Abstract
During animal development, tissues and organs are partitioned into compartments that do not intermix. This organizing principle is essential for correct tissue morphogenesis. Given that cell sorting defects during compartmentalization in humans are thought to cause malignant invasion and congenital defects such as cranio-fronto-nasal syndrome, identifying the molecular and cellular mechanisms that keep cells apart at boundaries between compartments is important. In both vertebrates and invertebrates, transcription factors and short-range signalling pathways, such as EPH/Ephrin, Hedgehog, or Notch signalling, govern compartmental cell sorting. However, the mechanisms that mediate cell sorting downstream of these factors have remained elusive for decades. Here, we review recent data gathered in Drosophila that suggest that the generation of cortical tensile forces at compartmental boundaries by the actomyosin cytoskeleton could be a general mechanism that inhibits cell mixing between compartments.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-011-0668-8) contains supplementary material, which is available to authorized users.
Keywords: Compartment, Boundary, Lineage restriction, Cell sorting, Cell mixing, Actin cytoskeleton, Nonmuscle Myosin II, Drosophila
Introduction
During development, tissues or organs are divided into compartments, in other words into groups of cells of distinct identity, that do not intermix. When the state of expression of selector genes within one compartment is clonally inherited, active mechanisms known as “lineage restriction” physically sort cells and keep them separated at compartmental boundaries ([1, 2], see [3–5] for reviews). Compartments and lineage restriction boundaries are conserved features during evolution. They were initially discovered in the imaginal wing disc of the fruit fly Drosophila melanogaster: this epithelial tissue is divided into anterior and posterior (A/P) as well as dorsal and ventral (D/V) compartments (Fig. 1a) [2, 6, 7]. Since then, they have been found in various tissues of numerous species. In arthropods, lineage restriction is at work in epithelia such as the abdomen of Oncopeltus [8], the imaginal genital disc [9], or the embryonic trunk in Drosophila (Fig. 1b) [10]. In vertebrates, lineage restriction boundaries are found in the ectoderm and mesenchyme of the limbs [11, 12], between the notochord and somites (a fissure eventually forms at the position corresponding to this initial boundary) [13], and in the fore-, mid-, and hindbrain [14–19].
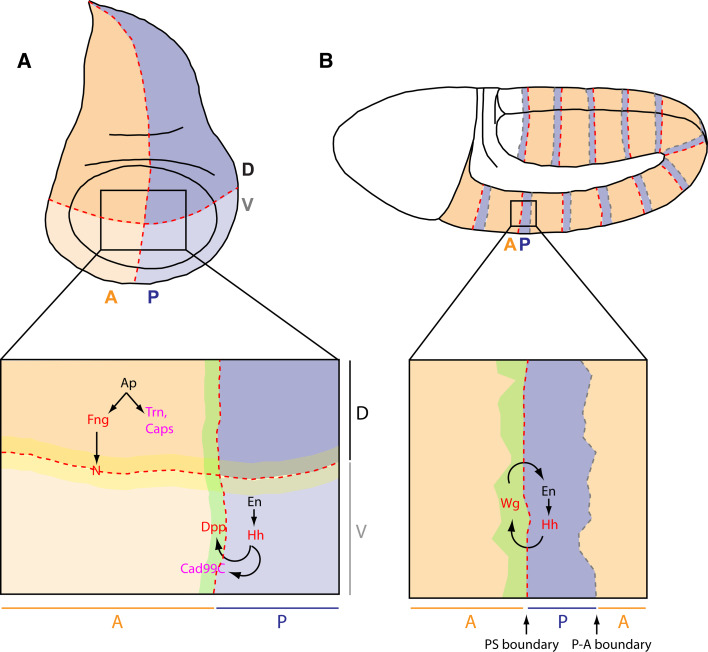
Fig. 1a, b.
Compartmental boundaries in Drosophila. a In the Drosophila imaginal wing disc, lineage restriction boundaries form between anterior (A) and posterior (P) compartments (orange and blue, respectively), as well as between dorsal (D) and ventral (V) compartments (dark and light shades, respectively). The Notch receptor is activated in the cells immediately ventral and dorsal to the D/V boundary (yellow stripe). b In Drosophila stage 8–11 embryos, anterior and posterior compartments meet at two interfaces in each metameric unit. The anterior-posterior interface is the parasegmental (PS) boundary, while the posterior-anterior interface (P-A boundary) corresponds to where segmental folds will form later in embryogenesis. Only parasegmental boundaries are lineage restriction boundaries. a, b Red and gray dashed lines represent lineage restriction boundaries and morphogen-dependent boundaries, respectively. Transcription factors are shown in black, components of cell-cell signalling pathways in red and adhesion molecules in pink. Arrows indicate the relationship between the genes involved in compartmentalization. N Notch, Fng Fringe, Ap Apterous, Trn Tartan, Caps Capricious, En Engrailed, Hh Hedgehog, Dpp Decapentaplegic, Cad99C Cadherin 99C, Wg Wingless
Compartmental boundaries are particularly important to localize signalling centers that pattern and trigger the differentiation of surrounding tissues during development [20]. For example, expression of the long-range morphogens Wingless/Wnt (Wg) and Decapentaplegic/TGFβ (Dpp) are activated in boundary cells between dorsal/ventral and anterior/posterior compartments, respectively, in the Drosophila wing disc [20], while numerous organizers colocalize with lineage restriction boundaries in the vertebrate brain [17]. Another property of lineage restriction boundaries is that they produce straight, sharp interfaces between compartments [3–5]. Because they often localize diffusible signalling molecules, the regularity of boundaries might be important for the stereotypical differentiation of surrounding tissues [3]. In humans, the development of cranio-fronto-nasal syndrome or of metastatic progression in organs such as the prostate has been attributed to defective cell sorting, highlighting the importance of functional compartmental boundaries [21–23].
Researchers in the last 10 years have elucidated signalling mechanisms required for the formation of lineage restriction boundaries in several tissues. In the imaginal wing discs of Drosophila, selector genes have been shown to cooperate with short-range signalling pathways to position the boundaries of lineage restriction. The Engrailed transcription factor, which confers posterior identity, positions the A/P boundary in cooperation with Hedgehog signalling (Fig. 1a) [24–27]. Note that Dpp/TGFβ, a long-range signal, is also required to maintain this boundary [28]. For D/V compartmentalization, the transcription factor Apterous—the determinant of dorsal identity—acts in conjunction with Notch signalling to position the boundary (Fig. 1a) [29–33]. In the vertebrate hindbrain, Notch and Eph-Ephrin bidirectional signalling are necessary for correct cell sorting at rhombomere boundaries [34–37]. Surprisingly, the downstream mechanisms by which these signalling pathways and selector genes implement cell sorting have remained rather elusive. Several hypotheses have been proposed to explain the basis of cell sorting in vivo. The main one, derived from the differential adhesion hypothesis (DAH) formulated by Steinberg [38, 39], stipulates that differential expression of adhesion molecules between two compartments, or expression of the same adhesion molecule at different levels, could sort cells at compartmental boundaries (Supplementary Fig. 1) [1, 3–5, 40]. In support of this hypothesis, several adhesion molecules have been found to be expressed in only one of two adjacent compartments. In the Drosophila wing disc, the LRR transmembrane molecules Tartan and Capricious are expressed in the dorsal compartment [41], and Hedgehog triggers Cadherin 99C (Cad99C) expression in one row of cells along the A/P boundary (Fig. 1a) [42]. In the mouse telencephalon, R-cadherin and cadherin-6 are complementarily expressed in two neighboring compartments [15]. Compartmentalization is disrupted by overexpression of any of these adhesion molecules [15, 41, 42] as well as by overexpression of the ubiquitous adhesion molecules E-Cadherin or Echinoid in the Drosophila wing disc [24, 43]. Surprisingly however, their loss of function does not alter compartmental cell sorting [15, 24, 41–43]. Moreover, two independent genetic screens failed to identify any adhesion molecules required for cell sorting at A/P and D/V boundaries in the Drosophila wing disc [44, 45]. To explain these negative results, it has been suggested that several adhesion molecules act redundantly at compartmental boundaries [45]. Such molecules remain to be identified. Alternatively, nonadhesion-based mechanisms could act redundantly with differential adhesion, or differential adhesion may not be the primary mechanism driving compartmental cell sorting.
Other hypotheses than the DAH have been proposed to explain compartmental cell sorting. In a growing tissue, the main cause of cell mixing at compartment boundaries is likely to be cell proliferation. A potential mechanism to stop proliferation from disrupting boundaries is if the axis of cell division is oriented so that the boundary cells systematically divide parallel to the boundary (Supplementary Fig. 1) [46]. Alternatively, cell division could be inhibited in a region straddling the boundary (Supplementary Fig. 1) [47]. Folds or grooves could also form between compartments and be filled by extracellular matrix to act as a physical fence (Supplementary Fig. 1) [5]. More recently, the actomyosin cytoskeleton was proposed to form a barrier to cell mixing at the Drosophila wing disc D/V boundary [46, 48]. Below, we review recent evidence showing that the actomyosin cytoskeleton is enriched at cell-cell contacts not only at the D/V boundary but also at embryonic and wing disc A/P boundaries in Drosophila [49, 50]. These studies suggest a general strategy to sort cells at compartmental boundaries: actomyosin cables could act as local barriers of increased cortical tension to correct cell mixing caused by division or intercalation of cells close to the boundary. We also discuss the mechanisms that could concentrate actomyosin at compartmental boundaries and propose a model for compartmental cell sorting based on these recent findings.
Downstream mechanisms of cell sorting at compartmental boundaries: a role for actomyosin barriers
Actomyosin cables at compartmental boundaries
In the past 5 years, investigation of cellular morphology and of the localization of cytoskeletal components at compartmental boundaries in Drosophila has suggested a role for the actomyosin cytoskeleton. In the imaginal wing disc, enrichments of filamentous actin (F-actin) and nonmuscle Myosin II (hereafter referred to as Myosin II) have been described at the interface between dorsal-ventral and anterior-posterior compartments [46, 48, 49]. The actin regulator Enabled, a member of the Ena/VASP family, is also upregulated at the D/V boundary interface [46]. As these enrichments are found at the cortex of each cell-cell contact at the boundary, they form cable-like structures that partition the tissue.
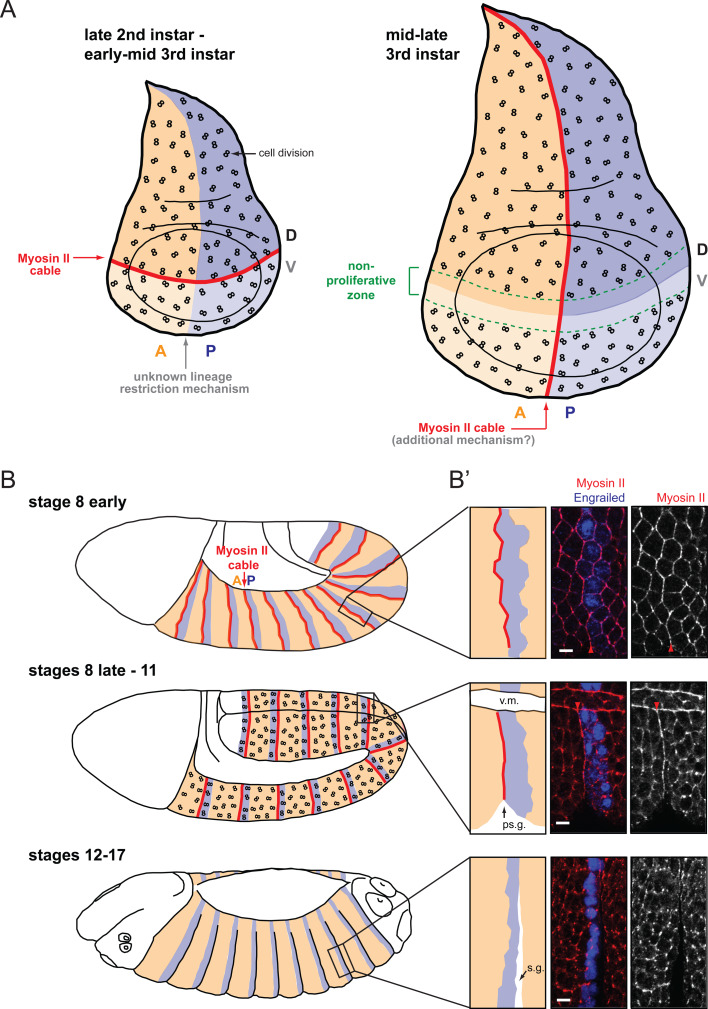
At the D/V boundary, actomyosin cables have been observed from early to mid third instar larvae (L3) [72–96 h after egg laying (AEL)] (Fig. 2a) [46]. At this boundary, evidence for lineage restriction was found from late second instar to late third instar stages [2, 6, 7]. Therefore, actomyosin cables could be responsible for sorting cells at this boundary in late second/early third instar stages, but a second independent mechanism has to take over in late third instar larvae (96–120 h AEL). At the wing disc A/P boundary, the presence of cables was reported at late third instar stages (96–120 h AEL) [46, 49], but not earlier (Fig. 2a) [46]. This temporal pattern indicates that an additional mechanism might sort cells early at the wing A/P boundary. Note that whereas the anterior and posterior compartments are established at embryonic stages, dorsoventral compartmentalization occurs during wing disc formation [2, 7, 51].
Fig. 2a–b′.
Actomyosin cables at compartmental boundaries. a In the Drosophila wing disc, an actomyosin cable is present at the D/V boundary from late second instar to mid third instar (red line). At that time, the boundary is challenged by cell division (represented by “8” symbols). The D/V actomyosin cable is dismantled in mid third instar larvae (around 96 h AEL) when a nonproliferating zone forms that straddles the boundary (green dashed lines). At the same time, another actomyosin cable forms at the A/P boundary. The mechanism that implements cell sorting at the A/P boundary at earlier stages is currently unknown. b, b′ In the embryo, parasegmental actomyosin cables are detectable by early stage 8, before cell division resumes in the epidermis at stage 8. They are dismantled by stage 12, once cell division ceases in the epidermis. When parasegmental actomyosin cables form at early stage 8, the boundary is not straight yet, but it straightens by stage 9 (top and middle panels in b′). This is consistent with the in silico modelling of local increase in tension between two cell populations performed by Landsberg and coworkers [49]. Also, consistent with increased tension at the parasegmental boundary, a “parasegmental” groove (ps.g.) forms at stage 10 that correlates spatially and temporally with parasegmental actomyosin cables (middle panel in b′). Scale bar in b′: 5 μm. v.m. Ventral midline, s.g. segmental groove. b′ is adapted by permission from Macmillan Publishers Ltd: Nature Cell Biology [50], copyright 2010
In contrast to the wing disc, each embryonic segment harbors not one but two boundaries between anterior and posterior compartments, at the anterior and posterior interfaces of each stripe of Engrailed-expressing cells (Fig. 1b). The boundaries forming on the anterior side of the Engrailed stripe are the parasegment boundaries [52], which have been demonstrated to stop cells crossing between stages 8 and 11 [10]. By contrast, boundaries on the posterior side of the Engrailed stripe (i.e., where segmental folds will form at stage 12 [53]) do not restrict cell movement at these early stages but rather are morphogen-dependent boundaries (Supplementary Fig. 2) [10]. Actomyosin enrichments are found at the parasegmental boundaries, but not at the more posterior morphogen-dependent boundaries, in stages 8–11 embryos (Fig. 2b, b′) [50]. High-magnification microscopy revealed that both anterior and posterior cells accumulate Myosin II at the parasegmental boundary interface [50]. Targeted expression of Myosin II-GFP in anterior or posterior compartments showed accumulation of the GFP-fusion protein at the parasegmental boundary in either case, confirming that both anterior and posterior cells contribute to the actomyosin cable [50]. So in the embryo, actomyosin accumulates symmetrically at the cortex of boundary cells on either side of the boundary interface.
Co-immunostaining with E-Cadherin demonstrates that, for the three boundaries studied, the boundary-specific enrichment of F-actin and Myosin II occurs at the level of the adherens junctions along the apico-basal axis of the cell [46, 48–50]. Careful analysis has shown that boundary cells minimize contact along this subapical region corresponding to the adherens junctions, both at embryonic and wing disc A/P boundaries [49, 50]. Since minimized cell-cell contact is a manifestation of cell sorting, these observations suggest a correlation between lineage restriction mechanisms at compartmental boundaries and the presence of actomyosin cables.
Cell proliferation: a major challenge to compartmental boundaries
In order to understand the mechanisms that drive compartmental cell sorting, it is important to consider the events that could disrupt compartmental organization. In the Drosophila larval wing disc, cells are highly proliferative, but clonal analysis shows that the progeny of a given cell tend to stay tightly clustered, indicating that there are no long-distance cell movements during disc growth [51]. This suggests that the main challenge to compartmental boundaries must be cell division. Interestingly, at the D/V boundary, the actomyosin cable is dismantled by 96 h AEL (mid-third instar) when a nonproliferative zone forms (Fig. 2a) [46, 47, 54, 55]. This suggests that the role of the D/V actomyosin cable could be to contain dividing cells in their compartment of origin, and that this structure is no longer required once cell division is locally abolished. A link between actomyosin barrier and cell proliferation is less clear for the A/P boundary. O’Brotcha and Bryant reported that a nonproliferative zone did not exist at this boundary [47]. However, good resolution pictures of BrdU incorporation published more recently suggest that a narrow region of nonproliferation might be present after all at the A/P boundary in late wing discs (schematized in Supplementary Fig. 3) [54, 55]. More detailed analysis is necessary to better understand the relationship between cell sorting, cell division, and actomyosin barriers at these boundaries.
In the embryo, epidermal cells undergo on average three rounds of cell division, and these occur between stages 8 and 11 [56], which correspond to the developmental period when lineage restriction is active at parasegmental boundaries [10, 57]. Interestingly, actomyosin cables are detectable at parasegmental boundaries at stage 8 at the time of the first epidermal divisions, and they are dismantled by stage 12 once cell divisions have stopped in the embryonic epidermis (Fig. 2b, b′) [50]. Remarkably, actomyosin cables are not dismantled in dividing boundary cells. The cables follow the dividing cell’s motion, moving slightly more basally within the epithelium [50]. Actomyosin cables are also not dismantled in the adjoining nondividing cells on the other side of the boundary. Time-lapse imaging in living embryos showed that dividing boundary cells can transiently deform their portion of actomyosin cable and invade the adjacent compartment [50]. However, approximately when cytokinesis starts, the cells return to their compartment of origin, as if pushed back by the contracting actomyosin cable.
Other cell behaviors, especially cell intercalation, may also challenge parasegmental boundaries. Analysis of fixed samples suggests that intercalation of boundary cells can lead to compartmental invasion, but that invasion could be corrected in a similar way as for cell division [50]. As in cell division, actomyosin cables are not dismantled when cells intercalate at parasegmental boundaries [50]. However, while cell intercalation is very active earlier during axis extension (stages 6–7), it is much rarer after stage 8, suggesting that cell intercalation is a lesser challenge to embryonic boundaries than cell division [58–61]. Together, these observations suggest that, both in Drosophila wing disc and embryo, cell division is the main challenge to compartmental boundaries and that actomyosin cables might correct, rather than prevent, invasion of a neighboring compartment.
Actomyosin cables sort cells at compartmental boundaries
The observations reported above suggest that the actomyosin cytoskeleton could provide a fence at compartmental boundaries. Experiments have therefore been designed to determine whether the actomyosin cytoskeleton is necessary for compartmental cell sorting. Myosin II is a multiprotein complex composed of two heavy chains (MHC), two essential light chains, and two regulatory light chains (MRLC) encoded, respectively, by zipper (zip), mlc-c, and spaghetti-squash (sqh) in Drosophila [62–64]. In wing discs mutant for zipper, the straightness of the A/P and D/V boundaries is lost [48, 49]. Major and Irvine [46] also reported irregular D/V boundaries following loss of function of capulet, a regulator of the actin cytoskeleton. In the embryo, inhibition of Myosin II activity by expression of dominant-negative forms of MHC or injection of drugs inhibiting Rho kinase (Drok) leads to irregular A/P boundaries and cell mixing [50]. Moreover, compartmentalization defects induced by Drok inhibition were rescued by expression of a constitutively activated form of MRLC. Together, these experiments in wing discs and embryos identify Myosin II as a critical regulator of cell sorting in Drosophila. In the embryo, compartmentalization defects following Myosin II inactivation were similar to a complete loss of compartmentalization during embryogenesis, suggesting that at least in this tissue, compartmental cell sorting might rely solely on Myosin II activity [50]. This is the first time that loss of function of an effector molecule has revealed a requirement for compartmental cell sorting.
Myosin II is ubiquitous and can be engaged in diverse subcellular pools in a given cell. Therefore, it is questionable whether the whole-tissue Myosin II loss-of-function experiments reported above reveal a direct requirement for actomyosin cables in sorting cells at compartmental boundaries. To test whether actomyosin cables are the structures that sort cells at boundaries, a laser-based method known as chromophore-assisted laser inactivation (CALI) [65, 66] has been used to directly inactivate the pool of Myosin II localized at the cable in Drosophila embryos [50]. In CALI, intense illumination of a chromophore (here GFP) produces deleterious reactive oxygen species with a short half-life. If a protein of interest is sufficiently close to the chromophore, the reactive oxygen species can damage it, through cleavage and/or crosslinking of its peptide backbone [65, 67–69]. In this experiment, a fusion between GFP and MRLC was used to inactivate Myosin II by CALI. In dividing boundary cells, CALI inactivation of Myosin II at the parasegmental cable produces irregular boundaries [50]. Time-lapse analysis showed that daughter cells produced by the division of boundary cells are no longer pushed back in their compartment of origin, therefore causing cell mixing between compartments. In control experiments where GFP is fused to an actin reporter that has no cellular function (MoesinABD-GFP), illumination of the boundary in the presence of challenging divisions does not disrupt cell sorting. This rules out phototoxic effects that the laser might cause or nonspecific damage by the reactive oxygen species. Together, these experiments demonstrate that, in the Drosophila embryo, actomyosin cables are required to inhibit cell mixing at compartmental boundaries [50]. Given the similarities between embryonic and wing disc actomyosin cables, it is likely that the actomyosin cables found at the wing disc boundaries are the structures that sort cells in this tissue as well.
Actomyosin contractility at compartmental boundaries
The best-characterized function of Myosin II consists in generating cortical tension by moving actin filaments in an ATP-dependent fashion [70, 71]. Do compartmental actomyosin cables sort cells by creating a fence of increased cortical tension at the boundary interface? Landsberg and collaborators directly tested this hypothesis by measuring cortical tension at the boundary cell-cell interfaces in the wing disc A/P boundary [49]. Using a UV laser beam, they ablated the cortical actomyosin cytoskeleton between two vertices and measured their displacement immediately after ablation. This approach allowed them to prove that cortical tension is uniform in the wing disc, apart from the A/P boundary interface where tension is increased by a factor of 2.5. Inactivation of Myosin II by inhibition of Drok decreased the velocity of vertex displacement following laser ablation of boundary interfaces, supporting the idea that the increased cortical tension observed at the A/P boundary is generated by the A/P actomyosin cable [49].
To determine whether an increased cortical tension at the boundary between two compartments is sufficient to promote cell sorting, Landsberg and collaborators carried out in silico simulations where they fined-tuned tension at the interface between two groups of proliferative cells [49]. While a tension that is similar at the boundary interface and elsewhere in the field of cells leads to a very irregular boundary, increasing tension at the boundary interface is sufficient to straighten it and hence to promote cell sorting. This simulation is consistent with the dynamic shape of parasegmental boundaries that are irregular at stage 8 when the actomyosin cable forms, but then straighten rapidly to form smooth interfaces by stage 9 (Fig. 2b′) [50].
In these computer simulations, however, while a 4-fold increased tension at the boundary interface segregates the two cell populations and produces a smooth interface, a factor of 2.5 is not sufficient to produce an interface as smooth as the one observed in wing discs in vivo. Possibly, tension measurements following laser ablation may have been underestimated. However, in late third instar larvae wing discs, a second mechanism could act redundantly with the local increase in cortical tension to inhibit cell mixing at the A/P boundary. This mechanism could be the one that promotes cell sorting at this boundary at earlier larval stages. Additional work is therefore required in this system to determine whether a local increase in cortical tension is sufficient to drive cell sorting at late larval stages or if an additional mechanism is involved.
Measures of cortical tension have not been reported for embryonic parasegmental and wing disc D/V boundaries. However, the formation of indentations or folds at the position corresponding to lineage boundaries might be a consequence of increased cortical tension. For example, the formation of the morphogenetic furrow in the Drosophila imaginal eye disc has been linked to the activation of Myosin II at the apical cortex of a row of cells [72]. All the cells in this row reduce their apical surface while shortening their apico-basal axis, producing the typical shape of the morphogenetic eye furrow (Supplementary Fig. 4). Suggesting a similar mechanism, the epidermis forms a fold at the wing disc D/V boundary [73]. Parasegmental boundaries are also associated with small epidermal indentations known as “parasegmental grooves” which are first seen at stage 10, after actomyosin cables are established, and which disappear when actomyosin cables are dismantled by stage 12 (see Fig. 2b′) [74]. Note that, in contrast to the cells of the morphogenetic furrow, only one side of the apical cortex in parasegmental boundary cells upregulates Myosin II and moves basally, producing a more subtle indentation (Supplementary Fig. 4). It is therefore conceivable that folds or grooves at compartmental boundaries are a signature of tension generated by actomyosin upregulation.
However, the wing disc A/P boundary, which is under tension as seen above [49], is not associated with a groove [73]. So, are grooves a relevant readout of actomyosin cable-dependent tension? Interestingly, loss of function of the Tbx transcription factor Optomotor-blind (Omb) leads to an increase in F-actin accumulation and to the formation of a deep groove at the wing disc A/P boundary [75, 76]. The depth of grooves might depend on the level of cortical tension generated by the actomyosin cytoskeleton. The cortical tension generated at the wing disc A/P boundary could be sufficient to drive cell sorting but not to form a groove. Alternatively, an actomyosin cable unable to induce the formation of a groove might not be sufficient per se to drive cell sorting, but it could cooperate with an additional mechanism. As discussed above, this could be the case for the wing disc A/P boundary.
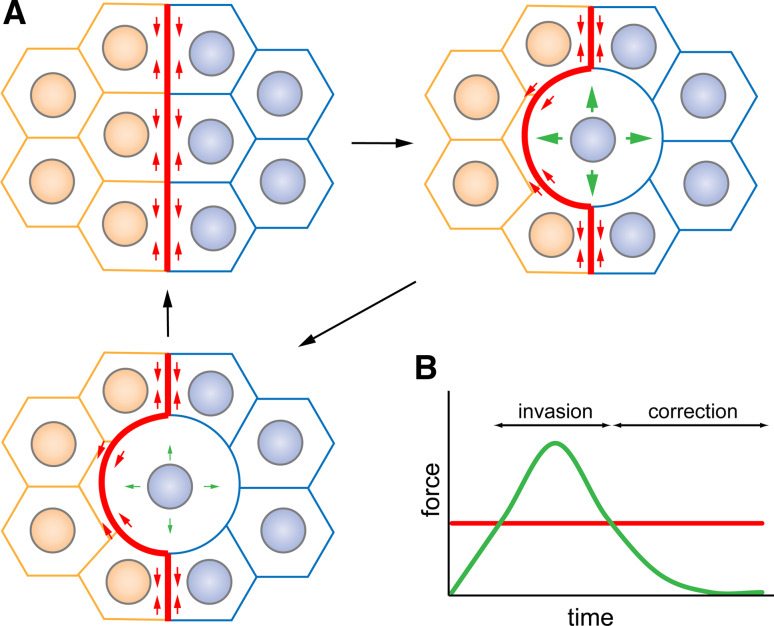
Time-lapse analysis of cell behavior at the vicinity of compartmental boundaries in the embryo has revealed that dividing cells can transiently invade the opposite compartment [50]. How can dividing cells cross the boundary if the actomyosin fence is under tension? Interestingly, prior to cell division, mother cells change their shape in a dramatic fashion: the Moesin-dependent stiffening of the cell’s cortex leads to their rounding up [77]. It is therefore conceivable that the cortical stiffening generated during cell division overcomes cortical tensile forces produced by the actomyosin cable at boundary interfaces, allowing dividing cells to cross the boundary and invade the opposite compartment. When forces generated by cell division decrease while division proceeds, cortical forces produced by the actomyosin cable would override them and push back dividing cells in their compartment of origin, thereby correcting transient cell mixing (Fig. 3).
Fig. 3a, b.
Correction of cell mixing by a fence of increased cortical tension. a The actomyosin cable at the boundary interface could continuously generate cortical tension (small red arrows). These forces could be overridden by forces produced by cortex stiffening during the division of a boundary cell (large green arrows), leading to invasion of the neighboring compartment by the dividing cell. Once forces produced by cell division become weaker (small green arrows) than the forces produced by the actomyosin cable, the actomyosin cable would push back the dividing cell into its compartment of origin. b Schematization of the interplay between forces generated by the actomyosin cable (red) and the forces produced by cortex stiffening in dividing boundary cells (green)
To summarize, in silico modelling suggests that a local increase in cortical tension is sufficient to generate straight boundaries, and consistent with this model, compartmental actomyosin cables do produce a local increase in cortical tension, as demonstrated for the wing disc A/P boundary [49]. It will be informative to measure cortical tension at the embryonic A/P and wing disc D/V boundaries. The increase in cortical tension at the boundary relative to the surrounding tissue might be higher at the parasegmental boundary compared to the AP boundary in the wing disc, since cell sorting at the embryonic boundary might rely only on actomyosin cables [50].
Building up actomyosin cables: regulation of actomyosin dynamics at boundaries
Together, studies from Major and Irvine, Landsberg and coworkers, and Monier and coworkers establish the importance of actomyosin barriers to inhibit cell mixing at compartmental boundaries [46, 48–50]. An open question is what are the links between the signals known to be required for boundary formation [3–5] and actomyosin enrichment at boundary interfaces.
Control of actomyosin cable formation/maintenance by signalling pathways
Cell sorting at the wing disc D/V boundary relies on activation of the Notch receptor in dorsal and ventral boundary cells and on the transcription factor Apterous, which confers dorsal identity to wing disc cells (Fig. 1a) [29–33]. Apterous could promote D/V cell sorting by two mechanisms: directly through targets proposed to be adhesion molecules and indirectly by inducing expression of the Notch regulator fringe in dorsal cells, a prerequisite to activate Notch in boundary cells. Whether actomyosin cabling is compromised in apterous mutant wing discs has not been investigated. However, it has been shown that Notch activity is required in both dorsal and ventral boundary cells to trigger actomyosin accumulation at the interface between dorsal and ventral compartments [46, 48]. Loss of Notch activity achieved using Notch temperature-sensitive mutants or by modulating expression of fringe compromises actomyosin cabling at the D/V boundary. On the contrary, ectopic activation of Notch in clones of cells, especially following ectopic expression of the Notch ligand Delta in dorsal cells, leads to actomyosin cabling at the periphery of the clones. Based on expression of truncated forms of Notch, Major and Irvine proposed that Notch acts through a nontranscriptional pathway to recruit actomyosin at the boundary interface [46]. These experiments prove that Notch signalling is important to trigger actomyosin cabling at the D/V boundary, but the mechanism downstream of Notch remains to be deciphered.
At the wing disc A/P boundary, cell sorting is mainly implemented by cooperation between the transcription factor Engrailed that confers posterior identity and the Hedgehog morphogen secreted by posterior cells (Fig. 1a) [24–28]. Posterior cells are refractory to Hedgehog signalling. Since the Hedgehog pathway acts at short range, only anterior cells close to the boundary receive the signal, turning up Dpp/TGFβ expression in one row of anterior cells (Fig. 1a) [78, 79]. Dpp/TGFβ signalling then cooperates with Engrailed and Hedgehog signalling to maintain the A/P boundary [24, 26–28]. Actomyosin cabling downstream of these factors has not been directly evaluated. However, boundary cells display a specific morphology: their apical area is larger than in other wing disc cells, and the boundary interface is straighter than other columns of interfaces elsewhere in the tissue [49]. Landsberg and co-workers induced clones of cells expressing Hedgehog in the anterior compartment [49]. As these clone cells were also mutant for the Hedgehog receptor smoothened they could not elicit a response to Hedgehog signalling. Therefore, this mimicked Hedgehog asymmetric signalling observed at the A/P boundary. Interestingly, the two cell properties characteristic of A/P boundary cells, large apical area and minimization of cell-cell contacts, are reproduced at the interface of wild-type and Hedgehog-expressing cells. Since apposition of anterior cells and cells expressing Hedgehog is sufficient to confer a boundary-like cell morphology, it is likely that this apposition also induces actomyosin cabling. This possibility needs to be directly tested. Nevertheless, this putative link is consistent with the reports that Hedgehog signalling controls cell shape changes via the regulation of Myosin II activity during morphogenetic furrow formation in the Drosophila eye disc [72, 80]. Also, if Myosin II cabling is induced at the endogenous boundary between anterior and posterior cells, it will be interesting to determine whether cabling is restricted to one cell subpopulation or if, as shown in the embryo [50], both cell types contribute to cabling. If the latter is true, Hedgehog might direct cabling only in anterior cells, due to its known asymmetric activity at the boundary, while another signaling molecule may trigger cabling in posterior cells.
In the Drosophila embryo, we reported recently the requirement of Wingless signalling for cell sorting and actomyosin cabling at parasegmental boundaries [50]. Actomyosin cables form but are not maintained in wingless mutant embryos. Wingless, which is secreted by anterior cells, may be involved only in maintaining cables once they are formed. Alternately, an additional signal could act redundantly with Wingless. Bidirectional signalling is necessary to implement cell sorting at the Drosophila wing disc D/V boundary (Notch/Notch) and A/P boundary (Dpp/Hh), as well as at vertebrate rhombomeres boundaries (Eph/Ephrin) [24, 26–30, 35–37]. Because actomyosin accumulates asymmetrically at the interfaces of parasegmental boundary cells [50], bidirectional signalling might be involved here as well. Given that the segment-polarity gene hedgehog, whose expression is dependent on Wingless signalling, is expressed in posterior compartments and controls cell sorting at wing disc A/P boundaries [24, 26, 27], it is tempting to speculate that Wg/Hh bidirectional signalling controls cell sorting at embryonic A/P boundaries. This hypothesis will, however, require experimental support in the future. Furthermore, it will be interesting to determine whether additional anterior/posterior patterning genes such as pair-rule or segment polarity genes also act as temporal and spatial cues to direct parasegmental actomyosin accumulation.
Is differential adhesion required to build actomyosin cables?
By which mechanism could actomyosin cabling be implemented downstream of cell-cell signalling receptors? Differential adhesion, conferred by differential expression of adhesion molecules under the control of signalling pathways, could conceivably trigger actomyosin accumulation at compartmental boundaries. This is based on the observation that apposition of clones of cells expressing and nonexpressing the adhesion molecule Echinoid, a Nectin homologue, causes actomyosin cabling at the clone interface in the Drosophila wing disc and follicular epithelia [43, 81]. A similar phenomenon could happen at compartmental boundaries.
As already mentioned, apterous should control cell sorting at the D/V boundary through fringe-dependent Notch activation and through a more direct mechanism thought to involve differential affinity [5, 31–33]. Identification of the dorsally expressed LRR transmembrane proteins Tartan and Capricious as apterous targets gave support to this theory. However, their loss of function does not disrupt boundary integrity [41], suggesting redundancy with additional adhesion molecules or other mechanisms. An alternative hypothesis could be considered: similarly to Echinoid, apposition of cells expressing and nonexpressing Tartan and/or Capricious could induce actomyosin cabling at the interface between the two cell populations. If true, apterous would control D/V boundary actomyosin enrichment both directly (via restricted expression of tartan and capricious in dorsal cells) and indirectly (because apterous-dependent restriction of fringe expression to the dorsal compartment induces Notch activation at the boundary, which in turn induces actomyosin cabling via a still unknown mechanism). Interestingly, the expression of tartan and capricious is restricted to the dorsal part of wing discs in second instar larvae [41], i.e., at the stage when D/V actomyosin cabling is thought to take place. At mid-late third instar, once the D/V actomyosin cable is dismantled, their expression becomes unrelated to compartmentalization [41]. Therefore, their dynamics of expression correlates well with the timing of presence of the D/V actomyosin cable. Intriguingly, we have reported recently that embryonic parasegmental boundaries are irregular in embryos homozygous for a deficiency removing capricious, amongst other genes [50]. Together, these observations open the possibility that capricious and tartan promote actomyosin cabling at the interface between boundary cells.
Is there a relationship between surface tension and actomyosin cables in boundary cells?
While studying the behavior of dissociated cells from zebrafish gastrulating embryos, Krieg et al. [82] recently observed that polymerized actin accumulates more strongly at the surface of ectoderm, mesoderm, or endoderm explants than at intercellular junctions. In this case, locally modified cell surface tension (the parameter that leads to cell sorting of dissociated cells; see legend of Supplementary Fig. 1 for details), which is due to the absence of adhesion at the explants-culture medium interface, could potentially lead to the local recruitment of the actomyosin cytoskeleton. In vivo, apposition of groups of cells expressing and nonexpressing a given adhesion molecule or set of adhesion molecules, such as tartan/capricious dorsal expression or echinoid mutant clones in the Drosophila wing disc, could for example lead to locally modified surface tension at the compartmental or clonal interface. Alternatively, adhesion could be fine-tuned only at the compartmental boundary interface. It is therefore possible that regulation of adhesion, potentially only at the boundary interface, acts as the primary driving force to promote cell sorting, and that it does so by inducing the formation of actomyosin cables, which would act as the final effector to inhibit cell mixing. Moreover, study of cell mechanics during convergence-extension of the early Drosophila embryo has recently shown that the linkage of Myosin II-enriched cell-cell interfaces increases Myosin II accumulation through a positive feedback loop [83]. In other words, tension recruits tension. Such a mechano-sensitive feedback mechanism could help accumulating Myosin II at compartmental boundary interfaces once locally altered surface tension has initiated the formation of a Myosin II cable.
Myosin II contraction at the cell cortex produces forces that tend to shrink the cell’s surface area while adhesion molecules at the plasma membrane produce opposite forces that lead to membrane expansion [84]. Surface tension is therefore the consequence of the balance between adhesion and contractility [85], and modulation of one of these parameters will impinge on the other one. If surface tension is modified at the compartmental boundary interface in vivo because of local down-regulation of adhesion, not only could it be a means to promote actomyosin cabling as seen above, but it could also locally enhance the cortical tension generated by the actomyosin cable, thus reinforcing the fence that inhibits cell mixing.
In the Drosophila wing disc, it has been shown that the Par3 homologue Bazooka is depleted from the adherens junctions of both A/P and D/V boundaries (its localization has not been determined at parasegmental boundaries) [48, 49]. This complementary pattern of actomyosin and Bazooka/Par3 localization is reminiscent of the planar polarity of these key molecules during germ-band extension of the early Drosophila embryo (at stages prior to parasegment boundary formation): actin and Myosin II are enriched in junctions parallel to the D/V axis, while Bazooka/Par3 is enriched in junctions that are perpendicular [58, 60, 61, 86]. If Bazooka/Par3 stabilizes the adherens junctions, its depletion at the compartmental boundary could locally decrease adhesion, therefore increasing tension at the interface between compartments and, possibly, trigger actomyosin accumulation at the boundary interface in the first place. It will be interesting in the future to characterize the timing of Myosin II accumulation and Bazooka/Par3 depletion at compartmental boundary interfaces and determine which event occurs first.
A model for the establishment and the maintenance of cell sorting at compartmental boundaries
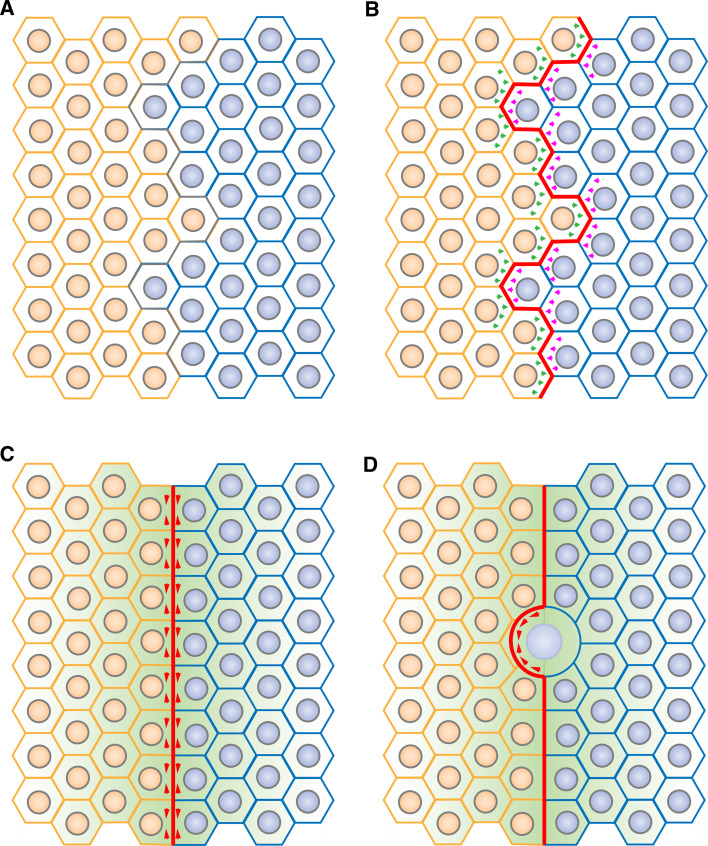
Taken together, studies of compartmentalization in Drosophila [3–5, 46, 48–50] suggest a model for boundary formation and function. Tissue patterning would first lead to the establishment of two distinct populations of cells separated by an irregular interface (Fig. 4a). Bidirectional signalling across the boundary would trigger actomyosin cabling at the compartmental interface through a yet unknown mechanism that could involve regulation of adhesion (Fig. 4b). Local cortical tension generated by the actomyosin cable would sharpen the boundary interface (Fig. 4c). The boundary cells could then express signalling factors that act as a secondary organizer to trigger differentiation of the surrounding tissue. The actomyosin barrier would then maintain a stable and straight organizer by correcting cell mixing due to local challenges such as those imposed by cell division (Fig. 4d). Actomyosin barriers would therefore be key components of two distinct steps of compartmentalization: boundary formation and boundary maintenance.
Fig. 4a–d.
A model for cell sorting at compartmental boundaries: role of contractile actomyosin barriers. a Patterning of fields of cells (such as segmentation in the Drosophila embryo or in the vertebrate hindbrain) leads to the apposition of two compartments. b Bidirectional signalling across the boundary (green and pink arrows) induces actomyosin cabling (red) at the compartmental boundary interface. c Cortical tension generated by the actomyosin cable (red arrowheads) at the level of adherens junctions straightens the compartmental boundary, leading to a segregation of cells based on their identity. A secondary organizer (green gradient) then triggers differentiation of the surrounding tissue. d Next, actomyosin cable-dependent cortical tensile forces correct local cell invasion, in particular following cell division, thereby maintaining a straight boundary and stable compartmentalization
This model might be transposable to more complex organisms. At vertebrate rhombomere boundaries, cell sorting relies on Eph-Ephrin and Notch signalling [34–37]. As Eph-Ephrin signalling modulates actomyosin cytoskeleton dynamics during axonal pathfinding and in the vascular system of vertebrates [87, 88] and Notch signalling triggers actomyosin assembly at the Drosophila wing disc D/V boundary [46, 48], it is tempting to speculate that actomyosin barriers might sort cells at rhombomere boundaries as well. Actomyosin barriers might also form at the zona limitans intrathalamica (ZLI) boundaries where cell sorting relies on lunatic fringe-dependent regulation of Notch signalling [17]. It is now important to determine whether the use of actomyosin barriers is an evolutionary conserved strategy that sorts cells in developing vertebrate epithelia as well as in Drosophila tissues.
Concluding remarks and perspectives
Our understanding of cell sorting mechanisms in vivo has long been based on the “DAH” initially formulated by Steinberg [1, 38, 39] to explain the behavior of aggregated cells following the cell dissociation experiments performed by Townes and Holfreter [89] (see legend of Supplementary Fig. 1A for additional details). However, cell dissociation experiments create an artificial situation, as cells are not initially misallocated to a wrong compartment during normal development. The identification of actomyosin cytoskeleton-based barriers to maintain the integrity of compartmental boundaries in Drosophila supports the idea that cell sorting in vivo is the consequence of local inhibition of cell mixing by specialized boundary cells rather than through active cell sorting as observed in dissociation experiments.
Interestingly, Harris predicted in 1976 that differential cell cortex contractility could promote cell sorting between two populations of dissociated cells [90]. This theory found some support recently with the experiments by Krieg and co-workers who reported that cell sorting of dissociated cells from distinct germ layers relies on differential contractility of the actomyosin cytoskeleton rather than on differential adhesion during zebrafish gastrulation [82]. Therefore, both phenomena (i.e., cell sorting of dissociated cells from different germ layers and compartmental cell sorting in vivo) rely on the same driving force: actomyosin cytoskeleton contractility.
Given the requirement of actomyosin cables for cell sorting at compartmental boundaries, one important issue consists in deciphering the molecular control of actin and Myosin II dynamics at boundaries. How is cell-cell signalling translated into a three-dimensional actomyosin structure at the boundary interface? Answering this question will require accurate description of the structure and identification of molecules enriched or depleted from compartmental boundary interfaces. If it is confirmed that grooves are a signature of tensile compartmental actomyosin cables, identifying mutations that affect their formation or disappearance should be informative. Additionally, as in other systems such as mesoderm invagination, dorsal closure, or cell intercalation, one pending question concerns how force is transmitted throughout the tissue [70]. In this case, how does the force propagate along the boundary? Also, what is the basis of actomyosin cable partitioning when a boundary cell divides parallel to the boundary? In such a case, cytokinesis occurs perpendicular to the cable, which is then split in two, with the two portions making a link again in the two daughter cells. Like force transmission along the boundary, this situation should require coupling between the actomyosin cable and adherens junctions components such as E-Cadherin. Additional work is required to decipher their relationship when implementing cell sorting at compartmental boundaries.
To conclude, identification of actomyosin barriers as a means to prevent mixing between populations of cells of distinct identities is an important step forward in our understanding of the mechanics of developing tissues, but much remains to be done to understand the complete mechanism underlying cell sorting at compartmental boundaries and to determine whether actomyosin barriers prevent cell mixing at vertebrate boundaries as well.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure S1: Mechanisms that could implement cell sorting at compartmental boundaries. a Differential adhesion: based on the analogy that liquids such as oil and water are immiscible due to distinct surface tension, it has been suggested that dissociated cells of distinct identities could sort according to their surface tension [1, 2]. It has been proposed that differential surface tension could be conferred by distinct adhesion molecules or by different levels of the same adhesion molecule. This hypothesis was validated in vitro by varying the levels or the nature of cadherins transfected in cultured cells [3]. This theory has been extrapolated to compartmental cell sorting [4–7]: here, the two cell populations express two distinct adhesion molecules: red and blue, respectively. However, one might note that additional parameters might come in the interplay to produce surface tension. In particular, surface tension probably relies on a balance between adhesion at the plasma membrane and contractibility at the cell cortex [8]. In other words, differential expression of adhesion molecules might not be the only strategy to produce differential surface tension between two groups of cells. b Extracellular matrix fence: in a tissue, two compartments could be kept separated by a groove filled with extracellular matrix that would act as a physical fence. c Nonproliferative zone: in a mitotically active epithelium, division of boundary cells could lead to an irregular boundary between two compartments. Inhibition of cell division a few rows of cells on each side of the compartmental boundary could prevent the boundary from being challenged, and thus maintain stable compartmentalization. In the Drosophila wing disc, Notch and Wingless signalling inhibits cell division close to the D/V boundary [9, 10]. d Local orientation of cell division axis: alternatively to c, the mitotic spindle of dividing boundary cells could be oriented so that boundary cells would divide parallel to the boundary. In this case, the progeny of dividing boundary cells would never be found in the opposite compartment, thereby preventing cell mixing. (TIFF 5778 kb)
Supplementary Figure S2: Cell behavior at morphogen-dependent boundaries. a Compartmentalization can be maintained by morphogen-dependent boundaries. Cell identity (blue) relies on reception of a given morphogen (green), and it changes when cells move too far away from the morphogen source. Stable compartmentalization maintained by morphogen-dependent boundaries does not involve physical segregation of cells. An example of morphogen-dependent boundary can be found in the Drosophila wing disc where the wing/notum boundary relies on reception of EGF signalling [11]. b In the Drosophila embryo at stages 8–11, anterior and posterior compartments are separated by parasegmental boundaries and by posterior-anterior (P-A) boundaries. P-A boundaries, at the level of which the segmental grooves will develop after stage 12, are morphogen-dependent boundaries: engrailed expression in posterior cells relies on reception of the Wingless morphogen secreted by cells of the anterior compartment that face the parasegmental boundary [12–15]. (TIFF 1752 kb)
Supplementary Figure S3: A narrow nonproliferative zone at theDrosophilawing disc A/P boundary? Schematization of the pattern of cell proliferation at boundaries in the Drosophila wing pouch showing the broad nonproliferative zone at the D/V boundary. A nonproliferative zone, although narrow, could also form at the A/P boundary in late third instar wing discs. The color-code is similar to the one used in figure 1, green indicates BrdU-positive cells of unknown identity. Schematization is based on the pattern of BrdU incorporation reported in Figure 1b of Johnston and Edgar (1998) [10]. A similar pattern was also reported in Figure 2A of Herranz and coworkers (2008) [9]. In the initial analysis by O’Brochta and Bryant (1985) [16], a nonproliferative zone was not identified at the A/P boundary. High resolution images of the pattern of BrdU incorporation in the more recent reports suggest that such a nonproliferative zone might exist at the A/P boundary after all, although its presence is not discussed by the authors [9, 10]. Additional experiments are required to clarify this situation. (TIFF 721 kb)
Supplementary Figure S4: Influence of the pattern of Myosin II activation upon groove formation. Top views (top) and cross-section (bottom) of the eye disc epithelium (left) and of the embryonic epithelium (right) showing the pattern of Myosin II activation (green) and the shape of the associated groove. (TIFF 2427 kb)
Acknowledgments
This work has been supported by ARC (Association pour la Recherche contre le Cancer) and Herchel Smith fellowships to B.M., by an EMBO fellowship to A.P.-M. and by HSFP and Wellcome Trust grants to B.S. A.P.-M. would like to thank Andrea Brand for her support.
Abbreviations
- AEL
After egg laying
- A/P
Anterior-posterior
- Cad99C
Cadherin 99C
- CALI
Chromophore-assisted laser inactivation
- DAH
Differential adhesion hypothesis
- Dpp
Decapentaplegic
- D/V
Dorsal-ventral
- F-actin
Filamentous actin
- Hh
Hedgehog
- LRR
Leucine rich repeat
- L2
Second instar larva
- L3
Third instar larva
- MHC
Myosin II Heavy Chain
- MRLC
Myosin II Regulatory Light Chain
- MyoII
Nonmuscle Myosin II
- Omb
Optomotor-blind
- sqh
spaghetti-squash
- Wg
Wingless
- zip
zipper
- ZLI
Zona limitans intrathalamica
Contributor Information
Bruno Monier, Phone: +33-4-91269611, FAX: +33-4-91820682, Email: bruno.monier@univmed.fr.
Bénédicte Sanson, Phone: +44-1223-333893, FAX: +44-1223-333840, Email: bs251@cam.ac.uk.
References
- 1.Garcia-Bellido A. Genetic control of wing disc development in Drosophila. Ciba Found Symp. 1975;0:161–182. doi: 10.1002/9780470720110.ch8. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol. 1973;245:251–253. doi: 10.1038/245251a0. [DOI] [PubMed] [Google Scholar]
- 3.Dahmann C, Basler K. Compartment boundaries: at the edge of development. Trends Genet. 1999;15:320–326. doi: 10.1016/S0168-9525(99)01774-6. [DOI] [PubMed] [Google Scholar]
- 4.Irvine KD, Rauskolb C. Boundaries in development: formation and function. Annu Rev Cell Dev Biol. 2001;17:189–214. doi: 10.1146/annurev.cellbio.17.1.189. [DOI] [PubMed] [Google Scholar]
- 5.Tepass U, Godt D, Winklbauer R. Cell sorting in animal development: signalling and adhesive mechanisms in the formation of tissue boundaries. Curr Opin Genet Dev. 2002;12:572–582. doi: 10.1016/S0959-437X(02)00342-8. [DOI] [PubMed] [Google Scholar]
- 6.Bryant PJ. Cell lineage relationships in the imaginal wing disc of Drosophila melanogaster. Dev Biol. 1970;22:389–411. doi: 10.1016/0012-1606(70)90160-0. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalization in the dorsal mesothoracic disc of Drosophila. Dev Biol. 1976;48:132–147. doi: 10.1016/0012-1606(76)90052-X. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence PA. A clonal analysis of segment development in Oncopeltus (Hemiptera) J Embryol Exp Morphol. 1973;30:681–699. [PubMed] [Google Scholar]
- 9.Chen EH, Baker BS. Compartmental organization of the Drosophila genital imaginal discs. Development. 1997;124:205–218. doi: 10.1242/dev.124.1.205. [DOI] [PubMed] [Google Scholar]
- 10.Vincent JP, O’Farrell PH. The state of engrailed expression is not clonally transmitted during early Drosophila development. Cell. 1992;68:923–931. doi: 10.1016/0092-8674(92)90035-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altabef M, Clarke JD, Tickle C. Dorso-ventral ectodermal compartments and origin of apical ectodermal ridge in developing chick limb. Development. 1997;124:4547–4556. doi: 10.1242/dev.124.22.4547. [DOI] [PubMed] [Google Scholar]
- 12.Arques CG, Doohan R, Sharpe J, Torres M. Cell tracing reveals a dorsoventral lineage restriction plane in the mouse limb bud mesenchyme. Development. 2007;134:3713–3722. doi: 10.1242/dev.02873. [DOI] [PubMed] [Google Scholar]
- 13.Reintsch WE, Habring-Mueller A, Wang RW, Schohl A, Fagotto F. Beta-catenin controls cell sorting at the notochord-somite boundary independently of cadherin-mediated adhesion. J Cell Biol. 2005;170:675–686. doi: 10.1083/jcb.200503009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser S, Keynes R, Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990;344:431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Tanaka T, Takeichi M, Chisaka O, Nakamura S, Osumi N. Role of cadherins in maintaining the compartment boundary between the cortex and striatum during development. Development. 2001;128:561–569. doi: 10.1242/dev.128.4.561. [DOI] [PubMed] [Google Scholar]
- 16.Larsen CW, Zeltser LM, Lumsden A. Boundary formation and compartition in the avian diencephalon. J Neurosci. 2001;21:4699–4711. doi: 10.1523/JNEUROSCI.21-13-04699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6:553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- 18.Langenberg T, Brand M. Lineage restriction maintains a stable organizer cell population at the zebrafish midbrain-hindbrain boundary. Development. 2005;132:3209–3216. doi: 10.1242/dev.01862. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez-Guri E, Udina F, Colas JF, Sharpe J, Padron-Barthe L, Torres M, Pujades C. Clonal analysis in mice underlines the importance of rhombomeric boundaries in cell movement restriction during hindbrain segmentation. PLoS One. 2010;5:e10112. doi: 10.1371/journal.pone.0010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence PA, Struhl G. Morphogens, compartments, and pattern: lessons from Drosophila? Cell. 1996;85:951–961. doi: 10.1016/S0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- 21.Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:1763–1776. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Pomares JM, Foty RA. Tissue fusion and cell sorting in embryonic development and disease: biomedical implications. Bioessays. 2006;28:809–821. doi: 10.1002/bies.20442. [DOI] [PubMed] [Google Scholar]
- 23.Twigg SR, Kan R, Babbs C, Bochukova EG, Robertson SP, Wall SA, Morriss-Kay GM, Wilkie AO. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc Natl Acad Sci USA. 2004;101:8652–8657. doi: 10.1073/pnas.0402819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahmann C, Basler K. Opposing transcriptional outputs of Hedgehog signaling and Engrailed control compartmental cell sorting at the Drosophila A/P boundary. Cell. 2000;100:411–422. doi: 10.1016/S0092-8674(00)80677-7. [DOI] [PubMed] [Google Scholar]
- 25.Morata G, Lawrence PA. Control of compartment development by the engrailed gene in Drosophila. Nature. 1975;255:614–617. doi: 10.1038/255614a0. [DOI] [PubMed] [Google Scholar]
- 26.Blair SS, Ralston A. Smoothened-mediated Hedgehog signalling is required for the maintenance of the anterior-posterior lineage restriction in the developing wing of Drosophila. Development. 1997;124:4053–4063. doi: 10.1242/dev.124.20.4053. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez I, Basler K. Control of compartmental affinity boundaries by Hedgehog. Nature. 1997;389:614–618. doi: 10.1038/39343. [DOI] [PubMed] [Google Scholar]
- 28.Shen J, Dahmann C. The role of Dpp signaling in maintaining the Drosophila anteroposterior compartment boundary. Dev Biol. 2005;279:31–43. doi: 10.1016/j.ydbio.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Micchelli CA, Blair SS. Dorsoventral lineage restriction in wing imaginal discs requires Notch. Nature. 1999;401:473–476. doi: 10.1038/46779. [DOI] [PubMed] [Google Scholar]
- 30.Rauskolb C, Correia T, Irvine KD. Fringe-dependent separation of dorsal and ventral cells in the Drosophila wing. Nature. 1999;401:476–480. doi: 10.1038/46786. [DOI] [PubMed] [Google Scholar]
- 31.Milan M, Cohen SM. Notch signaling is not sufficient to define the affinity boundary between dorsal and ventral compartments. Mol Cell. 1999;4:1073–1078. doi: 10.1016/S1097-2765(00)80235-X. [DOI] [PubMed] [Google Scholar]
- 32.Milan M, Cohen SM. A re-evaluation of the contributions of Apterous and Notch to the dorsoventral lineage restriction boundary in the Drosophila wing. Development. 2003;130:553–562. doi: 10.1242/dev.00276. [DOI] [PubMed] [Google Scholar]
- 33.O’Keefe DD, Thomas JB. Drosophila wing development in the absence of dorsal identity. Development. 2001;128:703–710. doi: 10.1242/dev.128.5.703. [DOI] [PubMed] [Google Scholar]
- 34.Cheng YC, Amoyel M, Qiu X, Jiang YJ, Xu Q, Wilkinson DG. Notch activation regulates the segregation and differentiation of rhombomere boundary cells in the zebrafish hindbrain. Dev Cell. 2004;6:539–550. doi: 10.1016/S1534-5807(04)00097-8. [DOI] [PubMed] [Google Scholar]
- 35.Cooke JE, Kemp HA, Moens CB. EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr Biol. 2005;15:536–542. doi: 10.1016/j.cub.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 36.Mellitzer G, Xu Q, Wilkinson DG. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. [DOI] [PubMed] [Google Scholar]
- 37.Xu Q, Mellitzer G, Robinson V, Wilkinson DG. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- 38.Steinberg MS. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science. 1963;141:401–408. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg MS. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool. 1970;173:395–433. doi: 10.1002/jez.1401730406. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence PA, Casal J, Struhl G. The Hedgehog morphogen and gradients of cell affinity in the abdomen of Drosophila. Development. 1999;126:2441–2449. doi: 10.1242/dev.126.11.2441. [DOI] [PubMed] [Google Scholar]
- 41.Milan M, Weihe U, Perez L, Cohen SM. The LRR proteins Capricious and Tartan mediate cell interactions during DV boundary formation in the Drosophila wing. Cell. 2001;106:785–794. doi: 10.1016/S0092-8674(01)00489-5. [DOI] [PubMed] [Google Scholar]
- 42.Schlichting K, Demontis F, Dahmann C. Cadherin Cad99C is regulated by Hedgehog signaling in Drosophila. Dev Biol. 2005;279:142–154. doi: 10.1016/j.ydbio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Wei SY, Escudero LM, Yu F, Chang LH, Chen LY, Ho YH, Lin CM, Chou CS, Chia W, Modolell J, et al. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev Cell. 2005;8:493–504. doi: 10.1016/j.devcel.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Bejarano F, Luque CM, Herranz H, Sorrosal G, Rafel N, Pham TT, Milan M. A gain-of-function suppressor screen for genes involved in dorsal-ventral boundary formation in the Drosophila wing. Genetics. 2008;178:307–323. doi: 10.1534/genetics.107.081869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vegh M, Basler K. A genetic screen for Hedgehog targets involved in the maintenance of the Drosophila anteroposterior compartment boundary. Genetics. 2003;163:1427–1438. doi: 10.1093/genetics/163.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Major RJ, Irvine KD. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development. 2005;132:3823–3833. doi: 10.1242/dev.01957. [DOI] [PubMed] [Google Scholar]
- 47.O’Brochta DA, Bryant PJ. A zone of non-proliferating cells at a lineage restriction boundary in Drosophila. Nature. 1985;313:138–141. doi: 10.1038/313138a0. [DOI] [PubMed] [Google Scholar]
- 48.Major RJ, Irvine KD. Localization and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev Dyn. 2006;235:3051–3058. doi: 10.1002/dvdy.20966. [DOI] [PubMed] [Google Scholar]
- 49.Landsberg KP, Farhadifar R, Ranft J, Umetsu D, Widmann TJ, Bittig T, Said A, Julicher F, Dahmann C. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr Biol. 2009;19:1950–1955. doi: 10.1016/j.cub.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 50.Monier B, Pelissier-Monier A, Brand AH, Sanson B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat Cell Biol. 2010;12:60–65. doi: 10.1038/ncb2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen SM (1993) Imaginal disc development. In: Bate MAE (ed) The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 747–841
- 52.Martinez-Arias A, Lawrence PA. Parasegments and compartments in the Drosophila embryo. Nature. 1985;313:639–642. doi: 10.1038/313639a0. [DOI] [PubMed] [Google Scholar]
- 53.Larsen CW, Hirst E, Alexandre C, Vincent JP. Segment boundary formation in Drosophila embryos. Development. 2003;130:5625–5635. doi: 10.1242/dev.00867. [DOI] [PubMed] [Google Scholar]
- 54.Herranz H, Perez L, Martin FA, Milan M. A Wingless and Notch double-repression mechanism regulates G1-S transition in the Drosophila wing. EMBO J. 2008;27:1633–1645. doi: 10.1038/emboj.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnston LA, Edgar BA. Wingless and Notch regulate cell-cycle arrest in the developing Drosophila wing. Nature. 1998;394:82–84. doi: 10.1038/27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. 2. Berlin: Spinger; 1997. pp. 287–308. [Google Scholar]
- 57.Martinez-Arias A (1993) Development and patterning of the larval epidermis of Drosophila. In: Bate MAE (ed) The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 517–608
- 58.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 59.Butler LC, Blanchard GB, Kabla AJ, Lawrence NJ, Welchman DP, Mahadevan L, Adams RJ, Sanson B. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat Cell Biol. 2009;11:859–864. doi: 10.1038/ncb1894. [DOI] [PubMed] [Google Scholar]
- 60.Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/S1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 61.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Edwards KA, Chang XJ, Kiehart DP. Essential light chain of Drosophila nonmuscle myosin II. J Muscle Res Cell Motil. 1995;16:491–498. doi: 10.1007/BF00126433. [DOI] [PubMed] [Google Scholar]
- 63.Karess RE, Chang XJ, Edwards KA, Kulkarni S, Aguilera I, Kiehart DP. The regulatory light chain of nonmuscle myosin is encoded by spaghetti-squash, a gene required for cytokinesis in Drosophila. Cell. 1991;65:1177–1189. doi: 10.1016/0092-8674(91)90013-O. [DOI] [PubMed] [Google Scholar]
- 64.Young PE, Richman AM, Ketchum AS, Kiehart DP. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 1993;7:29–41. doi: 10.1101/gad.7.1.29. [DOI] [PubMed] [Google Scholar]
- 65.Jacobson K, Rajfur Z, Vitriol E, Hahn K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 2008;18:443–450. doi: 10.1016/j.tcb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jay DG. Selective destruction of protein function by chromophore-assisted laser inactivation. Proc Natl Acad Sci USA. 1988;85:5454–5458. doi: 10.1073/pnas.85.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horstkotte E, Schroder T, Niewohner J, Jay DG, Henning SW. Toward understanding the mechanism of chromophore-assisted laser inactivation—evidence for the primary photochemical steps. Photochem Photobiol. 2005;81:358–366. doi: 10.1562/2004-07-22-RA-240.1. [DOI] [PubMed] [Google Scholar]
- 68.Liao JC, Roider J, Jay DG. Chromophore-assisted laser inactivation of proteins is mediated by the photogeneration of free-radicals. Proc Natl Acad Sci USA. 1994;91:2659–2663. doi: 10.1073/pnas.91.7.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan P, Xiong Y, Chen B, Negash S, Squier T, Mayer MU. Fluorophore-assisted light inactivation of Calmodulin involves singlet-oxygen mediated cross-linking and methionine oxidation. Biochemistry. 2006;45:4736–4748. doi: 10.1021/bi052395a. [DOI] [PubMed] [Google Scholar]
- 70.Martin AC. Pulsation and stabilization: contractile forces that underlie morphogenesis. Dev Biol. 2010;341:114–125. doi: 10.1016/j.ydbio.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 71.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Escudero LM, Bischoff M, Freeman M. Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev Cell. 2007;13:717–729. doi: 10.1016/j.devcel.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Brower D, Smith R, Wilcox M. Cell shapes on the surface of the Drosophila wing imaginal disc. J Embryol Exp Morphol. 1982;67:137–151. [Google Scholar]
- 74.Larsen C, Bardet PL, Vincent JP, Alexandre C. Specification and positioning of parasegment grooves in Drosophila. Dev Biol. 2008;321:310–318. doi: 10.1016/j.ydbio.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 75.Shen J, Dorner C, Bahlo A, Pflugfelder GO. Optomotor-blind suppresses instability at the A/P compartment boundary of the Drosophila wing. Mech Dev. 2008;125:233–246. doi: 10.1016/j.mod.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Umemori M, Takemura M, Maeda K, Ohba K, Adachi-Yamada T. Drosophila T-box transcription factor Optomotor-blind prevents pathological folding and local overgrowth in wing epithelium through confining Hh signal. Dev Biol. 2007;308:68–81. doi: 10.1016/j.ydbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 77.Kunda P, Pelling AE, Liu T, Baum B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr Biol. 2008;18:91–101. doi: 10.1016/j.cub.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 78.Masucci JD, Miltenberger RJ, Hoffmann FM. Pattern-specific expression of the Drosophila decapentaplegic gene in imaginal disks is regulated by 3′ cis-regulatory elements. Genes Dev. 1990;4:2011–2023. doi: 10.1101/gad.4.11.2011. [DOI] [PubMed] [Google Scholar]
- 79.Padgett RW, St Johnston RD, Gelbart WM. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature. 1987;325:81–84. doi: 10.1038/325081a0. [DOI] [PubMed] [Google Scholar]
- 80.Corrigall D, Walther RF, Rodriguez L, Fichelson P, Pichaud F. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Dev Cell. 2007;13:730–742. doi: 10.1016/j.devcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 81.Laplante C, Nilson LA. Differential expression of the adhesion molecule echinoid drives epithelial morphogenesis in Drosophila. Development. 2006;133:3255–3264. doi: 10.1242/dev.02492. [DOI] [PubMed] [Google Scholar]
- 82.Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 83.Fernandez-Gonzalez R, Simoes Sde M, Roper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 85.Manning ML, Foty RA, Steinberg MS, Schoetz EM. Coaction of intercellular adhesion and cortical tension specifies tissue surface tension. Proc Natl Acad Sci USA. 2010;107:12517–12522. doi: 10.1073/pnas.1003743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simoes Sde M, Blankenship JT, Weitz O, Farrell DL, Tamada M, Fernandez-Gonzalez R, Zallen JA. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev Cell. 2010;19:377–388. doi: 10.1016/j.devcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huber AB, Kolodkin AL, Ginty DD, Cloutier J-Fo. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 88.Ogita H, Kunimoto S, Kamioka Y, Sawa H, Masuda M, Mochizuki N. EphA4-mediated rho activation via Vsm-RhoGEF expressed specifically in vascular smooth muscle cells. Circ Res. 2003;93:23–31. doi: 10.1161/01.RES.0000079310.81429.C8. [DOI] [PubMed] [Google Scholar]
- 89.Townes PL, Holtfreter J. Directed movements and selective adhesion of embryonic amphibian cells. J Exp Zool. 1955;128:53–120. doi: 10.1002/jez.1401280105. [DOI] [PubMed] [Google Scholar]
- 90.Harris AK. Is cell sorting caused by differences in the work of intercellular adhesion? A critique of the Steinberg hypothesis. J Theor Biol. 1976;61:267–285. doi: 10.1016/0022-5193(76)90019-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Mechanisms that could implement cell sorting at compartmental boundaries. a Differential adhesion: based on the analogy that liquids such as oil and water are immiscible due to distinct surface tension, it has been suggested that dissociated cells of distinct identities could sort according to their surface tension [1, 2]. It has been proposed that differential surface tension could be conferred by distinct adhesion molecules or by different levels of the same adhesion molecule. This hypothesis was validated in vitro by varying the levels or the nature of cadherins transfected in cultured cells [3]. This theory has been extrapolated to compartmental cell sorting [4–7]: here, the two cell populations express two distinct adhesion molecules: red and blue, respectively. However, one might note that additional parameters might come in the interplay to produce surface tension. In particular, surface tension probably relies on a balance between adhesion at the plasma membrane and contractibility at the cell cortex [8]. In other words, differential expression of adhesion molecules might not be the only strategy to produce differential surface tension between two groups of cells. b Extracellular matrix fence: in a tissue, two compartments could be kept separated by a groove filled with extracellular matrix that would act as a physical fence. c Nonproliferative zone: in a mitotically active epithelium, division of boundary cells could lead to an irregular boundary between two compartments. Inhibition of cell division a few rows of cells on each side of the compartmental boundary could prevent the boundary from being challenged, and thus maintain stable compartmentalization. In the Drosophila wing disc, Notch and Wingless signalling inhibits cell division close to the D/V boundary [9, 10]. d Local orientation of cell division axis: alternatively to c, the mitotic spindle of dividing boundary cells could be oriented so that boundary cells would divide parallel to the boundary. In this case, the progeny of dividing boundary cells would never be found in the opposite compartment, thereby preventing cell mixing. (TIFF 5778 kb)
Supplementary Figure S2: Cell behavior at morphogen-dependent boundaries. a Compartmentalization can be maintained by morphogen-dependent boundaries. Cell identity (blue) relies on reception of a given morphogen (green), and it changes when cells move too far away from the morphogen source. Stable compartmentalization maintained by morphogen-dependent boundaries does not involve physical segregation of cells. An example of morphogen-dependent boundary can be found in the Drosophila wing disc where the wing/notum boundary relies on reception of EGF signalling [11]. b In the Drosophila embryo at stages 8–11, anterior and posterior compartments are separated by parasegmental boundaries and by posterior-anterior (P-A) boundaries. P-A boundaries, at the level of which the segmental grooves will develop after stage 12, are morphogen-dependent boundaries: engrailed expression in posterior cells relies on reception of the Wingless morphogen secreted by cells of the anterior compartment that face the parasegmental boundary [12–15]. (TIFF 1752 kb)
Supplementary Figure S3: A narrow nonproliferative zone at theDrosophilawing disc A/P boundary? Schematization of the pattern of cell proliferation at boundaries in the Drosophila wing pouch showing the broad nonproliferative zone at the D/V boundary. A nonproliferative zone, although narrow, could also form at the A/P boundary in late third instar wing discs. The color-code is similar to the one used in figure 1, green indicates BrdU-positive cells of unknown identity. Schematization is based on the pattern of BrdU incorporation reported in Figure 1b of Johnston and Edgar (1998) [10]. A similar pattern was also reported in Figure 2A of Herranz and coworkers (2008) [9]. In the initial analysis by O’Brochta and Bryant (1985) [16], a nonproliferative zone was not identified at the A/P boundary. High resolution images of the pattern of BrdU incorporation in the more recent reports suggest that such a nonproliferative zone might exist at the A/P boundary after all, although its presence is not discussed by the authors [9, 10]. Additional experiments are required to clarify this situation. (TIFF 721 kb)
Supplementary Figure S4: Influence of the pattern of Myosin II activation upon groove formation. Top views (top) and cross-section (bottom) of the eye disc epithelium (left) and of the embryonic epithelium (right) showing the pattern of Myosin II activation (green) and the shape of the associated groove. (TIFF 2427 kb)