Abstract
Genomic imprinting is an epigenetic gene silencing phenomenon that is specific to eutherians in the vertebrate lineage. The acquisition of both placentation and genomic imprinting has spurred interest in the possible evolutionary link for many years. In this review we examine the genetic evidence and find that while many imprinted domains are anchored by genes required for proper placenta development in a parent of origin fashion, an equal number of imprinted genes have no apparent function that depends on imprinting. Examination of recent data from studies of molecular and genetic mechanisms points to a maternal control of the selection and maintenance of imprint marks, reinforcing the importance of the oocyte in the healthy development of the placenta and fetus.
Keywords: Genomic imprinting, Placentation, Maternal effect, Differential methylation, Targeted mutation, Conflict hypothesis, Trophoblast, Oocyte
Introduction
Genomic imprinting was discovered in mammals 30 years ago with the publication of two seminal papers describing the same experiment: pronuclear exchange between fertilized zygotes generated two kinds of abnormal embryos—androgenotes (two paternal genomes) and gynogenotes (two maternal genomes) [1, 2]. Development of both kinds of embryo proceeded relatively normally to the blastocyst stage; however after implantation, embryogenesis in both groups began to go awry, but in different ways. Andogenotes that survived to mid-gestation possessed hyperplastic extraembryonic tissues of trophoblast origin to the exclusion of embryonic structures, while gynogenetic survivors at mid-gestation were characterized by poor to non-existent extraembryonic tissues of trophoblast origin and small, although normally developed embryos. The vast majority of androgenotes and gynogenotes die at or shortly after implantation [3], with occasional survival up to mid-gestation [4].
The results of these experiments suggested that one set of genes is silenced on the maternal allele, while another is silenced on the paternal allele [5, 6]. In the intervening 30 years, this prediction has been proven correct with the discovery of approximately 100 imprinted genes in mice and 50 in humans (the two most extensively studied species). Importantly, the discovery of genomic imprinting provided an explanation for several human genetic diseases whose inheritance patterns had stumped investigators for many years. The ensuing ramping up of research in this area provided the stimulus, both intellectual and financial, for studies in other fields of epigenetics, with the result that great progress in our understanding has been achieved, in part because technological strides have made it possible to address very complex questions in very specific ways.
The restriction of genomic imprinting in vertebrates to mammals is highly suggestive of an evolutionary link to placentation. Indeed, the phenotypes of androgenetic and gynogenetic embryos, with major defects in trophoblast, clearly indicate that at least early acting imprinted genes are involved in placenta development. These observations prompted proposals in the 1990s that genomic imprinting arose as an evolutionary protective mechanism to counter the potentially lethal effects of excessive placentation [7], or of ectopic trophoblast in females [8]. Other ideas about the evolution of imprinting have focused on functions outside of the extraembryonic tissues, including the brain [9, 10]. Widely cited is the popular conflict hypothesis [11] that has dominated thinking in the field for several decades. In this review we will examine the evidence that has accumulated in the past 30 years, with an emphasis on the roles played by imprinted genes in placenta development and function revealed by genetic manipulation.
Genomic imprinting has been studied in greatest depth in mice; while most of the studies in humans have been largely correlative, more recent high-throughput analyses in pathological placentas [12] or rare genome wide uniparental disomy (UPD) samples [13] revealed several novel imprinted genes, indicating that significant species differences probably exist. Studies in other species have been much more limited, although important observations of genomic imprinting in marsupials have provided insight into the evolutionary mechanisms [14]. This review will focus on the genetic evidence gathered in murine studies, with mention of other species where appropriate. Readers are also referred to a review by Tunster et al. [15].
Organization of imprinted genes and regulation of expression
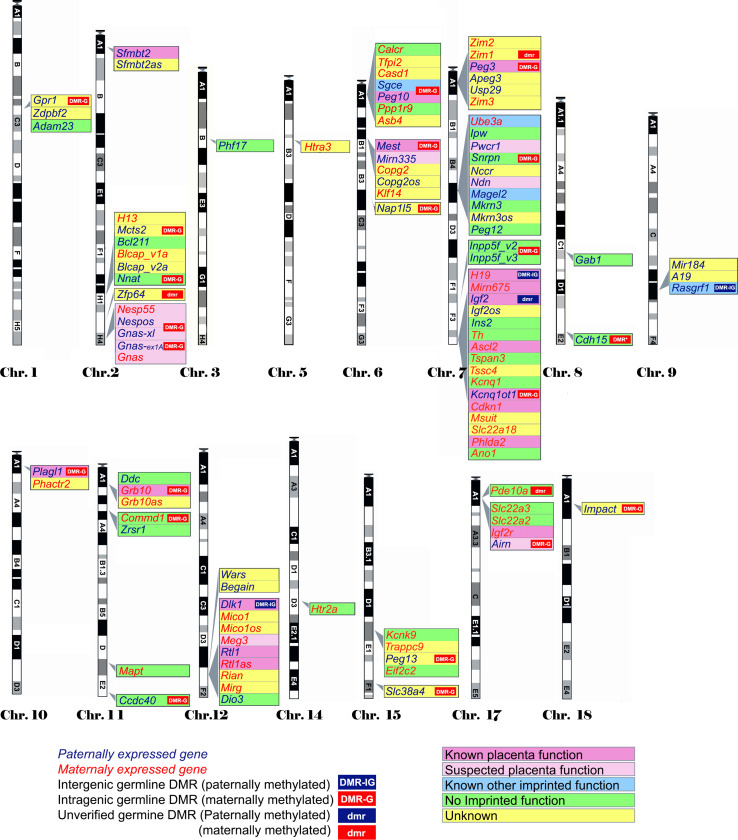
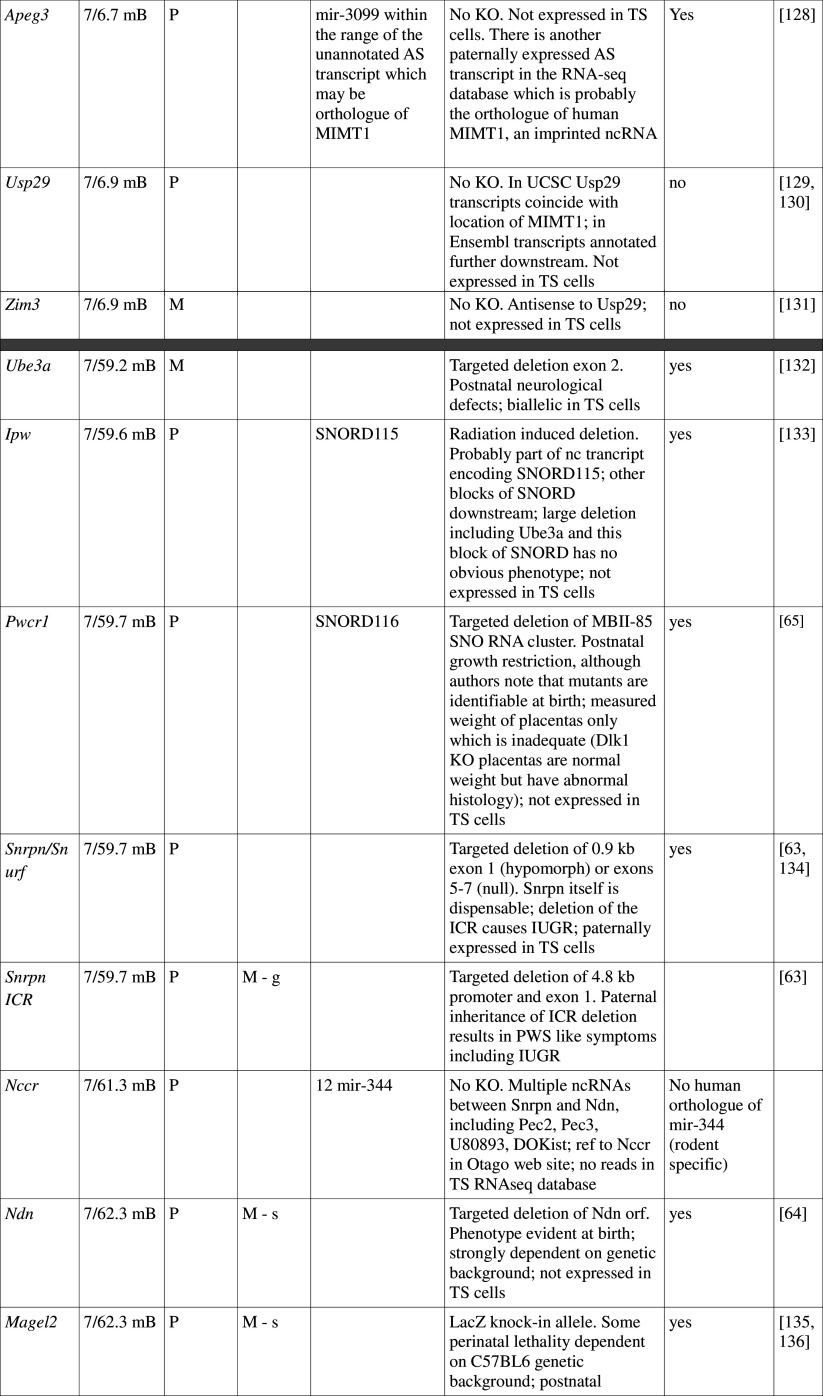
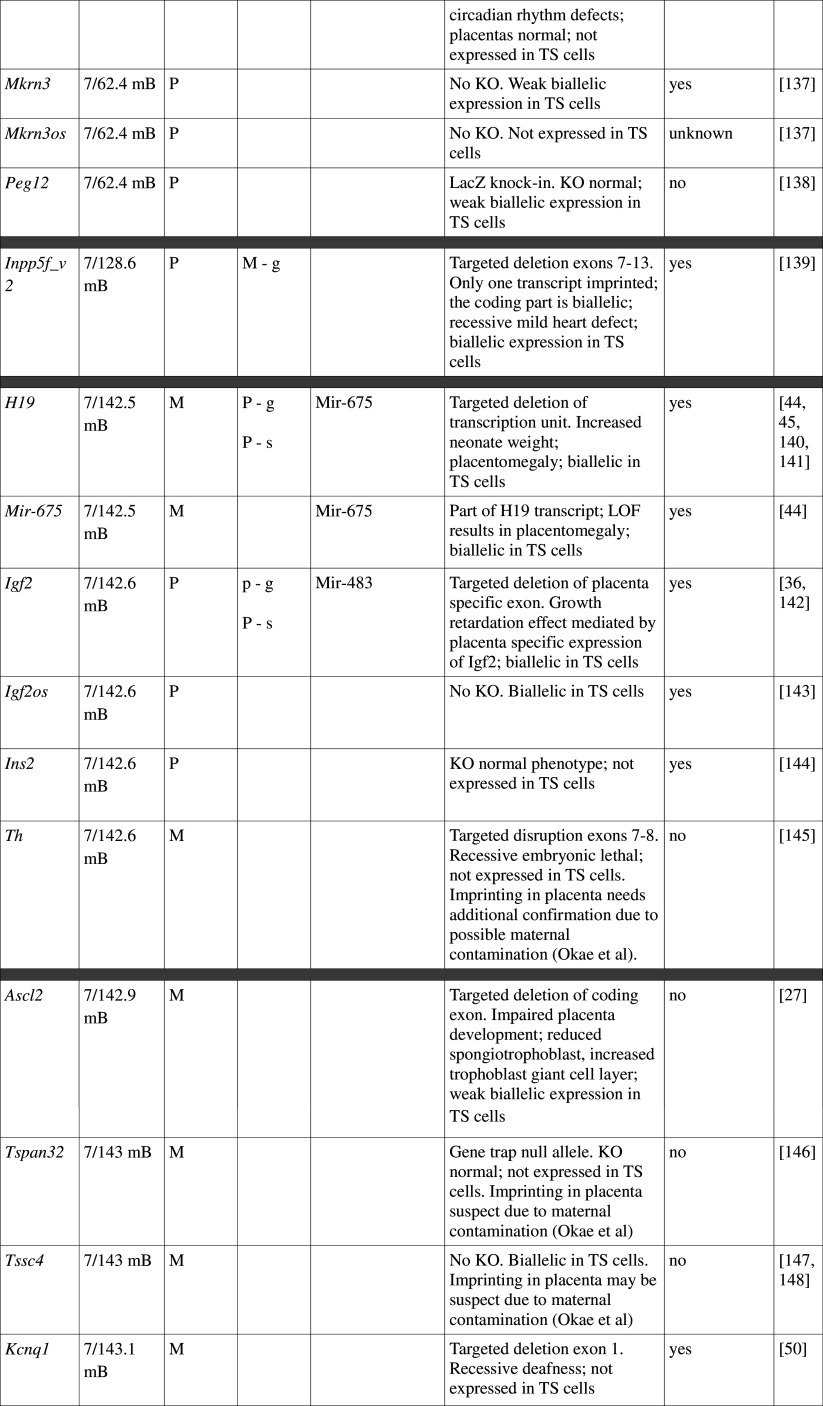
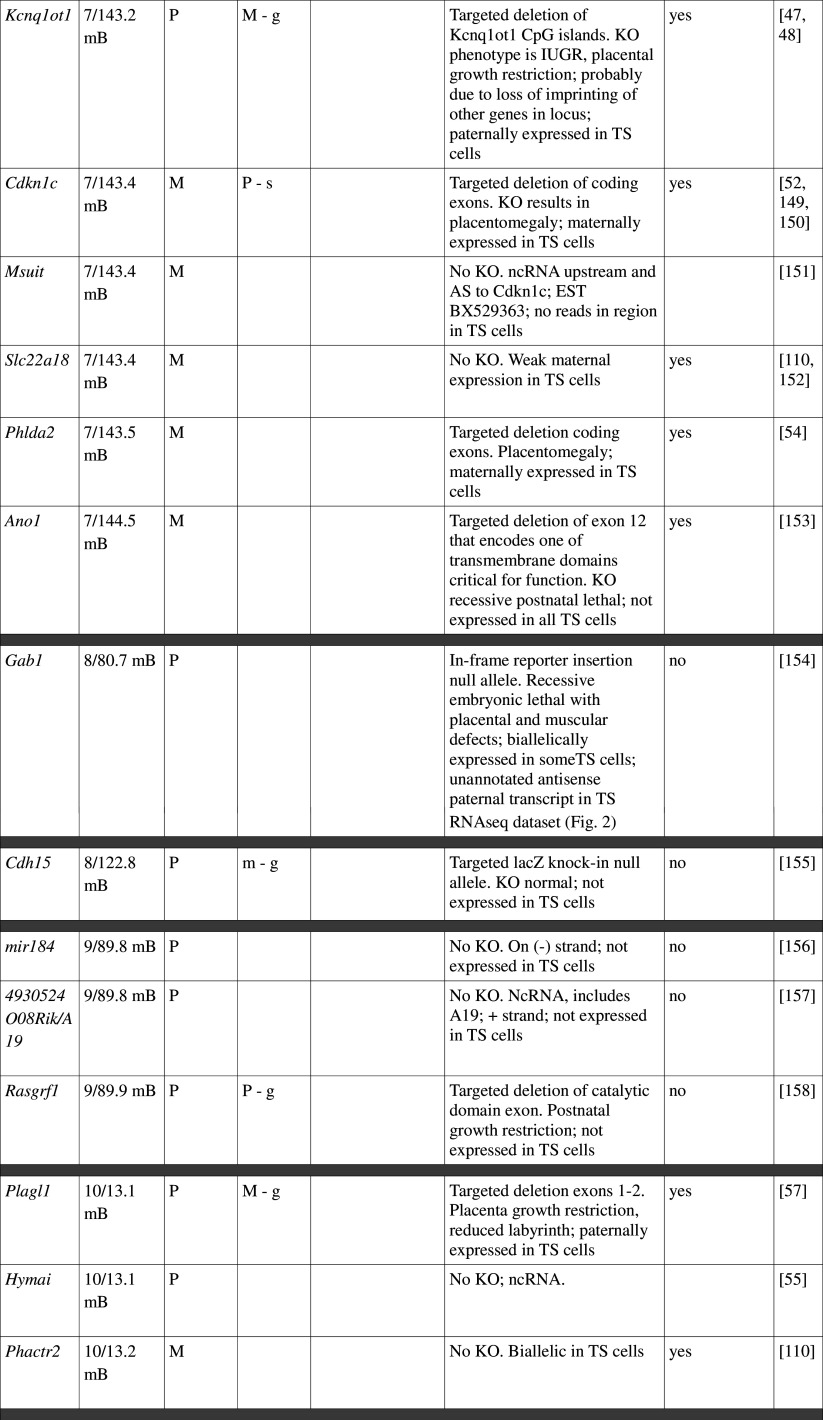
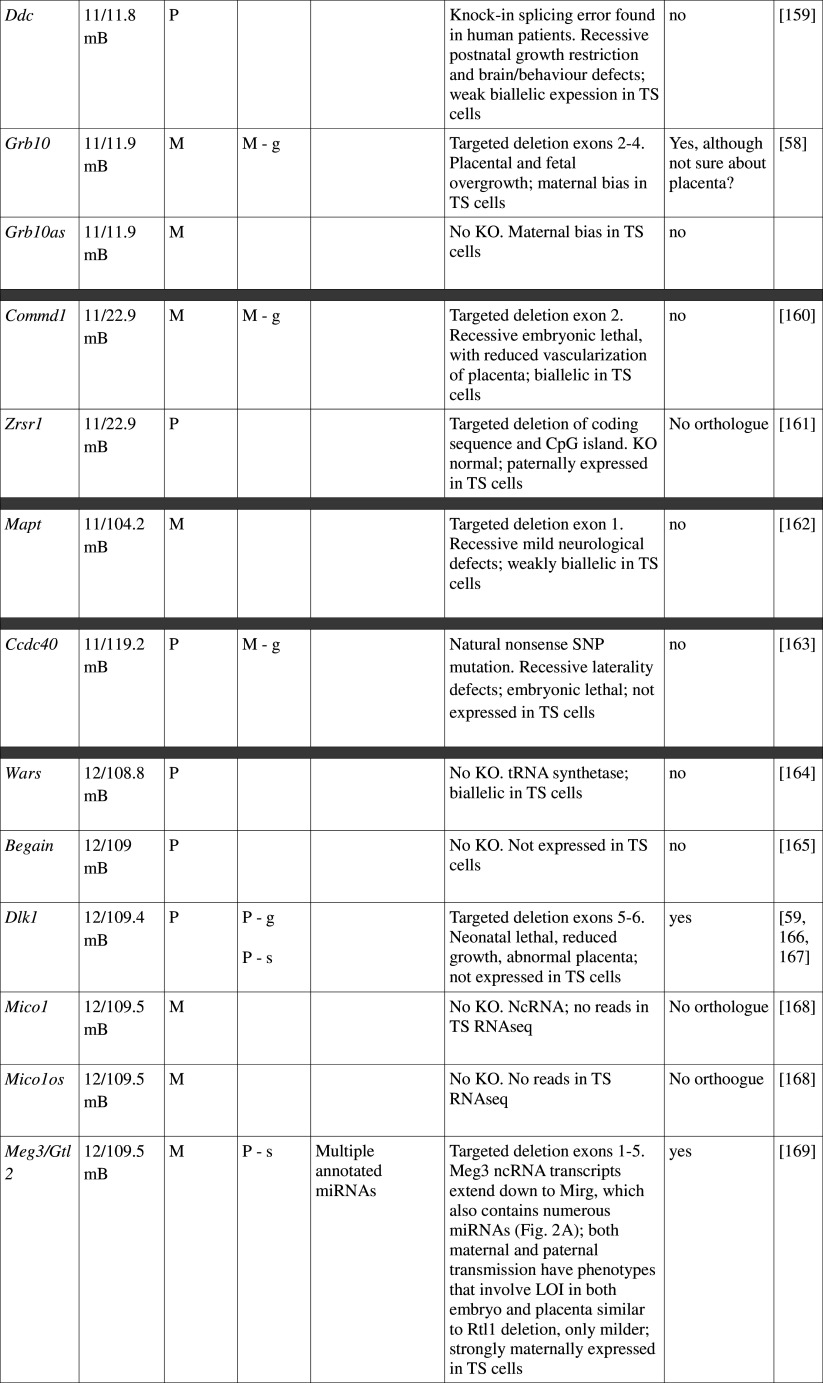
Many imprinted genes reside in clusters. However, this is not carved in stone. Of 31 imprinted gene loci in mice, 12 are represented by a single gene; imprinting of 10 is not conserved with humans, at least in the tissues assayed. This, and recent high-throughput data from human studies [12, 13], supports the idea that genomic imprinting is evolutionarily dynamic (see review by Renfree et al. [14]). Most, although not all, imprinted domains are regulated by a germline differentially methylated region (gDMR). Of the 23 confirmed gDMRs, 20 are methylated on the maternal allele, while 3 are paternally methylated (Fig. 1; Table 1). Multi-gene imprinted domains can contain genes that are maternally expressed, paternally expressed and biallelic, indicating that the gDMRs regulate chromatin structure over large distances rather than merely shutting off expression of a single target gene.
Fig. 1.
Map of imprinted genes. Known imprinted genes are displayed on a map of the mouse genome. Color coding highlights features discussed in the text, including known placental function (dark pink), suspected placental function (pale pink), non-placental imprinted function (blue), no imprinted function (green) and unknown (yellow). The location of verified gDMRs is indicated by capital letters and for unverified gDMRs by lowercase letters, with maternal DMRs represented by red boxes and paternal DMRs by blue boxes. Several domains do not possess gDMRs
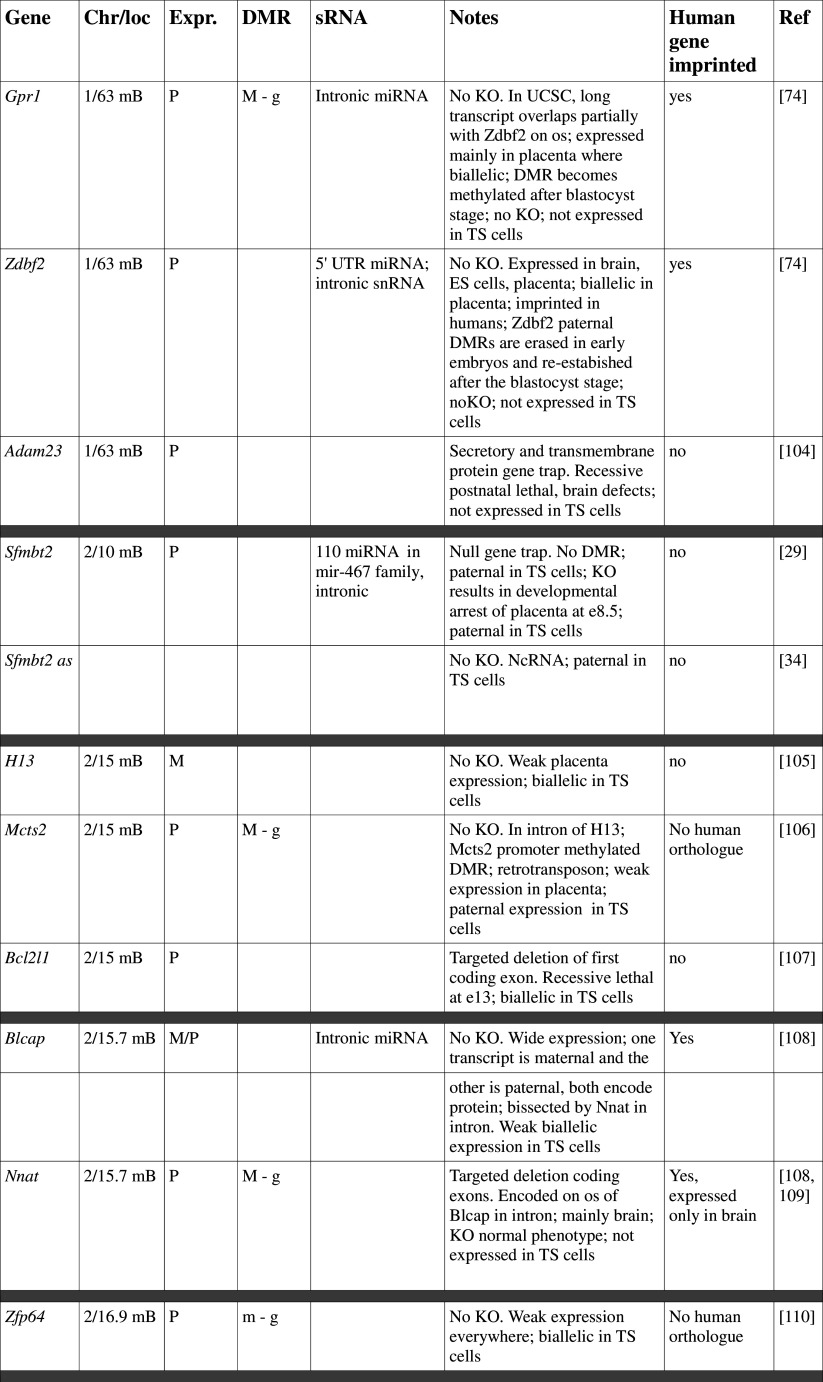
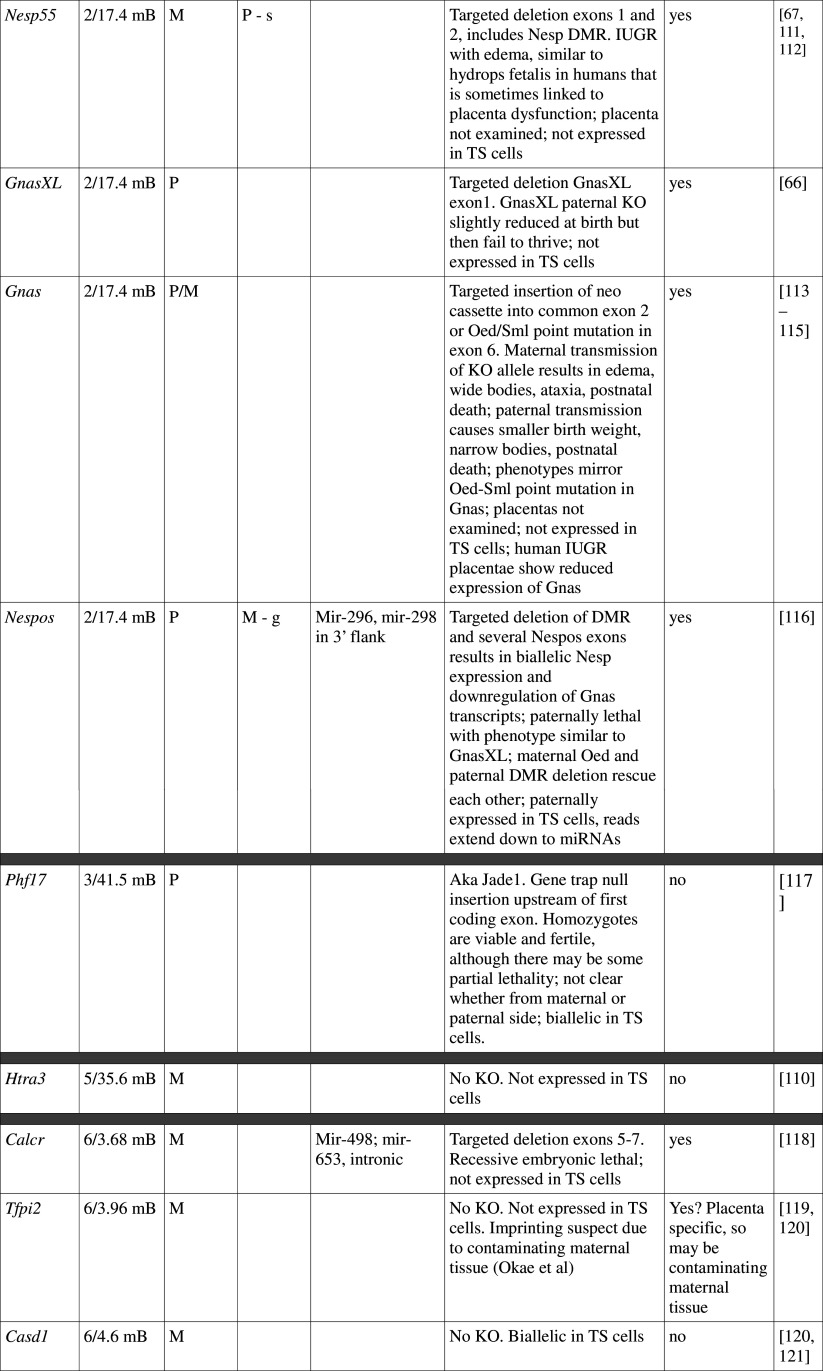
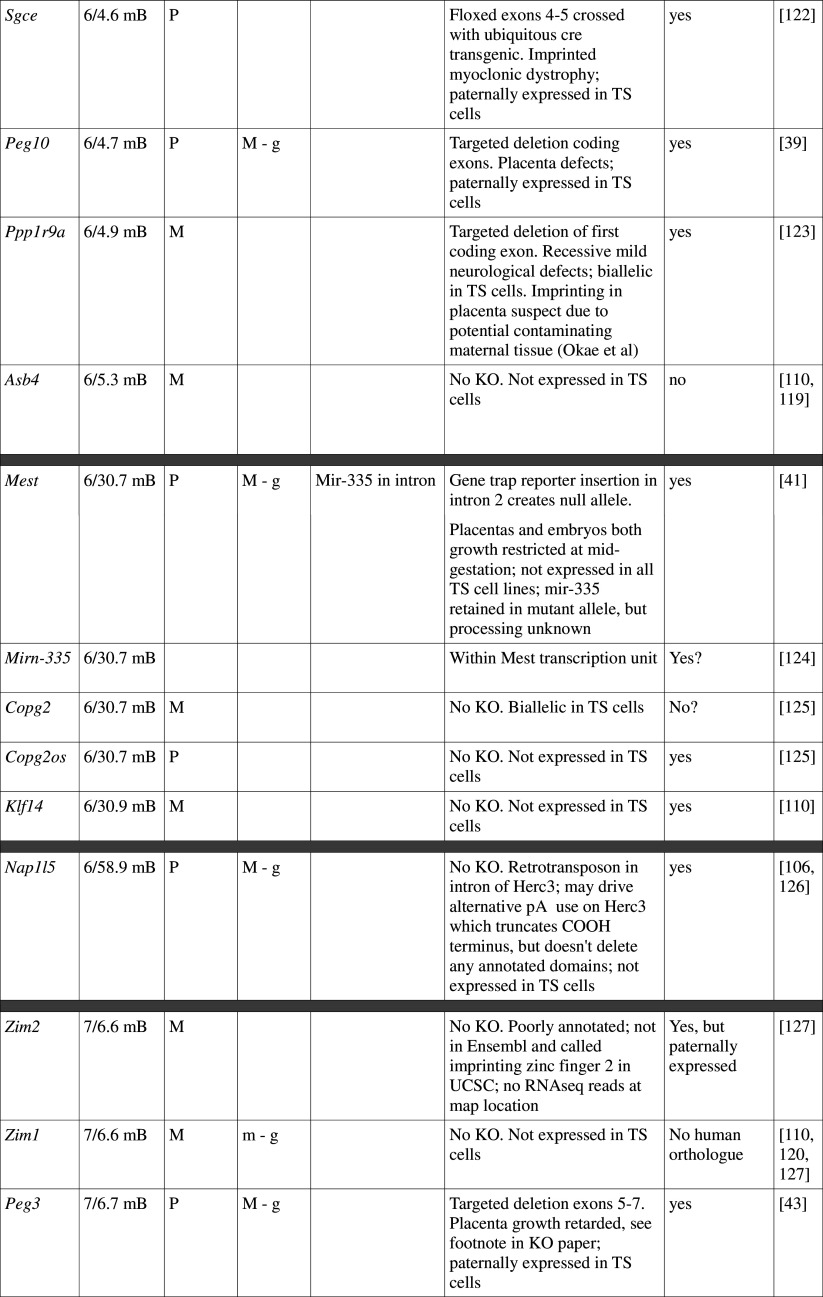
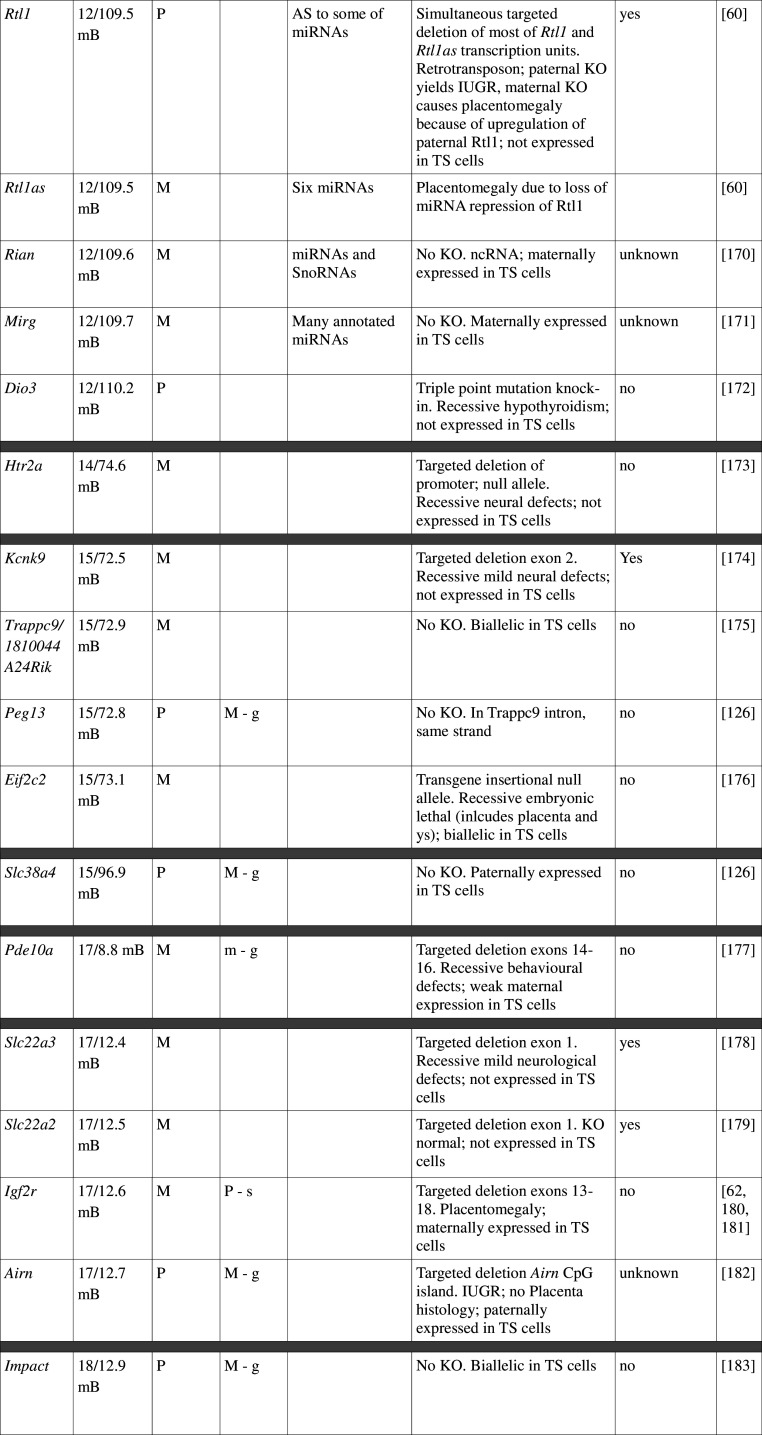
Table 1.
Imprinted gene function
Imprinted genes listed here were obtained from the Otago Catalogue of Parent of Origin Effects website (http://igc.otago.ac.nz/home.html). Where imprinting of a gene is listed as “provisional” or “other”, it has been left out of the table. TS cell expression data were obtained from the dataset published by Calabrese et al. [34]. DMRs with lowercase letters are derived from Kobayashi et al. [74] that have not been confirmed experimentally; “g” denotes germline; “s” denotes somatic. Annotated miRNAs are named unless there are too many. Domains are separated by grey bars
Maternal gDMRs are all associated with CpG islands near the transcriptional start sites (TSS) of protein-coding genes, while paternal gDMRs are intergenic. In general, methylation of the TSS CpG island is associated with repression of the target gene, whereas paternal intergenic gDMRs are associated with activation of at least one gene in the cluster, in two cases (H19 and Rasgrf1) through action of a CTCF boundary element [16, 17]. Interestingly, oocyte-specific knockdown of CTCF leads to methylation of the maternal H19 DMR, suggesting that methylation of this element may be the default state [18]. Indeed, germline-specific methylation as a default may be a general rule unrelated to imprinting, not surprising given the major differences between sperm and egg [19–21]. This point will be returned to later in the review.
Not all imprinted domains possess an identifiable gDMR. For example, the CpG island spanning the TSS of the paternally expressed murine Sfmbt2 gene is unmethylated [22]. This gene, imprinted only in old world rodents, is distinguished from orthologs in other species by the presence of a large block of miRNAs in intron 10. A similar situation exists in the older primate-specific C19M locus, although in this case a gDMR has been identified [23]. The correlation between the presence of the miRNAs and imprinting of the locus is suggestive of an RNA-based mechanism driving imprinting at this gene. Interestingly, imprinted genes are sixfold more likely to be closely associated with an miRNA than other biallelic genes. Two other imprinted domains are characterized by the presence of large blocks of either miRNAs or SnoRNAs, or both: the Dlk1 locus and the Snrpn locus. Interestingly, all but three—mir-296-5p, mir-483 and mir-494 (GEO accession GSE17966)—of the miRNAs associated with imprinted genes, regardless of their allelic expression bias later in development, are silent in oocytes. In addition, several loci are regulated by ncRNAs (Kcnq1ot1, Airn, Xist, H19); additional loci may be found to be similarly regulated as high-throughput datasets reveal unknown non-coding transcripts (e.g., Fig. 2).
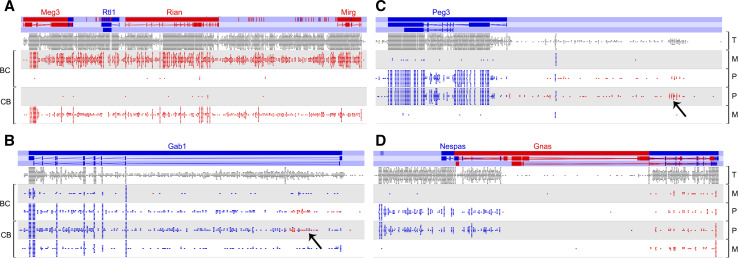
Fig. 2.
TS cell RNA-seq allele-specific expression. RNA-seq SNP specific reads from Calabrese et al. [34] are displayed using SeqMonk Mapped Sequence Data Analyser (http://www.bioinformatics.babraham.ac.uk/projects), version 0.24.1, onto the NCBIM37 genome. It should be noted that there is variability among TS cell lines in expression of genes, including imprinted genes [29]. Forward strand is red; reverse strand is blue. Arrows highlight unannotated antisense transcripts. The top two tracks of each panel are from C57BL6 X Cast TS cells (BC); the bottom two tracks are from Cast X C57BL6 TS cells (CB); T total, M maternal, P paternal. a Meg3 to Mirg interval, including all of the miRNAs at the locus. Note the lack of expression of Rtl1. b Gab1 is biallelic except for an unannotated antisense transcript that overlaps the region assayed by Okae et al. [31]. c Peg3. d Gnas locus. Images are screenshots of SeqMonk output and are not to scale
Imprinted genes in placenta
The notion that imprinting and placentation are evolutionarily linked is reinforced by the observation that a number of genes display extraembryonic tissue-only imprinting/expression; this includes both placenta and yolk sac. The most compelling evidence comes from a series of targeted mutations of imprinted genes (Table 1). In particular, genes closest to the gDMR, which may be the primary target of imprinting at a multi-gene domain (referred to below as the anchoring gene), often show a placenta phenotype. For example, Peg10, whose DMR regulates a domain on proximal Chr. 6, is required for the development of the spongiotrophoblast layer of the placenta; loss of function (LOF) is embryonic lethal because of failed placentation. A similar scenario can be described for several other genes that anchor imprinted domains (see below).
A recent analysis of imprinted genes restricted to extraembryonic tissues (EXEL) suggested that imprinting in the placenta and yolk sac may be governed by a different epigenetic mechanism [24, 25] than the traditional gDMR-regulated silencing, in line with an earlier study of genes at the Kcnq1 locus [26]. A similar interpretation was made following the discovery of several novel placenta-specific imprinted genes in humans [13]. However, some caution should be used in the interpretation of the data, especially allelic expression in placenta, which is “contaminated” with maternal tissue. Examination of the data in mice, for example, reveals that of 16 so-called EXEL genes, strong evidence exists for imprinting of only five (Ascl2 [27]: Ins2 [28]; Sfmbt2 [29]; Slc22a2; and Slc22a3 [30]); for most of the rest, imprinting can be discounted due to contamination by maternal tissue, or very weak maternal bias [31]. In the human studies, which focused on differential methylation [12, 13], the number of DMRs far outnumbered the number of genes showing consistent monoallelic expression in placenta, and between the two studies there was minimal overlap, suggesting that methodology may also introduce artifacts. As an example of the latter, the initial report of the murine Sfmbt2 gene described differential methylation of the maternal allele in placenta [32], based on PCR–RFLP analysis of genomic DNA that had been digested with methylation-sensitive restriction enzymes. However, in a later report [22], bisulfite sequence analysis clearly demonstrated very low methylation levels of both alleles in placenta, not surprising given the resistance of extraembryonic lineages to loss of DNA methyltransferases [33]. The most robust test of imprinting is derived from genetic analysis of targeted mutations, either reporter constructs in which expression is visualized following transmission from only one parent, or transcriptional null alleles in which expression of the endogenous gene can be assessed following transmission from either the mother or father.
Many imprinted genes (29) appear to be “innocent bystanders” [8]. Their loss is either without any effect (e.g., Nnat, Peg12) or, while they are clearly required for survival, this function is not dependent on imprinted expression, since it can only be observed when both alleles are mutated (e.g., Commd1, Dcn, Eif2c2), suggesting that the key expression is in a tissue or at a stage of development when both alleles are expressed and the imprints have been reprogrammed during development. There remains the possibility that a subtle imprinted effect has been overlooked in the phenotypic analysis of these mutations.
Targeted mutations in several anchoring imprinted genes have known phenotypes affecting the placenta. In some additional cases, placenta function can only be inferred from the reported phenotype, such as intrauterine growth restriction (IUGR), because the authors did not perform any placental analysis, or limited examination to measurement of weight. Many genes also have functions in later stages of development or in postnatal animals, in particular in the brain. This review will discuss only the placenta or early development functions of imprinted gene mutations. Given that almost the entire X chromosome is imprinted in trophoblast [34], we will only discuss autosomal genes.
Imprinted genes with known placenta function
Of the 31 imprinted domains, 10 are anchored by genes with known function in the placenta and 2 by genes with suspected placental function. The remaining 20 domains harbor genes with no known function or with no imprinted function (see Fig. 1). The relevance of these domains will be discussed below. Some large domains contain more than one gene required for proper placenta development and can be expressed either maternally or paternally. The following are brief descriptions of what we know about their roles in placentation. Analysis of placenta development and function is variable as there are no standard protocols, although several laboratories have adopted some sophisticated methodologies that allow examination of more subtle phenotypes [35], including physiological adaptation to reduced placental mass [36], demonstrating the capacity of the placenta to work overtime when pressed, and altered nutrient management [37]. This kind of thorough examination is warranted especially in cases where the phenotype does not “jump up off the bench and hit you on the head”.
Sfmbt2 is part of a murine-specific domain on proximal Chr. 2 consisting of the protein-coding gene and an antisense non-coding transcript (ncRNA); both are expressed from the paternal allele in extraembryonic tissues. Sfmbt2 encodes a PcG protein required for maintaining the trophoblast stem cell (TS) pool; its loss results in severe reduction of all trophoblast layers of the placenta and is accompanied by embryonic developmental arrest and death at mid-gestation [29]. Murine Sfmbt2 lacks a DMR [22], but possesses a large block of miRNAs in intron 10.
Peg10 is a retrotransposon-derived gene on proximal Chr. 6 that is paternally expressed and regulated by a maternal gDMR. Its loss results in severe reduction of the labyrinthine layer and almost complete loss of the spongiotrophoblast layer of the placenta by early mid-gestation. PEG10 protein may be involved in protection against apoptosis [38]. Placenta dysfunction is accompanied by embryonic developmental arrest and death at mid-gestation [39]. Peg10 is one of the earliest imprinted genes to arise during mammalian evolution [40] and is an example of retrotransposon-driven imprinting. It anchors a domain that contains six additional imprinted genes.
Mest is a paternally expressed epoxide hydrolase gene on Chr. 6 that is regulated by a maternal gDMR and anchors a domain containing four additional genes, one of which is an ncRNA. Mest is expressed in embryonic and extraembryonic mesoderm (e.g., allantois); its loss results in reduced growth of both the fetus and the placenta and in behavioral defects in females inheriting a paternal null allele [41]. Mest harbors an intronic mir-335, which has been linked to mesendoderm development through targeting of several transcription factors [42]. While the Mest mutant allele, a reporter construct inserted downstream of mir-335, has the potential to express mir-335, it is unknown whether the miRNA is processed from the mutant transcript. Unpublished data from the Lefebvre laboratory indicates that mir-335 is processed from the mutant allele, but not at the same levels as the wild-type allele (L. Lefebvre, personal communication).
Peg3 is a paternally expressed Zn finger gene regulated by a maternal gDMR and anchoring a domain containing five additional genes, one of which is a paternally expressed ncRNA anti-sense to Peg3; a second antisense transcript can be seen in the 3’ flank of Peg3 in the RNA-seq data from TS cells that is likely orthologous with the imprinted human ncRNA MIMT1 [34], (Fig. 2c). While the authors of the knockout paper focused on behavioral defects in females inheriting a paternal null allele, they note in the footnotes that placentae are 30 % smaller, suggesting that Peg3 is involved in placenta growth/function [43]. It is expressed robustly in TS cells.
H19 is a maternally expressed ncRNA that harbors a miRNA now known to regulate placenta growth and development [44]; loss of H19/mir-675 results in placentomegaly. H19 is regulated by a paternal intergenic gDMR that is thought to act as a boundary element through the activity of CTCF binding. Knockdown of CTCF in oocytes results in methylation of maternal DMR CpGs, suggesting that methylation is the default state for this element [18]. The H19 DMR anchors an imprinted domain consisting of four additional imprinted genes, including another ncRNA. An earlier study of H19 reported increased weight of neonates, but did not examine the placenta [45]; re-examination of this mutant line revealed placentomegaly [44], illustrating the utility of thorough analysis.
Igf2 is a paternally expressed growth factor that is reciprocally regulated by the H19 intergenic DMR on Chr. 7 [46]. Loss of a placenta-specific alternative transcript (P0) results in both placental and fetal growth restriction; this mirrors the phenotype described for the original knockout, suggesting that the placental function of Igf2 is the critical factor in how the gene regulates fetal growth [36].
Ascl2 (Mash2) is a maternally expressed transcription factor gene on distal Chr. 7 that is required for the development of the spongiotrophoblast layer of the placenta. Embryos inheriting a mutant allele from their mothers cease development and die at mid-gestation [27]. Ascl2 is regulated by the Kcnq1ot1 maternal gDMR which controls imprinting of a cluster of nine genes.
Kcnq1ot1 is a paternally expressed ncRNA in the intron of the Kcnq1 gene. Its promoter comprises the maternal gDMR that regulates the domain [47]. Fetuses inheriting a deletion of the Kcnq1ot1 DMR from their fathers are growth restricted [47]; this is reflected by growth restriction of the placenta [48], thought to reflect loss of imprinting/overexpression of maternally expressed genes in the cluster, such as Phlda2 and Cdkn1c. A transcription termination mutation at Kcnq1ot1 that results in partial loss of imprinting (LOI) of Cdkn1c in placenta but not somatic tissues produces a milder growth retardation phenotype [49]. However, no histological analysis of mutant placentas was undertaken in either the complete or partial Kcnq1ot1 mutants [47, 49]. Interestingly, the phenotype of the Kcnq1 mutant—recessive deafness—suggests that the function of this gene is unaffected by imprinting [50].
Cdkn1c is a maternally expressed cell cycle protein gene that is regulated by the Kcnq1ot1 gDMR. Maternal loss of function is generally lethal, although on a mixed genetic background growth-restricted mutant pups can survive [51]. Mutant placentas exhibit placentomegaly [52], and females pregnant with Cdkn1c mutant embryos show symptoms of pre-eclampsia [53].
Phlda2 encodes a pleckstrin homology domain protein that is maternally expressed; it is regulated by the Kcnq1ot1 gDMR and is one of the genes responsible for the Kcnq1ot1 deletion phenotype. Loss of Phlda2 results in placentomegaly, with no effect on fetal growth or survival [54].
Plagl1 encodes a paternally expressed Zn finger protein and at least two ncRNAs of unknown function [55]. It is regulated by a maternal gDMR that spans its transcriptional start site (TSS) [56] and anchors a domain with one other gene. Loss of Plagl1 results in IUGR up to 25 % and, while the authors claim there is minimal effect on the placenta, the data in the supplementary files indicate reduced placental weights and significantly reduced labyrinthine layer development [57]. A more thorough analysis of placenta in Plagl1 KO mice is warranted.
Grb10 encodes a maternally expressed pleckstrin homology domain protein that is regulated by a maternal gDMR near one of its TSS. Loss of maternal Grb10 results in placenta and fetal overgrowth [58]. Grb10 anchors a domain containing two other genes, one of which is a Grb10 antisense ncRNA.
Dlk1 is part of the complex Dlk1 imprinted domain that is regulated by the paternal intergenic gDMR. Dlk1 encodes a paternally expressed Delta-like protein, containing EGF domains. Its loss results in IUGR with reduced and abnormal labyrinth layer, although with no change in placenta weight. Conditional knockouts in pancreas, pituitary and endothelial cells were normal [59].
Rtl1 is another retrotransposon-derived gene that is expressed paternally. It is part of the complex Dlk1 locus on Chr. 12 that contains several protein-coding genes as well as numerous ncRNAs and large blocks of miRNA and SnoRNA genes, all of which are expressed in TS cells (Fig. 2a). One of the ncRNAs—Rtl1AS—antisense to Rtl1, is maternally expressed and encodes several miRNAs that directly target Rtl1 for RNAi-mediated repression. Deletion of part of the Rtl1 coding sequence simultaneously deletes both Rtl1 sequence and some of the miRNAs encoded by Rtl1AS. Paternal transmission of this deletion, which generates Rtl1 null embryos, results in IUGR and placental infarcts in the labyrinth due to excessive phagocytosis of the fetal endothelial cells that normally express Rtl1; maternal transmission, which generates Rtl1 overexpressing embryos, results in placentomegaly but normal fetal growth [60]. While the function of RTL1 protein remains to be established, the phenotype is suggestive of a role in protection against cell death, possibly similar to PEG10.
Igf2r is a maternally expressed Igf2 receptor that is thought to act as a sink for Igf2. It is regulated by the maternal gDMR that overlaps the promoter of the antisense ncRNA Airn, whose transcription through the Igf2r TSS represses Igf2r transcription [61]. Maternal transmission of a null allele results in placentomegaly and fetal overgrowth [62].
Potential placenta function
Several imprinted genes result in phenotypes that are likely to involve the placenta, but that were never analyzed thoroughly for placental function. For example, the Snrpn locus is of great interest because of the link with Prader Willi syndrome/Angelmann syndrome (PWS/AS) in humans. PWS is characterized by IUGR, neonatal respiratory distress and later developmental abnormalities, including neurological impairment and eating disorders, which likely have a root cause in defects of neural development during gestation. The Snrpn locus comprises several protein-coding genes (Ube3a, Snrpn, Ndn, Mkrn3, Magel2 and Peg12) and a large block of SnoRNAs encoded by several long ncRNAs. The mouse locus also harbors a block of rodent-specific miRNAs. Imprinting of the locus is regulated by a maternal gDMR near the Snrpn TSS. Intragenic knockout of Snrpn is without phenotypic consequence [63]; however, deletion of the gDMR results in IUGR and neonatal lethality, and is associated with loss of expression of several genes in the cluster. This phenotype is mimicked in part by targeted deletion of Ndn [64]. Deletion of one of the SnoRNA clusters (SNORD116/MBII-85) results in neonatal growth restriction that persisted into postnatal life; the authors reported no effect on placenta weight, but did not examine the histology [65]. It should be noted that the Dlk1 knockout results in abnormal placentas with normal weight.
Another locus that warrants closer examination of placenta function is the complex GNAS suite of genes. The Gnas locus generates multiple transcripts that encode different proteins or are non-coding; some (Gnas-XL, Gnas-exon1A, NespAS) are expressed from the paternal allele, some (Nesp55) are expressed from the maternal allele and others (Gnas) are biallelic in some tissues and monoallelic in others. The NespAS ncRNA may encode two miRNAs; NespAS reads in the TS cell RNA-seq dataset extend down to the region of the two miRNAs [34] (Fig. 2d). Inheritance of a paternal null allele of Gnas-XL results in pups with morphological body-type defects that fail to thrive and die in early postnatal stages [66]; these defects clearly arose during development as they were present at birth. Inheritance of a maternal deletion of Nesp55 leads to IUGR with edema [67], a phenotype that is similar to hydrops fetalis in humans, which can be associated with placental dysfunction [68–70]. Paternal transmission of a deletion of the gDMR associated with NespAS results in partial LOI at the locus and generates a phenotype similar to GnasXL mutants. Mutations in Gnas result in phenotypes that differ depending on the direction of transmission: maternal inheritance results in edema, wide bodies and failure to thrive, while paternal inheritance results in low birth weight, narrow bodies and failure to thrive. The edema and the lower birth weights are symptoms associated with dysfunctional placentas, although Okae et al. [31] reported biallelic expression of Gnas in whole transcriptome analysis of dissected placenta.
Finally, Airn deletion results in biallelic expression of Igf2r [71]. Fetal weights were reduced, but placental weights were unaffected, leading the authors to conclude there was no link between placenta function and fetal weight. However, the Airn deletion rescues maternal deletion of Igf2r, which is known to cause placentomegaly. It would be interesting to know whether placentomegaly is also rescued in this genetic system.
Evolutionary significance of imprinting
While many imprinted genes are either directly or potentially involved in placenta function, only five have imprinted functions that do not, and of these only one (Rasgrf1) represents a separate domain; the other four are located in clusters that are regulated by gDMRs anchored by genes that have demonstrated or potential placenta phenotypes. The genomic evidence suggests that imprinting starts out with a placenta-specific gene and then spreads to other genes in the locale [40], lending weight to the notion that imprinting arose to regulate placenta development [7, 8]. However, the dominant hypothesis in the field of genomic imprinting—the conflict hypothesis—still guides thinking among researchers [11]. How does the evidence stack up?
The conflict hypothesis in its simplest form posits that paternal genomes strive to increase fitness of their offspring by increasing their growth/size (“bigger is better”), while maternal genomes strive to spread the resources among all offspring by dampening growth. This has led investigators to analyze the effects of imprinted genes on growth. The fact that paternally expressed genes are often found to be required for growth, as assessed by the effects of loss of function (LOF) mutations, is seen as strong evidence in support of the hypothesis. However, there is an internal flaw in this argument that is often overlooked, i.e., that mammalian development is accompanied by growth; LOF mutations in genes essential for development will, a priori, result in either arrest or delay in development and consequently in growth. The effect of paternal genes as regulators of development cannot be distinguished from any role in conflict-driven growth enhancement. This means that the flip side of the argument, the maternal growth-dampening effect, is the only means of assessing the validity of the conflict hypothesis. Of 27 maternally expressed genes for which LOF mutations are available (Table 1), only three fit the hypothesis: H19, Grb10 and Igf2r (which, along with Igf2, inspired the conflict hypothesis more than 20 years ago). All three have compromised placentae associated with fetal overgrowth, so these examples also support hypotheses directed at maternal control of placentation. Placental defects do not always translate into fetal overgrowth; Phlda2 and Rtl1AS mutations both result in placentomegaly with no alteration in fetal growth; Cdkn1c results in fetal growth retardation with pathological effects on placenta, including evidence of pre-eclampsia; Nesp55 KO results in edema, thought to be a result of placental dysfunction. The evidence is inconclusive regarding the validity of the conflict hypothesis. However, the support for hypotheses directed at maternal control of placentation driving genomic imprinting, while strong, is not overwhelming. A glance at the map of imprinted domains illustrates that more than half do not seem to be doing much of anything (Fig. 1; Table 1). Why, therefore, are they imprinted? Part of the answer to this question may come from analyses of the mechanisms of imprinting.
Mechanism matters
Genomic imprinting is a gene-silencing phenomenon. Research over the past 30 years has revealed the great depth and breadth of epigenetic regulation in nature. In mammals, gene silencing is accomplished by a range of mechanisms that include DNA methylation, histone modifications, chromatin protein associations and RNA-based transcriptional and post-transcriptional gene silencing. All of these mechanisms have been co-opted to mediate genomic imprinting [69, 70].
The differentiation between the two parental genomes must take place while they are apart. The obvious place is in the germline. Significant effort has been expended on assessing the molecular differences between oocyte and sperm genomes, in spite of technical challenges. Until new tools became available, older technology limited analysis to DNA methylation. Consequently, there is a wealth of data on differential methylation between the maternal and paternal genomes. Exhaustive searches near imprinted genes have revealed that DMRs can be either germline or somatic, with the latter arising after fertilization and probably reflecting chromatin remodeling during development. Germline DMRs become methylated during germ cell development [72, 73]; spermatogonia acquire DNA methylation imprints during embryogenesis and before the onset of meiosis, while oocytes acquire their methylated DMRs during postnatal oocyte maturation. The number of imprinted genes is very small, so the results from a recent methylome analysis revealing the presence of several thousand germline DMRs that survived to the blastocyst stage was quite surprising [74]. They were found on every chromosome, including those shown to not harbor imprinted genes with a developmental function, suggesting that many may be ephemeral. It is tempting to speculate that roughly 30 genes that display no imprinted function retain their imprints, because there is no need to remodel them, except perhaps in select tissues. Also, striking from the data in this study were two other observations: sperm DNA is very heavily methylated everywhere, and both oocyte and sperm DNA have some mechanism that protects CpG islands from methylation. The rare exceptions are germline DMRs.
The heavy methylation of the paternal genome is likely necessary to aid the tight packaging required to fit the DNA into a tiny sperm head. We now know that this methylation is largely erased shortly after fertilization by maternal TET3 [75, 76]. The burning question is how the gDMRs escape the remodeling that occurs during the first cell cycle [77]. One likely mechanism is protection by other factors, such as ZFP57, which has been shown to bind to methylated gDMRs containing the consensus hexanucleotide sequence (TGCCGC) [78]. Maternal loss of Zfp57 results in embryonic failure due to LOI [79]. Another maternal protection factor is DPPA3/PGC7, which has been shown to bind DMRs where it inhibits TET3-mediated demethylation [76, 80]. Maternal DPPA3/PGC7 binds H3K9Me2 in the maternal pronucleus; depletion of H3K9Me2 leads to loss of both DPPA3/PGC7 and DNA methylation from the maternal pronucleus [76]. Interestingly, maternal LOF of DPPA3/PGC7 results in loss of methylation at both maternal and paternal gDMRs in zygotes [81].
Other maternal factors have been shown to play significant roles in both establishment and maintenance of gDMRs. Maternal effect mutations in several genes disrupt imprinting: Dnmt1o [82], Dnmt3l [83], Dnmt3A [84], Pgc7 [81] and to a lesser extent Zfp57 [79]. In other species, maternal effect mutations in human NLRP7 and KHDC3L result in biparental hydatidiform molar pregnancies as a result of catastrophic loss of imprinting in zygotes [85, 86]; interspecific hybrids involving Peromyscus (deer mice) result in placental defects related to imprinting dysregulation that can be traced to maternal effect genes [87].
Not all imprinted genes possess a gDMR (Sfmbt2, [22]), or are sensitive to loss of maintenance methylation (Ascl2, [88]). This suggests that other epigenetic marks regulate imprinted expression. Reports of chromatin marks such as H3K4Me2, H3K9Me2 and H3K27Me3 at imprinted loci at the Kcnq1 locus in placenta are difficult to interpret, given that half of the genes tested are likely not imprinted [26, 89]; the same technical problem with ChIP in placenta as was demonstrated for expression [31]—contamination with maternal tissues—confounds interpretation of results. De novo methylation may also play a minimal role in placenta-specific imprinting. Maternal loss of Dnmt3l [90], while lethal, has only subtle effects on placenta development. It does not preclude establishment/maintenance of TS cells, in spite of widespread loss of methylation at gDMRs [83]; Sfmbt2, required for TS cell maintenance [29], retains imprinted expression in Dnmt3l and Dnmt3a mutants [31].
Finally, the role played by ncRNAs in imprinting at some loci (e.g., Airn/Igf2r [61]; Kcnq1ot1/Kcnq1 [49]) and by oocyte transcription across gDMRs [91] suggests that some aspect of RNA biochemistry may be involved in silencing genes/domains in the maternal germline. The emerging picture points to initial marks on both maternal and paternal genomes being selectively maintained by the zygote after fertilization. The second burning question is: “Why those particular sites?”
When things go wrong
The consequences of LOI can be devastating. BWS, PWS/AS and Russell Silver syndrome (RSS) diseases are, in many cases, caused by LOI. In some BWS and RSS patients, global LOI is seen at multiple imprinted loci, suggesting that an early breakdown in the imprinting machinery has occurred [92–97], sometimes associated with conception via assisted reproductive technology (ART). A similar breakdown is thought to underly familial hydatidiform moles, caused by maternal effect mutations in two genes—NLRP7 and KHDC3L—and resulting in recurrent molar pregnancies [85, 86, 98]. Expression of both genes during oocyte development coincides with the timing of maternal gDMR methylation [73, 86, 99]. The connection between ART-induced LOI and maternal effect mutation-induced LOI strongly links oocyte health and proper imprinting. The ART studies also implicate the environment as a major factor in epigenetic health. Most studies focusing on diet, especially folate metabolism, are limited to gestation and lactation (e.g., [100]), although there have been some recent studies of periconceptional maternal diet [101, 102], possibly in response to a study of the Dutch Famine cohort [103] which demonstrated a more dramatic effect of diet during an unspecified periconceptional period compared with late gestation. Given that gDMR methylation occurs during oocyte development, starting around the secondary follicle stage, the need to ensure good nutrition in girls and women starting in childhood to ensure a healthy next generation warrants closer investigation.
The third burning question is: “Why does LOI have such devastating effects?” The stock answer is that there is a need to regulate gene dosage (to fulfill the requirements of the conflict hypothesis). However, organisms have evolved much better mechanisms to regulate the levels of gene products over the past 4 or 5 billion years, and mammals make use of all options. For example, expression from one allele of Sfmbt2 in extraembryonic tissues is about 50-fold higher than that from two alleles in somatic tissues [32]. Gene dosage is not a very satisfactory explanation. An alternative reason for the severe developmental abnormalities accruing from LOI is ectopic expression, either plus or minus, and spatial or temporal, or both, brought about by altered chromatin structure. Expression (or lack) of a key gene during development in cells where it is normally silent/active can wreak havoc. Thorough unbiased investigation of all tissues during development in imprinted gene mutations may yield some unexpected treasures.
References
- 1.Barton SC, Surani MAH, Norris ML. Role of paternal and maternal genomes in mouse development. Nature. 1984;311:374–376. doi: 10.1038/311374a0. [DOI] [PubMed] [Google Scholar]
- 2.McGrath J, Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell. 1984;37:179–183. doi: 10.1016/0092-8674(84)90313-1. [DOI] [PubMed] [Google Scholar]
- 3.Varmuza S, Mann M, Rogers I. Site of action of imprinted genes revealed by phenotypic analysis of parthenogenetic embryos. Dev Genet. 1993;14:239–248. doi: 10.1002/dvg.1020140310. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman MH, Barton SC, Surani MA. Normal postimplantation development of mouse parthenogenetic embryos to the forelimb bud stage. Nature. 1977;265:53–55. doi: 10.1038/265053a0. [DOI] [PubMed] [Google Scholar]
- 5.Surani MA, Barton SC, Norris ML. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature. 1984;308:548–550. doi: 10.1038/308548a0. [DOI] [PubMed] [Google Scholar]
- 6.Cattanach BM, Kirk M. Differential activity of maternal and paternally derived chromosome regions in mice. Nature. 1985;315:496–498. doi: 10.1038/315496a0. [DOI] [PubMed] [Google Scholar]
- 7.Hall JG. Genomic imprinting. Arch Dis Child. 1990;65:1013–1015. doi: 10.1136/adc.65.10_spec_no.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varmuza S, Mann M. Genomic imprinting—defusing the ovarian time bomb. Trends Genet. 1994;10:118–123. doi: 10.1016/0168-9525(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 9.Keverne EB. Importance of the matriline for genomic imprinting, brain development and behaviour. Philos Trans R Soc B Biol Sci. 2012;368:20110327. doi: 10.1098/rstb.2011.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer HG, Clark AG. Non-conflict theories for the evolution of genomic imprinting. Heredity. 2014 doi: 10.1038/hdy.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haig D, Westoby M. Parent-specific gene expression and the triploid endosperm. Am Nat. 1989;134:147–155. [Google Scholar]
- 12.Yuen RK, Jiang R, Peñaherrera MS, et al. Genome-wide mapping of imprinted differentially methylated regions by DNA methylation profiling of human placentas from triploidies. Epigenetics Chromatin. 2011;4:10. doi: 10.1186/1756-8935-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Court F, Tayama C, Romanelli V, et al. Genome-wide parent-of-origin DNA methylation analysis reveals the intricacies of human imprinting and suggests a germline methylation-independent mechanism of establishment. Genome Res. 2014;24:554–569. doi: 10.1101/gr.164913.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renfree MB, Suzuki S, Kaneko-Ishino T. The origin and evolution of genomic imprinting and viviparity in mammals. Philos Trans R Soc B Biol Sci. 2012;368:20120151. doi: 10.1098/rstb.2012.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tunster SJ, Jensen AB, John RM. Imprinted genes in mouse placental development and the regulation of fetal energy stores. Reproduction. 2013;145:R117–R137. doi: 10.1530/REP-12-0511. [DOI] [PubMed] [Google Scholar]
- 16.Bell AC, Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature. 2000;405:482–485. doi: 10.1038/35013100. [DOI] [PubMed] [Google Scholar]
- 17.Yoon B, Herman H, Hu B, et al. Rasgrf1 imprinting is regulated by a CTCF-dependent methylation-sensitive enhancer blocker. Mol Cell Biol. 2005;25:11184–11190. doi: 10.1128/MCB.25.24.11184-11190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedoriw AM. Transgenic RNAi reveals essential function for CTCF in H19 gene imprinting. Science. 2004;303:238–240. doi: 10.1126/science.1090934. [DOI] [PubMed] [Google Scholar]
- 19.Smallwood SA, Tomizawa S, Krueger F, et al. Dynamic CpG island methylation landscape in oocytes and preimplantation embryos. Nat Genet. 2011;43:811–814. doi: 10.1038/ng.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna CW, Kelsey G. The specification of imprints in mammals. Heredity. 2014 doi: 10.1038/hdy.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelsey G, Feil R. New insights into establishment and maintenance of DNA methylation imprints in mammals. Philos Trans R Soc Lond B Biol Sci. 2013;368:20110336. doi: 10.1098/rstb.2011.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Chow J, Hong J, et al. Recent acquisition of imprinting at the rodent Sfmbt2 locus correlates with insertion of a large block of miRNAs. BMC Genom. 2011;12:204. doi: 10.1186/1471-2164-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noguer-Dance M, Abu-Amero S, Al-Khtib M, et al. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum Mol Genet. 2010;19:3566–3582. doi: 10.1093/hmg/ddq272. [DOI] [PubMed] [Google Scholar]
- 24.Hudson QJ, Seidl CIM, Kulinski TM, et al. Extra-embryonic-specific imprinted expression is restricted to defined lineages in the post-implantation embryo. Dev Biol. 2011;353:420–431. doi: 10.1016/j.ydbio.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulinski TM, Barlow DP, Hudson QJ. Imprinted silencing is extended over broad chromosomal domains in mouse extra-embryonic lineages. Curr Opin Cell Biol. 2013;25:297–304. doi: 10.1016/j.ceb.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis A, Mitsuya K, Umlauf D, et al. Imprinting on distal chromosome 7 in the placenta involves repressive histone methylation independent of DNA methylation. Nat Genet. 2004;36:1291–1295. doi: 10.1038/ng1468. [DOI] [PubMed] [Google Scholar]
- 27.Guillemot F, Caspary T, Tilghman SM, et al. Genomic imprinting of Mash2, a mouse gene required for trophoblast development. Nat Genet. 1995;9:235–242. doi: 10.1038/ng0395-235. [DOI] [PubMed] [Google Scholar]
- 28.Duvillié B, Bucchini D, Tang T, et al. Imprinting at the mouse Ins2 locus: evidence for cis- and trans-allelic interactions. Genomics. 1998;47:52–57. doi: 10.1006/geno.1997.5070. [DOI] [PubMed] [Google Scholar]
- 29.Miri K, Latham K, Panning B, et al. (2013) The imprinted polycomb group gene Sfmbt2 is required for trophoblast maintenance and placenta. Development. doi:10.1242/dev.096511 [DOI] [PMC free article] [PubMed]
- 30.Zwart R, Sleutels F, Wutz A, et al. Bidirectional action of the Igf2r imprint control element on upstream and downstream imprinted genes. Genes Dev. 2001;15:2361–2366. doi: 10.1101/gad.206201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okae H, Hiura H, Nishida Y, et al. Re-investigation and RNA sequencing-based identification of genes with placenta-specific imprinted expression. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr488. [DOI] [PubMed] [Google Scholar]
- 32.Kuzmin A, Han Z, Golding MC, et al. The PcG gene Sfmbt2 is paternally expressed in extraembryonic tissues. Gene Expr Patterns GEP. 2008;8:107–116. doi: 10.1016/j.modgep.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaue M, Ohta H, Kumaki Y, et al. DNA methylation is dispensable for the growth and survival of the extraembryonic lineages. Curr Biol CB. 2010;20:1452–1457. doi: 10.1016/j.cub.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 34.Calabrese JM, Sun W, Song L, et al. Site-specific silencing of regulatory elements as a mechanism of X inactivation. Cell. 2012;151:951–963. doi: 10.1016/j.cell.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fowden AL, Coan PM, Angiolini E, et al. Imprinted genes and the epigenetic regulation of placental phenotype. Prog Biophys Mol Biol. 2011;106:281–288. doi: 10.1016/j.pbiomolbio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Constância M, Hemberger M, Hughes J, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 37.Tunster SJ, Tycko B, John RM. The imprinted Phlda2 gene regulates extraembryonic energy stores. Mol Cell Biol. 2010;30:295–306. doi: 10.1128/MCB.00662-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okabe H, Satoh S, Furukawa Y, et al. Involvement of PEG10 in human hepatocellular carcinogenesis through interaction with SIAH1. Cancer Res. 2003;63:3043–3048. [PubMed] [Google Scholar]
- 39.Ono R, Nakamura K, Inoue K, et al. Deletion of Peg10, an imprinted gene acquired from a retrotransposon, causes early embryonic lethality. Nat Genet. 2006;38:101–106. doi: 10.1038/ng1699. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki S, Ono R, Narita T, et al. Retrotransposon silencing by DNA methylation can drive mammalian genomic imprinting. PLoS Genet. 2007;3:e55. doi: 10.1371/journal.pgen.0030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lefebvre L, Viville S, Barton SC, et al. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet. 1998;20:163–169. doi: 10.1038/2464. [DOI] [PubMed] [Google Scholar]
- 42.Yang D, Lutter D, Burtscher I, et al. miR-335 promotes mesendodermal lineage segregation and shapes a transcription factor gradient in the endoderm. Dev Camb Engl. 2014;141:514–525. doi: 10.1242/dev.104232. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Keverne EB, Aparicio SA, et al. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science. 1999;284:330–333. doi: 10.1126/science.284.5412.330. [DOI] [PubMed] [Google Scholar]
- 44.Keniry A, Oxley D, Monnier P, et al. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol. 2012;14:659–665. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ripoche MA, Kress C, Poirier F, Dandolo L. Deletion of the H19 transcription unit reveals the existence of a putative imprinting control element. Genes Dev. 1997;11:1596–1604. doi: 10.1101/gad.11.12.1596. [DOI] [PubMed] [Google Scholar]
- 46.Weaver JR, Bartolomei MS. Chromatin regulators of genomic imprinting. Biochim Biophys Acta BBA Gene Regul Mech. 2014;1839:169–177. doi: 10.1016/j.bbagrm.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fitzpatrick GV, Soloway PD, Higgins MJ. Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. Nat Genet. 2002;32:426–431. doi: 10.1038/ng988. [DOI] [PubMed] [Google Scholar]
- 48.Salas M, John R, Saxena A, et al. Placental growth retardation due to loss of imprinting of Phlda2. Mech Dev. 2004;121:1199–1210. doi: 10.1016/j.mod.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 49.Shin J-Y, Fitzpatrick GV, Higgins MJ. Two distinct mechanisms of silencing by the KvDMR1 imprinting control region. EMBO J. 2008;27:168–178. doi: 10.1038/sj.emboj.7601960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee MP, Ravenel JD, Hu R-J, et al. Targeted disruption of the <i> Kvlqt1 </i> gene causes deafness and gastric hyperplasia in mice. J Clin Invest. 2000;106:1447–1455. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Susaki E, Nakayama K, Yamasaki L, Nakayama KI. Common and specific roles of the related CDK inhibitors p27 and p57 revealed by a knock-in mouse model. Proc Natl Acad Sci. 2009;106:5192–5197. doi: 10.1073/pnas.0811712106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi K, Kobayashi T, Kanayama N. p57(Kip2) regulates the proper development of labyrinthine and spongiotrophoblasts. Mol Hum Reprod. 2000;6:1019–1025. doi: 10.1093/molehr/6.11.1019. [DOI] [PubMed] [Google Scholar]
- 53.Kanayama N, Takahashi K, Matsuura T, et al. Deficiency in p57Kip2 expression induces preeclampsia-like symptoms in mice. Mol Hum Reprod. 2002;8:1129–1135. doi: 10.1093/molehr/8.12.1129. [DOI] [PubMed] [Google Scholar]
- 54.Frank D, Fortino W, Clark L, et al. Placental overgrowth in mice lacking the imprinted gene Ipl. Proc Natl Acad Sci. 2002;99:7490–7495. doi: 10.1073/pnas.122039999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iglesias-Platas I, Martin-Trujillo A, Cirillo D, et al. Characterization of novel paternal ncRNAs at the Plagl1 locus, including Hymai, predicted to interact with regulators of active chromatin. PLoS ONE. 2012;7:e38907. doi: 10.1371/journal.pone.0038907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arima T, Wake N. Establishment of the primary imprint of the <i> HYMAI/PLAGL1 </i> imprint control region during oogenesis. Cytogenet Genome Res. 2006;113:247–252. doi: 10.1159/000090839. [DOI] [PubMed] [Google Scholar]
- 57.Varrault A, Gueydan C, Delalbre A, et al. Zac1 regulates an imprinted gene network critically involved in the control of embryonic growth. Dev Cell. 2006;11:711–722. doi: 10.1016/j.devcel.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Charalambous M, Smith FM, Bennett WR, et al. Disruption of the imprinted Grb10 gene leads to disproportionate overgrowth by an Igf2-independent mechanism. Proc Natl Acad Sci USA. 2003;100:8292–8297. doi: 10.1073/pnas.1532175100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Appelbe OK, Yevtodiyenko A, Muniz-Talavera H, Schmidt JV. Conditional deletions refine the embryonic requirement for Dlk1. Mech Dev. 2013;130:143–159. doi: 10.1016/j.mod.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sekita Y, Wagatsuma H, Nakamura K, et al. Role of retrotransposon-derived imprinted gene, Rtl1, in the feto-maternal interface of mouse placenta. Nat Genet. 2008;40:243–248. doi: 10.1038/ng.2007.51. [DOI] [PubMed] [Google Scholar]
- 61.Latos PA, Pauler FM, Koerner MV, et al. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science. 2012;338:1469–1472. doi: 10.1126/science.1228110. [DOI] [PubMed] [Google Scholar]
- 62.Lau MM, Stewart CE, Liu Z, et al. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 1994;8:2953–2963. doi: 10.1101/gad.8.24.2953. [DOI] [PubMed] [Google Scholar]
- 63.Yang T, Adamson TE, Resnick JL, et al. A mouse model for Prader–Willi syndrome imprinting-centre mutations. Nat Genet. 1998;19:25–31. doi: 10.1038/ng0598-25. [DOI] [PubMed] [Google Scholar]
- 64.Gérard M, Hernandez L, Wevrick R, Stewart CL. Disruption of the mouse necdin gene results in early post-natal lethality. Nat Genet. 1999;23:199–202. doi: 10.1038/13828. [DOI] [PubMed] [Google Scholar]
- 65.Skryabin BV, Gubar LV, Seeger B, et al. Deletion of the MBII-85 snoRNA gene cluster in mice results in postnatal growth retardation. PLoS Genet. 2007;3:e235. doi: 10.1371/journal.pgen.0030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plagge A, Gordon E, Dean W, et al. The imprinted signaling protein XLαs is required for postnatal adaptation to feeding. Nat Genet. 2004;36:818–826. doi: 10.1038/ng1397. [DOI] [PubMed] [Google Scholar]
- 67.Frohlich LF, Mrakovcic M, Steinborn R, et al. Targeted deletion of the Nesp55 DMR defines another Gnas imprinting control region and provides a mouse model of autosomal dominant PHP-Ib. Proc Natl Acad Sci. 2010;107:9275–9280. doi: 10.1073/pnas.0910224107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braun T, Brauer M, Fuchs I, et al. Mirror syndrome: a systematic review of fetal associated conditions, maternal presentation and perinatal outcome. Fetal Diagn Ther. 2010;27:191–203. doi: 10.1159/000305096. [DOI] [PubMed] [Google Scholar]
- 69.Barlow DP. Genomic imprinting: a mammalian epigenetic discovery model. Annu Rev Genet. 2011;45:379–403. doi: 10.1146/annurev-genet-110410-132459. [DOI] [PubMed] [Google Scholar]
- 70.Barlow DP, Bartolomei MS. Genomic imprinting in mammals. Cold Spring Harb Perspect Biol. 2014;6:a018382. doi: 10.1101/cshperspect.a018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wutz A, Theussl HC, Dausman J, et al. Non-imprinted Igf2r expression decreases growth and rescues the Tme mutation in mice. Development. 2001;128:1881–1887. doi: 10.1242/dev.128.10.1881. [DOI] [PubMed] [Google Scholar]
- 72.Lucifero D, Mertineit C, Clarke HJ, et al. Methylation dynamics of imprinted genes in mouse germ cells. Genomics. 2002;79:530–538. doi: 10.1006/geno.2002.6732. [DOI] [PubMed] [Google Scholar]
- 73.Hiura H, Obata Y, Komiyama J, et al. Oocyte growth-dependent progression of maternal imprinting in mice. Genes Cells. 2006;11:353–361. doi: 10.1111/j.1365-2443.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 74.Kobayashi H, Sakurai T, Imai M, et al. Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish oocyte-specific heritable marks. PLoS Genet. 2012;8:e1002440. doi: 10.1371/journal.pgen.1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gu T-P, Guo F, Yang H, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477:606–610. doi: 10.1038/nature10443. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura T, Liu Y-J, Nakashima H, et al. PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature. 2012 doi: 10.1038/nature11093. [DOI] [PubMed] [Google Scholar]
- 77.Santos F, Peters AH, Otte AP, et al. Dynamic chromatin modifications characterise the first cell cycle in mouse embryos. Dev Biol. 2005;280:225–236. doi: 10.1016/j.ydbio.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 78.Quenneville S, Verde G, Corsinotti A, et al. In embryonic stem cells, ZFP57/KAP1 recognize a methylated hexanucleotide to affect chromatin and DNA methylation of imprinting control regions. Mol Cell. 2011;44:361–372. doi: 10.1016/j.molcel.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X, Ito M, Zhou F, et al. A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev Cell. 2008;15:547–557. doi: 10.1016/j.devcel.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bian C, Yu X. PGC7 suppresses TET3 for protecting DNA methylation. Nucleic Acids Res. 2014;42:2893–2905. doi: 10.1093/nar/gkt1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakamura T. PGC7/Stella protects against DNA demethylation in early embryogenesis. Nat Cell Biol. 2007;9:64–71. doi: 10.1038/ncb1519. [DOI] [PubMed] [Google Scholar]
- 82.Howell CY, Bestor TH, Ding F, et al. Genomic imprinting disrupted by a maternal effect mutation in the <i> Dnmt1 </i> Gene. Cell. 2001;104:829–838. doi: 10.1016/s0092-8674(01)00280-x. [DOI] [PubMed] [Google Scholar]
- 83.Bourc’his D, Xu G-L, Lin C-S, et al. Dnmt3L and the establishment of maternal genomic imprints. Science. 2001;294:2536–2539. doi: 10.1126/science.1065848. [DOI] [PubMed] [Google Scholar]
- 84.Kaneda M, Okano M, Hata K, et al. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004;429:900–903. doi: 10.1038/nature02633. [DOI] [PubMed] [Google Scholar]
- 85.Murdoch S, Djuric U, Mazhar B, et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat Genet. 2006;38:300–302. doi: 10.1038/ng1740. [DOI] [PubMed] [Google Scholar]
- 86.Parry DA, Logan CV, Hayward BE, et al. Mutations causing familial biparental hydatidiform mole implicate C6orf221 as a possible regulator of genomic imprinting in the human oocyte. Am J Hum Genet. 2011;89:451–458. doi: 10.1016/j.ajhg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loschiavo M, Nguyen QK, Duselis AR, Vrana PB. Mapping and identification of candidate loci responsible for Peromyscus hybrid overgrowth. Mamm Genome. 2007;18:75–85. doi: 10.1007/s00335-006-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka M, Puchyr M, Gertsenstein M, et al. Parental origin-specific expression of Mash2 is established at the time of implantation with its imprinting mechanism highly resistant to genome-wide demethylation. Mech Dev. 1999;87:129–142. doi: 10.1016/s0925-4773(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 89.Wagschal A, Sutherland HG, Woodfine K, et al. G9a histone methyltransferase contributes to imprinting in the mouse placenta. Mol Cell Biol. 2008;28:1104–1113. doi: 10.1128/MCB.01111-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arima T, Hata K, Tanaka S, et al. Loss of the maternal imprint in Dnmt3Lmat−/− mice leads to a differentiation defect in the extraembryonic tissue. Dev Biol. 2006;297:361–373. doi: 10.1016/j.ydbio.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Chotalia M. Transcription is required for establishment of germline methylation marks at imprinted genes. Genes Dev. 2009;23:105–117. doi: 10.1101/gad.495809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rossignol S, Steunou V, Chalas C, et al. The epigenetic imprinting defect of patients with Beckwith-Wiedemann syndrome born after assisted reproductive technology is not restricted to the 11p15 region. J Med Genet. 2006;43:902–907. doi: 10.1136/jmg.2006.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kou YC, Shao L, Peng HH, et al. A recurrent intragenic genomic duplication, other novel mutations in NLRP7 and imprinting defects in recurrent biparental hydatidiform moles. Mol Hum Reprod. 2008;14:33–40. doi: 10.1093/molehr/gam079. [DOI] [PubMed] [Google Scholar]
- 94.Cerrato F, Sparago A, Verde G, et al. Different mechanisms cause imprinting defects at the IGF2/H19 locus in Beckwith–Wiedemann syndrome and Wilms’ tumour. Hum Mol Genet. 2008;17:1427–1435. doi: 10.1093/hmg/ddn031. [DOI] [PubMed] [Google Scholar]
- 95.Azzi S, Rossignol S, Steunou V, et al. Multilocus methylation analysis in a large cohort of 11p15-related foetal growth disorders (Russell Silver and Beckwith Wiedemann syndromes) reveals simultaneous loss of methylation at paternal and maternal imprinted loci. Hum Mol Genet. 2009;18:4724–4733. doi: 10.1093/hmg/ddp435. [DOI] [PubMed] [Google Scholar]
- 96.Bliek J, Verde G, Callaway J, et al. Hypomethylation at multiple maternally methylated imprinted regions including PLAGL1 and GNAS loci in Beckwith-Wiedemann syndrome. Eur J Hum Genet. 2008;17:611–619. doi: 10.1038/ejhg.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim D, Bowdin SC, Tee L, et al. Clinical and molecular genetic features of Beckwith–Wiedemann syndrome associated with assisted reproductive technologies. Hum Reprod Oxf Engl. 2009;24:741–747. doi: 10.1093/humrep/den406. [DOI] [PubMed] [Google Scholar]
- 98.Hayward BE, De Vos M, Talati N, et al. Genetic and epigenetic analysis of recurrent hydatidiform mole. Hum Mutat. 2009;30:E629–E639. doi: 10.1002/humu.20993. [DOI] [PubMed] [Google Scholar]
- 99.Zhang P, Dixon M, Zucchelli M, et al. Expression analysis of the NLRP gene family suggests a role in human preimplantation development. PLoS ONE. 2008;3:e2755. doi: 10.1371/journal.pone.0002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ivanova E, Chen J-H, Segonds-Pichon A, et al. DNA methylation at differentially methylated regions of imprinted genes is resistant to developmental programming by maternal nutrition. Epigenetics. 2012;7:1200–1210. doi: 10.4161/epi.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cooper WN, Khulan B, Owens S, et al. DNA methylation profiling at imprinted loci after periconceptional micronutrient supplementation in humans: results of a pilot randomized controlled trial. FASEB J. 2012;26:1782–1790. doi: 10.1096/fj.11-192708. [DOI] [PubMed] [Google Scholar]
- 102.Dominguez-Salas P, Moore SE, Baker MS, et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun. 2014 doi: 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tobi EW, Lumey LH, Talens RP, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mitchell KJ, Pinson KI, Kelly OG, et al. Functional analysis of secreted and transmembrane proteins critical to mouse development. Nat Genet. 2001;28:241–249. doi: 10.1038/90074. [DOI] [PubMed] [Google Scholar]
- 105.Wood AJ, Schulz R, Woodfine K, et al. Regulation of alternative polyadenylation by genomic imprinting. Genes Dev. 2008;22:1141–1146. doi: 10.1101/gad.473408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wood AJ, Roberts RG, Monk D, et al. A screen for retrotransposed imprinted genes reveals an association between X chromosome homology and maternal germ-line methylation. PLoS Genet. 2007;3:e20. doi: 10.1371/journal.pgen.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Motoyama N, Wang F, Roth KA, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 108.Schulz R, McCole RB, Woodfine K, et al. Transcript- and tissue-specific imprinting of a tumour suppressor gene. Hum Mol Genet. 2008;18:118–127. doi: 10.1093/hmg/ddn322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang T, Li L, Tang J, et al. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol. 2010;28:749–755. doi: 10.1038/nbt.1644. [DOI] [PubMed] [Google Scholar]
- 110.Wang X, Soloway PD, Clark AG. A survey for novel imprinted genes in the mouse placenta by mRNA-seq. Genetics. 2011;189:109–122. doi: 10.1534/genetics.111.130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coombes C, Arnaud P, Gordon E, et al. Epigenetic properties and identification of an imprint mark in the Nesp-Gnasxl domain of the mouse Gnas imprinted locus. Mol Cell Biol. 2003;23:5475–5488. doi: 10.1128/MCB.23.16.5475-5488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu J, Yu S, Litman D, et al. Identification of a methylation imprint mark within the mouse Gnas locus. Mol Cell Biol. 2000;20:5808–5817. doi: 10.1128/mcb.20.16.5808-5817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu S, Yu D, Lee E, et al. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein α-subunit (Gsα) knockout mice is due to tissue-specific imprinting of the Gsα gene. Proc Natl Acad Sci. 1998;95:8715–8720. doi: 10.1073/pnas.95.15.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Skinner JA, Cattanach BM, Peters J. the imprinted oedematous-small mutation on mouse chromosome 2 identifies new roles for Gnas and Gnasxl in development. Genomics. 2002;80:373–375. doi: 10.1006/geno.2002.6842. [DOI] [PubMed] [Google Scholar]
- 115.Dunk CE, Roggensack AM, Cox B, et al. A distinct microvascular endothelial gene expression profile in severe IUGR placentas. Placenta. 2012;33:285–293. doi: 10.1016/j.placenta.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 116.Williamson CM. Identification of an imprinting control region affecting the expression of all transcripts in the Gnas cluster. Nat Genet. 2006;38:350–355. doi: 10.1038/ng1731. [DOI] [PubMed] [Google Scholar]
- 117.Tzouanacou E, Tweedie S, Wilson V. Identification of Jade1, a gene encoding a PHD zinc finger protein, in a gene trap mutagenesis screen for genes involved in anteroposterior axis development. Mol Cell Biol. 2003;23:8553–8562. doi: 10.1128/MCB.23.23.8553-8562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dacquin R. Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo. J Cell Biol. 2004;164:509–514. doi: 10.1083/jcb.200312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Monk D, Wagschal A, Arnaud P, et al. Comparative analysis of human chromosome 7q21 and mouse proximal chromosome 6 reveals a placental-specific imprinted gene, TFPI2/Tfpi2, which requires EHMT2 and EED for allelic-silencing. Genome Res. 2008;18:1270–1281. doi: 10.1101/gr.077115.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Okae H, Hiura H, Nishida Y, et al. Re-investigation and RNA sequencing-based identification of genes with placenta-specific imprinted expression. Hum Mol Genet. 2012;21:548–558. doi: 10.1093/hmg/ddr488. [DOI] [PubMed] [Google Scholar]
- 121.Babak T, Deveale B, Armour C, et al. Global survey of genomic imprinting by transcriptome sequencing. Curr Biol CB. 2008;18:1735–1741. doi: 10.1016/j.cub.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 122.Yokoi F, Dang MT, Mitsui S, Li Y. Exclusive paternal expression and novel alternatively spliced variants of epsilon-sarcoglycan mRNA in mouse brain. FEBS Lett. 2005;579:4822–4828. doi: 10.1016/j.febslet.2005.07.065. [DOI] [PubMed] [Google Scholar]
- 123.Allen PB, Zachariou V, Svenningsson P, et al. Distinct roles for spinophilin and neurabin in dopamine-mediated plasticity. Neuroscience. 2006;140:897–911. doi: 10.1016/j.neuroscience.2006.02.067. [DOI] [PubMed] [Google Scholar]
- 124.Royo H, Cavaillé J. Non-coding RNAs in imprinted gene clusters. Biol Cell. 2008;100:149–166. doi: 10.1042/BC20070126. [DOI] [PubMed] [Google Scholar]
- 125.Lee YJ, Park CW, Hahn Y, et al. Mit1/Lb9 and Copg2, new members of mouse imprinted genes closely linked to Peg1/Mest(1) FEBS Lett. 2000;472:230–234. doi: 10.1016/s0014-5793(00)01461-7. [DOI] [PubMed] [Google Scholar]
- 126.Smith RJ, Dean W, Konfortova G, Kelsey G. Identification of novel imprinted genes in a genome-wide screen for maternal methylation. Genome Res. 2003;13:558–569. doi: 10.1101/gr.781503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim J, Bergmann A, Lucas S, et al. Lineage-specific imprinting and evolution of the zinc-finger gene ZIM2. Genomics. 2004;84:47–58. doi: 10.1016/j.ygeno.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 128.Choo JH, Kim JD, Kim J. Imprinting of an evolutionarily conserved antisense transcript gene APeg3. Gene. 2008;409:28–33. doi: 10.1016/j.gene.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim J, Noskov VN, Lu X, et al. Discovery of a novel, paternally expressed ubiquitin-specific processing protease gene through comparative analysis of an imprinted region of mouse chromosome 7 and human chromosome 19q13.4. Genome Res. 2000;10:1138–1147. doi: 10.1101/gr.10.8.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Szeto IY, Li LL, Surani MA. Ocat, a paternally expressed gene closely linked and transcribed in the opposite direction to Peg3. Genomics. 2000;67:221–227. doi: 10.1006/geno.2000.6251. [DOI] [PubMed] [Google Scholar]
- 131.Kim J, Bergmann A, Wehri E, et al. Imprinting and evolution of two Kruppel-type zinc-finger genes, ZIM3 and ZNF264, located in the PEG3/USP29 imprinted domain. Genomics. 2001;77:91–98. doi: 10.1006/geno.2001.6621. [DOI] [PubMed] [Google Scholar]
- 132.Jiang Y, Armstrong D, Albrecht U, et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- 133.Ding F, Prints Y, Dhar MS, et al. Lack of Pwcr1/MBII-85 snoRNA is critical for neonatal lethality in Prader–Willi syndrome mouse models. Mamm Genome. 2005;16:424–431. doi: 10.1007/s00335-005-2460-2. [DOI] [PubMed] [Google Scholar]
- 134.Bressler J, Tsai T-F, Wu M-Y, et al. The SNRPN promoter is not required for genomic imprinting of the Prader–Willi/Angelman domain in mice. Nat Genet. 2001;28:232–240. doi: 10.1038/90067. [DOI] [PubMed] [Google Scholar]
- 135.Kozlov SV, Bogenpohl JW, Howell MP, et al. The imprinted gene Magel2 regulates normal circadian output. Nat Genet. 2007;39:1266–1272. doi: 10.1038/ng2114. [DOI] [PubMed] [Google Scholar]
- 136.Prickett AR, Barkas N, McCole RB, et al. Genome-wide and parental allele-specific analysis of CTCF and cohesin DNA binding in mouse brain reveals a tissue-specific binding pattern and an association with imprinted differentially methylated regions. Genome Res. 2013;23:1624–1635. doi: 10.1101/gr.150136.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jong MT, Carey AH, Caldwell KA, et al. Imprinting of a RING zinc-finger encoding gene in the mouse chromosome region homologous to the Prader–Willi syndrome genetic region. Hum Mol Genet. 1999;8:795–803. doi: 10.1093/hmg/8.5.795. [DOI] [PubMed] [Google Scholar]
- 138.Van Amerongen R. Frat is dispensable for canonical Wnt signaling in mammals. Genes Dev. 2005;19:425–430. doi: 10.1101/gad.326705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhu W, Trivedi CM, Zhou D, et al. Inpp5f is a polyphosphoinositide phosphatase that regulates cardiac hypertrophic responsiveness. Circ Res. 2009;105:1240–1247. doi: 10.1161/CIRCRESAHA.109.208785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bartolomei MS, Webber AL, Brunkow ME, Tilghman SM. Epigenetic mechanisms underlying the imprinting of the mouse H19 gene. Genes Dev. 1993;7:1663–1673. doi: 10.1101/gad.7.9.1663. [DOI] [PubMed] [Google Scholar]
- 141.Sasaki H, Ferguson-Smith AC, Shum AS, et al. Temporal and spatial regulation of H19 imprinting in normal and uniparental mouse embryos. Dev Camb Engl. 1995;121:4195–4202. doi: 10.1242/dev.121.12.4195. [DOI] [PubMed] [Google Scholar]
- 142.Feil R, Walter J, Allen ND, Reik W. Developmental control of allelic methylation in the imprinted mouse Igf2 and H19 genes. Dev Camb Engl. 1994;120:2933–2943. doi: 10.1242/dev.120.10.2933. [DOI] [PubMed] [Google Scholar]
- 143.Moore T, Constancia M, Zubair M, et al. Multiple imprinted sense and antisense transcripts, differential methylation and tandem repeats in a putative imprinting control region upstream of mouse Igf2. Proc Natl Acad Sci USA. 1997;94:12509–12514. doi: 10.1073/pnas.94.23.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Duvillié B, Cordonnier N, Deltour L, et al. Phenotypic alterations in insulin-deficient mutant mice. Proc Natl Acad Sci. 1997;94:5137–5140. doi: 10.1073/pnas.94.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kobayashi K, Morita S, Sawada H, et al. Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J Biol Chem. 1995;270:27235–27243. doi: 10.1074/jbc.270.45.27235. [DOI] [PubMed] [Google Scholar]
- 146.Tarrant JM, Groom J, Metcalf D, et al. The absence of Tssc6, a member of the tetraspanin superfamily, does not affect lymphoid development but enhances in vitro t-cell proliferative responses. Mol Cell Biol. 2002;22:5006–5018. doi: 10.1128/MCB.22.14.5006-5018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Paulsen M, El-Maarri O, Engemann S, et al. Sequence conservation and variability of imprinting in the Beckwith–Wiedemann syndrome gene cluster in human and mouse. Hum Mol Genet. 2000;9:1829–1841. doi: 10.1093/hmg/9.12.1829. [DOI] [PubMed] [Google Scholar]
- 148.Umlauf D, Goto Y, Cao R, et al. Imprinting along the Kcnq1 domain on mouse chromosome 7 involves repressive histone methylation and recruitment of Polycomb group complexes. Nat Genet. 2004;36:1296–1300. doi: 10.1038/ng1467. [DOI] [PubMed] [Google Scholar]
- 149.Bhogal B, Arnaudo A, Dymkowski A, et al. Methylation at mouse Cdkn1c is acquired during postimplantation development and functions to maintain imprinted expression. Genomics. 2004;84:961–970. doi: 10.1016/j.ygeno.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 150.Wood MD, Hiura H, Tunster SJ, et al. Autonomous silencing of the imprinted Cdkn1c gene in stem cells. Epigenetics Off J DNA Methylation Soc. 2010;5:214–221. doi: 10.4161/epi.5.3.11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Onyango P, Miller W, Lehoczky J, et al. Sequence and comparative analysis of the mouse 1-megabase region orthologous to the human 11p15 imprinted domain. Genome Res. 2000;10:1697–1710. doi: 10.1101/gr.161800. [DOI] [PubMed] [Google Scholar]
- 152.Dao D, Frank D, Qian N, et al. IMPT1, an imprinted gene similar to polyspecific transporter and multi-drug resistance genes. Hum Mol Genet. 1998;7:597–608. doi: 10.1093/hmg/7.4.597. [DOI] [PubMed] [Google Scholar]
- 153.Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321:141–149. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 154.Sachs M, Brohmann H, Zechner D, et al. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J Cell Biol. 2000;150:1375–1384. doi: 10.1083/jcb.150.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hollnagel A, Grund C, Franke WW, Arnold H-H. The Cell Adhesion Molecule M-cadherin is not essential for muscle development and regeneration. Mol Cell Biol. 2002;22:4760–4770. doi: 10.1128/MCB.22.13.4760-4770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nomura T, Kimura M, Horii T, et al. MeCP2-dependent repression of an imprinted miR-184 released by depolarization. Hum Mol Genet. 2008;17:1192–1199. doi: 10.1093/hmg/ddn011. [DOI] [PubMed] [Google Scholar]
- 157.De la Puente A, Hall J, Wu Y-Z, et al. Structural characterization of Rasgrf1 and a novel linked imprinted locus. Gene. 2002;291:287–297. doi: 10.1016/s0378-1119(02)00601-7. [DOI] [PubMed] [Google Scholar]
- 158.Itier JM, Tremp GL, Léonard JF, et al. Imprinted gene in postnatal growth role. Nature. 1998;393:125–126. doi: 10.1038/30120. [DOI] [PubMed] [Google Scholar]
- 159.Lee N-C, Shieh Y-D, Chien Y-H, et al. Regulation of the dopaminergic system in a murine model of aromatic l-amino acid decarboxylase deficiency. Neurobiol Dis. 2013;52:177–190. doi: 10.1016/j.nbd.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 160.Van de Sluis B, Muller P, Duran K, et al. Increased activity of hypoxia-inducible factor 1 is associated with early embryonic lethality in Commd1 null mice. mol cell Biol. 2007;27:4142–4156. doi: 10.1128/MCB.01932-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sunahara S, Nakamura K, Nakao K, et al. The oocyte-specific methylated region of the U2afbp-rs/U2af1-rs1 gene is dispensable for its imprinted methylation. Biochem Biophys Res Commun. 2000;268:590–595. doi: 10.1006/bbrc.2000.2189. [DOI] [PubMed] [Google Scholar]
- 162.Harada A, Oguchi K, Okabe S, et al. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- 163.Becker-Heck A, Zohn IE, Okabe N, et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat Genet. 2011;43:79–84. doi: 10.1038/ng.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.DeVeale B, van der Kooy D, Babak T. Critical evaluation of imprinted gene expression by RNA-Seq: a new perspective. PLoS Genet. 2012;8:e1002600. doi: 10.1371/journal.pgen.1002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tierling S, Gasparoni G, Youngson N, Paulsen M. The Begain gene marks the centromeric boundary of the imprinted region on mouse chromosome 12. Mamm Genome Off J Int Mamm Genome Soc. 2009;20:699–710. doi: 10.1007/s00335-009-9205-6. [DOI] [PubMed] [Google Scholar]
- 166.Takada S, Tevendale M, Baker J, et al. Delta-like and gtl2 are reciprocally expressed, differentially methylated linked imprinted genes on mouse chromosome 12. Curr Biol CB. 2000;10:1135–1138. doi: 10.1016/s0960-9822(00)00704-1. [DOI] [PubMed] [Google Scholar]
- 167.Schmidt JV, Matteson PG, Jones BK, et al. The Dlk1 and Gtl2 genes are linked and reciprocally imprinted. Genes Dev. 2000;14:1997–2002. [PMC free article] [PubMed] [Google Scholar]
- 168.Labialle S, Croteau S, Bélanger V, et al. Novel imprinted transcripts from the Dlk1-Gtl2 intergenic region, Mico1 and Mico1os, show circadian oscillations. Epigenetics Off J DNA Methylation Soc. 2008;3:322–329. doi: 10.4161/epi.3.6.7109. [DOI] [PubMed] [Google Scholar]
- 169.Zhou Y, Cheunsuchon P, Nakayama Y, et al. Activation of paternally expressed genes and perinatal death caused by deletion of the Gtl2 gene. Development. 2010;137:2643–2652. doi: 10.1242/dev.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hatada I, Morita S, Obata Y, et al. Identification of a new imprinted gene, Rian, on mouse chromosome 12 by fluorescent differential display screening. J Biochem (Tokyo) 2001;130:187–190. doi: 10.1093/oxfordjournals.jbchem.a002971. [DOI] [PubMed] [Google Scholar]
- 171.Seitz H, Royo H, Bortolin M-L, et al. A large imprinted microRNA gene cluster at the mouse Dlk1-Gtl2 domain. Genome Res. 2004;14:1741–1748. doi: 10.1101/gr.2743304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hernandez A. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116:476–484. doi: 10.1172/JCI26240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fiorica-Howells E, Hen R, Gingrich J, et al. 5-HT2A receptors: location and functional analysis in intestines of wild-type and 5-HT2A knockout mice. Am J Physiol-Gastrointest Liver Physiol. 2002;282:G877–G893. doi: 10.1152/ajpgi.00435.2001. [DOI] [PubMed] [Google Scholar]
- 174.Mulkey DK, Talley EM, Stornetta RL, et al. TASK channels determine ph sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang X, Sun Q, McGrath SD, et al. Transcriptome-wide identification of novel imprinted genes in neonatal mouse brain. PLoS ONE. 2008 doi: 10.1371/journal.pone.0003839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu J. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 177.Siuciak JA, McCarthy SA, Chapin DS, et al. Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: evidence for altered striatal function. Neuropharmacology. 2006;51:374–385. doi: 10.1016/j.neuropharm.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 178.Rappold PM, Cui M, Chesser AS, et al. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc Natl Acad Sci. 2011;108:20766–20771. doi: 10.1073/pnas.1115141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jonker JW, Wagenaar E, van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol. 2003;23:7902–7908. doi: 10.1128/MCB.23.21.7902-7908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hu JF, Balaguru KA, Ivaturi RD, et al. Lack of reciprocal genomic imprinting of sense and antisense RNA of mouse insulin-like growth factor II receptor in the central nervous system. Biochem Biophys Res Commun. 1999;257:604–608. doi: 10.1006/bbrc.1999.0380. [DOI] [PubMed] [Google Scholar]
- 181.Stöger R, Kubicka P, Liu CG, et al. Maternal-specific methylation of the imprinted mouse Igf2r locus identifies the expressed locus as carrying the imprinting signal. Cell. 1993;73:61–71. doi: 10.1016/0092-8674(93)90160-r. [DOI] [PubMed] [Google Scholar]
- 182.Wutz A, Theussl HC, Dausman J, et al. Non-imprinted Igf2r expression decreases growth and rescues the Tme mutation in mice. Dev Camb Engl. 2001;128:1881–1887. doi: 10.1242/dev.128.10.1881. [DOI] [PubMed] [Google Scholar]
- 183.Hagiwara Y, Hirai M, Nishiyama K, et al. Screening for imprinted genes by allelic message display: identification of a paternally expressed gene impact on mouse chromosome 18. Proc Natl Acad Sci USA. 1997;94:9249–9254. doi: 10.1073/pnas.94.17.9249. [DOI] [PMC free article] [PubMed] [Google Scholar]