Abstract
Actin filament-associated protein (AFAP) plays a critical role in the regulation of actin filament integrity, formation and maintenance of the actin network, function of focal contacts, and cell migration. Here, we show that endogenous AFAP was present not only in the cytoskeletal but also in the cytosolic fraction. Depolymerization of actin filaments with cytochalasin D or latrunculin A increased AFAP in the cytosolic fraction. AFAP harbors an actin-binding domain (ABD) in its C-terminus. AFAPΔABD, an AFAP mutant with selective ABD deletion, was mainly in the cytosolic fraction when overexpressed in the cells, which was associated with a disorganized cytoskeleton with reduced stress fibers, accumulation of F-actin on cellular membrane, and formation of actin-rich small dots. Cortactin, a well-known podosome marker, was colocalized with AFAPΔABD in these small dots at the ventral surface of the cell, indicating that these small dots fulfill certain criteria of podosomes. However, these podosome-like small dots did not digest gelatin matrix. This may be due to the reduced interaction between AFAPΔABD and c-Src. When AFAPΔABD-transfected cells were stimulated with phorbol ester, they formed podosome-like structures with larger sizes, less numerous and longer life span, in comparison with wild-type AFAP-transfected cells. These results indicate that the association of AFAP with F-actin through ABD is crucial for AFAP to regulate cytoskeletal structures. The AFAPΔABD, as cytosolic proteins, may be more accessible to the cellular membrane, podosome-like structures, and thus be more interactive for the regulation of cellular functions.
Keywords: Adaptor protein, Cytoskeleton, Matrix degradation, Cell invasion, c-Src activation
Introduction
Actin filament-associated protein (AFAP) was first identified as a substrate of the non-receptor tyrosine kinase v-Src [1]. AFAP harbors one Src Homology 3 (SH3) domain-binding motif and several putative SH2 domain-binding motifs [2, 3]. It contains two pleckstrin homology domains, a leucine zipper motif, and multiple putative serine/threonine phosphorylation sites. It interacts with actin filaments directly via an actin-binding domain (ABD) located in its C-terminus [2, 3]. AFAP functions as an adaptor protein, linking Src tyrosine kinase family members and/or other signaling proteins to actin filaments [4]. AFAP plays an essential role in regulating actin filament integrity [1, 5–8], cytoskeleton organization [9], function of focal contacts, and cell migration [10]. In MDA-MB-231 breast cancer cells, AFAP is required for actin stress fiber formation and cell adhesion [5]. In prostate cancer cells, AFAP is highly expressed and contributes to tumorigenic growth by regulating focal contact formation and dynamics [11]. In mouse embryo fibroblasts, PI3 K inhibitor LY294002 blocks protein kinase C (PKC) activation-directed colocalization between AFAP and c-Src, subsequent c-Src activation and cell migration [10].
Podosomes are recently discovered actin-rich structures that protrude into the extracellular matrix, resulting in localized remodeling activities with enhanced invasiveness [12, 13]. Podosomes not only establish close contact with substratum but also degrade components of extracellular matrix to assist motile cells to cross tissue boundaries. AFAP is required to mediate PKCα-induced activation of the tyrosine kinase c-Src and the subsequent formation of podosomes [14]. AFAP is a substrate of PKCα, and PKCα-AFAP interactions direct podosome formation in vascular smooth muscle A7r5 cells. The phosphorylation of AFAP at Ser277 by PKCα affects podosome lifespan [15]. Tks5, a scaffolding protein, is localized to podosomes in v-Src-transformed fibroblasts and in A7r5 cells, and serves as a specific recruiting adapter for AFAP, p190RhoGAP, and cortactin during podosome formation [16].
We cloned genes encoding rat [3] and human AFAP [17] and found that AFAP is distributed along the actin filaments in fetal rat lung epithelial cells and fibroblasts. Mechanical stretch increases the tyrosine phosphorylation of rat AFAP and the binding between AFAP and c-Src [3]. AFAP can directly activate c-Src through binding to its SH3 and/or SH2 domains [17]. Mutations at these specific binding sites on AFAP block the mechanical stretch-induced c-Src activation [17]. Recently, we reported that the interaction between AFAP and c-Src may lead to SRE/AP-1 transcriptional activation [18]. As a c-Src-binding partner, association with filamentous actin structures makes AFAP a very good candidate as an intracellular transducer for mechanotransduction [19].
Sawada and Sheetz reported that after removing cytoplasm with Triton extraction, the cytoskeletal structure can still sense mechanical stretch by activating protein tyrosine kinases [20]. We suspected that AFAP could be an important mediator to this type of cellular process. In the present study, when we separated the cytosolic fraction from the cytoskeletal fraction, a portion of AFAP was found in the cytosolic fraction, that is, it was not associated with F-actin. We hypothesized that the association of AFAP with F-actin is a dynamic process. AFAP associated with or free from F-actin may have different roles in the regulation of cellular function. To test this hypothesis, we selectively deleted the actin-binding domain from AFAP and compared its function with wild-type AFAP in actin filament organization and podosome function.
Materials and methods
Reagents, constructs, and antibodies
Src (clone GD11) monoclonal antibody (mAb), phosphotyrosine (4G10) mAb, cortactin (p80/85, clone 4F11) mAb, and horseradish peroxidase (HRP)-conjugated goat anti-mouse or anti-rabbit secondary antibodies were from Upstate Biotechnology Inc. (Lake Placid, NY). Src phospho-Tyr416 and phospho-Tyr527 polyclonal antibodies were from Biosource International (Camarillo, CA). FAK polyclonal antibody was from Cell Signaling (Danvers, MA). Anti-GFP antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-α-tubulin and anti-γ-tubulin mAbs were from Sigma (St. Louis, MO). AFAP (clone F1) polyclonal antibody, constructs for AFAP wild-type (WT), and the pCMV expression vector for c-Src were gifts from Dr. Daniel C. Flynn (West Virginia University). Alexa Fluor 594 labeled secondary antibodies, Oregon Green 488 phalloidin, Rhodamine phalloidin, TRITC and Hoechst dye 33342 were from Invitrogen Molecular Probes (Carlsbad, CA). All other reagents, unless indicated, were purchased from Sigma (St. Louis, MO).
AFAP∆ABD expression vector
pEGFP-c3-AFAP∆ABD construct was generated from chicken full-length pEGFP-c3-AFAP by deleting 2,062–2,112 bp (actin-binding domain), with QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) [11]. The forward primer is 5′-CGT GCT GCC ATT GAA GTC GAA GAG GAA TGT AAG ACG-3′ and the reverse primer is 5′-CGT CTT ACA TTC CTC TTC GAC TTC AAT GGC AGC ACG-3′ [18].
Cell culture
COS7, C3H10T1/2, and human normal bronchial epithelial BEAS2B cells were maintained in low-glucose Dulbecco’s modified Eagle’s medium (GIBCO, Rockville, MD) with 10% fetal bovine serum. Culture medium also contained penicillin (1 mg/ml) and streptomycin (1 mg/ml) (GIBCO) as previously described [3, 11, 17].
Biochemical studies
The detailed procedures for cell transfection, immunoprecipitation, and Western blotting have been described [11, 17, 21].
Preparation of Triton X-100 soluble and insoluble fraction
The Triton X-100 soluble and insoluble fractions of cell lysates were prepared with a protocol modified from Sawada and Sheetz [20, 22]. Briefly, each cell culture plate was overlaid with 5 ml of hot gelatin solution (2.5%), incubated at room temperature for 2 min, and the extra gelatin solution was then removed. Ice-cold PBS (5 ml/plate) was added to coated plates at room temperature for 5 min. C3H10T1/2 cells were cultured on the coated plates with transfection of pEGFP vector, pEGFP-AFAP, or pEGFP-AFAPΔABD. Twenty-four hours after transfection, the cells were washed with PBS and then treated with 500 μl of PBS containing 0.25% Triton X-100 and a cocktail of protease inhibitors for 1 min [20, 22]. The Triton soluble fraction was collected. The remaining cellular structures, as the Triton X-100 insoluble portion, were collected with regular cell lysis buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 2 mM EGTA, 2 mM EDTA, 1% Triton X-100, 100 μg/ml soybean trypsin inhibitor, 100 μg/ml benzamidine hydrochloride, 1 mM PMSF, 50 μg/ml aprotinin, 50 μg/ml leupeptin, 50 μg/ml pepstatin A, 50 μg/ml antipain, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, and 10 mM sodium orthovanadate). To determine the distribution of endogenous AFAP, non-transfected cells were treated with cytochalasin D or latrunculin A at different concentrations for 2 h and Triton soluble and insoluble fractions were then collected. Protein concentrations were measured by the Bradford Protein Assay (Bio-Rad Laboratories, Mississauga, Ontario). Equal amounts of total protein were subjected to SDS-PAGE, followed by Western blotting.
Immunofluorescent staining and in situ zymography
Cells were cultured on glass cover slips (22 × 22 mm, VWR Scientific Inc, West Chester, PA) in a six-well plate (Sarstedt, Rommelsdorfer, Germany). Cells were transiently transfected with pEGFP vector, pEGFP-AFAP, or pEGFP-AFAPΔABD. The staining protocol and microscopic condition have been described in detail [23, 24]. Briefly, cells were fixed with 4% paraformaldehyde in PBS for 10 min, and then permeabilized with 0.1% Triton X-100 in PBS for 30 min. The cells were stained with Rhodamine phalloidin and Hoechst dye 33342 for actin filaments and nuclei localization, respectively. The cover slips were mounted on microscope slides with Fluorescent Mounting Medium (Dako Cytomation Inc, Carpinteria, CA).
For staining of AFAP associated with Triton X-100 insoluble cytoskeleton, after removing the Triton X-100 soluble fraction, the remaining cellular components were washed with PBS and fixed with 4% paraformaldehyde in PBS for 10 min, triple stained with primary and Alexa Fluor 594 labeled secondary antibody for AFAP, Oregon Green 488 Phalloidin for F-actin, and Hoechst dye 33342 for nuclei.
For in situ zymography, glass cover slips were pre-cleaned with nitric acid, sterilized with ethanol, treated with 50 μg/ml poly-l-lysine, and fixed with 0.5% glutaraldehyde. The cover slips were coated with a base layer of gelatin sucrose gel and a layer of TRITC-conjugated gelatin on top. The residual reactive groups in the gelatin matrix were quenched with 5 mg/ml sodium borohydride. Cells were seeded on the coated cover slips in six-well plates. The cell culture and transfection were done under the same conditions described above. Foci of degraded matrix were observed as dark areas (diameter ~2 μm) under or near a cell on the red fluorescent gelatin matrix [23, 24].
Cell imaging was collected with an inverted laser scanning fluorescence confocal microscope (Olympus FluoView FV1000-ASW, Model 1X81) equipped with a 60× UPlanApo water immersion objective [numerical aperture (NA), 1.20] and a data acquisition system.
Live cell imaging
BEAS2B cells were transfected with designated plasmids expressing pEGFP, pEGFP-AFAP, or pEGFP-AFAPΔABD, using Lipofectamine 2000 (Invitrogen) as the transfection reagent according to the manufacturer’s instructions [11, 17]. After 48 h, the transfected cells were subjected to live cell imaging [23]. The time-lapse series of photographs was taken using the confocal microscope mentioned above, equipped with a computer-driven camera and autofocus system in a humidified chamber with 5% CO2 at 37°C. Differential interference contrast (DIC) and fluorescence images were taken every 5 s for up to 25 min. Original digital images were converted to movies with 30 frames/s by Olympus FluoView FV10-ASW Version 01.07.02 software.
GFP-positive cell sorting, cell invasion, and gelatin zymograph assay
BEAS-2B cells were cultured in a 100-mm culture dish until the density reached approximately 85% confluence and then transfected with 10 μg GFP plasmids and 20 μl Fugene 6 HD (Roche Diagnostic, Laval, Canada) for 6 h. GFP-positive cells were sorted with a cell sorter MoFlo-XDP (Beckman Coulter Canada, Inc, Mississauga, Canada) 42 h post-transfection. GFP-positive cells (5 × 104) were seeded into each invasion chamber of 24-well plate (Millipore, Billerica, MA) and cultured in 300 μl DMEM with DMSO (as vehicle control) or 500 nM PDBu for 18 h. The invading cells attaching to the chamber membrane were stained and counted. Cell culture media (25 μl for each sample) were analyzed with 10% zymogram gelatin gel (Invitrogen, Burlington, Canada) as described previously [24].
Statistics
Each experiment was performed at least three times. The quantification of each value represents the mean ± standard deviation. Significance was determined by Student’s t test or by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc analysis where appropriate.
Results
Endogenous AFAP is found not only associated with cytoskeleton but also in the cytoplasm
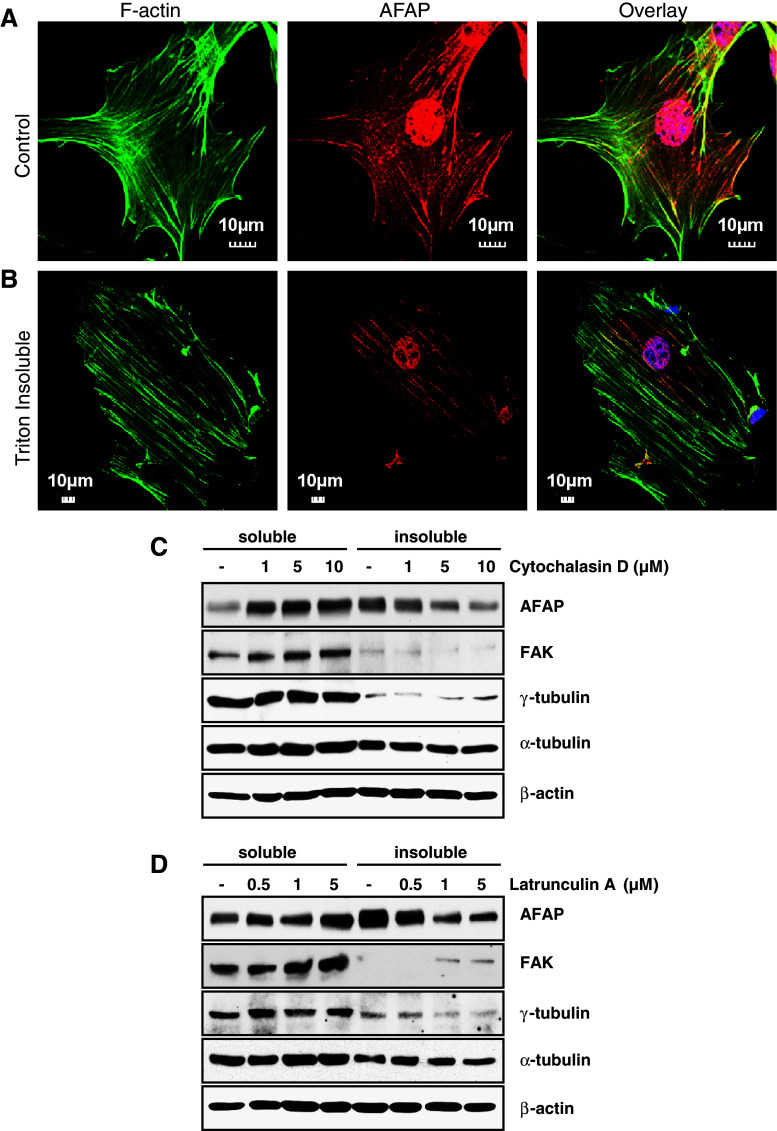
AFAP is well known for its distribution along stress fibers [2, 17, 25]. The C3H10T1/2 cell line established from C3H mouse embryo [26] is functionally similar to mesenchymal stem cells [27]. These cells display fibroblastic morphology in culture and have been used to study the interaction between AFAP and F-actin [6, 8, 17, 28]. When C3H10T1/2 cells were cultured on gelatin-coated plates and stained with Oregon Green 488 Phalloidin (Green) for F-actin, anti-AFAP (Red), and Hoechst dye (Blue) for nuclei, the endogenous AFAP was distributed along the F-actin fibers with nuclear staining in some cells (Fig. 1a). After the cells were treated with 0.25% Triton buffer for 1 min to remove the cytosolic fraction, AFAP staining was still found in the Triton-insoluble cytoskeleton portion, tightly bound to stress fibers (Fig. 1b). When the Triton-soluble and -insoluble fractions were subjected to Western blotting, AFAP, as well as cytoskeletal proteins, β-actin and α-tubulin, were found in both fractions (Fig. 1c, d). FAK has been found mainly in the cytosolic fraction of L929 cells with Triton extraction [20, 22]. We found similar results in C3H10T1/2 cells. Similarly, γ-tubulin was found mainly in the Triton-soluble fraction (Fig. 1c, d).
Fig. 1.
Endogenous AFAP is present in both cytoskeletal and cytosolic fractions. a Association of endogenous AFAP with the cytoskeletal fraction. C3H10T1/2 cells were cultured on gelatin-coated cover slips and stained with Oregon Green 488 Phalloidin (green), anti-AFAP (red) and Hoechst dye for nuclei (blue). b After cells were treated with 0.25% Triton X-100 for 1 min to remove cytosolic portion, AFAP was still seen associated with F-actin. c, d Depolymerization of actin filaments leads to a shift of AFAP from the Triton X-100 insoluble to soluble fraction. C3H10T1/2 cells were cultured on gelatin-coated plates and treated with cytochalasin D (c) or latrunculin A (d) at the indicated concentrations for 2 h. Triton X-100 soluble and insoluble fractions of cell lysates were collected and analyzed by Western blotting for AFAP distribution. FAK, γ-tubulin, α-tubulin and β-actin were also probed for comparison
To determine whether the distribution of AFAP between the cytoskeletal and cytosolic fractions can be regulated, C3H10T1/2 cells were treated with microfilament de-polymerization reagents, cytochalasin D, or latrunculin A, for 2 h. AFAP was redistributed from the Triton-insoluble cytoskeleton fraction to the soluble fraction in a cytochalasin D or latrunculin A concentration-dependent manner. In contrast, FAK, γ-tubulin, α-tubulin and β-actin did not show dramatic translocation between the two fractions (Fig. 1c, d). This suggested that endogenous AFAP was not only in cytoskeletal but also in the cytosolic fraction, and the distribution of AFAP between these two fractions can be regulated by the polymerization status of F-actin.
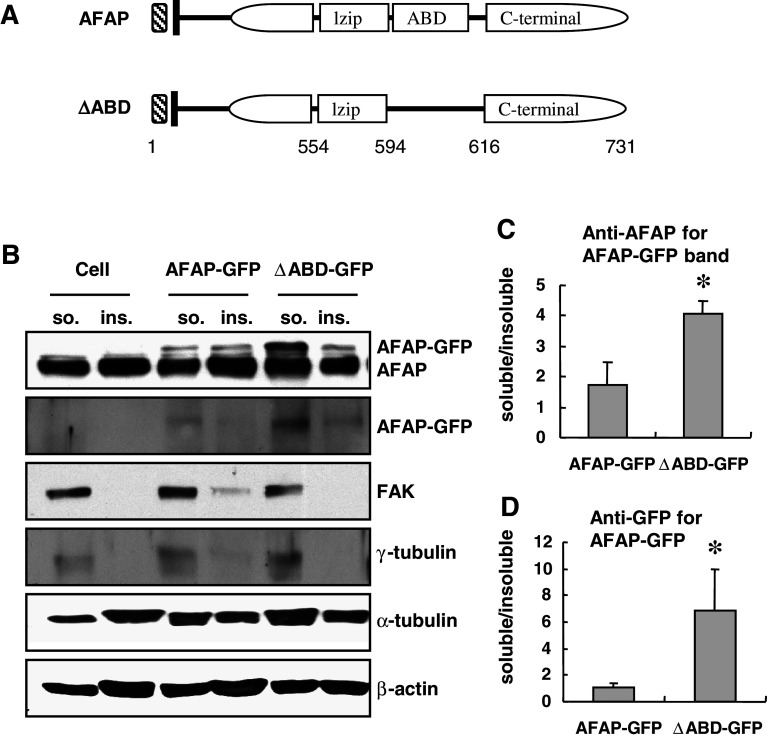
Actin-binding domain deletion in AFAP reduces its capability in associating with actin filament
Flynn and coworkers have identified an actin-binding domain in the C-terminus of AFAP between amino acids 594 and 616 [8]. We selectively deleted this domain from AFAP and generated an AFAPΔABD construct fused with GFP (Fig. 2a). C3H10T1/2 cells were transfected with pEGFP-AFAP or pEGFP-AFAP∆ABD, and extracted with Triton buffer. AFAP-GFP fusion proteins were determined by anti-AFAP or anti-GFP antibody. The GFP-AFAP fusion protein detected by either antibody was much more intense in the Triton-soluble fraction from the pEGFP-AFAP∆ABD-transfected cells than that from cells transfected with pEGFP-AFAP (Fig. 2b). The GFP-AFAP or GFP bands in both fractions were quantified through densitometry. The ratio of GFP-AFAP expressed in the Triton-soluble over Triton-insoluble fraction was significantly higher in the pEGFP-AFAP∆ABD-transfected cells than that in the pEGFP-AFAP-transfected cells (Fig. 2c, d).
Fig. 2.
Actin-binding domain deletion in AFAP reduces its capability in associating with actin filament. a Schematic representation of wild-type and the actin-binding domain deletion mutant (ΔABD) of AFAP. lzip leucine zipper motif, ABD actin-binding domain. b AFAP ΔABD was found to be enriched in the Triton soluble cytosolic fraction. C3H10T1/2 cells were transfected with pEGFP-AFAP or pEGFP-AFAP∆ABD. Triton-soluble and insoluble fractions of cell lysates were blotted with different antibodies as indicated. AFAP-GFP proteins were detected with anti-AFAP and anti-GFP antibodies. The molecular weight of AFAP-GFP is higher than endogenous AFAP. The ratio of AFAP-GFP in Triton-soluble versus insoluble fractions was quantified from anti-AFAP blots (c) or anti-GFP blots (d). *p < 0.05 versus AFAP-GFP group
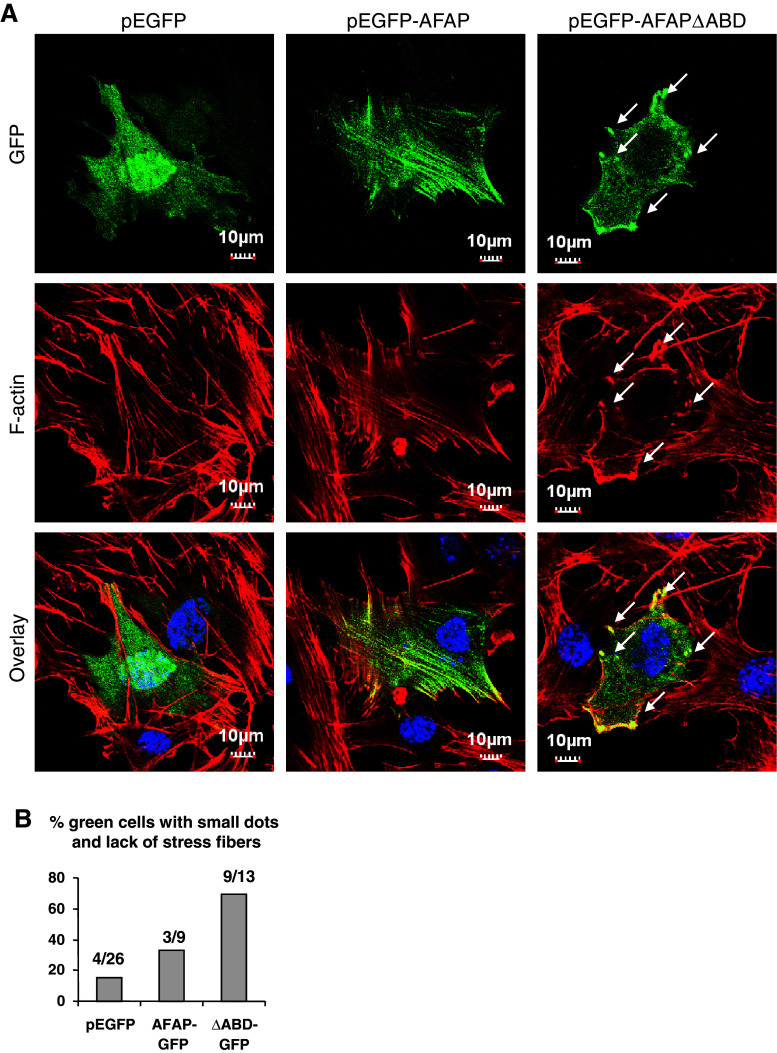
Overexpression of AFAP∆ABD induces cytoskeleton reorganization in C3H10T1/2 cells
To investigate the intracellular distribution of AFAPΔABD, C3H10T1/2 cells were transiently transfected with pEGFP vector, pEGFP-AFAP, or pEGFP-AFAP∆ABD, and then stained for F-actin. The pEGFP vector-transfected cells clearly displayed long F-actin stress fibers and the GFP signal appeared to be distributed in the cytoplasm (Fig. 3, left column). In pEGFP-AFAP-transfected cells, GFP-AFAP was well associated with F-actin stress fibers (Fig. 3, middle column). In pEGFP-AFAPΔABD-transfected cells, however, the long stress fibers disappeared and a few actin-rich small dots (shown by arrows) were found at the periphery of the cells. AFAPΔABD was found in the cytoplasm and was co-localized with these actin-rich small dots (Fig. 3, right column). These results suggest that the actin-binding domain of AFAP is involved in the regulation of actin filament structure. Overexpression of AFAPΔABD may compete with endogenous AFAP and interfere with the organization of stress fibers.
Fig. 3.
Expression of AFAPΔABD induces cytoskeleton disorganization in C3H10T1/2 cells. Over-expression of AFAPΔABD mutant interfered with actin filamentous structure. C3H10T1/2 cells were transfected with pEGFP vector, pEGFP-AFAP, or pEGFP-AFAPΔABD and stained with Rhodamine phalloidin for F-actin (red) and Hoechst dye for nuclei (blue)
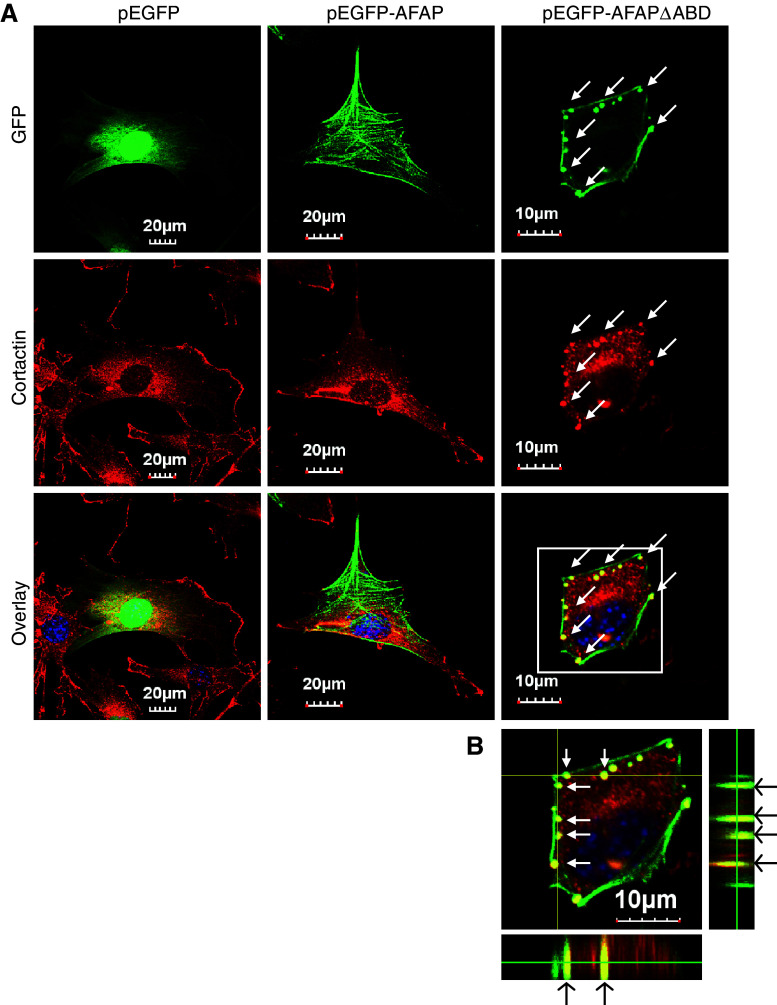
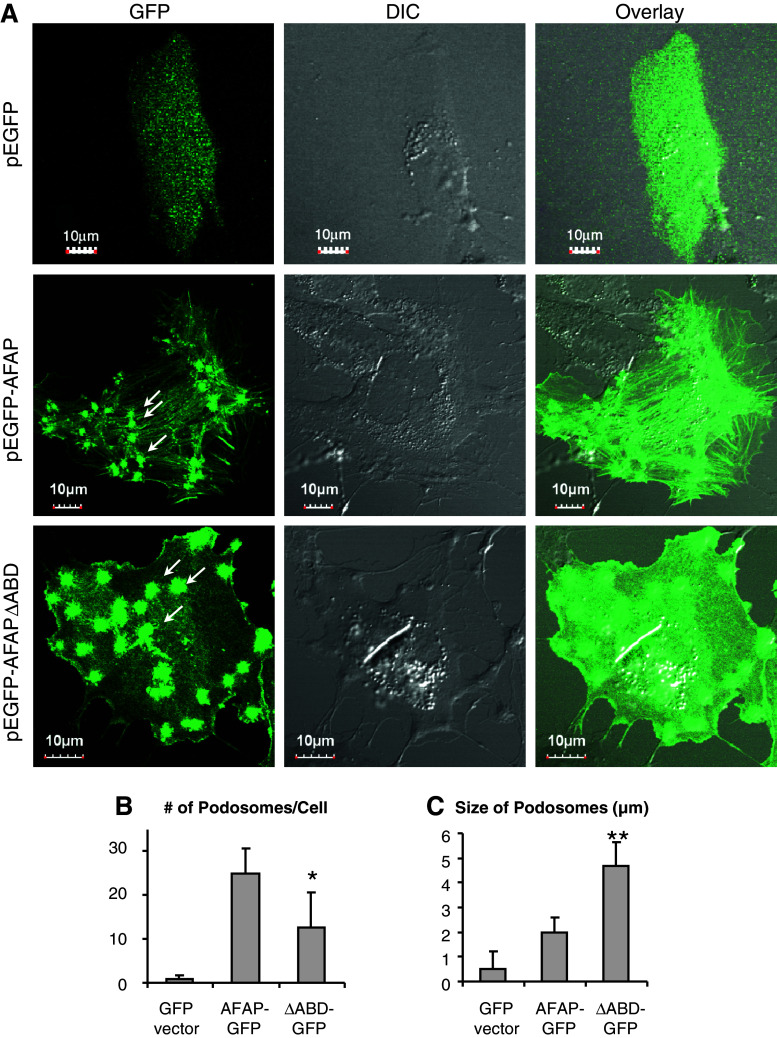
AFAPΔABD overexpression results in podosome-like structure formation in C3H10T1/2 cells
In v-Src transformed NIH3T3 fibroblasts, AFAP has been found in F-actin rich rosettes [2], also called podosomes, a specialized cellular structure for cell invasion [29]. AFAP also plays an important role in PKC activation-induced podosome formation [14]. To determine whether these F-actin rich small dots in pEGFP-AFAPΔABD-transfected cells share features of podosomes, C3H10T1/2 cells were transfected with pEGFP, pEGFP-AFAP, or pEGFP-AFAPΔABD, and stained with antibody for cortactin, a well-known podosome marker [29]. pEGFP-AFAPΔABD was found to be colocalized with cortactin in the small dots (Fig. 4a). Z-stack confocal microscopy demonstrated that these small dots are located at the ventral surface of the cells as inverted columns into the cytoplasm (Fig. 4b), which is a typical characteristic of podosome-like structures. The lack of binding to F-actin stress fiber by AFAPΔABD may facilitate formation of the podosome-like structures and AFAPΔABD may be more accessible to these structures.
Fig. 4.
AFAPΔABD is co-localized with cortactin in podosome-like structure in C3H10T1/2 cells. a C3H10T1/2 cells were transfected with pEGFP vector, pEGFP-AFAP or pEGFP-AFAPΔABD, and then stained for cortactin (red), a podosome marker. AFAPΔABD and cortactin are co-localized at podosome-like small dots (arrows). b Z-stack confocal microscopy images indicate that these small dots are inverted columns at the ventral surface of the cell
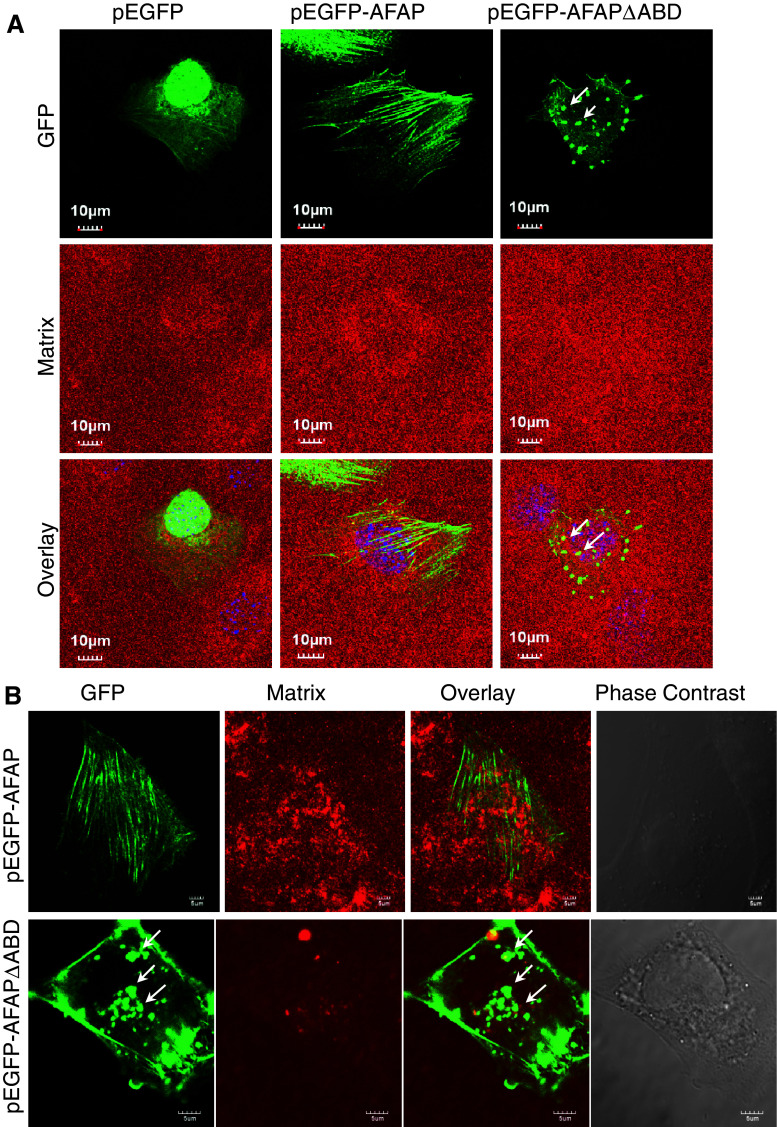
AFAPΔABD-induced podosome-like structures do not digest matrix
Recently, a study reported that MMP-14-deficient mouse bone marrow cells and spleen dendritic cells exhibit podosomes but lose the ability to degrade extracellular matrix after activation of the Toll-like receptor-signaling pathway [30]. We also found that PKC activation-induced podosome assembly and podosome-related ECM degradation can be decoupled in human lung bronchial epithelial BEAS2B cells [24]. Therefore, we examined the proteolytic activity of the AFAPΔABD-induced podosome-like structure with an in situ zymography assay. Interestingly, these actin-rich small dots did not digest the gelatin matrix in C3H10T1/2 cells (Fig. 5a). For comparison, we also studied BEAS2B cells transfected with pEGFP-AFAP or pEGFP-AFAPΔABD. GFP-AFAP was mainly distributed along stress-fibers in most of the cells (Fig. 5b, top panel). By contrast, GFP-AFAPΔABD was mainly along the cell membrane and as small dots in the cytoplasm (Fig. 5b, second panel). No matrix degradation was observed in these cells. These results suggest that although overexpression of this mutant may promote formation of podosome-like structures, these structures cannot digest gelatin matrix.
Fig. 5.
AFAPΔABD-induced podosome-like structures do not digest extracellular matrix. Cells were cultured on TRITC-conjugated gelatin matrix and transfected with pEGFP vector, pEGFP-AFAP, or pEGFP-AFAPΔABD. No matrix digestion occurred, as shown with in situ zymography in C3H10T1/2 cells (a) or in BEAS2B cells (b)
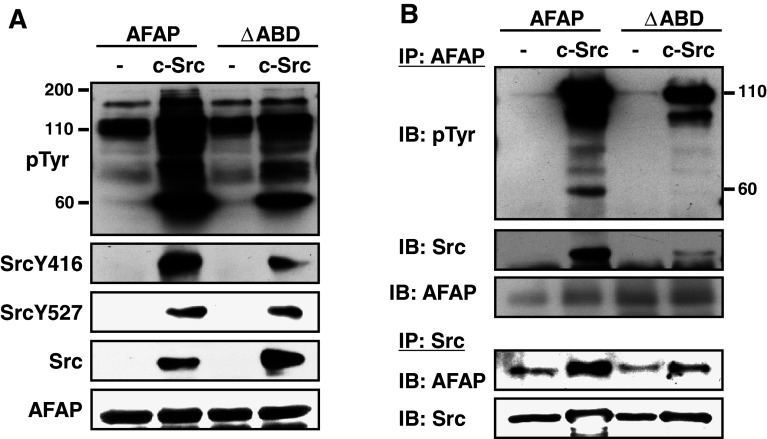
AFAPΔABD reduces c-Src activation and AFAP/c-Src interaction
Src tyrosine kinase is very important for podosome formation and function [14, 31, 32]. To determine why AFAPΔABD-induced podosome-like structures do not digest the gelatin matrix, we studied the role of the ABD of AFAP in c-Src-induced protein tyrosine phosphorylation. We co-expressed c-Src with AFAP or AFAP∆ABD in COS7 cells. Compared to c-Src/AFAP group, total protein tyrosine phosphorylation and phosphorylation of SrcY416 (a sign of Src activation) in the c-Src/AFAP∆ABD group was lower (Fig. 6a). When the cell lysates were immunoprecipitated with AFAP antibody and immunoblotted with anti-phosphotyrosine antibody, the bands around 110 kDa (mainly AFAP) and around 60 kDa (mainly c-Src) were much weaker, and the c-Src in the immunoprecipitates of anti-AFAP was less in the c-Src/AFAP∆ABD group than that in the c-Src/AFAP group (Fig. 6b). When the cell lysates were immunoprecipitated with anti-Src antibody, less AFAP was found in the c-Src/AFAP∆ABD group than that in the c-Src/AFAP group (Fig. 6b). These observations suggest that this mutant is less capable of binding to c-Src and enhancing its activation.
Fig. 6.
AFAPΔABD reduces c-Src activation and AFAP/c-Src interaction. a Co-expression of AFAP/c-Src-induced total protein tyrosine phosphorylation and SrcY416 phosphorylation were reduced when AFAP was replaced with its ΔABD mutant. Whole-cell lysates of COS7 cells expressing AFAP or AFAPΔABD with or without c-Src were immunoblotted with the indicated antibodies. b The phosphorylation of AFAP and c-Src and the interaction between AFAP and c-Src were reduced when ΔABD mutant was used to replace AFAP. The cell lysates were immunoprecipitated with anti-AFAP and immunoblotted with designated antibodies. The cell lysates were also immunoprecipitated with anti-Src antibody and immunoblotted with anti-AFAP and anti-Src antibodies
PKC activation-induced podosome-like structures formed by AFAPΔABD in BEAS2B cells are larger, faster, and longer lasting
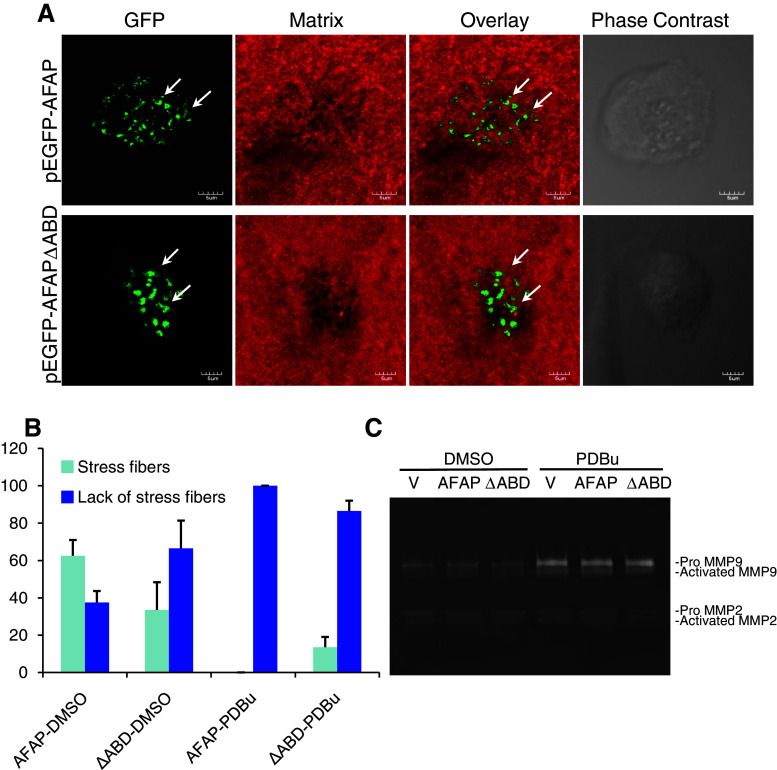
We then used PKC activation-induced podosomes in BEAS2B cells as a model system to compare the roles of wild-type AFAP and AFAPΔABD in podosome assembly and function. We transiently transfected BEAS2B cells with pEGFP, pEGFP-AFAP, or pEGFP-AFAPΔABD, and then challenged the cells with PDBu (500 nM, 30 min). In the pEGFP vector-transfected cells, the GFP signal was evenly distributed in the cytoplasm (Fig. 7a, top panel). In the pEGFP-AFAP-transfected cells, AFAP was localized at stress fibers and actin-rich podosomal dots (Fig. 7a, middle panel). In pEGFP-AFAPΔABD-transfected cells, AFAP appeared mainly as small dots (Fig. 7a, bottom panel). We quantified the number of podosome dots per cell and the size of each podosome dot. Although there were less podosomes in pEGFP-AFAPΔABD-transfected cells (Fig. 7b), they were larger in size, in comparison to that in pEGFP-AFAP-transfected cells (Fig. 7c).
Fig. 7.
AFAPΔABD forms irregular podosome-like structures in BEAS2B cells. a Expression of AFAPΔABD changes the morphology of PDBu-induced podosome-like structures in BEAS2B cells. Cells were transiently transfected with pEGFP vector, pEGFP-AFAP, or pEGFP-AFAPΔABD. Cells were photographed 30 min after adding PBDu (500 nM). b AFAPΔABD expression significantly reduced the number of podosome-like structures per cell. c AFAPΔABD expression significantly increased the size of podosome-like structures. *p < 0.05, **p < 0.01 compared to the AFAP-GFP group
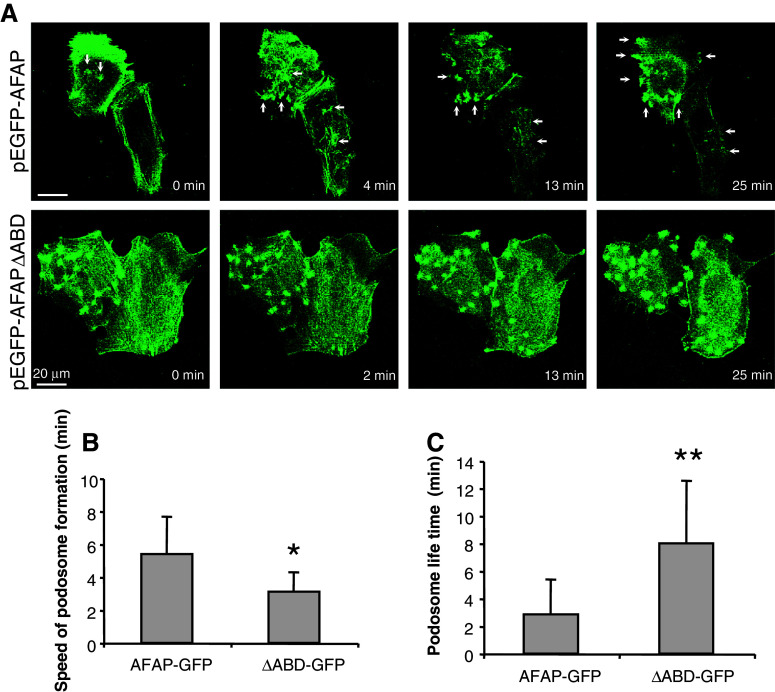
To compare the rate of podosome formation and the turnover of podosomes, we performed live cell-imaging analyses. The speed of PDBu-induced podosome formation in the pEGFP-AFAPΔABD-transfected cells was faster than in the pEGFP-AFAP-transfected cells (Fig. 8a, b). The lifespan of AFAPΔABD-induced podosome-like structures was longer (Fig. 8a, c).
Fig. 8.
Podosome-like structures formed by AFAPΔABD in BEAS2B cells are faster and have longer lifespan. a Spontaneously formed small dots were seen in cells transfected with pEGFP-AFAPΔABD, and PDBu-induced podosome-like structures appeared faster and lasted longer in these cells. BEAS2B cells were transiently transfected with pEGFP-AFAP or pEGFP-AFAPΔABD. Live cell imaging was performed and photographed after adding PBDu (500 nM). b AFAPΔABD expression significantly increases the speed of the formation of podosome-like structures. c AFAPΔABD expression significantly increases the lifespan of podosome-like structures. *p < 0.05, **p < 0.01 compared with AFAP-GFP group
To determine whether PKC activation can lead these podosome-like structures containing AFAPΔABD to digest gelatin matrix, we cultured BEAS2B cells on fluorescent-labeled gelatin matrix and transfected with pEGFP-AFAP or pEGFP-AFAPΔABD. The cells were stimulated with PDBu (500 nM, 4 h) or with DMSO (as a vehicle control), and matrix degradation was determined using the in situ zymography assay. After PDBu stimulation, stress fiber-like distribution of pEGFP-AFAP disappeared and podosome-like small dots were found in almost all cells (Fig. 9a). Similarly, in the AFAPΔABD group, podosome-like small dots were the predominant observation (Fig. 9a). Gelatin matrix degradation was equally effective in both groups. The morphological features of GFP-positive cells are summarized in Fig. 9b.
Fig. 9.
Podosome-like structures formed by AFAPΔABD in BEAS2B cells digested gelatin matrix after PKC activation. a PDBu stimulation induced formation of podosome-like small dots with significant matrix digestion. BEAS2B cells were seeded on fluorescent-labeled gelatin (red), and transiently transfected with pEGFP-AFAP or pEGFP-AFAPΔABD. Cells were stimulated with PBDu (500 nM) or DMSO (as vehicle control) for 4 h, and matrix digestion was determined with in situ zymography. b Effects of AFAPΔABD expression and PDBu stimulation on cytoskeletal structures were quantified. Data shown are mean ± standard deviation from two separate experiments with approximately 50 cells analyzed in each group. Stress fiber: GFP distribution along stress fibers. Lack of stress fibers: GFP signal was found on cell membrane or as small dots. c AFAPΔABD expression did not alter MMP-9 and MMP-2 protein expression and activation either under control condition or after PDBu stimulation, in comparison to GFP vector or GFP-AFAP-transfected cells. BEAS2B cells were transfected with pEGFP vector, pEGFP-AFAP, or pEGFP-AFAPΔABD. GFP-positive cells were sorted with flow cytometry and then challenged with PDBu (500 nM) or DMSO (vehicle control) for 18 h. Culture media were analyzed by gelatin zymography
To further test whether the small dots found in AFAPΔABD-transfected cells can affect cell invasion and MMP activities, BEAS2B cells were transfected with pEGFP, pEGFP-AFAP, or pEGFP-AFAPΔABD. We then sorted GFP-positive cells and performed a cell-invasion assay. In DMSO control groups, neither GFP-AFAP nor GFP-AFAPΔABD affected cell invasion, in comparison to GFP vector-transfected cells (data not shown). PDBu stimulation (500 nM, 18 h) increased cell invasion by 2.8-fold; however, there is no significant difference among the three groups. We then analyzed MMP protein expression and activities in the culture medium collected from GFP-positive cells treated with PDBu or DMSO with gelatin zymography. No difference was found in terms of protein levels and activities of MMP-9 and MMP-2 among DMSO-treated cells. PDBu stimulation increased expression and activities of MMP-9; however, it was not affected by expression of GFP-AFAP or GFP-AFAPΔABD (Fig. 9c).
Discussion
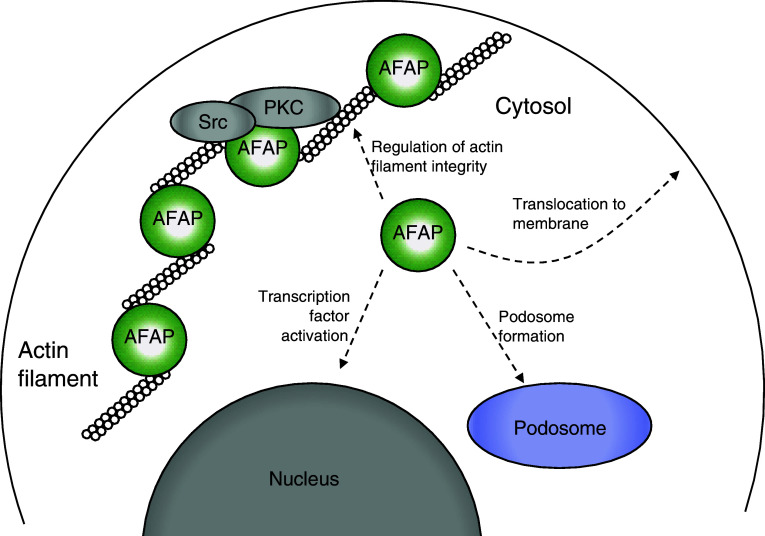
The distribution of AFAP in cells is predominately limited to stress filaments. Interestingly, in the present study, we found endogenous AFAP present in the cytosolic fraction. The association of AFAP with F-actin may be a dynamic process, as depolymerization of F-actin resulted in a redistribution of endogenous AFAP from the cytoskeletal to the cytosolic fraction. This phenomenon seems to be specific because several cytoskeletal proteins we examined showed less re-distribution. AFAP modulates changes in actin filament integrity in different cell types, such as fibroblasts, epithelial, endothelial cells, and cells in hematopoietic lineages [1]. Since the AFAPΔABD mutant cannot bind actin filaments well, they are more abundant in the cytosolic fraction. Overexpression of this mutant may represent the phenomenon of an increase of AFAP in the cytoplasm, which may change the ratio of AFAP between cytoskeletal and cytosolic fraction, and interrupt the integrity of stress fiber. Our results also suggest that these cytosolic AFAP may translocate to the cellular membrane and promote formation of podosome-like structures. We have recently shown that AFAPΔABD may more effectively mediate AP-1/SRE transactivation initiated by Src or other signaling mechanisms [18]. Therefore, cytosolic AFAP may participate in multiple cellular functions (Fig. 10).
Fig. 10.
Proposed function of AFAP in the cytosolic fraction. The ratio between cytoskeletal and cytosolic AFAP may be essential for the regulation of actin filament integrity. Cytosolic AFAP may be more accessible to cellular membrane, podosome, nucleus, and mediate signal transduction and other cellular functions
Anti-AFAP antibody also picked up signals in the nucleus in the C3H10T1/2 cells. AFAP has been proposed as one of the nuclear proteins in the AceView database (http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/av.cgi?db=human&term=AFAP1). Perhaps, it is easier for cytosolic AFAP to move into the nucleus. The function of AFAP in the nucleus, however, is unknown. Flynn’s group has also found AFAP in both Triton-soluble and insoluble fractions in Cos-1 cells overexpressing AFAP, which was not altered by co-expression of c-Src or constitutively active SrcY527F mutant [1]. Since the Triton-soluble fraction also contains some membrane-bound cytoskeletal component, we suspect that AFAP in this fraction may be still able to bind actin. Under the experimental conditions used in the present study, the depolymerization reagents, cytochalasin D and latrunculin A, did not change the distribution of actin between cytoskeletal and cytosolic fractions. This suggests that these treatments may affect the actin polymerization dynamics without significantly altering the net polymerization. These phenomena should be investigated further.
Dorfleutner et al. [5] used shRNA to downregulate the expression of endogenous AFAP in MDA-MB-231 breast cancer cells. Cells with reduced AFAP lost stress fiber; F-actin staining was mainly at the cell membrane and small dots, similar to what we observed in the present study. It seems that the expression level of AFAP is important in maintaining the filamentous structure of the cytoskeleton. In the present study, C3H10T1/2 cells transfected with pEGFP-AFAPΔABD showed disorganized cytoskeleton with less stress fibers and small F-actin dots at the cell periphery. Cortactin, a well-known podosome marker, was co-localized with AFAPΔABD in these small dots at the ventral surface of the cell, which indicated that these dots fulfilled certain criteria of podosomes. In human bronchial epithelial BEAS2B cells, expression of pEGFP-AFAPΔABD also disrupted stress filaments and led to formation of podosome-like small dots. Overexpression of AFAPΔABD did not reduce endogenous AFAP, but it may function as a dominant negative mutant, and competitively inhibit the function of endogenous AFAP.
Interestingly, the actin-rich dots formed in C3H10T1/2 or BEAS2B cells expressing AFAPΔABD did not digest the gelatin matrix. The protein levels and activities of MMP-9 and MMP-2 were not increased in these cells, and cell invasion activity was also not enhanced. Src PTK activation is crucial for podosome formation and function. Overexpression of a constitutively active SrcY527F induced podosome formation [31, 33], whereas overexpression of a dominant negative Src inhibited podosome formation [33]. AFAPΔABD was mainly in the cytosolic fraction with reduced affinity to c-Src compared to the full-length AFAP. Phosphorylation of AFAP, SrcY416, and other proteins was lower when AFAPΔABD was co-expressed with c-Src, suggesting that a lower association with stress fibers reduced the interaction between AFAP and Src kinase. Flynn’s group also found that tyrosine-phosphorylated AFAP was mainly in Triton-insoluble fraction, indicating cytoskeletal-associated AFAP has more interaction with protein tyrosine kinase [1]. Therefore, the lack of proteolytic activity of podosome-like structures in the pEGFP-AFAPΔABD-transfected cells may be due to a lower level of activation of c-Src tyrosine kinase at these structures, or due to the reduced recruitment of c-Src to these structures.
Previous studies have demonstrated that podosome assembly and subsequent matrix degradation can be uncoupled and regulated by distinct signaling pathways. In human bronchial epithelial BEAS2B cells, Rottlerin, PKCδ/μ/ζ siRNA, PKCζ pseudosubstrate inhibitor, and MMP-9/-14 siRNA can inhibit matrix degradation of PDBu-stimulated podosomes without affecting podosome assembly [24]. MMP-14-deficient mouse bone marrow cells and spleen dendritic cells produce podosomes but lose the ability to degrade extracellular matrix [30]. In CA1D cells (a Ras-transformed invasive variant of the MCF10A human breast cell line), the organization of the actin core of podosomes requires a signaling pathway through PI3 K and Src kinases upon TGF-β treatment. In contrast, the activation of MMP-9 through Smad2/3 that leads to the degradation of extracellular matrix requires ERK signaling [34]. In v-Src transformed NIH3T3 cells, Tks5 is required for podosome formation, while Tks4 is required for MMP-14 recruitment and extracellular matrix degradation [35]. Therefore, podosome formation and matrix degradation activity of podosomes could be regulated by different molecules.
As we reported recently [23, 24], when BEAS2B cells were stimulated with PDBu, an activator of PKC, the cell invasion, matrix degradation and MMP-9 expression and activities were all significantly increased. However, there were no significant differences between pEGFP-AFAPΔABD and pEGFP-AFAP expressing cells. AFAP is required for PKC-induced c-Src activation and podosome formation [14]. AFAP binds c-Src through a stretch of proline-rich sequences within its N-terminus; a single amino acid mutation from proline to alanine in this region is able to significantly reduce the interaction between these two proteins [14, 17]. Overexpression of this mutant alone did not cause rearrangement of the cytoskeleton [36], but this mutant can block PKC-induced Src activation and podosome formation [14]. Although we did not further mutate this Src-binding site in AFAPΔABD, the binding between AFAPΔABD and Src was reduced. Why were, under PDBu stimulation, the gelatin matrix degradation and cell invasion activities similar between pEGFP-AFAPΔABD and pEGFP-AFAP expressing cells? We have found that PDBu dramatically increased activities of Src, PI3 K, Akt, MEK/ERK, and JNK in BEAS2B cells (manuscript under revision). These overwhelming signals may activate MMP-9 and promote gelatin matrix degradation and cell invasion.
In summary, we demonstrated the importance of actin-binding domain of AFAP in mediating its function. Using AFAPΔABD as a surrogate of cytosolic AFAP, we propose that this group of AFAP may be an important reservoir for AFAP to participate in multiple cellular functions (Fig. 10).
Acknowledgments
We thank Dr. Daniel C. Flynn (West Virginia University) for providing AFAP antibody and plasmids. We thank Ms. Serisha Moodley for technical assistance and Ms. Lauren Turrell for proofreading the manuscript. This work was supported by Canadian Institutes of Health Research operating grants MOP-13270 and MOP-42546. HX was supported by Peterborough K. M. Hunter Graduate Studentship for Cancer Research.
References
- 1.Baisden JM, Qian Y, Zot HM, Flynn DC. The actin filament-associated protein AFAP-110 is an adaptor protein that modulates changes in actin filament integrity. Oncogene. 2001;20:6435–6447. doi: 10.1038/sj.onc.1204784. [DOI] [PubMed] [Google Scholar]
- 2.Flynn DC, Leu TH, Reynolds AB, Parsons JT. Identification and sequence analysis of cDNAs encoding a 110-kilodalton actin filament-associated pp60src substrate. Mol Cell Biol. 1993;13:7892–7900. doi: 10.1128/mcb.13.12.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodyga M, Bai XH, Mourgeon E, Han B, Keshavjee S, Liu M. Molecular cloning of actin filament-associated protein: a putative adaptor in stretch-induced Src activation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L265–L274. doi: 10.1152/ajplung.00492.2001. [DOI] [PubMed] [Google Scholar]
- 4.Qian Y, Baisden JM, Westin EH, Guappone AC, Koay TC, Flynn DC. Src can regulate carboxy terminal interactions with AFAP-110, which influence self-association, cell localization and actin filament integrity. Oncogene. 1998;16:2185–2195. doi: 10.1038/sj.onc.1201753. [DOI] [PubMed] [Google Scholar]
- 5.Dorfleutner A, Stehlik C, Zhang J, Gallick GE, Flynn DC. AFAP-110 is required for actin stress fiber formation and cell adhesion in MDA-MB-231 breast cancer cells. J Cell Physiol. 2007;213:740–749. doi: 10.1002/jcp.21143. [DOI] [PubMed] [Google Scholar]
- 6.Qian Y, et al. Analysis of the role of the leucine zipper motif in regulating the ability of AFAP-110 to alter actin filament integrity. J Cell Biochem. 2004;91:602–620. doi: 10.1002/jcb.10725. [DOI] [PubMed] [Google Scholar]
- 7.Baisden JM, Gatesman AS, Cherezova L, Jiang BH, Flynn DC. The intrinsic ability of AFAP-110 to alter actin filament integrity is linked with its ability to also activate cellular tyrosine kinases. Oncogene. 2001;20:6607–6616. doi: 10.1038/sj.onc.1204802. [DOI] [PubMed] [Google Scholar]
- 8.Qian Y, Baisden JM, Zot HG, Van Winkle WB, Flynn DC. The carboxy terminus of AFAP-110 modulates direct interactions with actin filaments and regulates its ability to alter actin filament integrity and induce lamellipodia formation. Exp Cell Res. 2000;255:102–113. doi: 10.1006/excr.1999.4795. [DOI] [PubMed] [Google Scholar]
- 9.Harder J, Xu X, Letourneau P, Lanier LM. The actin cross-linking protein AFAP120 regulates axon elongation in a tyrosine phosphorylation-dependent manner. Neurosci Lett. 2008;444:132–136. doi: 10.1016/j.neulet.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker VG, et al. PI3 K activation is required for PMA-directed activation of cSrc by AFAP-110. Am J Physiol Cell Physiol. 2007;293:C119–C132. doi: 10.1152/ajpcell.00525.2006. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, et al. XB130, a novel adaptor protein for signal transduction. J Biol Chem. 2007;282:16401–16412. doi: 10.1074/jbc.M701684200. [DOI] [PubMed] [Google Scholar]
- 12.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 13.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/S0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 14.Gatesman A, Walker VG, Baisden JM, Weed SA, Flynn DC. Protein kinase Calpha activates c-Src and induces podosome formation via AFAP-110. Mol Cell Biol. 2004;24:7578–7597. doi: 10.1128/MCB.24.17.7578-7597.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorfleutner A, Cho Y, Vincent D, Cunnick J, Lin H, Weed SA, Stehlik C, Flynn DC. Phosphorylation of AFAP-110 affects podosome lifespan in A7r5 cells. J Cell Sci. 2008;121:2394–2405. doi: 10.1242/jcs.026187. [DOI] [PubMed] [Google Scholar]
- 16.Crimaldi L, Courtneidge SA, Gimona M. Tks5 recruits AFAP-110, p190RhoGAP, and cortactin for podosome formation. Exp Cell Res. 2009;315:2581–2592. doi: 10.1016/j.yexcr.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Han B, Bai XH, Lodyga M, Xu J, Yang BB, Keshavjee S, Post M, Liu M. Conversion of mechanical force into biochemical signaling. J Biol Chem. 2004;279:54793–54801. doi: 10.1074/jbc.M406880200. [DOI] [PubMed] [Google Scholar]
- 18.Han B, Xiao H, Lodyga M, Bai XH, Jin T, Liu M. Actin filament-associated protein mediated c-Src related SRE/AP-1 transcriptional activation. FEBS Lett. 2011;585:471–477. doi: 10.1016/j.febslet.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Han B, Lodyga M, Liu M. Ventilator-induced lung injury: role of protein-protein interaction in mechanosensation. Proc Am Thorac Soc. 2005;2:181–187. doi: 10.1513/pats.200501-008AC. [DOI] [PubMed] [Google Scholar]
- 20.Sawada Y, Sheetz MP. Force transduction by triton cytoskeletons. J Cell Biol. 2002;156:609–615. doi: 10.1083/jcb.200110068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiozaki A, et al. XB130, a novel adaptor protein, promotes thyroid tumor growth. Am J Pathol. 2011;178:391–401. doi: 10.1016/j.ajpath.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Xiao H, Eves R, Yeh C, Kan W, Xu F, Mak AS, Liu M. Phorbol ester-induced podosomes in normal human bronchial epithelial cells. J Cell Physiol. 2009;218:366–375. doi: 10.1002/jcp.21609. [DOI] [PubMed] [Google Scholar]
- 24.Xiao H, Bai XH, Kapus A, Lu WY, Mak AS, Liu M. The protein kinase C cascade regulates recruitment of matrix metalloprotease 9 to podosomes and its release and activation. Mol Cell Biol. 2010;30:5545–5561. doi: 10.1128/MCB.00382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodyga M, Bai XH, Kapus A, Liu M. Adaptor protein XB130 is a Rac-controlled component of lamellipodia that regulates cell motility and invasion. J Cell Sci. 2010;123:4156–4169. doi: 10.1242/jcs.071050. [DOI] [PubMed] [Google Scholar]
- 26.Reznikoff CA, Brankow DW, Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973;33:3231–3238. [PubMed] [Google Scholar]
- 27.Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA. 2004;101:9607–9611. doi: 10.1073/pnas.0403100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian Y, et al. PKC phosphorylation increases the ability of AFAP-110 to cross-link actin filaments. Mol Biol Cell. 2002;13:2311–2322. doi: 10.1091/mbc.E01-12-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 30.West MA, Prescott AR, Chan KM, Zhou Z, Rose-John S, Scheller J, Watts C. TLR ligand-induced podosome disassembly in dendritic cells is ADAM17 dependent. J Cell Biol. 2008;182:993–1005. doi: 10.1083/jcb.200801022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhopadhyay UK, Eves R, Jia L, Mooney P, Mak AS. p53 suppresses Src-induced podosome and rosette formation and cellular invasiveness through the upregulation of caldesmon. Mol Cell Biol. 2009;29:3088–3098. doi: 10.1128/MCB.01816-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatin F, Varon C, Genot E, Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci. 2006;119:769–781. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- 33.Chellaiah MA, Kuppuswamy D, Lasky L, Linder S. Phosphorylation of a Wiscott–Aldrich syndrome protein-associated signal complex is critical in osteoclast bone resorption. J Biol Chem. 2007;282:10104–10116. doi: 10.1074/jbc.M608957200. [DOI] [PubMed] [Google Scholar]
- 34.Mandal S, Johnson KR, Wheelock MJ. TGF-beta induces formation of F-actin cores and matrix degradation in human breast cancer cells via distinct signaling pathways. Exp Cell Res. 2008;314:3478–3493. doi: 10.1016/j.yexcr.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Buschman MD, Bromann PA, Cejudo-Martin P, Wen F, Pass I, Courtneidge SA. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol Biol Cell. 2009;20:1302–1311. doi: 10.1091/mbc.E08-09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, et al. AFAP-110 is overexpressed in prostate cancer and contributes to tumorigenic growth by regulating focal contacts. J Clin Invest. 2007;117:2962–2973. doi: 10.1172/JCI30710. [DOI] [PMC free article] [PubMed] [Google Scholar]