Abstract
Arsenic is frequently used in alternative medicine, and it is critical to promptly identify and treat suspected arsenic toxicity in patients. In a case study, a female patient presented with several symptoms, including nausea, vomiting, bilateral tinnitus, hearing loss, vertigo, and other associated complaints. After admission, the patient showed lethargy, and topical application of Chinese herbal medicine was found on her left breast, along with visible pigmentation on her torso. Examination revealed severe bilateral sensorineural deafness, liver and kidney injury, and pancytopenia. Due to the presence of broken skin, toxicological analysis detected elevated levels of arsenic in both blood (113 ng/mL) and urine (865.4 ng/mL). The patient was diagnosed with arsenic poisoning and received symptomatic treatment, including detoxification. Unfortunately, the patient died due to long-term exposure to arsenic. Therefore, early identification of the etiology is crucial for managing cases of arsenic poisoning.
Keywords: Arsenic poisoning, sensorineural hearing loss, gangrene, and treatment
Introduction
Arsenide is a well-documented toxicant that affects multiple organs, was present in many traditional therapies, and is now used to treat acute promyelocytic leukemia. Arsenic poisoning is a rare disease and even rarer with severe sensorineural deafness in both ears as the first symptom.
Case Presentation
Informed consent was obtained before the writing of this case report.
The 37-year-old female patient was admitted to the hospital on March 10, 2023, presenting with unexplained nausea and vomiting for the past 3 days, bilateral tinnitus, hearing loss lasting 2 days, persistent tinnitus characterized by a “buzzing” quality, vertigo onset within the previous 14 hours, and worsening symptoms upon self-reported leftward head tilt. Pure-tone audiometry demonstrated severe bilateral sensorineural hearing loss (Figure 1), while sound transmission analysis disclosed an “A”-shaped curve in both tympanic membranes, suggesting the potential for bilateral sudden deafness. Upon physical examination, a 10 × 10 cm ulceration was discovered on the left breast where Chinese herbal medicine had been applied externally. The ulcer’s surface appeared contaminated with a small amount of exudate (Figure 2). Generalized edema and scattered pigmentation were also observed. Liver and kidney function tests conducted on March 11, 2023, revealed increased levels of alanine aminotransferase (381.1 U/L) and aspartate aminotransferase (337 U/L), decreased albumin levels (30.0 g/L), elevated creatinine levels (111.7 µmol/L), and increased urea levels (16.80 mmol/L). Blood test results from the same date showed a low white blood cell count of 1.74 × 109/L, red blood cell count of 2.45 × 1012/L, and hemoglobin level of 72 g/L. The chest computed tomography results on March 11, 2023, revealed bilateral pleural effusion with potential interstitial pulmonary edema. Cardiac ultrasound results from March 12, 2023, detected a small amount of pericardial effusion. Although symptomatic support treatment, such as nerve nourishment, circulation improvement, and supplementation of human blood albumin, was given, the patient’s condition did not significantly improve. On March 13, 2023, the patient experienced sudden unconsciousness and ventricular fibrillation and was subsequently transferred to the intensive care unit. In the later stages, the patient frequently experienced ventricular tachycardia (Figure 3). Upon reviewing the patient’s medical history, it was discovered that she had been treated with Chinese herbal medicine containing arsenic components for over 20 days. Hematuria samples taken on March 15, 2023, revealed 113 ng arsenic/mL and 865.4 ng arsenic/mL in the urine, indicating that the patient suffered from arsenic poisoning and a concurrent breast infection. Dynamic reassessment of routine blood tests showed a progressive decline in the 3 systems. Treatment included the addition of a human granulocyte-stimulating hematopoietic factor and upgrading antibiotics to meropenem in combination with vancomycin for anti-infection purposes. Renal function results from March 21, 2023, were creatinine at 175.5 µmol/L and urea at 15.70 mmol/L. Liver function results from March 22, 2023, showed alanine aminotransferase at 102.6 U/L, aspartate aminotransferase at 108 U/L, and albumin at 24.6 g/L. Blood routine results from the same date indicated a leukocyte count of 0.58 × 109/L, red blood cell count of 3.01 × 1012/L, platelet count of 17 × 109 g/L, and hemoglobin of 90 g/L. Interleukin-6 levels exceeded 5000 pg/mL, while procalcitonin measured 23.47 ng/mL. On March 22, 2023, the patient’s blood pressure dropped to 60/25 mmHg, and after 40 minutes, her heart rate and blood pressure could not be stabilized. The patient was subsequently declared deceased.
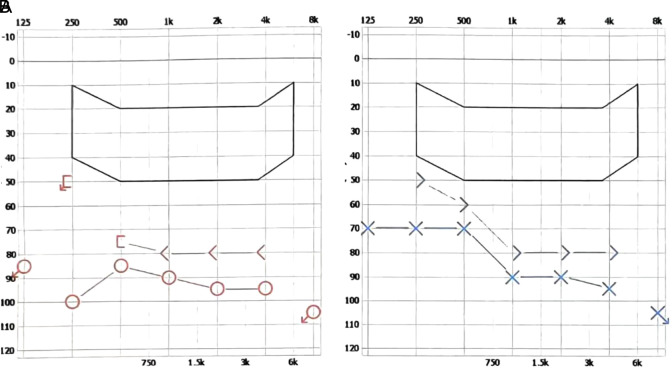
Figure 1.
Pure-tone audiometry demonstrated severe bilateral sensorineural hearing loss. (A) The mean ratio clarity index for pure-tone hearing thresholds was 90 for air conduction in the right ear and 78 for bone conduction in the right ear. (B) The pure-tone hearing threshold mean to clarity index was 83 for the left ear air conduction and 73 for the left ear bone conduction.
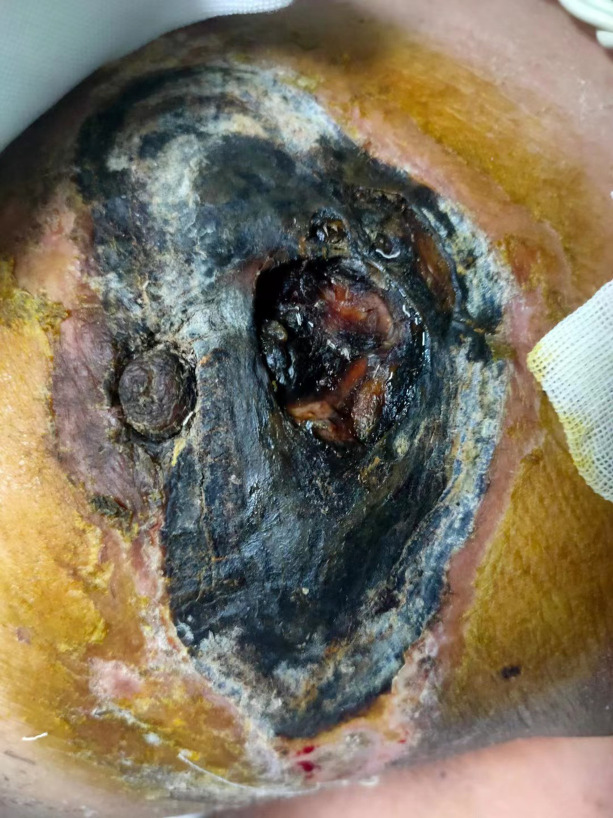
Figure 2.
Gangrene of the skin arsenicals causes tissue necrosis followed by infection by putrefactive bacteria with a black peculiar morphological change.
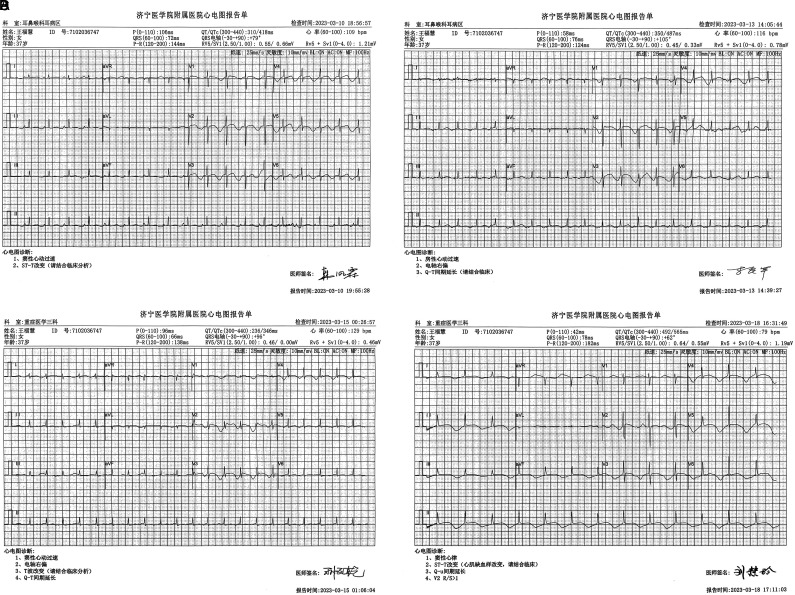
Figure 3.
Electrocardiographic changes in the patient after arsenic poisoning. (A) Electrocardiogram (ECG) findings on March 10, 2023, suggest sinus tachycardia and ST-T changes. (B) ECG findings on March 13, 2023, suggest atrial tachycardia and prolonged Q-T interval. (C) ECG findings on March 5, 2023, indicating sinus tachycardia with T wave changes and prolonged Q-T interval. (D) ECG findings on March 18, 2023, suggest ST-T changes (myocardial ischemia-like changes) and a prolonged Q-U interval.
Discussion
Arsenic is an ancient poison that can be absorbed through the respiratory tract, digestive tract, and skin mucosa. It binds to sulfhydryl groups, interfering with numerous enzyme systems involved in cellular respiration, DNA synthesis, and repair, inactivating up to 200 enzymes. Unbound arsenic is also toxic due to the production of reactive oxygen species during REDOX and metabolic activation, causing lipid peroxidation and DNA damage.1 Arsenic poisoning can damage the skin, heart, liver, kidney, lung, peripheral nervous system, and hematopoietic system, leading to various functional disorders.2,3 Acute poisoning may present with gastrointestinal symptoms, liver and kidney damage, and acute encephalopathy, while chronic poisoning typically affects peripheral nerves and skin.4 In this case, gastrointestinal symptoms and liver and kidney damage were indicative of acute poisoning, while skin gangrene, polyneuritis, and myelopoietic dysplasia suggested chronic exposure. Although the patient was diagnosed with arsenic poisoning and treated with hemoperfusion and sodium dimercaptopropane sulfonate detoxification in the later stage, she succumbed to the accumulated toxicity due to prolonged exposure and severe infection. The causes of death included toxic shock syndrome, arsenic poisoning, multiple organ dysfunction syndrome, and toxic myocardial damage.
The causes of death, in this case, were analyzed as follows: At first, arsenic poisoning was absorbed into the blood through skin wounds, resulting in atypical symptoms such as nausea, vomiting, bilateral tinnitus, hearing loss, and vertigo. Sudden deafness is more common with unilateral hearing loss; however, this case involved binaural tinnitus accompanied by hearing loss. Although initially identified as sudden deafness, the suspicion of systemic disease or poisoning symptoms could not be confirmed in a timely manner, and the patient’s severe sensorineural deafness and communication disorders further delayed the diagnosis. Secondly, chronic arsenic poisoning is prone to misdiagnosis.5 In this instance, scattered desquamating erythema and pigmentation across the body were misdiagnosed as simple eczema. Peripheral blood hematological abnormalities caused by arsenic poisoning include leukopenia, anemia, and thrombocytopenia,6-12 with some reports of chronic arsenic poisoning, misdiagnosed as pernicious anemia.13 Thirdly, in 1958, Martindale’s British Handbook of Pharmaceutical and Therapeutic Products listed indications for arsenic, including leukemia, skin diseases (psoriasis, dermatitis herpetiformis, and eczema), infantile stomatitis and gingivitis, and angina.14 Arsenic is used in traditional remedies to treat certain diseases, but it is still challenging to precisely control the dosage. In this case, the patient and their family were informed upon admission that the ototoxicity and hepatorenal toxicity might be caused by the Chinese herbal medicine applied. However, they continued to use it, unaware of its toxicity and potential harm, leading to irreversible consequences. A comparison of the patient’s examination indexes before admission and at the time of death revealed that although the liver function improved through symptomatic treatment, kidney damage progressively worsened to renal failure, and albumin and the 3 cell lines continuously decreased. Supplementation of human blood albumin and the addition of human granulocyte-stimulating hematopoietic factors could not reverse the situation. The patient’s white blood cell count was extremely low before death, and the inflammatory index was exceedingly high. As sequelae, sepsis occurred, and upgraded antibiotics could not control the severe infection, ultimately resulting in the patient’s death.
The patient’s death was related to the nature of arsenic, exposure time, concentration, and individual factors. The key to successful treatment is early identification of etiological factors, prompt intervention, and timely treatment to improve outcomes and reduce mortality. This case suggests that the possibility of heavy metal poisoning should be considered in the differential diagnosis of otolaryngological symptoms such as bilateral tinnitus and deafness, olfactory dysfunction, and respiratory symptoms like sore throat, cough, and wheezing.15 Furthermore, the dosage of medical arsenics should be carefully controlled. For patients with skin gangrene caused by arsenic compounds, dead tissue should be removed, and infection should be prevented and controlled with antibacterial drugs. For dermatitis, dimercaptopropyl ointment can be applied. Adrenocorticosteroids, vitamins B1, B6, B12, and C, among others, can be used to treat multiple neuritis. In addition to sulfhydryl detoxification for chronic arsenic poisoning, a daily injection of 10 mL of 10% sodium thiosulfate solution can promote arsenic excretion. In recent years, cases of arsenic poisoning have been relatively rare, resulting in limited experience in treating this type of disease. It is hoped that this case can provide valuable insights and lessons.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Informed Consent: Informed consent was obtained from the patient who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.S., X.Y.; Design – A.S.; Supervision – X.Y.; Resources – H.N., Materials – Y.W.; Data Collection and/or Processing – A.S., Y.W.; Analysis and/or Interpretation – A.S.; Literature Search – H.N., Y.W., X.Y.; Writing – A.S.; Critical Review – A.S., Y.W., H.N., X.Y.
Declaration of Interests: The authors have no conflict of interest to declare.
References
- 1. Cobo JM, Castiñeira M. Oxidative stress, mitochondrial respiration, and glycemic control: clues from chronic supplementation with Cr3+ or As3+ to male Wistar rats. Nutrition. 1997;13(11-12):965 970. ( 10.1016/s0899-9007(97)00338-9) [DOI] [PubMed] [Google Scholar]
- 2. Shokoohi R, Khazaei M, Karami M, et al. The relationship between chronic exposure to arsenic through drinking water and hearing function in exposed population aged 10-49 years: A cross-sectional study. Ecotoxicol Environ Saf. 2021;211:111939. ( 10.1016/j.ecoenv.2021.111939) [DOI] [PubMed] [Google Scholar]
- 3. Guo JX, Hu L, Yand PZ, Tanabe K, Miyatalre M, Chen Y. Chronic arsenic poisoning in drinking water in Inner Mongolia and its associated health effects. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007;42(12):1853 1858. ( 10.1080/10934520701566918) [DOI] [PubMed] [Google Scholar]
- 4. Agarwal A, Kumar Kn S, Jorwal P, Quadri JA, Gupta G, Biswas A. Arsenic intoxication with renal failure managed with hemodialysis alone: A case report. Drug Discov Ther. 2022;16(1):49 51. ( 10.5582/ddt.2021.01110) [DOI] [PubMed] [Google Scholar]
- 5. Dani SU, Walter GF. Chronic arsenic intoxication diagnostic score (CAsIDS). J Appl Toxicol. 2018;38(1):122 144. ( 10.1002/jat.3512) [DOI] [PubMed] [Google Scholar]
- 6. Terada H, Sasagawa T, Saito H, Shirata H, Sekiya T. Chronic arsenical poisoning and hematopoietic organs. Acta Med Biol (Niigata). 1962;9:279 292. [PubMed] [Google Scholar]
- 7. Kyle RA, Pease GL. Hematologic aspects of arsenic intoxication. N Engl J Med. 1965;273:18 23. ( 10.1056/NEJM196507012730104) [DOI] [PubMed] [Google Scholar]
- 8. Selzer PM, Ancel MA. Chronic arsenic poisoning masquerading as pernicious anemia. West J Med. 1983;139(2):219 220. [PMC free article] [PubMed] [Google Scholar]
- 9. Westhoff DD, Samaha RJ, Barnes A. Arsenic intoxication as a cause of megaloblastic anemia. Blood. 1975;45(2):241 246. [PubMed] [Google Scholar]
- 10. Lerman BB, Ali N, Green D. Megaloblastic, dyserythropoietic anemia following arsenic ingestion. Ann Clin Lab Sci. 1980;10(6):515 517. [PubMed] [Google Scholar]
- 11. van Tongeren JH, Kunst A, Majoor CL, Schillings PH. Folic-acid deficiency in chronic arsenic poisoning. Lancet. 1965;1(7389):784 786. ( 10.1016/s0140-6736(65)92956-9) [DOI] [PubMed] [Google Scholar]
- 12. Rezuke WN, Anderson C, Pastuszak WT, Conway SR, Firshein SI. Arsenic intoxication presenting as a myelodysplastic syndrome: a case report. Am J Hematol. 1991;36(4):291 293. ( 10.1002/ajh.2830360415) [DOI] [PubMed] [Google Scholar]
- 13. Heaven R, Duncan M, Vukelja SJ. Arsenic intoxication presenting with macrocytosis and peripheral neuropathy, without anemia. Acta Haematol. 1994;92(3):142 143. ( 10.1159/000204205) [DOI] [PubMed] [Google Scholar]
- 14. Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad Med J. 2003;79(933):391 396. ( 10.1136/pmj.79.933.391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sugiyama T, Ishii N, Ebihara Y, Shiomi K, Mochizuki H. Detailed analysis of neurological symptoms and sensory disturbances due to chronic arsenic exposure in Toroku, Japan. Int J Environ Res Public Health. 2021;18(20). ( 10.3390/ijerph182010749) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a