Abstract
Heparanase is the sole mammalian endoglycosidase that cleaves heparan sulfate, the key polysaccharide of the extracellular matrix and basement membranes. Enzymatic cleavage of heparan sulfate profoundly affects a variety of physiological and pathological processes, including morphogenesis, neovascularization, inflammation, and tumorigenesis. Critical involvement of heparanase in colorectal tumor progression and metastatic spread is widely documented; however, until recently a role for heparanase in the initiation of colon carcinoma remained underappreciated. Interestingly, the emerging data that link heparanase to chronic inflammatory bowel conditions, also suggest contribution of the enzyme to colonic tumor initiation, at least in the setting of colitis-associated cancer. Highly coordinated interplay between intestinal heparanase and immune cells (i.e., macrophages) preserves chronic inflammatory conditions and creates a tumor-promoting microenvironment. Here we review the action of heparanase in colon tumorigenesis and discuss recent findings, pointing to a role for heparanase in sustaining immune cell-epithelial crosstalk that underlies intestinal inflammation and the associated cancer.
Keywords: Extracellular matrix, Tumor microenvironment, Macrophages, Heparan sulfate, Heparanase, Colitis, Colon carcinoma
Introduction
Mammalian heparanase cleaves heparan sulfate (HS) glycosaminoglycans, ubiquitous polysaccharides found at the cell surface and in the extracellular matrix (ECM) [1–5]. HS chains bind to and assemble ECM proteins, thus playing important roles in ECM integrity, barrier function, cell–ECM interactions [1, 5], and providing a structural framework for proper tissue organization and architecture. In addition, the HS chains regulate the activity of a wide variety of bioactive molecules, especially cytokines and growth factors, at the cell surface and in the ECM [1, 6–9]. HS and its enzymatic degradation have long been shown to profoundly affect a variety of physiological and pathological processes, including morphogenesis, neovascularization, inflammation, cell motility, and tumorigenesis [1–3, 5, 9–12].
A role for HS-degrading heparanase in human cancer was originally suggested owing to a preferential expression of the enzyme in various tumor types. Cancer patients bearing tumors with high levels of heparanase mRNA and/or protein had a significantly shorter postoperative survival time as compared to patients whose tumors contained relatively low levels of heparanase [13–19]. Although a causative role for the enzyme is now well established in cancer, due to both heparanase over-expression [20, 21] and silencing [21, 22] studies, a uniform model for the role of heparanase activity in tumorigenesis remains incomplete. Apart of the well-documented catalytic feature of the enzyme (discussed in detail in the following sections of this review), heparanase was reported to exert biological functions independent of its enzymatic activity, such as enhanced cell adhesion [23, 24] and activation of several signaling pathways [24–28]. Despite the wealth of data suggesting a role for heparanase in tumor progression and metastatic spread, until recently there was little evidence to support a causative role for heparanase in the initiation of tumorigenesis. Interestingly, the emerging data linking heparanase to chronic inflammatory processes (i.e., inflammatory bowel disease) described in several recent papers, suggests a contribution of the enzyme to tumor initiation as well, at least in the inflammation-related colorectal cancer [29, 30]. In both tumor initiation and progression, the observations indicate that heparanase is exerting its effects owing to the aberrant activation of its expression. However, the cellular interpretation and outcome of this aberrant heparanase activity depends on contextual cues and interactions with other components of tumor microenvironment. In the following sections, we discuss how heparanase modulates the inflammatory phenotype in chronic colitis and how the inappropriate activation of heparanase affects formation and progression of colorectal tumors.
Heparan sulfate and heparanase
Heparan sulfate proteoglycans
The basic HS structure consists of a repeating disaccharide, constituted by uronic acid and hexosamine residues with various degrees of O- and N-sulfation. Several linear HS chains are covalently bound to a core protein, comprising heparan sulfate proteoglycan (HSPG). Two main types of cell-surface HSPG core proteins have been identified: the transmembrane syndecan with four isoforms [2], and the glycosylphosphatidyl inositol-linked glypican with six isoforms [31]. The major types of ECM-bound HSPGs include agrin and perlecan, endowed with a widespread tissue distribution and a complex modular structure [3]. HSPGs play key roles in numerous biological settings, including developmental processes, cytoskeleton organization, cell–cell and cell–ECM interactions [9, 32, 33]. HSPG moieties exert their multiple functional repertoires via several distinct mechanisms that combine structural, biochemical, and regulatory aspects. By interacting with laminin, fibronectin, and collagens I and IV, HSPGs contribute to the self-assembly and insolubility of the ECM and basement membrane [2, 34, 35]. Accumulating evidence indicates that HSPGs act to preserve proper tissue organization and inhibit cellular invasion by promoting tight cell–cell and cell–ECM interactions, and by maintaining the structural integrity of the ECM [36, 37]. Down-regulation of glycosaminoglycan biosynthesis, especially of the HS chains, is one of the characteristics of malignant transformation [36, 37]. Low levels of cell surface HS also correlate with high metastatic capacity of many tumors. Biochemically, HSPGs often facilitate the biological activity of bound ligands by actively participating in receptor-ligand complex formation [38]. In other cases, HSPGs mediate cellular uptake and catabolism of selected ligands [38], and/or their sequestration to the ECM and cell surface, generally as an inactive reservoir [7, 8, 39]. Cleavage of HSPGs would ultimately release these proteins and convert them into bioactive mediators, ensuring rapid tissue response to local or systemic cues.
Mammalian heparanase
Alterations in HSPGs levels under several pathological conditions (cancer, inflammation) raised the topic of how this ECM constituent is regulated. The decreased content of HSPGs may be due to either decreased HSPG synthesis or increased enzymatic degradation. Heparanase (endo-β-glucuronidase) is the only known endoglycosidase enzyme capable of HS cleavage. Heparanase degrades HS side chains presumably at sites of low sulfation, releasing saccharide products with appreciable size (4–7 kDa) that can still associate with protein ligands and facilitate their biological potency. Mammalian cells express primarily a single dominant functional heparanase enzyme (heparanase-1) [26, 40]. A second heparanase (heparanase-2) has been cloned and sequenced but has not been shown to have HS-degrading activity [41].
Regulation of heparanase
Enzymatic degradation of HS leads to disassembly of the ECM and is therefore involved in fundamental biological phenomena associated with tissue remodeling and cell migration, including inflammation, angiogenesis, and metastasis [26, 40]. Because of the potential tissue damage that could result from non-deliberate cleavage of HS, heparanase activity is kept tightly regulated. Under physiological conditions, heparanase levels are relatively low. Expression of heparanase in normal tissues is restricted to the placenta, activated immune cells, and keratinocytes. However, when physiological homeostasis is disrupted (cancer, inflammation, clot formation) heparanase expression levels, activation, and secretion are up-regulated.
Regulation of heparanase gene transcription represents one type of control mechanism. While in non-cancerous cells and tissues heparanase promoter is constitutively inhibited and the gene is not expressed, it is overexpressed in essentially all human tumors examined (reviewed in [26]). Promoter methylation status appears to play an important role in control of heparanase gene transcription. Tumor-derived cell lines that exhibit no heparanase expression or activity were found to harbor fully methylated alleles. Treating these cells with demethylating agents induced heparanase expression [42] restored heparanase activity, and was accompanied by augmented metastatic capacity in vivo [43]. A significantly higher promoter methylation, inversely correlating with heparanase expression, was found in benign tissue samples as compared to carcinomas [44, 45]. In other studies, wild-type p53 was shown to inhibit transcription of the heparanase gene by direct binding to its promoter [46]. Notably, mutated variants of p53 lose this inhibitory ability and in some cases even up-regulate heparanase expression [46]. These results suggest that under normal conditions the heparanase gene is constitutively inhibited and that epigenetic changes or mutational inactivation of p53 during cancer development may lead to activation of heparanase expression, providing a possible molecular mechanism for the frequent increase in heparanase levels observed in the course of tumorigenesis. Members of SP1 and Ets transcription factor families were associated with basal activity of heparanase promoter [47–49], while early growth response 1 (EGR1) was implicated in inducible transcription of the heparanase gene [44, 45, 50, 51]. Heparanase expression is stimulated by high glucose, reactive oxygen species [52, 53], estrogens [54, 55], and inflammatory cytokines [29, 56, 57].
Post-translational processing of latent heparanase pro-enzyme represents an additional key regulatory mechanism. The heparanase mRNA encodes a 61.2-kDa protein with 543 amino acids. This pro-enzyme is post translationally cleaved into 8- and 50-kDa subunits that non-covalently associate to form the active heparanase [58, 59]. Heterodimer formation is essential for heparanase enzymatic activity [58, 60]. Site-directed mutagenesis revealed that similar to other glycosyl hydrolases, heparanase has a common catalytic mechanism that involves two conserved acidic residues, a putative proton donor at Glu225 and a nucleophile at Glu343 [61]. Cellular processing of the latent 65-kDa pro-heparanase into its active 8 + 50-kDa heterodimer involves removal a 6-kDa linker segment and is inhibited by a cell permeable inhibitor of cathepsin L [62]. Moreover, multiple site-directed mutagenesis and cathepsin L gene silencing and knockout experiments indicate that cathepsin L is the predominant enzyme responsible for processing and activation of pro-heparanase [63]. Applying a structural model, it has been demonstrated that the linker segment, or even a small 1-kDa portion at its C-terminus, render the active site inaccessible to the HS substrate [63].
Another possible regulatory mechanism of heparanase enzymatic activity may be mediated by several endogenous molecules. Heparin (produced exclusively by mast cells [64]) and eosinophilic major basic protein (MBP) are potent inhibitors of heparanase [65, 66]. Both heparin and MBP are stored in granules and released from activated mast cells and eosinophils (in accordance) upon activation.
Heparanase in malignancy
In the course of early studies on heparanase, its enzymatic activity was shown to be associated with the metastatic potential of tumor-derived cells [65, 67]. Almost three decades of research provided direct evidence for a causal role of heparanase in tumor progression, by demonstration of an accelerated tumor growth [20, 21, 68–70] and increased metastatic ability [21, 71] of myeloma, colon, breast, and prostate carcinoma cells following over-expression of the heparanase gene, as well as by a marked decrease in the tumorigenic/metastatic potential of cancer cells following heparanase silencing [21, 22, 72]. These studies clearly indicated that heparanase not only enhances cell dissemination but also promotes the establishment of a vascular network that accelerates primary tumor growth and provides a gateway for invading metastatic cells [10, 26, 73]. The role of heparanase in sustaining the pathology of malignant tumors was further confirmed by demonstration of preferential over-expression of the enzyme in human carcinomas of various origins, including colon [74, 75], gastric [17, 76], liver [77], and pancreas [16, 78, 79].
Evidence indicates that during tumor progression, heparanase not only assists in the breakdown of ECM but also is involved in regulating the bioavailability and activity of growth factors. Various heparin-binding growth factors (i.e., bFGF, VEGF, HGF), are sequestered by HS in the ECM, providing a localized, readily accessible depot, protected from proteolytic degradation [8, 9], yet available to activate cells after being released by heparanase. In addition, heparanase enzymatic activity generates HS fragments which potentiate growth factor—receptor binding, dimerization and signaling [10]. There is evidence that the fragments of HS generated by heparanase are more biologically active than the native HS chain from which they are derived [6, 10]. Thus, heparanase acts as an “activator” of HSPGs creating a supportive microenvironment for tumor progression. Indeed, augmented levels of heparanase in tumors were often associated with reduced patients’ survival post-operation, increased tumor metastasis and higher microvessel density [8, 26, 73].
Heparanase in colorectal cancer
Colorectal carcinoma is a major cause of cancer morbidity and mortality. It is the third most commonly diagnosed cancer and one of the leading causes of cancer-related deaths in the United States and other Western countries [80, 81]. The pathogenic mechanisms underlying colorectal cancer development appear to be complex and heterogeneous. The main risk factors for developing the disease are age, diet, inherited and somatic mutations, as well as chronic gut inflammatory conditions. Overexpression of heparanase is regarded as a characteristic feature of colon cancer [18, 19, 70, 74, 75]. Both the heparanase mRNA and protein are expressed already at early stages of neoplasia, but are practically undetectable in the adjacent normal-looking colon epithelium [19, 74]. Gradually increasing expression of heparanase was reported during progression from severe dysplasia through well-differentiated to poorly differentiated colon carcinoma [70, 74]. The presence of heparanase protein in colon carcinoma is strongly associated with local invasion, lymph vessel infiltration, tumor angiogenesis, and metastasis [19, 74, 75], and correlated with TNM stage and Dukes stage [19]. A high heparanase expression was also found in colon carcinoma metastases to lung, liver, and lymph nodes [74]. Furthermore, heparanase is a significant independent risk factor for metastasis and a marker for poor prognosis in patients with colon cancer [18, 19, 75]. Transfection of the heparanase gene into colon carcinoma cell lines resulted in activation of the Akt/PKB signaling, increased tumor growth [70], and significantly augmented invasive ability of the cells [19].
Heparanase in inflammation
Induction of heparanase was reported in several inflammatory conditions, often associated with degradation of HS, remodeling of the ECM, facilitation of inflammatory cells migration towards the injury sites and release of chemokines anchored within the ECM network and cell surfaces [12]. In the studies performed prior to cloning of the heparanase gene, heparanase activity originating in immunocytes (neutrophils, activated T-lymphocytes) has been investigated and found to contribute to their ability to penetrate blood vessel walls and accumulate in target organs [82–87]. Presently, it becomes increasingly clear that immunocytes are not the sole source of the enzyme in inflammation. Up-regulation of heparanase, locally expressed (i.e., by epithelial cells, vascular endothelium) at the site of inflammation, was demonstrated in the mouse models of delayed type hypersensitivity [57], chronic colitis [29], psoriasis (our unpublished results), as well as in several auto-immune and auto-inflammatory human disorders, including rheumatoid arthritis [88], type 2 diabetes [89], ulcerative colitis, and Crohn’s disease [29, 30].
Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a chronic condition of the gastrointestinal tract resulting from inappropriate and exaggerated mucosal immune response believed to arise from a multitude of factors including genetic alterations, epithelial defects, and luminal flora composition [90–92]. Ulcerative colitis (UC) and Crohn’s disease represent the two major forms of IBD, together affecting ~1 in 250 people in Europe, North America, and Australia [93]. UC (which will be the main focus for further discussion) is a severe ulcerating inflammatory disease that is limited to the colon and rectum and extends only into the mucosa and submucosa. In contrast, Crohn’s disease, which has also been referred to as regional enteritis (because of frequent ileal involvement) may involve any area of the gastrointestinal tract and is typically transmural [91, 92].
Although the distinct causes of UC remain unknown, the disease is thought to result from inappropriate and continuous response of the mucosal immune system to commensal gut flora in genetically susceptible individuals [90, 92]. Under normal conditions, the gastrointestinal immune system keeps a delicate balance between the immune response to microbial pathogens and tolerance to the normal flora. In patients with UC, the mucosal barrier may become leaky, leading to the uncontrolled uptake of antigens and pro-inflammatory substances, such as bacteria and bacterial products from the gut lumen [90, 92, 94]. Cytokines (i.e., tumor necrosis factor (TNF)-α and other immune-mediated signals further direct the intestinal epithelium to increase tight junction permeability, which causes an increase in the flux of luminal flora [92, 95]. These events may establish a self-amplifying cycle in which an insult to the epithelial barrier triggers immune activation, cytokine release, and further barrier dysfunction [96].
The most feared long-term complication of UC is colon carcinoma [97–100], as the UC patients have a hazard of colorectal cancer which is an order of magnitude higher than the normal population. UC lasting more than 20 years increases the risk for colorectal cancer as much as do inherited cancer syndromes such as familial adenomatous polyposis [98]. In fact, colitis-associated cancer is widely recognized as a paradigm for the association between inflammation and tumorigenesis [101, 102]. Key components of tumor-promoting colonic inflammation include master transcription factors (for example, NF-κB), proinflammatory, and cancer-promoting cytokines (i.e., TNF-α, IL-1, IL-6, IL-23), signal transducer and activator of transcription 3 (STAT3), as well as innate immunity receptors and signaling molecules, such as Toll-like receptor 4 (TLR4) and MyD88 [103–108].
Different subsets of immune cells have been implicated in pathogenesis of UC and the associated cancer. Early experimental models have shown that UC-like inflammation can occur as a result of either excessive effector T-cell function, or deficient regulatory T-cell function (reviewed in [91]). More recently, the innate immune cells (in particular macrophages) were shown to be paramount for IBD development and its progression to colonic cancer [92, 109–113]. The gastrointestinal mucosa represents the largest reservoir of macrophages in the body. Intestinal macrophages are derived from blood monocytes that are recruited to the lamina propria by endogenous chemoattractants in the non-inflamed mucosa and by inflammatory chemokines and bacterial products during inflammation. Among the subsets of innate immune cells that monitor the luminal contents in the non-inflamed mucosa, resident macrophages show attenuated proliferation and chemotactic activity in response to either microbial ligands or host cytokines/chemokines [113]. In active inflammatory bowel disease, there is an increase in the mucosal macrophage population, derived from circulating monocytes [92, 110, 113]. These recruited macrophages are phenotypically different from the resident population and play a major role in mediating the chronic mucosal inflammation seen in IBD patients [92, 109, 113]. Unlike resident macrophages, they express Nod-like receptors (NLRs), Toll-like receptors (TLRs), and release numerous proinflammatory and pro-cancerous cytokines, such as TNF-α, IL-1, IL-6, IL-8, IL-12, IL-18, along with reactive metabolites of oxygen and nitrogen [90, 109, 113, 114]. Direct support for the macrophage involvement in the regulation of IBD has been obtained from analysis of mice with selective disruption of STAT3 in their macrophages [112]. TNF-α, produced by non-lymphoid cells, mostly macrophages, was found to be essential for the development of colitis using the adoptive T-cell model of colitis induction. Recent studies have also shown that depletion of macrophages in the Il10−/− mouse prevents development of colitis, which otherwise occurs owing to unregulated production of IL-12 and IL-23 by macrophages [111].
Heparan sulfate and heparanase in IBD
In the normal intestinal tract, HS represents a key polysaccharide of the ECM and is extensively found in the basal lamina of the intestinal mucosa [115, 116], where it provides a structural framework for gut tissue organization and functions as an electrostatic/mechanical barrier involved in the regulation of vascular and ECM permeability [117–120]. HS moieties were also detected at basolateral location on intestine epithelial cell surfaces [121]. Due to its specific interaction with other ECM components, HS is important for maintaining the structural integrity of the gut wall [117, 120]. Alterations in HS may significantly affect both the permeability of the colon and immune/inflammatory reactions. In IBD patients, an uncontrolled and sustained inflammatory cascade leads to breakdown of the basal lamina, destruction of bowel tissue architecture and lesion development. Analysis of glycosaminoglycan content in normal colonic tissue and colons of IBD patients revealed a substantial loss of HS from the subepithelial BM and from the vascular endothelium in submucosa [122–124]. Moreover, disruption of HS was found even in the non-inflamed areas of intestinal samples obtained from IBD patients [117]. Given the key importance of HS in maintaining BM supramolecular architecture and perm-selective characteristics, it is plausible that disintegration of basal lamina HS represent an important determinant in IBD pathogenesis and contributes to increased vascular permeability, immunocyte/protein extravasation, and tissue remodeling. Of note, abnormal increase in mucosal HS was reported in IBD [117, 122, 125], suggesting a compensatory mechanism for the restoration of the integrity of the intestinal mucosa [117].
Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis
Despite the progress in identifying genes that contribute to UC susceptibility [92, 93] and improved surveillance/therapy of IBD, the risk of colorectal cancer in the setting of ulcerative colitis remains substantial [97]. During the last decade, significant advancement has been made in deciphering the role of inflammatory cytokines (TNF-α, IL-1β, IL-6) and their downstream transcription factors (NF-κB, STAT3) in tumor-stimulating crosstalk between immune and epithelial cells [101, 103, 104, 107, 126–128]. The role of ECM-degrading enzymes in this cross talk remains less clear. Recently, based on preferential expression of heparanase in inflammation [30, 57, 88], and well-documented involvement of the enzyme in colon tumorigenesis [18, 19, 70, 74, 75], we proposed that heparanase may represent an important mechanistic link coupling colitis and cancer [29, 57]. Indeed, heparanase is constantly overexpressed by the colonic epithelium both in UC and experimental colitis [29, 30]. Moreover, the recent data from experiments utilizing heparanase-overexpressing transgenic mice in a model of colitis-associated cancer induced by the carcinogen azoxymethane (AOM), followed by the inflammatory agent dextran sodium sulfate (DSS), suggest that heparanase activity preserves chronic inflammatory conditions in DSS colitis and creates a tumor-promoting microenvironment, characterized by enhanced NF-κB signaling, STAT3 induction and increased vascularization. Furthermore, it appears that pathogenesis of chronic colitis and the associated colon tumorigenesis may involve a vicious cycle through which heparanase of epithelial origin, acting synergistically with the local flora and cytokine milieu, facilitates abnormal activation of innate immune cells (i.e., macrophages), which in turn stimulate further production/activation of the enzyme in the inflamed colon. A detailed analysis of the functional importance of heparanase in modulating inflammatory responses and sustaining immune-epithelial crosstalk underlying the pathogenesis of colitis-associated tumorigenesis is presented below.
Immunohistochemical staining revealed that while heparanase expression was practically not detected in healthy colon epithelium [30, 74], in UC specimens the epithelial lining showed a marked overexpression of heparanase [30]. Importantly, increased levels of heparanase were detected in both the acute and chronic phases of the disease [29, 30]. During all phases, inflammatory cells in the involved areas, showed little or no heparanase expression [29, 30]. Analogous (although not identical) temporo-spatial pattern of heparanase expression was observed in experimental DSS-induced colitis [29]. DSS causes an acute inflammatory reaction and ulceration in the colon and when three cycles of DSS administration are applied (typically each cycle lasts for 5–7 days, with 14-day intervals between the cycles), acute inflammation is followed by chronic colitis [129]. Moreover, three cycles of DSS subsequent to a single pretreatment by the carcinogen azoxymethane (AOM) result in development of colon tumors in ~100% of the treated mice [130], representing a well-established model of colitis-associated cancer. In addition, DSS alone may cause colonic tumors in almost 50% of the treated mice when the DSS cycles are repeatedly administered nine times [131], further demonstrating the relevance of this model to inflammation-induced tumorigenesis. Increased levels of heparanase mRNA, protein and enzymatic activity were found in the mouse colon during DSS-induced colitis [29]. However, unlike in UC patients, in mouse DSS colitis, heparanase levels gradually decrease during the chronic phase [29]. Application of AOM-DSS protocol in heparanase transgenic (Hpa-tg) mice [132], exhibiting colonic heparanase expression pattern closely resembling that of UC patients, enabled to highlight the role of heparanase in colitis and associated tumorigenesis [29]. Heparanase over-expression markedly increased the incidence and severity of colitis-associated tumors formed in Hpa-tg mice. Moreover, three DSS cycles administered without AOM pretreatment result in colonic tumors developed in 90% of the Hpa-tg mice, but not in wt mice. Further research revealed that exacerbated chronic inflammatory phenotype is responsible for the increased tumor incidence/severity in Hpa-tg colon. Although having little effect on the acute DSS colitis, heparanase over-expression profoundly affected the chronic phase of DSS-induced colitis, as demonstrated by microscopic and biochemical analyses of inflammatory phenotypes preserved in Hpa-tg but not wt mouse colon 1 month after cessation of DSS treatment [29]. In particular, augmented recruitment and continuous activation of macrophages was detected in Hpa-tg colons. In fact, macrophages are known to have a dual role in inflammation. In the scenario of inflammation resolution (an active process leading to normal structural and functional state and regulated by anti-inflammatory and reparative molecules including IL-10, TGF-β, glucocorticoids, lipoxins, resolvins and protectins [133, 134]) macrophages perform phagocytosis and produce anti-inflammatory cytokines [134, 135], thereby preventing inflammatory responses from lasting too long. However, if inflammation resolution is deregulated, macrophage response switches to the pattern of chronic inflammation [133]. Macrophages dominate in chronic inflammatory foci and generate significant amounts of growth factors, cytokines, and reactive oxygen species [101, 136]. The increase in mucosal macrophage population is well documented in UC patients [109]. Recruitment and activation of macrophages within the intestinal mucosa play a key role in the pathogenesis of both human UC [109, 137] and experimental colitis [110, 138]. Furthermore, macrophages are candidate linking cells between inflammation and cancer [136, 139, 140]. For instance, the tumor-promoting cytokines IL-1, IL-6, and TNF-α [105, 107] are produced mainly by activated macrophages and, along with macrophage-derived growth factors and reactive oxygen species, foster tumor initiation and progression [136, 137]. Our recent data [29] suggest that elevated levels of heparanase found in chronically inflamed Hpa-tg mouse colon (similar to colonic tissue of UC patients [30]), bring about continuous activation of TNF-α-producing macrophages, enhanced NF-κB signaling and increased expression of NF-κB regulated genes, including cyclooxygenase-2 (COX-2), IL-1, IL-6, macrophage inflammatory protein-1 (MIP-1) and MIP-2, antiapoptotic Bcl-XL, as well as activation of STAT3, critical effectors responsible for induction and progression of colitis-associated tumors [103, 104, 127, 128]. Collectively, these events lead to enhanced colonic tumor formation in heparanase-rich microenvironments [29].
It should be noted that in addition to macrophages, dendritic cells (DC) represent the major subset of innate immune cells involved in the pathogenesis of IBD [141]. A possible link between heparanase and DC maturation has been suggested in one publication [142]; however, it remains to be investigated whether heparanase activity affects either protective or pathogenic roles of DC in the setting of chronic intestinal inflammation.
Macrophages: a cellular target for heparanase action and regulators of heparanase expression/activity in chronic colitis
Preservation of the inflammatory profile of macrophages infiltrating the Hpa-tg colon for more than 1 month after termination of DSS treatment [29] led to the assumption that heparanase over-expression directly affects macrophage activation. This assumption was further supported by in vitro observations: when mouse macrophages were stimulated with lipopolysaccharide (LPS, a specific stimulator of TLR4 signaling [143]) in the absence or presence of active heparanase (recapitulating UC-conditions, i.e., a heparanase-rich environment and abundant microbial flora), heparanase strongly sensitized macrophages to activation by LPS in vitro, as indicated by a marked increase in TNF-α, IL-6, and IL-12p35 [29], cytokines known to be induced by TLR4 signaling and tightly involved in the pathogenesis of UC [137]. In fact, it was previously shown that intact extracellular HS inhibits LPS-mediated TLR4 signaling and macrophage activation, and that its removal relieves this inhibition [144]. Given that one of the unique aspects of colitis-associated cancer development is the involvement of lumenal flora and TLR signaling [145, 146], the ability of heparanase to sensitize macrophages to LPS activation is of particular significance in light of the increased epithelial permeability to lumenal microbial products, characteristic of UC [90, 92, 94]. Thus, in a UC setting, heparanase may preserve inflammatory conditions by reprogramming macrophage response from resolution of inflammation to unresolved chronic colitis.
On the other hand, activated macrophages are capable of inducing heparanase expression in intestinal epithelium, most likely through TNF-α-mediated stimulation of transcription factor EGR1, a powerful inducer of heparanase transcription in colonic cells [29, 51]. Notably, TNF-α was shown to stimulate EGR1 expression in IBD patients [147]. Furthermore, in chronic colitis, macrophages appear to be involved not only in regulation of heparanase expression but also in post-translational activation of the heparanase proenzyme in the inflamed colon. As mentioned above, cathepsin L (CatL) is the only known protease capable of proper proteolytic activation of 65-kDa latent heparanase [62, 63]. Of note, it has been previously shown that CatL plays an important pathophysiological role in colonic inflammation and that macrophages are the primary cellular source of inducible CatL expression in inflamed colon of UC patients and in DSS-induced mouse colitis [114]. Moreover, macrophages are unique in their ability to secrete mature CatL and allow extracellular accumulation of the active enzyme [148]. Our recent data directly confirmed that macrophages are responsible for proteolytic processing of the proheparanase in DSS colitis, providing a pool of extracellular active CatL [29].
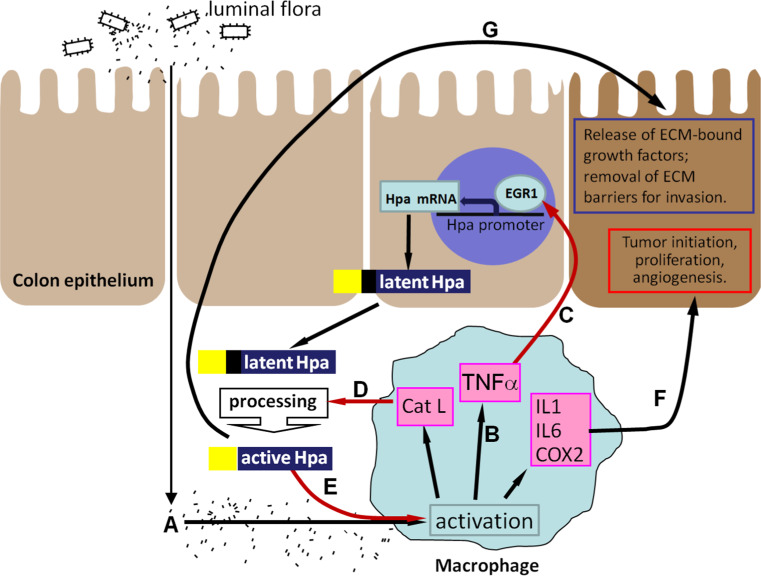
Thus, macrophages not only represent a cellular target for heparanase action but also decisively regulate heparanase in chronic colitis, both at the transcriptional and posttranslational levels. These findings demonstrate and highlight the cooperation between colonic epithelial and stromal compartments in heparanase regulation during colonic inflammation and associated tumorigenesis. Heparanase appears to generate a self-sustaining inflammatory circuit that can fuel chronic colitis and the associated tumorigenesis (Fig. 1). Briefly, macrophages activated by the influx of the luminal flora due to barrier function defect (Fig. 1A) secrete TNF-α and stimulate production of heparanase by the colon epithelium (Fig. 1B). The secreted latent heparanase is processed into its active form by Cath L, supplied by the activated macrophages (Fig. 1C). Enzymatically active heparanase sensitizes macrophages to further activation by microbial flora (Fig. 1D), thus preventing inflammation resolution, switching macrophage responses to the chronic inflammation pattern and creating a tumor-inducing inflammatory environment (Fig. 1E). In addition, heparanase promotes tumor development via stimulation of angiogenesis, release of ECM-bound growth factors and bioactive HS fragments, and removal of extracellular barriers for invasion (Fig. 1F). The suggested heparanase-powered vicious cycle may explain a yet poorly understood “multiplier effect” in IBD inflammation, in which even a small initial elevation in ‘initiating’ inflammatory stimuli gives rise to large increases in downstream cytokines [91]. Therefore, disruption of the heparanase-driven chronic inflammation might be highly relevant to the design of therapeutic interventions in colitis and the associated cancer.
Fig. 1.
A model of heparanase-driven vicious cycle that powers colitis and the associated tumorigenesis. Macrophages (activated by excessive exposure to the luminal flora (A) due to epithelial barrier function defects characteristic for UC) produce increased levels of TNF-α (B) and induce heparanase expression in colon epithelium via an EGR1-dependent mechanism (C). D The secreted 62-kDa latent heparanase is processed into its enzymatically-active (8 + 50 kDa) form by Cath L (which is supplied by the activated macrophages), and in turn sensitizes macrophages to further activation by luminal flora (E), thus preventing inflammation resolution, switching macrophage responses to the chronic inflammation pattern and creating a tumor-initiating inflammatory environment (F). In addition, heparanase promotes tumor take and progression via stimulation of angiogenesis, release of ECM-bound growth factors and bioactive HS fragments, and removal of extracellular barriers for invasion (G)
Heparanase inhibitors
Reports demonstrating clinical relevance of heparanase activity in cancer and other disorders, as well as several proof-of-concept studies utilizing heparanase gene silencing/knockdown approaches [21, 22, 57, 149–151], sparked considerable interest in heparanase inhibitors [152, 153]. With the availability of recombinant heparanase, a variety of inhibitory compounds have been developed, including small-molecule inhibitors and polyanionic molecules such as laminaran sulfate, suramin, phosphomannopentaose sulfate (PI-88), and modified species of heparin [152–158]. Recently, a novel heparanase inhibitor PG545, a synthetic fully sulfated HS mimetic, has entered Phase I trials for advanced cancer [158]. Heparin, another potent heparanase inhibitor, has been previously shown to exert beneficial effects in UC patients [159–161], although the precise mechanism of its action in UC is not fully understood. Importantly, a recent report, utilizing DSS model in syndecan-1-deficient mice, indicates that heparin therapy could be particularly efficient for UC patients with decreased colonic syndecan-1 content [162]. Furthermore, heparin (in particular the low-molecular-weight (LMW) heparin species) has been shown to increase the survival of cancer patients [163–166]. Inhibition of heparanase is an attractive explanation for these effects: as an analog of the natural substrate of the enzyme, heparin competes with HS for the heparanase active site and efficiently inhibits its activity [167]. However, the anti-coagulant properties of heparin limit its use as anti-inflammatory/anti-tumor agents [12, 153]. In addition, some recent studies reported no beneficial effect of LMW heparin in active ulcerative colitis [168, 169]. Although the biological reason for the lack of significant clinical advantage of LMW heparin in these studies was not clearly determined, additional bio-effects of heparin (e.g., anticoagulation, release and activation of ECM-bound pro-angiogenic growth factors [157]), as well as the route of administration, may play a role. In the current clinical applications, heparin is administered parenterally, as its size and charge are believed to preclude absorption from the gastrointestinal tract [170]. Although this route of administration is suitable for short-term treatment, longer-term heparin therapy appropriate for chronic conditions (i.e., IBD) may require engineering of optimal pharmacokinetic properties and oral bioavailability. In fact, the defects of intestine epithelial barrier function, characteristic for IBD, can turn highly advantageous for oral administration of heparins (as well as other types of heparanase inhibitors) in this particular clinical setting.
Elimination of anticoagulant activity represents an additional key step toward design of clinically relevant heparin-based therapies for IBD and the associated cancer. Importantly, glycol-splitting of either preexisting and/or chemically generated nonsulfated uronic acids dramatically increases the heparanase-inhibiting activity of N-acetylated species of LMW heparin, while it abolishes its anti-coagulant and pro-angiogenic activities [157, 171]. These chemically modified LMW heparin species can be given at higher doses and are safer to use when there are concerns of bleeding complications. The effectiveness of chemically modified LMW heparin devoid of anticoagulant activity in suppressing the biological effects of heparanase has been demonstrated applying in vivo models of several heparanase-driven disorders other than UC, such as DTH inflammation [57], diabetic nephropathy [172], melanoma, multiple myeloma, and pancreatic carcinoma progression [173–175]. However, the ability of LMW heparin species to stimulate TLR-dependent activation of macrophages (mimicking the stimulatory effect of soluble HS on TLR4 signaling [144]) may limit their therapeutic effects in the setting of chronic colitis, emphasizing further need for rationally designed specific inhibitory small molecules.
In conclusion, heparanase represents a highly relevant, but equally challenging therapeutic target in inflammatory bowel disease and the associated cancer. Findings cited in this review and elsewhere warrant further systematic analysis and continuous searching for the effective heparanase-inhibiting compound toward future translation to the clinical setting. In parallel, elucidation of the molecular mechanisms underlying heparanase involvement in colitis and the associated cancer will help to better define target patient populations in which future anti-heparanase therapies could be particularly beneficial.
Acknowledgments
We thank Prof. Israel Vlodavsky (Cancer and Vascular Biology Research Center, the Rappaport Faculty of Medicine, Technion, Haifa, Israel) for his continuous help and collaboration. This work was supported by grants from the German-Israel Research Foundation (GIF), Israel Science Foundation (grant 593/10), and Chief Scientist Office—Israeli Ministry of Health.
Footnotes
E. Hermano and I. Lerner contributed equally to this work.
References
- 1.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 2.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 3.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 4.Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 5.Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010;277:3904–3923. doi: 10.1111/j.1742-4658.2010.07800.x. [DOI] [PubMed] [Google Scholar]
- 6.Kato M, Wang H, Kainulainen V, Fitzgerald ML, Ledbetter S, et al. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat Med. 1998;4:691–697. doi: 10.1038/nm0698-691. [DOI] [PubMed] [Google Scholar]
- 7.Vlodavsky I, Bar-Shavit R, Ishai-Michaeli R, Bashkin P, Fuks Z. Extracellular sequestration and release of fibroblast growth factor: a regulatory mechanism? Trends Biochem Sci. 1991;16:268–271. doi: 10.1016/0968-0004(91)90102-2. [DOI] [PubMed] [Google Scholar]
- 8.Vlodavsky I, Miao HQ, Medalion B, Danagher P, Ron D. Involvement of heparan sulfate and related molecules in sequestration and growth promoting activity of fibroblast growth factor. Cancer Metastasis Rev. 1996;15:177–186. doi: 10.1007/BF00437470. [DOI] [PubMed] [Google Scholar]
- 9.Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat Rev Cancer. 2002;2:521–528. doi: 10.1038/nrc842. [DOI] [PubMed] [Google Scholar]
- 10.Elkin M, Ilan N, Ishai-Michaeli R, Friedmann Y, Papo O, et al. Heparanase as mediator of angiogenesis: mode of action. Faseb J. 2001;15:1661–1663. doi: 10.1096/fj.00-0895fje. [DOI] [PubMed] [Google Scholar]
- 11.Gotte M. Syndecans in inflammation. Faseb J. 2003;17:575–591. doi: 10.1096/fj.02-0739rev. [DOI] [PubMed] [Google Scholar]
- 12.Li JP, Vlodavsky I. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb Haemost. 2009;102:823–828. doi: 10.1160/TH09-02-0091. [DOI] [PubMed] [Google Scholar]
- 13.Bar-Sela G, Kaplan-Cohen V, Ilan N, Vlodavsky I, Ben-Izhak O. Heparanase expression in nasopharyngeal carcinoma inversely correlates with patient survival. Histopathology. 2006;49:188–193. doi: 10.1111/j.1365-2559.2006.02469.x. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Izhak O, Kaplan-Cohen V, Ilan N, Gan S, Vlodavsky I, et al. Heparanase expression in malignant salivary gland tumors inversely correlates with long-term survival. Neoplasia. 2006;8:879–884. doi: 10.1593/neo.06382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doweck I, Kaplan-Cohen V, Naroditsky I, Sabo E, Ilan N, et al. Heparanase localization and expression by head and neck cancer: correlation with tumor progression and patient survival. Neoplasia. 2006;8:1055–1061. doi: 10.1593/neo.06577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koliopanos A, Friess H, Kleeff J, Shi X, Liao Q, et al. Heparanase expression in primary and metastatic pancreatic cancer. Cancer Res. 2001;61:4655–4659. [PubMed] [Google Scholar]
- 17.Takaoka M, Naomoto Y, Ohkawa T, Uetsuka H, Shirakawa Y, et al. Heparanase expression correlates with invasion and poor prognosis in gastric cancers. Lab Invest. 2003;83:613–622. doi: 10.1097/01.lab.0000067482.84946.bd. [DOI] [PubMed] [Google Scholar]
- 18.Naomoto Y, Takaoka M, Okawa T, Nobuhisa T, Gunduz M, et al. The role of heparanase in gastrointestinal cancer (Review) Oncol Rep. 2005;14:3–8. [PubMed] [Google Scholar]
- 19.Nobuhisa T, Naomoto Y, Ohkawa T, Takaoka M, Ono R, et al. Heparanase expression correlates with malignant potential in human colon cancer. J Cancer Res Clin Oncol. 2005;131:229–237. doi: 10.1007/s00432-004-0644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen I, Pappo O, Elkin M, San T, Bar-Shavit R, et al. Heparanase promotes growth, angiogenesis and survival of primary breast tumors. Int J Cancer. 2006;118:1609–1617. doi: 10.1002/ijc.21552. [DOI] [PubMed] [Google Scholar]
- 21.Lerner I, Baraz L, Pikarsky E, Meirovitz A, Edovitsky E, et al. Function of heparanase in prostate tumorigenesis: potential for therapy. Clin Cancer Res. 2008;14:668–676. doi: 10.1158/1078-0432.CCR-07-1866. [DOI] [PubMed] [Google Scholar]
- 22.Edovitsky E, Elkin M, Zcharia E, Peretz T, Vlodavsky I. Heparanase gene silencing, tumor invasiveness, angiogenesis, and metastasis. J Natl Cancer Inst. 2004;96:1219–1230. doi: 10.1093/jnci/djh230. [DOI] [PubMed] [Google Scholar]
- 23.Goldshmidt O, Zcharia E, Cohen M, Aingorn H, Cohen I, et al. Heparanase mediates cell adhesion independent of its enzymatic activity. Faseb J. 2003;17:1015–1025. doi: 10.1096/fj.02-0773com. [DOI] [PubMed] [Google Scholar]
- 24.Zetser A, Bashenko Y, Miao HQ, Vlodavsky I, Ilan N. Heparanase affects adhesive and tumorigenic potential of human glioma cells. Cancer Res. 2003;63:7733–7741. [PubMed] [Google Scholar]
- 25.Gingis-Velitski S, Zetser A, Flugelman MY, Vlodavsky I, Ilan N. Heparanase induces endothelial cell migration via protein kinase B/Akt activation. J Biol Chem. 2004;279:23536–23541. doi: 10.1074/jbc.M400554200. [DOI] [PubMed] [Google Scholar]
- 26.Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38:2018–2039. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Zetser A, Bashenko Y, Edovitsky E, Levy-Adam F, Vlodavsky I, et al. Heparanase induces vascular endothelial growth factor expression: correlation with p38 phosphorylation levels and Src activation. Cancer Res. 2006;66:1455–1463. doi: 10.1158/0008-5472.CAN-05-1811. [DOI] [PubMed] [Google Scholar]
- 28.Cohen-Kaplan V, Doweck I, Naroditsky I, Vlodavsky I, Ilan N. Heparanase augments epidermal growth factor receptor phosphorylation: correlation with head and neck tumor progression. Cancer Res. 2008;68:10077–10085. doi: 10.1158/0008-5472.CAN-08-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerner I, Hermano E, Zcharia E, Rodkin D, Bulvik R, et al. Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. J Clin Invest. 2011;121:1709–1721. doi: 10.1172/JCI43792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waterman M, Ben-Izhak O, Eliakim R, Groisman G, Vlodavsky I, et al. Heparanase upregulation by colonic epithelium in inflammatory bowel disease. Mod Pathol. 2007;20:8–14. doi: 10.1038/modpathol.3800710. [DOI] [PubMed] [Google Scholar]
- 31.Fransson LA, Belting M, Cheng F, Jonsson M, Mani K, et al. Novel aspects of glypican glycobiology. Cell Mol Life Sci. 2004;61:1016–1024. doi: 10.1007/s00018-004-3445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer KL, Yost HJ. Heparan sulfate core proteins in cell–cell signaling. Annu Rev Genet. 2003;37:461–484. doi: 10.1146/annurev.genet.37.061103.090226. [DOI] [PubMed] [Google Scholar]
- 33.Iozzo RV. Heparan sulfate proteoglycans: intricate molecules with intriguing functions. J Clin Invest. 2001;108:165–167. doi: 10.1172/JCI13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hacker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Natl Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 35.Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–624. doi: 10.1016/S0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- 36.Sanderson RD. Heparan sulfate proteoglycans in invasion and metastasis. Semin Cell Dev Biol. 2001;12:89–98. doi: 10.1006/scdb.2000.0241. [DOI] [PubMed] [Google Scholar]
- 37.Timar J, Lapis K, Dudas J, Sebestyen A, Kopper L, et al. Proteoglycans and tumor progression: Janus-faced molecules with contradictory functions in cancer. Semin Cancer Biol. 2002;12:173–186. doi: 10.1016/S1044-579X(02)00021-4. [DOI] [PubMed] [Google Scholar]
- 38.Belting M. Heparan sulfate proteoglycan as a plasma membrane carrier. Trends Biochem Sci. 2003;28:145–151. doi: 10.1016/S0968-0004(03)00031-8. [DOI] [PubMed] [Google Scholar]
- 39.Patel VN, Knox SM, Likar KM, Lathrop CA, Hossain R, et al. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development. 2007;134:4177–4186. doi: 10.1242/dev.011171. [DOI] [PubMed] [Google Scholar]
- 40.Parish CR, Freeman C, Hulett MD. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471:M99–M108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- 41.McKenzie E, Tyson K, Stamps A, Smith P, Turner P, et al. Cloning and expression profiling of Hpa2, a novel mammalian heparanase family member. Biochem Biophys Res Commun. 2000;276:1170–1177. doi: 10.1006/bbrc.2000.3586. [DOI] [PubMed] [Google Scholar]
- 42.Ateeq B, Unterberger A, Szyf M, Rabbani SA. Pharmacological inhibition of DNA methylation induces proinvasive and prometastatic genes in vitro and in vivo. Neoplasia. 2008;10:266–278. doi: 10.1593/neo.07947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shteper PJ, Zcharia E, Ashhab Y, Peretz T, Vlodavsky I, et al. Role of promoter methylation in regulation of the mammalian heparanase gene. Oncogene. 2003;22:7737–7749. doi: 10.1038/sj.onc.1207056. [DOI] [PubMed] [Google Scholar]
- 44.Ogishima T, Shiina H, Breault JE, Tabatabai L, Bassett WW, et al. Increased heparanase expression is caused by promoter hypomethylation and up-regulation of transcriptional factor early growth response-1 in human prostate cancer. Clin Cancer Res. 2005;11:1028–1036. [PubMed] [Google Scholar]
- 45.Ogishima T, Shiina H, Breault JE, Terashima M, Honda S, et al. Promoter CpG hypomethylation and transcription factor EGR1 hyperactivate heparanase expression in bladder cancer. Oncogene. 2005;24:6765–6772. doi: 10.1038/sj.onc.1208811. [DOI] [PubMed] [Google Scholar]
- 46.Baraz L, Haupt Y, Elkin M, Peretz T, Vlodavsky I. Tumor suppressor p53 regulates heparanase gene expression. Oncogene. 2006;25:3939–3947. doi: 10.1038/sj.onc.1209425. [DOI] [PubMed] [Google Scholar]
- 47.Jiang P, Kumar A, Parrillo JE, Dempsey LA, Platt JL, et al. Cloning and characterization of the human heparanase-1 (HPR1) gene promoter: role of GA-binding protein and Sp1 in regulating HPR1 basal promoter activity. J Biol Chem. 2002;277:8989–8998. doi: 10.1074/jbc.M105682200. [DOI] [PubMed] [Google Scholar]
- 48.Lu WC, Liu YN, Kang BB, Chen JH. Trans-activation of heparanase promoter by ETS transcription factors. Oncogene. 2003;22:919–923. doi: 10.1038/sj.onc.1206201. [DOI] [PubMed] [Google Scholar]
- 49.Rao G, Liu D, Xing M, Tauler J, Prinz RA, et al. Induction of heparanase-1 expression by mutant B-Raf kinase: role of GA binding protein in heparanase-1 promoter activation. Neoplasia. 2010;12:946–956. doi: 10.1593/neo.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Mestre AM, Khachigian LM, Santiago FS, Staykova MA, Hulett MD. Regulation of inducible heparanase gene transcription in activated T cells by early growth response 1. J Biol Chem. 2003;278:50377–50385. doi: 10.1074/jbc.M310154200. [DOI] [PubMed] [Google Scholar]
- 51.de Mestre AM, Rao S, Hornby JR, Soe-Htwe T, Khachigian LM, et al. Early growth response gene 1 (EGR1) regulates heparanase gene transcription in tumor cells. J Biol Chem. 2005;280:35136–35147. doi: 10.1074/jbc.M503414200. [DOI] [PubMed] [Google Scholar]
- 52.Maxhimer JB, Quiros RM, Stewart R, Dowlatshahi K, Gattuso P, et al. Heparanase-1 expression is associated with the metastatic potential of breast cancer. Surgery. 2002;132:326–333. doi: 10.1067/msy.2002.125719. [DOI] [PubMed] [Google Scholar]
- 53.Rao G, Ding HG, Huang W, Le D, Maxhimer JB, et al. Reactive oxygen species mediate high glucose-induced heparanase-1 production and heparan sulphate proteoglycan degradation in human and rat endothelial cells: a potential role in the pathogenesis of atherosclerosis. Diabetologia. 2011;54:1527–1538. doi: 10.1007/s00125-011-2110-z. [DOI] [PubMed] [Google Scholar]
- 54.Elkin M, Cohen I, Zcharia E, Orgel A, Guatta-Rangini Z, et al. Regulation of heparanase gene expression by estrogen in breast cancer. Cancer Res. 2003;63:8821–8826. [PubMed] [Google Scholar]
- 55.Xu X, Ding J, Rao G, Shen J, Prinz RA, et al. Estradiol induces heparanase-1 expression and heparan sulphate proteoglycan degradation in human endometrium. Hum Reprod. 2007;22:927–937. doi: 10.1093/humrep/del483. [DOI] [PubMed] [Google Scholar]
- 56.Chen G, Wang D, Vikramadithyan R, Yagyu H, Saxena U, et al. Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression. Biochemistry. 2004;43:4971–4977. doi: 10.1021/bi0356552. [DOI] [PubMed] [Google Scholar]
- 57.Edovitsky E, Lerner I, Zcharia E, Peretz T, Vlodavsky I, et al. Role of endothelial heparanase in delayed-type hypersensitivity. Blood. 2006;107:3609–3616. doi: 10.1182/blood-2005-08-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levy-Adam F, Miao HQ, Heinrikson RL, Vlodavsky I, Ilan N. Heterodimer formation is essential for heparanase enzymatic activity. Biochem Biophys Res Commun. 2003;308:885–891. doi: 10.1016/S0006-291X(03)01478-5. [DOI] [PubMed] [Google Scholar]
- 59.McKenzie E, Young K, Hircock M, Bennett J, Bhaman M, et al. Biochemical characterization of the active heterodimer form of human heparanase (Hpa1) protein expressed in insect cells. Biochem J. 2003;373:423–435. doi: 10.1042/BJ20030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nardella C, Lahm A, Pallaoro M, Brunetti M, Vannini A, et al. Mechanism of activation of human heparanase investigated by protein engineering. Biochemistry. 2004;43:1862–1873. doi: 10.1021/bi030203a. [DOI] [PubMed] [Google Scholar]
- 61.Hulett MD, Hornby JR, Ohms SJ, Zuegg J, Freeman C, et al. Identification of active-site residues of the pro-metastatic endoglycosidase heparanase. Biochemistry. 2000;39:15659–15667. doi: 10.1021/bi002080p. [DOI] [PubMed] [Google Scholar]
- 62.Abboud-Jarrous G, Rangini-Guetta Z, Aingorn H, Atzmon R, Elgavish S, et al. Site-directed mutagenesis, proteolytic cleavage, and activation of human proheparanase. J Biol Chem. 2005;280:13568–13575. doi: 10.1074/jbc.M413370200. [DOI] [PubMed] [Google Scholar]
- 63.Abboud-Jarrous G, Atzmon R, Peretz T, Palermo C, Gadea BB, et al. Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J Biol Chem. 2008;283:18167–18176. doi: 10.1074/jbc.M801327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of glycobiology. 2008;40:29606–29613. [PubMed] [Google Scholar]
- 65.Nakajima M, Irimura T, Di Ferrante N, Nicolson GL. Metastatic melanoma cell heparanase. Characterization of heparan sulfate degradation fragments produced by B16 melanoma endoglucuronidase. J Biol Chem. 1984;259:2283–2290. [PubMed] [Google Scholar]
- 66.Temkin V, Aingorn H, Puxeddu I, Goldshmidt O, Zcharia E, et al. Eosinophil major basic protein: first identified natural heparanase-inhibiting protein. J Allergy Clin Immunol. 2004;113:703–709. doi: 10.1016/j.jaci.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 67.Vlodavsky I, Fuks Z, Bar-Ner M, Ariav Y, Schirrmacher V. Lymphoma cell-mediated degradation of sulfated proteoglycans in the subendothelial extracellular matrix: relationship to tumor cell metastasis. Cancer Res. 1983;43:2704–2711. [PubMed] [Google Scholar]
- 68.Mahtouk K, Hose D, Raynaud P, Hundemer M, Jourdan M, et al. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 2007;109:4914–4923. doi: 10.1182/blood-2006-08-043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Y, Macleod V, Bendre M, Huang Y, Theus AM, et al. Heparanase promotes the spontaneous metastasis of myeloma cells to bone. Blood. 2005;105:1303–1309. doi: 10.1182/blood-2004-06-2141. [DOI] [PubMed] [Google Scholar]
- 70.Doviner V, Maly B, Kaplan V, Gingis-Velitski S, Ilan N, et al. Spatial and temporal heparanase expression in colon mucosa throughout the adenoma-carcinoma sequence. Mod Pathol. 2006;19:878–888. doi: 10.1038/modpathol.3800603. [DOI] [PubMed] [Google Scholar]
- 71.Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, et al. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 72.Roy M, Reiland J, Murry BP, Chouljenko V, Kousoulas KG, et al. Antisense-mediated suppression of heparanase gene inhibits melanoma cell invasion. Neoplasia. 2005;7:253–262. doi: 10.1593/neo.04493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vreys V, David G. Mammalian heparanase: what is the message? J Cell Mol Med. 2007;11:427–452. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friedmann Y, Vlodavsky I, Aingorn H, Aviv A, Peretz T, et al. Expression of heparanase in normal, dysplastic, and neoplastic human colonic mucosa and stroma : evidence for its role in colonic tumorigenesis [In Process Citation] Am J Pathol. 2000;157:1167–1175. doi: 10.1016/S0002-9440(10)64632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato T, Yamaguchi A, Goi T, Hirono Y, Takeuchi K, et al. Heparanase expression in human colorectal cancer and its relationship to tumor angiogenesis, hematogenous metastasis, and prognosis. J Surg Oncol. 2004;87:174–181. doi: 10.1002/jso.20097. [DOI] [PubMed] [Google Scholar]
- 76.Tang W, Nakamura Y, Tsujimoto M, Sato M, Wang X, et al. Heparanase: a key enzyme in invasion and metastasis of gastric carcinoma. Mod Pathol. 2002;15:593–598. doi: 10.1038/modpathol.3880571. [DOI] [PubMed] [Google Scholar]
- 77.El-Assal ON, Yamanoi A, Ono T, Kohno H, Nagasue N. The clinicopathological significance of heparanase and basic fibroblast growth factor expressions in hepatocellular carcinoma. Clin Cancer Res. 2001;7:1299–1305. [PubMed] [Google Scholar]
- 78.Kim AW, Xu X, Hollinger EF, Gattuso P, Godellas CV, et al. Human heparanase-1 gene expression in pancreatic adenocarcinoma. J Gastrointest Surg. 2002;6:167–172. doi: 10.1016/S1091-255X(01)00087-7. [DOI] [PubMed] [Google Scholar]
- 79.Rohloff J, Zinke J, Schoppmeyer K, Tannapfel A, Witzigmann H, et al. Heparanase expression is a prognostic indicator for postoperative survival in pancreatic adenocarcinoma. Br J Cancer. 2002;86:1270–1275. doi: 10.1038/sj.bjc.6600232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 81.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 82.Matzner Y, Bar-Ner M, Yahalom J, Ishai-Michaeli R, Fuks Z, et al. Degradation of heparan sulfate in the subendothelial extracellular matrix by a readily released heparanase from human neutrophils. Possible role in invasion through basement membranes. J Clin Invest. 1985;76:1306–1313. doi: 10.1172/JCI112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fridman R, Lider O, Naparstek Y, Fuks Z, Vlodavsky I, et al. Soluble antigen induces T lymphocytes to secrete an endoglycosidase that degrades the heparan sulfate moiety of subendothelial extracellular matrix. J Cell Physiol. 1987;130:85–92. doi: 10.1002/jcp.1041300113. [DOI] [PubMed] [Google Scholar]
- 84.Vlodavsky I, Eldor A, Haimovitz-Friedman A, Matzner Y, Ishai-Michaeli R, et al. Expression of heparanase by platelets and circulating cells of the immune system: possible involvement in diapedesis and extravasation. Invasion Metastasis. 1992;12:112–127. [PubMed] [Google Scholar]
- 85.Lider O, Baharav E, Mekori YA, Miller T, Naparstek Y, et al. Suppression of experimental autoimmune diseases and prolongation of allograft survival by treatment of animals with low doses of heparins. J Clin Invest. 1989;83:752–756. doi: 10.1172/JCI113953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naparstek Y, Cohen IR, Fuks Z, Vlodavsky I. Activated T lymphocytes produce a matrix-degrading heparan sulphate endoglycosidase. Nature. 1984;310:241–244. doi: 10.1038/310241a0. [DOI] [PubMed] [Google Scholar]
- 87.Lider O, Mekori YA, Miller T, Bar-Tana R, Vlodavsky I, et al. Inhibition of T lymphocyte heparanase by heparin prevents T cell migration and T cell-mediated immunity. Eur J Immunol. 1990;20:493–499. doi: 10.1002/eji.1830200306. [DOI] [PubMed] [Google Scholar]
- 88.Li RW, Freeman C, Yu D, Hindmarsh EJ, Tymms KE, et al. Dramatic regulation of heparanase activity and angiogenesis gene expression in synovium from patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:1590–1600. doi: 10.1002/art.23489. [DOI] [PubMed] [Google Scholar]
- 89.Shafat I, Ilan N, Zoabi S, Vlodavsky I, Nakhoul F. Heparanase levels are elevated in the urine and plasma of type 2 diabetes patients and associate with blood glucose levels. PLoS One. 2011;6:e17312. doi: 10.1371/journal.pone.0017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 91.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 92.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 93.Anderson CA, Boucher G, Lees CW, Franke A, D’Amato M, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, et al. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6:205–214. [PubMed] [Google Scholar]
- 95.Mullin JM, Snock KV. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res. 1990;50:2172–2176. [PubMed] [Google Scholar]
- 96.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 97.Clevers H. Colon cancer–understanding how NSAIDs work. N Engl J Med. 2006;354:761–763. doi: 10.1056/NEJMcibr055457. [DOI] [PubMed] [Google Scholar]
- 98.Lashner BA. Colorectal cancer in ulcerative colitis patients: survival curves and surveillance. Cleve Clin J Med. 1994;61:272–275. doi: 10.3949/ccjm.61.4.272. [DOI] [PubMed] [Google Scholar]
- 99.Herszenyi L, Miheller P, Tulassay Z. Carcinogenesis in inflammatory bowel disease. Dig Dis. 2007;25:267–269. doi: 10.1159/000103898. [DOI] [PubMed] [Google Scholar]
- 100.Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 102.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 103.Bollrath J, Greten FR. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009;10:1314–1319. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010;29:3313–3323. doi: 10.1038/onc.2010.109. [DOI] [PubMed] [Google Scholar]
- 105.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 106.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(2101–2114):e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 109.Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:21–33. doi: 10.1002/ibd.3780060105. [DOI] [PubMed] [Google Scholar]
- 110.Krieglstein CF, Cerwinka WH, Sprague AG, Laroux FS, Grisham MB, et al. Collagen-binding integrin alpha1beta1 regulates intestinal inflammation in experimental colitis. J Clin Invest. 2002;110:1773–1782. doi: 10.1172/JCI200215256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900–6908. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 112.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/S1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 113.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 114.Menzel K, Hausmann M, Obermeier F, Schreiter K, Dunger N, et al. Cathepsins B, L and D in inflammatory bowel disease macrophages and potential therapeutic effects of cathepsin inhibition in vivo. Clin Exp Immunol. 2006;146:169–180. doi: 10.1111/j.1365-2249.2006.03188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beaulieu JF, Vachon PH, Chartrand S. Immunolocalization of extracellular matrix components during organogenesis in the human small intestine. Anat Embryol (Berl) 1991;183:363–369. doi: 10.1007/BF00196837. [DOI] [PubMed] [Google Scholar]
- 116.Simon-Assmann P, Bouziges F, Vigny M, Kedinger M. Origin and deposition of basement membrane heparan sulfate proteoglycan in the developing intestine. J Cell Biol. 1989;109:1837–1848. doi: 10.1083/jcb.109.4.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Belmiro CL, Souza HS, Elia CC, Castelo-Branco MT, Silva FR et al (2005) Biochemical and immunohistochemical analysis of glycosaminoglycans in inflamed and non-inflamed intestinal mucosa of patients with Crohn’s disease. Int J Colorectal Dis [DOI] [PubMed]
- 118.Bode L, Eklund EA, Murch S, Freeze HH. Heparan sulfate depletion amplifies TNF-alpha-induced protein leakage in an in vitro model of protein-losing enteropathy. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1015–G1023. doi: 10.1152/ajpgi.00461.2004. [DOI] [PubMed] [Google Scholar]
- 119.Bode L, Murch S, Freeze HH. Heparan sulfate plays a central role in a dynamic in vitro model of protein-losing enteropathy. J Biol Chem. 2006;281:7809–7815. doi: 10.1074/jbc.M510722200. [DOI] [PubMed] [Google Scholar]
- 120.Bode L, Salvestrini C, Park PW, Li JP, Esko JD, et al. Heparan sulfate and syndecan-1 are essential in maintaining murine and human intestinal epithelial barrier function. J Clin Invest. 2008;118:229–238. doi: 10.1172/JCI32335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oshiro M, Ono K, Suzuki Y, Ota H, Katsuyama T, et al. Immunohistochemical localization of heparan sulfate proteoglycan in human gastrointestinal tract. Histochem Cell Biol. 2001;115:373–380. doi: 10.1007/s004180100271. [DOI] [PubMed] [Google Scholar]
- 122.Murch SH, MacDonald TT, Walker-Smith JA, Levin M, Lionetti P, et al. Disruption of sulphated glycosaminoglycans in intestinal inflammation. Lancet. 1993;341:711–714. doi: 10.1016/0140-6736(93)90485-Y. [DOI] [PubMed] [Google Scholar]
- 123.Day R, Forbes A. Heparin, cell adhesion, and pathogenesis of inflammatory bowel disease. Lancet. 1999;354:62–65. doi: 10.1016/S0140-6736(98)09267-8. [DOI] [PubMed] [Google Scholar]
- 124.Day R, Ilyas M, Daszak P, Talbot I, Forbes A. Expression of syndecan-1 in inflammatory bowel disease and a possible mechanism of heparin therapy. Dig Dis Sci. 1999;44:2508–2515. doi: 10.1023/A:1026647308089. [DOI] [PubMed] [Google Scholar]
- 125.Symonds DA. The glycosaminoglycans of the human colon in inflammatory and neoplastic conditions. Arch Pathol Lab Med. 1978;102:146–149. [PubMed] [Google Scholar]
- 126.Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA, et al. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. doi: 10.4161/cc.4.2.1413. [DOI] [PubMed] [Google Scholar]
- 127.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jarnicki A, Putoczki T, Ernst M. Stat3: linking inflammation to epithelial cancer—more than a “gut” feeling? Cell Div. 2010;5:14. doi: 10.1186/1747-1028-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 130.Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39:87–92. doi: 10.1136/gut.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okayasu I, Yamada M, Mikami T, Yoshida T, Kanno J, et al. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J Gastroenterol Hepatol. 2002;17:1078–1083. doi: 10.1046/j.1440-1746.2002.02853.x. [DOI] [PubMed] [Google Scholar]
- 132.Zcharia E, Metzger S, Chajek-Shaul T, Aingorn H, Elkin M, et al. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. Faseb J. 2004;18:252–263. doi: 10.1096/fj.03-0572com. [DOI] [PubMed] [Google Scholar]
- 133.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 134.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 135.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 136.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 139.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 141.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Benhamron S, Nechushtan H, Verbovetski I, Krispin A, Abboud-Jarrous G, et al. Translocation of active heparanase to cell surface regulates degradation of extracellular matrix heparan sulfate upon transmigration of mature monocyte-derived dendritic cells. J Immunol. 2006;176:6417–6424. doi: 10.4049/jimmunol.176.11.6417. [DOI] [PubMed] [Google Scholar]
- 143.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 144.Brunn GJ, Bungum MK, Johnson GB, Platt JL. Conditional signaling by Toll-like receptor 4. Faseb J. 2005;19:872–874. doi: 10.1096/fj.04-3211fje. [DOI] [PubMed] [Google Scholar]
- 145.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fukata M, Hernandez Y, Conduah D, Cohen J, Chen A, et al. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm Bowel Dis. 2009;15:997–1006. doi: 10.1002/ibd.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Subbaramaiah K, Yoshimatsu K, Scherl E, Das KM, Glazier KD, et al. Microsomal prostaglandin E synthase-1 is overexpressed in inflammatory bowel disease. Evidence for involvement of the transcription factor Egr-1. J Biol Chem. 2004;279:12647–12658. doi: 10.1074/jbc.M312972200. [DOI] [PubMed] [Google Scholar]
- 148.Fiebiger E, Maehr R, Villadangos J, Weber E, Erickson A, et al. Invariant chain controls the activity of extracellular cathepsin L. J Exp Med. 2002;196:1263–1269. doi: 10.1084/jem.20020762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang ZH, Chen Y, Zhao HJ, Xie CY, Ding J, et al. Silencing of heparanase by siRNA inhibits tumor metastasis and angiogenesis of human breast cancer in vitro and in vivo. Cancer Biol Ther. 2007;6:587–595. doi: 10.4161/cbt.6.4.3888. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Y, Li L, Wang Y, Zhang J, Wei G, et al. Downregulating the expression of heparanase inhibits the invasion, angiogenesis and metastasis of human hepatocellular carcinoma. Biochem Biophys Res Commun. 2007;358:124–129. doi: 10.1016/j.bbrc.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 151.Zheng LD, Jiang GS, Pu JR, Mei H, Dong JH, et al. Stable knockdown of heparanase expression in gastric cancer cells in vitro. World J Gastroenterol. 2009;15:5442–5448. doi: 10.3748/wjg.15.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ferro V, Hammond E, Fairweather JK. The development of inhibitors of heparanase, a key enzyme involved in tumour metastasis, angiogenesis and inflammation. Mini Rev Med Chem. 2004;4:693–702. doi: 10.2174/1389557043403729. [DOI] [PubMed] [Google Scholar]
- 153.McKenzie EA. Heparanase: a target for drug discovery in cancer and inflammation. Br J Pharmacol. 2007;151:1–14. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miao HQ, Elkin M, Aingorn E, Ishai-Michaeli R, Stein CA, et al. Inhibition of heparanase activity and tumor metastasis by laminarin sulfate and synthetic phosphorothioate oligodeoxynucleotides. Int J Cancer. 1999;83:424–431. doi: 10.1002/(SICI)1097-0215(19991029)83:3<424::AID-IJC20>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 155.Pan W, Miao HQ, Xu YJ, Navarro EC, Tonra JR, et al. 1-[4-(1H-Benzoimidazol-2-yl)-phenyl]-3-[4-(1H-benzoimidazol-2-yl)-phenyl]- urea derivatives as small molecule heparanase inhibitors. Bioorg Med Chem Lett. 2006;16:409–412. doi: 10.1016/j.bmcl.2005.09.069. [DOI] [PubMed] [Google Scholar]
- 156.Simizu S, Ishida K, Osada H. Heparanase as a molecular target of cancer chemotherapy. Cancer Sci. 2004;95:553–558. doi: 10.1111/j.1349-7006.2004.tb02485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Naggi A, Casu B, Perez M, Torri G, Cassinelli G, et al. Modulation of the heparanase-inhibiting activity of heparin through selective desulfation, graded N-acetylation, and glycol splitting. J Biol Chem. 2005;280:12103–12113. doi: 10.1074/jbc.M414217200. [DOI] [PubMed] [Google Scholar]
- 158.Dredge K, Hammond E, Handley P, Gonda TJ, Smith MT, et al. PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models. Br J Cancer. 2011;104:635–642. doi: 10.1038/bjc.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gaffney PR, Doyle CT, Gaffney A, Hogan J, Hayes DP, et al. Paradoxical response to heparin in 10 patients with ulcerative colitis. Am J Gastroenterol. 1995;90:220–223. [PubMed] [Google Scholar]
- 160.Torkvist L, Thorlacius H, Sjoqvist U, Bohman L, Lapidus A, et al. Low molecular weight heparin as adjuvant therapy in active ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1323–1328. doi: 10.1046/j.1365-2036.1999.00599.x. [DOI] [PubMed] [Google Scholar]
- 161.Evans RC, Wong VS, Morris AI, Rhodes JM. Treatment of corticosteroid-resistant ulcerative colitis with heparin–a report of 16 cases. Aliment Pharmacol Ther. 1997;11:1037–1040. doi: 10.1046/j.1365-2036.1997.00252.x. [DOI] [PubMed] [Google Scholar]
- 162.Floer M, Gotte M, Wild MK, Heidemann J, Gassar ES, et al. Enoxaparin improves the course of dextran sodium sulfate-induced colitis in syndecan-1-deficient mice. Am J Pathol. 2010;176:146–157. doi: 10.2353/ajpath.2010.080639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kakkar AK. An expanding role for antithrombotic therapy in cancer patients. Cancer Treat Rev. 2003;29(Suppl 2):23–26. doi: 10.1016/S0305-7372(03)80006-3. [DOI] [PubMed] [Google Scholar]
- 164.Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130–2135. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 165.Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost. 2007;5:729–737. doi: 10.1111/j.1538-7836.2007.02427.x. [DOI] [PubMed] [Google Scholar]
- 166.Lee AY, Rickles FR, Julian JA, Gent M, Baker RI, et al. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol. 2005;23:2123–2129. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- 167.Casu B, Vlodavsky I, Sanderson RD. Non-anticoagulant heparins and inhibition of cancer. Pathophysiol Haemost Thromb. 2008;36:195–203. doi: 10.1159/000175157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bloom S, Kiilerich S, Lassen MR, Forbes A, Leiper K, et al. Low molecular weight heparin (tinzaparin) vs. placebo in the treatment of mild to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2004;19:871–878. doi: 10.1111/j.1365-2036.2004.01926.x. [DOI] [PubMed] [Google Scholar]
- 169.de Bievre MA, Vrij AA, Schoon EJ, Dijkstra G, de Jong AE, et al. Randomized, placebo-controlled trial of low molecular weight heparin in active ulcerative colitis. Inflamm Bowel Dis. 2007;13:753–758. doi: 10.1002/ibd.20085. [DOI] [PubMed] [Google Scholar]
- 170.Lever R, Page CP. Novel drug development opportunities for heparin. Nat Rev Drug Discov. 2002;1:140–148. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- 171.Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;13:2057–2073. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- 172.Gil N, Goldberg R, Neuman T, Garsen M, Zcharia E, et al. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012;61:208–216. doi: 10.2337/db11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hostettler N, Naggi A, Torri G, Ishai-Michaeli R, Casu B, et al. P-selectin- and heparanase-dependent antimetastatic activity of non-anticoagulant heparins. Faseb J. 2007;21:3562–3572. doi: 10.1096/fj.07-8450com. [DOI] [PubMed] [Google Scholar]
- 174.Yang Y, MacLeod V, Dai Y, Khotskaya-Sample Y, Shriver Z, et al. The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood. 2007;110:2041–2048. doi: 10.1182/blood-2007-04-082495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Meirovitz A, Hermano E, Lerner I, Zcharia E, Pisano C, et al. Role of heparanase in radiation-enhanced invasiveness of pancreatic carcinoma. Cancer Res. 2011;71:2772–2780. doi: 10.1158/0008-5472.CAN-10-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]