Abstract
Nucleotides are of crucial importance as carriers of energy in all organisms. However, the concept that in addition to their intracellular roles, nucleotides act as extracellular ligands specifically on receptors of the plasma membrane took longer to be accepted. Purinergic signaling exerted by purines and pyrimidines, principally ATP and adenosine, occurs throughout embryologic development in a wide variety of organisms, including amphibians, birds, and mammals. Cellular signaling, mediated by ATP, is present in development at very early stages, e.g., gastrulation of Xenopus and germ layer definition of chick embryo cells. Purinergic receptor expression and functions have been studied in the development of many organs, including the heart, eye, skeletal muscle and the nervous system. In vitro studies with stem cells revealed that purinergic receptors are involved in the processes of proliferation, differentiation, and phenotype determination of differentiated cells. Thus, nucleotides are able to induce various intracellular signaling pathways via crosstalk with other bioactive molecules acting on growth factor and neurotransmitter receptors. Since normal development is disturbed by dysfunction of purinergic signaling in animal models, further studies are needed to elucidate the functions of purinoceptor subtypes in developmental processes.
Keywords: ATP, Cell death, CNS, Differentiation, Heart, Muscle, Proliferation
Embryonic development
Most early studies of the roles of nucleotides in development have been discussed in terms of their intracellular roles and as a source of energy. However, since it is now generally accepted that purines and pyrimidines have potent extracellular actions mediated by the activation of specific membrane receptors (see [1]), many of these previous studies can now be reinterpreted. ATP and adenosine play key roles from the very beginning of life. Extracellular ATP promotes a rapid increase in Na+ permeability of the fertilized egg membrane through the activation of a specific ATP receptor [2]. Mg2+-ATPase activity has been localized on the entire surface of unfertilized eggs and in pre- and post-implantation embryos [3, 4]. Together with the demonstration that ATP-activated spermatozoa show very high success rates in fertilization tests [5], this strongly suggests that ATP is a key sperm-to-egg signal in the process of fertilization.
In this article, the extracellular roles of purines and pyrimidines will be considered as signaling molecules in embryological development in a wide variety of systems in amphibians, birds and mammals, including humans. Readers are referred to reviews of the earlier literature about the involvement of purinergic signaling in embryonic development [1, 6] and very good reviews specifically about the development of the nervous system [7, 8].
Early embryos
Frog embryos
The nicotinic channels in myotomal muscle cells cultured from Xenopus embryos at stages 19–22 were shown to be opened by micromolar concentrations of exogenous ATP [9], following the earlier demonstration that ATP increases the sensitivity of receptors in adult frog skeletal muscles without increasing the affinity of acetylcholine (ACh) for the receptor or acetylcholinesterase [10]. Since then, there have been a number of studies of the actions of ATP in developing Xenopus neuromuscular synapses (see [11]). Extracellular applications of ATP to developing Xenopus neuromuscular synapses in culture potentiate ACh responses of developing muscle cells during the early phase of synaptogenesis [12–14]. The possibility that extracellular ATP coreleased with ACh, may serve as a positive trophic factor at developing neuromuscular synapses has also been raised [11, 12]. It is further suggested that calcitonin gene-related peptide (CGRP) and ATP coreleased with ACh from the nerve terminal may act together to potentiate postsynaptic ACh channel activity during the early phase of synaptogenesis [15]; it is claimed that CGRP actions are mediated by cyclic adenosine monophosphate- (cAMP)-dependent protein kinase A, while ATP exerts its effects via protein kinase. In the most recent report from this group [16], they present results that suggest that endogenously released ATP, acting in concert with various protein kinases, is involved in the maintenance and/or development of the quantum size of synaptic vesicles at embryonic neuromuscular synapses.
A novel P2Y purinoceptor (X1P2Y or P2Y8) was cloned and sequenced that is expressed (as seen by Northern blots and in situ hybridization) in the neural plate of Xenopus embryos from stages 13–18 and again at stage 28 when secondary neurulation occurs in the tail bud [17]. It differs from other members of the P2Y purinoceptor family in that it has an intracellular C terminus with 216 amino acid residues (compared to 16–67 in P2Y1–8). When expressed as a recombinant receptor in Xenopus oocytes, it shows equipotent responses to the triphosphates ATP, UTP, ITP, CTP, and GTP and smaller responses to diphosphates and tetraphosphates, but is not responsive to inorganic phosphates. Responses to activation of the X1P2Y receptor have a long duration (40–60 min). These data suggest that this novel P2Y receptor may be involved in the early formation of the nervous system.
Suramin and Trypan blue, both substances that are known to be antagonists at P2 receptors (see [18]), as well as having other actions, have been shown to interfere with gastrulation [19]. If injected early when the dorsal lip is first invaginating, the Xenopus embryo develops no head or trunk and sometimes no tail; somites and notocord are also missing. If they are injected midway in gastrulation, embryos develop without heads, but with trunks and tails, while if injected at the end of gastrulation, the embryo is completely unaffected.
Regulation of rhythmic movements by purinergic transmitters in frog embryos has been described [20]. It was shown that ATP is released during swimming that activates P2Y receptors to reduce voltage-gated K+ currents and cause an increase in the excitability of the spinal motor circuits. It was also shown that adenosine, resulting from the breakdown of ATP, acts on P1 receptors to reduce the voltage-gated Ca2+ currents to lower excitability of the motor circuits thereby opposing the actions of ATP [21]. The authors suggest that a gradually changing balance between ATP and adenosine underlies the run-down of the motor pattern for swimming in Xenopus. In a later study, Dale [22] presented evidence to suggest that delayed production of adenosine underlies temporal modulation of swimming in the frog embryo and is likely to result from feed-forward inhibition of the 5′-ectonucleotidase in the spinal cord. A Xenopus homologue of apyrase has been identified during early development [23].
Chick embryos
Together with muscarinic cholinergic receptors, extracellular receptors to ATP were shown to be the first functionally active membrane receptors in chick embryo cells at the time of germ layer formation [24]. It was also shown that in gastrulating chick embryo, ATP caused rapid accumulation of inositol trisphosphate (IP3) and Ca2+ mobilization in a similar way and to the same extent as ACh, whereas other neuroendocrine substances such as insulin and noradrenaline (NA) had much weaker effects. This suggests that, alongside ACh, other phylogenetically old and universal regulators of cell metabolism such as ATP (and perhaps nitric oxide) might play a leading role in the functional regulation of gastrulation via the activation of specific receptors triggering Ca2+ mobilization.
ATP has been shown to induce precocious evagination of the embryonic chick thyroid, an event which has been hypothesized to be involved in the formation of the thyroid gland from the thyroid primordium [25]. The requirement for ATP was very precise, since it could not be replaced by pyrophosphate, AMP, or ADP nor by GTP, suggesting a high degree of specificity of the ATP-induced effect.
ATP acts on embryonic and developing cells of both nervous and non-nervous systems by increasing intracellular Ca2+ concentrations. Release of Ca2+ from intracellular stores is evoked in the otocyst epithelium of the early embryonic chick, incubated for 3 days (stage 18–19) [26] (Fig. 1), in developing chick myotubes [27] and in dissociated cells from whole early embryonic chicks [24, 28].
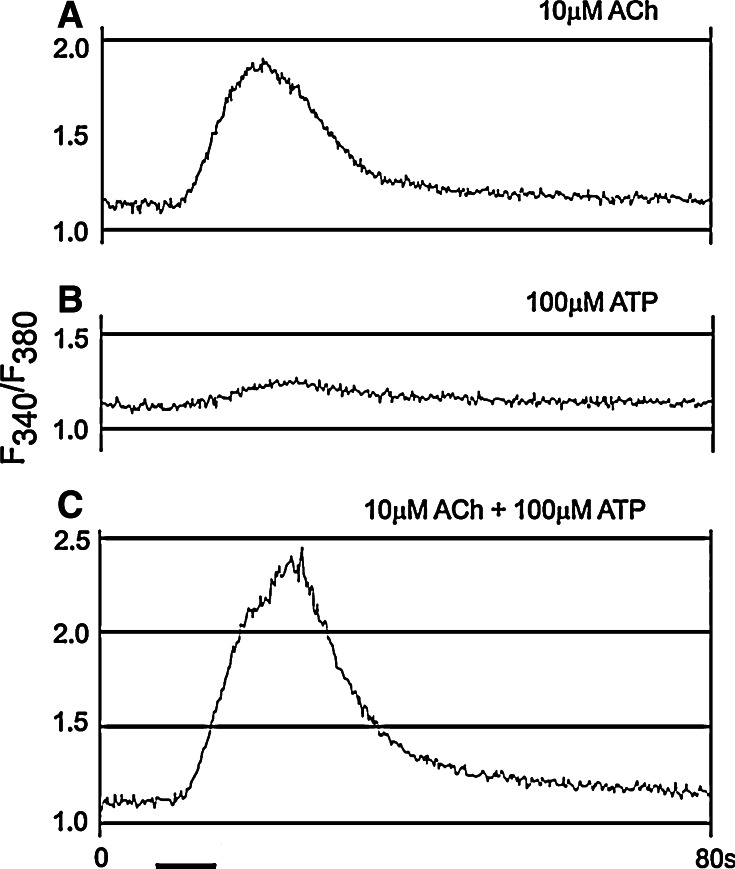
Fig. 1.
Interaction between acetylcholine (ACh) and ATP recorded in an otocyst from chick embryo. a The response to 10 μM acetylcholine. b The response to 100 μM ATP. c The response to the co-application of 10 μM acetylcholine and 100 μM ATP. The records in a–c were taken in this order at 5-min intervals. The bath solutions contained 25 mM Ca2+ (reproduced form [26] with permission from John Wiley and Sons Ltd.)
In a study, the expression of the G protein-coupled P2Y1 receptor during embryonic development of the chick was described [29]. During the first 10 days of embryonic development, the P2Y1 receptor is expressed in a developmentally regulated manner in the limb buds, mesonephros, brain, somites, and facial primordia (Fig. 2), suggesting that there may be a role for ATP and P2Y1 receptors in the development of these systems.
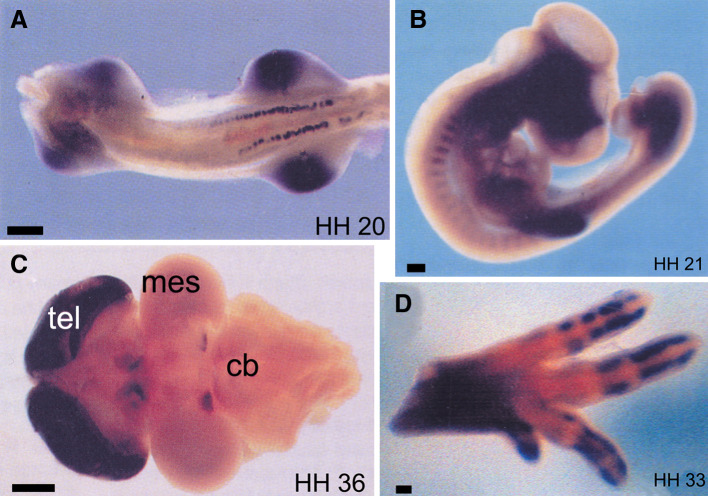
Fig. 2.
Expression of P2Y1 receptors during embryonic development of the chick as visualized by whole-mount in situ hybridization. Stages of development are shown in bottom right corner. a Ventral view of stage 20 embryo showing P2Y1 expression in mesonephros and limb buds (scale bar 200 μm). b Lateral view of the chick somite at stage 21 showing P2Y1 expression in the anterior region. The dark area in the head region is due to an artefact of photography (scale bar 200 μm). c Dorsal view of stage 36 brain (anterior to the left), showing increased levels of expression in telecephlon (tel), dorsal diencephlon and posterior midbrain. Mes mesencephalon, cb cerebellum (scale bar 1 mm). d An anterior-uppermost view of a leg at embryonic stage 33. Expression of P2Y1 is seen in the digits, but not in areas of joint formation. The same expression pattern is also seen in the wing (scale bar 100 μm) (reproduced from [29] with permission from Wiley-Liss, Inc.)
Adenosine has been implicated in growth regulation of the vascular system in the chick embryo [30], in common with a similar role claimed for experimental angiogenesis in the chorio-allantoic membrane [31–33].
Mammalian embryos
Puff-applied ATP has been shown to have two main effects on a mouse mesodermal stem cell line: an increase in intracellular Ca2+ concentrations and a subsequent hyperpolarization due to Ca2+-activated K+ conductance [34] (Fig. 3). The author speculates that the transient increase in intracellular Ca2+ may influence mesodermal cell differentiation, particularly in relation to muscle differentiation. In a later paper [35], two myoblastic cell lines, one from rat, the other from mouse, showed similar properties to those of the myogenic clonal cells derived from the mouse mesodermal stem cell line described above.
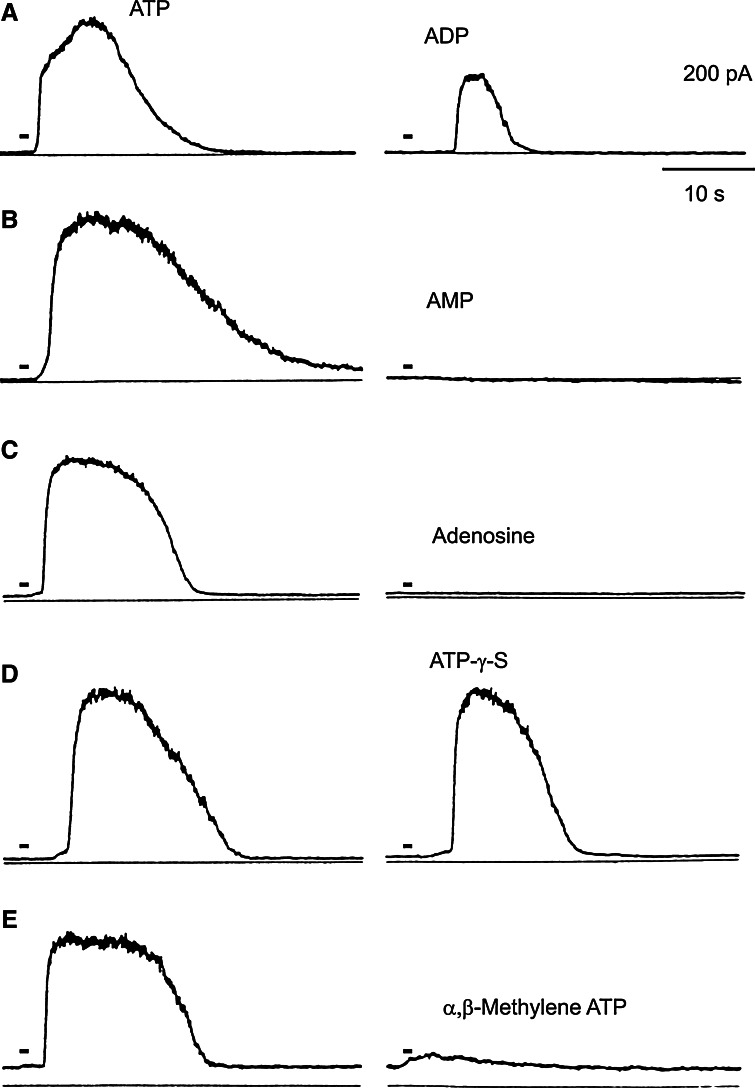
Fig. 3.
K+ responses to ATP analogues of a mouse mesodermal cell line. Each of the two traces was obtained from the same cell (a–e). The responses induced by ATP (left traces) and ATP analogues (right traces) are shown. The names of the analogues are shown near the traces. Each drug was applied at 20 μM, and the holding potentials were 0 mV (reproduced from [34] with permission from John Wiley and Sons)
ATP and ADP have been shown to enhance, reduce, or have no effect (depending on the dose used) on the incidence of Trypan blue-induced teratogenic malformations in the rat fetus at day 20 [36]. Concomitant administration of ATP and cortisone in mice either decrease the teratogenic effect of cortisone (50 μg ATP) or enhance its teratogenic effect (>100 μg ATP) [37]. Mouse heads of embryos from 14 to 24 pairs of body somites exposed to an ATP-containing medium have been demonstrated to undergo rapid epithelial thickening and invagination, a process that appears to take part in the shaping of nasal pits and formation of primary palate [38]. The trophoblast appears at the blastocyst stage of embryogenesis and contributes to placentation. ATP increases [Ca2+]i via a P2Y receptor in proliferating bovine trophobast cells [39].
Besides ATP, a number of reports implicate adenosine as one of the endogenous effectors that can selectively modulate cell growth during embryonic development. For example, adenosine is shown to potentiate the delaying effect of dibutyryl cAMP (a membrane-permeable analogue of cAMP) on meiosis resumption in denuded mouse oocytes [40]. The role of adenosine has been particularly well characterized in the morphogenetic outgrowth of vertebrate limb buds [41]. Embryonic limb development in the mouse is driven by rapid mesenchymal cell proliferation induced by trophic substances secreted by the apical ectodermal ridge. This interaction can be restricted experimentally by pharmacological agents that elevate intracellular cAMP levels, or physiologically by the onset of “programmed cell death” triggered by naturally occurring negative regulators of growth. Mutations that affect the pattern of limb/bud outgrowth provide invaluable experimental means to investigate these growth-regulatory processes. Knudsen and Elmer [41] studied the regulation of polydactylous outgrowth (an expression of the Hemimelia-extra toe (HmX/+) mutant phenotype) in hind-limb buds explanted into a serum-free in vitro system at stage 18 of gestation. Its expression was promoted by exposure to exogenous adenosine-deaminase, the enzyme that catalyzes the inactivation of endogenous adenosine, and conversely suppressed by co-exposure to hydrolysis-resistant adenosine analogues. Adenosine-induced effects were mediated by activation of specific extracellular receptors, since the P1 receptor antagonist, caffeine, could completely prevent suppression of polydactylous outgrowth. Measurement of both adenosine and adenosine deaminase levels in embryonal plasma and whole embryos argued against an endocrine mechanism of adenosine secretion, in favor of an autocrine (self-regulatory) or paracrine (proximate-regulatory) mechanism. These results suggest that the in vitro outgrowth of the prospective polydactylous region is induced upon escape from the local growth-inhibitory influence of extracellular adenosine.
Micromolar concentrations of adenosine, inosine, and hypoxanthine (but not guanosine) block the second or third cleavage of mouse embryos developing in vitro [42]. Zygotes or early two-cell embryos, cultured in a purine-containing medium for 24 h, resume development following transfer to purine-free conditions. The precise mechanism of the purine-sensitive process is not known, but embryos conceived in vivo are sensitive until approximately 28–30 h after fertilization and are no longer sensitive by 34 h [43]. However, a later study by this group has shown that the purine-induced block can be reversed by compounds that elevate cAMP [44]. Changes in expression of adenosine deaminase have been described in lymphoid tissues of rat embryos [45]. In the thymus, cortical lymphocytes begin to express significant amounts of the enzyme at 17 days of gestation; in the spleen and lymph node, adenosine deaminase was initially detected in T cell areas, but not primary follicles; in the duodenum, epithelial cells of villi and the neck of crypts showed positive staining; in the cartilage of 15-day fetuses, positive staining was seen in perinchondrial and hypertrophic cells; while Kupffer cells in the liver and vascular endothelial cells showed positive staining for adenosine deaminase at every autogenetic stage studied. In mouse embryonic development adenosine deaminase increased 74-fold between days 7 and 9; deoxyadenosine kinase increased 5.4-fold during the same period; adenosine kinase, deoxyguanosine kinase and purine nucleoside phosphorylase exhibited less than twofold changes in activity between days 7 and 13 [46]. The authors concluded that while phosphorylation of adenosine was the principal route of metabolism up to day 9, after there is a switch to deamination. The possible role of ectoadenosine deaminase in the development of the nervous system and the neurological abnormalities that occur in adenosine deaminase-deficient patients is discussed in a review by Franco et al. [47].
Human embryos
A few studies have been made of receptors to purines and pyrimidines in human embryos. Human embryonic kidney cells (HEK293) endogenously express P2Y1 and P2Y2 receptors [48]. These embryonic cells have also been shown to express an endogenous A2B adenosine receptor [49]. ATP and adenosine-5′-O-3-thiotriphosphate (ATPγS) were shown to stimulate DNA synthesis in human fetal astrocyte cultures [50]. In addition, ATP stimulated a mitogen-activated protein kinase (MAPK) termed ERK (extracellular signal-regulated protein), a key component of signal transduction pathways involved in cellular proliferation and differentiation. The activation of MAPK was mediated, at least in part, by P2 receptors since suramin produced 50% block.
There has been a study of plasma ATP levels in the fetus at the time of obstetrical delivery, with samples being collected immediately after clamping of the cord [51]. The results showed that the plasma ATP was significantly higher in arterial compared to venous or maternal venous blood. It was suggested therefore that the ATP in arterial blood was of fetal origin and that the levels decrease in response to stress during vaginal delivery and correlate with the oxygen supplied from the placenta.
In a study of human fibroblasts, differential sensitivity to adenosine was demonstrated in donors of different ages [52]. Fetal fibroblasts were the most sensitive to adenosine, which produced inhibition of growth and RNA synthesis; in contrast, fibroblasts taken from 4-year-old donors showed growth stimulation to adenosine. Activation of A2 receptors by adenosine stimulates l-arginine transport and nitric oxide synthase in human fetal endothelial cells [53].
Cardiovascular system
Heart
Studies of the development of pharmacological sensitivity to adenosine analogues in embryonic chick heart [54, 55] show that pharmacological sensitivity to A1 receptor agonists begins at embryonic day 7 and then increases continuously to day 12, when the atria became fully responsive. Ligand binding shows that A1 receptors are present at days 5 and 6, but are not responsive to adenosine, and the author concluded that the development of sensitivity to A1 adenosine receptor-mediated negative chronotropic responses was not paralleled by developmental changes in adenosine inhibition of adenylyl cyclase, or by the development of sympathetic and parasympathetic innervation. Chronic exposure of the embryonic chick heart (15–17 days old) to R-N 6-2-phenylisopropyladenosine (R-PIA) produces down-regulation of A1 adenosine receptors and desensitization of the negative inotropic response to adenosine [56]. A study of ventricular cells cultured from chick embryos 14 days in ovo showed that a functional A2A receptor is expressed and mediates augmentation of myocyte contractility [57]. The A2A receptor coexists with an A2B receptor, although it has 50-fold higher affinity, and the authors suggest that high affinity A2A receptors play an important modulatory role in the presence of low levels of adenosine, while the low affinity A2B receptor becomes functionally important when the adenosine level is high.
In fetal sheep, centrally administered adenosine influences cardiac function [58]. The ontogeny of A1 adenosine receptors was studied in rats with binding assays using [3H]1,3-dipropyl-8-cyclopentylxanthine (DPCPX), an A1 antagonist, and by in situ hybridization of mRNA [59]. In a later study of mouse embryo cardiac function [60], adenosine, via A1 receptors, was shown to potently regulate heart rate via multiple effector systems at very early stages of prenatal development (9–12 days post-conception). At gestational days 8–11, mRNA expression for A1 receptors was detected in the atrium (one of the earliest G protein-coupled receptor genes to be expressed in the heart), but not in other fetal structures, while at gestational day 14, A1 mRNA was present in the CNS (thalamus, ventral horn of spinal cord), as well as the atrium; by gestational age 17, patterns of A1 receptor expression in the brain were similar to those observed in adults [61, 62]. Determination of A1 receptor density in developing rat heart using [3H]DPCPX, showed that functional A1 receptors are present in greater numbers in the immature perinatal heart than in the adult heart [63].
Intravenous infusion of adenosine analogues into fetal lambs produced dose-dependent bradycardia and hypotension [64–66]. In contrast, in the newborn, 5′-N-ethylcarboxamidoadenosine produced dose-dependent tachycardia, while R-PIA and cyclohexyladenosine produced dose-dependent bradycardia. Although adenosine causes cardiovascular changes in pregnant ewes, the effects are well tolerated and do not significantly affect the cardiorespiratory status of the fetus [67].
P2 receptors are widely expressed in human fetal heart [68]. Sequence analysis demonstrated P2X1, P2X3, and P2X4 receptor subtypes, as well as P2Y2, P2Y4, and P2Y6 receptors were present. It has been claimed that a new subunit of the P2X receptor family had been isolated from cardiomyocytes and brain from 14-day-old chick embryos; the primary sequence shares 75% identity with the rat and human P2X4 receptor, suggesting that the cDNA isolated may be the corresponding chick isoform and the pharmacological properties of the receptor expressed in Xenopus oocytes was consistent with this view [69].
Skeletal muscle and neuromuscular junction
A transmitter-like action of ATP on patched membranes of myoblasts and myotubes cultured from 12-day-old chicken embryos was first demonstrated by Kolb and Wakelam [70]. Using biochemical methods, ATP-induced cation influx was later demonstrated in myotubes prepared from 11-day-old chick embryos and shown to be additive to cholinergic agonist action [71]. Later papers from this group claimed that the myotube P2 purinoceptor triggers phosphoinositide turnover [27, 72] and alters Ca2+ influx through dihydropyridine-sensitive channels [73]. ATP has a potent depolarizing action on myotubes derived from pectoral muscle cultured from 11-day-old chick embryos [74] and its physiological and pharmacological properties have been described in a series of papers [75–78]. The myotube P2 purinoceptor is not activated by ADP, AMP, adenosine, or the non-hydrolyzable ATP analogues α,β-methylene ATP (α,β-meATP) or β,γ-methylene ATP [74]. A single class of ATP-activated ion channel conducts both cations and anions in the myotube [76] and the P2 purinoceptors involved showed marked desensitization [77]. The sensitivity of extracellular ATP has been tested at various stages of development of different muscles [79]. At embryonic day 6 (stage 30 of Hamburger and Hamilton [80]) ATP (50–100 μM) elicits vigorous contractions in all the muscles tested, but by embryonic day 17 (stage 43) none of the muscles contract in response to ATP (Fig. 4). However, denervation of muscles in newly hatched chicks leads to the reappearance of sensitivity to ATP, suggesting that the expression of ATP receptors is regulated by motor neurons. An immunohistochemical study of the distribution of 5′-nucleotidase during the development of chick striated muscle shows that the adult exhibits a more restricted distribution compared to the embryo [81]. An orthologue of the mammalian P2X1 receptor has been identified in embryonic chicken skeletal muscle, perhaps forming heteromultimers with P2X4 and P2X5 receptor subunits [82]. P2X5 and P2X6 receptors were identified in developing chick skeletal muscles [29, 83].
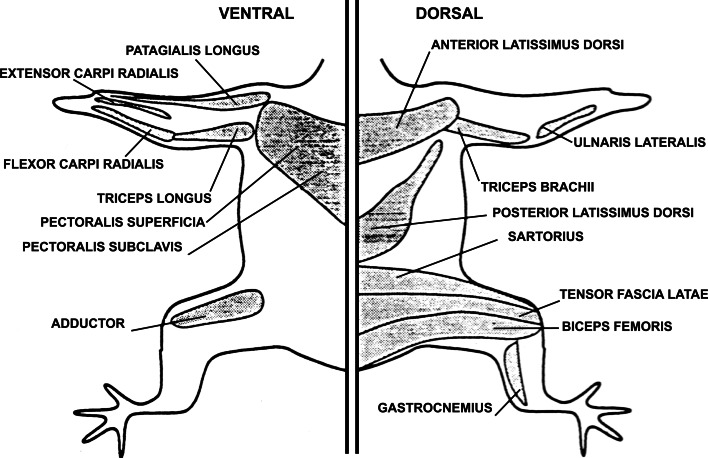
Fig. 4.
Location of the muscles of the chick embryo that were responsive to ATP. Three chick embryos from stages 35–37 were killed, and each muscle was identified and tested in at least two of the three embryos. All muscles tested in embryos of these ages contracted in response to ATP. By embryo day 17 (stage 43), none of the muscles contracted in response to ATP (reproduced from [79] with permission from Wiley-Liss, Inc.)
Purinoceptors have been characterized in mouse C2C12 myotubes [84–87]. Adenosine-sensitive P1 receptors activating cAMP formation were identified and a P2 purinoceptor was also postulated, sensitive to ATP, ADP, and ATPγS. The P2 receptor was also sensitive to UTP, but not α,β-meATP, 2-methylthio ATP (2-MeSATP), GTP or CTP, thus resembling the P2Y2 (or P2Y4) purinoceptor identified in mammals. The response to ATP and UTP was biphasic, a transient hyperpolarization being followed by a slowly declining depolarization; the hyperpolarization was blocked by apamin and suramin and abolished under Ca2+-free conditions. Occupation of the receptor by ATP or UTP led to formation of IP3 and release of Ca2+ from internal stores, as well as from the extracellular space. The responses to ATP of myotubes prepared from E18 mouse embryos from normal and mutant mdg/mdg mice with muscular dysgenesis were studied by Tassin et al. [88]. Using Fura-2 as a probe, they showed that many of the mdg/mdg myotube preparations showed little or no increase in cytoplasmic Ca2+ levels.
Functional studies have been described, which are consistent with the presence of P2X receptors in freshly isolated skeletal muscle cells from prenatal mice [89]. ATP released at the neuromuscular junction is involved in regulation of skeletal muscle development and proliferation. P2Y1 receptors appear to modulate muscle development via dual signaling mechanisms i.e., IP3 receptor-modulated Ca2+ transients and Ca2+-insensitive phosphorylation of ERK1/2 [90]. Transient changes in responsiveness to ATP [79] and in P2 receptor expression have been described in developing skeletal muscle [29, 91, 92]. In particular, P2X5, P2X6, and P2X2 receptors were expressed in a sequential manner. P2X5 and P2X6 receptors appear to be associated in the development of the myotube, while P2X2 and P2Y1 receptors appear to be involved in the formation of the skeletal neuromuscular junction [92–95].
There have been a number of studies of the actions of ATP in developing Xenopus neuromuscular synapses by Fu and colleagues [11, 16]. Extracellular applications of ATP to developing Xenopus neuromuscular synapses in culture potentiate ACh responses of developing muscle cells during the early phase of synaptogenesis. The possibility that extracellular ATP, coreleased with ACh, may serve as a positive trophic factor at developing neuromuscular synapses was also raised. It was further suggested that CGRP and ATP coreleased with ACh from the nerve terminal may act together to potentiate postsynaptic ACh channel activity during the early phase of synaptogenesis. CGRP actions are mediated by cAMP-dependent protein kinase A, while ATP exerts its effects via protein kinase C (PKC). It was suggested that endogenously released ATP, acting in concert with various protein kinases, is involved in the maintenance and/or development of the quantum size of synaptic vesicles at embryonic neuromuscular synapses.
Central nervous system
Radioligand-binding studies provided early information about the development of A1 receptors in guinea-pig and rat brain, in particular the forebrain and cerebellum [96–98]. In guinea-pig forebrain, it appears that A1 receptors are present from embryonic day 19, with adult binding levels achieved about 25 days postpartum. In guinea-pig cerebellum, however, A1 receptor binding is low until just prior to birth, when a dramatic increase in binding is observed, which then continues to increase up to adulthood. A similar development is seen in rat forebrain and cerebellum with A1 receptor binding changing very gradually in the forebrain, whereas binding in the cerebellum increases markedly after birth [99, 100]. The developmental appearance of A1 receptor gene expression was examined in rats by in situ hybridization [101]. Expression of A1 receptor mRNA in brain was first detected on gestation day 14, and was restricted to portions of neuroepithelium caudate putamen, piriform cortex, hypoglossal nucleus, and ventral horn of spinal cord; by gestational age 17 patterns of A1 receptor expression in the brain were similar to those observed in adults. The ontogeny of adenosine uptake sites in the guinea-pig brain has been described [102]. A1 receptor down-regulation has been shown in fetal brain after caffeine or theophylline treatment of pregnant rats [103]. A1 receptor activation mediates ethanol-induced inhibition of stimulated glutamate release in the hippocampus of near-term fetal guinea-pig [104].
There are a number of reports about changes in the distribution of the ectoenzymes involved in the breakdown of ATP and adenosine in the brain during fetal and neonatal development. 5′-Nucleotidase shows a marked redistribution during development of the cat visual cortex and is thought to be involved in the remodeling of ocular dominance columns [105]. A later electron microscopic study by the same group has suggested that synapse-bound 5′-nucleotidase activity plays a role in synaptic malleability during development; its later association with glial cell profiles may reflect other functions for this enzyme [106]. At 30 and 35 days of gestation of fetal guinea-pigs, 5′-nucleotidase levels were low, but increased rapidly during the 40 to 60-day period; in contrast, adenosine deaminase was present at 30 days gestation and remained at the same level until 60 days [107]. Complex changes in the activity of adenosine deaminase in the different regions of the developing rat brain suggest that there are important roles for purines in very early stages of development from 15 to 18 days of gestation in specific regions of the brain, namely the hypoglossal motor nucleus, cingulate, retrosplenial and visual cortex, posterior basal hypothalamus and in the facial motor nucleus [108–110]. Adenosine deaminase-containing neurons were seen in the olfactory cortex of rat embryos as early as E15; this was suggested to indicate precocious development of purinergic neurotransmission within this system [111]. A histochemical study of Ca2+-ATPase in the rat spinal cord during embryonic development demonstrated intense activity in the roof and floor plates, rather than in the basal and lateral plates at embryonic day 12, indicating a possible role for Ca2+-ATPase in early differentiation of neuroepithelial cells [112, 113]. ATP induces rises in intracellular Ca2+ in embryonic spinal cord astrocytes [114].
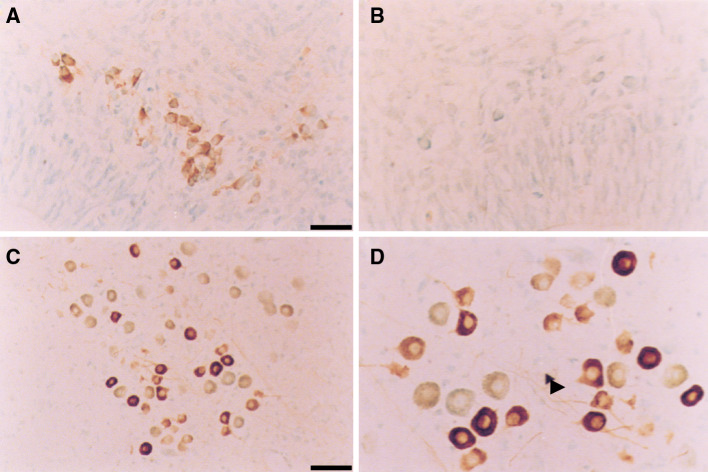
An immunohistochemical study has revealed intense labeling of P2X3 receptors in the embryonic rat brain [115] (Fig. 5). The staining was restricted to the hind brain at E16, in particular the mesencephalic trigeminal nucleus, the superior and inferior olives, the intermediate reticular zone, the spinal trigeminal tract and the prepositus hypoglossal nucleus. Other areas labeled included the mandibular nerves to the teeth, the auditory nerve from the inner ear and the trigeminal ganglion. In the E19 rat embryo P2X7 receptor mRNA was detected by in situ hybridization in brain ependyma but not neurons [116]. Primary cultures of human fetal astrocytes express low levels of P2X7 receptor mRNA and protein [117].
Fig. 5.
Immunoreactivity for P2X3 receptors in the trigeminal nucleus (Me5) of E16 (A, B) and P7 (C, D) rats. Immunohistochemistry was performed with a polyclonal antibody raised to a nine-amino-acid peptide identical to the carboxy terminus of the rat P2X3 receptor. The sections were counter-stained using Methyl Green. Labeled cell bodies can be detected for the majority of the Me5 neurons in the E16 rat (a, b) while in the P7 rat (c, d) a diminishing subpopulation of cells was labeled. Fibers and processes can clearly be seen in the P7 animal (arrowhead d). No staining was seen in control sections incubated with pre-immune serum in place of the P2X3 antibody (b). Scale bars 25 μm (a, b, d), 50 μm (c) (reproduced from [115] with permission from Elsevier)
Utilization of green fluorescent protein-tagged P2X2 receptors on embryonic hippocampal neurons has led to the claim that ATP application can lead to changes in dendritic morphology and receptor distribution [118]. P2X2 receptors were identified on Purkinje neurons in the neonatal cerebellum and with the aid of RT-PCR technology, mRNAs for P2X1-4 and P2X6 subunits were identified in the cerebellum during the first postnatal week with coexpression of two units in Purkinje cells demonstrated with patch-clamping [119].
A combined immunohistochemical and physiological study of purinergic signaling on precursor cells, neuroglial progenitors and differentiating neurons during neurogenesis of embryonic rat neocortex was carried out [120]. Neuroglial progenitors from the ventricular and subventricular zones prominently exhibited Ca2+ response to ATP. A detailed expression pattern for the P2X3 receptor in embryonic neurogenesis has been published [121]. P2X3 receptors first appeared in the hindbrain neural tube and sensory ganglia in E11–11.5 embryos; at E14.5 they appeared in the optic tract, NTS mesencephalic trigeminal nucleus, but P2X3 immunoreactivity was down-regulated in early postnatal brain stem. The P2X3 receptor was coexpressed with the P2X2 receptor in neurons in NTS and sensory ganglia.
P2Y receptors (particularly the P2Y1 subtype) were widely expressed in the embryonic rat brain as early as day 11 [122]. There was marked decrease in mRNA to P2Y1 receptors and upregulation of mRNA for P2Y2 receptors on freshly isolated astrocytes during development of rat hippocampus [123]. P2Y receptor proteins were strongly expressed transiently in structures that do not have correlates in the adult animal, suggesting that these receptors were likely to be involved in functions specific to embryonic development. For example, P2Y4 receptors disappeared from the brain stem and ventricle spinal cord postnatally.
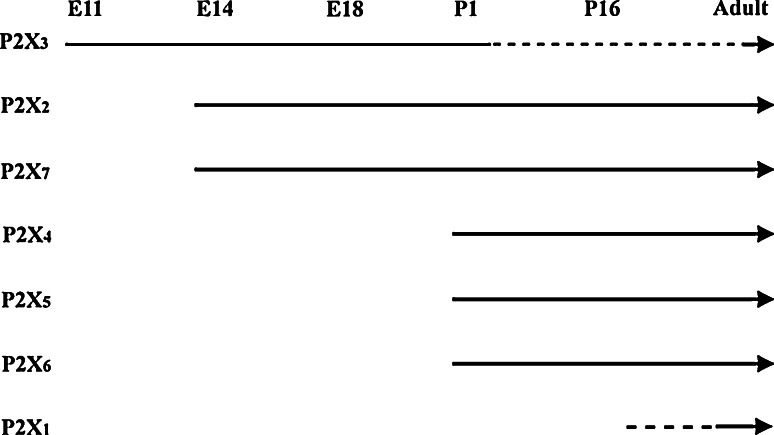
The sequential expression of P2X receptor subtypes during embryonic rat brain development was examined [124] (Fig. 6). P2X3 receptors appeared first at E11, P2X2 and P2X7 receptors at E14, while P2X4, P2X5 and P2X6 receptors did not appear until birth and P2X1 receptors even later.
Fig. 6.
Summary of the sequential expression of P2X receptor mRNA and protein during neurogenesis in the rat brain. P2X receptors are arranged from top to bottom according to the chronological order of expression during rat brain development from E11 to adult. While P2X3 receptors appeared early, they declined in the stages that followed (represented by dotted line). P2X2 and P2X7 receptors were expressed from the same day (E14) onwards, while P2X4, P2X5, and P2X6 receptors were expressed from P1 onwards. Initial dotted line for P2X1 receptor represents unknown starting point, since expression of P2X1 receptor was not observed in any of the developmental ages examined in this study. (reproduced from [124] with permission from Wiley-Liss Inc.)
In this study, ATP was shown to inhibit motor axon outgrowth during early embryonic neurogenesis, most likely via the P2X3 receptor and it was speculated that P2X7 receptors might be involved in programmed cell death during embryogenesis. At E9.5, P2X3 immunostaining was found in the hindbrain, midbrain, diencephalon, and forebrain neuroectoderm of mouse brain and in the marginal layer of diencephalon, midbrain, and hindbrain at E10.5 [125]. However, P2X3 receptor immunoreactivity disappeared from the marginal and mantle layers of the ventral horn by E14.5, although retained in the dorsal horn.
A subset of spontaneous and evoked postsynaptic currents in embryonic chick hypothalamus appear to arise from the concurrent activation of both GABA and P2X receptors [126]. The radial glial cell is a transient embryonic cell type known for its crucial role in neuronal migration and a progenitor cell for most cortical pyramidal neurons. It has been shown that calcium waves propagate through radial glial cells in the proliferative central ventricular zone and this requires both P2Y1 receptors and connexin hemichannels [127]. ATP has been shown to induce proliferation of human neural stem cells cultured from telecephalon tissues from a 15-week gestational age embryo [128].
Several studies of purinoceptors in the embryonic development of the brain of non-mammalian vertebrates have contributed to the field. For example, a novel P2Y receptor (p2y8) has been cloned and sequenced that is expressed (as seen by Northern blots and in situ hybridization) in the neural plate of Xenopus embryos from stages 13 to 18 and again at stage 28 when secondary neurulation occurs in the tail bud [17]. It differs from other members of the P2Y purinoceptor family in that it has an intracellular C terminus with 216 amino acid residues (compared to 16–67 in P2Y1–7). When expressed as a recombinant receptor in Xenopus oocytes, it shows equipotent responses to triphosphates ATP, UTP, ITP, CTP, and GTP and smaller responses to diphosphates and tetraphosphates, but is not responsive to inorganic phosphates. Responses to activation of the p2y8 receptor have a long duration (40–60 min). These data suggest that this novel P2Y receptor may be involved in the early formation of the nervous system. Regulation of rhythmic movements by purinergic transmitters in frog embryos has been described [129]. It was shown that ATP is released during swimming that activates P2Y receptors to reduce voltage-gated K+ currents and cause an increase in the excitability of the spinal motor circuits. It was also shown that adenosine, resulting from the breakdown of ATP, acts on P1 receptors to reduce the voltage-gated Ca2+ currents to lower excitability of the motor circuits, thereby opposing the actions of ATP. The author suggests that a gradually changing balance between ATP and adenosine underlies the run-down of the motor pattern for swimming in Xenopus. The cloning and functional characterization of a P2X receptor subunit in embryonic chick brain has been reported, which is highly homologous to the mammalian P2X4 receptor (human and rat) with approximately 75% sequence identity [69]. P2X3 receptors are expressed in the trigeminal ganglia of zebrafish from a very early stage of development, most likely in neural crest-derived trigeminal cells rather than placode-derived cells [130]. P2X3 receptors were also expressed in the spinal sensory Rohan-Beard cells and in the putative lateral line ganglion in the early development of zebrafish.
In a study of 48-h-old cultured ciliary ganglia and confluent peripheral and central nervous system glial cultures taken from 12 to 14-day-old embryonic chicks, Meghji et al. [131] concluded that adenosine is formed intracellularly and exported out of the cell by the nucleoside transporter; the participation of ecto-5′-nucleotidase was excluded. Adenosine transport into primary cultures of neurons and glial cells from chick embryonic brain has also been demonstrated [132, 133]. A2-like adenosine receptors were postulated to be present in glial cell membranes of chick embryonic brains [134]. Widespread programmed cell death has been demonstrated in proliferative regions of chick optic tectum during early development, particularly in the ventricular zone between stages E7.5 and E8 [135]. This is of particular interest since purinoceptors (particularly P1 and P2X7) are apoptosis-signaling molecules (see [136–138]).
Ganglia
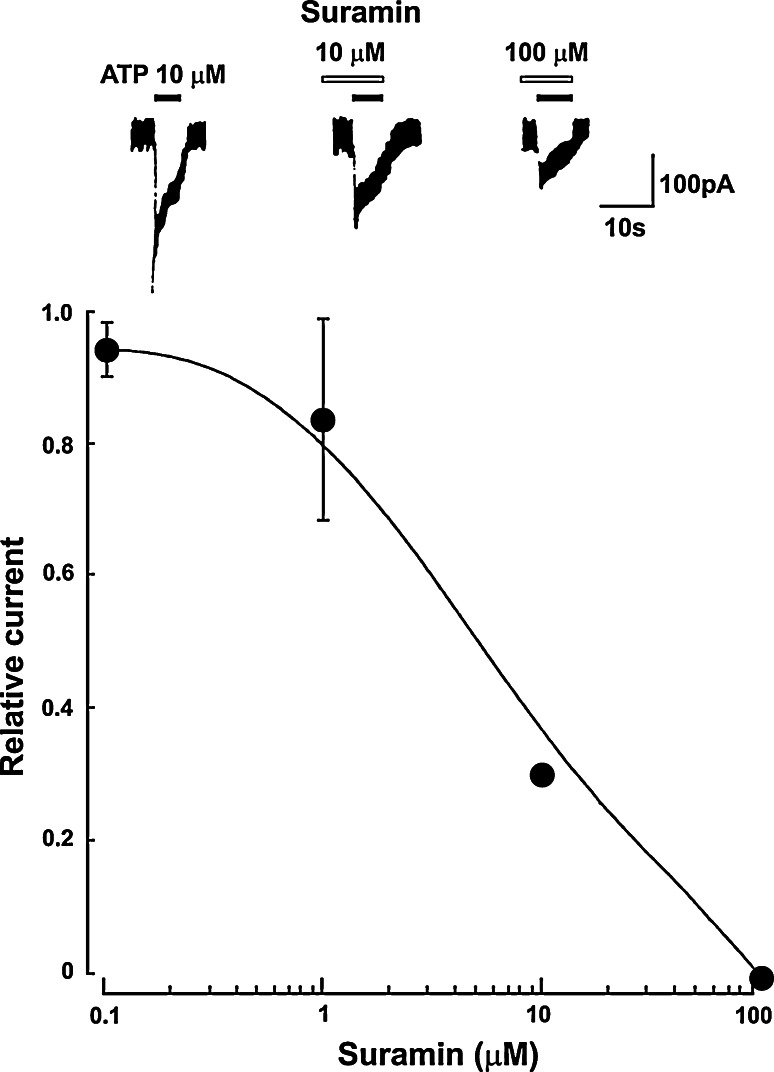
Responses to ATP have been described in ciliary neurons acutely dissociated from embryonic chick ciliary ganglia taken at day 14 [139]. The relative potency of agonists in producing transient inward currents with patch recording is ATP > 2-MeSATP > ADP; neither adenosine, AMP nor α,β-meATP are effective, but suramin is an antagonist (Fig. 7). The authors suggest that the P2 receptor subtype involved might be P2Y, but in view of more recent knowledge about the functional properties of cloned subtypes of the P2 receptor family, it seems more likely to belong to the P2X receptor family. ATP-evoked currents in cultured dorsal root ganglion (DRG) neurons from rat embryos modulate spontaneous glutamate release [140].
Fig. 7.
Chick embryo (day 14) ciliary ganglion cells: the inhibition of ATP-induced inward current by suramin. The neurons were pretreated with suramin of various concentrations for 2 min. In the upper panel the filled and open horizontal bars indicate the periods of application of ATP and suramin, respectively. In the lower panel, the responses in the presence of suramin are normalized to the peak current amplitude induced by 10 μM alone. Each point is the average of four neurons, and the vertical bars indicate standard error of the mean (reproduced from [139] with permission from Elsevier)
Adenosine inhibits neurite outgrowth of chick sympathetic neurons taken from 11-day chick embryos and kills by apoptosis about 80% of sympathetic nerves supported by growth factor over the next 2 days in culture [138]. Specific A1 or A2 agonists are not neurotoxic. The toxic effects of adenosine are not antagonized by aminophylline, but are prevented by an adenosine transporter or adenosine deaminase inhibitor, suggesting an intracellular site of action for the toxic effects of adenosine. The authors conclude that adenosine and its breakdown enzymes play an important role in the regulation of growth and development of sympathetic neurons. In follow-up experiments, the authors suggest that adenosine induces apoptosis by inhibiting mRNA and protein synthesis [141].
During early embryological development, the neural ectoderm folds to form the neural tube. Cells in the overlying ectoderm (the neural crest) then migrate within ectoderm and into the mesoderm. The cells that follow this latter pathway differentiate and mature to become glial cells and neurons. Some become primary afferent neurons of the DRG, while others become the post-ganglionic neurons of the sympathetic and parasympathetic ganglia. A third group of cells go on to form the enteric nervous system. One group of potential sympathetic neurons become surrounded by developing adrenal cortical cells and develop into adrenomedullary chromaffin cells. The sensory neurons of cranial nerves, including those of nodose, petrosal and trigeminal ganglia, however, are derived partly or entirely from the neural placodes.
ATP-gated currents activated in cultured embryonic rat DRG neurons show heterogeneity of time-courses comparable to that seen in different adult subpopulations of dissociated adult DRG neurons associated with the immunohistochemical demonstration of expression of P2X2 and P2X3 subunits [140]. Activation of P2X receptors on cultured embryonic DRG neurons results in the release of substance P [142]. Uniform immunostaining of P2X3 receptors found in most neurons was observed in embryonic mouse trigeminal and DRG, in contrast to adult ganglia, which express P2X3 receptors only on small-diameter neurons [121, 125, 143]. Nearly all sensory neurons in mouse DRG, trigeminal and nodose ganglia expressed P2X3 receptors at embryonic day 14, but after birth there was a gradual decline to about 50% of neurons showing positive staining [143]. ATP augments peptide release from neurons in embryonic DRG through activation of P2Y receptors [144].
Sympathetic neurons of the rat superior cervical ganglion (SCG) are more responsive to ATP and α,β-meATP at E18, birth and during the early postnatal period, with sustained inward currents via P2X2/3 heteromultimer receptors, but these responses are much reduced in mature rats [145]. Since this change in P2X receptor expression occurs at a time when synaptogenesis is taking place in the SCG, this might indicated a role for purinergic signaling in this process. IB4-binding DRG neurons (that express P2X3 receptors) switch from nerve growth factor to glial cell-derived neurotrophic factor dependence in early postnatal life [146]. A study of P2 receptors modulating NA release from chick sympathetic neurons cultured from 12-day-old embryos suggested that two different P2 receptor subtypes were involved: a facilitatory receptor and an inhibitory receptor [147]. In a sister paper, the authors postulated that the ATP receptor involved in NA release acted via a subclass of the nicotinic cholinoceptor [148].
Retina
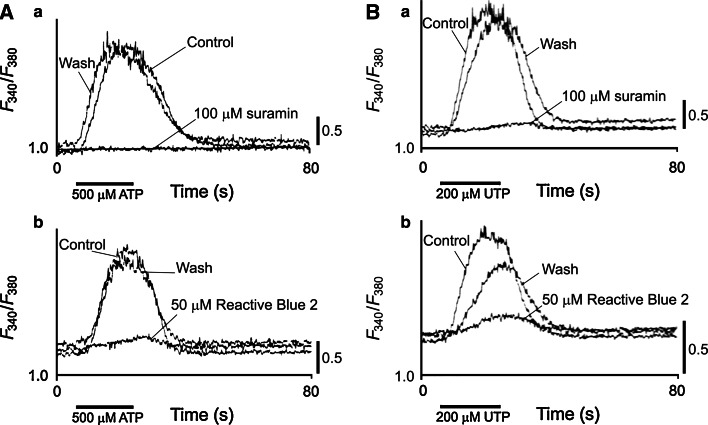
While there are many studies of purinergic signaling in the retina of adult mammals there are only a few reports about embryonic retina. Spontaneous waves of excitation in the developing mammalian retina are believed to play an important role in activity-dependent visual development of retinogeniculate connectivity [149]. The earliest age at which spontaneous waves were detected in rabbit retina was E22 and the possibility of an involvement of purinergic receptor activation in these waves was investigated [150]. Suramin blocked the wave, but pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) did not have a consistent antagonist action. A study of embryonic chick neural retina [151] (Fig. 8) has shown that the ATP-induced rise in intercellular Ca2+ is mediated by P2U (= P2Y2 or P2Y4) purinoceptors and that there is a dramatic decline of the ATP-induced rise in intracellular Ca2+ just before synaptogenesis. Suramin and Reactive blue 2 almost completely blocked these responses. These authors also reported that injection of Reactive blue 2 into early embryonic chicks produced severe effects in embryogenesis. While both the muscarinic and purinergic Ca2+-mobilizations utilize IP3-sensitive Ca2+ stores, different signal transduction pathways were involved [152]. P2 purinoceptor activated by autocrine or paracrine release of ATP have been claimed to be involved in the regulation of DNA synthesis in the neural retina at early embryonic stages [153]. ATP increased [3H]thymidine incorporation in retinal cultures from embryonic day 3 (E3) and suramin and PPADS inhibited these activities in a dose-dependent manner; the concentration of ATP increased 25-fold in the medium of E3 retinal organ cultures within 1 h of incubation and was maintained for at least 24 h (3 μM ATP at E3, declined to 0.15 μM at E7). In a review it was suggested that the change in Ca2+ signaling mediated by P2U receptors during development may underlie the differentiation of neuroepithelial cells or undifferentiated progenitor cells into neurons [154]. ATP acting on P2 receptors is involved in the regulation of retinal progenitor cell proliferation at early embryonic stages perhaps in collaboration with growth factors [153]. ATP, probably via P2Y1 receptors coupled to PLC, PKC, and MAP kinases, stimulates proliferation of both bipolar and Müller cells in early developing chick retina at embryonic days 6–8 [155]. Guanine nucleotides block agonist-driven Ca2+ influx in chick embryo retinal explants [156].
Fig. 8.
Effects of P2 receptor antagonists on the Ca2+ responses to ATP and UTP in embryonic (E3) chick neural retinas. A The effects of suramin (100 μM; A a) and Reactive blue 2 (50 μM; A b) on the response to 500 μM ATP. The records in the presence of suramin or Reactive blue were taken 7 min after changing the bath solutions to the antagonist-containing medium. The recovery controls (Wash) were taken after washing suramin for 7 min or Reactive blue for 25 min. The duration of ATP application (20 s) is indicated by the bars. All records were taken in the bath solutions containing 2.5 mM Ca2+. B The effects of suramin (100 μM; B a) and Reactive blue 2 (50 μM; B b) on the response to 200 μM UTP. The records in the presence of suramin or Reactive blue were taken 7 min after changing the bath solutions to the antagonist-containing medium. The recovery controls were taken after washing suramin for 7 min or Reactive blue for 15 min. The duration of UTP application (20 s) is indicated by the bars. All records were taken in the bath solutions containing 2.5 mM Ca2+ (reproduced from [151] with permission from John Wiley and Sons Ltd.)
Adenosine has also been implicated in chick retinal development. Adenosine induction of cAMP increased strongly from the 14th to the 17th embryonic day, P1 (A1) subtype receptors modulating D1 dopamine receptor-mediated stimulation of adenylate cyclase activity [157–159]. A1 receptors were localized predominantly in plexiform regions by E12. They were absent in the retina at E8, but were detected at E12 in the ganglion cell layer, as well as cells in the nuclear cell layer and photoreceptors. It was suggested that A1 receptors may have different functions in the embryonic retina as compared to mature chick retina, and the localization of A1 receptors and uptake sites in the developing chick retina were examined [160].
A fascinating paper has shown that purine-mediated signaling triggers eye development in Xenopus [161]. The authors have shown that over expression of ecto-NTPDase2 (that converts ATP to ADP) caused ectopic eye-like structures and occasionally complete duplication of the eye and increased expression of Pax6, a key gene in eye development. Downregulation of ecto-NTPDase2 decreases Pax6 expression. Further, evidence that ADP is acting via P2Y1 receptors was presented.
Gastrointestinal tract
In the gastrointestinal tract, non-adrenergic, non-cholinergic (NANC) nerve-mediated effects have been observed before birth in rat stomach [162] and in mouse and rabbit small intestine [163]. Also, quinacrine fluorescence, which indicates the presence of high levels of bound ATP, was observed before birth in enteric neurons of rabbit ileum and stomach, about 3 days before catecholamine fluorescence was detected in enteric nerves [164]. NANC inhibitory and cholinergic excitatory innervation appear simultaneously in the rabbit at 17 days of gestation and both were present in the mouse by the 16th day of gestation; however, the development of adrenergic innervation lagged far behind the other two components, clearly establishing that the intrinsic innervation of the gut is not adrenergic [163, 165].
An electrophysiological study of developmental changes in the innervation of the guinea-pig taenia coli has been carried out [166]. The non-adrenergic (largely purinergic) inhibitory system appeared before and matured faster than the cholinergic excitatory system. The NANC inhibitory system was present by 8 weeks of gestation, while cholinergic excitatory transmission was not seen until birth. Responses to α,β-meATP were also recorded in the fetal taenia coli.
Lung
ATP and UTP evoke [Ca2+]i signals in rat fetal lung epithelial cells, but only if grown into functionally polarized epithelia on permeable supports; moreover, RT-PCR identification of the mRNA for the P2Y2 receptor was clearly expressed in the polaroid cells [167]. In another study of epithelia explanted from fetal rat lung, receptors to adenosine, ATP and UTP were present on apical membranes throughout the lung; basolateral receptors for these agonists in distal lung and ATP/UTP receptors in trachea function later in gestation [168]. In E19 rat embryos P2X7 mRNA was detected by in situ hybridization in bronchial epithelium (as well as salivary gland, liver, bone marrow and brain endyma) [116].
ATP, ADP and adenosine are claimed to be important mediators of oxygen-induced pulmonary vasodilatation in fetal lambs [169, 170], probably via both A2A and P2Y receptors [171]. Vagal sensory nerve terminals in rat lung express P2X3 receptors from the first moment that they make contact with neuroepithelial bodies (NEBs) a few days before birth [172]. This is consistent with the important function of NEBs as oxygen sensors perinatally before the carotid body O2-sensory system is fully developed at about 2 weeks after birth. It has been claimed that adenosine plays a central role in modulating ventilation in the newborn piglet and is involved in the diphasic ventilatory responses to hypoxia.
Urinary bladder
In fetal human bladder, expression of P2X1 receptor transcripts was much lower than in adult bladder; P2X4 and P2X7 receptors were also present in the fetus [173]. With increasing gestation, the P2 receptor expression shifted from the dome to the body of the bladder. Obstruction of the fetal male sheep bladder leads to enlarged, hypocontractile and compliant bladder; however, there was no clear evidence for changes in purinergic (or in cholinergic or nitrergic) neurotransmitter effects [174].
Inner ear
During embryonic development of the rat inner ear, P2X2 receptor mRNA expression was present in the precursors of the cells bordering the cochlear endolymphatic compartment at E12, as well as spinal and vestibular ganglia [175]. Both inner and outer hair cells did not exhibit P2X2 receptor mRNA until after P10 through P12, concomitant with the onset of hearing. These data are consistent with roles for the P2X2 receptor both in the process of labyrinthine development and in the regulation of auditory and vestibular sensory transduction. A later paper from this group showed that P2X1 receptors provide the signal transduction pathway for development of afferent and efferent innervation of the sensory hair cells and purinergic influence on cochlea morphogenesis [176]. P2X3 receptor expression has been characterized in the mouse cochlea from E16 using confocal immunofluorescence [177]. From E18 to P6, spiral ganglion neuron cell bodies and peripheral neurites projecting to the inner and outer hair cells were labeled for P2X3 receptor protein, but diminished around P6, and were no longer detected at the onset of hearing (around P11). These data suggest a role for P2X3 receptor-mediated purinergic signaling in cochlea synaptic reorganization and establishment of neurotransmission that occurs just prior to the onset of hearing function [178].
Other organs
Chondrocytes, isolated from the cephalic region of day 19 chick embryo sterna, release nucleotides into the extracellular milieu, although they are rapidly degraded; it is claimed that they are involved in both chondrocyte maturation and matrix mineralization [179]. Extracellular ATP modulates [Ca2+]i in retinoic acid-treated chondrocytes isolated the cephalic portion of day 14 chick embryonic sterna [180]. They speculate that immature chondrocytes may generate adenine nucleotides that then act in a paracrinal manner on chondrocytes that are at a later stage of maturation. Rapid deamination of adenosine in cultures of fetal mouse calvarial bones was shown and taken to account, at least in part, for the failure to observe effects of adenosine in bone metabolism in culture [181]. Cells of osteogenic and chondrogenic lineage derived from fetal metatarsal bones were exposed to ATP4−; cells of hemopoietic origin were permeabilized and killed, while cells of non-hemopoietic origin (e.g. osteoblasts, chondrocytes) were insensitive to ATP4− and survived [182]. This system allows the study of the properties and functions of osteogenic or chondrogenic cells without interference by the presence of cells of hemopoietic origin. ATP pyrophosphohydrolase has been purified and partially characterized from fetal bovine epiphyseal cartilage of patients with chondrocalcinosis [183]. The most prominent location of P2X7 receptor mRNA in E19 rat embryos is bone marrow; but bone marrow cells from mouse femur also showed strong immunoreactivity for P2X receptors [116].
In the skin, Merkel cells appear in the epidermus of planum nasale or rat fetuses from the 16th day of intrauterine development and nerve fibres form close association with them by day 20 [184]. This is of interest since it is known that Merkel cells contain high levels of peptide-bound ATP and are in close association with sensory fibres expressing P2X3 receptors (see [185]).
Taken together, these results point to a role for purines in both physiological fertilization and normal development and also underline that alterations of the purinergic regulation of embryonal growth might be involved in the onset of morphological malformations. Depending upon the purine derivative, and probably upon the purinoceptor involved as well, ATP and adenosine can act as both positive and negative regulators of growth. This is also consistent with data obtained from in vitro cell lines, which implicates purines in cell proliferation, differentiation and apoptotic cell death. Further studies are needed to better characterize the receptor subtypes involved and also to identify more precisely the developmental events specifically controlled by purines.
Stem cells
Understanding the mechanisms underlying development, including cell proliferation, differentiation and death, has raised much interest over recent years. Stem cell models are being used to study mechanisms of differentiation in simplified conditions, as the investigation of mechanisms of development is difficult to perform in vivo. These studies aim to develop in vitro protocols for stem cell culture and differentiation into defined specialized cell types. Moreover, the investigation of stem cell biology will help to elucidate the molecular basis of phenotype determination and how endogenous stem cells are mobilized for tissue repair. Such knowledge is important for therapeutic applications of cell therapy, since optimization of culture conditions and verification of adequate endogenous stem cell niche conditions are essential for successful transplantation.
Stem cells can characterize different stages of development. Pluripotent stem cells (embryonic carcinoma and embryonic stem cells) have characteristics of early embryonic cells [186, 187] and differentiate into the three primary germ layers of the embryo. Embryonic stem cells originate tissue-specific stem cells, which are present in the fetal, as well as in the adult tissue. These fetal and adult tissue-specific stem cells have often a limited potential in giving rise to different cell types. However, it is known that endogenous stem progenitor cells are present throughout life and are recruited by specific signaling events for replacement of damaged tissue. The importance of purinergic signaling in stem cell biology, including regulation of proliferation, differentiation and cell death, has become evident [7, 188]. In the following text, we will discuss the expression of the purinergic system in embryonic and adult stem cells and its participation in induction of differentiation.
Induction of stem cells to neural differentiation
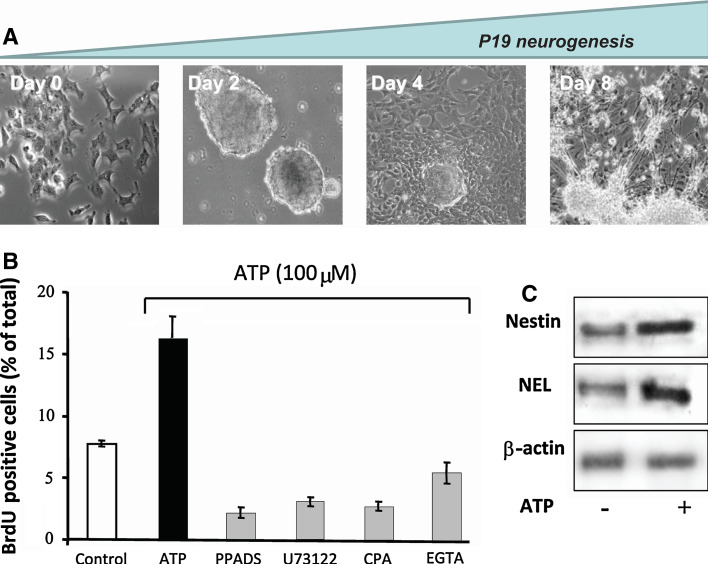
The P19 murine embryonal carcinoma cell line has been used for decades for studying mechanisms of early neurogenesis and resembles the developmental stages that are encountered during neuronal development [189]. When induced to neuronal differentiation in the presence of retinoic acid and kept in suspension culture, P19 cells form tri-dimensional cell aggregates, denominated embryonic bodies, which resemble the blastula stage of embryonic development. When collected after 48 h of suspension culture and re-plated in adherent culture dishes, cells on day 4 of differentiation express nestin, specific for neural-progenitor cells. Differentiation into neuronal cells is completed on day 8 (Fig. 9a), while glial cells predominate in the culture on day 14. Purinergic receptor activity was essential for proliferation and the progress of neurogenesis (Fig. 9b, c). Exposure of differentiating P19 cells at the neural progenitor stage to blockers of P2Y and P2X receptor-mediated calcium signal transduction resulted in inhibition of proliferation (Fig. 9b).
Fig. 9.
Participation of purinergic receptors in neurogenesis of P19 embryonal carcinoma cells. a. Cell differentiation: In vitro differentiation of P19 embryonal carcinoma cells resembles processes occurring during early neuroectodermal differentiation. Stages of differentiation: Pluripotent P19 are treated with 1 μM retinoic acid in defined serum-free medium and cultured for 48 h in non-adherent flasks for formation of embryonic bodies as described previously [246–248]. On the third day, embryonic bodies are collected and re-plated for neural differentiation to take place. Progenitor cells migrating from embryonic bodies express nestin, a specific marker for neural progenitors. Differentiation into neurons is complete on day 8 when cells reveal neuronal morphology, express β-3-tubulin and neuron-specific enolase (NEL) and form neuronal networks. Glial cells were eliminated from differentiating neuronal cultures by addition of cytosine–arabinoside [190]. b ATP-induced proliferation and differentiation. Prior to proliferation assays, P19 cells at the neural progenitor cell stage were kept in the absence or presence of the purinergic receptor antagonist PPADS (10 μM), cyclopiazonic acid (10 μM) for depletion of intracellular calcium pools or EGTA for chelating extracellular calcium (10 mM). Then 100 μM ATP was added and BrdU-incorporation was determined following 48 h of culture as a measure of cell proliferation. c Neural progenitors from day 4 of differentiation were incubated for 48 in the presence (+) or absence (−) of 100 μM ATP. Nestin, NEL and β-actin expression in cell extracts was determined by Western-blot analysis. (b and c are reproduced from [191] with permission from Elsevier)
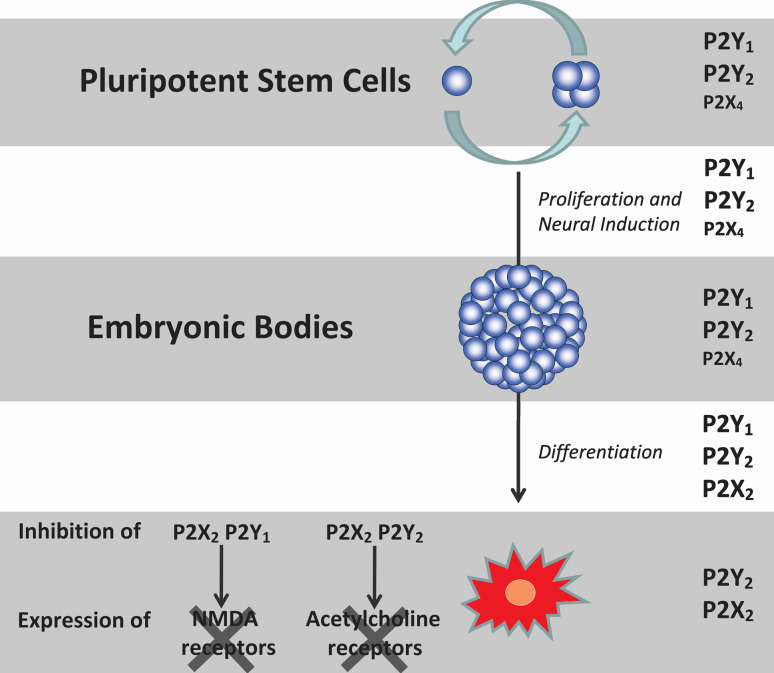
Gene and protein expression of P2X2, P2X6, P2Y2, and P2Y6 receptors increased during the course of differentiation, whereas P2X3, P2X4, P2Y1, and P2Y4 receptor expression was high in embryonic P19 cells and then decreased following induction of differentiation [190]. A further published work revealed that P2X4/6 and P2X2/6 heteromultimer receptors may also be expressed on P19 cells and it was concluded that the P2Y1 receptor is the major subtype involved in regulating cell proliferation and differentiation, followed by the P2Y2 receptor subtype and with a minor role for the P2X4 receptor [191] (Fig. 10). These conclusions were based on functional assays performed with the various purinergic receptor agonists and antagonists that have distinct pharmacological profiles. Recent, as yet unpublished, data showed that induction of proliferation was affected in the presence of siRNA for down-regulation of P2X4 receptors. In addition to its participation in neuronal differentiation, purinergic receptor activity is also important for neuronal phenotype definition. For instance, chronic inhibition of P2Y1 and possibly P2X2 receptor activity along differentiation of P19 cells led to a loss of NMDA receptor activity in neuronal-differentiated cells, whereas blockade of P2Y2 and possibly P2X2 receptors led to inhibition of cholinergic receptor responses. Figure 10 summarizes the major functions of purinergic receptor subtypes along neuronal differentiation of P19 cells.
Fig. 10.
Neurogenesis and phenotype determination of P19 cells involves sequential P2X and P2Y receptor activities. P19 embryonal carcinoma cells were induced to neuronal differentiation by addition of retinoic acid and embryonic body formation. Glial cells were eliminated from differentiating neuronal cultures by addition of cytosine-arabinoside [190]. Metabotropic P2Y1 and P2Y2 receptors, with a minor role of P2X4 receptors, participate in induction of proliferation of embryonic cells and embryonic body formation [191]. P2Y1, P2Y2, and P2X2 receptor activities are important for later differentiation and final phenotype determination, since, when these receptors were inhibited during P19 neurogenesis, no NMDA-glutamate and cholinergic receptor activities were detected in differentiated cells [190]. Neuronal-differentiated P19 cells express functional P2Y2 and P2X2 receptors
Neural stem cells are self-renewing multipotent progenitor cells, whose daughter cells can differentiate into both neurons and glia [192]. Isolated from fetal brain or neurogenic areas of adult brain, cells proliferate as free-floating spheric expansions, denominated neurospheres, expressing the neural-progenitor marker nestin. When induced to differentiation by removal of growth factors, cells of the outer layers of the neurosphere migrate and differentiate into three major neural phenotypes of the brain, neurons, astrocytes and oligodendrocytes ([193] reviewed in [194]) (Fig. 11).
Fig. 11.
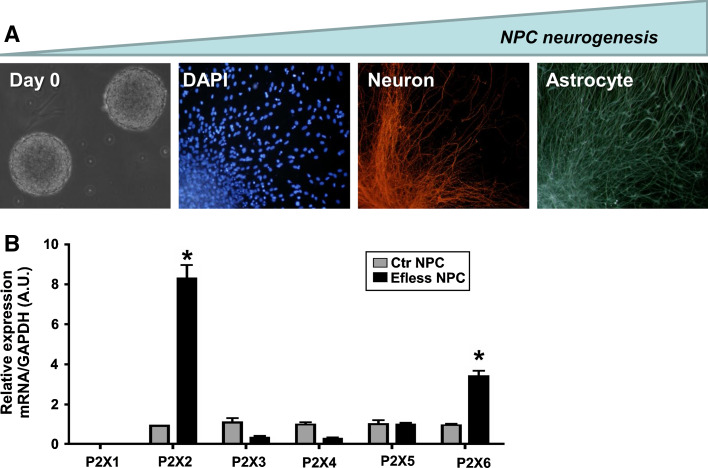
Purinergic P2X receptor subunit expression during in vitro neurogenesis of embryonic rat brain neural progenitor cells. A Cell differentiation: Neural stem and progenitor cells (NPC) are obtained by dissection of fetal rat telencephalon from embryonic day 14. NPC proliferation is maintained for 10 days in the presence of epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF-2) for formation of neurospheres as clonal expansions of a single precursor cell (left panel, day 0). Neurogenesis is induced by removal of growth factors and plating in adherent cell culture dishes pre-coated with poly-l-lysine and laminin [193]. Neurosphere differentiation is complete following 7 days of induction. Cells migrate and differentiate into neurons and glial cells (panels from left to right: cell nuclei staining by 4′,6′-diamidino-2-phenylindole (DAPI); neuronal and astroglial differentiation detected by immunofluorescence staining against β-3-tubulin and glial fibrillary acidic protein, respectively). b Changes of P2X receptor expression in NPC induced to neurogenesis by growth factor deprivation. Following neurosphere expansion, half of the population was kept for 7 days in complete culture containing EGF and FGF-2 (Control group—Ctr NPC), while the other half was maintained in the absence of growth factors for induction of neurogenesis (EFless NPC). Gene expression of P2X1–P2X6 receptor subunits in Ctr and EFless NPC was determined by real-time PCR. Relative gene expression levels were obtained by comparing them to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels, which are not supposed to vary under the chosen experimental conditions. Increased neurogenesis was accompanied by elevated P2X2 and P2X6 receptor subunit expression (*p < 0.05, determined by Student′s t test) (b reproduced from [195], with permission from Springer)
Neurospheres obtained from fetal rat brain expressed P2X2-7 receptor subunits, as well as P2Y1, 2, 4, 6 metabotropic receptors ([195] and unpublished data). In conditions of differentiation favoring neurogenesis, upregulation of P2X2 and P2X6 receptor subunits was observed (Fig. 11), being in agreement with data obtained in P19 cells [190, 191], pointing at the importance of P2X2 and P2X2/6 heteromeric receptors in this process. In agreement, P2X6 receptor subunit expression increases during pre- and postnatal mouse brain development [196].
ATP induces proliferation of human neural stem cells and activation of P2 receptors was coupled to release of [Ca2+]i from thapsigargin-sensitive intracellular stores [128], indicating mediation via P2Y receptors. It was further shown that ATP-induced proliferation was through the P13-kinase-dependent P70S6 kinase signaling pathway and it was suggested, therefore, that extracellular ATP plays an important physiological role during embryonic mammalian brain development.
The major challenge for scientists in the stem cell therapy field is to control cell fate and develop ideal culture conditions for neural progenitor cells (NPC) expansion in vitro, without altering their features. Transplantation of ex vivo-expanded human neural stem/progenitor cells is being used for treating intractable neuronal diseases and injuries. The stem cells are selectively expanded as free-floating neurospheres in serum-free culture medium containing fibroblast growth factor 2 (FGF-2) and/or epidermal growth factor (EGF). It has been suggested that NPC have a great potential for the treatment of neurological disorders including amyotrophic lateral sclerosis, Parkinson′s disease and epilepsy. NPC could provide a source of new neurons to alleviate neural injury. However, despite the initial euphoria, it soon turned out that transplantation of neurospheres did not result in any significant recovery of neuronal activity and function as, for example, investigated in a spinal cord injury model [197]. It became clear that successful integration of in vitro differentiated neural stem cells depends on favorable conditions in the stem cell niche, such as the availability of neurotrophic factors. Measurement of the ATP content of differentiating stem cells has been used to optimize culture conditions for the best clinical performance [198] indicating that ATP is essential for the stem cell niche. ATP increased survival of PC12 cells and induced neuritogenesis in vitro, while inhibition of purinergic receptors abolished ATP- and nerve growth factor-induced effects on neurite outgrowth [199].
One possible positive regulator of neurogenesis is ATP liberated in conditions of neuronal injury and death or from gentle mechanical deformation of local cells, which then could mobilize endogenous stem cells and induce them to undergo proliferation and neuronal differentiation. Purinergic activation has been associated with proliferation and neurogenesis in neonatal and adult mouse olfactory epithelium [200]. ATP-induced neurogenesis was mediated by induction of synthesis and liberation of neuropeptide Y (NPY), a well-known neurotrophic factor. Later on, Jia and Hegg [201] provided evidence for in vivo induction of neurogenesis by ATP. Intranasal application of ATP led to an increase in the number of NPY+ cells and BrdU+ incorporation in adult mouse epithelium. ATP-promoted neurogenesis was blocked in the presence of the purinergic receptor antagonist PPADS.
Functional purinergic receptors were also detected in other neurogenic areas of the brain. In more than 70% of undifferentiated progenitor cells from adult rat hippocampus, ATP and BzATP evoked an inward current and membrane depolarization, whereas ACh, NA, glutamate and GABA had no detectable effect; differentiated cells, however, possessed functional glutamate, GABA and glycine receptors, as well as P2X receptors [202]. Functional P2 receptors and NTPDase 2 were identified in adult hippocampal progenitor cells from both the subventricular zone (type B cells) and the dentate gyrus of the hippocampus [203]. ATP, ADP and to a lesser extent UTP evoked rapid Ca2+ transients in neurospheres mediated by P2Y1 and P2Y2 receptors and augmented cell proliferation in the presence of growth factors, suggesting synergistic activation of mitogenic growth factors and purinergic receptor-mediated signaling pathways [204]. The authors suggest that this supported the notion that extracellular nucleotides contribute to the control of adult neurogenesis.
UTP, combined with the mitogens FGF-2 and EGF, boosted proliferation of human midbrain mesencephalic stem/progenitor cells and stimulated dopaminergic differentiation [205]. Nucleotide agonists increased [Ca2+]i in the rank order of potency ATP > ADP > UTP > UDP in human midbrain-derived neural progenitor cells and it was also shown that UTP evoked the release of ATP from the cells, probably via P2Y2 receptors [206].
Ventricular zone neural stem and progenitor cells express P2Y receptors, occupation of which leads to increase in [Ca2+]i [207]. The progenitor cells themselves release ATP in episodic bursts. P2Y receptor antagonists suppressed proliferation and permitted differentiation into neurons and glia, while subsequent removal of purinergic inhibition restored progenitor cell expansion. Primary neurospheres prepared from the subventricular zone expressed mRNA for P2X4 and P2X7 receptors, all P2Y receptor subtypes (with the exception of P2Y4) and A1, A2A and A2B adenosine receptors [208]. Further, P2Y1 and A2A receptor antagonists reduced the size and frequency of primary neurospheres.
P2X7 receptors have been identified on neural progenitor cells, prepared from the striatum of mice at E14.5 day and prolonged occupation of these receptors by ATP or BzATP leads to cell death [209]. The authors conclude that their data supports the notion that high levels of extracellular ATP in inflammatory CNS lesions may delay the successful graft of neural progenitor cells used to replace cells and repair CNS damage. Induction of neuronal differentiation of Neuro-2a cells was accompanied by down-regulation of P2X7 receptor expression. Down-regulation of P2X7 receptor expression by siRNA induced neuronal differentiation [210].
The adult subventricular zone contains neural stem cells that generate neuroblasts migrating to the olfactory bulb and differentiate into interneurons. Nucleotides and EGF induce the formation of stress fibres, an increase in the cortical actin cytoskeleton and in cell migration [211]. GABAergic neurons derived from mouse embryonic stem cells liberate [Ca2+]i predominately via the activation of P2X2, P2X4 and P2Y1 receptors [212]. Adenosine A1 receptor agonists stimulate proliferation of neural stem cells derived from the subventricular zone of mouse brain via MEK/ERK and Akt signaling pathways [213]. Adenosine-releasing embryonic stem cells have been generated by disruption of both alleles of adenosine kinase (AdK −/−) [214]. When grafted into the lateral brain ventricles they have been claimed to suppress seizure activity [215, 216]. The same adenosine-releasing brain implants have been used to provide neuroprotection in ischemic mouse brain [217].
Human neural stem cells and their differentiated cultures have been used to evaluate the mechanisms involved in rotenone- and camptothecin-induced cytotoxicity [218]. Time-dependent ATP depletion occurred and it was suggested that intracellular ATP levels may play an important role in determining whether neural progenitors or their differentiated cells follow a caspase 9/3-dependent or -independent pathway in response to acute insults from neural toxicants.
In summary, data discussed in this review indicate that ATP-mediated effects on neural stem cells mobilize endogenous stem cells by inducing proliferation, thereby increasing the pool of cells which undergo differentiation. ATP-promoted neurogenesis, measured as increased proliferation of neural marker proteins in vitro and augmented production of neurotrophic factors in vivo, could be due to activation of neural-specific transcription programs, which promote progression to the next stage of differentiation and/or increase of cell survival by inhibition of apoptosis.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) have been taken from a variety of tissues, including bone marrow stromal cells, adipose tissue, dental pulp, skin, and umbilical cord.
Non-hematopoietic human bone marrow-derived stromal MSCs have the potential to develop into several types of cells. ATP autocrine/paracrine release via hemichannels (and perhaps vesicular exocytosis) [219] provides a signaling pathway involving Ca2+ oscillations mediated by P2Y1 receptors [220]. ATP released from human MSCs not only modulates their proliferation rate, but appears to act via P2Y1 receptors as one of the early factors determining their cell fate [221], including osteoblasts, chondrocytes, and adipocytes [219]. The order of potency of nucleotide agonists acting to increase [Ca2+]i in bone marrow stromal cells was ATP = UTP > ADP > UDP and RT-PCR and immunohistochemical studies identified P2Y2 receptors on these cells [222]. Adenosine A2A receptors are claimed to play an active role in mouse bone marrow-derived MSC development in tissue repair [223]. Evidence has been presented for adenosine receptor regulation of osteogenesis versus adipogenesis in MSCs derived from bone marrow [224]. Adenosine inhibits chemotaxis and induces hepatocyte-specific genes in bone marrow MSCs [225]. It was reported that bone marrow stromal cells that produce adenosine (perhaps after breakdown of released ATP) express CD73 and all four adenosine receptor subtypes that may be involved in regulation of progenitor cell differentiation and thereby contribute to bone formation and resorption [226].
Human MSCs derived from human skin biopsies increased [Ca2+]i in response to ATP, probably via P2X receptors [227]. Human MSCs generated from umbilical cord secrete trophic molecules, such as vascular endothelial growth factor A, hepatocyte growth factor and transforming growth factor-β, which enhance intracellular ATP content and insulin secretary function of human pancreatic islets from cadaveric donors [228]. Human MSCs derived from adipose tissue, which can differentiate into adipocytes and osteoblasts, express both P2X and P2Y receptors, which are involved in the selective differentiation process [229]. Human MSCs derived from bone marrow have been used largely for bone formation, but have also been used successfully in models of stroke [230], traumatic brain injury [231] and epilepsy [232]. As there is at present no conclusive evidence for the trans-differentiation of MSCs, it is believed that transplanted MSCs secrete neurotrophic factors, which recruit endogenous NPC.
Hematopoietic stem cells
The survival, proliferation and development of hematopoietic stem cells (HSCs) in vitro requires the presence of specific growth factors, such as interleukin 3 (IL-3) [233]. ADP receptors were claimed to be present on both human and rat hematopoietic cell lines [234]. High concentrations of ATP induced irreversible damage of leukemic cells, without injuring normal HSCs, offering the possibility of purging residual tumor cells in HSCs used for bone marrow transplantation [235]. Adult human (CD34+) HSCs from transplant donors were shown to express functional P2X7, P2Y1 and P2Y2 receptors and UTP shown to enhance proliferation of HSCs [236]. UTP is also a potent inducer of migration of HSCs [237]. RT-PCR analysis of murine bone marrow-derived HSPs confirmed the presence of P2X7 receptors and showed that occupation of these receptors could lead to cell death [238].
Adenosine potentiates the stimulatory effect of growth factors and cytokines on hematopoietic progenitor cells from granulocytes and macrophages [239]. An A3 receptor agonist caused proliferation of hematopoietic precursor cells during the depletion phase, while an A1 receptor agonist suppressed proliferation in the regenerative phase [240]. Activation of A3 receptors potentiates the stimulatory effects if IL-3 and stem cell factor on these cells [241]. Adenosine A2A receptor activation limits graft-versus-host disease after allogenic HSC transplantation [242].
Future perspectives
Roles for purinergic receptors (P2X, P2Y, and P1 adenosine receptors) have been defined in most developmental processes, including fertilization, embryological development and differentiation of various tissue types. The alteration of expression of purinergic receptors during development indicates specific functions for progression of differentiation and final phenotype specification. Studies carried out in vitro with cell lines, as well as in vivo with Xenopus embryos, point to the importance of purinergic signaling in early development, e.g., induction of differentiation was abolished in the presence of purinergic receptor antagonists. Furthermore, the trophic actions of purines on migration and differentiation of stem and progenitor cells and axonal growth of neuronal cells were observed. These differentiation-promoting effects may be further explored for optimizing transplantation procedures and recruiting quiescent endogenous stem cells and NPC for tissue repair. Uridine and its nucleotide metabolites activate brain P2Y receptors, which control neuronal differentiation and synaptic protein synthesis. For instance, activation of brain P2Y receptors promotes neuronal differentiation and synaptic protein synthesis. A preparation containing these compounds is currently under clinical evaluation for treating Alzheimer’s disease [243].
It is important to note that the same purinergic receptor subtypes do not necessarily induce similar effects in different cell types and/or developmental stages. For instance, the P2Y1 receptor has been implicated to function in proliferation induction and progress of development in different tissues and organisms, as detailed in this review. However, in contrast to its apparent function of promoting cell survival and proliferation, the receptor in distinct cellular conditions may also promote apoptosis of astrocytes [244]. The divergence in cellular responses by the same receptor subtype is based on the possible activation of multiple intracellular signaling pathways. Moreover, purinergic receptors interact with a variety of growth factors and neurotransmitter receptors (the nicotinic ACh receptor is a classical example), which may modulate ATP- or adenosine-induced responses.
Further studies are needed to better characterize the receptor subtypes involved and also to identify more precisely the developmental events specifically controlled by purines. It is already known that purinergic receptor dysfunction can affect the progress of normal development. For instance, adenosine A1 receptor activation potently inhibits the development of axons and can lead to leukomalacia [245] and ATP-derived ADP signaling via P2Y1 receptors has been implicated in triggering the gene expression necessary for formation of the eye [8, 161].
The major draw-back in relating purinergic receptor activity with normal and pathophysiological developmental processes is the lack of specific agonists and antagonists for some purinergic receptor subtypes. In view of this, the elucidation of purinergic receptor function will rely on the use of animal models carrying knock-out constructs for individual receptor subtypes, as well as the use of RNA interference in vitro and in vivo. Purinergic receptors with evidenced functions in developmental diseases will be subject to structure- and combinatorial library screening-based discovery of novel specific ligands that may reveal therapeutic approaches for treatment of developmental diseases resulting from purinoceptor dysfunction.
References
- 1.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 2.Kupitz Y, Atlas D. A putative ATP-activated Na + channel involved in sperm-induced fertilization. Science. 1993;261:484–486. doi: 10.1126/science.8392753. [DOI] [PubMed] [Google Scholar]
- 3.Smith R, Koenig C, Pereda J. Adenosinetriphosphatase (Mg-ATPase) activity in the plasma membrane of preimplantation mouse embryo as revealed by electron microscopy. Anat Embryol (Berl) 1983;168:455–466. doi: 10.1007/BF00304281. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa T, Seguchi H. Localization of Mg++-dependent adenosine triphosphatase and alkaline phosphatase activities in the postimplantation mouse embryos in day 5 and 6. Anat Embryol (Berl) 1985;173:7–11. doi: 10.1007/BF00707299. [DOI] [PubMed] [Google Scholar]
- 5.Foresta C, Rossato M, Di Virgilio F. Extracellular ATP is a trigger for the acrosome reaction in human spermatozoa. J Biol Chem. 1992;267:1–5. [PubMed] [Google Scholar]
- 6.Burnstock G. Purinergic signalling in development. In: Abbracchio MP, Williams M, editors. Handbook of experimental pharmacology, vol 151/I. Purinergic and pyrimidinergic signalling I: molecular nervous and urinogenitary system function. Berlin Heidelberg New York: Springer; 2001. pp. 89–127. [Google Scholar]
- 7.Zimmermann H. Nucleotide signaling in nervous system development. Pflugers Arch. 2006;452:573–588. doi: 10.1007/s00424-006-0067-4. [DOI] [PubMed] [Google Scholar]
- 8.Dale N. Dynamic ATP signalling and neural development. J Physiol. 2008;586:2429–2436. doi: 10.1113/jphysiol.2008.152207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igusa Y. Adenosine 5′-triphosphate activates acetylcholine receptor channels in cultured Xenopus myotomal muscle cells. J Physiol. 1988;405:169–185. doi: 10.1113/jphysiol.1988.sp017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akasu T, Hirai K, Koketsu K. Increase of acetylcholine-receptor sensitivity by adenosine triphosphate: a novel action of ATP on ACh-sensitivity. Br J Pharmacol. 1981;74:505–507. doi: 10.1111/j.1476-5381.1981.tb09997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu WM. Regulatory role of ATP at developing neuromuscular junctions. Prog Neurobiol. 1995;47:31–44. doi: 10.1016/0301-0082(95)00019-r. [DOI] [PubMed] [Google Scholar]
- 12.Fu WM, Poo MM. ATP potentiates spontaneous transmitter release at developing neuromuscular synapses. Neuron. 1991;6:837–843. doi: 10.1016/0896-6273(91)90179-4. [DOI] [PubMed] [Google Scholar]
- 13.Fu WM. Potentiation by ATP of the postsynaptic acetylcholine response at developing neuromuscular synapses in Xenopus cell cultures. J Physiol. 1994;477:449–458. doi: 10.1113/jphysiol.1994.sp020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu WM, Huang FL. Potentiation by endogenously released ATP of spontaneous transmitter secretion at developing neuromuscular synapses in Xenopus cell cultures. Br J Pharmacol. 1994;111:880–886. doi: 10.1111/j.1476-5381.1994.tb14820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu B, Fu WM. Regulation of postsynaptic responses by calcitonin gene related peptide and ATP at developing neuromuscular junctions. Can J Physiol Pharmacol. 1995;73:1050–1056. doi: 10.1139/y95-149. [DOI] [PubMed] [Google Scholar]
- 16.Fu WM, Chen YH, Lee KF, Liou JC. Regulation of quantal transmitter secretion by ATP and protein kinases at developing neuromuscular synapses. Eur J Neurosci. 1997;9:676–685. doi: 10.1111/j.1460-9568.1997.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 17.Bogdanov YD, Dale L, King BF, Whittock N, Burnstock G. Early expression of a novel nucleotide receptor in the neural plate of Xenopus embryos. J Biol Chem. 1997;272:12583–12590. doi: 10.1074/jbc.272.19.12583. [DOI] [PubMed] [Google Scholar]
- 18.Burnstock G. P2 purinoceptors: historical perspective and classification. In: Chadwick DJ, Goode JA, editors. P2 purinoceptors: localization, function and transduction mechanisms. Ciba Foundation Symposium vol 198. Chichester: Wiley; 1996. pp. 1–29. [DOI] [PubMed] [Google Scholar]
- 19.Gerhart J, Danilchik M, Doniach T, Roberts S, Rowning B, Stewart R. Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development. 1989;107(Suppl):37–51. doi: 10.1242/dev.107.Supplement.37. [DOI] [PubMed] [Google Scholar]
- 20.Dale N, Gilday D. Regulation of rhythmic movements by purinergic neurotransmitters in frog embryos. Nature. 1996;383:259–263. doi: 10.1038/383259a0. [DOI] [PubMed] [Google Scholar]
- 21.Brown P, Dale N. Adenosine A1 receptors modulate high voltage-activated Ca2+ currents and motor pattern generation in the Xenopus embryo. J Physiol. 2000;525:655–667. doi: 10.1111/j.1469-7793.2000.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dale N. Delayed production of adenosine underlies temporal modulation of swimming in frog embryo. J Physiol. 1998;511:265–272. doi: 10.1111/j.1469-7793.1998.265bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devader C, Webb RJ, Thomas GM, Dale L. Xenopus apyrase (xapy), a secreted nucleotidase that is expressed during early development. Gene. 2006;367:135–141. doi: 10.1016/j.gene.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Laasberg T. Ca2+-mobilizing receptors of gastrulating chick embryo. Comp Biochem Physiol C. 1990;97:9–12. doi: 10.1016/0742-8413(90)90164-5. [DOI] [PubMed] [Google Scholar]
- 25.Hilfer SR, Palmatier BY, Fithian EM. Precocious evagination of the embryonic chick thyroid in ATP-containing medium. J Embryol Exp Morphol. 1977;42:163–175. [Google Scholar]
- 26.Nakaoka Y, Yamashita M. Ca2+ responses to acetylcholine and adenosine triphosphate in the otocyst of chick embryo. J Neurobiol. 1995;28:23–34. doi: 10.1002/neu.480280104. [DOI] [PubMed] [Google Scholar]
- 27.Häggblad J, Heilbronn E. P2-purinoceptor-stimulated phosphoinositide turnover in chick myotubes. Calcium mobilization and the role of guanyl nucleotide-binding proteins. FEBS Lett. 1988;235:133–136. doi: 10.1016/0014-5793(88)81248-1. [DOI] [PubMed] [Google Scholar]
- 28.Lohmann F, Drews U, Donié F, Reiser G. Chick embryo muscarinic and purinergic receptors activate cytosolic Ca2+ via phosphatidylinositol metabolism. Exp Cell Res. 1991;197:326–329. doi: 10.1016/0014-4827(91)90441-v. [DOI] [PubMed] [Google Scholar]
- 29.Meyer MP, Clarke JDW, Patel K, Townsend-Nicholson A, Burnstock G. Selective expression of purinoceptor cP2Y1 suggests a role for nucleotide signalling in development of the chick embryo. Dev Dyn. 1999;214:152–158. doi: 10.1002/(SICI)1097-0177(199902)214:2<152::AID-AJA5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Adair TH, Montani JP, Strick DM, Guyton AC. Vascular development in chick embryos: a possible role for adenosine. Am J Physiol. 1989;256:H240–H246. doi: 10.1152/ajpheart.1989.256.1.H240. [DOI] [PubMed] [Google Scholar]
- 31.Fraser RA, Ellis EM, Stalker AL. Experimental angiogenesis in the chorio-allantoic membrane. Bibl Anat. 1979;18:25–27. [PubMed] [Google Scholar]
- 32.Teuscher E, Weidlich V. Adenosine nucleotides, adenosine and adenine as angiogenesis factors. Biomed Biochim Acta. 1985;44:493–495. [PubMed] [Google Scholar]
- 33.Dusseau JW, Hutchins PM, Malbasa DS. Stimulation of angiogenesis by adenosine on the chick chorioallantoic membrane. Circ Res. 1986;59:163–170. doi: 10.1161/01.res.59.2.163. [DOI] [PubMed] [Google Scholar]
- 34.Kubo Y. Properties of ionic currents induced by external ATP in a mouse mesodermal stem cell line. J Physiol. 1991;442:691–710. doi: 10.1113/jphysiol.1991.sp018815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubo Y. Electrophysiological and immunohistochemical analysis of muscle differentiation in a mouse mesodermal stem cell line. J Physiol. 1991;442:711–741. doi: 10.1113/jphysiol.1991.sp018816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaudoin AR. Effect of adenosine triphosphate and adenosine diphosphate on the teratogenic action of Trypan blue in rats. Neonatology. 1976;28:133–139. [Google Scholar]
- 37.Gordon HW, Tkaczyk W, Peer LA, Bernhard WG. The effect of adenosine triphosphate and its decomposition products on cortisone induced teratology. J Embryol Exp Morphol. 1963;11:475–482. [PubMed] [Google Scholar]
- 38.Smuts MS. Rapid nasal pit formation in mouse embryos stimulated by ATP-containing medium. J Exp Zool. 1981;216:409–414. doi: 10.1002/jez.1402160309. [DOI] [PubMed] [Google Scholar]
- 39.Nakano H, Shimada A, Imai K, Takahashi T, Hashizume K. ATP-evoked increase in intracellular calcium via the P2Y receptor in proliferating bovine trophoblast cells. Cell Tissue Res. 2003;313:227–236. doi: 10.1007/s00441-003-0754-9. [DOI] [PubMed] [Google Scholar]
- 40.Petrungaro S, Salustri A, Siracusa G. Adenosine potentiates the delaying effect of dbcAMP on meiosis resumption in denuded mouse oocytes. Cell Biol Int Rep. 1986;10:993. doi: 10.1016/0309-1651(86)90121-9. [DOI] [PubMed] [Google Scholar]
- 41.Knudsen TB, Elmer WA. Evidence for negative control of growth by adenosine in the mammalian embryo: induction of Hmx/+ mutant limb outgrowth by adenosine deaminase. Differentiation. 1987;33:270–279. doi: 10.1111/j.1432-0436.1987.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 42.Nureddin A, Epsaro E, Kiessling AA. Purines inhibit the development of mouse embryos in vitro. J Reprod Fertil. 1990;90:455–464. doi: 10.1530/jrf.0.0900455. [DOI] [PubMed] [Google Scholar]
- 43.Loutradis D, John D, Kiessling AA. Hypoxanthine causes a 2-cell block in random-bred mouse embryos. Biol Reprod. 1987;37:311–316. doi: 10.1095/biolreprod37.2.311. [DOI] [PubMed] [Google Scholar]
- 44.Fissore R, O’Keefe S, Kiessling AA. Purine-induced block to mouse embryo cleavage is reversed by compounds that elevate cyclic adenosine monophosphate. Biol Reprod. 1992;47:1105–1112. doi: 10.1095/biolreprod47.6.1105. [DOI] [PubMed] [Google Scholar]
- 45.Chechik BE, Sengupta S, Hibi T, Fernandes B. Immunomorphological localization of adenosine deaminase in rat tissues during ontogeny. Histochem J. 1985;17:153–170. doi: 10.1007/BF01003215. [DOI] [PubMed] [Google Scholar]
- 46.Jenuth JP, Mably ER, Snyder FF. Modelling of purine nucleoside metabolism during mouse embryonic development: relative routes of adenosine, deoxyadenosine, and deoxyguanosine metabolism. Biochem Cell Biol. 1996;74:219–225. doi: 10.1139/o96-022. [DOI] [PubMed] [Google Scholar]
- 47.Franco R, Casado V, Ciruela F, Saura C, Mallol J, Canela EI, Lluis C. Cell surface adenosine deaminase: much more than an ectoenzyme. Prog Neurobiol. 1997;52:283–294. doi: 10.1016/s0301-0082(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 48.Schachter JB, Sromek SM, Nicholas RA, Harden TK. HEK293 human embryonic kidney cells endogenously express the P2Y1 and P2Y2 receptors. Neuropharmacology. 1997;36:1181–1187. doi: 10.1016/s0028-3908(97)00138-x. [DOI] [PubMed] [Google Scholar]
- 49.Cooper J, Hill SJ, Alexander SP. An endogenous A2B adenosine receptor coupled to cyclic AMP generation in human embryonic kidney (HEK 293) cells. Br J Pharmacol. 1997;122:546–550. doi: 10.1038/sj.bjp.0701401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neary JT, McCarthy M, Kang Y, Zuniga S. Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neurosci Lett. 1998;242:159–162. doi: 10.1016/s0304-3940(98)00067-6. [DOI] [PubMed] [Google Scholar]
- 51.Fukuda S, Katoh S, Yamamoto K, Hashimoto M, Kitao M. Correlation between levels of plasma adenosine triphosphate and stress to the fetus at delivery. Biol Neonate. 1990;57:150–154. doi: 10.1159/000243185. [DOI] [PubMed] [Google Scholar]
- 52.Bynum JW. Differential adenosine sensitivity in fibroblasts from different age donors. Exp Gerontol. 1980;15:217–225. doi: 10.1016/0531-5565(80)90027-3. [DOI] [PubMed] [Google Scholar]
- 53.Sobrevia L, Yudilevich DL, Mann GE. Activation of A2-purinoceptors by adenosine stimulates l-arginine transport (system y+) and nitric oxide synthesis in human fetal endothelial cells. J Physiol. 1997;499:135–140. doi: 10.1113/jphysiol.1997.sp021916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blair TA, Parenti M, Murray TF. Development of pharmacological sensitivity to adenosine analogs in embryonic chick heart: role of A1 adenosine receptors and adenylyl cyclase inhibition. Mol Pharmacol. 1989;35:661–670. [PubMed] [Google Scholar]
- 55.Hatae J, Sperelakis N, Wahler GM. Development of the response to adenosine during organ culture of young embryonic chick hearts. J Dev Physiol. 1989;11:342–345. [PubMed] [Google Scholar]
- 56.Shryock J, Patel A, Belardinelli L, Linden J. Downregulation and desensitization of A1-adenosine receptors in embryonic chicken heart. Am J Physiol. 1989;256:H321–H327. doi: 10.1152/ajpheart.1989.256.2.H321. [DOI] [PubMed] [Google Scholar]
- 57.Liang BT, Haltiwanger B. Adenosine A2a and A2b receptors in cultured fetal chick heart cells. High- and low-affinity coupling to stimulation of myocyte contractility and cAMP accumulation. Circ Res. 1995;76:242–251. doi: 10.1161/01.res.76.2.242. [DOI] [PubMed] [Google Scholar]
- 58.Egerman RS, Bissonnette JM, Hohimer AR. The effects of centrally administered adenosine on fetal sheep heart rate accelerations. Am J Obstet Gynecol. 1993;169:866–869. doi: 10.1016/0002-9378(93)90017-d. [DOI] [PubMed] [Google Scholar]
- 59.Rivkees SA. The ontogeny of cardiac and neural A1 adenosine receptor expression in rats. Brain Res Dev Brain Res. 1995;89:202–213. doi: 10.1016/0165-3806(95)00120-3. [DOI] [PubMed] [Google Scholar]
- 60.Hofman PL, Hiatt K, Yoder MC, Rivkees SA. A1 adenosine receptors potently regulate heart rate in mammalian embryos. Am J Physiol. 1997;273:R1374–R1380. doi: 10.1152/ajpregu.1997.273.4.R1374. [DOI] [PubMed] [Google Scholar]
- 61.Weber RG, Jones CR, Lohse MJ, Palacios JM. Autoradiographic visualization of A1 adenosine receptors in rat brain with [3H]8-cyclopentyl-1,3-dipropylxanthine. J Neurochem. 1990;54:1344–1353. doi: 10.1111/j.1471-4159.1990.tb01968.x. [DOI] [PubMed] [Google Scholar]
- 62.Reppert SM, Weaver DR, Stehle JH, Rivkees SA. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol Endocrinol. 1991;5:1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- 63.Cothran DL, Lloyd TR, Taylor H, Linden J, Matherne GP. Ontogeny of rat myocardial A1 adenosine receptors. Biol Neonate. 1995;68:111–118. doi: 10.1159/000244226. [DOI] [PubMed] [Google Scholar]
- 64.Yoneyama Y, Power GG. Plasma adenosine and cardiovascular responses to dipyridamole in fetal sheep. J Dev Physiol. 1992;18:203–209. [PubMed] [Google Scholar]
- 65.Konduri GG, Woodard LL, Mukhopadhyay A, Deshmukh DR. Adenosine is a pulmonary vasodilator in newborn lambs. Am Rev Respir Dis. 1992;146:670–676. doi: 10.1164/ajrccm/146.3.670. [DOI] [PubMed] [Google Scholar]
- 66.Koos BJ, Mason BA, Ducsay CA. Cardiovascular responses to adenosine in fetal sheep: autonomic blockade. Am J Physiol. 1993;264:H526–H532. doi: 10.1152/ajpheart.1993.264.2.H526. [DOI] [PubMed] [Google Scholar]
- 67.Mason BA, Ogunyemi D, Punla O, Koos BJ. Maternal and fetal cardiorespiratory responses to adenosine in sheep. Am J Obstet Gynecol. 1993;168:1558–1561. doi: 10.1016/s0002-9378(11)90798-4. [DOI] [PubMed] [Google Scholar]
- 68.Bogdanov YD, Wildman SS, Clements MP, King BF, Burnstock G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Special report. Br J Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruppelt A, Liang BT, Soto F. Cloning, functional characterization and developmental expression of a P2X receptor from chick embryo. Prog Brain Res. 1999;120:81–90. doi: 10.1016/s0079-6123(08)63547-5. [DOI] [PubMed] [Google Scholar]
- 70.Kolb HA, Wakelam MJ. Transmitter-like action of ATP on patched membranes of cultured myoblasts and myotubes. Nature. 1983;303:621–623. doi: 10.1038/303621a0. [DOI] [PubMed] [Google Scholar]
- 71.Häggblad J, Eriksson H, Heilbronn E. ATP-induced cation influx in myotubes is additive to cholinergic agonist action. Acta Physiol Scand. 1985;125:389–393. doi: 10.1111/j.1748-1716.1985.tb07734.x. [DOI] [PubMed] [Google Scholar]
- 72.Häggblad J, Heilbronn E. Externally applied adenosine-5′-triphosphate causes inositol triphosphate accumulation in cultured chick myotubes. Neurosci Lett. 1987;74:199–204. doi: 10.1016/0304-3940(87)90149-2. [DOI] [PubMed] [Google Scholar]
- 73.Eriksson H, Heilbronn E. Extracellularly applied ATP alters the calcium flux through dihydropyridine-sensitive channels in cultured chick myotubes. Biochem Biophys Res Commun. 1989;159:878–885. doi: 10.1016/0006-291x(89)92190-6. [DOI] [PubMed] [Google Scholar]
- 74.Hume RI, Hönig MG. Excitatory action of ATP on embryonic chick muscle. J Neurosci. 1986;6:681–690. doi: 10.1523/JNEUROSCI.06-03-00681.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hume RI, Thomas SA. Multiple actions of adenosine 5′-triphosphate on chick skeletal muscle. J Physiol. 1988;406:503–524. doi: 10.1113/jphysiol.1988.sp017393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas SA, Hume RI. Permeation of both cations and anions through a single class of ATP-activated ion channels in developing chick skeletal muscle. J Gen Physiol. 1990;95:569–590. doi: 10.1085/jgp.95.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomas SA, Hume RI. Irreversible desensitization of ATP responses in developing chick skeletal muscle. J Physiol. 1990;430:373–388. doi: 10.1113/jphysiol.1990.sp018296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomas SA, Zawisa MJ, Lin X, Hume RI. A receptor that is highly specific for extracellular ATP in developing chick skeletal muscle in vitro. Br J Pharmacol. 1991;103:1963–1969. doi: 10.1111/j.1476-5381.1991.tb12360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wells DG, Zawisa MJ, Hume RI. Changes in responsiveness to extracellular ATP in chick skeletal muscle during development and upon denervation. Dev Biol. 1995;172:585–590. doi: 10.1006/dbio.1995.8062. [DOI] [PubMed] [Google Scholar]
- 80.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 81.Mehul B, Doyennette-Moyne MA, Aubery M, Codogno P, Mannherz HG. Enzymatic activity and in vivo distribution of 5′-nucleotidase, an extracellular matrix binding glycoprotein, during the development of chicken striated muscle. Exp Cell Res. 1992;203:62–71. doi: 10.1016/0014-4827(92)90040-f. [DOI] [PubMed] [Google Scholar]
- 82.Soto F, Krause U, Borchardt K, Ruppelt A. Cloning, tissue distribution and functional characterization of the chicken P2X1 receptor. FEBS Lett. 2003;533:54–58. doi: 10.1016/s0014-5793(02)03751-1. [DOI] [PubMed] [Google Scholar]
- 83.Ruppelt A, Ma W, Borchardt K, Silberberg SD, Soto F. Genomic structure, developmental distribution and functional properties of the chicken P2X5 receptor. J Neurochem. 2001;77:1256–1265. doi: 10.1046/j.1471-4159.2001.00348.x. [DOI] [PubMed] [Google Scholar]
- 84.Henning RH, Nelemans A, Van den Akker J, Den Hertog A. The nucleotide receptors on mouse C2C12 myotubes. Br J Pharmacol. 1992;106:853–858. doi: 10.1111/j.1476-5381.1992.tb14424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henning RH, Duin M, Den Hertog A, Nelemans A. Activation of the phospholipase C pathway by ATP is mediated exclusively through nucleotide type P2-purinoceptors in C2C12 myotubes. Br J Pharmacol. 1993;110:747–752. doi: 10.1111/j.1476-5381.1993.tb13875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henning RH, Duin M, Den Hertog A, Nelemans A. Characterization of P2-purinoceptor mediated cyclic AMP formation in mouse C2C12 myotubes. Br J Pharmacol. 1993;110:133–138. doi: 10.1111/j.1476-5381.1993.tb13782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henning RH, Duin M, van Popta JP, Nelemans A, Den Hertog A. Different mechanisms of Ca2+-handling following nicotinic acetylcholine receptor stimulation, P2U-purinoceptor stimulation and K+-induced depolarization in C2C12 myotubes. Br J Pharmacol. 1996;117:1785–1791. doi: 10.1111/j.1476-5381.1996.tb15355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tassin AM, Haggblad J, Heilbronn E. Receptor-triggered polyphosphoinositide turnover produces less cytosolic free calcium in cultured dysgenic myotubes than in normal myotubes. Muscle Nerve. 1990;13:142–145. doi: 10.1002/mus.880130210. [DOI] [PubMed] [Google Scholar]
- 89.Collet C, Strube C, Csernoch L, Mallouk N, Ojeda C, Allard B, Jacquemond V. Effects of extracellular ATP on freshly isolated mouse skeletal muscle cells during pre-natal and post-natal development. Pflugers Arch. 2002;443:771–778. doi: 10.1007/s00424-001-0758-9. [DOI] [PubMed] [Google Scholar]
- 90.May C, Weigl L, Karel A, Hohenegger M. Extracellular ATP activates ERK1/ERK2 via a metabotropic P2Y1 receptor in a Ca2+ independent manner in differentiated human skeletal muscle cells. Biochem Pharmacol. 2006;71:1497–1509. doi: 10.1016/j.bcp.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Ryten M, Hoebertz A, Burnstock G. Sequential expression of three receptor subtypes for extracellular ATP in developing rat skeletal muscle. Dev Dyn. 2001;221:331–341. doi: 10.1002/dvdy.1147. [DOI] [PubMed] [Google Scholar]
- 92.Ryten M, Dunn PM, Neary JT, Burnstock G. ATP regulates the differentiation of mammalian skeletal muscle by activation of a P2X5 receptor on satellite cells. J Cell Biol. 2002;158:345–355. doi: 10.1083/jcb.200202025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ryten M, Koshi R, Knight GE, Turmaine M, Dunn PM, Cockayne DA, Ford APDW, Burnstock G. Abnormalities in neuromuscular junction structure and skeletal muscle function in mice lacking the P2X2 nucleotide receptor. Neuroscience. 2007;148:700–711. doi: 10.1016/j.neuroscience.2007.06.050. [DOI] [PubMed] [Google Scholar]
- 94.Choi HB, Hong SH, Ryu JK, Kim SU, McLarnon JG. Differential activation of subtype purinergic receptors modulates Ca2+ mobilization and COX-2 in human microglia. Glia. 2003;43:95–103. doi: 10.1002/glia.10239. [DOI] [PubMed] [Google Scholar]
- 95.Ling KK, Siow NL, Choi RC, Tsim KW. ATP potentiates the formation of AChR aggregate in the co-culture of NG108–15 cells with C2C12 myotubes. FEBS Lett. 2005;579:2469–2474. doi: 10.1016/j.febslet.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 96.Morgan PF, Montgomery P, Marangos PJ. Ontogenetic profile of the adenosine uptake sites in rat forebrain. J Neurochem. 1987;49:852–855. doi: 10.1111/j.1471-4159.1987.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 97.Morgan PF, Deckert J, Nakajima T, Daval JL, Marangos PJ. Late ontogenetic development of adenosine A1 receptor coupling to associated G-proteins in guinea pig cerebellum but not forebrain. Mol Cell Biochem. 1990;92:169–176. doi: 10.1007/BF00218134. [DOI] [PubMed] [Google Scholar]
- 98.Nicolas F, Daval JL. Expression of adenosine A1 receptors in cultured neurons from fetal rat brain. Synapse. 1993;14:96–99. doi: 10.1002/syn.890140113. [DOI] [PubMed] [Google Scholar]
- 99.Marangos PJ, Patel J, Stivers J. Ontogeny of adenosine binding sites in rat forebrain and cerebellum. J Neurochem. 1982;39:267–270. doi: 10.1111/j.1471-4159.1982.tb04732.x. [DOI] [PubMed] [Google Scholar]
- 100.Geiger JD, LaBella FS, Nagy JI. Ontogenesis of adenosine receptors in the central nervous system of the rat. Brain Res. 1984;13:97–104. doi: 10.1016/0165-3806(84)90080-4. [DOI] [PubMed] [Google Scholar]
- 101.Weaver DR. A1-adenosine receptor gene expression in fetal rat brain. Brain Res Dev Brain Res. 1996;94:205–223. [PubMed] [Google Scholar]
- 102.Deckert J, Morgan PF, Daval JL, Nakajima T, Marangos PJ. Ontogeny of adenosine uptake sites in guinea pig brain: differential profile of [3H]nitrobenzylthioinosine and [3H]dipyridamole binding sites. Brain Res. 1988;470:313–316. doi: 10.1016/0165-3806(88)90251-9. [DOI] [PubMed] [Google Scholar]
- 103.León D, Albasanz JL, Ruiz MA, Fernandez M, Martin M. Adenosine A1 receptor down-regulation in mothers and fetal brain after caffeine and theophylline treatments to pregnant rats. J Neurochem. 2002;82:625–634. doi: 10.1046/j.1471-4159.2002.01008.x. [DOI] [PubMed] [Google Scholar]
- 104.Reynolds JD, Brien JF. The role of adenosine A1 receptor activation in ethanol-induced inhibition of stimulated glutamate release in the hippocampus of the fetal and adult guinea pig. Alcohol. 1995;12:151–157. doi: 10.1016/0741-8329(94)00078-6. [DOI] [PubMed] [Google Scholar]
- 105.Schoen SW, Leutenecker B, Kreutzberg GW, Singer W. Ocular dominance plasticity and developmental changes of 5′-nucleotidase distributions in the kitten visual cortex. J Comp Neurol. 1990;296:379–392. doi: 10.1002/cne.902960304. [DOI] [PubMed] [Google Scholar]
- 106.Schoen SW, Kreutzberg GW, Singer W. Cytochemical redistribution of 5′-nucleotidase in the developing cat visual cortex. Eur J Neurosci. 1993;5:210–222. doi: 10.1111/j.1460-9568.1993.tb00487.x. [DOI] [PubMed] [Google Scholar]
- 107.Mishra OP, Wagerle LC, Delivoria Papadopoulos M. 5’-Nucleotidase and adenosine deaminase in developing fetal guinea pig brain and the effect of maternal hypoxia. Neurochem Res. 1988;13:1055–1060. doi: 10.1007/BF00973150. [DOI] [PubMed] [Google Scholar]
- 108.Geiger JD, Nagy JI. Ontogenesis of adenosine deaminase activity in rat brain. J Neurochem. 1987;48:147–153. doi: 10.1111/j.1471-4159.1987.tb13139.x. [DOI] [PubMed] [Google Scholar]
- 109.Senba E, Daddona PE, Nagy JI. Transient expression of adenosine deaminase in facial and hypoglossal motoneurons of the rat during development. J Comp Neurol. 1987;255:217–230. doi: 10.1002/cne.902550206. [DOI] [PubMed] [Google Scholar]
- 110.Senba E, Daddona PE, Nagy JI. Development of adenosine deaminase-immunoreactive neurons in the rat brain. Brain Res. 1987;428:59–71. doi: 10.1016/0165-3806(87)90083-6. [DOI] [PubMed] [Google Scholar]
- 111.Senba E, Daddona PE, Nagy JI. Adenosine deaminase-containing neurons in the olfactory system of the rat during development. Brain Res Bull. 1987;18:635–648. doi: 10.1016/0361-9230(87)90133-x. [DOI] [PubMed] [Google Scholar]
- 112.Yoshioka T, Inoata K, Tanaka O. Cytochemistry of Ca2+-ATPase in the rat spinal cord during embryonic development. Acta Histochem Cytochem. 1987;20:511–526. [Google Scholar]
- 113.Yoshioka T. Histochemical examination of adenosine nucleotidases in the developing rat spinal cord: possible involvement in enzymatic chain of ATP degradation. Acta Histochem Cytochem. 1989;22:685–694. [Google Scholar]
- 114.Salter MW, Hicks JL. ATP causes release of intracellular Ca2+ via the phospholipase Cβ/IP3 pathway in astrocytes from the dorsal spinal cord. J Neurosci. 1995;15:2961–2971. doi: 10.1523/JNEUROSCI.15-04-02961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kidd EJ, Miller KJ, Sansum AJ, Humphrey PPA. Evidence for P2X3 receptors in the developing rat brain. Neuroscience. 1998;87:533–539. doi: 10.1016/s0306-4522(98)00294-2. [DOI] [PubMed] [Google Scholar]
- 116.Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 117.Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1β transiently enhances P2X7 receptor expression and function in human astrocytes. Glia. 2005;49:245–258. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khakh BS, Smith WB, Chiu CS, Ju D, Davidson N, Lester HA. Activation-dependent changes in receptor distribution and dendritic morphology in hippocampal neurons expressing P2X2-green fluorescent protein receptors. Proc Natl Acad Sci USA. 2001;98:5288–5293. doi: 10.1073/pnas.081089198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.García-Lecea M, Sen RP, Soto F, Miras-Portugal MT, Castro E. P2 receptors in cerebellar neurons: molecular diversity of ionotropic ATP receptors in Purkinje cells. Drug Dev Res. 2001;52:104–113. [Google Scholar]
- 120.Maric D, Maric I, Chang YH, Barker JL. Stereotypical physiological properties emerge during early neuronal and glial lineage development in the embryonic rat neocortex. Cereb Cortex. 2000;10:729–747. doi: 10.1093/cercor/10.8.729. [DOI] [PubMed] [Google Scholar]
- 121.Cheung K-K, Burnstock G. Localisation of P2X3 and co-expression with P2X2 receptors during rat embryonic neurogenesis. J Comp Neurol. 2002;443:368–382. doi: 10.1002/cne.10123. [DOI] [PubMed] [Google Scholar]
- 122.Cheung K-K, Ryten M, Burnstock G. Abundant and dynamic expression of G protein-coupled P2Y receptors in mammalian development. Dev Dyn. 2003;228:254–266. doi: 10.1002/dvdy.10378. [DOI] [PubMed] [Google Scholar]
- 123.Zhu Y, Kimelberg HK. Developmental expression of metabotropic P2Y1 and P2Y2 receptors in freshly isolated astrocytes from rat hippocampus. J Neurochem. 2001;77:530–541. doi: 10.1046/j.1471-4159.2001.00241.x. [DOI] [PubMed] [Google Scholar]
- 124.Cheung K-K, Chan WY, Burnstock G. Expression of P2X receptors during rat brain development and their inhibitory role on motor axon outgrowth in neural tube explant cultures. Neuroscience. 2005;133:937–945. doi: 10.1016/j.neuroscience.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 125.Boldogköi Z, Schütz B, Sallach J, Zimmer A. P2X3 receptor expression at early stage of mouse embryogenesis. Mech Dev. 2002;118:255–260. doi: 10.1016/s0925-4773(02)00280-0. [DOI] [PubMed] [Google Scholar]
- 126.Jo YH, Role LW. Cholinergic modulation of purinergic and GABAergic co-transmission at in vitro hypothalamic synapses. J Neurophysiol. 2002;88:2501–2508. doi: 10.1152/jn.00352.2002. [DOI] [PubMed] [Google Scholar]
- 127.Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 128.Ryu JK, Choi HB, Hatori K, Heisel RL, Pelech SL, McLarnon JG, Kim SU. Adenosine triphosphate induces proliferation of human neural stem cells: role of calcium and p70 ribosomal protein S6 kinase. J Neurosci Res. 2003;72:352–362. doi: 10.1002/jnr.10507. [DOI] [PubMed] [Google Scholar]
- 129.Dale N. Resetting intrinsic purinergic modulation of neural activity: an associative mechanism? J Neurosci. 2002;22:10461–10469. doi: 10.1523/JNEUROSCI.22-23-10461.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Norton WHJ, Rohr KB, Burnstock G. Embryonic expression of a P2X3 receptor encoding gene in zebrafish. Mech Dev. 2000;99:149–152. doi: 10.1016/s0925-4773(00)00472-x. [DOI] [PubMed] [Google Scholar]
- 131.Meghji P, Tuttle JB, Rubio R. Adenosine formation and release by embryonic chick neurons and glia in cell culture. J Neurochem. 1989;53:1852–1860. doi: 10.1111/j.1471-4159.1989.tb09252.x. [DOI] [PubMed] [Google Scholar]
- 132.Thampy KG, Barnes EM., Jr Adenosine transport by cultured glial cells from chick embryo brain. Arch Biochem Biophys. 1983;220:340–346. doi: 10.1016/0003-9861(83)90422-8. [DOI] [PubMed] [Google Scholar]
- 133.Thampy KG, Barnes EM., Jr Adenosine transport by primary cultures of neurons from chick embryo brain. J Neurochem. 1983;40:874–879. doi: 10.1111/j.1471-4159.1983.tb08061.x. [DOI] [PubMed] [Google Scholar]
- 134.Barnes EM, Jr, Thampy KG. Subclasses of adenosine receptors in brain membranes from adult tissue and from primary cultures of chick embryo. J Neurochem. 1982;39:647–652. doi: 10.1111/j.1471-4159.1982.tb07941.x. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Z, Galileo DS. Widespread programme death in early developing chick optic tectum. Neuroreport. 1998;9:2797–2801. doi: 10.1097/00001756-199808240-00021. [DOI] [PubMed] [Google Scholar]
- 136.Di Virgilio F, Zanovello P, Zambon A, Bronte V, Pizzo P, Murgia M. Cell membrane receptors for extracelluar ATP: a new family of apoptosis-signalling molecules. Fundam Clin Immunol. 1995;3:80–81. [Google Scholar]
- 137.Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, Baricordi OR. Cytolytic P2X purinoceptors. Cell Death Differ. 1998;5:191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- 138.Wakade TD, Palmer KC, McCauley R, Przywara DA, Wakade AR. Adenosine-induced apoptosis in chick embryonic sympathetic neurons: a new physiological role for adenosine. J Physiol. 1995;488:123–138. doi: 10.1113/jphysiol.1995.sp020951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Abe Y, Sorimachi M, Itoyama Y, Furukawa K, Akaike N. ATP responses in the embryo chick ciliary ganglion cells. Neuroscience. 1995;64:547–551. doi: 10.1016/0306-4522(94)00415-2. [DOI] [PubMed] [Google Scholar]
- 140.Labrakakis C, Gerstner E, MacDermott AB. Adenosine triphosphate-evoked currents in cultured dorsal root ganglion neurons obtained from rat embryos: desensitization kinetics and modulation of glutamate release. Neuroscience. 2000;101:1117–1126. doi: 10.1016/s0306-4522(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 141.Kulkarni JS, Przywara DA, Wakade TD. Adenosine induces apoptosis by inhibiting mRNA and protein synthesis in chick embryonic sympathetic neurons. Neurosci Lett. 1998;248:187–190. doi: 10.1016/s0304-3940(98)00369-3. [DOI] [PubMed] [Google Scholar]
- 142.Nakatsuka T, Mena N, Ling J, Gu JG. Depletion of substance P from rat primary sensory neurons by ATP, an implication of P2X receptor-mediated release of substance P. Neuroscience. 2001;107:293–300. doi: 10.1016/s0306-4522(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 143.Ruan H-Z, Moules E, Burnstock G. Changes in P2X purinoceptors in sensory ganglia of the mouse during embryonic and postnatal development. Histochem Cell Biol. 2004;122:539–551. doi: 10.1007/s00418-004-0714-9. [DOI] [PubMed] [Google Scholar]
- 144.Huang H, Wu X, Nicol GD, Meller S, Vasko MR. ATP augments peptide release from rat sensory neurons in culture through activation of P2Y receptors. J Pharmacol Exp Ther. 2003;306:1137–1144. doi: 10.1124/jpet.103.052951. [DOI] [PubMed] [Google Scholar]
- 145.Dunn PM, Gever J, Ruan H-Z, Burnstock G. Developmental changes in heteromeric P2X2/3 receptor expression in rat sympathetic ganglion neurons. Dev Dyn. 2005;234:505–511. doi: 10.1002/dvdy.20466. [DOI] [PubMed] [Google Scholar]
- 146.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 147.Allgaier C, Wellmann H, Schobert A, von Kügelgen I. Cultured chick sympathetic neurons: modulation of electrically evoked noradrenaline release by P2-purinoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:17–24. doi: 10.1007/BF00169185. [DOI] [PubMed] [Google Scholar]
- 148.Allgaier C, Wellmann H, Schobert A, Kurz G, von Kügelgen I. Cultured chick sympathetic neurons: ATP-induced noradrenaline release and its blockade by nicotinic receptor antagonists. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:25–30. doi: 10.1007/BF00169186. [DOI] [PubMed] [Google Scholar]
- 149.Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- 150.Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol. 2004;560:533–549. doi: 10.1113/jphysiol.2004.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sugioka M, Fukuda Y, Yamashita M. Ca2+ responses to ATP via purinoceptors in the early embryonic chick retina. J Physiol. 1996;493:855–863. doi: 10.1113/jphysiol.1996.sp021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sakai Y, Fukuda Y, Yamashita M. Muscarinic and purinergic Ca2+ mobilisation in the neural retina of early embryonic chick. Int J Dev Neurosci. 1996;14:691–699. doi: 10.1016/s0736-5748(96)00074-3. [DOI] [PubMed] [Google Scholar]
- 153.Sugioka M, Zhou WL, Hofmann HD, Yamashita M. Involvement of P2 purinoceptors in the regulation of DNA synthesis in the neural retina of chick embryo. Int J Dev Neurosci. 1999;17:135–144. doi: 10.1016/s0736-5748(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 154.Yamashita M, Sugioka M. Calcium mobilization systems during neurogenesis. News Physiol Sci. 1998;13:75–79. doi: 10.1152/physiologyonline.1998.13.2.75. [DOI] [PubMed] [Google Scholar]
- 155.Sanches G, de Alencar LS, Ventura AL. ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated kinase cascade. Int J Dev Neurosci. 2002;20:21–27. doi: 10.1016/s0736-5748(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 156.Burgos JS, Barat A, Ramirez G. Guanine nucleotides block agonist-driven 45Ca2+ influx in chick embryo retinal explants. Neuroreport. 2000;11:2303–2305. doi: 10.1097/00001756-200007140-00047. [DOI] [PubMed] [Google Scholar]
- 157.Paes de Carvalho R, De Mello FG. Adenosine-elicited accumulation of adenosine 3′, 5′-cyclic monophosphate in the chick embryo retina. J Neurochem. 1982;38:493–500. doi: 10.1111/j.1471-4159.1982.tb08655.x. [DOI] [PubMed] [Google Scholar]
- 158.Paes de Carvalho R, De Mello FG. Expression of A1 adenosine receptors modulating dopamine-dependent cyclic AMP accumulation in the chick embryo retina. J Neurochem. 1985;44:845–851. doi: 10.1111/j.1471-4159.1985.tb12892.x. [DOI] [PubMed] [Google Scholar]
- 159.de Mello MC, Ventura AL, Paes de Carvalho R, Klein WL, De Mello FG. Regulation of dopamine- and adenosine-dependent adenylate cyclase systems of chicken embryo retina cells in culture. Proc Natl Acad Sci USA. 1982;79:5708–5712. doi: 10.1073/pnas.79.18.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Paes de Carvalho R, Braas KM, Alder R, Snyder SH. Developmental regulation of adenosine A1 receptors, uptake sites and endogenous adenosine in the chick retina. Brain Res Dev Brain Res. 1992;70:87–95. doi: 10.1016/0165-3806(92)90106-7. [DOI] [PubMed] [Google Scholar]
- 161.Massé K, Bhamra S, Eason R, Dale N, Jones EA. Purine-mediated signalling triggers eye development. Nature. 2007;449:1058–1062. doi: 10.1038/nature06189. [DOI] [PubMed] [Google Scholar]
- 162.Ito S, Ohta T, Kimura A, Ohga A. Development of substance P- and vasoactive intestinal polypeptide-containing neurones in the rat stomach. Q J Exp Physiol. 1988;73:729–736. doi: 10.1113/expphysiol.1988.sp003192. [DOI] [PubMed] [Google Scholar]
- 163.Gershon MD, Thompson EB. The maturation of neuromuscular function in a multiply innervated structure: development of the longitudinal smooth muscle of the foetal mammalian gut and its cholinergic excitatory, adrenergic inhibitory, and non-adrenergic inhibitory innervation. J Physiol. 1973;234:257–277. doi: 10.1113/jphysiol.1973.sp010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Crowe R, Burnstock G. Perinatal development of quinacrine-positive neurons in the rabbit gastrointestinal tract. J Auton Nerv Syst. 1981;4:217–230. doi: 10.1016/0165-1838(81)90046-1. [DOI] [PubMed] [Google Scholar]
- 165.Miyazaki H, Ohga A, Saito K. Development of motor response to intramural nerve stimulation and to drugs in rat small intestine. Br J Pharmacol. 1982;76:531–540. doi: 10.1111/j.1476-5381.1982.tb09251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zagorodnyuk V, Hoyle CHV, Burnstock G. An electrophysiological study of developmental changes in the innervation of the guinea-pig taenia coli. Pflugers Arch. 1993;423:427–433. doi: 10.1007/BF00374937. [DOI] [PubMed] [Google Scholar]
- 167.Clunes MT, Collett A, Baines DL, Bovell DL, Murphie H, Inglis SK, McAlroy HL, Olver RE, Wilson SM. Culture substrate-specific expression of P2Y2 receptors in distal lung epithelial cells isolated from foetal rats. Br J Pharmacol. 1998;124:845–847. doi: 10.1038/sj.bjp.0701942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barker PM, Gatzy JT. Effects of adenosine, ATP, and UTP on chloride secretion by epithelia explanted from fetal rat lung. Pediatr Res. 1998;43:652–659. doi: 10.1203/00006450-199805000-00014. [DOI] [PubMed] [Google Scholar]
- 169.Konduri GG, Gervasio CT, Theodorou AA. Role of adenosine triphosphate and adenosine in oxygen-induced pulmonary vasodilation in fetal lambs. Pediatr Res. 1993;33:533–539. doi: 10.1203/00006450-199305000-00022. [DOI] [PubMed] [Google Scholar]
- 170.Konduri GG, Mital S, Gervasio CT, Rotta AT, Forman K. Purine nucleotides contribute to pulmonary vasodilation caused by birth-related stimuli in the ovine fetus. Am J Physiol. 1997;272:H2377–H2384. doi: 10.1152/ajpheart.1997.272.5.H2377. [DOI] [PubMed] [Google Scholar]
- 171.Konduri GG, Forman K, Mital S. Characterization of purine receptors in fetal lamb pulmonary circulation. Pediatr Res. 2000;47:114–120. doi: 10.1203/00006450-200001000-00020. [DOI] [PubMed] [Google Scholar]
- 172.Brouns I, Van Genechten J, Hayashi H, Gajda M, Gomi T, Burnstock G, Timmermans J-P, Adriaensen D. Dual sensory innervation of pulmonary neuroepithelial bodies. Am J Respir Cell Mol Biol. 2003;28:275–285. doi: 10.1165/rcmb.2002-0117OC. [DOI] [PubMed] [Google Scholar]
- 173.O’Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, Rymer JM, McMahon SB. A quantitative analysis of purinoceptor expression in human fetal and adult bladders. J Urol. 2001;165:1730–1734. [PubMed] [Google Scholar]
- 174.Thiruchelvam N, Wu C, David A, Woolf AS, Cuckow PM, Fry CH. Neurotransmission and viscoelasticity in the ovine fetal bladder after in utero bladder outflow obstruction. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1296–R1305. doi: 10.1152/ajpregu.00688.2002. [DOI] [PubMed] [Google Scholar]
- 175.Housley GD, Marcotti W, Navaratnam D, Yamoah EN. Hair cells: beyond the transducer. J Membr Biol. 2006;209:89–118. doi: 10.1007/s00232-005-0835-7. [DOI] [PubMed] [Google Scholar]
- 176.Nikolic P, Housley GD, Luo L, Ryan AF, Thorne PR. Transient expression of P2X1 receptor subunits of ATP-gated ion channels in the developing rat cochlea. Brain Res Dev Brain Res. 2001;126:173–182. doi: 10.1016/s0165-3806(00)00149-8. [DOI] [PubMed] [Google Scholar]
- 177.Huang LC, Greenwood D, Thorne PR, Housley GD. Developmental regulation of neuron-specific P2X3 receptor expression in the rat cochlea. J Comp Neurol. 2005;484:133–143. doi: 10.1002/cne.20442. [DOI] [PubMed] [Google Scholar]
- 178.Huang LC, Ryan AF, Cockayne DA, Housley GD. Developmentally regulated expression of the P2X3 receptor in the mouse cochlea. Histochem Cell Biol. 2006;125:681–692. doi: 10.1007/s00418-005-0119-4. [DOI] [PubMed] [Google Scholar]
- 179.Hatori M, Teixeira CC, Debolt K, Pacifici M, Shapiro IM. Adenine nucleotide metabolism by chondrocytes in vitro: role of ATP in chondrocyte maturation and matrix mineralization. J Cell Physiol. 1995;165:468–474. doi: 10.1002/jcp.1041650304. [DOI] [PubMed] [Google Scholar]
- 180.Hung CT, Allen FD, Mansfield KD, Shapiro IM. Extracellular ATP modulates [Ca2+]i in retinoic acid-treated embryonic chondrocytes. Am J Physiol. 1997;272:C1611–C1617. doi: 10.1152/ajpcell.1997.272.5.C1611. [DOI] [PubMed] [Google Scholar]
- 181.Fredholm BB, Lerner U. Metabolism of adenosine and 2′-deoxy-adenosine by fetal mouse calvaria in culture. Med Biol. 1982;60:267–271. [PubMed] [Google Scholar]
- 182.Modderman WE, Weidema AF, Vrijheid-Lammers T, Wassenaar AM, Nijweide PJ. Permeabilization of cells of hemopoietic origin by extracellular ATP4−: elimination of osteoclasts, macrophages, and their precursors from isolated bone cell populations and fetal bone rudiments. Calcif Tissue Int. 1994;55:141–150. doi: 10.1007/BF00297190. [DOI] [PubMed] [Google Scholar]
- 183.Hsu HH. Purification and partial characterization of ATP pyrophosphohydrolase from fetal bovine epiphyseal cartilage. J Biol Chem. 1983;258:3463–3468. [PubMed] [Google Scholar]
- 184.Pác L. Contribution to ontogenesis of Merkel cells. Z Mikrosk Anat Forsch. 1984;98:36–48. [PubMed] [Google Scholar]
- 185.Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 186.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 187.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Majumder P, Trujillo CA, Lopes CG, Resende RR, Gomes KN, Yuahasi KK, Britto LR, Ulrich H. New insights into purinergic receptor signaling in neuronal differentiation, neuroprotection, and brain disorders. Purinergic Signal. 2007;3:317–331. doi: 10.1007/s11302-007-9074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McBurney MW. P19 embryonal carcinoma cells. Int J Dev Biol. 1993;37:135–140. [PubMed] [Google Scholar]
- 190.Resende RR, Majumder P, Gomes KN, Britto LR, Ulrich H. P19 embryonal carcinoma cells as in vitro model for studying purinergic receptor expression and modulation of N-methyl-d-aspartate-glutamate and acetylcholine receptors during neuronal differentiation. Neuroscience. 2007;146:1169–1181. doi: 10.1016/j.neuroscience.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 191.Resende RR, Britto LR, Ulrich H. Pharmacological properties of purinergic receptors and their effects on proliferation and induction of neuronal differentiation of P19 embryonal carcinoma cells. Int J Dev Neurosci. 2008;26:763–777. doi: 10.1016/j.ijdevneu.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 192.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 193.Martins AH, Alves JM, Trujillo CA, Schwindt TT, Barnabé GF, Motta FL, Guimarães AO, Casarini DE, Mello LE, Pesquero JB, Ulrich H. Kinin-B2 receptor expression and activity during differentiation of embryonic rat neurospheres. Cytometry A. 2008;73:361–368. doi: 10.1002/cyto.a.20519. [DOI] [PubMed] [Google Scholar]
- 194.Trujillo CA, Schwindt TT, Martins AH, Alves JM, Mello LE, Ulrich H. Novel perspectives of neural stem cell differentiation: from neurotransmitters to therapeutics. Cytometry A. 2009;75:38–53. doi: 10.1002/cyto.a.20666. [DOI] [PubMed] [Google Scholar]
- 195.Schwindt TT, Trujillo CA, Negraes PD, Lameu C, Ulrich H (2010) Directed differentiation of neural progenitors into neurons is accompanied by altered expression of P2X purinergic receptors. J Mol Neurosci [Epub ahead of print, July 9] [DOI] [PubMed]
- 196.da Silva RL, Resende RR, Ulrich H. Alternative splicing of P2X6 receptors in developing mouse brain and during in vitro neuronal differentiation. Exp Physiol. 2007;92:139–145. doi: 10.1113/expphysiol.2006.921304. [DOI] [PubMed] [Google Scholar]
- 197.Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisén J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 198.Kanemura Y, Mori H, Nakagawa A, Islam MO, Kodama E, Yamamoto A, Shofuda T, Kobayashi S, Miyake J, Yamazaki T, Hirano S, Yamasaki M, Okano H. In vitro screening of exogenous factors for human neural stem/progenitor cell proliferation using measurement of total ATP content in viable cells. Cell Transplant. 2005;14:673–682. [PubMed] [Google Scholar]
- 199.D’Ambrosi N, Murra B, Cavaliere F, Amadio S, Bernardi G, Burnstock G, Volonté C. Interaction between ATP and nerve growth factor signalling in the survival and neuritic outgrowth from PC12 cells. Neuroscience. 2001;108:527–534. doi: 10.1016/s0306-4522(01)00431-6. [DOI] [PubMed] [Google Scholar]
- 200.Jia C, Doherty JP, Crudgington S, Hegg CC. Activation of purinergic receptors induces proliferation and neuronal differentiation in Swiss Webster mouse olfactory epithelium. Neuroscience. 2009;163:120–128. doi: 10.1016/j.neuroscience.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jia C, Hegg CC. NPY mediates ATP-induced neuroproliferation in adult mouse olfactory epithelium. Neurobiol Dis. 2010;38:405–413. doi: 10.1016/j.nbd.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hogg RC, Chipperfield H, Whyte KA, Stafford MR, Hansen MA, Cool SM, Nurcombe V, Adams DJ. Functional maturation of isolated neural progenitor cells from the adult rat hippocampus. Eur J Neurosci. 2004;19:2410–2420. doi: 10.1111/j.0953-816X.2004.03346.x. [DOI] [PubMed] [Google Scholar]
- 203.Shukla V, Zimmermann H, Wang L, Kettenmann H, Raab S, Hammer K, Sévigny J, Robson SC, Braun N. Functional expression of the ecto-ATPase NTPDase2 and of nucleotide receptors by neuronal progenitor cells in the adult murine hippocampus. J Neurosci Res. 2005;80:600–610. doi: 10.1002/jnr.20508. [DOI] [PubMed] [Google Scholar]
- 204.Mishra SK, Braun N, Shukla V, Füllgrabe M, Schomerus C, Korf HW, Gachet C, Ikehara Y, Sévigny J, Robson SC, Zimmermann H. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development. 2006;133:675–684. doi: 10.1242/dev.02233. [DOI] [PubMed] [Google Scholar]
- 205.Milosevic J, Brandt A, Roemuss U, Arnold A, Wegner F, Schwarz SC, Storch A, Zimmermann H, Schwarz J. Uracil nucleotides stimulate human neural precursor cell proliferation and dopaminergic differentiation: involvement of MEK/ERK signalling. J Neurochem. 2006;99:913–923. doi: 10.1111/j.1471-4159.2006.04132.x. [DOI] [PubMed] [Google Scholar]
- 206.Rubini P, Milosevic J, Engelhardt J, Al-Khrasani M, Franke H, Heinrich A, Sperlagh B, Schwarz SC, Schwarz J, Nörenberg W, Illes P. Increase of intracellular Ca2+ by adenine and uracil nucleotides in human midbrain-derived neuronal progenitor cells. Cell Cal. 2009;45:485–498. doi: 10.1016/j.ceca.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 207.Lin JH, Takano T, Arcuino G, Wang X, Hu F, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M. Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev Biol. 2007;302:356–366. doi: 10.1016/j.ydbio.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Stafford MR, Bartlett PF, Adams DJ. Purinergic receptor activation inhibits mitogen-stimulated proliferation in primary neurospheres from the adult mouse subventricular zone. Mol Cell Neurosci. 2007;35:535–548. doi: 10.1016/j.mcn.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 209.Delarasse C, Gonnord P, Galante M, Auger R, Daniel H, Motta I, Kanellopoulos JM. Neural progenitor cell death is induced by extracellular ATP via ligation of P2X7 receptor. J Neurochem. 2009;109:846–857. doi: 10.1111/j.1471-4159.2009.06008.x. [DOI] [PubMed] [Google Scholar]
- 210.Wu PY, Lin YC, Chang CL, Lu HT, Chin CH, Hsu TT, Chu D, Sun SH. Functional decreases in P2X7 receptors are associated with retinoic acid-induced neuronal differentiation of Neuro-2a neuroblastoma cells. Cell Signal. 2009;21:881–891. doi: 10.1016/j.cellsig.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 211.Grimm I, Ullsperger SN, Zimmermann H. Nucleotides and epidermal growth factor induce parallel cytoskeletal rearrangements and migration in cultured adult murine neural stem cells. Acta Physiol (Oxf) 2010;199:181–189. doi: 10.1111/j.1748-1716.2010.02092.x. [DOI] [PubMed] [Google Scholar]
- 212.Khaira SK, Pouton CW, Haynes JM. P2X2, P2X4 and P2Y1 receptors elevate intracellular Ca2+ in mouse embryonic stem cell-derived GABAergic neurons. Br J Pharmacol. 2009;158:1922–1931. doi: 10.1111/j.1476-5381.2009.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Migita H, Kominami K, Higashida M, Maruyama R, Tuchida N, McDonald F, Shimada F, Sakurada K. Activation of adenosine A1 receptor-induced neural stem cell proliferation via MEK/ERK and Akt signaling pathways. J Neurosci Res. 2008;86:2820–2828. doi: 10.1002/jnr.21742. [DOI] [PubMed] [Google Scholar]
- 214.Fedele DE, Koch P, Scheurer L, Simpson EM, Möhler H, Brüstle O, Boison D. Engineering embryonic stem cell derived glia for adenosine delivery. Neurosci Lett. 2004;370:160–165. doi: 10.1016/j.neulet.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 215.Guttinger M, Fedele D, Koch P, Padrun V, Pralong WF, Brüstle O, Boison D. Suppression of kindled seizures by paracrine adenosine release from stem cell-derived brain implants. Epilepsia. 2005;46:1162–1169. doi: 10.1111/j.1528-1167.2005.61804.x. [DOI] [PubMed] [Google Scholar]
- 216.Li T, Steinbeck JA, Lusardi T, Koch P, Lan JQ, Wilz A, Segschneider M, Simon RP, Brüstle O, Boison D. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007;130:1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- 217.Pignataro G, Studer FE, Wilz A, Simon RP, Boison D. Neuroprotection in ischemic mouse brain induced by stem cell-derived brain implants. J Cereb Blood Flow Metab. 2007;27:919–927. doi: 10.1038/sj.jcbfm.9600422. [DOI] [PubMed] [Google Scholar]
- 218.Li J, Spletter ML, Johnson DA, Wright LS, Svendsen CN, Johnson JA. Rotenone-induced caspase 9/3-independent and -dependent cell death in undifferentiated and differentiated human neural stem cells. J Neurochem. 2005;92:462–476. doi: 10.1111/j.1471-4159.2004.02872.x. [DOI] [PubMed] [Google Scholar]
- 219.Riddle RC, Taylor AF, Rogers JR, Donahue HJ. ATP release mediates fluid flow-induced proliferation of human bone marrow stromal cells. J Bone Miner Res. 2007;22:589–600. doi: 10.1359/jbmr.070113. [DOI] [PubMed] [Google Scholar]
- 220.Kawano S, Otsu K, Kuruma A, Shoji S, Yanagida E, Muto Y, Yoshikawa F, Hirayama Y, Mikoshiba K, Furuichi T. ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Cal. 2006;39:313–324. doi: 10.1016/j.ceca.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 221.Coppi E, Pugliese AM, Urbani S, Melani A, Cerbai E, Mazzanti B, Bosi A, Saccardi R, Pedata F. ATP modulates cell proliferation and elicits two different electrophysiological responses in human mesenchymal stem cells. Stem Cells. 2007;25:1840–1849. doi: 10.1634/stemcells.2006-0669. [DOI] [PubMed] [Google Scholar]
- 222.Ichikawa J, Gemba H. Cell density-dependent changes in intracellular Ca2+ mobilization via the P2Y 2 receptor in rat bone marrow stromal cells. J Cell Physiol. 2009;219:372–381. doi: 10.1002/jcp.21680. [DOI] [PubMed] [Google Scholar]
- 223.Katebi M, Soleimani M, Cronstein BN. Adenosine A2A receptors play an active role in mouse bone marrow-derived mesenchymal stem cell development. J Leukoc Biol. 2009;85:438–444. doi: 10.1189/jlb.0908520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gharibi B, Elford C, Lewis BM, Ham J, Evans BAJ. Evidence for adenosine receptor regulation of osteogenesis versus adipogenesis in mesenchymal stem cells. Calcif Tissue Int. 2008;83:9–10. [Google Scholar]
- 225.Mohamadnejad M, Sohail MA, Watanabe A, Krause DS, Swenson ES, Mehal WZ. Adenosine inhibits chemotaxis and induces hepatocyte-specific genes in bone marrow mesenchymal stem cells. Hepatology. 2010;51:963–973. doi: 10.1002/hep.23389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Evans BA, Elford C, Pexa A, Francis K, Hughes AC, Deussen A, Ham J. Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. J Bone Miner Res. 2006;21:228–236. doi: 10.1359/JBMR.051021. [DOI] [PubMed] [Google Scholar]
- 227.Orciani M, Mariggiò MA, Morabito C, Di Benedetto G, Di Primio R. Functional characterization of calcium-signaling pathways of human skin-derived mesenchymal stem cells. Skin Pharmacol Physiol. 2010;23:124–132. doi: 10.1159/000270383. [DOI] [PubMed] [Google Scholar]
- 228.Park KS, Kim YS, Kim JH, Choi BK, Kim SH, Oh SH, Ahn YR, Lee MS, Lee MK, Park JB, Kwon CH, Joh JW, Kim KW, Kim SJ. Influence of human allogenic bone marrow and cord blood-derived mesenchymal stem cell secreting trophic factors on ATP (adenosine-5′-triphosphate)/ADP (adenosine-5′-diphosphate) ratio and insulin secretory function of isolated human islets from cadaveric donor. Transplant Proc. 2009;41:3813–3818. doi: 10.1016/j.transproceed.2009.06.193. [DOI] [PubMed] [Google Scholar]
- 229.Scholze NJ, Zippel N, Müller CA, Pansky A, Tobiasch E. P2X and P2Y receptors in human mesenchymal stem cell differentiation. Tiss Engineer Part A. 2009;15:698–699. [Google Scholar]
- 230.Andrews EM, Tsai SY, Johnson SC, Farrer JR, Wagner JP, Kopen GC, Kartje GL. Human adult bone marrow-derived somatic cell therapy results in functional recovery and axonal plasticity following stroke in the rat. Exp Neurol. 2008;211:588–592. doi: 10.1016/j.expneurol.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurg. 2005;57:1026–1031. doi: 10.1227/01.neu.0000181369.76323.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Boison D. Engineered adenosine-releasing cells for epilepsy therapy: human mesenchymal stem cells and human embryonic stem cells. Neurotherapeut. 2009;6:278–283. doi: 10.1016/j.nurt.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Whetton AD, Huang SJ, Monk PN. Adenosine triphosphate can maintain multipotent haemopoietic stem cells in the absence of interleukin 3 via a membrane permeabilization mechanism. Biochem Biophys Res Commun. 1988;152:1173–1178. doi: 10.1016/s0006-291x(88)80408-x. [DOI] [PubMed] [Google Scholar]
- 234.Kalambakas SA, Robertson FM, O’Connell SM, Sinha S, Vishnupad K, Karp GI. Adenosine diphosphate stimulation of cultured hematopoietic cell lines. Blood. 1993;81:2652–2657. [PubMed] [Google Scholar]
- 235.Hatta Y, Aizawa S, Itoh T, Baba M, Horie T. Cytotoxic effect of extracellular ATP on L1210 leukemic cells and normal hemopoietic stem cells. Leuk Res. 1994;18:637–641. doi: 10.1016/0145-2126(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 236.Lemoli RM, Ferrari D, Fogli M, Rossi L, Pizzirani C, Forchap S, Chiozzi P, Vaselli D, Bertolini F, Foutz T, Aluigi M, Baccarani M, Di Virgilio F. Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood. 2004;104:1662–1670. doi: 10.1182/blood-2004-03-0834. [DOI] [PubMed] [Google Scholar]
- 237.Rossi L, Manfredini R, Bertolini F, Ferrari D, Fogli M, Zini R, Salati S, Salvestrini V, Gulinelli S, Adinolfi E, Ferrari S, Di Virgilio F, Baccarani M, Lemoli RM. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood. 2007;109:533–542. doi: 10.1182/blood-2006-01-035634. [DOI] [PubMed] [Google Scholar]
- 238.Yoon MJ, Lee HJ, Lee YS, Kim JH, Park JK, Chang WK, Shin HC, Kim DK. Extracellular ATP is involved in the induction of apoptosis in murine hematopoietic cells. Biol Pharm Bull. 2007;30:671–676. doi: 10.1248/bpb.30.671. [DOI] [PubMed] [Google Scholar]
- 239.Hofer M, Vacek A, Pospisil M, Weiterova L, Hola J, Streitova D, Znojil V. Adenosine potentiates stimulatory effects on granulocyte-macrophage hematopoietic progenitor cells in vitro of IL-3 and SCF, but not those of G-CSF, GM-CSF and IL-11. Physiol Res. 2006;55:591–596. doi: 10.33549/physiolres.930854. [DOI] [PubMed] [Google Scholar]
- 240.Hofer M, Pospisil M, Znojil V, Holá J, Streitová D, Vacek A. Homeostatic action of adenosine A3 and A1 receptor agonists on proliferation of hematopoietic precursor cells. Exp Biol Med (Maywood) 2008;233:897–900. doi: 10.3181/0802-RM-43. [DOI] [PubMed] [Google Scholar]
- 241.Hofer M, Vacek A, Pospisil M, Hola J, Streitova D, Znojil V. Activation of adenosine A3 receptors potentiates stimulatory effects of IL-3, SCF, and GM-CSF on mouse granulocyte-macrophage hematopoietic progenitor cells. Physiol Res. 2009;58:247–252. doi: 10.33549/physiolres.931454. [DOI] [PubMed] [Google Scholar]
- 242.Lappas CM, Liu PC, Linden J, Kang EM, Malech HL. Adenosine A2A receptor activation limits graft-versus-host disease after allogenic hematopoietic stem cell transplantation. J Leukoc Biol. 2010;87:345–354. doi: 10.1189/jlb.0609388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wurtman RJ, Cansev M, Sakamoto T, Ulus IH. Use of phosphatide precursors to promote synaptogenesis. Annu Rev Nutr. 2009;29:59–87. doi: 10.1146/annurev-nutr-080508-141059. [DOI] [PubMed] [Google Scholar]
- 244.Mamedova LK, Gao ZG, Jacobson KA. Regulation of death and survival in astrocytes by ADP activating P2Y1 and P2Y12 receptors. Biochem Pharmacol. 2006;72:1031–1041. doi: 10.1016/j.bcp.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rivkees SA, Zhao Z, Porter G, Turner C. Influences of adenosine on the fetus and newborn. Mol Genet Metab. 2001;74:160–171. doi: 10.1006/mgme.2001.3217. [DOI] [PubMed] [Google Scholar]
- 246.Tarnok A, Ulrich H. Characterization of pressure-induced calcium response in neuronal cell lines. Cytometry. 2001;43:175–181. doi: 10.1002/1097-0320(20010301)43:3<175::aid-cyto1046>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 247.Martins AH, Resende RR, Majumder P, Faria M, Casarini DE, Tarnok A, Colli W, Pesquero JB, Ulrich H. Neuronal differentiation of P19 embryonal carcinoma cells modulates kinin B2 receptor gene expression and function. J Biol Chem. 2005;280:19576–19586. doi: 10.1074/jbc.M502513200. [DOI] [PubMed] [Google Scholar]
- 248.Ulrich H, Majumder P. Neurotransmitter receptor expression and activity during neuronal differentiation of embryonal carcinoma and stem cells: from basic research towards clinical applications. Cell Prolif. 2006;39:281–300. doi: 10.1111/j.1365-2184.2006.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]