Abstract
The presence of tubulin in human erythrocytes was demonstrated using five different antibodies. Tubulin was distributed among three operationally distinguishable pools: membrane, sedimentable structure and soluble fraction. It is known that in erythrocytes from hypertensive subjects (HS), the Na+, K+-ATPase (NKA) activity is partially inhibited as compared with erythrocytes from normal subjects (NS). In erythrocytes from HS the membrane tubulin pool is increased by ~150%. NKA was found to be forming a complex with acetylated tubulin that results in inhibition of enzymes. This complex was also increased in erythrocytes from HS. Treatment of erythrocytes from HS with nocodazol caused a decrease of acetylated tubulin in the membrane and stimulation of NKA activity, whereas taxol treatment on erythrocytes from NS had the opposite effect. These results suggest that, in erythrocytes from HS, tubulin was translocated to the membrane, where it associated with NKA with the consequent enzyme inhibition.
Keywords: Na+, K+-ATPase; Acetylated tubulin; Detyrosinated tubulin; Hypertension; Human erythrocytes
Introduction
Flexibility and resistance of the human erythrocyte plasma membrane depend on the cytoskeleton that underlies the membrane and connects to it at various points. The erythrocyte cytoskeleton is comprised mainly of microfilaments, whose principal proteins are actin, adducin, α and β spectrin, ankyrin, band 3, protein 4.1 and others [1]. In addition, the erythrocyte membrane in vertebrates (except mammals) is reinforced by a bundle of microtubules, termed the marginal band, which circumscribes the cortical membrane in the horizontal plane of the cell. The marginal band is thought to provide structural support for the cell membrane and the elliptical cell morphology typical of vertebrate erythrocytes [2, 3]. Electron microscopy studies indicated that human erythrocytes contain neither microtubules nor marginal band [4]. In view of this finding, further studies on tubulins of human erythrocytes were not performed, and many researchers presumed that they do not exist [2, 3, 5]. However, recent proteomics studies [6] showed the presence of various isoforms of tubulin in human erythrocytes.
Tubulins are a group of cytosolic structural proteins that become associated with the plasma membrane through interaction with other proteins, acquiring hydrophobic properties [7–10]. The superfamily comprises various isoforms of α and β tubulins, which are differentially expressed in tissues of eukaryotic cells [11]. The isoforms undergo various post-translational modifications, which tend to be conserved during evolution. These modifications primarily involve the carboxy-terminal domain of α and β tubulin, which is located externally on the microtubule, facilitating its interaction with other proteins [12]. The post-translational modifications are all reversible. They include polyglycylation, polyglutamylation, detyrosination, tyrosination, phosphorylation, palmitoylation and acetylation [13]. Tubulins display a well-regulated capacity to polymerize into a cylindrical polymeric structure, the microtubule [14]. Several drugs, including nocodazole and colcemid, disrupt microtubule polymerization, whereas taxol is a microtubule-stabilizing compound.
In this study, using five different anti-tubulin antibodies, we confirmed the presence of tubulin in human erythrocytes and showed that it is distributed in three distinct intracellular pools that are separable by simple differential centrifugation. In addition, alteration of tubulin distribution was described in erythrocytes from hypertensive patients that resulted in modification of Na+, K+-ATPase activity.
Na+, K+-ATPase (NKA; the “sodium pump”) is a plasma membrane protein whose basic function is to maintain high Na+ and K+ gradients across cell membranes, utilizing energy from ATP hydrolysis. This pump is the major determinant of the cytoplasmic Na+ content [15]. Deficiency of NKA activity has been claimed to be the cause of the increased Na+ content observed in erythrocytes of hypertensive patients [16, 17]. NKA, together with the Na+/Ca2+ exchanger protein, is considered responsible for regulation of intracellular Na+ concentration [18, 19]. Reduced NKA activity has been observed in erythrocytes from spontaneously hypertensive rats [20] and from human patients with essential hypertension [21], and claimed to be the major reason for Na+ accumulation in various cells [22, 23]. Increased Na+ content has been reported in both erythrocytes [16, 24] and white blood cells [25, 26] from hypertensive patients.
The only explanation so far proposed for the reduced NKA activity in erythrocytes from hypertensive subjects is the absence or deficient expression of adducin, an intracellular activator of NKA [27]. In hypertensive rats, the gene that encodes adducin is mutated so that it cannot activate the enzyme [28]. However, only ~10% of human hypertension cases can be explained on this basis; in the majority of such cases the mechanism of NKA inhibition is unclear.
In a series of previous studies we showed that tubulin, the cytoplasmic protein constituent of microtubules, interacts with NKA and inhibits ATP hydrolysis and K+ uptake [9, 10, 29–31]. The acetylated isotype of tubulin is required for this interaction [10, 32]. Formation of the acetylated tubulin/NKA complex is reversible, and dissociation of the complex restores NKA activity. In the present study, we analyzed the presence and distribution of various tubulin isotypes in erythrocytes from hypertensive human patients and tested the possibility that reduced NKA activity in erythrocytes from these patients is due to its interaction with acetylated tubulin. Our results show that the membrane tubulin and sedimentable tubulin pools are involved in regulation of NKA activity in erythrocyte membranes.
Materials and methods
Materials
Nitrocellulose membrane, phenylmethyl-sulfonyl-fluoride (PMSF), nocodazole, paclitaxel (taxol), Triton X-100, SDS, and Tween were from Sigma Chemical Co. The LumigenTM PS-3 detection kit and high performance chemiluminescence film were from GE Healthcare Life Sciences.
Antibodies
Mouse mAb 6-11B-1 versus acetylated tubulin, mouse mAb 1A2 versus tyrosinated tubulin, mouse mAb DM1A specific to α-tubulin, mAb Tub2.1 specific to β-tubulin, mouse IgG conjugated with peroxidase, rabbit IgG conjugated with peroxidase, mouse IgG conjugated with fluorescein and rabbit IgG conjugated with rhodamine were from Sigma. Rabbit polyclonal Ab H-300 specific to α-subunit of NKA was from Santa Cruz Biotechnology. Rabbit polyclonal Ab versus detyrosinated tubulin (anti-Glu) was prepared in our laboratory as described by Gundersen et al. [33], and displayed specificity and titers similar to those of samples provided by this author.
Subjects and preparation of erythrocytes
Male and female patients at the Hospital Regional de Río Cuarto (Córdoba, Argentina) were recruited for this study using an informed consent protocol approved by the hospital’s Human Studies Committee. Demographic and hemodynamic data of the patients are shown in Table 1. Fresh blood samples were collected from healthy volunteer subjects (25–40 years of age) in Vacutainer tubes (Becton–Dickinson, Plymouth, UK), using 1 mg/ml EDTA as an anticoagulant. After storing blood at 4°C for 96 h, erythrocytes were isolated by conventional centrifugal separation and used immediately. Hypertensive patients were selected on the basis of a personal history of consistent blood pressure equal to or higher than 160/110 mmHg.
Table 1.
Demographic and hemodynamic data of the studied population
| Parameters | H (n = 50) | N (n = 20) | P |
|---|---|---|---|
| Age (years) | 65.4 ± 10.2 | 67.3 ± 9.3 | – |
| Sex, male/female | 32/18 | 9/11 | – |
| Weight (kg) | 77 ± 12 | 74 ± 11 | NS |
| Systolis BP (mmHg) | 161 ± 22 | 127 ± 13 | <0.05 |
| Diastolic BP (mmHg) | 102 ± 10 | 85 ± 11 | <0.05 |
| Triglycerides (mg/dl) | 254 ± 12 | 108 ± 7 | <0.01 |
| Diabetes (yes/no) | 0/50 | 0/20 | – |
| Hypercholesterolemia (yes/no) | 4/46 | 0/20 | – |
| Antihypertensive treatment (yes/no) | 26/24 | – | – |
| Urea (mg/dl) | 35 ± 9 | 33 ± 6 | NS |
| Creatinine (mg/dl) | 0.79 ± 0.15 | 0.72 ± 0.22 | NS |
| Na+ (mM) | 140 ± 12 | 143 ± 9 | NS |
| K+ (mM) | 5 ± 1 | 4 ± 2 | NS |
H hypertensive patients, N normotensive patients, NS not significant
Isolation of three tubulin pools
Erythrocytes isolated from 2 ml human blood were resuspended in 3 ml lysis buffer (7.5 mM sodium phosphate buffer, pH 7.5, containing 1 mM EDTA and 20 μg/ml PMSF) and incubated for 15 min at room temperature. The lysate was centrifuged (20,000×g) for 20 min at 30°C. The pellet was washed three times with 6 ml lysis buffer without PMSF, resuspended in 3 ml lysis buffer (Membrane tubulin, Mem-tub), and stored at −20°C until use. The supernatant fraction from the 20,000×g centrifugation was immediately centrifuged at 100,000×g for 30 min at 30°C. The pellet was resuspended in 3 ml lysis buffer (sedimentable tubulin, Sed-tub), and the supernatant fraction was loaded onto a phosphocellulose column for purification of cytosolic tubulin (Sol-tub) as described below.
Purification of cytosolic tubulin from supernatant fraction of erythrocytes
The 100,000×g supernatant fraction of erythrocytes obtained as described above contained a high level of hemoglobin. It was necessary to separate tubulin from hemoglobin before attempting to further characterize tubulin in this fraction. For this purpose, the fraction was chromatographed onto a phosphocellulose column (equilibrated with 100 mM Mes buffer, pH 6.8, containing 1 mM MgCl2) as described previously [34, 35]. After loading, hemoglobin remained bound to the column, while tubulin was eluted with the same buffer. Fractions containing tubulin (as detected by Western blot using mAb DM1A) were mixed and stored at −20°C for further analysis (soluble tubulin, Sol-tub).
Immunofluorescence microscopy
Erythrocytes were fixed on coverslips with anhydrous methanol at −20°C. Samples were washed, incubated with 2% (w/v) bovine serum albumin in NaCl/Pi (PBS) for 30 min and stained by indirect immunofluorescence as described by DeWitt et al. [36]. Two primary antibodies were used: rabbit polyclonal Ab H-300 (diluted 1:50) to visualize α-subunit of NKA and mouse mAb DM1A (diluted 1:100) to determine α-tubulin. Fluorescein-conjugated anti-mouse IgG and rhodamine-conjugated anti-rabbit immunoglobulin, at 1:50 dilution, were used as respective secondary antibodies. Coverslips were mounted in FluorSave and observed with an LSM 5 Pascal confocal microscope (Zeiss, Jena, Germany) using dual channel filters for simultaneous viewing of rhodamine and fluorescein isothiocyanate fluorochromes.
Immunoblotting
Proteins were separated by SDS-PAGE on 12% polyacrylamide slab gels, by the procedure of Laemmli [37], and the gel was transferred to a nitrocellulose sheet. Blots were reacted with mAb DM1A (dilution 1:1,000), Tub2.1 (dilution 1:1,000), 6-11B-1 (dilution 1:1,000), Tub 1A2 (dilution 1:1,000), polyclonal Ab anti-Glu (dilution 1:200), or rabbit polyclonal Ab H-300 (diluted 1:500) to visualize the α-subunit of NKA. Sheets were reacted with the corresponding anti-IgG conjugated with peroxidase and stained using the 4-chloro-1-naphthol method or ECL system. Band intensities were quantified using the Scion Image program.
Na+, K+-ATPase activity assay
Enzyme activity was determined by the method of Salvador and Mata [38]. Erythrocyte membranes (5–10 μg protein) were added to the reaction mixture (50 mM Tris-HCl, pH 7.4, 20 mM KCl, 100 mM NaCl, 2.5 mM MgCl2, 0.5 mM EGTA, 0.16 mM NADH, 1 mM phosphoenolpyruvate, 2.5 IU pyruvate kinase, 2.5 IU lactate dehydrogenase) in a final volume of 340 μl. The mixture was kept for 10 min at room temperature, and the reaction was started by addition of 1 mM ATP. NADH oxidation was measured for 15 min at room temperature at 340 nm, using a recording spectrophotometer. Control cuvettes were prepared without enzyme or with heat-denatured enzyme. Enzyme activity was estimated as the difference between samples incubated in the absence versus presence of 1 mM ouabain. Experiments were performed in quadruplicate, and the mean value is presented.
Preparation of antibody linked to Sepharose
mAb 6-11B-1 and anti-phosphatidylinositol 3-kinase p110 were covalently bound to cyanogen bromide-activated Sepharose 4B as described by Hubbert et al. [39], with slight modification. Sepharose beads were washed with 100 vol 0.001 M HCl at 21°C. Packed beads (1 ml) were mixed with antibody (2.5 mg protein) in 1 ml coupling buffer (0.5 M NaCl containing 0.2 M NaHCO3, pH 8.2). The mixture was agitated on a rocking platform 4 h at 21°C and loaded into a small chromatographic column. Unbound antibodies were removed by washing with 5 ml coupling buffer. Antibody-coated beads were transferred to a beaker and suspended in 1 ml coupling buffer containing 0.2 M glycine to block unreacted sepharose sites. The mixture was agitated 2 h at 21°C, and unbound glycine was removed by washing the beads with 10 ml coupling buffer. Resulting antibody-coated beads were washed with 1.5 ml 0.01 mM Tris-HCl, pH 8, containing 0.14 M NaCl and 0.025% NaN3, and stored at 4°C until use (maximum 2 days).
Immunoprecipitation
One volume of membrane preparation (5 mg protein/ml) was mixed with one volume of NaCl/Tris containing 1% Triton X-100 (NaCl/Tris-Triton) and centrifuged to eliminate residual insoluble material. Aliquots (0.3 ml) were mixed with 0.15 ml packed antibody-sepharose beads prepared as described above and incubated 4 h at 20°C with gentle agitation. Samples were centrifuged, and the precipitated material was washed five times with NaCl/Tris-Triton. Fractions (50 μl) of packed beads were resuspended in 50 μl Laemmli sample buffer, heated at 50°C for 15 min and centrifuged. Aliquots (20 μl) of soluble fractions were subjected to SDS-PAGE. A control was run in parallel using mAb phosphatidylinositol 3-kinase–sepharose instead of mAb 6-11B-1 antibody–sepharose.
Protein determination
Protein concentration was determined by the method of Bradford [40].
Statistical analysis
Results were expressed as mean ± SD. Student’s t test was used for comparison of two populations. Differences in results were considered statistically significant for P < 0.05.
Results
Presence of tubulin in human erythrocytes
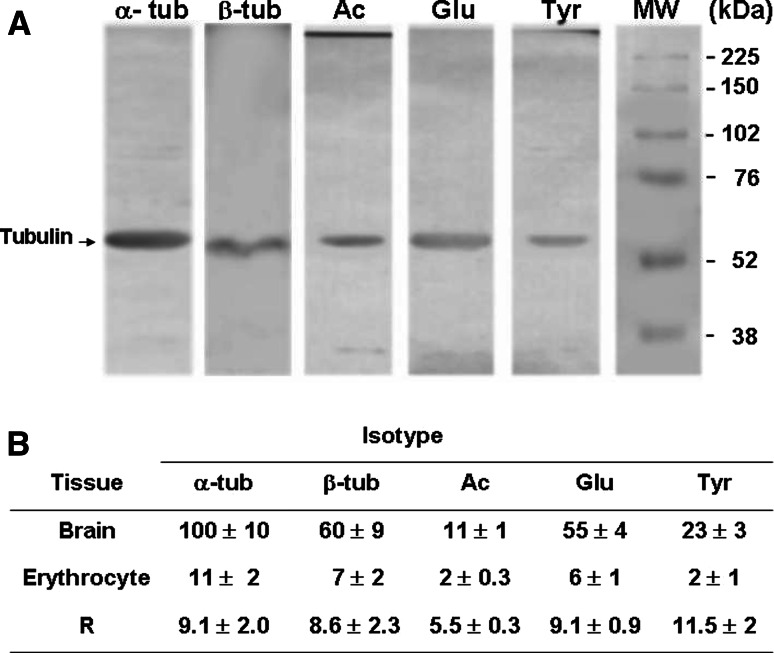
mAb DM1A is specific to α-tubulin, does not discriminate between various post-translational modifications and is therefore useful for estimating the amount of total α-tubulin. SDS-PAGE followed by immunoblotting with this mAb clearly revealed the presence of α-tubulin in human erythrocytes (Fig. 1a). Post-translational modifications of tubulin in eukaryotic cells include tyrosination/detyrosination and acetylation/deacetylation. To clarify the above finding, we performed immunoblot assays on hypotonic shock lysates (see following section) of erythrocytes, using antibodies specific to total α-tubulin (DM1A), β-tubulin (Tub2.1), tyrosinated tubulin (Tub 1A2), detyrosinated tubulin (Glu antibody), and acetylated tubulin (6-11B-1). Visual examination of bands from immunoblotting with each of these antibodies (Fig. 1a) confirmed the presence of tubulin in human erythrocytes. Comparative immunoblotting experiments showed that the proportion of total tubulin relative to total protein was ninefold lower in human erythrocytes than in rat brain tissue (data summarized in Fig. 1b). The proportion of acetylated tubulin was slightly higher in human erythrocytes than in rat brain, but proportions of the other three tubulin isoforms determined by the above antibodies were not significantly different (Fig. 1b).
Fig. 1.
Presence of tubulin in human erythrocytes. Sedimented erythrocytes prepared from 2 ml blood were lysed by resuspension in 3 ml lysis buffer containing 1% Triton X-100. a Aliquots (250 μg protein) of lysate were analyzed by SDS-PAGE and immunoblotted using antibodies specific to total α-tubulin (α-tub), β-tubulin (β-tub), tyrosinated tubulin (Tyr-tub), detyrosinated tubulin (Glu-tub) and acetylated tubulin (Ac-tub). b Tubulin bands were quantified (optical density in arbitrary units) using the Scion Image program, with optical density of the total α-tubulin band defined as 100%. Values are expressed as mean ± SD from three independent experiments. Homogenate samples prepared from 30-day-old rat brain were processed in parallel for comparative purposes. The brain sample is not shown in a
Distribution of tubulin in human erythrocytes
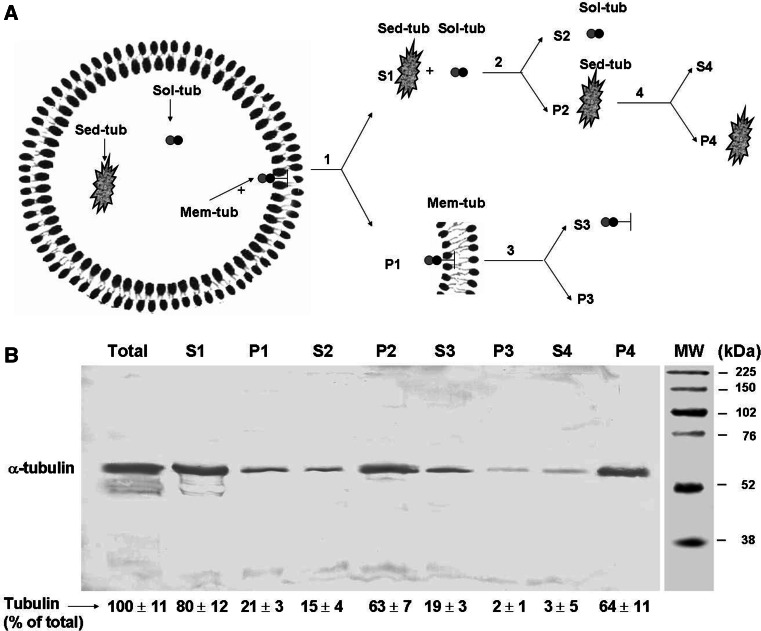
Several authors reported the absence of a marginal band, or any kind of microtubules, in human erythrocytes [3–5]. For this reason, the possible presence of tubulin in these cells was not studied for a number of years. In 2007, Goodman et al. [6] reported the presence of various isoforms of α- and β-tubulins in human erythrocytes, based on proteomics studies. We were interested to confirm this finding. Previous studies from our laboratory and others indicated that tubulin may be localized heterogeneously within erythrocytes. To analyze such subcellular distribution, human erythrocytes were disrupted by suspension in lysis buffer (“hypotonic shock”) and subjected to a differential centrifugation scheme (shown schematically in Fig. 2a), with subsequent monitoring of tubulin in each fraction. The lysate was centrifuged at 20,000×g for 20 min, followed by separation of the precipitate (P1) containing the membrane fraction and the supernatant fraction (S1). Tubulin present in P1 was termed “Mem-tub” (Mem-tub). S1 was further centrifuged at 100,000×g, and two types of tubulin pool were found: one remaining in the second (100,000×g) supernatant fraction (S2), termed “soluble tubulin” (Sol-tub), and the other found in the corresponding pellet (P2), termed “sedimenta ble tubulin” (Sed-tub). S2 was processed as described in Materials and methods to free soluble tubulin from hemoglobin. Each of the fractions obtained during this procedure (total lysate, S1, P1, S2, P2, S3, P3) was subjected to immunoblot using anti-total α-tubulin mAb DM1A (Fig. 2b). The sum of tubulin proportions in P1 (Mem-tub) plus S2 (Sol-tub) plus P2 (Sed-tub) should account for total tubulin. These values were, respectively, 21 ± 3, 15 ± 4 and 63 ± 7 (Fig. 2b).
Fig. 2.
Differential centrifugation scheme for isolation of three different tubulin pools from lysate of human erythrocytes. a Erythrocytes freshly obtained from 20 ml human blood were lysed by hypotonic shock as described in “Materials and methods.” The lysate was centrifuged at 20,000 ×g for 20 min at 30°C, the supernatant fraction (S1) was removed, and the sedimented fraction (P1) was washed three times with 60 ml lysis buffer without PMSF, resuspended in 3 ml lysis buffer (containing “membrane-bound tubulin” or “Mem-Tub”) and stored at −20°C. Fraction S1 was centrifuged at 100,000×g for 30 min at 30°C, and sedimented (P2) (containing “sedimentable tubulin” or “Sed-tub”) and supernatant (S2) (containing “soluble tubulin” or “Sol-tub”) fractions were separated. The Sol-tub fraction was loaded onto a chromatographic column to free soluble tubulin from hemoglobin, as described in “Materials and methods.” Aliquots of Mem-tub (P1) and Sed-tub (P2) fractions were treated with lysis buffer in the presence of 1% Triton X-100. Both mixtures were centrifuged at 100,000×g for 30 min. The supernatant and sedimented fractions from P1 were termed S3 and P3, and those from P2 were termed S4 and P4. b Aliquots of each fraction, corresponding to 100 μl whole blood, were subjected to immunoblot with antibody DM1A to estimate the amount of α-tubulin. Optical density (arbitrary units) of tubulin bands was quantified using the Scion Image program. Values are mean ± SD from three independent experiments, expressed as percentage of the amount of tubulin in the whole lysate
In order to rule out the possibility that Sed-tub was merely a membranous fraction that sedimented at 100,000×g but not at 20,000×g, we obtained P1 (Mem-tub) and P2 (Sed-tub) fractions, resuspended both with lysis buffer containing 1% Triton X-100, centrifuged at 100,000×g for 30 min, and analyzed the resulting supernatant and pellet fractions for total α-tubulin (Fig. 2a, b). The detergent treatment resulted in 100% solubilization of tubulin for Mem-tub (compare S3 with P3), but no tubulin solubilization for Sed-tub (compare S4 with P4). Thus, the interactions of Mem-tub with membranous elements are different in nature from those of Sed-tub. Taken together, these experiments demonstrate that tubulin is present in human erythrocytes in at least three different pools (termed Mem-tub, Sed-tub and Sol-tub) separable by differential centrifugation.
Localization of tubulin within the erythrocyte was further studied using confocal immunofluorescence microscopy. Localization of Na+, K+-ATPase, as a membrane marker, was monitored using a specific antibody. Tubulin was found to be dispersed in the cytoplasm (Fig. 3). In many cells, a relatively bright zone was seen at the periphery, suggesting that tubulin may be concentrated near the membrane. A minor part of tubulin appeared to co-localize with the membrane (Fig. 3c, c′).
Fig. 3.
Subcellular distribution of tubulin in human erythrocytes. Human erythrocytes were fixed on coverslips and analyzed by confocal immunofluorescence microscopy using mouse mAb DM1A for α-tubulin (green, a) and rabbit polyclonal antibody H-300 for α-subunit of Na+, K+-ATPase (red, b), as described in “Materials and methods.” c, merge. a′, b′ and c′: Enlargement of the area indicated by white rectangle in a, b and c, respectively
Membrane tubulin is eliminated from the membrane by alkaline or nocodazole treatment
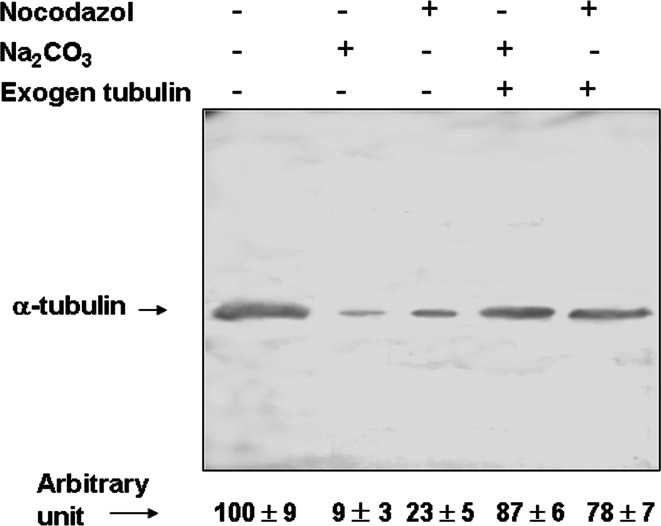
Alkaline treatment of membranes (Na2CO3, pH ≥11.5) results in dissociation of peripheral but not integral proteins, and is frequently used to distinguish these two types of proteins [41]. We used this approach to study the interaction of tubulin with plasma membrane of human erythrocytes. Membranes were treated with Na2CO3 and centrifuged, and the tubulin content in the pellet fraction was determined by Western blot. When membranes were treated with 0.1 M Na2CO3, pH ≥ 11.5, 10 min, at 4°C, 90% of Mem-tub was eliminated (Fig. 4). When partially purified brain tubulin was incubated (30 min, 37°C) with a tubulin-depleted membrane preparation, re-association of tubulin with membranes was observed (Fig. 4), indicating that the erythrocyte membrane contains some component(s) able to bind tubulin. Previous work with brain, cultured astrocytes and other types of cells (29, 30) demonstrated that a membrane component with the ability to associate with tubulin was NKA. This association was shown to be disrupted by treatment with the microtubule depolymerizing agent, nocodazole. We therefore tested the effect of nocodazole (50 μM, 30 min, room temperature) on erythrocyte membranes and obtained similar results (Fig. 4). Again, incubation of purified tubulin with erythrocyte membranes depleted of tubulin by nocodazole treatment resulted in re-association of tubulin to membranes (Fig. 4). Taken together, these findings indicate that erythrocyte membrane tubulin is a peripheral (not an integral) protein that binds to NKA.
Fig. 4.
Effects of nocodazol and Na2CO3 on membrane tubulin. Erythrocyte membranes (1 mg protein) were incubated in the presence (+) or absence (−) of 0.1 M Na2CO3, pH 11.5, for 15 min at 4 °C, or with 50 μM nocodazole for 30 min at room temperature, and centrifuged at 100,000×g for 30 min. The pellets were analyzed for total tubulin or, alternatively, were resuspended in TBS (300 μl) incubated with 300 μg/ml purified tubulin for 30 min at 37°C and centrifuged again at 100,000×g for 30 min. Aliquots of pellet fractions (250 μg protein) were analyzed for total tubulin using mAb DM1A. Optical density of tubulin bands was quantified using the Scion Image program. Values are shown in the lower panel as mean ± SD from three independent experiments, expressed as percentage relative to value from non-treated membranes
Sedimentable tubulin is moved to soluble fraction by nocodazole treatment
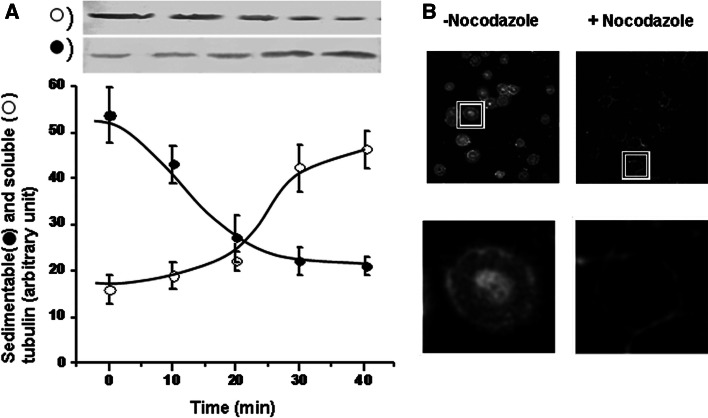
As described above, human erythrocytes contain a type of tubulin that we operationally termed “sedimentable tubulin” (Sed-tub). It would be interesting to characterize the structure that maintains tubulin as a sedimentable entity, but this was not a major objective of the present study. We simply examined the effect of nocodazole on this structure. It seemed unlikely that sedimentable tubulin is structured as microtubules, since previous reports seemed to indicate that microtubules are not present in erythrocytes. Surprisingly, we found that the pool of Sed-tub decreased as a function of incubation time in the presence of nocodazole, whereas the pool of Sol-tub increased (Fig. 5). Tubulin content in the sedimentable fraction decreased by >50% during 30 min of nocodazole treatment. When immunofluorescence microscopy was applied, nocodazole treatment was seen to eliminate (or reduce) tubulin in cytoplasmic locations (compare + versus − nocodazole in Fig. 5b).
Fig. 5.
Effect of nocodazol on Sed-tub pool. a Human blood (12 ml) was incubated in the presence of 50 μM nocodazole; 2 ml aliquots were taken at the indicated times, and erythrocytes were isolated by centrifugation. Sedimentable and soluble fractions were obtained as described in “Materials and methods.” Aliquots of each fraction (corresponding to 100 μl whole blood) were subjected to immunoblot with mAb DM1A to determine the amount of α-tubulin. Upper panel shows results from a typical experiment. Optical density of tubulin bands was quantified, and values (arbitrary units) are shown in the lower panel as mean ± SD from three independent experiments. b Human erythrocytes incubated in the presence or absence of 50 μM nocodazol for 40 min were fixed on coverslips and analyzed by confocal immunofluorescence microscopy using mAb DM1A. Lower images: Enlargement of area indicated by rectangle in upper images
Alterations of the tubulin distribution in human erythrocytes from hypertensive subjects
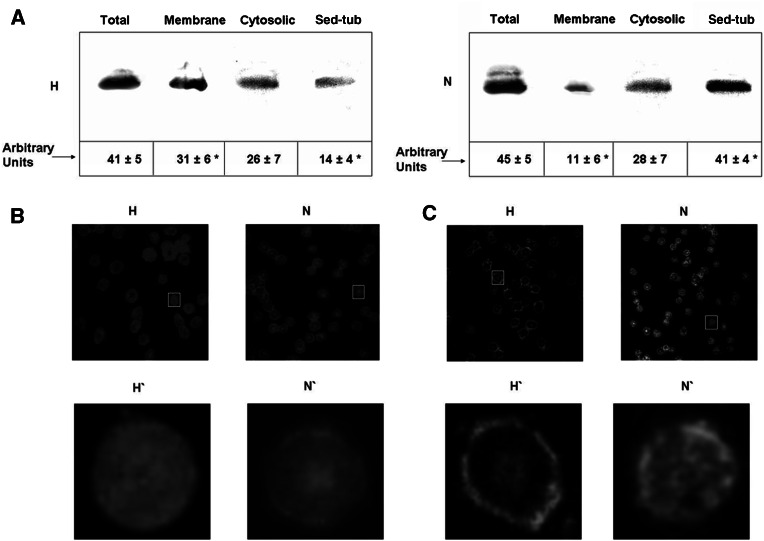
We studied the presence and distribution of tubulin in human erythrocytes from normotensive versus hypertensive subjects and found tubulin in all three of the above pools for both groups. The study included 20 normotensive subjects (N) and 50 patients with essential hypertension (H). Table 1 shows demographic and hemodynamic data in the N and H groups. The amount of total tubulin (that is, without distinction of pools) in erythrocytes, measured by Western blot analysis, did not differ significantly between the two groups (Fig. 6a). Cytosolic tubulin content was also the same in the two groups. However, Sed-tub content was 65% lower and membrane tubulin content was 200% higher in the hypertensive group compared to the normotensive group. Thus, erythrocytes from hypertensive subjects displayed a redistribution of tubulin from a cytoplasmic structure such as the Sed-tub pool toward the membrane.
Fig. 6.
Tubulin distribution in erythrocytes of hypertensive and normotensive subjects. a Tubulin in total, membrane, cytosolic and Sed-tub fractions was obtained from 2 ml human blood under microtubule-stabilizing conditions as described in “Materials and methods.” An aliquot of each fraction was used to determine the amount of α-tubulin by immunoblot using DM1A. Volumes loaded on each well were calculated to be representative of the same amount of erythrocytes. Tubulin bands were quantified using the Scion Image program. Values (means ± SD from triplicate experiments are expressed as arbitrary units. Erythrocytes of hypertensive (H) and normotensive (N) subjects were fixed on coverslips and analyzed by indirect (b) and confocal (c) immunofluorescence microscopy using DM1A antibody. (H′) and (N′): Enlargement of the area indicated by the rectangle in (H) and (N), respectively. *P < 0.01
Indirect immunofluorescence of erythrocytes from normotensive subjects showed a thin peripheral fluorescent ring (Fig. 6b, N and N′), whereas in erythrocytes from hypertensive subjects, uniformly distributed fluorescence (Fig. 6b, H and H′) was observed. On the other hand, confocal microscopy (Fig. 6c) showed that erythrocytes from normal subjects (N and N′) contain tubulin near the membrane and also in the cytoplasmic region, whereas those from hypertensive patients (H and H′) contain a small amount of tubulin in the cytoplasm, but it is increased at the periphery, colocalizing the membrane. These results are coincident with the idea that in erythrocytes from hypertensive subjects a tubulin displacement occurred from the cytoplasm to membrane as suggested by the Western blot experiments (Fig. 6a).
Content of different tubulin isotypes in Mem-tub and Sed-tub pools in erythrocytes from normotensive and hypertensive subjects
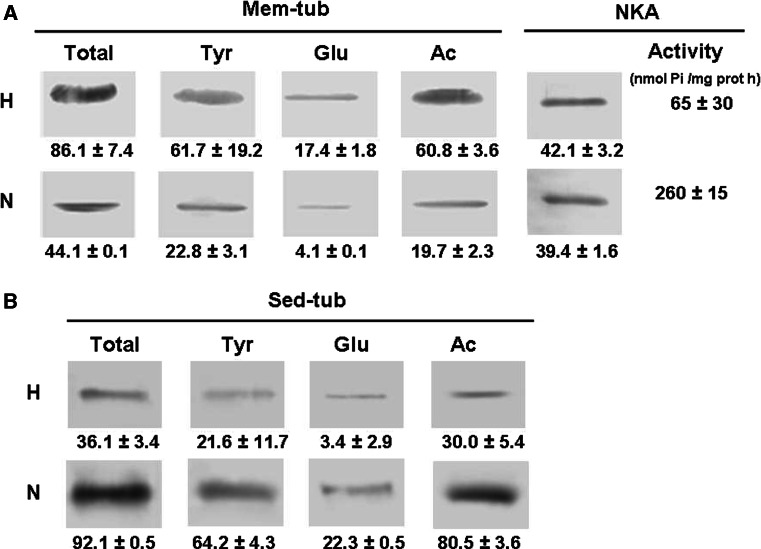
Amounts of total, acetylated, tyrosinated and detyrosinated tubulin in Mem-tub and Sed-tub fractions from erythrocytes isolated from normal (N) and hypertensive (H) subjects were determined by Western blotting using specific antibodies (Fig. 7). Total tubulin in this case refers to α-tubulin measured with an antibody (DM1A) that does not discriminate among different isotypes. Total tubulin was higher in erythrocyte membranes from H than from N subjects (Fig. 7a). The same was true for tyrosinated, detyrosinated and acetylated tubulin, although it should be pointed out that the increments of detyrosinated and acetylated tubulin were greater than for total tubulin. The amount of NKA in the membrane, as measured by Western blot, was similar for erythrocytes from N versus H subjects (Fig. 7a), whereas NKA activity was 75% less for cells from hypertensive subjects. In Sed-tub (Fig. 7b), total tubulin as well as the different isospecies was lower in erythrocytes isolated from H than those from N subjects.
Fig. 7.
Content of tubulin isotypes in membrane and sedimentable tubulin fractions: comparison between hypertensive (H) and normotensive (N) subjects. Membrane (Mem-tub, a) and sedimentable (Sed-tub, b) tubulin fractions from erythrocytes were extracted from 2 ml whole blood as described in “Materials and methods.” An aliquot of each fraction was used to determine the tubulin isotype and NKA by immunoblot using specific antibodies. Volumes loaded on each well were calculated to be representative of the same amount of erythrocytes. Tubulin and NKA bands were quantified using the Scion Image program, and values (arbitrary unit) are shown below the corresponding images. NKA activity was measured also in Mem-tub fractions (panel A) as described in “Materials and methods.” Values shown are mean ± SD from three independent experiments. For each tubulin isospecies and for NKA activity differences between H and N groups were statistically significant (P < 0.01)
Quantification of acetylated tubulin/NKA complex
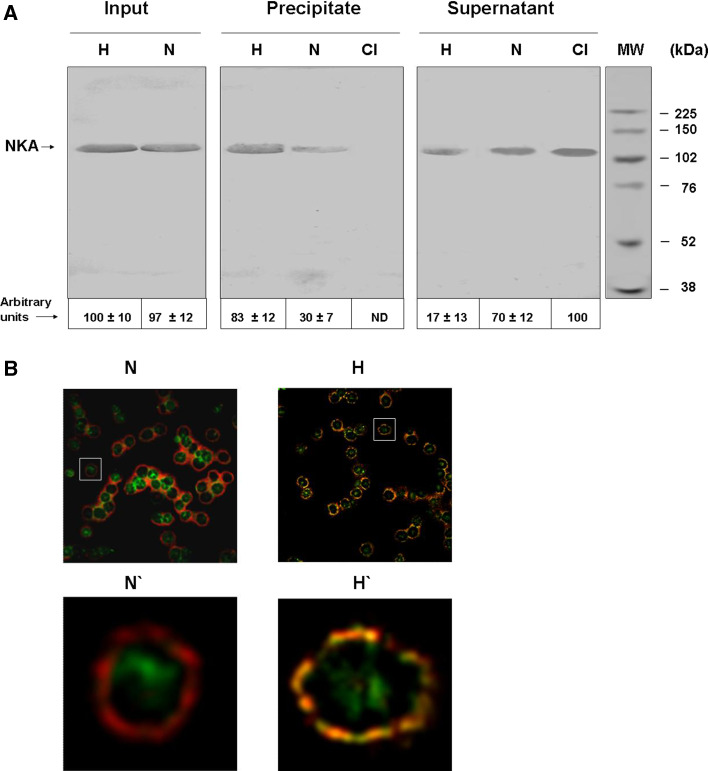
Results of our previous studies (see Introduction) suggested that displacement of tubulin from ST to the membrane pool might be correlated with increased formation of acetylated tubulin/NKA complex and inhibition of NKA activity. To test this possibility, we determined the amount of such complex in erythrocyte membranes from normotensive and hypertensive subjects by immunoprecipitation using anti-acetylated tubulin antibody (6-11B-1) linked to Sepharose beads. The NKA level in the precipitate was analyzed by Western blot using a specific antibody (rabbit polyclonal Ab H-300). NKA amounts were similar in the normotensive and hypertensive groups (Fig. 8a, “imput”). NKA activity was higher for hypertensive (Fig. 3a, “precipitate,” lane H) than for normotensive subjects (Fig. 8a, “precipitate,” lane N), indicating a higher amount of acetylated tubulin/NKA complex in membranes from the former group. NKA was not precipitated by Sepharose beads linked to an irrelevant antibody (Fig. 3a, “precipitate,” lane CI). Western blot analysis of non-precipitated fractions showed consistent results (Fig. 8a, supernatant). Means and SD for each experiment are shown below panels of Fig. 8a. Percent of total NKA associated with acetylated tubulin in erythrocyte membranes was ~83% for hypertensive subjects, but only~30% for normotensive subjects.
Fig. 8.
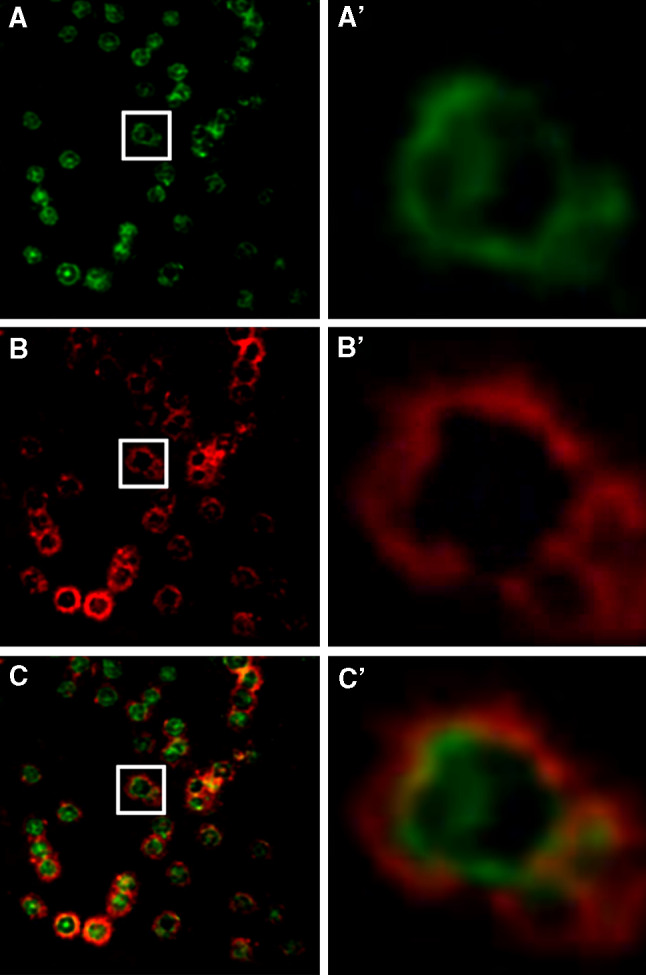
Acetylated tubulin/Na+, K+-ATPase complex in erythrocyte membranes of hypertensive (H) and normotensive (N) subjects. a Input: membranes were solubilized with 0.5% Triton X-100 and analyzed by immunoblot with M7-PB-E9 antibody, specific to NKA. Precipitate: membranes solubilized in detergent were immunoprecipited with Sepharose linked to 6-11B-1 antibody, specific to acetylated tubulin, or CI antibody, specific to phosphatidyl inositol 3-kinase as control. Immunoprecipitate was analyzed by immunoblot with rabbit polyclonal Ab H-300. Supernatant: upper fraction of immunoprecipitate. An equal quantity of membrane protein was loaded in all lanes. NKA bands were quantified using the Scion Image program. Values (mean ± SD from triplicate experiments) are shown below panel A as arbitrary units. ND, not detected. b Co-localization of α-tubulin and NKA. Erythrocytes from normotensive (N) and hypertensive (H) subjects were fixed on coverslips and subjected to double immunofluorescence using antibodies specific to NKA (red) and to α-tubulin (green). Co-localization is observed as yellow. (N′) and (H′): enlargement of area indicated by rectangle in (N) and (H), respectively
The higher level of acetylated tubulin/NKA complex in hypertensive subjects was confirmed by co-localization experiments. Confocal immunofluorescence microscopy showed a small amount of NKA co-localized with membrane tubulin in erythrocytes from normotensive subjects (Fig. 8b, N and N′) and a much larger amount for hypertensive subjects (Fig. 8b, H and H′).
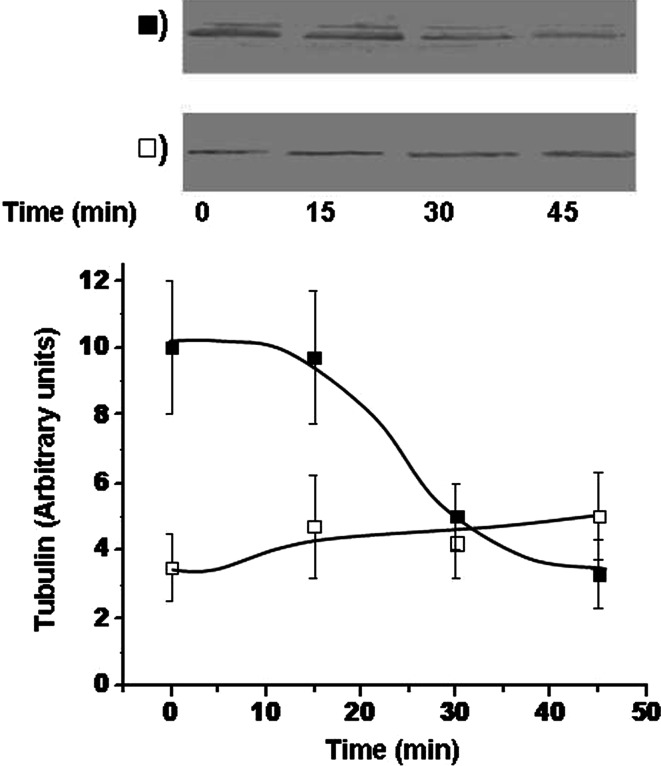
Sedimentable tubulin and Na+, K+-ATPase activity in erythrocytes
Tubulin bound to Sed-tub showed a sensitivity to nocodazole similar to that described for microtubules, that is, when erythrocytes from normotensive subjects were treated with nocodazole for 30 min, ~60% of tubulin from the Sed-tub pool was dissociated, and the cytosolic (soluble) pool was correspondingly increased (Fig. 5). When nocodazole sensitivity of Sed-tub fractions of erythrocytes was compared, we found that Sed-tub was dissociated for the normotensive group, but none was dissociated for the hypertensive group (Fig. 9). This finding suggested that the lower tubulin content in the Sed-tub pool for the hypertensive group (Fig. 6) might result from translocation of tubulin from the Sed-tub to the membrane pool. Perhaps this process involves an intermediate step via the Sol-tub pool. Tubulin in erythrocytes from normotensive subjects shows greater dynamics, which is consistent with the possibility that the Sed-tub pool is the source of tubulin for the increased membrane pool in hypertensive subjects (Fig. 6).
Fig. 9.
Dissociation of tubulin from Sed-tub in erythrocytes of hypertensive subjects; 2 ml blood from hypertensive (open square) and normotensive (filled square) subjects was incubated with 50 μM nocodazol for the indicated times. Erythrocytes were isolated by centrifugation, and Sed-tub fractions were obtained as described in “Materials and methods.” The α-tubulin content of the Sed-tub fraction was analyzed by immunoblot with DM1A antibody. Tubulin bands (results from a representative experiment are shown in upper panels) were quantified, and values (arbitrary units) are shown in the lower panel as mean ± SD from three independent experiments
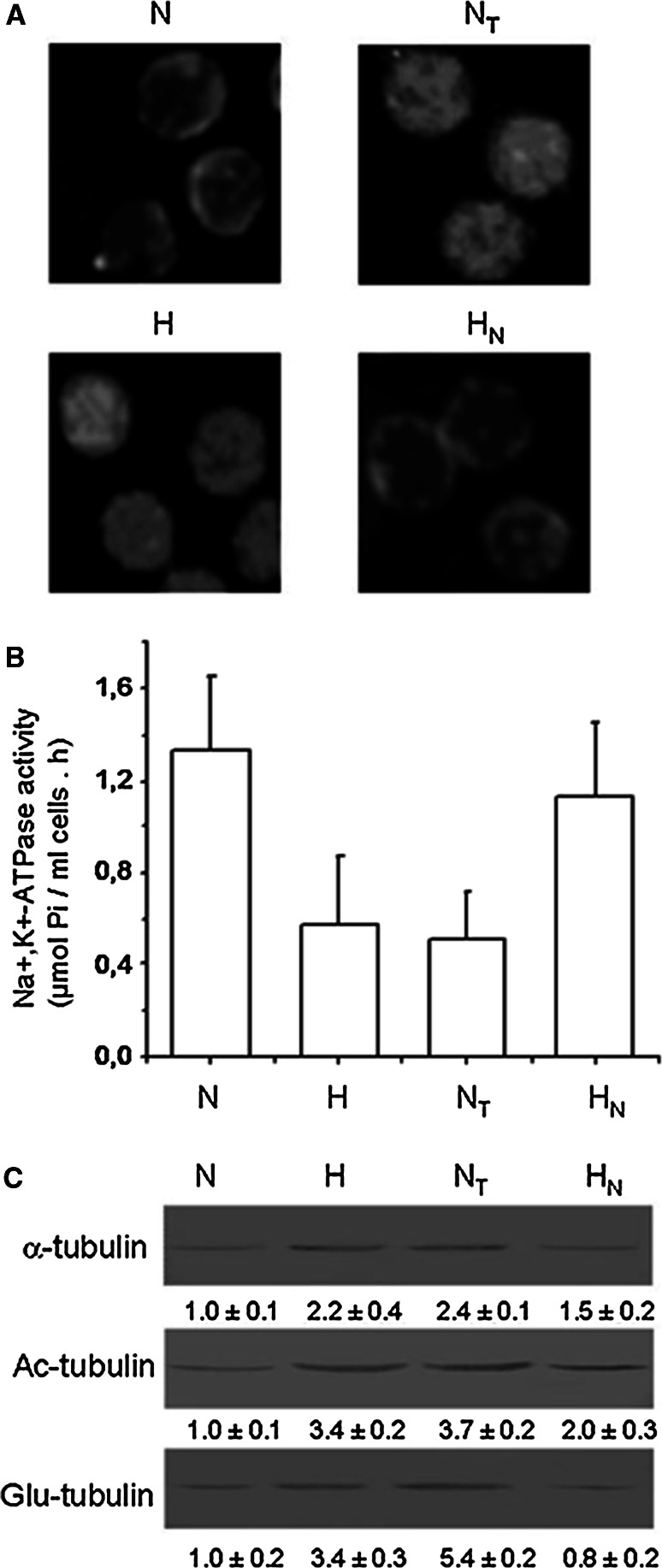
In view of our previous finding that nocodazole treatment dissociates tubulin from the membrane pool of various cell types [30], we investigated whether this also occurs for erythrocytes of hypertensive patients with concomitant recovery of NKA activity. Nocodazole treatment reduced the content of total tubulin in membranes (as well as acetylated and detyrosinated tubulin, Fig. 10c), with a corresponding 110% increase in NKA activity (Fig. 10b) while taxol treatment has no effect (data not shown). Immunofluorescence images show that tubulin was displaced from the membrane (Fig. 10a). On the other hand, treatment of erythrocytes from normotensive subjects with taxol, the microtubule stabilizer, resulted in a 2.4-fold increase of total tubulin content (Fig. 10c) and 60% reduction of NKA activity (Fig. 10b), whereas nocodazole treatment had no effect (data not shown). The immunofluorescence images show that tubulin is redistributed to the membrane (Fig. 10a) .
Fig. 10.
Effects of taxol and nocodazol on Na+, K+-ATPase activity, and tubulin isotype composition in erythrocyte membranes of hypertensive and normotensive subjects. Blood of normotensive subjects was incubated in the presence (NT) or absence (N) of 5 μM taxol for 2 h at 37°C. Blood of hypertensive subjects was incubated in the presence (HN) or absence (H) of 50 μM nocodazol for 2 h at 37°C. Blood was then centrifuged at 100×g for 10 min, and erythrocytes were resuspended in drug-free serum and incubated for 1 h at 37°C. a Erythrocytes were fixed on coverslips and analyzed by indirect immunofluorescence microscopy using mAb DM1A. b Other aliquots of erythrocyte membranes were obtained as described in “Materials and methods” and subjected to determination of NKA activity (7 μg protein). Data are mean ± SD from three independent experiments, expressed as arbitrary units. c Quantification of α-tubulin, acetylated and detyrosinated tubulin bands from a representative experiment. Data shown under blots are mean ± SD from three independent experiments, expressed as arbitrary units
Discussion
In 2007, Goodman et al. [6] compiled a comprehensive list of erythrocyte proteins identified by proteomics technology. The 751 proteins on that list, identified by mass spectrometry, included two alpha chains and one beta chain of tubulin, i.e., α6, α3 and β1. In the present study, we confirmed the presence of α- and β-tubulin (Fig. 1), observed three post-translational modifications of tubulin (detyrosination, tyrosination and acetylation) and found that tubulin is distributed in three operationally distinguishable pools (membrane-associated, sedimentable and soluble).
In view of the reported absence of microtubules in human erythrocytes [4], when these cells were lysed by hypotonic shock and subjected to differential centrifugation, we expected to find tubulin in two subcellular fractions, one bound to membrane (as in other types of cells) and the other in dimeric form in the 100,000×g soluble fraction. We were surprised to find a third tubulin pool (soluble at 20,000×g, sedimentable at 100,000×g), distinct from the membrane and soluble pools. The possibility that this “Sed-tub” was merely a membrane fraction of reduced size was ruled out since detergent treatment of the two fractions gave different results; i.e., tubulin from the membrane fraction that sedimented at 20,000×g was dissociated, whereas tubulin from the sedimentable pool was not. The existence of the Sed-tub pool was confirmed by consistent results in repeated experiments and analysis by confocal immunofluorescence microscopy (Fig. 3). More intriguing was the observed conversion from Sed-tub to Sol-tub caused by nocodazol treatment. Since nocodazole is known to depolymerize (disassemble) microtubules, this finding suggested that the Sed-tub pool might be forming microtubules. This idea is contrary to previous reports that human erythrocytes do not contain microtubules. However, we can consider the possibilities that (1) Sed-tub is arranged in short microtubules that were not detected in the previous studies; (2) tubulin interacts with an unknown sedimentable structure, and this interaction is disrupted by nocodazole. Studies along this line are in progress in our laboratory.
We demonstrated the presence of tyrosinated, detyrosinated and acetylated forms of tubulin in human erythrocytes, using specific antibodies (Fig. 1). At this point, we can only speculate regarding possible functions of these modified tubulins in erythrocytes. We have not investigated whether the tyrosination/detyrosination and the acetylation/deacetylation cycles are active in these cells. One possibility is that enzymes involved in these post-translational modifications continue to control proportions of the isoforms and modulate yet-unknown cell functions. A second possibility is that these tubulin isoforms were produced during the maturation process in precursor erythroblasts and simply remain in mature erythrocytes as residues. A third possibility, in view of our observations that acetylated tubulin interacts with NKA in neural and non-neural cells, resulting in inhibition of the enzyme activity [9, 10, 29–32], is that acetylated tubulin in human erythrocytes has a similar NKA-regulating function. This idea is consistent with our finding that erythrocyte membranes depleted of tubulin by nocodazole or alkaline treatment are able to subsequently re-associate with tubulin; we previously demonstrated the same property in membranes from neural and non-neural cells [29, 30]. The activity of NaKA in human erythrocytes is inhibited by more than 50% in hypertensive patients, and this could be the reason why there is an accumulation of intracellular Na+. As in other cells [29, 30], the enzyme activity of the sodium pump is regulated by acetylated tubulin. We speculate that the human erythrocyte tubulin could be involved in regulating the activity of NKA. In this regard we hope that tubulin plays an important role in the regulation of the enzyme, especially in erythrocytes of patients with hypertension. This being the case, we find alternative ways of regulation from the standpoint of pharmacological activity of the sodium pump.
Results of the present study suggest that NKA in the human erythrocyte membrane is regulated by acetylated tubulin, whose association or dissociation with the enzyme results in inhibition or re-activation, respectively. This conclusion is based on (1) the inverse correlation between NKA activity and tubulin amount observed in the comparison of erythrocyte membranes from hypertensive and normotensive subjects (Fig. 6,7); (2) the much higher proportion of NKA/acetylated tubulin complex in erythrocyte membranes from hypertensive subjects (83%) compared with normotensive subjects (30%) (Fig. 8). In other cell types, nocodazole treatment results in dissociation of this complex and concomitant recovery of NKA activity [9, 29–31]. Nocodazole treatment of erythrocytes from hypertensive subjects gave similar results (Fig. 10), which reinforces the idea that NKA in these cells is regulated by its interaction with acetylated tubulin.
Taxol is known as a microtubule-stabilizing agent. Since human erythrocytes do not contain microtubules, they were not expected to be affected by taxol treatment. Surprisingly, taxol treatment of erythrocytes from normotensive subjects caused increased membrane tubulin and reduced NKA activity (Fig. 10). Immunofluorescence studies (Fig. 10a) suggest that taxol causes re-distribution (translocation) of tubulin from the Sed-tub pool to the membrane pool. This finding is consistent with the concept that increased levels of NKA/acetylated tubulin complex cause decreased NKA activity; i.e., NKA is regulated via its interaction with acetylated tubulin. Translocation of tubulin from the Sed-tub pool to membrane pool, similar to that seen in Fig. 5B, is also observed in erythrocytes from hypertensive subjects (Fig. 6).
The mechanism for such translocation remains to be determined. One possibility is induction by a plasma factor that appears at a certain point in the individual’s life or is generated during erythroblast genesis or maturation. Since tubulin must be acetylated in order to associate with NKA [32], another possibility is that alteration in the equilibrium between acetyl transferase and deacetylase is responsible for the increased level of acetylated tubulin/NKA complex in the membrane.
The commonly used strategy to reduce blood pressure is administration of drugs that increase diuresis. Accordingly, hypertension and cardiovascular diseases are often treated with inhibitors of the Na+/Ca2+ exchanger or with an ouabain antagonist to increase the flow of Na+ out of the cell [42]. Some of these drugs, such as PST2238 and SEA0400, are being clinically studied as therapeutic agents [42, 43].
The relationship between altered distribution of acetylated tubulin in erythrocytes from hypertensive subjects and hypertension itself remains to be determined. It is certainly conceivable that reduced activity of NKA due to its interaction with acetylated tubulin is involved in alteration of some property of erythrocytes that leads to increased blood pressure. It is likely that the increase in membrane tubulin modifies rheological properties of erythrocytes, such as deformability and viscosity, and this affects blood pressure. Studies along this line are in progress in our laboratory.
Acknowledgments
We thank Dr. S. Anderson for the English editing. This study was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica de la Secretaría de Ciencia y Tecnología del Ministerio de Cultura y Educación en el marco del Programa de Modernización Tecnológica (BID 802 OC/AR, BID 1728/OC-AR), Consejo Nacional de Investigaciones Científicas y Técnicas (Conicet), Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto y de la Universidad Nacional de Córdoba y Ministerio de Ciencia y Técnica de la Provincia de Córdoba.
Abbreviations
- PMSF
Phenylmethyl-sulfonyl-fluoride
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
- 1.Cohen WD. The cytoskeletal system of nucleated erythrocytes. Int Rev Cytol. 1991;130:37–84. doi: 10.1016/S0074-7696(08)61501-6. [DOI] [PubMed] [Google Scholar]
- 2.Cohen WD, Cohen MF, Tyndale-Biscoe CH, VandeBerg JL, Ralston GB. The cytoskeletal system of mammalian primitive erythrocytes: studies in developing marsupials. Cell Motil Cytoskeleton. 1990;16:133–145. doi: 10.1002/cm.970160207. [DOI] [PubMed] [Google Scholar]
- 3.Liao EC, Paw BH, Peters LL, Zapata A, Pratt S, Do C, Lieschke G, Zon L. Hereditary spherocytosis in zebrafish riesling illustrates evolution of erythroid α-spectrin structure, and function in red cell morphogenesis and membrane stability. Development. 2000;127:5123–5132. doi: 10.1242/dev.127.23.5123. [DOI] [PubMed] [Google Scholar]
- 4.van Deurs B, Behnke O. The microtubule marginal band of mammalian red blood cells. Z Anat Entwicklungsgesch. 1973;143:43–47. doi: 10.1007/BF00519909. [DOI] [PubMed] [Google Scholar]
- 5.Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Ann Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- 6.Goodman SR, Kurdia A, Ammann L, Kakhniashvili D, Daescu O. The human red blood cell proteome and interactome. Exp Biol Med (Maywood) 2007;232:1391–1408. doi: 10.3181/0706-MR-156. [DOI] [PubMed] [Google Scholar]
- 7.Nuñez Fernandez M, Beltramo DM, Alonso AC, Barra HS. Conversion of hydrophilic tubulin into a hydrophobic compound. Evidence for the involvement of membrane proteins. Mol Cell Biochem. 1997;170:91–98. doi: 10.1023/A:1006861416448. [DOI] [PubMed] [Google Scholar]
- 8.Chae YC, Lee S, Lee HY, Heo K, Kim JH, Kim JH, Suh PG, Ryu SH. Inhibition of muscarinic receptor-linked phospholipase D activation by association with tubulin. J Biol Chem. 2005;280:3723–3730. doi: 10.1074/jbc.M406987200. [DOI] [PubMed] [Google Scholar]
- 9.Arce CA, Casale CH, Barra HS. Submembraneous microtubule cytoskeleton: regulation of ATPases by interaction with acetylated tubulin. FEBS J. 2008;275:4664–4674. doi: 10.1111/j.1742-4658.2008.06615.x. [DOI] [PubMed] [Google Scholar]
- 10.Zampar GG, Chesta ME, Carbajal A, Chanaday NL, Díaz NM, Casale CH, Arce CA. Acetylated tubulin associates with the fifth cytoplasmic domain of Na(+)/K(+)-ATPase: possible anchorage site of microtubules to the plasma membrane. Biochem J. 2009;422:129–137. doi: 10.1042/BJ20082410. [DOI] [PubMed] [Google Scholar]
- 11.Dustin P. Microtubules. Sci Am. 1980;243:66–76. doi: 10.1038/scientificamerican0880-66. [DOI] [PubMed] [Google Scholar]
- 12.Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 13.Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nature Rev. 2003;4:938–947. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]
- 14.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 15.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerve. Biochim Biophys Acta. 1957;23:394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- 16.Sudhakar K, Sujatha M, Devi CV, Reddy PP. Erythrocyte sodium and Na+, K+-ATPase activity in untreated hypertensives and their first degree relatives. Indian J Biochem Biophys. 1998;35:382–384. [PubMed] [Google Scholar]
- 17.Kim M, Kwon J, Suh S, Suh J, Jung J, Lee S. Transgenic overexpression of translationally controlled tumor protein induces systemic hypertension via repression of Na+, K+-ATPase. J Mol Cell Cardiol. 2008;44:151–159. doi: 10.1016/j.yjmcc.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 18.de Mendonca M, Grichois ML, Garay RP, Sassard J, Ben-Ishay D, Meyer P. Abnormal net Na+, and K+ fluxes in erythrocytes of three varieties of genetically hypertensive rats. Proc Natl Acad Sci USA. 1980;77:4283–4286. doi: 10.1073/pnas.77.7.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garay RP, Dagher G, Pernollet MG, Devynck MA, Meyer P. Inherited defect in a Na+, K+-co-transport system in erythrocytes from essential hypertensive patients. Nature. 1980;284:281–283. doi: 10.1038/284281a0. [DOI] [PubMed] [Google Scholar]
- 20.Rykdelski D, Kropp D, Duran NN. Hypertension and the Na+–K+ pump. Fed Proc. 1981;40:611. [Google Scholar]
- 21.Aderounmu AF, Salako LA. Abnormal cation composition and transport in erythrocytes from hypertensive patients. Eur J Clin Invest. 1979;9:369–375. doi: 10.1111/j.1365-2362.1979.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 22.Zicha J, Negrin CD, Dobesová Z, Carr F, Vokurková M, McBride MW. Altered Na+–K+ pump activity and plasma lipids in salt-hypertensive Dahl rats: relationship to Atp1a1 gene. Physiol Genomics. 2001;6:99–104. doi: 10.1152/physiolgenomics.2001.6.2.99. [DOI] [PubMed] [Google Scholar]
- 23.Vokurková M, Nováková O, Dobešová Z, Kuneš J, Zicha J. Relationships between membrane lipids and ion transport in red blood cells of Dahl rats. Life Sci. 2005;77:1452–1464. doi: 10.1016/j.lfs.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Lijnen P, M’Buyamba-Kabangu J, Fagard R, Staessen J, Amery A. Erythrocyte concentrations and transmembrane fluxes of sodium and potassium in essential hypertension: Role of intrinsic and environmental factors. Cardiovasc Drugs Ther. 1990;4:321–333. doi: 10.1007/BF02603172. [DOI] [PubMed] [Google Scholar]
- 25.Ambrosini E, Costa FV, Montebugnoli L, Tartagni F, Magnani B. Increased intralymphocytic sodium content in essential hypertension. Clin Sci. 1981;61:181–186. doi: 10.1042/cs0610181. [DOI] [PubMed] [Google Scholar]
- 26.Boon NA, Harper C, Aronson JK, Grahame-Smith DG. Cation transport functions in vitro in patients with untreated essential hypertension: A comparison of erythrocytes and leucocytes. Clin Sci. 1985;68:511–515. doi: 10.1042/cs0680511. [DOI] [PubMed] [Google Scholar]
- 27.Ferrandi M, Salardi S, Tripodi G, Barassi P, Rivera R, Manunta P. Evidence for an interaction between adducin and Na(+)-K(+)-ATPase: relation to genetic hypertension. Am J Physiol. 1999;277:H1338–H1349. doi: 10.1152/ajpheart.1999.277.4.H1338. [DOI] [PubMed] [Google Scholar]
- 28.Bianchi G, Tripodi G, Casari G, Salardi S, Barber BR, Garcia R. Two point mutations within the adducin genes are involved in blood pressure variation. Proc Natl Acad Sci USA. 1994;91:3999–4003. doi: 10.1073/pnas.91.9.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casale CH, Previtali G, Barra HS. Involvement of acetylated tubulin in the regulation of Na+, K+-ATPase activity in cultured astrocytes. FEBS Lett. 2003;534:115–118. doi: 10.1016/S0014-5793(02)03802-4. [DOI] [PubMed] [Google Scholar]
- 30.Casale CH, Previtali G, Serafino JJ, Arce CA, Barra HS. Regulation of acetylated tubulin/Na+, K+-ATPase interaction by L-glutamate in non-neural cells: involvement of microtubules. Biochim Biophys Acta. 2005;1721:185–192. doi: 10.1016/j.bbagen.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Casale CH, Alonso AC, Barra HS. Brain plasma membrane Na+, K+-ATPase is inhibited by acetylated tubulin. Mol Cell Biochem. 2001;216:85–92. doi: 10.1023/A:1011029125228. [DOI] [PubMed] [Google Scholar]
- 32.Santander VS, Bisig CG, Purro SA, Casale CH, Arce CA, Barra HS. Tubulin must be acetylated in order to form a complex with membrane Na+, K+-ATPase and to inhibit its enzyme activity. Mol Cell Biochem. 2006;291:167–174. doi: 10.1007/s11010-006-9212-9. [DOI] [PubMed] [Google Scholar]
- 33.Gundersen GG, Kalnoski MH, Bulinski JC. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- 34.Shelanski ML, Gaskin F, Cantor CR. Microtubule assembly in the absence of added nucleotides. Proc Nat Acad Sci USA. 1973;70:765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sloboda RD, Rosenbaum JL. Purification and assay of microtubule associated proteins (MAPs) Methods Enzymol. 1982;85:409–416. doi: 10.1016/0076-6879(82)85041-6. [DOI] [PubMed] [Google Scholar]
- 36.DeWitt ND, dos Santos CF, Allen KE, Slayman CW. Phosphorylation region of the yeast plasma membrane H+-ATPase. Role in protein folding and biogenesis. J Biol Chem. 1998;273:21744–21751. doi: 10.1074/jbc.273.34.21744. [DOI] [PubMed] [Google Scholar]
- 37.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 38.Salvador JM, Mata AM. Purification of the synaptosomal plasma membrane (Ca2+/Mg2+)-ATPase from pig brain. Biochem J. 1996;315:183–187. doi: 10.1042/bj3150183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 40.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principles of dye-protein binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 41.Beltramo DM, Nuñez M, Alonso AD, Barra HS. The relationship of hydrophobic tubulin with membranes in neural tissue. Mol Cell Biochem. 1994;141:57–63. doi: 10.1007/BF00935591. [DOI] [PubMed] [Google Scholar]
- 42.Iwamoto T, Watanabe Y, Kita S, Blaustein MP. Na+/Ca2+ exchange inhibitors: a new class of calcium regulators. Cardiovasc Hematol Disord Drug Targets. 2007;7:188–198. doi: 10.2174/187152907781745288. [DOI] [PubMed] [Google Scholar]
- 43.Manunta P, Ferrandi M, Messaggio E, Ferrari P. A new antihypertensive agent that antagonizes the prohypertensive effect of endogenous ouabain and adducing. Cardiovasc Hematol Agents Med Chem. 2006;4:61–66. doi: 10.2174/187152506775268811. [DOI] [PubMed] [Google Scholar]