Abstract
Nitrogen-bisphosphonates (n-BP), such as zoledronate, are the main class of drugs used for the prevention of osteoporotic fractures and the management of cancer-associated bone disease. However, long-term or high-dose use has been associated with certain adverse drug effects, such as osteonecrosis of the jaw and the loss of peripheral of blood Vγ9Vδ2 T cells, which appear to be linked to drug-induced immune dysfunction. In this report we show that neutrophils present in human peripheral blood readily take up zoledronate, and this phenomenon is associated with the potent immune suppression of human peripheral blood Vγ9Vδ2 T cells. Furthermore, we found this zoledronate-mediated inhibition by neutrophils could be overcome to fully reconstitute Vγ9Vδ2 T cell proliferation by concomitantly targeting neutrophil-derived hydrogen peroxide, serine proteases, and arginase I activity. These findings will enable the development of targeted strategies to mitigate some of the adverse effects of n-BP treatment on immune homeostasis and to improve the success of immunotherapy trials based on harnessing the anticancer potential of peripheral blood γδ T cells in the context of n-BP treatment.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1495-x) contains supplementary material, which is available to authorized users.
Keywords: Nitrogen-bisphosphonates, Osteoporosis therapy, Adverse drug effects, γδ T cells, Cancer therapy
Introduction
Nitrogen-bisphosphonates (n-BP) are the most widely prescribed class of anti-bone resorptive agents used to treat conditions such as osteoporosis, Paget’s disease, hypercalcemia and malignant osteolytic bone-disease [36]. n-BP are analogues of pyrophosphates, which confers their ability to inhibit the activity of farnesyl pyrophosphate synthase (FPPS), a key enzyme in the mevalonate pathway for isoprenoid synthesis in eukaryotic cells, and it is this property that is believed to be the mechanism by which n-BP prevent bone resorption by osteoclasts [30]. The natural substrate of FPPS, isopentenyl pyrophosphate (IPP), is also the prototypical endogenous antigen recognized specifically by γδ T cells that bear the canonical Vγ9Vδ2 T cell receptor, a unique subset of innate T cells found only in the peripheral blood of humans and some other primates such as the rhesus monkey [34]. Vγ9Vδ2 T cells typically comprise between 1 and 10 % of the circulating T cell pool in healthy individuals in industrialized countries [9]. Unlike conventional αβ T cells, γδ T cells respond to stress-associated antigens that have no requirement for processing or presentation by classical MHC molecules [3, 8, 22].
We previously showed that there is convincing evidence that some of the unusual serious adverse effects of n-BP therapy, such as osteonecrosis of the jaw, may be linked to their effect on the immune system [10]. Patients with simple osteoporosis and no other serious malignancy being treated with n-BP were found to become significantly deficient in circulating Vγ9Vδ2 T cells, and this loss was particularly striking in those receiving the drug intravenously who were typically deficient within 18 months of continuous treatment [10]. This finding corroborates the lack of success of cancer immunotherapy trials that have attempted to harness the anticancer potential of Vγ9Vδ2 T cells with the bone-sparing effect of n-BP. In contrast to the robust response observed in vitro using purified peripheral blood mononuclear cells (PBMC), it has been disappointingly noted that repeated administration of zoledronate with interleukin-2 (IL-2) in cancer patients actually reduced the number of Vγ9Vδ2 T cells in vivo and impaired expansion in vitro after the first cycle of therapy [18]. In the present study, we reveal a surprising and previously unrecognized role of neutrophils as potent suppressors of Vγ9Vδ2 T cells following treatment with zoledronate, which is likely to be one of the main mechanisms leading to the stark loss of these cells observed in patients on n-BP treatment and may be a contributing source to n-BP-associated adverse effects.
Materials and methods
Study approval
The use of peripheral blood from healthy donors for in vitro analysis was approved by the appropriate local ethics committee (UKSH D 405/10).
Visualizing uptake of zoledronate by neutrophils in whole blood
Zoledronate (kind gift from Novartis Pharma AG, Basel, Switzerland) was conjugated to carboxyfluorescein (CFSE) using a linker strategy similar to that described by Kashemirov et al. [12] to fluorescently label heterocyclic n-BP while retaining their FPPS binding property, which we refer to as FluorZol. Whole blood collected in EDTA tubes was diluted 1:2 in complete medium and incubated with various concentrations of FluorZol. Cell cultures were subsequently subjected to red blood cell (RBC) lysis using BD FACS Lysing Solution (BD Biosciences, Heidelberg, Germany), stained for surface markers, fixed with 1 % PFA solution and analyzed by flow cytometry.
Isolation of leukocytes and cell culture conditions
Column-free magnetic separation using CD14 Dynabeads (Life Technologies GmbH, Darmstadt, Germany) was used to deplete monocytes. Flow cytometry was used to confirm success of depletion, which was routinely >98 %. RBC were lysed with 1× RBC lysis buffer (BioLegend, Fell, Germany), and leukocytes (1 × 106 cells/ml) were cultured in complete medium (RPMI 1640 medium, glutamine, 1 % penicillin/streptomycin and 10 % FCS; Gibco, Life Technologies, Invitrogen, Darmstadt, Germany) in 96-well round-bottom tissue culture plates. For experiments using only highly purified neutrophils (>99 % purity), the EasySep Human Neutrophil Enrichment Kit (STEMCELL Technologies, Grenoble, France) was used for negative selection following RBC lysis of whole blood. CD14 MACS Microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) were used to isolate monocytes from PBMC obtained following Ficoll-Hypaque (Biochrom AG, Berlin, Germany) density centrifugation.
2 μM zoledronate and 300 nM bromohydrin pyrophosphate (BrHPP; generous gift from Innate Pharma, Marseille, France) were used for stimulation assays; 50 IU of recombinant human IL-2 (Novartis, Basel, Switzerland) was added to all cultures. Lovastatin (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was used at 1 μM. Prolastin (α1-antitrypsin; Baxter Germany GmbH, Unterschleißheim, Germany) was used at 0.25 mg/ml. NG-Hydroxy-l-arginine (NOHA) monoacetate salt (Calbiochem®, Merck KGaA, Darmstadt, Germany) was used to block the activity of neutrophil arginase I and 4,000 Units/ml of catalase from human erythrocytes (Sigma-Aldrich) was used to inhibit neutrophil-derived hydrogen peroxide.
Flow cytometry analysis
The following fluorescently conjugated mouse anti-human antibodies were used for flow cytometry: anti-Vδ2-fluorescein isothiocyanate (FITC) (clone IMMU389, Beckman Coulter GmbH, Krefeld, Germany); anti-CD3-phycoerythrin (PE) (clone SK7); anti-CD14-FITC (clone MoP9), anti-CD25-PE (clone 2A3), anti-CD69-PE (clone L78), anti-IFN-γ-PE (clone 4S.B3) and isotype controls mouse IgG1-FITC (clone X40) and mouse IgG2a-PE (clone X39) were all from BD Biosciences (Heidelberg, Germany); anti-CD66b-FITC and -PE (clone G10F5) and anti-CD277-PE (clone BT3.1 PE) were purchased from BioLegend (Fell, Germany). Dead cells were gated out using LIVE/DEAD® Fixable Far Red Dead Cell Stain Kit (Invitrogen, Life Technologies GmbH, Darmstadt, Germany). Intracellular cytokine staining for interferon-gamma (IFNγ) was performed using BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit with BD Golgistop (BD Biosciences) according to the manufacturer’s directions. Golgistop was added for 4 h on day 4 of culture following the respective experimental treatments. Data were acquired on a FACSCalibur (BD Biosciences, Heidelberg, Germany) equipped with CellQuest Software (CellQuest, Tampa, Florida).
Assessment of BTN3A isoform expression in neutrophils and monocytes
Purified neutrophils and monocytes (1 × 106 cells each) were pelleted and resuspended in Prisure (Promolgene, Berlin, Germany) and stored at −80 °C for subsequent RNA extraction and cDNA synthesis. The various BTN3A isoforms were analyzed by real-time qPCR using a Rotor-Gene 3000 system (LTF Labortechnik, Wasserburg, Germany). SYBR green-based qPCR mix and primers for the housekeeping genes (β-actin, beta-2-microglobulin and 18S) were purchased from Promolgene. Two different sets of primers for each of the three isoforms of BTN3A (Supplementary Table 1) were designed using the Web-based primer3 software (http://primer3.wi.mit.edu/) and synthesized by TIB MOLBIOL (Berlin, Germany). PCR conditions: initial denaturation 10 min at 95 °C, followed by 40 cycles of denaturation (95 °C, 20 s) annealing (60 °C, 20 s) and elongation (72 °C, 20 s). Data analysis was performed according to the ΔCt-method 13 using the mean Ct value of three housekeeping genes. Fold changes of the expression levels of the analyzed genes were calculated as described previously [24] with the expression level in monocytes set as the control comparative group for neutrophils.
Detection of cytokines in cell culture supernatants
Cytokines in cell culture supernatants (collected and stored at −20 °C until time of the assay) were assessed using Luminex technology on a Bio-Plex 100 machine with Bio-Plex Manager Version 4.1: IL-2 (171-B5003M), IL-8 (171-B5008M), granulocyte macrophage colony-stimulating factor (GM-CSF) (171-B5018M), IFNγ (171-B5019M) and tumor necrosis factor-alpha (TNFα) (171-B5026M). Curve-fitting analysis was done using Bio-Plex Manager software, version 5.0. Instrument and reagents were from Bio-Rad Laboratories GmbH (Munich, Germany).
Detection of hydrogen peroxide/peroxidase in cell culture supernatants
The Oxiselect Hydrogen Peroxide/Peroxidase Assay Kit (Cell Biolabs Inc, BIOCAT, GmbH, Heidelberg, Germany) was used to determine the levels of hydrogen peroxide in cell culture supernatants.
Statistical analysis
Differences among treatment conditions and leukocyte populations were assessed by two-way ANOVA followed by Bonferroni multiple comparisons test. One-way repeated-measures ANOVA followed by Tukey’s multiple comparison test was used to assess the contribution of each treatment condition alone and together in experiments using the different inhibitors of neutrophil-derived factors that influenced γδ T cell expansion. All tests were two-tailed with α set at 0.05. Graphical presentation and statistical analysis of the data were performed using GraphPad Prism v5.03 (GraphPad Software, La Jolla, CA, USA).
Results
Neutrophils, like monocytes, readily take up zoledronate
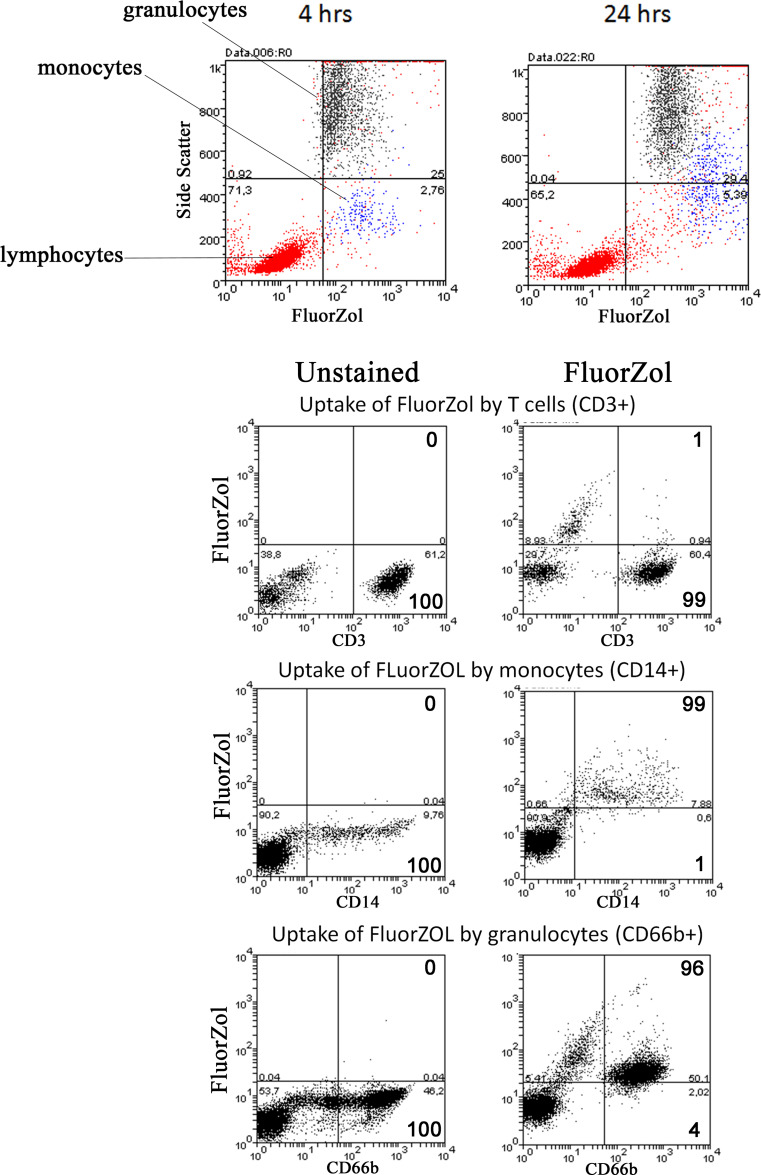
To track the uptake of n-BP by all peripheral blood leukocytes, we fluorescently labeled zoledronate (FluorZol) in a manner similar to that used to label risedronate (a heterocyclic n-BP like zoledronate), which retains its ability to block FPPS activity [12]. As previously noted [29], monocytes were able to take up the fluorescently labeled n-BP; whereas, lymphocytes, in particular T cells, were relatively poor at ingesting the molecule (Fig. 1). Unlike previous studies that used primarily only PBMC fractions for their studies, we additionally observed that granulocytes (of which neutrophils make up the majority) efficiently ingested FluorZol as well (Fig. 1).
Fig. 1.
Neutrophils in whole blood take up CFSE-labeled zoledronate (FluorZol). Flow cytometry analysis demonstrated that neutrophils (black) and monocytes (blue), but not lymphocytes (red) take up FluorZol (x-axis) shown for 4 and 24 h following treatment (top panel, representative of n = 10). Bottom three panels show the FluorZol-treated and untreated cells using specific markers for T cells (CD3), monocytes (CD14), and neutrophils (CD66b) that have taken up the molecule following 6 h of incubation
The presence of neutrophils in leukocyte cultures treated with zoledronate appears to inhibit Vγ9Vδ2 T cell proliferation
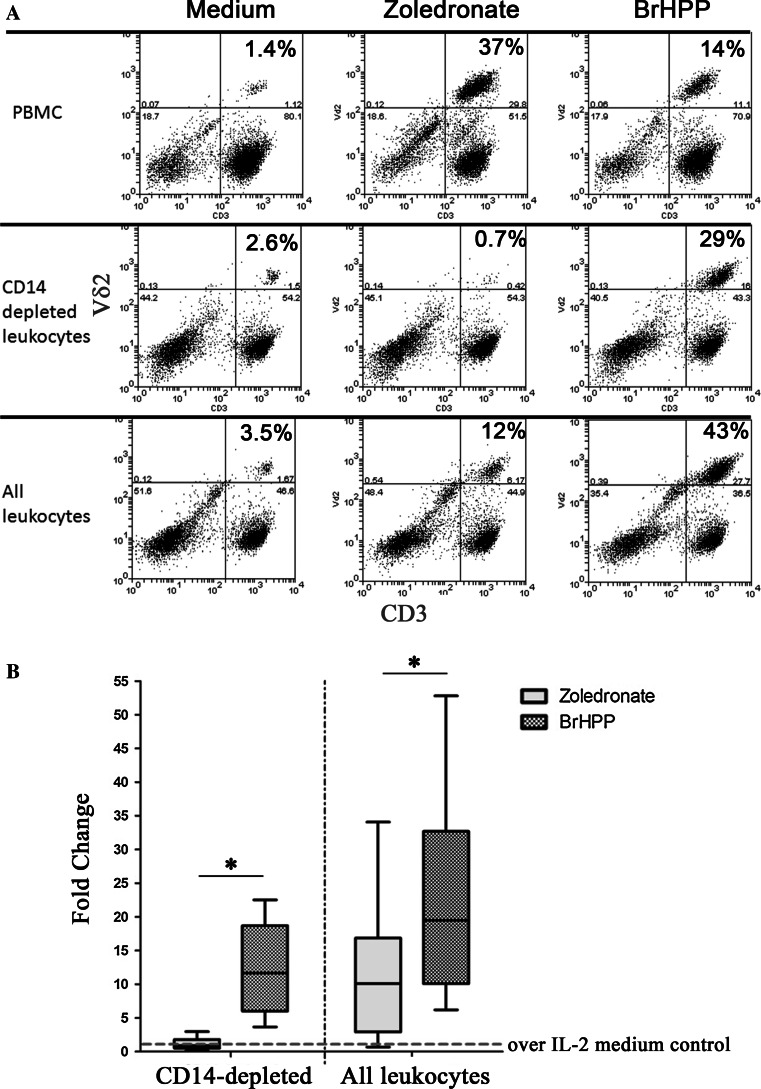
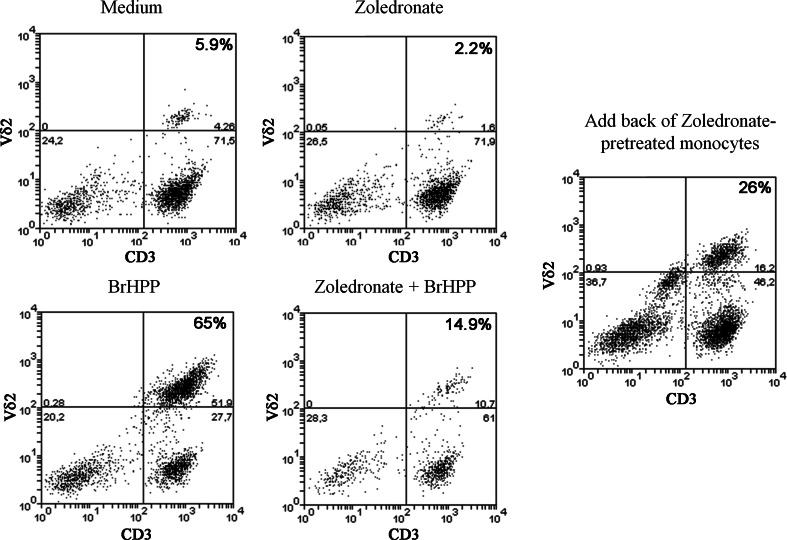
Zoledronate, and other n-BP, potently induces Vγ9Vδ2 T cell expansion in the presence of IL-2 using monocyte-derived cells as antigen-presenting cells or PBMC that are devoid of neutrophils [14, 21, 29]. The efficacy of Vγ9Vδ2 T cell expansion is at least equal to, if not greater than, their synthetic phospho-antigen, BrHPP, when γδ T cells are in the presence of monocytes treated with zoledronate but in the absence of neutrophils, as shown in the upper panel of Fig. 2a. To assess the outcome of the converse situation in which peripheral blood γδ T cells are in the presence of neutrophils treated with zoledronate in the absence of monocytes, we depleted leukocytes of monocytic cells using an antibody against their cell surface receptor, CD14. γδ T cells in CD14-depleted leukocytes were completely inhibited from proliferating following treatment with zoledronate, but they were still able to respond normally to BrHPP (Fig. 2a, middle panel; b). Zoledronate-mediated γδ T cell expansion was also significantly impaired relative to BrHPP when using all leukocytes that contained both neutrophils and monocytes present together in the same proportion as peripheral blood (Fig. 2a, lower panel; b). The observed inhibition by the presence of neutrophils was not due to zoledronate-induced neutrophil cell-death as the viability and proportion of neutrophils in cultures treated with zoledronate was similar in all treatment groups.
Fig. 2.
The presence of neutrophils is inhibitory to zoledronate-mediated expansion of Vδ2 T cells. a Flow cytometry plots showing the comparative expansion of Vδ2 T cells in PBMC (upper panel), monocyte (CD14)-depleted peripheral blood leukocytes (middle panel), and all leukocytes (lower panel) upon treatment with 2 μM of zoledronate (middle column) or 300 nM of BrHPP (right column) over 7 days. Percentage given in the top right box corresponds to the proportion of Vγ9Vδ2 T cells of the total T cell pool present under each experimental condition. b Box-plot analysis of Vδ2 T cell expansion (expressed as fold change over IL-2-containing medium) with 2 μM of zoledronate or 300 nM BrHPP in leukocytes either in the absence of monocytes (CD14-depleted, left panel) or their presence (right panel). There was a significant effect of both treatment, F(2,54) = 23.6, p < 0.0001, and leukocyte population, F(1,54) = 11.3, p = 0.0014, in influencing the expansion of Vγ9Vδ2 T cells (two-way ANOVA). Bonferroni multiple comparisons test verified that with the presence of neutrophils in leukocytes, zoledronate was significantly less able to induce Vγ9Vδ2 T cell expansion in comparison to BrHPP treatment (* p < 0.05). Whiskers represent the minimum and maximum responses; n = 10
Despite the observed inhibition on expansion in the presence of neutrophils that have taken up zoledronate, γδ T cells show signs of activation
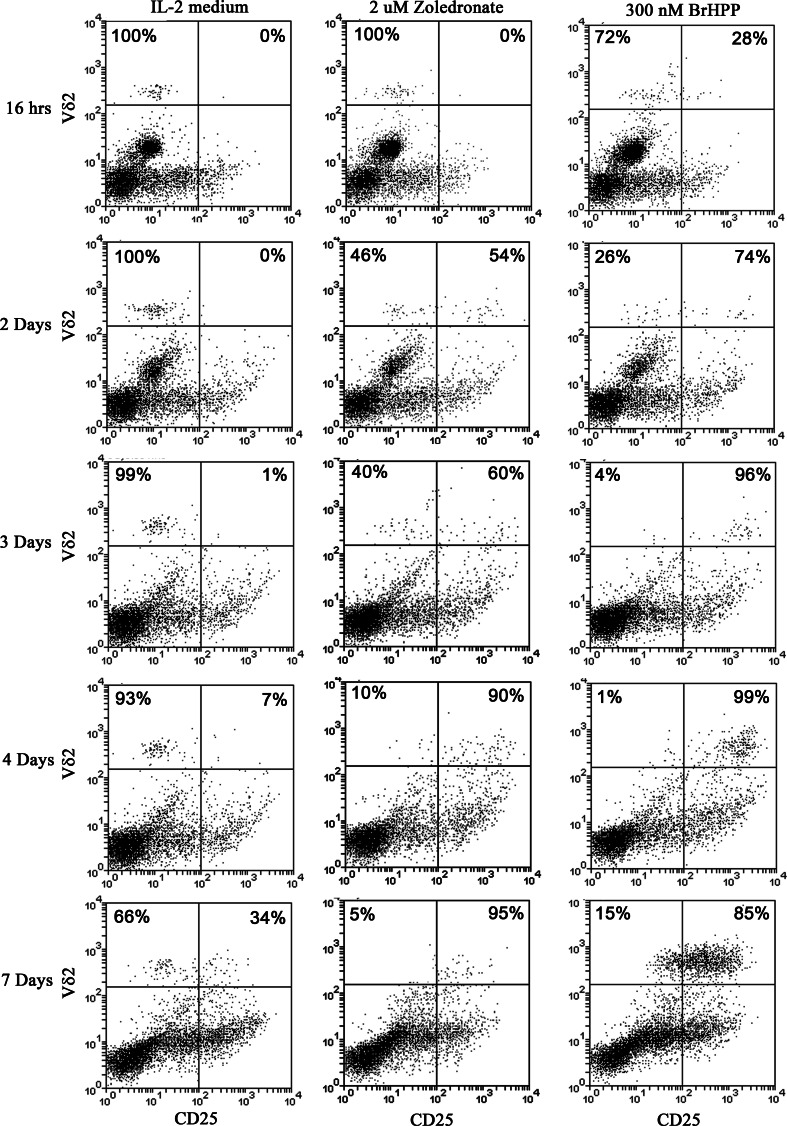
We assessed the up-regulation of CD25, the IL-2 receptor α-chain and T cell activation marker, over a 7-day time course to determine whether the observed failure to expand in response to neutrophils that have taken up zoledronate could be due to a lack of activation. Figure 3 shows that Vγ9Vδ2 T cells in CD14-depleted leukocytes start expressing CD25 by day 2 following treatment with zoledronate, and, similar to BrHPP treatment, the majority are CD25 positive by day 4 of culture. However, in contrast to the clear expansion of γδ T cells by day 7 in BrHPP-treated cells, there was a consistent failure of Vγ9Vδ2 T cells to similarly expand in response to zoledronate in the presence of neutrophils and absence of monocytes (Fig. 3), and in more than half the donors tested (n = 14) there were actually fewer γδ T cells than baseline (median proportion relative to baseline = 0.9, range = 0.09–3.0) under this experimental condition. Both the up-regulation of CD25 and the loss of Vγ9Vδ2 T cells could be largely prevented by the addition of 1 μM of lovastatin (Supplementary Fig. 1), which blocks the mevalonate pathway upstream of IPP synthesis, suggesting that the uptake of zoledronate by neutrophils activate γδ T cells through the accumulation of IPP, and this same event may also be linked to their observed loss.
Fig. 3.
CD25 is up-regulated on Vγ9Vδ2 T cells in the absence of monocytes but presence of neutrophils treated with zoledronate, but they fail to proliferate. Time-course analyses of CD25 expression (y-axis) over a period of 7 days in CD14-depleted leukocytes cultured in IL-2 medium (left panel), IL-2 medium with 2 μM of zoledroate (middle panel), or IL-2 medium with 300 nM of BrHPP (right panel) revealed that CD25 expression on Vδ2 T cells (x-axis) was initiated by day 2 following stimulation with zoledronate, similar to BrHPP-treated cells, and the majority were CD25 positive by day 4. However, where a notable expansion of Vδ2 T cells could be observed in the BrHPP-treated cells by day 7, there was a clear lack of expansion in those cultures treated with zoledronate and, instead, fewer Vδ2 T cells were remaining. Percentages given in the top quadrants are the proportion of γδ T cells gated to be either CD25-positive (upper right quadrant) or CD25-negative (upper left quadrant)
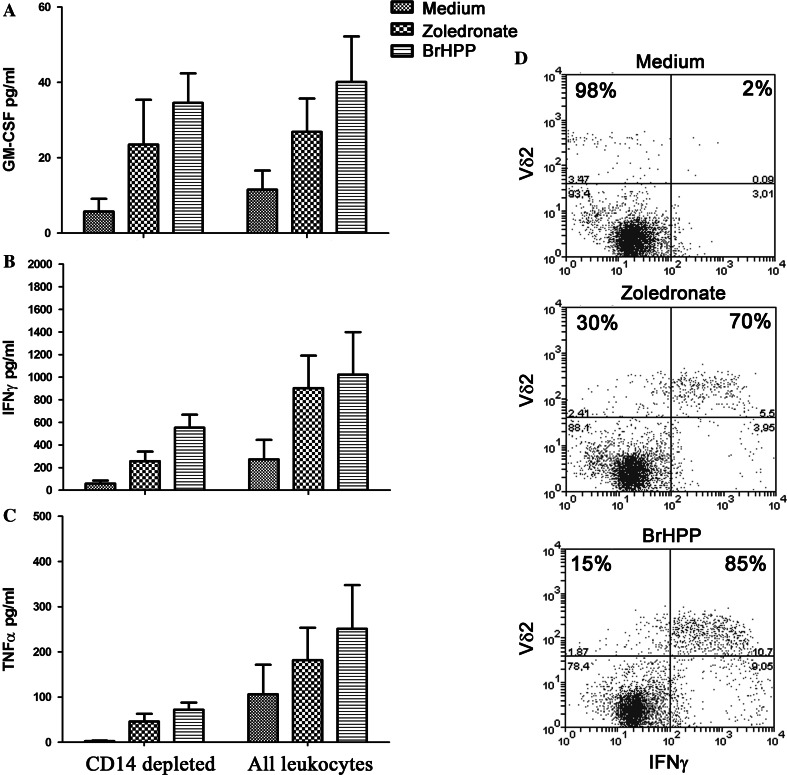
In addition to the induction of CD25 on γδ T cells, CD69 was also up-regulated on γδ T cells in the presence of neutrophils following treatment with either zoledronate or BrHPP, which was similar to what was observed in their absence using PBMC (Supplementary Fig. 2), and IFNγ and GM-CSF were detectable in supernatants of zoledronate and BrHPP-treated cultures in the presence of neutrophils (Fig. 4). Intracellular cytokine staining by flow cytometry confirmed that the majority of Vγ9Vδ2 T cells produced IFNγ in response to these treatments and were the major source of this cytokine (Fig. 4d). However, CD14-depletion lowered the overall baseline levels of IFNγ and significantly decreased the levels of TNFα (two-way ANOVA, F(1,18) = 9.10, p = 0.0074). The potent inhibition of γδ T cell proliferation was not due to IL-2 degradation as all culture supernatants still had similarly high levels. Purified neutrophils treated with zoledronate, however, did not produce detectable levels of IL-8 or TNFα.
Fig. 4.
Despite potent inhibition on γδ T cell proliferation by neutrophils that had taken up zoledronate, cytokines could still be detected in culture supernatants. a GM-CSF and b IFNγ were elevated in response to zoledronate and BrHPP in both CD14-depleted and undepleted leukocyte cultures; two-way ANOVA indicated that there was no significant difference in the magnitude of the response between the two groups over untreated cells. There was a significant difference (two-way ANOVA, F(1,18) = 9.10, p = 0.0074) in the levels of (c) TNFα. The removal of monocytes significantly lowered the overall TNFα levels under all treatment conditions and accounted for 29.2 % of the variance between the treatment groups. Error bars denote SD; n = 4. d Intracellular cytokine staining for IFNγ on day 4 following treatment confirmed that γδ T cells were a major source of this cytokine (percentages in the two top quadrants correspond to the proportion of γδ T cells that were either negative, left, or positive, right, for IFNγ)
Neutrophils express BTN3A1, a molecule that plays a role in activating Vγ9Vδ2 T cells in response to phospho-antigens
BTN3A (also referred to as CD277) facilitates activation of γδ T cells in response to phospho-antigens, such as IPP [7]. Of the three human isoforms of BTN3A, BTN3A1 is the primary one that fulfills this stimulatory function for γδ T cells [7]. The inability of neutrophils to support γδ T cell proliferation in response to zoledronate was not attributable to the lack of expression of this molecule as both their surface expression of CD277 and relative gene expression of BTN3A1 was at least equal to, if not greater than, monocytes (Supplementary Fig. 3).
Neutrophil-mediated inhibition of γδ T cell expansion in leukocytes treated with zoledronate could be overcome by blocking neutrophil-derived hydrogen peroxide, serine proteases, and arginase I activity
Given that γδ T cells were activated in the presence of neutrophils that had taken up zoledronate, there was access to sufficient IL-2 for T cell proliferation (which is required for optimal activation of γδ T cells by their phospho-antigens) but yet these innate T cells were potently inhibited from proliferating and their numbers were reduced when in the presence of neutrophils that had taken up zoledronate (particularly evident in the absence of monocytes) - we hypothesized that the uptake of zoledronate by neutrophils may be inducing the release of specific T-cell suppressive factors. We tested this hypothesis by adding zoledronate to CD14-depleted leukocytes treated with BrHPP; Fig. 5 shows that the addition of zoledronate completely suppressed the ability of Vγ9Vδ2 T cells to expand normally to BrHPP. Importantly, when we took freshly purified monocytes, incubated them with zoledronate, subsequently washed and added them back to these CD14-depleted leukocytes—γδ T cells were able to expand in the presence of neutrophils that had not taken up the zoledronate themselves (Fig. 5, right panel). Similarly, when neutrophils were added back to PBMC, they reduced the response to zoledronate specifically, and not to BrHPP; preincubation of these neutrophils with lovastatin partially reduced this inhibition (Supplementary Fig. 4). However, prior exposure of neutrophils to n-BP, subsequent washing, and adding them back to PBMC cultures, did not influence the expansion of γδ T cells in response to BrHPP nor did it further reduce their expansion to zoledronate in comparison to neutrophils that had not been previously exposed to n-BP (Supplementary Fig. 4), suggesting such handling removed or diluted most of the suppressive factors that they may be producing upon zoledronate uptake.
Fig. 5.
The uptake of zoledronate by neutrophils results in the suppression of Vγ9Vδ2 T cells to proliferate normally in response to BrHPP. CD14-depleted leukocytes were cultured over 7 days in medium control, zoledronate, BrHPP, or BrHPP+zoledronate. In the absence of monocytes, the clear inhibition by neutrophils by zoledronate treatment was made clear by the potent suppression of their response to BrHPP when combined with zoledronate. When purified monocytes were treated with zoledronate, washed, and subsequently added back to these CD14-depleted leukocyte cultures, γδ T cells were able to expand in the presence of neutrophils that had not taken up zoledronate themselves (far right panel). Percentages in the top right quadrant represent the proportion of Vδ2 T cells of the total T cell population
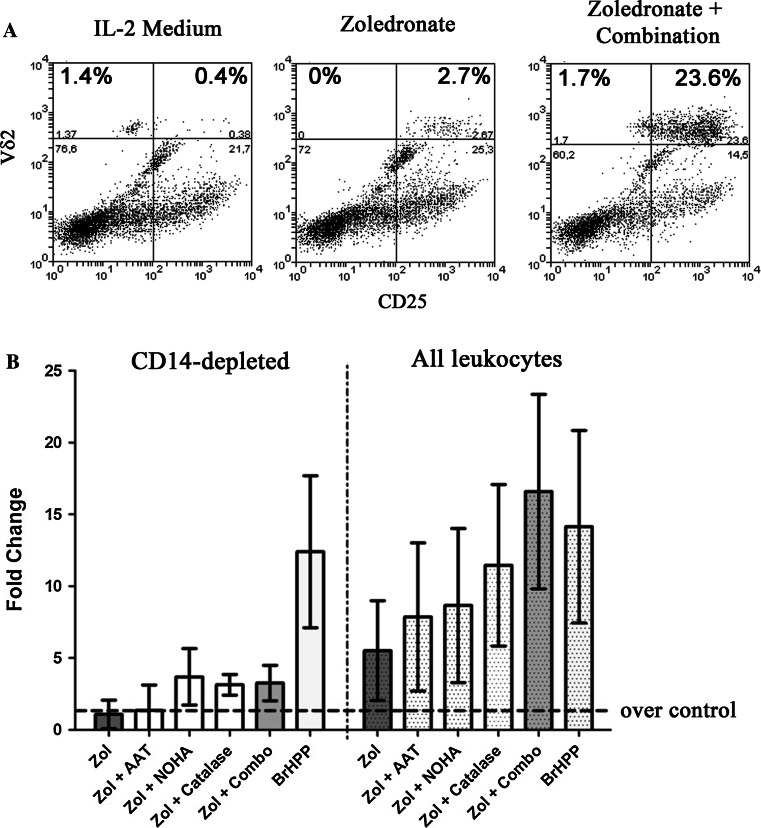
In an attempt to uncover the potential mechanism(s) contributing to this observed neutrophil-mediated inhibition following treatment with zoledronate, we used targeted strategies to systematically block individual neutrophilic factors. This approach revealed the contribution of three specific neutrophil derivatives—serine proteases [15, 26], arginase I [23, 31, 33], and hydrogen peroxide (H2O2) [5, 6, 25, 37] - to the observed γδ T cell inhibition. Figure 6 shows that when we concomitantly neutralized all three of these neutrophil-derived factors using 0.25 mg/ml of α-1 antitrypsin (AAT) to inhibit serine proteases, 500 μM NOHA to specifically block arginase I activity, and 4,000 Units/ml of catalase to decompose H2O2, we were able to completely reverse the immunosuppressive effects of neutrophils on γδ T cell expansion and support their survival following treatment with zoledronate—restoring it to levels equal to or greater than BrHPP in their presence. However, the presence of monocytes was required for their full proliferative potential. Catalase was the single most potent agent used to reverse the immune suppression, and in half the donors, catalase plus AAT performed similar to the complete combination of inhibitors of neutrophil function. However, we could not detect a difference in the overall oxidative environment when we measured H2O2 in culture supernatants despite the strong restorative effect of catalase for γδ T cell proliferation in the presence of neutrophils treated with zoledronate (not shown).
Fig. 6.
Reversal of the suppressive influence of neutrophils on γδ T cell expansion by zoledronate. a Complete reconstitution of Vγ9Vδ2 T cell expansion was achieved in peripheral blood leukocytes treated with 2 μM of zoledronate with the simultaneous addition of catalase, α1-antitrypsin (AAT) and the specific arginase I inhibitor, NOHA (combination). b This combination performed at least as well as 300 nM BrHPP for the expansion of Vγ9Vδ2 T cells. One-way repeated measures ANOVA, F(6,3) = 12.74, p < 0.0001, followed by a Tukey’s multiple comparisons test showed the addition of the complete combination of inhibitors with zoledronate more efficiently induced expansion of Vγ9Vδ2 T cells in comparison to zoledronate alone (p < 0.001), zoledronate+AAT (p < 0.01), zoledronate+NOHA (p < 0.5). However, monocytes were needed for the full expansion of γδ T cells, as these treatments failed to restore complete proliferative potential in their absence (left panel). n = 4
Discussion
Nitrogen-bisphosphonates first came into clinical use shortly prior to 1990, and they are now the leading treatment of choice for a wide array of disorders of bone fragility—in particular post-menopausal osteoporosis and the management of cancer-associated bone disease [32]. The discovery of the serendipitous ability of n-BP to activate human peripheral blood γδ T cells came almost a decade after approval of their clinical application. The revelation was made when determining the cause of the acute-phase response experienced by some patients initiating intravenous administration of n-BP [17], and it has led to great enthusiasm for the immunotherapeutic potential of the seemingly perfect marriage of n-BP and the mobilization of the cytolytic effector functions of γδ T cells for the treatment of diseases such as cancer and HIV [1, 2, 13, 19, 20, 28]. Many of the clinical trials that have been initiated towards harnessing this synergistic potential relied on in vitro studies using density gradient-separated PBMC treated with n-BP, such as zoledronate, to assess feasibility. These studies convincingly demonstrated the potent ability of n-BP to expand and activate human peripheral blood Vγ9Vδ2 T cells for targeted immunotherapy. However, the in vivo application of this approach has met with little success. The disappointing results of the cancer immunotherapy trials to date have largely been attributed to the immunosuppressive environment within the cancer patients themselves [11]. However, we recently observed that patients on n-BP therapy for simple osteoporosis who had no underlying malignancy also become deficient in peripheral Vγ9Vδ2 T cells—especially those receiving intravenous administration [10].
Roelofs et al. [29] had demonstrated that in PBMC, monocytes are the primary cell type that take up zoledronate and activate γδ T cells through the production of IPP, and lymphocytes seem to be generally incapable of fulfilling this function. By including all peripheral blood leukocytes in our assays and not just PBMC, we found that neutrophils also readily ingest n-BP. Relatively little is currently known about the immuno-regulatory influence of neutrophils on human γδ T cells; therefore, the strikingly different consequences of n-BP uptake by monocytes and neutrophils, respectively, on γδ T cells and immune function were unexpected. Where monocytes strongly support Vγ9Vδ2 T cell expansion and survival following treatment with zoledronate, neutrophils are surprisingly effective in preventing γδ T cells from proliferating despite being highly activated and appear to promote their loss—a phenomenon that has not been described previously. We were able to completely reverse this specific suppression by concomitantly counter-acting neutrophil-derived hydrogen peroxide, arginase I activity and serine proteases following n-BP treatment. Intriguingly, neutrophils alone were unable to provide the requisite support γδ T cells required to meet their full proliferative capacity in response to zoledronate—even after neutralizing the effect of all the immune-modulatory factors that were found to contribute to the observed inhibition; this was achieved only with the help of monocytes. A similar observation was made by Davey et al. [4] who found that neutrophils that had phagocytosed bacteria released the metabolite (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate, a highly potent microbial phospho-antigen for Vγ9Vδ2 T cells, but their full activation required cell-to-cell contact with monocytes. This monocyte-associated factor is unlikely to be CD277 (BTN3A), the molecule recently determined to play a pivotal role in activating γδ T cells in response to endogenous IPP [7], as we verified that the expression of the relevant isotype of BTN3A was relatively high in neutrophils. Our findings support the recent observation that monocytes in particular are central for the activation of Vγ9Vδ2 T cells and the promotion of the acute phase response following treatment with zoledronate [35].
In addition to the deleterious consequence on peripheral blood γδ T cells, the effect of zoledronate on neutrophils is likely to have wider implications on immune homeostasis. A recent murine study found that treatment with either pamidronate or zoledronate led to impaired neutrophil chemotaxis and increased neutrophil NADPH oxidase activity, which suggested that prolonged n-BP use could lead to a depressed immune system [16]. This is congruent with our observation that neutrophils that have taken up zoledronate fail to secrete IL-8 or TNFα, but instead locally release hydrogen peroxide, a downstream metabolite of the superoxide anion produced by NADPH oxidase. The inhibition of γδ T cells by neutrophil H2O2 appears to be a confined phenomenon as we did not detect an overall reduced oxidative environment. This is in line with the findings of Pillay and colleagues who showed that neutrophils suppressed T cell proliferation during systemic inflammation by delivering H2O2 through local synapses, and increased levels of H2O2 could not be detected in the overall culture medium despite the efficacy of catalase in partially alleviating the inhibition in their system [27]. The accumulation of IPP by neutrophils that have taken up zoledronate would specifically lure Vγ9Vδ2 T cells to these local synaptic junctions as the deleterious effect appears to be specific for this subset of T cells. There were no observable changes in the proportion of other T cells either in vitro or in vivo in our previous clinical study investigating the immune effects of n-BP therapy [10]. Furthermore, we show treatment with a statin, which would inhibit IPP production by neutrophils, largely prevented the zoledronate-mediate loss of γδ T cells—supporting the notion that these innate T cells require to be drawn into a close interaction with neutrophils to be subjected to oxidative damage.
Collectively, we anticipate this new insight on the consequence of zoledronate uptake by neutrophils and the resulting suppression of γδ T cells will provide new and improved strategies for the successful implementation of the therapeutic potential of n-BP and provide a means to mitigate or reduce some of the adverse effects of n-BP treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
SK was supported by a Fellowship from the Alexander von Humboldt Foundation of Germany and a Faculty of Medicine Grant from Christian-Albrechts University of Kiel. DK acknowledges grant support from the Deutsche Forschungsgemeinschaft (Ka 502/10-2 and “Inflammation-at-Interfaces” Cluster of Excellence). We would like to thank Hilke Clasen (Department of Immunology) for technical assistance, and Dr. Millan Patel (University of British Columbia) for review of the manuscript.
Conflict of interest
All authors state that they have no conflicts of interest.
Contributor Information
Shirin Kalyan, Phone: +49-431-5973380, FAX: +49-431-5973335, Email: kalyan@immunologie.uni-kiel.de.
Dieter Kabelitz, Email: kabelitz@immunologie.uni-kiel.de.
References
- 1.Bouet-Toussaint F, Cabillic F, Toutirais O, Le Gallo M, Thomas de la Pintiere C, Daniel P, Genetet N, Meunier B, Dupont-Bierre E, Boudjema K, Catros V. Vgamma9Vdelta2 T cell-mediated recognition of human solid tumors. Potential for immunotherapy of hepatocellular and colorectal carcinomas. Cancer Immunol Immunother. 2008;57:531–539. doi: 10.1007/s00262-007-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabillic F, Toutirais O, Lavoue V, de La Pintiere CT, Daniel P, Rioux-Leclerc N, Turlin B, Monkkonen H, Monkkonen J, Boudjema K, Catros V, Bouet-Toussaint F. Aminobisphosphonate-pretreated dendritic cells trigger successful Vgamma9Vdelta2 T cell amplification for immunotherapy in advanced cancer patients. Cancer Immunol Immunother. 2010;59:1611–1619. doi: 10.1007/s00262-010-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 4.Davey MS, Lin CY, Roberts GW, Heuston S, Brown AC, Chess JA, Toleman MA, Gahan CG, Hill C, Parish T, Williams JD, Davies SJ, Johnson DW, Topley N, Moser B, Eberl M. Human neutrophil clearance of bacterial pathogens triggers anti-microbial gammadelta T cell responses in early infection. PLoS Pathog. 2011;7:e1002040. doi: 10.1371/journal.ppat.1002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139:2406–2413. [PubMed] [Google Scholar]
- 6.el-Hag A, Lipsky PE, Bennett M, Clark RA. Immunomodulation by neutrophil myeloperoxidase and hydrogen peroxide: differential susceptibility of human lymphocyte functions. J Immunol. 1986;136:3420–3426. [PubMed] [Google Scholar]
- 7.Harly C, Guillaume Y, Nedellec S, Peigne CM, Monkkonen H, Monkkonen J, Li J, Kuball J, Adams EJ, Netzer S, Dechanet-Merville J, Leger A, Herrmann T, Breathnach R, Olive D, Bonneville M, Scotet E. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T cell subset. Blood. 2012;120:2269–2279. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabelitz D. Small molecules for the activation of human gammadelta T cell responses against infection. Recent Pat Anti-Infect Drug Discov. 2008;3:1–9. doi: 10.2174/157489108783413218. [DOI] [PubMed] [Google Scholar]
- 9.Kalyan S, Kabelitz D. Defining the nature of human gammadelta T cells: a biographical sketch of the highly empathetic. Cell Mol Immunol. 2013;10:21–29. doi: 10.1038/cmi.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalyan S, Quabius ES, Wiltfang J, Monig H, Kabelitz D. Can peripheral blood gammadelta T cells predict osteonecrosis of the jaw? An immunological perspective on the adverse drug-effects of aminobisphosphonate therapy. J Bone Miner Res. 2013;28:728–735. doi: 10.1002/jbmr.1769. [DOI] [PubMed] [Google Scholar]
- 11.Kalyan S, Wesch D, Kabelitz D. Aminobisphosphonates and Toll-like receptor ligands: recruiting Vgamma9Vdelta2 T cells for the treatment of hematologic malignancy. Curr Med Chem. 2011;18:5206–5216. doi: 10.2174/092986711798184280. [DOI] [PubMed] [Google Scholar]
- 12.Kashemirov BA, Bala JL, Chen X, Ebetino FH, Xia Z, Russell RG, Coxon FP, Roelofs AJ, Rogers MJ, McKenna CE. Fluorescently labeled risedronate and related analogues: “magic linker” synthesis. Bioconjugate Chem. 2008;19:2308–2310. doi: 10.1021/bc800369c. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Tanaka Y, Yagi J, Minato N, Tanabe K. Phase I/II study of adoptive transfer of gammadelta T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1075–1084. doi: 10.1007/s00262-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K, Sato S, Sato K, Hosoi A, Nakajima J, Yoshida Y, Shiraishi K, Nakagawa K, Kakimi K. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10:842–856. doi: 10.1080/14653240802419328. [DOI] [PubMed] [Google Scholar]
- 15.Korkmaz B, Horwitz MS, Jenne DE, Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev. 2010;62:726–759. doi: 10.1124/pr.110.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiper JW, Forster C, Sun C, Peel S, Glogauer M. Zoledronate and pamidronate depress neutrophil functions and survival in mice. Br J Pharmacol. 2012;165:532–539. doi: 10.1111/j.1476-5381.2011.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 18.Lang JM, Kaikobad MR, Wallace M, Staab MJ, Horvath DL, Wilding G, Liu G, Eickhoff JC, McNeel DG, Malkovsky M. Pilot trial of interleukin-2 and zoledronic acid to augment gammadelta T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1447–1460. doi: 10.1007/s00262-011-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother. 2007;56:1285–1297. doi: 10.1007/s00262-007-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, La Mendola C, Guggino G, D’Asaro M, Orlando V, Scarpa F, Roberts A, Caccamo N, Stassi G, Dieli F, Hayday AC. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyagawa F, Tanaka Y, Yamashita S, Minato N. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human gamma delta T cells by aminobisphosphonate antigen. J Immunol. 2001;166:5508–5514. doi: 10.4049/jimmunol.166.9.5508. [DOI] [PubMed] [Google Scholar]
- 22.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 23.Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, Kropf P, Mueller I, Kolb A, Modolell M, Ho AD. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108:1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 24.Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- 25.Patterson DA, Rapoport R, Patterson MA, Freed BM, Lempert N. Hydrogen peroxide-mediated inhibition of T-cell response to mitogens is a result of direct action on T cells. Arch Surg. 1988;123:300–304. doi: 10.1001/archsurg.1988.01400270034004. [DOI] [PubMed] [Google Scholar]
- 26.Pham CT. Neutrophil serine proteases fine-tune the inflammatory response. Int J Biochem Cell Biol. 2008;40:1317–1333. doi: 10.1016/j.biocel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P, Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poccia F, Gioia C, Martini F, Sacchi A, Piacentini P, Tempestilli M, Agrati C, Amendola A, Abdeddaim A, Vlassi C, Malkovsky M, D’Offizi G. Zoledronic acid and interleukin-2 treatment improves immunocompetence in HIV-infected persons by activating Vgamma9Vdelta2 T cells. AIDS. 2009;23:555–565. doi: 10.1097/QAD.0b013e3283244619. [DOI] [PubMed] [Google Scholar]
- 29.Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–250. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–6230s. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 31.Rotondo R, Bertolotto M, Barisione G, Astigiano S, Mandruzzato S, Ottonello L, Dallegri F, Bronte V, Ferrini S, Barbieri O. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J Leukoc Biol. 2011;89:721–727. doi: 10.1189/jlb.1109737. [DOI] [PubMed] [Google Scholar]
- 32.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Sippel TR, White J, Nag K, Tsvankin V, Klaassen M, Kleinschmidt-DeMasters BK, Waziri A. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clin Cancer Res. 2011;17:6992–7002. doi: 10.1158/1078-0432.CCR-11-1107. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Lee HK, Bukowski JF, Li H, Mariuzza RA, Chen ZW, Nam KH, Morita CT. Conservation of nonpeptide antigen recognition by rhesus monkey V gamma 2 V delta 2 T cells. J Immunol. 2003;170:3696–3706. doi: 10.4049/jimmunol.170.7.3696. [DOI] [PubMed] [Google Scholar]
- 35.Welton JL, Morgan MP, Marti S, Stone MD, Moser B, Sewell AK, Turton J, Eberl M. Monocytes and γδ T cells control the acute phase response to intravenous zoledronate: insights from a phase IV safety trial. J Bone Miner Res. 2013;28:464–471. doi: 10.1002/jbmr.1797. [DOI] [PubMed] [Google Scholar]
- 36.Wimalawansa SJ. Insight into bisphosphonate-associated osteomyelitis of the jaw: pathophysiology, mechanisms and clinical management. Expert Opin Drug Saf. 2008;7:491–512. doi: 10.1517/14740338.7.4.491. [DOI] [PubMed] [Google Scholar]
- 37.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Abraham E. Antiinflammatory effects of hydrogen peroxide in neutrophil activation and acute lung injury. Am J Respir Crit Care Med. 2009;179:694–704. doi: 10.1164/rccm.200806-851OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.