correlated with the disease stage. Therefore, soluble BAG-6 is a mediator of tumor immune escape and thus represents a promising target for therapeutic intervention strategies. The functional differences between soluble and membrane-associated BAG-6 are most likely explained by the different degree of multivalency and related differences in apparent affinity due to avidity. Incubation of healthy donors’ NK cells with CLL plasma resulted in reduced cytotoxicity by down regulation of the surface receptors CD16 and CD56. Depletion of BAG-6 from CLL plasma via anti-BAG-6 antibodies could rescue NK cell cytotoxicity. In conclusion, this study suggests that soluble BAG-6 and membrane-associated BAG-6 are functionally different.
Interestingly, the exosomes isolated from plasma of CLL patients do not contain BAG-6 [67], whereas other cell types release BAG-6-positive exosomes, which activate NK cell cytotoxicity [18, 19]. These studies suggest that soluble but not exosomal BAG-6 is a mediator of tumor immune escape reminiscent of observations made for the NKG2D/NKG2D-ligand system of NK cells [69, 72]. Nevertheless, the molecular details of the BAG-6-NKp30 interaction are largely unknown but are required to understand NK cell function and to develop NK cell-based therapies. We therefore, investigated the interaction of NKp30 and BAG-6 on the molecular level [73]. Within these studies, we have localized the binding site of NKp30 on BAG-6 to a sequence stretch of 250 amino acids within the C-terminal half of BAG-6 (BAG-6686–936). Interestingly, recombinant BAG-6686–936 forms homo-dimers and inhibits NKp30-dependent signaling in reporter cells and NK cells, thereby demonstrating the inhibitory function of soluble BAG-6 on molecular level. Furthermore, these data demonstrate that the BAG-6686–936 domain is essential and sufficient for inhibition of NKp30-dependent cytotoxicity. Notably, a recent study showed that full-length BAG-6 forms oligomers [74], therefore, we suggest that BAG-6686–936 represents the corresponding binding interface.
Conclusions and future perspectives
As outlined in the current review, BAG-6 is a fascinating multifunctional protein, which is involved in many non-related cellular pathways ranging from regulation of gene expression to immune escape of malignantly transformed cells. However, many molecular details of these pathways remain to be discovered. The recent findings about BAG-6 function in the regulation of immune responses and in the context of innate immunity towards malignantly transformed cells opens up new perspectives for cancer therapy focusing on improved recognition of membrane-associated BAG-6 as a tumor antigen or to circumvent tumor immune escape by scavenging soluble BAG-6 variants present in patients’ plasma. Moreover, this soluble BAG-6 might be a promising diagnostic and even prognostic marker protein to adjust the type and timing of conventional therapies. However, detailed patient cohorts need to be analyzed to get insights into the potential of soluble BAG-6 as a target and to determine which cancer types might benefit from counteractions against soluble BAG-6.
Acknowledgments
We thank Adelheid Cerwenka for helpful discussions and critical reading of the manuscript. The laboratory of J.K. is supported by institutional funds of the Georg-Speyer-Haus and by grants from LOEWE Center for Cell and Gene Therapy Frankfurt funded by: Hessisches Ministerium für Wissenschaft und Kunst (HMWK) funding reference number: III L 4- 518/17.004 (2010) and the Wilhelm-Sander Stiftung (2010.104.1). The Georg-Speyer-Haus is funded jointly by the German Federal Ministry of Health (BMG) and the Ministry of Higher Education, Research and the Arts of the State of Hessen (HMWK).
References
- 1.Banerji J, Sands J, Strominger JL, Spies T. A gene pair from the human major histocompatibility complex encodes large proline-rich proteins with multiple repeated motifs and a single ubiquitin-like domain. Proc Natl Acad Sci USA. 1990;87(6):2374–2378. doi: 10.1073/pnas.87.6.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thress K, Henzel W, Shillinglaw W, Kornbluth S. Scythe: a novel reaper-binding apoptotic regulator. EMBO J. 1998;17(21):6135–6143. doi: 10.1093/emboj/17.21.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thress K, Evans EK, Kornbluth S. Reaper-induced dissociation of a Scythe-sequestered cytochrome c-releasing activity. EMBO J. 1999;18(20):5486–5493. doi: 10.1093/emboj/18.20.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minami R, Shimada M, Yokosawa H, Kawahara H. Scythe regulates apoptosis through modulating ubiquitin-mediated proteolysis of the Xenopus elongation factor XEF1AO. Biochem J. 2007;405(3):495–501. doi: 10.1042/BJ20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikukawa Y, Minami R, Shimada M, Kobayashi M, Tanaka K, Yokosawa H, Kawahara H. Unique proteasome subunit Xrpn10c is a specific receptor for the antiapoptotic ubiquitin-like protein Scythe. FEBS J. 2005;272(24):6373–6386. doi: 10.1111/j.1742-4658.2005.05032.x. [DOI] [PubMed] [Google Scholar]
- 6.Wu YH, Shih SF, Lin JY. Ricin triggers apoptotic morphological changes through caspase-3 cleavage of BAT3. J Biol Chem. 2004;279(18):19264–19275. doi: 10.1074/jbc.M307049200. [DOI] [PubMed] [Google Scholar]
- 7.Preta G, Fadeel B. Scythe cleavage during Fas (APO-1)-and staurosporine-mediated apoptosis. FEBS Lett. 2012;586(6):747–752. doi: 10.1016/j.febslet.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Desmots F, Russell HR, Michel D, McKinnon PJ. Scythe regulates apoptosis-inducing factor stability during endoplasmic reticulum stress-induced apoptosis. J Biol Chem. 2008;283(6):3264–3271. doi: 10.1074/jbc.M706419200. [DOI] [PubMed] [Google Scholar]
- 9.Desmots F, Russell HR, Lee Y, Boyd K, McKinnon PJ. The reaper-binding protein scythe modulates apoptosis and proliferation during mammalian development. Mol Cell Biol. 2005;25(23):10329–10337. doi: 10.1128/MCB.25.23.10329-10337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki T, Gan EC, Wakeham A, Kornbluth S, Mak TW, Okada H. HLA-B-associated transcript 3 (Bat3)/Scythe is essential for p300-mediated acetylation of p53. Genes Dev. 2007;21(7):848–861. doi: 10.1101/gad.1534107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manchen ST, Hubberstey AV. Human Scythe contains a functional nuclear localization sequence and remains in the nucleus during staurosporine-induced apoptosis. Biochem Biophys Res Commun. 2001;287(5):1075–1082. doi: 10.1006/bbrc.2001.5701. [DOI] [PubMed] [Google Scholar]
- 12.Wu W, Song W, Li S, Ouyang S, Fok KL, Diao R, Miao S, Chan HC, Wang L. Regulation of apoptosis by Bat3-enhanced YWK-II/APLP2 protein stability. J Cell Sci. 2012;125(Pt 18):4219–4229. doi: 10.1242/jcs.086553. [DOI] [PubMed] [Google Scholar]
- 13.Krenciute G, Liu S, Yucer N, Shi Y, Ortiz P, Liu Q, Kim BJ, Odejimi AO, Leng M, Qin J, Wang Y. Nuclear BAG6-UBL4A-GET4 complex mediates DNA damage signaling and cell death. J Biol Chem. 2013;288(28):20547–20557. doi: 10.1074/jbc.M112.443416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen P, Bar-Sela G, Sun L, Bisht KS, Cui H, Kohn E, Feinberg AP, Gius D. BAT3 and SET1A form a complex with CTCFL/BORIS to modulate H3K4 histone dimethylation and gene expression. Mol Cell Biol. 2008;28(21):6720–6729. doi: 10.1128/MCB.00568-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakeman TP, Wang Q, Feng J, Wang XF. Bat3 facilitates H3K79 dimethylation by DOT1L and promotes DNA damage-induced 53BP1 foci at G1/G2 cell-cycle phases. EMBO J. 2012;31(9):2169–2181. doi: 10.1038/emboj.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamper N, Kessler J, Temme S, Wegscheid C, Winkler J, Koch N. A novel BAT3 sequence generated by alternative RNA splicing of exon 11B displays cell type-specific expression and impacts on subcellular localization. PLoS One. 2012;7(4):e35972. doi: 10.1371/journal.pone.0035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Liu Y, Soetandyo N, Baek K, Hegde R, Ye Y. A ubiquitin ligase-associated chaperone holdase maintains polypeptides in soluble states for proteasome degradation. Mol Cell. 2011;42(6):758–770. doi: 10.1016/j.molcel.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Boll B, Simhadri VL, Borchmann P, McKinnon PJ, Hallek M, Engert A. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27(6):965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A, Pogge von Strandmann E. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One. 2008;3(10):e3377. doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013;34(4):182–191. doi: 10.1016/j.it.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Thress K, Song J, Morimoto RI, Kornbluth S. Reversible inhibition of Hsp70 chaperone function by Scythe and Reaper. EMBO J. 2001;20(5):1033–1041. doi: 10.1093/emboj/20.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corduan A, Lecomte S, Martin C, Michel D, Desmots F. Sequential interplay between BAG6 and HSP70 upon heat shock. CMLS. 2009;66(11–12):1998–2004. doi: 10.1007/s00018-009-9198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki T, Marcon E, McQuire T, Arai Y, Moens PB, Okada H. Bat3 deficiency accelerates the degradation of Hsp70-2/HspA2 during spermatogenesis. J Cell Biol. 2008;182(3):449–458. doi: 10.1083/jcb.200802113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3(10):E237–E241. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- 25.Doong H, Vrailas A, Kohn EC. What’s in the ‘BAG’?—a functional domain analysis of the BAG-family proteins. Cancer Lett. 2002;188(1–2):25–32. doi: 10.1016/S0304-3835(02)00456-1. [DOI] [PubMed] [Google Scholar]
- 26.Naishiro Y, Adachi M, Okuda H, Yawata A, Mitaka T, Takayama S, Reed JC, Hinoda Y, Imai K. BAG-1 accelerates cell motility of human gastric cancer cells. Oncogene. 1999;18(21):3244–3251. doi: 10.1038/sj.onc.1202661. [DOI] [PubMed] [Google Scholar]
- 27.Rosati A, Graziano V, De Laurenzi V, Pascale M, Turco MC. BAG3: a multifaceted protein that regulates major cell pathways. Cell Death Dis. 2011;2:e141. doi: 10.1038/cddis.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Q, Ozawa F, Friess H, Zimmermann A, Takayama S, Reed JC, Kleeff J, Buchler MW. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett. 2001;503(2–3):151–157. doi: 10.1016/S0014-5793(01)02728-4. [DOI] [PubMed] [Google Scholar]
- 29.Tang SC, Shehata N, Chernenko G, Khalifa M, Wang X. Expression of BAG-1 in invasive breast carcinomas. J Clin Oncol. 1999;17(6):1710–1719. doi: 10.1200/JCO.1999.17.6.1710. [DOI] [PubMed] [Google Scholar]
- 30.Rabu C, Schmid V, Schwappach B, High S. Biogenesis of tail-anchored proteins: the beginning for the end? J Cell Sci. 2009;122(Pt 20):3605–3612. doi: 10.1242/jcs.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefanovic S, Hegde RS. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell. 2007;128(6):1147–1159. doi: 10.1016/j.cell.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 32.Schuldiner M, Metz J, Schmid V, Denic V, Rakwalska M, Schmitt HD, Schwappach B, Weissman JS. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell. 2008;134(4):634–645. doi: 10.1016/j.cell.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang YW, Chuang YC, Ho YC, Cheng MY, Sun YJ, Hsiao CD, Wang C. Crystal structure of Get4-Get5 complex and its interactions with Sgt2, Get3, and Ydj1. J Biol Chem. 2010;285(13):9962–9970. doi: 10.1074/jbc.M109.087098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mariappan M, Mateja A, Dobosz M, Bove E, Hegde RS, Keenan RJ. The mechanism of membrane-associated steps in tail-anchored protein insertion. Nature. 2011;477(7362):61–66. doi: 10.1038/nature10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mariappan M, Li X, Stefanovic S, Sharma A, Mateja A, Keenan RJ, Hegde RS. A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature. 2010;466(7310):1120–1124. doi: 10.1038/nature09296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leznicki P, Clancy A, Schwappach B, High S. Bat3 promotes the membrane integration of tail-anchored proteins. J Cell Sci. 2010;123(Pt 13):2170–2178. doi: 10.1242/jcs.066738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, Cai M, Yang Y, Huang L, Ye Y. SGTA recognizes a noncanonical ubiquitin-like domain in the Bag6-Ubl4A-Trc35 complex to promote endoplasmic reticulum-associated degradation. Cell Rep. 2012;2(6):1633–1644. doi: 10.1016/j.celrep.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winnefeld M, Grewenig A, Schnolzer M, Spring H, Knoch TA, Gan EC, Rommelaere J, Cziepluch C. Human SGT interacts with Bag-6/Bat-3/Scythe and cells with reduced levels of either protein display persistence of few misaligned chromosomes and mitotic arrest. Exp Cell Res. 2006;312(13):2500–2514. doi: 10.1016/j.yexcr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 39.Minami R, Hayakawa A, Kagawa H, Yanagi Y, Yokosawa H, Kawahara H. BAG-6 is essential for selective elimination of defective proteasomal substrates. J Cell Biol. 2010;190(4):637–650. doi: 10.1083/jcb.200908092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metzger MB, Maurer MJ, Dancy BM, Michaelis S. Degradation of a cytosolic protein requires endoplasmic reticulum-associated degradation machinery. J Biol Chem. 2008;283(47):32302–32316. doi: 10.1074/jbc.M806424200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reits EA, Vos JC, Gromme M, Neefjes J. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 2000;404(6779):774–778. doi: 10.1038/35008103. [DOI] [PubMed] [Google Scholar]
- 42.Khan S, de Giuli R, Schmidtke G, Bruns M, Buchmeier M, van den Broek M, Groettrup M. Cutting edge: neosynthesis is required for the presentation of a T cell epitope from a long-lived viral protein. J Immunol. 2001;167(9):4801–4804. doi: 10.4049/jimmunol.167.9.4801. [DOI] [PubMed] [Google Scholar]
- 43.Koch J, Tampe R. The macromolecular peptide-loading complex in MHC class I-dependent antigen presentation. CMLS. 2006;63(6):653–662. doi: 10.1007/s00018-005-5462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hessa T, Sharma A, Mariappan M, Eshleman HD, Gutierrez E, Hegde RS. Protein targeting and degradation are coupled for elimination of mislocalized proteins. Nature. 2011;475(7356):394–397. doi: 10.1038/nature10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirsch C, Gauss R, Horn SC, Neuber O, Sommer T. The ubiquitylation machinery of the endoplasmic reticulum. Nature. 2009;458(7237):453–460. doi: 10.1038/nature07962. [DOI] [PubMed] [Google Scholar]
- 46.Claessen JH, Ploegh HL. BAT3 guides misfolded glycoproteins out of the endoplasmic reticulum. PLoS One. 2011;6(12):e28542. doi: 10.1371/journal.pone.0028542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwak JH, Kim SI, Kim JK, Choi ME. BAT3 interacts with transforming growth factor-beta (TGF-beta) receptors and enhances TGF-beta1-induced type I collagen expression in mesangial cells. J Biol Chem. 2008;283(28):19816–19825. doi: 10.1074/jbc.M802285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ensminger AW, Isberg RR. E3 ubiquitin ligase activity and targeting of BAT3 by multiple Legionella pneumophila translocated substrates. Infect Immun. 2010;78(9):3905–3919. doi: 10.1128/IAI.00344-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Broderick P, Webb E, Wu X, Vijayakrishnan J, Matakidou A, Qureshi M, Dong Q, Gu X, Chen WV, Spitz MR, Eisen T, Amos CI, Houlston RS. Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat Genet. 2008;40(12):1407–1409. doi: 10.1038/ng.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Degli-Esposti MA, Abraham LJ, McCann V, Spies T, Christiansen FT, Dawkins RL. Ancestral haplotypes reveal the role of the central MHC in the immunogenetics of IDDM. Immunogenetics. 1992;36(6):345–356. doi: 10.1007/BF00218041. [DOI] [PubMed] [Google Scholar]
- 51.Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet. 2010;42(9):781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsieh YY, Lin YJ, Chang CC, Chen DY, Hsu CM, Wang YK, Hsu KH, Tsai FJ. Human lymphocyte antigen B-associated transcript 2, 3, and 5 polymorphisms and haplotypes are associated with susceptibility of Kawasaki disease and coronary artery aneurysm. J Clin Lab Anal. 2010;24(4):262–268. doi: 10.1002/jcla.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vandiedonck C, Beaurain G, Giraud M, Hue-Beauvais C, Eymard B, Tranchant C, Gajdos P, Dausset J, Garchon HJ. Pleiotropic effects of the 8.1 HLA haplotype in patients with autoimmune myasthenia gravis and thymus hyperplasia. Proc Natl Acad Sci USA. 2004;101(43):15464–15469. doi: 10.1073/pnas.0406756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harney SM, Vilarino-Guell C, Adamopoulos IE, Sims AM, Lawrence RW, Cardon LR, Newton JL, Meisel C, Pointon JJ, Darke C, Athanasou N, Wordsworth BP, Brown MA. Fine mapping of the MHC Class III region demonstrates association of AIF1 and rheumatoid arthritis. Rheumatology. 2008;47(12):1761–1767. doi: 10.1093/rheumatology/ken376. [DOI] [PubMed] [Google Scholar]
- 55.Ivanov I, Lo KC, Hawthorn L, Cowell JK, Ionov Y. Identifying candidate colon cancer tumor suppressor genes using inhibition of nonsense-mediated mRNA decay in colon cancer cells. Oncogene. 2007;26(20):2873–2884. doi: 10.1038/sj.onc.1210098. [DOI] [PubMed] [Google Scholar]
- 56.Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, Angin M, Wakeham A, Greenfield EA, Sobel RA, Okada H, McKinnon PJ, Mak TW, Addo MM, Anderson AC, Kuchroo VK. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat Med. 2012;18(9):1394–1400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamper N, Franken S, Temme S, Koch S, Bieber T, Koch N. gamma-Interferon-regulated chaperone governs human lymphocyte antigen class II expression. FASEB J. 2012;26(1):104–116. doi: 10.1096/fj.11-189670. [DOI] [PubMed] [Google Scholar]
- 58.Grover A, Izzo AA. BAT3 regulates mycobacterium tuberculosis protein ESAT-6-mediated apoptosis of macrophages. PLoS One. 2012;7(7):e40836. doi: 10.1371/journal.pone.0040836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 60.Pai RK, Askew D, Boom WH, Harding CV. Regulation of class II MHC expression in APCs: roles of types I, III, and IV class II transactivator. J Immunol. 2002;169(3):1326–1333. doi: 10.4049/jimmunol.169.3.1326. [DOI] [PubMed] [Google Scholar]
- 61.Derrick SC, Morris SL. The ESAT6 protein of Mycobacterium tuberculosis induces apoptosis of macrophages by activating caspase expression. Cell Microbiol. 2007;9(6):1547–1555. doi: 10.1111/j.1462-5822.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- 62.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276(2):1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 63.Kagan VE, Gleiss B, Tyurina YY, Tyurin VA, Elenstrom-Magnusson C, Liu SX, Serinkan FB, Arroyo A, Chandra J, Orrenius S, Fadeel B. A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J Immunol. 2002;169(1):487–499. doi: 10.4049/jimmunol.169.1.487. [DOI] [PubMed] [Google Scholar]
- 64.Preta G, Fadeel B. AIF and Scythe (Bat3) regulate phosphatidylserine exposure and macrophage clearance of cells undergoing Fas (APO-1)-mediated apoptosis. PLoS One. 2012;7(10):e47328. doi: 10.1371/journal.pone.0047328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferlazzo G, Morandi B, D’Agostino A, Meazza R, Melioli G, Moretta A, Moretta L. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol. 2003;33(2):306–313. doi: 10.1002/immu.200310004. [DOI] [PubMed] [Google Scholar]
- 66.Groth A, Kloss S, von Strandmann EP, Koehl U, Koch J. Mechanisms of tumor and viral immune escape from natural killer cell-mediated surveillance. J Innate Immun. 2011;3(4):344–354. doi: 10.1159/000327014. [DOI] [PubMed] [Google Scholar]
- 67.Reiners KS, Topolar D, Henke A, Simhadri VR, Kessler J, Sauer M, Bessler M, Hansen HP, Tawadros S, Herling M, Kronke M, Hallek M, Pogge von Strandmann E. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood. 2013;121(18):3658–3665. doi: 10.1182/blood-2013-01-476606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reiners KS, Kessler J, Sauer M, Rothe A, Hansen HP, Reusch U, Hucke C, Kohl U, Durkop H, Engert A, von Strandmann EP. Rescue of impaired NK cell activity in Hodgkin lymphoma with bispecific antibodies in vitro and in patients. Mol Ther. 2013;21(4):895–903. doi: 10.1038/mt.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernandez-Messina L, Ashiru O, Boutet P, Aguera-Gonzalez S, Skepper JN, Reyburn HT, Vales-Gomez M. Differential mechanisms of shedding of the glycosylphosphatidylinositol (GPI)-anchored NKG2D ligands. J Biol Chem. 2010;285(12):8543–8551. doi: 10.1074/jbc.M109.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, Reyburn HT. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70(2):481–489. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 72.Ullrich E, Koch J, Cerwenka A, Steinle A (2013) New prospects on the NKG2D/NKG2D-ligand system for oncology. Oncoimmunology 2(10):e26097. doi:10.4161/onci.26097 [DOI] [PMC free article] [PubMed]
- 73.Binici J, Hartmann J, Herrmann J, Schreiber C, Beyer S, Guler G, Vogel V, Tumulka F, Abele R, Mantele W, Koch J. A soluble fragment of the tumor antigen BCL2-associated athanogene 6 (BAG-6) is essential and sufficient for inhibition of NKp30-dependent cytotoxicity of natural killer cells. J Biol Chem. 2013 doi: 10.1074/jbc.M113.483602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Y, Liu Y, Lee JG, Ye Y. A ubiquitin-like domain recruits an oligomeric chaperone to a retrotranslocation complex in endoplasmic reticulum-associated degradation. J Biol Chem. 2013;288(25):18068–18076. doi: 10.1074/jbc.M112.449199. [DOI] [PMC free article] [PubMed] [Google Scholar]