Abstract
Background
Mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) is increasingly recognized as a clinicoradiological syndrome. Its etiology is diverse, encompassing a variety of triggers, including infections and metabolic abnormalities. Uniquely, MERS may present with psychiatric symptoms, such as delirium, visual hallucinations, and catatonia, posing diagnostic challenges. The variability of these neuropsychiatric symptoms necessitates early diagnosis through magnetic resonance imaging (MRI) to avoid prolonged antipsychotic treatment.
Case Presentation
This report details a case of MERS in a 39‐year‐old male. The patient initially presented with headache, sore throat, and abnormal laboratory results: leukocytosis, neutrophilia with a left shift, elevated C‐reactive protein (CRP) levels, and hyponatremia. On the fourth day of admission, he developed severe anxiety and restlessness, exhibited thoughts of death, and reported experiencing vivid hallucinations upon closing his eyes. MRI revealed a hyperintense lesion in the corpus callosum. A lumbar puncture showed no increase in cell count or protein. The patient showed a positive response to treatment with antibiotics and olanzapine, demonstrating rapid symptomatic improvement. A follow‐up MRI on the 11th day showed complete resolution of the brain lesions. Six months later, no neurological or psychiatric sequelae were noted. The patient's clinical progression and imaging findings led to a definitive diagnosis of MERS.
Conclusion
The early presentation of symptoms such as restlessness, hallucinations, and death ideation played a critical role in diagnosing MERS, with early MRI examination being instrumental in both diagnosis and preventing prolonged antipsychotic medication use.
Keywords: mild encephalitis/encephalopathy with reversible splenial lesion (MERS), MRI, tonsillitis, psychosis, closed‐eye visual hallucination
This report elucidates a rare presentation of MERS in a 39‐year‐old male, triggered by an episode of tonsillitis, with significant psychiatric manifestations. Although the early psychiatric manifestations of this case, particularly restlessness, hallucinations, and death ideation, were considered psychotic, timely MRI testing played an important role in the diagnosis and avoidance of long‐term use of antipsychotic medications. This case is noteworthy for its atypical presentation and the fact that a multidisciplinary approach to diagnosis and treatment resulted in a favorable outcome.
BACKGROUND
Mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) is increasingly recognized as a clinicoradiological syndrome. Its etiology is diverse, encompassing a variety of triggers, including infections and metabolic abnormalities. 1 Generally, MERS resolves spontaneously; however, certain etiologies necessitate specific symptomatic treatment. 2 Uniquely, MERS may present with psychiatric symptoms, such as delirium, 2 visual hallucinations, 3 and catatonia, 4 posing diagnostic and treatment challenges. The variability of these neuropsychiatric symptoms necessitates early diagnosis through magnetic resonance imaging (MRI) to avoid prolonged antipsychotic treatment.
In this report, we discuss a 39‐year‐old man who developed MERS following tonsillitis, presenting severe psychiatric symptoms that required antipsychotic treatment. This case underscores the importance of vigilance for MERS in patients exhibiting similar symptoms, particularly those with a recent history of infectious or inflammatory diseases. It also highlights the significance of early detection and management of MERS to prevent unnecessary long‐term antipsychotic drug administration.
CASE PRESENTATION
A 39‐year‐old male with no significant medical history presented to the outpatient clinic with tonsillitis symptoms. Despite initial oral antibiotic treatment, his headache and severe sore throat persisted. He was admitted to the otorhinolaryngology department 3 days later for further evaluation, as depicted in Figure 1.
Figure 1.
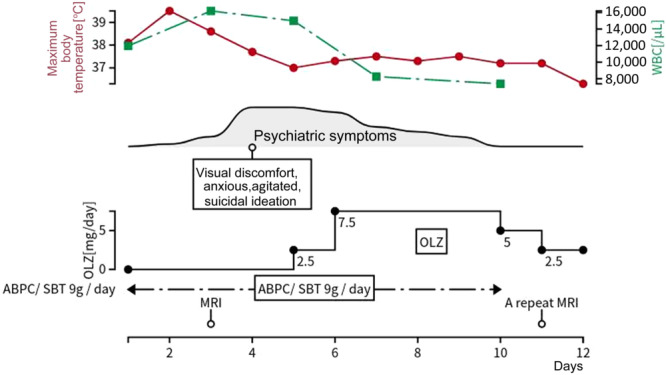
The patient's clinical course. ABPC/SBT, ampicillin‐sulbactam; OLZ, olanzapine; WBC, white blood cell count.
Upon admission (Day 1), the patient exhibited a fever of 38.1°C, tachycardia (>90 b.p.m.), and an enlarged right tonsil. Laboratory findings included a white blood cell count of 11,960/µL with elevated neutrophils (89%) and a marked left shift. He also showed hyponatremia (128 mEq/L), elevated creatine kinase (CK) at 4376 U/L, and increased serum liver enzyme levels: aspartate transaminase (AST) 87 U/L, alanine transaminase (ALT) 82 U/L, and γ‐glutamyl transferase (γ‐GTP) 91 U/L, indicative of a systemic inflammatory response syndrome (SIRS).
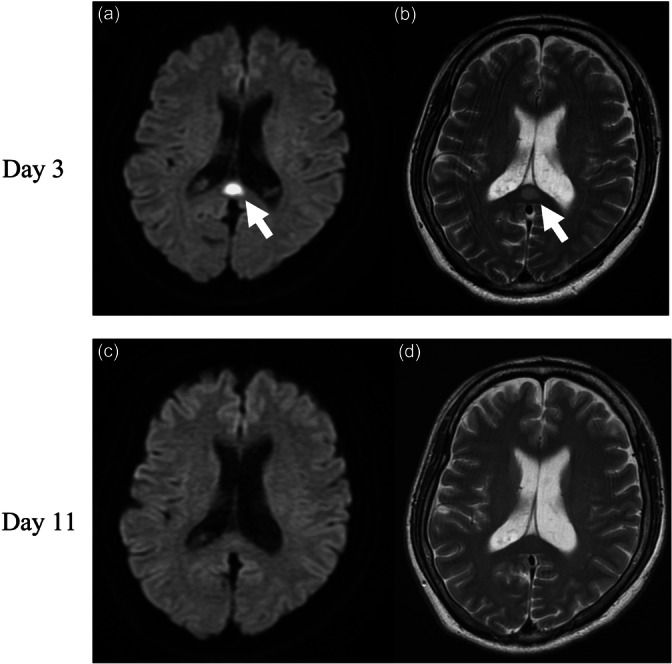
The patient was diagnosed with acute tonsillitis complicated by SIRS and started on intravenous ampicillin‐sulbactam (ABPC/SBT) 3 g every 8 h. On Day 4, he exhibited psychiatric symptoms including visual discomfort, anxiety, and suicidal ideation, accompanied by vivid visual memories when closing his eyes. He expressed a desire to die and attempted to leave the hospital. His impaired visual processing led to disorientation, and he was subsequently found lost in the hospital lobby. On the following day, a neurologist and our team commenced an intervention. The patient reported experiencing anxiety, exhibited signs of tension, and described encountering vivid hallucinations upon closing his eyes. Suspecting encephalitis or meningitis, a cerebrospinal fluid (CSF) examination was performed. The CSF was clear and colorless, with a cell count of 2/μL, protein concentration of 17 mg/dL, and glucose level of 89 mg/dL. MRI revealed hyperintense lesions in the splenium, evident on both diffusion‐weighted (DW) and T2‐weighted (T2W) images, as shown in Figure 2a,b.
Figure 2.
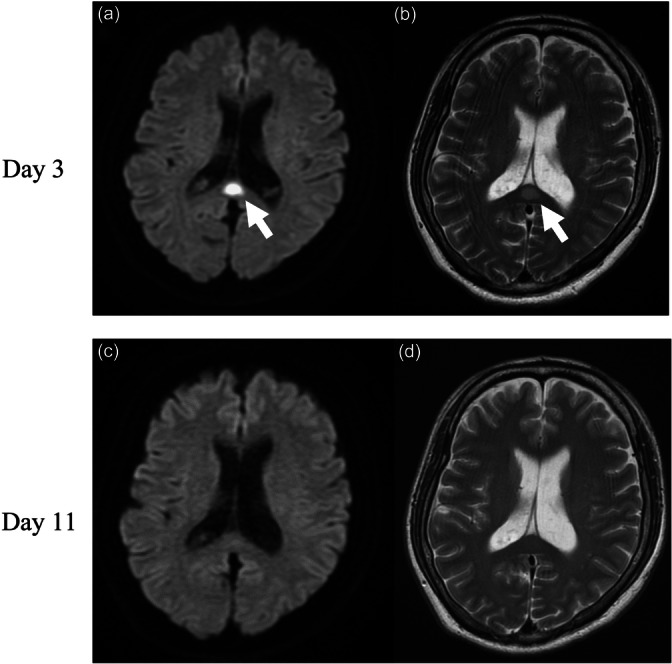
(a) Diffusion‐weighted and (b) T2‐weighted magnetic resonance imaging (MRI) on Day 4. (c) Diffusion‐weighted and (d) T2‐weighted MRI on Day 11. Arrows indicate regions of a high‐intensity splenial region of the corpus callosum.
Given these findings, the patient was suspected of having MERS as seen on MRI. Nonetheless, the diagnosis remained unconfirmed. Considering the potential differential diagnosis of schizophrenia spectrum disorder and delirium, olanzapine was administered, leading to an improvement in the patient's psychiatric symptoms. By Day 11, a follow‐up MRI showed complete resolution of the brain lesions (Figure 2c,d). On Day 12, he was discharged, and the administration of olanzapine was gradually reduced. Olanzapine was terminated on Day 16. At the 6‐month follow‐up, the patient showed no signs of neurological or psychiatric sequalae.
DISCUSSION
In Japan, MERS affects an estimated 80–140 children per year, making it the second most common syndrome of acute encephalopathy in children, following convulsive acute encephalopathy, which affects approximately 130–230 children annually. 5 In contrast, MERS in adults is less common, with fewer reported cases. The etiologies of the disease include infections, metabolic abnormalities, and drug reactions, among others. 6 , 7 Initial reports on MERS predominantly associated the condition with encephalitis and encephalopathy. 2 However, subsequent case studies have expanded the potential causes to include viral, bacterial, and parasitic infections; epilepsy; drug effects; withdrawal from antiepileptic drugs; cancer; cerebrovascular diseases; head trauma; and COVID‐19. 8 , 9 , 10 , 11 , 12 Diagnosis of MERS is based on clinical presentation and MRI findings, particularly lesions in the splenium. Differential diagnosis encompasses a range of conditions, such as ischemic infarction, acute disseminated encephalomyelitis (ADEM), multiple sclerosis (MS), Marchiafava–Bignami disease (MBD), osmotic demyelinating syndrome (ODS), and other diseases causing extensive lesions. 13 These conditions can be excluded based on clinical and MRI findings. Treatment of MERS largely involves supportive and symptomatic care, with antibiotics and antivirals for bacterial and viral infections, respectively. Prognosis is generally favorable with most patients recovering fully within days to weeks after appropriate treatment. 1 However, some patients may experience residual neurological impairments. 14
The current case report illustrates a unique presentation of MERS in a patient triggered by tonsillitis, presenting with severe psychotic symptoms, visual hallucinations with vivid visual memories when closing his eyes, and suicidal ideation that necessitated antipsychotic treatment, yet culminated in complete recovery. There was no relapse of symptoms after medication was terminated. Without the diagnosis of MERS, the patient might have been treated for a schizophrenia spectrum disorder, potentially leading to prolonged psychiatric care and continuous medication due to the risk of relapse or recurrence. Psychiatric manifestations, although uncommon, have been reported in several MERS cases. 4 , 15 , 16 The pathophysiology of MERS is not fully elucidated but is thought to involve transient cytotoxic edema of the corpus callosum due to inflammation. 6 , 7 , 17 , 18 The corpus callosum, crucial for interhemispheric communication and integration of linguistic and visual information, 19 can be disrupted by lesions, potentially resulting in abnormal language and visuospatial information transmission. The vulnerability of the splenium of the corpus callosum might be related to high concentrations of cytokines, glutamate, and aquaporin 4 (AQP4) receptors in neurons and glial cells. 20 Additionally, the dual blood supply to the splenium of the corpus callosum 19 may increase its susceptibility to cytokine‐induced cytotoxic edema. In this case, tonsillitis, a common infection leading to SIRS, 21 may have increased blood–brain barrier permeability, allowing inflammatory mediators into the CNS. 22 Hyponatremia could have predisposed the patient to closed‐eye visual hallucinations, typically associated with alcohol withdrawal and general anesthesia. 23 , 24 , 25 The disruption of information transmission combined with a predisposition for hallucinations might have triggered vivid visual memories upon eye closure. Further research is required to understand these mechanisms and their clinical implications fully.
CONCLUSIONS
This case report underscores the importance of considering MERS in the differential diagnosis of acute encephalopathy, even in adults. Early MRI is crucial for expediting diagnosis and guiding appropriate management. Further research is needed to fully elucidate the pathophysiology of MERS and its potential psychiatric manifestations.
AUTHOR CONTRIBUTIONS
Aki Tsuchida treated the patient and drafted the manuscript. Ken Sawada critically reviewed the draft and revised it. Both authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS APPROVAL STATEMENT
The publication of this case report received approval from the Clinical Research Ethics Committee at the Kochi Health Sciences Center.
PATIENT CONSENT STATEMENT
Informed written consent and a signed release were obtained from the patient for publication of this report and any accompanying images.
CLINICAL TRIAL REGISTRATION
Not applicable as this is a case report.
ACKNOWLEDGMENTS
The authors thank the patient for their participation.
Tsuchida A, Sawada K. Mild encephalitis/encephalopathy with a reversible splenial lesion (MERS). Psychiatry Clin Neurosci Rep. 2024;3:e191. 10.1002/pcn5.191
DATA AVAILABILITY STATEMENT
N/A
REFERENCES
- 1. Yuan J, Yang S, Wang S, Qin W, Yang L, Hu W. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) in adults‐a case report and literature review. BMC Neurol. 2017;17:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tada H, Takanashi J, Barkovich AJ, Oba H, Maeda M, Tsukahara H, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004;63:1854–1858. [DOI] [PubMed] [Google Scholar]
- 3. Le Soudéer L, Truong J, Le Gal J, Escoda S. Shigella‐associated mild encephalitis with reversible splenial lesion in Hospital Center Delafontaine, Saint‐Denis, France: a case report. BMC Pediatr. 2022;22:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karoui M, Bouhlel E, Maatouk O, Labbene E, Ben Mohamed D, Bouaziz M. Adult mild encephalitis with reversible splenial lesion and catatonia: a case report. Heliyon. 2022;8:e10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kasai M, Shibata A, Hoshino A, Maegaki Y, Yamanouchi H, Takanashi J, et al. Epidemiological changes of acute encephalopathy in Japan based on national surveillance for 2014‐2017. Brain Dev. 2020;42:508–514. [DOI] [PubMed] [Google Scholar]
- 6. Ka A, Britton P, Troedson C, Webster R, Procopis P, Ging J, et al. Mild encephalopathy with reversible splenial lesion: an important differential of encephalitis. Eur J Paediatr Neurol. 2015;19:377–382. [DOI] [PubMed] [Google Scholar]
- 7. Li XL, Han J, Yan ZR, Zhang BW, Wang HY. Mild encephalitis/encephalopathy with a reversible splenial lesion associated with respiratory syncytial virus infection in infants. J Neurovirol. 2021;27:638–643. [DOI] [PubMed] [Google Scholar]
- 8. Bulakbasi N, Kocaoglu M, Tayfun C, Ucoz T. Transient splenial lesion of the corpus callosum in clinically mild influenza‐associated encephalitis/encephalopathy. AJNR Am J Neuroradiol. 2006;27:1983–1986. [PMC free article] [PubMed] [Google Scholar]
- 9. Kosami K, Kenzaka T, Sagara Y, Minami K, Matsumura M. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion caused by methicillin‐sensitive Staphylococcus aureus bacteremia with toxic shock syndrome: a case report. BMC Infect Dis. 2016;16:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mawatari M, Kobayashi T, Yamamoto S, Takeshita N, Hayakawa K, Kutsuna S, et al. Mild encephalitis/encephalopathy with a reversible splenial lesion due to Plasmodium falciparum malaria: a case report. Trop Med Health. 2018;46:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi B, Li J, Jiang J, Li M, Zhang J, Shang X. Mild encephalitis/encephalopathy with a reversible splenial lesion secondary to encephalitis complicated by hyponatremia: a case report and literature review. Medicine. 2019;98:e17982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayashi M, Sahashi Y, Baba Y, Okura H, Shimohata T. COVID‐19‐associated mild encephalitis/encephalopathy with a reversible splenial lesion. J Neurol Sci. 2020;415:116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pan JJ, Zhao Y, Lu C, Hu Y, Yang Y. Mild encephalitis/encephalopathy with a reversible splenial lesion: five cases and a literature review. Neurol Sci. 2015;36:2043–2051. [DOI] [PubMed] [Google Scholar]
- 14. Takanashi J, Barkovich AJ, Shiihara T, Tada H, Kawatani M, Tsukahara H, et al. Widening spectrum of a reversible splenial lesion with transiently reduced diffusion. AJNR Am J Neuroradiol. 2006;27:836–838. [PMC free article] [PubMed] [Google Scholar]
- 15. Bellani M, Zanette G, Zovetti N, Barillari M, Del Piccolo L, Brambilla P. Adult mild encephalitis with reversible splenial lesion associated with delirious mania: a case report. Front Psychiatry. 2020;11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Udaya SC, Chauhan BN, Philip VJ. Bright splenium of a psychotic mind. Ann Indian Acad Neurol. 2015;18:80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aksu Uzunhan T, Maraş Genç H, Kutlubay B, Kalın S, Bektaş G, Yapıcı Ö, et al. Cytotoxic lesions of the corpus callosum in children: etiology, clinical and radiological features, and prognosis. Brain Dev. 2021;43:919–930. [DOI] [PubMed] [Google Scholar]
- 18. Starkey J, Kobayashi N, Numaguchi Y, Moritani T. Cytotoxic lesions of the corpus callosum that show restricted diffusion: mechanisms, causes, and manifestations. Radiographics. 2017;37:562–576. [DOI] [PubMed] [Google Scholar]
- 19. Knyazeva MG. Splenium of corpus callosum: patterns of interhemispheric interaction in children and adults. Neural Plast. 2013;2013:639430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tetsuka S. Reversible lesion in the splenium of the corpus callosum. Brain Behav. 2019;9:e01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. [DOI] [PubMed] [Google Scholar]
- 22. Banks WA. Physiology and pathology of the blood‐brain barrier: implications for microbial pathogenesis, drug delivery and neurodegenerative disorders. J Neurovirol. 1999;5:538–555. [DOI] [PubMed] [Google Scholar]
- 23. Peck T, Mercogliano C, York E. Closed‐eye visualizations in the setting of hyponatremia. Case Rep Med. 2018;2018:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Desai S, Toma AE, Sunik A. Closed‐eye visual hallucinations preceding severe alcohol withdrawal. Cureus. 2021;13:e17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fisher CM. Visual hallucinations and racing thoughts on eye closure after minor surgery. Arch Neurol. 1991;48:1091–1092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A


