Abstract
Central nervous system myelination requires recognition and signalling processes between neuronal axons and oligodendrocytes. Complex cellular rearrangements occur in myelination-competent oligodendrocytes requiring spatio-temporal control mechanisms. Although the molecular repertoire is becoming increasingly transparent, the signalling mechanisms governing myelination initiation are only poorly understood. The non-receptor tyrosine kinase Fyn has been implicated in axon–glial signal transduction and in several cellular processes required for oligodendrocyte maturation and myelination. Here, we review oligodendroglial Fyn signalling and discuss the role of Fyn in axon–glia interaction mediating myelination.
Keywords: Axon–glia interaction, Src family kinase, Myelin, L1-CAM, Translational control, hnRNP-A2, Integrin, Contactin
Introduction
In the central nervous system (CNS), oligodendrocytes myelinate neuronal axons and thereby facilitate saltatory conduction of action potentials at high velocities and limited energy requirements. Furthermore, oligodendrocytes provide trophic support for neurons and both cells form a sophisticated functional unit, depending on continuous bidirectional axon–glia interaction [1, 2]. From a cell biological point of view, the myelination process per se is as fascinating as it is complex. Oligodendrocytes recognise target axons and wrap their processes around several axonal segments followed by a compaction process ultimately resulting in multilamellar membrane stacks [3, 4]. This requires targeted protein and lipid delivery towards the axon–glial contact site that needs to be regulated in a temporal and localised manner adapting myelin formation to axonal specifications.
The protein and lipid composition of the myelin membrane is unique, containing proteolipid protein (PLP) and myelin basic protein (MBP) as the most abundant myelin proteins in the mammalian CNS [5]. In general, the process of myelination is remarkably resistant towards the genetic deletion of its structural protein components. While most major myelin proteins, including PLP, are not essential for myelination to proceed, the absence of functional MBP leads to severe myelination deficits as demonstrated by the naturally occurring shiverer mouse or long evans shaker rat [6, 7]. Among myelin proteins, MBP thus has a unique role in the process of myelin formation.
Intriguingly, the association of both major myelin proteins with the glial plasma membrane is promoted by neuronal signals. Transport of PLP from endosomal pools to the plasma membrane is regulated by a neuron-derived soluble signal [8]. MBP mRNA is transported in RNA-granules towards the distal tips of the oligodendrocyte membrane where it is translated into protein locally [9] and associates with the plasma membrane as a peripheral membrane protein. It has recently been demonstrated that the local translation of MBP is controlled by specific axon–glia interaction mediating activation of the Src-family kinase Fyn [10]. Here, we review a growing number of studies that imply a pivotal function of oligodendroglial Fyn kinase in translating axon–glial communication into different cellular responses directing CNS myelination.
Domain structure and regulation of Fyn kinase
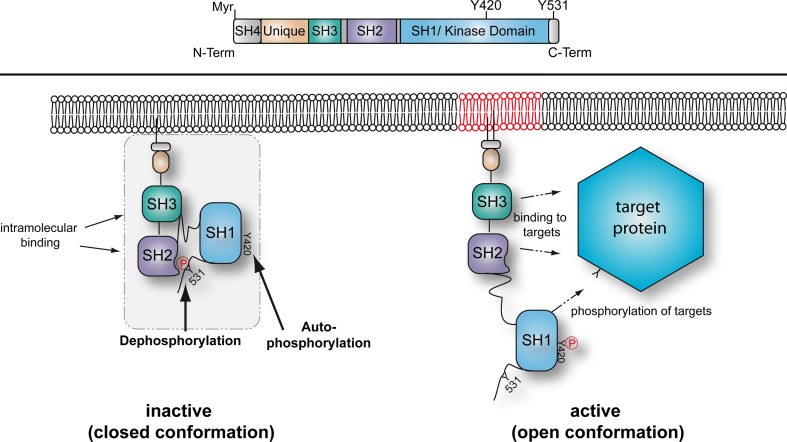
Fyn kinase is a 59-kD protein of the Src-family of non-receptor tyrosine kinases. These consist of an N-terminal Src-homology (SH)4 region with acylation sites, a unique domain, an SH3 and an SH2 protein binding domain, an SH1 or kinase domain and a C-terminal regulatory tail (Fig. 1) [11]. The most N-terminal glycine and cysteine residue can be myristoylated and palmitoylated, respectively. Myristoylation occurs co-translationally on free polysomes and this acylation anchors the kinase to the plasma membrane. Here, palmitoylation can occur which may target the kinase to lipid raft microdomains. Interestingly, this cysteine acylation seems to direct the remaining SH4 region away from the membrane, which may be important for the interaction of Fyn with target proteins [12]. Two Fyn protein isoforms have been identified which result from alternative splicing of exon 7. Exon 7a containing FynB is expressed in the brain and other tissues whereas Exon 7b containing FynT appears specific for T cells. The primary sequence of FynB is 3 amino acids longer and differs from FynT in approximately 50 amino acids between the end of the SH2 domain and the beginning of the kinase domain. FynT is encoded by two transcripts that vary in the 5′ UTR and in an alternate in-frame exon in the coding region. The functional differences between both isoforms are only poorly understood and their activity does not seem to differ largely [13].
Fig. 1.
Domain organization of Fyn kinase. Top Fyn kinase contains an N-terminal SH4 domain anchoring the protein in the plasma membrane by myristoylation (Myr), the SH3 and SH2 protein interaction domains, an SH1 or kinase domain and a C-terminus with the conserved regulatory tyrosine residue 531 (Y531). Bottom In the inactive or basal state the kinase is in a closed conformation due to intramolecular binding of the SH2 domain with the phosphorylated Y531 and the SH3 domain with a linker region between SH2 and SH1/kinase domain. Upon dephosphorylation of the C-terminal tyrosine or binding of external ligands to SH2 or SH3 domains, the conformation changes to an open form that is regarded as the active state. Intermolecular autophosphorylation of tyrosine 420 stabilises the active state of the kinase. Note that palmitoylation in the SH4 domain can direct Fyn into lipid raft microdomains
The activity of Src-family kinases such as Fyn is regulated by a combination of regulatory events (Fig. 1). In the inactive state, the regulatory tyrosine at the C-terminus (Y531 in mouse FynB) is phosphorylated and forms an intramolecular bond with the SH2 domain of the kinase. This phosphorylation is regulated by tyrosine kinases such as Csk (C-terminal Src kinase) and negatively regulates kinase activity [14]. Furthermore, the SH3 domain binds intramolecularly to a linker region between the SH2 and the kinase domain. This keeps the kinase in an inactive closed conformation in which both protein binding domains are occupied. Upon dephosphorylation of the C-terminal regulatory tyrosine residue by tyrosine phosphatases, the intramolecular binding to the SH2 domain is abolished. Loss of SH2-dependent folding alters the conformation of the kinase to a more open form making the SH2 and SH3 domains available for downstream protein interactions. Furthermore, the open conformation enables an intermolecular auto-phosphorylation of tyrosine 420 (Y420) which stabilises the active state of the catalytic site [15]. Src kinases can also be stimulated by interfering with the inhibitory intramolecular binding of SH2 and SH3 domains which can both bind to external ligands leading to an activation of the kinase [16, 17].
In the brain, Src-kinases exhibit a broad expression pattern. Oligodendrocytes express the Src-family members Fyn, Lyn and Src [18] among which Fyn is most prominent and Src is only expressed in small amounts.
Fyn integrates multiple axonal signals and mediates distinct oligodendroglial responses
The functional involvement of Fyn kinase signalling in CNS myelin formation has been recognised upon analysis of Fyn-deficient mice lacking either the complete protein or expressing a kinase inactive single amino acid mutant form [19–21]. These mice are affected by a marked hypomyelination in the forebrain region at all developmental stages, although the cervical spinal cord appears unaffected. In the optic nerve and corpus callosum, the number of myelinated fibres is drastically reduced (largely small diameter axons), while myelin ultrastructure appears normal. On the cellular level, Fyn-inactivation interferes with oligodendroglial maturation and in particular process outgrowth [22, 23]. Fyn expression and kinase activity is upregulated during oligodendroglial differentiation, peaking with the early and most active stages of myelination [19, 22, 24]. Initially, the large isoform of myelin-associated glycoprotein (L-MAG), a glial cell adhesion molecule located in direct apposition to the axon during the initiation phase of myelination, was proposed to activate Fyn, as evidenced by antibody-mediated crosslinking of L-MAG in cultured cells [19]. However, the native axonal ligand in this pathway remains undefined. MAG-deficient mice exhibit a myelin phenotype distinct from Fyn-mutants largely characterised by ultrastructural myelin abnormalities, and mice lacking both molecules are affected by more severe hypomyelination compared to single mutants [20]. If both molecules were part of the same signalling pathway, a combined Fyn/MAG knockout should reveal a similar phenotype to the single knockouts. Hence, this finding indicates the importance of MAG-independent pathways leading to Fyn activation. Indeed, over the past years, several upstream molecules modulating Fyn kinase activity have been identified that apparently operate at all stages of oligodendroglial development and feed into a complex network of downstream signalling pathways. Although expressed at low levels in OPCs, Fyn kinase mediates PDGF-dependent migration of OPCs by activation of cdk5, which modulates actin dynamics by phosphorylation of WAVE2 [25]. However, as anticipated by its developmental activity pattern, Fyn appears to exert its major functions at later stages, when OPCs have ceased migration and further differentiate to oligodendrocytes with many processes, establishing axonal contacts and initiating the myelination programme. By signalling to diverse downstream molecules, Fyn mediates distinct cellular responses with the common purpose of target-controlled morphological differentiation and timing of myelin formation (Fig. 2).
Fig. 2.
The role of Fyn as central integrator and mediator of axon–glia signalling. Axon-derived signals are sensed by oligodendroglial membrane receptors that modulate Fyn kinase activity. Fyn mediates downstream signalling that can be divided into three major pathways: (1) the RhoA/Cdc42/Rac1-dependent pathway modulates actin dynamics and mediates cell survival and morphological differentiation; (2) recruitment of the microtubule cytoskeleton contributes to cell polarisation and may facilitate axon-directed cargo transport; and (3) activated Fyn controls localised myelin protein synthesis by affecting mRNA transport, stability, and translational regulation. In summary, these pathways integrate axonal signals to spatiotemporally regulate myelin formation. See text for details
Fyn signalling to Rho GTPases: regulation of glial survival and morphological differentiation
The differentiation of migrating bipolar OPCs to multibranched myelinating oligodendrocytes is accompanied by rearrangement of the cytoskeleton and extensive membrane remodelling. Blocking Fyn kinase activity with selective inhibitors or overexpression of dominant-negative Fyn mutants constrains oligodendroglial process formation [22]. This function of Fyn in controlling oligodendrocyte morphogenesis largely appears to be mediated by downstream signalling to Rho-family GTPases, which are known regulators of actin cytoskeleton dynamics and furthermore seem to balance endocytic recycling of myelin proteins in oligodendrocytes [26]. Two GTPase-activating proteins, p190RhoGAP and p250RhoGAP, are phosphorylated by Fyn promoting the formation of Rho-GDP and thus Rho inactivation [27, 28]. Consistently, expression of dominant-negative RhoA leads to hyperextension of oligodendroglial processes, while expression of constitutively active RhoA interferes with process formation [29]. In contrast, the other Rho-family members, Cdc42 and Rac1, exhibit opposing activation patterns by switching to the active GTP-bound state to facilitate process formation in response to Fyn activity. The actin-binding kelch-family protein Mayven has also been shown to bind to Fyn and to regulate oligodendrocyte process outgrowth [30, 31].
Fyn activation in respect to the RhoGTPase downstream signalling pathway has primarily been assigned to the oligodendroglial surface receptors α6β1-integrin and Dcc (deleted in colorectal carcinoma). The integrin α6β1 is engaged by the extracellular matrix (ECM) protein laminin-2 (composed of α2β1γ1 chains) that is presented to oligodendroglial cells along axon tracts [32]. Fyn associates with α6β1-integrin in oligodendrocytes and interference with β1-integrin function using blocking antibodies or by expression of a dominant-negative chimeric integrin mutant inhibits Fyn activity and reduces the expansion of myelin-like membrane sheets in cultured cells [29, 33]. Moreover, exposure to laminin-2 substrates enhances the sensitivity of oligodendrocytes for growth factor-mediated cell survival signals and drives their differentiation, depending on functional Fyn kinase [18]. Fyn activation in response to laminin-2 exposure triggers tyrosine phosphorylation of focal adhesion kinase (FAK), which appears instrumental in the activation of Cdc42 and Rac1 [34]. Together, these findings imply a model of target-dependent control of glial survival and morphological differentiation mediated by α6β1-integrin-sensing axon-associated signals. This activates Fyn kinase, subsequently signalling to RhoGTPases via FAK. However, laminin-2 is not ubiquitous in myelinated fibre tracts and β1-integrin is not essential for CNS myelination to proceed [32, 35], implicating other ligand–receptor interactions in place. Recent work demonstrated that binding of the laminin-family member netrin-1 to the surface receptor Dcc regulates oligodendroglial process extension by activating Fyn and inactivation of RhoA [36]. Netrin-1 is a chemorepellent for migrating oligodendrocyte precursors and is expressed by myelinating oligodendrocytes at later stages in development. Netrin-1 acts independently of β1-integrin and operates in two modes: firstly, in a paracrine manner to support process outgrowth of premyelinating oligodendrocytes, and secondly, in an autocrine and paracrine manner to control sheet formation and process branching in mature cells, respectively. The oligodendroglial signalling cascade in response to netrin-1 involves recruitment of Fyn to a complex of Dcc, FAK, and N-WASP. Interestingly, Fyn activation downstream of netrin-1/Dcc interaction selectively triggers RhoA inactivation while Cdc42 and Rac1 activity remains unchanged. This selective downstream signalling of netrin-1 may explain the restriction of its effects on oligodendroglial morphogenesis as opposed to the broader effects of laminin-2 that include survival. It thus appears that distinct upstream activators of Fyn selectively signal through specific downstream pathways. Laminin-2/β1-integrin-dependent stimulation of Fyn-kinase may preferentially elicit Cdc42/Rac1 activation, while netrin-1/Dcc-dependent Fyn signalling may favour downregulation of RhoA activity.
The ability of different surface receptors to signal through distinct downstream pathways may be a consequence of compartmentalised actions of Fyn. Fyn can associate with lipid raft microdomains by palmitoylation. Stimulation of certain upstream receptors, including β1-integrin and F3/contactin (see below), triggers Fyn kinase activity preferentially within these domains [18, 24]. It is conceivable that other surface receptors mediate Fyn activation outside lipid rafts. The subcellular localisation of the upstream receptor and the availability of interacting proteins may then define the downstream signalling pathway and the resulting cellular response.
Active Fyn recruits the microtubule cytoskeleton
The Fyn SH2 and SH3 domains are protein-binding domains mediating downstream interactions of the kinase. It has been demonstrated that α-tubulin interacts with both the SH2 and SH3 domains of Fyn. Moreover, the microtubule-associated protein Tau specifically binds to the SH3 domain [37]. Tau regulates the assembly and stabilisation of microtubules. Specific disruption of the endogenous Fyn–Tau interaction in oligodendroglial cells by overexpression of a dominant-negative Tau deletion mutant lacking an SH3 interaction motif alters the length and number of oligodendroglial processes. Fyn kinase activity is retained, thus the morphological defect is a result of abolished Fyn–Tau interaction and not due to a side effect mediating inactivation of the kinase. Moreover, oligodendroglial cells expressing a similar mutant form of Tau transplanted into a demyelinated lesion remyelinate less efficiently than control cells [38]. Fyn interaction with Tau and α-tubulin has also been implicated in a signalling pathway mediating oligodendroglial differentiation in response to the application of apotransferrin [39].
Binding of Tau and α-tubulin depends on the activity status of Fyn because of the increased availability of the SH2 and SH3 domains in the open conformation and has not been linked to specific upstream receptors. Thus, active Fyn kinase recruits components of the microtubule network stabilising the cytoskeleton and most likely promoting microtubule-based transport processes towards the site of activation. A recent study showed that microtubule-associated Tau inhibits kinesin motor activity and induces its detachment from microtubules [40], facilitating cargo release and availability. In oligodendroglial cells, such a function of Tau may facilitate the site-directed release of myelin cargo.
Neuron-induced activation of oligodendroglial Fyn mediates myelin protein synthesis and myelination
Faultless myelination requires the presence of functional MBP. The isolation of MBP mRNA and ribosomes from myelin preparations has led to the assumption that MBP mRNA is transported from the cell soma to the periphery and the protein is synthesised at sites of active myelination [41]. Hence, the intracellular transport of MBP mRNA has been extensively studied in oligodendrocytes [9, 42]. The RNA binding protein heterogeneous nuclear ribonucleoprotein A2 (hnRNP A2) has been identified as a trans-acting factor mediating the transport of MBP mRNA from the nucleus towards the distal tips of oligodendroglial processes in RNA granules that contain many, if not all, elements of the translation machinery [9, 43, 44]. Translational repression during granule transport seems to be mediated by hnRNP E1 which is recruited to the granule by hnRNP A2 [45]. A neuron-induced signalling pathway involving Fyn kinase leading to a de-repression and MBP translation at the oligodendrocyte plasma membrane was recently proposed. Binding of the axonal cell adhesion molecule L1 to the oligodendroglial cell surface leads to phosphorylation of hnRNP A2 by Fyn resulting in dissociation of the RNA transport granule allowing translation of the released MBP mRNA [10]. The GPI-anchored protein F3/contactin is an oligodendroglial surface receptor for L1 and mediates Fyn activation selectively within lipid-raft microdomains [10, 24]. In the oligodendroglial membrane, β1-integrin acts as a co-receptor of F3/contactin and both receptors cooperate in Fyn activation enhancing both oligodendroglial survival and myelin synthesis in response to L1 and laminin-2 [46]. The stimulation of localised MBP protein synthesis by axon–glial signalling may thus initiate the formation of myelin at specific timepoints and axonal regions depending on the presentation of the appropriate axonal ligands. As mentioned above, activated Fyn recruits the microtubule cytoskeleton and may hence direct MBP mRNA containing RNA-granules towards the axon–glial contact site where localised translation occurs. Moreover, Fyn activity seems to result in increased MBP transcription that may reflect a positive response to ensure adequate levels of mRNA cargo [47].
An additional connection between extracellular signalling and intracellular localised protein synthesis has recently been shown in neurons. Here, Dcc interacts with the translational machinery and binding of Netrin to Dcc triggers translation initiation allowing spatially controlled protein synthesis [48]. It is unknown if Dcc-mediated activation of Fyn contributes to the initiation of MBP translation in addition to the aforementioned effects on the morphology of cultured oligodendrocytes. Interestingly, the RNA-binding Protein QKI has been shown to be involved in the homeostasis of several oligodendroglial mRNAs including MBP. Fyn-dependent phosphorylation of QKI leads to dissociation of QKI from MBP mRNA and its destabilisation [49, 50]. QKI-deficient mice show a severe hypomyelination phenotype in the CNS which is rescued by transgenic introduction of a specific QKI isoform [51]. It seems likely that MBP mRNA localisation and/or translational control is regulated at least in part by concerted actions of hnRNP A2 and QKI.
Although a physiological ligand has not been identified, oligodendroglial common γ-chain of immunoglobulin Fc receptors (FcRγ) seems to increase the expression of MBP via Fyn signalling. Whether MBP expression is augmented on a transcriptional or translational level remains to be clarified. The importance of this signalling cascade is demonstrated by the hypomyelinated phenotype of FcRγ knockout mice and FcRγ/Fyn double knockout mice [52]. Antibody-mediated activation of this signalling pathway may play a role in the context of neuroinflammatory myelin degeneration such as multiple sclerosis.
Balancing Fyn-kinase activity
Fyn activation involves the action of tyrosine phosphatases mediating dephosphorylation of Y531 (Fig. 1). Thus, they occupy a modulating role in Fyn activation acting in concert with upstream activating receptors. Receptor-like protein-tyrosine phosphatase alpha (PTPα) has been implicated in the regulation of Fyn activity in several cell types, including oligodendrocytes [53], and has been found in a complex with F3/contactin in neurons [54]. Fyn activity is reduced in the absence of oligodendroglial PTPα and downstream targets of Fyn are less phosphorylated. Interestingly, p190RhoGAP phosphorylation is not affected, leading to the assumption that residual Fyn activity may suffice to target this molecule or that p190RhoGAP can be phosphorylated by a compensating kinase. PTPα knockout mice show a myelination deficit consistent with its putative role in Fyn-mediated myelination events in vivo. In addition to PTPα, protein tyrosine phosphatase CD45 was implicated in Fyn-dependent myelination processes. CD45 immunoprecipitates with Fyn in vitro, CD45-deficient cultured oligodendrocytes fail to differentiate and CD45 knockout mice show dysmyelination in the CNS [55].
Balancing Fyn activity is most likely required to counteract overproduction of myelin. It has been demonstrated that LINGO-1 inhibits oligodendrocyte differentiation and myelination and LINGO-1 antagonists promote myelination in animal models of de- and remyelination [56, 57]. On the molecular level, this seems to be mediated by Fyn kinase as overexpression of full length LINGO-1 constructs in oligodendrocytes leads to decreased Fyn levels and activity whereas the presence of dominant negative LINGO-1 results in increased amounts and activity of Fyn. Consequently, attenuation of LINGO-1 function by application of LINGO-1-Fc fusion protein to oligodendrocytes reduces the levels of Rho-GTP which is most likely a downstream effect of increased Fyn activity as Fyn phosphorylates and activates RhoGAPs mediating the conversion from Rho-GTP to Rho-GDP (see above). It is unknown whether the reduction of Fyn-activity by LINGO involves the action of Csk and Cbp, both of which are expressed by oligodendrocytes and negatively regulate Fyn activity. Transmembrane Cbp recruits Csk to the membrane and this interaction leads to a phosphorylation of Fyn at the regulatory tyrosine Y531 [58].
In vivo analysis of the Fyn signalling pathway: a network safeguarding myelination?
Several of the individual players within the Fyn signalling pathway have been examined with respect to their role in myelination in vivo. Transgenic deletion of the cell adhesion molecule L1 and F3/contactin results in complex neural phenotypes largely attributable to their neuronal functions [59, 60]. While F3/contactin-deficient mice exhibit myelin abnormalities (F. Fernandes, U. Bergstrom, K. Murai, B. Ranscht, Society for Neuroscience, Neuroscience Meeting 2007. Abstr. 459), dysmyelination has not been described in L1-deficient mice, although the stimulating role of L1 on myelin formation in vitro is documented [46, 61]. However, several alternative cell adhesion molecules, including MAG, are still available to establish stable axon–glia contacts possibly compensating for the loss of L1. Moreover, the presence of laminin-2 and β1-integrin may be sufficient to activate Fyn and initiate myelination. In the CNS of laminin α2-subunit-deficient mice, oligodendrocyte maturation is delayed and hypomyelination is observed [58, 62]. The affected brain regions appear similar to Fyn-deficient mice, and hyperphosphorylation of the regulatory Y531 of Fyn accompanied by elevated levels of the responsible kinase Csk and its regulator Cbp have been detected. This suggests that dysregulation of Fyn is responsible for hypomyelination in these mice [58].
Interference with integrin function in vivo, however, conveys a more confusing picture. Mice deficient in α6-integrin die at birth, but by embryonic day E18.5, when OPCs populate the CNS and start differentiating, survival of newly formed oligodendrocytes appears reduced [32]. Conditional ablation of β1-integrin in oligodendrocytes utilising different Cre mouse-lines for gene recombination has led to inconsistent observations, one study showing reduced oligodendroglial survival but normal myelination [35], and another reporting mild hypomyelination and normal oligodendroglial differentiation [63]. Moreover, mice expressing two different versions of dominant negative β1-integrin in oligodendrocytes present a transient failure to initiate myelination of small-diameter axons during development of the optic nerve [64], or exhibit hypomyelination of specific brain areas not mimicking the pattern observed in Fyn-deficient mice [65]. The chosen experimental strategies may have led to varying phenotypes which are to be discussed in detail elsewhere. However, it needs to be emphasised that numerous upstream activators of Fyn exist and, despite a distinct role of β1-integrin in the signalling pathway, it may well not be essential for Fyn-mediated myelination initiation. L1-F3/contactin-mediated Fyn activation may occur in the absence of β1-integrin. This notion is supported by the finding that F3/contactin ligation induces Y420 phosphorylation required for kinase activation, while β1-integrin mediates Y531 dephosphorylation leading to maximal kinase activity when both molecules cooperate [46]. Moreover, PTPα action may be sufficient to dephosphorylate Y531 [53] in the absence of β1-integrin. Taken together, it appears that laminin-2/β1-integrin and L1-F3/contactin synergistically activate Fyn integrating ECM- and axon-derived signals, each capable of compensating the in vivo loss of function of the other. The fact that hypomyelination appears more pronounced in laminin α2-subunit-deficient compared to β1-integrin-deficient mice is most likely due to dystroglycan acting as second laminin-2 receptor with a reported role in CNS myelination [66].
Conditional oligodendroglia-specific ablation of effectors acting downstream in the Fyn signalling pathway, such as the Rho-GTPases Rac1 and Cdc42 or FAK, was also reported to produce specific dysmyelinating phenotypes. Surprisingly, neither Cdc42 and Rac1 are required for oligodendrocyte differentiation (as one might have anticipated from the in vitro studies), but their ablation results in abnormal accumulation of cytoplasm within myelin and myelin outfoldings [67]. FAK-deletion in oligodendroglia results in a transient hypomyelination that is compensated during development indicating that FAK controls efficiency and timing of myelination during initial stages, similar to what has been reported for mice expressing dominant-negative β1-integrin [64, 68]. Whether these phenotypes coincide with abnormal Fyn-signalling or may be related to other Fyn-independent functions of these molecules has not been analysed.
In summary, at first glimpse, the transgenic studies revealed surprisingly non-uniform phenotypes and in some cases were not able to substantiate the conclusions drawn from in vitro studies. Nonetheless, a global reconsideration of the Fyn signalling pathway reveals a broad spectrum of upstream and downstream effectors that inherently comprise a high level of redundancy among the individual players. While none of the components appear essential for myelination to proceed, the compensation of loss of function among the effectors is safeguarding an elementary process such as myelination within the nervous system. The central and integrative role of Fyn kinase within the pathway is underscored by the pronounced hypomyelination phenotype observed in response to genetic ablation. Region-specific variations in requirement of Fyn signalling may depend on the emphasis of other signalling pathways with similar influence on myelination in distinct brain regions, such as the PI3K/Akt pathway, that acts downstream of neuregulin-1/ErbB [69, 70] and appears to be linked to the Fyn pathway by laminin-2-β1-integrin signalling [62, 63]. Interestingly, MBP deficiency results in complete block of myelination, thus control of MBP translation by Fyn kinase activity may be a rate-limiting factor for myelination to proceed.
Conclusions
Oligodendroglial Fyn kinase integrates target-derived axonal signals feeding into multiple downstream signalling pathways that serve to adapt and fine-tune oligodendroglial differentiation and myelin formation to neuronal properties. The widespread and partially redundant signalling pathways provide a robust foundation to myelin formation, compensating the loss of function of individual players. Considering the central role of Fyn in axon–glia signalling, the fact that Fyn-deficient mice are still capable of synthesising myelin to a certain degree may appear surprising. Most likely, Fyn-independent axon–glia signalling pathways exist and the multi-modular organisation of the Fyn signalling pathway explains why myelin appears to be relatively normal upon single deletion of molecules involved in axon–glia recognition. This reveals myelination per se to be a remarkably robust process. Possibly, other as yet undefined downstream signalling components or crosstalks to other pathways further enhance the complexity of the pathway. While the molecular basis of the requirement of MBP for myelin formation remains unclear, the control of MBP protein synthesis may actually constitute the rate-limiting function of Fyn in myelination.
Acknowledgments
We thank Constantin Gonsior and Christine Winterstein for critical comments on the manuscript. The authors are grateful to Jacqueline Trotter and Heiko Luhmann for continuous support. E-M. K-A. would like to acknowledge support from the European Leukodystrophy Association and R.W. shows gratitude to the University of Mainz for start-up funding level 1.
Abbreviations
- A2RE
hnRNP-A2 response element
- Cbp
CSK binding protein
- CNS
Central nervous system
- Csk
C-terminal Src kinase
- Dcc
Deleted in colorectal carcinoma
- ECM
Extracellular matrix
- FAK
Focal adhesion kinase
- FcRγ
γ chain of immunoglobulin Fc receptors
- hnRNP
Heterogeneous nuclear ribonucleoprotein
- MAG
Myelin-associated glycoprotein
- MBP
Myelin basic protein
- OPC
Oligodendrocyte precursor cell
- PDGF
Platelet-derived growth factor
- PLP
Proteo-lipid protein
- PTP
Receptor-like protein-tyrosine phosphatase
- SH
Src-homology domain
- WAVE2
Wiskott–Aldrich syndrome protein family member 2
Contributor Information
Eva-Maria Krämer-Albers, Phone: +49-613-13926257, FAX: +49-613-13923840, Email: emkraemer@uni-mainz.de.
Robin White, Phone: +49-613-13927170, FAX: +49-613-13926071, Email: white@uni-mainz.de.
References
- 1.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- 2.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 3.Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-K. [DOI] [PubMed] [Google Scholar]
- 4.Simons M, Trotter J. Wrapping it up: the cell biology of myelination. Curr Opin Neurobiol. 2007;17:533–540. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Jahn O, Tenzer S, Werner HB. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol. 2009;40:55–72. doi: 10.1007/s12035-009-8071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwiecien JM, O’Connor LT, Goetz BD, Delaney KH, Fletch AL, Duncan ID. Morphological and morphometric studies of the dysmyelinating mutant, the Long Evans shaker rat. J Neurocytol. 1998;27:581–591. doi: 10.1023/A:1006922227791. [DOI] [PubMed] [Google Scholar]
- 7.Readhead C, Hood L. The dysmyelinating mouse mutations shiverer (shi) and myelin deficient (shimld) Behav Genet. 1990;20:213–234. doi: 10.1007/BF01067791. [DOI] [PubMed] [Google Scholar]
- 8.Trajkovic K, Dhaunchak AS, Goncalves JT, Wenzel D, Schneider A, Bunt G, Nave KA, Simons M. Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. J Cell Biol. 2006;172:937–948. doi: 10.1083/jcb.200509022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carson JH, Barbarese E. Systems analysis of RNA trafficking in neural cells. Biol Cell. 2005;97:51–62. doi: 10.1042/BC20040083. [DOI] [PubMed] [Google Scholar]
- 10.White R, Gonsior C, Kramer-Albers EM, Stohr N, Huttelmaier S, Trotter J. Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. J Cell Biol. 2008;181:579–586. doi: 10.1083/jcb.200706164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2:467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 12.Rawat A, Nagaraj R. Determinants of membrane association in the SH4 domain of fyn: roles of N-terminus myristoylation and side-chain thioacylation. Biochim Biophys Acta. 2010;1798:1854–1863. doi: 10.1016/j.bbamem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Resh MD. Fyn, a Src family tyrosine kinase. Int J Biochem Cell Biol. 1998;30:1159–1162. doi: 10.1016/S1357-2725(98)00089-2. [DOI] [PubMed] [Google Scholar]
- 14.Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, Okada M, Aizawa S. Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell. 1993;73:1125–1135. doi: 10.1016/0092-8674(93)90642-4. [DOI] [PubMed] [Google Scholar]
- 15.Roskoski R., Jr Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun. 2004;324:1155–1164. doi: 10.1016/j.bbrc.2004.09.171. [DOI] [PubMed] [Google Scholar]
- 16.Ostareck-Lederer A, Ostareck DH, Cans C, Neubauer G, Bomsztyk K, Superti-Furga G, Hentze MW. c-Src-mediated phosphorylation of hnRNP K drives translational activation of specifically silenced mRNAs. Mol Cell Biol. 2002;22:4535–4543. doi: 10.1128/MCB.22.13.4535-4543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sette C, Paronetto MP, Barchi M, Bevilacqua A, Geremia R, Rossi P. Tr-kit-induced resumption of the cell cycle in mouse eggs requires activation of a Src-like kinase. EMBO J. 2002;21:5386–5395. doi: 10.1093/emboj/cdf553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colognato H, Ramachandrappa S, Olsen IM, ffrench-Constant C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 2004;167:365–375. doi: 10.1083/jcb.200404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- 20.Biffiger K, Bartsch S, Montag D, Aguzzi A, Schachner M, Bartsch U. Severe hypomyelination of the murine CNS in the absence of myelin-associated glycoprotein and fyn tyrosine kinase. J Neurosci. 2000;20:7430–7437. doi: 10.1523/JNEUROSCI.20-19-07430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, Scherer SS, McMorris FA. A unique role for Fyn in CNS myelination. J Neurosci. 2001;21:2039–2047. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol. 1999;145:1209–1218. doi: 10.1083/jcb.145.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperber BR, McMorris FA. Fyn tyrosine kinase regulates oligodendroglial cell development but is not required for morphological differentiation of oligodendrocytes. J Neurosci Res. 2001;63:303–312. doi: 10.1002/1097-4547(20010215)63:4<303::AID-JNR1024>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.Kramer EM, Klein C, Koch T, Boytinck M, Trotter J. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J Biol Chem. 1999;274:29042–29049. doi: 10.1074/jbc.274.41.29042. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto Y, Yamauchi J, Tanoue A. Cdk5 phosphorylation of WAVE2 regulates oligodendrocyte precursor cell migration through nonreceptor tyrosine kinase Fyn. J Neurosci. 2008;28:8326–8337. doi: 10.1523/JNEUROSCI.1482-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kippert A, Trajkovic K, Rajendran L, Ries J, Simons M. Rho regulates membrane transport in the endocytic pathway to control plasma membrane specialization in oligodendroglial cells. J Neurosci. 2007;27:3560–3570. doi: 10.1523/JNEUROSCI.4926-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniguchi S, Liu H, Nakazawa T, Yokoyama K, Tezuka T, Yamamoto T. p250GAP, a neural RhoGAP protein, is associated with and phosphorylated by Fyn. Biochem Biophys Res Commun. 2003;306:151–155. doi: 10.1016/S0006-291X(03)00923-9. [DOI] [PubMed] [Google Scholar]
- 28.Wolf RM, Wilkes JJ, Chao MV, Resh MD. Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J Neurobiol. 2001;49:62–78. doi: 10.1002/neu.1066. [DOI] [PubMed] [Google Scholar]
- 29.Liang X, Draghi NA, Resh MD. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J Neurosci. 2004;24:7140–7149. doi: 10.1523/JNEUROSCI.5319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang S, Avraham HK, Park SY, Kim TA, Bu X, Seng S, Avraham S. Process elongation of oligodendrocytes is promoted by the Kelch-related actin-binding protein Mayven. J Neurochem. 2005;92:1191–1203. doi: 10.1111/j.1471-4159.2004.02946.x. [DOI] [PubMed] [Google Scholar]
- 31.Williams SK, Spence HJ, Rodgers RR, Ozanne BW, Fitzgerald U, Barnett SC. Role of Mayven, a kelch-related protein in oligodendrocyte process formation. J Neurosci Res. 2005;81:622–631. doi: 10.1002/jnr.20588. [DOI] [PubMed] [Google Scholar]
- 32.Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, ffrench-Constant C. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4:833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- 33.Relvas JB, Setzu A, Baron W, Buttery PC, LaFlamme SE, Franklin RJ, ffrench-Constant C. Expression of dominant-negative and chimeric subunits reveals an essential role for beta1 integrin during myelination. Curr Biol. 2001;11:1039–1043. doi: 10.1016/S0960-9822(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 34.Hoshina N, Tezuka T, Yokoyama K, Kozuka-Hata H, Oyama M, Yamamoto T. Focal adhesion kinase regulates laminin-induced oligodendroglial process outgrowth. Genes Cells. 2007;12:1245–1254. doi: 10.1111/j.1365-2443.2007.01130.x. [DOI] [PubMed] [Google Scholar]
- 35.Benninger Y, Colognato H, Thurnherr T, Franklin RJ, Leone DP, Atanasoski S, Nave KA, Ffrench-Constant C, Suter U, Relvas JB. Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J Neurosci. 2006;26:7665–7673. doi: 10.1523/JNEUROSCI.0444-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajasekharan S, Baker KA, Horn KE, Jarjour AA, Antel JP, Kennedy TE. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development. 2009;136:415–426. doi: 10.1242/dev.018234. [DOI] [PubMed] [Google Scholar]
- 37.Klein C, Kramer EM, Cardine AM, Schraven B, Brandt R, Trotter J. Process outgrowth of oligodendrocytes is promoted by interaction of fyn kinase with the cytoskeletal protein tau. J Neurosci. 2002;22:698–707. doi: 10.1523/JNEUROSCI.22-03-00698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belkadi A, LoPresti P. Truncated Tau with the Fyn-binding domain and without the microtubule-binding domain hinders the myelinating capacity of an oligodendrocyte cell line. J Neurochem. 2008;107:351–360. doi: 10.1111/j.1471-4159.2008.05600.x. [DOI] [PubMed] [Google Scholar]
- 39.Perez MJ, Ortiz EH, Roffe M, Soto EF, Pasquini JM. Fyn kinase is involved in oligodendroglial cell differentiation induced by apotransferrin. J Neurosci Res. 2009;87:3378–3389. doi: 10.1002/jnr.21962. [DOI] [PubMed] [Google Scholar]
- 40.Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colman DR, Kreibich G, Frey AB, Sabatini DD. Synthesis and incorporation of myelin polypeptides into CNS myelin. J Cell Biol. 1982;95:598–608. doi: 10.1083/jcb.95.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carson JH, Blondin N, Korza G. Rules of engagement promote polarity in RNA trafficking. BMC Neurosci. 2006;7(Suppl 1):S3. doi: 10.1186/1471-2202-7-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ainger K, Avossa D, Diana AS, Barry C, Barbarese E, Carson JH. Transport and localization elements in myelin basic protein mRNA. J Cell Biol. 1997;138:1077–1087. doi: 10.1083/jcb.138.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ainger K, Avossa D, Morgan F, Hill SJ, Barry C, Barbarese E, Carson JH. Transport and localization of exogenous myelin basic protein mRNA microinjected into oligodendrocytes. J Cell Biol. 1993;123:431–441. doi: 10.1083/jcb.123.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosturko LD, Maggipinto MJ, Korza G, Lee JW, Carson JH, Barbarese E. Heterogeneous nuclear ribonucleoprotein (hnRNP) E1 binds to hnRNP A2 and inhibits translation of A2 response element mRNAs. Mol Biol Cell. 2006;17:3521–3533. doi: 10.1091/mbc.E05-10-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laursen LS, Chan CW, ffrench-Constant C. An integrin–contactin complex regulates CNS myelination by differential Fyn phosphorylation. J Neurosci. 2009;29:9174–9185. doi: 10.1523/JNEUROSCI.5942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umemori H, Kadowaki Y, Hirosawa K, Yoshida Y, Hironaka K, Okano H, Yamamoto T. Stimulation of myelin basic protein gene transcription by Fyn tyrosine kinase for myelination. J Neurosci. 1999;19:1393–1397. doi: 10.1523/JNEUROSCI.19-04-01393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tcherkezian J, Brittis PA, Thomas F, Roux PP, Flanagan JG. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell. 2010;141:632–644. doi: 10.1016/j.cell.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Z, Ku L, Chen Y, Feng Y. Developmental abnormalities of myelin basic protein expression in Fyn knock-out brain reveal a role of Fyn in posttranscriptional regulation. J Biol Chem. 2005;280:389–395. doi: 10.1074/jbc.M405973200. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Lu Z, Ku L, Chen Y, Wang H, Feng Y. Tyrosine phosphorylation of QKI mediates developmental signals to regulate mRNA metabolism. EMBO J. 2003;22:1801–1810. doi: 10.1093/emboj/cdg171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao L, Tian D, Xia M, Macklin WB, Feng Y. Rescuing qkV dysmyelination by a single isoform of the selective RNA-binding protein QKI. J Neurosci. 2006;26:11278–11286. doi: 10.1523/JNEUROSCI.2677-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakahara J, Seiwa C, Tan-Takeuchi K, Gotoh M, Kishihara K, Ogawa M, Asou H, Aiso S. Signaling via immunoglobulin Fc receptors induces oligodendrocyte precursor cell differentiation. Dev Cell. 2003;4:841–852. doi: 10.1016/S1534-5807(03)00155-2. [DOI] [PubMed] [Google Scholar]
- 53.Wang PS, Wang J, Xiao ZC, Pallen CJ. Protein-tyrosine phosphatase alpha acts as an upstream regulator of Fyn signaling to promote oligodendrocyte differentiation and myelination. J Biol Chem. 2009;284:33692–33702. doi: 10.1074/jbc.M109.061770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeng L, D’Alessandri L, Kalousek MB, Vaughan L, Pallen CJ. Protein tyrosine phosphatase alpha (PTPalpha) and contactin form a novel neuronal receptor complex linked to the intracellular tyrosine kinase fyn. J Cell Biol. 1999;147:707–714. doi: 10.1083/jcb.147.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakahara J, Seiwa C, Tan-Takeuchi K, Gotoh M, Kishihara K, Ogawa M, Asou H, Aiso S. Involvement of CD45 in central nervous system myelination. Neurosci Lett. 2005;379:116–121. doi: 10.1016/j.neulet.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 56.Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, Hession C, Sah D, Trapp B, He Z, Jung V, McCoy JM, Pepinsky RB. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- 57.Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, Zhang Y, Shields CB, Zhang Y, Miklasz S, Shea D, Mason J, Franklin RJ, Ji B, Shao Z, Chedotal A, Bernard F, Roulois A, Xu J, Jung V, Pepinsky B. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009;65:304–315. doi: 10.1002/ana.21581. [DOI] [PubMed] [Google Scholar]
- 58.Relucio J, Tzvetanova ID, Ao W, Lindquist S, Colognato H. Laminin alters fyn regulatory mechanisms and promotes oligodendrocyte development. J Neurosci. 2009;29:11794–11806. doi: 10.1523/JNEUROSCI.0888-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet. 1997;17:346–349. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- 60.Berglund EO, Murai KK, Fredette B, Sekerkova G, Marturano B, Weber L, Mugnaini E, Ranscht B. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron. 1999;24:739–750. doi: 10.1016/S0896-6273(00)81126-5. [DOI] [PubMed] [Google Scholar]
- 61.Coman I, Barbin G, Charles P, Zalc B, Lubetzki C. Axonal signals in central nervous system myelination, demyelination and remyelination. J Neurol Sci. 2005;233:67–71. doi: 10.1016/j.jns.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 62.Chun SJ, Rasband MN, Sidman RL, Habib AA, Vartanian T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol. 2003;163:397–408. doi: 10.1083/jcb.200304154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barros CS, Nguyen T, Spencer KS, Nishiyama A, Colognato H, Muller U. Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development. 2009;136:2717–2724. doi: 10.1242/dev.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Camara J, Wang Z, Nunes-Fonseca C, Friedman HC, Grove M, Sherman DL, Komiyama NH, Grant SG, Brophy PJ, Peterson A, ffrench-Constant C. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J Cell Biol. 2009;185:699–712. doi: 10.1083/jcb.200807010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee KK, de Repentigny Y, Saulnier R, Rippstein P, Macklin WB, Kothary R. Dominant-negative beta1 integrin mice have region-specific myelin defects accompanied by alterations in MAPK activity. Glia. 2006;53:836–844. doi: 10.1002/glia.20343. [DOI] [PubMed] [Google Scholar]
- 66.Colognato H, Galvin J, Wang Z, Relucio J, Nguyen T, Harrison D, Yurchenco PD, Ffrench-Constant C. Identification of dystroglycan as a second laminin receptor in oligodendrocytes, with a role in myelination. Development. 2007;134:1723–1736. doi: 10.1242/dev.02819. [DOI] [PubMed] [Google Scholar]
- 67.Thurnherr T, Benninger Y, Wu X, Chrostek A, Krause SM, Nave KA, Franklin RJ, Brakebusch C, Suter U, Relvas JB. Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. J Neurosci. 2006;26:10110–10119. doi: 10.1523/JNEUROSCI.2158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forrest AD, Beggs HE, Reichardt LF, Dupree JL, Colello RJ, Fuss B. Focal adhesion kinase (FAK): a regulator of CNS myelination. J Neurosci Res. 2009;87:3456–3464. doi: 10.1002/jnr.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siefke C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave KA. Elevated phosphatidylinositol 3, 4, 5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010;30:8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]