Abstract
BACKGROUND
Shoulder subluxation caused by paralysis after stroke is a serious issue affecting shoulder pain and functional prognosis. However, its preventive treatment has not been fully investigated.
AIM
To investigate the effects of repetitive peripheral magnetic stimulation (rPMS) on the prevention of shoulder subluxation.
DESIGN
A single-center, parallel-group, prospective randomized, open-blinded, end-point study.
SETTING
Convalescent rehabilitation ward.
POPULATION
We included 50 inpatients in the convalescent rehabilitation ward with post-stroke, having upper limb paralysis, and the acromio-humeral interval (AHI) was within 1/2 finger-breadth.
METHODS
A blinded computer-based allocation system was used to randomly assign patients into two groups: 1) conventional rehabilitation plus rPMS therapy (rPMS group, N=25); and 2) conventional rehabilitation alone (control group, N=25). Blinded assessors evaluated the patients before the intervention (T0), 6 weeks after (T1), and 12 weeks after (T2). The primary outcome was the change in AHIs from T0 to T1 between the groups. In contrast, the secondary outcomes were shoulder pain, spasticity, active range of motion, and Fugl-Meyer Assessment upper extremity (FMA-UE) score.
RESULTS
Twenty-two patients in the rPMS group and 24 in the control group completed T1, whereas 16 in the rPMS group and 11 in the control group completed T2. The change in AHI was significantly lower in the rPMS group than in the control group ([95% CI, -5.15 to -0.390], P=0.023). Within-group analysis showed that AHI in the rPMS group did not change significantly, whereas it increased in the control group (P=0.004). There were no significant differences between T1 and T2 within or between the groups. Moreover, AHI did not show differences in patients with severe impairment but decreased in the rPMS group in patients with mild impairment (P=0.001).
CONCLUSIONS
The rPMS may be a new modality for preventing shoulder subluxation. The association between motor impairment and the sustained effect needs to be further examined.
CLINICAL REHABILITATION IMPACT
Applying rPMS to the muscles of the paralyzed shoulder after a stroke may prevent shoulder subluxation.
Key words: Magnetic field therapy, Electric stimulation, Shoulder dislocation, Prevention and control, Stroke
Shoulder subluxation on the paralyzed side after stroke is a condition in which the scapulohumeral joint is out of alignment because of the relaxation of the supraspinatus and deltoid muscles and continuous downward traction by the mass of the upper limb caused by paralysis.1 A recent study reported that 46 of 239 patients with stroke (19%) had shoulder subluxation.2 Moreover, sustained elongation of the shoulder muscles and capsule or shoulder joint motion causes shoulder pain.3 Patients with shoulder pain show worse functional prognoses and longer hospital stays than those without pain.4 Therefore, shoulder subluxation is a serious condition that affects not only pain but also functional prognosis of the individual.
Various treatment modalities have been investigated to improve shoulder subluxation.5-8 According to the Guidelines for Adult Stroke Rehabilitation and Recovery by the American Stroke Association,9 the positioning and use of supportive devices and slings are classified as Level C, Class IIa. An arm sling is often used in clinical practice, but it does not prevent pain and shoulder subluxation and may inhibit active correction.8 Neuromuscular electrical stimulation (NMES) is also a good treatment for individuals with minimal volitional movement within the first few months of a stroke or shoulder subluxation (Level A, Class IIa).9 The Japanese Guidelines for the Management of Stroke 2021 state that NMES is recommended for improving the range of motion of the shoulder joints and for treating shoulder subluxation in paralyzed limbs, but the effect is not long-lasting (Grade B).10
A systematic review indicated that the collar-and-cuff sling was the most commonly used sling for preventing shoulder subluxation after a stroke, but it may be ineffective.11 In a study using NMES, 40 patients with acute stroke within 48 h of stroke onset were randomized to receive NMES on the supraspinatus and deltoid (posterior) muscles for 1 h a day, 5 days a week, for 4 weeks, in addition to conventional rehabilitation. The intervention prevented shoulder subluxation, but the effect did not persist until eight weeks after the end of treatment.12 In another study, 48 patients with acute stroke within 48 h of onset underwent NMES to the supraspinatus, deltoid (middle), and deltoid (posterior) muscles for 1 h per day on weekdays during hospitalization, in addition to treatment based on the Bobath concept. After approximately 12 days of hospitalization, nine patients (37.5%) in the treatment group, based on the Bobath concept without NMES, developed shoulder subluxation, but none in the combined group. Shoulder joint alignment displacement values were also significantly higher in the control group, indicating the prevention of shoulder subluxation, but the retention effect was not examined.13 Moreover, 1.5-6.0 hours/day of stimulation is given in previous reports,5, 12 and at least 30−60 min stimulation per day is recommended.7 NMES requires time-consuming preparation by exposing the skin to electrode placement and causes pain and discomfort during stimulation.
Recently, a new modality using repetitive peripheral magnetic stimulation (rPMS) to treat shoulder subluxation has been reported.14 An rPMS is a surface system that induces eddy currents via electromagnetic induction. rPMS activates peripheral nerves and muscles without stimulating skin nociceptors, strengthening muscle forces and facilitating nerves while limiting pain.15-20 Furthermore, patients are not expected to expose their skin because magnetic stimulation passes through nonmetallic objects. The clinical applications of various somatosensory and motor disorders have been investigated.21-25
We hypothesized that rPMS would be effective in preventing shoulder subluxation. Therefore, this study examined the efficacy of rPMS in preventing shoulder subluxation.
Materials and methods
This prospective study was conducted according to the guidelines of the Declaration of Helsinki approved by the Fujita Health University Certified Review Board (no. CRB4180003) prior to initiation, and was registered with the UMIN Clinical Trials Registry (no. UMIN000031957), and the Japan Registry of Clinical Trials (no. jRCT s042180043).
Participants
The inclusion criteria were as follows: inpatients with stroke admitted to the convalescent rehabilitation ward after initial treatment at acute-care hospitals, age 20 years or older, first-time stroke, and upper limb paralysis (Brunnstrom recovery stage ≤V) without obvious shoulder subluxation. The examiner palpated the acromion and the superior aspect of the head of the humerus with the index and middle fingers.26 When the acromion-humerus interval (AHI)27 was more than 1/2 finger breadth, the patients were excluded from the study. In addition, patients with brainstem stroke, subarachnoid hemorrhage, the simultaneous presence of other neuromuscular disorders, unstable conditions, inability to sit on a chair, a history of epilepsy, cardiac pacemaker use, pregnancy, or magnetic materials near the intended stimulation site were excluded. Patients who were expected to be discharged within six weeks were also excluded. All the patients or proxies provided written informed consent.
Study protocol
This was a single-center, parallel-group, prospective, randomized, open-blinded end-point study. The patients were randomly assigned by computer (balanced prerandomization [1:1]) to receive conventional rehabilitation plus rPMS therapy (rPMS group) or conventional rehabilitation alone (control group). The conventional rehabilitation comprised joint mobilization, muscle stretching, muscle strengthening, gait, balance exercises, and task-related training in activities of daily living (ADL), depending on the function of the patients, for 180 min/day, 7 days/week. Additionally, both groups underwent conventional rehabilitation until discharge.
rPMS therapy
We used a commercially available peripheral magnetic stimulator (PathleaderTM, IFG, Sendai, Japan) for the rPMS treatment. The stimulator generated biphasic 350 µs with magnetic gradients up to 15 kT/s. The participants were placed in a sitting position, and the paralyzed upper limb was positioned on a pillow placed in the lap. Magnetic stimulation was applied to the supraspinatus and posterior deltoid/infraspinatus muscles (Figure 1).
Figure 1.

—rPMS therapy to the supraspinatus muscle.
We simultaneously stimulated the posterior deltoid and infraspinatus muscles because they overlap. The rPMS was administered for 2 s at 30 Hz with a 3-s off time, based on a previous study.14 We administered 6000 pulses for the supraspinatus and 6000 pulses for the posterior deltoid/infraspinatus muscles daily for 6 weeks. Therefore, it took 1000 s (approximately 17 min). During the stimulation, we confirmed the raising and external rotation of the humeral head by palpation. In principle, the stimulus intensity was set to the maximum intensity (approximately 0.9T) without inducing pain or discomfort. Sometimes, it was reduced to 70% of the maximum (0.65 T), depending on the subject’s discomfort.
Study endpoints
Participants were evaluated at three-time points: before the intervention (T0), 6 weeks after (T1), and 12 weeks after (T2) (Figure 2). Assessors and data analysts were blinded to the treatment allocation.
Figure 2.
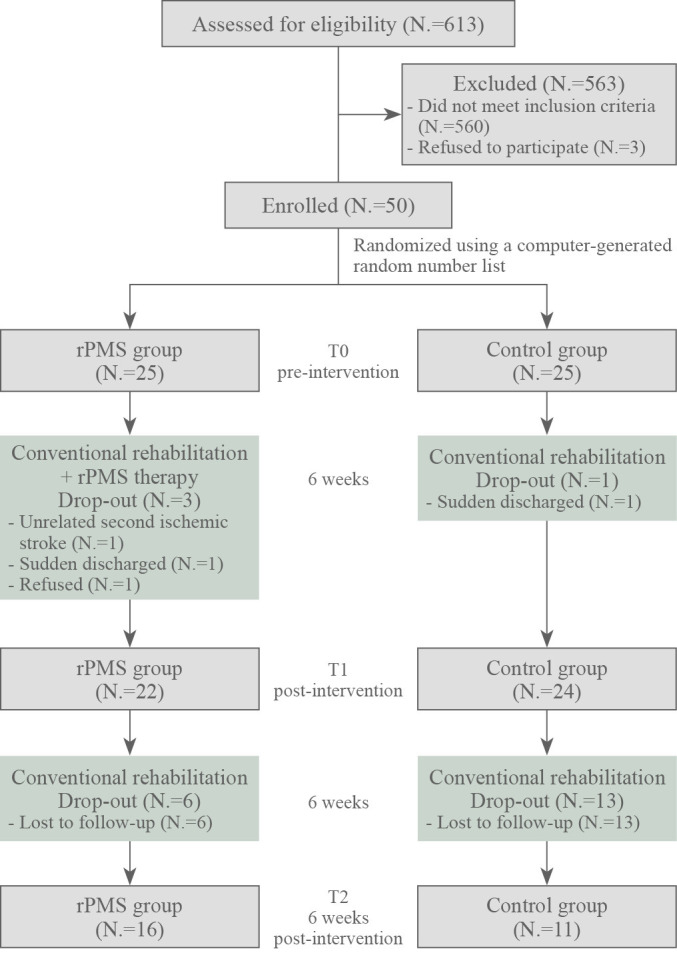
—Design and flow of participants through the study.
The primary outcome of the study was the change in AHIs from T0 to T1 between the groups. AHIs were calculated from plain anteroposterior radiographs of the shoulder joints obtained in the sitting position with the upper extremity hanging down, using the Adobe Acrobat Reader DC Version 2022.002.20191 (Adobe Systems Incorporated). The secondary outcomes of the study were changes in AHI from T0 to T1 in each group, shoulder pain at rest and during rPMS, spasticity of the shoulder adductor and elbow flexor muscles, active range of motion of shoulder abduction, and upper limb function. Shoulder pain was evaluated from 0 to 10 using a Numerical Rating Scale (0 = no pain; 10 = the most severe pain).28 Spasticity was assessed using the modified Ashworth scale.29 Finally, upper limb function was assessed using the subscales A (shoulder/elbow/forearm), B (wrist), C (hand), and D (coordination/speed) of the upper extremity Fugl-Meyer Assessment scale (FMA-UE).30 The maximum scores for subscales A, B, C, and D were 36, 10, 14, and 6, respectively, for a total of 66.
Statistical analysis and sample calculation
Based on a previous NMES study on the supraspinatus muscle for shoulder subluxation,6 the effect size was calculated 0.92. Assuming α=0.05, a power of 0.85, and a dropout rate of 10%, the sample size was estimated as 50 cases using G*Power 3.1 software (v. 3.1.9.6; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany).31
Fisher’s exact test was used to compare the sex, lesion type, and paretic side. Normality was checked using the Shapiro-Wilk test. An unpaired t-test was used to compare the primary outcome of change in AHI from T0 to T1 between groups. The Mann-Whitney U Test was used to compare each secondary outcome between groups. The Wilcoxon signed-rank test was used for within-group comparisons; P values were Bonferroni corrected for comparisons involving up to T2.
The subanalysis was based on the severity classification of upper limb motor function32 and compared changes in AHI between the two groups: FMA-UE total score <35 (severe) and FMA-UE total score ≥35 (mild).
For the statistical analysis, MAS scores of 1+, 2, 3, and 4 were converted to 2, 3, 4, and 5, respectively. All values are expressed as the mean±standard deviation (median). Statistical analyses were performed using SPSS version 27 (IBM, Armonk, NY, USA). Statistical significance was set at P<0.05.
Results
Patients
A total of 613 consecutive patients with stroke admitted to the convalescent rehabilitation ward were screened between September 2018 and November 2021. Five-hundred and sixty patients did not meet the inclusion criteria. Written informed consent was obtained from 50 of the 53 patients. They were randomly assigned to either the rPMS or control group. Each group included 25 patients who completed a baseline assessment (T0). However, one had another ischemic stroke, one refused to continue the study, and one suddenly discharged before T1 assessment in the rPMS group. In the control group, one was suddenly discharged before the T1 assessment. Therefore, 22 patients in the rPMS group and 24 in the control group completed the T1 evaluation and were included in subsequent analyses. Between the T1 and T2 evaluations, six patients in the rPMS group and 13 patients in the control group were lost to follow-up; hence, 16 patients in the rPMS group and 11 patients in the control group completed the T2 evaluation (Figure 2). None of the participants in either group experienced a serious adverse event during the study.
Baseline characteristics
Table I lists the baseline characteristics. The mean age of the patients was 65 years (range: 39-89 years). The study included 31 men and 15 women. The etiology was cerebral infarction in 23 patients and cerebral hemorrhage in 23 patients, including 19 with right and 27 with left hemiplegia. Not all data, except for age and AHI in the control group, showed a normal distribution. Thus, the Mann-Whitney U Test was used to compare groups. However, there were no statistically significant differences between the groups.
Table I. —Patients’ characteristics.
| Characteristics | rPMS group (N.=22) | Control group (N.=24) | P value |
|---|---|---|---|
| Gender, male / female | 14 / 8 | 17 / 7 | 0.755 a |
| Age (years) | 69±13 (71) | 61±15 (60) | 0.084 b |
| Lesion type, ischemic / hemorrhagic | 12 / 10 | 11 / 13 | 0.768 a |
| Days from stroke onset (days) | 34±23 (31) | 41±20 (36) | 0.135 b |
| Paretic side, right / left | 10 / 12 | 9 / 15 | 0.765 a |
| AHI (mm) | 12.4±4.0 (10.5) | 11.6±3.6 (11.1) | 0.553 b |
| NRS for shoulder pain at rest | 0.9±2.7 (0) | 0.6±1.9 (0) | 0.610 b |
| NRS for shoulder pain at movement | 3.9±4.2 (1.5) | 3.3±3.4 (3) | 0.631 b |
| MAS of shoulder adductors | 0.8±0.9 (1) | 0.6±0.9 (0) | 0.342 b |
| MAS of elbow flexors | 1.0±0.9 (1) | 0.8±1.1 (0.5) | 0.439 b |
| FMA-UE total score | 24.9±23.7 (10) | 30.2±23.1 (23) | 0.303 b |
| A, shoulder / elbow / forearm | 14.9±12.5 (8) | 19.0±12.2 (19) | 0.266 b |
| B, wrist | 3.1±4.1 (0) | 3.7±4.1 (1.5) | 0.540 b |
| C, hand | 5.5±6.0 (3) | 6.0±5.7 (3.5) | 0.642 b |
| D, coordination / speed | 1.4±2.2 (0) | 1.6±2.1 (0) | 0.520 b |
| A-ROM of shoulder abduction (degree) | 61.1±72.8 (15) | 81.3±73.0 (65) | 0.275 b |
Data are described as mean±SD (median). AHI: acromio-humeral interval; NRS: Numerical Rating Scale; MAS: Modified Ashworth scale; FMA-UE: Fugl-Meyer Assessment scale (upper extremity); A-ROM: active range of motion. a Fisher’s Exact Test; b Mann-Whitney U Test.
Primary outcome
The AHI changes from T0-T1 showed a normal distribution in both the rPMS and control groups. However, it was significantly decreased in the rPMS group compared to the control group by the unpaired t-test ([95% CI, -5.15 to -0.390], P=0.023) (Table II).
Table II. —Change in outcomes each group from baseline (T0) to the end of the intervention period (T1).
| Outcomes | Groups | T0 | T1 | Difference within groups | Difference between groups |
|---|---|---|---|---|---|
| P value a | P value b, c | ||||
| AHI (mm) | rPMS | 12.4±4.0 (10.5) | 11.6±4.6 (9.8) | 0.200 | 0.023* b |
| Control | 11.6±3.6 (11.1) | 13.5±4.0 (13.1) | 0.004** | ||
| NRS for shoulder pain at rest | rPMS | 0.9±2.7 (0) | 0.3±1.3 (0) | 0.109 | 0.232 c |
| Control | 0.6±1.9 (0) | 0.7±1.9 (0) | 0.854 | ||
| NRS for shoulder pain at movement | rPMS | 3.9±4.2 (1.5) | 3.8±3.4 (3.5) | 0.719 | 0.991 c |
| Control | 3.3±3.4 (3.0) | 2.9±3.6 (1.5) | 0.218 | ||
| MAS of shoulder adductors | rPMS | 0.8±0.9 (1) | 0.8±1.1 (0) | 1.000 | 0.746 c |
| Control | 0.6±0.9 (0) | 0.5±0.8 (0) | 0.480 | ||
| MAS of elbow flexors | rPMS | 1.0±0.9 (1) | 1.0±1.1 (1) | 0.564 | 0.559 c |
| Control | 0.8±1.1 (0.5) | 0.8±0.9 (1) | 0.527 | ||
| FMA-UE total score | rPMS | 24.9±23.7 (10) | 31.2±25.5 (20) | <0.001** | 0.680 c |
| Control | 30.2±23.1 (23) | 39.8±22.9 (47.5) | <0.001** | ||
| A, shoulder / elbow / forearm | rPMS | 14.9±12.5 (8) | 18.6±13.2 (16) | 0.001** | 0.972 c |
| Control | 19.0±12.2 (19) | 23.4±11.8 (28.5) | 0.001** | ||
| B, wrist | rPMS | 3.1±4.1 (0) | 3.9±4.2 (2) | 0.180 | 0.250 c |
| Control | 3.7±4.1 (1.5) | 5.3±4.3 (6.5) | 0.010* | ||
| C, hand | rPMS | 5.5±6.0 (3) | 6.8±6.3 (4) | 0.006* | 0.106 c |
| Control | 6.0±5.7 (3.5) | 8.8±5.3 (10) | <0.001** | ||
| D, coordination / speed | rPMS | 1.4±2.2 (0) | 2.0±2.6 (0) | 0.066 | 0.272 c |
| Control | 1.6±2.1 (0) | 2.3±2.2 (3) | 0.006* | ||
| A-ROM of shoulder abduction (degree) | rPMS | 61.1±72.8 (15) | 72.3±69.6 (70) | 0.074 | 0.430 c |
| Control | 81.3±73.0 (65) | 106.7±73.7 (125) | 0.004** |
Data are described as mean±SD (median). AHI: acromio-humeral interval; NRS: Numerical Rating Scale; MAS: Modified Ashworth scale; FMA-UE: Fugl-Meyer Assessment scale (upper extremity); A-ROM: active range of motion. a Wilcoxon signed-rank test; b unpaired t-test; c Mann-Whitney U Test; *P<0.05, **P<0.01.
Secondary outcomes
All data, except for AHI at T0, showed a non-normal distribution. Within-group analysis showed that AHI in the rPMS group did not change significantly, whereas it increased in the control group (P=0.004). The rPMS group had significantly improved total scores on the FMA-UE, Category A, and Category C. The total score of FMA-UE, categories A, B, C, and D, and the active range of motion of shoulder abduction significantly improved in the control group (Table II).
To confirm the retention effect of rPMS, we compared the AHI between T1 and T2 in 16 rPMS groups and 11 control group patients for whom T2 evaluation was possible. The characteristics of patients who underwent both T1 and T2 point evaluation are shown in Table III.
Table III. —Characteristics of patients who underwent both T1 and T2 evaluations.
| Characteristics | rPMS group (N.=16) | Control group (N.=11) | P value |
|---|---|---|---|
| Gender, male / female | 10 / 6 | 7 / 4 | 1.000 a |
| Age (years) | 66±13 (68) | 60±17 (57) | 0.331 b |
| Lesion type, ischemic / hemorrhagic | 7 / 9 | 6 / 5 | 0.704 a |
| Days from stroke onset (days) | 24±10 (25) | 42±15 (40) | 0.001 ** b |
| Paretic side, right / left | 6 / 10 | 3 / 8 | 0.692 a |
| AHI (mm) | 12.3±4.1 (10.4) | 11.7±4.1 (10.8) | 0.716 b |
| NRS for shoulder pain at rest | 0.6±2.0 (0) | 0.6±1.4 (0) | 0.981 b |
| NRS for shoulder pain at movement | 4.0±4.0 (3) | 3.5±2.8 (4) | 0.716 b |
| MAS of shoulder adductors | 0.8±0.9 (1) | 0.5±0.9 (0) | 0.394 b |
| MAS of elbow flexors | 0.9±0.8 (1) | 0.9±1.2 (1) | 0.753 b |
| FMA-UE total score | 24.3±24.3 (9) | 29.2±19.7 (23) | 0.512 b |
| A [Shoulder/Elbow/Forearm] | 14.9±13.1 (7) | 19.4±11.3 (21) | 0.451 b |
| B [Wrist] | 2.6±3.9 (0) | 2.9±3.4 (1) | 0.577 b |
| C [Hand] | 5.4±6.0 (2.5) | 5.5±4.8 (5) | 0.610 b |
| D [Coordination/Speed] | 1.4±2.4 (0) | 1.4±1.8 (0) | 0.753 b |
| A-ROM of shoulder abduction (degree) | 57.8±76.0 (15) | 71.8±76.1 (60) | 0.481 b |
Data are described as mean±SD (median). AHI: acromio-humeral interval; NRS: Numerical Rating Scale; MAS: Modified Ashworth scale; FMA-UE: Fugl-Meyer Assessment scale (upper extremity); A-ROM: active range of motion. a Fisher’s Exact Test; b Mann-Whitney U Test; **P<0.01.
The number of days from stroke onset to admission in the convalescent rehabilitation ward was significantly lower in the rPMS group than that in the control group. The AHIs at T1 and T2 were 11.9±4.4 (9.8) and 11.8±5.1 (10.3) in the rPMS group, while 13.5±3.8 (12.6) and 14.4±6.7 (12.1) in the control group, respectively (Figure 3). There were no significant differences within or between the groups.
Figure 3.
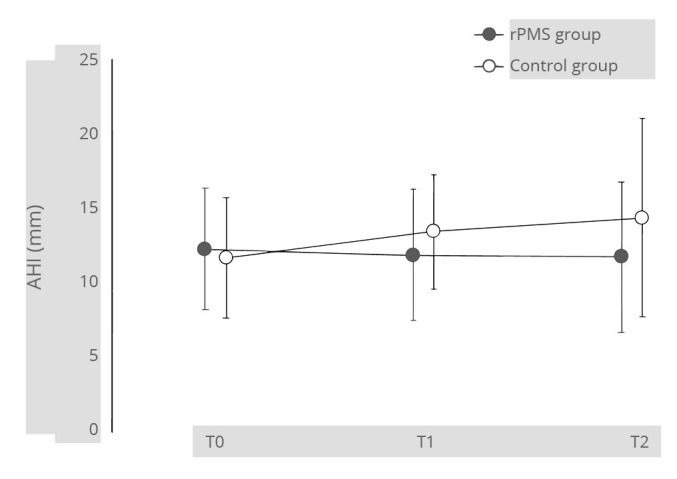
—The AHIs from T0 to T2 in 16 rPMS and 11 control group patients for whom T2 evaluation was possible.
Among the 22 rPMS group patients who completed the T1 evaluation, 13 had a severe impairment, and nine had mild impairment at T0. In contrast, 13 patients in the control group had a severe impairment, and 11 had mild impairment cases at T0 point in the control group. At T0, AHI was 13.5±3.5 (13.2) in 26 severe cases and 10.0±3.2 (9.4) in 20 mild cases. The AHI was significantly greater in severe cases (P<0.001). The change in AHI between T0 and T1 for severe impairment cases was -0.1±6.1 (-2.7) in the rPMS group and 2.4±3.4 (2.6) in the control group. However, for mild cases, it was -1.9±2.5 (-0.6) in the rPMS group and 1.4±2.2 (2.1) in the control group. In the between-group comparison, there was no significant difference between the rPMS and control groups in patients with severe impairment (P=0.281). Nevertheless, the AHI decreased significantly in the rPMS group in patients with mild impairment (P=0.001) (Figure 4).
Figure 4.
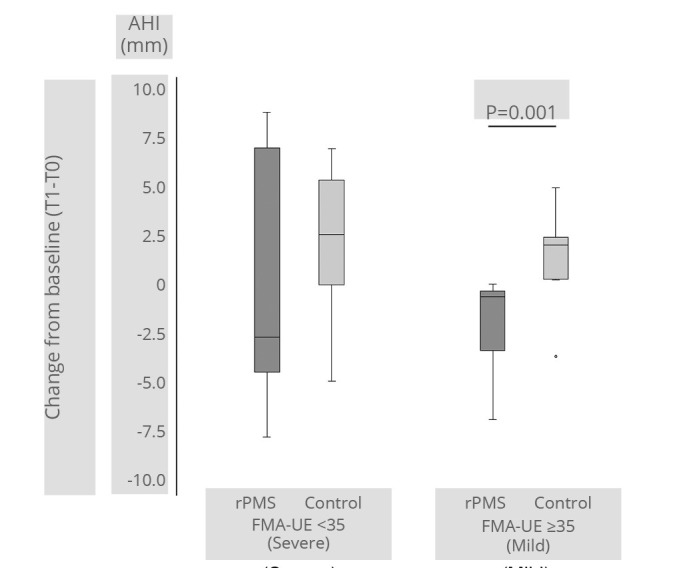
—AHI change from T0 to T1 between severe and mild function measured by the upper extremity Fugl-Meyer Assessment scale (FMA-UE).
Discussion
This randomized controlled trial investigated whether six weeks of rPMS to the supraspinatus and infraspinatus/deltoid (posterior) muscles effectively prevents shoulder subluxation in stroke patients. The results indicated that changes in AHI were significantly smaller in the rPMS group than in the control group. This was maintained in the rPMS group and significantly increased in the control group. Furthermore, this effect tended to be maintained for six weeks. This study suggests that rPMS effectively prevent shoulder subluxation after stroke.
This is the first study to examine the effect of rPMS in preventing shoulder subluxation after stroke. Previous NMES studies stimulated the supraspinatus and deltoid (posterior) muscles,5, 12, 13 whereas this study involved the infraspinatus muscle as a target muscle. The infraspinatus plays an important role in stabilizing the glenohumeral joint as a rotator cuff muscle.33, 34 Moreover, some of the upper fibers of the infraspinatus muscle fuse with the supraspinatus muscle and attach to the greater tuberosity to raise the humeral head.35 However, there are no reports of stimulation of the infraspinatus muscle by NMES,7 probably because this muscle is located deep in the skin. Many patients dropped out between T1 and T2, and the number of days from stroke onset to admission differed significantly between the two groups. As many participants lived far from our university hospital, it was difficult for them to visit our hospital again for T2 evaluation after discharge. Nevertheless, the AHIs at T1 and T2 did not exhibit significant differences within or between the groups. The efficacy of rPMS in preventing shoulder subluxation may be maintained up to 6 weeks after intervention.
While previous NMES studies12, 13 included stroke patients in the early stages, this study included patients in the convalescent rehabilitation ward. Shoulder subluxation is most likely to occur during the acute stages of the post-stroke period13 and in patients with severe motor impairments.36 In our subanalysis, AHI at T0 was greater in patients with severe impairment. Shoulder subluxation occurs when the upper extremity is exposed to gravity and continuously pulled downward.1, 37 In fact, AHI in the control group deteriorated between T0 and T1. Although rPMS to the periprosthetic shoulder muscles prevented the AHI increase, the subanalysis showed no difference between the rPMS and control groups in patients with severe upper motor dysfunction. The number of cases was small, but the effect of rPMS may be limited in such patients. The normal range of AHI is reported to be 7-14 mm.27 Therefore, at T0, some patients may have experienced mild shoulder subluxation. As shoulder subluxation after stroke often worsens during the time course,5, 6, 38, 39 this study may include the treatment effect of rPMS in addition to the effect of preventing subluxation.
All patients in this study were in the convalescent stage, and motor function improved significantly from T0 to T1 in both groups. Since there were no significant differences in the improvement of motor function between the groups, we hypothesize that rPMS for preventing shoulder subluxation cannot promote changes beyond the expected recovery of motor function. There were no significant changes in shoulder pain and spasticity in both groups. Shoulder pain is caused by a variety of factors, including rotator cuff disorders, adhesive capsulitis, and shoulder subluxation.40 A systematic review found that 7 of 14 studies reported no significant correlation between shoulder subluxation and shoulder pain.41 In this study, some patients had shoulder pain at T0 when no shoulder subluxation existed, suggesting that the shoulder pain may have arisen from other sources.
Limitations of the study
Although the results of this study support our hypothesis, we must acknowledge some limitations. First, it was not double-blinded because sham stimulation was not used in the control group. A comparison with sham stimulation may provide additional confidence regarding the effect of rPMS. We did not compare the effect of rPMS with that of other treatment modalities, such as NMES. Furthermore, many patients dropped out at the T2 evaluation, making it difficult to determine whether the effect of rPMS in preventing shoulder subluxation persists after intervention. Finally, the sample size was small to test the effect of rPMS in preventing shoulder subluxation after stroke. Future multicenter trials with larger samples are recommended.
Conclusions
Applying rPMS to the muscles of the paralyzed shoulder after a stroke may prevent shoulder subluxation. However, the sustained effects and the relationship to motor impairment need further investigation. rPMS may become a new modality for preventing shoulder subluxation.
Acknowledgements
The authors thank Fumika Nakao (OTR) and Manami Harada (OTR) from the Department of Rehabilitation at Fujita Health University Hospital for their assistance with the measurements used to collect data for this study. We also thank Assistant Professor Takuma Ishihara at the Center for Innovative Clinical Research, Gifu University Hospital, for his advice as a biostatistician regarding the statistical analysis.
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding: This work was partly supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI, grant no. JP19K19928.
References
- 1.Griffin C. Management of the hemiplegic shoulder complex. Top Stroke Rehabil 2014;21:316–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25150663&dopt=Abstract 10.1310/tsr2104-316 [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Yang S, Cui L, Bao Y, Gu L, Pan H, et al. Prevalence, risk factor and outcome in middle-aged and elderly population affected by hemiplegic shoulder pain: an observational study. Front Neurol 2023;13:1041263. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36712437&dopt=Abstract 10.3389/fneur.2022.1041263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aras MD, Gokkaya NK, Comert D, Kaya A, Cakci A. Shoulder pain in hemiplegia: results from a national rehabilitation hospital in Turkey. Am J Phys Med Rehabil 2004;83:713–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15314536&dopt=Abstract 10.1097/01.PHM.0000138739.18844.88 [DOI] [PubMed] [Google Scholar]
- 4.Barlak A, Unsal S, Kaya K, Sahin-Onat S, Ozel S. Poststroke shoulder pain in Turkish stroke patients: relationship with clinical factors and functional outcomes. Int J Rehabil Res 2009;32:309–15. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19077723&dopt=Abstract 10.1097/MRR.0b013e32831e455f [DOI] [PubMed] [Google Scholar]
- 5.Faghri PD, Rodgers MM, Glaser RM, Bors JG, Ho C, Akuthota P. The effects of functional electrical stimulation on shoulder subluxation, arm function recovery, and shoulder pain in hemiplegic stroke patients. Arch Phys Med Rehabil 1994;75:73–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8291967&dopt=Abstract https://doi.org/ 10.1016/0003-9993(94)90341-7 [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi H, Onishi H, Ihashi K, Yagi R, Handa Y. Reduction in subluxation and improved muscle function of the hemiplegic shoulder joint after therapeutic electrical stimulation. J Electromyogr Kinesiol 1999;9:327–36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10527214&dopt=Abstract 10.1016/S1050-6411(99)00008-5 [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Baker LL, Johnson RE, Tilson JK. Effectiveness of neuromuscular electrical stimulation for management of shoulder subluxation post-stroke: a systematic review with meta-analysis. Clin Rehabil 2017;31:1431–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28343442&dopt=Abstract 10.1177/0269215517700696 [DOI] [PubMed] [Google Scholar]
- 8.van Bladel A, Lambrecht G, Oostra KM, Vanderstraeten G, Cambier D. A randomized controlled trial on the immediate and long-term effects of arm slings on shoulder subluxation in stroke patients. Eur J Phys Rehabil Med 2017;53:400–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28145396&dopt=Abstract 10.23736/S1973-9087.17.04368-4 [DOI] [PubMed] [Google Scholar]
- 9.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research . Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:e98–169. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27145936&dopt=Abstract 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 10.Stroke Guideline Committee of the Japan Stroke Association. Japanese guideline for the management of stroke 2021 [in Japanese]. Tokyo: Kyowa Kikaku; 2021. [Google Scholar]
- 11.Foongchomcheay A, Ada L, Canning CG. Use of devices to prevent subluxation of the shoulder after stroke. Physiother Res Int 2005;10:134–45. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16245754&dopt=Abstract 10.1002/pri.3 [DOI] [PubMed] [Google Scholar]
- 12.Linn SL, Granat MH, Lees KR. Prevention of shoulder subluxation after stroke with electrical stimulation. Stroke 1999;30:963–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10229728&dopt=Abstract 10.1161/01.STR.30.5.963 [DOI] [PubMed] [Google Scholar]
- 13.Fil A, Armutlu K, Atay AO, Kerimoglu U, Elibol B. The effect of electrical stimulation in combination with Bobath techniques in the prevention of shoulder subluxation in acute stroke patients. Clin Rehabil 2011;25:51–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20702513&dopt=Abstract 10.1177/0269215510375919 [DOI] [PubMed] [Google Scholar]
- 14.Fujimura K, Kagaya H, Endou C, Ishihara A, Nishigaya K, Muroguchi K, et al. Effects of repetitive peripheral magnetic stimulation on shoulder subluxations caused by stroke: A preliminary study. Neuromodulation 2020;23:847–51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32840021&dopt=Abstract 10.1111/ner.13064 [DOI] [PubMed] [Google Scholar]
- 15.Barker AT. An introduction to the basic principles of magnetic nerve stimulation. J Clin Neurophysiol 1991;8:26–37. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2019648&dopt=Abstract 10.1097/00004691-199101000-00005 [DOI] [PubMed] [Google Scholar]
- 16.Cohen D, Cuffin BN. Developing a more focal magnetic stimulator. Part I: some basic principles. J Clin Neurophysiol 1991;8:102–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2019645&dopt=Abstract 10.1097/00004691-199101000-00013 [DOI] [PubMed] [Google Scholar]
- 17.Han TR, Shin HI, Kim IS. Magnetic stimulation of the quadriceps femoris muscle: comparison of pain with electrical stimulation. Am J Phys Med Rehabil 2006;85:593–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16788390&dopt=Abstract 10.1097/01.phm.0000223239.93539.fe [DOI] [PubMed] [Google Scholar]
- 18.Struppler A, Angerer B, Havel P. Modulation of sensorimotor performances and cognition abilities induced by RPMS: clinical and experimental investigations. Suppl Clin Neurophysiol 2003;56:358–67. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14677412&dopt=Abstract 10.1016/S1567-424X(09)70239-9 [DOI] [PubMed] [Google Scholar]
- 19.Struppler A, Binkofski F, Angerer B, Bernhardt M, Spiegel S, Drzezga A, et al. A fronto-parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: A PET-H2O15 study. Neuroimage 2007;36(Suppl 2):T174–86. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17499165&dopt=Abstract 10.1016/j.neuroimage.2007.03.033 [DOI] [PubMed] [Google Scholar]
- 20.Beaulieu LD, Massé-Alarie H, Camiré-Bernier S, Ribot-Ciscar É, Schneider C. After-effects of peripheral neurostimulation on brain plasticity and ankle function in chronic stroke: the role of afferents recruited. Neurophysiol Clin 2017;47:275–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28314519&dopt=Abstract 10.1016/j.neucli.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 21.Beaulieu LD, Schneider C. Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. A review. Neurophysiol Clin 2013;43:251–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24094911&dopt=Abstract 10.1016/j.neucli.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 22.Beaulieu LD, Schneider C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: A literature review on parameters of application and afferents recruitment. Neurophysiol Clin 2015;45:223–37. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26363684&dopt=Abstract 10.1016/j.neucli.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 23.Kagaya H, Ogawa M, Mori S, Aoyagi Y, Shibata S, Inamoto Y, et al. Hyoid bone movement at rest by peripheral magnetic stimulation of suprahyoid muscles in normal individuals. Neuromodulation 2019;22:593–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29608796&dopt=Abstract 10.1111/ner.12777 [DOI] [PubMed] [Google Scholar]
- 24.Obayashi S, Takahashi R. Repetitive peripheral magnetic stimulation improves severe upper limb paresis in early acute phase stroke survivors. NeuroRehabilitation 2020;46:569–75. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32508342&dopt=Abstract 10.3233/NRE-203085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimura K, Kagaya H, Tanikawa H. Kinematic analysis for repetitive peripheral magnetic stimulation of the intrinsic muscles of the hand. Appl Sci (Basel) 2022;12:9015. 10.3390/app12189015 [DOI] [Google Scholar]
- 26.Hall J, Dudgeon B, Guthrie M. Validity of clinical measures of shoulder subluxation in adults with poststroke hemiplegia. Am J Occup Ther 1995;49:526–33. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7645665&dopt=Abstract 10.5014/ajot.49.6.526 [DOI] [PubMed] [Google Scholar]
- 27.Weiner DS, Macnab I. Superior migration of the humeral head. A radiological aid in the diagnosis of tears of the rotator cuff. J Bone Joint Surg Br 1970;52:524–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=5455085&dopt=Abstract 10.1302/0301-620X.52B3.524 [DOI] [PubMed] [Google Scholar]
- 28.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. European Palliative Care Research Collaborative (EPCRC) . Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011;41:1073–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21621130&dopt=Abstract 10.1016/j.jpainsymman.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 29.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67:206–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3809245&dopt=Abstract 10.1093/ptj/67.2.206 [DOI] [PubMed] [Google Scholar]
- 30.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1135616&dopt=Abstract 10.2340/1650197771331 [DOI] [PubMed] [Google Scholar]
- 31.Faul F, Erdfelder E, Lang AG, Buchner A. G.*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17695343&dopt=Abstract 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 32.Woytowicz EJ, Rietschel JC, Goodman RN, Conroy SS, Sorkin JD, Whitall J, et al. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch Phys Med Rehabil 2017;98:456–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27519928&dopt=Abstract 10.1016/j.apmr.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop Relat Res 1996;(330):3–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8804269&dopt=Abstract 10.1097/00003086-199609000-00002 [DOI] [PubMed] [Google Scholar]
- 34.Ward SR, Hentzen ER, Smallwood LH, Eastlack RK, Burns KA, Fithian DC, et al. Rotator cuff muscle architecture: implications for glenohumeral stability. Clin Orthop Relat Res 2006;448:157–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16826111&dopt=Abstract 10.1097/0000214443.87645.bb [DOI] [PubMed] [Google Scholar]
- 35.Clark JM, Harryman DT, 2nd. Tendons, ligaments, and capsule of the rotator cuff. Gross and microscopic anatomy. J Bone Joint Surg Am 1992;74:713–25. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1624486&dopt=Abstract 10.2106/00004623-199274050-00010 [DOI] [PubMed] [Google Scholar]
- 36.Stolzenberg D, Siu G, Cruz E. Current and future interventions for glenohumeral subluxation in hemiplegia secondary to stroke. Top Stroke Rehabil 2012;19:444–56. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22982832&dopt=Abstract 10.1310/tsr1905-444 [DOI] [PubMed] [Google Scholar]
- 37.Najenson T, Pikielny SS. Malalignment of the gleno-humeral joint following hemıplegia. A review of 500 cases. Ann Phys Med 1965;8:96–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14337049&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 38.Wang RY, Chan RC, Tsai MW. Functional electrical stimulation on chronic and acute hemiplegic shoulder subluxation. Am J Phys Med Rehabil 2000;79:385–90, quiz 391–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10892625&dopt=Abstract 10.1097/00002060-200007000-00011 [DOI] [PubMed] [Google Scholar]
- 39.Wang RY, Yang YR, Tsai MW, Wang WT, Chan RC. Effects of functional electric stimulation on upper limb motor function and shoulder range of motion in hemiplegic patients. Am J Phys Med Rehabil 2002;81:283–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11953546&dopt=Abstract 10.1097/00002060-200204000-00007 [DOI] [PubMed] [Google Scholar]
- 40.Kalichman L, Ratmansky M. Underlying pathology and associated factors of hemiplegic shoulder pain. Am J Phys Med Rehabil 2011;90:768–80. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21430513&dopt=Abstract 10.1097/PHM.0b013e318214e976 [DOI] [PubMed] [Google Scholar]
- 41.Kumar P, Saunders A, Ellis E, Whitlam S. Association between glenohumeral subluxation and hemiplegic shoulder pain in patients with stroke. Phys Ther Rev 2013;18:90–100. 10.1179/108331913X13608385943254 [DOI] [Google Scholar]


