Abstract
Glacier and permafrost shrinkage and land-use intensification threaten mountain wildlife and affect nature conservation strategies. Here, we present paleometagenomic records of terrestrial and aquatic taxa from the southeastern Tibetan Plateau covering the last 18,000 years to help understand the complex alpine ecosystem dynamics. We infer that steppe-meadow became woodland at 14 ka (cal BP) controlled by cryosphere loss, further driving a herbivore change from wild yak to deer. These findings weaken the hypothesis of top-down control by large herbivores in the terrestrial ecosystem. We find a turnover in the aquatic communities at 14 ka, transitioning from glacier-related (blue-green) algae to abundant nonglacier-preferring picocyanobacteria, macrophytes, fish, and otters. There is no evidence for substantial effects of livestock herding in either ecosystem. Using network analysis, we assess the stress-gradient hypothesis and reveal that root hemiparasitic and cushion plants are keystone taxa. With ongoing cryosphere loss, the protection of their habitats is likely to be of conservation benefit on the Tibetan Plateau.
The threat of cryosphere loss to Tibetan alpine ecosystems is inferred from sedimentary ancient DNA spanning 18,000 years.
INTRODUCTION
High mountain regions harbor a unique biodiversity on which human living and diverse cultures depend (1). To what extent warming, glacier retreat, permafrost thaw, and land use shape the assembly of alpine terrestrial and aquatic communities is heavily debated (2–4). Compared with other mountain areas, upland warming rates are most pronounced on the Tibetan Plateau (5). The glacier and permafrost extent (6) and thereby the world’s largest alpine ecosystem are strongly related to temperature in this region (7, 8). Global warming threatens the unique Tibetan pastoral lifestyles (9) and biodiversity hotspots, such as the Hengduan Mountains on the southeastern Tibetan Plateau (10). Extensive cryosphere loss or even complete disappearance is predicted for the mountains of the eastern Tibetan Plateau by 2100 CE (11, 12). Likewise, the Tibetan Plateau lost a substantial part of its cryosphere during the last deglaciation (19 to 11.7 ka) (13, 14). Hence, discerning the range of ecological responses to past changes of climate, cryosphere, and land use will improve our knowledge and ability to predict future alpine ecosystem changes.
Ecological reconstructions, mainly based on pollen data, have documented a shift from alpine steppe to forest-shrub steppe/meadow on the Tibetan Plateau during the late glacial (approximately 14.7 to 11.7 ka) (15). Hitherto, climate change was assumed to be the main direct driver of this vegetation shift, while cryosphere-driven ecological change was not considered. Also, the impact of large wild herbivores as “keystone” species and/or “top-down” engineers, such as ascribed for the Eurasian glacial mammoth steppes (16–18), has not yet been regarded as a major ecological factor for the Tibetan Plateau. Phylogenetic evidence based on modern animals indicates megafaunal migrations and population expansion on the Tibetan Plateau during the late Pleistocene by wild yak (Bos mutus), the largest Tibetan herbivore (19). To know whether and to what extent herbivory shaped vegetation in the past is essential for the implementation of “natural climate solutions” to vulnerable ecosystems in the future (20). However, there is no information now available on megafaunal compositional shifts during the late glacial period on the Tibetan Plateau. It is still unknown whether changes in vegetation respond directly to changes in climate, cryosphere, or megafaunal composition.
The earliest human activities at high elevations are dated to 40 to 30 ka in the Nwya Devu archaeological site [4600 m above sea level (a.s.l.)] on the southern Tibetan Plateau (21). On the basis of archaeological records, year-round habitation at high elevations (>3500 m a.s.l.) has been widely established since 3.6 ka (22). Phylogenetic analyses infer that yak (Bos grunniens), the most important herding animal for Tibetans, was domesticated at 7.3 ka from its wild counterpart (B. mutus), and population size increased sixfold between 3.6 and 0 ka (23). Similarly, pollen indicators for livestock grazing such as Sanguisorba filiformis and Rumex-type are high in records from that time (24, 25). Accordingly, there is an ongoing debate about the extent to which prehistoric land use caused the present Tibetan alpine meadow ecosystem. The mainstream perspective emphasizes that livestock grazing caused the typical Tibetan lawn vegetation (24, 26–28). The alternative viewpoint highlights the importance of abiotic drivers of past and ongoing vegetation change (29, 30). Despite the debate, land management policies aimed at restricting or removing livestock grazing have been strictly implemented at the cost of livelihoods on the Tibetan Plateau over the past 20 years (31). Moreover, since domestic herbivores (e.g., B. grunniens) and their ancestors (e.g., B. mutus) generally share the same habitats (9), it is hard to distinguish the influence of livestock from that of wildlife grazing on vegetation composition. Accordingly, we still lack basic knowledge on the relative contribution of temperature, the cryosphere, natural herbivory, and land use on vegetation change since the last glacial period. This massively limits our ability to predict ecosystem state shifts and to provide guidance for maintaining and restoring ecological functions.
Likewise, little is known about the long-term changes of Tibetan Plateau lake communities, including macrophytes (32, 33), microbes, fish, and mammals, although the Tibetan Plateau is rich in lakes which represent unique biodiversity hotspots (34). Most studies suppose a direct response of aquatic organisms to lake water levels (attributed to climate changes) during the late glacial period (32, 35, 36). Recently, some studies argue that glaciers, via runoff, directly contribute to a lake’s microbial composition as revealed by aquatic biomarker (n-alkanes) concentrations from Lake Hala Hu (northeastern Tibetan Plateau) (37) and microbial sedimentary DNA from Lake Yamzhog Yumco (southern Tibetan Plateau) (38). However, there is little knowledge of shifts in aquatic communities, particularly how those taxa poorly represented in the (micro)fossil records responded to climate changes and related glacier retreats. Furthermore, during the late Holocene, human impacts on lake ecosystems are frequently inferred from a few taxa in individual assemblages such as zooplankton, algae, and submerged macrophytes. There is thus a knowledge deficit on the extent to which glacier dynamics and human-relevant activities contribute to ecosystem-level turnover in high-alpine environments.
Temporal species co-occurrence (coexistence) information is required to understand species assembly processes, including environmental filtering and biotic interactions (39, 40). According to the stress-gradient hypothesis—a major concept in ecology—positive interactions between species will become more common as environmental stress increases (41). For instance, facilitation is repeatedly reported to support the persistence of terrestrial alpine plants via microclimatic modifications from cushion plants (42, 43), a conclusion with high relevance when climate is warming (44). Such cushion plants, from Saussurea and Saxifraga genera, are characterized by a ground-hugging mat or dense stem structure, enabling them to trap heat and soil while also providing habitable conditions for other species within their crown area. However, aside from vegetation, the stress-gradient hypothesis has been little investigated at the ecosystem level. Even more, studies supporting this hypothesis almost exclusively originate from space-for-time assessments despite species assembly being a dynamically complex process. Further, other studies from the Tibetan Plateau have shown that major ecosystem attributes such as plant richness are not analogous in space and time (45).
Lake sediments are the most suitable archives for tracking temporal species-environment relationships (46). Compared to the traditional proxies (e.g., pollen and diatoms), sedimentary ancient DNA (sedaDNA) extracted from lake sediments has become a powerful tool for retrieving more detailed assemblages of past plants, animals, and microbes within lakes and their catchments (47). SedaDNA metagenomics (shotgun sequencing) has been increasingly used for ecosystem-level investigations and shows reliable taxonomic classification down to the genus level due to advancements in bioinformatic analysis and reference databases (48, 49). To date, times-series studies of lake sedaDNA studies investigating taxa composition and co-occurrence at the ecosystem level are not available for the Tibetan Plateau.
We reconstruct terrestrial and aquatic communities by shotgun sequencing on 40 sedaDNA samples covering 17.7 to 0 ka extracted from Lake Naleng, located in the Hengduan Mountains, southeastern Tibetan Plateau (Fig. 1A). Its surrounding landscape has been influenced by Quaternary glaciation and/or permafrost (Fig. 1, A and B, and fig. S1). The sedaDNA results reveal diverse mammals with a dominance of bovid species (e.g., wild yak) in the pre-14 ka period when steppe-meadow was well established. The Yak steppe-meadow ecosystem collapsed at 14 ka and shifted to woodlands supporting cervid species (e.g., red deer). No evidence supports large wild herbivores’ top-down control of vegetation shifts. Rather, warming-induced cryosphere loss directly triggered the vegetation changes that further forced the mammalian composition turnover. Likewise, we find that glacier mass loss at 14 ka strongly shaped the aquatic ecosystem from glacial microbes to a variety of interglacial organisms such as picocyanobacteria, submerged plants, fish, and otters. Overall, we infer only a limited impact of land use. After 3.6 ka, land use may have reduced mammals’ occurrences and contributed to the establishment of a picocyanobacterial turbid lake system. Through network analysis, partial correlations, controlling the influence of cryosphere changes, land use, and mediator taxa, act as surrogate representations of the direct associations among taxa. We find a high number of positive links during the glacial period (pre-14 ka, a high-stress phase) in both ecosystems, directly supporting the stress gradient hypothesis over a long time span.
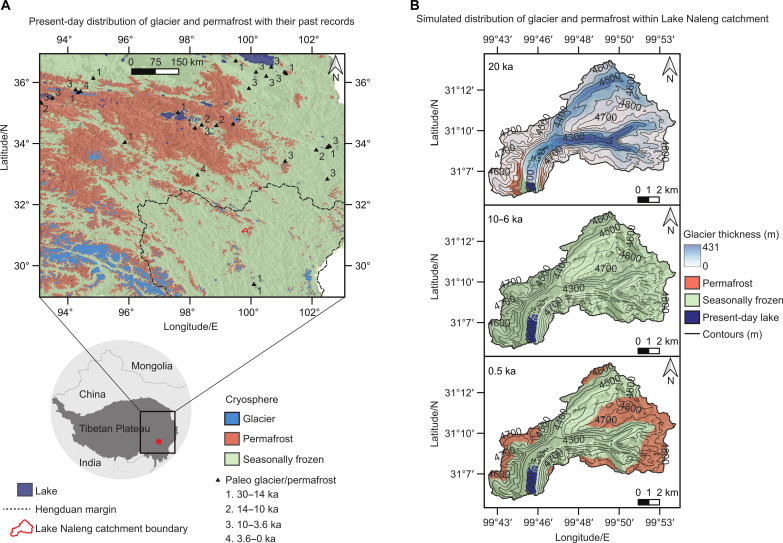
Fig. 1. Lake Naleng, located on the southeast Tibetan Plateau (Hengduan Mountains), a global biodiversity hotspot, is influenced by the past and modern cryosphere.
(A) Modern glacier and permafrost distributions (6) with their past records (14) indicate a southeastward advance of the cryosphere until 14 ka and a northeastward retreat afterward. (B) The simulated glaciers and permafrost distributions (Materials and Methods and fig. S1) indicate that Lake Naleng with its catchment was strongly influenced by the cryosphere during the late glacial period while without impact during the early-to-mid Holocene. Permafrost, but not glaciers, recovered during the late Holocene, mostly in the highlands in the east of the lake catchment.
RESULTS AND DISCUSSION
Ecosystem-level sedaDNA record of the past terrestrial and aquatic biosphere
Sequencing yielded 2,512,713,391 reads for bioinformatic analyses, of which 123,786,174 reads came from 40 samples and 12 controls which underwent taxonomic data cleaning and filtering (Materials and Methods). Consequently, 1,067,557 reads of 317 terrestrial and aquatic taxa, composed of seed-bearing plants, mammals, macrophytes, fish, algae, and Cyanobacteria (also called blue-green algae), with the best identity of ≥95% against the National Center for Biotechnology Information (NCBI) Reference Sequence Database, were used for ecosystem reconstruction. The ancient origin of the reads was confirmed by read length distribution (fig. S2) and characteristic C-to-T substitution at the 5′ and 3′ end with sufficient reads (Materials and Methods and figs. S3 and S4). The comparable DNA preservation and provenance conditions across sediments reinforce the accurate inference of biological information from our shotgun record (Supplementary Text and figs. S5 and S6).
The shotgun sequencing approach recovered 167 genera from 81 families of seed-bearing plants (data S1). Among these, the dominant ones are species rich on the Tibetan Plateau and are also abundant and detected by metabarcoding and pollen analyses (25, 45, 50) from the same sedimentary core (table S1). The mammals, which have less biomass than plants, were traced by the shotgun sequencing approach in all samples (data S1). Most mammalian reads were identified to even-toed ungulate mammals such as Bovidae and Cervus, followed by Ochotona, a rodent-like mammal dwelling on mountains. For aquatic communities (data S2), the reads assigned to genera of aquatic macrophytes are abundant in Potamogeton (Potamogetonaceae) and Myriophyllum (Haloragaceae). Salmonidae and Cyprinidae dominated the fish assemblage, with Cyprinidae being more abundant in the high-elevation regions of the current Tibetan Plateau (51). Few reads were classified to Lutra, which is now one of the endangered top predators in aquatic ecosystems (52). Most algal reads were assigned to Monodopsidaceae, specifically its genus Nannochloropsis, which contains species that can adapt to harsh environments such as the Last Glacial Maximum (53). As cyanobacterial blooms relate to lake ecosystem health (54), we focus on bacterial reads assigned to genera and families of Cyanobacteriota, among which Leptolyngbya, Pseudanabaena, and Synechococcus are the dominant genera.
Our results indicate that a single shotgun dataset can depict the biotic community at an ecosystem level. Our results further indicate that terrestrial and aquatic changes can concurrently be traced using the same approach. Even more, our time-series data suggest that a more continuous proxy signal can be retrieved from lake sediments compared with, for example, permafrost sediments (55). This is despite the fact that the majority of cellular organisms’ reads (98.6%) did not match any Eukaryota, which is consistent with previous environmental shotgun studies (56–58): Even a small proportion of the reads (1.4%) can provide a substantial amount of taxonomic information. The value of our dataset may increase in the future when more comprehensive databases are used for taxonomic assignment.
Terrestrial ecosystem shifts from late glacial Tibetan Yak steppe-meadow to Holocene deer woodland
Overall, our results indicate an abrupt collapse of alpine steppe-meadow at 14 ka, followed by advance of subalpine shrubland during 14 to 3.6 ka and alpine steppe-meadow reexpansion since 3.6 ka (Fig. 2, A and B).
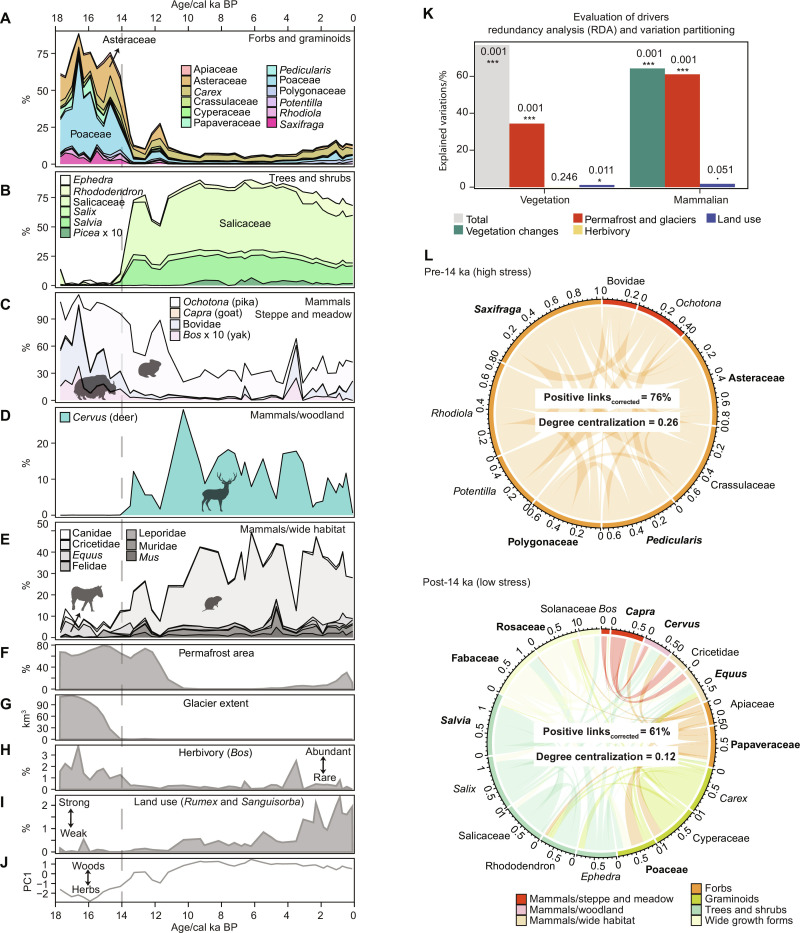
Fig. 2. Long-term trends in shotgun-based (metagenomics) main terrestrial taxa recorded in Lake Naleng compared with temporal changes of environmental factors.
(A and B) The shotgun-based relative abundance of the common terrestrial vegetation community indicates a transition from steppe-meadow pre-14 ka to woodland post-14 ka. (C to E) The shotgun-based relative abundance of the common terrestrial mammalian community shows a loss of wild megafauna since 14 ka. (F) The percentages of simulated glacier-free permafrost extent within the lake catchment (Materials and Methods). (G) The modeled glacier extent within the lake catchment (45). (H) The shotgun-based relative abundance of Bos relative to mammalian community as a signal of herbivory. (I) The percentages of Rumex and Sanguisorba relative to the pollen grains of terrestrial seed-bearing plants recorded in Lake Naleng as an indicator of intensity of land use (25, 50). (J) The first axis scores of a principal component analysis (PCA) of the terrestrial vegetation changes show a transition from steppe-meadow with dominant herbs and graminoids in the pre-14 ka period to woodland dominated by willow shrubs and trees. (K) Evaluation of drivers based on redundancy analysis (RDA) and variation partitioning. Explained variations are represented by percentages of adjusted r2 values, obtained from RDA for joint explanation and variation partitioning for unique explanation. (L) Weighted network analyses based on positive partial correlations. Taxa with Kleinberg’s hub centrality score ≥ 0.8 (normalized into an arbitrary range of 0 to 1 by taking maximum score into account) are considered keystone taxa and marked in bold. The percentage of positive links has been adjusted to the number of taxa. The chord thickness represents the positive partial correlation. The proportions of Picea (B) and Bos (C) are aggregated 10 times for better visibility. Significance codes of F test with 999 permutations are ***0.001, *0.05, and ·0.1.
The pre-14 ka vegetation is characterized by alpine forbs and graminoids including taxa dominant today in cold-dry places (e.g., Saxifraga, Asteraceae, and Poaceae), on moist stream banks, and in meadows (e.g., Carex, Pedicularis, and Ranunculaceae) of the Tibetan highlands. During 14 to 3.6 ka, the abundance of woody taxa increased, with the main components being the Salicaceae taxa (e.g., Salix) and Rhododendron, as well as a portion of Picea during 10 to 3.6 ka (Fig. 2B). Although Salix is usually overrepresented in sed(a)DNA spectra, its high values together with Rhododendron well reflect the subalpine shrub communities of the eastern Tibetan Plateau (59). Since 3.6 ka, the abundance of some forbs and graminoids (e.g., Asteraceae, Polygonaceae, and Poaceae) increased (Fig. 2A) although woody taxa (e.g., Salicaceae and Rhododendron) were still abundant. Accordingly, the lake catchment experienced a cold-dry climate during the glacial period, followed by a moderate-to-warm and moist climate between 14 and 3.6 ka, and then returned to cold conditions afterward. The main vegetation characteristics and climate conditions agree with studies of metabarcoding and pollen analyses from the same sedimentary core (fig. S7A) as well as regional pollen records (fig. S7B).
Our shotgun data indicate that the main terrestrial mammals (Fig. 2, C to E) are medium- to large-sized ungulate herbivores (>45 kg) and small lagomorphs (<0.3 kg). In general, mammalian composition shifted from the steppe-meadow–adapted bovid community in the pre-14 ka period (Fig. 2, A and C) to a mesic-adapted woodland cervid community post-14 ka (Fig. 2, B and D). However, a compositional shift back did not co-occur with steppe-meadow reestablishment during the late Holocene (Fig. 2, A and C). In the pre-14 ka period, herbivores (Bos and other bovids) that now inhabit alpine ecosystems in the northern and western plateau were abundant. Ochotona (pika), which shares similar habitats, occurred as well (Fig. 2C). In the post-14 ka period, Cervus occurred (Fig. 2D); while all Bovidae species had very low proportions except for evidently increased numbers at 3.5 ka only (Fig. 2C). Meanwhile, our data also record a higher amount of Cricetidae sedaDNA after 14 ka (Fig. 2E), which frequently occurs in the present-day moist meadows on the Tibetan Plateau.
We investigated the key drivers of compositional changes for plants and mammals at the terrestrial community level using constrained ordination analyses (Materials and Methods). Assessed drivers include cryosphere (Fig. 2, F and G; permafrost and glacier distribution simulated using temperature change and validated by sedaDNA-based permafrost and glacier microbiota changes, see Materials and Methods), herbivory (Fig. 2H; Bos%, excluded for mammalian constrained ordination), land use (Fig. 2I, Rumex and Sanguisorba%) (24, 25), and vegetation [Fig. 2J; PC1 of principal component analysis (PCA) site score, excluded for vegetation constrained ordination]. All variables together explain a high amount of variation in the vegetation composition (Fig. 2K; 73%, P = 0.001). Variation partitioning (Fig. 2K) indicates that the cryosphere alone explains most of the vegetation variation (34.4%, P = 0.001) compared with the variance exclusively explained by herbivory (0.1%, P = 0.246) and land use (1.2%, P = 0.011). Such results give no support for the hypothesis of a top-down regulated plant community by large herbivores (16, 17). Furthermore, we find that the compositional change of mammals is strongly explained by vegetation changes (64.2%, P = 0.001) and cryosphere (unique 61.1%, P = 0.001) but poorly by land use (unique 1.8%, P = 0.051, Fig. 2K). Accordingly, the millennial-scale findings undermine the long-term argument of a human-driven ecosystem change on the Tibetan Plateau at a millennial timescale (28, 60).
An increasing number of studies have reported that co-occurrence networks can offer more information than composition on community organization, and a few studies have focused on the ecosystem level (61). We investigated species’ co-occurrence patterns at the ecosystem level (Materials and Methods) to understand direct interactions (e.g., facilitation and competition) among coexisting taxa in two different regimes, pre-14 ka (high stress) and post-14 ka (low stress). We specifically focus on positive partial correlation as an indicator of facilitation among taxa. Our aim is to assess the applicability of the stress gradient hypothesis on a millennial scale. This assessment serves as an analogy for understanding shifts in facilitation within the context of ongoing cryosphere loss and land-use changes in mountain regions.
The whole terrestrial community is clustered into two modules (Fig. 2L) by taking cryosphere changes and land use as predictors (fig. S8A), separating common taxa of the pre-14 ka community (e.g., Asteraceae, Bovidae, and Ochotona) from those dominating the post-14 ka community (e.g., Salix, Cervus, and Cricetidae). The percentage of positive links within the terrestrial community is higher in the pre-14 ka period compared with the post-14 ka interval (76 and 61%, respectively; Fig. 2L). This strongly supports the stress-gradient hypothesis (61) which proposes that positive interactions are promoted under stressful conditions such as the cold glacial period in our study. Furthermore, we used Kleinberg’s hub centrality score, which ranges from 0 to 1, to identify the keystone taxa, as it measures the extent of a taxon’s connections to other important taxa in the overall network (62). A taxon with a high hub centrality score is more likely to be a keystone taxon that is particularly important to the functioning of ecosystems (63, 64). We find that Bovidae and Bos (wild yak) show lower Kleinberg’s hub centrality scores (0.76 and 0.632) than their main food resources in their respective modules (Asteraceae: 1; Poaceae: 0.909; table S2), thus refuting the hypothesis of large herbivores as keystone taxa in the Tibetan terrestrial ecosystem.
A major finding is that the cryosphere governed the terrestrial Tibetan ecosystem characteristics until 14 ka by promoting a forb-dominated steppe-meadow (Fig. 2K) that spread in the glacier-free catchment areas on permafrost soils (Fig. 1B and fig. S1). Our results furthermore suggest that such steppe-meadow composition was “bottom-up” controlled, with plants such as Pedicularis, Saxifraga, and Asteraceae species as keystone taxa, as indicated by their high Kleinberg’s hub centrality scores (Fig. 2L and table S2). Pedicularis, a root-hemiparasitic genus, can enhance grassland diversity by reducing the competitive advantage of grasses and legumes (which are preferred hosts) compared to forbs and sedges on the Tibetan Plateau (65). Asteraceae contains a variety of species from genera (e.g., Saussurea and Senecio) that form cushions, which can protect diverse terrestrial alpine plants against the harsh environment on an ecosystem level by creating a favorable microclimate through their dense, low-lying cushion structure, as well as stabilizing soil, retaining water, and contributing to nutrient cycling (43, 66). Similar to Saxifraga, the cushion life form contributes to the diversification of other species-rich alpine genera in the Tibetan Plateau (67). These findings indicate that facilitation rather than competition shaped the glacial terrestrial Tibetan ecosystem. It represents an assessment of the stress-gradient hypothesis over millennial timescales.
A further key finding suggested by our shotgun data is that abundant large herbivores such as wild yaks co-occurred with grass- and forb-dominated steppe-meadow (Fig. 2, A and C) extending across permafrost soils (Fig. 2F). This suggests that they frequently consumed protein-rich forbs rather than exclusively or heavily depending on grasses (68). The Lake Naleng’s non-pollen palynomorphs record supports this inference, indicating high percentages of Glomus before 14.5 ka (69). Given their association with erosion events and the fact that their hosts, such as Asteraceae, are frequently consumed by yaks during winter, an increased input of Glomus spores might originate from the dung of bovids after foraging on these plants (70, 71). Such consumers’ preferences are similar to the Eurasian “mammoth steppe” supporting a variety of mammals during the late Pleistocene at high latitudes (58, 72). Furthermore, vegetation shifts drive changes in mammalian communities in our study area (explained 64.2%; Fig. 2K), which may emphasize that resource stress (e.g., less available foraging habitats) could also pressure herbivores to facilitation rather than competition (positive partial correlations among mammals in the post-14 ka; Fig. 2L) which is typically less considered (73).
Overall, we suggest that temperature-driven cryosphere changes (Fig. 2, F and G) controlled vegetation turnover on the Tibetan Plateau at 14 ka, which, in turn, promoted a decline of large herbivory. This contrasts with the proposition of megafauna as keystone engineers of the mammoth steppe ecosystem of Glacial Siberia (74). Our permafrost simulation using a generalized linear model (Materials and Methods) shows that permafrost thawed at 14 ka in the U-shaped valley around Lake Naleng and along streams in response to the warming climate (Fig. 1B and fig. S1), and this is confirmed by time-series data for permafrost bacteria (fig. S9, A to C). This likely promoted the development of alluvial soils, thereby facilitating riparian woody vegetation (Salix) (75) as indicated in our sedaDNA record. This vegetation change may have limited the protein intake of large herbivores (e.g., wild yaks) (76), presumably forcing them to migrate to the restricted permafrost upslope or farther away to the northeastern Tibetan Plateau, where forbs still dominate today (15). Similarly, a decline of megafaunal grazers is reported to be coeval with dwarf willow expanding northward into the mammoth steppe of northeast Siberia and North Alaska around 14.5 to 13.5 ka (77).
Another major result of our study suggests that cryosphere changes and not human impact (Fig. 2K) were the main driver of terrestrial ecosystem change on the Tibetan Plateau at a millennial timescale. Even the partial reestablishment of steppe-meadow during the late Holocene (Fig. 2A), which has often been related to human impact (e.g., livestock grazing) (28) could be best explained by cooling-related permafrost reestablishment in the upper Lake Naleng catchment area. Unexpectedly, a steady reoccupation by Bovidae species such as wild yak did not co-occur with the reestablishment of steppe-meadow habitats (Fig. 2, A and C), nor did our record indicate any substantial herding activity post-3.6 ka. This contrasts with the co-abundance of bovid taxa and grassy alpine taxa pre-14 ka. Although crop agriculture was introduced into the southeastern Tibetan Plateau and spread up to 3600 m a.s.l. from 3.6 ka (22), cold and dry weather in this area probably forced the farmers to supplement their diets by hunting wild animals (78), and this may have contributed to a herbivore decline in contrast to the inferred high population levels associated with the pre-14 ka analog vegetation composition.
Aquatic ecosystem shifts from a glacially affected microbial system to a warm macrophyte fish-otter system
The aquatic ecosystem is characterized by a microbial community dominated by green algae and cyanobacteria during the cold, glaciated period before 14 ka (Fig. 3A), by the co-occurrences of picocyanobacteria and submerged macrophytes, fish, and otters during the warm and glacier-free early and mid-Holocene (Fig. 3, B to D), and by the dominance of nonglacially adapted picocyanobacteria after 3.6 ka (Fig. 3B). Evaluated drivers include glacier mass (Fig. 3E) and land use [Fig. 3F; Rumex and Sanguisorba%; (24, 25)], which explain 61% (P = 0.001) of the variation of the aquatic communities at the ecosystem level. Variation partitioning indicates that compositional turnover is strongly related to glacier mass changes (47.5%, P = 0.001; Fig. 3E), followed by land use (6.6%, P = 0.001; Fig. 3F). These findings suggest that glacier dynamics may be the main driver of the lake’s community composition change on the Tibetan Plateau (37, 38).
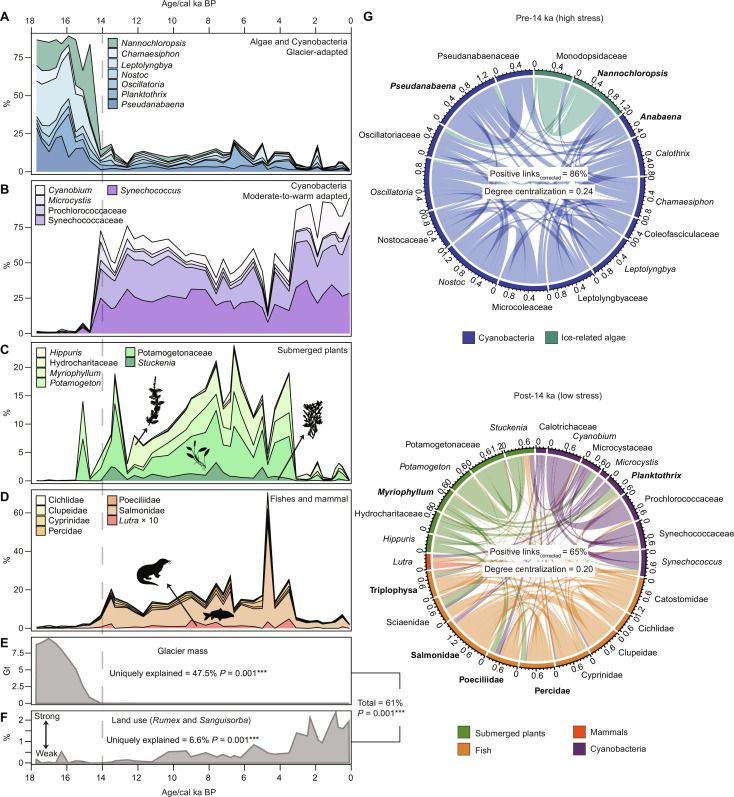
Fig. 3. Long-term changes in shotgun-based (metagenomics) aquatic communities recorded in Lake Naleng compared with temporal changes of environmental factors.
(A to D) Changes in the relative abundance of the common taxa indicate a shift from abundant glacier-adapted microbes (algae and cyanobacteria) in the pre-14 ka period to nonglacier-adapted picocyanobacteria, submerged plants, fish, and fish-eating otters until 3.6 ka, followed by overabundance of picocyanobacteria in the post-3.6 ka interval. (E and F) Evaluation of environmental factors suggests a high contribution of glacier mass on aquatic community composition changes. Explained variations are represented by adjusted r2 values, obtained from RDA for joint explanation and variation partitioning for unique explanation. (G) Weighted network analyses based on positive partial correlations classify two modules, characterized by common taxa in the pre-14 ka and post-14 ka, showing a high percentage of positive links in in the glacial period (pre-14 ka) while lower in the interglacial period (14 to 0 ka). Such structures support the stress-gradient hypothesis that positive interactions among organisms become prevalent under stressful environments. Further, more taxa connected via mediators developed a centralized system in the glacial phase. Taxa with Kleinberg’s hub centrality score ≥ 0.8 (normalized into an arbitrary range of 0 to 1 by taking the maximum score into account) are considered keystone taxa and marked in bold. The percentage of positive links has been adjusted to the number of taxa. The chord thickness represents the positive partial correlation. Significance code of F test with 999 permutations is ***0.001. Gt, gigatonne.
Network analyses taking glacier mass changes and land use as predictors (fig. S8B) group aquatic taxa into two modules (Fig. 3G), typified by a unique community in the pre-14 ka period (e.g., Nannochloropsis, Pseudanabaena, and Anabaena) and post-14 ka (e.g., Myriophyllum, Triplophysa, Salmonidae, Poeciliidae, Percidae, and Planktothrix). The aquatic ecosystem exhibited a higher relative number of positive links in the pre-14 ka period (Fig. 3G), supporting the stress gradient hypothesis. Apart from glacier loss, land use drives partial correlations among taxa (fig. S8B), suggesting that Lake Naleng’s productivity and nutrient status play a role in the co-occurrence pattern of the whole lake ecosystem.
Lake community inferred from shotgun data was dominated by microbial taxa, including Nannochloropsis (green algae), Leptolyngbya, and Pseudanabaena until 14 ka (Fig. 3A), when the lake catchment became glacier-free (Fig. 3E). These taxa or their congeners are known from cryoconite holes on a glacier’s surface in the Arctic and Asian mountains (79) and have been retrieved from Arctic lake sediments from the glacial period (53). Our results confirm that a glacier’s microbial communities are able to colonize downstream and that glacier ecosystem changes can be traced by lake sediments (80–82). Further, the high relative abundance of Oscillatoria, the genus hosting rich toxin-producing strains (54, 83), in the pre-14 ka period (Fig. 3A) possibly forced microbes into engaging in positive associations (84, 85). Yet, whether such biotic-relevant stressors can trigger lake communities to interact positively and override the effects of abiotic stress (e.g., glacier) on millennial scales will require further evaluation.
Obviously, glacier disappearance (Fig. 3E) induced a transition from glacier-preferring (blue-green) algae in the pre-14 ka period to picocyanobacteria communities during post-14 ka, including Synechococcus and Cyanobium taxa (Fig. 3B). Their relative abundance declined when other submerged plants such as Potamogeton, Stuckenia, and Myriophyllum (Fig. 3C) notably increased in response to warmer water conditions (86), which, in turn, favored the invasion from lower elevations and successful reproduction of fish (87, 88) including Salmonidae and Cyprinidae (Fig. 3D). These fish promoted the establishment of piscivores including otters (89). In addition, the submerged macrophytes can efficiently maintain water quality by releasing anticyanobacterial fatty acids (90). The key roles of submerged plants and fish are further inferred from co-occurrence patterns (Fig. 3G and table S3).
Two lake states likely coexist in one aquatic ecosystem post-14 ka, either spatially (Supplementary Text) or temporally separated, i.e., a macrophyte-dominated clear-water lake system at the lake shore and a blue-green algae-dominated turbid lake system. Hence, a complex subalpine lake ecosystem was established during the warm phase (post-14 ka) characterized by a low degree of centralization (Fig. 3G). Probably initiated by a decline in temperature-lowering submerged macrophytes, the lake ecosystem completely collapsed, leaving picocyanobacteria (Synechococcus and Cyanobium taxa; Fig. 2B) as the main component, which, in turn, posed a further threat to submerged plants by reducing light and oxygen (91). To what extent this change was enhanced by human impact is uncertain, but it is rather unlikely as we find no clear traces of herding (e.g., livestock DNA) in the mammalian community (Fig. 2, C to E). This finding highlights the importance of taking a long-term perspective when assessing the effects of natural and human-induced factors on lake state transitions, particularly before predicting the presence of critical transitions (92) and implementing sufficient lake restoration strategies to mitigate cyanobacteria blooms (54).
Conclusions and lessons learned for risks in a warmer future and restoration if temperatures cool again
We find that climate and cryosphere-induced vegetation changes affect mammal abundance and composition not vice versa; hence, there is no support for herbivory as a major ecosystem driver. Accordingly, managing large herbivores may not represent a conservation option; instead, only lowering the temperature by reducing global carbon emissions and preserving the cryosphere will help the conservation or restoration of the Tibetan alpine ecosystem.
We infer that Pedicularis and cushion plants (Asteraceae and Saxifraga), in particular, are keystone taxa for the Yak steppe-meadow ecosystem and should accordingly be the focus of protection measures, as they support high biodiversity at the ecosystem level by common facilitations. The way to protect them is to conserve their habitats, with priority given to areas not invaded by shrubs.
We deduce that greater grazing during the late glacial compared with the late Holocene did not destabilize the terrestrial ecosystem. By analogy, relaxed pasture management (e.g., moderate livestock reduction policy) does not represent a risk to the present Tibetan Plateau alpine ecosystem but can be recommended to sustain contemporary livelihoods in the highlands of the Tibetan Plateau (93, 94). To stay abreast of ongoing warming and human activities, adaptive management requires more studies (e.g., along a grazing intensity gradient) to achieve a full understanding of human-driven turnover in Tibetan alpine meadow ecosystems (95).
Pronounced shifts in the dominance of aquatic taxa, particularly microbial communities, occurred synchronously with the substantial decay of glaciers at 14 ka, while the glacial lake ecosystem was not restored in the cold late Holocene (3.6 to 0 ka) presumably because glaciers did not reestablish. Our findings call for more effort to inspect the glacier-lake connectivities of microbial communities as a base for developing and implementing appropriate conservation strategies. However, it is important to note that the specific aspects of glacial microbes that should be protected may vary depending on the ecosystem and the goals of conservation efforts. As our study does not investigate this aspect, we cannot offer specific recommendations for conservation actions related to glacial microbes in Tibetan glacial lakes.
Warmth-related aquatic ecosystems composed of submerged macrophytes and blue-green algae support fish and otters (main predators of fish). Warmth increased the complexity of the lake ecosystem with the coexistence of two states and less common positive associations. The lake ecosystem shifted to a turbid water state with few eukaryotes primarily due to cold-related macrophyte loss. It is unlikely that the external loading of nutrients (e.g., from husbandry) caused such a state shift as no similar signal has been observed from the terrestrial mammalian community.
We find that terrestrial and aquatic species co-occurrence patterns respond to the loss of the Tibetan cryosphere. A structure with fewer facilitative interactions suggested by positive partial correlations is detected for a permafrost-free terrestrial ecosystem and a glacier-free aquatic ecosystem. Our findings have broader implications beyond our study site, as we investigated a typical alpine lake with a catchment that includes elevational ranges typical of the southeastern Tibetan alpine ecosystem.
MATERIALS AND METHODS
Modern site setting
Lake Naleng (31.10°N, 99.75°E; 4200 m a.s.l.) is situated in a glacier-formed basin on the southeast Tibetan Plateau, which is a biodiversity hotspot (also referred to as the Hengduan Mountains). This lake is classified as a mesotrophic lake based on its pH value (8.11), Secchi depth (2.9 m), and dissolved oxygen content (6.86 mg/liter) (measured at noon on September 2009) (69). The catchment area is about 120 km2 and characterized by steep slopes and a narrow floor (U-shaped glacial trough) with Miocene granite and granodiorite rocks (96). More specifically, it has simple bedrock geology, primarily composed of aluminosilicate minerals (e.g., mica, quartz, and feldspars), covered by glacial deposits that were physically eroded from these rocks (96). The study area is mainly influenced by the South Asian summer monsoon and the East Asian winter monsoon. Warm and humid air masses occur in summer, while cold and dry air masses dominate in winter.
Modern vegetation types on the upper Tibetan Plateau mainly reflect the distribution of cryosphere characteristics (permafrost, snow fields, and glaciers) (97). Vegetation in adjacent mountains along an elevational gradient is characterized by farmland (legume, rice, and wheat) below ~3000 m a.s.l., Tsuga forest intermixed with other conifers (e.g., Abies ernestii, Picea wilsonii, and Betula albo-sinensis) up to 3200 m a.s.l., and coniferous forest (e.g., Abies squamata and Picea likiangensis) up to 4400 m a.s.l. with a secondary canopy composed mainly of Betula and Rhododendron (25).
Within the lake catchment, two patches of coniferous forest are found on the north-facing slope near the lake. The shrubby communities, such as Rhododendron telmateium and Salix vaccinoides, occupy wet slopes (north-facing), while Juniperus and Quercus occur on dry slopes (south-facing) (25). Above the shrubby zone, the dominant vegetation is alpine meadow, composed of Carex (syn. Kobresia) (98) and Polygonum species (50). The cushion and rosette plants (e.g., Saussurea and Brassicaceae) are mainly found in the subnival belt around 4900 to 5200 m a.s.l (50) on adjoining mountains. Human influence is livestock grazing (yaks and Tibetan sheep) in alpine meadows during the summer in the lake catchment (25).
Late Pleistocene/Holocene site setting
The regional and local climate was generally cold and dry during the late glacial period (15), with moraines by the lake’s outlet dating to 21.5 to 17.5 ka (99), suggesting that glaciers shaped the lake basin during and even earlier than this period. During the Holocene, a warm and humid climate has been widely recorded for the southeastern Tibetan Plateau (100) as well as our lake catchment (25, 96). These environmental settings are well captured by our cryospheric simulation (Supplementary Text) and sedaDNA metagenomics (fig. S9, A to C). Human impacts are assumed to have intensified at high elevation when the agropastoral economy expanded up to 3500 m a.s.l. around 3.6 ka (22). On the basis of the C/N ratios (varying between 2 and 16) and δ13Corg values (varying between −31 and −25‰) of the Naleng core (96), it is suggested that the lake sediments have received very little input of terrestrial plant materials through time (101).
Core, chronology, and sedaDNA material
The core collection, dating, and age-depth model are described in a previous study (69, 96). Because of a lack of macrofossils, 16 samples of bulk organic carbon were dated by accelerator mass spectrometer (AMS) 14C at the Leibniz Institute Kiel.
The 1-cm-thick sediment samples were stored at 4°C until subsampling for sedaDNA isolation that was performed using a PowerMax Soil DNA Isolation kit (Mo Bio Laboratories Inc., USA) with a modified protocol. A full description of the procedures is provided in (45).
Library preparation and shotgun sequencing
A total of 40 sedaDNA samples spanning 17.7 to 0 ka was used for library preparation. Each library batch contains sedimentary DNA isolates (15 ng), at least one DNA extraction blank, and one library blank. The libraries were prepared following the established protocol of single-stranded DNA library preparation (102) with incubation of second ligation (CL53/CL72) on a thermomixer. Then, all libraries with 50 μl each were frozen at −20°C. These libraries were subjected to qualification, indexing purification, and initial quality controls (103). Four sample libraries underwent agarose gel purification to minimize the impact of adapters on targeted DNA fragments (Supplementary Text).
Initially, libraries of 38 sediment samples and nine blanks were equimolarly pooled into four final pools of 10 nM each, with a molarity ratio of 10:1 between samples and blanks. They were sequenced on a HiSeq 2500 platform (2 × 125 bp with High-Output V4 mode) and an Illumina NovaSeq 6000 (two pools on 2 × 125 bp and one pool on 2 × 150 bp), respectively. Afterward, we decided to increase the sequencing depth for 16 sedimentary libraries and sequence two additional sediments to obtain better temporal resolution during the mid-Holocene. The 18 sediment samples with four blanks were equimolarly pooled into two pools of 10 nM each (with a molarity ratio as above) and sequenced on an Illumina NovaSeq 6000 (2 × 150 bp). The sequencing was performed by Fasteris SA (Switzerland) (data S3). Last, libraries of 40 lake sediments and 12 controls yielded 279.7 GB.
Bioinformatic analyses
We used FastQC (v 0.7.11) (104) to assess the quality of the raw reads (2,512,713,391) and then applied clumpify (BBMAP, https://github.com/BioInfoTools/BBMap/blob/master/sh/clumpify.sh, v. 0.20.1) with default settings, except for the “dedupe = t” setting, to remove duplicate raw reads. Subsequently, we used Fastp (v. 0.11.9; https://github.com/OpenGene/fastp) (105) for adapter trimming and merging of paired-end reads in parallel. The deduplicated reads were trimmed for adapter filter (-a auto) when R1/R2 lacked overlaps, poly-G ends (--ploy_g_min_len 10), poly-X ends (--ploy_x_min_len 10), quality filter (--qualified_quality_phred 15, --unqualified_percent_limit 40, and --n_base_limit 5), length filter (--length_required 30), and lower complexity filter (--low_complexity_filter 30). Overlapped reads were merged (-m --merged_out) based on overlapping detection with a minimal overlapped length of 30 bp (overlap_len_require 30), a maximum mismatch limit of 5 (overlap_diff_limit 5), and a maximum percentage of mismatch of 20 (overlap_diff_percent_limit 20). Then, the outputs were evaluated by FastQC (v 0.7.11) (104) for quality check.
The merged reads (1,724,066,613) were used for taxonomic assignment through end-to-end alignment in Bowtie2 (version 2.5.1) (106) and hereafter using ngsLCA (v. 1.0.5) (49). We established a taxonomic reference database incorporating all available RefSeq genomes from the NCBI (downloaded on 14 August 2023). Following the HOLI pipeline (48), this database was constructed using Bowtie2 (version 2.5.1) (106) with a default setting. The merged reads were aligned against the RefSeq reference genome database to identify a maximum of 1000 valid and unique alignments (-k 1000). The resulting possible alignments were sorted and hereafter classified using ngsLCA (v. 1.0.5) (49) with a minimal identity of 95%.
SedaDNA authentication and effect of a DNA damage
Short DNA fragmentation and cytosine deamination, indicated by high frequency of C > T substitutions in the 5′ and 3′ ends (figs. S2 and S3), characterize our metagenomic data originating from ancient sources (107). MapDamage2 (v. 2.2.1) was applied on taxonomically classified reads with settings of –rescale and --single-stranded (108). Twenty-six common taxa were authenticated with sufficient reads (fig. S3) as representations of terrestrial mammalian community (Bos, Cervus, Equus, Ochotona, and Cricetidae), terrestrial vegetation (Poaceae, Asteraceae, Carex, Rhodiola, Saxifraga, Potentilla, Pedicularis, Salix, and Salvia), aquatic vegetation (Myriophyllum and Potamogeton), fish (Cyprinidae and Salmonidae), and aquatic microbes (Nannochloropsis, Chamaesiphon, Leptolyngbya, Nostoc, Oscillatoria, Pseudanabaena, Planktothrix, and Cyanobium). We further observed an increase in the frequency of C > T deamination with age (fig. S4), indicating an effectively closed lake system that prevents DNA from being influenced by redeposition and environmental variables.
Taxonomic data cleaning and filtering
We obtained 123,786,174 reads assigned to cellular organisms, of which 107,648,162 reads classified to taxa at family or genus level (fig. S10A). These classified taxa are generally from Bacteria, followed by Eukaryotes and Archaea (fig. S10B). We detected a few proportions of reads in the blanks (in the range of 0.001 to 0.2%; table S4), while none of them shared similar taxa composition with the samples (fig. S11). Niche conservatism is evident in organisms on the Tibetan Plateau, particularly in plants adapted to harsh environmental conditions above 3000 m a.s.l. in our study region (109–111). The observed niche conservatism suggests that species within families and genera tend to preserve ancestral traits, showcasing consistent adaptive strategies for survival in challenging alpine environments over time. To detect the direct associations among taxa above species level, we kept reads belonging to natural seed-bearing plants, macrophytes, fish, and mammals (excluding Hominidae) if their families are recorded above 3000 m a.s.l. in the Tibetan Plateau. If a read is identified at the genus level but cannot be confidently matched to a known occurrence, then we assigned it to the family level, assuming that the best-matched genus was absent in the taxonomic reference database. The list of referred families and genera was compiled beforehand (Supplementary Text). Meanwhile, we kept Cyanobacteria and Monodopsidaceae (green algae) common in lake. Hereafter, we kept taxa that occur in at least two samples and have a total read count of ≥5 across all samples (49). Consequently, 94% reads of our targeted taxa groups were retained (fig. S10C).
Glacier and permafrost simulation
The spatial glacier extent and thickness were simulated using the numerical GC2D ice-flow model with specific settings for our study area, which have been reported in our previous study (45). The modeled glacier dynamics compare well with the changes in clay content (<2 μm) of Lake Naleng sediments that suggest decreasing influences of glaciers after 14.5 ka (96). We further calculated the percentage of glacier extent in the lake catchment (fig. S12A) and glacier mass from 22 to 0 ka at 500-year intervals (fig. S12B). We interpolated permafrost distribution through time (fig. S12, C to E) from the present-day relationship between permafrost distribution and annual mean air temperature. This present-day relationship was built by a generalized linear model and showed a significant P value < 2 × 10−16 (t test with SEs = 4.03 × 10−05). We compared permafrost distribution at 0 ka with present-day to correct permafrost fields across time and assessed the reliability of the simulated results. We found simulated permafrost dynamics (fig. S9A) matching well with the relative abundance of those bacteria known from permafrost soils and active layers on the Tibetan Plateau (fig. S9, B and C). Likewise, the simulated results are consistent with regional geological evidence (14). The detailed simulation processes and authentication are provided in Supplementary Text.
Taxonomic composition and ordination analyses
The filtered reads (1,067,557) belonging to 317 taxa were grouped into three count datasets: terrestrial vegetation, terrestrial mammalian, and aquatic ecosystem. There was a higher number of filtered reads in glacial samples than in interglacial samples for the terrestrial and aquatic datasets (fig. 10C). We calculated the relative abundance to obtain the compositionally equivalent information.
The negative binomial distributions (high mean values of read counts also have high variances) were detected in the read count datasets (fig. S13A). To address heterogeneity, the count datasets were normalized using the “regularized log” (rlog) transformation and the variance stabilizing transformation with default setting in the “DESeq2” package (112). Both methods take sampling depth and composition into account, achieving approximately homoscedastic distributions (fig. S13, B and C). Rlog-based counts, in particular, exhibit smaller SDs and were used for PCA, redundancy analysis (RDA), and variation partitioning analysis.
We linearly interpolated the reconstructed past temperature (113, 114), simulated permafrost extent covering glacier-free lake catchment, and simulated glacier mass to match the temporal resolution of the shotgun data. We applied PCA to rlog-based terrestrial plant counts using “rda(scale = FALSE)” and extracted PC1 site scores as an indicator of terrestrial vegetation turnover. To estimate the contribution of big herbivores to terrestrial vegetation changes, we extracted the percentage of Bos (relative to terrestrial mammalian taxa) recorded by Lake Naleng as an herbivore intensity proxy. Rumex and Sanguisorba are considered robust indicators of land-use intensity in mountain regions (115–118) because they are commonly found in soils with high levels of nitrogen (Rumex) or can withstand grazing and trampling by livestock due to their basal rosette growth habit (Sanguisorba). Therefore, we summed up the pollen percentage of both taxa (relative to pollen grains of terrestrial seed-bearing taxa) archived in Lake Naleng as an indicator of land-use intensity.
All environmental factors were standardized using the “decostand(method = ‘standardize’),” ensuring their transformation into variables approximating a normal distribution and achieving dimensional homogeneity (uniform unit variance). Subsequently, three rlog-transformed count datasets (terrestrial vegetation, terrestrial mammalian, and aquatic ecosystem) were separately linked to corresponding environmental variables using the “rda” function. RDA, a linear method, was chosen because the length of the first ordination axis was ≤1 SD as indicated by detrended correspondence analysis using the “decorana” function. Given temperature as a predictor variable for the cryosphere components’ simulation, the multicollinearity tests were implemented on the RDA results using the “vif” function in the “car” package (119) to select the independent environmental drivers (vif score ≤ 3). Then, those independent variables (tables S5 and S6) were used to calculate the unique explanation of variation [= adjusted r2 value; (120)] with variation partitioning analysis using the “varpart” function.
We further verified the consistency of conclusions drawn from the ordination analyses by comparing results based on both the entire set of taxa and a subset of common taxa identified through network analysis (as explained in the next section). Both of them yielded reproducible argumentations for terrestrial vegetation, terrestrial mammalian, and aquatic ecosystem (tables S5 and S6 and fig. S14, A to C). Unless otherwise noted, functions are inherent to the “vegan” package (121).
Co-occurrence networks
Multiple taxa may co-occur due to responding to common environments, mediators (e.g., similar abundance changes due to shared consumers), and direct associations (e.g., facilitation) (122). To explore the direct associations, we performed a Gaussian copula graphical model to the read count datasets of terrestrial (including vegetation and mammals) and aquatic ecosystems. This involved using “stackedsdm” to fit the marginal regression model, “cord” for latent factor analysis, and “cgr” for copula graph (also referred to as a network) generation. These functions are available in the ecoCopula package (123). This modeling approach comprises two essential steps: (i) examining conditional dependence relationships (partial correlation obtained from the inverse of the covariance matrix) between pairs of taxa while considering other correlations across the marginal models; (ii) identifying latent drivers and discerning co-occurrence patterns resulting from direct associations from those mediated by environmental factors and mediators (123, 124). Hence, in this study, partial correlations, after accounting for the influence of cryosphere changes, land use, and mediator taxa, serve as representatives of the direct associations among taxa.
For each ecosystem, marginal regression models were established both without (H0) and with changes in cryosphere and land use (H1): H0 = stackedsdm{Y, ~1 + offset[log(sizeFactors)], data = envi, family = “negative.binomial”} and H1 = stackedsdm{Y, ~X + offset[log(sizeFactors)], data = envi, family = “negative.binomial”}. Here, Y represents the raw count data, and X represents the combination of cryosphere and land use. An offset was incorporated to adjust for variations in sampling effort, estimated using “estimateSizeFactors(type = ‘ratio’)” in the DESeq2 package (112). Dunn-Smyth residuals and normal quantile plots suggest no violation of the assumptions of negative binomial distribution and generalized linear models for both H0 and H1 (figs. S15 and S16). Applying latent factor analysis using the “cord(nlv = 2, n.samp = 500, seed = 123)” revealed three distinct clusters of sites based on H0, which were not evident based on H1 (fig. S8, A and B). This suggests that misspecification in H1 has contributed minimally to the identification of direct interactions.
Subsequently, graphical models were constructed from H0 using the “cgr(method = ‘BIC’, seed = 123, n.samp = 500, n.lambda = 100),” while for H1, the specific parameter “n.lambda” was set to the optimized lambda value obtained from H0$all_graphs$lambda.opt. So, both models are comparable. Notably, rare taxa tend to exhibit fewer and weaker partial correlations, making it challenging to fit a completely dense co-occurrence network (one margin to infer the best network). To address this, we implemented a thresholding approach, ranging from 0 to 5000 with a step of 50, to exclude the rare taxa. The upper limit, retaining the top 5% very dominant taxa, is data dependent and not a universal recommendation. The lowest threshold required to generate the first marginal graphs was chosen for subsequent analysis.
We assumed that positive associations (e.g., facilitation) among taxa in terrestrial and aquatic ecosystems would be more prevalent during glacial periods compared to interglacial periods, in line with the stress gradient hypothesis (61). To test this assumption, we extracted the positive links from the partial correlation matrices of the best graphs of H0 and H1 for each ecosystem. Each best graph was converted to a data frame using “as_data_frame(graph_from_adjacency_matrix(H$best_graph$part, mode = ‘undirected,’ weighted = TRUE))” in the igraph package (125). Here, H represents either H0 or H1. The network structure was computed using “cluster_louvain” with a resolution ranging from 0 to 1 in increments of 0.01. The lowest resolution value was chosen on the basis of two criteria: (i) identical for both H0 and H1, and (ii) the modules detected for each network were limited to 2.
Together, we determined an abundance threshold of 2450 and a resolution of 0.69 for the terrestrial ecosystem and an abundance threshold of 50 with a resolution of 0.52 for the aquatic ecosystem. Accordingly, 27 (694,398 reads) of 274 terrestrial taxa (760,283 reads) and 39 (307,198 reads) of 43 aquatic taxa (307,274 reads) were used (referred to as common taxa).
Then, we calculated five general network properties, including nodes (taxa), edges (links between taxa), degree centralization, module (community), and Kleinberg’s hub centrality scores. The positive links were adjusted as follows: the number of positive links divided by the number of full links of all taxa. We used degree centralization based on the degree of centrality scores of taxa within the module to assess how centralized the network is around a few highly connected taxa. The degree centralization score per module was calculated using “centr_degree(normalized = TRUE).” High degree centralization score indicates that a few taxa within the module have substantially higher degrees (associations) than others. The loss of these taxa would substantially affect ecosystem functioning. To assess the taxon importance, we calculated Kleinberg’s hub centrality scores using “hub_score(scale = TRUE)” and considered a score of 0.8 as a threshold for being a keystone species. Such hub centrality scores are defined by the principal eigenvector of A*AT and rescaled between 0 and 1 by the maximum score (62). All analyses and data visualization were performed under the R environment v.4.2.2 (126), and, unless specified otherwise, default settings were used.
Acknowledgments
We thank H. H. Zimmermann for the suggestion of initial network analysis, B. Cao for valuable comments on permafrost simulation, W. Jia for the suggestion regarding the DNA-sediment relationship, and C. Jenks for English proofreading.
Funding: This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, grants 410561986 to S.K. and Mi 730/1-1,2 to S.M.) and China Scholarship Council (grant 201606180048 to S.L.).
Author contributions: U.H. and S.L. conceived this study. S.L. and U.H. led the interpretation and drafted the manuscript. S.L. did the genetic lab work under the guidance of K.R.S. and L.S. S.L. and L.H. performed the bioinformatic analyses with suggestions by K.R.S., L.S., and U.H. S.L. performed the multivariate and network analyses with input by U.H. S.L. and S.K. modeled the paleoclimate change with input by U.H. S.L. simulated the spatiotemporal distribution of permafrost. S.L. performed the data visualization. S.M. and C.Z. performed the fieldwork and provided the samples. All coauthors commented on the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data required to reproduce and evaluate the results and conclusions are provided in the Supplementary Materials and/or deposited in open access platforms. More specifically, the raw metagenomics (shotgun sequencing) data are available in European Nucleotide Archive (ENA) with BioProject accession PRJEB74036 (https://ebi.ac.uk/ena/browser/view/PRJEB74036). The source data containing the raw read counts and environmental variables to reproduce the taxonomic profiles, ordination, and network analyses for terrestrial and aquatic ecosystems are available in data S1 and S2, respectively. The information on metagenomics (shotgun sequencing) data of sediments is provided in data S3. The three datasets are also deposited on Zenodo (10.5281/zenodo.10843474) together with the source codes for bioinformatics, ancient DNA damage pattern analysis, taxonomic profiles, ordination analysis, network analysis, and past permafrost area simulation. A backup of the source codes is archived on GitHub (https://github.com/sisiliu-research/sedaDNA_Naleng).
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S16
Tables S1 to S6
Legends for data S1 to S3
References
Other Supplementary Material for this manuscript includes the following:
Data S1 to S3
REFERENCES AND NOTES
- 1.C. Körner, “Mountain biodiversity, its causes and function: An overview” in Mountain Biodiversity, C. Körner, E. M. Spehn, Eds. (Routledge, 2019), pp. 3–20. [Google Scholar]
- 2.Stibal M., Bradley J. A., Edwards A., Hotaling S., Zawierucha K., Rosvold J., Lutz S., Cameron K. A., Mikucki J. A., Kohler T. J., Šabacká M., Anesio A. M., Glacial ecosystems are essential to understanding biodiversity responses to glacier retreat. Nat. Ecol. Evol. 4, 686–687 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Jin X., Jin H., Iwahana G., Marchenko S. S., Luo D., Li X., Liang S., Impacts of climate-induced permafrost degradation on vegetation: A review. Adv. Clim. Change Res. 12, 29–47 (2021). [Google Scholar]
- 4.Li M., Wu J., Feng Y., Niu B., He Y., Zhang X., Climate variability rather than livestock grazing dominates changes in alpine grassland productivity across Tibet. Front. Ecol. Evol. 9, 631024 (2021). [Google Scholar]
- 5.Mountain Research Initiative EDW Working Group , Elevation-dependent warming in mountain regions of the world. Nat. Clim. Change 5, 424–430 (2015). [Google Scholar]
- 6.Zou D., Zhao L., Sheng Y., Chen J., Hu G., Wu T., Wu J., Xie C., Wu X., Pang Q., Wang W., Du E., Li W., Liu G., Li J., Qin Y., Qiao Y., Wang Z., Shi J., Cheng G., A new map of permafrost distribution on the Tibetan Plateau. Cryosphere 11, 2527–2542 (2017). [Google Scholar]
- 7.Yang M., Wang X., Pang G., Wan G., Liu Z., The Tibetan Plateau cryosphere: Observations and model simulations for current status and recent changes. Earth Sci. Rev. 190, 353–369 (2019). [Google Scholar]
- 8.Verrall B., Pickering C. M., Alpine vegetation in the context of climate change: A global review of past research and future directions. Sci. Total Environ. 748, 141344 (2020). [DOI] [PubMed] [Google Scholar]
- 9.D. Rhode, D. B. Madsen, P. Jeffrey Brantingham, T. Dargye, “Yaks, yak dung, and prehistoric human habitation of the Tibetan Plateau” in Late Quaternary Climate Change and Human Adaptation in Arid China, D. B. Madsen, F. Chen, X. Gao, Eds. (Developments in Quaternary Sciences, Elsevier, 2007), pp. 205–224. [Google Scholar]
- 10.Tang Z., Wang Z., Zheng C., Fang J., Biodiversity in China’s mountains. Front. Ecol. Environ. 4, 347–352 (2006). [Google Scholar]
- 11.Rounce D. R., Hock R., Shean D. E., Glacier mass change in high mountain Asia through 2100 using the open-source Python Glacier Evolution Model (PyGEM). Front. Earth Sci. 7, 331 (2020). [Google Scholar]
- 12.Zhang G., Nan Z., Hu N., Yin Z., Zhao L., Cheng G., Mu C., Qinghai-Tibet Plateau permafrost at risk in the late 21st century. Earth’s Future 10, e2022EF002652 (2022). [Google Scholar]
- 13.S. Zhou, J. Li, J. Zhao, J. Wang, J. Zheng, “Quaternary glaciations: Extent and chronology in China” in Quaternary Glaciations - Extent and Chronology, J. Ehlers, P. L. Gibbard, P. D. Hughes, Eds. (Developments in Quaternary Sciences, Elsevier, 2011), pp. 981–1002. [Google Scholar]
- 14.Jin H., Vandenberghe J., Luo D., Harris S. A., He R., Chen X., Jin X., Wang Q., Zhang Z., Spektor V., Wu Q., Wang S., Quaternary permafrost in China: Framework and discussions. Quaternary 3, 32 (2020). [Google Scholar]
- 15.Tang L., Shen C., Lu H., Li C., Ma Q., Fifty years of Quaternary palynology in the Tibetan Plateau. Sci. China Earth Sci. 64, 1825–1843 (2021). [Google Scholar]
- 16.Zimov S. A., Chuprynin V. I., Oreshko A. P., Chapin F. S. III, Reynolds J. F., Chapin M. C., Steppe-tundra transition: A herbivore-driven biome shift at the end of the Pleistocene. Am. Nat. 146, 765–794 (1995). [Google Scholar]
- 17.Zimov S. A., Zimov N. S., Tikhonov A. N., Chapin F. S., Mammoth steppe: A high-productivity phenomenon. Quat. Sci. Rev. 57, 26–45 (2012). [Google Scholar]
- 18.Malhi Y., Doughty C. E., Galetti M., Smith F. A., Svenning J.-C., Terborgh J. W., Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. U.S.A. 113, 838–846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z., Shen X., Liu B., Su J., Yonezawa T., Yu Y., Guo S., Ho S. Y. W., Vilà C., Hasegawa M., Liu J., Phylogeographical analyses of domestic and wild yaks based on mitochondrial DNA: New data and reappraisal. J. Biogeogr. 37, 2332–2344 (2010). [Google Scholar]
- 20.Macias-Fauria M., Jepson P., Zimov N., Malhi Y., Pleistocene Arctic megafaunal ecological engineering as a natural climate solution? Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190122 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X. L., Ha B. B., Wang S. J., Chen Z. J., Ge J. Y., Long H., He W., Da W., Nian X. M., Yi M., Zhou X.-Y., Zhang P. Q., Jin Y., Bar-Yosef O., Olsen J. W., Gao X., The earliest human occupation of the high-altitude Tibetan Plateau 40 thousand to 30 thousand years ago. Science 362, 1049–1051 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Chen F. H., Dong G. H., Zhang D. J., Liu X. Y., Jia X., An C. B., Ma M. M., Xie Y. W., Barton L., Ren X. Y., Zhao Z. J., Wu X. H., Jones M. K., Agriculture facilitated permanent human occupation of the Tibetan Plateau after 3600 B.P. Science 347, 248–250 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Qiu Q., Wang L., Wang K., Yang Y., Ma T., Wang Z., Zhang X., Ni Z., Hou F., Long R., Abbott R., Lenstra J., Liu J., Yak whole-genome resequencing reveals domestication signatures and prehistoric population expansions. Nat. Commun. 6, 10283 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlütz F., Lehmkuhl F., Holocene climatic change and the nomadic Anthropocene in Eastern Tibet: Palynological and geomorphological results from the Nianbaoyeze Mountains. Quat. Sci. Rev. 28, 1449–1471 (2009). [Google Scholar]
- 25.Kramer A., Herzschuh U., Mischke S., Zhang C., Holocene treeline shifts and monsoon variability in the Hengduan Mountains (southeastern Tibetan Plateau), implications from palynological investigations. Palaeogeogr. Palaeoclimatol. Palaeoecol. 286, 23–41 (2010). [Google Scholar]
- 26.Miehe G., Schleuss P.-M., Seeber E., Babel W., Biermann T., Braendle M., Chen F., Coners H., Foken T., Gerken T., Graf H.-F., Guggenberger G., Hafner S., Holzapfel M., Ingrisch J., Kuzyakov Y., Lai Z., Lehnert L., Leuschner C., Li X., Liu J., Liu S., Ma Y., Miehe S., Mosbrugger V., Noltie H. J., Schmidt J., Spielvogel S., Unteregelsbacher S., Wang Y., Willinghöfer S., Xu X., Yang Y., Zhang S., Opgenoorth L., Wesche K., The Kobresia pygmaea ecosystem of the Tibetan highlands – Origin, functioning and degradation of the world’s largest pastoral alpine ecosystem: Kobresia pastures of Tibet. Sci. Total Environ. 648, 754–771 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Xiao X., Yao A., Hillman A., Shen J., Haberle S. G., Vegetation, climate and human impact since 20 ka in central Yunnan Province based on high-resolution pollen and charcoal records from Dianchi, southwestern China. Quat. Sci. Rev. 236, 106297 (2020). [Google Scholar]
- 28.Miehe G., Hasson S. u., Glaser B., Mischke S., Böhner J., van der Knaap W. O., van Leeuwen J. F. N., Duo L., Miehe S., Haberzettl T., Föhn, fire and grazing in Southern Tibet? A 20,000-year multi-proxy record in an alpine ecotonal ecosystem. Quat. Sci. Rev. 256, 106817 (2021). [Google Scholar]
- 29.Lehnert L. W., Wesche K., Trachte K., Reudenbach C., Bendix J., Climate variability rather than overstocking causes recent large scale cover changes of Tibetan pastures. Sci. Rep. 6, 24367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian F., Qin W., Zhang R., Herzschuh U., Ni J., Zhang C., Mischke S., Cao X., Palynological evidence for the temporal stability of the plant community in the Yellow River source area over the last 7,400 years. Veg. Hist. Archaeobotany 31, 549–558 (2022). [Google Scholar]
- 31.Bauer K., Nyima Y., Laws and regulations impacting the enclosure movement on the Tibetan Plateau of China. HIMALAYA J. Assoc. Nepal Himal. Stud. 30, 23–37 (2011). [Google Scholar]
- 32.Aichner B., Herzschuh U., Wilkes H., Vieth A., Böhner J., δD values of n-alkanes in Tibetan lake sediments and aquatic macrophytes – A surface sediment study and application to a 16ka record from Lake Koucha. Org. Geochem. 41, 779–790 (2010). [Google Scholar]
- 33.Herzschuh U., Mischke S., Meyer H., Plessen B., Zhang C., Lake nutrient variability inferred from elemental (C, N, S) and isotopic (δ13C, δ15N) analyses of aquatic plant macrofossils. Quat. Sci. Rev. 29, 2161–2172 (2010). [Google Scholar]
- 34.Baiping Z., Xiaodong C., Baolin L., Yonghui Y., Biodiversity and conservation in the Tibetan Plateau. J. Geogr. Sci. 12, 135–143 (2002). [Google Scholar]
- 35.Herzschuh U., Zhang C., Mischke S., Herzschuh R., Mohammadi F., Mingram B., Kürschner H., Riedel F., A late Quaternary lake record from the Qilian Mountains (NW China): Evolution of the primary production and the water depth reconstructed from macrofossil, pollen, biomarker, and isotope data. Glob. Planet. Change 46, 361–379 (2005). [Google Scholar]
- 36.Saini J., Günther F., Aichner B., Mischke S., Herzschuh U., Zhang C., Mäusbacher R., Gleixner G., Climate variability in the past ∼19,000 yr in NE Tibetan Plateau inferred from biomarker and stable isotope records of Lake Donggi Cona. Quat. Sci. Rev. 157, 129–140 (2017). [Google Scholar]
- 37.Aichner B., Wünnemann B., Callegaro A., van der Meer M. T. J., Yan D., Zhang Y., Barbante C., Sachse D., Asynchronous responses of aquatic ecosystems to hydroclimatic forcing on the Tibetan Plateau. Commun. Earth Environ. 3, 3 (2022). [Google Scholar]
- 38.Huo S., Zhang H., Wang J., Chen J., Wu F., Temperature and precipitation dominates millennium changes of eukaryotic algal communities in Lake Yamzhog Yumco, Southern Tibetan Plateau. Sci. Total Environ. 829, 154636 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Tulloch A. I. T., Chadès I., Dujardin Y., Westgate M. J., Lane P. W., Lindenmayer D., Dynamic species co-occurrence networks require dynamic biodiversity surrogates. Ecography 39, 1185–1196 (2016). [Google Scholar]
- 40.Muscente A. D., Prabhu A., Zhong H., Eleish A., Meyer M. B., Fox P., Hazen R. M., Knoll A. H., Quantifying ecological impacts of mass extinctions with network analysis of fossil communities. Proc. Natl. Acad. Sci. U.S.A. 115, 5217–5222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertness M. D., Callaway R., Positive interactions in communities. Trends Ecol. Evol. 9, 191–193 (1994). [DOI] [PubMed] [Google Scholar]
- 42.Wang Y., Sun J., Liu B., Wang J., Zeng T., Cushion plants as critical pioneers and engineers in alpine ecosystems across the Tibetan Plateau. Ecol. Evol. 11, 11554–11558 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y.-Z., Qian L.-S., Chen X.-F., Sun L., Sun H., Chen J.-G., Diversity patterns of cushion plants on the Qinghai-Tibet Plateau: A basic study for future conservation efforts on alpine ecosystems. Plant Divers. 44, 231–242 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anthelme F., Cavieres L. A., Dangles O., Facilitation among plants in alpine environments in the face of climate change. Front. Plant Sci. 5, 387 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S., Kruse S., Scherler D., Ree R. H., Zimmermann H. H., Stoof-Leichsenring K. R., Epp L. S., Mischke S., Herzschuh U., Sedimentary ancient DNA reveals a threat of warming-induced alpine habitat loss to Tibetan Plateau plant diversity. Nat. Commun. 12, 2995 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.J. P. Smol, H. J. B. Birks, A. F. Lotter, S. Juggins, “The march towards the quantitative analysis of palaeolimnological data” in Tracking Environmental Change Using Lake Sediments, H. J. B. Birks, A. F. Lotter, S. Juggins, J. P. Smol, Eds. (Springer Netherlands,, 2012), vol. 5, chap. 1. [Google Scholar]
- 47.Jia W., Anslan S., Chen F., Cao X., Dong H., Dulias K., Gu Z., Heinecke L., Jiang H., Kruse S., Kang W., Li K., Liu S., Liu X., Liu Y., Ni J., Schwalb A., Stoof-Leichsenring K. R., Shen W., Tian F., Wang J., Wang Y., Wang Y., Xu H., Yang X., Zhang D., Herzschuh U., Sedimentary ancient DNA reveals past ecosystem and biodiversity changes on the Tibetan Plateau: Overview and prospects. Quat. Sci. Rev. 293, 107703 (2022). [Google Scholar]
- 48.Kjær K. H., Winther Pedersen M., De Sanctis B., De Cahsan B., Korneliussen T. S., Michelsen C. S., Sand K. K., Jelavić S., Ruter A. H., Schmidt A. M. A., Kjeldsen K. K., Tesakov A. S., Snowball I., Gosse J. C., Alsos I. G., Wang Y., Dockter C., Rasmussen M., Jørgensen M. E., Skadhauge B., Prohaska A., Kristensen J. Å., Bjerager M., Allentoft M. E., Coissac E., PhyloNorway Consortium, Rouillard A., Simakova A., Fernandez-Guerra A., Bowler C., Macias-Fauria M., Vinner L., Welch J. J., Hidy A. J., Sikora M., Collins M. J., Durbin R., Larsen N. K., Willerslev E., A 2-million-year-old ecosystem in Greenland uncovered by environmental DNA. Nature 612, 283–291 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y., Korneliussen T. S., Holman L. E., Manica A., Pedersen M. W., ngsLCA—A toolkit for fast and flexible lowest common ancestor inference and taxonomic profiling of metagenomic data. Methods Ecol. Evol. 13, 2699–2708 (2022). [Google Scholar]
- 50.Kramer A., Herzschuh U., Mischke S., Zhang C., Late glacial vegetation and climate oscillations on the southeastern Tibetan Plateau inferred from the Lake Naleng pollen profile. Quatern. Res. 73, 324–335 (2010). [Google Scholar]
- 51.Kang B., Deng J., Wu Y., Chen L., Zhang J., Qiu H., Lu Y., He D., Mapping China’s freshwater fishes: Diversity and biogeography. Fish Fish. 15, 209–230 (2014). [Google Scholar]
- 52.Zhang L., Wang Q., Yang L., Li F., Chan B. P. L., Xiao Z., Li S., Song D., Piao Z., Fan P., The neglected otters in China: Distribution change in the past 400 years and current conservation status. Biol. Conserv. 228, 259–267 (2018). [Google Scholar]
- 53.Lammers Y., Heintzman P. D., Alsos I. G., Environmental palaeogenomic reconstruction of an Ice Age algal population. Commun. Biol. 4, 200 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huisman J., Codd G. A., Paerl H. W., Ibelings B. W., Verspagen J. M. H., Visser P. M., Cyanobacterial blooms. Nat. Rev. Microbiol. 16, 471–483 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Courtin J., Perfumo A., Andreev A. A., Opel T., Stoof-Leichsenring K. R., Edwards M. E., Murton J. B., Herzschuh U., Pleistocene glacial and interglacial ecosystems inferred from ancient DNA analyses of permafrost sediments from Batagay megaslump, East Siberia. Environ. DNA 4, 1265–1283 (2022). [Google Scholar]
- 56.Pedersen M. W., Ruter A., Schweger C., Friebe H., Staff R. A., Kjeldsen K. K., Mendoza M. L. Z., Beaudoin A. B., Zutter C., Larsen N. K., Potter B. A., Nielsen R., Rainville R. A., Orlando L., Meltzer D. J., Kjær K. H., Willerslev E., Postglacial viability and colonization in North America’s ice-free corridor. Nature 537, 45–49 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Parducci L., Alsos I. G., Unneberg P., Pedersen M. W., Han L., Lammers Y., Salonen J. S., Väliranta M. M., Slotte T., Wohlfarth B., Shotgun environmental DNA, pollen, and macrofossil analysis of Lateglacial lake sediments from southern Sweden. Front. Ecol. Evol. 7, 189 (2019). [Google Scholar]
- 58.Wang Y., Pedersen M. W., Alsos I. G., De Sanctis B., Racimo F., Prohaska A., Coissac E., Owens H. L., Merkel M. K. F., Fernandez-Guerra A., Rouillard A., Lammers Y., Alberti A., Denoeud F., Money D., Ruter A. H., McColl H., Larsen N. K., Cherezova A. A., Edwards M. E., Fedorov G. B., Haile J., Orlando L., Vinner L., Korneliussen T. S., Beilman D. W., Bjørk A. A., Cao J., Dockter C., Esdale J., Gusarova G., Kjeldsen K. K., Mangerud J., Rasic J. T., Skadhauge B., Svendsen J. I., Tikhonov A., Wincker P., Xing Y., Zhang Y., Froese D. G., Rahbek C., Nogues D. B., Holden P. B., Edwards N. R., Durbin R., Meltzer D. J., Kjær K. H., Möller P., Willerslev E., Late Quaternary dynamics of Arctic biota from ancient environmental genomics. Nature 600, 86–92 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li K., Stoof-Leichsenring K. R., Liu S., Jia W., Liao M., Liu X., Ni J., Herzschuh U., Plant sedimentary DNA as a proxy for vegetation reconstruction in eastern and northern Asia. Ecol. Indic. 132, 108303 (2021). [Google Scholar]
- 60.Wende Z., Hou G., Gao J., Chen X., Jin S., Lancuo Z., Reconstruction of cultivated land in the northeast margin of Qinghai–Tibetan Plateau and anthropogenic impacts on palaeo-environment during the mid-Holocene. Front. Earth Sci. 9, 681995 (2021). [Google Scholar]
- 61.Adams A. E., Besozzi E. M., Shahrokhi G., Patten M. A., A case for associational resistance: Apparent support for the stress gradient hypothesis varies with study system. Ecol. Lett. 25, 202–217 (2022). [DOI] [PubMed] [Google Scholar]
- 62.Kleinberg J. M., Authoritative sources in a hyperlinked environment. J. ACM 46, 604–632 (1999). [Google Scholar]
- 63.Yang G., Ryo M., Roy J., Lammel D. R., Ballhausen M.-B., Jing X., Zhu X., Rillig M. C., Multiple anthropogenic pressures eliminate the effects of soil microbial diversity on ecosystem functions in experimental microcosms. Nat. Commun. 13, 4260 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson C. V., Porter T. M., Maitland V. C., Wright M. T. G., Hajibabaei M., Multi-marker metabarcoding resolves subtle variations in freshwater condition: Bioindicators, ecological traits, and trophic interactions. Ecol. Indic. 145, 109603 (2022). [Google Scholar]
- 65.Bao G., Suetsugu K., Wang H., Yao X., Liu L., Ou J., Li C., Effects of the hemiparasitic plant Pedicularis kansuensis on plant community structure in a degraded grassland. Ecol. Res. 30, 507–515 (2015). [Google Scholar]
- 66.Cavieres L. A., Badano E. I., Do facilitative interactions increase species richness at the entire community level? J. Ecol. 97, 1181–1191 (2009). [Google Scholar]
- 67.Ebersbach J., Schnitzler J., Favre A., Muellner-Riehl A. N., Evolutionary radiations in the species-rich mountain genus Saxifraga L. BMC Evol. Biol. 17, 119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jing X., Ding L., Zhou J., Huang X., Degen A., Long R., The adaptive strategies of yaks to live in the Asian highlands. Anim. Nutr. 9, 249–258 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kramer A., Herzschuh U., Mischke S., Zhang C., Late Quaternary environmental history of the south-eastern Tibetan Plateau inferred from the Lake Naleng non-pollen palynomorph record. Veg. Hist. Archaeobotany 19, 453–468 (2010). [Google Scholar]
- 70.Guo N., Wu Q., Shi F., Niu J., Zhang T., Degen A. A., Fang Q., Ding L., Shang Z., Zhang Z., Long R., Seasonal dynamics of diet–gut microbiota interaction in adaptation of yaks to life at high altitude. Npj Biofilms Microbiomes 7, 38 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Basumatary S. K., Singh H., van Asperen E. N., Tripathi S., McDonald H. G., Pokharia A. K., Coprophilous and non-coprophilous fungal spores of Bos mutus modern dung from the Indian Himalaya: Implications to temperate paleoherbivory and paleoecological analysis. Rev. Palaeobot. Palynol. 277, 104208 (2020). [Google Scholar]
- 72.Willerslev E., Davison J., Moora M., Zobel M., Coissac E., Edwards M. E., Lorenzen E. D., Vestergård M., Gussarova G., Haile J., Craine J., Gielly L., Boessenkool S., Epp L. S., Pearman P. B., Cheddadi R., Murray D., Bråthen K. A., Yoccoz N., Binney H., Cruaud C., Wincker P., Goslar T., Alsos I. G., Bellemain E., Brysting A. K., Elven R., Sønstebø J. H., Murton J., Sher A., Rasmussen M., Rønn R., Mourier T., Cooper A., Austin J., Möller P., Froese D., Zazula G., Pompanon F., Rioux D., Niderkorn V., Tikhonov A., Savvinov G., Roberts R. G., MacPhee R. D. E., Gilbert M. T. P., Kjær K. H., Orlando L., Brochmann C., Taberlet P., Fifty thousand years of Arctic vegetation and megafaunal diet. Nature 506, 47–51 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Barrio I. C., Hik D. S., Bueno C. G., Cahill J. F., Extending the stress-gradient hypothesis – is competition among animals less common in harsh environments? Oikos 122, 516–523 (2013). [Google Scholar]
- 74.Murchie T. J., Monteath A. J., Mahony M. E., Long G. S., Cocker S., Sadoway T., Karpinski E., Zazula G., MacPhee R. D. E., Froese D., Poinar H. N., Collapse of the mammoth-steppe in central Yukon as revealed by ancient environmental DNA. Nat. Commun. 12, 7120 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Politti E., Bertoldi W., Gurnell A., Henshaw A., Feedbacks between the riparian Salicaceae and hydrogeomorphic processes: A quantitative review. Earth Sci. Rev. 176, 147–165 (2018). [Google Scholar]
- 76.Yang C., Yan T., Sun Y., Hou F., Shrub cover impacts on yak growth performance and herbaceous forage quality on the Qinghai-Tibet Plateau, China. Rangel. Ecol. Manag. 75, 9–16 (2021). [Google Scholar]
- 77.Monteath A. J., Gaglioti B. V., Edwards M. E., Froese D., Late Pleistocene shrub expansion preceded megafauna turnover and extinctions in eastern Beringia. Proc. Natl. Acad. Sci. U.S.A. 118, e2107977118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.d’Alpoim Guedes J., Rethinking the spread of agriculture to the Tibetan Plateau. Holocene 25, 1498–1510 (2015). [Google Scholar]
- 79.Rozwalak P., Podkowa P., Buda J., Niedzielski P., Kawecki S., Ambrosini R., Azzoni R. S., Baccolo G., Ceballos J. L., Cook J., Di Mauro B., Ficetola G. F., Franzetti A., Ignatiuk D., Klimaszyk P., Łokas E., Ono M., Parnikoza I., Pietryka M., Pittino F., Poniecka E., Porazinska D. L., Richter D., Schmidt S. K., Sommers P., Souza-Kasprzyk J., Stibal M., Szczuciński W., Uetake J., Wejnerowski Ł., Yde J. C., Takeuchi N., Zawierucha K., Cryoconite-From minerals and organic matter to bioengineered sediments on glacier’s surfaces. Sci. Total Environ. 807, 150874 (2022). [DOI] [PubMed] [Google Scholar]
- 80.Liu K., Liu Y., Han B., Xu B., Zhu L., Ju J., Jiao N., Xiong J., Bacterial community changes in a glacial-fed Tibetan lake are correlated with glacial melting. Sci. Total Environ. 651, 2059–2067 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Liu K., Liu Y., Hu A., Wang F., Zhang Z., Yan Q., Ji M., Vick-Majors T. J., Fate of glacier surface snow-originating bacteria in the glacier-fed hydrologic continuums. Environ. Microbiol. 23, 6450–6462 (2021). [DOI] [PubMed] [Google Scholar]
- 82.Kleinteich J., Hanselmann K., Hildebrand F., Kappler A., Zarfl C., Glacier melt-down changes habitat characteristics and unique microbial community composition and physiology in alpine lake sediments. FEMS Microbiol. Ecol. 98, fiac075 (2022). [DOI] [PubMed] [Google Scholar]
- 83.Bruno M., Gucci P. M. B., Pierdominici E., Sestili P., Ioppolo A., Sechi N., Volterra L., Microcystin-like toxins in different freshwater species of Oscillatoria. Toxicon 30, 1307–1311 (1992). [DOI] [PubMed] [Google Scholar]
- 84.Hammarlund S. P., Harcombe W. R., Refining the stress gradient hypothesis in a microbial community. Proc. Natl. Acad. Sci. U.S.A. 116, 15760–15762 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hesse E., O’Brien S., Luján A. M., Sanders D., Bayer F., van Veen E. M., Hodgson D. J., Buckling A., Stress causes interspecific facilitation within a compost community. Ecol. Lett. 24, 2169–2177 (2021). [DOI] [PubMed] [Google Scholar]
- 86.Stoof-Leichsenring K. R., Huang S., Liu S., Jia W., Li K., Liu X., Pestryakova L. A., Herzschuh U., Sedimentary DNA identifies modern and past macrophyte diversity and its environmental drivers in high-latitude and high-elevation lakes in Siberia and China. Limnol. Oceanogr. 67, 1126–1141 (2022). [Google Scholar]
- 87.Tao J., He D., Kennard M. J., Ding C., Bunn S. E., Liu C., Jia Y., Che R., Chen Y., Strong evidence for changing fish reproductive phenology under climate warming on the Tibetan Plateau. Glob. Chang. Biol. 24, 2093–2104 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Liu C., Comte L., Xian W., Chen Y., Olden J. D., Current and projected future risks of freshwater fish invasions in China. Ecography 42, 2074–2083 (2019). [Google Scholar]
- 89.Ayres C., García P., Features of the predation of the Eurasian otter upon toads in north-western Spain. Mamm. Biol. 76, 90–92 (2011). [Google Scholar]
- 90.Nakai S., Yamada S., Hosomi M., Anti-cyanobacterial fatty acids released from Myriophyllum spicatum. Hydrobiologia 543, 71–78 (2005). [Google Scholar]
- 91.Mohamed Z. A., Macrophytes-cyanobacteria allelopathic interactions and their implications for water resources management—A review. Limnologica 63, 122–132 (2017). [Google Scholar]
- 92.Randsalu-Wendrup L., Conley D. J., Carstensen J., Fritz S. C., Paleolimnological records of regime shifts in lakes in response to climate change and anthropogenic activities. J. Paleolimnol. 56, 1–14 (2016). [Google Scholar]
- 93.Lu X., Kelsey K. C., Yan Y., Sun J., Wang X., Cheng G., Neff J. C., Effects of grazing on ecosystem structure and function of alpine grasslands in Qinghai–Tibetan Plateau: A synthesis. Ecosphere 8, e01656 (2017). [Google Scholar]
- 94.Hopping K. A., Knapp A. K., Dorji T., Klein J. A., Warming and land use change concurrently erode ecosystem services in Tibet. Glob. Chang. Biol. 24, 5534–5548 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Wang Y., Lv W., Xue K., Wang S., Zhang L., Hu R., Zeng H., Xu X., Li Y., Jiang L., Hao Y., Du J., Sun J., Dorji T., Piao S., Wang C., Luo C., Zhang Z., Chang X., Zhang M., Hu Y., Wu T., Wang J., Li B., Liu P., Zhou Y., Wang A., Dong S., Zhang X., Gao Q., Zhou H., Shen M., Wilkes A., Miehe G., Zhao X., Niu H., Grassland changes and adaptive management on the Qinghai–Tibetan Plateau. Nat. Rev. Earth Environ. 3, 668–683 (2022). [Google Scholar]
- 96.Opitz S., Zhang C., Herzschuh U., Mischke S., Climate variability on the south-eastern Tibetan Plateau since the Lateglacial based on a multiproxy approach from Lake Naleng – comparing pollen and non-pollen signals. Quat. Sci. Rev. 115, 112–122 (2015). [Google Scholar]
- 97.Wang Z., Wang Q., Zhao L., Wu X., Yue G., Zou D., Nan Z., Liu G., Pang Q., Fang H., Wu T., Shi J., Jiao K., Zhao Y., Zhang L., Mapping the vegetation distribution of the permafrost zone on the Qinghai-Tibet Plateau. J. Mt. Sci. 13, 1035–1046 (2016). [Google Scholar]
- 98.Larridon I., A linear classification of Cyperaceae. Kew Bull. 77, 309–315 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Strasky S., Graf A. A., Zhao Z., Kubik P. W., Baur H., Schlüchter C., Wieler R., Late Glacial ice advances in southeast Tibet. J. Asian Earth Sci. 34, 458–465 (2009). [Google Scholar]
- 100.Chen F., Zhang J., Liu J., Cao X., Hou J., Zhu L., Xu X., Liu X., Wang M., Wu D., Huang L., Zeng T., Zhang S., Huang W., Zhang X., Yang K., Climate change, vegetation history, and landscape responses on the Tibetan Plateau during the Holocene: A comprehensive review. Quat. Sci. Rev. 243, 106444 (2020). [Google Scholar]
- 101.Meyers P. A., Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem. Geol. 114, 289–302 (1994). [Google Scholar]
- 102.Gansauge M.-T., Gerber T., Glocke I., Korlević P., Lippik L., Nagel S., Riehl L. M., Schmidt A., Meyer M., Single-stranded DNA library preparation from highly degraded DNA using T4 DNA ligase. Nucleic Acids Res. 45, e79 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gansauge M. T., Meyer M., Single-stranded DNA library preparation for the sequencing of ancient or damaged DNA. Nat. Protoc. 8, 737–748 (2013). [DOI] [PubMed] [Google Scholar]
- 104.S. Andrews, FastQC: A quality control tool for high throughput sequence data. (2010). https://bioinformatics.babraham.ac.uk/projects/fastqc/.
- 105.Chen S., Zhou Y., Chen Y., Gu J., Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Langmead B., Salzberg S. L., Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dabney J., Meyer M., Pääbo S., Ancient DNA Damage. Cold Spring Harb. Perspect. Biol. 5, a012567 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jónsson H., Ginolhac A., Schubert M., Johnson P. L. F., Orlando L., MapDamage2.0: Fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics 29, 1682–1684 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He J., Lin H., Wang R., Dai C., Yu H., Tu J., Yu J., Jiang H., Joint effects of environmental filtering and dispersal limitation on the species assemblage of the Tibetan Plateau. J. Biogeogr. 49, 640–653 (2022). [Google Scholar]
- 110.Li X.-H., Zhu X.-X., Niu Y., Sun H., Phylogenetic clustering and overdispersion for alpine plants along elevational gradient in the Hengduan Mountains Region, southwest China. J. Syst. Evol. 52, 280–288 (2014). [Google Scholar]
- 111.Xu J., Chen Y., Zhang L., Chai Y., Wang M., Guo Y., Li T., Yue M., Using phylogeny and functional traits for assessing community assembly along environmental gradients: A deterministic process driven by elevation. Ecol. Evol. 7, 5056–5069 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shakun J. D., Clark P. U., He F., Marcott S. A., Mix A. C., Liu Z., Otto-Bliesner B., Schmittner A., Bard E., Global warming preceded by increasing carbon dioxide concentrations during the last deglaciation. Nature 484, 49–54 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Marcott S. A., Shakun J. D., Clark P. U., Mix A. C., A reconstruction of regional and global temperature for the past 11,300 years. Science 339, 1198–1201 (2013). [DOI] [PubMed] [Google Scholar]
- 115.Xie Z., Le Roux X., Wang C., Gu Z., An M., Nan H., Chen B., Li F., Liu Y., Du G., Feng H., Ma X., Identifying response groups of soil nitrifiers and denitrifiers to grazing and associated soil environmental drivers in Tibetan alpine meadows. Soil Biol. Biochem. 77, 89–99 (2014). [Google Scholar]
- 116.Court-Picon M., Buttler A., de Beaulieu J.-L., Modern pollen/vegetation/land-use relationships in mountain environments: An example from the Champsaur valley (French Alps). Veg. Hist. Archaeobotany 15, 151–168 (2006). [Google Scholar]
- 117.Ma M., Walck J. I., Ma Z., Wang L., Du G., Grazing disturbance increases transient but decreases persistent soil seed bank. Ecol. Appl. 28, 1020–1031 (2018). [DOI] [PubMed] [Google Scholar]
- 118.Pauler C. M., Isselstein J., Braunbeck T., Schneider M. K., Influence of highland and production-oriented cattle breeds on pasture vegetation: A pairwise assessment across broad environmental gradients. Agric. Ecosyst. Environ. 284, 106585 (2019). [Google Scholar]
- 119.J. Fox, S. Weisberg, “Diagnosing problems in linear and generalized linear models” in An R Companion to Applied Regression (SAGE Publications Inc., ed. 2, 2011), pp. 285–328. [Google Scholar]
- 120.Peres-Neto P. R., Legendre P., Dray S., Borcard D., Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology 87, 2614–2625 (2006). [DOI] [PubMed] [Google Scholar]
- 121.J. Oksanen, F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs, H. Wagner, vegan: Community Ecology Package. (2019). https://CRAN.R-project.org/package=vegan.
- 122.Valladares F., Bastias C. C., Godoy O., Granda E., Escudero A., Species coexistence in a changing world. Front. Plant Sci. 6, 866 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Popovic G. C., Warton D. I., Thomson F. J., Hui F. K. C., Moles A. T., Untangling direct species associations from indirect mediator species effects with graphical models. Methods Ecol. Evol. 10, 1571–1583 (2019). [Google Scholar]
- 124.Popovic G. C., Hui F. K. C., Warton D. I., A general algorithm for covariance modeling of discrete data. J. Multivar. Anal. 165, 86–100 (2018). [Google Scholar]
- 125.Csardi G., Nepusz T., The igraph software package for complex network research. InterJournal Complex Syst. 1695, 1–9 (2006). [Google Scholar]
- 126.R Core Team, R: A Language and Environment for Statistical Comput. Secur., version 4.1.2 (2021). https://R-project.org/.
- 127.Li X., Wang L., Chen D., Yang K., Xue B., Sun L., Near-surface air temperature lapse rates in the mainland China during 1962-2011. J. Geophys. Res. Atmospheres 118, 7505–7515 (2013). [Google Scholar]
- 128.GBIF.org, GBIF occurrence download: Tracheophyta, (2022). 10.15468/dl.8ruwan. [DOI]
- 129.Brach A. R., Song H., eFloras: New directions for online floras exemplified by the Flora of China Project. TAXON 55, 188–192 (2006). [Google Scholar]
- 130.Yu H., Favre A., Sui X., Chen Z., Qi W., Xie G., Mapping the genetic patterns of plants in the region of the Qinghai–Tibet Plateau: Implications for conservation strategies. Divers. Distrib. 25, 310–324 (2019). [Google Scholar]
- 131.Yu H., Miao S., Xie G., Guo X., Chen Z., Favre A., Contrasting Floristic Diversity of the Hengduan Mountains, the Himalayas and the Qinghai-Tibet Plateau Sensu Stricto in China. Front. Ecol. Evol. 8, 136 (2020). [Google Scholar]
- 132.Zhang Y., Li B., Zheng D., Datasets of the boundary and area of the Tibetan Plateau. Acta Geogr. Sin. 64, 164–168 (2014). [Google Scholar]
- 133.Li X., Fu W., Shen H., Huang C., Zhang L., Monitoring snow cover variability (2000–2014) in the Hengduan Mountains based on cloud-removed MODIS products with an adaptive spatio-temporal weighted method. J. Hydrol. 551, 314–327 (2017). [Google Scholar]
- 134.Pebesma E., Roger S. B., S classes and methods for spatial data: The sp package. R News 5, 9–13 (2005). [Google Scholar]
- 135.R. Bivand, E. J. Pebesma, V. Gómez-Rubio, “Further methods for handling spatial data” in Applied Spatial Data Analysis with R (Use R!, Springer,, ed. 2, 2013), pp. 140–142. [Google Scholar]
- 136.R. J. Hijmans, raster: Geographic data analysis and modeling. R package version 31-5 (2020). https://rspatial.org/raster.
- 137.QGIS.org, QGIS geographic information system, version 3.24.1-Tisler, QGIS Association (2022). http://qgis.org.
- 138.Chamberlain S. A., Szöcs E., Taxize: Taxonomic search and retrieval in R. F1000Res. 2, 191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.A. T. Smith, Y. Xie, R. S. Hoffmann, F. Gemma, Eds., Mammals of China (Princeton University Press, 2013).
- 140.GBIF.org, GBIF occurrence download: Mammalia, (2022). 10.15468/dl.7473xu. [DOI]
- 141.Xing Y., Zhang C., Fan E., Zhao Y., Freshwater fishes of China: Species richness, endemism, threatened species and conservation. Divers. Distrib. 22, 358–370 (2016). [Google Scholar]
- 142.GBIF.org, GBIF occurrence download: Freshwater fish, (2023). 10.15468/dl.cq3ytx. [DOI]
- 143.Yang M., Nelson F. E., Shiklomanov N. I., Guo D., Wan G., Permafrost degradation and its environmental effects on the Tibetan Plateau: A review of recent research. Earth Sci. Rev. 103, 31–44 (2010). [Google Scholar]
- 144.Lu Q., Zhao D., Wu S., Simulated responses of permafrost distribution to climate change on the Qinghai–Tibet Plateau. Sci. Rep. 7, 3845 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hu G., Zhao L., Li R., Wu X., Wu T., Pang Q., Liu G. y., Xie C., A model for obtaining ground temperature from air temperature in permafrost regions on the Qinghai-Tibetan Plateau. Catena 189, 104470 (2020). [Google Scholar]
- 146.Fick S. E., Hijmans R. J., WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017). [Google Scholar]
- 147.Watanabe T., Soil Erosion on Yak-Grazing Steps in the Langtang Himal, Nepal. Mt. Res. Dev. 14, 171–179 (1994). [Google Scholar]
- 148.Chen Y., Deng Y., Ding J., Hu H., Xu T., Li F., Yang G., Yang Y., Distinct microbial communities in the active and permafrost layers on the Tibetan Plateau. Mol. Ecol. 26, 6608–6620 (2017). [DOI] [PubMed] [Google Scholar]
- 149.Freeman C. L., Dieudonné L., Agbaje O. B. A., Žure M., Sanz J. Q., Collins M., Sand K. K., Survival of environmental DNA in sediments: Mineralogic control on DNA taphonomy. Environ. DNA 5, 1691–1705 (2023). [Google Scholar]
- 150.Sand K. K., Jelavić S., Kjær K. H., Prohaska A., Importance of eDNA taphonomy and sediment provenance for robust ecological inference: Insights from interfacial geochemistry. Environ. DNA 6, e519 (2024). [Google Scholar]
- 151.Zhang C., Mischke S., A Lateglacial and Holocene lake record from the Nianbaoyeze Mountains and inferences of lake, glacier and climate evolution on the eastern Tibetan Plateau. Quat. Sci. Rev. 28, 1970–1983 (2009). [Google Scholar]
- 152.Wang Y., Liu X., Mischke S., Herzschuh U., Environmental constraints on lake sediment mineral compositions from the Tibetan Plateau and implications for paleoenvironment reconstruction. J. Paleolimnol. 47, 71–85 (2012). [Google Scholar]
- 153.Janssen A. B. G., Teurlincx S., An S., Janse J. H., Paerl H. W., Mooij W. M., Alternative stable states in large shallow lakes? J. Great Lakes Res. 40, 813–826 (2014). [Google Scholar]
- 154.Laug A., Schwarz A., Lauterbach S., Engels S., Schwalb A., Ecosystem shifts at two mid-Holocene tipping points in the alpine Lake Son Kol (Kyrgyzstan, Central Asia). Holocene 30, 1410–1419 (2020). [Google Scholar]
- 155.Kanbar H. J., Olajos F., Englund G., Holmboe M., Geochemical identification of potential DNA-hotspots and DNA-infrared fingerprints in lake sediments. Appl. Geochem. 122, 104728 (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S16
Tables S1 to S6
Legends for data S1 to S3
References
Data S1 to S3