Abstract
Polar transport of the plant hormone auxin controls many aspects of plant growth and development. A number of synthetic compounds have been shown to block the process of auxin transport by inhibition of the auxin efflux carrier complex. These synthetic auxin transport inhibitors may act by mimicking endogenous molecules. Flavonoids, a class of secondary plant metabolic compounds, have been suggested to be auxin transport inhibitors based on their in vitro activity. The hypothesis that flavonoids regulate auxin transport in vivo was tested in Arabidopsis by comparing wild-type (WT) and transparent testa (tt4) plants with a mutation in the gene encoding the first enzyme in flavonoid biosynthesis, chalcone synthase. In a comparison between tt4 and WT plants, phenotypic differences were observed, including three times as many secondary inflorescence stems, reduced plant height, decreased stem diameter, and increased secondary root development. Growth of WT Arabidopsis plants on naringenin, a biosynthetic precursor to those flavonoids with auxin transport inhibitor activity in vitro, leads to a reduction in root growth and gravitropism, similar to the effects of synthetic auxin transport inhibitors. Analyses of auxin transport in the inflorescence and hypocotyl of independent tt4 alleles indicate that auxin transport is elevated in plants with a tt4 mutation. In hypocotyls of tt4, this elevated transport is reversed when flavonoids are synthesized by growth of plants on the flavonoid precursor, naringenin. These results are consistent with a role for flavonoids as endogenous regulators of auxin transport.
A critical determinant in controlling plant growth is the appropriate distribution of plant hormones. One class of hormones, the auxins, has been implicated in regulating the rate of organ elongation, photo- and gravitropism, and morphology (Lomax et al., 1995; Palme and Galweiler, 1999). Auxin moves from cell to cell in a polar fashion, with a basipetal polarity in stems and a more complex polarity in roots (Lomax et al., 1995). Polar auxin transport is controlled by several types of proteins, including auxin influx carriers and auxin efflux carriers, which pump auxin into and out of plant cells, respectively. Biochemical and genetic approaches have provided much information on the auxin efflux carrier (Palme and Galweiler, 1999; Muday, 2000) and the auxin influx carrier (Bennett et al., 1998; Marchant et al., 1999), yet the mechanisms by which transport is modulated during plant growth and development are largely unclear (Lomax et al., 1995). As a number of synthetic auxin transport inhibitors that act at the site of auxin efflux have been characterized (Rubery, 1990), one intriguing possibility is that naturally occurring small molecules regulate the activity of the auxin efflux carrier. The synthesis of these endogenous auxin transport inhibitors may be modulated by environmental or developmental changes to provide one level of regulation of the process of auxin transport (Lomax et al., 1995). One class of compounds that may act as such endogenous regulators is the flavonoids (Jacobs and Rubery, 1988).
A number of lines of experimentation have suggested that specific classes of flavonoid compounds may act as auxin transport inhibitors in vitro. The idea that phenolic compounds might block auxin transport was first proposed in the 1970s (Stenlid, 1976; Marigo and Boudet, 1977). Plants grown on quinic acid accumulated phenolic compounds, including but not limited to flavonoids, and had reduced auxin transport (Marigo and Boudet, 1977). Also, some flavonoids reduce polar auxin transport in zucchini hypocotyls (Jacobs and Rubery, 1988). A range of flavonoid compounds have been screened for their ability to block binding of a synthetic auxin transport inhibitor, naphthylphthalamic acid (NPA) and to inhibit auxin movement from hypocotyl segments (Jacobs and Rubery, 1988; Rubery and Jacobs, 1990). A strong correlation was found between the activity of flavonoid derivatives in these two assays (Jacobs and Rubery, 1988). Quercetin, the most active flavonoid in the studies of Jacobs and Rubery (1988), is a competitive inhibitor of NPA binding, suggesting that the two compounds may bind to the same protein. In addition, quercetin, kaempferol, and bestatin displace NPA binding to membranes isolated from Arabidopsis plants, and auxin transport is altered in young Arabidopsis seedlings that produce no flavonoids (Murphy et al., 2000). One report (Fischer et al., 1997) indicates that quercetin produces developmental alterations that are similar to those produced by NPA in wheat embryos. In vivo data demonstrating that changes in endogenous flavonoid concentration lead to changes in auxin transport would strengthen the hypothesis that flavonoids are endogenous regulators of auxin transport.
Flavonoids are reasonable candidates for endogenous regulators of auxin transport for several reasons that have been discussed previously (Jacobs and Rubery, 1988; Rubery and Jacobs, 1990). Endogenous regulators should be widely distributed throughout the plant kingdom to function as regulators of auxin transport in a diversity of species. Flavonoids have such a distribution (Stafford, 1990). There should be a tight relationship between structure and function that allows a narrow subset of chemical modifications to lead to auxin transport inhibitor activity. With flavonoids, the basic chemical backbone shows a diversity of modifications that may lead to compounds with a diversity of functions (Stafford, 1990). The synthesis of endogenous regulators should be tied to environmental and/or developmental changes that result in alterations in auxin transport. Flavonoid biosynthesis is highly regulated by environmental factors and changes through development such that these changes could lead to altered auxin transport. Light, wounding, pathogens, symbiotic bacteria, and development (Schmid et al., 1990; Feinbaum et al., 1991; Kubasek et al., 1992; Yang et al., 1992; Shirley et al., 1995; Shirley, 1996; Sakuta, 2000) regulate the synthesis of enzymes that control flavonoid biosynthesis. Several genes that encode enzymes in the flavonoid biosynthetic pathway, including chalcone synthase (CHS), the first committed step of the pathway, are induced by UV and blue light (Feinbaum and Ausubel, 1988; Feinbaum et al., 1991; Jackson et al., 1995). UV and blue light also affect seedling morphology and development (von Arnim and Deng, 1996). The resulting changes in flavonoid concentration and distribution in response to changes in light or other environmental factors could regulate auxin transport to allow growth changes in response to differing environmental conditions. The localization of endogenous auxin transport inhibitors must be in the tissues and cellular compartments in which auxin transport is regulated. In Arabidopsis, the CHS gene is expressed in roots and shoots, and inflorescence tissues (Chory and Peto, 1990; Brown, 1998). Flavonoids are localized to the tissues that transport auxin (Murphy et al., 2000; Peer et al., 2001) and to the plasma membrane (Peer et al., 2001), where the auxin transport inhibitor binding site is localized (Dixon et al., 1996). Therefore, flavonoids have the characteristics that make them suitable as endogenous regulators of auxin transport.
This manuscript contains experiments that support the hypothesis that flavonoids are endogenous negative regulators of auxin transport. Arabidopsis plants were grown on agar plates containing the flavonoid precursor, naringenin, with the goal of elevating the concentration of flavonoids. Growth on naringenin led to inhibition of root elongation and gravitropism, just as in plants grown on synthetic auxin transport inhibitors. Phenotypic analysis of mutant Arabidopsis plants defective in flavonoid biosynthesis indicates growth and developmental changes that are consistent with elevated auxin transport. In addition, Arabidopsis mutants with genetic lesions leading to the lack of flavonoid biosynthesis have higher levels of auxin transport in the inflorescence and the hypocotyl. Together, these results are consistent with flavonoids acting as endogenous negative regulators of auxin transport.
RESULTS
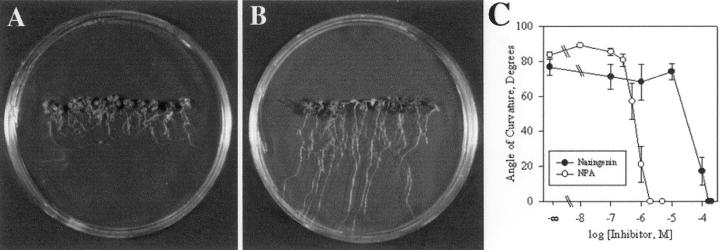
One characteristic expected of an endogenous regulator of polar auxin transport is the ability to inhibit root growth and gravitropism in a fashion similar to synthetic auxin transport inhibitors. Naringenin is an early intermediate in the flavonoid biosynthetic pathway that is taken up by Arabidopsis roots and is converted into later products in the pathway (Shirley et al., 1995). In Figure 1, A and B, the growth of wild-type (WT) Arabidopsis roots on 100 μm naringenin and control medium is compared. It is apparent that prolonged growth (12 d) at this concentration resulted in reduced elongation. In addition, roots grown on naringenin are agravitropic, as indicated by the absence of downward growth. When root gravitropism is inhibited by application of auxin transport inhibitors, Arabidopsis roots of the Columbia ecotype grow with a curve or slant in a consistent direction. Similar inhibition of root gravitropism is shown in Figure 1A, when plants are grown on the flavonoid precursor naringenin.
Figure 1.
The effect of naringenin and NPA on Arabidopsis root development. Photographs showing seedlings grown for 12 d on 100 μm naringenin (A) or 0.1% (v/v) ethanol control (B). C, Roots were grown for 12 d on naringenin and NPA. Gravity response was measured 24 h after reorientation of roots 90 degrees relative to the gravity vector. Values represent the average and se of 10 seedlings per data point.
Auxin transport inhibitors lead to a dose-dependent decrease in root growth and gravitropism. Therefore, the effects of a range of concentrations of naringenin and the synthetic auxin transport inhibitor, NPA, on root growth and gravitropism were compared. Plants were grown vertically on media containing a range of concentrations of NPA or naringenin for 24 h, after which the plants were reoriented by 90° to horizontal. Root gravitropic angles, 24 h after reorientation, were measured and are reported in Figure 1C. In the absence of NPA or naringenin, root gravitropism is at an angle of approximately 80°, whereas treatment with high con-centrations of naringenin or NPA abolishes gravity response completely. Primary root gravitropism was reduced at naringenin concentrations of 100 μm and higher and by NPA at concentrations of 0.5 μm and higher. Root growth was also inhibited by NPA or naringenin, in a dose-dependent fashion (data not shown) such that the concentration for 50% inhibition (IC50) could be calculated. The IC50 for growth and gravitropism were calculated for naringenin and are 66 and 39 μm, respectively, for this representative experiment. IC50 values have been previously reported for NPA and are 4.8 and 0.5 μm for growth and gravity inhibition, respectively (Rashotte et al., 2000). For NPA and naringenin, the gravity response was inhibited at lower concentrations than the growth response, yet both responses were more sensitive to inhibition by NPA than naringenin. The high levels of naringenin that are required to reduce growth and gravitropism may be due to several mechanisms. Higher concentrations of flavonoids may be necessary for inhibition of auxin transport, as suggested by in vitro experiments (Jacobs and Rubery, 1988). Also, greater retention of flavonoids in the agar matrix or cell wall resulting in less penetrance of these compounds into the seedlings may also account for these differences, as discussed previously (Jacobs and Rubery, 1988; Rubery and Jacobs, 1990).
Transparent Testa 4(tt4)(2YY6) Has Altered Growth and Development
In testing the hypothesis that flavonoids regulate auxin transport in vivo, the focal point of this study was the analysis of flavonoid-deficient plants with mutations in a gene encoding a flavonoid biosynthetic enzyme. The tt4(2YY6) allele has been shown through a variety of molecular and biochemical analyses to be a null mutant for flavonoid biosynthesis (Burbulis et al., 1996; Saslowsky et al., 2000). The analyses of flavonoid-deficient mutants included phenotypic analysis and direct measurement of auxin transport in comparison with parental strains.
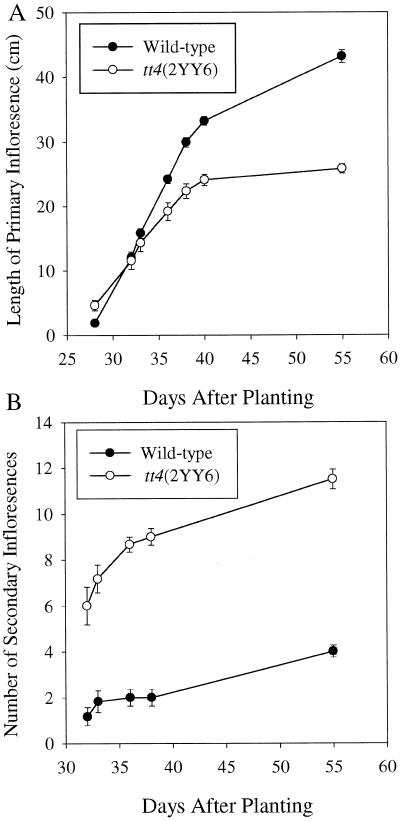
If flavonoids are endogenous negative regulators of auxin transport, then altered growth characteristics consistent with elevated auxin transport are expected in mutant plants that do not synthesize flavonoids. When compared with WT Columbia plants, tt4(2YY6) plants have distinct aerial and root phenotypes that are apparent, as can be seen in Figure 2. The mature tt4(2YY6) plants lack anthocyanins, have reduced apical dominance, and a reduced primary inflorescence length as compared with WT plants, as shown in Figure 2A. The length of the primary inflorescence was quantified over time for WT and tt4(2YY6) in Figure 3A. Although inflorescence lengths are initially similar, tt4(2YY6) plants have slower inflorescence growth rates. Reduced apical dominance was indicated by the increased number of secondary inflorescence stems and lateral branches in tt4(2YY6), as shown in Figure 3B. Root branching, which includes adventitious and lateral root formation, was increased in tt4(2YY6), as shown in Figure 2B. In tt4(2YY6) seedlings, lateral and adventitious roots initiated earlier and were 2- and 2.8-fold more abundant, respectively, when compared with WT seedlings. Primary root length was slightly longer in tt4(2YY6) seedlings than WT seedlings, as shown in Table I.
Figure 2.
Comparison of phenotype of WT and tt4(2YY6) plants. A, The aerial phenotype of representative WT (left) and tt4(2YY6) (right) plants were compared 37 d after planting. B, Secondary root development of three WT seedlings (left) and three tt4(2YY6) seedlings (right) grown under continuous light for 13 d are compared.
Figure 3.
Quantification of inflorescence phenotypes of WT and tt4 (2YY6) plants. A, Primary inflorescence height was monitored from d 28 until d 55 after planting. B, The number of secondary inflorescences were measured over time.
Table I.
Phenotypic analysis of WT and tt4(2YY6) Arabidopsis plants
| WT | tt4(2YY6)a | |
|---|---|---|
| Adult aerial phenotypeb | ||
| Primary inflorescence length (cm) | 43 ± 1 | 29 ± 1** |
| Number of lateral branches | 2.5 ± 0.2 | 1.8 ± 0.2* |
| Inflorescence stem diameter (mm) | 1.1 ± 0.04 | 0.70 ± 0.02** |
| No. of 2° inflorescence stems | 4.0 ± 0.3 | 12.0 ± 0.4** |
| Anthocyanins | Yes | No |
| Root developmentc | ||
| Adventitious root no. | 0.8 ± 0.2 | 2.2 ± 0.3** |
| Lateral root no. | 4.5 ± 0.5 | 8.8 ± 0.9** |
| Primary root length (mm) | 35 ± 0.8 | 38 ± 1.1* |
The phenotypic characteristics were compared for WT and tt4(2YY6) using a two-tailed Student's t test and the P values are reported.
, P < 0.05;
, P < 0.001.
Fifty-five-day-old plants were grown in a 21°C incubator programmed for 16 h of light (90 μmol s−1 m−2) followed by 8 h of dark. Reported values are the average and se of six individual plants per genotype.
Seven-day-old seedlings were grown under continuous white light (80–90 μmol s−1 m−2) at room temperature. Reported values are the average and se of 10 seedlings per genotype.
The possibility that the phenotypic differences in tt4(2YY6) are due to another unlinked mutation was addressed in two ways. The tt4(2YY6) plants were backcrossed twice to minimize the chance of another unlinked mutation. The phenotypes quantified above cosegregated with the anthocyanin-deficient phenotype of the tt4(2YY6) mutation. When another tt4 allele, tt4(85), was examined, many of these phenotypic differences were also found between tt4(85) and Landsberg erecta (Ler; data not shown).
Plants with Two tt4 Alleles Have Elevated Inflorescence Basipetal Auxin Transport
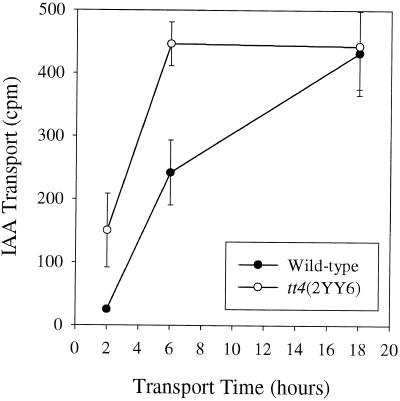
To correlate phenotypic data with alterations in auxin transport, it was necessary to compare basipetal auxin transport in each of the two alleles of tt4 with their WT parent. [3H]Indole-3-acetic acid (IAA) transport was measured in WT and tt4(2YY6) plants and is plotted as function of the duration of the transport assay in Figure 4. IAA transport is elevated in tt4(2YY6) relative to WT plants and the differences between WT and tt4(2YY6) were more pronounced at earlier time points. Maximum transport differences were observed at 6 h, with IAA transport being 2-fold greater in tt4(2YY6). In WT, transport of [3H]IAA increased linearly over time; however, transport of IAA in tt4(2YY6) became saturated at 6 h (Fig. 4). At 18 h, transport of [3H]IAA in WT and tt4(2YY6) segments was similar, which suggested that physiological changes may have taken place within the excised segments that limited the maximum amount of transport, or that transport had saturated after 18 h in WT and the mutant plants. Most measurements of auxin transport in the inflorescence of Arabidopsis use an 18-h transport period (Okada et al., 1991); however, estimated IAA transport rates of 5 to 20 mm h−1 have been reported for most tissues (Lomax et al., 1995). Because the segments used in these experiments measured 2.5 cm in length, one would have expected [3H]IAA to be detectable at the unsubmerged end of the segment between 1.25 and 5 h. Therefore, assays with shorter duration are practically and theoretically more appropriate for comparisons of auxin transport between WT and mutant Arabidopsis plants.
Figure 4.
Comparison of basipetal IAA transport over time in WT and tt4(2YY6) plants. Transport at 2, 6, or 18 h was measured using 28 nm [3H]IAA. Each value represents the average and se of four segments.
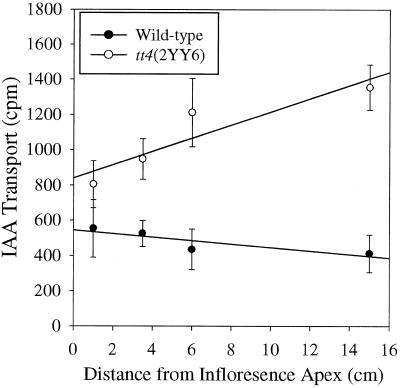
To identify the region of the inflorescence stem most appropriate for comparisons of transport between WT and tt4(2YY6), auxin transport was measured in adjacent segments down the length of the inflorescence stem, as shown in Figure 5. As IAA transport in adjacent stem segments of WT were compared, it became apparent that basipetal IAA transport in WT remained fairly equivalent between apical segments and decreased slightly as the distance from the inflorescence apex increased. Transport was 25% lower in basal segments when compared with apical segments, for the representative experiment shown in Figure 5. Thus, auxin transport was decreased slightly in basal segments of WT.
Figure 5.
Comparison of [3H]IAA transport measurements down the inflorescence stem of WT and tt4(2YY6) plants. Transport was measured on adjacent segments down the stem after 9 h in 134 nm [3H]IAA. Data represent the average and se of five segments. IAA transport in the presence of NPA was similar between WT and tt4(2YY6), averaging 98 and 96 cpm, respectively.
In tt4(2YY6), auxin transport was 170% higher in basal segments than in apical segments. As a consequence, the most prominent difference in auxin transport between WT and tt4(2YY6) was observed in basal inflorescence segments where tt4(2YY6) exhibited an almost 300% greater IAA transport than in WT, in the representative experiment shown in Figure 5. When the magnitude of the difference between WT and tt4(2YY6) is averaged for five separate experiments, the magnitude of the difference is even greater at 380%. Therefore, further experimentation was performed using basal segments, as these showed the largest differences between WT and tt4(2YY6).
The amount of basipetal [3H]IAA transport in the inflorescence of tt4(2YY6) and tt4(85) after 5 h was compared with that of the WT parents. This analysis was performed with tt4(2YY6) seeds that had been subjected to two backcrosses. As shown in Table II, there is a clear and quantitative increase in IAA transport in tt4(2YY6) and tt4(85), relative to WT. There is close to 200% more transport in both alleles of tt4, and this increase is statistically significant, as judged by Student's t test. Parental strains Ler and Columbia had similar levels of transport. The lower amount of IAA transport in WT Columbia and tt4(2YY6) in the presence of NPA is due to higher amounts of NPA used. When 10 μm NPA was used in assays with WT Columbia and tt4(2YY6), the values were similar to those with Ler and tt4(85) (data not shown).
Table II.
Basipetal IAA transport in Arabidopsis inflorescence segments
| Basipetal IAA Transporta
|
||||
|---|---|---|---|---|
| Columbiab | tt4(2YY6)b | Lerc | tt4(85)c | |
| cpm | ||||
| Minus NPAd | 667 ± 100 | 1176 ± 114 | 675 ± 41 | 1131 ± 41 |
| Plus NPAd | 15 ± 1 | 17 ± 1 | 254 ± 36 | 255 ± 37 |
IAA transport was measured on basal inflorescence segments after 5 h using 100 nm [3H]-IAA.
Reported values are average and se of 30 to 40 replicates and transport was measured in the presence and absence of 100 μm NPA.
Reported values are the average and se of 10 replicates and transport was measured in the presence and absence of 10 μm NPA.
Comparison of basipetal IAA transport in the absence of NPA indicates that the difference between Columbia and tt4(2YY6) and between Ler and tt4(85) are statistically different with P values < 0.001, as determined by Student's t test analysis. In the presence of NPA there is no significant difference between basipetal transport in Columbia and tt4(2YY6) or Ler and tt4(85) with P values > 0.2, as determined by Student's t test.
Auxin Transport Is Elevated in tt4 Seedlings and the Elevation Is Reversed by Naringenin
Auxin transport in the tt4(85) seedlings in the Ler background was also examined. For these assays [14C]IAA was applied in a drop to the top of hypocotyls of Ler and tt4(85) seedlings and after 5 h, a 2-mm segment at the base of the hypocotyl and a 2-mm basal root segment were excised and the radioactivity in each segment was determined separately. Auxin transport into the lower hypocotyl or root of tt4(85) was elevated 130% and 200%, respectively, compared with Ler, as shown in Table III. Supplying the tt4(85) seedlings with a 10 nm concentration of the flavonoid precursor naringenin reduced auxin transport in the hypocotyl to WT levels (508 versus 517 cpm), whereas the addition of naringenin to the WT plants reduced auxin transport to the level observed in seedlings treated with 10 nm NPA (354 versus 354 cpm).
Table III.
Basipetal IAA transport in WT Ler and tt4(85) seedlings
| Basipetal IAA Transporta
|
||||
|---|---|---|---|---|
| Hypocotyl
|
Root
|
|||
| Ler | tt4(85) | Ler | tt4(85) | |
| cpm | ||||
| Control mediumb | 517 ± 33 | 683 ± 38 | 136 ± 2 | 274 ± 4 |
| Naringenin | 354 ± 44 | 508 ± 14 | 66 ± 10 | 52 ± 6 |
| NPA | 354 ± 16 | 385 ± 23 | 66 ± 4 | 89 ± 17 |
IAA transport was measured in hypocotyl and root segments grown on control medium in the presence or absence of 10 nm naringenin or NPA. Reported values are the average and sd of 10 replicates.
Comparison of basipetal IAA transport in the absence of NPA indicates that the difference between WT Ler and tt4(85) is statistically different with a P < 0.005, as determined by Student's t test analysis.
When auxin transport into the roots of these plants is examined, there are several important differences. The magnitude of the increase in transport in tt4(85) relative to Ler is larger than in hypocotyls, being greater than 200%. The other difference is in the effectiveness of naringenin in reducing auxin transport to basal levels. Growth of Ler or tt4(85) on 10 nm naringenin reduces transport into the root to levels equivalent or lower than levels with treatment with 10 nm NPA. These results suggest a role for flavonoids in controlling the amount of auxin moving from the shoot into the root, which is consistent with the altered root phenotypes of the tt4 mutants, shown above, and localized flavonoid accumulation at the root shoot junction (Murphy et al., 2000).
Similar experiments performed using the tt4(2YY6) allele also indicated an elevation of hypocotyl auxin transport, as compared with WT Columbia, and identified that naringenin could reduce tt4(2YY6) transport to WT levels (data not shown). It should be noted that much less naringenin was needed in this transport assay to reverse the tt4 phenotype than to inhibit root growth and gravitropism (Fig. 1). In Table III, much less naringenin was used, as the goal was to restore flavonoid synthesis in tt4 plants to WT levels. In Figure 1, the goal was to elevate flavonoids to levels above normal to determine if flavonoids had similar effects to exogenously supplied synthetic auxin transport inhibitors. An additional difference is the method by which naringenin was supplied to plants in these two experiments. The dose-response curve for these two types of applications are parallel, but much more naringenin is needed when it is added to agar than when seedlings are grown on filter paper. This may be due to interactions between naringenin and the agar matrix that reduce naringenin availability to the plant, which have been reported previously (Rubery and Jacobs, 1990). In addition, Suc concentrations and salts in the media influence naringenin uptake and conversion to flavonoids (A.S. Murphy and W.A. Peer, unpublished data).
WT and tt4(2YY6) Plants Are Equally Sensitive to NPA
To rule out the possibility that there were differences in the auxin efflux carrier complex in tt4(2YY6) seedlings, the ability of NPA to inhibit auxin transport in tt4(2YY6) seedlings and the NPA sensitivity of tt4(2YY6) roots was compared with WT in several ways. The ability of NPA to inhibit root elongation and gravitropism in WT and tt4(2YY6) was compared (Fig. 1; data not shown). The IC50 values for root gravitropism inhibition were similar for WT and tt4(2YY6), indicating no significant difference in NPA sensitivity.
Comparison of the effect of NPA on IAA transport in WT and tt4(2YY6) inflorescences did not reveal any differences in the ability of NPA to regulate transport. Tables II and III show that in the presence of NPA, basipetal IAA transport was reduced to similar levels in WT and tt4(2YY6) or tt4(85). This indicates that the sensitivity of transport to inhibition by NPA was not altered in either tt4 mutant.
NPA binding to microsomal membranes from WT and tt4(2YY6) was assayed. Analysis of NPA binding data confirmed that NPA binding constants were similar in microsomal membranes prepared from WT and tt4(2YY6) rosettes, as shown in Table IV. These results together suggest that changes in auxin transport in tt4(2YY6) were not correlated with changes in abundance of NPA binding activity or the ability of NPA to regulate transport. Therefore, no functional differences in the auxin efflux carrier complex were detectable in the tt4 mutants.
Table IV.
Comparison of [3H]-NPA binding constants in WT and tt4(2YY6) rosette microsomal membranes
Reported values were derived from double-reciprocal plots and represent the average and se of four experiments using six concentrations of [3H]-NPA. Kd is calculated as −1/x intercept and is reported in nanomoles; Bmax is defined as 1/y intercept and is reported as picomoles per milligram.
Student's t test analysis revealed no significant difference in Kd or Bmax values between WT and tt4(2YY6) microsomal membranes.
Determination of Free IAA Concentrations in WT and tt4(2YY6) Plants
Although there are clear differences in IAA transport in the two tt4 mutants as compared with their parental strains, it is possible that flavonoids may alter IAA homeostasis. The endogenous levels of free IAA in the inflorescence tissue of tt4(2YY6) were compared with WT. Segments of the apex and base of the inflorescence of tt4(2YY6) and WT were prepared from tissues of similar age to those used for the transport assays in Table III. The free IAA levels were quantified by isotope dilution followed by gas chromatography-mass spectroscopy (Cohen et al., 1986; Chen et al., 1988). The results are shown in Table V and indicate that the free IAA concentrations are very similar in tt4(2YY6) and WT. The slightly lower values in tissues from the tt4(2YY6) inflorescence apex and higher values in the tt4(2YY6) inflorescence base are consistent with higher rates of auxin movement down the inflorescence stem in these plants as compared with the WT.
Table V.
Free IAA in Arabidopsis inflorescence segments
| Free IAAa
|
||
|---|---|---|
| Inflorescence apex | Inflorescence base | |
| ng/g fresh wt | ||
| WT | 43 ± 7 | 18 ± 6 |
| tt4(2YY6) | 38 ± 4 | 26 ± 12 |
The reported values are the average and se of three samples each containing 200 or 500 mg of inflorescence tissue.
DISCUSSION
The goal of this work was to test the hypothesis that flavonoids act as endogenous regulators of auxin transport in vivo. The first approach was to compare growth phenotypes in WT Arabidopsis plants and plants with two tt4 mutations, which encodes CHS, the first enzyme of flavonoid biosynthesis. The tt4(2YY6) plants had shorter inflorescence stems and increased branching of inflorescence and roots structures, consistent with altered auxin distribution (Fig. 2; Table I). Similar phenotypic alterations were also found in the tt4(85) allele in the Ler background (data not shown). These branching phenotypes are opposite to those in tir3 and pin1 mutants, which have reduced levels of auxin transport and reduced inflorescence and root branching (Okada et al., 1991; Ruegger et al., 1997). The tt4 phenotypic alterations are suggestive of changes in auxin transport, although more direct evidence is necessary to implicate flavonoids as endogenous auxin transport inhibitors.
The second approach was to grow plants in the presence of naringenin, an early intermediate in the flavonoid biosynthetic pathway. WT plants grown on naringenin show a dose-dependent inhibition of root growth and gravitropism that parallels the effect of synthetic auxin transport inhibitors (Fig. 1). The agravitropic phenotype is a relatively specific defect linked to auxin transport inhibition. Agravitropic growth is identifiable in response to treatment with synthetic auxin transport inhibitors (Katekar, 1976; Rashotte et al., 2000) and in plants with mutations in genes that encode proteins involved in auxin transport such as agr1 and aux1 (Chen et al., 1998; Marchant et al., 1999). Although root gravitropism and root elongation are sensitive to inhibition by naringenin, more of this compound was required for 50% inhibition of these process than with synthetic auxin transport inhibitors such as NPA. This difference in sensitivity may be due to less efficient uptake and/or conversion of naringenin to a flavonoid that is able to inhibit auxin transport, or to lower binding affinity of a natural compound than its synthetic counterpart.
The third approach to test the role of flavonoids in regulating auxin transport was to directly measure auxin transport in tt4 and WT Arabidopsis plants. Auxin transport was compared in the inflorescence stem and found to be elevated in tt4(2YY6) and tt4(85) plants over parental strains by about 2-fold (Table II). The magnitude of the difference in auxin transport between the WT and tt4(2YY6) mutant depended upon the duration of the assay and the position along the inflorescence that is examined. The elevation in auxin transport in tt4(2YY6) plants was greater in assays of shorter duration or when basal inflorescence segments were used.
The amount of auxin transport in hypocotyls of tt4(85) was also compared with WT for several reasons. First, it was important to determine if transport in the absence of flavonoids was also elevated over the WT parental line in younger tissue. Second, as plants used for this assay are grown on agar plates or moistened filter paper, it is possible to grow the plants on naringenin to chemically complement the mutation. The amount of IAA moving into basal hypocotyl or root segments was elevated to statistically significant levels in tt4(85) and this elevation was reversible by growth of plants on naringenin. Growth of WT Ler plants on naringenin reduced auxin transport in the hypocotyl, suggesting that the elevated flavonoid levels in this plant are reducing auxin transport.
An alternative hypothesis that could explain the elevated transport in the tt4 mutants is that flavonoids affect IAA metabolism. It has been reported that IAA oxidase activity is modulated by flavonoids (Mumford et al., 1961; Furuya et al., 1962; Stenlid, 1963). Although IAA oxidation is not the predominant method of regulating IAA levels (Normanly et al., 1995), it is also possible that flavonoids could act in a different way to regulate IAA catabolism. If IAA metabolism is altered by the absence of flavonoids, this could indirectly affect the measurements of auxin transport since elevated IAA concentration positively regulates the amount of auxin transported (Rayle et al., 1969). The amount of free IAA in the inflorescence stem of tt4(2YY6) plants is similar to the levels in WT plants, with slight changes that are consistent with greater auxin transport in the tt4(2YY6) plants. Therefore, the elevated auxin transport in tt4(2YY6) plants is not due to elevated free IAA. Flavonoids do not appear to have biologically significant effects on free IAA concentration.
It is not yet clear how synthetic or naturally occurring auxin transport inhibitors act to control auxin transport. The simplest possibility is that binding of these compounds leads to conformational changes in a protein that prevents auxin efflux from cells. Although there is a tight linkage between the ability of inhibitors to displace NPA binding and to block auxin efflux (Jacobs and Rubery, 1988; Rubery, 1990), there is no direct evidence for a simple conformational change upon binding of auxin transport inhibitors resulting in inhibition of auxin movements. In an alternate manner, it is possible that binding of auxin transport inhibitors to the NPA binding protein activates a signaling cascade. Compounds that act as protein kinase inhibitors in mammalian cells, including several flavonoid derivatives, have been found to reduce NPA binding (Bernasconi, 1996). This has lead to the suggestion that the NPA binding protein could be a kinase that acts to regulate auxin efflux by phosphorylation (Bernasconi, 1996). In addition, the Arabidopsis mutant rcn1 (roots curl in NPA) has altered growth characteristics that are suggestive of alterations in auxin transport, and this mutation has been found to reside within a gene encoding a protein phosphatase regulatory subunit (Garbers et al., 1996; Deruère et al., 1999). Direct tests of these two hypothetical modes of auxin transport inhibitor action are now possible with the recent identification of genes that are predicted to encode proteins that control auxin transport including AUX1 (Bennett et al., 1998) and the PIN gene family (Palme and Galweiler, 1999).
If flavonoids are acting as endogenous negative regulators of auxin transport, it may be possible to dissect the specific physiological roles of these compounds by combining the phenotypic and IAA transport analyses of the tt4 mutants with information on the localization of specific flavonoids. The distribution of flavonoids has been examined in detail in Arabidopsis plants at a cellular and tissue level (Peer et al., 2001). One striking aspect of the localization of flavonoids is the high level of accumulation at the root shoot junction (Murphy et al., 2000; Peer et al., 2001). In roots of Arabidopsis and other plants, there are two distinct polar movements of auxin (Reed et al., 1998; Rashotte et al., 2000). Auxin moves basipetally (from the root apex toward the base) in cortical or epidermal cells (Mitchell and Davies, 1975; Tsurumi and Ohwaki, 1978) and this polarity of auxin movement has been tied to root gravity response (Rashotte et al., 2000). Auxin also moves acropetally from the shoot toward the root tip, through cells of the central cylinder (Tsurumi and Ohwaki, 1978). As auxin moving from the shoot into the root has been implicated in controlling the number of elongated lateral roots (Reed et al., 1998), the role of this local in tt4(2YY6) flavonoid accumulation may be to control root branching. The increased number of lateral and adventitious roots are in tt4(2YY6) consistent with the absence of an endogenous auxin transport inhibitor that would normally block auxin movement into the root. There is intriguing evidence suggesting that formation of root nodules is tied to the synthesis of specific flavonoid derivatives, which may act to block auxin movement and to raise auxin concentration through local inhibition of auxin transport (Hirsch et al., 1989; Yang et al., 1992; Hirsch and Fang, 1994; Mathesius et al., 1998).
In conclusion, three separate lines of experimentation suggest that flavonoids are acting as endogenous negative regulators of auxin transport. A phenotypic analysis of Arabidopsis plants with mutations in flavonoid biosynthesis indicates altered growth patterns consistent with altered auxin transport. Auxin transport measurements in the inflorescence and the hypocotyl of two different tt4 mutants, which block flavonoid biosynthesis, indicate that auxin transport is elevated in the absence of endogenous flavonoids. Growth of plants on naringenin, an early intermediate in flavonoid biosynthesis, leads to growth and gravity inhibition consistent with inhibition of auxin transport, as well as a direct reduction in auxin transport in hypocotyl transport assays. These in vivo results combined with the previous in vitro evidence (Jacobs and Rubery, 1988) make a strong case for flavonoids acting as endogenous regulators of auxin transport.
MATERIALS AND METHODS
Reagents, Chemicals, and Radiochemicals
3-[5(n)-3H]Indolylacetic acid (25 Ci mmol−1) was purchased from Amersham International (Buckinghamshire, UK) and [14C]IAA (9.6 mCi mmol−1) was purchased from Sigma (St. Louis). [2,3,4,5(n)- 3H]NPA (58 Ci mmol−1) was obtained from American Radiolabeled Chemicals (St. Louis). [13C6]IAA acid was purchased from Cambridge Isotopes (Andover, MA). Naringenin was purchased from Indofine (Somerville, NJ). NPA and norflurazon were purchased from Chemical Services (West Chester, PA). All other chemicals were purchased from Sigma or from Fisher Scientific (Pittsburgh).
Seed Sterilization and Growth Conditions
Arabidopsis seeds of the Columbia and Ler ecotypes and tt4(2YY6) were generously provided by Dr. Brenda Winkel of The Virginia Polytechnic Institute and State University (Blacksburg, VA). The seeds for tt4(85), in the Ler background, were obtained from the Arabidopsis Biological Resource Center at Ohio State University (Columbus). The tt4 ethyl methanesulfonate mutant allele (2YY6) is in the Columbia background and is a null mutation in the CHS gene (Burbulis et al., 1996; Saslowsky et al., 2000). To reduce the probability of other unlinked mutations in the tt4(2YY6) plants, two subsequent backcrosses were performed.
Arabidopsis seeds were surface sterilized by allowing the seeds to imbibe water for at least 30 min, followed by 5 min in 95% (v/v) ethanol, and then 5 min in 20% (v/v) Clorox/0.01% (v/v) Triton X-100. Seeds were then washed five times in sterile distilled water. Seeds were plated onto medium consisting of 1× Murashige and Skoog salts, pH 6.0, 0.8% (w/v) agar, 1.5% (w/v) Suc, 1.0 μg mL−1 thiamine, 0.5 μg mL−1 pyroxidine HCl, 0.5 μg mL−1 nicotinic acid, and filter-sterilized ampicillin at 50 μg mL−1. Plates were then oriented vertically under continuous white light (80–90 μmol s−1 m−2) at room temperature (23°C), unless otherwise noted.
For plants grown in soil, seeds were allowed to imbibe water for at least 30 min and were directly dispensed onto previously watered Metromix 220 purchased from Scotts (Marysville, OH). Flats or pots were placed in a 21°C incubator under continuous light (approximately 50 μmol s−1 m−2), unless otherwise noted. For phenotypic analysis of aerial structures of WT and tt4(2YY6) plants, seeds were planted in 5-inch plastic pots as described above and were then placed in a 21°C incubator (approximately 90 μmol s−1 m−2) with a 16-h day/8-h night cycle. After germination, seedlings were thinned to one plant per pot. Growth of the seedlings into mature plants was monitored over time, with d 0 being the day of planting.
Seedling Development on Media Containing Naringenin and NPA
To determine the effect of NPA or naringenin on the growth of Arabidopsis seedlings, the agar media described above was supplemented with 10 nm to 5 μm NPA or was prepared with 3% (w/v) Suc and supplemented with 100 nm to 200 μm naringenin dissolved in 95% (w/v) ethanol according to previous procedures (Shirley et al., 1995). For NPA plates, the final dimethyl sulfoxide concentration was 0.1% (v/v) and for naringenin plates, the final ethanol concentration was 0.1% (v/v). Control plates had equivalent dimethyl sulfoxide or Suc and ethanol concentration as experimental plates.
Four-day-old light-grown Arabidopsis seedlings were transferred from control plates to plates containing inhibitor. Ten seedlings were transferred to each plate and root tips were aligned for new root growth to be recorded. After 24 h under continuous light (80–90 μmol s−1 m−2), plates were turned 90°. After an additional 24 h of growth, the angle of gravitropic curvature and the amount of root growth after 48 h were measured.
Polar Auxin Transport Measurements
Polar auxin transport in inflorescence stems was measured using a modification of a previously published procedure (Okada et al., 1991). Primary inflorescence stems were grown for 32 to 34 d at 75 to 100 μmol s−1 m−2 at 21°C until they averaged 15 to 20 cm in length. For data in Figure 4 and Table II, a 2.5-cm segment was excised that spanned from 5 to 7.5 cm above the base of the inflorescence. For the data in Table II, multiple 2.5-cm segments were excised and the distance from the apex of the upper end of each segment is reported. The segments reported are from 1 to 3.5, 3.5 to 6.0, 6.0 to 8.5, and 15 to 17.5 cm from the inflorescence apex. The final 15 to 17.5-cm segment is equivalent to the segments used in Figure 4 and Table II. Segments were placed into a 1.5-mL microcentrifuge tube with one end submerged in 30 μL of MES [2-(N-morpholino)-ethane-sulfonic acid] buffer (5 mm MES, 1% [w/v] Suc, pH 5.5) containing 1.45 μm total IAA with 100 nm of [3H]IAA, in the presence or absence of 10 or 100 μm NPA.
Based on the orientation of the inflorescence segment within the tube, basipetal or acropetal auxin transport was measured. The segments were incubated with one end submerged in the radiolabeled buffer at room temperature in darkness for the indicated time. After incubation, the segment was removed and the last 5 mm of the non-submerged end was excised and placed into 2.5 mL of scintillation fluid. The samples were allowed to sit for at least 18 h before being counted in a liquid scintillation counter. The amount of [3H]IAA transported to the end of the segment was reported as cpm and is reported directly or after background cpm (cpm due to segment with no added radioactivity) are subtracted. The reported data are the average and se of five segments per treatment, unless otherwise noted.
Auxin transport studies in young seedlings of Ler and tt4(85) were as previously described (Murphy et al., 2000). Plants were grown on filter paper that was saturated in 0.25× Murashige and Skoog salts for 4.5 d at 21°C and 80 μmol s−1 m−2 as described previously (Murphy and Taiz, 1995). Plants were grown for an additional day under similar conditions in media alone or supplemented with 10 nm NPA or naringenin. The transport assay was performed by application of a small drop (0.2 μL) of 10 nm [14C]IAA in ethanol (50 nCi μL−1) to the apical tip of each 5.5-d-old seedling. After a 4-h transport period, hypocotyls were rinsed, the upper hypocotyl and cotyledons were removed, and a 2-mm section of the hypocotyl immediately above the transition zone was excised, as well as a 2-mm section from the basal part of the root. The radioactivity in these segments was determined by scintillation counting and the experiment was repeated three times.
Microsomal Membrane Preparation and [3H]-NPA Binding Assays
Seeds were surface sterilized and plated in dense lines on germination media (1× Murashige-Skoog salts, 0.5 μg mL−1 MES, 1% [w/v] Suc, 0.8% [w/v] agar, and 50 μg mL−1 ampicillin, pH 5.8). Plates were placed at 4°C for 2 to 3 d in the dark to enhance germination, and were then oriented upright under continuous light in a 21°C incubator for approximately 3 weeks. Rosette and root microsomes were prepared according to previously published methods (Dixon et al., 1996; Ruegger et al., 1997) with the following modification. Due to the small yield of root tissue, roots were harvested and homogenized in NPA binding buffer (NBB; 20 mm sodium citrate, 1.0 mm MgCl2, and 0.25 mm Suc, pH 5.3) using a 15-mL ground glass homogenizer. The homogenate was filtered through two layers of Miracloth (Calbiochem, San Diego) prior to centrifugation. Protein concentration of the microsomal membrane preparations was determined using a bicinchoninic acid protein assay (Smith et al., 1985).
[3H]NPA binding assays were performed in a 200-μL total volume of NBB with protein at a final concentration of 0.2 mg mL−1. Microsomes were incubated with [3H]NPA concentrations ranging from 2 to 20 nm in the presence or absence of 10 μm NPA. The addition of unlabeled NPA allowed measurement of background or non-specific binding. Samples were incubated at 4°C with shaking for 1 h. After incubation, samples were filtered over 0.3% (v/v) polyethylenimine-treated GF/B filters and were washed with 5 mL of cold NBB. The filters were placed into 2.5 mL of scintillation fluid and were counted using a liquid scintillation counter. [3H]NPA binding activity was analyzed using double-reciprocal, Scatchard, and saturation plots.
Free IAA Determinations
Plants were grown for 33 d in soil and segments were excised from the top 2 cm of the inflorescence apex and the basal 2 cm of the inflorescence. Approximately 150 segments were pooled, the fresh weight was determined, and the samples were frozen in liquid nitrogen and stored at −80°C. Each sample contained approximately 200 or 500 mg fresh weight. Free IAA was purified and quantified using a previously published procedure (Chen et al., 1988). Tissue that had been frozen in liquid nitrogen and stored at −80°C was ground in a mortar and pestle using ice-cold IAA extraction buffer (65% [w/v] isopropanol and 35% [w/v] 0.2 m imidazole buffer, pH 7.0). [13C6]-IAA was used as an internal standard with a ratio of 50 ng g−1 fresh weight of tissue. [3H]IAA was added as a radiotracer at approximately 50,000 dpm for each sample. IAA was purified by an amino column (Prep Sep, Fisher Scientific), as described in Chen et al. (1988), with several organic washes and was then eluted in methanol that was 5% (w/v) acetic acid. After concentration, the sample was purified by reverse phase HPLC, methylated using ethereal diazomethane, and then analyzed by gas chromatography-single ion monitoring-mass spectroscopy. The gas chromatography-single ion monitoring-mass spectroscopy was used for selected ion measurements to quantify the free IAA concentrations in the inflorescence extracts relative to the [13C6]-IAA internal standard.
ACKNOWLEDGMENTS
We appreciate the generosity of Brenda Winkel in sharing Arabidopsis seeds and ideas, and the assistance of Jennifer Waters Shuler with image analysis.
Footnotes
This work was supported by Sigma Xi (grant to D.E.B.), by the National Aeronautical and Space Administration (grant no. NAG2 1203 to G.K.M.), by the National Aeronautical and Space Administration Specialized Center for Research and Training at North Carolina State University (grants to G.K.M. and A.M.R.), by the U.S. Department of Agriculture (grant no. 94–37100–0755 to A.S.M. and L.T.), and by the National Science Foundation (grant no. MCB-9870798 to J.N.). The Wake Forest University Research and Publications Fund supported the publication costs.
LITERATURE CITED
- Bennett M, Marchant A, May S, Swarup R. Going the distance with auxin: unravelling the molecular basis of auxin transport. Philos Trans R Soc Lond B. 1998;353:1511–1515. doi: 10.1098/rstb.1998.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi P. Effect of synthetic and natural protein tyrosine kinase inhibitors on auxin efflux in zucchini (Cucurbita pepo) hypocotyls. Physiol Plant. 1996;96:205–210. [Google Scholar]
- Brown D. Testing the hypothesis that flavonoids regulate polar auxin transport in vivo using Arabidopsis thaliana. MS thesis. Winston-Salem, NC: Wake Forest University; 1998. [Google Scholar]
- Burbulis IE, Iacobucci M, Shirley BW. A null mutation in the first enzyme of flavonoid biosynthesis does not affect male fertility in Arabidopsis. Plant Cell. 1996;8:1013–1025. doi: 10.1105/tpc.8.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K-H, Miller AN, Patterson GW, Cohen JD. A rapid and simple procedure for purification of indole-3-acetic acid prior to GC-SIM-MS analysis. Plant Physiol. 1988;86:822–825. doi: 10.1104/pp.86.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J, Peto CA. Mutations in the DET1 gene affect cell-type-specific expression of light-regulated genes and chloroplast development in Arabidopsis. Proc Natl Acad Sci USA. 1990;87:8776–8780. doi: 10.1073/pnas.87.22.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Baldi B, Slovin J. 13C6-[Benzene ring]-indole-3-acetic acid: a new internal standard for quantitative mass spectral analysis of indole-3-acetic acid in plants. Plant Physiol. 1986;80:14–19. doi: 10.1104/pp.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deruère J, Jackson K, Garbers C, Soll D, Delong A. The RCN1-encoded A subunit of protein phosphatase 2A increases phosphatase activity in vivo. Plant J. 1999;20:389–399. doi: 10.1046/j.1365-313x.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- Dixon MW, Jacobson JA, Cady CT, Muday GK. Cytoplasmic orientation of the naphthylphthalamic acid-binding protein in zucchini plasma membrane vesicles. Plant Physiol. 1996;112:421–432. doi: 10.1104/pp.112.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinbaum RL, Ausubel FM. Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol Cell Biol. 1988;8:1985–1992. doi: 10.1128/mcb.8.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinbaum RL, Storz G, Ausubel FM. High intensity and blue light regulated expression of chimeric chalcone synthase genes in transgenic Arabidopsis thaliana plants. Mol Gen Genet. 1991;226:449–456. doi: 10.1007/BF00260658. [DOI] [PubMed] [Google Scholar]
- Fischer C, Speth V, Fleig-Eberenz S, Neuhaus G. Induction of zygotic polyembryos in wheat: influence of auxin polar transport. Plant Cell. 1997;9:1767–1780. doi: 10.1105/tpc.9.10.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M, Galston AW, Stowe BB. Isolation from peas of co-factors and inhibitors of indolyl-3-acetic acid oxidase. Nature. 1962;193:456–457. doi: 10.1038/193456a0. [DOI] [PubMed] [Google Scholar]
- Garbers C, DeLong A, Deruere J, Bernasconi P, Soll D. A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 1996;15:2115–2124. [PMC free article] [PubMed] [Google Scholar]
- Hirsch AM, Bhuvaneswari TV, Torrey JG, Bisseling T. Early nodulin genes are inducible in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc Natl Acad Sci USA. 1989;86:1244–1248. doi: 10.1073/pnas.86.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AM, Fang Y. Plant hormones and nodulation: what's the connection? Plant Mol Biol. 1994;26:5–9. doi: 10.1007/BF00039514. [DOI] [PubMed] [Google Scholar]
- Jackson JA, Fuglevand G, Brown BA, Shaw MJ, Jenkins GI. Isolation of Arabidopsis mutants altered in the light-regulation of chalcone synthase gene expression using a transgenic screening approach. Plant J. 1995;8:369–380. doi: 10.1046/j.1365-313x.1995.08030369.x. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH. Naturally occurring auxin transport regulators. Science. 1988;241:346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Katekar GF. Inhibitors of the geotropic response in plants: a correlation of molecular structures. Phytochemistry. 1976;15:1421–1424. [Google Scholar]
- Kubasek WL, Shirley BW, McKillop A, Goodman HM, Briggs W, Ausubel FM. Regulation of flavonoid biosynthetic genes in germinating Arabidopsis seedlings. Plant Cell. 1992;4:1229–1236. doi: 10.1105/tpc.4.10.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery P. Auxin transport. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry, and Molecular Biology. Norwell, The Netherlands: Kluwer Academic Press; 1995. pp. 509–530. [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo G, Boudet P. Relations polyphenols-croissance: mise en evidence d'un effet inhibiteur des composes phenoliques sur le transport polarise de l'auxine. Physiol Plant. 1977;41:197–202. [Google Scholar]
- Mathesius U, Schlaman H, RM, Spaink H, P, Sautter C, Rolfe B, G, Djordjevic M, A. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 1998;14:23–34. doi: 10.1046/j.1365-313X.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- Mitchell EK, Davies PJ. Evidence for three different systems of movement of indoleacetic acid in intact roots of Phaseolus coccineus. Physiol Plant. 1975;33:290–294. doi: 10.1007/BF00385573. [DOI] [PubMed] [Google Scholar]
- Muday G. Interactions between the actin cytoskeleton and an auxin transport protein. In: Staiger CJ, Baluska F, Volkmann D, Barlow P, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht, The Netherlands: Kluwer Academic Press; 2000. pp. 541–556. [Google Scholar]
- Mumford F, Smith D, Castle J. An inhibitor of indoleacetic acid oxidase from pea tips. Plant Physiol. 1961;36:752–756. doi: 10.1104/pp.36.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A, Peer W, Taiz L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta. 2000;211:315–324. doi: 10.1007/s004250000300. [DOI] [PubMed] [Google Scholar]
- Murphy A, Taiz L. A new vertical mesh transfer technique for metal tolerance studies in Arabidopsis: ecotypic variation and copper-sensitive mutants. Plant Physiol. 1995;108:29–38. doi: 10.1104/pp.108.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J, Slovin JP, Cohen JD. Rethinking auxin biosynthesis and metabolism. Plant Physiol. 1995;107:1–7. doi: 10.1104/pp.107.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K, Gälweiler L. PIN-pointing the molecular basis of auxin transport. Curr Opin Plant Biol. 1999;2:375–381. doi: 10.1016/s1369-5266(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Peer WA, Murphy AS, Brown DE, Tague BW, Muday GK, Taiz L. Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiol. 2001;126:536–548. doi: 10.1104/pp.126.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte A, Brady S, Reed R, Ante S, Muday G. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 2000;122:481–490. doi: 10.1104/pp.122.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle DL, Ouitrakul R, Hertel R. Effect of auxins on the auxin transport system in coleoptiles. Planta. 1969;87:49–53. doi: 10.1007/BF00386963. [DOI] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998;118:1369–1378. doi: 10.1104/pp.118.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery P, Jacobs M. Auxin transport and its regulation by flavonoids. In: Pharis R, Rood S, editors. Plant Growth Substances 1988. Berlin: Springer-Verlag; 1990. pp. 428–440. [Google Scholar]
- Rubery PH. Phytotropins: receptors and endogenous ligands. Symp Soc Exp Biol. 1990;44:119–146. [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuta M. Transcriptional control of chalcone synthase by environmental stimuli. J Plant Res. 2000;113:327–333. [Google Scholar]
- Saslowsky DE, Dana CD, Winkel-Shirley B. An allelic series for the chalcone synthase locus in Arabidopsis. Gene. 2000;255:127–138. doi: 10.1016/s0378-1119(00)00304-8. [DOI] [PubMed] [Google Scholar]
- Schmid J, Doerner PW, Clouse SD, Dixon RA, Lamb CJ. Developmental and environmental regulation of a bean chalcone synthase promoter in transgenic tobacco. Plant Cell. 1990;2:619–631. doi: 10.1105/tpc.2.7.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley B. Flavonoid biosynthesis: “new” functions for an “old” pathway. Trends Plant Sci. 1996;1:377–382. [Google Scholar]
- Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, Goodman HM. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995;8:659–671. doi: 10.1046/j.1365-313x.1995.08050659.x. [DOI] [PubMed] [Google Scholar]
- Smith P, Krohn R, Hermanson G, Mallia A, Gartner F, Provenzano M, Fuyimoto E, Geoke N, Olson B, Klenk D. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–86. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stafford HA. Flavonoid Metabolism. Boca Raton, FL: CRC Press; 1990. [Google Scholar]
- Stenlid G. The effects of flavonoid compounds on oxidative phosphorylation and on the enzymatic destruction of indole-acetic acid. Physiol Plant. 1963;16:110–120. [Google Scholar]
- Stenlid G. Effects of flavonoids on the polar transport of auxins. Physiol Plant. 1976;38:262–266. [Google Scholar]
- Tsurumi S, Ohwaki Y. Transport of 14C-labeled indoleacetic acid in Vicia root segments. Plant Cell Physiol. 1978;19:1195–1206. [Google Scholar]
- von Arnim A, Deng X-W. Light control of seedling development. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:215–243. doi: 10.1146/annurev.arplant.47.1.215. [DOI] [PubMed] [Google Scholar]
- Yang W-C, Cremers HCJ, Hogendijk P, Katinakis P, Wijffelman CA, Franssen H, Kammen AV, Bisseling T. In-situ localization of chalcone synthase mRNA in pea root nodule development. Plant J. 1992;2:143–151. [Google Scholar]