Abstract
Hepatitis C virus (HCV) infection is associated with hepatic iron overload and elevated serum iron that correlate to poor antiviral responses. Hepcidin (HAMP), a 25-aa cysteine-rich liver-specific peptide, controls iron homeostasis. Its expression is up-regulated in inflammation and iron excess. HCV-mediated hepcidin regulation remains controversial. Chronic HCV patients possess relatively low hepcidin levels; however, elevated HAMP mRNA has been reported in HCV core transgenic mice and HCV replicon-expressing cells. We investigated the effect of HCV core protein on HAMP gene expression and delineated the complex interplay of molecular mechanisms involved. HCV core protein up-regulated HAMP promoter activity, mRNA, and secreted protein levels. Enhanced promoter activity was abolished by co-transfections of core with HAMP promoter constructs containing mutated/deleted BMP and STAT binding sites. Dominant negative constructs, pharmacological inhibitors, and silencing experiments against STAT3 and SMAD4 confirmed the participation of both pathways in HAMP gene regulation by core protein. STAT3 and SMAD4 expression levels were found increased in the presence of HCV core, which orchestrated SMAD4 translocation into the nucleus and STAT3 phosphorylation. To further understand the mechanisms governing the core effect, the role of the JAK/STAT-activating kinase CK2 was investigated. A CK2-dominant negative construct, a CK2-specific inhibitor, and RNAi interference abrogated the core-induced increase on HAMP promoter activity, mRNA, and protein levels, while CK2 acted in synergy with core to significantly enhance HAMP gene expression. Therefore, HCV core up-regulates HAMP gene transcription via a complex signaling network that requires both SMAD/BMP and STAT3 pathways and CK2 involvement.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-014-1621-4) contains supplementary material, which is available to authorized users.
Keywords: Hepatitis C virus, Iron, Hepcidin, SMAD4, STAT3, CK2
Introduction
The antimicrobial peptide hepcidin, encoded by the HAMP gene, is a 25-amino acid cysteine-rich peptide present in human serum and urine. It is mainly synthesized by the liver as an 84-amino acid precursor protein and released in circulation upon maturation [1]. It regulates iron homeostasis by binding to the only known cell surface iron exporter ferroportin and causing its internalization and degradation, thereby inhibiting iron efflux [2]. Ferroportin is expressed in duodenal enterocytes, macrophages, and hepatocytes. Therefore, hepcidin expression inhibits iron absorption by the duodenum, iron recycling by the macrophages, and iron mobilization from the hepatic stores [3]. Several studies have linked abnormalities in hepcidin expression with iron disorders in humans. Thus, mutations in the HAMP gene or its positive regulators HJV, TfR2, or HFE result in diminished hepcidin expression that leads to severe iron deposition in tissues and liver failure [4]. Conversely, mutations in the hepcidin-negative regulator matriptase-2 (TMPRSS6) cause increased hepcidin concentrations [5, 6]. Hepcidin expression is up-regulated by iron and inflammation and down-regulated by low body iron stores, anemia, hypoxia, and increased erythropoietic activity [7]. In response to elevated systemic iron, hepcidin expression is increased via the BMP signaling pathway. BMPs bind BMP receptors type I and type II, which phosphorylate the SMAD1/5/8 proteins intracellularly. These bind to the common mediator SMAD4 and translocate to the nucleus in order to initiate hepcidin transcription [8]. Hepcidin is also induced in response to infection or inflammation, mostly by IL-6, through the STAT3 pathway [9]. Other mechanisms of HAMP gene expression have been reported and involve a variety of transcription factors implicated in liver gene transcription, hypoxia, cell differentiation, and tumorigenesis, such as C/EBPα, AP1, NF-κB, HIF-1α, p53, USF1/2, SMAD7, and GATA-4 [10–18].
Hepatitis C virus (HCV) is a single-stranded, positive-sense RNA virus of the Flaviviridae family. It encodes a polyprotein precursor of approximately 3,000 amino acids, which is processed by cellular and viral proteases to yield at least ten structural and non-structural proteins (C, E1, E2, p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B) [19]. HCV infection is endemic to most parts of the world, with approximately 180 million infected individuals, worldwide [20]. The burden of HCV-related liver disease is substantial due to its high chronicity rate: an estimated 65–80 % of those infected will develop persistent infection, which is associated with widespread liver inflammation and fibrosis. The hallmark of the disease is the hepatic steatosis observed in 40–80 % of the patients and is a major determinant of the liver damage progression in chronic hepatitis C (CHC). As many as 20–50 % of CHC patients will develop cirrhosis over a three-decade time span and 5 % of patients with HCV-positive cirrhosis will develop hepatocellular carcinoma (HCC) every year [21]. CHC infection has been associated with elevated serum iron and ferritin, as well as increased intrahepatic iron stores [22]. Both iron overload and the viral infection itself have detrimental effects on the liver, including oxidative stress induction, organelle dysfunction, onset of liver fibrosis, hepatocyte injury, and aberrant growth [23]. Extensive research in CHC patients has shown that iron homeostasis is deregulated and hepcidin levels are low [24–27]. Remarkably, hepcidin expression levels in the very early stages of HCV infection have not yet been clearly established.
HCV core protein is involved in nucleocapsid formation and plays a crucial role in viral infection, virion assembly, and trafficking [28]. Standing at the forefront of virus–host interactions, core directs and participates in numerous pathophysiological cellular processes, such as insulin and lipid metabolism, apoptosis, oxidative stress, cytokine production, cell differentiation and proliferation, and signaling through the JAK/STAT, MAPKs, and TGF-β/SMAD pathways [29, 30]. It is the first viral protein encountered by the cell and has been shown to regulate crucial host immune responses, such as the acute phase response (APR) [31]. The APR collectively describes cellular and systemic changes that promote healing in response to injury, infection, and inflammation [32]. APR is characterized by differential modulation of the expression of acute phase proteins, which aim to restore homeostasis, and is mainly orchestrated by IL-6, IL-1, and TNF-α [33]. Since iron is crucial to pathogen invasion and infection [34], host antimicrobial mechanisms have been in place to limit its availability. In this respect, hepcidin, an IL-6-regulated type II acute phase protein [2, 35], causes hypoferremia by blocking ferroportin from transporting iron out of the cell [36–38].
Despite the link between CHC infection, iron homeostasis, and hepcidin, so far it is unclear how the virus triggers iron overload in the liver and whether HCV core protein, the first to be expressed following infection, interacts with the iron regulatory pathway. The severity of liver disease stemming from the virally induced iron overload dictates that more research is carried out on the causes and effects of such interactions. With these questions in mind, we sought to investigate the putative effect of HCV core on the master iron regulator hepcidin and delineate the molecular mechanisms involved.
Materials and methods
Materials
The SMAD1/5/8 inhibitor dorsomorphin was purchased from BioVision (Milpitas, California, USA), the CK2 inhibitor quinalizarin was from EMD Millipore (Billerica, Massachusetts, USA), and the STAT3 Inhibitor VII from Calbiochem (Billerica, Massachusetts, USA). All other chemicals were from Sigma (St. Louis, Missouri, USA).
Plasmids
The DNA plasmids used in this study are: the full-length HAMP gene promoter 3.1 kb [39], the truncated HAMP gene promoter −960 [40], the mutant HAMP gene promoters −942mBMP and −942ΔSTAT [9], the 3x-Ly6e promoter construct and dominant negative STAT3 [41], the dominant negative SMAD4 [42], the dominant negative CK2 [43], and the CK2 expression plasmid [44]. The expression plasmid pHPI1430 that codes for full-length core (c191) has been described elsewhere [45].
Mutations on the (−84/−79) BMP cis-acting element of the −3.1 kb HAMP promoter was carried out using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, California, USA) according to the manufacturer’s instructions. The BMP site was abolished without altering local promoter topology, according to transcription factor prediction analysis with TESS software. The primers used for mutagenesis are shown herein with the mutated bases in bold: F:5′-GCT TAT CTC TCC CGC CTT TTC AGA ACC ACC ACC TTC TTG G-3′ and R:5′-CCA AGA AGG TGG TGG TTC TGA AAA GGC GGG AGA GAT AAG C-3′. The resulting construct was subjected to sequencing analysis.
Cells and transfection assays
The hepatoma Huh7 and HepG2 cell lines were maintained in low-glucose DMEM supplemented with 2 mM glutamine, 10 % (v/v) heat-inactivated FCS, and 100 U/ml penicillin/streptomycin. The construction and maintenance of HCV-1a core-expressing HepG2 Tet-On cell line (C2-3) and empty vector control cell line (pTRE) has been described elsewhere [45]. Core expression was induced by adding 10 μg/ml doxycycline for 48 h. HCV core antigen in cell lysates was quantified by the Architect HCV Ag assay (Abbott, Chicago, Illinois, USA), according to manufacturer’s instructions and modifications described previously [48]. Moreover, in all cases, the control cell line pTRE was subjected to the same treatment with doxycycline and used for comparison purposes instead of doxycycline-free C2-3 cells, due to the slight leakiness on protein expression, often observed with the Tet-On system in the absence of doxycycline. Upon inhibitor treatment, the cells were pre-treated with the appropriate dose for 1.5 h, at which time doxycycline was added for a further 48 h.
For DNA transfections, 100,000 cells/well were seeded in 48-well plates 24 h prior to the experiment. Transient transfections were carried out using JetPEI (Polyplus, Illkirch, France) and 0.5 μg promoter-luciferase DNA constructs, the appropriate amount of expression plasmid for co-transfection experiments and 0.05 μg of CMV-β-galactosidase plasmid to provide an internal control for transfection efficiency. After 6 h, the cells were washed with phosphate-buffered saline and left for 48 h in fresh culture medium. Cell lysates were subjected to luciferase and β-galactosidase activity determination with commercially available kits (Promega, Fitchburg, Wisconsin, USA). Luciferase activity was normalized to β-galactosidase activity in order to yield relative luciferase activity (RLA). In all figures, the RLA vector control value (mean ± SD: standard deviation) was set as 100 % or fold-change (black or white histogram) and all other values were depicted as a ratio of this (hatched or dotted histograms). Each transfection was carried out at least three times in triplicate.
mRNA expression analysis
Total RNA was isolated from cells using RNAzol B (Wak-Chemie Medical, Steinbach, Germany) according to the manufacturer’s instructions with the following modification; 1 μg/μl glycogen was used to encourage RNA precipitation from isopropanol solutions, before the final wash step in 75 % (v/v) ethanol. Reverse transcription reactions were carried out using 1 µg RNA and MMLV reverse transcriptase (Promega). The prepared cDNA was subjected to qPCR using the Maxima SYBR green qPCR mix (Fermentas, Vilnius, Lithuania) in a Mini Opticon PCR machine (Bio-Rad, Hercules, California, USA). The gene-specific primers used were: HAMPF: 5′-CCA CAA CAG ACG GGA CAA CTT-3′ and HAMPR: 5′ AGT GGG TGT CTC GCC TCC TT 3′. Primers for 18SrRNA, IL-6, FGB, and SAA1 have been described elsewhere [46–49]. Results were analyzed with the internal standard-curve method and normalized to 18S rRNA to provide the relative mRNA expression. In all PCR experiments, unless otherwise stated, the relative mRNA expression control value (mean ± SD) was set as 100 % or fold-change (black histogram) and all other values were depicted as a ratio of this (hatched histograms). All experiments were performed in triplicate.
Protein analysis
Cells were washed twice with PBS and were either harvested in whole-cell extract lysis buffer (10 mM Tris–HCl pH 7.05, 50 mM NaCl, 1 % (w/v) Triton X-100, 0.5 mM PMSF and protease/phospho-protease inhibitor cocktail by Roche) or subjected to subcellular fractionation using the Proteojet Cytoplasmic and Nuclear Protein Extraction Kit (Fermentas) according to the manufacturer’s instructions. Protein concentrations were measured with the MicroBCA assay (Thermo Scientific, Waltham, Massachusetts, USA). Cell lysates (50 μg) were resolved in 10 % (v/v) SDS-PAGE gels and transferred onto nitrocellulose membranes. After blocking, membranes were incubated overnight with primary antibodies. Membranes were then washed and incubated with the appropriate secondary antibody for 90 min at room temperature. Chemiluminescence was detected using Pierce ECL Western-blotting substrate (Thermo Scientific). Individual gel photographs presented in each figure panel depict results from samples that were derived from the same experiment and processed in parallel. Additionally, loading controls were always run on the same blot as the primary antibodies.
The following antibodies were used in this study: STAT3 (sc-483), PARP1/2 (sc-7150), and CK2α (sc-9030) by Santa Cruz (Dallas, Texas, USA); pSer727STAT3 (#9136), pTyr705STAT3 (#9131), SMAD4 (#9515) by Cell Signaling (Danvers, Massachusetts, USA); TMPRSS6 (ab56180), TfR2 (ab83810) by Abcam (Cambridge, UK); TfR1 (#13-6800) by Invitrogen (Carlsbad, California, USA); β-actin (MAB1501) by Millipore (Billerica, Massachusetts, USA); Ferroportin 1 (MTP11) by Alpha Diagnostics (San Antonio, Texas, USA) and ferritin (A0133) by Dako (Glostrup, Denmark). The polyclonal HCV-1a core antibody used in this study has been referenced elsewhere [43].
CK2 activity was measured in cell extracts (2.5 μg) with a synthetic peptide substrate, RRRADDSDDDDD. Assays were performed for 10 min at 30 °C in a final reaction mixture volume of 30 μl containing 50 mM Tris–HCl (pH 7.5), 12 mM MgCl2, 10 μM ATP (specific activity ~1,500 cpm/pmol) and 0.5 mM substrate peptide. The reactions were terminated by spotting 25 μl of the reaction mixture onto P81 phosphocellulose filters. Then, filters were washed three times in 0.5 % (v/v) phosphoric acid and counted in scintillation fluid.
Enzyme-linked immunosorbent assays (ELISA)
Quantification of secreted hepcidin levels was performed by homemade competitive ELISA, as earlier reported [50]. The percent change in protein expression of the control cells was set as 100 % (black histogram) and all other values were a percentage of the control (hatched histograms). The assay was repeated at least three times in quadruplicates.
siRNA analysis
siRNA-mediated knock-down analysis was carried out using DharmaFECT Duo transfection reagent to deliver 50 nM siRNA oligonucleotides against CK2 (#6389) or STAT3 (#6582) (Cell Signaling) or SMAD4 (s8405) (Ambion, Austin, Texas, USA) or Allstars Negative control (Qiagen, Hilden, Germany) non-silencing oligonucleotides and the core-expression plasmid pHPI1430, according to the manufacturer’s protocols (Dharmacon RNA Technologies, Lafayette, Colorado, USA). At 48–65 h post-transfection, the cell supernatants were collected and subjected to ELISA for hepcidin protein measurements. Evaluation of siRNA knock-down efficiency was performed in the harvested whole-cell extracts by Western blotting.
Immunofluorescence analysis
HCV core expression following induction of the C2-3 cell clone, was examined by immunofluorescence with an HCV core polyclonal antibody, as previously described [51, 52].
Statistical analysis
Statistical analysis was performed using Student’s t test with p ≤ 0.05 considered as statistically significant (*p value ≤0.05; **p value ≤0.005). Unless otherwise shown, statistical analysis was carried out between control and treated cells.
Results
HCV core up-regulates HAMP gene expression, hepcidin secretion, and modulates iron homeostasis
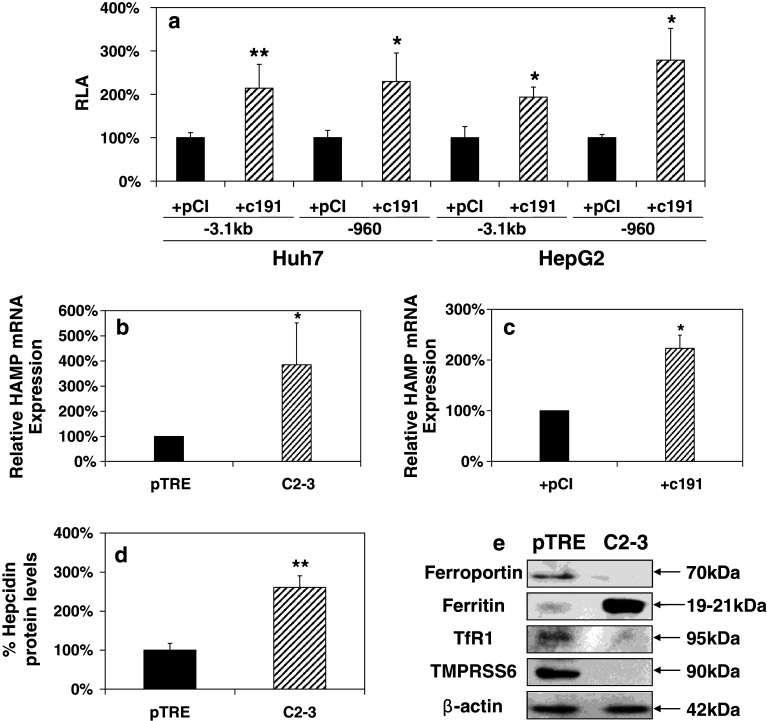
In order to investigate the putative regulation of HAMP gene expression by HCV core, we evaluated HAMP promoter activity by co-transfecting either the full-length −3.1 kb HAMP promoter or a truncated construct containing the first 960 bp with c191 in Huh7 and HepG2 cells. Figure 1a shows that compared to the empty vector control, HCV core increased the activity of both HAMP promoters by 2–3-fold in hepatoma cell lines Huh7 and HepG2. Subsequently, we used the core-expressing HepG2-based Tet-On cell line C2-3 and the control cell line pTRE to assess HAMP mRNA levels. Induction of core expression was confirmed by immunofluorescence and Western blotting (Suppl. Fig. 1a1, a2). Quantification of core showed that core antigen was similarly expressed in C2-3 cell lysates and HCV-infected hepatoma cells (~65 versus ~58 fmol/l at 48 h post-induction and post-infection, respectively). Figure 1b reveals an approximately 3 to 4-fold increase in endogenous HAMP mRNA levels in the presence of HCV core. Comparable results in endogenous HAMP mRNA levels were obtained with Huh7 cells, transiently transfected with c191 (Fig. 1c). The smaller increase in mRNA levels observed in this case may be attributed to less amounts of expressed core protein due to limitations in cell transfectability. Moreover, hepcidin peptide levels were determined by ELISA in the supernatants of pTRE and C2-3 cells. Figure 1d shows a 2 to 3-fold increase in hepcidin secreted from C2-3 cells as compared to control cells. The observed hepcidin concentration was found to be 18.6 ± 3.2 ng/ml for pTRE cells, elevated to 48.9 ± 11.5 ng/ml in C2-3 supernatants. Experiments carried out in both pTRE and C2-3 cells in the absence of doxycycline, showed no induction of hepcidin expression, while a similar fold-increase was observed in another clone of the HCV core-expressing cell line (data not shown). Taken together, these data clearly point towards an HCV core-mediated transcriptional regulation of HAMP.
Fig. 1.
HCV core up-regulates HAMP gene expression and differentially modulates components of the iron regulatory pathway. a Huh7 and HepG2 cells were transiently co-transfected with the full-length (−3.1 kb) and a truncated form (−960) of HAMP promoter and expression plasmids coding for vector pCI or c191. Promoter activity was measured using a commercially available luciferase assay. HAMP mRNA levels in b pTRE and C2-3 cells and c Huh7 cells transfected with expression plasmids coding for c191 or empty vector (pCI). Total RNA was isolated and subjected to qRT-PCR with HAMP gene-specific primers. d Secreted HAMP protein levels from pTRE and C2-3 supernatants measured by a competitive ELISA assay. e Western-blot analysis of whole-cell extracts from pTRE and C2-3 cells with antibodies against various components of the iron regulatory pathway. β-actin was used as an internal control. Polypeptide molecular weights are given on the side in kDa
Finally, the role of hepcidin in iron homeostasis is of such importance that one could expect perturbations in the expression of other proteins involved in the iron regulation network. Therefore, we performed Western-blot analysis in whole-cell extracts from core-expressing C2-3 cells and their controls. Consistently with their role in iron homeostasis and the increase in hepcidin transcription and secretion, we observed a dramatic decrease in ferroportin, TfR1, and the negative HAMP regulator TMPRSS6, while the increased ferritin reflected the elevated intracellular iron (Fig. 1e). TfR2 expression was also tested in the presence of core and found to be largely unchanged (data not shown). The robust cellular adaptation reported here, as a result of HCV core expression, suggests that HCV may play a critical role in cellular iron metabolism regulation.
HCV core elevates the activity of the HAMP gene promoter via both BMP/SMAD and STAT cis-acting elements
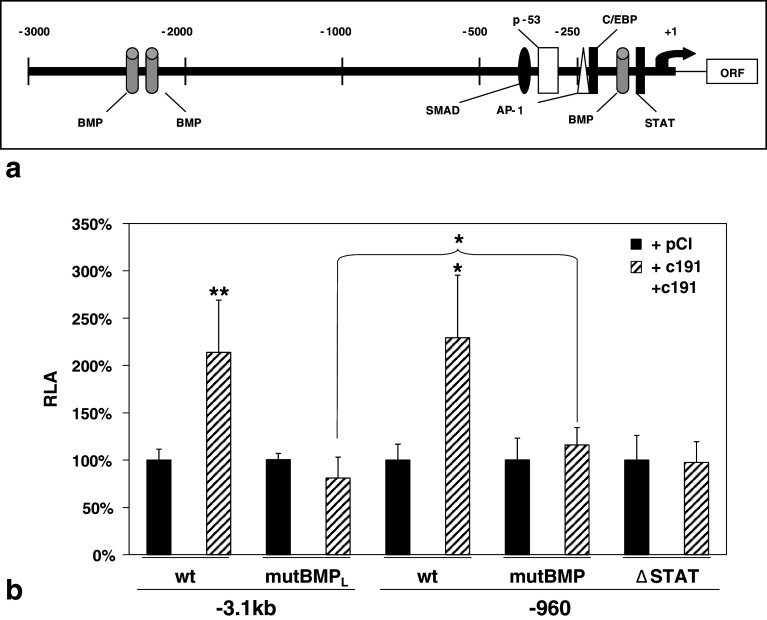
Previous studies described the association of STAT3 and BMP/SMAD signaling with HCV core protein [53–55] and the IL-6-mediated regulation of HAMP gene promoter [56]. Figure 2a depicts a simplified diagram of the STAT, BMP, and various other cis-acting elements located on the human HAMP promoter. In order to examine the participation of these elements in the HCV core-mediated regulation of HAMP promoter, we co-transfected the −960 and −3.1 kb HAMP promoter constructs and their respective mutants that contain a mutated proximal BMP site (−84/−79, mutBMP), a deleted STAT site (−72/−64, ΔSTAT), or the mutated BMP site in the context of the full-length −3.1 kb HAMP promoter (mutBMPL), together with c191 in Huh7 cells. The latter was prepared because it has been shown that there are two distal BMP sites in HAMP promoter located in the region between −2.3/−2.5 kb and at least one of them affects BMP/SMAD-mediated HAMP responsiveness to extracellular stimuli (iron or/and IL-6), possibly through co-operation with the proximal BMP site [39]. Figure 2b demonstrated that loss of either the STAT or the proximal BMP DNA binding sequence was enough for eradication of the HCV core-mediated up-regulation of the −960 and −3.1 kb HAMP promoters. Additionally, mutation of the proximal BMP site in the context of full-length HAMP promoter not only abolished the HCV core response, but also down-regulated HAMP promoter slightly but significantly (p value = 0.0026), suggesting that there might be some kind of collaboration between the distal and proximal BMP elements in the context of the full-length HAMP promoter.
Fig. 2.
Delineation of DNA-binding elements responsible for the HCV core-mediated regulation of HAMP promoter activity. a Schematic diagram of the HAMP promoter region with transcription factor binding sites. Black arrow: transcription initiation start. ORF: hepcidin open reading frame. b Transient co-transfections of Huh7 cells with full-length (black histogram) and truncated HAMP promoters (open histogram) harboring mutations and deletions in the (−84/−79) BMP and the (−72/−64) STAT cis-acting elements, with expression plasmids encoding either full-length core (c191) or the empty vector (pCI). Promoter activity was measured using a commercially available luciferase assay
The HCV core-dependent regulation of hepcidin involves both the BMP/SMAD and STAT3 signaling pathways
Next we examined whether the observed regulation of HAMP gene expression via both BMP and STAT3 DNA regulatory elements was followed by activation of the corresponding cellular signaling pathways. Two different approaches were used to test this hypothesis. Firstly, we carried out pathway-specific pharmacological inhibition, utilizing the small organic molecule dorsomorphin (D), a pharmacological inhibitor of the BMP/SMADs [57], and the STAT inhibitor VII (S), which specifically blocks STAT3 target gene transcription (Calbiochem). Secondly, we adopted a genetic approach using SMAD4 and STAT3 dominant-negative constructs in order to confirm HAMP promoter activity modulation by these pathways.
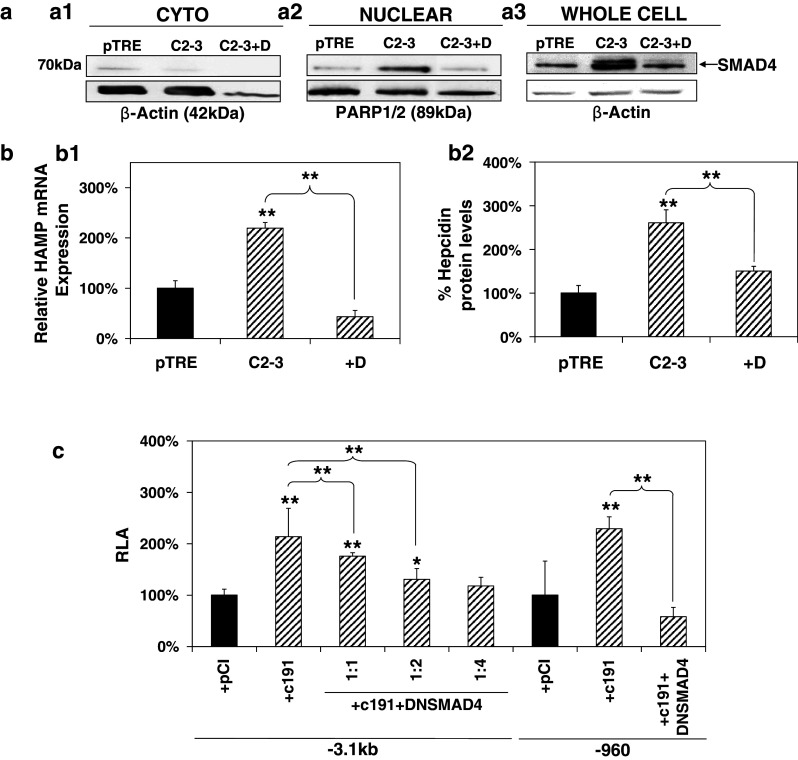
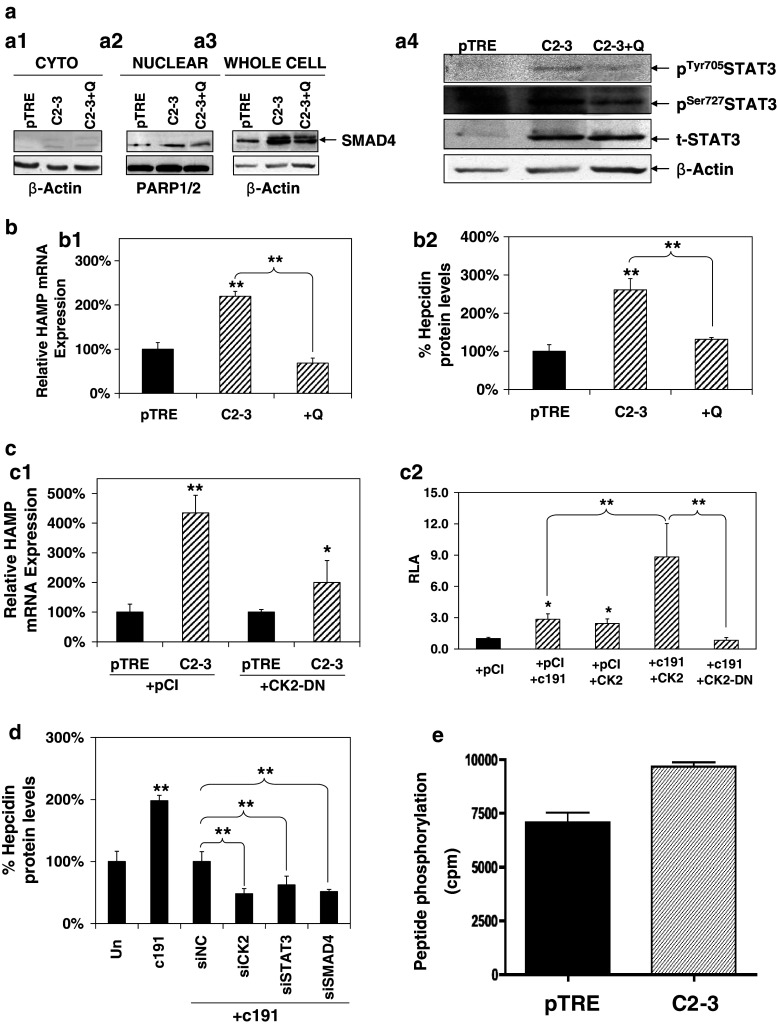
The involvement of the BMP/SMAD pathway in the HCV core-mediated effect was investigated by monitoring SMAD4 behavior in the presence of HCV core. SMAD4, otherwise known as the co-SMAD, is responsible for driving all BMP/SMAD signals to the nucleus through heterodimerization with the receptor-regulated SMAD members (R-SMADs) [58]. It has been shown that SMAD4 is constitutively phosphorylated [58]; therefore, translocation of the SMAD4 protein to the nucleus can be taken as an indication for pathway activation. Thus, we performed subcellular fractionation with control pTRE cells and HCV core-expressing C2-3 cells treated or not with dorsomorphin. PARP1/2 and β-actin were used as markers for the nuclear and cytoplasmic fractions, respectively, and together with whole-cell extracts were subjected to Western blotting with an anti-SMAD4 antibody. Figure 3a demonstrates a clear shift in SMAD4 localization from the cytoplasm (Fig. 3a1) to the nucleus (Fig. 3a2), which is blocked by the inhibitor. Additionally, it is shown for the first time that in our system, HCV core regulates SMAD4 expression by elevating its steady-state protein levels (Fig. 3a3). The observed increase of SMAD4 was reduced in the presence of dorsomorphin. Furthermore, in C2-3 core-expressing cells, the presence of dorsomorphin was able to abrogate the HCV core-mediated up-regulation of HAMP steady-state mRNA levels (Fig. 3b1), while the corresponding cell supernatants showed that hepcidin peptide production was reduced to background levels in cells treated with dorsomorphin (Fig. 3b2).
Fig. 3.
The BMP/SMAD pathway is involved in HCV core-mediated regulation of HAMP gene expression. a HCV core mediates changes in localization and expression of SMAD4 protein that is blocked by the BMP/SMAD inhibitor Dorsomorphin. Cytoplasmic (a1) and nuclear (a2) fractions of pTRE and C2-3 cells, as well as whole-cell extracts (a3), treated with 10 μM Dorsomorphin or vehicle control, were subjected to immunoblotting with an anti-SMAD4 antibody. β-actin and PARP1/2 were used as loading controls. b HCV core-mediated increase in b1 HAMP mRNA levels is abolished by the use of BMP/SMAD inhibitors. Following overnight starvation with 0.5 %(v/v) FCS, pTRE, and C2-3 cells were treated with 10 μM Dorsomorphin (D) or vehicle control and cells were harvested for RNA isolation and qRT-PCR with HAMP-specific primers. b2 HAMP protein levels return to normal following incubation with the BMP/SMAD inhibitor dorsomorphin. Supernatants from the experiment described in a1 were used for HAMP protein determination with a competitive ELISA. c SMAD4 is involved in the HCV core-mediated modulation of the HAMP gene promoter. Huh7 cells were co-transfected with the full-length and truncated HAMP promoter reporter constructs, expression plasmid for core or empty vector and a dominant negative construct for SMAD4 at various ratios. Promoter activity was measured using a commercially available luciferase assay
Furthermore, an expression plasmid coding for a dominant negative form of SMAD4 (DNSMAD4) that was able to down-regulate the −960 HAMP promoter constitutive activity in Huh7 cells (Suppl. Fig. 1c) was used. The −3.1 kb HAMP promoter was co-transfected with c191 and DNSMAD4 at mass ratios 1:1, 1:2, and 1:4. A dose-dependent effect, possibly due to the existence of the distal BMP sites, was observed with the −3.1 kb HAMP promoter, whereby the 1:4 ratio eradicated the HCV core-mediated up-regulation of HAMP promoter activity (Fig. 3c). The 1:1 ratio between the dominant negative and c191 expression plasmids was enough to eliminate HAMP promoter activity increase for the truncated −960 HAMP promoter.
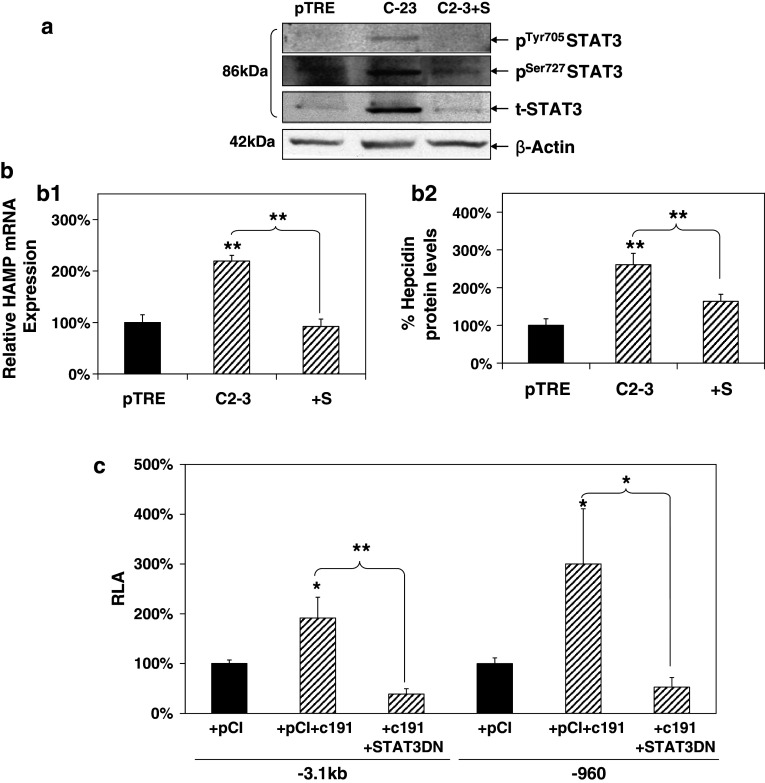
In order to assess STAT3 pathway involvement in HCV core-mediated modulation of hepcidin, a similar methodology as the one described above for SMAD4 was used. STAT3 phosphorylation status and expression in the presence of core was investigated by Western-blot analysis with whole-cell extracts from C2-3 and control pTRE cells in the presence of STAT inhibitor VII. As shown in Fig. 4a, STAT3 was found to be phosphorylated at both Tyr705 and Ser727 residues and also up-regulated in core-expressing cells, while STAT3 inhibitor managed to block the core-induced regulation of STAT3 activation and expression. The use of the STAT-specific inhibitor abolished the HCV core-mediated increase in HAMP mRNA levels (Fig. 4b1). Hepcidin protein levels also dropped to the background (Fig. 4b2) in the corresponding cell supernatants.
Fig. 4.
STAT3 is involved in HCV core-mediated regulation of HAMP gene expression. a HCV core mediates changes in activation and expression of STAT3 protein that is blocked by STAT3 Inhibitor VII. Whole-cell extracts, as well as nuclear and cytoplasmic fractions of pTRE and C2-3 cells treated with 4 μM STAT3 inhibitor VII or vehicle control, were subjected to immunoblotting with anti-total (t) and anti-phospho (p) STAT3 antibodies. β-actin was used as a loading control. b HCV core-mediated increase in b1 HAMP mRNA levels is abolished by the use of STAT and BMP/SMAD inhibitors. Following overnight starvation with 0.5 % (v/v) FCS, pTRE and C2-3 cells were treated with 4 μM STAT3 inhibitor VII (S) or vehicle control and cells were harvested for RNA isolation and qRT-PCR with HAMP-specific primers. b2 HCV core-mediated elevation in HAMP protein levels was abolished following incubation with STAT3 and/or BMP/SMAD inhibitors. Supernatants from the experiment described in a1 were used for HAMP protein determination with a competitive ELISA. c STAT3 is involved in the HCV core-mediated modulation of the HAMP gene promoter. Huh7 cells were co-transfected with the full-length and truncated HAMP promoter reporter constructs, expression plasmid for core or empty vector and a dominant negative construct for STAT3 in a 1:2:2 ratio. Promoter activity was measured using a commercially available luciferase assay
The use of a dominant negative STAT3 (STAT3DN) expression plasmid coding for a form of the protein that cannot be phosphorylated in Tyr705 was used in co-transfection experiments with c191 and the full-length or truncated HAMP promoter constructs in Huh7 cells. In both cases, failure to activate the STAT3 pathway eradicated HAMP modulation by core (Fig. 4c). Functionality of the plasmid used can be seen in Supplementary Figure 1c, where it succeeded in abolishing IL-6-mediated enhancement of activity in a STAT1/STAT3-driven promoter. Given that STAT3-mediated regulation of hepcidin has been attributed to IL-6, the main effector of the APR, we carried out mRNA expression analysis in Huh7 cells transfected with the full-length HCV core, in order to assess IL-6 expression. Indeed, IL-6 mRNA levels were found up-regulated 3.5-fold in the presence of HCV core (Suppl. Fig. 1b), thereby suggesting that even ectopic expression of HCV core may be enough to initiate the APR via IL-6 production in Huh7 cells and the concomitant changes in APP regulation, as suggested by simultaneous up-regulation of mRNA of IL-6-regulated APPs serum amyloid A1 [59] and fibrinogen [33] (Suppl. Fig 1b).
Taken together, the above results indicate that HCV core up-regulates HAMP gene expression through BMP/SMAD signaling and activation of the STAT3 pathway appears to be part of this mechanism.
HCV core-mediated regulation of HAMP gene expression occurs through activation of both STAT3 and BMP/SMAD pathways by CK2 kinase
CK2 is pleiotropic kinase that interacts with and modulates several pathways, such as the JAK/STAT and the BMP/SMAD [60, 61]. In the light of this and in order to comprehend the mechanisms governing the HCV core effect, we examined the involvement of CK2 in the HCV core-mediated regulation of HAMP and investigated a possible interaction with both STAT3 and SMAD4 proteins. A potent and selective CK2 inhibitor, quinalizarin (Q) [62], was used in a series of experiments with HCV core-expressing C2-3 and their control pTRE cells, in order to assess a putative inhibitory effect on STAT3 and SMAD4 activation and the up-regulation of HAMP gene expression itself, in the presence of core. Figure 5a shows that in cytoplasmic (Fig. 5a1) and nuclear (Fig. 5a2) fractions, as well as whole-cell extracts (Fig. 5a3) from cells incubated with quinalizarin for 48 h, the inhibitor blocked marginally the translocation of SMAD4 to the nucleus and the core-mediated increase in SMAD4 expression. Most interestingly, it inhibited phosphorylation of both STAT3 Tyr and Ser residues and repressed STAT3 expression moderately (Fig. 5a4). Collectively, the use of quinalizarin was enough to repress HAMP mRNA up-regulation (Fig. 5b1) and consequently secreted hepcidin peptide levels (Fig. 5b2).
Fig. 5.
a The CK2 inhibitor quinalizarin (Q) blocks HCV core-mediated changes in localization and expression of SMAD4 (a1, a2, a3) and activation of STAT3 (a4). Following overnight starvation with 0.5 % (v/v) FCS, pTRE and C2-3 cells were treated with 1 μM quinalizarin or vehicle control and subjected to subcellular fractionation or lysis for whole-cell extract preparation. Then, they were used in immunoblotting with an anti-SMAD4, anti-total (t) and anti-phospho (p) STAT3 antibodies. β-actin and PARP1/2 were used as loading controls. b HCV core-mediated increase in b1 HAMP mRNA levels is abolished by the use of CK2 inhibitor quinalizarin. RNA isolated from the experiment described in a was subjected to qRT-PCR with HAMP-specific primers. b2 HCV core-mediated elevation in HAMP protein levels was abolished following incubation with the CK2 inhibitor quinalizarin. Supernatants from the experiment described in a1 were used for HAMP protein determination with a competitive ELISA. c CK2 is involved in the HCV core-mediated modulation of the HAMP gene expression and potentiates the core effect. c1 A CK2 dominant negative expression plasmid (CK2-DN) transfected in pTRE and C2-3 cells abolishes HCV core-mediated increase in HAMP endogenous mRNA levels, compared to transfection with an empty vector control (pCI). c2 Huh7 cells were co-transfected with the full-length HAMP promoter in combination with expression plasmids for core (c191) and/or CK2 and/or empty vector (pCI) and/or a dominant negative CK2 construct. Promoter activity was measured using a commercially available luciferase assay. d siRNA knock-down analysis using CK2, SMAD4, and STAT3 or scrambled (NC) oligonucleotides in Huh7 cells transfected with empty vector (Un) or full-length core (c191). The supernatants were collected for secreted hepcidin protein measurements by ELISA 48–65 h post-transfection. e CK2 kinase activity is up-regulated in the presence of core. CK2 activity assay in pTRE and C2-3 cells, measured towards the peptide substrate, is shown as cpm incorporated [mean values (±SD) of three determinations]
Furthermore, we tested the effect of CK2 on HAMP promoter activity modulated by HCV core. A dominant negative CK2 (CK2-DN) expression plasmid that codes for a kinase-inactive mutant was transfected in core-expressing C2-3 and control pTRE cells and HAMP mRNA levels were evaluated by qRT-PCR. As seen in Fig. 5c1, CK2-DN reduced the core-mediated up-regulation of HAMP mRNA by half. When Huh7 cells were used in transient transfection assays with both the CK2-DN and a constitutively active CK2 expression plasmid (CK2), we observed that the core-mediated increase of HAMP full-length promoter was completely abolished with the former plasmid, while at the same time HCV core and CK2 acted in synergy and produced a dramatic 9 to 10-fold elevation in HAMP promoter activity levels. The synergistic effect of HCV core and CK2 was evident, since CK2 effect on HAMP gene promoter constitutive levels was similar to the up-regulation noticed by core alone (Fig. 5c2). Functionality of the dominant negative plasmid used can be seen in Supplementary Figure 1c, where it succeeded in abolishing IFN-γ-mediated enhancement of activity in a STAT1/STAT3-driven promoter. Finally, the use of RNA interference assays for CK2, SMAD4, and STAT3 followed by ELISA measurements of hepcidin protein levels in Huh7 cells transfected with full-length core (Fig. 5d), as well as in pTRE/C2-3 cells (data not shown), demonstrated that the interplay of all three pathways is crucial for the HCV core-mediated regulation of HAMP gene expression. Knock-down of the corresponding proteins was confirmed by Western-blot analysis (Supp. Fig. 1e1, e2, e3).
In order to probe the relationship between HCV core and CK2 a bit further, we investigated whether HCV core activates CK2 directly. Indeed, CK2 kinase activity was up-regulated in the presence of core in C2-3 cells measured by CK2 activity assay (Fig. 5e). Additionally, CK2 protein levels were found elevated in cell extracts from Huh7 cells transfected with c191 (Supp. Fig. 1d).
Discussion
HCV-mediated hepcidin regulation remains a controversial issue at best. Chronic HCV infection in humans is characterized by relatively low hepcidin expression [24, 25, 27, 63], although questions about hepcidin levels in acute infection have not yet been addressed adequately. Transgenic mice expressing the HCV polyprotein exhibited increased serum iron levels and reduced hepcidin expression, when compared to naive animals [64], while Huh7.5 cells bearing the HCV replicon system or HCV core protein expressed from an adenoviral vector, were shown to produce lower hepcidin levels than control cells [65]. In contrast, other studies reported elevated HAMP mRNA in HCV core transgenic mice [66] and replicon cells based on the parental Huh7 cell line [67]. The above conflicting data may be due to the different cellular systems used in these studies and be parts of a multifaceted phenomenon that governs hepcidin regulation during viral infection.
This study provides clear evidence for HCV core-mediated transcriptional up-regulation of HAMP gene expression in Huh7 and HepG2 cells. In our system, IL-6 levels were also found up-regulated by at least 2 to 3-fold in core-expressing Huh7 cells. This finding agrees with previous results that implicate HCV core in IL-6 regulation [68, 69] and may be the initial step in the observed HCV core-mediated up-regulation of hepcidin expression. One could speculate that hepcidin up-regulation results in modulation of cellular iron homeostasis, since we demonstrated for the first time that iron-regulated proteins ferroportin, TfR1, and matriptase-2 were diminished, while ferritin was elevated in the presence of HCV core, as expected in the case of increased hepcidin. Given that the increase of hepcidin by core results in enhanced iron levels intracellularly, one would expect mobilization of the IRE-IRP regulatory system and subsequent post-transcriptional regulation of these genes, in order to accommodate for the changes in iron status [70–73].
Several studies provide evidence that hepcidin is regulated by the BMP/SMAD pathway. BMP2, BMP4, BMP9, and more recently BMP6, have been implicated in HAMP gene modulation and iron regulation [8, 74–77]. Co-transfections of a HAMP promoter construct with a mutant proximal BMP site with HCV core in Huh7 cells showed that this pathway is involved in the HCV core-mediated regulation of hepcidin. The observed promoter activity increase was matched accordingly by HAMP mRNA and protein, which returned to background levels following incubation of the core-expressing cells with the BMP/SMAD-specific inhibitor dorsomorphin. SMAD4 involvement was verified with a SMAD4 dominant negative construct and introduction of SMAD4 siRNA oligonucleotides, which managed to abrogate HAMP promoter activity and secreted peptide up-regulation in Huh7 cells transfected with c191. Fractionation experiments with pTRE/C2-3 cells demonstrated increased SMAD4 trafficking to the nucleus. Surprisingly, this change in localization was accompanied by an up-regulation of SMAD4 protein levels, while both were blocked by dorsomorphin, as expected. Notably, Wang and colleagues reported the direct involvement of SMAD4 in HAMP constitutive expression, which we also noticed when co-transfection of the dominant negative SMAD4 construct with the HAMP promoter led to a dramatic decrease in promoter activity. Inducible HAMP gene expression was impaired in SMAD4-defficient hepatocytes, since it did not respond to extracellular stimuli known to elevate HAMP expression, such as IL-6, BMPs or iron [77]. Interestingly, mice bearing liver-specific knock-down of SMAD4 were found to have dramatically reduced HAMP levels and suffer from severe hepatic iron overload, thereby pointing towards the importance of SMAD4 in hepcidin regulation and iron homeostasis in vivo [78]. Furthermore, HCV interaction with the BMP/SMAD pathway has been demonstrated, as HCV replication is suppressed by TGF-β and BMP7 in a SMAD-dependent manner [79, 80]. However, to our knowledge, with the exception of tumor-derived core isolates [81], neither the effect of HCV nor a putative involvement of individual viral proteins on SMAD4 gene expression have ever been studied before. The aforementioned existing literature findings corroborate our results, which stress the important role of the BMP/SMAD pathway and specifically SMAD4 on HCV core-mediated transcriptional regulation of hepcidin in the early steps of viral infection.
Next, we demonstrated that HCV core-mediated up-regulation of HAMP gene promoter activity in both Huh7 and HepG2 hepatoma cell lines, as well as mRNA and protein levels also occurred through STAT3. Enhanced promoter activity returned to background levels with the deletion of a STAT site in the proximal HAMP promoter, as well as the co-transfection of a STAT3 dominant negative construct with core. Additionally, the use of a specific STAT3 inhibitor was enough to abolish the HCV core-mediated increase of hepcidin mRNA and protein. Phosphorylation of both tyrosine and serine STAT3 residues and a profound increase in total STAT3 protein indicated the activation of STAT3 pathway in core-expressing cells. These effects of core on STAT3 were specifically abrogated by the STAT3 inhibitor. Finally, RNAi interference against STAT3 in Huh7 cells transfected with c191 was able to decrease the HCV core-mediated elevation in secreted hepcidin.
Regulation of HAMP gene expression by STAT3 is very well documented [9, 16, 82]. In these studies, the common effector was IL-6, provided either exogenously or in the form of conditioned medium from macrophage cultures, mimicking inflammation and the APR. IL-6 has been known to transactivate STAT3 by inducing its phosphorylation in both tyrosine and serine residues [83]. IL-6 signaling occurred through the STAT site in the proximal HAMP promoter, which was also found responsible for constitutive HAMP expression [9]. Moreover, Pietrangelo et al. [84] showed that IL-6-mediated modulation of HAMP gene expression was not possible in mice bearing a liver-specific deletion of the IL-6 transducing receptor gp130. Finally, prohepcidin was recently shown to be responsible for hepcidin auto-regulation through localization in the nucleus and binding to the STAT site in the HAMP gene promoter [85]. STAT3 was also shown to be involved in HAMP gene modulation in response to tissue hypoxia. Under hypoxic conditions, STAT3 as well as C/EBPα, a positive hepcidin regulator [12], would be dislodged from the HAMP gene promoter, thereby causing down-regulation of hepcidin expression [86, 87]. On the other hand, the production of ROS in persistent inflammation has been demonstrated to increase hepcidin in a STAT3-dependent manner, possibly as a way to induce hypoferremia and limit tissue damage that could result from iron-generated free radicals [88]. Furthermore, STAT3 interacts with and is activated by HCV core through phosphorylation [55, 69, 89], while it may also increase STAT3 gene expression [55], a finding that agrees with our own data. In our series of experiments, we observed that in core-expressing cells the BMP/SMAD inhibitor dorsomorphin was able to block the core-mediated increase in STAT3 expression and phosphorylation (data not shown), thereby indicating that the core action on STAT3 may involve members of the BMP/SMAD cascade. Given that inhibition of an individual pathway eradicated the HCV core effect on HAMP, and therefore pathway redundancy is not an issue in the core response, our data strongly point towards a need for interplay between STAT3 and BMP/SMADs for the regulation of HAMP by HCV core. This seems to be generally true for inducible HAMP regulation by inflammatory stimuli, systemic iron, and erythropoiesis. Some of these studies have shown that inhibition of the BMP/SMAD pathway and SMAD4 blocks IL-6-mediated regulation of hepcidin [37, 90–92], while Wang et al. [77] suggested that SMAD4 may facilitate it by opening condensed chromatin structure so that STAT3 has better access to the HAMP promoter.
In order to understand the molecular mechanisms that govern the HCV core effect on HAMP gene regulation, we investigated the putative role of protein kinase CK2 (CK2). CK2 is a ubiquitously expressed serine/threonine kinase that circulates in the form of a tetramer, which contains two catalytic subunits (CK2α, CK2α′, or their combination) and two regulatory subunits (CK2β). It is a pleiotropic kinase, implicated in a plethora of pathophysiological cellular processes. It is a pro-survival molecule with increased activity and expression in many human cancers, including HCC [93], and is known to interact with the NF-κB, PI3 K, and Wnt pathways [94]. Interestingly, there is a growing volume of literature that implicates this kinase in viral biology. Viral proteins of HIV-1, CMV and oncogenic EBV, HPV-16, HTLV, and HCV have been shown to be phosphorylated by CK2, in order to achieve efficient replication and other important viral functions [95]. Specifically, HCV NS5A was demonstrated to be phosphorylated by CK2 at a Ser residue through N-terminus association [96]. Zheung et al. [61] showed that CK2 is constitutively associated with JAK1 and JAK2 and promotes STAT3 activation through JAK2 phosphorylation. The use of CK2 inhibitors has been shown to block STAT3 activation and lead cancer cells to apoptosis [97, 98]. In addition, CK2 has been shown to mediate the regulatory action of various cytokines [99]. More importantly, IL-6 employs CK2 to activate STAT3 through phosphorylation of both Ser and Tyr residues on the STAT3 polypeptide [98]. The relationship between CK2 and IL-6 goes both ways, since it has been shown that CK2 can up-regulate IL-6 gene expression in breast cancer [100]. Recently, there was evidence that CK2 may be required for BMP-mediated regulation in neurons and CK2 inhibitors specifically blocked SMAD phosphorylation, thereby hinting at CK2 involvement in the BMP/SMAD pathway activation [101].
In the light of those studies, we considered CK2 as a possible link between the STAT3 and BMP/SMAD pathways, employed by HCV core to alter HAMP gene expression. We used a CK2-specific inhibitor in core-expressing cells and demonstrated that blocking CK2 activity was enough to inhibit activation and increased expression of both STAT3 and SMAD4. Thus, CK2 seemed to orchestrate the HCV core response by switching on these signaling cascades. The inhibitor quinalizarin managed to eradicate HCV core-mediated up-regulation of HAMP mRNA and protein expression. The involvement of CK2 was validated further with a dominant negative CK2 construct, which following transfection in pTRE/C2-3 cells diminished the HCV core-induced regulation of HAMP mRNA expression. At the same time, it abolished the up-regulation of HAMP gene promoter when transfected in Huh7 cells. Similarly, following siRNA analysis with CK2 siRNA oligonucleotides in Huh7 cells transfected with full-length core, the elevated hepcidin secreted peptide levels were reduced by half. Interestingly, co-transfection of a CK2α expression plasmid with c191 in Huh7 cells increased dramatically HAMP promoter activity in a synergistic way with core. Moreover, we provide evidence for the first time that HCV core modulates CK2 activity and expression as CK2 activity assay revealed up-regulation of CK2 activity in the presence of HCV core, which was accompanied by an increase in CK2 protein levels in Huh7 cells transfected with c191. Notably, while this work was in progress, two studies demonstrated that CK2 may interact with HCV core. In the first one, Ngo and colleagues used functional proteomics and protein array analysis to identify cellular partners of core protein. CK2α′ was included in the list of approximately 100 proteins that were found to interact with HCV core, however the authors did not verify or study this relationship further [53]. The second study described an up-regulation of the CK2β subunit protein levels in the liver of transgenic mice expressing HCV core and HCV ARFP proteins, which the authors linked to increased Wnt signaling observed in these mice [102].
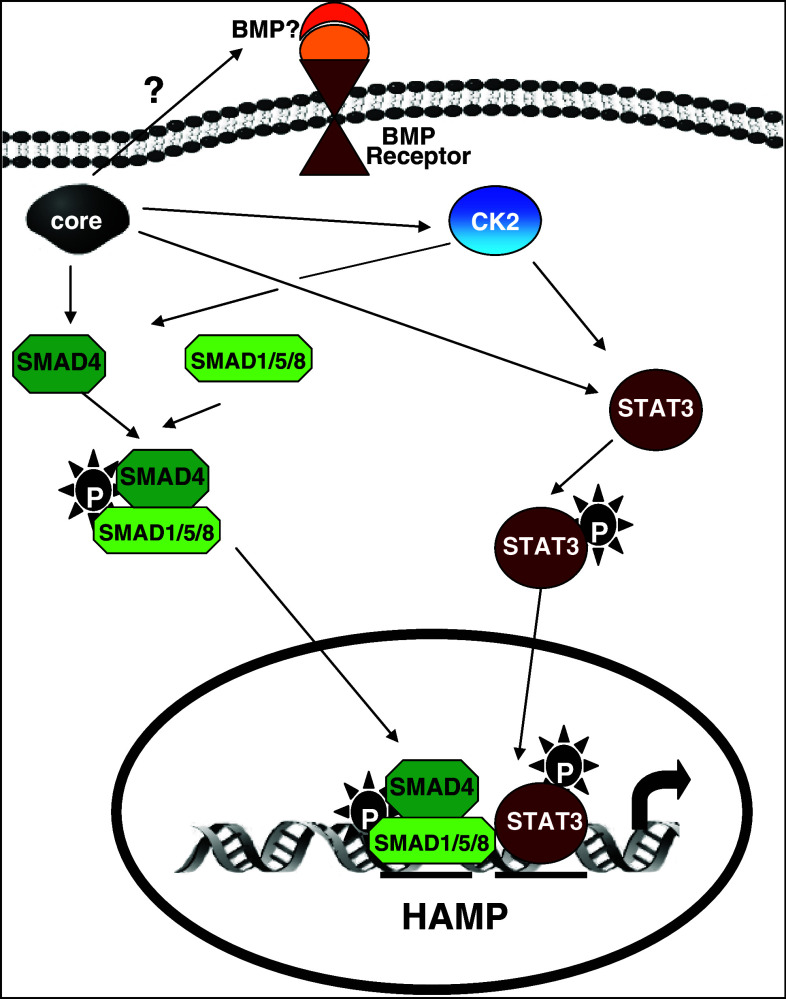
In conclusion, in this study we demonstrated that the key regulator of iron homeostasis and acute phase protein, hepcidin, is up-regulated by HCV core via a complex signaling network that employs enhanced expression and activation of STAT3, SMAD4, and CK2 (Fig. 6). To our knowledge, this is the first study to suggest involvement of CK2 in hepcidin regulation and importantly to provide evidence on the CK2-mediated activation of the STAT3 and BMP/SMAD pathways by HCV core. This interplay between HCV core and CK2 may involve multiple levels of regulation of the CK2 subunits and needs to be further explored, especially in the light of CK2 involvement in HCC that render it a promising therapeutic target.
Fig. 6.
Schematic diagram of the proposed model of HCV-mediated transcriptional regulation of the HAMP gene. HCV core up-regulates HAMP gene expression through interaction with CK2 that confers activation of the STAT3 and BMP/SMAD signaling pathways. The respective transcription factors enter the nucleus and occupy the STAT and BMP cis-acting elements in HAMP proximal promoter, resulting in elevated HAMP gene expression promoter activity, mRNA, and protein levels. P phosphorylation events, ? unknown reaction steps
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are grateful to Dr. P. Lee (The Scripps Research Institute, USA), Prof. M.U. Muckenthaler (University of Heidelberg, Germany), Dr. J.E. Darnell (Rockefeller University, NY, USA), Dr. R. Derynck (University of California at San Francisco) and Dr. C. Cochet (INSERM U1036, Grenoble, France) for providing promoter constructs and expression plasmids used in this study. Financial support was provided in the context of project 09SYN-12-682, which is implemented under the auspices of NSRF and the National Range Action “COOPERATION” and co-funded by the Greek Government and the European Union - European Regional Development Fund.
Footnotes
P. Foka and A. Dimitriadis contributed equally to this work and should be regarded as joint first authors.
Contributor Information
Urania Georgopoulou, Email: uraniag@pasteur.gr.
Avgi Mamalaki, Email: amamalaki@pasteur.gr.
References
- 1.Valore EV, Ganz T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells Mol Dis. 2008;40(1):132–138. doi: 10.1016/j.bcmd.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1(3):191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Camaschella C, Poggiali E. Inherited disorders of iron metabolism. Curr Opin Pediatr. 2011;23(1):14–20. doi: 10.1097/MOP.0b013e3283425591. [DOI] [PubMed] [Google Scholar]
- 5.Finberg KE. Regulation of systemic iron homeostasis. Curr Opin Hematol. 2013;20(3):208–214. doi: 10.1097/MOH.0b013e32835f5a47. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101(7):2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 7.Pietrangelo A. Hepcidin in human iron disorders: therapeutic implications. J Hepatol. 2011;54(1):173–181. doi: 10.1016/j.jhep.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, Lin HY. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38(5):531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 9.Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109(1):353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 10.Bayele HK, McArdle H, Srai SK. Cis and trans regulation of hepcidin expression by upstream stimulatory factor. Blood. 2006;108(13):4237–4245. doi: 10.1182/blood-2005-07-027037. [DOI] [PubMed] [Google Scholar]
- 11.Casanovas G, Mleczko-Sanecka K, Altamura S, Hentze MW, Muckenthaler MU. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J Mol Med (Berl) 2009;87(5):471–480. doi: 10.1007/s00109-009-0447-2. [DOI] [PubMed] [Google Scholar]
- 12.Courselaud B, Pigeon C, Inoue Y, Inoue J, Gonzalez FJ, Leroyer P, Gilot D, Boudjema K, Guguen-Guillouzo C, Brissot P, Loreal O, Ilyin G. C/EBPalpha regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. Cross-talk between C/EBP pathway and iron metabolism. J Biol Chem. 2002;277(43):41163–41170. doi: 10.1074/jbc.M202653200. [DOI] [PubMed] [Google Scholar]
- 13.Island ML, Fatih N, Leroyer P, Brissot P, Loreal O. GATA-4 transcription factor regulates hepatic hepcidin expression. Biochem J. 2011;437(3):477–482. doi: 10.1042/BJ20110225. [DOI] [PubMed] [Google Scholar]
- 14.Mleczko-Sanecka K, Casanovas G, Ragab A, Breitkopf K, Muller A, Boutros M, Dooley S, Hentze MW, Muckenthaler MU. SMAD7 controls iron metabolism as a potent inhibitor of hepcidin expression. Blood. 2010;115(13):2657–2665. doi: 10.1182/blood-2009-09-238105. [DOI] [PubMed] [Google Scholar]
- 15.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117(7):1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truksa J, Lee P, Beutler E. The role of STAT, AP-1, E-box and TIEG motifs in the regulation of hepcidin by IL-6 and BMP-9: lessons from human HAMP and murine Hamp1 and Hamp2 gene promoters. Blood Cells Mol Dis. 2007;39(3):255–262. doi: 10.1016/j.bcmd.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weizer-Stern O, Adamsky K, Margalit O, Ashur-Fabian O, Givol D, Amariglio N, Rechavi G. Hepcidin, a key regulator of iron metabolism, is transcriptionally activated by p53. Br J Haematol. 2007;138(2):253–262. doi: 10.1111/j.1365-2141.2007.06638.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu S, Zhang K, Lv C, Wang H, Cheng B, Jin Y, Chen Q, Lian Q, Fang X. Nuclear factor-kappaB mediated lipopolysaccharide-induced mRNA expression of hepcidin in human peripheral blood leukocytes. Innate Immun. 2012;18(2):318–324. doi: 10.1177/1753425911405087. [DOI] [PubMed] [Google Scholar]
- 19.Poenisch M, Bartenschlager R. New insights into structure and replication of the hepatitis C virus and clinical implications. Semin Liver Dis. 2010;30(4):333–347. doi: 10.1055/s-0030-1267535. [DOI] [PubMed] [Google Scholar]
- 20.Adinolfi LE, Durante-Mangoni E, Zampino R, Ruggiero G. Review article: hepatitis C virus-associated steatosis–pathogenic mechanisms and clinical implications. Aliment Pharmacol Ther. 2005;22(Suppl 2):52–55. doi: 10.1111/j.1365-2036.2005.02597.x. [DOI] [PubMed] [Google Scholar]
- 21.Lonardo A, Loria P, Carulli N. Dysmetabolic changes associated with HCV: a distinct syndrome? Intern Emerg Med. 2008;3(2):99–108. doi: 10.1007/s11739-008-0127-1. [DOI] [PubMed] [Google Scholar]
- 22.Metwally MA, Zein CO, Zein NN. Clinical significance of hepatic iron deposition and serum iron values in patients with chronic hepatitis C infection. Am J Gastroenterol. 2004;99(2):286–291. doi: 10.1111/j.1572-0241.2004.04049.x. [DOI] [PubMed] [Google Scholar]
- 23.Isom HC, McDevitt EI, Moon MS. Elevated hepatic iron: a confounding factor in chronic hepatitis C. Biochim Biophys Acta. 2009;1790(7):650–662. doi: 10.1016/j.bbagen.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Fujita N, Sugimoto R, Takeo M, Urawa N, Mifuji R, Tanaka H, Kobayashi Y, Iwasa M, Watanabe S, Adachi Y, Kaito M. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13(1–2):97–104. doi: 10.2119/2006-00057.Fujita. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girelli D, Pasino M, Goodnough JB, Nemeth E, Guido M, Castagna A, Busti F, Campostrini N, Martinelli N, Vantini I, Corrocher R, Ganz T, Fattovich G. Reduced serum hepcidin levels in patients with chronic hepatitis C. J Hepatol. 2009;51(5):845–852. doi: 10.1016/j.jhep.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanov AV, Bartosch B, Smirnova OA, Isaguliants MG, Kochetkov SN, Ivanov AV, Smirnova OA, Ivanova ON, Masalova OV, Kochetkov SN, Isaguliants MG. HCV and oxidative stress in the liver hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. Viruses. 2013;5(2):439–469. [Google Scholar]
- 27.Tsochatzis E, Papatheodoridis GV, Koliaraki V, Hadziyannis E, Kafiri G, Manesis EK, Mamalaki A, Archimandritis AJ. Serum hepcidin levels are related to the severity of liver histological lesions in chronic hepatitis C. J Viral Hepat. 2010;17(11):800–806. doi: 10.1111/j.1365-2893.2009.01244.x. [DOI] [PubMed] [Google Scholar]
- 28.Polyak SJ, Klein KC, Shoji I, Miyamura T, Lingappa JR. Assemble and Interact: Pleiotropic Functions of the HCV Core Protein. In: Tan SL, editor. Source Hepatitis C Viruses: Genomes and Molecular Biology, Chap 3. Horizon Bioscience: Norfolk; 2006. [PubMed] [Google Scholar]
- 29.Koike K. Steatosis, liver injury, and hepatocarcinogenesis in hepatitis C viral infection. J Gastroenterol. 2009;44(Suppl 19):82–88. doi: 10.1007/s00535-008-2276-4. [DOI] [PubMed] [Google Scholar]
- 30.McLauchlan J. Properties of the hepatitis C virus core protein: a structural protein that modulates cellular processes. J Viral Hepat. 2000;7(1):2–14. doi: 10.1046/j.1365-2893.2000.00201.x. [DOI] [PubMed] [Google Scholar]
- 31.Ait-Goughoulte M, Banerjee A, Meyer K, Mazumdar B, Saito K, Ray RB, Ray R. Hepatitis C virus core protein interacts with fibrinogen-beta and attenuates cytokine stimulated acute-phase response. Hepatology. 2010;51(5):1505–1513. doi: 10.1002/hep.23502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bode JG, Albrecht U, Haussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins—regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur J Cell Biol. 2012;91(6–7):496–505. doi: 10.1016/j.ejcb.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181(3):257–266. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 34.Johnson EE, Wessling-Resnick M. Iron metabolism and the innate immune response to infection. Microbes Infect. 2012;14(3):207–216. doi: 10.1016/j.micinf.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci USA. 2005;102(6):1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118(15):4129–4139. doi: 10.1182/blood-2011-04-351957. [DOI] [PubMed] [Google Scholar]
- 37.Fleming RE. Hepcidin activation during inflammation: make it STAT. Gastroenterology. 2007;132(1):447–449. doi: 10.1053/j.gastro.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 38.Park SO, Kumar M, Gupta S. TGF-beta and iron differently alter HBV replication in human hepatocytes through TGF-beta/BMP signaling and cellular microRNA expression. PLoS One. 2012;7(6):18. doi: 10.1371/journal.pone.0039276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truksa J, Lee P, Beutler E. Two BMP responsive elements, STAT, and bZIP/HNF4/COUP motifs of the hepcidin promoter are critical for BMP, SMAD1, and HJV responsiveness. Blood. 2009;113(3):688–695. doi: 10.1182/blood-2008-05-160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braliou GG, Verga Falzacappa MV, Chachami G, Casanovas G, Muckenthaler MU, Simos G. 2-Oxoglutarate-dependent oxygenases control hepcidin gene expression. J Hepatol. 2008;48(5):801–810. doi: 10.1016/j.jhep.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE., Jr Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18(5):2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998;394(6696):909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
- 43.Heriche JK, Lebrin F, Rabilloud T, Leroy D, Chambaz EM, Goldberg Y. Regulation of protein phosphatase 2A by direct interaction with casein kinase 2alpha. Science. 1997;276(5314):952–955. doi: 10.1126/science.276.5314.952. [DOI] [PubMed] [Google Scholar]
- 44.Salvi M, Sarno S, Marin O, Meggio F, Itarte E, Pinna LA. Discrimination between the activity of protein kinase CK2 holoenzyme and its catalytic subunits. FEBS Lett. 2006;580(16):3948–3952. doi: 10.1016/j.febslet.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 45.Katsarou K, Tsitoura P, Georgopoulou U. MEK5/ERK5/mef2: a novel signaling pathway affected by hepatitis C virus non-enveloped capsid-like particles. Biochim Biophys Acta. 2011;10:1854–1862. doi: 10.1016/j.bbamcr.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Foka P, Pourchet A, Hernandez-Alcoceba R, Doumba PP, Pissas G, Kouvatsis V, Dalagiorgou G, Kazazi D, Marconi P, Foschini M, Manservigi R, Konstadoulakis MM, Koskinas J, Epstein AL, Mavromara P. Novel tumour-specific promoters for transcriptional targeting of hepatocellular carcinoma by herpes simplex virus vectors. J Gene Med. 2010;12(12):956–967. doi: 10.1002/jgm.1519. [DOI] [PubMed] [Google Scholar]
- 47.Keller C, Keller P, Marshal S, Pedersen BK. IL-6 gene expression in human adipose tissue in response to exercise—effect of carbohydrate ingestion. J Physiol. 2003;550(Pt 3):927–931. doi: 10.1113/jphysiol.2003.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moriguchi M, Terai C, Koseki Y, Uesato M, Nakajima A, Inada S, Nishinarita M, Uchida S, Kim SY, Chen CL, Kamatani N. Influence of genotypes at SAA1 and SAA2 loci on the development and the length of latent period of secondary AA-amyloidosis in patients with rheumatoid arthritis. Hum Genet. 1999;105(4):360–366. doi: 10.1007/s004399900150. [DOI] [PubMed] [Google Scholar]
- 49.Tsitoura E, Thomas J, Cuchet D, Thoinet K, Mavromara P, Epstein AL. Infection with herpes simplex type 1-based amplicon vectors results in an IRF3/7-dependent, TLR-independent activation of the innate antiviral response in primary human fibroblasts. J Gen Virol. 2009;90(Pt 9):2209–2220. doi: 10.1099/vir.0.012203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koliaraki V, Marinou M, Samiotaki M, Panayotou G, Pantopoulos K, Mamalaki A. Iron regulatory and bactericidal properties of human recombinant hepcidin expressed in Pichia pastoris . Biochimie. 2008;90(5):726–735. doi: 10.1016/j.biochi.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Katsarou K, Lavdas AA, Tsitoura P, Serti E, Markoulatos P, Mavromara P, Georgopoulou U. Endocytosis of hepatitis C virus non-enveloped capsid-like particles induces MAPK-ERK1/2 signaling events. Cell Mol Life Sci. 2010;67(14):2491–2506. doi: 10.1007/s00018-010-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsitoura P, Georgopoulou U, Petres S, Varaklioti A, Karafoulidou A, Vagena D, Politis C, Mavromara P. Evidence for cellular uptake of recombinant hepatitis C virus non-enveloped capsid-like particles. FEBS Lett. 2007;581(21):4049–4057. doi: 10.1016/j.febslet.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 53.Ngo HT, Pham LV, Kim JW, Lim YS, Hwang SB. Modulation of mitogen-activated protein kinase-activated protein kinase 3 by hepatitis C virus core protein. J Virol. 2013;87(10):5718–5731. doi: 10.1128/JVI.03353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selimovic D, El-Khattouti A, Ghozlan H, Haikel Y, Abdelkader O, Hassan M. Hepatitis C virus-related hepatocellular carcinoma: an insight into molecular mechanisms and therapeutic strategies. World. 2012;4(12):342–355. doi: 10.4254/wjh.v4.i12.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan XB, Chen Z, Brechot C. Associations among genotype 1b hepatitis C virus core protein, protein kinase R, and signal transducer and activator of transcription 3. Hepat Mon. 2010;10(4):275–284. [PMC free article] [PubMed] [Google Scholar]
- 56.Verga Falzacappa MV, Casanovas G, Hentze MW, Muckenthaler MU. A bone morphogenetic protein (BMP)—responsive element in the hepcidin promoter controls HFE2-mediated hepatic hepcidin expression and its response to IL-6 in cultured cells. J Mol Med (Berl) 2008;86(5):531–540. doi: 10.1007/s00109-008-0313-7. [DOI] [PubMed] [Google Scholar]
- 57.Anderson GJ, Darshan D. Small-molecule dissection of BMP signaling. Nat Chem Biol. 2008;4(1):15–16. doi: 10.1038/nchembio0108-15. [DOI] [PubMed] [Google Scholar]
- 58.Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, ten Dijke P. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16(17):5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hagihara K, Nishikawa T, Isobe T, Song J, Sugamata Y, Yoshizaki K. IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system. Biochem Biophys Res Commun. 2004;314(2):363–369. doi: 10.1016/j.bbrc.2003.12.096. [DOI] [PubMed] [Google Scholar]
- 60.Bragdon B, Thinakaran S, Moseychuk O, King D, Young K, Litchfield DW, Petersen NO, Nohe A. Casein kinase 2 beta-subunit is a regulator of bone morphogenetic protein 2 signaling. Biophys J. 2010;99(3):897–904. doi: 10.1016/j.bpj.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng Y, Qin H, Frank SJ, Deng L, Litchfield DW, Tefferi A, Pardanani A, Lin FT, Li J, Sha B, Benveniste EN. A CK2-dependent mechanism for activation of the JAK-STAT signaling pathway. Blood. 2011;118(1):156–166. doi: 10.1182/blood-2010-01-266320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cozza G, Mazzorana M, Papinutto E, Bain J, Elliott M, di Maira G, Gianoncelli A, Pagano MA, Sarno S, Ruzzene M, Battistutta R, Meggio F, Moro S, Zagotto G, Pinna LA. Quinalizarin as a potent, selective and cell-permeable inhibitor of protein kinase CK2. Biochem J. 2009;421(3):387–395. doi: 10.1042/BJ20090069. [DOI] [PubMed] [Google Scholar]
- 63.Nagashima M, Kudo M, Chung H, Ishikawa E, Hagiwara S, Nakatani T, Dote K. Regulatory failure of serum prohepcidin levels in patients with hepatitis C. Hepatol Res. 2006;36(4):288–293. doi: 10.1016/j.hepres.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, Furutani T, Sakai A, Okuda M, Hidaka I, Okita K, Sakaida I. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134(1):226–238. doi: 10.1053/j.gastro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 65.Miura K, Taura K, Kodama Y, Schnabl B, Brenner DA. Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology. 2008;48(5):1420–1429. doi: 10.1002/hep.22486. [DOI] [PubMed] [Google Scholar]
- 66.Moriya K, Miyoshi H, Shinzawa S, Tsutsumi T, Fujie H, Goto K, Shintani Y, Yotsuyanagi H, Koike K. Hepatitis C virus core protein compromises iron-induced activation of antioxidants in mice and HepG2 cells. J Med Virol. 2010;82(5):776–792. doi: 10.1002/jmv.21661. [DOI] [PubMed] [Google Scholar]
- 67.Miyachi H, Kobayashi Y, Relja B, Fujita N, Iwasa M, Gabazza EC, Takei Y. Effect of suppressor of cytokine signaling on hepcidin production in hepatitis C virus replicon cells. Hepatol Res. 2011;41(4):364–374. doi: 10.1111/j.1872-034X.2011.00777.x. [DOI] [PubMed] [Google Scholar]
- 68.Kochlios E, Foka P, Tsitoura E, Doumba PP, Koskinas J, Mavromara P (2010) Effect of HCV core and core +1/S on pro- and anti-inflammatory cytokine and chemokine gene expression. In: 8th Joint Conference of the International Cytokine Society and the International Society for Interferon and Cytokine Research Chicago (USA), October 3–7, 2010, pp 21–24
- 69.Tacke RS, Tosello-Trampont A, Nguyen V, Mullins DW, Hahn YS. Extracellular hepatitis C virus core protein activates STAT3 in human monocytes/macrophages/dendritic cells via an IL-6 autocrine pathway. J Biol Chem. 2011;286(12):10847–10855. doi: 10.1074/jbc.M110.217653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aydemir F, Jenkitkasemwong S, Gulec S, Knutson MD. Iron loading increases ferroportin heterogeneous nuclear RNA and mRNA levels in murine J774 macrophages. J Nutr. 2009;139(3):434–438. doi: 10.3945/jn.108.094052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato J, Kobune M, Ohkubo S, Fujikawa K, Tanaka M, Takimoto R, Takada K, Takahari D, Kawano Y, Kohgo Y, Niitsu Y. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35(6):879–887. doi: 10.1016/j.exphem.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 72.Ramsay AJ, Hooper JD, Folgueras AR, Velasco G, Lopez-Otin C. Matriptase-2 (TMPRSS6): a proteolytic regulator of iron homeostasis. Haematologica. 2009;94(6):840–849. doi: 10.3324/haematol.2008.001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang AS, Anderson SA, Wang J, Yang F, DeMaster K, Ahmed R, Nizzi CP, Eisenstein RS, Tsukamoto H, Enns CA. Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase of matriptase-2 protein. Blood. 2011;117(5):1687–1699. doi: 10.1182/blood-2010-06-287292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41(4):482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Corradini E, Meynard D, Wu Q, Chen S, Ventura P, Pietrangelo A, Babitt JL. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011;54(1):273–284. doi: 10.1002/hep.24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci USA. 2006;103(27):10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, Deng CX. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2(6):399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 78.Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H, Roth MP. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112(4):1503–1509. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 79.Murata T, Ohshima T, Yamaji M, Hosaka M, Miyanari Y, Hijikata M, Shimotohno K. Suppression of hepatitis C virus replicon by TGF-beta. Virology. 2005;331(2):407–417. doi: 10.1016/j.virol.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 80.Sakamoto N, Yoshimura M, Kimura T, Toyama K, Sekine-Osajima Y, Watanabe M, Muramatsu M. Bone morphogenetic protein-7 and interferon-alpha synergistically suppress hepatitis C virus replicon. Biochem Biophys Res Commun. 2007;357(2):467–473. doi: 10.1016/j.bbrc.2007.03.167. [DOI] [PubMed] [Google Scholar]
- 81.Pavio N, Battaglia S, Boucreux D, Arnulf B, Sobesky R, Hermine O, Brechot C. Hepatitis C virus core variants isolated from liver tumor but not from adjacent non-tumor tissue interact with Smad3 and inhibit the TGF-beta pathway. Oncogene. 2005;24(40):6119–6132. doi: 10.1038/sj.onc.1208749. [DOI] [PubMed] [Google Scholar]
- 82.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schuringa JJ, Jonk LJ, Dokter WH, Vellenga E, Kruijer W. Interleukin-6-induced STAT3 transactivation and Ser727 phosphorylation involves Vav, Rac-1 and the kinase SEK-1/MKK-4 as signal transduction components. Biochem J. 2000;347(Pt 1):89–96. [PMC free article] [PubMed] [Google Scholar]
- 84.Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132(1):294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 85.Pandur E, Sipos K, Grama L, Nagy J, Poor VS, Setalo G, Miseta A, Fekete Z. Prohepcidin binds to the HAMP promoter and autoregulates its own expression. Biochem J. 2013;451(2):301–311. doi: 10.1042/BJ20121466. [DOI] [PubMed] [Google Scholar]
- 86.Choi SO, Cho YS, Kim HL, Park JW. ROS mediate the hypoxic repression of the hepcidin gene by inhibiting C/EBPalpha and STAT-3. Biochem Biophys Res Commun. 2007;356(1):312–317. doi: 10.1016/j.bbrc.2007.02.137. [DOI] [PubMed] [Google Scholar]
- 87.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Millonig G, Ganzleben I, Peccerella T, Casanovas G, Brodziak-Jarosz L, Breitkopf-Heinlein K, Dick TP, Seitz HK, Muckenthaler MU, Mueller S. Sustained submicromolar H2O2 levels induce hepcidin via signal transducer and activator of transcription 3 (STAT3) J Biol Chem. 2012;287(44):37472–37482. doi: 10.1074/jbc.M112.358911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M, Yoshimura A. Activation of STAT3 by the hepatitis C virus core protein leads to cellular transformation. J Exp Med. 2002;196(5):641–653. doi: 10.1084/jem.20012127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang H, Constante M, Layoun A, Santos MM. Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli. Blood. 2009;113(15):3593–3599. doi: 10.1182/blood-2008-08-173641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun CC, Vaja V, Babitt JL, Lin HY. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am J Hematol. 2012;87(4):392–400. doi: 10.1002/ajh.23110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4(1):33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sass G, Klinger N, Sirma H, Hashemolhosseini S, Hellerbrand C, Neureiter D, Wege H, Ocker M, Tiegs G. Inhibition of experimental HCC growth in mice by use of the kinase inhibitor DMAT. Int J Oncol. 2011;39(2):433–44294. doi: 10.3892/ijo.2011.1037. [DOI] [PubMed] [Google Scholar]
- 94.Ruzzene M, Pinna LA. Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim Biophys Acta. 2010;3:499–504. doi: 10.1016/j.bbapap.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 95.Bretana NA, Lu CT, Chiang CY, Su MG, Huang KY, Lee TY, Weng SL. Identifying protein phosphorylation sites with kinase substrate specificity on human viruses. PLoS One. 2012;7(7):23. doi: 10.1371/journal.pone.0040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim J, Lee D, Choe J. Hepatitis C virus NS5A protein is phosphorylated by casein kinase II. Biochem Biophys Res Commun. 1999;257(3):777–781. doi: 10.1006/bbrc.1999.0460. [DOI] [PubMed] [Google Scholar]
- 97.Lin YC, Hung MS, Lin CK, Li JM, Lee KD, Li YC, Chen MF, Chen JK, Yang CT. CK2 inhibitors enhance the radiosensitivity of human non-small cell lung cancer cells through inhibition of stat3 activation. Cancer Biother Radiopharm. 2011;26(3):381–388. doi: 10.1089/cbr.2010.0917. [DOI] [PubMed] [Google Scholar]
- 98.Piazza FA, Ruzzene M, Gurrieri C, Montini B, Bonanni L, Chioetto G, Di Maira G, Barbon F, Cabrelle A, Zambello R, Adami F, Trentin L, Pinna LA, Semenzato G. Multiple myeloma cell survival relies on high activity of protein kinase CK2. Blood. 2006;108(5):1698–1707. doi: 10.1182/blood-2005-11-013672. [DOI] [PubMed] [Google Scholar]
- 99.Harvey EJ, Li N, Ramji DP. Critical role for casein kinase 2 and phosphoinositide-3-kinase in the interferon-gamma-induced expression of monocyte chemoattractant protein-1 and other key genes implicated in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(4):806–812. doi: 10.1161/01.ATV.0000258867.79411.96. [DOI] [PubMed] [Google Scholar]
- 100.Drygin D, Ho CB, Omori M, Bliesath J, Proffitt C, Rice R, Siddiqui-Jain A, O’Brien S, Padgett C, Lim JK, Anderes K, Rice WG, Ryckman D. Protein kinase CK2 modulates IL-6 expression in inflammatory breast cancer. Biochem Biophys Res Commun. 2011;415(1):163–167. doi: 10.1016/j.bbrc.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 101.Chaverneff F, Barrett J. Casein kinase II contributes to the synergistic effects of BMP7 and BDNF on Smad 1/5/8 phosphorylation in septal neurons under hypoglycemic stress. J Neurochem. 2009;109(3):733–743. doi: 10.1111/j.1471-4159.2009.05990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu WT, Li HC, Lee SK, Ma HC, Yang CH, Chen HL, Lo SY. Both core and F proteins of hepatitis C virus could enhance cell proliferation in transgenic mice. Biochem Biophys Res Commun. 2013;435(1):147–152. doi: 10.1016/j.bbrc.2013.04.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.