Abstract
The inner nuclear membrane harbors a unique set of membrane proteins, many of which interact with nuclear intermediate filaments and chromatin components and thus play an important role in nuclear organization and gene expression regulation. These membrane proteins have to be constantly transported into the nucleus from their sites of synthesis in the ER to match the growth of the nuclear membrane during interphase. Many mechanisms have evolved to enable translocation of these proteins to the nucleus. The full range of mechanisms goes from rare autophagy events to regulated translocation using the nuclear pore complexes. Though mechanisms involving nuclear pores are predominant, within this group an enormous mechanistic range is observed from free diffusion through the peripheral channels to many distinct mechanisms involving different nucleoporins and other components of the soluble protein transport machinery in the central channels. This review aims to provide a comprehensive insight into this mechanistic diversity.
Keywords: Nuclear envelope, Inner nuclear membrane, Outer nuclear membrane, Endoplasmic reticulum, Translocation, Nuclear pore complex, Membrane trafficking, Phenylalanine-glycine (FG)
Introduction
Since the nuclear envelope (NE) first appeared roughly 3 billion years ago, the cell has had to deal with the problem of getting proteins into the nucleus. This problem was solved for soluble proteins through evolution of the nuclear pore complexes (NPCs), greater than 60 MDa structures containing roughly 30 distinct proteins in multiple copies to create a channel for regulated transport of macromolecules in and out of the nucleus (reviewed in [1]). Considerable work on transport of soluble cargos through the NPC has revealed a wide range of transport receptors that interact with different cargos via nuclear localization signals (NLSs) on the cargos. Though there are many different transport receptors, they all appear to function through a common mechanism. In contrast, the mechanism underlying translocation of transmembrane proteins has only recently come under scrutiny, but there appears to be a much wider range of translocation mechanisms for membrane proteins than there is for soluble transport.
The physical barrier of the NE is far stronger and more complex than that of the plasma membrane. While the plasma membrane is a single membrane, the NE is a membrane system with outer and inner nuclear membranes (ONM and INM) separated by a lumen rather like the moat of a castle. The ONM is continuous with the ER [2] and forms the outer cover of the nucleus facing the cytoplasm (Fig. 1). The ONM covers the nucleus completely, except for areas where the NPCs are inserted [2]. At these sites the ONM bends with both convex and concave curvature to form a unique channel structure at what is sometimes called the pore membrane. The pore membrane flows into the inner line of defense for the NE, the INM. Both INM and ONM contain partially unique sets of membrane proteins called NETs for nuclear envelope transmembrane proteins (NETs). Most NETs accumulate particularly in the INM and many interact with the lamin intermediate filament polymer, which lines the INM and gives structure to the NE (reviewed in [3, 4]). The 3 billion years of evolutionary pressures since the first eukaryotes were formed by the appearance of the NE has given this structure ample time to evolve many complex and redundant mechanisms for transport of these NETs.
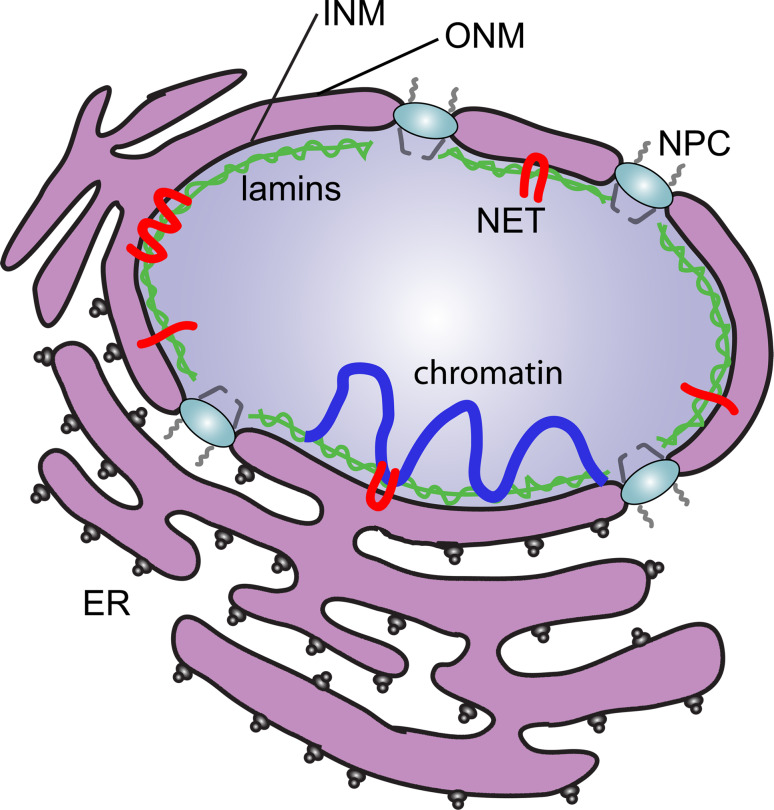
Fig. 1.
The organization of the NE. The NE is a double-membrane system. The ONM is continuous with the ER and fuses with the INM where the NPCs are inserted. Underlying the INM is the lamin intermediate filament polymer (green). NE transmembrane proteins (NETs; red) of the INM interact with both lamins and chromatin (blue) that is proximal to the NE
The NE breaks down during mitosis in higher eukaryotes: so NETs could gain access at this time. However, the density of NETs remains relatively constant during interphase while the nucleus and correspondingly the NE grows three to fourfold during this time to accommodate transcription and replication. Thus NETs clearly need a mechanism to enter the nucleus during interphase. NETs, like all proteins, are synthesized in the ER and as transmembrane proteins most should be co-translationally inserted into the ER membrane. Some tail-anchored NETs may be post-translationally inserted, but this needs to be directly tested on a case-by-case basis, as many aspects of NET targeting and topology are not well understood. As the ONM is continuous with the ER the NETs embedded in the ER membranes should in theory be able to freely diffuse in the membrane until they reach the ONM. At this point they still have to cross the NE barrier to reach the INM. Considering the hermetic nature of the NE, which covers the nucleus completely except where NPCs are inserted, there are only four theoretically possible routes for a NET to access the INM: (1) regulated vesicle fusion through the two membranes (Fig. 2a), (2) membrane ruptures such as in autophagy (Fig. 2b), (3) insertion via a chaperone-translocon mechanism similar to that in mitochondria (Fig. 2c), or (4) translocation via the NPCs (Fig. 2d).
Fig. 2.
Theoretically possible routes for a NET to access the INM. a NETs could be transported from the ONM to the INM by membrane vesicles deriving from the ONM, which would fuse with the INM. b NETs could reach the INM by membrane ruptures. When the NE is ruptured, the ruptured piece could be re-integrated in an inverted fashion (the INM piece could be incorporated into the ONM and the ONM piece into the INM) to repair the NE. In this case, a NET residing in the ONM piece would thus be integrated into the INM. c NETs could be transported from the ONM into the INM by channels residing in both the ONM and the INM of the NE similar to inner and outer membrane channels in mitochondria. d NETs could use the NPC sites to translocate from the ONM into the INM
For the first, translocation by vesicle fusion events has been extensively studied in the plasma membrane, the ER and the Golgi apparatus. These events are energy-, temperature- and calcium-dependent (reviewed in [5–7]). Fusion events in these membrane compartments have been also linked to specific proteins that promote vesicle fusion such as SNAREs and NSF. Two important proteins responsible for vesicle fusion events are p97 and p47 [8, 9], which were shown to be important for NE assembly and growth in one system. This system assembles vesicles from Xenopus oocyte extracts onto demembraned sperm chromatin [10]. This forms a fully functional NE around the chromatin that can also grow when chromatin is decondensed and induced to replicate [11]. In this system depletion of p97 blocked NE assembly and depletion of p47 blocked nuclear growth [12], suggesting that vesicle fusion events could also play a role in NET access during interphase. However, unlike the in vitro Xenopus system, the ER is not vesiculated in intact interphase cells. Thus it is generally thought that interphase NE growth in intact cells is more likely to be due to ER membrane streaming into the adjacent NE and correspondingly it would be less likely for NET translocation to occur by this mechanism. Indeed, studies where vesicle fusion was blocked in cultured cells [13] and even intact organisms [14] did not yield any notable NET translocation defects in the time frame analyzed. Nonetheless, recent large-scale proteomic analyses of the NE [15–17] have identified many proteins involved both in membrane synthesis and in vesicle fusion at the NE. The former suggests that the nuclear membrane may grow by synthesizing its own lipids rather than from ER lipids flowing into the INM and the latter indicates that vesicle fusion mechanisms at least stand in reserve to function for NE membrane growth and NET translocation.
The second theoretically possible route would likely involve autophagy (reviewed in [18]), which may have been commonly used in early eukaryotes as it is the most ancient system in terms of the proteins involved. Nuclear autophagy is a normal process activated by DNA damage [19] and appears to now be principally a mechanism for cleaning nuclei of damaged material or extra chromosomes resulting from inaccurate mitoses. Mutants defective in nuclear autophagy show gene amplification, increased DNA damage, chromosome instability, and aneuploidy [20, 21]. A recent study on NE ruptures due to an autophagy-like mechanism revealed that in standard tissue culture cells transient NE breaks are readily repaired [22]. A wide range of proteins is exchanged in both directions between the nucleus and the cytoplasm/ER when these breaks occur. These ruptures occur at greater frequency when cells carry lamin mutations [22] and when the NE is attacked during virus infection [23–25]. While this is clearly not a continuous active transport mechanism, the full machinery for both rupture and repair is active at the NE and capable of functioning when defects occur in the main translocation pathways.
The third route would involve passage through the membrane in the absence of membrane fusion or rupturing events. Such a mechanism is well characterized in mitochondria where proteins of the inner mitochondrial membrane are unfolded and kept from aggregating by chaperone proteins that then facilitate their threading through small channels in the outer membrane. The proteins are then refolded and properly integrated at the inner mitochondrial membrane (reviewed in [26]). This route is not very likely at the NE because only a small subset of mitochondrial channel related proteins were found in NE proteomics datasets and they appear to be of low abundance, consistent with their being contaminants [15–17].
The fourth theoretical route for NET transport into the INM is to go through the NPCs as these are the only places in the NE where gaps occur in the covering membrane. Note though that there is no actual gap in the lipid bilayers here because the ONM and INM bend with both convex and concave curvature at these sites to fuse forming the pore membrane, thus keeping the lumen of the NE completely sealed. Careful NPC reconstruction by cryoelectron microscopy indicates that there are peripheral channels of ~10 nm between the core NPC structure and the pore membrane (Fig. 3) [27, 28]. Though three core NPC proteins are inserted in the membrane, these are spaced so that the peripheral channels could accommodate a protein diffusing in the membrane with a globular nucleoplasmic mass of <60 kDa [29–31]. This contrasts with translocation through the ~39-nm-diameter central channel of the NPC that can accommodate even assembled ribosomes and virus particles [32, 33]. Thus for membrane proteins, two paths can be envisioned: to be extracted from the membrane and go through the central channel of the NPC or to diffuse in the membrane in the peripheral channels around the NPC. Within these two paths there appears to be several distinct mechanisms functioning, including one that seems to combine the two; thus the rest of this review will focus on the range of translocation mechanisms through or around the NPCs.
Fig. 3.
Two distinct channels in the NPC. Each NPC has one central channel with a diameter of ~39 nm. In addition, each NPC harbors two peripheral channels, which reside between the pore membrane and the structural components of the NPC
Licensing or free diffusion—how do NETs get to the translocation site after synthesis?
Before the actual translocation step, some NETs might need to be licensed for recognition by the NPC machinery. After synthesis and membrane insertion in the ER, NETs should be able to freely diffuse in the lipid bilayer throughout the ER and so eventually find themselves in the ONM (Fig. 4b), which appears to be continuous with the ER with no obstructions [2]. Indeed free diffusion in the membrane not just between the ER and the ONM but also through to the INM was proposed nearly 20 years ago to be the main mechanism for nuclear translocation of NETs [34, 35]. Once in the ONM, based on average NPC density in a cell [36, 37] NETs should encounter an NPC within less than 1 s [38]. However, it is possible that some mechanism exists to direct NETs inwards, towards the ONM as opposed to the Golgi or at least for NETs to be recognized and retained at the NPCs in order to increase the probability of a translocation event. Such mechanisms could involve signals in the NET sequence, a special post-translational modification, or partner proteins that bind co-translationally.
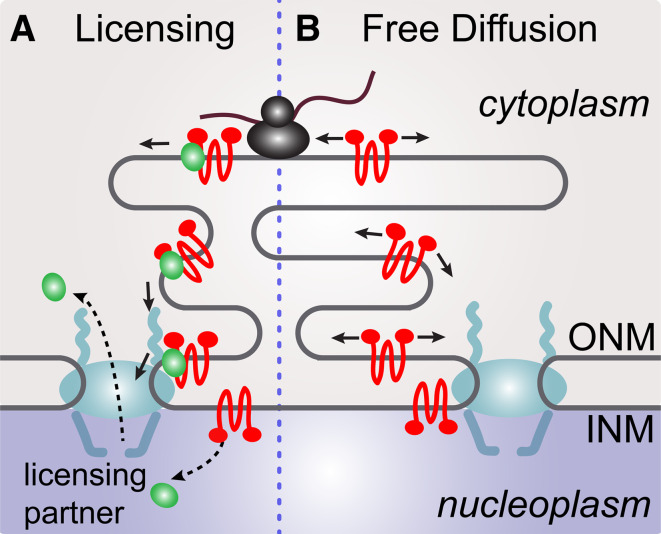
Fig. 4.
Transport of NETs from the sites of synthesis to the sites of translocation. After synthesis in the ER NETs need to reach the ONM to further translocate into the INM. a The NET can reach the site of translocation by a licensing step where it is actively delivered to the site of translocation. b Another way to reach the site of translocation would be by simple and undirected diffusion
A recent study that directly compared the diffusion characteristics of a large set of NETs under identical conditions found that in the ER two diffusional mobilities could be observed: one with a FRAP t 1/2 at ~6 s and the other ~10 s [38]. The faster set thus likely moves freely within the ER without any constraints while the slow set may be clustered with other proteins, perhaps in raft-like assemblies to slow the diffusion. Indeed, recent work suggests that some NETs have two populations with one functioning in the nucleus and the other functioning in the ER/ONM. For example, emerin binds lamins and transcriptional regulators in the nucleus and interacts with the centrosome outside the nucleus [39]. Another reason for the slower diffusion could be association with proteins that have roles in targeting such as transport receptors to license the NETs for INM translocation. This association raises an important concern for interpretation of data from studies analyzing the targeting of proteins to the INM and may apply for some of the studies described in this review: there is a possibility that association signals such as NLSs at the amino-terminus of a tail-anchored transmembrane protein might have sufficient time to bind to transport receptors prior to the membrane insertion and compete with the insertion machinery. In this case, the proteins may not be inserted into the membrane until after they have been translocated into the nucleus. This would also require having machinery for membrane insertion in the INM, which has not been determined yet: some such proteins were identified in NE proteomic datasets [15–17], but they could be in either the INM or the ONM. If in the ONM their function would not be distinct from that in the ER as the ONM is continuous with the ER and is studded with ribosomes.
Some NETs undergo licensing steps that navigate them to the NE (Fig. 4a). Several studies focusing on early stages of membrane insertion of nascent NETs found that NETs interact with ER components responsible for integration of nascent membrane proteins into the ER membrane [40]. Moreover, sorting motifs were found in NETs suggesting that NETs could be distinguished from membrane proteins destined for other cellular compartments. The sorting motifs would also direct the NET trafficking from the ER towards the translocation sites at the NE as additionally supported by the finding that a membrane-associated importin isoform, importin-α-16 (KPNA-4-16), interacted with NETs during and beyond their co-translational insertion into the ER membrane [40, 41]. SUN proteins also have such sorting motifs [42]. Though the evidence is strong for such licensing, thus far it has only been observed for a few NETs: LBR, nurim, Heh2, and Unc84A [42–44].
Like active sorting towards the destined compartment, other signals could prevent NETs from being retained in non-NE membrane compartments. In support of this, a recent study found a Golgi retrieval signal on the NET SUN2, which when mutated caused an accumulation of SUN2 in the Golgi [45].
Different mechanisms promote the ONM to INM translocation step
Nuclear envelope transmembrane proteins must be transported constantly to the INM because the NE surface grows three to fourfold during interphase while its protein density remains largely the same [46, 47]. The 10-nm peripheral channels of the NPC [27, 28] should enable transport of proteins in interphase while embedded in the membrane, provided their nucleoplasmic mass is less than 60 kDa. That a mechanism must exist for translocation of INM proteins was supported by observations that viral membrane proteins can diffuse freely between the ER/Golgi and the INM [48] and that INM proteins could move between nuclei in fused heterokaryons when protein synthesis was blocked [49].
The lateral diffusion–retention hypothesis
These observations were the basis of the “lateral diffusion–retention hypothesis”. The lateral diffusion part of this hypothesis postulated that both NETs and ER proteins diffuse laterally in the membrane without obstruction. In this way, they could move between the ER/ONM and INM at equilibrium without being extracted from the membrane using the peripheral (also termed lateral) NPC channels (Fig. 5a). However, for the retention part of the hypothesis NETs would accumulate in the INM because of their ability to bind nuclear components such as lamins and chromatin. This hypothesis was tested by increasing the size of the nucleoplasmic domain of a reporter using sequences known to bind to lamins [34, 35].
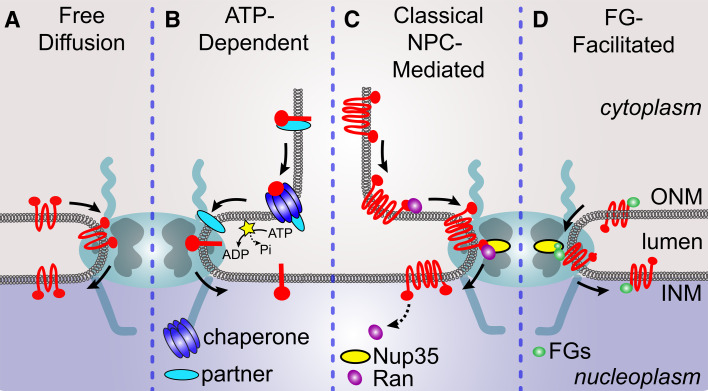
Fig. 5.
NETs translocate from the ONM into the INM by four distinct mechanisms. a NETs translocate by simple lateral diffusion using the peripheral channels of the NPC. b NETs require ATP prior to translocation. The ATP could be needed for a potential disassociation of other proteins from the NET in the ER, for example chaperones, which would release the NET prior to its translocation into the INM. c In order to translocate, NETs utilize classical NPC-mediated nuclear import machinery components such as the GTPase Ran and several transport receptors. This process requires nucleoporins such as the peripheral channel FG nucleoporin Nup35 (in yeast Nup53). d Translocation of NETs is facilitated by the presence of FGs in the NET sequence. This mechanism additionally requires the FG nucleoporin Nup35
To test the retention part of the hypothesis a study used the lamin B receptor (LBR), which binds lamin B through its nucleoplasmic domain [50, 51]. This region of LBR was fused to the transmembrane span of chicken hepatic leptin, which is a membrane protein that normally resides in the ER and the plasma membrane but not in the INM [52]. Fusing the LBR lamin-binding fragment enabled the hepatic lectin to accumulate in the INM, arguing that retention in the INM is the dominant factor in its targeting [34]. For this to be the case, the protein must sample both the ER/ONM and the INM until it finds a binding/retention site. Two further studies obtained similar results using lamin-binding regions of the NETs LAP2β [53] and MAN1 [54], respectively. The retention part of the hypothesis was additionally supported by the fact that FRAP on NETs in the NE never reached prebleach fluorescence levels [38, 54–60]. Thus some of the NET pools must be highly immobile, presumably due to interactions with chromatin and/or lamins. The opposite experiment also supported this retention postulate: when the nucleoplasmic retention partner (lamin A) of the NET emerin was absent, emerin became more mobile [58].
To test the peripheral channel diffusion part of the hypothesis, the LBR nucleoplasmic region was multiplied in tandem. This region is roughly 22 kDa and so is well below the predicted 60-kDa diffusion limit. Two copies of this region are also below the predicted diffusion limit, and accordingly this construct also translocated to the INM. However, three copies is above the diffusion limit and this construct did not accumulate in the INM [35]. Later studies confirmed this observation and further demonstrated that approaching the 60-kDa limit reduced INM accumulation without blocking it completely [13, 54]. In further support of the 60-kDa size exclusion limit, hundreds of transmembrane proteins from NE proteomic datasets exhibit a significant difference between the nucleoplasmic/cytoplasmic mass (which would in theory migrate in the peripheral channels) and the lumenal mass (which should not be affected by the size of the peripheral channels). In contrast, there is no such difference between these two masses for other transmembrane proteins encoded by the human genome (Fig. 6a).
Fig. 6.
Bioinformatic analysis on a large set of NETs supports the use of the peripheral channels and the use of FGs in translocation. Human proteins that had at least one annotated transmembrane domain in Ensembl 64 were examined. To ensure no bias was given to genes with multiple transcripts, only the largest protein per gene was analyzed. This set of proteins was divided into two subsets: those that were detected or had an ortholog detected in NEs isolated from liver [16], blood [15], and muscle tissue [17] (NETs) and those that were not detected in NEs. a Median nucleoplasmic mass of NETs is much smaller than the diffusion limit of the peripheral channels and also much smaller than their lumenal mass. TMHMM 2.0c was run on the dataset to determine lumenal (Lum) and nucleoplasmic (Nuc) regions of NETs and lumenal (Lum) and cytoplasmic (Cyt) regions of membrane proteins not residing in the NE. The number of amino acids in each region was recorded for each protein and represented by a Tukey’s boxplot. NETs exhibit a large difference between the median nucleoplasmic mass and the lumenal mass. In contrast, very little difference is observed between the cytoplasmic and the lumenal mass for other transmembrane proteins encoded by the human genome. b FGs are enriched in NETs. The number of FG amino acid pairings was counted in each protein and classified into one of the three groups shown (0, 1–4, or >4 FGs). Analysis of FG content in NETs identified by proteomics reveals an enrichment of FGs compared to all other membrane proteins encoded by the human genome
ATP- and temperature-dependence for NET translocation
The unrestrained lateral diffusion–retention hypothesis went unchallenged until 10 years later a study suggested that NET translocation involves a more complex mechanism that requires energy [13]. This study took advantage of the fact that FRB and FKBP bind at high affinity in the presence of the drug rapamycin [61, 62] to develop a reporter assay in which nuclear retention could be rapidly induced. For this a NET transmembrane region was fused to GFP and FRB while a soluble fragment containing the NET lamin-binding region was fused to FKBP. Thus the change in dynamics from the steady state of the transmembrane protein could be observed live upon linking the membrane-spanning segment to the nuclear retention fragment. At steady state, the reporter was mostly distributed throughout the ER with a weak nuclear rim whereas upon addition of rapamycin a much stronger nuclear rim signal was observed [13]. The INM accumulation of the reporter construct was inhibited by decreasing temperature, but not by inhibiting vesicle fusion. Thus the temperature dependence likely indicated an energy-dependent step, and accordingly INM accumulation was inhibited when cells were depleted of ATP.
This energy requirement is not general, however, because only two of six endogenous NETs tested for energy dependence in a later study yielded defects in INM accumulation upon ATP depletion [38]. Moreover, these two NETs also had reduced mobility in the ER upon ATP depletion, suggesting that energy could be needed for a licensing step such as those described earlier [40, 41, 43, 44] or possibly for chaperone-mediated disassociation of NET dimers (e.g., SUN proteins self-interact [63]) in order for the total mass to be small enough to pass through the size-limited peripheral channels (Fig. 5b).
A classical NPC-mediated mechanism for NET translocation
The finding that a transport receptor isoform was involved in targeting of NETs to the INM together with the finding that an NPC protein is involved in this process suggested that the NPC actively facilitates membrane protein translocation into the nucleus using components of the soluble nuclear transport machinery (Fig. 5c). This was further tested for several transport factors and nucleoporins using yeast as a model system. Two NLS-containing NETs that normally reside in the INM, Heh1 and Heh2, failed to localize to the INM when the import receptors importin-α and importin-β were depleted. This suggested that transport receptors, similar to the nuclear import of soluble proteins, could also be responsible for NET targeting to the INM via binding to the NLSs on the cargos [64]. Supporting this idea the NET translocation was NLS-dependent, with their NET failing to localize to the INM when the NLS was mutated or deleted.
Other transport factors in addition to transport receptors are also involved in NET translocation. The NLS-containing NET LBR, for example, binds Ran [65] and importins [43], both of which are components of the classical NPC-mediated import pathway for soluble proteins, and LBR translocation was inhibited when Ran function was impaired [38]. Additionally, another NLS-containing NET, SUN2, was found to bind importins [45]. The fact that both LBR and SUN2 contained NLSs led to the speculation that NLSs could be the main characteristic for a requirement to use the classical NPC-mediated pathway [66]. However, fusion of a classical NLS to several mammalian NETs failed to confer the dependence on Ran characteristic of this classical transport pathway [38]. Clearly, the function of NLSs in transport of transmembrane proteins is more complex than originally indicated. This is further supported by findings that mutation of the NLS in full-length SUN2 did not significantly alter the NE accumulation, but when combined with other mutations the NLS mutation greatly reduced NE accumulation [45]. Correspondingly, another study found that an NLS could confer INM targeting to a transmembrane protein when combined with an unstructured region [67]. These findings together indicate that an NLS is insufficient in itself to target the NET for translocation through this pathway using classical soluble transport components and emphasizes the need to check each NET individually regardless of the prediction of an NLS.
Phenylalanine-glycine (FG)-facilitated translocation
Though details of the mechanism remain highly contentious [68, 69] it is widely accepted that the nuclear import of soluble proteins occurs via interactions of phenylalanine-glycine (FG) motifs on both transport receptors and nucleoporins [70]. Presumably, the FGs of nucleoporins interact somehow to create a hydrophobic environment in the central channel and coating cargos with transport receptors that present FGs on the outer surface of the cargo-receptor complex enables the cargos to negotiate this hydrophobic environment.
Though most amino acid pairings will occur many times in an average protein, the FG pairing is rather unusual: ~3% of all proteins encoded in the human genome carry five or more FG pairings. FGs on transport receptors occur on the surface, but without any special context. In contrast, the FGs on nucleoporins tend to favor a context with particular amino acids preceding them such as FxFG or GLFG. They also tend to be in unstructured domains with prolines and glycines between them. Though most FG-containing nucleoporins reside in the central channel of the NPC, the most accurate model to date for the positioning of NPC proteins places some also in the peripheral channels [71]. This would suggest that NETs might also translocate with bound receptors through the peripheral channels whilst remaining embedded in the membrane; however, the size of most transport receptors even without cargo is greater than the exclusion limit of the peripheral channels. A bioinformatic comparison of hundreds of NETs identified by proteomics revealed a strong enrichment of FGs compared to the rest of the transmembrane proteins encoded in the genome (Fig. 6b; [38, 72]).
This suggested the possibility that NETs containing FGs translocate through the peripheral channels functioning as their own transport receptors. To test this, FGs were added to the nucleoplasmic region of NETs. This increased their rate of translocation to the INM (Fig. 5d; [38]). For this pathway to function there must also be a contribution of peripheral channel FG nucleoporins and indeed Nup35 (in yeast Nup53) is indicated to occur in the peripheral channels [71]. Knockdown of Nup35 blocked the increase in the rate of translocation for the NETs with the added FGs but did not affect the rate of translocation for the same NET lacking the added FGs. Thus FGs on NETs appear to provide a novel mechanism for transmembrane protein translocation to the INM.
Do all mechanisms exist in parallel, and if so, which pathways are dominant?
Most of the studies mentioned above tested just one or two NETs and focused experiments on the specific mechanism they were studying. Moreover, in some cases different organisms and cell lines were used. Thus it was possible, for example, that the mechanism using soluble transport receptors also used ATP and so forth. One recent study addressed this question by directly comparing a large set of NETs using identical experimental parameters and testing the entire set for multiple mechanisms.
This study made it clear that all the mechanisms mentioned above exist in cells and function independently from one another with different groups of NETs favoring one or another mechanism [38]. Out of six NETs tested in this study, only one used a Ran-dependent mechanism and two used an ATP-dependent mechanism, while the rest appeared to translocate by unrestrained lateral diffusion in the membrane. Although the only way to certainly determine the relative use of each mechanism is to directly test many more NETs, some hints can be obtained by analysis of the sequence characteristics of the many hundreds of NETs identified thus far by proteomic analyses of NEs [15–17, 73]. Over 50 of these NETs have been confirmed to accumulate in the INM [15–17, 50, 59, 74–80] and, as only a few of those tested have so far been found only in the ONM [81], trends in the larger datasets likely reflect the properties of NETs that translocate to the INM.
First, mechanisms involving recognition of NLSs on NETs are likely to function for only a small set of NETs. Though some NETs have strongly predicted NLSs [66], their function in translocation has only been tested for Heh2 and LBR where an effect was shown [38, 64] and for SUN2 where the NLS had no effect [45]. That only some of the strongly predicted NLSs on NETs are functional suggests that many NLS predictions for NETs will prove false. This should not be surprising because most predicted NLSs are stretches of basic residues that could instead be involved in binding DNA. In support of this, many NETs are known to interact with chromatin/DNA and the larger set of NETs identified by proteomics had a tendency for high isoelectric points [3, 38, 82]. Moreover, within this larger proteomic set of NETs there were only 21% that had an NLS prediction score above 0 including pat4 and pat7 basic NLSs and bipartite NLSs using the NLS prediction algorithm PSORTII [38, 83].
The finding that so many NETs in the larger datasets had FGs argues for this to be a more prevalent mechanism [38, 72]; however, it is important to remember that this was only tested in the positive direction. It still needs to be tested whether the loss of FGs on those NETs that have them yields a deficit in translocation. It is also important to note that while the unrestrained and unaided lateral diffusion mechanism was the most abundant based on the testing in the direct comparison study [38], it is possible that additional as yet undiscovered mechanisms exist that will reduce the fraction currently ascribed to the unrestrained lateral diffusion mechanism.
Involvement of NPC proteins in NET translocation
All of the mechanisms described in detail above have one thing in common: they all require either structural or functional aspects of the NPC for NET translocation. It is therefore not surprising that several of the studies found that specific nucleoporins were required for this process (Table 1; Fig. 7). Though the positional information for nucleoporin organization within the NPC has not yet reached atomic resolution (though getting there), it is reasonably certain with respect to whether a nucleoporin would face the central or peripheral channels [71]. Some nucleoporin positions were consistent with involvement of the peripheral channels but others were consistent with the central channels playing a role.
Table 1.
Nucleoporins involved in NET targeting
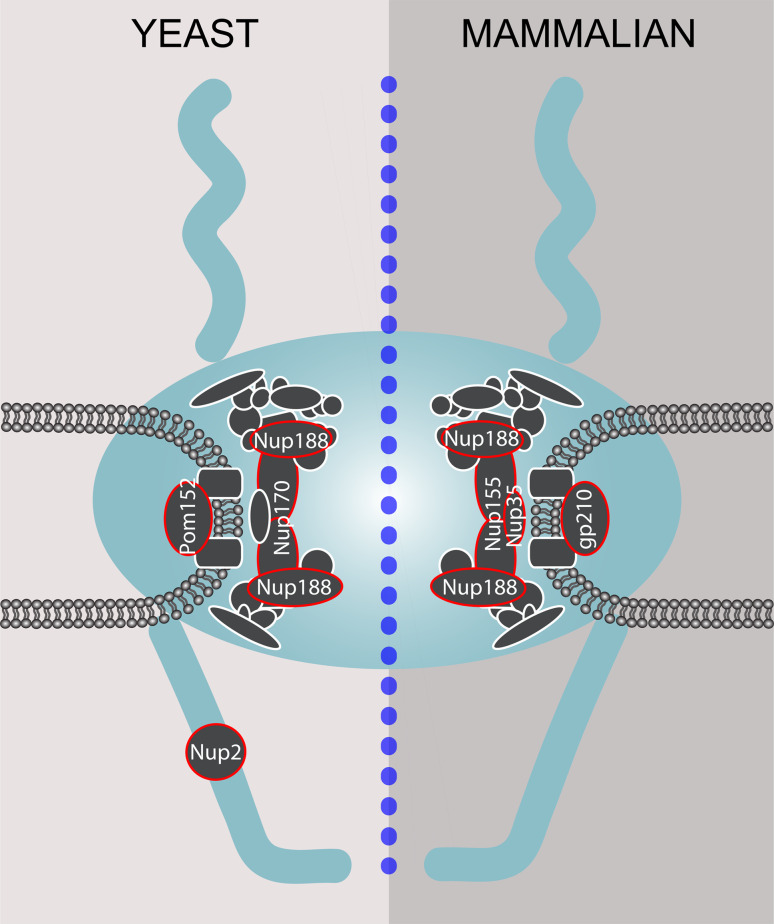
Fig. 7.
Nucleoporins involved in NET translocation. Many NPC proteins have been implicated in the translocation of NETs from the ONM into the INM. The schematic displays the core NPC components with nucleoporins implied in NET translocation in both yeast and mammalian cells highlighted in red
The use of the peripheral channels is strongly supported by the finding that depletion of Nup35 inhibited both LBR translocation and the FG-facilitated mode of translocation using artificially added FGs to NETs [38]. According to the yeast nucleoporin organization Nup53 (the Nup35 yeast homolog) should be central to the cytoplasmic–nucleoplasmic axis, but extending into the peripheral channels [71]. Though the overall position of nucleoporins has not been worked out as well for the mammalian NPC, Nup35 was found to bind lamin [84], which would place it more at the nucleoplasmic face but still at the peripheral channels. Nup155 was also found to be important for NET translocation. Depletion of Nup155 in mammalian cells inhibited the INM targeting of several NETs including LBR, LEM2, and LAP2β [85]. This effect seemed to be specific for transmembrane proteins because depletion of Nup155 did not affect targeting of lamin B, which is not a transmembrane protein. Nup155 is the mammalian homolog of yeast Nup170, which in the yeast nucleoporin positioning study was in the peripheral channel of the NPC [71].
In contrast, Nup188 should be in the central channel according to the nucleoporin positioning study [71] and it was necessary for translocation of the yeast NET Doa10 [86]. However, its depletion had the opposite effect on two other NETs. In Xenopus oocyte extracts depletion of Nup188 actually enhanced the translocation of NETs [87]. Subsequent depletion of Nup188 in intact mammalian cells also facilitated NET INM targeting [88]. One possible caveat to these studies is that Nup188 is part of a complex with yeast Nup170 (the mammalian Nup155) and as such it may reduce the overall volume of the NPC, thus allowing NETs to translocate with less steric hindrance. Nup188 had no effect on translocation of the NETs Heh1 and Heh2 [64].
The involvement of the transmembrane nucleoporins provides the most compelling argument for use of the peripheral channels, but these studies also intriguingly support use of the central channels for NET translocation. Antibodies to gp210 inhibited translocation of a reporter transmembrane cargo [13] and with most of its mass in the NE lumen this could only involve the peripheral channels. Similarly, the transmembrane nucleoporin Pom152 was required for translocation of the NET Doa10 [86]. However, the yeast NETs Heh1 and Heh2 were also tested for involvement of Pom152, finding no effect [64]. Although it is possible that other nucleoporins were unwittingly co-depleted in these studies, several determined that transport of other cargos was unaffected by the nucleoporin depletion. Thus, the global functional architecture of the NPC could not have been significantly disrupted.
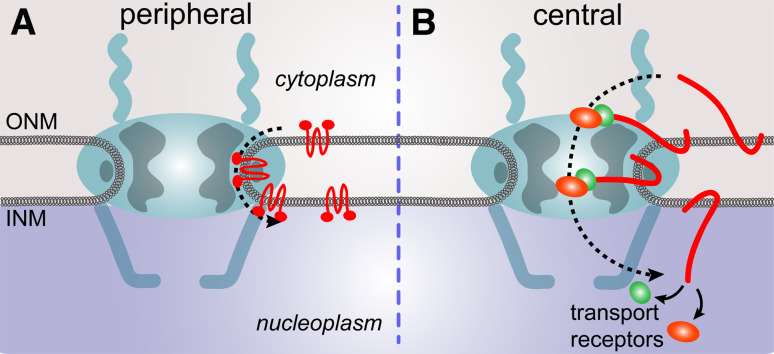
Do NETs translocate through the peripheral or central channels?
The majority of experimental data pointed strongly towards utilization of peripheral but not the central channels of the NPC for NET INM translocation (Fig. 8a). At the same time the involvement of both peripheral and central nucleoporins in itself argues for translocation mechanisms using either channel. However, one recent study strongly supports a mechanism that could use both peripheral and central channels simultaneously ([67], Fig. 8b). As discussed previously, the 10-nm peripheral channels can theoretically only accommodate a globular protein of not more than 60 kDa. The binding of transport receptors to NETs, however, would propel the nucleoplasmic mass of the import cargo complex for most NETs to far more than 100 kDa, making translocation trough the peripheral NPC channels physically impossible at a first glance. However, recent efforts to generate crystal structures for many nucleoporins suggest that these are highly unstructured proteins (reviewed in [89]) that might in theory be able to change conformation transiently to accommodate a cargo that was relatively unstructured in a region that might stick through the NPC into the central channels. Moreover, FRAP studies on nucleoporins indicated that some were highly mobile, including the transmembrane nucleoporin gp210 [90]. As this would have been thought to be one of the most stable nucleoporins as a membrane anchor, this would suggest the possibility that at least some parts of the NPC are very dynamic.
Fig. 8.
Are NETs translocating through the peripheral or central NPC channels? a The vast majority of experimental data today suggests that NETs translocate through the peripheral NPC channels. b Recent studies suggest that NET translocation could also occur through the central channel of the NPC in such way that the NET is retained in the membrane on one end while the other part of the NET is stretching through the NPC where it is bound to transport receptors
Support for the idea that NETs with unstructured nucleoplasmic domains might essentially slice through the NPC while interacting with transport receptors that would interact with central channel nucleoporins came from a study that added a long flexible linker to a reporter construct [67]. Both the reporter and a central channel nucleoporin were fused to the components of the FRB–FKBP system so that they should interact upon addition of rapamycin only if the NET reporter construct had access to the central channel. This is what was observed. Thus, yet another distinct mechanism is supported for NETs that would have the characteristic of a highly unstructured domain that would provide a thin segment to slice through the meta-stable NPC.
Perspectives
We are clearly only at the beginning of working out the details of NET translocation to the INM, but one compelling observation stands out. Translocation of soluble proteins through the central channels utilizes similar principles while giving specificity through a complexity of transport receptors to bind a wide range of cargos, whereas for membrane protein translocation there is great complexity and diversity even among the core mechanistic principles. Mechanisms clearly exist for directed vesicle fusion, autophagy, use of the peripheral channels and use of the central channels. It also appears that a mechanism may exist for use of both peripheral and central channels simultaneously. Some NPC-mediated mechanisms require particular nucleoporins and others require particular transport receptors, yet others appear to serve as their own transport receptors, and the remainder appears to translocate by unrestrained diffusion with no NPC requirement other than to shape the pore membrane.
The NPCs seem to have evolved from the same precursors as the COP vesicles involved in ER trafficking, using a combination of α-sollenoid and β-propeller structures to bend membranes [91]. It is reasonable to speculate that the original pores may have been just small channels formed by progenitor proteins carrying these structural domains. In this case, the FG-mediated mechanism may have first arisen with membrane bound transport receptors that subsequently evolved into soluble transport receptors. Though there is no evidence for it yet, future studies might reveal that some of the FG-containing NETs (still) serve as transport receptors to give directionality for trafficking of small soluble cargos. Similarly, they might function as a backup mechanism to get information to the nucleus if the central channels were to become blocked.
Acknowledgments
The authors thank Vlastimil Srsen, Jose de las Heras and Michael Robson for critically reading the manuscript. Funding for this work was provided by Wellcome Trust grants 095209 to ECS and 092076 for the Centre for Cell Biology. Tuition for NZ was also supported by the University of Edinburgh Staff Scholarship Scheme.
Abbreviations
- NE
Nuclear envelope
- INM
Inner nuclear membrane
- ONM
Outer nuclear membrane
- FG
Phenylalanine-glycine
- NPC
Nuclear pore complex
- NET
Nuclear envelope transmembrane protein
- LBR
Lamin B receptor
- NLS
Nuclear localization signal
- Nup
Nucleoporin
- FRAP
Fluorescence recovery after photobleaching
References
- 1.Suntharalingam M, Wente SR. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev Cell. 2003;4(6):775–789. doi: 10.1016/S1534-5807(03)00162-X. [DOI] [PubMed] [Google Scholar]
- 2.Callan HG, Randall JT, Tomlin SG. An electron microscope study of the nuclear membrane. Nature. 1949;163(4138):280. doi: 10.1038/163280a0. [DOI] [PubMed] [Google Scholar]
- 3.Mattout-Drubezki A, Gruenbaum Y. Dynamic interactions of nuclear lamina proteins with chromatin and transcriptional machinery. Cell Mol Life Sci. 2003;60(10):2053–2063. doi: 10.1007/s00018-003-3038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schirmer EC, Foisner R. Proteins that associate with lamins: many faces, many functions. Exp Cell Res. 2007;313(10):2167–2179. doi: 10.1016/j.yexcr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal R, Clague MJ, Durell SR, Epand RM. Membrane fusion. Chem Rev. 2003;103(1):53–69. doi: 10.1021/cr000036+. [DOI] [PubMed] [Google Scholar]
- 6.Lippincott-Schwartz J, Roberts TH, Hirschberg K. Secretory protein trafficking and organelle dynamics in living cells. Ann Rev Cell Dev Biol. 2000;16:557–589. doi: 10.1146/annurev.cellbio.16.1.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372(6501):55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 8.Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 9.Sudhof TC. The synaptic vesicle cycle: a cascade of protein–protein interactions. Nature. 1995;375(6533):645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 10.Lohka MJ, Maller JL. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol. 1985;101(2):518–523. doi: 10.1083/jcb.101.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newport J. Nuclear reconstitution in vitro: stages of assembly around protein-free DNA. Cell. 1987;48(2):205–217. doi: 10.1016/0092-8674(87)90424-7. [DOI] [PubMed] [Google Scholar]
- 12.Hetzer M, Meyer HH, Walther TC, Bilbao-Cortes D, Warren G, Mattaj IW. Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly. Nat Cell Biol. 2001;3(12):1086–1091. doi: 10.1038/ncb1201-1086. [DOI] [PubMed] [Google Scholar]
- 13.Ohba T, Schirmer EC, Nishimoto T, Gerace L. Energy- and temperature-dependent transport of integral proteins to the inner nuclear membrane via the nuclear pore. J Cell Biol. 2004;167(6):1051–1062. doi: 10.1083/jcb.200409149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poteryaev D, Squirrell JM, Campbell JM, White JG, Spang A. Involvement of the actin cytoskeleton and homotypic membrane fusion in ER dynamics in Caenorhabditis elegans . Mol Biol Cell. 2005;16(5):2139–2153. doi: 10.1091/mbc.E04-08-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korfali N, Wilkie GS, Swanson SK, Srsen V, Batrakou DG, Fairley EA, Malik P, Zuleger N, Goncharevich A, Las Heras J, Kelly DA, Kerr AR, Florens L, Schirmer EC. The leukocyte nuclear envelope proteome varies with cell activation and contains novel transmembrane proteins that affect genome architecture. Mol Cell Proteomics. 2010;9(12):2571–2585. doi: 10.1074/mcp.M110.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schirmer EC, Florens L, Guan T, Yates JRr, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301(5638):1380–1382. doi: 10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- 17.Wilkie GS, Korfali N, Swanson SK, Malik P, Srsen V, Batrakou DG, de las Heras J, Zuleger N, Kerr AR, Florens L, Schirmer EC. Several novel nuclear envelope transmembrane proteins identified in skeletal muscle have cytoskeletal associations. Mol Cell Proteomics. 2011;10(1):M110–003129. doi: 10.1074/mcp.M110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mijaljica D, Prescott M, Devenish RJ. The intricacy of nuclear membrane dynamics during nucleophagy. Nucleus. 2010;1(3):213–223. doi: 10.4161/nucl.1.3.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6(12):1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 20.Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21(13):1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21(11):1367–1381. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vos WH, Houben F, Kamps M, Malhas A, Verheyen F, Cox J, Manders EM, Verstraeten VL, van Steensel MA, Marcelis CL, van den Wijngaard A, Vaux DJ, Ramaekers FC, Broers JL. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum Mol Genet. 2011;20(21):4175–4186. doi: 10.1093/hmg/ddr344. [DOI] [PubMed] [Google Scholar]
- 23.de Noronha CM, Sherman MP, Lin HW, Cavrois MV, Moir RD, Goldman RD, Greene WC. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science. 2001;294(5544):1105–1108. doi: 10.1126/science.1063957. [DOI] [PubMed] [Google Scholar]
- 24.Radsak K, Schneider D, Jost E, Brucher KH. Alteration of nuclear lamina protein in human fibroblasts infected with cytomegalovirus (HCMV) Arch Virol. 1989;105(1–2):103–112. doi: 10.1007/BF01311120. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds AE, Liang L, Baines JD. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes U(L)31 and U(L)34. J Virol. 2004;78(11):5564–5575. doi: 10.1128/JVI.78.11.5564-5575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138(4):628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinshaw JE, Carragher BO, Milligan RA. Architecture and design of the nuclear pore complex. Cell. 1992;69(7):1133–1141. doi: 10.1016/0092-8674(92)90635-P. [DOI] [PubMed] [Google Scholar]
- 28.Reichelt R, Holzenburg A, Buhle EL, Jr, Jarnik M, Engel A, Aebi U. Correlation between structure and mass distribution of the nuclear pore complex and of distinct pore complex components. J Cell Biol. 1990;110(4):883–894. doi: 10.1083/jcb.110.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greber UF, Senior A, Gerace L. A major glycoprotein of the nuclear pore complex is a membrane-spanning polypeptide with a large lumenal domain and a small cytoplasmic tail. EMBO J. 1990;9(5):1495–1502. doi: 10.1002/j.1460-2075.1990.tb08267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallberg E, Wozniak RW, Blobel G. An integral membrane protein of the pore membrane domain of the nuclear envelope contains a nucleoporin-like region. J Cell Biol. 1993;122(3):513–521. doi: 10.1083/jcb.122.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansfeld J, Guttinger S, Hawryluk-Gara LA, Pante N, Mall M, Galy V, Haselmann U, Muhlhausser P, Wozniak RW, Mattaj IW, Kutay U, Antonin W. The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol Cell. 2006;22(1):93–103. doi: 10.1016/j.molcel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Lill Y, Lill MA, Fahrenkrog B, Schwarz-Herion K, Paulillo S, Aebi U, Hecht B. Single hepatitis-B virus core capsid binding to individual nuclear pore complexes in HeLa cells. Biophys J. 2006;91(8):3123–3130. doi: 10.1529/biophysj.106.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nissan TA, Bassler J, Petfalski E, Tollervey D, Hurt E. 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. EMBO J. 2002;21(20):5539–5547. doi: 10.1093/emboj/cdf547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soullam B, Worman HJ. The amino-terminal domain of the lamin B receptor is a nuclear envelope targeting signal. J Cell Biol. 1993;120(5):1093–1100. doi: 10.1083/jcb.120.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soullam B, Worman HJ. Signals and structural features involved in integral membrane protein targeting to the inner nuclear membrane. J Cell Biol. 1995;130(1):15–27. doi: 10.1083/jcb.130.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeshima K, Iino H, Hihara S, Funakoshi T, Watanabe A, Nishimura M, Nakatomi R, Yahata K, Imamoto F, Hashikawa T, Yokota H, Imamoto N. Nuclear pore formation but not nuclear growth is governed by cyclin-dependent kinases (Cdks) during interphase. Nat Struct Mol Biol. 2010;17(9):1065–1071. doi: 10.1038/nsmb.1878. [DOI] [PubMed] [Google Scholar]
- 37.Ribbeck K, Gorlich D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 2001;20(6):1320–1330. doi: 10.1093/emboj/20.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuleger N, Kelly DA, Richardson AC, Kerr AR, Goldberg MW, Goryachev AB, Schirmer EC. System analysis shows distinct mechanisms and common principles of nuclear envelope protein dynamics. J Cell Biol. 2011;193(1):109–123. doi: 10.1083/jcb.201009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salpingidou G, Smertenko A, Hausmanowa-Petrucewicz I, Hussey PJ, Hutchison CJ. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J Cell Biol. 2007;178(6):897–904. doi: 10.1083/jcb.200702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saksena S, Shao Y, Braunagel SC, Summers MD, Johnson AE. Cotranslational integration and initial sorting at the endoplasmic reticulum translocon of proteins destined for the inner nuclear membrane. Proc Natl Acad Sci USA. 2004;101(34):12537–12542. doi: 10.1073/pnas.0404934101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saksena S, Summers MD, Burks JK, Johnson AE, Braunagel SC. Importin-alpha-16 is a translocon-associated protein involved in sorting membrane proteins to the nuclear envelope. Nat Struct Mol Biol. 2006;13(6):500–508. doi: 10.1038/nsmb1098. [DOI] [PubMed] [Google Scholar]
- 42.Tapley EC, Ly N, Starr DA. Multiple mechanisms actively target the SUN protein UNC-84 to the inner nuclear membrane. Mol Biol Cell. 2011;22(10):1739–1752. doi: 10.1091/mbc.E10-08-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braunagel SC, Williamson ST, Ding Q, Wu X, Summers MD. Early sorting of inner nuclear membrane proteins is conserved. Proc Natl Acad Sci USA. 2007;104(22):9307–9312. doi: 10.1073/pnas.0703186104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu D, Wu X, Summers MD, Lee A, Ryan KJ, Braunagel SC. Truncated isoforms of Kap60 facilitate trafficking of Heh2 to the nuclear envelope. Traffic. 2010;11(12):1506–1518. doi: 10.1111/j.1600-0854.2010.01119.x. [DOI] [PubMed] [Google Scholar]
- 45.Turgay Y, Ungricht R, Rothballer A, Kiss A, Csucs G, Horvath P, Kutay U. A classical NLS and the SUN domain contribute to the targeting of SUN2 to the inner nuclear membrane. EMBO J. 2010;29(14):2262–2275. doi: 10.1038/emboj.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Angelo MA, Anderson DJ, Richard E, Hetzer MW. Nuclear pores form de novo from both sides of the nuclear envelope. Science. 2006;312(5772):440–443. doi: 10.1126/science.1124196. [DOI] [PubMed] [Google Scholar]
- 47.Maul GG, Maul HM, Scogna JE, Lieberman MW, Stein GS, Hsu BY, Borun TW. Time sequence of nuclear pore formation in phytohemagglutinin-stimulated lymphocytes and in HeLa cells during the cell cycle. J Cell Biol. 1972;55(2):433–447. doi: 10.1083/jcb.55.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torrisi MR, Lotti LV, Pavan A, Migliaccio G, Bonatti S. Free diffusion to and from the inner nuclear membrane of newly synthesized plasma membrane glycoproteins. J Cell Biol. 1987;104(3):733–737. doi: 10.1083/jcb.104.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powell L, Burke B. Internuclear exchange of an inner nuclear membrane protein (p55) in heterokaryons: in vivo evidence for the interaction of p55 with the nuclear lamina. J Cell Biol. 1990;111(6 Pt 1):2225–2234. doi: 10.1083/jcb.111.6.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Worman HJ, Yuan J, Blobel G, Georgatos SD. A lamin B receptor in the nuclear envelope. Proc Natl Acad Sci USA. 1988;85(22):8531–8534. doi: 10.1073/pnas.85.22.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye Q, Worman HJ. Primary structure analysis and lamin B and DNA binding of human LBR, an integral protein of the nuclear envelope inner membrane. J Biol Chem. 1994;269(15):11306–11311. [PubMed] [Google Scholar]
- 52.Chiacchia KB, Drickamer K. Direct evidence for the transmembrane orientation of the hepatic glycoprotein receptors. J Biol Chem. 1984;259(24):15440–15446. [PubMed] [Google Scholar]
- 53.Furukawa K, Fritze CE, Gerace L. The major nuclear envelope targeting domain of LAP2 coincides with its lamin binding region but is distinct from its chromatin interaction domain. J Biol Chem. 1998;273(7):4213–4219. doi: 10.1074/jbc.273.7.4213. [DOI] [PubMed] [Google Scholar]
- 54.Wu W, Lin F, Worman HJ. Intracellular trafficking of MAN1, an integral protein of the nuclear envelope inner membrane. J Cell Sci. 2002;115(Pt 7):1361–1371. doi: 10.1242/jcs.115.7.1361. [DOI] [PubMed] [Google Scholar]
- 55.Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ, Lippincott-Schwartz J. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J Cell Biol. 1997;138(6):1193–1206. doi: 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ostlund C, Ellenberg J, Hallberg E, Lippincott-Schwartz J, Worman HJ. Intracellular trafficking of emerin, the Emery-Dreifuss muscular dystrophy protein. J Cell Sci. 1999;112(Pt 11):1709–1719. doi: 10.1242/jcs.112.11.1709. [DOI] [PubMed] [Google Scholar]
- 57.Ostlund C, Folker ES, Choi JC, Gomes ER, Gundersen GG, Worman HJ. Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J Cell Sci. 2009;122(Pt 22):4099–4108. doi: 10.1242/jcs.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ostlund C, Sullivan T, Stewart CL, Worman HJ. Dependence of diffusional mobility of integral inner nuclear membrane proteins on A-type lamins. Biochemistry. 2006;45(5):1374–1382. doi: 10.1021/bi052156n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rolls MM, Stein PA, Taylor SS, Ha E, McKeon F, Rapoport TA. A visual screen of a GFP-fusion library identifies a new type of nuclear envelope membrane protein. J Cell Biol. 1999;146(1):29–44. doi: 10.1083/jcb.146.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimi T, Koujin T, Segura-Totten M, Wilson KL, Haraguchi T, Hiraoka Y. Dynamic interaction between BAF and emerin revealed by FRAP, FLIP, and FRET analyses in living HeLa cells. J Struct Biol. 2004;147(1):31–41. doi: 10.1016/j.jsb.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 61.Chen J, Zheng XF, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA. 1995;92(11):4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klemm JD, Beals CR, Crabtree GR. Rapid targeting of nuclear proteins to the cytoplasm. Curr Biol. 1997;7(9):638–644. doi: 10.1016/S0960-9822(06)00290-9. [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Du X, Cai Z, Greene MI. Characterization of the structures involved in localization of the SUN proteins to the nuclear envelope and the centrosome. DNA Cell Biol. 2006;25(10):554–562. doi: 10.1089/dna.2006.25.554. [DOI] [PubMed] [Google Scholar]
- 64.King MC, Lusk CP, Blobel G. Karyopherin-mediated import of integral inner nuclear membrane proteins. Nature. 2006;442(7106):1003–1007. doi: 10.1038/nature05075. [DOI] [PubMed] [Google Scholar]
- 65.Ma Y, Cai S, Lv Q, Jiang Q, Zhang Q, Sodmergen, Zhai Z, Zhang C (2007) Lamin B receptor plays a role in stimulating nuclear envelope production and targeting membrane vesicles to chromatin during nuclear envelope assembly through direct interaction with importin beta. J Cell Sci 120(Pt 3):520–530 [DOI] [PubMed]
- 66.Lusk CP, Blobel G, King MC. Highway to the inner nuclear membrane: rules for the road. Nat Rev Mol Cell Biol. 2007;8(5):414–420. doi: 10.1038/nrm2165. [DOI] [PubMed] [Google Scholar]
- 67.Meinema AC, Laba JK, Hapsari RA, Otten R, Mulder FA, Kralt A, van den Bogaart G, Lusk CP, Poolman B, Veenhoff LM. Long unfolded linkers facilitate membrane protein import through the nuclear pore complex. Science. 2011;333(6038):90–93. doi: 10.1126/science.1205741. [DOI] [PubMed] [Google Scholar]
- 68.Frey S, Richter RP, Gorlich D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science. 2006;314(5800):815–817. doi: 10.1126/science.1132516. [DOI] [PubMed] [Google Scholar]
- 69.Lim RY, Fahrenkrog B, Koser J, Schwarz-Herion K, Deng J, Aebi U. Nanomechanical basis of selective gating by the nuclear pore complex. Science. 2007;318(5850):640–643. doi: 10.1126/science.1145980. [DOI] [PubMed] [Google Scholar]
- 70.Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995;83(5):683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- 71.Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, Rout MP, Sali A. Determining the architectures of macromolecular assemblies. Nature. 2007;450(7170):683–694. doi: 10.1038/nature06404. [DOI] [PubMed] [Google Scholar]
- 72.Kerr AR, Schirmer EC. FG repeats facilitate integral protein trafficking to the inner nuclear membrane. Commun Integr Biol. 2011;4(5):557–559. doi: 10.4161/cib.4.5.16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dreger M, Bengtsson L, Schoneberg T, Otto H, Hucho F. Nuclear envelope proteomics: novel integral membrane proteins of the inner nuclear membrane. Proc Natl Acad Sci USA. 2001;98(21):11943–11948. doi: 10.1073/pnas.211201898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brachner A, Reipert S, Foisner R, Gotzmann J. LEM2 is a novel MAN1-related inner nuclear membrane protein associated with A-type lamins. J Cell Sci. 2005;118(Pt 24):5797–5810. doi: 10.1242/jcs.02701. [DOI] [PubMed] [Google Scholar]
- 75.Foisner R, Gerace L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell. 1993;73(7):1267–1279. doi: 10.1016/0092-8674(93)90355-T. [DOI] [PubMed] [Google Scholar]
- 76.Hodzic DM, Yeater DB, Bengtsson L, Otto H, Stahl PD. Sun2 is a novel mammalian inner nuclear membrane protein. J Biol Chem. 2004;279(24):25805–25812. doi: 10.1074/jbc.M313157200. [DOI] [PubMed] [Google Scholar]
- 77.Lin F, Blake DL, Callebaut I, Skerjanc IS, Holmer L, McBurney MW, Paulin-Levasseur M, Worman HJ. MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J Biol Chem. 2000;275(7):4840–4847. doi: 10.1074/jbc.275.7.4840. [DOI] [PubMed] [Google Scholar]
- 78.Malik P, Korfali N, Srsen V, Lazou V, Batrakou DG, Zuleger N, Kavanagh DM, Wilkie GS, Goldberg MW, Schirmer EC. Cell-specific and lamin-dependent targeting of novel transmembrane proteins in the nuclear envelope. Cell Mol Life Sci. 2010;67(8):1353–1369. doi: 10.1007/s00018-010-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manilal S, Nguyen TM, Sewry CA, Morris GE. The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum Mol Genet. 1996;5(6):801–808. doi: 10.1093/hmg/5.6.801. [DOI] [PubMed] [Google Scholar]
- 80.Senior A, Gerace L. Integral membrane proteins specific to the inner nuclear membrane and associated with the nuclear lamina. J Cell Biol. 1988;107(6 Pt 1):2029–2036. doi: 10.1083/jcb.107.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171(5):799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ulbert S, Platani M, Boue S, Mattaj IW. Direct membrane protein-DNA interactions required early in nuclear envelope assembly. J Cell Biol. 2006;173(4):469–476. doi: 10.1083/jcb.200512078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakai K, Horton P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci. 1999;24(1):34–36. doi: 10.1016/S0968-0004(98)01336-X. [DOI] [PubMed] [Google Scholar]
- 84.Hawryluk-Gara LA, Shibuya EK, Wozniak RW. Vertebrate Nup53 interacts with the nuclear lamina and is required for the assembly of a Nup93-containing complex. Mol Biol Cell. 2005;16(5):2382–2394. doi: 10.1091/mbc.E04-10-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mitchell JM, Mansfeld J, Capitanio J, Kutay U, Wozniak RW. Pom121 links two essential subcomplexes of the nuclear pore complex core to the membrane. J Cell Biol. 2010;191(3):505–521. doi: 10.1083/jcb.201007098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deng M, Hochstrasser M. Spatially regulated ubiquitin ligation by an ER/nuclear membrane ligase. Nature. 2006;443(7113):827–831. doi: 10.1038/nature05170. [DOI] [PubMed] [Google Scholar]
- 87.Theerthagiri G, Eisenhardt N, Schwarz H, Antonin W. The nucleoporin Nup188 controls passage of membrane proteins across the nuclear pore complex. J Cell Biol. 2010;189(7):1129–1142. doi: 10.1083/jcb.200912045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antonin W, Ungricht R, Kutay U. Traversing the NPC along the pore membrane: targeting of membrane proteins to the INM. Nucleus. 2011;2(2):87–91. doi: 10.4161/nucl.2.2.14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoelz A, Debler EW, Blobel G. The structure of the nuclear pore complex. Annu Rev Biochem. 2011;80:613–643. doi: 10.1146/annurev-biochem-060109-151030. [DOI] [PubMed] [Google Scholar]
- 90.Rabut G, Doye V, Ellenberg J. Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat Cell Biol. 2004;6(11):1114–1121. doi: 10.1038/ncb1184. [DOI] [PubMed] [Google Scholar]
- 91.Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, Sali A, Rout MP. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2004;2(12):e380. doi: 10.1371/journal.pbio.0020380. [DOI] [PMC free article] [PubMed] [Google Scholar]