Abstract
Over several decades, the approach to treating dyslipidaemias during pregnancy remains essentially unchanged. The lack of advancement in this field is mostly related to the fact that we lack clinical trials of pregnant patients both with available as well as new therapies. While there are numerous novel therapies developed for non-pregnant patients, there are still many limitations in dyslipidaemia treatment during pregnancy. Besides pharmacotherapy and careful clinical assessment, the initiation of behavioural modifications as well as pre-conception management is very important. Among the various lipid-lowering medications, bile acid sequestrants are the only ones officially approved for treating dyslipidaemia in pregnancy. Ezetimibe and fenofibrate can be considered if their benefits outweigh potential risks. Statins are still considered contraindicated, primarily due to animal studies and human case reports. However, recent systematic reviews and meta-analyses as well as data on familial hypercholesterolaemia (FH) in pregnant patients have indicated that their use may not be harmful and could even be beneficial in certain selected cases. This is especially relevant for pregnant patients at very high cardiovascular risk, such as those who have already experienced an acute cardiovascular event or have homozygous or severe forms of heterozygous FH. In these cases, the decision to continue therapy during pregnancy should weigh the potential risks of discontinuation. Bempedoic acid, olezarsen, evinacumab, evolocumab and alirocumab, and inclisiran are options to consider just before and after pregnancy is completed. In conclusion, decisions regarding lipid-lowering therapy for pregnant patients should be personalized. Despite the challenges in designing and conducting studies in pregnant women, there is a strong need to establish the safety and efficacy of dyslipidaemia treatment during pregnancy.
Keywords: Dyslipidaemia, Pregnancy, Bile acid sequestrants, Statins, Familial hypercholesterolaemia
Graphical Abstract
Graphical Abstract.
Introduction
The increase of lipid and lipoprotein levels during pregnancy is important for the proper growth and development of the foetus.1,2 During the first trimester, lipids accumulate in the mother’s body to support foetal development, with this process starting around the 7th week of pregnancy and peaking by the end of the second trimester.3,4 In late pregnancy, stored lipids serve as a reservoir for fatty acid synthesis in placental tissue.3–5 The most significant lipid changes occur during the second and third trimesters.6 Total cholesterol (TC) and triglyceride (TG) levels rise, driven by increased lactogen, oestrogen, and progesterone.7 Triglycerides undergo the most significant increase, reaching two to four times their pre-pregnancy values by the third trimester, typically rising 2.5–3 times.8 Simultaneously, the composition, buoyancy, and size of low-density lipoprotein (LDL) particles change.9,10 Studies indicate a decrease in LDL particle size, leading to an increase in small-dense LDL (sdLDL) concentration, which is considered particularly atherogenic.9,10 This unfavourable effect can be mitigated by elevated levels of high-density lipoprotein cholesterol (HDL-C) and apolipoprotein A-I (Apo A-I), which peak during the second trimester and may offer potential protection against atherogenic lipid fractions.10 However, HDL-C functionality during pregnancy also depends on various factors, including systemic inflammatory tone, obesity, diabetes, chronic kidney disease, and hypertension.11–13
It is crucial to note that all pregnant women experience a natural increase in lipid levels, including those with pre-existing dyslipidaemia. The additional rise is particularly significant for this group, as discontinuation of lipid-lowering therapy during pregnancy leads to a gradual elevation in LDL-C levels, resulting in increased exposure of the arterial vasculature to cholesterol over time.14 While ∼80% of dyslipidaemias are influenced by factors such as diet and lifestyle choices,15 women with a genetic predisposition to dyslipidaemias may face heightened cardiovascular risks.16,17 Consequently, the impact of familial hypercholesterolaemia (FH) on cardiovascular health is more pronounced in women than in men.18
Research by Amundsen et al. comparing plasma lipid concentrations between women with and without FH revealed that the relative increase in lipid fractions was similar. In the FH group, TC and LDL-C increased by 28.7% and 29.6%, respectively, while in healthy pregnant women, it was 25.4% and 34.2%, respectively.18 Triglycerides showed an even greater increase, reaching 116% in the FH group, compared to 103.4% in healthy patients (P < 0.05). Notably, HDL did not exhibit significant differences between the two groups (P < 0.05). These observations were made between the 17th–20th week of pregnancy and the 36th week. Lipid levels returned to normal 3–6 months post-partum. Importantly, despite significantly higher baseline lipid levels, the authors found no significant differences in terms of preterm births, hypertension prevalence, gestational duration, body weight, body length, and head circumference between patients with FH and those without FH before pregnancy.19
Although elevated lipid values during pregnancy are considered physiological, some evidence suggests potential associations with adverse events, such as pregnancy-induced hypertension, pre-eclampsia, gestational diabetes mellitus (GDM), preterm delivery, and macrosomia.19–25 After delivery, these lipid changes may lead to post-partum impaired glucose tolerance26,27 and post-partum dyslipidaemia in mothers.28,29 Napoli et al.30 reported a link between maternal hypercholesterolaemia and early atherosclerosis development in children, demonstrating that maternal dyslipidaemia leads to foetal aortic lesions, rendering children susceptible to fatty-streak formation and atherosclerosis development.
In addition, it is essential to consider the role of lipoprotein(a) [Lp(a)] during pregnancy. Lp(a) levels show a significant increase during pregnancy in all women. In fact, it can nearly double between the 10th and 35th weeks of pregnancy.31 Women with initially elevated Lp(a) levels will undergo a similar increase, and due to their higher baseline levels, they may develop very high Lp(a) levels during pregnancy. Lp(a) is an inflammatory lipoprotein that could induce endothelial dysfunction in the systemic vasculature, including those in the placenta. This could compromise placental arterial function, potentially resulting in high blood pressure in the mother and posing risks to the baby. Elevated Lp(a) values are observed in as many as 20–30% of pregnant women, which can impact prognosis, increasing the risk of pre-eclampsia, preterm delivery, or low birth weight.2,32 Genetically elevated Lp(a) levels promote endothelial dysfunction, possibly potentiating the development of pre-eclampsia.33 Moreover, Lp(a), with its antifibrinolytic properties, contributes to a prothrombotic state, which contributes to low birth weight and preterm labour.34 It may also play a role in reducing bleeding during childbirth.35 Based on these considerations, recent guidelines from six Polish scientific societies recommend the measurement of Lp(a) for all pregnant women (IIb C).16
Another critical concern during pregnancy is severe hypertriglyceridaemia, which can be a life-threatening condition for both the mother and child. With triglycerides steadily increasing during each trimester, there is a high risk of severe disturbances in women with pre-pregnancy elevated triglyceride levels ≥ 500 mg/dL (5.6 mmol/L), which can lead to acute pancreatitis,36 posing risks to both the foetus and mother.
Practical approach in the management of lipid disturbances during pregnancy
Pre-conception management and lipid screening during pregnancy
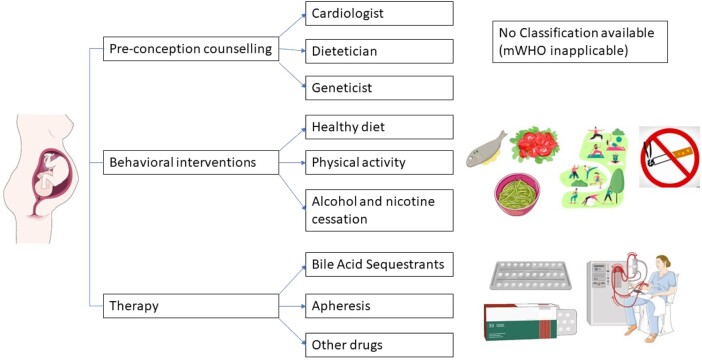
Before considering pregnancy in all patients with previously diagnosed dyslipidaemia, pre-conception referral to a cardiologist, clinical dietitian, and geneticist is recommended. The main purpose of such an approach is the need for careful evaluation of potential pros and cons of the discontinuation of lipid-lowering treatment. Even though lipid disturbances were not included in the modified World Health Organization (mWHO) classification,37 careful assessment of maternal cardiovascular risk should be performed together with the measurement of lipid levels and Lp(a). The monitoring of lipid parameters should be further continued during the whole pregnancy. We are lacking data regarding the frequency of such measurements; thus, it should be individualized. The timing of lipid screening during pregnancy is also not established. However, the benefits of such an approach are promising. Incorporating lipid testing to the routine prenatal check-up may lead to the improvement of a dyslipidaemia diagnosis. Golwala et al.38 assessed 445 pregnant women among whom 236 (66%) performed lipid testing showing abnormal results in 25% patients (n = 59). Incorporation of a lipid panel to the routine prenatal check-up during the first trimester of pregnancy identified one woman with FH. Despite the fact that this constitutes 0.4% of all tested women, it should be emphasized that it has repercussions for both the mother and the child. The authors claim that lipid testing during pregnancy is feasible; however, it requires patient counselling that may be a potential barrier.
Behavioural interventions
Before contemplating the initiation of pharmacological interventions, one must prioritize the integration of behavioural interventions for each pregnant woman afflicted with dyslipidaemia. This imperative arises from the stark realization that a mere 0.1% of pregnant women adhere to an optimal dietary regimen.39 Regrettably, there exist no tailored dietary directives for pregnant women with lipid disorders. Nevertheless, the choice of an appropriate dietary regimen should parallel that recommended for the average dyslipidemic patient, all while duly acknowledging the constraints imposed by pregnancy.
The primary dietary counsel endures unaltered: the avoidance of animal-derived foods, saturated fatty acids (while accentuating the intake of omega-3 polyunsaturated fatty acids), the elimination of trans fats, and highly processed food products, all concomitant with increased consumption of vegetables, fruits, soy products, grains, plant sterols, stanols, nuts, vegetable oils, and legumes.40 Intervention based on the Mediterranean diet (MedDiet) was proved to reduce the frequency of metabolic disorders post-pregnancy. In a study by Melero et al., dietary modification was implemented before the 12th week of pregnancy, and patients were observed at two endpoints post-delivery: at 3 months and 3 years. Up to 3 years post-partum, a group of women who were randomized to diet including extra virgin olive oil > 40 mL per day and nuts (MedDiet) presented with better glycaemic and lipid profiles as well as lower body mass index compared to women without such treatment.41
A considerable portion of the guidelines pertaining to a healthful gestational diet mirror those of a dyslipidemic dietary approach. Nevertheless, pregnant women should avoid overly restrictive diets, and those with severe dyslipidaemia should consult a clinical dietitian.
Alcohol and tobacco use are strictly contraindicated during pregnancy. Regular exercise and lifestyle adjustments enhance treatment efficacy. While general physical activity guidelines are well-established, data specific to gestation remain less clear. In recent years, numerous randomized controlled trials (RCTs) have addressed physical activity during pregnancy. A meta-analysis of these trials, encompassing over 12 000 patients, conducted by Rogozińska et al.,42 has demonstrated a statistically significant reduction in weight gain during pregnancy following the implementation of dietary or physical activity interventions. Moreover, these lifestyle interventions have not precipitated any untoward events. Though, on an individual scale, the decrease of 0.7 kg in gestational weight gain may appear marginal, its collective impact on the broader population is indeed advantageous.43 Physical activity programmes are beneficial for glucose and HbAc1 control in women with GDM, which is also linked to dyslipidaemia.44
In conclusion, it is imperative to institute both lifestyle interventions and concomitantly scrutinize gynaecological and cardiovascular outcomes. The vigilant monitoring of atherosclerotic disease progression, as well as concurrent cardiovascular comorbidities and the risk of pre-eclampsia, preterm labour, and intrauterine growth retardation, assume a position of paramount importance.45 Moreover, more general strategies should be implemented to improve population health, such as the regulation of price and availability of healthy and unhealthy foods. Such strategies would reach entire populations including pregnant women with or without dyslipidaemia, obesity, and other risk factors.
Pharmacotherapy
Despite the established association between dyslipidaemia during pregnancy and adverse events, recent European Society of Cardiology (ESC) guidelines on cardiovascular diseases management during pregnancy have largely overlooked this topic. The guidelines provide only limited information, primarily in a short paragraph about statin therapy.40 Similarly, no recommendations are available from the Centers for Disease Control and Prevention (CDC).46 The ESC guidelines on cardiovascular disease prevention in clinical practice advise against the use of statins in women of fertile age considering pregnancy.47
This underscores the need for greater attention to the issue of dyslipidaemia during pregnancy, particularly as the number of pregnant patients with various cardiovascular risk factors, including lipid disorders, continues to rise. Moreover, due to limited data, treatment options for these conditions remain relatively scarce.
The limited availability of drugs for use during pregnancy primarily stems from the common practice of excluding pregnant women from clinical trials. This scarcity of available data extends to the safety of widely used anti-hyperlipidemic medications. It is crucial to recognize that the treatment regimen impacts both the pregnant mother and the developing foetus, necessitating a careful evaluation of the therapy’s potential benefits weighed against potential risks. The US Food and Drug Administration (FDA) emphasizes the evaluation of drugs using the Pregnancy and Lactation Labelling Rule (PLLR), which offers a comprehensive assessment, including detailed descriptions of associated risks and findings from both animal and human studies.45 A similar classification system has recently been introduced in the ESC guidelines for cardiovascular disease management during pregnancy.48 The ESC guidelines on dyslipidaemias state that lipid-lowering medications should be avoided when pregnancy is being planned, during pregnancy, or while breastfeeding. Nevertheless, in the case of individuals with severe FH, healthcare providers may contemplate the use of bile acid sequestrants (which are not absorbed by the gastrointestinal tract) and/or LDL apheresis.39
Bile acid sequestrants
Among the various available classes of medications for lowering lipid levels, only bile acid sequestrants (BAS), such as cholestyramine, colestipol, and colesevelam, have received approval for treating dyslipidaemia during pregnancy. This approval is linked to the mechanism of action of these drugs. BAS function by disrupting the enterohepatic circulation of bile acids, binding them in the intestinal lumen, and leading to their excretion in stool.46 Consequently, fewer bile acids return to the liver, which activates hepatic bile acid production, a reaction that consumes cholesterol. This results in increased hepatic cholesterol biosynthesis and an increase in LDL receptor expression on the surface of hepatocytes. BAS are known to lower total cholesterol levels and have a modest impact on reducing LDL-C levels.49
The effectiveness of BAS is dosage-dependent. When used as monotherapy, these resins reduce LDL-C by ∼20–30%, and when combined with statins, they contribute an additional reduction of ∼10%.50 However, their effect on triglycerides is limited and may even lead to triglyceride and VLDL elevation, making them contraindicated in individuals with hypertriglyceridaemia (>400 mg/dL).51
It is essential to use BAS alongside dietary modifications. Published data have confirmed the efficacy of BAS in reducing the progression of atherosclerosis, which is associated with the decrease in LDL-C levels.49,50 The maximum effect of BAS is typically observed after one month of therapy, and after discontinuation, LDL levels return to baseline values in approximately one month.51
Currently, the BAS are considered the safest lipid-lowering drugs for use in pregnant women. However, their adherence remains suboptimal due to associated adverse effects, including constipation, abdominal pain, loss of appetite, indigestion, bloating, vomiting, and heartburn, which may exacerbate symptoms already present during normal pregnancy.46 Another important consideration is that the use of BAS can impede the absorption of fat-soluble vitamins, such as vitamin K, potentially increasing the risk of neonatal cerebral bleeding, necessitating appropriate supplementation.37 Furthermore, to prevent reduced absorption of other medications, ion exchange resins should be taken either 4 h before or 1 h after other medications. Based on available data, colesevelam (Cholestagel) appears to be the best-tolerated resin.46
Omega-3 fatty acids
Another viable treatment option during pregnancy is the use of omega-3 fatty acids, which can be safely supplemented during pregnancy. Omega-3 fatty acids reduce triglyceride levels by 20–30% and result in a slight decrease in non-HDL-C and apolipoprotein B levels. The effectiveness of omega-3 fatty acids is dose-dependent and influenced by baseline lipid values.52–55 With larger doses, even up to 4 g/day, they have the potential to reduce the risk of cardiovascular events, as demonstrated in significant trials such as the Japan EPA lipid intervention study (JELIS), and particularly the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) and its numerous sub-analyses.56,57 However, these findings are not specifically related to lipid lowering during pregnancy, as there are no data for this state.
Beyond their triglyceride-lowering effects, the use of omega-3 fatty acids during pregnancy has been shown to reduce the risk of preterm birth (<37 weeks), early preterm birth (<34 weeks), perinatal death, and low birthweight babies. However, it is important to note that omega-3 fatty acids may slightly increase the risk of large-for-gestational-age babies.58 Additionally, their use may elevate the risk of atrial fibrillation.54–57
In summary, omega-3 fatty acids may serve as an effective option for patients with severe hypertriglyceridaemia, especially those at risk of pancreatitis (e.g. individuals with very high levels > 500 mg/dL who are symptomatic or have a history of pancreatitis), even when used in combination with fenofibrate, provided the benefits outweigh the risks.
Statins
In the realm of medical science, HMG-CoA reductase inhibitors, which are commonly used in the treatment of dyslipidaemia, have demonstrated substantial benefits in preventing cardiovascular events. However, their usage during pregnancy is not recommended due to a lack of safety data, the potential decrease in cholesterol synthesis and other lipid-derived substances in newborns,58 and most of all concerns of teratogenicity.59–61 It is worth noting that this recommendation primarily relies on animal studies, which have exhibited findings such as gastroschisis and skeletal malformations in rats,59 reduced birth weight in rabbits and rats,59 and a single human case involving a child with multiple inborn malformations.61 Furthermore, the doses of statins administered in these animal studies exceeded typical human prescription usage.59,60
Some human studies have also suggested potential adverse birth outcomes following statin exposure. Edison et al. reported a series of 20 cases involving malformations in infants exposed to statins during the first trimester, including limb deficiencies (five cases) and central nervous system defects (five cases). It is noteworthy that all malformations occurred in infants whose mothers were exposed to lipophilic statins, while no adverse birth outcomes were reported for infants whose mothers used hydrophilic statins such as pravastatin.62 An analysis by Bateman et al. did not show significant teratogenic complications in a group of 1152 women using statins in the first trimester of pregnancy. In initial unadjusted analyses, the incidence of malformations in offspring from women who used statins during the first trimester was 6.34%, compared to 3.55% in offspring of non-statin users (RR 1.79, 95% CI 1.43–2.23). However, adjusting for confounding factors, particularly pre-existing diabetes, nullified this heightened risk (1.07, 0.85–1.37). Furthermore, there were no statistically significant increases observed in any specific organ malformations upon adjustment for confounders.63
A recently published meta-analysis of one case–control study and five cohort studies revealed no statistically significant elevations in the incidence of major congenital anomalies when comparing the statin-exposed cohort to the control group (OR = 1.27; 95% CI 0.80–2.04; aOR = 1.05; 95% CI 0.84–1.31). However, an increase in the risk of cardiac anomalies was observed in individuals exposed to statins when unadjusted ORs were aggregated (OR = 2.47; 95% CI 1.36–4.49). Upon further examination using adjusted ORs, there was no significant rise in the risk of cardiac anomalies among the statin-exposed cohort compared to the controls (aOR 1.24; 95% CI 0.93–1.66). A significantly diminished rate of live births (OR 0.60, 95% CI 0.49–0.75) and an elevated incidence of spontaneous abortions (OR 1.36; 95% CI 1.06–1.75) were noted in the statin-exposed cohort. However, these observations may be linked with other unadjusted issues and with maternal comorbidities.64
The Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) reported the results of a real-world pharmacovigilance study, showing that pregnancy-related statin adverse events were present in 477 patients.65 Compared to all other drugs, lovastatin was linked with a higher risk of foetal complications and pravastatin with a higher risk of preterm birth and low birth weight (OR = 2.45; 95% CI 1.22–4.95; OR = 4.89; 95% CI 3.65–6.54; and OR = 9.60; 95% CI 5.56–16.56, respectively). Statins were not associated with the risk of spontaneous abortion or foetal death.65 McGrogan et al. showed that in pregnancies potentially exposed to a statin, 25.27% ended in spontaneous loss, contrasting with 20.81% in unexposed pregnancies. Employing time-to-event analysis with exposure as a time-dependent covariate yielded an adjusted hazard ratio of 1.64 (95% CI 1.10–2.46) for spontaneous pregnancy loss in the statin-exposed cohort.66 Subsequent studies have provided evidence of the neutral effect of statins prescribed during pregnancy.67,68
Additionally, statins, owing to their pleiotropic effects, have shown potential in improving placental vascular remodelling. Pre-eclampsia, a complication of pregnancy associated with maternal vascular inflammation, may be mitigated by statin therapy, reducing the risk of its development.69 Nevertheless, the recent multicentre, double-blind, placebo-controlled STATIN trial with pravastatin did not demonstrate an impact on the incidence of pre-eclampsia.70 No significant differences were observed between the pravastatin group (548 patients) and the placebo group (543 patients) in terms of pre-eclampsia (14.6% vs. 13.6%), gestational hypertension, intrauterine growth retardation, neonatal morbidity, mortality, or stillbirth. Moreover, pravastatin was well-tolerated with good adherence to the therapy.70 Despite potentially positive results, current guidelines generally advise discontinuing statin therapy in most pregnant patients.37 However, a recent statement from the FDA removed the strongest warning against statins and suggested that for patients at very high risk of cardiovascular events, the decision about treatment should be individualized. In this context, the most suitable group of pregnant women with dyslipidaemia for statin treatment appears to be those with FH. Botha et al. retrospectively analysed data from 39 pregnancies in females with homozygous FH (HoFH). Among them, 19 patients were treated with statins before or during pregnancy. The authors reported no statistically significant differences in pregnancy complications between HoFH and healthy patients. In this FH patient group, 84% of all pregnancies reached full term. The rates of miscarriages and premature deliveries were both 8%. The authors concluded that statin therapy appears to be safe for both the mother and child, offering a valuable therapeutic option for severe hypercholesterolemic patients, including those with HoFH.71
A systematic review and meta-analysis conducted by Vahedian-Azimi et al. included 23 studies with 1 276 973 participants. Their review did not provide a clear association between statin therapy and an increased rate of birth defects. The meta-analysis indicated that statin treatment did not result in a higher total rate of birth defects (OR 1.48, 95% CI 0.90–2.42, P = 0.509). There were no significant links observed in separate analyses for cardiac anomalies and other congenital anomalies (respectively: OR 2.53, 95% CI 0.81–7.93, P = 0.112 and OR 1.19, 95% CI 0.70–2.03, P = 0.509).72 Another systematic review and meta-analysis by the same research group, comprising nine studies, assessed the effect of statins on the incidence of stillbirth (including 2350 participants), foetal abortion (8422 participants), and preterm delivery (483 participants). The results of the meta-analysis showed a correlation between statin use and the rate of spontaneous abortion (OR 1.36; 95% CI 1.10–1.68, P = 0.004). Conversely, the authors revealed that statin therapy was not associated with an increased incidence of stillbirth (OR 1.30; 95% CI 0.56–3.02, P = 0.54), induced abortion (OR 2.08; 95% CI 0.81–5.36, P = 0.129), or elective abortion (OR 1.37; 95% CI 0.68–2.76, P = 0.378).73
In conclusion, it appears that statin therapy may be a therapeutic option for patients with severe hypercholesterolaemia, especially those with severe FH and those at very high or extremely high cardiovascular disease risk with a history of acute coronary syndromes or strokes.
Fibrates
Alternative options for managing dyslipidaemia during pregnancy typically lack official approval. According to current guidelines, there are inadequate human data supporting the use of fibrates, and nicotinic acid has been reported in only a limited number of case studies. As a result, these drugs are not recommended for use during pregnancy, with the ESC guidelines for 2018 explicitly stating that they should only be considered when the benefits clearly outweigh the risks. Animal studies involving fenofibrate have shown various complications, including delayed delivery, reduced birth weight, increased post-implantation loss, skeletal and visceral abnormalities, abortions, and foetal deaths.37,74 The AHA Scientific Statement for Cardiovascular Considerations in Caring for Pregnant Patients proposes the consideration of fenofibrate or gemfibrozil in the second trimester if triglycerides are >500 mg/dL despite lifestyle modifications.75 The AHA/American College of Obstetricians and Gynecologists (ACOG) Presidential Advisory states that pregnant patients with a history of pancreatitis may benefit from the use of fenofibrate when triglyceride levels are >1000 mg/dL.76 The use of fibrates during the second trimester is after embryogenesis occurs reducing the risk. Studies in animals have found no increased risk of congenital malformations.77
Ezetimibe
Insufficient data are available regarding the use of ezetimibe, although it is considered a safe drug. Animal studies, in which ezetimibe was administered either alone or in combination with statins, demonstrated a higher incidence of skeletal changes in rats and extra ribs in rabbit thoraxes, with no confirmed lethal embryo effects in available animal studies.37,78 Consequently, its use during pregnancy is also limited to individual cases in which the potential benefits clearly outweigh the potential risks. It’s important to note that the lipid-lowering capacity of these drugs may be insufficient to attain the treatment goals for severe hypercholesterolaemia during pregnancy, even when used in conjunction with other lipid-lowering therapies, such as BAS (Tables 1 and 2).
Table 1.
Comparison of classifications indicating the possibility of application of lipid-lowering drugs during pregnancy
| Former FDA classificationa/ESC guidelines 2018 classification | PLLR | ESC guidelines 2018 classification | |
|---|---|---|---|
| BAS (e.g. colesevelam, colestipol, and cholestyramine) | C | Animal studies—failure to reveal evidence of foetal harm. Presence of infrequent reports of pregnancy in the post-marketing period; a causal association with congenital anomalies has not been established. No controlled data in human pregnancy. | Placenta permeable: unknown Transfer to breast milk: yes Clinical safety data: possible impairment of absorption of fat-soluble vitamins, e.g., vitamin K—cerebral bleeding (neonatal) |
| Ezetimibe | — | AU TGA category B3. Higher risk of malformation and other direct or indirect harmful effects on the human foetus. | Placenta permeable: yes (studies in rats and rabbits, no human data) Transfer to breast milk: unknown (data in nursing rats) Clinical safety data: inadequate human data; use only when benefit outweighs risk (data in rats and rabbits) |
| Fibrates (fenofibrate) | C | AU TGA category B3. Can be used only if clearly needed and the benefit outweighs the risk. | Placenta permeable: yes Transfer to breast milk: yes Clinical safety data: inadequate human data; use only when benefit outweighs risk (data in rats and rabbits |
| Niacin | C | ||
| Statins | X | AU TGA category D. Contraindicated | Placenta permeable: yes Transfer to breast milk: unknown Clinical safety data: congenital anomalies |
| PCSK9i | — | AU TGA category B1: Drugs taken only by a limited number of pregnant women and women of childbearing age; no increase in the frequency of malformation or other direct or indirect harmful effects on the human foetus. | Placenta permeable: yes (for evolocumab data in monkeys, no human data) Transfer to Breast milk: unknown Clinical safety data: inadequate human data; not recommended |
| Inclisiran | — | Not included in UA FDA recommendations. No data available. | Not included in ESC recommendations. No data available. |
| Bempedoic acid | — | Not included in UA FDA recommendations. No data available. | Not included in ESC recommendations. No data available. |
| Evinacumab | — | Not included in UA FDA recommendations. No data available. | Not included in ESC recommendations. No data available. |
| Lomitapide | X | Contradicted during pregnancy—the risk of foetal toxicity developmental in animal studies. | Not included in ESC recommendations. No data available. |
aDefinition of classification: C—animal reproduction studies have shown an adverse effect on the foetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks; X—studies in animals or humans have demonstrated foetal abnormalities and/or there is positive evidence of human foetal risk based on adverse reaction data from investigational or marketing experience, and the risks involved in use of the drug in pregnant women clearly outweigh potential benefits.
Table 2.
Management of severe hypercholesterolaemia during pregnancy
| Non-pharmaceutical interventions | Pre-conception plan Diet, physical activity |
In each pregnancy |
|---|---|---|
| Bile acid sequestrants | Approved during pregnancy May lead to hypertriglyceridaemia Poorly tolerated systemic side effects |
In each pregnancy |
| Ezetimibe | When the benefits outweigh the risks | Individual decision |
| Fibrates | When the benefits outweigh the risks | Individual decision |
| Omega-3 fatty acids | When the benefits outweigh the risks Reduce triglycerides |
Individual decision |
| Statins | Generally contraindicated For severe hypercholesterolaemia, the decision should be individualized |
Individual decision in severe hypercholesterolaemia |
| PCSK9i | Limited data | Lacking data |
| Inclisiran | No data regarding pregnancy available Hypothetically, administration before pregnancy may give a mean 41% reduction of LDL-C |
Lacking data, potentially useful in severe hypercholesterolaemia |
| Apheresis | The potential benefits and risks associated with the procedure should be considered Available in specialized centres In some cases, the only one available therapeutic option |
In each pregnancy Limited availability |
Other lipid-lowering medications
Limited or single studies have been conducted to assess proprotein convertase subtilisin:kexin type 9 (PCSK9) inhibitors (evolocumab, alirocumab), bempedoic acid, lomitapide, and inclisiran during pregnancy.37,79 In the case of evolocumab, studies showed a reduction in T cell-dependent responses following immunization with KLH in monkeys. Additionally, a recently published case report documented corpus callosum agenesis in a child whose mother, diagnosed with FH, was using alirocumab, statins, and ezetimibe at maximum tolerated doses up to the 6th week of an unplanned pregnancy. However, it was suggested that a direct causal link between these factors may not exist.37,80 Other available reports indicated that the administration of PCSK9 inhibitors had no effect on foetal growth and development in monkeys. Nevertheless, data from human studies remain insufficient. Consequently, due to the lack of an established safety profile, expectations for observational studies to provide data have arisen.37,81,82 However, two observational studies on evolocumab were prematurely terminated in December 2020, and a similar decision was made for the alirocumab registry during pregnancy (NCT03379558) in November 2020 due to changes in post-marketing requirements.37 Bempedoic acid, due to the limited amount of data regarding its use during pregnancy, is contradicted.
PCSK9 plays fundamental roles in cellular differentiation and proliferation.83 There is justifiable concern with respect to using agents that inhibit PCSK9 during foetal development. Ardissino et al.84 have introduced a highly innovative approach to assessing whether or not it is plausible that PCSK9 inhibition might be harmful to the developing foetus by using genome-wide association studies including ∼1.3 million patients. Using instrumental variants of PCSK9 that impact serum levels of LDL, these investigators showed that genetically proxied LDL-lowering through PCSK9 correlates with a higher odds of malformations the skin (OR 2.23, 95% CI 1.33–3.75, P = 0.007), and the vertebral, anorectal, cardiovascular, tracheo-oesophageal, renal, and limbs (OR 1.51, 95% CI 1.16–1.96, P = 0.007). Perhaps similar LDL proxies can be used to predict foetal harm in the setting of inhibiting such therapeutic targets as Niemann Pick C1-like protein (ezetimibe), angiopoietin-like protein 3 (evinacumab), apoprotein C3 (olezarsen), or ATP citrate lyase (bempedoic acid).
Novel lipid-lowering drugs, including volanesorsen and lomitapide, have no available data during pregnancy, and are therefore classified as ‘C’—contraindicated during pregnancy. Volanesorsen should be discontinued before attempting conception and lomitapide carries a risk of foetal toxicity. Data regarding other new drugs are very limited and thus far are not recommended.85,86
Finally, a new and highly effective lipid-lowering drug, inclisiran, an RNA silencing oligonucleotide, requires administration only twice a year, has become available since 2021. Notably, data from the ORION-1 study have indicated that a single administration may be associated with a mean 41% reduction in LDL-C levels after 9 months. Despite its unique safety profile (with no apparent safety concerns compared to a placebo, apart from local side effects due to injection), no data on its use in pregnant women and their foetuses are available. Therefore, it is purely hypothetical to consider employing inclisiran in high-risk patients before and immediately after pregnancy, with the expectation of achieving ∼40–50% LDL-C reduction.87,88 Nevertheless, no data currently exist to substantiate this assumption. Moreover, consistent with the analysis by Ardissini noted above, there is at least predicted potential for harm to the foetus using an agent that inhibits PCSK9.
Lipoprotein apheresis
An alternative therapeutic option for hyperlipidaemia during pregnancy is lipoprotein apheresis (LA), a mechanical method designed to remove atherogenic lipoproteins [LDL-C and Lp(a)] from plasma. This procedure involves filtering LDL, VLDL, Lp(a), alpha-2-macroglobulin, and coagulation factors, after which the plasma is returned to the bloodstream.89 This process has been shown to be safe for pregnant women.
The primary clinical indication for lipoprotein apheresis in pregnant patients is HoFH. Pregnant patients with HoFH, particularly those with exceptionally high LDL-C levels and less-than-optimal responses to lipid-lowering therapy, should be offered bi-weekly LDL apheresis. Ogura et al. reported 10 successful deliveries in seven patients with HoFH, whereas two pregnant patients with HoFH who did not receive lipid apheresis died during pregnancy.90 Importantly, lipoprotein apheresis has been employed in cases of severe hypertriglyceridaemia to prevent pancreatitis.91–93
Decisions regarding the use of lipoprotein apheresis should be made carefully, considering the potential benefits and risks associated with the procedure.92 However, this treatment method is only available in specialized centres equipped with apheresis machines, making it less widely accessible.94,95 Nonetheless, in certain cases, it represents the sole available therapeutic option.
Disparities in cardiovascular care for women, whether pregnant or not
Significant disparities in the quality of cardiovascular care exist for women when compared to men and women continue to be under-represented in clinical trials involving cardiovascular care.96 Effort is being made world-wide to correct this. In some ways, it is understandable that our knowledge of using lipid-modifying agents in pregnant women is relatively poor and under-informed. There is great trepidation in using pharmacologic agents in the setting of pregnancy, out of safety concerns for both mother and foetus. Cardiovascular disease remains the leading cause of pregnancy-related mortality and progress is being made in recognizing the unique needs of pregnant women as evidenced by the emergence of Cardio-Obstetrics.97 There is also no question that lipid disorders and especially FH are under-diagnosed and markedly undertreated in women when compared to men in both the primary and secondary prevention settings.98 Having been untreated or undertreated well into adulthood certainly magnifies risk when a woman is pregnant and her LDL-C and Lp(a) rise markedly. There is also harm to the foetus. It has been shown that when comparing normocholesterolemic to hypercholesterolemic pregnant mothers, the foetuses of the hypercholesterolemic mothers have significantly more and larger numbers of aortic fatty streaks. In a multivariate analysis of the French National Registry of FH, it was shown that maternal inheritance of the FH gene is associated with an increase in CAC scores by 86% (95% CI, 23–170%; P = 0.003), a 1.81-fold higher risk of having a CAC score ≥ 100 Agatston units (95% CI, 1.06–3.11; P = 0.03), and a 2.72-fold risk of having a CAC score ≥ 400 Agatston units (95% CI, 1.39–5.51; P = 0.004) compared with paternal inheritance.99 Given the apparent safety of statins in pregnancy, Graham and Raal100 have recommended that women homozygous for FH or with a defined atherosclerotic vessel or aortic disease be treated with statins during pregnancy if lipoprotein apheresis is not a therapeutic option.
Conclusions
Dyslipidaemia and the management of lipid and lipoprotein levels during pregnancy remain significant concerns, as they may lead to adverse outcomes for both the mother and the child. Current guidelines recommend discontinuing lipid-lowering treatment, except for BAS, one to two months before planned pregnancy or as soon as the pregnancy is detected, with no specific clinical guidance for severely hypercholesterolemic women, those with a high risk of cardiovascular disease, or those who have already experienced a cardiovascular event.95
However, a proportion of pregnancies remain unplanned and therefore lipid-lowering treatment may be continued after conception, leading to an increase in statin exposure during pregnancy. Moreover, we observe an increase in the age at which women experience their first pregnancy, leading to a higher number of pregnant women with a diagnosis of atherosclerotic cardiovascular disease (ASCVD) or a high risk of ASCVD.101 Pregnancy represents a period of heightened susceptibility to the progression of atherosclerosis. This vulnerability arises from the physiological increase in LDL-C, which is further exacerbated by the cessation of cholesterol-lowering treatment. Consequently, healthcare providers are increasingly faced with the challenge of assessing the risks and benefits of initiating or withholding lipid-lowering treatment. Hence, it appears that pre-conception counselling is taking on a growing role. On the other hand, there are data suggesting that temporary discontinuation of treatment has no adverse consequences. A retrospective review by Nangrahary et al. of 13 women with heterozygous FH (HeFH) proved good pregnancy outcome despite loss of statin treatment. In that group, the cessation of cholesterol-lowering therapy ranged between 12 months and 3.5 years. However, despite positive results of pregnancy outcome, the authors claim that in high-risk patients, continuation of statin therapy may be justified.102
Currently, the approved treatment methods are limited to behavioural interventions, including adopting a healthy lifestyle and diet, as well as the use of BAS, omega-3 fatty acids, and LDL apheresis. Fenofibrate and ezetimibe might be considered in some cases, but only when the potential benefits clearly outweigh the risks. The treatment options are constrained due to the reported adverse birth outcomes associated with certain drug categories.
On the other hand, it is well recognized that initiating lipid-lowering therapy as early as possible is critical for effective cardiovascular disease prevention in high-risk patients.103–105 Furthermore, there are patient groups for whom the aforementioned interventions may not be sufficient to achieve the necessary reduction in lipid levels, such as those with severe HeFH or HoFH. As a result, we believe that, in certain individual cases, statin therapy may be considered as a therapeutic option, particularly for patients with severe hypercholesterolaemia, those with HoFH, and those at a very high or extremely high risk after experiencing a cardiovascular event. Additionally, all pregnant patients with severe dyslipidaemia should receive close monitoring by a team including lipidologists, cardiologists, clinical dietitians, and obstetricians. This is consistent with the current guidance of the International Atherosclerosis Society,106 which recommend that statins and other cholesterol-lowering drugs be discontinued and that of bile acid sequestrants be initiated 3 months before a planned pregnancy. In patients with FH who become pregnant while taking statins, ezetimibe, PCSK9 inhibitors, or other lipid-modifying therapies should be discontinued. Patients should be reassured that stopping these therapies is unlikely to harm the foetus. The guidance recommends a different approach for women with HoFH and clinical atherosclerotic cardiovascular disease, when statin continuation should be considered despite pregnancy. Other lipid-modifying therapies can be considered especially after the first trimester when the LDL-C goal is not achieved, and lipoprotein apheresis is not available or feasible to initiate.106 The recommendations of the European Atherosclerosis Society (EAS) on HoFH management are similar. According to the 2023 update EAS Consensus Statement, women with HoFH should be offered weekly or fortnightly lipoprotein apheresis during pregnancy. If LA is unavailable, the continuation of statin therapy should be considered or reintroduction of a statin plus other lipid-lowering therapy from the second trimester onwards. Evidence suggests safety in this approach.107 The FDA has recognized the favourable risk/benefit ratio of statins in high-risk pregnant women, especially those with HoFH.108
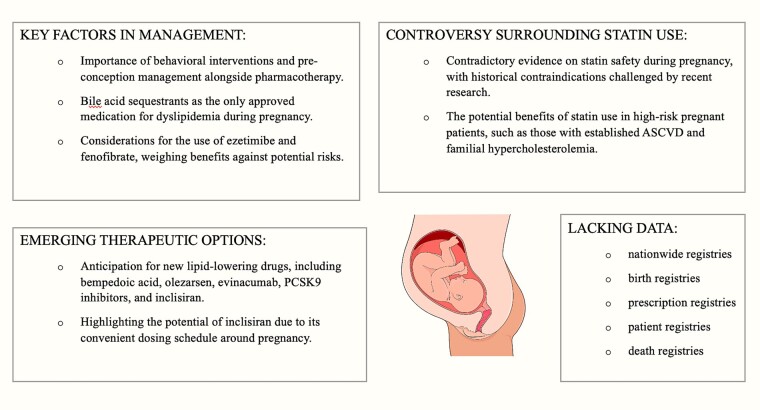
In summary, pregnancy is a unique clinical state in which the well-being of both the mother and the child is of paramount importance. Therefore, before altering guidelines that offer limited recommendations for pharmacotherapy, the safety of drugs must be thoroughly established. However, it should be emphasized that it is insufficient to enrol pregnant women in RCTs since these will be underpowered to detect any statistically significant differences in the rarest pregnancy outcomes such as congenital malformations. ‘More safety data’ mean that we need nationwide registries that can monitor entire populations throughout life: birth registries, prescription registries, patient registries, death registries, and other health related databases. The lack of studies on this topic during pregnancy, coupled with ethical considerations, complicates the issue of lipid-lowering therapy during pregnancy. Nevertheless, despite these obstacles, they serve as a strong motivation for further research into lipid management during pregnancy (Figure 1).109–111 We believe that, to a certain extent, the potential benefits of dyslipidaemia treatment may outweigh the risks, and this issue should receive more attention in ongoing research.
Figure 1.
Lipid management approach during pregnancy (images from: Dall-e and smart.servier.com).
Contributor Information
Joanna Lewek, Department of Preventive Cardiology and Lipidology, Medical University of Lodz (MUL), Rzgowska 281/289, 93-338 Lodz, Poland; Department of Cardiology and Congenital Diseases of Adults, Polish Mother’s Memorial Hospital Research Institute (PMMHRI), Rzgowska 281/289, 93-338 Lodz, Poland.
Agata Bielecka-Dąbrowa, Department of Preventive Cardiology and Lipidology, Medical University of Lodz (MUL), Rzgowska 281/289, 93-338 Lodz, Poland; Department of Cardiology and Congenital Diseases of Adults, Polish Mother’s Memorial Hospital Research Institute (PMMHRI), Rzgowska 281/289, 93-338 Lodz, Poland.
Peter P Toth, The Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins University School of Medicine, 600 N. Wolfe St, Carnegie 591, Baltimore, MD 21287, USA.
Maciej Banach, Department of Preventive Cardiology and Lipidology, Medical University of Lodz (MUL), Rzgowska 281/289, 93-338 Lodz, Poland; Department of Cardiology and Congenital Diseases of Adults, Polish Mother’s Memorial Hospital Research Institute (PMMHRI), Rzgowska 281/289, 93-338 Lodz, Poland; The Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins University School of Medicine, 600 N. Wolfe St, Carnegie 591, Baltimore, MD 21287, USA; Cardiovascular Research Centre, Zyty 28, 65-417 Zielona Góra, Poland.
Lead author biography

Prof. Maciej Banach is a head of the Department of Preventive Cardiology and Lipidology at the Medical University of Lodz, head of the Cardiovascular Research Centre at the University of Zielona Gora, and professor consultant at the Department of Cardiology and Congenital Diseases of Adults at the PMMHRI in Lodz, Poland. He is also an adjunct professor at the Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, and senior research fellow at the Liverpool Centre for Cardiovascular Sciences. He is a secretary of the European Atherosclerosis Society, founder and head of the Polish Lipid Association, founder of the Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group, and President of the International Lipid Expert Panel (ILEP). Prof. Banach has published >1500 original papers in the field of hypertension, dyslipidaemia, cardiology, and risk stratification (citations: 83 581, IH = 100 acc. Web of Science Core Collection, 145 716, IH = 129 acc. Google Scholar)-being within 1% the highest cited scientists in the world according to Essential Science Indicators by Clarivate (with 63 TOP Papers). He is a laureate of several prizes and award, including: the Most Influential Person in Medicine in Poland for year 2023 on the Top 100 list by Puls Medycyny journal (2024), and five recognitions of Doctor Honoris Causa (at the universities in Zagreb, Kosice, Bucharest, Kiev, and Timisoara).
Data availability
No data were generated or analysed for this manuscript.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding
None declared.
References
- 1. Hadden DR, McLauglin C. Normal and abnormal maternal metabolism during pregnancy. Semin Fetal Neonatal Med 2009;14:401. [DOI] [PubMed] [Google Scholar]
- 2. Vrijkotte TG, Krukziener N, Hutten BA, Vollebregt KC, van Eijsden M, Twickler MB. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study. J Clin Endocrinol Metab 2012;97:3917–3925. [DOI] [PubMed] [Google Scholar]
- 3. Pipe NG, Smith T, Halliday D, Edmonds CJ, Williams C, Coltart TM. Changes in fat, fat-free mass and body water in human normal pregnancy. Br J Obstet Gynaecol 1979;86:929–940. [DOI] [PubMed] [Google Scholar]
- 4. Clapp JF, Seaward BL 3rd, Sleamaker RH, Hiser J. Maternal physiologic adaptations to early human pregnancy. Am J Obstet Gynecol 1988;159:1456–1460. [DOI] [PubMed] [Google Scholar]
- 5. Kopp-Hoolihan LE, van Loan MD, Wong WW, King JC. Fat mass deposition during pregnancy using a four-component model. J Appl Physiol 1999;87:196–202. [DOI] [PubMed] [Google Scholar]
- 6. Aguilar Cordero MJ, Baena García L, Sánchez López AM, Guisado Barrilao R, Hermoso Rodríguez E, Mur Villar N. Nivel De Triglicéridos Como Factor De Riesgo Durante El Embarazo; Modelado Biológico; Revisión Sistemática [Triglyceride levels as a risk factor during pregnancy; biological modeling; systematic review]. Nutr Hosp 2015;32:517–527. [DOI] [PubMed] [Google Scholar]
- 7. Nsioudis D, Doulaveris G, Kanninen TT. Dyslipidemia in pregnancy and maternal–fetal outcome. Minerva Ginecol 2019;71:155–162. [DOI] [PubMed] [Google Scholar]
- 8. Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, Kiru G, Murphy J, Banach M, De Servi S, Gaita D, Gouni-Berthold I, Hovingh GK, Jozwiak JJ, Jukema JW, Kiss RG, Kownator S, Iversen HK, Maher V, Masana L, Parkhomenko A, Peeters A, Clifford P, Raslova K, Siostrzonek P, Romeo S, Tousoulis D, Vlachopoulos C, Vrablik M, Catapano AL, Poulter NR; DA VINCI study . EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol 2021;28:1279–1289. [DOI] [PubMed] [Google Scholar]
- 9. Brizzi P, Tonolo G, Esposito F, Puddu L, Dessole S, Maioli M, Milia S. Lipoprotein metabolism during normal pregnancy. Am J Obstet Gynecol 1999;181:430–434. [DOI] [PubMed] [Google Scholar]
- 10. Belo L, Caslake M, Gaffney D, Santos-Silva A, Pereira-Leite L, Quintanilha A. Changes in LDL size and HDL concentration in normal and preeclamptic pregnancies. Atherosclerosis 2001;162:425–432. [DOI] [PubMed] [Google Scholar]
- 11. Dong Y, Lin Y, Liu W, Zhang W, Jiang Y, Song W. Atrial natriuretic peptide inhibited ABCA1/G1-dependent cholesterol efflux related to low HDL-C in hypertensive pregnant patients. Front Pharmacol 2021;12:715302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bacchetti T, Morresi C, Vignini A, Tiano L, Orlando P, Montik N, Ciavattini A, Ferretti G. HDL functionality in follicular fluid in normal-weight and obese women undergoing assisted reproductive treatment. J Assist Reprod Genet 2019;36:1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang GH, Jin J, Liu YQ, Yang FY, Shi D, Zhang Y, Zhao YM, Wang Y. The changes of Lp-PLA2 in patients with gestational diabetes and its clinical significance. Medicine (Baltimore) 2021;100:e26786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holven KB, van Lennep JR. Sex differences in lipids: a life course approach. Atherosclerosis 2023;384:117270. doi: 10.1016/j.atherosclerosis.2023.117270. [DOI] [PubMed] [Google Scholar]
- 15. Banach M, Burchardt P, Chlebus K, Dobrowolski P, Dudek D, Dyrbuś K, Gąsior M, Jankowski P, Jóźwiak J, Kłosiewicz-Latoszek L, Kowalska I, Małecki M, Prejbisz A, Rakowski M, Rysz J, Solnica B, Sitkiewicz D, Sygitowicz G, Sypniewska G, Tomasik T, Windak A, Zozulińska-Ziółkiewicz D, Cybulska B. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch Med Sci 2021;17:1447–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC) . Global perspective of familial hypercholesterolaemia: a cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet 2021;398:1713–1725. [DOI] [PubMed] [Google Scholar]
- 17. Amundsen AL, Khoury J, Iversen PO, Bergei C, Ose L, Tonstad S, Retterstøl K. Marked changes in plasma lipids and lipoproteins during pregnancy in women with familial hypercholesterolemia. Atherosclerosis 2006;189:451–457. [DOI] [PubMed] [Google Scholar]
- 18. Roeters van Lennep JE, Tokgozoglu LS, Badimon L, Dumanski SM, Gulati M, Hess CN, Holven KB, Kavousi M, Kayıkçıoğlu M, Lutgens E, Michos ED, Prescott E, Stock JK, Tybjaerg-Hansen A, Wermer MJH, Benn M. Women, lipids, and atherosclerotic cardiovascular disease: a call to action from the European Atherosclerosis Society. Eur Hear J 2023;44:4157–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wizniter A, Mayer A, Novack V, Sheiner E, Gilutz H, Malhotra A, Novack L. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus population based study. Am J Obstet Gynecol 2009;201:482.e1–482.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen H, Liu X, Chen Y, He B, Cheng W. Associations of lipid levels during gestation with hypertensive disorders of pregnancy and gestational diabetes mellitus: a prospective longitudinal cohort study. BMJ Open 2016;6:e013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang J, Moore D, Subramanian A, Cheng KK, Toulis KA, Qiu X, Saravanan P, Price MJ, Nirantharakumar K. Gestational dyslipidaemia and adverse birthweight outcomes: a systematic review and meta-analysis. Obes Rev 2018;19:1256–1268. [DOI] [PubMed] [Google Scholar]
- 22. Jin WY, Lin SL, Hou RL, Chen XY, Han T, Jin Y, Tang L, Zhu Z-W, Zhao Z-Y. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth 2016;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharami SH, Gholipour M, Milani F, Kazemnejad E, Heirati SFD, Ranjbar ZA. The association between dyslipidemia and preterm birth: a prospective cohort study in the north of Iran. Endocr Metab Immune Disord Drug Targets 2020;20:227–233. [DOI] [PubMed] [Google Scholar]
- 24. Retnakaran R, Wen SW, Tan H, Zhou S, Ye C, Shen M, Smith GN, Walker MC. Maternal pre-gravid cardiometabolic health and infant birthweight: a prospective pre-conception cohort study. Nutr Metab Cardiovasc Dis 2017;27:723–730. [DOI] [PubMed] [Google Scholar]
- 25. Qiao L, Wattez JS, Lim L, Rozance PJ, Hay WW Jr, Shao J. Prolonged prepregnant maternal high-fat feeding reduces fetal and neonatal blood glucose concentrations by enhancing fetal β-cell development in C57BL/6 mice. Diabetes 2019;68:1604–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang D, Ding W, Xu S, Chen H, Liu B, Wang Z. The relationship between total cholesterol and postpartum impaired glucose tolerance in women with gestational diabetes mellitus. Lipids Health Dis 2020;19:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiao H, Chen Y, Pei L. Influence of dyslipidemia during pregnancy on postpartum glucose and lipid metabolism in GDM patients. J Sun Yat-Sen Univer (Med Sci) 2020;41:479–484. [Google Scholar]
- 28. Adank MC, Benschop L, van Streun SP, Smak Gregoor AM, Mulder MT, Steegers EAP, Schalekamp-Timmermans S, Roeters van Lennep JE. Gestational lipid profile as an early marker of metabolic syndrome in later life: a population-based prospective cohort study. BMC Med 2020;18:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pei L, Xiao H, Lai F, Li Z, Li Z, Yue S, Chen H, Li Y, Cao X. Early postpartum dyslipidemia and its potential predictors during pregnancy in women with a history of gestational diabetes mellitus. Lipids Health Dis 2020;19:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Napoli C, Glass CK, Witztum JI, Deutsch R, Armiento FPD, Palinski W. Influence of maternal hypercholesterolemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet 1999;354:1234–1241. [DOI] [PubMed] [Google Scholar]
- 31. Fanshawe AE, Ibrahim M. The current status of lipoprotein(a) in pregnancy: a literature review. J Cardiol 2013;61:99–106. [DOI] [PubMed] [Google Scholar]
- 32. Cybulska B, Kłosiewicz-Latoszek L, Penson PE, Banach M. What do we know about the role of lipoprotein(a) in atherogenesis 57 years after its discovery? Prog Cardiovasc Dis 2020;63:219–227. [DOI] [PubMed] [Google Scholar]
- 33. Manten GTR, van der Hoek YY, Sikkema JM, Voorbij HAM, Hameetemean TM, Visser GHA, Franx A. The role of lipoprotein(a) in pregnancies complicated by pre-eclampsia. Med Hypotheses 2005;64:162–169. [DOI] [PubMed] [Google Scholar]
- 34. Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet 2003;361:901–908. [DOI] [PubMed] [Google Scholar]
- 35. Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, Orringer CE. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol 2019;13:374–392. [DOI] [PubMed] [Google Scholar]
- 36. Sanderson SL, Iverius PH, Wilson DE. Successful hyperlipemic pregnancy. JAMA 1991;265:1858–1860. [PubMed] [Google Scholar]
- 37. The International Weight Management in Pregnancy (i-WIP) Collaborative Group . Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomized trials. BMJ 2017;358:j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Golwala S, Dolin CD, Nemiroff R, Soffer D, Denduluri S, Jacoby D, Lewey J. Feasibility of lipid screening during first trimester of pregnancy to identify women at risk of severe dyslipidemia. J Am Heart Assoc 2023;12:e028626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knight M. Diet and exercise in pregnancy. BMJ 2017;358:j3283. [DOI] [PubMed] [Google Scholar]
- 40. Banach M. Where are the recommendations on healthy lifestyle and cardiovascular disease prevention for pregnant women? JAHA 2020;9:e016052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Melero V, Arnoriaga M, Barabash A, Valerio J, Del Valle L, Martin O'Connor R, de Miguel MP, Diaz JA, Familiar C, Moraga I, Duran A, Cuesta M, Torrejon MJ, Martinez-Novillo M, Moreno M, Romera G, Runkle I, Pazos M, Rubio MA, Matia-Martín P, Calle-Pascual AL. An early Mediterranean-based nutritional intervention during pregnancy reduces metabolic syndrome and glucose dysregulation rates at 3 years postpartum. Nutrients 2023;15:3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rogozińska E, Chamillard M, Hitman GA, Khan KS, Thangaratinam S. Nutritional manipulation for the primary prevention of gestational diabetes mellitus: a meta-analysis of randomised studies. PLoS One 2015;10:e0115526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pernia S, DeMaagd G. The new pregnancy and lactation labelling rule. PT 2016;41:713–715. [PMC free article] [PubMed] [Google Scholar]
- 44. Laredo-Aguilera JA, Gallardo-Bravo M, Rabanales-Sotos JA, Cobo-Cuenca AI, Carmona-Torres JM. Physical activity programs during pregnancy are effective for the control of gestational diabetes mellitus. Int J Environ Res Public Health 2020;17:6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lent-Schochet D, Jialal I. Antilipemic agent bile acid sequestrants. [updated 2021 Sep 28], eds. Statpearls [internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549906/. [PubMed] [Google Scholar]
- 46. Guidelines and Recommendations for Treating and Managing Health Conditions during Pregnancy. https://www.cdc.gov/pregnancy/meds/treatingfortwo/treatment-guidelines.html (15 October 2021).
- 47. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B; ESC National Cardiac Societies, ESC Scientific Document Group . 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–3337. [DOI] [PubMed] [Google Scholar]
- 48. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, Rosano GMC, Seeland U, Simoncini T, Swan L, Warnes CA, Deaton C, Simpson IA, Aboyans V, Agewall S, Barbato E, Calda P, Coca A, Coman IM, De Backer J, Delgado V, Di Salvo G, Fitzsimmons S, Fitzsimons D, Garbi M, Gevaert S, Hindricks G, Jondeau G, Kluin J, Lionis C, McDonagh TA, Meier P, Moons P, Pantazis A, Piepoli MF, Rocca B, Roffi M, Rosenkranz S, Sarkozy A, Shlyakhto E, Silversides CK, Sliwa K, Sousa-Uva M, Tamargo J, Thorne S, Van de Velde M, Williams B, Zamorano JL, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Zamorano JL, Hammoudi N, Piruzyan A, Mascherbauer J, Samadov F, Prystrom A, Pasquet A, Caluk J, Gotcheva N, Skoric B, Heracleous H, Vejlstrup N, Maser M, Kaaja RJ, Srbinovska-Kostovska E, Mounier-Vehier C, Vakhtangadze T, Rybak K, Giannakoulas G, Kiss RG, Thrainsdottir IS, Erwin RJ, Porter A, Geraci G, Ibrahimi P, Lunegova O, Mintale I, Kadri Z, Benlamin H, Barysiene J, Banu CA, Caruana M, Gratii C, Haddour L, Bouma BJ, Estensen M-E, Hoffman P, Petris AO, Moiseeva O, Bertelli L, Tesic BV, Dubrava J, Koželj M, Prieto-Arévalo R, Furenäs E, Schwerzmann M, Mourali MS, Ozer N, Mitchenko O, Nelson-Piercy C; ESC Scientific Document Group . 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy: the task force for the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 49. Ast M, Frishman WH. Bile acid sequestrants. J Clin Pharmacol 1990;30:99–106. [DOI] [PubMed] [Google Scholar]
- 50. Levy RI, Brensike JF, Epstein SE, Kelsey SF, Passamani ER, Richardson JM, Loh IK, Stone NJ, Aldrich RF, Battaglini JW. The influence of changes in lipid values induced by cholestyramine and diet on progression of coronary artery disease: results of NHLBI Type II Coronary Intervention study. Circulation 1984;69:325–37. [DOI] [PubMed] [Google Scholar]
- 51. Brensike JF, Levy RI, Kelsey SF, Passamani ER, Richardson JM, Loh IK, Stone NJ, Aldrich RF, Battaglini JW, Moriarty DJ. Effects of therapy with cholestyramine on progression of coronary arteriosclerosis: results of the NHLBI Type II Coronary Intervention study. Circulation 1984;69:313–324. [DOI] [PubMed] [Google Scholar]
- 52. Nordgren TM, Lyden E, Anderson-Berry A, Hanson C. Omega-3 fatty acid intake of pregnant women and women of childbearing age in the United States: potential for deficiency?. Nutrients 2017;9:e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Skulas-Ray AC, West SG, Davidson MH, Kris-Etherton PM. Omega-3 fatty acid concentrates in the treatment of moderate hypertriglyceridemia. Expert Opin Pharmacother 2008;9:1237–1248. [DOI] [PubMed] [Google Scholar]
- 54. Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J; ASCEND Study Collaborative Group . Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018;379:1540–1550. [DOI] [PubMed] [Google Scholar]
- 55. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE; VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K; Japan EPA Lipid Intervention Study (JELIS) Investigators . Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–1098. [DOI] [PubMed] [Google Scholar]
- 57. Bhatt DL, Steg PG, Miller M; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 58. Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev 2018;11:CD003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Döbert M, Varouxaki AN, Mu AC, Syngelaki A, Ciobanu A, Akolekar R, De Paco Matallana C, Cicero S, Greco E, Singh M, Janga D, del Mar Gil M, Jani JC, Bartha JL, Maclagan K, Wright D, Nicolaides KH. Pravastatin versus placebo in pregnancies at high risk of term preeclampsia. Circulation 2021;144:670–679. [DOI] [PubMed] [Google Scholar]
- 60. Dostal LA, Schardein JL, Anderson JA. Developmental toxicity of the HMG-CoA reductase inhibitor, atorvastatin, in rats and rabbits. Teratology 1994;50:387–394. [DOI] [PubMed] [Google Scholar]
- 61. Minsker DH, MacDonald JS, Robertson RT, Bokelman DL. Mevalonate supplementation in pregnant rats suppresses the teratogenicity of mevinolinic acid, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme a reductase. Teratology 1983;28:449–456. [DOI] [PubMed] [Google Scholar]
- 62. Ghidini A, Sicherer S, Willner J. Congenital abnormalities (VATER) in baby born to mother using lovastatin. Lancet 1992;339:1416–1417. [DOI] [PubMed] [Google Scholar]
- 63. Bateman BT, Hernandez-Diaz S, Fischer MA, Seely EW, Ecker JL, Franklin JM, Desai RJ, Allen-Coleman C, Mogun H, Avorn J, Huybrechts KF. Statins and congenital malformations: cohort study. BMJ 2015;350:h1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Karadas B, Uysal N, Erol H, Acar S, Koc M, Kaya-Temiz T, Koren G, Kaplan YC. Pregnancy outcomes following maternal exposure to statins: a systematic review and meta-analysis. Br J Clin Pharmacol 2022;88:3962–3976. [DOI] [PubMed] [Google Scholar]
- 65. Edison RJ, Muenke M. Central nervous system and limb anomalies in case reports of first-trimester statin exposure. N Engl J Med 2004;350:1579–1582. [DOI] [PubMed] [Google Scholar]
- 66. McGrogan A, Snowball J, Charlton RA. Statins during pregnancy: a cohort study using the General Practice Research Database to investigate pregnancy loss. Pharmacoepidemiol Drug Saf 2017;26:843–852. [DOI] [PubMed] [Google Scholar]
- 67. Wu T, Shi Y, Zhu B, Li D, Li Z, Zhao Z, Zhang Y. Pregnancy-related adverse events associated with statins: a real-world pharmacovigilance study of the FDA Adverse Event Reporting System (FAERS). Expert Opin Drug Saf 2023:1–9. [DOI] [PubMed] [Google Scholar]
- 68. Karalis DG, Hill AN, Clifton S, Wild RA. The risks of statin use in pregnancy: a systematic review. J Clin Lipidol 2016;10:1081–1090. [DOI] [PubMed] [Google Scholar]
- 69. Zarek J, Koren G. The fetal safety of statins: a systematic review and meta-analysis. J Obstet Gynaecol Can 2014;36:506–509. [DOI] [PubMed] [Google Scholar]
- 70. Maierean SM, Mikhailidis DP, Toth PP, Grzesiak M, Mazidi M, Maciejewski M, Banach M. The potential role of statins in preeclampsia and dyslipidemia during gestation: a narrative review. Expert Opin Investig Drugs 2018;27:427–435. [DOI] [PubMed] [Google Scholar]
- 71.FDA requests removal of strongest warning against using cholesterol-lowering statins during pregnancy; still advises most pregnant patients should stop taking statins. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-removal-strongest-warning-against-using-cholesterol-lowering-statins-during-pregnancy (14 October 2021).
- 72. Botha TC, Pilcher GJ, Wolmarans K, Blom DJ, Raal FJ. Statins and other lipid-lowering therapy and pregnancy outcomes in homozygous familial hypercholesterolemia: a retrospective review of 39 pregnancies. Atherosclerosis 2018;277:502–507. [DOI] [PubMed] [Google Scholar]
- 73. Vahedian-Azimi A, Makvandi S, Banach M, Reiner Z, Sahebkar A. Fetal toxicity associated with statins: a systematic review and meta-analysis. Atherosclerosis 2021;327:P59–P67. [DOI] [PubMed] [Google Scholar]
- 74. Vahedian-Azimi A, Bianconi V, Makvandi S, Banach M, Mohammadi SM, Pirro M, Sahebkar A. A systematic review and meta-analysis on the effects of statins on pregnancy outcomes. Atherosclerosis 2021;336:1–11. [DOI] [PubMed] [Google Scholar]
- 75.Ezetimibe Pregnancy and Breastfeeding Warnings. https://www.drugs.com/pregnancy/ezetimibe.html (15 October 2021).
- 76. Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, Safdar B, Sharma G, Wood M, Valente AM, Volgman AS; American Heart Association Council on Clinical C, Council on Arteriosclerosis T, Vascular B, Council on C, Stroke N, Stroke C . Cardiovascular considerations in caring for pregnant patients: a scientific statement from the American Heart Association. Circulation 2020;141:e884–e903. [DOI] [PubMed] [Google Scholar]
- 77. Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol 2013;28:1–19. [DOI] [PubMed] [Google Scholar]
- 78. Liebeskind A, Thompson J, Wilson D. Reproductive health and its impact on lipid management in adolescent and young adult females. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Hofland J, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, and Wilson DP, eds. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc; 2022. [Google Scholar]
- 79. Blom DJ, Fayad ZA, Kastelein JJ, Larrey D, Makris L, Schwamlein C, Bloeden L, Underberg J; LOWER investigators . LOWER, a registry of lomitapide-treated patients with homozygous familial hypercholesterolemia: rationale and design. J Clin Lipidol 2016;10:273–282. [DOI] [PubMed] [Google Scholar]
- 80. Vuignier Y, Beaud F, Kosinski C, Panchaud A, Lebon S, Baud D, Kissling S, Collet T-H. Exposure to alirocumab during the first trimester of pregnancy: a case report. Birth Defects Res 2021;113:1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evolocumab Pregnancy Exposure Registry. https://clinicaltrials.gov/ct2/show/NCT02957604. (15 October 2021).
- 82. Ray KK, Stoekenbroek RM, Kallend D, Nishikido T, Leiter LA, Landmesser U, Wright RS, Wijngaard PLJ, Kastelein JJP. Effect of 1 or 2 doses of inclisiran on low-density lipoprotein cholesterol levels: one-year follow-up of the ORION-1 randomized clinical trial. JAMA Cardiol 2019;4:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Roudaut M, Idriss S, Caillaud A, Girardeau A, Rimbert A, Champon B, David A, Lévêque A, Arnaud L, Pichelin M, Prieur X, Prat A, Seidah NG, Zibara K, Le May C, Cariou B, Si-Tayeb K. PCSK9 regulates the NODAL signaling pathway and cellular proliferation in hiPSCs. Stem Cell Reports 2021;16:2958–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ardissino M, Slob EAW, Reddy RK, Morley AP, Schuermans A, Hill P, Williamson C, Honigberg MC, de Marvao A, Ng FS. Genetically proxied low-density lipoprotein cholesterol lowering via PCSK9-inhibitor drug targets and risk of congenital malformations. Eur J Prev Cardiol 2024. doi: 10.1093/eurjpc/zwad402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Study to Evaluate the Safety of Repatha® in Pregnancy. ClinicalTrials.gov Identifier: NCT02906124. Available from: http://clinicaltrials.gov/ct2/show/NCT02906124. (15 October 2020).
- 86. Kolovou G, Kolovou V, Katsiki N. Volanesorsen: a new era in the treatment of severe hypertriglyceridemia. J Clin Med 2022;11:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cefalu AB, D'Erasmo L, Iannuzzo G, Noto D, Giammanco A, Montali A, Zambon A, Forte F, Suppressa P, Giannini S, Barbagallo CM, Ganci A, Nardi E, Vernuccio F, Caldarella R, Ciaccio M, Arca M, Averna M. Efficacy and safety of lomitapide in familial chylomicronaemia syndrome. Atherosclerosis 2022;359:13–19. [DOI] [PubMed] [Google Scholar]
- 88. Stefanutti C, Julius U. Lipoprotein apheresis: state of the art and novelties. Atheroscler Suppl 2013;14:19–27. [DOI] [PubMed] [Google Scholar]
- 89. Henney NC, Banach M, Penson PE. RNA silencing in the management of dyslipidemias. Curr Atheroscler Rep 2021;23:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. France M, Rees A, Datta D, Thompson G, Capps N, Ferns G, Ramaswami U, Seed M, Neely D, Cramb R, Shoulders C, Barbir M, Pottle A, Eatough R, Martin S, Bayly G, Simpson B, Halcox J, Edwards R, Main L, Payne J, Soran H. HEART UK statement on the management of homozygous familial hypercholesterolaemia in the United Kingdom. Atherosclerosis 2016;255:128–139. [DOI] [PubMed] [Google Scholar]
- 91. Klingel R, Gohlen B, Schwarting A, Himmelsbach F, Straube R. Differential indication of lipoprotein apheresis during pregnancy. Ther Apher Dial 2003;7:359–364. [DOI] [PubMed] [Google Scholar]
- 92. Perrone G, Brunelli R. Prevention and treatment of cardiovascular disease in women: the obstetric-gynecologist’s point of view. Ther Apher Dial 2013;17:162–168. [DOI] [PubMed] [Google Scholar]
- 93. Basar R, Uzum AK, Canbaz B, Dogansen SC, Kalayoglu-Besisik S, Altay-Dadin S, Aral F, Ozbey NC. Therapeutic apheresis for severe hypertriglyceridemia in pregnancy. Arch Gynecol Obstet 2013;287:839–843. [DOI] [PubMed] [Google Scholar]
- 94. Russi G. Severe dyslipidemia in pregnancy: the role of therapeutic apheresis. Transfus Apher Sci 2015;53:283–287. [DOI] [PubMed] [Google Scholar]
- 95. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2019;73:3168–3209. [DOI] [PubMed] [Google Scholar]
- 96. Burgess SN. Understudied, under-recognized, underdiagnosed, and undertreated: sex-based disparities in cardiovascular medicine. Circulation: Cardiovascular Interventions 2022;15:e011714. [DOI] [PubMed] [Google Scholar]
- 97. Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, Safdar B, Sharma G, Wood M, Valente AM, Volgman AS. Cardiovascular considerations in caring for pregnant patients: a scientific statement from the American Heart Association. Circulation 2020;141:e884–e903. [DOI] [PubMed] [Google Scholar]
- 98. Klevmoen M, Mulder J, Roeters van Lennep JE, Holven KB. Sex differences in familial hypercholesterolemia. Curr Atheroscler Rep 2023;25:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mourre F, Giorgi R, Gallo A, Boccara F, Bruckert E, Carrié A, Hankard R, Inamo J, Laboureau S, Moulin P, Valéro R, Béliard S, Angoulvant D, Beliard S, Boccara F, Bruckert E, Cariou B, Carreau V, Carrie A, Charrieres S, Cottin Y, Di Filippo M, Ducluzeau PH, Dulong S, Durlach V, Farnier M, Ferrari E, Ferrieres D, Ferrieres J, Gallo A, Hankard R, Inamo J, Kalmykova O, Krempf M, Lemale J, Moulin P, Paillard F, Peretti N, Perrin A, Pradignac A, Rabes JP, Rigalleau V, Schiele F, Sultan A, Tounian P, Valero R, Verges B, Yelnik C, Ziegler O. Maternal inheritance of familial hypercholesterolemia gene mutation predisposes to coronary atherosclerosis as assessed by calcium score in adulthood. Arteriosclerosis. Thrombosis, and Vascular Biology 2023;43:e94–e103. [DOI] [PubMed] [Google Scholar]
- 100. Graham DF, Raal FJ. Management of familial hypercholesterolemia in pregnancy. Curr Opin Lipidol 2021;32:370–377. [DOI] [PubMed] [Google Scholar]
- 101. Christensen JJ, Bogsrud MP, Holven KB, Retterstøl K, Veierød MB, Nordeng H. Use of statins and other lipid-modifying agents across pregnancy: a nationwide drug utilization study in Norway in 2005–2018. Atherosclerosis 2023;368:25–34. [DOI] [PubMed] [Google Scholar]
- 102. Nangrahary M, Graham DF, Pang J, Barnett W, Watts GF. Familial hypercholesterolaemia in pregnancy: Australian case series and review. Aust N Z J Obstet Gynaecol 2023;63:402–408. [DOI] [PubMed] [Google Scholar]
- 103. Banach M, Surma S. A look to the past—what has had the biggest impact on lipids in the last four decades? A personal perspective. Arch Med Sci 2023;19:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Banach M, Kaźmierczak J, Mitkowski P, Wita K, Broncel M, Gąsior M, Gierlotka M, Gil R, Jankowski P, Niewada M, Witkowski A. Which patients at risk of cardiovascular disease might benefit the most from inclisiran? Polish experts’ opinion. The compromise between EBM and possibilities in healthcare. Arch Med Sci 2022;18:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Banach M, Reiner Z, Cicero AFG, Sabouret P, Viigimaa M, Sahebkar A, Postadzhiyan A, Gaita D, Pella D, Penson PE. 2022: the year in cardiovascular disease—the year of upfront lipid lowering combination therapy. Arch Med Sci 2022;18:1429–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Watts GF, Gidding SS, Hegele RA, Raal FJ, Sturm AC, Jones LK, Sarkies MN, Al-Rasadi K, Blom DJ, Daccord M, de Ferranti SD, Folco E, Libby P, Mata P, Nawawi HM, Ramaswami U, Ray KK, Stefanutti C, Yamashita S, Pang J, Thompson GR, Santos RD. International Atherosclerosis Society guidance for implementing best practice in the care of familial hypercholesterolaemia. Nat Rev Cardiol 2023;20:845–869. [DOI] [PubMed] [Google Scholar]
- 107. Cuchel M, Raal FJ, Hegele RA, Al-Rasadi K, Arca M, Averna M, Bruckert E, Freiberger T, Gaudet D, Harada-Shiba M, Hudgins LC, Kayikcioglu M, Masana L, Parhofer KG, Roeters van Lennep JE, Santos RD, Stroes ESG, Watts GF, Wiegman A, Stock JK, Tokgözoğlu LS, Catapano AL, Ray KK. 2023 update on European Atherosclerosis Society consensus statement on homozygous familial hypercholesterolaemia: new treatments and clinical guidance. Eur Heart J 2023;44:2277–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. US Food and Drug Administration Drug Safety Communication 7–20–2021. FDA requests removal of strongest warning against using cholesterol-lowering statins during pregnancy; still advises most pregnant patients should stop taking statins. 2021. (12 January 2024).
- 109. Kane JP, Malloy MJ, Tun P, Phillips NR, Freedman DD, Williams ML, Rowe JS, Havel RJ. Normalization of low-density-lipoprotein levels in heterozygous familial hypercholesterolemia with a combined drug regimen. N Engl J Med 1981;304:251–258. [DOI] [PubMed] [Google Scholar]
- 110. Ewald N, Kloer H-U. Treatment options for severe hypertriglyceridemia (SHTG): the role of apheresis. Clin Res Cardiol Suppl 2012;7:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, John Chapman M, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O; ESC Scientific Document Group . 2019 ESC/EAS guidelines for the management of dyslipidemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for this manuscript.