Abstract
The non-protein amino acid β-aminobutyric acid (BABA) protects numerous plants against various pathogens. Protection of Arabidopsis plants against virulent pathogens involves the potentiation of pathogen-specific defense responses. To extend the analysis of the mode of action of BABA to necrotrophs we evaluated the effect of this chemical on Arabidopsis plants infected with the gray mold fungus Botrytis cinerea. BABA-treated Arabidopsis were found to be less sensitive to two different strains of this pathogen. BABA protected mutants defective in the jasmonate and ethylene pathways, but was inactive in plants impaired in the systemic acquired resistance transduction pathway. Treatments with benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester, a functional analog of salicylic acid (SA), also markedly reduced the level of infection. Moreover, BABA potentiated mRNA accumulation of the SA-associated PR-1, but not the jasmonate/ethylene-dependent PDF1.2 gene. Thus, besides jasmonate/ethylene-dependent defense responses, SA-dependent signaling also contributes to restrict B. cinerea infection in Arabidopsis. Our results also suggest that SA-dependent signaling is down-regulated after infection by B. cinerea. The observed up-regulation of the PDF1.2 gene in mutants defective in the SA-dependent signaling pathway points to a cross-talk between SA- and jasmonate/ethylene-dependent signaling pathways during pathogen ingress.
Plants have developed a battery of complex defense mechanisms to escape infection by pathogens. Besides constitutive barriers, a number of mechanisms are induced upon recognition of the pathogen by the host. Depending on the pathogen, specific signal transduction pathways are induced, leading to the expression of sets of defense responses, which include rapid programmed cell death (hypersensitive response, HR), strengthening of the cell wall, or expression of antimicrobial genes (Hammond-Kosack and Jones, 1996). In many cases resistance is expressed locally and systemically in response to necrotizing pathogens or root-colonizing soil bacteria. Systemic acquired resistance (SAR) induced by pathogens is in many cases dependent on the endogenously synthesized signal salicylic acid (SA; Sticher et al., 1997). Other pathogens can induce defense responses characterized by jasmonic acid- (JA) and ethylene-dependent signal transduction pathways (Thomma et al., 1998, 1999). Furthermore, the spectrum of resistance is different depending on the signal transduction pathway. In SA-controlled SAR, plants deploy barriers that are effective against pathogens such as Peronospora parasitica (Thomma et al., 1998) or Pseudomonas syringae (Summermatter et al., 1995), whereas JA- or ethylene-controlled resistance leads to protection against Alternaria brassicicola or Botrytis cinerea (Thomma et al., 1998, 1999). These results made it clear that induced defense responses are mediated by multiple signal transduction pathways. In addition, these signaling pathways are not simple linear and isolated cascades, but can crosstalk with each other (Reymond and Farmer, 1998; Genoud and Métraux, 1999).
Defense responses induced by a primary infection can be expressed before the contact with a secondary challenging organism. A primary infection can also lead to a faster activation of defense responses after challenge inoculation, a phenomenon known as potentiation. Tissue priming or conditioning and the resulting potentiation of local defense responses was demonstrated in parsley cells treated with SA, 2,6-dichloroisonicotinic acid or benzo-(1, 2, 3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH). These primed cells show enhanced elicitation of the oxidative burst (Kauss and Jeblick, 1995), the secretion of cell wall phenolics (Kauss et al., 1993), phytoalexin production (Kauss et al., 1992), and activation of defense genes (Mur et al., 1996; Thulke and Conrath, 1998). In the latter case, a dual mechanism was observed: some genes such as the pathogenesis-related (PR) genes are directly induced, whereas some local defense genes are only potentiated. It has recently been proposed that an ubiquitin-proteasome system may play a role in potentiation processes (Becker et al., 2000).
Synthetic and natural compounds called inducers can effectively trigger induced resistance responses (Kessmann et al., 1994; Sticher et al., 1997). Some of the best characterized examples are 2,6-dichloroisonicotinic acid and BTH. These compounds induce the same spectrum of resistance as pathogen-induced SAR with concomitant activation of SA-dependent PR genes (Uknes et al., 1992; Lawton et al., 1996). The non-protein amino acid β-aminobutyric acid (BABA) has been shown to protect Arabidopsis against P. parasitica through activation of defense mechanisms such as callose deposition, HR, and the formation of trailing necroses (Jakab et al., 2000; Zimmerli et al., 2000). BABA is fully active against P. parasitica in transgenic plants or mutants impaired in the SA, JA, and ethylene signaling pathways. Although BABA did not induce the accumulation of mRNA of the SA-associated PR-1 or the JA- and ethylene-dependent PDF1.2 genes, it potentiated the accumulation of PR-1 mRNA after attack by virulent pathogenic bacteria. In the case of bacterial pathogens, BABA protected mutants insensitive to JA and ethylene, but was inactive in plants impaired in the SAR transduction pathway. Thus, BABA protects Arabidopsis against different virulent pathogens by potentiating pathogen-specific plant resistance mechanisms.
Here we have evaluated the effect of BABA in Arabidopsis infected by the necrotrophic fungus B. cinerea. We have found that BABA-treated Arabidopsis are protected against infection by B. cinerea. BABA also potentiates the accumulation of PR-1, but not PDF1.2 mRNA after infection. Our results indicate that the SAR signaling pathway contributes to restrict B. cinerea infection in Arabidopsis. Furthermore, we have shown that components of the SA-dependent signaling pathway inhibit the expression of JA/ethylene-dependent defense responses after B. cinerea infection.
RESULTS
BABA Enhances Arabidopsis Resistance to B. cinerea Infection
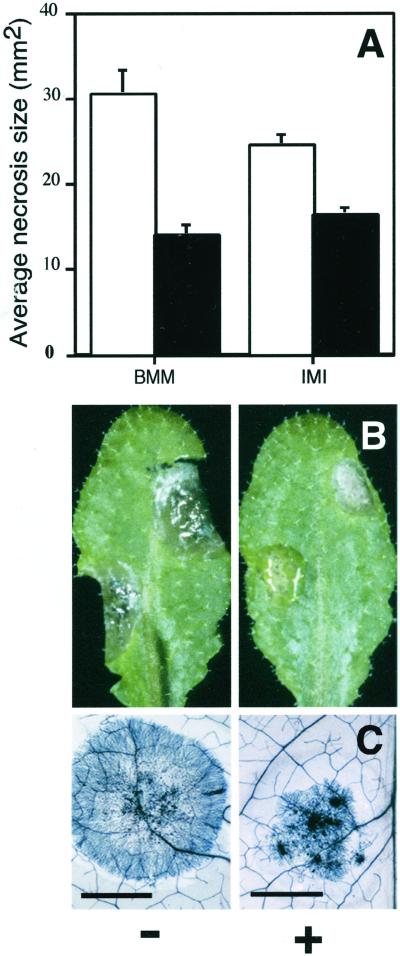
BABA protects Arabidopsis plants against P. parasitica and P. syringae pv tomato DC 3000 (Zimmerli et al., 2000). These two pathogens activate the SA-dependent signal transduction pathway in Arabidopsis. In this paper, our analysis was extended to pathogens inducing defense responses via the JA/ethylene signal transduction pathway. Soil drench treatment with 30 μg mL−1 BABA 1 d prior to the deposition of 3-μL droplets containing 75 conidia of B. cinerea each led to a significant reduction of the surface of the necroses, as observed 3 d after inoculation. Furthermore, BABA was effective against both strains of the gray mold fungus tested here (Fig. 1A). Fungal hyphae grew concentrically from the site of inoculation, resulting in a visible necrosis 3 d after inoculation. Necroses were smaller in BABA-treated Arabidopsis plants compared with the untreated controls (Fig. 1B). B. cinerea hyphae developed similarly in water- and BABA-treated Arabidopsis, as shown by microscopical observations, but the surface invaded by B. cinerea hyphae was smaller in BABA-treated Arabidopsis plants compared with untreated controls (Fig. 1C). As a consequence, the macroscopic symptoms reflect the level of infection in treated and untreated plants.
Figure 1.
Protection of Arabidopsis by BABA against infection with B. cinerea. A, Average size of necroses formed 3 d after inoculation on 5- to 6-week-old Arabidopsis Col-0 plants drop-inoculated with B. cinerea. Plants were soil-drenched with water (white bars) or 30 μg mL−1 BABA (black bars) 1 d prior to inoculation with strains BMM (BMM1) and IMI (IMI169558). The experiment was repeated at least three times with similar results. B, Symptoms observed 3 d after inoculation on leaves of 5-week-old Arabidopsis Col-0 drop-inoculated with B. cinerea strain BMM1. Plants were soil drenched with water (−) or 30 μg mL−1 BABA (+) 1 d prior to inoculation. C, Microscopical aspect of B. cinerea strain BMM1 infection. Micrographs show leaves stained with lactophenol-trypan blue (Keogh et al., 1980). Inoculation and treatment were performed as in B. Bar = 20 mm.
BABA Protects Arabidopsis Mutants Impaired in the JA/Ethylene-Dependent Signal Transduction Pathway, But Not Mutants or Transgenic Arabidopsis Defective in the SA-Dependent Signal Transduction Pathway
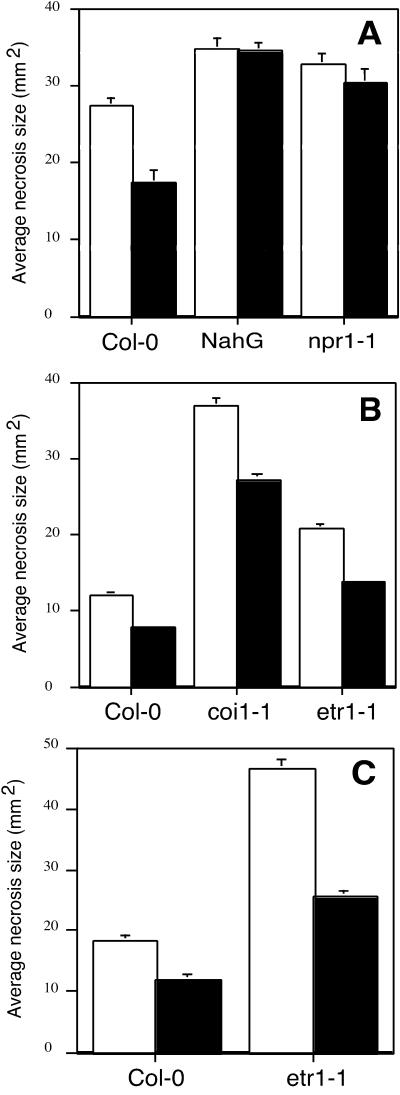
The mode of action of BABA was analyzed using transgenic Arabidopsis or mutants impaired in the signal transduction pathway to infection. nahG-expressing Arabidopsis unable to accumulate SA (Lawton et al., 1995) and npr1-1, a mutant non-responsive to inducers of SAR (Cao et al., 1994), were used to probe the SA pathway. It was interesting that BABA did not enhance the resistance against B. cinerea strain BBM1 in any of these plants (Fig. 2A). Analysis of the contribution of the JA/ethylene signal transduction pathway to BABA-mediated protection of Arabidopsis against B. cinerea was investigated with the ethylene-insensitive etr1-1 mutant (Bleecker and Kende, 1988) and with coi1-1, a mutant affected in the JA response pathway (Feys et al., 1994). Although colonization by B. cinerea was more extensive in both mutants compared with wild-type plants, BABA-treatment led to a similar reduction of fungal growth in all genotypes as measured 2 d after inoculation (Fig. 2B). Furthermore, etr1-1 mutants were also protected 3 d after inoculation (Fig. 2C). The fact that B. cinerea hyphae had already completely invaded the leaves of the untreated coi1-1 plants 3 d after inoculation did not allow us to analyze the level of infection of this highly sensitive mutant at this late timepoint. Hence, BABA protects Arabidopsis against B. cinerea through SA-dependent defense responses.
Figure 2.
Impact of BABA on disease development of Arabidopsis lines altered in their response to pathogens. A, Average size of necroses formed 3 d after drop-inoculation with B. cinerea strain BMM1. Five- to 6-week-old Arabidopsis wild-type control (Col-0) and transgenic line (nahG) or mutant (npr1-1) defective in the SA-dependent defense responses were soil-drenched with water (white bars) or 30 μg mL−1 BABA (black bars) 1 d prior to inoculation. The experiment was repeated three times with similar results. B, Average size of necroses formed 2 d after drop inoculation with B. cinerea strain BMM1. Arabidopsis wild-type control (Col-0) and mutants defective in the JA (coi1-1)/ethylene (etr1-1)-dependent signaling pathway were inoculated and treated as in A. C, Average size of necroses formed 3 d after drop inoculation on Arabidopsis wild-type control (Col-0) and etr1-1 mutants. Plants were inoculated and treated as in A.
BTH Protects Arabidopsis against B. cinerea
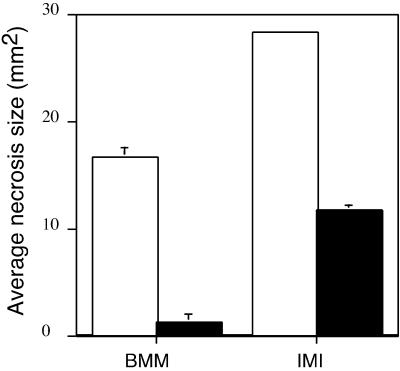
Since SA-dependent defense responses are involved in the BABA-mediated protection of Arabidopsis against B. cinerea, we evaluated the effect of BTH, a functional analog of SA, on the protection of Arabidopsis to infection to B. cinerea. A soil drench application of 0.33 10−3 M BTH 1 d prior to inoculation with two different strains of B. cinerea is sufficient to drastically slow down the infection (Fig. 3). This confirms the implication of SA-dependent defense responses in the protection of Arabidopsis against B. cinerea.
Figure 3.
Effect of BTH on the infection process of B. cinerea in Arabidopsis. Average size of lesions formed on Arabidopsis Col-0 leaves 3 d after drop inoculation with B. cinerea strain BMM (BMM1) and IMI (IMI169558). Plants were treated with water (white bars) or 0.33 10−3 M BTH (black bars) 1 d before inoculation. The experiments were repeated twice with similar results.
BABA Potentiates PR-1 But Not PDF 1.2 mRNA Accumulation
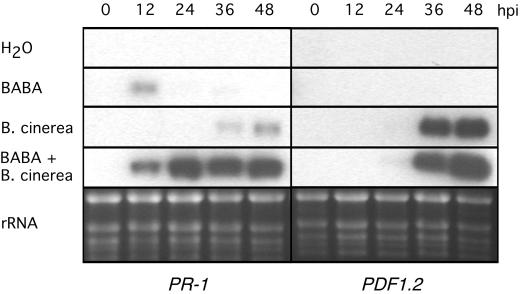
BABA treatment has been shown to prime the SA-dependent defense response pathway through potentiation of PR-1 mRNA accumulation after infection with virulent pathogenic bacteria (Zimmerli et al., 2000). JA/ethylene-dependent defense responses are essential for resistance against B. cinerea (Thomma et al., 1998, 1999). Moreover, treatment of Arabidopsis plants with these two plant hormones induces the accumulation of the plant defensin gene PDF1.2 (Penninckx et al., 1996). However, treatment with BABA potentiated the plant to induce PR-1 mRNA more rapidly and more intensively, but no differences were observed for PDF 1.2 mRNA accumulation (Fig. 4). PDF 1.2 transcripts accumulation was recorded starting 36 h post-inoculation in treated and untreated Arabidopsis. Thus, SA-dependent defense responses are boosted in BABA-treated Arabidopsis plants during infection with B. cinerea.
Figure 4.
Effect of BABA treatment on the time-course of the expression of defense genes in Arabidopsis infected with B. cinerea. Arabidopsis Col-0 plants were soil drenched with water or 30 μg mL−1 BABA 1 d prior to inoculation. Total RNA was extracted at different hours post-inoculation with B. cinerea strain BMM1. Each time point represents nine infected leaves harvested from three different plants. RNA blots were hybridized with PR-1 and PDF1.2 probes. Ethidium bromide staining of the RNA gel (rRNA) was used to show equal loading.
Pathogen-Induced Expression of PR-1 and PDF 1.2 mRNA in nahG, npr1-1, etr1-1, and coi1-1 Plants
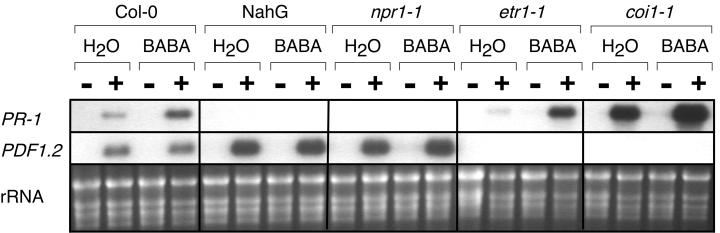
BABA-primed defense responses after B. cinerea infection were investigated by analyzing the expression of PR-1 and PDF 1.2 mRNA in nahG, npr1-1, etr1-1, and coi1-1 plants. As expected, potentiation of PR-1 transcript accumulation was observed in etr1-1 and coi1-1 mutants, but not in nahG and npr1-1 plants. It was interesting that coi1 plants showed a higher PR-1 transcript accumulation than the wild-type plants. Moreover, PDF 1.2 mRNA accumulated in nahG and npr1-1 plants, but not in etr1-1 and coi1-1 mutants (Fig. 5). Therefore, potentiation of PR-1 mRNA accumulation is dependent of a functional SAR defense pathway, whereas expression of PDF1.2 mRNA during B. cinerea infection is linked to a functional ETR1 and COI1. Furthermore, nahG and npr1-1 plants accumulated more PDF1.2 mRNA 2 d after inoculation with B. cinerea than the wild-type Columbia (Col-0) plants, confirming that defective SAR signaling provoked altered sensitivity to JA/ethylene signaling (Fig. 5).
Figure 5.
BABA-mediated induction of defense genes in Arabidopsis signal transduction mutants infected with B. cinerea. Arabidopsis nahG, npr1-1, coi1-1, and etr1-1 plants were soil drenched with water or 30 μg mL−1 BABA 1 d prior to inoculation. For each treatment, total RNA was extracted from nine infected leaves harvested from three different plants. Leaves were collected 48 h after inoculation with potato dextrose broth (PDB; −) or PDB containing spores of B. cinerea strain BMM1 (+). RNA blots were hybridized with PR-1 and PDF1.2 probes. Ethidium bromide staining of the RNA gel (rRNA) was used to show equal loading.
DISCUSSION
The effect of BABA on necrotrophic pathogens is still largely unknown. We have shown here that this chemical protects Arabidopsis plants against two different races of the gray mold fungus B. cinerea. The infection process was not completely stopped by BABA, but the disease incidence was clearly reduced, leading to smaller lesions 3 d after inoculation (Fig. 1). The nahG lines and the npr1-1 mutants were not protected by BABA, indicating that SA and NPR1 are involved in the BABA-mediated protection of Arabidopsis against B. cinerea. This also demonstrates that a direct antibiotic effect of BABA on B. cinerea can be excluded. The increased susceptibility of nahG and npr1 plants toward infection by B. cinerea compared with control plants is in contrast to previous observation made by Thomma et al. (1998), where no differences in infection levels between the mutants and the wild type were observed. This discrepancy in the results might be due to the fact that in our experiments the plants were kept in constant high air humidity, thus strongly favoring the infection process. This situation might lead to a greater disadvantage for plants such as nahG and npr1 impaired in defense mechanisms. It is interesting that coi1-1 and etr1-1 mutants, defective in the JA and ethylene pathways, respectively, were protected at a level similar to the wild-type control Col-0. In agreement with previous results (Thomma et al., 1998, 1999), mutants defective in the JA/ethylene-dependent defense responses are more sensitive than wild types to B. cinerea infection. Thus, BABA can inhibit infection even in mutants highly sensitive to B. cinerea, confirming the independence of BABA-mediated defense mechanisms on JA and ethylene signaling. The dependence of BABA on the SA pathway was further evaluated in Arabidopsis plants treated with BTH, an activator of the SAR signal transduction pathway (Lawton et al., 1996). BTH-treated Arabidopsis plants showed a reduction of the size of the necroses on both strains tested (Fig. 3). This is in contrast with observations on tobacco where BTH does not induce resistance against B. cinerea (Friedrich et al., 1996).
The action of BABA against B. cinerea is not based on a direct fungitoxic effect (see above; Zimmerli et al., 2000). Rather, it seems to act like an inducer of plant resistance mechanisms. We have, therefore, investigated the effect of BABA on the expression of defense-related genes. We have given a special attention to JA/ethylene-dependent genes, since they are associated with resistance against necrotrophic pathogens such as A. brassicicola and B. cinerea (Thomma et al., 1998, 1999). However, unlike the effect of BABA on the potentiation of PR-1 gene after infection with virulent bacteria (Zimmerli et al., 2000), accumulation and potentiation of PDF1.2 gene was not observed in BABA-treated plants after B. cinerea inoculation. By contrast, potentiation of PR-1 transcript accumulation was observed during B. cinerea infection (Fig. 4). As a consequence, BABA stimulates the SA-dependent, but not the JA/ethylene-dependent, signaling pathway in Arabidopsis infected with widely diverse pathogens.
The expression of the PDF1.2 gene in response to B. cinerea infection was enhanced in plants with a defective SA-dependent signaling pathway (Fig. 5). On the other hand, potentiation of PR1 mRNA accumulation was stronger in mutants defective in the JA/ethylene-dependent signaling pathway. However, in this case the up-regulation of PR-1 gene expression is probably due to a more extensive fungal colonization. Since the same expression level of PDF1.2 gene was observed in water- and in the less-infected BABA-treated Col-0 plants, the level of PDF1.2 mRNA accumulation did not reflect the rate of fungal colonization. Up-regulation of PDF1.2 gene expression has also been observed in mutants defective in the SA-dependent signaling after inoculation with A. brassicicola (Penninckx et al., 1996) or treatments with rose bengal or methyl JA (Gupta et al., 2000). All these data indicate that SA-dependent signaling interferes with JA/ethylene-dependent defense responses.
BABA enhances resistance through potentiation of SA-dependent defense responses leading to restriction of B. cinerea growth and spread. A. brassicicola and B. cinerea infection of water-treated Arabidopsis leads to a weak and delayed PR-1 mRNA accumulation, whereas PDF1.2 mRNA is strongly induced (Thomma et al., 1998; this work). Furthermore, coi1-1 and etr1-1 mutants defective in the JA and ethylene signaling pathway, respectively, are more sensitive to these two necrotrophs and fail to express PDF1.2 upon infection (Thomma et al., 1998; this work). As a consequence, SA- and JA/ethylene-dependent defense responses are involved in protection to B. cinerea. The question arises why B. cinerea fails to induce a strong SAR response. B. cinerea might down-regulate the SA-dependent signaling pathway or, alternatively, fail to induce it due to a defective recognition or signal transduction, leading to a delayed expression of PR-1 gene. In such a situation, effective defense would depend more on the JA/ethylene pathway. BABA might counteract or shortcut such inhibitory mechanisms and allow the expression of the SA-dependent signaling pathway after B. cinerea infection. In a similar manner, it was demonstrated recently that necrotrophs can exploit a host defense mechanism such as HR for their pathogenicity (Govrin and Levine, 2000). In an alternate manner, induction of PR-1 gene results from tissue damage inflicted by B. cinerea and this is potentiated by BABA. A small necrosis would be sufficient to induce PR-1 gene expression in BABA-treated plants, whereas in water-treated controls, a larger lesion would be required for the expression of SA-dependent genes.
These observations document the action of the chemical inducer BABA against necrotrophic pathogens. BABA acts by potentiation of a normally underexpressed pathway. Our results also suggest how a pathogen might modulate the network of defense pathways to its advantage.
MATERIALS AND METHODS
Biological Material
The transgenic Arabidopsis line harboring the nahG gene (Lawton et al., 1995) was obtained from J. Ryals (Novartis, Research Triangle Park, NC). The Col-0 ecotype mutants npr1-1, etr1-1, and coi1-1 were provided by X. Dong (Duke University, Durham, NC), the Nottingham Arabidopsis Stock Center, and J. Turner (University of East Anglia, Norwich, UK), respectively. Arabidopsis accessions Col-0 were obtained from Lehle Seeds (Round Rock, TX). Plants were grown on a pasteurized soil mix of commercial potting soil:perlite (3:1) at a 22°C day/18°C night temperature with 12 h of light per 24 h. Botrytis cinerea (strains BMM1, isolated from Pelargonium zonale; and IMI169558, International Mycology Institute, Kew, UK) were grown on 19.5 g L−1 potato dextrose agar (Difco, Detroit) at 20°C for 10 d. The conidia were collected and suspended in sterile PDB (12 g L−1; Difco).
Chemical Treatment and Plant Inoculation
Chemicals were all dissolved in water and applied as soil drench. Evaluation of symptoms was done on 30 5- to 6-week-old soil-grown Arabidopsis plants. Treatments were performed 1 d before inoculation with B. cinerea. The three smallest leaves (nos. 5, 6, and 7 from the apex) able to support two 3-μL droplets of a suspension of 2.5 × 104 conidia mL−1 in PDB (12 g L−1) were used for inoculation. Droplets were deposited on fixed positions left and right from the midvein.
For the time course experiments and analysis of defense genes expression in different genotypes, soil drench treatment of 30 μg mL−1 BABA was done 1 d before inoculation. Inoculation time corresponds to the time 0. Tissue was harvested at the times indicated for RNA extraction and analysis. Inoculation was performed by spraying a suspension of 2.5 × 104 conidia mL−1 in PDB (12 g L−1). For all the experiments, each time point represents a pool of nine leaves coming from three different plants. To ensure infection, inoculated plants were kept at 100% relative humidity during all the infection process, at 19°C/17°C day/night temperatures with 12 h of light per 24 h.
Monitoring Susceptibility to B. cinerea
Susceptibility to B. cinerea was evaluated by macroscopic observation of the diameter of the necroses. Since B. cinerea hyphae developed concentrically, results were expressed as necrosis size in square millimeters.
RNA Extraction and Analysis
RNA was isolated from frozen tissue samples as described previously (Zimmerli et al., 2000). Total RNA samples (6 μg) were separated through formaldehyde-agarose gels and were blotted to a Nylon membrane (Hybond-N, Amersham Pharmacia Biotech, Little Chalfont, UK). 32P-Labeled cDNA probes of PR genes PR-1 and PDF1.2 were synthesized by random priming of isolated insert DNA using the random primers DNA labeling system (RadPrime DNA Labeling System, Life Technologies, Merelbeke, Belgium). Equal loading of samples was shown by ethidium bromide staining of the rRNA.
ACKNOWLEDGMENTS
We thank Drs. C. Nawrath and G. Jakab for critical reading of the manuscript and helpful discussions. We are grateful to Drs. B.P.H.J. Thomma and W.F. Broekaert for providing B. cinerea strain IMI 169558.
Footnotes
This work was supported by the Swiss National Foundation (grant nos. 3100–049279.96 to B.M.M. and 3100–055662.98 J.-P.M.) and by the office fédéral de l'éducation et de la science (grant no. 96.0233 to B.M.M. and J.-P.M.).
LITERATURE CITED
- Becker J, Kempf R, Jeblick W, Kauss H. Induction of competence for elicitation of defense responses in cucumber hypocotyls requires proteasome activity. Plant J. 2000;21:311–316. doi: 10.1046/j.1365-313x.2000.00677.x. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong XN. Characterization of an Arabidopsis mutant that is non-responsive to inducers of systemic acquired resistance. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich L, Lawton K, Ruess W, Masner P, Specker N, Rella MG, Meier B, Dincher S, Staub T, Uknes S. A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J. 1996;10:61–71. doi: 10.1046/j.1365-313x.1996.10010071.x. [DOI] [PubMed] [Google Scholar]
- Genoud T, Métraux JP. Crosstalk in plant cell signaling: structure and function of the genetic network. Trends Plant Sci. 1999;4:503–507. doi: 10.1016/s1360-1385(99)01498-3. [DOI] [PubMed] [Google Scholar]
- Govrin EM, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 2000;10:751–757. doi: 10.1016/s0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- Gupta V, Willits MG, Glazebrook J. Arabidopsis thaliana EDS4 contributes to salicylic acid (SA)-dependent expression of defense responses: evidence for inhibition of jasmonic acid signaling by SA. Mol Plant-Microbe Interact. 2000;13:503–511. doi: 10.1094/MPMI.2000.13.5.503. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G, Cottier V, Toquin V, Rigoli G, Zimmerli L, Métraux J-P, Mauch-Mani B. β-Aminobutyric acid-induced resistance in plants. Eur J Plant Pathol. 2001;107:29–37. [Google Scholar]
- Kauss H, Franke R, Krause K, Conrath U, Jeblick W, Grimmig B, Matern U. Conditioning of parsley (Petroselinum crispum L.) suspension cells increases elicitor-induced incorporation of cell wall phenolics. Plant Physiol. 1993;102:459–466. doi: 10.1104/pp.102.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Jeblick W. Pretreatment of parsley suspension cultures with salicylic acid enhances spontaneous and elicited production of H2O2. Plant Physiol. 1995;108:1171–1178. doi: 10.1104/pp.108.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Theisingerhinkel E, Mindermann R, Conrath U. Dichloroisonicotinic and salicylic acid, inducers of systemic acquired resistance, enhance fungal elicitor responses in parsley cells. Plant J. 1992;2:655–660. [Google Scholar]
- Keogh RC, Deverall BJ, McLeod S. Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Trans Br Mycol Soc. 1980;74:329–333. [Google Scholar]
- Kessmann H, Staub T, Hofmann C, Maetzke T, Herzog J, Ward E, Uknes S, Ryals J. Induction of systemic acquired resistance in plants by chemicals. Annu Rev Phytopathol. 1994;32:439–459. doi: 10.1146/annurev.py.32.090194.002255. [DOI] [PubMed] [Google Scholar]
- Lawton K, Weymann K, Friedrich L, Vernooij B, Uknes S, Ryals J. Systemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Mol Plant-Microbe Interact. 1995;8:863–870. doi: 10.1094/mpmi-8-0863. [DOI] [PubMed] [Google Scholar]
- Lawton KA, Friedrich L, Hunt M, Weymann K, Delaney T, Kessmann H, Staub T, Ryals J. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J. 1996;10:71–82. doi: 10.1046/j.1365-313x.1996.10010071.x. [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Naylor G, Warner SAJ, Sugars JM, White RF, Draper J. Salicylic acid potentiates defense gene expression in tissue exhibiting acquired resistance to pathogen attack. Plant J. 1996;9:559–571. [Google Scholar]
- Penninckx IAMA, Eggermont K, Terras FR, Thomma BP, De Samblanx GW, Buchala A, Métraux JP, Manners JM, Broeckaert WF. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol. 1998;1:404–411. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- Sticher L, Mauch-Mani B, Métraux J-P. Systemic acquired resistance. Annu Rev Phytopathol. 1997;35:235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- Summermatter K, Sticher L, Métraux JP. Systemic responses in Arabidopsis thaliana infected and challenged with Pseudomonas syringae pv syringae. Plant Physiol. 1995;108:1379–1385. doi: 10.1104/pp.108.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Tierens KFMJ, Broekaert WF. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999;121:1093–1101. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulke O, Conrath U. Salicylic acid has a dual role in the activation of defense-related genes in parsley. Plant J. 1998;14:35–42. doi: 10.1046/j.1365-313X.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J. Acquired resistance in Arabidopsis. Plant Cell. 1992;4:645–656. doi: 10.1105/tpc.4.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli L, Jakab G, Métraux J-P, Mauch-Mani B. Potentiation of pathogen-specific defense mechanisms in Arabidopsis by β-aminobutyric acid. Proc Natl Acad Sci USA. 2000;97:12920–12925. doi: 10.1073/pnas.230416897. [DOI] [PMC free article] [PubMed] [Google Scholar]