Abstract
Amplification of the MycN oncogene characterizes a subset of highly aggressive neuroblastomas, the most common extracranial solid tumor of childhood. However, the significance of MycN amplification for tumor cell survival is controversial, since down-regulation of MycN was found to decrease markedly neuroblastoma sensitivity towards conventional anticancer drugs, cisplatin, and doxorubicin. Here, we show that a redox-silent analogue of vitamin E, α-tocopheryl succinate (α-TOS), which triggers apoptotic cell death via targeting mitochondria, can kill tumor cells irrespective of their MycN expression level. In cells overexpressing MycN, as well as cells in which MycN was switched off, α-TOS stimulated rapid entry of Ca2+ into the cytosol, compromised Ca2+ buffering capacity of the mitochondria and sensitized them towards mitochondrial permeability transition and subsequent apoptotic cell death. Prevention of mitochondrial Ca2+ accumulation or chelation of cytosolic Ca2+ rescued the cells. Thus, targeting mitochondria might be advantageous for the elimination of tumor cells with otherwise dormant apoptotic pathways.
Keywords: Mitochondria, Apoptosis, Calcium, MycN
Introduction
Neuroblastoma (NB) is the most common extracranial solid tumor in childhood. Amplification of the MycN oncogene characterizes a subset of highly aggressive NBs and correlates with poor prognosis in patients. MycN belongs to the Myc family of transcription factors that play a key role in the regulation of a variety of cellular processes, such as cell proliferation, differentiation, and apoptosis. The Myc gene is deregulated in a host of different tumors, including lung cancer, osteosarcoma, glioblastoma, breast and cervix carcinomas, myeloid and plasma cell leukemias, Burkitt’s lymphoma and NB [1]. The views on the role and significance of MycN amplification for tumor cell survival are controversial. On the one hand, amplification of MycN characterizes a subset of rapidly proliferating and aggressive NBs. On the other, the ability of MycN to sensitize cells to apoptosis is well documented. Thus, downregulation of MycN markedly decreased NB cell sensitivity towards the DNA-damaging anticancer drugs cisplatin and doxorubicin, which makes MycN targeting in the treatment of patients problematic [2]. Identifying novel compounds that are equally efficient in tumor cells with different levels of MycN and/or p53 expression is therefore of critical importance for antitumor therapy.
Killing tumor cells is the ultimate aim of anticancer treatment. Among the different forms of cell death, the molecular mechanisms of apoptosis are so far the best characterized. Apoptosis is a gene-regulated mode of cell death responsible for cell deletion during embryogenesis and maintenance of tissue homeostasis in the adult organism. Efficient anticancer therapy is based on the ability to stimulate dormant apoptotic pathways in tumor cells. Although it appears that different signals trigger distinct pathways leading to cell death, they often merge at a common regulator—the mitochondria. In particular, the release of pro-apoptotic proteins from the mitochondrial intermembrane space is regarded as a key event in apoptosis induction. Among these proteins are cytochrome c, apoptosis inducing factor (AIF), Smac/Diablo, and Omi (reviewed in [3]). Considering their role as key participants in the cell-death process, targeting of the mitochondria could contribute to tumor cell elimination. Therefore, sensitization of the mitochondria via manipulation of their energy metabolism, stimulation of reactive oxygen species (ROS) generation (or suppression of antioxidant capacity), or disruption of intracellular Ca2+ homeostasis might all contribute to more effective anticancer therapy [4].
Recently, a range of compounds, named mitocans (an abbreviation formed from MITOchondria and CANcer), were shown to cause cell death via targeting mitochondria [5]. α-Tocopheryl succinate (α-TOS), a derivative of tocopherol, is one of the mitocans. In 1982, Prasad and Edwards-Prasad [6] reported for the first time that this redox-silent analogue of vitamin E induced morphological changes and growth inhibition in mouse melanoma cells. Further, α-TOS was shown to inhibit the proliferation of avian reticuloendotheliosis virus-transformed lymphoblastoid cells in a dose-dependent manner, blocking the cells in the G2/M cell cycle phase, and to induce apoptosis [7]. α-TOS was also found to destabilize mitochondria, stimulating their production of ROS and to kill malignant cells at concentrations non-toxic to normal cells and tissues [8]. Although α-tocopherol is known as an important chain-breaking antioxidant in cells, its derivative, α-TOS, is apparently unable to act as an antioxidant unless the succinate moiety is cleaved off. It has been reported that non-malignant cells have the ability to hydrolyze α-TOS by means of esterases, gradually releasing α-tocopherol to prevent membrane oxidative damage [9], whereas in malignant cells the hydrolysis of α-TOS is suppressed due to lower esterase activity [8, 10].
In the present study, we show that targeting mitochondria by α-TOS overcomes the resistance of NB cells to treatment caused by MycN downregulation. α-TOS caused mitochondrial destabilization followed by permeabilization of the outer mitochondrial membrane (OMM), release of pro-apoptotic proteins and activation of the downstream caspase cascade, irrespective of the cellular MycN or p53 expression levels. These findings support the use of α-TOS as a tool in tumor cell elimination.
Materials and methods
Cells
Cells were cultured in RPMI 1640 complete medium supplemented with 10% (w/v) heat-inactivated fetal calf serum and penicillin/streptomycin (100 U/ml). For Tet21 N cells, 100 μg/ml hygromycin and 200 μg/ml geneticine was additionally added to the medium. Cells were grown in a humidified air/CO2 (5%) atmosphere at 37°C and maintained in a logarithmic growth phase for all experiments. A stock solution of α-TOS (50 mM) was prepared by dissolving α-TOS in ethanol. In order to analyze the significance of MycN for the sensitivity of Tet21 N cell towards apoptosis inducers, MycN expression was switched off by adding 0.1 μg/ml of doxycycline (MycN(−) cells) [11].
Estimation of mitochondrial activity in digitonin-permeabilized cells
Mitochondrial accumulation of Ca2+ was monitored with a Ca2+-sensitive electrode (Thermo Scientific, Beverly, MA, USA). Cells were harvested and resuspended in 400 μl of buffer (150 mM KCl, 5 mM KH2PO4, 1 mM MgSO4, 5 mM succinate, 5 mM Tris, pH 7.4) and added to the Ca2+ electrode chamber. Following a 2-min stabilization period, cells were permeabilized with digitonin (5 μg/106 cells) and sequential pulses of Ca2+ (20 nmol each) were added to the permeabilized cells until mitochondrial permeability transition (MPT) was induced and accumulated Ca2+ released. The total amount of Ca2+ causing MPT was expressed as nmoles of Ca2+/106 cells.
Assessment of cytochrome c release
Cells were digitonin-permeabilized and fractionated into supernatant and pellet. Samples were mixed with Laemmli’s loading buffer, boiled for 5 min, and subjected to 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 40 mA followed by electroblotting to nitrocellulose for 2 h at 120 V. Membranes were blocked for 1 h with 5% nonfat milk in phosphate-buffered saline (PBS) at room temperature and subsequently probed overnight with a mouse anti-cytochrome c antibody (BD Biosciences, San Jose, CA). The membranes were rinsed and incubated with a horseradish peroxidase-conjugated secondary antibody (1:10,000). Blots were visualized by ECL™ (Amersham Biosciences, Buckinghamshire, UK) and X-ray film.
Measurement of caspase-3-like activity
The measurement of DEVD-AMC (Peptide Institute, Osaka, Japan) cleavage was performed using a modified version of a fluorometric assay. Cells were pelleted and washed once with PBS. After centrifugation, cells were resuspended in PBS at a concentration of 2 × 106 cells/100 μl; 25 μl of the suspension were added to a microtiter plate and mixed with the appropriate peptide substrate dissolved in a standard reaction buffer (100 mM HEPES, 10% sucrose, 5 mM DTT, 0.001% NP-40 and 0.1% CHAPS, pH 7.25). Cleavage of the fluorogenic peptide substrate was monitored by AMC liberation in a Fluoroscan II plate reader (Labsystems, Stockholm, Sweden) using 355-nm excitation and 460-nm emission wavelengths.
Flow cytometry
Cell death was assessed using the Annexin V-FLUOS staining kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions.
Morphological assessment of apoptosis
Cells were seeded on coverslips, fixed for 20 min in 4% formaldehyde at 4°C, and then washed with PBS. Nuclei were stained with HOECHST (2 μg/ml) by 10-min incubation at room temperature. Stained slides were mounted using Vectashield H-1000 (Vector Laboratories Inc., Burlingame, CA, USA) and examined under a Zeiss LSM 510 META confocal laser scanner microscope.
Live cell imaging
Tet21 N cells were incubated for 30 min (37°C, 5% CO2) in Krebs–Ringer solution containing 5 μM Fluo-4/AM (Invitrogen-Molecular Probes, Eugene, OR). The Krebs–Ringer solution contained 119 mM NaCl, 2.5 mM KCl, 1.0 mM NaH2PO4 (monobasic), 2.5 mM CaCl2 2H2O, 1.3 mM MgCl2 6H2O, 20 mM HEPES, 11 mM d-glucose (dextrose) C6H12O6 and was adjusted to a pH of 7.4. After the incubation, cells were washed once with Krebs–Ringer solution and subsequently examined with a Zeiss LSM 510 META confocal laser scanning microscope. Images were acquired at 0.2 Hz and all drugs were bath-applied.
The amount of cytosolic H2O2 in Tet21 N cells was assessed using the genetically encoded, targeted to cytosol fluorescent indicator pHyPer-dCyto (Evrogen, Moscow, Russia). Cells were seeded on coverslips and, on the following day, transiently transfected with pHyPer-dCyto using the Lipofectamine LTX/Plus reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. All experiments were performed using a Zeiss LSM 510 META confocal laser scanning microscope (Zeiss, Jena, Germany). For the time lapse, cells were cultivated in the POC-R cell cultivation system (Zeiss, Jena, Germany) at 37°C and humidified air/CO2 (5%) atmosphere. α-TOS was applied before start of the time lapse and fluorescence recorded for 16 h. Measurements were repeated three times and the H2O2 production at 16 h after treatment expressed as x-fold increase in comparison with the starting point of the experiment.
Results
Downregulation of MycN attenuates apoptosis induced by cisplatin
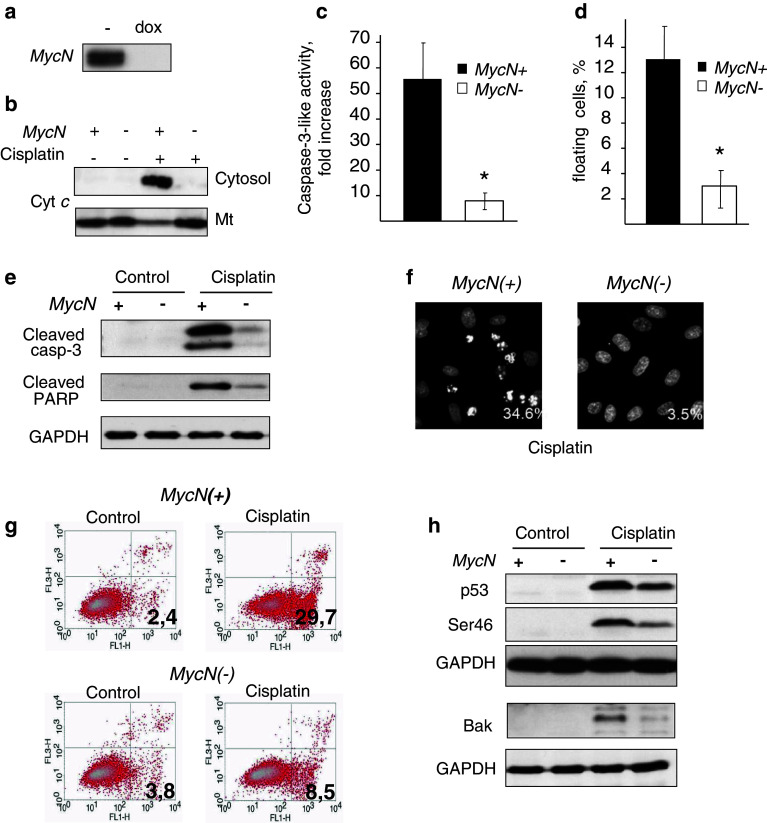
Treatment of Tet21 N cells expressing MycN (MycN(+) cells) with 0.1 μg/ml of doxycycline prevented the expression of MycN (MycN(−) cells) (Fig. 1a). Analysis of the response of Tet21 N cells to cisplatin revealed a marked difference between MycN(+) cells and MycN(−) cells. Switching off MycN prominently suppressed cisplatin-induced apoptosis, assessed by cytochrome c release (Fig. 1b), stimulation of caspase-3-like activity (Fig. 1c), and number of floating cells (Fig. 1d). Switching off MycN suppressed caspase-3 processing and cleavage of its downstream target PARP (Fig. 1e). In addition, downregulation of MycN markedly decreased the number of cisplatin-treated cells with apoptotic morphology (Fig. 1f). The dependence of cisplatin-induced apoptosis on MycN expression was confirmed by the analysis of phosphatidyl serine (PS) externalization, by staining with Annexin V and PI (Fig. 1g). Switching off MycN noticeably decreased the number of cells exposing PS on the outer surface of the plasma membrane (29.7 vs. 8.3%).
Fig. 1.
Effect of MycN downregulation on apoptotic manifestations in Tet21 N cells treated with 10 µg/ml cisplatin for 16 h. a Incubation of Tet21 N cells with 0.1 μg/ml doxycycline blocks expression of MycN oncogene; b switching off MycN suppresses cytochrome c release, caspase-3-like activity (c), number of floating cells (d), processing of caspase-3 and PARP cleavage in response to 10 μg/ml cisplatin (e), black bars: MycN overexpressing cells, white bars: MycN non-expressing Tet21 N cells, *p < 0.05; f analysis of apoptotic morphology in cisplatin-treated MycN(+) and MycN(−) Tet21 N cells; numbers show the percentage of cells with apoptotic nuclei. Number of counted cells: 720 for MycN(+) cells and 450 for MycN(−) cells; g analysis of PS externalization in MycN(+) and MycN(−) Tet21 N cells in response to 10 μg/ml cisplatin; h upper blot: 10 μg/ml cisplatin-stimulated expression of p53 in MycN(+) and MycN(−) cells; middle blot: phosphorylation of p53; lower blot: cisplatin-induced expression of Bak
In order to clarify the mechanism of apoptosis suppression in MycN(−) cells, we analyzed the expression level of the transcription factor p53, which was shown to be a direct transcriptional target of MycN [12]. p53 is capable of launching an apoptotic program that includes direct transcriptional activation of death-inducing genes, such as Noxa, Puma, and Bax (reviewed in [13]). Treatment with cisplatin stimulated the expression of p53 in both MycN(+) and MycN(−) cells, although the level of p53 was distinctly lower in the MycN(−) cells (Fig. 1h). Further, analysis of the p53 phosphorylation status revealed a lower phosphorylation level of the serine 46 residue in MycN(−) cells, which is in agreement with recently published data on the significance of p53 phosphorylation for apoptosis in MycN overexpressing cells [14]; the extent of phosphorylation was correlated with the level of total p53 (Fig. 1h). Further, the expression of Bak, a pro-apoptotic Bcl-2 family protein, was also significantly lower in cisplatin-treated MycN(−) cells (Fig. 1h, lower blot). This may explain the suppression of cytochrome c release (Fig. 1b) as well as the processing and activation of caspase-3 and PARP cleavage in MycN(−) cells.
Downregulation of MycN does not affect apoptosis induced by α-TOS
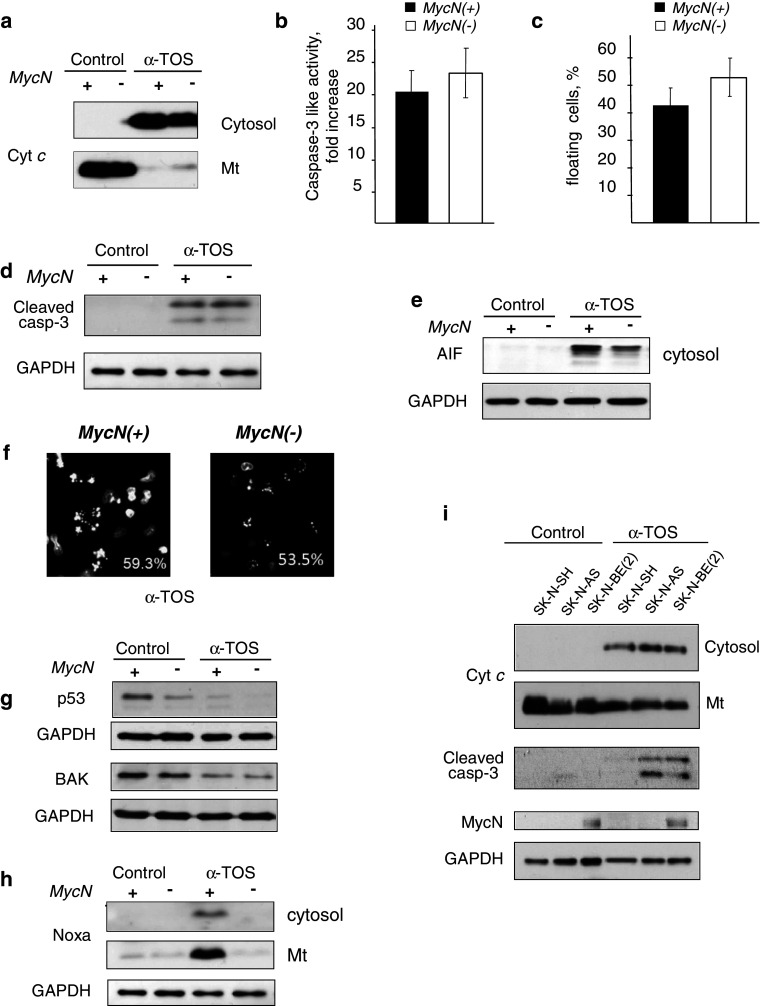
In contrast to cisplatin, α-TOS induced apoptosis in Tet21 N cells irrespective of the status of MycN. Release of cytochrome c, caspase-3 processing and activity, as well as the number of floating cells were comparable, or even modestly enhanced, after switching MycN off (Fig. 2a–d). In addition to cytochrome c, release of AIF from mitochondria after α-TOS treatment was also detected (Fig. 2e). Accordingly, the number of cells with apoptotic morphology was similar in both cell types: 55–60% (Fig. 2f).
Fig. 2.
Effect of MycN downregulation on apoptotic manifestations in Tet21 N cells treated with 60 μM α-TOS for 16 h. a α-TOS equally stimulates cell death assessed by the release of cytochrome c (a), caspase-3-like activity (b), the number of floating cells (c), and processing of caspase-3 (d) in MycN(+) (black bars) and MycN(−) (white bars) Tet21 N cells; e α-TOS-induced release of AIF from mitochondria in MycN(+) and MycN(−) Tet21 N cells; f analysis of apoptotic morphology in α-TOS-treated MycN(+) and MycN(−) Tet21 N cells. Numbers show the percentage of cells with apoptotic nuclei. Number of counted cells: 560 for MycN(+) cells and 260 for MycN(−) cells; g upper blot: p53 expression, lower blot: Bak expression in α-TOS treated MycN(+) and MycN(−) Tet21 N cells; h switching off MycN attenuated α-TOS-induced expression of Noxa; i α-TOS-induced cytochrome c release, caspase-3 cleavage, and MycN expression in various NB cells. Lower blot: loading control
α-TOS did not stimulate p53 expression as cisplatin did (Fig. 2g). Incubation with α-TOS caused suppression in p53 expression. Accordingly, the content of Bak was also reduced in both cell lines. It has been shown that α-TOS-induced permeabilization of the OMM involves formation of Bax [10] or Bak channels, and that this process is modulated by Noxa, which is upregulated transcriptionally in a p53-independent manner [15]. Noxa can facilitate OMM permeabilization by displacing anti-apoptotic Bcl-2 family members from their complexes with other pro-apoptotic members, e.g., Bax. Incubation with α-TOS stimulated the expression of Noxa more prominently in MycN(+) cells, as compared to MycN(−) cells (Fig. 2h). In spite of this, there was almost no difference in apoptosis manifestation between MycN(+) and MycN(−) cells. In addition, analysis of apoptotic changes in various NB cell lines with different levels of MycN expression [SK-N-AS, SK-N-SH, and SK-N-BE (2)] confirmed that OMM permeabilization, followed by release of cytochrome c and processing of caspase-3, was not dependent on MycN expression (Fig. 2i).
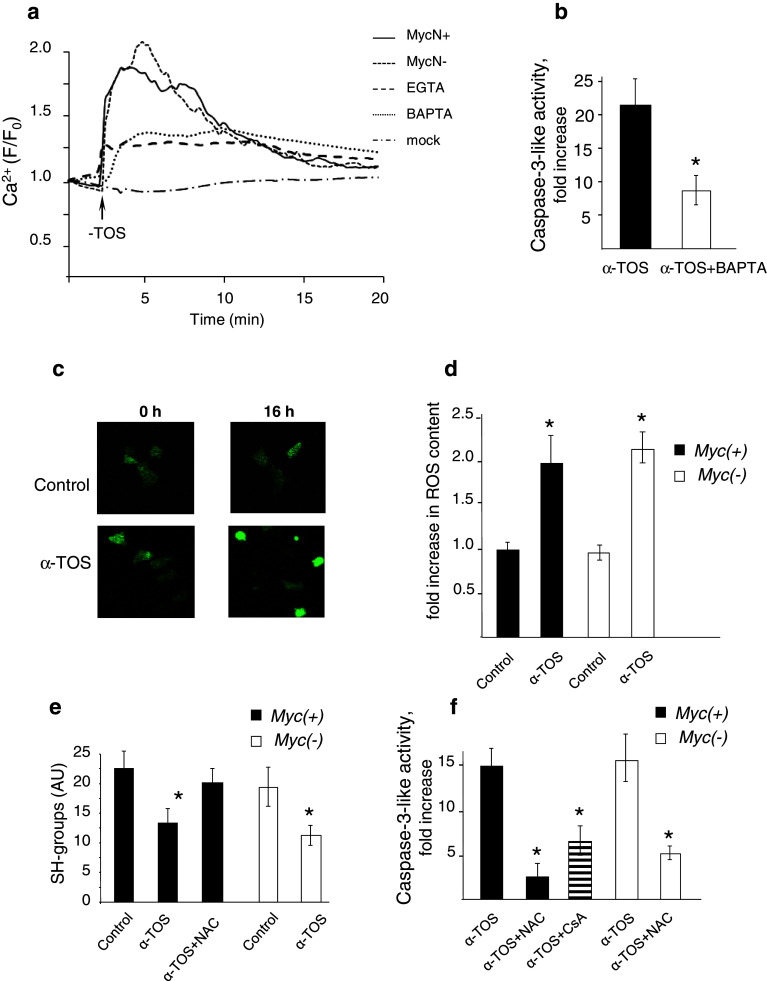
Another mechanism of OMM permeabilization, which can lead to the release of pro-apoptotic proteins from the mitochondrial intermembrane space, is the induction of MPT due to opening of a non-specific pore (MPT pore) in the inner mitochondrial membrane (IMM) [16]. A prerequisite step for pore opening is the accumulation of Ca2+ by the mitochondria; thus, an increase in cytosolic Ca2+ level facilitates MPT induction. We have shown earlier that α-TOS can stimulate cellular Ca2+ uptake, resulting in MPT induction [17]. Analysis of α-TOS-induced cytosolic Ca2+ transients did not reveal any difference between MycN(+) and MycN(−) cells (Fig. 3a). In both cell types, the addition of α-TOS led to a rapid increase in cytosolic Ca2+ concentration with subsequent normalization within a few minutes. Chelation of cytosolic Ca2+ by BAPTA markedly decreased the amplitude of the Ca2+ response to α-TOS. When extracellular Ca2+ was quenched by EGTA, the cytosolic Ca2+ increase diminished markedly, but not completely, indicating that release of Ca2+ from the endoplasmic reticulum might contribute to the α-TOS-induced Ca2+ transients. Dissipation of the mitochondrial membrane potential (the main driving force for Ca2+ accumulation) by the uncoupler CCCP prevented normalization of the Ca2+ level in the cytosol, indicating that it was dependent on Ca2+ accumulation by the mitochondria [17]. This was found to be critical for the apoptotic response, since chelation of cytosolic Ca2+ by BAPTA significantly suppressed the α-TOS-induced caspase-3-like activity (Fig. 3b).
Fig. 3.
Alteration of cytosolic and mitochondrial Ca2+ homeostasis in NB cells. a 60 μM α-TOS stimulates Ca2+ transients in MycN(+) and MycN(−) cells. b Chelation of intracellular Ca2+ by BAPTA AM attenuates caspase-3-like activity in Tet21 N cells, *p < 0.05; c ROS accumulation in MycN(+) cells treated with 60 μM α-TOS for 16 h. d Fold increase in ROS content after 16 h incubation of MycN(+) and MycN(−) cells with 60 μM α-TOS, *p < 0.05. The results were calculated as average from at least ten responding cells. e Incubation with 60 μM α-TOS for 16 h decreases the content of SH-groups in NB cells; NAC, 5 mM, *p < 0.05; f NAC and cyclosporin A (5 μM) prevent α-TOS-mediated stimulation of caspase-3-like activity
A powerful factor sensitizing mitochondria to MPT is oxidative stress. In view of the ability of α-TOS to stimulate ROS production [8], analysis of ROS production in MycN(+) and MycN(−) cells was performed using genetically encoded, targeted to cytosol fluorescent indicator pHyPer-dCyto, as described in “Materials and methods”. Figure 3c demonstrates confocal images of cells treated with 60 μM α-TOS for 16 h, and Fig. 3d shows a fold increase in ROS content in MycN(+) and MycN(−) cells. In both cases, α-TOS-induced oxidative stress was comparable. In addition, the depletion of thiols was assessed in MycN(+) and MycN(−) cells as a marker of oxidative stress. Incubation of cells with α-TOS for 16 h decreased the level of thiols in both cell lines to the same extent; this effect was preventable by the antioxidant N-acetylcysteine (NAC). (Fig. 3e). α-TOS-induced oxidative stress is important for apoptosis induction since NAC prominently decreased α-TOS-mediated caspase-3 activation (Fig. 3f). Similarly, caspase-3-like activity in α-TOS-treated cells was diminished by cyclosporin A (CsA), an inhibitor of the MPT pore (Fig. 3f, dashed bar), indicating that execution of apoptosis involves MPT induction.
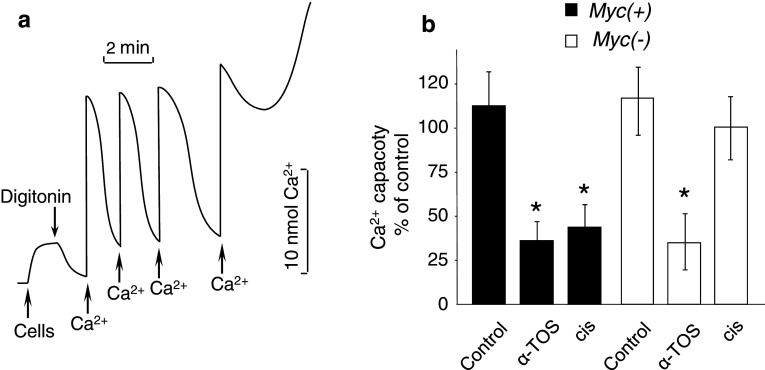
It appears from these findings that exposure to α-TOS is likely to make mitochondria more sensitive to MPT pore opening. Indeed, analysis of mitochondrial Ca2+ buffering capacity revealed that treatment with α-TOS markedly reduced the threshold level of Ca2+, required for MPT induction. In these experiments, cells were treated with 60 μM α-TOS or 10 µg/ml cisplatin for 16 h, harvested, and resuspended in KCl-based buffer containing succinate as a mitochondrial substrate (see “Materials and methods”). After a 2-min period of stabilization, the plasma membrane was permeabilized with digitonin (5 μg/106 cells). Addition of Ca2+ to the permeabilized cells led to a rapid increase in the level of this cation in the incubation buffer followed by a return to the initial level (Fig. 4a) as mitochondria accumulated the added Ca2+. Mitochondria took up sequential additions of Ca2+ until MPT was induced and Ca2+ was released. The threshold level of Ca2+ required for MPT induction (calcium capacity) was calculated by summation of all Ca2+ pulses. Administration of α-TOS caused similar suppression of the mitochondrial Ca2+ capacity in both MycN(+) and MycN(−) Tet21 N cells (Fig. 4b). In contrast, MycN(−) cells treated with cisplatin did not show any significant decrease in Ca2+ capacity. The difference between cisplatin-treated MycN(+) and MycN(−) cells correlates with the amount of cytochrome c released from their mitochondria. These results demonstrate that treatment with α-TOS sensitizes mitochondria to MPT induction, and that the mitochondria are equally susceptible to Ca2+-mediated pore opening irrespective of MycN status. This is in accordance with the finding of comparable amounts of cytochrome c release from the mitochondria in α-TOS-treated MycN(+) and MycN(−) Tet21 N cells.
Fig. 4.
Mitochondrial Ca2+ accumulation in NB cells with different level of MycN expression. a Accumulation of Ca2+ by mitochondria in digitonin-permeabilized MycN(+) cells. Pulses of Ca2+ (20 nmol) were added sequentially until MPT was induced and the accumulated Ca2+ was released; b effect of 60 μM α-TOS and 10 μg/ml cisplatin on mitochondrial Ca2+ capacity (the threshold level of Ca2+ required for MPT induction) in MycN(+) and MycN(−) Tet21 N cells, *p < 0.05
Discussion
The results presented here show that the consequences of MycN downregulation for the susceptibility of tumor cells to undergo apoptosis depend on the type of apoptosis inducer. In case of cisplatin, downregulation of MycN attenuates cell death, whereas α-TOS-induced apoptosis is not affected.
Two main mechanisms of OMM permeabilization—Bax/Bak-dependent and MPT-dependent—can be responsible for the release of mitochondrial pro-apoptotic proteins during apoptosis. In case of cisplatin, damage to DNA leads to stimulation of p53 expression and subsequent transcription of a number of pro-apoptotic proteins, including those involved in OMM permeabilization. Switching off MycN suppresses cisplatin-induced p53 expression and, as a result thereof, attenuates apoptotic manifestations.
Pro-apoptotic Bcl-2 family proteins have been reported to also be involved in α-TOS-induced apoptosis. Thus, α-TOS-stimulated production of ROS was suggested to be responsible for the formation of disulfide bridges between cytosolic Bax monomers, facilitating OMM permeabilization and cytochrome c release [10]. Moreover, α-TOS was reported to cause conformational changes in the pro-apoptotic protein Bak, involving its oligomerization, in various cell types [15]. In addition, α-TOS stimulated the expression of Noxa, a pro-apoptotic member of the Bcl-2 family [18], in a FoxO1-dependent manner [19]. Noxa facilitates OMM permeabilization by displacing anti-apoptotic Bcl-2 proteins from their complexes with pro-apoptotic family members. Switching off MycN suppressed the α-TOS-induced expression of Noxa. However, the lower expression of Noxa in MycN(−) cells, as compared to MycN(+) cells, did not attenuate α-TOS-induced apoptosis, suggesting that in addition to Noxa expression, other mechanisms of OMM permeabilization might be more important for the outcome of α-TOS treatment.
Recently, we reported that α-TOS also activates the MPT-dependent pathway of OMM permeabilization [17]. Together with Bax/Bak-mediated pore formation, this mechanism might be responsible for α-TOS-mediated OMM permeabilization; in particular, release of cytochrome c and stimulation of caspase-3-like activity were observed in mouse embryonic fibroblasts lacking both Bax and Bak. Hence, α-TOS-mediated Ca2+ influx into NB cells and its subsequent accumulation in the mitochondria was suggested to destabilize these organelles and facilitate MPT. The mitochondrial Ca2+ accumulation was important for apoptosis progression, as inhibition of mitochondrial Ca2+ uptake by CCCP, or chelation of intracellular Ca2+ by BAPTA (Fig. 3b), significantly mitigated the apoptotic response.
Which mechanism is predominant in OMM permeabilization is cell-type dependent. We believe that both mechanisms are important for OMM permeabilization. Moreover, there is a certain coordination between them. Thus, as we have shown earlier, treatment of isolated rat liver mitochondria with Bax resulted in stimulation of MPT [20]. In digitonin-permeabilized Tet21 N cells tBid-mediated permeabilization of OMM sensitized mitochondria to Ca2+ loading [17]. Apparently, Bax does not participate directly in Ca2+-mediated permeability transition [21], but Bax and MPT were shown to cooperate in the release of cytochrome c during endoplasmic reticulum stress-induced apoptosis [22]. Collaboration of the two pathways in OMM permeabilization has been confirmed recently by Brustovetsky et al. [23]. The authors showed that recombinant Bax readily integrates and oligomerizes in the OMM in isolated brain mitochondria, but produces only a minute release of cytochrome c. In contrast, Ca2+ and Bax together caused CsA-preventable substantial release of cytochrome c, while Ca2+ alone was not effective. Accumulation of Ca2+ is a prerequisite step for MPT induction. Indeed, perturbation of intracellular Ca2+ homeostasis can cause cytotoxicity and trigger either apoptotic or necrotic cell death [24]. However, MPT might also occur under normal physiological conditions, especially in mitochondria located in close proximity to calcium “hot spots”, microdomains in which the local concentration of ionized calcium by far exceeds the average concentration measured throughout the cytosol [25]. This local Ca2+ concentration might be high enough to induce mitochondrial Ca2+ overload and subsequent pore opening. Further, compounds affecting mitochondrial function, e.g., mitochondrial respiration and production of ROS, or interacting with components of the MPT pore machinery, can compromise mitochondrial Ca2+ buffering capacity and decrease the threshold level of Ca2+ that is normally required for MPT induction. Various compounds, such as ceramide metabolites [26] or palmitate [27], can sensitize mitochondria towards permeability transition. In such conditions, MPT induction and initiation of cell death might also occur in the absence of any significant disruption of intracellular Ca2+ homeostasis [28].
α-TOS triggered cellular Ca2+ uptake by NB cells, which was unaffected by the level of MycN expression. The influx of Ca2+ was followed by its accumulation in mitochondria, induction of MPT, and OMM permeabilization. In addition, the degree of thiol oxidation was similar in α-TOS-treated MycN(+) and MycN(−) Tet21 N cells; oxidative stress sensitizes mitochondria towards permeability transition [29]. Therefore, it appears that MPT largely contributes to OMM permeabilization in α-TOS-induced apoptosis. This is triggered by the influx of extracellular Ca2+ and its subsequent accumulation in the mitochondria. MPT induction is further facilitated by the enhanced ROS production and inhibition of maximal respiratory chain capacity by α-TOS. None of these effects of α-TOS treatment is dependent on the MycN status of the cells, which also does not influence their susceptibility to undergo α-TOS-induced apoptosis.
Although the precise mechanisms of α-TOS action remain to be further investigated, the results obtained so far make it an attractive candidate for antitumor therapy. It has been demonstrated that α-TOS can affect the mitochondrial respiratory chain through interaction with the CoQ-binding site of Complex II. Targeting of Complex II by α-TOS was demonstrated in experiments with xenografts derived from Chinese hamster lung fibroblasts with functional, dysfunctional, and reconstituted Complex II, which revealed that the growth of Complex II-functional and Complex II-reconstituted tumors was strongly suppressed by α-TOS, and that this was accompanied by a high level of apoptosis induction in the tumor cells [30]. Hence, targeting mitochondria by mitocans might be advantageous for the elimination of tumor cells with otherwise dormant apoptotic pathways.
Acknowledgments
The authors are indebted to Prof. Marie Henriksson (Karolinska Institutet, Stockholm) and Dr. John Inge Johnsen (Karolinska Institutet, Stockholm) for providing the cell lines used in the study. The work was supported by grants from the Swedish Research Council, the Swedish and the Stockholm Cancer Societies, the Swedish Childhood Cancer Foundation, the EC FP-6 (Chemores), the Russian Ministry of High Education and Science (11.G34.31.0006), and the EC FP7 (Apo-Sys) programs.
References
- 1.Cole MD, Henriksson M. 25 years of the c-Myc oncogene. Semin Cancer Biol. 2006;16:241. doi: 10.1016/j.semcancer.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Fulda S, Lutz W, Schwab M, Debatin KM. MycN sensitizes neuroblastoma cells for drug-induced apoptosis. Oncogene. 1999;18:1479–1486. doi: 10.1038/sj.onc.1202435. [DOI] [PubMed] [Google Scholar]
- 3.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006;1757:639–647. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Hagland H, Nikolaisen J, Hodneland LI, Gjertsen BT, Bruserud O, Tronstad KJ. Targeting mitochondria in the treatment of human cancer: a coordinated attack against cancer cell energy metabolism and signalling. Expert Opin Ther Targets. 2007;11:1055–1069. doi: 10.1517/14728222.11.8.1055. [DOI] [PubMed] [Google Scholar]
- 5.Ralph SJ, Neuzil J. Mitocans, a class of emerging anti-cancer drugs. Mol Nutr Food Res. 2009;53:7–8. doi: 10.1002/mnfr.200890054. [DOI] [PubMed] [Google Scholar]
- 6.Prasad KN, Edwards-Prasad J. Effects of tocopherol (vitamin E) acid succinate on morphological alterations and growth inhibition in melanoma cells in culture. Cancer Res. 1982;42:550–555. [PubMed] [Google Scholar]
- 7.Qian M, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate induces apoptosis in avian retrovirus-transformed lymphoid cells. Nutr Cancer. 1996;25:9–26. doi: 10.1080/01635589609514424. [DOI] [PubMed] [Google Scholar]
- 8.Ottino P, Duncan JR. Effect of alpha-tocopherol succinate on free radical and lipid peroxidation levels in BL6 melanoma cells. Free Radic Biol Med. 1997;22:1145–1151. doi: 10.1016/S0891-5849(96)00529-1. [DOI] [PubMed] [Google Scholar]
- 9.Fariss MW, Nicholls-Grzemski FA, Tirmenstein MA, Zhang JG. Enhanced antioxidant and cytoprotective abilities of vitamin E succinate is associated with a rapid uptake advantage in rat hepatocytes and mitochondria. Free Radic Biol Med. 2001;31:530–541. doi: 10.1016/S0891-5849(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 10.Neuzil J, Wang XF, Dong LF, Low P, Ralph SJ. Molecular mechanism of ‘mitocan’-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins. FEBS Lett. 2006;580:5125–5129. doi: 10.1016/j.febslet.2006.05.072. [DOI] [PubMed] [Google Scholar]
- 11.Lutz W, Stohr M, Schurmann J, Wenzel A, Lohr A, Schwab M. Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene. 1996;13:803–812. [PubMed] [Google Scholar]
- 12.Chen L, Iraci N, Gherardi S, Gamble LD, Wood KM, Perini G, Lunec J, Tweddle DA. p53 is a direct transcriptional target of MYCN in neuroblastoma. Cancer Res. 2010;70:1377–1388. doi: 10.1158/0008-5472.CAN-09-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Junttila MR, Evan GI. p53–a Jack of all trades but master of none. Nat Rev Cancer. 2009;9:821–829. doi: 10.1038/nrc2728. [DOI] [PubMed] [Google Scholar]
- 14.Petroni M, Veschi V, Prodosmo A, Rinaldo C, Massimi I, Carbonari M, Dominici C, McDowell HP, Rinaldi C, Screpanti I, Frati L, Bartolazzi A, Gulino A, Soddu S, Giannini G. MYCN sensitizes human neuroblastoma to apoptosis by HIPK2 activation through a DNA damage response. Mol Cancer Res. 2011;9:67–77. doi: 10.1158/1541-7786.MCR-10-0227. [DOI] [PubMed] [Google Scholar]
- 15.Prochazka L, Dong LF, Valis K, Freeman R, Ralph SJ, Turanek J, Neuzil J. alpha-Tocopheryl succinate causes mitochondrial permeabilization by preferential formation of Bak channels. Apoptosis. 2010;15:782–794. doi: 10.1007/s10495-010-0482-z. [DOI] [PubMed] [Google Scholar]
- 16.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. doi: 10.1042/0264-6021:3410233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gogvadze V, Norberg E, Orrenius S, Zhivotovsky B. Involvement of Ca2 + and ROS in alpha-tocopheryl succinate-induced mitochondrial permeabilization. Int J Cancer. 2010;127:1823–1832. doi: 10.1002/ijc.25204. [DOI] [PubMed] [Google Scholar]
- 18.Valis K, Prochazka L, Boura E, Chladova J, Obsil T, Rohlena J, Truksa J, Dong LF, Ralph SJ, Neuzil J. Hippo/Mst1 stimulates transcription of the proapoptotic mediator NOXA in a FoxO1-dependent manner. Cancer Res. 2011;71:946–954. doi: 10.1158/0008-5472.CAN-10-2203. [DOI] [PubMed] [Google Scholar]
- 19.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 20.Gogvadze V, Robertson JD, Zhivotovsky B, Orrenius S. Cytochrome c release occurs via Ca2+-dependent and Ca2+-independent mechanisms that are regulated by Bax. J Biol Chem. 2001;276:19066–19071. doi: 10.1074/jbc.M100614200. [DOI] [PubMed] [Google Scholar]
- 21.De Marchi U, Campello S, Szabo I, Tombola F, Martinou JC, Zoratti M. Bax does not directly participate in the Ca(2 +)-induced permeability transition of isolated mitochondria. J Biol Chem. 2004;279:37415–37422. doi: 10.1074/jbc.M314093200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, Armstrong JS. Bax and the mitochondrial permeability transition cooperate in the release of cytochrome c during endoplasmic reticulum-stress-induced apoptosis. Cell Death Differ. 2007;14:703–715. doi: 10.1038/sj.cdd.4402072. [DOI] [PubMed] [Google Scholar]
- 23.Brustovetsky T, Li T, Yang Y, Zhang JT, Antonsson B, Brustovetsky N. BAX insertion, oligomerization, and outer membrane permeabilization in brain mitochondria: role of permeability transition and SH-redox regulation. Biochim Biophys Acta. 2010;1797:1795–1806. doi: 10.1016/j.bbabio.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Natl Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 25.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 26.Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koshkin V, Dai FF, Robson-Doucette CA, Chan CB, Wheeler MB. Limited mitochondrial permeabilization is an early manifestation of palmitate-induced lipotoxicity in pancreatic beta-cells. J Biol Chem. 2008;283:7936–7948. doi: 10.1074/jbc.M705652200. [DOI] [PubMed] [Google Scholar]
- 28.Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Roy SS, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J Biol Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 30.Dong LF, Freeman R, Liu J, Zobalova R, Marin-Hernandez A, Stantic M, Rohlena J, Valis K, Rodriguez-Enriquez S, Butcher B, Goodwin J, Brunk UT, Witting PK, Moreno-Sanchez R, Scheffler IE, Ralph SJ, Neuzil J. Suppression of tumor growth in vivo by the mitocan alpha-tocopheryl succinate requires respiratory complex II. Clin Cancer Res. 2009;15:1593–1600. doi: 10.1158/1078-0432.CCR-08-2439. [DOI] [PubMed] [Google Scholar]