Abstract
In cross-sectional analyses, early institutional care is associated with shorter stature but not obesity during puberty in children adopted into US families. We examined whether shorter stature and leaner body composition in youth adopted internationally from institutions would continue as puberty progressed. We also examined whether current psychosocial stress would moderate the association between early institutional deprivation and growth during adolescence. Using an accelerated longitudinal design and linear mixed-effects models, we examined the height and body mass index (BMI) of 132 previously institutionalized (PI) and 176 nonadopted (NA) youth. We examined youth aged 7–15 at the beginning of the study three times across 2 years. Nurses assessed anthropometrics and pubertal status. Current psychosocial stress was measured using the Youth Life Stress Interview. Our results indicated that PI youth remained shorter and leaner across three assessments than NA youth. However, age-and-sex-adjusted BMI increased faster in PI youth. Psychosocial stress during puberty predicted greater age-and-sex-adjusted BMI, but this effect did not differ by group. The gap in BMI but not height appears to close between PI and NA youth. Higher psychosocial stress was associated with higher BMI during puberty.
Keywords: BMI, early-life stress, growth, height, puberty
1 |. INTRODUCTION
It has been long recognized that adverse early-life experiences profoundly impact human development. During sensitive periods (Greenough et al., 1987; Nelson, 2000), early-life stress (ELS) may program body morphology, increasing the risks of both short stature and obesity (Bennett et al., 2010; Danese & Tan, 2014; Lissau & Sorensen, 1994; Tang et al., 2018). For example, ELS in the form of a variable for-aging demand paradigm has been found to be associated with greater weight, BMI, and abdominal circumference in peripubertal juvenile Bonnet macaques (Kaufman et al., 2007). Additionally, research in rodents finds that male rodents exposed to postweaning stress exhibit reduced growth rate during adolescence and predisposition to higher levels of adiposity in adulthood (Schipper et al., 2020). In humans, neglectful parental behavior has been associated with an elevated risk of obesity in childhood (Whitaker et al., 2007) and adolescence (Shin & Miller, 2012). Childhood sexual and physical abuse is associated with increased risks of obesity in adulthood (Bentley & Widom, 2009; Noll et al., 2007). Long-term neglect and emotional abuse have also been found to predict growth failure in height and weight in preschool-aged boys (Oliván, 2003).
As experienced in institutional care, early caregiving deprivation has been associated with a higher risk of growth stunting in children at the time of adoption into families in the United States, Great Britain, and Western Europe (Miller & Hendrie, 2000; Reid et al., 2017; Rutter, 1998; Van IJzendoorn et al., 2007). However, once removed from deprivation and placed in families, children adopted from orphanage-like institutions exhibit rapid catchup growth and are typically within normal anthropometric parameters for age within a year of adoption or fostering (Le Mare & Audet, 2006; Miller et al., 2010; Palacios et al., 2014; Pomerleau et al., 2005; Van IJzendoorn et al., 2007).
One issue with the current literature is that most previous studies on children with histories of ELS from orphanage/institutional rearing have focused on children’s development during infancy and childhood but ignored puberty (Van IJzendoorn et al., 2007). In one of the few studies in humans examining puberty, catchup growth in height and weight of Romanian children adopted into English families was almost complete by age 6. However, a follow-up study found a marked deceleration in linear growth rate in adopted children relative to youth without adverse histories, resulting in greater physical differences between the Romanian-adopted and comparison youth at 15 years compared to 6 years (Barke et al., 2010). The youth in that study were adopted from what was described as severe and global deprivation. Thus, it appeared that severe early deprivation negatively impacted growth during puberty. As there is increasing evidence that early growth faltering and catchup growth are associated with later obesity and cardiovascular risk (Adair & Cole, 2003; Ekelund et al., 2006; Kelishadi et al., 2015; Ong et al., 2000; Singhal, 2017), it is essential to determine if the pattern noted in the early Romanian adoptees holds in other populations of children who experience early deprivation due to rearing in institutions.
Another question that remains unanswered is the heterogeneity in children’s developmental outcomes. While multiple studies found catchup growth in height and weight in children adopted into stable and nurturing families in high-income countries such as Sweden, the United States, and the United Kingdom, the rate of children’s catchup growth and overall developmental outcomes vary between individuals (Johnson, 2002; Proos et al., 1991). For instance, in a sample of Indian girls adopted into Sweden, 45% of the girls showed fast catchup in height while 55% of them showed slow or zero catchup growth. In terms of weight, only 17% of the girls caught up rapidly, leaving the rest catching up slowly (35%) or showing no catchup (48%). During the final assessment of the study, 8% of the girls were still severely growth stunted (Proos et al., 1991). An analysis of Romanian adoptees showed a similar result. While growth was promising in children adopted after 6 months of age, 13% were still within the 5th percentile in height and 6% were within the 5th percentile in weight (Benoit et al., 1996).
We wondered whether children’s current psychosocial stress could explain different rates and outcomes of children’s physical development during puberty. In children reared in families, chronic psychosocial stress caused by family conflict has been associated with slow growth in children up to 7 years of age (Montgomery et al., 1997). Early childhood familial disruption, including parental death and divorce, has also been associated with shorter height in males and females in the long term (Sheppard et al., 2015). In children with histories of early institutional care, their physical development may be even more affected. Notably, there is evidence of altered stress and defensive functioning in these children consistent with exposure to stress early in life (Gee et al., 2013; Tarullo & Gunnar, 2006). Children who experienced institutional care exhibit increased fear and behavioral inhibition (Stellern et al., 2014), anxious phenotypes (Tottenham et al., 2010), and dysfunctional stress systems. These stress systems include hyperresponsivity to threat stimuli in the amygdala (Tottenham, 2012) and the hyporeactivity of the hypothalamic—pituitary–adrenal (HPA) axis (e.g., flatter diurnal rhythms and blunted cortisol responses) (Gunnar & Vazquez, 2001). These effects have been noted when PI youth are exposed to current life psychosocial stressors (Gunnar & Vazquez, 2001; Tottenham, 2012). However, the impact of current psychosocial stress on children’s physical growth has not yet been examined.
In the present study, we collected and examined anthropometric data, including height, weight, and body mass index (BMI), and calculated age-and-sex-adjusted World Health Organization (WHO) BMI and height Z-scores, as well as assessed chronic psychosocial stress via the Youth Life Stress Interview (Adrian & Hammen, 1993; Rudolph & Hammen, 1999) three times over 2 years on PI and NA youth. Our goal was to describe the growth trajectories of BMI and height during puberty in children with and without histories of early institutional deprivation and examine whether current psychosocial stress would influence these growth trajectories. We formed three hypotheses. First, based on an early cross-sectional analysis of the present sample (Reid et al., 2017) and previous findings on the significant growth gap between youth with and without histories of institutional care (Benoit et al., 1996; Groze & Ileana, 1996; Rutter, 1996), we expected the PI youth to be shorter and leaner than the comparison youth. Second, as the difference in growth between youth who experienced and did not experience early institutional deprivation was greater in adolescence than in childhood (Barke et al., 2010), we hypothesized that the height and BMI differences between the PI and NA youth would increase with increasing puberty. Third, given the potential influences of psychosocial stress on developmental outcomes in children (Montgomery et al., 1997; Sheppard et al., 2015), we predicted that the heterogeneity in patterns of growth for the PI youth would, in part, be related to variations in current-life stress.
2 |. METHODS
2.1 |. Participants
One hundred and thirty-two previously institutionalized (PI) youth who were adopted internationally from orphanage-like institutions (88 females and 44 males; age rangeat-time-1, 7.08–15.12 years; mean ageat-time-1 = 11.31, SDage = 2.40 years) and 176 youth born and raised in families comparable to those who adopt internationally (nonadopted, NA; 92 females and 84 males; age rangeat-time-1, 7.27–14.99 years; mean ageat-time-1 = 11.18, SDage = 2.28 years) participated in the present study. Demographic details are provided in Table 1. Briefly, family environment (e.g., parents’ age, education, and household income) did not differ by group. Preadoption conditions as reported by the adoptive parents are shown in Table 2. PI youth were excluded if they were adopted after 60 months of age, or were diagnosed with fetal alcohol exposure, or had neurodevelopmental, congenital, and endocrine disorders. NA youth were recruited from a registry of research-interested families whose household income and parental education level were comparable to the PI group. Exclusion criteria for NA children were diagnoses of neurodevelopmental disorders and early adverse experiences, including childhood maltreatment and neglect. All participants were currently residing within driving distance of our research laboratory in a major metropolitan city at the time of assessment.
TABLE 1.
Demographics for previously institutionalized (PI) and nonadopted (NA) youth at baseline
| Previously institutionalized, PI (n = 132) | Nonadopted, NA (n = 176) | |
|---|---|---|
| Female | n = 88 (67%) | n = 92 (52%) |
| Age (years) at time 1 | 11.31 ± 2.40 | 11.18 ± 2.28 |
| Age range (years) | 7.08–15.12 | 7.27–14.99 |
| Region of origin | ||
| Russia/Europe | 57 | - |
| Asia | 51 | - |
| Latin America/Caribbean | 13 | - |
| Other | 11 | - |
| United States | - | 176 |
| Race | ||
| Asian | 54 | 1 |
| White | 51 | 157 |
| Native American | 13 | - |
| African American/African | 6 | 3 |
| Multiracial | 5 | 13 |
| Unknown | 3 | 1 |
| Parents’ mean age (years) | 49.99 | 43.2 |
| Percentage of parents who are White | 95.45 | 93.18 |
| Parents’ education | ||
| Mother | Master’s degree | Four-year college |
| Father | Four-year college | Four-year college |
| Annual household income | $150,000–$200,000 | $150,000–$200,000 |
TABLE 2.
Parent-reported preadoption conditions
| Percentage of cases reported | Mean | SD | Max | Median | Min | |
|---|---|---|---|---|---|---|
| Neglect of physical needs | 28.03 | - | - | - | - | - |
| Neglect of social needs | 60.60 | - | - | - | - | - |
| Physical abuse | 6.06 | - | - | - | - | - |
| Age of adoption (years) | - | 1.61 | 1.05 | 4.92 | 1.25 | 0.46 |
| Time spent in institutions (i years) | - | 1.52 | 0.96 | 4.75 | 1.25 | 0.33 |
2.2 |. Measurements and protocol
Data collection was conducted during annual research visits spanning 2 years (Figure 1). Participants were aged between 7 and 15 at the start of data collection, with an average age of 11.7 months between research visits. During each session/visit, each participant underwent a nurse’s exam for anthropometrics and pubertal staging (Marshall & Tanner, 1969, 1970) and participated in an in-person Youth Life Stress Interview (Adrian & Hammen, 1993; Rudolph & Hammen, 1999). All procedures were approved by the Institutional Review Boards of the University of Minnesota-Twin Cities and the state of Minnesota. All participants and their parents provided informed consent and assent for the study. Participants were compensated for their time.
FIGURE 1.
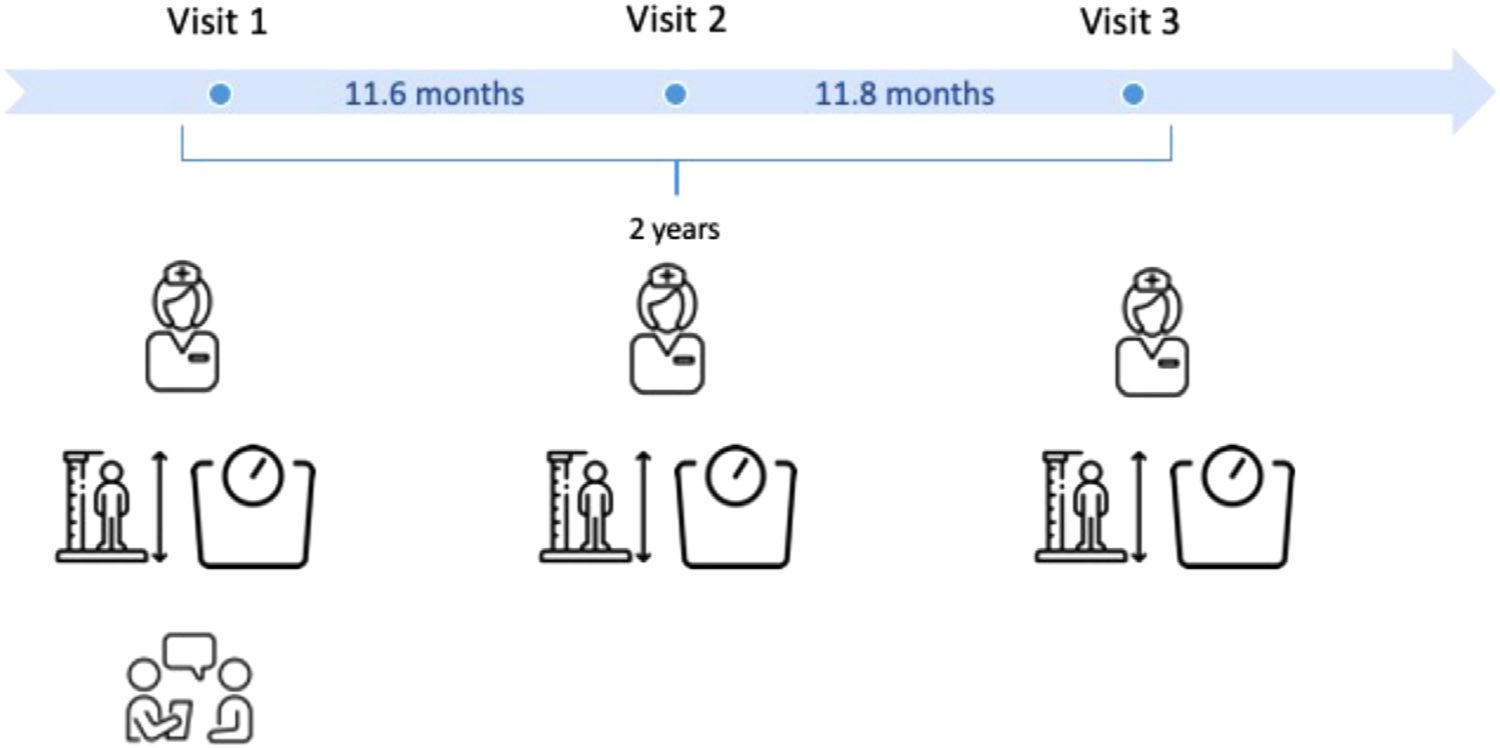
Study procedure
2.2.1 |. Anthropometrics
Participants’ height (in centimeters) and weight (in kilograms) were measured in triplicate by a nurse trained in auxanology. Height was assessed to the nearest 0.1 cm using a calibrated stadiometer (SECA Model 216, Seca Hanover, MD), and weight was measured to the nearest 0.1 kg based on a calibrated scale (Health-O-Meter Professional 349KLX professional Medical Weight Scale, Pelstar LLC, McCook, IL). All measurements were collected when participants wore light clothing. The mean score of the measures was calculated and used when there was a discrepancy between the three assessments. BMI (weight in kilograms per height in square meters) was calculated using the measurements above. Age–and-sex-adjusted BMI Z-score and height Z-score were then computed based on the 2007 WHO’s global child growth standards, which comprise growth data from various countries and regions (Johnson et al., 2016).
2.2.2 |. Current psychosocial stress
Current psychosocial stress was assessed through the Youth Life Stress Interview (Adrian & Hammen, 1993; Rudolph & Hammen, 1999), a detailed semistructured interview that assesses chronic strain in multiple life domains (school, behavior, peer relationship, opposite-sex platonic relationship, sibling relationship, parent–child relationship, and parents’ marital relationship). The interview length ranged from 45 to 120 min, during which participants answered questions about their life within the past 12 months. Based on participants’ responses to the interview questions, two reliable coders (interrater reliability: intraclass correlation coefficient (ICC) = 0.90) first assessed current psychosocial stress separately on a 1 (superior or no stress) to 5 (severe stress) scale and then jointly decided on the best rating for each domain (interrater reliability: ICC = 0.90). As items held together well each assessment (α ranged .76–.79), an overall stress score for each interview session was later computed as the average of all scales.
2.2.3 |. Pubertal stage
Pubertal stage was determined through the nurse exam using Tanner criteria (Marshall & Tanner, 1969, 1970). One of three nurses conducted the assessment in each session. As detailed elsewhere (DePasquale et al., 2019; Gunnar et al., 2019; Reid et al., 2017), reliability in assessment was determined by having two nurses perform exams on the same child for 10% of exams (κ = .889 across all three annual sessions). In girls, pubertal stage was determined by the pubertal breast stage by visual and tactile examination. In boys, it was determined by testicular volume using a Prader orchidometer. If a child reached stage 5 before their third annual assessment, they were presumed to remain at stage 5 and did not complete pubertal staging again (session 2, n = 19 children; session 3, n = 35 children). Twenty-two children refused the physical exam at session 1, 17 at session 2, and 23 at session 3. Missing pubertal stage values were imputed based on parent report on Petersen Pubertal Development Scale (PDS) (Petersen et al., 1988). The PDS scores were converted to Tanner scores following guidelines from Shirtcliff et al. (2009). Tanner and PDS scores were highly correlated at all sessions (r = .81–.83, p < .001). Details about Tanner scores at three sessions can be found in Tables S1 and S2.
2.3 |. Data analysis
2.3.1 |. Age–and-sex-adjusted BMI Z-score
An independent t-test was conducted to examine the initial difference in age–and-sex-adjusted BMI Z-score between PI and NA groups. An unconditional random intercepts model was then fit, in which the ICC was computed. We found that an estimated 89.4% of the total variation in BMI Z-score was attributable to differences between participants, and 10.6% of the total variation was attributable to differences between time points (within participants). Having partitioned the total variation into within subjects and between subjects, and given the longitudinal nature of our dataset, we believed that the mixed-effects model was the most appropriate one for the present study. This technique permits the inclusion of multiple measurements per individual, missing data, and irregular assessment intervals to enhance statistical power and control for within-subject variation (Pinheiro & Bates, 2000).
Given the nature of the longitudinal study, we first explored how BMI Z-scores changed as a function of time. Here we used three annual study sessions as indicators of time. An unconditional means model, a model that included the linear effect of time (i.e., session), and a model including a quadratic effect of time (i.e., session) was formed using the maximum likelihood (ML) estimation. Then Akaike information criterion (AIC) was used to compare the models and select the best structure (Akaike, 1973, 1974; Long, 2012). The evidence indicated that the model that included the linear effect of time (i.e., session) should be adopted. Visual inspection showed that BMI Z-score in youth varied at the first time of assessment and varied in growth trajectories across time. Therefore, we then fitted the random intercepts and random slopes model and compared it with the random intercepts-only model. The AIC showed that the random intercepts and slopes model should be adopted (level-1 model).
For level-2 analysis, our focal predictors included session, group (PI vs. NA), child sex, current psychosocial stress, and pubertal stage. Note that we used the baseline current psychosocial stress to predict children’s growth over time. While pubertal stage was initially categorized into five levels, it was later dichotomized such that pubertal stage included early (stages 1 and 2) versus late (stages 3–5) stages, which renders the results readily interpretable and allows for direct comparison between two groups. Then, using ML, each of the predictors was taken to fit an unconditional growth model, an unconditional growth model with the intercept, and an unconditional growth model with the intercept and slope. These models were compared using AIC, and we selected the predictors from the best fitting models to form the final model. As a result, our final model included the effect of session, group (PI vs. NA), current psychosocial stress, pubertal stage, and the interaction of Session × Group.
2.3.2 |. Age- andsex-adjusted height Z-score
The same statistical procedure was conducted to compare the initial difference between PI and NA groups and to select the best fitting model for height Z-scores in youth, thus was not reiterated here. The final model for height Z-score was an unconditional model, suggesting no linear relationship between session and height Z-score. In other words, although height for age and sex in PI youth was initially lower than NA youth, neither of the PI and NA groups increased in their standard scores across three assessments. Note that analyses for raw data of BMI and height are provided in the Supporting Information. All data were analyzed using R studio Version 1.1.456.
3 |. RESULTS
3.1 |. Age–and-sex-adjusted BMI Z-score and height Z-score at baseline
While both PI and NA groups were within the normal and healthy range, we found a significant difference in BMI Z-score (df = 254.58, p = .00) and height Z-score (df = 283.88, p = .00) (Table 3) at the initial assessment, indicating that PI youth were significantly thinner and shorter.
TABLE 3.
World Health Organization (WHO) age–and-sex-adjusted BMI Z-score and height Z-score across three sessions
| Previously institutionalized, PI (n = 132) | Nonadopted, NA (n = 176) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | df | p | ||
| Malea | Session 1 BMI-Z | 0.19 ± 1.29 | 0.34 ± 1.20 | 82.65 | .50 |
| Session 2 BMI-Z | 0.40 ± 1.29 | 0.31 ± 1.23 | 65.84 | .73 | |
| Session 3 BMI-Z | 0.53 ± 1.31 | 0.27 ± 1.23 | 60.82 | .34 | |
| Femaleb | Session 1 BMI-Z | −0.18 ± 1.23 | 0.35 ± 0.93 | 161.56 | 00*** |
| Session 2 BMI-Z | −0.19 ± 1.20 | 0.25 ± 1.03 | 148.07 | .02* | |
| Session 3 BMI-Z | −0.02 ± 1.13 | 0.31 ± 1.22 | 130.60 | .11 | |
| Total | Session 1 BMI-Z | −0.06 ± 1.26 | 0.35 ± 1.06 | 254.58 | .00** |
| Session 2 BMI-Z | −0.00 ± 1.26 | 0.28 ± 1.13 | 226.61 | .06 | |
| Session 3 BMI-Z | 0.16 ± 1.21 | 0.29 ± 1.22 | 215.56 | .43 | |
| Malea | Session 1 Height-Z | −0.23 ± 1.04 | 0.53 ± 1.10 | 92.00 | 00*** |
| Session 2 Height-Z | −0.24 ± 1.05 | 0.47 ± 0.97 | 63.94 | 00*** | |
| Session 3 Height-Z | −0.16 ± 1.14 | 0.50 ± 0.90 | 55.07 | 01** | |
| Femaleb | Session 1 Height-Z | −0.37 ± 1.00 | 0.18 ± 0.93 | 175.02 | .00*** |
| Session 2 Height-Z | −0.38 ± 1.05 | 0.16 ± 1.02 | 150.95 | .00*** | |
| Session 3 Height-Z | −0.45 ± 1.05 | 0.17 ± 0.98 | 132.71 | .00*** | |
| Total | Session 1 Height-Z | −0.32 ± 1.01 | 0.34 ± 1.02 | 283.88 | 00*** |
| Session 2 Height-Z | −0.34 ± 1.05 | 0.32 ± 1.00 | 235.40 | 00*** | |
| Session 3 Height-Z | −0.35 ± 1.08 | 0.33 ± 0.96 | 202.70 | 00*** |
p ≤ .05;
p ≤ .01;
p ≤ .001.
n = 126.
n = 179.
3.2 |. BMI Z-score
Model coefficients for focal predictors of the final model are shown in Table 4. The final model included BMI Z-score as the outcome with session, group, current psychosocial stress, and the Session × Group interaction as predictors. We found that PI youth were initially thinner than NA youth. However, there was a significant interaction of Session × Group with PI youth having a higher slope of the BMI Z-score trajectory than NA youth, which suggested that the gap between these two groups tended to narrow across three assessments (Figure 2). The main effect of current psychosocial stress was significant, indicating that youth who experienced higher current psychosocial stress would manifest higher BMI for age and sex. However, this effect of stress did not differ by group. In terms of the growth in raw BMI, the final model included session, group, current stress, and pubertal stage (Table S4). The effect of session was significant, indicating that both groups grew in BMI across the 2 years. There was a significant group effect, suggesting that PI youth, on average, had a lower BMI than the NA youth. Later pubertal stage was associated with higher BMI in youth.
TABLE 4.
Model coefficients (standard errors) for final model predicting World Health Organization (WHO) age–and-sex-adjusted BMI Z-score and age–and-sex-adjusted height Z-score in youth
| Intercept | Fixed effect | Pubertal stage | Session × Group | Random effects | ||||
|---|---|---|---|---|---|---|---|---|
| Session | Group | Stress | Intercept | Residual | ||||
| BMI Z-score | −0. 36 (0. 31) | −0.00 (0.03) | −0.49 (0.14)*** | 0.29 (0.13)* | 0.11 (0.06) | 0.08 (0.04)* | 1.19 (1.09) | 0.09 (0.30) |
| Height Z-score | 0.05 (0.06) | - | - | - | - | 1.12 (1.06) | 0.05 (0.23) | |
Note: Results are reported based on fitting each model using maximum likelihood (ML) estimation.
p ≤ .05;
p ≤ .001.
FIGURE 2.
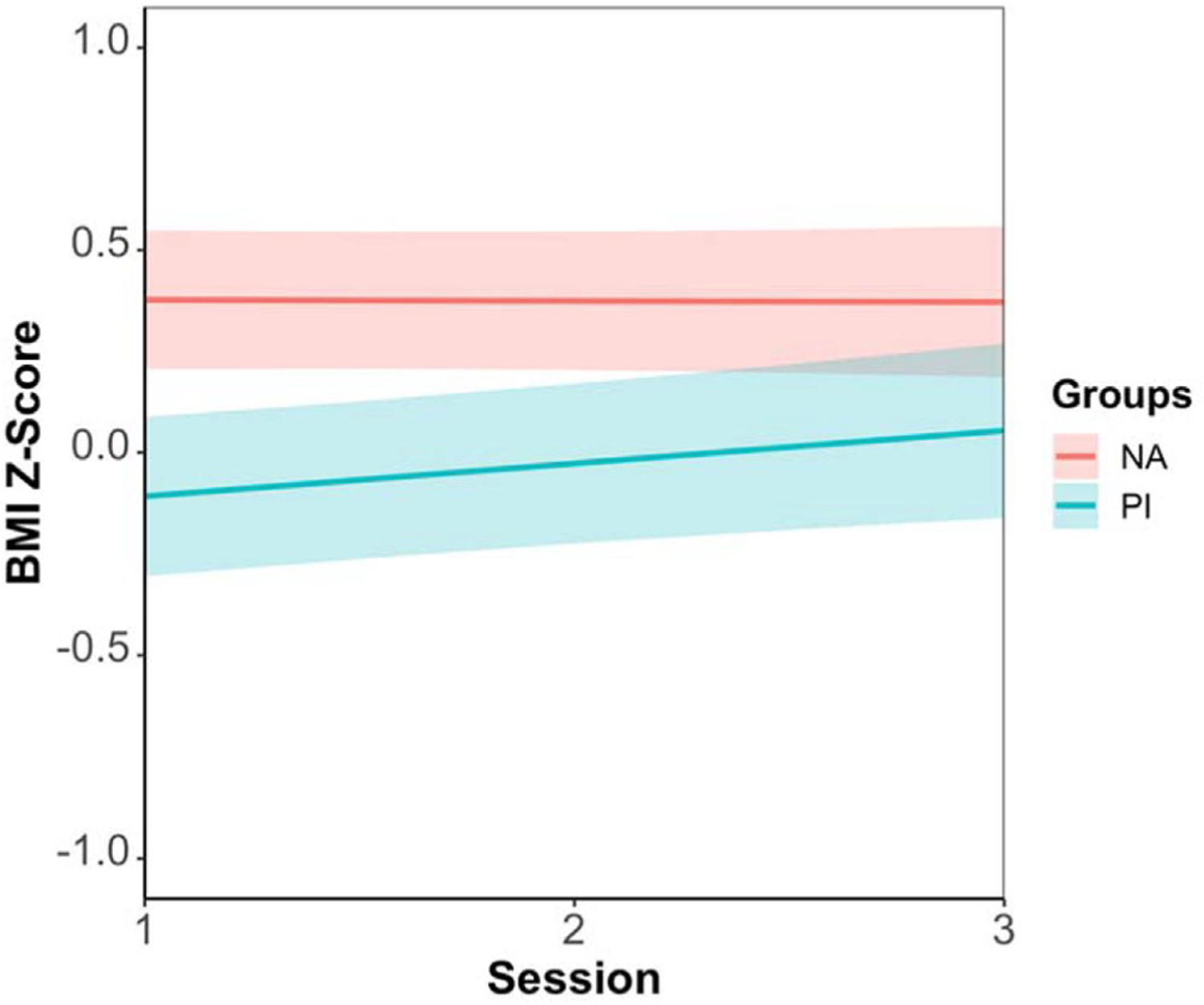
A significant interaction of Session × Group was found in age–and-sex-adjusted BMI Z-score. Previously institutionalized (PI) youth had a higher slope of the BMI Z-score trajectory than nonadopted (NA) youth, thus the gap between these two groups became narrower across three times of assessment.
3.3 |. Height Z-score
Model coefficients for focal predictors of the final model are also shown in Table 4. Our results showed that in the beginning, PI youth were shorter. However, no linear relationship was found between session and height Z-score in the final model, suggesting that PI and NA youth remained the same in height for age and sex over three sessions. Regarding the height growth in raw measurement, PI and NA youth both grew across the 2 years, while the difference between the two groups remained the same (Figure 3; Tables S3 and S4). Note that figures demonstrating raw data points of BMI Z-score and height are shown in Figures S2 and S3.
FIGURE 3.
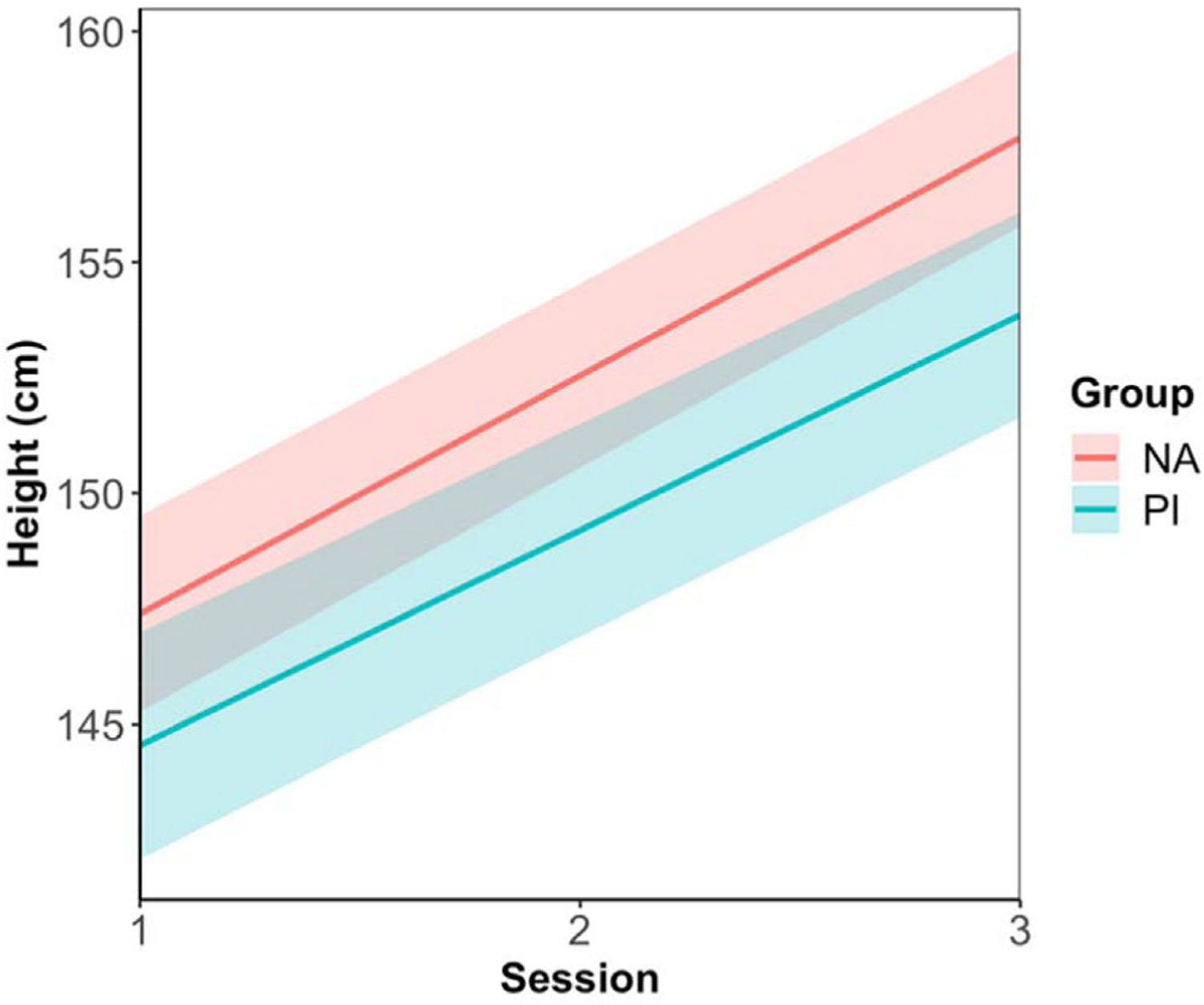
Previously institutionalized (PI) youth were shorter than nonadopted (NA) youth at the initial assessment and the difference between these two groups remained the same as puberty progressed.
4 |. DISCUSSION
The present study characterized the effect of ELS in the form of orphanage/institutional care in the first years of life and current-life stress on the growth trajectories in BMI and height in youth during puberty. Our first hypothesis that children adopted internationally from institutional care would be shorter and leaner than the comparison youth was supported. Findings showed that at our first assessment, when youth were between 7 and 15 years of age, PI youth were leaner in body composition than the family-reared group, and this was the case even though the comparison group exhibited a healthy BMI (Table S3). Thus, while still within the normal range, PI youth were generally on the lower end of the weight spectrum (Anderson et al., 2017). Though this replicates previous cross-sectional work in this cohort of PI youth (Reid et al., 2017), this finding revealed the opposite effect from what has often been seen in childhood maltreatment and trauma: childhood maltreatment and trauma exposure have been associated with the heightened risk for being overweight and obese (Shin & Miller, 2012; Whitaker et al., 2007). Two reasons may explain these contradictory findings. Child maltreatment and early deprivation may lead to different behavioral responses, diverting individuals to two opposite ends on the weight spectrum. For instance, overeating is a common coping and protective mechanism in cases of child abuse (Felitti, 1993). It could also be that in previous studies, the continuation of adverse experiences throughout childhood, not just in the first few years, was vital in supporting the emergence of obesogenic patterns of behavior. In this study, children were adopted early at approximately 1.5 years of age and spent approximately 9 years in an enriching home.
It is noteworthy that while PI youth had lower BMI scores on average than the NA group, they had faster growth in BMI (albeit within the normal range) from our first assessment over the subsequent two annual assessments. Thus, the gap in age- and-sex-adjusted BMI between PI and NA youth narrowed over time. This developmental trajectory of BMI Z-score demonstrated that catchup growth in PI youth occurred beyond infancy and early childhood (Van IJzendoorn et al., 2007), which suggested that puberty could be another period of plasticity. We also found the main effect of pubertal psychosocial stress on youth’s growth in body composition. Consistent with previous findings (Stenhammar et al., 2010; Whitaker et al., 2007), our study showed that higher psychosocial stress during puberty was associated with higher BMI for age and sex. This stress effect did not differ by group, which did not support our hypothesis that the heterogeneity in growth patterns for the PI youth would be correlated with variations in current-life stress. The lack of support for our hypothesis could be due to the lack of severe self-reported stress in both the PI and comparison youth (Figure S1). Though PI youth in this study experienced higher current-life stress than the NA group, their scores were relatively normative. Significant ongoing psychosocial stress may be necessary for ELS to result in significantly higher growth in BMI for age during the peripubertal period. Another possibility is that with years of nurturing in emotionally committed and well-resourced families, the stress and neuroendocrine systems (e.g., HPA axis) in PI youth were able to recalibrate to a level that their responsivities to life stress became comparable to family-reared nonadopted children (Gunnar et al., 2019). Therefore, the bodily reaction under stress was similar.
Using the raw measurement of BMI, youth exhibited higher BMI as they progressed through advanced stages of puberty, which is consistent with previous literature that growth accelerated as children transitioned into adolescence (Onis et al., 2007). However, there was no significant interaction between session and group, confirming that PI youth did not grow faster than the NA youth. Our results should be interpreted with some caution, as the PI group contained more female youth, who, on average, had lower BMI (see Table S3).
In terms of height, our study revealed that the height for age and sex in PI youth remained the same during puberty while BMI for age and sex showed a faster growth, which suggested that PI youth tended to grow faster in weight than height during the pubertal period. The raw height score analyses indicated that PI youth started shorter than NA youth and remained shorter in height across the three assessments (note that mean height for age was within the normal range). This finding was consistent with previous studies on growth in height among PI youth during infancy and childhood (Reid et al., 2017) and suggested that ELS could continue to impact children’s physical growth in the pubertal period. However, there was no interaction of time (session) and growth in raw height, suggesting that PI’s growth rate was similar to NA youth during puberty. Thus, the gap between PI and NA youth in height development neither increased nor decreased. Combined with our findings on BMI Z-scores, our hypothesis of an increased growth gap during puberty between children with and without ELS was not supported. It may be because PI children in the current sample received excellent care in their adoptive homes, which may have buffered the consequence of early deprived caregiving. Thus, these children could maintain catchup or typical speed of growth as they transitioned into adolescence. Additionally, while previous study showed an increasing difference in growth in children with and without histories of early institutional care, the gap was found in middle adolescence (Barke et al., 2010). In contrast, we did not find a significant interaction between puberty, session, and group. Further, our oldest youth were assessed at 15 and again at 16 and 17 years. Thus, while a faster increase in BMI-Z scofor the PI group might be found if our study continued into adulthood, we cannot test that based on the current data.
These findings should be considered in light of their limitations. First, common with longitudinal studies, this study had attrition in two follow-up data collections. However, the statistical procedures we used were robust to missingness. Additionally, we do not have detailed histories of the participants’ preadoption experiences. Relatedly, we have limited information about the birth parents and family conditions of the PI children; thus, we could not eliminate the likelihood of intergenerational effects on children’s physical growth. For example, birth parents or grandparents of PI children could have come from socioeconomically disadvantaged families who suffered from poverty and malnutrition. These adversities could affect the physical growth in multiple generations and mark children’s development even after conditions improve (Hock et al., 2020; Kuzawa & Fried, 2017). Therefore, we remind our readers of the effect of intergenerational transmissions of adversity on children’s growth when interpreting the current findings. Moreover, we did not have information about other contemporary variables, such as nutrition, associated with children’s growth outcomes. However, since dietary intake has been consistently related to socioeconomic status (Mead et al., 2010; Wardle & Steptoe, 2003), and demographics between the adoptive and comparison homes were very similar, we speculate that the nutritional environments between the PI and non-adoptive groups should not be very different.
Another point to consider is the heterogeneity of PI origin countries and races/ethnicities, which could have impacted our results. For example, some ethnicities (e.g., Hispanic) have been associated with higher risks in physical development than others (Burgard, 2002; Zilanawala et al., 2015). However, previous examinations of this issue in this sample suggest that the main findings are generalizable (Reid et al., 2017), and a sensitivity analysis of the current data showed that our results held when only the White youth in both groups were examined, thus eliminating differences in race (see Table S5).
Finally, while BMI is strongly correlated with gold standard body fat measures, it may not be an unbiased weight status and health indicator. For example, BMI cannot distinguish between lean and fat mass and does not indicate body fat distribution (Adab et al., 2018). Additionally, it does not reflect muscle composition, bone size, or age-related changes in body composition (Kurbel et al., 2008; Rothman, 2008). Therefore, our findings on BMI growth should be interpreted with caution. Future studies could include hormonal data documenting puberty status and growth curve data prior to and following participation in the study that would allow understanding of peak height velocity, peak BMI prior to puberty, and degree of catchup growth following adoption. Additionally, given the relative lack of animal models specifically examining pubertal growth trajectories after ELS, cross-species animal models would help elucidate the mechanistic underpinnings of the findings presented herein.
Despite the limitations, this study has several strengths. First, our study not only compared the initial differences in growth between PI and NA youth but also characterized developmental changes over time. This provided us with a clearer picture of whether and how children’s developmental trajectories diverge and emphasize the importance of early prevention and intervention for youth with histories of ELS. Additionally, unlike previous studies focusing on children’s development during infancy and early childhood, our study examined children’s physical growth in puberty. As puberty is a period of rapid growth and increased risk for developing obesity later in life (Simmonds et al., 2016), it is an important period of plasticity to examine the lingering effect of early deprivation. Finally, this study raised questions about whether early institutional deprivation and child maltreatment by parents/caregivers might affect obesogenic processes differently.
5 |. CONCLUSION
In conclusion, we show that PI children adopted into well-resourced homes had lower height and BMI than comparison children. During puberty, catchup growth was found in age-and-sex-adjusted BMI in the adopted youth. Psychosocial stress also affected BMI growth in youth during puberty, but this effect did not differ by group. We believe the present study adds key longitudinal data showing the long-term effect of early institutional deprivation on children’s physical growth and suggests a modification to hypotheses about the effects of early-life stress on growth stunting and obesity risk in children.
Supplementary Material
ACKNOWLEDGMENTS
We would like to express our gratitude to the families that make our research possible, the Minnesota International Adoption Project, and the Center for Neurobehavioral Development at the University of Minnesota. We also thank Tori Simenec, Bao Moua, Lea Neumann, and Heather Taylor for their assistance with the study; our nurses Janet Goodwalt, Terri Jones, and Melissa Stoll for Tanner staging; and Dr. Lorah Dorn for providing training in pubertal assessment. This study was funded by a grant R01 HD075349 (to MG) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institute of health (NIH) and supported in part by the Center for Neurobehavioral Development, University of Minnesota. This work was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number T32HD101392 (fellow: Dr. BM Reid). The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
Funding information
Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) of the National Institute of Health (NIH), Grant/Award Numbers: R01HD075349, T32HD101392
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adab P, Pallan M, & Whincup PH (2018). Is BMI the best measure of obesity? British Medical Journal, 360, 361. [DOI] [PubMed] [Google Scholar]
- Adair LS, & Cole TJ (2003). Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension, 41(3), 451–456. [DOI] [PubMed] [Google Scholar]
- Adrian C, & Hammen C (1993). Stress exposure and stress generation in children of depressed mothers. Journal of Consulting and Clinical Psychology, 61(2), 354–359. [DOI] [PubMed] [Google Scholar]
- Akaike H (1973). Information theory as an extension of the maximum likelihood principle. Proceedings of the 2nd International Symposium on Information Theory (pp. 267–281). In Petrov BN, & Csaki F (Eds.). Akademiai Kiado. [Google Scholar]
- Akaike H (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6), 716–723. [Google Scholar]
- Anderson LN, Carsley S, Lebovic G, Borkhoff CM, Maguire JL, Parkin PC, & Birken CS (2017). Misclassification of child body mass index from cut-points defined by rounded percentiles instead of Z-scores. BMC Research Notes, 10(1), 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barke E, Schlotz W, & Rutter M VII. (2010). Physical growth and maturation following early severe institutional deprivation: Do they mediate specific psychopathological effects? Monographs of the Society for Research in Child Development, 75(1), 143–166. [DOI] [PubMed] [Google Scholar]
- Bennett DS, Sullivan M, Thompson SM, & Lewis M (2010). Early child neglect: does it predict obesity or underweight in later childhood? Child Maltreatment, 15(3), 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit TC, Jocelyn LJ, Moddemann DM, & Embree JE (1996). Romanian adoption: The Manitoba experience. Archives of Pediatrics & Adolescent Medicine, 150(12), 1278–1282. [DOI] [PubMed] [Google Scholar]
- Bentley T, & Widom CSA (2009). 30-year follow-up of the effects of child abuse and neglect on obesity in adulthood. Obesity, 17(10), 1900–1905. [DOI] [PubMed] [Google Scholar]
- Burgard S (2002). Does race matter? Children’s height in Brazil and South Africa. Demography, 39(4), 763–790. [DOI] [PubMed] [Google Scholar]
- Danese A, & Tan M (2014). Childhood maltreatment and obesity: Systematic review and meta-analysis. Molecular Psychiatry, 19(5), 544. [DOI] [PubMed] [Google Scholar]
- DePasquale CE, Donzella B, & Gunnar MR (2019). Pubertal recalibration of cortisol reactivity following early life stress: A cross-sectional analysis. Journal of Child Psychology and Psychiatry, 60(5), 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund U, Ong K, Linné Y, Neovius M, Brage S, Dunger DB, Wareham NJ, & Rössner S (2006). Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: The Stockholm Weight Development Study (SWEDES). American Journal of Clinical Nutrition, 83(2), 324–330. [DOI] [PubMed] [Google Scholar]
- Felitti VJ (1993). Childhood sexual abuse, depression, and family dysfunction in adult obese patients: A case control study. Southern Medical Journal, 86(7), 732–736. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, & Tottenham N (2013). Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences, 110(39), 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Black JE, & Wallace CS (1987). Experience and brain development. Child Development, 539–559. [PubMed] [Google Scholar]
- Groze V, & Ileana D (1996). A follow-up study of adopted children from Romania. Child and Adolescent Social Work Journal, 13(6), 541–565. [Google Scholar]
- Gunnar MR, DePasquale CE, Reid BM, Donzella B, & Miller BS (2019). Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proceedings of the National Academy of Sciences, 116(48), 23984–23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, & Vazquez DM (2001). Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology, 13(3), 515–538. [DOI] [PubMed] [Google Scholar]
- Hock RS, Rabinowitz AG, Bryce CP, Fitzmaurice GM C PT Jr, & Galler JR (2020). Intergenerational effects of childhood maltreatment and malnutrition on personality maladaptivity in a Barbadian longitudinal cohort. Psychiatry Research, 113016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE (2002). Adoption and the effect on children’s development. Early Human Development, 68(1), 39–54. [DOI] [PubMed] [Google Scholar]
- Johnson W, Onuma O, Owolabi M, & Sachdev S (2016). Stoke: a global response is needed. Bulletin of the World Health Organization, 94(9), 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D, Banerji MA, Shorman I, Smith EL, Coplan JD, Rosenblum LA, & Kral JG (2007). Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes, 56(5), 1382–1386. [DOI] [PubMed] [Google Scholar]
- Kelishadi R, Haghdoost AA, Jamshidi F, Aliramezany M, & Moosazadeh M (2015). Low birthweight or rapid catch-up growth: which is more associated with cardiovascular disease and its risk factors in later life? a systematic review and cryptanalysis. Paediatrics and Child Health, 35(2), 110–123. [DOI] [PubMed] [Google Scholar]
- Kurbel S, Zucić D, Vrbanec D, & Pleština S (2008). Comparison of BMI and the body mass/body surface ratio: Is BMI a biased tool? Collegium Antropologicum, 32(1), 299–301. [PubMed] [Google Scholar]
- Kuzawa CW, & Fried RL (2017). Intergenerational memories of past nutritional deprivation: The phenotypic inertia model. In Sherry DS, Jasienska G, & Holmes DJ, (Eds.), The arc of life: Evolution and health over the life course. Harvard University Press. [Google Scholar]
- Le Mare L, & Audet K (2006). A longitudinal study of the physical growth and health of postinstitutionalized Romanian adoptees. Paediatrics and Child Health, 11(2), 85–91. [PMC free article] [PubMed] [Google Scholar]
- Lissau I, & Sorensen TI (1994). Parental neglect during childhood and increased risk of obesity in young adulthood. Lancet, 343(8893), 324–327. [DOI] [PubMed] [Google Scholar]
- Long JD (2012). Longitudinal data analysis for the behavioral sciences using R. Sage. [Google Scholar]
- Marshall WA, & Tanner JM (1969). Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood, 44(235), 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, & Tanner JM (1970). Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood, 45(239), 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead E, Gittelsohn J, Roache C, & Sharma S (2010). Healthy food intentions and higher socioeconomic status are associated with healthier food choices in an Inuit population. Journal of Human Nutrition and Dietetics, 23, 83–91. [DOI] [PubMed] [Google Scholar]
- Miller BS, Kroupina MG, Mason P, Iverson SL, Narad C, Himes JH, Johnson DE, & Petryk A (2010). Determinants of catch-up growth in international adoptees from Eastern Europe. International Journal of Pediatric Endocrinology, 2010(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LC, & Hendrie NW (2000). Health of children adopted from China. Pediatrics, 105(6), e76–e76. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Bartley MJ, & Wilkinson RG (1997). Family conflict and slow growth. Archives of Disease in Childhood, 77(4), 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA (2000). Neural plasticity and human development: The role of early experience in sculpting memory systems. Developmental Science, 3(2), 115–136. [Google Scholar]
- Noll JG, Zeller MH, Trickett PK, & Putnam FW (2007). Obesity risk for female victims of childhood sexual abuse: A prospective study. Pediatrics, 120(1), e61–e67. [DOI] [PubMed] [Google Scholar]
- Oliván G (2003). Catch-up growth assessment in long-term physically neglected and emotionally abused preschool age male children. Child Abuse and Neglect, 27(1), 103–108. [DOI] [PubMed] [Google Scholar]
- Ong KK, Ahmed ML, Emmett PM, Preece MA, & Dunger DB (2000). Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. British Medical Journal, 320(7240), 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onis MD, Onyango AW, Borghi E, Siyam A, Nishida C, & Siekmann J (2007). Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World Health Organization, 85, 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios J, Román M, Moreno C, León E, & Peñarrubia MG (2014). Differential plasticity in the recovery of adopted children after early adversity. Child Development Perspectives, 8(3), 169–174. [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, & Bates DM (2000). Linear mixed-effects models: Basic concepts and examples. In Mixed-effects models in S and S-Plus, statistics and computing (pp. 3–56). Springer. [Google Scholar]
- Pomerleau A, Malcuit G, Chicoine J-F, Séguin R, Belhumeur C, Germain P, Amyot I, & Jéliu G (2005). Health status, cognitive and motor development of young children adopted from China, East Asia, and Russia across the first 6 months after adoption. International Journal of Behavioral Development, 29(5), 445–457. [Google Scholar]
- Proos LA, Hofvander Y, & Tuvemo T (1991). Menarcheal age and growth pattern of Indian girls adopted in Sweden: I. Menarcheal age. Acta Paediatr, 80(8–9), 852–858. [DOI] [PubMed] [Google Scholar]
- Reid BM, Miller BS, Dorn LD, Desjardins C, Donzella B, & Gunnar M (2017). Early growth faltering in post-institutionalized youth and later anthropometric and pubertal development. Pediatric Research, 82(2), 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ (2008). BMI-related errors in the measurement of obesity. International Journal of Obesity, 32(3), S56–S59. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, & Hammen C (1999). Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Development, 70(3), 660–677. [DOI] [PubMed] [Google Scholar]
- Rutter M (1996). Romanian orphans adopted early overcome deprivation. Brown University Child and Adolescent Behavior Letter, 12(6), 1–3. [Google Scholar]
- Rutter M (1998). Developmental catch-up, and deficit, following adoption after severe global early privation. Journal of Child Psychology and Psychiatry, 39(4), 465–476. [PubMed] [Google Scholar]
- Schipper L, van Heijningen S, Karapetsas G, van der Beek EM, & van Dijk G (2020). Individual housing of male C57BL/6J mice after weaning impairs growth and predisposes for obesity. PloS One, 15(5), e0225488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard P, Garcia JR, & Sear R (2015). Childhood family disruption and adult height: Is there a mediating role of puberty? Evolution, Medicine and Public Health, 2015(1), 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, & Miller DP (2012). A longitudinal examination of childhood maltreatment and adolescent obesity: Results from the National Longitudinal Study of Adolescent Health (AddHealth) Study. Child Abuse and Neglect, 36(2), 84–94. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, & Pollak SD (2009). Pubertal development: Correspondence between hormonal and physical development. Child Development, 80(2), 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds M, Llewellyn A, Owen CG, & Woolacott N (2016). Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obesity Reviews, 17(2), 95–107. [DOI] [PubMed] [Google Scholar]
- Singhal A (2017). Long-term adverse effects of early growth acceleration or catch-up growth. Annals of Nutrition & Metabolism, 70(3), 236–240. [DOI] [PubMed] [Google Scholar]
- Stellern S, Esposito E, Mliner S, Pears K, & Gunnar M (2014). Increased freezing and decreased positive affect in postinstitutionalized children. Journal of Child Psychology and Psychiatry, 55(1), 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenhammar C, Olsson G, Bahmanyar S, Hulting AL, Wettergren B, Edlund B, & Montgomery S (2010). Family stress and BMI in young children. Acta Paediatrica, 99(8), 1205–1212. [DOI] [PubMed] [Google Scholar]
- Tang A, Slopen N, Nelson CA, Zeanah CH, Georgieff MK, & Fox NA (2018). Catch-up growth, metabolic, and cardiovascular risk in post-institutionalized Romanian adolescents. Pediatric Research, 84(6), 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, & Gunnar MR (2006). Child maltreatment and the developing HPA axis. Hormones and Behavior, 50(4), 632–639. [DOI] [PubMed] [Google Scholar]
- Tottenham N (2012). Human amygdala development in the absence of species-expected caregiving. Developmental Psychobiology, 54(6), 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, & Casey BJ (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1), 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ, & Juffer F (2007). Plasticity of growth in height, weight, and head circumference: meta-analytic evidence of massive catch-up after international adoption. Journal of Developmental and Behavioral Pediatrics, 28(4), 334–343. [DOI] [PubMed] [Google Scholar]
- Wardle J, & Steptoe A (2003). Socioeconomic differences in attitudes and beliefs about healthy lifestyles. Journal of Epidemiology and Community Health, 57(6), 440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker RC, Phillips SM, Orzol SM, & Burdette HL (2007). The association between maltreatment and obesity among preschool children. Child Abuse and Neglect, 31(11–12), 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilanawala A, Davis-Kean P, Nazroo J, Sacker A, Simonton S, & Kelly Y (2015). Race/ethnic disparities in early childhood BMI, obesity and overweight in the United Kingdom and United States. International Journal of Obesity, 39(3), 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study are available from the corresponding author upon reasonable request.


