Abstract
Aim
Healthy older drivers may be at high risk of fatal traffic accidents. Our recent study showed that volumetric alterations in gray matter in the brain regions within the dorsal attention network (DAN) were strongly related to the risk of unsafe driving in healthy older people. However, the relationship between white matter (WM) structural connectivity and driving ability in healthy older people is still unclear.
Methods
We used diffusion tensor imaging to examine the association between microstructural alterations in the DAN and the risk of unsafe driving among healthy older people. We enrolled 32 healthy older individuals aged over 65 years and screened unsafe drivers using an on‐road driving test. We then determined the pattern of WM aberrations in unsafe drivers using tract‐based spatial statistics.
Results
The analysis demonstrated that unsafe drivers had significantly higher axial diffusivity values in nine WM clusters compared with safe drivers. These results were primarily observed bilaterally in the dorsal superior longitudinal fasciculus, which is involved in the DAN. Furthermore, correlation analyses showed that higher axial diffusivity values in the superior longitudinal fasciculus were associated with lower Trail Making Test A scores within unsafe drivers. This result suggests that functionally, WM microstructural alterations in the DAN are associated with attention problems, which may contribute to the risk of unsafe driving among healthy older people.
Conclusion
Our findings may elucidate the neurobiological mechanisms underlying the increased risk of unsafe driving in healthy older people, potentially facilitating the development of new interventions to prevent fatal accidents.
Keywords: diffusion tensor imaging, distracted driving, healthy older people, microstructural changes, tract‐based spatial statistics (TBSS)
INTRODUCTION
In developed countries, the number of older drivers is rapidly increasing as the population ages. This poses significant challenges for public health, as older drivers have a relatively higher risk of causing fatal traffic accidents. 1 , 2 The higher crash rates among older drivers may be related to age‐related medical conditions, such as dementia, stroke, Parkinson's disease, and other neurodegenerative disorders. 3 In fact, many studies have shown that these conditions can lead to a decline in driving ability. 4 , 5 Despite the large number of health conditions that can adversely affect driving ability, the percentage of older drivers who are considered healthy still represents the largest segment. Surprisingly, the latest National Police Agency report covering annual traffic accidents in Japan showed that cognitively healthy older people caused nearly half of all fatal accidents involving older drivers. 6 However, no standardized screening method to identify older drivers who are cognitively normal but carry a high risk of causing fatal traffic accidents has been established. 7 Although on‐road driving tests are recognized as the gold standard for measuring driving ability, they are costly, making it impractical to administer such tests to all older drivers. 8 Therefore, an objective evaluation of driving ability in healthy older people based on biological markers of unsafe driving is needed. Such assessments could use neuropsychological tests or neurobiological markers identified using brain magnetic resonance imaging (MRI) data.
Previous studies using neuropsychological tests have shown that the Maze Navigation Time Test and the Trail‐Making Test (TMT) are useful for screening driving ability on the basis of their positive correlation with on‐road driving evaluations. 9 , 10 However, these previous studies lacked objectivity in terms of assessments of driving ability. Specifically, on‐road driving ability was measured using the scores provided by driving instructors, making these studies vulnerable to interrater variability and inconsistent definitions of rating scores. 11 To overcome these problems, we developed a new method for objectively evaluating on‐road driving ability in healthy older people based on vehicle behavior. Furthermore, to clarify the neuropsychological functions associated with unsafe driving, we built a reliable classification model utilizing a machine‐learning approach to dissociate unsafe drivers from safe drivers. In a previous study, our model had an accuracy of 84.8% based on neuropsychological assessments. 7 In this model, the results of an attentional function task had the greatest contribution to the classification, indicating that attentional dysfunction is strongly associated with the risk of unsafe driving in healthy older people.
To our knowledge, no previous studies have investigated how brain structural alterations affect on‐road driving ability. Thus, based on our previous study, we hypothesized that structural abnormalities in brain regions involved in attentional functions might be associated with the risk of unsafe driving among healthy older drivers. Previously, we exploratively built a classification model that could dissociate unsafe drivers from safe drivers with an accuracy of 87.5% using gray matter volume data. 12 Our analysis of the features that contributed to this model showed that gray matter volume in the frontal eye field and inferior parietal lobule (IPL) were strongly associated with a high risk of unsafe driving among healthy older people. 12 Since the frontal eye field and IPL constitute the dorsal attention network (DAN), this finding suggested that the DAN is more strongly involved in the risk of unsafe driving in healthy older people than other attention‐related networks, such as the ventral attention network (VAN) and the default mode network (DMN). Moreover, previous diffusion tensor imaging (DTI) studies have shown that attentional dysfunction in older people was associated with white matter (WM) microstructural alterations. 13 , 14 Thus, it may be possible to assess the risk of unsafe driving in healthy older people according to WM microstructural alterations in the DAN. However, to the best of our knowledge, no studies have used DTI to directly examine the relationship between WM microstructure and the risk of unsafe driving in healthy older people.
In the present study, we hypothesized that WM microstructural alterations in the DAN would be associated with the risk of unsafe driving among healthy older people. To test this hypothesis, we used a whole‐brain, voxel‐wise DTI approach to characterize WM microstructural properties associated with a high risk of unsafe driving among healthy older people.
METHODS
Participants
The present study included 32 healthy individuals aged over 65 years. They were recruited from the local community through online advertisements at the University of Tokyo and the Musashisakai Driving School (Tokyo, Japan). All participants were diagnosed as “cognitively normal” based on the Alzheimer's Disease Neuroimaging Initiative (ADNI) diagnostic classification: (1) Mini‐Mental State Examination (MMSE) score between 24 and 30; (2), Clinical Dementia Rating (CDR) score of 0; and (3) normal memory function measured by education‐adjusted scores on the Logical Memory II subscale (delayed paragraph recall) from the Wechsler Memory Scale‐Revised (WMS‐R). 15 No participants had been diagnosed with psychiatric or neurological conditions. They all had a valid driver's license and were actively driving at the time of the study. After receiving an extensive description of the study, all participants provided written informed consent prior to enrollment. The study protocol was approved by the ethics committees of the University of Tokyo and Keio University and prepared in accordance with the ethical standards of the Declaration of Helsinki. All of the participants first took an on‐road driving test at the Musashisakai Driving School, and then travelled to Keio University Hospital where they underwent cognitive assessments and an MRI scan.
Measurements
Cognitive assessment
We administered the Raven's Colored Progressive Matrices (RCPM) test to all participants as an estimate of general cognitive function. We then further evaluated memory and visuospatial function using the Rey Auditory Verbal Learning Test (RAVLT), the Rey–Osterrieth Complex Figure Test (ROCFT), the Clock Drawing Test (CDT), and the Everyday Memory Checklist (EMC); attentional/executive function using the Stroop Test (ST), the Trail Making Test (TMT) A and B, and the Dysexecutive Questionnaire (DEX); and subjective driving ability using the Driving Behavior Questionnaire (DBQ). 16 We assessed handedness using the Edinburgh Handedness Inventory. 17 The CDT was scored using a five‐point system adopted from the cognitive assessment component. 18 Clinical neuropsychologists (M. Y. and K. K.) administered all cognitive assessments in an environment with adequate lighting and reduced noise conditions.
On‐road driving test and classification of safe and unsafe drivers
To evaluate on‐road driving ability on the basis of vehicle behaviors, we administered an on‐road driving test using an instrumented automatic vehicle with a data recorder and charge‐coupled device (CCD) cameras. 19 During the on‐road driving test, we were able to collect vehicle information, including the speed, acceleration, position, and location. Based on this vehicle information, we classified all participants as unsafe or safe drivers. 7 , 12 Specifically, we evaluated participant driving ability at intersections with a stop sign, because older drivers most frequently cause traffic accidents at intersections without traffic lights. 20 The on‐road driving tests were conducted at the Musashisakai Driving School in suburban Tokyo. All participants drove the instrumented vehicle on the same course in a city area located near the driving school for 30 min. To ensure participant safety, a driving instructor accompanied participants in the car while they drove the vehicle.
MRI acquisition
All MRI data were acquired using a 3.0 Tesla MRI scanner (MAGNETOM Verio; Siemens Healthineers) equipped with an eight‐channel head coil. Diffusion‐weighted images (DWI) were collected using an echo‐planar imaging (EPI) spin echo sequence with b0‐images and 30 directions of diffusion gradients (in‐plane resolution, 2.0 × 2.0 mm; 2‐mm slice thickness with no gap; repetition time, 15.0 s; echo time, 91 ms; b‐value, 1000 s/mm2; matrix size, 114 × 114; phase encoding, anterior‐to‐posterior; 75 transversal slices; number of excitations, 1).
Preprocessing
First, DWI data were denoised using the “dwidenoise” tool from the MRtrix3 package (http://www.mrtrix.org). 21 , 22 , 23 The data were then corrected for Gibbs ringing artifacts using the “mrdegibbs” tool from MRtrix3, 23 corrected for eddy currents and head motion using the “dwifslpreproc” tool from MRtrix3, 24 and corrected for B1 field inhomogeneity using the “dwibiascorrect” tool with the “‐ants” option from MRtrix3. 25 Next, we generated a whole brain mask for each image using the “dwi2mask” tool from MRtrix3. 26 Finally, fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) maps were calculated using the DTIFIT tool implemented in FMRIB Software Library (FSL; version 6). 27
Tract‐based spatial statistics
The FA images from all participants were registered to a template created by averaging the FA images (FMRIB‐58) in Montreal Neurological Institute (MNI) space using the FNIRT tool from FSL. After registration, all of the FA images were averaged to generate a mean FA image. Then, the mean FA image was thinned to create a mean FA skeleton of WM tracts, and an FA value of 0.2 was used as a threshold to distinguish WM from gray matter. 28 Next, the FA images for each subject were projected onto the mean FA skeleton. In a similar manner, other DTI parameters, including MD, RD, and AD, were projected onto the mean FA skeleton. Differences in DTI parameters between unsafe and safe drivers were analyzed in a voxel‐wise fashion using the Randomise function in FSL with 5000 permutations and age and sex as covariates. 29 Statistical significance was set at a family‐wise error rate (α = 0.05)‐corrected threshold‐free cluster enhancement (TFCE) of p < 0.05, with a minimum cluster size of 10 voxels. 30
Correlation of DTI parameters in the superior longitudinal fasciculus with TMT A scores among unsafe drivers
We examined correlations between microstructural alterations in the superior longitudinal fasciculus (SLF) and visuospatial attentional function in unsafe drivers, because the SLF was the main WM tract where the group differences were observed in the tract‐based spatial statistics (TBSS) analyses. Specifically, we analyzed the correlation between the DTI parameters of the SLF and total scores of the TMT A. 31 In this analysis, we first identified the right/left SLF in the TBSS skeleton based on the Johns Hopkins University WM tractography atlas. 32 Subsequently, the voxels within the SLF that showed significant differences between unsafe and safe drivers in the TBSS analysis were defined as regions of interest. The mean DTI parameters of the regions of interest were then extracted from each participant and plotted against the corresponding TMT A scores.
Statistical analysis
For the clinical data, we adopted a two‐tailed t‐test, χ 2 test, or multivariate analysis of variance for the group comparisons between safe drivers and unsafe drivers. We used IBM SPSS Statistics 25 software for Mac OS (IBM) for the statistical analysis. We used a liberal statistical threshold of p < 0.05.
RESULTS
Demographics and cognitive measures
We classified 21 participants as safe drivers and 11 participants as unsafe drivers (Table 1). There were significant differences between the groups in terms of the DEX and the EMC scores. There were no differences in the sex ratio, age, duration of education, driving experience, or handedness between the groups.
Table 1.
Demographics and cognitive measures
| Subscale | Safe drivers | Unsafe drivers | F or T | p Value | |
|---|---|---|---|---|---|
| n | 21 | 11 | |||
| Demographic data | |||||
| Sex male/female | 20/1 | 10/1 | 0.631 | ||
| Age (years) | 74.9 ± 3.7 | 77.9 ± 4.1 | 2.02 | 0.052 | |
| Handedness | 89.4 ± 34.3 | 100 ± 0.0 | 0.99 | 0.330 | |
| Education (years) | 14.4 ± 2.1 | 14.5 ± 1.9 | 0.10 | 0.925 | |
| Driving experience (years) | 51.0 ± 6.9 | 47.0 ± 14.5 | 0.83 | 0.424 | |
| Neuropsychological tests | |||||
| MMSE total | 27.5 ± 2.2 | 27.8 ± 1.5 | 0.38 | 0.708 | |
| Logical memory section of the WMS‐R | 0.54 | 0.586 | |||
| Immediate recall | 19.4 ± 5.1 | 17.2 ± 7.4 | |||
| Delayed recall | 15.1 ± 5.4 | 12.8 ± 6.2 | |||
| RCPM | 29.3 ± 2.9 | 31.0 ± 2.9 | 1.53 | 0.135 | |
| RAVLT | 1.58 | 0.183 | |||
| Immediate recall, 1st trial | 5.2 ± 1.8 | 4.2 ± 1.5 | |||
| Immediate recall, 2nd trial | 7.2 ± 1.9 | 7.2 ± 2.0 | |||
| Immediate recall, 3rd trial | 8.8 ± 2.4 | 8.5 ± 1.8 | |||
| Immediate recall, 4th trial | 9.8 ± 2.6 | 10.0 ± 2.0 | |||
| Immediate recall, 5th trial | 10.8 ± 2.3 | 10.5 ± 2.3 | |||
| Interference | 4.6 ± 1.5 | 4.3 ± 1.8 | |||
| Delayed recall | 8.8 ± 3.1 | 6.7 ± 3.5 | |||
| Recognition correct | 14.0 ± 0.9 | 13.2 ± 3.9 | |||
| Recognition false positive | 1.1 ± 1.8 | 0.6 ± 1.1 | |||
| Recognition false negative | 1.0 ± 0.9 | 1.8 ± 3.9 | |||
| ROCFT | 1.98 | 0.156 | |||
| Copy | 35.0 ± 1.3 | 35.5 ± 0.8 | |||
| Delayed recall | 20.0 ± 5.0 | 23.9 ± 5.0 | |||
| ST | Completion time | 0.64 | 0.598 | ||
| Part I (s) | 17.1 ± 2.7 | 18.1 ± 3.8 | |||
| Part II (s) | 20.0 ± 3.8 | 21.8 ± 4.6 | |||
| Part III (s) | 28.8 ± 11.2 | 29.3 ± 6.3 | |||
| Number of errors | 0.32 | 0.813 | |||
| Part I | 0.1 ± 0.3 | 0.1 ± 0.3 | |||
| Part II | 0.2 ± 0.5 | 0.3 ± 0.4 | |||
| Part III | 1.2 ± 1.4 | 0.7 ± 1.1 | |||
| TMT | 0.55 | 0.585 | |||
| A | 100.7 ± 33.9 | 97.3 ± 20.2 | |||
| B | 158.9 ± 79.2 | 133.8 ± 55.9 | |||
| CDT | 0.77 | 0.388 | |||
| Copy | 5.0 ± 0.0 | 5.0 ± 0.0 | |||
| Free‐drawn | 4.9 ± 0.3 | 4.7 ± 0.4 | |||
| DEX* | 10.5 ± 7.3 | 17.2 ± 9.4 | 2.15 | 0.040 | |
| EMC* | 6.7 ± 3.8 | 10.8 ± 3.8 | 2.82 | 0.008 | |
| DBQ | 69.5 ± 14.5 | 73.2 ± 15.2 | 0.65 | 0.518 | |
Abbreviations: CDT, Clock Drawing Test; DBQ, Driving Behavior Questionnaire; DEX, Dysexecutive Questionnaire; EMC, Everyday Memory Checklist; MMSE, Mini‐Mental State Examination; RAVLT, Rey Auditory Verbal Learning Test; RCPM, Raven's Colored Progressive Matrices; ROCFT, Rey–Osterrieth Complex Figure Test; SD, standard deviation; ST, Stroop Test; TMT, Trail Making Test; WMS‐R, Logical Memory II subscale of the Wechsler Memory Scale–Revised.
*Statistical significance between groups.
TBSS
TBSS demonstrated that unsafe drivers had significantly higher AD values in nine WM clusters compared with safe drivers (TFCE‐corrected p < 0.05; Figure 1; Table 2). These results were primarily observed bilaterally in the SLF. Specifically, the nine clusters contained a total of 1804 voxels, of which 384 voxels were placed in the right SLF and 286 voxels were located in the left SLF. Other clusters with significantly higher AD were bilaterally distributed in the anterior thalamic radiation (ATR), inferior longitudinal fasciculus, and inferior fronto‐occipital fasciculus. No clusters exhibited significant group differences in FA, RD, or MD (TFCE‐corrected p < 0.05).
Figure 1.
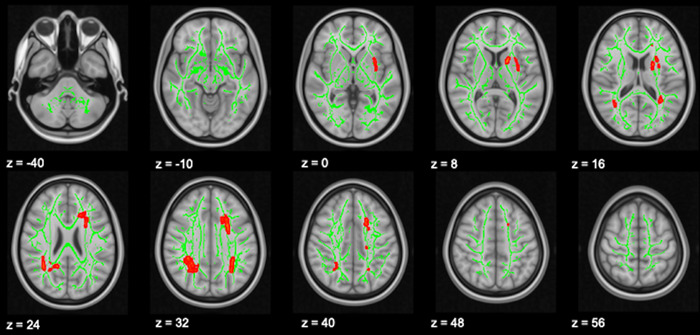
Voxels showing significant higher axial diffusivity (AD) values in unsafe drivers compared with safe drivers (threshold‐free cluster enhancement [TFCE]‐corrected p < 0.05). An AD increase in unsafe drivers relative to safe drivers is shown in red. The whole‐brain skeleton is shown in green. The results are mapped onto a standard T1 Montreal Neurological Institute (MNI) 152 template. Images are presented using the right/left radiological convention with MNI z coordinates in millimeters.
Table 2.
Clusters with significant higher AD values in unsafe drivers (TFCE‐corrected p < 0.05)
| Cluster ID | Tract(s) | p | T max | voxels | X | Y | z |
|---|---|---|---|---|---|---|---|
| 1 | R anterior thalamic radiation, cingulate gyrus, hippocampus, forceps major, inferior fronto‐occipital fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus | 0.033 | 5.05 | 705 | 24 | −55 | 29 |
| 2 | L anterior thalamic radiation, cingulate gyrus, forceps minor, inferior fronto‐occipital fasciculus, superior longitudinal fasciculus, uncinate fasciculus | 0.036 | 4.78 | 565 | −21 | 15 | 35 |
| 3 | L anterior thalamic radiation, cingulate gyrus, inferior fronto‐occipital fasciculus, inferior longitudinal fasciculus, superior longitudinal fasciculus | 0.047 | 3.13 | 147 | −32 | −47 | 31 |
| 4 | L anterior thalamic radiation, inferior fronto‐occipital fasciculus | 0.045 | 3.75 | 125 | −20 | 3 | 15 |
| 5 | L superior longitudinal fasciculus | 0.045 | 4.01 | 83 | −33 | −4 | 6 |
| 6 | L inferior fronto‐occipital fasciculus, superior longitudinal fasciculus, uncinate fasciculus | 0.047 | 3.53 | 74 | −32 | 4 | 1 |
| 7 | L anterior thalamic radiation, cingulate gyrus, inferior fronto‐occipital fasciculus, superior longitudinal fasciculus, uncinate fasciculus | 0.047 | 3.29 | 53 | −27 | 12 | 12 |
| 8 | L anterior thalamic radiation, inferior fronto‐occipital fasciculus, superior longitudinal fasciculus | 0.048 | 4.32 | 36 | −33 | −50 | 15 |
| 9 | L corticospinal tract | 0.050 | 3.01 | 16 | −17 | −21 | 35 |
Abbreviations: AD, axial diffusivity; TFCE, threshold‐free cluster enhancement; T max, maximum T statistic within the cluster at X Y Z MNI coordinates in millimeters; R, right; L, left.
Correlation between AD values in the SLF and TMT A scores among unsafe drivers
We found significant positive correlations between AD values in the SLF and TMT A scores within unsafe drivers (left: R 2 = 0.62, p = 0.0037; right: R 2 = 0.50, p = 0.015) (Figure 2).
Figure 2.
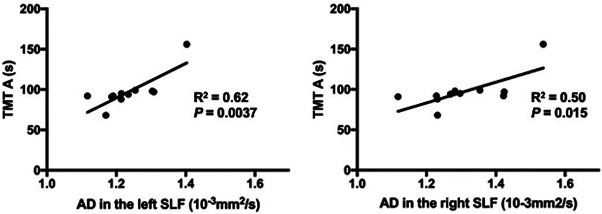
Scatter plots of axial diffusivity (AD) values in the superior longitudinal fasciculus (SLF) against Trail Making Test (TMT) A scores in unsafe drivers. AD values in the SLF were significantly and positively correlated with TMT A scores. Statistical significance threshold: p < 0.05 for the left and right SLF (Bonferroni correction).
DISCUSSION
The aim of the present study was to characterize WM microstructural properties associated with a high risk of unsafe driving among healthy older people using a whole‐brain, voxel‐wised DTI approach. We found that unsafe drivers had significantly higher AD values in nine WM clusters compared with safe drivers. These results were primarily observed bilaterally in the SLF. In contrast, we found no group differences in other DTI parameters, such as FA, RD, and MD. Furthermore, correlation analyses showed that AD values in the SLF were positively correlated with TMT A scores.
The SLF is an extensive WM tract that mainly connects the frontal and parietal lobes, and is anatomically divided into three segments: dorsal, ventral, and posterior. 33 In detail, the dorsal SLF links the IPL to the superior frontal gyrus (SFG), the ventral SLF originates from the supramarginal gyrus and terminates in the inferior frontal gyrus, and the posterior SLF connects the middle temporal gyrus to the angular gyrus. 34 Functionally, each segment of the SLF has been shown to be involved in different cognitive functions. Specifically, the dorsal SLF is involved in visuospatial attention, 35 , 36 the ventral SLF is responsible for verbal working memory and auditory language comprehension, 37 , 38 and the posterior SLF is related to prosodic processing and social cognition. 39 , 40 In general, it is technically infeasible to determine the segments of the SLF that exhibit the strongest WM microstructural alterations between groups. Thus, we were unable to perform this type of analysis in the present study. However, we assumed that the dorsal SLF was the main WM segment that was disrupted for the following two reasons. First, anatomically, most of the voxels showing WM microstructural alterations were located around the SFG and IPL, which constitute the dorsal SLF. Second, functionally, the findings of our correlation analyses showed that higher AD values were associated with worse scores on the TMT‐A task in the unsafe drivers group. Thus, we assumed that the microstructure of the dorsal SLF was the most strongly affected based on its functional role in visuospatial attention.
Intriguingly, a recent brain imaging study combining functional MRI and network tractography showed that the dorsal SLF plays major roles in the DAN. 41 In general, the DAN is involved in the goal‐directed control of perceptual processing (i.e., top‐down attention). 42 , 43 , 44 Top‐down attention improves the detection of targets such as road signs by prioritizing goal‐relevant information in the visual cortex prior to the appearance of the target. 45 For older drivers, top‐down attention compensates for age‐related reductions in road hazard detection. Correspondingly, declines in top‐down attention have been shown to lead to motor vehicle accidents involving older drivers. 46 WM microstructural disruptions in the dorsal SLF are thus associated with reduced top‐down attention, and may be associated with the risk of unsafe driving in older drivers. However, no previous DTI studies have directly examined the relationship between on‐road driving ability and WM microstructural alterations in healthy older individuals. Therefore, the present results extend the previous findings and provide the first neurobiological evidence that changes in WM microstructures involved in top‐down attention are associated with a greater risk of unsafe driving in healthy older people.
In general, different DTI parameters reflect distinct aspects of WM microstructure; so investigating the DTI parameters that vary between groups could elucidate the mechanisms of WM microstructural alterations. 47 In the present study, the AD value was the most sensitive parameter that could be used to characterize group differences. Specifically, unsafe drivers had significantly higher AD values compared with safe drivers. An increased AD represents elevated water diffusivity parallel to axonal fiber tracts, 48 which reflects axonal swelling and a consequent narrowing of the space between myelin fiber bundles. 49 , 50 Although the underlying neuropathological mechanisms of our findings are unclear, age‐related chronic ischemia could cause axonal swelling leading to increased AD values. Previous postmortem brain studies have shown that axonal edema is caused by diseases associated with chronic ischemia. Specifically, autopsy findings using electron microscopy in patients with reversible posterior leukoencephalopathy syndrome 51 and Fabry's disease 52 demonstrated hydropic swelling of axons in the cerebral deep WM. Furthermore, a recent in vitro study using mouse models showed that chronic ischemia induces axonal swelling because of water influx via α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionate (AMPA) receptors. 53 In summary, axonal edema caused by chronic ischemia may underlie the enhanced risk of unsafe driving in healthy older people.
Using TBSS, we found that unsafe drivers had significantly higher AD values in WM tracts, including the bilateral SLF and ATR. Increased AD values in the SLF and ATR have repeatedly been shown to be associated with normal aging. For example, a TBSS study comparing 63 healthy older subjects with 80 young healthy subjects showed that the older group had increased AD values in WM tracts, including the bilateral SLF and ATR. 54 In addition, another TBSS study exploring WM structural changes in healthy older adults during a 2‐year period revealed a significant increase in AD values in multiple brain regions, including the SLF and ATR, at 2 years compared with the baseline. 55 According to these observations, increased AD values in unsafe drivers may represent age‐related WM microstructural alterations, suggesting that the effect of aging on WM microstructure might have been more pronounced in unsafe versus safe drivers.
LIMITATIONS
This study has several limitations. First, given the cross‐sectional nature of our study design, future longitudinal studies are needed to establish the causal relationship suggested by the current results. A longitudinal design with two or more data‐collection time points would allow us to more precisely assess the relationship between WM microstructural changes and the risk of unsafe driving. Second, although we evaluated on‐road driving ability on the basis of vehicle behavior, we only assessed one aspect of driving ability. Examining driver behaviors when turning right at an intersection or making a lane change, for example, might yield useful results. Third, the number of participants per group was relatively small, which might have caused our study to be underpowered. In other words, due to the limited sample size, there might have been no clusters exhibiting significant group differences in FA, MD, or RD. Therefore, our findings require additional confirmation by future studies with larger samples at multiple sites.
CONCLUSION
In conclusion, we revealed for the first time that WM microstructural alterations are associated with the risk of unsafe driving in healthy older people. Specifically, unsafe drivers exhibited increased AD values mainly in the dorsal SLF, which is involved in DAN. The current findings enhance our understanding of the mechanisms underlying an increased risk of unsafe driving in healthy older people, which may facilitate the development of new interventions to prevent fatal accidents.
AUTHOR CONTRIBUTIONS
Yasuharu Yamamoto, Jinichi Hirano, Motoki Shino, Masaru Mimura, and Bun Yamagata designed the study. Yasuharu Yamamoto, Jinichi Hirano, Hiroshi Yoshitake, Motoki Shino, Mika Yamagishi, Mariko Kimura, Kei Kamiya, and Bun Yamagata acquired the data. Yasuharu Yamamoto, Jinichi Hirano, Ryo Ueda, Hiroshi Yoshitake, Motoki Shino, and Bun Yamagata analyzed and interpreted the results of the data. Yasuharu Yamamoto, Jinichi Hirano, Masaru Mimura, and Bun Yamagata drafted the manuscript. All authors contributed to the article and approved the submitted version.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS APPROVAL STATEMENT
Research procedures involving human participants were reviewed and approved by the ethics committees of the University of Tokyo and Keio University.
PATIENT CONSENT STATEMENT
The participants provided their written informed consent to participate in this study.
ACKNOWLEDGMENTS
We thank Tomomi Kikuya for contributing to the data collection. This work was supported by The General Insurance Association of Japan (to B. Y.); JSPS KAKENHI Grant Number 20H03607 (to B. Y.); JSPS KAKENHI Grant Number 22K15770 (to Y. Y.); “Research and development of technology for enhancing functional recovery of elderly and disabled people based on non‐invasive brain imaging and robotic assistive devices”, commissioned research of the National Institute of Information and Communications Technology, Japan (to M. M.); JSPS KAKENHI JP16H03130 (to M. S.); and The Mitsui Sumitomo Insurance Welfare Foundation (to M. S.).
Yamamoto Y, Hirano J, Ueda R, Yoshitake H, Yamagishi M, Kimura M, et al. White matter alterations in the dorsal attention network contribute to a high risk of unsafe driving in healthy older people. Psychiatry Clin Neurosci Rep. 2022;1:e45. 10.1002/pcn5.45
Jinichi Hirano and Bun Yamagata contributed equally to this work.
Contributor Information
Jinichi Hirano, Email: hjinichi@gmail.com.
Bun Yamagata, Email: yamagata@a6.keio.jp.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request to the corresponding author.
REFERENCES
- 1. Evans L. Risks older drivers face themselves and threats they pose to other road users. Int J Epidemiol. 2000;29:315–22. [DOI] [PubMed] [Google Scholar]
- 2. Dattoma LL. Evaluation of the older driver. Prim Care. 2017;44:457–67. [DOI] [PubMed] [Google Scholar]
- 3. McGwin G, Brown DB. Characteristics of traffic crashes among young, middle‐aged, and older drivers. Accid Anal Prev. 1999;31:181–98. [DOI] [PubMed] [Google Scholar]
- 4. Drachman DA, Swearer JM. Driving and Alzheimer's disease: the risk of crashes. Neurology. 1993;43:2448–56. [DOI] [PubMed] [Google Scholar]
- 5. Frittelli C, Borghetti D, Iudice G, Bonanni E, Maestri M, Tognoni G, et al. Effects of Alzheimer's disease and mild cognitive impairment on driving ability: a controlled clinical study by simulated driving test. Int J Geriatr Psychiatry. 2009;24:232–8. [DOI] [PubMed] [Google Scholar]
- 6. National Police Agency. Survey of Driver's License in 2019 . 2020; [cited 2021 Dec 2]. Available from: https://www.npa.go.jp/publications/statistics/koutsuu/menkyo.html
- 7. Yamamoto Y, Hirano J, Yoshitake H, Negishi K, Mimura M, Shino M, et al. Machine‐learning approach to predict on‐road driving ability in healthy older people. Psychiatry Clin Neurosci. 2020;74:488–95. [DOI] [PubMed] [Google Scholar]
- 8. Langford J, Fitzharris M, Koppel S, Newstead S. Effectiveness of mandatory license testing for older drivers in reducing crash risk among urban older Australian drivers. Traffic Inj Prev. 2004;5:326–35. [DOI] [PubMed] [Google Scholar]
- 9. Whelihan WM, DiCarlo MA, Paul RH. The relationship of neuropsychological functioning to driving competence in older persons with early cognitive decline. Arch Clin Neuropsychol. 2005;20:217–28. [DOI] [PubMed] [Google Scholar]
- 10. Anstey KJ, Wood J, Lord S, Walker JG. Cognitive, sensory and physical factors enabling driving safety in older adults. Clin Psychol Rev. 2005;25:45–65. [DOI] [PubMed] [Google Scholar]
- 11. Withaar FK, Brouwer WH, van Zomeren AH. Fitness to drive in older drivers with cognitive impairment. J Int Neuropsychol Soc. 2000;6:480–90. [DOI] [PubMed] [Google Scholar]
- 12. Yamamoto Y, Yamagata B, Hirano J, Ueda R, Yoshitake H, Negishi K, et al. Regional gray matter volume identifies high risk of unsafe driving in healthy older people. Front Aging Neurosci. 2020;12:592979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011;77:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bennett IJ, Motes MA, Rao NK, Rypma B. White matter tract integrity predicts visual search performance in young and older adults. Neurobiol Aging. 2012;33(433):e21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakai H, Takahara M, Honjo NF, Doi S, Sadato N, Uchiyama Y. Regional frontal gray matter volume associated with executive function capacity as a risk factor for vehicle crashes in normal aging adults. PLoS One. 2012;7:e45920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 18. Iwatsubo T, Iwata A, Suzuki K, Ihara R, Arai H, Ishii K, et al. Japanese and North American Alzheimer's Disease Neuroimaging Initiative studies: harmonization for international trials. Alzheimer's Dement. 2018;14:1077–87. [DOI] [PubMed] [Google Scholar]
- 19. Shino M, Nakanishi M, Imai R, Yoshitake H, Fujita Y. Investigation of driving behavior and cognitive ability concerning planning process during driving of elderly drivers. Int J Automot Eng. 2018;9:138–44. [Google Scholar]
- 20. Lombardi DA, Horrey WJ, Courtney TK. Age‐related differences in fatal intersection crashes in the United States. Accid Anal Prev. 2017;99:20–9. [DOI] [PubMed] [Google Scholar]
- 21. Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137. [DOI] [PubMed] [Google Scholar]
- 22. Veraart J, Novikov DS, Christiaens D, Ades‐aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magn Reson Med. 2016;76:1574–81. [DOI] [PubMed] [Google Scholar]
- 24. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off‐resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–90. [DOI] [PubMed] [Google Scholar]
- 28. Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract‐based spatial statistics: voxelwise analysis of multi‐subject diffusion data. Neuroimage. 2006;31:1487–505. [DOI] [PubMed] [Google Scholar]
- 29. Möller C, Hafkemeijer A, Pijnenburg YAL, Rombouts SARB, van der Grond J, Dopper E, et al. Joint assessment of white matter integrity, cortical and subcortical atrophy to distinguish AD from behavioral variant FTD: a two‐center study. NeuroImage: Clin. 2015;9:418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamagata B, Ueda R, Tasato K, Aoki Y, Hotta S, Hirano J, et al. Widespread white matter aberrations are associated with phonemic verbal fluency impairment in chronic traumatic brain injury. J Neurotrauma. 2020;37:975–81. [DOI] [PubMed] [Google Scholar]
- 31. Hird MA, Egeto P, Fischer CE, Naglie G, Schweizer TA. A systematic review and meta‐analysis of on‐road simulator and cognitive driving assessment in Alzheimer's disease and mild cognitive impairment. J Alzheimers Dis. 2016;53:713–29. [DOI] [PubMed] [Google Scholar]
- 32. Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract‐specific quantification. Neuroimage. 2008;39:336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakajima R, Kinoshita M, Shinohara H, Nakada M. The superior longitudinal fascicle: reconsidering the fronto‐parietal neural network based on anatomy and function. Brain Imaging Behav. 2020;14:2817–30. [DOI] [PubMed] [Google Scholar]
- 34. Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT‐MRI study. Cerebral Cortex. 2005;15:854–69. [DOI] [PubMed] [Google Scholar]
- 35. Nakajima R, Kinoshita M, Miyashita K, Okita H, Genda R, Yahata T, et al. Damage of the right dorsal superior longitudinal fascicle by awake surgery for glioma causes persistent visuospatial dysfunction. Sci Rep. 2017;7:17158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thiebaut De Schotten M, Tomaiuolo F, Aiello M, Merola S, Silvetti M, Lecce F, et al. Damage to white matter pathways in subacute and chronic spatial neglect: a group study and 2 single‐case studies with complete virtual “in vivo” tractography dissection. Cerebral Cortex. 2014;24:691–706. [DOI] [PubMed] [Google Scholar]
- 37. van Geemen K, Herbet G, Moritz‐Gasser S, Duffau H. Limited plastic potential of the left ventral premotor cortex in speech articulation: evidence from intraoperative awake mapping in glioma patients. Hum Brain Mapp. 2014;35:1587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Papagno C, Comi A, Riva M, Bizzi A, Vernice M, Casarotti A, et al. Mapping the brain network of the phonological loop. Hum Brain Mapp. 2017;38:3011–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maldonado IL, Moritz‐Gasser S, de Champfleur NM, Bertram L, Moulinié G, Duffau H. Surgery for gliomas involving the left inferior parietal lobule: new insights into the functional anatomy provided by stimulation mapping in awake patients: clinical article. J Neurosurg. 2011;115:770–9. [DOI] [PubMed] [Google Scholar]
- 40. Nakajima R, Yordanova YN, Duffau H, Herbet G. Neuropsychological evidence for the crucial role of the right arcuate fasciculus in the face‐based mentalizing network: a disconnection analysis. Neuropsychologia. 2018;115:179–87. [DOI] [PubMed] [Google Scholar]
- 41. Allan PG, Briggs RG, Conner AK, O'Neal CM, Bonney PA, Maxwell BD, et al. Parcellation‐based tractographic modeling of the dorsal attention network. Brain Behav. 2019;9:e01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ptak R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist. 2012;18:502–15. [DOI] [PubMed] [Google Scholar]
- 43. Tamber‐Rosenau BJ, Asplund CL, Marois R. Functional dissociation of the inferior frontal junction from the dorsal attention network in top‐down attentional control. J Neurophysiol. 2018;120:2498–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meehan TP, Bressler SL, Tang W., Astafiev S v., Sylvester CM, Shulman GL, et al. Top‐down cortical interactions in visuospatial attention. Brain Struct Funct. 2017;222:3127–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top‐down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci. 2008;28:10056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feng J, Choi H, Craik FIM, Levine B, Moreno S, Naglie G, et al. Adaptive response criteria in road hazard detection among older drivers. Traffic Inj Prev. 2018;19:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pierpaoli C, Jezzard P, Basser PJ, Barnett A, di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–48. [DOI] [PubMed] [Google Scholar]
- 48. Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion‐weighted images. NMR Biomed. 1995;8:333–44. [DOI] [PubMed] [Google Scholar]
- 49. Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, et al. Aging of cerebral white matter. Ageing Res Rev. 2017;34:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain‐a review. NBM. 2002;15:561–9. [DOI] [PubMed] [Google Scholar]
- 51. Okeda R, Kawamoto T, Tanaka E, Shimizu H. An autopsy case of drug‐induced diffuse cerebral axonopathic leukoencephalopathy: the pathogenesis in relation to reversible posterior leukoencephalopathy syndrome. Neuropathology. 2007;27:364–70. [DOI] [PubMed] [Google Scholar]
- 52. Okeda R, Nisihara M. An autopsy case of Fabry disease with neuropathological investigation of the pathogenesis of associated dementia. Neuropathology. 2008;28:532–40. [DOI] [PubMed] [Google Scholar]
- 53. Cui Y, Jin X, Choi DJ, Choi JY, Kim HS, Hwang DH, et al. Axonal degeneration in an in vitro model of ischemic white matter injury. Neurobiol Dis. 2020;134:104672. [DOI] [PubMed] [Google Scholar]
- 54. Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li SC, Lindenberger U, et al. Age‐related differences in white matter microstructure: region‐specific patterns of diffusivity. Neuroimage. 2010;49:2104–12. [DOI] [PubMed] [Google Scholar]
- 55. Stephen R, Solomon A, Ngandu T, Levälahti E, Rinne JO, Kemppainen N, et al. White matter changes on diffusion tensor imaging in the FINGER randomized controlled trial. J Alzheimer's Dis. 2020;78:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request to the corresponding author.


