Abstract
The ability of cardiomyocytes to detect mechanical and humoral stimuli is critical for adaptation of the myocardium in response to new conditions and for sustaining the increased workload during stress. While certain stimuli mediate a beneficial adaptation to stress conditions, others result in maladaptive remodelling, ultimately leading to heart failure. Specific signalling pathways activating either adaptive or maladaptive cardiac remodelling have been identified. Paradoxically, however, in a number of cases, the transduction pathways involved in such opposing responses engage the same signalling proteins. A notable example is the Raf–MEK1/2–ERK1/2 signalling pathway that can control both adaptive and maladaptive remodelling. ERK1/2 signalling requires a signalosome complex where a scaffold protein drives the assembly of these three kinases into a linear pathway to facilitate their sequential phosphorylation, ultimately targeting specific effector molecules. Interestingly, a number of different Raf–MEK1/2–ERK1/2 scaffold proteins have been identified, and their role in determining the adaptive or maladaptive cardiac remodelling is a promising field of investigation for the development of therapeutic strategies capable of selectively potentiating the adaptive response.
Keywords: Scaffold proteins, ERK1/2 pathway, Cardiac remodelling, Intracellular signalling
Introduction
Heart failure is a complex disease due to inherited gene mutations or to acquired abnormalities in heart function mainly due to chronic exposure to work overload. This condition is usually detected by the heart, with the subsequent activation of a process called adaptive heart remodelling, a complex and coordinated reaction of the entire organ that includes cardiomyocyte hypertrophy, metabolic changes, and modifications in calcium handling and contractile function. The vascular bed and stromal tissue also undergo important remodelling to support the increased metabolic need and increased mechanical strain of the organ. This initial adaptive response can, however, progress toward contractile dysfunction and heart dilation due to cardiomyocyte death, inflammatory reactions and heart fibrosis. Heart remodelling is triggered by the activation of mechanical signals, cytokine growth factors and neurohumoral stimuli. While some of these stimuli mainly mediate an adaptive heart remodelling response, others result in maladaptive changes and the actual myocardial function is determined by the balance between these different stimuli [1]. This is due to the fact that different stimuli activate specific intracellular signalling cascades, leading to unique responses in term of cardiac cell metabolism, hypertrophy and survival. Numerous intracellular signalling pathways have been characterized as being involved in heart remodelling. Analyses of genetically modified mice overexpressing or lacking specific signalling components have greatly contributed to the concept that heart remodelling can be beneficial or maladaptive depending on the specific pathways involved [1–3]. It is well known that neurohumoral stimuli, such as angiotensin and endothelin-1, via Gq-coupled seven transmembrane receptor signalling, trigger a maladaptive cardiac hypertrophic response, leading to heart failure [4, 5]. Conversely, the PI3 K/AKT/GSK3β signalling cascade, activated by growth factors, such as IGF-1, via tyrosine kinase receptors, is known to induce a compensatory hypertrophic response in the absence of fibrosis and systolic dysfunction, and protect cardiomyocytes from apoptotic death [6–9].
Current understanding of the biochemical pathways triggered by different stress stimuli on the heart indicates that, in some instances, the same signalling molecules are activated both during adaptive and maladaptive remodelling generating confusion in the comprehension of the molecular mechanisms involved in cardiac remodelling and casting doubts on the possible uses of these signalling molecules as drug targets [10, 11]. The Ras-Raf–MEK1/2–ERK1/2 MAPK signalling pathway is a paradigmatic example of this dual role as it is involved both in maladaptive and beneficial remodelling.
In the ERK1/2 pathway, Ras GTPase stimulates the activity of Raf kinase, which phosphorylates MEK1/2 that sequentially phosphorylate and activate the downstream kinases ERK1/2. Once activated, ERK1/2 phosphorylate multiple intracellular targets, both in the cytoplasm and in the nucleus. Cytoplasmic substrates include approximately 70 proteins, among them EGFr, SOS, RSK1, cPLA2, p70 S6 kinase and phosphodiesterase 4D (PDE4) [12]. In the nucleus, ERK1/2 phosphorylates numerous transcription factors that induce reprogramming of cardiac gene expression.
Many reports in the literature support the beneficial role of ERK1/2 signalling in the heart. For instance, raf-1 null mice show left ventricular systolic dysfunction and heart dilatation without cardiac hypertrophy, and increased cardiomyocyte apoptosis [13]. Accordingly, transgenic mice with cardiac-specific expression of a dominant negative form of Raf-1 show blunted hypertrophic remodelling, high levels of cardiomyocyte apoptosis and increased mortality in response to pressure overload [14]. Furthermore, transgenic mice expressing activated MEK1 in cardiomyocytes develop stable concentric hypertrophy devoid of interstitial cell fibrosis [15]. In another study, Erk1 null and Erk2−/+ heterozygous mice display heart decompensation and failure after long-term pressure overload due to an increase in cardiomyocyte apoptosis [16].
On the other hand, other studies point to ERK1/2 as a maladaptive signalling pathway in heart remodelling. Expression of a constitutively active Ras in the mouse heart promotes hypertrophic cardiomyopathy characterized by myofilament disarray and interstitial fibrosis [17, 18]. However, given that Ras activates several signalling cascades in addition to the MEK–ERK1/2 pathway, the involvement of other pathways cannot be excluded [19]. A well-known negative effect of ERK1/2 signalling in human heart function is highlighted by the fact that different mutations able to increase ERK1/2 pathway activationlead to cardiac pathologies in patients with Noonan and related syndromes, such as Costello, LEOPARD and CFC (cardio-facio-cutaneous) syndromes [15, 16, 20–25]. Knock-in mice expressing the Noonan syndrome-associated Raf1 L613V mutation exhibit enhanced ERK1/2 signalling, eccentric cardiac hypertrophy in basal conditions and an accelerated transition toward heart failure in response to pressure overload. Interestingly, postnatal treatment with MEK inhibition normalizes cardiac defects [26]. A further example is represented by the mouse model of Emery–Dreifuss muscular dystrophy, carrying a mutation in the A-type lamin gene and causing dilated cardiomyopathy. These mice show abnormal activation of ERK1/2 signalling in the heart [27] and treatment with MEK inhibitors prevents the development of left ventricular dilatation [28] and improves heart performance if administered after the onset of cardiac disease [29]. Furthermore, Lorenz et al. demonstrated that G protein-coupled receptor-induced ERK1/2 autophosphorylation of Thr188 causes localization of ERK1/2 to the nucleus, thus enhancing phosphorylation of nuclear targets of ERK1/2 [30]. Interestingly, Thr188 phosphorylation of ERK1/2 does not affect its kinase activity, but does mediate ERK1/2 nuclear accumulation. Transgenic mice expressing a Thr188 phosphomimetic Erk2 mutant (Erk2T188D) are normal under basal conditions, but show enhanced hypertrophic remodelling in response to pressure overload associated with reduced fractional shortening and cardiac dysfunction [30].
The results discussed above clearly demonstrate that activation of the ERK1/2 pathway can result in either beneficial or maladaptive cardiac remodelling, which can be ascribed to different modes of activation.
ERK1/2 MAPK scaffold proteins in the heart
ERK signalling requires the spatial and temporal organization of three different kinases, Raf, MEK1/2 and ERK1/2, which act in sequential cascade, an event controlled by scaffold molecules. A number of scaffold molecules regulating the Raf–MEK1/2–ERK1/2 pathway have been identified [31]. These include β-arrestin, Gβγ subunits, the four-and-a-half LIM domain- protein 1(FHL1), Ras GTPase-activating-like protein 1 (IQGAP-1), kinase suppressor of Ras 1 (KSR1), mitogen-activated protein kinase organizer 1 (MORG1) and MEK Partner 1 (MP1), paxillin and G protein-coupled receptor (GPCR)-kinase interacting protein-1 (GIT1) [32] and dual-specificity tyrosine-phosphorylated and regulated kinase 1A (DYRK1A) [33].
A major function of molecular scaffolds is to bind different components of the signal transduction pathway keeping these molecules in the location and orientation needed for their reciprocal interactions and activation/deactivation. A second important function of scaffold molecules is to localise the signalling complexes to a given cellular sub-compartment in order to place them in close proximity to specific effectors and, therefore, to achieve signalling specificity. Moreover, scaffold molecules can often bind proteins of multiple signalling pathways, thus, contributing to the crosstalk between pathways and the organisation of the signalling network typical of a specific stimulus in a given cellular context [34].
Some of the Raf–MEK1/2–ERK1/2 scaffold molecules mentioned above have been studied for their role in cardiac tissue, while others, despite being expressed in the heart, have mainly been characterised in other organs.
Here, we discuss the involvement of scaffold proteins in mediating the assembly and spatiotemporal activation of specific Raf–MEK1/2–ERK1/2 signalling complexes and their impact on beneficial or maladaptive cardiac remodelling.
β-arrestins and Gβγ subunits
The seven transmembrane receptors, such as angiotensin and adrenergic receptors, activate the ERK1/2 signalling by different mechanisms involving at least two different scaffold proteins: a G protein-independent mechanism involving the β-arrestins [35, 36] and a G protein-dependent pathway involving Gβγ subunits [30, 37]. An additional G protein-dependent mechanism involves the Gα subunit which can activate the ERK1/2 pathway via classical GPCR downstream effectors, such as PKA and PKC [38, 39], possibly through the FHL1 scaffold protein [41], as will be discussed below.
β-arrestin 1 and 2 are multifunctional adaptor proteins, classically known for their role in G protein-coupled receptor (GPCR) desensitisation. In particular, recruitment of β-arrestins to the GPCR prevents further G protein coupling of the receptor, thereby leading to desensitisation [36]. β-arrestins also function as a scaffold, linking GPCR activation to several downstream effectors such as Src, the ubiquitin ligase, Mdm2 and MAPK cascades (ERK and JNK) [41]. In particular, β-arrestins bind Raf–MEK1/2–ERK1/2, aligning them in the appropriate orientation to activate each other [35]. β-arrestin–mediated scaffolding directs ERK1/2 activity to their cytoplasmic substrates, protecting cells from apoptosis [42]. Based on this property, it has been suggested that β-arrestins are cardioprotective in two different ways, namely by activating a G protein independent ERK1/2 pathway and by blocking the deleterious G protein pathway [43].
Gβγ complex downstream of the seven transmembrane receptors can also act as a Raf–MEK1/2–ERK1/2 scaffold and mediates the G protein-dependent ERK1/2 maladaptive signalling [30]. G scaffolding mediates ERK1/2 autophosphorylation at a specific site, Thr188, which is ultimately linked to detrimental remodelling, characterised by excessive hypertrophy and ventricle dilation in response to pressure overload [30, 37]. Thr188 phosphorylation strongly increases ERK1/2 nuclear localisation, raising the possibility that the ERK1/2 scaffold-driven nuclear localisation is at least partially responsible for maladaptive remodelling.
The four-and-a-half LIM domains protein 1 (FHL1)
The FHL family, composed of FHL 1, 2, 3, 4 and ACT, is a group of LIM-only proteins characterised by an N-terminal half LIM domain followed by four complete LIM domains [44, 45]. FHL1 is highly expressed in striated muscles and moderately expressed in cardiac muscle and other tissues [44]. FHL1 mutations have been found in hypertrophic cardiomyopathies [46] and are responsible for four distinct skeletal muscle diseases [44]. Moreover, FHL1 is upregulated in mouse hearts in response to pressure overload [47, 48] as well as in hearts of human patients with hypertrophic cardiomyopathy [49–51]. From a molecular point of view, FHL1 plays an important role in biomechanical stress responses involved in cardiac hypertrophy. FHL1 deficiency in mice prevents both pressure overload-induced and Gαq-mediated cardiomyopathy, suggesting that FHL1 coordinates maladaptive signalling downstream of Gαq [40]. FHL1 functions as a scaffold for the Raf–MEK1/2–ERK1/2 pathway in the heart by regulating its subcellular localisation at the I band and by regulating intensity and/or duration of ERK1/2 activation in response to pressure overload [40], negatively impacting on muscle compliance [52]. In summary, FHL1-mediated Gαq-dependent ERK1/2 activation leads to detrimental hypertrophic cardiac remodelling.
Ras GTPase-activating-like protein 1 (IQGAP-1)
IQGAP-1 is a ubiquitously expressed multi-domain protein originally identified as a putative GAP for CDC42. More recently, it has been shown that IQGAP-1 binds to Raf, MEK1/2 and ERK1/2, functioning as a scaffoldand activating the ERK1/2 pathway in response to EGF in fibroblasts and epithelial cells [53, 54]. Indeed, in the heart, IQGAP-1 is expressed both in cardiomyocytes and in fibroblasts and acts as a Raf, MEK1/2 and ERK1/2 scaffold molecule required for activating a temporally delayed wave of ERK1/2 signalling in response to pressure overload [55]. Interestingly, ERK1/2 activation at early time points after pressure overload is independent from IQGAP-1 [55], underlying the importance of scaffold proteins in determining the temporal specificity of signalling pathway activation. Moreover, IQGAP-1 is also able to bind Akt [56], and is required for Akt activation in response to pressure overload [55], highlighting its ability to orchestrate different signalling pathways. Overall, the absence of IQGAP-1 causes an accelerated transition towards left ventricle dilation and impaired contractility, indicating a role for IQGAP-1 in sustaining functional remodelling upon chronic pressure overload [55].
Interestingly, IQGAP-1 not only binds c-Raf, MEK1/2 and ERK1/2 in the heart but also interacts with the heat shock protein, Hsp90, and melusin, a muscle-specific small molecular chaperone regulating signal transduction [57, 58]. Chaperone molecules play an important role in assisting conformational changes required for activation and stabilisation of signalling proteins [59], suggesting that a cooperation of scaffolds and chaperones is required to organise a fully active signalosome complex.
Additional Raf-MEK-ERK scaffolds
The ERK scaffold proteins briefly discussed below have been characterised in several cellular systems, but their role in cardiac remodelling has not yet been addressed, despite the fact that they are expressed in the heart.
Kinase suppressor of Ras 1 (KSR1)
KSR1 is one of the best characterised scaffold proteins of the ERK pathway in yeast as well as in mammalian cells [31, 60]. In resting cells, KSR1 is retained in the cytoplasm and interacts with 14-3-3 proteins [61] and with the E3 ubiquitin ligase IMP (impedes mitogenic signal propagation) [62]. Cell stimulation with growth factors induces disassembly of the complexes, resulting in localisation of KSR1 to the plasma membrane where it facilitates signalling from c-Raf to MEK1/2 and to ERK1/2 [63]. Recent studies using cells obtained from KSR1-null mice revealed that KSR1 modulates the intensity and duration of Raf–MEK1/2–ERK1/2 signalling, promoting cell differentiation and oncogene-induced senescence [64, 65]. KSR1 can also function as a scaffold for iNOS in association with the heat shock protein, Hsp90 [66], thus suggesting that KSR1 can act as an integrator of multiple signalling pathways.
Mitogen-activated protein kinase organiser 1 (MORG1) and MEK Partner 1 (MP1)
MP1 can associate with both MEK1/2 and ERK1/2 and target this complex to late endosomes, resulting in localisation of ERK1/2 signalling to this intracellular compartment. MP1 also binds to MORG1 that in turn binds c-Raf and co-localises with vesicles in cells. The MORG1-bound Raf–MEK1/2–ERK1/2 complex is selectively stimulated by lysophosphatidic acid through G protein-coupled receptors, but is not activated through tyrosine kinase receptors in response to epidermal growth factor (EGF) or platelet-derived growth factor (PDGF). By contrast, Raf–MEK1/2–ERK1/2 complexes coordinated by KSR1 scaffold protein at the cell membrane respond to both G protein-coupled and tyrosine kinase receptors [67], further highlighting the key role of scaffolds in determining specific signalling modality.
Paxillin
Paxillin has been reported to function as a scaffold protein for c-Raf, MEK1/2, and ERK1/2, downstream of the hepatocyte growth factor receptor Met. ERK1/2 binding to paxillin is thought to control cell motility by phosphorylation of paxillin on S83, and promoting its binding to focal adhesion kinase, FAK. This results in localisation of ERK1/2 signalling to focal adhesions, activation of the Rho family GTPase Rac, and extension of lamellipodia [68].
G protein-coupled receptor (GPCR)-kinase interacting protein-1 (GIT1)
GIT1 is a scaffold protein for the MEK1–ERK1/2 pathway [69] known to bind paxillin and GRK2 (G protein-coupled receptor kinase 2). GIT1 is required for sustained activation of MEK1-ERK1/2 after stimulation with angiotensin II or epidermal growth factor [69], being involved both in G protein-coupled and tyrosine kinase receptor signalling [69]. Interestingly, GIT1 null mice show enhanced cardiomyocyte apoptosis and cardiac hypertrophy that progressed to heart failure due to impaired mitochondrial biogenesis and function [70]. However, the involvement of GIT1-dependent ERK1/2 signalling in the cardiac phenotype remains to be determined.
Dual-specificity tyrosine-phosphorylated and regulated kinase 1A (DYRK1A)
DYRK protein kinases phosphorylate serine and threonine residues on many substrates, as well as possessing autophosphorylation activity on tyrosine residues [71]. Mammalian DYRK1A is ubiquitously expressed in adult and foetal tissues with high expression in brain and heart during development. DYRK1A has been reported to function as a scaffold protein for Ras, B-Raf and MEK1, facilitating the formation of Ras–B-Raf–MEK1 multi-protein complexes, and prolonging the kinetics of ERK activation in PC12 neuronal cells in a cell-specific manner [33]. In addition, DYRK1A interacts with several transcription factors and its ability to phosphorylate NFAT has been reported [72, 73]. In cardiomyocytes DYRK1A acts as a negative regulator of the calcineurin–NFAT pathway by mediating NFAT nuclear export which leads to reduced pro-hypertrophic gene transcription [74]. Although DYRK1A has an important function in heart remodelling via the calcineurin–NFAT pathway [74–76], its potential role as an ERK1/2 scaffold molecule in cardiomyocytes has not yet been demonstrated.
Conclusions
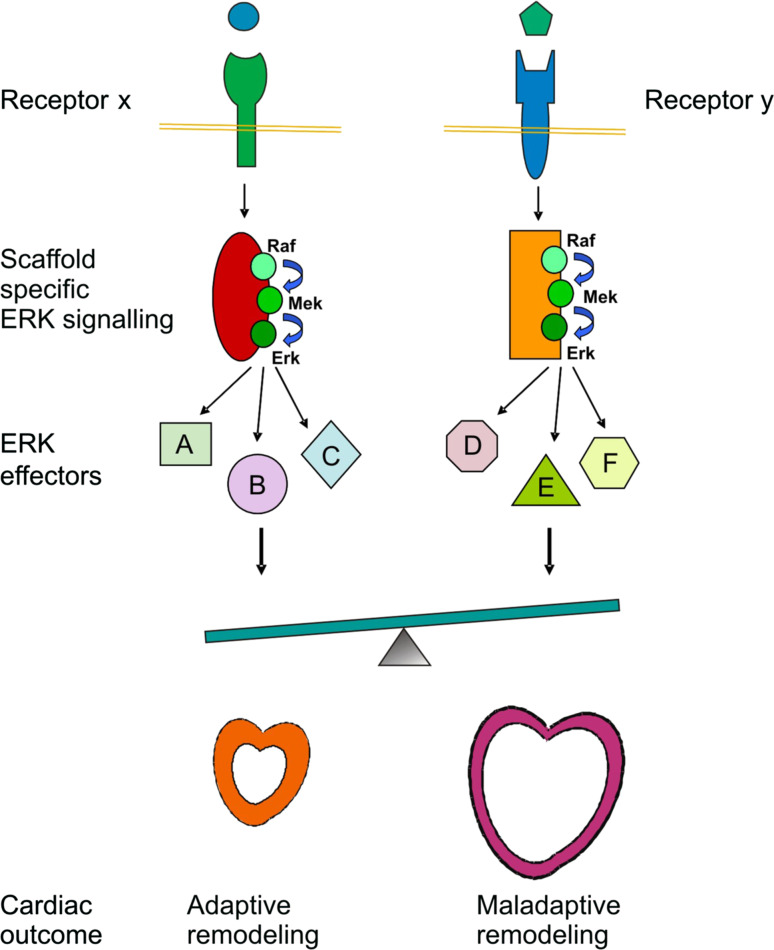
As discussed above, several scaffold proteins can organise ERK1/2 signalling in the heart in response to different stimuli leading to differential impacts on heart remodelling. This is likely due to scaffold protein-mediated spatial regulation of ERK1/2 activation that in turn determines substrate specificity [12]. Adaptive remodelling is mainly regulated by β-arrestins and IQGAP-1, while Gβγ and FHL1 determine a maladaptive heart response. However, in the stressed heart, multiple stimuli act in combination, resulting in the simultaneous activation of multiple ERK1/2 pathways via different scaffold proteins (Fig. 1). In addition, the same receptor can activate multiple ERK1/2 pathways via different scaffolds (Fig. 2). Thus, the balance among different pathways defines the ultimate outcome on cardiac remodelling. In this scenario, the general inhibition of ERK1/2 pathway as a potential therapeutic approach is undesirable, as it will impair both detrimental and beneficial signalling. Therefore, understanding how signalling specificity is achieved is an urgent challenge of molecular cardiology, for the development of therapeutic strategies capable of selectively interfering with the correct target. It is clear that the scaffold component involved in the activation of the signalling pathway is a crucial element for selectively determining either adaptive or maladaptive signalling. In principle, the downregulation of a specific scaffold can be helpful in reducing a particular maladaptive signal, as exemplified by FHL1 knockout mice [40]. However, scaffold molecules are often multifunctional proteins involved in different signalling pathways. Detailed knowledge of the scaffold/kinase signalosome complexes will enable the use of peptide sequences or other compounds to displace specific kinases from a specific scaffold molecule resulting in interruption of a particular signalling cascade. This strategy has been adopted in a number of cases for selective attenuation of a signalling pathway [77, 78], but needs to be translated in vivo in order to evaluate the impact on heart remodelling.
Fig. 1.
Multiple stimuli can act in combination via different receptors, resulting in the simultaneous activation of multiple ERK1/2 pathways via different scaffold proteins
Fig. 2.
The same receptor can activate multiple ERK1/2 pathways via different scaffold proteins
Acknowledgments
We wish to thank R. Srinivasan for manuscript revision. This work was supported by grants from the Regione Piemonte POR F.E.S.R.2007/2013 “DRUIDI: Piattaforme Innovative per le Scienze della Vita” to G.T. and M.B., Telethon GGP12047 to G.T., FIRB RBFR10L0GK to M.S., PRIN 2010J8RYS7 to M.B. and PRIN 2010RNXM9C to G.T.
Conflict of interest
None declared.
References
- 1.Selvetella G, Hirsch E, Notte A, Tarone G, Lembo G. Adaptive and maladaptive hypertrophic pathways: points of convergence and divergence. Cardiovasc Res. 2004;63(3):373–380. doi: 10.1016/j.cardiores.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 3.McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34(4):255–262. doi: 10.1111/j.1440-1681.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- 4.Sakata Y, Hoit BD, Liggett SB, Walsh RA, Dorn GW., 2nd Decompensation of pressure-overload hypertrophy in G alpha q-overexpressing mice. Circulation. 1998;97(15):1488–1495. doi: 10.1161/01.CIR.97.15.1488. [DOI] [PubMed] [Google Scholar]
- 5.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105(1):85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 6.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA. 2002;99(19):12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19(11):2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277(25):22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 9.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3 K-PTEN signaling pathways. Cell. 2002;110(6):737–749. doi: 10.1016/S0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 10.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90(4):1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: targeting Raf/MEK/ERK1/2-signaling. Int J Biochem Cell Biol. 2009;41(12):2351–2355. doi: 10.1016/j.biocel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Casar B, Pinto A, Crespo P. ERK dimers and scaffold proteins: unexpected partners for a forgotten (cytoplasmic) task. Cell Cycle. 2009;8(7):1007–1013. doi: 10.4161/cc.8.7.8078. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi O, Watanabe T, Nishida K, Kashiwase K, Higuchi Y, Takeda T, Hikoso S, Hirotani S, Asahi M, Taniike M, Nakai A, Tsujimoto I, Matsumura Y, Miyazaki J, Chien KR, Matsuzawa A, Sadamitsu C, Ichijo H, Baccarini M, Hori M, Otsu K. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J Clin Invest. 2004;114(7):937–943. doi: 10.1172/JCI20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110(6):718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 15.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19(23):6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci USA. 2007;104(35):14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter JJ, Tanaka N, Rockman HA, Ross J, Jr, Chien KR. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995;270(39):23173–23178. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 18.Zheng M, Dilly K, Dos Santos Cruz J, Li M, Gu Y, Ursitti JA, Chen J, Ross J, Jr, Chien KR, Lederer JW, Wang Y. Sarcoplasmic reticulum calcium defect in Ras-induced hypertrophic cardiomyopathy heart. Am J Physiol Heart Circ Physiol. 2004;286(1):H424–H433. doi: 10.1152/ajpheart.00110.2003. [DOI] [PubMed] [Google Scholar]
- 19.Kehat I, Molkentin JD. Extracellular signal-regulated kinase 1/2 (ERK1/2) signaling in cardiac hypertrophy. Ann NY Acad Sci. 2010;1188:96–102. doi: 10.1111/j.1749-6632.2009.05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoki Y, Niihori T, Narumi Y, Kure S, Matsubara Y. The RAS/MAPK syndromes: novel roles of the RAS pathway in human genetic disorders. Hum Mutat. 2008;29(8):992–1006. doi: 10.1002/humu.20748. [DOI] [PubMed] [Google Scholar]
- 21.Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, Bos JM, Ommen SR, Esposito G, Lepri F, Faul C, Mundel P, Lopez Siguero JP, Tenconi R, Selicorni A, Rossi C, Mazzanti L, Torrente I, Marino B, Digilio MC, Zampino G, Ackerman MJ, Dallapiccola B, Tartaglia M, Gelb BD. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39(8):1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Colbert M, Krenz M, Molkentin JD, Hahn HS, Dorn GW, 2nd, Robbins J. Mediating ERK 1/2 signaling rescues congenital heart defects in a mouse model of Noonan syndrome. J Clin Invest. 2007;117(8):2123–2132. doi: 10.1172/JCI30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bogoyevitch MA, Sugden PH. The role of protein kinases in adaptational growth of the heart. Int J Biochem Cell Biol. 1996;28(1):1–12. doi: 10.1016/1357-2725(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 24.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91(9):776–781. doi: 10.1161/01.RES.0000038488.38975.1A. [DOI] [PubMed] [Google Scholar]
- 25.Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115(3):527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Simpson J, Hong JH, Kim KH, Thavarajah NK, Backx PH, Neel BG, Araki T. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1 (L613 V) mutation. J Clin Invest. 2011;121(3):1009–1025. doi: 10.1172/JCI44929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muchir A, Pavlidis P, Decostre V, Herron AJ, Arimura T, Bonne G, Worman HJ. Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J Clin Invest. 2007;117(5):1282–1293. doi: 10.1172/JCI29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muchir A, Shan J, Bonne G, Lehnart SE, Worman HJ. Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum Mol Genet. 2009;18(2):241–247. doi: 10.1093/hmg/ddn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu W, Muchir A, Shan J, Bonne G, Worman HJ. Mitogen-activated protein kinase inhibitors improve heart function and prevent fibrosis in cardiomyopathy caused by mutation in lamin A/C gene. Circulation. 2011;123(1):53–61. doi: 10.1161/CIRCULATIONAHA.110.970673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15(1):75–83. doi: 10.1038/nm.1893. [DOI] [PubMed] [Google Scholar]
- 31.Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6(11):827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 32.Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26(22):3185–3202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- 33.Kelly PA, Rahmani Z. DYRK1A enhances the mitogen-activated protein kinase cascade in PC12 cells by forming a complex with Ras, B-Raf, and MEK1. Mol Biol Cell. 2005;16(8):3562–3573. doi: 10.1091/mbc.E04-12-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332(6030):680–686. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308(5721):512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 36.Tilley DG. G protein-dependent and G protein-independent signaling pathways and their impact on cardiac function. Circ Res. 2011;109(2):217–230. doi: 10.1161/CIRCRESAHA.110.231225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidal M, Wieland T, Lohse MJ, Lorenz K. Beta-adrenergic receptor stimulation causes cardiac hypertrophy via a Gbetagamma/Erk-dependent pathway. Cardiovasc Res. 2012 doi: 10.1093/cvr/cvs249. [DOI] [PubMed] [Google Scholar]
- 38.Esposito G, Prasad SV, Rapacciuolo A, Mao L, Koch WJ, Rockman HA. Cardiac overexpression of a G(q) inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH(2)-terminal kinase activity in in vivo pressure overload. Circulation. 2001;103(10):1453–1458. doi: 10.1161/01.CIR.103.10.1453. [DOI] [PubMed] [Google Scholar]
- 39.Belcheva MM, Coscia CJ. Diversity of G protein-coupled receptor signaling pathways to ERK/MAP kinase. Neurosignals. 2002;11(1):34–44. doi: 10.1159/000057320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA, Zheng M, Liang X, Zhang T, Yajima T, Gu Y, Dalton ND, Mahata SK, Dorn GW, 2nd, Heller-Brown J, Peterson KL, Omens JH, McCulloch AD, Chen J. An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest. 2008;118(12):3870–3880. doi: 10.1172/JCI34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel PA, Tilley DG, Rockman HA. Beta-arrestin-mediated signaling in the heart. Circ J. 2008;72(11):1725–1729. doi: 10.1253/circj.CJ-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9(5):373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noor N, Patel CB, Rockman HA. Beta-arrestin: a signaling molecule and potential therapeutic target for heart failure. J Mol Cell Cardiol. 2011;51(4):534–541. doi: 10.1016/j.yjmcc.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowling BS, Cottle DL, Wilding BR, D’Arcy CE, Mitchell CA, McGrath MJ. Four and a half LIM protein 1 gene mutations cause four distinct human myopathies: a comprehensive review of the clinical, histological and pathological features. Neuromuscul Disord. 2011;21(4):237–251. doi: 10.1016/j.nmd.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Lee SM, Tsui SK, Chan KK, Garcia-Barcelo M, Waye MM, Fung KP, Liew CC, Lee CY. Chromosomal mapping, tissue distribution and cDNA sequence of four-and-a-half LIM domain protein 1 (FHL1) Gene. 1998;216(1):163–170. doi: 10.1016/S0378-1119(98)00302-3. [DOI] [PubMed] [Google Scholar]
- 46.Friedrich FW, Wilding BR, Reischmann S, Crocini C, Lang P, Charron P, Muller OJ, McGrath MJ, Vollert I, Hansen A, Linke WA, Hengstenberg C, Bonne G, Morner S, Wichter T, Madeira H, Arbustini E, Eschenhagen T, Mitchell CA, Isnard R, Carrier L. Evidence for FHL1 as a novel disease gene for isolated hypertrophic cardiomyopathy. Hum Mol Genet. 2012;21(14):3237–3254. doi: 10.1093/hmg/dds157. [DOI] [PubMed] [Google Scholar]
- 47.Chu PH, Ruiz-Lozano P, Zhou Q, Cai C, Chen J. Expression patterns of FHL/SLIM family members suggest important functional roles in skeletal muscle and cardiovascular system. Mech Dev. 2000;95(1–2):259–265. doi: 10.1016/S0925-4773(00)00341-5. [DOI] [PubMed] [Google Scholar]
- 48.Gaussin V, Tomlinson JE, Depre C, Engelhardt S, Antos CL, Takagi G, Hein L, Topper JN, Liggett SB, Olson EN, Lohse MJ, Vatner SF, Vatner DE. Common genomic response in different mouse models of beta-adrenergic-induced cardiomyopathy. Circulation. 2003;108(23):2926–2933. doi: 10.1161/01.CIR.0000101922.18151.7B. [DOI] [PubMed] [Google Scholar]
- 49.Hwang DM, Dempsey AA, Wang RX, Rezvani M, Barrans JD, Dai KS, Wang HY, Ma H, Cukerman E, Liu YQ, Gu JR, Zhang JH, Tsui SK, Waye MM, Fung KP, Lee CY, Liew CC. A genome-based resource for molecular cardiovascular medicine: toward a compendium of cardiovascular genes. Circulation. 1997;96(12):4146–4203. doi: 10.1161/01.CIR.96.12.4146. [DOI] [PubMed] [Google Scholar]
- 50.Hwang DM, Dempsey AA, Lee CY, Liew CC. Identification of differentially expressed genes in cardiac hypertrophy by analysis of expressed sequence tags. Genomics. 2000;66(1):1–14. doi: 10.1006/geno.2000.6171. [DOI] [PubMed] [Google Scholar]
- 51.Lim DS, Roberts R, Marian AJ. Expression profiling of cardiac genes in human hypertrophic cardiomyopathy: insight into the pathogenesis of phenotypes. J Am Coll Cardiol. 2001;38(4):1175–1180. doi: 10.1016/S0735-1097(01)01509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raskin A, Lange S, Banares K, Lyon RC, Zieseniss A, Lee LK, Yamazaki KG, Granzier HL, Gregorio CC, McCulloch AD, Omens JH, Sheikh F. A novel mechanism involving four-and-a-half lim domain protein-1 and extracellular signal-regulated kinase-2 regulates titin phosphorylation and mechanics. J Biol Chem. 2012;287(35):29273–29284. doi: 10.1074/jbc.M112.372839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren JG, Li Z, Sacks DB. IQGAP1 modulates activation of B-Raf. Proc Natl Acad Sci USA. 2007;104(25):10465–10469. doi: 10.1073/pnas.0611308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy M, Li Z, Sacks DB. IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol Cell Biol. 2005;25(18):7940–7952. doi: 10.1128/MCB.25.18.7940-7952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sbroggio M, Carnevale D, Bertero A, Cifelli G, De Blasio E, Mascio G, Hirsch E, Bahou WF, Turco E, Silengo L, Brancaccio M, Lembo G, Tarone G. IQGAP1 regulates ERK1/2 and AKT signalling in the heart and sustains functional remodelling upon pressure overload. Cardiovasc Res. 2011;91(3):456–464. doi: 10.1093/cvr/cvr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen F, Zhu HH, Zhou LF, Wu SS, Wang J, Chen Z. IQGAP1 is overexpressed in hepatocellular carcinoma and promotes cell proliferation by Akt activation. Exp Mol Med. 2010;42(7):477–483. doi: 10.3858/emm.2010.42.7.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sbroggio M, Bertero A, Velasco S, Fusella F, De Blasio E, Bahou WF, Silengo L, Turco E, Brancaccio M, Tarone G. ERK1/2 activation in heart is controlled by melusin, focal adhesion kinase and the scaffold protein IQGAP1. J Cell Sci. 2011;124(Pt 20):3515–3524. doi: 10.1242/jcs.091140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferretti R, Sbroggio M, Di Savino A, Fusella F, Bertero A, Michowski W, Tarone G, Brancaccio M. Morgana and Melusin: Two fairies chaperoning signal transduction. Cell Cycle. 2011;10(21):3678–3683. doi: 10.4161/cc.10.21.18202. [DOI] [PubMed] [Google Scholar]
- 59.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11(7):515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 60.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 61.Muller J, Ory S, Copeland T, Piwnica-Worms H, Morrison DK. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol Cell. 2001;8(5):983–993. doi: 10.1016/S1097-2765(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 62.Matheny SA, Chen C, Kortum RL, Razidlo GL, Lewis RE, White MA. Ras regulates assembly of mitogenic signalling complexes through the effector protein IMP. Nature. 2004;427(6971):256–260. doi: 10.1038/nature02237. [DOI] [PubMed] [Google Scholar]
- 63.Cacace AM, Michaud NR, Therrien M, Mathes K, Copeland T, Rubin GM, Morrison DK. Identification of constitutive and ras-inducible phosphorylation sites of KSR: implications for 14–3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol Cell Biol. 1999;19(1):229–240. doi: 10.1128/mcb.19.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kortum RL, Johnson HJ, Costanzo DL, Volle DJ, Razidlo GL, Fusello AM, Shaw AS, Lewis RE. The molecular scaffold kinase suppressor of Ras 1 is a modifier of RasV12-induced and replicative senescence. Mol Cell Biol. 2006;26(6):2202–2214. doi: 10.1128/MCB.26.6.2202-2214.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kortum RL, Costanzo DL, Haferbier J, Schreiner SJ, Razidlo GL, Wu MH, Volle DJ, Mori T, Sakaue H, Chaika NV, Chaika OV, Lewis RE. The molecular scaffold kinase suppressor of Ras 1 (KSR1) regulates adipogenesis. Mol Cell Biol. 2005;25(17):7592–7604. doi: 10.1128/MCB.25.17.7592-7604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Li X, Carpinteiro A, Goettel JA, Soddemann M, Gulbins E. Kinase suppressor of Ras-1 protects against pulmonary Pseudomonas aeruginosa infections. Nat Med. 2011;17(3):341–346. doi: 10.1038/nm.2296. [DOI] [PubMed] [Google Scholar]
- 67.Vomastek T, Schaeffer HJ, Tarcsafalvi A, Smolkin ME, Bissonette EA, Weber MJ. Modular construction of a signaling scaffold: MORG1 interacts with components of the ERK cascade and links ERK signaling to specific agonists. Proc Natl Acad Sci USA. 2004;101(18):6981–6986. doi: 10.1073/pnas.0305894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ishibe S, Joly D, Liu ZX, Cantley LG. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell. 2004;16(2):257–267. doi: 10.1016/j.molcel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Yin G, Haendeler J, Yan C, Berk BC. GIT1 functions as a scaffold for MEK1-extracellular signal-regulated kinase 1 and 2 activation by angiotensin II and epidermal growth factor. Mol Cell Biol. 2004;24(2):875–885. doi: 10.1128/MCB.24.2.875-885.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pang J, Xu X, Getman MR, Shi X, Belmonte SL, Michaloski H, Mohan A, Blaxall BC, Berk BC. G protein coupled receptor kinase 2 interacting protein 1 (GIT1) is a novel regulator of mitochondrial biogenesis in heart. J Mol Cell Cardiol. 2011;51(5):769–776. doi: 10.1016/j.yjmcc.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Himpel S, Tegge W, Frank R, Leder S, Joost HG, Becker W. Specificity determinants of substrate recognition by the protein kinase DYRK1A. J Biol Chem. 2000;275(4):2431–2438. doi: 10.1074/jbc.275.4.2431. [DOI] [PubMed] [Google Scholar]
- 72.Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, Okamura H, Bolton D, Feske S, Hogan PG, Rao A. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441(7093):646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- 73.Arron JR, Winslow MM, Polleri A, Chang CP, Wu H, Gao X, Neilson JR, Chen L, Heit JJ, Kim SK, Yamasaki N, Miyakawa T, Francke U, Graef IA, Crabtree GR. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441(7093):595–600. doi: 10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- 74.Kuhn C, Frank D, Will R, Jaschinski C, Frauen R, Katus HA, Frey N. DYRK1A is a novel negative regulator of cardiomyocyte hypertrophy. J Biol Chem. 2009;284(25):17320–17327. doi: 10.1074/jbc.M109.006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.da Costa Martins PA, Salic K, Gladka MM, Armand AS, Leptidis S, el Azzouzi H, Hansen A, Coenen-de Roo CJ, Bierhuizen MF, van der Nagel R, van Kuik J, de Weger R, de Bruin A, Condorelli G, Arbones ML, Eschenhagen T, De Windt LJ. MicroRNA-199b targets the nuclear kinase DYRK1A in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol. 2010;12(12):1220–1227. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- 76.Grebe C, Klingebiel TM, Grau SP, Toischer K, Didie M, Jacobshagen C, Dullin C, Hasenfuss G, Seidler T. Enhanced expression of DYRK1A in cardiomyocytes inhibits acute NFAT activation but does not prevent hypertrophy in vivo. Cardiovasc Res. 2011;90(3):521–528. doi: 10.1093/cvr/cvr023. [DOI] [PubMed] [Google Scholar]
- 77.Calvo F, Agudo-Ibanez L, Crespo P. The Ras-ERK pathway: understanding site-specific signaling provides hope of new anti-tumor therapies. BioEssays. 2010;32(5):412–421. doi: 10.1002/bies.200900155. [DOI] [PubMed] [Google Scholar]
- 78.Matallanas D, Crespo P. New druggable targets in the Ras pathway? Curr Opin Mol Ther. 2010;12(6):674–683. [PubMed] [Google Scholar]