Abstract
There are many neuroimaging studies of mild behavioral impairment (MBI), but the results have been somewhat inconsistent. Moreover, it remains unclear whether MBI is a risk factor or prodromal symptom of dementia. Therefore, a systematic review was conducted to summarize the results of neuroimaging studies of MBI and consider whether MBI is a prodromal symptom of dementia in terms of its neural correlates. A systematic review supported by a JSPS Grant‐in‐Aid for Scientific Research (C) was conducted using MBI neuroimaging studies identified using PubMed, PsycINFO, CINAHL, and Google Scholar on November 1, 2022. The inclusion criteria were (i) neuroimaging study; (ii) research on human subjects; (iii) papers written in English; and (iv) not a case study, review, book, comments, or abstract only. Joanna Briggs Institute critical appraisal checklists were used to assess the quality of selected studies, and 23 structural and functional imaging studies were ultimately included in the systematic review. The structural studies suggested an association of MBI with atrophy in the hippocampus, parahippocampal gyrus, entorhinal cortex, and temporal lobe, whereas the functional studies indicated involvement of an altered default mode network, frontoparietal control network, and salience network in MBI. A limitation in many studies was the use of region‐of‐interest analysis. The brain areas detected as neural correlates of MBI are considered to be alterations in the early stage of each dementia. Therefore, MBI may emerge against a background of pathological changes in dementia.
Keywords: default mode network, frontoparietal control network, medial temporal lobe, mild behavioral impairment, salience network
The brain areas detected as neural correlates of MBI are considered to be alterations in the early stage of each dementia. Therefore, MBI may emerge against a background of pathological changes in dementia. Each brain region may also be linked to conversion from MBI to each dementia.
INTRODUCTION
The concept of mild behavioral impairment (MBI) was originally proposed as an early symptom of frontotemporal dementia (FTD), 1 , 2 based on: (i) presence of persistent behavioral changes and mild psychiatric symptoms, especially disinhibition; (ii) no severe memory impairment; (iii) no difficulty in activities of daily living; and (iv) no dementia. 1 , 2 However, behavioral changes and psychiatric symptoms also appear in dementia other than FTD, and this has led to modification of the concept of MBI. 3 Thus, the new criteria for MBI include behavior or personality changes that (i) begin after age 50 and persist for at least 6 months; (ii) cause minimal disability, at least in interpersonal relationships, social functions other than interpersonal relationships, and ability to work in the workspace; (iii) cannot be explained by other diseases; and (iv) do not meet diagnostic criteria for dementia. 3
Symptoms of MBI are divided into five domains: decreased motivation, affective dysregulation, impulse dyscontrol, social inappropriateness, and abnormal perception or thought content. On the behavioral disturbance axis, MBI is a predementia stage, and the behavioral disturbance that occurs after onset of dementia is defined as behavioral and psychological symptoms of dementia (BPSD). 4 On the cognitive impairment axis, mild cognitive impairment (MCI) and subjective cognitive decline (SCD) are in the predementia stage, 4 and MBI and MCI or SCD can coexist. 3 , 4 A meta‐analysis found a prevalence of MBI of 33.5% in the community and in memory and psychiatric clinics, with increased prevalence with progression of cognitive impairment: normal cognition (NC) 17.0%, SCD 35.8%, and MCI 45.5%. 5
In a meta‐analysis of the five MBI domains, the prevalence of affective dysregulation was highest (32.84%), followed by impulse dyscontrol (26.67%), decreased motivation (12.58%), social inappropriateness (6.05%), and abnormal perception or thought content (2.81%). 6 MBI increases the risk of dementia 7 , 8 and the annual rates of conversion are 14.7% from MBI to dementia and 2.5% from MBI to NC. 9 Since the rates in MCI without neuropsychiatric symptoms are 8.3% and 5.3%, MBI carries a greater risk of dementia than MCI without neuropsychiatric symptoms. 9 MBI can convert to FTD, and to dementia with Lewy bodies (DLB), vascular dementia (VaD), and Alzheimer's disease (AD). 10 The conversion rate to dementia ranges from 27% to 70% over a 2‐ to 15‐year period, and the types of dementia include FTD, AD, and DLB. 8 , 11 , 12 , 13 Two studies found mainly FTD, 11 , 12 while AD was dominant in other studies. 8 , 13 Interestingly, Rouse found that the hazard ratio (HR) for conversion to FTD (10.787) was higher than that for conversion to AD (3.179) and DLB (2.518) in NC with MBI. 13 In MCI with MBI, the HR for conversion to DLB (3.023) was the highest, followed by those for conversion to FTD (2.595) and AD (1.770). 13
Factors related to MBI include AD biomarkers, 14 , 15 , 16 , 17 , 18 , 19 , 20 brain‐derived neurotrophic factor (BDNF) Val66Met polymorphism, 21 cognitive impairment, 22 , 23 , 24 , 25 dual‐task gait, 26 activities of daily living, 25 frailty, 27 , 28 diabetes mellitus, 29 low vitamin D, 30 high serum triglyceride, 30 hearing impairment, 31 and male gender. 28 , 31 , 32 , 33 Many neuroimaging studies of MBI have also been performed, but the results are inconsistent. Moreover, it is unclear whether MBI is a risk factor or prodromal symptom of dementia. Therefore, a systematic review of neuroimaging studies of MBI was conducted to examine these issues in terms of neural correlates.
METHODS
Literature search
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 statement. 34 A search using PubMed, PsycINFO, and CINAHL was performed on November 1, 2022. The search item was (“mild behavioral impairment” OR “mild behavioural impairment”). We also searched in Google Scholar to identify studies not found in these databases. In the Google Scholar search, the term allintitle; “mild behavioral impairment” or allintitle; “mild behavioural impairment” was used, and patents and quotes were excluded. The inclusion criteria were (i) neuroimaging study; (ii) research on human subjects; (iii) papers written in English; and (iv) not a case study, review, book, comment or abstract only. The searches were performed separately by two authors (T.M. and A.I.). If the search results were different, the two authors discussed the findings and reached a final conclusion. The PRISMA flow diagram is shown in Figure 1. The protocol of this systematic review was not registered because of the short study period. The Joanna Briggs Institute critical appraisal checklists for cross‐sectional studies, cohort studies, and case control studies 35 were used to assess the quality of selected studies (Supporting Information: Tables 1, 2, and 3). This quality assessment was also performed separately by T.M. and A.I., with a subsequent discussion used to reach a final conclusion.
Figure 1.
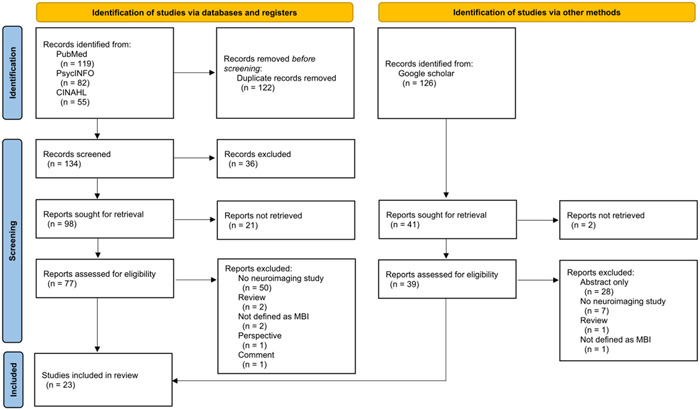
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 flow diagram for systematic reviews, including searches of databases, registers and other sources. MBI, mild behavioral impairment.
RESULTS
Characteristics of selected studies
The systematic review of the literature identified 23 studies that met the defined criteria (Table 1). Most of these studies targeted NC, SCD, or MCI, 13 , 14 , 15 , 17 , 37 , 40 , 41 , 42 , 43 , 44 , 46 , 47 while some focused on MBI 2 , 11 , 12 or Parkinson's disease (PD). 36 , 38 , 49 , 50 , 51 Three studies also included patients with AD. 39 , 45 , 48 MBI was assessed using the MBI‐Checklist 52 in most studies. 14 , 17 , 36 , 38 , 40 , 41 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 51 Three studies used MBI criteria. 2 , 11 , 12 The Neuropsychiatric Inventory (NPI) or NPI‐Questionnaire (NPI‐Q) was used in six studies, 13 , 15 , 37 , 39 , 42 , 50 in which a formula to convert the NPI score to a MBI score 53 was used. The NPI assesses neuropsychiatric symptoms for 1 month, which is a disadvantage in use of the NPI for assessment of MBI. Three studies overcame this problem by conducting NPI at two time points at least 6 months apart, 13 , 37 , 42 and one study used 6 months as a reference range for NPI. 50 The quality of all the studies was high (Supporting Information: Tables 1, 2, and 3), but some 2 , 11 , 46 did not deal with confounding factors in neuroimaging analysis.
Table 1.
Neuroimaging studies of MBI.
| Author | Participants (N) | Age (years) | Sex, male/female | Assessment of MBI | Imaging type | Image analysis | Covariates | Main findings |
|---|---|---|---|---|---|---|---|---|
| Taragano et al. 2 | MBI without complaint of cognitive impairment by patient or caregiver (119) | 72.9 ± 8.9 | 56/63 | MBI criteria (Taragano et al.) 2 | MRI or CT | ROI analysis | None | Prevalences of general atrophy, focal atrophy, and leukoaraiosis did not differ significantly in MBI and MCI. |
| MCI (239) | 72.3 ± 7.8 | 98/141 | SPECT | Prevalence of decreased perfusion in frontal or temporal lobe greater in MBI than in MCI. | ||||
| Taragano et al. 11 | MBI without complaint of cognitive impairment by patient or caregiver (96) | 71.9 ± 8.9 | 44/52 | MBI criteria (Taragano et al.) 11 | MRI or CT | ROI analysis | None | Prevalences of general atrophy, focal atrophy, and leukoaraiosis did not differ significantly in MBI and MCI. |
| MCI (87) | 71.1 ± 7.7 | 34/53 | ||||||
| Yoon et al. 36 | PD‐MBI (20) | 71.3 ± 6.5 | 15/5 | MBI‐C | MRI | Vertex‐by‐ vertex analysis | Age | Thickness and volume of right middle temporal cortex significantly lower in PD‐MBI than in PD‐noMBI. |
| PD‐noMBI (40) | 70.2 ± 6.2 | 27/13 | Education | |||||
| NC (29) | 68.7 ± 5.9 | 14/15 | UPDRS‐III | Volume of right middle temporal cortex negatively correlated with MBI total score in PD. | ||||
| TIV | Thickness of left parahippocampal cortex, volume and surface area of right precuneus, and volume of right lingual cortex and lateral frontal pole significantly lower in PD‐MBI than in NC. | |||||||
| Gill et al. 37 | NC (102) | Median: 75.0, IQR: 71.0–80.0 | 52/50 | NPI‐Q | MRI | ROI analysis | Age | MBI total score and left hippocampus volume at baseline required in optimal machine learning binary classification (NC vs. MCI/AD at final diagnosis) |
| MCI (238) | Median: 74.0, IQR: 70.0–80.0 | 154/84 | Sex | |||||
| Education | MBI total score, MBI impulse dyscontrol score, MBI affective dysregulation score, left hippocampus volume, cortical thickness and volume of left entorhinal cortex, and cortical thickness of left middle temporal gyrus at baseline required in optimal machine learning three‐class model (NC vs. MCI vs. AD at final diagnosis). | |||||||
| Lang et al. 38 | PD‐MBI (21) | 71.8 ± 6.4 | 15/6 | MBI‐C | fMRI | Atlas‐based analysis | Education | FC between striatum and DMN significantly lower in PD‐MBI than in PD‐noMBI and NC. |
| PD‐noMBI (53) | 70.4 ± 5.8 | 34/19 | MRI | Seed‐based analysis | UPDRS‐III | |||
| NC (28) | 69.8 ± 6.7 | 13/15 | MoCA | FC between striatum and SAN significantly lower in PD‐MBI than in PD‐noMBI and NC. | ||||
| Average thickness of SAN significantly lower in PD‐MBI than in PD‐noMBI and NC. | ||||||||
| FC of left caudate head with dorsal anterior cingulate cortex and left middle frontal gyrus, FC of left dorsal putamen with left inferior temporal gyrus, and FC of right caudate head with precuneus, superior occipital cortex, dorsal anterior cingulate, left supramarginal gyrus, left angular gyrus, and right precentral gyrus negatively correlated with MBI‐C total score. | ||||||||
| FC of right caudate head with left posterior hippocampal gyrus and right cerebellum positively correlated with MBI‐C total score. | ||||||||
| Lussier et al. 14 | NC (96) | 71.5 ± 6.0 | 38/58 | MBI‐C | MRI | VBM | Age | Amyloid PET SUVR in left frontal cortex, left posterior cingulate cortex, left caudate nucleus, and left thalamus significantly positively correlated with MBI‐C total score. |
| Amyloid PET | Sex | |||||||
| Education | ||||||||
| Tau PET | APOE ε4 status | Tau PET SUVR and GM volume not correlated with MBI‐C total score. | ||||||
| Orso et al. 12 | Group AMBI‐dementia nonconverters (38) | 65.4 ± 7.9 | 22/16 | MBI criteria (Ismail et al. 3 Taragano et al. 11 ) | MRI | ROI analysis | Age | In group A, atrophy of frontal lobe significantly greater in MBI‐dementia converters than in nonconverters. |
| 64.5 ± 6.3 | 11/7 | Sex | ||||||
| 66.6 ± 6.4 | 23/21 | Education | ||||||
| and converters (18)Group BMBI‐dementia nonconverters (44) and converters (13) | 65.3 ± 6.2 | 7/6 | MMSE | In Group B, proportion of cases with frontal atrophy significantly higher in MBI‐dementia converters (10/3) than in nonconverters (3/41). | ||||
| NPI | ||||||||
| Gill et al. 39 | NC (70) | 73.3 ± 6.7 | 111/92 | NPI‐Q | MRI | ROI analysis | Age | FA in fornix and superior fronto‐occipital fasciculus lower in cases with MBI impulse dyscontrol symptoms. |
| MCI (95) | Sex | |||||||
| AD (38) | Education | MD, AxD, and RD in fornix, AxD in cingulum, and RD in superior fronto‐occipital fasciculus higher in cases with MBI impulse dyscontrol symptoms. | ||||||
| Diagnosis | ||||||||
| Smaller cortical thickness and greater surface area in the parahippocampal gyrus associated with MBI impulse dyscontrol symptoms. | ||||||||
| Matsuoka et al. 40 | NC (30) | 76.1 ± 5.7 | 12/18 | MBI‐C | fMRI | ROI‐to‐ROI analysis | Age | FC of left posterior parietal cortex with right middle frontal gyrus negatively correlated with MBI‐C total score. |
| MCI (13) | 78.8 ± 5.4 | 8/5 | MRI | Independent component analysis | Sex | Reduced voxel‐based FC in left frontal pole and superior frontal gyrus linked to higher MBI‐C total score. | ||
| Education | ||||||||
| MMSE score | ||||||||
| VBM analysis | TIV | |||||||
| Similar results for MBI‐C affective dysregulation score. | ||||||||
| Volume of left superior temporal gyrus negatively correlated with MBI‐C impulse dyscontrol score. | ||||||||
| Reduced voxel‐based FC in left superior frontal gyrus, left frontal pole, and right cerebellum, and increased voxel‐based FC in bilateral precentral gyri linked to higher MBI‐C impulse dyscontrol score. | ||||||||
| Matuskova et al. 41 | SCD (37) | 66.4 ± 6.7 | 15/22 | MBI‐C | MRI | ROI analysis | Age | Thickness in entorhinal cortex negatively correlated with MBI‐C total score and MBI‐C impulse dyscontrol score. |
| MCI (79) | 71.1 ± 8.4 | 44/35 | Sex | |||||
| Education | Hippocampus volume negatively correlated with MBI‐C impulse dyscontrol score and MBI‐C decreased motivation score. | |||||||
| Miao et al. 42 | MCI (768) | 72.8 ± 8.0 | 325/443 | NPI | MRI | WMH volume measured by automated segmentation algorithm | Age | MBI associated with higher WMH volume. |
| Sex | MBI emotional dysregulation, impulse dyscontrol, and apathy domains tended to be associated with higher WMH volume. | |||||||
| Education | ||||||||
| TIV | ||||||||
| MMSE score | ||||||||
| Rouse. 13 | NC with MBI (150) | 69.5 ± 8.5 | 58/92 | NPI‐Q | MRI | ROI analysis | Age | Reduced parietal lobe volume linked to impairment of attention in MBI. |
| NC without MBI (1044) | 69.4 ± 9.8 | 376/668 | Sex | |||||
| 73.5 ± 8.2 | 126/75 | Race | Volume of rostral anterior cingulate cortex associated with performance on attention, with strongest relationship in NC with MBI. | |||||
| MCI with MBI (201) | 74.2 ± 7.9 | 118/119 | Education | |||||
| MCI without MBI (237) | TIV | |||||||
| Cognitive status | Atrophy in total brain gray matter, temporal lobe, occipital lobe, hippocampus, parahippocampal gyrus, and entorhinal cortex associated with episodic memory impairment in MBI. | |||||||
| Volume reduction in total brain gray matter, total cerebrum gray matter, temporal lobe, and rostral anterior cingulate cortex involved in impairment of language in MBI. | ||||||||
| Reduced volume of total brain gray matter, parietal lobe, temporal lobe, and occipital lobe associated with visuospatial deficits in MBI. | ||||||||
| Greater volume of temporal lobe, hippocampus, and parahippocampal gyrus linked to worse processing speed in MBI. | ||||||||
| Shu et al. 43 | NC with MBI (32) | 67.3 ± 6.6 | 12/20 | MBI‐C | MRI | Regional network analysis | Age | Nodal betweenness centrality in left middle frontal gyrus, right opercular part of inferior frontal gyrus, and left Heschl gyrus lower in MBI than in NC. |
| NC without MBI (38) | 66.3 ± 7.3 | 20/18 | Sex | |||||
| Education | ||||||||
| Network hub analysis | Mean overall GM volume | Nodal betweenness centrality in left gyrus rectus, right insula, bilateral precuneus, and left thalamus greater in MBI than in NC. | ||||||
| Right orbital part of superior frontal gyrus, left middle frontal gyrus, right Rolandic operculum, left gyrus rectus, right insula, left anterior cingulate and paracingulate gyri, right postcentral gyrus, right superior parietal gyrus, bilateral precuneus, and right paracentral lobule form network hub in MBI. | ||||||||
| Shu et al. 44 | NC with MBI (16) | 67.3 ± 6.7 | 6/10 | MBI‐C | MRI | VBM | Age | Volume of left brainstem, right temporal transverse gyrus, left superior temporal gyrus, left inferior temporal gyrus, left middle temporal gyrus, right occipital pole, right thalamus, left precentral gyrus, and left middle frontal gyrus significantly lower in MBI than in NC. |
| NC without MBI (18) | 66.7 ± 7.2 | 10/8 | Sex | |||||
| Education | ||||||||
| TIV | ||||||||
| Volume of left postcentral gyrus, right exterior cerebellum, and left superior frontal gyrus correlated with MBI‐C total score. | ||||||||
| Cassidy et al. 45 | NC (118) | 72.3 ± 5.7 | 36/82 | MBI‐C | Neuromelanin‐sensitive MRI | ROI analysis | Age | Middle caudal locus coeruleus signal predicted severity of MBI, especially MBI impulse dyscontrol, in tau‐positive cases. |
| MCI (44) | 73.2 ± 5.4 | 21/23 | Sex | |||||
| AD (28) | 67.4 ± 8.9 | 12/16 | MRI | Tau burden in temporal lobe | ||||
| Tau PET | Cortical amyloid burden | |||||||
| Amyloid PET | Cortical gray matter volume | |||||||
| CDR | ||||||||
| Stella et al. 46 | NC (15) | 72.3 ± 8.5 | 5/10 | MBI‐C | MRI | ROI analysis | None | MBI‐C total score not associated with periventricular hyperintensity on Fazekas scale. |
| aMCI (21) | 74.5 ± 7.2 | 7/14 | FDG‐PET | |||||
| mdMCI (44) | 74.3 ± 6.8 | 10/34 | MBI not related to medial temporal atrophy, global cerebral atrophy, frontal atrophy, posterior atrophy, and FDG‐PET hypometabolism. | |||||
| Yang et al. 47 | NC with low WMH (35) | 65.8 ± 7.8 | 14/21 | MBI‐C | MRI | VBM and SBM analysis | Age | Cases with high WMH had higher MBI‐C total score, and decreased motivation, affective dysregulation and impulse dyscontrol scores compared to people with low WMH. |
| Sex | ||||||||
| NC with high WMH (25) | 74.6 ± 6.7 | 15/10 | Education | |||||
| TIV | ||||||||
| In cases with high WMH and high MBI‐C total score (>8), gray matter volume in left anterior insula and left thalamus proper, and thickness of right posterior cingulate were negatively correlated with MBI‐C total score. These brain alterations partially mediated the association of WMH volume with MBI‐C total score. | ||||||||
| Lussier. 48 | NC (173) | 70.8 ± 7.7 | 59/114 | MBI‐C | Amyloid PET | ROI analysis | None | Cases with amyloid burden had significantly higher MBI‐C total scores. |
| MCI (77) | 71.1 ± 7.2 | 35/42 | ||||||
| AD (63) | 68.2 ± 9.1 | 28/35 | Tau PET | Cases with higher Braak stage had higher MBI‐C total scores. | ||||
| Cognitively impaired people (91) | 71.0 ± 7.2 | 44/47 | MBI‐C | Amyloid PET | ROI analysis | Age | Neocortical amyloid SUVR significantly positively correlated with MBI‐C decreased motivation score. | |
| VBM analysis | Sex | |||||||
| Tau PET | Education | |||||||
| MRI | APOEε4 status | Tau SUVR in ROIs associated with Braak I–II, III–IV, and V–VI significantly positively correlated with MBI‐C total score and MBI‐C decreased motivation score. | ||||||
| Amyloid SUVR in left putamen and bilateral lingual gyri significantly positively correlated with MBI‐C decreased motivation score. | ||||||||
| Tau SUVR in bilateral orbitofrontal cortex, posterior cingulate, precuneus, cuneus, and superior temporal lobes significantly positively correlated with MBI‐C total score. | ||||||||
| Tau SUVR in bilateral cuneus and temporal lobes significantly positively correlated with MBI‐C decreased motivation score. | ||||||||
| Tau SUVR in right cuneus and temporal lobe significantly positively correlated withMBI‐C affective dysregulation score. | ||||||||
| Tau SUVR in orbitofrontal cortex and right parietal lobe significantly positively correlated with MBI‐C impulse dyscontrol score. | ||||||||
| Tau SUVR in orbitofrontal cortex, posterior cingulate, and precuneus significantly positively correlated with MBI‐C social inappropriateness score. | ||||||||
| Tau SUVR in orbitofrontal cortex and right superior temporal lobe significantly positively correlated with MBI‐C abnormal perception or thought content score. | ||||||||
| Gray matter density in bilateral precuneus significantly negatively correlated with MBI‐C total score. | ||||||||
| Gray matter density in bilateral posterior cingulate and precuneus significantly negatively correlated with MBI‐C decreased motivation score. | ||||||||
| NC (94) | 71.2 ± 6.2 | 36/58 | MBI‐C | Amyloid PET | ROI analysis | Age | Cases with amyloid burden had higher rate of annual change in MBI‐C total score than those without amyloid burden. | |
| MCI (35) | 72.0 ± 6.1 | 16/19 | Tau PET | VBM analysis | Sex | |||
| AD (22) | 70.1 ± 7.8 | 10/12 | Education | |||||
| APOEε4 status | Braak V–VI cases had significantly higher rate of annual change in MBI‐C total score than those in Braak 0, I–II, and III–IV stages | |||||||
| Diagnostic category | Neocortical amyloid SUVR significantly positively correlated with annual change in MBI‐C total score. | |||||||
| Tau SUVR in bilateral precuneus, bilateral temporal lobes, right anterior and posterior cingulate, and dorsal medial frontal cortex significantly positively correlated with annual change in MBI‐C total score. | ||||||||
| Lang et al. 49 | Non‐demented PD (74) | 70.8 ± 6.0 | 49/25 | MBI‐C | fMRI | Commonality analysis | Age | FC between caudate and dorsal anterior cingulate cortex, between caudate and right DLPFC, and between right DLPFC and right inferior parietal lobe found in MBI. |
| Sex | ||||||||
| Levodopa equivalent dose | ||||||||
| Yoo et al. 50 | PD‐MBI (89) | 68.7 ± 8.3 | 41/48 | NPI | [18F] N‐3‐fluoropropyl‐2β‐carbomethoxy‐3β‐(4‐iodophenyl) Nortropane PET | ROI analysis | Age | Lower DAT availability in anterior caudate and anterior putamen linked to MBI. |
| PD‐noMBI (186) | 66.3 ± 8.9 | 97/89 | Sex | |||||
| Education | ||||||||
| Sun et al. 15 | NC with MBI (89) | 71.7 ± 6.1 | 37/52 | NPI‐Q | Amyloid PET | ROI analysis | Age | In the cross‐sectional study, greater amyloid PET SUVR and lower FDG‐PET linked to severer MBI. |
| NC without MBI (497) | 72.2 ± 6.3 | 218/279 | Sex | |||||
| MCI with MBI (296) | 71.7 ± 7.6 | 186/110 | Tau PET | Education | ||||
| MCI without MBI (247) | 72.5 ± 7.2 | 126/121 | FDG‐PET | Diagnosis | Amyloid burden mediated the relationship of MBI with impairment of global cognition, memory, executive function, and language, while tau burden and decreased FDG‐PET did not mediate this relationship. | |||
| APOE4 status | In the longitudinal study, higher MBI total scores calculated by NPI‐Q predicted greater amyloid burden in total samples. | |||||||
| Higher MBI total score associated with faster amyloid burden elevation in MCI and NC. | ||||||||
| MBI did not predict tau burden or decreased FDG‐PET. | ||||||||
| Yoon et al. 51 | PD‐MBI (21) | 70.9 ± 6.6 | 16/5 | MBI‐C | fMRI | Whole brain | Age | Activation in right dorsolateral PFC and inferior parietal lobule significantly lower in PD‐MBI than in NC and PD‐noMBI when planning the set‐shift. |
| PD‐noMBI (38) | 69.9 ± 6.3 | 23/15 | analysis | UPDRS‐III | ||||
| NC (26) | 68.6 ± 6.1 | 10/16 | ROI analysis | Levodopa equivalent daily dose | ||||
| Activation in left lateral frontopolar area significantly lower in PD‐MBI than in NC when executing the set‐shift. | ||||||||
| In ROI analysis, activation in hippocampus during planning the set‐shift significantly positively correlated with MBI‐C total score in all PD cases. | ||||||||
| Johansson et al. 17 | Amyloid positive cognitively unimpaired people (50) | 72.3 ± 9.7 | 25/25 | MBI‐C | tau‐PET | ROI analysis | Age | Higher tau PET SUVR in entorhinal cortex and hippocampus associated with higher MBI‐C total score. |
| Whole‐brain voxel‐ | Sex | |||||||
| Education | ||||||||
| based analysis | White matter lesions volume | Same result for MBI‐C affective dysregulation and impulse dyscontrol scores. | ||||||
| In whole‐brain voxel‐based analysis, tau PET SUVR in entorhinal cortex, hippocampus, and anterior fusiform gyrus significantly positively correlated with MBI‐C total score. |
Abbreviations; AD, Alzheimer's disease; aMCI, amnestic mild cognitive impairment; APOE, apolipoprotein E; AxD, axial diffusivity; CT, computed tomography; DAT, dopamine transporter; CDR, clinical dementia rating; DLPFC, dorsolateral prefrontal cortex; DMN, default mode network; FA, fractional anisotropy; FC, functional connectivity; FDG, 18F‐fluorodeoxyglucose; fMRI, functional magnetic resonance imaging; GM, gray matter; IQR, interquartile range; MBI, mild behavioral impairment; MBI‐C, mild behavioral impairment checklist; MCI, mild cognitive impairment; MD, mean diffusivity; md MCI, multiple‐domain amnestic mild cognitive impairment; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging; NC, normal cognition; NPI‐Q, Neuropsychiatric Inventory‐Questionnaire; PD, Parkinson's disease; PET, positron emission tomography; PFC, prefrontal cortex; RD, radial diffusivity; ROI, region of interest; SAN, salience network; SBM, surface‐based morphometry; SCD, subjective cognitive decline; SPECT, single photon emission computed tomography; SUVR, standardized uptake value ratio; TIV, total intracranial volume; UPDRS, Unified Parkinson's Disease Rating Scale; VBM, voxel based morphometry; WMH, white matter hyperintensity.
Structural imaging
Magnetic resonance imaging (MRI) was performed in 18 studies 2 , 11 , 12 , 13 , 14 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 and suggested associations of MBI with atrophy in the temporal lobe, 13 , 36 , 40 , 43 , 44 hippocampus, 13 , 41 parahippocampal gyrus, 13 , 36 , 39 and entorhinal cortex. 13 , 41 Atrophy in brain regions related to the salience network (SAN) was associated with MBI in PD. 38 Use of regional network analysis and network hub analysis showed that MBI was linked to brain regions including the left middle frontal gyrus, bilateral precuneus, and right insula, which are associated with the frontoparietal control network (FPCN), default mode network (DMN), and SAN. 43 The left middle frontal gyrus, 44 precuneus, 36 , 48 and left insula 47 have also been associated with MBI, and three studies 43 , 44 , 47 reported involvement of the thalamus in MBI.
In contrast, some studies did not identify a relationship between MBI and brain atrophy, 2 , 11 , 14 , 46 and one using diffusion tensor imaging suggested an association between altered white matter integrity, including the fornix, superior fronto‐occipital fasciculus, and cingulum, and the MBI impulse dyscontrol domain. 39 Results on involvement of vascular lesions in MBI have been inconsistent, with two studies finding no significant difference in the prevalence of leukoaraiosis between MBI and MCI. 2 , 11 Periventricular hyperintensity assessed by the Fazekas score may not be linked to MBI, 46 but an assessment using image analysis software revealed a relationship of MBI with white matter hyperintensity (WMH) volume. 42 WMH has been found to show a tendency to be involved in the MBI affective dysregulation, impulse dyscontrol, and apathy domains, 42 and high WMH assessed by the Fazekas scale and a lesion segmentation tool has been associated with the MBI‐C total score and scores for decreased motivation, affective dysregulation, and impulse dyscontrol. 47 Atrophy in the left anterior insula, left thalamus proper, and right posterior cingulate partially mediated the relationship between WMH volume and MBI‐C total score in people with NC with high WMH and MBI. 47
Studies of conversion from MBI to dementia using MRI 12 , 37 have identified MBI total score and left hippocampal volume as predictors of conversion to MCI or AD using machine learning, 37 and involvement of frontal lobe atrophy in conversion from MBI to dementia, especially behavioral variant FTD (bvFTD). 12 An examination of the relationship between gray matter volume reduction and cognitive impairment in MBI 13 suggested involvement of the temporal lobe, hippocampus, and parahippocampal gyrus in episodic memory and processing speed in MBI. The temporal lobe was also associated with language and visuospatial function in MBI, and relationships were found for the parietal lobe with attention and visuospatial function, the rostral anterior cingulate cortex with attention and language, and the occipital lobe with episodic memory and visuospatial function. 13 A study using neuromelanin‐sensitive MRI found an association between the middle caudal locus coeruleus signal and MBI, especially impulse dyscontrol in tau‐positive participants. 45
Functional imaging
Functional imaging using single photon emission computed tomography (SPECT), 18F‐fluorodeoxyglucose (FDG)‐positron emission tomography (PET), functional MRI (fMRI), and dopamine transporter (DAT) PET was reviewed in eight studies. 2 , 15 , 38 , 40 , 46 , 49 , 50 , 51 The findings indicated an association of reduced functional connectivity (FC) between the striatum and DMN, 38 SAN, 38 , 49 and FPCN 49 with MBI in PD; a link of reduced FC in the FPCN with MBI, and especially the MBI affective dysregulation domain 40 and performance of a set‐shifting task in PD‐MBI 51 ; and reduced deactivation in the hippocampus in PD‐MBI. 51 DAT PET showed a relationship between reduced DAT in the anterior caudate and putamen and MBI. 50 In conflicting results, MBI has been related to decreased perfusion in the frontal or temporal lobe 2 and FDG‐PET hypometabolism, 15 but also found to not be associated with FDG‐PET hypometabolism. 46
Other neuroimaging studies
Several studies have used amyloid PET and/or tau PET. 14 , 15 , 17 , 48 Amyloid deposits in the left frontal lobe, posterior cingulate, caudate, and thalamus in people with NC have been suggested to be associated with MBI, whereas tau deposits and atrophy did not show this association. 14 In contrast, in amyloid‐positive NC, tau deposits in the entorhinal cortex and hippocampus were correlated with MBI, especially the MBI affective dysregulation and impulse dyscontrol domains. 17 In people with cognitive impairment (mean Mini‐Mental State Examination score 24.0 ± 6.4; Clinical Dementia Rating global score: 0.76 ± 0.46), the neocortical amyloid burden was positively related to the MBI‐C decreased motivation score, and tau burden in regions related to Braak Stages I–VI were significantly positively correlated with MBI‐C total scores and MBI‐C decreased motivation scores. 48 Moreover, in people with NC, MCI, and AD, amyloid and tau deposits in the brain were linked to a higher MBI‐C total score and the annual change in MBI‐C total score. 48 The amyloid burden has been suggested to mediate the relationship between MBI and cognitive impairment of global cognitive function, memory, executive function, and language. 15 MBI predicted a greater amyloid burden in this longitudinal study, 15 but did not predict tau burden or decreased FDG‐PET.
DISCUSSION
This review included 23 neuroimaging studies of the neural correlates of MBI. Structural imaging suggested an association of MBI with atrophy, mainly in the hippocampus, parahippocampal gyrus, entorhinal cortex, and temporal lobe (Figure 2); while functional imaging indicated that MBI involves alterations in the DMN, FPCN, and SAN (Figure 3).
Figure 2.
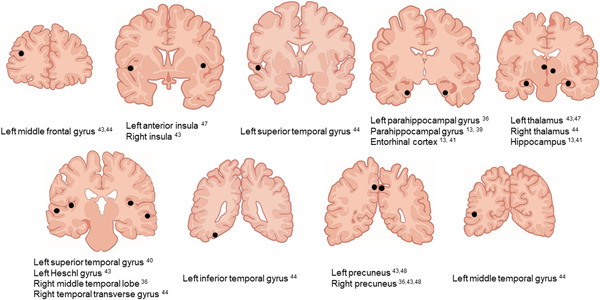
Main brain regions with a detected association with mild behavioral impairment in studies using structural neuroimaging.
Figure 3.
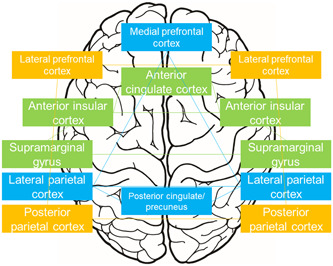
Main brain regions related to mild behavioral impairment in studies using functional neuroimaging. Light blue, default mode network; orange, frontoparietal control network; green, salience network.
The brain regions (hippocampus, parahippocampal gyrus, entorhinal cortex, and temporal lobe) identified as neural correlates of MBI 13 , 36 , 39 , 40 , 41 , 43 , 44 (Figure 2) are known to show atrophy in the early stage of AD. 54 Tau burden in the entorhinal cortex and hippocampus has been linked to MBI in amyloid‐positive NC, 17 but not in NC with an unknown amyloid status. 14 Since tau deposits in the hippocampus and entorhinal cortex correspond to Braak Stages I–II, 55 early neuropathological changes of AD may be involved in MBI. Moreover, increased amyloid and tau brain accumulation may be related to more severe MBI. 48 Therefore, MBI might reflect the AD pathological background. On the other hand, since frontal atrophy in people with MBI is linked to conversion to bvFTD, 12 MBI with frontal atrophy might reflect the FTD pathological background.
Cerebrovascular lesions may be associated with MBI, but the results are inconsistent. Links have been proposed between WMH and MBI, especially for decreased motivation, affective dysregulation, and impulse dyscontrol, based on detailed assessment of WMH using automatic image analysis software. 42 , 47 Lifestyle diseases, such as diabetes mellitus 29 and hyperlipidemia, 30 are also involved in MBI, which suggests that cerebrovascular lesions may affect MBI. Alternatively, WMH might be involved in MBI via brain atrophy. 47
Functional imaging 38 , 40 , 49 , 51 has suggested dysfunction in the DMN, FPCN, and SAN as neural correlates of MBI (Figure 3). Relationships between MBI and atrophy in brain regions related to the SAN, 38 , 43 , 47 DMN, 36 , 43 , 48 and FPCN 43 , 44 have also been proposed (Figure 2). FC reduction in the DMN and FPCN is associated with AD 56 ; SAN dysfunction is involved in bvFTD 57 ; and MBI in PD has been associated with the DMN, SAN, and FPCN. 38 , 49 , 51 These results indicate that alterations of these networks due to neuropathological changes in AD, FTD, and DLB might cause MBI. The neural correlates of MBI may also differ in each MBI domain. The MBI impulse dyscontrol domain has been linked mainly to alterations in the hippocampus, 17 , 41 parahippocampal gyrus, 39 and entorhinal cortex. 17 , 41 There are only a few studies of the neural basis of other MBI domains. 17 , 40 , 41 , 48
There are several limitations in this review. First, many studies used region‐of‐interest (ROI) analysis, 2 , 11 , 12 , 13 , 15 , 37 , 39 , 40 , 41 , 43 , 45 , 46 , 49 , 50 including specific ROIs in the frontal lobe, temporal lobe, hippocampus, parahippocampal gyrus, entorhinal cortex, anterior cingulate, and posterior cingulate, 2 , 11 , 12 , 13 , 15 , 39 , 41 , 46 while others 40 , 43 , 49 used many ROIs (range: 78–164). The results of these ROI analyses may have some bias, although the findings were partially consistent with those in whole brain analysis. 14 , 17 , 36 , 44 , 48 , 51 Second, various participants and assessments of MBI were used. Third, many studies were cross‐sectional, 13 , 14 , 17 , 36 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 49 , 50 , 51 and the relationship between neural correlates of MBI and conversion from MBI to dementia was not completely clear. Fourth, statistical analysis was not possible because the study methods varied. Fifth, we did not examine sex differences in neural correlates of MBI. The prevalence and symptoms of MBI differ between the sexes 28 , 31 , 32 , 33 and thus, the neural correlates of MBI may also differ. Sixth, a few studies used amyloid and tau PET, and MBI may reflect prodromal symptoms of tauopathies, including argyrophilic grain disease, progressive supranuclear palsy, and corticobasal degeneration.
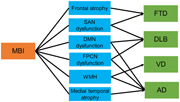
In conclusion, neuroimaging indicates that the neural correlates of MBI include atrophy in the medial and lateral temporal lobes and frontal lobe, WMH, and dysfunction in the SAN, DMN, and FPCN. These brain areas are thought to have alterations in the early stage of each dementia. Therefore, MBI may emerge in a background of pathological changes in dementia; that is, MBI may reflect prodromal symptoms of dementia. Each brain region may also be linked to conversion from MBI to each dementia (Figure 4). MBI with frontal atrophy or SAN dysfunction may be associated with conversion to FTD, and SAN, DMN, or FPCN dysfunction may be involved in conversion to DLB. DMN or FPCN dysfunction or medial temporal atrophy may be related to conversion to AD, while WMH might be correlated with conversion to VD or AD. Further prospective studies are needed to investigate the associations between brain alterations in MBI and conversion to dementia.
Figure 4.
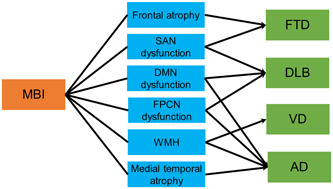
Hypothesis for relationships among mild behavioral impairment (MBI), brain changes, and conversion to dementia. AD, Alzheimer's disease; DLB, dementia with Lewy bodies, DMN, default mode network; FPCN, frontoparietal control network; FTD, frontotemporal dementia; SAN, salience network; VD, vascular dementia; WMH, white matter hyperintensity.
AUTHOR CONTRIBUTIONS
Teruyuki Matsuoka designed the study, searched the literature, assessed the quality of selected studies, and wrote the article. Ayu Imai searched the literature, assessed the quality of selected studies, and assisted with writing of the article. Jin Narumoto assisted with the systematic review and writing of the article. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS APPROVAL STATEMENT
N/A.
PATIENT CONSENT STATEMENT
N/A.
CLINICAL TRIAL REGISTRATION
N/A.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study is based on work supported by a JSPS Grant‐in‐Aid for Scientific Research (C) (21K07505).
Matsuoka T, Imai A, Narumoto J. Neuroimaging of mild behavioral impairment: A systematic review. Psychiatry Clin Neurosci Rep. 2023;2:e81. 10.1002/pcn5.81
DATA AVAILABILITY STATEMENT
N/A.
REFERENCES
- 1. Taragano FE, Allegri RF. Mild behavioral impairment: The early diagnosis. The Eleventh International Congress of the International Psychogeriatric Association, Chicago, USA, 18 August 2003.
- 2. Taragano FE, Allegri RF, Krupitzki H, Sarasola DR, Serrano CM, Loñ L, et al. Mild behavioral impairment and risk of dementia: a prospective cohort study of 358 patients. J Clin Psychiatry. 2009;70:584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimer's Dement. 2016;12:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ismail Z, McGirr A, Gill S, Hu S, Forkert ND, Smith EE. Mild behavioral impairment and subjective cognitive decline predict cognitive and functional decline. J Alzheimers Dis. 2021;80:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pan Y, Shea YF, Li S, Chen R, Mak HKF, Chiu PKC, et al. Prevalence of mild behavioural impairment: a systematic review and meta‐analysis. Psychogeriatrics. 2021;21:100–11. [DOI] [PubMed] [Google Scholar]
- 6. Pan Y, Shea YF, Ismail Z, Mak HKF, Chiu PKC, Chu LW, et al. Prevalence of mild behavioural impairment domains: a meta‐analysis. Psychogeriatrics. 2022;22:84–98. [DOI] [PubMed] [Google Scholar]
- 7. Mortby ME, Burns R, Eramudugolla R, Ismail Z, Anstey KJ. Neuropsychiatric symptoms and cognitive impairment: understanding the importance of co‐morbid symptoms. J Alzheimer's Dis. 2017;59:141–53. [DOI] [PubMed] [Google Scholar]
- 8. Matsuoka T, Ismail Z, Narumoto J. Prevalence of mild behavioral impairment and risk of dementia in a psychiatric outpatient clinic. J Alzheimer's Dis. 2019;70:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGirr A, Nathan S, Ghahremani M, Gill S, Smith EE, Ismail Z. Progression to dementia or reversion to normal cognition in mild cognitive impairment as a function of late‐onset neuropsychiatric symptoms. Neurology. 2022;98:e2132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taragano FE, Allegri RF, Lyketsos C. Mild behavioral impairment: a prodromal stage of dementia. Dement Neuropsychol. 2008;2:256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taragano FE, Allegri RF, Heisecke SL, Martelli MI, Feldman ML, Sánchez V, et al. Risk of conversion to dementia in a mild behavioral impairment group compared to a psychiatric group and to a mild cognitive impairment group. J Alzheimer's Dis. 2018;62:227–38. [DOI] [PubMed] [Google Scholar]
- 12. Orso B, Mattei C, Arnaldi D, Massa F, Serafini G, Plantone D, et al. Clinical and MRI predictors of conversion from mild behavioural impairment to dementia. Am J Geriatr Psychiatry. 2020;28:755–63. [DOI] [PubMed] [Google Scholar]
- 13. Rouse HJ. Early indicators of cognitive dysfunction: the role of mild behavioral impairment. PhD Thesis. University of South Florida; 2021.
- 14. Lussier FZ, Pascoal TA, Chamoun M, Therriault J, Tissot C, Savard M, et al. Mild behavioral impairment is associated with β‐amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimer's Dementia. 2020;16:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun Y, Xu W, Chen KL, Shen XN, Tan L, Yu JT. Mild behavioral impairment correlates of cognitive impairments in older adults without dementia: mediation by amyloid pathology. Transl Psychiatry. 2021;11:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miao R, Chen HY, Gill S, Naude J, Smith EE, Ismail Z. Plasma β‐amyloid in mild behavioural impairment–neuropsychiatric symptoms on the alzheimer's continuum. J Geriatr Psychiatry Neurol. 2022;35:434–41. [DOI] [PubMed] [Google Scholar]
- 17. Johansson M, Stomrud E, Insel PS, Leuzy A, Johansson PM, Smith R, et al. Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer's disease. Transl Psychiatry. 2021;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andrews SJ, Ismail Z, Anstey KJ, Mortby M. Association of Alzheimer's genetic loci with mild behavioral impairment. Am J Med Genet, Part B. 2018;177:727–35. [DOI] [PubMed] [Google Scholar]
- 19. Creese B, Arathimos R, Brooker H, et al. Genetic risk for Alzheimer's disease, cognition, and mild behavioral impairment in healthy older adults. Alzheimers Dement. 2021;13:e12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naude JP, Gill S, Hu S, McGirr A, Forkert ND, Monchi O, et al. Plasma neurofilament light: a marker of neurodegeneration in mild behavioral impairment. J Alzheimer's Dis. 2020;76:1017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramezani M, Ruskey JA, Martens K, Kibreab M, Javer Z, Kathol I, et al. Association between BDNF Val66Met polymorphism and mild behavioral impairment in patients with parkinson's disease. Front Neurol. 2021;11:587992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Creese B, Brooker H, Ismail Z, Wesnes KA, Hampshire A, Khan Z, et al. Mild behavioral impairment as a marker of cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry. 2019;27:823–34. [DOI] [PubMed] [Google Scholar]
- 23. Rouse HJ, Small BJ, Schinka JA, Loewenstein DA, Duara R, Potter H. Mild behavioral impairment as a predictor of cognitive functioning in older adults. Int Psychogeriatr. 2020;33(3):285–93. [DOI] [PubMed] [Google Scholar]
- 24. Kassam F, Chen H, Nosheny RL, McGirr A, Williams T, Ng N, et al. Cognitive profile of people with mild behavioral impairment in Brain Health Registry participants. Int Psychogeriatr. 2022;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Botero‐Rodríguez F, Córdoba Sastoque AM, Santacruz Escudero JM, Santamaría‐García H. Neuropsychiatric symptoms in patients with neurocognitive disorder and their performance between mild and major stages. J Alzheimer's Dis. 2022;85:1735–44. [DOI] [PubMed] [Google Scholar]
- 26. Guan DX, Chen HY, Camicioli R, Montero‐Odasso M, Smith EE, Ismail Z. Dual‐task gait and mild behavioral impairment: the interface between non‐cognitive dementia markers. Exp Geront. 2022;162:111743. [DOI] [PubMed] [Google Scholar]
- 27. Fan S, Liang X, Yun T, Pei Z, Hu B, Ismail Z, et al. Mild behavioral impairment is related to frailty in non‐dementia older adults: a cross‐sectional study. BMC Geriatr. 2020;20:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guan DX, Rockwood K, Smith EE, Ismail Z. Sex Moderates the association between frailty and mild behavioral impairment. J Prev Alzheimers Dis. 2022;9:692–700. [DOI] [PubMed] [Google Scholar]
- 29. Soo SA, Ng KP, Wong F, Saffari SE, Yatawara C, Ismail Z, et al. The association between diabetes mellitus and mild behavioral impairment among mild cognitive impairment: findings from Singapore. J Alzheimer's Dis. 2021;82:411–20. [DOI] [PubMed] [Google Scholar]
- 30. Rao A, Thakral M, Saini M, Chatterjee P, Dey A. Blood biomarkers in older subjects with mild behavioral impairment: a cross‐sectional study from the memory clinic, all india institute of medical sciences, India. J Indian Acad Geriatr. 2020;16:91–4. [Google Scholar]
- 31. Gosselin P, Guan DX, Chen HY, Pichora‐Fuller MK, Phillips N, Faris P, et al. The Relationship between hearing and mild behavioral impairment and the influence of sex: a study of older adults without dementia from the COMPASS‐ND study. J Alzheimers Dis Rep. 2022;6:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wolfova K, Creese B, Aarsland D, et al. Gender/sex differences in the association of mild behavioral impairment with cognitive aging. J Alzheimers Dis. 2022;88: 345–355. [DOI] [PubMed] [Google Scholar]
- 33. Mortby ME, Ismail Z, Anstey KJ. Prevalence estimates of mild behavioral impairment in a population‐based sample of pre‐dementia states and cognitively healthy older adults. Int Psychogeriatr. 2018;30:221–32. [DOI] [PubMed] [Google Scholar]
- 34. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. JBI; 2020. Available from: https://synthesismanual.jbi.global [Google Scholar]
- 36. Yoon EJ, Ismail Z, Hanganu A, Kibreab M, Hammer T, Cheetham J, et al. Mild behavioral impairment is linked to worse cognition and brain atrophy in Parkinson disease. Neurology. 2019;93:e766–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gill S, Mouches P, Hu S, Rajashekar D, MacMaster FP, Smith EE, et al. Using machine learning to predict dementia from neuropsychiatric symptom and neuroimaging data. J Alzheimer's Dis. 2020;75:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lang S, Yoon EJ, Kibreab M, Kathol I, Cheetham J, Hammer T, et al. Mild behavioral impairment in Parkinson's disease is associated with altered corticostriatal connectivity. NeuroImage: Clin. 2020;26:102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gill S, Wang M, Mouches P, Rajashekar D, Sajobi T, MacMaster FP, et al. Neural correlates of the impulse dyscontrol domain of mild behavioral impairment. Int J Geriatr Psychiatry. 2021;36:1398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsuoka T, Ueno D, Ismail Z, Rubinstein E, Uchida H, Mimura M, et al. Neural correlates of mild behavioral impairment: a functional brain connectivity study using resting‐state functional magnetic resonance imaging. J Alzheimer's Dis. 2021;83:1221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matuskova V, Ismail Z, Nikolai T, Markova H, Cechova K, Nedelska Z, et al. Mild behavioral impairment is associated with atrophy of entorhinal cortex and hippocampus in a memory clinic cohort. Front Aging Neurosci. 2021;13:643271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miao R, Chen HY, Robert P, Smith EE, Ismail Z, MEMENTO Study Group . White matter hyperintensities and mild behavioral impairment: findings from the MEMENTO cohort study. Cereb Circ‐Cogn Behav. 2021;2:100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shu J, Qiang Q, Yan Y, Ren Y, Wei W, Zhang L. Aberrant topological patterns of structural covariance networks in cognitively normal elderly adults with mild behavioral impairment. Front Neuroanat. 2021;15:738100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shu J, Qiang Q, Yan Y, Wen Y, Ren Y, Wei W, et al. Distinct patterns of brain atrophy associated with mild behavioral impairment in cognitively normal elderly adults. Int J Med Sci. 2021;18:2950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cassidy CM, Therriault J, Pascoal TA, Cheung V, Savard M, Tuominen L, et al. Association of locus coeruleus integrity with Braak stage and neuropsychiatric symptom severity in Alzheimer's disease. Neuropsychopharmacology. 2022;47:1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stella F, Pais MV, Loureiro JC, Radanovic M, Forlenza OV. Neuropsychiatric symptoms and cerebrovascular risk in non‐demented elders: cross‐sectional study using the mild behavioural impairment checklist (MBI‐C). Psychogeriatrics. 2022;22:55–66. [DOI] [PubMed] [Google Scholar]
- 47. Yang L, Shu J, Yan A, Yang F, Xu Z, Wei W. White matter hyperintensities‐related cortical changes and correlation with mild behavioral impairment. Adv Med Sci. 2022;67:241–9. [DOI] [PubMed] [Google Scholar]
- 48. Lussier F. Alzheimer's disease pathophysiological correlates of the syndrome of mild behavioral impairment across the spectrum of the disease. MSc Thesis. McGill University; 2022.
- 49. Lang S, Ismail Z, Kibreab M, Kathol I, Sarna J, Monchi O. Common and unique connectivity at the interface of motor, neuropsychiatric, and cognitive symptoms in Parkinson's disease: a commonality analysis. Hum Brain Mapp. 2020;41:3749–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yoo HS, Lee S, Chung SJ, Ye BS, Sohn YH, Yun M, et al. Clinical and striatal dopamine transporter predictors of mild behavioral impairment in drug‐naive parkinson disease. Clin Nucl Med. 2020;45:e463–8. [DOI] [PubMed] [Google Scholar]
- 51. Jin Yoon E, Ismail Z, Kathol I, Kibreab M, Hammer T, Lang S, et al. Patterns of brain activity during a set‐shifting task linked to mild behavioral impairment in Parkinson's disease. NeuroImage: Clin. 2021;30:102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ismail Z, Agüera‐Ortiz L, Brodaty H, Cieslak A, Cummings J, Fischer CE, et al. The Mild Behavioral Impairment Checklist (MBI‐C): a rating scale for neuropsychiatric symptoms in pre‐dementia populations. J Alzheimer's Dis. 2017;56:929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sheikh F, Ismail Z, Mortby ME, Barber P, Cieslak A, Fischer K, et al. Prevalence of mild behavioral impairment in mild cognitive impairment and subjective cognitive decline, and its association with caregiver burden. Int Psychogeriatr. 2018;30:233–44. [DOI] [PubMed] [Google Scholar]
- 54. Planche V, Manjon JV, Mansencal B, Lanuza E, Tourdias T, Catheline G, et al. Structural progression of Alzheimer's disease over decades: the MRI staging scheme. Brain Commun. 2022;4:fcac109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease‐associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao Q, Sang X, Metmer H, Swati ZNK, Lu J. Functional segregation of executive control network and frontoparietal network in Alzheimer's disease. Cortex. 2019;120:36–48. [DOI] [PubMed] [Google Scholar]
- 57. Rosenberg PB, Nowrangi MA, Lyketsos CG. Neuropsychiatric symptoms in Alzheimer's disease: what might be associated brain circuits? Mol Aspects Med. 2015;43‐44:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
N/A.


