Abstract
The pro-oncogene FBI-1, encoded by Zbtb7a, is a transcriptional repressor that belongs to the POK (POZ/BTB and Krüppel) protein family. In this study, we investigated a potential interaction between androgen receptor (AR) signaling and FBI-1 and demonstrated that overexpression of FBI-1 inhibited ligand-dependent AR activation. A protein–protein interaction was identified between FBI-1 and AR in a ligand-dependent manner. Furthermore, FBI-1, AR and SMRT formed a ternary complex and FBI-1 enhanced the recruitment of NCoR and SMRT to endogenous PSA upstream sequences. Our data also indicated that the FBI-1-mediated inhibition of AR transcriptional activity is partially dependent on HDAC. Interestingly, FBI-1 plays distinct roles in regulating LNCaP (androgen-dependent) and PC-3 cell (androgen-independent) proliferation.
Keywords: Androgen receptor, FBI-1, Gene expression, POZ domain, Co-repressor
Introduction
The androgen receptor (AR) is critical for the proliferation, differentiation, maintenance and functioning of prostate cells. Like other members of the nuclear receptor superfamily, AR has four major functional regions including an N-terminal transactivation domain (NTD), a DNA-binding domain (DBD), a C-terminal ligand-binding domain (LBD), and a hinge region connecting the DBD and LBD [1, 2]. Two transactivation functions, a constitutively active activation function (AF-1) in the NTD and a ligand-dependent activation function (AF-2) in the LBD, are required for the transcriptional activity of nuclear receptors [3].
In the absence of androgens, the AR always resides in the cytosol as a protein complex bound by chaperones. Upon binding with androgens, the AR dissociates from chaperones, dimerizes, and translocates into the nucleus, where it binds to androgen response elements (AREs) located in the promoter of the target genes [4, 5]. The AR dimer recruits co-activators such as p160 members, and thus facilitates the formation of an active pre-initiation complex and interacts with the basal transcription machinery to trigger the transcription of the target genes. When the AR recruits co-repressors such as NCoR and SMART, it leads to transcription repression presumably due to the chromatin compaction [6].
FBI-1 is a transcriptional repressor that belongs to the POK protein family [7]. The POZ domain (for Poxvirus and zinc finger, also known as the BTB domain), originally identified in Drosophila and Poxvirus, is an evolutionary conserved protein–protein interaction domain. The most striking and common property of POZ domain transcriptional factors is their ability to repress transcription via their POZ domain [8]. FBI-1 was originally identified as a protein that binds specifically to the inducer of short transcripts (IST) element on the HIV-1 promoter and was subsequently identified as a homologue of PLZF [9, 10]. It was recently reported to be an important proto-oncogene that is highly expressed in breast, thymus, colon, bladder cancer and lymphoma. Overexpression of FBI-1 can reduce RB and ARF gene expression, which in turn gives rise to p53 degradation and oncogenic transformation. Conversely, knockout of FBI-1 not only suppresses oncogenic transformation but also induces cell senescence and apoptosis [7, 11, 12].
Given the evidence that the AR plays an important role in the development of prostate cancer and that FBI-1 is overexpressed in prostate LNCaP cancer cells treated with androgen [13], we hypothesized that FBI-1 might also be involved in the transcriptional regulation of AR target genes. In this study, we found that FBI-1 physically interacts with AR in vitro and in vivo. Overexpression of FBI-1 inhibited AR-mediated transcriptional activity in a ligand-dependent manner, whereas knockdown of endogenous FBI-1 with small interfering RNA (siRNA) significantly enhanced AR transcriptional activity. Multiple lines of evidence have suggested that FBI-1 inhibits AR transcriptional activity via recruitment of NCoR and SMRT. Moreover, overexpression of FBI-1 repressed LNCaP cell proliferation in the presence of R1881.
Materials and methods
Cells and culture
LNCaP and PC-3 prostate cancer cells were cultured in RPMI1640 medium containing 10% FBS (Hyclone). 293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS. All cell types were cultured at 37°C in a humidified incubator containing 5% CO2. LNCaP and PC-3 cells were cultured in phenol red-free RPMI 1640 plus 10% charcoal-stripped FBS for 3 days before treatment with a synthetic androgen R1881 (Sigma).
Plasmids
The plasmid pcDNA3.0-AR expressing the full-length AR was generated by PCR amplification from a prostate cDNA library (Clontech). The reporter plasmid pPSA-LUC, containing the luciferase gene under the control of a 6.1-kb promoter fragment of the human PSA gene, was constructed by PCR amplification using LNCaP cell DNA as the template [14]. FLAG-NCoR and FLAG-SMRT were kindly provided by Dr. Glass (University of California, USA) FLAG-AR and truncated mutants were generated by PCR amplification using pcDNA3.0-AR as a template and the PCR products were then cloned into pcDNA 3.0 linked with FLAG at the amino terminus. To construct pcDNA3.0-FBI-1 and FLAG-FBI-1, full-length human FBI-1 was obtained by standard PCR amplification from LNCaP cell cDNA and was then cloned into pcDNA3.0 and pcDNA3.0 linked with FLAG at the amino terminus. To construct Myc-FBI-1 and the corresponding truncated mutants, the amplified full-length FBI-1, truncated mutants Myc-POZ (residues 1–130) and Myc-FBI-1ΔPOZ (residues 131–584) were cloned into pcMV-Myc vector (Clontech). To construct the siRNA expression vector, a DNA fragment containing an inverted repeat of the target sequence was synthesized and cloned under control of the U6 promoter into the BamHI/HindIII sites of pSilencer2.1-U6 (Ambion). The target sequences as follows: FBI-1 siRNA: 5′-GCTGGACCTTGTAGATCAA-3′. NCoR and SMRT siRNA were constructed according to a previous report [15]. Plasmid pSilencer2.1-U6 negative control (Ambion) was used as a control vector. This control vector produces universal scramble siRNA that has no significant homology to mouse, rat, or human gene sequences.
Transient transfections and reporter gene assays
LNCaP and PC-3 cells were grown in RPMI1640 media supplemented with 10% FBS. For transfection, the cells were plated in 24-well plates containing phenol red-free RPMI 1640 medium supplemented with 10% charcoal-stripped FBS (Hyclone), and the plasmids were transfected with Lipofectamine 2000 (Invitrogen). Following transfection, the cells were treated with R1881 or ethanol for 24 h and then harvested for the dual luciferase assay. The dual luciferase reporter assay system (Promega) was employed to measure the luciferase activity.
GST-pull down assay
FBI-1 were expressed as GST-fusion proteins in E. coli strain BL21 and bound to glutathione-Sepharose beads purified as described by the manufacturer (Amersham Biosciences). The expression plasmid for AR was used for in vitro transcription and translation in the TNT system (Promega). The 35S-labeled TNT-expressed AR was incubated with about 1 μg of GST alone or GST-FBI-1 fusion protein bound to glutathione-Sepharose beads in 500 μl of binding buffer (50 mM Tris–HCl, pH 7.4, 100 mM NaCl, 5 mM MgCl2, 0.1% Nonidet P-40, 10% glycerol, 1 mM dithiothreitol and protease inhibitor tablets from Roche Applied Science) at 4°C or 4 h. The beads were precipitated, washed three times with binding buffer, and subjected to SDS-PAGE and autoradiography.
Coimmunoprecipitation
293T or PC-3 cells were transfected with the indicated plasmids using Lipofectamine 2000 (Invitrogen) and treated with 1 nM R1881 or ethanol for 1 h before harvest. Cells were washed with phosphate-buffered saline (PBS), lysed in 0.5 ml lysis buffer (50 mM Tris, pH 8.0, 250 mM NaCl, 0.25% NP-40, 1 mM DTT and protease inhibitor tablets from Roche), and immunoprecipitated with anti-FLAG-agarose beads (Sigma) for 4 h at 4°C. The beads were centrifuged, washed four times with the lysis buffer, and eluted in of SDS-PAGE sample buffer. The eluted proteins were separated by SDS-PAGE, followed by immunoblotting with anti-FBI-1 (Sigma), anti-Myc (Santa Cruz Biotech) or anti-FLAG (Sigma) according to the standard procedures. For reimmunoprecipitation, the immune complexes precipitated with anti-FLAG were eluted by a competition with 3× FLAG peptide according to the manufacturer’s instructions (Sigma). The eluate was precleared with 20 μL of 50% protein A agarose beads (Santa Cruz Biotech) for 30 min. Proteins were reprecipitated with anti-FBI-1 or control IgG (Santa Cruz Biotech) plus 20 μL of protein A agarose beads. Reprecipitates were washed four times with lysis buffer, eluted by boiling in SDS-PAGE sample buffer, and resolved by SDS-PAGE, followed by immunoblotting.
RT-PCR and real-time PCR
Total RNA was isolated from LNCaP cells stably expressing FBI-1 or FBI-1 siRNA-1 using TRIzol reagent according to the manufacturer’s instructions (Invitrogen). Total RNA from each sample was reverse transcribed with random primers using a reverse transcriptase kit (Takara) followed by quantitative PCR. Primers for amplification of the PSA mRNA were 5′-GCCCACCCAGGAGCCAGCACT-3′ and 5′-GGCCCCCAGAATCACCCGAGCAG-3′ [16]. Primers for amplification of the GAPDH mRNA were 5′-GGGCTCTCCAGAACATCAT-3′ and 5′-AGCCCCAGCGTCAAAGGTG-3′. PCR products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. Real-time PCR was performed with SYBR Green PCR Master mix Reagents using an ABI Prism 7700 Sequence Detection System (Applied Biosystems). The primers used were as follows: PSA forward primer: 5′-GACCACCTGCTACGCCTCA-3′ and reverse primer: 5′-GGAGGTCCACACTGAAGTTTC-3′; GAPDH forward primer: 5′-TGCACCACCAACTGCTTAGC-3′ and reverse primer: 5′-GGCATGGACTGTGGTCATGAG-3′.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) analysis was performed following a protocol provided by the ChIP kit (Upstate,). In brief, 2 × 109 LNCaP cells were fixed by adding formaldehyde to the medium to a final concentration of 1%. After cross-linking for 10 min at 22°C, glycine was added to a final concentration of 125 mM, and then the cells were harvested with lysis buffer. The nuclei were pelleted by centrifugation and resuspended in nuclear lysis buffer. The nuclear lysates were sonicated to generate an average DNA size of 0.5–1 kb, and then immunoprecipitation was performed with anti-AR (Cell Signaling Technology), anti-NCoR, anti-SMRT (Santa Cruz Biotechnology) or anti-FBI-1 antibodies (Sigma), respectively. Real-time PCR amplification was performed with DNA extracted from the immunoprecipitates and primers flanking the PSA promoter. The primers used, AREI (located in the promoter) and ARE III (located in the enhancer), are as follows: AREI forward, 5′-CCTAGATGAAGTCTCCATG-3′ and reverse, 5′-AACCTTCATTCCCCAGGACT-3′ [17]; and ARE III forward, 5′-GCAAACCTGCTCAGCCTT and reverse, 5′-GCTTGCTTACTGTCCTAGAT-3′ [18].
Cell proliferation assays
The proliferation of LNCaP cells stably expressing either FLAG-FBI-1 or FBI-1 siRNA was determined using a CellTiter 96® nonradioactive cell proliferation assay kit (Promega) according to the manufacturer’s instructions.
Results
FBI-1 inhibits ligand-dependent transcriptional activity of AR
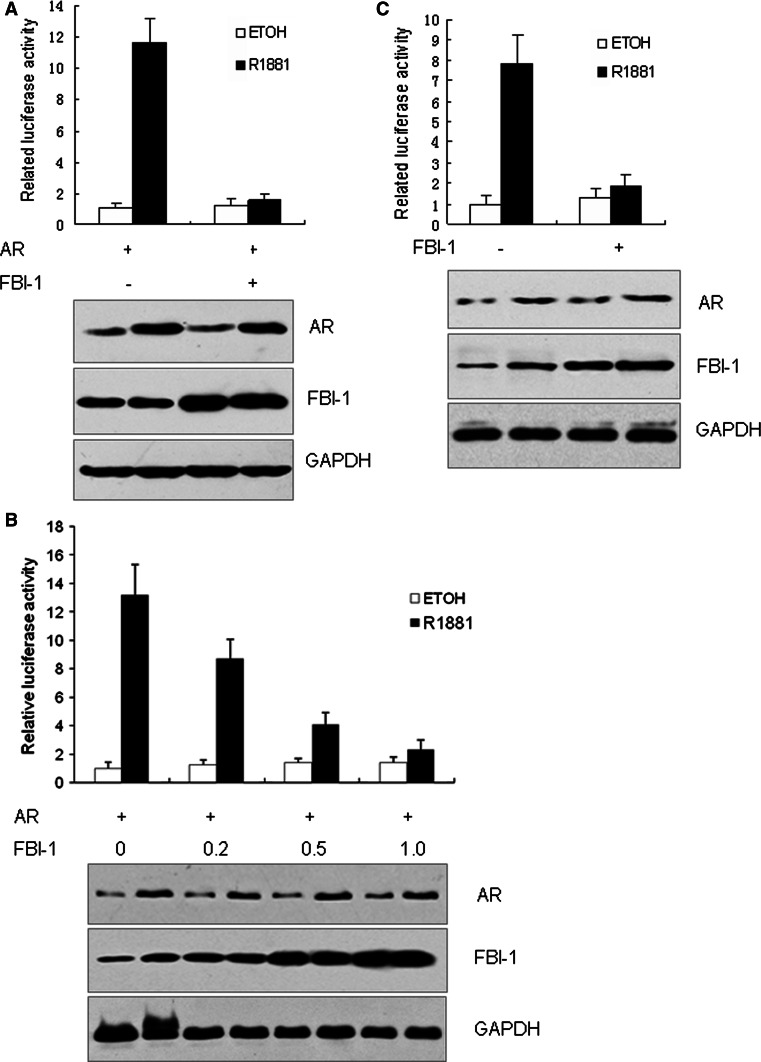
Since FBI-1 is a central regulator in oncogenesis and its expression can be induced by androgens in prostate cancer, we hypothesized that FBI-1 might be involved in the AR-mediated signaling. Thus, a PSA promoter-luciferase reporter was transfected into PC-3 cells together with the AR and FBI-1 expression plasmids. The cells were then treated with R1881 or ethanol. As shown in Fig. 1a, an approximately 10-fold higher induction was observed in the presence of 1 nM R1881 compared with vehicle treatment. Co-transfection of the FBI-1 expression plasmid significantly inhibited the AR transcriptional activity in the presence of R1881. However, FBI-1 did not affect the AR activity in the absence of R1881.
Fig. 1.
FBI-1 inhibits ligand-dependent transcriptional activity of AR. a PC-3 cells were co-transfected with PSA-LUC, PRL-TK (internal control), AR expression vector and pcDNA3.0-FBI-1 or pcDNA3.0 (negative control). b FBI-1 inhibited AR activity in a dose-dependent manner. PC-3 cells were co-transfected with vectors indicated as (a) except with increasing amount of pcDNA3.0-FBI-1 as indicated. c LNCaP cells were co-transfected with vectors indicated as (a) without AR expression vector. Following transfection, cells were stimulated with 1 nM R1881 or 0.1% ethanol vehicle (ETOH) for 24 h. Cells were harvested for the luciferase assay. Western blotting (bottom) showed the expression level of AR and FBI-1. The values are the mean ± SE of three independent experiments performed in triplicate, and are normalized to Renilla luciferase activity
In order to identify whether the effect of FBI-1 on AR transcriptional activity is dose-dependent, various amounts of FBI-1 were transfected into PC-3 cells and then cells were treated with R1881. As shown in Fig. 1b, FBI-1 decreased AR activity in a dose-dependent way in the presence of R1881. The inhibition of the AR activity by FBI-1 was also observed in the AR positive LNCaP cell line (Fig. 1c). These results indicate that FBI-1 can inhibit the ligand-dependent transcriptional activity of the AR.
Knockdown of endogenous FBI-1 can enhance the AR transcriptional activity
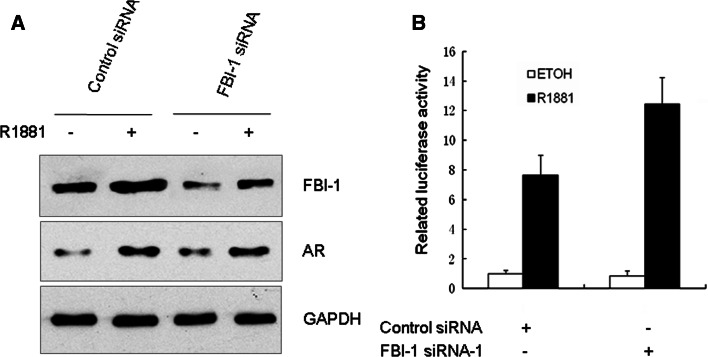
To determine if endogenous FBI-1 may be involved in AR transactivation, we used siRNA to diminish FBI-1 expression. LNCaP cells stably expressing FBI-1 siRNAs or universal scramble siRNA (control) were generated. As expected, FBI-1 siRNA effectively inhibited the expression of FBI-1 protein, whereas universal scramble siRNA had no effect (Fig. 2a). The FBI-1 siRNA did not affect the expression of AR, which indicated that FBI-1 regulated the AR transcriptional activity not via altering AR protein level. As shown in Fig. 2b, suppression of the normal expression of FBI-1 in LNCaP cells by the specific FBI-1 siRNAs significantly enhanced AR transcriptional activity. These results further suggest that FBI-1 inhibits the AR transcriptional activity.
Fig. 2.
Knockdown of endogenous FBI-1 enhances AR transcriptional activity in LNCaP cells stably express FBI-1 siRNA-1 or scramble siRNA (control). a Western blotting with various antibodies showing the specific knockdown effect of FBI-1 siRNA on the endogenous FBI-1 protein level. Cells were treated with 1 nM R1881 or 0.1% ethanol vehicle (ETOH) for 24 h. Whole-cell extracts were prepared and probed with anti-FBI-1, AR, or GAPDH antibody. b Luciferase reporter assays in the control and FBI-1 knockdown cells. LNCaP cells were co-transfected with PSA-LUC. Cells were treated and analyzed as described in the legend to Fig. 1c
Interaction between AR and FBI-1 in vitro and in vivo
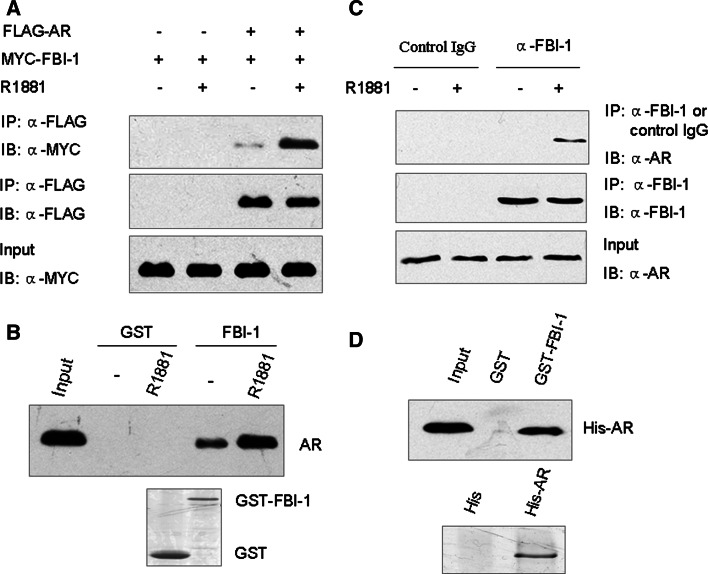
Next, we examined the possible interaction between AR and FBI-1. N-terminally FLAG-tagged full-length AR and Myc-FBI-1 were expressed in 293T cells and treated with R1881 or ethanol. FLAG-AR was immunoprecipitated from cell lysates by an anti-FLAG antibody and analyzed for FBI-1 binding by western blotting analysis. As shown in Fig. 3a, FBI-1 was co-immunoprecipitated and detected in the presence of R1881. However, a very weak signal was detected in the absence of R1881. This result demonstrates that in vivo FBI-1 interacts with AR in vivo in a ligand-dependent manner.
Fig. 3.
FBI-1 can interact with AR in vitro and in vivo. a Interaction of exogenous FBI-1 with AR in vivo. FLAG-tagged AR and Myc-tagged FBI-1 or empty vector were co-transfected into 293T cells. Cell lysates were immunoprecipitated (IP) by anti-FLAG M2 monoclonal antibody, and the precipitates were then immunoblotted with anti-Myc polyclonal antibody. b In vitro interaction of FBI-1 with AR. Glutathione-Sepharose beads bound with GST-FBI-1 or with GST was incubated with 35S-labeled AR in the presence or absence of 1 nM R1881. After washing the beads, the bound proteins were eluted and subjected to SDS-PAGE and autoradiography. c Interaction of endogenous FBI-1 with AR in vivo. LNCaP cells were treated without and with 1 nM R1881 for 1 h. Cell lysates were immunoprecipitated with either anti-FBI-1 antibody or control IgG. The precipitates were analyzed by western blot using anti-AR antibody (Cell Signaling Technology). d Direct interaction of FBI-1 with AR. Escherichia coli expressing His-AR were purified, and incubated with purified GST or GST-FBI-1 immobilized on glutathione-Sepharose beads. Bound proteins were analyzed by immunoblotting using anti-His antibody (upper panel). SDS-PAGE analysis of the purified His-AR protein is shown at the bottom
To identify the interaction between FBI-1 and AR in vitro, we used a GST-FBI-1 fusion protein incubated with AR in a GST pull-down experiment. As shown in Fig. 3b, the binding of AR to GST-FBI-1, but not to GST, was observed both in the absence and in the presence of R1881. Moreover, R1881 significantly increased the interaction of AR with GST-FBI-1.
To ascertain the interaction of FBI-1 with AR in a more physiological context, the endogenous FBI-1 protein from LNCaP cells was immunoprecipitated with anti-FBI-1 antibody. Subsequent blotting with anti-AR antibody indicated that the endogenous AR was co-precipitated with FBI-1 in the presence of, but not in the absence of, R1881 (Fig. 3c). In the negative control experiment, control IgG did not immunoprecipitate AR. Taken together, these data strongly suggest that FBI-1 interacts with AR under physiological condition.
As it is still possible that FBI-1 binds to AR indirectly, it is necessary to explore whether FBI-1 can interact directly with AR in vitro. To this end, purified GST-FBI-1 was incubated with purified His-tagged AR in a GST pull-down assay. As expected, a stable interaction between these two proteins was readily detectable by anti-His antibody (Fig. 3d). This observation confirmed that FBI-1 binds directly to AR.
Mapping the AR domains interacting with FBI-1
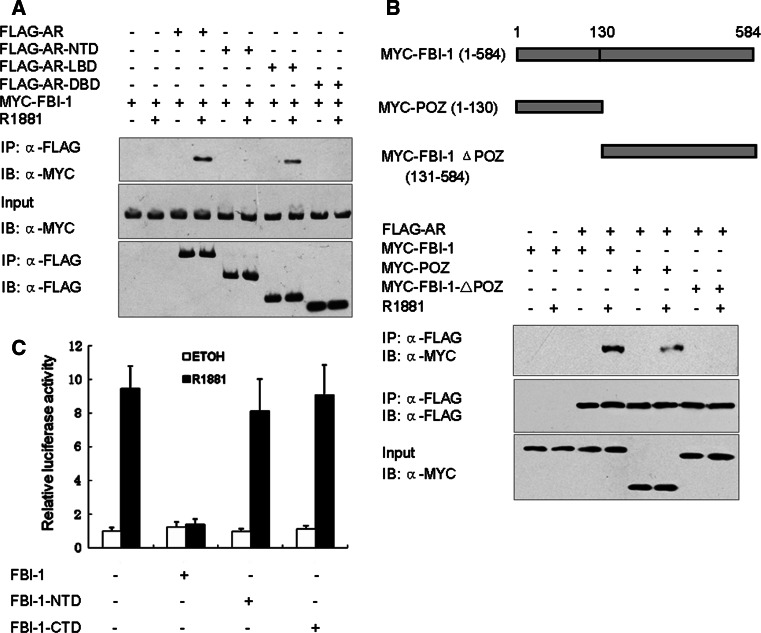
To delineate the domains in the AR that mediate the protein–protein interaction with FBI-1 in vivo, three AR truncated mutants, NH2-terminal (AR-NTD, residues 1–500), DBD alone (AR-DBD, residues 501–660) and LBD (AR-LBD, residues 661–920) were constructed into the pcDNA3.0-FLAG vector. 293T cells were transfected with FLAG-tagged AR or three truncated mutants and Myc-FBI-1 and then cultured in the presence of 1 nM R1881 or 0.1% ethanol for 1 h. Then, cells were harvested for co-immunoprecipitation experiments. Figure 4a demonstrated that FBI-1 was co-immunoprecipitated in a ligand-dependent manner in the presence of FLAG-AR and FLAG-AR-LBD but not the FLAG-tagged empty vector. However, in the presence of FLAG-AR-NTD and DBD, FBI-1 was not co-immunoprecipitated. This result suggests that the LBD in the AR is responsible for mediating the interaction with FBI-1.
Fig. 4.
Mapping of the FBI-1 and AR interacting regions. a 293T cells co-transfected with FLAG-AR or truncated mutants and Myc-tagged full-length FBI-1 as indicated were lysed and immunoprecipitated with anti-FLAG antibody. Western blotting analysis was then performed using anti-FLAG or anti-Myc antibody. b Immunoprecipitation was repeated with FLAG-AR and Myc-tagged full-length FBI-1 or truncated mutants as indicated. Schematic diagram of the FBI-1 deletion construct is shown at the top. c Luciferase reporter assays in cells transfected with FBI-1 or truncated mutants. LNCaP Cells were co-transfected with PSA-LUC, PRL-TK and FBI-1 or truncated mutants as indicated. Cells were treated and analyzed as described in the legend to Fig. 1c
The POZ domain of FBI-1 is responsible for the interaction with AR
The POZ domain is a protein–protein interaction motif and is responsible for transcription repression in several POZ domain proteins [19]. Thus, we identify whether the POZ domain is required for the interaction of FBI-1 with AR. Two FBI-1 truncated mutants, NH2-terminal (residues 1–130, POZ domain), and C-terminal (residues 131–584) were constructed and co-immunoprecipitation experiments were performed to identify the interaction between the two truncated mutants and AR. As shown in Fig. 4b, in the presence of R1881, AR interacted with full-length FBI-1 and POZ domain, but not with FBI-1 C-terminal fragment (FBI-1 ΔPOZ). Then, the effects of two truncated mutants on AR transcriptional activity were detected. Consistent with the result described above, the full-length FBI-1 inhibited the AR transcriptional activity in the presence, but not in the absence, of R1881. Importantly, both POZ domain and FBI-1 C-terminal fragment did not inhibit AR transcriptional activity in the presence or absence of R1881 (Fig. 4c). These results suggest that POZ domain is responsible for the interaction with AR and full-length FBI-1 is required for the repression of the AR transcriptional activity.
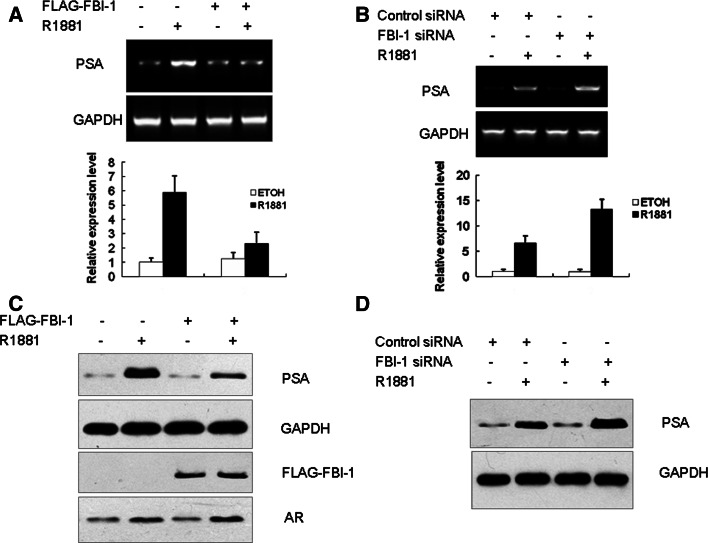
FBI-1 can repress the expression of endogenous PSA
While the above experiments (see Fig. 2b) demonstrated that modulation of endogenous FBI-1 levels in LNCaP cells affects AR activity on a transiently transfected reporter plasmid, they do not monitor changes in endogenous gene expression. To corroborate the results of the luciferase reporter assay, the effect of FBI-1 on the expression of endogenous PSA was examined. LNCaP cells stably expressing either the empty vector or FBI-1 were treated with 1 nM R1881 or ethanol for 24 h. Total RNA was isolated and RT-PCR was performed using PSA specific primers. As expected, an R1881-dependent increase in PSA expression was observed, and FBI-1 repressed the expression of PSA mRNA in the presence of R1881 (Fig. 5a). Subsequently, we determined whether the inhibitory effect on PSA mRNA levels was translated on protein expression. Total protein extract were prepared and western blotting was performed using anti-human PSA antibody. As a result, the repression by FBI-1 on PSA expression in the presence of R1881 was also observed at the protein level (Fig. 5c). Conversely, knockdown of FBI-1 led to an increase of PSA expression in both mRNA and protein level in the presence of R1881 (Fig. 5b, d). These results suggest FBI-1 represses the expression of endogenous PSA in a ligand-dependent manner.
Fig. 5.
FBI-1 can repress the expression of endogenous PSA. LNCaP cells stably transfected with FBI-1 or empty vector (a, c) or FBI-1 siRNA or control siRNA as indicated (b, d) were cultured in phenol red-free RPMI 1640 medium and treated with control (0.1% ethanol) vehicle or 1 nM R1881 for 24 h. a, b Cells were harvested, and total RNA was extracted. Semiquantitative RT-PCR was performed as described under “Materials and methods”. GAPDH served as an internal control. The results from real-time PCR are shown at the bottom. b, d cells were harvested and whole cell lysate was used for western blot analysis of the expression of AR,FBI-1 PSA and GAPDH with various antibodies. GAPDH served as a loading control
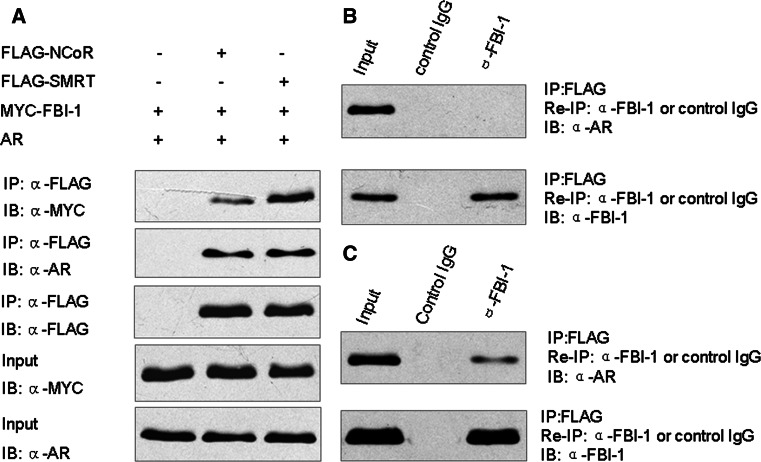
FBI-1, AR and SMRT can form a ternary complex
Previous studies showed that FBI-1 can interact with NCoR and SMRT, whereas NCoR and SMRT are important co-repressors of AR [12, 19]. Thus, we deduced that the observed repression of AR transcriptional activity by FBI-1 was associated with the recruitment of NCoR and SMRT complexes. In order to address the possibility, we examined the interactions between NCoR, SMRT, AR and FBI-1 in a cellular context. N-terminal FLAG-tagged NCoR or SMRT was co-transfected in PC-3 cells together with Myc-tagged FBI-1 and pcDNA3.0-AR. Before harvesting, cells were treated with R1881 for 1 h. FLAG-NCoR and SMRT were immunoprecipitated from cell lysates by anti-FLAG antibody and analyzed for FBI-1 and AR binding by western blotting. As shown in Fig. 6a transient expression of FLAG-tagged NCoR and SMRT, but not control FLAG vector, was accompanied by interactions with FBI-1 and AR. These results suggest that NCoR and SMRT interact with AR and FBI-1 in vivo. Notably, FBI-1 exhibited a much stronger interaction with SMTR compared to that with NCoR. To examine whether FBI-1, AR and NCoR or SMRT formed a ternary complex, the immune complexes precipitated with FLAG antibody were eluted with FLAG peptide and then subjected to a second immunoprecipitation with FBI-1 antibody. The reprecipitates were then subjected to immunoblotting with AR antibody to detect AR protein. The result showed that AR was present after sequential immunoprecipitation when cells were transfected with FLAG-tagged SMRT (Fig. 6c). However, AR was absent after sequential immunoprecipitation when cells were transfected with FLAG-tagged NCoR (Fig. 6b). In contrast, AR was also absent after a second immunoprecipitation with control IgG. These data suggest that FBI-1, AR and SMRT but not NCoR together can form a ternary complex in vivo.
Fig. 6.
SMRT can form a ternary complex with FBI-1 and AR. a NCoR and SMRT can interact with both FBI-1 and AR in vivo. PC-3 cells were co-transfected with AR and treated with 1 nM R1881 for 1 h, Myc-tagged FBI-1, and FLAG-tagged NCoR or SMRT or control vector as indicated. The cell extracts were immunoprecipitated with anti-FLAG monoclonal antibody followed by immunoblotting with the indicated antibodies. b PC-3 cells were transfected with AR, Myc-tagged FBI-1, and FLAG-tagged NCoR or control vector and treated with 1 nM R1881 for 1 h. c PC-3 cells were transfected with AR, Myc-tagged FBI-1, and FLAG-tagged SMRT or control vector and then treated with 1 nM R1881 for 1 h. Immune complexes described in Fig. 6a were eluted with FLAG peptide and re-immunoprecipitated (re-IP) using anti-FBI-1 antibody and normal rabbit serum as a negative control. The resulting precipitates were resolved by SDS-PAGE followed by immunoblotting with the indicated antibodies
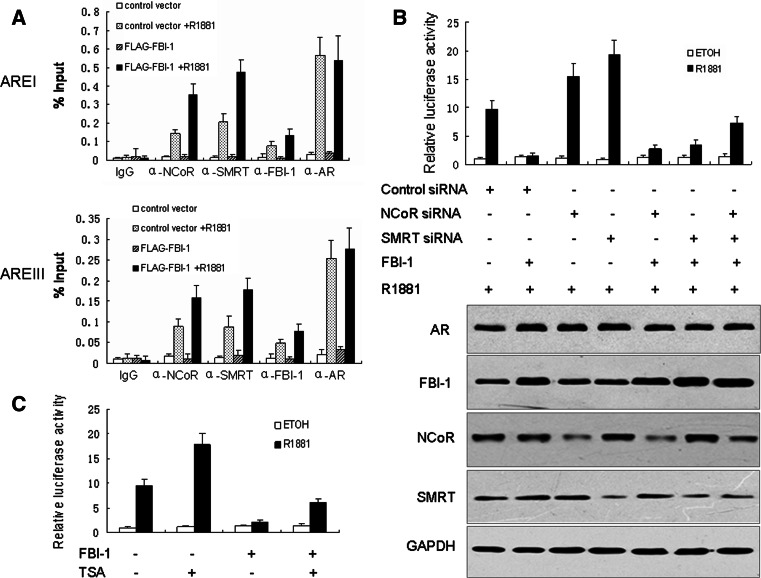
FBI-1 enhances the recruitment of NCoR and SMRT to PSA upstream sequence
Previously studies showed that NCoR and SMRT can be recruited to endogenous AREs in the presence of agonist. In order to examine the possibility that FBI-1 can affect the recruitment of NCoR and SMRT to the PSA promoter and enhancer, DNA occupancy at the ARE element within the endogenous PSA promoter and enhancer by NCoR, SMRT and AR was detected by ChIP assay. Consistent with a previous report, AR, NCoR and SMRT displayed a clear recruitment to the PSA promoter and enhancer in the presence but not the absence of R1881 [20]. Importantly, FBI-1 significantly enhanced the recruitment of NCoR and SMRT to the PSA upstream sequence, whereas FBI-1 showed no effect on the recruitment of AR. The specificity of AR, NCoR and SMRT association within the PSA promoter and enhancer was confirmed by ChIP analysis using control IgG which failed to immunoprecipitate the PSA promoter and enhancer sequences (Fig. 7a). These results suggest that FBI-1 inhibites the AR transcriptional activity by enhancing the recruitment of NCoR and SMRT to endogenous ARE elements.
Fig. 7.
The mechanism of FBI-1 suppression of AR activity. a FBI-1 can enhance the recruitment of NCoR and SMRT to the PSA promoter and enhancer. LNCaP cells stably transfected with FBI-1 or empty vector were cultured in phenol red-free medium and treated with control vehicle or 1 nM R1881 for 1 h and then soluble chromatin was prepared and subjected to immunoprecipitation by using IgG (negative control) or antibodies for AR, NCoR and SMRT. Immunoprecipitated DNA was quantified by real-time PCR. b NCoR and SMRT mediates FBI-1 suppression of R1881 induced AR activity. LNCaP cells were co-transfected with PSA-LUC, FBI-1 expression vector, NCoR siRNA, SMRT siRNA vector or control vector as indicated. Following transfection, cells were stimulated with 1 nM R1881 or 0.1% ethanol (ETOH) for 24 h. Cells were harvested for the luciferase assay. The values are the mean ± SE of three independent experiments performed in triplicate, and are normalized to Renilla luciferase activity. Western blot (bottom) indicates the expression level of proteins. c Treatment of LNCaP cells with the specific HDAC inhibitor TSA causes a drastical relief of FBI-1 mediated repression of AR transcriptional activity. LNCaP cells were co-transfected with PSA-LUC, expression plasmid for FBI-1 or control vector. Cells were then treated with control vehicle (0.1% ethanol), 1 nM R1881 or 50 nM TSA as indicated. The luciferase activity obtained on transfection of PSA-LUC without exogenous FBI-1 in the absence of R1881 and TSA was set as 1. The values are the mean ± SE of three independent experiments performed in triplicate, and are normalized to Renilla luciferase activity
NCoR and SMRT mediates FBI-1 suppression of R1881 induced AR activity
We next tested the involvement of NCoR and SMRT in the suppression of AR activity by FBI-1. LNCaP cells were co-transfected with NCoR or SMRT siRNA together with FBI-1 or control vector. As shown in Fig. 7b, the R1881 induced AR activity was enhanced in LNCaP cells after transfection of NCoR or SMRT siRNA which was effective in knocking down NCoR and SMRT (data not shown). More importantly, knockdown of NCoR or SMRT effectively inhibited the repressive effect of FBI-1 on AR transcriptional activity in the presence of R1881. However, FBI-1 can hardly affect the AR transcriptional activity in the presence of combined siRNAs against both NCoR and SMRT. This result suggests that NCoR and SMRT mediate the FBI-1 suppressive effect on R1881 induced AR activity.
FBI-1 inhibition is partially dependent on HDACs
Previous studies by our group and others showed that FBI-1 can interact with NCoR, SMRT and histone deacetylases (HDACs), which raises the possibility that the repression of AR function by FBI-1 may be HDAC-dependent. To this end, we examined the effect of TSA, a specific inhibitor of HDAC enzyme, on FBI-1-mediated repression of PSA transcription in LNCaP cells. The result showed that treatment with TSA resulted in a significant increase in AR transcriptional activity and partially reversed FBI-1 mediated repression of AR functions in the presence of R1881(Fig. 7c). These data suggest that FBI-1 may recruit a co-repressor complex harboring HDACs to repress AR transcriptional activity.
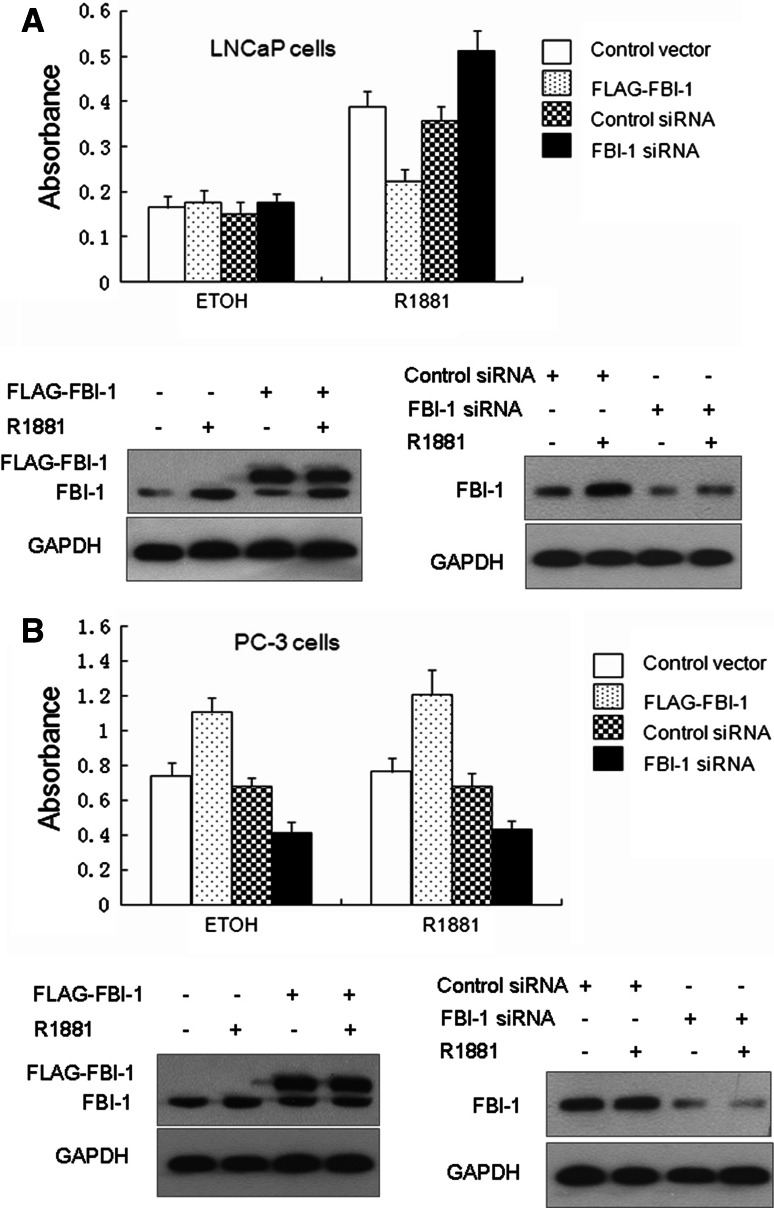
FBI-1 affects R1881 induced LNCaP cell proliferation
The data above strongly suggest that FBI-1 inhibites AR ligand-dependent transcriptional activity which potentially provides a novel approach to regulate the LNCaP cell proliferation by altering the endogenous levels of FBI-1 in the cells. Therefore, we generated stable cell lines expressing FBI-1 or FBI-1 siRNA in LNCaP and PC-3 cells. Through cell growth assay, we found that LNCaP cells overexpressing FBI-1 displayed a reduced rate of proliferation in comparison with that of the control in the presence of R1881. However, the growth of cells expressing FBI-1 siRNA showed a significant advantage. In the absence of R1881, the growth of these cells showed no significant increase (Fig. 8a). Interestingly, either in the presence or absence of R1881, PC-3 cell proliferation was dramatically inhibited in stable cell lines expressing FBI-1 siRNA in comparison with the control siRNA cell lines (Fig. 8b). Conversly, PC-3 cells overexpressing FBI-1 displayed an elevated rate of proliferation. These data indicate that FBI-1 plays distinct roles in LNCaP and PC-3 cell proliferation.
Fig. 8.
FBI-1 can affect LNCaP and PC-3 cell proliferation. Equal numbers of LNCaP cells (a) and PC-3 cells (b) stably transfected with FBI-1 or control vector, FBI-1 siRNA-1 or control siRNA were seeded in complete medium lacking androgen and R1881 (0.1 nM) or ethanol (ETOH) was added. After 5 days, cells were harvested and cell number was determined by MTT assay. Western blotting (bottom) showed the expression level of FBI-1 and FLAG-FBI-1. The addition of a FLAG epitope and other encoding sequences in vector increases the size of the encoded protein by ~2 kDa (19 amino acids)
Discussion
In the present study, we have identified the transcription factor FBI-1 as a new AR-interacting partner. The physical interaction has been validated by a number of assays, including in vitro GST pull-down and in vivo co-immunoprecipitation. Importantly, the direct interaction of FBI-1 and AR was also confirmed. Overexpression of FBI-1 repressed the transcriptional activity of AR in a ligand-dependent manner. In sharp contrast, knockdown of endogenous FBI-1 enhances the transcriptional activity of AR, suggesting that FBI-1 plays an important role in AR signaling. Furthermore, we have shown that FBI-1 can enhance the recruitment of NCoR and SMRT to endogenous PSA promoter and enhancer. Knockdown of endogenous NCoR and SMRT can dramatically reverse the inhibitory effect of FBI-1 on AR activity. These data suggest that FBI-1 functions as a co-repressor of AR via interactions with NCoR and SMRT. Moreover, overexpression of FBI-1 can repress the proliferation of LNCaP cells in the presence of R1881, which indicates that FBI-1 plays an important role in the regulation of LNCaP cell proliferation.
The pro-oncogene FBI-1, encoded by Zbtb7a, is a transcriptional repressor that belongs to the POK protein family. The N-terminal POZ/BTB domain is known to mediate homodimerization and heterodimerization plus recruitment of co-repressors/HDAC complex to the protein [9, 19]. Thus, FBI-1 fulfills its role in repressing transcription via its POZ domain. Increasing evidence suggests that FBI-1 represses the transcription of several genes such as P21, ARF, RB and ADH5/FDH. However, whether FBI-1 could regulate the signaling of nuclear receptors such as AR and ER was still unclear [9, 11, 12, 21]. Recently, zinc finger protein 131 (ZN131), a typical member of the BTB/POZ family, was found to function as a repressor of estrogen receptor alpha (ERα) and repress ligand-dependent ERα transactivitation and the expression of pS2, an ERα target gene [22]. PLZF, the homolog of FBI-1, is an androgen-responsive gene and can inhibit LNCaP proliferation. A fusion protein between PLZF and AR can repress the AR transcriptional activity and the androgen-regulated growth of LNCaP prostate cancer cells [23, 24]. These reports suggest the POZ domain may be involved in the regulation of AR and ER signaling. In the presence of androgen, the expression of FBI-1 significantly increased both at the mRNA and protein level which led us to speculate that FBI-1 might affect the AR transcriptional activity [13]. Indeed, our experimental results suggest that FBI-1 can significantly inhibit the AR transcriptional activity in the presence of R1881.
To date, many co-regulators of AR have been identified and characterized. Compared with the co-activators, the identified AR co-repressors are relatively fewer in number and less well-characterized. Co-repressors that trigger chromatin condensation and/or chromatin modifications typically recruit HDACs to the AR complex [25, 26]. The well-characterized co-repressors NCoR and SMRT can interact directly with multiple HDACs and recruit them to AR [27]. Usually, POZ zinc finger transcription factors repress promoter activity by binding to a regulatory element and subsequently recruiting a co-repressor complex with HDAC activity [19]. Recently, it was reported that FBI-1 can interact with NCoR and SMRT and involves a co-repressor-HDAC complex to fulfill its ability for transcriptional repression [12, 21]. In this study, our results confirmed the interaction of FBI-1 with NCoR and SMRT in vivo. More importantly, SMRT but not NCoR can form a ternary complex with AR and FBI-1. We deduced that the interaction of NCoR with FBI-1 was much weaker than that of SMRT, and for this reason we could not detect the existence of the ternary complex containing NCoR.
NCoR and SMRT have been shown to inhibit AR transcriptional activity and to be recruited to the PSA promoter in the presence of AR antagonist but not agonist. Conversely, several studies implicated that in the presence of agonist, NCoR and SMRT can inhibit the AR transcriptional activity and be recruited to the PSA promoter and enhancer [16, 20, 28]. In fact, our data showed that knockdown of NCoR and SMRT increased R1881 stimulated transcriptional activity of AR but did not affect the basal transcriptional activity. More importantly, knockdown of NCoR or SMRT effectively inhibited the repression effect of FBI-1 on the AR transcriptional activity. However, FBI-1 has little effect on AR transcriptional activity in the presence of combined siRNA against both NCoR and SMRT. In order to further identify the association of the repression effect of FBI-1 with NCoR and SMRT, ChIP assays were performed. Our ChIP data showed that NCoR and SMRT can be recruited to the PSA promoter and enhancer in the presence of R1881. Interestingly, FBI-1 significantly enhanced the recruitment of NCoR and SMRT to the PSA promoter and enhancer. It is currently held that NCoR and SMRT are class I HDAC-containing co-repressor complexes and the HDAC inhibitor TSA can reverse their transcriptional repression [27]. Several recent studies suggest that FBI-1 performs its transcriptional repression via recruiting HDACs [21, 29]. In this study, treatment of LNCaP cells with TSA significantly affected transcriptional repression on AR by FBI-1, which implicated the involvement of HDACs in transcriptional repression by FBI-1. It was notable that TSA could not completely counteract the transcriptional repression of FBI-1 on AR, which indicated that alternative mechanisms may be involved. These multiple lines of evidence support that FBI-1 acts through NCoR and SMRT to inhibit the AR transcriptional activity.
It is well understood that oncogenes affecting AR signaling often function as co-activators of AR and promote the progression of prostate cancer cells such as insulin-like growth factor 1 (IGF1), CDK6, HBx and cyclin E. However, studies have suggested that several oncogenes may function as co-repressors of AR [30–33]. Cyclin D1, which is a prerequisite for cellular proliferation, was shown to function as a co-repressor to inhibit ligand-dependent AR transcriptional activity and repress cell cycle progression in androgen-dependent LNCaP cells [34, 35]. Another proto-oncogene, c-Jun, was also reported to be able to repress androgen-dependent LNCaP cell proliferation [36]. Recently, it was reported that CDC25A is upregulated in prostate cancer and its expression is also correlated with the tumor stage and metastasis, and that CDC25A can function as a co-repressor of AR in prostate cancer cells [37]. PLZF, the closet homolog of FBI-1, is also an androgen-responsive gene and can repress LNCaP cell proliferation [24, 38]. A recent study suggested that FBI-1 can repress cell proliferation via inhibiting the expression of CyclinA and FBI-1 acts as a dual regulator in adipogenesis [29]. In this study, we found that proto-oncogene FBI-1 functions as a co-repressor of AR and inhibits LNCaP cell proliferation in the presence of R1881. Interestingly, FBI-1 can enhance the proliferation of PC-3 cell, an androgen-independent cancer cell line. These data indicate that FBI-1 plays distinct roles in regulating androgen-dependent and -independent prostate cancer cells via different mechanisms. Since prostate cancer cells depend on the AR as the primary mediator of growth and survival during androgen-dependent progression, the inhibition of AR activity by FBI-1 may lead to a reduced rate of cell proliferation. During androgen-independent progression, prostate cancer cells develop a variety of cellular pathways to survive and flourish in an androgen-depleted environment. Inactivation of the tumor suppressor gene phosphatase and tensin (PTEN) is one way in which androgen-independent prostate cancer cells escape apoptosis [39, 40]. Thus, we deduced that, in AR-independent prostate cancer cells, FBI-1 may act as a positive cell proliferation regulator via inhibiting tumor suppressor genes expression such as ARF. While FBI-1 is constitutively up-regulated by androgen in LNCaP cells, overexpression of FBI-1 showed a reduced rate of proliferation in the presence of androgen. Being similar to PLZF, FBI-1 induction may contribute to the growth inhibition of LNCaP cells by high doses of androgens [38].
In summary, we demonstrate that FBI-1 functions as a novel co-repressor of AR. As the expression of this transcriptional repressor plays distinct roles in regulating androgen-dependent and -independent prostate cancer cells, FBI-1 might be an interesting target in the future for the treatment of prostate cancer.
Acknowledgments
We thank Dr. Glass for kindly providing FLAG-NCoR and FLAG-SMRT. This work was supported by the grant from the National Natural Science Foundation of China (No. 30772001 and No. 30671927) and Beijing Municipal Natural Science Foundation (No. 7102126).
Footnotes
J. Cui, Y. Yang and C. Zhang were contributed equally to this work.
Contributor Information
Chuanfu Zhang, Phone: +86-10-66948475, FAX: +86-10-66948475, Email: zhangcfu@gmail.com.
Hongbin Song, Phone: +86-10-66948475, FAX: +86-10-66948475, Email: hongbsong@yahoo.com.
References
- 1.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 2.White R, Parker MG. Molecular mechanisms of steroid hormone action. Endocr Relat Cancer. 1998;5:1–14. doi: 10.1677/erc.0.0050001. [DOI] [Google Scholar]
- 3.Lavery DN, McEwan IJ. The human androgen receptor AF1 transactivation domain: interactions with transcription factor IIF and molten-globule-like structural characteristics. Biochem Soc Trans. 2006;34:1054–1057. doi: 10.1042/BST0341054. [DOI] [PubMed] [Google Scholar]
- 4.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Nomura M, Morinaga H, Matsubara E, Okabe T, Goto K, Yanase T, Zheng H, Lu J, Nawata H. Modulation of androgen receptor transactivation by FoxH1, a newly identified androgen receptor corepressor. J Biol Chem. 2005;280:36355–36363. doi: 10.1074/jbc.M506147200. [DOI] [PubMed] [Google Scholar]
- 6.Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/er.23.2.175. [DOI] [PubMed] [Google Scholar]
- 7.Maeda T, Hobbs RM, Pandolfi PP. The transcription factor Pokemon: a new key player in caner pathogenesis. Cancer Res. 2005;65:8575–8578. doi: 10.1158/0008-5472.CAN-05-1055. [DOI] [PubMed] [Google Scholar]
- 8.Koh DI, Choi WI, Jeon BN, Lee CE, Yun CE, Hur MW. A novel POK family transcription factor, ZBTB5, represses transcription of p21CIP1 gene. J Biol Chem. 2009;284:19856–19866. doi: 10.1074/jbc.M109.025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DK, Suh D, Edenberg HJ, Hur MW. POZ domain transcription factor, FBI-1, repress transcription of ADH5/FDH by interacting with the zinc finger and interfering with DNA binding activity of SP1. J Biol Chem. 2002;277:26761–26768. doi: 10.1074/jbc.M202078200. [DOI] [PubMed] [Google Scholar]
- 10.Maeda T, Merghoub T, Hobbs RM, Dong L, Maeda M, Zakrzewski J, van den Brink MR, Zelent A, Shigematsu H, Akashi K. Regulation of B versus T lymphoid lineage fate decision by the proto-oncogene LRF. Science. 2007;316:860–866. doi: 10.1126/science.1140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda T, Hobbs RM, Merghoub T, Guernah I, Zelent A, Cardon-Cardo C, Teruya-Feldstein J, Pandolfi PP. Role of the proto-oncogene Pokemon in cellular transformation and ARF repression. Nature. 2005;433:278–285. doi: 10.1038/nature03203. [DOI] [PubMed] [Google Scholar]
- 12.Jeon BN, Yoo JY, Choi WI, Lee CE, Yoon HG, Hur MW. Proto-oncogene FBI-1 (Pokemon/ZBTB7A) represses transcription of the tumor suppressor Rb gene via binding competition with Sp1 and recruitment of co-repressors. J Biol Chem. 2008;283:33199–33210. doi: 10.1074/jbc.M802935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi WI, Jeon BN, Park H, Yoo JY, Kim YS, Koh DI, Kim MH, Kim YR, Lee CE, Kim KS. Proto-oncogene FBI-1 (Pokemon) and SREBP-1 synergistically activate transcription of fatty-acid synthase gene (FASN) J Biol Chem. 2008;283:29341–29354. doi: 10.1074/jbc.M802477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleutjens KB, van der Korput HA, van Eekelen CC, van Rooij HC, Faber PW, Trapman J. An androgen response element in a far upstream enhancer region is essential for high androgen regulated activity of the prostate specific antigen promoter. Mol Endocrinol. 1997;11:148–161. doi: 10.1210/me.11.2.148. [DOI] [PubMed] [Google Scholar]
- 15.Yu C, Markan K, Temple KA, Deplewski D, Brady MJ, Cohen RN. The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor γ transcriptional activity and repress 3T3-L1 adipogenesis. J Biol Chem. 2005;280:13600–13605. doi: 10.1074/jbc.M409468200. [DOI] [PubMed] [Google Scholar]
- 16.Yoon HG, Wong J. The corepressors silencing mediator of retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist and antagonist regulated transcription by androgen receptor. Mol Endocrinol. 2006;20:1048–1060. doi: 10.1210/me.2005-0324. [DOI] [PubMed] [Google Scholar]
- 17.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/S1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 18.Louie MC, Yang HQ, Ma AH, Xu W, Zou JX, Kung HJ, Chen HW. Androgen-induced recruitment of RNA polymerase II to a nuclear receptor p160 coactivator complex. Proc Natl Acad Sci USA. 2003;100:2226–2230. doi: 10.1073/pnas.0437824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh KD, Bardwell VJ. The BCL-6 POZ domain and other POZ domains interact with the co-repressors NCoR and SMRT. Oncogene. 1998;17:2473–2484. doi: 10.1038/sj.onc.1202197. [DOI] [PubMed] [Google Scholar]
- 20.Ma QP, Fu W, Li PF, Nicosia SV, Jenster G, Zhang XH, Bai WL. FoxO1 Mediates PTEN suppression of androgen receptor N- and C-terminal interactions and coactivator recruitment. Mol Endocrinol. 2009;23:213–215. doi: 10.1210/me.2008-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi WI, Jeon BN, Kim PH, Kim SE, Choi KY, Kim SH, Hur MW. Proto-oncogene FBI-1 represses transcription of p21CIP1 by inhibition of transcription activation by p53 and Sp1. J Biol Chem. 2009;284:12633–12644. doi: 10.1074/jbc.M809794200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han X, JH GUO, Deng WW, Zhang CY, Du P, Shi TP, Ma DL. High-throughput cell based screening reveals a role for ZNF131 as a repressor of ER alpha signaling. BMC Genomics. 2008;9:476–485. doi: 10.1186/1471-2164-9-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang F, Wang Z. Identification and characterization of PLZF as a prostatic androgen responsive gene. Prostate. 2004;59:426–435. doi: 10.1002/pros.20000. [DOI] [PubMed] [Google Scholar]
- 24.Pike J, Holmes D, Kamalati T, Davies D, Tolhurst R, Mazhar D, Fishpool S, Jehani R, Waxman J, Zelent A, Lemoine NR, Ali S, Buluwela L. Silencing of androgen regulated genes using a fusion of AR with the PLZF transcriptional repressor. Oncogene. 2004;23:7561–7570. doi: 10.1038/sj.onc.1208030. [DOI] [PubMed] [Google Scholar]
- 25.Burd CJ, Morey LM, Knudsen KE. Androgen receptor corepressors and prostate cancer. Endocr Relat Cancer. 2006;13:979–994. doi: 10.1677/erc.1.01115. [DOI] [PubMed] [Google Scholar]
- 26.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 27.Guenther MG, Barak O, Lazar MA. The SMRT and NCoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol. 2001;21:6091–6101. doi: 10.1128/MCB.21.18.6091-6101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodgson MC, Astapova I, Cheng S, Lee LJ, Verhoeven MC, Choi E, Balk SP, Hollenberg AN. The androgen receptor recruits nuclear receptor corepressor (NCoR) in the presence of mifepristone via its N and C termini revealing a novel molecular mechanism for androgen receptor antagonists. J Biol Chem. 2005;280:6511–6519. doi: 10.1074/jbc.M408972200. [DOI] [PubMed] [Google Scholar]
- 29.Laudes M, Bilkovski R, Oberhauser F, Droste A, Gomolka M, Lesser U, Udelhoven M, Krone W. Transcription factor FBI-1 acts as a dual regulator in adipogenesis by coordinated regulation of cyclin-A and E2F-4. J Mol Med. 2008;86:597–608. doi: 10.1007/s00109-008-0326-2. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto A, Hashimoto Y, Kohri K, Ogata E, Kato S, Ikeda K, Nakanishi M. Cyclin E as a coactivator of the androgen receptor. J Cell Biol. 2000;150:873–880. doi: 10.1083/jcb.150.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim JT, Mansukhani M, Weinstein IB. Cyclin-dependent kinase 6 associates with the androgen receptor and enhances its transcriptional activity in prostate cancer cells. Proc Natl Acad Sci USA. 2005;102:5156–51561. doi: 10.1073/pnas.0501203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan WQ, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H, Fukamizu A, Kato S, Takayanagi R, Nawata H. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329–7338. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y, Chen WL, Ma WL, Chang C, Ou JH. Enhancement of gene transactivation activity of androgen receptor by hepatitis B virus X protein. Virology. 2007;363:454–461. doi: 10.1016/j.virol.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petre CE, Wetherill YB, Danielsen M, Knudsen KE. Cyclin D1: mechanism and consequence of androgen receptor co-repressor activity. J Biol Chem. 2002;277:2207–2215. doi: 10.1074/jbc.M106399200. [DOI] [PubMed] [Google Scholar]
- 35.Petre CE, Williams EB, Burd CJ, Gladden A, Moghadam H, Meller J, Diehl JA, Knudsen KE. A central domain of cyclin D1 mediates nuclear receptor corepressor activity. Oncogene. 2005;24:431–444. doi: 10.1038/sj.onc.1208200. [DOI] [PubMed] [Google Scholar]
- 36.Chen SY, Cai C, Fisher CJ, Zheng Z, Omwancha J, Hsieh CL, Shemshedini L. c-Jun enhancement of androgen receptor transactivation is associated with prostate cancer cell proliferation. Oncogene. 2006;25:7212–7223. doi: 10.1038/sj.onc.1209705. [DOI] [PubMed] [Google Scholar]
- 37.Chiu YT, Han HY, Leung SC, Yuen HF, Chau CW, Guo ZY, Qiu Y, Chan KW, Wang XH, Wong YC. CDC25A functions as a novel AR corepressor in prostate cancer cells. J Mol Biol. 2009;385:446–456. doi: 10.1016/j.jmb.2008.10.070. [DOI] [PubMed] [Google Scholar]
- 38.Jiang F, Wang Z. Identification and characterization of PLZF as a prostatic androgen-responsive gene. Prostate. 2004;59:426–435. doi: 10.1002/pros.20000. [DOI] [PubMed] [Google Scholar]
- 39.Pienta CJ, Bradley D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 40.Pienta CJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1665–1671. doi: 10.1158/1078-0432.CCR-06-0067. [DOI] [PubMed] [Google Scholar]