Abstract
Aim
The aim of this study is to examine the long‐term impact of early menarche with adult depression, and to assess whether this association was explained by childhood traumatic experience and socioeconomic condition in early adulthood.
Methods
The data were derived from World Mental Health Survey Japan Second, a cross‐sectional survey conducted among Japanese community residents between 2013 and 2015. We used the data of female respondents aged 20–75 years (N = 1171). Hazard ratio (HR) of the onset of major depression up to 40 years was calculated for an early‐menarche group and a non‐early‐menarche group, respectively. Kaplan–Meier curve and log–rank statistics were used to examine the difference in failure. Cox proportional hazard models were administered for the association of major depression with early‐menarche and early‐life psychosocial factors.
Results
Risk for major depressive disorders were three to four times higher in an early‐menarche group, and the differences in survival functions were significant (p < 0.001). HR of early menarche was 2.79 (95% CI = 1.29–6.02), and was slightly changed when childhood traumatic experience and socioeconomic conditions in young adulthood were added in the model (HR = 2.88, 95% CI = 1.30–6.38; HR = 3.19, 95% CI = 1.41–7.21).
Conclusion
Early menarche was significantly associated with increased risk for depression by the age of 40 years. Childhood trauma and socioeconomic hardship in early adulthood did not account for the association. Both physical and psychosocial risk factors in early life need to be addressed for preventing women's depression.
Keywords: child adversities, depression, Japan, menarche, women
Women who had menarche at an earlier age were at higher risk for depression up to the age of 40 years. This association was independent of psychosocial stressors in early life, such as childhood trauma and socioeconomic hardship in early adulthood. Biological factors that affect the central nervous system during early puberty may be a potential mediator between early menarche and adult depression

INTRODUCTION
The global burden of diseases caused by depressive disorders has been increasing, being ranked among the top 10 leading causes of disease burden in 2019, as well as fourth among those aged 10–24 years and sixth among those aged 25–49 years. 1 Considering the fact that depressive disorders pose a 1.5‐times greater burden on women than they do on men, 2 gender‐specific factors, either biological or psychosocial, may exist behind the onset of depression. 3
Early menarche is defined as the onset of menarche before the standard age for menarche in that population. The age criteria for early menarche ranges from 10 to 14 years old in existing studies. Early menarche has been hypothesized to be a gender‐specific risk factor of women's depression. 4 , 5 , 6 , 7 , 8 , 9 A large‐scale prospective study in the United States found that early menarche was associated with an increased risk for depressive symptoms up to the age of 27 years, 4 while other studies found that the association became nonsignificant before they reached adulthood. 6 , 8 The prolonged effect of early menarche by 40 years' old throughout women's reproductive ages has yet to be known.
One of most prominent theoretical explanations for the pathway between early menarche and later depression is the psychosocial stressors model. This model addresses childhood exposure to recurrent stressors in and around the family and their impact on pubertal timing. 10 , 11 , 12 , 13 Psychosocial acceleration theory specifically addresses the fact that sexual maturation of children experiencing recurrent traumatic events are likely to be accelerated, resulting in early sexual initiation, unstable pair bonds, and internalizing and externalizing disorders in adolescence. 10 , 11 , 14 , 15 , 16 This psychosocial vulnerability among adolescents could further lead them to socioeconomic disadvantages, such as early pregnancy, educational underachievement and unstable jobs, 11 which could increase the risk for depression throughout their life.
Empirical studies supported this view by demonstrating the association of early menarche with several indicators in early life: childhood social disadvantage, 17 , 18 , 19 parental separation, 20 parental psychopathology, 21 family conflict and abuse, 21 , 22 , 23 early sexual initiation and pregnancy, 9 , 22 , 24 , 25 , 26 health risk behaviors, 25 , 26 externalizing and internalizing disorders, 5 , 27 and antisocial behaviors. 4 However, many of these studies were conducted among adolescents, 5 , 17 , 19 , 20 , 24 , 25 , 27 and included a limited number of early‐life indicators. Because early‐life adversities often co‐occurred and had a complex interweaving effect on later mental health, 28 , 29 a wider range of childhood indicators need to be considered for a more rigorous assessment of the prolonged impact of early menarche.
This cross‐sectional study aims at examining the association of early menarche with history of major depressive disorder by age 40 years and assessing whether this association is independent of the early‐life psychosocial stressors of childhood traumatic experience and early‐life socioeconomic conditions. We used the data from World Mental Health Japan Survey Second (WMHJ2) that provided representative data of Japanese community residents. 30 We expected that early menarche would have a significant association with adult depression and that the association would be partly explained by childhood traumatic experience and early socioeconomic conditions. We controlled the analytical models with parental mental disorders that were identified as being associated with both early menarche and later depression. 21
METHODS
Study population
Analysis was conducted on the data from the WMHJ2, 30 which was a cross‐sectional survey conducted from 2013 to 2015 in collaboration with the World Health Organization World Mental Health Survey. 31 Survey participants were Japanese community residents aged 20–75 years, who were selected by a two‐stage stratified random sampling method. The primary sampling unit was the survey area and the secondary unit was the survey participants. Respondents were excluded from participation if they were (1) deceased or institutionalized, (2) had moved from the survey site, or (3) could not speak Japanese. The final sample was 2450 (response rate 43.4%). We analyzed the data of female participants who responded to the item that asked the timing of menarche (N = 1171).
Survey instrument
Data were collected by face‐to‐face interviews using the computer‐assisted personal interviews (CAPI) and a self‐administered questionnaire. The questionnaire built in CAPI was the Japanese version of the World Mental Health (WMH) Survey Initiative version of the World Health Organization Composite International Diagnostic Interview (WMH‐CIDI), a fully structured diagnostic interview that is to be conducted by trained interviewers. 32 CIDI has demonstrated acceptable reliability and validity as a clinical assessment tool of common mental disorders. 33 A self‐administered questionnaire was developed for use in the WMHJ2, which included a women's section that asked information related to reproductive health.
Measures
Major depressive disorder
Respondents' lifetime experience of major depressive disorder between 10 and 40 years' old was assessed based on the DSM‐IV adopted in the WMH‐CIDI. 32 Depressive symptoms caused by illness, injury, and medication and that appeared only during the presence of other mental disorders were excluded. The age at which respondents experienced depressive symptoms that met diagnostic criteria for the first time was used to define the age of onset.
Early menarche
Timing of menarche was based on the following retrospective self‐report that asked about the first occurrence of their menstruation: “How old were you when you had a first menstruation?” The onset of menstruation at the age of 10 or younger was coded as early menarche. 16
Covariates
Childhood traumatic experience
Five types of life‐threating traumatic events during childhood were included in the analysis: physical abuse by parents or caretakers, sexual abuse, witnessing domestic violence between parents, neglect, and separation from parents. Respondents were asked how often they experienced the following acts of parents until they had grown up: (1) pushing, grabbing, or shoving; (2) throwing an object at each other; and (3) slapping, hitting, or punching. 34 Response options were often, sometimes, rarely, and never. Those who responded with often or sometimes to any of the three questions were coded as physically abused. A positive response to another dichotomous question that asked whether the respondent had been beaten up by parents or caretakers was also used to define physical abuse. Childhood sexual abuse was coded as positive, if the respondents had experienced repeated sexual assault, molestation, or rape during their childhood. 35 Witnessing interparental violence was defined as positive when respondents witnessed any of the following acts either sometimes or often between the parents or caretakers while growing up: slapping, hitting, pushing, grabbing, shoving, or throwing an object at each other. Neglect was assessed by questions about being made to do chores that were too difficult or dangerous, being left alone or unsupervised, and not being provided school supplies, meals, and or/medical treatment during one's childhood. 36 Those who responded often to any of these questions were coded as positive, while those who answered sometimes, never, or rarely were coded as negative.
Separation from parents was coded as positive if they responded yes to the following dichotomous items: parental death, parental divorce, and any other separation from one or both parents due to parent's absence (e.g., hospitalization and desertion) or respondent's absence (e.g., adoption, boarding school, foster care, and left home before age 16 years).
Disadvantageous socioeconomic conditions in young adulthood
Disadvantageous early socioeconomic conditions included the following three types: teenage pregnancy, lower educational attainment, and early employment. Teenage pregnancy was coded as positive if the first pregnancy was at the age of 19 years or younger. Lower educational attainment was defined as junior high school graduates or lower. Early employment was defined as the first employment at the age 17 years or younger.
Parental mental disorders during childhood
Parental mental disorder during childhood was measured by a modified version of the Family History Research Diagnostic Criteria Interview in the CIDI. 37 Major depressive disorder of a parent was coded as positive if she or he ever had been sad or depressed most of the time for more than 2 weeks AND if she or he had other symptoms, such as low energy, changes in sleep or appetite, and problems with concentration during that time. General anxiety disorder was coded as positive if she or he had ever been constantly nervous, edgy, or anxious AND if she or he had other symptoms, such as being restless, irritable, easily tired, and difficulty falling asleep during that time. Panic disorder was coded as positive if she or he had ever had an anxiety attack when she or he felt frightened, anxious, or panicky. Substance abuse was coded as positive if she or he had a problem with alcohol or drugs. The four types of mental disorders described above were assessed individually for the mother and father. Having any of the disorders was coded as “mother's mental disorders” and “father's mental disorders,” respectively, and included in the analysis.
Age
All the models were adjusted by age. Age was arranged into three groups: 20–39, 40–59, and 60–75 years.
Analysis
First, a descriptive analysis was conducted to see the prevalence of major depressive disorder, childhood traumatic experience, socioeconomic conditions in young adulthood, and parental mental disorders in the total sample and those who had early menarche. The difference in the prevalence between early‐menarche and non‐early‐menarche groups was examined by the Fisher's exact test.
We drew a Kaplan–Meier curve for the failure time from 10 to 40 years by the timing of menarche. The curve showed the cumulative probability of major depressive disorder. Log–rank statistics were calculated to test the difference in failure curve. Incidence of the onset of major depressive disorders by 5 years from 10 to 40 years old was calculated for the early‐menarche and non‐early‐menarche groups, respectively. We also calculated incidence rate ratio (IRR) and the 95% confidence interval (95% CI) by the age interval to quantify the difference in incidence between the two groups.
Cox proportional hazard models were used to examine the association between the failure time for depressive disorders and early‐menarche with the adjustment of early‐life factors. As a basic model, we calculated the HR of early menarche (reference = not early menarche) on the onset of depressive disorders adjusted by age (Model 1). Variables of childhood traumatic experience (Model 2), socioeconomic conditions in young adulthood (Model 3), and parental mental disorders (Model 4) were cumulatively added to the basic model to assess the effect of these early‐life factors on the association between early‐menarche and onset of depressive disorders. Analyses were performed using STATA (Version 15; Stat Corp.). Statistical significance was consistently tested at a 0.05 level, two‐sided.
RESULTS
Sample characteristics
Table 1 shows the characteristics of the sample and the prevalence of early menarche. Among the 1171 respondents, 52 had early menarche (4.4%). Early menarche was more prevalent in younger age groups (p < 0.001), from 9.4% in those aged 20–39 years to 0.3% in those aged 60–76 years. The prevalence of early menarche was significantly higher among those who experienced sexual abuse (p = 0.011) and among those who experienced major depressive disorder (p = 0.002) than in their counterparts. The timing of menarche did not differ by other childhood traumatic experience and socioeconomic conditions in young adulthood.
Table 1.
Sample characteristics and the prevalence of early menarche in WMHJ2
| Total sample (N = 1171) | Prevalence of early menarche by sample characteristics | ||
|---|---|---|---|
| N (%) | Early menarchea n = 52 (prevalenceb, %) | Fisher's exact p value | |
| Age (mean, SD) | >0.001*** | ||
| 20–39 | 340 (29.0) | 32 (9.4) | |
| 40–59 | 459 (39.2) | 19 (4.1) | |
| 60–75 | 372 (31.8) | 1 (0.3) | |
| Childhood traumatic experience | |||
| Physical abuse | 102 (9.3) | 3 (2.9) | 0.471 |
| Sexual abuse | 4 (0.3) | 2 (50.0) | 0.011* |
| Witness of DV | 64 (5.8) | 2 (3.1) | 0.764 |
| Neglect | 21 (1.8) | 0 (0) | 1 |
| Separation from parents | 55 (5.0) | 2 (3.6) | 1 |
| Socioeconomic conditions in young adulthood | |||
| Teen pregnancy | 43 (3.7) | 1 (2.3) | 1 |
| Junior high school or below | 92 (7.9) | 4 (4.4) | 0.139 |
| Employment at 17 or younger | 196 (17.2) | 7 (3.6) | 0.703 |
| Parental mental disorders | |||
| Mother's mental disorders | 24 (2.1) | 3 (12.5) | 0.087 |
| Father's mental disorders | 21 (1.8) | 1 (4.8) | 0.618 |
| History of DSM‐IV major depressive disorder by age 40 | 53 (4.5) | 8 (15.1) | 0.002** |
Abbreviation: WMHJ2, World Mental Health Japan Survey Second.
Early menarche, having the first menstruation at the age of 10 years or younger.
Prevalence, percentage of responders who experienced early menarche to the total sample of each characteristics' variables.
p < 0.05; **p < 0.01; ***p < 0.00.
Onset of major depressive disorder at age intervals by the timing of menarche
The Kaplan–Meier curve (Figure 1) shows the cumulative probability of major depressive disorder by the timing of menarche. The cumulative probability of early menarche exceeded that of the others at the age of 20 years and the difference enlarged as they grew older. As a result of the log–rank test, the survival function significantly differed between the two groups (χ 2 = 19.59, DF = 1, p < 0.001). The hazard ratio from those aged 10 to 40 years was 4.47 (95% CI = 1.82–9.58). Incidence of major depressive disorder by the timing of menarche was presented at 5‐year age intervals (Table 2). The IRR was the highest at the age‐interval of 36–40 years' old (IRR = 5.58), although the wide 95% CI included 1.0 (95% CI = 0.12–43.41), possibly due to the small sample size.
Figure 1.
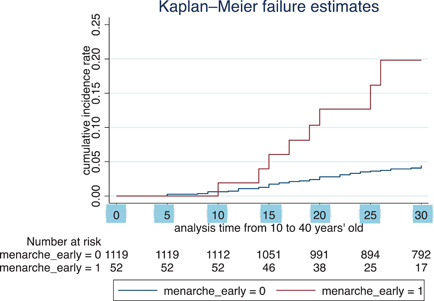
Kaplan–Meier curve for the occurrence of major depressive disorder from 10 to 40 years by the timing of menarche. Kaplan–Meier curves of an early menarche group (red) and the non‐early‐menarche group (blue) that show cumulative incidence rate of major depressive disorders between the age of 10 and 40 years.
Table 2.
Incidence rate and incidence rate ratio of the onset of DSM‐IV major depressive disorder at each time interval among the early‐menarche group and the non‐early‐menarche group (N = 1171)
| Early menarche (n = 52) | Others (n = 1119) | IRR | 95% CI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Person‐year | MDD (n) | Incidence rate | 95% CI | Person‐year | MDD (n) | Incidence rate | 95% CI | ||||
| 10–15 years | 260 | 0 | – | – | 5595 | 3 | 0.54 | 0.17–1.66 | – | – | |
| 16–20 years | 260 | 1 | 3.85 | 0.54–27.30 | 5575 | 4 | 0.72 | 0.27–1.91 | 3.13 | 0.06–31.58 | |
| 21–25 years | 241 | 2 | 8.30 | 2.08–33.18 | 5375 | 12 | 2.23 | 1.27–3.93 | 1.77 | 0.19–7.93 | |
| 26–30 years | 213 | 3 | 14.08 | 4.54–43.67 | 5076 | 11 | 2.17 | 1.20–3.91 | 1.38 | 0.25–5.23 | |
| 31–35 years | 144 | 1 | 6.94 | 0.98–49.30 | 4683 | 8 | 1.71 | 0.85–3.42 | 1.27 | 0.03–9.49 | |
| 36–40 years | 95 | 1 | 10.53 | 1.48–74.73 | 4182 | 7 | 1.67 | 0.80–3.51 | 5.58 | 0.12–43.41 | |
Abbreviations: IRR, incidence rate ratio; MDD, major depressive disorder.
Association of early‐menarche with the onset of depressive disorders and its possible mechanisms
Table 3 shows the HRs of early menarche and other possible risk factors in early life as estimated by the Cox proportional‐hazards model. HR of early menarche in the basic model was 2.79 (95% CI = 1.29–6.02) (Model 1), which did not change significantly with the adjustment of childhood traumatic experience in Model 2 (HR = 2.88, 95% CI = 1.30–6.38). The HR slightly changed after adding socioeconomic conditions in young adulthood (HR = 3.19, 95% CI = 1.41–7.21) (Model 3) and parental mental disorders (HR = 3.23, 95% CI = 1.43–7.30) (Model 4).
Table 3.
Hazard ratio of early menarche on the onset of DSM‐IV major depressive disorder by age 40 estimated by Cox hazard model
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p‐value | HR | 95% CI | p‐value | HR | 95% CI | p‐value | HR | 95% CI | p‐value | |||
| Early menarches | 2.79 | 1.29–6.02 | 0.009* | 2.88 | 1.30–6.38 | 0.009** | 3.19 | 1.41–7.21 | 0.005** | 3.23 | 1.43–7.30 | 0.005** | ||
| Traumatic experience | – | – | – | |||||||||||
| Physical abuse | 3.09 | 1.57–6.08 | 0.001** | 3.00 | 1.47–6.14 | 0.003** | 2.66 | 1.28–5.54 | 0.009** | |||||
| Sexual abuse | 1.15 | 0.14–9.08 | 0.896 | 1.54 | 0.19–12.65 | 0.688 | 1.12 | 0.13–9.77 | 0.921 | |||||
| Witness DV | 1.06 | 0.39–2.83 | 0.915 | 0.91 | 0.31–2.69 | 0.859 | 0.72 | 0.23–2.25 | 0.568 | |||||
| Neglect | 2.38 | 0.68–8.28 | 0.172 | 2.72 | 0.77–9.60 | 0.121 | 2.88 | 0.79–10.44 | 0.108 | |||||
| Separation | 2.18 | 0.83–5.70 | 0.112 | 1.58 | 0.52–4.76 | 0.418 | 1.58 | 0.52–4.74 | 0.418 | |||||
| Socioeconomic conditions | – | – | ||||||||||||
| Junior high school or below | 0.29 | 0.06–1.35 | 0.114 | 0.32 | 0.07–1.51 | 0.152 | ||||||||
| Early employment | 2.81 | 1.50–5.27 | 0.001** | 2.88 | 1.54–5.39 | 0.001** | ||||||||
| Teen pregnancy | 1.13 | 0.34–3.73 | 0.840 | 1.13 | 0.34–3.76 | 0.841 | ||||||||
| Parental mental disorders | ||||||||||||||
| Mother's disorders | 2.67 | 1.00–7.17 | 0.051 | |||||||||||
| Father's disorders | 2.07 | 0.60–7.11 | 0.249 | |||||||||||
Notes: Model 1 was adjusted for age. N = 1171.
Model 2 was adjusted for age and childhood adversity. N = 1100.
Model 3 was adjusted for age, childhood adversity, and socioeconomic conditions in young adulthood. N = 1070.
Model 4 was adjusted for age, childhood adversity, socioeconomic conditions in young adulthood, and parental disorders. N = 1070.
Abbreviation: HR, hazard ratio.
p < 0.05; **p < 0.01.
DISCUSSION
Increased risk of major depressive disorder among those who had early menarche
The present study found that women with early menarche were at higher risk for major depressive disorder in later life by the age of 40 years. This result extends the existing evidence, 4 , 9 highlighting the prolonged impact of early menarche throughout their reproductive age. Increased risk for major depressive disorder among those with early menarche emerged after 15 years of age, which echoes existing evidence that the effect of early menarche on depression was detectable after 12 years old with the time lag from the first menstruation of the early developers. 5
Mechanisms of the association between early menarche and major depressive disorder
Our results did not support the hypothesis that childhood traumatic experience and early socioeconomic conditions contributed to the higher risk for adult depression among women with early menarche. A previous study among adolescent girls in the United States found that 6.2% of the association between childhood trauma and depression was explained by early menarche. 38 However, the link between childhood trauma and early menarche was not observed in our Japanese sample. The contradictory results suggest that the association between early menarche and psychosocial stressors in early life have a sociocultural variation. In a cultural group, where risky behaviors in reproductive health were suppressed or controlled, the link between early menarche and childhood psychosocial stressors may not be evident. 24
An alternative pathway between early menarche and adult major depressive disorder is biological. Existing studies addressed that gonadal steroid hormone could have direct effect on the central nervous system especially during puberty, which can affect psychopathology in later life. 5 , 12 In addition, the fact that a gender gap in the occurrence of depression became observable around the age of 13 years indicated that gonadal hormone plays a critical role in increased risk of depression. 3 The long‐term mental health effect of gonadal steroid hormone and its mechanism has yet to be examined in relation to early menarche.
Limitations
Our study had the following limitations. First, the data were derived from a cross‐sectional study, and the participants' age ranged from 20 to 75 years. An historical trend of acceleration of menarche timing 12 was also observed in our sample, which implied different cutoff points for early menarche between the younger and the older. In addition, self‐assessment of psychosocial stressors may vary as they grew older, which could obscure the association between early menarche and psychosocial stressors. Although the accuracy of self‐report on the timing of menarche was reported as acceptable, 39 recall bias may occur depending on educational level and factors associated with reproductive health. 40 Second, the sample size may not be sufficient for examining the effect of sexual abuse. Childhood abuse, especially sexual abuse, has been consistently a significant risk factor for early menarche in many studies. 12 , 21 , 41 Lack of association in our study may be due to the small sample size, as we have only four respondents who reported having been sexually abused. A large‐scale cohort data would mitigate these biases. Third, psychosocial stressors directly resulted from early menarche, such as physical‐maturity gap and social experience of being “off‐time,” 10 , 12 was not examined in this study. Although the limited mediating effect of these stressors was reported in the literature, 14 negative self‐image and stressful social interaction caused in adolescence might be associated with later depression.
CONCLUSION
We found that women who had menarche at an earlier age were at higher risk for depression by the age of 40 years. Psychosocial stressors in early life, such as childhood trauma and socioeconomic hardship in early adulthood, did not account for the association. Biological factors that affect the central nerve system during early puberty may be a potential mediator between early menarche and adult depression. Cultural variation for the effect of early menarche is worth investigating with the use of a larger cohort sample from other countries. Although biological factors during adolescence have not attracted much attention in predicting adult psychopathology, our findings suggest that early menarche serves as a robust predictor for adult depression. These results suggest that preventive efforts for women's depression start from the early stage of their lives, and the assessment of both physical and psychosocial risk factors is essential.
AUTHOR CONTRIBUTIONS
All the authors have read and contributed to the manuscript preparation. All the authors took part in study design and project administration. Maki Umeda and Norito Kawakami conceptualized the study. Maki Umeda conducted the analysis and prepared the first draft. Norito Kawakami, Hisateru Tachimori, Tadashi Takehisa, Haruki Shimoda, Miyamoto Karin, and Hanako Ishikawa provided critical review and added important intellectual contents for interpretation of results. All the authors agreed with the final manuscript and the submission to this journal.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DISCLOSURE STATEMENT
The funding sources had no role in any process of the research design, data collection, data analysis and interpretation, and submission of the manuscript.
ETHICS APPROVAL STATEMENT
The contents and procedure of this study were approved by the Research Ethics Committees of Graduate School of Medicine/Faculty of Medicine, The University of Tokyo (10131‐ (2), (3), and (4)). This research met the ethical standards laid down in the Declaration of Helsinki and ethical guidelines for epidemiological studies.
PATIENT CONSENT STATEMENT
All participants gave their written informed consent prior to their participation. The participation in this study was completely voluntary and confidentiality was assured.
ACKNOWLEDGMENTS
We thank all the members and contributors of the World Mental Health Japan Survey Second (WMHJ2) and the World Health Organization World Mental Health Survey Initiative. The WMHJ2 was supported by the Grant for Research on Psychiatric and Neurological Diseases and Mental Health from the Japan Ministry of Health, Labour, and Welfare (H25‐SEISHIN‐IPPAN006), and by the Research and Development Grants for Comprehensive Research for Persons with Disabilities from Japan Agency for Medical Research and Development (15dk0310020h0003). The overall WMHS was supported by the US National Institute of Mental Health (grant R01 MH070884), the MacArthur Foundation (grant R13‐MH066849), the Pfizer Foundation (grant R01MH069864), the US Public Health Service (grant R01 DA016558), the Fogarty International Centre (grant R03TW006481), the Pan American Health Organization, Eli Lilly and Company, Ortho‐McNeil Pharmaceutical Inc., Glaxo Smith Kline, Bristol Myers Squibb, and Shire.
Umeda M, Kawakami N, Shimoda H, Miyamoto K, Ishikawa H, Tachimori H, et al. Early menarche and adult major depressive disorder among Japanese women: the role of childhood traumatic experience and socioeconomic conditions in young adulthood. Psychiatry Clin Neurosci Rep. 2022;1:e16. 10.1002/pcn5.16
DATA AVAILABILITY STATEMENT
The data for this study are not publicly available due to privacy and ethical restrictions, and will be available on request from the corresponding author. When a reasonable request is received, the data will be made available after approval by the ethics committee of the University of Tokyo.
REFERENCES
- 1. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990‐2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rehm J, Shield KD. Global Burden of Disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21(2):10. [DOI] [PubMed] [Google Scholar]
- 3. Maughan B. Depression and psychological distress: a life course perspective. In: Kuh D, Hardy R editors. A life course approach to women's health. New York: Oxford; 2002. p. 161–76. [Google Scholar]
- 4. Mendle J, Ryan RM, McKone KMP. Age at menarche, depression, and antisocial behavior in adulthood. Pediatrics. 2018;141(1): e20171703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Copeland W, Shanahan L, Miller S, Costello EJ, Angold A, Maughan B. Outcomes of early pubertal timing in young women: a prospective population‐based study. Am J Psychiatry. 2010;167(10):1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deng F, Tao FB, Wan YH, Hao JH, Su PY, Cao YX. Early menarche and psychopathological symptoms in young Chinese women. J Womens Health (Larchmt). 2011;20(2):207–13. [DOI] [PubMed] [Google Scholar]
- 7. Kowalski AJ, Addo OY, Kramer MR, Martorell R, Norris SA, Waford RN, et al. Longitudinal associations of pubertal timing and tempo with adolescent mental health and risk behavior initiation in urban South Africa. J Adolesc Health. 2021;69(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lien L, Haavet OR, Dalgard F. Do mental health and behavioural problems of early menarche persist into late adolescence? A three year follow‐up study among adolescent girls in Oslo, Norway. Soc Sci Med. 2010;71(3):529–33. [DOI] [PubMed] [Google Scholar]
- 9. Boden JM, Fergusson DM, Horwood LJ. Age of menarche and psychosocial outcomes in a New Zealand birth cohort. J Am Acad Child Adolesc Psychiatry. 2011;50(2):132–40e5. [DOI] [PubMed] [Google Scholar]
- 10. Ellis BJ. Timing of pubertal maturation in girls: an integrated life history approach. Psychol Bull. 2004;130(6):920–58. [DOI] [PubMed] [Google Scholar]
- 11. Forman MR, Mangini LD, Thelus‐Jean R, Hayward MD. Life‐course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graber JA. Pubertal timing and the development of psychopathology in adolescence and beyond. Horm Behav. 2013;64(2):262–9. [DOI] [PubMed] [Google Scholar]
- 13. Mishra GD, Cooper R, Tom SE, Kuh D. Early life circumstances and their impact on menarche and menopause. Womens Health (Lond). 2009;5(2):175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golub MS, Collman GW, Foster PM, Kimmel CA, Rajpert‐De Meyts E, Reiter EO, et al. Public health implications of altered puberty timing. Pediatrics. 2008;121(Suppl 3):S218–30. [DOI] [PubMed] [Google Scholar]
- 15. Yermachenko A, Dvornyk V. Nongenetic determinants of age at menarche: a systematic review. BioMed Res Int. 2014;2014:371583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu EJ, Choe SA, Yun JW, Son M. Association of early menarche with adolescent health in the setting of rapidly decreasing age at menarche. J Pediatr Adolesc Gynecol. 2020;33(3):264–70. [DOI] [PubMed] [Google Scholar]
- 17. Ellis BJ, Essex MJ. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Dev. 2007;78(6):1799–817. [DOI] [PubMed] [Google Scholar]
- 18. James‐Todd T, Tehranifar P, Rich‐Edwards J, Titievsky L, Terry MB. The impact of socioeconomic status across early life on age at menarche among a racially diverse population of girls. Ann Epidemiol. 2010;20(11):836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun Y, Mensah FK, Azzopardi P, Patton GC, Wake M. Childhood social disadvantage and pubertal timing: a national birth cohort from Australia. Pediatrics. 2017;139(6):e20164099. [DOI] [PubMed] [Google Scholar]
- 20. Steppan M, Whitehead R, McEachran J, Currie C. Family composition and age at menarche: findings from the International Health Behaviour in School‐Aged Children study. Reprod Health. 2019;16(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henrichs KL, McCauley HL, Miller E, Styne DM, Saito N, Breslau J. Early menarche and childhood adversities in a nationally representative sample. Int J Pediatr Endocrinol. 2014;2014(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jorm AF, Christensen H, Rodgers B, Jacomb PA, Easteal S. Association of adverse childhood experiences, age of menarche, and adult reproductive behavior: does the androgen receptor gene play a role? Am J Med Genet B Neuropsychiatr Genet. 2004;125b(1):105–11. [DOI] [PubMed] [Google Scholar]
- 23. Romans SE, Martin JM, Gendall K, Herbison GP. Age of menarche: the role of some psychosocial factors. Psychol Med. 2003;33(5):933–9. [DOI] [PubMed] [Google Scholar]
- 24. Dunbar J, Sheeder J, Lezotte D, Dabelea D, Stevens‐Simon C. Age at menarche and first pregnancy among psychosocially at‐risk adolescents. Am J Public Health. 2008;98(10):1822–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gaudineau A, Ehlinger V, Vayssiere C, Jouret B, Arnaud C, Godeau E. Factors associated with early menarche: results from the French Health Behaviour in School‐aged Children (HBSC) study. BMC Public Health. 2010;10:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ibitoye M, Choi C, Tai H, Lee G, Sommer M. Early menarche: a systematic review of its effect on sexual and reproductive health in low‐ and middle‐income countries. PLoS One. 2017;12(6):e0178884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Platt JM, Colich NL, McLaughlin KA, Gary D, Keyes KM. Transdiagnostic psychiatric disorder risk associated with early age of menarche: a latent modeling approach. Compr Psychiatry. 2017;79:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta‐analysis. Lancet Public Health. 2017;2(8):e356–66. [DOI] [PubMed] [Google Scholar]
- 29. Wiehn J, Hornberg C, Fischer F. How adverse childhood experiences relate to single and multiple health risk behaviours in German public university students: a cross‐sectional analysis. BMC Public Health. 2018;18(1):1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishikawa H, Tachimori H, Takeshima T, Umeda M, Miyamoto K, Shimoda H, et al. Prevalence, treatment, and the correlates of common mental disorders in the mid 2010's in Japan: the results of the world mental health Japan 2nd survey. J Affect Disord. 2018;241:554–62. [DOI] [PubMed] [Google Scholar]
- 31. Kessler R, Aguilar‐Gaxiola S, Alonso J, Angermeyer M, Anthony J, Berglund P, et al. Lifetime prevalence and age of onset distributions of mental disorders in the World Mental Health Survey initiative. In: Ronald CK, Ustun TB, editors. The WHO World Mental Health surveys: global perspectives on the epidemiology of mental disorders. New York: Cambridge University Press; 2008. p. 511–21. [Google Scholar]
- 32. Kessler RC, Ustün TB. The World Mental Health (WMH) survey initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res. 2004;13(2):93–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haro JM, Arbabzadeh‐Bouchez S, Brugha TS, de Girolamo G, Guyer ME, Jin R, et al. Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health surveys. Int J Methods Psychiatr Res. 2006;15(4):167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Straus MA, Douglas EM. A short form of the Revised Conflict Tactics Scales, and typologies for severity and mutuality. Violence Vict. 2004;19(5):507–20. [DOI] [PubMed] [Google Scholar]
- 35. Scott KM, Von Korff M, Alonso J, Angermeyer MC, Benjet C, Bruffaerts R, et al. Childhood adversity, early‐onset depressive/anxiety disorders, and adult‐onset asthma. Psychosom Med. 2008;70(9):1035–43. [DOI] [PubMed] [Google Scholar]
- 36. Courtney ME, Piliavin I, Grogan‐Kaylor A, Nesmith A. Foster youth transitions to adulthood: a longitudinal view of youth leaving care. Child Welfare. 2001;80(6):685–717. [PubMed] [Google Scholar]
- 37. Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34(10):1229–35. [DOI] [PubMed] [Google Scholar]
- 38. Colich NL, Platt JM, Keyes KM, Sumner JA, Allen NB, McLaughlin KA. Earlier age at menarche as a transdiagnostic mechanism linking childhood trauma with multiple forms of psychopathology in adolescent girls. Psychol Med. 2020;50(7):1090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson‐Hughes B, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155(7):672–9. [DOI] [PubMed] [Google Scholar]
- 40. Cooper R, Blell M, Hardy R, Black S, Pollard TM, Wadsworth ME, et al. Validity of age at menarche self‐reported in adulthood. J Epidemiol Community Health. 2006;60(11):993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Magnus MC, Guyatt AL, Lawn RB, Wyss AB, Trajanoska K, Küpers LK, et al. Identifying potential causal effects of age at menarche: a Mendelian randomization phenome‐wide association study. BMC Med. 2020;18(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study are not publicly available due to privacy and ethical restrictions, and will be available on request from the corresponding author. When a reasonable request is received, the data will be made available after approval by the ethics committee of the University of Tokyo.


