Abstract
Reactive oxygen species (ROS) production by the phagocyte NADPH oxidase is essential for host defenses against pathogens. ROS are very reactive with biological molecules such as lipids, proteins and DNA, potentially resulting in cell dysfunction and tissue insult. Excessive NADPH oxidase activation and ROS overproduction are believed to participate in disorders such as joint, lung, vascular and intestinal inflammation. NADPH oxidase is a complex enzyme composed of six proteins: gp91phox (renamed NOX2), p22phox, p47phox, p67phox, p40phox and Rac1/2. Inhibitors of this enzyme could be beneficial, by limiting ROS production and inappropriate inflammation. A few small non-peptide inhibitors of NADPH oxidase are currently used to inhibit ROS production, but they lack specificity as they inhibit NADPH oxidase homologues or other unrelated enzymes. Peptide inhibitors that target a specific sequence of NADPH oxidase components could be more specific than small molecules. Here we review peptide-based inhibitors, with particular focus on a molecule derived from gp91phox/NOX2 and p47phox, and discuss their possible use as specific phagocyte NADPH oxidase inhibitors.
Keywords: NADPH-oxidase, ROS, Neutrophils, Peptide inhibitors, gp91phox, NOX2, p22phox, p47phox
Introduction
Reactive oxygen species (ROS) production by phagocytes such as neutrophils, monocytes and macrophages are essential for host defenses against bacteria and fungi, by reacting with pathogen DNA, proteins and lipids [1–3]. ROS are produced in large quantities when phagocytes are stimulated by pro-inflammatory agents or by particles such as bacteria. Superoxide anion (O·−2), the precursor of the other ROS species, is first produced by the enzyme NADPH oxidase. Superoxide anion (O·−2) is unstable and gives rise to hydrogen peroxide (H2O2), hydroxyl radical (OH·) and hypochlorous acid (HOCl) [2]. The vital role of phagocyte NADPH oxidase in host defenses against pathogens is illustrated by a human genetic disorder called chronic granulomatous disease, which is associated with life-threatening recurrent bacterial and fungal infections [3, 4]. However, excessive ROS production by neutrophils could result in ROS leakage from the phagosome and is believed to cause direct tissue insult in a broad range of inflammatory diseases, including rheumatoid arthritis, inflammatory bowel diseases, acute respiratory distress syndrome, sepsis, diabetic complications, cardiovascular disease, ischemic tissue injury and neurodegenerative diseases [5, 6]. Pharmacological inhibition of the phagocyte NADPH oxidase might therefore be beneficial in these disorders. To develop specific inhibitors of the phagocyte NADPH oxidase, one strategy is to use peptide-based inhibitors derived from specific subunits of the enzyme. In this review we will first describe the structure of the NADPH oxidase and its activation, underlining the advantages of the cell free system for assessing peptide inhibitors. Finally, we will describe existing NADPH oxidase inhibitors, with a special focus on peptide inhibitors and the use of cell-penetrating peptides for delivery to intact cells.
Structure of the phagocyte NADPH oxidase and its homologues
The active phagocyte NADPH oxidase is a multicomponent enzyme complex composed of six proteins: p22phox (phox: phagocyte oxidase), gp91phox/NOX2, p47phox, p67phox, p40phox and the small G-protein Rac1 or Rac2 [7–9]. In resting cells, p47phox, p67phox, p40phox and Rac1/2 are localized in the cytosol, whereas p22phox and gp91phox are located in the plasma membrane and membranes of specific granules. Together, these two membranous proteins form the flavocytochrome b558. When cells are activated, the cytosolic components migrate to the membranes where they associate with the membrane-bound components to assemble the catalytically active oxidase [7, 8]. The flavocytochrome b558 is the central membrane-bound component of NADPH oxidase with catalytic activity [9]. Human cytochrome b558 is composed of a 1:1 complex between a glycosylated 91-kDa protein subunit (gp91phox) of 570 amino acids and a non-glycosylated 22-kDa subunit (p22phox) of 195 amino acids. Flavocytochrome b558 contains one FAD and two hemes, and forms the NADPH oxidase electron transfer chain [9]. Also it serves as the central docking site for the cytosolic components via numerous interaction sites [7, 8].
In resting cells the cytosolic components p47phox, p67phox and p40phox may form a complex or remain free in the cytosol. Human p47phox is a protein composed of 390 amino acids that contains one phox homology (PX) domain in the N-terminal region, two src-homology 3 (SH3) domains, a sequence called the auto-inhibitory region (AIR) and a proline-rich region. P47phox binds to the flavocytochrome b558 during NADPH oxidase activation and is the subunit responsible for transporting the cytosolic complex (p47phox-p67phox-p40phox) from the cytosol to the membrane during oxidase activation, and for organizing the NADPH oxidase complex. Human p67phox is composed of 526 amino acids, with four tetratricopeptide-rich regions, two SH3 domains, a PB1/PC domain and a proline-rich region [7]. During activation, p67phox interacts with Rac1/2 and flavocytochrome b558, and can regulate its catalytic activity via a sequence called the activation domain [10]. Human p40phox is a 339-amino-acid protein that was initially identified through its binding to p67phox [11]. It contains one PX domain, one SH3 domain and one PB1/PC domain. Rac2 is the most abundant rac protein in human neutrophils, while rac1 is the most abundant rac protein in monocytes and macrophages. In resting cells, Rac1 and Rac2 bind GDP and are part of a tight cytosolic complex with RhoGDI.
Five homologues of gp91phox have been identified in human tissues and in various cell types [6, 12]. They are called NOX1 (NOX for NADPH oxidase), NOX2 (formerly gp91phox), NOX3, NOX4 and NOX5. Two homologues of gp91phox and myeloperoxidase (MPO) have been identified and are named Duox, for dual oxidase (Duox1 and Duox2) [13, 14]. These new findings concerning NOX family proteins suggest that ROS might be involved in several cellular functions such as local tissue-specific bactericidal activity (in colon and lung) and intracellular signaling. Concerning their regulation, NOX1, NOX3 and NOX4, like NOX2, are associated with p22phox and require Rac1 for their activation [12]. NOX1 and NOX3 are regulated by two homologues of p47phox and p67phox, called NOX organizer 1 (NOXO1) and NOX activator 1 (NOXA1) [15–18]. Like p47phox, NOXO1 ‘organizes’ the assembly of the fully active NOX1 enzyme complex at the plasma membrane.
Activation of the phagocyte NADPH oxidase in intact cells versus cell-free systems
Activation of NADPH oxidase in phagocytes can be induced by a large number of inflammatory stimuli such as opsonized bacteria, opsonized zymosan, bacterial formylated peptides such as formyl-Met-Leu-Phe (fMLF), C5a and platelet-activating factor, and also by pharmacological agents such as calcium ionophores A23127, ionomycin and PKC activators such as phorbol myristate acetate (PMA) [19]. In intact cells, NADPH oxidase activation is accompanied by phosphorylation of p47phox, p67phox, p40phox, p22phox and gp91phox, along with several protein-protein interactions [20–24]. P47phox is phosphorylated on multiple sites located in its carboxy-terminal portion, including serines 303–379, which play a central role in NADPH oxidase activation and regulation [19]. In human neutrophils, various protein kinases have been implicated in the activation of NADPH oxidase, among which the PKC and MAP kinase families appear to play a major role [19]. Parallel to these events, Rac1/2 replace GDP by GTP and translocate to the plasma membranes (Fig. 1). During neutrophil activation, 10–20 % of cytosolic NADPH oxidase components migrate to the plasma membrane, where they bind to flavocytochrome b558 [7–9].
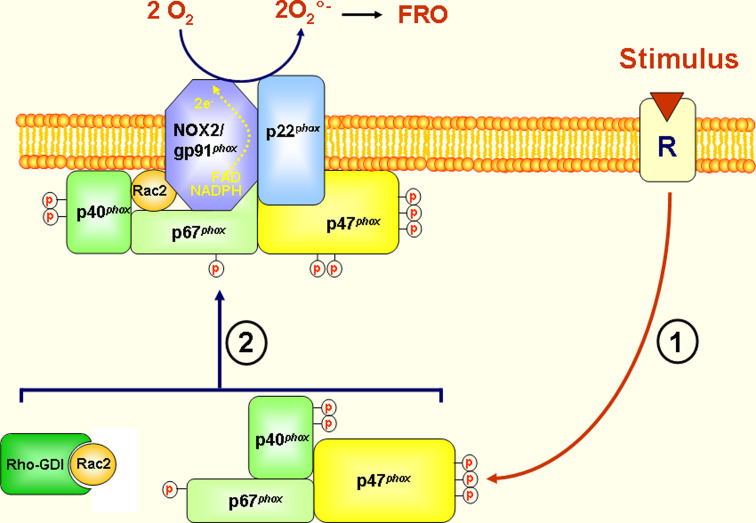
Fig. 1.
NADPH oxidase/NOX2 activation in intact cells. The active phagocyte NADPH oxidase is a multicomponent enzyme complex composed of six proteins: p22phox (phox: phagocyte oxidase), gp91phox/NOX2, p47phox, p67phox, p40phox and the small G-protein Rac1 or Rac2. In resting cells p47phox, p67phox, p40phox and Rac1/2 are localized in the cytosol, and p22phox and gp91phox are at the plasma membrane and membranes of specific granules to form flavocytochrome b558. When cells are activated by a receptor-dependent agonist, the cytosolic components are phosphorylated and migrate to the membranes, where they associate with the membrane-bound components to assemble the catalytically active oxidase (FRO=ROS)
NADPH oxidase can also be activated in a cell-free system (Fig. 2) containing cytosol and plasma membranes from resting cells (neutrophils or monocytes or macrophages) in the presence of Mg++, GTP and an anionic amphiphile, such as arachidonic acid or sodium/lithium dodecyl sulfate (SDS, LDS) [25, 26]. Anionic amphiphiles are believed to mimic phosphorylation by providing negative charges. Active NADPH oxidase can also be reconstituted from recombinant or highly purified cytochrome b558, p47phox, p67phox and Rac proteins. However, p40phox is not required for NADPH oxidase activation in a cell-free system. Use of this cell-free system led to major advances in enzymologic characterization of the enzyme and identification of NADPH oxidase components, and has been used to assess the effect of various peptides and macromolecules on NADPH oxidase activation, thus allowing the identification of several NADPH oxidase peptide inhibitors.
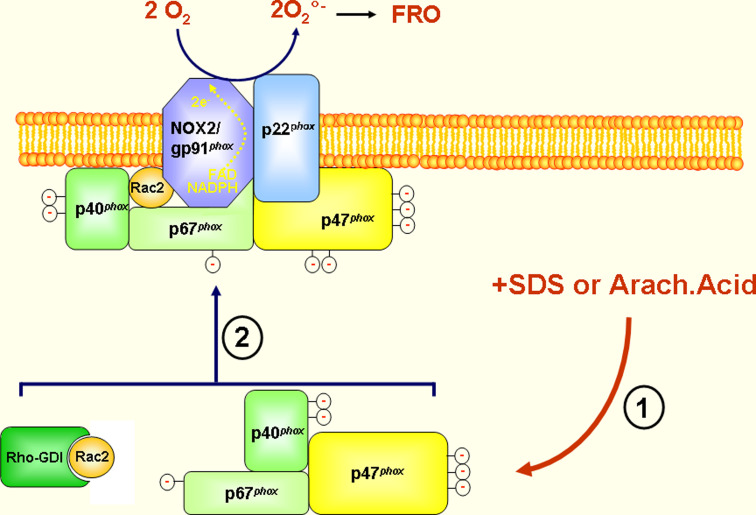
Fig. 2.
NADPH oxidase/NOX2 activation in the cell-free system. The phagocyte NADPH oxidase can be activated in a reaction mix containing cytosol, which provides cytosolic components (p47phox, p67phox and Rac1/2), plasma membranes (which provide cytochrome b558) isolated from resting cells (neutrophils, monocytes or macrophages), Mg++, GTP and an anionic amphiphile, such as arachidonic acid or sodium/lithium dodecyl sulfate (SDS, LDS). Although p40phox is present in the cytosol and in the complex, this protein is not necessary for NADPH oxidase activation in the cell-free system (FRO=ROS)
Phagocyte NADPH oxidase inhibitors
Non peptide inhibitors
The best-known inhibitor of NADPH oxidase is diphenylene iodonium (DPI), which directly inhibits the activity of gp91phox/NOX2 [27]. This molecule targets the FAD binding sequence found in other flavoproteins and is therefore not specific for NOX2. Indeed, DPI inhibits not only all NOXs and DUOXs, but also NO synthase and other flavoproteins [28]. The second NADPH oxidase inhibitor is apocynin (4-hydroxy-3-methoxyacetophenone-substituted), a natural molecule structurally related to vanillin [29, 30]. Apocynin is believed to act on p47phox, and its effect requires a peroxidase such as MPO [31]. It might also inhibit NOX1 via NOXO1 in the presence of peroxidase, and also scavenges ROS, making it non-specific for NOX2 [32]. Many other small molecules have been shown to inhibit the phagocyte NADPH oxidase and other NOXs [33–36], but there are currently no specific chemical inhibitors of the phagocyte NADPH oxidase. Peptide inhibitors could be more specific than chemical inhibitors because they are derived from specific subunit sequences. The next part of this review describes existing peptide inhibitors and examines their specificity.
Peptide inhibitors
The cell-free activation system has been used to examine the effect of various macromolecules such as peptides on NADPH oxidase activity. This system notably helped to identify 12 peptides derived from gp91phox/NOX2, 7 peptides derived from p22phox, 13 peptides derived from p47phox, 6 peptides derived from Rac1 and a few peptides not derived from NADPH oxidase components [37]. As p22phox is a protein common to NOX1, NOX2, NOX3 and NOX4. As Rac1, in addition to its requirement for NOX1, NOX2, NOX3 and NOX4, is involved in a multitude of other functions. Peptides derived from p22phox and Rac1 cannot be specific for NOX2. For this reason, p22phox- and Rac1-derived peptides will not be examined here. To our knowledge no peptide sequences derived from p67phox or p40phox have yet been shown to inhibit the phagocyte NADPH oxidase. P67phox sequences such as the activation domain [10] might be used to develop new competitive inhibitory peptides. The reason for the lack of p40phox-derived peptides may be that p40phox is not required for NADPH oxidase activation in the cell-free system, making it more difficult to assess the effect of p40phox-derived peptides in intact cells.
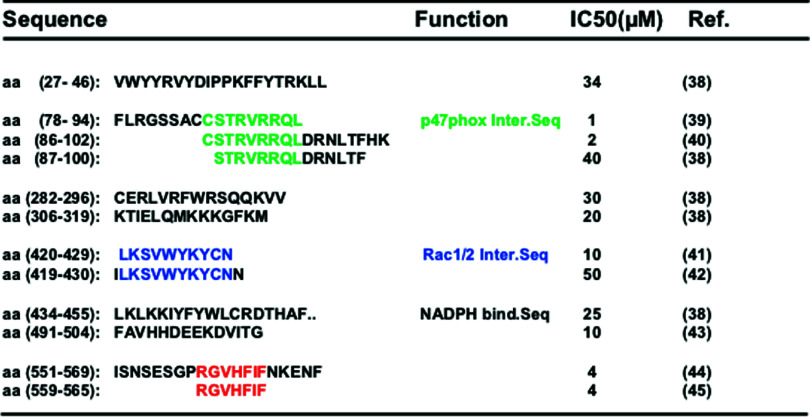
Peptide inhibitors derived from gp91phox/NOX2 are located throughout the protein, from the N-terminal to C-terminal (Table 1). The peptide concentrations that inhibit 50 % of the NADPH oxidase activity in cell-free systems (IC50) range from 1 to 50 μM for peptides that target three main sequences: a p47phox interaction sequence, a Rac1/2 interaction sequence and the NADPH binding consensus sequence. The NOX2 carboxy-terminal peptide RGVHFIF (amino acids 559–565) inhibits NADPH oxidase activation in the cell-free system, but its mechanism of action remains unknown. Most NOX-derived peptide inhibitors also lie in, or share homology with, sequences of NOX1, NOX3 or NOX4, and cannot thus be specific for NOX2. The reason for this lack of specificity is that these peptides were characterized before the discovery of the new NOX2 homologues. However, other NOX2 sequences could be used to develop new specific peptide inhibitors.
Table 1.
Peptide inhibitors derived from human gp91phox/NOX2
IC50 corresponds to the peptide concentration required to achieve 50 % NADPH oxidase inhibition in the cell-free system. The colors correspond to different sequences with different functions
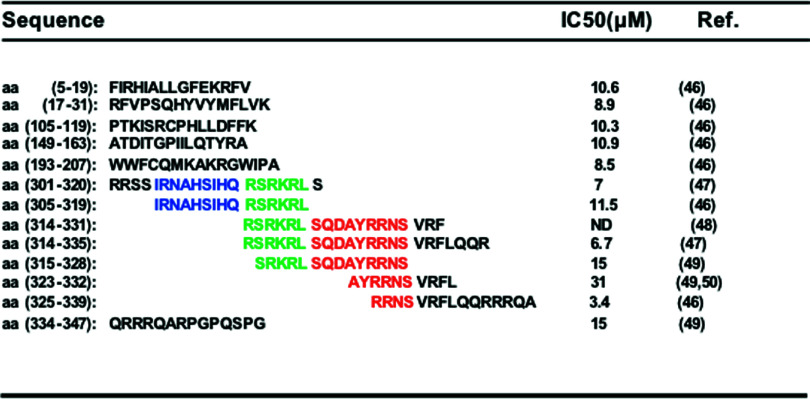
Peptide inhibitors derived from p47phox are also located throughout the protein (Table 2). In the cell-free system these peptides have IC50 s of between 3 and 31 μM. They are derived from the PX domain, the SH3 domain, the AIR domain and the PRR. These peptides could exert their effect by inhibiting the binding of p47phox to membrane phospholipids or to p22phox or p67phox, or alternatively by inhibiting p47phox phosphorylation by inhibiting protein kinase activity. The PX-, SH3- and PRR-derived peptide inhibitors share homology with sequences of the p47phox homologue NOXO, except for AIR-derived peptides, which are specific for p47phox and could thus specifically inhibit NOX2.
Table 2.
Peptide inhibitors derived from human p47phox
IC50 corresponds to the peptide concentration required to achieve 50 % NADPH oxidase inhibition in the cell-free system. The colors correspond to sequences found in different peptides
Delivering NADPH oxidase peptide inhibitors into living cells
The main problem with the use of peptides to inhibit NADPH oxidase in intact living cells is their inability to cross the plasma membrane lipid bilayer. The use of cell-penetrating peptides (CPP) or protein transduction domains (PTD) is an attractive solution to this problem [51–53]. The most widely used CPPs are derived from the human immunodeficiency virus (HIV1) transactivator protein TAT (amino acid sequence 47–59: YGRKKRRQRRRPP), the Drosophila transcription factor antennapedia protein, also called penetratin (amino acid sequence 43–58: RQIKIWFQNRRMKWKK), a peptide called transportan (GWTLNSAGYLLGKINLKALAALAKKIL), a polyarginine peptide called R9 (RRRRRRRRR) and the herpes simplex virus (HSV) type 1 protein VP22. Although the exact mechanism of peptide transduction is not entirely clear, these peptides can deliver biologically active proteins, DNA and RNA across plasma membranes [51–53]. The peptide most widely used for neutrophils is TAT, which has been shown to localize in the cytosol of human neutrophils [54]. Several NADPH oxidase peptide inhibitors coupled to the TAT peptide have been shown to inhibit ROS production in vitro and in vivo [42, 55–57]. The most widely used is gp91ds-tat, corresponding to the TAT sequence and the amino acid sequence 85–93 of the mouse gp91phox/NOX2 (RKKRRQRRR-CSTRIRRQL) [55]. Although this peptide does not inhibit ROS production very efficiently in neutrophils (35 % inhibition at the highest peptide concentration of 100 μM), it is widely used as a NOX2 inhibitor in vitro and in animal models [58, 59]. It is noteworthy that this gp91phox/NOX2 sequence is conserved in NOX1 and NOX4, meaning that it is not specific for the phagocyte NADPH oxidase/NOX2.
Another inhibitory peptide of NADPH oxidase in cell-free systems was coupled to TAT peptide and was shown to inhibit NADPH oxidase in neutrophils. This peptide contains the Rac interaction site within NOX2 and corresponds to amino acids 419–430 (ILKSVWYKYCNN) [42]. When used at 20 μM, this peptide inhibited ROS production in neutrophils stimulated by fMLF or PMA. This NOX2-Rac binding domain is also conserved in NOX1 and NOX3 but not in NOX4, NOX5 or DUOXs, making it selective for NOX1, NOX2 and NOX3.
Other strategies have been used to inhibit the phagocyte NADPH oxidase in intact cells. One was designed to inhibit NOX2 hyperactivation by targeting a TNFα-induced p47phox phosphorylation site [56]. This peptide, containing the Ser345 sequence (amino acids 334–347) and a TAT sequence, inhibited NADPH oxidase hyper-activation induced by TNFα in human neutrophils and also hyperactivation of neutrophils isolated from synovial fluid of rheumatoid arthritis patients, while preserving the physiological ability of bacterial N-formyl peptide to activate neutrophils [56].
Another strategy was designed to specifically target p47phox expression. A cell-permeable dominant negative peptide (DN-Ets-1-TAT) derived from Ets-1, a transcriptional regulator of p47phox, was found to lower angiotensin II-induced p47phox expression and ROS production in vitro, and also to attenuate medial hypertrophy of the thoracic aorta in mice [57]. This strategy could be used to target p47phox expression in monocytes, macrophages or other cells which continuously express p47phox.
Conclusion
Excessive ROS production by the phagocyte NADPH oxidase NOX2 has been implicated in many inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease, acute respiratory distress syndrome, sepsis, diabetic complications, cardiovascular diseases, ischemic tissue injury and neurodegenerative diseases, in which ROSs are believed to cause direct tissue injury, thus contributing to inflammatory reactions. Pharmacological targeting of the phagocyte NADPH oxidase might therefore be beneficial as an anti-inflammatory strategy. Peptide-based inhibitors show promise, provided the target sequence is specific for a particular phagocyte NADPH oxidase component. The best targets for NADPH oxidase inhibitors are gp91phox/NOX2, p47phox and p67phox, because their expression is more specific for phagocytes and some sequences are specific for these proteins. The p47phox-AIR sequence is not found in its homologue NOXO, making AIR-derived peptides more specific than other sequences. This tends to rule out the use of peptides derived from p22phox and Rac1/2, which are components common to all NOXs.
Peptide inhibitors could be more specific than small-molecule inhibitors. The main problem with inhibitory peptides is their cell delivery, but this can now be achieved by coupling to cell-permeable peptides such as TAT or antennapedia peptides. Several cell-permeant peptide antagonists of NOX2 have already been shown to inhibit ROS production in vitro and in vivo. The other problem with peptides is their stability in live organisms. This problem can be solved by the use of peptides synthesized with amino acid stereoisomers. Subcutaneous or intravenous infusion of NOX2-inhibitory peptides has been shown to attenuate vascular disorders in experimental animals. Oral administration is unlikely to provide efficient peptide delivery because of the hostile gastrointestinal environment. Identification of new stable peptides and new delivery strategies such as nanotechnologies could improve the efficiency of peptide inhibitors in vivo.
Acknowledgments
This work was supported by grants from Agence Nationale de la Recherche (ANR), Arthritis Fondation Courtin, INSERM and CNRS.
Conflict of interest
The authors have no competing interests to declare.
References
- 1.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 2.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 3.El-Benna J, Dang PM, Gougerot-Pocidalo MA, Elbim C. Phagocyte NADPH oxidase: a multicomponent enzyme essential for host defenses. Arch Immunol Ther Exp (Warsz) 2005;53:199–206. [PubMed] [Google Scholar]
- 4.Roos D, De Boer M, Kuribayashi F, Meischl C, Weening RS, Segal AW, Ahlin A, Nemet K, Hossle JP, Bernatowska-Matusskiewicz E, middleton-Price H. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87:1663–1681. [PubMed] [Google Scholar]
- 5.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/S0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 6.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 9.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han CH, Freeman JL, Lee T, Motalebi SA, Lambeth JD. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox) J Biol Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- 11.Matute JD, Arias AA, Dinauer MC, Patino PJ. p40phox: the last NADPH oxidase subunit. Blood Cells Mol Dis. 2005;35:291–302. doi: 10.1016/j.bcmd.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 13.Dupuy C, Ohayon R, Valent A, Nol-Hudson MS, Dème D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 14.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 15.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 16.Bánfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 17.Kroviarski Y, Debbabi M, Bachoual R, Périanin A, Gougerot-Pocidalo MA, El-Benna J, Dang PM. Phosphorylation of NADPH oxidase activator 1 (NOXA1) on serine 282 by MAP kinases and on serine 172 by protein kinase C and protein kinase A prevents NOX1 hyperactivation. FASEB J. 2010;24:2077–2092. doi: 10.1096/fj.09-147629. [DOI] [PubMed] [Google Scholar]
- 18.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 19.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 20.El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regier DS, Waite KA, Wallin R, McPhail LC. A phosphatidic acid-activated protein kinase and conventional protein kinase C isoforms phosphorylate p22(phox), an NADPH oxidase component. J Biol Chem. 1999;274:36601–36608. doi: 10.1074/jbc.274.51.36601. [DOI] [PubMed] [Google Scholar]
- 22.Raad H, Paclet MH, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PM, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. FASEB J. 2009;23:1011–1022. doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang PM, Morel F, Gougerot-Pocidalo MA, Benna JE. Phosphorylation of the NADPH oxidase component p67(PHOX) by ERK2 and P38MAPK: selectivity of phosphorylated sites and existence of an intramolecular regulatory domain in the tetratricopeptide-rich region. Biochemistry. 2003;42:4520–4526. doi: 10.1021/bi0205754. [DOI] [PubMed] [Google Scholar]
- 24.Bouin AP, Grandvaux N, Vignais PV, Fuchs A. p40(phox) is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase. Implication of a protein kinase C-type kinase in the phosphorylation process. J Biol Chem. 1998;273:30097–30103. doi: 10.1074/jbc.273.46.30097. [DOI] [PubMed] [Google Scholar]
- 25.Bromberg Y, Pick E. Activation of NADPH-dependent superoxide production in a cell-free system by sodium dodecyl sulfate. J Biol Chem. 1985;260:13539–13545. [PubMed] [Google Scholar]
- 26.Dagher MC, Pick E. Opening the black box: lessons from cell-free systems on the phagocyte NADPH-oxidase. Biochimie. 2007;89:1123–1132. doi: 10.1016/j.biochi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Hancock JT, Jones OT. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987;242:103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuehr DJ, Fasehun OA, Kwon NS, Gross SS, Gonzalez JA, Levi R, Nathan CF. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. 1991;5:98–103. doi: 10.1096/fasebj.5.1.1703974. [DOI] [PubMed] [Google Scholar]
- 29.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 30.Stefanska J, Pawliczak R (2008) Apocynin: molecular aptitudes. Mediators Inflamm 2008:106507. Article ID 106507. doi:10.1155/2008/106507 [DOI] [PMC free article] [PubMed]
- 31.Ximenes VF, Kanegae MP, Rissato SR, Galhiane MS. The oxidation of apocynin catalyzed by myeloperoxidase: proposal for NADPH oxidase inhibition. Arch Biochem Biophys. 2007;457:134–141. doi: 10.1016/j.abb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 33.Le Cabec V, Maridonneau-Parini I. Complete and reversible inhibition of NADPH oxidase in human neutrophils by phenylarsine oxide at a step distal to membrane translocation of the enzyme subunits. J Biol Chem. 1995;270:2067–2073. doi: 10.1074/jbc.270.5.2067. [DOI] [PubMed] [Google Scholar]
- 34.Diatchuk V, Lotan O, Koshkin V, Wikstroem P, Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem. 1997;272:13292–13301. doi: 10.1074/jbc.272.20.13292. [DOI] [PubMed] [Google Scholar]
- 35.Lambeth JD, Krause KH, Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 36.Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 37.El-Benna J, Dang PM, Périanin A. Peptide-based inhibitors of the phagocyte NADPH oxidase. Biochem Pharmacol. 2010;80:778–785. doi: 10.1016/j.bcp.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Park MY, Imajoh-Ohmi S, Nunoi H, Kanegasaki S. Synthetic peptides corresponding to various hydrophilic regions of the large subunit of cytochrome b558 inhibit superoxide generation in a cell-free system from neutrophils. Biochem Biophys Res Commun. 1997;234:531–536. doi: 10.1006/bbrc.1997.6672. [DOI] [PubMed] [Google Scholar]
- 39.DeLeo FR, Yu L, Burritt JB, Loetterle LR, Bond CW, Jesaitis AJ, Quinn MT. Mapping sites of interaction of p47-phox and flavocytochrome b with random-sequence peptide phage display libraries. Proc Natl Acad Sci USA. 1995;92:7110–7114. doi: 10.1073/pnas.92.15.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu L, Cross AR, Zhen L, Dinauer MC. Functional analysis of NADPH oxidase in granulocytic cells expressing a delta488-497 gp91(phox) deletion mutant. Blood. 1999;94:2497–2504. [PubMed] [Google Scholar]
- 41.Tsuchiya T, Imajoh-Ohmi S, Nunoi H, Kanegasaki S. Uncompetitive inhibition of superoxide generation by a synthetic peptide corresponding to a predicted NADPH binding site in gp91-phox, a component of the phagocyte respiratory oxidase. Biochem Biophys Res Commun. 1999;257:124–128. doi: 10.1006/bbrc.1999.0428. [DOI] [PubMed] [Google Scholar]
- 42.Kao YY, Gianni D, Bohl B, Taylor RM, Bokoch GM. Identification of a conserved Rac-binding site on NADPH oxidases supports a direct GTPase regulatory mechanism. J Biol Chem. 2008;283:12736–12746. doi: 10.1074/jbc.M801010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leusen JH, de Boer M, Bolscher BG, Hilarius PM, Weening RS, Ochs HD, Roos D, Verhoeven AJ. A point mutation in gp91-phox of cytochrome b558 of the human NADPH oxidase leading to defective translocation of the cytosolic proteins p47-phox and p67-phox. J Clin Invest. 1994;93:2120–2126. doi: 10.1172/JCI117207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakanishi A, Imajoh-Ohmi S, Fujinawa T, Kikuchi H, Kanegasaki S. Direct evidence for interaction between COOH-terminal regions of cytochrome b558 subunits and cytosolic 47-kDa protein during activation of an O(2-)-generating system in neutrophils. J Biol Chem. 1992;267:19072–19074. [PubMed] [Google Scholar]
- 45.Rotrosen D, Kleinberg ME, Nunoi H, Leto T, Gallin JI, Malech HL. Evidence for a functional cytoplasmic domain of phagocyte oxidase cytochrome b558. J Biol Chem. 1990;265:8745–8750. [PubMed] [Google Scholar]
- 46.Morozov I, Lotan O, Joseph G, Gorzalczany Y, Pick E. Mapping of functional domains in p47(phox) involved in the activation of NADPH oxidase by “peptide walking”. J Biol Chem. 1998;273:15435–15444. doi: 10.1074/jbc.273.25.15435. [DOI] [PubMed] [Google Scholar]
- 47.Huang J, Kleinberg ME. Activation of the phagocyte NADPH oxidase protein p47(phox). Phosphorylation controls SH3 domain-dependent binding to p22(phox) J Biol Chem. 1999;274:19731–19737. doi: 10.1074/jbc.274.28.19731. [DOI] [PubMed] [Google Scholar]
- 48.Labadia ME, Zu YL, Huang CK. A synthetic peptide containing a predominant protein kinase C site within p47phox inhibits the NADPH oxidase in intact neutrophils. J Leukoc Biol. 1996;59:116–122. doi: 10.1002/jlb.59.1.116. [DOI] [PubMed] [Google Scholar]
- 49.DeLeo FR, Nauseef WM, Jesaitis AJ, Burritt JB, Clark RA, Quinn MT. A domain of p47phox that interacts with human neutrophil flavocytochrome b558. J Biol Chem. 1995;270:26246–26251. doi: 10.1074/jbc.270.44.26246. [DOI] [PubMed] [Google Scholar]
- 50.Nauseef WM, McCormick S, Renee J, Leidal KG, Clark RA. Functional domain in an arginine-rich carboxyl-terminal region of p47phox. J Biol Chem. 1993;268:23646–23651. [PubMed] [Google Scholar]
- 51.Jarver P, Langel U. The use of cell-penetrating peptides as a tool for gene regulation. Drug Discov Today. 2004;9:395–402. doi: 10.1016/S1359-6446(04)03042-9. [DOI] [PubMed] [Google Scholar]
- 52.Brooks H, Lebleu B, Vivès E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Noguchi H, Matsumoto S. Protein transduction technology: a novel therapeutic perspective. Acta Med Okayama. 2006;60:1–11. doi: 10.18926/AMO/30757. [DOI] [PubMed] [Google Scholar]
- 54.Choi M, Rolle S, Wellner M, Cardoso MC, Scheidereit C, Luft FC, Kettritz R. Inhibition of NF-kappaB by a TAT-NEMO-binding domain peptide accelerates constitutive apoptosis and abrogates LPS-delayed neutrophil apoptosis. Blood. 2003;102:2259–2267. doi: 10.1182/blood-2002-09-2960. [DOI] [PubMed] [Google Scholar]
- 55.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res. 2001;31:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 56.Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox-serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–2043. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ni W, Zhan Y, He H, Maynard E, Balschi JA, Oettgen P. Ets-1 is a critical transcriptional regulator of reactive oxygen species and p47(phox) gene expression in response to angiotensin II. Circ Res. 2007;101:985–994. doi: 10.1161/CIRCRESAHA.107.152439. [DOI] [PubMed] [Google Scholar]
- 58.Zhou MS, Hernandez Schulman I, Pagano PJ, Jaimes EA, Raij L. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension. 2006;47:81–86. doi: 10.1161/01.HYP.0000197182.65554.c7. [DOI] [PubMed] [Google Scholar]
- 59.Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]